UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1−K
ANNUAL REPORT PURSUANT TO REGULATION A
OF THE SECURITIES ACT OF 1933
For the fiscal year ended December 31, 2021
Oracle Health, Inc.
(Exact name of issuer as specified in its charter)
| Delaware | | 84-1730527 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
910 Woodbridge Court, Safety Harbor, FL 34695
(Full mailing address of principal executive offices)
(727) 470-3466
(Issuer’s telephone number, including area code)
Common Stock
Title of each class of securities issued pursuant to Regulation A
TABLE OF CONTENTS
Use of Terms
Except as otherwise indicated by the context and for the purposes of this report only, references in this report to “we,” “us,” “our,” “our company” or “Future Cardia” refer to Oracle Health, Inc., a Delaware corporation.
Special Note Regarding Forward Looking Statements
Certain information contained in this report includes forward-looking statements. The statements herein which are not historical reflect our current expectations and projections about our company’s future results, performance, liquidity, financial condition, prospects and opportunities and are based upon information currently available to our company and our management and our interpretation of what is believed to be significant factors affecting the businesses, including many assumptions regarding future events.
Forward-looking statements are generally identifiable by use of the words “may,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” or “project” or the negative of these words or other variations on these words or comparable terminology. Actual results, performance, liquidity, financial condition, prospects and opportunities could differ materially from those expressed in, or implied by, these forward-looking statements as a result of various risks, uncertainties and other factors. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Item 1. Business─Risk Factors” below, and matters described in this report generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this report will in fact occur.
Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
ITEM 1. BUSINESS
Overview
Founded in May 2019, we are a medical device technology startup focusing on the development of a tiny insertable cardiac monitoring device to monitor heart failure. Our monitoring device is designed to detect signs of heart failure early enough so that non-hospital treatments can be administered and, thus, hospitalizations that typically result from heart failure can be reduced and prevented. Our tiny insertable cardiac monitor utilizes a multi-sensor approach and cloud-based pattern recognition (machine learning) to monitor chronic heart failure. We filed a provisional patent application that covers the technology related to our insertable cardiac device, software dashboard, smartphone app and data accumulation techniques in May 2019, and a non-provisional utility patent application in May 2020 (Patent application No. 62/853,899). We expect to submit our insertable cardiac device to the FDA for review under the FDA’s 510k framework around mid-2023, and we are on schedule for a patient ready insertable cardiac device for human implant under the first in man clinical study by December 2022.
Our tiny insertable monitoring device, which has not yet been cleared by the FDA, will offer a long-term solution to heart failure monitoring that, we believe, features simplicity, improved accuracy, high patient protocol compliance and hospital economics. Our device is equipped with multi-sensors to track trending changes in heart performance, including heart rhythms, electrocardiogram (ECG or EKG), and heart and lung sounds and activities, to monitor heart failure using telemedicine and machine learning technology.
Our Industry
The U.S. boasts the largest medical device market in the world, with Select USA reporting that the market reached $156 billion in size in 2017. By 2023, industry experts project the U.S. market to grow to $208 billion. The market is comprised of articles, instruments, apparatuses, or machines that are used for preventative, diagnostic, or treatment purposes. According to Select USA, this industry includes nearly two million direct and indirect jobs in the U.S., with over 80% of medical devices companies operating at under 50 employees.
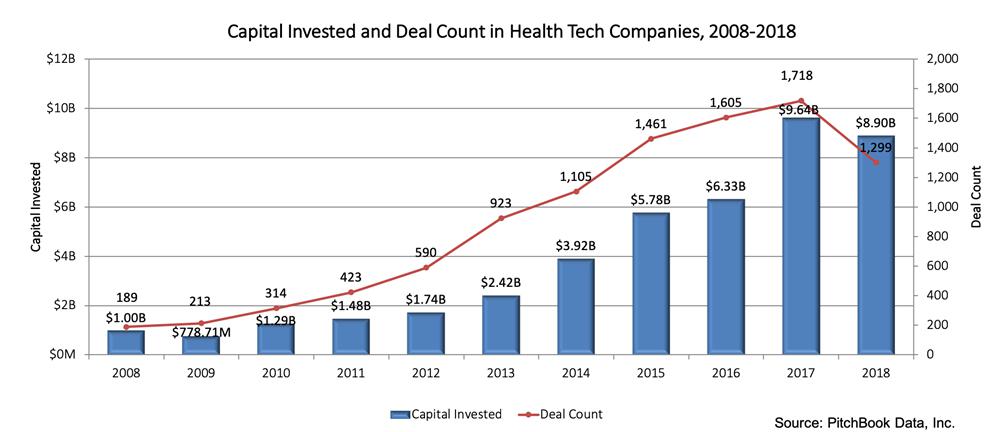
In 2018, venture capital in health tech companies reached $8.9 billion across 1,299 total deals. Health tech companies received record highs in investment amount ($9.64 billion) and deal count (1,718) in 2017. From 2016 to 2017, there was a 52% year-over-year increase in capital invested. According to PitchBook Data, between 2008 and 2018, more than $43 billion was invested across 9,840 venture capital deals in health tech companies.
The U.S. Center for Disease Control and Prevention, or the CDC, estimates that roughly 5.7 million adults in the U.S. have heart failure, which is defined as a chronic, progressive condition in which the heart cannot pump enough blood and oxygen to support other organs in the body. The CDC also reported that heart failure costs the U.S. approximately $30.7 billion each year in health care services, medications, and missed days of work from patients with this condition. An Emory University study shows that there are nearly 550,000 new cases of heart failure diagnosed in the U.S. each year. According to the Emory University Study, this condition is responsible for 11 million physician visits each year, and even more hospitalizations than all forms of cancer combined.
According to St. Jude Medical, heart failure market revenue exceeded $4 billion in 2016.
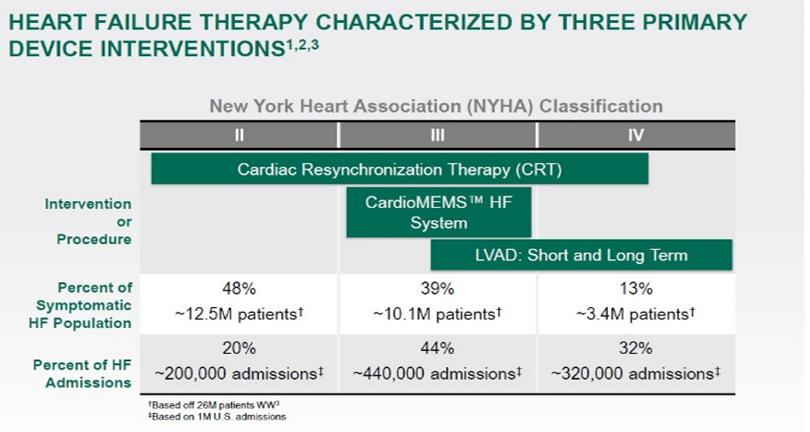
The chart below, presented at the St. Jude Medical 2016 Analyst and Investor Meeting, shows certain measurements relating to the effect of two types of device intervention therapies and one monitoring solution, the CardioMEMS HF System (then owned by St. Jude) on the three classifications of heart failure established by the New York Heart Association, or NYHA (i.e., Class II, mild symptoms of cardiac disease resulting in slight limitation on physical activities, Class III, moderate symptoms, and Class IV, severe symptoms).
With the cardiac resynchronization, or CRT, therapy, specialized pacemakers or defibrillators with three wires (or leads) were implanted in the chests of patients to restore the normal timing pattern of the heartbeat; with left ventricular assist device, or LVAD, therapy, mechanical pumps were implanted inside patients’ chests to help weakened hearts pump blood; and with the CardioMEMS HF System, a small pressure-sensing device was implanted directly into the patients’ pulmonary arteries and, without needing to visit the hospital of doctor’s office, information was regularly sent wirelessly to the patients’ care teams.
Based on the NYHA’s data, it can be seen from the chart that approximately 25 million heart failure patients, across the three heart failure classifications, were symptomatic and could benefit from some type of therapy. However, despite the three referenced available therapy solutions, of the symptomatic patients, approximately one million still needed to be hospitalized - 20% of whom were categorized as Class II, 44% as Class III and 32% as Class IV, we believe, due to lack of viable monitoring solutions. The conclusion that we draw from this chart is that there is a need for additional monitoring solutions for the three classes of cardiac disease patients.
Reducing heart failure related hospital visits through the use of implantable cardiac monitors is a first step in addressing this national health problem (Multi-Sense and CardioMems data). Research and Markets reports that the global implantable cardiac monitors market is projected to reach $682 million by 2023, growing at a 7.6% compound annual growth rate, or CAGR, from 2017 to 2023 (this report only focused on arrhythmia monitoring devices). According to Research and Markets, growth is expected to be driven by the miniaturization of these devices, as well as the growing demand for cardiac monitors that can continuously monitor heart health and detect any abnormalities.
Wearable devices have grown in popularity among consumers for their ability to simply monitor activity and heart rates for fitness purposes. Transparency Market Research valued the global wearable medical devices market at $6.8 billion in 2017. According to Transparency Market Research, the market is forecasted to grow at a CAGR of 17% from 2018 to 2026, in part due to the expansion of the health care industry, government initiatives that promote wearables, additional health care expenditure, and increased product approvals.
In addition, consumers have grown increasingly comfortable with digital health services and technologies. Not only are consumers going digital out of curiosity or for general fitness and well-being, but with the intention to address and treat real, concrete health needs. According to Rock Health, these consumers are using digital health solutions to manage diagnoses, connect with providers, and make critical healthcare decisions. We expect that this trend might accelerate as a result of the COVID-19 pandemic.
88% of respondents to a Rock Health survey reported using a digital health tool in 2018, up from 80% in 2015. The most widely used digital health tools – online health information, online provider reviews, mobile tracking, wearables, and live video telemedicine – also saw increased adoption year-over-year in 2018. In particular, live video telemedicine surged in 2018, increasing more than 100% year-over-year from 2017 to 2018 according to Rock Health. The chart below presents this data in graphic form.

According to the Rock Health survey results, the following number of patients/users were surveyed: in 2015, 4,017 patients; in 2016, 4,015 patients; in 2017, 3,997 patients; and in 2018, 4,000 patients.
Our Market
Heart failure is a major public health problem affecting more than 6 million patients in the United States and more than 23 million patients worldwide. Patients with heart failure have high morbidity and mortality rates. According to the American Heart Association, one in five persons in the U.S. will develop heart failure in their lifetime and 50% of those who develop heart failure will die within 5 years. The projected cost to the U.S. healthcare system is expected to reach $70 billion by 2030 and the burden of heart failure related hospitalizations represents 80% of costs attributed to heart failure care. Thus, accurate monitoring and timely detection of worsening heart failure may reduce heart failure admissions.
Current Approaches
Patients’ self-tracking of daily weights, a lagging indicator of impending heart failure decompensation (that is, the inability of the heart to maintain adequate circulation) has not proven to be effective in preventing episodes of decompensation (See Figure 1, below). Traditional physiologic markers such as abnormal increases in patient weight (that is, fluid accumulation and/or swelling of the lower extremities) occur late in the decompensation process (approximately five days prior to hospitalization, according to Figure 1 below) and leave little time to react before hospitalization. The ineffectiveness of self-tracking of daily weight changes is due to the reactive nature of having to act quickly on late-in-the-cycle data that is represented by the weight gain information. By the time the patient is presented with notable weight gain, the patient is already at the heart failure decompensation stage, requiring immediate intervention.
Numerous studies that evaluated the decompensation parameter’s ability to reduce heart failure related hospitalizations have indicated that this methodology has failed. Traditional clinical observations, and intrathoracic impedance monitoring (measuring the liquid accumulation in the lungs) have also failed to provide sufficient insight into patient decompensation and have not led to a decrease in hospital readmissions according to Link-HF (study 1, 2 and 3).
The development of wearable monitors, which have gained in popularity in recent years and have been thought to provide a simple to implement heart monitoring solution, have not resulted in an improvement in patient outcomes. Although a few of the devices have shown promise in the short-term (days to weeks), these devices have not proven beneficial for the long-term chronic management of heart failure as they suffer from low accuracy, poor patient compliance, lack of sustainability and lack of physician adoption. Additionally, despite improved accuracy, invasive catheterization, or Cardiac Cath Lab, procedures are complex and expensive and have suffered from low adoption by physicians and hospitals.

Figure 1. Adapted from Emani. Current Heart Failure Rep. 2017:14(1):40-7
Note: The dotted white lines in Figure 1 represent the points in time prior to hospitalization that the indicated biomarkers begin to show.
It has been hypothesized that the development of physiological parameters, while associated with more variations in a population, may provide improved sensitivity and specificity, given that an individual’s baseline measurements are used to evaluate future events. Studies (e.g., Characterization of Cardiac Acoustic Biomarkers in Patients with Heart Failure (the “Burkhoff Study”) - https://onlinelibrary.wiley.com/doi/full/10.1111/anec.12717) have shown that early detection of signs related to acute worsening of heart failure through the use of heart sounds as a cardiac acoustic biomarker may provide insight regarding the timing of treatment interventions, leading to a decrease in hospitalizations. In the Burkhoff study, 1060 patients with heart failure were tested using a wearable cardioverter defibrillator (WCD). This WCD device recorded ECG and cardiohemic vibrations (i.e., heart sounds) as well other notable cardiac data that can be algorithmically combined to provide cardiac acoustic biomarkers of heart failure patients. The Burkhoff Study provided critical information about typical values in heart failure patients, intra-subject variability, circadian rhythms, and changes over time of these parameters. The Burkhoff Study concluded that short term noninvasive cardiac acoustic biomarkers offer the possibility to assess parameters associated with heart function, clinical status, and other aspects of cardiovascular physiology that differ between normal and heart failure states.
A recent Multi-Sense clinical trial, which enrolled 900 patients suffering from heart failure, who each received a cardiac resynchronization therapy implantable cardioverter defibrillator device provided the initial proof that a multi-parameter approach in an implantable device improved the care of the heart failure patient and reduced future heart failure admissions. The HeartLogic (Boston Scientific) index, which combines data from multiple implantable cardioverter defibrillator (ICD)-based sensors, was able to detect 70% of the impending heart failure events with a median 34 days’ warning. Additional evidence for physiologic (hemodynamic) assessment of the heart failure patient was demonstrated in the CHAMPION trial where one-year remote monitoring of a CardioMEMS Champion heart failure monitoring system resulted in an $11,260 reduction for Heart failure related hospitalizations compared with the year before the device implant trial. These two successful trials clearly demonstrated that the use of the “physiologic data” approach is superior to that of patient weights and intrathoracic impedance.
The burden of employing devices such as those referenced above lies in the cost and invasive placement procedure and the complexities of addressing the needs of different heart failure populations. Patient populations include those requiring correction of left bundle branch block, or LBBB, with a low ejection fraction, or EF (a measurement, expressed as a percentage, of how much blood the left ventricle pumps out with each contraction), those who suffered a myocardial infarction (a heart attack), or MI, or who are thought to be at risk for this event and those patients with heart failure and normal conduction physiology and no previous MI, that is, heart failure with preserved (or normal) ejection fraction, or HFpEF. Currently these devices are not indicated for patients with HFpEF.
Our Solution
The market for insertable cardiac monitors needs a solution that brings simplicity, improved accuracy, high patient protocol compliance and hospital economics to heart failure monitoring. Based on the criteria discussed below and equipped with strong clinical evidence on heart sounds as a biomarker, we are developing a subcutaneously (SQ, under the skin) insertable device with multi-sensors to check for trending changes in heart performance to monitor heart failure using telemedicine and machine learning technology.
Our insertable cardiac monitoring device will monitor the following heart related functions with the indicated tools:
| ● | heart rhythms (electrocardiogram (ECG)); |
| ● | heart and lung sounds (phonocardiogram (PCG)); and |
| ● | body motion, activity and orientation (three axis accelerometer) |
The current unmet need in the HFpEF market segment is significant and growing in terms of patient numbers and heart failure admissions. Along with this current unmet need is the issue of “treatment solutions for HFpEF.” Segments or phenotypes of the HFpEF population may be served with similar algorithms to those that treat heart failure with reduced (that is, abnormal) ejection fraction, or HFrEF (also referred to as systolic heart failure). However, the disease process associated with HFpEF is very distinct from that associated with HFrEF.
The development of solutions for HFpEF has led investigators to take a look at the phenotypes that may present. A meeting of experts in this space took place at National Institute of Health, the NIH, in June 2017. Several key insights developed. First, should clinical trials enrolling HFpEF patients focus on all HPpEF patients or focus on smaller subpopulations (phenotypes). Most clinical trials adopt broad inclusion criteria, to ensure that the primary objective of the trial has adequate statistical power. The downside of this approach, to enroll all patients with the HFpEF but presenting with a variety of phenotypes, is the resulting uncertainty as to the primary factor(s) causing heart failure in each phenotype. This may lead to trials that include additional variables, that while well understood for the HFrEF population, remain to be evaluated in the HFpEF segment. For instance, elevated B-type natriuretic peptide (BNP) levels are prognostic for HFpEF but are lower in the HFrEF population for any level of this specific heart failure. Including BNP in a broad inclusion HFpEF trial may lead to data that cannot be quantified and analyzed, without a very large sample size. Similarly, the phenomenon of patients with HFrEF that does not respond to cardiac resynchronization therapy (CRT) has resulted in more than 500 published papers. These papers have focused on possible mechanisms responsible for the non-responder issue. As a result of this effort, new pre-implant and post-implant solutions have been successfully identified and solutions have been developed during the 19 years that CRT has been on the market. At the time of the CRT launch, insufficient knowledge into the complex nature of heart failure with LBBB prevented “phenotype” based tools from being used to improve the responder rate. In regard to HFpEF, the scientific knowledge specific to the phenotype is more mature than that known for HFrEF and CRT. It is reasonable that the use of a multi-sensor device such as our insertable cardiac monitor will allow greater care of the HFpEF population compared to the early days of device management in the HFrEF population.
More than 35 trials have been developed to evaluate a possible “solution” for HFpEF. These studies and other investigations have concluded that the unique pathophysiology may include a variety of phenotypes; 1) at-risk metabolic disorders, 2) pulmonary vascular disease, 3) elevated atrial pressure 4) those presenting with coronary microvascular dysfunction, and alterations in titin physiology, to name a few. These phenotypes may or may not confound a trial’s primary objective.
Many HFrEF trials included improvements in quality of life and functional capacity as primary or secondary endpoints. These measures were not necessarily correlated with morbidity and mortality. The improvements in quality of life and/or functional capacity did not lead to a similar reduction in morbidity and mortality rate. The HFrEF population may have “lived” more comfortably but did not live longer. The HFpEF disease appears at a later age than the HFrEF and improvements addressing this population appear to be focused on an improvement in functional capacity. As current pharmaceutical treatments for cardiovascular disease (CVD) do not improve this group’s functional capacity, reducing the impact of the disease variables on this functional capacity parameter is key. Symptoms commonly observed in the HFpEF population, including reduced exercise tolerance (impacting daily activities), dyspnea (impacting respiratory rate) and declining cardiac function (heart sounds) are the focus on the Future Cardia device. A combination of pharmaceutical therapies tailored to these “physiologic markers” may reduce the need for heart failure admissions. The number one goal for health care providers is to prevent morbidity and mortality. From a patient perspective, their goal, while to stay alive, is really to live with a reasonable functional capacity. We believe that our insertable cardiac monitoring device can help achieve this objective.
Our Product Features
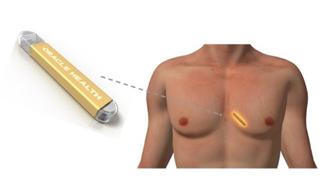
Future Cardia’s insertable cardiac monitor is designed to be a super-minimally invasive solution that can effectively help cardiologists monitor and treat their heart failure patients. Our insertable cardiac monitor is comprised of the following features:
| ● | Under the skin insertable device transmits secured data to a smartphone with advanced antenna and sensor technologies; |
| ● | Smartphone automatically pushes the data up to cloud-based pattern recognition software (machine learning); and, |
| ● | Clinicians log on to a portal to assess cardiac performance. |
The diagram below illustrates this structure.

Insertion Procedure
Future Cardia’s insertable cardiac monitor utilizes commonly used insertion procedure. This is a 2-minute office-based procedure commonly used by thousands of cardiologists every day.
 |
| | | | | |
Cardiologist numbs a small region and
then makes ¼ inch incision. | | Cardiologist inserts the device,
just under the skin. | | After device is inserted, physician then
covers the incision area with
a special sterile bandage. |
Heart Monitoring
After the minimally invasive insertion process, our insertable cardiac monitor is able to listen to heart sounds and record electrocardiography (ECG). Based on currently available batter technology and longevity, which we intend to incorporate into our monitoring device, we expect our device to be able to continuously transmit monitoring signals for up to three years. Cardiologists can monitor patient data and trends in heart performance using our proprietary software dashboard. Our insertable cardiac monitoring platform will use cloud-based machine learning to analyze all of a patient’s data and help cardiologists efficiently determine the next steps in patient care.
Our Competition
Our primary competitors are Abbott, Medtronic, Sensible Medical Innovations, VitalConnect and ReThink Medical. The following is a description of each of these competitors.
Abbott Laboratories (NYSE: ABT): Abbott Laboratories is an Illinois-based healthcare company that sells medical devices, diagnostics, medicines, and nutritional products to treat a wide range of health problems, including cardiovascular diseases. The cardiovascular disease division at Abbott has many internal divisions that are designed to solve specific heart problems. Organizations within the cardiovascular disease division include structural heart, heart failure, cardiac rhythm management, electrophysiology, peripheral intervention, vessel closure, carotid intervention, and coronary intervention. The heart failure organization has over five products that are designed to help physicians and patients more effectively monitor and manage heart failure. The CardioMems HF System is a monitoring device that is implanted directly into a patient’s pulmonary artery that then sends information wirelessly to the patient’s doctor. Abbott reports that this device has been clinically proven to reduce hospital admission by 58% over an average of 12 months. In 2018, Abbott Laboratories reported that its Heart Failure division earned $646 million in total revenue, of which about 72% came from sales made in the U.S.
Medtronic (NYSE: MDT): Founded in 1949, Medtronic is a medical device company that designs and sells devices for a range of medical uses. Heart failure and cardiac rhythm is one such medical issue that Medtronic sells devices to treat, with the company offering implantable cardiac pacemakers, implantable cardioverter defibrillators, implantable cardiac resynchronization therapy devices, AF ablation product, insertable cardiac monitoring systems, and mechanical circulatory support products. The Medtronic LINQ is the company’s flagship Insertable Cardiac Monitoring system product, which is designed to record the heart’s electrical activity before, during, and after transient symptoms, as well as assist in diagnosis. In the 2019 fiscal year (which ended on April 26, 2019), Medtronic reported $5.84 billion in revenue from its cardiac rhythm and heart failure group, down from $5.94 billion in 2018.
Sensible Medical Innovations: Sensible Medical Innovations is an Israeli company that aims to lead a new standard of care in heart failure. Initially used in the military, the company’s medical radar (ReDS) monitoring technology has been adapted for medical use to help physicians deliver a non-invasive solution to heart failure patients. By implementing this product, Sensible Medical claims that healthcare professionals are able to measure a patient’s lung fluid, which is a key data point for heart failure patients. Additionally, Sensible Medical’s solution can be used both at home and in a clinic. In May 2019, Sensible Medical entered into an agreement with Bayer for the use of Sensible’s ReDS technology. Sensible Medical raised a $20 million financing round led by Boston Scientific in November 2013, which was the company’s last disclosed funding.
VitalConnect: Headquartered in Silicon Valley, California, VitalConnect is developing an external sticky patch device to monitor cardiac functions. The company was founded in 2011, has received total funding of $86 million and has completed a 100 patient clinical trial of its sticky patch device in the U.S. (LINK HF).
ReThink Medical: San Francisco-based ReThink Medical is a health tech startup that has designed medical devices to help monitor heart failure. The company’s wrist-worn monitoring device is equipped with technology that is designed to predict and prevent heart failure hospitalizations. The device uses machine learning, artificial intelligence, and continuous physiologic monitoring to detect worsening symptoms. In May 2017, ReThink Medical raised a $3 million Series A round led by Emergent Medical Partners.
Currently there are several medical device companies competing in this field. However, we believe our product will bring simplicity, improved accuracy, high patient protocol compliance and hospital economics to the monitoring of heart failure compared to other devices in the market.
Our Competitive Advantages
We believe that the following characteristics of our product will provide us with a competitive advantage once our implantable monitoring device is fully developed and FDA cleared:
| ● | Simple Procedure – Our monitoring device, which can be inserted under the skin in a two minute office visit procedure, features a simpler approach than other devices currently on the market that require femoral vein access or a sensor implantation inside the heart or pulmonary artery. |
| ● | More Accurate Data and Comprehensive Diagnostics – Our heart monitoring device utilizes a unique set of multi-sensors (ECG, acoustic sensor for heart & lung sounds, and activity sensor/accelerometer), and it has been shown that heart failure monitoring with multiple sensors yields improvements in accuracy (sensitivity) of up to 70% (based on clinical data provided in Boston Scientific’s Multi-Sense clinical trial) compared to more traditional methods of monitoring. We expect that our multiple sensor heart monitoring device will also display such improved accuracy. |
| ● | High Patient Protocol Compliance – Patients do not need to wear any equipment or worry about following a detailed or cumbersome long term regiment such as those that are required in connection with the use of currently available wearable or implantable heart monitoring solutions. |
| ● | Telemedicine – Using smartphone, WiFi and cellular networks, all device output data is securely transmitted by the inserted device to a smartphone, then to a cloud based AI system for data analytics. |
| ● | Existing Healthcare Reimbursements – Our device leverages existing Current Procedural Terminology (CPT code 33285) to streamline the healthcare reimbursement process. |
Our Development Highlights
2019
| ● | Completed ZeroTo510 Med Tech Accelerator (GAN Accelerator) |
| ● | Proof of concept device developed in August 2019 for animal lab and human testing |
| ● | Accepted to Johnson & Johnson’s life sciences incubator, or JLABS, in November 2019 |
| ● | Signed research agreement with Maastricht University for animal and human testing |
2020
| ● | 8 heart failure patient data studies completed (Maastricht University study- proof of concept device that records ECG and measures heart sounds through an acoustic sensor in a non-invasive, external approach) |
| ● | Non-provisional utility patent filed in May 2020 |
| ● | Accepted to Tampa Bay Wave Accelerator (GAN Accelerator) in May 2020 |
| ● | In discussions with nationally recognized heart failure clinics for planned human trials |
| ● | Hardware and software team identified |
| ● | Dr. Dan Burkhoff, MD, PhD, and Dr. Kevin Heist, MD, PhD, joined our advisory team in July 2020 |
| ● | In discussions with a leading medical microelectronics development, design and manufacturing company for the acquisition of our implantable battery solution |
| ● | In contract discussions with ISO certified medical device manufacturer with expertise in implantable cardiac devices for the manufacture of our heart failure device |
| ● | Accepted to four week “Boot Camp” at the TMCx Accelerator of the Texas Medical Center, in October 2020. |
2021
| ● | In January 2021, we started feasibility testing of our technology and upon positive review, we proceeded to R&D phase for human implant. |
| ● | In February 2021, we obtained a key battery technology to expedite our development. |
| ● | In July 2021, we filed for a second patent that encompasses the wireless antenna technology (Patent application No. 17/443,899). We commenced engineering related to this patent in January 2021 with a contract design firm that specializes in implantable cardiac devices. |
| ● | In summer 2021, we were accepted into the “YCombinator – Summer Batch 2021.” However, we declined participation in favor of our capital raise with investors on the Republic platform then and other venture firms and potential strategic partners. |
| ● | By September 2021, we had identified and made arrangements with engineering and data analysis partners for the commercialization of our device. |
2022 Q1
| ● | In January 2022, we were accepted into the Stanford StartX accelerator program. Stanford StartX is the premier accelerator program in the world with one of the highest success rates, with ten (10) startups achieving unicorn status in the past 12 years. StartX companies have a combined valuation of over $30 billion and 92% survival rate. Over 300 Silicon Valley mentors participate in the program to create one of the most powerful networks of entrepreneurs, professors, scientists and investors. |
| ● | In January 2022, we had selected our key engineering and manufacturing facilities. |
| ● | In February 2022, we had identified a principal investigator who will lead the first in human implant clinical trial. |
| ● | In March 2022, we had successfully implanted our prototype device in animal lab to demonstrate near real world scenario. This testing showed the device’s embodied antenna and sensor technology as well as data acquisitions and analytics capabilities. This will allow us to conduct additional testings before expected human implant in December 2022. |
Our Maastricht University Research Collaboration
On September 21, 2019, we entered into a research collaboration agreement with Maastricht University, more specifically with the Faculty of Health, Medicine and Life Sciences/School for Cardiovascular Diseases, or CARIM, to investigate to what extent the analysis of heart sounds is comparable or complementary to standard care non-invasive assessments in diagnosing HFpEF and whether heart sounds may contribute to better classification within the HFpEF population. This research collaboration entails a study of a proof of concept model of our heart monitoring device in 30 patients referred to the HFpEF outpatient clinic at Maastricht University Medical Center. The Maastricht proof of concept device is about the size of a 9V battery and is equipped with an ECG recorder and an acoustic sensor to monitor and measure heart rhythm and heart sounds. Data that is externally recorded by the device during a treadmill test is wirelessly transmitted to a smartphone for analysis. This testing has enabled us to acquire heart failure patients’ cardiac data (ECG and heart sounds in a digital format) for input into a non-proprietary, generally available machine learning analysis program.
Although information provided by the testing of eight heart failure individuals is only anecdotal, after treadmill exercises and the recording of ECG and heart sounds, we were able to observe, as hypothesized, acute cardiac performance changes in this test group that are not seen in persons with normal heart function. Shortly after the treadmill exercise, the diseased heart showed that it is not able to pump effectively. The pumping amplitude was reduced, and the frequency of each pumping (width) was widened in contrast to healthy hearts.
The term of the Maastritch agreement is for one year; however, the agreement will remain in effect, upon written agreement between the parties, until the study, in progress, is completed. To date we have collected data for over 35 patients and are analyzing this data for publication of the findings. Under the terms of this collaboration, we have agreed to pay CARIM a fee of 5,000 Euro for the execution of the study and the transfer of the study output data to us. All rights resulting from developments connected to the research project as performed by us will vest in us, and ownership of any invention, modification or improvement developed during the research project and relating to our device will vest in us as well. We will be responsible for all patent process costs if we determine to file any patent applications based on the research project outcomes.
Our Product Launch Roadmap
We plan to launch our product as soon as practicable by taking the following steps:
| ● | complete patient ready device development (design and engineering) for an early feasibility study and first in man implant; |
| ● | complete 30 implants along with data analysis - Because our heart monitor is a 510k Class II device, we are not required to show effectiveness in humans in order to obtain 510k clearance. In the resubmission meeting with the FDA, FDA staff suggested conducting an observational clinical trial with a small number of clinic centers; |
| ● | submit Pre-Sub to FDA followed by obtaining FDA 510k clearance; |
| ● | penetrate Texas and Florida accounts (two of the highest implant volume states in the US); |
| ● | commercialize and expand to other high-volume regions outside of Texas and Florida, and further refine the product; and |
| ● | solidify commercialization and earn market share from competitors and explore the application of our device in respiratory space. |
Our Sales and Marketing
We intend to operate a multi-distribution model for the sales of our device. We will use internal sales managers who will work directly with hospitals and independent pacer representatives who then sell medical devices to cardiologists. Initially, we expect to focus on cardiologists in Texas and Florida where we already have pre-existing relationships.
Our Product Engineering
In January 2021, we entered into a confidentiality agreement with a top U.S. medical device manufacturer for the manufacturing design and production process for our implantable medical device. We estimate a per unit manufacturing cost of approximately $1,000, and we anticipate that our device will retail for approximately $5,300.
In January 2022, we established an engineering design team and selected implantable medical devices manufacturing firms to solidify our development for human implant.
Our Intellectual Property
We acquired the intellectual property for our implantable heart failure monitoring device from our founder and CEO, Jaeson Bang for consideration of $100. On April 19, 2021, we entered into an assignment agreement with Mr. Bang, pursuant to which Mr. Bang transferred all of his right, title and interest in the intellectual property assets, including the patent applications listed in the table below and the inventions described and embodied therein to Oracle Health, Inc. for $100 in cash. The foregoing description of the assignment agreement does not purport to be complete and is qualified in its entirety by reference to Exhibit 6.1 to this annual report on Form 1-K.
| Docket Number | | Title | | Application No. | | Patent No. | | Status |
| OCL-001-PCT | | Implantable Cardiac Monitor* | | 62/853,899 | | N/A | | Pending |
| OCL-001-PR1 | | Implantable Cardiac Monitor* | | PCT/US20/35171 | | N/A | | Pending |
| * | In May 2019, a provisional patent application that covers the technology related to our implantable cardiac device, software dashboard, smartphone app and data accumulation techniques was filed by Jaeson Bang with the United States Patent and Trademark Office, or USPTO. In October 2019, we completed a patentability assessment. In May 2020, Jaeson Bang, R. Maxwell Flaherty, and J. Christopher Flaherty, the inventors, filed a non-provisional utility patent application with the USPTO under the PCT (patent cooperation treaty). On June 19, 2020, the inventors entered into an assignment agreement, under which they assigned the entire right, title and interest in this patent application to our company. On September 9, 2020 we entered into an assignment agreement assigning all of these rights to our founder, Jaeson Bang. On April 19, 2021, Jaeson Bang assigned all of these rights back to our company pursuant to an assignment agreement as described above. |
In July 2021, our company, as the applicant, filed for a patent that encompasses the wireless antenna technology, as shown in the table below. David Nghiem and Jaeson Bang were listed as inventors in the application for this patent.
| Docket Number | | Title | | Application No. | | Patent No. | | Status |
| 5886.001US1 | | Implantable Antenna and Sensor Configurations | | 17/443,899 | | N/A | | Pending |
We and Mr. Bang intend to file additional patent applications to strengthen our intellectual property portfolio.
On February 18, 2020, we filed a trademark application with the USPTO for the mark “Voice of the Heart.” On June 9, 2020, this application was published for opposition.
On September 14, 2021, we registered “Future Cardia” as our trade name or “d/b/a” with the State of Florida where our principal executive officers are located.
We also own the URL https://futurecardia.com/.
Employees and Advisors
We currently have one (1) full-time employee, our Chief Executive Officer and ten (10) part-time employees. We are also working with two contract engineering firms.
In addition, we have engaged the following team of distinguished industry experts and researchers with substantive experience and extensive credentials as our advisors:
Dr. Dan Burkhoff MD PhD. Dr. Burkhoff is a renowned heart failure expert with multiple medical tech startup experience. He has participated in one medical tech company’s $1.1 billion exit to Medtronic. He is currently the director of the Cardiovascular Research Foundation.
Randolph Armstrong. Mr. Armstrong has more than 30 years of experience in medical device development. He has participated in a medical tech company’s $1.3 billion exit to Boston Scientific. He has executive-level experience gained at multiple medical tech companies such as Boston Scientific and Medtronic, and he also worked for NASA.
Dimitrios Georgakopoulous PhD. Dr. Georgakopoulous has been the chief science officer at multiple venture-backed biotechnology companies. He completed his post-doctoral fellowship in Cardiology at Johns Hopkins Hospital and has authored numerous public research papers. He received a PhD in Cardiovascular Physiology and Biomedical Engineering from Johns Hopkins University.
Dr. Kevin Heist MD PhD. Dr. Heist works at Massachusetts General Hospital. He is an associate professor of Medicine at Harvard Medical School. He received a PhD and an MD from the Stanford University School of Medicine.
Dr. Toshimasa Okabe MD. Dr. Okabe works at the Department of Clinical Cardiac Electrophysiology and Cardiovascular Disease at the Ohio State University Medical Center. He received an MD from the University of Tokyo.
Professor Frits Prinzen PhD. Dr. Prinzen is a Professor of Physiology, with a focus on “Electro-mechanics of the heart,” at Maastricht University, The Netherlands. He received his Medical Biology degree from University of Utrecht.
Dr. David Kraus MD. Dr. Kraus works at Stern Cardiovascular in Memphis, TN. He is an associate director at Cardiac Laboratories at Baptist Memorial Hospital. He received an MD from the University of Tennessee Center for the Health Sciences.
Anatoly Yakovlev PhD. Dr. Yakovlev received a PhD in Electrical Engineering from Stanford University and is an expert in machine learning.
Properties
Our main office is located at 910 Woodbridge Court, Safety Harbor, FL 34695. Our operational offices are located at the Johnson & Johnson JLABS facility at the Texas Medical Center in Houston, Texas. We pay the Texas Medical Center an annual rental rate of approximately $6,000 to utilize the JLABS space which rate increases by 3% annually.
We lease the JLABS space under a license agreement with the Texas Medical Center. Under this license, we are allowed to conduct laboratory research in a dedicated workstation area using laboratory facilities and equipment provided by the Texas Medical Center in their laboratory building located at 2450 Holcombe Boulevard, Houston, Texas 77021-2040.
We believe that all our properties are adequately maintained, are generally in good condition, and are suitable and adequate for our business.
We do not currently lease or own any other real property.
Government Regulation and the FDA Review Process
Government regulations can stimulate or slow growth of medical technology companies based on how governmental agencies evaluate products. The breadth of government regulations is comprehensive, governing areas such as medical device design and development, clinical testing, premarket clearance and approval, listing, manufacturing, labeling, advertising, storage, promotions, sales and distribution, and post-market surveillance.
In the U.S., the Food and Drug Administration, or FDA, typically oversees many of these regulations. Two pathways exist to propose a new medical device, the investigative device exemption, or IDE, requiring a premarket approval application, or PMA, and the 510k premarket submission. The 510k process requires the manufacturer to show that a device is “substantially equivalent” to an existing device that is already legally marketed. We are now in the process of completing that analysis. The FDA occasionally requires clinical data in a 510k application review, and often takes between ninety days to one year to complete the application process. We plan to submit our insertable cardiac device for FDA review under a pre-submission filing, or Pre-Sub, in the first quarter of 2021. Currently, we are working with regulatory experts to prepare the required documents to submit the Pre-Sub to the FDA for their opinion and guidance. We are also working to complete the development and engineering of our device. Following the receipt of FDA guidance which we should receive sometime after the Pre-Sub filing, we expect to set an appointment with the FDA for a Pre-Sub meeting, to gain clarification regarding their guidance, so that we can then proceed with their recommendations. We intend to file our application for FDA clearance under the 510k framework following completion of this Pre-Sub process. We expect that when we file our 510k application, although our device will not be fully developed, we will be ready to start the “pilot phase” of our product development, which will include animal testing of our device for safety and functionality, with a focus on quality and biocompatibility testing, the operability of device management systems, and risk control and effectiveness validation and verification procedures as mandated by the FDA. Following this pilot phase and prior to FDA 510k clearance, we will enter into a “release phase” in which we plan to conduct an additional risk management review of or device and “release” our device for an in-human early feasibility study clinical trial (although not required for 510k clearance). Once expected 510k clearance is obtained, we will begin the commercial sale of our device.
We believe we are in compliance with all material government regulations which apply to our product and operations. However, we are not able to predict the nature of any future laws, regulations, interpretations or applications, nor can we predict what effect future changes would have on our business.
Legal Proceedings
We know of no existing or pending legal proceedings against us, nor are we involved as a plaintiff in any proceeding or pending litigation. There are no proceedings in which any of our directors, officers or any of their respective affiliates is an adverse party or has a material interest adverse to our interest.
Risk Factors
Investing in our securities involves a significant degree of risk. In evaluating our company and an investment in our securities, careful consideration should be given to the following risk factors, in addition to the other information included in this report. Each of these risk factors could materially adversely affect our business, operating results or financial condition, as well as adversely affect the value of an investment in our securities. The following is a summary of the most significant factors. We are still subject to all the same risks that all companies in our industry, and all companies in the economy, are exposed to. These include risks relating to economic downturns, political and economic events and technological developments (such as cyber-security). Additionally, early-stage companies are inherently riskier than more developed companies. You should consider general risks as well as specific risks when deciding whether to invest.
Risks Related to our Business and Industry
We have a limited operating history upon which you can evaluate our performance, and accordingly, our prospects must be considered in light of the risks that any new company encounters.
We were incorporated in May 2019 and accordingly, we have a limited history upon which an evaluation of our prospects and future performance can be made. Our proposed operations are subject to all business risks associated with new enterprises. The likelihood of our creation of a viable business must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with the inception of a business, operation in a competitive industry, and the continued development of advertising, promotions, and a corresponding client base. In order to succeed, our company will need to attract additional capital and additional personnel, and there can be no assurances that our company will be able to attract the needed capital and personnel.
We have a history of operating losses and there can be no assurance that we can achieve or maintain profitability.
We have experienced net losses since inception. As of December 31, 2021, we had an accumulated deficit of $2,556,048. We had net losses in the amount of $1,738,609 and $727,320 for the years ended December 31, 2021 and 2020, respectively. There can be no assurance that we will achieve or maintain profitability. If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable would impair our ability to sustain operations and adversely affect the price of our common stock, once trading, and our ability to raise capital. Our operating expenses may increase as we spend resources on growing our business, and if our revenue does not correspondingly increase, our operating results and financial condition will suffer. You must consider our business and prospects in light of the risks and difficulties we will encounter as business with an early-stage technology in a new and rapidly evolving industry. We may not be able to successfully address these risks and difficulties, which could significantly harm our business, operating results and financial condition.
The COVID-19 pandemic may have an adverse impact on our business.
The COVID-19 pandemic has negatively impacted the U.S. economy, disrupted supply chains, created significant volatility and disruption in financial markets, increased unemployment levels and decreased consumer confidence generally. In addition, the pandemic has resulted in temporary closures of many businesses and the enforcement of social distancing in many states and communities.
The extent of the impact of the COVID-19 pandemic on our business, operations, and prospects will depend on a number of evolving factors, including:
| ● | The duration, extent, and severity of the pandemic. COVID-19 has not been contained and could affect significantly more households and businesses. The duration and severity of the pandemic continue to be impossible to predict. |
| ● | The response of governmental and nongovernmental authorities. Many of the actions taken by authorities have been directed at curtailing personal and business activity to contain COVID-19 while simultaneously deploying fiscal-and monetary-policy measures to assist in mitigating the adverse effects on individuals and businesses. These actions are not consistent across jurisdictions but, in general, have been evolving in scope and intensity. |
| ● | The effect on our targeted markets. COVID-19 and its associated consequences and uncertainties may affect individuals, households, and businesses differently and unevenly. In the near term if not longer, we generally expect that our targeted market may be adversely impacted. We also cannot predict if the impact will be short-lived or long-lasting. |
The duration of these business interruptions and related impacts on our proposed business and operations, which will depend on future developments, are highly uncertain and cannot be reasonably estimated at this time. Even after COVID-19 has subsided, we may continue to experience materially adverse impacts to our business as a result of the virus’s global economic impact, including the availability of credit, adverse impacts on our liquidity and any recession that has occurred or may occur in the future.
The forecasts of market growth included in this report may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, we cannot assure you our business will grow at similar rates, if at all.
Growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. The forecasts contained in this report, some of which reflecting pre-COVID-19 data, may prove to be inaccurate. Even if these markets experience the forecasted growth described in this report, we may not grow our business at similar rates, or at all. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties, including our ability to raise sufficient capital. Accordingly, the forecasts of market growth included in this report should not be taken as indicative of our future growth.
Our future profitability is uncertain.
We have incurred losses since the beginning of our operations and we will continue to have losses in the future as we incur additional expenses to execute our business plan, fuel our potential growth and conduct further research and development. We expect to make significant expenditures to commercialize our product and further develop our business. We will have to begin to generate and sustain and increase revenues to achieve or maintain profitability. We may not generate sufficient revenues to achieve or maintain profitability in the future. We may incur significant losses in the future for a number of reasons, including those discussed in other risk factors and factors that we cannot foresee.
We will need additional financing to execute our business plan which we may not be able to secure on acceptable terms, or at all.
We currently rely on external financing to fund our operations. We expect capital outlays and operating expenditures to increase over the next few years as we expand our infrastructure, commercial operations, development activities and establish offices.
Our future funding requirements will depend on many factors, including but not limited to the following:
| ● | The cost of expanding our operations; |
| ● | The financial terms and timing of any collaborations, licensing or other arrangements into which we may enter; |
| ● | The rate of progress and cost of development activities; |
| ● | The need to respond to technological changes and increased competition; |
| ● | The costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| ● | The cost and delays in product development that may result from changes in regulatory requirements applicable to our products; |
| ● | Sales and marketing efforts to bring our products to market; |
| ● | Unforeseen difficulties in establishing and maintaining an effective sales and distribution network; and |
| ● | Lack of demand for and market acceptance of our products and technologies. |
We may have difficulty obtaining additional funding and we cannot assure you that additional capital will be available to us when needed, if at all, or if available, will be obtained on terms acceptable to us. If we raise additional funds by issuing additional debt securities, such debt instruments may provide for rights, preferences or privileges senior to our equity securities. In addition, the terms of debt securities that we might issue could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. If adequate funds are not available, we may have to delay, scale back, or eliminate some of our operations or our research development and commercialization activities. Under these circumstances, if our company is unable to acquire additional capital or is required to raise it on terms that are less satisfactory than desired, it may have a material adverse effect on its financial condition.
In order for us to compete and grow, we must attract, recruit, retain and develop the necessary personnel who have the needed experience.
Recruiting and retaining highly qualified personnel is critical to our success. These demands may require us to hire additional personnel and will require our existing management personnel to develop additional expertise. We face intense competition for personnel. The failure to attract and retain personnel or to develop such expertise could delay or halt the sales and licensing of our product. If we experience difficulties in hiring and retaining personnel in key positions, we could suffer from delays in our development, loss of customers and sales and diversion of management resources, which could adversely affect operating results. Our future consultants may be employed by third parties and may have commitments under consulting or advisory contracts with third parties that may limit their availability to us.
Our success depends on the services of our founder and Chief Executive Officer, the loss of whom could disrupt our business; although we rely on this individual, we do not have key man life insurance.
We depend to a large extent on the services of our founder and Chief Executive Officer, Jaeson Bang. Given his knowledge and experience, he is important to our future prospects and development as we rely on his expertise in developing our business strategies and maintaining our operations. Because we are a start-up dependent on the vision of our founder, it will be critical to our prospects and successful development that he remains with us to help establish, develop and grow our business. The loss of the service of Mr. Bang and the failure to find timely replacements with comparable experience and expertise could disrupt and adversely affect our business. Additionally, we have not purchased an insurance policy with respect to Mr. Bang in the event of his death or disability. Therefore, if Mr. Bang dies or becomes disabled, we will not receive any compensation to assist us with such person’s absence.
Our internal control over financial reporting may be ineffective.
We do not have the internal infrastructure necessary, and are not required, to complete an attestation about our financial controls that would be required under Section 404 of the Sarbanes-Oxley Act of 2002. There can be no assurances that there are no significant deficiencies or material weaknesses in the quality of our financial controls. We expect to incur additional expenses and diversion of management’s time when it becomes necessary to perform the system and process evaluation, testing and remediation required to comply with the management certification and auditor attestation requirements.
We rely on various intellectual property rights, including patents and trademarks in order to operate our business.
Such intellectual property rights, however, may not be sufficiently broad or otherwise may not provide us a significant competitive advantage. In addition, the steps that we have taken to maintain and protect our intellectual property may not prevent it from being challenged, invalidated, circumvented or designed-around. In some circumstances, enforcement may not be available to us because an infringer has a dominant intellectual property position or for other business reasons. Our failure to obtain or maintain intellectual property rights that convey competitive advantage, adequately protect our intellectual property or detect or prevent circumvention or unauthorized use of such property, could adversely impact our competitive position and results of operations. We may also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements will adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets or other proprietary rights.
As we expand our business, protecting our intellectual property will become increasingly important. The protective steps we have taken may be inadequate to deter our competitors from using our proprietary information. In order to protect or enforce our patent rights, we may be required to initiate litigation against third parties, such as infringement lawsuits. Also, these third parties may assert claims against us with or without provocation. These lawsuits could be expensive, take significant time and could divert management’s attention from other business concerns. The law relating to the scope and validity of claims in the technology field in which we operate is still evolving. We cannot assure you that we will prevail in any of these potential suits or that the damages or other remedies awarded, if any, would be commercially valuable.
We will be subject to income taxes as well as non-income based taxes, such as payroll, sales, use, value-added, net worth, property and goods and services taxes in the U.S.
Significant judgment is required in determining our provision for income taxes and other tax liabilities. In the ordinary course of our business, there are many transactions and calculations where the ultimate tax determination is uncertain. Although we believe that our tax estimates are reasonable: (i) there is no assurance that the final determination of tax audits or tax disputes will not be different from what is reflected in our income tax provisions, expense amounts for non-income based taxes and accruals and (ii) any material differences could have an adverse effect on our financial position and results of operations in the period or periods for which determination is made.
The development and commercialization of our insertable cardiac monitor is highly competitive.
We face competition in the cardiac monitors market. Our competitors may have significantly greater financial, technical and human resources than we have and superior expertise in research and development and thus may be better equipped than us to develop and commercialize cardiac monitoring technologies. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Accordingly, our competitors may commercialize products more rapidly or effectively than we are able to, which would adversely affect our competitive position.
Industry consolidation may result in increased competition.
Some of our competitors have made or may make acquisitions or may enter into partnerships or other strategic relationships to offer more comprehensive services than they individually had offered or achieve greater economies of scale. In addition, new entrants not currently considered to be competitors may enter our market through acquisitions, partnerships or strategic relationships. We expect these trends to continue as companies attempt to strengthen or maintain their market positions. The potential entrants may have competitive advantages over us, such as greater name recognition, longer operating histories, more varied services and larger marketing budgets, as well as greater financial, technical and other resources. The companies resulting from combinations or that expand or vertically integrate their business to include the market that we address may create more compelling product offerings and may offer greater pricing flexibility than we can or may engage in business practices that make it more difficult for us to compete effectively, including on the basis of price, sales and marketing programs, technology or service functionality.
Successful development of our products is uncertain.
Our development of current and future product candidates is subject to the risks of failure and delay inherent in the development of new products and products based on new technologies, including:
| ● | delays in product development, clinical testing, or manufacturing; |
| ● | unplanned expenditures in product development, clinical testing, or manufacturing; |
| ● | failure to receive regulatory approvals; |
| ● | inability to manufacture on our own, or through any others, product candidates on a commercial scale; |
| ● | failure to achieve market acceptance; and |
| ● | emergence of superior or equivalent products. |
Because of these risks, our research and development efforts may not result in any commercially viable products. If a significant portion of these development efforts are not successfully completed, required regulatory approvals are not obtained, or any approved products are not commercially successfully, our business, financial condition, and results of operations may be materially harmed.
We could be adversely affected by health care reform legislation.
Third-party payers for medical products and services, including state, federal and foreign governments, are increasingly concerned about escalating health care costs and can indirectly affect the pricing or the relative attractiveness of our products by regulating the maximum amount of healthcare reimbursement they will provide for our products. Following years of increasing pressure, during 2010 the U.S. government enacted comprehensive health care reform with the enactment of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, which made changes that significantly impact the pharmaceutical and medical device industries. The Protecting Access to Medicare Act of 2014 imposes additional limitations on Medicare reimbursement rates. These statutes may restrict Medicare reimbursement rates for our products, which may adversely affect our business, financial condition and results of operations. If healthcare reimbursement amounts for our products decrease further in the future, such decreases may reduce the amount that will be reimbursed to hospitals or physicians and consequently, could place constraints on the levels of overall pricing, which could have a material effect on our revenues.
The 2.3% medical device tax originally established as part of the U.S. health care reform legislation through December 31, 2015 is now repealed. We are unable to predict any future legislative changes or developments related to this excise tax or any other excise tax. Additional state and federal health care reform measures may be adopted in the future, any of which could have a material adverse effect on our ability to successfully commercialize our products and on our industry in general. For example, the United States government has in the past considered, is currently considering and may in the future consider, health care policies and proposals intended to curb rising health care costs, including those that could significantly affect both private and public reimbursement for health care services. Further, state and local governments are also considering or have adopted similar types of policies. Future significant changes in the health care system in the United States or elsewhere, and current uncertainty about whether and how changes may be implemented, could have a negative impact on the demand for our products. We are unable to predict whether health care policies, including policies stemming from legislation or regulations affecting our business, may be proposed or enacted in the future, what effect such policies would have on our business, or the effect that ongoing uncertainty about these matters will have on the purchasing decisions of our customers.
Changes to government health care programs that reduce payments under Medicare and Medicaid may negatively impact our revenues.
Previous legislative changes have resulted in, and future legislative changes may result in, limitations on and reduced levels of payment and healthcare reimbursement for a substantial portion of hospital procedures and costs. Current or future health care reform and deficit reduction efforts, changes in laws or regulations regarding government health care programs, other changes in the administration of government health care programs and changes to commercial third-party payers in response to health care reform and other changes to government health care programs could have a material, adverse effect on our financial position and results of operations.
If third-party payors do not provide adequate coverage and healthcare reimbursement for the use of our products, our revenues will be negatively impacted.
Our success in marketing our products depends in large part on whether U.S. and international government health administrative authorities, private health insurers and other organizations will adequately cover and reimburse customers for the cost of our products. In the United States, a third-party payor’s decision to provide coverage for our products does not imply that an adequate healthcare reimbursement rate will be obtained. Further, one third-party payor’s decision to cover our products does not assure that other payors will also provide coverage for the products or provide coverage at an adequate healthcare reimbursement rate. Healthcare reimbursement systems in international markets vary significantly by country and by region within some countries, and healthcare reimbursement approvals must be obtained on a country-by-country basis. In many international markets, a product must be approved for healthcare reimbursement before it can be approved for sale in that country. Further, many international markets have government-managed healthcare systems that control healthcare reimbursement for new devices and procedures. In most markets there are private insurance systems as well as government-managed systems. If sufficient coverage and healthcare reimbursement is not available for our current or future products, in either the United States or internationally, the demand for our products and our revenues will be adversely affected.
Privacy laws and regulations could restrict our ability or the ability of our customers to obtain, use or disseminate patient information, or could require us to incur significant additional costs to re-design our products.
State, federal and foreign laws, such as the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), regulate the confidentiality of sensitive personal information and the circumstances under which such information may be released. These and future laws could have an adverse impact on our results of operations. Other health information standards, such as regulations under HIPAA, establish standards regarding electronic health data transmissions and transaction code set rules for specified electronic transactions, for example transactions involving claims submissions to third party payors. These also continue to evolve and are often unclear and difficult to apply. In addition, under the federal Health Information Technology for Economic and Clinical Health Act (HITECH Act), which was passed in 2009, many businesses that were previously only indirectly subject to federal HIPAA privacy and security rules became directly subject to such rules because the businesses serve as “business associates” to our customers. On January 17, 2013, the Office for Civil Rights of the Department of Health and Human Services released a final rule implementing the HITECH Act and making certain other changes to HIPAA privacy and security requirements. Compliance may increase the requirements applicable to our business. Failure to maintain the confidentiality of sensitive personal information in accordance with the applicable regulatory requirements, or to abide by electronic health data transmission standards, could expose us to breach of contract claims, fines and penalties, costs for remediation and harm to our reputation.
The healthcare industry is highly regulated.
We are subject to regulation in the U.S. at both the federal and state levels. In addition, the U.S. federal and state governments have allocated greater resources to the enforcement of these laws. If we fail to comply with these regulatory requirements, or if allegations are made that we failed to comply, our results of operations and financial condition could be adversely affected.
Products that we will manufacture, source, distribute or market are required to comply with regulatory requirements.
To lawfully operate our business, we are required to hold permits, licenses and other regulatory approvals from, and to comply with operating and security standards of, governmental bodies. Failure to maintain or renew necessary permits, licenses or approvals, or noncompliance or concerns over noncompliance may result in suspension of our ability to distribute or manufacture products, product recalls or seizures, or criminal and civil sanctions and could have an adverse effect on our results of operations and financial condition.
The manufacture, distribution, marketing and use of our products are subject to extensive regulation and increased scrutiny by the Food and Drug Administration (FDA) and other regulatory authorities.
Any new product must undergo lengthy and rigorous testing and other extensive, costly and time-consuming procedures mandated by the FDA and foreign regulatory authorities. Changes to current products may be subject to vigorous review, including additional 510(k) and other regulatory submissions, and approvals are not certain. Once we start manufacturing, failure to comply with the requirements of the FDA or other regulatory authorities, including a failed inspection or a failure in our adverse event reporting system, could result in adverse inspection reports, warning letters, product recalls or seizures, monetary sanctions, injunctions to halt the manufacture and distribution of products, civil or criminal sanctions, refusal of a government to grant approvals or licenses, restrictions on operations or withdrawal of existing approvals and licenses. Any of these actions could cause a loss of customer confidence in us and our products, which could adversely affect our sales and results of operations.
The sales, marketing and pricing of products and relationships that pharmaceutical and medical device companies have with healthcare providers are under increased scrutiny by federal and state government agencies.
Compliance with the Anti-Kickback Statute, False Claims Act, Food, Drug and Cosmetic Act (including as these laws relate to off-label promotion of products) and other healthcare related laws, as well as competition, data and patient privacy and export and import laws is under increased focus by the agencies charged with overseeing such activities, including FDA, Office of Inspector General (OIG), Department of Justice (DOJ) and the Federal Trade Commission. The DOJ and the Securities and Exchange Commission have also increased their focus on the enforcement of the U.S. Foreign Corrupt Practices Act (FCPA), particularly as it relates to the conduct of pharmaceutical companies, which may adversely impact our future global expansions.
Federal and state laws pertaining to healthcare fraud and abuse could adversely affect our business.
We are subject to various federal and state laws targeting fraud and abuse in the healthcare industry, including anti-kickback laws, false claims laws, laws constraining the sales, marketing and other promotional activities of manufacturers of medical devices by limiting the kinds of financial arrangements we may enter into with physicians, hospitals, laboratories and other potential purchasers of medical devices, laws requiring the reporting of certain transactions between us and healthcare professionals and HIPAA, as amended by HITECH, which governs the conduct of certain electronic healthcare transactions and protects security and privacy of protected health information. Violations of these laws are punishable by criminal or civil sanctions, including substantial fines, imprisonment and exclusion from participation in government healthcare programs such as Medicare and Medicaid. Many of the existing requirements are new and have not been definitively interpreted by state authorities or courts, and available guidance is limited. Unless and until we are in full compliance with these laws, we could face enforcement action and fines and other penalties, and could receive adverse publicity, all of which could materially harm our business. In addition, changes in or evolving interpretations of these laws, regulations, or administrative or judicial interpretations, may require us to change our business practices or subject our business practices to legal challenges, which could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to educate physicians on the safe and effective use of our products, we may be unable to achieve our expected growth.
An important part of our sales process will include the education of physicians on the safe and effective use of our products. There is a learning process for physicians to become proficient in the use of our products and it typically takes several procedures for a physician to become comfortable using our device. If a physician experiences difficulties during an initial procedure or otherwise, that physician may be less likely to continue to use our product, or to recommend it to other physicians. It is critical to the success of our commercialization efforts to educate physicians on the proper use of the insertable heart monitor, and to provide them with adequate product support during clinical procedures. It is important for our growth that these physicians advocate for the benefits of our products in the broader marketplace. If physicians are not properly trained, they may misuse or ineffectively use our products. This may also result in unsatisfactory patient outcomes, patient injuries, negative publicity or lawsuits against us, any of which could have an adverse effect on our business.
The design, manufacture and marketing of our medical devices entail an inherent risk of product liability claims.
Manufacturing and marketing of our products, and clinical testing of our products under development, may expose us to product liability and other tort claims. Although we intend to maintain, liability insurance, the coverage limits of our insurance policies may not be adequate and one or more successful claims brought against us may have a material adverse effect on our business and results of operations. There are a number of factors that could result in an unsafe condition or injury to, or death of, a patient with respect to these or other products which we will sell, including component failures, manufacturing flaws, design defects or inadequate disclosure of product-related risks or product-related information. Product liability claims may be brought by individuals or by groups seeking to represent a class. The outcome of litigation, particularly class action lawsuits, is difficult to assess or quantify. Plaintiffs in these types of lawsuits often seek recovery of very large or indeterminate amounts, and the magnitude of the potential loss relating to such lawsuits may remain unknown for substantial periods of time. Any costs (the material components of which are settlements, judgments, legal fees and other related defense costs) not covered under the product liability insurance policies and future reserves could have a material adverse effect on our revenues, financial position and cash flows. Additionally, product liability claims could negatively affect our reputation, continued product sales, and our ability to obtain and maintain regulatory approval for our products.
Risks Related to Ownership of Our Securities
There is no public market for our common stock. You cannot be certain that an active trading market or a specific share price will be established, and you may not be able to resell your securities at or above the public offering price.
There has been no public trading market for our securities. There can be no assurance that a public trading market for our common stock will develop or that a public trading market, if developed, will be sustained. If an active market for our common stock does not develop or is not maintained, it may be difficult for you to sell our common stock. An inactive trading market also may impair our ability to raise capital to continue to fund operations by selling shares. The lack of an active market also may reduce the fair market value of our common stock.
Future issuances of our common stock or securities convertible into our common stock could cause the market price of our common stock to decline and would result in the dilution of your shareholding.
Future issuances of our common stock or securities convertible into our common stock could cause the market price of our common stock to decline, should such a public trading market for our common stock exist. We cannot predict the effect, if any, of future issuances of our common stock or securities convertible into our common stock on the price of our common stock. In all events, future issuances of our common stock would result in the dilution of your shareholding. In addition, the perception that new issuances of our common stock, or other securities convertible into our common stock, could occur, could adversely affect the market price of our common stock.
Future issuances of debt securities, which would rank senior to our capital stock upon our bankruptcy or liquidation, and future issuances of equity securities may adversely affect the level of return you may be able to achieve from an investment in our securities.
In the future, we may attempt to increase our capital resources by offering debt securities. Upon bankruptcy or liquidation, holders of our debt securities, and lenders with respect to other borrowings we may make, would receive distributions of our available assets prior to any distributions being made to holders of our capital stock. Moreover, if we issue additional equity securities, the holders of such equity securities could be entitled to preferences over existing holders of common stock and equity securities in respect of the payment of dividends and the payment of liquidating distributions. Because our decision to issue debt or preferred securities in any future offering, or borrow money from lenders, will depend in part on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of any such future offerings or borrowings. You must bear the risk that any future offerings we conduct or borrowings we make may adversely affect the level of return you may be able to achieve from an investment in our securities.
We have never paid cash dividends on our stock and we do not intend to pay dividends for the foreseeable future.
We have paid no cash dividends on any class of our stock to date and we do not anticipate paying cash dividends in the near term. For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and we do not anticipate paying any cash dividends on our stock. Accordingly, investors must be prepared to rely on sales of their shares after price appreciation to earn an investment return, which may never occur. Investors seeking cash dividends should not purchase our shares. Any determination to pay dividends in the future will be made at the discretion of our board of directors and will depend on our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our Board deems relevant.
Our Chief Executive Officer exercises significant control over us, which will limit your ability to influence corporate matters and could delay or prevent a change in corporate control.
Our officers and directors have significant control over stockholder matters, and the minority stockholders will have little or no control over our affairs. Our founder and Chief Executive Officer owns approximately 83.79% of our outstanding common stock, without giving effect to shares of our common stock issuable upon conversion of outstanding convertible notes and SAFEs. Accordingly, our founder and Chief Executive Officer has control over stockholders matters, such as the election of directors, amendments to our certificate of incorporation, and approval of significant corporate transactions. Given the substantial equity interest held by our Chief Executive Officer, he will be able to elect directors who may be in favor of higher executive compensation packages for himself and other officers of our company than independent directors would be. As a result, our minority stockholders will have little or no control over our affairs.
Certain provisions of our amended certificate of incorporation may make it more difficult for a third party to effect a change-of-control.
Our certificate of incorporation as amended authorizes our board of directors to issue up to 10,000,000 shares of preferred stock. The preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by the stockholders. These terms may include voting rights including the right to vote as a series on particular matters, preferences as to dividends and liquidation, conversion rights, redemption rights and sinking fund provisions. The issuance of any preferred stock could diminish the rights of holders of existing shares, and therefore could reduce the value of such shares. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of our board of directors to issue preferred stock could make it more difficult, delay, discourage, prevent or make it costlier to acquire or effect a change-in-control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the value of our common stock.
We are subject to ongoing public reporting requirements that are less rigorous than rules for more mature public companies, and our stockholders receive less information.
We are required to publicly report on an ongoing basis under the reporting rules set forth in Regulation A for Tier 2 issuers. The ongoing reporting requirements under Regulation A are more relaxed than for public companies reporting under the Securities Exchange Act of 1934, as amended, or the Exchange Act. The differences include, but are not limited to, being required to file only annual and semiannual reports, rather than annual and quarterly reports. Annual reports are due within 120 calendar days after the end of the issuer’s fiscal year, and semiannual reports are due within 90 calendar days after the end of the first six months of the issuer’s fiscal year.
We may elect to become a public reporting company under the Exchange Act. If we elect to do so, we will be required to publicly report on an ongoing basis as an emerging growth company (as defined in the JOBS Act) under the reporting rules set forth under the Exchange Act. For so long as we remain an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are applicable to other Exchange Act reporting companies that are not emerging growth companies, including but not limited to:
| | ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| | ● | being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and |
| | ● | being exempt from the requirement to hold a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the “Securities Act”), for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. If we elect to take advantage of the benefits of this extended transition period, our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
We would expect to take advantage of these reporting exemptions until we are no longer an emerging growth company. We would remain an emerging growth company for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our total annual gross revenues exceed $1.07 billion, (ii) the date that we become a large accelerated filer as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
If we decide to apply for the quotation of our common stock on the OTCQB or OTCQX market, we will be subject to the OTC Market’s Reporting Standards, which can be satisfied in a number of ways, including by remaining in compliance with (i) the SEC reporting requirements, if we elect to become a public reporting company under the Exchange Act, or (ii) Regulation A reporting requirements, if we elect not to become a reporting company under the Exchange Act.
In either case, we will be subject to ongoing public reporting requirements that are less rigorous than Exchange Act rules for companies that are not emerging growth companies, and our stockholders could receive less information than they might expect to receive from more mature public companies.
If our common stock becomes subject to the penny stock rules, it would become more difficult to trade our common stock.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not obtain and retain a listing or quotation of our common stock and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
we are a medical device technology startup focusing on the development of a tiny insertable cardiac device to monitor heart failure. Our monitoring device is designed to detect signs of heart failure early enough so that non-hospital treatments can be administered and, thus, hospitalizations that typically result from heart failure can be reduced and prevented. Our tiny insertable device, which has not yet been cleared by the FDA, will offer a long-term solution to heart failure monitoring that, we believe, features simplicity, improved accuracy, high patient protocol compliance and hospital economics. Our device is equipped with multi-sensors to track trending changes in heart performance (heart rhythms (ECG), and heart and lung sounds and activities) and monitors heart failure using telemedicine and a cloud-based pattern recognition (machine learning technology).
In summer 2019, we completed the ZeroTo510 Medical Tech Accelerator program (a GAN accelerator) operated by MidSouth Sustainable Energy Solutions, Inc. in Memphis, TN. In August 2019, our concept device was approved by Maastricht University in The Netherlands for animal lab and human testing, and in September 2019, we entered into a research collaboration agreement with Maastricht University for the animal and human testing of our device. In November 2019, we were accepted to Johnson & Johnson’s life sciences incubator, or JLABS, located in the Texas Medical Center in Houston, where we maintain our research office and development facilities under license with the Texas Medical Center.
In May 2020, we were accepted to Tampa Bay Wave Accelerator program (a GAN accelerator). In July 2020, we began discussions with a number of nationally recognized heart failure clinics to discuss the possibility of human trials at one of those clinics. In October 2020, we were accepted to the “bootcamp” program of the TMCx Accelerator at the Texas Medical Center.
We filed a provisional patent application that covers the technology related to our insertable cardiac device, software dashboard, smartphone app and data accumulation techniques in May 2019, and a non-provisional utility patent application was submitted in May 2020 (Patent application No. 62/853,899).
In January 2021, we commenced engineering of an implantable battery to be used in our device with a top U.S. medical device manufacturer. In February 2021, we obtained a key battery technology to expedite our development. In July 2021, we filed for a second patent that encompasses the wireless antenna technology (Patent application No. 17/443,899) to be employed in our device. In summer 2021, we were accepted into the “YCombinator – Summer Batch 2021.” However, we declined participation in favor of our capital raise with investors on the Republic platform then and other venture firms and potential strategic partners. By September 2021, we had identified and made arrangements with engineering and data analysis partners for the commercialization of our device.
Since our inception, we have devoted substantially all of our efforts to research and development, testing, product engineering and raising capital. Accordingly, we are considered to be in the development stage. We have not generated revenue from operations to date. As of March 31, 2022, we have raised approximately $5.3 million through private placements and equity crowd funding as described in more detail below.
Recent Developments
| ● | In January 2022, we were accepted into the Stanford StartX accelerator program. Stanford StartX is the premier accelerator program in the world with one of the highest success rates, with ten (10) startups achieving unicorn status in the past 12 years. Over 300 Silicon Valley mentors participate in the program to create one of the most powerful networks of entrepreneurs, professors, scientists and investors. |
| ● | In January 2022, we had selected key engineering and manufacturing facilities, established an engineering design team and selected implantable medical devices manufacturing firms to solidify the development of our device for human implant. |
| ● | In February 2022, we had identified a principal investigator who will lead the first in human implant clinical trial. |
| ● | On February 18, 2022, we filed a Certificate of Amendment of the Amended and Restated Certificate of Incorporation with the Secretary of the State of Delaware to reduce the total number of shares of common stock that the Company is authorized to issue from 200,000,000 to 50,000,000 shares and the total number of shares of preferred stock that the Company is authorized to issue from 50,000,000 to 10,000,000 shares. |
| ● | In March 2022, we had successfully implanted our prototype device in animal lab to demonstrate near real world scenario. This testing showed the embodied antenna and sensor technology as well as data acquisitions and analytics capabilities. This will allow us to conduct additional testings before expected human implant in December 2022. |
Principal Factors Affecting our Financial Performance
Our operating results are primarily affected by the following factors:
| ● | our ability to access additional capital and the size and timing of subsequent financings; |
| ● | the rate of progress and cost of development activities; |
| ● | costs of third-party laboratories to conduct our clinical studies; |
| ● | the financial terms and timing of any collaborations, licensing or other arrangements into which we may enter; |
| ● | the cost and delays in product development that may result from changes in regulatory requirements applicable to our products; |
| ● | personnel and facilities costs as we expand our operations; |
| ● | the costs of sales, marketing, and customer acquisition; |
| ● | willingness of healthcare providers to prescribe our device and the fees charged by them to do so; |
| ● | the costs of compliance with any unforeseen regulatory obstacles or governmental mandates in any states or countries in which we seek to operate; and |
| ● | the costs of any additional clinical studies which are deemed necessary for us to remain viable and competitive in other regions of the world. |
Results of Operations
The following table sets forth key components of our results of operations during the years ended December 31, 2021 and 2020.
| | | Years Ended
December 31, | | | Change | |
| | | 2021 | | | 2020 | | | $ | | | % | |
| Revenues | | $ | - | | | | - | | | | - | | | | - | % |
| Cost of revenues | | | - | | | | - | | | | - | | | | - | % |
| Gross profit | | | - | | | | - | | | | - | | | | - | % |
| Operating expenses | | | | | | | | | | | | | | | | |
| Research and development | | | 1,180,838 | | | | 16,712 | | | | 1,164,126 | | | | 6,965.8 | % |
| Sales, general and administrative | | | 542,240 | | | | 657,314 | | | | (115,074 | ) | | | 520.3 | % |
| Total operating expenses | | | 1,723,078 | | | | 674,026 | | | | 1,049,052 | | | | 155.6 | % |
| Loss from operations | | | (1,723,078 | ) | | | (674,026 | ) | | | (1,049,052 | ) | | | (155.6 | )% |
| Total other income (expense) | | | (15,531 | ) | | | (53,294 | ) | | | (53,294 | ) | | | (70.9 | )% |
| Net loss | | $ | (1,738,609 | ) | | | (727,320 | ) | | | (727,320 | ) | | | 139.0 | % |
Revenues.
The Company has not realized revenue as of December 31, 2021 and 2020.
Cost of revenues.
The Company has not realized revenue and the related cost of revenues as of December 31, 2021 and 2020.
Gross profit and gross margin.
The Company has not realized revenue or gross profit as of December 31, 2021 and 2020.
Sales, general and administrative expenses.
The Company had sales, general and administrative expenses of $542,240 and $657,314 as of December 31, 2021 and 2020, respectively. Sales, general and administrative expenses consisted primarily of stock based compensation, salaries and wages, professional fees, sales and marketing expenses and general and administrative expenses.
Research and development expenses.
The Company had research and development expenses of $1,180,838 and $16,712 as of December 31, 2021 and 2020, respectively. Research and development expenses consist primarily of services and costs related to the development of our insertable cardiac device.
Net loss.
The Company had a net loss of $1,738,609 and $727,320 as of December 31, 2021 and 2020, respectively. The increase in net loss was primarily a result of increased research and development activities.
Liquidity and Capital Resources
We have not generated any revenue from operations to date. Our sources of cash have historically included private placements of our securities and equity crowd funding. Our historical cash outflows have primarily been associated with cash used for operating activities such as research and development activities and other working capital needs. We intend to fund our operations through capital raised from investors in the near term.
Summary of Cash Flows
As of December 31, 2021, we had approximately $2,391,353 in cash. The following table presents a summary of our cash flows for the periods indicated:
| | | Years Ended
December 31, | |
| | | 2021 | | | 2020 | |
| Net cash used in operating activities | | $ | (1,635,060 | ) | | $ | (288,708 | ) |
| Net cash used in investing activities | | | (5,913 | ) | | | (25,030 | ) |
| Net cash provided by financing activities | | | 3,703,958 | | | | 642,106 | |
| Net increase in cash | | | 2,062,985 | | | | 328,368 | |
| Cash at beginning of period | | | 328,368 | | | | - | |
| Cash at end of period | | $ | 2,391,353 | | | $ | 328,368 | |
Net cash used in operating activities was $1,635,060 for the year ended December 31, 2021, as compared to $288,708 for the year ended December 31, 2020. The principal use of cash for operating activities was to fund our operating expenses during our development of the Company.
Net cash used in investing activities was $5,913 for the year ended December 31, 2021, as compared to $25,030 net cash used in investing activities for the year ended December 31, 2020. Net cash used in investing activities for the year ended December 31, 2021 and 2020 was used primarily to reimburse a shareholder for expenses paid on behalf of the Company.
Net cash provided by financing activities was $3,703,958 for the year ended December 31, 2021, as compared to $642,106 for the year ended December 31, 2020. Net cash provided by financing activities for the year ended December 31, 2021 consisted of $2,973,958 from the net proceeds from the Regulation A offering and proceeds of $730,000 from issuance of convertible notes and, while net cash provided by financing activities for the year ended December 31, 2020 consisted of proceeds of $350,000 from the issuance of convertible notes and net proceeds of $292,106 from the issuance of SAFE Agreements.
Regulation A Offering
On January 13, 2021, we launched an offering on the Republic platform, with OpenDeal Broker LLC as our broker of record, under Regulation A of Section 3(b) of the Securities Act, for Tier 2 offerings, whereby we offered up to 4,000,000 shares of common stock at an offering price of $2.00 per share. On August 25, 2021, we closed the offering on the Republic platform in which we sold 1,644,779 shares of common stock for gross proceeds of $3,289,558.
On December 20, 2021, we moved this Regulation A offering to the StartEngine platform after changing our broker of record for this offering to StartEngine Primary, LLC. This Regulation A offering will continue until the earliest of (i) June 30, 2022 (which date may be extended one or more times by us, in our discretion), (ii) the date when all of the shares offered under this offering, or 4,000,000 shares, are sold, or (iii) such earlier time as we may determine in our sole discretion.
Capital Expenditures
The Company had no capital expenditures for the years ended December 31, 2021 and 2020.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with United States generally accepted accounting principles, or U.S. GAAP, requires our management to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments. We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements:
Equity Based Compensation
The Company accounts for stock options issued to employees under ASC 718 (Stock Compensation). Under ASC 718, share-based compensation cost to employees is measured at the grant date, based on the estimated fair value of the award, and is recognized as an item of expense ratably over the employee’s requisite vesting period. The Company has elected early adoption of ASU 2018-07, which permits measurement of stock options at their intrinsic value, instead of their fair value. An option’s intrinsic value is defined as the amount by which the fair value of the underlying stock exceeds the exercise price of an option. In certain cases, this means that option compensation granted by the Company may have an intrinsic value of $0.
The Company measures compensation expense for its non-employee stock-based compensation under ASC 505 (Equity). The fair value of the option issued or committed to be issued is used to measure the transaction, as this is more reliable than the fair value of the services received. The fair value is measured at the value of the Company’s common stock on the date that the commitment for performance by the counterparty has been reached or the counterparty’s performance is complete. The fair value of the equity instrument is charged directly to expense and credited to additional paid-in capital.
Income Taxes
The Company applies ASC 740 Income Taxes (“ASC 740”). Deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial statement reported amounts at each period end, based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax expense for the period, if any and the change during the period in deferred tax assets and liabilities. ASC 740 also provides criteria for the recognition, measurement, presentation and disclosure of uncertain tax positions. A tax benefit from an uncertain position is recognized only if it is “more likely than not” that the position is sustainable upon examination by the relevant taxing authority based on its technical merit.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board, or FASB, or other standard setting bodies and adopted by the Company as of the specified effective date. The Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its financial position or results of operations upon adoption.
In May 2014, the FASB issued ASU, 2014-09—Revenue from Contracts with Customers (Topic 606), or ASU 2014-09, and further updated through ASU 2016-12, or ASU 2016-12, which amends the existing accounting standards for revenue recognition. ASU 2014-09 is based on principles that govern the recognition of revenue at an amount to which an entity expects to be entitled to when products are transferred to customers. This guidance is effective for annual reporting periods, and interim periods within those years, beginning December 15, 2018 for non-public entities. The new revenue standard may be applied retrospectively to each prior period presented or retrospectively with the cumulative effect recognized as of the date of adoption. The adoption of ASU 2014-09 had no material impact on the Company’s financial statements and related disclosures.
In June 2018, the FASB issued ASU No. 2018-07, Compensation - Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which simplifies the accounting for share-based payments granted to nonemployees for goods and services. Under the ASU, most of the guidance on such payments to nonemployees would be aligned with the requirements for share-based payments granted to employees. As a result, most of the guidance in ASC 718 associated with employee share-based payments, including most requirements related to classification and measurement, applies to nonemployee share-based payment arrangements. This standard is effective beginning in the first quarter of 2019, with early adoption permitted. The adoption of ASU 2018-07 is not expected to have a material effect on the Company’s financial statements.
ITEM 3. DIRECTORS AND OFFICERS
Directors and Executive Officers
The following table sets forth the name and position of each of our current executive officers, directors and significant employees.
| Name | | Position | | Age | | Term of Office | | Approximate hours per week
for part-time employees |
| Jaeson Bang | | Chief Executive Officer, President, Treasurer, Secretary and Director | | 50 | | From May 2019 | | N/A |
| Lauren Iaslovits | | Director | | 56 | | From October 2021 | | N/A |
Jaeson Bang is the founder of our company and has served as our Chief Executive Officer, President, Secretary, Treasurer and our sole director since our inception. Jaeson founded our company in May 2019, after approximately four years at EBR Systems, a Silicon Valley-based medical technology startup. While at EMR, Jae worked cross functionally with the CTO and R&D engineers on device development, as well as leading a national team of therapy development managers and field clinician engineers. Prior to that, he spent time consulting the business and clinical operations team at Keystone Heart, ltd., a venture-backed Israeli medical technology company. Throughout his career, Jae has worked at startups that operate at the intersection of medicine, technology, and business. These companies have been funded by investors such as Johnson & Johnson and New Enterprise Associates (NEA). Jae graduated from the Northwestern University – Kellogg School of Management with his Executive MBA in 2015.
Lauren Iaslovits has served as a member of the Board since October 2021. Ms. Iaslovits has over 30 years of experience in private equity financing and manufacturing industry with multiple successful exits. She brings a wealth of knowledge and insight in the venture capital and private equity investment landscape to guide our company to the next stage of growth.
Directors are elected until their successors are duly elected and qualified.
There are no agreements or understandings for any of our executive officers or directors to resign at the request of another person and no officer or director is acting on behalf of nor will any of them act at the direction of any other person.
Family Relationships
There are no family relationships between any director, executive officer, person nominated or chosen to become a director or executive officer or any significant employee.
Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past five years:
| ● | been convicted in a criminal proceeding (excluding traffic violations and other minor offences); or |
| ● | had any petition under the federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing. |
Corporate Governance
Our board of directors currently consists of two members, Jaeson Bang and Lauren Iaslovits. We currently do not have standing audit, nominating or compensation committees. Our entire board of directors handles the functions that would otherwise be handled by each of the committees.
Compensation of Directors and Executive Officers
Summary Compensation Table
The following table sets forth the annual compensation of each of the three highest paid persons who were executive officers or directors during our last completed fiscal year:
| Name | | Capacities in which compensation was received | | Cash Compensation ($) | | | Other Compensation ($) | | | Total Compensation ($) | |
| Jaeson Bang | | Chief Executive Officer | | $ | 150,000 | | | $ | 0 | | | $ | 150,000 | |
Cash compensation for the fiscal year ended December 31, 2021 consisted of base salary of $150,000. Mr. Bang had not received any other compensation during 2021.
Employment Agreement
On February 1, 2021, we entered into an executive employment agreement with Jaeson Bang as our Chief Executive Officer, a copy of which is filed as Exhibit 6.6 to this annual report on Form 1-K. Under this employment agreement, Mr. Bang is entitled to an annual base salary of $150,000, which may be increased annually by no less than 5% or such greater amount as is determined by our board of directors. The term of this agreement is for one year and it automatically renews for additional one year periods. Mr. Bang is entitled to three weeks paid vacation for the first year and four weeks thereafter and expense reimbursement, and he is eligible to participate in all employee benefit plans, policies and practices now or hereafter maintained by us commensurate with his position with us.
Mr. Bang’s employment agreement contains restrictive covenants prohibiting him from owning or operating a business that competes with our company or soliciting our customers or employees for up to six months following the termination of his employment.
On January 7, 2022, we entered into a new executive employment agreement (the “2022 Executive Employment Agreement”) with Mr. Bang which contains the same terms and provisions as the prior executive employment agreement dated February 1, 2021 except that the annual base salary increases from $150,000 to $175,000. A copy of the 2022 Executive Employment Agreement is filed as Exhibit 6.7 to this annual report on Form 1-K.
Director Compensation
During 2021, our non-executive director did not receive any cash or equity compensation for serving as a director.
Outstanding Options
As of December 31, 2021, under our 2020 equity incentive plan as described below, we had granted options to certain of our advisors to acquire an aggregate of 700,000 shares of our common stock.
2020 Equity Incentive Plan
On February 1, 2020, our board of directors and our stockholders approved the Company’s 2020 Equity Incentive Plan, or our 2020 plan. The 2020 plan is a stock-based compensation plan that provides for discretionary grants of stock options, stock awards and stock unit awards to key employees, non-employee directors and consultants. The purpose of our 2020 plan is to attract, motivate, and retain directors, employees, and others in a position to affect the financial and operational performance of our company and to recognize contributions made to our company by these persons and to provide them with additional incentive to achieve the objectives of our company. The following is a summary of our 2020 plan.
Administration. Our 2020 plan will be administered by our board of directors, unless we establish a committee of the board of directors for this purpose (we refer to the body administering our 2020 plan as the administrator). The administrator will have full authority to select the individuals who will receive awards under our 2020 plan, determine the form and amount of each of the awards to be granted and establish the terms and conditions of awards.
Number of Shares of Common Stock. The number of shares of the common stock that may be issued under our 2020 plan is 1,500,000. Shares issuable under our 2020 plan may be authorized but unissued shares or treasury shares. If there is a lapse, forfeiture, expiration, termination or cancellation of any award made under our 2020 plan for any reason, the shares subject to the award will again be available for issuance. Any shares subject to an award that are delivered to us by a participant, or withheld by us on behalf of a participant, as payment for an award or payment of withholding taxes due in connection with an award will not again be available for issuance, and all such shares will count toward the number of shares issued under our 2020 plan. The number of shares of common stock issuable under our 2020 plan is subject to adjustment, in the event of any reorganization, recapitalization, stock split, stock distribution, merger, consolidation, split-up, spin-off, combination, subdivision, consolidation or exchange of shares, any change in the capital structure of our company or any similar corporate transaction. In each case, the administrator has the discretion to make adjustments it deems necessary to preserve the intended benefits under our 2020 plan. No award granted under our 2020 plan may be transferred, except by will, the laws of descent and distribution.
Eligibility. All employees designated as key employees, including consultants, for purposes of our 2020 plan and all non-employee directors and advisors are eligible to receive awards under our 2020 plan.
Awards to Participants. The Plan provides for discretionary awards of stock options, stock awards and stock unit awards to participants. Each award made under our 2020 plan will be evidenced by a written award agreement specifying the terms and conditions of the award as determined by the administrator in its sole discretion, consistent with the terms of our 2020 plan.
Stock Options. The administrator has the discretion to grant non-qualified stock options or incentive stock options to participants and to set the terms and conditions applicable to the options, including the type of option, the number of shares subject to the option and the vesting schedule; provided that the exercise price of each stock option will be the fair market value (as defined in the 2020 Plan) of the common stock on the date on which the option is granted, except that the exercise price per share under a non-qualified stock option may be less than 100% of the fair market value of such shares on the date such option is granted provided that, and only if, the board of directors approves a lower price after consideration of the application of Section 409A of the internal revenue code, each option will expire no later than ten years from the date of grant and no dividend equivalents may be paid with respect to stock options. It is intended that stock options qualify as “performance-based compensation” under Section 162(m) of the internal revenue code and thus be fully deductible by us for federal income tax purposes, to the extent permitted by law.
In addition, an incentive stock option granted to a key employee is subject to the following rules: (i) the aggregate fair market value (determined at the time the option is granted) of the shares of common stock with respect to which incentive stock options are exercisable for the first time by a key employee during any calendar year (under all incentive stock option plans of our company and its subsidiaries) cannot exceed $100,000, and if this limitation is exceeded, that portion of the incentive stock option that does not exceed the applicable dollar limit will be an incentive stock option and the remainder will be a non-qualified stock option; (ii) if an incentive stock option is granted to a key employee who owns stock possessing more than 10% of the total combined voting power of all class of stock of our company, the exercise price of the incentive stock option will be 110% of the fair market value of the common stock on the date of grant and the incentive stock option will expire no later than five years from the date of grant; and (iii) no incentive stock option can be granted after ten years from the date our 2020 plan was adopted.
Stock Awards. The administrator has the discretion to grant stock awards to participants. Stock awards will consist of shares of common stock granted without any consideration from the participant or shares sold to the participant for appropriate consideration as determined by the Board. The number of shares awarded to each participant, and the restrictions, terms and conditions of the award, will be at the discretion of the administrator. Subject to the restrictions, a participant will be a shareholder with respect to the shares awarded to him or her and will have the rights of a shareholder with respect to the shares, including the right to vote the shares and receive dividends on the shares; provided that dividends otherwise payable on any performance-based stock award will be held by us and will be paid to the holder of the stock award only to the extent the restrictions on such stock award lapse, and the administrator in its discretion can accumulate and hold such amounts payable on any other stock awards until the restrictions on the stock award lapse.
Stock Units. The administrator has the discretion to grant stock unit awards to participants. Each stock unit entitles the participant to receive, on a specified date or event set forth in the award agreement, one share of common stock or cash equal to the fair market value of one share on such date or event, as provided in the award agreement. The number of stock units awarded to each participant, and the terms and conditions of the award, will be at the discretion of the administrator. Unless otherwise specified in the award agreement, a participant will not be a shareholder with respect to the stock units awarded to him prior to the date they are settled in shares of common stock. The award agreement may provide that until the restrictions on the stock units lapse, the participant will be paid an amount equal to the dividends that would have been paid had the stock units been actual shares; provided that dividend equivalents otherwise payable on any performance-based stock units will be held by us and paid only to the extent the restrictions lapse, and the administrator in its discretion can accumulate and hold such amounts payable on any other stock units until the restrictions on the stock units lapse.
Payment for Stock Options and Withholding Taxes. The administrator may make one or more of the following methods available for payment of any award, including the exercise price of a stock option, and for payment of the minimum required tax obligation associated with an award: (i) cash; (ii) cash received from a broker-dealer to whom the holder has submitted an exercise notice together with irrevocable instructions to deliver promptly to us the amount of sales proceeds from the sale of the shares subject to the award to pay the exercise price or withholding tax; (iii) by directing us to withhold shares of common stock otherwise issuable in connection with the award having a fair market value equal to the amount required to be withheld; and (iv) by delivery of previously acquired shares of common stock that are acceptable to the administrator and that have an aggregate fair market value on the date of exercise equal to the exercise price or withholding tax, or certification of ownership by attestation of such previously acquired shares.
Provisions Relating to a “Change in Control” of our Company. Notwithstanding any other provision of our 2020 plan or any award agreement, in the event of a “Change in Control” of our company, the administrator has the discretion to provide that all outstanding awards will become fully exercisable, all restrictions applicable to all awards will terminate or lapse, and performance goals applicable to any stock awards will be deemed satisfied at the highest target level. In addition, upon such Change in Control, the administrator has sole discretion to provide for the purchase of any outstanding stock option for cash equal to the difference between the exercise price and the then fair market value of the common stock subject to the option had the option been currently exercisable, make such adjustment to any award then outstanding as the administrator deems appropriate to reflect such Change in Control and cause any such award then outstanding to be assumed by the acquiring or surviving corporation after such Change in Control.
Effect of Termination of Employment; Company Repurchase Right. The right to exercise an option (to the extent that it is vested) following termination of a participant’s employment or service with our company will expire thirty (30) days following the termination of employment or service, except (i) to the extent any longer period is permitted under the rules of section 422 of the internal revenue code with respect to a participant’s death or disability, and (ii) if a participant’s employment or service with our company is terminated for cause, as that term is defined in our 2020 plan, then, immediately upon the termination of the participant’s employment or service with us, all vested and unvested awards granted to participant shall be immediately forfeited and automatically terminate. With respect to an award of our restricted common stock, upon a death or disability, all of the shares of restricted common stock subject to an award shall become immediately vested. Upon the termination of a participant’s employment or service with our company for any reason, we will have the right, but not the obligation, until the first anniversary of the termination of the participant’s employment or service to repurchase some or all of the vested shares and/or the vested options from the participant, the participant’s estate (in the case of the participant’s death), or any permitted transferee of such vested shares and/or vested options. When exercising this right, we shall pay the participant an amount per share equal to the lesser of (i) the price per share paid by the participant for such shares and (ii) the lesser of the fair market value of the shares as of the date of termination of the participant’s employment with us and the date we exercise the repurchase right.
Amendment of Award Agreements; Amendment and Termination of our 2020 plan; Term of our 2020 plan. The administrator may amend any award agreement at any time, provided that no amendment may adversely affect the right of any participant under any agreement in any material way without the written consent of the participant, unless such amendment is required by applicable law, regulation or stock exchange rule.
The Board may terminate, suspend or amend our 2020 plan, in whole or in part, from time to time, without the approval of the shareholders, unless such approval is required by applicable law, regulation or stock exchange rule, and provided that no amendment may adversely affect the right of any participant under any outstanding award in any material way without the written consent of the participant, unless such amendment is required by applicable law, regulation or rule of any stock exchange on which the shares are listed.
Notwithstanding the foregoing, neither our 2020 plan nor any outstanding award agreement can be amended in a way that results in the repricing of a stock option. Repricing is broadly defined to include reducing the exercise price of a stock option or cancelling a stock option in exchange for cash, other stock options with a lower exercise price or other stock awards. (This prohibition on repricing without shareholder approval does not apply in case of an equitable adjustment to the awards to reflect changes in the capital structure of our company or similar events.)
No awards may be granted under our 2020 plan on or after the tenth anniversary of the effective date of our 2020 plan.
Except as set forth above, we do not have any ongoing plan or arrangement for the compensation of directors and executive officers.
ITEM 4. SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table sets forth information regarding beneficial ownership of our voting stock as of April 20, 2022 (i) by each of our executive officers and directors who beneficially owns more than 10% of any class of our voting securities; (ii) by all of our executive officers and directors as a group; and (iii) by each person who is known by us to beneficially own more than 10% of any class of our voting securities. Unless otherwise specified, the address of each of the persons set forth below is in care of our company at 910 Woodbridge Court, Safety Harbor, FL 34695.
| Title of Class | | Name and address of beneficial owner | | Amount and nature of beneficial ownership | | | Amount and nature of beneficial ownership acquirable(1) | | | Percent of class(2) | |
| Common Stock | | Jaeson Bang | | | 8,500,000 | | | | 0 | | | | 83.79 | % |
| Common Stock | | Lauren Iaslovits | | | - | | | | - | | | | * | |
| Common Stock | | All directors and officers as a group (2 persons) | | | 8,500,000 | | | | 0 | | | | 83.79 | % |
| (1) | Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has direct ownership of and sole voting power and investment power with respect to the shares. For each beneficial owner above, any securities acquirable within 60 days have been included in the denominator in accordance with SEC Rule 13d-3(d)(1). |
| (2) | Based on 10,144,779 shares of our Common Stock outstanding as of April 20, 2022. We have no preferred stock outstanding as of April 20, 2022. |
ITEM 5. INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
The following includes a summary of transactions since the beginning of our 2020 fiscal year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 and one percent of the average of our total assets at year-end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Item 3. Directors and Officers—Compensation of Directors and Executive Officers”). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
We licensed the intellectual property for our implantable heart failure monitoring device from our founder and CEO, Jaeson Bang, from September 21, 2020 to April 18, 2021. Under our license agreement with Mr. Bang dated September 21, 2020, we had a nonexclusive, nontransferable, royalty free, perpetual, worldwide, irrevocable and non-assignable license to commercially utilize the intellectual property relating to the implantable heart monitoring device to develop and commercialize the implantable heart monitoring device for human use during the license period. We had not paid Mr. Bang any royalties or other fees under this license agreement.
On April 19, 2021, we entered into an assignment agreement with Mr. Bang, pursuant to which Mr. Bang transferred all of his right, title and interest in the intellectual property assets described by and embodied in the patent applications (62/853,899/ PCT/US20/35171) relating to the implantable heart monitoring device to our company for $100 in cash.
ITEM 6. OTHER INFORMATION
We have no information to disclose that was required to be in a report on Form 1-U during the last six months of the fiscal year ended December 31, 2021, but was not reported.
ITEM 7. FINANCIAL STATEMENTS
INDEX TO FINANCIAL STATEMENTS
Independent Auditors’ Report
Your Vision Our Focus

Independent Auditors’ Report
To the shareholders of
Oracle Health, Inc.
We have audited the accompanying financial statements of Oracle Health, Inc. (the Company), which comprise the balance sheets as of December 31, 2021 and 2020, and the related statements of operations, stockholders’ deficit and cash flows for the years then ended, and the related notes to the financial statements.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditors’ judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Turner, Stone & Company, L.L.P. Accountants and Consultants 12700 Park Central Drive, Suite 1400
Dallas, Texas 75251
Telephone: 972-239-1660 ⁄ Facsimile: 972-239-1665
Toll Free: 877-853-4195 Web site: turnerstone.com | |  INTERNATIONAL ASSOCIATION OF ACCOUNTANTS AND AUDITORS |
Opinion
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and cash flows for the each of the years then ended in accordance with accounting principles generally accepted in the United States of America.
Emphasis of Matter
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has a incurred net losses from inception and has a working capital deficit all of which raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Turner, Stone & Company, L.L.P.
Certified Public Accountants
Dallas, Texas
April 20, 2022
ORACLE HEALTH, INC.
BALANCE SHEETS
| | | December 31, | | | December 31, | |
| | | 2021 | | | 2020 | |
| Assets | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash | | $ | 2,391,353 | | | $ | 328,368 | |
| Total current assets | | | 2,391,353 | | | | 328,368 | |
| | | | | | | | | |
| Total Assets | | $ | 2,391,353 | | | $ | 328,368 | |
| | | | | | | | | |
| Liabilities and stockholders' deficit | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable | | $ | 35,114 | | | $ | 142 | |
| Accrued interest | | | 38,566 | | | | 4,298 | |
| Total current liabilities | | | 73,680 | | | | 4,440 | |
| | | | | | | | | |
| Long-term Liabilities | | | | | | | | |
| Convertible notes payable | | | 1,080,000 | | | | 350,000 | |
| SAFE Agreements | | | 412,106 | | | | 412,106 | |
| Total Liabilities | | | 1,565,786 | | | | 766,546 | |
| | | | | | | | | |
| Commitments and Contingencies (Note 7) | | | | | | | | |
| | | | | | | | | |
| Stockholders’ Deficit | | | | | | | | |
| Preferred stock, $0.00001 par value, 50,000,000 shares authorized, no shares issued and outstanding | | | - | | | | - | |
| Common stock, $0.00001 par value, 200,000,000 shares authorized, 10,144,779 and 8,500,000 shares issued and outstanding as of December 31, 2021 and 2020, respectively | | | 101 | | | | 85 | |
| Additional paid in capital | | | 3,381,514 | | | | 379,176 | |
| Accumulated deficit | | | (2,556,048 | ) | | | (817,439 | ) |
| Total Stockholders’ Deficit | | | 825,567 | | | | (438,178 | ) |
| Total Liabilities and Stockholders’ Deficit | | $ | 2,391,353 | | | $ | 328,368 | |
The accompanying footnotes are an integral part of these financial statements
ORACLE HEALTH, INC.
STATEMENTS OF OPERATIONS
| | | For the
Year Ended
December 31,
2021 | | | For the
Year Ended
December 31,
2020 | |
| Revenue | | $ | - | | | $ | - | |
| | | | | | | | | |
| Operating expenses: | | | | | | | | |
| Stock based compensation | | | 28,396 | | | | 379,176 | |
| Salaries and wages | | | 150,000 | | | | 120,000 | |
| Professional fees | | | 222,691 | | | | 114,265 | |
| Sales and marketing | | | 52,672 | | | | 12,948 | |
| General and administrative | | | 88,481 | | | | 30,925 | |
| Research and development | | | 1,180,838 | | | | 16,712 | |
| Total operating expenses | | | 1,723,078 | | | | 674,026 | |
| | | | | | | | | |
| Operating loss | | | (1,723,078 | ) | | | (674,026 | ) |
| | | | | | | | | |
| Other (expense): | | | | | | | | |
| Interest expense | | | (34,268 | ) | | | (4,298 | ) |
| Unrealized loss on due from shareholder | | | (5,913 | ) | | | (54,996 | ) |
| Other income | | | 24,650 | | | | 6,000 | |
| Total other (expense) | | | (15,531 | ) | | | (53,294 | ) |
| | | | | | | | | |
| Net loss | | $ | (1,738,609 | ) | | $ | (727,320 | ) |
| | | | | | | | | |
| Per share information: | | | | | | | | |
| Basic weighted average shares outstanding | | | 8,973,726 | | | | 8,500,000 | |
| Diluted weighted average shares outstanding | | | 8,973,726 | | | | 8,500,000 | |
| | | | | | | | | |
| Net loss per share - basic and diluted | | $ | (0.19 | ) | | $ | (0.09 | ) |
The accompanying footnotes are an integral part of these financial statements
ORACLE HEALTH, INC.
STATEMENTS OF STOCKHOLDERS' (DEFICIT) EQUITY
For the Years Ended December 31, 2021 and 2020
| | | Common Stock | | | Additional
Paid In | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Total | |
| Balance, January 1, 2020 | | | 8,500,000 | | | $ | 85 | | | $ | - | | | $ | (90,119 | ) | | $ | (90,034 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Vesting of stock options | | | - | | | | - | | | | 379,176 | | | | - | | | | 379,176 | |
| Net loss | | | - | | | | - | | | | - | | | | (727,320 | ) | | | (727,320 | ) |
| Balance, December 31, 2020 | | | 8,500,000 | | | $ | 85 | | | $ | 379,176 | | | | (817,439 | ) | | $ | (438,178 | ) |
| | | Common Stock | | | Additional
Paid In | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Total | |
| Balance, January 1, 2021 | | | 8,500,000 | | | $ | 85 | | | $ | 379,176 | | | $ | (817,439 | ) | | $ | (438,178 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Sale of common stock | | | 1,644,779 | | | | 16 | | | | 2,973,942 | | | | | | | | 2,973,958 | |
| Vesting of stock options | | | - | | | | - | | | | 28,396 | | | | - | | | | 28,396 | |
| Net loss | | | - | | | | - | | | | - | | | | (1,738,609 | ) | | | (1,738,609 | ) |
| Balance, December 31, 2021 | | | 10,144,779 | | | $ | 101 | | | $ | 3,381,514 | | | $ | (2,556,048 | ) | | $ | 825,567 | |
The accompanying footnotes are an integral part of these financial statements
ORACLE HEALTH, INC.
STATEMENTS OF CASH FLOWS
| | | For the
Year Ended | | | For the
Year Ended | |
| | | December 31,
2021 | | | December 31,
2020 | |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (1,738,609 | ) | | $ | (727,320 | ) |
| Adjustment to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Stock based compensation | | | 28,396 | | | | 379,176 | |
| Unrealized loss on due from shareholder | | | 5,913 | | | | 54,996 | |
| Change in operating assets and liabilities: | | | | | | | | |
| Accounts payable | | | 34,972 | | | | 142 | |
| Accrued interest | | | 34,268 | | | | 4,298 | |
| Net cash used in operating activities | | | (1,635,060 | ) | | | (288,708 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Due from shareholder, net | | | (5,913 | ) | | | (25,030 | ) |
| Net cash used in investing activities | | | (5,913 | ) | | | (25,030 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from sale of common stock | | | 2,973,958 | | | | - | |
| Proceeds from issuance of SAFE Agreements | | | - | | | | 292,106 | |
| Proceeds from issuance of convertible note payable | | | 730,000 | | | | 350,000 | |
| Net cash provided by financing activities | | | 3,703,958 | | | | 642,106 | |
| | | | | | | | | |
| Net change in cash | | | 2,062,985 | | | | 328,368 | |
| Cash, beginning of period | | | 328,368 | | | | - | |
| Cash, end of year | | $ | 2,391,353 | | | $ | 328,368 | |
| | | | | | | | | |
| Supplemental disclosure of cash flow information | | | | | | | | |
| Cash paid for interest | | $ | - | | | $ | - | |
| Cash paid for income taxes | | $ | - | | | $ | - | |
The accompanying footnotes are an integral part of these financial statements
ORACLE HEALTH, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2021
NOTE 1 – NATURE OF OPERATIONS, BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Oracle Health, Inc. (the “Company”) was incorporated in Delaware on May 9, 2019. The Company is a healthcare technology company that is developing a digital cardiac monitor. The Company’s products will exploit certain proprietary research carried out by Jaeson Bang, its founder.
Throughout this report, the terms “our,” “we,” “us,” and the “Company” refer to Oracle Health, Inc.
Basis of Presentation
The accompanying financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The Company’s fiscal year ends December 31.
Significant Risks and Uncertainties
The Company is subject to customary risks and uncertainties associated with development of new technology including, but not limited to, the need for protection of intellectual property, dependence on key personnel, costs of services provided by third parties, the need to obtain additional financing, and limited operating history.
The Company currently has no developed products for commercialization and there can be no assurance that the Company’s research and development will be successfully commercialized. Developing and commercializing a product requires significant capital, and based on the current operating plan, the Company expects to continue to incur operating losses as well as cash outflows from operations in the near term.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates made in connection with the accompanying financial statements include the estimate of valuation of stock-based compensation, and valuation allowances against net deferred tax assets.
Financial Instruments
Our financial instruments consist of cash, accounts payable, SAFE agreements and convertible notes. The carrying values of these instruments approximate fair value due to the short-term maturities of these instruments.
Fair Value Measurements
Financial Accounting Standards Board (“FASB”) ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”), provides a comprehensive framework for measuring fair value and expands disclosures which are required about fair value measurements. Specifically, ASC 820 sets forth a definition of fair value and establishes a hierarchy prioritizing the inputs to valuation techniques, giving the highest priority to quoted prices in active markets for identical assets and liabilities and the lowest priority to unobservable value inputs. ASC 820 defines the hierarchy as follows:
Level 1 - Quoted prices are available in active markets for identical assets or liabilities as of the reported date. The types of assets and liabilities included in Level 1 are highly liquid and actively traded instruments with quoted prices.
Level 2 - Pricing inputs are other than quoted prices in active markets but are either directly or indirectly observable as of the reported date. The types of assets and liabilities in Level 2 are typically either comparable to actively traded securities or contracts or priced with models using highly observable inputs.
Level 3 - Significant inputs to pricing that are unobservable as of the reporting date. The types of assets and liabilities included in Level 3 are those with inputs requiring significant management judgment or estimation, such as complex and subjective models and forecasts used to determine the fair value of financial transmission rights.
The Company’s financial instruments consist of cash, accounts payable and convertible notes. The estimated fair value of these financial instruments approximates their carrying amounts due to the short-term nature of these instruments.
Certain non-financial assets are measured at fair value on a nonrecurring basis. Accordingly, these assets are not measured and adjusted to fair value on an ongoing basis but are subject to periodic impairment tests. These items primarily include long-lived assets and other intangible assets.
Cash and Cash Equivalents
Cash include all cash balances, and highly liquid investments with maturities of three months or less when purchased.
Revenue
ASC Topic 606, “Revenue from Contracts with Customers” establishes principles for reporting information about the nature, amount, timing and uncertainty of revenue and cash flows arising from the entity’s contracts to provide goods or services to customers. Revenues are recognized when control of the promised goods or services are transferred to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. The Company applies the following five steps in order to determine the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its agreements: 1) identify the contract with a customer; 2) identify the performance obligations in the contract; 3) determine the transaction price; 4) allocate the transaction price to performance obligations in the contract; and 5) recognize revenue as the performance obligation is satisfied.
Property and Equipment
The Company capitalizes assets with an expected useful life of one year or more, and an original purchase price of $1,000 or more. Depreciation is calculated on a straight-line basis over management’s estimate of each asset’s useful life.
Rent
The Company is party to a non-cancellable lease agreement for office space which commenced in January 2020 and had a term of eleven months. The Company is currently on a month-to-month basis with this lease agreement at $515 per month.
Advertising
The Company records advertising expenses in the period incurred.
Research & Development
The Company records research & development expenses in the period incurred.
Stock Compensation
The Company accounts for stock options issued to employees under ASC 718 Stock Compensation. Under ASC 718, share-based compensation cost to employees is measured at the grant date, based on the estimated fair value of the award, and is recognized as an item of expense ratably over the employee’s requisite vesting period. The Company recognizes stock-based compensation for all share-based payment awards made to employees based on the estimated fair values, using the Black-Scholes option pricing model.
Non-employee stock-based compensation is accounted for based on the fair value of the related stock or options. The fair value of options to be granted are estimated on the date of each grant using the Black-Scholes option pricing model and amortized ratably over the option’s vesting periods, which approximates the service period.
The Company measures compensation expense for its non-employee stock-based compensation under ASC 718. The fair value of the option issued or committed to be issued is used to measure the transaction, as this is more reliable than the fair value of the services received. The fair value is measured at the value of the Company’s common stock on the date that the commitment for performance by the counterparty has been reached or the counterparty’s performance is complete. The fair value of the equity instrument is charged directly to expense and credited to additional paid-in capital.
Loss Per Share
We calculate net loss per share in accordance with ASC Topic 260, Earnings per Share. Basic net loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding for the period, and diluted earnings per share is computed by including common stock equivalents outstanding for the period in the denominator. For the years ended December 31, 2021 and 2020, any equivalents would have been anti-dilutive as we had a loss for the periods then ended. For the years ended December 31, 2021 and 2020, the following number of potentially dilutive shares have been excluded from diluted net income (loss) since such inclusion would be anti-dilutive:
| | | Years Ended
December 31, | |
| | | 2021 | | | 2020 | |
| Stock options outstanding | | | 700,000 | | | | 651,000 | |
| | | | | | | | | |
| Total | | | 700,000 | | | | 651,000 | |
Income Taxes
Income taxes are provided based upon the liability method of accounting pursuant to the ASC Topic 740 Income Taxes. Under this approach, deferred income taxes are recorded to reflect the tax consequences in future years of differences between the tax basis of assets and liabilities and their financial reporting amounts at each year-end. A valuation allowance is recorded against the deferred tax asset if management does not believe the Company has met the “more likely than not” standard to allow recognition of such an asset.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued that we adopt as of the specified effective date. We believe that the impact of recently issued standards that are not yet effective may have an impact on our results of operations and financial position.
NOTE 2 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
As of December 31, 2021, the Company had an accumulated deficit of $2,556,048 and working capital of $2,317,673. During the year ended December 31, 2021, the Company used cash in operating activities of $1,635,060. As of December 31, 2021, the Company had cash of $2,391,353. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The Company recognizes it will need to raise additional capital in order to fund operations and meet its payment obligations. There is no assurance that additional financing will be available when needed or that management will be able to obtain financing on terms acceptable to the Company and whether the Company will generate revenues, become profitable and generate positive operating cash flow. If the Company is unable to raise sufficient additional funds on favorable terms, it will have to develop and implement a plan to further extend payables and to raise capital through the issuance of debt or equity on less favorable terms until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
Accordingly, the accompanying financial statements have been prepared in conformity with U.S. GAAP, which contemplates continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily represent realizable or settlement values. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 3 – RELATED PARTY TRANSACTIONS
Due from Shareholder
Our founding shareholder, who is also our CEO, periodically receives advances and reimbursements from the Company for expenses incurred on behalf of the Company. The total net advances due from our founding shareholder as of December 31, 2021 and December 31, 2020 were $5,913 and $25,030, respectively.
The Company recorded a full valuation allowance against the due from shareholder as of December 31, 2021, recording an unrealized loss on due from shareholder of $5,913 and reducing the net balance due of $5,913 to $0. Additionally, the Company recorded a full valuation allowance against the due from shareholder as of December 31, 2020, recording an unrealized loss on due from shareholder of $54,996 and reducing the net balance due of $54,996 to $0. Our founding shareholder does not anticipate taking additional advances in the future.
SAFE Agreement
On May 6, 2019, the Company entered into a SAFE Agreement with our CEO for proceeds of $30,000 which was outstanding and reflected as a long-term liability as of December 31, 2021 and 2020 (see Note 4).
Employment Agreement
The Company entered into an informal employment agreement with its CEO on January 1, 2020, which includes an annual base salary of $120,000. On January 30, 2021, the board of directors approved a formal Employment Agreement which was executed on February 1, 2021, which increased his base annual salary to $150,000 effective January 1, 2021.
NOTE 4 – NOTES PAYABLE
Convertible Notes Payable
On February 21, 2020, the Company issued a convertible promissory note in exchange for $100,000. The convertible note bears an interest rate of 5% per annum and will mature in thirty-six months at which the principle and all accrued interest will be due.
On November 12, 2020, the Company issued a convertible promissory note in exchange for $200,000. The convertible note bears an interest rate of 6% per annum and will mature in thirty-six months at which the principle and all accrued interest will be due.
On November 25, 2020, the Company issued a convertible promissory note in exchange for $50,000. The convertible note bears an interest rate of 6% per annum and will mature in thirty-six months at which the principle and all accrued interest will be due.
On August 23, 2021, the Company issued a convertible promissory note in exchange for $200,000. The convertible note bears an interest rate of 6% per annum and will mature in thirty-six months at which the principle and all accrued interest will be due.
On August 23, 2021, the Company issued a convertible promissory note in exchange for $530,000. The convertible note bears an interest rate of 6% per annum and will mature in thirty-six months at which the principle and all accrued interest will be due.
The above convertible notes payable will convert under certain pre-defined condition such as a Qualified Equity Financing or Change of Control. Upon conversion, the convertible notes payable will convert to common shares of the Company at the lesser of (i) eighty percent (80%) of the price per share paid by the other purchasers of Next Round Securities in the Qualified Equity Financing and (ii) the price obtained by dividing $5,000,000 by the number of outstanding shares of common stock of the Company immediately prior to the Qualified Equity Financing.
The Company accrued $38,566 and $4,298 in interest associated with these convertible notes payable as of December 31, 2021 and 2020, respectively.
SAFE Agreements
During the period from May 9, 2019 (inception) through December 31, 2020, the Company issued Simple Agreements for Future Equity (“SAFE”). The SAFE agreements have no maturity date and bear no interest. The SAFE agreements provide a right to the holder to future equity in the Company in the form of SAFE Preferred Stock. SAFE Preferred Stock are shares of a series of Preferred Stock issued to the investor in an equity financing, having identical rights, privileges, preferences and restrictions as the shares of standard Preferred Stock offered to non-holders of SAFE agreements other than with respect to: (i) the per share liquidation preference and the conversion price for purposes of price-based anti-dilution protection, which will equal the Safe price (price per share equal to the valuation capitalization divided by the total capitalization of the Company); and (ii) the basis for any dividend rights, which will be based on the conversion price. The number of shares issued to the holder is determined by either (1) the face value of the SAFE agreement divided by the price per share of the standard preferred stock issued, if the pre-money valuation is less than or equal to the valuation capitalization (ranging from $1,666,666 and $5,000,000); or (2) a number of shares of SAFE Preferred Stock equal to the face value of the SAFE agreement divided by the price per share equal to the valuation cap divided by the total capitalization of the company immediately prior to an equity financing event. Total capitalization of the company includes all shares of capital stock issued and outstanding and outstanding vested and unvested options as if converted.
If there is a liquidity event (as defined in the SAFE agreements), the investor will, at their option, either (i) receive a cash payment equal to the face value of the SAFE agreement (“Purchase Amount”) or (ii) automatically receive from the Company a number of shares of common stock equal to the Purchase Amount divided by the price per share equal to the valuation cap divided by the Liquidity Capitalization (“Liquidity Price”) (as defined in the SAFE agreements). If there are not enough funds to pay the holders of SAFE agreements in full, then all of the Company’s available funds will be distributed with equal priority and pro-rata among the SAFE agreement holders in proportion to their Purchase Amounts and they will automatically receive the number of shares of common stock equal to the remaining unpaid Purchase Amount divided by the Liquidity Price.
If there is a dissolution event (as defined in the SAFE agreements), the Company will pay an amount equal to the Purchase Amount, due and payable to the investor immediately prior to, or concurrent with, the consummation of the dissolution event. The Purchase Amount will be paid prior and in preference to any distribution of any of the assets of the Company to holders of outstanding capital stock. If immediately prior to the consummation of the dissolution event, the assets of the Company legally available for distribution to all SAFE holders, are insufficient to permit the payment to their respective Purchase Amounts, then all of the assets of the Company legally available for distribution will be distributed with equal priority and pro-rata among the SAFE holders as a single class.
The SAFE agreements will expire and terminate upon either (i) the issuance of shares to the investor pursuant to an equity financing event or (ii) the payment, or setting aside for payment, of amounts due to the investor pursuant to a liquidity or dissolution event.
As of December 31, 2021, no SAFE agreements had been converted into equity, nor had any terminated or expired based on the terms of the agreements.
The Company had $412,106 and $412,106 of SAFE obligations outstanding as of December 31, 2021 and 2020, respectively, which includes a SAFE obligation for $30,000 to our Chief Executive Officer, with valuation caps ranging from $1,666,666 and $5,000,000.
The Company accounts for the SAFE agreements under ASC 480 (Distinguishing Liabilities from Equity), which requires that they be recorded at fair value as of the balance sheet date. Any changes in fair value are to be recorded in the statements of operations. The Company has determined that the fair value at the date of issuance, and as of December 31, 2021 are both consistent with the proceeds received at issuance, and therefore there is no mark-to-market fair value adjustments required or reflected in income for the year ended December 31, 2021.
CARES Act and Grant
In 2020, the Company received an Economic Injury Disaster Loan Advance under the Paycheck Protection Program provision of the CARES Act. The advance does not have to be repaid and bears no interest. The Company received $4,650 and $4,000 from this program during the years ended December 31, 2021 and 2020, respectively, which is included as other income on the statement of operations.
The Company also received a local city grant of $2,000 in July 2020.
NOTE 5 – STOCKHOLDERS’ DEFICIT
Preferred stock
As per our Amended and Restated Certificate of Articles of Incorporation dated October 22, 2020, we are authorized to issue up to 50,000,000 shares of preferred stock. Our certificate of incorporation authorize our board of directors to issue these shares in one or more series, to determine the designations and the powers, preferences and rights and the qualifications, limitations and restrictions thereof, including the dividend rights, conversion or exchange rights, voting rights (including the number of votes per share), redemption rights and terms, liquidation preferences, sinking fund provisions and the number of shares constituting the series. Our board of directors could, without stockholder approval, issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of common stock and which could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
Common stock
Under our Amended and Restated Certificate of Articles of Incorporation, the Company is authorized to issue 200,000,000 shares of $0.00001 par value Common Stock. Common shareholders have the right to vote on certain items of Company business at the rate of one vote per share of stock.
On May 9, 2019, the Company issued 8,500,000 common shares to its founder at an aggregate price of $85.
On April 28, 2021, the Company received the initial proceeds from the sale of its common stock at $2.00 per share under the Offering Circular of the Company dated January 13, 2021, relating to the Company’s public offering under Regulation A of Section 3(6) of the Securities Act of 1933, as amended, for Tier 2 offerings, pursuant to which the Company offered up to 4,000,000 shares of common stock at an offering price of $2.00 per share for aggregate maximum gross proceeds of $8,000,000 (the “Offering”). On August 25, 2021, we closed the Offering in which we sold an aggregate of 1,644,779 shares of common stock for gross proceeds of approximately $3,300,000, or net proceeds of $2,973,958.
As of December 31, 2021 and December 31, 2020, the number of common shares issued and outstanding was 10,144,779 and 8,500,000, respectively.
NOTE 6 – STOCK OPTIONS
In 2020, the Board of Directors adopted the 2020 Equity Incentive Plan (“the Plan”). The Plan provides for the grant of equity awards to employees, and consultants, including stock options, stock purchase rights and restricted stock units to purchase shares of common stock. Up to 1,500,000 shares of common stock may be issued pursuant to awards granted under the Plan. The Plan is administered by the Board of Directors, and expires ten years after adoption, unless terminated earlier by the Board.
During 2020, the Company granted 700,000 stock options under the Plan to seven of our advisors. The granted options had an exercise price of $0.59 (the fair market value of our outstanding stock on the date of grant as determined by our sole director), expire in ten years, and ranged from 100% immediate vesting to vesting over a four-year period.
The total grant date fair value of options granted was $407,573.
The following is a summary of outstanding stock options issued as of December 31, 2021:
| | | Number of
Options | | | Exercise Price
per Share | | | Average Remaining
Term in Years | |
| Outstanding, December 31, 2020 | | | 700,000 | | | $ | 0.59 | | | | 9.15 | |
| Granted | | | - | | | | - | | | | - | |
| Exercised | | | - | | | | - | | | | - | |
| Outstanding, December 31, 2021 | | | 700,000 | | | $ | 0.59 | | | | 8.65 | |
| | | | | | | | | | | | | |
| Vested and expected to vest, December 31, 2021 | | | 700,000 | | | $ | 0.59 | | | | 8.65 | |
| Exercisable, December 31, 2021 | | | 700,000 | | | $ | 0.59 | | | | 8.65 | |
Stock option expense for the years ended December 31, 2021 and 2020 was $28,396 and $379,176, respectively. No unamortized stock compensation remains as of December 31, 2021.
The stock options were valued using the Black-Scholes pricing model as indicated below:
| Expected life (years) | | | 5.19 | |
| Risk-free interest rate | | | 1.37 | % |
| Expected volatility | | | 217 | % |
| Annual dividend yield | | | 0 | % |
The risk-free interest rate assumption for options granted is based upon observed interest rates on the United States government securities appropriate for the expected term of the Company’s employee stock options.
The expected term of employee stock options is calculated using the simplified method which takes into consideration the contractual life and vesting terms of the options.
The Company determined the expected volatility assumption for options granted using the historical volatility of comparable public company’s common stock. The Company will continue to monitor peer companies and other relevant factors used to measure expected volatility for future stock option grants, until such time that the Company’s common stock has enough market history to use historical volatility.
The dividend yield assumption for options granted is based on the Company’s history and expectation of dividend payouts. The Company has never declared or paid any cash dividends on its common stock, and the Company does not anticipate paying any cash dividends in the foreseeable future.
NOTE 7 – COMMITMENTS AND CONTINGENCIES
The Company is party to a non-cancellable operating lease agreement for office space. The lease commenced in January 2020 with a term of eleven months at $500 per month and is currently on a month-to-month basis at $515 per month beginning January 2021.
On January 8, 2021, the Company paid $100,000 pursuant to a development agreement with a consultant dated January 5, 2021. The agreement includes design work by the consultant over the following 18 months at a total cost of approximately $2 million to deliver an implantable device suitable for patients under FDA guidelines.
NOTE 8 – INCOME TAXES
The Company accounts for income taxes under ASC 740 – Income Taxes (“ASC 740”), which provides for an asset and liability approach of accounting for income taxes. Under this approach, deferred tax assets and liabilities are recognized based on anticipated future tax consequences, using currently enacted tax laws, attributed to temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts calculated for income tax purposes.
Significant components of the Company’s deferred tax assets as of December 31, 2021 and 2020 are summarized below.
| | | 2021 | | | 2020 | |
| Deferred tax assets: | | | | | | | | |
| Net operating loss carryforwards | | $ | 618,000 | | | $ | 96,000 | |
| Total deferred tax asset | | | 618,000 | | | | 96,000 | |
| Valuation allowance | | | (618,000 | ) | | | (96,000 | ) |
| | | $ | - | | | $ | - | |
The Company recognizes deferred tax assets to the extent that it believes that these assets are more likely than not to be realized. In making such a determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. The Company assessed the need for a valuation allowance of $618,000 and $96,000 required as of December 31, 2021 and 2020, respectively, as the Company has no history of generating taxable income. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible.
A reconciliation of the federal income tax rate to the Company’s effective tax rate is as follows at December 31, 2021 and 2020:
| | | 2021 | | | 2020 | |
| Statutory federal income tax rate | | | 21.0 | % | | | 21.0 | % |
| State taxes, net of federal tax benefit | | | 3.5 | % | | | 3.5 | % |
| Change in valuation allowance | | | (24.5 | )% | | | (24.5 | )% |
| Income tax provision | | | — | % | | | — | % |
As of December 31, 2021, the Company has approximately $2.5 million of U.S. federal and state net operating loss carryovers, which do not expire.
The Company has evaluated its income tax positions and has determined that it does not have any uncertain tax positions. The Company will recognize interest and penalties related to any uncertain tax positions through its income tax expense.
The Company files income tax returns in the U.S. federal jurisdiction and in various state and local jurisdictions and is subject to examination by the various taxing authorities.
The Company is subject to franchise tax filing requirements in the State of Delaware and accrued approximately $23,500 for such tax liability as of December 31, 2021.
NOTE 9 – SUBSEQUENT EVENTS
Management considered events subsequent to the end of the period but before the date that the financial statements were available to be issued. Based on this evaluation, other than below, no additional material events were identified which require adjustment or disclosure in the financial statements.
On February 17, 2022, the Company amended the Company’s current Amended and Restated Certificate of Incorporation to reduce the total number of shares of common stock that the Company is authorized to issue from 200,000,000 to 50,000,000 shares and the total number of shares of preferred stock that the Company is authorized to issue from 50,000,000 to 10,000,000 shares.
On January 28, 2022, we issued two convertible notes to two investors in the principal amount of $150,000 and $200,000, respectively. On February 10, 2022, we issued one convertible note to one investor in the principal amount of $100,000. These new notes have the same terms as the previous Notes except that they have a valuation cap of $20,000,000 instead of $5,000,000 (see Note 4).
ITEM 8. Exhibits
| Exhibit No. | | Description |
| 1.1 | | Listing Agreement, dated January 7, 2021, between OpenDeal Broker LLC and Oracle Health, Inc. (incorporated by reference to Exhibit 6.8 to Amendment No. 3 to the Offering Statement on Form 1-A/A filed on January 12, 2021) |
| 1.2 | | Posting Agreement dated June 25, 2021 between the Company and StartEngine Primary, LLC (incorporated by reference to Exhibit 6.1 to a Current Report on Form 1-U filed on December 20, 2021) |
| 2.1 | | Amended and Restated Certificate of Incorporation of Oracle Health, Inc. (incorporated by reference to Exhibit 2.1 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 2.2* | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of Oracle Health, Inc. |
| 2.3 | | Amended and Restated Bylaws of Oracle Health, Inc. (incorporated by reference to Exhibit 2.2 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 3.1 | | Form of Simple Agreement for Future Equity (SAFE) (incorporated by reference to Exhibit 3.1 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 3.2 | | Form of Convertible Note (incorporated by reference to Exhibit 3.2 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 3.3 | | Form of Crowd Note (incorporated by reference to Exhibit 3.3 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 3.4 | | Oracle Health, Inc. Equity Incentive Plan (incorporated by reference to Exhibit 15.1 to a Semiannual Report on Form 1-SA filed on September 28, 2021) |
| 4.1 | | Form of Subscription Agreement for the Regulation A Offering (incorporated by reference to Exhibit 4.1 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 6.1 | | Patent Assignment Agreement, dated April 19, 2021, between Jaeson Bang and Oracle Health, Inc. (incorporated by reference to Exhibit 6.4 to the semiannual report on Form 1-SA filed on September 28, 2021) |
| 6.2 | | License Agreement, dated November 18, 2019, between Oracle Health, Inc. and Texas Medical Center (i.e., Johnson & Johnson Innovation LLC (JLABS) License Agreement) (incorporated by reference to Exhibit 6.4 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 6.3 | | Research Collaboration Agreement dated September 21, 2019, between Oracle Health, Inc. and Maastricht University (incorporated by reference to Exhibit 6.5 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 6.4 | | Agreement between Tampa By Wave and Oracle Health, Inc. dated April 27, 2020 (incorporated by reference to Exhibit 6.6 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 6.5 | | TMCx Accelerator Program Participation Agreement, dated October 5, 2020 (incorporated by reference to Exhibit 6.9 to the Offering Statement on Form 1-A filed on October 29, 2020) |
| 6.6 | | Executive Employment Agreement dated February 1, 2021 between Oracle Health, Inc. and Jaeson Bang (incorporated by reference to Exhibit 6.10 to a Semiannual Report on Form 1-SA filed on September 28, 2021) |
| 6.7* | | Executive Employment Agreement dated January 7, 2022 between Oracle Health, Inc. and Jaeson Bang |
| 6.8 | | Services Agreement dated June 29, 2021 between the Company and StartEngine Crowdfunding, Inc. (incorporated by reference to Exhibit 6.2 to a Current Report on Form 1-U filed on December 20, 2021) |
| 6.9 | | Credit Card Services Agreement dated June 29, 2021 between the Company and StartEngine Crowdfunding, Inc. (incorporated by reference to Exhibit 6.3 to a Current Report on Form 1-U filed on December 20, 2021) |
| 6.10 | | Form of Stock Option Agreement (Oracle Health, Inc. Equity Incentive Plan) (incorporated by reference to Exhibit 15.2 to a Semiannual Report on Form 1-SA filed on September 28, 2021) |
| 8.1 | | Escrow Services Agreement, dated January 11, 2021, between Prime Trust LLC and Oracle Health, Inc. (incorporated by reference to Exhibit 8.1 to Amendment No. 4 to the Offering Statement on Form 1-A/A filed on January 12, 2021) |
| 8.2 | | Escrow Services Agreement dated November 30, 2021 among the Company, Prime Trust, LLC and StartEngine Primary, LLC. (incorporated by reference to Exhibit 8.1 to a Current Report on Form 1-U filed on December 20, 2021) |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: April 21, 2022 | Oracle Health, INC. |
| | |
| | /s/ Jaeson Bang |
| | Name: | Jaeson Bang |
| | Title: | Chief Executive Officer |
| | | (Principal Executive Officer and Principal Financial and Accounting Officer) |
Pursuant to the requirements of Regulation A, this report has been signed below by the following persons on behalf of the issuer and in the capacities and on the dates indicated.
| SIGNATURE | | TITLE | | DATE |
| | | | | |
| /s/ Jaeson Bang | | CEO, President and Director | | April 21, 2022 |
| Jaeson Bang | | (principal executive officer and principal financial and accounting officer) | | |
| | | | | |
| /s/ Lauren Iaslovits | | Director | | April 21, 2022 |
| Lauren Iaslovits | | | | |