Registration File No. 333-239395


| | | Page | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| • | the audited consolidated financial statements of Amryt as of and for the years ended December 31, 2019 and December 31, 2018, prepared in accordance with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”); |
| • | the audited financial statements of Aegerion as of and for the years ended December 31, 2018 and December 31, 2017 prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”); and |
| • | the unaudited interim financial statements of Aegerion as of June 30, 2019, prepared in accordance with U.S. GAAP. |
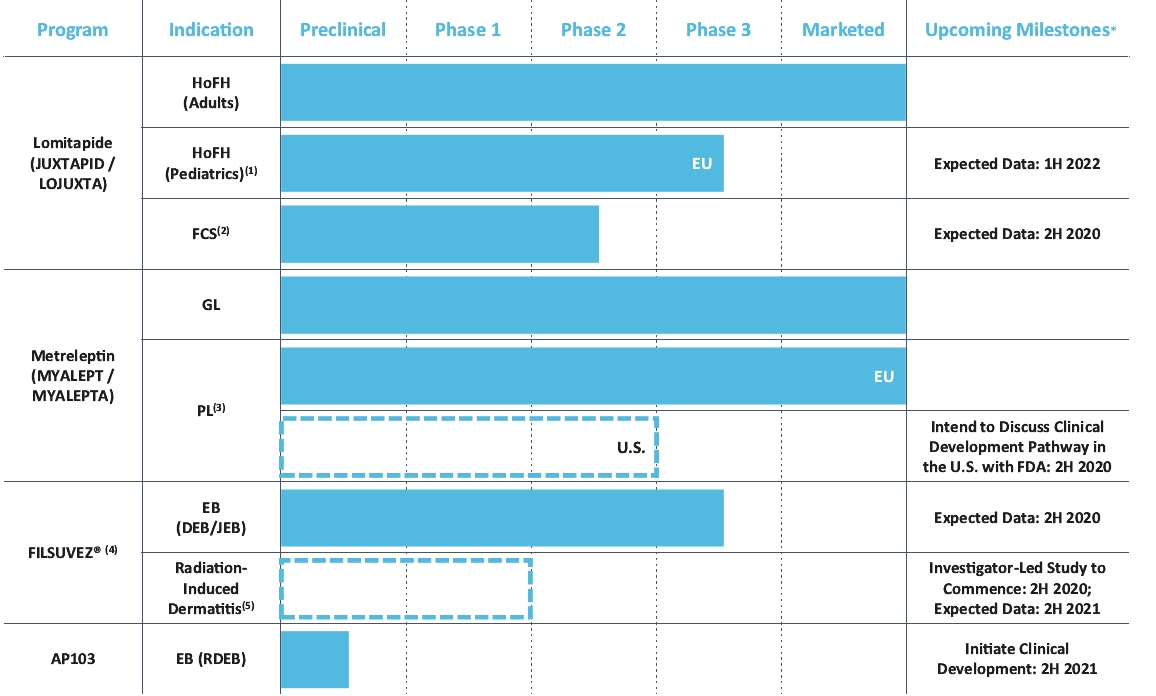
| * | Upcoming clinical milestones are subject to the impact of COVID-19 on our business. |
| (1) | We are conducting a Phase 3 study of HoFH in children and adolescents in Europe, Middle East and Africa (“EMEA”) as part of our European Medicines Agency (“EMA”) post-approval commitments. |
| (2) | The familial chylomicronemia syndrome Phase 2 trial is an open-label investigator-led study. |
| (3) | The dotted line segment indicates we have not yet commenced any clinical trials in the United States for metreleptin for the treatment of PL. |
| (4) | AP101 was approved in 2016 by the EMA for the treatment of partial thickness wounds in adults, but has not been commercially launched. |
| (5) | The dotted line segment indicates we have not yet commenced any clinical trials for radiation-induced dermatitis. This planned radiation-induced dermatitis Phase 2 trial is an investigator-led study. |
| • | Revenue-generating commercial products. We currently generate revenue, including royalties, from global sales of lomitapide and metreleptin. This revenue stream provides us with financial flexibility to fund the continued development and potential commercialization of our existing development candidates as well as the potential acquisition or in-license of additional rare disease products and late-stage product candidates. We have retained worldwide development and commercial rights to all of our programs, excluding Japan for lomitapide, where we receive royalties, and Japan, South Korea and Taiwan for metreleptin. |
| • | Late-stage clinical program in severe EB. We are conducting a global pivotal Phase 3 trial of AP101 for the treatment of cutaneous manifestations of severe EB and we expect to report data in the second half of 2020. This Phase 3 trial is the largest EB study conducted to date. Based on our conversations with the FDA and the EMA, we believe that positive results from this trial would allow us to apply for marketing approval for AP101 in both the United States and Europe. |
| • | Existing, scalable global commercial and medical infrastructure. We sell lomitapide and metreleptin in the Americas, Europe and the Middle East through our existing rare disease commercial infrastructure. Our commercial expertise includes market access, marketing, sales managers and sales representatives and is supported by our experienced medical affairs team with medical science liaisons, patient advocates and dieticians in the field. We also leverage our network of third-party distributors in other key markets throughout the world. We believe we will be able to leverage our existing global infrastructure and expertise to efficiently and expeditiously commercialize additional products we may acquire or develop, including our lead product candidate, AP101, if approved. |
| • | Proven track record of building a diversified rare disease product portfolio. We acquired AP101 through the acquisition of Birken AG in 2016, in-licensed LOJUXTA in December 2016, in-licensed our gene therapy platform, including AP103, in March 2018 and acquired metreleptin and the remaining rights to lomitapide through the Acquisition in September 2019. |
| • | Strong patent protection and regulatory exclusivity. We believe our intellectual property portfolio as well as protection afforded by regulatory exclusivity provide us with a substantial competitive advantage in marketing our current products and also protect our development programs. Our lomitapide patent portfolio includes patents that provide protection from 2025 to 2027 in the United States and into 2025 in the European Union, with supplementary protection granted to extend patent protection in major EU countries into 2028. The metreleptin patent portfolio includes patents that provide protection from 2022 to 2027 in the United States and into 2022 in the European Union and orphan exclusivity in the European Union into 2028. The AP101 patent portfolio includes patents that provide protection in both the United States and the European Union into 2025 and 2030 and a further international patent application directed to the clinical formulation and methods of manufacturing and treatment with AP101 which, if granted, would provide worldwide protection into 2039. We have also submitted additional patent applications to further strengthen our intellectual property portfolio. |
| • | Experienced management team comprised of industry leaders in rare diseases. Our management team has extensive expertise in the acquisition, development and commercialization of rare disease assets. We believe that the breadth of experience and successful track record of our management team and our Board, combined with our broad network of established relationships with leaders in the industry and medical community, provide us with strong drug development and commercialization capabilities. |
| • | Drive revenue growth for our existing commercial products. We intend to continue to focus on growing the sales of lomitapide and metreleptin in the markets and indications we currently sell them. We also intend to expand the market opportunity by seeking approval for the use of lomitapide to treat pediatric HoFH and for the treatment of FCS and for the use of metreleptin to treat PL in the United States. |
| • | Complete development and commercialize our lead product candidate, AP101, for the treatment of severe EB. AP101 is currently in a pivotal Phase 3 trial for the treatment of cutaneous manifestations of severe EB and we expect to report data in the second half of 2020. If the trial is successful, we intend to apply for approval of AP101 and commercialize it in the United States and the European Union. If approved by the FDA, we are eligible to apply for a PRV that we can use, sell or transfer. |
| • | Leverage our global commercial and medical infrastructure. We intend to leverage our existing global infrastructure and expertise to commercialize our development-stage pipeline, including our lead product candidate, AP101, if approved, and any rare disease assets we may acquire or in-license in the future. |
| • | Continue developing our gene therapy product candidate, AP103, for the treatment of RDEB. AP103 is currently in preclinical development for the treatment of RDEB. We intend to initiate clinical development in the second half of 2021. |
| • | Continue evaluating opportunities to expand our rare disease product portfolio and pipeline. We believe we are well positioned to continue to opportunistically acquire or in-license rare disease assets that we believe we can efficiently sell through our existing commercial infrastructure. |
| • | As a result of the Acquisition, our future results are likely to differ materially from our historical performance. |
| • | We may not be successful in our efforts to build a pipeline of product candidates and develop additional marketable products. |
| • | We have significant payment obligations under the terms of our long-term debt, $206.6 million of which was outstanding as of December 31, 2019. |
| • | The terms of our debt and any requirements to incur further indebtedness or refinance our indebtedness in the future, including restrictive covenants in certain of the agreements and instruments governing our indebtedness, could have a material adverse effect on our business and results of operations. |
| • | We may be subject to ongoing financial liabilities and other obligations that we assumed upon Aegerion’s emergence from bankruptcy. |
| • | Adverse events involving any of our products and product candidates may lead the FDA, the EMA or other regulatory authorities to delay or deny clearance for our products or result in product recalls that could harm our reputation, business and financial results. |
| • | The Acquisition exposes us, and any future acquisitions we make may expose us, to risks that could adversely affect our business, and we may not achieve the anticipated benefits of acquisitions of businesses or technologies. |
| • | Our products may not gain market acceptance, in which case we may not be able to generate product revenues. |
| • | If we are unable to complete clinical development of AP101, or experience significant delays in doing so, our business could be materially harmed. |
| • | We rely on third parties for distribution services around the world, and a failure to manage these third parties could harm our business. |
| • | It may be challenging or costly for us to obtain, maintain, enforce and defend our intellectual property rights. |
| • | The outbreak of the novel strain of coronavirus disease, COVID-19, could adversely impact our business, including our preclinical studies and clinical trials. |
| • | the option to present only two years of audited financial statements and only two years of related management’s discussion and analysis of financial condition and results of operations in this prospectus; |
| • | not being required to have our registered independent public accounting firm attest to management’s assessment of our internal control over financial reporting; |
| • | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); |
| • | not being required to submit certain executive compensation matters to shareholder advisory votes, such as “say-on-pay,” “say-on-frequency” and “say-on-golden parachutes;” and |
| • | not being required to disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation. |
| • | the rules under the Exchange Act requiring domestic filers to issue financial statements prepared under U.S. GAAP; |
| • | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| • | the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| • | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specific information, or current reports on Form 8-K, upon the occurrence of specified significant events. |
| • | up to 48,343,750 ordinary shares that may be issued upon conversion of the $125 million aggregate principal amount of our 5% senior unsecured convertible notes (“Convertible Notes”); |
| • | ordinary shares that may be issued in full satisfaction of the contingent value rights (“CVRs”) issued to holders of ordinary shares and employee option holders prior to the Acquisition, assuming all relevant milestones are achieved; |
| • | up to 17,154,554 ordinary shares issuable upon the exercise of share options with a weighted average exercise price of £1.1721 per share; |
| • | warrants to purchase 345,542 ordinary shares at a strike price of £1.44 per share; |
| • | 4,864,656 ordinary shares held as treasury shares; and |
| • | options to purchase an aggregate of 1,320,000 ordinary shares that we intend to grant to our non-executive directors immediately after the effectiveness of the registration statement of which this prospectus forms a part. |
| • | no conversion of the Convertible Notes; and |
| • | no exercise of the warrants, CVRs or options. |
| • | Summary consolidated statements of comprehensive loss of Amryt for the years ended December 31, 2018 and 2019 and a summary consolidated statement of financial position of Amryt as of December 31, 2019, which have been derived from our audited consolidated financial statements included elsewhere in this prospectus. |
| • | Summary condensed consolidated statements of comprehensive loss of Amryt for the three-month periods ended March 31, 2020 and 2019 and a summary condensed consolidated statement of financial position of Amryt as of March 31, 2019, which have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus. |
| • | Summary consolidated statements of comprehensive loss of Aegerion for the years ended December 31, 2017 and 2018, which have been derived from Aegerion’s audited consolidated financial statements included elsewhere in this prospectus. Aegerion’s audited consolidated financial statements have been prepared in accordance with U.S. GAAP. |
| • | Summary consolidated statements of comprehensive loss of Aegerion for the six months ended June 30, 2019 and a summary consolidated statement of financial position of Aegerion as of June 30, 2019, which have been derived from Aegerion’s unaudited interim consolidated financial statements included elsewhere in this prospectus. Aegerion’s unaudited interim consolidated financial statements have been prepared in accordance with U.S. GAAP. The accounting principles applied in Aegerion’s unaudited interim financial statements are consistent with those used in Aegerion’s annual audited financial statements. In the opinion of management, the unaudited financial data reflects all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the financial information in those statements. |
| | | Three Months Ended March 31, | | | Year Ended December 31, | |||||||
| | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | (unaudited) | | | | | ||||||
| | | (In thousands, except per share data) | ||||||||||
Statement of comprehensive loss data: | | | | | | | | | ||||
Revenue | | | $44,574 | | | 4,542 | | | $58,124 | | | $17,095 |
Cost of sales | | | (32,620) | | | (1,830) | | | (42,001) | | | (6,266) |
Gross Profit | | | 11,954 | | | 2,712 | | | 16,123 | | | 10,829 |
Total administrative, selling and marketing expenses | | | (19,151) | | | (3,987) | | | (36,339) | | | (18,163) |
Research and development expenses | | | (8,934) | | | (1,505) | | | (15,827) | | | (10,703) |
Impairment charge | | | — | | | — | | | (4,670) | | | — |
Restructuring and acquisition costs | | | (853) | | | — | | | (13,038) | | | — |
Operating loss before finance expense | | | (16,984) | | | (2,780) | | | (53,751) | | | (18,037) |
Non-cash change in fair value of contingent consideration | | | (2,906) | | | (1,938) | | | (6,740) | | | (10,566) |
Non-cash contingent value rights finance expense | | | (1,448) | | | — | | | (1,511) | | | — |
Net finance expense — other | | | (9,416) | | | (661) | | | (4,759) | | | (1,841) |
Loss on ordinary activities before taxation | | | (30,754) | | | (5,379) | | | (66,761) | | | (30,444) |
Tax credit/(charge) on loss on ordinary activities | | | 1,857 | | | (6) | | | 1,226 | | | (43) |
Loss for the period/year attributable to the equity holders of the Company | | | (28,897) | | | (5,385) | | | (65,535) | | | (30,487) |
Exchange translation differences which may be reclassified through profit or loss | | | (13) | | | 80 | | | 781 | | | (77) |
Total other comprehensive (loss) / income | | | (13) | | | 80 | | | 781 | | | (77) |
Total comprehensive loss for the period/year attributable to the equity holders of the Company | | | $(28,910) | | | (5,305) | | | $(64,754) | | | $(30,564) |
Loss per share - basic and diluted | | | $(0.19) | | | (0.12) | | | $(0.86) | | | $(0.67) |
Selected Other Data (unaudited): | | | | | | | | | ||||
Adjusted EBITDA(1) | | | $5,358 | | | (2,598) | | | $(12,180) | | | $(16,849) |
| (1) | In addition to analyzing our operating results on an IFRS basis, management also reviews our results on an “Adjusted EBITDA” basis. Adjusted EBITDA is defined as net loss before income taxes, non-cash change in fair value of contingent consideration, non-cash contingent value rights finance expense, net finance expense – other, amortization expense, depreciation expense, share-based payments, impairment charges, and restructuring and acquisition costs related to the acquisition of Aegerion. Adjusted EBITDA is not a measure of performance in accordance with IFRS and should not be considered as an alternative to net income/loss or operating cash flows determined in accordance with IFRS. We believe that the inclusion of Adjusted EBITDA in this prospectus is appropriate to provide additional information to investors because securities analysts, investors and other interested parties use this non-IFRS measure to assess our operating performance across periods on a consistent basis and to evaluate the relative risk of an investment in our securities. However, Adjusted EBITDA has limitations as an analytical tool and should not be considered in isolation or as a substitute for analysis of our results as reported under IFRS. Some of these limitations are: |
| • | although depreciation and amortization are non-cash charges, the assets being depreciated or amortized will often have to be replaced in the future, and Adjusted EBITDA does not reflect any cash requirements for such replacements; |
| • | Adjusted EBITDA does not reflect our cash expenditures, or future requirements for capital expenditures or contractual commitments; |
| • | Adjusted EBITDA does not reflect changes in, or cash requirements for, our working capital needs; and |
| • | Adjusted EBITDA does not reflect the significant interest expense, or the cash requirements necessary to service interest or principal payments, on our significant amount of indebtedness. |
| | | Three Months Ended March 31, | | | Year Ended December 31, | |||||||
| | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | (unaudited) | | | ||||||||
| | | (In thousands) | ||||||||||
Loss for the year attributable to the equity holders of the Company | | | $(28,897) | | | $(5,385) | | | $(65,535) | | | $(30,487) |
Income taxes | | | (1,857) | | | 6 | | | (1,226) | | | 43 |
Non-cash change in fair value of contingent consideration | | | 2,906 | | | 1,938 | | | 6,740 | | | 10,566 |
Non-cash contingent value rights finance expense | | | 1,448 | | | 3 | | | 1,511 | | | — |
Net finance expense - other | | | 9,416 | | | 661 | | | 4,759 | | | 1,841 |
Amortization of inventory fair value step-up | | | 9,503 | | | — | | | 10,367 | | | — |
Amortization expense - other | | | 11,160 | | | 13 | | | 11,957 | | | 50 |
Depreciation expense | | | 81 | | | 78 | | | 698 | | | 317 |
Share-based payments(a) | | | 745 | | | 91 | | | 841 | | | 821 |
Impairment charge | | | — | | | — | | | 4,670 | | | — |
Restructuring and acquisition costs | | | 853 | | | — | | | 13,038 | | | — |
Adjusted EBITDA | | | $5,358 | | | $(2,598) | | | $(12,180) | | | $(16,849) |
| (a) | This is a non-cash item that represents share-based compensation expense. |
| | | As of March 31, 2020 | | | As of December 31, 2019 | |
| | | (unaudited) | | | ||
| | | (In thousands) | ||||
Statement of financial position data: | | | | | ||
Cash and cash equivalents | | | $68,067 | | | $67,229 |
Trade and other receivables | | | 41,179 | | | 36,387 |
Inventories | | | 33,904 | | | 43,623 |
Working capital(1) | | | 31,443 | | | 47,025 |
Total assets | | | 518,229 | | | 534,347 |
Secured Credit Facility | | | 82,989 | | | 81,610 |
Convertible Notes, net of equity component | | | 97,872 | | | 96,856 |
Total liabilities | | | 417,072 | | | 405,025 |
Accumulated deficit | | | 162,569 | | | 133,674 |
Total equity | | | 101,157 | | | 129,322 |
| (1) | We define working capital as current assets less current liabilities. |
| | | Six Months Ended June 30, 2019 | | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | ||||
| | | (In thousands) | |||||||
Statement of comprehensive loss data: | | | | | | | |||
Net revenues | | | $95,857 | | | $130,432 | | | $138,438 |
Cost of product sales | | | 35,364 | | | 59,697 | | | 77,220 |
Operating expenses: | | | | | | | |||
Selling, general and administrative | | | 43,424 | | | 64,437 | | | 77,793 |
Research and development | | | 13,946 | | | 38,064 | | | 44,895 |
Restructuring charges | | | — | | | 2,171 | | | 121 |
Related party expense (income), net | | | 397 | | | 942 | | | (177) |
Total operating expenses | | | 57,767 | | | 105,614 | | | 122,632 |
Income (loss) from operations | | | 2,726 | | | (34,879) | | | (61,414) |
Reorganization items, net | | | (2,145) | | | — | | | — |
Interest expense, net | | | (29,681) | | | (50,746) | | | (39,467) |
Interest expense due to Novelion | | | (1,182) | | | (2,987) | | | (1,089) |
Loss on extinguishment of debt | | | — | | | (4,333) | | | –– |
Other expense, net | | | (224) | | | (1,888) | | | (836) |
Loss before provision for income taxes | | | (30,506) | | | (94,833) | | | (102,806) |
Provision for income taxes | | | (369) | | | (1,705) | | | (594) |
Net loss | | | $(30,875) | | | $(96,538) | | | $(103,400) |
| | | As of June 30, 2019 | |
| | | (In thousands) | |
Statement of financial position data: | | | |
Cash and cash equivalents | | | $36,080 |
Trade and other receivables | | | 26,408 |
Inventories | | | 51,792 |
Total assets | | | 322,634 |
Total current liabilities | | | 67,434 |
Provision for legal settlements - non-current | | | 11,962 |
Other non-current liabilities | | | 1,444 |
Total liabilities not subject to compromise | | | 80,840 |
Liabilities subject to compromise | | | 420,651 |
Total liabilities | | | 501,491 |
Total equity | | | (178,857) |
| (1) | Debtor in possession as of June 30, 2019 but not as of December 31, 2017 and 2018. |
| • | the ability to continue to maintain and grow market acceptance for lomitapide and metreleptin among healthcare professionals and patients in the United States, European Union and other key markets for the treatment of approved indications; |
| • | continuing market demand and medical need for these products; |
| • | maintaining regulatory approvals without onerous restrictions or limitations in key markets and securing regulatory approvals in additional markets on a timely basis and with commercially feasible labels, and pricing and reimbursement approvals at adequate levels, where required, on a timely basis; |
| • | side effects or other safety issues associated with the use of lomitapide and metreleptin could require us or our collaborators to modify or halt commercialization of these products or expose us to product liability lawsuits which will harm our business; |
| • | we may be required by regulatory agencies to conduct additional studies regarding the safety and efficacy of lomitapide and metreleptin, which we have not planned or anticipated; |
| • | generating revenues in markets that allow for sales of pharmaceutical products without regulatory approval based solely on the approvals of such products in the United States or European Union, and in which no promotion or commercialization activities are permitted; and |
| • | adequately investing in the manufacturing, sales, marketing, market access, medical affairs and other functions that are supportive of our commercialization efforts. |
| • | make it more difficult for us to pay or refinance debts as they become due; |
| • | require us to use a larger portion of cash flow for debt service, reducing funds available for other purposes; |
| • | limit our ability to pursue business opportunities, such as potential acquisitions, and to react to changes in market or industry conditions; |
| • | reduce the funds available for other purposes, such as implementing our strategy, funding capital expenditures and making distributions to shareholders; |
| • | increase our vulnerability to adverse economic, industry or competitive developments; |
| • | affect our ability to obtain additional financing, particularly as substantially all of our assets (including our intellectual property) are subject to liens securing indebtedness under our Secured Credit Facility; |
| • | decrease our profitability, if we become profitable, or cash flow, or require us to dispose of significant assets in order to satisfy debts and other obligations if we are not able to satisfy these obligations using cash from operations or other sources; and |
| • | disadvantage us compared to competitors. |
| • | decreased demand or coverage for our products; |
| • | impairment of our business reputation and exposure to adverse publicity; |
| • | warnings on product labels; |
| • | withdrawal of clinical trial participants; |
| • | substantial monetary awards to trial participants or patients; |
| • | significant time and costs to defend the related litigation; |
| • | distraction of management’s attention from our primary business; |
| • | substantial monetary awards to patients or other claimants; |
| • | loss of revenues; and |
| • | the inability to successfully commercialize our products. |
| • | unsuccessful and/or untimely completion of preclinical and clinical development of our product candidates and any other future candidates, as well as the associated costs, including any unforeseen costs we may incur as a result of preclinical study or clinical trial delays; |
| • | delays or difficulties in initiating, enrolling, conducting or completing our planned and ongoing clinical trials; |
| • | risk that participants enrolled in our clinical trials will acquire COVID-19 while the clinical trial is ongoing, which could impact the results of the clinical trial, including by increasing the number of observed adverse events; |
| • | existing patients with serious diseases included in our clinical trials may die as a result of contracting COVID-19 or suffer other adverse medical events for reasons that may not be related to our products or candidates; |
| • | delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff; |
| • | healthcare budgets may be adversely affected and as a result, funding may not be available to pay for our products; |
| • | diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials; |
| • | interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal, state or local governments, employers and others or interruption of clinical trial subject visits and study procedures (such as pre-planned clinical trial assessments), which may impact the integrity of subject data and clinical study endpoints; |
| • | limitations in employee resources that would otherwise be focused on the conduct of our preclinical studies and clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; |
| • | interruption or delays in the operations of the FDA or other regulatory authorities, which may impact review and approval timelines; |
| • | delays in receiving approval from local regulatory authorities to initiate our planned clinical trials; |
| • | delays in clinical sites receiving the supplies and materials needed to conduct our clinical trials due to staffing shortages, production slowdowns or stoppages and disruptions in delivery systems; |
| • | suspension or termination of a clinical trial by us, by the Institutional Review Boards (“IRBs”) of the institutions in which such trial is being conducted, by a DSMB for such trial or by the FDA, the EMA or comparable foreign regulatory authorities due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, the EMA or comparable foreign regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects; |
| • | refusal of the FDA to accept data from clinical trials in affected geographies outside the United States; |
| • | changes in local regulations as part of a response to the COVID-19 pandemic which may require us to change the ways in which our clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether; |
| • | delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; |
| • | impairment of our operations, including among others, employee mobility and productivity, availability of facilities, conduct of clinical trials, manufacturing and supply capacity, disruption of our supply chain, availability of shipping and distribution channels, restrictions on import and export regulations and the availability and productivity of third party service suppliers; |
| • | incurrence of delays in the delivery of our products or our inability to deliver products to our patients; |
| • | interruption in global shipping that may affect the transport of clinical trial materials, such as investigational drug product used in our clinical trials; and |
| • | disruption and volatility in the global capital markets, which increases the cost of capital and adversely impacts access to capital should we have specific strategic considerations which require it. |
| • | limited support and user knowledge for legacy systems of acquired companies; |
| • | problems maintaining uniform procedures, controls and policies with respect to our financial accounting systems; |
| • | difficulties in managing geographically dispersed operations, including risks associated with entering foreign markets in which we have no or limited prior experience; |
| • | underperformance of any acquired technology, product or business relative to our expectations and the price we paid; |
| • | negative near-term impacts on financial results after an acquisition, including acquisition-related earnings charges; |
| • | the potential loss of key employees, customers and strategic partners of acquired companies; |
| • | claims by terminated employees and shareholders of acquired companies or other third parties related to the transaction; |
| • | the assumption or incurrence of additional debt obligations or expenses, or use of substantial portions of our cash; |
| • | the issuance of equity securities to finance or as consideration for any acquisitions that dilute the ownership of our shareholders; |
| • | any collaboration, strategic alliance and licensing arrangement may require us to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us; |
| • | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; |
| • | diversion of management’s attention and company resources from existing operations of the business; |
| • | inconsistencies in standards, controls, procedures and policies; |
| • | the impairment of intangible assets as a result of technological advancements, or worse-than-expected performance of acquired companies; |
| • | assumption of, or exposure to, historical liabilities of the acquired business, including unknown contingent or similar liabilities that are difficult to identify or accurately quantify; |
| • | our inability to generate revenue from acquired technology or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs; and |
| • | risks associated with acquiring intellectual property, including potential disputes regarding acquired companies’ intellectual property. |
| • | whether clinicians and potential patients perceive product candidates to have better efficacy, safety, tolerability profile and ease of use, when compared with the products marketed by our competitors and the prevailing standard of care (“SOC”); |
| • | the timing and location of market introduction of any approved products; |
| • | our ability to provide acceptable evidence of safety and efficacy; |
| • | the frequency and severity and causal relationships of any side effects and a continued acceptable safety profile following approval; |
| • | relative convenience and ease of administration; |
| • | cost effectiveness; |
| • | patient diagnostics and screening infrastructure in each market; |
| • | marketing and distribution support; |
| • | the availability of healthcare coverage, reimbursement and adequate payment from health maintenance organizations and other third-party payers, both public and private; and |
| • | competition from other therapies. |
| • | we may experience a negative impact on market acceptance and increased dropout rates; |
| • | regulatory authorities may suspend, withdraw or alter their approval of the relevant product; |
| • | regulatory authorities may require the addition of labeling statements, such as warnings or contraindications or distribution and use restrictions such as, for example, the modifications to the JUXTAPID label to include language instructing patients to cease therapy upon the occurrence of severe diarrhea; |
| • | regulatory authorities may issue, or require us to issue additional specific communications such as safety alerts, field alerts, or “Dear Doctor” letters to healthcare professionals; |
| • | regulatory authorities may require us to recall, withdraw, or stop selling a product or take other enforcement action; |
| • | we may receive negative publicity; |
| • | we may be required to change the way the relevant product is administered, conduct additional preclinical studies or clinical trials or restrict the distribution or use of the relevant product; |
| • | patients could suffer harm, and we could be sued and held liable for harm caused to patients; |
| • | the regulatory authorities may require us to amend the relevant REMS program, Risk Management Plan or comparable equivalent; and |
| • | our reputation may suffer. |
| • | increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care; and |
| • | requiring drug manufacturers to provide a 50% discount on Medicare Part D brand name prescription drugs sold to Medicare beneficiaries whose prescription drug costs cause the beneficiaries to be subject to the Medicare Part D coverage cap (i.e., the so-called donut hole). |
| • | the EMA, the FDA or any other comparable regulatory agency may disagree with the design or implementation of clinical trials or interpretation of data from non-clinical trials or clinical trials; |
| • | the population studied in the clinical program may not be sufficiently broad or representative to ensure that the clinical data can be relied on safely in the full population for which we are seeking approval; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to support a finding that has statistically significant clinical meaningfulness or support the submission of a new drug application or other submission, or to obtain regulatory approval in relevant jurisdictions, such as the European Union and the United States; |
| • | we may be unable to demonstrate to the EMA, the FDA or any other comparable regulatory agency that a product candidate’s risk-benefit ratio for its proposed indication is acceptable; |
| • | the EMA, the FDA or any other comparable regulatory agency may fail to approve the manufacturing processes, test procedures and specifications or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| • | the approval policies or regulations of the EMA, the FDA or any other comparable regulatory agency may significantly change in a manner rendering clinical data insufficient for approval. |
| • | product, composition of matter, formulation and method of use patents in the European Union, the United States and other key global markets for existing and future products; |
| • | Orphan Drug exclusivity granted to our products because they aim to treat rare diseases and conditions, which entitle us to exclusivity protections for a period of up to seven years after approval in the United States (although metreleptin should also qualify for a 12-year period of exclusivity from biosimilar or interchangeable products) and up to ten years in the European Union and Japan as well as certain financial incentives. The ten-year Orphan Drug exclusivity period in the European Union can be extended a further two years upon successful completion of a PIP. Conversely, the ten-year exclusivity period may be reduced to six years, if at the end of the fifth year, it is established that a product no longer fulfills the criteria for Orphan Drug Designation; |
| • | medicinal products granted a marketing authorization in the European Union entitles us to eight years’ data exclusivity after approval, and up to ten years’ market exclusivity protection which can be extended for a further year if a new indication is granted; and |
| • | available extensions to the terms of our Orphan Drug exclusivity, product and methods of use patents in the European Union and United States. |
| • | put one or more of our patents at risk of being invalidated, rendered unenforceable or interpreted narrowly; |
| • | adversely impact the patentability of our inventions relating to our products; |
| • | result in monetary damages, injunctive relief or otherwise harm our competitive position, including by limiting marketing and selling activities, increasing the risk for generic competition, limiting development and commercialization activities or requiring us to obtain licenses to use the relevant technology (which licenses may not be available on commercially reasonable terms, if at all); and |
| • | otherwise negatively impact the enforceability, validity or scope of protection offered by the patents relating to the products. |
| • | incur substantial monetary damages; |
| • | encounter significant delays in expanding the market of our products; and |
| • | be precluded from manufacturing or selling any products; |
| • | we will be able to successfully develop or commercialize our product before some or all of the relevant patents or regulatory exclusivity expire, or in countries where we do not have patent protection or exclusivity; |
| • | we or our licensors were the first to make the inventions covered by each of the pending patent applications and patents; |
| • | we or our licensors were the first to file patent applications for these inventions; |
| • | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | any of our pending patent applications or those that we have licensed will result in issued patents; |
| • | any of our patents or those we have licensed will be valid or enforceable; |
| • | we will be able to license the patents or pending patent applications necessary or desirable to enforce or protect our patent rights on commercially reasonable terms or at all; |
| • | any patents issued to us or our licensors or collaborators will provide a basis for any additional commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| • | we will be able to develop additional proprietary technologies that are patentable; |
| • | Orphan Drug exclusivity marketing rights for our products in the United States will be maintained, if, for example, the FDA determines in the future that the request for Orphan Drug Designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. In addition, the FDA, and the EMA for the European Union, may subsequently approve products with the same active moiety for the same condition if the FDA or the EMA concludes that the later drug is safer, more effective, or makes a major contribution to patient care. Orphan Drug Designation neither shortens the development time nor regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process; or |
| • | the patents of others will not have an adverse effect on our business. |
| • | actual or anticipated variations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | announcements of technological partnerships, innovations or new products by us or our competitors; |
| • | the success of competitive products or technologies; |
| • | changes in management and members of our Board; |
| • | changes in financial estimates or recommendations by securities analysts; |
| • | changes in the trading volume of our ADSs on the Nasdaq and of our ordinary shares on AIM and EGE; |
| • | sales of our ADSs or ordinary shares by executive officers or future holders of our equity securities; |
| • | announcements or expectations of additional debt or equity financing efforts; |
| • | unanticipated losses or gains due to unexpected events, including events related to the success of our clinical trials or regulatory approvals; |
| • | significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving us or our competitors; |
| • | changes in our accounting policies or practices; |
| • | disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • | failure to integrate successfully the Aegerion business with ours or to realize anticipated benefits from the integration; |
| • | changes in government regulations, including any changes that may affect pricing or reimbursement; and |
| • | conditions in the financial markets or changes in general economic conditions. |
| • | only being required to present two years of audited financials and related discussion in Management’s Discussion & Analysis; |
| • | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| • | not being required to comply with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
| • | reduced disclosure obligations regarding executive compensation; and |
| • | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
| • | the last day of the fiscal year during which we have total annual gross revenues of $1.07 billion (as such amount is indexed for inflation every five years by the SEC) or more; |
| • | the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; |
| • | the date on which we are deemed to be a “large accelerated filer,” as defined in Rule 12b-2 of the Exchange Act, which would occur if the market value of our ordinary shares and ADSs that are held by non-affiliates exceeds $700 million as of the last day of our most recently completed second fiscal quarter; and |
| • | the last day of the fiscal year following the fifth anniversary of the completion of our first sale of common equity securities pursuant to this registration statement under the Securities Act, or December 31, 2025. |
| • | We do not intend to follow Nasdaq Rule 5620(c) regarding quorum requirements applicable to meetings of shareholders. Such quorum requirements are not required under English law. In accordance with generally accepted business practice, our Articles of Association provide alternative quorum requirements that are generally applicable to meetings of shareholders. |
| • | We do not intend to follow Nasdaq Rule 5605(b)(2), which requires that independent directors regularly meet in an executive session, where only independent directors are present. The independent directors may choose to meet in an executive session at their discretion. |
| • | at least 75% of its gross income is “passive income,” or |
| • | at least 50% of the value, determined on the basis of a quarterly average, of its gross assets is attributable to assets that produce or are held for the production of “passive income.” |
| • | As an ADS holder, we will not treat you as one of our shareholders and you will not be able to exercise shareholder rights, except through the depositary as permitted by the deposit agreement. |
| • | Distributions on the ordinary shares represented by your ADSs will be paid to the depositary, and before the depositary makes a distribution to you on behalf of your ADSs, any withholding taxes that must be paid will be deducted. Additionally, if the exchange rate fluctuates during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution. |
| • | We and the depositary may amend or terminate the deposit agreement without the ADS holders’ consent in a manner that could prejudice ADS holders. |
| • | our significant operating losses since our inception and ability to obtain and maintain profitability in the future; |
| • | our commercial products, including statements regarding the expected strategies and profitability thereof; |
| • | our product candidates, including statements regarding the expected initiation, timing, progress and availability of data from clinical trials, all of which may be adversely impacted by the rapidly evolving COVID-19 global pandemic; |
| • | our ability to successfully commercialize, or enter into strategic relationships with third parties to commercialize, our products or product candidates, if approved; |
| • | our ability to acquire or in-license new product candidates; |
| • | our competition, most of whom have far greater resources than we have, which may make it more difficult for us to achieve significant market penetration; |
| • | the size of our addressable markets and market trends; |
| • | potential strategic relationships; |
| • | our ability to obtain and maintain intellectual property rights; |
| • | the impact of potential fluctuations in foreign currency exchange rates; |
| • | estimates regarding expenses, future revenues, capital requirements and the need for additional financing; and |
| • | risks associated with the COVID-19 pandemic, which may adversely impact our business, preclinical studies and clinical trials. |
| | | As of March 31, 2020 | |
| | | (in thousands) | |
Cash and cash equivalents | | | $68,067 |
Long-term debt | | | |
Secured Credit Facility | | | $82,989 |
Convertible Notes, principal amount | | | 125,000 |
Total long-term debt | | | 207,989 |
Equity: | | | |
Share capital | | | 11,918 |
Share premium | | | 2,422 |
Other reserves | | | 249,386 |
Accumulated deficit | | | (162,569) |
Total equity | | | 101,157 |
Total capitalization | | | $309,146 |
| • | Selected consolidated statements of comprehensive loss of Amryt for the years ended December 31, 2018 and 2019 and a selected consolidated statement of financial position of Amryt as of December 31, 2019, which have been derived from our audited consolidated financial statements included elsewhere in this prospectus. |
| • | Selected condensed consolidated statements of comprehensive loss of Amryt for the three-month periods ended March 31, 2020 and 2019 and a selected condensed consolidated statement of financial position of Amryt as of March 31, 2019, which have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus. |
| • | Selected consolidated statements of comprehensive loss of Aegerion for the years ended December 31, 2017 and 2018, which have been derived from Aegerion’s audited consolidated financial statements included elsewhere in this prospectus. Aegerion’s audited consolidated financial statements have been prepared in accordance with U.S. GAAP. |
| • | Selected consolidated statements of comprehensive loss of Aegerion for the six months ended June 30, 2019 and a selected consolidated statement of financial position of Aegerion as of June 30, 2019, which have been derived from Aegerion’s unaudited interim consolidated financial statements included elsewhere in this prospectus. Aegerion’s unaudited interim consolidated financial statements have been prepared in accordance with U.S. GAAP. The accounting principles applied in Aegerion’s unaudited interim financial statements are consistent with those used in Aegerion’s annual audited financial statements. In the opinion of management, the unaudited data reflects all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the financial information in those statements. |
| | | Three Months Ended March 31 | | | Year Ended December 31, | |||||||
| | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | (unaudited) | | | | | ||||||
| | | (In thousands, except per share data) | ||||||||||
Statement of comprehensive income/(loss) data: | | | | | | | | | ||||
Revenue | | | $44,574 | | | 4,542 | | | $58,124 | | | $17,095 |
Cost of sales | | | (32,620) | | | (1,830) | | | (42,001) | | | (6,266) |
Gross profit | | | 11,954 | | | 2,712 | | | 16,123 | | | 10,829 |
Total administrative, selling and marketing expenses | | | (19,151) | | | (3,987) | | | (36,339) | | | (18,163) |
Research and development expenses | | | (8,934) | | | (1,505) | | | (15,827) | | | (10,703) |
Impairment charge | | | — | | | — | | | (4,670) | | | — |
Restructuring and acquisition costs | | | (853) | | | — | | | (13,038) | | | — |
Operating loss before finance expense | | | (16,984) | | | (2,780) | | | $(53,751) | | | $(18,037) |
Non-cash change in fair value of contingent consideration | | | (2,906) | | | (1,938) | | | (6,740) | | | (10,566) |
Non-cash contingent value rights finance expense | | | (1,448) | | | — | | | (1,511) | | | — |
Net finance expense — other | | | (9,416) | | | (661) | | | (4,759) | | | (1,841) |
Loss on ordinary activities before taxation | | | (30,754) | | | (5,379) | | | $(66,761) | | | $(30,444) |
Tax credit/(charge) on loss on ordinary activities | | | 1,857 | | | (6) | | | 1,226 | | | (43) |
Loss for the period/year attributable to the equity holders | | | (28,897) | | | (5,385) | | | (65,535) | | | (30,487) |
Total other comprehensive (loss)/income | | | (13) | | | 80 | | | 781 | | | (77) |
Total comprehensive loss for the period/year attributable to the equity holders | | | $(28,910) | | | (5,305) | | | $(64,754) | | | $(30,564) |
Loss per share — basic and diluted | | | $(0.19) | | | (0.12) | | | $(0.86) | | | $(0.67) |
| | | As of March 31, 2020 | | | As of December 31, 2019 | |
| | | (unaudited) | | | ||
| | | (In thousands) | ||||
Statement of financial position data: | | | | | ||
Cash and cash equivalents | | | $68,067 | | | $67,229 |
Trade and other receivables | | | 41,179 | | | 36,387 |
Inventories | | | 33,904 | | | 43,623 |
Working capital(1) | | | 31,443 | | | 47,025 |
Total assets | | | 518,229 | | | 534,347 |
Secured Credit Facility | | | 82,989 | | | 81,610 |
Convertible Notes, net of equity component | | | 97,872 | | | 96,856 |
Total liabilities | | | 417,072 | | | 405,025 |
Accumulated deficit | | | 162,569 | | | 133,674 |
Total equity | | | 101,157 | | | 129,322 |
| (1) | We define working capital as current assets less current liabilities. |
| | | Six Months Ended June 30, 2019 | | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | ||||
| | | (In thousands) | |||||||
Statement of comprehensive loss data: | | | | | | | |||
Net revenues | | | $95,857 | | | $130,432 | | | $138,438 |
Cost of product sales | | | 35,364 | | | 59,697 | | | 77,220 |
Operating expenses: | | | | | | | |||
Selling, general and administrative | | | 43,424 | | | 64,437 | | | 77,793 |
Research and development | | | 13,946 | | | 38,064 | | | 44,895 |
Restructuring charges | | | — | | | 2,171 | | | 121 |
Related party expense (income), net | | | 397 | | | 942 | | | (177) |
Total operating expenses | | | 57,767 | | | 105,614 | | | 122,632 |
Loss from operations | | | 2,726 | | | (34,879) | | | (61,414) |
Reorganization items, net | | | (2,145) | | | — | | | — |
Interest expense, net | | | (29,681) | | | (50,746) | | | (39,467) |
Interest expense due to Novelion | | | (1,182) | | | (2,987) | | | (1,089) |
Loss on extinguishment of debt | | | — | | | (4,333) | | | — |
Other expense, net | | | (224) | | | (1,888) | | | (836) |
Loss before provision for income taxes | | | (30,506) | | | (94,833) | | | (102,806) |
Provision for income taxes | | | (369) | | | (1,705) | | | (594) |
Net loss | | | $(30,875) | | | $(96,538) | | | $(103,400) |
| | | As of June 30, 2019 | |
| | | (In thousands) | |
Statement of financial position data: | | | |
Cash and cash equivalents | | | $36,080 |
Trade and other receivables | | | 26,408 |
Inventories | | | 51,792 |
Total assets | | | 322,634 |
Total current liabilities | | | 67,434 |
Provision for legal settlements - non-current | | | 11,962 |
Other non-current liabilities | | | 1,444 |
Total liabilities not subject to compromise | | | 80,840 |
Liabilities subject to compromise | | | 420,651 |
Total liabilities | | | 501,491 |
Total equity | | | (178,857) |
| (1) | Debtor in possession as of June 30, 2019 but not as of December 31, 2017 and 2018. |
| • | Prior to consummation of the Acquisition, we conducted a private placement of ordinary shares to certain accredited investors, which generated gross proceeds of approximately $8 million. Proceeds from this issuance were used primarily to pay for transaction costs incurred in connection with the Acquisition. |
| • | Prior to consummation of the Acquisition, we also issued the CVRs to holders of ordinary shares and to employee option holders entitling them to proceeds of up to $85 million upon the occurrence of specified milestones related to the regulatory approval and commercialization of AP101. |
| • | On September 24, 2019, we completed a $60 million fundraising by way of the issue of (i) new ordinary shares at a price of $1.79 per ordinary share, and (ii) zero cost warrants to new and existing investors, and certain creditors of Aegerion. Proceeds from this issuance were used to fund development of our product pipeline and potential new indications for our late stage product candidates and for general corporate purposes. |
| • | On September 24, 2019, Aegerion issued $125 million in aggregate principal amount of the Convertible Notes, to certain of its pre-bankruptcy creditors. The Convertible Notes were issued in satisfaction of the creditors’ claims against Aegerion pursuant to Section 1145 of the Bankruptcy Code. |
| • | On September 24, 2019, we entered into a new five-year $81 million first-lien Secured Credit Facility guaranteed by certain of our subsidiaries and bearing an interest rate of (x) 11% per annum paid in cash, or (y) 6.5% per annum paid in cash plus 6.5% per annum paid in kind, in each case, on a quarterly basis. |
| | | Amryt consolidated loss for the year ended December 31, 2019 | | | Aegerion consolidated loss for the period to June 30, 2019 | | | Aegerion consolidated profit for the period from July 1 to September 24, 2019 | | | Adjustments | | | Notes | | | Pro forma consolidated loss for the year ended December 31, 2019 | |
| | | IFRS | | | U.S. GAAP | | | U.S. GAAP | | | | | IFRS | |||||
| | | (In thousands, except per share data) | ||||||||||||||||
Net revenues | | | $58,124 | | | $67,362 | | | $33,742 | | | $(2,463) | | | 3a | | | $156,765 |
Upfront license fee | | | — | | | 28,495 | | | — | | | — | | | | | 28,495 | |
Total Revenues | | | 58,124 | | | 95,857 | | | 33,742 | | | (2,463) | | | | | 185,260 | |
Cost of product sales | | | (19,803) | | | (22,209) | | | (11,127) | | | 2,463 | | | 3a | | | (50,676) |
Amortization of acquired intangibles | | | (11,831) | | | (12,548) | | | (5,856) | | | (9,772) | | | 3b | | | (40,007) |
Amortization of inventory fair value step-up | | | (10,367) | | | (607) | | | (1,359) | | | — | | | | | (12,333) | |
Total cost of sales | | | (42,001) | | | (35,364) | | | (18,342) | | | (7,309) | | | | | (103,016) | |
Gross profit | | | 16,123 | | | 60,493 | | | 15,400 | | | (9,772) | | | | | 82,244 | |
Selling, general and administrative expenses | | | (35,498) | | | (45,607) | | | (29,244) | | | 50,172 | | | 3c | | | (60,177) |
Research and development expenses | | | (15,827) | | | (13,676) | | | (8,628) | | | 5 | | | | | (38,126) | |
Restructuring and acquisition costs | | | (13,038) | | | — | | | — | | | 13,038 | | | 3d | | | — |
Shared based payment expense | | | (841) | | | (703) | | | 20 | | | (704) | | | 3e | | | (2,228) |
Impairment of intangible assets | | | (4,670) | | | — | | | — | | | — | | | | | (4,670) | |
Operating (loss)/profit before finance expense | | | (53,751) | | | 507 | | | (22,452) | | | 52,739 | | | | | (22,957) | |
Net finance (expense)/income | | | (4,759) | | | (31,012) | | | 77,281 | | | (60,382) | | | 3f | | | (18,872) |
Non-cash change in fair value of contingent consideration | | | (6,740) | | | — | | | — | | | — | | | | | (6,740) | |
Non-cash contingent value rights finance expense | | | (1,511) | | | — | | | — | | | (3,860) | | | 3g | | | (5,371) |
(Loss)/profit on ordinary activities before taxation | | | (66,761) | | | (30,505) | | | 54,829 | | | (11,503) | | | | | (53,940) | |
Tax credit/(charge) on ordinary activities | | | 1,226 | | | (369) | | | 26 | | | — | | | | | 883 | |
(Loss)/profit for the year attributable to the equity holder of the company | | | $(65,535) | | | $ (30,874) | | | $54,855 | | | $(11,503) | | | | | $(53,057) | |
Loss per share – basic and diluted, attributable to ordinary equity holders of the parent (US$) | | | $(0.86) | | | — | | | — | | | — | | | | | $(0.34) | |
Weighted average number of ordinary shares in issue | | | 75,871,562 | | | — | | | — | | | — | | | | | 154,498,887 | |
| 1. | Basis of preparation |
| 2. | Accounting policy conformity change |
| 3. | Pro forma adjustments |
| a) | Adjustment to eliminate inter-company transactions between Amryt and Aegerion. Revenues have been reduced to reflect the royalty income of $2.4 million recognized by Aegerion prior to the Acquisition, and cost of product sales has been reduced by the same amount to reflect the cost in Amryt’s historical financial statements. |
| b) | Adjustment to amortization of acquired intangibles of $9.8 million consists of: |
| i) | amortization expense in the historical financial statements of Aegerion converted to IFRS, resulting in a reduction in amortization expense of $0.6 million; |
| ii) | elimination of the historical amortization expense on the Aegerion intangible assets acquired by Amryt, including the IFRS adjustment above, of $18.4 million; and |
| iii) | recognition of a revised amortization expense of $28.2 million, based on the fair value of intangible assets acquired. Amortization is calculated on a straight-line basis. |
| c) | Adjustment to selling, general and administrative expenses of $50.2 million consists of: |
| i) | the elimination of Aegerion non-recurring restructuring, acquisition, severance and bankruptcy expenses, totalling $50.4 million. These costs were incurred by Aegerion prior to the Acquisition and are excluded on the basis that they are not expected to have a continuing impact on our combined results following the Acquisition; and |
| ii) | additional selling, general and administrative expenses of $0.2 million arising from reclassification of $0.7 million of expenses and $0.5 million of income from finance expenses to selling, general and administrative expenses. |
| d) | Adjustment to restructuring and acquisition expenses to eliminate Amryt’s non-recurring restructuring and acquisition expenses. These expenses are excluded on the basis that they are not expected to have a continuing impact on our combined results following the Acquisition. |
| e) | Increase in share based payment expenses of $0.7 million to reflect the increase in Aegerion’s share based payment expenses under IFRS 2. |
| f) | Adjustment made for the following: |
| i) | elimination of finance charges, unamortized debt discounts and debt issuance costs totalling $39.4 million and gains on the extinguishment of debt of $86.2 million, recognized by Aegerion in its historical financial statements; |
| ii) | elimination of finance charges of $2.8 million relating to the EIB loan facility held by Amryt which was fully repaid in connection with the Acquisition; |
| iii) | inclusion of interest expenses of $8.1 million relating to the new Secured Credit Facility and $7.6 million relating to the Convertible Notes, assuming that these liabilities were incurred on January 1, 2019; |
| iv) | reclassification of $0.5 million included in interest income in the historical financial statements of Aegerion to selling, general and administrative expenses; and |
| v) | additional net finance charges of $0.1 million to reflect $0.8 million booked as an IFRS adjustment for amortization of Aegerion’s debt issuance costs, partially offset by a reclassification of $0.7 million to selling, general and administrative expenses relating to interest expense on prepetition claims and interest on liability settlements in Aegerion’s historical financial statements. |
| g) | Adjustment for non-cash financing expenses, assuming that the CVRs were issued on January 1, 2019. |
| 4. | Exclusion from Pro Forma Adjustments |
| • | lomitapide, an approved treatment in the United States and the European Union for adult patients with HoFH; |
| • | metreleptin, an approved treatment in the United States for GL and in the European Union for GL and PL; |
| • | AP101, our lead development asset, which is currently in a pivotal Phase 3 trial as a potential treatment for severe EB (if AP101 is approved for this indication, we intend to market it under the name FILSUVEZ); |
| • | AP103, our first product candidate utilizing our novel polymer-based topical gene therapy delivery platform, which is in preclinical development as a potential treatment for patients with EB and other topical indications; and |
| • | Imlan, a range of derma-cosmetic products marketed solely in Germany as a treatment for sensitive, allergy-prone and dry skin. |
| • | Prior to consummation of the Acquisition, we conducted a private placement of ordinary shares to certain accredited investors, which generated gross proceeds of approximately $8 million. Proceeds from this issuance were used primarily to pay for transaction costs incurred in connection with the Acquisition. |
| • | Prior to consummation of the Acquisition, we also issued the CVRs to holders of ordinary shares and to employee option holders entitling them to proceeds of up to $85 million upon the occurrence of specified milestones related to the regulatory approval and commercialization of AP101. |
| • | On September 24, 2019, we completed a $60 million fundraising by way of the issue of (i) new ordinary shares at a price of $1.79 per ordinary share, and (ii) zero cost warrants to new and existing investors, and certain creditors of Aegerion. Proceeds from this issuance are being used to fund development of our product pipeline and potential new indications for our late stage product candidates and for general corporate purposes. |
| • | On September 24, 2019, we issued $125 million in aggregate principal amount of the Convertible Notes, to certain of Aegerion’s pre-bankruptcy creditors. The Convertible Notes were issued in satisfaction of the creditors’ claims against Aegerion pursuant to Section 1145 of the Bankruptcy Code. |
| • | On September 24, 2019, we entered into a five-year $81 million Secured Credit Facility, guaranteed by certain of our subsidiaries and bearing an interest rate of (x) 11% per annum paid in cash, or (y) 6.5% per annum paid in cash plus 6.5% per annum paid in kind, in each case, on a quarterly basis. |
| | | Three months ended March 31, | ||||
Statement of comprehensive loss data: | | | 2020 | | | 2019 |
| | | (Unaudited) | ||||
| | | (In thousands, except per share data) | ||||
Revenue | | | $44,574 | | | $4,542 |
Cost of sales | | | (32,620) | | | (1,830) |
Gross profit | | | 11,954 | | | 2,712 |
Research and development expenses | | | (8,934) | | | (1,505) |
Selling, general and administrative expenses | | | (18,406) | | | (3,896) |
Acquisition and severance related costs | | | (853) | | | — |
Share based payment expenses | | | (745) | | | (91) |
Operating loss before finance expense | | | (16,984) | | | (2,780) |
Non-cash change in fair value of contingent consideration | | | (2,906) | | | (1,938) |
Non-cash contingent value rights finance expense | | | (1,448) | | | — |
Net finance expense — other | | | (9,416) | | | (661) |
Loss on ordinary activities before taxation | | | (30,754) | | | (5,379) |
Tax credit / (charge) on loss on ordinary activities | | | 1,857 | | | (6) |
Loss for the period attributable to the equity holders of the Company | | | (28,897) | | | (5,385) |
Exchange translation differences which may be reclassified through profit or loss | | | (13) | | | 80 |
Total other comprehensive (loss)/income | | | (13) | | | 80 |
Total comprehensive loss for the period attributable to the equity holders of the Company | | | $(28,910) | | | $(5,305) |
Loss per share – basic and diluted, attributable to ordinary equity holders of the parent | | | $(0.19) | | | $(0.12) |
| | | Three months March 31, | | | Increase / (Decrease) | |||||||
Revenues: | | | 2020 | | | 2019 | | |||||
| | | Unaudited | | | Unaudited | | | | | |||
| | | $000 | | | $000 | | | % | ||||
Metreleptin | | | $26,927 | | | $— | | | $26,927 | | | 100% |
Lomitapide | | | 17,421 | | | 4,419 | | | 13,002 | | | 294.2% |
Other | | | 26 | | | 123 | | | 103 | | | 83.7% |
Total revenues | | | $44,574 | | | $4,542 | | | $40,032 | | | 881.4% |
| • | maintain existing patients on therapy; |
| • | continue to build market acceptance for metreleptin in the United States; |
| • | build market acceptance in the European Union following the approval by the EMA in July 2018, and continue to obtain pricing and reimbursement approvals in key markets in the European Union; |
| • | secure regulatory approval from the FDA for the treatment of PL in the United States; and |
| • | obtain regulatory approvals for metreleptin in new markets for the treatment of GL and PL based on the data package which secured approval in Europe. |
| • | maintain existing patients on therapy; |
| • | continue to build market acceptance for lomitapide in existing markets; |
| • | continue to support patient access programs in all territories; |
| • | obtain pricing and reimbursement approvals in new markets in the European Union; and |
| • | secure regulatory approval for lomitapide in Brazil and other key markets. |
| | | Three months ended March 31, | | | Increase / (Decrease) | |||||||
Cost of Sales: | | | 2020 | | | 2019 | | | | | ||
| | | Unaudited | | | | | ||||||
| | | $000 | | | $000 | | | % | ||||
Cost of product sales | | | $6,247 | | | $1,053 | | | $5,194 | | | 493.3% |
Amortization of acquired intangibles | | | 11,092 | | | — | | | 11,092 | | | 100% |
Amortization of inventory fair value step-up | | | 9,503 | | | — | | | 9,503 | | | 100% |
Royalty expenses | | | 5,778 | | | 777 | | | 5,001 | | | 643.6% |
Total cost of sales | | | $32,620 | | | $1,830 | | | $30,790 | | | 1682.5% |
| | | Year ended December 31, | ||||
Statement of comprehensive loss data: | | | 2019 | | | 2018 |
| | | (In thousands, except per share data) | ||||
Revenue | | | $58,124 | | | $17,095 |
Cost of sales | | | (42,001) | | | (6,266) |
Gross profit | | | 16,123 | | | 10,829 |
Research and development expenses | | | (15,827) | | | (10,703) |
Selling, general and administrative expenses | | | (35,498) | | | (17,342) |
Restructuring and acquisition costs | | | (13,038) | | | — |
Share based payment expenses | | | (841) | | | (821) |
Impairment charge | | | (4,670) | | | — |
Operating loss before finance expense | | | (53,751) | | | (18,037) |
Non-cash change in fair value of contingent consideration | | | (6,740) | | | (10,566) |
Non-cash contingent value rights finance expense | | | (1,511) | | | — |
Net finance expense — other | | | (4,759) | | | (1,841) |
Loss on ordinary activities before taxation | | | (66,761) | | | (30,444) |
Tax credit/(charge) on ordinary activities | | | 1,226 | | | (43) |
Loss for the year attributable to the equity holders of the Company | | | (65,535) | | | (30,487) |
Total other comprehensive profit/(loss) | | | 781 | | | (77) |
Total comprehensive loss for the year attributable to the equity holders of the Company | | | $(64,754) | | | $(30,564) |
Loss per share – basic and diluted, attributable to ordinary equity holders of the parent | | | $(0.86) | | | $(0.67) |
| | | Year ended December 31, | | | Increase / (Decrease) | |||||||
Revenues: | | | 2019 | | | 2018 | | |||||
| | | $000 | | | $000 | | | % | ||||
Metreleptin | | | $25,088 | | | $— | | | $25,088 | | | 100% |
Lomitapide | | | 32,260 | | | 16,110 | | | 16,150 | | | 100.2% |
Other | | | 776 | | | 985 | | | (209) | | | (21.2%) |
Total revenues | | | $58,124 | | | $17,095 | | | $41,029 | | | 240.0% |
| • | maintain existing patients on therapy; |
| • | continue to build market acceptance for metreleptin in the United States; |
| • | build market acceptance in the European Union following the recent approval by the EMA in July 2018, and continue to obtain pricing and reimbursement approvals in key markets in the European Union; |
| • | secure regulatory approval from the FDA for the treatment of PL in the United States; and |
| • | obtain regulatory approvals for metreleptin in new markets for the treatment of GL and PL based on the data package which secured approval in Europe. |
| • | maintain existing patients on therapy; |
| • | continue to build market acceptance for lomitapide in existing markets; |
| • | continue to support patient access programs in all territories; |
| • | obtain pricing and reimbursement approvals in new markets in the European Union; and |
| • | secure regulatory approval for lomitapide in Brazil and other key markets. |
| | | Year ended December 31, | | | Increase / (Decrease) | |||||||
Cost of Sales: | | | 2019 | | | 2018 | | |||||
| | | $000 | | | $000 | | | % | ||||
Cost of product sales | | | $11,384 | | | $3,588 | | | $7,796 | | | 217.3% |
Amortization of acquired intangibles | | | 11,831 | | | — | | | 11,831 | | | 100.0% |
Amortization of inventory fair value step-up | | | 10,367 | | | — | | | 10,367 | | | 100.0% |
Royalty expenses | | | 8,419 | | | 2,678 | | | 5,741 | | | 214.3% |
Total cost of sales | | | $42,001 | | | $6,266 | | | $35,735 | | | 570.3% |
| | | Six Months Ended June 30, | | | $ Increase / (Decrease) | ||||
| | | 2019 | | | 2018 | | |||
| | | (in thousands) | |||||||
Net revenues | | | $95,857 | | | $59,388 | | | $36,469 |
Cost of product sales | | | 35,364 | | | 29,208 | | | 6,156 |
Operating expenses: | | | | | | | |||
Selling, general and administrative | | | 43,424 | | | 38,568 | | | 4,856 |
Research and development | | | 13,946 | | | 21,113 | | | (7,167) |
Related party expense, net | | | 397 | | | 617 | | | (220) |
Total operating expenses | | | 57,767 | | | 60,298 | | | (2,531) |
Income (loss) from operations | | | 2,726 | | | (30,118) | | | (32,844) |
Reorganization items, net | | | (2,145) | | | — | | | 2,145 |
Interest expense, net | | | (29,681) | | | (22,628) | | | 7,053 |
Interest expense due to Novelion | | | (1,182) | | | (1,406) | | | (224) |
Other expense, net | | | (224) | | | (1,054) | | | (830) |
Loss before provision for income taxes | | | (30,506) | | | (55,206) | | | (24,700) |
Provision for income taxes | | | (369) | | | (1,205) | | | (836) |
Net loss | | | $(30,875) | | | $(56,411) | | | $(25,536) |
| | | Six Months Ended June 30, | | | Increase / (Decrease) | ||||
Revenues: | | | 2019 | | | 2018 | | ||
| | | (in thousands) | |||||||
Metreleptin | | | $38,915 | | | $29,751 | | | $9,164 |
Lomitapide | | | 56,942 | | | 29,637 | | | 27,305 |
Total net revenues | | | $95,857 | | | $59,388 | | | $36,469 |
| | | Year ended December 31, | | | $ Increase / (Decrease) | ||||
| | | 2018 | | | 2017 | | |||
| | | (in thousands) | |||||||
Net revenues | | | $130,432 | | | $138,438 | | | $(8,006) |
Cost of sale | | | 59,697 | | | 77,220 | | | (17,523) |
Operating expenses: | | | | | | | |||
Selling, general and administrative expenses | | | 64,437 | | | 77,793 | | | (13,356) |
Research and development expenses | | | 38,064 | | | 44,895 | | | (6,831) |
Restructuring charges | | | 2,171 | | | 121 | | | 2,050 |
Related party expenses (income), net | | | 942 | | | (177) | | | 1,119 |
Total operating expenses | | | 105,614 | | | 122,632 | | | (17,018) |
Loss from operations | | | (34,879) | | | (61,414) | | | (26,535) |
Interest expense, net | | | (50,746) | | | (39,467) | | | 11,279 |
Interest expense due to Novelion | | | (2,987) | | | (1,089) | | | 1,898 |
Loss on extinguishment of debt | | | (4,333) | | | — | | | 4,333 |
Other expense, net | | | (1,888) | | | (836) | | | 1,052 |
Loss before provision for income taxes | | | (94,833) | | | (102,806) | | | (7,973) |
Provision for income taxes | | | (1,705) | | | (594) | | | 1,111 |
Net loss | | | $(96,538) | | | $(103,400) | | | $(6,862) |
| | | Year ended December 31, | | | $ Increase / (Decrease) | ||||
| | | 2018 | | | 2017 | | |||
| | | (in thousands) | |||||||
Metreleptin | | | $71,360 | | | $66,308 | | | $5,052 |
Lomitapide | | | 59,072 | | | 72,130 | | | (13,058) |
Total net revenues | | | $130,432 | | | $138,438 | | | $(8,006) |
| | | Three months ended March 31, | | | Year ended December 31, | |||||||
| | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | Unaudited | | | Unaudited | | | | | |||
| | | (in thousands) | ||||||||||
Net cash flow from / (used in) operating activities | | | $6,187 | | | $(5,441) | | | $(37,497) | | | $(15,454) |
Net cash used in investing activities | | | (13) | | | (4) | | | 24,425 | | | (229) |
Net cash (used in) / flow from financing activities | | | (1,506) | | | 5,675 | | | 65,942 | | | 3,265 |
Exchange and other movements | | | (3,830) | | | (74) | | | 3,133 | | | (767) |
Net change in cash and cash equivalents | | | $838 | | | $156 | | | $56,003 | | | $(13,185) |
| | | Payments due by period | |||||||||||||
(in thousands) | | | Less than 1 year | | | 1 to 3 years | | | 3 to 5 years | | | More than 5 years | | | Total |
Principal debt obligations | | | $11,957 | | | $24,796 | | | $136,927 | | | $128,125 | | | $301,805 |
Operating leases obligations | | | 969 | | | 916 | | | 143 | | | 20 | | | 2,048 |
Contingent consideration and contingent value rights | | | — | | | 99,559 | | | 27,998 | | | — | | | 127,557 |
Other liabilities | | | 15,722 | | | 3,928 | | | — | | | — | | | 19,650 |
Total | | | $28,648 | | | $129,199 | | | $165,068 | | | $128,145 | | | $451,060 |
| • | a requirement to include only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure in the registration statement of which this prospectus forms a part; and |
| • | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act. |
| • | the last day of the first fiscal year in which our annual revenues were at least $1.07 billion; |
| • | the last day of the fiscal year following the fifth anniversary of the effectiveness of the registration statement of which this prospectus forms a part; |
| • | the date on which we have issued more than $1 billion of non-convertible debt securities over a three-year period; and |
| • | the last day of the fiscal year during which we meet the following conditions: (i) the worldwide market value of our common equity securities held by non-affiliates as of our most recently completed second fiscal quarter is at least $700 million, (ii) we have been subject to U.S. public company reporting requirements for at least 12 months and (iii) we have filed at least one annual report as a U.S. public company. |
| • | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| • | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| • | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. |

| * | Upcoming clinical milestones are subject to the impact of COVID-19 on our business. |
| (1) | We are conducting a Phase 3 study of HoFH in children and adolescents in Europe, Middle East and Africa (“EMEA”) as part of our European Medicines Agency (“EMA”) post-approval commitments. |
| (2) | The familial chylomicronemia syndrome Phase 2 trial is an open-label investigator-led study. |
| (3) | The dotted line segment indicates we have not yet commenced any clinical trials in the United States for metreleptin for the treatment of PL. |
| (4) | AP101 was approved in 2016 by the EMA for the treatment of partial thickness wounds in adults, but has not been commercially launched. |
| (5) | The dotted line segment indicates we have not yet commenced any clinical trials for radiation-induced dermatitis. This planned radiation-induced dermatitis Phase 2 trial is an investigator-led study. |

| • | Revenue-generating commercial products. We currently generate revenue, including royalties, from global sales of lomitapide and metreleptin. This revenue stream provides us with financial flexibility to fund the continued development and potential commercialization of our existing development candidates as well as the potential acquisition or in-license of additional rare disease products and late-stage product candidates. We have retained worldwide development and commercial rights to all of our programs, excluding Japan for lomitapide, where we receive royalties, and Japan, South Korea and Taiwan for metreleptin. |
| • | Late-stage clinical program in severe EB. We are conducting a global pivotal Phase 3 trial of AP101 for the treatment of cutaneous manifestations of severe EB and we expect to report data in the second |
| • | Existing, scalable global commercial and medical infrastructure. We sell lomitapide and metreleptin in the Americas, Europe and the Middle East through our existing rare disease commercial infrastructure. Our commercial expertise includes market access, marketing, sales managers and sales representatives and is supported by our experienced medical affairs team with medical science liaisons, patient advocates and dieticians in the field. We also leverage our network of third-party distributors in other key markets throughout the world. We believe we will be able to leverage our existing global infrastructure and expertise to efficiently and expeditiously commercialize additional products we may acquire or develop, including our lead product candidate, AP101, if approved. |
| • | Proven track record of building a diversified rare disease product portfolio. We acquired AP101 through the acquisition of Birken AG in 2016, in-licensed LOJUXTA in December 2016, in-licensed our gene therapy platform, including AP103, in March 2018 and acquired metreleptin and the remaining rights to lomitapide through the Acquisition in September 2019. |
| • | Strong patent protection and regulatory exclusivity. We believe our intellectual property portfolio as well as protection afforded by regulatory exclusivity provide us with a substantial competitive advantage in marketing our current products and also protect our development programs. Our lomitapide patent portfolio includes patents that provide protection from 2025 to 2027 in the United States and into 2025 in the European Union, with supplementary protection granted to extend patent protection in major EU countries into 2028. The metreleptin patent portfolio includes patents that provide protection from 2022 to 2027 in the United States and into 2022 in the European Union and orphan exclusivity in the European Union into 2028. The AP101 patent portfolio includes patents that provide protection in both the United States and the European Union into 2025 and 2030 and a further international patent application directed to the clinical formulation and methods of manufacturing and treatment with AP101 which, if granted, would provide worldwide protection into 2039. We have also submitted additional patent applications to further strengthen our intellectual property portfolio. |
| • | Experienced management team comprised of industry leaders in rare diseases. Our management team has extensive expertise in the acquisition, development and commercialization of rare disease assets. We believe that the breadth of experience and successful track record of our management team and our Board, combined with our broad network of established relationships with leaders in the industry and medical community, provide us with strong drug development and commercialization capabilities. |
| • | Drive revenue growth for our existing commercial products. We intend to continue to focus on growing the sales of lomitapide and metreleptin in the markets and indications we currently sell them. We also intend to expand the market opportunity by seeking approval for the use of lomitapide to treat pediatric HoFH and for the treatment of FCS and for the use of metreleptin to treat PL in the United States. |
| • | Complete development and commercialize our lead product candidate, AP101, for the treatment of severe EB. AP101 is currently in a pivotal Phase 3 trial for the treatment of cutaneous manifestations of severe EB and we expect to report data in the second half of 2020. If the trial is successful, we intend to apply for approval of AP101 and commercialize it in the United States and the European Union. If approved by the FDA, we are eligible to apply for a PRV that we can use, sell or transfer. |
| • | Leverage our global commercial and medical infrastructure. We intend to leverage our existing global infrastructure and expertise to commercialize our development-stage pipeline, including our lead product candidate, AP101, if approved, and any rare disease assets we may acquire or in-license in the future. |
| • | Continue developing our gene therapy product candidate, AP103, for the treatment of RDEB. AP103 is currently in preclinical development for the treatment of RDEB. We intend to initiate clinical development in the second half of 2021. |
| • | Continue evaluating opportunities to expand our rare disease product portfolio and pipeline. We believe we are well positioned to continue to opportunistically acquire or in-license rare disease assets that we believe we can efficiently sell through our existing commercial infrastructure. Our Commercial Products |
| • | the development, validation and implementation of a ligand binding assay to supplement the neutralizing bioassay that tests for the presence of neutralizing antibodies in serum samples from patients with GL; |
| • | testing all banked clinical samples from the GL clinical program for the presence of neutralizing antibodies against leptin using the ligand binding assay and to correlate neutralizing antibodies with clinical events; and |
| • | a prospective study to assess the immunogenicity of metreleptin in patients receiving metreleptin. |
| • | a non-interventional, prospective, observational study (product exposure registry) of GL and PL patients initiating treatment with metreleptin. This study will be open to all patients treated with metreleptin and will continue to the life-span of the product; |
| • | a post-approval efficacy study in PL patients; and |
| • | an integrated analysis of immunogenicity that includes testing, using validated assays, on samples from historical studies, as well as the registry, the pediatric investigation plan (“PIP”) and the post-approval efficacy study in PL patients. |
| | Patient (wound #) | | | Blinded Efficacy Assessment Experts | | | Result | | |||
| | Reviewer 1 | | | Reviewer 2 | | ||||||
| | 1 | | | Non-adhesive wound dressing | | | AP101 | | | Undecided | |
| | 2 | | | AP101 | | | Equal | | | AP101 | |
| | 3 | | | AP101 | | | AP101 | | | AP101 | |
| | 4 | | | AP101 | | | Equal | | | AP101 | |
| | 5 | | | Equal | | | Equal | | | Undecided | |
| | 6 | | | AP101 | | | AP101 | | | AP101 | |
| | 7 | | | Equal | | | Equal | | | Undecided | |
| | 8 | | | AP101 | | | Non-adhesive wound dressing | | | Undecided | |
| | 9 (1) | | | AP101 | | | AP101 | | | AP101 | |
| | 9 (2) | | | Equal | | | AP101 | | | AP101 | |
| | 10 (1) | | | AP101 | | | AP101 | | | AP101 | |
| | 10 (2) | | | AP101 | | | AP101 | | | AP101 | |
| | | n (%) | | | 95% CI | | | p value(1) | |
Patients with earlier healing of SOC treated wound half | | | 5 (14.3) | | | 4.8, 30.3 | | | |
Patients with earlier healing of AP101 treated wound half | | | 30 (85.7) | | | 69.7, 95.2 | | | < 0.0001 |
| (1) | Based on one-sided, exact binomial test evaluating the rate of superiority of AP101 being > 0.5. |
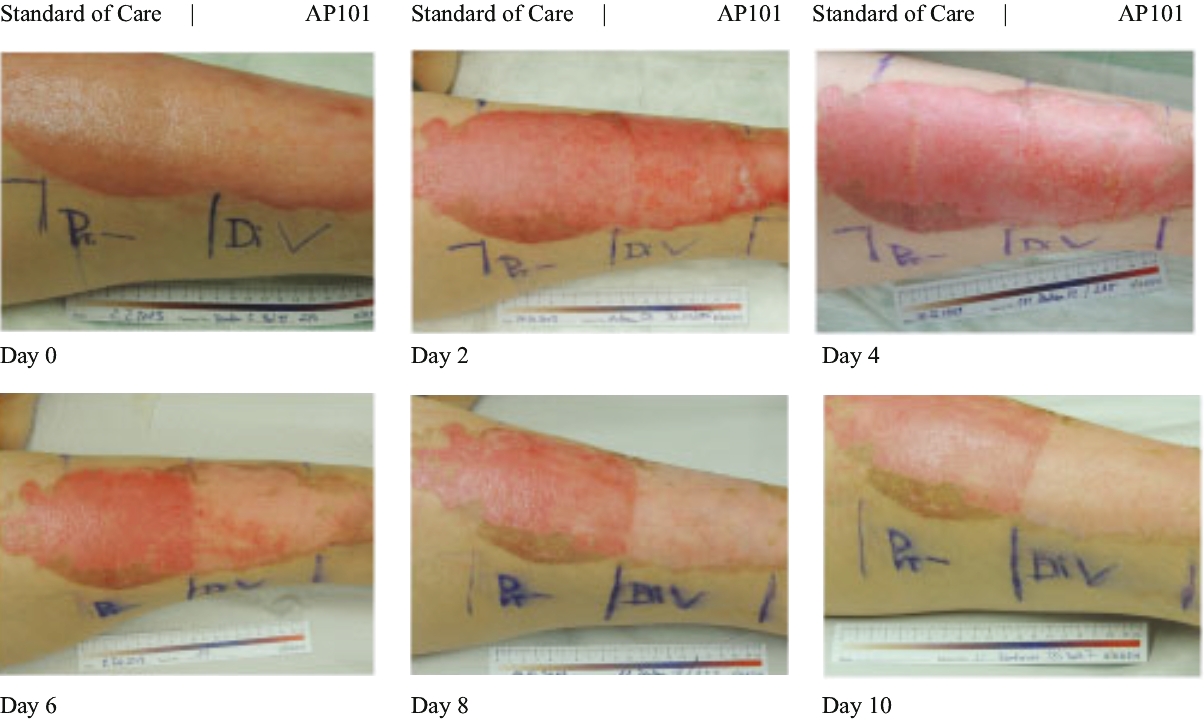
Study | | | n | | | Median | | | Min, Max | | | Mean | | | 95% CI | | | p value(2) |
BSH-12 | | | 107 | | | -0.3 | | | -10.0, 2.3 | | | -1.4 | | | -1.8, -0.9 | | | < 0.001 |
BSG-12 | | | 110 | | | 0.0 | | | -18.3, 12.3 | | | -0.8 | | | -1.5, -0.1 | | | 0.0232 |
Pooled | | | 217 | | | -0.3 | | | -18.3, 12.3 | | | -1.1 | | | -1.5, -0.7 | | | < 0.0001 |
| (1) | Difference in time to wound closure was set to 0 for photos rated as not evaluable. If wound closure was not observed, wound closure was set to 1 day after last photograph of the series (= “conservative +1 day approach”). |
| (2) | Two-sided paired t-test evaluating the mean difference as different from 0. |
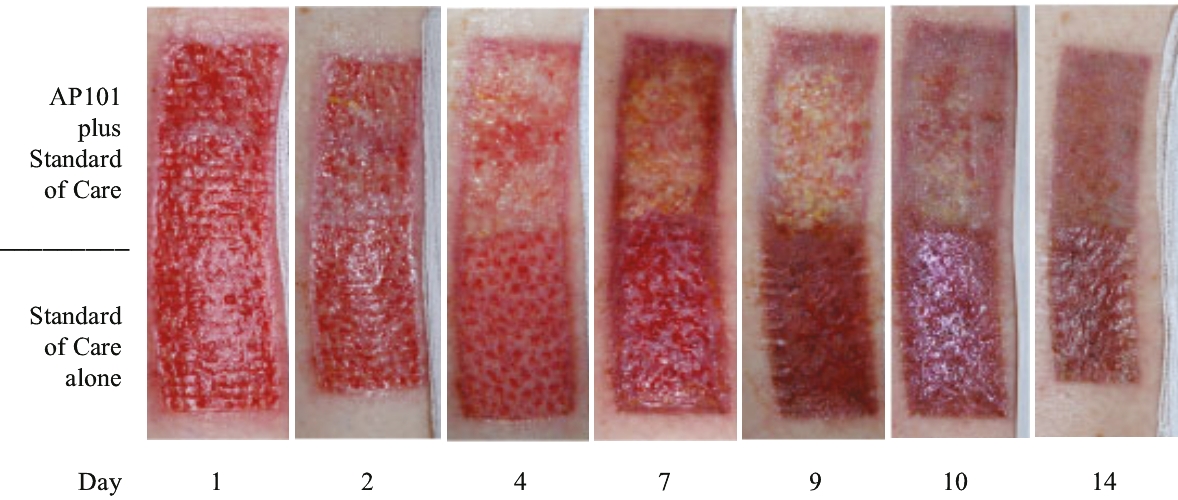
| • | Time to first complete closure of the EB target wound as evidenced by clinical assessment until the end of the double-blind phase (day 90±7 days) |
| • | Proportion of patients with first complete closure of the EB target wound at day 90±7 days based on clinical assessment by the investigator |
| • | The incidence of wound infection between baseline and day 90±7 days as evidenced by adverse events and/or use of topical and/or systemic antibiotics (related to wound infection) |
| • | The maximum severity of wound infection between baseline and day 90±7 days as evidenced by adverse events and/or use of topical and/or systemic antibiotics (related to wound infection) |
| • | Change from baseline in total body wound burden as evidenced by clinical assessment using Section I of the EB Disease Activity and Scarring Index at day 90±7 days |
| • | Change from baseline in itching using the Itch Man Scale in patients ≥ 4 years and up to 13 years of age and the Leuven Itch Scale in patients ≥ 14 years of age before wound dressing changes at day 90±7 days |
| • | Incidence, severity and relatedness of adverse events |
| • | Local tolerability as judged by the investigator |
| • | Safety laboratory data |
| • | Systemic exposure to betulin |
| • | Lomitapide: A significant competitor product to our lomitapide product is a class of drugs known as PCSK9 inhibitors. One PCSK9 inhibitor, an Amgen, Inc. (“Amgen”) product, is currently approved and commercialized in the European Union and the United States for the same treatment indication, HoFH, as lomitapide. Sales of this PCSK9 inhibitor compete with sales of the lomitapide product and we expect that this product will continue to compete with lomitapide. However, because many therapies, including PCSK9 inhibition products, act by increasing the activity of LDL-R, HoFH patients often have an inadequate response due to their impaired LDL-R function. In addition, other companies may succeed in developing, acquiring or licensing additional pharmaceutical products that are introduced into the market and that are more effective or less costly than our products. For example, we are aware of several companies developing products that could potentially compete with lomitapide, including Regeneron Pharmaceuticals, Inc. and REGENXBIO Inc. |
| • | Metreleptin: Our competitors are also developing products, which, if approved and depending on the labelled indication, could potentially compete with metreleptin, including Regeneron Pharmaceuticals, Inc. and Akcea Therapeutics, Inc. Although MYALEPT is the first and only product approved in the United States for the treatment of complications of leptin deficiency in patients with GL, there are a number of therapies approved to treat these complications independently that are not specific to GL. MYALEPTA also faces competition in the European Union, both for the treatment of GL and PL. |
| • | AP101: Although there are no approved products in the United States or the European Union for the treatment of EB, some of our competitors are developing products which, if approved, and depending on the labelled indication, could potentially compete with AP101. Some companies, including Krystal Biotech, Inc. and Abeona Therapeutics Inc., are developing gene therapy treatments, while other companies, including Castle Creek Pharmaceuticals Holdings, Inc. and Wings Therapeutics, Inc., are developing non-gene therapy treatments. |
| • | €3 million within 30 days of net ex-factory sales on or after marketing approval of at least €100,000; |
| • | €10 million once net ex-factory sales/net revenue in any calendar year exceed €50 million; |
| • | €15 million once net ex-factory sales/net revenue in any calendar year exceed €100 million; and |
| • | €10 million on receipt of marketing approval by the EMA or the FDA for the treatment of EB. |
| • | €100,000 on the successful completion of a Phase 2a proof of concept study; |
| • | €100,000 on the successful completion of a Phase 2b study; |
| • | €200,000 upon the first commercial sale of a gene therapy product incorporating or utilizing the licensed technology; and |
| • | €200,000 upon the first commercial sale of each subsequent product requiring a separate marketing authorization. |
| • | if we are in material breach of any provision of the agreement and, after receiving notice from University College Dublin identifying a material breach, we fail to cure said material breach within 60 business days, University College Dublin may issue a notice of default. If we do not cure the material breach within 60 business days from receipt of the notice of default, then University College Dublin may terminate the agreement; |
| • | if we become insolvent, or if an interim order is applied for or made, or a voluntary arrangement approved, or a voluntary arrangement is proposed or approved or an administration order is made, or a receiver or administrative receiver is appointed for any of our assets or undertaking or a winding-up resolution or petition is passed or presented (otherwise than for the purposes of reconstruction or amalgamation), or if any circumstances arise which entitle the court or a creditor to appoint a receiver, administrative receiver or administrator or to prevent a winding-up petition or make a winding-up order, or other similar or equivalent action is taken against or by us by reason of our insolvency or in consequence of debt, or if we make any arrangement with our creditors; |
| • | if we or our affiliates challenge the validity of the patent applications when granted, or assist any third party to commence legal proceedings to challenge such validity; |
| • | if, according to an independent expert’s judgment, we have failed to use commercially reasonable efforts to develop, use and exploit the intellectual property licensed under the agreement, and six months after the independent expert’s judgment we have still failed to take the specific actions to develop, use and exploit the intellectual property licensed, then University College Dublin may at any time within three months after the end of that six-month period, provide at least three months’ notice to us to terminate the agreement; |
| • | if we fail to pay any amount due under the agreement within 30 business days having received written notice from University College Dublin of our failure to pay, and such failure to pay is not subject to a good faith dispute between the parties; and |
| • | if we dispose of all, or a substantial part of our business involving the licensing of the intellectual property under the agreement in circumstances where we do not enter into a novation agreement pursuant to us becoming insolvent or any other circumstance described above. |
| • | we have not achieved certain agreed performance milestones; |
| • | we have willfully made a false statement of, or willfully omitted, a material fact in the license application or in any report required by the agreement; |
| • | we have committed a material breach of a covenant or agreement contained in the agreement that has not been remedied within 90 days; |
| • | we have not been keeping the licensed products or the licensed processes reasonably available to the public after commercial use commences; or |
| • | we cannot reasonably satisfy unmet health and safety needs. |
| • | completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices and other applicable regulations; |
| • | submission to the FDA of an investigational new drug (“IND”) application, which must become effective before human clinical trials may begin; |
| • | performance of human clinical trials, including adequate and well-controlled trials, according to Good Clinical Practice to establish the safety and efficacy of the proposed drug for its intended use, or the safety, purity and potency of a biological product; |
| • | approval by an independent IRB, representing each clinical site before each clinical trial may be initiated; |
| • | submission to the FDA of an NDA or BLA; |
| • | completion of registration batches and validation of the manufacturing process to show that the producer is capable of consistently producing quality batches of product; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA or BLA. |
| �� | Phase 1. The investigational drug is initially introduced into healthy human subjects, and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing may be conducted in patients with the target diseases. |
| • | Phase 2. This phase involves trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3. This phase involves trials undertaken after preliminary evidence of effectiveness has been obtained and is intended to further evaluate dosage and clinical efficacy and safety of the drug, or the safety, purity, and potency of a biological product, in an expanded patient population, often at geographically dispersed clinical trial sites. These trials are intended to establish the overall risk/benefit ratio of the product, and to provide an adequate basis for product approval and product labelling. |
| (i) | prescribers are educated about the approved indication for JUXTAPID, the risk of hepatotoxicity associated with the use of JUXTAPID; and the need to monitor patients during treatment with JUXTAPID as per product labeling, |
| (ii) | JUXTAPID is dispensed only to patients with a clinical or laboratory diagnosis consistent with homozygous familial hypercholesterolemia (HoFH), and |
| (iii) | patients are informed about the risk of hepatotoxicity associated with the use of JUXTAPID and the need for baseline and periodic monitoring. |
| 1. | the risks of serious adverse sequelae (such as severe infections, excessive weight gain, glucose intolerance, diabetes mellitus) due to the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or MYALEPT, and |
| 2. | the risk of lymphoma by: |
| • | Educating prescribers about the development of neutralizing anti-metreleptin antibodies, the serious adverse sequelae that may result from these antibodies, and the risk for lymphoma associated with MYALEPT. |
| • | Limiting the population exposed to MYALEPT by requiring prescriber certification, pharmacy certification, and prescriber attestation that each patient has a diagnosis consistent with the approved indication. |
| • | issue safety alerts, “Dear Doctor” letters, press releases or other communications containing warnings about such product; |
| • | mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; |
| • | require that we conduct post-marketing studies; |
| • | require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; |
| • | seek an injunction or impose civil or criminal penalties or monetary fines; |
| • | suspend marketing of, withdraw regulatory approval of or recall such product; |
| • | suspend any ongoing clinical studies; |
| • | refuse to approve pending applications or supplements to applications filed by us; |
| • | suspend or impose restrictions on operations, including costly new manufacturing requirements; or |
| • | seize or detain products, refuse to permit the import or export of products or require us to initiate a product recall. |
| • | The U.S. Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2025 unless additional Congressional action is taken. |
| • | The U.S. American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. |
| • | The U.S. Middle Class Tax Relief and Job Creation Act of 2012 required that the CMS reduce the Medicare clinical laboratory fee schedule by 2% in 2013, which served as a base for 2014 and subsequent years. In addition, effective January 1, 2014, CMS also began bundling the Medicare payments for certain laboratory tests ordered while a patient received services in a hospital outpatient setting. |
| • | increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care; |
| • | requiring drug manufacturers to provide a 50% discount on Medicare Part D brand name prescription drugs sold to Medicare beneficiaries whose prescription drug costs cause the beneficiaries to be subject to the Medicare Part D coverage cap (i.e., the so-called donut hole); and |
| • | expanding programs designed to test innovative payment models, service delivery models, or value-based arrangements. |
Name | | | Age | | | Position |
Executive Officers | | | | | ||
Dr. Joseph A. Wiley | | | 49 | | | Chief Executive Officer |
Rory P. Nealon | | | 52 | | | Chief Financial Officer and Chief Operating Officer |
| | | | | |||
Non-Executive Directors | | | | | ||
Raymond T. Stafford | | | 73 | | | Chairman of the Board |
George P. Hampton, Jr. | | | 50 | | | Non-executive Director |
Dr. Alain H. Munoz | | | 71 | | | Non-executive Director |
Donald K. Stern | | | 74 | | | Non-executive Director |
Dr. Patrick V.J.J. Vink | | | 57 | | | Non-executive Director |
Stephen T. Wills | | | 63 | | | Non-executive Director |
| • | Exemption from filing quarterly reports on Form 10-Q and current reports on Form 8-K disclosing significant events within four days of their occurrence. |
| • | Exemption from Section 16 rules regarding sales of ordinary shares by insiders, which will provide less data in this regard than to shareholders of U.S. companies that are subject to the Exchange Act. |
| • | Exemption from the Nasdaq rules applicable to domestic issuers requiring disclosure within four business days of any determination to grant a waiver of the code of business conduct and ethics to directors and officers. Although we will require approval from our Board of any such waiver, we may choose not to disclose the waiver in the manner set forth in the Nasdaq rules, as permitted by the foreign private issuer exemption. |
| • | Exemption from the requirements that director nominees are selected, or recommended for selection by our Board, either by (1) independent directors constituting a majority of our Board’s independent directors in a vote in which only independent directors participate, or (2) a committee comprised solely of independent directors, and that a formal written charter or board resolution, as applicable, addressing the nominations process is adopted. |
| • | monitoring the integrity of our financial statements, including our annual and half-yearly reports, interim management statements, preliminary results announcements and any other formal announcement relating to our financial performance; |
| • | advising on and recommending the appointment of the independent auditors; |
| • | evaluating our independent auditors’ qualifications, performance and independence, and presenting its conclusions to the full Board on at least an annual basis; |
| • | reviewing and challenging where necessary our accounting policies, methods and applications of accounting standards; |
| • | ensuring that we maintain an effective system of internal and external audit and financial control; |
| • | risk management; and |
| • | reviewing and considering the scope of the annual audit and the extent of the non-audit work undertaken by our independent auditors. |
| • | proposing and recommending specific remuneration packages for each of the executive directors, including pension rights and any compensation payments; |
| • | recommending and monitoring the level and structure of remuneration for senior management, and the implementation of share option, or other performance-related schemes; |
| • | determining and monitoring policy on and setting levels of remuneration for executive directors and senior management; |
| • | determining policy on termination of senior management; |
| • | determining performance-related pay, pension arrangements, share incentive plans and reporting and disclosure; |
| • | reviewing the Company’s arrangements for its employees to raise concerns, in confidence, about possible wrongdoing in financial reporting or other matters; and |
| • | establishing the selection criteria for, selecting, appointing and setting the terms of reference for, any remuneration consultants who advise the Remuneration Committee. |
| | | Base Salary and Fees | | | Bonus | | | Pension Contributions | | | Other Benefits | | | 2019 Total | |
| | | US$ in thousands | |||||||||||||
Raymond T. Stafford | | | $61 | | | $— | | | $— | | | $— | | | $61 |
Dr. Joseph A. Wiley | | | 588 | | | 703 | | | 50 | | | 31 | | | 1,372 |
George P. Hampton, Jr.(1) | | | 17 | | | — | | | — | | | — | | | 17 |
Dr. Alain H. Munoz(1) | | | 15 | | | — | | | — | | | — | | | 15 |
Donald K. Stern(1) | | | 21 | | | — | | | — | | | — | | | 21 |
Dr. Patrick V.J.J. Vink(1) | | | 16 | | | — | | | — | | | — | | | 16 |
Stephen T. Wills(1) | | | 23 | | | — | | | — | | | — | | | 23 |
Rory P. Nealon(2) | | | 411 | | | 583 | | | 37 | | | 18 | | | 1,049 |
Harry Stratford(2) | | | 82 | | | — | | | — | | | — | | | 82 |
James Culverwell(2) | | | 58 | | | — | | | — | | | — | | | 58 |
Markus Zeiner(2) | | | 47 | | | — | | | — | | | — | | | 47 |
Total(2) | | | $1,339 | | | $1,286 | | | $87 | | | $49 | | | $2,761 |
| (1) | Mr. Hampton, Mr. Munoz, Mr. Stern, Dr. Vink and Mr. Wills were all appointed to the Board on September 24, 2019 and their salaries reflect the period from the date of appointment to December 31, 2019. |
| (2) | Mr. Nealon, Mr. Statford, Mr. Culverwell and Mr. Zeiner resigned from the Board on September 24, 2019 upon completion of the Acquisition. Mr. Nealon’s remuneration is for the full financial year as he continued in his roles as Chief Financial Officer and Chief Operating Officer, and the salaries of the other directors that resigned on September 24, 2019 reflect their salaries from January 1, 2019 to September 24, 2019. |
| • | on death, one year from the date of death; |
| • | on the option holder ceasing to meet the requirements of a participant in the Equity Incentive Plan due to retirement or resignation or early retirement due to disability or ill health, on the expiration of one year after having resigned or retired; or |
| • | on resignation, retirement or dismissal for reasons other than termination summarily for serious misconduct, 90 days after such event. On termination summarily for serious misconduct, options lapse immediately on such termination. |
Name | | | Ordinary Shares | | | % Ownership of Share Capital | | | Share Options | | | Grant Date | | | Exercise Price Stg£ | | | Expiration Date |
Dr. Joseph A. Wiley | | | 3,507,080 | | | 2.0% | | | 343,521 | | | Nov 28, 2017 | | | 1.2072 | | | Nov 28, 2024 |
| | | | | | 316,039 | | | May 21, 2019 | | | 0.758 | | | May 21, 2026 | ||||
| | | | | | 5,777,900 | | | Nov 5, 2019 | | | 1.215 | | | Nov 5, 2026 | ||||
Rory P. Nealon | | | 1,610,770 | | | 0.9% | | | 137,408 | | | Nov 28, 2017 | | | 1.2072 | | | Nov 28, 2024 |
| | | | | | 251,915 | | | May 21, 2019 | | | 0.758 | | | May 21, 2026 | ||||
| | | | | | 4,437,500 | | | Nov 5, 2019 | | | 1.215 | | | Nov 5, 2026 | ||||
George P. Hampton | | | — | | | — | | | — | | | — | | | — | | | — |
Dr. Alain H. Munoz | | | — | | | — | | | — | | | — | | | — | | | — |
Raymond T. Stafford | | | 1,301,001 | | | 0.8% | | | — | | | — | | | — | | | — |
Donald K. Stern | | | — | | | — | | | — | | | — | | | — | | | — |
Stephen T. Wills | | | — | | | — | | | — | | | — | | | — | | | — |
| • | directors, alternate directors, officers or employees of a group company; or |
| • | trustees of any pension fund in which our employees or employees of any other group company are interested, including in each case insurance against any liability incurred by such persons in respect of any act or omission in the actual or purported execution and/or discharge of their duties and/or in the exercise or purported exercise of their powers and/or otherwise in relation to their duties, powers or offices in relation to us or any other group company or pension fund. |
| • | We were not required to take any action which is prohibited or restricted by the Takeover Code in relation to any alternative transaction. Similarly, we were not required to refrain from any action which is required by the Takeover Code in relation to any alternative transaction. |
| • | Following the approval by the U.S. Bankruptcy Court of the Plan Funding Agreement Approval Motion on June 28, 2019, Aegerion had a 55-day period to solicit an alternative transaction. Subject to the limitations of the Plan Funding Agreement¸ Aegerion was also entitled to respond to unsolicited proposals that Aegerion determined reasonably likely to result in a superior transaction. |
| • | At any time prior to and after the completion of the 55-day period, Aegerion was entitled to receive offers from competing bidders and was able to respond to any such offer if its board deemed that the offer constituted or was likely to constitute a superior proposal. |
| • | a subscription agreement between Amryt, Highbridge MSF International Ltd. and Highbridge SCF Special Situations SPV, L.P. (together, “Highbridge Subscribers”) pursuant to which the Highbridge Subscribers agreed to subscribe for 907,193 ordinary shares for an aggregate subscription price of $1 million and, subject to the GM Resolutions being passed, a further 2,765,901 ordinary shares for an aggregate subscription price of $3 million; |
| • | a subscription agreement between Amryt and Raymond Stafford pursuant to which Raymond Stafford agreed to subscribe for 918,273 Amryt ordinary shares for an aggregate subscription price of $1 million; and |
| • | a subscription agreement between Amryt and Stonepine Capital, LP pursuant to which Stonepine Capital, LP agreed to subscribe for 459,136 Amryt ordinary shares for an aggregate subscription price of $500,000. |
| • | each person, or group of affiliated persons, known to us to beneficially own 5% or more of our outstanding ordinary shares; |
| • | each member of our Board and each of our executive officers; and |
| • | all Board members and executive officers as a group. |
| | | Ordinary Shares Beneficially Owned Prior to this Offering | | | Number of Shares Being Registered in the Offering | | | Ordinary Shares Beneficially Owned After this Offering** | |||||||
Name of beneficial owner | | | Number | | | Percentage | | | | | Number | | | Percentage | |
5% or greater shareholders (not including current directors or officers): | | | | | | | | | | | |||||
Athyrium Funds(1) | | | 64,796,247 | | | 37.7% | | | 64,796,247 | | | 0 | | | — |
Highbridge(2) | | | 36,342,967 | | | 21.2% | | | 36,342,967 | | | 0 | | | — |
UBS(3) | | | 13,932,851 | | | 8.1% | | | 13,932,851 | | | 0 | | | — |
Novelion Therapeutics Inc. | | | 12,490,250 | | | 7.3% | | | 12,490,250 | | | 0 | | | — |
Edgepoint Capital | | | 12,126,650 | | | 7.1% | | | 12,126,650 | | | 0 | | | — |
Software AG-Stiftung | | | 10,212,153 | | | 5.9% | | | 10,212,153 | | | 0 | | | — |
Executive officers and directors: | | | | | | | | | | | |||||
Dr. Joseph A. Wiley(4) | | | 3,757,850 | | | 2.2% | | | 3,507,080 | | | 250,770 | | | * |
Rory P. Nealon(5) | | | 1,742,453 | | | 1.0% | | | 1,610,770 | | | 131,083 | | | * |
Ray T. Stafford | | | 1,363,501 | | | * | | | 1,363,501 | | | 0 | | | — |
Donald K. Stern | | | — | | | — | | | | | | | |||
Dr. Alain H. Munoz | | | — | | | — | | | | | | | |||
George P. Hampton | | | — | | | — | | | | | | | |||
Dr. Patrick V.J.J. Vink | | | — | | | — | | | | | | | |||
Stephen T. Wills | | | — | | | — | | | | | | | |||
All executive officers and directors as a group (eight persons) | | | 6,801,304 | | | 4.0% | | | 6,481,351 | | | 381,853 | | | * |
Other Registered Holder | | | | | | | | | | | |||||
Axa Investments | | | 6,494,164 | | | 3.8% | | | 6,494,164 | | | 0 | | | — |
| * | Less than 1%. |
| ** | Unlike an initial public offering, any disposition by the Registered Holders of the Registered Shares represented by ADSs is not being underwritten by any investment bank. The Registered Holders may or may not elect to dispose of Registered Shares represented by ADSs as and to the extent that they may individually determine. Such dispositions, if any, will be made through brokerage transactions on Nasdaq or other securities exchanges in the United States at prevailing market prices, and the post-offering ownership figures in these columns represent the lowest level of ownership that would exist if the Registered Holders sold 100% of the Registered Shares owned by them, which may or may not happen. |
| (1) | Consists of an aggregate of 64,796,247 ordinary shares that are registered hereby, including 43,286,346 ordinary shares and 21,509,901 ordinary shares issuable upon conversion of Convertible Notes held by the following affiliates of Athyrium: Athyrium Opportunities II Acquisition 2 LP, Athyrium Opportunities III Acquisition 2 LP, Athyrium Opportunities II Acquisition LP and Athyrium Opportunities III Acquisition LP. |
| (2) | Consists of an aggregate of 36,342,967 ordinary shares that are registered hereby, including 10,954,293 ordinary shares, 12,422,154 ordinary shares issuable upon conversion of Convertible Notes, and 12,966,520 ordinary shares issuable upon exercise of zero cost warrants held by the following affiliates of Highbridge: Highbridge MSF International Ltd., Highbridge SCF Special Situations SPV, L.P. and Highbridge Tactical Credit Master Fund, L.P. |
| (3) | Consists of an aggregate of 13,932,851 ordinary shares that are registered hereby, including 6,309,224 ordinary shares, 3,393,874 ordinary shares issuable upon conversion of Convertible Notes, and 4,229,753 ordinary shares issuable upon exercise of zero cost warrants held by the following affiliates of UBS: Nineteen77 Global Multi-Strategy Alpha Master Limited and Nineteen77 Global Convertible Bond Master Limited. |
| (4) | Consists of 3,507,080 ordinary shares that are registered hereby, as well as (i) 171,760 share options exercisable at £1.2072 per share, which expire on November 28, 2024 and (ii) 79,010 share options exercisable at £0.758 per share, which expire on May 21, 2026, that are not registered hereby. |
| (5) | Consists of 1,610,770 ordinary shares that are registered hereby, as well as (i) 68,704 share options exercisable at £1.2072 per share, which expire on November 28, 2024 and (ii) 62,979 share options exercisable at £0.758 per share, which expire on May 21, 2026, that are not registered hereby. |
| • | On the date of our incorporation, being July 17, 2019, we issued one ordinary share. |
| • | On September 12, 2019, we issued one redeemable share with a nominal value of £49,999.94. This has subsequently been redeemed on November 7, 2019 and, upon redemption, the redeemable share was cancelled and our issued share capital was diminished accordingly by the nominal value of the redeemable share. |
| • | On September 24, 2019, we issued 53,149,070 ordinary shares to former holders of Amryt Pharma Holdings Limited. The ordinary shares were issued by resolution of the Board, as authorized pursuant to a resolution passed by the holders of our ordinary shares at the general meeting held on September 23, 2019. |
| • | On September 24, 2019, we issued 77,027,423 ordinary shares (including 48,739,975 ordinary shares represented by 9,747,995 ADSs) and 8,065,000 zero cost warrants to former creditors of Aegerion as consideration for the Acquisition. The ordinary shares and warrants were issued by resolution of the Board, as authorized pursuant to a resolution passed by the holders of our ordinary shares at the general meeting held on September 23, 2019. |
| • | On September 24, 2019, we issued 27,541,944 ordinary shares (including 1,693,275 ordinary shares represented by 338,655 ADSs) and 5,911,722 zero cost warrants in connection with a $60 million equity offering to new and existing investors and former creditors of Aegerion. The ordinary shares and warrants were issued by resolution of the Board, as authorized pursuant to a resolution passed by the holders of our ordinary shares at our general meeting held on September 23, 2019. |
| • | On November 14, 2019, we repurchased 4,864,656 ordinary shares from Highbridge Tactical Master Fund L.P., Highbridge SCF Special Situations SPV, L.P. and Nineteen77 Global Multi Strategy Alpha Master Limited. In exchange for the ordinary shares, these institutions were issued an equivalent number of zero cost warrants. Each warrant entitles the holder to subscribe for one ordinary share at zero cost. The repurchased ordinary shares are now held as treasury shares. On December 19, 2019, these holders elected to exercise 1,645,105 warrants in exchange for 1,645,105 ordinary shares. |
| • | On December 19, 2019, Highbridge MSF International Ltd exercised 1,645,105 warrants in exchange for 1,645,105 ordinary shares. |
| • | subject to any special rights or restrictions as to voting attached to any shares, on a show of hands every holder who (being an individual) is present in person or by proxy not being himself or herself a holder or (being a corporation) is present by a representative or by proxy not being himself or herself a holder shall have one vote and on a poll every holder who is present in person or by proxy shall have one vote for every share of which he or she is the holder; and |
| • | holders have the right to receive notice of, attend and vote at general meetings; the right to participate in our profits and receive such dividends as are recommended by the directors, pro rata according to the amount paid up on the shares during the period in respect of which the dividend is paid; and, on a winding up or return of capital or otherwise, the right to repayment of the amounts paid up or credited as paid up on the shares and the right to participate pro rata in any excess assets. |
| • | the name of any person, without sufficient cause, is entered in or omitted from our register of holders; or |
| • | there is a default or unnecessary delay in entering on the register the fact of any person having ceased to be a holder. |
| • | Warrants issued on September 24, 2019 to purchase 345,542 ordinary shares with a weighted average exercise price of £1.44 per ordinary share. These warrants generally lapse after five years from the date of the grant. These warrants were issued to Shore Capital and Davy in exchange for warrants granted to such parties on April 19, 2016 in connection with the placement undertaken by us on that date. |
| • | Warrants to purchase 17,196,273 ordinary shares, with an exercise price of zero. These warrants were issued (i) to certain Aegerion creditors who expressed a wish to restrict their percentage share interest in our issued share capital and who elected to take warrants in lieu of ordinary shares, and (ii) to certain investors in exchange for the repurchase of 4,864,656 ordinary shares by us from these investors on November 14, 2019 and who subsequently, on December 19, 2019, elected to exercise 1,645,105 warrants in exchange for 1,645,105 ordinary shares. |
| • | 57,457,870 EMA CVRs, which entitle their holders to a payment of up to $15 million in the aggregate (in satisfaction of which we may elect to issue loan notes or ordinary shares) upon the EMA issuing a qualifying approval (i.e., an approval or marketing authorization issued by the EMA in relation to the sale by us of AP101 to consumers for medical purposes which satisfies a certain criteria in respect of AP101) if such qualifying approval is obtained by December 31, 2021. The amount payable reduces on a straight-line basis if the qualifying approval is obtained after December 31, 2021 but prior to July 1, 2022; |
| • | 57,457,870 FDA CVRs, which entitle their holders to a payment of up to $35 million in the aggregate (in satisfaction of which we may elect to issue loan notes or ordinary shares) upon the FDA issuing a qualifying approval (i.e., an approval or marketing authorization issued by the FDA in relation to the sale by us of AP101 to consumers for medical purposes which satisfies a certain criteria in respect of AP101) if qualifying approval is obtained by December 31, 2021. The amount payable reduces on a straight-line basis if the qualifying approval is obtained after December 31, 2021 but prior to July 1, 2022; and |
| • | 57,457,870 Revenue CVRs, which entitle their holders to a payment of up to $35 million in the aggregate (in satisfaction of which we may elect to issue loan notes or ordinary shares) upon the generation of certain revenues from sales of AP101 in trailing 12-month revenues in any period prior to June 30, 2024. |
| • | consolidate and divide all or any of our share capital into shares of larger nominal value than our existing shares; |
| • | cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken; and |
| • | sub-divide our shares into shares of smaller nominal value than our existing shares. |
| • | any call or other sum presently payable by him or her to us in respect of the shares remains unpaid; or |
| • | the holder has been served with a notice under section 793 of the Companies Act and he or she has failed to provide us with information concerning interests in those shares required to be provided under the Companies Act. |
| • | relating to the giving of any security, guarantee or indemnity: |
| • | to him or her in relation to money lent or obligations incurred by him or her at the request of or for the benefit of us or any of our subsidiaries; or |
| • | to a third party in relation to our or any of our subsidiaries’ debt or obligation for which he himself or she herself has assumed responsibility in whole or part by the giving of security under a guarantee or indemnity; |
| • | where we or any of our subsidiaries is offering securities in which offer the director is or is to be interested directly or as a participant in the underwriting or sub-underwriting; |
| • | relating to another company in which he or she does not hold an interest in shares representing 1% or more of either class of the equity share capital, or the voting rights in such company; |
| • | relating to a superannuation fund, retirement benefits scheme, share option scheme or share incentive scheme under which he or she may benefit; or |
| • | concerning the purchase and/or maintenance of any insurance policy under which he or she may benefit. |
| • | directors, alternate directors, officers or employees of a group company; or |
| • | trustees of any pension fund in which our employees or employees of any other group company are interested, including in each case insurance against any liability incurred by such persons in respect of any act or omission in the actual or purported execution and/or discharge of their duties and/or in the exercise or purported exercise of their powers and/or otherwise in relation to their duties, powers or offices in relation to us or any other group company or pension fund. |
| • | to be interested in our shares; or |
| • | to have been so interested at any time in the three years immediately preceding the date on which the notice is to be issued. |
| • | confirm that fact or (as the case may be) to state whether or not it is the case; and |
| • | if he or she holds, or has during that time held, any such interest, to give such further information as may be required in accordance with section 793 of the Companies Act (including particulars of the interest (past or present) and the identity of the persons interested in the shares in question). |
| • | if, at the time that the distribution is made, the amount of its net assets (that is, the aggregate of the company’s assets less the aggregate of its liabilities) is no less than the aggregate of its called up share capital and undistributable reserves; and |
| • | if, and to the extent that, the distribution itself, at the time that it is made, does not reduce the amount of the net assets to less than that total. |
| • | acquires an interest in our shares which, when taken together with shares in which he or she or persons acting in concert with him or her are interested, carries 30% or more of the voting rights of our shares; or |
| • | who, together with persons acting in concert with him or her, is interested in shares that in the aggregate carry not less than 30% and not more than 50% of the voting rights of our shares, and such persons, or any person acting in concert with him or her, acquires additional interests in shares that increase the percentage of shares carrying voting rights in which that person is interested, |
| | | England and Wales | | | Delaware | |||||||
Number of Directors | | | Under the Companies Act, a public limited company must have at least two directors and the number of directors may otherwise be fixed by or in the manner provided in the company’s articles of association. | | | Under Delaware law, a corporation must have at least one director and the number of directors shall otherwise be fixed by or in the manner provided in the bylaws. | ||||||
| | | | | | | | | |||||
Removal of Directors | | | Under the Companies Act, shareholders may remove a director without cause by an ordinary resolution (which is passed by a simple majority of those voting in person or by proxy at a general meeting) irrespective of any provisions of any service contract the director has with the company, provided 28 clear days’ notice of the resolution has been given to the company and the company must, where practicable, give its shareholders notice of such resolution in the same manner and at the same time as it gives notice of the meeting. Where that is not practicable, the company must give its shareholders notice at least 14 days before the meeting. On receipt of notice of an intended resolution to remove a director, the company must forthwith send a copy of the notice to the director concerned. Certain other procedural requirements under the Companies Act must also be followed such as allowing the director to make representations against his or her removal either at the meeting or in writing. | | | Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (a) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, shareholders may effect such removal only for cause, or (b) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his or her removal would be sufficient to elect him or her if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he or she is a part. | ||||||
| | | | | | | | | |||||
Vacancies on the Board of Directors | | | Under English law, the procedure by which directors, other than a company’s initial directors, are appointed is generally set out in the company’s articles of association, provided that where two or more persons are appointed as directors of a public limited company by resolution of the shareholders, resolutions appointing each director must be voted on individually. | | | Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (a) otherwise provided in the certificate of incorporation or by-laws of the corporation or (b) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. | ||||||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Annual General Meeting | | | Under the Companies Act, a public limited company must hold an annual general meeting in each six-month period beginning with the day following the company’s annual accounting reference date. | | | Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. | ||||||
| | | | | | | | | |||||
General Meeting | | | Under the Companies Act, a general meeting of the shareholders of a public limited company may be called by the directors. In addition, shareholders holding at least 5% of the paid-up capital of the company carrying voting rights at general meetings (excluding any paid-up capital held as treasury shares) can require the directors to call a general meeting and, if the directors fail to do so within a certain period, the requisitionists (or any of them representing more than one-half of the total voting rights of all of them) may convene a general meeting. | | | Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. | ||||||
| | | | | | | | | |||||
Notice of General Meetings | | | Under the Companies Act, at least 21 clear days’ notice must be given for an annual general meeting and any resolutions to be proposed at the meeting, subject to a company’s articles of association providing for a longer period. Subject to a company’s articles of association providing for a longer period, at least 14 clear days’ notice is required for any other general meeting. In addition, certain matters, such as the removal of directors or auditors, require special notice, which is 28 clear days’ notice. The shareholders of a company may in all cases consent to a shorter notice period, the proportion of shareholders’ consent required being 100% of those entitled to attend and vote in the case of an annual general meeting and, in the case of any other general meeting, a majority in number of the members having a right to attend and vote at the meeting, being a majority who together hold not less than 95% in nominal value of the shares giving a right to attend and vote at the meeting (excluding any shares held in the company as treasury shares). | | | Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour and purpose or purposes of the meeting. | ||||||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Quorum | | | Subject to the provisions of a company’s articles of association, the Companies Act provides that two shareholders present at a meeting (in person, by proxy or authorized representative under the Companies Act) shall constitute a quorum for companies with more than one shareholder. | | | The certificate of incorporation or bylaws may specify the number of shares, the holders of which shall be present or represented by proxy at any meeting in order to constitute a quorum, but in no event shall a quorum consist of less than one-third of the shares entitled to vote at the meeting. In the absence of such specification in the certificate of incorporation or bylaws, a majority of the shares entitled to vote, present in person or represented by proxy, shall constitute a quorum at a meeting of stockholders. | ||||||
| | | | | | | | | |||||
Proxy | | | Under the Companies Act, a shareholder may appoint another person to attend, speak and vote at any general meeting on their behalf by proxy. | | | Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director. | ||||||
| | | | | | | | | |||||
Preemptive Rights | | | Under the Companies Act, “equity securities,” being (i) shares in the company other than shares that, with respect to dividends and capital, carry a right to participate only up to a specified amount in a distribution, referred to as ordinary shares, or (ii) rights to subscribe for, or to convert securities into, ordinary shares, proposed to be allotted for cash must be offered first to the existing holders of ordinary shares in the company in proportion to the respective nominal value of their holdings, unless an exception applies or a special resolution disapplying such preemptive rights has been passed by shareholders in a general meeting or the articles of association provide for the disapplication of such preemptive rights in each case in accordance with the provisions of the Companies Act. | | | Under Delaware law, shareholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation. | ||||||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Authority to Allot | | | Under the Companies Act, the directors of a public limited company must not allot shares or grant rights to subscribe for or to convert any security into shares unless an exception applies or an ordinary resolution has been passed by shareholders in a general meeting authorizing such allotment or the articles of association provide for such authorization, in each case subject to a specified maximum nominal value and in accordance with the provisions of the Companies Act. | | | Under Delaware law, if the corporation’s charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. The board of directors may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. | ||||||
| | | | | | | | | |||||
Liability of Directors and Officers | | | Under the Companies Act, any provision, whether contained in a company’s articles of association or any contract or otherwise, that purports to exempt a director of a company, to any extent, from any liability that would otherwise attach to him or her in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. | | | Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its stockholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for: | ||||||
| | | | | | | | | |||||
| | | In addition, any provision by which a company directly or indirectly provides an indemnity, to any extent, for a director of the company or of an associated company against any liability attaching to him or her in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he or she is a director is also void except as permitted by the Companies Act, which provides exceptions for the company to (a) purchase and maintain insurance against such liability; (b) provide a “qualifying third party indemnity” (being an indemnity against liability incurred by the director to a person other than the company or an associated company or criminal proceedings in which he or she is convicted); and (c) provide a “qualifying pension scheme indemnity” (being an indemnity against liability incurred in connection with the company’s activities as trustee of an occupational pension plan). | | | • | | | any breach of the director’s duty of loyalty to the corporation or its stockholders; | ||||
| | | | ||||||||||
| | • | | | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; | ||||||||
| | | | ||||||||||
| | • | | | intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or | ||||||||
| | | | ||||||||||
| | • | | | any transaction from which the director derives an improper personal benefit. | ||||||||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Voting Rights | | | For an English company it is usual for the articles of association to provide that, unless a poll is demanded by the shareholders of a company or is required by the chairman of the meeting or the company’s articles of association, shareholders shall vote on all resolutions on a show of hands. On a show of hands every shareholder has one vote (regardless of the number of ordinary shares held) and, subject to the company's articles of association, every proxy appointed by more than one shareholder has one vote unless they have been instructed by different shareholders to vote in different ways (in which case they will have one vote for and one vote against a resolution). Under the Companies Act, a provision of a company's articles is void if it has the effect of making ineffective a demand for a poll by (a) not fewer than five shareholders having the right to vote on the resolution; (b) any shareholder(s) representing not less than 10% of the total voting rights of all the shareholders having the right to vote on the resolution (excluding any voting rights attaching to treasury shares); or (c) any shareholder(s) holding shares in the company conferring a right to vote on the resolution (excluding any voting rights attaching to treasury shares) being shares on which an aggregate sum has been paid up equal to not less than 10% of the total sum paid up on all the shares conferring that right. A company’s articles of association may provide more extensive rights for shareholders to call a poll. Under English law, ordinary resolutions require the affirmative vote of a simple majority (more than 50%) of the votes cast by shareholders present, in person or by proxy, at the meeting. Special resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present, in person or by proxy, at the meeting. | | | Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder. | ||||||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Shareholder Vote on Certain Transactions | | | The Companies Act provides for schemes of arrangement, which are arrangements or compromises between a company and any class of shareholders or creditors and used in certain types of reconstructions, amalgamations, capital reorganizations or takeovers. These arrangements require: | | | Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires: | ||||||
| | | | | | | | | |||||
| | | • | | | the approval at a shareholders’ or creditors’ meeting convened by order of the court, of a majority in number of shareholders or creditors or a class thereof representing 75% in value of the capital held by, or debt owed to, the class of shareholders or creditors, or class thereof present and voting, either in person or by proxy; and | | | • | | | the approval of the board of directors; and | |
| | | | ||||||||||
| | • | | | approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of the corporation entitled to vote on the matter. | ||||||||
| | | | | | | | | |||||
| | | • | | | the approval of the court. | | | | | |||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Standard of Conduct for Directors | | | Under English law, a director owes various statutory and fiduciary duties to the company, including: | | | Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the stockholders. Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself or herself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he or she reasonably believes to be in the best interests of the corporation. He or she must not use his corporate position for personal gain or advantage. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation. In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders. | ||||||
| | | | | |||||||||
| | • | | | to act in the way he or she considers, in good faith, would be most likely to promote the success of the company for the benefit of its members as a whole (and in doing so have regard (amongst other matters) to: (i) the likely consequences of any decision in the long-term, (ii) the interests of the company’s employees, (iii) the need to foster the company’s business relationships with suppliers, customers and others, (iv) the impact of the company’s operations on the community and the environment, (v) the desirability to maintain a reputation for high standards of business conduct and (vi) the need to act fairly as between members of the company); | | |||||||
| | | | | |||||||||
| | • | | | to avoid a situation in which he or she has, or can have, a direct or indirect interest that conflicts, or possibly conflicts, with the interests of the company; | | |||||||
| | | | | |||||||||
| | • | | | to act in accordance with the company’s constitution and only exercise his or her powers for the purposes for which they are conferred; | | |||||||
| | | | | |||||||||
| | • | | | to exercise independent judgment; | | |||||||
| | | | | |||||||||
| | • | | | to exercise reasonable care, skill and diligence; | | |||||||
| | | | | |||||||||
| | • | | | not to accept benefits from a third party conferred by reason of his or her being a director or doing, or not doing, anything as a director; and | | |||||||
| | | | | |||||||||
| | • | | | a duty to declare any interest that he or she has, whether directly or indirectly, in a proposed or existing transaction or arrangement with the company. | | |||||||
| | | | | | | | | |||||
| | | England and Wales | | | Delaware | |||||||
Stockholder Suits | | | Under English law, generally, the company, rather than its shareholders, is the proper claimant in an action in respect of a wrong done to the company or where there is an irregularity in the company’s internal management. Notwithstanding this general position, the Companies Act provides that (i) a court may allow a shareholder to bring a derivative claim (that is, an action in respect of and on behalf of the company) in respect of a cause of action arising from a director’s negligence, default, breach of duty or breach of trust and (ii) a shareholder may bring a claim for a court order where the company’s affairs have been or are being conducted in a manner that is unfairly prejudicial to some of its shareholders. | | | Under Delaware law, a stockholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must: | ||||||
| | | | ||||||||||
| | • | | | state that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter devolved on the plaintiff by operation of law; and | ||||||||
| | | | ||||||||||
| | • | | | allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or | ||||||||
| | | | ||||||||||
| | • | | | state the reasons for not making the effort. | ||||||||
| | | | | | | | | |||||
| | | | | | | Additionally, the plaintiff must remain a stockholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. | ||||||
| • | no timely request that the rights be distributed to you is received or if we request that the rights not be distributed to you; |
| • | we fail to deliver satisfactory documents to the depositary; or |
| • | it is not reasonably practicable to distribute the rights. |
| • | we do not timely request that the property be distributed to you or if we request that the property not be distributed to you; |
| • | we do not deliver satisfactory documents; or |
| • | the depositary determines that all or portion of the distribution to you is not reasonably practicable. |
| • | the ordinary shares are duly authorized, validly issued, fully paid, non-assessable and legally obtained; |
| • | all preemptive (and similar) rights, if any, with respect to such ordinary shares have been validly waived or exercised; |
| • | you are duly authorized to deposit the ordinary shares; |
| • | the ordinary shares presented for deposit are free and clear of any lien, encumbrance, security interest, charge, mortgage or adverse claim, and are not, and the ADSs issuable upon such deposit will not be, “restricted securities” (as defined in the deposit agreement); |
| • | the ordinary shares presented for deposit have not been stripped of any rights or entitlements; and |
| • | the deposit of shares does not violate any applicable provision of English law. |
| • | ensure that the surrendered ADR is properly endorsed or otherwise in proper form for transfer; |
| • | provide such proof of identity and genuineness of signatures as the depositary deems appropriate; |
| • | provide any transfer stamps required by the State of New York or the United States; and |
| • | pay all applicable fees, charges, expenses, taxes and other government charges payable by ADR holders pursuant to the terms of the deposit agreement, upon the transfer of ADRs. |
| • | temporary delays that may arise because (i) the transfer books for the ordinary shares or ADSs are closed, or (ii) ordinary shares are immobilized on account of a shareholders’ meeting or a payment of dividends; |
| • | obligations to pay fees, taxes and similar charges; |
| • | restrictions imposed because of laws or regulations applicable to ADSs or the withdrawal of securities on deposit; and |
| • | other circumstances specifically contemplated by Section I.A.(I) of the General Instructions to Form F-6 (as such General Instructions may be amended from time to time). |
| • | If voting at the shareholders’ meeting by show of hands: The depositary will vote (or cause the custodian to vote) all the securities represented by ADSs in accordance with the voting instructions received from a majority of the ADS holders who provided voting instructions. |
| • | If voting at the shareholders’ meeting by poll: The depositary will vote (or cause the custodian to vote) the securities represented by ADSs in accordance with the voting instructions received from the holders of ADSs. |
Service | | | Fees |
Issuance of ADSs (e.g., an issuance of ADS upon a deposit of ordinary shares, upon a change in the ADS(s)-to-ordinary shares ratio, or for any other reason), excluding ADS issuances as a result of distributions of ordinary shares) | | | Up to U.S. 5¢ per ADS issued |
| | | ||
Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property, upon a change in the ADS(s)-to-ordinary shares ratio, or for any other reason) | | | Up to U.S. 5¢ per ADS cancelled |
| | | ||
Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements) | | | Up to U.S. 5¢ per ADS held |
| | | ||
Distribution of ADSs pursuant to (i) stock dividends or other free stock distributions, or (ii) exercise of rights to purchase additional ADSs | | | Up to U.S. 5¢ per ADS held |
| | | ||
Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off) | | | Up to U.S. 5¢ per ADS held |
| | | ||
ADS services | | | Up to U.S. 5¢ per ADS held on the applicable record date(s) established by the depositary bank |
| | | ||
Registration of ADS transfers (e.g., upon a registration of the transfer of registered ownership of ADSs, upon a transfer of ADSs into DTC and vice versa, or for any other reason) | | | Up to U.S. 5¢ per ADS (or fraction thereof) transferred |
| | |
Service | | | Fees |
Conversion of ADSs of one series for ADSs of another series (e.g., upon conversion of Partial Entitlement ADSs for Full Entitlement ADSs, or upon conversion of Restricted ADSs (each as defined in the deposit agreement) into freely transferable ADSs, and vice versa). | | | Up to U.S. 5¢ per ADS (or fraction thereof) converted |
| • | taxes (including applicable interest and penalties) and other governmental charges; |
| • | the registration fees as may from time to time be in effect for the registration of ordinary shares on the share register and applicable to transfers of ordinary shares to or from the name of the custodian, the depositary bank or any nominees upon the making of deposits and withdrawals, respectively; |
| • | certain cable, telex and facsimile transmission and delivery expenses; |
| • | the fees, expenses, spreads, taxes and other charges of the depositary bank and/or service providers (which may be a division, branch or affiliate of the depositary bank) in the conversion of foreign currency; |
| • | the reasonable and customary out-of-pocket expenses incurred by the depositary bank in connection with compliance with exchange control regulations and other regulatory requirements applicable to ordinary shares, ADSs and ADRs; and |
| • | the fees, charges, costs and expenses incurred by the depositary bank, the custodian, or any nominee in connection with the ADR program. |
| • | We and the depositary are obligated only to take the actions specifically stated in the deposit agreement without negligence or bad faith. |
| • | The depositary disclaims any liability for any failure to carry out voting instructions, for any manner in which a vote is cast or for the effect of any vote, provided it acts in good faith and in accordance with the terms of the deposit agreement. |
| • | The depositary disclaims any liability for any failure to determine the lawfulness or practicality of any action, for the content of any document forwarded to you on our behalf or for the accuracy of any translation of such a document, for the investment risks associated with investing in ordinary shares, for the validity or worth of the ordinary shares, for any tax consequences that result from the ownership of ADSs, for the credit-worthiness of any third party, for allowing any rights to lapse under the terms of the deposit agreement, for the timeliness of any of our notices or for our failure to give notice. |
| • | We and the depositary will not be obligated to perform any act that is inconsistent with the terms of the deposit agreement. |
| • | We and the depositary disclaim any liability if we or the depositary are prevented or forbidden from or subject to any civil or criminal penalty or restraint on account of, or delayed in, doing or performing any act or thing required by the terms of the deposit agreement, by reason of any provision, present or future of any law or regulation, or by reason of present or future provision of any provision of our Articles of Association, or any provision of or governing the securities on deposit, or by reason of any act of God or war or other circumstances beyond our control. |
| • | We and the depositary disclaim any liability by reason of any exercise of, or failure to exercise, any discretion provided for in the deposit agreement or in our Articles of Association or in any provisions of or governing the securities on deposit. |
| • | We and the depositary further disclaim any liability for any action or inaction in reliance on the advice or information received from legal counsel, accountants, any person presenting ordinary shares for deposit, any holder of ADSs or authorized representatives thereof, or any other person believed by us in good faith to be competent to give such advice or information. |
| • | We and the depositary also disclaim liability for the inability by a holder to benefit from any distribution, offering, right or other benefit that is made available to holders of ordinary shares but is not, under the terms of the deposit agreement, made available to you. |
| • | We and the depositary may rely without any liability upon any written notice, request or other document believed to be genuine and to have been signed or presented by the proper parties. |
| • | We and the depositary also disclaim liability for any consequential or punitive damages for any breach of the terms of the deposit agreement. |
| • | No disclaimer of any Securities Act liability is intended by any provision of the deposit agreement. |
| • | Nothing in the deposit agreement gives rise to a partnership or joint venture, or establishes a fiduciary relationship, among us, the depositary bank and you as ADS holder. |
| • | Nothing in the deposit agreement precludes Citibank (or its affiliates) from engaging in transactions in which parties adverse to us or the ADS owners have interests, and nothing in the deposit agreement obligates Citibank to disclose those transactions, or any information obtained in the course of those transactions, to us or to the ADS owners, or to account for any payment received as part of those transactions. |
| • | Convert the foreign currency to the extent practical and lawful and distribute the U.S. dollars to the holders for whom the conversion and distribution is lawful and practical. |
| • | Distribute the foreign currency to holders for whom the distribution is lawful and practical. |
| • | Hold the foreign currency (without liability for interest) for the applicable holders. |
| • | banks and other financial institutions; |
| • | insurance companies; |
| • | regulated investment companies or real estate investment trusts; |
| • | dealers or traders in securities or currencies that use a mark-to-market method of accounting; |
| • | broker-dealers; |
| • | tax exempt organizations, retirement plans, individual retirement accounts and other tax deferred accounts; |
| • | persons holding the ADSs as part of a straddle, hedging, conversion or integrated transaction for U.S. federal income tax purposes; |
| • | U.S. expatriates; |
| • | U.S. Holders whose functional currency is not the U.S. dollar; |
| • | any entity or arrangement classified as partnership for U.S. federal income tax purposes or investors therein; |
| • | persons who own or are deemed to own, directly or constructively, 10% or more of the total combined voting power of all classes of our voting stock or 10% or more of the total value of shares of all classes of our stock; |
| • | persons subject to special tax accounting rules as a result of any item of gross income with respect to the ADSs being taken into account in an applicable financial statement; and |
| • | persons that held, directly, indirectly or by attributions, ownership interest in us prior to the effectiveness of the registration statement of which this prospectus forms a part. |
| • | a citizen or individual resident of the United States; |
| • | a corporation (or other entity treated as a corporation) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions of the trust or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
| • | at least 75% of its gross income for such year is “passive income” for purposes of the PFIC rules; or |
| • | at least 50% of the value of its assets (determined based on a quarterly average) during such year is attributable to assets that produce or are held for the production of passive income. |
| • | the excess distribution or recognized gain would be allocated ratably over the U.S. Holder’s holding period for the ADSs; |
| • | the amount of the excess distribution or recognized gain allocated to the taxable year of distribution or gain, and to any taxable years in the U.S. Holder’s holding period prior to the first taxable year in which we were treated as a PFIC, would be treated as ordinary income; and |
| • | the amount of the excess distribution or recognized gain allocated to each other taxable year would be subject to the highest tax rate in effect for individuals or corporations, as applicable, for each such year and the resulting tax will be subject to the interest charge generally applicable to underpayments of tax. |
| • | only applies to the absolute beneficial owners of the ADSs and any dividends paid in respect of the ordinary shares represented by the ADSs where the dividends are regarded for UK tax purposes as that person’s own income (and not the income of some other person); and |
| • | (a) only addresses the principal UK tax consequences for investors who hold the ADSs as capital assets/investments, (b) does not address the tax consequences that may be relevant to certain special classes of investor such as dealers, brokers or traders in shares or securities and other persons who hold the ADSs otherwise than as an investment, (c) does not address the tax consequences for holders that are financial institutions, insurance companies, collective investment schemes, pension schemes, charities or tax-exempt organizations, (d) assumes that the holder is not an officer or employee of the Company (or of any related company) and has not (and is not deemed to have) acquired the ADSs by reason of an office or employment, and (e) assumes that the holder does not control or hold (and is not deemed to control or hold), either alone or together with one or more associated or connected persons, directly or indirectly (including through the holding of the ADSs), an interest of 10% or more in the issued share capital (or in any class thereof), voting power, rights to profits or capital of the Company, and is not otherwise connected with the Company. |
| • | the ordinary shares are admitted to trading on AIM but are not listed on any market (with the term “listed” being construed in accordance with section 99A of the UK Finance Act 1986); and |
| • | AIM continues to be accepted as a “recognized growth market” (as construed in accordance with section 99A of the UK Finance Act 1986). |
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions and offshore transactions; |
| • | settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part; |
| • | broker-dealers may agree with the Registered Holders to sell a specified number of such shares at a stipulated price per share; |
| • | through the writing or settlement of options or other hedging transactions, whether such options are listed on an options exchange or otherwise; |
| • | a combination of any such methods of disposition; and |
| • | any other method permitted pursuant to applicable law. |
Item | | | Amount to be Paid |
| | | (In thousands) | |
SEC registration fee | | | $40 |
Nasdaq Global Select Market listing fee | | | 175 |
Printing and engraving expenses | | | 70 |
Legal fees and expenses | | | 1,315 |
Accounting fees and expenses | | | 581 |
Miscellaneous expenses | | | 5 |
Total | | | $2,186 |
| • | recognize or enforce judgments of United States courts obtained against it or its directors or officers predicated upon the civil liabilities provisions of the securities laws of the United States or any state in the United States; or |
| • | entertain original actions brought in England and Wales against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States. |
| • | the appropriate procedural requirements in England and Wales for recognition and enforcement of such a judgment were followed; |
| • | the relevant U.S. court had jurisdiction over the original proceedings according to English conflicts of laws principles at the time when proceedings were initiated; |
| • | the courts of England and Wales had jurisdiction over the matter on enforcement, and the defendant either submitted to such jurisdiction or was resident or carrying on business within such jurisdiction and was duly served with process; |
| • | the U.S. judgment was final and conclusive on the merits in the sense of being final and unalterable in the court that pronounced it and being for a definite sum of money; |
| • | the judgment given by the courts was not in respect of penalties, taxes, fines or similar fiscal or revenue obligations (or otherwise based on a U.S. law that an English court considers to relate to a penal, revenue or other public law); if, on the contrary, this were the case, an English court may sever the relevant part from an otherwise unenforceable judgment; |
| • | the judgment was not procured by fraud; |
| • | recognition or enforcement of the judgment in England and Wales would not be contrary to public policy or the Human Rights Act 1998; |
| • | the proceedings pursuant to which judgment was obtained were not contrary to natural justice or incompatible with the principles of notice in England and Wales; |
| • | the U.S. judgment was not obtained in breach of an anti-suit injunction or an applicable alternative dispute resolutions provision; |
| • | the U.S. judgment was not arrived at by doubling, trebling or otherwise multiplying a sum assessed as compensation for the loss or damages sustained and not being otherwise in breach of Section 5 of the UK Protection of Trading Interests Act 1980, or is a judgment based on measures designated by the Secretary of State under Section 1 of that Act; if, on the contrary, this were the case, an English court may sever the relevant part from an otherwise unenforceable judgment; |
| • | there is not a prior decision of an English court or the court of another jurisdiction on the issues in question between the same parties; and |
| • | the English enforcement proceedings were commenced within the limitation period. |
| | | Page | |
Financial Information of Amryt Pharma plc | | | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | Page | |
Financial Information of Aegerion Pharmaceuticals, Inc. | | | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | Page | |
Financial Information of Aegerion Pharmaceuticals, Inc. (Debtor-in-Possession) (Unaudited) | | | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | | | Year ended December 31, | |||||
| | | | | 2019 | | | 2018 | ||
| | | Note | | | US$’000 | ||||
Assets | | | | | | | |||
Non-current assets | | | | | | | |||
Goodwill | | | 12 | | | $30,813 | | | $— |
Intangible assets | | | 12 | | | 350,953 | | | 60,297 |
Property, plant and equipment | | | 13 | | | 3,036 | | | 1,098 |
Other non-current assets | | | | | 2,306 | | | 149 | |
Total non-current assets | | | | | 387,108 | | | 61,544 | |
Current assets | | | | | | | |||
Trade and other receivables | | | 14 | | | 36,387 | | | 5,927 |
Inventories | | | 15 | | | 43,623 | | | 2,137 |
Cash and cash equivalents, including restricted cash | | | 16 | | | 67,229 | | | 11,226 |
Total current assets | | | | | 147,239 | | | 19,290 | |
Total assets | | | | | 534,347 | | | 80,834 | |
| | | | | | | ||||
Equity and liabilities | | | | | | | |||
Equity attributable to owners of the parent | | | | | | | |||
Share capital | | | 17 | | | 11,918 | | | 25,198 |
Share premium | | | 17 | | | 2,422 | | | 68,233 |
Other reserves | | | | | 248,656 | | | (24,865) | |
Accumulated deficit | | | | | (133,674) | | | (72,263) | |
Total equity | | | | | 129,322 | | | (3,697) | |
Non-current liabilities | | | | | | | |||
Contingent consideration and contingent value rights | | | 6 | | | 102,461 | | | 47,316 |
Deferred tax liability | | | 18 | | | 18,921 | | | 6,161 |
Long term loan | | | 19 | | | 81,610 | | | 19,011 |
Convertible notes | | | 20 | | | 96,856 | | | — |
Provisions and other liabilities | | | 22 | | | 4,963 | | | — |
Total non-current liabilities | | | | | 304,811 | | | 72,488 | |
Current liabilities | | | | | | | |||
Trade and other payables | | | 21 | | | 76,596 | | | 12,043 |
Provisions and other liabilities | | | 22 | | | 23,618 | | | — |
Total current liabilities | | | | | 100,214 | | | 12,043 | |
Total liabilities | | | | | 405,025 | | | 84,531 | |
Total equity and liabilities | | | | | $534,347 | | | $80,834 | |
| | | | | Year ended December 31, | |||||
| | | | | 2019 | | | 2018 | ||
| | | Note | | | US$’000 | ||||
Revenue | | | 3 | | | $58,124 | | | $17,095 |
Cost of sales | | | 4 | | | (42,001) | | | (6,266) |
Gross profit | | | | | 16,123 | | | 10,829 | |
Research and development expenses | | | | | (15,827) | | | (10,703) | |
Selling, general and administrative expenses | | | | | (35,498) | | | (17,342) | |
Restructuring and acquisition costs | | | 6 | | | (13,038) | | | — |
Share based payment expenses | | | 5 | | | (841) | | | (821) |
Impairment charge | | | 12 | | | (4,670) | | | — |
Operating loss before finance expense | | | 7 | | | (53,751) | | | (18,037) |
Non-cash change in fair value of contingent consideration | | | 6 | | | (6,740) | | | (10,566) |
Non-cash contingent value rights finance expense | | | 6 | | | (1,511) | | | — |
Net finance expense — other | | | 9 | | | (4,759) | | | (1,841) |
Loss on ordinary activities before taxation | | | | | (66,761) | | | (30,444) | |
Tax credit/(charge) on loss on ordinary activities | | | 10 | | | 1,226 | | | (43) |
Loss for the year attributable to the equity holders of the Company | | | | | (65,535) | | | (30,487) | |
Exchange translation differences which may be reclassified through profit or loss | | | | | 781 | | | (77) | |
Total other comprehensive profit/(loss) | | | | | 781 | | | (77) | |
Total comprehensive loss for the year attributable to the equity holders of the Company | | | | | $(64,754) | | | $(30,564) | |
| | | | | | | ||||
Loss per share | | | | | | | |||
Loss per share - basic and diluted, attributable to ordinary equity holders of the parent (US$) | | | 11 | | | $(0.86) | | | $(0.67) |
| | | | | Year ended December 31, | |||||
| | | | | 2019 | | | 2018 | ||
| | | Note | | | US$’000 | ||||
Cash flows from operating activities | | | | | | | |||
Loss on ordinary activities after taxation | | | | | $(65,535) | | | $(30,487) | |
Net finance expense — other | | | 9 | | | 4,759 | | | 1,841 |
Depreciation and amortization | | | 12, 13 | | | 12,655 | | | 367 |
Amortization of inventory fair value step-up | | | 4, 7 | | | 10,367 | | | — |
Loss on disposal of fixed assets | | | | | 43 | | | — | |
Share based payment expenses | | | 5 | | | 841 | | | 821 |
Non-cash change in fair value of contingent consideration | | | 6 | | | 6,740 | | | 10,566 |
Non-cash contingent value rights finance expense | | | 6 | | | (1,511) | | | — |
Impairment of intangible asset | | | 12 | | | 4,670 | | | — |
Deferred taxation credit | | | | | (1,665) | | | — | |
Movements in working capital and other adjustments: | | | | | | | |||
Change in trade and other receivables | | | 14 | | | (4,732) | | | (532) |
Change in trade and other payables | | | 21 | | | (6,356) | | | 3,051 |
Change in provision and other liabilities | | | 22 | | | 4,922 | | | — |
Change in inventories | | | 15 | | | (5,894) | | | (928) |
Change in non-current assets | | | | | 177 | | | (153) | |
Net cash flow used in operating activities | | | | | (37,497) | | | (15,454) | |
| | | | | | | ||||
Cash flow from investing activities | | | | | | | |||
Net cash received on acquisition of subsidiary | | | 6 | | | 24,985 | | | — |
Payments for property, plant and equipment | | | 13 | | | (578) | | | (80) |
Payments for intangible assets | | | 12 | | | (74) | | | (155) |
Deposit interest received | | | | | 92 | | | 6 | |
Net cash flow from (used in) investing activities | | | | | 24,425 | | | (229) | |
| | | | | | | ||||
Cash flow from financing activities | | | | | | | |||
Proceeds from issue of equity instruments - net of expenses | | | 17 | | | 63,009 | | | — |
Proceeds from long term borrowings net of debt issue costs | | | 19 | | | 31,176 | | | 5,914 |
Repayment of long term debt | | | 19 | | | (21,990) | | | — |
Interest paid | | | 19 | | | (6,253) | | | (283) |
Payment of deferred consideration | | | | | — | | | (2,366) | |
Net cash flow from financing activities | | | | | 65,942 | | | 3,265 | |
| | | | | | | ||||
Exchange and other movements | | | | | 3,133 | | | (767) | |
Net change in cash and cash equivalents | | | | | 56,003 | | | (13,185) | |
Cash and cash equivalents at beginning of year | | | | | 11,226 | | | 24,411 | |
Restricted cash at end of year | | | 16 | | | 2,032 | | | 1,362 |
Cash at bank available on demand at end of year | | | 16 | | | 65,197 | | | 9,864 |
Total cash and cash equivalents at end of year | | | 16 | | | $67,229 | | | $11,226 |
| | | | | Share capital | | | Share premium | | | Warrant reserve | | | Treasury shares | | | Share based payment reserve | | | Merger reserve | | | Reverse acquisition reserve | | | Equity component of convertible notes | | | Other distributable reserves | | | Currency translation reserve | | | Accumulated deficit | | | Total | ||
| | | Note | | | US$'000 | ||||||||||||||||||||||||||||||||||
Balance at January 1, 2018 | | | | | $25,198 | | | $68,233 | | | $— | | | $— | | | $5,659 | | | $42,627 | | | $(73,914) | | | $— | | | $— | | | $26 | | | $(41,783) | | | $26,046 | |
Loss for the year | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (30,487) | | | (30,487) | |
Foreign exchange translation reserve | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (77) | | | — | | | (77) | |
Total comprehensive loss | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (77) | | | (30,487) | | | (30,564) | |
Transactions with owners | | | | | | | | | | | | | | | | | | | | | | | | | | | |||||||||||||
Share based payment expense | | | 5 | | | — | | | — | | | — | | | — | | | 821 | | | — | | | — | | | — | | | — | | | — | | | — | | | 821 |
Share based payment expense – Lapsed | | | | | — | | | — | | | — | | | — | | | (7) | | | — | | | — | | | — | | | — | | | — | | | 7 | | | — | |
Total transactions with owners | | | | | — | | | — | | | — | | | — | | | 814 | | | — | | | — | | | — | | | — | | | — | | | 7 | | | 821 | |
Balance at December 31, 2018 | | | | | 25,198 | | | 68,233 | | | — | | | — | | | 6,473 | | | 42,627 | | | (73,914) | | | — | | | — | | | (51) | | | (72,263) | | | (3,697) | |
Balance at January 1, 2019 | | | | | 25,198 | | | 68,233 | | | — | | | — | | | 6,473 | | | 42,627 | | | (73,914) | | | — | | | — | | | (51) | | | (72,263) | | | (3,697) | |
Loss for the year | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (65,535) | | | (65,535) | |
Foreign exchange translation reserve | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 781 | | | — | | | 781 | |
Total comprehensive loss | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 781 | | | (65,535) | | | (64,754) | |
Transactions with owners | | | | | | | | | | | | | | | | | | | | | | | | | | | |||||||||||||
Share consolidation | | | 17 | | | (21,262) | | | 21,262 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Issue of shares in August 2019 equity fund raise | | | 17 | | | 533 | | | 7,467 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 8,000 |
Issue costs associated with August 2019 equity fund raise | | | 17 | | | — | | | (1,886) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,886) |
Acquisition of subsidiary without a change of control | | | 17 | | | (495) | | | (3,726) | | | — | | | — | | | — | | | — | | | — | | | — | | | (2,969) | | | 7,190 | | | — | | | — |
Issue of shares and warrants in consideration of Aegerion Acquisition | | | 17 | | | 5,759 | | | 132,392 | | | 14,464 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 152,615 |
Issue of shares and warrants in equity fund raise | | | 17 | | | 2,059 | | | 47,338 | | | 10,603 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 60,000 |
Issue costs associated with September 2019 equity fund raise | | | 17 | | | — | | | (2,575) | | | (530) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (3,105) |
Issue of convertible notes | | | 20 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 29,210 | | | — | | | — | | | — | | | 29,210 |
Issue of contingent value rights | | | 6 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (47,902) | | | — | | | — | | | (47,902) |
Transfer to distributable reserves | | | 17 | | | — | | | (268,505) | | | — | | | — | | | — | | | — | | | — | | | — | | | 268,505 | | | — | | | — | | | — |
Treasury shares acquired in consideration for additional warrants | | | 17 | | | — | | | — | | | 7,534 | | | (7,534) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Issue of shares in exchange for warrants in December 2019 | | | 17 | | | 126 | | | 2,422 | | | (2,548) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — |
Share based payment expense | | | 5 | | | — | | | — | | | — | | | — | | | 841 | | | — | | | — | | | — | | | — | | | — | | | — | | | 841 |
Share based payment expense – Lapsed | | | | | — | | | — | | | — | | | — | | | (4,124) | | | — | | | — | | | — | | | — | | | — | | | 4,124 | | | — | |
Total transactions with owners | | | | | (13,280) | | | (65,811) | | | 29,523 | | | (7,534) | | | (3,283) | | | — | | | — | | | 29,210 | | | 217,634 | | | 7,190 | | | 4,124 | | | 197,773 | |
Balance at December 31, 2019 | | | | | $11,918 | | | $2,422 | | | $29,523 | | | $(7,534) | | | $3,190 | | | $42,627 | | | $(73,914) | | | $29,210 | | | $217,634 | | | $7,920 | | | $(133,674) | | | $129,322 | |
| • | assets and liabilities were translated from their Euro functional currency to U.S. dollars using the exchange rate in effect at the balance sheet date; |
| • | income and expenditure was translated at the average rate of exchange prevailing for the relevant period; and |
| • | opening shareholders’ equity at January 1, 2018 was translated at the historic rate on that date and any other movements in shareholders’ equity during the year have been translated using the rates prevailing on the date of the transaction. |
Foreign currency units to 1 US$ | | | € | | | £ | | | CHF | | | SEK | | | NOK | | | DKK |
Average period to December 31, 2019 | | | 0.8932 | | | 0.7836 | | | 0.9938 | | | 9.4533 | | | 8.7976 | | | 6.6690 |
At December 31, 2019 | | | 0.8929 | | | 0.7624 | | | 0.971 | | | 9.3282 | | | 8.8046 | | | 6.6698 |
Foreign currency units to 1 US$ | | | € | | | £ | | | CHF | | | SEK | | | NOK | | | DKK |
Average period to December 31, 2018 | | | 0.8455 | | | 0.7485 | | | 0.9763 | | | 8.6784 | | | 8.1289 | | | 6.2997 |
At December 31, 2018 | | | 0.8739 | | | 0.7833 | | | 0.9976 | | | 9.0855 | | | 8.5654 | | | 6.5700 |
| • | IAS 19 Employee Benefits (Amendment on Employee Benefits Plan, Amendment, Curtailment or Settlement) |
| • | IFRIC 23 Uncertainty over Income Tax Payments |
| • | IFRS 9 Prepayment Features with Negative Compensation (Amendment to IFRS 9) |
| • | IAS 28 Long-term Interests in Associates and Joint Ventures (Amendment to IAS 28) |
| • | Annual improvements to IFRS 2015-2017 Cycle |
| • | Definition of Business (Amendment to IFRS 3 Business Combination) |
| • | IFRS 17 Insurance Contracts |
| • | Definition of Material (Amendments to IAS 1 and 8) |
| • | Conceptual Framework for Financial Reporting |
| • | loan term and maturity; |
| • | repayment profile during the loan term other than interest; |
| • | level of loan security; and |
| • | principal amount of the loan. |
| • | estimates of revenues and operating profits related to the products or product candidates; |
| • | the probability of success for unapproved product candidates considering their stages of development; |
| • | the time and resources needed to complete the development and approval of product candidates; |
| • | projecting regulatory approvals; |
| • | developing appropriate discount rates and probability rates by project; and |
| • | tax implications, including the forecasted effective tax rate. |
| • | estimates of saleable inventory and non-saleable inventory, which was determined by a sales forecast and production timeline; and |
| • | expected selling price and estimated costs of disposal. |
| • | expected timing of achievement of the two milestones (U.S. Food and Drug Administration (“FDA”) approval and European Medicines Agency approval) related to AP101; |
| • | probabilities of achievements; |
| • | revenue forecast related to AP101; and |
| • | the appropriate discount rate selected to measure the risks inherent in the future cash flows. |
| • | completing the asset is technically feasible so that the asset will be available for use or sale; |
| • | there is an intention to complete the asset and use or sell it; |
| • | there is an ability to use or sell the asset; |
| • | the asset will generate probable future economic benefits and demonstrate the existence of a market or the usefulness of the asset if it is to be used internally; |
| • | adequate technical, financial and other resources are available to complete the development of the asset and to use or sell it; and |
| • | there is an ability to measure reliably the expenditure attributable to the intangible asset. |
| • | identifying the contract with a customer; |
| • | identifying the performance obligations; |
| • | determining the transaction price; |
| • | allocating the transaction price to the performance obligations; and |
| • | recognizing revenue when/as performance obligation(s) are satisfied. |
| • | the Group is primarily responsible for fulfilling the promise to provide the promised goods; |
| • | the Group bears the inventory risk before or after the goods have been ordered by the customer, during shipping or on return; |
| • | the Group has the discretion in establishing the selling price of the goods to customers. The distributors’ consideration in these contracts is either the margin fee or commission; and |
| • | the Group is exposed to the credit risk for the amounts receivable from the customers. |
| • | amortized cost; |
| • | fair value through profit or loss (“FVTPL”); and |
| • | fair value through other comprehensive income (“FVOCI”). |
| • | the Group’s business model for managing the financial asset; and |
| • | the contractual cash flow characteristic of the financial asset. |
| • | they are held within a business model whose objective is to hold the financial assets and collect its contractual cash flows; and |
| • | the contractual terms of the financial assets give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding. |
| • | the underlying asset is identified in the contract; and |
| • | the customer has both the right to direct the identified asset’s use and to obtain substantially all the economic benefits from that use. |
| • | fixed lease payments (including in-substance fixed payments), less any lease incentives receivable; |
| • | variable lease payments (linked to an index or interest rate); |
| • | expected payments under residual value guarantees; |
| • | the exercise price of purchase options, where exercise is reasonably certain; |
| • | lease payments in optional renewal periods, where exercise of extension options is reasonably certain; and |
| • | penalty payments for the termination of a lease, if the lease term reflects the exercise of the respective termination option. |
| • | the initial lease liability amount; |
| • | initial direct costs incurred when entering into the lease; |
| • | (lease) payments before commencement date of the respective lease; |
| • | an estimate of costs to dismantle and remove the underlying asset; and |
| • | less any lease incentives received. |
• | | | Property, plant and machinery | | | 5 to 15 years |
• | | | Office equipment | | | 3 to 10 years |
• | | | Software and hardware | | | 3-10 years |
• | | | Website development | | | 5-10 years |
| | | December 31, 2019 | ||||||||||
| | | U.S. | | | EMEA | | | Other | | | Total | |
| | | US$'000 | ||||||||||
Metreleptin | | | $14,944 | | | $8,048 | | | $2,096 | | | $25,088 |
Lomitapide | | | 10,616 | | | 18,985 | | | 2,659 | | | 32,260 |
Other | | | — | | | 671 | | | 105 | | | 776 |
Total revenue | | | $25,560 | | | $27,704 | | | $4,860 | | | $58,124 |
| | | December 31, 2018 | ||||||||||
| | | U.S. | | | EMEA | | | Other | | | Total | |
| | | US$'000 | ||||||||||
Metreleptin | | | $— | | | $— | | | $— | | | $— |
Lomitapide | | | — | | | 15,132 | | | 978 | | | 16,110 |
Other | | | — | | | 928 | | | 57 | | | 985 |
Total revenue | | | $— | | | $16,060 | | | $1,035 | | | $17,095 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Cost of product sales | | | $11,384 | | | $3,588 |
Amortization of acquired intangibles (see Note 12) | | | 11,831 | | | — |
Amortization of inventory fair value step-up (see Note 15) | | | 10,367 | | | — |
Royalty expenses | | | 8,419 | | | 2,678 |
Total cost of sales | | | $42,001 | | | $6,266 |
| | | Share Options | | | Warrants | |||||||
| | | Units | | | Weighted average exercise price (Sterling pence) | | | Units | | | Weighted average exercise price (Sterling pence) | |
Balance at January 1, 2018 (pre share consolidation) | | | 19,696,586 | | | 19.16p | | | 23,103,481 | | | 24.74p |
Balance at January 1, 2018 (restated for 6:1 share consolidation) | | | 3,282,764 | | | 114.96p | | | 3,850,580 | | | 148.44p |
Lapsed | | | (31,909) | | | 142.50p | | | (32,255) | | | 672.00p |
Outstanding at December 31, 2018 | | | 3,250,855 | | | 115.20p | | | 3,818,325 | | | 144.00p |
Exercisable at December 31, 2018 | | | 1,327,406 | | | 116.83p | | | 3,818,325 | | | 144.00p |
| | | Share Options | | | Warrants | |||||||
| | | Units | | | Weighted average exercise price (Sterling pence) | | | Units | | | Weighted average exercise price (Sterling pence) | |
Balance at January 1, 2019 (pre share consolidation) | | | 3,250,855 | | | 115.20p | | | 3,818,325 | | | 144.00p |
Granted | | | 11,330,641 | | | 117.01p | | | 18,841,378 | | | — |
Lapsed | | | (99,776) | | | 197.66p | | | (3,472,783) | | | 144.00p |
Exercised | | | — | | | — | | | (1,645,105) | | | — |
Outstanding at December 31, 2019 | | | 14,481,720 | | | 116.00p | | | 17,541,815 | | | 0.03p |
Exercisable at December 31, 2019 | | | 2,468,310 | | | 109.08p | | | 17,541,815 | | | 0.03p |
| | | 2019 Options Inputs | | | 2019 Warrant Inputs | | | 2018 Options Inputs | | | 2018 Warrant Inputs | |
Days to Expiration | | | 2,555 | | | — | | | — | | | — |
Volatility | | | 27% - 48% | | | — | | | — | | | — |
Risk free interest rate | | | 0.38% - 0.83% | | | — | | | — | | | — |
Share price at grant | | | 75.84p – 121.5p | | | — | | | — | | | — |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$’000 | ||||
Share option expense | | | $841 | | | $821 |
Total share option expense | | | $841 | | | $821 |
| | | Provisional Fair Value at date of acquisition | |
| | | US$’000 | |
Assets | | | |
Non-current assets | | | |
Property, plant and equipment | | | $276 |
Right of use assets | | | 924 |
Intangible Assets | | | 308,374 |
Other assets | | | 2,334 |
Total non-current assets | | | 311,908 |
| | | ||
Current assets | | | |
Cash and cash equivalents | | | 24,985 |
Trade and other receivables | | | 23,259 |
Inventory | | | 45,959 |
Prepaid expenses and other assets | | | 2,469 |
Total current assets | | | 96,672 |
| | | ||
Total assets | | | 408,580 |
| | | Provisional Fair Value at date of acquisition | |
| | | US$’000 | |
Current liabilities | | | |
Accounts payable | | | 5,137 |
Accrued liabilities | | | 64,088 |
Lease liabilities – current | | | 384 |
Provision for legal settlements – current | | | 14,916 |
Total current liabilities | | | 84,525 |
| | | ||
Non-current liabilities | | | |
Lease liabilities - long term | | | 538 |
Long term debt | | | 54,469 |
Convertible notes debt and equity components - long term | | | 125,000 |
Provision for legal settlements - long term | | | 7,821 |
Deferred tax liability | | | 14,425 |
Total non-current liabilities | | | 202,253 |
| | | ||
Total liabilities | | | 286,778 |
| | | ||
Total identifiable net assets at fair value | | | 121,802 |
Goodwill arising on acquisition | | | 30,813 |
Consideration | | | 152,615 |
| | | ||
Consideration | | | |
Issue of fully paid up ordinary shares and zero cost warrants | | | 152,615 |
Total consideration | | | $ 152,615 |
| • | The total CVR payable is up to US$85,000,000 |
| • | This is divided into three milestones which are related to the success of AP101 (the Group’s lead development asset, currently in Phase 3 clinical trials) |
| • | FDA approval |
| ○ | US$35,000,000 upon FDA approval |
| ○ | 100% of the amount due if approval is obtained before December 31, 2021, with a sliding scale on a linear basis to zero if before July 1, 2022 |
| • | EMA approval |
| ○ | US$15,000,000 upon EMA approval |
| ○ | 100% of the amount due if approval is obtained before December 31, 2021, with a sliding scale on a linear basis to zero if before July 1, 2022 |
| • | Revenue targets |
| ○ | US$35,000,000 upon AP101 revenues exceeding US$75,000,000 in any 12-month period prior to June 30, 2024 |
| • | Payment can at the Board’s discretion be in the form of either: |
| ○ | 120-day loan notes (effectively cash), or |
| ○ | Shares valued using the 30 day / 45-day VWAP. |
| • | Milestone payments of: |
| ○ | €10,000,000 on receipt of first marketing approval by the EMA of Episalvan, paid on the completion date (April 18, 2016); |
| ○ | Either (i) €5,000,000 once net ex-factory sales of Episalvan have been at least €100,000 or (ii) if no commercial sales are made within 24 months of EMA first marketing approval (being January 14, 2016), €2,000,000 24 months after receipt of such approval, which was paid in January 2018, and €3,000,000 following the first commercial sale; |
| ○ | €10,000,000 on receipt of marketing approval by the EMA or the FDA of a pharmaceutical product containing Betulin as its API for the treatment of EB; |
| ○ | €10,000,000 once net ex-factory sales/net revenue in any calendar year exceed €50,000,000; |
| ○ | €15,000,000 once net ex-factory sales/ net revenue in any calendar year exceed €100,000,000; |
| • | Cash consideration of €150,000, due and paid on the completion date (April 18, 2016); and |
| • | Royalties of 9% on sales of Episalvan products for 10 years from first commercial sale. |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Fees payable to the Group’s auditor and their associates | | | $611 | | | $106 |
Changes in inventory expensed (excluding fair value step-up) | | | 11,335 | | | 1,700 |
Amortization of inventory fair value step-up | | | 10,367 | | | — |
Research and development expenses | | | 15,827 | | | 10,703 |
Share based payments | | | 841 | | | 821 |
Pension costs | | | 769 | | | 583 |
Depreciation of property, plant and equipment | | | 698 | | | 317 |
Amortization of intangible assets | | | 11,957 | | | 50 |
Operating lease rentals | | | 170 | | | 300 |
Foreign exchange (gains) losses | | | $(3,750) | | | $223 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Wages and salaries | | | $17,268 | | | $7,249 |
Social security costs | | | 2,037 | | | 1,005 |
Pension costs - employees | | | 769 | | | 583 |
Directors' remuneration | | | 2,555 | | | 1,565 |
Shared based payments - directors | | | 510 | | | 175 |
Shared based payments - employees/consultants | | | 331 | | | 646 |
Total employee costs | | | $23,470 | | | $11,223 |
| | | December 31, 2019 | |||||||
Director | | | Number | | | Exercise price (Sterling pence) | | | Expiration Date |
Joseph Wiley | | | 6,437,460 | | | 0.76p - 121.50p | | | November 27, 2024 - November 4, 2026 |
| | | December 31, 2018 | |||||||
Director | | | Number | | | Exercise price (Sterling pence) | | | Expiration Date |
Joseph Wiley | | | 343,521 | | | 120.72p | | | November 27, 2024 |
Rory Nealon | | | 137,409 | | | 120.72p | | | November 27, 2024 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Interest on loans | | | $8,481 | | | $1,603 |
Charges and fees paid | | | 120 | | | 20 |
Interest received | | | (92) | | | (5) |
Foreign exchange losses (gains) | | | (3,750) | | | 223 |
Total | | | $4,759 | | | $1,841 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$’000 | ||||
Loss before tax | | | $(66,760) | | | $(30,444) |
Tax credit at Irish corporation tax rate of 12.5% | | | (8,345) | | | (3,806) |
Effect of: | | | | | ||
Movement in unrecognized deferred tax assets | | | 3,508 | | | 4,182 |
Permanent differences | | | 6,474 | | | 43 |
Differences in overseas taxation rates | | | (2,863) | | | (376) |
Total tax (credit)/charge on loss on ordinary activities | | | $(1,226) | | | $43 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$’000 | ||||
Ireland | | | $53,266 | | | $36,428 |
United States | | | 36,334 | | | — |
Germany | | | 26,228 | | | 27,236 |
United Kingdom | | | 16,828 | | | 7,812 |
ROW | | | — | | | 315 |
Total | | | $132,656 | | | $71,791 |
| | | Number of shares | | | Weighted average shares | |
December 31, 2019 | | | 154,498,887 | | | 75,871,562 |
December 31, 2018 | | | 274,817,283 | | | 274,817,283 |
December 31, 2018, as adjusted | | | 45,802,880 | | | 45,802,880 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
Loss after tax attributable to equity holders of the Company (US$'000) | | | $(65,535) | | | $(30,487) |
Weighted average number of ordinary shares in issue | | | 75,871,562 | | | 45,802,880 |
Fully diluted average number of ordinary shares in issue | | | 75,871,562 | | | 45,802,880 |
Basic and diluted loss per share (US$) | | | $(0.86) | | | $(0.67) |
| | | Developed technology - metreleptin | | | Developed technology - lomitapide | | | In process R&D | | | Other intangible assets | | | Total intangible assets | | | Goodwill | |
| | | US$'000 | ||||||||||||||||
Cost | | | | | | | | | | | | | ||||||
At January 1, 2018 | | | $— | | | $— | | | $62,498 | | | $114 | | | $62,612 | | | $— |
Additions | | | — | | | — | | | — | | | 155 | | | 155 | | | — |
Disposals | | | — | | | — | | | — | | | (1) | | | (1) | | | — |
Foreign exchange movement | | | — | | | — | | | (2,407) | | | (10) | | | (2,417) | | | — |
At December 31, 2018 | | | — | | | — | | | 60,091 | | | 258 | | | 60,349 | | | — |
Additions | | | — | | | — | | | — | | | 74 | | | 74 | | | — |
Acquired assets | | | 185,000 | | | 123,000 | | | — | | | 374 | | | 308,374 | | | 30,813 |
Impairment charge | | | — | | | — | | | (4,670) | | | — | | | (4,670) | | | — |
Foreign exchange movement | | | — | | | — | | | (1,160) | | | (5) | | | (1,165) | | | — |
At December 31, 2019 | | | $185,000 | | | $123,000 | | | $54,261 | | | $701 | | | $362,962 | | | $30,813 |
| | | | | | | | | | | | | |||||||
Accumulated amortization | | | | | | | | | | | | | ||||||
At January 1, 2018 | | | — | | | — | | | — | | | 5 | | | 5 | | | — |
Amortization charge | | | — | | | — | | | — | | | 50 | | | 50 | | | — |
Amortization charge on disposals | | | — | | | — | | | — | | | (1) | | | (1) | | | — |
Foreign exchange movement | | | — | | | — | | | — | | | (2) | | | (2) | | | — |
At December 31, 2018 | | | — | | | — | | | — | | | 52 | | | 52 | | | — |
Amortization charge | | | 7,688 | | | 4,143 | | | — | | | 126 | | | 11,957 | | | — |
Foreign exchange movement | | | — | | | — | | | — | | | — | | | — | | | — |
At December 31, 2019 | | | $7,688 | | | $4,143 | | | $— | | | $178 | | | $12,009 | | | $— |
Net book value | | | | | | | | | | | | | ||||||
At December 31, 2018 | | | $— | | | $— | | | $60,091 | | | $206 | | | $60,297 | | | $— |
At December 31, 2019 | | | $177,312 | | | $118,857 | | | $54,261 | | | $523 | | | $350,953 | | | $30,813 |
| | | Metreleptin | | | Lomitapide | |
Years Ending | | | US$'000 | |||
2020 | | | $28,831 | | | $15,537 |
2021 | | | 28,831 | | | 15,537 |
2022 | | | 28,831 | | | 15,537 |
2023 | | | 28,831 | | | 15,537 |
2024 | | | 28,831 | | | 15,537 |
Thereafter | | | 33,157 | | | 41,172 |
Total intangible assets subject to amortization | | | $177,312 | | | $118,857 |
| • | In the event that there was a variation of 10% in the assumed level of future growth in revenues, which would, in management’s view, represent a reasonably likely range of outcomes, this variation would not result in an impairment loss at December 31, 2019. |
| • | In the event there was a 10% increase in the discount rate used in the value in use model which would in management’s view represent a reasonably likely range of outcomes, this variation would not result in an impairment loss at December 31, 2019. |
| | | Property | | | Plant and Machinery | | | Office Equipment | | | Right-of-use Asset | | | Total | |
| | | US$'000 | |||||||||||||
Cost | | | | | | | | | | | |||||
At January 1, 2018 | | | $401 | | | $1,077 | | | $389 | | | $— | | | $1,867 |
Additions | | | — | | | 11 | | | 69 | | | — | | | 80 |
Disposals | | | — | | | (7) | | | (21) | | | — | | | (28) |
Foreign exchange movement | | | (15) | | | (42) | | | (16) | | | — | | | (73) |
At December 31, 2018 | | | $386 | | | $1,039 | | | $421 | | | $— | | | $1,846 |
| | | Property | | | Plant and Machinery | | | Office Equipment | | | Right-of-use Asset | | | Total | |
| | | US$'000 | |||||||||||||
Additions | | | 6 | | | 253 | | | 167 | | | 152 | | | 578 |
Impact of IFRS 16 adoption | | | — | | | — | | | — | | | 874 | | | 874 |
Acquired assets | | | — | | | 276 | | | — | | | 924 | | | 1,200 |
Disposals | | | — | | | (114) | | | (32) | | | — | | | (146) |
Foreign exchange movement | | | (9) | | | (22) | | | (9) | | | 50 | | | 10 |
At December 31, 2019 | | | $383 | | | $1,432 | | | $547 | | | $2,000 | | | $4,362 |
| | | Property | | | Plant and Machinery | | | Office Equipment | | | Right-of-use Asset | | | Total | |
| | | US$'000 | |||||||||||||
Accumulated Depreciation | | | | | | | | | | | |||||
At January 1, 2018 | | | $176 | | | $201 | | | $109 | | | $— | | | $486 |
Depreciation charge | | | 103 | | | 137 | | | 77 | | | — | | | 317 |
Depreciation charged on disposals | | | — | | | (7) | | | (21) | | | — | | | (28) |
Foreign exchange movement | | | (10) | | | (12) | | | (5) | | | — | | | (27) |
At December 31, 2018 | | | 269 | | | 319 | | | 160 | | | — | | | 748 |
Depreciation charge | | | 90 | | | 162 | | | 64 | | | 382 | | | 698 |
Depreciation charged on disposals | | | — | | | (71) | | | (32) | | | — | | | (103) |
Foreign exchange movement | | | (6) | | | (6) | | | (5) | | | — | | | (17) |
At December 31, 2019 | | | $353 | | | $404 | | | $187 | | | $382 | | | $1,326 |
Net book value | | | | | | | | | | | |||||
At December 31, 2018 | | | 117 | | | 720 | | | 261 | | | — | | | 1,098 |
At December 31, 2019 | | | $30 | | | $1,028 | | | $360 | | | $1,618 | | | $3,036 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Trade receivables | | | $28,607 | | | $3,572 |
Accrued income and other debtors | | | 5,934 | | | 2,326 |
VAT recoverable | | | 1,846 | | | 29 |
Trade and other receivables | | | $36,387 | | | $5,927 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Raw materials | | | $17,689 | | | $303 |
Work in progress | | | 2,488 | | | 782 |
Finished goods | | | 23,446 | | | 1,052 |
Inventories | | | $43,623 | | | $2,137 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Cash at bank available on demand | | | $65,197 | | | $9,864 |
Restricted cash | | | 2,032 | | | 1,362 |
Total cash and cash equivalents | | | $67,229 | | | $11,226 |
Date | | | Number of ordinary shares | | | Number of deferred shares | | | Total Share Capital US$'000 | | | Total Share Premium US$'000 |
At December 31, 2019 | | | 159,363,543 | | | — | | | $11,918 | | | $2,422 |
At December 31, 2018 | | | 274,817,283 | | | 43,171,134 | | | $25,198 | | | $68,233 |
| • | 77,027,423 ordinary shares and 8,065,000 warrants for a consideration of US$152,615,000 were issued as part of the Aegerion acquisition whereby the company acquired the entire share capital of Aegerion. |
| • | 27,541,944 ordinary shares and 5,911,722 warrants were issued as part of a US$60,000,000 fund raising. |
| • | Distribution of the share premium amount on November 6, 2019 of US$268,505,000. |
| • | A deemed distribution of US$47,902,000 arising from the issuance of CVRs. |
| • | A deemed distribution of US$2,969,000 arising from the scheme of arrangement in September 2019 whereby Amryt Pharma plc, which was incorporated in July 2019, became a 100% shareholder of Amryt Pharma Holdings Limited (formerly named Amryt Pharma plc) (the “Acquisition of subsidiary without a change of control”). |
| | | Total | |
| | | US$'000 | |
At January 1, 2018 | | | $6,161 |
Movement during the year | | | — |
At December 31, 2018 | | | 6,161 |
Net movement during the year | | | 12,760 |
At December 31, 2019 | | | $18,921 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Long term loan | | | $81,610 | | | $17,164 |
Long term loan interest | | | — | | | 1,847 |
Long term loan and interest | | | $81,610 | | | $19,011 |
| | | Total | |
Changes in long term loans from financing activities: | | | US$'000 |
At January 1, 2019 | | | $19,011 |
Cash-flows | | | |
Proceeds from loans and borrowings | | | 31,176 |
Repayment of loans and borrowings | | | (21,990) |
Liability related | | | |
Effect of changes in foreign exchange rates | | | 797 |
Acquired loans and borrowings | | | 54,469 |
Interest accrual | | | (1,853) |
At December 31, 2019 | | | $81,610 |
| | | December 31, 2019 | |
| | | US$'000 | |
Issuance of convertible notes | | | $125,000 |
Amount classified as equity | | | (29,210) |
Accreted interest | | | 1,066 |
Total convertible notes | | | $96,856 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Trade payables | | | $23,418 | | | $5,339 |
Accrued expenses | | | 52,382 | | | 6,204 |
Social security costs and other taxes | | | 796 | | | 500 |
Trade and other payables | | | $76,596 | | | $12,043 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Non-current liabilities | | | | | ||
Provisions and other liabilities | | | $3,910 | | | $— |
Leases due greater than 1 year | | | 1,053 | | | — |
| | | 4,963 | | | — | |
Current liabilities | | | | | ||
Provisions and other liabilities | | | 23,047 | | | — |
Leases due less than 1 year | | | 571 | | | — |
| | | 23,618 | | | — | |
Total provisions and other liabilities | | | $28,581 | | | $— |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Short-term employee benefits | | | $1,049 | | | $803 |
Performance related bonus | | | 1,286 | | | 420 |
Post-employment benefits | | | 86 | | | 76 |
Share-based compensation benefits | | | 510 | | | 175 |
Total compensation | | | $2,931 | | | $1,474 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
| | | US$'000 | ||||
Financial assets (all at amortized cost): | | | | | ||
Cash and cash equivalents | | | $67,229 | | | $11,226 |
Trade receivables | | | 28,607 | | | 3,572 |
Total financial assets | | | 95,836 | | | 14,798 |
| | | | | |||
Financial liabilities: | | | | | ||
At amortized cost | | | | | ||
Trade payables and accrued expenses | | | 75,800 | | | 11,543 |
Lease liabilities | | | 1,624 | | | — |
Other liabilities | | | 19,457 | | | — |
Convertible notes | | | 96,856 | | | — |
Long term loan | | | 81,610 | | | 19,011 |
Contingent value rights | | | 49,413 | | | — |
At fair value | | | | | ||
Contingent consideration | | | 53,048 | | | 47,316 |
Total financial liabilities | | | 377,808 | | | 77,870 |
Net | | | $(281,972) | | | $(63,072) |
| • | Level 1: fair value evaluations using prices listed on active markets (not adjusted) of identical assets or liabilities. |
| • | Level 2: fair value evaluations using input data for the asset or liability that are either directly observable (as prices) or indirectly observable (derived from prices), but which do not constitute listed prices pursuant to Level 1. |
| • | Level 3: fair value evaluations using input data for the asset or liability that are not based on observable market data (unobservable input data). |
| • | Contingent consideration relating to the acquisition of Amryt GmbH (see Note 6, Business combinations and asset acquisitions) that was measured at US$53,048,000 as at December 31, 2019 (2018: US$47,316,000). The fair value comprises royalty payments which was determined using probability weighted revenue forecasts and the fair value of the milestones payments which was determined using probability adjusted present values. It also included a revision to the discount rate used, and revenue and costs forecasts have been amended to reflect management’s current expectations. |
| • | An increase of 10% in estimated revenue forecasts would result in an increase to the fair value of US$3,710,000. A decrease would have the opposite effect. |
| • | A 5% increase in the discount factor used would result in a decrease to the fair value of US$9,761,000. A decrease of 5% would result in an increase to the fair value of US$13,312,000. |
| • | A six-month delay in the launch date for AP101 for EB would result in a decrease to the fair value of US$4,313,000. |
| | | Less than 1 month | | | Between 1 and 3 months | | | Between 3 and 6 months | | | Total | |
December 31, 2019 | | | US$'000 | |||||||||
Trade payables | | | $17,995 | | | $3,272 | | | $2,151 | | | $23,418 |
| | | Less than 1 month | | | Between 1 and 3 months | | | Between 3 and 6 months | | | Total | |
December 31, 2018 | | | US$'000 | |||||||||
Trade payables | | | $4,344 | | | $— | | | $995 | | | $5,339 |
| | | Less than 1 year | | | Between 1 and 3 years | | | Between 3 and 5 years | | | Greater than 5 years | | | Total | |
December 31, 2019 | | | US$'000 | ||||||||||||
Lease liabilities | | | $969 | | | $916 | | | $143 | | | $20 | | | $2,048 |
Other liabilities | | | 15,722 | | | 3,928 | | | — | | | — | | | 19,650 |
| | | $16,691 | | | $4,844 | | | $143 | | | $20 | | | $21,698 | |
| | | Less than 1 year | | | Between 1 and 3 years | | | Between 3 and 5 years | | | Greater than 5 years | | | Total | |
December 31, 2019 | | | US$'000 | ||||||||||||
Long term loan | | | $5,585 | | | $12,296 | | | $124,427 | | | $— | | | $142,308 |
Convertible notes | | | 6,372 | | | 12,500 | | | 12,500 | | | 128,125 | | | 159,497 |
| | | $11,957 | | | $24,796 | | | $136,927 | | | $128,125 | | | $301,805 | |
| | | Less than 1 year | | | Between 1 and 3 years | | | Between 3 and 5 years | | | Greater than 5 years | | | Total | |
December 31, 2018 | | | US$'000 | ||||||||||||
Long term loan | | | $— | | | $— | | | $19,358 | | | $— | | | $19,358 |
| | | Less than 1 year | | | Between 1 and 3 years | | | Between 3 and 5 years | | | Greater than 5 years | | | Total | |
December 31, 2019 | | | US$'000 | ||||||||||||
Contingent consideration and contingent value rights | | | $— | | | $99,559 | | | $27,998 | | | $— | | | $127,557 |
| | | Less than 1 year | | | Between 1 and 3 years | | | Between 3 and 5 years | | | Greater than 5 years | | | Total | |
December 31, 2018 | | | US$'000 | ||||||||||||
Contingent consideration and contingent value rights | | | $— | | | $14,875 | | | $28,607 | | | $— | | | $43,482 |
Subsidiary | | | Ownership | | | Activities | | | Company Number | | | Incorporation | | | 2019 % Holding | | | 2018 % Holding |
Amryt Pharma Holdings Ltd. | | | Direct | | | Holding company and management services | | | 5316808 | | | UK | | | 100 | | | 100 |
Amryt Pharmaceuticals DAC | | | Indirect | | | Holding company and management services | | | 566448 | | | Ireland | | | 100 | | | 100 |
Amryt Research Limited | | | Indirect | | | Pharmaceuticals R&D | | | 571411 | | | Ireland | | | 100 | | | 100 |
Amryt Endocrinology Limited | | | Indirect | | | Pharmaceuticals R&D | | | 572984 | | | Ireland | | | 100 | | | 100 |
Amryt Lipidology Limited | | | Indirect | | | Licensee for Lojuxta | | | 593833 | | | Ireland | | | 100 | | | 100 |
Amryt Genetics Limited | | | Indirect | | | Pharmaceutical R&D | | | 622577 | | | Ireland | | | 100 | | | 100 |
Amryt Pharma (UK) Limited | | | Indirect | | | Management services | | | 10463152 | | | UK | | | 100 | | | 100 |
Amryt Pharma France | | | Indirect | | | Dormant | | | 824 418 156 00017 | | | France | | | 100 | | | 100 |
Amryt Pharma Italy SRL | | | Indirect | | | Management services | | | 2109476 | | | Italy | | | 100 | | | 100 |
Amryt Pharma Spain SL | | | Indirect | | | Management services | | | B67130567 | | | Spain | | | 100 | | | 100 |
Amryt GmbH (previously Amryt AG) | | | Indirect | | | Product Sales and Pharmaceuticals R&D | | | HRB 711487 | | | Germany | | | 100 | | | 100 |
SomPharmaceuticals SA | | | Indirect | | | Pharmaceuticals R&D and management services | | | CHE-435.396.568 | | | Switzerland | | | 100 | | | 100 |
SomTherapeutics, Corp | | | Indirect | | | License holder | | | P14000071235 | | | USA | | | 100 | | | 100 |
Aegerion Pharmaceuticals, Inc. | | | Indirect | | | Holding company and management services | | | 3922075 | | | USA | | | 100 | | | Not applicable |
Aegerion International Ltd. | | | Indirect | | | Management services | | | 52048 | | | Bermuda | | | 100 | | | Not applicable |
Aegerion Securities Corporation | | | Indirect | | | Management services | | | 464215084 | | | USA | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals Holdings, Inc. | | | Indirect | | | Management services | | | 5213687 | | | USA | | | 100 | | | Not applicable |
Aegerion Argentina S.R.L. | | | Indirect | | | Management services | | | 901-709682-0 | | | Argentina | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals (Canada) Ltd. | | | Indirect | | | Management services | | | 85134 5132 RT0001 | | | Canada | | | 100 | | | Not applicable |
Aegerion Colombia S.A.S. | | | Indirect | | | Management services | | | R048196625 | | | Colombia | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals K.K. | | | Indirect | | | Management services | | | 0104-01-107816 | | | Japan | | | 100 | | | Not applicable |
Aegerion Brasil Comercio E Importacao De Medicamentos LTDA | | | Indirect | | | Management services | | | 3522602510-1 | | | Brazil | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals Ltd. | | | Indirect | | | Management services | | | 46134 | | | Bermuda | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals Limited | | | Indirect | | | Management services | | | 8114919 | | | UK | | | 100 | | | Not applicable |
Amryt Pharmaceuticals, SAS | | | Indirect | | | Management services | | | 534 195 59900012 | | | France | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals S.r.l. | | | Indirect | | | Management services | | | 1166250 | | | Italy | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals GmbH | | | Indirect | | | Management services | | | HRB 95895 | | | Germany | | | 100 | | | Not applicable |
Aegerion İlaç Ticaret Limited Şirketi | | | Indirect | | | Management services | | | 907292 | | | Turkey | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals SARL | | | Indirect | | | Management services | | | CHE-497.494.599 | | | Switzerland | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals B.V. | | | Indirect | | | Management services | | | 69859647 | | | Netherlands | | | 100 | | | Not applicable |
Aegerion Pharmaceuticals Spain, S.L. | | | Indirect | | | Management services | | | B88019161 | | | Spain | | | 100 | | | Not applicable |
Company | | | Registered Office Address |
Amryt Pharma Holdings Ltd | | | Dept 920a 196 High Road, Wood Green, London, United Kingdom, N22 8HH |
Amryt Pharmaceuticals DAC | | | 90 Harcourt Street, Dublin 2 |
Amryt Research Limited | | | 90 Harcourt Street, Dublin 2 |
Amryt Endocrinology Limited | | | 90 Harcourt Street, Dublin 2 |
Amryt Lipidology Limited | | | 90 Harcourt Street, Dublin 2 |
Amryt Genetics Limited | | | 90 Harcourt Street, Dublin 2 |
Amryt Pharma (UK) Limited | | | 3rd Floor 1 Ashley Road, Altrincham, Cheshire, United Kingdom, WA14 2DT |
Amryt Pharma France | | | 17 Avenue George V, 75008 Paris |
Amryt Pharma Italy SRL | | | Milano (MI)-Via Dell'Annunciata 23/4 |
Amryt Spain SL | | | Barcelona, calle Diputacio, number 260 |
Amryt GmbH (previously Amryt AG) | | | Streiflingsweg 11, 75223 Niefern-Öschelbronn |
SomPharmaceuticals SA | | | Bahnofstrasse 21, 6300 Zug |
SomTherapeutics, Corp | | | 3795 Coventry Lane, Boca Raton, FL 33496 |
Aegerion Pharmaceuticals Inc. | | | 245 First Street, Riverview II, 18th Floor, Cambridge, MA 02142 |
Aegerion International Ltd. | | | Clarendon House, 2 Church Street, Hamilton, HM11 |
Aegerion Securities Corporation | | | 245 First Street, Riverview II, 18th Floor, Cambridge, MA 02142 |
Aegerion Pharmaceuticals Holdings, Inc. | | | 245 First Street, Riverview II, 18th Floor, Cambridge, MA 02142 |
Aegerion Argentina S.R.L. | | | Avda. Camacua 421, Suite 102, Olivos, Vicente Lopez, 1636 |
Aegerion Pharmaceuticals Canada (Ltd). | | | 5300 Commerce Court West, 199 Bay Street, Toronto, ON M5L 1B9 |
Aegerion Colombia S.A.S. | | | CR 12 89 33 P 5, Bogota DC, Bogota 110111 |
Aegerion Pharmaceuticals K.K. | | | 12F, Ark Mori Building, 1-12-32 Akasaka, Minato-ku, Tokyo |
Aegerion Brazil Comercio E Importacao De Medicamentos. LTDA | | | Rua Joseefina, 200-Guarulhos City, Sao Paulo |
Aegerion Pharmaceuticals Ltd. | | | Clarendon House, 2 Church Street, Hamilton, HM11 |
Aegerion Pharmaceuticals Limited | | | Royal Albert House, Sheet Street, Windsor, UK SL4 1BE |
Amryt Pharmaceuticals, SAS | | | 235, Avenue Le Jour se Leve, Boulogne-Billancourt, 92 100 |
Aegerion Pharmaceuticals, S.r.l. | | | Viale Abruzzi n. 94, Milano, 20131 |
Aegerion Pharmaceuticals GmbH | | | Maximilianstrasse 35A, Munich, Germany, 80539 |
Aegerion ILac Ticaret Limited Sirketi | | | Orjin Maslak, Eski Buyukdere Caddesi No: 27 K:11, Maslak, Istanbul, 34485 |
Aegerion Pharmaceuticals SARL | | | Rue de Rive 5, Nyon, Switzerland 1260 |
Aegerion Pharmaceuticals B.V. | | | Atrium Building, 8th Floor, Strawinskylaan 3127, 8e verdieping, Amsterdam |
Aegerion Pharmaceuticals Spain, S.L. | | | Calle Josep Coroleu, 83 2-2, Vilanova I la Geltru, Barcelona 08800 |
| | | | | As at, | |||||
| | | | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | ||
| | | Note | | | US$’000 | ||||
Assets | | | | | | | |||
Non-current assets | | | | | | | |||
Goodwill | | | 7 | | | $30,813 | | | $30,813 |
Intangible assets | | | 7 | | | 339,094 | | | 350,953 |
Property, plant and equipment | | | | | 2,862 | | | 3,036 | |
Other non-current assets | | | | | 2,310 | | | 2,306 | |
Total non-current assets | | | | | 375,079 | | | 387,108 | |
Current assets | | | | | | | |||
Trade and other receivables | | | 8 | | | 41,179 | | | 36,387 |
Inventories | | | | | 33,904 | | | 43,623 | |
Cash and cash equivalents, including restricted cash | | | 9 | | | 68,067 | | | 67,229 |
Total current assets | | | | | 143,150 | | | 147,239 | |
Total assets | | | | | 518,229 | | | 534,347 | |
| | | | | | | ||||
Equity and liabilities | | | | | | | |||
Equity attributable to owners of the parent | | | | | | | |||
Share capital | | | 10 | | | 11,918 | | | 11,918 |
Share premium | | | 10 | | | 2,422 | | | 2,422 |
Other reserves | | | | | 249,386 | | | 248,656 | |
Accumulated deficit | | | | | (162,569) | | | (133,674) | |
Total equity | | | | | 101,157 | | | 129,322 | |
Non-current liabilities | | | | | | | |||
Contingent consideration and contingent value rights | | | 5 | | | 106,145 | | | 102,461 |
Deferred tax liability | | | | | 17,345 | | | 18,921 | |
Long term loan | | | 11 | | | 82,989 | | | 81,610 |
Convertible notes | | | 12 | | | 97,872 | | | 96,856 |
Provisions and other liabilities | | | 13 | | | 1,014 | | | 4,963 |
Total non-current liabilities | | | | | 305,365 | | | 304,811 | |
Current liabilities | | | | | | | |||
Trade and other payables | | | | | 87,575 | | | 76,596 | |
Provisions and other liabilities | | | 13 | | | 24,132 | | | 23,618 |
Total current liabilities | | | | | 111,707 | | | 100,214 | |
Total liabilities | | | | | 417,072 | | | 405,025 | |
Total equity and liabilities | | | | | $518,229 | | | $534,347 | |
| | | | | Three months ended March 31, | |||||
| | | | | 2020 (unaudited) | | | 2019 (unaudited) | ||
| | | Note | | | US$’000 | ||||
Revenue | | | 3 | | | $44,574 | | | $4,542 |
Cost of sales | | | | | (32,620) | | | (1,830) | |
Gross profit | | | | | 11,954 | | | 2,712 | |
Research and development expenses | | | | | (8,934) | | | (1,505) | |
Selling, general and administrative expenses | | | | | (18,406) | | | (3,896) | |
Acquisition and severance related costs | | | | | (853) | | | — | |
Share based payment expenses | | | 4 | | | (745) | | | (91) |
Operating loss before finance expense | | | | | (16,984) | | | (2,780) | |
Non-cash change in fair value of contingent consideration | | | 5 | | | (2,906) | | | (1,938) |
Non-cash contingent value rights finance expense | | | 5 | | | (1,448) | | | — |
Net finance expense - other | | | | | (9,416) | | | (661) | |
Loss on ordinary activities before taxation | | | | | (30,754) | | | (5,379) | |
Tax credit/(charge) on loss on ordinary activities | | | | | 1,857 | | | (6) | |
Loss for the period attributable to the equity holders of the Company | | | | | (28,897) | | | (5,385) | |
Exchange translation differences which may be reclassified through profit or loss | | | | | (13) | | | 80 | |
Total other comprehensive (loss)/income | | | | | (13) | | | 80 | |
Total comprehensive loss for the period attributable to the equity holders of the Company | | | | | $(28,910) | | | $(5,305) | |
| | | | | | | ||||
Loss per share | | | | | | | |||
Loss per share - basic and diluted, attributable to ordinary equity holders of the parent (US$) | | | 6 | | | $(0.19) | | | $(0.12) |
| | | | | Three months ended March 31, | |||||
| | | | | 2020 (unaudited) | | | 2019 (unaudited) | ||
| | | Note | | | US$’000 | ||||
Cash flows from operating activities | | | | | | | |||
Loss on ordinary activities after taxation | | | | | $(28,897) | | | $(5,385) | |
Net finance expense - other | | | | | 9,416 | | | 661 | |
Depreciation and amortization | | | | | 11,241 | | | 91 | |
Amortization of inventory fair value step-up | | | | | 9,503 | | | — | |
Share based payment expenses | | | 4 | | | 745 | | | 91 |
Non-cash change in fair value of contingent consideration | | | 5 | | | 2,906 | | | 1,938 |
Non-cash contingent value rights finance expense | | | 5 | | | 1,148 | | | |
Deferred taxation credit | | | | | (1,576) | | | — | |
Movements in working capital and other adjustments: | | | | | | | |||
Change in trade and other receivables | | | | | (4,792) | | | (754) | |
Change in trade and other payables | | | | | 9,416 | | | (1,902) | |
Change in provision and other liabilities | | | 13 | | | (3,435) | | | — |
Change in inventories | | | | | 216 | | | (255) | |
Change in non-current assets | | | | | (4) | | | 74 | |
Net cash flow from (used in) operating activities | | | | | 6,187 | | | (5,441) | |
| | | | | | | ||||
Cash flow from investing activities | | | | | | | |||
Payments for property, plant and equipment | | | | | (79) | | | (4) | |
Deposit interest received | | | | | 66 | | | — | |
Net cash used in investing activities | | | | | (13) | | | (4) | |
| | | | | | | ||||
Cash flow from financing activities | | | | | | | |||
Increase in long term debt | | | | | — | | | 5,679 | |
Interest paid | | | | | (1,506) | | | (4) | |
Net cash (used in) flow from financing activities | | | | | (1,506) | | | 5,675 | |
| | | | | | | ||||
Exchange and other movements | | | | | (3,830) | | | (74) | |
Net change in cash and cash equivalents | | | | | 838 | | | 156 | |
Cash and cash equivalents at beginning of the period | | | | | 67,229 | | | 11,226 | |
Restricted cash at end of the period | | | | | 1,093 | | | — | |
Cash at bank available on demand at end of the period | | | | | 66,974 | | | 11,382 | |
Total cash and cash equivalents at end of the period | | | | | $68,067 | | | $11,382 | |
| | | | | Share capital | | | Share premium | | | Warrant reserve | | | Treasury shares | | | Share based payment reserve | | | Merger reserve | | | Reverse acquisition reserve | | | Equity component of convertible notes | | | Other distributable reserves | | | Currency translation reserve | | | Accumulated deficit | | | Total | ||
| | | Note | | | US$’000 | ||||||||||||||||||||||||||||||||||
Balance at January 1, 2020 (audited) | | | | | $11,918 | | | $2,422 | | | $29,523 | | | $(7,534) | | | $3,190 | | | $42,627 | | | $(73,914) | | | $29,210 | | | $217,634 | | | $7,920 | | | $(133,674) | | | $129,322 | |
Loss for the period | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (28,897) | | | (28,897) | |
Foreign exchange translation reserve | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (13) | | | — | | | (13) | |
Total comprehensive loss | | | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (13) | | | (28,897) | | | (28,910) | |
Transactions with owners | | | | | | | | | | | | | | | | | | | | | | | | | | | |||||||||||||
Share based payment expense | | | 4 | | | — | | | — | | | — | | | — | | | 745 | | | — | | | — | | | — | | | — | | | — | | | — | | | 745 |
Share based payment expense – Lapsed | | | | | — | | | — | | | — | | | — | | | (2) | | | — | | | — | | | — | | | — | | | — | | | 2 | | | — | |
Total transactions with owners | | | | | — | | | — | | | — | | | — | | | 743 | | | — | | | — | | | — | | | — | | | — | | | 2 | | | 745 | |
Balance at March 31, 2020 (unaudited) | | | | | $11,918 | | | $2,422 | ��� | | $29,523 | | | $(7,534) | | | $3,933 | | | $42,627 | | | $(73,914) | | | $29,210 | | | $217,634 | | | $7,907 | | | $(162,569) | | | $101,157 | |
| | | | | Share capital | | | Share premium | | | Share based payment reserve | | | Merger reserve | | | Reverse acquisition reserve | | | Currency translation reserve | | | Accumulated deficit | | | Total | ||
| | | Note | | | US$’000 | ||||||||||||||||||||||
Balance at January 1, 2019 (audited) | | | | | $25,198 | | | $68,233 | | | $6,473 | | | $42,627 | | | $(73,914) | | | $(51) | | | $(72,263) | | | $(3,697) | |
Loss for the period | | | | | — | | | — | | | — | | | — | | | — | | | — | | | (5,385) | | | (5,385) | |
Foreign exchange translation reserve | | | | | — | | | — | | | — | | | — | | | — | | | 80 | | | — | | | 80 | |
Total comprehensive loss | | | | | — | | | — | | | — | | | — | | | — | | | 80 | | | (5,385) | | | (5,305) | |
Transactions with owners | | | | | | | | | | | | | | | | | | | |||||||||
Share based payment expense | | | 4 | | | — | | | — | | | 91 | | | — | | | — | | | — | | | — | | | 91 |
Total transactions with owners | | | | | — | | | — | | | 91 | | | — | | | — | | | — | | | — | | | 91 | |
Balance at March 31, 2019 (unaudited) | | | | | $25,198 | | | $68,233 | | | $6,564 | | | $42,627 | | | $(73,914) | | | $29 | | | $(77,648) | | | $8,911 | |
Foreign currency units to 1 US$ | | | € | | | £ | | | CHF | | | SEK | | | NOK | | | DKK |
Average period to March 31, 2019 (unaudited) | | | 0.8804 | | | 0.7683 | | | 0.9967 | | | 9.1704 | | | 8.5802 | | | 6.5711 |
At March 31, 2019 (unaudited) | | | 0.8915 | | | 0.7673 | | | 0.9953 | | | 9.2979 | | | 8.6271 | | | 6.6550 |
Foreign currency units to 1 US$ | | | € | | | £ | | | CHF | | | SEK | | | NOK | | | DKK |
Average period to December 31, 2019 (audited) | | | 0.8932 | | | 0.7836 | | | 0.9938 | | | 9.4533 | | | 8.7976 | | | 6.6690 |
At December 31, 2019 (audited) | | | 0.8929 | | | 0.7624 | | | 0.971 | | | 9.3282 | | | 8.8046 | | | 6.6698 |
Foreign currency units to 1 US$ | | | € | | | £ | | | CHF | | | SEK | | | NOK | | | DKK |
Average period to March 31, 2020 (unaudited) | | | 0.9068 | | | 0.7809 | | | 0.9679 | | | 9.6618 | | | 9.4731 | | | 6.7750 |
At March 31, 2020 (unaudited) | | | 0.9043 | | | 0.8068 | | | 0.9570 | | | 9.9977 | | | 10.5721 | | | 6.7517 |
| | | Three months ended March 31, 2020 (unaudited) | ||||||||||
| | | U.S. | | | EMEA | | | Other | | | Total | |
| | | US$’000 | ||||||||||
Metreleptin | | | $14,914 | | | $8,628 | | | $3,385 | | | $26,927 |
Lomitapide | | | 9,470 | | | 5,233 | | | 2,718 | | | 17,421 |
Other | | | — | | | 226 | | | — | | | 226 |
Total revenue | | | $24,384 | | | $14,087 | | | $6,103 | | | $44,574 |
| | | Three months ended March 31, 2019 (unaudited) | ||||||||||
| | | U.S. | | | EMEA | | | Other | | | Total | |
| | | US$’000 | ||||||||||
Metreleptin | | | $— | | | $— | | | $— | | | $— |
Lomitapide | | | — | | | 4,419 | | | — | | | 4,419 |
Other | | | — | | | 123 | | | — | | | 123 |
Total revenue | | | $— | | | $4,542 | | | $— | | | $4,542 |
| | | Share Options | | | Warrants | |||||||
| | | Units | | | Weighted average exercise price (Sterling pence) | | | Units | | | Weighted average exercise price (Sterling pence) | |
Balance at January 1, 2019 (restated for 6:1 share consolidation) | | | 3,250,855 | | | 115.20p | | | 3,818,325 | | | 144.00p |
Granted | | | 11,330,641 | | | 117.01p | | | 18,841,378 | | | — |
Lapsed | | | (99,776) | | | 197.66p | | | (3,472,783) | | | 144.00p |
Exercised | | | — | | | — | | | (1,645,105) | | | — |
Outstanding at December 31, 2019 (audited) (unaudited) | | | 14,481,720 | | | 116.00p | | | 17,541,815 | | | 0.03p |
Exercisable at December 31, 2019 (audited) | | | 2,468,310 | | | 109.08p | | | 17,541,815 | | | 0.03p |
| | | | | | | | | |||||
Balance at January 1, 2020 | | | 14,481,720 | | | 116.00p | | | 17,541,815 | | | 0.03p |
Granted | | | 2,687,000 | | | 123.50p | | | — | | | — |
Lapsed | | | (14,166) | | | 75.84p | | | — | | | — |
Exercised | | | — | | | — | | | — | | | — |
Outstanding at March 31, 2020 (unaudited) | | | 17,154,554 | | | 117.21p | | | 17,541,815 | | | 0.03p |
Exercisable at March 31, 2020 (unaudited) | | | 2,712,679 | | | 109.25p | | | 17,541,815 | | | 0.03p |
| | | March 31, 2020 Options Inputs (unaudited) | | | March 31, 2020 Warrant Inputs (unaudited) | | | December 31, 2019 Options Inputs (audited) | | | December 31, 2019 Warrant Inputs (audited) | |
Days to Expiration | | | 2,555 | | | — | | | 2,555 | | | — |
Volatility | | | 33% | | | — | | | 27% – 48% | | | — |
Risk free interest rate | | | 0.46% | | | — | | | 0.38% – 0.83% | | | — |
Share price at grant | | | 123.5p | | | — | | | 75.84p – 121.5p | | | — |
| | | Three months ended March 31, | ||||
| | | 2020 (unaudited) | | | 2019 (unaudited) | |
| | | US$’000 | ||||
Share option expense | | | $745 | | | $91 |
Total share option expense | | | $745 | | | $91 |
| • | The total CVR payable is up to US$85,000,000 |
| • | This is divided into three milestones which are related to the success of AP101 (the Group’s lead development asset, currently in Phase 3 clinical trials) |
| • | FDA approval |
| ○ | US$35,000,000 upon FDA approval |
| ○ | 100% of the amount due if approval is obtained before December 31, 2021, with a sliding scale on a linear basis to zero if before July 1, 2022 |
| • | EMA approval |
| ○ | US$15,000,000 upon EMA approval |
| ○ | 100% of the amount due if approval is obtained before December 31, 2021, with a sliding scale on a linear basis to zero if before July 1, 2022 |
| • | Revenue targets |
| ○ | US$35,000,000 upon AP101 revenues exceeding US$75,000,000 in any 12-month period prior to June 30, 2024 |
| • | Payment can, at the Board’s discretion, be in the form of either: |
| ○ | 120-day loan notes (effectively cash), or |
| ○ | Shares valued using the 30 day / 45-day VWAP. |
| • | Milestone payments of: |
| ○ | €10,000,000 on receipt of first marketing approval by the EMA of Episalvan, paid on the completion date (April 18, 2016); |
| ○ | Either (i) €5,000,000 once net ex-factory sales of Episalvan have been at least €100,000 or (ii) if no commercial sales are made within 24 months of EMA first marketing approval (being January 14, 2016), €2,000,000 24 months after receipt of such approval, which was paid in January 2018, and €3,000,000 following the first commercial sale; |
| ○ | €10,000,000 on receipt of marketing approval by the EMA or FDA of a pharmaceutical product containing Betulin as its API for the treatment of EB; |
| ○ | €10,000,000 once net ex-factory sales/net revenue in any calendar year exceed €50,000,000; |
| ○ | €15,000,000 once net ex-factory sales/ net revenue in any calendar year exceed €100,000,000; |
| • | Cash consideration of €150,000, due and paid on the completion date (April 18, 2016); and |
| • | Royalties of 9% on sales of Episalvan products for 10 years from first commercial sale; |
| | | Number of shares | | | Weighted average shares | |
March 31, 2020 (unaudited) | | | 154,498,887 | | | 154,498,887 |
March 31, 2019 (unaudited) | | | 274,817,283 | | | 274,817,283 |
March 31, 2019, as adjusted (unaudited) | | | 45,802,880 | | | 45,802,880 |
| | | Three months ended March 31, | ||||
| | | 2020 (unaudited) | | | 2019 (unaudited) | |
Loss after tax attributable to equity holders of the Company (US$’000) | | | $(28,897) | | | $(5,385) |
Weighted average number of ordinary shares in issue | | | 154,498,887 | | | 45,802,880 |
Fully diluted average number of ordinary shares in issue | | | 154,498,887 | | | 45,802,880 |
Basic and diluted loss per share (US$) | | | $(0.19) | | | $(0.12) |
| | | Developed technology - metreleptin | | | Developed technology - lomitapide | | | In process R&D | | | Other intangible assets | | | Total intangible assets | | | Goodwill | | |||
| | | US$’000 | |||||||||||||||||||
Cost | | | | | | | | | | | | | | ||||||||
At January 1, 2019 | | | | ||||||||||||||||||
(audited) | | | $— | | | $— | | | $60,091 | | | $258 | | | $60,349 | | | $— | | ||
Additions | | | — | | | — | | | — | | | 74 | | | 74 | | | — | | ||
Acquired assets | | | 185,000 | | | 123,000 | | | — | | | 374 | | | 308,374 | | | 30,813 | | ||
Impairment charge | | | — | | | — | | | (4,670) | | | — | | | (4,670) | | | — | | ||
Foreign exchange movement | | | — | | | — | | | (1,160) | | | (5) | | | (1,165) | | | — | | ||
At December 31, 2019 (audited) | | | 185,000 | | | 123,000 | | | 54,261 | | | 701 | | | 362,962 | | | 30,813 | | ||
Foreign exchange movement | | | — | | | — | | | (685) | | | (11) | | | (696) | | | — | | ||
At March 31, 2020 (unaudited) | | | $185,000 | | | $123,000 | | | $53,576 | | | $690 | | | $362,266 | | | $30,813 | | ||
| | | | | | | | | | | | | | |||||||||
Accumulated amortization | | | | | | | | | | | | | | ||||||||
At January 1, 2019 (audited) | | | — | | | — | | | — | | | 52 | | | 52 | | | — | | ||
Amortization charge | | | 7,688 | | | 4,143 | | | — | | | 126 | | | 11,957 | | | — | | ||
Foreign exchange movement | | | — | | | — | | | — | | | — | | | — | | | — | | ||
| | | Developed technology - metreleptin | | | Developed technology - lomitapide | | | In process R&D | | | Other intangible assets | | | Total intangible assets | | | Goodwill | | |||
| | | US$’000 | |||||||||||||||||||
At December 31, 2019 (audited) | | | 7,688 | | | 4,143 | | | — | | | 178 | | | 12,009 | | | — | | ||
Amortization charge | | | 7,208 | | | 3,884 | | | — | | | 68 | | | 11,160 | | | — | | ||
Foreign exchange movement | | | — | | | — | | | — | | | 3 | | | 3 | | | — | | ||
At March 31, 2020 (unaudited) | | | $14,896 | | | $8,027 | | | $— | | | $249 | | | $23,172 | | | $— | | ||
| | | | | | | | | | | | | | |||||||||
Net book value | | | | | | | | | | | | | | ||||||||
At December 31, 2019 (audited) | | | $177,312 | | | $118,857 | | | $54,261 | | | $523 | | | $350,953 | | | $30,813 | | ||
At March 31, 2020 (unaudited) | | | $170,104 | | | $114,973 | | | $53,576 | | | $441 | | | $339,094 | | | $30,813 | | ||
| | | As at | | ||||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | | |||
| | | US$’000 | |||||||
Trade receivables | | | $32,276 | | | $28,607 | | ||
Accrued income and other debtors | | | 5,556 | | | 5,934 | | ||
VAT recoverable | | | 3,347 | | | 1,846 | | ||
Trade and other receivables | | | $41,179 | | | $36,387 | | ||
| | | As at | ||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | |
| | | US$’000 | ||||
Cash at bank available on demand | | | $66,974 | | | $65,197 |
Restricted cash | | | 1,093 | | | 2,032 |
Total cash and cash equivalents | | | $68,067 | | | $67,229 |
Date | | | Number of ordinary shares | | | Total Share Capital US$’000 | | | Total Share Premium US$’000 |
At March 31, 2020 (unaudited) | | | 159,363,543 | | | $11,918 | | | $2,422 |
At December 31, 2019 (audited) | | | 159,363,543 | | | $11,918 | | | $2,422 |
| • | Distribution of the share premium amount on November 6, 2019 of US$268,505,000. |
| • | A deemed distribution of US$47,902,000 arising from the issuance of CVRs. |
| • | A deemed distribution of US$2,969,000 arising from the scheme of arrangement in September 2019 whereby Amryt Pharma plc, which was incorporated in July 2019, became a 100% shareholder of Amryt Pharma Holdings Limited (formerly named Amryt Pharma plc) (the “Acquisition of subsidiary without a change of control”). |
| | | As at | ||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | |
| | | US$’000 | ||||
Long term loan principal | | | $81,021 | | | 81,021 |
Accrued unpaid interest | | | 2,789 | | | 1,435 |
Unamortized debt issuance costs | | | (821) | | | (846) |
Long term loan | | | $82,989 | | | $81,610 |
| | | As at | ||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | |
| | | US$’000 | ||||
Issuance of convertible notes | | | $125,000 | | | $125,000 |
Amount classified as equity | | | (29,210) | | | (29,210) |
Accreted interest | | | 2,082 | | | 1,066 |
Total convertible notes | | | $97,872 | | | $96,856 |
| | | As at | ||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | |
| | | US$’000 | ||||
Non-current liabilities | | | | | ||
Provisions and other liabilities | | | $— | | | $3,910 |
Leases due greater than 1 year | | | 1,014 | | | 1,053 |
| | | 1,014 | | | 4,963 | |
Current liabilities | | | | | ||
Provisions and other liabilities | | | 23,670 | | | 23,047 |
Leases due less than 1 year | | | 462 | | | 571 |
| | | 24,132 | | | 23,618 | |
Total provisions and other liabilities | | | $25,146 | | | $28,581 |
| | | As at | ||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | |
| | | US$’000 | ||||
Financial assets (all at amortized cost): | | | | | ||
Cash and cash equivalents | | | $68,067 | | | $67,229 |
Trade receivables | | | 32,276 | | | 28,607 |
Total financial assets | | | 100,343 | | | 95,836 |
| | | As at | ||||
| | | March 31, 2020 (unaudited) | | | December 31, 2019 (audited) | |
| | | US$’000 | ||||
Financial liabilities: | | | | | ||
At amortized cost | | | | | ||
Trade payables and accrued expenses | | | 87,460 | | | 75,800 |
Lease liabilities | | | 1,476 | | | 1,624 |
Other liabilities | | | 16,169 | | | 19,457 |
Convertible notes | | | 97,872 | | | 96,856 |
Long term loan | | | 82,989 | | | 81,610 |
Contingent value rights | | | 50,861 | | | 49,413 |
At fair value | | | | | ||
Contingent consideration | | | 55,284 | | | 53,048 |
Total financial liabilities | | | 392,111 | | | 377,808 |
Net | | | $(291,768) | | | $(281,972) |
| • | Level 1: fair value evaluations using prices listed on active markets (not adjusted) of identical assets or liabilities. |
| • | Level 2: fair value evaluations using input data for the asset or liability that are either directly observable (as prices) or indirectly observable (derived from prices), but which do not constitute listed prices pursuant to Level 1. |
| • | Level 3: fair value evaluations using input data for the asset or liability that are not based on observable market data (unobservable input data). |
| • | Contingent consideration relating to the acquisition of Amryt GmbH (see Note 5, Business combinations and asset acquisitions) that was measured at US$55,284,000 as at March 31, 2020 (December 31, 2019: US$53,048,000). The fair value comprises royalty payments which was determined using probability weighted revenue forecasts and the fair value of the milestones payments which was determined using probability adjusted present values. |
| • | An increase of 10% in estimated revenue forecasts would result in an increase to the fair value of US$3,900,000. A decrease would have the opposite effect. |
| • | A 5% increase in the discount factor used would result in a decrease to the fair value of US$9,822,000. A decrease of 5% would result in an increase to the fair value of US$13,296,000. |
| • | A six-month delay in the launch date for AP101 for EB would result in a decrease to the fair value of US$4,540,000. |
| | | December 31, | ||||
| | 2018 | | | 2017 | ||
Assets | | | | | ||
Current assets: | | | | |||
Cash and cash equivalents | | | $31,881 | | | $14,307 |
Accounts receivable, net | | | 28,912 | | | 22,191 |
Inventories - current | | | 12,745 | | | 15,886 |
Prepaid expenses and other current assets | | | 15,292 | | | 10,499 |
Total current assets | | | 88,830 | | | 62,883 |
Inventories - non-current | | | 36,202 | | | 33,940 |
Property and equipment, net | | | 1,397 | | | 2,572 |
Intangible assets, net | | | 200,176 | | | 225,272 |
Other non-current assets | | | 1,209 | | | 2,247 |
Total assets | | | $327,814 | | | $326,914 |
| | | | | |||
Liabilities and shareholders’ deficit | | | | | ||
Current liabilities: | | | | | ||
Accounts payable | | | $4,995 | | | $13,182 |
Accrued liabilities | | | 42,356 | | | 36,197 |
Payable due to Novelion | | | 11,003 | | | — |
Short-term debt | | | 73,677 | | | — |
Short-term debt due to Novelion | | | 37,264 | | | — |
Convertible notes, net | | | 274,815 | | | — |
Provision for legal settlements - current | | | 11,689 | | | 8,596 |
Total current liabilities | | | 455,799 | | | 57,975 |
Convertible notes, net | | | — | | | 258,538 |
Long-term debt due to Novelion | | | — | | | 23,500 |
Provision for legal settlements - non-current | | | 19,391 | | | 31,016 |
Payable due to Novelion | | | — | | | 4,760 |
Other non-current liabilities | | | 795 | | | 595 |
Total liabilities | | | 475,985 | | | 376,384 |
Commitments and contingencies (Note 15) | | | | | ||
Shareholders’ equity: | | | | | ||
Common shares, without par value, 30,301 shares issued and outstanding at December 31, 2018 and 2017, respectively | | | — | | | — |
Additional paid-in-capital | | | 59,381 | | | 59,381 |
Accumulated deficit | | | (206,217) | | | (109,679) |
Accumulated other comprehensive (loss) income | | | (1,335) | | | 828 |
Total shareholders’ deficit | | | (148,171) | | | (49,470) |
Total liabilities and shareholders’ deficit | | | $327,814 | | | $326,914 |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
Net revenues | | | $130,432 | | | $138,438 |
Cost of product sales | | | 59,697 | | | 77,220 |
Operating expenses: | | | | | ||
Selling, general and administrative | | | 64,437 | | | 77,793 |
Research and development | | | 38,064 | | | 44,895 |
Restructuring charges | | | 2,171 | | | 121 |
Related party expenses (income), net | | | 942 | | | (177) |
Total operating expenses | | | 105,614 | | | 122,632 |
Loss from operations | | | (34,879) | | | (61,414) |
Interest expense, net | | | (50,746) | | | (39,467) |
Interest expense due to Novelion | | | (2,987) | | | (1,089) |
Loss on extinguishment of debt | | | (4,333) | | | — |
Other expense, net | | | (1,888) | | | (836) |
Loss before provision for income taxes | | | (94,833) | | | (102,806) |
Provision for income taxes | | | (1,705) | | | (594) |
Net loss | | | $(96,538) | | | $(103,400) |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
Net loss | | | $(96,538) | | | $(103,400) |
Other comprehensive (loss) income: | | | | | ||
Foreign currency translation | | | (2,163) | | | 1,209 |
Other comprehensive (loss) income | | | (2,163) | | | 1,209 |
Comprehensive loss | | | $(98,701) | | | $(102,191) |
| | | Common Shares | | | Additional Paid-In Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive (Loss) Income | | | Total Shareholders Equity (Deficit) | ||||
| | | Shares | | | Amount | | ||||||||||||
Balance at January 1, 2017 | | | 30,301,444 | | | $— | | | $59,381 | | | $(6,279) | | | $(381) | | | $52,721 |
Net loss | | | — | | | — | | | — | | | (103,400) | | | — | | | (103,400) |
Foreign currency translation adjustment | | | — | | | — | | | — | | | — | | | 1,209 | | | 1,209 |
Balance at December 31, 2017 | | | 30,301,444 | | | — | | | 59,381 | | | (109,679) | | | 828 | | | (49,470) |
Net loss | | | — | | | — | | | — | | | (96,538) | | | — | | | (96,538) |
Foreign currency translation adjustment | | | — | | | — | | | — | | | — | | | (2,163) | | | (2,163) |
Balance at December 31, 2018 | | | 30,301,444 | | | $— | | | $59,381 | | | $(206,217) | | | $(1,335) | | | $(148,171) |
| | | Year Ended December 31, | ||||
Cash used in operating activities | | | 2018 | | | 2017 |
Net loss | | | $(96,538) | | | (103,400) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | ||
Depreciation | | | 1,577 | | | 1,865 |
Amortization of intangible assets | | | 25,091 | | | 25,052 |
Stock-based compensation | | | 1,465 | | | 2,145 |
Non-cash interest expense | | | 39,670 | | | 32,954 |
Non-cash interest expense due to Novelion | | | 2,987 | | | 1,089 |
Provision for inventory excess and obsolescence | | | 2,062 | | | 18,814 |
Unrealized foreign exchange loss (gain) | | | 2,908 | | | (818) |
Amortization of debt issuance costs and debt discount | | | 3,251 | | | — |
Deferred income taxes | | | 858 | | | 138 |
Other non-cash operating activities | | | (13) | | | 24 |
Loss on extinguishment of Shareholder Term Loans | | | 4,025 | | | — |
Changes in assets and liabilities: | | | | | ||
Accounts receivable | | | (6,745) | | | (13,032) |
Inventories | | | (1,700) | | | 6,081 |
Prepaid expenses and other assets | | | (4,876) | | | 19,282 |
Accounts payable | | | (10,517) | | | (207) |
Payable due to Novelion | | | 448 | | | (406) |
Accrued and other liabilities | | | (2,715) | | | (23,970) |
Net cash used in operating activities | | | (38,762) | | | (34,389) |
Cash used in investing activities | | | | | ||
Purchase of property and equipment | | | (442) | | | (372) |
Net cash used in investing activities | | | (442) | | | (372) |
Cash provided by financing activities | | | | | ||
Net proceeds from Bridge Loans | | | 70,000 | | | — |
Net proceeds from Novelion Loan | | | 15,000 | | | 22,000 |
Repayment of Shareholder Term Loans | | | (20,000) | | | — |
Repayment of Novelion Loan | | | (3,500) | | | — |
Payment of debt issuance costs | | | (2,966) | | | — |
Net cash provided by financing activities | | | 58,534 | | | 22,000 |
Exchange rate effect on cash | | | (1,756) | | | 2,032 |
Net increase (decrease) in cash and cash equivalents | | | 17,574 | | | (10,729) |
Cash and cash equivalents, beginning of period | | | 14,307 | | | 25,036 |
Cash and cash equivalents, end of period | | | $31,881 | | | 14,307 |
Supplemental disclosures of cash flow information | | | | | ||
Cash paid for interest | | | $8,182 | | | $6,514 |
Cash paid for taxes, net | | | $42 | | | $1,671 |
Non-cash investing activities | | | | | ||
Purchases of property and equipment included in accounts payable | | | $10 | | | $122 |
Non-cash financing activities | | | | | ||
Refinance from Roll Up Loans | | | $22,500 | | | $— |
Retirement of Convertible Notes | | | $(22,500) | | | $— |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Work-in-process | | | $26,676 | | | $22,579 |
Finished goods | | | 22,271 | | | 27,247 |
Total | | | 48,947 | | | 49,826 |
Less: Inventories - current | | | (12,745) | | | (15,886) |
Inventories - non-current | | | $36,202 | | | $33,940 |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Leasehold improvements | | | $1,730 | | | $1,686 |
Office furniture and equipment | | | 798 | | | 810 |
Computer and office equipment | | | 2,482 | | | 1,975 |
Construction in progress | | | — | | | 123 |
Property and equipment, at cost | | | 5,010 | | | 4,594 |
Less accumulated depreciation | | | (3,613) | | | (2,022) |
Property and equipment, net | | | $1,397 | | | $2,572 |
| | | December 31, 2018 | |||||||
| | | Gross Carrying Value | | | Accumulated Amortization | | | Net Carrying Value | |
| | | (in thousands) | |||||||
Developed technology - metreleptin | | | $210,158 | | | $(44,084) | | | $166,074 |
Developed technology - lomitapide | | | 42,300 | | | (8,198) | | | 34,102 |
Total intangible assets | | | $252,458 | | | $(52,282) | | | $200,176 |
| | | December 31, 2017 | |||||||
| | | Gross Carrying Value | | | Accumulated Amortization | | | Net Carrying Value | |
| | | (in thousands) | |||||||
Developed technology - metreleptin | | | $210,158 | | | $(22,924) | | | $187,234 |
Developed technology - lomitapide | | | 42,300 | | | (4,262) | | | 38,038 |
Total intangible assets | | | $252,458 | | | $(27,186) | | | $225,272 |
| | | Amount | |
Years Ending December 31, | | | (in thousands) |
2019 | | | $25,095 |
2020 | | | 25,095 |
2021 | | | 25,095 |
2022 | | | 25,095 |
2023 | | | 25,095 |
Thereafter | | | 74,701 |
Total intangible assets subject to amortization | | | $200,176 |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Accrued employee compensation and related costs | | | $2,108 | | | $5,182 |
Accrued professional fees | | | 592 | | | 1,952 |
Accrued allowances: government rebates | | | 19,637 | | | 13,471 |
Accrued royalties | | | 5,112 | | | 3,588 |
Other accrued liabilities | | | 14,907 | | | 12,004 |
Total | | | $42,356 | | | $36,197 |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Beginning balance | | | $13,471 | | | $7,849 |
Provision | | | 24,646 | | | 23,087 |
Payments | | | (18,375) | | | (17,465) |
Other adjustments | | | (105) | | | — |
Ending balance | | | $19,637 | | | $13,471 |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Beginning balance | | | $10 | | | $118 |
Costs incurred | | | 2,171 | | | 121 |
Payments | | | (1,813) | | | (229) |
Other adjustments | | | (9) | | | — |
Ending balance | | | $359 | | | $10 |
| | | December 31, 2018 | ||||||||||
| | | New Money Loans | | | Roll Up Loans | | | Novelion Loan | | | Total | |
| | | (in thousands) | ||||||||||
Short-term principal and commitment fee | | | $51,000 | | | $22,500 | | | $37,777 | | | $111,277 |
Exit fee payable | | | 1,500 | | | — | | | — | | | 1,500 |
Accrued unpaid interest | | | 826 | | | 66 | | | 2,987 | | | 3,879 |
Unamortized debt issuance costs | | | (1,054) | | | — | | | — | | | (1,054) |
Debt discount | | | (1,161) | | | — | | | — | | | (1,161) |
Repayment | | | — | | | — | | | (3,500) | | | (3,500) |
Total short-term debt | | | $51,111 | | | $22,566 | | | $37,264 | | | $110,941 |
| | | December 31, | |
| | | Novelion Loan | |
| | | (in thousands) | |
Principal | | | $22,411 |
Accrued unpaid interest | | | 1,089 |
Total short-term debt | | | $23,500 |
| • | The Convertible Notes are senior unsecured obligations of Aegerion and bear interest at a rate of 2.0% per year, payable semi-annually in arrears on February 15 and August 15 of each year, beginning on February 15, 2015. The Convertible Notes matured on August 15, 2019, unless earlier repurchased or converted. |
| • | After Novelion’s acquisition of Aegerion, the Convertible Notes became convertible into Novelion’s common shares at a conversion rate of 4.9817 common shares per $1,000 principal amount of the Convertible Notes. If the holders elect to convert the Convertible Notes, Aegerion can settle the conversion of the Convertible Notes through payment or delivery of cash, common shares, or a combination of cash and common shares, in its discretion. |
| • | On or after February 15, 2019 until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or any portion of their Convertible Notes, in multiples of $1,000 principal amount, at the option of the holder. |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Principal | | | $302,498 | | | $324,998 |
Less: debt discount | | | (27,683) | | | (66,460) |
Net carrying amount | | | $274,815 | | | $258,538 |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Contractual interest expense | | | $6,435 | | | $6,500 |
Amortization of debt discount | | | 38,778 | | | 32,954 |
Total interest expense | | | $45,213 | | | $39,454 |
| | | Amount | |
| | | (in thousands) | |
Matured on August 15, 2019 | | | $308,548 |
| | | 308,548 | |
Less amount representing interest | | | (6,050) |
Less debt discount, net | | | (27,683) |
Net carrying amount of Convertible Notes as of December 31, 2018 | | | $274,815 |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
Expected stock price volatility | | | 45.10% | | | 38.38% |
Risk-free interest rate | | | 2.72% | | | 1.99% |
Expected life of options (years) | | | 5.64 | | | 6.25 |
Expected dividend yield | | | — | | | — |
| | | Number of Stock Options | | | Weighted- Average Exercise Price Per Share | | | Weighted Average Remaining Contractual Life (years) | | | Aggregate Intrinsic Value | |
Outstanding at January 1, 2018 | | | 844,203 | | | $9.40 | | | 7.84 | | | $— |
Granted | | | 1,080,999 | | | 3.42 | | | | | ||
Exercised | | | — | | | — | | | | | ||
Forfeited/cancelled | | | (613,484) | | | 7.73 | | | | | ||
Outstanding at December 31, 2018 | | | 1,311,718 | | | $5.25 | | | 9.00 | | | $— |
Vested and expected to vest at December 31, 2018 | | | 1,311,718 | | | $5.25 | | | 9.00 | | | $— |
Exercisable at December 31, 2018 | | | 161,882 | | | $9.27 | | | 7.54 | | | $— |
| | | Number of RSUs | | | Weighted- Average Grant Date Fair Value | |
Outstanding at January 1, 2018 | | | 219,752 | | | $11.67 |
Granted | | | 182,830 | | | 4.52 |
Vested | | | (122,014) | | | 11.87 |
Forfeited/cancelled | | | (169,804) | | | 7.85 |
Outstanding at December 31, 2018 | | | 110,764 | | | $5.51 |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Selling, general and administrative | | | $1,028 | | | $1,591 |
Research and development | | | 437 | | | 554 |
Total stock-based compensation expense | | | $1,465 | | | $2,145 |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
U.S. | | | $(67,858) | | | $(69,842) |
Other Foreign | | | (26,975) | | | (32,964) |
Loss before provision for income taxes | | | $(94,833) | | | $(102,806) |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Current benefit (provision): | | | | | ||
U.S. | | | $52 | | | $106 |
Other Foreign | | | (899) | | | (647) |
| | | (847) | | | (541) | |
Deferred provision: | | | | | ||
Other Foreign | | | (858) | | | (53) |
Provision for income taxes | | | $(1,705) | | | $(594) |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
U.S. statutory tax rates | | | 21% | | | 35% |
Loss before income taxes | | | $(94,833) | | | $(102,806) |
Expected income tax benefit | | | 19,915 | | | 35,982 |
Net increase in valuation allowance | | | (19,553) | | | (4,458) |
Tax credits | | | 891 | | | (176) |
Stock-based compensation | | | 22 | | | (11) |
Foreign rate differential | | | (3,043) | | | (8,091) |
Tax rate change | | | (680) | | | (22,654) |
Non-taxable expenditures | | | (1,550) | | | (723) |
Change in uncertain tax positions | | | (215) | | | (20) |
Return to provisions | | | 838 | | | (2,185) |
State tax | | | 1,670 | | | 1,742 |
Provision for income taxes | | | $(1,705) | | | $(594) |
| | | December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Deferred tax assets: | | | | | ||
Net operating loss carryforwards | | | $24,664 | | | $21,227 |
Research and development credits | | | 1,836 | | | 187 |
Stock-based compensation | | | 724 | | | 478 |
Capitalized research expenses | | | 716 | | | 1,028 |
Depreciable and amortizable assets | | | 10,811 | | | 9,727 |
Business interest expense limitation | | | 11,609 | | | — |
Other temporary differences | | | 13,093 | | | 11,689 |
Total gross deferred tax assets | | | 63,453 | | | 44,336 |
Valuation allowance | | | (63,453) | | | (43,479) |
Net deferred tax assets | | | $— | | | $857 |
| | | Year Ended December 31, | ||||
| | | 2018 | | | 2017 | |
| | | (in thousands) | ||||
Total provision for UTP as of January 1, | | | $1,029 | | | $911 |
Increases related to current year tax positions | | | 655 | | | 56 |
Changes in tax positions of prior periods | | | (1,044) | | | 62 |
Total provision for UTP as of December 31, | | | 640 | | | 1,029 |
Deferred tax assets available to offset provision for UTP | | | — | | | (612) |
Total provision for UTP as of December 31, | | | $640 | | | $417 |
| | | Year Ended December 31, 2018 | |||||||||||||
| | | U.S. | | | Japan | | | Brazil | | | Other Foreign Countries | | | Total | |
| | | (in thousands) | |||||||||||||
Metreleptin | | | $47,942 | | | $945 | | | $5,170 | | | $17,303 | | | $71,360 |
Lomitapide | | | 35,461 | | | 10,822 | | | 388 | | | 12,401 | | | 59,072 |
Total net revenues | | | $83,403 | | | $11,767 | | | $5,558 | | | $29,704 | | | $130,432 |
| | | Year Ended December 31, 2017 | |||||||||||||
| | | U.S. | | | Japan | | | Brazil | | | Other Foreign Countries | | | Total | |
| | | (in thousands) | |||||||||||||
Metreleptin | | | $50,972 | | | $788 | | | $6,837 | | | $7,711 | | | $66,308 |
Lomitapide | | | 46,431 | | | 5,836 | | | 6,659 | | | 13,204 | | | 72,130 |
Total net revenues | | | $97,403 | | | $6,624 | | | $13,496 | | | $20,915 | | | $138,438 |
| | | Lease Commitments | |
| | | (in thousands) | |
Years Ending December 31,: | | | |
2019 | | | $1,246 |
2020 | | | 432 |
2021 | | | 190 |
2022 | | | 134 |
2023 | | | 97 |
Thereafter | | | 41 |
Total | | | $2,140 |
| • | New Money Loans and Roll Up Loans − the full amount including the accrued unpaid interest, was discharged. Each holder of the New Money Loans received secured credit facility issued by Aegerion at the Closing on a dollar for dollar basis on account of its New Money Loan claim: each holder of the Roll Up Loans received new convertible notes issued by Aegerion at the Closing on a dollar for dollar basis on account of its Roll Up Loan claim. |
| • | Novelion Loan – the full amount, including the accrued unpaid interest, was discharged. Novelion received American depositary receipts representing approximately 14.0 million Ordinary Shares, as discussed below. |
| • | Convertible Notes – the outstanding unpaid principal and interest were discharged. Each Convertible Noteholders received its pro rata share of the net remaining new convertible notes and certain Amryt common stocks. |
| • | Outstanding settlements due to DOJ and SEC – The Company will continue to pay the outstanding settlements to DOJ and SEC in accordance with payment terms as set forth in the original settlement agreements. |
| • | Ongoing trade transactions – The Company will continue to follow the respective payment terms with each vendor. Any outstanding amount that was associated to the pre-Petition period but was put on hold for payment during the bankruptcy period was fully paid in cash in September 2019, plus post-petition interest at the applicable interest rate. |
| • | Payables due to Novelion – the outstanding payables due to Novelion were discharged. |
| | | June 30, 2019 | | | December 31, 2018 | |
Assets | | | | | ||
Current assets: | | | | | ||
Cash and cash equivalents | | | $36,080 | | | $31,881 |
Accounts receivable, net | | | 26,408 | | | 28,912 |
Inventories - current | | | 13,486 | | | 12,745 |
Prepaid expenses and other current assets | | | 17,153 | | | 15,292 |
Total current assets | | | 93,127 | | | 88,830 |
Inventories - non-current | | | 38,306 | | | 36,202 |
Property and equipment, net | | | 754 | | | 1,397 |
Intangible assets, net | | | 187,629 | | | 200,176 |
Other non-current assets | | | 2,818 | | | 1,209 |
Total assets | | | $322,634 | | | $327,814 |
| | | | | |||
Liabilities and shareholders’ deficit | | | | | ||
Current liabilities: | | | | | ||
Accounts payable | | | $3,615 | | | $4,995 |
Accrued liabilities | | | 49,749 | | | 42,356 |
Payable due to Novelion | | | — | | | 11,003 |
Short-term debt | | | — | | | 73,677 |
Short-term debt due to Novelion | | | — | | | 37,264 |
Convertible notes, net | | | — | | | 274,815 |
Provision for legal settlements - current | | | 14,070 | | | 11,689 |
Total current liabilities | | | 67,434 | | | 455,799 |
Provision for legal settlements - non-current | | | 11,962 | | | 19,391 |
Other non-current liabilities | | | 1,444 | | | 795 |
Total liabilities not subject to compromise | | | 80,840 | | | 475,985 |
Liabilities subject to compromise | | | 420,651 | | | — |
Total liabilities | | | 501,491 | | | 475,985 |
Commitments and contingencies (Note 13) | | | | | ||
Shareholders’ deficit: | | | | | ||
Common shares, without par value, 30,301 shares issued and outstanding at June 30, 2019 and December 31, 2018, respectively | | | — | | | — |
Additional paid-in-capital | | | 59,381 | | | 59,381 |
Accumulated deficit | | | (237,092) | | | (206,217) |
Accumulated other comprehensive loss | | | (1,146) | | | (1,335) |
Total shareholders’ deficit | | | (178,857) | | | (148,171) |
Total liabilities and shareholders’ deficit | | | $322,634 | | | $327,814 |
| | | Six Months Ended June 30, | ||||
| | | 2019 | | | 2018 | |
Net revenues | | | $95,857 | | | $59,388 |
Cost of product sales | | | 35,364 | | | 29,208 |
Operating expenses: | | | | | ||
Selling, general and administrative | | | 43,424 | | | 38,568 |
Research and development | | | 13,946 | | | 21,113 |
Related party expense, net | | | 397 | | | 617 |
Total operating expenses | | | 57,767 | | | 60,298 |
Income (loss) from operations | | | 2,726 | | | (30,118) |
Reorganization items, net | | | (2,145) | | | — |
Interest expense, net (contractual interest of $690) | | | (29,681) | | | (22,628) |
Interest expense due to Novelion (contractual interest of $352) | | | (1,182) | | | (1,406) |
Other expense, net | | | (224) | | | (1,054) |
Loss before provision for income taxes | | | (30,506) | | | (55,206) |
Provision for income taxes | | | (369) | | | (1,205) |
Net loss | | | $(30,875) | | | $(56,411) |
| | | Six Months Ended June 30, | ||||
| | | 2019 | | | 2018 | |
Net loss | | | $(30,875) | | | $(56,411) |
Other comprehensive income (loss): | | | | | ||
Foreign currency translation | | | 189 | | | (1,335) |
Other comprehensive income (loss) | | | 189 | | | (1,335) |
Comprehensive loss | | | $(30,686) | | | $(57,746) |
| | | Six Months Ended June 30, 2019 | |||||||||||||||||||
| | | Common Shares | | | Additional Paid-In Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive Loss | | | Total Shareholders’ Deficit | | ||||||
| | | Shares | | | Amount | | |||||||||||||||
Balance at December 31, 2018 | | | 30,301,444 | | | $— | | | $59,381 | | | $(206,217) | | | $(1,335) | | | $(148,171) | | ||
Net loss | | | — | | | — | | | — | | | (30,875) | | | — | | | (30,875) | | ||
Foreign currency translation adjustment | | | — | | | — | | | — | | | — | | | 189 | | | 189 | | ||
Balance at June 30, 2019 | | | 30,301,444 | | | $— | | | $59,381 | | | $(237,092) | | | $(1,146) | | | $(178,857) | | ||
| | | Six Months Ended June 30, 2018 | |||||||||||||||||||
| | | Common Shares | | | Additional Paid-In Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income/(Loss) | | | Total Shareholders’ Deficit | | ||||||
| | | Shares | | | Amount | | |||||||||||||||
Balance at December 31, 2017 | | | 30,301,444 | | | $— | | | $59,381 | | | $(109,679) | | | $828 | | | $(49,470) | | ||
Net loss | | | — | | | — | | | — | | | (56,411) | | | — | | | (56,411) | | ||
Foreign currency translation adjustment | | | — | | | — | | | — | | | — | | | (1,335) | | | (1,335) | | ||
Balance at June 30, 2018 | | | 30,301,444 | | | $— | | | $59,381 | | | $(166,090) | | | $(507) | | | $(107,216) | | ||
| | | Six Months Ended June 30, | ||||
| | | 2019 | | | 2018 | |
Cash used in operating activities | | | | | ||
Net loss | | | $(30,875) | | | $(56,411) |
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | ||
Depreciation | | | 490 | | | 842 |
Non-cash lease expense | | | 888 | | | — |
Amortization of intangible assets | | | 12,548 | | | 12,548 |
Stock-based compensation | | | 703 | | | 933 |
Non-cash interest expense | | | 24,214 | | | 19,146 |
Non-cash interest expense due to Novelion | | | 1,182 | | | 1,406 |
Unrealized foreign exchange (gain) loss | | | (31) | | | 1,161 |
Amortization of debt issuance costs and debt discount | | | 2,215 | | | 127 |
Deferred income taxes | | | — | | | 919 |
Other non-cash operating activities | | | (8) | | | 9 |
Loss on disposal of property and equipment | | | 140 | | | — |
Changes in assets and liabilities: | | | | | ||
Accounts receivable | | | 2,565 | | | 3,811 |
Inventories | | | (2,355) | | | (1,433) |
Prepaid expenses and other assets | | | (2,391) | | | (3,738) |
Accounts payable | | | 1,819 | | | (3,958) |
Accrued liabilities and other liabilities | | | 1,093 | | | (84) |
Payable due to Novelion | | | (2,951) | | | 235 |
Net cash provided by (used in) operating activities | | | 9,246 | | | (24,487) |
Cash used in investing activities | | | | | ||
Purchases of property and equipment | | | — | | | (384) |
Net cash used in investing activities | | | — | | | (384) |
Cash provided by financing activities | | | | | ||
Net proceeds from Shareholder Term Loans, net of debt discount | | | — | | | 19,977 |
Proceeds from Novelion Loan | | | — | | | 15,000 |
Repayment of Bridge Loans | | | (2,996) | | | — |
Repayment of Novelion Loan | | | (2,106) | | | — |
Payment of issuance costs | | | — | | | (698) |
Net cash (used in) provided by financing activities | | | (5,102) | | | 34,279 |
Exchange rate effect on cash | | | 55 | | | (862) |
Net increase in cash and cash equivalents | | | 4,199 | | | 8,546 |
Cash and cash equivalents, beginning of period | | | 31,881 | | | 14,307 |
Cash and cash equivalents, end of period | | | $36,080 | | | $22,853 |
Supplemental disclosures of cash flow information | | | | | ||
Cash paid for interest | | | $3,393 | | | $3,422 |
Cash paid (received) for taxes, net | | | $352 | | | $(71) |
Non-cash investing activities and financing activities: | | | | | ||
Purchases of property and equipment included in accounts payable | | | $— | | | $35 |
Right-of-use assets obtained in exchange for operating lease obligation | | | $1,945 | | | $— |
Lease liabilities arising in exchange for right of use assets | | | $1,950 | | | $— |
| • | Amryt acquiring 100% of the outstanding new equity interests in recapitalized Aegerion; |
| • | Ordinary equity of Amryt representing 61.4% of the outstanding ordinary shares of Amryt, after giving effect to the Restructuring Transactions but before giving effect to shares underlying the New Convertible Notes, the Deal Equity Raise (each as described below), ordinary shares that may be issuable in satisfaction of the contingent value right if the relevant milestones are achieved, and equity that is reserved for issuance under any management equity compensation plan adopted by Amryt, will be distributed to certain existing creditors of Aegerion in complete or partial satisfaction of their claims, including in partial satisfaction of the claims of the holders of the existing Convertible Notes and in complete satisfaction of the outstanding Novelion Loan; |
| • | The equity interests of Aegerion held by Novelion being terminated; |
| • | Aegerion issuing $125 million of new 5% convertible notes (“New Convertible Notes”). The New Convertible Notes will be issued to certain existing creditors of Aegerion in satisfaction of their claims (and not for cash), including in satisfaction of a portion of the existing Convertible Notes, the approximately $22 million of Roll Up Loans under the Bridge Loans, and any amounts drawn down under Aegerion’s DIP Financing (defined below) that are not otherwise satisfied in cash at the closing of the Restructuring Transactions; |
| • | Aegerion’s existing Bridge Loans in the original principal amount of $50 million, held by certain funds managed by and Athyrium Capital Management, LP (“Athyrium”) and Highbridge Capital Management, LLC (“Highbridge”), as well as Amryt’s existing approximately €20 million (in principal) of secured debt, will be converted into new first-lien secured debt of Aegerion and Amryt, which will have a cash interest rate of 6.5% per annum and an additional 6.5% PIK (Paid in Kind) and mature five years from the closing date of the Restructuring Transactions; |
| • | In connection with the closing of the Restructuring Transactions, Amryt plans to raise $60 million through the issuance of new equity of Amryt (“Deal Equity Raise”). The proceeds from the Deal Equity Raise will be used as provided in the plan of reorganization to pay certain expenses and for general corporate purposes. The new equity will be priced at a 20 percent discount to Amryt’s implied valuation pro forma to the Restructuring Transaction with $18 million of the new equity offered to certain investors of Amryt and $42 million to certain creditors of Aegerion on a pro rata basis, including Novelion; |
| • | Aegerion intends to, and the plan of reorganization provides that Aegerion will, continue to fully honor all obligations to the U.S. Department of Justice (“DOJ”), the U.S. Securities and Exchange Commission (“SEC”) and other U.S. and state government agencies and courts, which obligations will not be impaired by the Restructuring Transactions; |
| • | Aegerion intends to continue to pay all trade and other ordinary operating expenses that arise during the course of the Chapter 11 Cases and, upon consummation of the Restructuring Transactions, repay 100% of the trade claims; |
| | | June 30, 2019 | |
| | | (in thousands) | |
Short-term debt | | | $75,213 |
Short-term debt due to Novelion | | | 36,340 |
Convertible notes, net | | | 296,712 |
Accounts Payable | | | 3,379 |
Payable due to Novelion | | | 9,007 |
Total liabilities subject to compromise | | | $420,651 |
| | | Six Months Ended June 30, 2019 | |
| | | (in thousands) | |
Professional fees | | | $2,145 |
Total | | | $2,145 |
| | | Amount | |
| | | (in thousands) | |
Balance as of December 31, 2018 | | | $19,637 |
Provision | | | 15,679 |
Payments | | | (18,239) |
Balance as of June 30, 2019 | | | $17,077 |
| • | License: The consideration for the license performance obligation consists of the $30.0 million in one-time, fixed payments as well as a reduction related to the 60% of net sales benefit to Recordati during the transition period, from the Transaction Effective Date through the Completion Date (“the |
| • | JUXTAPID supply on hand at the Completion Date: The consideration for JUXTAPID supply on hand at the Completion Date, pursuant to the transitional services agreement, was variable consideration dependent on the inventory on hand at the Completion Date multiplied by the cost plus markup. Revenue allocated to the JUXTAPID supply on hand at the Completion Date will be recognized when such supply is made available to Recordati at the manufacturing facility engaged by the Company. During the six months ended June 30, 2019, the Company earned $1.4 million of revenue from Recordati for the sales of JUXTAPID supply on hand at the Completion Date. |
| • | Supply of JUXTAPID to Recordati: The consideration for the supply of JUXTAPID to Recordati will be variable consideration dependent on the inventory ordered pursuant to the supply agreement multiplied by the cost plus markup. Revenue allocated to the JUXTAPID supply will be recognized when such supply is made available to Recordati at the manufacturing facility engaged by the Company. During the six months ended June 30, 2019, there was no such supply sales made to Recordati. |
| | | June 30, 2019 | | | December 31, 2018 | |
| | | (in thousands) | ||||
Work-in-process | | | $26,727 | | | $26,676 |
Finished goods | | | 25,065 | | | 22,271 |
Total | | | 51,792 | | | 48,947 |
Less: Inventories - current | | | (13,486) | | | (12,745) |
Inventories - non-current | | | $38,306 | | | $36,202 |
| | | June 30, 2019 | |||||||
| | | Gross Carrying Value | | | Accumulated Amortization | | | Net Carrying Value | |
| | | (in thousands) | |||||||
Developed technology - metreleptin | | | $210,158 | | | $(54,664) | | | $155,494 |
Developed technology - lomitapide | | | 42,300 | | | (10,165) | | | 32,135 |
Total intangible assets | | | $252,458 | | | $(64,829) | | | $187,629 |
| | | December 31, 2018 | |||||||
| | | Gross Carrying Value | | | Accumulated Amortization | | | Net Carrying Value | |
| | | (in thousands) | |||||||
Developed technology - metreleptin | | | $210,158 | | | $(44,084) | | | $166,074 |
Developed technology - lomitapide | | | 42,300 | | | (8,198) | | | 34,102 |
Total intangible assets | | | $252,458 | | | $(52,282) | | | $200,176 |
| | | Amount | |
Years Ending December 31, | | | (in thousands) |
2019 (remaining 6 months) | | | $12,549 |
2020 | | | 25,095 |
2021 | | | 25,095 |
2022 | | | 25,095 |
2023 | | | 25,095 |
Thereafter | | | 74,700 |
Total intangible assets subject to amortization | | | $187,629 |
| | | June 30, 2019 | | | December 31, 2018 | |
| | | (in thousands) | ||||
Accrued employee compensation and related costs | | | $2,110 | | | $2,108 |
Accrued professional fees | | | 3,838 | | | 592 |
Accrued allowances: government rebates | | | 17,077 | | | 19,637 |
Accrued royalties | | | 9,950 | | | 5,112 |
Other accrued liabilities | | | 16,774 | | | 14,907 |
Total | | | $49,749 | | | $42,356 |
| | | Six Months Ended June 30, 2019 | |
| | | (in thousands) | |
Operating lease expense | | | $931 |
Variable lease expense | | | 20 |
Short-term lease expense | | | 92 |
Total lease expense | | | $1,043 |
| | | Amount | |
Year Ending December 31, | | | (in thousands) |
2019 (remaining 6 months) | | | $245 |
2020 | | | 451 |
2021 | | | 211 |
2022 | | | 138 |
2023 | | | 98 |
Thereafter | | | 41 |
Total lease payments | | | 1,184 |
Less: Present value adjustment using incremental borrowing rate | | | 122 |
Present value of operating lease liabilities | | | $1,062 |
| | | Amount | |
Years Ending December 31, | | | (in thousands) |
2019 | | | $1,246 |
2020 | | | 432 |
2021 | | | 190 |
2022 | | | 134 |
2023 | | | 97 |
Thereafter | | | 41 |
Total | | | $2,140 |
| | | June 30, 2019 | ||||||||||
| | | New Money Loans | | | Roll Up Loans | | | Novelion Loan | | | Total | |
| | | (in thousands) | ||||||||||
Short-term principal and commitment fee | | | $51,000 | | | $22,500 | | | 37,264 | | | $110,764 |
Exit fee payable | | | 1,500 | | | — | | | — | | | 1,500 |
Accrued unpaid interest | | | 2,967 | | | 242 | | | 1,182 | | | 4,391 |
Repayment of short-term principal, including exit fee | | | (2,996) | | | — | | | (2,106) | | | (5,102) |
Total short-term debt | | | $52,471 | | | $22,742 | | | $36,340 | | | $111,553 |
| | | December 31, 2018 | ||||||||||
| | | New Money Loans | | | Roll Up Loans | | | Novelion Loan | | | Total | |
| | | (in thousands) | ||||||||||
Short-term principal and commitment fee | | | $51,000 | | | $22,500 | | | $37,777 | | | $111,277 |
Exit fee payable | | | 1,500 | | | — | | | — | | | 1,500 |
Accrued unpaid interest | | | 826 | | | 66 | | | 2,987 | | | 3,879 |
Unamortized debt issuance costs | | | (1,054) | | | — | | | — | | | (1,054) |
Debt discount | | | (1,161) | | | — | | | — | | | (1,161) |
Repayment of principal | | | — | | | — | | | (3,500) | | | (3,500) |
Total short-term debt | | | $51,111 | | | $22,566 | | | $37,264 | | | $110,941 |
| | | Six Months Ended June 30, | ||||
| | | 2019 | | | 2018 | |
| | | (in thousands) | ||||
Contractual interest expense | | | $3,025 | | | $3,250 |
Amortization of debt discount | | | 21,897 | | | 18,605 |
Total | | | $24,922 | | | $21,855 |
| | | Amount | |
| | | (in thousands) | |
Principal | | | $302,498 |
Interest | | | 3,025 |
Total | | | $305,523 |
| | | Six Months Ended June 30, 2019 | ||||||||||||||||
| | | U.S. | | | Japan | | | Germany | | | Brazil | | | Other Foreign Countries | | | Total | |
| | | (in thousands) | ||||||||||||||||
Metreleptin | | | $24,891 | | | $62 | | | $5,191 | | | $— | | | $8,771 | | | $38,915 |
Lomitapide | | | 18,710 | | | 32,642 | | | 79 | | | — | | | 5,511 | | | 56,942 |
Total | | | $43,601 | | | $32,704 | | | $5,270 | | | $— | | | $14,282 | | | $95,857 |
| | | Six Months Ended June 30, 2018 | ||||||||||||||||
| | | U.S. | | | Japan | | | Germany | | | Brazil | | | Other Foreign Countries | | | Total | |
| | | (in thousands) | ||||||||||||||||
Metreleptin | | | $22,248 | | | $48 | | | $787 | | | $1,170 | | | $5,498 | | | $29,751 |
Lomitapide | | | 19,100 | | | 4,803 | | | — | | | — | | | 5,734 | | | 29,637 |
Total | | | $41,348 | | | $4,851 | | | $787 | | | $1,170 | | | $11,232 | | | $59,388 |
| | | June 30, 2019 | |
| | | (In thousands) | |
Assets | | | |
Current assets: | | | |
Cash and cash equivalents | | | $28,462 |
Accounts receivable, net | | | 16,138 |
Inventories - current | | | 3,779 |
Prepaid expenses and other current assets | | | 4,896 |
Total current assets | | | 53,275 |
Inventories - non-current | | | 10,733 |
Property and equipment, net | | | 429 |
Intangible assets, net | | | 120,754 |
Other non-current assets | | | 1,731 |
Investment in subsidiaries | | | 100,830 |
Intercompany receivable | | | 56,763 |
Total assets | | | $344,515 |
| | | ||
Liabilities and shareholders’ deficit | | | |
Current liabilities: | | | |
Accounts payable | | | $500 |
Accrued liabilities | | | 39,075 |
Provision for legal settlements - current | | | 14,070 |
Total current liabilities | | | 53,645 |
Long-term liabilities: | | | |
Provision for legal settlements - non-current | | | 11,962 |
Other non-current liabilities | | | 32 |
Intercompany payables | | | 8,708 |
Total liabilities not subject to compromise | | | 74,347 |
Liabilities subject to compromise | | | 420,651 |
Total liabilities | | | 494,998 |
Commitments and contingencies (Note 13) | | | |
Shareholders’ deficit: | | | |
Total shareholders’ deficit | | | (150,483) |
Total liabilities and shareholders’ deficit | | | $344,515 |
| | | Six Months Ended June 30, 2019 | |
| | | (In thousands) | |
Net revenues | | | $81,551 |
Cost of product sales | | | 34,887 |
Operating expenses: | | | |
Selling, general and administrative | | | 37,298 |
Research and development | | | 10,256 |
Intercompany expense, net | | | 546 |
Related party expense, net | | | 397 |
Total operating expenses | | | 48,497 |
Loss from operations | | | (1,833) |
Reorganization items, net | | | (2,145) |
Interest expense, net (contractual interest of $690) | | | (29,672) |
Interest expense due to Novelion (contractual interest of $352) | | | (1,182) |
Other income, net | | | 289 |
Loss before provision for income taxes | | | (34,543) |
Provision for income taxes | | | (186) |
Net loss | | | $(34,729) |
Comprehensive loss | | | $(34,729) |
| | | Six Months Ended June 30, 2019 | |
| | | (In thousands) | |
Cash provided by (used in) operating activities | | | |
Net loss | | | $(34,729) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | |
Depreciation | | | 461 |
Non-cash lease expense | | | 686 |
Amortization of intangible assets | | | 8,614 |
Stock-based compensation | | | 676 |
Non-cash interest expense | | | 24,214 |
Non-cash interest expense due to Novelion | | | 1,182 |
Unrealized foreign exchange (gain) loss | | | (414) |
Amortization of debt issuance costs and debt discount | | | 2,215 |
Other non-cash operating activities | | | (8) |
Loss on disposal of property and equipment | | | 140 |
Changes in assets and liabilities: | | | |
Accounts receivable | | | (42) |
Inventories | | | 1,246 |
Prepaid expenses and other assets | | | 1,898 |
Intercompany activities | | | 4,461 |
Accounts payable | | | 1,781 |
Accrued liabilities and other liabilities | | | (200) |
Payable due to Novelion | | | (2,659) |
Net cash provided by operating activities | | | 9,522 |
Net cash used in investing activities | | | — |
Cash used in financing activities | | | |
Repayment of Novelion Loan | | | (2,106) |
Repayment of Bridge Loans | | | (2,996) |
Net cash used in financing activities | | | (5,102) |
Exchange rate effect on cash | | | — |
Net decrease in cash and cash equivalents | | | 4,420 |
Cash and cash equivalents, beginning of period | | | 24,042 |
Cash and cash equivalents, end of period | | | $28,462 |
| • | New Money Loans and Roll Up Loans − the full amount, including the accrued unpaid interest, was discharged. Each holder of the New Money Loans received secured credit facility issued by Aegerion at the Closing on a dollar for dollar basis on account of its New Money Loan claim; each holder of the Roll Up Loans received new convertible notes issued by Aegerion at the Closing on a dollar for dollar basis on account of its Roll Up Loan claim. |
| • | Novelion Loan – the full amount, including the accrued unpaid interest, was discharged. Novelion received American depositary receipts representing approximately 14.0 million Ordinary Shares, as discussed below. |
| • | Convertible Notes – the outstanding unpaid principal and interest were discharged. Each Convertible Noteholders received its pro rata share of the net remaining new convertible notes and certain Amryt common stock. |
| • | Outstanding settlements due to DOJ and SEC – The Company will continue to pay the outstanding settlements to DOJ and SEC in accordance with payment terms as set forth in the original settlement agreements. |
| • | Ongoing trade transactions – The Company will continue to follow the respective payment terms with each vendor. Any outstanding amount that was associated to the pre-Petition period but was put on hold for payment during the bankruptcy period was fully paid in cash in September 2019, plus post-petition interest at the applicable interest rate. |
| • | Payables due to Novelion – the outstanding payables due to Novelion were discharged. |
