About This Prospectus
The prospectus that is included in this registration statement is in two parts: first, this prospectus supplement, which sets forth the principal terms of the Up BTOX PharmaShares and the Down BTOX PharmaShares, and, second, the base prospectus, which describes PharmaShares Advisors, LLC, in its capacity as depositor and administrative agent, and the PharmaShares Trust, and includes detailed disclosure about the terms applicable to each Series of the PharmaShares Trust and the related risks and other considerations that are important for investors in any of these Series.
You should rely only on the information contained in this prospectus supplement and the base prospectus. We have not, and no Authorized Participant has, authorized any person to provide you with information that is different from that contained in this prospectus supplement and the base prospectus. We are offering to sell, and seeking offers to buy, up to the maximum registered dollar amount of Up BTOX PharmaShares and Down BTOX PharmaShares specified on the cover page of this registration statement only in states or other jurisdictions where such offers and sales are permitted.
Table of Contents
NOTE ABOUT CERTAIN INFORMATION CONTAINED IN THIS PROSPECTUS SUPPLEMENTiii
WHERE YOU CAN FIND MORE INFORMATIONiii
REPORTS TO SHAREHOLDERSiii
FORWARD-LOOKING STATEMENTSiv
REFERENCE PRODUCT OVERVIEW1
SUMMARY OF PRINCIPAL TERMS2
RISK FACTORS RELATED TO YOUR SERIES9
THE TERMS OF YOUR SERIES12
The Issuer and the Securities Offered12
Use of Proceeds13
The Administrative Agent13
The Marketing Agent13
The Trustee13
The Calculation Agent14
Daily Reporting14
The Series Assets15
The Reference Value16
The Reference Product17
The Projected Breakpoint19
Periodic Distributions24
Final Distributions27
Paired Optional Redemptions28
Paired Issuances28
Termination Triggers29
Fees and Expenses29
Form of the Shares31
Listing31
U.S. Federal Income Tax Considerations32
ERISA Considerations32
CUSIP and ISIN Numbers32
Experts32
Plan of Distribution32
Appendices
Appendix A: Hypothetical Returns Based on Broker Reporting Group Projections A-1
Appendix B: Hypothetical Underlying Value CalculationsB-1
Appendix C: Projected Breakpoint for Current Calendar QuarterC-1
Appendix F: Financial Statements for the PharmaShares TrustF-1
ii
Unless otherwise indicated, all references in this prospectus supplement to the “administrative agent,” “we,” “us,” “our,” or similar terms refer to PharmaShares Advisors, LLC.
We include cross-references in this prospectus supplement to sections where you can find further related discussions. The preceding table of contents provides the pages on which these sections begin. We also include cross-references in this prospectus supplement to sections within the base prospectus where you can find information relevant to the Up BTOX PharmaShares and the Down BTOX PharmaShares.
NOTE ABOUT CERTAIN INFORMATION CONTAINED IN THIS PROSPECTUS SUPPLEMENT
The information contained in this prospectus supplement in the sections entitled “REFERENCE PRODUCT OVERVIEW,” “SUMMARY OF PRINCIPAL TERMS — Reporting Verticals of BOTOX®” and “THE TERMS OF YOUR SERIES — The Reference Product” is based on information obtained from sources that we believe to be reliable. However, we have not independently verified the accuracy or completeness of such information.
PharmaSharesTM are not sponsored, endorsed, sold or promoted by AbbVie Inc. or any of its subsidiaries or affiliates or any other entity (referred to as a “related entity”) that is involved in the intellectual property ownership, development, production, marketing and/or sales of BOTOX®. None of AbbVie Inc., any of its subsidiaries or affiliates or any related entity has made any representation or warranty, express or implied, to the owners of a class of PharmaShares of Series BTOX, or any member of the public regarding the advisability of investing in securities generally or in any class of PharmaShares issued by Series BTOX particularly or the investor returns that may be obtained by comparing actual net revenues generated by BOTOX® over a defined period to revenue projections made by a select group of brokers. None of AbbVie Inc., its subsidiaries or affiliates or any related entity has any relationship to PharmaShares Advisors, LLC. None of AbbVie Inc., any of its subsidiaries or affiliate or any related entity is responsible for or has participated in determining the pricing, quantities or timing of any issuance or sale of Series BTOX 2020-1 or any other Series of PharmaShares by the PharmaShares Trust or the calculation of underlying value for Series BTOX 2020-1 or any other Series.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the United States Securities and Exchange Commission, or the “SEC,” a registration statement under the United States Securities Act of 1933, as amended, which we refer to as the “Securities Act,” with respect to the shares offered pursuant to the base prospectus and this prospectus supplement for the Up BTOX PharmaShares, Series BTOX-2020 and the Down BTOX PharmaShares, Series BTOX-2020. Each of the base prospectus and this prospectus supplement contains summaries of the material terms of the documents it refers to, but does not contain all of the information set forth in the registration statement of which the base prospectus and this prospectus supplement are part. For further information, we refer you to the registration statement. The SEC maintains an internet website that contains our registration statement, as well as reports, information statements and other information that we file electronically with the SEC. You may access the website at http://www.sec.gov. You may also access the registration statement and related documents at http://www.pharmashares.net.
The base prospectus and this prospectus supplement together constitute the offering document for the Up BTOX PharmaShares, Series BTOX-2020 and the Down BTOX PharmaShares, Series BTOX-2020. The base prospectus and this prospectus supplement do not constitute an offer of shares to any person in any state or other jurisdiction in which such offer would be unlawful.
You may inspect (1) copies of the master trust agreement for the PharmaShares Trust and the Series BTOX-2020 Trust Supplement to that master trust agreement, or the “Series BTOX Trust Supplement,” and (2) the records maintained by the trustee, the calculation agent and the administrative agent on behalf of Series BTOX at the office of the respective service provider during regular business hours upon two (2) business days’ prior notice. The office of the trustee and calculation agent is located at [●]. The office of the administrative agent is located at 36 Vincent Street, Chatham, New Jersey 07928.
REPORTS TO SHAREHOLDERS
We will prepare and file with the SEC, in accordance with the requirements of the United States Exchange Act of 1934, as amended, quarterly reports on Form 10-Q, annual reports on Form 10-K and current reports on Form
iii
8-K for the PharmaShares Trust and Series BTOX. You may contact your broker to obtain paper copies of these reports.
FORWARD-LOOKING STATEMENTS
The SEC encourages issuers to disclose forward-looking information so that investors can better understand the future prospects of their investments and make informed investment decisions. This prospectus supplement and the base prospectus contains these types of statements. Words such as “anticipates,” “estimates,” “expects,” “projects,” “intends,” “plans,” “believes” and words or terms of similar substance used in connection with any discussion of the future performance of the shares offered in this prospectus supplement are forward-looking statements. All forward-looking statements reflect our present expectation of future events and the realization of these future events is subject to a number of important variables that could cause actual results to differ materially from those described in the forward-looking statements. The “RISK FACTORS” section of the base prospectus and “RISK FACTORS RELATED TO YOUR SERIES” in this prospectus supplement provide examples of these variables. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this prospectus supplement and the base prospectus. Except for our ongoing obligation to disclose material information under the federal securities laws, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
iv
REFERENCE PRODUCT OVERVIEW
The following information is taken from historic public filings made by Allergan plc (“Allergan”) and, after Allergan was acquired by AbbVie Inc. (“AbbVie”), from public filings made by AbbVie. Additional information about BOTOX® is contained in those public filings.
Existing Indications
Product | Therapeutic Area | Active Ingredient | Therapeutic Classification |
BOTOX® Cosmetic | Facial Aesthetics | Onabotulinum Toxin A | Acetylcholine release inhibitor |
BOTOX® Hyperhidrosis | Medical Dermatology | Onabotulinum Toxin A | Acetylcholine release inhibitor |
BOTOX® Therapeutics | Neuroscience & Urology | Botulinum toxin | Musculoskeletal agent |
Potential New Indications/Delivery
Product | Therapeutic Area | Indication | Expected Launch Year | Phase |
BOTOX® | Medical Aesthetics | Platysma/Masseter | 2025/2023 | II |
Year of United States Food and Drug Administration (FDA) approval for therapeutic application: 1989
First year of commercial sales for therapeutic application: 1990
Year of FDA approval for cosmetic application: 2002
First year of commercial sales for BOTOX® Cosmetic: 2003
Initial year of patent expiry: 11/28/2014
Drug administration: Injection
Ownership profile of drug: AbbVie ownership of intellectual property and manufacturing rights, following AbbVie’s acquisition of Allergan.
Royalties or out-licensing: BOTOX® has been exclusively sold by Allergan and it is not out-licensed for sale. The license will continue to be held by Allergan, Inc., as subsidiary of AbbVie, or may need to be transferred to AbbVie in some regions.
1
SUMMARY OF PRINCIPAL TERMS
Reference Product and Value Reference Product | BOTOX® (OnabotulinumtoxinA) |
Reference Value
Geography covered
Indications covered
Out-licensed sales not covered | Net revenue for BOTOX® reported in U.S. dollars that are generated:
worldwide
for all indications
no exclusions |
Reporting Verticals of BOTOX® | Facial Aesthetics (BOTOX® Cosmetic), Medical Dermatology (BOTOX® Hyperhidrosis) and Neuroscience & Urology (BOTOX® Therapeutics) |
Reference pharmaceutical company | AbbVie Inc. BOTOX® was developed and manufactured by Allergan plc which was acquired and merged into AbbVie Inc. as of May 8, 2020. |
Reporting source of BOTOX® net revenue data Publication Dates | AbbVie Inc. periodic public filings on Form 10-Q and Form 10-K made with the United States Securities and Exchange Commission (“SEC”). The dates on which the reference pharmaceutical company files its periodic public reports. Historically, AbbVie Inc. has filed its 10-Q between 33 to 40 days following the end of Q1, Q2 and Q3 of each year and it has filed its 10-K between 47 to 58 days following the end of Q4. Publication dates may occur up to 45 days following the end of Q1, Q2 and Q3 and up to 90 days following the end of Q4. |
2
Calculation of Projected Breakpoints | |
Broker Reporting Group | The group of brokers who research and produce performance projections for BOTOX® and make their projections available to a market research integration service for the purpose of generating a consensus composite projection. The market research integration service will be Visible Alpha, FactSet or another investment technology firm that generates consensus models based on research models shared by participating brokers. |
Methodology for Calculating Projected Breakpoint |
The absolute value of the difference between projected net revenues for the Current Calendar Quarter and actual net revenues for the Base Calendar Quarter, divided by those actual net revenues.
Projected net revenues will be the median composite net revenues projection calculated by the market research integration service after removing the highest (maximum) and lowest (minimum) broker projections. The Projected Breakpoint will be compared against the Actual Percentage Change to determine the Up and Down Underlying Values on each Publication Date. |
Actual Percentage Change | The absolute value of the difference between the Reference Value for the Current Calendar Quarter and the Reference Value for the Base Calendar Quarter, divided by the Reference Value for the Base Calendar Quarter. |
Date of determination for each
Broker Reporting Group projection |
The median composite published most recently by the market research integration service but not earlier than two (2) business days prior to the first day of each calendar quarter.
|
Projected Breakpoint for Current Calendar Quarter |
See Appendix C.
|
3
Median composite, mean composite, standard deviation and high/low of broker projections for the Current Calendar Quarter |
See Appendix C.
|
Current Calendar Quarter | For each Publication Date and the ensuing Calculation Period, the calendar quarter for which the Reference Value was published on that Publication Date.
See Appendix C for the Current Calendar Quarter with respect to the current Projected Breakpoint. |
Base Calendar Quarter | The same calendar quarter as the Current Calendar Quarter but occurring 12 months prior.
See Appendix C for the Base Calendar Quarter with respect to the current Projected Breakpoint. |
Underlying Value Calculations | |
Up Underlying Value | The assets on deposit in Series BTOX to which the holders of the Up BTOX PharmaShares are entitled on any date of determination. On each Publication Date, the Up Underlying Value will be calculated as the product of (1) the Up Allocation Factor and (2) the Up Investment Amount. On the Periodic Calculation Date that follows each Publication Date, any increase in the Up Underlying Value will be declared as a distribution on the Up BTOX PharmaShares, together with an Equalization Payment. The Up Underlying Value will be reduced by the entire amount of this Periodic UV Performance Distribution made on the Up BTOX PharmaShares and will remain unchanged for the remainder of the current Calculation Period, except for the addition of any Up Daily Net Income Accruals. Up Daily Income Accruals will be added to the Up Underlying Value on each day of a Calculation Period if Series BTOX is earning net treasury income on its treasury securities during that Calculation Period. |
4
Down Underlying Value | The assets on deposit in Series BTOX to which the holders of the Down BTOX PharmaShares are entitled on any date of determination. On each Publication Date, the Down Underlying Value will be calculated as the product of (1) the Down Allocation Factor and (2) the Down Investment Amount. On the Periodic Calculation Date that follows each Publication Date, any increase in the Down Underlying Value will be declared as a distribution on the Down BTOX PharmaShares, together with an Equalization Payment. The Down Underlying Value will be reduced by the entire amount of this Periodic UV Performance Distribution made on the Down BTOX PharmaShares and will remain unchanged for the remainder of the current Calculation Period, except for the addition of any Down Daily Net Income Accruals. Down Daily Income Accruals will be added to the Down Underlying Value on each day of a Calculation Period if Series BTOX is earning net treasury income on its treasury securities during that Calculation Period. |
Per Share Underlying Value | For any date of determination, the Up Underlying Value divided by the number of outstanding Up BTOX PharmaShares. For any date of determination, the Down Underlying Value divided by the outstanding number of Down BTOX PharmaShares. |
Up Investment Amount | For any Calculation Period, an amount equal to one-half of all cash and treasuries on deposit in Series BTOX on the last Periodic Calculation Date.
The Up Investment Amount will always be equal to the Up Aggregate Par Amount, unless losses are realized on the treasury securities during any Calculation Period.
|
Down Investment Amount | An amount equal to one-half of all cash and treasuries on deposit in Series BTOX on the last Periodic Calculation Date. The Down Investment Amount will always be equal to the Down Aggregate Par Amount, unless losses are realized on the treasury securities during any Calculation Period.
|
5
Up Allocation Factor | A factor calculated on each Publication Date that reflects a comparison of the Projected Breakpoint to the Actual Percentage Change in net revenues from the Base Calendar Quarter to the Current Calendar Quarter. The Up Allocation Factor, when multiplied by the Up Investment Amount, will reflect the Up Underlying Value on that Publication Date.
Please see the Up Allocation Factor calculation and the Up Underlying Value formula, starting on page 21. |
Down Allocation Factor | A factor calculated on each Publication Date that reflects a comparison of the Projected Breakpoint to Actual Percentage Change in net revenues from the Base Calendar Quarter to the Current Calendar Quarter. The Down Allocation Factor, when multiplied by the Down Investment Amount, will reflect the Down Underlying Value on that Publication Date. Please see the Down Allocation Factor calculation and the Down Underlying Value formula, beginning on page 22. |
Up Daily Income Accruals and
Down Daily Income Accruals | Net treasury income for each Calculation Period, divided equally among all outstanding Up and Down BTOX PharmaShares. Net treasury income will be equal to the aggregate income earned on the treasury securities owned by Series BTOX, less the fees and expenses of Series BTOX, which will accrue at an accrual rate determined for each Calculation Period by the administrative agent, not to exceed an annual rate of 2.50% of the Series Investment Amount. |
Adjustments to Up and Down Underlying Value | Up Leverage Factor: [1]x
Down Leverage Factor: [3]x [No leverage] applies to the Up BTOX PharmaShares. The returns on the Down BTOX PharmaShares will be tripled by the Down Leverage Factor of [3]x. Up Periodic Return Cap: 15%
Down Periodic Return Cap: 15% The returns on both the Up and Down BTOX PharmaShares will be limited to 15% for each Calculation Period. The increase in the Up or Down Underlying Value, as applicable, for any Calculation Period will be reduced to equal 15% if such increase exceeds the Up or Down Periodic Return Cap. |
Distributions | |
6
Periodic Calculation Dates | Quarterly, on the fifth (5th) business day following each Publication Date. Periodic Income Distributions and Periodic UV Performance Distributions will be calculated and declared on each Periodic Calculation Date and distributed to holders two business days later. |
Calculation Period | The period that begins on but excludes the preceding Publication Date for Series BTOX and ends on and includes the next scheduled Publication Date. The first Calculation Period will begin on [●], 2020. |
Periodic Income Distributions | On each Periodic Calculation Date, a distribution equal to the sum of the Up Daily Income Accruals for the related Calculation Period will be declared on the Up BTOX PharmaShares. On each Periodic Calculation Date, a distribution equal to the sum of the Down Daily Income Accruals for the related Calculation Period will be declared on the Down BTOX PharmaShares. |
Periodic UV Performance Distributions | If an increase in the Up Underlying Value has occurred during a Calculation Period based on the actual performance of the Reference Value compared to the Projected Breakpoint, a distribution equal to that increase plus an Equalization Payment will be declared on the Up BTOX PharmaShares. If an increase in the Down Underlying Value has occurred during a Calculation Period based on the actual performance of the Reference Value compared to the Projected Breakpoint, a distribution equal to that increase plus an Equalization Payment will be declared on the Down BTOX PharmaShares. The Underlying Value of the class of PharmaShares that experienced an increase in Underlying Value will be reduced by the amount of the related Periodic UV Performance Distribution. The Underlying Value of the class of PharmaShares that experienced a decrease in Underlying Value in will be reduced by the amount of that decrease. Each Periodic UV Performance Distribution will decrease the amount of assets on deposit in Series BTOX and the potential investment returns that holders may earn in the future unless they purchase additional shares. |
7
Equalization Payments | Each Periodic UV Performance Distribution on the Up BTOX PharmaShares will consist of the increase in the Up Underlying Value for the related Calculation Period, which will be distributed out of the Down Investment Amount, and an Equalization Payment that will be equal to that increase in Underlying Value, but will be distributed out of the Up Investment Amount. Each Periodic UV Performance Distribution on the Down BTOX PharmaShares will consist of the increase in the Down Underlying Value for the related Calculation Period, which will be distributed out of the Up Investment Amount, and an Equalization Payment that will be equal to that increase in Underlying Value, but will be distributed out of the Down Investment Amount. The purpose of each Equalization Payment is to maintain the Up/Down Ratio. |
Up/Down Ratio | The proportion of the Up Investment Amount to the Down Investment Amount, which must be one to one (1:1) for so long as the Paired BTOX PharmaShares are outstanding. |
Fees and Expenses | |
Administrative Agent Fee | 1.25% per annum of the Series Investment Amount |
Trustee Fee | [●]% per annum of the Series Investment Amount |
Calculation Agent Fee | [●]% per annum of the Series Investment Amount |
Marketing Agent Fee | 0.35% of the Series Investment Amount |
Selling Commissions (initial offering) | $[●] |
Series BTOX formation costs and other fixed fees and expenses |
$[1,500,000]
|
Series Investment Amount | All assets on deposit in Series BOTX. Also, the sum of the Up and the Down Investment Amount. |
Daily Fee Accrual Rate | The daily rate determined for each Calculation Period by the administrative agent at which fees will accrue during that period, not to exceed an annualized rate of 2.50% per year. |
8
RISK FACTORS RELATED TO YOUR SERIES
An investment in the Up BTOX PharmaShares or the Down BTOX PharmaShares involves significant risks. You should carefully review the information contained in this section, as well as under “RISK FACTORS” in the base prospectus, before making an investment decision.
The acquisition of Allergan by AbbVie may affect the future net revenue performance of BOTOX®. On June 25, 2019, Allergan plc (“Allergan”), which was headquartered in Dublin, Ireland, and Abbvie Inc. (“Abbvie”), which is domiciled in the United States, announced that they had entered into an agreement pursuant to which Abbvie intended to acquire all of Allergan’s stock in exchange for stock of AbbVie and a cash payment, together reflecting a total valuation of $63 billion dollars. The acquisition was consummated on May 8, 2020, after AbbVie completed the process of obtaining regulatory approvals from all relevant government authorities, including the Federal Trade Commission and the Irish High Court. The combined company will continue to be incorporated as “AbbVie Inc.” in the State of Delaware and have its principal executive offices in North Chicago, Illinois.
It is difficult to predict how the acquisition will affect AbbVie, its developmental pipeline of drugs and the manufacturing and marketing support for AbbVie’s and Allergan’s respective on-market drugs, including BOTOX®. As with any merger of two companies, it will take significant time and effective and dedicated management efforts to integrate the operations of AbbVie and Allergan. If such integration is not successful or if AbbVie does not devote sufficient resources to support the manufacturing, market positioning or sales of BOTOX®, net revenues from BOTOX® may be adversely affected or may experience unpredictable fluctuations.
AbbVie currently publishes net revenue numbers as separate line items for 15 of its pharmaceutical products. BOTOX®, which had global net revenues of over $2.7 billion as of the nine months ended on September 30, 2019, will represent a major source of revenue for AbbVie. Accordingly, we expect that AbbVie will publish global net revenues for BOTOX® as a separate line item (or several line items that sum to equal such revenue) in its public reports on Form 10-Q and 10-K. However, if AbbVie were to cease publishing BOTOX® global net revenues on a quarterly basis as a separate line item or items for two consecutive calendar quarters, a termination trigger would occur and a final distribution equal to the Up underlying value of the Up BTOX PharmaShares and the Down underlying value of the Down BTOX PharmaShares would be distributed on the early termination date that followed the occurrence of this termination trigger.
The license to sell BOTOX® in the United States and other jurisdictions may be required to be transferred to AbbVie. During the period when that transfer is being effected, AbbVie will not be able to sell BOTOX®, which will result in a reduction in BOTOX® net revenues. If this reduction is significant enough such that there is a deviation from expected sales of more than 50% compared to the Reference Value reported on the last publication date prior to the effective date of the acquisition, it will qualify as a “major market disruption,” as described under “REFERENCE PRODUCTS AND REFERENCE VALUES” in the base prospectus. As a result, there will be deemed to be no change in the Reference Value on each publication date on which net revenues from BOTOX® indicate that a major market disruption is in effect. For so long as a major market disruption persists, the underlying value of the Up and Down BTOX PharmaShares will remain the same as it was on the last publication date prior to that disruption.
Demand for BOTOX® is seasonal. Sales of aesthetics products have historically been higher during the second and fourth quarters, presumably in advance of the summer vacation and holiday seasons. Fluctuations in BOTOX® sales are also impacted by the effect of promotional activity, which cause non-seasonal variability in sales trends. The consistent calendar quarter, year-over-year tracking method used for the Up BTOX PharmaShares and the Down BTOX PharmaShares may mitigate the impact of seasonality but may not fully eliminate the effect on your returns of seasonal fluctuations in demand for BOTOX®. It may be difficult to predict the effect of the seasonal demand for BOTOX® on the trading price of the Up BTOX PharmaShares and/or Down BTOX PharmaShares.
Demand for BOTOX® is likely to be impacted by the global pandemic. In addition to the risks discussed under “RISK FACTORS — The global pandemic may affect liquidity in the market for your shares, the ability of your Series to acquire treasuries and the performance of your reference drug” in the base prospectus, you should consider that sales of aesthetics products and drugs that are used to treat conditions which are not life-threatening
9
such as, in the case of BOTOX®, migraines, are likely to decrease during the COVID-19 global pandemic and any future pandemics. The population of certain states or countries may be required to quarantine at home and may be discouraged by government authorities or be individually fearful of seeking medical treatment, especially for conditions that are not life threatening. Further, a significant amount of B OTOX® sales and administration of the drug occurs in offices of dermatologists for aesthetic purposes. The delivery of a non-essential, injectable drug product within physician locations may be limited by city, county and state regulations during the pandemic. Additionally, the demographic of patients receiving non-essential injections may be one that voluntarily reduces certain treatments during the pandemic; thus, demand for such non-essential products may decrease during this period. After the re-opening of pandemic-related restrictions, sales of BOTOX® may experience a notable increase due to deferred demand. It may be difficult to predict the effect of these fluctuations in the demand for BOTOX® on the trading price of the Up BTOX PharmaShares and/or Down BTOX PharmaShares.
Factors that affect pricing and sale volume of BOTOX®. BOTOX® unit pricing and volume may fluctuate significantly. BOTOX® is sold for both cosmetic and therapeutic indications. A portion of BOTOX® sales for therapeutic application may be eligible for reimbursement by private or federal insurance providers, and such payment may be at a lower negotiated rate. The percentage reimbursement rate may impact the number of units sold, and the timing of sales during the course of a year due to an increasing number of beneficiaries with high deductible plans. In contrast, cosmetic indications for BOTOX® are generally not subject to pricing pressure from contracts with insurance providers. AbbVie may use rebates and other incentives during certain periods which may result in an increase in demand during those periods. General economic conditions in markets in which BOTOX® Cosmetic is sold may have an impact on both the volume of units sold and their price. Further, sales of BOTOX® for all indications are affected by competing products in the market. Some of the foregoing variables may increase sales of BOTOX® while others may reduce sales. The combined impact of these variables on net revenues from BOTOX® over time may be difficult to predict.
There are various risks associated with how BOTOX® is manufactured. Allergan manufactured BOTOX® exclusively in Ireland and it is expected that BOTOX® will continue to be manufactured there after the AbbVie acquisition. The manufacturing process utilizes sophisticated equipment, which requires a significant amount of time to obtain and install. The business would suffer if some part or all of the manufacturing facilities were to become inoperable for a period of time. This could occur for various reasons, including catastrophic events, such as natural disasters or terrorism, unexpected equipment failures or delays in obtaining spare parts, contamination by microorganisms or viruses, labor disputes or shortages, contractual disputes with suppliers and contract manufacturers, as well as construction delays or defects and other events, both within and outside of the control of AbbVie. In the event of a disruption in manufacturing processes, AbbVie may need to build or locate replacement facilities, as well as seek and obtain the necessary regulatory approvals for these facilities. The inability to manufacture BOTOX® in sufficient quantities to meet demand could have a material adverse effect on net revenues, but the magnitude and timing of such adverse effect may be difficult to predict.
Claims, consumer actions and government investigations may cause reputational harm to AbbVie and its products. As stated in their public filings, both AbbVie and Allergan, prior to its acquisition by AbbVie, have been subject to a variety of claims, proceedings, investigations and litigation related to its pharmaceutical products that have been initiated by government agencies and third parties. These claims, proceedings and investigations involve compliance matters, product regulation and safety, taxes, employee benefit plans, employment discrimination, health and safety, environmental, antitrust, customs, import/export, government contract compliance, financial controls and reporting, intellectual property, allegations of misrepresentation, false claims or false statements, commercial claims, claims regarding promotion of products and services, or other matters. With respect to BOTOX®, consumer groups and certain plaintiffs have alleged that certain uses of BOTOX®, including “off-label” uses, have caused patient injuries and death and that Allergan failed to adequately warn patients of the risks relating to BOTOX® use. Such allegations may directly adversely impact BOTOX® net revenue. Private claims and government investigations relating to other products manufactured by Allergan and AbbVie may indirectly have a negative impact on BOTOX® net revenues as a result of reputational harm suffered by AbbVie, for its own products, as well the products previously manufactured and marketed by Allergan. For example, Allergan was a manufacturer of breast implants. Reports about the quality and safety of breast implant devices are published from time to time, including reports that have suggested a link between cancer and breast implants, as well as negative reports from European regulatory authorities involving a breast implant manufacturer that was not affiliated with Allergan. Negative publicity, whether accurate or inaccurate, about the efficacy, safety or side effects of certain products or product categories, whether involving AbbVie, Allergan or a competitor, could materially reduce market acceptance of
10
AbbVie’s products, including BOTOX®, cause consumers to seek alternatives to these products and result in product withdrawals. Negative publicity could also result in an increased number of product liability claims, whether or not these claims have a basis in scientific fact. Any such claims, proceedings, investigations or litigation, regardless of the merits, might result in substantial restrictions on product use or sales, and cause volatility or cause harm to AbbVie’s business, including sales of BOTOX® the impact of which may be difficult to quantify in advance.
There has been litigation relating to BOTOX®. A class action complaint was filed in federal court in California on February 24, 2015, and amended May 29, 2015, alleging unlawful market allocation in violation of Section 1 of the Sherman Act, 15 U.S.C. § 1; agreement in restraint of trade in violation of 15 U.S.C. § 1 of the Sherman Act; unlawful maintenance of monopoly market power in violation of Section 2 of the Sherman Act, 15 U.S.C. § 2; violations of California’s Cartwright Act, Section 16700 et seq. of the California Business and Professions Code; and violations of California’s unfair competition law, Section 17200 et seq. of the California Business and Professions Code. In the complaint, plaintiffs seek an unspecified amount of treble damages. On July 19, 2016, plaintiffs filed a motion for class certification. On October 14, 2016, Allergan filed an opposition to plaintiffs’ motion for class certification. On June 13, 2017, the court granted plaintiff’s motion for class certification. In September 2017, the parties filed cross motions for summary judgment, which were heard by the court on October 27, 2017. On November 30, 2017, the parties reached a tentative settlement. Future litigation involving BOTOX® based upon drug marketing strategies or safety of product use may have an impact on sales and such impact may be difficult to quantify.
There are pending government investigations relating to certain BOTOX® indications. On April 18, 2017, Allergan received a Civil Investigative Demand (CID), dated April 12, 2017, from the United States Department of Justice seeking information relating to Allergan’s BOTOX® sales and marketing practices relating to urology practices. In its public filings, Allergan stated that it is cooperating fully with the United States Department of Justice requests. For so long as this investigation continues, its impact on the net revenues of BOTOX® may be difficult to predict.
Allergan Merger with Actavis Led to Atypical Sales Results for second and third quarters of 2015; AbbVie Acquisition May Also Result in a Temporary Disruption in Sales. On March 17, 2015, Actavis plc, or “Actavis,” acquired Allergan, Inc. for approximately $77.0 billion, including outstanding indebtedness assumed of $2.2 billion, cash consideration of $40.1 billion and equity consideration of $34.7 billion. As a result of the merger, Allergan, Inc. became a wholly-owned, indirect subsidiary of Actavis. On June 15, 2015, Actavis adopted the Allergan name and became known as Allergan plc. Following the merger, Allergan, Inc.’s existing licenses to sell its products were required to be transferred to Allergan plc. The licensing transfer process was not completed until post-merger and during this process, BOTOX® was not allowed to be sold. The inconsistent results during the period after the Actavis merger and any inconsistent results that may result during any required license transfer from Allergan to AbbVie may impact the accuracy of predictions about future BOTOX® net revenues.
11
THE TERMS OF YOUR SERIES
This is a prospectus supplement to the base prospectus for the offering of the Up BTOX PharmaShares and the Down BTOX PharmaShares. You should read this prospectus supplement in conjunction with the base prospectus, including the “RISK FACTORS” and “FORWARD-LOOKING STATEMENTS” sections in the base prospectus, before making an investment decision.
Please note that when we refer in this section to the “Underlying Value” that is represented by your Up BTOX PharmaShares or your Down BTOX PharmaShares on any date, we mean the portion of the net asset value of Series BTOX that your shares represent on that date and also the final distribution that you would be entitled to receive if that date were an early termination date or the final scheduled termination date. Such a distribution is, however, merely hypothetical and we refer to it solely for the purpose of explaining the meaning of Underlying Value.
This prospectus supplement uses defined terms. Terms are defined the first time they appear in this prospectus supplement, but you can also find definitions of important terms used in this prospectus supplement under “GLOSSARY OF DEFINED TERMS” beginning on page 105 of the base prospectus.
The Issuer and the Securities Offered
We are PharmaShares Advisors, Inc. and we have formed Series BTOX-2020 of the PharmaShares Trust, which is referred to in this prospectus supplement as “Series BTOX,” for the purpose of issuing the Up BTOX PharmaShares, Series BTOX-2020, or the “Up BTOX PharmaShares,” and the Down BTOX PharmaShares, Series BTOX-2020, or the “Down BTOX PharmaShares.” As a holder of Up BTOX PharmaShares or a holder of Down BTOX PharmaShares, your rights will be governed by the “Master Trust Agreement” entered into among us, as depositor and administrative agent, [●], as trustee and calculation agent, and [●], as marketing agent, pursuant to which we formed the PharmaShares Trust on [●], 2020, and the “Series BTOX Trust Supplement” to that agreement, pursuant to which we formed Series BTOX on [●], 2020.
For more information about the PharmaShares Trust, see “THE PHARMASHARES TRUST” in the base prospectus. For a description of the terms of the Master Trust Agreement and the Series BTOX Trust Supplement, see “DESCRIPTION OF THE TRUST AGREEMENT” in the base prospectus.
The Up BTOX PharmaShares and the Down BTOX PharmaShares are referred to as the “Paired BTOX PharmaShares” and represent undivided beneficial interests in the assets of Series BTOX. The trustee will deposit the proceeds of the initial sale and each subsequent issuance of the Paired BTOX PharmaShares in a segregated Series account and hold those funds for the exclusive benefit of the shareholders of Series BTOX, separate from the assets and liabilities of all other Series of the PharmaShares Trust. Pursuant to the Master Trust Agreement and the Series BTOX Trust Supplement, the assets of Series BTOX will not be available to satisfy the liabilities of, or to make distributions on the shares issued by, any other Series, and the assets of other Series of the PharmaShares Trust will not be available to make distributions on the Paired BTOX PharmaShares.
The Paired BTOX PharmaShares must be created and redeemed concurrently in PharmaShares Units consisting of an Aggregate Par Amount of Up BTOX PharmaShares equal to $1,000,000 and an Aggregate Par Amount of Down BTOX PharmaShares equal to $1,000,000. These ongoing paired creations are referred to as “Paired Issuances” and the paired redemptions as “Paired Optional Redemptions.” The Aggregate Par Amount of the Up BTOX PharmaShares and the Aggregate Par Amount of the Down BTOX PharmaShares will be equal to, as of any date of determination, respectively:
·the product of (1) the aggregate number of issued Up BTOX PharmaShares that remain outstanding on that date and (2) the par amount of one Up BTOX PharmaShare on that date; and
·the product of (1) the aggregate number of issued Down BTOX PharmaShares that remain outstanding on that date and (2) the par amount of one Down BTOX PharmaShare on that date.
12
Periodic distributions consisting of increases in the Up or Down Underlying Value will decrease the amount of assets in Series BTOX. As a result, the par amount of each Up BTOX PharmaShare and each Down BTOX PharmaShare will be recalculated on each Periodic Calculation Date after any Periodic UV Performance Distribution is made on those shares. The new par amount per Up BTOX PharmaShare will be the Up Investment Amount after the distribution has been made, divided by the number of outstanding Up BTOX PharmaShares. The new par amount per Down BTOX PharmaShare will be the Down Investment Amount after the distribution has been made, divided by the number of outstanding Down BTOX PharmaShares.
The declining par amount of your Up BTOX PharmaShares or Down BTOX PharmaShares reflects the decreasing amount of your investment in those shares as a result of Periodic UV Performance Distributions. The only way to maintain a consistent level of investment in your Up or Down BTOX PharmaShares is to purchase additional shares, as discussed under “RISK FACTORS — The amount of your investment in your PharmaShares and your potential gains will decline over time” in the base prospectus.
Use of Proceeds
The trustee will apply the proceeds received by Series BTOX in connection with the initial and each subsequent Paired Issuance to acquire, in accordance with the directions of the administrative agent and on behalf of Series BTOX, U.S. treasury securities and overnight repurchase agreements collateralized by United States Treasury securities, of the type and tenor described under “THE PHARMASHARES TRUST — United States Treasury Obligations” in the base prospectus. PharmaShares Advisors, LLC will pay all of the costs associated with the formation of Series BTOX and the issuance, registration, marketing and offering of the Paired BTOX PharmaShares. PharmaShares Advisors, LLC will reimburse itself for these costs out of the treasury income realized by Series BTOX, subject to a maximum fee rate, as discussed under “— Fees and Expenses” in this prospectus supplement.
The Administrative Agent
We are PharmaShares Advisors, LLC, a Delaware limited liability company, and we are acting as “administrative agent” for the PharmaShares Trust and Series BTOX pursuant to the Master Trust Agreement and the Series BTOX Trust Supplement. Our duties as administrative agent are described in the base prospectus under “DESCRIPTION OF THE TRUST AGREEMENT — The Administrative Agent.” For performing our duties as administrative agent for Series BTOX, we will receive a fee equal to an annual rate of 1.25% accrued on the Series Investment Amount on each calendar day, which will be payable in arrears on each Periodic Calculation Date.
The Marketing Agent
[●], a [●], will act as “marketing agent” for Series BTOX pursuant to the terms of the Master Trust Agreement and the Series BTOX Trust Supplement. Its duties as marketing agent are described under “DESCRIPTION OF THE TRUST AGREEMENT — The Marketing Agent” in the base prospectus. For performing the duties of marketing agent for Series BTOX, [●] will receive a fee equal to an annual rate of 0.35% accrued on the Series Investment Amount on each calendar day, which will be payable in arrears on each Periodic Calculation Date. [●] may engage other parties from time to time to act as additional marketing agents for Series BTOX. [●] will compensate these parties from the fee payable to it as a marketing agent.
The Trustee
[●], a [●], is acting as “trustee” for the PharmaShares Trust and Series BTOX pursuant to the terms of the Master Trust Agreement and the Series BTOX Trust Supplement. The trustee will perform, on behalf of Series BTOX, the duties described in the base prospectus under “DESCRIPTION OF THE TRUST AGREEMENT — Duties of the Trustee and the Calculation Agent.”
For performing its duties as trustee for Series BTOX, the trustee will receive a fee equal to an annual rate of [●]% accrued on the Series Investment Amount on each calendar day, or an annual minimum fee of $[●], whichever is greater, which will be payable in arrears on each Periodic Calculation Date.
13
The Calculation Agent
[●] will also act as “calculation agent” for the PharmaShares Trust and Series BTOX pursuant to the terms of the Master Trust Agreement and the Series BTOX Trust Supplement. The calculation agent will perform, on behalf of Series BTOX, the duties described in the base prospectus under “THE PHARMASHARES TRUST — Daily Reporting” and “DESCRIPTION OF THE TRUST AGREEMENT — Duties of the Trustee and the Calculation Agent” and under “ — Daily Reporting” below.
For performing its duties as calculation agent for Series BTOX, the calculation agent will receive a fee equal to an annual rate of [●]% accrued on the Series Investment Amount on each calendar day, which will be payable in arrears on each Periodic Calculation Date.
Daily Reporting
The calculation agent will calculate (1) the Per Share Underlying Value of the Up BTOX PharmaShares and the Per Share Underlying Value of the Down BTOX PharmaShares on each Publication Date after a new Reference Value is published, (2) the Per Share Underlying Value of the Up BTOX PharmaShares and the Down BTOX PharmaShares on each Periodic Calculation Date, after any increase in the Up or Down Underlying Value has been distributed as a Periodic UV Performance Distribution, and (3) on each day of a Calculation Period that follows the Periodic Calculation Date, the Up and Down Underlying Value plus the Up or Down Daily Net Income Accruals for each day, if any. At the close of each business day, the calculation agent will perform these calculations for that business day or, if that business day is followed by one or more intervening non-business days, for that business day and each such intervening non-business day. It will provide these calculations to the administrative agent for posting on the website maintained by the administrative agent at http://www.pharmashares.net not later than one hour prior to the commencement of trading on NYSE Arca on the next business day that follows the day of calculation. The administrative agent will also calculate and post on its website on each business day, not later than one hour prior to commencement of trading on NYSE Arca, (1) the premium or discount of the midpoint of the bid/offer price spread for one Up BTOX PharmaShare at the close of the preceding trading day over the Per Share Underlying Value of one Up BTOX PharmaShare on that day and (2) the premium or discount of the midpoint of the bid/offer price spread for one Down BTOX PharmaShare at the close of the preceding trading day over the Per Share Underlying Value of one Down BTOX PharmaShare on that day.
The calculation agent will base its calculation of the Up Underlying Value and the Down Underlying Value on each Publication Date on the Reference Value that was reported on that Publication Date and the Projected Breakpoint for the preceding calendar quarter. On the Periodic Calculation Date that follows each Publication Date, the calculation agent will calculate the Periodic UV Performance Distribution that will be made on the class which experienced an increase in its Underlying Value. The calculation agent will also calculate the Up and Down Underlying Values after that Periodic UV Performance Distribution has been made, decreasing the Underlying Value of the class that receives that distribution by the entire amount of the distribution. On each remaining calendar day of the Calculation Period, the Up and Down Underlying Value will remain the same, except that the calculation agent will add any Up Daily Net Income Accruals to the Up Underlying Value and any Down Daily Net Income Accruals to the Down Underlying Value. All treasury income earned on the assets in Series BTOX, less the fees and expenses of Series BTOX, will be allocated equally among the Up and Down BTOX PharmaShares.
Public companies are required to report their earnings on a quarterly basis and, accordingly, Publication Dates for the Reference Value will occur no more frequently than quarterly. As a result, the Underlying Value of the Up BTOX PharmaShares and the Underlying Value of the Down BTOX PharmaShares will remain the same throughout each Calculation Period except for the addition of Up Daily Net Income Accruals and Down Daily Net Income Accruals to that Underlying Value.
Information reported by or about AbbVie or BOTOX® during the course of each calendar quarter, including third party data and information services that provide prescription trend data on a weekly or monthly basis for various pharmaceutical products, may affect the expected Underlying Value of the Up and Down BTOX PharmaShares on the next Publication Date. In anticipation of that future impact on Underlying Value, the current trading price of the Up and/or the Down BTOX PharmaShares during the current Calculation Period may be affected. However, such market knowledge and expectations will not be reflected in the current Up Underlying
14
Value or Down Underlying Value calculation. See “RISK FACTORS — Fluctuations in the per share underlying value of your PharmaShares and other factors may affect their trading price” in the base prospectus.
The Projected Breakpoint Increase or Projected Breakpoint Decrease for each calendar quarter will be calculated and disclosed not later than 8 p.m. E.T. on the last business day preceding the first day of each calendar quarter by means of the filing of an updated registration statement. The following variables required to calculate the Projected Breakpoint will also be disclosed: (1) the median composite projection, calculated after eliminating the highest and lowest projections, that was published by the market research integration service most recently, but not later than the second business date prior to the beginning of each calendar quarter, (2) the list of all brokers whose projections are eligible to be included by the market research integration service in any composite, based upon such service’s established guidelines, (3) the subset of brokers who made projections for the Current Calendar Quarter and are included in the current composite, and (4) actual net revenues reported for the applicable Base Calendar Quarter. The Projected Breakpoint and its related inputs will be disclosed for each calendar quarter in an updated Appendix C to this prospectus supplement.
The Series Assets
Series BTOX will hold its assets in segregated accounts solely for the benefit of its shareholders and will not make such assets available to the creditors or shareholders of any other Series. Series BTOX will have no rights with respect to the assets held by any other Series of the PharmaShares Trust.
The assets of Series BTOX consist of:
·U.S. treasury securities of the type and tenor described in the base prospectus under “THE PHARMASHARES TRUST — United States Treasury Obligations,” and what we refer to as “income” on those securities, consisting of stated interest on treasury notes and bonds and the discount that is realized when the par amount received on a treasury bill, note or bond at maturity exceeds the purchase price at which that treasury security was acquired on behalf of Series BTOX;
·treasury repurchase agreements with the characteristics described in the base prospectus under “THE PHARMASHARES TRUST — United States Treasury Obligations,” and what we refer to as “income” on those agreements consisting of the difference between the purchase price and the repurchase price for the treasury securities borrowed under those agreements;
·the rights of Series BTOX under the Master Trust Agreement and the Series BTOX Trust Supplement to rely on the services provided by the trustee, the calculation agent, the administrative agent and the marketing agent;
·a securities account into which all of the treasury securities acquired from time to time on behalf of Series BTOX are deposited for the benefit of its shareholders;
·a distribution account into which all income and maturity proceeds realized on the treasury securities are deposited and used to make (1) required deposits into the fee payment account and (2) periodic distributions to the holders of the Up and Down BTOX PharmaShares; and
·a fee payment account into which all or a portion of the income realized on the treasury securities will be deposited on each Periodic Calculation Date and applied to pay the fees and expenses of Series BTOX.
The treasury securities and treasury repurchase agreements, referred to generically as “treasury securities,” purchased on behalf of Series BTOX in connection with each Periodic Calculation Date or each issuance will have the terms described in the base prospectus, including that each treasury security must mature or terminate prior to the next scheduled Periodic Calculation Date. The procedures that must be followed by the administrative agent and the trustee in acquiring treasury securities for Series BTOX and selecting treasury securities for delivery in redemptions are described in the base prospectus. See “THE PHARMASHARES TRUST — United States Treasury Obligations” in the base prospectus.
15
The Reference Value
The Paired BTOX PharmaShares track (x) the projected change in the Reference Value from the Base Calendar Quarter to the Current Calendar Quarter, calculated based upon projections for the Reference Value that were made by the Broker Reporting Group for the Current Calendar Quarter, compared to (y) the actual change in the Reference Value between the same two calendar quarters. The “Reference Value,” is the net revenue generated by worldwide sales of BOTOX® for all indicated uses of the drug, as reported by AbbVie on each Publication Date in its income statement prepared in accordance with U.S. GAAP and filed with the SEC as part of AbbVie’s periodic public reports under the Exchange Act. The sole reporting source of BOTOX® net revenue data will be AbbVie’s quarterly reports on Form 10-Q and its annual reports on Form 10-K.
Series BTOX uses year-over-year net revenue tracking, from the Current Calendar Quarter to the same quarter of the preceding year, which is referred to as the “Base Calendar Quarter.” The “Publication Date” for each calendar quarter will occur within 45 days, or, in the case of the fourth quarter of each year, within 90 days, following the last day of that calendar quarter. AbbVie typically files its periodic reports between 33 to 40 days after the end of each of the first three (3) calendar quarters and 47 to 58 days following year end, but it may elect at any time to file its reports for a particular quarter at some earlier or later date that occurs prior to the expiration of the 45 or 90 days, as applicable, that is permitted under the Exchange Act. Each “Calculation Period” for Series BTOX will begin on and include a Publication Date and end on but exclude the following Publication Date. Accordingly, the length of each Calculation Period may vary.
The entity that manufactured and sold BOTOX® worldwide was Allergan, Inc., which was a wholly-owned, indirect subsidiary of Allergan plc. Allergan plc was acquired by, and merged into, by AbbVie on May 8, 2020. BOTOX®, its indicated uses, AbbVie, as its new manufacturer and sales agent, and other facts about the reference drug that may be relevant to you as an investor in the Up or Down BTOX PharmaShares are described below under “ — The Reference Product.”
After a Periodic UV Performance Distribution has been declared on any Periodic Calculation Date, neither the Reference Value nor the calculation of Underlying Value will be retroactively adjusted to take into account any revisions made to the calculation of BOTOX® net revenues by AbbVie.
We, the PharmaShares Trust, Series BTOX, any other Series created from time to time, our affiliates and our respective directors, managers, officers, members, trustees, employees, attorneys and advisors are not liable for (a) any losses to you resulting from, the methodologies used in calculating, and the actual calculation of, net revenues of BOTOX®, or the resulting Reference Value, or (b) any losses to you for any revisions to the methodology for calculating net revenues, any subsequent revisions made to published net revenues, any alleged or actual inaccuracies or inherent limitations of the net revenues calculation, any delays in net revenue reporting or publication, any errors in the underlying data used to calculate net revenues, or the impact of any of the foregoing factors on the yield realized by you as a holder of Up BTOX PharmaShares or Down BTOX PharmaShares.
None of (1) AbbVie, (2) the brokers included in the Broker Reporting Group from time to time, (3) the market research integration service, or (4) the respective affiliates and respective directors, officers, members, trustees, employees, attorneys and advisors of any of the entities listed in (1), (2) and (3), are affiliated with us and no such entity or person has been or will in any way be involved with the PharmaShares Trust, Series BTOX, any other Series created from time to time or the issuance of the Paired BTOX PharmaShares and will not be liable for (a) any disclosure contained in the base prospectus or this prospectus supplement, (b) any losses to you resulting from, the methodologies used in calculating, and the actual calculation of, net revenues of BOTOX®, the resulting Reference Value, the Projected Breakpoint or the Underlying Value of the Up or Down BTOX PharmaShares, or (c) any losses to you for any revisions to the methodology for calculating net revenues, any subsequent revisions made to published net revenues, any alleged or actual inaccuracies or inherent limitations of the net revenue calculation, any delays in net revenue reporting or publication, any errors in the underlying data used to calculate net revenues, or the impact of any of the foregoing factors on the yield realized by you as a holder of Up BTOX PharmaShares or Down BTOX PharmaShares.
16
The Reference Product
BOTOX® is the Reference Product for Series BTOX. BOTOX®, also known as Onabotulinum Toxin A, is an acetylcholine release inhibitor and a neuromuscular blocking agent. It is delivered by injection for intramuscular, intradetrusor, or intradermal use.
BOTOX® Medical
BOTOX® is available by prescription only for the following indicated conditions:
·to treat overactive bladder symptoms such as a strong need to urinate with leaking or wetting accidents (urgent urinary incontinence), a strong need to urinate right away (urgency), and urinating often (frequency) in adults 18 years and older when another type of medicine (anticholinergic) does not work well enough or cannot be taken;
·to treat leakage of urine (incontinence) in adults 18 years and older with overactive bladder due to neurologic disease who still have leakage or cannot tolerate the side effects after trying an anticholinergic medication;
·to prevent headaches in adults with chronic migraine who have 15 or more days each month with headache lasting four (4) or more hours each day in people 18 years or older;
·to treat increased muscle stiffness in elbow, wrist, finger, thumb, ankle, and toe muscles in people 18 years and older with upper and lower limb spasticity;
·to treat the abnormal head position and neck pain that happens with cervical dystonia (CD) in people 16 years and older;
·to treat certain types of eye muscle problems (strabismus) or abnormal spasm of the eyelids (blepharospasm) in people 12 years and older; and
·to treat the symptoms of severe underarm sweating (severe primary axillary hyperhidrosis) in people 18 years and older when topical medicines used on the skin do not work well enough.
It is not known whether BOTOX® and BOTOX® Cosmetic are safe or effective to prevent headaches in patients with migraines who have 14 or fewer headache days each month (episodic migraine).
It is not known whether BOTOX® is safe or effective to treat increased stiffness in upper limb muscles other than those in the elbow, wrist, fingers, and thumb, or in lower limb muscles other than those in the ankle and toes. BOTOX® has not been shown to help people perform task-specific functions with their upper limbs or increase movement in joints that are permanently fixed in position by stiff muscles. BOTOX® is not meant to replace existing physical therapy or other rehabilitation that may have been prescribed.
It is not known whether BOTOX® and BOTOX® Cosmetic are safe or effective for severe sweating anywhere other than in the armpits.
BOTOX® Cosmetic
BOTOX® Cosmetic is available by prescription only for the following cosmetic indications in adults:
·to temporarily improve the look of moderate to severe frown lines between the eyebrows (glabellar lines);
·to temporarily improve the look of moderate to severe crow’s feet lines (lateral canthal lines); and
·to temporarily improve the look of forehead lines.
17
Patent History on BOTOX®
Patent Number | Priority Date | Use |
US6667041B2 | 7/15/1997 | Use of neurotoxin therapy for treatment of urologic and related disorders |
US7001602B2 | 7/15/1997 | Use of botulinum toxin therapy for urinary incontinence and related disorders |
US7429387B2 | 7/15/1997 | Use of botulinum toxin therapy for treatment of recalcitrant voiding dysfunction |
US7449192B2 | 7/15/1997 | Use of neurotoxin therapy for treatment of urologic and related disorders related to neurogenic bladder dysfunction |
US7968104B2 | 7/15/1997 | Use of neurotoxin therapy for treatment of urologic and related disorders |
US8057807B2 | 7/15/1997 | Use of botulinum toxin therapy for treatment of recalcitrant voiding dysfunction |
US8062643B2 | 7/15/1997 | Use of neurotoxin therapy for treatment of urologic and related disorders |
US8501195B2 | 3/30/2010 | Injection paradigm for administration of botulinum toxins |
Reference Pharmaceutical Company
Until it was acquired by AbbVie, Allergan, Inc. manufactured and sold BOTOX®. Allergan, Inc. was formed in 1948, incorporated in 1950 and became a public company in 1970. Allergan, Inc. operated as a global pharmaceutical company focused on eye care, neurosciences, medical dermatology, medical aesthetics, breast enhancement, obesity intervention and urologics. On March 17, 2015, Actavis plc, a global pharmaceutical company, acquired Allergan, Inc. in a merger transaction. As a result of the merger, Allergan, Inc. became a wholly-owned, indirect subsidiary of Actavis plc, or “Actavis.” On June 15, 2015, Actavis adopted the “Allergan” name and became known as Allergan plc. In connection with the merger, Allergan, Inc.’s existing licenses to sell its products were required to be transferred to Allergan plc. The licensing transfer process was not completed until post-merger and during this process, BOTOX® was not allowed to be sold. The inconsistent results during this historical period may impact the accuracy of predictions of BOTOX® future net revenue.
AbbVie completed its acquisition of Allergan plc on May 8, 2020. AbbVie is a research-based bio-pharmaceutical company that was established in January of 2013, when Abbott Laboratories spun off its drug division to concentrate on its medical devices business. AbbVie has announced its intention to market the cosmetic indications of BOTOX® under a new global subsidiary, Allergan Aesthetics, while integrating medical BOTOX® directly into AbbVie. The final structure of AbbVie as Allergan and its subsidiaries are merged into it will be determined over the course of the remainder of 2020 and possibly beyond. Depending upon how Allergan is merged into AbbVie, the license to market BOTOX® may not need to be transferred to a new entity or may need to be transferred only in certain regions of the world.
For a discussion of the risks related to the AbbVie acquisition, see “RISK FACTORS — The acquisition of Allergan by AbbVie may affect the future net revenue performance of BOTOX®.”
Net Revenue for the Reference Product
“Net revenue” means the gross worldwide sales of BOTOX® for all indicated uses of the drug, adjusted for sales-related deductions, including, but not limited to, chargebacks, trade discounts, sale returns and allowances, commercial and government rebates, customer loyalty programs and fee-for-service arrangements with certain distributors, collectively referred to as “sales allowances.” For a discussion of how sales allowances are applied to adjust net revenue, see “REFERENCE PRODUCTS AND REFERENCE VALUES” in the base prospectus.
18
The Projected Breakpoint
The Up Underlying Value and Down Underlying Value will determine the amount of distributions that will be declared on each Periodic Calculation Date on the Up or Down BTOX PharmaShares and the final distribution that will be made in redemption of the Up and Down BTOX PharmaShares. The Up and Down Underlying Value will be calculated by comparing
·the Projected Breakpoint Increase or Projected Breakpoint Decrease from the Base Calendar Quarter to the Current Calendar Quarter, to
·the actual percentage increase or decrease in BOTOX® net revenues from the Base Calendar Quarter to the Current Calendar Quarter.
The "Projected Breakpoint Increase" or "Projected Breakpoint Decrease" (also generically referred to as the "Projected Breakpoint") is the percentage change calculated by subtracting actual net revenues for BOTOX® for the Base Calendar Quarter from projected net revenues for the Current Calendar Quarter and dividing the absolute value of the result by actual net revenues. If projected revenues for the Current Calendar Quarter exceed actual revenues for the Base Calendar Quarter, there will be a Projected Breakpoint Increase. If projected revenues for the Current Calendar Quarter are less than actual revenues for the Base Calendar Quarter, there will be a Projected Breakpoint Decrease.
For Series BTOX, the projected net revenues for each calendar quarter will be calculated based on the revenue projections made by a Broker Reporting Group.
The “Broker Reporting Group” for Series BTOX will consist of the group of brokers who make their research models for BOTOX® available to Visible Alpha, FactSet or a similar company, referred to in this prospectus supplement as a “market research integration service.” Each broker is registered with FINRA and employs analysts who have historically published and continue to publish net revenue projections for BOTOX®. Members of the Broker Reporting Group are generically referred to as “brokers.”
Not all broker projections will be included in the composite number for any particular calendar quarter, because a projection may be stale, as defined by the market research integration service, or a broker may not have published a BOTOX® projection for the Current Calendar Quarter. Due to the seasonal demand for BOTOX®, many brokers publish projections for only the higher-demand second and fourth calendar quarters.
The hypothetical returns in Appendix A to this prospectus supplement illustrate the performance of the Up and Down BTOX PharmaShares over several consecutive calendar quarters based on historical BOTOX® net revenue performance and historical composite numbers published by a market research integration service. The historical composite numbers are based on a Broker Reporting Group consisting of the brokers who reported for BOTOX® for each past calendar quarter that is shown. The hypothetical returns are based on no leverage (1x) on the Up BTOX PharmaShares and tripled (3x) returns on the Down BTOX PharmaShares. The actual leverage applicable to each class of the PharmaShares will be determined at closing and reflect investor demand for each class of the PharmaShares and the requirement that an equal number of Up and Down BTOX PharmaShares must be issued.
The Broker Reporting Group for the Paired BTOX PharmaShares must at all times have the following characteristics:
·there must be at least five (5) brokers in the Broker Reporting Group who make their projections available to the market research integration service for the purpose of allowing it to generate the composite projection; however, so long as there are at least five (5) brokers, the market research integration service may add, remove or substitute brokers in the group at any time in its sole discretion;
·at least three (3) projections from brokers in the Broker Reporting Group must be used by the market research integration service to calculate the composite for each calendar quarter; and
19
·if fewer than five (5) brokers are included in the Broker Reporting Group during any calendar quarter or fewer than three (3) projections are used for any composite calculation, then the Underlying Values of the Paired BTOX PharmaShares will remain unchanged at the end of the affected calendar quarter.
The composite generated by the same market research integration service will be used to calculate the Projected Breakpoint throughout the term of the Paired BTOX PharmaShares unless the service ceases to generate a composite for BOTOX®. A Termination Trigger will occur if, on two consecutive calendar quarters, the market research integration service fails to generate the composite projection for BOTOX® and we are unable to identify and appoint a replacement market research integration service.
Average Revenue Projection Methodology
The market research integration service will calculate and publish, for each calendar quarter, (1) the median of the broker projections (both with the highest and lowest projections included and after removing them), (2) the mean of the broker projections (both with the highest and lowest projections included and after removing them), and (3) the standard deviation of the broker projections. If there is an even number of projections made for any calendar quarter, the median will be calculated as the average of the two middle projections. For Series BTOX, the Projected Breakpoint will be calculated using the median of the Broker Reporting Group projections (after high/low projections are removed).
The calculation agent will use the composite median projection published by the market research integration service on the most recent date that precedes but is not less than two (2) business days prior to the beginning of the Current Calendar Quarter. The calculation agent will calculate the Projected Breakpoint for the Current Calendar Quarter by subtracting actual net revenues for the Base Calendar Quarter from the composite median projection and dividing the absolute value of the result by those actual net revenues.
Reporting
The Projected Breakpoint Increase or Decrease for each calendar quarter will be calculated and announced not later than 8 p.m. EST on the business day preceding each calendar quarter by means of the filing of an updated registration statement. The Projected Breakpoint will be disclosed in an updated Appendix C to this prospectus supplement. The mean, median, high, low and standard deviation published by the market research integration service for BOTOX® net revenues and the number of brokers whose projections were used in those calculations will also be disclosed. Revised guidance from any broker who is a member of the Broker Reporting Group after the start of any calendar quarter will not be used to recalculate any previously determined Projected Breakpoint.
As noted above, the composite projection may include only that subset of the brokers in the Broker Reporting Group for any particular calendar quarter that actually published projections for that quarter. Neither the identity of the brokers in this subset nor their respective projections will be disclosed for any calendar quarter. However, if the market research integration service adds or removes any members of its broker group as a whole, the updated group will be disclosed in Appendix C. The actual projections published by each broker as part of its research on BOTOX® may be accessed by subscription to various financial information services and platforms, including Bloomberg, FactSet, Eikon and PitchBook.
Calculation of Underlying Value
To calculate Underlying Value on any Publication Date in this year-over-year tracking Series, the calculation agent first determines the “Actual Percentage Change” by subtracting the Reference Value for the Base Calendar Quarter from the Reference Value for the Current Calendar Quarter that was published on the most recent Publication Date and dividing the absolute value of the result by the Reference Value for the Base Calendar Quarter. The resulting Actual Percentage Change is then compared against the Projected Breakpoint Increase or Decrease that was calculated at the beginning of the Current Calendar Quarter based on actual results for the Base Calendar Quarter and projected revenues for the Current Calendar Quarter. The formulas for calculating the Up Underlying Value and the Down Underlying Value are described below.
The Up Underlying Value and Down Underlying Value calculated on each Publication Date will determine the amount of the Periodic UV Performance Distributions that will be declared on the next Periodic Calculation
20
Date. If the Up Underlying Value has increased, the Up BOTX PharmaShares will receive a Periodic UV Performance Distribution equal to that increase plus an Equalization Payment. Conversely, if the Down Underlying Value has increased, the Down BTOX PharmaShares will receive a Periodic UV Performance Distribution equal to that increase plus an Equalization Payment.
After a Periodic UV Performance Distribution is made on the Up BTOX PharmaShares, the Up Underlying Value will be reduced by the entire amount of that distribution. Conversely, after a Periodic UV Performance Distribution is made on the Down BTOX PharmaShares, the Down Underlying Value will be reduced by the entire amount of that distribution. After each Periodic UV Performance Distribution has been made, the Up and Down Underlying Values will be the same and will remain unchanged during the ensuing Calculation Period, except for the addition to those values of Up and Down Daily Net Income Accruals, if any.
On each Periodic Calculation Date, the “Up Leverage Factor” of [1]x reflects that [no leverage] will be applied to returns on the Up BTOX PharmaShares and the “Down Leverage Factor” of [3]x reflects that returns on the Down BTOX PharmaShares will be [tripled]. On each Periodic Calculation Date, if the returns on the Up BTOX PharmaShares or the Down BTOX PharmaShares exceed 15% based on the performance of the Reference Value, those returns, as reflected in the increase in the Up Underlying Value or the increase in the Down Underlying Value, as applicable, will be reduced to equal not more than 15%, calculated based on the aggregate par amount of the Up and Down BTOX PharmaShares at the beginning of the related Calculation Period. These caps on returns are referred to as the “Up Periodic Return Cap” and the “Down Periodic Return Cap,” and either may generically be referred to as a “Periodic Return Cap.” The application of the Down Periodic Return Cap may eliminate the effect of the Down Leverage Factor.
The “Up Underlying Value” for the Up BTOX PharmaShares will be equal, on each Publication Date, to:
·the Up Investment Amount multiplied by the Up Allocation Factor that was calculated based on the Reference Value published on that Publication Date and the Projected Breakpoint for the preceding calendar quarter
plus
·the sum of the Up Daily Net Income Accruals for each day that has elapsed during the current Calculation Period, up to and including the current calendar day.
The “Down Underlying Value” for the Down BTOX PharmaShares will be equal, on each Publication Date, to:
·the Down Investment Amount multiplied by the Down Allocation Factor that was calculated based on the Reference Value published on that Publication Date and the Projected Breakpoint for the preceding calendar quarter
plus
·the sum of the Down Daily Net Income Accruals for each day that has elapsed during the current Calculation Period, up to and including the current calendar day.
After the Periodic UV Performance Distribution is made on the Periodic Calculation Date that follows each Publication Date, the “Up Underlying Value” will equal, for the remainder of the current Calculation Period:
·the Up Underlying Value on the last Publication Date, as reduced by either the Periodic UV Performance Distribution made on the Up BTOX PharmaShares or the increase in the Down Underlying Value distributed on the Down BTOX PharmaShares
plus
·the sum of the Up Daily Net Income Accruals for each day that has elapsed during the current Calculation Period, up to and including the current calendar day,
21
and the “Down Underlying Value” will equal, for the remainder of the current Calculation Period:
·the Down Underlying Value on the last Publication Date, as reduced by either the Periodic UV Performance Distribution made on the Down BTOX PharmaShares or the increase in the Up Underlying Value distributed on the Up BTOX PharmaShares
plus
·the sum of the Down Daily Net Income Accruals for each day that has elapsed during the current Calculation Period, up to and including the current calendar day.
The Up Investment Amount and the Down Investment Amount will be reduced by redemptions as well as by Periodic UV Performance Distributions, as described below under “ — Periodic Distributions” and under “DESCRIPTION OF THE PHARMASHARES — Periodic Distributions” in the base prospectus. Reductions in the Up Investment Amount and the Down Investment Amount will reduce the amount of your investment and your potential gains, as discussed in the base prospectus under “RISK FACTORS — The amount of your investment in your PharmaShares and your potential gains will decline over time.” Purchasing additional Up BTOX PharmaShares or Down BTOX PharmaShares is the only means to maintain the size of your investment.
The “Up Allocation Factor” and “Down Allocation Factor” are determined as described below.
If a Projected Breakpoint Increase was projected by the Broker Reporting Group for the Current Calendar Quarter, because the Broker Reporting Group expected the Reference Value to increase relative to the Base Calendar Quarter, then:
·If the Actual Percentage Change is greater than the Projected Breakpoint Increase, the Up Underlying Value will increase as follows and there will be a corresponding decrease in the Down Underlying Value:
UUVΔ = Actual Percentage Change – Projected Breakpoint Increase
Up Allocation Factor = 1 + UUVΔ (the increase in the Up Underlying Value), and
Down Allocation Factor = 1 – UUVΔ (the increase in the Up Underlying Value)
·If the Actual Percentage Change reflects an increase that is less than the Projected Breakpoint Increase, the Down Underlying Value will increase as follows and there will be a corresponding decrease in the Up Underlying Value:
DUVΔ = Projected Breakpoint Increase – Actual Percentage Change
Down Allocation Factor = 1 + DUVΔ (the increase in the Down Underlying Value), and
Up Allocation Factor = 1 – DUVΔ (the increase in the Down Underlying Value)
·If the Actual Percentage Change reflects a decrease in the Reference Value when there is a Projected Breakpoint Increase, the Down Underlying Value will increase as follows and there will be a corresponding decrease in the Up Underlying Value:
DUVΔ = Actual Percentage Change + Projected Breakpoint Increase
Down Allocation Factor = 1 + DUVΔ (the increase in the Down Underlying Value), and
Up Allocation Factor = 1 – DUVΔ (the increase in the Down Underlying Value)
If a Projected Breakpoint Decrease was projected by the Broker Reporting Group for the Current Calendar Quarter, because the Broker Reporting Group expected the Reference Value to decrease relative to the Base Calendar Quarter, then:
22
·If the Actual Percentage Change reflects a decrease that is greater than the Projected Breakpoint Decrease, the Down Underlying Value will increase as follows and there will be a corresponding decrease in the Up Underlying Value:
DUVΔ = Actual Percentage Change – Projected Breakpoint Decrease
Down Allocation Factor = 1 + DUVΔ (the increase in the Down Underlying Value), and
Up Allocation Factor = 1 – DUVΔ (the increase in the Down Underlying Value)
·If the Actual Percentage Change reflects a decrease that is less than the Projected Breakpoint Decrease, the Up Underlying Value will increase as follows and there will be a corresponding decrease in the Down Underlying Value:
UUVΔ = Projected Breakpoint Decrease – Actual Percentage Change
Up Allocation Factor = 1 + UUVΔ (the increase in the Up Underlying Value), and
Down Allocation Factor = 1 – UUVΔ (the increase in the Up Underlying Value)
·If the Actual Percentage Change reflects an increase in the Reference Value when there is a Projected Breakpoint Decrease, the Up Underlying Value will increase as follows and there will be a corresponding decrease in the Down Underlying Value:
UUVΔ = Actual Percentage Change + Projected Breakpoint Decrease
Up Allocation Factor = 1 + UUVΔ (the increase in the Up Underlying Value), and
Down Allocation Factor = 1 – UUVΔ (the increase in the Up Underlying Value).
Each increase in the Up Underlying Value will be multiplied by the Up Leverage Factor, and then adjusted by the Up Periodic Return Cap if the leveraged returns exceed that Up Periodic Return Cap, as part of calculating the Up Allocation Factor. Each increase in the Down Underlying Value will be multiplied by the Down Leverage Factor, and then adjusted by the Down Periodic Return Cap if the leveraged returns exceed that Down Periodic Return Cap, as part of calculating the Down Allocation Factor.
As described in the base prospectus under “REFERENCE PRODUCTS AND REFERENCE VALUES,” there will be no change in the Up Underlying Value or the Down Underlying Value and no Periodic UV Performance Distributions will be declared if certain Major Market Disruptions impact the reporting of BOTOX® net revenue during a Calculation Period. There are no additional events that constitute Major Market Disruptions for Series BTOX other than those described in the base prospectus.
Hypothetical calculations of Underlying Value based upon the historical or hypothetical performance of the Reference Value and historical or hypothetical composite median projections are included for illustrative purposes in Appendix B to this prospectus supplement.
The initial proportion, or “Up/Down Ratio,” of the Up Investment Amount to the Down Investment Amount will be 1:1 for Series BTOX, without regard to the respective initial public offering prices or subsequent trading prices of the Up BTOX PharmaShares and Down BTOX PharmaShares. This initial proportion will be maintained for so long as the Paired BTOX PharmaShares are outstanding by virtue of the requirements that:
(1)if the Up BTOX PharmaShares or the Down BTOX PharmaShares receive a distribution of any increase in their Underlying Value in the form of a Periodic UV Performance Distribution, that Periodic UV Performance Distribution will also include an Equalization Payment that will restore the Up/Down Ratio of the Up Investment Amount to the Down Investment Amount;
(2)all redemptions and issuances must be effected in PharmaShares Units composed of an equal Aggregate Par Amount of Up BTOX PharmaShares and Down BTOX PharmaShares; and
(3)without regard to the respective Underlying Values at which the Paired BTOX PharmaShares are issued or redeemed, on the books and records of Series BTOX, one-half of the funds used to effect
23
a Paired Optional Redemption will always be allocated to reduce the Up Investment Amount and one-half will be allocated to reduce the Down Investment Amount; similarly, one-half of the funds received in connection with a Paired Issuance will always be allocated to increase the Up Investment Amount and one-half will always be allocated to increase the Down Investment Amount. The Up Investment Amount and the Down Investment Amount are variables in, respectively, the Up Underlying Value formula and the Down Underlying Value formula, as illustrated earlier in this section and in the “GLOSSARY OF DEFINED TERMS” in the base prospectus.
Maintaining the Up/Down Ratio ensures that the Underlying Value formula and the yield on the Up BTOX PharmaShares or the Down BTOX PharmaShares will not be affected by increases or decreases in the Up Investment Amount or Down Investment Amount due to Periodic UV Performance Distributions or due to Paired Optional Redemptions and Paired Issuances.
Periodic Distributions
Periodic Calculation Dates
A “Periodic Calculation Date” is scheduled to occur quarterly for the Up and Down BTOX PharmaShares on the fifth (5th) business day following each Publication Date, commencing in [●] of 2021. Periodic UV Performance Distributions and Periodic Income Distributions, referred to collectively or generically as “Periodic Distributions,” will be declared on each Periodic Calculation Date. Periodic UV Performance Distributions will reflect changes in the Up and Down Underlying Values during the preceding Calculation Period and Periodic Income Distributions will reflect net treasury income earned during the preceding Calculation Period.
Each “Calculation Period” will begin on, and include, the preceding Publication Date (or, in the case of the first Calculation Period, begin on the first date of issuance for the Paired BTOX PharmaShares) and end on, but exclude, the current Publication Date.
The Periodic Distributions declared on each Periodic Calculation Date will be paid to shareholders on the second (2nd) business day following that Periodic Calculation Date. Each shareholder who is a registered holder of Up BTOX PharmaShares or Down BTOX PharmaShares on the “Record Date,” which will be the [●] business day preceding each Periodic Calculation Date, will be entitled to receive the Periodic Distributions, if any, that were declared on the Up BTOX PharmaShares or the Down BTOX PharmaShares, respectively, on that Periodic Calculation Date.
Periodic Income Distributions
On each Periodic Calculation Date, the PharmaShares Trust will declare a “Periodic Income Distribution” equal to the net treasury income earned by Series BTOX during the preceding Calculation Period, if any. “Net treasury income” will be equal to the treasury income that remains on deposit in Series BTOX after it has (i) deposited the amounts described below under “— Fees and Expenses,” into the fee payment account to pay its fees and expenses for the preceding Calculation Date and (ii) acquired treasury securities with an aggregate purchase price equal to the sum of the Aggregate Par Amount of its outstanding Up BTOX PharmaShares and Down BTOX PharmaShares. The Periodic Income Distribution declared on any Periodic Calculation Date will be allocated equally among the Up BTOX PharmaShares and the Down BTOX PharmaShares. The Periodic Income Distribution made on the Up BTOX PharmaShares will be equal to the sum of the Up Daily Net Income Accruals for each day of the preceding Calculation Period. The Periodic Income Distribution made on the Down BTOX PharmaShares will be equal to the sum of the Down Daily Net Income Accruals for each day of the preceding Calculation Period.
The PharmaShares Trust will not make any Periodic Income Distributions on the Up BTOX PharmaShares or the Down BTOX PharmaShares until the earlier of the time when (i) average annual yield on the treasury securities held by Series BTOX exceeds 3% or (ii) the aggregate amount invested in Series BTOX exceeds $250 million.
24
Periodic UV Performance Distributions
On each Periodic Calculation Date, the Up Underlying Value and Down Underlying Value will be calculated based upon the Reference Value published on the Publication Date that occurred five (5) business days prior to that Periodic Calculation Date and the Projected Breakpoint calculated at the beginning of the preceding calendar quarter.
If the Up Underlying Value has increased as a result of the performance of the Reference Value on a Publication Date, the Down Underlying Value will decrease by a corresponding amount and the PharmaShares Trust will declare the following “Periodic UV Performance Distribution” on the Up BTOX PharmaShares on the related Periodic Calculation Date:
·the amount of the increase in the Up Underlying Value (without regard to any portion of that Up Underlying Value that is attributable to Up Daily Net Income Accruals for the related Calculation Period, which will be separately distributed on the same date as a Periodic Income Distribution)
plus
·an “Equalization Payment” that is equal to the increase in the Up Underlying Value in order to bring the proportion of the Up Investment Amount to the Down Investment Amount back to the original Up/Down Ratio of 1:1,
divided by
·the aggregate number of outstanding Up BTOX PharmaShares.
No Periodic UV Performance Distribution will be declared on the Down BTOX PharmaShares on a Periodic Calculation Date on which the Up Underlying Value has increased.
In connection with each Periodic UV Performance Distribution, the trustee will reduce, on the books and records of Series BTOX, (1) the Down Investment Amount and the Down Aggregate Par Amount by an amount equal to the increase in the Up Underlying Value and (2) the Up Investment Amount and Up Aggregate Par Amount by the amount of the Equalization Payment that was made as part of that distribution. In addition, the Up Underlying Value will be reduced by the entire amount of the Periodic UV Performance Distribution and the Down Underlying Value will be reduced by the amount of the increase in the Up Underlying Value.
During any period when the yield on treasury securities held by Series BTOX are insufficient to pay all fees and expenses of the Series, an amount of up to 2.50% of that portion of any Periodic UV Performance Distribution that represents an increase in the Up Underlying Value will be deducted from the Periodic UV Performance Distribution on the Up BOTX PharmaShares and used to pay accrued fees and expenses of Series BTOX that remain unpaid after application of current treasury income earned by Series BTOX. The amount deducted for fees and expenses will never exceed 2.5% of the portion of any Periodic UV Performance Distribution that represents an increase in the Up Underlying Value. No amounts will be deducted from the Equalization Payment portion of any Periodic UV Performance Distribution on the Up BTOX PharmaShares.
If the Down Underlying Value has increased as a result of the performance of the Reference Value on a Publication Date, the Up Underlying Value will decrease by a corresponding amount and the PharmaShares Trust will declare the following “Periodic UV Performance Distribution” on the Down BTOX PharmaShares on the related Periodic Calculation Date:
·the amount of the increase in the Down Underlying Value (without regard to any portion of that Down Underlying Value that is attributable to Down Daily Net Income Accruals for the related Calculation Period, which will be separately distributed on the same date as a Periodic Income Distribution)
plus
25
·an “Equalization Payment” that is equal to the increase in the Down Underlying Value in order to bring the proportion of the Up Investment Amount to the Down Investment Amount back to the original Up/Down Ratio of 1:1,
divided by
·the aggregate number of outstanding Down BTOX PharmaShares.
No Periodic UV Performance Distribution will be declared on the Up BTOX PharmaShares on a Periodic Calculation Date on which the Down Underlying Value has increased.
In connection with each Periodic UV Performance Distribution, the trustee will reduce, on the books and records of Series BTOX, (1) the Up Investment Amount and the Up Aggregate Par Amount by an amount equal to the increase in the Down Underlying Value and (2) the Down Investment Amount and Down Aggregate Par Amount by the amount of the Equalization Payment that was made as part of that distribution. In addition, the Down Underlying Value will be reduced by the entire amount of the Periodic UV Performance Distribution and the Up Underlying Value will be reduced by the amount of the increase in the Down Underlying Value.
During any period when the yields on treasury securities held by Series BTOX are insufficient to pay the fees and expenses of the Series, an amount of up to 2.50% of that portion of the Periodic UV Performance Distribution that represents an increase in the Down Underlying Value will be deducted from the Periodic UV Performance Distribution made on the Down BTOX PharmaShares and used to pay accrued fees and expenses of Series BTOX that remain unpaid after application of current treasury income earned by Series BTOX. The amount deducted for fees and expenses will never exceed 2.5% of the portion of any Periodic UV Performance Distribution that represents an increase in the Down Underlying Value. No amounts will be deducted from the Equalization Payment portion of any Periodic UV Performance Distribution on the Down BTOX PharmaShares.
For a more detailed description of certain matters relating to the calculation of Underlying Value and Periodic Distributions, see “DESCRIPTION OF THE PHARMASHARES — Periodic Distributions” in the base prospectus.
Each Periodic UV Performance Distribution on your Up or Down BTOX PharmaShares will represent both (1) an investment gain equal to the increase in the Underlying Value of your Up or Down BTOX PharmaShares, and (2) a return of your invested capital in the form of the related Equalization Payment. Conversely, each Periodic UV Performance Distribution made to the paired class of BTOX PharmaShares will represent a loss on your investment and a gain for the paired class and the related Equalization Payment will represent a return of invested capital to the paired class. Each Periodic UV Performance Distribution that is made on your class of PharmaShares will reduce your investment by the amount of the Equalization Payment you receive to maintain the Up/Down Ratio. Each Periodic UV Performance Distribution that is made on the paired class of PharmaShares will reduce your investment by the amount of the investment loss you suffered equal to the decrease in the Underlying Value of your shares. Each Periodic UV Performance Distribution will decrease the amount of your investment in your shares and the potential gain on those shares that can be realized from the net assets of Series BTOX. The only way to maintain your desired exposure to your Up or Down BTOX PharmaShares is to acquire additional shares in the secondary market, including by placing bids for additional shares in the Grouped Paired Issuances. See “RISK FACTORS — The amount of your investment in your PharmaShares and your potential gains will decline over time” in the base prospectus.
If treasury yields were to become negative, the negative yield will result in a corresponding decrease in the Series Investment Amount that will be allocated equally among all shareholders. The decreased Up and Down Investment Amount will result in reduced Up and Down Underlying Values and reduced Periodic UV Performance Distributions by reducing the aggregate amount of assets in Series BTOX. Any future treasury income will first be applied to restore the Series Investment Amount to equal the Aggregate Par Amount of the Paired BTOX PharmaShares and then be used for fees and expenses.
26
Final Distributions
The PharmaShares Trust will declare a Final Distribution on all or a portion, as applicable, of the Up BTOX PharmaShares and the Down BTOX PharmaShares, on the earliest to occur of (i) the “Final Scheduled Termination Date” that will occur on [●], 2030, (ii) an Early Termination Date (as defined below under “ — Termination Triggers”) following the occurrence of a Termination Trigger, and (iii) on the “Redemption Order Date” on which an Authorized Participant places an order for a Paired Optional Redemption.
For purposes of determining the Final Distribution, the Up Underlying Value and Down Underlying Value will be calculated based upon the Projected Breakpoint for the preceding calendar quarter and the Reference Value reported on the last Publication Date that, in each case, precedes the Final Scheduled Termination Date, an Early Termination Date or the relevant Redemption Order Date. Any Periodic UV Performance Distribution declared on the last Periodic Calculation Date will be deducted from the Up and Down Underlying Value and any Up Daily Net Income Accruals or Down Daily Net Income Accruals, as applicable, accrued from the last Publication Date through and including the last calendar day preceding the Final Scheduled Termination Date, Early Termination Date or relevant Redemption Date will be added to the Up and Down Underlying Value.
On the final Redemption Date that follows the Final Scheduled Termination Date or Early Termination Date or on the Redemption Date that follows a Redemption Order Date, the PharmaShares Trust will distribute on each outstanding Up BTOX PharmaShare (in the case of a final Redemption Date) or on the portion of those shares that are being redeemed by Authorized Participants (in the case of a Redemption Date), a Final Distribution in cash equal to:
·the Up Underlying Value (including the Up Daily Net Income Accrual component of that Up Underlying Value)
divided by
·the aggregate number of Up BTOX PharmaShares that have been issued but not yet redeemed as of that date or the aggregate number of Up BTOX PharmaShares being redeemed by Authorized Participants, as applicable;
and on each outstanding Down BTOX PharmaShare (in the case of a final Redemption Date) or on the portion of those shares that are being redeemed by Authorized Participants (in the case of a Redemption Date), a Final Distribution in cash equal to:
·the Down Underlying Value (including the Down Daily Net Income Accrual component of that Down Underlying Value)
divided by
·the aggregate number of Down BTOX PharmaShares that have been issued but not yet redeemed as of that date or the aggregate number of Down BTOX PharmaShares being redeemed by Authorized Participants, as applicable.
During any period when the yield on the treasuries held by Series BTOX is below the rate necessary to pay the fees and expenses of Series BTOX, an amount up to 2.50% of that portion of any Final Distribution on the Final Scheduled Termination Date or an Early Termination Date that represents an increase in the Up or Down Underlying Value, as applicable, will be deducted from that distribution and used to pay the accrued fees and expenses of Series BTOX.
Upon receipt of a Final Distribution on the final Redemption Date, your Up BTOX PharmaShares and your Down BTOX PharmaShares will be considered to be redeemed in full and the PharmaShares Trust and Series BTOX will have no further obligations with respect to those shares even if the amount of the Final Distribution is less than the Aggregate Par Amount of those shares or less than the purchase price you paid for those shares.
27
See “RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution” in the base prospectus.
The Final Scheduled Termination Date or Early Termination Date for the Paired BTOX PharmaShares will be the last day of trading for those shares.
Paired Optional Redemptions
The Paired BTOX PharmaShares may be redeemed concurrently at the direction of Authorized Participants in PharmaShares Units in a “Paired Optional Redemption” on a “Redemption Date,” which is any business day prior the Final Scheduled Termination Date or an Early Termination Date. The Final Distribution in a Paired Optional Redemption, which may be delivered in the form of cash and/or treasuries, will always be equal to the respective Per Share Underlying Values of the Up BTOX PharmaShares and Down BTOX PharmaShares being redeemed. The Up and Down Per Share Underlying Values will be calculated based upon the Projected Breakpoint for the last calendar quarter and the Reference Value published on the last Publication Date, in each case, preceding the relevant Redemption Order Date. Any Periodic UV Performance Distribution declared on the last Periodic Calculation Date will be deducted from the Per Share Underlying Value and any Up Daily Net Income Accruals or Down Daily Net Income Accruals, as applicable, accrued from the last Publication Date through and including the last calendar day preceding the relevant Redemption Date will be added to the Per Share Underlying Value.
The trustee will allocate, on the books and records of Series BTOX, one-half of the aggregate funds delivered as a Final Distribution in the Paired Optional Redemption to reduce the Up Investment Amount and the other half to reduce the Down Investment Amount, without regard to the respective Per Share Underlying Values at which the Paired BTOX PharmaShares were redeemed, in order to preserve the one-to-one ratio of the Up Investment Amount and the Down Investment Amount.
Following receipt of the Final Distribution in a Paired Optional Redemption, the Up BTOX PharmaShares and the Down BTOX PharmaShares tendered for redemption will be redeemed in full and will cease to be outstanding. The PharmaShares Trust and Series BTOX will have no further obligations with respect to those shares, even if the amount of the Final Distribution was less than the Aggregate Par Amount of those shares or less than the purchase price at which those shares were acquired by the Authorized Participant. Paired Optional Redemptions must be effected in accordance with the rules and procedures described in the base prospectus under “DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions.”
Paired Issuances
On any business day prior to the Final Scheduled Termination Date or an Early Termination Date, an Authorized Participant may effect a “Paired Issuance” of Paired BTOX PharmaShares by directing the PharmaShares Trust to issue additional shares in one or more PharmaShares Units in accordance with the procedures that are described in the base prospectus under “DESCRIPTION OF THE PHARMASHARES — Paired Issuances.” Up BTOX PharmaShares and Down BTOX PharmaShares will always be issued at their respective Per Share Underlying Values, which will be calculated based upon the Projected Breakpoint for the last calendar quarter and the Reference Value published on the last Publication Date, in each case, preceding the relevant Issuance Order Date. Any Periodic UV Performance Distribution declared on the last Periodic Calculation Date will be deducted from the Per Share Underlying Value and any Up Daily Net Income Accruals or Down Daily Net Income Accruals, as applicable, accrued from the beginning of the current Calculation Period through and including the last calendar day preceding the relevant Issuance Date will be added to the Per Share Underlying Value.
The trustee will allocate, on the books and records of Series BTOX, one-half of the aggregate funds received by Series BTOX in connection with each Paired Issuance to the Up Investment Amount and the other half to the Down Investment Amount, without regard to the respective Per Share Underlying Values at which the Paired BTOX PharmaShares were issued, in order to preserve the one-to-one ratio of the Up Investment Amount and the Down Investment Amount.
For more information about Paired Issuances, see “DESCRIPTION OF THE PHARMASHARES — Paired Issuances” in the base prospectus.
28
Termination Triggers
The occurrence of any of the Termination Triggers identified in the base prospectus will cause an early redemption of the Up BTOX PharmaShares and the Down BTOX PharmaShares on the next Periodic Calculation Date that follows the Termination Trigger, referred to as an “Early Termination Date.” With regard to the Termination Trigger relating to a failure to publish the Reference Value on or prior to the 50th day following the end of the first three calendar quarters of each year or on or prior to the 100th day following the end of the fourth calendar quarter, if AbbVie Inc. either (1) fails to publish global net revenues for all indications of BOTOX® or (2) fails to publish global net revenues for all indications of BOTOX® as a separate line items (or several line items that together aggregate to global net revenues for all indications) in its income statement prepared in accordance with U.S. GAAP and filed on Form 10-Q or Form 10-K within the specified time periods for two (2) or more consecutive calendar quarters, a Termination Trigger will occur on the second (2nd) consecutive Publication Date on which such failure persists. The related Termination Trigger will be deemed to occur on the 50th day following quarter end for the first three calendar quarters and on the 100th day following quarter end for the fourth calendar quarter on which net revenues are not published for the second consecutive quarter and the next Periodic Calculation Date that occurs after that Publication Date will be an Early Termination Date. There will be no change in the Up Underlying Value or Down Underlying Value on any Publication Date on which the Reference Value is not published within the specified time period.
There are no other Termination Triggers for Series BTOX other than those described in the base prospectus and in the preceding paragraph.
Upon electing to declare a Termination Trigger following the occurrence of certain events, or obtaining knowledge or receiving notice of the occurrence of a Termination Trigger, we will file a Form 8-K with the SEC disclosing the Termination Trigger and announcing the applicable Early Termination Date.
On the next Periodic Calculation Date following the occurrence of a Termination Trigger, the PharmaShares Trust will declare a Final Distribution in redemption of all outstanding Paired BTOX PharmaShares equal to their respective Per Share Underlying Values. The calculation of the Up Underlying Value and the Down Underlying Value for purposes of the Final Distribution on an Early Termination Date will be based upon (1) the Reference Value published on the last Publication Date on which a Reference Value was published that precedes the date on which a Termination Trigger occurred or, in the case of a delay in publishing the Reference Value that occurs for two consecutive calendar quarters, on the last Publication Date on which the Reference Value was published within the specified time frames described above, (2) the Projected Breakpoint for the calendar quarter that precedes the relevant Publication Date, and (3) after deducting the Periodic UV Performance Distribution declared on the last Periodic Calculation Date and adding all accrued Up and Down Daily Net Income Accruals since the beginning of the current Calculation Period. Any Reference Value published after the occurrence of a Termination Trigger will not be taken into consideration. Following this Final Distribution, the Up BTOX PharmaShares and the Down BTOX PharmaShares will be considered to be redeemed in full and will cease to be outstanding.
For more information about Termination Triggers, see “DESCRIPTION OF THE PHARMASHARES — Termination Triggers” in the base prospectus.
Fees and Expenses
General
On each Periodic Calculation Date, the following fixed and variable fees of Series BTOX will be payable:
·a fee payable to us, in our capacity as the administrative agent, which will accrue on each calendar day at an annualized rate of 1.25% of the Series Investment Amount;
·a fee payable to the marketing agent, which will accrue on each calendar day at an annualized rate of 0.35% of the Series Investment Amount;
·a fee payable to the trustee, which will accrue on each calendar day at an annualized rate of [●]% of the Series Investment Amount or an annual minimum fee of $[●], whichever is greater;
29
·a fee payable to the calculation agent, which will accrue on each calendar day at an annualized rate of [●]% of the Series Investment Amount;
·annual fees payable to the accountants equal to $[●] per year, which will be reserved for at an annualized rate not greater than [●]% of the Series Investment Amount on each calendar day, to the extent that Series BTOX has realized sufficient treasury income;
·Series BTOX formation expenses of $1.5 million, which will be recovered at an annualized rate not greater than [●]% of the Series Investment Amount on each calendar day, to the extent that Series BTOX has realized sufficient treasury income;
·selling commissions payable to the initial Authorized Participants, as described under “ — Plan of Distribution” in this prospectus supplement equal to [●]% of the gross offering proceeds of all Up BTOX PharmaShares and Down BTOX PharmaShares, which will be recovered on each calendar day at an annualized rate not greater than [●]% of the Series Investment Amount, to the extent that Series BTOX has realized sufficient treasury income;
·ordinary, recurring fees and expenses incurred by, or allocable to, Series BTOX, including initial and continuous offering expenses, and any extraordinary expenses incurred by, or allocable to, Series BTOX; and
·fees and expenses accrued with respect to previous Calculation Periods that were paid on behalf of Series BTOX by us, in our capacity as administrative agent, which will be recovered on each calendar day at an annualized rate not greater than [●]% of the Series Investment Amount, to the extent that Series BTOX has realized sufficient treasury income.
Each of the fees that accrue at an annualized rate will be calculated on the basis of the actual number of days in the current year. The dollar amount of these variable fees will depend upon the aggregate amount of assets on deposit from time to time in Series BTOX. Any fees and expenses of the PharmaShares Trust that affect or benefit more than one Series will be allocated to each Series on the basis of its respective Series Investment Amount.
Allocation of Treasury Income to Pay Fees and Expenses
On each Periodic Calculation Date, Series BTOX is required to deposit all or a portion of its treasury income earned during the preceding Calculation Period into the fee payment account to pay the fees and expenses incurred by the Series during that Calculation Period. These fees and expenses will accrue or will be incurred during each Calculation Period and will be payable in arrears on each Periodic Calculation Date or, at the direction of the administrative agent, on any business day occurring during the Calculation Period in which they are incurred. The fees and expenses of Series BTOX will be allocated equally among all of the outstanding Paired BTOX PharmaShares by means of the deduction of an equal amount allocable to fees and expenses from the Up Daily Net Income Accrual and the Down Daily Net Income Accrual.
For so long as the Series Investment Amount is equal to or less than $250 million and average yields for treasury securities of the type that are permitted to be held by Series BTOX, as described under “THE PHARMASHARES TRUST — United States Treasury Obligations” in the base prospectus, are equal to or less than an average annual rate of 3%, all treasury income accrued during each Calculation Period will be used to pay the fees and expenses of Series BTOX. During this time, the fees payable to us, in our capacity as administrative agent, and the reimbursement of any fees and expenses paid by us on behalf of Series BTOX will be deferred in whole or in part. If, after such deferral, the treasury income of Series BTOX is still insufficient to pay its remaining fees and expenses, these fees and expenses will be paid by us on behalf of Series BTOX. We will seek to recoup any fees and expenses we have paid on behalf of Series BTOX out of the treasury income realized by Series BTOX during future Calculation Periods unless and until the Series Investment Amount exceeds $[●] for at least [●] Calculation Periods, after which we will waive our right to seek any further fee and expense reimbursement from Series BTOX.
The amount of treasury income, if available, that will be deposited into the Series BTOX fee payment account on each Periodic Calculation Date will be equal to the sum of, for each day during that Calculation Period, the Series Investment Amount on that day multiplied by an aggregate “Daily Fee Accrual Rate” equal to an annual
30
rate not to exceed 2.50%, divided by 365 or 366, depending upon the number of days in the current year, which will reflect the sum of (1) the variable fees described above plus (2) the rate at which fixed fees are being reimbursed plus (3) the rate at which the administrative agent is recovering any unreimbursed fees and expenses previously paid by it on behalf of Series BTOX. We will determine the Daily Fee Accrual Rate at the beginning of each Calculation Period. The Daily Fee Accrual Rate will always be capped at an annualized rate of 2.50%.
On any day on which the Daily Fee Accrual Rate exceeds the Daily Yield Rate, the full amount of treasury income earned for that day will be allocated toward the payment of fees and expenses and there will be no Up Daily Net Income Accrual or Down Daily Net Income Accrual for that day.
During any period when the Series Investment Amount for Series BTOX exceeds $250 million or average yields for treasury securities of the type that are permitted to be held by Series BTOX exceed 3% per year, Series BTOX will pay all of its own fees and expenses, subject to the 2.50% fee accrual cap.
Allocation of UV Performance Returns to Pay Fees and Expenses
In addition to allocating treasury income to pay fees and expenses, during any period when the average yield on the treasuries is below the rate necessary to pay the fees and expenses of Series BTOX, an amount up to 2.50% of that portion of any Periodic UV Performance Distribution or any Final Distribution on the Up or Down BTOX PharmaShares that represents an increase in the Up or Down Underlying Value, as applicable, will be deducted from that distribution. Deductions will only be made out of Final Distributions made on the Final Scheduled Termination Date, an Early Termination Date or a Redemption Date that occurs on a Periodic Calculation Date. This amount will be used to pay accrued fees and expenses of Series BTOX on the next Periodic Calculation Date that would otherwise remain unpaid because treasury income during the related Calculation Period was insufficient to pay those fees and expenses. However, the amount deducted for fees and expenses will never exceed 2.50% of that portion of any Periodic UV Performance Distribution or any Final Distribution that represents the increase in the Up or Down Underlying Value, as applicable, minus the actual treasury income earned during the last Calculation Period. No amounts will be deducted from the Equalization Payment portion of any Periodic UV Performance Distribution or from the portion of any Final Distribution that does not represent an increase in the Up Underlying Value or an increase in the Down Underlying Value, as applicable. The Daily Fee Accrual Rate will continue to be capped at 2.50% and any fees and expenses that remain unpaid after the allocations described in this paragraph will be paid by us. We will seek reimbursement of any fees and expenses we paid on behalf of Series BTOX out of treasury income earned during future Calculation Periods.
Form of the Shares
The Up BTOX PharmaShares and the Down BTOX PharmaShares will both be issued in the form of one or more global certificates registered in the name of Cede & Co., as the nominee of The Depository Trust Company, or “DTC,” and deposited with DTC in the United States or with Clearstream Banking, société anonyme or Euroclear Bank S.A./NV in Europe. If you are not a participant in DTC or in Clearstream or Euroclear, you may hold an interest in the Up BTOX PharmaShares and the Down BTOX PharmaShares only by opening an account with a participant or with certain banks, brokers, dealers, trust companies and other parties that maintain a custodial relationship with a DTC participant. You will not receive a physical certificate and you will not be considered the registered holder of the global certificate representing your Up BTOX PharmaShares or your Down BTOX PharmaShares. No minimum lot requirements are applicable to the Up BTOX PharmaShares or the Down BTOX PharmaShares.
For more information about book-entry registration, see “DESCRIPTION OF THE PHARMASHARES — Book-Entry Registration” in the base prospectus.
Listing
The Up BTOX PharmaShares will trade on NYSE Arca under the symbol [●]. The Down BTOX PharmaShares will trade on NYSE Arca under the symbol [●].
31
U.S. Federal Income Tax Considerations
Please see the base prospectus for a discussion of the U.S. federal income tax considerations that are applicable to an investment in the Up BTOX PharmaShares and the Down BTOX PharmaShares. See “U.S. FEDERAL INCOME TAX CONSIDERATIONS” in the base prospectus.
ERISA Considerations
Please see the base prospectus for a discussion of the ERISA considerations that are applicable to the Up BTOX PharmaShares and the Down BTOX PharmaShares. See “CERTAIN ERISA CONSIDERATIONS” in the base prospectus.
CUSIP and ISIN Numbers
The CUSIP for your Up BTOX PharmaShares is [●]. The ISIN number for your Up BTOX PharmaShares is [●]. The CUSIP for your Down BTOX PharmaShares is [●]. The ISIN number for your Down BTOX PharmaShares is [●].
Experts
The financial statements of the PharmaShares Trust, including Series BTOX which is consolidated with the PharmaShares Trust, will be appended to this prospectus supplement and will be so included in reliance on the report of [●], an independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing.
Plan of Distribution
Initial Offering and Auction
During the first 60 days following the effective date of this prospectus supplement and the base prospectus, the Up BTOX PharmaShares and the Down BTOX PharmaShares will be offered on a “best efforts basis” without any firm underwriting commitment by [●], [●] and [●], each of whom is referred to as an initial “Authorized Participant.” We and the PharmaShares Trust have entered into an Authorized Participants Agreement with each of the initial Authorized Participants and may enter into such agreements with other FINRA-registered broker dealers in the future. By executing an Authorized Participants Agreement, an Authorized Participant becomes part of the group of parties eligible to purchase PharmaShares Units from, and tender PharmaShares Units for redemption to, the PharmaShares Trust and Series BTOX. An Authorized Participant is under no obligation to create or redeem PharmaShares Units, and an Authorized Participant is under no obligation to offer to the public Up BTOX PharmaShares or Down BTOX PharmaShares of any PharmaShares Units it does create.
Series BTOX will only issue Up BTOX PharmaShares and Down BTOX PharmaShares to the initial Authorized Participants in PharmaShares Units and fractional parts of a Unit, in the case of Grouped Paired Issaunces. The Up BTOX PharmaShares and the Down BTOX PharmaShares will be issued at their respective Per Share Underlying Values at the time of their sale by Series BTOX to Authorized Participants. Beginning with the first Calculation Period during which the PharmaShares are offered, the Per Share Underlying Value of each of the Up and Down BTOX PharmaShares will be equal to their par amount of $[50] per share. On the first Publication Date, the Up and Down Underlying Value will change based upon the Reference Value published on that date relative to the Projected Breakpoint for the second quarter of 2021. After any increase in Up or Down Underlying Value is distributed to the holders of the Up or Down BTOX PharmaShares, as applicable, the Up and Down Underlying Value will be reduced by such distribution, as described under “PERIODIC DISTRIBUTIONS — Periodic UV Performance Distributions,” and the Per Share Underlying Value of the Up and Down BTOX PharmaShares will be adjusted accordingly. These new Per Share Underlying Values will be the respective prices at which the Paired Shares will be issued to Authorized Participants for the remainder of the second Calculation Period. Please see Appendix A for a hypothetical illustration of how Underlying Values (and Per Share Underlying Values) change over the course of each Calculation Period.
Each Authorized Participant will solicit bids for Up BTOX PharmaShares and/or Down BTOX PharmaShares during the initial offering period. Prospective investors who bid for PharmaShares must deposit
32
funds with their brokers or directly with an Authorized Participant in accordance with the rules and procedures specified by such broker or Authorized Participant. Bids will not be revocable. [●] will aggregate all bids obtained by it and each of the other Authorized Participants and will match up bids for Up BOTX PharmaShares and Down BTOX PharmaShares into PharmaShares Units consisting of $1,000,000 Aggregate Par Amount of Up BTOX PharmaShares and $1,000,000 Aggregate Par Amount of Down BTOX PharmaShares. All bids for Up BTOX PharmaShares and for Down BTOX PharmaShares that are combined into PharmaShares Units will be accepted by [●] and the sale of the shares will be executed by the applicable Authorized Participant or broker. A bid for an Up BTOX PharmaShare that cannot be paired with a bid for a Down BTOX PharmaShare such that the combined price of those Paired Shares is at least equal to their combined Underlying Value will be rejected.
Series BTOX will pay selling commissions to the initial Authorized Participants on a deferred basis out of its treasury income on each Periodic Calculation Date, in an amount equal to an annualized rate of 2.5% to 5% of the gross offering proceeds of all Up BTOX PharmaShares and Down BTOX PharmaShares sold by each Authorized Participant, as specified in each Authorized Participant Agreement. Selling commissions will only be payable out of any treasury income realized by Series BTOX and, to the extent such income is insufficient for any Calculation Period, out of Periodic UV Performance Distributions, in an amount not to exceed 2.50% of the portion of any such distribution that reflects the increase in Underlying Value. The aggregate fees and expenses payable by Series BTOX will be limited on each Payment Date to an annualized rate of 250 basis points of the Series Investment Amount, as described above under “ — Fees and Expenses” in this prospectus supplement. Subject to these limitations, funds will be allocated to pay selling commissions on each Periodic Calculation Date until they have been paid in full.
Ongoing Offering
After the initial offering of the Paired BTOX PharmaShares, the offering will continue. The PharmaShares Trust will issue Paired BTOX PharmaShares to Authorized Participants in PharmaShares Units (and, in the case of Grouped Paired Issuances, fractional parts of Units) on a continuous basis at their respective Per Share Underlying Values.
During the continuous offering that follows the initial offering, Authorized Participants may present orders for PharmaShares Units to the PharmaShares Trust as described under “ —Paired Issuances” in this prospectus supplement. In addition, in order to increase liquidity and facilitate price discovery for the Paired BTOX PharmaShares, weekly auctions will be held for “Grouped Paired Issuances” and “Grouped Paired Redemptions” on each Monday, or the first business day of the week. Investors may submit bids electronically from 5 p.m. E.T. on the preceding Friday through 7 a.m. E.T. on each Monday (or first business day of the week). Bids may not be withdrawn after 6 a.m. E.T. on Monday (or first business day) and bidders will be notified of the auction results by 9 a.m. E.T. on that same day. We may designate one of the Authorized Participants to administer the Grouped Paired Issuances and Grouped Paired Redemptions and to assist Foreside Financial Group or another service provider with coordinating the process of aggregating and pairing bids each week.
An existing holder may submit a bid to redeem, and a prospective investor may submit a bid to purchase, Up or Down BTOX PharmaShares. A valid bid must specify (1) the number of shares to be created or redeemed, which must be a minimum of 100 shares and (2) in the case of a redeeming bidder, the lowest price that bidder is willing to accept in the auction for its shares and, in the case of an acquiring bidder, the highest price at which that bidder is willing to purchase shares. A Grouped Paired Issuance will be effected only if the Up and Down BTOX PharmaShares for which bids were placed can be matched into pairs the combined bid price of which equals or exceeds the combined Per Share Underlying Value of such pair. A Grouped Paired Redemption will be effected only if the Up and Down BTOX PharmaShares tendered for redemption can be matched into pairs the combined redemption price for which does not exceed the combined Per Share Underlying Value of such pair. Once the creation and redemption bids have been paired and aggregated by the Authorized Participants into PharmaShares Units and fractional parts of PharmaShares Units, they will be fulfilled by the PharmaShares Trust using the same procedures as those described in “ —Paired Optional Redemptions” and “ —Paired Issuances” in this prospectus supplement.
Individual investors are not able to direct the PharmaShares Trust to effect creations or redemptions of the Paired BTOX PharmaShares, and must approach an Authorized Participant through their brokers to execute acquisitions. We and the PharmaShares Trust will have contractual arrangements with multiple Authorized
33
Participants and service providers that facilitate information-sharing, aggregating orders and transaction execution among Authorized Participants. An individual Authorized Participant may receive requests from existing shareholders or prospective investors for the redemption or creation of Up or Down BTOX PharmaShares that such Authorized Participant may not be able to match fully with the paired class of PharmaShares or aggregate with a sufficient number of other paired shares to constitute a PharmaShares Unit. Authorized Participants may coordinate with other Authorized Participants of the PharmaShares Trust to pair unmatched orders for creations and redemptions in order to aggregate sufficient orders to direct an issuance of a PharmaShares Unit or to tender a PharmaShares Unit for redemption. This coordination may take the form of direct discussions among Authorized Participants or the matching function may be executed through a private exchange accessible by Authorized Participants that assists with the process of matching issuance and redemption requests for Up and Down PharmaShares. We may enter into an agreement with Foreside Financial Group or another service provider to provide various services to us and the PharmaShares Trust, including creating a private exchange for our Authorized Participants to facilitate the matching and aggregation of Up and Down BTOX PharmaShares for Paired Issuances and Paired Optional Redemptions.
In light of the fact that new Up BTOX PharmaShares and new Down BTOX PharmaShares can be created and issued on an ongoing basis at any point for so long as Series BTOX exists, a “distribution,” as such term is used in the Securities Act, will be occurring continuously. Authorized Participants, other broker-dealers and other persons are cautioned that some of their activities will result in their being deemed participants in a distribution in a manner that will render them statutory underwriters and subject them to the prospectus-delivery and liability provisions of the Securities Act. An Authorized Participant, other broker-dealer firm or any of their respective clients will be deemed a statutory underwriter if any such person purchases a PharmaShares Unit, breaks it down into the constituent Up BTOX PharmaShares and Down BTOX PharmaShares and sells these shares to its customers; or if it chooses to couple the creation of a supply of new PharmaShares with an active selling effort involving solicitation of secondary market demand. A determination of whether a particular market participant is an underwriter must take into account all the facts and circumstances pertaining to the activities of the broker-dealer or its client in the particular case, and the examples mentioned above should not be considered a complete description of all the activities that would lead to designation as an underwriter and becoming subject to the prospectus-delivery and liability provisions of the Securities Act.
Authorized Participants that do offer to the public Up BTOX PharmaShares and Down BTOX PharmaShares from the PharmaShares Units they create will do so at a per share offering price that will vary depending upon, among other factors, the respective trading prices of the Up BTOX PharmaShares and the Down BTOX PharmaShares on NYSE Arca, the respective Per Share Underlying Values, the supply of and demand for the Up BTOX PharmaShares and the Down BTOX PharmaShares at the time of the offer and the results of the Grouped Paired Issuances and Grouped Paired Redemptions. Up BTOX PharmaShares initially comprising the same PharmaShares Unit but offered by an Authorized Participant to the public at different times may have different offering prices. Down BTOX PharmaShares initially comprising the same PharmaShares Unit but offered by an Authorized Participant to the public at different times may have different offering prices. The excess, if any, of the price at which an Authorized Participant sells an Up BTOX PharmaShare or a Down BTOX PharmaShare in respect of which it is acting as a statutory underwriter over the price paid by that Authorized Participant in connection with the creation of that Up BTOX PharmaShare or that Down BTOX PharmaShare in a PharmaShares Unit may be deemed to be underwriting compensation. However, such underwriting compensation, together with any other underwriting compensation received in connection with the offering (if any), will not exceed 10% of the gross proceeds in accordance with FINRA Conduct Rule 2310.
After the initial sixty-day offering period, the PharmaShares Trust will not pay a selling commission or any other compensation out of the funds on deposit in Series BTOX or any other source to any Authorized Participant in connection with the creation of PharmaShares Units. Investors that purchase Up BTOX PharmaShares and/or Down BTOX PharmaShares through a commission/fee-based brokerage account may, however, pay commissions/fees charged by the brokerage account. It is recommended that investors review the terms of their brokerage accounts for details on applicable charges.
Dealers that are not “underwriters” (including Authorized Participants that are not acting as underwriters) but are participating in a distribution (as contrasted to ordinary secondary trading transactions), and thus dealing with Up BTOX PharmaShares or Down BTOX PharmaShares that are part of an “unsold allotment” within the
34
meaning of Section 4(a)(3)(C) of the Securities Act, would be unable to take advantage of the prospectus-delivery exemption provided by Section 4(a)(3) of the Securities Act.
We intend that all sales be made through broker-dealers who are members of FINRA. Investors intending to create or redeem PharmaShares Units through Authorized Participants in transactions not involving a broker-dealer registered in any such investor’s state of domicile or residence should consult their legal advisor regarding applicable broker-dealer or any securities regulatory requirements under the state securities laws prior to such creation or redemption.
Any Authorized Participant may make a market in the Up BTOX PharmaShares or the Down BTOX PharmaShares; however, no Authorized Participant is obligated to do so, and any of them may cease any market-making efforts at any time without notice. No assurance can be given as to the liquidity of the trading market for the Up BTOX PharmaShares or the Down BTOX PharmaShares.
35
APPENDIX A
Hypothetical Returns Based on Broker Reporting Group Projections
The following examples show hypothetical returns on the Up BTOX PharmaShares and the Down BTOX PharmaShares based on historical BOTOX® net revenue performance and related Broker Reporting Group composite projections. The hypothetical returns are based on no leverage (1x) on the Up BTOX PharmaShares and tripled (3x) returns on the Down BTOX PharmaShares. The actual leverage applicable to each class of the PharmaShares will be determined at closing and reflect investor demand for each class of the PharmaShares and the requirement that an equal number of Up and Down BTOX PharmaShares must be issued.
NET INVESTMENT AMOUNT AND PERIODIC UNDERLYING VALUE PERFORMANCE DISTRIBUTIONS(1) PROJECTED BREAKPOINT | COMPOSITE INDEX(2) MEDIAN SCENARIO (EX-MIN / MAX)
| | | Botox Y/o/Y |
| | | Q1 17 | Q2 17 | Q3 17 | Q4 17 | Q1 18 | Q2 18 | Q3 18 | Q4 18 | Q1 19 | Q2 19 | Q3 19 | Q4 19 |
| | | 12.0% | 13.4% | 12.3% | 16.9% | 14.5% | 14.5% | 13.6% | 9.4% | 6.3% | 4.2% | 5.6% | 7.9% |
Up 1x / Down 3x / 15% Cap / Projected Breakpoint | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | Q1 17 | Q2 17 | Q3 17 | Q4 17 | Q1 18 | Q2 18 | Q3 18 | Q4 18 | Q1 19 | Q2 19 | Q3 19 | Q4 19 |
| | | | | | | | | | | | | | |
Up BTOX Return Scenario | | Botox Y/o/Y Actual Results | 12.0% | 13.4% | 12.3% | 16.9% | 14.5% | 14.5% | 13.6% | 9.4% | 6.3% | 4.2% | 5.6% | 7.9% |
| Projected Breakpoint | 7.6% | 10.5% | 14.0% | 12.6% | 7.6% | 10.6% | 10.3% | 9.4% | 7.0% | 6.5% | 5.7% | 4.7% |
| Change in Up Underlying Value | 4.4% | 2.9% | (5.1%) | 4.3% | 6.9% | 3.9% | 3.3% | 0.0% | (2.1%) | (6.8%) | (0.2%) | 3.2% |
| Up Allocation Factor | 1.044 | 1.029 | 0.949 | 1.043 | 1.069 | 1.039 | 1.033 | 1.000 | 0.979 | 0.932 | 0.998 | 1.032 |
| Beginning Up Investment Amount | $1,000 | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 |
| Gain from Down / (Loss from Up) Investment Amount | $44 | $28 | ($47) | $38 | $58 | $31 | $25 | $0 | ($15) | ($49) | ($2) | $21 |
| UV Before Periodic Distribution | $1,044 | $984 | $881 | $919 | $901 | $816 | $779 | $729 | $714 | $665 | $663 | $684 |
| Distributions: | | | | | | | | | | | | |
| Up Underlying Value Gain (3) | $44 | $28 | – | $38 | $58 | $31 | $25 | $0 | – | – | – | $21 |
| Equalization Payment | $44 | $28 | – | $38 | $58 | $31 | $25 | $0 | – | – | – | $21 |
| UV After Periodic Distribution (4) | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 | $642 |
| Cumulative UV Gain / (Loss) | $44 | $72 | $25 | $63 | $121 | $152 | $177 | $177 | $162 | $113 | $111 | $132 |
| Cumulative Equalization Payments | $44 | $72 | $72 | $110 | $168 | $199 | $224 | $224 | $243 | $224 | $224 | $245 |
| | | | | | | | | | | | | | |
Up 1x / Down 3x / 15% Cap / Projected Breakpoint | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | Q1 17 | Q2 17 | Q3 17 | Q4 17 | Q1 18 | Q2 18 | Q3 18 | Q4 18 | Q1 19 | Q2 19 | Q3 19 | Q4 19 |
| | | | | | | | | | | | | | |
Down BTOX Return Scenario | | Botox Y/o/Y Actual Results | 12.0% | 13.4% | 12.3% | 16.9% | 14.5% | 14.5% | 13.6% | 9.4% | 6.3% | 4.2% | 5.6% | 7.9% |
| Projected Breakpoint | 7.6% | 10.5% | 14.0% | 12.6% | 7.6% | 10.6% | 10.3% | 9.4% | 7.0% | 6.5% | 5.7% | 4.7% |
| Change in Down Underlying Value | (4.4%) | (2.9%) | 5.1% | (4.3%) | (6.9%) | (3.9%) | (3.3%) | (0.0%) | 2.1% | 6.8% | 0.2% | (3.2%) |
| Down Allocation Factor | 0.956 | 0.971 | 1.051 | 0.957 | 0.931 | 0.961 | 0.967 | 1.000 | 1.021 | 1.068 | 1.002 | 0.968 |
| Beginning Down Investment Amount | $1,000 | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 |
| Gain from Up / (Loss from Down) Investment Amount | ($44) | ($28) | $47 | ($38) | ($58) | ($31) | ($25) | ($0) | $15 | $49 | $2 | ($21) |
| UV Before Periodic Distribution | $956 | $928 | $975 | $843 | $785 | $754 | $729 | $729 | $744 | $763 | $667 | $642 |
| Distributions: | | | | | | | | | | | | |
| Down Underlying Value Gain (5) | – | – | $47 | – | – | – | – | – | $15 | $49 | $2 | – |
| Equalization Payment | – | – | $47 | – | – | – | – | – | $15 | $49 | $2 | – |
| UV After Periodic Distribution (6) | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 | $642 |
| Cumulative UV Gain / (Loss) | ($44) | ($72) | ($25) | ($63) | ($121) | ($152) | ($177) | ($177) | ($162) | ($113) | ($111) | ($132) |
| Cumulative Equalization Payments | $0 | $0 | $47 | $47 | $47 | $47 | $47 | $47 | $62 | $111 | $113 | $113 |
(1)The calculations in the tables above do not take into account any net treasury income distributions or any reductions in the Periodic UV Performance Distributions to pay fees and expenses due to insufficient treasury income or negative treasury yields. Numbers may not sum due to rounding.
(2)Composite Index represents Broker Reporting Group consensus projections (excluding min and max projections for each period).
(3)The Up Underlying Value Gain plus the Equalization Payment is equal to the Periodic UV Performance Distribution on the Up PharmaShares.
(4)The Up UV after the Periodic UV Performance Distribution is also equal to the new Up Investment Amount.
(5)The Down Underlying Value Gain plus the Equalization Payment is equal to the Periodic UV Performance Distribution on the Down PharmaShares.
(6)The Down UV after the Periodic UV Performance Distribution is also equal to the new Down Investment Amount.
Sources: Historic Botox Sales: AGN Company Filings Composite Index Consensus Projections (excluding min and max projections for each period): Visible Alpha
A-1
NET INVESTMENT AMOUNT AND PERIODIC UNDERLYING VALUE PERFORMANCE DISTRIBUTIONS POST-FEES(1) PROJECTED BREAKPOINT | COMPOSITE INDEX(2) MEDIAN SCENARIO (EX-MIN / MAX)
| | | Treasury Income |
| | | Q1 17 | Q2 17 | Q3 17 | Q4 17 | Q1 18 | Q2 18 | Q3 18 | Q4 18 | Q1 19 | Q2 19 | Q3 19 | Q4 19 |
| | Treasury Yield (3) | 0.60% | 0.94% | 1.05% | 1.24% | 1.61% | 1.91% | 2.11% | 2.37% | 2.40% | 2.12% | 1.88% | 1.55% |
| | Treasury earnings for Series BTOX | $12.00 | $17.97 | $19.50 | $21.85 | $27.16 | $30.01 | $31.85 | $34.58 | $35.00 | $30.27 | $25.02 | $20.58 |
| | Net Treasury Yield (% fees in excess of earnings) (4) | (1.90)% | (1.56)% | (1.45)% | (1.26)% | (0.89)% | (0.59)% | (0.39)% | (0.13)% | (0.10)% | (0.38)% | (0.62)% | (0.95)% |
| | Net Treasury Income (Fees in excess of earnings) ($) | (38.00) | (29.83) | (26.93) | (22.21) | (15.01) | (9.27) | (5.89) | (1.90) | (1.46) | (5.43) | (8.25) | (12.61) |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Up 1x / Down 3x / 15% Cap / Projected Breakpoint | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | Q1 17 | Q2 17 | Q3 17 | Q4 17 | Q1 18 | Q2 18 | Q3 18 | Q4 18 | Q1 19 | Q2 19 | Q3 19 | Q4 19 |
| | | | | | | | | | | | | | |
Up BTOX Return Scenario | | Botox Y/o/Y Actual Results | 12.0% | 13.4% | 12.3% | 16.9% | 14.5% | 14.5% | 13.6% | 9.4% | 6.3% | 4.2% | 5.6% | 7.9% |
| Projected Breakpoint | 7.6% | 10.5% | 14.0% | 12.6% | 7.6% | 10.6% | 10.3% | 9.4% | 7.0% | 6.5% | 5.7% | 4.7% |
| % Change in Up Underlying Value | 4.4% | 2.9% | (5.1%) | 4.3% | 6.9% | 3.9% | 3.3% | 0.0% | (2.1%) | (6.8%) | (0.2%) | 3.2% |
| Up Allocation Factor | 1.044 | 1.029 | 0.949 | 1.043 | 1.069 | 1.039 | 1.033 | 1.000 | 0.979 | 0.932 | 0.998 | 1.032 |
| Beginning Up Investment Amount | $1,000 | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 |
| Gain from Down / (Loss from Up) Investment Amount | $44 | $28 | ($47) | $38 | $58 | $31 | $25 | $0 | ($15) | ($49) | ($2) | $21 |
| UV Before Periodic Distribution | $1,044 | $984 | $881 | $919 | $901 | $816 | $779 | $729 | $714 | $665 | $663 | $684 |
| Distributions: | | | | | | | | | | | | |
| Up Underlying Value Gain (5) | $44.00 | $28.00 | – | $38.00 | $58.00 | $31.00 | $25.00 | $0.00 | – | – | – | $21.00 |
| Actual Fee Deduction from Gain (6) | ($1.10) | ($0.70) | – | ($0.95) | ($1.45) | ($0.78) | ($0.63) | ($0.00) | – | – | – | ($0.53) |
| Equalization Payment | $44.00 | $28.00 | – | $38.0 | $58.00 | $31.00 | $25.00 | $0.00 | – | – | – | $21.00 |
| UV After Periodic Distribution (7) | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 | $642 |
| Cumulative UV Gain / (Loss) After Fee Deduction ($) | 42.90 | 69.20 | 22.20 | 60.20 | 116.75 | 146.97 | 171.34 | 171.34 | 156.34 | 108.34 | 106.34 | 126.81 |
| Cumulative Equalization Payments | $44 | $72 | $72 | $110 | $168 | $199 | $224 | $224 | $224 | $224 | $224 | $245 |
| Cumulative Fees Deducted From Gain Distribution | ($1.10) | ($1.80) | ($1.80) | ($2.80) | ($4.25) | ($5.03) | ($5.66) | ($5.66) | ($5.66) | ($5.66) | ($5.66) | ($6.19) |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Up 1x / Down 3x / 15% Cap / Projected Breakpoint | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | Q1 17 | Q2 17 | Q3 17 | Q4 17 | Q1 18 | Q2 18 | Q3 18 | Q4 18 | Q1 19 | Q2 19 | Q3 19 | Q4 19 |
| | | | | | | | | | | | | | |
Down BTOX Return Scenario | | Botox Y/o/Y Actual Results | 12.0% | 13.4% | 12.3% | 16.9% | 14.5% | 14.5% | 13.6% | 9.4% | 6.3% | 4.2% | 5.6% | 7.9% |
| Projected Breakpoint | 7.6% | 10.5% | 14.0% | 12.6% | 7.6% | 10.6% | 10.3% | 9.4% | 7.0% | 6.5% | 5.7% | 4.7% |
| % Change in Down Underlying Value | (4.4%) | (2.9%) | 5.1% | (4.3%) | (6.9%) | (3.9%) | (3.3%) | (0.0%) | 2.1% | 6.8% | 0.2% | (3.2%) |
| Down Allocation Factor | 0.956 | 0.971 | 1.051 | 0.957 | 0.931 | 0.961 | 0.967 | 1.000 | 1.021 | 1.068 | 1.002 | 0.968 |
| Beginning Down Investment Amount | $1,000 | $956 | $928 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 |
| Gain from Up / (Loss from Down) Investment Amount | ($44) | ($28) | $47 | ($38) | ($58) | ($31) | ($25) | ($0) | $15 | $49 | $2 | ($21) |
| UV Before Periodic Distribution | $956 | $928 | $975 | $843 | $785 | $754 | $729 | $729 | $744 | $763 | $667 | $642 |
| Distributions: | | | | | | | | | | | | |
| Down Underlying Value Gain (8) | – | – | $47.00 | – | – | – | – | – | $15.00 | $49.00 | $2.00 | – |
| Actual Fee Deduction from Gain (6) | – | – | ($1.20) | – | – | – | – | – | ($0.38) | ($1.23) | ($0.05) | – |
| Equalization Payment | – | – | $47.00 | – | – | – | – | – | $15.00 | $49.00 | $2.00 | – |
| UV After Periodic Distribution (9) | $956 | $929 | $881 | $843 | $785 | $754 | $729 | $729 | $714 | $665 | $663 | $642 |
| Cumulative UV Gain / (Loss) After Fee Deduction ($) | (44) | (72) | (26.20) | (64.20) | (122.20) | (153.2) | (178.2) | (178.2) | (163.58) | (115.81) | (113.86) | (134.86) |
| Cumulative Equalization Payments | $0 | $0 | $47 | $47 | $47 | $47 | $47 | $47 | $62 | $111 | $113 | $113 |
| Cumulative Fees Deducted From Gain Distribution | – | – | ($1.20) | ($1.20) | ($1.20) | ($1.20) | ($1.20) | ($1.20) | ($1.58) | ($2.81) | ($2.86) | ($2.86) |
(1)The calculations in the tables above include net treasury income distributions and any reductions in the UV gain portion of Periodic UV Performance Distributions to pay fees and expenses due to insufficient treasury income or negative treasury yields. Numbers may not sum due to rounding.
(2)Composite Index represents Broker Reporting Group consensus projections (excluding min and max projections for each period).
(3)Treasury yields are based on the 3 Month U.S. Treasury Bill Rate. Series BTOX may invest only in treasuries and repurchase agreements for treasuries that mature, in each case, prior to the end of the current quarter.
(4)For purposes of the hypothetical yields shown in the table above, we have assumed the maximum permitted fee deduction rate of 2.5% of the Series Investment Amount. The actual fee deduction rate, as determined by the administrative agent for each period, may be lower (but never greater than 2.5%). No net treasury income was earned during any period shown in the tables above.
(5)For each period in which there is an increase in the Up Underlying Value, the sum of (a) the Up UV Gain, less any deduction for fees, plus (b) the Equalization Payment, plus (c) any net treasury income will be equal to the Periodic UV Performance Distribution on the Up PharmaShares.
(6)A fee deduction will be made equal to the lesser of actual fees in excess of treasury income and a maximum permitted fee deduction of 2.5% of the UV gain portion of any Periodic UV Performance Distribution on the Up or the Down PharmaShares; such a deduction will only be made if treasury income for the applicable period is insufficient to pay all fees and expenses.
(7)The Up UV after the Periodic UV Performance Distribution is also equal to the new Up Investment Amount.
(8)For each period in which there is an increase in the Down Underlying Value, the sum of (a) the Down UV Gain, less any deduction for fees, plus (b) the Equalization Payment, plus (c) any net treasury income will be equal to the Periodic UV Performance Distribution on the Down PharmaShares.
(9)The Down UV after the Periodic UV Performance Distribution is also equal to the new Down Investment Amount.
Source: Historic Botox Sales: AGN Company Filings. Composite Index Consensus Projections (excluding min and max projections for each period): Visible Alpha
A-2
APPENDIX B
Hypothetical Underlying Value Calculations
Example showing an Actual Percentage Change that Exceeds the Projected Breakpoint Increase.
The calculations for the example below are based on the following assumptions:
• Fourth calendar quarter of 2017 BOTOX® net revenues, published on 02/06/18: $864.3 million
• Fourth calendar quarter of 2016 BOTOX® net revenues, published on 02/15/17: $739.3 million
• Actual Percentage Change from Q4 of 2017 to Q4 2016 (net revenues for Q4 of 2017 minus net revenues for Q4 of 2016 divided by net revenues for Q4 of 2016): an increase of 16.9%
• Net revenue projections of $833.0 million (Visible Alpha median with high/low removed) was projected for the fourth quarter of 2017 compared to the fourth quarter of 2016 based on eleven (11) brokers’ projections (including high/low projections) as of 09/30/17. Accordingly, the Projected Breakpoint Increase is 12.6%.
• Applicable Leverage Factor for Up/Down: 1x/3x
• Periodic Return Cap on Up returns/Down returns: 15%/15%
Up Investment Amount: $1,000,000
Down Investment Amount: $1,000,000
Up Underlying Value at the beginning of the Calculation Period: $1,000,000
Down Underlying Value at the beginning of the Calculation Period: $1,000,000
• The Actual Percentage Change, as measured on the Publication Date occurring on February 6, 2018 was 16.9%, which was greater than the Projected Breakpoint Increase. The Up Underlying Value and Down Underlying Value would have been calculated as follows:
UUVΔ = Actual Percentage Change – Projected Breakpoint Increase1
UUVΔ = (16.9% – 12.6%)
UUVΔ = 4.3%
In this example, as a result of the 16.9% Actual Percentage Change, the Up Underlying Value would have increased by 4.3%.
After applying the Up Leverage Factor of 1x and the Up Periodic Return Cap of 15%, the Up Underlying Value increase would remain 4.3%.
Accordingly, the Up Allocation Factor | = 1 + the increase in the Up Underlying Value, |
| = 1 + 0.043 |
1 The appropriate formula was selected based on the fact that a Projected Breakpoint Increase was projected by the Broker Reporting Group for the Current Calendar Quarter and the Actual Percentage Change was greater than the Projected Breakpoint Increase. In such circumstances, the Up Underlying Value will increase as follows (and there will be a corresponding decrease in the Down Underlying Value):
UUVΔ = Actual Percentage Change – Projected Breakpoint Increase
Up Allocation Factor = 1 + the increase in the Up Underlying Value, and
Down Allocation Factor = 1– the increase in the Up Underlying Value
B-1
the Down Allocation Factor | = 1 – 0.043 |
| = 0.957 |
Up Underlying Value = 1,000,000 * 1.043 = $1,043,000
Down Underlying Value = 1,000,000 * 0.957 = $957,000
Periodic UV Performance Distribution for Up BTOX PharmaShares = $86,000
For this Calculation Period, disregarding any Net Treasury Income, the Up Underlying Value would have increased to $1,043,000 and the Down Underlying Value would have decreased to $957,000. The Up BTOX PharmaShares would have received a Periodic UV Performance Distribution equal to $86,000 (consisting of the $43,000 increase in the Up Underlying Value and an Equalization Payment equal to $43,000). The Down BTOX PharmaShares would not have received any Periodic UV Performance Distribution for this Calculation Period.
Example showing an Actual Percentage Change that exceeds the Projected Breakpoint Increase equal to the Periodic Return Cap for 4 consecutive Calculation Periods.
The calculations for the example below are based on the following assumptions, all of which are hypothetical net revenue numbers and hypothetical Projected Breakpoints. At no time during the past 4 years have actual net revenue results exceeded the Visible Alpha median projection (with high and low removed) by 15% or more:
First Calculation Period:
• Actual Percentage Change from Q1 of Year X to Q1 of Year X+1: 20.0%
• Projected Breakpoint Increase is 5.0%.
• Applicable Leverage Factor for Up/Down: 1x/3x
• Periodic Return Cap on Up returns/Down returns: 15%/15%
• Up Investment Amount: $1,000,000 / Down Investment Amount: $1,000,000
• Up Underlying Value / Down Underlying Value at start of Calculation Period: $1,000,000 / $1,000,000
• Treasury income average accrual rate: 2.0% / Fee accrual rate: max rate of 2.5%
• Excess fees: $2 million * 0.005% = $10,000
The Actual Percentage Change is 15% higher than the Projected Breakpoint Increase. The Up Underlying Value and Down Underlying Value would be calculated as follows:
UUVΔ = Actual Percentage Change – Projected Breakpoint Increase
UUVΔ = 20.0% – 5.0%
UUVΔ = 15.0%
In this example, as a result of the 20.0% Actual Percentage Change, the Up Underlying Value would increase by 15.0%.
After applying the Up Leverage Factor of 1x and the Up Periodic Return Cap of 15%, the Up Underlying Value increase would remain 15.0%.
Accordingly, the Up Allocation Factor | = 1 + the increase in the Up Underlying Value, |
| = 1 + 0.15 |
| = 1.15 |
B-2
the Down Allocation Factor | = 1 – 0.15 |
| = 0.85 |
Up Underlying Value prior to periodic distribution = 1,000,000 * 1.15 = $1,150,000
Down Underlying Value prior to periodic distribution = 1,000,000 * 0.85 = $850,000
Periodic UV Performance Distribution for Up BTOX PharmaShares
= Increase in UV – (lesser of (excess fees and 2.5% of Increase in UV)) + Equalization Payment
= $150,000 – (lesser of $10,000 and $3,750) + 150,000
= $296,250
For this Calculation Period, the Up Underlying Value would increase to $1,150,000 and the Down Underlying Value would decrease to $850,000. The Up BTOX PharmaShares would receive a Periodic UV Performance Distribution equal to $3000,000 (consisting of the $150,000 increase in the Up Underlying Value payable out of the Down Investment Amount and an Equalization Payment equal to $150,000 payable out of the Up Investment Amount) which would be reduced by $3,750, allocable to excess fees. The Down BTOX PharmaShares would not receive any Periodic UV Performance Distribution for this Calculation Period. Both the Up and the Down Underlying Value would be $850,000 after the Periodic UV Performance Distribution is made.
Second Calculation Period:
• Actual Percentage Change from Q2 of Year X to Q2 of Year X+1: 10.0%
• Projected Breakpoint Decrease is 5.0%.
• Applicable Leverage Factor for Up/Down: 1x/3x
• Periodic Return Cap on Up returns/Down returns: 15%/15%
• Up Investment Amount: $850,000 / Down Investment Amount: $850,000
• Up Underlying Value / Down Underlying Value at start of Calculation Period: $850,000 / $850,000
• Treasury income average accrual rate: 1.5% / Fee accrual rate: max rate of 2.5%
• Excess fees: Series Investment Amount of $1.7 million * 0.010% = $17,000
The Actual Percentage Change is a 10% increase when a 5% decrease was projected. The Up Underlying Value and Down Underlying Value would be calculated as follows:
UUVΔ = Actual Percentage Change + Projected Breakpoint Decrease
UUVΔ = 10.0% + 5.0%
UUVΔ = 15.0%
In this example, as a result of the 10.0% Actual Percentage Change when a 5% decrease was projected, the Up Underlying Value would increase by 15.0%.
After applying the Up Leverage Factor of 1x and the Up Periodic Return Cap of 15%, the Up Underlying Value increase would remain 15.0%.
Accordingly, the Up Allocation Factor | = 1 + the increase in the Up Underlying Value, |
| = 1 + 0.15 |
| = 1.15 |
B-3
the Down Allocation Factor | = 1 – 0.15 |
| = 0.85 |
Up Underlying Value prior to periodic distribution = 850,000 * 1.15 = $977,500
Down Underlying Value prior to periodic distribution = 850,000 * 0.85 = $722,500
Periodic UV Performance Distribution for Up BTOX PharmaShares
= Increase in UV – (lesser of (excess fees and 2.5% of Increase in UV)) + Equalization Payment
= $977,500-850,000 – (lesser of $17,000 and $3,187.50) + 127,500
= $251,812.50
For this Calculation Period, the Up Underlying Value would increase to $977,500 and the Down Underlying Value would decrease to $722,500. The Up BTOX PharmaShares would receive a Periodic UV Performance Distribution equal to $255,000 (consisting of the $127,500 increase in the Up Underlying Value payable out of the Down Investment Amount and an Equalization Payment equal to $127,500 payable out of the Up Investment Amount) which would be reduced by $3,187.50, allocable to excess fees. The Down BTOX PharmaShares would not receive any Periodic UV Performance Distribution for this Calculation Period. Both the Up and the Down Underlying Value would be $722,500 after the Periodic UV Performance Distribution is made.
Third Calculation Period:
• Actual Percentage Change from Q3 of Year X to Q3 of Year X+1: 25.0%
• Projected Breakpoint Increase is 7.0%.
• Applicable Leverage Factor for Up/Down: 1x/3x
• Periodic Return Cap on Up returns/Down returns: 15%/15%
• Up Investment Amount: $722,500 / Down Investment Amount: $722,500
• Up Underlying Value / Down Underlying Value at start of Calculation Period: $722,500 / $722,500
• Treasury income average accrual rate: 2.8% / Fee accrual rate: max rate of 2.5%
• Excess fees: $0
The Actual Percentage Change is a 25% increase when a 7% decrease was projected. The Up Underlying Value and Down Underlying Value would be calculated as follows:
UUVΔ = Actual Percentage Change - Projected Breakpoint Decrease
UUVΔ = 25.0% - 7.0%
UUVΔ = 18.0%
In this example, as a result of the 25.0% Actual Percentage Change when a 7.0% decrease was projected, the Up Underlying Value would increase by 18.0%.
After applying the Up Leverage Factor of 1x and the Up Periodic Return Cap of 15%, the Up Underlying Value increase would be reduced to 15.0%.
Accordingly, the Up Allocation Factor | = 1 + the increase in the Up Underlying Value, |
| = 1 + 0.15 |
| = 1.15 |
B-4
the Down Allocation Factor | = 1 – 0.15 |
| = 0.85 |
Up Underlying Value prior to periodic distribution = 722,500 * 1.15 = $830,875
Down Underlying Value prior to periodic distribution = 722,500 * 0.85 = $614,125
Periodic UV Performance Distribution for Up BTOX PharmaShares
= Increase in UV – (lesser of (excess fees and 2.5% of Increase in UV)) + Equalization Payment
= $830,875-722,500 – (lesser of $0 and $2709) + 108,375
= $216,750
For this Calculation Period, the Up Underlying Value would increase to $830875 and the Down Underlying Value would decrease to $614,125. The Up BTOX PharmaShares would receive a Periodic UV Performance Distribution equal to $216,750 (consisting of the $108,375 increase in the Up Underlying Value payable out of the Down Investment Amount and an Equalization Payment equal to $108,375 payable out of the Up Investment Amount). There are no excess fees. The Down BTOX PharmaShares would not receive any Periodic UV Performance Distribution for this Calculation Period. Both the Up and the Down Underlying Value would be $614,125 after the Periodic UV Performance Distribution is made.
Fourth Calculation Period:
• Actual Percentage Change from Q4 of Year X to Q4 of Year X+1: 18.0%
• Projected Breakpoint Increase is 2.0%.
• Applicable Leverage Factor for Up/Down: 1x/3x
• Periodic Return Cap on Up returns/Down returns: 15%/15%
• Up Investment Amount: $614,125 / Down Investment Amount: $614,125
• Up Underlying Value / Down Underlying Value at start of Calculation Period: $614,125 / $614,125
• Treasury income average accrual rate: 2.1% / Fee accrual rate: max rate of 2.5%
• Excess fees: Series Investment Amount of $1.2283 million * 0.004% = $4,913
The Actual Percentage Change is a 18% increase when a 2% decrease was projected. The Up Underlying Value and Down Underlying Value would be calculated as follows:
UUVΔ = Actual Percentage Change - Projected Breakpoint Decrease
UUVΔ = 18.0% + 2.0%
UUVΔ = 16.0%
In this example, as a result of the 18.0% Actual Percentage Change when a 2.0% increase was projected, the Up Underlying Value would increase by 16.0%.
After applying the Up Leverage Factor of 1x and the Up Periodic Return Cap of 15%, the Up Underlying Value increase would be reduced to 15.0%.
Accordingly, the Up Allocation Factor | = 1 + the increase in the Up Underlying Value, |
| = 1 + 0.15 |
| = 1.15 |
B-5
the Down Allocation Factor | = 1 – 0.15 |
| = 0.85 |
Up Underlying Value prior to periodic distribution = 614,125 * 1.15 = $706,243.75
Down Underlying Value prior to periodic distribution = 614,125 * 0.85 = $522,006.25
Periodic UV Performance Distribution for Up BTOX PharmaShares
= Increase in UV – (greater of (excess fees and 2.5% of Increase in UV)) + Equalization Payment
= $706,243.75-614,125 – (lesser of $4,913 and $2,303) + 92,118.75
= $181,934.50
For this Calculation Period, the Up Underlying Value would increase to $706,243.75 and the Down Underlying Value would decrease to $522,006.25. The Up BTOX PharmaShares would receive a Periodic UV Performance Distribution equal to $184,237.50 (consisting of the $92,118.75 increase in the Up Underlying Value payable out of the Down Investment Amount and an Equalization Payment equal to $92,118.75 payable out of the Up Investment Amount) which would be reduced by $2,303, allocable to excess fees. The Down BTOX PharmaShares would not receive any Periodic UV Performance Distribution for this Calculation Period. Both the Up and the Down Underlying Value would be $522,006.25 after the Periodic UV Performance Distribution is made.
Example showing an Actual Percentage Change that is Less Than the Projected Breakpoint Increase.
The calculations for the example below are based on the following assumptions:
• Second calendar quarter of 2018 BOTOX® net revenues, published on 07/26/2018: $935 million
• Second calendar quarter of 2019 BOTOX® net revenues, published on 08/6/2019: $974 million
• Actual Percentage Change (net revenues for 2nd Q of 2019 minus net revenues for 2nd Q of 2018 divided by net revenues for 2nd Q of 2018): 4.2%
• Projected Breakpoint Increase: A Projected Breakpoint Increase of 6.5% was projected for the second quarter of 2019 compared to the second quarter of 2018 based on 15 brokers’ projections as of 03/31/19.
• Applicable Leverage Factor for Up/Down: 1x/3x
• Periodic Return Cap on Up returns/Down returns: 15%/15%
Up Investment Amount: $1,000,000
Down Investment Amount: $1,000,000
Up Underlying Value at the beginning of the Calculation Period: $1,000,000
Down Underlying Value at the beginning of the Calculation Period: $1,000,000
• The Actual Percentage Change, as measured on the Publication Date for the second quarter of 2019 was 4.2%, which was less than the Projected Breakpoint Increase. The Up Underlying Value and Down Underlying Value would have been calculated as follows:
DUVΔ = Projected Breakpoint Increase – Actual Percentage Change2
DUVΔ = 6.5% – 4.2%
DUVΔ = 2.3%
2 The appropriate formula was selected based on the fact that a Projected Breakpoint Increase was projected by the Broker Reporting Group for the Current Calendar Quarter and the Actual Percentage Change was less than the Projected Breakpoint Increase. In such circumstances, the Down Underlying Value will increase as follows (and there will be a corresponding decrease in the Up Underlying Value):
DUVΔ = Projected Breakpoint Increase – Actual Percentage Change
Down Allocation Factor = 1 + the increase in the Down Underlying Value, and
Up Allocation Factor = 1 – the increase in the Down Underlying Value
B-6
In this example, as a result of the 4.2% Actual Percentage Change, the Down Underlying Value would have increased by 2.3%.
After applying the Down Leverage Factor of 3x, the Down Underlying Value would increase to 6.9%. Applying the Down Periodic Return Cap would have no effect.
Accordingly, the Up Allocation Factor | = 1 - the increase in the Down Underlying Value, |
| = 1 - 0.069 |
| = 0.931 |
the Down Allocation Factor | = 1 + the increase in the Down Underlying Value |
| = 1 + 0.069 |
| = 1.069 |
Up Underlying Value = 1,000,000 * 0.931 = $931,000
Down Underlying Value = 1,000,000 * 1.069 = $1,069,000
Periodic UV Performance Distribution for Down BTOX PharmaShares = $138,000
For this Calculation Period, disregarding any Net Treasury Income, the Up Underlying Value would have decreased to $931,000 and the Down Underlying Value would have increased to $1,069,000. The Down BTOX PharmaShares would have received a Periodic UV Performance Distribution equal to $138,000 (consisting of the $69,000 increase in the Down Underlying Value and an Equalization Payment equal to $69,000). The Up BTOX PharmaShares would not have received any Periodic UV Performance Distribution for this Calculation Period.
B-7
APPENDIX C
Underlying Value for Current Calculation Period; Projected Breakpoint for Current Calendar Quarter; Change in Underlying Value on Publication Date
Per Share Underlying Value of the Up PharmaShares at beginning of Calculation Period relating to Q2 2021: $[50]/share
Per Share Underlying Value of the Down PharmaShares at beginning of Calculation Period: relating to Q2 2021: $[50]/share Full list of brokers reporting for BOTOX® and
making their projections available
to a market research integration service
Projected Breakpoint for Q2/2021
| Atlantic Equities, Bank of America Merrill Lynch, Berenberg, Bank of Montreal, Citicorp (ICG), Cowen, Credit Suisse, Deutsche Bank, Goldman Sachs, Guggenheim, Jefferies, SVB Leerink, Morgan Stanley, Piper, Royal Bank of Canada, Société Générale, SunTrust, UBS, William Blair and Wolfe Projected Breakpoint [Increase][Decrease] of [●]% |
Composite Projection
Date Published
Mean
Median
High/Low Number of Brokers Included in Composite for Current Projected Breakpoint Average projected net revenues used for
Q2/2021 Projected Breakpoint Actual net revenues for Q2 of 2020 |
[●], 20[●]
[●] ([●] with high/low removed)
[●] ([●] with high/low removed)
[●]/[●]
[●]
$[●] (median with high and low removed)
$[●]
|
| |
| |
C-1
Actual Percentage Change
Q2/2020 vs. Q2/2021
Up Underlying Value [Increase][Decrease] Per Share
Down Underlying Value [Increase][Decrease] Per Share New Per Share Underlying Value of the Up PharmaShares on Publication Date: $[ ]/share New Per Share Underlying Value of the Down PharmaShares on Publication Date: $[ ]/share Periodic UV Performance Distribution
Per Share Underlying Value of the Up PharmaShares after Periodic UV Performance Distribution in made and for remainder of Calculation Period relating to Q3 2021
Per Share Underlying Value of the Down PharmaShares after Periodic UV Performance Distribution in made and for remainder of Calculation Period relating to Q3 2021 | [●]%
$[●]
$[●]
$[●]
$[●]
$[●] per [Up][Down] PharmaShare
$[●] per Up PharmaShare
$[●] per Down PharmaShare
|
C-2
APPENDIX F
Financial Statements of the PharmaShares Trust
and Series BTOX-2020
PharmaShares Trust
Series BTOX-2020
Statement of Financial Condition
[●][●], 2020
Assets: | |
Cash | $ [ ] |
| |
Capital: | |
Capital | $ [ ] |
The accompanying notes are an integral part of this financial statement.
F-1
PharmaShares Trust
Consolidated Statement of Financial Condition
[●][●], 2020
| Series BTOX | Series [Drug #2] | Series [Drug #3] | Series [Drug #4] | Series [Drug #5] | Series [Drug #6] | Series [Drug #7] | Series [Drug #8] | Series [Drug #9] | Series [Drug #10] | Series [Drug #11] | Series [Drug #12] | Consolidated Total |
Assets | | | | | | | | | | | | | |
Cash | $ | $ | $ | $ | $ | | | | | $ | $ | | $ |
| | | | | | | | | | | | | |
Capital | | | | | | | | | | | | | |
Capital | $ | $ | $ | $ | $ | | | | | $ | $ | | $ |
The accompanying notes are an integral part of this financial statement.
* The Consolidated Statement of Financial Condition of PharmaShares Trust is being provided solely to meet United States Securities and Exchange Commission regulatory requirements. The PharmaShares Trust does not have assets or liabilities separate from those of its Series. An investor in a Series of the PharmaShares Trust has an entitlement to the assets of that Series only and not to the assets of any other Series or the PharmaShares Trust as a whole.
F-2
PharmaShares Trust
Notes to the Financial Statement
NOTE 1 ORGANIZATION
The PharmaShares Trust is a Delaware statutory trust formed on [●][●], 2020 and organized into [●] separate series (each, a “Series”). PharmaShares Advisors, LLC (“PharmaShares Advisors”) acts as the depositor for the PharmaShares Trust and for each Series of that trust. PharmaShares Depositor will retain all of the residual interests in the PharmaShares Trust and each Series of that trust.
In connection with the issuance of each new Series of Paired PharmaShares, a new Series of the PharmaShares Trust will be established. The trustee will deposit and hold the proceeds of the initial sale and subsequent issuances of the Paired PharmaShares of each Series in a segregated account in which those funds will be held by the trustee for the exclusive benefit of the shareholders of that Series, separate from the assets and liabilities of all other Series of the PharmaShares Trust. The assets of a Series will not be available to satisfy the liabilities of, or to make distributions on the shares issued by, any other Series.
NOTE 2 SIGNIFICANT ACCOUNTING POLICIES
The following is a summary of significant accounting policies followed by each Series in preparation of its financial statements. These policies are in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
Use of Estimates & Indemnifications
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts and disclosures in these financial statements. Actual results could differ from those estimates.
In the normal course of business, the PharmaShares Trust enters into contracts that contain a variety of representations which provide general indemnifications. The PharmaShares Trust’s maximum exposure under these arrangements cannot be known; however, PharmaShares Advisors expects any risk of loss to be remote.
Income Taxes
For U.S. federal and applicable state and local income tax purposes, the PharmaShares Trust intends to treat (i) each Series as a separate taxable corporation, (ii) the shares of each Series as stock therein and (iii) each investor in a Series as a shareholder in such Series. Accordingly, each taxable year each Series will be subject to federal and applicable state and local income taxation at applicable corporate income tax rates on its net taxable income, if any.
NOTE 3 AGREEMENTS:
Master Trust Agreement and Trust Supplements and Authorized Participant Agreement
Administrative Agent Fee; Payment of Fees by, and Reimbursement of, PharmaShares Advisors
PharmaShares Advisors acts as the administrative agent for each Series pursuant to the Master Trust Agreement and each Trust Supplement. Each Series pays to PharmaShares Advisors an administrative fee, quarterly in arrears, in an amount equal to the product of (i) an annual rate of 1.25% and (ii) the daily Series Investment Amount of that Series, calculated on the basis of a 365-day year.
In the event that any Series does not have adequate treasury income to pay its fixed and variable fees and expenses on any Periodic Calculation Date, PharmaShares Advisors will reduce its administrative fee and, if there is still insufficient treasury income available to pay all fees and expenses after the administrative agent fee has been reduced to zero, PharmaShares Advisors will pay such fees and expenses on behalf of the applicable Series. PharmaShares Advisors will seek to reimburse itself for all fees and expenses it has paid on behalf of a Series out of
F-3
the treasury income of that Series, including fees and expenses that were paid as a result of the reduction of the administrative agent fee.
All fees and expenses of each Series, including any reimbursement amounts payable to PharmaShares Advisors, are subject to a cap equal to an annual rate of 2.50% on the daily Series Investment Amount, calculated on the basis of a 365-day year. Fees and expenses cannot be reimbursed to PharmaShares Advisors at a rate in excess of the cap, after taking into account all other current fees and expenses payable with respect to any Calculation Period.
In addition, at any time during which treasury yields are below the rate at which fees and expenses, including reimbursement of past fees and expenses paid by PharmaShares Advisors, are accruing on the Series Investment Amount of the applicable Series, a portion of any Periodic UV Performance Distributions payable on the Up or Down PharmaShares of that Series, not to exceed 2.5% of any such distribution, will be deducted to pay fees and expenses that remain unpaid after the application of treasury income.
The rate at which fees and expenses, include reimbursement of past fees and expenses, accrue on the Series Investment Amount for the applicable Series will be set by PharmaShares Advisors for each Calculation Period and may not exceed an annual rate of 2.5%.
For each Series, PharmaShares Advisors will waive reimbursement of expenses it has paid on behalf of such Series after the assets of such Series have exceeded a specified dollar amount for a specified number of Calculation Periods.
The Trustee and Calculation Agent
[●] will serve as the trustee and calculation agent for the PharmaShares Trust and each Series pursuant to the terms of the Master Trust Agreement and a Series Trust Supplement for each Series. The trustee will be responsible for the holding and safekeeping of assets delivered to it on behalf of each Series, and performing various administrative duties in accordance with the Master Trust Agreement and the related Trust Supplement. For acting as trustee for each Series, the trustee will receive a fee which will accrue at an annualized rate of [●]% of the daily Series Investment Amount for the applicable Series or an annual minimum fee of $[●], whichever is greater.
The calculation agent will perform the Underlying Value and Projected Breakpoint calculations for each Series. For acting as calculation agent for each Series, the calculation agent will receive a fee which will accrue at an annualized rate of [●]% of the daily Series Investment Amount for the applicable Series.
The fees of the trustee and calculation agent will be paid by each Series out of its treasury income.
The Marketing Agent
Pursuant to the Master Trust Agreement and a Series Trust Supplement for each Series, [●], in its capacity as marketing agent, will coordinate investor education. For acting as marketing agent for each Series, the marketing agent will receive a fee which will accrue at an annualized rate of 0.35% of the daily Series Investment Amount for the applicable Series. The fees of the marketing agent will be paid by each Series out of its treasury income.
Routine Operational, Administrative and Other Ordinary Expenses
The routine operational, administrative, and other ordinary expenses of each Series, including, but not limited to, computer services expenses, legal and accounting fees and expenses, filing fees, and printing, mailing and duplication costs will be borne by each Series. Ordinary expenses affecting the PharmaShares Trust as a whole or benefiting all Series will be prorated to each Series according to its respective Series Investment Amount at the time the fees are payable,
PharmaShares Advisors, LLC will pay all routine operational, administrative and other ordinary expenses on behalf of each Series and will seek reimbursement of those expenses out of the treasury income of the applicable Series. All fees and expenses of each Series, including reimbursement of formation expenses, are subject to a cap equal to an annual rate of 2.50% on the daily Series Investment Amount, calculated on the basis of a 365-day year.
F-4
Expenses, fees and taxes shall be accrued in advance for all non-business days at the end of the immediately preceding business day.
Non-Recurring Fees and Expenses
All formation expenses and any extraordinary and/or non-recurring expenses will be borne by the affected Series. Extraordinary fees and expenses affecting the PharmaShares Trust as a whole will be prorated to each Series according to its respective Series Investment Amount at the time the fees are payable. Extraordinary, non-recurring expenses include, without limitation, legal claims and liabilities, litigation costs or indemnification or other unanticipated expenses. Such fees and expenses, by their nature, are unpredictable with respect to timing and amount.
PharmaShares Advisors, LLC will pay the formation expenses as well as extraordinary, non-recurring expenses on behalf of each Series and will seek reimbursement of those expenses out of the treasury income of the applicable Series. All fees and expenses of each Series, including reimbursement of expenses to PharmaShares Advisors, are subject to a cap equal to an annual rate of 2.50% on the daily Series Investment Amount, calculated on the basis of a 365-day year.
For Series BTOX, PharmaShares Advisors expects to incur and pay at least $1,500,000 in formation expenses. It will seek to reimburse itself for this amount out of the treasury income of Series BTOX.
Brokerage Commissions and Fees
Under each Authorized Participant Agreement, each Series will be responsible for paying selling commissions for sales by Authorized Participants during the initial offering of the shares of that Series. Selling commissions will not be payable after the initial offering of the shares of each Series, which will conclude following the initial effective date of the registration statement for those shares and the commencement of the trading of those shares on a national stock exchange. The selling commissions will range from 2.5% to 5% of the aggregate amount of shares sold and will be payable on a deferred basis out of treasury income earned by the applicable Series. All fees and expenses of each Series, including any selling commissions, are subject to a cap equal to an annual rate of 2.50% on the daily Series Investment Amount, calculated on the basis of a 365-day year.
NOTE 4 INITIAL AND CONTINUOUS OFFERING; OFFERING COSTS
Series BTOX will only issue Up BTOX PharmaShares and Down BTOX PharmaShares to the initial Authorized Participants in PharmaShares Units. The Up BTOX PharmaShares and the Down BTOX PharmaShares will be issued at their respective Per Share Underlying Values, which will be calculated based upon the Reference Value published on the last Publication Date that precedes the initial issuance date and the Projected Breakpoint that was calculated at the beginning of the calendar quarter preceding the calendar quarter during which the initial issuance date occurs.
Each Authorized Participant will solicit bids for Up and/or Down BTOX PharmaShares during the initial offering. Prospective investors who bid for PharmaShares must deposit funds with their brokers or directly with an Authorized Participant in accordance with the rules and procedures specified by such broker or Authorized Participant. The lead manager will aggregate all bids obtained by each Authorized Participant and will match up bids for Up BOTX PharmaShares and Down BTOX PharmaShares into PharmaShares Units. All bids for Up and Down BTOX PharmaShares that are combined into PharmaShares Units will be accepted and the sale of the shares will be executed by the applicable Authorized Participant or broker. Bids for Up or Down BTOX PharmaShares that cannot be paired into PharmaShares Units because those bids are, in the aggregate, insufficient in number of shares bid for or price offered per share to compose and pay for a PharmaShares Unit, will be rejected.
After the initial offering of the Paired BTOX PharmaShares, an ongoing offering will continue. The PharmaShares Trust will issue Paired BTOX PharmaShares in PharmaShares Units to Authorized Participants on a continuous basis at their respective Per Share Underlying Values. The combined Per Share Underlying Value of a PharmaShares Unit will always be equal to the sum of the Up Aggregate Par Amount and the Down Aggregate Par Amount of the Paired BTOX PharmaShares that make up that PharmaShares Unit plus the Up and Down Daily Net Income Accruals for each day of the current Calculation Period through and including the calendar day preceding the Issuance Date for that PharmaShares Unit.
F-5
Normal and expected expenses incurred in connection with the initial offering and the continuous offering of shares of each Series will be paid by PharmaShares Advisors. PharmaShares Advisors will seek to reimburse itself for these expenses out of the treasury income of the applicable Series. All fees and expenses of each Series, including initial and continuous offering expenses, are subject to a cap equal to an annual rate of 2.50% on the daily Series Investment Amount, calculated on the basis of a 365-day year.
NOTE 5 PAIRED ISSUANCES AND PAIRED OPTIONAL REDEMPTIONS
Each Series will issue and redeem shares from time to time, only in one or more PharmaShares Units. A PharmaShares Unit is composed of $1,000,000 Aggregate Par Amount of Up PharmaShares and $1,000,000 Aggregate Par Amount of Down PharmaShares of the applicable Series. PharmaShares Units may be created or redeemed only by Authorized Participants. Authorized Participants may sell the shares included in the PharmaShares Units they purchase from the applicable Series to other investors in the secondary market.
Except when aggregated in PharmaShares Units, the shares are not redeemable securities. Retail investors, therefore, generally will not be able to purchase or redeem shares directly from or with the Series. Rather, most retail investors will purchase or sell shares in the secondary market with the assistance of a broker.
Authorized Participants will pay a transaction fee of $2,000 per paired redemption or paired issuance order for PharmaShares Units to the trustee. The transaction fee is intended to defray the cost for processing the issuance and redemption orders. The transaction fee may be reduced, increased or otherwise changed by the trustee in its sole discretion.
NOTE 6 DISTRIBUTIONS
Periodic Income Distributions
Each Series will declare Periodic Income Distributions on each Periodic Calculation Date equal to the net treasury income earned by the applicable Series during the preceding Calculation Period, if any. Net Treasury Income will be equal to the treasury income that remains on deposit in the applicable Series after it has paid its fees and expenses and purchased treasury securities with an aggregate purchase price equal to the sum of the Aggregate Par Amount of its outstanding Up and Down PharmaShares, to be held during the next Calculation Period. The Periodic Income Distribution declared on any Periodic Calculation Date will be allocated equally among the Up and Down PharmaShares of the applicable Series or in accordance with some other allocation method specified in the related prospectus supplement.
The PharmaShares Trust will not make any Periodic Income Distributions on the Up or Down PharmaShares of a Series until the earlier of the time when (i) average annual yield on the treasury securities held by that Series exceeds the minimum yield specified in the related prospectus supplement or (ii) the aggregate amount invested in that Series exceeds the minimum amount specified in the related prospectus supplement.
Periodic UV (Underlying Value) Performance Distributions
If the Underlying Value of one class of the Paired PharmaShares has increased as a result of the performance of the Reference Value compared to the Projected Breakpoint for a particular calendar quarter, the Underlying Value of the paired class will decrease by a corresponding amount and the PharmaShares Trust will declare a Periodic UV Performance Distribution on the class of PharmaShares that experienced the Underlying Value increase on the related Periodic Calculation Date. The Periodic UV Performance Distribution will be equal to the sum of (1) the amount of the increase in the Underlying Value and (2) an Equalization Payment that is equal to that increase or some fraction or multiple of that increase, as specified in the related prospectus supplement. The purpose of the Equalization Payment is to maintain the proportion of the Up Investment Amount to the Down Investment Amount in the original Up/Down Ratio for the applicable Series.
In connection with each Periodic UV Performance Distribution on the Up PharmaShares, the trustee will reduce, on the books and records of the applicable Series, (1) the Down Investment Amount and the Down Aggregate Par Amount by an amount equal to the increase in the Up Underlying Value and (2) the Up Investment Amount and Up Aggregate Par Amount by the amount of the Equalization Payment that was made as part of that distribution. In connection with each Periodic UV Performance Distribution on the Down PharmaShares, the trustee will reduce, on
F-6
the books and records of the applicable Series, (1) the Up Investment Amount and the Up Aggregate Par Amount by an amount equal to the increase in the Down Underlying Value and (2) the Down Investment Amount and Down Aggregate Par Amount by the amount of the Equalization Payment that was made as part of that distribution.
During any period when the yield on treasury securities held by the applicable Series are insufficient to pay all fees and expenses of the Series, an amount of up to 2.50% of that portion of any Periodic UV Performance Distribution that represents an increase in the Underlying Value will be deducted from the Periodic UV Performance Distribution on the applicable class of PharmaShares and used to pay accrued fees and expenses of Series that remain unpaid after application of current treasury income. The amount deducted for fees and expenses will never exceed 2.5% of the portion of any Periodic UV Performance Distribution that represents an increase in the Underlying Value. No amounts will be deducted from the Equalization Payment portion of any Periodic UV Performance Distribution.
Final Distributions
The PharmaShares Trust will declare a Final Distribution on all or a portion, as applicable, of the Up and Down PharmaShares of a Series on the earliest to occur of (i) the Final Scheduled Termination Date for that Series, (ii) an Early Termination Date following the occurrence of certain events, and (iii) a Redemption Order Date on which an Authorized Participant places an order for a Paired Optional Redemption.
For purposes of determining the Final Distribution, the Up Underlying Value and Down Underlying Value will be calculated based upon the Reference Value reported on the last Publication Date that precedes the Final Scheduled Termination Date, an Early Termination Date or the relevant Redemption Order Date and the Projected Breakpoint determined for the calendar quarter that preceded the current calendar quarter. The final distribution on the Up PharmaShares of a Series will be equal to the Up Underlying Value and the final distribution on the Down PharmaShares of a Series will be equal to the Down Underlying Value.
During any period when the yield on treasury securities held by the applicable Series are insufficient to pay all fees and expenses of the Series, an amount of up to 2.50% of that portion of any Final Distribution that represents an increase in the Underlying Value for the last Calculation Period will be deducted from that Final Distribution on the applicable class of PharmaShares and used to pay accrued fees and expenses of Series that remain unpaid after application of current treasury income.
NOTE 7 SUBSEQUENT EVENTS
PharmaShares Advisors has evaluated the possibility of subsequent events existing in the financial statements of the Series through the date the financial statements were issued. PharmaShares Advisors has determined that there are no material events that would require disclosure in the Series’ financial statements through this date.
F-7
Report of Independent Registered Public Accounting Firm
[TO BE PROVIDED]
F-8
TABLE OF CONTENTS
i
ii
We are providing information to you in this base prospectus that may be applicable to all or only certain Series of PharmaShares. You should rely only on the information contained in this base prospectus and in the prospectus supplement for your Series of PharmaShares, which we refer to as "your prospectus supplement." References to the "related prospectus supplement" are to your prospectus supplement. The information in this base prospectus will be supplemented and modified by the information contained in the prospectus supplement for each Series with specific details and terms of that Series. However, should there be any inconsistency between the contents of this base prospectus and any prospectus supplement, the contents of the relevant prospectus supplement will, to the extent of any such inconsistency, prevail.
Unless otherwise indicated, all references in this base prospectus to the "administrative agent," "we," "us," "our," or similar terms refer to PharmaShares Advisors, LLC.
We include cross-references in this base prospectus to sections where you can find further related discussions. The preceding table of contents provides the pages on which these sections begin. We also include cross-references in this base prospectus to sections within the prospectus supplement for your Series where you can find information specific to your Series of PharmaShares.
NOTE ABOUT CERTAIN INFORMATION CONTAINED IN THIS BASE PROSPECTUS
The information contained in this base prospectus in the sections entitled "DESCRIPTION OF THE PHARMACEUTICAL INDUSTRY" and "REFERENCE PRODUCTS AND REFERENCE VALUES" is based on information obtained from sources that we believe to be reliable. However, we have not independently verified the accuracy or completeness of such information.
PharmaShares are not sponsored, endorsed, sold or promoted by any pharmaceutical company or other related entity that is involved in the development, production, marketing and/or sales of any reference drug. No such pharmaceutical company or related entity has made any representation or warranty, express or implied, to the owners of PharmaShares or any member of the public regarding the advisability of investing in securities generally or in PharmaShares particularly or the accuracy of using any Reference Value to generate investor returns that track the revenue performance of any reference drug or basket of drugs. No pharmaceutical company or related entity has any relationship to PharmaShares Advisors, LLC. No pharmaceutical company or other related entity is responsible for or has participated in determining the pricing, quantities or timing of any issuance or sale of PharmaShares by PharmaShares Advisors, LLC or the assessment or method of settlement calculation therefor. No pharmaceutical company has provided to PharmaShares Advisors, LLC or any of its affiliates any information, whether public or non-public, relating to any Reference Product and no pharmaceutical company is responsible for updating the information in this base prospectus. Additional information about any Reference Product may be found in public filings made by the applicable pharmaceutical company or in the general public domain.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the United States Securities and Exchange Commission, or the "SEC," a registration statement under the United States Securities Act of 1933, as amended, which we refer to as the "Securities Act," with respect to the shares offered pursuant to this base prospectus and the prospectus supplement for each Series of PharmaShares. Each of this base prospectus and the prospectus supplement for each Series contains summaries of the material terms of the documents it refers to, but does not contain all of the information set forth in the registration statement of which this base prospectus and your prospectus supplement are part. For further information, we refer you to the registration statement. The SEC maintains an internet website that contains our registration statement, as well as reports, information statements and other information that we file electronically with the SEC. You may access the website at http://www.sec.gov. You may also access the registration statement and related documents at http://www.pharmashares.net.
This base prospectus and the prospectus supplement for each Series together constitute the offering document for the Up PharmaShares and the Down PharmaShares of that Series. This base prospectus and the related prospectus supplement do not constitute an offer of shares to any person in any state or other jurisdiction in which such offer would be unlawful.
iii
You may inspect (1) copies of the master trust agreement and the applicable series trust supplement for your Series of PharmaShares, and (2) the records maintained by the trustee, the calculation agent and the administrative agent on behalf of your Series at the office of the respective service provider during regular business hours upon two business days' prior notice. The office of the trustee and calculation agent is located at [●]. The office of the administrative agent is located at 36 Vincent Street, Chatham New Jersey 07928.
REPORTS TO SHAREHOLDERS
We will prepare and file with the SEC, in accordance with the requirements of the United States Securities Exchange Act of 1934, as amended, referred to as the "Exchange Act," quarterly reports on Form 10-Q, annual reports on Form 10-K and current reports on Form 8-K for each Series of the PharmaShares Trust. You may contact your broker to obtain paper copies of these reports.
FORWARD-LOOKING STATEMENTS
The SEC encourages issuers to disclose forward-looking information so that investors can better understand the future prospects of their investments and make informed investment decisions. This base prospectus contains these types of statements. We make these statements directly in this base prospectus. Words such as "anticipates," "estimates," "expects," "projects," "intends," "plans," "believes" and words or terms of similar substance used in connection with any discussion of the future performance of the shares offered in this base prospectus are forward-looking statements. All forward-looking statements reflect our present expectation of future events and the realization of these future events is subject to a number of important variables that could cause actual results to differ materially from those described in the forward-looking statements. The "RISK FACTORS" section of this base prospectus as well as the risk factors section in your prospectus supplement provide examples of these variables. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this base prospectus. Except for our ongoing obligation to disclose material information under the federal securities laws, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
iv
TRANSACTION DIAGRAM
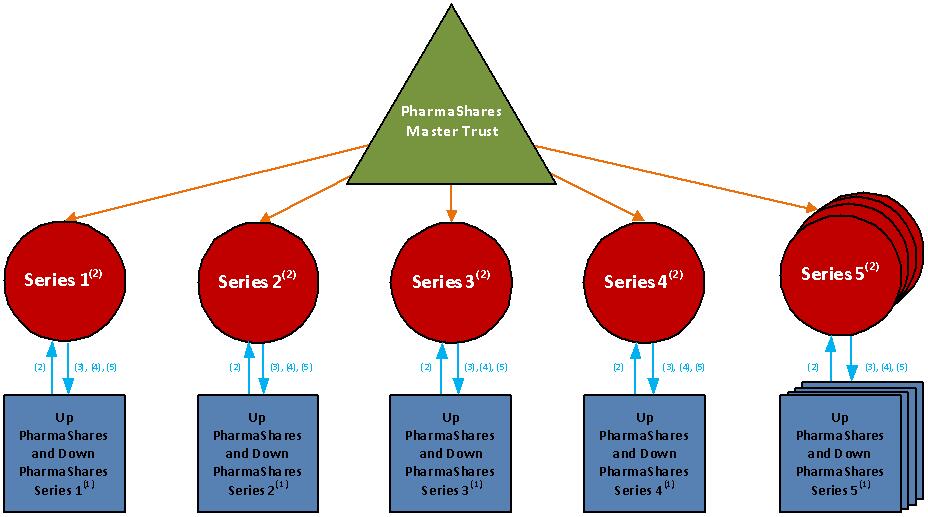
(1)Investors purchase Up PharmaShares or Down PharmaShares issued by a Series of the PharmaShares Master Trust. The Up PharmaShares and Down PharmaShares of each Series will only be issued concurrently in PharmaShares Units.
(2)Funds invested by shareholders and held on deposit in each Series will be invested in treasury securities, including bills, bonds and notes issued and guaranteed by the United States Treasury and repurchase agreements collateralized by treasury securities.
(3)If any income is realized on the treasuries held by each Series, net of the fees and expenses of that Series, that net income will be declared as a periodic income distribution on both the Up PharmaShares and the Down PharmaShares on each periodic calculation date.
(4)Each Series will make periodic UV (or underlying value) performance distributions based on the Reference Value for that Series that is published on the last publication date that precedes each periodic calculation date. Only the holders of the class of PharmaShares that has realized an increase in its underlying value will receive a periodic UV performance distribution.
(5)A Series will make a final distribution on the redemption date that follows any date on which a redemption order is delivered to that Series by an authorized participant or on the final redemption date that follows either an early termination date or the final scheduled termination date. The final distribution will be equal to the respective underlying values of the Up PharmaShares and the Down PharmaShares on the redemption order date.
v
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this base prospectus, but may not include all of the information that may be important to you. You should read this entire base prospectus carefully, including the "RISK FACTORS" and "FORWARD-LOOKING STATEMENTS" sections, as well as the entire prospectus supplement for your Series of PharmaShares, before making an investment decision.
This is a base prospectus for the offering of one or more issuances of Up PharmaShares and Down PharmaShares, each issued by the PharmaShares Trust and each constituting a separate "Series." In this base prospectus, we discuss matters relating to the PharmaShares Trust to the extent that these matters are relevant to you, as a holder of the Up PharmaShares or Down PharmaShares of any particular Series. Only the Up PharmaShares and Down PharmaShares of one Series are being offered by this base prospectus, together with the related prospectus supplement for that Series. Other Series of Up PharmaShares and Down PharmaShares may concurrently be offered pursuant to this base prospectus, together with the related prospectus supplement for each such other Series.
Please note that when we refer in this summary to the "per share underlying value" that is represented by your Up PharmaShares or Down PharmaShares on any date, we mean the portion of the net asset value of your Series that your shares represent on that date and also the final distribution that you would be entitled to receive if that date were an early termination date or the final scheduled termination date. Such a distribution is, however, merely hypothetical and we refer to it solely for the purpose of explaining the meaning of underlying value.
The Issuer and the Securities Offered
On [●] [●], 2020, the PharmaShares Master Trust, which is referred to in this base prospectus as the "PharmaShares Trust," was formed by us, in our capacity as depositor, as a statutory trust organized in series, under the provisions of the Delaware Statutory Trust Act. A master trust agreement entered into by the depositor, the administrative agent, the marketing agent, the calculation agent and the trustee, governs the PharmaShares Trust and provides for the establishment of one or more trusts as series of the PharmaShares Trust, each of which is referred to as a "Series." Each Series will issue two classes of shares: the Up PharmaShares and the Down PharmaShares, which are referred to as the "paired PharmaShares." The formation of each Series and the issuance by that Series of paired PharmaShares, which will represent undivided beneficial interests in the assets of that Series, will be governed by a series trust supplement to the master trust agreement.
For more information about the PharmaShares Trust, see "THE PHARMASHARES TRUST" in this base prospectus. For a description of the terms of the master trust agreement and the expected terms of each series trust supplement, see "DESCRIPTION OF THE TRUST AGREEMENT."
In connection with the issuance of each new Series of paired PharmaShares, a new Series of the PharmaShares Trust will be established. The trustee will deposit the proceeds of the initial sale and subsequent issuances of the paired PharmaShares of each Series in a segregated account in which those funds will be held by the trustee for the exclusive benefit of the shareholders of that Series, separate from the assets and liabilities of all other Series of the PharmaShares Trust. Each Series will issue Up PharmaShares and Down PharmaShares with the characteristics described in this base prospectus and the related prospectus supplement. The assets of your Series will not be available to satisfy the liabilities of, or to make distributions on the shares issued by, any other Series. The assets of other Series of the PharmaShares Trust will not be available to make distributions on your PharmaShares.
The Depositor
We are PharmaShares Advisors, LLC and we are acting as "depositor" for the PharmaShares Trust and each Series of that trust. We were established as a limited liability company in the State of Delaware on May 24, 2019. All of the outstanding membership interests of PharmaShares Advisors, LLC are owned by PharmaShares Manager, LLC, which is, in turn, majority-owned by PharmaShares Manager Holding, LLC. See “PharmaShares Advisors, LLC” in this base prospectus.
1
In our capacity as depositor, our principal duties consist of forming the PharmaShares Trust and each Series of that trust. For more information about us, see "PHARMASHARES ADVISORS, LLC" in this base prospectus
The Administrative Agent
We are also acting as the "administrative agent" for the PharmaShares Trust and each Series of that trust under the master trust agreement and the applicable series trust supplement for each Series.
As administrative agent, we will perform or oversee the performance of a number of duties on behalf of each Series, of which the following are important to you, as the holder of the Up PharmaShares or Down PharmaShares of a Series:
·directing the trustee on each periodic calculation date and each issuance date with respect to the acquisition of new treasury securities for your Series, including placing, or directing a designated service provider to place, the purchase orders for such treasuries, in accordance with the acquisition guidelines that are specified in the master trust agreement and described in this base prospectus under "THE PHARMASHARES TRUST — United States Treasury Obligations;"
·processing redemption and issuance orders from Authorized Participants;
·directing the trustee in effecting redemptions and issuances;
·selecting treasuries to be delivered to redeeming Authorized Participants in connection with paired optional redemptions in accordance with the rules specified in the master trust agreement;
·maintaining the website at http://www.pharmashares.net where you can obtain information about the performance of your Series of PharmaShares;
·performing certain calculations that we are required to provide to the trustee or to post on http://www.pharmashares.net;
·preparing and filing all periodic reports required to be filed by your Series;
·maintaining the listing of your class of PharmaShares on a national stock exchange; and
·providing notification of the occurrence of certain termination triggers.
For performing our duties as administrative agent, we will be compensated as described under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
The Trustee
[●], a [●], will act as "trustee" for the PharmaShares Trust under the master trust agreement and a series trust supplement for each Series of PharmaShares. Acting in the capacity of trustee, [●] will perform a number of duties on behalf of each Series, of which the following are important to you, as the holder of the Up PharmaShares or Down PharmaShares of your Series:
·administering the PharmaShares Trust and each Series of that trust;
·effecting paired optional redemptions and paired issuances in accordance with the directions of the administrative agent;
·paying periodic distributions and the final distribution to the holders of the Up PharmaShares and Down PharmaShares;
·acting as the custodian for the treasury securities and all other assets of your Series;
2
·settling purchase orders for treasury securities that are placed on behalf of your Series by the administrative agent, in accordance with the directions of the administrative agent; and
·providing notification of the occurrence of certain termination triggers (as described under "― Termination Triggers" below).
For performing their duties under the master trust agreement and the series trust supplement for your Series, the trustee and calculation agent will be compensated as described under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
The Calculation Agent
[●], a [●], will act as "calculation agent" for the PharmaShares Trust under the master trust agreement and a series trust supplement for each Series of PharmaShares. Acting in the capacity of calculation agent, [●] will perform a number of duties on behalf of each Series, of which the following are important to you, as the holder of the Up PharmaShares or Down PharmaShares of your Series:
·calculating the respective daily per share underlying values of one Up PharmaShare and one Down PharmaShare of your Series and providing such values to the administrative agent on each business day for posting on the website maintained by the administrative agent at http://www.pharmashares.net;
·calculating, for each periodic calculation date, the amount of treasury income on deposit in your Series after deducting its fees and expenses and the periodic income distribution to be declared on the PharmaShares of your Series;
·calculating, for each periodic calculation date, the respective underlying values of the Up and Down PharmaShares of your Series and the periodic UV performance distribution to be declared on either the Up PharmaShares or the Down PharmaShares on that periodic calculation date;
·calculating, following each periodic calculation date, the respective underlying values of the Up and Down PharmaShares of your Series after the periodic UV performance distribution has been made;
·calculating, in connection with each redemption order date, any early termination date and the final scheduled termination date for your Series, the respective underlying values of the Up and Down PharmaShares and the respective final distributions to be declared on the Up and Down PharmaShares on those dates;
·if your Series has a Broker Reporting Group, calculating the average of the broker projections of net revenue and the resulting Projected Breakpoint for each calendar quarter; and
·if your Series has a Breakpoint Auction Reset, calculating the average of the net revenue projections made by investors and the resulting Projected Breakpoint for each calendar quarter.
For performing its duties under the master trust agreement and the series trust supplement for your Series, the calculation agent will be compensated as described under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
The Marketing Agent
[●] will act as the "marketing agent" for the PharmaShares Trust and each Series. Under the terms of the master trust agreement, the duties of the marketing agent will include developing a marketing plan for each Series, preparing marketing materials, organizing investor presentations, coordinating investor education and general advertising. For performing its duties for your Series, the marketing agent will be compensated as described under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
3
Daily Reporting
The calculation agent, with the assistance of the administrative agent, will calculate the per share underlying value of the Up PharmaShares and the per share underlying value of the Down PharmaShares of each Series:
·on each publication date, based on the Reference Value published on that day and the Projected Breakpoint for the applicable current calendar quarter,
·on the periodic calculation date that follows each publication date, after the periodic UV performance distribution is declared on the Up or Down PharmaShares and both the Up and Down underlying value has been reduced accordingly,
·for each calendar day during the remainder of that calculation period, adding any daily net income accruals to the Up and Down underlying value,
·prior to the start of each trading day, the premium or discount of the midpoint of the bid/offer price spread for an Up PharmaShare at the close of the preceding trading day over the per share underlying value of that Up PharmaShare on that preceding day,
·prior to the start of each trading day, the premium or discount of the midpoint of the bid/offer price spread for a Down PharmaShare at the close of the preceding trading day over the per share underlying value of that Down PharmaShare on that preceding day, and
·either on the last business day preceding the first day of each calendar quarter or on or immediately following each publication date, as specified in the prospectus supplement for each Series, the Projected Breakpoint Increase or Projected Breakpoint Decrease for the current calendar quarter.
The calculation agent will perform the underlying value calculations as soon as practicable on each publication date and each periodic calculation date. In the case of all other business days, the calculation agent will perform the underlying value calculations for that business day after its close. If that business day is followed by one or more intervening non-business days, the calculation will be made for that business day and each such intervening non-business day. After the periodic UV performance distribution is declared on the periodic calculation date that occurs at the beginning of each calculation period, the respective underlying values of the Up and Down PharmaShares will remain the same for the remainder of that calculation period except for the addition of daily net income accruals.
The administrative agent will post all calculations on the website maintained by it at http://www.pharmashares.net not later than one hour prior to the commencement of trading on the national stock exchange on which the Up PharmaShares and Down PharmaShares of each Series are listed. The Projected Breakpoint for each calendar quarter will be disclosed by means of the filing of an updated registration statement for each Series.
Information reported by or about the Reference Product or the reference pharmaceutical company during the course of each calendar quarter, including third party data and information services that provide prescription trend data on a weekly or monthly basis for various pharmaceutical products, may affect investor expectations about the change in the Underlying Value of the Up and Down PharmaShares of a Series on the next scheduled publication date and, as a result, the trading price of those PharmaShares during that quarter. However, such market knowledge and expectations will not be reflected in the underlying value calculation for any Series of PharmaShares. See "RISK FACTORS — Fluctuations in the per share underlying value of your PharmaShares and other factors may affect their trading price" in this base prospectus.
4
The Assets of Each Series
Each Series will hold its assets solely for the benefit of the holders of its Up PharmaShares and Down PharmaShares and will not commingle such assets with the assets of any other Series of the PharmaShares Trust. The assets of each Series are expected to consist of:
·U.S. treasury securities and what we refer to as "income" on those securities, consisting of stated interest on treasury notes and bonds and the discount that is realized when the par amount received on a treasury bill, note or bond at maturity exceeds the purchase price at which that Series acquired that treasury security;
·treasury repurchase agreements and what we refer to as "income" on those agreements consisting of the difference between the purchase price and the repurchase price for the treasury securities borrowed under those agreements;
·the rights of that Series under the master trust agreement and the applicable Series trust supplement to rely on the services provided by the administrative agent, the marketing agent, the trustee and the calculation agent;
·a securities account to which all of the treasury securities acquired from time to time by that Series will be credited for the benefit of its shareholders;
·a distribution account into which all income and maturity proceeds realized on the treasury securities of that Series will be deposited and used to make (1) required deposits into the fee payment account and (2) periodic income distributions, periodic UV performance distributions and the final distribution to the holders of the Up PharmaShares and Down PharmaShares of that Series; and
·a fee payment account into which all or a portion of the income realized on the treasury securities will be deposited on each periodic calculation date and applied to pay the fees and expenses of that Series.
The "treasury securities" purchased on behalf of each Series may consist of bills, notes and bonds of varying maturities and repurchase agreements of varying terms that are fully collateralized by treasury securities and entered into with counterparties that meet specified capital requirements or are deemed creditworthy by the administrative agent. Each treasury security and treasury repurchase agreement must mature or terminate prior to the next scheduled periodic calculation date. When we refer to "treasuries" in this base prospectus, the term includes both treasuries and treasury repurchase agreements on treasuries. On each periodic calculation date, except for the final scheduled termination date or an early termination date, the administrative agent will direct the trustee to reinvest the proceeds of the maturity of the treasuries held by each Series in new treasuries. The administrative agent will also direct the trustee to invest in treasuries all funds delivered to it in connection with each paired issuance.
On each periodic calculation date and each issuance order date, the administrative agent will use commercially reasonable efforts to identify and direct the trustee to purchase, on behalf of the applicable Series, treasuries in accordance with the acquisition guidelines specified in the master trust agreement and described in more detail in this base prospectus under "THE PHARMASHARES TRUST — United States Treasury Obligations." Although the administrative agent will use commercially reasonable efforts to direct the trustee to keep all funds on deposit in each Series invested in treasuries, a portion of the assets of a Series may from time to time be held in the form of cash, due to mismatches between the maturity profiles of treasuries available for purchase and the length of time between periodic calculation dates. In addition, any treasuries delivered to Authorized Participants in connection with a paired optional redemption will be selected by the administrative agent on a "last in, first out" basis. If interest rates are increasing and funds received in connection with paired issuances are being invested in higher-yielding treasuries, this method of selection may result in relatively higher-yielding treasuries being delivered to redeeming Authorized Participants and relatively lower-yielding treasuries remaining in the Series, thereby causing a decrease in the daily yield rates realized by that Series. Conversely, if interest rates are decreasing and funds received in connection with paired issuances are being invested in lower-yielding treasuries, the "last in, first
5
out" method of selection may result in the relatively lower-yielding treasuries being delivered to redeeming Authorized Participants. The treasuries selected by the administrative agent to be delivered as the final distribution in a paired optional redemption will be distributed ratably, by type, to each redeeming Authorized Participant. See "RISK FACTORS — The return on your PharmaShares is uncertain — Treasury securities may be delivered instead of cash in a paired optional redemption" in this base prospectus. For more information about the assets of each Series, see "THE PHARMASHARES TRUST — The Assets of Each Series" and "— United States Treasury Obligations" in this base prospectus and "THE TERMS OF YOUR SERIES — The Series Assets" in your prospectus supplement.
The Reference Value
Each Series of PharmaShares will track (x) the projected change in the Reference Value from a "base calendar quarter" that occurred in the past to an ongoing or just ended "current calendar quarter," calculated based upon net revenue projections for a Reference Product for the current calendar quarter made by the Broker Reporting Group or determined via a Breakpoint Auction Reset, compared to (y) the actual change in the Reference Value between the same two calendar quarters.
When we refer to a "change in net revenue for the Reference Product" throughout this base prospectus, we intend to cover each of the following three possible Reference Value scenarios, as specified in the prospectus supplement for your Series:
(1)the change in net revenue of a specified drug, a basket of drugs or a pharmaceutical device or devices (each referred to as a "Reference Product");
(2)the change in net revenues allocable to a specified division of a publicly traded pharmaceutical company; or
(3)the change in net revenues allocable to a specified entity that is party to a collaboration agreement, distribution agreement, joint venture or other profit-sharing arrangement relating to the sale of a Reference Product.
When we refer to the "Reference Value" for a Series on any date of determination, we are referring to the net revenue in U.S. dollars for the Reference Product that is publicly reported by the reference pharmaceutical company on an income statement prepared in accordance with United States generally accepted accounting principles, or "U.S. GAAP." Net revenue will not be expressed in unit sales unless otherwise noted in your prospectus supplement. For purposes of calculating the net revenue allocable to a pharmaceutical company division or individually reported business segment that are being used as the basis for Reference Value, each product sold by that division or business segment that is a material asset or a revenue-generating product for which revenue is publicly reported on an income statement prepared in accordance with U.S. GAAP will be included in the related net revenue calculation. Net revenues for the Reference Product may be presented as net aggregated global sales and may be shown in a single line item or in separate line items that show net sales by various geographic regions and/or therapeutic indications for the Reference Product. The Reference Value for most Series of PharmaShares will be equal to or based upon net aggregated global sales, but certain Series may reference net sales for only a specified geographic region or a specified therapeutic indication, as specified in your prospectus supplement.
"Net revenue" for any Reference Product will generally mean, unless otherwise stated in your prospectus supplement, the gross sales of a Reference Product adjusted for sales-related deductions, including but not limited to, chargebacks, trade discounts, sale returns and allowances, commercial and government rebates, customer loyalty programs and fee-for-service arrangements with certain distributors, collectively referred to as "sales allowances." Pharmaceutical companies will generally make an estimate of sales allowances and apply that estimate to reduce the gross sales price at the time of sale based upon historical experience, sales agreements in place and estimates for claims incurred but not yet paid. In some cases, this gross-to-net estimate for a calendar quarter will deviate from the actual reduction that should have been applied to revenues for that period and such deviation may be significant enough to require the reference pharmaceutical company to adjust its reported net revenue and make an accounting restatement. If such a restatement is made after the periodic calculation date that follows a publication date, the Reference Value that will be used to calculate underlying value will be the net revenue originally published and not the restated net revenue. If that same period is later used as a base calendar quarter, the adjusted net revenue number
6
will be used to calculate Reference Value. However, for purposes of any current period, neither the Reference Value nor the calculation of underlying value will be retroactively adjusted to take into account any revisions or restatements made to net revenues after the original publication date by the related pharmaceutical company. For a discussion of the reasons why an investment in securities which reference the earnings performance of a specified Reference Product may differ from a direct investment in such Reference Product or the pharmaceutical company that manufactures and sells that product, see "RISK FACTORS — An investment in Up PharmaShares may not resemble a direct investment in the Reference Product; an investment in Down PharmaShares may not be an effective hedge against exposure to the Reference Product" in this base prospectus.
The "publication date" for the Reference Value of any Series will be the date on which the pharmaceutical company that manufactures and sells the Reference Product complies with its periodic reporting obligations under the Exchange Act as a public reporting company by filing a quarterly report on Form 10-Q or an annual report on Form 10-K with the SEC. Each publication date is generally expected to occur on or prior to the 45th day following the end of each calendar quarter in the case of quarterly reports and on or prior to the 90th day following the end of each calendar year in the case of annual reports. Some pharmaceutical companies seek to file their reports within the same number of days following the end of each quarter, but we cannot control when a company will actually make its filing. As a result, publication dates and the length of each calculation period may vary from quarter to quarter. See "RISK FACTORS — The return on your PharmaShares is uncertain — The changes in the underlying value of your PharmaShares from period to period depend upon the Reference Value and the Projected Breakpoint" in this base prospectus.
Generally, Reference Value for a Series of PharmaShares will be tracked from the current calendar quarter to:
·the same calendar quarter of the preceding calendar year in PharmaShares Series that track changes from year to year, referred to as "year-over-year tracking Series," which will typically be suitable for drugs that have a seasonality aspect to their sales, that may be sold with larger first quarter discounts before insurance deductibles are satisfied and/or that are at a more mature point in their lifecycle with less sequential quarter variability in sales levels;
·the immediately preceding calendar quarter in quarter over quarter PharmaShares Series, referred to as "sequential tracking Series," which will typically be suitable for drugs that have been recently launched or that have the potential to experience significant changes in sales from quarter to quarter; or
·some other comparative base quarter, as specified in your prospectus supplement.
The starting Reference Value is published on the publication date that follows the base calendar quarter, and the ending Reference Value is published on the publication date that follows the current calendar quarter. Each periodic calculation date and each redemption order date, any early termination date and the final scheduled termination date on which distributions are declared on the PharmaShares of a Series will occur after a publication date on which net revenue for the current calendar quarter is reported. For example, for a year-over-year tracking Series, if the ending Reference Value is net revenue for the Reference Product for the first quarter ended March 31 of year X, as reported on the related publication date occurring not later than May 15 of Year X, then the starting Reference Value would be net revenue for the Reference Product for the first quarter of Year X-1, as reported not later than May 15 of Year X-1. For a sequential tracking Series, if the ending Reference Value is net revenue for the Reference Product for the second calendar quarter ended June 30 of Year X, as reported on the related publication date not later than August 15 of Year X, then the starting Reference Value would be net revenue for the Reference Product for the first quarter of Year X, as reported not later than May 15 of Year X.
The calculation agent will calculate the underlying value of the Up PharmaShares and the Down PharmaShares of each Series for each day of a calculation period based on the Reference Value reported on the publication date that occurred on the first day of that calculation period. Calculation periods begin and end on successive publication dates for the Reference Product. See "—Calculation of Underlying Value" below.
If the Reference Value for your Series is not reported publicly by the reference pharmaceutical company in an income statement prepared in accordance with U.S. GAAP for any reason on one or more consecutive publication
7
dates, there will be deemed to be no change in Reference Value for the related calculation period and no distributions will be declared on your PharmaShares on the related periodic calculation date. If no Reference Value is available on one or more publication dates, an early termination date may occur on the next scheduled periodic calculation date, as specified in your prospectus supplement. See "DESCRIPTION OF THE PHARMASHARES — Termination Triggers" in this base prospectus and "THE TERMS OF YOUR SERIES — Termination Triggers" in your prospectus supplement.
No director, officer, member, manager, employee, legal counsel, accountant or advisor of any reference pharmaceutical company or any of its affiliates, or any other person who has access to the earnings data used to calculate the Reference Value of any Series before such data becomes publicly available, will be permitted to invest in the Up PharmaShares or the Down PharmaShares of that Series.
We, the PharmaShares Trust and each Series of the PharmaShares Trust that may be established from time to time, our respective affiliates and our respective directors, officers, members, trustees, managers, employees, attorneys and advisors are not liable for:
·any losses to you resulting from the methodologies used in calculating, and the actual calculation of, net revenues of any Reference Product, or net revenues of a specified division of a pharmaceutical company or the net revenue allocations for any drug subject to a collaboration or joint venture agreement, or the resulting Reference Value in the case of any of the foregoing; or
·any losses to you for any revisions to the methodology for calculating net revenues, any subsequent revisions made to published net revenues, any alleged or actual inaccuracies or inherent limitations of the net revenues calculation, any delays in net revenue reporting and publication, any errors in the underlying data used to calculate net revenues, or the impact of any of the foregoing factors on the yield realized by you as a holder of PharmaShares or otherwise.
The Projected Breakpoint
The Up underlying value and Down underlying value will determine the amount of periodic distributions and the final distribution that will be made on the Up PharmaShares and Down PharmaShares of a Series. Underlying value for the Up PharmaShares and the Down PharmaShares of each Series will be calculated by comparing
·the Projected Breakpoint Increase or Projected Breakpoint Decrease from the base calendar quarter to the current calendar quarter, to
·the actual percentage increase or decrease in net revenues for the Reference Product from the base calendar quarter to the current calendar quarter, based upon either year-over-year tracking or sequential tracking (or some other tracking method).
The "Projected Breakpoint Increase" or "Projected Breakpoint Decrease" (also generically referred to as the "Projected Breakpoint") is the percentage increase or percentage decrease, as applicable, calculated by dividing (1) the absolute value of the difference between projected net revenues for the Reference Product for the current calendar quarter and actual net revenues for the base calendar quarter by (2) actual net revenues for the base calendar quarter. The projected net revenues are calculated based upon the net revenue projections of a Broker Reporting Group or determined by means of a Projected Breakpoint Auction Reset, as specified in your prospectus supplement.
Broker Reporting Group
A Series may use a Broker Reporting Group to establish the Projected Breakpoint, if so specified in your prospectus supplement. The "Broker Reporting Group" for a Series of PharmaShares will consist of a group of investment banks, asset managers and other broker-dealers registered with the Financial Industry Regulatory Authority, Inc., or "FINRA," all of whom are generically referred to as "brokers," that employ analysts who publish net revenue projections for the Reference Product. A Broker Reporting Group may include (1) individual brokers selected by us, in our capacity as administrative agent, whose projections are published on Bloomberg or a similar
8
financial data service available by subscription, (2) a composite projection number generated by a third-party service that aggregates and integrates the market research and models provided to it by many different brokers, such as Visible Alpha, FactSet or a similar service specified in your prospectus supplement (a “market research integration service”), or (3) a combination of individual brokers and a composite projection number. The broker revenue projections will be obtained from research reports released by each individual broker or, in the case of the composite projection, generated by the market research integration service, on the most recent date that precedes but is not less than two (2) business days prior to the “BRG projected revenue determination date” which will be either (1) the first day of each calendar quarter or (2) each publication date for the Reference Product for your Series, as specified in your prospectus supplement.
A Broker Reporting Group, the members of which will be selected by us, in our capacity as the administrative agent, will have the following characteristics:
·it may consist of a composite projection number published by a market research integration service so long as such service considers the projections of a minimum of five (5) brokers in generating the composite for a Reference Product; and, further, for each calendar quarter or year, the service must calculate the composite using not less than three (3) projections from the various brokers included in its broker group; if there are less than five (5) brokers in the Broker Reporting Group or less than three (3) projections are used in any composite calculation, then the underlying values of the paired PharmaShares will remain unchanged at the end of the affected calendar quarter;
·the identities of the entire group of eligible brokers whose projections may be used by the market research integration service to generate the composite number for the Reference Product will be disclosed in your prospectus supplement; the projections of only a subset of these brokers may be used by the service to generate the composite projection for any particular calendar quarter and the number of brokers in this subset (but not the identity of those brokers) will be disclosed, along with the Projected Breakpoint, in an updated prospectus supplement for your Series; the market research integration service may, in its sole discretion, add, remove and substitute brokers from the group whose projections it uses to generate the composite at any time; any such change to the group of eligible brokers used to calculate the composite will be disclosed in an updated prospectus supplement for your Series;
·it may also include, in addition to the composite number, any number of individual brokers whose projections are not used in the composite number by the market research integration service but who employ analysts publishing projections for the Reference Product; each individual broker will be identified in your prospectus supplement and will be included in the Broker Reporting Group until the final scheduled maturity date of your Series, unless the broker ceases to employ analysts who publish projections for the Reference Product; individual brokers in a Broker Reporting Group that includes a composite number need not publish projections for every consecutive calendar quarter to be part of the group, but any quarterly projections they do publish must be included in the calculation of the Projected Breakpoint for the relevant quarter;
·it may consist of individual brokers only, so long as there are not less than three (3) such brokers and each of them has been publishing quarterly projections for the Reference Product for at least the last four consecutive calendar quarters; each individual broker will be identified in your prospectus supplement and will be included in the Broker Reporting Group until the final scheduled maturity date of your Series, unless the broker ceases to employ analysts who publish projections for the Reference Product or ceases publishing projections not less frequently than quarterly; if an individual broker in a Broker Reporting Group composed exclusively of individual brokers is removed because its analysts either cease tracking the Reference Product or cease publishing quarterly net revenue projections for the Reference Product, that broker will be reinstated if it employs a new analyst who resumes publishing quarterly revenue projections for the Reference Product within four calendar quarters of the broker’s removal; if there are fewer than three (3) individual brokers in the group or less than three (3) projections are published by
9
the brokers for any calendar quarter, then the underlying values of the paired PharmaShares of the applicable Series will remain unchanged at the end of the affected calendar quarter;
·the composite projection for a calendar quarter and/or each projection for a calendar quarter made by an individual broker who is part of the Broker Reporting Group must be included in the calculation of the Projected Breakpoint for that calendar quarter; the calculation agent will source the composite projection from the market research integration service and each projection, if any, that was made by an individual member of the Broker Reporting Group, from Bloomberg or a similar financial data service; and
·in the event that, with respect to more than one calculation period, an individual broker stops publishing quarterly projections or the composite projection number is no longer generated by the market research integration service or otherwise becomes unavailable and there are less than the required minimum number of brokers remaining in the Broker Reporting Group or less than three (3) projections are available for any calendar quarter, the administrative agent may select a replacement market research integration service and/or individual brokers to replace brokers who have ceased publishing projections and who meet the criteria for inclusion in the Broker Reporting Group discussed above; if such replacements cannot be made or the administrative agent elects not to make them, a termination trigger will occur within the number of publication dates specified in your prospectus supplement.
No broker acting as an Authorized Participant for a Series of PharmaShares or any of its affiliates may be selected as an individual broker, but any such related broker may be included in a composite projection that is determined using at least 5 or more brokers, including such Authorized Participants. No authorized participant has any obligation to consider the interests of the holders of Up or Down PharmaShares of any Series in making projections for any Reference Product. See “RISK FACTORS — Broker dealers who act as authorized participants for your Series may make projections for your Reference Product.”
The same Broker Reporting Group will be used throughout the term of a Series, except that replacements may be made as described above. The same methodology for calculating the composite number and the average of the net revenue projections of the Broker Reporting Group, as described in the next section, will be used throughout the term of a Series.
Average Revenue Projection Methodology for Broker Reporting Groups
The market research integration service that generates the composite will calculate and publish the high and low broker projections, the median of the broker projections (both with the high and low projections included and after removing those projections), the mean of the broker projections (both with the high and low projections included and after removing those projections), and the standard deviation of the broker projections. In the case of a Broker Reporting Group that includes only a composite projection, the average revenue projection number that will be used to calculate the Projected Breakpoint will be the mean or median of the broker projections, calculated by the market research integration service after the high and low projections are eliminated. The prospectus supplement for your Series will specify whether the composite mean or median will be used.
In the case of any Broker Reporting Group that includes both a composite projection and individual brokers, the calculation agent will, first, identify the composite mean or median calculated by the market research integration service, as described above and specified in your prospectus supplement, and, second, calculate the weighted, arithmetic mean of the projected revenues for the entire Broker Reporting Group. Each broker, including each broker included in the composite mean or median number, after removal of the high and low projections, will be weighted as “1.” For example, if 10 brokers are included in the composite number and there are 2 individual brokers, the composite number will have a weighting of 10/12 and each individual broker will have a weighting of 1/12.
In the case of any Broker Reporting Group that includes only individual brokers, the calculation agent will calculate the arithmetic mean of the projections published by each broker in the group. In the case of any Broker Reporting Group with only the minimum three (3) individual brokers (and no composite projection), the calculation
10
agent will calculate, for each calendar quarter or year, the arithmetic mean of the projections subject to a cap and a floor equal to a 20% deviation from the mean, in the following manner:
·first, the arithmetic mean is computed;
·second, the highest projection is excluded and an adjusted mean of the two remaining projections is computed. The highest projection is then reduced to equal a number that represents not more than a 20% positive variance from the adjusted mean;
·third, the lowest projection is excluded and an adjusted mean of the two remaining projections is computed. The lowest projection is then increased to equal to a number that represents not more than a 20% negative variance from the adjusted mean; and
·fourth, the arithmetic mean of the projections, with the highest and lowest projections adjusted as described above, is recalculated.
Breakpoint Auction Reset
A Series may employ a public auction to determine the Projected Breakpoint if so specified in your prospectus supplement. Auctions will be held at 8:00 am ET on the “auction date” which will be specified in your prospectus supplement and which may occur on either (1) the date that is two business days prior to the first day of trading for each calendar quarter or (2) the first business day following each publication date for a Series. Bids may be submitted electronically beginning 5 business days prior to the applicable auction date. Bids may not be withdrawn after 6:00 am ET on the applicable auction date and bidders will be notified of results by 9:00 am ET on the auction date. The auction process is referred to as a “Breakpoint Auction Reset.”
Only existing holders of Up or Down PharmaShares and committed bidders for Up or Down PharmaShares may participate in the Breakpoint Auction Reset, but no existing holder will be obligated to participate. A qualified bid must include the number of Up PharmaShares or Down PharmaShares the bidder is committing to acquire. Each participating existing holder and each committed bidder must submit its maximum and minimum projected net revenue estimates for the Reference Product for the upcoming calendar quarter. The range between the maximum and minimum projected net revenue estimates may not exceed the percentage specified in your prospectus supplement.
Each bidder will be committing to purchase the Up or Down PharmaShares for which it is bidding so long as the projected net revenues for the upcoming calendar quarter determined via the Breakpoint Auction Reset is within the range of the maximum and minimum projected net revenues specified by the bidder. Each bidder will be committing to purchase Up or Down PharmaShares at the per share underlying value of those shares, as determined on the publication date immediately preceding the auction date or at the per share underlying value prevailing during the current calculation period, in the case of an auction date occurring on the first trading day of a calendar quarter. Committed bidders must deposit funds equal to the aggregate underlying value of the Up or Down PharmaShares for which it is bidding with a designated escrow agent in accordance with the procedures of their broker or an Authorized Participant. Escrowed funds related to bids that were rejected because the projected revenue number set by the Breakpoint Auction Reset is outside of the range of projected net revenues specified by the bidder will be returned in accordance with the procedures established by each bidder’s broker or the applicable Authorized Participant.
One of the Authorized Participants will be designated as an auction agent and will pair all bids for Up PharmaShares with bids for Down PharmaShares. The escrowed funds relating to accepted bids will be delivered to the PharmaShares Trust, which will issue Up and Down PharmaShares to the Authorized Participants who obtained those bids.
The calculation agent will use the midpoint of the range of projected net revenues submitted by each committed bidder for Up PharmaShares and each participating existing holder of Up PharmaShares, to calculate the weighted arithmetic mean of all submissions made with respect to the Up PharmaShares of a Series, based upon the number of shares owned by an existing holder or bid for by a committed bidder. The calculation agent will use the midpoint of the range of projected net revenues submitted by each committed bidder for Down PharmaShares and
11
each participating existing holder of Down PharmaShares, to calculate the weighted arithmetic mean of all submissions made with respect to the Down PharmaShares of a Series, based upon the number of shares owned by an existing holder or bid for by a committed bidder. Finally, the calculation agent will calculate the projected net revenue for the upcoming calendar quarter as the midpoint of the median of the Up submissions and the median of the Down submissions. The projected net revenue number determined for a calendar quarter by means of the Breakpoint Auction Reset will then be used to calculate the Projected Breakpoint for that quarter.
Reporting
The Projected Breakpoint Increase or Decrease for each calendar quarter will be calculated and announced not later than 8 p.m. EST on the auction date or the BRG projected revenue determination date by means of the filing of an updated registration statement pursuant to Rule 424(b) of the Securities Act. Revised guidance from any broker who is a member of the Broker Reporting Group after the start of any calendar quarter will not be used to recalculate previously determined Projected Breakpoints. The following inputs required to calculate the Projected Breakpoint and related inputs will also be disclosed:
·the identity of each individual broker included in the Broker Reporting Group, the identity of all of the brokers whose projections may be used by the market research integration service to generate the composite and the number of brokers in the subset of brokers whose projections are actually included in the composite projection for the current calendar quarter (but not the identity of those brokers);
·the composite projection published by the market research integration service (but not the individual projections included in that composite) and the projection that was published by each individual broker member of the Broker Reporting Group (but not which individual broker published which particular projection);
·the weighting for each broker projection and for the composite projection based upon the total number of individual brokers included in the Broker Reporting Group and the number of brokers whose projections were used to calculate the composite for the current calendar quarter;
·with respect to a Breakpoint Auction Reset, the weighted arithmetic mean of all submissions made with respect to the Up PharmaShares of a Series, the arithmetic mean of all submission made with respect to the Down PharmaShares of the same Series, and the midpoint of the median of the Up submissions and the median of the Down submissions; and
·actual net revenues reported for the applicable base calendar quarter.
Calculation of Underlying Value
The respective underlying values of the Up PharmaShares and Down PharmaShares of a Series is calculated on each calendar day based upon the Reference Value published on the most recent publication date and the Projected Breakpoint for the most recently elapsed calendar quarter. Although the relevant calendar quarter is referred to throughout this base prospectus as the “current calendar quarter,” underlying value cannot be determined until the current calendar quarter has elapsed and the Reference Value for that quarter is reported by the reference pharmaceutical company.
The respective underlying values of the Up and Down PharmaShares represent the aggregate amount of the assets on deposit in that Series to which the holders of the Up PharmaShares and the Down PharmaShares, respectively, would be entitled if final distributions were to be made on those shares on that calendar day.
The PharmaShares Trust will declare periodic UV (or underlying value) performance distributions on each periodic calculation date for the class of PharmaShares which has experienced an increase in its underlying value, based on the Reference Value published on the last publication date that preceded the applicable periodic calculation date and the Projected Breakpoint for the most recently elapsed calendar quarter.
12
The PharmaShares Trust will make a final distribution on the Up PharmaShares and Down PharmaShares of a Series equal to the respective underlying values of those shares on the applicable redemption order date, early termination date or the final scheduled termination date.
The PharmaShares Trust will make periodic income distributions of the treasury income of a Series, net of the Series fees and expenses, which may be distributed equally among the holders of the Up and Down PharmaShares of that Series or it may be allocated based upon the respective underlying values of those Up and Down PharmaShares, as specified in your prospectus supplement.
To calculate underlying value on any date of determination, the Reference Value for the most recently elapsed calendar quarter is compared against (a) the Reference Value for the calendar quarter that preceded the most recently elapsed quarter in a sequential tracking Series, (b) the same calendar quarter that occurred one year ago in a year-over-year tracking Series or (c) such other base calendar quarter as may be specified in your prospectus supplement. The resulting actual percentage change in Reference Value is then compared against the Projected Breakpoint Increase or Projected Breakpoint Decrease that applied to the most recently elapsed calendar quarter.
If a Projected Breakpoint Increase is specified for the current calendar quarter, because the Reference Value was projected to increase compared to the Reference Value for the applicable base calendar quarter, then
–an increase in the Reference Value for the current calendar quarter relative to the Reference Value for the base calendar quarter that is greater than this Projected Breakpoint Increase will result in an increase in the underlying value of the Up PharmaShares and a corresponding decrease in the underlying value of the Down PharmaShares of a Series; and
–an increase in the Reference Value that is less than the Projected Breakpoint Increase or any decrease in the Reference Value relative to the Reference Value for the base calendar quarter will result in a decrease in the underlying value of the Up PharmaShares and a corresponding increase in the underlying value of the Down PharmaShares of a Series.
If a Projected Breakpoint Decrease is specified for the current calendar quarter, because the Reference Value was projected to decrease compared to the Reference Value for the applicable base calendar quarter, then
–a decrease in the Reference Value relative to the Reference Value for the base calendar quarter that is greater than this Projected Breakpoint Decrease will result in an increase in the underlying value of the Down PharmaShares and a corresponding decrease in the underlying value of the Up PharmaShares of a Series; and
–a decrease in the Reference Value that is less than the Projected Breakpoint Decrease or any increase in the Reference Value relative to the Reference Value for the base calendar quarter will result in a decrease in the underlying value of the Down PharmaShares and a corresponding increase in the underlying value of the Up PharmaShares of a Series.
If the rise or fall in Reference Value for any current calendar quarter relative to the base calendar quarter is equal to the Projected Breakpoint Increase or Projected Breakpoint Decrease for the current calendar quarter, there will be no change in the underlying value of the Up or Down PharmaShares of a Series, other than for the addition to that underlying value of any Up and Down daily net income accruals.
The underlying value calculation will be adjusted by any leverage factors and periodic return caps applicable to returns on the Up PharmaShares and Down PharmaShares of a Series, as described below.
For some Series, the calculation of underlying value will be a two-step process in which it will first be determined whether the actual percentage change in Reference Value, as defined below, has exceeded the Projected Breakpoint Increase or Projected Breakpoint Decrease and, if it has, then, either (1) underlying value will be calculated by subtracting from the actual percentage change in revenue the Projected Breakpoint Increase or Projected Breakpoint Decrease such that the underlying value reflects only the net change or (2) underlying value
13
will be calculated based on the full actual percentage change in revenue, not merely the net change. The details of the calculations that will be made for such a Series will be specified in your prospectus supplement.
If certain major market disruptions occur during the course of a calendar quarter and materially affect sales of the Reference Product, there will be deemed to be no change in the Reference Value for that calendar quarter, as discussed under "REFERENCE PRODUCTS AND REFERENCE VALUES" in this base prospectus.
"Underlying value" for the Up PharmaShares of each Series, also referred to as the "Up underlying value," is calculated on each calendar day of a calculation period for that Series as follows:
–the "Up investment amount" multiplied by the "Up Allocation Factor" that was calculated based on the Reference Value published on the most recent publication date and the Projected Breakpoint for the most recently elapsed calendar quarter
minus
–if the Up PharmaShares experienced an increase in the Up underlying value on the last publication date, the amount of the equalization payment distributed as part of the periodic UV performance distribution made on the Up PharmaShares on the periodic calculation date that followed that publication date and, if the Up PharmaShares experienced a decrease in the Up underlying value on the last publication date, the amount of such decrease
plus
–the sum of the "Up daily net income accruals" for each day that has elapsed during the current calculation period, up to and including the current calendar day.
"Underlying value" for the Down PharmaShares of each Series, also referred to as the "Down underlying value," is calculated on each calendar day of a calculation period for that Series as follows:
–the "Down investment amount" multiplied by the "Down Allocation Factor" that was calculated based on the Reference Value published on the most recent publication date and the Projected Breakpoint for the most recently elapsed calendar quarter
minus
–if the Down PharmaShares experienced an increase in the Down underlying value on the last publication date, the amount of the equalization payment distributed as part of the periodic UV performance distribution made on the Down PharmaShares on the periodic calculation date that followed that publication date and, if the Down PharmaShares experienced a decrease in the Down underlying value on the last publication date, the amount of such decrease
plus
–the sum of the "Down daily net income accruals" for each day that has elapsed during the current calculation period, up to and including the current calendar day.
The terms used in the underlying value definition have the following meanings:
The "Up investment amount" will generally be equal to the aggregate par amount of the Up PharmaShares. The "Down investment amount" will generally be equal to the aggregate par amount of the Down PharmaShares. The Up investment amount and Down investment amount will be reduced by redemptions as well as by periodic distributions to shareholders of increases in the Up underlying value or the Down underlying value, as described below under "— Periodic Distributions" and under "DESCRIPTION OF THE PHARMASHARES — Periodic Distributions" in this base prospectus. Reductions in the Up investment amount and Down investment amount will reduce the amount of your investment and your potential gains, as discussed under "RISK FACTORS — The amount
14
of your investment in your PharmaShares and your potential gains will decline over time." Purchasing additional Up or Down PharmaShares is the only means to maintain the size of your investment.
The "Up Allocation Factor" and "Down Allocation Factor" are determined as described in the sample calculations below.
For each day of a current calculation period, the Up PharmaShares and Down PharmaShares of a Series will be entitled to receive the net treasury income accrued for that day on the treasuries held by that Series. The entitlement of the Up PharmaShares and Down PharmaShares to the Series net treasury income may be adjusted by an allocation rule specified in your prospectus supplement. We refer to the daily income additions to underlying value as the "Up daily net income accruals" and the "Down daily net income accruals."
A synthetic ratchet, referred to as a "leverage factor," of between 0.5x and 3x may be applied to the returns on the Up PharmaShares and/or the paired Down PharmaShares of a Series for each calculation period. Your prospectus supplement will specify the leverage factor, if any, that applies to the Up and/or the Down PharmaShares of your Series. Your prospectus supplement will also specify any cap that applies on the periodic returns on the Up and/or the Down PharmaShares of your Series, referred to as a "periodic return cap." The amount of a periodic return cap, if any, will depend upon past and projected changes in net revenues, with higher periodic return caps generally used for Reference Products the sales of which have in the past experienced, and/or which are projected by the Broker Reporting Group to experience, significant future fluctuations. As indicated in the examples below, underlying value will be calculated in the following order:
first,the Actual Percentage Change is calculated;
second,the Actual Percentage Change is compared against the Projected Breakpoint to determine which class has experienced an underlying value increase and which class has experienced a decrease;
third,the percentage change in underlying value of the class of PharmaShares that has experienced an increase in its underlying value is multiplied by the applicable leverage factor, if any;
fourth,the applicable periodic return cap, if any, is applied to adjust the resulting increase in underlying value such that the increase does not exceed that cap; and
finally,the Up and Down allocation factors are determined.
If so described in your prospectus supplement, any applicable leverage factor and periodic return cap may be adjusted during the term of your Series in the event that net revenues or the percentage change in revenues over a specified period of time exceeds or falls below a specified value also disclosed in your prospectus supplement.
Please see "RISK FACTORS — The return on your shares is uncertain — The return on some Series of PharmaShares will be capped," and "— A Series may terminate early" in this base prospectus.
We refer to the period between publication dates, beginning on (but excluding) the preceding publication date and ending on (and including) the current publication date, as a "calculation period." On each publication date, the Reference Value and the resulting Up underlying value and Down underlying value will be determined. The initial launch of a Series of PharmaShares may not coincide with the beginning of a calendar quarter or a publication date, which may result in an initial period during which the respective underlying values of the paired PharmaShares of a Series diverge significantly from the trading price of those shares. Further, reference pharmaceutical companies may not publish the relevant Reference Value on the same publication date and, as a result, the length of each calculation period may vary.
The "Up Allocation Factor" and the "Down Allocation Factor" are calculated on each publication date as follows:
(i)If a Projected Breakpoint Increase was predicted for the current calendar quarter and:
(a)if the Actual Percentage Change is greater than the Projected Breakpoint Increase, there will be an Up underlying value increase equal to:
15
UUVΔ = Actual Percentage Change – Projected Breakpoint Increase;
the Up Allocation Factor will equal 1 + the Up underlying value increase, and
the Down Allocation Factor will equal 1 – the Up underlying value increase;
(b)if the Actual Percentage Change reflects an increase that is less than the Projected Breakpoint Increase, the Down underlying value will increase as follows:
DUVΔ = Projected Breakpoint Increase – Actual Percentage Change;
the Down Allocation Factor will equal 1+ the Down underlying value increase, and
the Up Allocation Factor will equal 1 – the Down underlying value increase; or
(c)if the Actual Percentage Change reflects a decrease for a current calendar quarter with a Projected Breakpoint Increase, the Down underlying value will increase as follows:
DUVΔ = Actual Percentage Change + Projected Breakpoint Increase;
the Down Allocation Factor will equal 1 + the Down underlying value increase, and
the Up Allocation Factor will equal 1 – the Down underlying value increase.
(ii)If a Projected Breakpoint Decrease was predicted for the current calendar quarter and:
(a)if the Actual Percentage Change reflects a decrease that is greater than the Projected Breakpoint Decrease, the Down underlying value will increase as follows:
DUVΔ = Actual Percentage Change – Projected Breakpoint Decrease;
the Down Allocation Factor will equal 1 + the Down underlying value increase, and
the Up Allocation Factor will equal 1 – the Down underlying value increase;
(b)if the Actual Percentage Change reflects a decrease that is less than the Projected Breakpoint Decrease, the Up underlying value will increase as follows:
UUVΔ = Projected Breakpoint Decrease – Actual Percentage Change;
the Up Allocation Factor will equal 1 + the Up underlying value increase, and
the Down Allocation Factor will equal 1 – Up underlying value increase; or
(c)if the Actual Percentage Change reflects an increase for a current calendar quarter with a Projected Breakpoint Decrease, the Up underlying value will increase as follows:
UUVΔ = Actual Percentage Change + Projected Breakpoint Decrease;
the Up Allocation Factor will equal 1 + the Up underlying value increase, and
the Down Allocation Factor will equal 1 – the Up underlying value increase.
The initial proportion of the Up investment amount to the Down investment amount, or the "Up/Down ratio," for each Series will initially be 1:1, or such other proportion as may be specified in your prospectus supplement, without regard to the respective initial public offering prices or subsequent trading prices of the Up PharmaShares and Down PharmaShares of any Series. The initial Up/Down ratio will be maintained for so long as the PharmaShares of a Series are outstanding by virtue of the requirements that:
(1)if the Up PharmaShares or Down PharmaShares receive a distribution of any increase in their underlying value that was declared on any periodic calculation date, that class of PharmaShares will also receive an equalization payment that will restore the Up/Down ratio; and
(2)all redemptions and issuances must be effected in PharmaShares Units composed of an equal aggregate par amount (or other specified proportion) of Up PharmaShares and Down PharmaShares of a Series.
16
Maintaining the initial Up/Down ratio for each Series ensures that the underlying value formula and the yield on your Up PharmaShares or Down PharmaShares will not be affected by increases or decreases in the Up or Down investment amount due to periodic UV performance distributions or due to redemptions and subsequent issuances.
Periodic Distributions
Periodic Calculation Dates
The "periodic calculation dates" for your Series of PharmaShares will occur quarterly on the fifth (5th) business day following each publication date unless otherwise specified in your prospectus supplement. On each periodic calculation date, the calculation agent will calculate a "periodic distribution" for the Up PharmaShares and Down PharmaShares of your Series, which will consist of a periodic income distribution, if there is any net treasury income available for that purpose, and a periodic UV performance distribution, which will depend on the net revenue performance of the Reference Product relative to the Projected Breakpoint. Most Series of PharmaShares are expected to have quarterly periodic calculation dates and calculation periods consisting of approximately 90 days, but periodic distributions may occur annually or at some other interval specified in your prospectus supplement.
"Publication dates" will occur within 45 days following the last day of each calendar quarter or, in the case of the fourth quarter of each calendar year, within 90 days of the end of that quarter. The actual publication date may vary from quarter to quarter for each Reference Product. The "calculation period" related to each periodic calculation date will be specified in your prospectus supplement and will generally be the period between the last two publication dates that occurred prior to that periodic calculation date. The chart below shows the relative timing of publication dates, periodic calculation dates and calculation periods.
The Reference Value published on the publication date that begins each calculation period will determine (1) the periodic UV performance distribution, if any, that will be declared on either the Up PharmaShares or the Down PharmaShares on the periodic calculation date that follows that publication date and (2) the Up underlying value and Down underlying value for the entire duration of that calculation period, as reduced, in the case of one class of PharmaShares, by any decrease in underlying value experienced by that class, and, in the case of the other class, any equalization payment distributed on that class as part of its periodic UV performance distribution, and as increased by any Up and Down daily net income accruals for each day of that calculation period.
On the second (2nd) business day following each periodic calculation date, the trustee will pay any declared periodic distribution to the holders of the Up PharmaShares and Down PharmaShares, unless otherwise specified in your prospectus supplement.
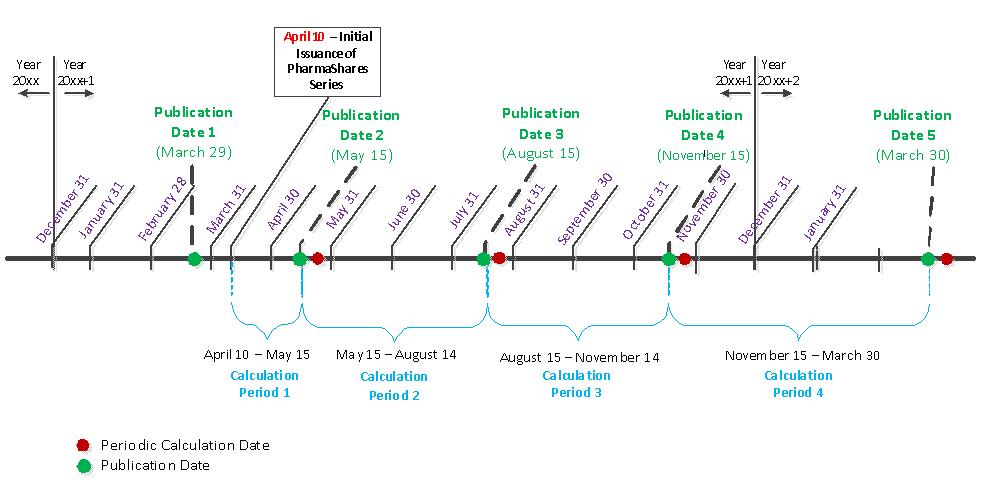
Periodic Income Distributions
On each periodic calculation date, the PharmaShares Trust may declare a "periodic income distribution" on the Up PharmaShares and Down PharmaShares of your Series out of the income, if any, that your Series has realized on its treasury securities during the preceding calculation period and which is remaining after your Series has:
·deposited sufficient funds into the fee payment account to pay its fees and expenses, which will consist of the fees and expenses described generally in this base prospectus under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series" and specified in your prospectus under "THE TERMS OF YOUR SERIES — Fees and Expenses"; and
·acquired new treasury securities with an aggregate purchase price equal to the aggregate par amount of the outstanding paired PharmaShares of your Series.
The income on deposit in your Series after the deduction of fees and expenses and the required reinvestment in new treasuries is referred to as its "net treasury income" for the applicable calculation period. On each day of a calculation period, the net treasury income of a Series is allocated between the Up PharmaShares and Down PharmaShares in accordance with the allocation mechanism specified in your prospectus supplement. Each outstanding PharmaShare of a Series may be entitled to an equal share of the net treasury income of its Series, its entitlement may be based on its per share underlying value or its entitlement may be determined based on some other allocation mechanism specified in your prospectus supplement. The income entitlement of each PharmaShare for each day of a calculation period is referred to as its "daily net income accrual." The portion of the underlying value of your Up PharmaShares or Down PharmaShares that is attributable to their income entitlement is referred to as the "net income component of underlying value" for those PharmaShares.
On each periodic calculation date, your Series will declare a "periodic income distribution" on each outstanding Up PharmaShare equal to:
·the Up daily net income accruals realized during each day of the preceding calculation period
multiplied by
·a fraction, the numerator of which is one Up PharmaShare and the denominator of which is the aggregate number of outstanding Up PharmaShares on that periodic calculation date.
17
On each periodic calculation date, your Series will declare a "periodic income distribution" on each outstanding Down PharmaShare equal to:
·the Down daily net income accruals realized during each day of the preceding calculation period
multiplied by
·a fraction, the numerator of which is one Down PharmaShare and the denominator of which is the aggregate number of outstanding Down PharmaShares on that periodic calculation date.
The declared periodic income distribution will be paid to shareholders on the second business day following each periodic calculation date, unless otherwise specified in your prospectus supplement.
An amount equal to the series aggregate par amount, as calculated after any periodic distributions being declared on each periodic calculation date are determined, will always be set aside on that date to be reinvested by the trustee, at the direction of the administrative agent, in new treasuries unless that periodic calculation date is related to the final scheduled termination date or an early termination date. We refer to the product of the aggregate number of outstanding Up PharmaShares issued by your Series and the stated par amount per share specified in your prospectus supplement as the "Up aggregate par amount" and to the product of the aggregate number of outstanding Down PharmaShares issued by your Series and the stated par amount per share as the "Down aggregate par amount." The stated par amount per share of the PharmaShares of any Series does not affect in any way the underlying value of those shares, their public offering price or their trading price at any time. The underlying value of each class of paired PharmaShares represents the entitlement of that class to the assets of its Series. The stated par amount per share represents, on any date of determination, each share's proportional interest in the underlying value of its class. For a description of how periodic UV performance distributions decrease the Up and Down aggregate par amounts, see "— Periodic UV Performance Distributions" below.
No Series of the PharmaShares Trust will make any periodic income distributions on its PharmaShares until the earlier of the time when (i) average treasury yields prevailing in the market exceed 3% or (ii) that Series has achieved an aggregate amount of invested assets at least equal to the minimum amount specified in your prospectus supplement.
If, as of any periodic calculation date, your Series does not have any net treasury income, it will not make any periodic income distributions to its shareholders. Your Series is not required to make periodic income distributions in any stated amount and if no funds are available to make such a distribution as of any periodic calculation date, your Series will not be obligated to make such distribution on any subsequent date. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" and "— Income on the treasuries may be insufficient to make periodic income distributions" in this base prospectus.
Periodic UV Performance Distributions
If the underlying value of the Up PharmaShares increases based on the Reference Value published on a publication date relative to the Projected Breakpoint, the PharmaShares Trust will declare a periodic UV performance distribution on the Up PharmaShares equal to that increase, plus an equalization payment, on the periodic calculation date that follows that publication date. No periodic UV performance distribution will be declared on the paired Down PharmaShares on that periodic calculation date. Conversely, if the underlying value of the Down PharmaShares increases on a publication date relative to the Projected Breakpoint, the PharmaShares Trust will declare a periodic UV performance distribution on the Down PharmaShares equal to that increase, plus an equalization payment, on that periodic calculation date. No periodic UV performance distribution will be declared on the paired Up PharmaShares on that periodic calculation date. The purpose of periodic UV performance distributions is to distribute any increase in the Up underlying value to the holders of the Up PharmaShares and any increase in the Down underlying value to the holders of the Down PharmaShares. Each declared periodic UV performance distribution will be paid to the applicable shareholders on the second business day following each periodic calculation date.
Periodic UV performance distributions include equalization payments in order to maintain the initial Up/Down ratio of the Up investment amount and the Down investment amount, which will be 1:1 in the case of
18
most Series. In a 1:1 Series, the equalization payment that will be included as part of any periodic UV performance distribution will be equal to the increase in the Up underlying value or the Down underlying value, as applicable. In a Series with a different Up/Down ratio, equalization payments will be calculated as specified in your prospectus supplement.
The distribution of (1) increases in the Down underlying value and (2) the equalization payments that accompany distributions of increases in the Up underlying value will both be paid from funds in your Series that are allocated, on the books and records of your Series, to the Up investment amount and will reduce that Up investment amount. The distribution of (1) increases in the Up underlying value and (2) the equalization payments that accompany distributions of increases in the Down underlying value will both be paid from funds in your Series that are allocated, on the books and records of your Series, to the Down investment amount and will reduce that Down investment amount. See "RISK FACTORS — The amount of your investment in your PharmaShares and your potential gains will decline over time."
If there was an increase in the Up underlying value of your Series based on the Reference Value published on a publication date relative to the Projected Breakpoint, the PharmaShares Trust will declare the following periodic UV performance distribution on each Up PharmaShare of that Series on the periodic calculation date that follows that publication date:
·the amount of the increase in the Up underlying value (not including the net income component of that underlying value, which will be separately declared on the same date as a periodic income distribution)
plus
·an equalization payment equal to the amount required to maintain the original Up/Down ratio for that Series;
divided by
·the aggregate number of Up PharmaShares outstanding on that periodic calculation date.
If there was an increase in the Down underlying value of your Series based on the Reference Value published on a publication date relative to the Projected Breakpoint, the PharmaShares Trust will declare the following periodic UV performance distribution on each Down PharmaShare of that Series on the periodic calculation date that follows that publication date:
·the amount of the increase in the Down underlying value (not including the net income component of that underlying value, which will be separately declared on the same date as a periodic income distribution)
plus
·an equalization payment equal to the amount required to maintain the original Up/Down ratio for that Series;
divided by
·the aggregate number of Down PharmaShares outstanding on that periodic calculation date.
During any period when the yields on the treasury securities held by a Series are insufficient to pay the fees and expenses of that Series, a percentage of that portion of the next scheduled periodic UV performance distribution that represents the increase in the Up or Down underlying value, as applicable, will be deducted from that distribution and used to pay the accrued fees and expenses of that Series that remain unpaid after application of current treasury income. The amount deducted for fees and expenses will never exceed the percentage specified in the prospectus supplement for your Series. No amount will be deducted from the equalization payment portion of any periodic UV performance distribution on either class of PharmaShares.
19
If the underlying value of your PharmaShares consistently declines as a result of the performance of the Reference Value for your Series relative to Projected Breakpoints, and you are unable to sell those shares, you may lose your entire investment. Even if returns on the paired PharmaShares for your Series, and therefore your losses, are capped on each periodic calculation date, if the Reference Value for your Series consistently fails to exceed Projected Breakpoint Increases, in the case of the Up PharmaShares, or consistently fails to exceed Projected Breakpoint Decreases, in the case of the Down PharmaShares, or if the paired class of PharmaShares consistently does exceed Projected Breakpoint Increases or Decreases, as applicable, the underlying value of your PharmaShares will continue to decrease and you may lose a substantial portion of your investment in those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution."
Final Distributions
The PharmaShares Trust will declare a final distribution on all of the Up PharmaShares and Down PharmaShares of a Series or, in the case of a redemption, on the portion being redeemed, on the earliest to occur of:
·the "final scheduled termination date" specified in your prospectus supplement;
·an "early termination date," which is the next periodic calculation date that follows the occurrence of an event or events that constitute a termination trigger for your Series of PharmaShares; and
·a "redemption order date," which is any business day on which an Authorized Participant places an order for a paired optional redemption of all or a portion of the paired PharmaShares of your Series.
The final distribution will be payable on the "final redemption date" which will be the second (2nd) business day that follows the final scheduled termination date or early termination date or, in the case of a paired optional redemption, on the "redemption date," which is the next business day that follows the related redemption order date.
The "final distribution" declared by the PharmaShares Trust on, respectively, the Up PharmaShares and Down PharmaShares of a Series on the final scheduled termination date, an early termination date or any redemption order date will depend upon the underlying value of the Up PharmaShares and the underlying value of the Down PharmaShares of that Series, immediately preceding the final scheduled termination date, the early termination date or the relevant redemption order date. The final distribution will consist of the entire underlying value of your Up or Down PharmaShares, not merely any increase in that underlying value that would be declared as a periodic UV performance distribution on a periodic calculation date.
On the final redemption date that follows the final scheduled termination date or early termination date, the trustee will pay the final distribution to each holder of the outstanding Up PharmaShares and each holder of the outstanding Down PharmaShares, in redemption of those shares. On the redemption date that follows a redemption order date, the trustee will pay the final distribution to each redeeming Authorized Participant in redemption of the paired PharmaShares it tendered for redemption. On any redemption date or final redemption date, any daily net income accruals that have accrued with respect to each class of PharmaShares during the current calculation period, up to and including the last calendar day preceding the applicable redemption date, will be included in the final distribution made on that redemption date or final redemption date.
The redemption of a portion of the paired PharmaShares in a paired optional redemption may only be effected in PharmaShares Units and may only be directed by an Authorized Participant who is the beneficial holder of those shares. As discussed in greater detail later in this summary, unless you are an Authorized Participant, you will not have a right to direct a redemption of your PharmaShares. Consequently, you will only be able to liquidate your investment in the PharmaShares prior to the final scheduled termination date or an early termination date by selling them to an investor who is willing to purchase them from you, including any Authorized Participant who may wish to acquire those shares in order to direct a paired optional redemption. The market price that you are able to obtain for your PharmaShares may be less than the price you paid for those shares and less than the per share underlying value that is represented by those shares for the reasons discussed in this base prospectus under "RISK FACTORS — Fluctuations in the per share underlying value of your PharmaShares and other factors may affect their trading price."
20
Final Distributions on the Final Scheduled Termination Date or an Early Termination Date
On the final scheduled termination date or an early termination date for a Series, the PharmaShares Trust will declare a final distribution in redemption of the Up and Down PharmaShares using all of the funds held by the Series on that date, which will consist of all interest, discount, principal and any other amounts realized by the Series upon the maturity of its treasury securities at the end of the final calculation period, net of fees and expenses. The final distribution will include the net income accrual component of the underlying value of the paired PharmaShares that would have been declared as a periodic income distribution if the early termination date or final scheduled termination date had been an ordinary periodic calculation date.
On the final redemption date that follows the final scheduled termination date or early termination date, your Series will distribute:
on each outstanding Up PharmaShare a final distribution in cash equal to,
·the Up underlying value on that final scheduled termination date or that early termination date
divided by
·the aggregate number of Up PharmaShares outstanding as of that date, and
on each outstanding Down PharmaShare a final distribution in cash equal to,
·the Down underlying value on that final scheduled termination date or that early termination date
divided by
·the aggregate number of Down PharmaShares outstanding as of that date.
The final scheduled termination date or early termination date for your PharmaShares will be the last day of trading for those shares.
During any period when the yields on the treasury securities held by a Series are insufficient to pay the fees and expenses of that Series, a percentage of that portion of any final distribution made on an early termination date or the final scheduled termination date that represents the increase in the Up or Down underlying value, as applicable, will be deducted from that distribution and used to pay the accrued fees and expenses of that Series that remain unpaid after application of current treasury income. The amount deducted for fees and expenses will never exceed the percentage specified in the prospectus supplement for your Series. No amount will be deducted from the equalization payment portion of any final distribution on either class of PharmaShares. No amounts will be deducted for the payment of fees and expenses in the case of any early termination date on which there was no change in the underlying value of the paired PharmaShares.
Upon receipt of a final distribution on the final redemption date, your PharmaShares will be considered to be redeemed in full and the PharmaShares Trust and your Series will have no further obligations with respect to those shares even if the amount of the final distribution is less than the aggregate par amount of your PharmaShares or less than the purchase price you paid for those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" in this base prospectus.
Final Distributions in Paired Optional Redemptions
On each redemption order date on which an Authorized Participant orders a paired optional redemption in accordance with the procedures described in this base prospectus under "DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions," and such redemption order is accepted, the PharmaShares Trust will declare a final distribution on the Up PharmaShares and Down PharmaShares being redeemed. This final
21
distribution will be paid out of all funds held by the Series on that date multiplied by the "redemption percentage," equal to the paired PharmaShares being redeemed divided by all outstanding paired PharmaShares.
On the next business day that follows a redemption order date, which is referred to as the "redemption date," your Series will distribute:
·on the Up PharmaShares being redeemed, the Up underlying value of those Up PharmaShares on the redemption order date; and
·on the Down PharmaShares being redeemed, the Down underlying value of those Down PharmaShares on the redemption order date.
The final distribution made on a redemption date will include any Up daily net income accruals or Down daily net income accruals, as applicable, accrued from the first day of the current calculation period through and including the last calendar day preceding the relevant redemption date that would have otherwise been declared as part of the periodic income distribution on the Up or the Down PharmaShares on the next periodic calculation date.
No amounts will ever be deducted from the final distribution made in any paired optional redemption to cover unpaid fees and expenses, because the increase in the Up or Down underlying value for the preceding calculation period will already have been declared on the last periodic calculation date and distributed to shareholders.
Upon receipt of a final distribution on a redemption date, the tendered paired PharmaShares will be considered to be redeemed in full and the PharmaShares Trust and the applicable Series will have no further obligations with respect to those shares even if the amount of the final distribution is less than the aggregate par amount of those PharmaShares or less than the purchase price paid by the redeeming Authorized Participant for those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" in this base prospectus.
Paired Optional Redemptions
On any business day prior to the final scheduled termination date or an early termination date, the Up PharmaShares and Down PharmaShares of a Series may be redeemed concurrently in PharmaShares Units at the direction of Authorized Participants in what we refer to as a "paired optional redemption." A "PharmaShares Unit" for each Series will consist of a specified aggregate par amount of Up PharmaShares and a specified aggregate par amount of Down PharmaShares, as disclosed in your prospectus supplement. A tender of paired PharmaShares for redemption will be irrevocable.
The paired PharmaShares of any Series may only be redeemed by Authorized Participants. However, the discussion which follows describing paired optional redemptions and the final distribution that will be made on the related redemption date is relevant to you as a holder of the PharmaShares of any Series even if you are not an Authorized Participant, because it explains the rules that Authorized Participants must follow in order to effect paired optional redemptions and their ability to effect these redemptions may affect the demand for your PharmaShares. See "RISK FACTORS — The return on your PharmaShares is uncertain — The right to redeem the PharmaShares is limited" in this base prospectus.
In order to be an "Authorized Participant," an entity must:
(1)be a registered broker-dealer and a member in good standing with FINRA, or a participant in the securities markets such as a bank or other financial institution that is not required to register as a broker-dealer or be a member of FINRA in order to engage in securities transactions;
(2)be a participant in The Depository Trust Company, or "DTC," or have indirect access to the clearing facilities of DTC by virtue of a custodial relationship with a DTC participant; and
22
(3)have entered into a "participants agreement" with the PharmaShares Trust, the trustee and the administrative agent which specifies procedures for the issuance and redemption of paired PharmaShares.
The amount of cash and/or treasuries that will be delivered on the redemption date in a paired optional redemption will always be equal to the Up underlying value of the Up PharmaShares being redeemed and the Down underlying value of the Down PharmaShares being redeemed on the related redemption order date.
If all conditions to effecting a paired optional redemption have been satisfied, the trustee will deliver cash and/or treasuries in accordance with the instructions of the administrative agent to the redeeming Authorized Participant by 3:00 p.m. New York City time on the redemption date. Following receipt of the final distribution on the relevant redemption date, the Up BTOX PharmaShares and the Down BTOX PharmaShares tendered for redemption will be considered to be redeemed in full and will cease to be outstanding.
Following a paired optional redemption, the trustee will record a reduction in the aggregate number of Up PharmaShares and Down PharmaShares of the redeeming Series that are outstanding. If PharmaShares Units are being both redeemed and issued on the same day at the direction of several Authorized Participants, the trustee will record such reduction only if a net decrease in the aggregate par amount of paired PharmaShares has occurred.
Orders placed by Authorized Participants for paired optional redemptions may be rejected or the fulfillment of those orders may be suspended or postponed for the reasons described in this base prospectus under "RISK FACTORS — Redemption and issuance orders are subject to postponement, suspension or rejection in certain circumstances."
For more information about paired optional redemptions, see "DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions" in this base prospectus.
Paired Issuances
On any business day prior to the final scheduled termination date or an early termination date, an Authorized Participant may effect a "paired issuance" by directing the PharmaShares Trust to issue additional paired PharmaShares of a Series in the aggregate par amount that constitutes at least one PharmaShares Unit. Following receipt of an issuance order, the PharmaShares Trust will issue additional paired PharmaShares to the Authorized Participant on the next business day, referred to as the "issuance date," if the applicable conditions to fulfilling the issuance order have been satisfied. Up and Down PharmaShares of a Series will always be issued at their respective per share underlying values on the relevant issuance order date and will include any daily net income accruals from the beginning of the current calculation period through and including the last calendar day preceding the relevant issuance date.
For Series with a 1:1 Up/Down ratio, the trustee will allocate, on the books and records of your Series, one-half of the aggregate funds received by it in connection with an issuance of paired PharmaShares to the Up investment amount and the other half to the Down investment amount, without regard to the respective per share underlying values at which the Up PharmaShares and Down PharmaShares were issued, in order to preserve the 1:1 Up/Down ratio. If the Up/Down ratio specified in your prospectus supplement is other than 1:1, then a different allocation of funds will be made, as disclosed in your prospectus supplement.
Issuance orders for new PharmaShares Units will be processed through a manual clearing process operated by DTC. By 3:00 p.m. New York City time on the issuance date, the administrative agent will instruct the trustee to deliver to the Authorized Participant's account at DTC the Up and Down PharmaShares that were created in the paired issuance.
Orders placed by Authorized Participants for paired issuances may be rejected or the fulfillment of those orders may be suspended or postponed for the reasons described in this base prospectus under "RISK FACTORS — Redemption and issuance orders are subject to postponement, suspension or rejection in certain circumstances."
For more information about paired issuances, see "DESCRIPTION OF THE PHARMASHARES — Paired Issuances" in this base prospectus.
23
Termination Triggers
The occurrence of specified events, which we refer to as "termination triggers," will cause an early redemption of all Series of paired PharmaShares or of only a single affected Series. The early redemption will occur on the related "early termination date," which is the first periodic calculation date that follows the occurrence of the event or, in the case of several related events, the final event that caused the termination trigger. The following events will cause the PharmaShares Trust to declare an early redemption of all outstanding Series of paired PharmaShares:
·the PharmaShares Trust becomes required to register as an "investment company" under the United States Investment Company Act of 1940, as amended, and we elect not to, or we are unable to, promptly cause such registration, but elect, instead, to terminate all outstanding Series;
·DTC becomes unwilling or unable to act as depository and no suitable replacement is willing or able to assume the duties of a depository for the PharmaShares Trust;
·the administrative agent, the trustee and/or the calculation agent resign or are unable to perform their duties under the master trust agreement and the Series trust supplements, or become bankrupt or insolvent, and no suitable replacement is willing and able to assume those duties;
·we elect to terminate all outstanding Series and holders of 66 and 2/3% of the Up PharmaShares of each Series, voting in the aggregate, and 66 and 2/3% of the Down PharmaShares of each Series, voting in the aggregate, consent to such termination;
·the PharmaShares Trust is adjudged to be bankrupt or insolvent or becomes involved in voluntary or involuntary insolvency or similar proceedings that are not dismissed within 90 days; and
·we determine in our good faith discretion that there is a material risk that the PharmaShares Trust is subject to withholding tax liability, and we elect, in our discretion, to terminate all outstanding Series.
The following events will constitute "termination triggers" for only the affected Series:
·the reference pharmaceutical company fails to publish net revenues or such other Reference Value metric as may be applicable to a particular Series in its income statement prepared in accordance with U.S. GAAP and filed with the SEC on Form 10-Q or Form 10-K for one or more publication dates, as specified in your prospectus supplement;
·the Up PharmaShares and/or the paired Down PharmaShares of a Series are delisted by the securities exchange on which they were listed and no other listing can be obtained within a reasonable period of time;
·a particular Series is adjudged to be bankrupt or insolvent or becomes involved in voluntary or involuntary insolvency or similar proceedings that are not dismissed within 90 days; and
·the amount of cash and treasuries on deposit in a Series is less than $50,000,000 (or such other amount as may be specified in your prospectus supplement) on any business day and we elect, in our discretion, to terminate that Series.
Upon obtaining knowledge or receiving notice of the occurrence of a termination trigger, we will file a current report on Form 8-K for each affected Series describing the termination trigger and announcing the applicable early termination date.
On the applicable early termination date, the affected Series or each Series will declare a final distribution in redemption of all of its outstanding shares equal to their respective underlying values on that early termination date. Following this final distribution, the Up and Down PharmaShares of each affected Series will be considered to be redeemed in full and will cease to be outstanding. The final distribution to be made by your Series may be
24
subject to delays pending the resolution of bankruptcy proceedings if the relevant termination trigger was the voluntary or involuntary bankruptcy of the PharmaShares Trust or a Series of the PharmaShares Trust. No bankruptcy court has previously adjudicated the bankruptcy of a statutory trust organized in series, as discussed under "RISK FACTORS — Limitations on inter-series liability for Delaware Series trusts have not been reviewed by the courts."
Authorized Participants may continue to direct paired optional redemptions and paired issuances at the respective per share underlying values of the paired PharmaShares of a Series after the occurrence of a termination trigger. The last redemption order or issuance order may be placed on the last business day prior to the early termination date.
For more information about termination triggers, see "DESCRIPTION OF THE PHARMASHARES — Termination Triggers" in this base prospectus and for a description of any additional termination triggers that may apply to your Series, see "THE TERMS OF YOUR SERIES — Termination Triggers" in your prospectus supplement.
Fees and Expenses
On each periodic calculation date, your Series is required to deposit an amount equal to its fees and expenses accrued during the preceding calculation period or, if its treasury income is less than its fees and expenses, then all of its treasury income, into the fee payment account to be applied to the payment of those fees and expenses. The fees and expenses of your Series are described in your prospectus supplement. Ongoing and extraordinary expenses of the PharmaShares Trust that benefit all Series will be allocated among them based upon the assets held by each Series.
The fees and expenses payable by your Series will accrue during each calculation period and will be payable in arrears on each periodic calculation date or, at the direction of the administrative agent, on any business day occurring during a calculation period. Unless otherwise stated in your prospectus supplement, in the event that that treasury income of your Series is insufficient to cover in full its fees and expenses for any calculation period, we will defer our fee as administrative agent and, further, we will pay any remaining deficiency with our own funds on behalf of your Series. We will also pay the formation expenses of your Series. We will seek reimbursement for these formation expenses, our deferred fees and any amounts we paid on behalf of your Series on any past periodic calculation date from future treasury income realized by your Series that is in excess of the amount required to pay current fees and expenses, subject to a maximum fee accrual rate cap specified in your prospectus supplement on the amount of fees, expenses and reimbursement amounts that may be required to be paid by your Series on any periodic calculation date. We will waive our right to seek reimbursement for deferred fees and amounts paid on behalf of a Series after the assets on deposit in that Series exceed the dollar amount specified in your prospectus supplement.
For more information about fees and expenses, see "THE PHARMASHARES TRUST — Fees and Expenses of each Series" in this base prospectus and "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
Form of the Shares
The Up and Down PharmaShares of each Series will be issued in the form of one or more global certificates registered in the name of Cede & Co., as the nominee of DTC, and deposited with DTC in the United States or with Clearstream Banking, société anonyme or Euroclear Bank S.A./NV in Europe. If you are not a participant in DTC or in Clearstream or Euroclear, you may hold an interest in the PharmaShares of any Series only by opening an account with a participant or with certain banks, brokers, dealers, trust companies and other parties that maintain a custodial relationship with a DTC participant. You will not receive a physical certificate and you will not be considered the registered holder of the global certificate representing your PharmaShares.
The PharmaShares of each Series may only be issued to, or redeemed at the direction of, Authorized Participants and only in PharmaShares Units. All other investors may hold and exchange the PharmaShares of any Series in the secondary market. No minimum lot requirements are applicable to the PharmaShares of any Series.
25
For more information about the form of your shares, see "DESCRIPTION OF THE PHARMASHARES — Book-Entry Registration" in this base prospectus.
Listing
The Up and Down PharmaShares of your Series will trade on the listing exchange specified in your prospectus supplement. See "THE TERMS OF YOUR SERIES — Listing" in your prospectus supplement.
U.S. Federal Income Tax Considerations
The consequences of holding the Up PharmaShares or Down PharmaShares of a Series are discussed in this base prospectus under "U.S. FEDERAL INCOME TAX CONSIDERATIONS."
ERISA Considerations
We anticipate that each Series of Up PharmaShares and Down PharmaShares will meet the criteria for "publicly-offered securities" under the Plan Assets Regulation issued by the U.S. Department of Labor.
We expect that:
·there will be no restrictions imposed on the transfer of the Up PharmaShares or Down PharmaShares of any Series as described in the Plan Assets Regulation under the U.S. Employee Retirement Income Security Act of 1974, as amended, or "ERISA";
·the Up PharmaShares of each Series will be held by at least 100 independent investors and the Down PharmaShares of each Series will be held by at least 100 independent investors; and
·the Up PharmaShares and Down PharmaShares of each Series will be sold as part of an offering pursuant to an effective registration statement under the Securities Act and then will be timely registered under the Exchange Act.
However, it is not known at this time when the Up PharmaShares of your Series will be held by at least 100 independent investors for purposes of the Plan Assets Regulation and when the Down PharmaShares of your Series will be held by at least 100 independent investors for purposes of the Plan Assets Regulation. Plans (as defined in "GLOSSARY OF DEFINED TERMS") may not invest in the Up or Down PharmaShares of any Series and each investor will be deemed to have represented that it is not, and is not investing assets of, a Plan unless and until we confirm, by means of a filing made on Form 8-K with the SEC, that, as applicable, the Up PharmaShares of that Series are held by at least 100 independent investors or the Down PharmaShares of that Series are held by at least 100 independent investors.
If either class of any Series of PharmaShares were to fail to meet the criteria of publicly-offered securities, the assets of the applicable Series could be deemed under ERISA to include the assets of any Plans that invested in such Series. In that event, transactions involving the assets of the affected Series and parties in interest or disqualified persons with respect to plans that invested in the PharmaShares of that Series will be prohibited under ERISA and the U.S. Internal Revenue Code of 1986, as amended, unless another exception to the Plan Assets Regulation or a statutory or administrative exception to the prohibited transaction rules applies.
CUSIP and ISIN Numbers
The CUSIP and ISIN numbers assigned to the Up PharmaShares and Down PharmaShares of your Series, which are securities identification numbers used to facilitate clearing and settlement of your PharmaShares, are specified in your prospectus supplement.
Plan of Distribution
For a discussion of the plan of distribution for your Series of PharmaShares, see “THE TERMS OF YOUR SERIES — Plan of Distribution” in your prospectus supplement.
26
RISK FACTORS
An investment in the Up PharmaShares or the Down PharmaShares of any Series involves significant risks. You should carefully review the information contained in this section before making an investment decision.
Please note that when we refer in this section to the "per share underlying value" that is represented by each PharmaShare on any date, we mean the amount that you would be entitled to receive as a final distribution on that share if your Series were to redeem your PharmaShares on that date. Such a final distribution is, however, merely hypothetical and we refer to it solely for the purpose of explaining the meaning of underlying value and the terms of your PharmaShares. As a holder of Up PharmaShares or Down PharmaShares of any Series, you are entitled to receive a final distribution only on the final redemption date that follows the final scheduled termination date or an early termination date or, if you are an Authorized Participant, on the redemption date that follows your direction to redeem those shares if you satisfy all of the conditions of a paired optional redemption. You must sell your PharmaShares in order to liquidate your investment in those shares on any other date. The trading price of your Up PharmaShares or your Down PharmaShares may diverge from their per share underlying value.
The return on your PharmaShares is uncertain.
Your pre-tax return depends on several factors. Your pre-tax return on an investment in the Up PharmaShares or the Down PharmaShares of any Series will depend upon:
·the purchase price you paid for your shares;
·the number of periodic UV performance distributions that are declared on your class of PharmaShares and the amount of each such distribution, which will consist of both a gain on your class of PharmaShares and a partial return of your capital;
·the number of periodic UV performance distributions that are declared on the paired PharmaShares of your Series and the amount of each such distribution, which will represent a loss on your class of PharmaShares;
·the amount and timing of treasury income realized by your Series on its treasuries;
·the amount of periodic income distributions declared on your PharmaShares on each periodic calculation date, which will be determined by the net income of your Series after its fees and expenses are deducted and the entitlement of your class of PharmaShares to that net income, which may be equivalent for each PharmaShare issued by a Series or may be based upon the Reference Value or some other allocation rule specified in your prospectus supplement;
·if treasury income is insufficient to pay all of the fees and expenses of your Series, any deductions from the periodic UV performance distributions to which your class of PharmaShares is entitled to pay for those fees and expenses;
·the length of time that your PharmaShares are outstanding;
·if you sell your PharmaShares, the price that you are able to obtain for your shares; and
·the underlying value of your class of PharmaShares on the last calendar day preceding the final scheduled termination date or early termination date on which you receive the final distribution on your shares.
The changes in the underlying value of your PharmaShares from period to period depend upon the Reference Value and the Projected Breakpoint. The underlying value of your PharmaShares will be calculated by reference to the change in the Reference Value from a designated base calendar quarter to the current calendar quarter, as reported on the publication date that follows the current calendar quarter. This change in Reference
27
Value is then compared to the Projected Breakpoint Increase or the Projected Breakpoint Decrease, as applicable, that was calculated based on the average projections of a Broker Reporting Group for the current calendar quarter or by means of a Breakpoint Auction Reset. The base calendar quarter may be the quarter that preceded the current calendar quarter, the same calendar quarter occurring during the preceding calendar year, or some other base calendar quarter specified in your prospectus supplement. Publication dates will generally occur after the end of each calendar quarter when public pharmaceutical companies report net revenues from sales of their products, including from the relevant Reference Product for your Series, on an income statement filed as part of their quarterly and annual reports. Although public companies generally publish their periodic reports on or around the same date following the end of each calendar quarter, publication dates may occur as long as 45 days after the end of each of the first three (3) calendar quarters, when quarterly reports on Form 10-Q are due, and as long as 90 days after the last calendar quarter, when an annual report on Form 10-K is due. The length of each calculation period will be determined by the period of time between publication dates and the market value of your PharmaShares may fluctuate significantly if a publication date occurs later than expected.
Projected Breakpoints for your Series may be calculated based upon the most recent research reports published, or composite number generated, by members of the Broker Reporting Group either prior to each calendar quarter or immediately following each Reference Value publication date. Revised estimates of net revenues for a Reference Product by members of the Broker Reporting Group after the start of each quarter will not be used to revise the Projected Breakpoint. The formula for calculating the respective underlying values of the Up PharmaShares and Down PharmaShares of a Series is described in detail under "DESCRIPTION OF THE PHARMASHARES — Calculation of Underlying Value" in this base prospectus and in the prospectus supplement for your Series. Hypothetical calculations of underlying value are included for illustrative purposes under "DESCRIPTION OF THE PHARMASHARES — Calculation of Underlying Value" in this base prospectus and in an appendix to the prospectus supplement for your Series. As discussed in the section entitled "DESCRIPTION OF THE PHARMACEUTICAL INDUSTRY," many complicated factors may affect the net revenue for a Reference Product. Further, predictions made by the analysts that are part of the Broker Reporting Group about how these factors, as well as general economic and political factors, will interact to affect future net revenues are based on their judgment and past experience. By investing in the PharmaShares of any Series, you are subject to the inherent uncertainties surrounding the future net revenue performance of the Reference Product underlying your Series and to the limitations of predicting such performance.
On each calendar day, the per share underlying value of your class of PharmaShares represents the amount of assets held by your Series which your class of PharmaShares would be entitled to receive as the final distribution if that day were the final scheduled termination date or an early termination date or, in the case of Authorized Participants, a redemption order date. The increase, if any, in the underlying value of the Up or Down PharmaShares during a calculation period plus an equalization payment will be the periodic UV performance distribution that your Series will declare on the applicable class of PharmaShares on the periodic calculation date that follows the end of each calculation period. However, your class of PharmaShares will not receive any periodic UV performance distributions with respect to any calculation period during which its underlying value decreases. If the underlying value of your class of PharmaShares continually declines during the time you hold your shares, you may lose (1) all or substantially all of your investment in your PharmaShares if your Series has no periodic return caps and (2) a significant portion of your investment if your Series has a periodic return cap on the returns of the paired class of PharmaShares. Although you can never lose more than the amount you invested in PharmaShares, your losses may be relatively greater if you purchased your shares at a premium over their per share underlying value.
Periodic income distributions may be based on Reference Value. Each class of the paired PharmaShares may be entitled to an equivalent portion of the net treasury income of their Series that accrues during each calculation period or such entitlement may depend upon their respective underlying values during that period. After a Series has reinvested an amount equal to the aggregate par amount of its Up and Down PharmaShares in new treasuries on each periodic calculation date and deducted the amount necessary to pay its fees and expenses (assuming it is not required to use all treasury income to pay fees and expenses in the circumstances discussed below under "— Income on the treasuries may be insufficient to make periodic income distributions"), the remaining net income will be allocated among the outstanding Up and Down PharmaShares of that Series based upon their respective underlying values. If the Reference Value increases by less than any Projected Breakpoint Increase or decreases or, in the case of a Projected Breakpoint Decrease, decreases by more than that Projected Breakpoint
28
Decrease, the Up PharmaShares of that Series will be entitled to a proportionally smaller periodic income distribution on the next periodic calculation date than the paired Down PharmaShares. Conversely, if the Reference Value decreases by less than any Projected Breakpoint Decrease or increases or, in the case of a Projected Breakpoint Increase, increases by more than that Projected Breakpoint Increase, the Down PharmaShares of that Series will be entitled to a proportionally smaller periodic income distribution on the next periodic calculation date than the paired Up PharmaShares. The allocation method for net treasury income is specified in the prospectus supplement for your Series.
The right to redeem the PharmaShares is limited. The PharmaShares of each Series may only be redeemed by Authorized Participants and only in paired optional redemptions in which the Up and Down PharmaShares constituting one or more PharmaShares Units are tendered for redemption. These requirements, particularly the rule that an Up PharmaShare may only be redeemed with a paired Down PharmaShare and a DownShare may only be redeemed with a paired Up PharmaShare, may significantly limit the ability of Authorized Participants to redeem the PharmaShares and, as a consequence, your ability to sell your PharmaShares in the market. Only Authorized Participants that participate in DTC and have signed a participants agreement with us, the PharmaShares Trust and the trustee will be entitled to present a PharmaShares Unit for redemption. Unless you are an Authorized Participant and hold the requisite aggregate par amount of paired PharmaShares, you will not be able to effect a paired optional redemption. As a result, you must be prepared to hold your PharmaShares until their final scheduled termination date or to sell them in the secondary market, if any exists, at the prevailing market price, which may be below their per share underlying value and below the price that you paid for those shares. There may be no Authorized Participants who are willing to purchase your PharmaShares when you desire to sell them, because, among other reasons, no Authorized Participant is able to satisfy the conditions for a paired optional redemption. Furthermore, if the paired PharmaShares are trading at a premium over their per share underlying value, the market price of your PharmaShares may be adversely affected, because Authorized Participants may be willing to acquire your shares only if you sell them at a discount from their underlying value. Due to the fact that shares are always redeemed at their per share underlying value, Authorized Participants would lose money in any paired optional redemption in connection with which they had to acquire the Up PharmaShares and/or Down PharmaShares at a premium to their respective per share underlying values without an off-setting discount on the paired class of PharmaShares. If you are unable to sell your shares in the secondary market to an Authorized Participant or to another investor, you may be forced to incur increasing losses if the underlying value of those shares continues to decrease.
Treasury securities may be delivered instead of cash in a paired optional redemption. The PharmaShares Trust will deliver cash in a paired optional redemption so long as the redeeming Series has cash available from the maturity proceeds of its treasury repurchase agreements or from funds delivered by Authorized Participants who are directing paired issuances on the same date on which redemptions are also occurring. On each periodic calculation date, the administrative agent will be required to adjust the amount of treasury repurchase agreements which it directs the trustee to acquire for each Series based on the actual redemption experience of that Series during the preceding twelve (12)-month period. However, if the redeeming Series does not have sufficient cash available to effect a paired optional redemption entirely in cash, then the PharmaShares Trust will satisfy all or a portion of its obligation to make the final distribution by delivering treasuries the Series has on deposit to the redeeming Authorized Participant. A Series is always required to deliver treasuries with a value equal to the underlying value of the shares that are being redeemed. For purposes of delivering treasuries in a paired optional redemption, each Series will value its treasuries at the acquisition cost of those treasuries. However, if the market price of the treasuries delivered in a paired optional redemption has decreased since the time that they were acquired by that Series, and if the redeeming Authorized Participant liquidates those treasuries immediately upon receipt, the amount of its final distribution will be less than if it had received a final distribution in cash or if it had held the treasuries to their maturity. The market risk on the treasuries to which Authorized Participants may be exposed when they effect paired optional redemptions may adversely affect the price they are willing to pay for your PharmaShares.
If your Series delivers treasuries in a paired optional redemption, the remaining holders of PharmaShares may realize somewhat reduced income accruals after the redemption, because the administrative agent will use a "last in, first out" method to select treasuries for delivery to redeeming Authorized Participants. As a result, if interest rates were to fall during a calculation period and several redemptions were followed by one or more issuances later during that same calculation period, the relatively higher-yielding treasuries that were acquired by the Series on the preceding periodic calculation date would be delivered to redeeming Authorized Participants and would be replaced with relatively lower-yielding treasuries following the paired issuances. Conversely, in a rising
29
interest rate environment, during a calculation period in which issuances were followed by redemptions, the relatively higher-yielding treasuries acquired in connection with the issuances would be delivered to redeeming Authorized Participants in the redemptions.
Moreover, a falling interest rate environment during any calculation period could create an incentive for Authorized Participants to effect a paired optional redemption for the purpose of receiving a delivery of the relatively higher-yielding treasuries held by the Series, which would adversely affect the remaining holders of the PharmaShares of that Series. Such an incentive would exist during a time when (1) the Series has no more cash available to distribute in connection with redemptions, (2) Authorized Participants could obtain both the Up and Down PharmaShares of that Series at market prices that, when combined, were equal to or less than the combined per share underlying value of those shares, (3) the transaction costs associated with effecting a paired optional redemption were exceeded by the value of the treasuries that were delivered, and (4) Authorized Participants were prepared to hold the treasuries they received to maturity in order to realize the full discount from par that was associated with those treasuries.
The periodic return on some Series of PharmaShares will be capped. The return on your PharmaShares for each calculation period may be subject to a periodic return cap, as specified in your prospectus supplement. As a result, even if the positive change in Reference Value greatly exceeds the Projected Breakpoint Increase, your return on the PharmaShares will be limited to the maximum return permitted by any periodic return cap that applies to your class. Further, unless you purchase additional PharmaShares, your investment and the capped return you are able to achieve will decrease as a result of each periodic UV performance distribution that is declared on your class or on the paired class of PharmaShares, as described below under "— The amount of your investment in your PharmaShares and your potential gains will decline over time." At any time when you seek to acquire additional PharmaShares, the trading price of those shares may have diverged from their per share underlying value and you may need to pay a premium over the per share underlying value, which will adversely affect the return you are able to achieve.
In addition, the return on the Up PharmaShares is also limited by the size of the Down investment amount and the return on the Down PharmaShares is limited by the size of the Up investment amount. Even if the return on your PharmaShares is not capped, you can never realize a return in excess of the assets invested in your Series by the shareholders of the paired class of PharmaShares.
A Series may terminate early. Your Series may terminate prior to its final scheduled termination date as a result of the occurrence of one of the termination triggers described in this base prospectus and your prospectus supplement. Following the occurrence of any termination trigger, the PharmaShares Trust will declare a final distribution on the next scheduled periodic calculation date which will be the early termination date. This early redemption may occur before you have had an opportunity to achieve the maximum return on your PharmaShares, or to reduce your existing losses. Moreover, you may not be able to identify a comparable investment referencing the net revenues of the Reference Product.
Many countervailing factors may affect the net revenue generated by a Reference Product. As discussed in the section entitled "DESCRIPTION OF THE PHARMACEUTICAL INDUSTRY," there are many factors that may cause growth in the Reference Value for a Series from period to period while other factors decrease that rate of growth or cause decreases in that Reference Value. The cumulative effect of these factors may be a significant degree of volatility in the Reference Value. Depending upon the accuracy of the Projected Breakpoints for each period, these countervailing factors may have an adverse effect on the underlying value and periodic UV performance distributions on, and the market price of, your PharmaShares. Moreover, if you intend to hold your PharmaShares until the final scheduled termination date or if you are unable to liquidate your investment in the PharmaShares, the return on your shares will depend upon the long-term revenue performance of the Reference Product for your Series and the accuracy of the Projected Breakpoints in predicting that performance from period to period.
30
You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution.
If there is an increase in the underlying value of the Up PharmaShares of a Series following the publication of a new Reference Value on any publication date, that Series will declare a periodic UV performance distribution equal to that increase on the next periodic calculation date plus an equalization payment discussed below under "― The amount of your investment in your PharmaShares and your potential gains will decline over time." Holders of the Up PharmaShares will receive that distribution on the second business day following the periodic calculation date. Conversely, if there is an increase in the underlying value of the Down PharmaShares of a Series, that increase plus an equalization payment will be paid to the Down shareholders. The portion of each periodic UV distribution made by a Series on a class of PharmaShares that is attributable to the increase in the underlying value of that class will represent the amount of the loss suffered by the paired class of PharmaShares of the same Series.
Each Series will declare a final distribution equal to the respective underlying values of its Up PharmaShares and its Down PharmaShares on the final scheduled termination date, an early termination date or any redemption order date. These underlying values will be based upon the Reference Value that was published on the last publication date that preceded each of those dates and the Projected Breakpoint determined for the last calendar quarter preceding those dates. If the underlying value of your class of PharmaShares preceding the date on which a Final Distribution is made on those shares is below the amount you invested in those shares, as reduced over time by periodic UV performance distributions, you will realize a loss on your remaining investment. Series in which the returns on the paired class of PharmaShares are not capped may result in a loss of your entire investment in your class of PharmaShares.
The Reference Value may increase or decrease or it may increase or decrease by a smaller percentage than anticipated by the market analysts who are included in the Broker Reporting Group. If you invest in Up PharmaShares and the Reference Value declines or increases by less than the Projected Breakpoint Increase consistently and significantly, you may lose all of your investment in your Up PharmaShares. Even if returns on the paired Down PharmaShares are capped and, as a result, the amount of losses you can suffer on your Up PharmaShares on any single periodic calculation date are also capped, if the Reference Value repeatedly and materially either (1) declines or (2) increases by less than the projected growth, this may, over time, result in significant losses on your Up PharmaShares. Conversely, if you invest in Down PharmaShares and the Reference Value increases or declines by less than the Projected Breakpoint Decrease consistently and significantly, you may lose all of your investment in the Down PharmaShares. Even if returns on the paired Up PharmaShares are capped and, as a result, the amount of losses you can suffer on your Down PharmaShares on any single periodic calculation date are also capped, if the Reference Value repeatedly and materially either (1) increases or (2) declines by less than the projected decrease, this may, over time, result in significant losses on your Down PharmaShares.
The PharmaShares Trust will also make periodic income distributions on your PharmaShares equal to the treasury income of your Series, net of its fees and expenses. Your Series may provide for an equal allocation of net treasury income among the Up and Down PharmaShares. Alternately, if your Series provides for allocation of income based upon Reference Value, then your periodic income distributions will also depend upon the performance of the Reference Value relative to the Projected Breakpoint from a past calendar quarter to the current calendar quarter, which will determine the entitlement of your class of PharmaShares to that income. Any periodic income distribution or the net income component of your final distribution may be reduced or eliminated as a result of the following factors:
·income on the treasuries in your Series must first be used to pay Series fees and expenses. The entire amount of the treasury income earned by your Series will be used to pay its fees and expenses for so long as (1) the dollar amount of funds invested in your Series is less than the minimum amount specified in your prospectus supplement, generally between $250 million and $500 million, and (2) yields on the types of treasuries that are permitted to be acquired by your Series are below the threshold annual percentage specified in your prospectus supplement, generally less than 3%. For so long as these conditions persist, the fees and expenses of a Series are expected to exceed its treasury income and no portion of that income will be distributed to its shareholders;
31
·after the funds invested in your Series exceed the specified minimum amount or treasury yields exceed the threshold annual percentage, treasury income will first be used to pay the fees and expense of the Series and then distributed to shareholders. Such fees and expenses (including any amounts reimbursable to us, as described in the next sentence) will be capped at the maximum rate specified in your prospectus supplement, generally an annual rate of not more than 2.50%. However, your Series will be required to reimburse us for the legal and administrative costs of establishing your Series that are disclosed in your prospectus supplement and for fees and expenses that we paid on behalf of the Series during any period when such fees and expenses exceeded the income of the Series. Such reimbursable amounts may be significant depending on the amount of excess fees and expenses and the length of the period during which we had to pay them on behalf of your Series and this reimbursement obligation will reduce the amount of the treasury income that is available to be declared as a periodic income distribution on your shares on any periodic calculation date; and
·if the underlying value of your class of PharmaShares has declined on any periodic calculation date as a result of the performance of the Reference Value during the preceding calculation period and if your Series provides for income to be allocated based upon underlying value, the paired class of PharmaShares will receive a greater share of the net income of your Series than your class.
The amount of your investment in your PharmaShares and your potential gains will decline over time.
When you receive a periodic UV performance distribution on your class of PharmaShares, you will receive both (1) an investment return equal to the increase in the underlying value of your class of PharmaShares , and (2) a return of capital in the form of an equalization payment. Conversely, each periodic UV performance distribution made to the paired class of PharmaShares of your Series (not including the related equalization payment) will represent a loss of value to you and a gain for shareholders who hold that paired class. However, each periodic UV performance distribution, including the equalization payment component of these distributions, that is made on your class of PharmaShares as well as on the paired class, will reduce your investment in the PharmaShares you hold. The only way to maintain your desired exposure to your class of PharmaShares is to acquire additional shares in the secondary market.
Equalization payments do not represent income or gain on your PharmaShares; instead, they are a mandatory return of a portion of your contributed capital to ensure that the Up investment amount and Down investment amount remain in the original proportion established for your Series. Each Series maintains, on its books and records, funds that are allocated to the investment made by holders of Up PharmaShares, referred to as the Up investment amount, and funds that are allocated to the investment made by holders of Down PharmaShares, referred to as the Down investment amount. The proportion of the Up investment amount to the Down investment amount, also referred to as the Up/Down ratio, will be 1:1 for most Series. The original Up/Down ratio must be maintained for so long as the shares of that Series are outstanding in order to preserve the nature of the investment for current and future investors in the Series.
The Up/Down ratio will be maintained for a 1:1 Series by observing the following rules: (1) one-half of the proceeds of any paired issuance will be allocated, on the books and records of the Series, to the Up investment amount and one-half to the Down investment amount, without regard to the Up underlying value and Down underlying at the time of the paired issuance or to the respective market prices of the Up PharmaShares and Down PharmaShares being issued, (2) one-half of the funds used to effect any redemption will be subtracted, on the books and records of the Series, from the Up investment amount and one-half from the Down investment amount, also without regard to respective underlying values or market prices of the shares being redeemed, and (3) an equalization payment will be made as part of each periodic UV performance distribution equal to the amount of the Up underlying value increase or Down underlying value increase, as applicable, that is being distributed. Equalization payments are always deducted, on the books and records of the Series, from the investment amount of the class of PharmaShares that is receiving the periodic UV performance distribution.
The periodic UV performance distributions made by your Series, including the equalization payments that are part of these distributions, will reduce the amount of assets in your Series and your opportunity for future income and gains (as well as limiting loss exposure) on your class of PharmaShares. You may increase your investment in
32
your class of PharmaShares only by acquiring additional PharmaShares in the open market, but there may not be enough PharmaShares available for purchase at the time you wish to purchase them or they may be trading at a premium over their per share underlying value.
There is currently no market for the PharmaShares of your Series, and no market may develop.
No market will exist for any Series of PharmaShares prior to their initial issuance. We will seek to have each Series of Up PharmaShares and Down PharmaShares listed on a national stock exchange and to maintain such listing for so long as that Series remains outstanding. However, we cannot guarantee that a secondary market will develop for either class of the PharmaShares of any Series or, if a secondary market does develop, that it will provide liquidity of investment or continue for the life of those shares. Any Authorized Participant may, but will not be obligated to, make a market in either or both classes of the paired PharmaShares of any Series. The Up PharmaShares and/or Down PharmaShares of your Series may experience price volatility due to the following factors: (1) there may be only a limited number of prospective buyers and sellers for the shares; (2) the amount of invested funds has declined such that changes in the Reference Value result in increasingly smaller dollar amount changes in underlying value, particularly in capped deals; and (3) in both capped and uncapped deals, termination triggers will occur once the assets fall below a specified dollar amount. Price volatility may affect the price that you are able to obtain for your shares and your ability to resell the shares. The lack of a market for the paired class of PharmaShares of your Series may adversely affect the development of a market for your class of PharmaShares. Due to the foregoing considerations, you must be prepared to hold your class of PharmaShares until their final scheduled termination date.
Fluctuations in the per share underlying value of your PharmaShares and other factors may affect their trading price.
The trading price of your PharmaShares will be determined by a number of different factors, including the current per share underlying value of those PharmaShares, which will depend upon changes in the Reference Value from a designated past calendar quarter to the current calendar quarter compared to a Projected Breakpoint for the same two quarters, as well as factors such as the prevailing pharmaceutical drug pricing environment, general economic conditions in the United States and, to a lesser extent, throughout the world, investor expectations about pharmaceutical product pricing and government regulation of such pricing, the expected growth and vitality of the U.S. economy, investor expectations about net revenue growth or decline of the Reference Product underlying your Series of PharmaShares and the supply and demand for your class of PharmaShares as well as for the paired class issued by your Series. The trading price of your shares on any date may differ from their per share underlying value as of that date for many reasons, including, but not limited to, the following:
·the Projected Breakpoint Increase or Projected Breakpoint Decrease will be calculated as the average of the net revenue projections made by the group of independent research analysts selected by us and may also include a composite projection generated by a market research integration service based on the projections of a specified group of brokers who provide their research to that service. However, the Broker Reporting Group will not include all research analysts that publicly publish revenue projections for the Reference Product and any single Projected Breakpoint may be based on only that subset of the brokers in the Broker Reporting Group who published projections for the current calendar quarter. The Broker Reporting Group's projections of net revenue for the Reference Product may be materially different from both the average of all research analysts and the actual net revenue that is reported at the end of the current period. As a result, the underlying value of your PharmaShares is highly dependent upon the net revenue projections made by the analysts who are included in the Broker Reporting Group;
·unlike commodities, securities or other underlying reference assets for exchange-traded products, no daily trading information will be available for the Reference Product underlying any Series of PharmaShares. During the calculation period that precedes each publication date, various sources may generate data relating to sales of the Reference Product, which may include information on total prescriptions and new prescriptions written and filled through certain distribution channels. These independent reporting sources are not operated or controlled by the reference pharmaceutical company or by us. In many cases, there is a subscription fee associated with
33
obtaining drug sales information from these sources. This third-party information will not be used in calculating the Reference Value and the underlying value of any Series of PharmaShares, but the information may influence the trading price of your PharmaShares between publication dates;
·over the course of a calendar quarter, investors may have cause to expect that the positive change in net revenue of the Reference Product for the defined measurement period will be less than any Projected Breakpoint Increase or will decline or, in the case of a Projected Breakpoint Decrease, that it will decline by more than that Projected Breakpoint Decrease, due to the occurrence of certain events, such as the impending launch of a competing product or discovery of an adverse side effect, and this expectation may cause a decrease in the trading price of your Up PharmaShares below their per share underlying value and a concurrent increase in the trading price of the paired Down PharmaShares above their per share underlying value; conversely, investors' expectations over the course of a calendar quarter that the positive change in net revenue will be greater than any Projected Breakpoint Increase or that any negative change in net revenue may not be as great as any Projected Breakpoint Decrease may cause a decrease in the trading price of your Down PharmaShares below their per share underlying value and a concurrent increase in the trading price of the paired Up PharmaShares above their per share underlying value;
·the Projected Breakpoint Increase or Decrease calculated for any period is at a level that does not generate equal demand for the Up and Down PharmaShares of a Series and the increased or decreased demand for one class of PharmaShares affects the trading price of both classes, including as a result of the impact on paired issuances and redemptions;
·the lack of availability of the paired class of PharmaShares in the secondary market may cause an authorized participant to have to pay a premium for those shares in order to be able to effect a paired optional redemption and, as a result, the price at which an authorized participant is willing to acquire your class of PharmaShares may decline;
·the expectation that a termination trigger will occur in the near future may result in increased trading volumes, increased creations and/or redemptions and market pricing that begins to diverge more significantly from underlying value as investors begin to attribute a value to the anticipated occurrence of the termination trigger that is independent of the respective underlying values of the paired PharmaShares of your Series;
·a large trade of either the Up PharmaShares or the Down PharmaShares of your Series may occur near the end of a trading day and no corresponding sale or acquisition of the paired Down or Up PharmaShares, as applicable, which aligns their market price with such trade occurs on that same day, resulting in an apparent divergence of the price of the paired PharmaShares which may be remedied by trading on ensuing days;
·the PharmaShares product structure may be unfamiliar to investors;
·the lack of an active trading market may decrease liquidity and depress the price of both your class of PharmaShares and the paired class; and
·the supply of your class of PharmaShares may exceed demand for such shares or demand may exceed supply.
Your PharmaShares can only be redeemed by Authorized Participants and only as part of a PharmaShares Unit consisting of the aggregate par amount of Up PharmaShares and the aggregate par amount of Down PharmaShares that is specified in your prospectus supplement. Consequently, in order to liquidate your investment in your PharmaShares, you may have to sell those shares at their prevailing market price, which may be below their per share underlying value for the reasons discussed above.
34
An investment in Up PharmaShares may not resemble a direct investment in the Reference Product; an investment in Down PharmaShares may not be an effective hedge against existing exposure to the Reference Product.
The investment return on the Up PharmaShares or the Down PharmaShares of any Series depends primarily upon (1) fluctuations in the underlying value of those shares resulting from changes in the applicable Reference Value over the defined measurement period compared to the Projected Breakpoint for that same period; (2) how long the Up PharmaShares or the Down PharmaShares are outstanding and receiving periodic UV performance distributions and periodic income distributions, if any; and (3) either the underlying value of your Up PharmaShares on the date you receive the final distribution on those shares or the price at which you sell those shares. It is currently not possible to invest directly in the net revenues of any of the products that we anticipate using as the Reference Product for any Series. However, if such an investment were possible, an investment in any Series of Up PharmaShares would likely not be comparable to such a direct investment in the applicable Reference Product due to the following factors:
·The underlying value of your PharmaShares and your return on those shares is not based on the absolute change in net revenue for a Reference Product from a base period to the current period; instead, your return will be based on the change in net revenue relative to a Projected Breakpoint or "hurdle." Accordingly, even if net sales of a Reference Product have increased substantially during the current period compared to a base period, the underlying value of your Up PharmaShares will not increase unless the actual percentage increase in sales is greater than the applicable Projected Breakpoint Increase for that period. Moreover, the increase in your Up underlying value will only be equal to the amount by which the percentage change in actual net sales exceeds the Projected Breakpoint Increase. Similarly, even if net sales of a Reference Product have decreased substantially during the current period compared to a base period, the underlying value of your Down PharmaShares will not increase unless the actual percentage decrease in sales is greater than the applicable Projected Breakpoint Decrease for that period. Moreover, the increase in your Down underlying value will only be equal to the amount by which the percentage change in actual net sales exceeds the Projected Breakpoint Decrease;
·Net revenue for any Reference Product will generally be adjusted by chargebacks, trade discounts, sale returns and allowances, commercial and government rebates, customer loyalty programs, distributor fee-for-service arrangements and other sales allowances. An estimate of sales allowances will generally be made at the time of sale based upon historical experience, sales agreements in place and an estimate for claims incurred but not yet paid. In some cases this gross-to-net estimate will be greater or lesser than the actual reduction, which may not be determined until after the relevant publication date on which Reference Value is reported. However, neither the Reference Value nor the calculation of underlying value and related periodic UV performance distributions will be retroactively adjusted to take into account any revisions or restatements made to net revenues by the related pharmaceutical company after any publication date;
·Developing, manufacturing and marketing a Reference Product generally involves a massive investment over an extended period of time. Even after a Reference Product has been patented and successfully marketed, there are many ongoing expected and contingent costs to the pharmaceutical company that manufactures that Reference Product related to sourcing raw materials, manufacturing and marketing costs, government agency oversight and potential legal claims from consumers. None of these initial investment and ongoing costs will be reflected in the underlying value or trading price of your Up PharmaShares, and such costs may be only partly and only in certain circumstances reflected in the underlying value and trading price of your Down PharmaShares, such as when a manufacturing problem results in lowered production volume and, consequently, reduced net revenues;
·The impact of changes in underlying value is multiplied by any leverage factor that may be applicable to the Up PharmaShares and/or to the Down PharmaShares, as specified in your prospectus supplement. The effect of a leverage factor is to multiply the impact on underlying
35
value of changes in the Reference Value relative to the Projected Breakpoint, making the per share underlying value and the trading price of your PharmaShares potentially more volatile than the net revenues of the relevant Reference Product. A leverage factor may cause an earlier termination of your Series and it may cause you to lose your investment earlier than you otherwise would have had such leverage factor not been used in the computation of underlying value;
·The impact of changes in underlying value is limited by any periodic return cap that may be applicable to the Up PharmaShares and/or the Down PharmaShares, as specified in your prospectus supplement. If a periodic return cap applies to your PharmaShares, the increase in underlying value that you will be able to realize on any periodic calculation date cannot exceed the limit set by that cap, regardless of the performance of the Reference Value during the preceding calendar quarter;
·For Series without a periodic return cap, the maximum underlying value of your Up PharmaShares is equal to the Up investment amount, representing 100% of the funds invested by the holders of the Up PharmaShares, plus the Down investment amount representing 100% of the funds invested by the holders of the Down PharmaShares; the value of an investment in your Up PharmaShares cannot exceed this maximum, even if the actual percentage change in Reference Value compared to the Projected Breakpoint Increase is greater than 100% for any period; conversely, the maximum underlying value of your Down PharmaShares is equal to the Down investment amount plus the Up investment amount, and the value of an investment in your Down PharmaShares cannot exceed this maximum, even if the actual percentage change in Reference Value compared to the Projected Breakpoint Decrease is greater than 100% for any period;
·The absolute size of any future potential gain (or loss) in your per share underlying value will decline as the assets in your Series are reduced as a result of being used to make periodic UV performance distributions. The only means to maintain the size of your investment in the PharmaShares of any Series are to acquire additional shares;
·The paired Down PharmaShares may be trading at a premium and an Authorized Participant wishing to effect a paired optional redemption will have to pay this premium in order to acquire those shares, which may decrease the price that Authorized Participants and other investors are willing to pay for your Up PharmaShares; conversely, the paired Up PharmaShares may be trading at a premium which may decrease the price that Authorized Participants and other investors are willing to pay for your Down PharmaShares;
·Following the occurrence of a termination trigger and the declaration of a final distribution in redemption of your PharmaShares on the related early termination date, we do not provide any guarantee that we will establish a new Series with the same Reference Product as the terminated Series and there is no guarantee that you will be able to invest the proceeds from your PharmaShares in an investment with a comparable yield or an investment that will continue to allow you to realize comparable gains from growth or declines, as applicable, in net revenues from the same Reference Product;
·If no termination trigger occurs, your Series will terminate on its final scheduled termination date, at which point the PharmaShares Trust will declare a final distribution on your PharmaShares in an amount equal to the per share underlying value represented by those shares on the last calendar day prior to the final scheduled termination date. This per share underlying value may be less than the par value of your shares (as such par value has been reduced by prior periodic UV performance distributions on your shares as well as on the paired PharmaShares) and less than the market price at which you acquired them; and
·As discussed above in the risk factor entitled "— The return on your PharmaShares is uncertain — Treasury securities may be delivered instead of cash in a paired optional redemption," if treasury securities instead of cash are delivered to redeeming Authorized Participants and the
36
market price of such treasury securities has declined since they were acquired by the applicable Series, then, if the Authorized Participants choose to liquidate those treasuries rather than hold them to their maturity, the amount of the final distribution realized by those Authorized Participants will be less than the per share underlying value of the shares being redeemed.
As a result of the foregoing structural factors, (1) the return on any Series of Up PharmaShares may not resemble the return that you may have achieved if an investment in the growth in net revenues of the applicable Reference Product were possible and you had made that investment, and (2) the return on any Series of Down PharmaShares may not resemble the return that you may have achieved if shorting an investment in the growth of net revenues of the applicable Reference Product were possible and you had entered into such a transaction.
Returns on the Up PharmaShares are not designed to track the stock performance of the reference pharmaceutical company that manufactures and sells the applicable Reference Product; returns on the Down PharmaShares are not designed to track an investment that shorts that stock performance.
A Series of PharmaShares tracks the difference in net revenue generated by a specified Reference Product between a past time period and a more recent time period, compares that change to the average net revenue change projections made by a selected group of market analysts, adjusted for the same time period, and then provides for distributions of the difference between the actual versus the projected revenue increase or decrease out of funds invested by the shareholders of that Series. Accordingly, investing in PharmaShares is materially different from investing in the stock of the pharmaceutical company that manufactures and sells the specified Reference Product, or shorting that stock. Moreover, some Series of PharmaShares will track changes in revenue year over year, which means that the current period net revenue will be compared to net revenue for the same calendar quarter occurring during the preceding year rather than the immediately preceding calendar quarter. The investment returns of year over year tracking Series are likely to diverge even further than those of sequential tracking Series from the investment returns that could be realized by investing in, or shorting, the stock of a pharmaceutical company.
If a single Reference Product marketed and manufactured by a pharmaceutical company represents a significant percentage of that company's total net revenues, then the revenue performance of that Reference Product may be a meaningful factor in determining the net revenues of the company as a whole. However, there are many additional factors that affect a pharmaceutical company's stock performance, as reflected in the appreciation or depreciation of share price and the amount of dividends paid on that stock, and these factors may cause the stock performance to diverge from the revenue performance of a single Reference Product. This may be the case even if a Series of PharmaShares is based on a "blockbuster" drug within the drug company's portfolio of products. A substantial portion of the net revenue associated with mature commercialized drugs manufactured and marketed by public pharmaceutical companies, less the associated costs of marketing, manufacturing, selling, allocations of general and administrative costs, interest on borrowed proceeds, taxes and other associated costs and expenses, result in available free cash flow that may be used to fund the required research and development costs of a pipeline of potential drug products that may not ever be approved and become available for sale. Cash flow from commercialized drug products may also be used to fund the purchase of other drug companies or drug products, or to acquire the licensing rights to market the drug products of unaffiliated companies. These acquisitions may not lead to meaningful cash flow and may not positively impact the share price of the acquiring pharmaceutical company. In addition, changes in interpretations of laws and regulations, accounting standards (including differences between the fair value measurement of assets and liabilities and their actual value), global taxation rules, product marketing application standards, the approval process for new pharmaceutical products, the application process for generic and biosimilar products, prevailing interest rates, changes in dividend policies, government regulation of drug pricing in the United States and in other jurisdictions where products are sold, and environmental laws could all impact a pharmaceutical company's stock price.
Calculation of underlying value for PharmaShares versus pharmaceutical company valuation. The underlying value of any Series of Up PharmaShares and Down PharmaShares will be calculated for each calendar quarter based upon changes in net revenue from a base period to the current period which are then compared to the Projected Breakpoint Increase or Projected Breakpoint Decrease, as applicable, for the same two periods, and potentially multiplied and/or limited by (i) any leverage factor applicable to the Up PharmaShares or the Down PharmaShares, and (ii) any periodic return cap that sets a limit on the maximum return that may be realized by the Up PharmaShares and/or the Down PharmaShares on each periodic calculation date. Thus the calculation of the
37
return on your Up PharmaShares or your Down PharmaShares will be a function of the relationship between the change in net revenue between two specified periods and the Projected Breakpoint for those periods, with a potential incremental increase in that return from treasury income realized by your Series. Further, any applicable leverage factor may not be the same multiple that public investors apply to the aggregate net sales of the pharmaceutical company selling the Reference Product for your Series in their assessment of stock valuation. In addition, any applicable leverage factor will multiply net revenues for the Reference Product only and not the total net revenues of a pharmaceutical company.
In contrast, the valuation of a pharmaceutical company may be based on a variety of factors, including a multiple of revenue, a multiple of earnings before interest, depreciation and amortization (EBITDA) or a multiple of total net income. The primary valuation metric used by research analysts and public investors to value the stock of a public pharmaceutical company will depend on the company's total net revenue, historical and projected net revenue growth, scope and number of commercialized products, life cycle of key commercialized products, pipeline of new products, dividend policy and outstanding debt. Investors and pharmaceutical stock research analysts may look at several valuation metrics to settle on a valuation range at any point in time for the stock of a public pharmaceutical company. Additionally, investors in pharmaceutical companies must consider the net impact on valuation of current cash spending for research and development and the projected, probability-adjusted future net revenue associated with the company's pipeline of products in development. Those pharmaceutical companies that are valued other than as a multiple of net revenue will have their stock value impacted by costs associated with research and development, manufacturing, sales and marketing and general overhead costs. Additionally, for pharmaceutical companies valued on a multiple of net income, non-cash expense items of depreciation and amortization plus cash expenses of interest and taxes will also be factors impacting stock valuation.
The activities of the PharmaShares Trust and each of its Series are passive and limited compared to pharmaceutical company operations. Mid-sized and large capitalized pharmaceutical companies (generally defined as those companies with market capitalizations exceeding $10 billion), including companies that may sell, manufacture and market a Reference Product, may spend $1 billion or more annually on developing new drug products and improving existing products and processes, without any certainty that their efforts and capital spending will result in commercially successful products. Conducting research, development and the trial process for new pharmaceutical products is time-intensive and carries with each of them a significant amount of inherent uncertainty, which can have a materially adverse effect on the financial condition of a pharmaceutical company. Products that appear promising in development may fail to reach the market for numerous reasons, including the following: failure to demonstrate effectiveness; safety concerns; superior safety or efficacy of competing therapies; failure to achieve positive clinical outcomes; inability to obtain necessary regulatory approvals; limited scope of approved uses; excessive manufacturing costs; failure to obtain or maintain intellectual property rights; infringement of the intellectual property rights of others; changing clinical preferences or industry standards and innovation by competitors; and/or a poorly coordinated launch of a new product.
Further, a pharmaceutical company may acquire other businesses, license rights to technologies or products, form alliances, or dispose of assets, which could impact the profitability of the company as a whole. Major transactions, such as mergers, acquisitions, technology licensing arrangements, strategic alliances, and reallocation of assets, can be time-intensive, difficult to integrate and expensive. Tax laws and regulations of different jurisdictions may change over time and may cause pharmaceutical companies to pursue transactions to lower income taxes at the expense of top line revenue growth. Moreover, certain corporate, tax and securities laws and certain provisions in the governance documents of pharmaceutical companies, such as voting provisions, special shareholder meetings, board composition and director removal, may prevent or delay an acquisition of another pharmaceutical company, which could decrease the trading price of both the acquiring company's and the target's common stock temporarily or permanently.
A pharmaceutical company's stock performance is subject to ancillary risk issues that may have no effect on the net revenue performance of the company's lead product. Product liability claims, lawsuits, safety alerts, and product recalls, regardless of their ultimate outcome, can significantly impact the stock price of a pharmaceutical company. These issues may relate to a pharmaceutical company's lead commercialized drug or another product. Manufacturing issues relating to any commercialized drug and/or distribution problems may result in damage to a company's reputation and could inhibit the company's ability to attract and retain customers. Consequences may include damage to reputation and brand loyalty for the pharmaceutical company, increase in the costs of modifying
38
the manufacturing process and exposure to future liability claims. Most product liability losses are self-insured, so this type of claim is likely to have a material adverse effect on profitability. Investing in PharmaShares may present investors with similar contingencies only for the relevant Reference Product, whereas an investment in a pharmaceutical company exposes an investor to such risks with respect to all commercialized and pipeline products of such company, as well as its acquired targets or assets.
Most pharmaceutical companies depend on wholesale distributors for the distribution of their products. In some cases, a limited number of wholesale distributors account for substantially all of the sales of a pharmaceutical company and/or its key product. Accordingly, the performance of the pharmaceutical company is significantly influenced by the economic stability and performance of its distributors. When distributors encounter financial or operational difficulties, a pharmaceutical company may be unable to collect the sales receipts that the distributor owes to it or distribution could be reduced company-wide. In addition, pharmaceutical companies outsource various functions to third parties, including the execution of all or a portion of a Phase I-III trial study for a pipeline product. Further, a portion of a pharmaceutical company's near-term pharmaceutical pipeline typically relies on collaborations with third parties. Disruptions in these collaborative relationships or breach of contract by third parties who provide various outsourced services could adversely affect the productivity and stock performance of the pharmaceutical company.
Pharmaceutical companies generally operate on a global basis. Many countries outside of the United States allow sales of pharmaceutical products under rules different from those in the United States for drug distribution and pricing. These countries may negotiate drug product pricing policies with a pharmaceutical company. When the government of a jurisdiction that is the source of significant sales for a pharmaceutical company modifies its funding for pharmaceuticals or its standards relating to safety, efficacy or labeling, a pharmaceutical company may experience resulting fluctuations in its revenue performance. A government may require pharmaceutical companies to amend the conditions of use for a product based on new government regulations. Moreover, a pharmaceutical company reliant on government contracts might experience significant gain or loss due to governmental budgetary actions, such as a reduction or increase in pharmaceutical rebates. Further, pharmaceutical companies are subject to increasing public and legislative pressure in the United States to reduce prices, as discussed in greater detail under "DESCRIPTION OF THE PHARMACEUTICAL INDUSTRY — Executive branch initiatives and political pressure for drug price controls." If operating expenses are not reduced at the same rate as any pricing pressure on drugs, the operating income and operating profit margins of pharmaceutical companies will suffer. These drug sales and pricing contingencies that affect net revenue in countries around the world also apply to PharmaShares with respect to the underlying Reference Product. However, the entire portfolio of a pharmaceutical company may potentially be affected and there may be a greater impact on stock performance as a result of the company's cost structure.
The capital structure of, and terms of periodic distributions on, a Series of PharmaShares versus financing of pharmaceutical companies. Equity issuances for capital market transactions or equity awards granted by a pharmaceutical company dilute the ownership share of its stockholders. Most pharmaceutical company employees have options to purchase shares of common stock. These awards dilute the company's earnings per share, which may adversely affect the market price of its common stock. In addition, many pharmaceutical companies can issue certain classes or series of preferred stock without stockholder approval. The terms of such preferred stock could dilute the voting power or reduce the value of a pharmaceutical company's common stock. Likewise, the repurchase, redemption or liquidation preferences a pharmaceutical company can grant to holders of its preferred stock could affect the residual value of its common stock. In contrast, a Series of PharmaShares will be tied to the net revenues of a specified Reference Product relative to the projected performance of that Reference Product and will not be affected by the number of outstanding shares or earnings per share of the pharmaceutical company that is manufacturing and selling that Reference Product.
Large pharmaceutical companies that own several commercialized drug products pay substantial dividends. These companies may pay dividends exceeding the rate at which they pay interest on certain portions of their debt as well as the yield rate on short-term treasury securities, and they may increase their dividend payments over time. Dividends are not guaranteed to be paid or to increase, but the prospect of receiving dividends is a factor considered by investors in holding these stocks. A Series of PharmaShares may make periodic income distributions if treasury income exceeds the fees and expense of that Series. Any distribution of net treasury income on a Series of PharmaShares may be substantially lower than the dividends paid on the stock of the pharmaceutical company manufacturing and selling the Reference Product for that Series.
39
The amount of debt incurred by a pharmaceutical company and the associated cash interest expense will impact its cash flow and dividend profile and its net income, which could adversely affect its stock price in the event it is valued on a price-to-earnings multiple. Interest payments required to be made on the debt will reduce cash flow available for research and development, acquisition, and dividends. Additionally, if a pharmaceutical company's cash flow is not sufficient to repay all its outstanding debts, it may need to borrow more money, enter into credit facilities with restrictive covenants, issue additional equity or sell assets to refinance its debt. A pharmaceutical company with excessive debt or one that has lost its investment grade credit rating may be unable to fund expansion, develop products or respond to competitive pressures, all of which could negatively affect its profitability and stock value. In comparison, neither the PharmaShares Trust nor any of its Series will borrow funds from any third parties for any purpose. All available cash for a Series is sourced from the investment made by the holders of the PharmaShares of that Series. This cash will not be used to acquire any operating assets; it will be invested in treasuries maturing prior to each periodic calculation date and used to make periodic income distributions and periodic UV performance distributions. However, a Series of PharmaShares may have a leverage factor applicable to its Up PharmaShares and/or Down PharmaShares that may increase gains or losses by up to three (3) times the base return. A Series may also have a periodic return cap that may limit the leveraged or unleveraged returns on its Up PharmaShares and/or Down PharmaShares for each calculation period.
The PharmaShares Trust will make distributions on the Up PharmaShares and Down PharmaShares of a Series solely from the assets held by that Series.
No Series will have any assets or sources of funds other than the treasuries purchased with the net proceeds of the initial offering and continuous creation of its Up and Down PharmaShares. The PharmaShares Trust will be the only entity obligated to make distributions on the PharmaShares issued by each Series and, pursuant to the terms of the master trust agreement and the related series trust supplements, it will be permitted to make those distributions solely out of the assets that are held by the Series that issued those shares and not out of the assets held by any other Series. The PharmaShares of each Series will not be insured or guaranteed by us, the Authorized Participants, the trustee, the calculation agent, the administrative agent or any of our or their respective affiliates. Holders of the PharmaShares of each Series will have no contractual recourse to any of these persons and no contractual or legal recourse to the holders of the PharmaShares of any other Series or to any other person or entity if their Series has insufficient assets to make any periodic income distributions, periodic UV performance distributions or the final distribution. Holders of the PharmaShares, by virtue of acquiring those shares, will be deemed to have agreed that they have no entitlement to the assets in any Series of the PharmaShares Trust other than the Series that issued their PharmaShares. Neither the PharmaShares Trust nor any trust created as a series of the PharmaShares Trust will be insured or guaranteed by the United States government or any government agency or instrumentality and will not represent investments in a money-market type fund.
The respective underlying values of the Up and Down PharmaShares represents their entitlement to the assets held by your Series. The respective underlying values of the Up and Down PharmaShares of a Series will fluctuate based on the change in the applicable Reference Value between two specified quarters compared to the Projected Breakpoint for the same quarters, as described in this base prospectus and your prospectus supplement. An increase in the underlying value of the Up or Down PharmaShares based on the Reference Value published on a publication date will result in the declaration of a periodic UV performance distribution on the class of PharmaShares that realized the underlying value increase on the next periodic calculation date. On an early termination date or the final scheduled termination date, the PharmaShares Trust will make a final distribution on both the Up and Down PharmaShares of the applicable Series equal to their respective underlying values as of the most recent calendar date, which will reflect (1) their underlying value on the most recent publication date, less (2) the increase in underlying value of one class distributed out of the assets allocable to the paired class or the equalization payment distributed out of the assets of the class that experienced such increase, as applicable, as part of any periodic UV performance distribution made since that date, and plus (3) the net income accrued on the PharmaShares since that date. The sole source of each periodic income distribution, each periodic UV performance distribution and the final distribution on the PharmaShares of any Series will be the cash and treasuries on deposit in that Series and the income accrued on these treasuries during each calculation period, net of the fees and expenses of that Series.
40
Income on the treasuries may be insufficient to make periodic income distributions.
The assets on deposit in each Series will consist entirely of treasuries that mature prior to each periodic calculation date and repurchase agreements collateralized by treasuries that terminate within twenty-four (24) hours of their execution which are rolled over into new repurchase agreements with yield rates then prevailing in the market. Periodic calculation dates will typically occur quarterly (but may occur less frequently for a particular Series) and, on each of these dates, a Series must reinvest the proceeds of its matured treasuries in new treasuries that will be trading at a discount or earning interest at a stated rate, in each case, based on the rates prevailing at the time of reinvestment. If prevailing interest rates or the discount rates offered on the treasuries decline, the interest income realized by a Series will decline and the aggregate amount of periodic income distributions made by that Series in future periods will be reduced. Furthermore, because each Series will be required to hold its treasuries to maturity, if interest rates increase or treasuries are being sold at higher discounts at any time during the period between periodic calculation dates, you will not be able to benefit from these changes until the next periodic calculation date. As a result you are exposed, as a holder of PharmaShares, to interest rate risk on the treasuries and treasury repurchase agreements held by your Series.
Each Series must first pay its fees and expenses on each periodic calculation date out of its treasury income. For so long as the amount of funds invested in your Series is below the minimum level specified in your prospectus supplement, generally between $250 million and $500 million, and average treasury yields realized by your Series are below a minimum threshold annual yield also specified in your prospectus supplement but generally equal to at least 3%, your Series will use all of its treasury income to pay its fees and expenses and will not make any periodic income distributions. The fees and expenses payable by your Series will be capped at the rate specified in your prospectus supplement and any fees and expenses in excess of that rate will be paid by us and reimbursed to us out of the future treasury income of your Series, if any. After the date on which, and continuing for so long as, the amount invested in your Series exceeds the specified minimum amount and/or treasury yields exceed the specified threshold yield, shareholders will receive as periodic income distributions any treasury income remaining after the fees and expenses of your Series have been paid and we have been reimbursed for Series formation expenses and for any fees and expenses we paid on behalf of your Series when its income was insufficient to pay such amounts, except that we may waive our right to be reimbursed for past expenses after a Series achieves a minimum dollar amount of invested assets, as specified in your prospectus supplement. Fees and expenses payable for each calculation period, including amounts reimbursable to us, will generally be capped so that these amounts do not exceed the maximum specified in your prospectus supplement, generally equal to 250 basis points.
During the past twelve (12) years, short-term (three (3)-month) treasury rates have been at relatively low levels compared to historical levels during the period from 1982 to 2007 (excluding 2001 through 2005 during which low rates, generally below 3%, prevailed). These rates, as low as 0.10% or less during 2014 through 2016 and slightly higher but still below 2.00% during 2016 through the second quarter of 2018 and increasing thereafter but currently below 3%, have prevailed as a result of the large-scale policies and programs adopted starting in 2007 by the U.S. federal government, the goal of which has been to support and stimulate capital market activity. These policies have included a series of coordinated actions by the U.S. Treasury and the Federal Reserve designed to alleviate severe credit market dislocations by injecting liquidity into the financial system. These monetary policies and government interventions to address the so-called "Great Recession" or "2008 Recession" have resulted in a huge increase in global demand for dollar-denominated, short-term, high-credit quality assets such as U.S. treasuries, which caused the yield on such assets to decrease significantly. The Federal Reserve allowed the federal funds rate to rise at the beginning of 2018; nevertheless, treasury rates remain below historical levels and it is uncertain for how long these market conditions will prevail and how government macroeconomic policies may change in the future. For so long as short-term treasury yields remain below 3% and the invested amount in your Series does not reach the minimum dollar amount specified in your prospectus supplement, you will not receive any periodic income distributions on your PharmaShares.
Your Series may incur losses in connection with treasuries delivered upon the default of a repurchase agreement counterparty.
We, in our capacity as the administrative agent, will instruct the trustee to invest a portion of the funds on deposit in your Series in overnight repurchase agreements for U.S. Treasury obligations. In the event that the seller under an overnight treasury repurchase agreement defaults on its obligation to repurchase from the applicable
41
portfolio the U.S. Treasury securities that were transferred under that agreement, the trustee will be required to deliver a default notice to the seller. After delivery of that notice, the trustee will be entitled to exercise all of the remedies permitted to be taken under the repurchase agreement, including retaining the transferred treasuries, in which case the trustee, acting at our direction, must liquidate these treasuries on behalf of the applicable Series. The seller in each repurchase agreement will be required to deliver treasuries with a market value, as measured on the date of transfer and discounted by the expected transaction costs which would be incurred if the Series had to liquidate such collateral following a default by the seller, that will be at least equal to the repurchase price specified in such repurchase agreement. However, in the event that the actual price that the affected Series is able to obtain for the treasuries is less than what those treasuries were valued at for purposes of the repurchase agreement, the amount of funds available to make periodic income distributions will be reduced. If the loss incurred upon a sale of the treasuries is large enough to reduce the Up and Down investment amounts, then the Series funds available to make periodic UV performance distributions and the final distribution will also be reduced.
Redemption and issuance orders are subject to postponement, suspension or rejection in certain circumstances.
We, in our capacity as the administrative agent, are required to suspend the right to effect a paired optional redemption or a paired issuance or postpone any redemption date or issuance date, (i) for any period during which NYSE Arca is closed or trading on the exchange is suspended or restricted, (ii) for any period during which an emergency exists as a result of which the delivery or acquisition of treasuries is not reasonably practicable or (iii) for such other period as we, in our capacity as the administrative agent, determine to be necessary for the protection of the shareholders. In addition, we, in our capacity as the administrative agent, may, in our sole discretion, reject any redemption order or issuance order (i) that is not in the proper form required by the participants agreements, (ii) if insufficient shares, in the case of a redemption order, or insufficient funds, in the case of a issuance order, are tendered to the trustee, (iii) if we determine, based in either case upon advice of counsel, that such order would have adverse tax or securities laws consequences for a Series or any holders of paired PharmaShares or that the fulfillment of such order may be unlawful, or (iv) if circumstances outside of our control make it impractical or not feasible to process the order. In addition, we, in our capacity as the administrative agent, will reject any issuance order if there are not sufficient paired PharmaShares registered pursuant to the Securities Act to fulfill the order. We and the trustee will not be liable to any person in any way for any loss or damage that may result from any such suspension, postponement or rejection. Any such postponement, suspension or rejection may adversely affect the ability of Authorized Participants to effect paired issuances or paired optional redemptions and the demand for and market price of your PharmaShares.
The global pandemic may affect liquidity in the market for your shares, the ability of your Series to acquire treasuries and the performance of your reference drug.
The current global pandemic caused by COVID-19 has affected, and any pandemic that may occur in the future will affect, liquidity in the ETF market and bid/ask spreads, which may decrease demand for, and trading in, your Series of PharmaShares and your ability to sell those shares at a price that reflects their underlying value. Further, the pandemic may result in short-term disruptions in the treasury market. As a result, we may not be able to identify sufficient amounts of treasuries that mature prior to the end of the next periodic calculation date for your Series in which to invest the Series assets and those assets may have to held in cash. Measures implemented by the federal government to alleviate the recession in economic activity due to the pandemic has affected, and may in the future continue to affect, yields on treasuries, resulting in minimal yields or even negative yields for an extended period of time. As a result, if your Series does not realize sufficient income on its treasuries to pay its fees and expenses and the amount of fees and expenses that will be reimbursable to the administrative agent will accumulate. In those circumstances, a portion of the periodic UV performance distributions to which you may be entitled on your shares, not to exceed a specified percentage of the portion of any distribution that constitutes an increase in the underlying value on your shares, would be deducted from that distribution to pay the unpaid fees and expenses of your Series, including amounts reimbursable to the administrative agent. Further, negative yields would reduce the Series investment amount and negatively impact the underlying value and trading price for your shares. All future treasury income for your Series would first be used to replenish the Series investment amount before being applied to pay fees and expenses or make periodic income distributions. Accordingly, an extended period of very low or negative treasury yields may adversely affect the investment yield and trading prices of your shares.
42
Any regional or global pandemic may impact the net revenues from a reference drug. In determining the degree of that impact, you should consider whether the reference drug is elective or non-elective, the form of delivery of the reference drug, e.g., via pill or injection, and the usual location of drug delivery, e.g., hospital, physician office or out-of-office. You must also consider the location where the reference drug is manufactured, the supply chain used by the reference pharmaceutical company and the jurisdiction in which the reference pharmaceutical company coordinates global sales of the reference drug. The prospectus supplement for your Series may contain additional considerations relating to the impact of the pandemic on the net revenues of the reference drug for your Series.
You should be aware of the tax consequence of your investment in the PharmaShares. For example, you may have U.S. federal income tax liabilities in advance, or in excess, of your periodic distributions.
The PharmaShares Trust intends, and will elect, to be classified as a corporation for U.S. federal income tax purposes. Accordingly, the PharmaShares Trust expects to be subject to U.S. federal income tax on its taxable income and holders of PharmaShares are expected to be treated as shareholders of a corporation for U.S. federal income tax purposes with the anticipated consequences described in "U.S. FEDERAL INCOME TAX CONSIDERATIONS." Moreover, by accepting PharmaShares, holders agree to report the income and loss from the PharmaShares consistent with such treatment.
The PharmaShares Trust and the PharmaShares have certain unique characteristics. There are no authorities, however, that address the U.S. federal income tax treatment of an entity such as the PharmaShares Trust or interests such as the PharmaShares. As a result, there can be no assurance that the U.S. Internal Revenue Service, or the "IRS," or a court will agree with the treatment noted above. If the IRS were to successfully challenge the intended treatment of the PharmaShares Trust as a corporation for U.S. federal income tax purposes, holders of PharmaShares could be required to recognize income, gains, losses, and deductions with respect to their PharmaShares in amounts and at times materially different from those that would be expected from an investment in corporate stock. For example, a holder could be currently subject to tax on a distribution that was expected to be treated as a return of capital. Each prospective investor should consult its tax advisor regarding the tax consequences of an investment in the PharmaShares in light of its particular circumstances. See "U.S. FEDERAL INCOME TAX CONSIDERATIONS" in this base prospectus for more information.
Recent and potential tax legislation may alter the tax consequences to holders of an investment in the PharmaShares of any Series.
Prospective investors should be aware that developments in the tax laws of the United States could have a material effect on tax consequences to the PharmaShares Trust and/or holders of PharmaShares. Such developments could affect holders even if not specifically targeted at such holders or vehicles such as the PharmaShares Trust. Moreover, the interpretation and application of tax laws and regulations by certain tax authorities may not be clear, consistent, or transparent. In addition, on December 22, 2017, the President of the United States signed into law a U.S. tax reform bill known as the "Tax Cuts and Jobs Act", or the "TCJA," which made significant changes to the U.S. tax system, including by changing tax rates and modifying certain rules relating to the use of losses and deductions. In some cases, there is uncertainty around the scope and application of the newly enacted legislation, which may be addressed in future IRS guidance. Such developments could be applied retroactively.
Each prospective investor should consult its tax advisor to determine the U.S. federal, state, local and non-U.S. income tax and other tax consequences of acquiring, holding, and disposing of PharmaShares in light of its particular circumstances, including as a result of changes made by the TCJA.
Limitations on interseries liability for Delaware series trusts have not been reviewed by the courts.
The master trust agreement for the PharmaShares Trust and each Series trust supplement will provide that the assets and liabilities of each Series will be maintained separate and apart from the assets of every other Series, including depositing the treasury securities and cash of each Series into segregated accounts and keeping separate and distinct records and accounting for each Series in compliance with Section 3804(a) of the Delaware Statutory Trust Act. In addition, each Series trust supplement will provide that, by acquiring the PharmaShares of that Series, each holder is deemed to agree that it shall have no right to the assets of any other Series of the PharmaShares Trust
43
and such assets will not be available to make any distributions on its PharmaShares or any other liabilities of its Series. Each participants agreement, subscription agreement or other contractual obligation into which a Series enters will also explicitly state that only the assets of that Series and not of any other Series or of the PharmaShares Trust generally will be available to satisfy the liabilities of that Series.
Section 3804(a) of the Delaware Statutory Trust Act provides that the parties to a trust agreement may agree to segregate the assets and liabilities of each series by explicitly providing for a limitation on interseries liability in the certificate of trust and governing instrument for a statutory trust organized in series. However, no court has had the occasion to review such a limitation or the record-keeping practices of any statutory trust organized in series. If a court were to undertake such a review in the future, it could determine that there are equitable exceptions to the statutory provisions that permit the limitation on interseries liability or that the record-keeping practices of the PharmaShares Trust does not full comply with the requirements of Section 3804(a). Furthermore, no federal bankruptcy court has adjudicated a case involving insolvency proceedings of one Series of a Delaware statutory trust. The master trust agreement and each Series trust supplement will provide that the trustee will not, acting on its own or at the direction of the shareholders of that Series, make a voluntary bankruptcy filing on behalf of a Series for so long as any PharmaShares of that Series remain outstanding. However, if your Series, any other Series or the PharmaShares Trust itself were nevertheless to become subject to voluntary or involuntary bankruptcy proceedings, it is not certain that a bankruptcy court would uphold the limitations on interseries liability, in which case the assets of your Series could be consolidated with the assets of other Series and made available to satisfy the liabilities of those other Series.
The PharmaShares do not confer upon their holders many of the rights normally associated with shares issued by a corporation.
Holders of the PharmaShares of any Series are not entitled to many of the rights typically exercised by shareholders in a corporation. By acquiring PharmaShares, you are not acquiring the right to elect directors, to vote on matters relating to your Series or the PharmaShares Trust, or to take other actions generally associated with the ownership of shares in a corporation. You will have only the limited rights described under "DESCRIPTION OF THE TRUST AGREEMENT — Voting Rights" and "— Modification and Waiver.
Broker dealers who act as authorized participants for your Series may make projections for your Reference Product.
Broker-dealers who act as authorized participants for a Series of PharmaShares may have affiliated research groups that deliver projections on various Reference Products. Although a broker who acts as an authorized participant for a Series may never be selected as an individual broker for the Broker Reporting Group of that Series, the projections of its research division may be included in a composite projection that is generated by an unaffiliated market research integration firm and is used to determine the Projected Breakpoint for your Series. Brokers who provide their projections to market research integration services do so in the ordinary course of their established relationships with those service firms and are not delivering projections for purposes of enabling the computation of the Projected Breakpoint. The brokers will not receive any direct compensation for allowing their projections on a Reference Product to be included in the composite. In addition, banks that have both a sales/trading department and an equity research department maintain strict firewalls between the two and are subject to rules that prohibit fraudulent practices. Each broker in a Broker Reporting Group, including as part of a composite number, must be a FINRA member.
Any composite number included in a Broker Reporting Group must have a minimum of 5 brokers and a minimum of three projections must be generated by those brokers for each period. If less than three projections are generated for any period, no Projected Breakpoint will be calculated for that period and the underlying value of the related PharmaShares will remain unchanged. Market research integration services typically include a dozen or more brokers in the group of brokers whose projections they use to generate a composite.
The prospectus supplement for your Series will specify whether any of the authorized participants for your Series will also be part of the Broker Reporting Group for that Series. A FINRA-regulated firm that generates projections for a Reference Product and is contracted by us to act as an authorized participant for the Series that tracks the performance of that Reference Product, will have no obligation to consider the interests of the Up or
44
Down shareholders of that Series (and may be prohibited by law from doing so) when making its Reference Product projections.
USE OF PROCEEDS
The trustee for each Series of PharmaShares will apply the proceeds received by that Series in connection with each Paired Issuance to acquire, in accordance with the directions of the administrative agent and on behalf of that Series, bills, bonds and notes issued and guaranteed by the United States Treasury that are scheduled to mature prior to each Periodic Calculation Date, and overnight repurchase agreements collateralized by United States Treasury securities, in accordance with the procedures described under "THE PHARMASHARES TRUST — United States Treasury Obligations." PharmaShares Advisors, LLC will pay for all of the costs associated with the formation of each Series of the PharmaShares Trust and the issuance, registration, marketing and offering of the paired PharmaShares issued by each Series. PharmaShares Advisors, LLC will seek to reimburse itself for costs incurred on behalf of a Series out of the treasury income realized by that Series, subject to the maximum fee rate specified in the prospectus supplement for each Series. For each Series, the fees and expenses of its third-party service providers, its regular, ongoing expenses and any extraordinary expenses, and any selling commissions payable to Authorized Participants in connection with the initial offering of its paired PharmaShares will be paid out of the Net Treasury Income of that Series on each Periodic Calculation Date. Please see the discussion under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series" in this base prospectus and under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
PHARMASHARES ADVISORS, LLC
We were established as a limited liability company in the State of Delaware on May 24, 2019, and are wholly-owned and managed by our parent and sole member, PharmaShares Manager, LLC. Of the outstanding membership interests of PharmaShares Manager, LLC, 86% are held by PharmaShares Manager Holding, LLC, which is, in turn, wholly-owned by Jon C. Ohrn. The two other members of PharmaShares Manager, LLC, David W. Jaffin and Dennis Purcell, each holds 7% of the company’s outstanding membership interests. Biographical information about the principals of PharmaShares Manager, LLC and PharmaShares Manager Holding, LLC is included below.
Our limited liability company agreement provides that we may conduct any lawful activities necessary or incidental to serving as depositor and administrative agent for each Series of the PharmaShares Trust and similar trusts formed by us in the future. We will hold the residual trust certificate for each Series of the PharmaShares Trust.
We have developed PharmaShares as a means of transforming the performance of individual products within the pharmaceutical industry into interests that can be acquired by individual investors in the form of publicly tradeable securities.
No director, officer, manager, member, employee, legal counsel, accountant or advisor of PharmaShares Advisors, LLC, PharmaShares Manager, LLC, PharmaShares Manager Holding, LLC or any affiliate of any of them will be permitted to invest in the Up or Down PharmaShares of any Series.
Jon C. Ohrn
Jon C. Ohrn is the founder and major equity owner of PharmaShares Advisors LLC. He has provided advisory services within the legal and investment banking sectors during the last 23 years. During his career, Mr. Ohrn has spent significant time designing debt and equity investment securities, including exchange traded products and ‘40 Act investment products. Mr. Ohrn’s advisory background includes clients engaged in operating areas of asset management and securities exchanges. He has also spent considerable time executing transactions within the healthcare industry. Mr. Ohrn has held legal and investment banking positions at White & Case LLP, Merrill Lynch, Deutsche Bank and SVB Leerink.
Mr. Ohrn received a B.A. from University of Washington in Accounting and Finance, and a J.D./L.L.M. in Taxation from New York University School of Law.
45
David W. Jaffin
David W. Jaffin is a partner with B2B CFO, which provides CFO and other senior advisory services to small and middle market companies. He is also Senior Advisor to Bradford Equities Management LLC, a private equity firm specializing in small to middle market leveraged buyouts, and is a director of a number of private companies. Most recently Mr. Jaffin was co-founder and CFO of Poliwogg Holdings, a startup creating innovative healthcare indices. Previously, he was Chief Financial and Operating Officer of XShares Advisors LLC, an issuer of exchange traded funds which was acquired by Deutsche Bank in 2010. Prior experience includes both operating and financial roles in a variety of financial and industrial companies.
Mr. Jaffin received a B.A. from Harvard College and an M.B.A. from New York University.
Dennis Purcell
Dennis Purcell is the founder of Aisling Capital LLC, where he was a Senior Managing Partner and Advisor. Prior to Aisling Capital, Mr. Purcell served as Managing Director of the Life Sciences Investment Banking Group at Chase H&Q (formerly Hambrecht & Quist, “H&Q”) for over five years. While at H&Q, he was directly involved with over two hundred completed transactions and supervised over $10 billion of financing and advisory assignments in the pharmaceutical, biotechnology and medical products industries. During his tenure, BioWorld and other industry publications cited H&Q as the leading underwriter of life sciences securities. Prior to joining H&Q, Mr. Purcell was a Managing Director in the Healthcare Group at PaineWebber, Inc.
Mr. Purcell is a frequent commentator on the biotechnology industry and has been honored in the “Biotech Hall of Fame” by Genetic Engineering News. He has also been named to the Biotechnology All-Stars list by Forbes ASAP, honored as one of the top 50 Irish-American businessmen and cited as one of the top 100 contributors to the biotechnology industry. Mr. Purcell has served on numerous private and public healthcare company boards. He currently sits on the Boards of Real Endpoints, Summus Global, Inc., BioScience Managers Pty Ltd., and Ichnos Sciences. He is on the Editorial Advisory Board at Life Science Leader Magazine. He is also a member of NYBIO Association and both the Investment Visiting Committee and the Biopharmaceutical Innovation Board, both at the University of Delaware.
Mr. Purcell received his M.B.A. from Harvard Business School and a B.S. in Accounting from the University of Delaware.
THE PHARMASHARES TRUST
Formation of the Master Trust and Each Series
On [●] [●], 2020, the PharmaShares Master Trust, which is referred to in this base prospectus as the "PharmaShares Trust," was formed by PharmaShares Advisors, LLC, in its capacity as depositor, as a statutory trust organized in series under the provisions of the Delaware Statutory Trust Act. The Master Trust Agreement governs the PharmaShares Trust and provides for the establishment of one or more series, each of which is referred to as a "Series." Each Series will issue two classes of shares: the Up PharmaShares and the Down PharmaShares, which are together referred to as the "Paired PharmaShares" of each Series. The formation of each Series and the issuance by that Series of Paired PharmaShares representing undivided beneficial interests in the assets of that Series will be governed by a separate Series Trust Supplement to the Master Trust Agreement.
For a description of the terms of the Master Trust Agreement and the expected terms of each Series Trust Supplement, see "DESCRIPTION OF THE TRUST AGREEMENT" in this base prospectus.
A new Series of the PharmaShares Trust will be established in connection with the issuance of each new series of Paired PharmaShares. The trustee will deposit the proceeds of each issuance and sale of the Paired PharmaShares of a Series into a segregated account for that Series in which those funds will be held by the trustee
46
for the exclusive benefit of the shareholders of that Series, separate from the assets and liabilities of each other Series of the PharmaShares Trust. Each Series will issue Up PharmaShares and Down PharmaShares with the characteristics described in this base prospectus and the related prospectus supplement. The Master Trust Agreement and your Series Trust Supplement will provide that the assets of your Series will not be available to satisfy the liabilities of, or to make distributions on the shares issued by, any other Series and the assets of other Series of the PharmaShares Trust will not be available to make distributions on your PharmaShares or satisfy any of the liabilities of your Series. Although the Delaware Statutory Trust Act permits trusts organized in series to limit interseries liability, no court has yet had the occasion to adjudicate the enforceability of, or exceptions to, such a limitation. See "RISK FACTORS — Limitations on interseries liability for Delaware series trusts have not been reviewed by the courts."
The Assets of Each Series
Each Series will hold its assets solely for the benefit of the holders of the Up PharmaShares and Down PharmaShares of that Series and will not commingle such assets with the assets of any other Series of the PharmaShares Trust. The assets of each Series are expected to consist of:
·United States treasury securities of the type described below under "— United States Treasury Obligations" and what we refer to as "income" on those securities, consisting of stated interest on treasury notes and bonds and the discount that is realized when the par amount received on a treasury bill, note or bond at maturity exceeds the purchase price at which that Series acquired that treasury;
·treasury repurchase agreements and what we refer to as "income" on those agreements consisting of the difference between the purchase price and the repurchase price for the treasury securities borrowed under those agreements;
·the rights of that Series under the Master Trust Agreement and its Series Trust Supplement to rely on the services provided by the trustee, the administrative agent, the marketing agent and the calculation agent, as described under "DESCRIPTION OF THE TRUST AGREEMENT;"
·a securities account created under its Series Trust Supplement into which all of the treasury securities acquired from time to time by that Series will be deposited for the benefit of its shareholders, segregated from the assets of each other Series;
·a distribution account created under its Series Trust Supplement, segregated from the assets of each other Series, into which all income and maturity proceeds realized on the treasury securities will be deposited and used to make (1) required deposits into the fee payment account and (2) Periodic Income Distributions, Periodic UV Performance Distributions and the Final Distribution to the holders of the Up PharmaShares and Down PharmaShares of that Series;
·a fee payment account created under its Series Trust Supplement, segregated from each other Series, into which all or a portion of the income realized on the treasury securities will be deposited on each Periodic Calculation Date for the purpose of paying the fees and expenses of that Series; and
·a netting account created under its Series Trust Supplement to which the trustee will credit (1) Paired PharmaShares of that Series that are being redeemed in Paired Optional Redemptions and nets such shares against any Paired PharmaShares being created in Paired Issuances on the same date, and (2) cash delivered by Authorized Participants in connection with Paired Issuances and net such cash against the Final Distribution to be made in connection with Paired Optional Redemptions being effected on the same date.
47
United States Treasury Obligations
On each Periodic Calculation Date, each Issuance Date, and any other date on which there is cash on deposit in a Series that is not being used to make Periodic Distributions or the Final Distribution to shareholders, all such cash will be invested by the trustee, acting in accordance with the directions of the administrative agent and on behalf of the applicable Series, in bills, notes and bonds issued and backed by the full faith and credit of the government of the United States of America, which mature prior to the next scheduled Periodic Calculation Date. Cash will also be invested in agreements for the sale and repurchase of, and collateralized by, U.S. Treasury securities, which qualify as "eligible repos," because (i) they are entered into with a seller that is bank with at least one billion U.S. dollars in assets or a registered securities dealer that is deemed creditworthy by the administrative agent, (ii) they terminate within one (1) business day following the date of their execution, (iii) they are denominated in U.S. dollars, and (iv) they are "collateralized fully," meaning that (A) the value of the assets collateralizing the repo (less transaction costs, including loss of interest, that the applicable Series reasonably could expect to incur if the seller were to default) is, and during the entire term of the repo remains, at least equal to the resale price payable by the seller under the repo, (B) title to the underlying collateral assets passes to the trust or, if the asset transfer is recharacterized as a secured loan, the trust will have a perfected first priority security interest in the assets securing the seller's obligations, (C) such assets are held by a custodian bank for the benefit of the applicable Series during the term of the repo, (D) such assets consist entirely of U.S. Treasury obligations, and (E) upon the insolvency of the seller, the repo would qualify under a provision of applicable insolvency law providing an exclusion from any automatic stay of creditors' rights against the seller. We collectively refer to the assets described in this paragraph as "treasury securities."
Each Series will invest its cash in treasury securities in order to generate income to pay its fees and expenses and, depending on treasury yield rates and the invested amount of the Series, to generate additional income for shareholders. Each Series will hold a portion of its assets in eligible overnight repos, because these agreements mature and convert to cash within one day, which will make it possible for the Series to have sufficient liquidity to be able to effect a Paired Optional Redemption in cash rather than by delivering treasuries. The administrative agent will initially direct the trustee to invest a maximum of 35% of the assets of a Series in eligible repos. On each subsequent Periodic Calculation Date, the administrative agent will be required to use commercially reasonable efforts to direct the investment of the funds of that Series in such a manner that the percentage of assets held in eligible repos will be equal to 10% plus the highest actual percentage of Paired PharmaShares of that Series that was redeemed on any one Redemption Date, relative to the total number of outstanding Paired PharmaShares on that Redemption Date, during the preceding twelve (12) months.
The repos which the administrative agent will select on behalf of each Series will be entered into by the trustee, on behalf of the applicable Series, acting as the "buyer," and a bank or securities dealer that will act as the "seller." The seller will transfer U.S. Treasury securities to the applicable Series in exchange for a cash payment by the Series and such seller will promise to repurchase these securities within one (1) business day following the date of the execution of the agreement. The seller must deliver to the applicable Series U.S. Treasury securities with a market value, as measured on the date of transfer and discounted by the expected transaction costs which would be incurred if that Series had to liquidate such securities following a default by the seller, that is at least equal to the repurchase price specified in such repurchase agreement. The repurchase price for the U.S. Treasury securities will be equal to the purchase price paid by the Series plus an additional amount, which will constitute the implicit interest that will be earned by the Series on the repo. Upon payment of the repurchase price, legal title to the underlying U.S. Treasury securities will be transferred back to the seller. However, the administrative agent expects to "roll-over" the cash proceeds of each day's repos into new overnight repos if these proceeds are not needed to effect redemptions. Accordingly, the U.S. Treasury securities that collateralize the repos will remain in the possession of the applicable Series until the repo arrangement with a particular seller is terminated. In the event that a seller were to default on its obligation to repurchase the U.S. Treasury securities from a Series, the trustee, acting on behalf of that Series, would be required to deliver a notice of default to the seller and, following the delivery of that notice, the trustee would be entitled to pursue any remedies permitted under the terms of the eligible repo, including retaining the underlying U.S. Treasury securities. Following a seller default, the applicable Series will have to liquidate these securities and will incur transaction costs and be exposed to market risk in connection with such liquidation. See "RISK FACTORS — Your Series may incur losses in connection with treasuries delivered upon the default of a repurchase agreement counterparty."
48
The principal terms of the eligible repos will be set forth in the Global Master Repurchase Agreement (2011 version) prepared and updated from time to time by International Capital Market Association. These terms will include (1) the delivery obligations of the seller, (2) the method of valuation of the U.S. Treasury securities that will collateralize the repo, and (3) rights and obligations of each party in the event of a default by the seller. The master agreement will be supplemented by a written confirmation setting forth the pricing terms for the repo which will be negotiated on behalf of each Series by the administrative agent. The pricing terms will consist of the maturity date of the repo, which will always be overnight, and the repurchase price or implicit yield to be earned by the applicable Series on the repo. Yield rates on repos are determined by the supply and demand for money, as reflected in the Federal funds rate, as well as the term of the repo and the creditworthiness of the seller; these rates do not depend upon the rates on the underlying U.S. Treasury securities. The administrative agent will enter into eligible repos in accordance with the acquisition guidelines described in the last two paragraphs of this section.
On each Periodic Calculation Date, except for the Final Scheduled Termination Date or an Early Termination Date, the administrative agent will direct the trustee to reinvest the proceeds received upon the maturity of the treasury securities held by each Series in new treasuries in the amount described under "DESCRIPTION OF THE PHARMASHARES — Periodic Distributions — Periodic Income Distributions." During each Calculation Period, the administrative agent will also direct the trustee to invest in treasuries all funds delivered to it in connection with each Paired Issuance and the maturity proceeds of any treasuries that mature during that Calculation Period. On the Final Redemption Date that follows the Final Scheduled Termination Date or an Early Termination Date, all of the proceeds of the treasury securities held by a Series will be used to declare the Final Distribution on each Paired PharmaShare. On any Redemption Date for all or any portion of the outstanding Paired PharmaShares that is also a Periodic Calculation Date, all or the allocable portion of the cash held by a Series will be delivered to the Authorized Participants who are redeeming Paired PharmaShares as the Final Distribution on those shares. On any Redemption Date for all or any portion of the outstanding Paired PharmaShares that is not also a Periodic Calculation Date, all or the allocable portion of the cash and/or treasuries held by a Series will be delivered to the Authorized Participants who are redeeming Paired PharmaShares as the Final Distribution on those shares. The administrative agent will select treasuries for delivery to Authorized Participants in accordance with the rule specified in Appendix B to this base prospectus.
On each Periodic Calculation Date and each Issuance Order Date for a Series, the administrative agent will use commercially reasonable efforts to identify and direct the trustee to purchase, on behalf of that Series, eligible treasury securities and eligible repos in accordance with the following acquisition guidelines which are contained in the Master Trust Agreement:
·dealers from whom each Series will purchase eligible treasury securities will be selected based on best execution;
·counterparties with whom each Series will enter into eligible repos will be selected based on best execution;
·no eligible repo may be entered into with, and no eligible treasury security may be purchased from, any person who is an Affiliated Person (as defined in Section 2(a)(3) of the United States Investment Company Act of 1940, as amended) with respect to PharmaShares, the PharmaShares Master Trust or any Series of that trust, the trustee, the administrative agent, any marketing agents or any Authorized Participant who qualifies as a statutory underwriter for the PharmaShares Master Trust; provided, that eligible repos and eligible treasury securities may be entered into with such Authorized Participants if they fall within a specified rate range of the best available yield and otherwise comply with the requirements and conditions specified for transactions with affiliated persons in Appendix B to this base prospectus; and
·a minimum of 65% of the funds of a Series during any Calculation Period will be invested in eligible treasury securities and a maximum of 35% of such funds may be invested in eligible repos during the first twelve (12) months following the date of initial issuance of Paired PharmaShares by a Series; thereafter, the administrative agent will use its commercially reasonable efforts to adjust this allocation so that the amount allocated for investment in eligible repos by the Series on each Periodic Calculation Date is equal to 10% plus the highest percentage of Paired
49
PharmaShares of that Series that was redeemed on any one Redemption Date during the preceding twelve-month period.
Although the administrative agent will use commercially reasonable efforts to direct the trustee to keep all funds held by each Series invested in treasuries, a portion of the assets of a Series may from time to time be held in the form of cash, due to (1) mismatches between the maturity profiles of treasury securities available for purchase and the length of time between Periodic Calculation Dates, and (2) longer than expected Calculation Periods that occur when a reference pharmaceutical company files its periodic report later than the date on which it customarily makes such filings following each calendar quarter. In addition, any treasury securities delivered to Authorized Participants in connection with a Paired Optional Redemption will be selected by the administrative agent on a "last in, first out" basis in accordance with the requirements set forth in Appendix B to this base prospectus. If interest rates are increasing and funds received in connection with Paired Issuances are being invested in higher-yielding treasuries, the "last in, first" out method of selection may result in the relatively higher-yielding treasuries being delivered to redeeming Authorized Participants and relatively lower-yielding treasuries remaining on deposit in a Series, thereby causing a decrease in its Daily Yield Rates. Conversely, if interest rates are decreasing and funds received in connection with Paired Issuances are being invested in lower-yielding treasuries, the "last in, first out" method of selection may result in the relatively lower-yielding treasuries being delivered to redeeming Authorized Participants. The treasury securities selected by the administrative agent to be delivered as the Final Distribution in a Paired Optional Redemption will be distributed ratably, by type, to each redeeming Authorized Participant. The treasuries will be valued at their acquisition cost plus accrued interest. In the event that the market value of the treasuries being delivered has declined since their acquisition by a Series, an Authorized Participant will receive a Final Distribution that is less than the Per Share Underlying Value of the shares it is redeeming, unless it holds the treasuries it received to their maturity. See "RISK FACTORS — The return on your shares is uncertain — Treasury securities may be delivered instead of cash in a paired optional redemption."
Daily Reporting
The calculation agent, with the assistance of the administrative agent, will calculate the Per Share Underlying Value of the Up PharmaShares and the Per Share Underlying Value of the Down PharmaShares of each Series:
·on each Publication Date, based on the Reference Value published on that day and the Projected Breakpoint for the applicable Current Calendar Quarter,
·on the Periodic Calculation Date that follows each Publication Date, after the Periodic UV Performance Distribution is declared on the Up or Down PharmaShares and both the Up and Down Underlying Value has been reduced accordingly,
·for each calendar day of the remainder of that Calculation Period, adding any Daily Net Income Accruals to the Up and Down Underlying Value,
·prior to the start of each trading day, the premium or discount of the midpoint of the bid/offer price spread for an Up PharmaShare at the close of the preceding trading day over the Per Share Underlying Value of that Up PharmaShare on that preceding day,
·prior to the start of each trading day, the premium or discount of the midpoint of the bid/offer price spread for a Down PharmaShare at the close of the preceding trading day over the Per Share Underlying Value of that Down PharmaShare on that preceding day, and
·either on the last business day preceding the first day of each calendar quarter or on or immediately following each Publication Date, as specified in the prospectus supplement for each Series, the Projected Breakpoint Increase or Projected Breakpoint Decrease for the Current Calendar Quarter.
The calculation agent will perform the underlying value calculations as soon as practicable on each Publication Date and each Periodic Calculation Date. In the case of all other business days, the calculation agent
50
will perform the underlying value calculations for that business day after its close. If that business day is followed by one or more intervening non-business days, the calculation will be made for that business day and each such intervening non-business day. After the Periodic UV Performance Distribution is declared on the Periodic Calculation Date that occurs at the beginning of each Calculation Period, the respective Underlying Values of the Up and Down PharmaShares will remain the same for the remainder of that Calculation Period except for the addition of Daily Net Income Accruals.
The administrative agent will post all calculations on the website maintained by it at http://www.pharmashares.net not later than one hour prior to the commencement of trading on the national stock exchange on which the Up PharmaShares and Down PharmaShares of each Series are listed. The Projected Breakpoint for each calendar quarter will be disclosed by means of the filing of an updated registration statement for each Series.
Information reported by or about the Reference Product or the reference pharmaceutical company during the course of each calendar quarter, including third party data and information services that provide prescription trend data on a weekly or monthly basis for various pharmaceutical products, may affect investor expectations about the change in the Underlying Value of the Up and Down PharmaShares of a Series on the next scheduled Publication Date and, as a result, the trading price of those PharmaShares during that quarter. However, such market knowledge and expectations will not be reflected in the Underlying Value calculation for any Series of PharmaShares. See "RISK FACTORS — Fluctuations in the per share underlying value of your PharmaShares and other factors may affect their trading price" in this base prospectus.
The Projected Breakpoint for each calendar quarter will be calculated and disclosed, as specified in your prospectus supplement, either (1) not later than 8 p.m. E.T. on the last business day preceding the first day of each calendar quarter or (2) on or immediately following each Publication Date, concurrently with the calculation of the Up and Down Underlying Value for the Current Calendar Quarter. The Projected Breakpoint for each calendar quarter will be disclosed by means of the filing of an updated registration statement for each Series. The following variables required to calculate the Projected Breakpoint in a Series with a Broker Reporting Group will also be disclosed: (1) the composite projection, if any, and the number of brokers whose projections were used by the market research integration service to calculate that composite (but not the projections made by each broker), (2) the identity of the individual brokers included in the Broker Reporting Group and each individual projection (but not which broker made which projection), (3) the weighting for each broker projection and for the composite projection, if any, based upon the sum of the number of individual brokers and the number of brokers whose projections were included in the composite, and (4) actual net revenues reported for the applicable Base Calendar quarter. Revised guidance from any broker who is a member of the Broker Reporting Group after the start of any calendar quarter will not be used to recalculate previously determined Projected Breakpoints.
Fees and Expenses of each Series
On each Periodic Calculation Date, treasury income of your Series will be allocated to pay the fixed and variable fees of that Series that are specified in your prospectus supplement. Ordinary and extraordinary expenses incurred by the PharmaShares Trust that affect or benefit all Series will be allocated among them based on the respective Series Investment Amount of each Series. On each Periodic Calculation Date, your Series will deposit all or a portion of its treasury income earned during the preceding Calculation Period into the fee payment account to be applied to the payment of its fees and expenses. These fees and expenses will accrue or will be incurred during each Calculation Period and will be payable in arrears on or immediately following each Periodic Calculation Date or, at the direction of the administrative agent, on any business day occurring during the Calculation Period in which they are incurred.
Each of the variable fees that accrue at an annualized rate will be calculated on the basis of the actual number of days in the current year. The dollar amount of these variable fees will depend upon the aggregate amount of assets on deposit from time to time in the applicable Series. The fees and expenses of your Series will be allocated equally among all of its outstanding Paired PharmaShares by means of the deduction from Net Treasury Income of the amount allocable to fees and expenses.
51
For so long as the Series Investment Amount on deposit in your Series is less than the minimum dollar amount specified in your prospectus supplement and treasury yields for treasury securities of the type and tenor that are permitted to be held by your Series, as described above under "— United States Treasury Obligations," are equal to or less than the average annual rate also specified in your prospectus supplement, generally equal to at least 3%, all of the treasury income of your Series will be used to pay its fixed and variable fees and expenses. During this time, the fees payable to PharmaShares Advisors, LLC, in its capacity as the administrative agent and the reimbursement of any formation expenses for your Series will be deferred. If, after such deferral, the treasury income of your Series is still insufficient to pay its remaining fees and expenses, these fees and expenses will be paid on behalf of your Series by PharmaShares Advisors, LLC. PharmaShares Advisors, LLC will seek to recoup any payments it made on behalf of your Series out of the treasury income realized by the Series on future Periodic Calculation Dates. After the dollar amount of assets invested in your Series exceeds the amount specified in your prospectus supplement for a specified period of time, PharmaShares Advisors, LLC will waive its right to seek reimbursement for past expenses it has paid on behalf of your Series.
The amount of treasury income, if available, that will be deposited into the fee payment account of your Series on each Periodic Calculation Date will be equal to the sum of, for each day of that Calculation Period, the Series Investment Amount on that day multiplied by an aggregate "Daily Fee Accrual Rate" equal to an annual rate not to exceed the percentage specified in your prospectus supplement, divided by 365 or 366, depending upon the number of days in the current year, which will reflect the sum of (1) the variable fees of your Series plus (2) the rate at which the accrued fixed fees of your Series are being reimbursed plus (3) the rate at which the administrative agent is recovering any formation expenses and unreimbursed fees previously paid by it on behalf of your Series. The Daily Fee Accrual Rate will be determined by the administrative agent at the beginning of each Calculation Period.
On any day on which the Daily Fee Accrual Rate exceeds the Daily Yield Rate on the Treasury Securities held by your Series, the full amount of treasury income earned for that day will be allocated to the payment of fees and expenses and there will be no Up Daily Net Income Accrual or Down Daily Net Income Accrual for that day.
During any period when the Series Investment Amount for your Series exceeds the dollar amount specified in your prospectus supplement or average yields for treasury securities of the type that are permitted to be held by your Series exceed the percentage specified in your prospectus supplement, your Series will pay all of its own fees and expenses. If the Daily Fee Accrual Rate determined by us for your Series for any Calculation Period would exceed the specified maximum fee cap, we will defer payment of our administrative agency fee and reimbursement amounts due to us such that the cap is not exceeded.
The trustee will receive transaction fees in connection with Paired Issuances and Paired Optional Redemptions. See "DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions" and "— Paired Issuances."
For information about the specific fees and expenses payable by your Series, see "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
DESCRIPTION OF THE PHARMASHARES
General
Each Up PharmaShare will have an initial stated par amount per share that will be disclosed in your prospectus supplement. The initial par amount per Up PharmaShare and the Up Aggregate Par Amount will decrease with each Periodic UV Performance Distribution that is made by your Series. The Up Aggregate Par Amount will also decrease with each Paired Optional Redemption and increase with each Paired Issuance. The entitlement of each holder of Up PharmaShares to any Periodic Distributions and the Final Distribution made by the related Series on its Up PharmaShares will be based on the Aggregate Par Amount of Up PharmaShares held by that holder and the proportion that this number bears to the Up Aggregate Par Amount of Up PharmaShares issued by that Series and outstanding on the relevant Periodic Calculation Date, Redemption Order Date, Early Termination Date or the Final Scheduled Termination Date.
52
Each Down PharmaShare will have an initial stated par amount per share that will be disclosed in your prospectus supplement. The initial par amount per Down PharmaShare and the Down Aggregate Par Amount will decrease with each Periodic UV Performance Distribution that is made by your Series. The Down Aggregate Par Amount will also decrease with each Paired Optional Redemption and increase with each Paired Issuance. The entitlement of each holder of Down PharmaShares to any Periodic Distributions and the Final Distribution made by the related Series on its Down PharmaShares will be based on the Aggregate Par Amount of Down PharmaShares held by that holder and the proportion that this number bears to the Down Aggregate Par Amount of Down PharmaShares issued by that Series and outstanding on the relevant Periodic Calculation Date, Redemption Order Date, Early Termination Date or the Final Scheduled Termination Date.
The Up PharmaShares and Down PharmaShares of each Series will be issued pursuant to a Series Trust Supplement to the Master Trust Agreement. Each Up PharmaShare and each Down PharmaShare will represent an undivided beneficial interest in the related Series. The Up PharmaShares and Down PharmaShares of each Series will not represent interests in or obligations of the administrative agent, the trustee, the calculation agent, the marketing agent, any Authorized Participant or any of our or their respective affiliates.
The Up PharmaShares and Down PharmaShares of each Series will be denominated in, and all distributions with respect to those shares will be payable in, United States dollars. No holding or transfer restrictions or minimum lot requirements will be applicable to the Up PharmaShares or Down PharmaShares of any Series.
The Up Underlying Value and Down Underlying Value will determine the amount of Periodic Distributions and the Final Distribution that are made on the Up PharmaShares and Down PharmaShares of a Series.
The PharmaShares Trust will declare Periodic UV Performance Distributions on each Periodic Calculation Date for the class of PharmaShares which has experienced an increase in its Underlying Value, based on the Reference Value published on the last Publication Date that precedes the applicable Periodic Calculation Date compared to the Projected Breakpoint for the preceding calendar quarter.
The PharmaShares Trust will make a Final Distribution on the Up PharmaShares and Down PharmaShares of a Series, which will be equal to the respective Underlying Values of those shares, based on the Reference Value measured on the Publication Date that precedes the applicable Redemption Order Date, Early Termination Date or the Final Scheduled Termination Date compared to the Projected Breakpoint for the preceding calendar quarter.
The PharmaShares Trust will make Periodic Income Distributions of the Net Treasury Income of a Series which may be distributed equally among the holders of the Up and Down PharmaShares of that Series or it may be allocated based upon the Up and Down Underlying Value, as specified in your prospectus supplement.
The respective Underlying Values of the Up and Down PharmaShares of a Series are calculated on each calendar day based upon the Reference Value published on the most recent Publication Date compared to the Projected Breakpoint for the preceding calendar quarter and represents the aggregate amount of the assets on deposit in that Series to which the holders of the Up PharmaShares and the Down PharmaShares, respectively, would be entitled if a Final Distribution were to be made on that calendar day.
Underlying Value for the Up PharmaShares and the Down PharmaShares of each Series will be calculated by comparing
·the Actual Percentage Change in net revenue generated by the Reference Product for your Series, expressed as a percentage change from a past Base Calendar Quarter to the Current Calendar Quarter based upon either year-over-year tracking or sequential tracking (or some other tracking method), to
·a Projected Breakpoint Increase or Projected Breakpoint Decrease that relates to the same Base Calendar Quarter and Current Calendar Quarter.
The Reference Value for each Series will always be published after the Current Calendar Quarter has ended. As a result, when calculating Underlying Value, reference is made to the Projected Breakpoint for the preceding calendar quarter. This “preceding” calendar quarter is the same as the defined Current Calendar Quarter.
53
The Projected Breakpoint
The "Projected Breakpoint Increase" or "Projected Breakpoint Decrease" (also generically referred to as the "Projected Breakpoint") is the percentage increase or percentage decrease, as applicable, calculated by dividing (1) the absolute value of the difference between projected net revenues for the Reference Product for the Current Calendar Quarter and actual net revenues for the Base Calendar Quarter by (2) actual net revenues for the Base Calendar Quarter. The projected net revenues are calculated based upon the net revenue projections of a Broker Reporting Group or determined by means of a Projected Breakpoint Auction Reset, as specified in your prospectus supplement.
Broker Reporting Group
A Series may use a Broker Reporting Group to establish the Projected Breakpoint, if so specified in your prospectus supplement.
The "Broker Reporting Group" for a Series of PharmaShares will consist of a group of investment banks, asset managers and other broker-dealers registered with FINRA, all of whom are generically referred to as "brokers," that employ analysts who publish net revenue projections for the Reference Product. A Broker Reporting Group may include (1) individual brokers selected by us, in our capacity as administrative agent, whose projections are published on Bloomberg or a similar financial data service available by subscription, (2) a composite projection number generated by a third-party service that aggregates and integrates the market research and models provided to it by many different brokers, such as Visible Alpha, FactSet or a similar service specified in your prospectus supplement and referred to as a “market research integration service,” or (3) a combination of individual brokers and a composite projection number. The broker revenue projections will be obtained from research reports released by each individual broker or, in the case of the composite projection, generated by the market research integration service, on the most recent date that precedes but is not less than two (2) business days prior to the “BRG projected revenue determination date” which will be either (1) the first day of each calendar quarter or (2) each Publication Date for the Reference Product for your Series, as specified in your prospectus supplement.
A Broker Reporting Group, the members of which will be selected by us, in our capacity as the administrative agent, will have the following characteristics:
·it may consist of a composite projection number published by a market research integration service so long as such service considers the projections of a minimum of five (5) brokers in generating the composite for a Reference Product; and, further, for each calendar quarter, the service must calculate the composite using not less than three (3) projections from the various brokers included in its broker group; if there are less than five (5) brokers in the Broker Reporting Group or less than three (3) projections are used in any composite calculation, then the Underlying Values of the Paired PharmaShares will remain unchanged at the end of the affected calendar quarter;
·the identities of the entire group of eligible brokers whose projections may be used by the market research integration service to generate the composite number for the Reference Product will be disclosed in your prospectus supplement; the projections of only a subset of these brokers may be included in any composite for any particular calendar quarter, because not all brokers in the group may publish a projection for that quarter, and the number of brokers in this subset (but not the identity of those brokers) will be disclosed, along with the Projected Breakpoint, in an updated prospectus supplement for your Series; the market research integration service may, in its sole discretion, add, remove and substitute brokers from the group whose projections it uses to generate the composite at any time; any such change to the group of eligible brokers used to calculate the composite will be disclosed in an updated prospectus supplement for your Series;
·it may also include, in addition to the composite number, any number of individual brokers whose projections are not used in the composite number by the market research integration service but
54
who employ analysts publishing projections for the Reference Product; each individual broker will be identified in your prospectus supplement and will be included in the Broker Reporting Group until the Final Scheduled Maturity Date of your Series, unless the broker ceases to employ analysts who publish projections for the Reference Product; individual brokers in a Broker Reporting Group that includes a composite number need not publish projections for every consecutive calendar quarter to be part of the group, but any quarterly projections they do publish must be included in the calculation of the Projected Breakpoint for the relevant quarter;
·it may consist of individual brokers only, so long as there are not less than three (3) such brokers and each of them has been publishing quarterly projections for the Reference Product for at least the last four consecutive calendar quarters; each individual broker will be identified in your prospectus supplement and will be included in the Broker Reporting Group until the Final Scheduled Maturity Date of your Series, unless the broker ceases to employ analysts who publish projections for the Reference Product or ceases publishing projections not less frequently than quarterly; if an individual broker in a Broker Reporting Group composed exclusively of individual brokers is removed because its analysts either cease tracking the Reference Product or cease publishing quarterly net revenue projections for the Reference Product, that broker will be reinstated if it employs a new analyst who resumes publishing quarterly revenue projections for the Reference Product within four calendar quarters of the broker’s removal; if there are fewer than three (3) individual brokers in the group or less than three (3) projections are published by the brokers for any calendar quarter, then the Underlying Values of the paired PharmaShares of the applicable Series will remain unchanged at the end of the affected calendar quarter;
·the composite projection for a calendar quarter and/or each projection for a calendar quarter made by an individual broker who is part of the Broker Reporting Group must be included in the calculation of the Projected Breakpoint for that calendar quarter; the calculation agent will source the composite projection from the market research integration service and each projection, if any, that was made by an individual member of the Broker Reporting Group, from Bloomberg or a similar financial data service; and
·in the event that, with respect to more than one calculation period, an individual broker stops publishing quarterly projections or the composite projection number is no longer generated by the market research integration service or otherwise becomes unavailable and there are less than the required minimum number of brokers remaining in the Broker Reporting Group or less than three (3) projections are available for any calendar quarter, the administrative agent may select a replacement market research integration service and/or individual brokers to replace brokers who have ceased publishing projections and who meet the criteria for inclusion in the Broker Reporting Group discussed above; if such replacements cannot be made or the administrative agent elects not to make them, a Termination Trigger will occur within the number of publication dates specified in your prospectus supplement.
No broker acting as an Authorized Participant for a Series of PharmaShares or any of its affiliates may be selected as an individual broker, but any such related broker may be included in a composite projection that is determined using the calculations of a group of at least five (5) or more brokers, including such Authorized Participants. No authorized participant has any obligation to consider the interests of the holders of Up or Down PharmaShares of any Series in making projections for any Reference Product. See “RISK FACTORS — Broker dealers who act as authorized participants for your Series may make projections for your Reference Product.”
The same Broker Reporting Group will be used throughout the term of a Series, except that replacements may be made as described above. The same methodology for calculating the composite number and the average of the net revenue projections of the Broker Reporting Group, as described in the next section, will be used throughout the term of a Series.
Average Revenue Projection Methodology for Broker Reporting Groups
55
The market research integration service that generates the composite will calculate and publish the high and low broker projections, the median of the broker projections (both with the high and low projections included and after removing those projections), the mean of the broker projections (both with the high and low projections included and after removing those projections), and the standard deviation of the broker projections. In the case of a Broker Reporting Group that includes only a composite projection, the average revenue projection number that will be used to calculate the Projected Breakpoint will be the mean or median of the broker projections, calculated by the market research integration service after the high and low projections are eliminated. The prospectus supplement for your Series will specify whether the composite mean or median will be used.
In the case of any Broker Reporting Group that includes both a composite projection and individual brokers, the calculation agent will, first, identify the composite mean or median calculated by the market research integration service, as described above and specified in your prospectus supplement, and, second, calculate the weighted, arithmetic mean of the projected revenues for the entire Broker Reporting Group. Each broker, including each broker included in the composite mean or median number, after removal of the high and low projections, will be weighted as “1.” For example, if 10 brokers are included in the composite number and there are 2 individual brokers, the composite number will have a weighting of 10/12 and each individual broker will have a weighting of 1/12.
In the case of any Broker Reporting Group that includes only individual brokers, the calculation agent will calculate the arithmetic mean of the projections published by each broker in the group. In the case of any Broker Reporting Group with only the minimum three (3) individual brokers (and no composite projection), the calculation agent will calculate, for each calendar quarter or year, the arithmetic mean of the projections subject to a cap and a floor equal to a 20% deviation from the mean, in the following manner:
·first, the arithmetic mean is computed;
·second, the highest projection is excluded and an adjusted mean of the two remaining projections is computed. The highest projection is then reduced to equal a number that represents not more than a 20% positive variance from the adjusted mean;
·third, the lowest projection is excluded and an adjusted mean of the two remaining projections is computed. The lowest projection is then increased to equal to a number that represents not more than a 20% negative variance from the adjusted mean; and
·fourth, the arithmetic mean of the projections, with the highest and lowest projections adjusted as described above, is recalculated.
Breakpoint Auction Reset
A Series may employ a public auction to determine the Projected Breakpoint if so specified in your prospectus supplement. Auctions will be held at 8:00 am ET on the “auction date” which will be specified in your prospectus supplement and which may occur on either (1) the date that is two business days prior to the first day of trading for each calendar quarter or (2) the first business day following each Publication Date for a Series. Bids may be submitted electronically beginning 5 business days prior to the applicable auction date. Bids may not be withdrawn after 6:00 am ET on the applicable auction date and bidders will be notified of results by 9:00 am ET on the auction date. The auction process is referred to as a “Breakpoint Auction Reset.”
Only existing holders of Up or Down PharmaShares and committed bidders for Up or Down PharmaShares may participate in the Breakpoint Auction Reset, but no existing holder will be obligated to participate. A qualified bid must include the number of Up PharmaShares or Down PharmaShares the bidder is committing to acquire. Each participating existing holder and each committed bidder must submit its maximum and minimum projected net revenue estimates for the Reference Product for the upcoming calendar quarter. The range between the maximum and minimum projected net revenue estimates may not exceed the percentage specified in your prospectus supplement.
56
Each bidder will be committing to purchase the Up or Down PharmaShares for which it is bidding so long as the projected net revenues for the upcoming calendar quarter determined via the Breakpoint Auction Reset is within the range of the maximum and minimum projected net revenues specified by the bidder. Each bidder will be committing to purchase Up or Down PharmaShares at the Per Share Underlying Value of those shares, as determined on the Publication Date immediately preceding the auction date or at the Per Share Underlying Value prevailing during the current Calculation Period, in the case of an auction date occurring on the first trading day of a calendar quarter. Committed bidders must deposit funds equal to the aggregate Underlying Value of the Up or Down PharmaShares for which it is bidding with a designated escrow agent in accordance with the procedures of their broker or an Authorized Participant. Escrowed funds related to bids that were rejected because the projected revenue number set by the Breakpoint Auction Reset is outside of the range of projected net revenues specified by the bidder will be returned in accordance with the procedures established by each bidder’s broker or the applicable Authorized Participant.
One of the Authorized Participants will be designated as an auction agent and will pair all bids for Up PharmaShares with bids for Down PharmaShares. The escrowed funds relating to accepted bids will be delivered to the PharmaShares Trust, which will issue Up and Down PharmaShares to the Authorized Participants who obtained those bids.
The calculation agent will use the midpoint of the range of projected net revenues submitted by each committed bidder for Up PharmaShares and each participating existing holder of Up PharmaShares, to calculate the weighted arithmetic mean of all submissions made with respect to the Up PharmaShares of a Series, based upon the number of shares owned by an existing holder or bid for by a committed bidder. The calculation agent will use the midpoint of the range of projected net revenues submitted by each committed bidder for Down PharmaShares and each participating existing holder of Down PharmaShares, to calculate the weighted arithmetic mean of all submissions made with respect to the Down PharmaShares of a Series, based upon the number of shares owned by an existing holder or bid for by a committed bidder. Finally, the calculation agent will calculate the projected net revenue for the upcoming calendar quarter as the midpoint of the median of the Up submissions and the median of the Down submissions. The projected net revenue number determined for a calendar quarter by means of the Breakpoint Auction Reset will then be used to calculate the Projected Breakpoint for that quarter.
Reporting
The Projected Breakpoint Increase or Decrease for each calendar quarter will be calculated and announced not later than 8 p.m. EST on the auction date or the BRG projected revenue determination date by means of the filing of an updated registration statement pursuant to Rule 424(b) of the Securities Act. Revised guidance from any broker who is a member of the Broker Reporting Group after the start of any calendar quarter will not be used to recalculate previously determined Projected Breakpoints. The following inputs required to calculate the Projected Breakpoint and related inputs will also be disclosed:
·the identity of each individual broker included in the Broker Reporting Group, the identity of all of the brokers whose projections may be used by the market research integration service to generate the composite and the number of brokers in the subset of brokers whose projections are actually included in the composite projection for the current calendar quarter (but not the identity of those brokers);
·the composite projection published by the market research integration service (but not the individual projections included in that composite) and the projection that was published by each individual broker member of the Broker Reporting Group (but not which individual broker published which particular projection);
·the weighting for each broker projection and for the composite projection based upon the total number of individual brokers included in the Broker Reporting Group and the number of brokers whose projections were used to calculate the composite for the Current Calendar Quarter;
57
·with respect to a Breakpoint Auction Reset, the weighted arithmetic mean of all submissions made with respect to the Up PharmaShares of a Series, the arithmetic mean of all submission made with respect to the Down PharmaShares of the same Series, and the midpoint of the median of the Up submissions and the median of the Down submissions; and
·actual net revenues reported for the applicable base Calendar Quarter.
Calculation of Underlying Value
To calculate Underlying Value on any date of determination, the Reference Value for the Current Calendar Quarter is compared against (a) the Reference Value for the immediately preceding quarter in a sequential tracking Series, (b) the same quarter of the preceding year in a year-over-year tracking Series or (c) such other Base Calendar Quarter as may be specified in your prospectus supplement. The resulting Actual Percentage Change in Reference Value is then compared against the Projected Breakpoint Increase or Projected Breakpoint Decrease that applies to the Current Calendar Quarter.
If a Projected Breakpoint Increase is specified for the Current Calendar Quarter, because the Reference Value was projected to increase compared to the Reference Value for the applicable Base Calendar Quarter, then
–an increase in the Reference Value for the Current Calendar Quarter relative to the Reference Value for the Base Calendar Quarter that is greater than this Projected Breakpoint Increase will result in an increase in the Underlying Value of the Up PharmaShares and a corresponding decrease in the Underlying Value of the Down PharmaShares of a Series; and
–an increase in the Reference Value that is less than the Projected Breakpoint Increase or any decrease in the Reference Value relative to the Reference Value for the Base Calendar Quarter will result in a decrease in the Underlying Value of the Up PharmaShares and a corresponding increase in the Underlying Value of the Down PharmaShares of a Series.
If a Projected Breakpoint Decrease is specified for the Current Calendar Quarter, because the Reference Value was projected to decrease compared to the Reference Value for the applicable Base Calendar Quarter, then
–a decrease in the Reference Value relative to the Reference Value for the Base Calendar Quarter that is greater than this Projected Breakpoint Decrease will result in an increase in the Underlying Value of the Down PharmaShares and a corresponding decrease in the Underlying Value of the Up PharmaShares of a Series; and
–a decrease in the Reference Value that is less than the Projected Breakpoint Decrease or any increase in the Reference Value relative to the Reference Value for the Base Calendar Quarter will result in a decrease in the Underlying Value of the Down PharmaShares and a corresponding increase in the Underlying Value of the Up PharmaShares of a Series.
If the rise or fall in Reference Value for any Current Calendar Quarter relative to the Base Calendar Quarter is equal to the Projected Breakpoint Increase or Projected Breakpoint Decrease for the Current Calendar Quarter, there will be no change in the Underlying Value of the Up or Down PharmaShares of a Series, other than for the addition to that Underlying Value of any Up and Down Daily Net Income Accruals.
The Underlying Value calculation will be adjusted by any Leverage Factors and Periodic Return Caps applicable to returns on the Up PharmaShares and Down PharmaShares of a Series, as described below.
For some Series, the calculation of Underlying Value will be a two-step process in which it will first be determined whether the Actual Percentage Change in Reference Value, as defined below, has exceeded the
58
Projected Breakpoint Increase or Projected Breakpoint Decrease and, if it has, then, either (1) Underlying Value will be calculated by subtracting from the Actual Percentage Change in revenue the Projected Breakpoint Increase or Projected Breakpoint Decrease such that the Underlying Value reflects only the net change or (2) Underlying Value will be calculated based on the full Actual Percentage Change in revenue, not merely the net change. The details of the calculations that will be made for such a Series will be specified in your prospectus supplement.
If certain major disruptions occur during the course of a calendar quarter and materially affect sales of the Reference Product, the change in Reference Value for the two relevant calendar quarters will be deemed to be zero. Events that qualify as major market disruptions are described under "REFERENCE PRODUCTS AND REFERENCE VALUES" in this base prospectus.
"Underlying Value" for the Up PharmaShares of each Series, also referred to as the "Up Underlying Value," is calculated on each calendar day of a Calculation Period for that Series as follows:
–the "Up Investment Amount" multiplied by the "Up Allocation Factor" that was calculated based on the Reference Value published on the most recent Publication Date relative to the Projected Breakpoint for the preceding calendar quarter
minus
–if the Up PharmaShares experienced an increase in the Up Underlying Value on the last Publication Date, the amount of the Equalization Payment distributed as part of the Periodic UV Performance Distribution made on the Up PharmaShares on the Periodic Calculation Date that followed that Publication Date and, if the Up PharmaShares experienced a decrease in the Up Underlying Value on the last Publication Date, the amount of such decrease
plus
–the sum of the "Up Daily Net Income Accruals" for each day that has elapsed during the current Calculation Period, up to and including the current calendar day.
"Underlying Value" for the Down PharmaShares of each Series, also referred to as the "Down Underlying Value," is calculated on each calendar day of a Calculation Period for that Series as follows:
–the "Down Investment Amount" multiplied by the "Down Allocation Factor" that was calculated based on the Reference Value published on the most recent Publication Date relative to the Projected Breakpoint for the preceding calendar quarter
minus
–if the Down PharmaShares experienced an increase in the Down Underlying Value on the last Publication Date, the amount of the Equalization Payment distributed as part of the Periodic UV Performance Distribution made on the Down PharmaShares on the Periodic Calculation Date that followed that Publication Date and, if the Down PharmaShares experienced a decrease in the Down Underlying Value on the last Publication Date, the amount of such decrease
plus
–the sum of the "Down Daily Net Income Accruals" for each day that has elapsed during the current Calculation Period, up to and including the current calendar day.
The terms used in the Underlying Value definition have the following meanings:
The "Up Investment Amount" will generally be equal to the Aggregate Par Amount of the Up PharmaShares. The "Down Investment Amount" will generally be equal to the Aggregate Par Amount of the Down
59
PharmaShares. If a Series suffers any loses on its treasury securities, its Up and Down Investment Amount will fall below the Up and Down Aggregate Par Amount, respectively, and this loss will be reflected in the Up and Down Underlying Value calculation. See "RISK FACTORS — Your Series may incur losses in connection with treasuries delivered upon the default of a repurchase agreement counterparty."
The Up Investment Amount and Down Investment Amount will be reduced by redemptions as well as by distributions to shareholders of increases in the Up Underlying Value or the Down Underlying Value, as described below under "— Periodic Distributions." Reductions in the Up Investment Amount and Down Investment Amount will reduce the amount of your investment and your potential gains, as discussed under "RISK FACTORS — The amount of your investment in your PharmaShares and your potential gains will decline over time." Purchasing additional Up PharmaShares or Down PharmaShares is the only means by which you can maintain a constant level of investment in your PharmaShares.
For each day of a current Calculation Period, the Up and Down PharmaShares of a Series will be entitled to receive the Net Treasury Income accrued for that day on the treasury securities held by that Series. The entitlement of the Up PharmaShares and Down PharmaShares of a Series to the Net Treasury Income of that Series may be adjusted by an allocation rule specified in your prospectus supplement. We refer to the daily income additions to Underlying Value as the "Up Daily Net Income Accruals" and the "Down Daily Net Income Accruals." These terms are defined in greater detail in the "Glossary" in this base prospectus.
The calculation of the Up Allocation Factor and the Down Allocation Factor is illustrated in the sample Underlying Value calculation that follows.
The following calculations illustrate the impact on Up Underlying Value and Down Underlying Value that occurs in six possible scenarios: (1) an increase in Reference Value that is greater than the Projected Breakpoint Increase; (2) an increase in Reference Value that is less than the Projected Breakpoint Increase; (3) a decrease in Reference Value during a quarter with a Projected Breakpoint Increase; (4) a decrease in Reference Value that is greater than the Projected Breakpoint Decrease; (5) a decrease in Reference Value that is less than the Projected Breakpoint Decrease; and (6) an increase in Reference Value during a quarter with a Projected Breakpoint Decrease.
The sample calculations of Underlying Value are based on a Series in which:
•a 1.5x leverage factor applies to returns on the Up PharmaShares,
•a 2x leverage factor applies to returns on the Down PharmaShares,
•a 15% periodic return cap applies to returns on the Up PharmaShares,
•an 18% periodic return cap applies to returns on the Down PharmaShares, and
•the Up/Down Ratio of the Up Investment Amount and the Down Investment Amount is 1:1.
If a Projected Breakpoint Increase was predicted for a particular calendar quarter, then the change in the Up Underlying Value and the Down Underlying Value will be calculated on the Publication Date that follows the end of that particular calendar quarter as follows:
·if the "Actual Percentage Change," equal to the absolute value of the difference between the Reference Value for the Current Calendar Quarter (RVt), as measured on the Publication Date that follows the end of that calendar quarter and the Reference Value for the Base Calendar Quarter (RV0), divided by the Reference Value for the Base Calendar Quarter (RV0), is greater than the Projected Breakpoint Increase, the Up Underlying Value will increase and the Down Underlying Value will decrease as follows:
For a calendar quarter in which the Projected Breakpoint Increase is 10%, if RVt is $1.3 billion and RV0 is $1 billion, resulting in an Actual Percentage Change of 30%, then the change in the Up Underlying Value would be:
UUVΔ = (Actual Percentage Change – Projected Breakpoint Increase)
UUVΔ = (0.30 – 0.10) = .20 or 20%
60
In this example, as a result of the 30% increase in the Reference Value, the Underlying Value of the Up PharmaShares would increase by 20%.
Applying the Up Leverage Factor of 1.5x would increase the Up Underlying Value change to 30% and then applying the 15% Up Periodic Return Cap would reduce the Up Underlying Value change to 15%.
On this Publication Date, there would be a 15% increase in the Up Underlying Value and a corresponding decrease of 15% in the Down Underlying Value. For purposes of calculating Underlying Value on each day of the Calculation Period that follows this Publication Date, the Up Allocation Factor would be 1 + the Up Underlying Value increase, or 1.15, and the Down Allocation Factor would be 1 – the Down Underlying Value decrease, or 0.85.
This increase in the Up Underlying Value, together with the related Equalization Payment, would be distributed to the holders of the Up PharmaShares on the second business day after the Periodic Calculation Date that follows this Publication Date.
·If the Actual Percentage Change reflects an increase that is less than the Projected Breakpoint Increase, the Down Underlying Value will increase and the Up Underlying Value will decrease as follows:
In the same example as above, if the RVt is $1.02 billion and RV0 is $1 billion, resulting in an Actual Percentage Change of 2%, then the change in the Down Underlying Value would be:
DUVΔ = (Projected Breakpoint Increase – Actual Percentage Change)
DUVΔ = (0.10 – 0.02) = 0.08 or 8%
In this example, as a result of the 2% increase in the Reference Value, which is less than the Projected Breakpoint Increase, the Underlying Value of the Down PharmaShares would increase by 8%.
Applying the Down Leverage Factor of 2x would increase the Down Underlying Value change to 16%; applying the 18% Down Periodic Return Cap would have no effect, so the Down Underlying Value change would be 16%.
On this Publication Date, there would be a 16% increase in the Down Underlying Value and a corresponding decrease of 16% in the Up Underlying Value. For purposes of calculating Underlying Value on each day of the Calculation Period that follows this Publication Date, the Down Allocation Factor would be 1 + the Down Underlying Value increase, or 1.16, and the Up Allocation Factor would be 1 – the Up Underlying Value decrease, or 0.84.
This increase in the Down Underlying Value, together with the related Equalization Payment, would be distributed to the holders of the Down PharmaShares on the second business day after the Periodic Calculation Date that follows this Publication Date.
·If the Actual Percentage Change reflects a decrease in the Reference Value over the Base Calendar Quarter when there is a Projected Breakpoint Increase, the Down Underlying Value will increase and the Up Underlying Value will decrease as follows:
In the same example as above, if the RVt is $0.9 billion and RV0 is $1 billion resulting in an Actual Percentage Change of 10%, then the change in the Down Underlying Value would be:
DUVΔ = (Actual Percentage Change + Projected Breakpoint Increase)
61
DUVΔ = (0.10 + 0.10) = 0.20 or 20%
In this example, as a result of the 10% decrease in the Reference Value, the Underlying Value of the Down PharmaShares would increase by 20%.
Applying the Down Leverage Factor of 2x would increase the Down Underlying Value change to 40% and then applying the 18% Down Periodic Return Cap would reduce the Down Underlying Value change to 18%.
On this Publication Date, there would be an 18% increase in the Down Underlying Value and a corresponding decrease of 18% in the Up Underlying Value. For purposes of calculating Underlying Value on each day of the Calculation Period that follows this Publication Date, the Down Allocation Factor would be 1 + the Down Underlying Value increase, or 1.18, and the Up Allocation Factor would be 1 – the Up Underlying Value decrease, or 0.82.
This increase in the Down Underlying Value, together with the related Equalization Payment, would be distributed to the holders of the Down PharmaShares on the second business day after the Periodic Calculation Date that follows this Publication Date.
If a Projected Breakpoint Decrease was predicted for a particular calendar quarter, then the change in the Up Underlying Value and the Down Underlying Value will be calculated on the Publication Date that follows the end of that particular calendar quarter as follows:
·If the Actual Percentage Change reflects a decrease that is greater than the Projected Breakpoint Decrease, the Down Underlying Value will increase and the Up Underlying Value will decrease as follows:
For a calendar quarter in which the Projected Breakpoint Decrease is 10%, if the RVt is $0.87 billion and RV0 is $1 billion resulting in an Actual Percentage Change of 13%, then the change in the Down Underlying Value would be:
DUVΔ = (Actual Percentage Change – Projected Breakpoint Decrease)
DUVΔ = (0.13 – 0.10) = 0.03 or 3%
In this example, as a result of the 13% decrease in the Reference Value, which is 3% in excess of the Projected Breakpoint Decrease, the Underlying Value of the Down PharmaShares would increase by 3%.
Applying the Down Leverage Factor of 2x would increase the Down Underlying Value change to 6%; applying the 18% Down Periodic Return Cap would have no effect, so the Down Underlying Value change would be 6%.
On this Publication Date, there would be a 6% increase in the Down Underlying Value and a corresponding decrease of 6% in the Up Underlying Value. For purposes of calculating Underlying Value on each day of the Calculation Period that follows this Publication Date, the Down Allocation Factor would be 1 + the Down Underlying Value increase, or 1.06, and the Up Allocation Factor would be 1 – the Up Underlying Value decrease, or 0.94.
This increase in the Down Underlying Value, together with the related Equalization Payment, would be distributed to the holders of the Down PharmaShares on the second business day after the Periodic Calculation Date that follows this Publication Date.
·If the Actual Percentage Change reflects a decrease that is less than the Projected Breakpoint Decrease, the Up Underlying Value will increase and the Down Underlying Value will decrease as follows:
62
In the same example as above, if the RVt is $0.93 billion and RV0 is $1 billion resulting in an Actual Percentage Change of 4% decrease, then the change in the Up Underlying Value would be:
UUVΔ = (Projected Breakpoint Decrease – Actual Percentage Change)
UUVΔ = (0.10 – 0.04) = 0.06 or 6%
In this example, as a result of the 4% decrease in the Reference Value, which is 6% less than the Projected Breakpoint Decrease, the Underlying Value of the Up PharmaShares would increase by 6%.
Applying the Up Leverage Factor of 1.5x would increase the Up Underlying Value change to 9%; then applying the 15% Up Periodic Return Cap would have no effect, so the Up Underlying Value change would be 9%.
On this Publication Date, there would be a 9% increase in the Up Underlying Value and a corresponding decrease of 9% in the Down Underlying Value. For purposes of calculating Underlying Value on each day of the Calculation Period that follows this Publication Date, the Up Allocation Factor would be 1 + the Up Underlying Value increase, or 1.09, and the Down Allocation Factor would be 1 – the Down Underlying Value decrease, or 0.91.
This increase in the Up Underlying Value, together with the related Equalization Payment, would be distributed to the holders of the Up PharmaShares on the second business day after the Periodic Calculation Date that follows this Publication Date.
·If the Actual Percentage Change reflects an increase in the Reference Value over the Reference Value of the Base Calendar Quarter when there is a Projected Breakpoint Decrease, the Up Underlying Value will increase and the Down Underlying Value will decrease as follows:
In the same example as above, if the RVt is $1.04 billion and RV0 is $1 billion resulting in an Actual Percentage Change of 104% (or a 4% increase), then the change in the Up Underlying Value would be:
UUVΔ = (Actual Percentage Change + Projected Breakpoint Decrease)
UUVΔ = (0.04 + 0.10) = 0.14 or 14%
In this example, as a result of the 4% increase in the Reference Value, the Underlying Value of the Up PharmaShares would increase by 14%.
Applying the Up Leverage Factor of 1.5x would increase the Up Underlying Value to 21%, and then applying the 15% Up Periodic Return Cap would reduce the Up Underlying Value to 15%.
On this Publication Date, there would be a 15% increase in the Up Underlying Value and a corresponding decrease of 15% in the Down Underlying Value. For purposes of calculating Underlying Value on each day of the Calculation Period that follows this Publication Date, the Up Allocation Factor would be 1 + the Up Underlying Value increase, or 1.15, and the Down Allocation Factor would be 1 – the Down Underlying Value decrease, or 0.85.
This increase in the Up Underlying Value, together with the related Equalization Payment, would be distributed to the holders of the Up PharmaShares on the second business day after the Periodic Calculation Date that follows this Publication Date.
63
A synthetic ratchet, referred to as a "Leverage Factor," of between 0.5x and 3x may apply to the returns on the Up PharmaShares and/or the paired Down PharmaShares of a Series for each Calculation Period. Your prospectus supplement will specify the Leverage Factor, if any, that applies to the Up and/or the Down PharmaShares of your Series. Your prospectus supplement will also specify any cap that applies to the periodic returns on the Up and/or the Down PharmaShares of your Series, referred to as a "Periodic Return Cap." The amount of a Periodic Return Cap, if any, will depend upon past and projected changes in net revenues, with higher Periodic Return Caps generally used for Reference Products the sales of which have in the past experienced, and/or which are projected by the Broker Reporting Group to experience, significant future fluctuations. As indicated in the preceding examples, Underlying Value will be calculated in the following order:
first,the Actual Percentage Change is calculated;
second,the Actual Percentage Change is compared against the Projected Breakpoint to determine which class has experienced an Underlying Value increase and the amount of that increase is calculated using one of the formulas specified in the preceding examples;
third,the percentage change in Underlying Value of the class of PharmaShares that has experienced an increase in its Underlying Value is multiplied by the applicable Leverage Factor for that class, if any;
fourth,the applicable Periodic Return Cap, if any, is applied to adjust the resulting leveraged increase in Underlying Value such that the increase does not exceed that cap; and
finally, the Up and Down Allocation Factors are determined based on the leveraged and capped Underlying Value increase of the applicable class of PharmaShares.
If so described in your prospectus supplement, any applicable Leverage Factor and Periodic Return Cap may be adjusted during the term of your Series in the event that net revenues or the percentage change in net revenues over a specified period of time exceeds or falls below a specified range also disclosed in your prospectus supplement. Please see "RISK FACTORS — The return on your shares is uncertain — The return on some Series of PharmaShares will be capped," and "— A Series may terminate early" in this base prospectus.
We refer to the period between Publication Dates, beginning on (but excluding) the preceding Publication Date and ending on (and including) the current Publication Date, as a "Calculation Period." On each Publication Date, the Reference Value will be published and a new Up Underlying Value and Down Underlying Value will be determined. On the Periodic Calculation Date that follows each Publication Date, the increase in the Up or Down Underlying Value and the related Equalization Payment will be declared as a Periodic UV Performance Distribution. After this distribution is made, the Up and Down Underlying Value will remain the same throughout the remainder of the Calculation Period, other than for the addition of Up Daily Net Income Accruals and Down Daily Net Income Accruals.
The initial launch of a Series of PharmaShares may not coincide with the beginning of a calendar quarter or a Publication Date, which may result in an initial period during which the respective Underlying Values of the Paired PharmaShares diverge significantly from the trading prices of those shares. Further, reference pharmaceutical companies may not publish the relevant Reference Value on the same Publication Date following each calendar quarter and, as a result, the length of each Calculation Period may vary.
The initial proportion of the Up Investment Amount to the Down Investment Amount for each Series, or its "Up/Down Ratio," will be 1:1, or such other proportion as may be specified in your prospectus supplement, without regard to the respective initial public offering prices or subsequent trading prices of the Up and Down PharmaShares of any Series. This initial Up/Down Ratio will be maintained for so long as the PharmaShares of a Series are outstanding by virtue of the requirements that:
(1)when the Up or Down PharmaShares receive a distribution of any increase in their Underlying Value, that class of PharmaShares will also receive an Equalization Payment that will restore the Up/Down Ratio;
64
(2)all redemptions and issuances must be effected in PharmaShares Units composed of an equal Aggregate Par Amount (or other specified proportion) of Up PharmaShares and Down PharmaShares of a Series; and
(3)without regard to the respective Underlying Values at which the Paired PharmaShares of a Series are issued or redeemed, on the books and records of a Series, one-half of the funds used to effect a Paired Optional Redemption is always allocated to reduce the Up Investment Amount and one-half is allocated to reduce the Down Investment Amount and one-half of the funds received in connection with a Paired Issuance is allocated to increase the Up Investment Amount and one-half is allocated to increase the Down Investment Amount. The Up Investment Amount and Down Investment Amount are variables in, respectively, the Up Underlying Value formula and the Down Underlying Value formula, as illustrated earlier in this section and in the "Glossary of Defined Terms."
Maintaining the initial Up/Down Ratio for your Series ensures that the Underlying Value formula and the yield on your Up or Down PharmaShares will not be modified as a result of Periodic UV Performance Distributions or as a result of Paired Optional Redemptions and Paired Issuances.
Periodic Distributions
Periodic Calculation Dates
"Periodic Calculation Dates" for your Series of PharmaShares will occur quarterly on the fifth (5th) business day following each Publication Date unless otherwise specified in your prospectus supplement. On each Periodic Calculation Date, the calculation agent will calculate a "Periodic Distribution" for the Up and Down PharmaShares of each Series, which will consist of a Periodic Income Distribution if there is any Net Treasury Income available for that purpose, and a Periodic UV Performance Distribution, which will depend on the net revenue performance of the Reference Product relative to the Projected Breakpoint. Most Series of PharmaShares are expected to have quarterly Periodic Calculation Dates and Calculation Periods consisting of approximately 90 days, but Periodic Distributions may occur annually or at some other interval specified in your prospectus supplement.
"Publication Dates" will occur within 45 days following the last day of each calendar quarter or, in the case of the fourth quarter of each calendar year, within 90 days of the end of that quarter. The actual Publication Date may vary from quarter to quarter for each Reference Product. The "Calculation Period" related to each Periodic Calculation Date will be specified in your prospectus supplement and will generally be the period between the last two Publication Dates that precede that Periodic Calculation Date. The length of each Calculation Period may vary if the reference pharmaceutical company does not make its Form 10-Q and 10-K filings within the same number of days after the end of each calendar quarter.
On the second (2nd) business day following each Periodic Calculation Date, the PharmaShares Trust will pay any Periodic Distribution to the holders of the Up and Down PharmaShares, unless otherwise specified in your prospectus supplement. Each shareholder who is a registered holder of the applicable Series of PharmaShares on the "Record Date" specified in your prospectus supplement will be entitled to receive the Periodic Distribution calculated and declared on the related Periodic Calculation Date.
Periodic Income Distributions
On each Periodic Calculation Date, the PharmaShares Trust will declare a "Periodic Income Distribution" on the Up and Down PharmaShares of your Series out of the income, if any, that your Series has realized on its treasury securities during the preceding Calculation Period and which remain on deposit after your Series has:
·deposited sufficient funds into the fee payment account to pay its fees and expenses, which will consist of the fees and expenses described generally in this base prospectus under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series" and specified under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement; and
65
·acquired new treasury securities with an aggregate purchase price equal to the Aggregate Par Amount of the outstanding PharmaShares of your Series.
The income on deposit in your Series after the deduction of fees and expenses and the required reinvestment in new treasuries is referred to as its "Net Treasury Income" for the applicable Calculation Period. On each day of a Calculation Period, the Net Treasury Income of a Series is allocated between the Up PharmaShares and Down PharmaShares in accordance with the allocation mechanism specified in your prospectus supplement. Each outstanding PharmaShare of a Series may be entitled to an equal share of the Net Treasury Income of its Series, its entitlement may be based on its Per Share Underlying Value or its entitlement may be determined based on some other allocation mechanism specified in your prospectus supplement. The income entitlement of each PharmaShare for each day of a Calculation Period is referred to as its "Daily Net Income Accrual." The portion of the Underlying Value of your Up or Down PharmaShares that is attributable to their income entitlement is referred to as the "net income component of Underlying Value" of those PharmaShares.
Income accruals, net of fee deductions, must be calculated for each Series on each day of a Calculation Period rather than at the end of that period due to (1) daily reporting requirements, (2) the need to calculate the net income component of Underlying Value for purposes of Paired Issuances and Paired Optional Redemptions which may occur on any business day during a Calculation Period, and (3) changing income accrual rates resulting from the changing composition of treasuries in your Series due to issuances and redemptions and due to the fact that the funds of a Series may remain uninvested for several days during any Calculation Period the length of which exceeds the typical length of a Calculation Period for your Series.
The administrative agent will always use a "last in, first out" method for selecting which treasuries to deliver in a redemption of PharmaShares. As a result, in both a rising and a falling interest rate environment, Paired Optional Redemptions that are effected during a Calculation Period may result in a decrease in the Daily Yield Rate that is earned on the treasuries by the remaining shareholders of a Series. See "RISK FACTORS — The return on your PharmaShares is uncertain — Treasury securities may be delivered instead of cash in a paired optional redemption" in this base prospectus.
On each Periodic Calculation Date, your Series will declare a "Periodic Income Distribution" on each outstanding Up PharmaShare equal to:
·the Up Daily Net Income Accruals realized during each day of the preceding Calculation Period, if any,
multiplied by
·a fraction, the numerator of which is one Up PharmaShare and the denominator of which is the aggregate number of outstanding Up PharmaShares on that Periodic Calculation Date.
On each Periodic Calculation Date, your Series will declare a "Periodic Income Distribution" on each outstanding Down PharmaShare equal to:
·the Down Daily Net Income Accruals realized during each day of the preceding Calculation Period, if any,
multiplied by
·a fraction, the numerator of which is one Down PharmaShare and the denominator of which is the aggregate number of outstanding Down PharmaShares on that Periodic Calculation Date.
The declared Periodic Income Distribution will be paid to shareholders on the second (2nd) business day following each Periodic Calculation Date, unless otherwise specified in your prospectus supplement.
Treasury income earned by each Series will first be applied to pay the fees and expenses of that Series. The annual fees and expenses of each Series will consist of the variable and fixed fees described under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series," and specified under "THE TERMS OF YOUR
66
SERIES — Fees and Expenses" in your prospectus supplement. If the fees and expenses of a Series exceed its treasury income during any Calculation Period, the net income component of Underlying Value of that Series will be equal to zero and it will not declare any Periodic Income Distribution on the related Periodic Calculation Date. See "RISK FACTORS — Income on the treasuries may be insufficient to make Periodic Income Distributions."
An amount equal to the Series Aggregate Par Amount, as calculated after any Periodic Distributions being declared on each Periodic Calculation Date are determined, will always be set aside on that date to be reinvested by the trustee, at the direction of the administrative agent, in new treasuries unless that Periodic Calculation Date is related to the Final Scheduled Termination Date or an Early Termination Date. If a redemption order is delivered on a Periodic Calculation Date or on the day preceding a Periodic Calculation Date, the Series Aggregate Par Amount will first be reduced by the Aggregate Par Amount of any PharmaShares being redeemed on that date. If, after the amount needed to pay fees and expenses is deposited into the fee payment account, the funds remaining on deposit in your Series on any Periodic Calculation Date are equal to, or less than, the Series Aggregate Par Amount, then all of these remaining funds must be reinvested in treasuries and your Series will have no Net Treasury Income on that date. If your Series has suffered losses on its treasury securities during one or more preceding Calculation Periods, treasury income may need to be reinvested in treasuries rather than being distributed to shareholders as Periodic Income Distributions in order to satisfy the requirement that an amount equal to the Series Aggregate Par Amount or, if less than this amount is available, then all assets on deposit in the Series, must be invested in treasuries during each Calculation Period.
We refer to the product of the aggregate number of outstanding Up PharmaShares issued by your Series and the stated par amount per share specified in your prospectus supplement as the "Up Aggregate Par Amount" and to the product of the aggregate number of outstanding Down PharmaShares issued by your Series and the stated par amount per share as the "Down Aggregate Par Amount." The stated par amount per share of the Up PharmaShares and Down PharmaShares of any Series does not affect in any way the respective Underlying Values of those shares, their respective public offering prices or their trading prices at any time. The stated par amount per share represents only, on any date of determination, each share's entitlement to the Underlying Value of its class of PharmaShares. For a description of how Periodic UV Performance Distributions decrease the Up and Down Aggregate Par Amount and the stated par amount per share for each class of PharmaShares, see "— Periodic UV Performance Distributions" below.
No Series of the PharmaShares Trust will make any Periodic Income Distributions on its PharmaShares until the earlier of the time when (i) treasury yields prevailing in the market for treasury securities of the type and tenor permitted to be acquired by that Series exceed an average annual rate specified in your prospectus supplement and generally expected to equal at least 3% or (ii) that Series has achieved an aggregate amount of invested assets at least equal to the minimum amount specified in your prospectus supplement.
If, as of any Periodic Calculation Date, your Series does not have any Net Treasury Income, it will not declare any Periodic Income Distribution. Your Series is not required to make Periodic Income Distributions in any stated amount and if no funds are available to make such a distribution as of any Periodic Calculation Date, your Series will not be obligated to make such distribution on any subsequent date. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" and "— Income on the treasuries may be insufficient to make Periodic Income Distributions" in this base prospectus.
Periodic UV Performance Distributions
If the Underlying Value of the Up PharmaShares increases based on the Reference Value published on a Publication Date relative to the Projected Breakpoint for the preceding calendar quarter, the PharmaShares Trust will declare a Periodic UV Performance Distribution on the Up PharmaShares equal to that increase, plus an Equalization Payment, on the Periodic Calculation Date that follows that Publication Date. No Periodic UV Performance Distribution will be declared on the paired Down PharmaShares on that Periodic Calculation Date. Conversely, if the Underlying Value of the Down PharmaShares increases on a Publication Date relative to the Projected Breakpoint for the preceding calendar quarter, the PharmaShares Trust will declare a Periodic UV Performance Distribution on the Down PharmaShares equal to that increase, plus an Equalization Payment, on that
67
Periodic Calculation Date. No Periodic UV Performance Distribution will be declared on the paired Up PharmaShares on that Periodic Calculation Date. The purpose of Periodic UV Performance Distributions is to distribute any increase in the Up Underlying Value to the holders of the Up PharmaShares and any increase in the Down Underlying Value to the holders of the Down PharmaShares. Periodic UV Performance Distributions include Equalization Payments in order to maintain the initial proportion of the Up Investment Amount and the Down Investment Amount, which will be 1:1 in the case of most Series. In a 1:1 Series, the Equalization Payment that will be included in any Periodic UV Performance Distribution will be equal to the increase in the Up Underlying Value or the Down Underlying Value, as applicable. In a Series with a different Up/Down Ratio, the Equalization Payments will be calculated as specified in your prospectus supplement.
The distribution of (1) increases in the Down Underlying Value and (2) Equalization Payments that accompany distributions of increases in the Up Underlying Value will be paid from funds in your Series that are allocated, on the books and records of your Series, to the Up Investment Amount and will reduce that Up Investment Amount. The distribution of (1) increases in the Up Underlying Value and (2) Equalization Payments that accompany distributions of increases in the Down Underlying Value will be paid from funds in your Series that are allocated, on the books and records of your Series, to the Down Investment Amount and will reduce that Down Investment Amount. See "RISK FACTORS — The amount of your investment in your PharmaShares and your potential gains will decline over time."
If there was an increase in the Up Underlying Value of your Series based on the Reference Value published on a Publication Date relative to the Projected Breakpoint, the PharmaShares Trust will declare the following Periodic UV Performance Distribution on each Up PharmaShare of that Series on the Periodic Calculation Date that follows that Publication Date:
·the amount of the increase in the Up Underlying Value (not including the net income component of that Underlying Value, which will be separately declared on the same date as a Periodic Income Distribution)
plus
·an Equalization Payment equal to the amount required to maintain the original Up/Down Ratio for that Series;
divided by
·the aggregate number of Up PharmaShares outstanding on that Periodic Calculation Date.
If there was an increase in the Down Underlying Value of your Series based on the Reference Value published on a Publication Date relative to the Projected Breakpoint, the PharmaShares Trust will declare the following Periodic UV Performance Distribution on each Down PharmaShare of that Series on the Periodic Calculation Date that follows that Publication Date:
·the amount of the increase in the Down Underlying Value (not including the net income component of that Underlying Value, which will be separately declared on the same date as a Periodic Income Distribution)
plus
·an Equalization Payment equal to the amount required to maintain the original Up/Down Ratio for that Series;
divided by
·the aggregate number of Down PharmaShares outstanding on that Periodic Calculation Date.
The declared Periodic UV Performance Distribution will be paid to shareholders on the second business day following each Periodic Calculation Date, unless otherwise specified in your prospectus supplement.
68
If the Underlying Value of your PharmaShares consistently declines as a result of the performance of the Reference Value for your Series relative to Projected Breakpoints, and you are unable to sell those shares, you may lose your entire investment. Even if returns on the Paired PharmaShares for your Series, and therefore your losses, are capped on each Periodic Calculation Date, if the Reference Value for your Series consistently fails to exceed Projected Breakpoint Increases, in the case of the Up PharmaShares, or consistently fails to exceed Projected Breakpoint Decreases, in the case of the Down PharmaShares, or if the paired class of PharmaShares consistently does exceed Projected Breakpoint Increases or Decreases, as applicable, the Underlying Value of your PharmaShares will continue to decrease and you may lose a substantial portion of your investment in those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution."
Final Distributions
The PharmaShares Trust will declare a Final Distribution on all of the Up PharmaShares and Down PharmaShares of a Series or, in the case of a redemption, on the portion being redeemed, on the earliest to occur of:
·the "Final Scheduled Termination Date" specified in your prospectus supplement;
·an "Early Termination Date," which is the next Periodic Calculation Date that follows the occurrence of an event or events that constitute a Termination Trigger for your Series of PharmaShares; and
·a "Redemption Order Date," which is any business day on which an Authorized Participant places an order for a Paired Optional Redemption of all or a portion of the Paired PharmaShares of your Series.
The Final Distribution will be payable on the "Final Redemption Date" which will be the second (2nd) business day that follows the Final Scheduled Termination Date or Early Termination Date. In the case of a Paired Optional Redemption, the Final Distribution will be payable on the "Redemption Date," which is the next business day that follows the related Redemption Order Date.
The "Final Distribution" declared by the PharmaShares Trust on, respectively, the Up and Down PharmaShares of a Series on the Final Scheduled Termination Date, an Early Termination Date or any Redemption Order Date will depend upon the Underlying Value of the Up PharmaShares and the Underlying Value of the Down PharmaShares of that Series, calculated based upon the Reference Value published on the last Publication Date that precedes the Final Scheduled Termination Date, the Early Termination Date or the relevant Redemption Order Date. The Final Distribution will consist of the entire Underlying Value of your Up or Down PharmaShares, not merely any increase in that Underlying Value that would be declared as a Periodic UV Performance Distribution on a Periodic Calculation Date.
On the Final Redemption Date that follows the Final Scheduled Termination Date or an Early Termination Date, the trustee will pay the final distribution to each holder of the outstanding Up PharmaShares and each holder of the outstanding Down PharmaShares, in redemption of those shares. Any Daily Net Income Accruals that have accrued with respect to each class of PharmaShares and that would have been distributed as a Periodic Income Distribution will be included in that Final Distribution. On the Redemption Date that follows a Redemption Order Date, the trustee will pay the Final Distribution to each redeeming Authorized Participant in redemption of the Paired PharmaShares it tendered for redemption. On each Redemption Date, any Daily Net Income Accruals that have accrued with respect to each class of PharmaShares during the current Calculation Period up to and including the last calendar day preceding the applicable Redemption Date will be included in the Final Distribution made on that Redemption Date.
The redemption of a portion of the Paired PharmaShares in a Paired Optional Redemption may only be effected in PharmaShares Units and may only be directed by an Authorized Participant who is the beneficial holder of those shares. As discussed in greater detail later in this section, unless you are an Authorized Participant, you will not have a right to direct a redemption of your PharmaShares. Consequently, you will only be able to liquidate your investment in the PharmaShares prior to the Final Scheduled Termination Date or an Early Termination Date by
69
selling them to an investor who is willing to purchase them from you, including any Authorized Participant who may wish to acquire those shares in order to direct a Paired Optional Redemption. The market price that you are able to obtain for your PharmaShares may be less than the price you paid for those shares and less than the Per Share Underlying Value that is represented by those shares for the reasons discussed in this base prospectus under "RISK FACTORS — Fluctuations in the per share Underlying Value of your PharmaShares and other factors may affect their trading price."
Final Distributions on the Final Scheduled Termination Date or an Early Termination Date
On an Early Termination Date or the Final Scheduled Termination Date for a Series, the PharmaShares Trust will declare a Final Distribution in redemption of the Up PharmaShares and Down PharmaShares using all of the funds held by the Series on that date, which will consist of all interest, discount, principal and any other amounts realized by the Series upon the maturity of its treasury securities at the end of the final Calculation Period, net of fees and expenses. The Final Distribution will include the net income accrual component of the Underlying Value of the Paired PharmaShares that would have been declared as a Periodic Income Distribution if that Final Scheduled Termination Date or Early Termination Date had been an ordinary Periodic Calculation Date.
On the Final Redemption Date that follows the Final Scheduled Termination Date or Early Termination Date, your Series will distribute:
on each outstanding Up PharmaShare a Final Distribution in cash equal to,
·the Up Underlying Value, calculated based upon the Reference Value published on the last Publication Date preceding that Final Scheduled Termination Date or that Early Termination Date and the Projected Breakpoint for the preceding calendar quarter
divided by
·the aggregate number of Up PharmaShares outstanding as of that date, and
on each outstanding Down PharmaShare a Final Distribution in cash equal to,
·the Down Underlying Value, calculated based upon the Reference Value published on the last Publication Date preceding that Final Scheduled Termination Date or that Early Termination Date and the Projected Breakpoint for the preceding calendar quarter
divided by
·the aggregate number of Down PharmaShares outstanding as of that date.
The Final Scheduled Termination Date or Early Termination Date for your PharmaShares will be the last day of trading for those shares.
Upon receipt of a Final Distribution on the Final Redemption Date, your PharmaShares will be considered to be redeemed in full and the PharmaShares Trust and your Series will have no further obligations with respect to those shares even if the amount of the Final Distribution is less than the Aggregate Par Amount of your PharmaShares or less than the purchase price you paid for those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" in this base prospectus.
Final Distributions in Paired Optional Redemptions
On each Redemption Order Date on which an Authorized Participant orders a Paired Optional Redemption in accordance with the procedures described below under "— Paired Optional Redemptions," and such redemption order is accepted, the PharmaShares Trust will declare a Final Distribution on the Up PharmaShares and Down
70
PharmaShares being redeemed. This Final Distribution will be paid out of all funds held by the Series on that date multiplied by the "Redemption Percentage," equal to the Paired PharmaShares being redeemed divided by all outstanding Paired PharmaShares. The Final Distribution will include the net income accrual component of the Underlying Value of the Paired PharmaShares that would have been declared as part of a Periodic Income Distribution on the next Periodic Calculation Date.
On the next business day that follows a Redemption Order Date, which is referred to as the "Redemption Date," your Series will distribute:
·on the Up PharmaShares being redeemed, the Up Underlying Value of those Up PharmaShares; and
·on the Down PharmaShares being redeemed, the Down Underlying Value of those Down PharmaShares.
The Up Underlying Value distributed as the Final Distribution on a Redemption Date will include any Up Daily Net Income Accruals from the first day of the current Calculation Period through and including the last calendar day preceding that Redemption Date. The Down Underlying Value distributed as the Final Distribution on a Redemption Date will include any Down Daily Net Income Accruals from the first day of the current Calculation Period through and including the last calendar day preceding that Redemption Date. In the case of a Paired Optional Redemption that is ordered on a Periodic Calculation Date or on the business day following a Periodic Calculation Date or on a Redemption Order Date that is separated from the related Redemption Date by one or more intervening non-business days, the amount delivered on the related Redemption Date will include the Daily Net Income Accruals for all intervening days between the Redemption Order Date and the Redemption Date.
Upon receipt of a Final Distribution on a Redemption Date, the tendered PharmaShares will be considered to be redeemed in full and the PharmaShares Trust and the applicable Series will have no further obligations with respect to those shares even if the amount of the Final Distribution is less than the Aggregate Par Amount of those PharmaShares or less than the purchase price paid by the redeeming Authorized Participant for those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" in this base prospectus.
Paired Optional Redemptions
On any business day prior to the Final Scheduled Termination Date or an Early Termination Date, the Up PharmaShares and Down PharmaShares of a Series may be redeemed concurrently in PharmaShares Units at the direction of Authorized Participants in what we refer to as a "Paired Optional Redemption." A "PharmaShares Unit" for each Series will consist of a specified aggregate par amount of Up PharmaShares and a specified aggregate par amount of Down PharmaShares, as disclosed in your prospectus supplement. A tender of Paired PharmaShares for redemption will be irrevocable.
The Paired PharmaShares of any Series may only be redeemed by Authorized Participants. However, the discussion which follows describing Paired Optional Redemptions and the Final Distribution that will be made on the related Redemption Date is relevant to you as a holder of the PharmaShares of any Series even if you are not an Authorized Participant, because it explains the rules that Authorized Participants must follow in order to effect Paired Optional Redemptions and their ability to effect these redemptions may affect the demand for your PharmaShares. See "RISK FACTORS — The return on your PharmaShares is uncertain — The right to redeem the PharmaShares is limited" in this base prospectus.
In order to be an "Authorized Participant," an entity must:
(1)be a registered broker-dealer and a member in good standing with the Financial Industry Regulatory Authority, Inc. ("FINRA"), or a participant in the securities markets such as a bank or other financial institution that is not required to register as a broker-dealer or be a member of FINRA in order to engage in securities transactions;
71
(2)be a participant in The Depository Trust Company ("DTC") or have indirect access to the clearing facilities of DTC by virtue of a custodial relationship with a DTC participant; and
(3)have entered into a "Participants Agreement" with the PharmaShares Trust, the trustee and the administrative agent which specifies procedures for the issuance and redemption of Paired PharmaShares.
The amount of cash and/or treasuries that will be delivered on the Redemption Date in a Paired Optional Redemption will always be equal to the Up Underlying Value of the Up PharmaShares being redeemed and the Down Underlying Value of the Down PharmaShares being redeemed, calculated based upon the Reference Value for the preceding calendar quarter that was published on the last Publication Date preceding the Redemption Order Date, relative to the Projected Breakpoint for that calendar quarter. The Up Underlying Value will include Up Daily Net Income Accruals for each day of the current Calculation Period, from and including the last Publication Date through and including the calendar day preceding the applicable Redemption Date. The Down Underlying Value will include Down Daily Net Income Accruals for the same time period. In the case of a Paired Optional Redemption that is ordered on a Periodic Calculation Date or on the business day following a Periodic Calculation Date or on a Redemption Order Date that is separated from the related Redemption Date by one or more intervening non-business days, the amount delivered on the related Redemption Date will include the Up and Down Daily Net Income Accruals for all intervening days between the Redemption Order Date and the Redemption Date.
Authorized Participants must place redemption orders with the administrative agent by 4:00 p.m. New York City time on any business day. Any redemption order that is not placed by or prior to that time may be rejected in the sole discretion of the administrative agent. A tender of Paired PharmaShares for redemption will be irrevocable.
The Authorized Participant will place its redemption order on the Redemption Order Date and the Paired Optional Redemption will be effected on the next business day, which is referred to as the "Redemption Date." Each redeeming Authorized Participant must deliver to the administrative agent a redemption order with the following information:
·the Authorized Participant's e-mail address and telephone number and the name and personal identification number of the authorized person who is submitting the redemption order on behalf of the Authorized Participant; and
·the number of PharmaShares Units being redeemed.
Not later than 10:00 a.m. New York City time on the Redemption Date, the Authorized Participant who placed the redemption order must deliver to the trustee:
·Up PharmaShares and Down PharmaShares in the Aggregate Par Amounts that constitute one or more PharmaShares Units being redeemed by such Authorized Participant;
·the applicable Redemption Cash Component, if applicable; and
·a transaction fee of $2,000, payable directly to the trustee by the redeeming Authorized Participant who placed the redemption order to compensate the trustee for services rendered in effecting the Paired Optional Redemption.
If all conditions to effecting a Paired Optional Redemption are satisfied, the trustee will effect the redemption by delivering cash and/or treasuries in accordance with the instructions of the administrative agent to the redeeming Authorized Participant by 3:00 p.m. New York City time on the Redemption Date. If the redemption order was placed on a Periodic Calculation Date, the redeeming Authorized Participant will receive cash. If there was a net increase in the Series Aggregate Par Amount on any Redemption Date that was also an Issuance Date, because more PharmaShares Units were created than redeemed, redeeming Authorized Participants will receive their Final Distribution in cash out of the funds delivered to the Series by the Authorized Participants who created Paired PharmaShares on the same date. If any Paired Issuances were effected on the Redemption Date, even if there was a
72
net decrease in the Series Aggregate Par Amount, redeeming Authorized Participants will receive a portion of their Final Distribution in cash out of the funds delivered by the creating Authorized Participants. All or a portion of the Final Distribution may also be made out of the cash proceeds of any eligible repos held by the Series. Any remaining portion of the Final Distribution for which cash is not available will be made by delivering treasury securities to the redeeming Authorized Participant.
In connection with a Final Distribution in a Paired Optional Redemption in which treasury securities must be delivered, the administrative agent will direct the trustee to select and segregate treasuries on a "last in, first out" basis such that the value of the segregated treasuries is equal to the product of (1) the applicable Redemption Percentage and the aggregate value of all of the treasuries held by the redeeming Series. The "value" of each treasury will be equal to the purchase price at which the Series acquired that treasury plus all interest and/or discount accrued on that treasury since its acquisition date. The "Redemption Percentage" in a Paired Optional Redemption is equal to a fraction, the numerator of which is the Aggregate Par Amount of Up PharmaShares and Down PharmaShares that are being redeemed and the denominator of which is the Aggregate Par Amount of Paired PharmaShares that are outstanding prior to the redemption. The treasuries selected by the administrative agent to be delivered as the Final Distribution will be distributed ratably, by type, to each redeeming Authorized Participant. The selection and delivery of treasuries as a Final Distribution must comply with the conditions specified in Appendix B to this base prospectus. For a discussion of the potential risks associated with the delivery of treasuries instead of cash as a final distribution, see "RISK FACTORS — The return on your PharmaShares is uncertain — Treasury securities may be delivered instead of cash in a Paired Optional Redemption" in this base prospectus.
Following a Paired Optional Redemption, the trustee will record a reduction in the Aggregate Par Amount of Up PharmaShares and the Aggregate Par Amount of Down PharmaShares of the redeeming Series that are outstanding. If PharmaShares Units are being both redeemed and issued on the same day at the direction of several Authorized Participants, the trustee will record such reduction only if a net decrease in the Series Aggregate Par Amount has occurred.
The trustee will reduce, on the books and records of your Series, the Up Investment Amount by the Aggregate Par Amount of Up PharmaShares that were redeemed and the Down Investment Amount by the Aggregate Par Amount of Down PharmaShares that were redeemed, without regard to the respective Per Share Underlying Values at which those Up PharmaShares and Down PharmaShares were redeemed, in order to preserve the one-to-one ratio of the Up Investment Amount and the Down Investment Amount. If the ratio specified in your prospectus supplement is other than one-to-one, then a different reduction in the Up Investment Amount and the Down Investment Amount will be made, as disclosed in your prospectus supplement.
Orders placed by Authorized Participants for Paired Optional Redemptions may be rejected or the fulfillment of those orders may be suspended or postponed for the reasons described in this base prospectus under "RISK FACTORS — Redemption and issuance orders are subject to postponement, suspension or rejection in certain circumstances."
Upon receipt of the Final Distribution in a Paired Optional Redemption, the Paired PharmaShares presented for redemption will be considered to be redeemed in full and your Series will have no further obligations with respect to those shares, even if the amount of the Final Distribution was less than the Aggregate Par Amount of those shares or the purchase price paid by an Authorized Participant for those shares. See "RISK FACTORS — You may lose your entire investment in the PharmaShares that you hold; there is no guarantee as to the amount of any periodic distribution or the amount of the final distribution" in this base prospectus.
Paired Issuances
On any business day prior to the Final Scheduled Termination Date or an Early Termination Date, an Authorized Participant may effect a "Paired Issuance" of a Series of Up PharmaShares and Down PharmaShares by directing the PharmaShares Trust to issue additional Paired PharmaShares in the Aggregate Par Amount that constitutes at least one PharmaShares Unit. Following receipt of an issuance order, the PharmaShares Trust will issue additional Paired PharmaShares to the Authorized Participant if the applicable conditions to fulfilling the issuance order have been satisfied. Up PharmaShares and Down PharmaShares of a Series will always be issued at their respective Per Share Underlying Values, which will be calculated based upon the Reference Value for the
73
preceding calendar quarter that was published on the last Publication Date preceding the relevant Issuance Date and the Projected Breakpoint for that calendar quarter and will include any Up Daily Net Income Accruals and Down Daily Net Income Accruals, as applicable, from that Publication Date through and including the last calendar day preceding the relevant Issuance Date, as defined below.
For Series with a 1:1 Up/Down Ratio, the trustee will allocate, on the books and records of your Series, one-half of the aggregate funds received by it in connection with a Paired Issuance to the Up Investment Amount and the other half to the Down Investment Amount, without regard to the respective Per Share Underlying Values at which the Up PharmaShares and Down PharmaShares were issued, in order to preserve the one-to-one Up/Down Ratio. If the Up/Down Ratio specified in your prospectus supplement is other than one-to-one, then a different allocation of funds will be made, as disclosed in your prospectus supplement.
Following a Paired Issuance for a Series, the trustee will record an increase in the Aggregate Par Amount of Up PharmaShares and Down PharmaShares of that Series that are outstanding. If PharmaShares Units are being both issued and redeemed on the same day at the direction of several Authorized Participants, the trustee will record an increase only if a net increase in the Series Aggregate Par Amount has occurred.
To create a new PharmaShares Unit, an Authorized Participant must place an issuance order with the administrative agent by 4:00 p.m. New York City time on any business day. Any issuance order that is not placed by or prior to that time may be rejected at the sole discretion of the administrative agent. The Authorized Participant will place its issuance order on the Issuance Order Date and the Paired Issuance will be effected on the next business day, which is referred to as the "Issuance Date." Each creating Authorized Participant must deliver to the administrative agent an issuance order with the following information:
·the Authorized Participant's e-mail address and telephone number and the name and personal identification number of the authorized person who is submitting the issuance order on behalf of the Authorized Participant; and
·the number of PharmaShares Units being created.
By 10:00 a.m. New York City time on the Issuance Date, the Authorized Participant must deposit immediately available funds in an amount equal to:
·the aggregate Per Share Underlying Value of the Up PharmaShares being created, as measured on the last calendar day preceding the Issuance Date;
·the aggregate Per Share Underlying Value of the Down PharmaShares being created, as measured on the last calendar day preceding the Issuance Date; and
·a transaction fee of $2,000, payable directly to the trustee by the Authorized Participant who placed the issuance order to compensate the trustee for services rendered in effecting the Paired Issuance.
Issuance orders for new PharmaShares Units will be processed through a manual clearing process operated by DTC. By 3:00 p.m. New York City time on the Issuance Date, the administrative agent will instruct the trustee to deliver to the Authorized Participant's account at DTC the Up PharmaShares and Down PharmaShares that were created in the Paired Issuance.
Orders placed by Authorized Participants for Paired Issuances may be rejected or the fulfillment of those orders may be suspended or postponed for the reasons described in this base prospectus under "RISK FACTORS — Redemption and issuance orders are subject to postponement, suspension or rejection in certain circumstances."
Termination Triggers
The occurrence of specified events, which we refer to as "Termination Triggers," will cause an early redemption of all Series of Paired PharmaShares or of only a single affected Series. The early redemption will occur on the related "Early Termination Date," which is the first Periodic Calculation Date that follows the occurrence of
74
the event or, in the case of several related events, the final event, that caused the Termination Trigger. Upon obtaining knowledge or receiving notice of the occurrence of a Termination Trigger, we will file a current report on Form 8-K with the SEC for each affected Series describing the Termination Trigger and announcing the applicable Early Termination Date. The following events will cause the PharmaShares Trust to declare an early redemption of all outstanding Series of Paired PharmaShares:
·the PharmaShares Trust becomes required to register as an "investment company" under the United States Investment Company Act of 1940, as amended, and we elect not to, or we are unable to, promptly cause such registration, but elect, instead, in our discretion, to terminate all outstanding Series;
·DTC becomes unwilling or unable to act as depository and no suitable replacement is willing or able to assume the duties of a depository for the PharmaShares Trust, and we elect, in our discretion, to terminate all outstanding Series;
·the administrative agent, the trustee and/or the calculation agent resign or are unable to perform their duties under the Master Trust Agreement and the Series Trust Supplements, or become bankrupt or insolvent, and no suitable replacement is willing and able to assume those duties;
·we elect to terminate all outstanding Series and holders of 66 and 2/3% of the Up PharmaShares of each Series, voting in the aggregate, and 66 and 2/3% of the Down PharmaShares of each Series, voting in the aggregate, consent to such termination;
·the PharmaShares Trust is adjudged to be bankrupt or insolvent or becomes involved in voluntary or involuntary insolvency or similar proceedings that are not dismissed within 90 days; and
·we determine in our good faith discretion that there is a material risk that the PharmaShares Trust is subject to withholding tax liability, and we elect, in our discretion, to terminate all outstanding Series.
The following events will constitute "Termination Triggers" for only the affected Series:
·the reference pharmaceutical company fails to publish net revenues or such other Reference Value metric as may be applicable to a particular Series in its income statement prepared in accordance with U.S. GAAP and filed with the SEC on Form 10-Q or Form 10-K for one or more Publication Dates, as specified in your prospectus supplement;
·the Up PharmaShares and/or the paired Down PharmaShares of a Series are delisted by the securities exchange on which they were listed and no other listing can be obtained within a reasonable period of time;
·a particular Series is adjudged to be bankrupt or insolvent or becomes involved in voluntary or involuntary insolvency or similar proceedings that are not dismissed within 90 days; and
·the Series Investment Amount of a Series is less than $50,000,000 (or such other amount as may be specified in your prospectus supplement) on any business day and we elect, in our discretion, to terminate that Series.
We, in our capacity as administrative agent, will be responsible for notifying the trustee and the calculation agent of the occurrence of series-specific Termination Triggers, unless otherwise stated in your prospectus supplement, as well as our resignation or bankruptcy, or the consent of all outstanding shareholders to an early termination of a Series following our election to effect such termination, and our decision to terminate all outstanding Series if any of the foregoing occurs: the PharmaShares Trust becomes required to register as an investment company or if there is a material risk that withholding tax liability may be imposed on the PharmaShares Trust. The trustee, upon obtaining knowledge of the occurrence of any of the other Termination Triggers described above, will be responsible for notifying us of such occurrence.
75
On the applicable Early Termination Date, the affected Series or each Series will declare a Final Distribution in redemption of all of its outstanding shares, calculated based upon the Reference Value for the preceding calendar quarter that was published on the last Publication Date preceding that Early Termination Date and the Projected Breakpoint for that calendar quarter. Following this Final Distribution, the Up PharmaShares and Down PharmaShares of each affected Series will be considered to be redeemed in full and will cease to be outstanding. The Final Distribution to be made by your Series may be subject to delays pending the resolution of bankruptcy proceedings if the relevant Termination Trigger was the voluntary or involuntary bankruptcy of the PharmaShares Trust or a Series of the PharmaShares Trust. No bankruptcy court has previously adjudicated the bankruptcy of a statutory trust organized in series, as discussed under "RISK FACTORS — Limitations on interseries liability for Delaware Series trusts have not been reviewed by the courts."
In the case of the Termination Trigger related to the bankruptcy of the PharmaShares Trust or any of its Series, the Early Termination Date will not occur until the Periodic Calculation Date that follows the ninetieth (90th) day after the date on which the PharmaShares Trust has been adjudged to be bankrupt or insolvent or on which it becomes involved in voluntary or involuntary insolvency or similar proceedings. In the case of Termination Triggers in which we, as administrative agent, have the option to elect to terminate the PharmaShares Trust, the Early Termination Date will not occur until after we have made such election and notified the trustee of that election. The respective Per Share Underlying Values of the Paired PharmaShares of an affected Series preceding the Early Termination Date may be higher or lower than the Per Share Underlying Values at the time of the initial event that resulted in the occurrence of the Termination Trigger, as described under "RISK FACTORS — The return on your PharmaShares is uncertain — A Series may terminate early." The Final Distribution will be determined as described in detail above under "— Final Distributions" and also under "THE TERMS OF YOUR SERIES — Final Distributions" in your prospectus supplement.
Authorized Participants may continue to direct Paired Optional Redemptions and Paired Issuances at the respective Per Share Underlying Values of the Paired PharmaShares of a Series after the occurrence of a Termination Trigger. The last redemption order or issuance order may be placed on the last business day prior to the Early Termination Date.
For a description of any additional termination triggers that may apply to your Series and a description of each Series-specific Termination Trigger, see "THE TERMS OF YOUR SERIES — Termination Triggers" in your prospectus supplement.
Book-Entry Registration
The Paired PharmaShares of each Series will be evidenced by one or more global certificates. We will deposit a global certificate representing the Up PharmaShares of a Series and a global certificate representing the Down PharmaShares of that Series with The Depository Trust Company in the United States or with Clearstream Banking, société anonyme or Euroclear Bank S.A./NV in Europe. We refer to The Depository Trust Company as "DTC," Clearstream Banking, société anonyme as "Clearstream" and the Euroclear system operated by Euroclear Bank S.A./NV as "Euroclear." Each global certificate will be registered in the name of Cede & Co. as DTC's nominee. Except as set forth below, a global certificate may be transferred, in whole or in part, only to another nominee of DTC or to a successor of DTC or its nominee.
Beneficial interests in a global certificate may be held directly or indirectly through DTC, Clearstream or Euroclear. Clearstream and Euroclear will hold omnibus positions on behalf of their participants through customers' securities accounts in Clearstream's and Euroclear's names on the books of their respective depositaries that in turn will hold such positions in customers' securities accounts in the depositaries' names on the books of DTC. [●] will act as the relevant depositary for Clearstream, and [●] will act as the relevant depositary for Euroclear. Transfers between participants will be effected in the ordinary way in accordance with DTC rules and will be settled in clearinghouse funds. The laws of some states require that certain persons take physical delivery of securities in definitive form. As a result, the ability to transfer beneficial interests in the global certificate to those persons may be limited.
Shareholders who are not participants may beneficially own interests in a global certificate held by DTC only through participants, or certain banks, brokers, dealers, trust companies and other parties that clear through or
76
maintain a custodial relationship with a participant, either directly or indirectly, which we refer to as "indirect participants." So long as Cede & Co., as the nominee of DTC, is the registered owner of a global certificate, Cede & Co. for all purposes will be considered the sole holder of the global certificates. Except as provided below, owners of beneficial interests in a global certificate will:
·not receive physical delivery of certificates in definitive registered form; and
·not be considered holders of the global certificate.
The trustee will make distributions on the shares to Cede & Co., as the registered owner of the global certificate, by wire transfer of immediately available funds on the second business day following each Periodic Calculation Date and on each settlement date for Paired Optional Redemptions. We and the trustee will not be liable for the accuracy of, and are not responsible for maintaining, supervising or reviewing DTC's records or any participant's records relating to the book-entry certificates. We and the trustee will not be responsible or liable for errors in payments made on account of the book-entry certificates, unless such error in payment was caused by an instruction error originating from us or the trustee.
Transfers between participants in DTC will be effected in accordance with DTC's procedures and will be settled in same-day funds, and transfers between participants in Euroclear and Clearstream will be effected in accordance with their respective rules and operating procedures.
Cross-market transfers between the participants in DTC, on the one hand, and Euroclear or Clearstream, on the other hand, will be effected through DTC in accordance with DTC's rules on behalf of Euroclear or Clearstream, as the case may be, by its respective depositary. These cross-market transactions, however, will require delivery of instructions to Euroclear or Clearstream, as the case may be, by the counterparty in that system in accordance with the rules and procedures and within the established deadlines (Brussels time) of that system. Euroclear or Clearstream will, if the transaction meets its settlement requirements, deliver instructions to its respective depositary to take action to effect final settlement on its behalf by delivering or receiving interest in the relevant global certificate in DTC, and making or receiving distributions in accordance with normal procedures for same-day funds settlement applicable to DTC. Euroclear or Clearstream participants may not deliver instructions directly to depositaries for Euroclear or Clearstream.
Although DTC has agreed to the foregoing procedures to facilitate transfers of interest in the global certificates among participants in DTC, it is under no obligation to perform or to continue to perform those procedures, and those procedures may be discontinued at any time. DTC has advised us that it will take any action permitted to be taken by a holder of shares, including the presentation of shares for redemption or exchange, only at the direction of one or more participants to whose account with DTC interests in the global certificate are credited, and only in respect of those shares represented by the global certificates as to which the participant or participants has or have given such direction.
DTC has advised us that it is:
·a limited purpose trust company organized under the laws of the State of New York and a member of the Federal Reserve System;
·a "clearing corporation" within the meaning of the Uniform Commercial Code; and
·a "clearing agency" registered pursuant to the provisions of Section 17A of the Exchange Act.
DTC was created to hold securities for its participants and to facilitate the clearance and settlement of securities transactions between participants through electronic book-entry changes to the accounts of its participants. Participants include securities brokers, dealers, banks, trust companies, clearing corporations and other organizations. Some of the participants or their representatives, together with other entities, own DTC. Indirect access to the DTC system is available to others such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a participant, either directly or indirectly.
77
Clearstream Banking, société anonyme, Luxembourg, has advised us that it is:
·incorporated under the laws of the Grand Duchy of Luxembourg as a professional depository; and
·subject to regulation by the Commission de Surveillance du Secteur Financier in Luxembourg.
Clearstream holds certificates for its participants. Clearstream facilitates the clearance and settlement of securities transactions between Clearstream participants through electronic book-entry changes in the accounts of Clearstream participants, eliminating the need for physical movement of securities. Clearstream provides to Clearstream participants, among other things, services for safekeeping, administration, clearance and settlement of internationally traded securities and securities lending and borrowing. Clearstream interfaces with domestic markets in several countries. Clearstream participants are recognized financial institutions around the world, including underwriters, securities brokers and dealers, banks, trust companies, clearing corporations and certain other organizations. Indirect access to Clearstream is also available to others, such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a Clearstream participant, either directly or indirectly.
Distributions, to the extent received by the relevant depositary for Clearstream, with respect to the securities held beneficially through Clearstream, will be credited to cash accounts of Clearstream participants in accordance with its rules and procedures.
Euroclear Bank S.A./NV has advised us that it is:
·licensed by the Belgian Banking and Finance Commission to carry out banking activities on a global basis; and
·regulated and examined by the Belgian Banking and Finance Commission.
Euroclear was created in 1968 to hold securities for its participants and to clear and settle transactions between Euroclear participants through simultaneous electronic book-entry delivery and payment. Transactions may be settled in any of 32 currencies, including United States dollars. Euroclear is operated by Euroclear Bank S.A./NV under contract with Euroclear Clearance Systems S.C., a Belgian cooperative corporation. Euroclear Bank S.A./NV conducts all operations. All Euroclear securities clearance accounts and Euroclear cash accounts are accounts with Euroclear Bank S.A./NV, not Euroclear Clearance Systems S.C. Euroclear Clearance Systems S.C. establishes policy for Euroclear on behalf of Euroclear participants. Euroclear participants include banks (including central banks), securities brokers and dealers and other professional financial intermediaries. Indirect access to Euroclear is also available to other firms that clear through or maintain a custodial relationship with a Euroclear participant, either directly or indirectly.
Securities clearance accounts and cash accounts with Euroclear Bank S.A./NV are governed by the terms and conditions governing use of Euroclear and the related operating procedures of the Euroclear system and applicable Belgian law. These terms and conditions, operating procedures and laws govern transfers of securities and cash within Euroclear, withdrawals of securities and cash from Euroclear, and receipts of distributions with respect to securities in Euroclear. All securities in Euroclear are held on a fungible basis without attribution of specific certificates to specific securities clearance accounts. Euroclear Bank S.A./NV acts under the terms and conditions only on behalf of Euroclear participants and has no record of or relationship with persons holding through Euroclear participants.
DTC, Clearstream and Euroclear have agreed to the foregoing procedures to facilitate transfers of interests in a global certificate among participants. However, DTC, Clearstream and Euroclear are under no obligation to perform or continue to perform these procedures and may discontinue these procedures at any time.
We will issue the shares in definitive certificated form if DTC notifies us that it is unwilling or unable to continue as depositary or DTC ceases to be a clearing agency registered under the Exchange Act, and a successor depositary is not appointed by us within 90 days. In addition, beneficial interests in a global certificate may be exchanged for definitive certificates upon request by or on behalf of DTC in accordance with customary procedures. We may determine at any time and in our sole discretion that shares shall no longer be represented by global
78
certificates, in which case we will issue shares in definitive form in exchange for the global certificates. If DTC becomes unwilling or unable to act as depository and no suitable replacement is willing or able to assume the duties of a depository for the PharmaShares Trust, we may elect to treat such event as a Termination Trigger and cause the early termination of all outstanding Series, as described above under "— Termination Triggers."
Listing
The Up PharmaShares and Down PharmaShares of your Series will trade on the listing exchange specified in your prospectus supplement. See "THE TERMS OF YOUR SERIES — Listing" in your prospectus supplement.
REFERENCE PRODUCTS AND REFERENCE VALUES
Each Series of PharmaShares is designed to track the performance of a Reference Value during a specified time period relative to the Projected Breakpoint for that Reference Value for the same time period. A "Reference Value" will consist of:
·the change in net revenue of a "Reference Product" which may be a specified drug, a basket of drugs or a pharmaceutical device or devices;
·the change in net revenues allocable to a specified division of a publicly traded pharmaceutical company; or
·the change in net revenues allocable to a specified entity that is party to a collaboration agreement, distribution agreement, joint venture or other profit-sharing arrangement relating to the sale of a Reference Product.
When we refer to the "change in net revenue for the Reference Product" throughout this base prospectus, we intend to cover each of the three possible Reference Value scenarios described above.
The Reference Value will be tracked from a starting point that occurred during a past calendar quarter, referred to as the "Base Calendar Quarter," to the present calendar quarter that is ongoing or has just ended, referred to as the "Current Calendar Quarter."
When we refer to the "Reference Value" of the Up PharmaShares or the Down PharmaShares of a Series on any date of determination, we are referring to the net revenue in U.S. dollars for the Reference Product that is publicly reported by the reference pharmaceutical company on an income statement prepared in accordance with United States generally accepted accounting principles, or "U.S. GAAP." Net revenue will not be expressed in unit sales unless otherwise noted in your prospectus supplement. For purposes of calculating the net revenues of a pharmaceutical company division or individually reported business segment that are being used as the basis for Reference Value, each product sold by that division or business segment that is a material asset or a revenue-generating product for which revenue is publicly reported on an income statement prepared in accordance with U.S. GAAP will be included in the related net revenue calculation. Net revenues for the Reference Product may be presented as net aggregated global sales and may be shown in one line item or in separate line items that show net sales by various geographic regions and/or therapeutic indications for the Reference Product. The Reference Value for most Series of PharmaShares will be equal to or based upon net aggregated global sales, but certain Series may reference net sales for only a specified geographic region or a specified therapeutic indication, as specified in your prospectus supplement.
"Net revenue" for any Reference Product will generally mean, unless otherwise stated in your prospectus supplement, the gross sales of a Reference Product adjusted for sales-related deductions, including but not limited to, chargebacks, trade discounts, sale returns and allowances, commercial and government rebates, customer loyalty programs and fee-for-service arrangements with certain distributors, collectively referred to as "sales allowances." Pharmaceutical companies will generally make an estimate of sales allowances and apply that estimate to reduce the gross sales price at the time of sale based upon historical experience, sales agreements in place and estimates for claims incurred but not yet paid. In some cases, this gross-to-net estimate for a calendar quarter will deviate from the actual reduction that should have been applied to revenues for that period and such deviation may be significant
79
enough to require the reference pharmaceutical company to adjust its reported net revenue and make an accounting restatement. If such a restatement is made after the Periodic Calculation Date that follows a Publication Date, the Reference Value that will be used to calculate Underlying Value will be the net revenue originally published and not the restated net revenue. If that same period is later used as a Base Calendar Quarter, the adjusted net revenue number will be used to calculate Reference Value. However, for purposes of any current period, after Periodic UV Performance Distributions have been calculated on the related Periodic Calculation Date, neither the Reference Value nor the calculation of Underlying Value will be retroactively adjusted to take into account any revisions or restatements made to net revenues after the original Publication Date by the related pharmaceutical company. For a discussion of the reasons why an investment in securities which reference the earnings performance of a specified Reference Product may differ from a direct investment in such Reference Product or the pharmaceutical company that manufactures and sells that product, see "RISK FACTORS — An investment in Up PharmaShares may not resemble a direct investment in the Reference Product; an investment in Down PharmaShares may not be an effective hedge against exposure to the Reference Product" in this base prospectus.
The "Publication Date" for the Reference Value of any Series will be the date on which the pharmaceutical company that manufactures and sells the Reference Product complies with its periodic reporting obligations under the Exchange Act as a public reporting company by filing a quarterly report on Form 10-Q or an annual report on Form 10-K with the SEC. Each Publication Date is generally expected to occur on or prior to the 45th day following the end of each calendar quarter in the case of quarterly reports and on or prior to the 90th day following the end of each calendar year in the case of annual reports. Some pharmaceutical companies seek to file their reports within the same number of days following the end of each quarter, but we cannot control when a company will actually make its filing. As a result, Publication Dates and the length of each Calculation Period may vary from quarter to quarter. See "RISK FACTORS — The return on your PharmaShares is uncertain — The changes in the underlying value of your PharmaShares from period to period depend upon the Reference Value and the Projected Breakpoint" in this base prospectus.
Generally, the Reference Value for a Series of PharmaShares will be tracked from the Current Calendar Quarter to:
·the same calendar quarter of the preceding calendar year in PharmaShares Series that track changes from year to year, referred to as "year-over-year tracking Series," which will typically be suitable for drugs that have a seasonality aspect to their sales, that may be sold with larger first quarter discounts before insurance deductibles are satisfied and/or that are at a more mature point in their lifecycle with less sequential quarter variability in sales levels;
·the immediately preceding calendar quarter in quarter over quarter PharmaShares Series, referred to as "sequential tracking Series," which will typically be suitable for drugs that have been recently launched or that have the potential to experience significant changes in sales from quarter to quarter; or
·some other comparative base quarter, as specified in your prospectus supplement.
The Current Calendar Quarter and the Base Calendar Quarter is specified in your prospectus supplement. The Reference Value tracking method for a series will not change for so long as that Series remains outstanding.
The starting Reference Value was determined on the Publication Date that occurred after the end of the Base Calendar Quarter and the ending Reference Value is determined on the last Publication Date that occurred after the end of the Current Calendar Quarter. Each Periodic Calculation Date and each Redemption Order Date, any Early Termination Date and the Final Scheduled Termination Date on which distributions are declared on the PharmaShares of a Series will refer back to the last Publication Date on which net revenue for the Current Calendar Quarter was reported. For example, for a year-over-year tracking Series, if the ending Reference Value is net revenue for the Reference Product for the first quarter ended March 31 of year X, as reported on the related Publication Date occurring not later than May 15 of Year X, then the starting Reference Value would be net revenue for the Reference Product for the first quarter of Year X-1, as reported not later than May 15 of Year X-1. For a sequential tracking Series, if the ending Reference Value is net revenue for the Reference Product for the second calendar quarter ended June 30 of Year X, as reported on the related Publication Date not later than August 15 of
80
Year X, then the starting Reference Value would be net revenue for the Reference Product for the first quarter of Year X, as reported not later than May 15 of Year X.
The calculation agent will calculate the Underlying Value of the Up PharmaShares and the Down PharmaShares of each Series for each day during a Calculation Period based on the Reference Value reported on the last Publication Date that occurred on the first day of that Calculation Period. See "DESCRIPTION OF THE PHARMASHARES —Calculation of Underlying Value" in this base prospectus.
If certain Major Market Disruptions occur during the course of a calendar quarter and materially affect sales of the Reference Product, the change in Reference Value for the applicable Measurement Period will be deemed to be zero. "Major Market Disruptions" consist of:
(1)a merger or pending merger involving the reference pharmaceutical company that requires the transfer of licenses to new entities and results in the Reference Product not being permitted to be sold for part or most of the Measurement Period and a resulting deviation from expected sales of more than 50% compared to the last Publication Date preceding the effective date of the merger agreement;
(2)a reported gross-to-net adjustment of more than 25 percent of net revenues or one that causes a delay in the release of U.S. GAAP earnings of more than 90 days;
(3)a failure on the part of the reference pharmaceutical company to report net revenues for the Reference Product; or
(4)such other event as may be specified in your prospectus supplement.
Material reductions in net revenues as a result of any of the following will not constitute a major disruption: (i) loss of exclusivity; (ii) introduction to the market of competing products, including generics; (iii) manufacturing problems or delays; (iv) a safety alert or recall; or (v) any other events relating to operational issues of the reference pharmaceutical company or changes in the competitive environment for the Reference Product.
If the Reference Value for your Series is not reported publicly by the reference pharmaceutical company in an income statement prepared in accordance with U.S. GAAP for any reason on one or more consecutive Publication Dates, there will be deemed to be no change in Reference Value for the related Calculation Period and no distributions will be declared on your PharmaShares on the related Periodic Calculation Date. If no Reference Value is available on one or more Publication Dates, an Early Termination Date may occur on the next scheduled Periodic Calculation Date, as specified in your prospectus supplement. See "DESCRIPTION OF THE PHARMASHARES — Termination Triggers" in this base prospectus and "THE TERMS OF YOUR SERIES — Termination Triggers" in your prospectus supplement.
No director, officer, member, employee, legal counsel, accountant or advisor of any reference pharmaceutical company or any of its affiliates, or any other person who has access to the earnings data used to calculate the Reference Value of any Series before such data becomes publicly available, will be permitted to invest in the Up PharmaShares or the Down PharmaShares of that Series. PharmaShares Advisors, LLC will not consult with any such person in calculating the Projected Breakpoint for any Series of PharmaShares.
We, the PharmaShares Trust and each Series of the PharmaShares Trust that may be established from time to time, our respective affiliates and our respective directors, officers, members, managers, trustees, employees, attorneys and advisors are not liable for:
·any losses to you resulting from the methodologies used in calculating, and the actual calculation of, net revenues of any Reference Product, or net revenues of a specified division of a pharmaceutical company or the net revenue allocations for any drug subject to a collaboration or joint venture agreement, or the resulting Reference Value in the case of any of the foregoing; or
81
·any losses to you for any revisions to the methodology for calculating net revenues, any subsequent revisions made to published net revenues, any alleged or actual inaccuracies or inherent limitations of the net revenues calculation, any delays in net revenue reporting and publication, any errors in the underlying data used to calculate net revenues, or the impact of any of the foregoing factors on the yield realized by you as a holder of PharmaShares or otherwise.
DESCRIPTION OF THE PHARMACEUTICAL INDUSTRY
Introduction
The sections below describe the lifecycle of a pharmaceutical product, pharmaceutical industry payors, factors that affect the net revenue that is generated by a pharmaceutical product and recent executive branch proposals and the general political climate relating to drug price controls. Not all of the matters described in this section may be directly relevant to the Reference Product for your Series of PharmaShares and there may be additional issues and concerns that relate to your Reference Product that are not discussed in detail in this base prospectus.
Typical Pharmaceutical Product Lifecycle
The following paragraphs describe the lengthy lifecycle of a pharmaceutical product from initial research and development to becoming commercialized, which means that it has received all regulatory approvals for sale to the public and has been successfully brought to market by the pharmaceutical company that manufactures the product. Most pharmaceutical products are never approved for commercial distribution and do not reach consumers. Failure at any step of the development or regulatory review of a drug can immediately end its lifecycle.
PharmaShares will be issued for existing, commercialized Reference Products that have at least one indicated use approved for sale. Generally, unless otherwise indicated in the related prospectus supplement, a Series of PharmaShares will reference all current and future indications of the reference drug. Accordingly, holders of PharmaShares will not be exposed to development risk for existing indications, but will be making an investment decision about the likelihood of successful future expansions of commercialized indications.
A Series of PharmaShares may reference a Reference Product that is a biopharmaceutical drug. The discovery, approval, exclusivity period and generic competition phases for these drug products may be different from chemical-based drugs. Biopharmaceutical drugs, which are also referred to as "biologics," are produced using biotechnology rather than direct extraction from a native, non-engineered biological source. The successful discovery, development, manufacture and sale of biologics is a long and expensive process with unique risks and challenges.
Most Series of PharmaShares are expected to cover exposure to pharmaceutical drug and biologic products; accordingly, most of the industry background in this base prospectus primarily addresses drug products. Industry-specific matters relating to other pharmaceutical products that may be covered by a Series of PharmaShares, such as medical devices, will be described more fully in the prospectus supplement for that Series.
The following diagram illustrates the phase of the typical drug lifecycle:
82
Discovery. The discovery of a potential medical application for a compound or substance can happen in the course of scientific research of a certain medical condition or by accidental insight. After discovery and some preliminary testing, a promising pharmaceutical product will move into the development phase.
Development and FDA Approval. In this phase, researchers conduct experiments to better understand and characterize the drug. Researchers focus on efficacy, tolerability, dose response, ADME (Absorption, Distribution, Metabolism, Excretion) and side effects. Preclinical research on non-human subjects focuses on ensuring that the drug will not be dangerous for human subjects. This usually involves in vitro and in vivo studies.
Clinical research in human subjects follows a successful preclinical trial. Before beginning clinical trials involving people, drug developers must submit an Investigational New Drug (IND) application to the United States Food and Drug Administration, or the "FDA." The FDA has 30 days to review these applications, which include information about previous animal studies, manufacturing, and clinical protocols. If the application is approved, the clinical research can commence.
Researchers design clinical trials to observe the impact of the drug in a controlled environment for a fixed period of time. Clinical trials usually start small and grow if the results of early trials are promising. Early studies aim to investigate drug safety and appropriate dosage while later studies assess drug effectiveness, often referred to as drug efficacy. In order for clinical development to continue, a drug must both be confirmed to be safe for patients and show signs of efficacy or desired clinical benefit for the indication under evaluation.
When human clinical trials produce evidence that a pharmaceutical product is both safe and effective, the pharmaceutical company will file an application to market the drug with the FDA. The New Drug Application (NDA) is required to include every detail about a new drug, including proposed labeling, drug abuse information, data from studies conducted outside the U.S. and other categories of data. An expert FDA review team carefully inspects every aspect of the NDA and makes a recommendation, usually within six to ten months. The final decision on whether to approve a drug is made by a senior FDA official. If the FDA approves a drug, the FDA works with the pharmaceutical company to develop and refine its labeling and prescribing information.
Manufacturing. At the same time that human clinical trials are being designed, the pharmaceutical company's manufacturing organization is engaged in scaling up production of the drug product for clinical trials. These production processes are fundamentally different for chemical compounds (small molecules) and biologic compounds (large molecules). Once the drug product can be manufactured in a stable and replicable modality, typically a pill or an injection, clinical trial lots are manufactured and the product is ready for human testing. Assuming the clinical trial(s) are successful, the pharmaceutical company develops a commercial manufacturing scale up plan to submit to the FDA as part of the overall submission.
83
The foregoing development phases are less relevant for PharmaShares which reference drugs that have already been approved for at least one indication, but should be considered in terms of the reference pharmaceutical company's other pipeline products and potential expansion of commercialized indications for the reference drug.
Setting Prices and Negotiating Reimbursement Rates. Beginning up to one year prior to FDA approval, pharmaceutical companies begin negotiating reimbursement rates with various payors, including insurers and pharmacy benefit managers (PBMs) who administer prescription drug programs for commercial health plans, self-insured employer plans, Medicare Part D plans, the Federal Employees Health Benefits Program and state government employee plans. In the U.S., there is a lack of price transparency under current regulatory policy and the multi-payer healthcare system that has resulted in a fragmented market for purchasing drugs, which reduces the ability of payors to negotiate prices. Unlike European government-run healthcare systems, Medicare, the single largest US payor for prescription drugs, cannot by law directly negotiate prices with drug manufacturers. This contrasts with most European systems, in which the decision to include a drug within a formulary, or list of preferred brand name and generic prescription drugs, is at the government's discretion. Medicare Part B is required to cover drugs and medical services deemed to be 'reasonable and necessary,' a standard that is susceptible to broad interpretation. In most instances, Medicare cannot refuse to provide coverage for a particular drug, no matter the cost, to patients. As a result, there may not be a direct correlation between how much a drug costs and its efficacy or safety profile.
However, healthcare as a service industry has been transitioning from a fee-for-service enterprise to fee-for-value, in which reimbursements are directly tied to standardized quality metrics and patient outcomes. This began with the 2012 Pioneer Accountable Care Organization (ACO) program and continued with the final rules for the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA). As a result of the unsustainability of ever-increasing drug prices in the pharmaceutical market, public backlash, and broader reimbursement trends in healthcare, payors and drug manufacturers have begun experimenting with value-based reimbursements. Some payors and benefits managers have negotiated variations of value-based contracts with pharmaceutical companies, coupling payments for a particular drug to corresponding indications and surrogate patient outcomes such as readmission rates and changes in blood count. The price of a drug varies depending on how well it performs within either a single patient or a given patient population. Payors and patients pay the premium price to manufacturers when the medication achieves desired outcomes. If the drug does not work as advertised, then manufacturers receive a lower price, or do not get paid at all.
Marketing and Sale. Once a drug receives FDA approval, the pharmaceutical company launches and begins marketing and selling the drug. Pharmaceutical companies advertise their drugs over a variety of media, including the internet, television, and print media and employ pharmaceutical representatives to deliver information about the drug directly to physicians and hospitals. The FDA regulates advertising and labeling to ensure that they are neither false nor misleading. Pharmaceutical companies are permitted to advertise a drug only for indications that have been approved by the FDA. Physicians may prescribe an FDA-approved drug for additional, "off-label" indications, but the amount of net revenue received for off label sales typically represents only a fraction of the net revenue for approved indications. A drug product may be approved for additional indications over time, but this requires a supplemental NDA (sNDA) to prove the drug is effective in this new indication. Net revenue received from later indications may be lower due to a shorter period of exclusivity to sell the drug for such indication before generic replacements are approved. Supplemental NDAs for an approved drug are also required to be filed for changes in manufacturing processes, minor reformulations such as new dosage or strength, new patient populations, changes in labelling and changes in the marketing of the drug.
Post-Market Drug Safety Monitoring. While the development and review process involve rigorous research, the time period is necessarily limited. If additional adverse effects of a drug are revealed over time after the drug has been approved, it could be taken off the market. Through MedWatch and MedSun, manufacturers, health professionals, and consumers report issues relating to approved drugs. Moreover, FDA officials continue monitoring manufacturing facilities in the U.S. and abroad to ensure that quality is maintained over time. Any time a pharmaceutical company wishes to reformulate or significantly change a drug, it must file a new IND to the FDA for approval.
Exclusivity Period. Generally, upon approval, drug products may be entitled to various types of exclusivity under applicable intellectual property and regulatory regimes. Pharmaceutical companies typically seek patent
84
protection, where available, in all significant markets and/or countries for each product in development. In the United States, the expiration date for patents is 20 years after the filing date. However, given that patents are often obtained early in the drug development process and given the significant amount of time needed to complete clinical trials and other development activities required for regulatory approval, the length of time between product launch and patent expiration is typically significantly less than 20 years. Applicable laws and regulations dictate the scope of any exclusivity to which a product is entitled upon its approval in any particular country. However, given the length of time required to complete clinical development of a pharmaceutical product, the periods of exclusivity that might be achieved in any individual case would not be expected to exceed a minimum of three years and a maximum of 14 years.
Pharmaceutical products may be entitled to other forms of legal or regulatory exclusivity which begins upon receiving regulatory approval. The scope, length, and requirements for each of these exclusivities vary both in the United States and in other jurisdictions. In the United States, if the FDA approves a drug product that contains an active ingredient not previously approved, the product is typically entitled to five years of non-patent regulatory exclusivity. Other products may be entitled to three years of exclusivity if approval was based on the FDA's reliance on new clinical studies essential to approval submitted by the NDA applicant. If the NDA applicant studies the product for use by children, the FDA may extend the longest exclusivity applicable to the product by an additional 180 days. For products that are either used to treat conditions that afflict less than 200,000 individuals in the United States or for which there is not a reasonable expectation that the research and development costs will be recovered, the FDA may designate the pharmaceutical as an orphan drug and grant it seven years of market exclusivity following approval of the drug during which period the FDA will not approve any other application for the same drug and for the same orphan disease or condition. Biologics may be entitled to exclusivity under the Biologics Price Competition and Innovation Act, which was passed on March 23, 2010 as Title VII to the Patient Protection and Affordable Care Act. The law provides a pathway for approval of biosimilars following the expiration of 12 years of exclusivity for the innovator biologic. Biologics are also eligible for the orphan drug exclusivity discussed above.
The Drug Price Competition and Patent Term Restoration Act of 1984 (commonly known as the Hatch-Waxman Act) permits a patent holder to seek a patent extension, commonly called a "patent term restoration," for patents on products (or processes for making the product) regulated by the Federal Food, Drug, and Cosmetic Act. The length of the patent extension is roughly based on 50 percent of the period of time from the filing of an Investigational New Drug Application (NDA) for a compound to the submission of the NDA for such compound, plus 100 percent of the time period from NDA submission to regulatory approval. The extension, however, cannot exceed five years and the patent term remaining after regulatory approval cannot exceed 14 years. Biologics licensed under the Public Health Service Act are similarly eligible for terms of patent restoration.
Both private parties and government authorities may challenge, invalidate or circumvent patents. For example, manufacturers of generic pharmaceutical products may file Abbreviated New Drug Applications with the FDA seeking to market generic forms of products prior to the expiration of related patents by asserting that the patents are invalid, unenforceable and/or would not be infringed. In addition, parties may file challenges to the validity of a patent under the 2011 Leahy-Smith America Invents Act, which created inter partes review and post grant review procedures for challenging patent validity in administrative proceedings at the United States Patent and Trademark Office. Some foreign governments have sought compulsory licenses to patents, which decrease or eliminate sales in their jurisdictions, to further their domestic policies or on the basis of national emergencies, such as HIV/AIDS.
Competition from Generics. Once a patent expires, any manufacturer may develop a drug in its "generic" version with the same dosage, strength, safety, quality, performance, and intended use. Because the safety and efficacy of the drug is already established, generic manufacturers do not have to conduct clinical trials and may file an abbreviated NDA for bio-equivalence studies. Lower-priced generics can quickly and significantly erode the market share for a drug that has lost its patent protection. Certain biopharmaceutical products, such as biologic drugs, may be less affected, because they cannot be readily substituted.
Reference Products may include drugs which face generic competition, because exclusivity has been lost for one or more of the indications for which that drug is used. This may be the case at the time of the initial issuance
85
of the related series of Up PharmaShares or at some time thereafter. Holders of Up PharmaShares should monitor the relevant patent period and any extensions.
Obsolescence. Some pharmaceutical products remain in the market place for an extended period of time while others become obsolete. A pharmaceutical product could be pushed out for any number of reasons: competition from generic or biosimilar drugs, shifting industry preferences for a new product, changing governmental regulations, discovery of untenable side effects, or increase in the price of raw materials needed to manufacture the drug. With more biotechnology than ever before, drug developers constantly discover new drugs, often causing older drugs to become outdated. A pharmaceutical company can make efforts either to rebrand or to stop producing an outdated drug.
Executive branch initiatives and political pressure for drug price controls.
Major developments during the past five to ten years, including the Affordable Care Act, the loss of patent exclusivity on successful products, and higher rebates and discounts offered by pharmaceutical companies in response to increased competition and the negotiating power of insurance companies and PBMs, have created an estimated $200 billion downward pressure on pharmaceutical industry revenues. Innovation is decreasing globally, while measures such as international price controls exacerbate the issue. The only remaining option to grow the pharmaceutical industry have been price increases, so small molecule and specialty drug prices have soared. Further, the pharmaceutical industry is beginning to focus on high-cost drugs with less competition, so that high launch prices can help compensate for the downward pressures on revenue. High-cost drugs currently account for 35 to 40 percent of health plan spending, and will likely increase to more than half of drug spending in the next five years. Consumers and certain other payors bear the bulk of the burden under the current system, in which growth in the pharmaceutical industry is driven by price increases rather than innovation. Little incentive currently exists to control list prices, as health plans and PBMs benefit from increasing list prices.
In an attempt to address the foregoing issues, the Trump Administration released the Department of Health and Human Services (HHS) Blueprint Plan, titled "American Patients First," on May 11, 2018. If the Blueprint Plan were to be implemented, pharmaceutical companies can anticipate that drug revenues will decline. The main concerns identified by the Blueprint Plan were (1) high list prices for drugs, (2) seniors and government programs overpaying for drugs, (3) high and rising out-of-pocket costs for consumers, and (4) foreign governments exploiting American innovation, research and development. The Blueprint Plan intends to use the following strategies in a private-market focused approach to counteract these concerns: (1) improved competition, (2) better negotiation, (3) creating incentives for lower list prices, and (4) lowering out-of-pocket costs.
The Blueprint Plan proposes a phased and gradual approach to lowering drug prices. Although the Blueprint Plan outlines some immediate action by HHS, it leaves space for feedback and adjustment to the plan in the future. For example, the Blueprint contemplates transitioning the payment model from a rebate system based on list prices to one based on fixed rates with upfront discounts. However, HHS would likely need Congressional approval to implement such a change in the safe harbor regulations.
To increase competition, the Blueprint Plan proposes the following immediate actions: (1) preventing manufacturer gaming of regulatory processes, (2) allowing the Centers for Medicare & Medicaid Services to innovate services and negotiate for lower drug prices, and (3) promoting innovation and competition in biologics. The expansion of the biologics market would facilitate future opportunities to promote the increased use of biosimilars. Additionally, proposed changes to the Medicare Prescription Drug Program in the 2019 Part C and D regulation would allow Medicare beneficiaries receiving low income subsidies to access biosimilars at a lower cost. To further increase competition, HHS would be directed to look for opportunities to encourage the sharing of samples for generic drug development.
One proposal to increase competition in the generics market is to accelerate FDA approval of generic drugs. In 2017, the FDA developed a Drug Competition Action Plan to focus the agency's efforts to enable patients to access more affordable medication by: (1) improving the efficiency of generic drug development, (2) maximizing scientific and regulatory clarity for complex generic drugs, and (3) closing loopholes which allow gaming of FDA rules. Moreover, the Fiscal Year 2019 Budget proposed that companies be prohibited from using their 180-day generic exclusivity under the theory that it delays real competition and consumer savings.
86
To facilitate better negotiation, the Blueprint Plan proposes: (1) experimenting with value-based purchasing in federal programs, (2) allowing more substitution, especially for single source generics, in Medicare Part D, (3) giving Medicare Part D plan sponsors more power in their negotiations with manufacturers, (4) assessing the problem of foreign free-riding, (5) leveraging the competitive Medicare Part B acquisition program, and (6) reporting on the details of negotiating lower Medicare Part B drug prices for Medicare Part D plans. Part B drugs, such as those used during chemotherapy and blood infusions, are expensive and normally administered in hospital outpatient settings and doctor's offices. Insurers compete for business in Part D and negotiate prices for their members, but there is no such price negotiation in Part B. Moving drugs covered under Medicare Part B into the Part D program and adding the ability to negotiate has the potential to reduce drug costs to the government (and revenues to pharmaceutical companies) by billions of dollars.
Beyond the Blueprint Plan, HHS has taken action to support better negotiation, such as finalizing the changes to the Medicare Prescription Drug Program in the 2019 Part C and D proposed regulation to allow for faster mid-year substitution of generic drugs onto formularies. HHS also aims to require site neutrality in payment and to develop a means to evaluate the accuracy of national drug spending data. Moreover, the Fiscal Year 2019 Budget included a number of proposals designed to facilitate negotiation, including: (1) clarifying the definition of brand name drugs, (2) a 5-part plan to modernize the Medicare Part D program, (3) the establishment of an inflation limit to address abusive drug pricing by pharmaceutical companies, (4) increasing the integrity and transparency of the Medicaid Drug Rebate Program, and (5) new demonstration authority for up to five states to test the reforms.
HHS plans to incentivize lower list prices by immediately updating Medicare's drug-pricing dashboard to increase transparency. Additionally, the FDA will evaluate the inclusion of list prices in advertising. Following these immediate aims, HHS seeks to (1) reform the rebate system and discount programs, (2) develop incentives to discourage Part B and D drug price increases, and (3) consider fiduciary status for PBMs.
The Blueprint Plan seeks to improve the usefulness of the Part D Explanation of Benefits statement by providing access to lower cost alternatives and taking action to prohibit Part D contracts from instructing pharmacists not to inform patients when they can pay less without using insurance. HHS plans to employ more measures to inform Part D and Part B beneficiaries of lower cost options and provide information on cost to Part D beneficiaries at least annually.
Changes in the benefits offered under insurance plans have resulted in greater exposure on the part of consumers to the increasing out-of-pocket cost of prescription drugs. In 2016, approximately 40 percent of adults with employer-sponsored insurance and more than half of adults with individually-purchased insurance enrolled in a high-deductible plan. This means that consumers who have not met their high deductible or are subject to co-insurance pay for drugs based on pharmacy list prices. These prices are unmitigated by PBM rebates, and, as a result, consumers do not get the benefit of the gap between list and net prices. Under the current system, plans may be incentivized to push utilization of higher cost drugs because doing so more quickly reduces the level of their liability and offers the plan increased profits if higher drug costs can be negotiated for higher rebates which exceed the plan's own forecasts. This increases costs to beneficiaries and the government while sponsors’ costs are reduced. The price controls proposed in the Blueprint Plan aim to alleviate these types of issues and mitigate system abuse.
The Affordable Care Act placed a new tax on branded prescription drug sales to government health care programs. In 2011, drug companies paid $2.5 billion in taxes; in comparison, they paid $4.1 billion in 2018. The Affordable Care Act also increased the mandatory rebate on brand name drugs to 23.1% and effectively doubled the number of Medicaid rebate-eligible drugs. These discount expansions may have placed pressure on list prices and caused drug manufacturers to raise prices overall. The Affordable Care Act also increased the demands of the 340B drug discount program and increased the number of 340B hospitals from 1,700 in 2011 to 2,479 in 2017. The billions of dollars in discounted sales and cross-subsidization from the 340B expansion have also caused drug manufacturers to try to offset reduced revenues or increase list prices.
International prices controls in Europe became more pronounced in 2008, during the global financial crisis. Most European countries used single-payor healthcare systems to impose drug price controls alongside increased co-payments and value-added tax rates on prescription drugs. International price controls paired with the threat of market lockout, as well as intellectual property concerns, prevented drug companies from charging market rates for their products in foreign markets and delayed the availability of innovative drugs to patients in countries with such
87
policies. The Blueprint Plan seeks to better understand price disparities in the international market by updating historical studies to analyze drug prices in Organisation for Economic Co-operation and Development member countries with the goal of justifying revision of the Centers for Medicare & Medicaid Services and HHS standards to allow various U.S. payors to negotiate drug price to the same extent as that permitted by foreign governments.
The Bipartisan Budget Act of 2018 ("BBA") impacted certain Medicare programs, including Part C (Medicare Advantage) and drug pricing under Medicare Part D, affecting both the pricing and sales volume of certain drug products. The BBA is evidence of the current political climate in which there is continued pressure to enact drug price controls. This climate, paired with the Blueprint Plan, if implemented, is likely adversely affect pharmaceutical company revenues.
Pharmaceutical Industry Payors
The net revenues from a pharmaceutical product are determined by its pricing and the volume of sales. Pharmaceutical products are purchased from pharmaceutical companies by a variety of payors, including private payors, third-party payors such as insurance companies, the U.S. federal government, state and public agency payors and foreign (government and private) payors. The ability of each such party to negotiate rates on drug products and volume discounts varies and, as a result, the pricing structure for each such payor will be different, especially in the case of government payors who have the ability to impose price controls on certain drug products. In addition, one or more series of Up PharmaShares may include Reference Products that are elective and are not covered by government or private payors. These drugs may have greater fluctuations in price and sales may be seasonal in nature.
Private Payors. Private payors include private health insurance plans, employers, wholesale distributors, and pharmacies. Private payors are sensitive to the cost of drugs and are increasingly seeking to implement cost containment measures to control, restrict access to, or influence the purchase of drugs, biologicals, and other health care products and services. Private payors have significant influence over the products their customers choose. For example, private health insurance plans restrict coverage of some products by means of (1) payer formularies under which only selected drugs are covered, (2) variable co-payments that make drugs that are not preferred by the payor more expensive for patients, and (3) utilization management controls, such as requirements for prior authorization or failure on another type of treatment. Private payors often impose these obstacles to coverage on higher-priced drugs, which result in patients becoming responsible for a higher percentage of the total cost of a given drug. This type of payor-driven restriction lowers the demand for a product if patients cannot afford the additional cost or do not have a strong preference for a particular product. While rebates related to government programs are created pursuant to laws and regulations, private payors create rebate and discount programs through contractual agreements with pharmaceutical companies. Various factors, particularly the ability of private payors to control patient access to products, provide private payors with leverage to negotiate rebates or discounts.
Pharmacy Benefit Managers. While not a direct link in the supply chain for pharmaceutical products, PBMs have become an integral part of most consumer drug purchases. PBMs work with third-party private payors, such as private insurers and self-funded employers, to manage consumer drug purchases by defining which drugs will be paid for and the portion payable to the pharmacy and the amount that the consumer must pay out-of-pocket when the prescription is filled.
U.S. Government. Sales of pharmaceutical products depend in significant part on the extent of coverage and reimbursement from government programs, such as Medicare and Medicaid. The Centers for Medicare & Medicaid Services, a branch of HHS, is the federal agency that runs the Medicare Program and monitors Medicaid programs offered by each state. These U.S. government agencies regulate reimbursement, pricing and coverage of products in order to control costs and utilization levels for certain products. Generally, under current law, U.S. government agencies do not have the ability to negotiate drug prices directly with pharmaceutical companies. This may change in the future if current rules are amended.
Medicare. Medicare, a U.S. federal government insurance program, covers individuals 65 years and older, individuals of any age with certain disabilities, and individuals with end-stage renal disease. The primary Medicare programs that affect reimbursement for pharmaceutical companies are Medicare Part B, which covers physician services and outpatient care, and Medicare Part D, which provides a voluntary outpatient prescription drug benefit.
88
Medicaid. Medicaid is a government health insurance program for low-income children, families, pregnant women, and people with disabilities. It is jointly funded by the federal government and individual state governments. Medicaid is administered by individual states within parameters established by the federal government, so coverage and reimbursement for drugs and biologics varies by state. Medicaid also includes the Drug Rebate Program under which pharmaceutical companies are required to pay a rebate to each state Medicaid program for the quantities of products that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program.
Rebates and Pricing for Government Payors. Pricing and rebate calculations vary among products and programs. The calculations are complex and are often subject to interpretation by pharmaceutical companies, governmental or regulatory agencies and the courts. Rebates and pricing from governmental payors can be unpredictable, particularly when the political climate is volatile or when a governmental agency revises its requirements. For example, the Budget Control Act of 2011 required Medicare payments for all items and services, including drugs and biologics, to be reduced by 2.0% under sequestration. Subsequent legislation extended the 2.0% reduction, on average, to 2025. This demonstrates the impact that federal and state legislation can have on revenue from governmental payors.
Federal Upper Limits for Medicaid pharmacy drug pricing, which are updated monthly, can be found at: https://www.medicaid.gov/medicaid/prescription-drugs/pharmacy-pricing/index.html.
Drug pricing for Medicare Part B, which is updated every few months, can be found at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/
McrPartBDrugAvgSalesPrice/2018ASPFiles.html.
Drug rebates required to be paid by pharmaceutical companies for Medicare Part D can be found at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Pharma.html.
Federal Supply Schedule. Pharmaceutical companies must participate in the Department of Veterans Affairs (VA) Federal Supply Schedule (FSS) pricing program to be eligible to receive federal funds from Medicare, Medicaid, grantees and other federal agencies. To participate, pharmaceutical companies are required to enter into an FSS contract with the VA, under which they must make covered drugs available to the VA, the Department of Defense, the Public Health Service, and the Coast Guard. Pricing under the FSS contract is capped pursuant to a statutory federal ceiling price formula set forth in Section 603 of the Veterans Health Care Act of 1992.
340B Drug Pricing Program. Federal law also requires that any company that participates in the Medicaid Drug Rebate Program also participate in the Public Health Service 340B drug pricing program. Under 340B, statutorily-defined covered entities are charged no more than the "ceiling price," which is calculated using a statutory formula based on the average manufacturer price and rebate amount for the outpatient drug. Entities covered by 340B include a variety of community health clinics, entities that receive health services grants from the Public Health Service, as well as hospitals that serve a disproportionate share of low-income patients.
Fraud and Abuse with Government Payors. Pharmaceutical companies participating in federal healthcare programs are subject to various U.S. federal and state laws pertaining to healthcare fraud and abuse. Violations of U.S. federal and state fraud and abuse laws may be punishable by criminal, civil and administrative sanctions, including fines, damages, civil monetary penalties and exclusion from federal healthcare programs. Applicable statutes include the Anti-Kickback Statute, False Claims Act, False Statements Statute, Civil Monetary Penalties Law, Health Insurance Portability and Accountability Act of 1996, and Open Payments Program. Federal and state authorities devote significant attention and resources to enforcement of fraud and abuse laws within the pharmaceutical industry. Private individuals have been active in alleging violations of the law and bringing suits on behalf of the government. Still, many of these laws and regulations contain ambiguous requirements that government officials have not yet clarified and that may be likely to lead to more claims of alleged breach.
Wholesale Distributors. Wholesale distributors purchase pharmaceutical products from manufacturers and distribute them to a variety of customers, including pharmacies, hospitals, and long-term care facilities. The wholesale distribution industry has gone through significant change and consolidation due in large part to the increasing pressures to lower costs. Between 1975 and 2000, the number of wholesale distributors in the U.S. has significantly declined and it appears that this decline may continue due to consolidation.
89
Pharmacies. There are several types of pharmacies, including independent pharmacies, chain drug stores, pharmacies in supermarkets and other large retail establishments, and mail-order pharmacies. Most pharmacies purchase their drug supply from a wholesale distributor, but many large institutional pharmacies, retail chain pharmacies, specialty pharmacies, and mail-order pharmacies obtain drugs directly from the manufacturer. These organizations can deal directly with manufacturers because they already possess the operational infrastructure (warehousing facilities, distribution vehicles, and inventory control systems) necessary to bypass wholesalers. Once a pharmacy takes possession of the drug products, it distributes the products to physicians or directly to consumers. The distribution of drug products through pharmacies is generally limited to oral tablet and liquid products, and excludes a full scope of injectable pharmaceutical products.
Non-U.S. Market Payor System. The requirements governing drug pricing and reimbursement vary widely from country to country. For instance, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. Pharmaceutical companies must engage with appropriate authorities in individual countries and regions on a continuous basis regarding the operational, reimbursement, price approval and funding processes that are separately required in each country.
Impact of high deductible insurance plans and rebates by pharmaceutical companies. The escalating cost of health insurance has led many families to elect private healthcare and prescription insurance coverage with high deductibles. This often results in lower amounts of filled prescriptions during the first quarter of a calendar year. In many cases, pharmaceutical companies will offer rebates to incentivize patients to remain current with their medication, particularly within the first quarter of a year. This may have a larger impact on drug products that do not have an immediately life threatening impact if doses are missed or delayed.
Factors Affecting Net Revenue for Reference Products
There are many variables that affect the net revenue performance of a Reference Product underlying a Series of PharmaShares.
Safety and Efficacy Issues. Significant safety or efficacy issues could arise with respect to a Reference Product (or a competitor of that Reference Product) and have a material adverse effect on the net revenues from that drug. Pharmaceutical companies receive regulatory approval for a drug based on data obtained in controlled clinical studies of limited duration. However, following regulatory approval, safety or efficacy issues may arise as patients use the drug over a longer term and as independent researchers continue to study the effects of the drug more comprehensively. If patients or researchers report new safety, efficacy, or misuse issues, a pharmaceutical company may be required to amend the conditions of use or sales might even be restricted by government agencies. Additionally, a safety issue may impact an entire class of drugs with similar molecular targets, even when the direct adverse side effect is observed from a single drug within the class. This action can be severe, and can even result in market withdrawal. These safety and efficacy considerations apply to all indications of a drug. As such, a drug that has not resulted in toxicity in an initial indication may encounter safety issues upon prescription for future indications.
Litigation and Regulatory Action. Product liability claims, lawsuits, safety alerts, and product recalls, regardless of their ultimate outcome, can significantly impact the net revenues from the affected reference drug. Consequences may include damage to reputation and brand loyalty for the affected reference drug, loss or delay of sales related to modifying the manufacturing process, adverse effect on the ability to attract and retain customers for the affected drug and loss of future sales due to cessation of marketing from litigation claim. This risk will be a consideration of both an investor in a pharmaceutical company and PharmaShares applicable to a Drug Product of a series, but the impact of such items may have different consequences to the amount of any associated loss of investment.
Biologics. Governmental agencies monitor, regulate and limit the sourcing, production and transport of ingredients derived from living animal or plant material that are used to make biologics. Manufacturing biologics, especially in large quantities, is often complex and costly due to the frequent use of innovative technologies in specifically designed facilities with sophisticated quality assurance and quality control procedures. The Biologics Price Competition and Innovation Act created a framework for the approval of biosimilar drugs in the United States, allowing competitors to access and rely upon data from already approved biologic products and making the process
90
of developing biologic drugs even more uncertain. Companies around the world are currently developing biosimilars that have the potential to compete with existing biologics that may serve as a reference drug. If competitors are able to obtain marketing approval for biosimilars, the reference drug will be subject to increased competition. Moreover, a biosimilar product may be less costly to bring to market, may be priced lower, and may result in a reduction in the pricing and reimbursement of the original biologic reference drug. In addition, the pharmaceutical company that developed and manufactures the existing biologic might face additional litigation and administrative proceedings relating to the validity and scope of its patent for the original biologic drug.
Manufacturing. The manufacture of drugs and other pharmaceutical products tends to be a complex process. Countless sources of error may arise during the manufacturing process, including: equipment malfunction, failure to follow specific protocols or strict regulatory requirements, problems with raw materials, delays related facilities, limits on manufacturing capacity, changes in the types of products produced, and environmental factors. Many pharmaceutical companies use products sourced from single suppliers in both their pharmaceutical and biologic manufacturing processes. Interruption in the supply or changes in trade regulation of those products could potentially halt the production of a reference drug. Even with business interruption insurance, errors in the manufacturing process or disruptions to supply could adversely impact the net revenue of a Reference Product.
Government Action. When a particular jurisdiction that is the source of significant sales for a Reference Product modifies the funding that is available to government agencies or programs in that jurisdiction for acquisition of that product or its standards relating to safety, efficacy or labeling that affect that product, the net revenues of the Reference Product may be adversely affected. Governments may require a pharmaceutical company to amend the conditions of use for a Reference Product based on new government regulations. Moreover, if the volume of sales of a Reference Product is dependent on government contracts, net revenues will be affected by governmental budgetary actions, such as a reduction or increase in pharmaceutical rebates. Further, ongoing public and legislative pressure to reduce drug prices, as discussed in greater detail under "– Executive branch initiatives and political pressure for drug price controls," if such pressure results in government-imposed price controls or voluntary price reductions, is likely to have an adverse effect on net revenues for an affected Reference Product.
DESCRIPTION OF THE TRUST AGREEMENT
General
The PharmaShares Master Trust will be governed by the Master Trust Agreement and each Series of the PharmaShares Master Trust will be governed by, and will issue its Paired PharmaShares pursuant to, a Series Trust Supplement. The Master Trust Agreement and each Series Trust Supplement are collectively referred to as the "Trust Agreement" for purposes of this section of the base prospectus.
The PharmaShares Master Trust will be composed of one or more Series constituting separate designated series of assets and shareholders in accordance with the Delaware Statutory Trust Act and each Series will be a separate series of the PharmaShares Master Trust within the meaning of the Delaware Statutory Trust Act. The debts, liabilities, obligations and expenses incurred, contracted for or otherwise existing with respect to each Series will be enforceable against the assets of that Series only, and not against the assets of the PharmaShares Master Trust generally or any other Series. Notice of these limitations on interseries liabilities has been set forth in the certificate of trust of the PharmaShares Master Trust filed in the Office of the Secretary of State of the State of Delaware, as required under the statutory provisions of the Delaware Statutory Trust Act relating to limitations on interseries liabilities. The enforceability of, and any equitable exceptions to, the concept of limiting interseries liability has not been adjudicated by any state court or federal bankruptcy court, as discussed under "RISK FACTORS — Limitations on interseries liability for Delaware series trusts have not been reviewed by the courts" in this base prospectus. Notwithstanding that each Series is a series of the PharmaShares Master Trust, each Series is intended to be treated as though it is a separate legal entity, and each Series will make and execute contracts and instruments, sue and be sued, and otherwise conduct its activities and affairs in its own name. The PharmaShares Master Trust itself will not have any assets or liabilities generally, and all assets and liabilities will be allocated to one or more Series.
91
The Trustee
[●], a [●], will act as trustee for each Series of the PharmaShares Trust. The office of the trustee is located at [●], and its telephone number is [●]. [●] will also act as the calculation agent.
For performing its duties under the Master Trust Agreement and the applicable Series Trust Supplement, the trustee will be compensated by that Series as described generally under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series." The fees and expenses payable by your Series to the trustee are specified under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
The Trust Agreement will provide that the trustee and any director, shareholder, officer, employee, agent, affiliate and subsidiary of the trustee will be indemnified by your Series in the circumstances and to the extent described below under " — Certain Matters Regarding Us and the Trustee." In addition, the Trust Agreement will provide that the trustee may be terminated and replaced by a successor trustee in the circumstances described below under "— Trustee Termination Events."
The trustee will make reasonable efforts to collect, on behalf of your Series, all payments under the treasury securities, but the trustee will not be obligated to fund any collection or legal fees or expenses out of its own funds or assets.
[●], and any successor trustee under the Trust Agreement, must satisfy the following eligibility criteria: (i) it must be a bank or trust company organized and doing business under the laws of the United States of America or in the State of Delaware, authorized under such laws to exercise corporate trust powers and subject to supervision or examination by federal or state authority, (ii) it must have a combined capital and surplus of at least $20,000,000, (iii) it or its parent must maintain any credit or deposit rating required by nationally recognized rating organizations (as of the date of this base prospectus, "A-1" for Standard & Poor's Rating Service or "P-1" for Moody's Investors Service, Inc.) and (iv) it must accept and act in the capacity of trustee for your Series and for the PharmaShares Trust. If at any time the trustee ceases to be eligible in accordance with these eligibility criteria, the trustee must resign immediately in the manner and with the effect specified in the Trust Agreement.
The trustee has no liability in its individual corporate capacity with respect to any contractual or other claim or obligation relating to the Trust Agreement and any party which is asserting any claims or seeking the payment or discharge of any liabilities relating to a Series must look solely to the assets of that Series.
Duties of the Trustee and the Calculation Agent
Under the Trust Agreement, the duties of the trustee and calculation agent will include:
·administering the PharmaShares Trust and each Series of that trust;
·calculating and paying the fees and expenses of each Series, on behalf of that Series;
·effecting Paired Issuances in accordance with the provisions described under "DESCRIPTION OF THE PHARMASHARES — Paired Issuances;"
·effecting Paired Optional Redemptions and delivering cash and treasury securities to redeeming shareholders on each Redemption Date in accordance with the provisions described under "DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions;"
·making Periodic Income Distributions, Periodic UV Performance Distributions and the Final Distribution to the holders of the Up PharmaShares and Down PharmaShares of each Series;
·acting as the custodian for the treasury securities and all other assets of each Series;
·settling purchase orders for treasury securities that are placed on behalf of each Series by the administrative agent, in accordance with the directions of the administrative agent;
92
·calculating the respective daily Per Share Underlying Values of one Up PharmaShare and one Down PharmaShare of each Series and providing such values to the administrative agent on each business day for posting on the website maintained by the administrative agent at http://www.pharmashares.net;
·calculating, for each Series on each Periodic Calculation Date, the amount of treasury income on deposit in that Series after deducting the Series fees and expenses and the Periodic Income Distribution to be made on the PharmaShares of that Series;
·calculating, for each Series and each Periodic Calculation Date, the respective Underlying Values of the Up PharmaShares and the Down PharmaShares of that Series and the Periodic UV Performance Distribution, including the applicable Equalization Payment, to be declared on either the Up PharmaShares or the Down PharmaShares of that Series on that Periodic Calculation Date;
·calculating, in connection with each Redemption Order Date, any Early Termination Date and the Final Scheduled Termination Date for each Series, the respective Underlying Values of the Up PharmaShares and Down PharmaShares and the Final Distribution to be declared on the PharmaShares on those dates, based on the Reference Value published on the last Publication Date that precedes the applicable Redemption Order Date, Early Termination Date or Final Scheduled Termination Date;
·preparing any notices required to be provided by the trustee under the Trust Agreement;
·providing notification of the occurrence of certain Termination Triggers; and
·performing all of the other obligations expressly required of it under the Trust Agreement and the other transaction documents.
The trustee may engage any other persons to assist it with its duties under the Trust Agreement, including its affiliates, but the trustee will remain liable to the shareholders of each Series for the performance of these duties to the extent expressly provided in the Trust Agreement.
The trustee will not make any representations or warranties as to the validity or sufficiency of various matters related to any Series, including, among other matters, the Trust Agreement, the shares issued by any Series, the assets in any Series, any licensing agreement or any other related agreement, document or instrument. In addition, the trustee makes no representation or warranty regarding federal securities laws or state securities or "blue sky" laws in connection with the distribution of the shares issued by any Series or their registration under applicable federal or state securities laws. The trustee is required to perform only those duties specifically enumerated under the Trust Agreement. Upon receipt of the various certificates, statements, reports or other instruments required to be furnished to it, the trustee is required to examine them to determine whether they are in the form which the Trust Agreement requires. However, the trustee is not responsible for the accuracy or content of any of these documents or information furnished to it under the Trust Agreement and is not required to perform any due diligence or investigation with respect to any documents, information or instructions by the administrative agent or any other authorized parties.
The trustee may be held liable under the Trust Agreement for its own grossly negligent action or failure to act, or for its own willful misconduct. However, the Trust Agreement limits the trustee's liabilities, including, among other matters, that the trustee will not be personally liable with respect to any action it takes, suffers or omits to take in good faith with respect to any Series in accordance with the depositor's directions or the directions of the administrative agent or holders of 66 and 2/3% of the Paired PharmaShares of any Series. The trustee is not required to expend or risk its own funds or otherwise incur any financial liability in the performance of any of its duties under the Trust Agreement, or in the exercise of any of its rights or powers, if it believes in good faith that repayment of those funds or adequate indemnity against any related risk or liability is not reasonably assured to it.
[●] is not the issuer of the Up PharmaShares or the Down PharmaShares of any Series under the Securities Act and has not been involved in the preparation or review of this base prospectus or the prospectus supplement for
93
any Series and assumes no responsibility for any misstatements or omissions contained in this base prospectus or the prospectus supplement for any Series or for compliance with the Securities Act or other applicable securities laws in connection with the offer or sale of the Up PharmaShares or Down PharmaShares of any Series or with respect to the provisions of the Exchange Act, or other applicable securities laws as they may relate to the PharmaShares Trust or any Series or any affiliated persons of the PharmaShares Trust or any Series.
The Depositor
We will act as "depositor" for the PharmaShares Trust and each Series of the PharmaShares Trust. Our principal duties as depositor consist of forming the PharmaShares Trust and each of its Series. For more information about us, please see "PharmaShares Advisors, LLC" in this base prospectus.
The Administrative Agent
We will be acting as the administrative agent for the PharmaShares Trust and for each Series of the PharmaShares Trust. In our capacity as administrative agent, we will perform or oversee the performance of a number of duties on behalf of each Series, including the following:
·directing the trustee on each Periodic Calculation Date and each Issuance Date in the acquisition of new treasury securities for each Series, including placing, or directing a designated service provider to place, purchase orders for such treasury securities, in accordance with the acquisition guidelines that are specified in the Trust Agreement and described in this base prospectus under "THE PHARMASHARES TRUST — United States Treasury Obligations;"
·processing redemption and issuance orders from Authorized Participants;
·directing the trustee in effecting Paired Optional Redemptions and Paired Issuances;
·selecting treasury securities to be delivered to redeeming Authorized Participants in connection with Paired Optional Redemptions in accordance with the rules specified in the Trust Agreement and described in this base prospectus under "THE PHARMASHARES TRUST — United States Treasury Obligations;"
·performing certain calculations which we are required to provide to the trustee or to post on the http://www.pharmashares.net website;
·selecting the individual brokers and the provider of a composite projection that will make up the Broker Reporting Group for each Series;
·calculating the Projected Breakpoint Increase or Projected Breakpoint Decrease for each Series prior to each Calculation Period;
·maintaining the website at http://www.pharmashares.net where investors can obtain information about the performance of each Series of PharmaShares;
·preparing and filing all periodic reports required to be filed by each Series;
·maintaining the listing of both classes of PharmaShares issued by each Series on a national stock exchange; and
·providing notification of the occurrence of certain Termination Triggers.
For performing our duties as administrative agent under the Trust Agreement for each Series, we will be compensated by that Series as described generally under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series." The fee payable by your Series to us as administrative agent is specified under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement.
94
The Marketing Agent
[·] will act as the marketing agent for the PharmaShares Trust and each Series of the PharmaShares Trust. The marketing agent will perform the following duties:
·develop a marketing plan for each Series;
·prepare marketing materials and organize investor presentations;
·coordinate investor education and advertising for the PharmaShares;
·hire additional marketing agents to assist with the marketing of the PharmaShares of one or more Series; and
·perform the additional duties, if any, described in the Series Trust Supplement for your Series.
For performing its duties under the Master Trust Agreement and the applicable Series Trust Supplement for each Series, the marketing agent will be compensated by that Series as described generally under "THE PHARMASHARES TRUST — Fees and Expenses of Each Series." The fees and expenses payable by your Series to the marketing agent are specified under "THE TERMS OF YOUR SERIES — Fees and Expenses" in your prospectus supplement. [·] will pay the fees of any additional marketing agents it may appoint from time to time out of its marketing fee.
Certain Matters Regarding Us and the Trustee
The Trust Agreement will provide that we, the trustee, in its capacity as trustee and as calculation agent, and any of our or the trustee's respective directors, officers, members, managers, employees and agents will not incur any liability for taking any action, or omitting to take action, in good faith pursuant to the Trust Agreement or for errors in judgment, provided that we and any person related to us, will not be protected against any liability that results:
·from gross negligence, willful misfeasance or bad faith in the performance of our duties; or
·by reason of reckless disregard of our obligations and duties under the Trust Agreement.
In addition, we, as administrative agent, will be required to compensate the applicable Series for any reduced income resulting from our violation of the requirements and conditions contained in Appendix B to this base prospectus relating to transactions with affiliated persons.
The Trust Agreement may also provide that we, the trustee, and any of our or the trustee's respective directors, officers, members, managers, shareholders, employees, agents, affiliates (as defined in Regulation S-X of the Securities Act) and subsidiaries (each of the foregoing, a "related person") will be:
·entitled to indemnification by each Series, individually and not jointly, for liabilities and claims related to matters involving that particular Series; and
·held harmless by each Series against any loss, liability or expense incurred in connection with any legal action or claim relating to the Trust Agreement and that particular Series and related transaction documents or the shares issued by that Series, unless the loss, liability or expense incurred was a result of our, the trustee's or a related person's gross negligence, willful misfeasance, willful misconduct or bad faith in the performance of our, the trustee's or the related person's respective duties and obligations or by reason of the reckless disregard of these duties and obligations.
The trustee will not be liable for any indemnification provided to any person by a Series or by us. Any indemnification expenses will be paid out of the treasury income of each Series or will be paid by us if the treasury income of a Series is insufficient. Any amounts payable to the trustee or related person in respect of indemnification
95
will be payable in advance or will be secured by a lien on the assets of the related Series and will include, among other costs and expenses, the costs and expenses (including counsel fees) of investigating and defending against all such claims and liabilities.
In addition, the Trust Agreement will provide that neither we nor the trustee will be under any obligation to appear in, prosecute or defend any legal action which is not incidental to our or the trustee's respective responsibilities under the Trust Agreement or which in our or its opinion may involve us or it in any expense or liability. We may, however, in our discretion take any such action which we may deem necessary or desirable with respect to the Trust Agreement and the rights and duties of the parties and the interests of the shareholders under the Trust Agreement.
Any person into or with which the trustee may be merged or consolidated, or any person succeeding to the business of the trustee, will be the successor of the trustee under the Trust Agreement so long as that successor is otherwise qualified and eligible under the provisions of the Trust Agreement.
Modification and Waiver
No amendment to the Master Trust Agreement and a Series Trust Agreement for a Series may:
·modify the formula for calculating Underlying Value for that Series or any defined terms related to that formula; or
·modify the Up/Down Ratio of the Up Investment Amount to the Down Investment Amount that is required to be maintained on the books and records of that Series.
Without the written consent of each shareholder of PharmaShares of a Series that would be adversely affected, and subject to the restrictions outlined above, no amendment to the related Series Trust Supplement may:
·modify the amount or timing of any distributions that are required to be made on the PharmaShares of that Series; or
·reduce the percentage of shareholders that are required to consent to the foregoing amendment and the amendments in the next paragraph.
Subject to the restrictions outlined above, with the written consent of shareholders representing a majority of the Up Aggregate Par Amount and a majority of the Down Aggregate Par Amount of a Series, we and the other parties to the related Series Trust Supplement may amend that supplement for the purpose of:
·adding any provisions to or changing in any manner or eliminating any of the provisions of that Series Trust Supplement; or
·modifying in any manner the rights of the shareholders of that Series.
Subject to the restrictions outlined above, we and the other parties to the Trust Agreement may amend the Master Trust Agreement and/or any or all of the Series Trust Supplements without notice to or consent of the shareholders:
·to cure any ambiguity or to correct or supplement any provision which may be defective or inconsistent with any other provision of the Trust Agreement;
·to conform the provisions of the Trust Agreement to this base prospectus or the applicable prospectus supplement, including the appendices to each of those documents;
·to add to the covenants, restrictions or obligations of any entity under the Trust Agreement for the benefit of the shareholders of one or more Series or to modify any provisions of the Trust Agreement so long as such modification does not adversely affect the interests of the shareholders of any Series in any material respect;
96
·to evidence and provide for the acceptance of appointment under the Trust Agreement of a successor trustee, a successor calculation agent, a successor administrative agent or any successor marketing agent;
·to modify the procedures for effecting Paired Issuances and Paired Optional Redemptions or for directing and settling issuance orders and redemption orders in connection with an amendment to a Participants Agreement for any one or more Series;
·to increase or decrease the Aggregate Par Amount of Up PharmaShares and Down PharmaShares that constitute a PharmaShares Unit so long as the original Up/Down Ratio is maintained;
·to modify the rules governing the administrative agent's selection of treasury securities and treasury repurchase agreements which are summarized under "THE PHARMASHARES TRUST — United States Treasury Obligations;" and
·to comply with any requirements imposed by the Code or any securities laws.
The trustee will not enter into any amendment or modification which would cause the PharmaShares Trust or any Series to be required to register as an investment company under the United States Investment Company Act of 1940, as amended. The trustee may rely on the advice of counsel or an opinion of counsel in taking any action or refraining from taking any action with respect to any amendment to the Trust Agreement or with respect to any request for its consent to the taking of any action or the refraining from any action by any other person under the terms of the Trust Agreement.
Voting Rights
Each holder of the PharmaShares of a Series will be entitled to vote under the Master Trust Agreement and the related Series Trust Supplement on the following matters:
·any amendments requiring the prior written consent of shareholders of that Series, as described above under "—Modification and Waiver;"
·the termination of the trustee under the Trust Agreement with respect to that Series; and
·the appointment of a successor trustee under the Trust Agreement with respect to that Series.
Each holder's voting rights as of any business day will be based on the Aggregate Par Amount of its PharmaShares relative to the Aggregate Par Amount of all PharmaShares of the same Series that are outstanding on that date.
As described in this base prospectus under "DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions" and "— Paired Issuances," only Authorized Participants may exercise the right to direct the issuance and redemption of the Paired PharmaShares.
Reports to Shareholders
We will prepare, on behalf of each Series of the PharmaShares Trust, quarterly reports on Form 10-Q with respect to each calendar quarter and annual reports on Form 10-K with respect to each calendar year. The reports on Form 10-K will include financial statements audited on behalf of each Series by [●], acting as the independent registered public accounting firm for each Series. We will also prepare current reports on Form 8-K. We will file all such reports with the SEC. You may contact your broker to obtain paper copies of these reports.
Trustee Termination Events
A "Trustee Termination Event" under the Trust Agreement will consist of the following:
97
·a failure to make any Periodic Income Distribution or Periodic UV Performance Distribution in the amount determined in accordance with the calculations required to be made under the applicable Series Trust Supplement, to the extent that funds are available in the applicable Series to make those distributions, which failure continues unremedied for a period of five (5) or more business days;
·a failure to distribute the available proceeds of all of the assets of a Series as a Final Distribution on an Early Termination Date or the Final Scheduled Termination Date or a failure to make a Final Distribution on any Redemption Date in the amount determined in accordance with the calculations required to be made under the applicable Series Trust Supplement, to the extent that funds are available in the applicable Series to make those distributions, which failure continues unremedied for a period of five (5) or more business days;
·a failure by the trustee to observe or perform in any material respect any of its other covenants or obligations under the Trust Agreement, which failure continues unremedied for thirty (30) days after the giving of written notice of such failure to the trustee by us or by not less than 25% of the shareholders of any Series, voting by Aggregate Par Amount; and
·the trustee becoming ineligible or incapable of acting as trustee under the Trust Agreement.
So long as a Trustee Termination Event remains unremedied, we may and, at the direction of the required percentage of shareholders of one or more affected Series, we will, terminate the trustee's rights and obligations under the Trust Agreement with respect to all Series. A successor trustee will succeed to all the responsibilities, duties and liabilities of the terminated trustee under the Trust Agreement with respect to the PharmaShares Trust and all Series and will be entitled to similar compensation arrangements. If no successor trustee has been appointed and has accepted the appointment within the period specified in the Trust Agreement after the delivery of a notice of removal, the terminated trustee may petition a court of competent jurisdiction for the appointment of a successor trustee with a net worth at the time of its appointment of at least $20,000,000 or such higher amount as we may specify. Pending the acceptance of that appointment, the terminated trustee is obligated to continue to act as trustee under the Trust Agreement. Without the consent of a majority of the shareholders of each Series, voting by Aggregate Par Amount, the compensation to be paid to the successor trustee may not be greater than the compensation paid to the terminated trustee under the Trust Agreement.
We may also elect to terminate the trustee without cause and appoint a successor trustee that meets or exceeds the requirements described above under "— The Trustee." Pending the acceptance of that appointment by the successor trustee, the terminated trustee is obligated to continue to act as trustee under the Trust Agreement.
Resignation of Trustee
The trustee may, upon written notice to us, resign at any time with respect to all Series of the PharmaShares Trust. If the trustee resigns we will be obligated to use our best efforts to appoint a successor trustee. If no successor trustee has been appointed and has accepted its appointment within a specified period, the resigning trustee may petition any court of competent jurisdiction to appoint a successor trustee. A resignation of the trustee will not become effective until a successor trustee has been appointed and has accepted its appointment.
Removal and Resignation of Administrative Agent
We may resign at any time as administrative agent upon written notice to the trustee. The administrative agent may also be removed, with cause, at any time by the trustee upon written notice. Our resignation or removal as administrative agent will not become effective until a successor administrative agent has been appointed and has accepted its appointment.
Insolvency of Depositor
If PharmaShares Advisors, LLC is adjudged to be insolvent or is liquidated or dissolved for any reason, this will not (1) result in a termination of the Trust Agreement, the PharmaShares Trust or any Series, (2) entitle the legal representatives or assigns of PharmaShares Advisors, LLC to petition any court to partition or wind up all or any
98
part of the PharmaShares Trust or any Series or any of their respective properties or (3) otherwise affect the rights, obligations and liabilities of the trustee or the shareholders of any Series.
U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a summary of the U.S. federal income tax considerations generally applicable to the purchase, ownership, and disposition of PharmaShares. This discussion is based on the Code, its legislative history, Treasury regulations, and published rulings and court decisions, all as in effect as of the date hereof, and all of which are subject to change, possibly with retroactive effect, which could alter the U.S. federal income tax considerations described below. No ruling from the Internal Revenue Service ("IRS") has been or will be sought on any of the issues discussed herein, and there can be no assurance that the IRS or a court will concur with the conclusions reached below.
This summary is addressed only to holders who hold their PharmaShares as capital assets and not as a hedge, a position in a "straddle" or other conversion transaction or as part of a "synthetic" security or other integrated financial transaction. This summary does not describe all of the tax considerations of purchasing, owning or disposing of shares that may be relevant to investors in light of their particular circumstances. For example, this summary does not address all U.S. federal income tax issues relating to shares that may be relevant to financial institutions, partnerships, tax-exempt organizations, insurance companies, dealers or traders in securities or currencies, persons whose functional currency is not the U.S. dollar, or certain former citizens or former long-term residents of the United States. This discussion does not address alternative minimum tax considerations, nor does it address any state, local or foreign tax considerations relating to the purchase, ownership, or disposition of shares.
Special features of any particular series will be address in the applicable prospectus supplement.
As used in this discussion, "U.S. Holder" means a beneficial owner of PharmaShares that is, for U.S. federal income tax purposes:
·a citizen or resident of the United States,
·a corporation (or other entity treated as a corporation) created or organized in, or under the laws of, the United States, any state of the United States, or the District of Columbia,
·an estate, the income of which is subject to U.S. federal income taxation regardless of its source, or
·a trust if (i) a court within the United States is able to exercise primary supervision over the trust's administration and one or more U.S. Persons have the authority to control all of the trust's substantial decisions or (ii) it has a valid election in effect under applicable Treasury regulations to be treated as a United States person.
"Non-U.S. Holder" means a beneficial owner of PharmaShares other than a U.S. Holder or an entity treated as a partnership for U.S. federal income tax purposes.
For U.S. federal income tax purposes, the treatment of any beneficial owner of an interest in any entity or arrangement treated as a partnership for U.S. federal income tax purposes will generally depend upon the status of the partner and the activities of the partnership. Partnerships and their partners should consult their tax advisors about the U.S. federal income tax consequences of purchasing, owning and disposing of PharmaShares.
There is no authority directly on point dealing with securities such as Up PharmaShares or the transactions of the type described in this base prospectus and no rulings with respect to related tax matters have been sought from the IRS. Accordingly, there can be no assurance that the IRS will not assert, or that a court would not sustain, a position different from any described herein.
Each prospective investor should consult with its tax advisor regarding the application of tax laws to its particular situation.
99
U.S. Federal Income Tax Classification of the PharmaShares Trust and PharmaShares
The PharmaShares Trust intends, and will elect, to be classified as a corporation for U.S. federal income tax purposes. Accordingly, the PharmaShares Trust is expected to be subject to U.S. federal income tax on its taxable income and holders of PharmaShares are expected to be treated for U.S. federal income tax purposes as shareholders of a corporation with the anticipated consequences described in this "U.S. FEDERAL INCOME TAX CONSIDERATIONS" section. Moreover, by accepting PharmaShares, holders agree to report the income and loss from such PharmaShares consistent with such treatment.
If the IRS were to successfully challenge the intended treatment of the PharmaShares Trust as a corporation for U.S. federal income tax purposes, holders of PharmaShares could be required to recognize income, gains, losses, and deductions with respect to their PharmaShares in amounts and at times materially different from those that would be expected from an investment in corporate stock. For example, a holder could be currently subject to tax on a distribution that was expected to be treated as a return of capital. Each prospective investor should consult its tax advisor regarding the tax consequences of an investment in the PharmaShares Trust in light of such investor's particular circumstances.
The remainder of this discussion assumes the PharmaTrust will be treated for U.S. federal income tax purposes as a separate corporation and that PharmaShares will be treated as equity interests in such corporation.
U.S. Holders
Distributions of Cash
Distributions of cash made by the PharmaShares Trust to a U.S. Holder (whether such cash represents a Periodic Income distribution, a Periodic UV Performance Distribution, an Equalization Payment, or a Final Distribution) will generally be includible by U.S. Holders as dividends to the extent paid out of the current or accumulated earnings and profits of the PharmaShares Trust, as determined under U.S. federal income tax principles. The amount of any such distribution that exceeds the current and accumulated earnings and profits of the PharmaTrust for a taxable year, the excess will be treated first as a return of capital to the extent of the U.S. Holder's adjusted basis in its PharmaShares, which would not be subject to tax, and then as capital gain. Any such capital gain will be long-term if the U.S. Holder's holding period for its PharmaShares exceeds one year at the time the distribution is received.
Sale, Exchange, or Other Taxable Disposition
A U.S. Holder will generally recognize gain or loss on the sale, exchange, or other taxable disposition of PharmaShares in an amount equal to the difference between the amount realized on the disposition and the U.S. Holder's tax basis in the PharmaShares. Any such gain or loss will generally be capital gain or loss and will be long-term if the U.S. Holder's holding period for the PharmaShares exceeds one year at the time of disposition. Non-corporate U.S. Holders may be eligible for preferential rates of U.S. taxation in respect of long-term capital gains. A U.S. Holder's ability to deduct capital losses is subject to certain limitations.
Redemptions
The redemption of a U.S. Holder's PharmaShares in exchange for cash will generally be treated as a sale or other taxable disposition of such PharmaShares with the consequences described above if the redemption (i) results in a "complete termination" of the U.S. Holder's interest in the PharmaShares Trust, (ii) is "substantially disproportionate" with respect to the U.S. Holder, or (iii) is "not essentially equivalent to a dividend." For these purposes, PharmaShares actually owned, as well as those treated for U.S. federal income tax purposes as constructively owned by the U.S. Holder must be taken into account. If none of the foregoing requirements for sale treatment are satisfied, cash received by a U.S. Holder in redemption of its PharmaShares will be treated as a distribution of cash with the consequences described above.
Each U.S. Holder should consult its tax advisor regarding the possibility that a redemption of PharmaShares could be treated as a distribution of cash and the consequences thereof.
100
Non-U.S. Holders
Distributions of Cash
Distributions of cash made by the PharmaShares Trust to a Non-U.S. Holder (whether such cash represents a periodic income distribution, a UV performance distribution, an Equalization Payment, or a Final Distribution) will generally be treated as dividends to the extent paid out of the current or accumulated earnings and profits of the PharmaShares Trust, as determined under U.S. federal income tax principles. Any such dividends will generally be subject to withholding at a rate of 30%, unless the Non-U.S. Holder certifies on an appropriate IRS Form W-8 that (i) it is entitled to the benefits of an applicable income tax treaty or (ii) the dividends are effectively connected with the Non-U.S. Holder's conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States).
A Non-U.S. Holder eligible for a reduced rate of withholding under an applicable income tax treaty may file an appropriate claim for refund of any excess amounts withheld with the IRS. Non-U.S. Holders should consult their tax advisors regarding their eligibility for benefits under applicable income tax treaties.
Dividends effectively connected with a Non-U.S. Holder's conduct of a trade or business in the United States will generally be subject to U.S. federal income tax on a net income basis at the same graduated rates as are applicable to U.S. Holders and, if the Non-U.S. Holder is a corporation, may also be subject to a "branch profits tax" at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
The amount of any distribution that exceeds the current and accumulated earnings and profits of the PharmaTrust for a taxable year, will be treated first as a return of capital to the extent of the Non-U.S. Holder's adjusted basis in its PharmaShares, which would not be subject to tax, and then as gain realized from the sale, exchange, or other disposition of PharmaShares, with the consequences described below.
Sale, Exchange, or Other Disposition
A Non-U.S. Holder will generally not be subject to U.S. federal income tax on gain realized on the sale, exchange, or other disposition of PharmaShares unless (i) the Non-U.S. Holder is an individual present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the Non-U.S. Holder will generally be subject to a flat income tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty), or (ii) the gain is effectively connected with the Non-U.S. Holder's conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment maintained by the Non-U.S. Holder in the United States), in which case such gain will generally be subject to U.S. federal income tax on a net income basis at the same graduated rates as are applicable to U.S. Holders and, if the Non-U.S. Holder is a corporation, may also be subject to a "branch profits tax" at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
Redemptions
The redemption of a Non-U.S. Holder's PharmaShares in exchange for cash will generally be treated as a sale or other disposition of such PharmaShares with the consequences described above if the redemption (i) results in a "complete termination" of the Non-U.S. Holder's interest in the PharmaShares Trust, (ii) is "substantially disproportionate" with respect to the Non-U.S. Holder, or (iii) is "not essentially equivalent to a dividend." For these purposes, PharmaShares actually owned, as well as those treated for U.S. federal income tax purposes as constructively owned by the Non-U.S. Holder must be taken into account. If none of the foregoing requirements for sale treatment are satisfied, cash received by a Non-U.S. Holder in redemption of its PharmaShares will be treated as a distribution of cash with the consequences described above.
Each U.S. Holder should consult its tax advisor regarding the possibility that a redemption of PharmaShares could be treated as a distribution of cash and the consequences thereof.
101
Additional Withholding Requirements
Sections 1471 through 1474 of the Code, together with the Treasury Regulations issued thereunder and other applicable guidance ("FATCA"), may require withholding at a rate of 30% on dividends paid to certain foreign financial institutions and certain non-financial foreign entities unless (1) in the case of a foreign financial institution, such institution enters into, and complies with, an agreement with the U.S. government to withhold on certain payments, and to collect and provide, on an annual basis, to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners), (2) in the case of a non‑financial foreign entity, such entity certifies to the withholding agent that it does not have any substantial U.S. owners or provides the withholding agent with a certification identifying the direct and indirect substantial U.S. owners of the entity, (3) the foreign financial institution or non‑financial foreign entity otherwise qualifies for an exemption from these rules or, if required under an intergovernmental agreement between the United States and an applicable foreign country, reports the information in clause (1) to its local tax authority, which will exchange such information with the U.S. authorities. If FATCA withholding is imposed, a beneficial owner that is not a foreign financial institution will generally be entitled to a refund of any amounts withheld by filing a U.S. federal income tax return (which may entail significant administrative burden). An intergovernmental agreement between the United States and an applicable foreign country, or future Treasury regulations, may modify these requirements. Accordingly, the entity through which PharmaShares are held will affect the determination of whether such withholding is required. Each prospective investor should consult its tax advisor regarding the possible implications of FATCA on an investment in PharmaShares in light of its particular circumstances.
STATE TAX CONSIDERATIONS
In addition to the U.S. federal income tax consequences described in "U.S. FEDERAL INCOME TAX CONSIDERATIONS," you should consider the state income tax consequences of the acquisition, ownership, and disposition of the shares. State income tax law may differ substantially from the corresponding federal law, and this discussion does not purport to describe any aspect of the income tax laws of any state. Therefore, you should consult your tax advisor with respect to the various state tax considerations of an investment in the shares.
CERTAIN ERISA CONSIDERATIONS
We anticipate that each Series of Up PharmaShares and Down PharmaShares will meet the criteria of "publicly-offered securities" pursuant to the Plan Assets Regulation issued by the Department of Labor.
We expect that:
·there will be no restrictions imposed on the transfer of any series of Up PharmaShares or Down PharmaShares that will cause a Series to not be "freely transferable" for purposes of the Plan Asset Regulations;
·the Up PharmaShares of each Series will be held by at least 100 independent investors and the Down PharmaShares of each Series will be held by at least 100 independent investors; and
·each Series of Up PharmaShares and Down PharmaShares will be sold as part of an offering pursuant to an effective registration statement under the Securities Act and then will be timely registered under the Exchange Act.
However, it is not known at this time when the Up PharmaShares and the Down PharmaShares of a particular Series will each be held by at least 100 independent investors for purposes of the Plan Assets Regulation. Consequently, Plans may not invest in a Series of PharmaShares and each investor will be deemed to have represented that it is not, and is not investing assets of, a Plan, unless and until we confirm for that Series, by means of a filing made on Form 8-K, that the Up PharmaShares of that Series are held by at least 100 independent investors and the Down PharmaShares of that Series are held by at least 100 independent investors.
102
If the Up PharmaShares or Down PharmaShares of a Series were to fail to meet the criteria of publicly-offered securities, the assets of that Series could be deemed under ERISA to include the assets of any plans that invested in that Series. In that event, among other things, transactions involving the affected Series' assets and parties in interest or disqualified persons with respect to plans that invested in that Series would be prohibited under ERISA and Section 4975 of the Code, unless another exception under the Plan Assets Regulation, or a statutory or administrative exception were to apply.
Prospective fiduciaries of a Plan considering the purchase of the Up PharmaShares or Down PharmaShares of any Series should consult with their legal advisors concerning the impact of ERISA and the Code and the potential consequences of making an investment in those shares with respect to their specific circumstances. Each Plan fiduciary should take into account, among other considerations:
·whether the fiduciary has the authority to make the investment,
·the composition of the Plan's portfolio with respect to diversification by type of asset,
·the Plan's funding objectives,
·the tax effects of the investment,
·whether the assets of the Series that are represented by the Up PharmaShares and the Down PharmaShares might be considered "plan assets," and
·whether, under the applicable general fiduciary standards of investment prudence and diversification an investment in the Up PharmaShares and the Down PharmaShares of a Series is appropriate for the Plan taking into account the overall investment policy of the Plan and the composition of the Plan's investment portfolio.
Any purchaser of any Up PharmaShares or Down PharmaShares of a Series that is an insurance company using the assets of an insurance company general account should consider the extent (if any) to which the assets of the general account may constitute "plan assets" for purposes of the fiduciary responsibility provisions of ERISA and Section 4975 of the Code to the extent such assets relate to certain contracts issued to employee benefit plans.
Certain employee benefit plans, such as governmental plans (as defined in Section 3(32) of ERISA) and certain church plans (as defined in Section 3(33) of ERISA), are not subject to the requirements of ERISA or Section 4975 of the Code. Accordingly, assets of such plans may be invested in any Series of Up PharmaShares or Down PharmaShares without regard to the ERISA considerations described in this base prospectus, subject to the provisions or other applicable federal and state law. However, any such plan that is qualified and exempt from taxation under Sections 401(a) and 501(a) of the Code is subject to the prohibited transaction rules set forth in Section 503 of the Code.
PLAN OF DISTRIBUTION
For a detailed discussion of the plan of distribution for your Series of Up PharmaShares and Down PharmaShares, see “THE TERMS OF YOUR SERIES — Plan of Distribution” in your prospectus supplement.
LEGAL MATTERS
Certain legal matters relating to the issuance of each Series of Paired PharmaShares will be passed upon for the PharmaShares Trust and each Series by BurgherGray LLP, special New York counsel to PharmaShares Advisors, LLC and the PharmaShares Trust. Certain legal matters relating to the federal tax consequences of the issuance of each series of Paired PharmaShares will be passed upon for the PharmaShares Trust and each Series by BurgherGray LLP, special New York tax counsel to PharmaShares Advisors, LLC and the PharmaShares Trust.
103
EXPERTS
The financial statements of the PharmaShares Trust and each Series of that trust at [●][●], 2020 and for the period then ended appearing in this base prospectus and the registration statement of which this base prospectus is a part have been audited by [●], an independent registered public accounting firm, and are included in reliance upon that report given on the authority of that firm as experts in accounting and auditing.
GLOSSARY OF DEFINED TERMS
"Actual Percentage Change" means, for a Series on any Publication Date, the percentage obtained by dividing (1) the absolute value of the difference between the Reference Value published for the Current Calendar Quarter on that Publication Date and the Reference Value for the Base Calendar Quarter by (2) the Reference Value for the Base Calendar Quarter.
"administrative agent" means, for each Series, PharmaShares Advisors, LLC or any successor thereto.
"Aggregate Par Amount" means, for a Series on any date of determination and as the context requires, (i) the Up Aggregate Par Amount on that date, (ii) the Down Aggregate Par Amount on that date, or (iii) the Aggregate Par Amount of a specified portion of the Up and/or Down PharmaShares outstanding on that date.
"Allocation Factor" means, for a Series, the Up Allocation Factor or the Down Allocation Factor, as applicable.
"Auction Date" means, a specified in the prospectus supplement for your Series, either (1) the date that is two business days prior to the first day of trading for each calendar quarter or (2) the first business day following each Publication Date for the Reference Product for your Series.
"Authorized Participant" means an entity that:
·is a registered broker-dealer and a member in good standing with FINRA, or a participant in the securities markets such as a bank or other financial institution that is not required to register as a broker-dealer or be a member of FINRA in order to engage in securities transactions;
·is a participant in DTC or has indirect access to the clearing facilities of DTC by virtue of a custodial relationship with a DTC participant; and
·has entered into a Participants Agreement with the PharmaShares Trust, the trustee for the PharmaShares Trust and the administrative agent.
"Base Calendar Quarter" means, for purposes of the calculation of Underlying Value for the PharmaShares of a Series on a Publication Date or any subsequent date occurring during the Calculation Period that follows that Publication Date, a calendar quarter that occurred in the past and the published Reference Value for which quarter is compared against the Reference Value published for the Current Calendar Quarter, in order to determine the Actual Percentage Change. For sequential tracking Series, the Base Calendar Quarter will be the calendar quarter that immediately precedes the Current Calendar Quarter. For year over year tracking Series, the Base Calendar Quarter will be the same calendar quarter as the Current Calendar Quarter but occurring during the preceding calendar year.
"BRG Projected Revenue Determination Date" means, as specified in your prospectus supplement, either (1) the first day of each calendar quarter or (2) each Publication Date for the Reference Product for your Series.
"Broker Reporting Group" means, for a Series and its Reference Product, a group of investment banks, asset managers and other broker-dealers registered with FINRA, that each employ analysts who publish net revenue projections for the Reference Product, or otherwise make their models containing such projections available to a third-party aggregation service that generates a composite projection for the Reference Product. The Broker Reporting Group must have the characteristics specified under "DESCRIPTION OF THE PHARMASHARES —
104
Calculation of Underlying Value" in this base prospectus for each Series and under "THE TERMS OF YOUR SERIES — Calculation of Underlying Value — Broker Reporting Group" in the prospectus supplement for your Series.
"business day" means any day other than a Saturday, a Sunday or a day on which banking institutions and stock exchanges in New York, New York or Wilmington, Delaware are authorized or required by law, regulation or executive order to close.
"Calculation Period" means, for a Series, the period beginning on (but excluding) the preceding Publication Date for the Reference Product of that Series (or, in the case of the first Calculation Period, beginning on the first Issuance Date) and ending on (and including) the current Publication Date for the Reference Product of that Series.
A "change in net revenue" means, for a Series and with respect to a Reference Product, (i) a change in the net revenue of the Reference Product for that Series, (ii) a change in the net revenues of a specified division of a publicly traded pharmaceutical company that is referenced by that Series, or (iii) the change in net revenues allocated to a specified entity referenced by that Series, which entity is party to a collaboration agreement, distribution agreement, joint venture or other profit-sharing arrangement relating to the sale of a Reference Product, as specified in the prospectus supplement for that Series.
"Code" means the United States Internal Revenue Code of 1986, as amended.
"Current Calendar Quarter" means, for purposes of the calculation of Underlying Value for the PharmaShares of a Series on a Publication Date or any subsequent date occurring during the Calculation Period that follows that Publication Date, the most recently elapsed calendar quarter for which the Reference Value that was published on that Publication Date is compared against the Reference Value published for the Base Calendar Quarter in order to determine the Actual Percentage Change.
"Daily Fee Accrual Rate" means, for a Series, the annual rate at which fees payable by that Series accrue, as disclosed in the prospectus supplement for that Series.
"Daily Net Income Accrual" means, for a Series, the entitlement of each PharmaShare to the Net Treasury Income earned by that Series on each day, which will be equal to the Up Daily Net Income Accrual for the Up PharmaShares of that Series and to the Down Daily Net Income Accrual for the Down PharmaShares of that Series.
"Daily Yield Rate" means, for a Series and each treasury security on deposit in that Series, the yield rate applicable to that treasury security divided by either 365 or 366, depending upon the actual number of days in the current calendar year. For purposes of this definition, the "yield rate" for each treasury security will be equal to its stated or implied interest rate, if any, or any applicable discount rate, based on the date of purchase and the purchase price at which the applicable Series acquired that treasury security and, in the case of any treasury repurchase agreements, the difference between its purchase price and its repurchase price.
"Delaware Statutory Trust Act" means Chapter 38 of Title 12 of the Delaware Code, 12 Del. C. § 3801, et seq.
"Down Aggregate Par Amount" means, for a Series and any date of determination, the amount determined by multiplying the aggregate number of Down PharmaShares issued by that Series on any Issuance Date that occurred prior to that date of determination, less the aggregate number of shares that were redeemed on any Redemption Date that occurred prior to that date, by the par amount of the Down PharmaShares on that date.
"Down Allocation Factor" means, for a Series, a number calculated on each Publication Date as follows:
(i)if a Projected Breakpoint Increase was predicted for the Current Calendar Quarter:
(a)if the Actual Percentage Change is greater than the Projected Breakpoint Increase, there will be an Up Underlying Value increase equal to:
UUVΔ = Actual Percentage Change – Projected Breakpoint Increase; and
105
the Down Allocation Factor will equal 1 – the Up Underlying Value increase;
(b)if the Actual Percentage Change reflects an increase that is less than the Projected Breakpoint Increase, the Down Underlying Value will increase as follows:
DUVΔ = Projected Breakpoint Increase – Actual Percentage Change; and
the Down Allocation Factor will equal 1 + the Down Underlying Value increase; or
(c)if the Actual Percentage Change reflects a decrease for a Current Calendar Quarter with a Projected Breakpoint Increase, the Down Underlying Value will increase as follows:
DUVΔ = Actual Percentage Change + Projected Breakpoint Increase; and
the Down Allocation Factor will be equal to 1+ the Down Underlying Value increase.
(ii)If a Projected Breakpoint Decrease was predicted for the Current Calendar Quarter:
(a)if the Actual Percentage Change reflects a decrease that is greater than the Projected Breakpoint Decrease, the Down Underlying Value will increase as follows:
DUVΔ = (Actual Percentage Change – Projected Breakpoint Decrease); and
the Down Allocation Factor will be equal to 1 + the Down Underlying Value increase;
(b)if the Actual Percentage Change reflects a decrease that is less than the Projected Breakpoint Decrease, the Up Underlying Value will increase as follows:
UUVΔ = (Projected Breakpoint Decrease – Actual Percentage Change); and
the Down Allocation Factor will be equal to 1 – the Up Underlying Value increase; or
(c)if the Actual Percentage Change reflects an increase for a Current Calendar Quarter with a Projected Breakpoint Decrease, the Up Underlying Value will increase as follows:
UUVΔ = (Actual Percentage Change + Projected Breakpoint Decrease); and
The Down Allocation Factor will be equal to 1 – the Up Underlying Value increase.
"Down Daily Net Income Accrual" means, for the Down PharmaShares of a Series and each calendar day occurring during any Calculation Period:
·the product of (A)(i) the sum of, for each treasury security on deposit in that Series on that day, the product of the purchase price at which that Series acquired that treasury security multiplied by the Daily Yield Rate applicable to that treasury security minus (ii) the Series Investment Amount multiplied by the Daily Fee Accrual Rate, divided by 365 or 366, depending upon the number of days in the current calendar year, and (B)(i) the Down Percentage if net income is being allocated equally among the Up and the Down PharmaShares of that Series, (ii) the Down Underlying Value for that Calculation Period divided by the Down Investment Amount for that Calculation Period, if net income is being allocated based upon the Up Underlying Value and Down Underlying Value, or (iii) such other allocation rule as may be specified in the prospectus supplement for that Series;
plus
·if that calendar day is also an Issuance Date on which a net increase in the Down Aggregate Par Amount has occurred after giving effect to all Paired Issuances and Paired Optional Redemptions effected on that date, the product of the number of Down PharmaShares of that Series created on such date constituting such net increase and the net income component of the Per Share Underlying Value of each such Down PharmaShare, which represents the Down Daily Net Income
106
Accruals allocable to such share during the period from the first day of the current Calculation Period to such Issuance Date, and
minus
·if that calendar day is also a Redemption Date on which a net decrease in the Down Aggregate Par Amount has occurred after giving effect to all Paired Optional Redemptions and Paired Issuances effected on that day, the product of the number of Down PharmaShares of that Series redeemed on such day constituting such net decrease and the income component of the Per Share Underlying Value of each such Down PharmaShare, which represents the Down Daily Net Income Accruals allocable to such share during the period from the first day of the current Calculation Period to such Redemption Date.
If the result of the foregoing calculation is a negative number, then the Down Daily Net Income Accrual for that day will be equal to zero. The treasury securities will be valued at their acquisition cost for purposes of calculating Down Daily Net Income Accruals, but will be valued at their amortized cost in the financial statements prepared for each Series.
"Down Investment Amount" means, for a Series on any Periodic Calculation Date, the amount of cash received from holders of the Down PharmaShares of that Series that was actually allocated for investment on behalf of the Series in treasury securities on that Periodic Calculation Date, which is required to equal the lesser of (x) the Down Aggregate Par Amount and (y) all funds on deposit in that Series as of that Periodic Calculation Date that are allocable to the Down PharmaShares, based upon the Up/Down Ratio. In a Series in which the Up/Down Ratio is 1:1, one-half of all invested funds will be allocated to the Down Investment Amount.
On any other day of a Calculation Period, the "Down Investment Amount" will equal the Down Aggregate Par Amount if the amount actually allocated for investment on the preceding Periodic Calculation Date was equal to the Down Aggregate Par Amount.
If less than the Down Aggregate Par Amount was allocated for investment on the last Periodic Calculation Date due to losses on the treasury securities or treasury repurchase agreements, then the "Down Investment Amount" for each day of the ensuing Calculation Period will equal the amount that was actually invested plus the Down Aggregate Par Amount of all Down PharmaShares created since the last Periodic Calculation Date and minus the product of (i)(A) the Down Investment Amount divided by (B) the number of Down PharmaShares outstanding on the date of determination and (ii) the number of Down PharmaShares redeemed since the last Periodic Calculation Date.
"Down Leverage Factor" means, with respect to a Series, the multiplier or ratchet that is applicable to returns on the Down PharmaShares of that Series that is disclosed in the prospectus supplement for that Series.
"Down Percentage" means, for a Series and any date of determination, the Down Aggregate Par Amount on that date divided by the Series Aggregate Par Amount on that date.
"Down Periodic Return Cap" means, with respect to a Series, the percentage limit on the returns that may be achieved by the Down PharmaShares of that Series on any Periodic Calculation Date that is disclosed in the prospectus supplement for that Series.
"Down PharmaShares" means, with respect to a Series, the class of shares titled the "Down PharmaShares" and having the rights and characteristics specified in the Series Trust Supplement pursuant to which such class was issued.
The "Down Underlying Value" of the Down PharmaShares of a Series will be equal on any Publication Date to the Down Investment Amount multiplied by the Down Allocation Factor, calculated based on the Reference Value for that Series that was reported on that Publication Date.
The "Down Underlying Value" of the Down PharmaShares of a Series will equal on any calendar day occurring during the Calculation Period that follows each Publication Date:
107
·the sum of the Down Daily Net Income Accruals for each day that has elapsed during the current Calculation Period, up to and including the current calendar day
plus
·the Down Investment Amount multiplied by the Down Allocation Factor, calculated based on the Reference Value for that Series that was reported on the most recent Publication Date.
"DTC" means The Depository Trust Company.
"Early Termination Date" means, for a Series, the Periodic Calculation Date following the occurrence of a Termination Trigger on which a Final Distribution is declared on the PharmaShares of that Series. Such Periodic Calculation Date may be removed from the occurrence of the Termination Trigger by one or more Calculation Periods, as specified for each Termination Trigger in this base prospectus and in the prospectus supplement for each Series.
"Equalization Payment" means, for a Series and each Periodic UV Performance Distribution, the additional payment included as part of that distribution in the amount that is needed to restore the Up/Down Ratio for that Series. In a Series with an Up/Down Ratio of 1:1, the Equalization Payment will always be exactly equal to the amount of the increase in Underlying Value. In Series with a different Up/Down Ratio, the Equalization Payment will be calculated as specified in the related prospectus supplement.
"ERISA" means the United States Employee Retirement Income Security Act of 1974, as amended.
"Exchange Act" means the United States Securities Exchange Act of 1934, as amended.
"Final Distribution" means (1) the distribution made in redemption of all of the Paired PharmaShares of a Series on the Final Redemption Date that follows a Final Scheduled Termination Date or an Early Termination Date or (2) the distribution made in redemption of all or a portion of the Paired PharmaShares of a Series on the Redemption Date that follows a Redemption Order Date. The Final Distribution will always be equal to the Per Share Underlying Value of the PharmaShares being redeemed on the last calendar day preceding the Final Scheduled Termination Date or Early Termination Date, as calculated based upon the Reference Value published on the last Publication Date that occurred prior to the Final Scheduled Termination Date or Early Termination Date.
"Final Redemption Date" means, for a Series, the second business day that follows the Final Scheduled Termination Date or an Early Termination Date for that Series, on which the Final Distribution that was declared on either of those dates will be paid to shareholders.
"Final Scheduled Termination Date" means, for a Series, the final Periodic Calculation Date that is scheduled to occur for that Series, as disclosed in the prospectus supplement for that Series, on which a Final Distribution in redemption of all outstanding Paired PharmaShares of that Series will be declared.
"FINRA" means the Financial Industry Regulatory Authority, Inc.
"Issuance Date" means, with respect to a Series, the business day following an Issuance Order Date on which Paired PharmaShares of that Series were ordered, on which business day those Paired PharmaShares are issued and delivered to the Authorized Participant who placed the related issuance order. In the case of any issuance order that is delivered on a Periodic Calculation Date or the business day following a Periodic Calculation Date, the "Issuance Date" will be the third business day following the Issuance Order Date.
"Issuance Order Date" means, for a Series, the business day on which an Authorized Participant delivers an issuance order to the administrative agent directing a Paired Issuance of the Paired PharmaShares of that Series.
"Leverage Factor" means, with respect to a Series, the Up Leverage Factor and/or the Down Leverage Factor for that Series.
"Major Market Disruption" has the meaning specified in "REFERENCE PRODUCTS AND REFERENCE VALUES" in this base prospectus.
108
"Master Trust Agreement" means the master trust agreement, dated as of [●], 2020, among the depositor, the administrative agent, the trustee, the marketing agent and the calculation agent, pursuant to which the PharmaShares Trust was formed and its activities are governed.
"net income component of Underlying Value" means, for a Series, any Calculation Period and the Up PharmaShares and Down PharmaShares of that Series, the portion of the Up Underlying Value of the Up PharmaShares or the portion of the Down Underlying Value of the Down PharmaShares, as applicable, as measured on any day of that Calculation Period, that is attributable to the entitlement of that class of PharmaShares to the Net Treasury Income of that Series.
"net revenue" means, if applicable for a Series, the gross sales of the Reference Product adjusted for sales-related deductions, including, but not limited to, chargebacks, trade discounts, sale returns and allowances, commercial and government rebates, customer loyalty programs and fee-for-service arrangements with certain distributors, collectively referred to as "sales allowances." The prospectus supplement for a Series will specify any additional components or deductions applicable to the "net revenue" of the Reference Product for that Series.
"Net Treasury Income" means, for a Series on each Periodic Calculation Date, the funds on deposit in that Series on that Periodic Calculation Date after the Series has (i) set aside the amount necessary to pay its fees and expenses and (ii) acquired, or allocated funds for the acquisition of, treasury securities with an aggregate purchase price equal to the Series Aggregate Par Amount as of that Periodic Calculation Date. Net Treasury Income for a Series for any Calculation Period will also be equal to the sum of the Up Daily Net Income Accruals for each day of that Calculation Period plus the sum of the Down Daily Net Income Accruals for each day of that Calculation Period.
"Paired Issuance" means, with respect to a Series, the issuance of Paired PharmaShares of that Series in PharmaShares Units as described in "DESCRIPTION OF THE PHARMASHARES — Paired Issuances" in this base prospectus.
"Paired Optional Redemption" means, with respect to a Series, a redemption of the Paired PharmaShares of that Series in PharmaShares Units as described in "DESCRIPTION OF THE PHARMASHARES — Paired Optional Redemptions" in this base prospectus.
"Paired PharmaShares" means, with respect to a Series, the Up PharmaShares and the Down PharmaShares of that Series.
"par amount" means, for each Up PharmaShare and each Down PharmaShare of a Series, the stated amount per share that is disclosed in the prospectus supplement for that Series, as reduced by Periodic UV Performance Distributions. The par amount per share for any PharmaShare of a Series on any calendar day will be equal to the Up Aggregate Par Amount or Down Aggregate Par Amount, as applicable, divided by the number of outstanding Up PharmaShares or Down PharmaShares, as applicable.
"Participants Agreements" means, with respect to a Series, the agreements entered into by the administrative agent, the PharmaShares Trust and the trustee for the PharmaShares Trust with each entity that is acting as an Authorized Participant for that Series, which specify procedures for Paired Issuances and Paired Optional Redemptions for that Series.
"Periodic Calculation Date" means, with respect to a Series, the date specified in the related prospectus supplement on which, among other actions, Periodic Distributions are declared, calculations of the Underlying Value and other items may be made, fees and expenses of service providers are deposited, and, if other than a Final Scheduled Termination Date or an Early Termination Date, treasury securities are acquired for deposit in that Series, which will generally be the fifth (5th) business day following each Publication Date for that Series, unless otherwise specified in your prospectus supplement.
"Periodic Distribution" means, with respect to a Series and each Periodic Calculation Date for that Series, (i) the Periodic UV Performance Distribution declared on the Up PharmaShares or the Down PharmaShares of that Series and (ii) the Periodic Income Distribution, if any, declared on each PharmaShare of that Series, in each case, on that Periodic Calculation Date.
109
"Periodic Income Distribution" means, with respect to a Series and each Periodic Calculation Date for that Series, the distribution of Net Treasury Income that is declared on the Up PharmaShares and/or Down PharmaShares of that Series on that date.
"Periodic Return Cap" means, with respect to a Series, the Up Periodic Return Cap and/or Down Periodic Return Cap.
"Periodic UV Performance Distribution" means, for a Series and each Periodic Calculation Date for that Series, (i) a distribution declared on the Up PharmaShares of that Series on that Periodic Calculation Date that will be equal to the increase in the Up Underlying Value, as calculated based upon the Reference Value published on the most recent Publication Date compared to the related Projected Breakpoint, plus an Equalization Payment, or (ii) a distribution declared on the Down PharmaShares of that Series on that Periodic Calculation Date that will be equal to the increase in the Down Underlying Value, as calculated based upon the Reference Value published on the most recent Publication Date compared to the related Projected Breakpoint, plus an Equalization Payment.
"Per Share Underlying Value" means, for a Series and each calendar day occurring during any Calculation Period, (1) with respect to the Up PharmaShares of that Series, an amount calculated by dividing the Up Underlying Value on that day by the number of the Up PharmaShares outstanding on that day, and (2) with respect to the Down PharmaShares of that Series, an amount calculated by dividing the Down Underlying Value on that day by the number of the Down PharmaShares outstanding on that day.
"PharmaShares" means, for a Series, as the context requires, the Up PharmaShares or Down PharmaShares of that Series or a generic reference to either or both classes of shares issued by that Series or some subset of those shares.
"PharmaShares Trust" means the PharmaShares Master Trust, a statutory trust organized in series under the provisions of the Delaware Statutory Trust Act that was formed pursuant to, and the activities of which are governed by, the Master Trust Agreement.
"PharmaShares Unit" means, with respect to a Series, the unit of creation or redemption consisting of a specified Aggregate Par Amount of Up PharmaShares of that Series and a specified Aggregate Par Amount of Down PharmaShares of that Series, as disclosed in the prospectus supplement for that Series.
"Plan" means (A) an employee benefit plan (as defined in Section 3(3) of ERISA), that is subject to Title I of ERISA, (B) a plan described in Section 4975 of the Code, that is subject to Section 4975 of the Code, or (C) any entity whose underlying assets include assets of a plan described in (A) or (B) above by reason of such plan’s or plans’ investment in such entity under ERISA and the Plan Assets Regulation.
"Plan Assets Regulation" means the United States Department of Labor's regulation set forth at 29 C.F.R. Section 2510.3 101, as modified by Section 3(42) of ERISA.
"Projected Breakpoint" means, with respect to a Series and each Publication Date, the Projected Breakpoint Increase or Projected Breakpoint Decrease, as applicable, that has been calculated with respect to that Publication Date. The Projected Breakpoint will be disclosed for a Series prior to each Calculation Period by means of the filing of an updated registration statement for that Series pursuant to Rule 424(b) under the Securities Act.
"Projected Breakpoint Decrease" means, with respect to a Series, the related Reference Product and a designated Base Calendar Quarter and Current Calendar Quarter, the absolute value of the percentage decrease in net revenues for the Reference Product between those two calendar quarters, based upon the projections of a Broker Reporting Group or the Breakpoint Reset Auction process.
"Projected Breakpoint Increase" means, with respect to a Series, the related Reference Product and a designated Base Calendar Quarter and Current Calendar Quarter, the absolute value of the percentage increase in net revenues for the Reference Product between those two calendar quarters, based upon the projections of a Broker Reporting Group or the Breakpoint Reset Auction process.
"Publication Date" means, for a Series, the date following the end of each calendar quarter on which the pharmaceutical company that manufactures and sells the Reference Product complies with its periodic reporting
110
obligations under the Exchange Act as a public reporting company by filing a quarterly report on Form 10-Q or an annual report on Form 10-K with the SEC.
"Record Date" means, with respect to a Series, the date prior to each Periodic Calculation Date that is disclosed in the prospectus supplement for that Series and on which the beneficial holders of record of the PharmaShares of that Series are determined by the trustee.
"Redemption Cash Component" means the funds that must be delivered by an Authorized Participant to the trustee in connection with any redemption in which treasury securities are being delivered to make the Final Distribution and these treasury securities, valued at their acquisition cost, represent a value in excess of the Per Share Underlying Value of the shares being redeemed.
"Redemption Date" means, for a Series, the next business day following the Redemption Order Date, which is the date on which a Paired Optional Redemption for the Paired PharmaShares of that Series is effected or, in the event that the Redemption Order Date is a Periodic Calculation Date or the business day following the Periodic Calculation Date, on the third business day after the Redemption Order Date.
"Redemption Order Date" means, for a Series, any business day on which an Authorized Participant places an order for a Paired Optional Redemption for the Paired PharmaShares of that Series.
"Redemption Percentage" means, for a Series:
·on any Redemption Order Date, a fraction, expressed as a percentage, the numerator of which is the Aggregate Par Amount of the Paired PharmaShares of that Series that are being redeemed and the denominator of which is Aggregate Par Amount of the Paired PharmaShares of that Series that are outstanding prior to that redemption; or
·on the Final Scheduled Termination Date or an Early Termination Date, a percentage equal to 100%.
"Reference Product" means a specified drug, a basket of drugs, a pharmaceutical device or several pharmaceutical devices sold, in each case, by a pharmaceutical company that reports a Reference Value for such drug or drugs or such pharmaceutical device or devices.
"Reference Value" means, for a Series, (i) the net revenue for a Reference Product; (ii) the change in net revenues of a specified division of a publicly traded pharmaceutical company; or (iii) the change in net revenues allocated to a specified entity that is party to a collaboration agreement, distribution agreement, joint venture or other profit-sharing arrangement relating to the sale of a Reference Product. Net revenue must be reported by the reference pharmaceutical company that manufactures and sells the Reference Product on its income statement prepared in accordance with U.S. GAAP and filed with the SEC on Form 10-Q or Form 10-K in satisfaction of its public reporting obligations under the Exchange Act.
"SEC" means the United States Securities and Exchange Commission.
"Securities Act" means the United States Securities Act of 1933, as amended.
"sequential tracking Series" means a Series of PharmaShares that tracks the Reference Value for that Series from the Current Calendar Quarter to the immediately preceding calendar quarter, which is the "Base Calendar Quarter." A sequential tracking Series is typically suitable for Reference Products that have been recently launched or that have the potential to experience significant changes in sales from quarter to quarter. The prospectus supplement for each Series states if it is a year over year tracking Series or a sequential tracking Series.
"Series" or "Series of PharmaShares" means, individually and collectively, each series of the PharmaShares Trust that is formed by the depositor under the laws of the State of Delaware and pursuant to the related Series Trust Supplement, each issuing one class of Up PharmaShares and one class of Down PharmaShares and having the Reference Product and other characteristics specified in the prospectus supplement for that series.
111
"Series Aggregate Par Amount" means, for a Series and any date of determination, the sum of the Up Aggregate Par Amount for that Series and the Down Aggregate Par Amount for that Series, in each case, on that date.
"Series Investment Amount" means, for a Series and any date of determination, the sum of the Up Investment Amount and the Down Investment Amount for that Series, in each case, on that date. The Series Investment Amount will always be equal to the Series Aggregate Par Amount for each Series unless losses are realized by a Series on its treasury securities or treasury repurchase agreements.
"Series Trust Agreement" means, for a Series, the Master Trust Agreement, together with the Series Trust Supplement for that Series.
"Series Trust Supplement" means, for a Series, the trust supplement to the Master Trust Agreement that governs that Series and specifies the terms of its Paired PharmaShares.
"Termination Triggers" means, with respect to a Series, the events described in this base prospectus under "DESCRIPTION OF THE PHARMASHARES — Termination Triggers" and in the prospectus supplement for that Series under "THE TERMS OF YOUR SERIES — Termination Triggers."
"treasury securities" are securities of the type and tenor described under "THE PHARMASHARES TRUST — United States Treasury Obligations" in this base prospectus.
"Underlying Value" means, for a Series, as the context requires, the Up Underlying Value of all of the outstanding Up PharmaShares of that Series or some portion of those Up PharmaShares, or the Down Underlying Value of the Down PharmaShares of that Series or some portion of those Down PharmaShares.
"Up Aggregate Par Amount" means, for a Series and any date of determination, the amount determined by multiplying the aggregate number of Up PharmaShares issued by that Series on any Issuance Date that occurred prior to that date of determination, less the aggregate number of shares that were redeemed on any Redemption Date that occurred prior to that date, by the par amount of the Up PharmaShares on that date.
"Up Allocation Factor" means, for a Series, a number calculated on each Publication Date as follows:
(i)if a Projected Breakpoint Increase was predicted for the Current Calendar Quarter:
(a)if the Actual Percentage Change is greater than the Projected Breakpoint Increase, there will be an Up Underlying Value increase equal to:
UUVΔ = (Actual Percentage Change – Projected Breakpoint Increase); and
the Up Allocation Factor will equal 1 + the Up Underlying Value increase;
(b)if the Actual Percentage Change reflects an increase that is less than the Projected Breakpoint Increase, the Down Underlying Value will increase as follows:
DUVΔ = (Projected Breakpoint Increase – Actual Percentage Change); and
the Up Allocation Factor will equal 1 – the Down Underlying Value increase; or
(c)if the Actual Percentage Change reflects a decrease for a Current Calendar Quarter with a Projected Breakpoint Increase, the Down Underlying Value will increase as follows:
DUVΔ = (Actual Percentage Change + Projected Breakpoint Increase); and
the Up Allocation Factor will be equal to 1 – the Down Underlying Value increase.
(ii)If a Projected Breakpoint Decrease was predicted for the Current Calendar Quarter:
112
(a)if the Actual Percentage Change reflects a decrease that is greater than the Projected Breakpoint Decrease, the Down Underlying Value will increase as follows:
DUVΔ = (Actual Percentage Change – Projected Breakpoint Decrease); and
the Up Allocation Factor will be equal to 1 – the Down Underlying Value increase;
(b)if the Actual Percentage Change reflects a decrease that is less than the Projected Breakpoint Decrease, the Up Underlying Value will increase as follows:
UUVΔ = (Projected Breakpoint Decrease – Actual Percentage Change)); and
the Up Allocation Factor will be equal to 1 + the Up Underlying Value increase; or
(c)if the Actual Percentage Change reflects an increase for a Current Calendar Quarter with a Projected Breakpoint Decrease, the Up Underlying Value will increase as follows:
UUVΔ = (Actual Percentage Change + Projected Breakpoint Decrease); and
The Up Allocation Factor will be equal to 1 + the Up Underlying Value increase.
"Up Daily Net Income Accrual" means, for the Up PharmaShares of a Series and each calendar day occurring during any Calculation Period:
•the product of (A)(i) the sum of, for each treasury security on deposit in that Series on that day, the product of the purchase price at which that Series acquired that treasury security multiplied by the Daily Yield Rate applicable to that treasury security minus (ii) the Series Investment Amount multiplied by the Daily Fee Accrual Rate, divided by 365 or 366, depending upon the number of days in the current calendar year, and (B)(i) the Up Percentage if net income is being allocated equally among the Up and the Down PharmaShares of that Series, (ii) the Up Underlying Value for that Calculation Period divided by the Up Investment Amount for that Calculation Period, if net income is being allocated based upon the Up Underlying Value and Down Underlying Value, or (iii) such other allocation rule as may be specified in the prospectus supplement for that Series;
plus
•if that calendar day is also an Issuance Date on which a net increase in the Up Aggregate Par Amount has occurred after giving effect to all Paired Issuances and Paired Optional Redemptions effected on that date, the product of the number of Up PharmaShares of that Series created on such date constituting such net increase and the net income component of the Per Share Underlying Value of each such Up PharmaShare, which represents the Up Daily Net Income Accruals allocable to such share during the period from the first day of the current Calculation Period to such Issuance Date, and
minus
•if that calendar day is also a Redemption Date on which a net decrease in the Up Aggregate Par Amount has occurred after giving effect to all Paired Optional Redemptions and Paired Issuances effected on that day, the product of the number of Up PharmaShares of that Series redeemed on such day constituting such net decrease and the income component of the Per Share Underlying Value of each such Up PharmaShare, which represents the Up Daily Net Income Accruals allocable to such share during the period from the first day of the current Calculation Period to such Redemption Date.
If the result of the foregoing calculation is a negative number, then the Up Daily Net Income Accrual for that day will be equal to zero. The treasury securities will be valued at their acquisition cost for purposes of calculating Up
113
Daily Net Income Accruals, but will be valued at their amortized cost in the financial statements prepared for each Series.
"Up/Down Ratio" means, for a Series, the proportion of the amount of funds held by that Series that are allocable to the Up PharmaShares to the amount of funds held by that Series that are allocable to the Down PharmaShares. The Up/Down Ratio is also referred to as the proportion of the Up Investment Amount to the Down Investment Amount. The Up/Down Ratio for most Series will be 1:1.
"Up Investment Amount" means, for a Series on any Periodic Calculation Date, the amount of cash received from holders of the Up PharmaShares of that Series that was actually allocated for investment on behalf of the Series in treasury securities on that Periodic Calculation Date, which is required to equal the lesser of (x) the Up Aggregate Par Amount and (y) all funds on deposit in that Series as of that Periodic Calculation Date that are allocable to the Up PharmaShares, based upon the Up/Down Ratio. In a Series in which the Up/Down Ratio is 1:1, one-half of all invested funds will be allocated to the Up Investment Amount.
On any other day of a Calculation Period, the "Up Investment Amount" will equal the Up Aggregate Par Amount if the amount actually allocated for investment on the preceding Periodic Calculation Date was equal to the Up Aggregate Par Amount.
If less than the Up Aggregate Par Amount was allocated for investment on the last Periodic Calculation Date due to losses on the treasury securities or treasury repurchase agreements, then the "Up Investment Amount" for each day of the ensuing Calculation Period will equal the amount that was actually invested plus the Up Aggregate Par Amount of all Up PharmaShares created since the last Periodic Calculation Date and minus the product of (i)(A) the Up Investment Amount divided by (B) the number of Up PharmaShares outstanding on the date of determination and (ii) the number of Up PharmaShares redeemed since the last Periodic Calculation Date.
"Up Leverage Factor" means, with respect to a Series, the multiplier or ratchet that is applicable to returns on the Up PharmaShares of that Series that is disclosed in the prospectus supplement for that Series.
"Up Percentage" means, for a Series and any date of determination, the Up Aggregate Par Amount on that date divided by the Series Aggregate Par Amount on that date.
"Up Periodic Return Cap" means, with respect to a Series, the percentage limit on the returns that may be achieved by the Up PharmaShares of that Series on any Periodic Calculation Date that is disclosed in the prospectus supplement for that Series.
"Up PharmaShares" means, with respect to a Series, the class of shares titled the "Up PharmaShares" and having the rights and characteristics specified in the Series Trust Supplement pursuant to which such class was issued.
The "Up Underlying Value" of the Up PharmaShares of a Series will be equal on any Publication Date to the Up Investment Amount multiplied by the Up Allocation Factor, calculated based on the Reference Value for that Series that was reported on that Publication Date.
The "Up Underlying Value" of the Up PharmaShares of a Series will equal on any calendar day occurring during the Calculation Period that follows each Publication Date:
·the sum of the Up Daily Net Income Accruals for each day that has elapsed during the current Calculation Period, up to and including the current calendar day
plus
·the Up Investment Amount multiplied by the Up Allocation Factor, calculated based on the Reference Value for that Series that was reported on the most recent Publication Date.
"U.S. GAAP" means United States generally accepted accounting principles.
114
"Value" means, with respect to each treasury security, the purchase price at which a Series acquired that treasury security plus all interest and/or discount accrued on that treasury security since its acquisition date.
"year-over-year tracking Series" means a Series of PharmaShares that tracks the Reference Value for that Series from the Current Calendar Quarter to the same calendar quarter of the preceding calendar year, which is the "Base Calendar Quarter." A "year over year tracking Series" is typically suitable for Reference Products that have a seasonality aspect to their sales, that may be sold with larger first quarter discounts before insurance deductibles are satisfied and/or that are at a more mature point in their lifecycle with less sequential quarter variability in sales levels. The prospectus supplement for each Series states if it is a year over year tracking Series or a sequential tracking Series.
115
Appendix A
HISTORICAL TREASURY YIELDS
Date | 3-month value | | Date | 3-month value | | Date | 3-month value | | Date | 3-month value | | Date | 3-month value |
1/31/1990 | 8.00 | | 1/31/1991 | 6.37 | | 1/31/1992 | 3.94 | | 1/29/1993 | 2.96 | | 1/31/1994 | 3.05 |
2/28/1990 | 8.04 | | 2/28/1991 | 6.22 | | 2/28/1992 | 4.03 | | 2/26/1993 | 3.01 | | 2/28/1994 | 3.47 |
3/30/1990 | 8.07 | | 3/28/1991 | 5.92 | | 3/31/1992 | 4.15 | | 3/31/1993 | 2.95 | | 3/31/1994 | 3.56 |
4/30/1990 | 8.07 | | 4/30/1991 | 5.68 | | 4/30/1992 | 3.79 | | 4/30/1993 | 2.97 | | 4/29/1994 | 3.97 |
5/31/1990 | 8.01 | | 5/31/1991 | 5.71 | | 5/29/1992 | 3.79 | | 5/28/1993 | 3.13 | | 5/31/1994 | 4.31 |
6/29/1990 | 8.00 | | 6/28/1991 | 5.71 | | 6/30/1992 | 3.65 | | 6/30/1993 | 3.10 | | 6/30/1994 | 4.26 |
7/31/1990 | 7.74 | | 7/31/1991 | 5.70 | | 7/31/1992 | 3.25 | | 7/30/1993 | 3.10 | | 7/29/1994 | 4.39 |
8/31/1990 | 7.63 | | 8/30/1991 | 5.49 | | 8/31/1992 | 3.23 | | 8/31/1993 | 3.08 | | 8/31/1994 | 4.68 |
9/28/1990 | 7.37 | | 9/30/1991 | 5.26 | | 9/30/1992 | 2.75 | | 9/30/1993 | 2.98 | | 9/30/1994 | 4.80 |
10/31/1990 | 7.34 | | 10/31/1991 | 4.96 | | 10/30/1992 | 3.03 | | 10/29/1993 | 3.10 | | 10/31/1994 | 5.20 |
11/30/1990 | 7.24 | | 11/29/1991 | 4.47 | | 11/30/1992 | 3.38 | | 11/30/1993 | 3.21 | | 11/30/1994 | 5.72 |
12/31/1990 | 6.63 | | 12/31/1991 | 3.96 | | 12/31/1992 | 3.15 | | 12/31/1993 | 3.07 | | 12/30/1994 | 5.68 |
| | | | | | | | | | | | | |
1/31/1995 | 6.00 | | 1/31/1996 | 5.05 | | 1/31/1997 | 5.15 | | 1/30/1998 | 5.19 | | 1/29/1999 | 4.48 |
2/28/1995 | 5.94 | | 2/29/1996 | 5.02 | | 2/28/1997 | 5.22 | | 2/27/1998 | 5.32 | | 2/26/1999 | 4.66 |
3/31/1995 | 5.88 | | 3/29/1996 | 5.13 | | 3/31/1997 | 5.35 | | 3/31/1998 | 5.16 | | 3/31/1999 | 4.49 |
4/28/1995 | 5.87 | | 4/30/1996 | 5.14 | | 4/30/1997 | 5.28 | | 4/30/1998 | 5.00 | | 4/30/1999 | 4.55 |
5/31/1995 | 5.81 | | 5/31/1996 | 5.18 | | 5/30/1997 | 4.96 | | 5/29/1998 | 5.03 | | 5/28/1999 | 4.66 |
6/30/1995 | 5.60 | | 6/28/1996 | 5.18 | | 6/30/1997 | 5.25 | | 6/30/1998 | 5.10 | | 6/30/1999 | 4.78 |
7/31/1995 | 5.60 | | 7/31/1996 | 5.32 | | 7/31/1997 | 5.25 | | 7/31/1998 | 5.10 | | 7/30/1999 | 4.75 |
8/31/1995 | 5.45 | | 8/30/1996 | 5.29 | | 8/29/1997 | 5.24 | | 8/31/1998 | 4.96 | | 8/31/1999 | 4.98 |
9/29/1995 | 5.40 | | 9/30/1996 | 5.14 | | 9/30/1997 | 5.06 | | 9/30/1998 | 4.37 | | 9/30/1999 | 4.88 |
10/31/1995 | 5.48 | | 10/31/1996 | 5.17 | | 10/31/1997 | 5.21 | | 10/30/1998 | 4.33 | | 10/29/1999 | 5.12 |
11/30/1995 | 5.48 | | 11/29/1996 | 5.13 | | 11/28/1997 | 5.22 | | 11/30/1998 | 4.57 | | 11/30/1999 | 5.30 |
12/29/1995 | 5.10 | | 12/31/1996 | 5.21 | | 12/31/1997 | 5.36 | | 12/31/1998 | 4.48 | | 12/31/1999 | 5.33 |
A-1
Date | 3-month value | | Date | 3-month value | | Date | 3-month value | | Date | 3-month value | | Date | 3-month value |
1/31/2000 | 5.76 | | 1/31/2001 | 4.99 | | 1/31/2002 | 1.76 | | 1/31/2003 | 1.18 | | 1/30/2004 | 0.92 |
2/29/2000 | 5.78 | | 2/28/2001 | 4.85 | | 2/28/2002 | 1.79 | | 2/28/2003 | 1.20 | | 2/27/2004 | 0.96 |
3/31/2000 | 5.88 | | 3/30/2001 | 4.30 | | 3/28/2002 | 1.79 | | 3/31/2003 | 1.14 | | 3/31/2004 | 0.95 |
4/28/2000 | 5.82 | | 4/30/2001 | 3.95 | | 4/30/2002 | 1.77 | | 4/30/2003 | 1.13 | | 4/30/2004 | 0.98 |
5/31/2000 | 5.63 | | 5/31/2001 | 3.63 | | 5/31/2002 | 1.74 | | 5/30/2003 | 1.11 | | 5/28/2004 | 1.08 |
6/30/2000 | 5.88 | | 6/29/2001 | 3.65 | | 6/28/2002 | 1.70 | | 6/30/2003 | 0.90 | | 6/30/2004 | 1.33 |
7/31/2000 | 6.27 | | 7/31/2001 | 3.54 | | 7/31/2002 | 1.71 | | 7/31/2003 | 0.96 | | 7/30/2004 | 1.45 |
8/31/2000 | 6.31 | | 8/31/2001 | 3.37 | | 8/30/2002 | 1.69 | | 8/29/2003 | 0.98 | | 8/31/2004 | 1.59 |
9/29/2000 | 6.23 | | 9/28/2001 | 2.40 | | 9/30/2002 | 1.57 | | 9/30/2003 | 0.95 | | 9/30/2004 | 1.71 |
10/31/2000 | 6.38 | | 10/31/2001 | 2.05 | | 10/31/2002 | 1.44 | | 10/31/2003 | 0.96 | | 10/29/2004 | 1.91 |
11/30/2000 | 6.21 | | 11/30/2001 | 1.78 | | 11/29/2002 | 1.22 | | 11/28/2003 | 0.93 | | 11/30/2004 | 2.23 |
12/29/2000 | 5.89 | | 12/31/2001 | 1.74 | | 12/31/2002 | 1.22 | | 12/31/2003 | 0.95 | | 12/31/2004 | 2.22 |
| | | | | | | | | | | | | |
1/31/2005 | 2.51 | | 1/31/2006 | 4.47 | | 1/31/2007 | 5.12 | | 1/31/2008 | 1.96 | | 1/30/2009 | 0.24 |
2/28/2005 | 2.76 | | 2/28/2006 | 4.62 | | 2/28/2007 | 5.16 | | 2/29/2008 | 1.85 | | 2/27/2009 | 0.26 |
3/31/2005 | 2.79 | | 3/31/2006 | 4.63 | | 3/30/2007 | 5.04 | | 3/31/2008 | 1.38 | | 3/31/2009 | 0.21 |
4/29/2005 | 2.90 | | 4/28/2006 | 4.77 | | 4/30/2007 | 4.91 | | 4/30/2008 | 1.43 | | 4/30/2009 | 0.14 |
5/31/2005 | 2.99 | | 5/31/2006 | 4.86 | | 5/31/2007 | 4.73 | | 5/30/2008 | 1.89 | | 5/29/2009 | 0.14 |
6/30/2005 | 3.13 | | 6/30/2006 | 5.01 | | 6/29/2007 | 4.82 | | 6/30/2008 | 1.90 | | 6/30/2009 | 0.19 |
7/29/2005 | 3.42 | | 7/31/2006 | 5.10 | | 7/31/2007 | 4.96 | | 7/31/2008 | 1.68 | | 7/31/2009 | 0.18 |
8/31/2005 | 3.52 | | 8/31/2006 | 5.05 | | 8/31/2007 | 4.01 | | 8/29/2008 | 1.72 | | 8/31/2009 | 0.15 |
9/30/2005 | 3.55 | | 9/29/2006 | 4.89 | | 9/28/2007 | 3.82 | | 9/30/2008 | 0.92 | | 9/30/2009 | 0.14 |
10/31/2005 | 3.98 | | 10/31/2006 | 5.08 | | 10/31/2007 | 3.94 | | 10/31/2008 | 0.46 | | 10/30/2009 | 0.05 |
11/30/2005 | 3.95 | | 11/30/2006 | 5.03 | | 11/30/2007 | 3.15 | | 11/28/2008 | 0.01 | | 11/30/2009 | 0.06 |
12/30/2005 | 4.08 | | 12/29/2006 | 5.02 | | 12/31/2007 | 3.36 | | 12/31/2008 | 0.11 | | 12/31/2009 | 0.06 |
Date | 3-month value | | Date | 3-month value | | Date | 3-month value | | Date | 3-month value | | Date | 3-month value |
1/29/2010 | 0.08 | | 1/31/2011 | 0.15 | | 1/31/2012 | 0.06 | | 1/31/2013 | 0.07 | | 1/31/2014 | 0.02 |
2/26/2010 | 0.13 | | 2/28/2011 | 0.15 | | 2/29/2012 | 0.08 | | 2/28/2013 | 0.11 | | 2/28/2014 | 0.05 |
3/31/2010 | 0.16 | | 3/31/2011 | 0.09 | | 3/30/2012 | 0.07 | | 3/28/2013 | 0.07 | | 3/31/2014 | 0.05 |
4/30/2010 | 0.16 | | 4/29/2011 | 0.04 | | 4/30/2012 | 0.10 | | 4/30/2013 | 0.05 | | 4/30/2014 | 0.03 |
5/28/2010 | 0.16 | | 5/31/2011 | 0.06 | | 5/31/2012 | 0.07 | | 5/31/2013 | 0.04 | | 5/30/2014 | 0.04 |
6/30/2010 | 0.18 | | 6/30/2011 | 0.03 | | 6/29/2012 | 0.09 | | 6/28/2013 | 0.04 | | 6/30/2014 | 0.04 |
7/30/2010 | 0.15 | | 7/29/2011 | 0.10 | | 7/31/2012 | 0.11 | | 7/31/2013 | 0.04 | | 7/31/2014 | 0.03 |
8/31/2010 | 0.14 | | 8/31/2011 | 0.02 | | 8/31/2012 | 0.09 | | 8/30/2013 | 0.03 | | 8/29/2014 | 0.03 |
9/30/2010 | 0.16 | | 9/30/2011 | 0.02 | | 9/28/2012 | 0.10 | | 9/30/2013 | 0.02 | | 9/30/2014 | 0.02 |
10/29/2010 | 0.12 | | 10/31/2011 | 0.01 | | 10/31/2012 | 0.11 | | 10/31/2013 | 0.04 | | 10/31/2014 | 0.01 |
11/30/2010 | 0.17 | | 11/30/2011 | 0.01 | | 11/30/2012 | 0.08 | | 11/29/2013 | 0.06 | | 11/28/2014 | 0.02 |
12/31/2010 | 0.12 | | 12/30/2011 | 0.02 | | 12/31/2012 | 0.05 | | 12/31/2013 | 0.07 | | 12/31/2014 | 0.04 |
| | | | | | | | | | | | | |
A-2
1/30/2015 | 0.02 | | 1/29/2016 | 0.33 | | 1/31/2017 | 0.52 | | 1/31/2018 | 1.46 | | 1/31/2019 | 2.41 |
2/27/2015 | 0.02 | | 2/29/2016 | 0.33 | | 2/28/2017 | 0.53 | | 2/28/2018 | 1.65 | | 2/28/2019 | 2.45 |
3/31/2015 | 0.03 | | 3/31/2016 | 0.21 | | 3/31/2017 | 0.76 | | 3/29/2018 | 1.73 | | 3/29/2019 | 2.40 |
4/30/2015 | 0.01 | | 4/29/2016 | 0.22 | | 4/28/2017 | 0.80 | | 4/30/2018 | 1.87 | | 4/30/2019 | 2.43 |
5/29/2015 | 0.01 | | 5/31/2016 | 0.34 | | 5/31/2017 | 0.98 | | 5/31/2018 | 1.93 | | 5/24/2019 | 2.35 |
6/30/2015 | 0.01 | | 6/30/2016 | 0.26 | | 6/30/2017 | 1.03 | | 6/29/2018 | 1.93 | | | |
7/31/2015 | 0.08 | | 7/29/2016 | 0.28 | | 7/31/2017 | 1.07 | | 7/31/2018 | 2.03 | | | |
8/31/2015 | 0.08 | | 8/31/2016 | 0.33 | | 8/31/2017 | 1.01 | | 8/31/2018 | 2.11 | | | |
9/30/2015 | 0.00 | | 9/30/2016 | 0.29 | | 9/29/2017 | 1.06 | | 9/28/2018 | 2.19 | | | |
10/30/2015 | 0.08 | | 10/31/2016 | 0.34 | | 10/31/2017 | 1.15 | | 10/31/2018 | 2.34 | | | |
11/30/2015 | 0.22 | | 11/30/2016 | 0.48 | | 11/30/2017 | 1.27 | | 11/30/2018 | 2.37 | | | |
A-3
Appendix B
TRANSACTIONS WITH AFFILIATED PERSONS
1.Acquisitions of U.S. Treasury Securities and Treasury Repurchase Agreements
(a)Subject to a best execution requirement, the administrative agent may direct the trustee to acquire U.S. treasury securities and treasury repurchase agreements from (i) an Authorized Participant or (ii) an Affiliated Person (as defined in clause (c) below) with respect to any Authorized Participant, only if such acquisition is within the Range of the Best Yield, each as defined in clause (c) below. The persons described in clauses (i) and (ii) above are referred to, for purposes hereof, as "AP Affiliated Persons," and, any acquisition by an Up PharmaShares Trust from an AP Affiliated Person is referred to as an "AP Acquisition."
(b)The available market yields for purposes of calculating Best Yield are based, for trades in U.S. treasury securities, on prices displayed on the applicable Bloomberg screen and, for trades in treasury repurchase agreements, general market offered yields obtained from Bloomberg or another third-party service provider for treasury repurchase agreements.
(c)"Affiliated Person" means, for purposes of this Appendix B, (i) any person directly or indirectly owning, controlling, or holding with power to vote, 5 percent or more of the outstanding voting securities of an Authorized Participant; (ii) any person 5 percent or more of whose outstanding voting securities are directly or indirectly owned, controlled, or held with power to vote, by an Authorized Participant; (iii) any person directly or indirectly controlling, controlled by, or under common control with, an Authorized Participant; (iv) any officer, director, partner, copartner, or employee of an Authorized Participant; (v) if the Authorized Participant is an investment company, any investment adviser thereof or any member of an advisory board thereof; and (vi) if the Authorized Participant is an unincorporated investment company not having a board of directors, the depositor thereof.
"Best Yield" means the average of the five highest yields offered by sellers with whom the administrative agent has a trading agreement and the "Range" is the difference between (i) the greater of (x) 0.10% and (y) 2% times the Best Yield, which is the bottom of the Range, and (ii) the Best Yield, which is the top of the range.
2.In-Kind Redemptions
Any "in-kind" Paired Optional Redemption by an AP Affiliated Person is executed by the trustee in accordance with instructions delivered to it by the administrative agent who employs a "last in, first out" methodology to select U.S. treasury securities for delivery to the AP Affiliated Person (each such Paired Optional Redemption, an "AP In-Kind Redemption").
3.Record Retention
(a)For each AP Acquisition, the administrative agent will retain a print-out of the Bloomberg screen at the time each U.S. treasury security purchase order is placed and a print-out of the Bloomberg or other pricing service yield information at the time each treasury repurchase agreement purchase order is placed (as well as, in each case, the corresponding trade ticket information relating to such AP Acquisition). Such print-outs will include all available offers at the time the applicable order was placed, as well as the time, date, amount, counterparty and implied yield or price of each transaction.
B-1
(b)For each AP Acquisition that was effected at a yield that was lower than the Best Yield, the administrative agent will record, contemporaneously with the transaction, why the AP Acquisition was effected at a yield that was lower than the Best Yield.
(c)For each AP In-Kind Redemption, the administrative agent will retain in its books and records, the date of the transaction, the name of the redeeming Authorized Participant, the U.S. treasury securities that were delivered in the redemption and the U.S. treasury securities that were on deposit in the applicable Up PharmaShares Trust immediately prior to the In-Kind Redemption (including on the relevant acquisition date), and the U.S. treasury securities which remained on deposit in that Up PharmaShares Trust after such redemption.
(d)The information recorded by the administrative agent pursuant to this Section 3 will be retained by the administrative agent for a period of one year from the date each AP Acquisition and AP In-Kind Redemption took place.
4.Independent Verification Procedures
(a)At the conclusion of each calendar quarter, the trustee, acting as verification agent (the "Verification Agent"), selects, randomly and without notifying the administrative agent, one business day in each week of the preceding quarter, and
(i)if any AP Acquisitions occurred on such day, the Verification Agent will verify that (1) the administrative agent properly identified and recorded all such AP Acquisitions; and (2) no transactions outside of the Range were executed with any AP Affiliated Person; and
(ii)if any AP In-Kind Redemptions occurred on such day, the Verification Agent will verify (1) that the administrative agent properly identified and recorded all such AP In-Kind Redemptions and (2) that the AP In-Kind Redemptions were executed in accordance to Section 2, hereof.
(iii)In the event that the Verification Agent has identified an AP Acquisition outside of the Range or an AP In-Kind Redemption that violates Section 2, hereof, (each such transaction, a "Prohibited Transaction"), the Verification Agent shall review all the information retained by the administrative agent in accordance with Sections 3(a) or 3(b) hereof, as applicable, during, (i) the six-month period that preceded such Prohibited Transaction; and (ii) the calendar quarter that follows the Prohibited Transaction, to determine whether any additional Prohibited Transactions occurred.
5.Remedy for Prohibited Transactions
(a)In the event that the Verification Agent identifies any Prohibited Transaction, the Verification Agent will notify the depositor and the administrative agent, and the administrative agent will be required to pay to the applicable Series of the PharmaShares Trust for each such Prohibited Transaction, within thirty days of such notification,
(i)if the Prohibited Transaction was an AP Acquisition, the difference between (x) the Best Yield that was available for such transaction occurred, less the Range and (y) the yield actually obtained in the Prohibited Transaction; and
(ii)if the Prohibited Transaction was an AP In-Kind Redemption, an amount equal to the yield to maturity of the U.S. treasury securities which were delivered minus the yield to maturity of the U.S. treasury securities which should have been delivered to the redeeming AP Affiliated Person.
B-2
These procedures apply to transactions between the PharmaShares Trust and any Authorized Participant or any Affiliated Person with regard to the such Authorized Participant, to the extent that such Authorized Participant meets the definition of "principal underwriter" with regard to the PharmaShares Trust that is set forth in Section 2(a)(29) of the Investment Company Act of 1940, as amended and as interpreted by the SEC and its Staff.
B-3
PART II
Item 13. Other Expenses of Issuance and Distribution
The following is an itemized list of the estimated expenses to be incurred in connection with the offering of the securities being offered hereunder other than underwriting discounts and commissions.
Legal fees and expenses | $[●] |
Accounting fees and expenses | [●] |
SEC/FINRA expenses | [●] |
Travel and road show | [●] |
NYSE Area listing and filing fees | [●] |
Directors and officers insurance | [●] |
Printing and engraving expenses | [●] |
Miscellaneous Fees and Expenses | [●] |
| |
Total offering expenses | $[●] |
| |
Item 14. Indemnification of Directors and Officers
Section 18-108 of the Delaware Limited Liability Company Act provides that, subject to such standards and restrictions, if any, as are set forth in its limited liability company agreement, a limited liability company may, and shall have the power to, indemnify and hold harmless any member or manager or other person from and against any and all claims and demands whatsoever.
The registrant was formed under the laws of the State of Delaware. The Limited Liability Company Agreement ("LLC Agreement") of the registrant provides, in effect, that, subject to certain limited exceptions, it will indemnify its member, officers, managers, employees and agents and the employees, representatives, agents and affiliates of its member (collectively, the "Covered Persons"), to the fullest extent permitted by applicable law, for any loss, damage or claim incurred by such Covered Person by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the registrant and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person by the LLC agreement, except that no Covered Person shall be entitled to be indemnified in respect of any loss, damage or claim incurred by such Covered Person by reason of its own gross negligence or willful misconduct with respect to such acts or omissions.
To the fullest extent permitted by applicable law, expenses (including legal fees) incurred by a Covered Person defending any claim, demand, action, suit or proceeding shall, from time to time, be advanced by the registrant prior to the final disposition of such claim, demand, action, suit or proceeding upon receipt by the registrant of an undertaking by or on behalf of the Covered Person to repay such amount if it shall be determined that the Covered Person is not entitled to be indemnified as authorized in the LLC Agreement.
A Covered Person shall be fully protected in relying in good faith upon the records of the registrant and upon such information, opinions, reports or statements presented to the registrant by any person as to matters the Covered Person reasonably believes are within such other person’s professional or expert competence and who has been selected with reasonable care by or on behalf of the registrant, including information, opinions, reports or statements as to the value and amount of the assets or liabilities of the registrant, or any other facts pertinent to the existence and amount of assets from which distributions to the member might properly be paid.
To the extent that, at law or in equity, a Covered Person has duties (including fiduciary duties) and liabilities relating thereto to the registrant or to any other Covered Person, a Covered Person acting under the LLC Agreement shall not be liable to the registrant or to any other Covered Person for its good faith reliance on the provisions of the LLC Agreement or any approval or authorization granted by the registrant or any other Covered Person. The provisions of the LLC Agreement, to the extent that they restrict the duties and liabilities of a Covered Person otherwise
1
existing at law or in equity, are agreed by the member to replace such other duties and liabilities of such Covered Person.
The undersigned registrant hereby undertakes that, insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
Each authorized participants agreement will generally provide that the authorized participant will indemnify the registrant and its directors, officers and controlling parties against specified liabilities, including liabilities under the Securities Act of 1933 relating to certain information provided or actions taken by the authorized participant. The registrant has been advised that in the opinion of the Securities and Exchange Commission this indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered Securities.
None.
Item 16. Exhibits and Financial Statement Schedules.
(a)Exhibits.
Exhibit Number | Description |
| |
[●] | LLC Agreement of PharmaShares Advisors, LLC |
[●] | Certificate of Trust Formation of PharmaShares Trust |
[●] | Master Trust Agreement |
[●] | Series Trust Supplement for Series BTOX-2019 |
[●] | Participants Agreement |
[●] | Opinion with respect to corporate matters |
[●] | Opinion with respect to tax matters |
[●] | Selling Agency Agreement |
[●] | Power of Attorney |
(b)Financial Statements. See page F-1 for an index to the financial statements and schedules included in the registration statement.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1)To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement . Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20
2
percent change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement; and
(iii)To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2)That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3)To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4)That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
If the Registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5)That, for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii)Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii)The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and
(iv)Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser.
(6)That insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the
3
securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, State of New York, on the twenty-fifth day of June, 2021.
PharmaShares Advisors, LLC, |
as sponsor of PharmaShares Master Trust |
| |
| |
By: | /s/ Jon C. Ohrn |
Name: | Jon C. Ohrn |
Title: | Member |
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities* and on the dates indicated.
| | | | | | |
Signature | | | | Title | | Date |
| | | |
/s/ Jon C. Ohrn | | Member | June 25, 2021 |
| | | | | | ____________ |
* The registrant is a trust and the signatory is signing in his capacity of a managing member of PharmaShares Advisors, LLC, the sponsor of the registrant.
4


