Exhibit 99.2

AMRYT VIRTUAL CAPITAL MARKETS EVENT September 13, 2021

DI S CLAIM ER 2 This presentation has been prepared by the Company . “Presentation” means this document, any oral presentation, any question and answer session and any written or oral material discussed or distributed during the meeting . By receiving this presentation and/or attending the meeting where this presentation is made, or by reading the presentation slides, you agree to be bound by the following limitations . This presentation is being made only to, and is directed only at, (i) persons having professional experience in matters relating to investments who fall within the definition of "investment professionals" in Article 19 ( 5 ) of the United Kingdom Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (as amended from time to time) (the “Order”) ; (ii) high net worth bodies corporate, unincorporated associations, partnerships and trustees of high value trusts as described in Article 49 ( 2 )(a) - (d) of the Order ; (iii) “overseas recipients” as defined in Article 12 of the Order ; (iv) persons in member states of the European Economic Area (the “EEA”) who are qualified investors, as defined in the Prospectus Regulation (Regulation (EU) 2017 / 1129 ) (as amended) ; or (v) persons to whom it would otherwise be lawful to distribute it (all such persons being “Relevant Persons”) . Persons who receive this communication (other than Relevant Persons) should not rely on or act upon the contents of this presentation and should return this document immediately . This presentation is being directed only at Relevant Persons and any investment or investment activity to which this presentation relates will be engaged in only with Relevant Persons . This presentation does not constitute or form part of any offer to sell or issue, or invitation to purchase or subscribe for, or any solicitation of any offer to purchase or subscribe for, any securities of the Company or any of its subsidiaries (together the “Group”) or in any other entity, nor shall this presentation or any part of it, or the fact of its presentation, form the basis of, or be relied on in connection with, any contract or investment activity (including within the meaning specified in section 21 of the United Kingdom Financial Services and Markets Act 2000 ), nor does it constitute a recommendation regarding the securities of the Group . Past performance, including the price at which the Company’s securities have been bought or sold in the past and the past yield on the Company’s securities, cannot be relied on as a guide to future performance . Nothing herein should be construed as financial, legal, tax, accounting, actuarial or other specialist advice and persons needing advice should consult an independent financial adviser or independent legal counsel . Neither this presentation nor any information contained in this presentation should be transmitted into, distributed in or otherwise made available in whole or in part by the recipients of the presentation to any other person in any jurisdiction which prohibits or restricts the same except in compliance with or as permitted by law or regulation . Recipients of this presentation are required to inform themselves of and comply with all restrictions or prohibitions in such jurisdictions . Accordingly, by requesting to receive and reviewing this document you represent that you are able to receive this document without contravention of any legal or regulatory restrictions applicable to you . No responsibility is accepted by and, to the fullest extent permitted by law, the Company, the Group, their affiliates and advisers and their respective directors, officers, partners, representatives, employees and agents expressly disclaim any and all liability, whether direct or indirect, express or implied, contractual, tortious, statutory or otherwise, as to the accuracy, fairness, reliability or completeness of the information contained herein or discussed verbally or as to the reasonableness of any assumptions on which any of the same is based or the use of any of the same or for any errors, omissions or misstatements in or from this presentation . No representations or warranties, express or implied, are given by the Company, the Group, their affiliates and advisers and their respective directors, officers, partners, representatives, employees and agents as to the accuracy, reliability or completeness of this presentation or any other written or oral information which has been or may be made available . Accordingly, no such person will be liable for any direct, indirect or consequential loss or damage suffered by any person resulting from the use of the information contained herein, or for any opinions expressed by any such person, or any errors, omissions or misstatements made by any of them . No duty of care is owed or will be deemed to be owed to any person in relation to the presentation . No reliance whatsoever may be placed on the presentation for any purpose . By accepting this presentation, you agree to use and maintain any such information in accordance with your contractual obligations and applicable laws, including all applicable securities laws . The information contained in this presentation has not been independently verified . The Amryt logo, Myalept®, Myalepta®, Juxtapid®, Lojuxta®, Filsuvez® and Mycapssa® and other trademarks or service marks of Amryt appearing in this presentation are the property of Amryt . This presentation includes trademarks, tradenames and service marks, certain of which belong to us and others that are the property of other organizations . Solely for convenience, trademarks, tradenames and service marks referred to in this presentation appear without the ®, TM and SM symbols, but the absence of those symbols is not intended to indicate, in any way, that we will not assert our rights or that the applicable owner will not assert its rights to these trademarks, tradenames and service marks to the fullest extent under applicable law . We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties . Filsuvez® (Oleogel - S 10 /birch triterpenes/formerly known as AP 101 ) is currently an investigational product and has not received regulatory approval by the FDA or EMA . Elements of this presentation only apply in the event that Filsuvez® is approved by the appropriate regulatory authorities . Mycapssa® has been approved by the FDA for the long - term maintenance treatment in patients with acromegaly who have responded to and tolerated treatment with octreotide or lanreotide but remains an investigational drug outside the US and for other indications . This presentation is intended only for communications with investors .

This presentation may contain forward - looking statements containing the words "expect", "anticipate", "intends", "plan", "estimate", "aim", "forecast", "project" and similar expressions (or their negative) identify certain of these forward - looking statements . The forward - looking statements in this communication are based on numerous assumptions and Amryt's present and future business strategies and the environment in which Amryt expects to operate in the future . Forward - looking statements involve inherent known and unknown risks, uncertainties and contingencies because they relate to events and depend on circumstances that may or may not occur in the future and may cause the actual results, performance or achievements to be materially different from those expressed or implied by such forward - looking statements, and actual results could differ materially from those currently anticipated due to a number of risks and uncertainties . These statements are not guarantees of future performance or the ability to identify and consummate investments . Many of these risks and uncertainties relate to factors that are beyond each of Amryt's ability to control or estimate precisely, such as future market conditions, the course of the COVID - 19 pandemic, currency fluctuations, the behaviour of other market participants, the outcome of clinical trials, the actions of regulators and other factors such as Amryt's ability to obtain financing, changes in the political, social and regulatory framework in which Amryt operates or in economic, technological or consumer trends or conditions . The delivery of this presentation shall not give rise to any implication that there have been no changes to the information and opinions contained in this presentation since the time specified . Subject to obligations under the AIM Rules for Companies published by London Stock Exchange plc, the Securities and Exchange Commission (“SEC”) and the Market Abuse Regulation (Regulation (EU) 596 / 2014 ) (each as amended from time to time), none of the Company, the Group, their affiliates and advisers and their respective directors, officers, partners, representatives, employees and agents, undertakes to publicly update or revise any such information or opinions, including without limitation, any forward - looking statement or any other statements contained in this presentation, whether as a result of new information, future events or otherwise . In giving this presentation none of the Company, the Group, their affiliates and advisers and their respective directors, officers, partners, representatives, employees and agents, undertakes any obligation to provide the recipient with access to any additional information or to update any additional information or to correct any inaccuracies in any such information which may become apparent . Certain industry and market data contained in this presentation has been obtained from third party sources . Third party industry publications, studies and surveys generally state that the data contained therein have been obtained from sources believed to be reliable, but that there is no guarantee of the accuracy or completeness of such data . While the Company believes that each of these publications, studies or surveys has been prepared by a reputable source, the Company has not independently verified the data contained therein . In addition, certain of the industry, scientific and market data contained in this presentation comes from the Company’s own internal case studies, research and estimates based on the knowledge and experience of the Company’s management in the market in which it operates . While the Company believes that such research, estimates and results from its case studies are reasonable and reliable, they, and their underlying methodology and assumptions, have not been verified by any independent source for accuracy or completeness unless otherwise stated and are subject to change without notice . Risks and uncertainties affecting the Company are outlined further in the Company SEC filings . 3 FORWARD - LOOKING STATEMENTS

DR. JOE WILEY, CEO - WELCOME & INTRODUCTION

AMRYT MANAGEMENT TEAM IN ATTENDANCE COMPRISED OF INDUSTRY LEADERS IN RARE DISEASES DR. JOE WILEY CEO RORY NEALON COO/CFO DR. MARK SUMERAY Chief Medical Officer DR. HELEN PHILLIPS Head Of Medical Affairs SHEILA FRAME President Americas DR. GERRY GILLIGAN VP Manufacturing Supply Chain PAUL GREENLAND President EMEA Region DR. TRACY CUNNINGHAM VP Head of Development 5

SEPTEMBER 13, 2021 - AGENDA 6 Time (Eastern) 1000 - 1005 Introduction & Overview Dr. Joe Wiley, CEO 1005 - 1025 KOL - Epidermolysis Bullosa Discussion Dr. Harper Price, MD, FAAD, FAAP, Phoenix Children’s Hospital 1025 - 1050 Oleogel - S10 Update • Overview of Clinical Data • Regulatory Pathways • Launch Plans Dr. Tracy Cunningham, VP Head of Development Sheila Frame, President, Americas 1050 - 1115 KOL - Acromegaly Discussion Dr. Maria Fleseriu, MD, FACE, Oregon Health & Science University 1115 - 1145 Mycapssa ® • Acromegaly Sheila Frame, President, Americas • Market Opportunity • US Expansion Plans • NET - Pipeline Opportunities Dr. Mark Sumeray, CMO • Regulatory Pathways • Synergy Achievement Plans Rory Nealon, CFO / COO 1145 - 1200 Q&A Session Dr. Joe Wiley, Dr. Mark Sumeray, Rory Nealon, Sheila Frame, Dr. Helen Philips, Dr. Tracy Cunningham, Paul Greenland, Dr. Gerry Gilligan

DR. HARPER PRICE – EPIDERMOLYSIS BULLOSA DISCUSSION

DR. HARPER PRICE 8 Harper Price, MD completed her dermatology training at Penn State Milton S . Hershey Medical Center in Hershey, Pennsylvania, and her pediatric dermatology fellowship at New York University Medical Center, New York . She has served at Phoenix Children’s Hospital (PCH) since 2009 where she provides the latest medications and technology available for the specialized treatment of infants, children, adolescents and young adults affected by large nevi . Shortly after joining PCH, she became the program director of their pediatric dermatology fellowship . She now serves as Associate Chief of the Division of Dermatology . While a student, Dr . Price worked closely with doctors at NYU to review all the major nevus registries in existence at the time and undertook to combine all the data into a single collection . Nevus Outreach invited Dr . Price to attend and assist at the 2010 conference . Based on the strength of her work, she was invited to speak at the 2011 International Expert Meeting for Large Congenital Melanocytic Nevi and Neurocutaneous Melanocytosis in Tübingen, Germany, where she was recruited to serve as Assistant Director of a team of world experts to oversee creation of an all - new world - wide nevus patient data repository under the direction of Dr . Veronica Kinsler at Great Ormond Street Hospital in London . Dr . Price is a regular speaker at national dermatology and pediatric conferences . Her work is published in textbooks and peer - reviewed journals .

Epidermolysis Bullosa: D i s ea se S t a t e A w a r en e ss & Bu r d e n Harper N. Price, MD, FAAD, FAAP

Epidermolysis bullosa was first described in 1870, yet there is no FDA approved treatment or effective clinical standard of care today. Debra.org 10
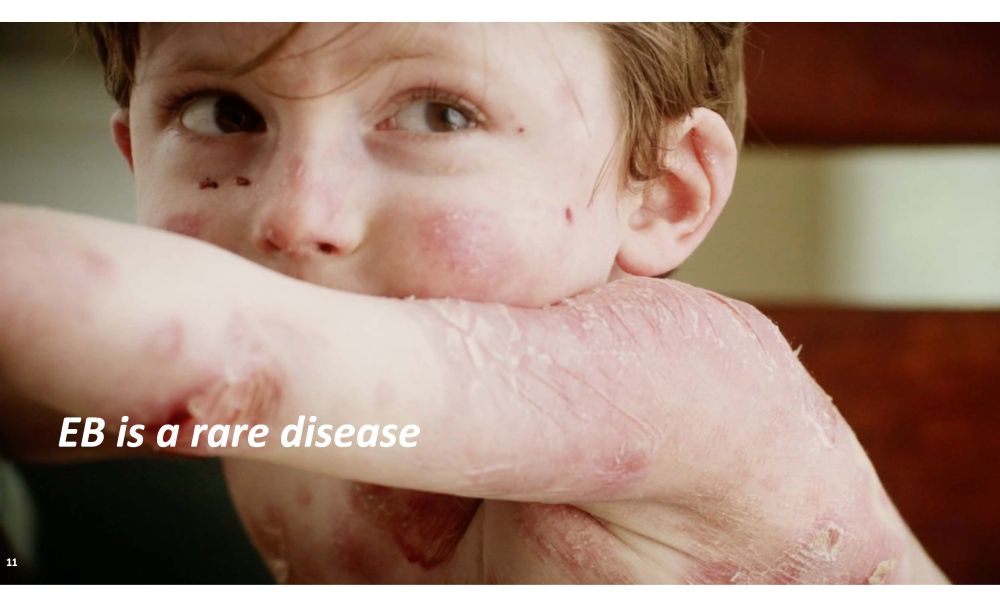
EB is a rare disease 11
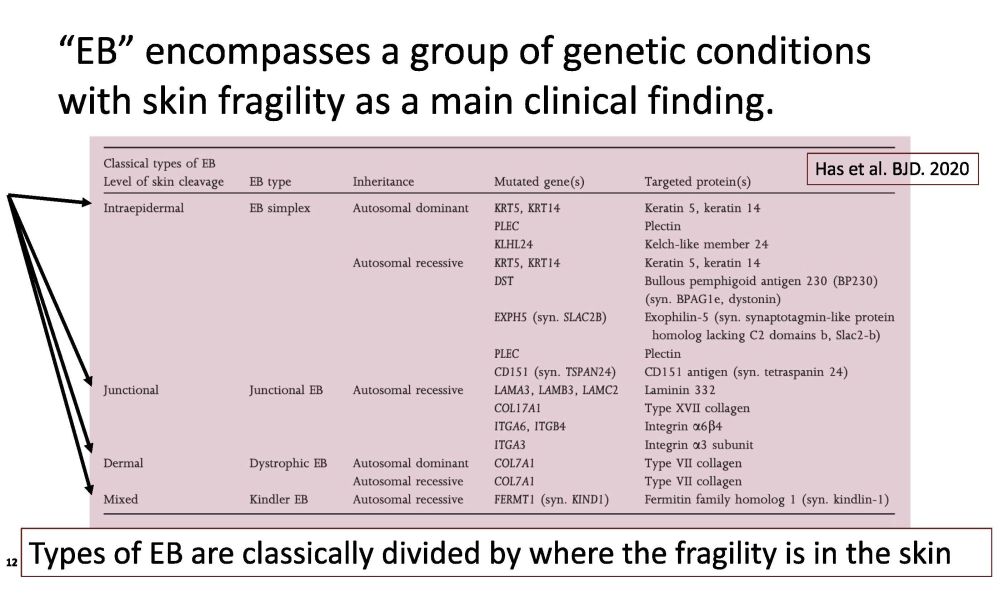
“EB” encompasses a group of genetic conditions with skin fragility as a main clinical finding. Types of EB are classically divided by where the fragility is in the skin Has et al. BJD. 2020 12
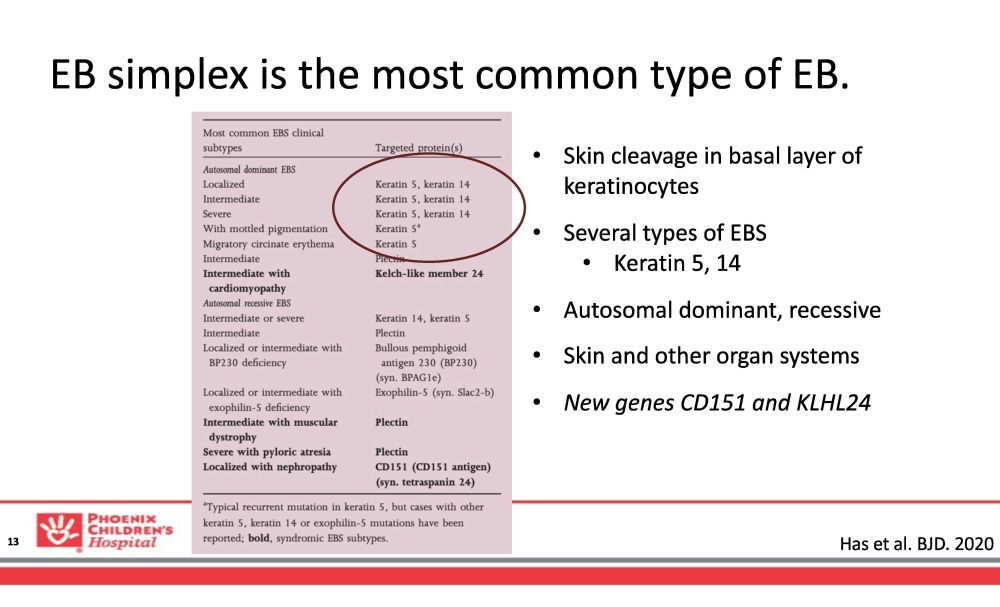
EB simplex is the most common type of EB. • Skin cleavage in basal layer of keratinocytes • Several types of EBS • Keratin 5, 14 • Autosomal dominant, recessive • Skin and other organ systems • New genes CD151 and KLHL24 Has et al. BJD. 2020 13

EB simplex blisters occur in the basal layer of the epidermis. 14 https:// www.britannica.com/science/human - skin
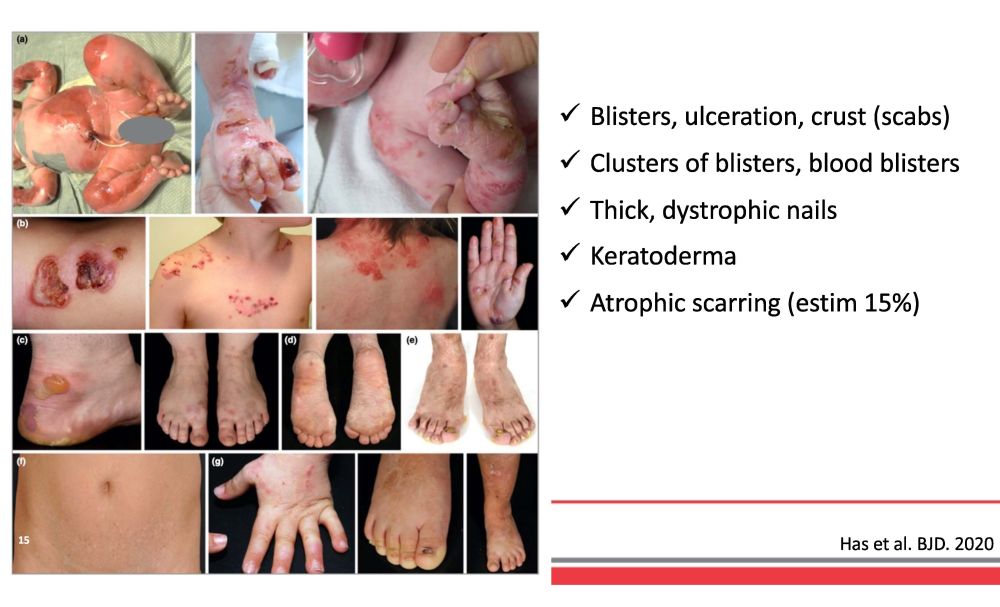
B li st e r s , u l ce r a t i o n , cru s t ( s c a b s) C l u st e r s o f b li st e r s , b l oo d b li st e r s Th i c k , d y s t r o ph i c na il s K e r at o de r m a A t r o ph i c s c arr i n g ( e s t i m 15% ) Has et al. BJD. 2020 15
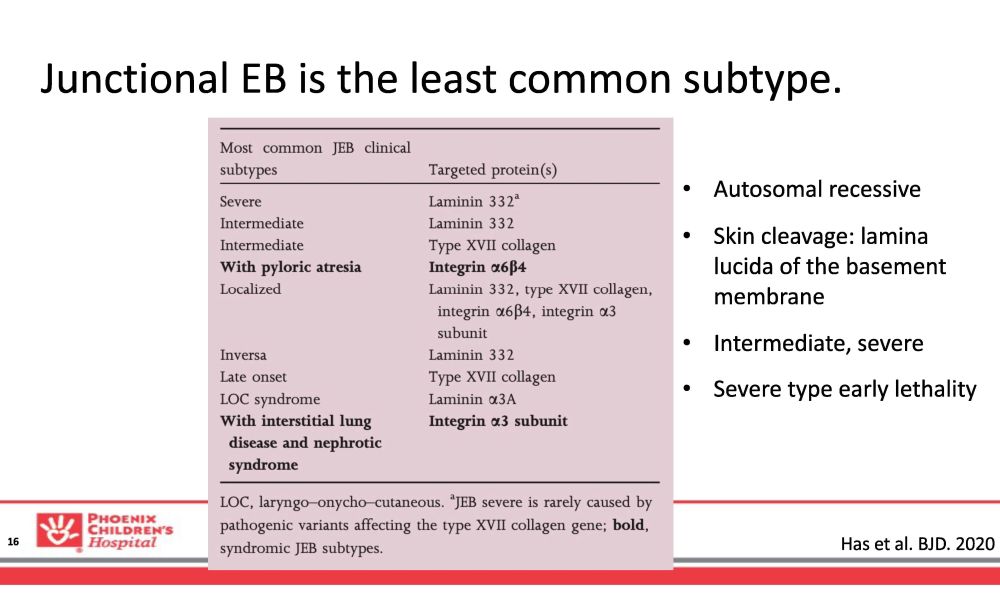
Junctional EB is the least common subtype. Has et al. BJD. 2020 16 • Autosomal recessive • Skin cleavage: lamina lucida of the basement membrane • Intermediate, severe • Severe type early lethality

JEB blisters occur in the lamina lucida. 17

G r anu l a t i o n t i ssu e o n th e d i st a l d i g i ts , f ace , ears H o a r s e c r y Skin blistering and crusts (scabs) Na i l l o s s an d d y s t r o p h y S c arr i n g A l o pec i a ( ha i r l o ss ) De n t a l ena m e l d e f ect s ( d i s c o l o r ed , p i t t e d teeth) Has et al. BJD. 2020 18

Dystrophic EB is caused by mutations in the gene encoding collagen VII , COL7A1 . Has et al. BJD. 2020 19 • AR and AD • RDEB is more severe • Overlap exists • Milia and scarring • Secondary c o m p li c a t i o n s
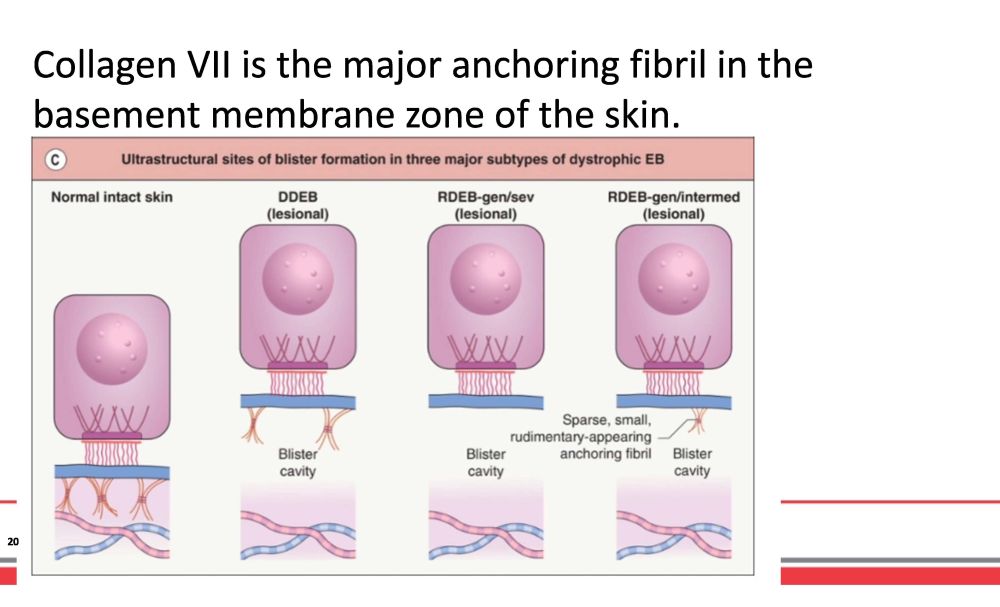
Collagen VII is the major anchoring fibril in the basement membrane zone of the skin. 20
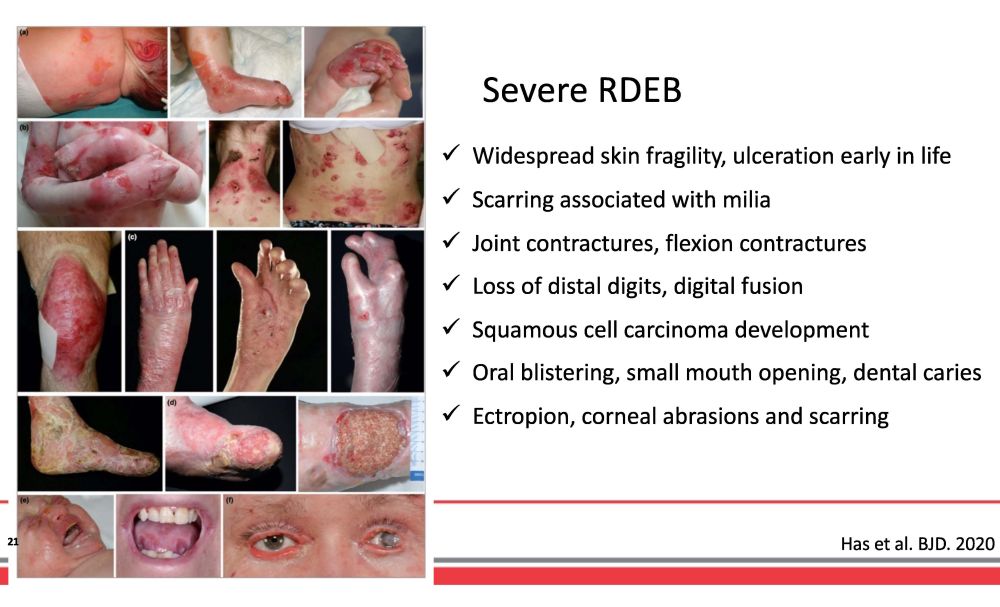
Widespread skin fragility, ulceration early in life Scarring associated with milia Joint contractures, flexion contractures Loss of distal digits, digital fusion Squamous cell carcinoma development Oral blistering, small mouth opening, dental caries Ectropion, corneal abrasions and scarring Severe RDEB Has et al. BJD. 2020 21

Dominant DEB, Intermediate RDEB 22 Often overlap in appearance Blisters over acral sites and bony areas Scarring, may be with milia Nail dystrophy or loss of nails Thickened skin on fingers, palms may cause contractures
Diagnosis is based on clinical information and molecular genetic testing. • Bedside exam where it starts • Family history, personal history • Presence or absence of clinical findings (scarring, milia) • Past induce a blister for biopsy X 2 – Immunomapping, electron microscopy (EM) • Current genetic testing (blood, cheek swab) 23

24
Molecular genetic testing for EB is [likely] the new gold standard. 25 • Allows for precise subtype information and prognosis – Clinical diagnosis is unreliable • Immunomapping and EM are not always accurate • Little phenotypic correlation and no subtype information previously • Allows for inclusion into clinical trials, treatments, natural history • Genetic counseling and prenatal diagnosis

EB disease process is complex. From: Prodinger, Bauer, Laimer. Exper Dermatol. 2020 26

The standard of care for EB is wound care and maximizing wound management. • Prevention and treatment of infection (localized to sepsis) • Protect from further injury, trauma • Facilitate wound healing • Limit, treat pain and itch • Monitor for skin cancer (RDEB) and stalled wounds • Lab monitoring for end organ damage, malnutrition 27
Many forms of EB do not just involve the skin. 28 • Eye involvement, blindness • Anemia (chronic/iron) • Urethral strictures • Elevated systemic inflammation • Malnutrition, vitamin deficiencies • Constipation, malabsorption, anal fissures • Esophageal strictures, reflux • Cardiomyopathy • Joint contractures • Oral disease, poor dentition • Airway disease • Kidney and lung disease • Delayed puberty • Osteopenia, osteoporosis

EB multidisciplinary care by a team is ideal. • Dermatologists (pediatric/adult) • Dedicated EB nurse • Clinic coordinator • Pain management • Occupational/Physical Therapy • Nutrition • Social worker/case management • Gynecology • Psychology/psychiatry • Palliative care • Surgical subspecialists • Gastroenterology • Hematology/oncology • Dental • Hospital medicine • Endocrinology • Rehab medicine • Ophthalmology • Nephrology/Urology 29

There are many unmet needs for patients with EB. • Limited access to testing and chronic need for supplies ($$$) • Few EB centers in USA and limited number of experts (transition?) • Localized treatments to heal wounds • Generalized disease modifying drugs – Not just for the skin! • Drugs to alleviate itch/pain 30
Financial impact for patients is burdensome. • Bandage coverage and cost is variable in the US – Out of pocket costs for wound care supplies $$$ • 26% respondents spent >$1000 monthly* – Limited insurance coverage • *73% Major or moderate financial impact (severe subtypes) *E. Gorrell at el. Pediatr Derm 2020 31

90 Question Survey 156 responses (patients/caregivers) Impact on: • Education • Career • Home life • QOL (neg) • Finances (neg) 32

NHS - 0482 - 2DC - 6N DERM & EB Nurse Patient and Family at EB center Social Work Case Manager Pain service and Psychology Hema t ology Oncology PT/OT Rehab GI and Nu t ri t ion Other medical subspecialis t s Patient support groups and resources Primary Care P rovider D E N T A L CLINICAL TRIALS & RESEARCH C O N SO R T IUM S Patient A dvisory Board 33

Clinical trials in EB — local to broad effects • Allogeneic cell therapies (keratinocytes, fibroblasts, stem cells) • Hematopoietic stem cell transplant (BMT) • Gene therapies -- ex vivo, in vivo • Disease modifying, symptom relief therapies – Address the inflammatory and fibrotic processes • Novel and re - purposed topical treatments 34

clinicaltrials.gov, 23 interventional studies* *Recruiting, Enrolling by invitation 35

clinicaltrials.gov, 13 active, not recruiting 36

How can clinical trials in EB potentially improve the patient’s disease burden? 37 • Clarify or confirm genetic diagnosis if testing is provided • More frequent visits and provider contact • No/less discrimination based on insurance, SES, location • Potential for short term relief from itch, pain, wound burden • Potential for long term open label access to drug

Lessons learned from an EB PI 38 • Patients (subjects) are often desperate to try anything • Deviation from typical wound care or increase burden of wound care at home is a barrier and burden • Wounds are dynamic, lots of factors to consider • Patient reported outcomes (QOL, itch, pain, etc) are critical • Beware of exhausting your own study populations

hprice@phoenixchildrens.com EPIDERMOLYSIS BULLOSA CLINICAL RESEARCH CONSORTIUM (EBCRC) 39 Thank You

OLEOGEL - S10 UPDATE DR. TRACY CUNNINGHAM, VP, HEAD OF DEVELOPMENT SHEILA FRAME, PRESIDENT, AMERICAS

OLEOGEL - S10 EASE PHASE 3 STUDY IN EB DOUBLE BLIND, RANDOMIZED, PLACEBO CONTROLLED, PHASE 3, EFFICACY AND SAFETY STUDY OF OLEOGEL - S10 IN PATIENTS WITH JUNCTIONAL AND DYSTROPHIC EB P r i mar y e ndpo i nt met , S e p tem b e r 202 0 p - va l ue = 0 . 013 Control Gel + dressing Randomisation 1:1 (stratified by EB subtype and target wound size) Oleogel - S10 + dressing Visit schedule 0 D a y M 90 3 Month Day D D D D 0 1 4 3 0 4 5 60 M 12 M 24 90 - D A Y D O UB L E - B L I ND PHASE 2 YEAR OPEN LABEL EXTENSION Primary Endpoint: proportion of target wounds healed by day 45 41 Oleogel - S10 + dressing LARGEST EVER GLOBAL PHASE 3 STUDY IN EB Oleogel - S10 (Filsuvez®) is an investigational product for the treatment of EB
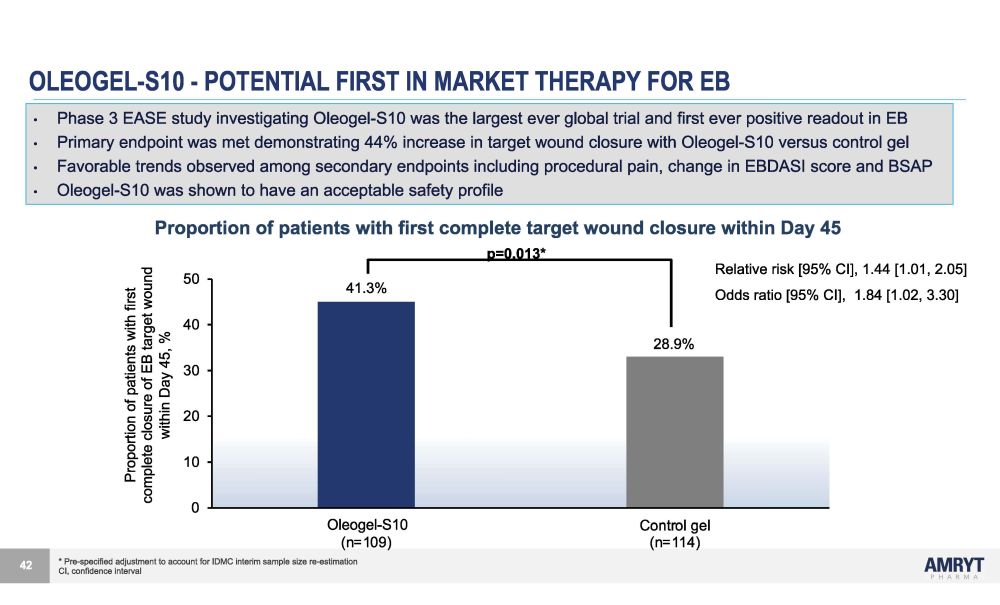
OLEOGEL - S10 - POTENTIAL FIRST IN MARKET THERAPY FOR EB * Pre - specified adjustment to account for IDMC interim sample size re - estimation CI, confidence interval Relative risk [95% CI], 1.44 [1.01, 2.05] Odds ratio [95% CI], 1.84 [1.02, 3.30] 30 20 10 0 40 50 O l eo g e l - S 1 0 Control gel (n=114) O l eoge l - S 1 0 (n=109) 41.3% 28.9% p=0.013* Proportion of patients with first complete closure of EB target wound within Day 45, % 42 • Phase 3 EASE study investigating Oleogel - S10 was the largest ever global trial and first ever positive readout in EB • Primary endpoint was met demonstrating 44% increase in target wound closure with Oleogel - S10 versus control gel • Favorable trends observed among secondary endpoints including procedural pain, change in EBDASI score and BSAP • Oleogel - S10 was shown to have an acceptable safety profile Proportion of patients with first complete target wound closure within Day 45
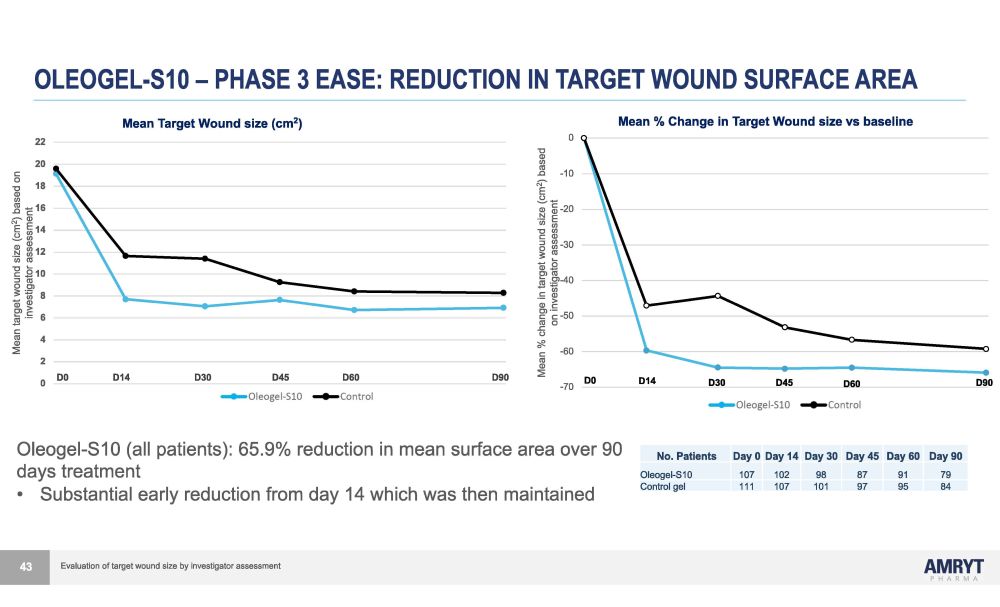
OLEOGEL - S10 – PHASE 3 EASE: REDUCTION IN TARGET WOUND SURFACE AREA D0 D14 D30 D45 D60 D90 0 2 4 6 8 10 12 14 16 18 20 22 Mean target wound size (cm 2 ) based on investigator assessment - 7 0 - 6 0 - 5 0 - 4 0 - 3 0 - 2 0 - 1 0 0 Mean % change in target wound size (cm 2 ) based on investigator assessment Oleogel - S10 (all) Control (all) D 6 0 Oleogel - S10 (all patients): 65.9% reduction in mean surface area over 90 days treatment • Substantial early reduction from day 14 which was then maintained No. Patients Day 0 Day 14 Day 30 Day 45 Day 60 Day 90 Oleogel - S10 107 102 98 87 91 79 Control gel 111 107 101 97 95 84 43 Mean Target Wound size (cm 2 ) D0 D 1 4 D 3 0 D 4 5 D 9 0 Mean % Change in Target Wound size vs baseline Evaluation of target wound size by investigator assessment
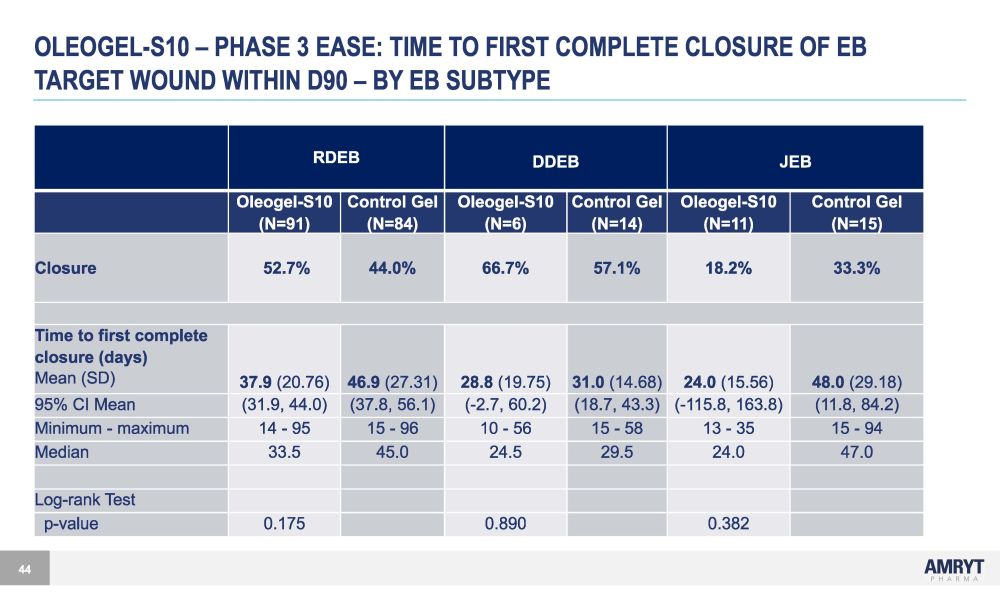
OLEOGEL - S10 – PHASE 3 EASE: TIME TO FIRST COMPLETE CLOSURE OF EB TARGET WOUND WITHIN D90 – BY EB SUBTYPE 44 RDEB DDEB JEB Oleogel - S10 (N=91) Control Gel (N=84) Oleogel - S10 (N=6) Control Gel (N=14) Oleogel - S10 (N=11) Control Gel (N=15) Closure 52.7% 44.0% 66.7% 57.1% 18.2% 33.3% Time to first complete closure (days) Mean (SD) 37.9 (20.76) 46.9 (27.31) 28.8 (19.75) 31.0 (14.68) 24.0 (15.56) 48.0 (29.18) 95% CI Mean (31.9, 44.0) (37.8, 56.1) ( - 2.7, 60.2) (18.7, 43.3) ( - 115.8, 163.8) (11.8, 84.2) Minimum - maximum 14 - 95 15 - 96 10 - 56 15 - 58 13 - 35 15 - 94 Median 33.5 45.0 24.5 29.5 24.0 47.0 Log - rank Test p - value 0.175 0.890 0.382
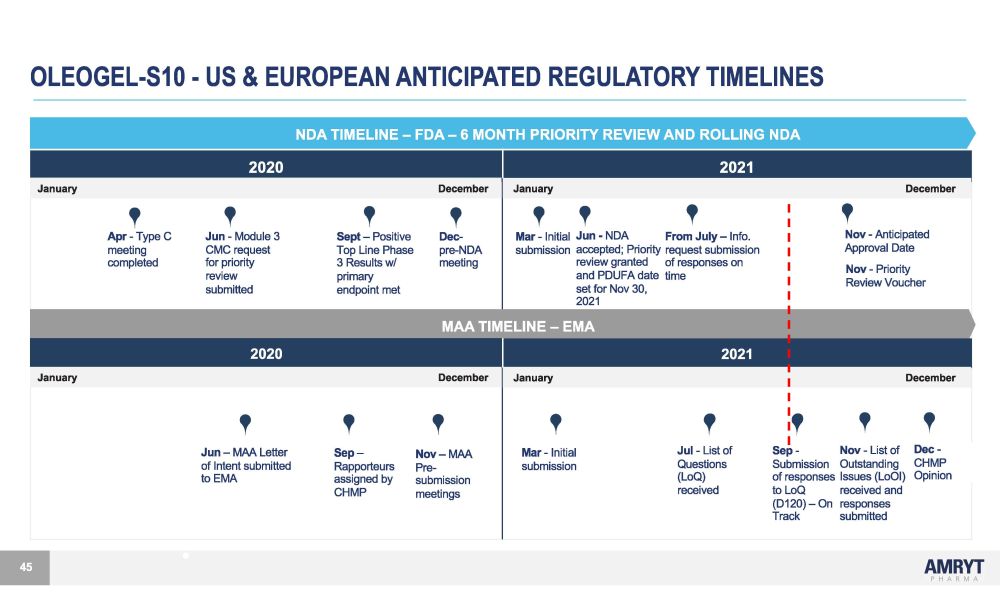
OLEOGEL - S10 - US & EUROPEAN ANTICIPATED REGULATORY TIMELINES NDA TIMELINE – FDA – 6 MONTH PRIORITY REVIEW AND ROLLING NDA 2020 2021 January December January December A p r - T yp e C J u n - M odu l e 3 S e p t – P os i t i v e D ec - M a r - I n i t i a l J u n - ND A F r o m J u l y – I n f o . meeting CMC request Top Line Phase pre - NDA submission accepted; Priority request submission co m p l e t e d f o r p r i o r i t y 3 R e s u l t s w / m ee t i n g r e v i e w g r an t e d o f r e s pon s e s on r e v i e w p r i m a r y an d PDUF A da t e t i m e sub m i tt e d endpo i n t m e t se t f o r N o v 30 , 2021 MAA TIMELINE – EMA N o v - A n t i c i pa t e d Approval Date N o v - P r i o r i t y R e v i e w V ou c he r 2020 2021 January December January December J u n – M A A Le tt e r o f I n t en t s ub m i tt ed to EMA S e p – R appo rt eu r s a ss i gne d by N o v �� M A A Pre - sub m i ss i o n M a r - I n i t i a l submission J u l - L i s t of Q ues t i on s (LoQ) S e p - N o v - L i s t o f D e c - Submission Outstanding CHMP of responses Issues (LoOI) Opinion CHMP meetings received t o Lo Q r e c e i v e d an d (D120) – On responses Track submitted 45

“I believe, and I know in my heart of hearts, that we will have at least one approved drug to treat EB by next Father’s Day.” Brett Kopelan, Executive of Debra of America Oleogel - S10 (Filsuvez®) is an investigational product for the treatment of EB 46 “ A big step in the right direction!” Michael P. Hund Chief Executive Officer, EB Research Partnership 7 8 D A Y S T O P DU F A – THE M A R KE T I S R EA DY… .A ND S O A RE WE POTENTIAL FIRST AND ONLY APPROVED PRODUCT TO TREAT EB

TEN CENTERS IN THE US APPEAR TO HAVE THE MAJORITY OF SEVERE EB PATIENTS C i n c i nna t i C H / U n i v . o f O h i o * S t an f o r d CH * K e y ce n t e r s C o l o r ad o C H / U n i v . o f C o l o r ad o M o r ga n S t an l e y C H / C o l u m b i a U n i v . U n i v . o f M i nne s o t a M a s on i c CH Lu r i e C H / N o rt h w e s t e r n U n i v . Phoenix CH / Univ. of Arizona. Jackson Memorial Hospital / Univ. of Miami U n i v . o f M a ss a c hu s e tt s M e m o r i a l CH H en r y F o r d H o s p i t a l O t he r cen t e r s tr ea t i n g E B pa t i en t s National centers M a j o r cen t e r s w i t h E B c li n i c Minor centers with strong pediatric dermatology, also seeing adults 47 Cincinnati CH is the largest EB center in the US with 150 patients but is strictly pediatric; Stanford is the only hospital with a dedicated adult EB clinic in addition to a pediatric clinic National cen t e r s o f reference f o r D eb r a
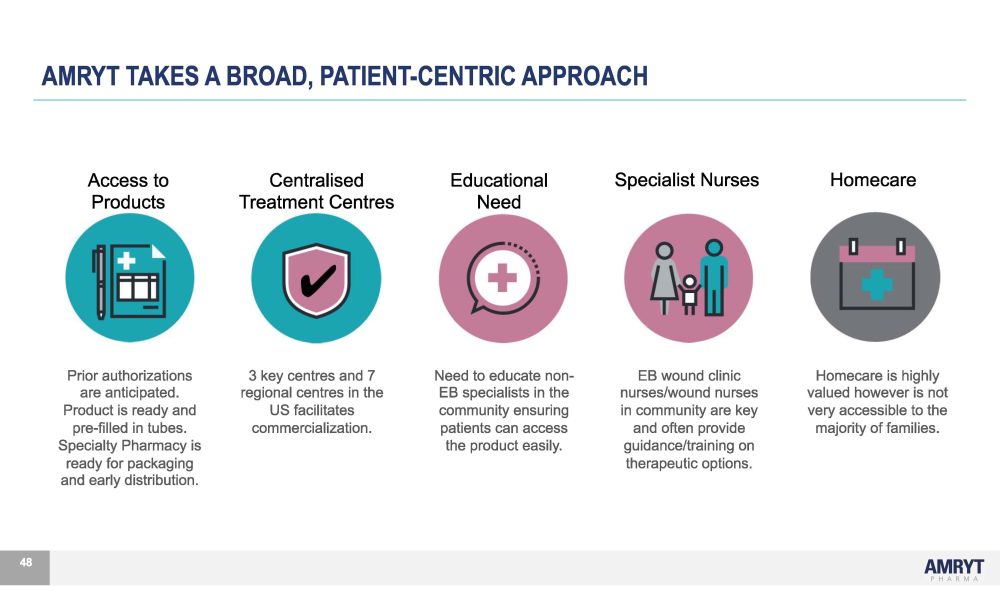
Prior authorizations are anticipated. Product is ready and pre - filled in tubes. Specialty Pharmacy is ready for packaging and early distribution. 48 3 key centres and 7 regional centres in the US facilitates commercialization. Need to educate non - EB specialists in the community ensuring patients can access the product easily. EB wound clinic nurses/wound nurses in community are key and often provide guidance/training on therapeutic options. Homecare is highly valued however is not very accessible to the majority of families. Access to Products Centralised Treatment Centres Edu c at i ona l Need Specialist Nurses Homecare AMRYT TAKES A BROAD, PATIENT - CENTRIC APPROACH

MAKING OLEOGEL - S10 THE BACKBONE OF TREATMENT IN SEVERE EB* 49 Preparing for Launch • Dedicated expert medical affairs team in field • Early payer engagement • Dedicated dermatology commercial team trained for day 1 launch • Exceptional patient support services • Easy to use and ready for distribution directly to patients to have on hand day 1 *Seeking FDA approval for the indication of accelerated healing of wounds in adult and pediatric patients with dystrophic (DEB) and junctional epidermolysis bullosa (JEB), two of the most severe forms of EB.
50 78 DAYS TO TARGET PDUFA – THE MARKET IS READY…AND SO ARE WE Oleogel - S10 demonstrated accelerated wound healing, with an acceptable safety profile, and conveniently fits into patients' daily routine at home. • Commercial launch plans at advanced stage • Dedicated medical affairs team in field • Dedicated commercial team in recruitment • Enthusiasm among the EB community • Strong partnership with patient advocacy organizations • Website launched: www.livingwitheb.com • Engage payers to achieve early and sustainable patient access by minimizing access barriers • Ensure seamless customer experience 50

DR. MARIA FLESERIU - ACROMEGALY DISCUSSION

DR. MARIA FLESERIU Maria Fleseriu, MD, FACE is a Professor of Medicine and Neurological Surgery and Director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon, USA and Past President of the Pituitary Society . Dr . Fleseriu has a long - standing clinical and research interest in the pathophysiology and treatment of pituitary and adrenal disorders and has been global principal investigator in many pituitary clinical trials . She is a frequent plenary guest speaker at national and international meetings and has authored over 200 manuscripts, including guidelines, consensus papers and book chapters . Major focus on research now is individualized treatment of acromegaly and Cushing’s . Dr . Fleseriu has been awarded the title of “Doctor Honors Causa” by the University of Medicine and Pharmacy “Carol Davila” Bucharest, she serves on the Board of Directors for Pituitary Society as Program Chair and she is past chair of the Endocrine Society Guidelines Committee and the Hypopituitarism task force . She has also served on several committees for the Endocrine Society, Pituitary Society, European Society of Endocrinology and American Association of Clinical Endocrinology . Dr . Fleseriu is Associate Editor for European Journal of Endocrinology , Chief Editor of Pituitary Endocrin ology for Frontiers in Endocrinology, Section Head for Pituitary and Neuroendocrine F 1000 , Associate Editor for Reviews in Endocrinology and Metabolism, Senior Editor for Endocrinology, Diabetes and Metabolism CR and a member of the editorial board of Pituitary . She has been involved in leaders hip positions of educational programs sponsored by the Endocrine Society, the Pituitary Society, and patient advocacy groups to teach physicians and patients about pituitary tumors and neuroendocrine disorders . She has served on multiple scientific advisory boards for biotechnology and pharmaceutical companies and participated in study design and has been global principal investigator for several Cushing’s and acromegaly studies . 52

Acromegaly updates and Oral Octreotide Clinical Trials (Focus on Chiasma OPTIMAL) Maria Fleseriu, MD, FACE Professor Medicine (Endocrinology) and Neurological Surgery Director Pituitary Center Oregon Health & Science University, Portland, OR 53
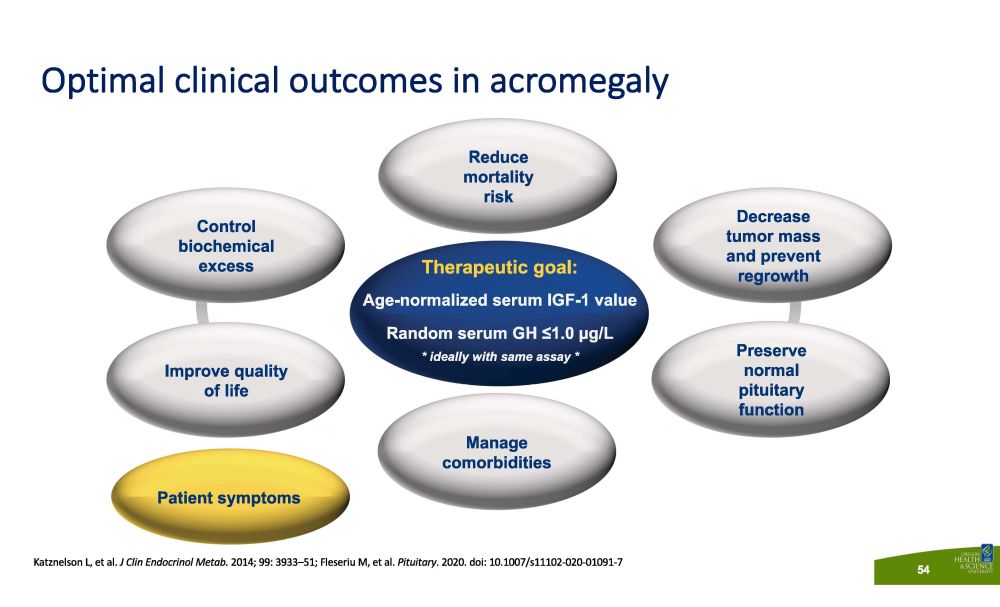
Katznelson L, et al. J Clin Endocrinol Metab. 2014; 99: 3933 – 51; Fleseriu M, et al. Pituitary . 2020. doi: 10.1007/s11102 - 020 - 01091 - 7 Optimal clinical outcomes in acromegaly 54 Therapeutic goal: Age - normalized serum IGF - 1 value Random serum GH ≤1.0 µg/L * ideally with same assay * Control b i oche m i cal excess Decrease tumor mass and prevent regrowth P r ese r v e normal pituitary function Manage co m o r b i d i t i es Improve quality of life Reduce m o rt a li ty risk Patient symptoms
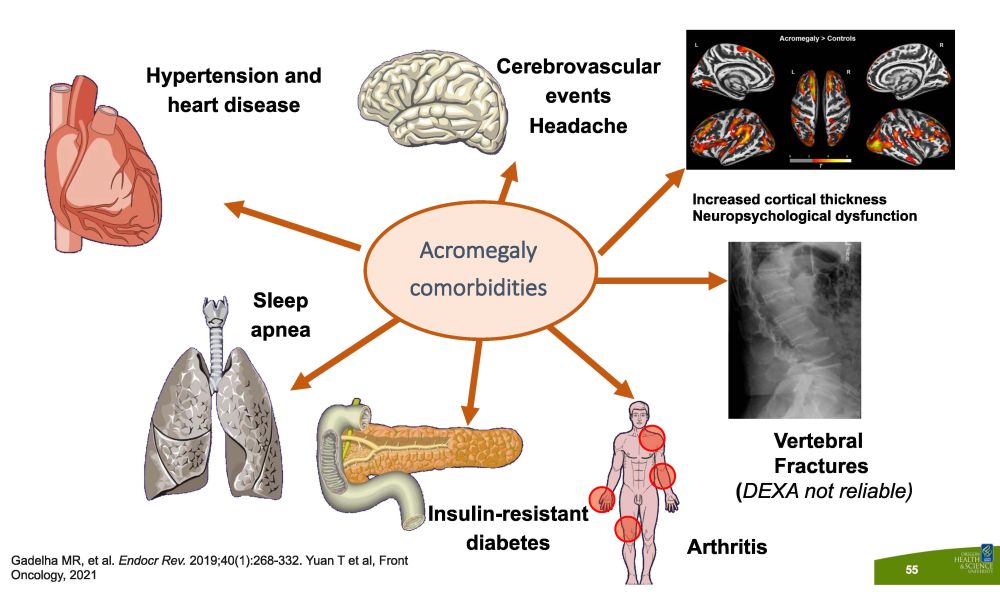
Vertebral F r ac t u r es ( DEXA not reliable) Hypertension and heart disease C ere b r o vasc u l a r events Headache Sleep apnea A rt h r i t i s I n s u li n - r es i s t ant diabetes Acromegaly c om or b i d i t i es Gadelha MR, et al. Endocr Rev. 2019;40(1):268 - 332. Yuan T et al, Front Oncology, 2021 Increased cortical thickness Neuropsychological dysfunction 55
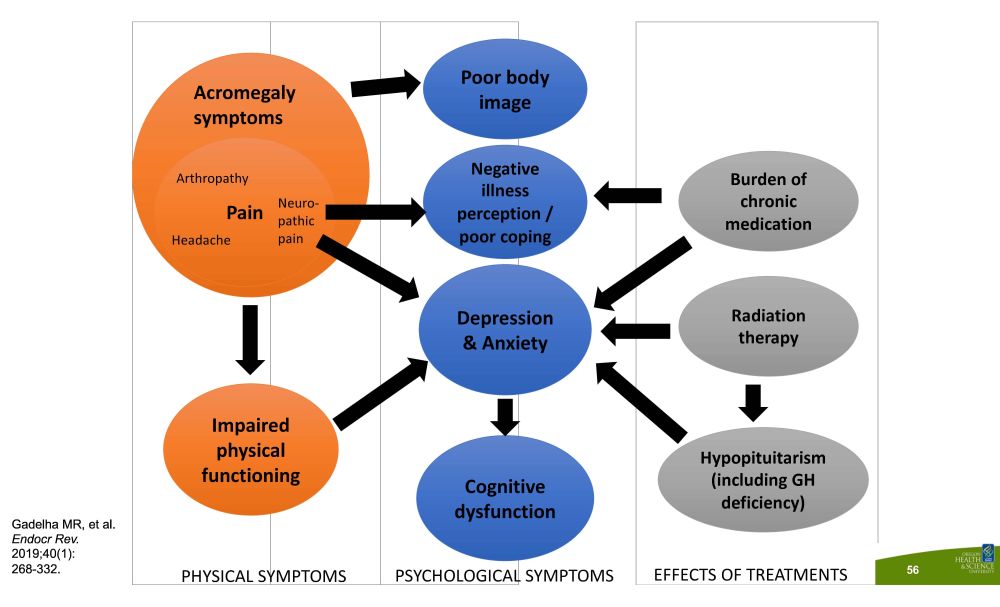
D e p r e ss i o n & Anxiety Cognitive d ys func t io n Poor body image Negative illness perception / poor coping Burden of chronic m e di c a t i o n Radi a t i o n therapy H ypopi t ui t ari s m (including GH deficiency) Impaired physical func t i on i ng N eu r o - pathic pain Arthropathy P ai n Headache PHYSICAL SYMPTOMS PSYCHOLOGICAL SYMPTOMS EFFECTS OF TREATMENTS Ac r om e g al y symptoms Gadelha MR, et al. Endocr Rev. 2019;40(1): 268 - 332. 56

Medical therapies: How do we decide what to use and when? Frequency of ad m i n i s tr a t i on Route of ad m i n i s tr a t i on Efficacy Sa f e ty Tumor volume Patient p r e f e r ence Availability Biomarkers M ed i c a l t he r ap y GH and IGF - 1 levels HCP - or self - adm i n is tered ? Giustina et al. Rev Endocr Metab Disord. 2 020; 21: 667 - 78; Giustina, et al. J Clin Endocrinol Metab. 2020, 105(4): e937 – 46. 57
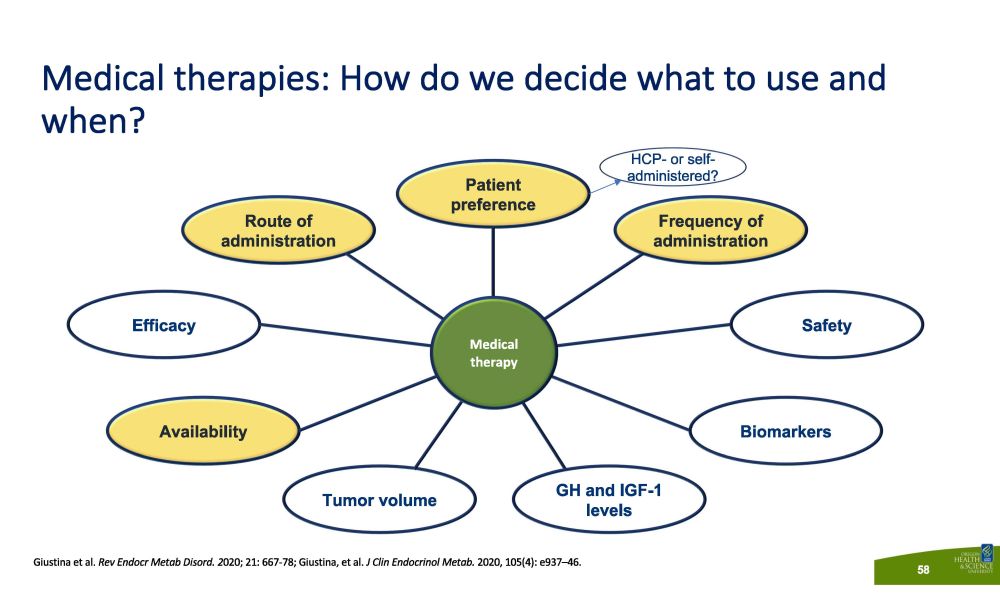
Medical therapies: How do we decide what to use and when? Frequency of ad m i n i s tr a t i on Route of ad m i n i s tr a t i on Efficacy Sa f e ty Tumor volume Patient p r e f e r ence Availability Biomarkers M ed i c a l t he r ap y GH and IGF - 1 levels HCP - or self - administered? Giustina et al. Rev Endocr Metab Disord. 2 020; 21: 667 - 78; Giustina, et al. J Clin Endocrinol Metab. 2020, 105(4): e937 – 46. 58
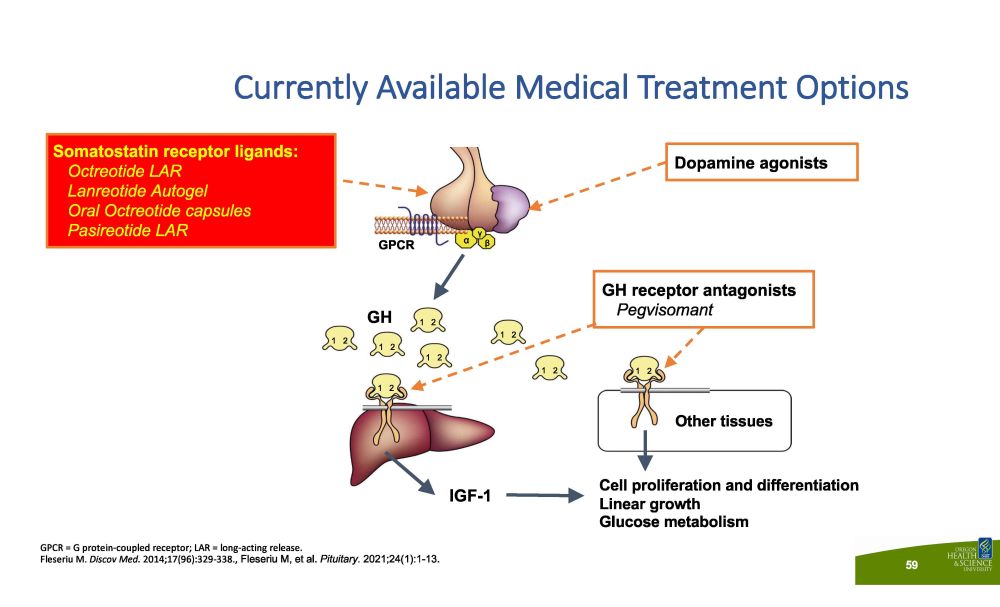
Currently Available Medical Treatment Options GPCR = G protein - coupled receptor; LAR = long - acting release. Fleseriu M. Discov Med. 2014;17(96):329 - 338., Fleseriu M, et al. Pituitary. 2021;24(1):1 - 13. IG F - 1 Other tissues G PC R 1 2 GH 1 2 1 2 1 2 1 2 1 2 1 2 1 2 Dopamine agonists Cell proliferation and differentiation Linear growth Glucose metabolism GH receptor antagonists Pegvisomant α γ β Somatostatin receptor ligands: Octreotide LAR Lanreotide Autogel Oral Octreotide capsules Pasireotide LAR 59
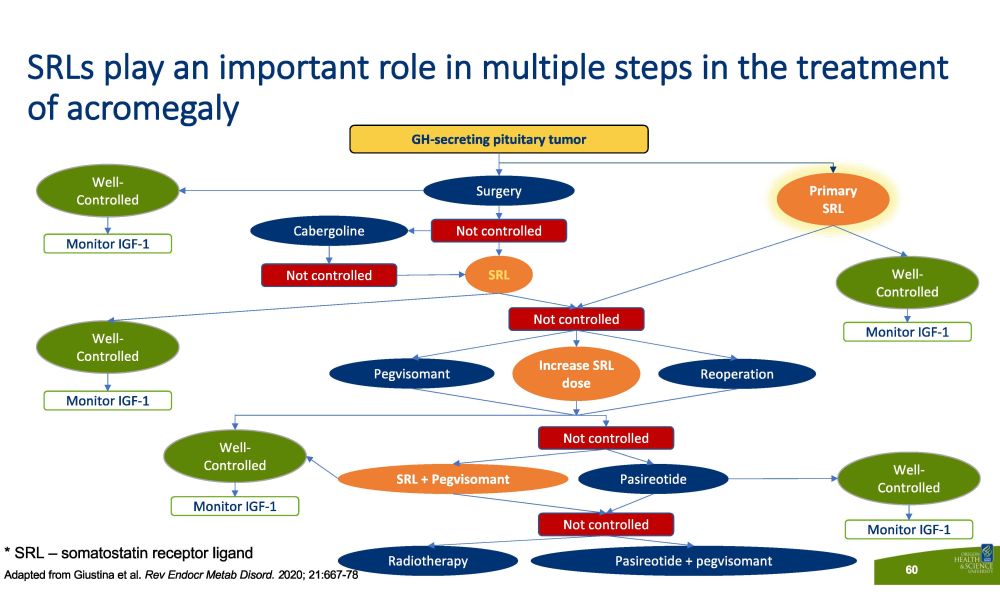
SRLs play an important role in multiple steps in the treatment of acromegaly GH - secreting pituitary tumor Not controlled S u r ge r y Well - C o n t ro lled Monitor IGF - 1 Pr i m a ry SRL Monitor IGF - 1 Monitor IGF - 1 Monitor IGF - 1 Monitor IGF - 1 Not controlled Not controlled Not controlled Not controlled Well - C o n t ro lled Well - C o n t ro lled Well - C o n t ro lled Well - C o n t ro lled S R L Cabergoline Pegvisomant Increase SRL dose Reoperation SRL + Pegvisomant Pasireotide R a di o t he r a py Pasireotide + pegvisomant * SRL – somatostatin receptor ligand Adapted from Giustina et al. Rev Endocr Metab Disord. 2 020; 21:667 - 78 60
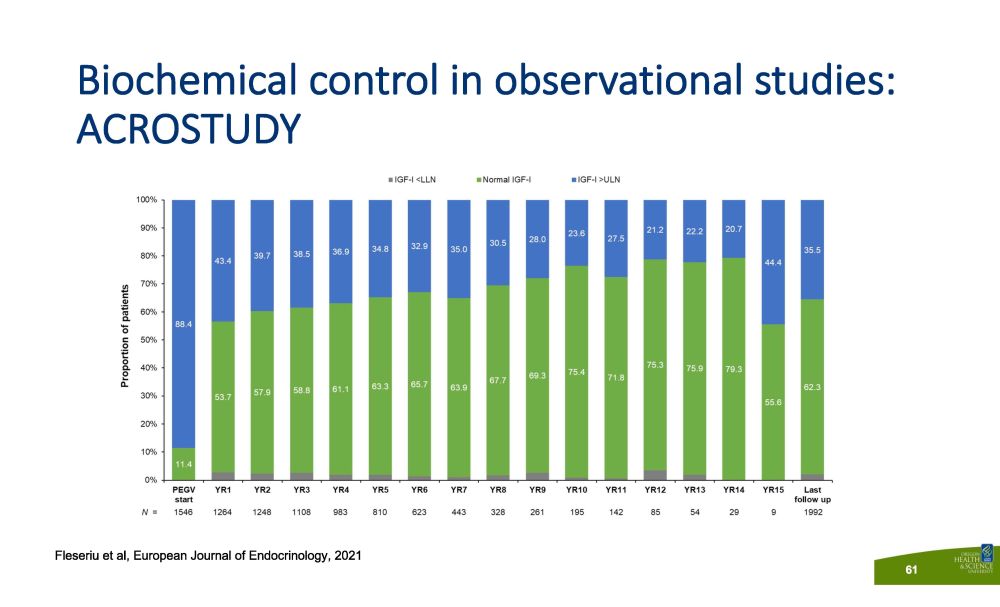
Biochemical control in observational studies: ACROSTUDY Fleseriu et al, European Journal of Endocrinology, 2021 61

OOC, oral octreotide capsules. 1. Strasburger CJ, et al. Eur J Endocrinol. 2016;174(3):355 - 362. 2. Fleseriu M et al, Pituitary 2020, Aug;23(4):347 - 358. Current SRL injection therapies carry significant treatment burdens 1,2 B r ea k th r ou gh Symptoms Injection Site Reactions Quality of Life P a i n Unmet need in the treatment of acromegaly To provide a potential option that could address challenges with injections, Oral Octreotide Capsules (OOC) were developed 62

Geer E, et al. Pituitary . 2019; https//doi.org/10.1007/s11102 - 019 - 0103 - 2. Concordance between patient - reported and HCP - reported treatment outcomes is low Patients reported more : • Headache • Excess sweating • Joint pain • Carpal tunnel syndrome • Vision problems • Swelling • Snoring • Acro - fog HCPs reported more : • Fatigue • Weakness • Feeling tired 63
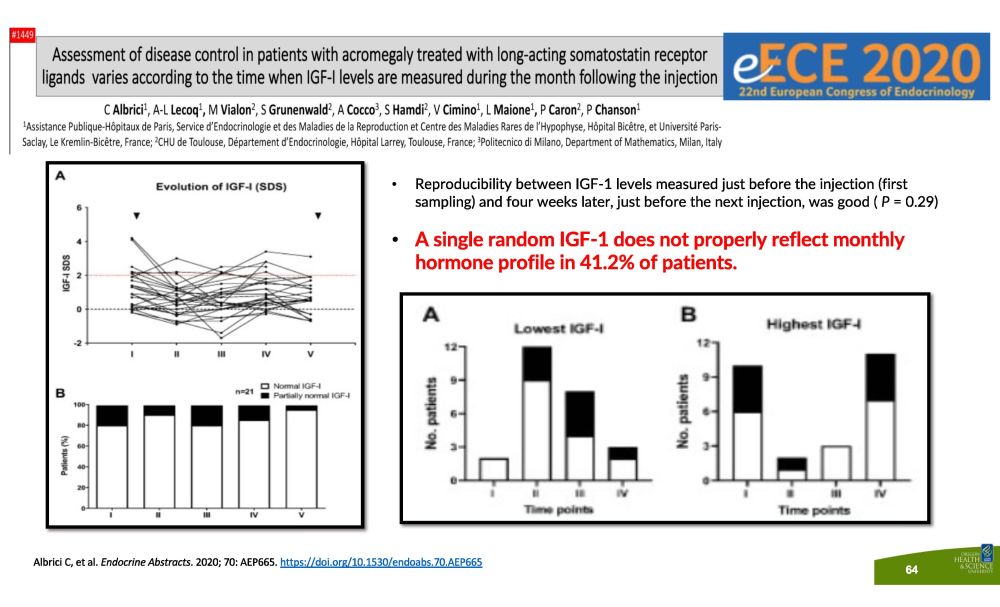
• Reproducibility between IGF - 1 levels measured just before the injection (first sampling) and four weeks later, just before the next injection, was good ( P = 0.29) • A single random IGF - 1 does not properly reflect monthly h o r m o n e p r o f i le i n 41 . 2 % o f p a t i e n t s . Albrici C, et al. Endocrine Abstracts . 2020; 70: AEP665. https://doi.org/10.1530/endoabs.70.AEP665 64
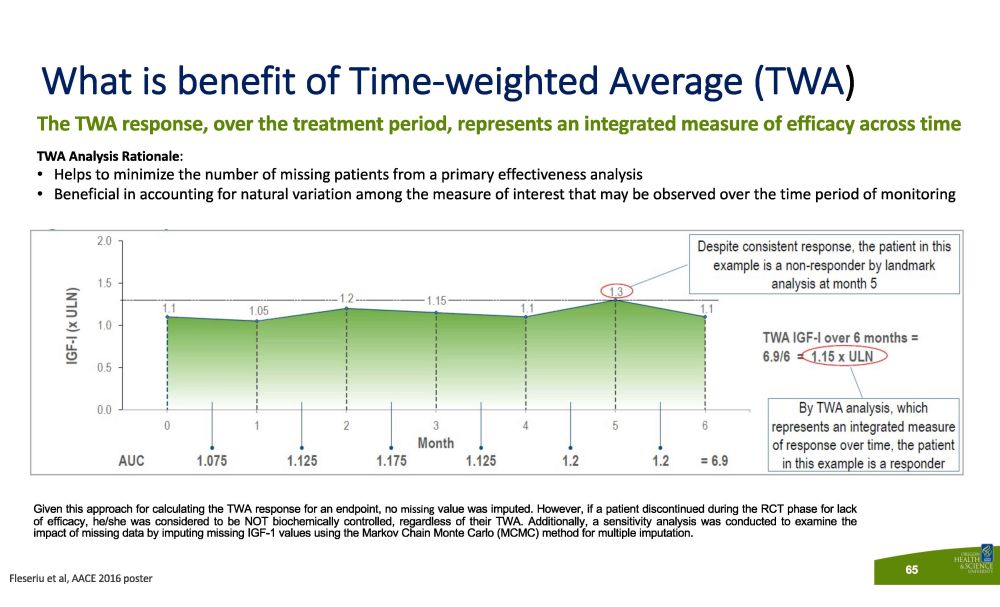
The TWA response, over the treatment period, represents an integrated measure of efficacy across time TWA Analysis Rationale : • Helps to minimize the number of missing patients from a primary effectiveness analysis • Beneficial in accounting for natural variation among the measure of interest that may be observed over the time period of monitoring Given this approach for calculating the TWA response for an endpoint, no missing value was imputed . However, if a patient discontinued during the RCT phase for lack of efficacy, he/she was considered to be NOT biochemically controlled, regardless of their TWA . Additionally, a sensitivity analysis was conducted to examine the impact of missing data by imputing missing IGF - 1 values using the Markov Chain Monte Carlo (MCMC) method for multiple imputation . What is benefit of Time - weighted Average (TWA ) Fleseriu et al, AACE 2016 poster 65
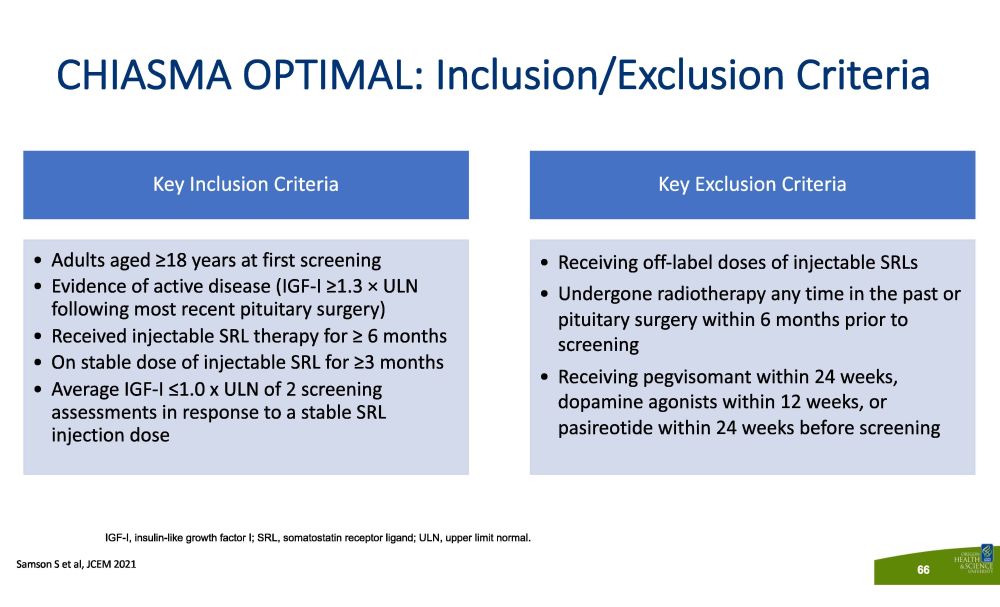
CHIASMA OPTIMAL: Inclusion/Exclusion Criteria S a m so n S e t a l , JCE M 202 1 66 Key Inclusion Criteria • Adults aged ≥18 years at first screening • Evidence of active disease (IGF - I ≥1.3 п ULN following most recent pituitary surgery) • Received injectable SRL therapy for ≥ 6 months • On stable dose of injectable SRL for ≥3 months • Average IGF - I ≤ 1 . 0 x ULN of 2 screening assessments in response to a stable SRL injection dose Key Exclusion Criteria • Receiving off - label doses of injectable SRLs • Undergone radiotherapy any time in the past or pituitary surgery within 6 months prior to screening • Receiving pegvisomant within 24 weeks, dopamine agonists within 12 weeks, or pasireotide within 24 weeks before screening IGF - I, insulin - like growth factor I; SRL, somatostatin receptor ligand; ULN, upper limit normal.

Open label extension (OLE) Ba s e li n e n=28 Placeb o n=28 Last somatostatin receptor ligand i njection Sc r een i n g Primary endpoint 34 36 wk Withdrawal IG F - I Oral Octreotide Capsules (OOC) Rescue injection Predefined withdrawal criteria (both arms) • IGF - I ≥1.3 x ULN for 2 consecutive visits on the highest dose and exacerbation of clinical signs/symptoms • Early terminated patients followed ≤ 36 weeks on injections, per protocol 40 mg 60 mg 80 mg 60 mg 8 0 mg 4 0 mg CHIASMA OPTIMAL: Study Design S a m so n S e t a l , JCE M 202 1 67 DPC, double - blind placebo - controlled; IGF - I, insulin - like growth factor I; OLE, open - label extension; OOC, oral octreotide capsules; ULN, upper limit of normal. Double - blind placebo - controlled (DPC) (36 weeks) Oral Octreotide Capsules (OOC)

CHIASMA OPTIMAL: Patient Disposition Screened (N=119) Randomized (n=56) Placebo (n=28) Early treatment discontinuation (n=19) Treatment failure (n=18) Adverse events (n=1) Completed on study drug (n=9) Oral Octreotide Capsules (n=28) Completed on study drug (n=21) 40 mg (n=6) 60 mg (n=2) 80 mg (n=13) Early treatment discontinuation (n=7) 40 mg (n=1) 80 mg (n=6) Treatment failure (n=5) Adverse events (n=2) Screen failure (n=63) S a m so n S e t a l , JCE M 202 1 68
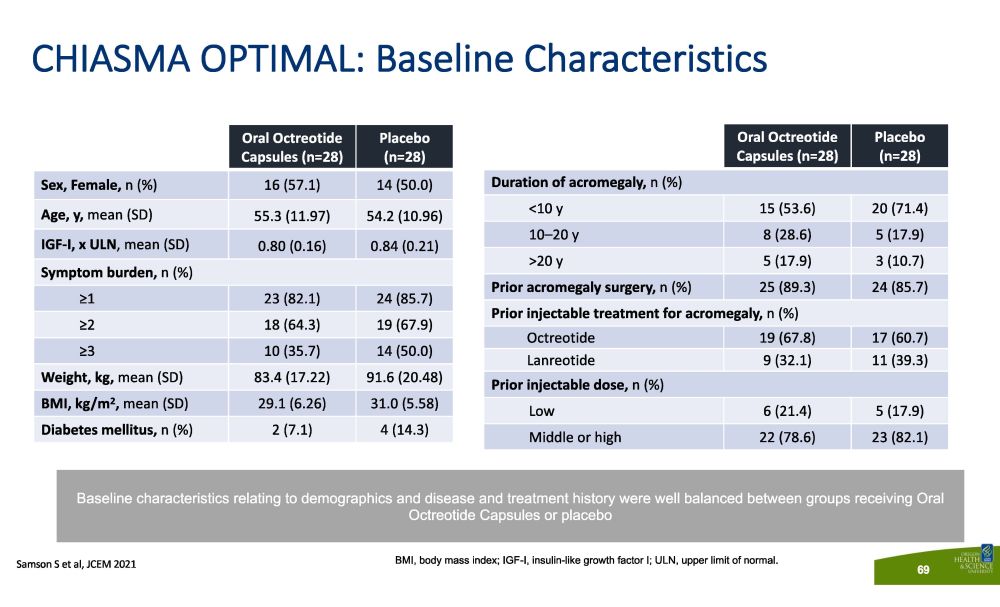
CHIASMA OPTIMAL: Baseline Characteristics O r a l Oc t r e ot id e Capsules (n=28) P l aceb o (n=28) Sex, Female, n (%) 16 (57.1) 14 (50.0) Age, y, mean (SD) 55.3 (11.97) 54.2 (10.96) IGF - I, x ULN , mean (SD) 0.80 (0.16) 0.84 (0.21) Symptom burden, n (%) ≥1 23 (82.1) 24 (85.7) ≥2 18 (64.3) 19 (67.9) ≥3 10 (35.7) 14 (50.0) Weight, kg, mean (SD) 83.4 (17.22) 91.6 (20.48) BMI, kg/m 2 , mean (SD) 29.1 (6.26) 31.0 (5.58) Diabetes mellitus, n (%) 2 (7.1) 4 (14.3) O r a l Oc t r e ot id e Capsules (n=28) P l aceb o (n=28) Duration of acromegaly, n (%) <10 y 15 (53.6) 20 (71.4) 10 – 20 y 8 (28.6) 5 (17.9) >20 y 5 (17.9) 3 (10.7) Prior acromegaly surgery, n (%) 25 (89.3) 24 (85.7) Prior injectable treatment for acromegaly, n (%) Octreotide 19 (67.8) 17 (60.7) Lanreotide 9 (32.1) 11 (39.3) Prior injectable dose, n (%) Low 6 (21.4) 5 (17.9) Middle or high 22 (78.6) 23 (82.1) BMI, body mass index; IGF - I, insulin - like growth factor I; ULN, upper limit of normal. Baseline characteristics relating to demographics and disease and treatment history were well balanced between groups receiving Oral Octreotide Capsules or placebo S a m so n S e t a l , JCE M 202 1 69
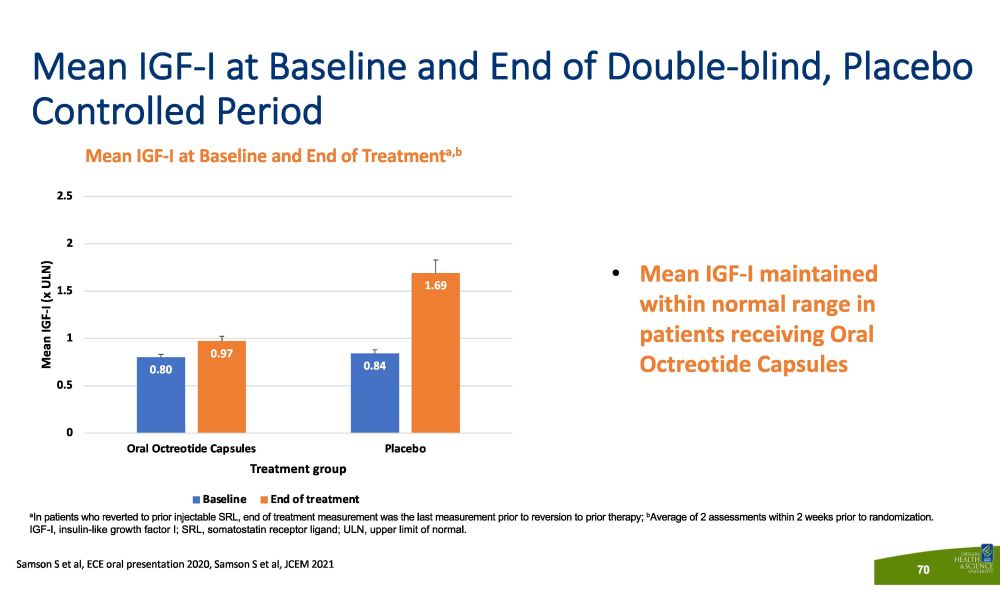
Mean IGF - I at Baseline and End of Double - blind, Placebo Controlled Period Mean IGF - I at Baseline and End of Treatment a,b a In patients who reverted to prior injectable SRL, end of treatment measurement was the last measurement prior to reversion to prior therapy; b Average of 2 assessments within 2 weeks prior to randomization. IGF - I, insulin - like growth factor I; SRL, somatostatin receptor ligand; ULN, upper limit of normal. • Mean IGF - I maintained within normal range in patients receiving Oral Octreotide Capsules 0.80 0.84 0.97 1.69 0 0 . 5 1 1 . 5 2 . 5 2 P l a ce b o Mean IGF - I (x ULN) Oral Octreotide Capsules Treatment group B a s e li n e E n d o f t r e a t m e n t Samson S et al, ECE oral presentation 2020, Samson S et al, JCEM 2021 70
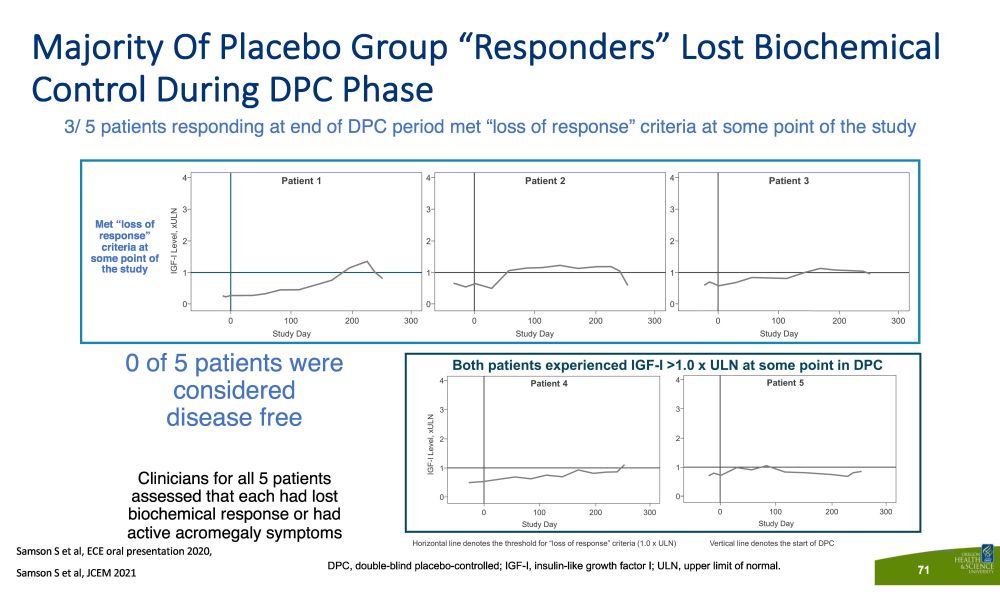
Majority Of Placebo Group “Responders” Lost Biochemical Control During DPC Phase 3/ 5 patients responding at end of DPC period met “loss of response” criteria at some point of the study 71 Met “loss of response” criteria at some point of the study DPC, double - blind placebo - controlled; IGF - I, insulin - like growth factor I; ULN, upper limit of normal. 0 of 5 patients were considered disease free Clinicians for all 5 patients assessed that each had lost biochemical response or had active acromegaly symptoms Samson S et al, ECE oral presentation 2020, S a m so n S e t a l , JCE M 202 1

ndary Endpoints Met Oral Octreotide Capsules (n=28) P l a c eb o (N=28) P Value Primary Endpoint Proportion maintaining IGF - I response 58% 19% a 0.008 Secondary Endpoints Proportion maintaining GH response 78% 30% 0.001 Median time to IGF - I >1.0 x ULN Not reached in 36 week DPC 16 weeks <0.001 Median time to IGF - I >1.3 x ULN Not reached in 36 week DPC 16 weeks <0.001 Reverted to prior injectable 25% 68% 0.003 S a m so n S e t a l , JCE M 202 1 72 CHIASMA OPTIMAL: Primary and All S e c o a All 19% of the placebo responders (n=5) continued in the OLE based on PI discretion, as they either had lost response at some point in the study, or had continuing acromegaly symptoms. DPC, double - blind placebo - controlled; IGF - I, insulin - like growth factor I; GH, growth hormone; OLE, open - label extension; PI, principal investigator; ULN, upper limit of normal.

CHIASMA OPTIMAL: Safety Results a TEAEs of special interest includes: headache, fatigue, perspiration, soft tissue swelling, arthralgia, dysglycemia, hypertension, or other signs in view of investigator as related to acromegaly. AESI, adverse event of special interest; DPC, double - blind placebo - controlled; SAE, serious adverse event; TEAE, treatment - emergent adverse event. Subjects With, n (%) Oral Oc t r e ot i d e Capsules (n=28) P l a c e b o (n=28) ≥1 Treatment Emergent Adverse Event (TEAE) 28 (100) 27 (96.4) ≥1 Treatment - related TEAE 18 (64.3) 15 (53.6) ≥1 Serious Adverse Event (SAE) 2 (7.1) 1 (3.6) ≥1 Treatment - related SAEs 0 0 ≥1 Maximum severity severe TEAE 3 (10.7) 7 (25.0) ≥1 TEAE leading to study drug discontinuation 2 (7.1) 1 (3.6) ≥1 Adverse event of special interest (AESI, acromegaly symptoms) a 15 (53.6) 26 (92.9) Most TEAEs were mild or moderate in intensity AESIs were more common in the placebo group S a m so n S e t a l , JCE M 202 1 73

CHIASMA OPTIMAL AE, adverse event; AESI, adverse event of special interest; DPC, double - blind placebo - controlled; IGF - I, insulin - like growth factor I; GI, gastrointestinal; OOC, oral octreotide capsules. Mean IGF - I was maintained within the normal range for patients receiving OOC 58% of patients receiving OOC maintained response at the end of the DPC period Median time to loss of response in the placebo group was 16 weeks; this was not met in the OOC group by 36 weeks 90% of the OOC group chose to continue in extension The safety profile of OOC was consistent with the known safety profile of octreotide No new/unexpected safety signals were observed Most AEs were mild to moderate in intensity; GI AEs were transient in nature AESIs were more common in the placebo group CHIASMA OPTIMAL met all primary and secondary endpoints Oral Octreotide Capsules (OOC) may be a potential option in the management of patients with acromegaly currently controlled by injectable SRLs 75% o f pa t i 7 e 5 n % t s o n OO C co m ple t ed 36 weeks without need for reversion to prior injectable therapy Samson S et al, JCEM 2021, Labadzhyan A et al, Pituitary 2021 74

MYCAPSSA® SHEILA FRAME , PRESIDENT, AMERICAS DR. MARK SUMERAY, CMO RORY NEALON, CFO/COO
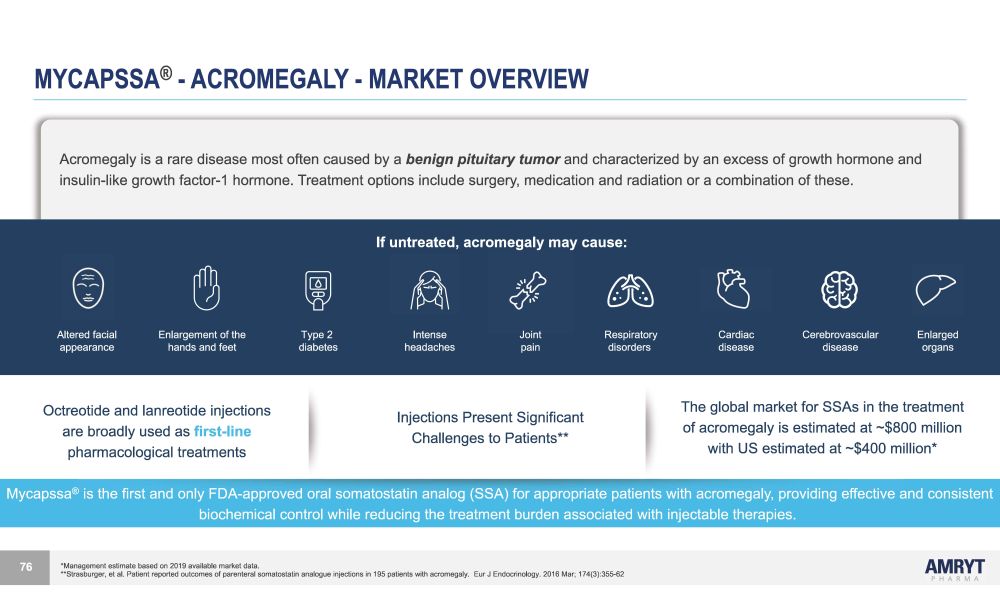
MYCAPSSA ® - ACROMEGALY - MARKET OVERVIEW Octreotide and lanreotide injections are broadly used as first - line pharmacological treatments Injections Present Significant Challenges to Patients** The global market for SSAs in the treatment of acromegaly is estimated at ~$800 million with US estimated at ~$400 million* Acromegaly is a rare disease most often caused by a benign pituitary tumor and characterized by an excess of growth hormone and insulin - like growth factor - 1 hormone. Treatment options include surgery, medication and radiation or a combination of these. If untreated, acromegaly may cause: *Management estimate based on 2019 available market data. **Strasburger, et al. Patient reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinology. 2016 Mar; 174(3):355 - 62 Altered facial Enlargement of the Type 2 Intense Joint Respiratory Cardiac Cerebrovascular Enlarged appearance hands and feet diabetes headaches pain disorders disease disease organs Mycapssa ® is the first and only FDA - approved oral somatostatin analog (SSA) for appropriate patients with acromegaly, providing effective and consistent biochemical control while reducing the treatment burden associated with injectable therapies. 76
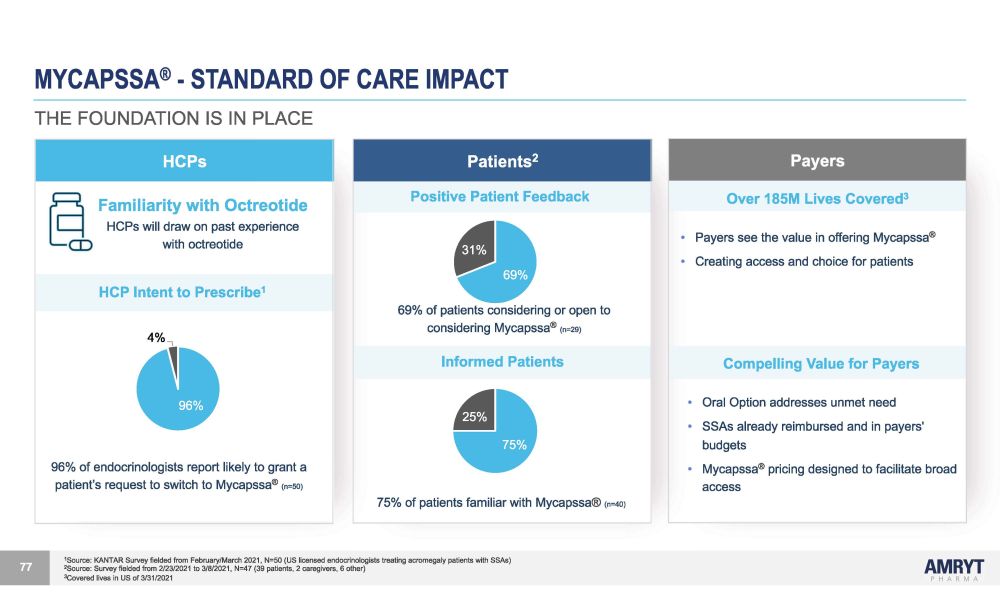
MYCAPSSA ® - STANDARD OF CARE IMPACT Patients 2 Positive Patient Feedback 31% 69% 69% of patients considering or open to considering Mycapssa ® (n=29) Informed Patients 25% 75% 75% of patients familiar with Mycapssa ® (n=40) 1 Source: KANTAR Survey fielded from February/March 2021, N=50 (US licensed endocrinologists treating acromegaly patients with SSAs) 2 Source: Survey fielded from 2/23/2021 to 3/8/2021, N=47 (39 patients, 2 caregivers, 6 other) 3 Covered lives in US of 3/31/2021 77 HCPs Familiarity with Octreotide HCPs will draw on past experience with octreotide HCP Intent to Prescribe 1 4% 96% 96% of endocrinologists report likely to grant a patient’s request to switch to Mycapssa ® (n=50) Payers Over 185M Lives Covered 3 • Payers see the value in offering Mycapssa ® • Creating access and choice for patients Compelling Value for Payers • Oral Option addresses unmet need • SSAs already reimbursed and in payers' budgets • Mycapssa ® pricing designed to facilitate broad access THE FOUNDATION IS IN PLACE
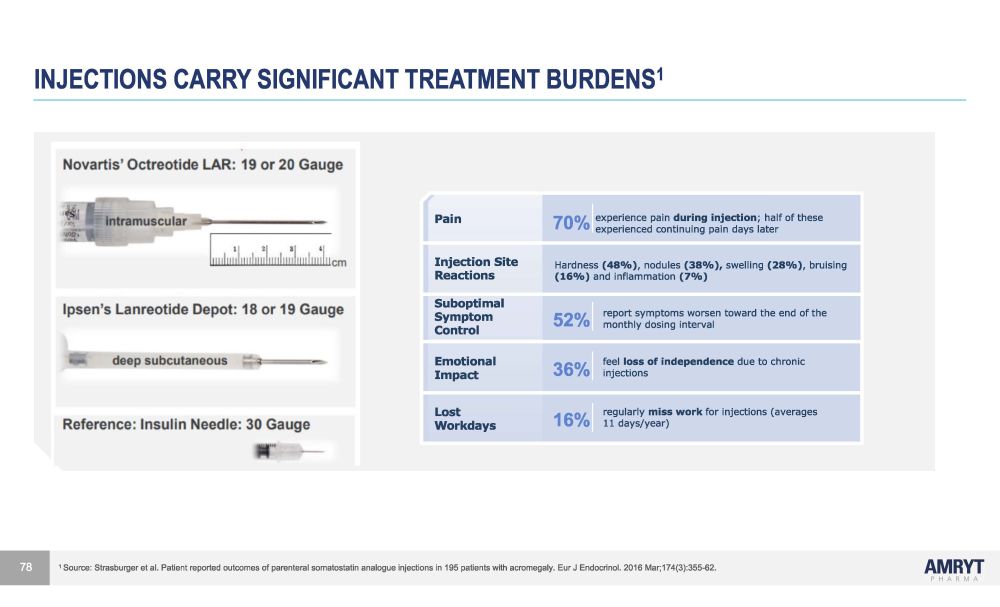
experienced continuing pain days later INJECTIONS CARRY SIGNIFICANT TREATMENT BURDENS 1 Pa i n 70% experience pain during injection ; half of these I n j ec t i o n S i t e Reactions Hardness (48%) , nodules (38%), swelling (28%) , bruising (16%) and inflammation (7%) Subop t i m a l Symptom Control 52% report symptoms worsen toward the end of the monthly dosing interval E m o t i ona l Impact 36% feel loss of independence due to chronic injections Lost W o r k da y s 16% regularly miss work for injections (averages 11 days/year) 1 Source: Strasburger et al. Patient reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol. 2016 Mar;174(3):355 - 62. 78
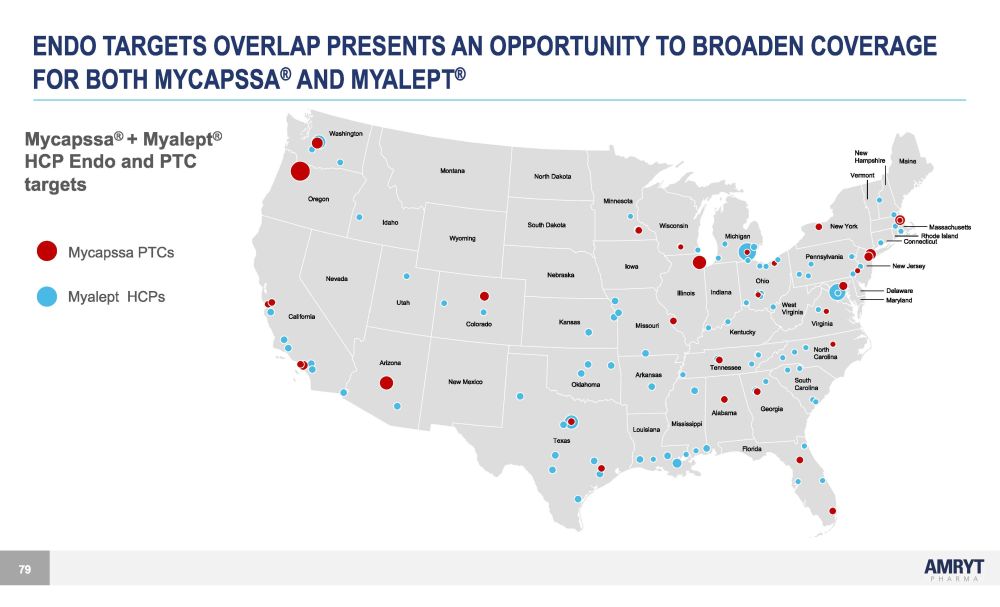
ENDO TARGETS OVERLAP PRESENTS AN OPPORTUNITY TO BROADEN COVERAGE FOR BOTH MYCAPSSA ® AND MYALEPT ® Mycapssa ® + Myalept ® HCP Endo and PTC targets Mycapssa PTCs Myalept HCPs Washington Oregon I daho Montana Wyoming U t ah Colorado Nevada California Arizona New Mexico T exas Florida Alabama Georgia Mississippi Louisiana South Carolina North Carolina Oklahoma Kansas Nebraska S ou t h Dako t a North Dakota I owa Missouri Arkansas Tennessee Illinois Minnesota Wisconsin O hio Pennsylvania New Y ork Indiana Michigan Kentucky Virginia West V irginia Maine New Hampshire Massachusetts Connecticut Delaware Maryland New Jersey Vermont Rhode I sland 79

MYCAPSSA ® - FOCUS TO ACCELERATE UPTAKE IN THE US • Leverage inside sales team to pre - screen open offices and secure stronger focus on community endocrinologist • Combine and train endocrinology commercial teams (Mycapssa ® + Myalept ® ) to extend reach and breadth by increasing the field sales team • Integrate the Medical Affairs endocrinology expertise by increasing the field medical team, to engage endocrinologists with emerging data • Build stronger patient support program and services to better support patient transition from injection to capsule. Work with patients and prescribers to appropriately manage initiation and titration for persistence to improve patient outcomes Mycapssa ® is the first and only somatostatin analogue (SSA) capsule for the management of appropriate patients with acromegaly 80

MYCAPSSA ® - DEVELOPMENT OPPORTUNITY IN NET • In the U.S. c. 24,000* Neuroendocrine Tumor (NET) patients are treated with SSA injections • Primarily non - pancreatic GI - NETs cause carcinoid syndrome in patients • Majority of these patients receive SSA to limit tumor progression and treat carcinoid syndrome • Injectable formulations of octreotide are the existing standard - of - care in the management of NET • Mycapssa ® is potentially well - positioned to address the unmet need in the SSA therapy arena for NET** • Patients (as with acromegaly) experience high treatment burden with injectables and HCPs / patients have expressed high interest in additional treatment options 81 * National Cancer Institute SEER Database; Halperin et al. 2017 https:// www.ncbi.nlm.nih.gov/pmc/articles/PMC6066284 **Further studies required

MYCAPSSA ® - DEVELOPMENT OPPORTUNITY IN NET CONT’D 82 • Oral SSA may provide more convenient and less painful delivery and can be administered at home • Mycapssa ® has potential for improved efficacy over current SoC with “continuous disease control” • Regulatory / HCP familiarity and comfort with octreotide • Mycapssa ® has demonstrated therapeutic equivalence with injectable formulations of octreotide in the management of acromegaly • The main risk to approvability is the extent to which oral administration achieves adequate and consistent within - patient bioavailability to provide adequate control of symptoms at a tolerated dose level

MYCAPSSA ® - REGULATORY PATHWAY FOR NET APPROVAL 83 • F D A A li gn m ent • In principle single Phase 3 NET registrational trial focused on carcinoid syndrome symptoms utilizing 505(b)(2) pathway • Target indication • Long - term treatment of severe diarrhea and flushing episodes associated with metastatic carcinoid tumors (same as Sandostatin LAR) • PIND Written Response Only FDA feedback (Nov and Dec 2020) • No additional pre - clinical work needed • DDI and PK studies needed in addition to pivotal Phase 3 trial

MYCAPSSA ® - REGULATORY PATHWAY FOR NET CONT’D 84 • Type C meeting with FDA June 2021 • Good progress with discussion on disease, treatment and complications • Agreed potential study design for Phase 3 • Plan to complete PK study to establish bioavailability of octreotide at higher doses of Mycapssa® vs injectable and Type C meeting with FDA Q4 2021 to finalize study design for Phase 3 • Plan to initiate study in 2022 and file NDA in 2024

TPE - VALIDATED DELIVERY TECHNOLOGY PLATFORM 85 Capsules with TPE technology have an enteric coating to protect against degradation in the stomach. Once in the small intestine, the capsule is designed to dissolve and release the TPE formulation. TPE technology induces the reversible expansion of tight junctions between intestinal epithelial cells, a natural process to absorb nutrients. Capsules containing TPE can allow drug therapies to enter systemic circulation while excluding toxins, bacteria and viruses. With the approval of Mycapssa ® , the TPE* represents a validated technology delivery platform for potential new development opportunities *TPE – Transient Permeability Enhancer

KEY AREAS OF FOCUS FOR SYNERGIES 86 Overall Objective – Migrate Chiasma retained employees to existing Amryt infrastructure • Headcount related savings • Reduction in duplication of roles • Day 1 leavers, including C - suite • Leverage off existing Amryt infrastructure which has bandwidth to take on an additional commercial asset, transitioning various Chiasma employees out over 1 - 12 months transition period • Migration of certain roles to Ireland, resulting in facilities and staff cost reductions • Future headcount recruitment savings

KEY AREAS OF FOCUS FOR SYNERGIES CONT’D 87 Other Operating Expenditure related savings • In - sourcing current elements of Chiasma operating expenses, including external regulatory, PV and clinical consulting costs • Leveraging off existing systems, with significant synergies to be achieved in IT and quality platforms • Eliminating duplicate vendors/ services, e.g. audit and tax fees, IR/ PR services, company secretarial/ listing fees • Leveraging Amryt management’s experience with new product launches and Amryt’s existing presence in endocrinology resulting in more focused and strategic commercial and medical affairs spend

A n sw e rs Q ue s t i on s &























































































