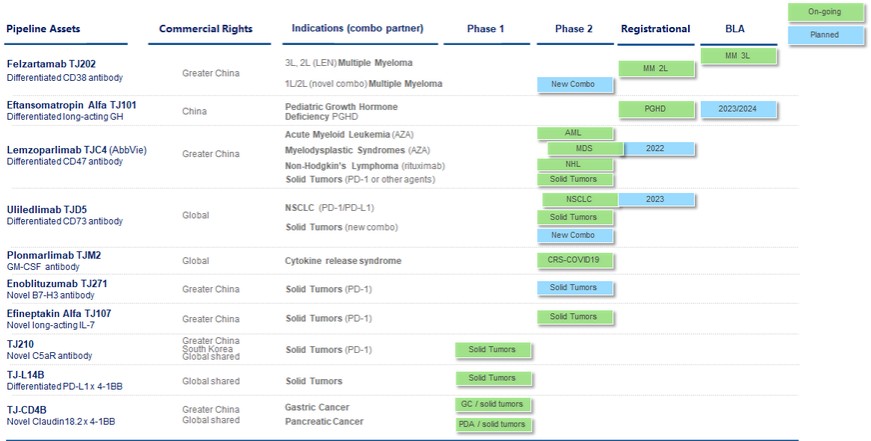
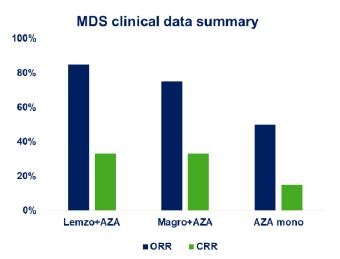
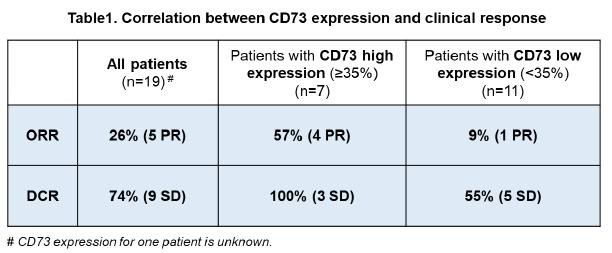
(2) | Preclinical assets and programs |
TJ-L1IF: A novel PD-L1/IFN-α antibody-cytokine fusion protein, which is specifically designed for the treatment of PD-1/PD-L1 resistant tumors through the addition of a strong immune adjuvant (interferon-alpha, IFN-α) to potentially convert “cold” tumor to “hot” tumor on top of a PD-L1 antibody to achieve superior anti-tumor activity than PD-(L)1 antibody monotherapy. Novel drug molecules with such design is badly needed to address the current clinical challenges where a majority of cancer patients do not or poorly respond to PD-1/PDL-1 therapies.
IFN-α was the first cytokine approved for cancer treatment, but its clinical use is highly limited due to considerable systemic toxicity. TJ-L1IF is composed of a PD-L1 VHH nanobody linked with the Fc of human IgG with an engineered IFN-α2b fused at the C-terminus. It is a prodrug in that the IFN-α2b moiety is masked by a PEG group through a protease-cleavable linker rendering the drug inactive in the systemic circulation, thus strongly reducing systemic toxicity. Once the drug accumulates at the tumor site by PD-L1 antibody targeting, the linker is cleaved by proteases that are highly expressed in the tumor environment to achieve specific activation only at the tumor site. This unique property of TJ-L1IF has been confirmed in a series of in vitro and in vivo studies, in which TJ-L1IF demonstrated plasma stability, good safety in cynomolgus monkeys, and superior anti-tumor activity in the PD-1/PD-L1 resistant tumor models, than that achieved by PD-L1 antibody or IFN-α used either alone or in combination. After the first dose of treatment, the active format of the drug was quickly detected and accumulated in the tumor but not in the periphery, confirming the local delivery and conversion to an active form of IFN-α at the tumor site (Figure 2). TJ-L1IF was developed using Affinity’s TMEA technology and is now under pre-clinical development.
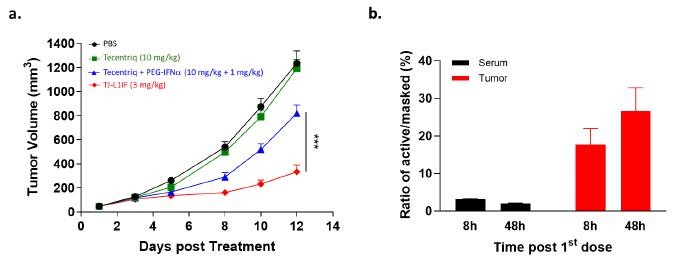
Figure 2. In vivo anti-tumor activity of TJ-L1IF in PD-L1 resistant tumor model. (a) NSG mice transplanted subcutaneously with colon cancer cell line were treated with Tecentriq (10 mg/kg) alone, Tecentriq (10 mg/kg) and PEG-IFNa (1 mg/kg) combination and TJ-L1IF (3 mg/kg) twice a week. (b) The concentration of PEG cleaved active and PEG masked L1IF was measured in tumor and serum, respectively at 8h and 48h post the first dosing. The ratio of the level of active to that of masked L1IF was calculated.
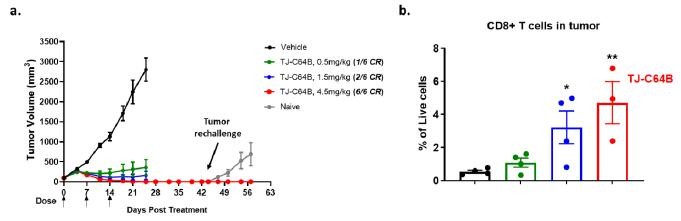
TJ-C64B: Our third bispecific molecule being developed by leveraging a conditional 4-1BB platform which has the advantage of minimizing liver toxicity with an increased therapeutic window. It is specifically designed to simultaneously target Claudin 6 (CLDN6), uniquely expressed in specific cancer types, including ovarian cancer cells, and 4-1BB expressed by T cells to mediate the T cell killing of CLDN6+ tumor cells. CLDN6 is hardly detectable in normal adult tissues to ensure treatment specificity for ovarian cancers. We have achieved candidate selection and is actively progressing the pre-clinical development of the candidate molecule.
TJ-C64B activates T cells through 4-1BB stimulation only upon CLDN6 engagement, providing a localized immune activation in tumors with expected efficacy and reduced systemic toxicity. Owing to a competent Fc, TJ-C64B has an added advantage of specifically depleting CLDN6-expressing tumor cells and intra-tumor regulatory T cells highly expressing 4-1BB, which differentiates it from other 4-1BB bispecific antibodies under clinical development. As published in AACR 2022, pre-clinical data showed that TJ-C64B enhances CLDN6-dependent T cell activation upon the engagement of cancer cell lines with different CLDN6 expression levels. In a syngeneic mouse model, TJ-C64B treatment induces strong anti-tumor activity with complete tumor regression in all tested mice at the dose of 4.5 mg/kg and long-term protection from tumor re-challenge through the immunological memory response. Further, ex vivo analysis confirms localized immune activation by TJ-C64B as evident by the increased CD8+ T cells, specifically those residing in tumors (Figure 3). TJ-C64B is now under pre-clinical development and we plan to submit an IND in the U.S. around mid-2023.