
Exhibit 99.1 BAUDAX BIO Corporate Presentation March 2023 Confidential: For Internal Use Only I

Forward Looking Statements This presentation contains forward-looking statements that involve risks and uncertainties. Such forward-looking statements reflect Baudax Bio’s expectations about its future performance and opportunities that involve substantial risks and uncertainties. When used herein, the words “anticipate,” “believe,” “estimate,” “may,” “upcoming,” “plan,” “target,” “goal,” “intend,” and “expect,” and similar expressions, as they relate to Baudax Bio or its management, are intended to identify such forward-looking statements. These forward-looking statements are based on information available to Baudax Bio as of the date of publication on this internet site and are subject to a number of risks, uncertainties, and other factors that could cause Baudax Bio’s performance to differ materially from those expressed in, or implied by, these forward-looking statements. These risks and uncertainties include, among other things, risks related to market and other conditions, the ongoing economic and social consequences of the COVID-19 pandemic, including on Baudax Bio’s supply chain and labor force, Baudax Bio’s ability to advance its current product candidate pipeline through pre-clinical studies and clinical trials, Baudax Bio’s ability to raise future financing for continued development of its product candidates such as BX1000, BX2000 and BX3000, Baudax Bio’s ability to pay its debt and satisfy conditions necessary to access future tranches of debt, Baudax Bio’s ability to comply with the financial and other covenants under its credit facility, Baudax Bio’s ability to manage costs and execute on its operational and budget plans, Baudax Bio’s ability to achieve its financial goals; Baudax Bio’s ability to maintain listing on the Nasdaq Capital Market, and Baudax Bio’s ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection. These forward-looking statements should be considered together with the risks and uncertainties that may affect Baudax Bio’s business and future results included in Baudax Bio’s filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to Baudax Bio, and Baudax Bio assumes no obligation to update any forward-looking statements except as required by applicable law. Confidential: For Internal Use Only I 2

Investor Highlights Addressing an estimated $4 billion global opportunity in growing acute care neuromuscular blocking agents (NMBs) segment Clinical stage NMB assets offer potential for improved patient management, cost reduction for procedures requiring NMBs BX-1000 key data readouts anticipated 1H 2023 • Second pre-planned interim analysis expected March 2023 • Top line phase 2 data readout anticipated by mid Q2 2023 BX-3000 key events • IND filing for NMB reversal agent anticipated summer 2023 Global commercial rights to NMBs and proprietary reversal agent Experienced leadership with history of successful approvals and commercialization Confidential: For Internal Use Only I 3

Our Team Significant drug development and commercial assessment expertise Gerri Henwood Randall Mack Stewart Mc Callum, MD President & CEO EVP, Development & Operations EVP, Medical Affairs Founder, President, CEO Recro EVP Development, Recro EVP Medical Affairs, Recro President of Malvern Consulting Group VP Development & Operations, Adolor Medical Director, GSK Founder, Pres., CEO Auxilium VP Development, Auxilium Assistant Professor of Urology Stanford University Founder, CEO IBAH (NASDAQ listed CRO) Director Venture Development, Abbott Labs Staff Surgeon, Stanford University & Palo Alto VA (now AbbVie) Medical Center >10 years at SK&F (now GSK) Diane Myers Mike Choi Jillian Dilmore SVP Regulatory & Quality Finance Corporate Controller SVP Regulatory & Quality, Recro VP Finance, Recro Director of Accounting, Recro SVP Regulatory Affairs & Quality Assurance, MCG VP Finance, Auxilium Assistant Controller, Royal DSM VP Regulatory Affairs & Quality, Auxilium VP Finance, IBAH Senior Auditor, Deloitte & Touche >15 years at GSK Confidential: For Internal Use Only I 4

Neuromuscular Blockers and Reversal Agent

NMB Overview ~400 million patients receive 1 NMB agents annually Used to induce rapid total paralysis to permit intubation and muscle relaxation during surgery or in ventilated patients Used in the operating room/ASC to optimize surgical conditions Additional use in ICU to facilitate mechanical ventilation Procedural use increasing with growth of laparoscopic abdominal procedures 1. IMS, MIDAS 2010. NMB = Neuromuscular blocking agents * Based on extrapolations from dose escalation study in Confidential: For Internal Use Only I 6 volunteers and on pre-clinical pharmacology data in animals
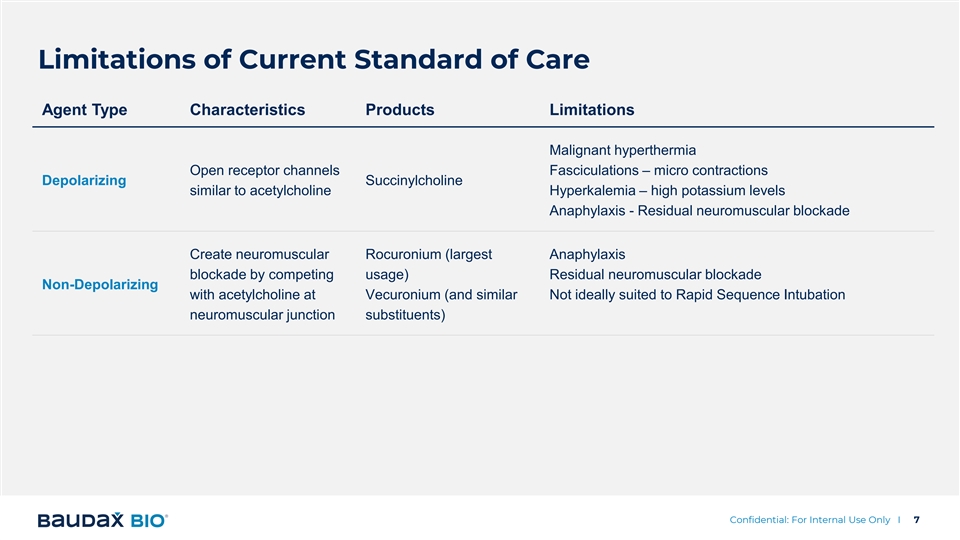
Limitations of Current Standard of Care Agent Type Characteristics Products Limitations Malignant hyperthermia Open receptor channels Fasciculations – micro contractions Depolarizing Succinylcholine similar to acetylcholine Hyperkalemia – high potassium levels Anaphylaxis - Residual neuromuscular blockade Create neuromuscular Rocuronium (largest Anaphylaxis blockade by competing usage) Residual neuromuscular blockade Non-Depolarizing with acetylcholine at Vecuronium (and similar Not ideally suited to Rapid Sequence Intubation neuromuscular junction substituents) Confidential: For Internal Use Only I 7
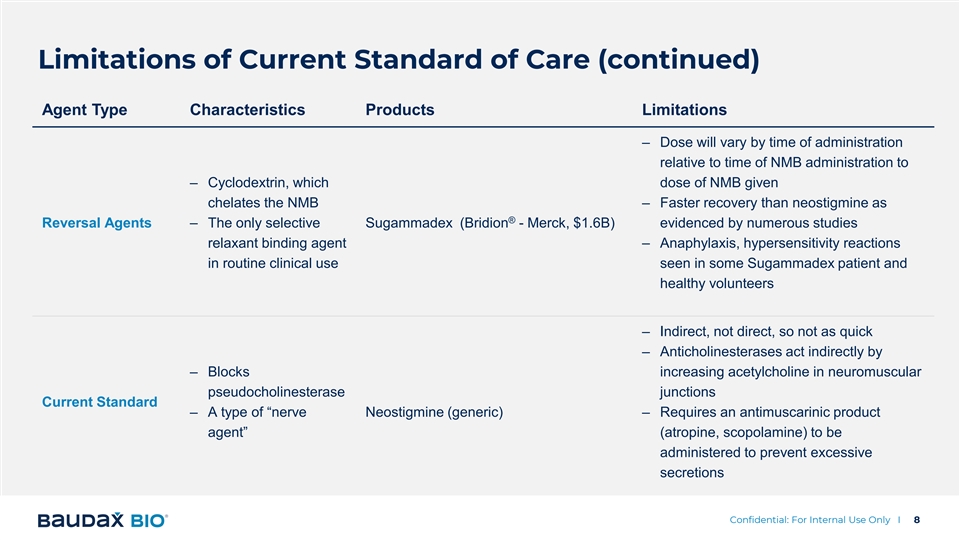
Limitations of Current Standard of Care (continued) Agent Type Characteristics Products Limitations – Dose will vary by time of administration relative to time of NMB administration to – Cyclodextrin, which dose of NMB given chelates the NMB – Faster recovery than neostigmine as ® Reversal Agents – The only selective Sugammadex (Bridion - Merck, $1.6B) evidenced by numerous studies relaxant binding agent – Anaphylaxis, hypersensitivity reactions in routine clinical use seen in some Sugammadex patient and healthy volunteers – Indirect, not direct, so not as quick – Anticholinesterases act indirectly by – Blocks increasing acetylcholine in neuromuscular pseudocholinesterase junctions Current Standard – A type of “nerve Neostigmine (generic) – Requires an antimuscarinic product agent” (atropine, scopolamine) to be administered to prevent excessive secretions Confidential: For Internal Use Only I 8
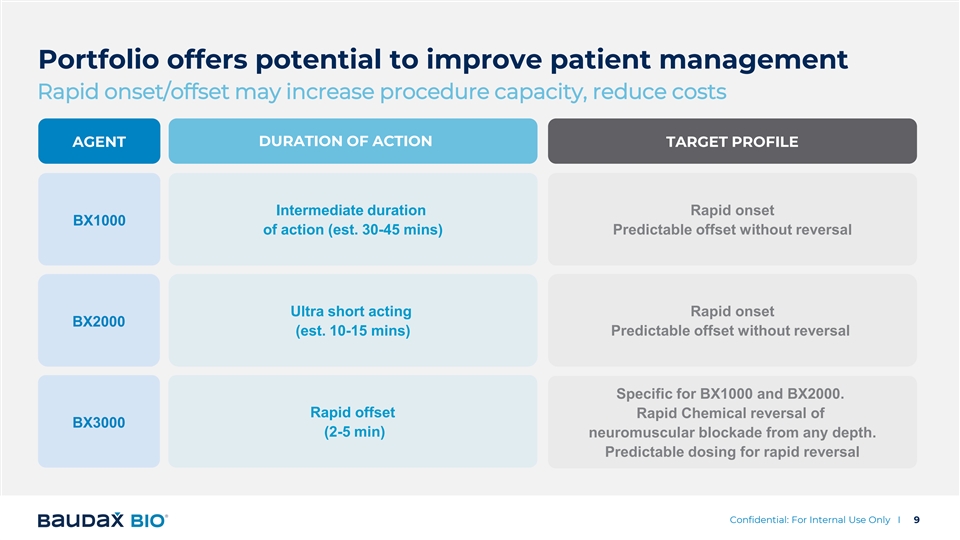
Portfolio offers potential to improve patient management Rapid onset/offset may increase procedure capacity, reduce costs AGENT DURATION OF ACTION TARGET PROFILE Intermediate duration Rapid onset BX1000 of action (est. 30-45 mins) Predictable offset without reversal Ultra short acting Rapid onset BX2000 (est. 10-15 mins) Predictable offset without reversal Specific for BX1000 and BX2000. Rapid offset Rapid Chemical reversal of BX3000 (2-5 min) neuromuscular blockade from any depth. Predictable dosing for rapid reversal Confidential: For Internal Use Only I 9
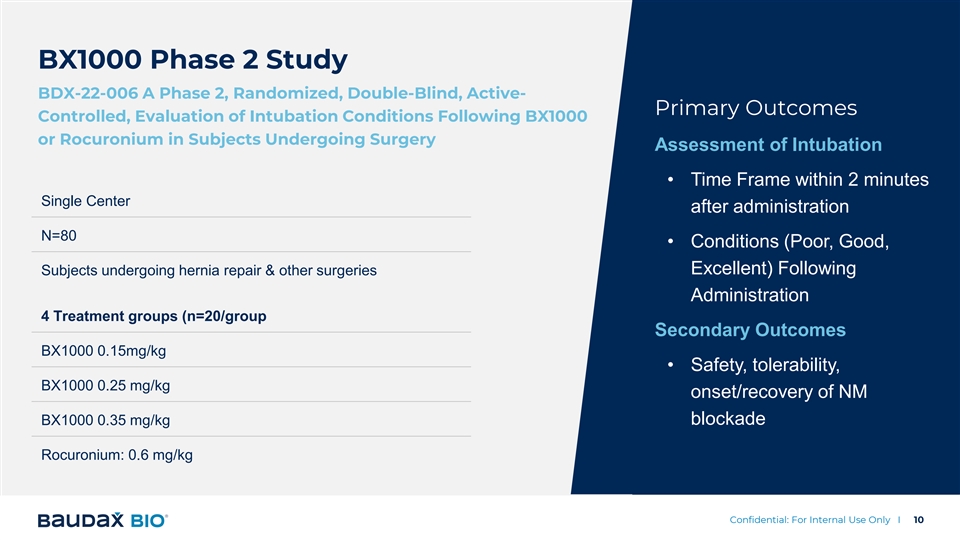
BX1000 Phase 2 Study BDX-22-006 A Phase 2, Randomized, Double-Blind, Active- Primary Outcomes Controlled, Evaluation of Intubation Conditions Following BX1000 or Rocuronium in Subjects Undergoing Surgery Assessment of Intubation • Time Frame within 2 minutes Single Center after administration N=80 • Conditions (Poor, Good, Excellent) Following Subjects undergoing hernia repair & other surgeries Administration 4 Treatment groups (n=20/group Secondary Outcomes BX1000 0.15mg/kg • Safety, tolerability, BX1000 0.25 mg/kg onset/recovery of NM BX1000 0.35 mg/kg blockade Rocuronium: 0.6 mg/kg Confidential: For Internal Use Only I 10
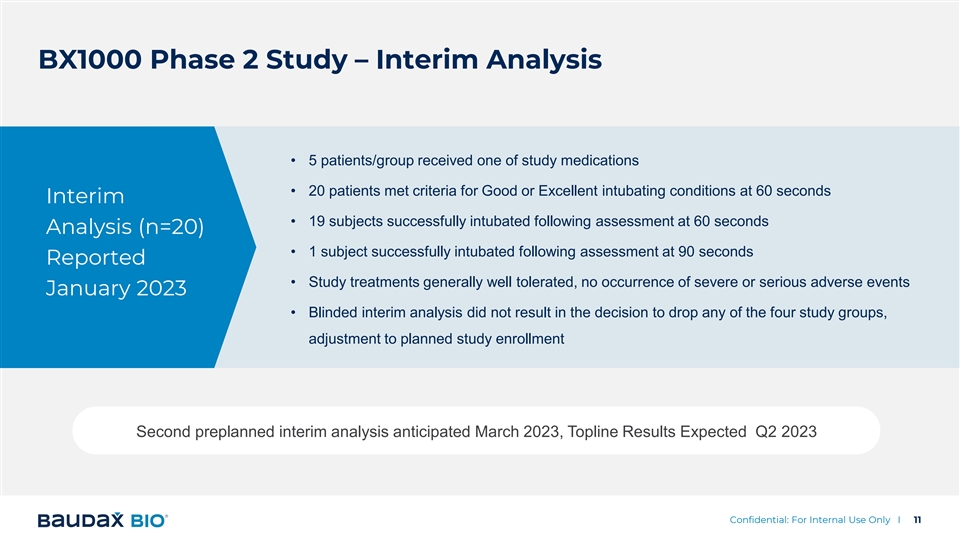
BX1000 Phase 2 Study – Interim Analysis • 5 patients/group received one of study medications • 20 patients met criteria for Good or Excellent intubating conditions at 60 seconds Interim • 19 subjects successfully intubated following assessment at 60 seconds Analysis (n=20) • 1 subject successfully intubated following assessment at 90 seconds Reported • Study treatments generally well tolerated, no occurrence of severe or serious adverse events January 2023 • Blinded interim analysis did not result in the decision to drop any of the four study groups, adjustment to planned study enrollment Second preplanned interim analysis anticipated March 2023, Topline Results Expected Q2 2023 Confidential: For Internal Use Only I 11

BX2000 Phase 1 Trial Phase 1, double-blind, placebo-controlled, single dose escalation BDX-20-004 Dose Range 10 cohorts First in human use 0.02 mg/kg to 0.64 mg/kg N=~80 Anticipate enrollment ~7 cohorts Active to placebo ratio (6:2) Objectives Evaluate the safety and tolerability of single ascending doses of BX2000 Characterize the pharmacokinetics Evaluate the neuromuscular blocking profile Each cohort’s data reviewed by a safety committee Confidential: For Internal Use Only I 12
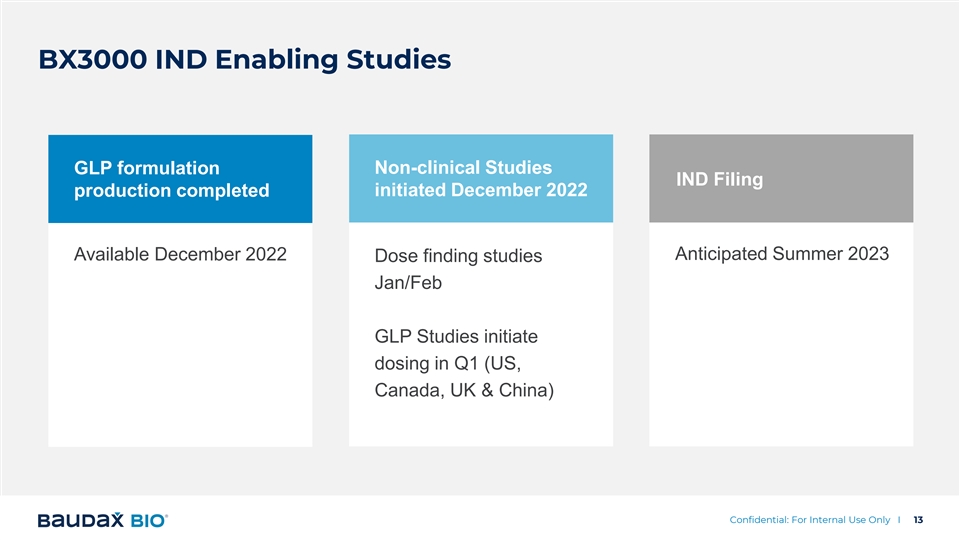
BX3000 IND Enabling Studies GLP formulation Non-clinical Studies IND Filing initiated December 2022 production completed Anticipated Summer 2023 Available December 2022 Dose finding studies Jan/Feb GLP Studies initiate dosing in Q1 (US, Canada, UK & China) Confidential: For Internal Use Only I 13
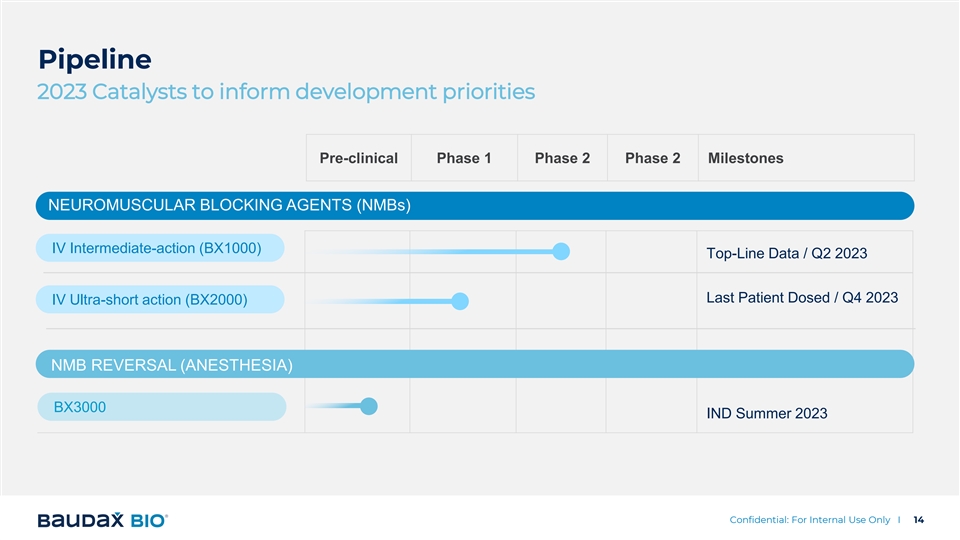
Pipeline 2023 Catalysts to inform development priorities Pre-clinical Phase 1 Phase 2 Phase 2 Milestones NEUROMUSCULAR BLOCKING AGENTS (NMBs) IV Intermediate-action (BX1000) Top-Line Data / Q2 2023 Last Patient Dosed / Q4 2023 IV Ultra-short action (BX2000) NMB REVERSAL (ANESTHESIA) BX3000 IND Summer 2023 Confidential: For Internal Use Only I 14

Commercial Update
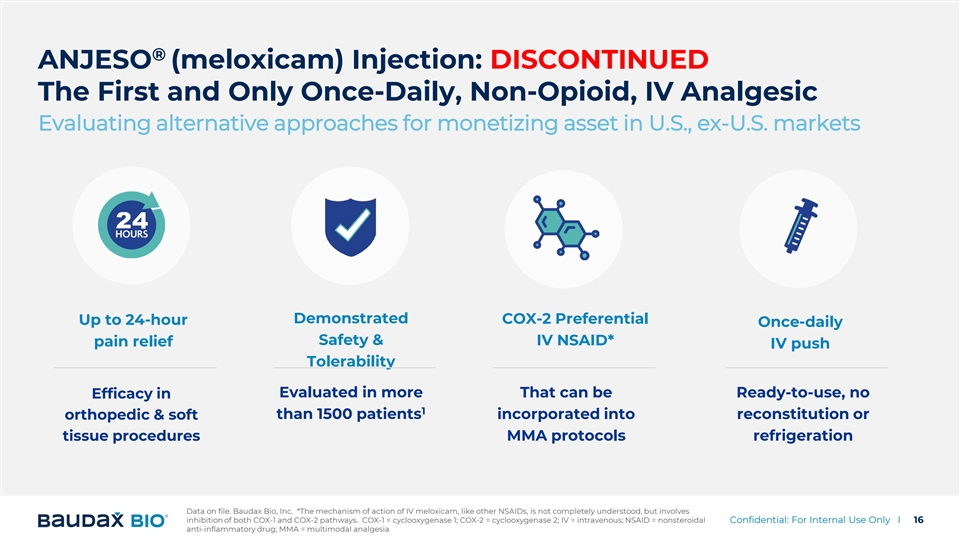
® ANJESO (meloxicam) Injection: DISCONTINUED The First and Only Once-Daily, Non-Opioid, IV Analgesic Evaluating alternative approaches for monetizing asset in U.S., ex-U.S. markets Demonstrated COX-2 Preferential Up to 24-hour Once-daily Safety & IV NSAID* pain relief IV push Tolerability Evaluated in more That can be Ready-to-use, no Efficacy in 1 than 1500 patients orthopedic & soft incorporated into reconstitution or tissue procedures MMA protocols refrigeration Data on file. Baudax Bio, Inc. *The mechanism of action of IV meloxicam, like other NSAIDs, is not completely understood, but involves inhibition of both COX-1 and COX-2 pathways. COX-1 = cyclooxygenase 1; COX-2 = cyclooxygenase 2; IV = intravenous; NSAID = nonsteroidal Confidential: For Internal Use Only I 16 anti-inflammatory drug; MMA = multimodal analgesia
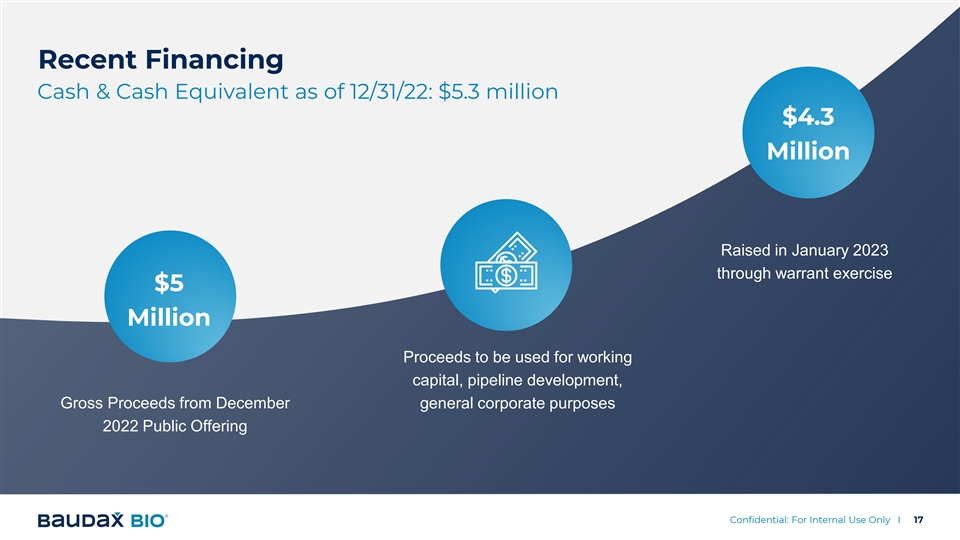
Recent Financing Cash & Cash Equivalent as of 12/31/22: $5.3 million $4.3 Million Raised in January 2023 through warrant exercise $5 Million Proceeds to be used for working capital, pipeline development, Gross Proceeds from December general corporate purposes 2022 Public Offering Confidential: For Internal Use Only I 17

THANK YOU! Confidential: For Internal Use Only I