Exhibit 99.1

Intranasal Live Attenuated Pneumococcus Vaccine to Protect Against Pneumonia & AOM and Potential Platform for Combination Vaccines NASDAQ: BWV October 13, 2022 Ali Fattom, Brian Price, Ron Cobb, and Jason Rosch* Bluewater Vaccines Inc. * St. Jude Children’s Research Hospital, Memphis, TN

The Presentation (the “Presentation”) has been prepared by Blue Water Vaccines, Inc. (the "Company"). Certain information con tai ned herein has been derived from sources prepared by third parties. While such information is believed to be reliable for the pur pos es used herein, the Company makes no representation or warranty with respect to the accuracy of such information. This Presentation does not constitute an offer to sell, or the solicitation of an offer to buy, any securities of the Company in any jurisdiction, domestic of foreign, where the offer, solicitation or sale is not permitted or would be unlawful prior to regis tra tion or qualification under the securities laws of any such state or jurisdiction. FORWARD LOOKING STATEMENTS : Certain statements in this presentation (the ”Presentation”) has been prepared by Blue Water Vaccines, Inc . (the “Company”) . This presentation contains forward - looking within the meaning of the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate,” “expect,” and “intend,” among others . These forward - looking statements are based on BWV’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to the development of BWV’s vaccine candidates, including, but not limited to BWV - 301 ; the failure to obtain FDA clearances or approvals and noncompliance with FDA regulations ; delays and uncertainties caused by the global COVID - 19 pandemic ; risks related to the timing and progress of clinical development of our product candidates ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and substantial competition . As with any vaccine under development, there are significant risks in the development, regulatory approval and commercialization of new products . BWV does not undertake an obligation to update or revise any forward - looking statement . Investors should read the risk factors set forth in BWV’s Annual Report on Form 10 - K for the fiscal year ended December 31 , 2021 , filed with the Securities and Exchange Commission (the “SEC”) on March 31 , 2022 , Quarterly Report on Form 10 - Q for the quarter ended June 30 , 2022 , filed with the SEC on August 15 , 2022 and periodic reports filed with the SEC on or after the date thereof . All of BWV’s forward - looking statements are expressly qualified by all such risk factors and other cautionary statements . The information set forth herein speaks only as of the date thereof . 2
3 Pneumococcus Vaccines: Success and Limitations × Protection is serotype specific × Efficacy was almost exclusive to invasive diseases; Bacteremia, sepsis, and meningitis × Emergence of non - vaccine type in the community × Cost and availability in LMIC × Poor protection against major infections: Pneumonia, AOM, and nasopharyngeal colonization Limitations x Introduction of the highly efficacious polysaccharide - conjugate vaccines (e.g., Prevnar series, Synflorix , etc.) reduced pneumococcus infections dramatically Success Problem: Pneumococcus causes serious diseases (e.g., acute otitis media, sinusitis, pneumonia, bacteremia, sepsis, meningitis, etc that cause high morbidity and mortality in children and elderly Moffitt &Malley, 2016 Daniels, C. C., Rogers, P. D., & Shelton, C. M. (2016). A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Rec omm endations and Future Protein Antigens. The journal of pediatric pharmacology and therapeutics : JPPT : the official journal of PPAG, 21(1), 27 – 35. https://do i.org/10.5863/1551 - 6776 - 21.1.27
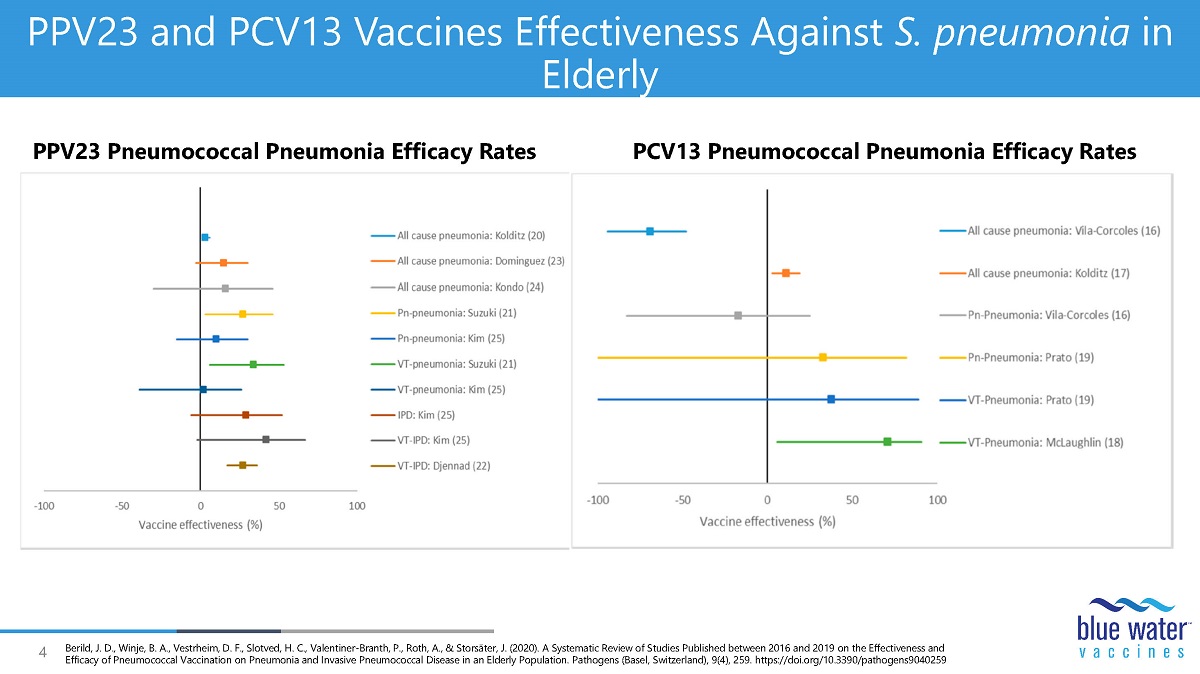
4 PPV23 and PCV13 Vaccines Effectiveness Against S. pneumonia in Elderly PPV23 Pneumococcal Pneumonia Efficacy Rates PCV13 Pneumococcal Pneumonia Efficacy Rates Berild , J. D., Winje , B. A., Vestrheim , D. F., Slotved , H. C., Valentiner - Branth , P., Roth, A., & Storsäter , J. (2020). A Systematic Review of Studies Published between 2016 and 2019 on the Effectiveness and Efficacy of Pneumococcal Vaccination on Pneumonia and Invasive Pneumococcal Disease in an Elderly Population. Pathogens (Base l, Switzerland), 9(4), 259. https://doi.org/10.3390/pathogens9040259

5 Characteristics of an Ideal, Safe, & Effective Vaccine x Highly cross - reactive and serotype independent (Conserved surface proteins ) x Highly immunogenic and elicits: x Mucosal Immunity: IgA, Th17, Homed B and T - cells x Systemic Immunity: Opsonic IgG, balanced Th1/Th2 x Efficacious against nasopharyngeal colonization, AOM, and pneumonia x Low cost (e.g., to ensure utilization in LMICs) x Easily delivered x Longevity of immune response x Localized long - term memory Berild , J. D., Winje , B. A., Vestrheim , D. F., Slotved , H. C., Valentiner - Branth , P., Roth, A., & Storsäter , J. (2020). A Systematic Review of Studies Published between 2016 and 2019 on the Effectiveness and Efficacy of Pneumococcal Vaccination on Pneumonia and Invasive Pneumococcal Disease in an Elderly Population. Pathogens (Base l, Switzerland), 9(4), 259. https://doi.org/10.3390/pathogens9040259
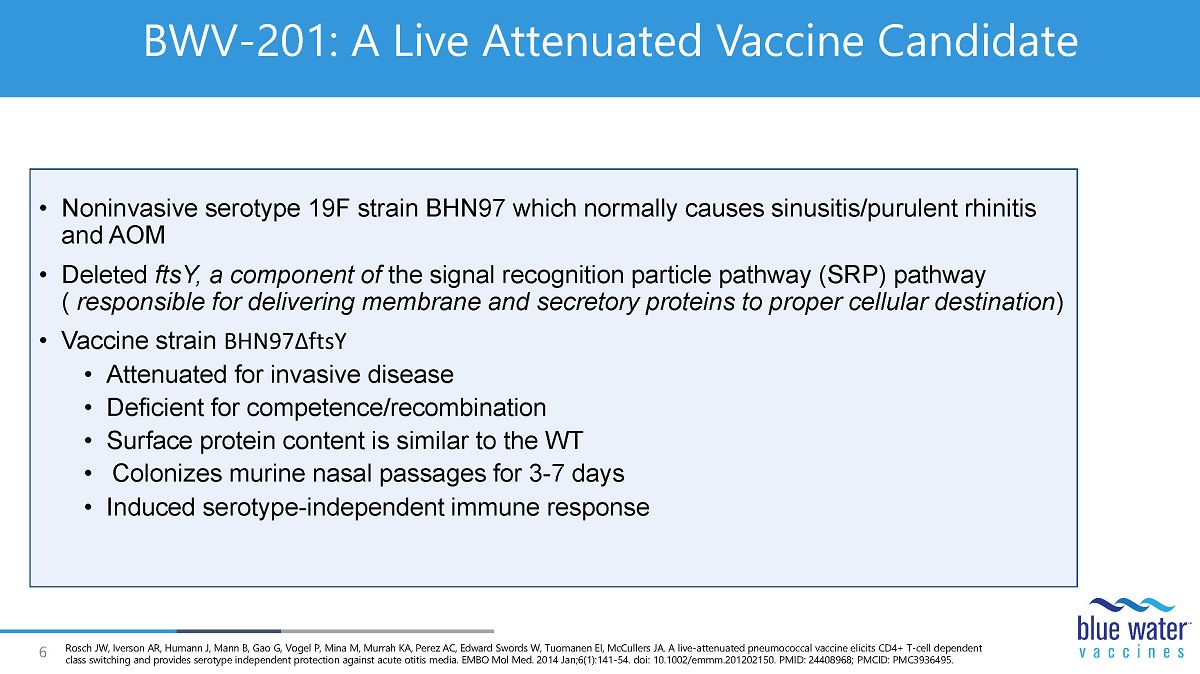
6 BWV - 201: A Live Attenuated Vaccine Candidate • N oninvasive serotype 19F strain BHN97 which normally causes sinusitis/purulent rhinitis and AOM • Deleted ftsY , a component of the signal recognition particle pathway (SRP) pathway ( responsible for delivering membrane and secretory proteins to proper cellular destination ) • Vaccine strain BHN97 ∆ ftsY • Attenuated for invasive disease • Deficient for competence/recombination • Surface protein content is similar to the WT • Colonizes murine nasal passages for 3 - 7 days • Induced serotype - independent immune response Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Edward Swords W, Tuomanen EI, McCullers JA. A live - attenuated pneumococcal vaccine elicits CD4+ T - cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med. 2014 Jan;6(1):141 - 54. doi : 10.1002/emmm.201202150. PMID: 24408968; PMCID: PMC3936495.
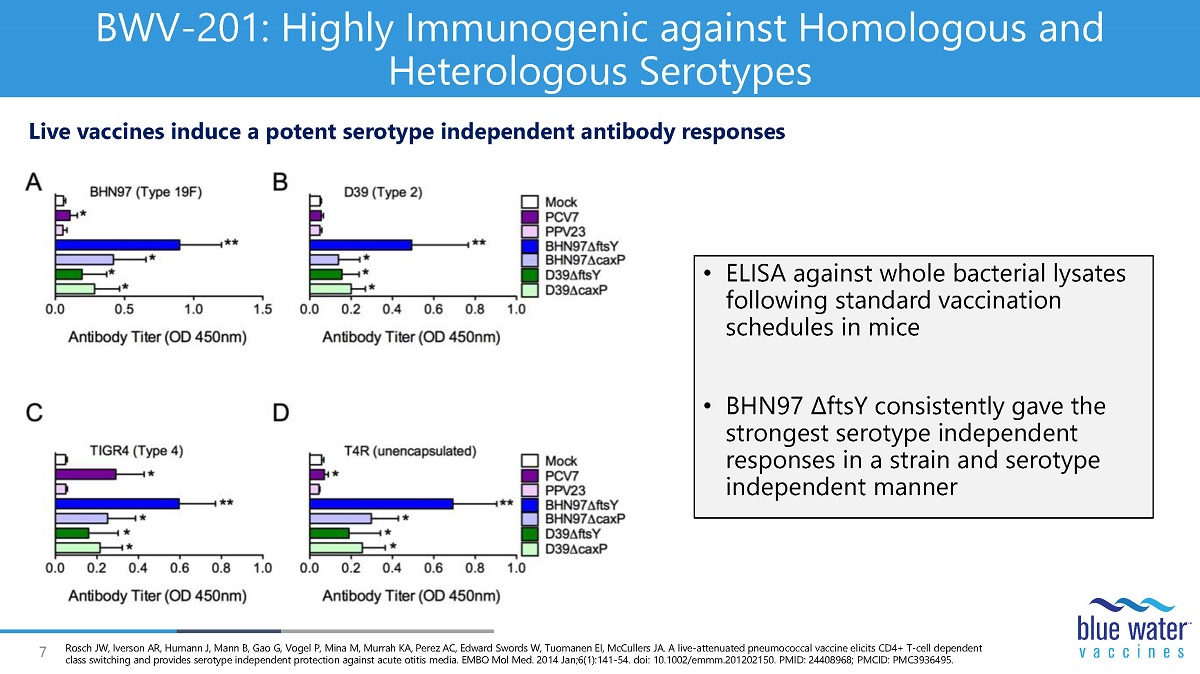
7 BWV - 201: Highly Immunogenic against Homologous and Heterologous Serotypes • ELISA against whole bacterial lysates following standard vaccination schedules in mice • BHN97 ∆ ftsY consistently gave the strongest serotype independent responses in a strain and serotype independent manner Live vaccines induce a potent serotype independent antibody responses Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Edward Swords W, Tuomanen EI, McCullers JA. A live - attenuated pneumococcal vaccine elicits CD4+ T - cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med. 2014 Jan;6(1):141 - 54. doi : 10.1002/emmm.201202150. PMID: 24408968; PMCID: PMC3936495.

8 BWV - 201: Induction of a Balanced Th1/Th2 and Mucosal Immunity Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Edward Swords W, Tuomanen EI, McCullers JA. A live - attenuated pneumococcal vaccine elicits CD4+ T - cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med. 2014 Jan;6(1):141 - 54. doi : 10.1002/emmm.201202150. PMID: 24408968; PMCID: PMC3936495.
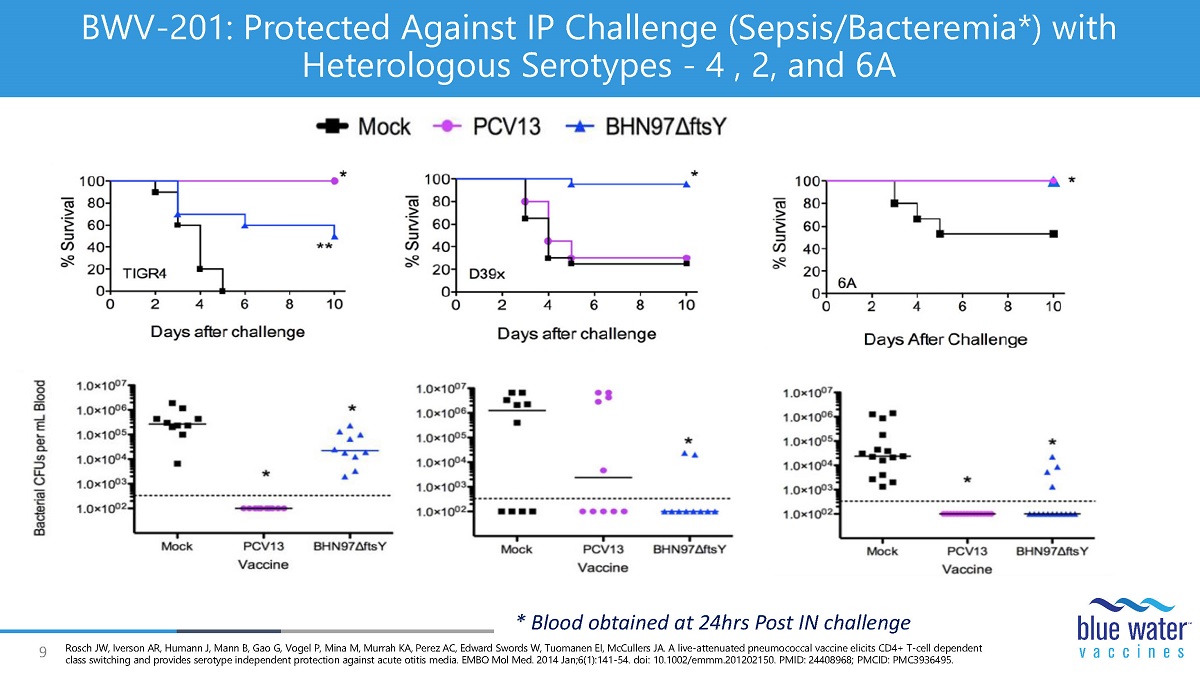
9 BWV - 201: Protected Against IP Challenge (Sepsis/Bacteremia*) with Heterologous Serotypes - 4 , 2, and 6A * Blood obtained at 24hrs Post IN challenge Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Edward Swords W, Tuomanen EI, McCullers JA. A live - attenuated pneumococcal vaccine elicits CD4+ T - cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med. 2014 Jan;6(1):141 - 54. doi : 10.1002/emmm.201202150. PMID: 24408968; PMCID: PMC3936495.
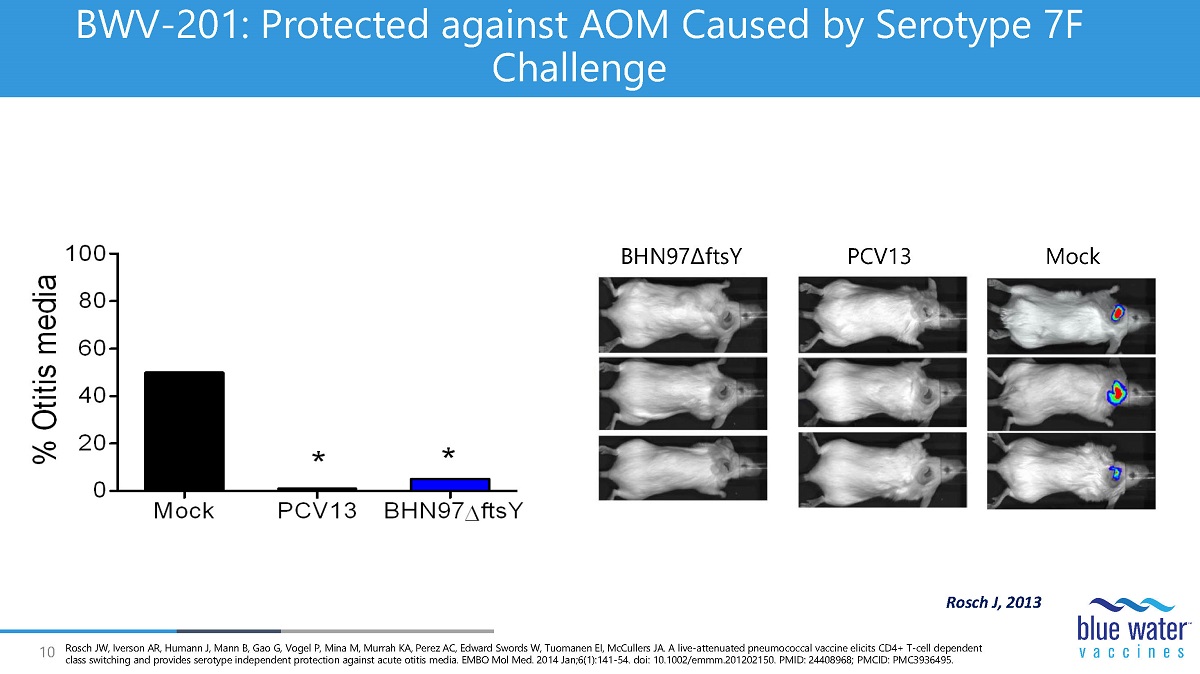
10 BWV - 201: Protected against AOM Caused by Serotype 7F Challenge BHN97 Δ ftsY PCV13 Mock Rosch J, 2013 Rosch JW, Iverson AR, Humann J, Mann B, Gao G, Vogel P, Mina M, Murrah KA, Perez AC, Edward Swords W, Tuomanen EI, McCullers JA. A live - attenuated pneumococcal vaccine elicits CD4+ T - cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol Med. 2014 Jan;6(1):141 - 54. doi : 10.1002/emmm.201202150. PMID: 24408968; PMCID: PMC3936495.
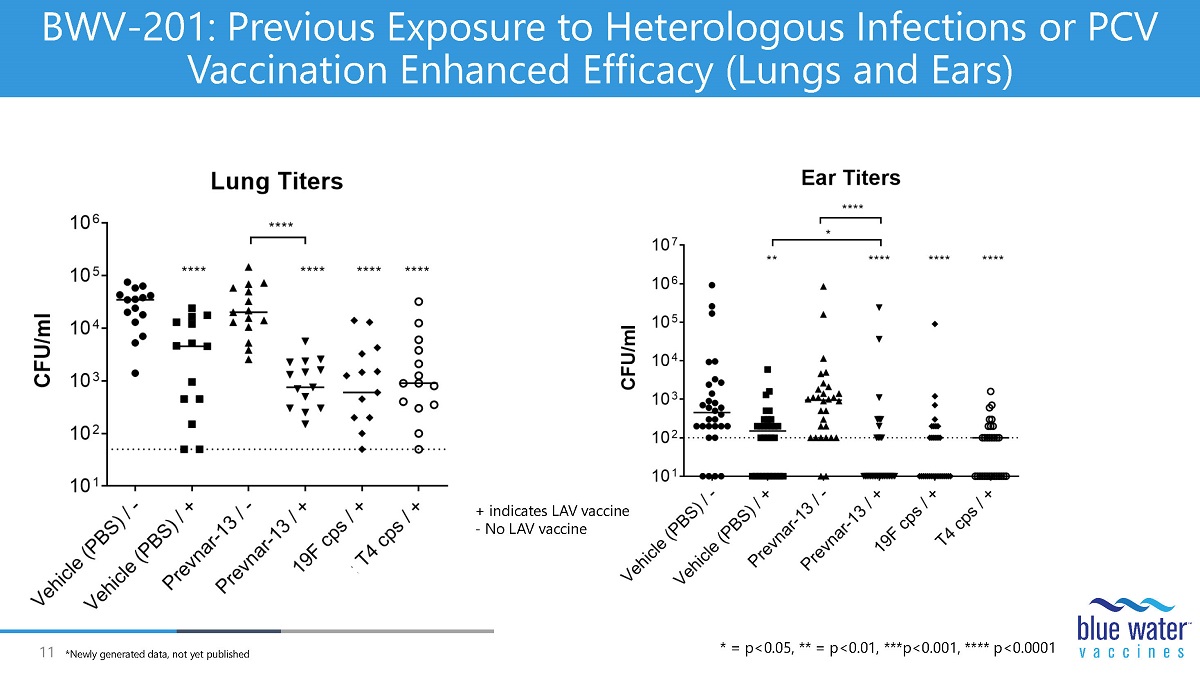
11 BWV - 201: Previous Exposure to Heterologous Infections or PCV Vaccination Enhanced Efficacy (Lungs and Ears) + indicates LAV vaccine - No LAV vaccine * = p<0.05, ** = p<0.01, ***p<0.001, **** p<0.0001 *Newly generated data, not yet published

12 BHN97 ∆ ftsY : A Potential Platform for Combination Vaccines Multiple Challenges: 1) Codon optimization and regulation strategies vary dramatically between species 2) Different strategies and mechanisms for protein sorting and localization between different bacterial species, particularly Gram - positive and Gram - negatives *Newly generated data, not yet published
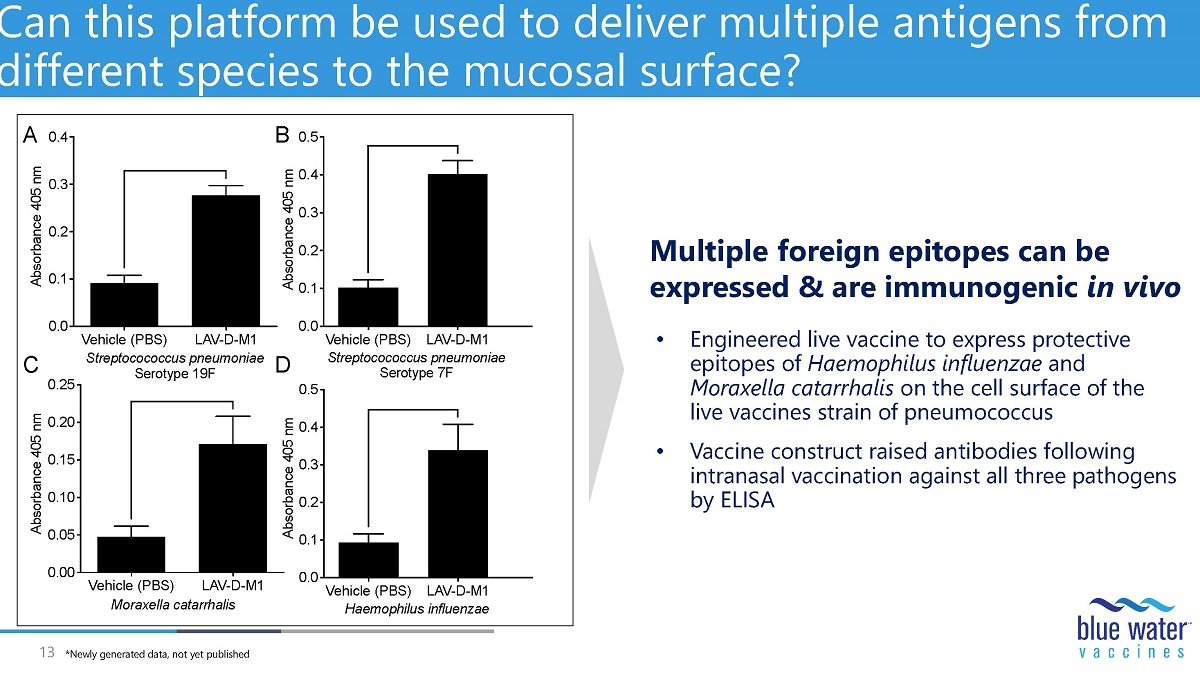
13 Can this platform be used to deliver multiple antigens from different species to the mucosal surface? • Engineered live vaccine to express protective epitopes of Haemophilus influenzae and Moraxella catarrhalis on the cell surface of the live vaccines strain of pneumococcus • Vaccine construct raised antibodies following intranasal vaccination against all three pathogens by ELISA Vehicle (PBS) LAV-D-M1 0.0 0.1 0.2 0.3 0.4 0.5 A b s o r b a n c e 4 0 5 n m Streptocococcus pneumoniae Serotype 7F Vehicle (PBS) LAV-D-M1 0.00 0.05 0.10 0.15 0.20 0.25 A b s o r b a n c e 4 0 5 n m Moraxella catarrhalis Vehicle (PBS) LAV-D-M1 0.0 0.1 0.2 0.3 0.4 A b s o r b a n c e 4 0 5 n m Streptocococcus pneumoniae Serotype 19F Vehicle (PBS) LAV-D-M1 0.0 0.1 0.2 0.3 0.4 0.5 A b s o r b a n c e 4 0 5 n m Haemophilus influenzae A B C D Multiple foreign epitopes can be expressed & are immunogenic in vivo *Newly generated data, not yet published
14 x Live attenuated pneumococcal vaccines elicited robust protection against both invasive (sepsis/bacteremia) and not invasive infections (AOM/pneumonia) media x Protection across heterologous serotypes x Existing immunity (vaccination or colonization) is synergistic and enhanced protection x BWV - 201 may serve as a platform to include other proteins from multiple bacterial species x Potential for combination vaccine with disease - specific indication AOM or Pneumonia caused by different pathogens Conclusions

Thank you! Follow us on: https://www.facebook.com/BlueWaterVaccines https://www.linkedin.com/company/blue - water - vaccines - inc/ https://twitter.com/vaccinesInc














