As filed on November 17, 2020 to the Securities and Exchange Commission
Registration No. 333-236797
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Amendment No. 4
To
Form F-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NLS Pharmaceutics Ltd.
(Exact name of registrant as specified in its charter)
| Switzerland | | 2834 | | Not Applicable |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
| Alexander Zwyer | | |
| Chief Executive Officer | | Puglisi & Associates |
| Alter Postplatz 2 | | 850 Library Ave., Suite 204 |
| CH-6370 Stans, Switzerland | | Newark, DE 19711 |
| Tel: +41.41.618.80.00 | | Tel: (302) 738-6680 |
| (Address, including zip code, and telephone number, | | (Name, address, including zip code, and telephone |
| including area code, of registrant’s principal executive offices) | | number, including area code, of agent for service) |
Copies to:
| Oded Har-Even, Esq. | | | | | | |
| Howard Berkenblit, Esq. | | | | Mitchell Nussbaum, Esq. | | Hans-Jakob Diem, Esq. |
| Ron Ben-Bassat, Esq. | | Pascal Honold, Esq. | | Angela M. Dowd, Esq. | | Patrick Schleiffer, Esq. |
| Sullivan & Worcester LLP | | Wenger & Vieli AG | | Loeb & Loeb LLP | | Lenz & Staehelin |
| 1633 Broadway | | Dufourstrasse 56 | | 345 Park Ave. | | Brandschenkestrasse 24 |
| New York, NY 10019 | | Zurich, Switzerland CH-8034 | | New York, NY 10154 | | Zurich, Switzerland CH-8027 |
| Tel: 212.660.5000 | | Tel: +41.58.958.58.58 | | Tel: 212.407.4000 | | Tel: +41.58.450.8000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date hereof.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☐
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards † provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
†The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED NOVEMBER 17, 2020 |

3,636,363 Units consisting of
3,636,363 Common Shares and Warrants to purchase up to 3,636,363 Common Shares
NLS Pharmaceutics Ltd. is offering units, or Units, each consisting of one of our common shares and one Warrant to purchase one of our common shares, or, each, a Warrant, in an initial public offering. No public market currently exists for our common shares or any security comprising the Units. The estimated initial public offering price per Unit is between $5.00 and $6.00 per Unit. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The common shares and Warrants are immediately separable and will be issued separately in this offering. Each Warrant offered hereby is immediately exercisable on the date of issuance at an exercise price of $ per common share (which shall not be less than 100% of the public offering price per Unit), and will expire five years from the date of issuance.
We have applied to list our common shares and Warrants on the Nasdaq Capital Market, or Nasdaq, under the symbol “NLSP” and “NLSPW,” respectively. There can be no assurance that we will be successful in listing our common shares or Warrants on Nasdaq.
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and will be subject to reduced public company reporting requirements.
Investing in our common shares involves a high degree of risk. See “Risk Factors” beginning on page 9.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | | Per Share | | | Total | |
| Initial public offering price | | $ | | | | $ | | |
| Underwriting discounts and commissions (1) | | $ | | | | $ | | |
| Proceeds to us (before expenses) | | $ | | | | $ | | |
| (1) | We have agreed to reimburse the underwriters for certain expenses. See the section titled “Underwriting” beginning on page 144 of this prospectus for additional disclosure regarding underwriter compensation and offering expenses. |
Certain existing officers, who are also shareholders, and a director nominee, have indicated an interest in purchasing up to an aggregate of $138,000 of Units in this offering at the initial public offering price per Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these persons, or any of these persons may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these persons as they will on any other Units sold to the public in this offering.
We have granted the representative of the underwriters an option, exercisable within 45 days from the date of this prospectus, to purchase from us, up to an additional 545,454 common shares at the public offering price of $ per common share and/or up to an additional 545,454 Warrants to purchase up to 545,454 common shares at the public offering price of $ per Warrant, less, in each case, the underwriting discounts and commissions, to cover over-allotments, if any. If the representative of the underwriters exercises the option in full, the total underwriting discounts and commissions payable will be $ , and the total proceeds to us, before expenses, will be $ .
The underwriters expect to deliver the Units to purchasers in the offering on or about ______, 2020.
Book Running Manager
Maxim Group LLC
Co-Manager
Brookline Capital Markets
a division of Arcadia Securities, LLC
The date of this prospectus is ________, 2020
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not authorized anyone to provide you with information that is different. We are offering to sell the Units, and seeking offers to buy the Units, only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of the Units.
We are organized under the laws of Switzerland and our registered office and domicile is located in Stans, Switzerland. Moreover, the majority of our directors and executive officers are not residents of the United States, and all or a substantial portion of the assets of such persons are located outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon us or upon such persons or to enforce against them judgments obtained in U.S. courts, including judgments in actions predicated upon the civil liability provisions of the federal securities laws of the United States. We have been advised by our Swiss counsel that there is doubt as to the enforceability in Switzerland of original actions, or in actions for enforcement of judgments of U.S. courts, of civil liabilities to the extent solely predicated upon the federal and state securities laws of the United States. See “Enforceability of Civil Liabilities” for additional information.
For investors outside of the United States: Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
In this prospectus, “we,” “us,” “our,” the “Company” and “NLS” refer, prior to the Reorganization, as defined in this prospectus, to NLS-0 Pharma AG, or NLS-0, NLS-1 Pharma AG, or NLS-1, and NLS Pharma AG, or NLS Pharma, and, after the Reorganization, to NLS Pharmaceutics Ltd.
All trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Our reporting currency and functional currency is the U.S. dollar. Unless otherwise expressly stated or the context otherwise requires, references in this prospectus to “dollars,” “USD” or “$” mean U.S. dollars, and references to “CHF” are to the Swiss Franc. U.S. dollar translations of CHF amounts presented in this prospectus were done on different dates in accordance with the date as of such entry in the Company’s books and are derived from our audited financial statements included elsewhere in this prospectus. U.S. dollar translations of CHF amounts presented in this prospectus that are not derived from our audited financial statements included elsewhere in this prospectus are translated using the rate of CHF 1.00 to $1.0326, based on the exchange rate provided by the Swiss Federal Tax Administration on December 31, 2019.
This prospectus includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we believe that these sources are reliable, we have not independently verified the information contained in such publications.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in the Units. Before you decide to invest in the Units, you should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and related notes appearing at the end of this prospectus.
Overview
We are an innovative biotechnology company engaged in the discovery and development of life-improving drug therapies to treat rare and complex central nervous system, or CNS, disorders, including narcolepsy, idiopathic hypersomnia, or IH, and other rare sleep disorders, as well as neurodevelopmental disorders, such as Attention Deficit Hyperactivity Disorder, or ADHD. Our lead compound mazindol, in a controlled release, or CR, formulation is a pan-monoaminergic reuptake inhibitor and partial orexin receptor 2 agonist, for the treatment of narcolepsy with cataplexy. We also believe that we may be able to use Mazindol CR as a compound in product candidates to treat other CNS disorders. CNS disorders are a diverse group of conditions that include neurological, psychiatric, and substance use disorders. According to the World Health Organization and based on data from the Global Burden of Disease Report, 25% of the world’s population suffers from at least one CNS disorder, resulting in a socio-economic burden of more than $317 billion annually in the United States alone. Additionally, CNS disorders are expected to account for approximately 15% of the global disease burden by 2020, the largest of any disease area. However, treatment options for these conditions are often limited, inadequate or nonexistent, and the development of new CNS treatments generally trails behind other therapeutic areas. We are pursuing the development of the next generation of CNS therapies with high medical impact to address this critical and growing unmet need. Our dual development strategy is designed to optimize the outcome of our clinical programs by developing new chemical entities from known molecules with strong scientific rationale, and also by re-defining previously approved molecules with well-established tolerability and safety profiles, as determined by applicable regulatory agencies. We believe that our streamlined clinical development approach has the potential to advance our product candidates rapidly through early-stage clinical trials, while carrying a lower development risk. We believe that lower development risk exists with respect to the development of our lead product candidates due to their use of mazindol, an active substance, which was previously approved and marketed in the United States, Japan and Europe for obesity, and is currently listed as a Schedule IV controlled substance as classified by the U.S. Drug Enforcement Administration, or the DEA, under the Controlled Substances Act of 1970, or CSA. The DEA defines Schedule IV controlled substances as those “with a low potential for abuse and a low risk of dependence.”
Our discovery platform currently focuses on single molecules that operate through multiple mechanisms designed to target the complexity of the CNS disease state, and as such, we believe that such a discovery platform may offer new treatment options for patients, including for those patients who are refractory to currently available treatments. Our current focus is in the therapeutic areas of rare hypersomnia disorders (condition highlighted by excessive daytime sleepiness, or EDS, or excessive daytime spent sleeping) and complex neurodevelopmental disorders, and includes our two lead product candidates: Quilience, for the treatment of EDS and cataplexy, the two main symptoms associated with narcolepsy, and Nolazol, for the treatment of ADHD. We have yet to conduct any clinical trials for Quilience; however, it is our intention to initiate Phase 2 and 3 clinical trials for Quilience in adults, provided that we receive permission to initiate these trials from the FDA and other applicable regulatory agencies, which may also require certain chemistry, manufacturing, and controls, or CMC, clinical, and non-clinical work prior to the initiation of such trials, the extent of which is expected to be determined after we meet with the FDA and other applicable regulatory agencies, with such meetings expected to occur after this offering (see “ – Our Solution: Quilience for Narcolepsy - A Well-Suited Approach for the Disease Pathology – Non-Clinical Development Strategy” for more information). We intend to seek entrance into an accelerated approval pathway during the fourth quarter of 2020, such as Breakthrough Therapy Designation, Fast Track Designation or PRIME scheme, and subject to acceptance into such accelerated pathway, we intend to begin a Phase 2 dose-finding clinical trial in the first quarter of 2021 while the Phase 3 clinical trial expected to occur in the fourth quarter of 2021, following an interim review of the data from the Phase 2 dose-finding clinical trial during the third quarter of 2021. We have completed a Phase 2 clinical trial evaluating the safety and efficacy of Nolazol in adults with ADHD in the U.S. and given the positive outcome of this trial, we may initiate Phase 3 clinical trials after we receive approval to commercialize Quilience. We intend to seek FDA and other regulatory approval for both product candidates for use in children, which requires additional non-clinical work, as well as staged clinical work in determining safe dosing and monitoring.
Quilience and Nolazol both contain mazindol as the active molecule in a proprietary controlled release formulation allowing for what we intend to be a once-a-day, single tablet use. Mazindol has a well-established long-term safety record from its extended history of clinical use across the United States and several countries in Europe, where mazindol was previously approved in an immediate release formulation for the short-term management of obesity. It was marketed for nearly 30 years, into the early 2000s, before being voluntarily withdrawn from the market for commercial reasons and is no longer available nor marketed in these regions. In addition to the 30-year period in which it was marketed, mazindol was also widely used off-label and prescribed under compassionate use for the treatment of narcolepsy for approximately four decades, during which time it demonstrated a well-tolerated safety profile in patients over long-term, chronic use of the drug.
Quilience for Narcolepsy
We believe that Quilience offers a meaningfully differentiated product profile over current treatment options for the following reasons:
| | ● | | Mechanism of action. If approved, Quilience would be the only partial orexin type 2 agonist approved by the FDA. Narcolepsy is caused by selective loss of the neurons producing orexins (also known as hypocretins). |
| | ● | | Established long-term use. Mazindol has a well-established long-term safety record from its extended history of clinical use across the United States and several countries in Europe, where it was previously approved in an immediate release formulation for the short-term management of obesity. In addition to the 30-year period in which it was marketed, mazindol was also widely used off-label and prescribed under compassionate use for the treatment of narcolepsy for approximately four decades, during which time it demonstrated a well-tolerated safety profile in patients over long-term, chronic use of the drug. |
| | ● | | Low potential for abuse and misuse and diversion. Mazindol is listed as a Schedule IV controlled substance as classified by the DEA. The DEA defines Schedule IV controlled substances as those “with a low potential for abuse and a low risk of dependence”. Unlike Xyrem (sodium oxybate), the top-selling treatment for narcolepsy in the United states, historically, for Mazindol, the FDA never required a risk evaluation and mitigation strategy, or REMS, to manage known or potential serious risks associated with its use. |
| | ● | | Quilience is expected to be developed as a monotherapy or to be administered concomitantly with other narcolepsy treatments. Narcolepsy is a difficult disorder to manage and the majority of narcolepsy patients often require multiple medications to treat their symptoms. A retrospective analysis (Nittur et.al, Sleep Med. 2013 Jan;14(1):30-6) showed that mazindol has a long-term, favorable benefit/risk ratio in 60% of drug-resistant hypersomniacs, including a clear benefit on cataplexy. |
| | ● | | Quilience is being developed as a once-daily oral tablet administered in the morning upon wakening. Patients have identified a need for treatment options that are easier to take and are dosed less frequently. We believe that once-daily dosing with Quilience may address this need and may help improve patient compliance and adherence with treatment. |
Product Candidates
Quilience and Nolazol, our two lead product candidates, are being developed as separate and uniquely differentiated controlled release pharmaceutical products. Our proprietary controlled-release formulations are being designed to optimize their pharmacokinetic and pharmacodynamic properties with a rapid onset of action and prolonged controlled therapeutic effect, allowing for a daily oral dose that effectively provides consistent and long acting symptom control and is designed to uniquely meet the needs of patients.
Leveraging our scientific insights and direct hands-on clinical experience, we are developing compounds that we believe have innovative mechanisms of action with positive therapeutic profiles and represent a differentiated treatment option to overcome the limitations of the current therapies. For example, we believe that Quilience and Nolazol are agents that differ from available treatments, given their apparent dual mechanism of action. In addition to their action as triple monoamine reuptake inhibitors that simultaneously block serotonin, norepinephrine, and dopamine transporters, and as such function as SNDRIs (serotonin–norepinephrine–dopamine reuptake inhibitors), our small molecule lead product candidates are designed to quickly penetrate the blood brain barrier and also target the orexin pathway as a selective (in relation to the orexin-1 receptor) partial agonist of the orexin-2 receptor, or OX2R. This activity could provide cortical regulation of the lower brain, and thereby lead to improved attention and inhibitory control, while also improving regulation of executive function and inhibitory control which would provide both “top down” and “bottom up” regulation of the brain. Orexin neurons (located in a part of the brain known as the hypothalamus) are multi-tasking neurons that serve as key modulators of several vital functions, including sleep-wake states, attention, feeding behavior, energy homeostasis, reward systems, cognition and mood throughout the cerebral cortex, and as such, the orexin system is a central promoter of wakefulness. A drug that targets orexin could potentially increase arousal in individuals with ADHD, which could be useful in individuals who have a low level of arousal or motivation, as is sometimes the case in individuals with ADHD. To the best of our knowledge, no currently available medications for ADHD or for the treatment of EDS and cataplexy, the two main symptoms associated with narcolepsy, have effects on the orexin system. In addition to narcolepsy and ADHD, we believe that our lead product candidates may have therapeutic potential across a range of diverse CNS conditions such as restless-leg-syndrome, or RLS, idiopathic hypersomnia, or IH, obstructive sleep apnea, or OSA, Kleine-Levin-syndrome, daytime sleepiness due to myotonic dystrophy Type 1 (DM1) and Prader Willi Syndrome, given their mechanism of action.
Company History and Management Team
NLS was formed in June 2015 in Stans, Switzerland and is a Swiss limited company. In 2016, we acquired several patents from Assistance Publique – Hopitaux de Paris, or AP-HP in France, including for our lead compound mazindol. Since our founding, we have assembled an experienced leadership team with a track record of developing and repurposing products to treat rare neurological disorders. Our Chief Executive Officer, Alexander Zwyer, has held a variety of senior leadership roles of increasing responsibility throughout his career including global roles in business development, marketing, commercial, operations and general management. We complement our management team with a group of scientific and clinical advisors that includes internationally recognized North American and European experts in CNS disorders, including the areas of our current focus, rare hypersomnolence and complex neurodevelopmental disorders.
Our Strategy
Our goal is to continue building a differentiated, global biotechnology company that is patient-focused in the development of transformative therapies that address critical unmet needs in rare and complex CNS and neurodevelopmental disorders, such as ADHD. As we navigate the competitive landscape of our industry, while focusing on development of our product candidates, we also intend to continually seek out-licensing and asset sale transactions that we believe will allow us to drive greater value for our shareholders. The key elements of our business strategy are to:
| | ● | globally develop Quilience for narcolepsy first in adults and then in children; |
| | | |
| | ● | pursue new indications beyond narcolepsy (e.g. Nolazol in ADHD); and |
| | ● | explore expansion of our growing product pipeline either through in-house innovation or in-licensing. |
Risks Associated with Our Business
Investing in our common shares involves substantial risk. Our ability to execute our strategy is also subject to certain risks. The risks described under the heading “Risk Factors” included elsewhere in this prospectus may cause us not to realize the full benefits of our strengths or may cause us to be unable to successfully execute all or part of our strategy. In particular, our risks include, but are not limited to, the following:
| | ● | the ongoing COVID-19 pandemic may adversely affect our planned clinical trials, operations and financial condition; |
| | ● | we may be unable to successfully use mazindol, on which we depend substantially, as the drug substance for each of our current lead product candidates, Quilience, for the treatment of narcolepsy, and Nolazol, for the treatment of ADHD, and which outcome could prove costly to our business and could prevent us from obtaining regulatory or marketing approval; |
| | ● | we may not be able to immediately initiate our Phase 2 dose-finding clinical trial in Quilience without additional pre-clinical studies, CMC work or early-stage clinical trials; |
| | ● | prior results of mazindol for the treatment of other indications may not be replicated in the clinical trials that we conduct for the treatment of narcolepsy or ADHD; |
| | ● | we require regulatory approvals prior to any attempt to commercialize our product candidates. Prior to commercialization of Quilience, we must receive approvals from the FDA and from the EMA in order to initiate our Phase 2 and 3 clinical trials. Similarly, the FDA previously accepted our investigational new drug, or IND, application in 2016 for Nolazol; however, before we may commercialize Nolazol for the treatment of ADHD, we must receive additional approvals from the FDA, including approval to conduct our Phase 3 clinical trials. Our planned clinical trials for Quilience and Nolazol must be successful if we are to seek and obtain regulatory marketing application through the submission of a marketing authorization application, or MAA, and new drug application, or NDA, with the EMA and FDA, respectively. Advanced clinical trials are often not successful even if prior trials were successful, and even if we are able to conduct advanced clinical trials and those trials are successful, we may not obtain necessary regulatory approvals for Quilience and Nolazol or we may be unable to successfully commercialize either, or both, even if we receive the necessary regulatory approvals; |
| | ● | our data from future clinical trials may not satisfy the FDA or other comparable regulatory agencies, or the FDA or other regulatory agencies may require additional time or studies to assess the safety and efficacy of Quilience, Nolazol, or other product candidates that we may seek to develop in the future; |
| | ● | to date, we have not generated revenue, and we do not expect to generate significant revenue unless and until we obtain marketing approval to commercialize Quilience or Nolazol. We are unable to predict the extent of future losses or when we will become profitable based on the sale of any product, if at all. Even if we succeed in developing and commercializing our product candidates, we may never generate sufficient revenue to sustain profitability. As of June 30, 2020, we had an accumulated deficit of approximately $28.1 million; |
| | ● | we have no sources of revenue and will need to raise substantial additional capital to successfully complete development and seek to commercialize Quilience, Nolazol, or other product candidates that we may seek to develop in the future, and such capital may not be available to us or available to us only on unfavorable terms; |
| | ● | the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern, which could prevent us from obtaining new financing on reasonable terms or at all; |
| | ● | if, and when, we seek to commercialize Quilience and Nolazol, we may be partially dependent upon prescriptions from physicians for the sale of both such product candidates, and therefore, the loss of a significant number of patient referrals by physicians prescribing Quilience or Nolazol may have an adverse effect on our future revenues, if any, which could have an adverse effect on our business, financial condition and results of operations; |
| | | |
| | ● | our Chief Executive Officer, directors and shareholders who own more than 5% of our outstanding common shares before this offering (including a certain lender controlled by persons that are also our shareholders, one of which is our Chairman) currently own approximately 86% of our outstanding common shares and will own approximately 61% of our common shares upon the completion of this offering (excluding any common shares that may be purchased by such persons in this offering, as disclosed herein) and will therefore be able to exert significant control over matters submitted to our shareholders for approval; |
| | ● | as a public company following the conclusion of this offering, we will need to comply with extensive additional U.S. and Swiss governmental and Nasdaq regulations, which will be expensive and which will require significant management attention; and |
| | ● | we have identified material weaknesses in our internal control over financial reporting and, if our remediation of the material weaknesses is not effective or if we identify additional material weaknesses in the future, we may not be able to accurately or timely report our financial results, or prevent fraud, and investor confidence in our Company and the market price of our common shares and Warrants, if a public market then exists for such securities, may be adversely affected. |
Corporate Information
NLS-1 and NLS Pharma were incorporated in June 2015 and NLS-0 was incorporated in April 2016, each in Switzerland. In March 2019, and pursuant to Swiss law, effective as of January 1, 2019, NLS-0 and NLS Pharma each merged with and into NLS-1. We refer to these transactions throughout the prospectus included in this registration statement collectively as the “Reorganization.” As part of the Reorganization, all assets and liabilities of NLS-0 and NLS Pharma were transferred to NLS-1 by way of universal succession (pursuant to which, under Swiss law, assets and liabilities are transferred as a whole and in one act), and NLS-1 was renamed NLS Pharmaceutics Ltd.
On September 14, 2020, our shareholders approved the adoption of an amendment to our articles of association, which reflects a 5,000 for 1 stock split of our common shares, effective as of September 15, 2020, or the Share Split.
Our registered office and principal executive offices are located at Alter Postplatz 2, CH-6370 Stans, Switzerland. Our telephone number in Switzerland is +41.41.618.8000. Our website address is https://nlspharma.com/. The information contained on, or that can be accessed through, our website is not part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
The Securities and Exchange Commission, or the SEC, also maintains an Internet website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings with the U.S. Securities and Exchange Commission, or the SEC, are also available to the public through the SEC’s website at http://www.sec.gov.
Implications of Being an “Emerging Growth Company”
We are an “emerging growth company,” as defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the JOBS Act. As such, we are eligible to, and intend to, take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not “emerging growth companies” such as not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and not being required to comply with any new or revised financial accounting standards until such date that a private company is otherwise required to comply with such new or revised accounting standards. We could remain an “emerging growth company” for up to five years, or until the earliest of (a) the last day of the first fiscal year in which our annual gross revenue exceeds $1.07 billion, (b) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which would occur if the market value of our common shares that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (c) the date on which we have issued more than $1 billion in nonconvertible debt during the preceding three-year period.
Implications of being a “Foreign Private Issuer”
We are subject to the information reporting requirements of the Exchange Act that are applicable to “foreign private issuers,” and under those requirements we file reports with the SEC. As a foreign private issuer, we are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. For example, we are not required to issue quarterly reports, proxy statements that comply with the requirements applicable to U.S. domestic reporting companies, or individual executive compensation information that is as detailed as that required of U.S. domestic reporting companies. We also have four months after the end of each fiscal year to file our annual report with the SEC and are not required to file current reports as frequently or promptly as U.S. domestic reporting companies. Our officers, directors and principal shareholders are exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. As a foreign private issuer, we are not subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act. In addition, as a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the Nasdaq Stock Market rules for domestic U.S. issuers and are not required to be compliant with all Nasdaq Stock Market rules as of the date of our initial listing on Nasdaq as would domestic U.S. issuers (See “Risk Factors—Risks Related to this Offering and the Ownership of Our Securities).” These exemptions and leniencies will reduce the frequency and scope of information and protections available to you in comparison to those applicable to a U.S. domestic reporting company. We intend to take advantage of the exemptions available to us as a foreign private issuer during and after the period we qualify as an “emerging growth company.”
THE OFFERING
| Units offered by us | | 3,636,363 Units, each consisting of one common share and one Warrant to purchase one common share. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The common shares and Warrants are immediately separable and will be issued separately in this offering. |
| | | |
Common shares issued and outstanding prior to this offering | | 6,960,000 common shares |
| | | |
| Common shares to be issued and outstanding after this offering | | 10,596,363 common shares, or 11,141,817 common shares assuming that the underwriters exercise their over-allotment option in full (assuming in each case, no exercise of the Warrants). |
| | | |
| Description of the Warrants | | The Warrants will have an exercise price of $ per common share, which shall not be less than 100% of the public offering price per Unit, will be immediately exercisable and will expire five years from the date of issuance. Each Warrant is exercisable for one common share, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common shares. A holder may not exercise any portion of a Warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of our outstanding common shares after exercise, as such ownership percentage is determined in accordance with the terms of the Warrants, except that upon notice from the holder to us, the holder may waive such limitation up to a percentage, not in excess of 9.99%. This prospectus also relates to the offering of the common shares issuable upon exercise of the Warrants. To better understand the terms of the Warrants, you should carefully read the “Description of Share Capital and Governing Documents” section of this prospectus. You should also read the form of Warrant, which is filed as an exhibit to the registration statement that includes this prospectus. |
| | | |
| Over-allotment option | | The underwriters have an option for a period of 45 days to purchase from us up to an additional 545,454 common shares, at the public offering price of $ , and/or up to an additional 545,454 Warrants to purchase up to 545,454 common shares at the public offering price of $ per Warrant, in each case less the underwriting discounts and commissions, solely to cover over-allotments, if any. |
| | | |
| Use of proceeds | | We estimate that the net proceeds from our issuance and sale of 3,636,363 Units in this offering will be approximately $17.5 million, assuming an offering price of $5.50 per Unit, the midpoint of the estimated price range set forth on the cover page of this prospectus, excluding any proceeds that may be received upon the exercise of the Warrants, and after deducting underwriting discounts and commissions and offering expenses payable by us, or the Estimated Net Proceeds. If the representative of the underwriters exercises the over-allotment option in full, we estimate that the net proceeds from this offering will be approximately $20.2 million, assuming an offering price of $5.50 per Unit, excluding any proceeds that may be received upon the exercise of the Warrants, and after deducting underwriting discounts and commissions and offering expenses payable by us. We currently expect to use the net proceeds from this offering for: |
| | | ● approximately $14 million to further develop Quilience (which may also benefit, in part, our development, if any, of Nolazol), to conduct pre- and non-clinical trials for Quilience and potentially reach the interim review of our Phase 2 dose-finding clinical trial; |
| | | |
| | | ● approximately $0.834 million, of which $0.223 million was incurred subsequent to June 30, 2020, in the aggregate to pay the deferred compensation of our Chief Executive Officer (including interest) and other interim executive officers; |
| | | |
| | | ● approximately $0.262 million (CHF 248,400) to repay the COVID-19 Loan (as defined in this prospectus); |
| | | |
| | | ● approximately $1.566 million, including $0.657 million of convertible debt, in the aggregate to repay each of the following types of indebtedness, each of which is scheduled to mature on December 31, 2020: CHF 500,000 borrowed under a certain 2020 Bridge Loan entered into in August 2020, or the 2020 Bridge Loan ($527,650, which carries an interest rate of 10% per year; approximately $5,071 accrued interest as of September 30, 2020), the Interest-Bearing Facility ($85,737, with no interest), the NLS Pharma Credit Facility ($150,000, with no interest), the Notes (approximately $527,650, each of which carries an interest rate of 10% per year; approximately $96,980 accrued interest as of September 30, 2020), the Convertible Note (approximately $24,293, which carries an interest rate of 10% per year; approximately $36,628 accrued interest as of September 30, 2020) and the Convertible Loan (as defined below) with a related party ($105,530,which carries an interest rate of 10% per year; approximately $6,485 accrued interest as of September 30, 2020). The $0.528 million Bridge Loan was entered into subsequent to June 30, 2020 and $0.025 million of interest was incurred subsequent to June 30, 2020. (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Financing Activities” and “Related Party Transactions – Shareholders and Officer and Director Loans” for additional information on each form of indebtedness); |
| | | ● payment to our Interim Chief Business Officer pursuant to the terms of his consulting agreement with us (to receive 1% of the net proceeds actually received by us in an initial public offering) (approximately $176,722 if we receive the Estimated Net Proceeds) (also referred to herein as the Transaction Fee); and |
| | | |
| | | ● the remainder, if any, for working capital and general corporate purposes. |
| | | |
| | | See “Use of Proceeds” for additional information. |
| | | |
| Proposed Nasdaq trading symbol and listing | | We have applied to list our common shares and Warrants on Nasdaq under the symbol “NLSP” and “NLSPW,” respectively. No assurance can be given that such listing will be approved or that a liquid trading market will develop for either our common shares or Warrants. |
| Lock-up | | Our directors, executive officers, and shareholders who own 5% or more of the outstanding shares of our common shares have agreed with the underwriters not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common shares or securities convertible into common shares for a period of six months commencing on the date of this prospectus. See “Underwriting.” |
| | | |
| Risk factors | | You should read the “Risk Factors” section starting on page 9 of this prospectus for a discussion of factors to consider carefully before deciding to invest in our common shares. |
The number of our common shares that are and will be outstanding immediately before and after this offering as shown above is based on 6,960,000 shares outstanding as of November 16, 2020, which, as used throughout this prospectus, unless otherwise indicated, excludes common shares, if any, issuable upon conversion of the (i) remaining outstanding amount of a certain convertible promissory note for CHF 550,000 (approximately $557,920) that we entered into in January 2019, and as amended thereafter, or the Convertible Note, which may be converted into common shares in this offering (ii) seven convertible loan agreements, one of which was with our Chief Executive Officer, in the aggregate amount of CHF 475,822 (approximately $501,358) that we entered into in December 2019 and February, March and June 2020, or, each, a Convertible Loan, (iii) up to 109,090 common shares issuable upon exercise in full of the Representative’s Warrants, excluding any such warrants that may be issued by us as a result of the exercise by the representative of the underwriters of the over-allotment option (as defined in this prospectus) and (iv) $50,000 of our common shares that we are contractually required to issue to a certain service provider (see “Underwriting – Other Relationships” for additional information).
Unless otherwise indicated, all information in this prospectus assumes no exercise by the representative of the underwriters of the over-allotment option nor the exercise of the Warrants.
Except as otherwise indicated, information in this prospectus reflects the 5,000 for 1 Share Split of our issued and outstanding common shares.
Certain existing shareholders, who are also shareholders, and a director nominee, have indicated an interest in purchasing up to an aggregate of $138,000 of Units in this offering at the initial public offering price per Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these persons, or any of these persons may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these persons as they will on any other Units sold to the public in this offering.
SUMMARY FINANCIAL DATA
The following table summarizes our financial data. We have derived the following statements of operations data for the years ended December 31, 2019 and 2018 and balance sheet data as of December 31, 2019 from our audited financial statements included elsewhere in this prospectus. We have derived the following statements of operations data for the six months ended June 30, 2020 and 2019 and balance sheet data as of June 30, 2020 from our unaudited interim financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. The following summary financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
Our financial statements included in this prospectus were prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP.
| | | Six Months Ended
June 30,
(unaudited) | | | Year Ended
December 31, | |
| U.S. dollars in thousands, except share and per share data | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | | | | | | | | | | | |
| Research and development expenses | | $ | 161 | | | $ | 980 | | | $ | 1,702 | | | $ | 1,437 | |
| General and administrative expenses | | | 921 | | | | 1,246 | | | | 2,495 | | | | 2,012 | |
| Total operating expenses | | | 1,082 | | | | 2,226 | | | | 4,197 | | | | 3,449 | |
| Operating loss | | | (1,082 | ) | | | (2,226 | ) | | | (4,197 | ) | | | (3,449 | ) |
| Other (expense) income, net | | | (62 | ) | | | (31 | ) | | | (65 | ) | | | 16 | |
| Interest expense | | | (37 | ) | | | | | | | (1 | ) | | | - | |
| Interest on related party loans | | | (51 | ) | | | (793 | ) | | | (819 | ) | | | (1,085 | ) |
| Loss on conversion of debt | | | - | | | | (365 | ) | | | (365 | ) | | | (629 | ) |
| Net loss | | $ | (1,232 | ) | | $ | (3,415 | ) | | $ | (5,447 | ) | | $ | (5,147 | ) |
| Other comprehensive loss: | | | | | | | | | | | | | | | | |
| Defined pension plan adjustments | | | - | | | | - | | | | (8 | ) | | | (1 | ) |
| Comprehensive loss | | | (1,232 | ) | | | (3,415 | ) | | | (5,455 | ) | | | (5,148 | ) |
| Basic and diluted net loss per common share | | $ | (0.18 | ) | | $ | (0.51 | ) | | $ | (0.80 | ) | | $ | (0.82 | ) |
| Weighted average common shares used in computing basic and diluted net loss per common share | | | 6,960,000 | | | | 6,715,000 | | | | 6,840,000 | | | | 6,240,000 | |
| U.S. dollars in thousands | | As of
June 30,
2020 (unaudited) | | | As of
December 31,
2019 | |
| Balance Sheet Data: | | | | | | |
| Cash and cash equivalents | | $ | 145 | | | $ | 220 | |
| Total assets | | | 640 | | | | 696 | |
| Total non-current liabilities | | | 3,253 | | | | 2,832 | |
| Accumulated deficit | | | (28,130 | ) | | | (26,898 | ) |
| Total shareholders’ deficit | | | (7,334 | ) | | | (6,171 | ) |
RISK FACTORS
An investment in our securities involves a high degree of risk. We operate in a dynamic and rapidly changing industry that involves numerous risks and uncertainties. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including the financial statements and the related notes included elsewhere in this prospectus, before deciding whether to invest in our securities. If any of the risks discussed below actually occur, our business, financial condition, operating results or cash flows could be materially adversely affected. This could cause the trading price of our common shares or Warrants to decline, and you may lose all or part of your investment.
Risks Related to Product Development, Regulatory Approval and Commercialization
We depend substantially on the success of our lead product candidates, Quilience and Nolazol. We cannot give any assurance that any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized.
We have invested almost all of our efforts and financial resources in general and administrative costs and the research and development of our lead product candidates, Quilience for the treatment of EDS and cataplexy associated with narcolepsy and Nolazol, for the treatment of ADHD. The process to develop, obtain regulatory approval for and commercialize pharmaceutical product candidates is long, complex, costly and inherently uncertain of outcome. We are not permitted to market any of our product candidates in the United States, European Union, or the EU, or any other jurisdiction until we receive the requisite regulatory approvals. We cannot give any assurance that our current clinical development plan will proceed as planned, or that our product candidates will receive regulatory approval, or that such regulatory approval, if received, will be within a timeframe that allows us to effectively compete with our competitors, or be successfully marketed and commercialized.
Our initiation of clinical studies for Quilience is dependent upon the review and approval of the relevant regulatory agencies and authorities and if we are required to conduct earlier-stage clinical trials, approval of an NDA or MAA, if any, for Quilience would be delayed and we may require additional capital as a result thereof.
Based in part on the prior use and FDA approval of mazindol for treatment of obesity, we believe that we will be able to commence our Phase 2 and 3 clinical trials for Quilience without having to do prior early-stage clinical trials, such as Phase 1 trials. No assurance can be given that the EMA or FDA will agree to allow us to initiate clinical trials for Quilience without conducting such earlier-stage clinical trials. If the FDA or EMA, or any other applicable regulatory agency, were to require us to conduct additional clinical trials, our planned development strategy for Quilience would be materially impacted and approval of an NDA or MAA, if any, for Quilience would be delayed and we may require additional capital as a result thereof.
In addition, we plan to request a PIP deferral in order delay conducting clinical trials for Quilience in children until after we receive an MAA from the EMA for the use of Quilience in adults. A PIP deferral, as can be agreed upon by the EMA, allows an applicant to delay studies in children until after there is sufficient data on use in adults; we anticipate that we will be able to receive a PIP deferral from the EMA for Quilience; however, there is no guarantee that this deferral will be granted, which could impact our planned development process and would make the product candidate approval process more costly.
The commencement and completion of clinical trials can be delayed or prevented for a number of reasons.
We may not be able to commence or complete the clinical trials that would support our submission of an NDA to the FDA or MAA to the EMA. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage of any of our clinical trials. Clinical trials can be delayed or prevented for a number of reasons, including:
| | ● | difficulties obtaining regulatory approval to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial; |
| | ● | delays in reaching or failing to reach agreement on acceptable terms with prospective contract research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| | ● | insufficient or inadequate supply or quality of a product candidate or other materials necessary to conduct our clinical trials; |
| | | |
| | ● | if the FDA or EMA elect to enact policy changes, as a result of the COVID-19 pandemic or otherwise; |
| | ● | difficulties obtaining institutional review board, or IRB, approval to conduct a clinical trial at a prospective site; and |
| | ● | challenges recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including fears resulting from the COVID-19 pandemic or due to government actions enacted and as a result of such pandemic, size and nature of patient population, proximity of patients to clinical sites, eligibility criteria for the trial, nature of trial protocol, the availability of approved effective treatments for the relevant disease and competition from other clinical trial programs for similar indications. |
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA, the IRBs at the sites where the IRBs are overseeing a trial, a data safety monitoring board overseeing the clinical trial at issue or by other regulatory authorities due to a number of factors, including:
| | ● | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| | ● | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities; |
| | ● | unforeseen safety issues (including those that result from the COVID-19 pandemic) or lack of effectiveness; and |
| | ● | lack of adequate funding to continue the clinical trial. |
The results of pre-clinical studies, early-stage clinical trials, data obtained from real-world use, and published third-party studies may not be indicative of results in future clinical trials and we cannot assure you that any planned or future clinical trials will lead to results sufficient for the necessary regulatory approvals.
The results of pre-clinical studies may not be predictive of the results of clinical trials, and the results of any completed clinical trials, including studies derived from real-world use and studies in published literature, or clinical trials we commence may not be predictive of the results of later-stage clinical trials. Additionally, interim results during a clinical trial do not necessarily predict final results. Later clinical trial results may not replicate earlier clinical trials for a variety of reasons, including differences in trial design, different trial endpoints (or lack of trial endpoints in exploratory studies), subject population, number of subjects, subject selection criteria, trial duration, drug dosage and formulation and lack of statistical power in the earlier studies There can be no assurance that any of our clinical trials will ultimately be successful or support further clinical development of any of our product candidates. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development could have a negative impact on our business.
We may find it difficult to enroll patients in our clinical trials. Difficulty in enrolling patients could delay or prevent clinical trials of our product candidates.
Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends in part on the speed at which we can recruit patients to participate in testing our product candidates, and we may experience delays in our clinical trials if we encounter difficulties in enrollment. The evolving COVID-19 pandemic may directly or indirectly impact the pace of enrollment in our clinical trials as patients may avoid or may not be able to travel to healthcare facilities and physicians’ offices unless due to a health emergency and clinical trial staff can no longer get to the clinic. Additionally, such facilities and offices have been and may continue to be required to focus limited resources on non-clinical trial matters, including treatment of COVID-19 patients, thereby decreasing availability, in whole or in part, for clinical trial services. See “Risks Related to Our Business Operations – We face business disruption and related risks resulting from the recent outbreak of COVID-19, which could have a material adverse effect on our business and results of operations” for additional information.
In addition, as a rare disorder, there is a limited patient pool from which to draw for our clinical trials for Quilience. Further, the eligibility criteria of our clinical trials will further limit the pool of available study participants as we will require that patients have specific characteristics that we can measure or to assure their disease is either severe enough or not too advanced to include them in a study.
Additionally, the process of finding patients may prove costly. We also may not be able to identify, recruit and enroll a sufficient number of patients to complete our clinical trials because of the perceived risks and benefits of the product candidate under study, the availability and efficacy of competing therapies and clinical trials, the proximity and availability of clinical trial sites for prospective patients and the patient referral practices of physicians. If patients are unwilling to participate in our studies for any reason, the timeline for recruiting patients, conducting studies and obtaining regulatory approval of potential product candidates will be delayed.
If we experience delays in the completion or termination of any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product candidate revenue from any of these product candidates could be delayed or prevented. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product candidate sales and generate revenue. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Obtaining approval of an NDA or a MAA even after clinical trials that are believed to be successful is an uncertain process.
We are not permitted to market Quilience or Nolazol in the United States or the EU until we receive regulatory approval of an NDA from the FDA or MAA from the EMA, or in any foreign countries until we receive the requisite approval from regulatory authorities in such countries. We have not received regulatory clearance to conduct the additional clinical trials that are necessary to be able to submit an NDA to the FDA for Nolazol. Similarly, we have not received regulatory clearance in the EU to conduct clinical trials that are necessary to receive approval of a MAA for Quilience in Europe. As such, we have not submitted an MAA for any of our product candidates.
Even if we complete our planned clinical trials and believe the results to be successful, all of which are uncertain, obtaining regulatory approval is an extensive, lengthy, expensive and uncertain process, and the FDA and EMA, and other regulatory authorities may delay, limit or deny approval of Quilience or Nolazol for many reasons, including, but not limited to:
| | ● | we may not be able to demonstrate to their satisfaction that the product candidate is a safe or effective treatment for a given indication; |
| | ● | the results of clinical trials may not meet the level of statistical significance or clinical significance required by the regulatory agencies; |
| | ● | disagreements regarding the number, design, size, conduct or implementation of our clinical trials, or with our interpretation of data from pre-clinical studies or clinical trials; |
| | ● | a lack of acceptance of the accuracy or sufficiency of the data generated at our clinical trial sites to demonstrate, among others, that clinical and other benefits outweigh its safety risks or to support the submission of an NDA or MAA; |
| | ● | difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee, or such other similar committee, may recommend against approval of our application or may recommend that such regulators require, as a condition of approval, additional pre-clinical studies or clinical trials, limitations on approved labeling, or distribution and use restrictions; |
| | ● | the requirement that we develop a REMS, as a condition of approval, which may or may not be feasible for us; |
| | ● | the identification of deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for clinical and commercial supplies; |
| | ● | changes in approval policies or the adoption of new regulations by such regulators; and |
| | ● | we may be unable to be granted a PIP deferral which we intend to request from the EMA for delayed clinical trials and subsequent approval in children; this may delay our clinical trial program or approvals for adults, or we may have successful clinical trial results for adults but not children (if we were required to conduct pediatric studies prior to the receipt of an NDA or MMA for use of our product candidates in adults), or vice versa. |
Before we can submit an NDA to the FDA, we must conduct Phase 3 clinical trials, in addition to human pharmacokinetic and bioavailability studies, that will be substantially broader than our Phase 2 trial for Nolazol. An NDA must be supported by extensive clinical and pre-clinical data, as well as extensive information regarding chemistry, manufacturing and controls to demonstrate the safety and effectiveness of the applicable product candidate. The number and types of pre-clinical studies and clinical trials that will be required varies depending on the product candidate, the disease or condition that the product candidate is designed to target and the regulations applicable to any particular product candidate. Obtaining approval of an NDA is a lengthy, expensive and uncertain process, and we may not be successful in obtaining approval. The FDA review processes can take years to complete and approval is never guaranteed.
In this respect, we will also need to agree on a protocol with the FDA for the Phase 3 clinical trials before commencing those trials. Phase 3 clinical trials frequently produce unsatisfactory results even though prior clinical trials were successful. Therefore, the results of the additional trials that we conduct may or may not be successful. The FDA may suspend all clinical trials or require that we conduct additional clinical, non-clinical, manufacturing validation or drug product quality studies and submit those data before it will consider or reconsider the NDA. Depending on the extent of these or any other studies, approval of any applications that we submit may be delayed by several years, or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve the NDA. If any of these outcomes occur, we would not receive approval for Quilience or Nolazol at such time, if any, that we seek FDA approval. We may face similar risks with respect to obtaining regulatory approval from the EMA for Quilience, and for Nolazol, at such time, if any, that we seek EMA approval. The risks that we face in obtaining applicable approvals from the FDA and EMA for Quilience and Nolazol, or any other product candidate that we may seek to develop, may also exist with other regulatory authorities, such as those in Latin America.
Even if we obtain FDA, EMA or other regulatory approval for Quilience or Nolazol, the approval might contain significant limitations related to use restrictions for certain age groups, warnings, precautions or contraindications, or may be subject to significant post-marketing studies or risk mitigation requirements. In addition, even if we obtain a MAA from the EMA for the use of Quilience in adults, there can be no guarantee that we will receive an MAA for Quilience for the use in children. If we are unable to successfully commercialize Quilience or Nolazol, we may be forced to cease operations.
Interim topline and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim topline or preliminary data from our clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. Adverse differences between preliminary or interim data and final data could significantly harm our reputation and business prospects.
The results of clinical trials conducted at clinical sites outside the United States may not be accepted by the FDA and the results of clinical trials conducted at clinical sites in the United States may not be accepted by international regulatory authorities.
We plan to conduct some of our clinical trials outside the United States. For example, we are planning to globally develop Quilience in the United States and the EU. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to certain conditions imposed by the FDA. For example, the clinical trial must be well-designed and conducted and performed by qualified investigators in accordance with ethical principles such as or IRB or ethics committee approval and informed consent. The study population must also adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deems clinically meaningful. Generally, the subject population for any clinical trials conducted outside of the United States must be representative of the U.S. population. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will be dependent upon its determination that the trials were conducted consistent with all applicable U.S. laws and regulations. There can be no assurance the FDA or international regulatory authorities will accept data from trials conducted outside of the United States or inside the United States, as the case may be, as adequate support of a marketing application. If the FDA does not accept the data from sites in our globally conducted clinical trials, or if international regulatory authorities do not accept the data from our U.S. clinical trials, it would likely result in the need for additional trials, which would be costly and time-consuming and could delay or permanently halt the development of one or more of our product candidates.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in significant negative consequences following regulatory approval, if obtained.
During the conduct of clinical trials, patients may experience changes in their health, including illnesses, injuries, discomforts or a fatal outcome. It is possible that as we develop Quilience and Nolazol, or other product candidates that we may seek to develop, in larger, longer and more extensive clinical trials as use of our product candidates becomes more widespread if they receive regulatory approval, illnesses, injuries, discomforts and other adverse events that were observed in earlier clinical trials, as well as conditions that did not occur or went undetected in previous clinical trials, will be reported by subjects. Many times, side effects are only detectable after investigational products are tested in larger scale, Phase 2 and 3 clinical trials or, in some cases, after they are made available to patients on a commercial scale after approval. If additional clinical experience indicates that Quilience or Nolazol, or other product candidates that we may seek to develop, have side effects or cause serious or life-threatening side effects, the development of the product candidate may fail or be delayed, or, if the product candidate has received regulatory approval, such approval may be revoked or limited.
Additionally, if any of our product candidates receives marketing approval, the FDA or EMA could require us to adopt a REMS to ensure that the benefits outweigh its risks, which may include, among other things, a medication guide outlining the risks of the product for distribution to patients, a communication plan to health care practitioners, and restrictions on how or where the product can be distributed, dispensed or used. Furthermore, if we or others later identify undesirable side effects caused by Quilience or Nolazol, several potentially significant negative consequences could result, including:
| | ● | regulatory authorities may suspend or withdraw approvals of such a product candidate; |
| | ● | regulatory authorities may require additional warnings on the label; |
| | ● | regulatory authorities may issue negative publicity regarding the affected product, including safety communications; |
| | ● | we may be required to change the way the product is distributed, dispensed or administered, or conduct additional pre-clinical studies or clinical trials; |
| | | |
| | ● | we may need to voluntarily recall our products; and |
| | ● | we could be sued and held liable for harm caused to patients. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate and could significantly harm our business, prospects, financial condition and results of operations.
Changes in methods of product candidate manufacturing or formulation may result in additional costs or delay.
As product candidates proceed through pre-clinical studies to late-stage clinical trials towards potential approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods and formulation, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives. Any of these changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the materials manufactured using altered processes. Such changes may also require additional testing, FDA or EMA notification or FDA approval. This could delay completion of clinical trials, require the conduct of bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidates and jeopardize our ability to commence sales and generate revenue.
We will need to obtain FDA approval of any proposed names for our product candidates that gain marketing approval, and any failure or delay associated with such naming approval may adversely impact our business.
Any name we intend to use for our product candidates will require approval from the FDA regardless of whether we have secured a formal trademark registration from the U.S. Patent and Trademark Office, or the U.S. PTO. The FDA typically conducts a review of proposed product names, including an evaluation of whether proposed names may be confused with the names of other drug products. The FDA may object to any product name we submit if it believes the name inappropriately implies medical claims. If the FDA objects to any of our proposed product names, we may be required to adopt an alternative name for our product candidates, which could result in further evaluation of proposed names with the potential for additional delays and costs.
Obtaining regulatory approval for clinical trials of Quilience and Nolazol in children will be more difficult than obtaining such approvals for adult clinical trials since the requirements for regulatory approval to conduct pediatric clinical trials are more stringent.
Pediatric drug development requires additional non-clinical work (such as animal studies in juvenile animals and additional reproductive toxicity work), as well as staged clinical work in determining safe dosing and monitoring. These additional tasks involve investment of significant additional resources beyond those needed for approval of the drug for adults. Approval of our current leading product candidates – Quilience and Nolazol – for children may be significantly delayed due to these additional requirements and this may have an adverse effect on the commercial prospects for Quilience and Nolazol and our ability to generate product revenue would be delayed, possibly materially. In addition, we do not know if as a result of the COVID-19 pandemic there will be a smaller pool of children from which we can enroll for our clinical trials. We cannot guarantee that we will receive any regulatory approvals to commercialize our product candidates in children.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which may result in necessary changes to clinical trial protocols, which could result in increased costs to us, delay our development timeline or reduce the likelihood of successful completion of our clinical trials.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, as a result of which we may need to amend clinical trial protocols. Amendments may require us to resubmit our clinical trial protocols to IRBs for review and approval, which may impact the cost, timing or successful completion of a clinical trial. If we experience delays in completion of, or if we terminate, any of our clinical trials, the commercial prospects for Quilience and Nolazol would be harmed and our ability to generate product revenue would be delayed, possibly materially.
Our development and regulatory strategy for our product candidates depends in part on published scientific literature and the FDA’s prior findings regarding the safety and efficacy of approved products containing mazindol. If the FDA does not conclude that our product candidates satisfy the requirements for the Section 505(b)(2) regulatory approval pathway, or if the requirements for our product candidates under Section 505(b)(2) are not as we expect, the approval pathway would likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated and in either case may not be successful.
The Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Amendments, added Section 505(b)(2) to the Federal Food, Drug, and Cosmetic Act, or FDCA, or Section 505(b)(2). Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. We intend to seek FDA approval through the Section 505(b)(2) regulatory pathway for Quilience and may seek this regulatory pathway for other product candidates that we seek to develop.
If the FDA does not allow us to pursue the Section 505(b)(2) regulatory pathway as anticipated, we may need to conduct additional clinical trials, provide additional data and information and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval, and complications and risks associated with FDA approval, would substantially increase. We may need to obtain additional funding, which could result in significant dilution to the ownership interests of our then existing shareholders to the extent we issue equity securities or convertible debt. We cannot assure you that we would be able to obtain such additional financing on terms acceptable to us, if at all. Moreover, inability to pursue the Section 505(b)(2) regulatory pathway could result in new competitive product candidates reaching the market faster than our product candidates, which could materially adversely impact our competitive position and prospects. Even if we are allowed to pursue the Section 505(b)(2) regulatory pathway, we cannot assure you that our product candidates will receive the requisite approvals for commercialization.
We may seek designations for our product candidates with the FDA and other comparable regulatory authorities that are intended to confer benefits such as a faster development process or an accelerated regulatory pathway, but there can be no assurance that we will successfully obtain such designations. In addition, even if one or more of our product candidates are granted such designations, we may not be able to realize the intended benefits of such designations.
The FDA, and other comparable regulatory authorities, offer certain designations for product candidates that are intended to encourage the research and development of pharmaceutical products addressing conditions with significant unmet medical need. These designations may confer benefits such as additional interaction with regulatory authorities, a potentially accelerated regulatory pathway and priority review. There can be no assurance that we will successfully obtain such designation for Quilience or Nolazol. In addition, while such designations could expedite the development or approval process, they generally do not change the standards for approval. Even if we obtain such designations for one or more of our product candidates, there can be no assurance that we will realize their intended benefits.
For example, we may seek a Breakthrough Therapy Designation from the FDA for one or more of our product candidates. A Breakthrough Therapy Designation is defined as a therapy that is intended, alone or in combination with one or more other therapies, to treat a serious or life-threatening disease or condition, if preliminary clinical evidence indicates that the therapy may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For therapies that have Breakthrough Therapy Designation, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Therapies with Breakthrough Therapy Designation from the FDA are also eligible for accelerated approval. Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for Breakthrough Therapy Designation, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a Breakthrough Therapy Designation for a product candidate may not result in a faster development process, review or approval compared to therapies considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify for Breakthrough Therapy Designation, the FDA may later decide that such product candidates no longer meet the conditions for qualification.
We may also seek Fast Track Designation from the FDA for some of our product candidates. If a therapy is intended for the treatment of a serious or life-threatening condition and the therapy demonstrates the potential to address unmet medical needs for this condition, the therapy sponsor may apply for Fast Track Designation. The FDA has broad discretion whether or not to grant this designation, so even if we believe a particular product candidate is eligible for this designation, there can be no assurance that the FDA would decide to grant it. Even if we do receive Fast Track Designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures, and receiving a Fast Track Designation does not provide assurance of ultimate FDA approval. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program.
Even if we obtain regulatory approval for a product candidate, our products will remain subject to ongoing regulatory oversight.
Even if we obtain any regulatory approval for our product candidates, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping and submission of safety and other post-market information. Any regulatory approvals that we receive for our product candidates also may be subject to a REMS, limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the quality, safety and efficacy of the product. The holder of an approved marketing application also must submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable federal and state laws.
In addition, product manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, or cGMP, requirements and adherence to commitments made in the NDA or foreign marketing application. If we, or a regulatory authority, discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured or disagrees with the promotion, marketing or labeling of that product, a regulatory authority may impose restrictions relative to that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements, a regulatory authority may:
| | ● | issue an untitled letter or warning letter that we are in violation of the law; |
| | | |
| | ● | seek an injunction or impose administrative, civil or criminal penalties or monetary fines; |
| | ● | suspend or withdraw regulatory approval; |
| | ● | suspend any ongoing clinical trials; |
| | ● | refuse to approve pending applications or supplements to applications; |
| | ● | restrict the marketing or manufacturing of the product; |
| | ● | seize or detain the products or require the withdrawal of the product from the market; |
| | | |
| | ● | refuse to permit the import or export of the products; or |
| | | |
| | ● | refuse to allow us to enter into supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and adversely affect our business, financial condition, results of operations and prospects.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States, Switzerland or the EU.
Other than our headquarters and other operations which are located in Switzerland (as further described below), we currently have limited international operations, but our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval of our product candidates. We may plan to maintain sales representatives and conduct physician and patient association outreach activities, as well as clinical trials, outside of the United States, Switzerland and Europe. If Quilience or Nolazol are approved for commercialization outside the United States, Switzerland, or the EU, we will likely enter into agreements with third parties to market the drugs in these additional global territories. We expect that we will be subject to additional risks related to entering into or maintaining international business relationships, including:
| | ● | different regulatory requirements for drug approvals in foreign countries; |
| | ● | differing United States and foreign drug import and export rules, tariffs and other trade barriers; |
| | ● | reduced protection for intellectual property rights in foreign countries; |
| | ● | failure by us to obtain regulatory approvals for the use of our products in various countries; |
| | ● | different reimbursement systems; |
| | ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| | ● | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; |
| | ● | complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems; |
| | ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations, which could result in increased operating expenses and reduced revenues; |
| | ● | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| | ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
| | | |
| | ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, or FCPA, its books and records provisions or its anti-bribery provisions. |
| | ● | potential liability resulting from development work conducted by these distributors; and |
| | ● | business interruptions resulting from a local or worldwide pandemic, such as COVID-19, geopolitical actions, including war and terrorism, or natural disasters. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
Even if any of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
The commercial success of Quilience and Nolazol will depend upon the acceptance of each product by the medical community, including physicians, patients and third-party payors. The degree of market acceptance of any approved product will depend on a number of factors, including:
| | ● | the efficacy and safety of the product; |
| | ● | the potential advantages of the product compared to available therapies; |
| | | |
| | ● | the convenience and ease of administration compared to alternative treatments; |
| | | |
| | ● | limitations or warnings, including use restrictions contained in the product’s approved labeling; |
| | ● | distribution and use restrictions imposed by the EMA, FDA or other regulatory authority or agreed to by us as part of a mandatory or voluntary risk management plan; |
| | ● | availability of alternative treatments, including, in the case of Nolazol, a number of competitive products already approved for the treatment of ADHD or expected to be commercially launched in the near future; |
| | ● | pricing and cost effectiveness in relation to alternative treatments; |
| | ● | if the product is included under physician treatment guidelines as a first-, second,- or third-line therapy; |
| | ● | the strength of sales, marketing and distribution support; |
| | | |
| | ● | the availability of third-party coverage and adequate reimbursement and the willingness of patients to pay out-of-pocket in the absence of coverage by third-party payors; |
| | ● | the strength of sales, marketing and distribution support; |
| | ● | the willingness of patients to pay for drugs out of pocket in the absence of third-party coverage; and |
| | | |
| | ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies. |
If Quilience or Nolazol is approved but does not achieve an adequate level of acceptance by physicians, third party payors and patients, we may not generate sufficient revenue from the product, and we may not become or remain profitable. In addition, our efforts to educate the medical community and third-party payors on the benefits of the product may require significant resources and may never be successful.
In addition, we may choose to collaborate with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If we enter into arrangements with third parties to perform sales, marketing and distribution services for our products, the resulting revenues or the profitability from these revenues to us are likely to be lower than if we had sold, marketed and distributed our products ourselves. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval. Depending on the nature of the third-party relationship, we may have little control over such third parties, and any of these third parties may fail to devote the necessary resources and attention to sell, market and distribute our products effectively. If we are not successful in commercializing our product candidates, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.
Even if we are able to commercialize any product candidates, the products may become subject to unfavorable pricing regulations or third-party coverage and reimbursement policies, any of which could harm our business.
Our ability to commercialize any product candidates successfully will depend, in part, on the extent to which coverage and reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and impact reimbursement levels.
Obtaining and maintaining adequate reimbursement for our products may be difficult. We cannot be certain if and when we will obtain an adequate level of reimbursement for our products by third party payors. Even if we do obtain adequate levels of reimbursement, third-party payors, such as government or private healthcare insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for, drugs. Reimbursement rates from private health insurance companies vary depending on the company, the insurance plan and other factors. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for drugs. We may also be required to conduct expensive pharmacoeconomic studies to justify coverage and reimbursement or the level of reimbursement relative to other therapies. If coverage and reimbursement are not available or reimbursement is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval, and the royalties resulting from the sales of those products may also be adversely impacted.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Our inability to promptly obtain coverage and adequate reimbursement rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drug products vary widely from country to country. Current and future legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be reimbursed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription drug pricing remains subject to continuing governmental control, including possible price reductions, even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval. There can be no assurance that our product candidates, if they are approved for sale in the United States or in other countries, will be considered medically necessary or cost-effective for a specific indication, or that coverage or an adequate level of reimbursement will be available.
The FDA and other regulatory authorities actively enforce the laws and regulations prohibiting the promotion of off-label uses.
If any of our product candidates are approved and we are found to have improperly promoted off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, if approved. In particular, while the FDA permits the dissemination of truthful and non-misleading information about an approved product, a manufacturer may not promote a product for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees, corporate integrity agreements or permanent injunctions under which specified promotional conduct must be changed or curtailed. If we cannot successfully manage the promotion of our product candidates, if approved, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
Our product candidates Quilience and Nolazol contain the active ingredient mazindol, which is a Schedule IV controlled substance under the CSA. Failure to maintain compliance with applicable requirements under the CSA or other applicable federal or state regulations could have an adverse effect on our operations and business.
Controlled substances are classified by the DEA as Schedule I, II, III, IV or V, or CI, CII, CIII, CIV, and CV, substances, with CI substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. The active ingredient of our lead product candidates is mazindol, which is classified as a Schedule IV controlled substance under the CSA, and regulations of the DEA. Under the CSA, subject to certain exemptions, every person who manufactures, distributes, dispenses, imports or exports any controlled substance must register with the DEA.
Although the CSA’s restrictions governing substances in CIV are not as stringent as those for substances in CI, CII or CIII, they could still limit our ability to market and commercialize Quilience and Nolazol, if approved for marketing. In addition, failure to maintain compliance with applicable requirements under the CSA, particularly as manifested in loss or diversion of regulated substances, can result in enforcement action that could include civil penalties, refusal to renew registrations or quotas, revocation of registrations or quotas or criminal proceedings, any of which could have a material adverse effect on our business, results of operations and financial condition. Individual states also regulate controlled substances, and we along with our contract manufacturers will be subject to state regulation on distribution of these products.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions. Our failure to obtain regulatory approval in foreign jurisdictions would prevent our product candidates from being marketed abroad, and any approval we are granted for our product candidates in the United States would not assure approval of product candidates in foreign jurisdictions.
In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding clinical trial design, safety and efficacy. The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of drugs are subject to extensive regulation by the FDA in the United States and other regulatory authorities in other countries. These regulations differ from country to country. Even if we obtain and maintain regulatory approval of our product candidates in one jurisdiction, such approval does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries.
Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional non-clinical studies or clinical trials as investigations conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval. These regulatory procedures can result in substantial delays in such countries. In other countries, product approval depends on showing superiority to an approved alternative therapy. This can result in significant expense for conducting complex clinical trials.
Finally, we do not have any products approved for sale in any jurisdiction, including international markets, and we do not have experience in obtaining regulatory approval. If we, or any third parties with whom we work, fail to comply with regulatory requirements in United States or international markets or to obtain and maintain required approvals or if regulatory approvals in international markets are delayed, our target market may be reduced and our ability to realize the full market potential of our products will likely be harmed. The inability to meet continuously evolving regulatory standards for approval may result in our failing to obtain regulatory approval to market our current product candidates, which could significantly harm our business, results of operations and prospects.
Additionally, on June 23, 2016, the electorate in the United Kingdom voted in favor of leaving the EU, commonly referred to as “Brexit.” On March 29, 2017, the country formally notified the EU of its intention to withdraw pursuant to Article 50 of the Lisbon Treaty, scheduled to be effective March 29, 2019. The withdrawal of the United Kingdom from the EU took effect on January 31, 2020. Since a significant proportion of the regulatory framework in the United Kingdom is derived from EU directives and regulations, the referendum could materially impact the regulatory regime with respect to the approval of our product candidates in the United Kingdom or the EU. Any delay in obtaining, or an inability to obtain, any regulatory approvals, as a result of Brexit or otherwise, would prevent us from commercializing our product candidates in the United Kingdom and/or the EU and restrict our ability to generate revenue and achieve and sustain profitability. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approval in the United Kingdom and/or EU for our product candidates, which could significantly and materially harm our business.
Our market is subject to intense competition, which may result in others commercializing products before or more successfully than us. If we are unable to compete effectively, Quilience and Nolazol may be rendered noncompetitive or obsolete, which may adversely affect our operating results.
The development and commercialization of new products is highly competitive. Our potential competitors include major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide with respect to Quilience or Nolazol or any future product candidate that we may seek to develop or commercialize. Our competitors may succeed in developing, acquiring or licensing technologies and products that are more effective, have fewer or more tolerable side effects or are more convenient or less costly than Quilience or Nolazol or any future product candidate we may develop, which could render any product candidates obsolete and noncompetitive. Our competitors also may obtain FDA or other marketing approval for their products before we are able to obtain approval for ours, which could result in competitors establishing a strong market position before we are able to enter the applicable market.
Many of our potential competitors, alone or with their strategic partners, have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining marketing approvals and commercializing approved products than we do. There is a trend toward consolidation in the pharmaceutical and biotechnology industry, and additional mergers and acquisitions in these industries may result in even more resources being concentrated among a smaller number of our competitors, which may adversely affect us.
Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials.
In addition, if we enter the markets of our product candidates, with such entrance remaining subject to various additional regulatory approvals, too late in the cycle, we may not achieve commercial success, or we may have to reduce our price in order to effectively compete, which would impact our ability to generate revenue, obtain profitability and adversely affect our operating results.
We may be unable to obtain orphan drug designation for our product candidates and, even if we obtain such designation, we may not be able to realize the benefits of such designation, including potential marketing exclusivity of our product candidates, if approved.
Regulatory authorities in some jurisdictions, including the United States and EU, may designate drugs for relatively small patient populations as “orphan drugs.” Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States or a patient population of greater than 200,000 individuals in the United States, but for which there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. In the EU, the European Commission grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the EU community. Additionally, designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the EU would be sufficient to justify the necessary investment in developing the drug.
The FDA granted orphan drug designation to mazindol for the treatment of narcolepsy in the United States. We also received orphan drug designation for mazindol in the EU. This designation of a product candidate as an orphan product does not mean that any regulatory agency will accelerate regulatory review of, or ultimately approve, that product candidate, nor does it limit the ability of any regulatory agency to grant orphan drug designation to product candidates of other companies that treat the same indications as our product candidates prior to our product candidates receiving exclusive marketing approval
Generally, if a product candidate with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the drug may be entitled to a period of marketing exclusivity, which precludes the FDA or the EMA from approving another marketing application for the same drug for that time period, except in limited circumstances. If another sponsor receives such approval before we do (regardless of our orphan drug designation), we will be precluded from receiving marketing approval for our product for the applicable exclusivity period. The applicable period is seven years in the United States and ten years in the EU, which may be extended by six months and two years, respectively, in the case of product candidates that have complied with the respective regulatory agency’s agreed upon PIP. The exclusivity period in the EU can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be revoked if any regulatory agency determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Even if we obtain orphan drug exclusivity for a product candidate, that exclusivity may not effectively protect the product candidate from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA may subsequently approve another drug for the same condition if the FDA concludes that the latter drug is not the same drug or is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In the EU, marketing authorization may be granted to a similar medicinal product for the same orphan indication if:
| | ● | the second applicant can establish in its application that its medicinal product, although similar to the orphan medicinal product already authorized, is safer, more effective or otherwise clinically superior; |
| | ● | the holder of the marketing authorization for the original orphan medicinal product consents to a second orphan medicinal product application; or |
| | ● | the holder of the marketing authorization for the original orphan medicinal product cannot supply sufficient quantities of orphan medicinal product. |
While orphan drug product candidates are typically sold at a high price relative to other medications, the market may not be receptive to high pricing of our product candidates.
We are developing our product candidates to treat rare CNS disorders with high-unmet medical needs, a space where medications are usually sold at high prices compared with other medications. Accordingly, even if regulatory authorities approve our product candidates, the market may not be receptive to, and it may be difficult for us to achieve, a per-patient per-year price high enough to allow us to realize a return on our investment.
If product liability lawsuits are brought against us, we may incur substantial liabilities, even if we have appropriate insurance policies, and we may be required to limit commercialization of our product candidates.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Currently, we have no products that have been approved for marketing or commercialization; however, the use of our product candidates in clinical trials, and the sale of these product candidates, if approved, in the future, may expose us to liability claims. Product liability claims may be brought against us or our partners by participants enrolled in our clinical trials, patients, health care providers, pharmaceutical companies, our collaborators or others using, administering or selling any of our future approved products. If we cannot successfully defend ourselves against any such claims, we may incur substantial liabilities, even if we have product liability or such other applicable insurance policies in effect. We may not be able to maintain adequate levels of insurance for these liabilities at reasonable cost and/or reasonable terms. Excessive insurance costs or uninsured claims would add to our future operating expenses and adversely affect our financial condition. As a result of such lawsuits and their potential results, we may be required to limit commercialization of our product candidates. Regardless of the merits or eventual outcome, liability claims may result in:
| | ● | decreased demand for our product candidates; |
| | ● | termination of clinical trial sites or entire trial programs; |
| | | |
| | ● | injury to our reputation and negative media attention; |
| | ● | product recalls or increased warnings on product labels; |
| | ● | withdrawal of clinical trial participants; |
| | ● | costs of to defend the related litigation; |
| | ● | diversion of management and our resources; |
| | ● | substantial monetary awards to, or costly settlements with, clinical trial participants, patients or other claimants; |
| | ● | higher insurance premiums; |
| | ● | loss of initiation of investigations by regulators or other authorities; and |
| | ● | the inability to successfully commercialize our product candidates, if approved. |
Risks Related to Our Financial Position and Capital Requirements
We have a limited operating history and we have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future.
We are an emerging biotechnology company with a limited operating history. To date, we have focused almost exclusively on developing product candidates using the active ingredient mazindol in a controlled release formulation. We have funded our operations to date primarily through proceeds from the private placement of common shares, convertible instruments, related party credit facilities and shareholder loans and a certain license agreement, which we refer to herein as the EF License Agreement. We have only a limited operating history upon which you can evaluate our business and prospects. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the pharmaceutical industry. To date, although we received an upfront payment of approximately $2.5 million pursuant to the EF License Agreement, we have not generated revenue from the sale of our product candidates (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” for additional information). We have incurred losses in each year since our inception. Our net loss attributable to holders of our common shares for the six months ended June 30, 2020 was approximately $1.2 million and for the years ended December 31, 2019 and 2018 was approximately $5.4 million and $5.1 million, respectively. As of June 30, 2020, we had an accumulated deficit of approximately $28.1 million. Substantially all of our operating losses resulted from costs incurred in connection with our development program and from general and administrative costs associated with our operations.
We expect our research and development expenses to increase in connection with our planned expanded clinical trials. In addition, if we obtain marketing approval for Quilience, Nolazol or any other current or future product candidate, we will likely incur significant sales, marketing and outsourced manufacturing expenses, as well as continued research and development expenses. Furthermore, in the period following this offering, we expect to incur additional costs associated with operating as a public company, which we estimate will be at least several hundred thousand dollars annually. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or when we will become profitable, if at all.
We expect to continue to incur significant losses until we are able to commercialize our product candidates, which we may not be successful in achieving. We anticipate that our expenses will increase substantially if and as we:
| | ● | continue the research and development of our product candidates; |
| | | |
| | ● | expand the scope of our current clinical studies for our product candidates; |
| | | |
| | ● | seek regulatory and marketing approvals for our product candidates that successfully complete clinical studies; |
| | | |
| | ● | establish a sales, marketing, and distribution infrastructure to commercialize our product candidates; |
| | | |
| | ● | seek to identify, assess, acquire, license, and/or develop other product candidates and subsequent generations of our current product candidates; |
| | | |
| | ● | seek to maintain, protect, and expand our intellectual property portfolio; |
| | | |
| | ● | seek to attract and retain skilled personnel; and |
| | | |
| | ● | create additional infrastructure to support our operations as a public company and our product candidate development and planned future commercialization efforts. |
We have not generated revenue from any product candidate and may never be profitable.
Our ability to become profitable depends upon our ability to generate revenue. To date, we have not generated any revenue from our development stage product candidates, Quilience and Nolazol, and we do not know when, or if, we will generate any such revenue. We do not expect to generate significant revenue unless or until we obtain marketing approval of, and commercialize, Quilience and/or Nolazol. Our ability to generate future revenue from product candidate sales depends heavily on our success in many areas, including but not limited to:
| | ● | obtaining favorable results from and progress the pre-clinical and clinical development of our product candidates, namely Quilience and Nolazol; |
| | | |
| | ● | developing and obtaining regulatory approval for registration studies protocols for our product candidates, namely Quilience and Nolazol; |
| | | |
| | ● | subject to successful completion of registration and clinical trials of Nolazol, applying for and obtaining marketing approval; |
| | | |
| | ● | establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products, and at acceptable costs, to support market demand for our product candidates, if marketing approval is received; |
| | ● | identifying, assessing, acquiring and/or developing new product candidates; |
| | | |
| | ● | accurately identifying demand for our product candidates; |
| | | |
| | ● | continued consumer interest in treatments to the symptoms of narcolepsy and ADHD; |
| | | |
| | ● | obtaining market acceptance of our product candidates, if approved for marketing, as viable treatment options; |
| | | |
| | ● | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; |
| | | |
| | ● | obtaining and maintaining CIV labeling (no FDA imposed boxed warning, commonly referred to as a “Black Box” warning) of our lead product candidates, Quilience and Nolazol; |
| | | |
| | ● | establishing and nurturing relationships with the leading prescribers of ADHD prescriptions in the United States; and |
| | | |
| | ● | attracting, hiring and retaining qualified personnel. |
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern, which could prevent us from obtaining new financing on reasonable terms or at all.
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm on our audited financial statements for each of the two years ended December 31, 2019 and 2018 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our audited financial statements do not include any adjustments that might result from the outcome of the uncertainty regarding our ability to continue as a going concern. This going concern opinion could materially limit our ability to raise additional funds through the issuance of equity or debt securities or otherwise. Further reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern. If we cannot continue as a going concern, our investors may lose their entire investment in our common shares and the Warrants. Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through debt or equity financing. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to our products.
Even if this offering is successful, we expect that we will need to raise substantial additional funding before we can expect to complete the development of Quilience or any other product candidate. This additional financing may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product candidate development efforts or other operations.
As of June 30, 2020, our cash and cash equivalents were approximately $0.1 million, and we had a working capital deficit of $4.5 million and an accumulated deficit of $28.1 million. Based upon our currently expected level of operating expenditures, we expect that our existing cash and cash equivalents, including the 2020 Bridge Loan, will only be sufficient to fund operations through December 2020. Even if this offering is completed, we expect that we will require substantial additional capital to commercialize our product candidates. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future funding requirements will depend on many factors, including but not limited to:
| | ● | our clinical trial results; |
| | | |
| | ● | the cost, timing and outcomes of seeking marketing approval of Quilience and Nolazol; |
| | ● | the cost of filing and prosecuting patent applications and the cost of defending our patents; |
| | | |
| | ● | the cost of prosecuting patent infringement actions against third parties; |
| | | |
| | ● | development of other early-stage development product candidates; |
| | | |
| | ● | the costs associated with commercializing Quilience and Nolazol if we receive marketing approval, including the cost and timing of establishing sales and marketing capabilities to market and sell Quilience and Nolazol; |
| | | |
| | ● | subject to receipt of marketing approval, revenue received from sales of approved products, if any, in the future; |
| | | |
| | ● | any product liability or other lawsuits related to our products; |
| | | |
| | ● | the expenses needed to attract and retain skilled personnel; and |
| | | |
| | ● | the costs associated with being a public company. |
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all, during or after the COVID-19 pandemic. Moreover, the terms of any financing may adversely affect the holdings or the rights of holders of our securities and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common shares or Warrants to decline. The incurrence of indebtedness could result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable, and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Even if we believe that we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the development or commercialization, if any, of any product candidates or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Raising additional capital would cause dilution to our existing shareholders, and may affect the rights of existing shareholders.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the issuance of equity (such as this offering) or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder. Future sales of our common shares or of securities convertible into our common shares, or the perception that such sales may occur, could cause immediate dilution and adversely affect the market price of our common shares or the Warrants.
Risks Related to Our Reliance on Third Parties
We are dependent on a sole manufacturer for mazindol. Any delay, price increase or unavailability of mazindol could materially adversely affect our ability to conduct clinical trials and, if this were to occur after we obtained commercialization and marketing approval, could cause us to cease operations.
Currently we rely on a single manufacturer for mazindol for our clinical trials conducted to date pursuant to purchase orders and do not have any existing contract with such manufacturer for future supplies. The FDA requires identification of raw material suppliers in applications for approval of drug products. If mazindol was unavailable from a specified manufacturer, FDA approval of a new manufacturer, assuming one is found, could delay the manufacture of the drug involved or delay any clinical trial we are then conducting or planning to conduct. Either such occurrence could have an adverse effect on our operations and reputation and could cause us to cease operations.
Furthermore, there is a risk of a sole approved manufacturer significantly raising prices. If prices for mazindol or other raw materials were to be significantly increased, our profit margins and sales, if any, would be greatly reduced and, assuming our products were approved for commercialization or marketing, delay product launches, or delay clinical trials at earlier stages of development. Such price increase occurrences could be resolved by the successful FDA approval of an alternate supplier; however, such approval process can be lengthy and costly. There can be no guarantee that a resolution would be reached in the event of a significant price increase by our supplier of mazindol.
We rely on third parties to conduct our pre-clinical and clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party CROs to monitor and manage data for our ongoing pre-clinical and clinical programs. We rely on these parties for execution of our pre-clinical and clinical studies, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs and other vendors are required to comply with current cGMP, Good Clinical Practices, or GCP, quality system requirements, or QSR, and Good Laboratory Practices, or GLP, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, and comparable foreign regulatory authorities for all of our product candidates in clinical development. Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators, study sites and other contractors. If we or any of our CROs or vendors fail to comply with applicable regulations, the clinical data generated in our clinical studies may be deemed unreliable and the FDA, EMA or comparable foreign regulatory authorities may require us to perform additional clinical studies before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical studies comply with GCP regulations. In addition, our clinical studies must be conducted with product candidates which are produced under cGMP regulations. These risks may be heightened as a result of the evolving COVID-19 pandemic. Our failure to comply with these regulations may require us to repeat clinical studies, which would delay the regulatory approval process.
We have no manufacturing capacity and anticipate reliance on third-party manufacturers for our products.
We do not currently operate manufacturing facilities for clinical production of Nolazol or Quilience. We do not intend to develop facilities for the manufacture of products for clinical trials or commercial purposes in the foreseeable future. We will rely on third-party manufacturers to produce bulk drug products required for our clinical trials and commercial sales, if any. We plan to continue to rely upon contract manufacturers and, potentially, collaboration partners to manufacture commercial quantities of our drug product candidates if and when approved for marketing by the applicable regulatory authorities. Our contract manufacturers have not completed process validation for the drug substance manufacturing process. Process validation involves a series of activities taking place over the lifecycle of the product and process. If our contract manufacturers are not approved by the FDA, or other regulatory bodies, our commercial supply of drug substance will be significantly delayed and may result in significant additional costs. If the FDA does not consider the result of the process validation or required testing to be satisfactory, regulatory approval and/or commercial supply after launch may be delayed. The FDA and similar foreign regulatory bodies may also implement new requirements, or change their interpretation and enforcement of existing requirements, for manufacturers, packaging or testing of products at any time. If we, or our contract manufacturers are unable to comply with such process validation or other requirements, we may be subject to regulatory, civil actions or penalties which could harm our business.
We purchase finished mazindol, the molecule in both of Quilience and Nolazol, from a single third party, currently under a development agreement with us. In addition, we do not have an agreement in place for, but we have identified, a secondary fill/finish supplier. There can be no guarantee that we will enter into an agreement with such fill/finish supplier or that an effort to enter into such an agreement will be on favorable terms. If we need to identify an additional fill/finish manufacturer, we would not be able to do so without delay and likely significant additional cost.
Our existing manufacturer and any future contract manufacturers may not perform as agreed or may not remain in the contract manufacturing business. In the event of a natural disaster, business failure, strike or other difficulty, such as difficulties involving production yields, quality control and quality assurance, we may be unable to replace a third-party manufacturer in a timely manner and the production of any product candidate or commercialized drug would be interrupted, resulting in delays and additional costs.
In addition, because the contract manufacturer of our drug substance is located outside of the United States, we may face difficulties in importing our drug substances into the United States as a result of, among other things, FDA import inspections, incomplete or inaccurate import documentation or defective packaging.
We and our collaborators and contract manufacturers are subject to significant regulation with respect to manufacturing our product candidates. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and have limited capacity.
Manufacturers and their facilities are required to comply with extensive regulatory requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs. These cGMP regulations cover all aspects of manufacturing relating to our product candidates. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational product candidates and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our product candidates that may not be detectable in final product testing. We, our collaborators or our contract manufacturers must supply all necessary documentation in support of an NDA or MAA on a timely basis and must adhere to GLP and cGMP QSR regulations enforced by the FDA and other regulatory authorities through their facilities inspection program. Some of our contract manufacturers have never produced a commercially approved pharmaceutical product and therefore have not obtained the requisite regulatory authority approvals to do so. The facilities and quality systems of some or all of our collaborators and third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates or any of our other potential product candidates. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or our other potential product candidates or the associated quality systems for compliance with the regulations applicable to the activities being conducted. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the regulatory requirements. If these facilities do not pass a pre-approval plant inspection, regulatory approval of the product candidates may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever. Moreover, if our contract manufacturer’s fail to achieve and maintain high manufacturing standards, in accordance with applicable regulatory requirements, or there are substantial manufacturing errors, this could result in patient injury or death, product shortages, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business.
Any collaboration arrangements that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our current and potential future product candidates.
We may seek collaboration arrangements with pharmaceutical or biotechnology companies for the development or commercialization of our current and potential future product candidates. We may enter into these arrangements on a selective basis depending on the merits of retaining commercialization rights for ourselves as compared to entering into selective collaboration arrangements with other pharmaceutical or biotechnology companies for each product candidate, both in the United States and internationally. We will face, to the extent that we decide to enter into collaboration agreements, significant competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements should we so choose to enter into such arrangements. The terms of any collaborations or other arrangements that we may establish may not be favorable to us.
Disagreements between parties to a collaboration arrangement regarding clinical development and commercialization matters can lead to delays in the development process or commercializing the applicable product candidate and, in some cases, termination of the collaboration arrangement. These disagreements can be difficult to resolve if neither of the parties has final decision-making authority.
Collaborations with pharmaceutical or biotechnology companies and other third parties often are terminated or allowed to expire by the other party. Any such termination or expiration could adversely affect us financially and could harm our business reputation.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to develop and manufacture our product candidates, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business.
Risks Related to Our Intellectual Property
We have filed multiple patent applications and have a very limited number of issued patents. There can be no assurance that any of our patent applications will result in issued patents. As a result, our ability to protect our proprietary technology in the marketplace may be limited.
We have filed patent applications in many countries worldwide. These applications cover a range of areas including: the therapeutic use of mazindol for the treatment of ADHD and combination therapies containing mazindol (add-ons with iron or stimulants) as well as sleep disorders such as narcolepsy or IH. Unless and until our pending patent applications are issued, their protective scope is impossible to determine. It is impossible to predict whether or how many of our patent applications will result in issued patents. Even if pending applications are issued, they may be issued with coverage significantly narrower than what we currently seek
Our proprietary position for our product candidates currently depends upon patents protecting the method of use, which may not prevent a competitor or other third party from using the same product candidate for another use.
The primary patent based intellectual property protection for our product candidates will be any patents granted on the method of use and formulation. We do not have patents or patent applications covering our products as a composition of matter (i.e., compound claims).
Composition of matter patent claims on the active pharmaceutical ingredient, or API, in pharmaceutical drug products are generally considered to be the favored form of intellectual property protection for pharmaceutical products, as such patents provide protection without regard to any particular method of use, manufacture or formulation of the API used. Method of use patent claims protect the use of a product for the specified method and dosing. These types of patent claims do not prevent a competitor or other third party from making and marketing an identical API for an indication that is outside the scope of the method claims or from developing a different dosing regimen. Moreover, even if competitors or other third parties do not actively promote their product for our targeted indications or uses for which we may obtain patents, physicians may recommend that patients use these products off-label, or patients may do so themselves. Although off-label use may infringe or contribute to the infringement of method of use patents, the practice is common and such infringement is difficult to prevent or prosecute.
Even if we are issued patents, because the patent positions of pharmaceutical products are complex and uncertain, we cannot predict the scope and extent of patent protection for our product candidates.
Any patents that may be issued to us will not ensure the protection of our intellectual property for a number of reasons, including without limitation the following:
| | ● | any issued patents may not be broad or strong enough to prevent competition from other drug products including identical or similar drug products; |
| | | |
| | ● | if we are not issued patents or if issued patents expire, there would be no protections against competitors making generic equivalents; |
| | | |
| | ● | there may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim; |
| | ● | there may be other patents existing, now or in the future, in the patent landscape for Quilience and Nolazol, or any other product candidates that we seek to commercialize or develop, if any, that will affect our freedom to operate; |
| | | |
| | ● | if our patents are challenged, a court could determine that they are not valid or enforceable; |
| | | |
| | ● | a court could determine that a competitor’s technology or product does not infringe our patents; |
| | | |
| | ● | our patents could irretrievably lapse due to failure to pay fees or otherwise comply with regulations, or could be subject to compulsory licensing; and |
| | | |
| | ● | if we encounter delays in our development or clinical trials, the period of time during which we could market our products under patent protection would be reduced. |
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the U.S. PTO and foreign patent agencies in several stages over the term of the patent. The U.S. PTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to office actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States and Switzerland can be less extensive than those in the United States and Switzerland. In addition, the laws of some foreign countries do not protect intellectual property to the same extent as laws in the United States and Switzerland. Consequently, we may not be able to seek to prevent third parties from practicing our inventions in all countries outside the United States and Switzerland, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors, for example, may use our technologies in jurisdictions where we have not obtained patents to develop their own products and further, may export otherwise infringing products to territories where we have patents, but enforcement is not as strong as that in the United States and Switzerland.
Many companies have encountered significant problems in protecting and defending intellectual property in foreign jurisdictions. The legal systems of certain countries, particularly China and certain other developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property, particularly those relating to medical devices and biopharmaceutical and biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. To date, we have not sought to enforce any issued patents in these foreign jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. The requirements for patentability may differ in certain countries, particularly developing countries. Certain countries in Europe and developing countries, including China and India, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In those countries, we and our licensors may have limited remedies if patents are infringed or if we or our licensors are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
If we are unable to maintain effective proprietary rights for our product candidates, we may not be able to compete effectively in our markets.
In addition to the protection afforded by any patents currently owned and that may be granted, historically, we have relied on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes that are not easily known, knowable or easily ascertainable, and for which patent infringement is difficult to monitor and enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data, trade secrets and intellectual property by maintaining physical security of our premises and physical and electronic security of our information technology systems. Agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets and intellectual property may otherwise become known or be independently discovered by competitors.
We cannot provide any assurances that our trade secrets and other confidential proprietary information will not be disclosed in violation of our confidentiality agreements or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Also, misappropriation or unauthorized and unavoidable disclosure of our trade secrets and intellectual property could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets and intellectual property are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secret.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability to develop, manufacture, market and sell our platform technology without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the medical device and pharmaceutical industries. While no such litigation has been brought against us and we have not been held by any court to have infringed a third party’s intellectual property rights, we cannot guarantee that our technology or use of our technology does not infringe third-party patents. It is also possible that we have failed to identify relevant third-party patents or applications. For example, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing, which is referred to as the priority date. Therefore, patent applications covering our technology could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our technology.
We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our technology, including inter parties review, interference, or derivation proceedings before the U.S. PTO and similar bodies in other countries. Third parties may assert infringement claims against us based on existing intellectual property rights and intellectual property rights that may be granted in the future.
If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our technology or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. In addition, we may receive less revenue from future products if any of our employees successfully claim for compensation for their work in developing our intellectual property, which in turn could impact our future profitability.
Under applicable employment laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees. In addition, employees may be entitled to seek compensation for their inventions irrespective of their agreements with us.
We generally enter into non-competition agreements with our employees and certain key consultants. These agreements prohibit our employees and certain key consultants, if they cease working for us, from competing directly with us or working for our competitors or clients for a limited period of time. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants developed while working for us. Under Swiss law, a non-compete agreement could be invalidated if, for example, the geographic scope of the non-compete agreement is too broad, or, alternatively, such an agreement could be deemed by a Swiss court to be an occupation ban. Such actions would make enforcing our non-competition agreements more challenging and could make it easier for our competitors to employee our previous employees.
In addition, under Swiss law, if we wish to obtain ownership over inventions developed by our employees, which inventions were developed while performing their employment activities, but outside the performance of their contractual duties, we are required to compensate the employee for the rights to their respective inventions. There can be no guarantee that we will be able to obtain any such inventions and the failure to obtain such ownership rights over employee inventions could have a material adverse effect on our operations and ability to effectively compete.
Risks Related to Our Business Operations
We manage our business through a small number of employees and key consultants.
Our key employees include our Chief Executive Officer, Mr. Alexander Zwyer, who co-founded us in 2015, as well as our Interim Chief Financial Officer, Robert Dickey IV, our Interim Chief Business Officer, Hervé Girsault, and our Interim Chief Medical Officer, Sandy Eisen. With the exception of our Chief Executive Officer, our interim executive officers are not engaged as full-time employees and we do not expect that other executive officers that join us after the completion of this offering will be engaged as full-time employees in the immediate future. Our future growth and success depend on our ability to recruit, retain, manage and motivate our employees and key consultants. The loss of the services of our Chief Executive Officer or any of our key executive officers or the inability to hire or retain experienced management personnel could adversely affect our ability to execute our business plan and harm our operating results. Although we expect to enter into employment agreements with management, these agreements will likely be terminable at will with minimal notice.
In addition, laws and regulations on executive compensation, including legislation in our home country, Switzerland, may restrict our ability to attract, motivate and retain the required level of qualified personnel. In Switzerland, legislation affecting public companies has been passed that, among other things, (i) imposes an annual binding shareholders’ “say-on-pay” vote with respect to the compensation of the members of (a) the executive committee and (b) the board of directors, (ii) prohibits severance, advances, transaction premiums and similar payments to executive officers and directors, and (iii) requires companies to specify various compensation-related matters in their articles of association, thus requiring them to be approved by a shareholders’ vote.
Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific and technical consultants. In particular, the loss of one or more of our executive officers or key consultants could be detrimental to us if we cannot recruit suitable replacements in a timely manner. We do not currently carry “key person” insurance on the lives of members of senior management. The competition for qualified personnel in the pharmaceutical field is intense. Due to this intense competition, we may be unable to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
Certain agreements that we entered into with our lenders contain, and future debt agreements may contain, restrictions that may limit our flexibility in operating our business.
Prior to the Reorganization, we entered into a credit facility with certain lenders, one of which is a member of our board, which contained restrictions, such as prohibiting that we merge into another company or change our accounting standards, that could limit our flexibility in operating our business. Documents governing our future indebtedness, or in connection with additional capital raises, if any, may contain, numerous financial and operating covenants that limit the discretion of management with respect to certain business matters. Restrictive covenants included in the above-mentioned credit facility include restrictions on, among others, our ability to:
| | ● | create or permit to subsist any security interest over any of our assets; |
| | | |
| | ● | sell, transfer or otherwise dispose of any or our receivables on recourse terms; |
| | | |
| | ● | pay dividends; |
| | | |
| | ● | buy back our own common shares; |
| | | |
| | ● | incur or permit additional indebtedness; |
| | | |
| | ● | merge or conduct any other corporate reconstruction; and |
| | | |
| | ● | change the nature of our business. |
Our ability to comply with these and other provisions of the existing debt agreements is dependent on our future performance, which will be subject to many factors, some of which are beyond our control. The breach of any negative covenants in our current or future agreements could result in an event of default, as may be defined in such agreements, thereby leading to a potential default interest rate or immediate repayment of any borrowed amounts. These restrictive covenants which may be in place from time to time and a lack of compliance by us could limit our flexibility in operating our business.
We face business disruption and related risks resulting from the recent outbreak of the COVID-19 pandemic, which could have a material adverse effect on our business and results of operations.
In December 2019, a novel strain of coronavirus, COVID-19, was identified in Wuhan, China. This virus continues to spread globally and, as of September 2020, has spread to approximately 200 countries, including the United States and Switzerland. The spread of COVID-19 from China to other countries has resulted in the World Health Organization declaring the outbreak of COVID-19 as a “pandemic,” or a worldwide spread of a new disease. Many countries around the world have imposed quarantines and restrictions on travel and mass gatherings to slow the spread of the virus. Authorities around the world have and may continue implementing similar restrictions on businesses and individuals in their jurisdictions. While COVID-19 is still spreading and the final implications of the pandemic are difficult to estimate at this stage, it is clear that it has affected the lives of a large portion of the global population.
Our operations and business have experienced disruption due to the unprecedented conditions surrounding the COVID-19 pandemic spreading throughout Switzerland and the world, including our decision to delay the offering of securities pursuant to this prospectus. Although we cut our operating expenses prior to the COVID-19 pandemic, there can be no assurance that this or other remedial measures that have been enacted will enable us to avoid part or all of any impact from the spread of COVID-19 or its consequences, including downturns in business sentiment generally or in our sector in particular. In addition, the impact of COVID-19 may cause delays to our future clinical trials, and may make it difficult for us to enroll patients to clinical trials.
It is not possible at this time to estimate the full impact that the COVID-19 pandemic, the continued spread of COVID-19, and any additional measures taken by governments, health officials or by us in response to such spread, could have on our business, including the timing of our planned clinical trials, results of operations and financial condition. The COVID-19 pandemic and mitigation measures have also negatively impacted global economic conditions, which, in turn, could adversely affect our business, including the timing of our planned clinical trials, results of operations and financial condition. We continue to monitor our operations and government recommendations. A significant reduction in our workforce and our compliance with instructions imposed by Swiss and other European authorities may harm our ability to continue operating our business and materially and adversely affect our operations and financial condition. Moreover, we cannot foresee whether the Swiss or other European authorities or the U.S. federal government will impose further restrictive instructions, which if implemented may lead to significant changes. The spread of COVID-19 may also result in the inability of our suppliers to deliver components or raw materials on a timely basis. The extent to which COVID-19 impacts our business, results of operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others. See “Risks Related to Product Development, Regulatory Approval and Commercialization – We may find it difficult to enroll patients in our clinical trials. Difficulty in enrolling patients could delay or prevent clinical trials of our product candidates” for additional information.
We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth.
We currently have a very limited number of employees and in order to commercialize our products, we will need to substantially increase our operations, including expanding our employee base of managerial, operational and financial personnel. Any future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. To that end, we must be able to:
| | ● | manage our clinical trials and the regulatory process effectively; |
| | ● | develop our administrative, accounting and management information systems and controls; |
| | ● | hire and train additional qualified personnel; and |
| | ● | integrate current and additional management, administrative, financial and sales and marketing personnel. |
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations may be directly or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act and physician sunshine laws and regulations. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| | ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| | ● | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
| | ● | the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| | ● | HIPAA, as amended by the Health Information Technology and Clinical Health Act, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
| | ● | the federal physician sunshine requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or the PPACA, requires manufacturers of drugs, devices and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; |
| | ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; and |
| | | |
| | ● | European and other foreign law equivalents of each of the laws, including reporting requirements detailing interactions with and payments to healthcare providers, and the European General Data Protection Regulation, or GDPR, which became effective in May 2018 and contains new provisions specifically directed at the processing of health information, higher sanctions and extra-territoriality measures intended to bring non-EU companies under the regulation, including companies like us that conduct clinical trials in the EU; we anticipate that over time we may expand our business operations to include additional operations in the EU and with such expansion, we would be subject to increased governmental regulation in the EU countries in which we might operate, including the GDPR. |
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other related governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, disgorgement, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, reputational harm, additional oversight and reporting obligations if we become subject to a corporate integrity agreement or similar settlement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to similar actions, penalties and sanctions. Efforts to ensure that our business arrangements comply with applicable healthcare laws and regulations, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from the business.
Healthcare legislative and regulatory reform measures may have a material adverse effect on our business and results of operations.
Our industry is highly regulated and changes in law may adversely impact our business, operations or financial results. The PPACA is a sweeping measure intended to, among other things, expand healthcare coverage within the United States, primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. Several provisions of the law may affect us and increase certain of our costs.
In addition, other legislative changes have been adopted since the PPACA was enacted. These changes include aggregate reductions in Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, following passage of the Bipartisan Budget Act of 2018, will remain in effect through 2027 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and, accordingly, our financial operations.
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the reimbursement our customers may receive for our products. Further, there have been, and there may continue to be, judicial and Congressional challenges to certain aspects of the PPACA. For example, the U.S. Tax Cuts and Jobs Act of 2017, or TCJA, includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Additional legislative and regulatory changes to the PPACA, its implementing regulations and guidance and its policies, remain possible in Congress and under the Trump administration. However, it remains unclear how any new legislation or regulation might affect the prices we may obtain for any of our product candidates for which regulatory approval is obtained. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
In addition, the delivery of healthcare in the European Union, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval.
We are currently unable to predict what additional legislation or regulation, if any, relating to the health care industry may be enacted in the future or what effect recently enacted federal legislation or any such additional legislation or regulation would have on our business. The pendency or approval of such proposals or reforms could result in a decrease in the price of our common shares or Warrants or limit our ability to raise capital or to enter into collaboration agreements for the further development and potential commercialization of our products.
The use of any of our product candidates could result in product liability or similar claims that could be expensive, damage our reputation and harm our business.
Our business exposes us to an inherent risk of potential product liability or similar claims. The pharmaceutical industry has historically been litigious, and we face financial exposure to product liability or similar claims if the use of any of our products were to cause or contribute to injury or death. There is also the possibility that defects in the design or manufacture of any of our products might necessitate a product recall. Although we plan to maintain product liability insurance, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration and a substantial diversion of management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations.
Failure in our information technology systems, including by cybersecurity attacks or other data security incidents, could significantly disrupt our operations.
Our operations depend, in part, on the continued performance of our information technology systems. Our information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. Failure of our information technology systems could adversely affect our business, profitability and financial condition. Although we have information technology security systems, a successful cybersecurity attack or other data security incident could result in the misappropriation and/or loss of confidential or personal information, create system interruptions, or deploy malicious software that attacks our systems. It is possible that a cybersecurity attack might not be noticed for some period of time. The occurrence of a cybersecurity attack or incident could result in business interruptions from the disruption of our information technology systems, or negative publicity resulting in reputational damage with our shareholders and other stakeholders and/or increased costs to prevent, respond to or mitigate cybersecurity events. In addition, the unauthorized dissemination of sensitive personal information or proprietary or confidential information could expose us or other third parties to regulatory fines or penalties, litigation and potential liability, or otherwise harm our business.
Unsuccessful compliance with certain European privacy regulations could have an adverse effect on our business and reputation.
The collection and use of personal health data in the EU is governed by the provisions of the Data Protection Directive, and as of May 2018 the GDPR. These directives impose several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, notification of data processing obligations to the competent national data protection authorities and the security and confidentiality of the personal data. The GDPR also extends the geographical scope of EU data protection law to non-EU entities under certain conditions, tightens existing EU data protection principles and creates new obligations for companies and new rights for individuals. Failure to comply with the requirements of the Data Protection Directive, the GDPR, and the related national data protection laws of the EU Member States may result in fines and other administrative penalties. The GDPR introduces new data protection requirements in the EU and substantial fines for breaches of the data protection rules. The GDPR regulations impose additional responsibility and liability in relation to personal data that we process and we intend to put in place additional mechanisms ensuring compliance with these and/or new data protection rules. In addition, other jurisdictions, including Switzerland, are currently discussing or implementing regulations similar to GDPR. Changes to these European privacy regulations (and similar regulations in other jurisdictions) and unsuccessful compliance may be onerous and adversely affect our business, financial condition, prospects, results of operations and reputation.
Risks Related to this Offering and Ownership of Our Securities
The market price of our common shares and Warrants may be highly volatile, and you may not be able to resell your shares or Warrants at or above the initial public offering price.
Prior to this offering, there has not been a public market for our securities, including Units. If an active trading market for our common shares or Warrants does not develop following this offering, you may not be able to sell your shares or Warrants quickly or at the market price. The initial public offering price for the Units will be determined by negotiations between us and representative of the underwriters and may not be indicative of prices that will prevail in the trading market.
The trading price of each of our common shares and Warrants is likely to be volatile. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of such securities:
| | ● | inability to obtain the approvals necessary to commence further clinical trials; |
| | | |
| | ● | unsatisfactory results of clinical trials; |
| | | |
| | ● | announcements of regulatory approval or the failure to obtain it, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| | | |
| | ● | announcements of therapeutic innovations or new products by us or our competitors; |
| | ● | adverse actions taken by regulatory authorities with respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
| | ● | changes or developments in laws or regulations applicable to the treatment of the symptoms of narcolepsy, ADHD, or any other indication that we may seek to develop, or the use of mazindol to treat any such indications; |
| | ● | any adverse changes to our relationship with manufacturers or suppliers; |
| | ● | any intellectual property infringement actions in which we may become involved; |
| | ● | announcements concerning our competitors or the pharmaceutical industry in general; |
| | ● | achievement of expected product sales and profitability or our failure to meet expectations; |
| | ● | our commencement of, or involvement in, litigation; |
| | ● | any major changes in our board of directors or management; |
| | | |
| | ● | our ability to recruit and retain qualified regulatory, research and development personnel; |
| | ● | legislation in the United States relating to the sale or pricing of pharmaceuticals; |
| | | |
| | ● | the depth of the trading market in our common shares and Warrants; |
| | | |
| | ● | termination of the lock-up agreements or other restrictions limiting our ability or that of any of our existing shareholders to sell our common shares (or any other securities that we may issue, if any) after this offering; |
| | | |
| | ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| | | |
| | ● | business interruptions resulting from a local or worldwide pandemic, such as COVID-19, geopolitical actions, including war and terrorism, or natural disasters; |
| | | |
| | ● | the granting or exercise of employee stock options or other equity awards; and |
| | | |
| | ● | changes in investors’ and securities analysts’ perception of the business risks and conditions of our business. |
In addition, the stock market in general, and the Nasdaq Stock Market in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of small companies. Broad market and industry factors may negatively affect the market price of our common shares and Warrants, regardless of our actual operating performance. Further, a systemic decline in the financial markets and related factors beyond our control may cause our share price to decline rapidly and unexpectedly.
We have identified material weaknesses in our internal control over financial reporting and, if our remediation of the material weaknesses is not effective or if we identify additional material weaknesses in the future, we may not be able to accurately or timely report our financial results, or prevent fraud, and investor confidence in our Company and the market price of our shares and Warrants may be adversely affected.
As a public company, we will be required to maintain internal control over financial reporting and to report any material weaknesses in such internal control. Section 404 of the Sarbanes-Oxley Act requires that we evaluate and determine the effectiveness of our internal control over financial reporting. A material weakness is a deficiency or combination of deficiencies in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis.
To date, we have had limited financial and accounting personnel, which has resulted in a limited segregation of duties to fully execute our accounting processes and address our internal control over financial reporting. In connection with the audits of our financial statements as of and for the years ended December 31, 2019 and 2018, we identified certain material weaknesses in our internal control over financial reporting.
Such material weaknesses relate to an improper cutoff of our accounts payable and accrued expenses in a previous period, and to the accounting for complex transactions, including intangible assets and a licensing agreement. We plan to take steps to address the internal control deficiencies that contributed to the material weaknesses, including the following:
| | ● | hiring of additional finance and accounting personnel with prior experience working for finance departments and technical accounting experience, supplemented by third-party resources; |
| | ● | documenting and formally assessing our accounting and financial reporting policies and procedures; and |
| | | |
| | ● | increasing the use of third-party consultants in assessing significant accounting transactions and other technical accounting and financial reporting issues, preparing accounting memoranda addressing these issues and maintaining these memoranda in our corporate records. |
While we believe that these efforts will improve our internal control over financial reporting, the implementation of these measures is ongoing and will require validation and testing of the design and operating effectiveness of internal controls over a sustained period of financial reporting cycles. We cannot assure you that the measures we have taken to date, and are continuing to implement, will be sufficient to remediate the material weaknesses we have identified or avoid potential future material weaknesses. If the steps we take do not correct the material weaknesses in a timely manner, we will be unable to conclude that we maintain effective internal control over financial reporting. Accordingly, there could continue to be a reasonable possibility that these deficiencies or others could result in a misstatement of our accounts or disclosures that would result in a material misstatement of our financial statements that would not be prevented or detected on a timely basis.
Participation in this offering by certain of our existing officers, who are also shareholders, and a director nominee, could reduce the public float for our shares.
Certain existing officers, who are also shareholders, and a director nominee, have indicated an interest in purchasing up to an aggregate of $138,000 of Units in this offering at the initial public offering price per Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these persons, or any of these persons may determine to purchase more, less or no Units in this offering.
If these persons are allocated all or a portion of the Units in which each has indicated an interest in this offering or are allocated more Units than each has indicated an interest in this offering, and these persons purchase any such shares, such purchase could reduce the available public float for our common shares if these persons hold the common shares purchased as part of the Units for an extended period of time.
The Warrants included in the Units are expected to be listed on Nasdaq separately upon the pricing of this offering, and may provide investors with an arbitrage opportunity that could adversely affect the trading price of our common shares.
Because the Units will never trade as a unit, and the Warrants are expected to be traded on Nasdaq, investors may be provided with an arbitrage opportunity that could depress the price of our common shares.
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their shares have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
Our Chief Executive Officer, directors and shareholders who own more than 5% of our outstanding common shares before this offering (including a certain lender controlled by persons that are also our shareholders, one of which is our Chairman) currently own approximately 86% of our outstanding common shares and will own approximately 61% of our common shares upon the completion of this offering (excluding any common shares that may be purchased by such persons in this offering, as disclosed herein). They will therefore be able to exert significant control over matters submitted to our shareholders for approval.
After this offering, our Chief Executive Officer and directors, and shareholders who own more than 5% of our outstanding common shares before this offering (including a certain lender controlled by persons that are also shareholders of ours) will, in the aggregate, beneficially own approximately 61% of our common shares (excluding any common shares that may be purchased by such persons in this offering, as disclosed herein) (assuming no exercise of the underwriters’ over-allotment option and no exercise of outstanding options). This significant concentration of share ownership may adversely affect the trading price for our common shares or Warrants because investors often perceive disadvantages in owning shares in companies with controlling shareholders. As a result, these shareholders, if they acted together, could significantly influence or even unilaterally approve matters requiring approval by our shareholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these shareholders may not always coincide with our interests or the interests of other shareholders.
The implementation of the share capital increase may be challenged or blocked.
Prior to this offering, we will need to obtain a shareholder resolution for, among other things, the increase in share capital necessary to issue the common shares to be sold in this offering (including, if exercised, the common shares to be sold under the over-allotment option). Even if we get this approval, as with all share capital increases in Switzerland, (i) a third party, such as shareholders or creditors, may at least temporarily block the registration of the capital increase in the commercial register of the Canton of Nidwalden and request the competent court to grant a preliminary injunction in a summary proceeding in which we would be entitled to appear, and (ii) a shareholder may challenge the underlying shareholders’ resolution within two months after such shareholders’ meeting and, therefore, prevent or delay the completion of this offering. There can be no assurance that the implementation of the share capital increase will not be challenged or blocked.
If you purchase Units in this offering, you will incur immediate and substantial dilution in the book value of your common shares.
The initial public offering price is substantially higher than the net tangible book value per share of our common shares. Investors purchasing Units, including our common shares, in this offering will pay a price per share that substantially exceeds the net tangible book value of our common shares. As a result, investors purchasing common shares in this offering will incur immediate dilution of $4.61 per common share, based on an assumed initial public offering price of $5.50 per Unit based on the midpoint of the estimated price range set forth on the cover page of this prospectus, and our pro forma net tangible book value as of June 30, 2020. The exercise of outstanding convertible securities or Warrants would result in additional dilution. As a result of this dilution, investors purchasing Units may receive significantly less than the purchase price paid in this offering in the event of liquidation. See “Dilution” for additional information.
Sales of a substantial number of shares of our common shares in the public market by our existing shareholders could cause our share price to fall.
Sales of a substantial number of shares of our common shares in the public market or the perception that these sales might occur, could depress the market price of our common shares and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common shares. While the common shares sold to non-affiliates in this offering will be freely tradable, without restriction, in the public market, substantially all of the shares owned prior to this offering or purchased in this offering by our existing shareholders are expected to be subject to lock-up agreements with the underwriters of this offering that restrict the ability of these shareholders’ to transfer our common shares held by them for at least six months from the date of this prospectus. These outstanding shares that are subject to lock-up agreements are expected to become eligible for unrestricted sale upon expiration of the lockup period, as described in the section of this prospectus entitled “Shares Eligible for Future Sale.” In addition, shares issued or issuable upon exercise of options and warrants vested as of the expiration of the lock-up period will be eligible for sale at that time. Sales of shares by these shareholders could have a material adverse effect on the trading price of our common shares.
The warrants are speculative in nature.
The Warrants offered in this offering do not confer any rights of common share ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire common shares at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the Warrants may exercise their right to acquire the common shares and pay an exercise price of $ per common share, which shall not be less than 100% of the public offering price per Unit, prior to five years from the date of issuance, after which date any unexercised Warrants will expire and have no further value.
Holders of the Warrants will have no rights as a common shareholder until they acquire our common shares.
Until holders of the Warrants acquire common shares upon exercise of the Warrants, the holders will have no rights with respect to the common shares issuable upon exercise of the Warrants. Upon exercise of the Warrants, the holder will be entitled to exercise the rights of a common shareholder as to the security exercised only as to matters for which the record date occurs after the exercise.
There is no established market for the Warrants to purchase our common shares being offered in this offering.
There is no established trading market for the Warrants. Although the we have applied to list the Warrants on Nasdaq there can be no assurance that there will be an active trading market for the Warrants. Without an active trading market, the liquidity of the Warrants will be limited.
Management will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
Our management will have broad discretion in the allocation of the net proceeds and could use them for purposes other than those contemplated at the time of this offering and as described in the section titled “Use of Proceeds.” Our management could spend the proceeds in ways that you do not agree with or that do not improve our results of operations or enhance the value of our common shares.
If we were to be characterized as a “passive foreign investment company” for U.S. tax purposes, U.S. holders of our common shares and Warrants could have adverse U.S. income tax consequences.
In general, we will be treated as a passive foreign investment company, or a PFIC, for U.S. federal income tax purposes in any taxable year in which either (1) at least 75% of our gross income is “passive income” or (2) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account. We do not believe that we will be deemed a PFIC for 2019. If we are a PFIC in any taxable year during which a U.S. taxpayer holds the common shares and Warrants, such U.S. taxpayer would be subject to certain adverse U.S. federal income tax rules. In particular, except with respect to the Warrants (unless exercised), if the U.S. taxpayer did not make an election to treat us as a “qualified electing fund,” or QEF, or make a “mark-to-market” election, then “excess distributions” to the U.S. taxpayer, and any gain realized on the sale or other disposition of the common shares by the U.S. taxpayer: (1) would be allocated ratably over the U.S. taxpayer’s holding period for the common shares; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the U.S. Internal Revenue Service, or the IRS, determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. taxpayer to make a timely QEF or mark-to-market election. U.S. taxpayers that have held the common shares during a period when we were a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. taxpayer who made a timely QEF or mark-to-market election. A U.S. taxpayer can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. We intend to make available to U.S. taxpayers upon request the information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. U.S. taxpayers that hold the common shares are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election with respect to the common shares in the event that we are a PFIC. See “Taxation—U.S. Federal Income Tax Consequences—Passive Foreign Investment Companies” for additional information.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or our shares or Warrants, the trading prices of our shares and Warrants and trading volume of our shares and Warrants could decline.
The trading market for our common shares and Warrants will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding our shares or Warrants, or provide more favorable relative recommendations about our competitors, the trading price of our shares or Warrants would likely decline. If any analyst who may cover us were to cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the trading price of our shares and Warrants and trading volume of our shares and Warrants to decline.
We have not paid, and do not intend to pay, dividends on our common shares and, therefore, unless our traded securities appreciate in value, our investors may not benefit from holding our common shares.
We have never declared or paid cash dividends on our common shares. We do not anticipate paying any cash dividends on our common shares in the foreseeable future. In addition, in March 2020, in response to the COVID-19 pandemic, the Swiss Federal Council issued the COVID-19 Ordinance on Joint and Several Guarantees, or the COVID-19 Ordinance, according to which, companies domiciled in Switzerland that are economically affected by the COVID-19 pandemic can apply for financial support in the form of emergency bank loans of up to 10% of their 2019 revenues (as shown in the relevant statutory financial statements) or a maximum of CHF 20 million, or the COVID-19 Loan. This COVID-19 loan is secured by the Swiss Confederation. The lending banks receive collateral in the form of joint and several guarantees. We received such a COVID-19 Loan from our bank for CHF 248,400 ($262,137), with 0% interest and a term of 60 months. We have drawn down the entire amount available to us under the COVID-19 Loan. The amount drawn under our COVID-19 Loan is equivalent to approximately 10% of our 2019 revenues as shown on our statutory standalone financials (which have been prepared in accordance with Swiss corporate law). The revenues reflected in such statutory financial statements relate to payments that we have received under the EF License Agreement in 2019. Unlike under U.S. GAAP, where no revenue was recognized for our 2019 financial year, the license income from the EF License Agreement is recognized as revenue in the Swiss statutory financial statements. Pursuant to the terms of the COVID-19 Loan, we may not pay any dividends until we repay such loan in full.
Moreover, Swiss Law imposes certain restrictions on our ability to declare and pay dividends. See “Dividend Policy” and “Description of Share Capital and Governing Documents—Dividends and Other Distributions” for additional information.
The requirements associated with being a public company will require significant company resources and management attention.
Following this offering, we will become subject to the reporting requirements of the Exchange Act, Nasdaq listing requirements and other applicable securities rules and regulations. The Exchange Act requires that we file periodic reports with respect to our business and financial condition and maintain effective disclosure controls and procedures and internal control over financial reporting. In addition, subsequent rules implemented by the SEC and the Nasdaq Stock Market may also impose various additional requirements on public companies. As a result, we will incur additional legal, accounting and other expenses that we did not incur as a nonpublic company, particularly after we are no longer an “emerging growth company” as defined in the JOBS Act. In the period following this offering, we estimate that these expenses will be at least several hundred thousand dollars annually. Further, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our development plans. We have made changes to our corporate governance standards, disclosure controls and financial reporting and accounting systems to meet our reporting obligations. The measures we take, however, may not be sufficient to satisfy our obligations as a public company, which could subject us to delisting of our common shares, fines, sanctions and other regulatory action and potentially civil litigation.
The JOBS Act allows us to postpone the date by which we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the SEC, which could undermine investor confidence in our Company and adversely affect the market price of our common shares and Warrants.
For so long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies” including:
| | ● | the provisions of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| | ● | Section 107 of the JOBS Act, which provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are electing to delay such adoption of new or revised accounting standards. As a result of this adoption, our financial statements may not be comparable to companies that comply with the public company effective date; |
| | ● | any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements; and |
| | ● | our ability to furnish two rather than three years of income statements and statements of cash flows in various required filings. |
We intend to take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the date of the completion of this offering, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, as defined in the rule under the Exchange Act, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
We cannot predict if investors will find our common shares or Warrants less attractive because we may rely on these exemptions. If some investors find our common shares or Warrants less attractive as a result, there may be a less active trading market for each such traded security, and the trading price of each may be more volatile and may decline.
As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of otherwise applicable SEC and Nasdaq requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.
Our status as a foreign private issuer exempts us from compliance with certain SEC laws and regulations and certain regulations of the Nasdaq Stock Market, including the proxy rules, the short-swing profits recapture rules, and certain governance requirements such as independent director oversight of the nomination of directors and executive compensation. In addition, we will not be required under the Exchange Act to file current reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act and we will generally be exempt from filing quarterly reports with the SEC. Furthermore, as a foreign private issuer, we are also not subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act. These exemptions and leniencies will reduce the frequency and scope of information and protections to which you are entitled as an investor. See “Management – Differences between Swiss Laws and Nasdaq Requirements” for additional information.
As a foreign private issuer, we are permitted, and intend, to phase-in our compliance with certain Nasdaq Listing Rules, as permitted by Nasdaq Listing Rule 5615(b)(1), instead of otherwise having to be in compliance with such rules as of the date of our initial listing on Nasdaq, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers and if we do not obtain compliance within the allotted time, we could become subject to delisting by Nasdaq.
In accordance with Nasdaq Listing Rule 5615(b)(1), as a foreign private issuer, we are permitted, and intend, to phase-in our compliance with certain Nasdaq Listing Rules, instead of otherwise having to be in compliance with such rules as of the date of our initial listing on Nasdaq. For instance, although we are a Foreign Private Issuer and have opted into following our home country rules, which are the laws of the State of Switzerland, in lieu of following certain Nasdaq Listing Rules, we are still required to have an audit committee that satisfies Nasdaq Listing Rule 5605(c)(3) and ensure that such audit committee members meet the independence requirement in Nasdaq Listing Rule 5605(c)(2)(A)(ii), provided, however, that in light of Nasdaq Listing Rule 5615(b)(1), we have up to one year from the date of our listing to have an audit committee and members who meet such requirements. This phased-in period of compliance may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers and if we do not obtain compliance within the allotted time, we could become subject to delisting by Nasdaq.
Risks Related to Swiss Law and Our Operations in Switzerland
We are a Swiss corporation. The rights of our shareholders may be different from the rights of shareholders in companies governed by the laws of U.S. jurisdictions.
We are a Swiss corporation. Our corporate affairs are governed by our articles of association and by the laws governing companies, including listed companies, incorporated in Switzerland. The rights of our shareholders and the responsibilities of members of our board of directors may be different from the rights and obligations of shareholders and directors of companies governed by the laws of U.S. jurisdictions. In the performance of its duties, our board of directors is required by Swiss law to consider the interests of our Company, our shareholders, our employees and other stakeholders, in all cases with due observation of the principles of reasonableness and fairness. It is possible that some of these parties will have interests that are different from, or in addition to, your interests as a shareholder. Swiss corporate law limits the ability of our shareholders to challenge resolutions made or other actions taken by our board of directors in court. Our shareholders generally are not permitted to file a suit to reverse a decision or an action taken by our board of directors but are instead only permitted to seek damages for breaches of fiduciary duty. However, if a board resolution has been invalidly formed, anyone, including the shareholders, may claim that such an invalid resolution shall be declared null and void. As a matter of Swiss law, shareholder claims against a member of our board of directors for breach of fiduciary duty would have to be brought in Stans, Switzerland, or where the relevant member of our board of directors is domiciled. In addition, under Swiss law, any claims by our shareholders against us must be brought exclusively in Stans, Switzerland. See “Description of Share Capital and Governing Documents” and “Comparison of Delaware Law and Swiss Law.”
Our common shares are issued under the laws of Switzerland, which may not protect investors in a similar fashion afforded by incorporation in a U.S. state.
We are organized under the laws of Switzerland. See “Description of Share Capital and Governing Documents” and “Comparison of Delaware Law and Swiss Law” for additional information. However, there can be no assurance that Swiss law will not change in the future or that it will serve to protect investors in a similar fashion afforded under corporate law principles in the United States, which could adversely affect the rights of investors.
Our status as a Swiss corporation may limit our flexibility with respect to certain aspects of capital management and may cause us to be unable to make distributions without subjecting our shareholders to Swiss withholding tax.
Swiss law allows our shareholders to authorize share capital that can be issued by the board of directors without additional shareholder approval. This authorization is limited to 50% of the existing registered share capital and the authorization must be renewed by the shareholders every two years; we expect to obtain authorization from our shareholders prior to this offering. Additionally, subject to specified exceptions, Swiss law grants pre-emptive subscription rights to existing shareholders to subscribe to any new issuance of shares. Swiss law also does not provide as much flexibility in the various terms that can attach to different classes of shares as the laws of some other jurisdictions. Swiss law also reserves for approval by shareholders certain corporate actions over which a board of directors would have authority in some other jurisdictions. For example, dividends must be approved by shareholders. These Swiss law requirements relating to our capital management may limit our flexibility, and situations may arise where greater flexibility would have provided substantial benefits to our shareholders.
Under Swiss law, a Swiss corporation may pay dividends only if the corporation has sufficient distributable profits from previous fiscal years, or if the corporation has distributable reserves, each as evidenced by its audited statutory balance sheet. Freely distributable reserves are generally booked either as “free reserves” or as “capital contributions” (apports de capital, contributions received from shareholders) in the “reserve from capital contributions.” Distributions may be made out of issued share capital—the aggregate nominal value of a company’s issued shares—only by way of a capital reduction. Upon completion of this offering, we expect the Company to have CHF 26.357 million (approximately $28.040 million) of qualifying capital contributions and CHF 211,927.26 of registered share capital (consisting of 10,596,363 common shares each with a nominal value of CHF 0.02 and no preferred shares)) on its audited statutory balance sheet (which audit will not be performed by our independent registered public accounting firm), as required pursuant to Swiss law. We will not be able to pay dividends or make other distributions to shareholders on a Swiss withholding tax-free basis in excess of that amount unless we increase our share capital or our reserves from capital contributions. We would also be able to pay dividends out of distributable profits or freely distributable reserves but such dividends would be subject to Swiss withholding taxes. There can be no assurance that we will have sufficient distributable profits, free reserves, reserves from capital contributions or registered share capital to pay a dividend or effect a capital reduction, that our shareholders will approve dividends or capital reductions proposed by us, or that we will be able to meet the other legal requirements for dividend payments or distributions as a result of capital reductions.
Generally, Swiss withholding tax of 35% is due on dividends and similar distributions to our shareholders, regardless of the place of residency of the shareholder, unless the distribution is made to shareholders out of (i) a reduction of nominal value or (ii) assuming certain conditions are met, qualifying capital contributions accumulated on or after January 1, 1997. A U.S. holder that qualifies for benefits under the Convention between the United States of America and the Swiss Confederation for the Avoidance of Double Taxation with Respect to Taxes on Income, which we refer to as the “U.S.-Swiss Treaty,” may apply for a refund of the tax withheld in excess of the 15% treaty rate (or in excess of the 5% reduced treaty rate for qualifying corporate shareholders with at least 10% participation in our voting shares, or for a full refund in the case of qualified pension funds).
There can be no assurance that we will have sufficient qualifying capital contributions to pay dividends free from Swiss withholding tax, or that Swiss withholding rules will not be changed in the future. In addition, we cannot provide assurance that the current Swiss law with respect to distributions out of qualifying capital contributions will not be changed or that a change in Swiss law will not adversely affect us or our shareholders, in particular as a result of distributions out of qualifying capital contributions becoming subject to additional corporate law or other restrictions. In addition, over the long term, the amount of par value available to us for nominal value reductions or qualifying capital contributions available to us to pay out as distributions is limited. If we are unable to make a distribution through a reduction in nominal value or out of qualifying capital contributions, we may not be able to make distributions without subjecting our shareholders to Swiss withholding taxes.
Pursuant to the COVID-19 Ordinance, we received a COVID-19 Loan. For as long as any amount is outstanding under the COVID-19 Loan, we are subject to certain restrictions regarding the use of funds.
On March 25, 2020, the Swiss Federal Council issued the COVID-19 Ordinance on joint and several guarantees. The COVID-19 Ordinance is part of a set of different measures enacted to minimize the economic impact of the COVID-19 pandemic. Pursuant to the COVID-19 Ordinance, companies domiciled in Switzerland that are economically affected by COVID-19 can apply for financial support in the form of emergency bank loans. The emergency loans are secured by the Swiss Confederation and the lending banks receive collateral in the form of joint and several guarantees from the Swiss Confederation. We received such a COVID-19 Loan from our bank for CHF 248,400 ($262,137), with 0% interest and a term of 60 months. We have drawn down the entire amount available to us under the COVID-19 Loan. Pursuant to the terms of the COVID-19 Ordinance, the following are prohibited for as long as any amount of the COVID-19 Loan is outstanding:
| | ● | making new investments in fixed assets which are not replacement investments, such as property or plant and equipment investments that serves to replace worn out or non-functional assets; |
| | ● | distributing dividends and bonuses and the reimbursement of capital contributions; |
| | ● | refinancing private loans and shareholder loans; |
| | ● | repayment of group loans; and |
| | ● | passing on of credit funds to foreign group companies (e.g. as part of a cash pooling system). |
If we were to undertake any of the foregoing actions while our COVID-19 Loan remains outstanding, we could be subject to a fine of up to CHF 100,000 ($105,530). Accordingly, until we repay the entire COVID-19 Loan amount, we do not expect to repay any of our outstanding credit facilities or convertible instruments. We currently expect, however, to repay the COVID-19 Loan with the proceeds raised from the Offering (see “Use of Proceeds” for additional information).
Our securities are not listed in Switzerland, our home jurisdiction. As a result, our shareholders will not benefit from certain provisions of Swiss law that are designed to protect shareholders in a public takeover offer or a change-of-control transaction and may not be protected in the same degree in a public takeover offer or a change-of-control transaction as are shareholders of certain U.S. companies or in a Swiss company listed in Switzerland.
Because our securities will be listed exclusively on Nasdaq and not in Switzerland, our shareholders will not benefit from the protection afforded by certain provisions of Swiss law that are designed to protect shareholders in the event of a public takeover offer or a change-of-control transaction. For example, Article 120 of the Swiss Financial Market Infrastructure Act and its implementing provisions require investors to disclose their interest in our company if they reach, exceed or fall below certain ownership thresholds. Similarly, the Swiss takeover regime imposes a duty on any person or group of persons who acquires more than one-third of a company’s voting rights to make a mandatory offer for all of the company’s outstanding listed equity securities. In addition, the Swiss takeover regime imposes certain restrictions and obligations on bidders in a voluntary public takeover offer that are designed to protect shareholders. However, these protections are applicable only to issuers that list their equity securities in Switzerland and, because our securities will be listed exclusively on Nasdaq, will not be applicable to us. Furthermore, since Swiss law restricts our ability to implement rights plans or U.S.-style “poison pills,” our ability to resist an unsolicited takeover attempt or to protect minority shareholders in the event of a change of control transaction may be limited. Therefore, our shareholders may not be protected in the same degree in a public takeover offer or a change-of-control transaction as are shareholders in certain U.S. companies or in a Swiss company listed in Switzerland.
U.S. shareholders may not be able to obtain judgments or enforce civil liabilities against us or our executive officers or members of our board of directors.
We are organized under the laws of Switzerland and our registered office and domicile is located in Stans, Switzerland. Moreover, a majority of our directors and executive officers are not residents of the United States, and all or a substantial portion of the assets of such persons are located outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon us or upon such persons or to enforce against them judgments obtained in U.S. courts, including judgments in actions predicated upon the civil liability provisions of the federal securities laws of the United States. We have been advised by our Swiss counsel that there is doubt as to the enforceability in Switzerland of original actions, or in actions for enforcement of judgments of U.S. courts, of civil liabilities to the extent solely predicated upon the federal and state securities laws of the United States. Original actions against persons in Switzerland based solely upon the U.S. federal or state securities laws are governed, among other things, by the principles set forth in the Swiss Federal Act on Private International Law. This statute provides that the application of provisions of non-Swiss law by the courts in Switzerland shall be precluded if the result is incompatible with Swiss public policy. Also, certain mandatory provisions of Swiss law may be applicable regardless of any other law that would otherwise apply.
Switzerland and the United States do not have a treaty providing for reciprocal recognition and enforcement of judgments in civil and commercial matters. The recognition and enforcement of a judgment of the courts of the United States in Switzerland is governed by the principles set forth in the Swiss Federal Act on Private International Law. This statute provides in principle that a judgment rendered by a non-Swiss court may be recognized in Switzerland only if:
| | ● | the non-Swiss court had jurisdiction pursuant to the Swiss Federal Act on Private International Law; |
| | ● | the judgment of such non-Swiss court has become final and non-appealable; |
| | ● | the judgment does not contravene Swiss public policy; |
| | ● | the court procedures and the service of documents leading to the judgment were in accordance with the due process of law; and |
| | ● | no proceeding involving the same position and the same subject matter was first brought in Switzerland, or adjudicated in Switzerland, or was earlier adjudicated in a third state and this decision is recognizable in Switzerland. |
Exchange rate fluctuations between the U.S. dollar and the Swiss Franc may negatively affect our results.
Under our existing agreements, we make a significant amount of payments in U.S. dollars and Swiss Francs. Since our reporting currency is the U.S. dollar, financial line items are converted into U.S. dollars at the applicable exchange rates. As a result, changes and fluctuations in currency exchange rates between the Swiss Franc the U.S. dollar could have a material adverse effect on our operating results.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere in this prospectus constitute forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” “intends” or “continue,” or the negative of these terms or other comparable terminology.
Forward-looking statements include, but are not limited to, statements about:
| | ● | the regulatory pathways that we may elect to utilize in seeking EMA, FDA and other regulatory approvals; |
| | ● | the use of Quilience in a compassionate use program, or CUP, and the results thereof; |
| | ● | obtaining EMA and FDA approval of, or other regulatory action in Europe or the United States and elsewhere with respect to, Quilience, Nolazol or other product candidates that we may seek to develop; |
| | ● | the commercial launch and future sales of Quilience, Nolazol or any other future product candidates; |
| | ● | the dosage of Quilience and Nolazol; |
| | ● | our expectations regarding the timing of commencing further clinical trials and the order of such trials with each of our product candidates or whether such trials will be conducted at all; |
| | ● | improved convenience relating to the prescription of and use of Nolazol for prescribers and patients (and their parents); |
| | ● | our expectations regarding the supply of mazindol; |
| | ● | third-party payor reimbursement for Quilience and Nolazol; |
| | ● | our estimates regarding anticipated expenses, capital requirements and our needs for additional financing; |
| | ● | changes to the narcolepsy patient market size and market adoption of Quilience by physicians and patients; |
| | ● | the timing, cost, regulatory approvals or other aspects of the commercial launch of Quilience and Nolazol; |
| | ● | submission of an MAA and NDA with the EMA and FDA for Quilience and Nolazol, respectively; |
| | ● | completion and receiving favorable results of clinical trials for Quilience and Nolazol; |
| | ● | our use of proceeds from this offering; |
| | ● | issuance of patents to us by the U.S. PTO and other governmental patent agencies; |
| | ● | new issuances of orphan drug designations; |
| | ● | the development and approval of the use of mazindol for additional indications other than narcolepsy and ADHD; |
| | | |
| | ● | the development and commercialization, if any, of any other product candidates that we may seek to develop; |
| | | |
| | ● | the use of our lead product candidates for treatment of additional indications other than narcolepsy and ADHD; |
| | ● | the ability of our management team to lead the development of our product candidates; |
| | ● | our expectations regarding licensing, acquisitions and strategic operations; and |
| | | |
| | ● | our expectations regarding the impact of the COVID-19 pandemic, including on our planned clinical trials, operations and financial position. |
These statements are only current predictions and are subject to known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading “Risk Factors” and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus.
USE OF PROCEEDS
We estimate that the net proceeds from our issuance and sale of 3,636,363 Units in this offering will be approximately $17.5 million, based on an assumed offering price of $5.50 per Unit, the midpoint of the estimated price range set forth on the cover page of this prospectus, excluding any proceeds that may be received upon the exercise of the Warrants, and after deducting underwriting discounts and commissions and offering expenses payable by us. If the representative of the underwriters exercises the over-allotment option in full, we estimate that the net proceeds from this offering will be approximately $20.2 million, assuming an offering price of $5.50 per Unit, the midpoint of the estimated price range set forth on the cover page of this prospectus, excluding any proceeds that may be received upon the exercise of the Warrants, and after deducting underwriting discounts and commissions and offering expenses payable by us.
A $1.00 increase or decrease in the assumed public offering price of $5.50 per Unit would increase or decrease the net proceeds from this offering by approximately $3.3 million, assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same, excluding any proceeds that may be received upon the exercise of the Warrants, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of 100,000 in the number of Units offered by us would increase or decrease our proceeds by approximately $0.5 million, assuming the assumed public offering price remains the same, excluding any proceeds that may be received upon the exercise of the Warrants, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently expect to use the net proceeds from this offering for:
| | ● | approximately $14 million to further develop Quilience (which may also benefit, in part, our development, if any, of Nolazol), to conduct pre- and non-clinical trials for Quilience and potentially reach the interim review of our Phase 2 dose-finding clinical trial; |
| | | |
| | ● | approximately $0.834 million, of which $0.223 million was incurred subsequent to June 30, 2020, in the aggregate to pay the deferred compensation of our Chief Executive Officer (including interest) and other interim executive officers; |
| | | |
| | ● | approximately $0.262 million (CHF 248,400) to repay the COVID-19 Loan (as defined in this prospectus); |
| | | |
| | ● | approximately $1.566 million, including $0.657 million of convertible debt, in the aggregate to repay each of the following types of indebtedness, each of which is scheduled to mature on December 31, 2020: CHF 500,000 borrowed under the 2020 Bridge Loan ($527,650, which carries an interest rate of 10% per year; approximately $5,071 accrued interest as of September 30, 2020), the Interest-Bearing Facility ($85,737, with no interest), NLS Pharma Credit Facility ($150,000, with no interest), the Notes (approximately $527,650, each of which carries an interest rate of 10% per year; approximately $96,980 accrued interest as of September 30, 2020), the Convertible Note (approximately $24,293, which carries an interest rate of 10% per year; approximately $38,628 accrued interest as of September 30, 2020) and the Convertible Loan with a related party ($105,530, which carries an interest rate of 10% per year; approximately $6,485 accrued interest as of September 30, 2020). The $0.528 million Bridge Loan was entered into subsequent to June 30, 2020 and $0.025 million of interest was incurred subsequent to June 30, 2020. (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Financing Activities” and “Related Party Transactions – Shareholders and Officer and Director Loans” for additional information on each form of indebtedness); |
| | ● | payment to our Interim Chief Business Officer pursuant to the terms of his consulting agreement with us (to receive 1% of the net proceeds actually received by us in an initial public offering) (approximately $176,722 if we receive the Estimated Net Proceeds) (also referred to herein as the Transaction Fee); and |
| | ● | the remainder, if any, for working capital and general corporate purposes. |
We incurred indebtedness under the 2020 Bridge Loan, the Convertible Loan with our Chief Executive Officer and COVID-19 Loan within the last year. We used the proceeds from these borrowings in order to maintain operational activity and for general and administrative expenses and expenses relating to this offering.
We intend to pay the Transaction Fee within 10 days of receipt of the actual net proceeds. In addition, we intend to account for the Transaction Fee as a reduction to additional paid-in capital as this expense is part of our financing activities.
We believe that the expected net proceeds from this offering, together with our existing cash and cash equivalents, will be sufficient to enable us to fund our operating expenses through at least the next 12 months from the date of this offering and potentially reach the interim review of our Phase 2 dose-finding clinical trial. It is difficult to predict the cost and timing required to complete our clinical trials due to, among other factors, the final number of patients and the rate of patient enrollment in our clinical trials, filing requirements with regulatory agencies, clinical trial results, and the actual costs of manufacturing and supplying our product candidates. The expected net proceeds from this offering, together with our existing cash and cash equivalents, will not be sufficient for us to fund any of our product candidates through regulatory approval, and, specifically, we will need to raise additional capital to complete clinical trials and development of Quilience even before we can complete our Phase 2 clinical trial.
We expect to finance our cash needs primarily through equity offerings and potentially through debt financings, collaborations, license and development agreements. We have based these estimates on assumptions that may prove to be incorrect, and we could expend our available capital resources at a rate greater than we currently expect.
The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our development and commercialization efforts, the status of and results from our clinical trials, whether or not we enter into strategic collaborations or partnerships and our operating costs and expenditures. Accordingly, our management will have significant flexibility in applying the net proceeds of this offering.
We have no current commitments or binding agreements with respect to any material acquisition of or investment in any technologies, products or companies.
Pending our use of the net proceeds from this offering, we may invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common shares and do not anticipate paying any cash dividends in the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our shareholders, upon proposal by our board of directors, and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant. In addition, pursuant to the COVID-19 Loan, we may not make any dividend payments until such loan is repaid in full.
Under Swiss law, any dividend must be proposed by our board of directors and approved by a shareholders’ meeting. In addition, our auditors must confirm that the dividend proposal of our board of directors conforms to Swiss statutory law and our articles of association. A Swiss corporation may pay dividends only if it has sufficient distributable profits brought forward from the previous business years (“Gewinnvortrag”) or if it has distributable reserves (“frei verfügbare Reserven”), each as evidenced by its audited standalone statutory balance sheet prepared pursuant to Swiss law and after allocations to reserves required by Swiss law and its articles of association have been deducted. Distributable reserves are generally booked either as “free reserves” (“freie Reserven”) or as “reserve from capital contributions” (“Reserven aus Kapitaleinlagen”). Distributions out of issued share capital, which is the aggregate nominal value of a corporation’s issued shares, may be made only by way of a share capital reduction. See “Description of Share Capital and Governing Documents” for additional information.
Payment of dividends may be subject to Swiss withholding taxes. See “Taxation—Switzerland Tax Considerations” for additional information.
CAPITALIZATION
The following table sets forth our cash and cash equivalents and our capitalization as of June 30, 2020:
| | ● | on a pro forma basis to give further effect to the issuance and sale of 3,636,363 Units in this offering at an assumed public offering price of $5.50 per Unit, the midpoint of the estimated price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, and to the application of the net proceeds as described in “Use of Proceeds,” as if the sale of the Units had occurred on June 30, 2020. |
The pro forma as adjusted information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
You should read this table in conjunction with the sections titled “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| | | As of June 30,
2020 | |
| U.S. dollars in thousands, except share data | | Actual | | | Pro Forma (1) | |
| | | | | | | |
| Cash and cash equivalents | | $ | 145 | | | | 15,328 | |
| | | | | | | | | |
| Convertible securities, net of debt discount of $98 | | | 956 | | | | 298 | |
| Total liabilities | | | 7,974 | | | | 6,085 | |
| | | | | | | | | |
| Shareholders’ deficit: | | | | | | | | |
| Common shares, par value of CHF 0.02 ($0.03), 6,960,000 shares registered: 6,960,000 shares issued, actual; 10,596,363 shares, pro forma | | | 145 | | | | 254 | |
| Additional paid in capital | | | 20,670 | | | | 37,694 | |
| Accumulated other comprehensive loss | | | (18 | ) | | | (18 | ) |
| Accumulated deficit | | | (28,130 | ) | | | (28,555 | ) |
| Total shareholders’ (deficit) equity | | | (7,334 | ) | | | 9,375 | |
| | | | | | | | | |
| Total capitalization | | $ | 640 | | | | 15,460 | |
| (1) | A $1.00 increase or decrease in the assumed public offering price of $5.50 per Unit would increase or decrease the amount of each of cash and cash equivalents and total shareholders’ deficit by approximately $3.3 million, assuming that the number of Units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of 100,000 in the number of Units offered by us would increase or decrease each of cash and cash equivalents and total shareholders’ deficit by approximately $0.5 million, assuming the assumed public offering price remains the same, after deducting estimated underwriting discounts and commissions and any estimated offering expenses payable by us. |
The number of our common shares to be outstanding immediately after this offering is 10,596,363 common shares based on 6,960,000 common shares outstanding as of November 16, 2020, and excludes (i) up to 4,417 common shares, if any, issuable upon conversion of the outstanding amount under the Convertible Note (at the assumed public offering price), (ii) up to 46,780 common shares, if any, issuable upon conversion of the Convertible Loans, as amended, (iii) up to 109,090 common shares issuable upon exercise in full of the Representative’s Warrants (as defined in this prospectus) and (iv) $50,000 of our common shares that we are contractually required to issue to a certain service provider (see “Underwriting – Other Relationships ” for additional information).
SELECTED FINANCIAL DATA
The following table summarizes our financial data. We have derived the following statements of operations and comprehensive loss for the years ended December 31, 2019 and 2018 and balance sheet data as of December 31, 2019 from our audited financial statements included elsewhere in this prospectus. We have derived the following statements of operations data for the six months ended June 30, 2020 and 2019 and balance sheet data as of June 30, 2019 from our unaudited interim financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. The following summary financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
Our financial statements included in this prospectus were prepared in accordance with U.S. GAAP.
| | | Six Months Ended
June 30,
(unaudited) | | | Year Ended
December 31, | |
| U.S. dollars in thousands, except share and per share data | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | | | | | | | | | | | |
| Research and development expenses | | $ | 161 | | | $ | 980 | | | $ | 1,702 | | | $ | 1,437 | |
| General and administrative expenses | | | 921 | | | | 1,246 | | | | 2,495 | | | | 2,012 | |
| Total operating expenses | | | 1,082 | | | | 2,226 | | | | 4,197 | | | | 3,449 | |
| Operating loss | | | (1,082 | ) | | | (2,226 | ) | | | (4,197 | ) | | | (3,449 | ) |
| Other (expense) income, net | | | (62 | ) | | | (31 | | | | (65 | | | | 16 | |
| Interest expense | | | (37 | ) | | | | | | | (1 | | | | - | |
| Interest on related party loans | | | (51 | ) | | | (793 | ) | | | (819 | ) | | | (1,085 | ) |
| Loss on conversion of debt | | | - | | | | (365 | | | | (365 | ) | | | (629 | ) |
| Net loss | | $ | (1,232 | ) | | $ | (3,415 | ) | | $ | (5,447 | ) | | $ | (5,147 | ) |
| Other comprehensive loss: | | | | | | | | | | | | | | | | |
| Defined pension plan adjustments | | | - | | | | - | | | | (8 | ) | | | (1 | ) |
| Comprehensive loss | | | (1,232 | ) | | | (3,415 | ) | | | (5,455 | ) | | | (5,148 | ) |
| Basic and diluted net loss per common share | | $ | (0.18 | ) | | $ | (0.51 | ) | | $ | (0.80 | ) | | $ | (0.82 | ) |
| Weighted average common shares used in computing basic and diluted net loss per common share | | | 6,960,000 | | | | 6,715,000 | | | | 6,840,000 | | | | 6,240,000 | |
| U.S. dollars in thousands | | As of
June 30,
2020 (unaudited) | | | As of
December 31,
2019 | |
| Balance Sheet Data: | | | | | | |
| Cash and cash equivalents | | $ | 145 | | | $ | 220 | |
| Total assets | | | 640 | | | | 696 | |
| Total non-current liabilities | | | 3,253 | | | | 2,832 | |
| Accumulated deficit | | | (28,130 | ) | | | (26,898 | ) |
| Total shareholders’ deficit | | | (7,334 | ) | | | (6,171 | ) |
DILUTION
If you invest in the Units, you will experience immediate and substantial dilution to the extent of the difference between the public offering price of our common shares and the pro forma as adjusted net tangible book value per share of our common shares immediately after the offering. At June 30, 2020, we had net tangible book value of negative $7,686,170, representing a net tangible book value of negative $1.10 per common share. Net tangible book value per common share represents the amount of our total tangible assets less our total liabilities, divided by the total number of common shares outstanding at June 30, 2020.
After giving effect to the sale of the Units offered by us in this offering and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value estimated at June 30, 2020 would have been approximately $9,375,043, representing $0.89 per common share. At the assumed public offering price for this offering of $5.50 per Unit, the midpoint of the estimated price range set forth on the cover page of this prospectus, this represents an immediate increase in historical net tangible book value of $1.99 per common share to existing shareholders and an immediate dilution in net tangible book value of $4.61 per common share to purchasers of common shares in this offering. Dilution for this purpose represents the difference between the price per common share paid by these purchasers and as adjusted net tangible book value per common share immediately after the completion of this offering.
The following table illustrates this dilution on a per share basis to new investors:
| Assumed public offering price per share, the midpoint of the estimated price range set forth on the cover page of this prospectus | | | | | | $ | 5.50 | |
| Historical net tangible book value per share as of June 30, 2020 | | $ | (1.10 | ) | | | | |
| Increase in net tangible book value per share attributable to new investors in this offering | | $ | 1.99 | | | | | |
| Pro forma as adjusted net tangible book value per share after offering | | | | | | $ | 0.89 | |
| Dilution in tangible book value per share to new investors | | | | | | $ | 4.61 | |
| Percentage of dilution in net tangible book value per share for new investors | | | | | | | 84 | % |
The dilution information set forth in the table above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
A $1.00 increase or decrease in the assumed initial public offering price of $5.50 per Unit would increase or decrease our as adjusted net tangible book value per common share after this offering by $0.31 and the dilution per common share to new investors by $0.69, assuming the number of common shares offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of common shares we are offering.
An increase or decrease of 100,000 in the number of Units offered by us would increase or decrease our as adjusted net tangible book value after this offering by approximately $0.5 million and the pro forma as adjusted net tangible book value per common share after this offering by $0.04 per common share and would increase or decrease the dilution per common share to new investors by $0.04, assuming the assumed public offering price remains the same, after deducting estimated underwriting discounts and estimated offering expenses payable by us.
The following table summarizes, on an as adjusted basis as of June 30, 2020, the differences between the number of common shares acquired from us as part of the Units, the total amount paid and the average price per common share paid by the existing holders of our common shares and by investors in this offering and based upon an assumed public offering price of $5.50 per Unit, the midpoint of the estimated price range set forth on the cover page of this prospectus.
| | | Shares | | | Total Consideration | | | Average
Price Per
Ordinary | |
| | | Number | | | Percent | | | Amount | | | Percent | | | Share | |
| Existing shareholders | | | 6,960,000 | | | | 65.7 | % | | $ | 16,177,113 | | | | 44.7 | % | | $ | 2.32 | |
| New investors | | | 3,636,363 | | | | 34.3 | % | | $ | 19,999,997 | | | | 55.3 | % | | $ | 5.50 | |
| Total | | | 10,596,363 | | | | 100.0 | % | | $ | 36,177,110 | | | | 100 | % | | $ | 3.41 | |
The number of our common shares to be outstanding immediately after this offering is 10,596,363 based on 6,960,000 common shares outstanding as of November 16, 2020 and excludes (i) up to 4,417 common shares, if any, issuable upon conversion of the outstanding amount under the Convertible Note (at the assumed public offering price), (ii) up to 46,780 common shares, if any, issuable upon conversion of the Convertible Loans, as amended, (iii) up to 109,090 common shares issuable upon exercise in full of the Representative’s Warrants (as defined in this prospectus) and (iv) $50,000 of our common shares that we are contractually required to issue to a certain service provider (see “Underwriting – Other Relationships ” for additional information).
If all of such issued and outstanding convertible securities had been exercised as of June 30, 2020, the number of common shares held by existing shareholders would increase to 7,011,197, or 65.8% of the total number of common shares outstanding after this offering, and the average price per common share paid by the existing shareholders would be $2.38.
To the extent that options are granted under our equity benefit plans, as may be implemented after this offering, there will be further dilution to investors purchasing common shares in this offering. In addition, any exercises of the Warrants could also further dilute your ownership in our Company.
If the representative of the underwriters exercises its over-allotment option to purchase additional common shares in full in this offering, the number of common shares held by new investors will increase to 4,181,817, or 37.5% of the total number of common shares outstanding after this offering and the percentage of common shares held by existing shareholders will decrease to 62.5% of the total common shares outstanding.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our financial statements and related notes included elsewhere in this prospectus. This discussion and other parts of the prospectus contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are an innovative biotechnology company engaged in the discovery and development of life-improving drug therapies to treat rare and complex CNS disorders, including narcolepsy, IH and other rare sleep disorders, as well as neurodevelopmental disorders, such as ADHD. Our lead compound mazindol, in a CR formulation, is a pan-monoaminergic reuptake inhibitor and partial orexin receptor 2 agonist, for the treatment of narcolepsy with cataplexy. We also believe that we may be able to use Mazindol CR as a compound in product candidates to treat other CNS disorders. CNS disorders are a diverse group of conditions that include neurological, psychiatric, and substance use disorders.
Components of Operating Results
Licensing Agreement
In February 2019, we entered into an exclusive license agreement, or the EF License Agreement, to develop and commercialize our product candidate, Nolazol, in Latin American countries with Eurofarma Laboratorios S.A., or Eurofarma, a Brazilian pharmaceutical company. The EF License Agreement covers the grant of non-transferable licenses, without the right to sublicense, to Eurofarma to develop and commercialize Nolazol in Latin America. The EF License Agreement also specifies our obligation to advance development activities with respect to Nolazol in the United States. A joint steering committee will oversee the development and regulatory activities directed towards marketing approval, manufacturing and commercialization phases. We believe that our participation in the joint steering committee is not of material significance to the licenses in the context of the EF License Agreement on the whole and, as such, management has excluded these activities in the determination of its performance obligation(s) under the EF License Agreement. The EF License Agreement also provides that the parties shall enter into a separate manufacturing and supply agreement during the term of the EF License Agreement.
Under the EF License Agreement, we received a non-refundable, upfront payment, of $2,500,000 and are further eligible to receive non-refundable milestone payments of up to $16,000,000, based on the achievement of milestones related to regulatory filings, regulatory approvals and the commercialization of Nolazol. The achievement and timing of the milestones depend on the success of development, approval and sales progress, if any, of Nolazol in the future. In addition, we are also eligible for tiered royalty payments.
Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as a current portion of deferred revenue in the balance sheets in our financial statements included elsewhere in this prospectus. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion. As of June 30, 2020, we have long-term deferred revenues of $2,500,000, which will be recognized when the development services of Nolazol are completed and the product candidate receives applicable regulatory approval in Latin America that allows Eurofarma to commence commercialization of Nolazol in accordance with the EF License Agreement.
Operating Expenses
Our current operating expenses consist of three components – research and development expenses, general and administrative expenses and depreciation and amortization.
Research and Development Expenses
Our research and development expenses consist primarily of salaries and related personnel expenses, costs of third-party clinical consultants and expenses related to conducting clinical and pre-clinical trials, travel expenses and other research and development expenses.
The following table discloses the breakdown of research and development expenses:
| | | For the Six Months Ended
June 30, | | | For the Year Ended
December 31, | |
| | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | | | | | | | | | | | |
| Development materials and expenses | | $ | 41,421 | | | $ | 168,598 | | | $ | 567,123 | | | $ | 324,097 | |
| Subcontractors | | | - | | | | 152,266 | | | | 275,890 | | | | 301,389 | |
| Legal expenses | | | 119,330 | | | | 618,467 | | | | 804,329 | | | | 728,747 | |
| Other | | | - | | | | 40,543 | | | | 54,775 | | | | 82,969 | |
| Total | | $ | 160,751 | | | $ | 979,874 | | | $ | 1,702,117 | | | $ | 1,437,202 | |
We expect that our research and development expenses will materially increase as we enter into the Phase 2/3 clinical development stage of our product candidates and recruit additional research and development employees.
Comparison of the Six Months Ended June 30, 2020 to the Six Months Ended June 30, 2019
Results of Operations
| | | For the Six Months Ended
June 30, | |
| | | 2020 | | | 2019 | |
| | | | | | | |
| Research and development expenses | | $ | 160,751 | | | $ | 979,874 | |
| General and administrative expenses | | | 920,943 | | | | 1,246,558 | |
| Operating loss | | | (1,081,694 | ) | | | (2,226,432 | ) |
| Other expense, net | | | (62,193 | ) | | | (31,386 | ) |
| Interest expense | | | (37,094 | ) | | | | |
| Interest on related party loans | | | (51,235 | ) | | | (792,486 | ) |
| Loss on conversion of debt | | | - | | | | (364,950 | |
| Net loss | | $ | (1,232,216 | ) | | $ | (3,415,254 | ) |
Research and Development Expenses
Our research and development expenses totaled $160,751 for the six months ended June 30, 2020, representing a decrease of $819,123, or 84%, compared to $979,874 for the six months ended June 30, 2019. The decrease was primarily attributable the Company postponing all non-essential business until after the completion of this offering.
General and Administrative Expenses
Our general and administrative expenses totaled $920,941 for the six months ended June 30, 2020, representing a decrease of $325,615, or 26%, compared to $1,246,558 for the six months ended June 30, 2019. The decrease was primarily attributable to the Company postponing all non-essential business until after the completion of this offering. The prior year period included additional costs in consulting and legal costs relating to (i) the Reorganization, (ii) conversion of the credit facilities, shareholder loans and the Convertible Note to equity, as described below, and (ii) increased marketing and communication expenses.
Operating Loss
As a result of the foregoing, our operating loss totaled $1,081,694 for the six months ended June 30, 2020, representing a decrease of $1,144,738, or 51%, compared to $2,226,432 for the six months ended June 30, 2019.
Other Expense
Other expense consists of exchange rate differences and financial expenses related to our credit card fees. We recognized other expense of $62,193 for the six months ended June 30, 2019, representing an increase of $30,807, or 98%, compared to $31,386 for the six months ended June 30, 2019. The increase was primarily attributable to exchange rate differences.
Interest Expense
Interest expense consists of interest and imputed interest expenses on the Convertible Loans. Interest expense was $37,094, including $21,261 of imputed interest, for the six months ended June 30, 2020. There was no such interest expense for the six months ended June 30, 2019. The increase was due to seven Convertible Loans entered into since the six months ended June 30, 2019.
Interest on Related Party Loans
Interest on related party loans consists of interest and imputed interest expenses on related party promissory notes, shareholder loans and credit facilities. Interest on related party loans was $51,235, including $15,403 of imputed interest, for the six months ended June 30, 2020, representing a decrease of $741,251, or 94%, compared to $792,486, including $752,152 of imputed interest, for the six months ended June 30, 2019. The decrease was mainly due to the amortization of imputed interest in full for the debts that were converted to equity during the six months ended June 30, 2019.
Loss on Conversion of Debt
The loss on conversion of debt of $364,950 for the six months ended June 30, 2019 was a result of the conversion of credit facilities, a shareholder bridge loan and four promissory notes with an aggregate principal amount of $7,658,250 in exchange for 580,000 common shares with a fair value of CHF 14 per share and an aggregate fair value of $8,023,200.
Net Loss
As a result of the foregoing, our net loss totaled $1,232,216 for the six months ended June 30, 2020, representing a decrease of $2,183,038, or 64%, compared to $3,415,254 for the six months ended June 30, 2019.
Comparison of the Year Ended December 31, 2019 to the Year Ended December 31, 2018
Results of Operations
| | | For the Year Ended
December 31, | |
| | | 2019 | | | 2018 | |
| Research and development expenses | | $ | 1,702,117 | | | $ | 1,437,202 | |
| General and administrative expenses | | | 2,494,922 | | | | 2,011,659 | |
| Operating loss | | | (4,197,039 | ) | | | (3,448,861 | ) |
| Interest and other financing income (expense), net | | | (66,437 | ) | | | 16,035 | |
| Interest on related party loans | | | (818,719 | ) | | | (1,084,708 | ) |
| Loss on conversion of debt | | | (364,950 | ) | | | (629,232 | ) |
| Net loss | | $ | (5,447,145 | ) | | $ | (5,146,766 | ) |
Research and Development Expenses
Our research and development expenses totaled $1,702,117 for the year ended December 31, 2019, representing an increase of $264,915, or 18%, compared to $1,437,202 for the year ended December 31, 2018. The increase was primarily attributable to an increase in development expenses and legal expenses related to patent work.
General and Administrative Expenses
Our general and administrative expenses totaled $2,494,922 for the year ended December 31, 2019, representing an increase of $483,263, or 24%, compared to $2,011,659 for the year ended December 31, 2018. The increase was primarily attributable to an increase in consulting and legal costs relating to (i) the Reorganization, (ii) conversion of the credit facilities, shareholder loans and the Convertible Note to equity, as described below, and (iii) increased marketing and communication expenses.
Operating Loss
As a result of the foregoing, our operating loss totaled $4,197,039 for the year ended December 31, 2019, representing an increase of $748,178, or 21%, compared to $3,448,861 for the year ended December 31, 2018.
Interest and Other Financing Income
Interest and other financing income consists of exchange rate differences and financial expenses related to our credit card fees. We recognized interest and other financing expenses of $66,437 for the year ended December 31, 2019, representing an increase of $82,472, or 514%, compared to income of $16,035 for the year ended December 31, 2018. The increase was primarily attributable to exchange rate differences.
Interest on Related Party Loans
Interest on related party loans was $818,719, including $754,734 of imputed interest, for the year ended December 31, 2019, representing a decrease of $265,989, or 25%, compared to $1,084,708, including $1,090,412 of imputed interest, for the year ended December 31, 2018. The decrease was mainly due to the amortization of imputed interest using the effective interest rate method on debt that was converted in the year ended December 31, 2018. The decrease was offset, in part, by an increase of promissory notes, namely, the Notes, during the year ended December 31, 2018 and the Convertible Loans entered into during the year ended December 31, 2019.
Loss on Conversion of Debt
The loss on conversion of debt of $364,950 for the year ended December 31, 2019 was a result of the conversion of credit facilities, a shareholder bridge loan and four promissory notes with an aggregate principal amount of $7,658,250 in exchange for 580,000 common shares with a fair value of CHF 14 per share and an aggregate fair value of $8,023,200. The loss on conversion of debt of $629,232 for the year ended December 31, 2018 was a result of the conversion of credit facilities with an aggregate principal amount of $3,418,520 in exchange for 260,000 common shares with a fair value of CHF 16 per share and an aggregate fair value of $4,047,752.
Net Loss
As a result of the foregoing, our loss totaled $5,447,145 for the year ended December 31, 2019, representing an increase of $300,379, or 6%, compared to $5,146,766 for the year ended December 31, 2018.
Critical Accounting Policies
We describe our significant accounting policies more fully in Note 2 to our financial statements included elsewhere in this prospectus. We believe that the accounting policies described below and in Note 2 are critical in order to fully understand and evaluate our financial condition and results of operations.
We prepare our financial statements in accordance with U.S. GAAP. At the time of the preparation of the financial statements, our management is required to use estimates, evaluations, and assumptions which affect the application of the accounting policy and the amounts reported for assets, obligations, income, and expenses. Any estimates and assumptions are continually reviewed. The changes to the accounting estimates are credited during the period in which the change to the estimate is made.
Revenue Recognition
As of June 30, 2020, we have not recognized any revenue from the EF License Agreement as the upfront payment we received has been deferred. The EF License Agreement provides for the development and commercialization of our product candidate, Nolazol, in Latin American countries with Eurofarma. The EF License Agreement is within the scope of Accounting Standards Codification, or ASC, 606, “Revenue from Contract with Customers,” or ASC 606.
Under ASC 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration which the entity expects to receive in exchange for those goods or services. To determine the appropriate amount of revenue to be recognized for arrangements determined to be within the scope of ASC 606, we perform the following five steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) we satisfy each performance obligation. We only apply the five-step model to contracts when it is probable that the entity will collect consideration it is entitled to in exchange for the goods or services it transfers to the customer.
Performance obligations are promised goods or services in a contract to transfer a distinct good or service to the customer and are considered distinct when (i) the customer can benefit from the good or service on its own or together with other readily available resources and (ii) the promised good or service is separately identifiable from other promises in the contract. In assessing whether promised goods or services are distinct, we consider factors such as the stage of development of the underlying intellectual property, the capabilities of the customer to develop the intellectual property on its own or whether the required expertise is readily available and whether the goods or services are integral or dependent to other goods or services in the contract.
We estimate the transaction price based on the amount expected to be received for transferring the promised goods or services in the contract. The consideration may include fixed consideration or variable consideration. At the inception of each arrangement that includes variable consideration, we evaluate the amount of potential payments and the likelihood that the payments will be received. We utilize either the most likely amount method or expected amount method to estimate the amount expected to be received based on which method best predicts the amount expected to be received. The amount of variable consideration which is included in the transaction price may be constrained and is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period.
We allocate the transaction price based on the estimated stand-alone selling price of each of the performance obligations. We must develop assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in a contract with a customer. We utilize key assumptions to determine the stand-alone selling price for service obligations, which may include other comparable transactions, pricing considered in negotiating the transaction and the estimated costs. Additionally, in determining the standalone selling price for material rights, we may reference comparable transactions, clinical trial success probabilities, and develop estimates of option exercise likelihood. Any variable consideration is allocated specifically to one or more performance obligations in a contract when the terms of the variable consideration relate to the satisfaction of the performance obligation and the resulting amounts allocated are consistent with the amounts we would expect to receive for the satisfaction of each performance obligation.
The consideration allocated to each performance obligation is recognized as revenue when control is transferred for the related goods or services. For performance obligations which consist of licenses and other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress. We evaluate the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Development and regulatory milestone payments are assessed under the most likely amount method and constrained if it is probable that a significant revenue reversal would occur. Milestone payments that are not within our control or the licensee’s control, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. At the end of each reporting period, we re-evaluate the probability of achievement of such development milestones and any related constraint, and if necessary, adjust our estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect license revenues in the period of adjustment. To date, we have not recognized any consideration related to the achievement of development, regulatory, or commercial milestone revenue resulting from the EF License Agreement.
For revenue related to sales-based royalties received from licensees, including milestone payments based on the level of sales, where the license is deemed to be the predominant item to which the royalties relate, we recognize revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, we have not recognized any consideration related to sales-based royalty revenue resulting from any of our license agreement.
To the extent we receive payments, including non-refundable payments, in excess of the recognized revenue such excess is recorded as deferred revenue until we perform our obligations under these arrangements. Amounts are recorded as accounts receivable when our right to consideration is unconditional.
Pension Obligations
We have a single insurance collective pension plan that is fully insured and operated by an insurance company which covers the employees. Both we and the participants provide monthly contributions to the pension plan that are based on the covered salary. A portion of the pension contribution is credited to employees’ savings accounts which earns interest at the rate provided in the plan. The pension plan provides for retirement benefits as well as benefits on long-term disability and death. The pension plan qualifies as a defined benefit plan in accordance with U.S. GAAP. As such, the cost of the defined pension arrangement is determined based on actuarial valuations. An actuarial valuation assumes the estimation of discount rates, estimated returns on assets, future salary increases, mortality figures and future pension increases. Because of the long-term nature of these pension plans, the valuation of these is subject to uncertainties.
Income Taxation
We incur tax loss carryforwards generating deferred tax assets against which a valuation allowance is recorded when it is not more likely than not that the tax benefit can be realized. Significant judgment is required in determining the use of tax loss carryforwards. Management’s current judgment is that it is not more likely than not that the tax benefits can be realized and a full valuation allowance is therefore recognized.
Liquidity and Capital Resources
Overview
Since our inception through June 30, 2020, we have funded our operations principally with $20.6 million from the sales of our common shares, convertible instruments, related party credit facilities and shareholder loans, COVID19 bank loan and the EF License Agreement. As of June 30, 2020, we had $145,404 in cash and cash equivalents.
The table below presents our cash flows for the periods indicated:
| | | For the Six Months Ended
June 30, | | | For the Year Ended
December 31, | |
| | | 2020 | | | 2019 | | | 2019 | | | 2018 | |
| | | | | | | | | | | | | |
| Cash provided by (used in) operating activities | | $ | (532,924 | ) | | $ | 712,956 | | | $ | (190,009 | ) | | $ | (2,732,804 | ) |
| Cash provided by financing activities | | $ | 439,563 | | | $ | 512,800 | | | $ | 407,479 | | | $ | 557,920 | |
| Effect of exchange rate changes on cash and cash equivalents | | | 18,498 | | | | (7,543 | ) | | | (3,881 | ) | | | (1,369 | ) |
| | | | | | | | | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | $ | (74,863 | ) | | $ | 1,218,213 | | | $ | 213,589 | | | $ | (2,176,253 | ) |
Operating Activities
Net cash used in operating activities of $532,924 during the six months ended June 30, 2020, was primarily related to the payment of $0.2 million mainly for clinical and regulatory legal expenses and an aggregate of $0.4 million in salary and consultant payments. Net cash provided by operating activities of $712,956 during the six months ended June 30, 2019, was primarily due to the receipt of a $2,500,000 upfront payment related to the EF Licensing Agreement, offset in part by the payment of $200,000 for clinical trials and other third-party expenses and an aggregate of $700,000 in salary and consultant payments. The remaining amount was for legal, administrative and other miscellaneous expenses.
Net cash used in operating activities of $190,009 during the year ended December 31, 2019, was primarily related to $1.7 million for clinical trials and other third-party expenses, including consulting and legal expenses, and an aggregate of $1.0 million in salary and consultant expenses. These expenses were offset by the receipt of the $2.5 million upfront payment related to the EF License Agreement. Net cash used in operating activities of $2,732,804 during the year ended December 31, 2018, was primarily related to $0.4 million for clinical trials and other third-party expenses and an aggregate of $1.4 million in salary and consultant expenses. The remaining amount was for legal, administrative and other miscellaneous expenses.
Financing Activities
Net cash provided by financing activities in the six months ended June 30, 2020, consisted of $279,745 of net proceeds from issuance of Convertible Loans, including one with a related party for $105,530, and $262,137 of net proceeds from the COVID-19 Loan, offset in part, by $102,319 of deferred offering costs relating to this offering. Net cash provided by financing activities in the six months ended June 30, 2019 consisted of $512,800 of net proceeds from the issuance of the Notes.
Net cash provided by financing activities in the year ended December 31, 2019, consisted of $512,800 of net proceeds from the issuance of the Notes and $154,890 of net proceeds of Convertible Loans, offset, in part, by $260,211 of deferred offering costs relating to this offering. Net cash provided by financing activities in the year ended December 31, 2018, consisted of $557,920 of net proceeds from issuance of Convertible Note.
In August 2015, NLS Pharma Ltd. and NLS-1 Ltd., and in March 2017, NLS-0 Ltd., each entered into credit facilities providing the Company with credit lines of $150,000 (non-interest bearing), $7.1 million ($500,000 of which bears interest at an annual rate of 10%, or the Interest-Bearing Facility) and approximately $3.44 million (non-interest bearing), respectively, or the NLS Pharma Credit Facility, the NLS-1 Credit Facility and the NLS-0 Credit Facility, respectively.
On March 2, 2017, we issued an aggregate of 210,000 common shares to three investors in a private offering, at a price per share of approximately CHF 16 (approximately $16) per common share for aggregate proceeds of CHF 3,279,300 (approximately $3,276,676). In connection with this private offering, we also issued, in the aggregate, 160,000 of our common shares to certain existing shareholders, Messrs. Hafner, Bauer, Stein and Ödman, or the Shareholders, in light of anti-dilution rights held by such shareholders and one share to a consultant as a finder’s fee.
In March 2017, we entered into a certain interest-free $2 million bridge loan, or the Bridge Loan, with the Shareholders. On March 12, 2019, simultaneously with the closing of the Reorganization, we issued an aggregate of 260,000 of our common shares to the Shareholders, in exchange for the consideration of the conversion of the entire Bridge Loan and the entire borrowed amount of $1.45 million under the NLS-0 Credit Facility, at a conversion price of $13 per common share.
On July 17, 2018, we issued an aggregate of 260,000 common shares to the Shareholders, in connection with the conversion of an aggregate amount of $3,418,519 borrowed by us pursuant to a $7.1 million credit facility, or the Credit Facility, at a conversion price of $13 per common share.
In January 2019, we issued four promissory notes, or the Notes, each for CHF 125,000 (approximately $128,200), with the Shareholders. Each Note carries an interest rate of 10% per year, compounded annually and originally matured as of the earlier of (i) April 30, 2019 and (ii) five days following such time as we have received aggregate financing, in a sole or series of transactions, exceeding CHF 500,000 (approximately $527,650) in the form of straight equity or convertible instrument investments or other means of proceeds from third parties. Pursuant to amendments to each of the Notes, the maturity dates have been extended to December 31, 2020.
On March 12, 2019, we conducted the Reorganization. In connection therewith, in addition to those 260,000 common shares issued in connection with the Bridge Loan and the NLS-0 Credit Facility, we issued:
| | ● | an aggregate of 280,000 of our common shares to the Shareholders, in connection with the conversion of the remaining $3,681,481 borrowed by us pursuant to the Credit Facility, at a conversion price of $13 per common share; |
| | ● | an aggregate of 40,000 of our common shares to Magnetic Rock Investment AG, or Magnetic Rock, a company that is controlled by the Shareholders, in connection with the conversion of the CHF 526,979.84 (approximately $526,769) Convertible Note at a conversion price of CHF 13 (approximately $13) per common share; and |
| | ● | an aggregate of 745,000 of our common shares to those holders of NLS-0 and NLS Pharma common shares. |
On September 16, 2019, we and the Shareholders amended the NLS Pharma Credit Facility to extend the maturity date to December 31, 2019. If we default on the NLS Pharma Credit Facility, we will be subject to a default interest rate of 5% per year. The Interest-Bearing Facility stopped accruing interest upon the Reorganization and the accrued interest stands at $85,737. The NLS-1 Credit Facility has been amended to extend the maturity date covering the Interest-Bearing Facility to December 31, 2020.
On December 23, 2019 and in February, March and June 2020, we entered into the Convertible Loans in the aggregate amount of CHF 475,822 (approximately $501,358). Each Convertible Loan carries an interest rate of 10% per year, compounded annually and mature between June 30, 2020 and January 31, 2022, unless converted into common shares or repaid by us prior to then. If we default on the Convertible Loans, we will be subject to a default interest rate of 15% per year. During September 2020, we amended two Convertible Loans entered into during December 2019, in the aggregate amount of CHF 210,000 ($221,613), such that our optional early repayment date of the Convertible Loans has been delayed by six months, or the Convertible Loan Amendment.
On March 26, 2020, we applied for and thereafter received a COVID-19 Loan from our bank for CHF 248,400 ($262,137), with 0% interest and a term of 60 months. We have drawn down the entire amount available to us under the COVID-19 Loan.
In August 2020, we received the first tranche of CHF 300,000 ($316,590) pursuant to the 2020 Bridge Loan and received the second tranche of CHF 200,000 (211,060) in September 2020. The borrowed amounts under this 2020 Bridge Loan carry an interest rate of 10% per year, compounded annually, and all borrowed sums, including interest, are scheduled to mature on December 31, 2020; provided, however, that we may repay such loan, including interest, prior to the scheduled maturity date. If we default on the 2020 Bridge Loan, we will be subject to a default interest rate of 15% per year.
As of November 16, 2020, the following loans and notes remain outstanding:
| | ● | CHF 500,000 ($527,650) under the 2020 Bridge Loan; |
| | | |
| | ● | CHF 248,400 ($262,137) under the COVID-19 Loan; |
| | | |
| | ● | $85,737 under the Interest-Bearing Facility; |
| | | |
| | ● | $150,000 under the NLS Pharma Credit Facility; |
| | | |
| | ● | CHF 500,000 ($527,650) under the Notes, each of which carries an interest rate of 10% per year; |
| | | |
| | ● | CHF 23,020 ($24,293) under the Convertible Note, which carries an interest rate of 10% per year; and |
| | | |
| | ● | CHF 475,086 (approximately $501,358) under the Convertible Loans, which each carry an interest rate of 10% per year. |
Current Outlook
We have financed our operations to date primarily through proceeds from sales of our common shares, convertible instruments, related party credit facilities and shareholder loans and the EF License Agreement. In April 2020, we also obtained additional capital through the COVID-19 Loan and in August and September 2020 we received the entire amount available to us under the 2020 Bridge Loan. We have incurred losses and generated negative cash flows from operations since inception in 2015. To date we have not generated revenue, and we do not expect to generate significant revenues from the sale of our product candidates in the near future.
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. At this time, there is no guarantee that we will be able to obtain an adequate level of financial resources required for the short and long-term support of our operations or that we will be able to obtain additional financing as needed, or meet the conditions of such financing, or that the costs of such financing may not be prohibitive. These conditions raise substantial doubt about our ability to continue as a going concern for a period within one year from the date of the financial statements included elsewhere in this prospectus.
As of June 30, 2020, our cash and cash equivalents were $145,404. We believe that our existing cash and cash equivalents, including the 2020 Bridge Loan, will only be sufficient to fund our projected cash requirements through December 2020 Therefore, we will require significant additional financing in the near future to fund our operations. As we continue to assess the effects of the COVID-19 pandemic, we do believe that it is possible that the COVID-19 pandemic may make financing opportunities scarcer or more difficult or, if such funds are available to us, that such additional financing may not be available in an amount that is sufficient to meet our needs. Our failure to raise capital or enter into such other arrangements when needed would have a negative impact on our financial condition and could force us to delay, reduce or terminate our planned or ongoing clinical trials or other operations, or grant rights to develop and commercialize product candidates that we would otherwise prefer to develop and commercialize ourselves.
We currently anticipate that we will require approximately $14 million for research and development activities over the course of the next 12 months. We also anticipate that we will require between $3 to $4 million for capital expenditures over such 12-month period, which consists primarily of expenditures for the manufacture of our product candidates for use in clinical trials and supporting pre-clinical studies required for obtaining approval to conduct such clinical studies and general and administration labor costs. In light of our financial condition, we reduced our operating expenses, including for those purposes listed above, before the start of the COVID-19 pandemic. As part of the cut to our operating expenses, our Chief Executive Officer and other interim executive officers have agreed to defer the receipt of payment of a portion or all of their respective compensation until we raise sufficient capital, including through the sale of Units in this offering. There can be no assurance that the analysis that we have undertaken or remedial measures that have been enacted will enable us to avoid part or all of any impact from the spread of COVID-19 or its consequences.
In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future capital requirements will depend on many factors, including:
| | ● | the length of the COVID-19 pandemic and its impact on our planned clinical trials, operations and financial condition; |
| | | |
| | ● | the progress and costs of our pre-clinical studies, clinical trials and other research and development activities; |
| | ● | the scope, prioritization and number of our clinical trials and other research and development programs; |
| | ● | any cost that we may incur under in- and out-licensing arrangements relating to our product candidate that we may enter into in the future; |
| | ● | the costs and timing of obtaining regulatory approval for our product candidates; |
| | ● | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; |
| | ● | the costs of, and timing for, strengthening our manufacturing agreements for production of sufficient clinical and commercial quantities of our product candidates; |
| | ● | the potential costs of contracting with third parties to provide marketing and distribution services for us or for building such capacities internally; and |
| | ● | the costs of acquiring or undertaking the development and commercialization efforts for additional, future therapeutic applications of our product candidates and the magnitude of our general and administrative expenses. |
Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through our existing cash, cash equivalents and short-term deposits, the net proceeds from this current offering, loans, debt or equity financings, or by out-licensing applications of our product candidates. As long as the COVID-19 Loan remains outstanding, we will also be restricted in our ability to repay any existing debt. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to, one or more applications of our product candidates. This may raise substantial doubts about our ability to continue as a going concern.
Off-Balance Sheet Arrangements
Except for standard operating leases, we have not engaged in any off-balance sheet arrangements, such as the use of unconsolidated subsidiaries, structured finance, special purpose entities or variable interest entities.
We do not believe that our off-balance sheet arrangements and commitments have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Quantitative and Qualitative Disclosure About Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our current investment policy is to invest available cash in bank deposits with banks that have a credit rating of at least A-. Accordingly, a substantial majority of our cash and cash equivalents is held in deposits that bear interest. Given the current low rates of interest we receive, we will not be adversely affected if such rates are reduced. Our market risk exposure is primarily a result of foreign currency exchange rates, which is discussed in detail in the following paragraph.
Foreign Currency Exchange Risk
Our results of operations and cash flow are subject to fluctuations due to changes in foreign currency exchange rates. As discussed above, the vast majority of our liquid assets is held in USD, and a certain portion of our expenses are denominated in CHF. For instance, in 2019, approximately 40% of our expenses were denominated in CHF. Changes of 5% and 10% in the USD/CHF exchange rate would have increased/decreased our operating expenses by 2% and 4%, respectively. However, these historical figures may not be indicative of future exposure, as we expect that the percentage of our CHF denominated expenses will materially decrease in the near future, therefore reducing our exposure to exchange rate fluctuations.
We do not hedge our foreign currency exchange risk. In the future, we may enter into formal currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
JOBS Act Accounting Election
We are an “emerging growth company.” Under the JOBS Act, an emerging growth company can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have irrevocably elected to avail ourselves of this exemption from new or revised accounting standards, and, therefore, will not be subject to the same new or revised accounting standards as public companies that are not emerging growth companies.
BUSINESS
Overview
We are an innovative biotechnology company engaged in the discovery and development of life-improving drug therapies to treat rare and complex CNS disorders, including narcolepsy, IH and other rare sleep disorders, as well as neurodevelopmental disorders, such as ADHD. Our lead compound mazindol, in a CR formulation, is a pan-monoaminergic reuptake inhibitor and partial orexin receptor 2 agonist, for the treatment of narcolepsy with cataplexy. We also believe that we may be able to use Mazindol CR as a compound in product candidates to treat other CNS disorders. CNS disorders are a diverse group of conditions that include neurological, psychiatric, and substance use disorders. According to the World Health Organization and based on data from the Global Burden of Disease Report, resulting in a socio-economic burden of more than $317 billion annually in the United States alone. Additionally, CNS disorders are expected to account for approximately 15% of the global disease burden by 2020, the largest of any disease area. However, treatment options for these conditions are often limited, inadequate or nonexistent, and the development of new CNS treatments generally trails behind other therapeutic areas. We are pursuing the development of the next generation of CNS therapies with high medical impact to address this critical and growing unmet need. Our dual development strategy is designed to optimize the outcome of our clinical programs by developing NCEs from known molecules with strong scientific rationale, and also by re-defining previously approved molecules with well-established tolerability and safety profiles, as determined by applicable regulatory agencies. We believe that our streamlined clinical development approach has the potential to advance our product candidates rapidly through early-stage clinical trials, while carrying a lower development risk. We believe that lower development risk exists with respect to the development of our lead product candidates due to their use of mazindol, an active substance, which was previously approved and marketed in the United States, Japan and Europe for obesity, and is currently listed as a CIV controlled substance as classified by the DEA under the CSA. The DEA defines CIV stimulants as those “with a low potential for abuse and a low risk of dependence.”
Our discovery platform currently focuses on single molecules that operate through multiple mechanisms designed to target the complexity of the CNS disease state, and as such, we believe that such a discovery platform may offer new treatment options for patients, including for those patients who are refractory to currently available treatments. Our current focus is in the therapeutic areas of rare hypersomnia disorders (condition highlighted by EDS, or excessive daytime spent sleeping) and complex neurodevelopmental disorders, and includes our two lead product candidates: Quilience, for the treatment of EDS and cataplexy, the two main symptoms associated with narcolepsy, and Nolazol, for the treatment of ADHD. We have yet to conduct any clinical trials for Quilience; however, it is our intention to initiate Phase 2 and 3 clinical trials for Quilience in adults, provided that we receive permission to initiate these trials from the FDA and other applicable regulatory agencies, which may also require certain CMC, clinical, and non-clinical work prior to the initiation of such trials, the extent of which is expected to be determined after we meet with the FDA and other applicable regulatory agencies, with such meetings expected to occur after this offering (see “ – Our Solution: Quilience for Narcolepsy - A Well-Suited Approach for the Disease Pathology – Non-Clinical Development Strategy” for more information). We intend to seek entrance into an accelerated approval pathway during the fourth quarter of 2020, such as Breakthrough Therapy Designation, Fast Track Designation or PRIME scheme, and subject to acceptance into such accelerated pathway, we intend to begin a Phase 2 dose-finding clinical trial in the first quarter of 2021 while the Phase 3 clinical trial expected to occur in the fourth quarter of 2021, following an interim review of the data from the Phase 2 dose-finding clinical trial during the third quarter of 2021. We have completed a Phase 2 clinical trial evaluating the safety and efficacy of Nolazol in adults with ADHD in the U.S. and given the positive outcome of this trial, we may initiate Phase 3 clinical trials after we receive approval to commercialize Quilience. We intend to seek FDA and other regulatory approval for both product candidates for use in children, which requires additional non-clinical work, as well as staged clinical work in determining safe dosing and monitoring.
Quilience and Nolazol both contain mazindol as the active molecule in a proprietary controlled release formulation allowing for what we intend to be a once-a-day, single tablet use. Mazindol has a well-established long-term safety record from its extended history of clinical use across the United States and several countries in Europe, where mazindol was previously approved in an immediate release formulation for the short-term management of obesity. It was marketed for nearly 30 years, into the early 2000s, before being voluntarily withdrawn from the market for commercial reasons and is no longer available nor marketed in these regions. In addition to the 30-year period in which it was marketed, mazindol was also widely used off-label and prescribed under compassionate use for the treatment of narcolepsy for approximately four decades, during which time it demonstrated a well-tolerated safety profile in patients over long-term, chronic use of the drug.
We believe that Quilience offers a meaningfully differentiated product profile over current treatment options for the following reasons:
| | ● | | Mechanism of action. If approved, Quilience would be the only partial orexin type 2 agonist approved by the FDA. Narcolepsy is caused by selective loss of the neurons producing orexins (also known as hypocretins). |
| | ● | | Established long-term use. Mazindol has a well-established long-term safety record from its extended history of clinical use across the United States and several countries in Europe, where it was previously approved in an immediate release formulation for the short-term management of obesity. In addition to the 30-year period in which it was marketed, mazindol was also widely used off-label and prescribed under compassionate use for the treatment of narcolepsy for approximately four decades, during which time it demonstrated a well-tolerated safety profile in patients over long-term, chronic use of the drug. |
| | ● | | Low potential for abuse and misuse and diversion. Mazindol is listed as a Schedule IV controlled substance as classified by the DEA. The DEA defines Schedule IV controlled substances as those “with a low potential for abuse and a low risk of dependence”. Unlike Xyrem (sodium oxybate), the top-selling treatment for narcolepsy in the United states, historically mazindol never required a REMS to manage known or potential serious risks associated with its use. |
| | ● | | Quilience is expected to be developed as a monotherapy or to be administered concomitantly with other narcolepsy treatments. Narcolepsy is a difficult disorder to manage and the majority of narcolepsy patients often require multiple medications to treat their symptoms. A retrospective analysis (Nittur et.al, Sleep Med. 2013 Jan;14(1):30-6) showed that mazindol has a long-term, favorable benefit/risk ratio in 60% of drug-resistant hypersomniacs, including a clear benefit on cataplexy. |
| | ● | | Quilience is being developed as a once-daily oral tablet administered in the morning upon wakening. Patients have identified a need for treatment options that are easier to take and are dosed less frequently. We believe that once-daily dosing with Quilience may address this need and may help improve patient compliance and adherence with treatment. |
Quilience and Nolazol, our two lead product candidates, are being developed as separate and uniquely differentiated controlled release pharmaceutical products. Our proprietary controlled-release formulations are being designed to optimize their pharmacokinetic and pharmacodynamic properties with a rapid onset of action and prolonged controlled therapeutic effect, allowing for a daily oral dose that effectively provides consistent and long acting symptom control and is designed to uniquely meet the needs of patients.
Leveraging our scientific insights and direct hands-on clinical experience, we are developing compounds that we believe have innovative mechanisms of action with positive therapeutic profiles and represent a differentiated treatment option to overcome the limitations of the current therapies. For example, Quilience and Nolazol are agents that differ from available treatments in their apparent dual mechanism of action. In addition to their action as a triple monoamine reuptake inhibitor that simultaneously blocks serotonin, norepinephrine, and dopamine transporters, and as such functioning as an SNDRI, our small molecule lead product candidates that quickly penetrate the blood brain barrier, also target the orexin pathway as a selective (in relation to the orexin-1 receptor) partial agonist of the OX2R. This activity could provide cortical regulation of the lower brain, and thereby lead to improved attention and inhibitory control, while also improving regulation of executive function and inhibitory control which would provide both “top down” and “bottom up” regulation of the brain. Orexin neurons (located in a part of the brain known as the hypothalamus) are multi-tasking neurons that serve as key modulators to several vital functions, including sleep-wake states, attention, feeding behavior, energy homeostasis, reward systems, cognition and mood throughout the cerebral cortex, and as such, the orexin system is a central promoter of wakefulness. A drug that targets orexin could potentially increase arousal in individuals with ADHD, which could be useful in individuals who have a low level of arousal or motivation, as is not infrequently the case in individuals with ADHD. To the best of our knowledge, no currently available medications for ADHD or for the treatment of EDS and cataplexy, the two main symptoms associated with narcolepsy, have effects on the orexin system. In addition to narcolepsy and ADHD, we believe that our lead product candidates may have therapeutic potential across a range of diverse CNS conditions such as RLS, IH, OSA, Kleine-Levin-syndrome, daytime sleepiness due to myotonic dystrophy Type 1 (DM1) and Prader Willi Syndrome, given their mechanism of action.
NLS was formed in June 2015 in Stans, Switzerland and is a Swiss limited company. In 2016 we acquired several patents from AP-HP in France, including for our lead compound mazindol. Since our founding, we have assembled an experienced leadership team with a track record of developing and repurposing products to treat rare neurological disorders and others. Our Chief Executive Officer, Alexander Zwyer, has held a variety of senior leadership roles of increasing responsibility throughout his career including global roles in business development, marketing, commercial, operations and general management. We complement our management team with a group of scientific and clinical advisors that includes internationally recognized North American and European experts in CNS disorders, including the areas of our current focus, rare hypersomnolence and complex neurodevelopmental disorders.
Our Development Pipeline
In addition to our lead product candidates, Quilience and Nolazol, we have early and late-stage compounds that we may seek to further develop in the future. We may seek to develop these other compounds, some of which are based on NCEs, in order to build a pipeline of product candidates at various stages of development that further complement our rare hypersomnia and complex neurodevelopmental disorder franchises. Additionally, we intend to continue to invest in our discovery research and development programs, with the goal of adding what we believe to be promising new compounds and indications to our product candidate development pipeline. See “Business – Research and Development” for additional information.
Our Research and Development Strategy
Our goal is to continue building a differentiated, global biotechnology company that is patient-focused in the development of transformative therapies that address critical unmet needs in rare and complex CNS disorders which are or may be classified as orphan disease and neurodevelopmental disorders, such as ADHD. As we navigate the competitive landscape of our industry, while focusing on development of our product candidates, we also intend to continually pursue out-licensing agreements and asset sale transactions that we believe will allow us to drive greater value for our shareholders. Key elements of our business strategy include the following:
| | ● | Efficiently advance our lead product candidates, Quilience and Nolazol, through marketing approval. Our goal is to expeditiously progress our lead product candidates, Quilience and Nolazol, into further clinical trials that may be required to receive marketing approval, as further determined by the FDA and other similar regulatory agencies. We plan to first advance Quilience into registrational studies to support approval of a MAA, followed by applicable studies in Nolazol in order to obtain an NDA. If successful, we plan to initially file for marketing approval in the United States and the EU for Quilience and subsequently in the United States for Nolazol. |
| | ● | Find partners for out-licensing and asset sale agreements. While we continue with our goal of progressing our lead product candidates, Quilience and Nolazol, on our own into further clinical trials that may be required in order to initiate commercialization of such product candidates, we may seek to enter into transactions to sell or out-license Quilience, Nolazol or certain other product candidates or intellectual property that we develop. This strategy allows us to potentially create value for our shareholders ahead of our timelines. |
| | | |
| | ● | Reduce clinical and regulatory risk, limit development costs, and accelerate time to market. Our lead product candidates, Quilience and Nolazol, incorporate a known molecule in a proprietary controlled release formulation. The former immediate release formulation of mazindol has a well-established safety record from its long history of clinical use across the United States and several countries in Europe and, as a result thereof, a well-characterized safety profile that has allowed us to rapidly begin conducting clinical development of Nolazol and to generate supportive phase 2 data in a study conducted in 85 patients with ADHD in the United States in May 2017. We believe that this strategy also allows us to potentially seek FDA approval using the 505(b)(2) regulatory pathway. |
| | ● | Develop products with differentiated pharmacological profiles. We are developing product candidates with dual mechanisms of action. For example, Quilience and Nolazol utilize a dual mechanism of action, resulting in a unique pharmacological profile targeting multiple CNS pathways which we believe are involved in the development and progression of the disorders targeted by our product candidates. We believe that products with clearly differentiated features, as compared to currently available drug therapies, will be attractive to patients and physicians and will provide us with a competitive commercial advantage. |
| | ● | Maximize the therapeutic potential of our existing targets and product candidates. Given the central physiological roles played by the distinct targets of our lead product candidates, we believe that there is significant potential for us to address multiple indications and our goal is to expand the therapeutic and commercial potential of our existing product candidates to additional indications. For example, we would plan to explore the development of Quilience for the treatment of IH, another rare CNS disorder for which Quilience may receive an orphan drug designation by the relevant regulatory bodies (see “ – Our Solution: Quilience for Narcolepsy - A Well-Suited Approach for the Disease Pathology - Quilience Label Expansion” for additional information). |
| | ● | Deploy our value-driven approach to broaden our product portfolio. Our team has extensive experience in CNS research and a strong record of publication in peer-reviewed journals and we plan to develop additional product candidates to treat indications with a high unmet medical need, and may seek to in-license from or collaborate with third parties to develop product candidates that we believe are highly differentiated, promising therapeutic candidates that address major unmet clinical needs. Our scientifically rigorous approach to evaluating new opportunities includes a robust asset evaluation of key factors, including the unmet medical need, biological rationale, safety profile, as determined by applicable regulatory agencies, feasibility of clinical development, potential for accelerated development path, regulatory approval, intellectual property position, competitive landscape and commercial potential. |
Relationship Between Narcolepsy and ADHD
Narcolepsy and psychiatric disorders have a significant but unrecognized relationship in which the two can coexist. However, narcolepsy is frequently misdiagnosed initially as a psychiatric condition, contributing to the protracted time for accurate diagnosis and treatment. Narcolepsy is a disabling neurological condition that carries a high risk for development of social and occupational dysfunction. Deterioration in function associated with narcolepsy may lead to the secondary development of psychiatric symptoms and inversely, the development of psychiatric symptoms can lead to the deterioration in function and quality of life. The overlap in treatments may further enhance the difficulty to distinguish between diagnoses.
ADHD is the most common neurobehavioral disorder characterized by symptoms of inattention, impulsivity and hyperactivity with an estimated prevalence rate of approximately 4-12% worldwide, as reported by the paper, “Understanding Attention Deficit/Hyperactivity Disorder From Childhood to Adulthood,” by Drs. Timothy E. Wilens and Thomas J. Spencer. On the surface, ADHD may appear to be the opposite of narcolepsy; however, there may actually be significant clinical similarity between the two. Cumulative data about sleep problems in children and adolescents with ADHD has shown that children with ADHD have had a higher rate of restless sleep, impaired sleep, and daytime sleepiness than children without ADHD. However, it is unclear whether EDS in ADHD is due to nocturnal sleep disturbances or primary vigilance disorders because shorter sleep onset latency is assessed by the Multiple Sleep Latency Test, or MSLT (an objective physiologic measure of sleepiness), in ADHD, rather than in the control group irrespective of the presence/absence of sleep disturbances.
On the other hand, problems with sleep may represent an intrinsic component of ADHD. The presence of ADHD symptoms in children and adolescents with narcolepsy has been found to be about two-fold higher than in the general control population and adults with narcolepsy have been found to have a much greater likelihood of having a diagnosis of ADHD in childhood compared to the general control population. Hyperactivity seen in ADHD may, in fact, be a compensatory response for individuals who are under-aroused or sleepy and ADHD symptoms contribute to poor quality of life and increased frequency of depressive symptoms, similar to narcolepsy. To the best of our knowledge, almost all of the treatments used in ADHD have mechanistic overlap with treatments used in narcolepsy for EDS and researchers suggest that the symptoms of EDS, fatigue, and sleep fragmentation may be the cause for ADHD symptoms, which is consistent with similar findings in other hypersomnia disorders.
Our Portfolio and Lead Product Candidates
Quilience for Treatment of EDS and Cataplexy in Narcolepsy
Narcolepsy is a rare chronic primary sleep disorder characterized by four main symptoms:
| | ● | EDS: In general, EDS interferes with normal activities on a daily basis, whether or not a person with narcolepsy has sufficient sleep at night. People with EDS report mental cloudiness, a lack of energy and concentration, memory lapses, a depressed mood, and/or extreme exhaustion. |
| | ● | Hallucinations: Usually, these delusional experiences are vivid and frequently frightening. The content is primarily visual, but any of the other senses can be involved. These are called hypnagogic hallucinations when accompanying sleep onset and hypnopompic hallucinations when they occur during awakening. |
| | ● | Sleep paralysis: This symptom involves the temporary inability to move or speak while falling asleep or waking up. These episodes are generally brief, lasting a few seconds to several minutes. After episodes end, people rapidly recover their full capacity to move and speak. |
| | ● | Cataplexy: This symptom consists of a sudden loss of muscle tone that leads to feelings of weakness and a loss of voluntary muscle control. It can cause symptoms ranging from slurred speech to total body collapse, depending on the muscles involved, and is often triggered by intense emotions such as surprise, laughter, or anger. |
Nearly all patients diagnosed with narcolepsy require lifelong treatment to combat the daily and debilitating symptoms, yet, most continue to suffer because of the current gap between their medical needs and effective treatments. We are aware of only one FDA approved treatment for both EDS and cataplexy associated with narcolepsy, sodium oxybate (Xyrem), and although marked with significant limitations, it generated revenues of over $1.64 billion in 2019 based on fourth quarter and full year financial results issued by Jazz Pharmaceuticals plc. According to a September 2019 press release from ResearchAndMarkets.com, the narcolepsy market generated $2.42 billion in 2018 and is projected to reach $5.36 billion by 2026, growing at a compound annual growth rate of 10.3% from 2019 to 2026.
As an agonist of the OX2R and a triple monoamine reuptake inhibitor, we believe that Quilience reflects a scientifically supported mechanism of action to address the shortcomings of the available therapies and the unmet needs of patients. Quilience is being designed as an oral, once-daily treatment, intended for long-term use. Through the use of mazindol in Quilience and the expected benefits of our proprietary controlled-release formulation, we hope that regulatory agencies will conclude that Quilience has a desirable balance of efficacy, safety and tolerability to support marketing authorization.
We also anticipate developing Quilience for broader hypersomnia indications, such as IH, where there is a critical unmet need and no currently approved treatments available, in addition to other disorders, such as neurocognitive disorders implicated by EDS.
Quilience has been granted orphan drug designation by both the FDA and European Commission for the treatment of narcolepsy, and if approved for marketing in adults, this designation is expected to provide 7 years and 10 years of market exclusivity in the United States and Europe, respectively, and with the potential for additional market exclusivity, if and when further developed and approved in pediatrics (extended for an aggregate of 7.5 years and 12 years in the United States and Europe, respectively). Additionally, we have filed an international patent application under the Patent Cooperation Treaty, or PCT, for proprietary controlled release formulation, and, if granted, may provide patent protection through 2037 in the United States. We held a pre-IND meeting with the FDA with written guidance in December 2016, and we plan to submit for an IND in the fourth quarter of 2020 along with a Scientific Advice and Protocol Assistance, or SAPA, to the EMA also in the fourth quarter of 2020. Additionally, we received scientific advice with protocol assistance from the EMA in March 2017 and are planning for the development of the Clinical Trial Agreement submission, which upon approval, will allow for clinical trials to begin in the EU. We plan to pursue an expedited development program with the FDA under the Breakthrough Therapy Designation and Fast Track Designation programs, as well as a similar program, the Priority Medicine, or PRIME, scheme with the EMA. Collectively, these programs are designed to expedite the development and review of drugs intended to treat serious conditions and fill an unmet medical need. The Breakthrough Therapy Designation, in particular, is reserved for product candidates with preliminary clinical evidence indicating the potential for a substantial improvement over available therapy. We believe Quilience may qualify for these programs based on (i) positive real-world evidence, namely, previous off-label use of Quilience’s active molecule, mazindol, in treating narcolepsy, which was prescribed under France’s Authorization Treatment Use program for 17 years in patients who failed to respond or could not tolerate the available approved treatments, (ii) its mechanism of action (a dual mechanism of action, including as a partial agonist of the OX2R, which we believe is crucial in addressing the underlying symptoms of EDS and cataplexy), resulting in a unique pharmacological profile targeting multiple CNS pathways), and (iii) the limited availability of therapies that successfully treat both cataplexy and EDS in narcolepsy.
Additionally, we are planning to leverage the demonstrated biological activity of the active molecule in Quilience to further expedite the clinical development, as well as drawing on the data from our ADHD program to support our clinical development efforts of Quilience and the FDA’s previous approval of mazindol (in its immediate release form in the pharmaceutical product “Sanorex,” as manufactured and distributed by Novartis (through its Sandoz division)) as safe in the management of obesity through the 505(b)(2) regulatory pathway. See “Business – Government Regulation and Product Approvals – Review and Approval of Medicinal Products in the EU” for additional information.
We believe that previous off-label use of Quilience’s active molecule, mazindol, in treating narcolepsy, which was prescribed under a regulatory authorized CUP in France for 17 years in patients who failed to respond or could not tolerate the available approved treatments, supports our development of Quilience. We believe this positive real-world evidence in improving both EDS and reducing cataplexy demonstrates the potential of Quilience as a potential treatment for patients suffering from narcolepsy, and notably, also the potential to provide a solution for patients who have not had a successful outcome with approved treatments, such as sodium oxybate, modafinil, methylphenidate-based, and amphetamine-based products. If approved, Quilience could be the first new therapy in nearly two decades approved by the FDA for the treatment of cataplexy and only the second therapy ever approved by the FDA for treating cataplexy since narcolepsy was first identified in the late 1800s.
In most patients, narcolepsy is caused by the permanent loss of orexin (also called hypocretin), which has been identified as the major regulator in the brain that modulates the stability of the sleep-wake cycle. Individuals with narcolepsy often emphasize that their symptoms go beyond falling asleep during the day and report feeling chronically tired, fatigued, or sleep deprived and also struggle with cognitive difficulties that interfere with their ability to perform at work or school and devastating cataplexy attacks that can be debilitating and frightening. These impairments are associated with an increased risk of accidents, trauma, and difficulties in maintaining personal relationships. The economic burden of narcolepsy is also extensive and people with narcolepsy have above-average rates of health care visits, medication use, and unemployment, and those employed have lower income levels. According to the Narcolepsy Network, narcolepsy affects an estimated 1 in every 2,000 people in the United States, which equates to 150,000 to 200,000 patients in the United States and 3 million worldwide; however, it is estimated that only 25% of those living with narcolepsy have been diagnosed and are receiving treatment. Most people with narcolepsy begin having symptoms in their teenage years, but the diagnosis is usually not made until adulthood, suggesting that narcolepsy is both an underdiagnosed and an undertreated disorder. According to the August 2018 National Know Narcolepsy Survey, most narcolepsy patients remain unsatisfied on current treatments.
While symptomatic improvement is possible, patients’ needs are usually not met and the therapeutic effects of the currently approved treatments remain inadequate for most patients, including lack of symptom control, variable efficacy, rebound sleepiness and rebound cataplexy, troublesome side effects, inconvenience, and high potential for abuse. Given the considerable burden of the condition, the adverse effects on the health of narcolepsy patients, and the limitations of available medications, there is a critical unmet need for additional treatment options. Quilience is a triple monoamine reuptake inhibitor (SNDRI) and partial agonist of the OX2R that may mimic the natural sleep-wake process by activating and further enhancing the brain mechanisms that promote and regulate wakefulness. We believe Quilience may bridge this considerable treatment gap and that the current narcolepsy landscape may provide an opportunity to establish ourselves as a leader in this space.
Real-World Evidence in Narcolepsy
The use of real-world evidence may improve the quality and efficiency of clinical development and clinical trial design, with the potential to accelerate the development of therapies that may provide meaningful benefits to patients. Real-world evidence is the analysis of real-world data, which can originate from sources such as CUPs, and may provide a more complete picture of patient experience to inform patient-focused drug development, and support the advancement of randomized, placebo-controlled clinical trials to support a marketing application.
While no longer commercially available in the United States or Europe, the active molecule in Quilience, mazindol, was previously widely used off-label and for several decades for the treatment of narcolepsy. Additionally, it was prescribed under a long-term CUP administered and regulated by ANSM (“L’agence nationale de sécurité du medicament et des produits de santé”), the national agency in France responsible for overseeing the safety of medicines and health products, helping to address the unmet needs of patients who did not initially respond, later failed or were unable to tolerate the approved available therapies, such as modafinil, methylphenidate, sodium oxybate, and amphetamine-based products. This CUP ran for 17 years, ending in 2016 due to unavailability of the product, and more than 200 patients with narcolepsy who were refractory to available treatments were prescribed mazindol for the treatment of EDS and/or cataplexy.
A retrospective, multi-center, observational study of this real-world data, financed by the French Health Ministry, and conducted in part by certain members of our team and members of our Scientific Advisory Board (prior to their work with us), was performed to evaluate the effectiveness and safety of mazindol in real-world, clinical practice. A total of 94 patients with narcolepsy, and suffering from cataplexy, including adults and children, with a mean 30 months of treatment exposure were included in the analysis to evaluate the effectiveness of mazindol on improving EDS. Mazindol noticeably improved EDS (p<0.0001), as measured by the Epworth Sleepiness Score, or ESS. The ESS is a validated patient-reported measure of the patients’ recent likelihood of falling asleep in everyday activities and is the same primary outcome measure widely used in other narcolepsy-related Phase 3 clinical trials. An analysis was also performed assessing the effectiveness of mazindol in controlling cataplexy in 62 patients and demonstrated a statistically significant reduction in the frequency of cataplexy episodes (p<0.0001). The ESS score before mazindol was 18.0 ± 3.1 and decreased to 13.6 ± 5 after mazindol, for a change of -4.2 (p<0.0001). In addition, the change in weekly cataplexy rate before and after mazindol changed from 4.6 ± 3.1 (before mazindol) to 2.0 ± 2.8 after mazindol (for a change of -2.7) (p<0.0001).
This retrospective study with analysis of real-world data, provides positive real-world evidence that the treatment was well-tolerated and effective, in terms of improvement in EDS and the reduction in cataplexy events, with a conclusion of an overall positive benefit-risk ratio in the majority of patients. In their report concluding the results of the study (Nittur et.al, Sleep Med. 2013 Jan;14(1):30-6), the researchers found that Mazindol has a long-term, favorable benefit/risk ratio in 60% of drug-resistant hypersomniacs, including a clear benefit on cataplexy and that the data, taken altogether, suggest that mazindol has a major effect on sleepiness, possibly greater than that of modafinil (Provigil).
Narcolepsy Overview and Market Opportunity
Narcolepsy is the inability to stay awake and arises from the dysregulation of the sleep-wake cycle, and in most individuals is thought to be caused by an immune system mediated destruction of brain cells that contain orexin. Orexin is a neuropeptide and is critical to the maintenance of wakefulness, continuity of sleep and coordination of the timing and features of REM sleep. Narcolepsy is often debilitating and incapacitating for those affected and with no known cure, it usually requires life-long symptomatic treatment. Narcolepsy symptoms significantly interfere with and cause a negative impact on cognitive, psychological and social functioning and can greatly affect daily activities.
The clinical hallmark of narcolepsy is EDS, which is present in all narcolepsy patients and is generally the first symptom to occur. EDS manifests as sudden irresistible bouts of sleep throughout the day, which can strike at any time, leading to inadvertently falling asleep during a variety of situations, such as while at work or at school, when having a conversation, playing a game, eating a meal, or, most dangerously, when driving or operating other types of machinery. A prominent and distinctive symptom of narcolepsy is cataplexy, which occurs in approximately 70% of those patients with narcolepsy. Although the severity of cataplexy is variable, it can be very frightening and usually causes additional disability. In addition to cataplexy, individuals with narcolepsy may also have symptoms indicative of altered REM sleep. REM sleep is normally characterized by dreaming and muscle paralysis that prevents an individual from acting out their dreams. In narcolepsy however, REM sleep can occur at any time of day and elements of REM sleep can mix into wake, manifesting as cataplexy, hallucinations that occur when going to sleep or upon waking, and sleep paralysis, a transitional state between wakefulness and sleep in which one is aware but cannot move, speak, or react.
Narcolepsy is classified into two subtypes, narcolepsy type 1 and narcolepsy type 2. Type 1 is characterized by cataplexy or an undetectable or low level of orexin in the cerebrospinal fluid, or CSF. In type 2, patients generally have less severe symptoms and do not experience cataplexy attacks. The underlying cause of narcolepsy type 2 is unknown and many patients have normal CSF orexin levels; however, 25% of patients with narcolepsy type 2 do have intermediate CSF orexin levels and these individuals are more likely to develop cataplexy and subsequently be diagnosed with narcolepsy type 1.
Cataplexy is a symptom that occurs almost exclusively in people with narcolepsy type 1, so its presence strongly suggests this diagnosis. However, cataplexy can sometimes be subtle or mild, making additional testing sometimes necessary, with the diagnosis depending on ruling out causes of secondary hypersomnia and performing an overnight sleep test with MSLT. On the MSLT, it takes less than eight minutes on average for people with narcolepsy type 1 to fall asleep, and REM sleep is observed on at least two naps. People with narcolepsy type 2 show the same results on MSLT as those with cataplexy.
In addition to EDS, cataplexy and other symptoms of altered REM sleep, people with narcolepsy type 1 are prone to gain significant weight, which may be due to the loss of orexin. Soon after narcolepsy onset, children can rapidly gain 5–15 kg (approximately 11 to 33 pounds) and adults with type 1 are often overweight or obese, despite normal caloric intake and activity levels. Given that the active molecule in Quilience was previously approved for the management of obesity and its multifunctional mechanism of action that targets two pathways known to be implicated in obesity, Quilience may also help to counter this negative effect of weight gain associated with narcolepsy.
Prevalence
According to the European Narcolepsy Network, narcolepsy affects only 20 to 40 out of every 100,000 people; however, it is estimated that only one out of four people living with narcolepsy have been properly diagnosed. Although it usually has an early age of onset during the adolescent years, a diagnostic delay that often exceeds 10 years from the time of symptom onset suggests that narcolepsy is both under diagnosed and under treated. This delay may result from several factors, including lack of clinician and patient recognition of the signs and symptoms of narcolepsy and leading to multiple physician visits before receiving a diagnosis, as well as misdiagnosis of narcolepsy as another condition, such as epilepsy, depression, or ADHD, which further delays treatment.
Current Treatment Landscape and Therapy Limitations
Until a cure is available, the current treatment of narcolepsy focuses on symptom control, with the goal of keeping the patient alert during the day, primarily by improving EDS and minimizing the occurrence of cataplexy. More than 90% of individuals with narcolepsy require chronic use of medication to manage symptoms, in order to allow for important everyday activities to be performed safely, such as attending school, going to work and caring for a child.
The current available treatment options require the careful balancing of the drug’s efficacy, convenience of administration, development of drug tolerance, adverse effects, comorbidities, monitoring for evidence of drug abuse, and new life circumstances, such as school, pregnancy and parenthood. In narcolepsy, several classes of drugs are used for the treatment of EDS, including a CNS depressive agent, wake-promoting agents, a histamine 3 receptor antagonist/inverse agonist, and CII controlled stimulants; however, only sodium oxybate (Xyrem) is approved by the FDA for the treatment of cataplexy.
Sodium oxybate (Xyrem) is the legally manufactured form of gamma hydroxybutyrate, an illicit drug of abuse. It is the only FDA approved treatment for cataplexy and is also approved for EDS, and as such, is often used as a first-line therapeutic. While it has been reported to have a positive impact for patients, sodium oxybate also has many challenges with significant limitations that can often impede its use. As a severe CNS depressant with a rapid onset of sedation with both hypnotic and amnestic effects, it is abused to incapacitate victims for sexual assault. Sodium oxybate is a CIII controlled drug and with the potential for an adverse outcome, it is further subject to higher CI controls for abuse and is only available through an FDA imposed restricted-access REMS program. It carries a Black Box warning for respiratory depression and abuse, which can lead to seizures, decreased consciousness, coma and death, and at doses lower than those used to treat narcolepsy. The occurrence of experiencing adverse effects is more common with sodium oxybate compared to other medications used in narcolepsy and adverse effects, even at recommended doses, include nausea, confusion, CNS and respiratory depression, neuropsychiatric depression and confusion, bed-wetting, sleepwalking, automatic behaviors, and involuntary movements. Sodium oxybate has a short half-life and is administered in a split dose, once at bedtime and again two and a half to four hours later, which can be difficult for patients to manage. In addition, generally, an extensive titration period is required, which can take upwards of seven months to achieve a complete optimal response.
Many patients with narcolepsy have cardiovascular risk concerns, including hypertension, and treatment with sodium oxybate contributes 1,100-1,640 milligrams, or mg, to an individual’s daily sodium intake, in comparison to a total daily intake of 1,500 mg as recommended by the American Heart Association. Additionally, life-style changes are also often needed when being treated with sodium oxybate, including the avoidance of alcohol and other medications that may cause sedation and due to its profound sedation and hypotonic effects, a change in living arrangements may be needed if living alone or the need to seek different and multiple treatment options when becoming a parent. Yet, despite these severe limitations, as the only FDA approved treatment for cataplexy, Xyrem continues to be the market leader in the United States.
Our Solution: Quilience for Narcolepsy - A Well-Suited Approach for the Disease Pathology
Narcolepsy is a debilitating neurological disorder and the currently available treatment options are not considered sufficiently effective for most patients. This is highlighted by the results of the recent 2018 “Know Narcolepsy Survey,” conducted by Versta Research, that emphasizes the continuing and substantial burden of narcolepsy with an astonishing 88% of patients indicating that their current treatments are not effectively managing their symptoms, while 94% and 93% stated that new treatment options are needed and expressed frustration with current treatment options, respectively.
Only one drug has been approved by the FDA for the treatment of cataplexy, and it is marked with significant limitations often impeding its use. In addition, we are aware of one other company developing an orexin-targeting agent – Takeda Pharmaceutical Company Limited. Quilience has a mechanism of action that is distinct from existing and emerging therapies and we believe that, if approved, Quilience may represent a substantial improvement to existing mono-therapy treatments due to the positive real-world evidence of its active molecule and its expected lower scheduling (CIV instead of CI, CII or CIII) under the CSA, which indicates the potential for abuse and risk of dependence of stimulants. The DEA defines CIV stimulants, such as mazindol, as those with a low potential for abuse and a low risk of dependence. Mazindol’s mechanism of action, which may restore orexin signaling in the brain and further enhance monoamine availability in promoting wakefulness and reducing cataplexy has the potential to be a breakthrough treatment and thereby offering a significant treatment advancement. Furthermore, in November 2019, the Swiss Narcolepsy Network endorsed Quilience as a potential novel treatment of narcolepsy. The Swiss Narcolepsy Network stated that its decision is based on several decades of highly promising off-label use and compassionate use of mazindol in patients with narcolepsy.
Quilience Label Expansion
Following our current focus on the development of Quilience for narcolepsy, we are also potentially planning to develop Quilience for the treatment of IH, a rare and chronic hypersomnia disorder for which there is currently no effective or approved treatments available. Its hallmark symptom is chronic EDS and a craving to sleep during the day, regardless of how many hours slept at night, which results in such persons taking daytime naps that are usually long and not refreshing. Individuals with IH struggle to wake, despite setting multiple alarms and may have difficulty rising from bed, called sleep inertia. Sleep inertia also includes feelings of grogginess upon waking and can result in impaired alertness and interfere with the ability to perform mental or physical tasks. Similar to narcolepsy, people with IH may also suffer from hallucinations and sleep paralysis when going to bed or upon waking.
The active molecule in Quilience was also prescribed under compassionate use for the treatment of IH, providing positive real-world evidence of its benefit in improving EDS specifically in patients with IH. We plan to request orphan drug designation from both the FDA and the European Commission in the fourth quarter of 2020 for the treatment of IH and, if granted and later approved for marketing, this designation is expected to provide us initially with 7 years and 10 years of market exclusivity in the United States and Europe, respectively. Additionally, we have filed an international patent application under the PCT for a proprietary controlled release formulation, and, if granted, may provide patent protection through 2037 in the United States.
Non-Clinical Development Strategy
We intend to conduct the necessary non-clinical pharmacology, pharmacokinetic, and toxicology studies required for regulatory approval of our lead product candidates in parallel with our clinical programs. In addition, we also intend to submit the NDA for Quilience under Section 505(b)(2) of the FDCA. The NDA pathway under Section 505(b)(2) was created, in part, to help avoid unnecessary duplication of studies, including non-clinical studies, already performed on an existing or previously approved drug. A NDA submitted pursuant to Section 505(b)(2) is one that relies, at least partially, upon data that a company does not own or have a right of reference to, including published literature. A 505(b)(2) application may also rely upon the FDA’s finding of safety and/or efficacy of a previously approved drug, and as such, we intend to rely, in part, on the FDA’s previous findings of safety of the listed drug. If successful, our non-clinical program may potentially be streamlined and/or reduced. We may also seek to license the innovator data, and if successful, this may potentially provide another source to streamline and/or reduce our non-clinical program. Our ability to rely on the FDA’s previous findings of safety, studies published in the scientific literature, or the extent to which licensed data may be utilized will depend on our ability to demonstrate a scientific bridge to Quilience and there can be no assurance whether, or to what extent, the FDA will accept a 505(b)(2) filling or that we will be successful in obtaining a right of reference to the innovator data. After this offering, we intend to meet with the FDA, and other regulatory agencies such as the EMA, on our proposed non-clinical program and obtain their feedback and agreement on the various program elements required to support approval of our lead product candidates.
Phase 2 and 3 Clinical Trial Development Strategy
After this offering, we intend to hold meetings with the FDA and EMA to confirm all necessary details, along with targeted alignment between these agencies, and we plan to initiate Phase 2 and 3 clinical trials, of which the Phase 2 clinical trial is expected to be conducted with the expected commercial formulation during the first quarter of 2021 and Phase 3 clinical trial expected to be initiated in the fourth quarter of 2021. Prior to commencing either of these trials, in addition to communicating with the applicable regulatory authorities, we will need to finalize formulation development and develop and validate metabolic synthesis and analytical methods as well as potentially carry out CMC work or non-clinical studies.
We intend to seek entrance into an accelerated approval pathway during the fourth quarter of 2020, such as Breakthrough Therapy Designation, Fast Track Designation or PRIME scheme, and subject to acceptance into such accelerated pathway, we intend to conduct our clinical trials in Quilience, as further described below.
The Phase 2 dose-finding clinical trial is planned to begin in the first quarter of 2021 at multiple centers in the United States and Europe. This trial is expected to be an eight-week randomized, double-blind, placebo controlled, parallel group, forced-dose titration study in approximately 200 adult narcolepsy patients (type 1) to evaluate three doses of Quilience, as compared to placebo, with primary outcome measures for EDS and cataplexy. As of now, the primary endpoints are expected to be the measure of change in the ESS as well as the change in the mean sleep latency on the Maintenance of Wakefulness Test, or MWT. The key secondary endpoints to be examined are expected to be the change in the number of weekly cataplexy attacks and the change in the Change in the Global Improvement of Severity Score, or CGI-S. CGI-S is a seven-point scale that requires the clinician to rate the severity of the patient’s illness at the time of assessment, relative to the clinician’s past experience with patients who have the same diagnosis. Below is a pictorial depiction highlighting the Phase 2 clinical trial for Quilience.
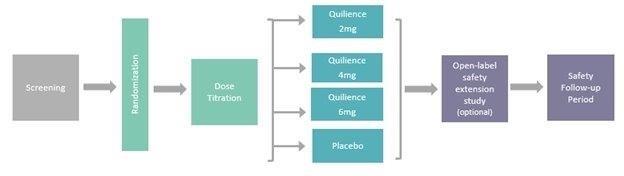
The Phase 3 clinical trial is planned to begin in the fourth quarter of 2021, following an interim review of the data from the Phase 2 clinical trial during the third quarter of 2021, to select the best dose for use in narcolepsy patients. This eight-week randomized double-blind study in 150 adult narcolepsy patients (both type 1 and type 2) is also expected to be conducted at multiple centers in the United States and Europe and is expected to evaluate EDS and cataplexy as compared to placebo and an active comparator, which we expect to determine after discussing with the applicable regulatory agencies. As of now, the primary endpoints are expected to be the measure of change in the ESS as well as the change in the mean sleep latency on the MWT. The key secondary endpoints to be examined are expected to be the change in the number of weekly cataplexy attacks and the change in the CGI-S. Below is a pictorial depiction highlighting the Phase 3 clinical trial for Quilience.

Following the start of the Phase 3 clinical trial, planned for the fourth quarter of 2021, we expect to receive the results of the Phase 2 clinical trial during the first quarter of 2022 followed by results of the Phase 3 clinical trial during the fourth quarter of 2022. If such results are successful, as deemed by regulatory authorities, such as the EMA and FDA, we would seek to obtain MAA and NDA approval in the third quarter of 2023.
Compassionate Use Objectives in Narcolepsy in Europe
While “compassionate use” originally was understood to imply that the drug product is supplied for free, there is a growing understanding with policymakers, payers and patient organizations, that paid-for CUPs are justifiable. In Europe and other regions of the word (excluding the United States), it is recognized that CUP funding can provide an early source of biopharma revenue and incentivize the increased availability of potentially transformative treatments, especially for rare disorders and orphan diseases. Given the extensive therapeutic experience with mazindol, including off-label in narcolepsy patients, we believe that there is ample precedence to justify a funded CUP in selected European countries for a defined group of patients.
We intend to conduct either or both a cohort program and a named patient program, or NPP, each of which is highlighted below in a variety of European countries. Although we have yet to determine which countries we will initiate CUPs in first, our current plan is to target the following countries in the following priorities (priority in each wave has yet to be determined):
| | ● | First Wave: Austria, Belgium, France, Italy and Switzerland; |
| | ● | Second Wave: Brazil and Latin America, the Czech Republic, Denmark, Spain and the United Kingdom; and |
| | ● | Third Wave: China, Germany, Japan, Sweden and Taiwan. |

In addition to the potential benefit of generating revenues before Quilience market authorization from the regulatory agencies, the collection of data within the CUP is a key potential benefit for the overall Quilience evidence generation strategy, as we believe that a CUP program for Quilience would:
| | ● | complement the clinical data package for regulatory submissions; |
| | ● | support primary regulatory approvals with longer-term follow up effectiveness and safety data and patient lived experience evidence; |
| | ● | support label expansions in broader populations than included in the Quilience late state trials (e.g., in juvenile narcolepsy); |
| | ● | potentially accelerate regulatory approval in China if we were to seek regulatory approval for Quilience; |
| | ● | enhance the evidence package for market access and pricing and reimbursement; |
| | ● | provide qualitative live-experience data and information on the unmet needs from the patient (and caregiver) perspective that may contextualize the net therapeutic benefits offered by Quilience within the narcolepsy management paradigm; |
| | ● | bridge patients from the end of Phase 2 and 3 clinical trials to commercialization (assure continued patient access); |
| | ● | allow us to generate hypotheses for label expansions in broader populations (as compared to clinical trials); |
| | ● | allow us to leverage real-world and patient-centric data collected (board populations, quality of life, satisfaction); and |
| | ● | allow us to professionally address patient and physician requests (formal published policy and process). |
In addition to these potential benefits of a CUP program for Quilience, commercial objectives of such a program would include the ability to (i) generate pre-licensing revenues in selected countries, (ii) receive post-authorization revenues in non-priority countries for commercial use, (iii) potentially establish an early market presence with a key opinion lead, and center of excellence strategy and (iv) more rapid revenue uptake (than a non-CUP program) upon commercial availability (patients already on product and treated).
Nolazol for the Treatment of ADHD
Nolazol is a triple monoamine reuptake inhibitor and orexin receptor-2 partial agonist and its unique pharmacological profile is expected to yield import benefits compared to existing treatments of ADHD. Enhancing the function of the three neurotransmitters well-known to be implicated in ADHD, norepinephrine, dopamine and serotonin, along with its activity on the orexin-2 receptor, Nolazol may produce an optimal reduction in ADHD symptoms over available treatments.
Nolazol is supported by a positive pilot clinical trial with mazindol in 24 children with ADHD and a positive Phase 2 clinical trial in 85 adults with ADHD. The Phase 2 clinical trial in adults met all primary and secondary study endpoints and was well-tolerated. A robust effect on ADHD symptoms was demonstrated with a large placebo-adjusted effect size of 1.09 in the investigator-rated ADHD symptom scores. See “Nolazol Clinical Trial Results” below for additional information.
Although more than 25 different products have been approved by the FDA since 1937 for the treatment of ADHD, many of which are no longer available, there still continues to be a large treatment gap with no optimal treatment currently available. Physicians, patients and their caregivers press for improved treatment options to address key shortcomings with currently available treatments, including the need for a more tolerable safety profile, more consistent efficacy with no rebound effect, and the need for lower risk of abuse, dependence, and misuse. Currently, doctors, patients, and caregivers must choose between treatments that may be effective, but come with significant safety liabilities, such as high potential for abuse and risk of diversion coupled with tight restrictions on writing and filling prescriptions; or treatments that are unscheduled, but are also typically less effective. We are seeking to develop Nolazol such that, if approved for marketing, it could be the drug product to close this treatment gap and address this unmet medical need.
ADHD Overview and Market Opportunity
ADHD is a chronic neurodevelopmental disorder affecting children, adolescents and adults and is characterized by an ongoing pattern of inattention and/or hyperactivity-impulsivity and is associated with clinically significant impairments in executive functioning. In addition, ADHD is one of the most commonly diagnosed neurodevelopmental disorders in school-age children and it often persists into adulthood. It is characterized by symptoms of inattention and/or hyperactivity-impulsivity, and affects cognitive, academic, behavioral, emotional, and social functioning. An estimated 8.4% of children and 5% of adults in the United States have a current diagnosis of ADHD, and if left untreated can lead to poor occupational and psychosocial outcomes. In the United States, the research firm GlobalData has reported that the ADHD market was worth $8.5 billion in 2018 (of which 95% was generated in the United States) and projected to reach a market value of $14.4 billion in 2023. Furthermore, based on a report by Grand View Research, Inc., ADHD is the 12th most prevalent therapeutic indication for dispensed prescriptions and is predicted to grow at a 6.4% compounded annual growth rate until 2030. Despite their CII classification and black box warnings and safety liabilities, revenues of the market leader Vyvanse (Shire plc), as reported by Shire, were $2.1 billion in 2017, and revenues of the leading methylphenidate product Concerta (Johnson & Johnson) were $1.1 billion in 2012, as reported by Johnson & Johnson. Furthermore, based on a report from LifeSci Capital, it is estimated that CNS stimulants – amphetamine and methylphenidate containing products, represent approximately 90% of all ADHD drug sales.
Across the lifespan, ADHD may impede cognitive functioning as well as mental health and development and can have a significant social impact on patient’s lives, causing disruption at school, work, and in relationships and can also be associated with risk-taking or criminal behavior and other far-reaching effects on wider society. Over the past eight years, ADHD diagnoses have increased 30% in the United States, a trend indicating the need to focus on the diagnosis and treatment for a growing number of patients.
The Centers for Disease Control and Prevention, or the CDC, reported that 6.1 million, or 9.4%, of children and adolescents in the United States have ever been diagnosed with ADHD and 5.4 million, or 8.4%, have a current diagnosis, and 62% take medication, while 47% receive behavioral therapy and 23% receive no treatment at all. Furthermore, the CDC reported in 2019 that among children aged 2-5 only 18% are receiving ADHD medication, in contrast to 60-70% of children aged 6-17. Additionally, ADHD is the second most impactful condition affecting children and adolescent health in the United States, as measured by the Blue Cross Blue Shield Health Index, and children diagnosed with ADHD struggle with paying attention, controlling impulses and being overly active. Social skills in children with ADHD often are significantly impaired. Problems with inattention may limit opportunities to acquire social skills or to attend to social cues necessary for effective social interaction, making it difficult to form friendships. Hyperactive and impulsive behaviors may result in peer rejection. The negative consequences of impaired social function, such as poor self-esteem, increased risk for depression and anxiety, may be long standing.
Once believed to only affect children and adolescents, ADHD is now well understood to be a lifespan disorder that persists into adulthood in up to 65% of patients affecting 1 out of 30 adults worldwide, as disclosed by the ADHD Institute, an educational platform developed and funded by Takeda, and, based on our own assessments of data from the U.S. census Bureau, there are approximately 11 million adults in the United States with ADHD. Research firm GlobalData reported that since 2015, the adult ADHD market has become larger and begun growing at a faster rate than the pediatric ADHD market. Adult ADHD is often characterized by recurrent problems with restlessness, impulsivity, problems with time management and finances, as well as problems regulating emotions. Rather than being hyperactive like children, adults with ADHD report experiencing an internal sense of fidgetiness and restlessness and with signs of inattention more apparent through problems communicating with others. Upon entering the job market, many adults also experience obstacles in employment, and are at increased risk to be terminated due to repeated tardiness or absenteeism. Such difficulties contribute to poorer employment outcomes and a lower likelihood of being employed in professional environments. Adults with ADHD often find it difficult to generate effective solutions to social problems and these deficits in social cognition can increase the likelihood of peer rejection, and social isolation, adding to struggles with depression and social anxiety.
Throughout an individual’s lifetime, untreated ADHD can increase the risk of psychiatric disorders, educational and occupational failure, accidents, criminality, social disability and addictions and ADHD treatment is usually initiated with stimulants, such as amphetamine and methylphenidate-based products. While these drugs may provide a generally effective treatment option for patients, they also have serious safety concerns and are misused recreationally with both diversion and abuse being a common and significant risk. As a result, they are controlled substances and classified under the CSA as CII stimulants. Consequently, they all carry an FDA imposed Black Box warning on the drug label to call attention to these serious or life-threating risks. Further, not all individuals respond optimally to or can tolerate CII stimulants and their use is contraindicated in numerous patients, including those with tics, anxiety and other certain psychiatric disorders, cardiovascular concerns, substance use disorder, or stimulant refusal. A few non-stimulant treatment options are available; however, their efficacy is not as robust, and their tolerability profile is not necessarily improved when compared to CII stimulants. In addition, the few non-stimulant treatments available are generally considered as a second-line treatments and are often used in conjunction with CII simulants rather than as a therapy that uses one type of treatment, commonly referred to as a mono-therapy.
The availability of a treatment that has robust efficacy on par with CII stimulants and that is well tolerated with lower potential for abuse represents what we believe is an important unmet need to persons with ADHD. The magnitude of the treatment effect demonstrated in our Phase 2 study is comparable to what is typically seen with the leading CII stimulants, and, mazindol, the active molecule in Nolazol, is currently a CIV controlled substance under the CSA, meaning it has an already established low risk of abuse. We believe Nolazol, whose active ingredient is mazindol, a CIV stimulant, may be the transformative treatment for ADHD that physicians and patients have been waiting for, by providing a treatment with what we believe will be similar efficacy as CII stimulants, but with a low potential for abuse.
We have a robust method of use patent for Nolazol for the treatment of ADHD expiring in August 2028 in the United States and in December 2027 in Europe. Additionally, we have filed an international patent application under the PCT, for a proprietary controlled release formulation, and, if granted, may provide patent protection through 2037 in the United States.
Diagnostics and Patient Sub-Types
Healthcare providers use the guidelines in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, or DSM-5, to help diagnose ADHD and this diagnostic standard helps ensure that people are appropriately diagnosed and treated for ADHD. The DSM-5 identifies three sub-types and each presentation is distinguished by a distinct set of symptoms that physicians use to diagnose the condition. The three presentations are: (1) Predominantly Inattentive; (2) Predominantly Hyperactive-Impulsive; and (3) Combined Presentation.
The diagnostic evaluation for ADHD includes a comprehensive medical, developmental, educational, and psychosocial evaluation. This comprehensive evaluation is necessary to confirm the presence, persistence, pervasiveness, and functional complications of core symptoms, exclude other explanations for core symptoms and identify coexisting emotional, behavioral, and medical disorders. In order to meet criteria for ADHD, core symptoms must also impair function in academic, social, or occupational activities.
Two-thirds of individuals with ADHD have at least one other neurodevelopmental, psychiatric, or other CNS disorder, including anxiety, depression, autism, and sleep disorders. Substance use disorder is a common comorbidity associated with ADHD and may have a direct underpinning in the pathophysiology of the disease, amplifying the need for treatment options with a lower risk of abuse.
Adults with ADHD, with a delayed diagnosis in adulthood, are often diagnosed after several attempts to find treatment for comorbid disorders, such as depression, substance abuse, sleep disturbances, or anxiety. The presentation in adults is typically related to problems with work, disorganization, and tendency to procrastinate, as well as anxiety, sleep disorders, and impulsivity.
We believe Nolazol could provide a transformative treatment option for all individuals with ADHD and further could draw substantial market share from patients who have failed or couldn’t tolerate stimulants; current patients, or their parents, with concerns with stimulant use; current patients seeking the convenience of a CIV product compared to a CII product, allowing for generally no limitations on quantity, the ability to refill, phone-in prescriptions, and less frequent office visits; individuals where abuse and/or diversion is a prominent concern; and individuals or parents of individuals diagnosed with ADHD who have avoided treatment due to stimulant concerns and the associated stigma.
Current Treatment Landscape and Therapy Limitations
Although there is no cure for ADHD, medications may help to reduce symptoms and improve functioning. The current treatment options for ADHD can be broadly classified as either amphetamine or methylphenidate-based stimulants or as non-stimulants. Based on data that we have collected, we believe that stimulants represent a majority of the ADHD drug market in the United States, with a market share of approximately 90%.
Amphetamine and methylphenidate-based products are all classified under the CSA as CII stimulants, due to their high potential for abuse and the risk of severe psychological or physical dependence. These drugs are heavily controlled under U.S. federal and state laws, and are subject to criminal sanctions for abuse, diversion and misuse and require a CII level prescription, and despite being a chronic disorder and need for daily medication, this limits the quantity to a 30-day supply and is also not refillable. Consequently, all CII stimulants contain Black Box warnings, similar to narcotics such as fentanyl and oxycodone, which are also CII substances. In addition, CII stimulants have the potential for numerous adverse effects, as indicated by their warnings of serious cardiovascular reactions such as sudden death, stroke, and heart attack, psychotic or manic symptoms in patients with no prior history, and are associated with peripheral vasculopathy, including Raynaud’s phenomenon. CII stimulants are also sleep-affecting drugs and shorten total sleep time, increase the time it takes to fall asleep, adversely impact the ability to stay asleep, and increase daytime sleepiness.
The long-term use of prescription stimulants has been widely reported to cause drug tolerance, which is the loss of efficacy over time and requires a complete change in treatment, or an increased dose of the existing treatment, or the add-on of another medication to the existing treatment in order to achieve therapeutic effectiveness. This leads to an increased risk of developing serious adverse effects that are unrelated to ADHD. Another concern in treating patients with CII stimulants is the potential “rebound effect” that occurs when the medication wears off, resulting in the return of ADHD symptoms and which may also occur in an amplified form. In children especially, this often triggers increased irritability and/or aggressive behavior and the rebound in children and adults may be exacerbated by multiple drug administrations, often used to obtain the desired duration of effect or to address drug tolerance. Additionally, studies have highlighted that primary limitations of CII stimulants are intolerable adverse effects that interfere with patient adherence rates and sub-optimal efficacy with the onset of drug tolerance.
According to the 2002 practice parameter for the use of stimulant medications from the American Academy of Child & Adolescent Psychiatry, approximately 30% of patients do not respond adequately to or have dose-limiting adverse effects with CII stimulants. Additionally, certain patients, or parents of patients, prefer not to use CII stimulants due to their stigma and known abuse potential. There are a few non-stimulant treatments available, such as atomoxetine (Strattera), clonidine (Kapvay), and guanfacine (Intuniv), that were developed to address this need; however, their efficacy is sub-optimal to stimulants and while unscheduled, their overall safety profile does not necessarily provide an improvement to CII stimulants.
Strattera, a norepinephrine reuptake inhibitor, was the first non-stimulant treatment option for ADHD and while its initial launch started strong, underscoring the demand for an alternative to CII stimulants, sales steadily declined as patients and physicians found it to not be nearly as effective as CII stimulants. It is now considered a second-line treatment and is typically used as an alternative to CII stimulants for patients who have a substance abuse problem, a family member(s) with a substance abuse problem, tics, or intolerable side effects with CII stimulants. Strattera carries a Black Box warning for increased risk of suicidal thoughts in children and adolescents and additional warning statements for liver damage. Moreover, Strattera takes four weeks to reach initial onset of action and six to ten weeks to achieve full clinical effectiveness, related both to the prolonged titration needed and the delay in the onset of action of the compound. Today Strattera (branded and generic) has about 3.6% market share, despite initially climbing to nearly 20% following launch on the basis of being an alternative to CII stimulants.
Clonidine and Guanfacine are also non-stimulants that are alpha-2 adrenergic receptor agonists, and were both initially approved for managing blood pressure. They have been approved for use in children and adolescents, generally in conjunction with CII stimulants as an add-on therapy. While they are used in children and adolescents, there is little study of their efficacy and safety in adults. As a mono-therapy, they are usually reserved for children and adolescents who respond poorly after several trials of stimulants and Strattera, have unacceptable side effects with stimulants or Strattera, or have significant comorbid conditions limiting the treatment options to these products. These two drugs have not had significant commercial success, with a combined peak market share of about 5%.
Our Solution: Nolazol – Next-Generation ADHD Therapeutic
We believe a large market opportunity exists for a non-CII stimulant, such as Nolazol, that uses mazindol controlled release as its active ingredient; mazindol has been classified as a CIV stimulant, due to its low risk of abuse and tolerance.
Given its unique binding profile (specifically, as an OX2R agonist), in addition to its classification as a CIV stimulant, we believe Nolazol could be transformative for the ADHD treatment landscape. Nolazol is a triple monoamine reuptake inhibitor and also a partial agonist of the OX2R, which we believe is an important, unique and differentiating factor relative to other ADHD treatments. In our clinical studies conducted to date, Nolazol has been well-tolerated and there were no treatment-related serious adverse effects or discontinuations. In terms of abuse potential, Nolazol has an already established low risk of abuse, as previously determined by the DEA when mazindol, its active ingredient, was scheduled as a CIV substance, underscoring the awareness and agreement that Nolazol has a lower risk of abuse than CII stimulants. In addition, based on the current DEA classification of mazindol as a CII stimulant, we expect that Nolazol will not have a Black Box warning, which is another important differentiator relative to both CII stimulants and the current non-stimulants in use today to treat ADHD.
Our Phase 2 trial showed significant improvement in ADHD symptoms met all primary and secondary study endpoints and was well-tolerated and with no clinically significant adverse effects over placebo. In light of its innovative mechanism of action and low potential for abuse, we believe Nolazol, if approved for marketing, could represent a highly differentiated alternative to the CII treatments in use today to treat ADHD.
Nolazol Clinical Trial Results
Phase 2 Clinical Trial
We completed a Phase 2 clinical trial in 2017 in the United States, in which Nolazol was well-tolerated and demonstrated statistically significant improvement over placebo. The clinical trial met the primary and all secondary endpoints and had a robust effect on ADHD symptoms with a large placebo-adjusted effect size of 1.09 in the investigator-rated ADHD symptom scores. We believe that the magnitude of this effect is comparable to currently leading CII stimulants and considerably larger than the available non-stimulant treatment options. The below table summarizes the results of our Phase 2 clinical trial in adults and shows the magnitude of the results.

Our Phase 2 study evaluated the efficacy, safety and tolerability of Nolazol in a randomized, double-blind, placebo-controlled, multi-center, parallel trial in 85 adults with a diagnosis of ADHD. Subjects were administered Nolazol or matching placebo once a day for six weeks, dosed flexibly during a three-week double-blind optimization period, followed by a three-week double-blind fixed dosing period. The figure below provides an overview of the study design.

Efficacy Results
The primary efficacy endpoint was the change from baseline in the total ADHD-RS score at Week 6, as compared to placebo, and measured by the clinician-administered ADHD Rating Scale with DSM-5 symptoms, or the ADHD-RS-5. ADHD-RS-5 is a standardized, “gold standard endpoint” validated test for measuring severity of ADHD symptoms and assessing response to treatment. The scale is based on the ADHD diagnostic criteria as defined in the DSM-5.
The primary endpoint was met, showing statistically significant improvement versus placebo, in favor of Nolazol. The least square, or LS, mean improvements in subjects’ scores from baseline were -18.9 for Nolazol and -5.7 for placebo, with a LS mean difference between Nolazol and placebo of -13.2 (95% CI,-18.7, -7.6). The results were found to be consistent across all sensitivity analyses, establishing that the large placebo-adjusted effect size of 1.09 was not biased by any of the statistical methods used.
In addition, Nolazol provided a significant reduction in ADHD-RS-5 scores starting as early as Week 1, which was also observed throughout the full treatment period. After six weeks of treatment, patients treated with Nolazol demonstrated more than three times the improvement in the ADHD-RS-5 total score symptoms as compared to placebo treated patients. The figures below summarize the results of the primary endpoint observed in our Phase 2 trial.


A p-value is a conventional statistical method for measuring the statistical significance of experimental results. A p-value of less than 0.05 is generally considered to represent statistical significance, meaning that there is a less than 5% likelihood that the observed results occurred by chance. In the figure above and all subsequent figures where p-values are included, a p-value of less than 0.05 is represented by “*.” P-values of less than 0.01 or less than 0.001 are represented by “**” or “***” respectively, and are considered to have higher statistical significance.
Secondary efficacy endpoints included patient response to treatment, as measured by the reduction of the ADHD-RS-5 score by at least 30% and by at least 50% from baseline, and as measured by the Clinical Global Impressions-Improvement, or CGI-I. The CGI-I is a standardized and validated assessment used by the clinician to rate the severity of a patient’s illness and improvement over time. There was a significant improvement in ADHD-RS-DSM5 scores by Week 6 for Nolazol compared with the placebo. At Week 6, 70% of the Nolazol-treated patients, compared with 21% of the placebo-treated patients, had at least a 30% reduction in their ADHD-RS-5 score (p < 0.001), and 55% of the Nolazol-treated patients, compared with 15.8% of the placebo-treated patients, had at least a 50% reduction in their ADHD-RS-5 score (p = 0.002). There were significantly more “responders,” defined as a ≥ 30% reduction from baseline in ADHD-RS-5 scores, present in those receiving Nolazol compared with placebo at the first assessment point Week 1, and at each subsequent assessment. Furthermore, there were significantly more “excellent” responders, defined as a ≥ 50% reduction from baseline in ADHD-RS-5 score, compared with placebo present by Week 2 and at each subsequent assessment point. The excellent response by Week 2 was also evident in the CGI-I analysis, which indicated significantly more CGI-I responders on Nolazol compared with placebo on Week 2 and at each subsequent visit (p ≤ 0.003). The sensitivity analyses resulted in a similar magnitude of difference between Nolazol and placebo for all responder definitions. The figure below summarizes the patient responder results.
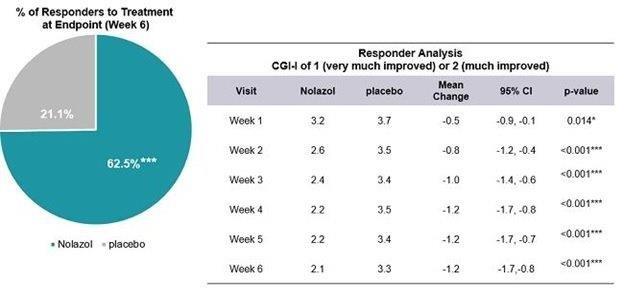
Safety and Tolerability
Nolazol was well-tolerated and there were no deaths or serious adverse events reported and no discontinuations in the Nolazol treated group due to adverse events or lack of efficacy. Adverse events reported were mild to moderate and the more prevalent reported events in the Nolazol treated group, as compared to placebo, included constipation, dry mouth, nausea, fatigue, somnolence, middle insomnia and heart rate increased. Relative to placebo, on Week 6, the Nolazol treated group had a minimal increase in diastolic and systolic blood pressure, a small increase in heart rate, and no significant changes in electrocardiography, or ECG, parameters. There were no remarkable findings on physical examination, hematology, serum chemistry, or urinalysis values from baseline to Week 6 between Nolazol and placebo groups. Mean weight loss of the Nolazol group was 1.73 kg, compared to a mean weight increase for the placebo group of 1.07 kg.
Phase 1 Clinical Trial
We completed a Phase 1 randomized, open-label, cross-over clinical trial in the United States in 2016, which characterized the pharmacokinetics and evaluated the safety and tolerability of Nolazol following a single dose in the fasted and fed state in normal healthy adult subjects. Subjects received a single, fixed dose of Nolazol under fasted conditions in one dosing period and the same single, fixed dose 30 minutes prior to a high-fat breakfast in another dosing period. Nolazol demonstrated a predictable, dose-dependent, and linear pharmacokinetics profile. This study is intended to serve as the basis of our Phase 3 clinical trials, if any, by providing a preliminary understanding of the concentration-time profile of Nolazol under the fed and fasted conditions.
Food administered 30 minutes after administration of Nolazol resulted in similar exposure and a slightly lower peak concentration compared to administration in the fasted state, while the time to reach the peak concentration was similar in the fasted and fed states. These results suggest that our Phase 3 trials will not need to include dosing restrictions in terms of meal timing relative to dose administration, which is an important feature for increased compliance in ADHD patients. A definitive food-effect study with the final formulation of Nolazol will be conducted during the Phase 3 program. The figure below shows the pharmacokinetic curve of Nolazol under the fed and fasted conditions utilized in this clinical trial.

Nolazol was well-tolerated and there were no deaths, serious adverse events or withdrawals due to adverse events. Adverse events were reported in 30% of the Nolazol-treated subjects and included dizziness and somnolence and there were no clinically significant changes in laboratory values, ECG, blood pressure, or heart rate.
Phase 2 Pediatric Clinical Trial
A Phase 2 open-label pilot study in France was conducted in 2015 by Eric Konofal, one of our founders, and others while at AP-HP. This study evaluated the efficacy, safety, and tolerability of mazindol in 24 children, aged 9 – 12 years, and diagnosed with ADHD. All enrolled patents had a low rate of response to methylphenidate, which is a first-line treatment in children with ADHD. All patients received the same fixed dose of mazindol daily for seven days, followed by a three-week drug-free safety observation period. The mean change from baseline in the parent rated and clinician rated ADHD-RS-IV total score after 7 days of treatment was -24.1, with >90% improvement in ADHD symptoms from baseline (p<0.0001), pointing to a viable long-acting treatment option, assuming it is shown to be safe, as determined by applicable regulatory agencies. Additionally, the mean change in the parent rated and clinician rated ADHD-RS-IV total score from end of treatment (Week 1) to the final observation visit (Week 4) demonstrated statistical significance (p<0.0001), indicating significant alteration in the level of symptomology of ADHD after mazindol withdrawal. The figure below summarizes the results of the primary endpoint.

Mazindol was well tolerated in children with ADHD. Adverse events included decreased appetite, headache and abdominal pain and there were no clinically significant changes in laboratory values, ECG, blood pressure, heart rate, or body weight. This clinical trial provided proof-of concept data, potential benefit, and supported the advancement into a more expansive Phase 2 trial.
Phase 3 Development Strategy
Having successfully met both the primary and secondary endpoints in our initial Phase 2 trial, we plan to further the development of Nolazol to support filing for marketing and commercialization approval initially in adults in the United States, followed by children and adolescents. We intend to have an End of Phase 2 meeting with the FDA no earlier than the fourth quarter of 2021.
Our Phase 3 clinical trial is planned to evaluate two doses of Nolazol in approximately 260 adults with ADHD, with subjects randomized to receive Nolazol or placebo for 6 weeks. The primary endpoint will be the change from baseline in the ADHD-RS-5 score, which was the primary endpoint in our Phase 2 study. Our Phase 3 clinical trial could be to evaluate three doses of Nolazol in children and adolescents, with an embedded placebo-controlled sub-study in a laboratory classroom setting for the children age group. A laboratory classroom study provides a simulation of a real academic environment, including the potential for interaction and distraction among children, and allows for assessment by trained observers over the course of a typical extended school day.
Commercialization
Given our stage of development, we do not currently have an established internal sales, marketing or distribution infrastructure. If our product candidates are approved for marketing, we intend to commercialize our product candidates either alone or in partnership with others, where appropriate, to maximize the value of our product candidates. We expect to build our commercial infrastructure using a focused and efficient approach, initially, establishing market access, sales and marketing capabilities in a targeted manner that is appropriate for the relevant product opportunity. We believe that this approach will allow us to effectively reach patients and physicians and to maximize the commercial potential of our product candidates
In February 2019, we entered into the EF License Agreement with Eurofarma, a Brazilian pharmaceutical company with a presence in 20 Latin American countries, to develop and commercialize our product candidate, Nolazol, in Latin American countries. Eurofarma operates in the areas of medical prescription, over-the-counter, generic, hospital, bids, oncology, veterinary and services for third parties’ segments. In Brazil, their portfolio is composed of more than 330 brands, with around 1,000 presentations covering 28 medical specialties and 48 therapeutic classes. See “License Agreements - Exclusive License Agreement with Eurofarma” for additional information.
Research and Development
We are conducting research and development activities to expand the commercial potential of both Quilience and Nolazol, while continuing to examine the development of other CNS disorders and compounds that could serve as effective treatments. We sponsor and conduct clinical research activities with investigators and institutions to measure key clinical outcomes that are necessary in order for us to be able to file an NDA with the FDA and equivalent filings with other regulatory authorities. Our research and development efforts are focused primarily in the following areas and serve as a basis for future development, if any, of a more diverse product pipeline, of which certain product candidate leads are in early development stages.

In addition to these ongoing efforts, in order to further our research and development efforts, in December 2019, we entered into a feasibility development agreement, or the Development Agreement, with Adare Pharmaceuticals, Inc., or Adare, pursuant to which we intend to utilize Adare’s proprietary modified release technologies in the development of mazindol for use in our product candidates used for the treatment of narcolepsy and ADHD, or the Adare Indications. The formulation to be developed by Adare under this Development Agreement is intended to be owned solely by us. If we and Adare complete the research and development activities proscribed for in the Development Agreement, the parties are expected to negotiate a separate manufacturing agreement, which is expected to provide Adare with the sole manufacturing and supply rights to us for the Adare Indications for 10 years, pursuant to which we will be required to pay certain milestone payments. We have yet to commence any activities with Adare under the Development Agreement, including the payment of upfront fees to Adare, and there is no guarantee that we will ever commence any of the activities provided for under the Development Agreement.
Manufacturing and Suppliers
We do not own or operate manufacturing or distribution facilities for the production of our product candidates and we currently rely, and expect to continue to rely, on third parties for the manufacturing, packaging, labeling and distribution of our product candidates for pre-clinical and clinical testing, as well as for future commercial manufacturing, if our product candidates receive marketing approval. We require all of our contract manufacturing organizations to conduct manufacturing activities in compliance with cGMP requirements and although we rely on manufacturers, we have engaged with consultants with significant technical, manufacturing, analytical, quality, regulatory, including cGMP, and project management experience to oversee our third-party manufacturers. This approach allows us to maintain a more efficient infrastructure while enabling us to focus our expertise on developing and commercializing our product candidates. Reliance on third-party providers may expose us to more risk than if we were to manufacture product candidates ourselves.
In December 2019, we entered into an agreement with Cambrex High Point for the production of our drug substance, pursuant to which, upon completion of purchase orders, Cambrex High Point may manufacture mazindol for us. We obtain our supply of the finalized drug product from another third-party manufacturer pursuant to purchase order between the parties. We do not currently have any contracts binding us to use supply or production services provided for under such agreements. We expect to continue to rely on third-party manufacturers to produce sufficient quantities of our product candidates and their component raw materials for use in our internal research efforts and clinical trials and in relation to any future commercialization of our product candidates. Our third-party manufacturers are responsible for obtaining the raw materials necessary to manufacture our product candidates, which we believe are readily available from more than one source. Additional third-party manufacturers are and will be used to formulate, fill, label, package and distribute investigational drug products and eventually our products, if and when our product candidates receive approval. This approach allows us to maintain a more efficient infrastructure while enabling us to focus our expertise on developing and commercializing our product candidates. We believe that our current supplier and manufacturers have the capacity to support both clinical supply and commercial-scale production, but we do not have any formal agreements at this time for such supply and production, and we may also elect to enter into agreements with additional or alternative parties in the future.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies and intense competition. While we believe that our knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize may compete with existing therapies and new therapies that may become available in the future.
Many of our competitors have far greater marketing and research capabilities than us. We also face potential competition from academic institutions, government agencies and private and public research institutions, among others, which may in the future develop products to treat those diseases that we currently or, in the future, seek to treat. All of these companies and institutions may have product candidates in development that are or may become superior to Quilience and Nolazol. Our commercial opportunity would be reduced significantly if our competitors develop and commercialize products that are safer, more effective, more convenient, have fewer side effects or are less expensive than either or both of Quilience or Nolazol. Public announcements regarding the development of competing drugs could adversely affect the commercial potential of either or both of Quilience and Nolazol.
Narcolepsy
We face competition from established pharmaceutical and biotechnology companies that currently market products for the treatment of symptoms in narcolepsy. There is no cure and many patients report that their medicines do not improve their complete range of symptoms. For the treatment of both EDS and cataplexy, we believe that currently our only competitor is Jazz Pharmaceuticals plc (Xyrem). Although only indicated for EDS, our competitors also include Novartis Ltd. (Ritalin), Teva Pharmaceutical Industries Ltd. (Modafinil/Armodafinil (Provigil/Nuvigil), Jazz Pharmaceuticals plc (Xyrem and Sunosi), and Harmony Biosciences LLC (Wakix) as well as amphetamines, such as Adderall and Dexedrine. Other early stage compounds currently under development include JZP-258 (Jazz Pharmaceuticals plc), TAK-925 (Takeda Pharmaceutical Company Ltd.) and Inexia/Orexia (Sosei Group Corp.).
The below table highlights the limitations of certain drug products approved for use in the United States by the FDA for treatment of EDS or cataplexy.
| Product | | Cataplexy Approval | | Risk of
Abuse
Diversion | | Limitations |
Amphetamines (Adderall, Dexedrine) | | No | | High: CII | | ● “Grandfathered” approval only (pre-1938 indication) for Immediate Release (IR) | | ● Tolerance and rebound hypersomnolence ● Short-acting |
Methylphenidate (Ritalin) | | No | | High: CII | | ● “Grandfathered” approval only (pre-1938 drug) for IR | | ● Tolerance and rebound hypersomnolence ● Short-acting |
Solriamfetol (Sunosi) | | No | | Low: CIV | | ● Efficacy for controlling cataplexy not available | | ● Risk for drug induced high-blood pressure that can go undetected |
Modafinil / Armodafinil (Provigil/Nuvigil) | | No | | Low: CIV | | ● Potential for serious rash, including Stevens-Johnson syndrome ● Cases of major fetal congenital malformations | | ● Substrate, inducer, and inhibitor of CYP450 isoenzymes, which significantly increases the risks for drug-drug interactions |
Pitolisant (Wakix, Bioprojet) | | No | | Not determined | | ● Approved by FDA in August 2019 but not yet available ● Efficacy for controlling cataplexy not replicated ● Pitolisant may reduce efficacy of oral contraceptives, and so alternative methods of contraception should be utilized | | ● Unclear efficacy; partially failed Phase 3 program |
Sodium oxybate (Xyrem, Jazz) | | Yes | | High: CIII, CI penalties for diversion | | ● Very short acting; requiring two nighttime doses ● Not effective against EDS, so used in combination with stimulants or modafinil ● Because of the risks of depression, abuse, and misuse, Xyrem is available only through a restricted distribution program called the Xyrem REMS Program ● Potential life-threatening adverse effects | | ● Can take up to 3+ months for response ● Known as the “date rape drug” ● Xyrem is a CIII controlled substance as sodium salt of gamma hydroxybutyrate (GHB), a Schedule I controlled substance. Abuse or misuse of illicit GHB is associated with CNS adverse reactions (acting as a CNS depressant), including seizures, respiratory depression, decreased consciousness, coma, and death |
The below table highlights the limitations of drug products approved for use in certain European countries by applicable regulators for treatment of EDS or cataplexy.
| Product | | Cataplexy Approval | | Risk of Abuse Diversion | | Limitations |
Modafinil (Provigil, Cephalon; now generic) Approved via centralized procedure, or CP, in 2005 Modafinil is indicated in adults for the treatment of excessive sleepiness associated with narcolepsy with or without cataplexy | | No | | Low: CIV | | ● Known potential for serious rashes, including Stevens-Johnson syndrome ● Use other than narcolepsy stopped by CHMP (EU Committee for Medicinal Products for Human Use) in 2010 for safety concerns, relating to psychiatric disorders, as well as skin and subcutaneous tissue reactions, significant off-label use and potential for abuse ● Associated with cases of major fetal congenital malformations (Dear Doctor letter June 2019, advises against use in pregnancy) | | ● Substrate, inducer, and inhibitor of CYP450 isoenzymes, which significantly increases the risks for drug-drug interactions ● In 2007, CHMP’s Pharmacovigilance Working Party (PhVWP) strengthened product warnings on thought disorders (suicidal thoughts, mania and symptoms of psychosis such as delusion) and skin reactions, including severe reactions such as Stevens-Johnson syndrome |
Sodium oxybate (Xyrem, UCB Pharma/Jazz) Approved CP 2005 Treatment of narcolepsy with cataplexy in adult patients | | Yes | | High: CII, CI penalties for diversion | | ● Very short acting; requiring two separated nighttime doses ● Not effective against EDS, in most cases associated with modafinil (Provigil) ● Nocturia warning added to SmPC (Summary of Product Characteristics) by the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in 2018 | | ● Can take up to 3+ months for response ● 78%-85% of patients in Phase 3 trials for Xyrem® required stimulant therapy to manage their symptoms ● Also known as the “date rape drug” |
Pitolisant (Wakix, Bioprojet) Approved CP 2016 Indicated in adults for the treatment of narcolepsy with or without cataplexy | | Yes | | Not determined | | ● Efficacy for controlling cataplexy not replicated ● Pitolisant may reduce efficacy of oral contraceptives, and so alternative methods of contraception should be utilized | | ● Unclear efficacy; partially failed Phase 3 program |
ADHD
We face competition from established pharmaceutical and biotechnology companies that currently market a range of CII stimulants to treat ADHD. Our primary competitors include Takeda Pharmaceutical Company Ltd. (Vyvanse, Adderall and Mydayis), Neos Therapeutics Ltd. (Adzenys XR-ODT and Contempla XR-ODT), Eli Lilly & Co. (Strattera), Novartis AG (Focalin) and Janssen Pharmaceutica N.V., a subsidiary of Johnson & Johnson (Concerta). The below table provides a more in depth breakdown of Nolazol against certain competing pharmaceutical products, based on the current scheduling of mazindol by the DEA.
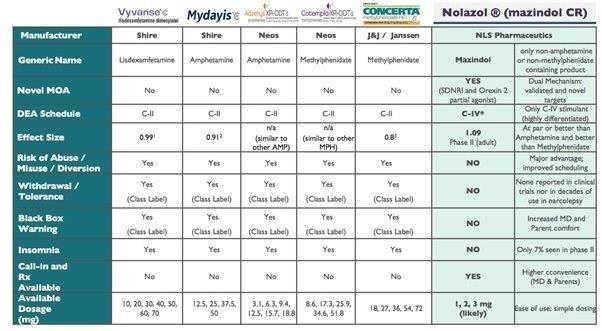
Intellectual Property
We seek patent protection in the United States and internationally for Quilience and Nolazol and other product candidates that we may seek to develop. Our policy is to pursue, maintain and defend patent rights developed internally and to protect the technology, inventions and improvements that are commercially important to the development of our business.
We have developed a robust patent portfolio in the United States, Europe, and other major countries (e.g., Canada, Australia, China, Japan, Latin America countries). Our patent portfolio for Quilience and Nolazol currently includes issued patents in the United States and Europe covering the use of mazindol for treatment of ADHD and patent applications filed in major countries to protect our proprietary controlled release formulation for treatment of ADHD and narcolepsy. One of our patents in the United States covering the use of mazindol for the treatment of ADHD received patent term adjustment, thereby extending the patent term of such patent to August 2028. Our patent covering mazindol controlled release, if issued, will expire in March 2037.
The following table provides a description of our key patents and patent applications and is not intended to represent an assessment of claims, limitations or scope of those patents listed. In some cases, a jurisdiction is listed as both pending and granted for a single patent family. This is due to pending continuation or divisional applications of the granted case.
| Patent Name and Application Number | | Pending Jurisdictions | | Granted Jurisdictions | | Expiry Date | | Type |
Mazindol combination in the treatment of ADHD PCT/EP2007/053512 | | United States China | | France United States Europe Canada Australia Israel New Zealand Morocco | | April 11, 2027 (August 22, 2028 for a U.S. patent receiving patent term adjustment of 499 days) | | Method of use |
Lauflumide and the enantiomers thereof, method for preparing same and therapeutic uses thereof PCT/EP2012/050881 | | | | France United States Europe Canada Israel | | January 20, 2032 | | Method of use (United States), compound
(ex-US) |
Phacetoperane for the treatment of ADHD PCT/FR2012/052749 | | United States | | France United States Europe Japan China Israel Canada | | November 29, 2032 | | Method of use |
A mazindol ir/sr multilayer tablet and its use for the treatment of ADHD* PCT/IB2017/000352 *includes narcolepsy and IH | | United States Europe Canada Australia Brazil China Israel Japan South Korea Mexico New Zealand Hong Kong Morocco | | Not Applicable | | March 8, 2037 | | Formulation |
| Mazindol treatment for heroin dependence and substance use disorder PCT/IB2018/001138 | | United States Europe Canada Brazil China Japan South Korea Mexico | | Not Applicable | | September 6, 2038 | | Method of use |
We cannot be sure that any patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, or that we will develop additional proprietary products that are patentable. There is also a significant risk that any issued patents will have substantially narrower claims than those that are currently sought or will not be challenged by any third parties, or that the patents of others will not prevent the commercialization of products incorporating our technology. Even with respect to any patents that may be issued to us, we cannot be sure that any such patents will be commercially useful in protecting our technology. Furthermore, we cannot assure that others will not independently develop similar products, duplicate any of our products, or design around our patents. U.S. patent applications are not immediately made public, so we might be surprised by the grant to someone else of a patent on a technology we are actively using.
In addition to our patents and patent applications, we also rely on unpatented trade secrets, know-how, and continuing technological innovation to develop and maintain our competitive position. We seek to protect our ownership of know-how and trade secrets through an active program of legal mechanism including invention assignments, confidentiality agreements, material transfer agreements, research collaborations and licenses to protect our product candidates. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions. For a more comprehensive discussion of the risks related to our intellectual property, please see “Risk Factors — Risks Related to Our Intellectual Property.”
License Agreements
Pegasus Advanced Research SAS License Agreement
In February 2015, Pegasus Advanced Research, then named NeuroLife Sciences SAS, or Pegasus, a company owned by certain of our founders, who are also shareholders, including Eric Konofal, Eric-Jean Desbois, Bruno Figadere and our Chief Executive Officer, Alexander Zwyer, or the Pegasus Founders, entered into a license agreement with AP-HP, or the AP-HP License Agreement, to obtain a worldwide, sub-licensable license, covering four different compounds, which included the use of mazindol for the treatment of ADHD, or the Licensed Compounds. As part of the AP-HP License Agreement, Pegasus was given an option to purchase the Licensed Compounds. In February 2016, after the AP-HP License Agreement was assigned and transferred to NLS-1, pursuant to an Assignment and Transfer Agreement, or the ATA, NLS-1 purchased the Licensed Compounds from AP-HP for an aggregate consideration of approximately 2.65 million euros, including reimbursement of certain expenses. On April 1, 2017 and September 20, 2019, the parties entered into subsequent amendments of the ATA, or the First Amendment to the ATA and the Second Amendment to the ATA, respectively. Under the Second Amendment to the ATA, NLS agreed to pay Pegasus a royalty of 1.8% of annual net sales (including sublicensee sales) realized upon the commercialization of products developed on the basis of the Licensed Compounds during the terms of their respective patents; provided, however, that under certain circumstances, the rate of the royalty payment will decrease. For instance, if a competing generic product using mazindol for the treatment of ADHD were to become available during the term of the patents covering the Licensed Compounds, there would be no royalties paid to Pegasus.
Exclusive License Agreement with Eurofarma
In February 2019, we entered into the EF License Agreement. The EF License Agreement provides Eurofarma with an exclusive, fee-bearing, non-transferrable (i) distribution right to distribute Nolazol in Latin America and an (ii) exclusive, fee-bearing, non-transferrable license to our patents and trademarks in connection with the commercialization, if any, of Nolazol in Latin America. The EF License Agreement is in effect until the later of either (i) ten years from the date of its execution, or until February 2029, or (ii) until the expiration of the last valid patent relating to Nolazol, subject to early termination under certain circumstances.
Pursuant to the terms of the EF License Agreement, we are responsible for obtaining regulatory approval to market and commercialize Nolazol in the United States and Eurofarma shall be responsible for obtaining regulatory approval in South America; provided, however, that Eurofarma shall inform us of any additional information that regulators in Latin America may require in order to seek marketing authorization which otherwise may not be required by the FDA, or the Supplemental U.S. Data. Although pursuant to the EF License Agreement we will undertake the efforts to generate the Supplemental U.S. Data, the parties have agreed to share the costs associated with generating such data. Eurofarma will be responsible for seeking and covering the costs relating to obtaining the required regulatory approvals in Latin America and, once approved on a country-by-country basis, for commercializing Nolazol in Latin America. Furthermore, the marketing approvals obtained in Latin America, if any, shall be owned by Eurofarma until the end of the term of the EF License Agreement.
Upon the execution of the EF License Agreement, Eurofarma paid us $2.5 million. In accordance with the EF License Agreement, we are eligible to receive milestone payments as well as royalties from Eurofarma, which consist of the following:
Clinical Milestones
| Upon successful completion by us of Phase 3 clinical trials for the treatment of ADHD in adults in the United States | | $ | 500,000 | |
| Upon successful completion by us of Phase 3 clinical trials for the treatment of ADHD in children in the United States | | $ | 500,000 | |
Regulatory Milestones
| Upon price approval of the licensed product in Brazil by the relevant agency | | $ | 1,000,000 | |
| Upon receipt of marketing approval by the relevant agency of the licensed product in any other country in Latin America | | $ | 1,000,000 | |
Sales Milestones (single payments)
| Upon reaching annual net sales of $10 million | | $ | 1,000,000 | |
| Upon reaching annual net sales of $50 million | | $ | 2,000,000 | |
| Upon reaching annual net sales of $75 million | | $ | 4,000,000 | |
| Upon reaching annual net sales of $100 million | | $ | 6,000,000 | |
Royalties
| Annual net sales in Latin America | | Royalties in percent of Net Sales | |
| Under $10 million | | | 7 | % |
| $10 million to less than $20 million | | | 8 | % |
| $20 million to less than $30 million | | | 9 | % |
| $30 million and above | | | 10 | % |
Government Regulation and Product Approvals
Government authorities in the United States, at the federal, state and local levels, and in other countries and jurisdictions, including the EU, extensively regulate, among other things, the research, development, testing, manufacture, sales, pricing, reimbursement, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of biopharmaceutical products. The processes for obtaining marketing approvals in the United States and in foreign countries and jurisdictions, along with compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Approval and Regulation of Drugs in the United States
In the United States, drug products are regulated under the FDCA and implementing regulations. Drug products are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable regulatory requirements at any time during the drug product development process, the approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions may include, but are not limited to, the FDA’s refusal to allow an applicant to proceed with clinical trials, refusal to approve pending applications, warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution, adverse publicity, injunctions, fines and civil or criminal investigations and penalties brought by the FDA, the Department of Justice or other government entities, including state agencies.
The process required by the FDA before a drug may be approved and marketed in the United States generally involves the following:
| | ● | completion of pre-clinical laboratory tests, pre-clinical animal studies and formulation studies, in accordance with the FDA’s GLP regulations and other applicable regulations; |
| | ● | submission of an IND, which must become effective before human clinical trials may begin; |
| | ● | approval by an IRB representing each clinical site before each clinical trial may be initiated; |
| | ● | performance of adequate and well-controlled human clinical trials in accordance with applicable regulations, including the FDA’s GCP regulations, to establish the safety and efficacy of the proposed drug for its proposed indication; |
| | ● | submission to the FDA of an NDA for a new drug; |
| | ● | satisfactory completion of an FDA advisory committee review, if applicable; |
| | ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities, where the drug is produced to assess compliance with the FDA’s cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
| | ● | potential FDA audit of pre-clinical and/or clinical trial sites that generated the data in support of the NDA; and |
| | ● | FDA review and approval of the NDA prior to any commercial marketing or sale of the drug in the United States. |
Before an applicant begins testing a product candidate with potential therapeutic value in humans, the product candidate enters the pre-clinical testing stage. Pre-clinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and toxicity of the product candidate. The conduct of the pre-clinical tests must comply with federal regulations and requirements, including GLPs. The sponsor must submit the results of the pre-clinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. An IND is a request for authorization from the FDA to administer an investigational drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human trials. Some pre-clinical testing may continue even after the IND is submitted.
The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions regarding the proposed clinical trials and places the IND on clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. The FDA may also impose clinical holds on a product candidate at any time before or during clinical trials due to safety concerns or non-compliance.
Clinical trials involve the administration of the product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety and assess efficacy. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries.
Human clinical trials are typically conducted in three phases that may overlap or be combined:
| | ● | Phase 1. The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion, the side effects associated with increasing doses and if possible, to gain early evidence of effectiveness. In the case of some product candidates for severe or life-threatening diseases, especially when the product candidate may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| | ● | Phase 2. The product candidate is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases or conditions and to determine dosage tolerance, optimal dosage and dosing schedule. |
| | ● | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety of the product candidate in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall benefit/risk ratio of the product candidate and provide an adequate basis for product candidate approval. Generally, at least two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA. |
Post-approval studies, or Phase 4 clinical trials, may be conducted after initial marketing approval is granted. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access to certain data from the trial.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
A sponsor may choose, but is not required, to conduct a foreign clinical study under an IND. When a foreign clinical study is conducted under an IND, all IND requirements must be met unless waived by the FDA. When a foreign clinical study is not conducted under an IND, the sponsor must ensure that the study complies with certain regulatory requirements of the FDA in order to use the study as support for an IND or NDA. Foreign clinical studies not conducted under an IND must be conducted in accordance with GCPs, including undergoing review and receiving approval by an independent ethics committee, or IEC, and seeking and receiving informed consent from subjects. The FDA’s regulations are intended to help ensure the protection of human subjects enrolled in non-IND foreign clinical studies, as well as the quality and integrity of the resulting data.
U.S. Review and Approval Processes
The results of product development, pre-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. Data may come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, such as literature and data from real-world use. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational drug product to the satisfaction of the FDA. The submission of an NDA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances.
In addition, the Pediatric Research Equity Act, or PREA, requires a sponsor to conduct pediatric clinical trials for most drugs, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs and supplements must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data needs to be collected before the pediatric clinical trials begin. The FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation. Unless otherwise required by regulation, the Pediatric Research Equity Act does not apply to any drug for an indication for which orphan designation has been granted. However, if only one indication for a product has orphan designation, a pediatric assessment may still be required for any applications to market that same product for the non-orphan indication(s).
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information rather than accepting an NDA for filing. The FDA must make a decision on accepting an NDA for filing within 60 days of receipt. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the Prescription Drug User Fee Act, or PDUFA, guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA is submitted to FDA because the FDA has approximately two months to make a “filing” decision after it the application is submitted The FDA does not always meet its PDUFA goal dates for standard and priority NDAs, and the review process is often significantly extended by FDA requests for additional information or clarification.
After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions and typically follows the advisory committee’s recommendations.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical sites to assure compliance with GCP requirements. After the FDA evaluates the application, manufacturing process and manufacturing facilities, it may issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application will not be approved in its present form. A Complete Response Letter usually describes all of the specific deficiencies in the NDA identified by the FDA. The Complete Response Letter may require additional clinical data and/or (an) additional Phase 3 clinical trial(s), and/or other significant and time-consuming requirements related to clinical trials, pre-clinical studies or manufacturing. If a Complete Response Letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling or may condition the approval of the NDA on other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post-market studies or clinical trials. For example, the FDA may require Phase 4 testing, which involves clinical trials designed to further assess a drug safety and effectiveness, and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. The FDA may also determine that a REMS is necessary to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS; the FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States or, if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making a drug product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan designation must be requested before submitting an NDA. After the FDA grants orphan designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity or inability to manufacture the product in sufficient quantities. The designation of such drug also entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If an orphan designated product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan exclusivity.
Expedited Development and Review Programs
The FDA’s Fast Track Designation program is intended to expedite or facilitate the process for reviewing new drug products that meet certain criteria, or Fast Track Designation. Specifically, new drugs are eligible for Fast Track Designation if they are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Unique to a product candidate seeking Fast Track Designation, the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
Any product submitted to the FDA for approval, including a product with a Breakthrough Therapy or Fast Track Designation, may also be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. A product is eligible for priority review if it has the potential to provide safe and effective therapy for a serious condition where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a serious condition compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug designated for priority review in an effort to facilitate the review. In addition, a product may be eligible for accelerated approval. Drug products intended to treat serious or life-threatening diseases or conditions may be eligible for accelerated approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Fast Track Designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
A sponsor may seek FDA designation of a product candidate for Breakthrough Therapy Designation if the drug is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes intensive FDA interaction and guidance. If a provided with Breakthrough Therapy Designation, the FDA will expedite the development and review of such drug. Breakthrough Therapy Designation includes all of the Fast Track Designation program features, as well as more intensive FDA interaction and guidance. The Breakthrough Therapy Designation is a distinct status from both accelerated approval and priority review, which can also be granted to the same drug if relevant criteria are met. If a product is designated with a Breakthrough Therapy Designation, the FDA will work to expedite the development and review of such drug.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. In addition, this designation may not provide a material commercial advantage.
Post-Approval Requirements
Any drug products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, restrictions on promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling (known as “off-label use”), limitations on industry-sponsored scientific and educational activities, and requirements for promotional activities involving the internet. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses.
In addition, quality control and manufacturing procedures must continue to conform to applicable manufacturing requirements after approval to ensure the long term stability of the drug product. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products in accordance with cGMP regulations. cGMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of records and documentation and the obligation to investigate and correct any deviations from cGMP. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved NDA, including, among other things, recall or withdrawal of the product from the market. In addition, changes to the manufacturing process are strictly regulated, and depending on the significance of the change, may require prior FDA approval before being implemented. Other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
The FDA also may require post-marketing testing, known as Phase 4 testing, and surveillance to monitor the effects of an approved product. Discovery of previously unknown problems with a product or the failure to comply with applicable FDA requirements can have negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties, among others. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
Expanded Access to an Investigational Drug for Treatment Use
Expanded access, also called “compassionate use,” is the use of investigational new drug products outside of clinical trials to treat patients with serious or immediately life-threatening diseases or conditions when there are no comparable or satisfactory alternative treatment options. The FDA regulations allow access to investigational drugs under an IND by the sponsor or the treating physician for treatment purposes on a case-by-case basis for individual patients, intermediate-size patient populations, and larger populations for use of the drug under a treatment protocol or Treatment IND Application.
The suitability of treating a patient or a group of patients under expanded access is determined by the following: if patient(s) have a serious or immediately life-threatening disease or condition, there is no comparable or satisfactory alternative therapy to diagnose, monitor, or treat the disease or condition, the potential patient benefit justifies the potential risks of the treatment and the potential risks are not unreasonable in the context or condition to be treated, and the expanded use of the investigational drug for the requested treatment will not interfere with the initiation, conduct or completion of clinical investigations that could support marketing approval of the product candidate or otherwise compromise the potential development of the product candidate.
In 2016, the 21st Century Cures Act established (and the 2017 Food and Drug Administration Reauthorization Act later amended) a requirement that sponsors of one or more investigational drugs for the treatment of a serious disease(s) or condition(s) make publicly available their policies for evaluating and responding to requests for expanded access for individual patients. This provision requires drug companies to make publicly available their policies for expanded access for individual patient access to products intended for serious diseases. Sponsors are required to make such policies publicly available upon the earlier of initiation of a Phase 2 or Phase 3 study or 15 days after the drug receives Breakthrough Therapy Designation, Fast Track Designation product, or regenerative medicine advanced therapy. Additionally, in 2018 the Right to Try Act, was signed into law. The law, among other things, provides a federal framework for certain patients to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA permission under the FDA expanded access program. There is no obligation for a drug manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act, but the manufacturer must develop an internal policy and respond to patient requests according to that policy.
Section 505(b)(2) NDAs
NDAs for most new drug products are based on two full clinical studies that must contain substantial evidence of the safety and efficacy of the proposed new drug product for the proposed indication. These applications are submitted under Section 505(b)(1) of the FDCA. The FDA is, however, authorized to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. This type of application allows the applicant to rely, in part, on the FDA’s previous findings of safety and efficacy for a similar drug product or on appropriately supporting literature, and therefore may provide an alternate and potentially more expeditious pathway to FDA approval for new formulations or new uses of previously approved drug products.
Specifically, Section 505(b)(2) applies to NDAs for a drug for which the investigations “were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted” and authorizes the FDA to approve an NDA based on safety and efficacy data that were not developed by or for the applicant. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, the applicant may eliminate the need to conduct certain pre-clinical or clinical studies of the new drug product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved drug product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced drug product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Hatch-Waxman Act, Congress established an abbreviated regulatory scheme authorizing the FDA to approve generic drugs that are shown to contain the same active moiety as, and to be bioequivalent to, drugs previously approved by the FDA pursuant to NDAs. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application, or ANDA, to the agency. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the API, bioequivalence, drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data and quality control procedures. ANDAs are “abbreviated” because they generally do not include pre-clinical or clinical data to demonstrate safety and effectiveness. Instead, in support of such applications, a generic manufacturer may rely on the pre-clinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference-listed drug, or RLD.
Specifically, in order for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect to the active moiety, the route of administration, the dosage form, the strength of the drug and the conditions of use of the drug. At the same time, the FDA must also determine that the generic drug is “bioequivalent” to the innovator drug. Under the statute, a generic drug is bioequivalent to a RLD if “the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug.” Upon approval of an ANDA, the FDA indicates whether the generic product candidate is “therapeutically equivalent” to the RLD in its publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” also referred to as the “Orange Book.” Physicians and pharmacists consider a therapeutic equivalent generic drug to be fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA’s designation of therapeutic equivalence often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or the patient.
Under the Hatch-Waxman Act, the FDA may not approve an ANDA until any applicable period of non-patent exclusivity for the RLD has expired. The FDCA provides a period of five years of non-patent data exclusivity for a new drug containing a NCE. For the purposes of this provision, an NCE is a drug that contains no active moiety that has previously been approved by the FDA in any other NDA. An active moiety is the molecule or ion responsible for the physiological or pharmacological action of the drug substance. In cases where such NCE exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product candidate approval.
The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication. Three-year exclusivity would be available for a drug product that contains a previously approved active moiety, provided the statutory requirement for a new clinical investigation is satisfied. Unlike five-year NCE exclusivity, an award of three-year exclusivity does not block the FDA from accepting ANDAs seeking approval for generic versions of the drug as of the date of approval of the original drug product. The FDA typically makes decisions about awards of data exclusivity shortly before a product candidate is approved.
The FDA must establish a priority review track for certain generic drugs, requiring the FDA to review a drug application within eight months for a drug that has three or fewer approved drugs listed in the Orange Book and is no longer protected by any patent or regulatory exclusivities, or is on the FDA’s drug shortage list. The new legislation also authorizes FDA to expedite review of “competitor generic therapies” or drugs with inadequate generic competition, including holding meetings with or providing advice to the drug sponsor prior to submission of the application.
Hatch-Waxman Act Patent Certification and the 30-Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s drug product or an approved method of using the drug product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the referenced drug product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved drug product in the Orange Book to the same extent that an ANDA applicant would.
Specifically, the applicant must certify with respect to each patent that:
| | ● | the required patent information has not been filed; |
| | ● | the listed patent has expired; |
| | ● | the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or |
| | ● | the listed patent is invalid, is unenforceable or will not be infringed by the new drug product. |
A certification that the new drug product will not infringe the already approved drug product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicates that it is not seeking approval of a patented method of use, the application will not be approved until all of the listed patents claiming the referenced drug product have expired (other than method of use patents involving indications for which the applicant is not seeking approval).
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earliest of 30 months after the receipt of the Paragraph IV notice, expiration of the patent and a decision in the infringement case that is favorable to the ANDA applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved drug product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a Section 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced drug product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of an NCE, listed in the Orange Book for the referenced drug product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earliest of 30 months, settlement of the lawsuit and a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Act, which permits a patent restoration of up to five years for patent term lost during product candidate development and FDA regulatory review. The restoration period granted on a drug patent covering is typically one-half of the time between the effective date of a clinical investigation involving human beings is begun and the submission date of an application, plus the time between the submission date of an application and the ultimate approval date. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the drug product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple drug products for which approval is sought can only be extended in connection with one of the approvals. The USPTO reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
Controlled Substances
The CSA and its implementing regulations establish a “closed system” of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA. The DEA is the federal agency responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
DEA registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized to be handled under that registration.
The DEA categorizes controlled substances into one of five schedules: CI, CII, CIII, CIV, or CV, with varying qualifications for listing in each schedule. CI substances by definition have a high potential for abuse, have no currently “accepted medical use” in treatment in the United States and lack accepted safety for use under medical supervision. They may be used only in federally approved research programs and may not be marketed or sold for dispensing to patients in the United States. Pharmaceutical products having a currently accepted medical use that are otherwise approved for marketing may be listed as CII, CIII, CIV or CV substances, with CII substances presenting the highest potential for abuse and physical or psychological dependence, and CV substances presenting the lowest relative potential for abuse and dependence. Mazindol, the substance in our lead product candidates Quilience and Nolazol, is listed by the DEA as a CIV substance under the CSA. The regulatory requirements are more restrictive for CII substances than CIII, CIV, and CV substances. For example, all CII drug prescriptions must be signed by a physician, physically presented to a pharmacist in most situations, and cannot be refilled.
The DEA typically inspects certain facilities to review their security controls, recordkeeping and reporting prior to issuing a registration. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to CI and CII substances. Security measures required by the DEA include background checks on employees and physical control of inventory through measures such as vaults, cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances, and periodic reports made to the DEA. Reports must also be made for thefts or losses of any controlled substance, suspicious orders, and to obtain authorization to destroy any controlled substance. In addition, special authorization and notification requirements may apply to imports and exports.
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within CI and CII that may be manufactured or produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. This limited aggregate amount that the DEA allows to be produced in the United States each year is allocated among individual companies, which, in turn, must annually apply to the DEA for individual manufacturing and procurement quotas. The quotas apply equally to the manufacturing of the API production of dosage forms. The DEA may adjust aggregate production quotas a few times per year, and individual manufacturing or procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments for individual companies.
The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Failure to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in enforcement action that could have a material adverse effect on our business, results of operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could result in criminal proceedings.
Individual states also regulate controlled substances, and we and our contract manufacturers will be subject to state regulation on distribution of these drug products, including licensing, recordkeeping, and security.
Controlled substances are also regulated pursuant to several international drug control treaties. The United States is a signatory to these treaties and thus must conform its laws and regulations to the international requirements, which generally include licensing, recordkeeping and reporting requirements. Mazindol is currently included in Schedule IV of the 1971 Convention on Psychotropic Substances. Any change in the international treaties regarding classification of mazindol could affect regulation of this substances in the United States and in other countries.
Health Care Laws and Regulations
Health care providers and third-party payors play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with providers, consultants, third-party payors and customers are subject to broadly applicable fraud and abuse, anti-kickback, false claims laws, patient privacy laws and regulations and other health care laws and regulations that may constrain business and/or financial arrangements. Restrictions under applicable federal and state health care laws and regulations include the following:
| | ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, paying, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid; |
| | ● | the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false, fictitious or fraudulent or knowingly making, using or causing to made or used a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| | ● | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program or making false statements relating to health care matters; |
| | ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their respective implementing regulations, including the Final Omnibus Rule published in January 2013, which impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| | ● | the federal false statements statute, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for health care benefits, items or services; |
| | ● | the FCPA which prohibits companies and their intermediaries from making, or offering or promising to make improper payments to non-U.S. officials for the purpose of obtaining or retaining business or otherwise seeking favorable treatment; |
| | ● | the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the PPACA, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services within the United States Department of Health and Human Services, information related to payments and other transfers of value made by that entity to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and |
| | ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to health care items or services that are reimbursed by non-government third-party payors, including private insurers. |
Further, some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. Additionally, some state and local laws require the registration of pharmaceutical sales representatives in the jurisdiction. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Pharmaceutical Insurance Coverage and Health Care Reform
In the United States and markets in other countries, patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated health care costs. Significant uncertainty exists as to the coverage and reimbursement status of drug products approved by the FDA and other government authorities. Thus, even if a product candidate is approved, sales of such product will depend, in part, on the extent to which third-party payors, including government health programs in the United States such as Medicare and Medicaid, commercial health insurers and managed care organizations, provide coverage and establish adequate reimbursement levels for, the product. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product once coverage is approved. Third-party payors are increasingly challenging the prices charged, examining the medical necessity and reviewing the cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product candidate that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable marketing approvals. Nonetheless, product candidates may not be considered medically necessary or cost effective. A decision by a third-party payor not to cover a product candidate could reduce physician utilization once the product candidate is approved. Additionally, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor.
The containment of health care costs also has become a priority of federal, state and foreign governments and the prices of products have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic drug products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit a company’s revenue generated from the sale of any approved drug products. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more drug products for which a company or its collaborators receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
There have been a number of federal and state proposals during the last few years regarding the pricing of pharmaceutical and biopharmaceutical products, limiting coverage and reimbursement for drugs and biologics and other medical products, government control and other changes to the health care system in the United States.
In March 2010, the United States Congress enacted the PPACA, which, among other things, included changes to the coverage and payment for drug products under government health care programs. The PPACA effected the following changes of importance to our potential product candidates:
| | ● | established an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
| | ● | expanded eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability; |
| | ● | expanded manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate for both branded and generic drugs and revising the definition of “average manufacturer price” for calculating and reporting Medicaid drug rebates on outpatient prescription drug prices; |
| | ● | addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; |
| | ● | expanded the types of entities eligible for the 340B drug discount program; |
| | ● | established the Medicare Part D coverage gap discount program by requiring manufacturers to provide a 70% point-of-sale discount off of the negotiated price of applicable brand drugs to eligible beneficiaries during their coverage gap period as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D; and |
| | ● | established a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
Other legislative changes have been proposed and adopted in the United States since the PPACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This included aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments, will remain in effect through 2027 unless additional congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Since enactment of the PPACA, there have been numerous legal challenges and congressional actions to repeal and replace provisions of the law. For example, with enactment of the TCJA, which was signed by the President on December 22, 2017, Congress repealed the “individual mandate.” The repeal of this provision, which requires most Americans to carry a minimal level of health insurance, will become effective in 2019. According to the Congressional Budget Office, the repeal of the individual mandate will cause 13 million fewer Americans to be insured in 2027 and premiums in insurance markets may rise. Additionally, on January 22, 2018, the President signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain PPACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, among other things, amended the PPACA, effective January 1, 2019, to increase from 50% to 70% the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”. Congress will likely consider other legislation to replace elements of the PPACA during the next congressional session.
The current presidential administration has also taken executive actions to undermine or delay implementation of the PPACA. In January 2017, the President signed an Executive Order directing federal agencies with authorities and responsibilities under the PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. In October 2017, the President signed a second Executive Order allowing for the use of association health plans and short-term health insurance, which may provide fewer health benefits than the plans sold through the PPACA exchanges. At the same time, the current presidential administration announced that it will discontinue the payment of cost-sharing reduction, or CSR, payments to insurance companies until Congress approves the appropriation of funds for such CSR payments. The loss of CSR payments is expected to increase premiums on certain policies issued by qualified health plans under the PPACA. A bipartisan bill to appropriate funds for CSR payments was introduced in the Senate, but the future of that bill is uncertain. In addition, the Centers for Medicare & Medicaid Services has recently proposed regulations that would give states greater flexibility in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the PPACA for plans sold through such marketplaces.
Further, there have been several recent U.S. congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the costs of drugs under Medicare and reform government program reimbursement methodologies for drug products. At the federal level, the current presidential administration’s budget proposal for fiscal year 2019 contained further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. While any proposed measures will require authorization through additional legislation to become effective, Congress and the current presidential administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs.
At the state level, individual states are increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs.
Review and Approval of Medicinal Products in the EU
In order to market any medicinal product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of medicinal products. Whether or not it obtains FDA approval for a product candidate, an applicant will need to obtain the necessary approvals by the comparable non-U.S. regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. Specifically, the process governing approval of medicinal products in the EU generally follows the same lines as in the United States. It entails satisfactory completion of pre-clinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. It also requires the submission to the relevant competent authorities of an MAA and granting of a marketing authorization by these authorities before the medicinal product can be marketed and sold in the EU.
Clinical Trial Approval
The Clinical Trials Directive 2001/20/EC, the Directive 2005/28/EC on Good Clinical Practice, and the related national implementing provisions of the individual EU Member States govern the system for the approval of clinical trials in the EU. Under this system, an applicant must obtain prior approval from the competent national authority of the EU Member States in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial at a specific study site after the competent ethics committee has issued a favorable opinion. The clinical trial application must be accompanied by, among other documents, an investigational medicinal product dossier (the Common Technical Document) with supporting information prescribed by Directive 2001/20/EC, Directive 2005/28/EC, where relevant the implementing national provisions of the individual EU Member States and further detailed in applicable guidance documents.
In April 2014, the new Clinical Trials Regulation, (EU) No 536/2014, or the Clinical Trials Regulation, was adopted. The Clinical Trials Regulation was published on June 16, 2014 but is not expected to apply until 2021. The Clinical Trials Regulation will be directly applicable in all the EU Member States, repealing the current Directive 2001/20/EC and replacing any national legislation that was put in place to implement the Directive. Conduct of all clinical trials performed in the EU will continue to be bound by currently applicable provisions until the new Clinical Trials Regulation becomes applicable. The extent to which ongoing clinical trials will be governed by the Clinical Trials Regulation will depend on when the Clinical Trials Regulation becomes applicable and on the duration of the individual clinical trial. If a clinical trial continues for more than three years from the day on which the Clinical Trials Regulation becomes applicable the Clinical Trials Regulation will at that time begin to apply to the clinical trial.
The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the EU. The main characteristics of the regulation include: a streamlined application procedure via a single-entry point, the “EU Portal and Database”; a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed by the appointed reporting EU Member State, whose assessment report is submitted for review by the sponsor and all other competent authorities of all EU Member States in which an application for authorization of a clinical trial has been submitted, or Concerned Member States. Part II is assessed separately by each Concerned Member State. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics committees in the assessment procedure will continue to be governed by the national law of the Concerned Member State. However, overall related timelines will be defined by the Clinical Trials Regulation.
PRIME Designation in the EU
In March 2016, the EMA launched an initiative to facilitate development of product candidates in indications, often rare, for which few or no therapies currently exist. The PRIME scheme is intended to encourage drug development in areas of unmet medical need and provides accelerated assessment of product candidates representing substantial innovation reviewed under the centralized procedure. Product candidates from small- and medium-sized enterprises, or SMEs, may qualify for earlier entry into the PRIME scheme than larger companies. Many benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and accelerated MAA assessment once a dossier has been submitted. Importantly, a dedicated contact and rapporteur from the Committee for Human Medicinal Products, or CHMP, or the Committee for Advanced Therapies is appointed early in the PRIME scheme facilitating increased understanding of the product candidate at EMA’s committee level. A kick-off meeting initiates these relationships and includes a team of multidisciplinary experts at the EMA to provide guidance on the overall development and regulatory strategies.
Marketing Authorization
To obtain a marketing authorization for a product candidate under the EU regulatory systems, an applicant must submit an MAA either under a centralized procedure administered by the EMA or one of the procedures administered by competent authorities in the EU Member States (either a decentralized procedure, national procedure or mutual recognition procedure). A marketing authorization may be granted only to an applicant established in the EU. Regulation (EC) No 1901/2006 provides that prior to obtaining a marketing authorization in the EU, applicants have to demonstrate compliance with all measures included in an EMA-approved PIP covering all subsets of the pediatric population, unless the EMA has granted: (i) a product-specific waiver, (ii) a class waiver or (iii) a deferral for one or more of the measures included in the PIP. We intend to seek such a deferral, specifically for Quilience; however, there is a risk the EMA may not provide us with such a PIP deferral, in which case we would be required to demonstrate compliance with an EMA-approved PIP prior to commercialization of the product candidate for which we sought such a waiver.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid across the European Economic Area (i.e. the EU, as well as Iceland, Liechtenstein and Norway). Pursuant to Regulation (EC) No. 726/2004, the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional. The centralized procedure may also be used in certain other cases at the request of the applicant. We anticipate that the centralized procedure will be mandatory for the product candidates we are developing.
Under the centralized procedure, the CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. If the CHMP accepts such request, the time limit of 210 days will be reduced to 150 days but it is possible that the CHMP can revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment. At the end of this period, the CHMP provides a scientific opinion on whether or not a marketing authorization should be granted in relation to a medicinal product. Within 15 calendar days of receipt of a final opinion from the CHMP, the European Commission must prepare a draft decision concerning an application for marketing authorization. This draft decision must take the opinion and any relevant provisions of EU law into account. Before arriving at a final decision on an application for centralized authorization of a medicinal product the European Commission must consult the Standing Committee on Medicinal Products for Human Use, or the Standing Committee. The Standing Committee is composed of representatives of the EU Member States and chaired by a non-voting European Commission representative. The European Parliament also has a related “droit de regard.” The European Parliament’s role is to ensure that the European Commission has not exceeded its powers in deciding to grant or refuse to grant a marketing authorization.
The European Commission may grant a so-called “marketing authorization under exceptional circumstances.” Such authorization is intended for product candidates for which the applicant can demonstrate that it is unable to provide comprehensive data on efficacy and safety under normal conditions of use, because the indications for which the product candidate in question is intended are encountered so rarely that the applicant cannot reasonably be expected to provide comprehensive evidence, or in the present state of scientific knowledge, comprehensive information cannot be provided, or it would be contrary to generally accepted principles of medical ethics to collect such information. Consequently, marketing authorization under exceptional circumstances may be granted subject to certain specific obligations, which may include the following:
| | ● | the applicant must complete an identified program of studies within a time period specified by the competent authority, the results of which form the basis of a reassessment of the benefit/risk profile; |
| | ● | the medicinal product in question may be supplied on medical prescription only and may in certain cases be administered only under strict medical supervision, possibly in a hospital and in the case of a radiopharmaceutical, by an authorized person; and |
| | ● | the package leaflet and any medical information must draw the attention of the medical practitioner to the fact that the particulars available concerning the medicinal product in question are as yet inadequate in certain specified respects. |
A marketing authorization under exceptional circumstances is subject to annual review to reassess the risk-benefit balance in an annual reassessment procedure. Continuation of the authorization is linked to the annual reassessment and a negative assessment could potentially result in the marketing authorization being suspended or revoked. The renewal of a marketing authorization of a medicinal product under exceptional circumstances, however, follows the same rules as a “normal” marketing authorization. Thus, a marketing authorization under exceptional circumstances is granted for an initial five years, after which the authorization will become valid indefinitely, unless the EMA decides that safety grounds merit one additional five-year renewal.
The European Commission may also grant a so-called “conditional marketing authorization” prior to obtaining the comprehensive clinical data required for an application for a full marketing authorization. Such conditional marketing authorizations may be granted for product candidates (including medicines designated as orphan medicinal products), if (i) the risk-benefit balance of the product candidate is positive, (ii) it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data, (iii) the product fulfills an unmet medical need and (iv) the benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required. A conditional marketing authorization may contain specific obligations to be fulfilled by the marketing authorization holder, including obligations with respect to the completion of ongoing or new studies and the collection of pharmacovigilance data. Conditional marketing authorizations are valid for one year, and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions and/or specific obligations. The timelines for the centralized procedure described above also apply with respect to the review by the CHMP of applications for a conditional marketing authorization.
Unlike the centralized authorization procedure, the decentralized marketing authorization procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU Member State in which the product candidate is to be marketed. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU Member State cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the European Commission, whose decision is binding on all EU Member States.
The mutual recognition procedure similarly is based on the acceptance by the competent authorities of the EU Member States of the marketing authorization of a medicinal product by the competent authorities of other EU Member States. The holder of a national marketing authorization may submit an application to the competent authority of an EU Member State requesting that this authority recognize the marketing authorization delivered by the competent authority of another EU Member State.
Regulatory Data Protection in the EU
In the EU, innovative medicinal products approved on the basis of a complete independent data package qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity pursuant to Directive 2001/83/EC. Regulation (EC) No 726/2004 repeats this entitlement for medicinal products authorized in accordance the centralized authorization procedure. Data exclusivity prevents applicants for authorization of generics of these innovative products from referencing the innovator’s data to assess a generic (abridged) application for a period of eight years. During an additional two-year period of market exclusivity, a generic MAA can be submitted and authorized, and the innovator’s data may be referenced, but no generic medicinal product can be placed on the EU market until the expiration of the market exclusivity. The overall 10-year period will be extended to a maximum of 11 years if, during the first eight years of those 10 years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be an NCE so that the innovator gains the prescribed period of data exclusivity, another company nevertheless could also market another version of the product if such company obtained marketing authorization based on an MAA with a complete independent data package of pharmaceutical tests, pre-clinical tests and clinical trials.
Periods of Authorization and Renewals
A marketing authorization has an initial validity for five years in principle. The marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU Member State. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. The European Commission or the competent authorities of the EU Member States may decide, on justified grounds relating to pharmacovigilance, to proceed with one further five-year period of marketing authorization. Once subsequently definitively renewed, the marketing authorization shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (in case of centralized procedure) or on the market of the authorizing EU Member State within three years after authorization ceases to be valid (the so-called sunset clause).
Pediatric Studies and Exclusivity
Prior to obtaining a marketing authorization in the EU, applicants must demonstrate compliance with all measures included in an EMA-approved PIP covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, a class waiver, or a deferral for one or more of the measures included in the PIP. The respective requirements for all marketing authorization procedures are laid down in Regulation (EC) No 1901/2006, commonly referred to as the Pediatric Regulation. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized. The Pediatric Committee of the EMA, or PDCO, may grant deferrals for some medicines, allowing a company to delay development of the medicine for children until there is enough information to demonstrate its effectiveness and safety in adults. The PDCO may also grant waivers when development of a medicine for children is not needed or is not appropriate, such as for diseases that only affect the elderly population. Before an MAA can be filed, or an existing marketing authorization can be amended, the EMA must determine that a company actually complied with the agreed studies and measures listed in each relevant PIP, unless the EMA has granted: (i) a product-specific waiver, (ii) a class waiver or (iii) a deferral for one or more of the measures included in the PIP. If an applicant obtains a marketing authorization in all EU Member States, or a marketing authorization granted in the Centralized Procedure by the European Commission, and the study results for the pediatric population are included in the drug product information, even when negative, the medicine is then eligible for an additional six-month period of qualifying patent protection through extension of the term of the Supplementary Protection Certificate.
Regulatory Requirements after a Marketing Authorization has been Obtained
In case an authorization for a medicinal product in the EU is obtained, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products. These include:
| | ● | Compliance with the EU’s stringent pharmacovigilance or safety reporting rules must be ensured. These rules can impose post-authorization studies and additional monitoring obligations. |
| | ● | The manufacturing of authorized medicinal products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the applicable EU laws, regulations and guidance, including Directive 2001/83/EC, Directive 2003/94/EC, Regulation (EC) No 726/2004 and the European Commission Guidelines for Good Manufacturing Practice, or EU cGMP. These requirements include compliance with EU cGMP standards when manufacturing medicinal products and APIs, including the manufacture of API outside of the EU with the intention to import the API into the EU. |
| | ● | The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the EU notably under Directive 2001/83EC, as amended, and E. Member State laws. Direct-to-consumer advertising of prescription medicines is prohibited across the EU. |
Brexit and the Regulatory Framework in the United Kingdom
On June 23, 2016, the electorate in the United Kingdom voted in favor of Brexit. Thereafter, on March 29, 2017, the country formally notified the EU of its intention to withdraw pursuant to Article 50 of the Lisbon Treaty. The withdrawal of the United Kingdom from the EU took effect on January 31, 2020. Since the regulatory framework for pharmaceutical products in the United Kingdom covering quality, safety and efficacy of pharmaceutical products, clinical trials, marketing authorization, commercial sales and distribution of pharmaceutical products is derived from EU directives and regulations, Brexit could materially impact the future regulatory regime which applies to drug products and the approval of product candidates in the United Kingdom. It remains to be seen how, if at all, Brexit will impact regulatory requirements for product candidates and approved drug products in the United Kingdom.
General Data Protection Regulation
The collection, use, disclosure, transfer or other processing of personal data of individuals in the EU, including personal health data, is governed by the GDPR, which became effective on May 25, 2018. The GDPR is wide-ranging in scope and imposes numerous requirements on companies that process personal data, including requirements relating to processing health and other sensitive data, obtaining consent of the individuals to whom the personal data relates, providing notice to individuals regarding data processing activities, implementing safeguards to protect the security and confidentiality of personal data, providing notification of data breaches, and taking certain measures when engaging third party processors. The GDPR also imposes strict rules on the transfer of personal data to countries outside the EU, including the United States, and permits data protection authorities to impose large penalties for violations of the GDPR, including potential fines of up to €20 million or 4% of annual global revenues, whichever is greater. The GDPR also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies and obtain compensation for damages. Compliance with the GDPR will be a rigorous and time-intensive process that may increase our cost of doing business.
Switzerland is currently in the process of amending its Data Protection Act, or the DPA. We expect the amended DPA to contain an equivalent of many of the provisions introduced in the EU through the GDPR. We currently expect that the amended DPA will be adopted by Swiss parliament in the course of 2020 with respective entry into force in early 2021.
Pricing Decisions for Approved Drug Products
In the EU, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies or so-called health technology assessments, in order to obtain reimbursement or pricing approval. For example, the EU provides options for the EU Member States to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. EU Member States may approve a specific price for a product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other EU Member States allow companies to fix their own prices for drug products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the EU have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage health care expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the EU. The downward pressure on health care costs in general, particularly with respect to prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new drug products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU Member States, and parallel trade, i.e., arbitrage between low-priced and high-priced EU Member States, can further reduce prices. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any drug products, if approved in those countries.
Employees
As of November 16, 2020, we have four executive officers, of which only our Chief Executive Officer is a full-time employee, while the additional three interim officers are part-time employees. In addition, we have one part-time employee and six consultants that we engage on a continual basis. None of our employees is represented by labor unions or covered by collective bargaining agreements. We believe that we maintain good relations with our employees.
At the time of the offering, all of our employment and consulting agreements will include employees’ and consultants’ undertakings with respect to non-competition and assignment to us of intellectual property rights developed in the course of employment and confidentiality.
Property and Facilities
Our principal executive office is located at Alter Postplatz 2, 6370 Stans, Canton of Nidwalden, Switzerland, and consists of approximately 25 square meters (approximately 270 square feet) of leased office space under an indefinite term lease that may be terminated by either party with six-months prior notice.
We consider that our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business; provided, however, that we may require additional space and facilities as our business expands.
Legal Proceedings
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
MANAGEMENT
Executive Officers and Directors
The following table sets forth information regarding our executive officers and directors as of November 16, 2020:
| Name | | Age | | Position |
| | | | | |
| Ronald Hafner(1)(3) | | 57 | | Chairman of the Board of Directors |
| | | | | |
| Alexander Zwyer | | 51 | | Chief Executive Officer and Director |
| | | | | |
| Pascal Brenneisen(1)(2)(3) | | 57 | | Director |
| | | | | |
| Myoung-Ok Kwon, Ph.D.(2)(3) | | 49 | | Director* |
| | | | | |
| Stig Løkke Pedersen(1)(2)(3) | | 59 | | Director* |
| | | | | |
| Robert Dickey IV | | 65 | | Interim Chief Financial Officer |
| | | | | |
| Hervé Girsault | | 61 | | Interim Chief Business Officer |
| | | | | |
| Sandy Eisen, M.D. | | 65 | | Interim Chief Medical Officer |
| | | | | |
| Mehdi Tafti, Ph.D. | | 62 | | Chief Scientific Officer* |
| | | | | |
| Geert Mayer, M.D. | | 71 | | Chief Development Officer* |
| | | | | |
| Silvia Panigone, Ph.D. | | 49 | | Chief Operating Officer* |
| (1) | Member of the Compensation, Nomination and Governance Committee |
| (2) | Member of the Audit Committee |
| (3) | Independent Director (as defined under Nasdaq Stock Market rules) |
| * | Expected to become a director or executive officer, as the case may be, after the completion of this offering. |
In general, members of our board of directors are elected by our shareholders and elected to serve for a one-year period. See “Description of Share Capital and Governing Documents – Articles of Association – Board of Directors” for additional information.
Ronald Hafner, Chairman of the Board of Directors
Ronald Hafner has been a member of our board and served as Chairman of the Board of Directors since August 2015. Mr. Hafner has been a partner at Deloitte Consulting AG since January 2016 and since 2018 he has served as the chairman of Magnetic Rock, a private investment company. From 2015 to 2016, Mr. Hafner served as an independent director of the Pingar Group Ltd., a private company developing artificial intelligence software solutions. Prior to serving in these capacities, Mr. Hafner served as the Chief Executive Officer of Infosys Consulting AG. Prior to Lodestone Management Consultants AG’s acquisition by Infosys Ltd. in 2012, Mr. Hafner was Chief Executive Officer and partner of Lodestone Management Consultants AG, where he was responsible for the company’s global operations since founding the business in 2005. Mr. Hafner has an M.B.A. and master’s degree in economics from the University of Basel/Switzerland.
Alexander Zwyer, Chief Executive Officer and Director
Alexander Zwyer has served as our Chief Executive Officer and as a Director since our incorporation in August 2015. Mr. Zwyer has over 25 years of international business experience. In 2007, prior to, and until founding NLS in 2015, Mr. Zwyer founded a startup in the high-end luxury food sector, and served as its chief executive officer until 2015 when he successfully sold the company. From 1991 and until 2007, Mr. Zwyer served in various positions with Viforpharma AG (SWX: VIFN) (then known as Vifor (International) Inc.), most notably serving as Executive Vice President (chief operating officer), leading the company’s global regulatory affairs, medical affairs, sales and marketing as well as business development teams. Mr. Zwyer speaks seven languages fluently. Mr. Zwyer holds a B.B.A. in business administration from Oekral, Zurich, Switzerland and an executive M.B.A. from GSBA/Lorange Institute of Business, Zurich, Switzerland and an M.B.A. from University at Albany SUNY.
Pascal Brenneisen, Director
Pascal Brenneisen has been a member of our board since September 2020. Mr. Brenneisen has a mix of expertise in general management of countries and regions, strategic business development, sales and marketing, operations, finance, operational auditing and digital transformation in the industries of pharmaceuticals, health care and fast-moving consumer goods. Mr. Brenneisen was Vice President Region Head for Eurasia, Middle East & Africa for Shire Biopharma from January 2018 to December 2019. Before that, from March 2016 to November 2017, Mr. Brenneisen was regional head of central and eastern Europe, with a focus on the life science and pharma industry for Hewlett Packard Enterprise Services (part of Hewlett Packard Inc.) and from 2012 to 2015, he was President of Novartis CH, part of Novartis International. In addition, since 2015, Mr. Brenneisen has served on the board of directors of several companies, including Topadur Pharma Ltd., Bottmedical Ltd. and BioCopy AG Allschwil. Mr. Brenneisen earned a law degree from the University of Basel, Switzerland and has since participated in numerous executive education programs.
Myoung-Ok Kwon, Director
Myoung-Ok Kwon, Ph.D. is expected to become a member of our board of directors in connection with the completion of this offering. Ms. Kwon has 16 years of experience in the healthcare venture capital and public investments and pharma research fields. Ms. Kwon is Head of Healthcare Investment at PMG Investment Solutions AG, or PMG, and Asset Manager of the PMG Global Biotech Fund. From 2004 to 2007, Ms. Kwon worked at Novartis AG as a Senior Research Planner and Scientific Assistant to the Head of Corporate Research. After leaving Novartis AG in 2007, she joined the Zurich-based venture capital fund Nextech Invest as managing partner. She led the investment team from 2007-2012 and made 10 biotech investments in the United States and Europe. Her Board of Directors and observer representation included Sunesis Pharmaceuticals, Inc. (Nasdaq: SNSS) (USA) (as an observer) from 2009 to 2012, MacroGenics Inc. (USA) (as an observer) From 2008 to 2012, TRACON Pharmaceuticals, Inc. (Nasdaq: MGNX) (USA) from 2011 to 2012, Telormedix SA (Switzerland) (as an observer) from 2008 to 2012, ImVisioN Therapeutics AG (Germany) from 2007 to 2012. She has been a venture partner of Arcus Ventures, a New York-based venture capital fund, since 2014. She is also the founder of Julier Partners, a healthcare consulting firm active since 2013 and built up the Biotech Core Value Fund, a Liechtenstein-based fund, as an investment advisor from 2014-2019. She holds a Ph.D. in biochemistry and molecular genetics from the University of Basel and the Friedrich Miescher Institute, which is part of the Novartis Research Foundation.
Stig Løkke Pedersen, Director
Stig Lokke Pedersen is expected to become a member of our board of directors in connection with the completion of this offering. Mr. Pedersen has over 35 years of business experience, having held numerous executive positions and board positions over such period of time. Since 2012, Mr. Pedersen has been a member of the investment board in the Danish private equity fund Catacap A/S and from 2001 to 2011, Mr. Pedersen served as a member of the executive management team at H. Lundbeck A/S (Denmark) (Nasdaq Copenhagen: LUN), one of Denmark’s largest public companies and a prominent, global CNS specialist pharmaceutical company. During his time as Chief Commercial Officer at H. Lundbeck A/S (Denmark), Mr. Pedersen was responsible of all business activities in the group, including operations in more than 60 countries worldwide, a turnover of $3 billion and around 4,500 employees. In addition, Mr. Pedersen has served on numerous boards since 1992, including Chairman of the board of directors of Nuevolution AB (Nasdaq Sweden: NUE) from 2001 to 2019 and Chairman of the board of directors of ChemoMetec A/S (Nasdaq Copenhagen: CHEMM) from 2009 to 2013. In addition, Mr. Pedersen currently sits on the board of directors of nine other companies, including the life science companies Stemform A/S, SSI Diagnostika A/S, TAP A/S, Moksha8 Ltd., Union Therapeutics A/S and Index Pharmaceuticals AB. Mr. Pedersen earned his MSc. (Econ.) from Aalborg University in Denmark.
Robert Dickey IV, Interim Chief Financial Officer
Robert Dickey IV has served as our Interim Chief Financial Officer since August 2019, and previously served as our Deputy Chief Financial Officer from February 2019. Mr. Dickey has over 25 years of experience leading life science and medical device companies, both private and public. Mr. Dickey currently serves as the Managing Director of Foresite Advisors, through which he is engaged with us. Mr. Dickey served as Chief Financial Officer of several companies, including Mofit Bio PLC (Nasdaq/AIM: MTFB) from 2017 to 2018, Tyme Technologies (Nasdaq: TYME) from 2015 to 2017 and NeoStem, Inc. (now, Caladrius Biosciences) (Nasdaq: CLBS) from 2013 to 2015. Since 2018, Mr. Dickey has served on the board of directors and as chairman of the audit committee of Emmaus Life Sciences, Inc. (Nasdaq: EMMA). Mr. Dickey previously spent 18 years in investment banking at Lehman Brothers and Legg Mason. Mr. Dickey holds a A.B. from Princeton University and M.B.A. from The Wharton School, University of Pennsylvania.
Hervé Girsault, Interim Chief Business Officer
Hervé Girsault has served as our Interim Chief Business Officer since December 2017, and previously served as our business development advisor from September 2016. Mr. Girsault has over 20 years of experience in the healthcare industry, serving in a variety of senior positions, including Chief Executive Officer of Synarc Inc. in the United States (now BioClinica Inc.), of Novartis Benelux and of Gerber France SA in France, and as head of mergers & acquisitions, business development & partnering for global companies, such as Novartis International AG and Novartis Consumer Health (also serving as its Chief Executive Officer), a division of Novartis AG, and as manager of mergers & acquisitions of the healthcare practice at Coopers & Lybrand Consultants. Prior to joining us, from 2013, Mr. Girsault served as the Chief Business officer of Piqur Therapeutics AG. Mr. Girsault co-founded TUROS Capital AG in 2018, and currently sits on its board of directors.
Sandy Eisen, M.D., Interim Chief Medical Officer
Sandy Eisen, M.D. has served as our Interim Chief Medical Officer since September 2019. Dr. Eisen has 30 years of experience working in the pharmaceutical industry and in pharmaceutical medicine and regulation, with time initially as a UK and EU regulator at the Medicines and Healthcare products Regulatory Agency and the EMA in London. Since 2011, Dr. Eisen has provided medical, regulatory and drug safety services to pharmaceutical and biotech companies through his privately held company, Frontline Pharma Consulting Ltd. From May 2013 to December 2015, Dr. Eisen also served as a non-executive director of Shield Therapeutics /Phosphate Therapeutics (LON: STX). From 2005 to 2011, Dr. Eisen served in a variety of roles at Teva Pharmaceuticals, including working as Chief Medical Officer for Teva Pharmaceuticals Europe from 2006 to 2011. Dr. Eisen studied medicine at Cambridge University and then at St. Bartholomew’s Hospital Medical School, London and in addition to a medical qualification, has postgraduate qualifications in surgery and in pharmaceutical medicine.
Mehdi Tafti, Ph.D., Chief Scientific Officer
Mehdi Tafti, Ph.D. is expected to become our Chief Scientific Officer following the completion of this offering. Mr. Tafti has been active in the field of sleep research and sleep medicine since 1985 (neurophysiology, neuroanatomy, molecular genetics, sleep apnea syndrome, sleepwalking, Kleine-Levin syndrome, and narcolepsy). Mr. Tafti is one of the world-leading specialists of narcolepsy and has worked with both human and narcoleptic dogs. He is also one of the few in the world with a dual expertise in the basic sleep research in animal models, and pharmacology and molecular genetics of sleep disorders in humans. Mr. Tafti has served as a full-time professor in the Faculty of Biology and Medicine of the University of Lausanne since 2011, previously serving as an associate from 2004 until 2011. In 2007, Mr. Tafti founded the Center for Investigation and Research in Sleep at the Vaud University Hospital. Prior to joining the University of Lausanne, Mr. Tafti spent time working at the Center for Narcolepsy Research at Stanford University and afterward he went on to establish what is believed to be the first global laboratory dedicated to the molecular genetics of sleep and sleep disorders at the Department of Psychiatry of the University Hospitals of Geneva (Switzerland). Mr. Tafti holds a Ph.D. in neurophysiology from the University of Montpellier, France and a Swiss accreditation as a clinical somnologist.
Geert Mayer, M.D., Chief Development Officer
Geert Mayer, M.D. is expected to become our Chief Development Officer following the completion of this offering. Dr. Mayer has been a member of the board of the German Sleep Society since 1996 and served as president of the society from 2006 to 2011. From 1998 to 2016, Dr. Mayer was a director of Hephata Clinic (Germany) (in the Department of Neurology, Psychiatry, Sleep Medicine and Chronobiology), and since 2016 he has been a senior director of the clinic. Dr. Mayer has also served as a professor in the Department of Psychiatry of the Georg-August University of Göttingen and from 2005, in the Department of Neurology at Philipps University of Marburg. Dr. Mayer previously served as Chairman of the Task Force "Clinical Education" of the European Sleep Research Society, Chairman of the Commission Sleep of the German Society of Neurology and Chairman of the Commission Polysomnography of the German Society for Neurophysiology and is currently a member of the European Sleep Research Society, the European Academy of Neurology and the European Narcolepsy Network, of which he is the founder. Dr. Mayer received his medical degree from Georg-August University Göttingen in 1974 and his special degree for neurology and psychiatry in 1984 from the State Medical Association of Hesse (Landesärztekammer Hessen).
Silvia Panigone, Ph.D., Chief Operating Officer
Silvia Panigone, Ph.D. is expected to become our Chief Operating Officer following the completion of this offering. Ms. Panigone combines a profound understanding of corporate finance and private investments with a deep knowledge of drug development research and development processes. Ms. Panigone founded in September 2013, and currently serves as Managing Director of, ADYA Consulting Sagl, a Swiss investment boutique firm in the life sciences sector that focuses on value extraction, development and implementation of strategic plans, deal structuring, capital raising and M&A transactions across the globe. From 2013 until 2018, Ms. Panigone served as a coach for life sciences companies though The Commission for Technology and Innovation, a body operating under the Swiss Confederation. Also from 2013 and until January 2016, Ms. Panigone spent time as Managing Director, Europe and a team member in the United States for I-Bankers Direct LLC and I-Bankers Securities, Inc. Ms. Panigone possesses a Molecular Biology degree from University of Milan (Italy), a Ph.D. in Molecular Oncology from National Cancer Institute, Milan in collaboration program with the Open University, London and a Master in Finance - Management of Economical Resources from SDA Bocconi, Milan, Italy.
Family Relationships
There are no family relationships among our executive officers and directors.
Arrangements Concerning Election of Directors and Members of Management
The members of our board of directors were appointed and duly elected to serve as such in accordance with a certain shareholders agreement that we previously entered into and which shall terminate upon our listing on Nasdaq. With the exception of such shareholders agreement, there are no other arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our directors or members of senior management were selected as such. See “Related Party Transactions – Shareholders Agreements” for additional information.
Compensation
The following table presents in the aggregate all compensation we paid to all of our directors and senior management as a group for the year ended December 31, 2019. The table does not include any amounts we paid to reimburse any of such persons for costs incurred in providing us with services during this period. Pursuant to Swiss law, at the time of this offering, we are not required to provide the compensation, on an individual basis, of our executive officers and directors.
All amounts reported in the table below reflect the cost to the Company, in thousands of U.S. Dollars, for the year ended December 31, 2019.
| | | Salary, Bonuses and Related Benefits | | | Pension, Retirement and Other Similar Benefits | | | Share Based Compensation | |
| All directors and senior management as a group, consisting of 7 persons (1)(2) | | $ | 898,978 | | | $ | 40,083 | | | $ | - | |
| | (1) | The amounts include $278,836 of deferred salary, bonus and related benefits and $6,275 of deferred pension, retirement and other similar benefits that have yet to be paid. |
| | (2) | The amounts do not include $2,496 in interest owed to our Chief Executive Officer. See “–Employment and Service Agreements with Executive Officers” below and “Related Party Transactions” for additional information. |
Employment and Service Agreements with Executive Officers
We have entered into a written employment agreement with our Chief Executive Officer. Our other interim executive officers are not engaged as full-time employees. Pursuant to the consulting agreement with our Interim Chief Business Officer, he is eligible to receive a 1% fee tied to the proceeds actually received by us in certain transactions, such as, but not limited to, an M&A transaction and an initial public offering of our securities. In connection with this offering, he shall receive a fee of 1% of the net proceeds actually received by us, or the Transaction Fee. We expect the Transaction Fee to be approximately $176,722 if we receive the Estimated Net Proceeds.
In addition, our Chief Executive Officer and other interim executive officers have agreed to defer the receipt of payment of a portion or all of their respective compensation until we raise sufficient capital, including through the sale of the Units in this offering. In addition, pursuant to an agreement between us and our Chief Executive Officer, compensation that is due and outstanding to him, but has yet to be paid, will accrue interest at a rate of 10% per year and is due to be repaid to him in full by December 31, 2020.
After this offering, we intend to enter into consulting or employment agreements with other suitable candidates. All of these agreements are expected to contain provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law and, our assignment of inventions agreements with our Swiss executive officers will contain terms and conditions customary under Swiss law. In addition, our executive officers will be subject to our directors’ and officers’ liability insurance policy, which we expect to obtain prior to or shortly after the completion of this offering. Members of our senior management are eligible for bonuses each year. The bonuses will be payable upon meeting objectives and targets that are set annually by our board of directors and compensation, nomination and governance committee, and, in certain circumstances, upon approval by our shareholders.
Directors’ Service Contracts
Other than with respect to Mr. Zwyer, who has an agreement to serve as our Chief Executive Officer, we do not have a written agreement with our other director providing for benefits upon the termination of his service with us or otherwise; however, our directors will be subject to our directors’ and officers’ liability insurance policy, which we expect to obtain prior to or shortly after the completion of this offering.
Differences between Swiss Laws and Nasdaq Requirements
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, require foreign private issuers, such as us, to comply with various corporate governance practices. In addition, following the listing of the common shares and Warrants on Nasdaq, we will be required to comply with the Nasdaq Stock Market Rules. Under those rules, we may elect to follow certain corporate governance practices permitted under the Swiss law in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Nasdaq Stock Market Rules for U.S. domestic registrants.
In accordance with Swiss law and practice and subject to the exemption set forth in Rule 5615 of the Nasdaq Stock Market Rules, as a foreign private issuer, we have elected to rely on home country governance requirements and certain exemptions thereunder rather than the Nasdaq Stock Market Rules, with respect to the following requirements:
| | ● | Composition of the board of directors. Swiss law does not require that a majority of our board of directors consist of independent directors. Our board of directors therefore may include fewer independent directors than would be required if we were subject to Nasdaq Listing Rule 5605(b)(1). In addition, we will not be subject to Nasdaq Listing Rule 5605(b)(2), which requires that independent directors must regularly have scheduled meetings at which only independent directors are present. |
| | ● | Quorum. In accordance with Swiss law and generally accepted business practices, our articles of association do not provide quorum requirements generally applicable to general meetings of shareholders. Our practice thus varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. According to Swiss law, for the approval of the management report and the consolidated annual financial statements, the Company’s auditor must be present at such a general meeting of shareholders, unless the general meeting of shareholders waives such attendance by unanimous decision. |
| | ● | Proxy Solicitations. There are currently three types of institutional proxies under Swiss Law: (i) the independent proxy, (ii) the board proxy and (iii) the custodian proxy, all which tend to vote in favor of the board’s proposals, because the default for the independent proxy is to vote with the board and by virtue of law the board and custodian proxies must vote with the board of directors. The Swiss Ordinance against Excessive Compensation in Listed Stock Companies, or the Compensation Ordinance, is applicable to companies limited by shares that are listed in Switzerland or abroad. According to the Compensation Ordinance, the solicitation of proxies by our board of directors or a custodian thereof is not permitted and only independent proxies are permitted. The independent proxy has to be elected by the general meeting of shareholders, must only vote pursuant to the specific instructions of the shareholders and must abstain from voting in the absence of specific instructions. However, Swiss law does not have a regulatory regime for the solicitation of proxies and company solicitation of proxies is prohibited for public companies in Switzerland; thus our practice will vary from the requirement of Nasdaq Listing Rule 5620(b), which sets forth certain requirements regarding the solicitation of proxies. |
| | ● | Share Issuances. Pursuant to Swiss law, prior to this offering, we will opt out of shareholder approval requirements by way of including authorized and conditional share capital (see “Description of Share Capital and Governing Documents—Articles of Association—Our Authorized Share Capital” and “Description of Share Capital and Governing Documents—Articles of Association—Our Conditional Share Capital”) for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of the Company and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Listing Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. |
| | ● | Compensation, Nomination and Governance Committee. The Compensation Ordinance requires that we adopt a compensation committee, so in accordance with Nasdaq Listing Rule 5615(a)(3), we will follow home country requirements with respect to the compensation committee. Under home country rules, the general meeting of shareholders has the authority to set up the basic principles of the compensation committee’s responsibilities in a company’s articles of association. In addition, the shareholders may decide to include in the articles of association a list of concrete responsibilities of the compensation committee. Unless the articles of association list concrete responsibilities of the compensation committee, the determination of the concrete responsibilities falls within the competence of the board of directors. Furthermore, the general meeting of shareholders must vote on the compensation report and the total of the remunerations of the board of directors, management and advisory committee. According to the doctrine, the strategic planning of the remuneration system and policy as well as the determination of the remuneration procedure is the inalienable task of the board of directors, unless a list of concrete responsibilities is included in the articles of association of the Company. Apart from that, the board of directors has the authority to determine whether the compensation committee shall have the decision-making power with regard to the individual salaries or whether it shall have only a request right to the board of directors. Furthermore, pursuant to Swiss law, both the number of committee members (the minimum and maximum number of compensation committee members) and the identity of the compensation committee members is determined by our shareholders. Our home country rules also do not require us to authorize a committee of our independent directors or alternatively hold a vote consisting of solely our independent directors in order to determine which persons shall be nominated for election by our shareholders. Our practice will therefore vary from the requirements of Nasdaq Listing Rule 5605(d), which sets forth certain requirements as to the responsibilities, composition and independence of compensation committees, and from the independent director oversight of director nominations requirements of Nasdaq Listing Rule 5605(e). Furthermore, we will be subject to the Compensation Ordinance (“Say on Pay” Rule). This means that the compensation of our board of directors and executive officers must be presented by the board of directors to our shareholders and our shareholders must vote on the proposed compensation. Furthermore, pursuant to the Compensation Ordinance, severance, advances, transaction premiums and similar payments to executive officers and director are prohibited. |
| | ● | Code of Business Conduct and Ethics. Pursuant to Swiss law, prior to this offering, we will adopt a code of business conduct and ethics applicable to all of our directors, officers and employees, which may not comply with the requirements of Nasdaq Listing Rule 5610. |
Committees of the Board of Directors
Our board of directors intends to establish an audit committee and a compensation, nomination and governance committee prior to the completion of this offering.
Under Nasdaq Listing Rule 5615(b)(1), a company listing in connection with its initial public offering is permitted to phase in its compliance with the independent committee requirements and the committee composition requirements. Accordingly, a company listing in connection with its initial public offering shall be permitted to phase in its compliance with the committee composition requirements set forth in Nasdaq Listing Rule 5605(d)(2) and (e)(1)(B) as follows: (1) one member must satisfy the requirement at the time of listing; (2) a majority of members must satisfy the requirement within 90 days of listing; and (3) all members must satisfy the requirement within one year of listing. We intend to rely on the phase-in schedules set forth in the aforementioned Nasdaq Listing Rule with respect to the composition of our audit committee.
Audit Committee
Under the Nasdaq Stock Market rules, we are required to maintain an audit committee consisting of at least three members, all of whom are independent and are financially literate and one of whom has accounting or related financial management expertise. We intend to phase-in our compliance with this requirement in accordance with Nasdaq Listing Rule 5615(b)(1).
Our audit committee is expected to initially include Pascal Brenneisen, who is “independent,” as such term is defined under Nasdaq Stock Market rules, and who currently serves as the chairman of the committee. Mr. Brenneisen meets the requirements for financial literacy under the Nasdaq Stock Market rules. In addition, subject to their election to our board of directors, Myoung-Ok Kwon and Stig Løkke Pedersen, each of whom meet the requirements for financial literacy under the Nasdaq Stock Market rules, are expected to become members of the audit committee. Our board of directors has determined that Mr. Brenneisen is an “audit committee financial expert” as defined by the SEC rules and has the requisite financial experience as defined by the Nasdaq Listing Rules.
The audit committee will be governed by a charter that complies with Nasdaq Stock Market rules. Upon the completion of this offering, the audit committee has the responsibility to, among other things:
| | ● | consider and make recommendations to the board of directors on our financial statements, review and discuss the financial statements and present its recommendations with respect to the financial statements to the board of directors prior to the approval of the financial statements by our board of directors; |
| | ● | oversee our independent auditor and engage and determine compensation or termination of engagement of our auditor; |
| | ● | determine and pre-approve the terms of certain audit and non-audit services provided by our auditor; |
| | ● | review and monitor, if applicable, legal matters with significant impact and findings of regulatory authorities, receive reports regarding irregularities and legal compliance, act according to our “whistleblower policy” and make recommendations to our board of directors if so required, and oversee our policies and procedures regarding compliance to applicable financial and accounting related standards, rules and regulations; and |
| | ● | review and assess the independent auditor’s report, management letters and take notice of all comments of the independent auditor on accounting procedures and systems of control and review the independent auditor’s reports with management. |
Compensation, Nomination and Governance Committee
The members of our compensation, nomination and governance committee are expected to include Pascal Brenneisen and Ronald Hafner. In addition, subject to his election to our board of directors and election to this committee by our shareholders, Stig Løkke Pedersen is expected to become a member of the compensation, nomination and governance committee. The compensation, nomination and governance committee will assist our board of directors in overseeing our cash compensation and equity award recommendations for our executive officers along with the rationale for such recommendations, as well as summary information regarding the aggregate compensation provided to our executive officers. Swiss law requires that we adopt a compensation committee, so in accordance with Nasdaq Listing Rule 5615(a)(3), we will follow home country requirements with respect to the compensation committee. We will be subject to the Compensation Ordinance. This means that the compensation of our board of directors and executive officers must be presented by the board of directors to our shareholders and our shareholders must vote on the proposed compensation. In addition, in accordance with Nasdaq Listing Rule 5615(a)(3), we will follow home country requirements with respect to the nomination and governance committee. With respect to the nomination of persons to our board of directors, our home country rules do not require us to authorize a committee of our independent directors or alternatively hold a vote consisting of solely our independent directors in order to determine which persons shall be nominated for election by our shareholders.
As a result, our practice will vary from the requirements of Nasdaq Listing Rule 5605(d) and 5605(e) which set forth certain requirements as to the responsibilities, composition and independence of compensation committees and the nomination of directors for election by shareholders, respectively. See “—Differences between Swiss Laws and Nasdaq Requirements.”
Equity Incentive Plan
Following the completion of this offering, we intend to adopt a new omnibus equity incentive plan under which we would have the discretion to grant a broad range of equity-based awards to eligible participants.
BENEFICIAL OWNERSHIP OF PRINCIPAL SHAREHOLDERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our common shares as of November 16, 2020 by:
| | ● | each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our outstanding common shares; |
| | ● | each of our current directors and executive officers; and |
| | ● | all of our current directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC, and includes voting or investment power with respect to common shares. Percentage of shares beneficially owned before this offering is based on 6,960,000 shares outstanding on November 16, 2020. The number of common shares deemed outstanding after this offering includes the common shares being offered for sale as part of the Units in this offering.
Certain existing officers, who are also shareholders, and a director nominee, have indicated an interest in purchasing up to an aggregate of $138,000 of Units in this offering at the initial public offering price per Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these persons, or any of these persons may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these persons as they will on any other Units sold to the public in this offering. The information in the table below does not reflect the anticipated purchase of any Units in this offering by our existing shareholders or director nominees.
Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. Unless otherwise noted below, each beneficial owner’s address is: c/o Alter Postplatz 2, CH-6370 Stans, Switzerland.
| | | No. of Shares Beneficially Owned Prior to this Offering | | | Percentage Owned Before this Offering(1) | | | Percentage Owned After this Offering | |
| Holders of more than 5% of our voting securities: | | | | | | | | | |
| Ronald Hafner✝ | | | 380,000 | | | | 5.5 | % | | | 3.6 | % |
| Alexander Zwyer✝(2) | | | 1,215,000 | | | | 17.2 | % | | | 11.5 | % |
| Eric Konofal* | | | 1,020,000 | | | | 14.7 | % | | | 9.6 | % |
| Eric-Jean Desbois* | | | 535,000 | | | | 7.7 | % | | | 5.0 | % |
| Magnetic Rock Investment AG (3)(4) | | | 2,849,417 | | | | 40.9 | % | | | 26.9 | % |
| Directors and executive officers who are not 5% holders: | | | | | | | | | | | | |
| Hervé Girsault | | | 25,000 | | | | ** | | | | ** | |
| Robert Dickey IV | | | - | | | | - | | | | | |
| Sandy Eisen | | | - | | | | - | | | | | |
| All directors and executive officers as a group (5 persons) | | | 1,620,000 | | | | 23.3 | % | | | 15.3 | % |
| ✝ | Indicates director of the Company. |
| * | Indicates founder and consultant to the Company. |
| ** | Less than 1%. |
| (1) | The percentages shown are based on 6,960,000 common shares issued and outstanding as of November 16, 2020. |
| (2) | Includes 10,000 common shares issuable pursuant to a Convertible Loan in the amount of CHF 100,000 (approximately $105,530). |
| (3) | Magnetic Rock is a private company owned in equal part by each of the Shareholders. Ronald Hafner serves on our board of directors. |
| (4) | Includes 4,417 common shares issuable pursuant to the remaining outstanding amount of CHF 23,020 ($24,293) under the Convertible Note (at the assumed public offering price). |
To our knowledge, Myoung-Ok Kwon and Stig Løkke Pedersen, who are expected to become directors following this offering, do not beneficially own any of our common shares.
Significant Changes in Percentage Ownership by Major Shareholders
During the past three years, there have been no significant percentage ownership changes in the ownership of our common shares by any major shareholder.
Record Holders
As of November 16, 2020, there was a total of 15 holders of record of our common shares, none of which had, to the best of our knowledge, a registered address in the United States.
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to us which would result in a change in control of the Company at a subsequent date.
RELATED PARTY TRANSACTIONS
Participation in this Offering
Certain existing officers, who are also shareholders, and a director nominee, have indicated an interest in purchasing up to an aggregate of $138,000 of Units in this offering at the initial public offering price per Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these persons, or any of these persons may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these persons as they will on any other Units sold to the public in this offering.
Employment and Consulting Agreements
We have entered into a written employment agreement with our Chief Executive Officer. Our other interim executive officers are not engaged as full-time employees and we do not expect that other executive officers that join us after the completion of this offering will be engaged as full-time employees in the immediate future. See “Management - Employment and Service Agreements with Executive Officers” for additional information. In addition, pursuant to the consulting agreement with our Interim Chief Business Officer, upon completion of this offering, he shall receive the Transaction Fee. We expect the Transaction Fee to be approximately $176,722 if we receive the Estimated Net Proceeds.
All employment agreements that we enter into are expected to contain provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law and, our assignment of inventions agreements with our Swiss executive officers will contain terms and conditions customary under Swiss law. In addition, our executive officers will be subject to our directors and officers insurance policy, which we expect to obtain prior to the completion of this offering. Members of our senior management are eligible for bonuses each year. The bonuses will be payable upon meeting objectives and targets that are set annually by our board of directors and compensation, nomination and governance committee, and, in certain circumstances, upon approval by our shareholders.
Shareholders Agreement
On August 31, 2015, and as amended in February 2017 and July 2017, we and our shareholders, which include Ronald Hafner, Chairman of the Board of Directors, our founders, including our Chief Executive Officer, as well as our Interim Chief Business Officer, entered into a certain shareholders agreement, as amended, or the Shareholders Agreement. The Shareholders Agreement provides for certain rights, including, the right of first refusal, co-sale rights and certain anti-dilution rights, and contains provisions governing the board of directors and other corporate governance matters. In August 2020, in connection with the 2020 Bridge Loan, our founders agreed to provide certain preferential liquidation preferences to a number of our shareholders upon the occurrence of a liquidation event, which specifically excludes the offering contemplated by this registration statement. This Shareholders Agreement, as amended, will automatically terminate upon our listing on Nasdaq. See – “2020 Bridge Loan and Liquidation Rights” below for additional information.
Transfer Pricing Agreement with Pegasus
On April 1, 2017, we and Pegasus, a company owned by certain of our shareholders, including our Chief Executive Officer, entered into the First Amendment to the ATA to provide certain milestone payments to the Pegasus Founders as an amendment to the remuneration previously owed to Pegasus for the transfer of the contractual rights of the AP-HP License Agreement that it assigned to us.
On September 20, 2019, we and Pegasus entered into the Second Amendment to the ATA whereby the milestone payments agreed upon in the First Amendment to the ATA were replaced by a royalty payment of 1.8% of the annual net sales (including sales made by sub-licensees) of the Licensed Compounds; provided, however, that such royalty payment could be further reduced to 0.9% in the event that the U.S. PTO were to issue a revised notice of allowance or expand the patent rights of the Licensed Compounds or be completely cancelled in the event that a competing generic product were to reach the market during the term of the patents covering the Licensed Compounds.
Cash Advances Between the Company and Pegasus
Since entering into the ATA, Pegasus, a company owned by certain of our shareholders, including our Chief Executive Officer, and the Company have advanced to each other certain amounts of cash to cover costs. We have offset the related receivables and payable to and from Pegasus for a combined net other long-term receivable of $56,191 and $54,472 for the six months ended June 30, 2020 and the year ended December 31, 2019, respectively.
Shareholders and Officer and Director Loans
We entered into the following loan agreements with Ronald Hafner, a member of our board, and the other Shareholders, and with Magnetic Rock, a company controlled by the Shareholders.
Credit Facilities
In August 2015, NLS Pharma Ltd. and NLS-1 Ltd., and in March, 2017, NLS-0 Ltd., each entered into the NLS Pharma Credit Facility, the NLS-1 Credit Facility and the NLS-0 Credit Facility, respectively.
The entire amount of the NLS Pharma Credit Facility remains outstanding and is currently scheduled to mature on December 31, 2020. If we default on the NLS Pharma Credit Facility, we will be subject to a default interest rate of 5% per year.
The entire principal amount of the NLS-1 Credit Facility has been converted into equity. In July 2018, we converted $3,418,519 of the principal amount (including the entire Interest-Bearing Facility) into 260,000 of our common shares, at a conversion price of $13 per common share and in March 2019, in connection with the Reorganization, the remaining $3,681,481 of the principal amount into 280,000 common shares at the same conversion price as the July 2018 conversion. The Interest-Bearing Facility stopped accruing interest upon the Reorganization and, as a result thereof, the accrued interest stands at $85,737. The NLS-1 Credit Facility is currently scheduled to mature on December 31, 2020.
We borrowed $1.45 million, or the Borrowed Amount, under the NLS-0 Credit Facility. In connection with the Reorganization, the Entire Borrowed Amount under the NLS-0 Loan and a certain $2 million bridge loan agreement with the Shareholders, or the Bridge Loan (as discussed more fully below), were converted together into an aggregate of 260,000 of our common shares, at a conversion price of $13 per common share.
Bridge Loan
In December 2017, in connection with the Bridge Loan, we received a loan of $2 million. The entire amount borrowed pursuant to the Bridge Loan was converted into shares of our common shares, together with the NLS-0 Credit Facility.
January 2019 Promissory Notes
In January 2019, we issued the four Notes, each for CHF 125,000 (approximately $128,200), with the Shareholders. Each Note carries an annual interest rate of 10% per year, compounded annually and is currently scheduled to mature on December 31, 2020.
January 2019 Convertible Promissory Note
In addition, on January 18, 2019, we issued the Convertible Note with Magnetic Rock for CHF 550,000 (approximately $557,920) pursuant to loan proceeds that were received in advance in each of August and December 2018 in the amount of CHF 500,000 ($507,200) and CHF 50,000 ($50,720). The Convertible Note carries an annual interest rate of 10% per year, compounded annually and was scheduled to matures on September 30, 2020. In October 2020, the Convertible Note was amended to extend the maturity date to December 31, 2020, or the Convertible Note Maturity Date. If the Convertible Note is not converted on or before Convertible Note Maturity Date, then on the Convertible Note Maturity Date, at the election of Magnetic Rock, either the outstanding amount under the Convertible Note shall be repaid in full or remain outstanding with a new maturity date to be set by Magnetic Rock.
If while the Convertible Note is outstanding, we conduct an equity financing, any such outstanding amounts shall convert, at the election of Magnetic Rock, into such number of our common shares equal to the amount of the Convertible Note then outstanding divided by the lower of (i) the price per share in the offering of such common shares or (B) the price per share to any lender of bridge loan financing who converts their loan into equity in such equity financing, or the Convertible Note Conversion. On March 12, 2019, in connection with the Reorganization, in exchange for the conversion of approximately CHF 526,980 (approximately $526,769) of debt into equity, we issued 40,000 of our common shares to Magnetic Rock at a price of CHF 13 (approximately $13) per common share. As of November 16, 2020, CHF 23,020 (approximately $24,293) of the principal amount remains outstanding.
Convertible Loans
On February 21, 2020, we entered into a Convertible Loan with our Chief Executive Officer, who is also a member of our board of directors, for an aggregate loan in the amount of CHF 150,000 (approximately $154,890). In September 2020, we amended the Convertible Loan with our Chief Executive Officer to decrease the amount of the loan to CHF 100,000 (approximately $105,530). The Convertible Loan carries an interest rate of 10% per year, compounded annually and originally matured on June 30, 2020, unless converted into common shares or repaid by us prior to then. The Convertible Loan with our Chief Executive Officer has been amended to extend the maturity date to December 31, 2020. If we default on this Convertible Loan, we will be subject to a default interest rate of 15% per year.
In addition, one Convertible Loan Amendment, in the amount of CHF 60,000 ($63,318) was with Ms. Silvia Panigone, who is expected to become our Chief Operating Officer following the completion of this offering. Ms. Panigone entered into a second Convertible Loan with us in the amount of CHF 20,000 ($21,106). Pursuant to a consulting agreement that we have with a company founded and managed by Ms. Panigone, Ms. Panigone was due consulting fees from us in the aggregate amount of CHF 129,779 ($136,956), of which CHF 10,770 ($11,366) has been paid as of November 16, 2020.
2020 Bridge Loan and Liquidation Rights
In August 2020, we received the first tranche of CHF 300,000 ($316,590) pursuant to the 2020 Bridge Loan. In September 2020, we received the second tranche of CHF 200,000 ($211,060) pursuant to the 2020 Bridge Loan, for an aggregate of CHF 500,000 ($527,650). The borrowed amounts under this 2020 Bridge Loan carry an interest rate of 10% per year, compounded annually, and all borrowed sums, including interest, were originally scheduled to mature on September 30, 2020; provided, however, that we may repay such loan, including interest, prior to the scheduled maturity date. If we default on the 2020 Bridge Loan, we will be subject to a default interest rate of 15% per year. The 2020 Bridge Loan has been amended to extend the maturity date to December 31, 2020.
In connection with and as a condition to the receipt of this loan, our founders agreed to provide preferential liquidation preferences to a number of our shareholders, including Magnetic Rock and Mr. Ronald Hafner, a member of our Board of Directors and also a part owner of Magnetic Rock. These preferential liquidation rights are scheduled to terminate upon the repayment of the 2020 Bridge Loan, including interest, which we expect to complete following this offering (see “Use of Proceeds” for additional information).
DESCRIPTION OF SHARE CAPITAL AND GOVERNING DOCUMENTS
The following description of our share capital and provisions of our articles of association are summaries and do not purport to be complete.
General
As of November 16, 2020, our authorized share capital consisted of 6,960,000 common shares, par value CHF 0.02 per share, all of which were issued and outstanding as of such date. All of our outstanding common shares have been validly issued, fully paid and non-assessable.
On September 14, 2020, our shareholders approved the adoption of an amendment to our articles of association, which reflected the Share Split, which became effective as of September 15, 2020. Following such Share Split and capital increase and upon completion of this offering, our issued fully paid-in share capital will consist of 10,596,363 common shares, each with a par value of CHF 0.02 per common share.
Common Shares
During the last three years we have issued an aggregate of 1,960,000 common shares, as further detailed below.
From September 2017 and until the Reorganization, we issued an aggregate of 635,000 common shares in private placements of common shares, due to anti-dilution provisions and in exchange for the conversion of certain amounts of debt, for aggregate net proceeds of approximately $6,691,998.
In connection with the Reorganization, we increased our share capital by 580,000 common shares in order to issue 580,000 of our common shares for the conversion of an aggregate of approximately $7,658,250 of debt, at a conversion price from approximately $13.
In addition, as a result of the Reorganization, the shareholders of NLS-0 received, in exchange for every one common share of NLS-0, 575.10 (rounded up to the nearest whole number) shares of our common shares and the shareholders of NLS Pharma received, in exchange for every one common share of NLS Pharma, 169.82 shares of our common shares, and as a result thereof, we issued, in the aggregate, 745,000 of our common shares.
All of our issued and outstanding common shares are duly authorized, validly issued, fully paid and non-assessable.
Convertible Securities
We issued the Convertible Note in January 2019. On March 12, 2019, in connection with the Reorganization, in consideration for converting CHF 526,979.84 ($526,769) into equity in lieu of repayment, we issued 40,000 of our common shares at a conversion price of CHF 13 (approximately $13) per common share. As of June 30, 2020, CHF 23,020 (approximately $24,293) remains outstanding. The Convertible Note matures on December 31, 2020 (also referred to herein as the Convertible Note Maturity Date). At the election of Magnetic Rock, it may elect to conduct the Convertible Note Conversion of the remaining outstanding amount prior to the Convertible Note Maturity Date.
We issued the Convertible Loans in December 2019 and February, March and June 2020 and entered into subsequent amendments. The Convertible Loans are in the aggregate amount of CHF 475,086 (approximately $501,358) and mature between December 31, 2020 and January 31, 2022, unless converted into common shares or repaid by us prior to then. Conversion of the Convertible Loans is at the discretion of each lender at a conversion price of CHF 9 ($10) per common share.
Warrants Offered in this Offering
The following summary of certain terms and provisions of the Warrants offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the form of Warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of Warrant.
The Warrants issued in this offering entitle the registered holder to purchase common shares at a price equal to $ per share, subject to adjustment as discussed below, immediately following the issuance of such Warrants and terminating at 5:00 p.m., New York City time, five years after the closing of this offering.
The exercise price and number of common shares issuable upon exercise of the Warrants may be adjusted in certain circumstances, including in the event of a stock dividend or recapitalization, reorganization, merger or consolidation. However, the Warrants will not be adjusted for issuances of common shares at prices below its exercise price.
Exercisability
The Warrants are exercisable immediately upon issuance and at any time up to the date that is five years from the date of issuance. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full for the number of common shares purchased upon such exercise. Each Warrant entitles the holder thereof to purchase one common share. Warrants are not exercisable for a fraction of a share and may only be exercised into whole numbers of shares. In lieu of fractional shares, we will, pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price and round down to the nearest whole share. Unless otherwise specified in the Warrant, the holder will not have the right to exercise the Warrants, in whole or in part, if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or 9.99% at the holder’s election) of the number of our common shares outstanding immediately after giving effect to the exercise, as such percentage is determined in accordance with the terms of the Warrant. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon at least 61 days’ prior notice from the holder to us.
Exercise Price
The exercise price per common share purchasable upon exercise of the Warrants is no less than 100% of the public offering price per Unit, and is subject to adjustments for stock splits, reclassifications, subdivisions, and other similar transactions. In addition to the exercise price per common share, and other applicable charges and taxes are due and payable upon exercise.
Warrant Agent; Global Certificate
The Warrants will be issued in registered form under a warrant agency agreement between a warrant agent and us. The Warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Listing; Transferability
We intend to apply for listing of the Warrants on Nasdaq. However, without an active trading market, the liquidity of the Warrants will be limited. We intend to have the Warrants issued in registered form under the warrant agency agreement between us and the warrant agent. Subject to applicable laws, the Warrants may be transferred at the option of the holders upon surrender of the Warrants to the warrant agent, together with the appropriate instruments of transfer.
Rights as a Shareholder
Except by virtue of such holder’s ownership of our common shares, the holder of Warrants does not have rights or privileges of a shareholder, including any voting rights, until the holder exercises such Warrant.
Articles of Association
Prior to the completion of this offering, we intend to adopt amended and restated articles of association. When we refer to our articles of association in this prospectus, unless the context provides otherwise, we refer to our amended and restated articles of association as currently expected to be in force immediately after the completion of this offering.
Ordinary Capital Increase, Authorized and Conditional Share Capital
Under Swiss law, we may increase our share capital (Aktienkapital) with a resolution of the general meeting of shareholders (ordinary capital increase) that must be carried out by the board of directors within three months in order to become effective. In the case of subscription and increase against payment of contributions in cash, a resolution passed by an absolute majority of the shares represented at the general meeting of shareholders is required. In the case of subscription and increase against contributions in kind or to fund acquisitions in kind, when shareholders’ statutory pre-emptive rights are withdrawn or where transformation of reserves into share capital is involved, a resolution passed by two-thirds of the shares represented at a general meeting of shareholders and the absolute majority of the nominal amount of the shares represented is required.
Under the Swiss Code of Obligations, or the CO, our shareholders, by a resolution passed by two-thirds of the shares represented at a general meeting of shareholders and the absolute majority of the nominal amount of the shares represented, may empower our board of directors to issue shares of a specific aggregate nominal amount up to a maximum of 50% of the share capital in the form of:
| | ● | authorized capital (“genehmigtes Kapital”) to be utilized by the board of directors within a period determined by the shareholders but not exceeding two years from the date of the shareholder approval; or |
| | ● | conditional capital for the purpose of issuing shares in connection with, among other things, (i) option and conversion rights granted in connection with warrants and convertible bonds of the Company or one of our subsidiaries or (ii) grants of rights to employees, members of our board of directors or consultants or our subsidiaries or other persons providing services to the Company or a subsidiary to subscribe for new shares (conversion or option rights). |
Our Authorized Share Capital
The articles of association authorize our board of directors to increase the share capital (within a period of no more than two years) and set forth the nominal amount by which the board of directors may increase the share capital (such authorized capital may not exceed one-half of the existing share capital).
Under our articles of association, as expected to be in place immediately before completing this offering, our board of directors is authorized at any time until September 14, 2022 to increase our share capital by a maximum aggregate amount of CHF 69,600.00 through the issuance of not more than 3,480,000 common shares, which would have to be fully paid-in, each with a par value of CHF 0.02 per common share. However, prior to the closing of this offering, we expect to amend our articles of association to authorize our board of directors, at any time until two years after the date of the amendment of the articles of association to increase our share capital by a maximum aggregate amount of CHF 105,963.62 through the issuance of not more than 5,298,181 common shares, which would have to be fully paid-in, each with a par value of CHF 0.02 per common share Increases in partial amounts are permitted. The board of directors determines the timing, issue price, the type of contributions and the date on which the dividend entitlement commences.
The articles of association also explicitly set forth the events for which a restriction or exclusion of the pre-emptive rights from the current shareholders is permitted.
Within the limits of Swiss law, a general meeting of shareholders may increase or alter the authorization granted to the board of directors. See “— Ordinary Capital Increase, Authorized and Conditional Share Capital.”
To affect any capital increase based on our authorized share capital, the Company will have to follow the relevant procedures under Swiss law. In particular, the Company’s board of directors will have to approve a general authorization resolution (“Ermächtigungsbeschluss”), issue a capital increase report (“Kapitalerhöhungsbericht”), approve a notarized confirmation resolution (“Feststellungsbeschluss”) on the capital increase and the articles of association, and obtain (i) duly executed subscription form(s) covering the subscription of the relevant number of new shares, (ii) a report of an audit firm relating to the withdrawal of the pre-emptive rights, as well as (iii) a banking confirmation confirming the payment of the aggregate nominal value of the respective number of new shares to a special Swiss bank account, all in accordance with Swiss law. The Company’s board of directors will subsequently have to file the relevant documentation accompanied by an application form with the competent commercial register. Any issuance of common shares based on such filing(s) is subject to the recording of the respective capital increase(s) in the commercial register in accordance with Swiss law.
The up to 545,454 shares and/or Warrants to purchase up to 545,454 shares to be sold and issued to the underwriters upon exercise of the over-allotment option would be issued based on our authorized share capital, unless the over-allotment option is exercised prior to the issuance and registration of the 3,636,363 common shares offered in connection with this offering, in which case such up to 545,454 common shares may be issued as part of the ordinary capital increase.
Our Conditional Share Capital
Conditional Share Capital for Warrants and Convertible Bonds and Advisors
Our nominal share capital may be increased by a maximum aggregate amount of CHF 105,963.62 through the issuance of not more than 5,298,181 common shares, which would have to be fully paid-in, with a nominal value of CHF 0.02 each, by the exercise of option and conversion rights granted in connection with warrants and convertible bonds of the Company or one of our subsidiaries. Shareholders will not have pre-emptive subscription rights in such circumstances. The holders of convertible bonds are entitled to the new shares upon the occurrence of the applicable conversion feature.
When issuing convertible bonds, the board of directors is authorized to withdraw or to limit the advance subscription right of shareholders to subscribe to the convertible bond issuance:
| | ● | for the purpose of financing or refinancing the acquisition of enterprises, divisions thereof, or of participations or of newly planned investments of the Company; or |
| | ● | if the issuance occurs in domestic or international capital markets, including private placements. |
To the extent that the advance subscription rights are withdrawn, (i) the convertible bonds are to be issued at market conditions; (ii) the term to exercise the option or conversion rights may not exceed ten years as of the date of the convertible bond issue and twenty years for conversion rights; and (iii) the exercise price for the new shares must at least correspond to the market conditions at the time of the convertible bond issuance.
Conditional Share Capital for Equity Incentive Plans
Our nominal share capital may, to the exclusion of the pre-emptive subscription rights of shareholders, be increased out of the condition share capital of the Company by a maximum aggregate amount of CHF 19,818.22 through the issuance of not more than 990,911 common shares, which would have to be fully paid-in, with no less than the nominal value of CHF 0.02 each, by the exercise of option or conversion rights that have been granted to employees, members of the board of directors or consultants of the Company or of one of our subsidiaries, if any, or other persons providing services to the Company or a subsidiary, if any, through one or more equity incentive plans created by the board of directors. If this conditional share capital is not sufficient for our future equity incentive plan, then we intend to rely on the authorized share capital, which we will have to dedicate for that purpose, or, alternatively, if such conditional or authorized share capital is not sufficient, then we intend to seek shareholder approval for the equity incentive plan(s) that we may seek to implement.
Pre-emptive Rights
Pursuant to the CO, shareholders have pre-emptive rights (“Bezugsrechte”) to subscribe for new issuances of shares. With respect to conditional capital in connection with the issuance of conversion rights, convertible bonds or similar debt instruments, shareholders have advance subscription rights (“Vorwegzeichnungsrechte”) for the subscription of conversion rights, convertible bonds or similar debt instruments.
A resolution passed at a general meeting of shareholders by two-thirds of the shares represented and the absolute majority of the nominal value of the shares represented may authorize our board of directors to withdraw or limit pre-emptive rights and/or advance subscription rights in certain circumstances. If pre-emptive rights are granted, but not exercised, the board of directors may allocate the pre-emptive rights as it elects.
Prior to the completion of this offering, we intend to convene a meeting of our shareholders to authorize our board of directors to withdraw or limit pre-emptive rights and/or advance subscription rights in certain circumstances, including as a result of this offering.
With respect to our authorized share capital, the board of directors is authorized by our articles of association to withdraw or to limit the pre-emptive rights of shareholders, and to allocate them to third parties or to us, in the event that the newly issued shares are used for a purpose set forth in our articles of association.
Uncertificated Securities
Our shares are uncertificated securities (“Wertrechte,” within the meaning of the CO) and, when administered by a financial intermediary (“Verwahrungsstelle,” within the meaning of the Federal Act on Intermediated Securities, or FISA), qualify as intermediated securities (“Bucheffekten,” within the meaning of the FISA). In accordance with art. 973c of the CO, we maintain a non-public register of uncertificated securities (“Wertrechtebuch”).
If registered in our share register, a shareholder may at any time request from us a written confirmation with respect to such person’s shares. The Company may issue certificates representing one or several shares at any time. Shareholders are not entitled, however, to request the printing and delivery of certificates.
Participation Certificates and Profit-sharing Certificates
The Company has not issued any non-voting equity securities, such as participation certificates (“Partizipationsscheine”) or profit sharing certificates (“Genussscheine”), nor has it issued any preference shares (“Vorzugsaktien”).
No Additional Capital Contributions
Under Swiss law, shareholders are not obliged to make any capital contribution in excess of the subscription amount, which can be under no circumstance less than the nominal value per share.
General Meeting of Shareholders: Meetings and Powers
The general meeting of shareholders is our supreme corporate body. Under Swiss law, an annual, ordinary general meeting of shareholders must be called on an annual basis and we may hold extraordinary general meetings of shareholders in addition to any annual general meeting. Under Swiss law, an ordinary general meeting of shareholders must be held annually within six months after the end of a corporation’s financial year. In our case, this means on or before the 30th day of June.
The following powers are vested exclusively in the general meeting of shareholders:
| | ● | adopting and amending our articles of association; |
| | | |
| | ● | electing the members of the board of directors, the chairman of the board of directors, the members of the compensation, nomination and governance committee, the auditors and the independent proxy holder (a person annually elected by the general meeting of shareholders and who may represent our shareholders at a general meeting of shareholders as a proxy solicitor); |
| | ● | approving the annual report, the annual statutory financial statements and the consolidated financial statements, and deciding on the allocation of profits as shown on the balance sheet, in particular with regard to dividends and bonus payments to members of the board of directors; |
| | ● | approving the compensation of members of the board of directors and executive management, which under Swiss law is not necessarily limited to the executive officers; |
| | ● | discharging the members of the board of directors and executive management from liability with respect to their tenure in the previous financial year; |
| | ● | dissolving the Company with or without liquidation; and |
| | ● | deciding matters reserved to the general meeting of shareholders by law or our articles of association or that are presented to it by the board of directors. |
An extraordinary general meeting of shareholders may be called by a resolution of the board of directors or, under certain circumstances, by our auditor, liquidator or bondholder (or the representatives of convertible bondholders), if any. In addition, in accordance with the CO, the board of directors is required to convene an extraordinary general meeting of shareholders if shareholders representing at least one-tenth of our share capital request such general meeting of shareholders in writing. Such request must set forth the items to be discussed and the proposals to be acted upon. According to the CO, the board of directors must convene an extraordinary general meeting of shareholders and propose financial restructuring measures if, based on our stand-alone annual statutory balance sheet, half of our share capital and reserves are not covered by our assets.
Voting and Quorum Requirements
Shareholder resolutions and elections (including elections of members of the board of directors) require the affirmative vote of the absolute majority of shares represented at the general meeting of shareholders, unless otherwise stipulated by law.
A resolution of the general meeting of the shareholders passed by two-thirds of the shares represented at the meeting, and the absolute majority of the nominal value of the shares represented is required for:
| | ● | any amendment of the Company’s objectives; |
| | ● | the introduction of shares with preferential voting rights; |
| | ● | any restriction on the transferability of registered shares; |
| | ● | creating authorized or conditional share capital; |
| | ● | a capital increase funded by equity, against contributions in kind or for the purpose of funding acquisitions in kind and the granting of special privileges; |
| | ● | any restriction or cancellation of the subscription right (i.e., pre-emptive rights); |
| | ● | a relocation of the seat (registered office) of the Company; and |
| | ● | the dissolution of the Company. |
The same voting requirements apply to resolutions regarding transactions among corporations based on Switzerland’s Federal Act on Mergers, Demergers, Transformations and the Transfer of Assets, or the Merger Act (including a merger, demerger or conversion of a corporation) see “— Compulsory Acquisitions; Appraisal Rights.”
In accordance with Swiss law and generally accepted business practices, our articles of association do not provide quorum requirements generally applicable to general meetings of shareholders. Additionally, before a resolution to approve the management report and consolidated accounts can be passed, our auditor must also be present at such general meeting of the shareholders, unless the general meeting of the shareholders waives such attendance by unanimous decision of those present. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock.
Notice
General meetings of shareholders must be convened by the board of directors at least twenty days before the date of the meeting. The general meeting of shareholders is convened by way of a notice appearing in our official publication medium, currently the Swiss Official Gazette of Commerce. Registered shareholders may also be informed by letter, facsimile or electronic mail. The notice of a general meeting of shareholders must state the items on the agenda, the proposals to be acted upon and, in case of elections, the names of the nominated candidates. Except in the limited circumstances listed below, a resolution may not be passed at a general meeting without proper notice. This limitation does not apply to proposals to convene an extraordinary general meeting of shareholders, the initiating of a special audit or the election of an auditor upon request of a shareholder. No previous notification is required for proposals concerning items included in the agenda or for debates that do not result in a vote. The notice period for a general meeting of shareholders may be waived if all shareholders are present or represented at such meeting (“Universalversammlung”).
Agenda Requests
Pursuant to Swiss law, one or more shareholders whose combined shareholdings represent the lower of (i) one tenth of the share capital or (ii) an aggregate nominal value of at least CHF 1,000,000, may request that an item be included in the agenda for an ordinary general meeting of shareholders. To be timely, the shareholder’s request must be received by us at least 30 calendar days in advance of the meeting. The request must be made in writing and contain, for each of the agenda items, the following information:
| | ● | a brief description of the business desired to be brought before the ordinary general meeting of shareholders and the reasons for conducting such business at the ordinary general meeting of shareholders; |
| | ● | the name and address, as they appear in the share register, of the shareholder proposing such business; and |
| | ● | all other information required under the applicable laws and stock exchange rules. |
In accordance with Swiss law, a business report, compensation report (as prepared by our board of directors) and an auditor’s report must be made available for inspection by the shareholders at our registered office no later than 20 days prior to the ordinary general meeting of shareholders. Shareholders of record are notified of this in writing.
Voting Rights
The right to vote, and the other rights of share ownership, may only be exercised by shareholders (including any nominees) or usufructuaries (a person who has the right to enjoy the use and advantages of another’s property short of the destruction or waste of its substance). who are entered in our share register at cut-off date determined by the board of directors, as authorized by our articles of association and Swiss law. Those entitled to vote in the general meeting of shareholders may be represented by the independent proxy holder (annually elected by the general meeting of shareholders), another registered shareholder or third person with written authorization to act as proxy or the shareholder’s legal representative. The chairman of the board of directors has the power to decide whether to recognize a power of attorney.
Dividends and Other Distributions
Our board of directors may propose to shareholders that a dividend or other distribution be paid but cannot itself authorize the distribution. Dividend payments require a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. In addition, our auditor must confirm that the dividend proposal of our board of directors conforms to Swiss statutory law and our articles of association.
Under Swiss law, we may only pay dividends if we have sufficient distributable profits brought forward from the previous business years (“Gewinnvortrag”), or if we have distributable reserves (“frei verfügbare Reserven”), each as evidenced by our audited stand-alone statutory balance sheet prepared pursuant to Swiss law, and after allocations to reserves required by Swiss law and the articles of association have been deducted. We are not permitted to pay interim dividends out of profit of the current business year.
Under our articles of association and in accordance with Swiss law, only our shareholders have the power to approve the payment of any dividends.
Distributable reserves are generally booked either as “free reserves” (“freie Reserven”) or as “reserve from capital contributions” (“Reserven aus Kapitaleinlagen”). Under the CO, if our general reserves (“allgemeine Reserve”) amount to less than 20% of our share capital recorded in the commercial register (i.e., 20% of the aggregate nominal value of our issued capital), then at least 5% of our annual profit must be retained as general reserves. The CO permits us to accrue additional general reserves. Further, a purchase of our own shares (whether by us or a subsidiary) reduces the distributable reserves in an amount corresponding to the purchase price of such own shares. Finally, the CO, under certain circumstances, requires the creation of revaluation reserves which are not distributable.
Distributions out of issued share capital (i.e. the aggregate nominal value of our issued shares) are not allowed and may be made only by way of a share capital reduction. Such a capital reduction requires a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. The resolution of the shareholders must be recorded in a public deed and a special audit report must confirm that claims of our creditors remain fully covered despite the reduction in the share capital recorded in the commercial register. The share capital may be reduced below CHF 100,000 only if and to the extent that at the same time the statutory minimum share capital of CHF 100,000 is reestablished by sufficient new fully paid-up capital. Upon approval by the general meeting of shareholders of the capital reduction, the board of directors must give public notice of the capital reduction resolution in the Swiss Official Gazette of Commerce three times and notify creditors that they may request, within two months of the third publication, satisfaction of or security for their claims. The reduction of the share capital may be implemented only after expiration of this time limit.
Our board of directors determines the date on which the dividend entitlement starts. Dividends are usually due and payable shortly after the shareholders have passed the resolution approving the payment; however, the shareholders, upon request of the board of directors, may resolve at the general meeting of shareholders to defer the due date of the dividend payments (e.g., in quarterly or other installments).
Transfer of Shares
Shares in uncertificated form (“Wertrechte”) may only be transferred by way of assignment. Shares that constitute intermediated securities (“Bucheffekten”) may only be transferred when a credit of the relevant intermediated securities to the acquirer’s securities account is made in accordance with the relevant provisions of the FISA. Article 4 of our articles of association provides that in the case of securities held with an intermediary such as a registrar, transfer agent, trust corporation, bank or similar entity, any transfer, grant of a security interest or usufructuary right in such intermediated securities and the appurtenant rights associated therewith requires the cooperation of the intermediary in order for such transfer, grant of a security interest or usufructuary right to be valid against us.
Voting rights may be exercised only after a shareholder has been entered in our share register (“Aktienbuch”) with his or her name and address (in the case of legal entities, the registered office) as a shareholder with voting rights. Any acquirer of our shares who is not registered in our share register as a shareholder with voting rights will, assuming the acquirer holds our shares as of the record date, still be entitled to dividends and other rights with financial value with respect to such shares.
Inspection of Books and Records
Under the CO, a shareholder has a right to inspect our share register with respect to his own shares and otherwise to the extent necessary to exercise his shareholder rights. No other person has a right to inspect our share register. Our books and correspondence may be inspected with the express authorization of the general meeting of shareholders or by resolution of the board of directors and subject to the safeguarding of our business secrets. See “Comparison of Swiss Law and Delaware Law — Inspection of Books and Records.”
Special Audit
If the shareholders’ inspection rights, as outlined above, prove to be insufficient in the judgment of the shareholder, any shareholder may propose to the general meeting of shareholders that specific facts be examined by a special audit in a special investigation. If the general meeting of shareholders approves the proposal, a company or any shareholder may, within 30 calendar days after the general meeting of shareholders, request a court in Stans, Switzerland, where our registered office is located, to appoint a special auditor. If the general meeting of shareholders rejects the request, one or more shareholders representing at least 10 percent of the share capital or holders of shares in an aggregate nominal value of at least CHF 2,000,000 may within three months’ request that the court appoint a special auditor. The court will issue such an order if the petitioners can demonstrate that the board of directors, any member of the board of directors or our executive management infringed the law or our articles of association and thereby caused damages to the Company or our shareholders.
Compulsory Acquisitions; Appraisal Rights
Business combinations and other transactions that are governed by the Swiss Merger Act (i.e. mergers, demergers, transformations and certain asset transfers) are binding on all shareholders. A statutory merger or demerger requires approval of two-thirds of the shares represented at a general meeting of shareholders and the absolute majority of the nominal value of the shares represented.
If a transaction under the Swiss Merger Act receives all of the necessary consents, there are no appraisal rights and all shareholders are compelled to participate.
Swiss corporations may be acquired by an acquirer through the direct acquisition of the share capital of the Swiss corporation. The Swiss Merger Act provides for the possibility of a so-called “cash-out” or “squeeze-out” merger if the acquirer controls 90% of the outstanding shares. In these limited circumstances, minority shareholders of the corporation being acquired may be compensated in a form other than through shares of the acquiring corporation (for instance, through cash or securities of a parent corporation of the acquiring corporation or of another corporation). Following a statutory merger or demerger, pursuant to the Merger Act, shareholders can file an appraisal action against the surviving company. If the consideration is deemed inadequate, the court will determine an adequate compensation payment.
In addition, under Swiss law, the sale of “all or substantially all of our assets” (“faktische Liquidation”) by us may require the approval of two-thirds of the number of shares represented at a general meeting of shareholders and the absolute majority of the nominal value of the common shares represented. Whether a shareholder resolution is required depends on the particular transaction, including whether the following test is satisfied:
| | ● | a core part of our business is sold without which it is economically impracticable or unreasonable to continue to operate the remaining business; |
| | | |
| | ● | our assets, after the divestment, are not invested in accordance with our statutory business purpose; and |
| | ● | the proceeds of the divestment are not earmarked for reinvestment in accordance with the Company’s business purpose but, instead, are intended for distribution to our shareholders or for financial investments unrelated to our business. |
Board of Directors
Pursuant to Swiss law and according to our articles of association, the board of directors shall consist of three or more members, none of which need to be shareholders. The current members of the board of directors are Ronald Hafner (Chairman of the board of directors), Alexander Zwyer and Pascal Brenneisen. Prior to the completion of this offering, we intend to expand the size of our board of directors.
The members of our board of directors are elected by the general meeting of shareholders for a term of one year. A year within the meaning of this provision is the period between two ordinary general meetings of shareholders. If a member of the board of directors retires or is replaced, his successor shall continue in office until the end of his predecessor’s term. Each member of our board of directors must be elected individually.
Powers
Our board of directors has the following non-delegable and inalienable powers and duties:
| | ● | the overall management of the company and the issuing of all necessary directives; |
| | | |
| | ● | determination of our appropriate management organization, including the power to define responsibilities and duties of our corporate bodies as well as our internal hierarchy; |
| | ● | the organization of the accounting, financial control and financial planning systems as required for management of the Company; |
| | | |
| | ● | the appointment and dismissal of persons entrusted with managing and representing the Company; |
| | ● | overall supervision of the persons entrusted with managing the Company, in particular with regard to compliance with the law, articles of association, operational regulations and directives; |
| | | |
| | ● | compilation and issuance of an annual report, preparation for the general meeting and implementation of its resolutions; and |
| | | |
| | ● | notification to the court in the event that the Company is over-indebted. |
According to Article 17 of our articles of association, the board of directors may assign the preparation and the implementation of its resolutions or the supervision of business transactions with regard to the non-transferable and inalienable duties to committees or individual members. In the event that our board of directors assigns duties in accordance with our articles of association and Swiss law, the board of directors shall design and implement reporting policies describing how such an assignment would be carried out by a committee or individual member.
Based on Article 18 of our articles of association, our board of directors may completely or partially delegate the power to manage the Company to one or more of its members (managing directors) or to third persons (managers).
Indemnification of Executive Management and Directors
According to Swiss Law and our articles of association, the general meeting of shareholders has the authority to grant discharge to the members of the board of directors from liability. The effect of the resolution of release (discharge) by the general meeting of shareholders is effective only for disclosed facts and only as against the Company and those shareholders who approved the resolution or who have since acquired their shares in full knowledge of the resolution. The right of action of other shareholders lapses six months after the resolution of release.
In addition, under general principles of Swiss employment law, an employer may be required to indemnify an employee against losses and expenses incurred by such employee in the proper execution of their duties under the employment agreement with the employer. See “Comparison of Swiss Law and Delaware Law—Indemnification of directors and executive management and limitation of liability.”
Conflict of Interest, Management Transactions
Swiss law does not provide for a general provision regarding conflicts of interest. However, the CO contains a provision that requires our directors and executive management to safeguard the Company’s interests and imposes a duty of loyalty and duty of care on our directors and executive management. This rule is generally understood to disqualify directors and executive management from participation in decisions that directly affect them. Our directors and executive officers are personally liable to us for a breach of these provisions. In addition, Swiss law contains provisions under which directors and all persons engaged in the Company’s management are liable to the Company, each shareholder and the Company’s creditors for damages caused by an intentional or negligent violation of their duties. Furthermore, Swiss law contains a provision under which payments made to any of the Company’s shareholders or directors or any person associated with any such shareholder or director, other than payments made at arm’s length, must be repaid to the Company if such shareholder or director acted in bad faith.
Principles of the Compensation of the Board of Directors and the Executive Management
Pursuant to Swiss law, our shareholders must annually, upon becoming a public company whose shares are listed for trade by the public, approve the compensation of the board of directors and the persons whom the board of directors has, fully or partially, entrusted with the management of the Company. The board of directors must issue, on an annual basis, a written compensation report that must be reviewed together with a report on our business by our auditor. The compensation report must disclose all compensation, loans and other forms of indebtedness granted by the Company, directly or indirectly, to current or former members of the board of directors and executive management to the extent related to their former role within the Company or not on customary market terms.
The disclosure concerning compensation, loans and other forms of indebtedness must include the aggregate amount for the board of directors and the executive management as well as the particular amount for each member of the board of directors and executive officer, specifying the name and function of each respective person.
Certain forms of compensation are prohibited for members of our board of directors and executive management, such as:
| | ● | severance payments provided for either contractually or in the articles of association (compensation due until the termination of a contractual relationship does not qualify as severance payment); |
| | | |
| | ● | advance compensation; |
| | ● | incentive fees for the acquisition or transfer of corporations or parts thereof by the Company or by companies being, directly or indirectly, controlled by us; |
| | | |
| | ● | loans, other forms of indebtedness, pension benefits not based on occupational pension schemes and performance-based compensation not provided for in the articles of association; and |
| | ● | equity securities and conversion and option rights awards not provided for in the articles of association. |
Compensation to members of the board of directors and executive management for activities in entities that are, directly or indirectly, controlled by the Company is prohibited if the compensation (i) would have been prohibited if it was paid directly by the Company, (ii) is not provided for in the articles of association or (iii) has not been approved by the general meeting of shareholders.
The general meeting of shareholders votes on the compensation received directly or indirectly by the board of directors, the executive management and the advisory board (“Beirat”). The general meeting of shareholders must vote annually on the compensation of its board of directors, executive management and the advisory board, and accordingly, at such a meeting, the vote of the general meeting of shareholders shall have a binding effect.
In the event that the general meeting of shareholders votes prospectively on the compensation of the executive management, the articles of association may provide for an additional amount for the compensation of the members of the executive management appointed after the vote.
The additional amount may only be used if the total amount of the compensation of the executive management decided by the general meeting of shareholders is not sufficient for the compensation of the new members until the next vote of the general meeting of the shareholders.
The general meeting of shareholders shall not vote on the additional amount of compensation.
Borrowing Powers
Neither Swiss law nor our articles of association restrict in any way our power to borrow and raise funds. The decision to borrow funds is made by or under the direction of our board of directors, and no approval by the shareholders is required in relation to any such borrowing.
Repurchases of Shares and Purchases of Own Shares and Other Limitations on the Rights to Own Securities
The CO limits our right to purchase and hold our own shares. We and our subsidiaries may purchase shares only if and to the extent that (i) freely disposable equity capital is available in the required amount; and (ii) the combined nominal value of all such shares does not exceed 10% of the share capital. Pursuant to Swiss law, where shares are acquired in connection with a transfer restriction set out in the articles of association, the foregoing upper limit is 20%. We currently do not have any transfer restriction in our articles of association. If we own shares that exceed the threshold of 10% of our share capital, the excess must be sold or cancelled by means of a capital reduction within a reasonable time.
Shares held by us or our subsidiaries are not entitled to vote at the general meeting of shareholders but are entitled to the economic benefits applicable to the shares generally, including dividends and pre-emptive rights in the case of share capital increases.
Swiss law and/or our articles of association do not impose any restrictions on the exercise of voting or any other shareholder rights by shareholders residing outside of Switzerland.
Notification and Disclosure of Substantial Share Interests
The disclosure obligations generally applicable to shareholders of Swiss corporations under the Swiss Financial Market Infrastructure Act do not apply to us since our shares are not listed on a Swiss exchange. Similarly, the Swiss takeover regime imposes a duty on any person or group of persons who acquires more than one-third of a company's voting rights to make a mandatory offer for all of the company's outstanding listed equity securities. However, these protections are applicable only to issuers that list their equity securities in Switzerland and, because our common shares will be listed exclusively on Nasdaq, will not be applicable to us.
Pursuant to Article 663c of the CO, Swiss corporations whose shares are listed on a stock exchange must specify the significant shareholders and their shareholdings in the notes to the balance sheet, where these are known or ought to be known. Significant shareholders are defined as shareholders and groups of shareholders linked through voting rights who own more than five percent of all voting rights.
Stock Exchange Listing
We have applied to list our common shares on the Nasdaq Capital Market under the symbol “NLSP.”
The Depository Trust Company
Initial settlement of any common shares to be issued pursuant to this prospectus will take place through DTC, in accordance with its customary settlement procedures for equity securities. Each person owning common shares held through DTC must rely on the procedures thereof and on institutions that have accounts therewith to exercise any rights of a holder of the shares.
Transfer Agent and Registrar of Shares
Our share register is currently kept by VStock Transfer LLC, which acts as transfer agent and registrar. The share register reflects only record owners of our common shares.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, no public market existed for our common shares or the Warrants. Sales of substantial amounts of our common shares following this offering, or the perception that these sales could occur, could adversely affect prevailing market prices of our common shares or Warrants and could impair our future ability to obtain capital, especially through an offering of equity securities. Assuming that the underwriters do not exercise their over-allotment option with respect to this offering and assuming no exercise of options outstanding following the offering, we will have an aggregate of 10,596,363 common shares outstanding upon completion of this offering. Of these shares, the common shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, unless purchased by “affiliates” (as that term is defined under Rule 144 of the Securities Act), who may sell only the volume of shares described below and whose sales would be subject to additional restrictions described below.
The remaining common shares will be held by our existing shareholders. Because substantially all of these shares were sold outside the United States to persons residing outside the United States at the time, they also will be freely tradable without restriction or further registration, except that shares held by affiliates must be sold pursuant to Rule 144 or another available exemption, and except for the lock-up restrictions described below. Further, substantially all of our common shares outstanding prior to this offering are subject to lock-up agreements described below.
Certain existing officers, who are also shareholders, and a director nominee, have indicated an interest in purchasing up to an aggregate of $138,000 of Units in this offering at the initial public offering price per Unit. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, less or no Units in this offering to any of these persons, or any of these persons may determine to purchase more, less or no Units in this offering. The underwriters will receive the same underwriting discount on any Units purchased by these persons as they will on any other Units sold to the public in this offering.
Lock-Up Agreements
We, all of our directors and executive officers and holders of all of our outstanding common shares are expected to sign lock-up agreements pursuant to which, subject to certain exceptions, they will agree not to sell or otherwise dispose of their common shares or any securities convertible into or exchangeable for common shares, including securities purchased in this offering, if any, for a period of at least 180 days after the date of the pricing of our initial public offering without the prior written consent of the representative of the underwriters.
Rule 144
In general, under Rule 144, any person who is not our affiliate (and has not been an affiliate of ours for the last three months) and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction as long as we continue to file required public information with the SEC. In addition, under Rule 144, any person who is not an affiliate of ours (and has not been an affiliate of ours for the last three months) and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the completion of this offering without regard to whether current public information about us is available.
A person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three-month period that does not exceed the greater of:
| | ● | one percent of the number of common shares then outstanding; or |
| | ● | the average weekly trading volume of our common shares on Nasdaq during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
If an affiliate acquires “restricted securities,” those securities will also be subject to holding period requirements.
Substantially all of our outstanding common shares are expected to be either unrestricted or to be eligible for sale under Rule 144. We cannot estimate the number of our common shares that our existing shareholders will elect to sell.
Regulation S
Regulation S under the Securities Act provides that securities owned by any person may be sold without registration in the United States, provided that the sale is affected in an offshore transaction and no directed selling efforts are made in the United States (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our common shares may be sold in some manner outside the United States without requiring registration in the United States.
Rule 701
In general, Rule 701 under the Securities Act, provides that each of our employees, consultants or advisors who received our common shares from us in connection with a compensatory share plan or other written agreement executed prior to the completion of this offering is eligible to resell such common shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144.
Form S-8 Registration Statement
Following the completion of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register common shares issued or reserved for issuance under an equity incentive plan that we intend to adopt. The registration statement on Form S-8 will become effective automatically upon filing. Common shares issued upon exercise of a share option and registered under the Form S-8 registration statement will, subject to vesting and lock-up provisions and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately unless they are subject to a lock-up, in which case, immediately after the term of the lock-up expires.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL SHARE TRANSFER RESTRICTION MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN LEGAL ADVISOR REGARDING THE PARTICULAR SECURITIES LAWS AND TRANSFER RESTRICTION CONSEQUENCES OF PURCHASING, HOLDING, AND DISPOSING OF THE COMMON SHARES INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
COMPARISON OF SWISS LAW AND DELAWARE LAW
The Swiss laws applicable to Swiss corporations and their shareholders differ from laws applicable to U.S. corporations and their shareholders. The following table summarizes significant differences in shareholder rights between the applicable provisions of the CO and the Compensation Ordinance (as a company incorporated in Switzerland and listed on a stock exchange) and the Delaware General Corporation Law, applicable to companies incorporated in Delaware and their shareholders. Please note that this is only a general summary of certain provisions applicable to companies in Delaware. Certain Delaware companies may be permitted to exclude certain of the provisions summarized below in their charter documents.
| DELAWARE CORPORATE LAW | | SWISS CORPORATE LAW |
| | | |
| Mergers and similar arrangements |
| |
| Under the Delaware General Corporation Law, with certain exceptions, a merger, consolidation, sale, lease or transfer of all or substantially all of the assets of a corporation must be approved by the board of directors and a majority of the outstanding shares entitled to vote thereon. A shareholder of a Delaware corporation participating in certain major corporate transactions may, under certain circumstances, be entitled to appraisal rights pursuant to which such shareholder may receive cash in the amount of the fair value of the shares held by such shareholder (as determined by a court) in lieu of the consideration such shareholder would otherwise receive in the transaction. The Delaware General Corporation Law also provides that a parent corporation, by resolution of its board of directors, may merge with any subsidiary of which it owns at least 90.0% of each class of capital stock without a vote by the shareholders of such subsidiary. Upon any such merger, dissenting shareholders of the subsidiary would have appraisal rights. | | Under Swiss law, with certain exceptions, a merger or a division of the corporation or a sale of all or substantially all of the assets of a corporation must be approved by two-thirds of the shares represented at the respective general meeting of shareholders as well as the absolute majority of the share capital represented at such shareholders’ meeting. The articles of association may increase the voting threshold. A shareholder of a Swiss corporation participating in a statutory merger or demerger pursuant to the Swiss Merger Act can file an appraisal right lawsuit against the surviving company. As a result, if the consideration is deemed “inadequate,” such shareholder may, in addition to the consideration (be it in shares or in cash) receive an additional amount to ensure that such shareholder receives the fair value of the shares held by such shareholder. Swiss law also provides that a parent corporation, by resolution of its board of directors, may merge with any subsidiary of which it owns at least 90.0% of the shares without a vote by shareholders of such subsidiary, if the shareholders of the subsidiary are offered the payment of the fair value in cash as an alternative to shares. |
| | | |
| Shareholders’ suits |
| |
| Class actions and derivative actions generally are available to shareholders of a Delaware corporation for, among other things, breach of fiduciary duty, corporate waste and actions not taken in accordance with applicable law. In such actions, the court has discretion to permit the winning party to recover attorneys’ fees incurred in connection with such action. | | Class actions and derivative actions as such are not available under Swiss law. Nevertheless, certain actions may have a similar effect. A shareholder is entitled to bring suit against directors for breach of, among other things, their fiduciary duties and claim the payment of the company’s damages to the corporation. Likewise, an appraisal lawsuit won by a shareholder will indirectly compensate all shareholders. Under Swiss law, the winning party is generally entitled to recover attorneys’ fees incurred in connection with such action, provided, however, that the court has discretion to permit the shareholder whose claim has been dismissed to recover attorneys’ fees incurred to the extent he acted in good faith. |
| DELAWARE CORPORATE LAW | | SWISS CORPORATE LAW |
| Shareholder vote on board and management compensation |
| |
| Under the Delaware General Corporation Law, the board of directors has the authority to fix the compensation of directors, unless otherwise restricted by the certificate of incorporation or bylaws. | | Pursuant to the Compensation Ordinance, applicable to Swiss companies traded on exchanges, such as Nasdaq, the general meeting of shareholders has the non-transferable right, amongst others, to vote separately (in a binding vote) on the aggregate compensation due to the board of directors, executive management and advisory boards. |
| | | |
| Annual vote on board renewal |
| |
| Unless otherwise specified in the certificate of incorporation or bylaws of the corporation, directors shall be elected by a plurality of votes of the shareholders on a date and at a time designated by or in the manner provided in the bylaws. Re-election is possible. | | The general meeting of shareholders elects annually (i.e., until the following general meeting of shareholders), by a plurality of votes, the members of the board of directors (including the chairman) and the members of the compensation committee individually for a term of office of one year. Re-election is possible. |
| | | |
| Indemnification of directors and executive management and limitation of liability |
| |
The Delaware General Corporation Law provides that a certificate of incorporation may contain a provision eliminating or limiting the personal liability of directors, officers, employees or agents of the corporation for monetary damages for breach of a fiduciary duty as a director, except no provision in the certificate of incorporation may eliminate or limit the liability of a director for: any breach of a director’s duty of loyalty to the corporation or its shareholders; acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; statutory liability for unlawful payment of dividends or unlawful share purchase or redemption; or any transaction from which the director derived an improper personal benefit. A Delaware corporation may indemnify any person who was or is a party or is threatened to be made a party to any proceeding, other than an action by or on behalf of the corporation, because the person is or was a director or officer, against liability incurred in connection with the proceeding if the director or officer acted in good faith and in a manner reasonably believed to be in, or not opposed to, the best interests of the corporation; and the director or officer, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. | | Under Swiss corporate law, an indemnification of a director or member of the executive management in relation to potential personal liability is not effective to the extent the director or member of the executive management intentionally or negligently violated his or her corporate duties towards the corporation (certain views advocate that at least a grossly negligent violation is required to exclude the indemnification). Most violations of corporate law are regarded as violations of duties towards the corporation rather than towards the shareholders. In addition, indemnification of other controlling persons is not permitted under Swiss corporate law, including shareholders of the corporation. Nevertheless, a corporation may enter into and pay for directors’ and officers’ liability insurance which typically covers negligent acts as well. |
| DELAWARE CORPORATE LAW | | SWISS CORPORATE LAW |
Unless ordered by a court, any foregoing indemnification is subject to a determination that the director or officer has met the applicable standard of conduct: by a majority vote of the directors who are not parties to the proceeding, even though less than a quorum; by a committee of directors designated by a majority vote of the eligible directors, even though less than a quorum; by independent legal counsel in a written opinion if there are no eligible directors, or if the eligible directors so direct; or by the shareholders. Moreover, a Delaware corporation may not indemnify a director or officer in connection with any proceeding in which the director or officer has been adjudged to be liable to the corporation unless and only to the extent that the court determines that, despite the adjudication of liability but in view of all the circumstances of the case, the director or officer is fairly and reasonably entitled to indemnity for those expenses which the court deems proper. | | |
| | | |
| Directors’ fiduciary duties |
| |
A director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the procedural fairness of the transaction, and that the transaction was of fair value to the corporation. | | A director of a Swiss corporation has a fiduciary duty to the corporation only. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent director would exercise under similar circumstances. Under this duty, a director must inform himself of, and disclose, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interest of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits in principle self-dealing by a director and mandates that the best interest of the corporation take precedence over any interest possessed by a director or officer. The burden of proof for a violation of these duties is with the corporation or with the shareholder bringing a suit against the director. Directors also have an obligation to treat shareholders equally proportionate to their share ownership. |
| DELAWARE CORPORATE LAW | | SWISS CORPORATE LAW |
| Shareholder action by written consent |
| |
| A Delaware corporation may, in its certificate of incorporation, eliminate the right of shareholders to act by written consent. | | Shareholders of a Swiss corporation may only exercise their voting rights in a general meeting of shareholders and may not act by written consent. |
| | | |
| Shareholder proposals |
| |
| A shareholder of a Delaware corporation has the right to put any proposal before the annual meeting of shareholders, provided it complies with the notice provisions in the governing documents. A special meeting may be called by the board of directors or any other person authorized to do so in the governing documents, but shareholders may be precluded from calling special meetings. | | At any general meeting of shareholders any shareholder may put proposals to the meeting if the proposal is part of an agenda item. Unless the articles of association provide for a lower threshold or for additional shareholders’ rights: one or several shareholders representing 10.0% of the share capital may ask that a general meeting of shareholders be called for specific agenda items and specific proposals; and one or several shareholders representing 10.0% of the share capital or CHF 1.0 million of nominal share capital may ask that an agenda item including a specific proposal be put on the agenda for a regularly scheduled general meeting of shareholders, provided such request is made with appropriate notice. Any shareholder can propose candidates for election as directors without prior written notice. In addition, any shareholder is entitled, at a general meeting of shareholders and without advance notice, to (i) request information from the Board on the affairs of the company (note, however, that the right to obtain such information is limited), (ii) request information from the auditors on the methods and results of their audit and (iii) request, under certain circumstances and subject to certain conditions, a special audit. |
| | | |
| Cumulative voting |
| |
| Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation provides for it. | | Cumulative voting is not permitted under Swiss corporate law. Pursuant to Swiss law, shareholders can vote for each proposed candidate, but they are not allowed to cumulate their votes for single candidates. An annual individual election of (i) all members of the board of directors, (ii) the chairperson of the board of directors, (iii) the members of the compensation committee, and (iv) the election of the independent proxy for a term of office of one year (i.e., until the following annual general meeting of shareholders), as well as the vote on the aggregate amount of compensation of the members of the board of directors, of the executive committee and of the members of any advisory board, is mandatory for listed companies. Re-election is permitted. |
| DELAWARE CORPORATE LAW | | SWISS CORPORATE LAW |
| Removal of directors |
| |
| Unless there is cumulative voting or there is a classified board, generally a director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors | | A Swiss corporation may remove, with or without cause, any director at any time with a resolution passed by an absolute majority of the shares represented at a general meeting of shareholders. The articles of association may provide for a qualified majority for the removal of a director. |
| | | |
| Transactions with interested shareholders |
| |
| The Delaware General Corporation Law generally prohibits a Delaware corporation from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or group who or which owns or owned 15.0% or more of the corporation’s outstanding voting shares within the past three years. | | No such rule applies to a Swiss corporation. |
| | | |
| Dissolution; winding up |
| |
| Unless the board of directors of a Delaware corporation approves the proposal to dissolve, dissolution must be approved by shareholders holding 100.0% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board. | | A dissolution and winding up of a Swiss corporation requires the approval by two-thirds of the shares represented as well as the absolute majority of the nominal value of the share capital represented at a general meeting of shareholders passing a resolution on such dissolution and winding up. The articles of association may increase the voting thresholds required for such a resolution (but only by way of a resolution with the majority stipulated by law). |
| | | |
| Variation of rights of shares |
| |
| A Delaware corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise. | | The general meeting of shareholders of a Swiss corporation may resolve that preference shares be issued or that existing shares be converted into preference shares with a resolution passed by a majority of the shares represented at the general meeting of shareholders. Where a company has issued preference shares, further preference shares conferring preferential rights over the existing preference shares may be issued only with the consent of both a special meeting of the adversely affected holders of the existing preference shares and of a general meeting of all shareholders, unless otherwise provided in the articles of association. Shares that are granted more voting power are not regarded as a special class for these purposes. |
| | | |
| Amendment of governing documents |
| |
| A Delaware corporation’s governing documents may be amended with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. | | By way of a public deed, the articles of association of a Swiss corporation may be amended with a resolution passed by an absolute majority of the shares represented at such meeting, unless otherwise provided in the articles of association. There are a number of resolutions, such as an amendment of the stated purpose of the corporation and the introduction of authorized and conditional capital, that require the approval by two-thirds of the votes and an absolute majority of the nominal value of the shares represented at a shareholders’ meeting. The articles of association may increase the voting thresholds. |
| DELAWARE CORPORATE LAW | | SWISS CORPORATE LAW |
| Inspection of Books and Records |
| |
| Shareholders of a Delaware corporation, upon written demand under oath stating the purpose thereof, have the right during the usual hours for business to inspect for any proper purpose, and to obtain copies of list(s) of shareholders and other books and records of the corporation and its subsidiaries, if any, to the extent the books and records of such subsidiaries are available to the corporation. | | Shareholders of a Swiss corporation may only inspect books and records if the general meeting of shareholders or the board of directors approved such inspection. The inspection right is limited in scope and only extends to information required for the exercise of shareholder rights and does not extend to confidential information. The right to inspect the share register is limited to the right to inspect that shareholder’s own entry in the share register. |
| | | |
| Payment of dividends |
| |
The board of directors may approve a dividend without shareholder approval. Subject to any restrictions contained in its certificate of incorporation, the board may declare and pay dividends upon the shares of its capital stock either: out of its surplus, or in case there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. | | Dividend payments are subject to the approval of the general meeting of shareholders. The board of directors may propose to shareholders that a dividend shall be paid but cannot itself authorize the distribution. Payments out of the Company’s share capital (in other words, the aggregate nominal value of the Company’s registered share capital) in the form of dividends are not allowed and may be made by way of a capital reduction only. Dividends may be paid only from the profits brought forward from the previous business years or if the Company has distributable reserves, each as will be presented on the Company’s audited annual stand-alone balance sheet. The dividend may be determined only after the allocations to reserves required by the law and the articles of association have been deducted. |
| | | |
| Creation and issuance of new shares |
| |
Shareholder approval is required to authorize capital stock in excess of that provided in the charter. The corporation must file a certificate of amendment to its certificate of incorporation before the creation of additional authorized shares may become effective. The board of directors may, without shareholder consent, authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation, or any combination thereof. | | All creation of shares requires a shareholders’ resolution documented by way of a public deed. The creation of authorized or conditional share capital requires at least two-thirds of the voting rights represented at the general meeting of shareholders and an absolute majority of the nominal value of shares represented at such meeting. The board of directors may issue shares out of the authorized share capital during a period of up to two years. Shares are created and issued out of conditional share capital through the exercise of options or of conversion rights that the board of directors may grant in relation to, e.g., debt instruments or to employees. |
TAXATION
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership or disposition of our common shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign, including Swiss, or other taxing jurisdiction.
SWITZERLAND TAX CONSIDERATIONS
This summary of material Swiss tax consequences is based on Swiss law and regulations and the practice of the Swiss tax administration as in effect on the date hereof, all of which are subject to change (or subject to changes in interpretation), possibly with retroactive effect. The summary does not purport to take into account the specific circumstances of any particular shareholder or potential investor and does not relate to persons in the business of buying and selling common shares or other securities. The summary is not intended to be, and should not be interpreted as, legal or tax advice to any particular potential shareholder/s, and no representation with respect to the tax consequences to any particular shareholder/s is made.
Current and prospective shareholders are advised to consult their own tax advisers in light of their particular circumstances as to the Swiss tax laws, regulations and regulatory practices that could be relevant for them in connection with the acquiring, owning and selling or otherwise disposing of common shares and receiving dividends and similar cash or in-kind distributions on common shares (including dividends on liquidation proceeds and stock dividends) or distributions on common shares based upon a capital reduction (“Nennwertrückzahlungen”) or reserves paid out of capital contributions (“Reserven aus Kapitaleinlagen”) and the consequences thereof under the tax laws, regulations and regulatory practices of Switzerland.
Taxation of NLS Pharmaceutics Ltd.
NLS is a Swiss based company, subject to taxation in the Canton of Nidwalden. The Company is taxed at a current effective income tax rate of approximately 12% (including direct federal as well as cantonal/communal taxes). In addition, an annual capital tax rate of approximately 0.01% is levied on the net equity of the Company.
Based on a vote by eligible Swiss voters in Switzerland on May 19, 2019, Switzerland reformed certain elements of its corporate tax law which impact the taxation of NLS (including the abolition of the mixed company privilege at cantonal/communal level). These new federal regulations went into effect as of January 1, 2020. Regarding the implementation of certain measures on the cantonal level, a referendum has been put up for a vote in the Canton of Nidwalden. It is expected that a vote will take place in the course of 2020.
Swiss Federal Withholding Tax on Dividends and other Distributions
Dividend payments and similar cash or in-kind distributions on the common shares (including dividends on liquidation proceeds and stock dividends) that the Company makes to shareholders are subject to Swiss federal withholding tax (“Verrechnungssteuer”) at a rate of 35% on the gross amount of the dividend. The Company is required to withhold the Swiss federal withholding tax from the dividend and remit it to the Swiss Federal Tax Administration. Distributions based upon a capital reduction (“Nennwertrückzahlungen”) and reserves paid out of capital contribution reserves (“Reserven aus Kapitaleinlagen”) are not subject to Swiss federal withholding tax.
The redemption of common shares in the Company may under certain circumstances (in particular, if the common shares in the Company are redeemed for subsequent cancellation) be taxed as a partial liquidation for Swiss federal withholding tax purposes, with the consequence that the difference between the repurchase price and the nominal value of the shares (“Nennwertprinzip”) plus capital contribution reserves (“Reserven aus Kapitaleinlagen”) is subject to Swiss federal withholding tax.
The Swiss federal withholding tax is refundable or creditable in full to a Swiss tax resident corporate and individual shareholder as well as to a non-Swiss tax resident corporate or individual shareholder who holds the common shares as part of a trade or business carried on in Switzerland through a permanent establishment or fixed place of business situated for tax purposes in Switzerland, if such person is the beneficial owner of the distribution and, in the case of a Swiss tax resident individual who holds the common shares as part of his private assets, duly reports the gross distribution received in his individual income tax return or, in the case of a person who holds the common shares as part of a trade or business carried on in Switzerland through a permanent establishment or fixed place of business situated for tax purposes in Switzerland, recognizes the gross dividend distribution for tax purposes as earnings in the income statements and reports the annual profit in the Swiss income tax return.
If a shareholder who is not a Swiss resident for tax purposes and does not hold the common shares in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes in Switzerland, receives a distribution from the Company, the shareholder may be entitled to a full or partial refund or credit of Swiss federal withholding tax incurred on a taxable distribution if the country in which such shareholder is resident for tax purposes has entered into a treaty for the avoidance of double taxation with Switzerland and the further prerequisites of the treaty for a refund have been met. Shareholders not resident in Switzerland should be aware that the procedures for claiming treaty benefits (and the time required for obtaining a refund or credit) may differ from country to country.
Individual and Corporate Income Tax on Dividends
Swiss resident individuals holding the common shares as part of their private assets who receive dividends and similar distributions (including stock dividends and liquidation proceeds), which are not repayments of the nominal value (“Nennwertrückzahlungen”) of the common shares or reserves paid out of capital contributions (“Reserven aus Kapitaleinlagen”) are required to report such payments in their individual income tax returns and are liable to Swiss federal, cantonal and communal income taxes on any net taxable income for the relevant tax period. Furthermore, for the purpose of the Direct Federal Tax, dividends, shares in profits, liquidation proceeds and pecuniary benefits from shares (including bonus shares) are included in the tax base for only 70% of their value (“Teilbesteuerung”), if the investment amounts to at least 10% of nominal share capital of the Company. All Swiss cantons have introduced partial taxation measures at cantonal and communal levels.
Swiss resident individuals as well as non-Swiss resident individual taxpayers holding the common shares in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are required to recognize dividends, distributions based upon a capital reduction (“Nennwertrückzahlungen”) and reserves paid out of capital contributions (“Reserven aus Kapitaleinlagen’) in their income statements for the relevant tax period and are liable to Swiss federal, cantonal and communal individual or corporate income taxes, as the case may be, on any net taxable earnings accumulated (including the payment of dividends) for such period. Furthermore, for the purpose of the Direct Federal Tax, dividends, shares in profits, liquidation proceeds and pecuniary benefits from shares (including bonus shares) are included in the tax base for only 70% (“Teilbesteuerung”), if the investment is held in connection with the conduct of a trade or business or qualifies as an opted business asset (“gewillkürtes Geschäftsvermögen”) according to Swiss tax law and amounts to at least 10% of nominal share capital of the Company. All cantons have introduced partial taxation measures at cantonal and communal levels.
Swiss resident corporate taxpayers as well as non-Swiss resident corporate taxpayers holding the common shares in connection with the conduct of a trade or business through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are required to recognize dividends, distributions based upon a capital reduction (“Nennwertrückzahlungen”) and reserves paid out of capital contributions (“Reserven aus Kapitaleinlagen”) in their income statements for the relevant tax period and are liable to Swiss federal, cantonal and communal corporate income taxes on any net taxable earnings accumulated for such period. Swiss resident corporate taxpayers as well as non-Swiss resident corporate taxpayers holding the common shares in connection with the conduct of a trade or business through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland may be eligible for participation relief (“Beteiligungsabzug”) in respect of dividends and distributions based upon a capital reduction (“Nennwertrückzahlungen”) and reserves paid out of capital contributions (“Reserven aus Kapitaleinlagen”) if the common shares held by them as part of a Swiss business have an aggregate market value of at least CHF 1 million or represent at least 10% of the nominal share capital of the Company or give entitlement to at least 10% of the profits and reserves of the Company, respectively.
Recipients of dividends and similar distributions on the common shares (including stock dividends and liquidation proceeds) who neither are residents of Switzerland nor during the current taxation year have engaged in a trade or business in Switzerland and who are not subject to taxation in Switzerland for any other reason are not subject to Swiss federal, cantonal or communal individual or corporate income taxes in respect of dividend payments and similar distributions because of the mere holding of the common shares.
Wealth and Annual Capital Tax on Holding of Common Shares or Warrants
Swiss resident individuals and non-Swiss resident individuals holding the common shares and/or Warrants in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are required to report their common shares as part of their wealth and will be subject to cantonal and communal wealth tax to the extent the aggregate taxable net wealth is allocable to Switzerland.
Swiss resident corporate taxpayers and non-Swiss resident corporate taxpayers holding the common shares and/or Warrants in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, will be subject to cantonal and communal annual capital tax on the taxable capital to the extent the aggregate taxable capital is allocable to Switzerland.
Individuals and corporate taxpayers not resident in Switzerland for tax purposes and not holding the common shares and/or Warrants in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are not subject to wealth or annual capital tax in Switzerland because of the mere holding of the common shares and/or Warrants.
Capital Gains on Disposal of Common Shares or Warrants
Swiss resident individuals who sell or otherwise dispose of the common shares and/or Warrants realize a tax-free capital gain, or a non-tax deductible capital loss, as the case may be, provided that they hold the common shares, as part of their private assets. Under certain circumstances, the sale proceeds may be requalified into taxable investment income (e.g., if the taxpayer is deemed to be a professional securities dealer).
Capital gains realized on the sale of the common shares and/or Warrants held by Swiss resident individuals, Swiss resident corporate taxpayers as well as non-Swiss resident individuals and corporate taxpayers holding the common shares and/or Warrants in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, will be subject to Swiss federal, cantonal and communal individual or corporate income tax, as the case may be. This also applies to Swiss resident individuals who, for individual income tax purposes, are deemed to be professional securities dealers for reasons of, inter alia, frequent dealing and debt-financed purchases. Capital gains realized by resident individuals who hold the common shares as business assets might be entitled to reductions or partial taxations similar to those mentioned above for dividends (“Teilbesteuerung”) if certain conditions are met (e.g. holding period of at least one year and participation of at least 10% of nominal share capital of the Company).
Swiss resident corporate taxpayers as well as non-Swiss resident corporate taxpayers holding the common shares and/or Warrants in connection with the conduct of a trade or business, through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are required to recognize such capital gain in their income statements for the relevant tax period. Corporate taxpayers may qualify for participation relief on capital gains (“Beteiligungsabzug”), if the common shares sold during the tax period represent at least 10% of the Company’s share capital or if the common shares sold give entitlement to at least 10% of the Company’s profits and reserves and were held for at least one year. The tax relief applies to the difference between the sale proceeds of common shares by the Company and the acquisition costs of the participation (“Gestehungskosten”).
Individuals and corporations not resident in Switzerland for tax purposes and not holding the common shares and/or Warrants in connection with the conduct of a trade or business in Switzerland through a permanent establishment or fixed place of business situated, for tax purposes, in Switzerland, are not subject to Swiss federal, cantonal and communal individual income or corporate income tax, as the case may be, on capital gains realized on the sale of the common shares and/or Warrants.
Gift and Inheritance Tax
Transfers of common shares and/or Warrants may be subject to cantonal and/or communal inheritance or gift taxes if the deceased or the donor or the recipient were resident in a Canton levying such taxes.
Swiss Issuance Stamp Duty
The Company is subject to paying to the Swiss Federal Tax Administration a 1% Swiss federal issuance stamp tax (“Emissionsabgabe”) on any increase of the nominal share capital of the Company (including capital surplus) or any other equity contributions received by the Company (with or without issuance of shares). Certain costs incurred in connection with the issuance of shares (if any) may be deductible. There are several exemptions from issuance stamp tax that may apply under certain circumstances (e.g., certain intercompany reorganizations).
Swiss Securities Transfer Tax
The purchase or sale (or other financial transfer) of the common shares, whether by Swiss residents or non-Swiss residents, may be subject to Swiss securities transfer tax of up to 0.15%, calculated on the purchase price or the proceeds if the purchase or sale occurs through or with a bank or other securities dealer in Switzerland or Liechtenstein as defined in the Swiss Federal Stamp Duty Act as an intermediary or party to the transaction unless an exemption applies.
Automatic Exchange of Information in Tax Matters
On November 19, 2014, Switzerland signed the multilateral competent authority agreement on the automatic exchange of financial account information, which is intended to ensure the uniform implementation of automatic exchange of information, or the AEOI.
The AEOI is being introduced in Switzerland through bilateral agreements or multilateral agreements. Switzerland has concluded a multilateral agreement with the EU on the AEOI in tax matters, or the AEOI Agreement. This AEOI Agreement entered into force as of January 1, 2017 and applies to all 28 member states as well as Gibraltar. Furthermore, on January 1, 2017, the multilateral competent authority agreement on the automatic exchange of financial account information and, based on such agreement, a number of bilateral AEOI agreements with other jurisdictions entered into force. The Federal Act on the International Automatic Exchange of Information in Tax Matters, or the AEOI Act, which is the primary legal basis for the implementation of the AEOI standard in Switzerland, entered into force on January 1, 2017 as well.
Based on such multilateral agreements and bilateral agreements and the implementing laws of Switzerland, Switzerland collects data in respect of financial assets, which may include Shares, held in, and income derived thereon and credited to, accounts or deposits with a paying agent in Switzerland for the benefit of individuals resident in a EU member state or in a treaty state since 2017, and exchanges it since 2018. Switzerland has signed and is expected to sign further bi- or multilateral AEOI in tax matter agreements with other countries, which entered into force on January 1, 2018 or January 1, 2019 or will enter into force at a later date.
A list of such multilateral agreements and bilateral agreements of Switzerland in effect or signed and becoming effective can be found on the website of the State Secretariat for International Finance (SIF).
On February 27, 2019, the Federal Council initiated the consultation on the revision of the AEOI Act and AEOI Ordinance. The consultation proposal takes account of recommendations from the Global Forum on Transparency and Exchange of Information for Tax Purposes. They concern, among other things, certain due diligence and registration obligations, the maintenance of a document retention obligation for reporting Swiss financial institutions, as well as definitions thereunder. Some exceptions have also been removed or adapted. The amendments to the AEOI Act and AEOI Ordinance are expected to enter into force on January 1, 2021.
Swiss Facilitation of the Implementation of the U.S. Foreign Account Tax Compliance Act
Switzerland has concluded an intergovernmental agreement with the U.S. to facilitate the implementation of the Foreign Account Tax Compliance Act (FATCA). The agreement ensures that the accounts held by U.S. persons with Swiss financial institutions are disclosed to the U.S. tax authorities either with the consent of the account holder or by means of group requests within the scope of administrative assistance. Information will not be transferred automatically in the absence of consent, and instead will be exchanged only within the scope of administrative assistance on the basis of the double taxation agreement between the U.S. and Switzerland. On October 8, 2014, the Swiss Federal Council approved a mandate for negotiations with the U.S. on changing the current direct-notification-based regime to a regime where the relevant information is sent to the Swiss Federal Tax Administration, which in turn provides the information to the U.S. tax authorities. The new regime may come into force earliest in 2020.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE PARTICULAR SWISS TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON SHARES, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.U.S. FEDERAL INCOME TAX CONSEQUENCES
THE FOLLOWING SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF COMMON SHARES AND AMERICAN DEPOSITARY SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the common shares. For this purpose, a “U.S. Holder” is a holder of common shares or Warrants that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our common shares. This summary generally considers only U.S. Holders that will own our common shares or Warrants as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, (including with respect to the TCJA), and the U.S./Switzerland Income Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the IRS with regard to the U.S. federal income tax treatment of an investment in our common shares by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity;” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our common shares or Warrants in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our common shares or Warrants as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, common shares representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities) or persons who hold common shares through a partnership or other pass-through entity are not addressed.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our common shares, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Exercise or Expiry of Warrants
No gain or loss will be realized on the exercise of a Warrant. When a Warrant is exercised, the U.S. Holder’s cost of the common share acquired thereby will be equal to the U.S. Holder’s adjusted cost basis of the Warrant plus the exercise price paid for the common share. The expiry of an unexercised Warrant will generally give rise to a capital loss equal to the adjusted cost basis to the U.S. Holder of the expired Warrant. The holding period of the common share acquired thru the exercise of a Warrant includes the holding period of the Warrant. See “Passive Foreign Investment Companies,” for the impact of the exercise of a Warrant on taxation of a U.S. Holder if we are a PFIC.
Taxation of Dividends Paid on Common Shares
We do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under the heading “Passive Foreign Investment Companies” below and the discussion of “qualified dividend income” below, a U.S. Holder, other than certain U.S. Holder’s that are U.S. corporations, will be required to include in gross income as ordinary income the amount of any distribution paid on common shares (including the amount of any Swiss tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution which exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the common shares to the extent thereof, and then capital gain. We do not expect to maintain calculations of our earnings and profits under U.S. federal income tax principles and, therefore, U.S. Holders should expect that the entire amount of any distribution generally will be reported as dividend income.
In general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, inter alia, dividends received from a “qualified foreign corporation.” A “qualified foreign corporation” is a corporation that is entitled to the benefits of a comprehensive tax treaty with the United States which includes an exchange of information program. The IRS has stated that the Switzerland/U.S. Tax Treaty satisfies this requirement and we believe we are eligible for the benefits of that treaty.
In addition, our dividends will be qualified dividend income if our common shares are readily tradable on Nasdaq or another established securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend is paid or in the prior year, as a PFIC, as described below under “Passive Foreign Investment Companies.” A U.S. Holder will not be entitled to the preferential rate: (1) if the U.S. Holder has not held our common shares for at least 61 days of the 121 day period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder is under an obligation to make related payments on substantially similar property. Any days during which the U.S. Holder has diminished its risk of loss on our common shares are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who elect to treat the dividend income as “investment income” pursuant to Code section 163(d)(4) will not be eligible for the preferential rate of taxation.
The amount of a distribution with respect to our common shares will be measured by the amount of the fair market value of any property distributed, and for U.S. federal income tax purposes, the amount of any Swiss taxes withheld therefrom. Cash distributions paid by us in NIS will be included in the income of U.S. Holders at a U.S. dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such NIS for U.S. federal income tax purposes equal to such U.S. dollar value. If the U.S. Holder subsequently converts the NIS into U.S. dollars or otherwise disposes of it, any subsequent gain or loss in respect of such NIS arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
Except as described below in “Passive Foreign Investment Companies,” ownership of the Warrants has no tax impact on U.S. Holders since holders of the Warrants do not receive distributions unless and until the Warrants are exercised and common shares are purchased. As described below, if we are a PFIC, ownership of the Warrants may impact the holding period of PFIC shares and impact elections under the PFIC tax rules.
Taxation of the Disposition of Common Shares and Warrants
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or other disposition of our common shares or the Warrants, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the common shares and Warrants in U.S. dollars and the amount realized on the disposition in U.S. dollar (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of common shares and Warrants will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition. Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses is subject to various limitations.
Passive Foreign Investment Companies
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares and warrants of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
| | ● | 75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or |
| | ● | At least 50% of our assets, averaged over the year and generally determined based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income. |
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income.
We do not believe that we will be a PFIC for the current taxable year although we have not determined whether we will be a PFIC in the future. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our common shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC.
If we currently are or become a PFIC, each U.S. Holder who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our common shares and Warrants at a gain: (1) have such distribution or gain allocated ratably over the U.S. Holder’s holding period for the common shares, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, when shares and warrants of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
A U.S. Holder of our Warrants is taxed in a manner similar to a U.S. holder of common shares if the holder realizes gain on the sale of the Warrants. If the holder of the Warrants exercises the Warrants to purchase common shares, the holding period over which any income realized is allocated includes the holding period of the Warrants. The U.S. Warrant holder is treated as a holder of PFIC stock taxable under the ordinary income allocation and interest charge regime described above.
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the common shares while we are a PFIC, provided that we comply with specified reporting requirements. A U.S. Holder of our Warrants may not make a QEF election regarding our Warrants. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. We do not intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year. In addition, we do not intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. Therefore, the QEF election will not be available with respect to our common shares.
In addition, except with respect to the Warrants (unless exercised), the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our common shares which are regularly traded on a qualifying exchange, including Nasdaq, can elect to mark the common shares to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the common shares and the U.S. Holder’s adjusted tax basis in the common shares. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our common shares and Warrants during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules.
Tax on Net Investment Income
U.S. Holders who are individuals, estates or trusts will generally be required to pay a 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition of our common shares), or in the case of estates and trusts on their net investment income that is not distributed. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Tax Consequences for Non-U.S. Holders of Common Shares and Warrants
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our common shares.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our common shares (but not Warrants) or gain from the disposition of our common shares and Warrants if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; or (2) in the case of a disposition of our common shares and Warrants, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our common shares if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding at a rate of 24% with respect to cash dividends and proceeds from a disposition of common shares. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Pursuant to recently enacted legislation, a U.S. Holder with interests in “specified foreign financial assets” (including, among other assets, our common shares, unless such common shares are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file a Report of Foreign Bank and Financial Accounts if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
UNDERWRITING
We are offering the Units described in this prospectus through the underwriters named below. Maxim Group LLC, or Maxim, is acting as representative of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase, and we have agreed to sell to the underwriters, the number of Units listed next to its name in the following table.
| Underwriters | | Number
of Units | |
| Maxim Group LLC | | | | |
| Brookline Capital Markets, a division of Arcadia Securities, LLC | | | | |
| | | | | |
| | | | | |
| Total | | | | |
The underwriting agreement provides that the underwriters must buy all of the Units being sold in this offering if they buy any of them. However, the underwriters are not required to take or pay for the Units covered by the underwriters’ option to purchase additional Units as described below.
Our Units are offered subject to a number of conditions, including:
| | ● | receipt and acceptance of the securities underlying the Units by the underwriters; and |
| | ● | the underwriters’ right to reject orders in whole or in part. |
We have been advised by Maxim that the underwriters intend to make a market in our Units but that they are not obligated to do so and may discontinue making a market at any time without notice.
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
Option to Purchase Additional Common Shares
We have granted the representative an option to buy up to 545,454 common shares, at the public offering price minus $ , and/or Warrants to purchase up to 545,454 common shares at a price of $ per Warrant, in each case less the underwriting discounts and commissions, solely to cover over-allotments, if any. The underwriters have 45 days from the date of this prospectus to exercise this option. If the underwriters exercise this option, they will each purchase additional common shares and/or Warrants approximately in proportion to the amounts specified in the table above.
Underwriting Discount
Units sold by the underwriters to the public will initially be offered at the initial offering price set forth on the cover of this prospectus. Any Units sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. The underwriters may offer the common shares through one or more of their affiliates or selling agents. If all the common shares are not sold at the initial public offering price, Maxim may change the offering price and the other selling terms. Upon execution of the underwriting agreement, the underwriters will be obligated to purchase the common shares at the prices and upon the terms stated therein.
The following table shows the per Unit and total underwriting discount we will pay to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase up to 545,454 additional common shares and/or additional Warrants to purchase up to 545,454 common shares solely to cover over-allotments, if any.
| | | Per Unit | | | Total without Over-Allotment Option | | | Total with Over-Allotment Option | |
| Public offering price | | $ | | | | $ | | | | $ | | |
| Underwriting discounts and commissions (8%) | | $ | | | | $ | | | | $ | | |
| Proceeds, before expenses to us | | $ | | | | $ | | | | $ | | |
We have agreed to pay Maxim’s out-of-pocket accountable expenses, including Maxim’s legal fees, up to a maximum amount of $125,000 if this offering is completed. We have paid $25,000 to Maxim as an advance to be applied towards reasonable out-of-pocket expenses, or the Advance. Any portion of the Advance shall be returned back to us to the extent not actually incurred.
We estimate that the total expenses of the offering payable by us, not including the underwriting discount or the Transaction Fee, will be approximately $0.9 million if we receive the Estimated Net Proceeds. We have also agreed to reimburse the underwriters for certain expenses incurred by them.
Representative’s Warrants
We have also agreed to issue to Maxim (or its permitted assignees) warrants to purchase a number of our shares equal to an aggregate of 3% of the total number of common shares sold in this offering, or the Representative’s Warrants. The Representative’s Warrants will have an exercise price equal to 125% of the offering price of the common shares sold in this offering and may be exercised on a cashless basis. The Representative’s Warrants are exercisable commencing six months after the effective date of the registration statement related to this offering, and will expire five years after the effective date of such registration statement. The Representative’s Warrants are not redeemable by us. We have agreed to a one time demand registration of the common shares underlying the Representative’s Warrants at our expense and an additional demand registration at the holders’ expense for a period of five years from the effective date of the registration statement related to this offering. The Representative’s Warrants also provide for unlimited “piggyback” registration rights at our expense with respect to the underlying common shares during the five year period commencing from the effective date of the registration statement related to this offering. The Representative’s Warrants and the common shares underlying the Representative’s Warrants, have been deemed compensation by the Financial Industry Regulatory Authority, or FINRA, and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The underwriters (or permitted assignees under the Rule) may not sell, transfer, assign, pledge or hypothecate the Representative’s Warrants or the securities underlying the Representative’s Warrants, nor will they engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the Representative’s Warrants or the underlying securities for a period of six months from the effective date of this offering, except to any FINRA member participating in the offering and their bona fide officers or partners. The Representative’s Warrants will provide for adjustment in the number and price of such Representative’s Warrants (and the common shares underlying such Representative’s Warrants) to prevent dilution in the event of a forward or reverse stock split, stock dividend or similar recapitalization.
Right of First Refusal
We have agreed to grant Maxim, for the 12 month period following the effective date of the registration statement related to this offering, a right of first refusal to act as lead manager and book runner for any and all future public or private equity, equity-linked and debt (excluding commercial bank) offerings or as exclusive financial advisor with respect to any merger, acquisition, or sale of stock or assets during such 12 month period by us, or any successor to or any subsidiary of our company subject to such procedures as agreed upon in the underwriting agreement.
Lock-Up Agreements
We and our directors, officers and any holder of 5% or more of our outstanding common shares as of the effective date of the registration statement related to this offering (and all holders of securities exercisable for or convertible into common shares) shall enter into customary “lock-up” agreements in favor of Maxim pursuant to which such persons and entities shall agree, for a period of six months after the effective date of the registration statement related to this offering, that they shall neither offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any of our securities without Maxim’s prior written consent, including the issuance of common shares upon the exercise of currently outstanding convertible securities.
Indemnification
We have agreed to indemnify the several underwriters against certain liabilities, including certain liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters may be required to make in respect of those liabilities.
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
We have agreed to issue to Livingston Securities, LLC, or Livingston, $50,000 of our common shares, or the Equity, with the total share allocation to be determined after our finalized planned increase of shares outstanding, pursuant to a certain engagement letter entered into between us and Livingston, dated as of October 21, 2019. The Equity will be issued within ten days after this offering at a price per share equivalent to that of the initial public offering.
The Equity has been deemed compensation by FINRA and is therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. Livingstone (or permitted assignees under the Rule) may not sell, transfer, assign, pledge or hypothecate the Equity, nor will they engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the Equity for a period of six months from the effective date of the registration statement of which this prospectus forms a part, except to any FINRA member participating in the offering and their bona fide officers or partners.
No Public Market
Prior to this offering, there has not been a public market for our securities in the U.S. and the public offering price for the Units will be determined through negotiations between us and the underwriters. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
We offer no assurances that the initial public offering price will correspond to the price at which our common shares will trade in the public market subsequent to this offering or that an active trading market for our common shares or Warrants will develop and continue after this offering.
Stock Exchange
We have applied to list our common shares and Warrants on the Nasdaq Capital Market under the symbol “NLSP” and “NLSPW,” respectively. There can be no assurance that we will be successful in listing our common shares on the Nasdaq Capital Market.
Electronic Distribution
A prospectus in electronic format may be made available on websites or through other online services maintained by one or more of the underwriters of this offering, or by their affiliates. Other than the prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common shares during and after this offering, including:
| | ● | stabilizing transactions; |
| | ● | purchases to cover positions created by short sales; |
| | ● | imposition of penalty bids; and |
| | ● | syndicate covering transactions. |
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common shares while this offering is in progress. Stabilization transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. These transactions may also include making short sales of our common shares, which involve the sale by the underwriters of a greater number of common shares than they are required to purchase in this offering and purchasing common shares on the open market to cover short positions created by short sales. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked short sales,” which are short positions in excess of that amount.
The underwriters may close out any covered short position by either exercising their option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option.
Naked short sales are short sales made in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common shares in the open market that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because Maxim has repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
These stabilizing transactions, short sales, purchases to cover positions created by short sales, the imposition of penalty bids and syndicate covering transactions may have the effect of raising or maintaining the market price of our common shares or preventing or retarding a decline in the market price of our common shares. As a result of these activities, the price of our common shares may be higher than the price that otherwise might exist in the open market. The underwriters may carry out these transactions on the Nasdaq, in the over-the-counter market or otherwise. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of the common shares. Neither we, nor any of the underwriters make any representation that the underwriters will engage in these stabilization transactions or that any transaction, once commenced, will not be discontinued without notice.
Determination of Offering Price
Prior to this offering, there was no public market for our common shares. The initial public offering price will be determined by negotiation between us and Maxim. The principal factors to be considered in determining the initial public offering price include, but are not limited to:
| | ● | the information set forth in this prospectus and otherwise available to Maxim; |
| | ● | our history and prospects and the history and prospects for the industry in which we compete; |
| | ● | our past and present financial performance; |
| | ● | our prospects for future earnings and the present state of our development; |
| | ● | the general condition of the securities market at the time of this offering; |
| | ● | the recent market prices of, and demand for, publicly traded shares of generally comparable companies; and |
| | ● | other factors deemed relevant by the underwriters and us. |
The estimated public offering price range set forth on the cover page of this prospectus is subject to change as a result of market conditions and other factors. Neither we nor the underwriters can assure investors that an active trading market will develop for our common shares or Warrants or that the common shares or Warrants will trade in the public market at or above the initial public offering price (taken individually or as a group).
Affiliations
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and their affiliates may from time to time in the future engage with us and perform services for us or in the ordinary course of their business for which they will receive customary fees and expenses. In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of us. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of these securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in these securities and instruments.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Canada. The Units, common shares and Warrants may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the Units, common shares and Warrants must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
European Economic Area. In relation to each Member State of the European Economic Area, or, each a Member State, no Units, common shares or Warrants have been offered or will be offered pursuant to an offer to the public in that Member State, except that offers of the Units, common shares and Warrants may be made to the public in that Member State at any time under the following exemptions under the Prospectus Regulation:
| (i) | to any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
| (ii) | to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of Maxim for any such offer; or |
| (iii) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of the Units, common shares and Warrants shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to any the Units, common shares and/or Warrants in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any the Units, common shares and/or Warrants to be offered so as to enable an investor to decide to purchase or subscribe for any the Units, common shares and/or Warrants, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
Switzerland. This Prospectus is not intended to constitute an offer or solicitation to purchase or invest in the Units, common shares and Warrants described herein. Neither the Units, nor the common shares nor the Warrants may not be publicly offered, sold or advertised, directly or indirectly, in or into Switzerland within the meaning of the Swiss Financial Services Act, or FinSA, except under the following exemptions under the FinSA:
| (i) | to any investor that qualifies as a professional client within the meaning of the FinSA; |
| (ii) | in any other circumstances falling within article 36 of the FinSA; |
provided, in each case, that no such offer of Units, common shares and Warrants referred to in (i) and (ii) above shall require the publication of a prospectus for offers of Units, common shares and Warrants and/or the publication of a key information document, or KID, (or an equivalent document) pursuant to the FinSA.
The Units, common shares and Warrants have not and will not be listed or admitted to trading on any trading venue in Switzerland.
Neither this prospectus nor any other offering or marketing material relating to the Units, common shares and Warrants constitutes a prospectus or a KID (or an equivalent document) as such terms are understood pursuant to the FinSA, and neither this prospectus nor any other offering or marketing material relating to the Units, common shares and Warrants may be distributed or otherwise made available in Switzerland in an manner which would require the publication of a prospectus or a KID (or an equivalent document) in Switzerland pursuant to the FinSA.
Neither this prospectus nor any other offering or marketing material relating to the Units, common shares and Warrants have been or will be filed with or approved by any Swiss regulatory authority.
United Kingdom. This prospectus is only being distributed to and is only directed at, and any offer subsequently made may only be directed at: (i) persons who are outside the United Kingdom; (ii) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order; or (iii) high net worth companies, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons falling within (1)-(3) together being referred to as “relevant persons”). The common shares are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire the common shares will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this prospectus or any of its contents.
People’s Republic of China. This prospectus may not be circulated or distributed in the People’s Republic of China, or PRC, and the Units, common shares and Warrants may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of the PRC except pursuant to applicable laws, rules and regulations of the PRC. For the purpose of this paragraph only, the PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
Hong Kong. The Units, common shares and Warrants may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules promulgated thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the ordinary shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to ordinary shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules promulgated thereunder.
EXPENSES
Set forth below is an itemization of the total expenses, excluding underwriting discounts, expected to be incurred in connection with the offer and sale of the Units by us. With the exception of the SEC registration fee (rounded down), the Nasdaq listing fee and the FINRA filing fee (rounded up), all amounts are estimates:
| SEC registration fee | | $ | 5,381 | |
| Nasdaq listing fee | | $ | 5,000 | |
| FINRA filing fee | | $ | 7,529 | |
| Transaction Fee | | $ | 176,722 | |
| Printer fees and expenses | | $ | 10,000 | |
| Legal fees and expenses | | $ | 500,000 | |
| Accounting fees and expenses | | $ | 75,000 | |
| Miscellaneous | | $ | 700 | |
| Total | | $ | 780,332 | |
LEGAL MATTERS
Certain legal matters concerning this offering will be passed upon for us by Sullivan & Worcester LLP, New York, New York. Certain legal matters with respect to the legality of the issuance of the securities offered by this prospectus will be passed upon for us by Wenger & Vieli AG, Zurich, Switzerland. Certain legal matters related to the offering will be passed upon for the underwriters by Loeb & Loeb LLP, New York, New York. Lenz & Staehelin, Zurich, Switzerland is representing the underwriters with respect to certain matters of Swiss law.
EXPERTS
The financial statements of NLS for its fiscal years ended December 31, 2019 and December 31, 2018 included herein have been audited by Marcum LLP, independent registered public accounting firm, as set forth in their report thereon, which includes an explanatory paragraph relating to our ability to continue as a going concern. Such financial statements are included herein in reliance upon such report given on the authority of such firm as experts in accounting and auditing. The address of Marcum LLP is 750 Third Avenue, New York, New York 10017, United States.
Enforceability of civil liabilities
We are incorporated under the laws of the State of Switzerland and our registered office and domicile is located in Stans, Switzerland. Moreover, a majority of our directors and executive officers are not residents of the United States, and all or a substantial portion of our assets are located outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon us or upon such persons or to enforce against them judgments obtained in U.S. courts, including judgments in actions predicated upon the civil liability provisions of the federal securities laws of the United States.
We have been advised by our Swiss counsel that there is doubt as to the enforceability in Switzerland of original actions, or in actions for enforcement of judgments of U.S. courts, of civil liabilities to the extent predicated upon the federal and state securities laws of the United States. Original actions against persons in Switzerland based solely upon the U.S. federal or state securities laws are governed, among other things, by the principles set forth in the Swiss Federal Act on International Private Law. This statute provides that the application of provisions of non-Swiss law by the courts in Switzerland shall be precluded if the result was incompatible with Swiss public policy. Also, mandatory provisions of Swiss law may be applicable regardless of any other law that would otherwise apply.
Switzerland and the United States do not have a treaty providing for reciprocal recognition and enforcement of judgments in civil and commercial matters. The recognition and enforcement of a judgment of the courts of the United States in Switzerland is governed by the principles set forth in the Swiss Federal Act on Private International Law. This statute provides in principle that a judgment rendered by a non-Swiss court may be enforced in Switzerland only if:
| | ● | the non-Swiss court had jurisdiction pursuant to the Swiss Federal Act on Private International Law; |
| | ● | the judgment of such non-Swiss court has become final and non-appealable; |
| | ● | the judgment does not contravene Swiss public policy; |
| | ● | the court procedures and the service of documents leading to the judgment were in accordance with the due process of law; and |
| | ● | no proceeding involving the same position and the same subject matter was first brought in Switzerland, or adjudicated in Switzerland, or was earlier adjudicated in a third state and this decision is recognizable in Switzerland. |
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act relating to this offering of our common shares. This prospectus does not contain all of the information contained in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself for a complete description of its terms.
The SEC maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
Upon completion of this offering, we will be subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those requirements will file reports with the SEC. Those other reports or other information may be inspected without charge at the locations described above. As a foreign private issuer, we will be exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information.
We maintain a corporate website at https://nlspharma.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
NLS PHARMACEUTICS LTD.
CONDENSED FINANCIAL STATEMENTS
SIX MONTHS ENDED JUNE 30, 2020 AND 2019
(UNAUDITED)
NLS PHARMACEUTICS LTD.
CONDENSED FINANCIAL STATEMENTS
NLS PHARMACEUTICS LTD.
CONDENSED BALANCE SHEETS
(Unaudited)
| | | June 30,
2020 | | | December 31,
2019 | |
| | | | | | | |
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 145,404 | | | $ | 220,267 | |
| Prepaid expenses and other current assets, including related party of $13,155 and $13,432 as of June 30, 2020 and December 31, 2019, respectively | | | 75,875 | | | | 161,479 | |
| Total current assets | | | 221,279 | | | | 381,746 | |
| | | | | | | | | |
| Deferred offering costs | | | 362,530 | | | | 260,211 | |
| Other receivables, net – related party | | | 56,191 | | | | 54,472 | |
| Total assets | | $ | 640,000 | | | $ | 696,429 | |
| | | | | | | | | |
| LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 2,756,084 | | | $ | 2,509,350 | |
| Interest payable | | | 223,915 | | | | 170,268 | |
| Convertible promissory notes – related parties | | | 551,944 | | | | 540,071 | |
| Current portion of credit facilities, subordinated – related parties | | | 150,000 | | | | 150,000 | |
| Current portion of convertible loan – related party, net of discount of $14,627 | | | 90,903 | | | | - | |
| Other accrued liabilities, including related party of $289,700 and $115,341 as of June 30, 2020 and December 31, 2019, respectively | | | 948,244 | | | | 665,415 | |
| Total current liabilities | | | 4,721,090 | | | | 4,035,104 | |
| | | | | | | | | |
| Convertible loans, net of discount of $83,023 and $64,788 as of June 30, 2020 and December 31, 2019, respectively | | | 312,805 | | | | 152,058 | |
| Swiss government loan | | | 262,137 | | | | - | |
| Deferred revenues | | | 2,499,969 | | | | 2,499,969 | |
| Accrued pension liability | | | 177,639 | | | | 180,248 | |
| Total liabilities | | | 7,973,640 | | | | 6,867,379 | |
| | | | | | | �� | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Shareholders’ deficit: | | | | | | | | |
| Common shares, CHF0.02 ($0.03) par value; 6,960,000 registered shares issued and outstanding at June 30, 2020 and December 31, 2019 | | | 145,139 | | | | 145,139 | |
| Additional paid-in capital | | | 20,670,397 | | | | 20,600,871 | |
| Accumulated deficit | | | (28,130,478 | ) | | | (26,898,262 | ) |
| Accumulated other comprehensive loss | | | (18,698 | ) | | | (18,698 | ) |
| Total shareholders’ deficit | | | (7,333,640 | ) | | | (6,170,950 | ) |
| Total liabilities and shareholders’ deficit | | $ | 640,000 | | | $ | 696,429 | |
The accompanying notes are an integral part of these condensed financial statements.
NLS PHARMACEUTICS LTD.
CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(UNAUDITED)
| | | For the Six Months Ended June 30, | |
| | | 2020 | | | 2019 | |
| | | | | | | |
| Operating expenses: | | | | | | |
| Research and development | | $ | 160,751 | | | $ | 979,874 | |
| General and administrative | | | 920,943 | | | | 1,246,558 | |
| Total operating expenses | | | 1,081,694 | | | | 2,226,432 | |
| | | | | | | | | |
| Operating loss | | | (1,081,694 | ) | | | (2,226,432 | ) |
| | | | | | | | | |
| Other expense, net | | | (62,193 | ) | | | (31,386 | ) |
| Interest expense | | | (37,094 | ) | | | - | |
| Interest on related party loans | | | (51,235 | ) | | | (792,486 | ) |
| Loss on conversion of debt | | | - | | | | (364,950 | ) |
| | | | | | | | | |
| Net and comprehensive loss | | $ | (1,232,216 | ) | | $ | (3,415,254 | ) |
| | | | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.18 | ) | | $ | (0.51 | ) |
| | | | | | | | | |
| Weighted average common shares used in computing basic and diluted net loss per common share | | | 6,960,000 | | | | 6,715,000 | |
The accompanying notes are an integral part of these condensed financial statements.
NLS PHARMACEUTICS LTD.
CONDENSED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
FOR THE SIX MONTHS ENDED JUNE 30, 2020 AND 2019
(UNAUDITED)
| | | Common Shares | | | Additional Paid in | | | Accumulated | | | Accumulated Other Comprehensive | | | | |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Loss | | | Total | |
| BALANCE, JANUARY 1, 2020 | | | 6,960,000 | | | $ | 145,139 | | | $ | 20,600,871 | | | $ | (26,898,262 | ) | | $ | (18,698 | ) | | $ | (6,170,950 | ) |
| Debt discount on convertible loans | | | - | | | | - | | | | 39,496 | | | | - | | | | - | | | | 39,496 | |
| Debt discount on related party convertible loan | | | - | | | | - | | | | 30,030 | | | | - | | | | - | | | | 30,030 | |
| Net loss | | | - | | | | - | | | | - | | | | (1,232,216 | ) | | | - | | | | (1,232,216 | ) |
| BALANCE, JUNE 30, 2020 | | | 6,960,000 | | | $ | 145,139 | | | $ | 20,670,397 | | | $ | (28,130,478 | ) | | $ | (18,698 | ) | | $ | (7,333,640 | ) |
| | | Common Shares | | | Additional Paid in | | | Accumulated | | | Accumulated Other Comprehensive | | | | |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Loss | | | Total | |
| BALANCE, JANUARY 1, 2019 | | | 6,380,000 | | | $ | 133,544 | | | $ | 12,515,118 | | | $ | (21,451,117 | ) | | $ | (11,254 | ) | | $ | (8,813,709 | ) |
| Issuance of common shares for conversion of related party debt to equity | | | 580,000 | | | | 11,595 | | | | 8,011,605 | | | | - | | | | - | | | | 8,023,200 | |
| Debt discount on related party loans | | | - | | | | - | | | | 8,540 | | | | - | | | | - | | | | 8,540 | |
| Net loss | | | - | | | | - | | | | - | | | | (3,415,254 | ) | | | - | | | | (3,415,254 | ) |
| BALANCE, JUNE 30, 2019 | | | 6,960,000 | | | $ | 145,139 | | | $ | 20,535,263 | | | $ | (24,866,371 | ) | | $ | (11,254 | ) | | $ | (4,197,223 | ) |
The accompanying notes are an integral part of these condensed financial statements.
NLS PHARMACEUTICS LTD.
CONDENSED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | | For the Six Months Ended June 30, | |
| | | 2020 | | | 2019 | |
| Cash Flows From Operating Activities: | | | | | | |
| Net loss | | $ | (1,232,216 | ) | | $ | (3,415,254 | ) |
| Adjustments to reconcile net loss to net (used in) provided by in operating activities: | | | | | | | | |
| Amortization of debt discount | | | 36,664 | | | | 752,152 | |
| Loss on conversion of debt | | | - | | | | 364,950 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Prepaid expenses and other current assets | | | 85,605 | | | | (9,963 | ) |
| Other receivables, net – related parties | | | (1,719 | ) | | | 204 | |
| Accounts payable | | | 246,734 | | | | 723,165 | |
| Interest payable | | | 51,789 | | | | 42,037 | |
| Deferred revenues | | | - | | | | 2,499,969 | |
| Other accrued liabilities | | | 280,219 | | | | (244,304 | ) |
| Net cash (used in) provided by operating activities | | | (532,924 | ) | | | 712,956 | |
| | | | | | | | | |
| Cash Flows From Financing Activities: | | | | | | | | |
| Proceeds from convertible loans | | | 174,215 | | | | - | |
| Proceeds from convertible loan – related party | | | 105,530 | | | | - | |
| Proceeds from Swiss government loan | | | 262,137 | | | | - | |
| Proceeds from convertible promissory notes – related parties | | | - | | | | 512,800 | |
| Deferred offering costs | | | (102,319 | ) | | | - | |
| Net cash provided by financing activities | | | 439,563 | | | | 512,800 | |
| | | | | | | | | |
| Effect of exchange rate on cash and cash equivalents | | | 18,498 | | | | (7,543 | ) |
| Change in cash and cash equivalents | | | (74,863 | ) | | | 1,218,213 | |
| Cash and cash equivalents at the beginning of period | | | 220,267 | | | | 6,678 | |
| Cash and cash equivalents at the end of period | | $ | 145,404 | | | $ | 1,224,891 | |
| | | | | | | | | |
| Supplemental disclosure of non-cash financing activities: | | | | | | | | |
| Issuance of common shares for settlement of related party credit facilities | | $ | - | | | $ | 8,023,200 | |
| Debt discount on related party loans | | $ | - | | | $ | 8,540 | |
| Debt discount on convertible loans | | $ | 39,496 | | | $ | - | |
| Debt discount on convertible loan – related party | | $ | 30,030 | | | $ | - | |
The accompanying notes are an integral part of these condensed financial statements.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 1
Background:
NLS Pharmaceutics Ltd. (the “Company”) is an emerging biotechnology company engaged in the discovery and development of life-improving drug therapies to treat central nervous system disorders, including narcolepsy, idiopathic hypersomnia and other rare sleep disorders, as well as neurodevelopmental disorders, such as Attention Deficit Hyperactivity (“ADHD”). The lead product candidates are Quilience, to treat narcolepsy (type 1 and type 2), and Nolazol, to treat ADHD.
On March 12, 2019, the Company merged NLS-0 Pharma Ltd. and NLS Pharma Ltd. into NLS-1 Pharma Ltd. (the “Merger”), the predecessor of the Company, retroactively and effective on January 1, 2019 and renamed the Company NLS Pharmaceutics Ltd. Due to the high degree of common ownership among the three companies and because individual investors’ ownership is in substance the same after the transaction, this Merger was deemed to be a non-substantive merger, with no step up in basis of the assets and liabilities in the Merger. The financial statements presented were prepared as if the Merger occurred on January 1, 2019. All intercompany transactions and balances were eliminated in the Merger.
Liquidity and Going Concern
As of June 30, 2020, the Company had an accumulated deficit of approximately $28.1 million and the Company incurred an operating loss for the six months ended June 30, 2020 of approximately $1.1 million. To date, the Company has dedicated most of its financial resources to general and administrative expenses, research and development and clinical studies associated with its ongoing biopharmaceutical business.
As of June 30, 2020, the Company’s cash and cash equivalents were $145,404 and working capital deficit was $4,499,811. The Company does not believe that its current cash on hand will be sufficient to fund its projected operating requirements. The Company expects that its existing cash and cash equivalents, including its bridge loan entered into in August 2020, will only be sufficient to fund operations through December 2020. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period within one year from the issuance of these financial statements. The Company has filed a registration statement on Form F-1 to register its common shares and warrants to purchase its common shares (the “Initial Public Offering”). However, there can be no assurance that such Initial Public Offering will be successfully completed.
The Company has historically financed its activities primarily with cash from the private placement of equity and debt securities and borrowings under credit facilities and shareholder loans. The Company intends to raise additional capital through private placements of debt and equity securities or through loans from third parties, but there can be no assurance that these funds will be available, or that they are available, that their availability will be on terms acceptable to the Company or in an amount sufficient to enable the Company to fully complete its development activities or sustain operations. Additionally, the negative impact of the COVID-19 outbreak, as discussed in Note 10, on the financial markets could interfere with the Company’s ability to access financing and to access it on favorable terms if at all. If the Company is unable to raise sufficient additional funds, it will have to develop and implement a plan to further extend payables and indebtedness, reduce overhead, or scale back its current business plan until sufficient additional capital is raised to support further operations or force the Company to grant rights to develop and commercialize product candidates that it would otherwise prefer to develop and commercialize on its own. There can be no assurance that such a plan will be successful.
Accordingly, the accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which contemplate continuation of the Company as a going concern for a period within one year from the issuance of these financial statements and the realization of assets and satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily purport to represent realizable or settlement values. The financial statements do not include any adjustment that might result from the outcome of this uncertainty.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 1A
Stock Split
On September 14, 2020, the Company filed an amendment to its Articles of Incorporation and effected a 5,000-for-1 stock split of its issued and outstanding common shares, whereby 1,392 outstanding common shares with a par value of CHF 100.00 per share were exchanged for 6,960,000 common shares with a par value of CHF 0.02 per share. All per share amounts and number of shares in the financial statements and related notes have been retroactively restated to reflect the share split.
Note 2
Summary of Significant Accounting Policies:
Basis of Presentation
The condensed interim financial statements have been prepared in accordance with U.S. GAAP for interim financial information and accordingly do not include all information and disclosures as required by U.S. GAAP for complete financial statements. The year-end condensed balance sheet data was derived from audited financial statements, but does not include all disclosures required by U.S. GAAP. These condensed financial statements should be read in conjunction with the financial statements as of and for the year ended December 31, 2019, included elsewhere in this prospectus.
In the opinion of management, these condensed interim financial statements reflect all adjustments necessary, which are of a normal recurring nature, to fairly state the balance sheets, statements of comprehensive loss, cash flows and changes in shareholders’ deficit for the interim periods presented.
Use of Estimates
The preparation of the condensed financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect amounts reported of assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting periods. Actual results could differ from those estimates and be based on events different from those assumptions. As part of these financial statements, the more significant estimates include (1) pension and other post-employment benefits, including certain asset values that are based on significant unobservable inputs; (2) the inputs used in determining the fair value of equity per share; (3) the beneficial conversion feature on the convertible loans and (4) valuation allowance related to the Company’s deferred tax assets.
Deferred Offering Costs
Specific incremental legal, accounting and other fees and costs directly attributable to a proposed or actual offering of securities may properly be deferred and charged against the gross proceeds of such an offering. In the event the Company’s planned Initial Public Offering does not occur or is significantly delayed, all of the costs will be expensed. As of June 30, 2020 and December 31, 2019, there were $362,530 and $262,211, respectively, of Initial Public Offering costs, primarily consisting of legal, accounting and printing fees, that were capitalized and included in other non-current assets on the balance sheet. The costs deferred exclude any management and general and administrative expenses and only include costs that are related to the current offering.
Segment Reporting
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The Company’s singular focus is on developing therapeutics for the treatment of neurobehavioral and neurocognitive disorders. All of the Company’s tangible assets are held in the United States.
Subsequent Events
Management has evaluated subsequent events that have occurred through the date these financial statements were issued. There were no events that require adjustment to or disclosure in the Company’s financial statements, except as disclosed.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 3
Prepaid Expenses and Other Current Assets:
The Company’s prepaid expenses and other current assets consisted of the following as of June 30, 2020 and December 31, 2019:
| | | June 30,
2020 | | | December 31,
2019 | |
| | | | | | | |
| Vendor overpayments | | $ | 15,542 | | | $ | 37,445 | |
| VAT recoverable and other current assets | | | 20,227 | | | | 33,579 | |
| Convertible loan funding | | | - | | | | 61,956 | |
| Prepaid expenses | | | 26,951 | | | | 15,067 | |
| Employee receivable | | | 13,155 | | | | 13,432 | |
| | | | | | | | | |
| Total prepaid expenses and other current assets | | $ | 75,875 | | | $ | 161,479 | |
The Company’s prepaid expenses and other current assets as of June 30, 2020 and December 31, 2019, consisted mainly of some vendor overpayments and value added tax (“VAT”) recoverable, that were returned to the Company during the following fiscal periods. Additionally, the Company recorded a receivable from the Chief Executive Officer (“CEO”) when Company travel cards include travel expenses. Subsequent to the six months ended June 30, 2020, the CEO receivable has been paid in full. The prepaid expenses and other current assets for the year ended December 31, 2019 also included funding of a convertible loan entered into on December 23, 2019, for which funding was received on January 6, 2020.
Note 4
Other Receivables, net – Related Party
The Company’s majority shareholders are also majority shareholders in Pegasus Advanced Research SAS, formerly NeuroLifeScienses SAS (“Pegasus”), and as such Pegasus is considered to be a related party to the Company. In December 2015, Pegasus assigned its exclusive global license to certain compounds involving mazindol to the Company (the “License Agreement”). The License Agreement includes a royalty payment of 1.8% of the annual net sales (including sales made by sub-licensees) of the licensed compounds covered thereunder; provided, however, that such royalty payment could be further reduced to 0.9% in the event that the U.S. Patent and Trademark Officer were to issue a revised notice of allowance or expand the patent rights of certain licensed compounds or be completely cancelled in the event that a competing generic product were to reach the market during the term of the patents covering such licensed compounds.
Since assigning the License Agreement to the Company, the Company and Pegasus have paid for some operating expenses for each other. The transactions between the companies are minimal and are not considered to be a funding source for either entity. The Company offsets the related receivables and payable to/from Pegasus for a combined net other long-term receivable of $56,191 and $54,472 for the six months ended June 30, 2020 and the year ended December 31, 2019, respectively.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 5
Convertible Promissory Notes – Related Parties:
The following summarized the Company’s convertible promissory notes with related parties as of June 30, 2020 and December 31, 2019.
| | | June 30,
2020 | | | December 31,
2019 | |
| | | (unaudited) | | | | |
| | | | | | | | | |
| Convertible promissory notes | | $ | 551,944 | | | $ | 540,071 | |
In January 2019, the Company entered into an agreement with Magnetic Rock Investment AG (“MRI”), which is owned by certain shareholders of the Company, to loan the Company CHF 550,000 ($557,920). The loan proceeds were received in advance of the agreement, in two payments, in each of August and December 2018 in the amount of CHF 500,000 ($507,200) and CHF 50,000 ($50,720), respectively. Management determined that no separate accounting or bifurcation was required for this conversion feature as it doesn’t meet the definition of a debenture because the net settlement criterion is not met. The note accrues interest at 10% per year and has a conversion feature such that upon a capital closing (as defined), MRI has the right to require conversion of its note to equity at the same rates as the capital closing. As of June 30, 2020, such capital closing (as defined) has not occurred, so the note has not converted. This note had an original maturity date of April 30, 2019 at which time if it wasn’t already converted, a new maturity date would be set at the discretion of MRI. The parties have amended the agreement to extend the maturity date to December 31, 2020. The Company has accrued interest payable of $37,060 and $33,374 for the note as of June 30, 2020 and December 31, 2019, respectively. Additionally, the Company recorded debt discount and a corresponding increase in additional paid in capital of $4,019 based on an imputed interest rate of 12%, the rate readily available to the Company, compared to the 10% interest stated in the loan agreement. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the six months ended June 30, 2020 and 2019, amortization of the debt discount of $0 and $1,548 was included in interest expense – related parties, respectively.
In March 2019, MRI and the Company agreed to convert an aggregate amount of CHF 526,980 ($526,769) of its loan into 40,000 of the Company’s common shares at a conversion price of approximately $13 per share. The common shares had a fair value of approximately CHF 14 ($14) per share and an aggregate fair value of $550,829 resulting in a $24,060 loss on conversion of the loan. Pursuant to an amendment to this loan, the maturity of the remaining loan of CHF 23,020 ($24,293) was extended to December 31, 2020. The Company determined that the extension of the loan which was past due or near maturity was in substance no different than issuing new financial instruments and accordingly, the Company did not recognize a gain or loss in connection with the extension.
In January 2019, the Company entered into four additional convertible promissory notes, on the same terms, with certain Company shareholders. The promissory notes were for CHF 125,000 ($128,200) each, accrue interest at 10% per year and have original maturity dates of the earlier of April 30, 2019 or when any investment into the Company of CHF 500,000 ($527,650) or greater occurs. The maturity of these notes was extended to December 31, 2020. The Company has accrued interest payable of $81,415 and $50,985 for the notes as of June 30, 2020 and December 31, 2019, respectively. The Company recorded debt discount and a corresponding increase in additional paid in capital of $8,540, based on an imputed interest rate of 12% compared to the 10% interest accrued on the loans. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the six months ended June 30, 2019 amortization of $4,647 was included in interest expense – related parties.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 6
Credit Facilities, Subordinated – Related Parties:
The following summarizes the Company’s credit facilities with related parties as of June 30, 2020 and December 31, 2019.
| | | June 30,
2020 | | | December 31,
2019 | |
| | | (unaudited) | | | | |
| | | | | | | | | |
| Second Credit Facility | | | 150,000 | | | | 150,000 | |
| | | | 150,000 | | | | 150,000 | |
| Less: current portion | | | (150,000 | ) | | | (150,000 | ) |
| Credit facilities, long-term | | $ | - | | | $ | - | |
In August 2015, the Company entered into a second credit facility agreement (the “Second Credit Facility”) with shareholders (the “Lenders”). The maximum borrowings under the Second Credit Facility are $500,000. The Second Credit Facility had a maturity date of December 31, 2018 and accrued interest at 0%. As the Second Credit Facility doesn’t accrue interest, management, upon issuance of the debt, recorded total debt discount and a corresponding increase in additional paid in capital based on an imputed interest at a rate of 12%. The debt discount was being amortized to interest expense using the effective interest rate method over the debt term.
In June 2018, the Company and the Lenders entered into a subordination agreement regarding the Second Credit Facility. The subordination agreement defers payments on the Second Credit Facility during the term of the agreement. The agreement expires if (i) the Lenders irrevocably waive the subordinated claims; or (ii) if the claims are converted to share capital or participation capital of the Company.
The remaining balance of the Second Credit Facility of $150,000 is currently outstanding and the parties have amended the agreement covering such credit facility to extend the maturity date to December 31, 2020. Additionally, the accrued interest of $85,737 on an expired credit facility remains unpaid and the parties have amended that credit facility to extend the maturity date to December 31, 2020 (“Credit Facility D”). The Company determined that the extension of these credit facilities which were past due or near maturity was in substance no different than issuing new financial instruments and accordingly, the Company did not recognize a gain or loss in connection with the extension.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 7
Convertible Loans:
The following summarizes the Company’s convertible loans as of June 30, 2020 and December 31, 2019.
| | | June 30,
2020 | | | December 31,
2019 | |
| | | (unaudited) | | | | |
| | | | | | | | | |
| Convertible loans, net of discount of $86,734 | | $ | 312,805 | | | $ | 152,058 | |
| Convertible loans – related party, net of discount of $10,916 | | | 90,903 | | | | - | |
| | | | 403,708 | | | | 152,058 | |
| Less: current portion | | | (90,903 | ) | | | - | |
| | | | | | | | | |
| Convertible loans, long term | | $ | 312,805 | | | $ | 152,058 | |
In December 2019, the Company entered into two loans for a total of CHF210,000 ($216,846) (the “Convertible Loans”). In February, March and June 2020, the Company entered into five additional convertible loans, on terms similar to those in December 2019, including one with a related party, for a total of CHF 265,086 ($279,745). The Convertible Loans accrue interest at 10% and have a conversion feature such that the lender can choose to convert the loan to equity at any time up to the maturity date of August 31, 2021. The conversion rate of CHF 9 ($10) per common share is at a discount to the CHF 12 ($13) per common share fair value of the Company. The beneficial conversion feature valued at CHF 63,537 ($65,608) for the December 2019 convertible loans and CHF 65,883 ($69,526) for the 2020 convertible loans was recorded as a discount to the Convertible Loans. On the date of issuance, the beneficial conversion feature value was calculated as the difference between the conversion price and the fair value per share, multiplied by the number of common shares into which the Convertible Loans are convertible. The embedded conversion feature was not bifurcated as it did not meet all of the elements of a derivative. For the six months ended June 30, 2020, amortization of $21,261 was included in interest expense and $15,403 was included in interest expense – related parties. The Company has accrued interest payable of $19,702 and $172 for the Convertible Loans as of June 30, 2020 and December 31, 2019, respectively.
Note 8
Swiss Government Loan:
In April 2020, in response to the COVID 19 pandemic, the Swiss Federal Council issued the COVID-19 Ordinance on Joint and Several Guarantees, according to which companies domiciled in Switzerland that are economically affected by the COVID-19 pandemic can apply for financial support in the form of emergency bank loans of up to 10% of their 2019 revenues or a maximum of CHF 20 million (the “COVID-19 Loan”). This COVID-19 Loan is secured by the Swiss Confederation. The lending banks receive collateral in the form of joint and several guarantees. The Company received a COVID-19 Loan from its bank for CHF 248,400 ($262,137), which carries a 0% interest rate and is due in 60 months. The loan can be drawn down over several installments and to date the Company has drawn down the entire amount. Pursuant to the terms of the COVID-19 Loan, the Company may not pay any dividends until it repays such loan in full.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 9
License Revenues:
In February 2019, the Company entered into the EF License Agreement, to develop and commercialize its product candidate, Nolazol, in Latin American countries with Eurofarma, a Brazilian pharmaceutical company. The EF License Agreement covers the grant of non-transferable licenses, without the right to sublicense, to Eurofarma to develop and commercialize Nolazol in Latin America. The EF License Agreement also specifies the Company’s obligation to advance ongoing development activities with respect to Nolazol in the United States. A joint steering committee will oversee the development and regulatory activities directed towards marketing approval, manufacturing and commercialization phases. The Company believes its participation in the joint steering committee is not of material significance to the licenses in the context of the EF License Agreement on the whole and, as such, management has excluded these activities in the determination of its performance obligation(s) under the EF License Agreement.
The EF License Agreement provides that the parties shall enter into a separate manufacturing and supply agreement during the term of the EF License Agreement.
Under the EF License Agreement, the Company received a non-refundable, upfront payment of $2,500,000 and is further eligible to receive non-refundable milestone payments of up to $16,000,000, based on the achievement of milestones related to regulatory filings, regulatory approvals and the commercialization of Nolazol. The achievement and timing of the milestones depend on the success of development, approval and sales progress, if any, of Nolazol in the future. In addition, the Company is also eligible for tiered royalty payments.
The Company identified the licenses granted to Eurofarma and its obligation to advance development activities with respect to Nolazol in the United States as the material promises under the EF License Agreement. For purposes of identifying the Company’s performance obligations under the EF License Agreement, management believes that while the exclusive licenses were granted to Eurofarma at the outset of the EF License Agreement, the grant of those licenses does not singularly result in the transfer of the Company’s broader obligation to Eurofarma under the EF License Agreement.
The Company is obligated under the EF License Agreement to advance its development activities in the United States and those activities precede Eurofarma’s necessary regulatory approvals for commercialization of Nolazol, in Latin American countries. The Company intends to apply its proprietary know-how to the ongoing development activities in the United States involving its intellectual property relating to Nolazol. These development activities are specific to the Company and the Company believes they are not capable of being distinct in the context of the EF License Agreement on the whole.
The licenses provided to Eurofarma are not transferable and without the right to sublicense therefore Eurofarma is not presently able to monetize its investment in Nolazol as clinical development in the United States or any Latin American countries has yet to be completed and Eurofarma has yet to seek or obtain regulatory approval in any Latin American country. The licenses to Eurofarma represent rights to use the Company’s intellectual property with respect to Nolazol for which revenue is recognized at a point in time which is when Eurofarma is able to use and benefit from the licenses. The licenses are considered of limited value without the Company’s development activities with respect to Nolazol in the United States. As such, the licenses are not capable of being distinct until after successful clinal development and regulatory approval and alone do not have standalone functionality to Eurofarma. Management has determined that the licenses, while capable of being distinct, are not distinct as they do not have stand-alone value to Eurofarma without the Company’s planned development activities in the United States and the approval for sale in Latin America.
Bundled together with the Company’s development activities of Nolazol in the United States, the licenses granted under the EF License Agreement will enable Eurofarma to seek regulatory approvals and ultimately seek to commercialize Nolazol in Latin America. Therefore, management believes the licenses bundled together with the Company’s development activities in the United States constitute a single distinct performance obligation under the EF License Agreement for accounting purposes, or (the “License Performance Obligation”).
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
The Company has initially estimated a total transaction price of $2,500,000, consisting of the fixed upfront payment determined to be an advance on the License Performance Obligation. Upon execution of the EF License Agreement and as of June 30, 2020, variable consideration consisting of milestone payments has been constrained and excluded from the transaction price given the significant uncertainty of achievement of the development and regulatory milestones.
The Company has allocated the transaction price entirely to the single License Performance Obligation and recorded the $2,500,000 as deferred revenue that is expected to be recognized upon Brazilian or other Latin American market approval or, in the event marketing approval in the United States and/or Latin America is not achieved, whether by failure in clinical development or otherwise, when the Company’s performance obligations are contractually complete or the EF License Agreement is terminated.
Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as a current portion of deferred revenue in the accompanying condensed balance sheets. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion. As of June 30, 2020 and December 31, 2019, the Company has long-term deferred revenues of $2,500,000, which will be recognized when the development services of Nolazol are completed and the product candidate receives applicable regulatory approval in Latin America that allows Eurofarma to commence commercialization of Nolazol in accordance with the EF License Agreement.
Note 10
Commitments and Contingencies:
Commitments
In December 2019, the Company entered into a feasibility development agreement (the “Development Agreement”) with Adare Pharmaceuticals, Inc. (“Adare”), pursuant to which the Company intends to utilize Adare’s proprietary modified release technologies in the development of mazindol for use in the Company’s product candidates used for the treatment of narcolepsy and ADHD. The formulation to be developed by Adare under this Development Agreement is intended to be owned solely by the Company. Upon completion of certain milestones, as defined in the Development Agreement, the Company is obligated to pay Adare up to $840,000. As the project hadn’t yet begun, the Company recorded no liability for the six months ended June 30, 2020 and the year ended December 31, 2019.
Litigation
The Company may become involved in miscellaneous litigation and legal actions, including product liability, consumer, commercial, tax and governmental matters, which can arise from time to time in the ordinary course of the Company’s business. Litigation and legal actions are inherently unpredictable, and excessive verdicts can result in such situations. The Company is not currently involved in any such matters.
COVID-19
In December 2019, a novel strain of coronavirus (“COVID-19”) emerged in Wuhan, China. Since then, it has spread to several other countries and infections have been reported around the world. On March 11, 2020, the World Health Organization declared the outbreak of COVID-19 a global pandemic. In response to the outbreak, governmental authorities in the United States, Canada and internationally, including in Switzerland, have introduced various recommendations and measures to try to limit the pandemic, including travel restrictions, border closures, non-essential business closures, quarantines, self-isolations, shelters-in-place and social distancing. The COVID-19 outbreak and the response of governmental authorities to try to limit it are having a significant impact on the private sector and individuals, including unprecedented business, employment and economic disruptions. The continued spread of COVID-19 in the United States, Canada and globally, including in Switzerland, could have an adverse impact on the Company’s business, operations and financial results, including through disruptions to its planned clinical and non-clinical activities, supply chains, as well as a deterioration of general economic conditions including a possible national or global recession. Shelter-in-place orders and social distancing practices designed to limit the spread of COVID-19 may affect the Company’s ability to conduct clinical studies. Due to the speed with which the COVID-19 situation is developing and the uncertainty of its magnitude, outcome and duration, it is not possible to estimate its impact on the Company’s business, operations or financial results; however, the impact could be material.
NLS PHARMACEUTICS LTD.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 11
Subsequent Events:
In August 2020, the Company received the first tranche of CHF 300,000 ($316,590) pursuant to a bridge loan (the “Bridge Loan”). In September 2020, the Company received the second tranche of CHF 200,000 ($211,060), which, pursuant to the Bridge Loan, became available to the Company upon successful filing of its registration statement on Form F-1, which occurred in August 2020. The total Bridge Loan of CHF 500,000 ($527,650) carries an interest rate of 10% annually and was due September 30, 2020 and since has been amended to December 31, 2020.
In September 2020, a Convertible Loan with a related party of CHF 150,000 ($158,295), which carries an interest rate of 10% per year, was amended such that the total loan value was reduced to CHF 100,000 ($105,530).
In October 2020, two Convertible Loans were amended such that the time period for conversion of the loan amounts into the Company’s common shares will be extended to January 23, 2021.
In October 2020, the following loans and notes were amended such that the maturity date of each instrument is currently scheduled to be December 31, 2020:
| ● | $85,737 under Credit Facility D; |
| ● | $150,000 under the Second Credit Facility; |
| ● | CHF 500,000 ($527,650) under the four convertible promissory notes from January 2019, each of which carries an interest rate of 10% per year; |
| ● | CHF 23,020 ($24,293) under the Convertible Note with MRI, which carries an interest rate of 10% per year; |
| ● | CHF 100,000 ($105,530) under the Convertible Loan with a related party, which carries an interest rate of 10% per year; and |
| ● | CHF 500,000 ($527,650) under the Bridge Loan, which carries an interest rate of 10% per year. |
NLS PHARMACEUTICS LTD.
FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
NLS Pharmaceutics Ltd.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of NLS Pharmaceutics Ltd. (the “Company”) as of December 31, 2019 and 2018, the related statements of operations and comprehensive loss, changes in shareholders’ deficit and cash flows for each of the two years in the period ended December 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1, the Company has a significant working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2019.
New York, NY
August 31, 2020, except for Note 1A, as to which the date is October 16, 2020
NLS PHARMACEUTICS LTD.
BALANCE SHEETS
| | | December 31, | |
| | | 2019 | | | 2018 | |
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| Cash and cash equivalents | | $ | 220,267 | | | $ | 6,678 | |
| Prepaid expenses and other current assets, including related party of $13,432 and $9,356 as of December 31, 2019 and 2018 | | | 161,479 | | | | 83,485 | |
| Total current assets | | | 381,746 | | | | 90,163 | |
| | | | | | | | | |
| Deferred offering costs | | | 260,211 | | | | - | |
| Other receivables, net – related party | | | 54,472 | | | | 55,472 | |
| Total assets | | $ | 696,429 | | | $ | 145,635 | |
| | | | | | | | | |
| LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 2,509,350 | | | $ | 1,130,805 | |
| Interest payable | | | 170,268 | | | | 103,109 | |
| Convertible promissory notes – related parties, net of debt discount of $0 and $237 as of December 31, 2019 and 2018, respectively | | | 540,071 | | | | 557,683 | |
| Current portion of credit facilities, subordinated – related parties | | | 150,000 | | | | 150,000 | |
| Other accrued liabilities, including related party of $115,341 and $30,149 as of December 31, 2019 and 2018, respectively | | | 665,415 | | | | 473,204 | |
| Total current liabilities | | | 4,035,104 | | | | 2,414,801 | |
| | | | | | | | | |
| Shareholder bridge loan – related parties, net of debt discount of $0 and $432,439 as of December 31, 2019 and 2018, respectively | | | - | | | | 1,567,561 | |
| Credit facilities, subordinated – related parties, net of debt discount of $0 and $313,519 as of December 31, 2019 and 2018, respectively | | | - | | | | 4,817,962 | |
| Convertible loans, net of discount of $64,788 as of December 31, 2019 | | | 152,058 | | | | - | |
| Deferred revenues | | | 2,499,969 | | | | - | |
| Accrued pension liability | | | 180,248 | | | | 159,020 | |
| Total liabilities | | | 6,867,379 | | | | 8,959,344 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Shareholders’ deficit: | | | | | | | | |
| Common shares, CHF 0.02 ($0.02) par value, 6,960,000 and 6,380,000 registered shares issued and outstanding at December 31, 2019 and 2018, respectively | | | 145,139 | | | | 133,544 | |
| Additional paid-in capital | | | 20,600,871 | | | | 12,515,118 | |
| Accumulated deficit | | | (26,898,262 | ) | | | (21,451,117 | ) |
| Accumulated other comprehensive loss | | | (18,698 | ) | | | (11,254 | ) |
| Total shareholders’ deficit | | | (6,170,950 | ) | | | (8,813,709 | ) |
| Total liabilities and shareholders’ deficit | | $ | 696,429 | | | $ | 145,635 | |
The accompanying notes are an integral part of these financial statements.
NLS PHARMACEUTICS LTD.
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| | | For the Years Ended
December 31, | |
| | | 2019 | | | 2018 | |
| | | | | | | |
| Operating expenses: | | | | | | |
| Research and development | | $ | 1,702,117 | | | $ | 1,437,202 | |
| General and administrative | | | 2,494,922 | | | | 2,011,659 | |
| Total operating expenses | | | 4,197,039 | | | | 3,448,861 | |
| | | | | | | | | |
| Operating loss | | | (4,197,039 | ) | | | (3,448,861 | ) |
| | | | | | | | | |
| Other (expense) income, net | | | (65,446 | ) | | | 16,035 | |
| Interest expense | | | (992 | ) | | | - | |
| Interest on related party loans | | | (818,719 | ) | | | (1,084,708 | ) |
| Loss on conversion of debt | | | (364,950 | ) | | | (629,232 | ) |
| | | | | | | | | |
| Net loss | | | (5,447,145 | ) | | | (5,146,766 | ) |
| | | | | | | | | |
| Other comprehensive loss: | | | | | | | | |
| Defined pension plan adjustments | | | (7,444 | ) | | | (1,261 | ) |
| Comprehensive loss | | $ | (5,454,589 | ) | | $ | (5,148,027 | ) |
| | | | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.80 | ) | | $ | (0.82 | ) |
| Weighted average common shares used in computing basic and diluted net loss per common share | | | 6,840,000 | | | | 6,240,000 | |
The accompanying notes are an integral part of these financial statements.
NLS PHARMACEUTICS LTD.
STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2019 AND 2018
| | | Common Shares | | | Additional
Paid in | | | Accumulated | | | Accumulated
Other
Comprehensive | | | | |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Loss | | | Total | |
| BALANCE, JANUARY 1, 2018 | | | 6,120,000 | | | $ | 128,344 | | | $ | 8,468,547 | | | $ | (16,304,351 | ) | | $ | (9,993 | ) | | $ | (7,717,453 | ) |
| Issuance of common shares for conversion of related party debt to equity | | | 260,000 | | | | 5,200 | | | | 4,042,552 | | | | - | | | | - | | | | 4,047,752 | |
| Debt discount on related party loans | | | - | | | | - | | | | 4,019 | | | | - | | | | - | | | | 4,019 | |
| Defined pension plan adjustments | | | - | | | | - | | | | - | | | | - | | | | (1,261 | ) | | | (1,261 | ) |
| Net loss | | | - | | | | - | | | | - | | | | (5,146,766 | ) | | | - | | | | (5,146,766 | ) |
| BALANCE, DECEMBER 31, 2018 | | | 6,380,000 | | | | 133,544 | | | | 12,515,118 | | | | (21,451,117 | ) | | | (11,254 | ) | | | (8,813,709 | ) |
| Issuance of common shares for conversion of related party debt to equity | | | 580,000 | | | | 11,595 | | | | 8,011,605 | | | | - | | | | - | | | | 8,023,200 | |
| Debt discount on related party loans | | | - | | | | - | | | | 8,540 | | | | - | | | | - | | | | 8,540 | |
| Debt discount on convertible loans | | | - | | | | - | | | | 65,608 | | | | - | | | | - | | | | 65,608 | |
| Defined pension plan adjustments | | | - | | | | - | | | | - | | | | - | | | | (7,444 | ) | | | (7,444 | ) |
| Net loss | | | - | | | | - | | | | - | | | | (5,447,145 | ) | | | | | | | (5,447,145 | ) |
| BALANCE, DECEMBER 31, 2019 | | | 6,960,000 | | | $ | 145,139 | | | $ | 20,600,871 | | | $ | (26,898,262 | ) | | $ | (18,698 | ) | | $ | (6,170,950 | ) |
The accompanying notes are an integral part of these financial statements.
NLS PHARMACEUTICS LTD.
STATEMENTS OF CASH FLOWS
| | | For the Years Ended
December 31, | |
| | | 2019 | | | 2018 | |
| Cash Flows From Operating Activities: | | | | | | |
| Net loss | | $ | (5,447,145 | ) | | $ | (5,146,766 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Periodic pension costs | | | 20,085 | | | | 26,566 | |
| Amortization of debt discounts | | | 755,555 | | | | 1,090,411 | |
| Loss on conversion of debt | | | 364,950 | | | | 629,232 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Prepaid expenses and other current assets | | | (16,038 | ) | | | 7,382 | |
| Other receivables - related parties | | | 1,000 | | | | (41,101 | ) |
| Accounts payable | | | 1,378,545 | | | | 565,851 | |
| Interest payable | | | 67,159 | | | | (5,252 | ) |
| Deferred revenues | | | 2,499,969 | | | | - | |
| Other accrued liabilities | | | 185,911 | | | | 140,873 | |
| Net cash used in operating activities | | | (190,009 | ) | | | (2,732,804 | ) |
| | | | | | | | | |
| Cash Flows From Financing Activities: | | | | | | | | |
| Proceeds from convertible loans | | | 154,890 | | | | - | |
| Proceeds from convertible promissory notes – related parties | | | 512,800 | | | | 557,920 | |
| Deferred offering costs | | | (260,211 | ) | | | - | |
| Net cash provided by financing activities | | | 407,479 | | | | 557,920 | |
| | | | | | | | | |
| Effect of exchange rate on cash and cash equivalents | | | (3,881 | ) | | | (1,369 | ) |
| Change in cash and cash equivalents | | | 213,589 | | | | (2,176,253 | ) |
| Cash and cash equivalents at the beginning of period | | | 6,678 | | | | 2,182,931 | |
| Cash and cash equivalents at the end of period | | $ | 220,267 | | | $ | 6,678 | |
| | | | | | | | | |
| Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | |
| Issuance of common shares for settlement of related party credit facilities | | $ | 8,023,200 | | | $ | 4,047,752 | |
| Debt discount on related party loans | | $ | 8,540 | | | $ | 4,019 | |
| Debt discount on convertible loans | | $ | 65,608 | | | $ | - | |
| Proceeds from convertible loans in transit | | $ | 61,956 | | | $ | - | |
The accompanying notes are an integral part of these financial statements.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Note 1
Background:
NLS Pharmaceutics Ltd. (the “Company”) is an emerging biotechnological company engaged in the discovery and development of life-improving drug therapies to treat rare and complex central nervous system disorders, including narcolepsy, idiopathic hypersomnia and other rare sleep disorders, and of neurodevelopmental disorders, such as Attention Deficit Hyperactivity Disorder (“ADHD”). The lead product candidates are Quilience, to treat narcolepsy (type 1 and type 2), and Nolazol, to treat ADHD.
On March 12, 2019, the Company merged NLS-0 Pharma Ltd. and NLS Pharma Ltd. into NLS-1 Pharma Ltd. (the “Merger”), the predecessor of the Company, retroactively and effective as per January 1, 2019 and renamed the Company NLS Pharmaceutics Ltd. Due to the high degree of common ownership among the three companies and because individual investors’ ownership is in substance the same after the transaction, this Merger was deemed to be a non-substantive merger, with no step-up in basis of the assets and liabilities in the Merger. The financial statements presented were prepared as if the Merger occurred on January 1, 2018. All intercompany transactions and balances were eliminated in the Merger.
Liquidity and Going Concern
As of December 31, 2019, the Company had an accumulated deficit of approximately $26.9 million and the Company incurred an operating loss for the year ended December 31, 2019 of approximately $4.2 million. To date, the Company has dedicated most of its financial resources to general and administrative expenses, research and development and clinical studies associated with its ongoing biotechnological business.
As of December 31, 2019, the Company’s cash and cash equivalents were $220,267 and working capital deficit was $3,653,358. The Company does not believe that its current cash on hand will be sufficient to fund its projected operating requirements. The Company expects that its existing cash and cash equivalents, including the Convertible Loans entered into in 2020, will only be sufficient to fund operations through November 2020. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period within one year from the issuance of these financial statements. The Company is filing a registration statement on Form F-1 to register its common shares and warrants to purchase its common shares (the “Initial Public Offering”). However, there can be no assurance that such Initial Public Offering will be successfully completed.
The Company has historically financed its activities primarily with cash from the private placements of equity and debt securities and borrowings under credit facilities and shareholder loans. The Company intends to raise additional capital through private placements of debt and equity securities or through loans from third parties, but there can be no assurance that these funds will be available, or that if they are available, that their availability will be on terms acceptable to the Company or in an amount sufficient to enable the Company to fully complete its development activities or sustain operations. If the Company is unable to raise sufficient additional funds, it will have to develop and implement a plan to further extend payables and indebtedness, reduce overhead, or scale back its current business plan until sufficient additional capital is raised to support further operations or force the Company to grant rights to develop and commercialize product candidates that it would otherwise prefer to develop and commercialize on its own. There can be no assurance that such a plan will be successful.
Accordingly, the accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which contemplate continuation of the Company as a going concern for a period within one year from the issuance of these financial statements and the realization of assets and satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily purport to represent realizable or settlement values. The financial statements do not include any adjustment that might result from the outcome of this uncertainty.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Note 1A
Stock Split
On September 14, 2020, the Company filed an amendment to its Articles of Incorporation and effected a 5,000-for-1 stock split of its issued and outstanding common shares, whereby 1,392 outstanding common shares with a par value of CHF 100.00 per share were exchanged for 6,960,000 common shares with a par value of CHF 0.02 per share. All per share amounts and number of shares in the financial statements and related notes have been retroactively restated to reflect the share split.
Note 2
Summary of Significant Accounting Policies:
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with U.S. GAAP. Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates
The preparation of the financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect amounts reported of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting periods. Actual results could differ from those estimates and be based on events different from those assumptions. As part of these financial statements, the Company’s significant estimates include (1) pension and other post-employment benefits, including certain asset values that are based on significant unobservable inputs; (2) the inputs used in determining the fair value of equity per share; (3) the beneficial conversion feature on the convertible loans and (4) valuation allowance relating to the Company’s deferred tax assets.
JOBS Act Accounting Election
The Company is an “emerging growth company” or “EGC”, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, an EGC can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. The Company deposits its cash primarily in checking, money market accounts, as well as certificates of deposit. The Company generally does not enter into investments for trading or speculative purposes, rather to preserve its capital for the purpose of funding operations.
Deferred Offering Costs
Specific incremental legal, accounting and other fees and costs directly attributable to a proposed or actual offering of securities may properly be deferred and charged against the gross proceeds of such an offering. In the event the Company’s planned Initial Public Offering does not occur or is significantly delayed, all of the costs will be expensed. As of December 31, 2019, there were $260,211 of Initial Public Offering costs, primarily consisting of legal, accounting and printing fees, that were capitalized in other non-current assets on the balance sheet. No deferred Initial Public Offering costs were capitalized as of December 31, 2018.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk include cash. At December 31, 2019 and 2018, substantially all of the cash balances are deposited in one banking institution. At various times, the Company has deposits in financial institutions which are in excess of federally insured limits.
Functional Currency
The Company has operations in the United States, certain European Union countries and Switzerland. The Company’s functional currency is the U.S. dollar (“USD”). The results of its non-USD based operations are translated to USD at the average exchange rates during the year. The Company’s assets and liabilities are translated using the current exchange rate as of the balance sheet date and shareholders’ equity is translated using historical rates. Foreign exchange transaction gains and losses are included in other income/expense in the Company’s results of operations.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Revenue Recognition
As of December 31, 2019, the Company has not recognized any revenue from its exclusive license agreement (the “EF License Agreement”), as the upfront payment the Company received has been deferred. The EF License Agreement is to develop and commercialize its product candidate, Nolazol, in Latin American countries with Eurofarma Laboratorios S.A. (“Eurofarma”), a Brazilian pharmaceutical company. The EF License Agreement is within the scope of ASC 606, “Revenue from Contract with Customers” (“ASC 606”).
Under ASC 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration which the entity expects to receive in exchange for those goods or services. To determine the appropriate amount of revenue to be recognized for arrangements determined to be within the scope of ASC 606, the Company performs the following five steps: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation. The Company only applies the five-step model to contracts when it is probable that the entity will collect consideration it is entitled to in exchange for the goods or services it transfers to the customer.
Performance obligations are promised goods or services in a contract to transfer a distinct good or service to the customer and are considered distinct when (i) the customer can benefit from the good or service on its own or together with other readily available resources and (ii) the promised good or service is separately identifiable from other promises in the contract. In assessing whether promised goods or services are distinct, the Company considers factors such as the stage of development of the underlying intellectual property, the capabilities of the customer to develop the intellectual property on its own or whether the required expertise is readily available and whether the goods or services are integral to or dependent on other goods or services in the contract.
The Company estimates the transaction price based on the amount expected to be received for transferring the promised goods or services in the contract. The consideration may include fixed consideration or variable consideration. At the inception of each arrangement that includes variable consideration, the Company evaluates the amount of potential payments and the likelihood that the payments will be received. The Company utilizes either the most likely amount method or expected amount method to estimate the amount expected to be received based on which method best predicts the amount expected to be received. The amount of variable consideration which is included in the transaction price may be constrained and is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period.
The Company allocates the transaction price based on the estimated stand-alone selling price of each of the performance obligations. The Company must develop assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in a contract with a customer. The Company utilizes key assumptions to determine the stand-alone selling price for service obligations, which may include other comparable transactions, pricing considered in negotiating the transaction and the estimated costs. Additionally, in determining the standalone selling price for material rights, the Company may reference comparable transactions, clinical trial success probabilities, and develop estimates of exercising the material rights. Any variable consideration is allocated specifically to one or more performance obligations in a contract when the terms of the variable consideration relate to the satisfaction of the performance obligation and the resulting amounts allocated are consistent with the amounts the Company would expect to receive for the satisfaction of each performance obligation.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
The consideration allocated to each performance obligation is recognized as revenue when control is transferred for the related goods or services. For performance obligations which consist of licenses and other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Development and regulatory milestone payments are assessed under the most likely amount method and constrained if it is probable that a significant revenue reversal would occur. Milestone payments that are not within the Company’s control or the licensee’s control, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. At the end of each reporting period, the Company re-evaluates the probability of achievement of such development milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect license revenues in the period of adjustment. To date, the Company has not recognized any consideration related to the achievement of development, regulatory, or commercial milestone revenue resulting from the EF License Agreement.
For revenue related to sales-based royalties received from licensees, including milestone payments based on the level of sales, where the license is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any consideration related to sales-based royalty revenue resulting from any of the Company’s license agreement.
To the extent the Company receives payments, including non-refundable payments, in excess of the recognized revenue, such excess is recorded as deferred revenue until the Company performs its obligations under these arrangements. Amounts are recorded as accounts receivable when the Company’s right to consideration is unconditional.
Research and Development
Costs for research and development (“R&D”) of products, including payroll, contractor expenses, and supplies, are expensed as incurred. Clinical trial and other development costs incurred by third parties are expensed as the contracted work is performed. Where contingent milestone payments are due to third parties under research and development arrangements, the obligations are recorded when the milestone results are probable of being achieved.
Fair Value Measurements
The Company measures and discloses fair value in accordance with ASC 820, “Fair Value,” which defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions there exists a three-tier fair-value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1 - unadjusted quoted prices are available in active markets for identical assets or liabilities that the Company has the ability to access as of the measurement date.
Level 2 - pricing inputs are other than quoted prices in active markets that are directly observable for the asset or liability or indirectly observable through corroboration with observable market data.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Level 3 - pricing inputs are unobservable for the non-financial asset or liability and only used when there is little, if any, market activity for the non-financial asset or liability at the measurement date. The inputs into the determination of fair value require significant management judgment or estimation. Fair value is determined using comparable market transactions and other valuation methodologies, adjusted as appropriate for liquidity, credit, market and/or other risk factors.
This hierarchy requires the Company to use observable market data, when available, and to minimize the use of unobservable inputs when determining fair value.
The Company’s cash and cash equivalents are carried at fair value, determined according to the fair value hierarchy described above. The carrying values of the Company’s accounts payable approximate their fair value due to the short-term nature of these liabilities. The fair value of the Company’s debt approximated its recorded value as the rate of interest is readily available to the Company.
Debt Issuance Costs and Debt Discount
Debt issuance costs related to a recognized debt liability are presented on the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts, and are amortized to interest expense over the term of the related debt using the effective interest method.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the financial statements or in the Company’s tax returns. Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. The Company assesses the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent it believes, based upon the weight of available evidence, that it is more likely than not that all or a portion of the deferred tax assets will not be realized, a valuation allowance is established through a charge to income tax expense. Potential for recovery of deferred tax assets is evaluated by estimating the future taxable profits expected and considering prudent and feasible tax planning strategies.
Due to the fact that the Company has a history of generating losses, and expects to generate losses in the foreseeable future, a full valuation allowance has been recorded as no deferred taxes have been recognized.
The Company accounts for uncertain tax positions in accordance with an amendment to ASC Topic 740-10, “Income Taxes (Accounting for Uncertainty in Income Taxes),” which clarified the accounting for uncertainty in tax positions. This amendment provides that the tax effects from an uncertain tax position can be recognized in the financial statements only if the position is “more-likely-than-not” to be sustained were it to be challenged by a taxing authority. The assessment of the tax position is based solely on the technical merits of the position, without regard to the likelihood that the tax position may be challenged. If an uncertain tax position meets the “more-likely-than-not” threshold, the largest amount of tax benefit that is more than 50% likely to be recognized upon ultimate settlement with the taxing authority is recorded.
Employee Benefits (including Post Retirement Benefits)
The Company operates the mandatory pension plan for its employees in Switzerland. The plan is generally funded through payments to insurance companies or trustee-administered funds. The Company has a pension plan designed to pay pensions based on accumulated contributions on individual savings accounts. However, this plan is classified as a defined benefit plan under ASC 960, “Plan Accounting – Defined Benefit Pension Plans.”
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
The net defined benefit liability is the present value of the defined benefit obligation at the balance sheet date minus the fair value of plan assets. The defined benefit obligation is in all material cases calculated annually by independent actuaries using the projected unit credit method, which reflects services rendered by employees to the date of valuation, incorporates assumptions concerning employees’ projected salaries, pension increases as well as discount rates of highly liquid corporate bonds which have terms to maturity approximating the terms of the related liability.
Remeasurements of the net defined benefit liability, which comprise actuarial gains and losses, and the return on plan assets (excluding interest), are recognized immediately in Other Comprehensive Loss. Past service costs, including curtailment gains or losses, are recognized immediately as a split in research and development and general and administrative expenses within the operating results. Settlement gains or losses are recognized in either research and development and/or general and administrative expenses within the operating results. The Company determines the net interest expense (income) on the net defined benefit liability for the period by applying the discount rate used to measure the defined benefit obligation at the beginning of the annual period or in case of any significant events between measurement dates to the then-net defined benefit liability, taking into account any changes in the net defined benefit liability during the period as a result of contributions and benefit payments. Net interest expense and other expenses related to defined benefit plans are recognized in the statement of operations and comprehensive loss.
Earnings per Share
Basic net loss per common share is computed by dividing the net loss applicable to common shareholders by the weighted-average number of common shares outstanding during the year. Diluted loss per common share is computed similar to basic loss per share, except that the denominator is increased to include the number of additional potential common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Potential common shares are excluded from the computation for a period in which a net loss is reported or if their effect is anti-dilutive. The Company’s potential common shares consist of convertible promissory notes and convertible loans with their potential dilutive effect considered using the “if-converted” method. For the year ended December 31, 2019, 20,000 shares related to the convertible loans were excluded from the computation.
Segment Reporting
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The Company’s singular focus is on developing therapeutics for the treatment of neurobehavioral and neurocognitive disorders. All of the Company’s tangible assets are held in the United States.
Accounting Pronouncements – Not yet Adopted
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842),” which requires lessees to recognize leases on-balance sheet and disclose key information about leasing arrangements. The new standard establishes a right-of-use model (“ROU”) that requires a lessee to recognize a ROU asset representing the right to use the underlying asset over the lease term and lease liability on the balance sheet for all leases with a term longer than 12 months. Lease obligations are to be measured at the present value of lease payments and accounted for using the effective interest method. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement. For finance leases, the leased asset is depreciated on a straight-line basis and recorded separately from the interest expense in the income statement resulting in higher expense in the earlier part of the lease term. For operating leases, the depreciation and interest expense components are combined, recognized evenly over the term of the lease, and presented as a reduction to operating income. The ASU requires that assets and liabilities be presented or disclosed separately and classified appropriately as current and noncurrent. The ASU further requires additional disclosure of certain qualitative and quantitative information related to lease agreements. In July 2018, the FASB issued new guidance that provided for a new optional transition method that allows entities to initially apply the new leases standard at the adoption date and recognize a cumulative-effect adjustment to opening retained earnings. Under this approach, comparative periods are not restated.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
The Company, an EGC, has deferred the adoption of this accounting standard. The guidance will become effective for the Company for fiscal years beginning after December 31, 2021 and interim periods within fiscal years beginning after December 15, 2022. The Company is still evaluating if this guidance will have a material impact on the Company’s financial statements.
Accounting Pronouncements – Recently Adopted
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers (Topic 606),” which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. This ASU has replaced the most historical revenue recognition guidance in U.S. GAAP when it became effective. The Company adopted this standard on January 1, 2019 using the modified retrospective transition method. The Company did not record a cumulative catch-up adjustment upon adoption, as there was no effect on the timing or amount of revenue recognized for existing contracts that were not completed as of the implementation date.
Commencing January 1, 2019, the Company adopted ASU No. 2016-18, Statement of Cash Flows (Topic 230), “Restricted Cash,” which requires companies to include amounts generally described as restricted cash and restricted cash equivalents in cash and cash equivalents when reconciling beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The amendments in this update were effective for non-public business entities for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. This ASU guidance had no material impact on the Company’s financial statements.
Subsequent Events
Management has evaluated subsequent events that have occurred through the date these financial statements were issued. There were no events that require adjustment to or disclosure in the Company’s financial statements, except as disclosed.
Note 3
Prepaid Expenses and Other Current Assets:
The Company’s prepaid expenses and other current assets consisted of the following as of December 31, 2019 and 2018:
| | | December 31, | |
| | | 2019 | | | 2018 | |
| | | | | | | |
| Vendor overpayments | | $ | 37,445 | | | $ | 46,592 | |
| VAT recoverable and other current assets | | | 33,579 | | | | 27,537 | |
| Convertible loan funding | | | 61,956 | | | | - | |
| Prepaid expenses | | | 15,067 | | | | - | |
| CEO receivable | | | 13,432 | | | | 9,356 | |
| | | | | | | | | |
| Total prepaid expenses and other current assets | | $ | 161,479 | | | $ | 83,485 | |
The Company’s prepaid expenses and other current assets as of December 31, 2019 included funding of a convertible loan entered into on December 23, 2019, for which funding was received on January 6, 2020. The balance of the prepaid expenses and other current assets for the years ended December 31, 2019 and 2018, consisted mainly of some vendor overpayments and value added tax (“VAT”) recoverable, that were returned to the Company during the following fiscal periods. Additionally, the Company recorded a receivable from the Chief Executive Officer (“CEO”) when Company travel cards include personal travel expenses. Subsequent to the year ended December 31, 2019, the CEO receivable has been paid in full.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Note 4
Other Receivables, net – Related Party:
The Company’s majority shareholders are also majority shareholders in Pegasus Advanced Research SAS, formerly NeuroLifeScienses SAS (“Pegasus”), and as such Pegasus is considered to be a related party to the Company. In December 2015, Pegasus assigned its exclusive global license to certain compounds involving mazindol to the Company (the “License Agreement”). The License Agreement includes a royalty payment of 1.8% of the annual net sales (including sales made by sub-licensees) of the licensed compounds covered thereunder; provided, however, that such royalty payment could be further reduced to 0.9% in the event that the U.S. Patent and Trademark Officer were to issue a revised notice of allowance or expand the patent rights of certain licensed compounds or be completely cancelled in the event that a competing generic product were to reach the market during the term of the patents covering such licensed compounds.
Since assigning the License Agreement to the Company, the Company and Pegasus have paid for some operating expenses for each other. The transactions between the companies are minimal and are not considered to be a funding source for either entity. The Company offsets the related receivables and payable to/from Pegasus for a combined net other long-term receivable of $54,472 and $55,472 for the years ended December 31, 2019 and 2018, respectively.
Note 5
Convertible Promissory Notes – Related Parties:
The following summarizes the Company’s convertible promissory notes with related parties as of December 31, 2019 and 2018:
| | | December 31, | |
| | | 2019 | | | 2018 | |
| Convertible promissory notes, current, net of debt discount of $0 and $237 | | $ | 540,071 | | | $ | 557,683 | |
| | | | | | | | | |
In January 2019, the Company entered into an agreement with Magnetic Rock Investment AG (“MRI”), which is owned by certain shareholders of the Company, to loan the Company CHF 550,000 ($557,920). The loan proceeds were received in advance of the agreement, in two payments, in each of August and December 2018 in the amount of CHF 500,000 ($507,200) and CHF 50,000 ($50,720), respectively. Management determined that no separate accounting or bifurcation was required for this conversion feature as it doesn’t meet the definition of a derivative because the net settlement criterion is not met. The note accrues interest at 10% per year and has a conversion feature such that upon a capital closing (as defined), MRI has the right to require conversion of its note to equity at the same rates as such capital closing. As of December 31, 2019, such capital closing (as defined) has not occurred, so the note has not converted. The note had an original maturity date of April 30, 2019 at which time if it wasn’t already converted, a new maturity date would be set at the discretion of MRI. The parties have amended the agreement to extend the maturity date to September 30, 2020. The Company has accrued interest payable of $33,374 for the note as of December 31, 2019. Additionally, the Company recorded debt discount of $4,019 based on an imputed interest rate of 12%, the rate readily available to the Company, compared to the 10% annual interest rate stated in the loan agreement. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the years ended December 31, 2019 and 2018, amortization of $237 and $3,782 was included in interest expense – related parties.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
In January 2019, the Company entered into four additional convertible promissory notes, on the same terms, with certain Company shareholders. The promissory notes were for CHF 125,000 ($128,200) each, accrue interest at 10% per year and have original maturity dates of the earlier of April 30, 2019 or when any investment into the Company of CHF 500,000 ($516,300) or greater occurs. The maturity of these notes was extended to September 30, 2020. The Company has accrued interest payable of $50,985 for the notes as of December 31, 2019. Additionally, the Company recorded debt discount of $8,540, based on an imputed interest rate of 12% compared to the 10% interest accrued on the loans. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the year ended December 31, 2019 amortization of $8,540 was included in interest expense – related parties.
In March 2019, MRI and the Company agreed to convert an aggregate amount of CHF 526,980 ($526,769) of its loan into 40,000 of the Company’s common shares at a conversion price of approximately $13 per share. The common shares had a fair value of CHF 14 ($14) per share and an aggregate fair value of $550,829, resulting in a $24,060 loss on conversion of the loan. Pursuant to an amendment to this loan, the maturity date of the remaining loan of CHF 23,020 ($23,771) was extended to September 30, 2020. The Company determined that the extension of the loan which was past due or near maturity was in substance no different than issuing new financial instruments and accordingly, the Company did not recognize a gain or loss in connection with the extension.
Note 6
Shareholder Bridge Loan – Related Parties:
The following summarizes the Company’s shareholder liabilities with related parties as of December 31, 2019 and 2018:
| | | December 31, | |
| | | 2019 | | | 2018 | |
| Shareholder liabilities, net of debt discount of $0 and $432,439, respectively | | $ | - | | | $ | 1,567,561 | |
In December 2017, the Company entered into an agreement with certain shareholders to borrow $2,000,000 (the “Bridge Loan”). There are no payments due under this Bridge Loan and the maturity date is subject to certain conditions; provided, however, that the maturity date would not be prior to June 30, 2019. The Bridge Loan was still considered to be long-term as of December 31, 2018 as the loan was converted to equity subsequent to year-end. The Bridge Loan has no specified interest rate. Accordingly, upon issuance of the debt, the Company recorded debt discount of $613,372 based on imputed interest at a rate of 12%. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the years ended December 31, 2019 and 2018, amortization of $432,439 and $179,546, respectively, was included in interest expense – related parties.
In March 2019, as part of the Merger, the parties to the Bridge Loan agreed to convert the entire Bridge Loan, in the amount of $2,000,000 into 150,000 of the Company’s common shares at a conversion price of approximately $13 per share. The common shares had a fair value of CHF 14 ($14) per share and an aggregate fair value of $2,091,348 resulting in a $91,348 loss on conversion of the Bridge Loan.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Note 7
Credit Facilities, Subordinated – Related Parties:
The following summarizes the Company’s credit facilities with related parties as of December 31, 2019 and 2018:
| | | December 31, | |
| | | 2019 | | | 2018 | |
| Credit Facility A, net of debt discount of $0 | | $ | - | | | $ | 3,000,000 | |
| Credit Facility B, net of debt discount of $0 | | | - | | | | 681,481 | |
| Second Credit Facility, net of debt discount of $0 | | | 150,000 | | | | 150,000 | |
| Third Credit Facility, net of debt discount of $0 and $313,519, respectively | | | - | | | | 1,136,481 | |
| | | | 150,000 | | | | 4,967,962 | |
| Less: current portion | | | (150,000 | ) | | | (150,000 | ) |
| Credit facilities, long-term | | $ | - | | | $ | 4,817,962 | |
The Company is a party to certain credit facility agreements with certain shareholders (the “Lenders”). The maximum borrowings under these credit facilities are $3,000,000 for credit facility A (“Credit Facility A”); $2,760,000 for credit facility B (“Credit Facility B”); $840,000 for credit facility C (“Credit Facility C”); and $500,000 for credit facility D (“Credit Facility D”) (together, the “Initial Credit Facility”) and a second credit facility for $150,000 (the “Second Credit Facility”). These credit facilities had a maturity date of December 31, 2018 and accrued interest at 0%, except Credit Facility D which accrues interest at 10% per year. Credit Facility A is secured by the founding shareholders’ common shares in the Company (60% ownership as of the date of the loan), while the remaining credit facilities are secured by all patents subject to the Company’s License Agreement. The Company has accrued interest payable of $85,737 related to Credit Facility D, as of December 31, 2019 and 2018. As Credit Facility A, B and C don’t accrue interest, management, upon issuance of the debt, recorded total debt discount of $2,253,601 based on imputed interest at a rate of 12%. Additionally, the Company recorded debt discount of $16,791 based on an imputed interest rate of 12% compared to the 10% interest accrued on the Credit Facility D. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the year ended December 31, 2018, amortization of $776,912, was included in interest expense – related parties.
In March 2017, the Company entered into a third credit facility with the Lenders (the “Third Credit Facility”). The maximum borrowing for this facility was $3,435,920, of which the Company borrowed $1,450,000 in 2017. The maturity date of this Third Credit Facility is December 31, 2020 and it accrues interest at 0%. This Third Credit Facility is not secured by any of the Company’s assets. As this credit facility doesn’t accrue interest, management, upon issuance of the debt, recorded a debt discount of $534,874 based on imputed interest at a rate of 12%. The debt discount is being amortized to interest expense using the effective interest rate method over the debt term. For the years ended December 31, 2019 and 2018, amortization of $313,518 and $130,171, respectively, was included in interest expense – related parties.
In June 2018, the Company and the Lenders entered into subordination agreements regarding the Initial Credit Facility, Second Credit Facility and Third Credit Facility. The subordination agreements defer payments on credit facilities during the term of their respective agreements. Any of these agreements expires if (i) the Lenders irrevocably waive the subordinated claims; or (ii) if the claims are converted to share capital or participation capital of the Company.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
In July 2018, the parties to the Initial Credit Facility agreed to convert an aggregate principal amount of $3,418,519 ($500,000 under Credit Facility D, $840,000 under Credit Facility C and $2,078,519 under Credit Facility B) into 260,000 of the Company’s common shares at a conversion price of approximately $13 per share. The common shares had a fair value of CHF 16 ($16) per share and an aggregate fair value of $4,047,752, resulting in a loss of $629,232 on conversion of the credit facilities included in the Statements of Operations and Comprehensive Loss. In March 2019, the parties to the Initial Credit Facility agreed to convert the remaining aggregate principal amount of the Initial Credit Facility, $3,681,481 ($681,481 under Credit Facility B and $3,000,000 under Credit Facility A) into 280,000 common shares at a conversion price of $13 per share. The common shares had a fair value of CHF 14 ($14) per share and an aggregate fair value of $3,864,796, resulting in a loss of $183,315 on conversion of the Initial Credit Facility. The remaining balance of the Second Credit Facility of $150,000 is currently outstanding and the parties have amended the agreement covering such credit facility to extend the maturity date to September 30, 2020. The accrued interest of $85,737 on Credit Facility D remains unpaid and the parties have amended the Initial Credit Facility to extend the maturity date to September 30, 2020. The Company determined that the extension of the notes which were past due or near maturity was in substance no different than issuing new financial instruments and accordingly, the Company did not recognize a gain or loss in connection with the extension.
Additionally, in March 2019, the Company and the Lenders agreed to amend the Third Credit Facility, pursuant to which, the entire borrowed amount of $1,450,000 was converted into 110,000 of the Company’s common shares at a conversion price of approximately $13 per share. The common shares had a fair value of CHF 14 ($14) per share and an aggregate fair value of $1,516,227, resulting in a $66,227 loss on conversion of the Third Credit Facility.
Note 8
Convertible Loans:
As of December 31, 2019, the Company has convertible loans outstanding in the aggregate amount of $152,058, net of debt discount of $64,788.
In December 2019, the Company entered into loans for CHF150,000 ($154,890) (“Loan 1”) and CHF 60,000 ($61,956) (“Loan 2”) (together, the “Convertible Loans”). The Convertible Loans accrue interest at 10% and have a conversion feature such that the lender can choose to convert the loan to equity at any time up to the maturity date of August 31, 2021. The Company has accrued interest payable of $172 for the Convertible Loans as of December 31, 2019. The conversion rate of CHF 9 ($10) per common share is at a discount to the CHF 13 ($13) per common share fair value of the Company. The beneficial conversion feature valued at CHF 47,653 ($49,206) for Loan 1 and CHF 15,884 ($16,402) for Loan 2 was recorded as a discount to the Convertible Loans. On the date of issuance, the beneficial conversion feature value was calculated as the difference between the conversion price and the fair value per share, multiplied by the number of common shares into which the Convertible Loans are convertible. The embedded conversion feature was not bifurcated as it did not meet all of the elements of a derivative. For the year ended December 31, 2019, amortization of $820 was included in interest expense.
Note 9
Pension Liability:
The Company joined a collective pension plan operated by an insurance company as of 2016 which covers the employees in Switzerland.
Both the Company and the participants provide monthly contributions to the pension plan which are based on the covered salary. The respective saving parts of premiums are credited to employees’ accounts. In addition, interest is credited to employees’ accounts at the rate provided in the plan. The pension plan provides for retirement benefits as well as benefits on long-term disability and death.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
The following table provides information on the pension plan for the years ended December 31, 2019 and 2018:
| | | 2019 | | | 2018 | |
| Service cost | | $ | 25,206 | | | $ | 25,617 | |
| Interest cost | | | 2,992 | | | | 2,190 | |
| Expected return on assets | | | (4,249 | ) | | | (3,294 | ) |
| Effect of settlement | | | (5,810 | ) | | | - | |
| Administrative expenses | | | 1,946 | | | | 2,053 | |
| | | | | | | | | |
| Net periodic pension cost | | $ | 20,085 | | | $ | 26,566 | |
The reconciliation of the projected benefit obligation and the changes to the fair value of the plan assets of the pension plan are shown in the following table:
| | | 2019 | | | 2018 | |
| Projected benefit obligation, beginning of period | | $ | 385,448 | | | $ | 243,783 | |
| Service cost | | | 25,206 | | | | 25,617 | |
| Interest cost | | | 2,992 | | | | 2,190 | |
| Transfers-in and (-out), net | | | (40,147 | ) | | | 123,244 | |
| Actuarial (gain)/ loss | | | 6,765 | | | | (6,413 | ) |
| Currency conversion adjustments | | | 5,800 | | | | (2,973 | ) |
| Projected benefit obligation, end of period | | $ | 386,064 | | | $ | 385,448 | |
| | | | | | | | | |
| Plan assets, beginning of period | | $ | 226,428 | | | $ | 95,529 | |
| Actual return on plan assets | | | 1,739 | | | | (4,380 | ) |
| Employer contributions | | | 16,867 | | | | 15,692 | |
| Participant contributions | | | 9,608 | | | | 9,177 | |
| Transfers-in and (-out), net | | | (49,755 | ) | | | 114,067 | |
| Administration expenses | | | (1,946 | ) | | | (2,053 | ) |
| Currency conversion adjustments | | | 2,875 | | | | (1,604 | ) |
| Plan assets, end of period | | $ | 205,816 | | | $ | 226,428 | |
| | | | | | | | | |
| Accrued pension liability | | $ | 180,248 | | | $ | 159,020 | |
As of December 31, 2019 and 2018, the Company recorded an accrued pension liability of $180,248 and $159,020, respectively, in non-current liabilities.
The pension assets are measured at fair value and are invested in a collective pension foundation with pooled investments. Plan assets mainly consist of cash and cash equivalents, equity funds, equity securities, corporate bonds, government bonds, and real estate funds classified as Level 1 and Level 2 under the fair value hierarchy.
The Company records net gains/losses, consisting of actuarial gains/losses, curtailment gains/losses and differences between expected and actual returns on plan assets, in other comprehensive income/loss. Such net gains/losses are amortized to the statements of operations to the extent that they exceed 10% of the greater of projected benefit obligations or pension assets. The Company further records prior service costs/credits from plan amendments in other comprehensive income/loss in the period of the respective plan amendment and amortizes such amounts to the statement of operations over the future service period of the plan participants. For the years ended December 31, 2019 and 2018, the amortization was $0. As of December 31, 2019 and 2018, the accumulated other comprehensive loss includes unrecognized pension costs of $18,698 and $11,254, respectively, consisting of net losses.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
The following table shows the components of unrecognized pension cost in accumulated other comprehensive income/loss that have not yet been recognized as components of net periodic pension cost:
| | | 2019 | | | 2018 | |
| Net loss, beginning of period | | $ | 11,254 | | | $ | 9,993 | |
| Other gain/loss during the period | | | 1,634 | | | | 1,261 | |
| Effect of settlement | | | 5,810 | | | | - | |
| Amortization of pension related net loss | | | - | | | | - | |
| Net loss, end of period | | | 18,698 | | | | 11,254 | |
| | | | | | | | | |
| Prior service cost, beginning of period | | | - | | | | - | |
| Amortization of prior service cost | | | - | | | | - | |
| Prior service cost end of period | | | - | | | | - | |
| | | | | | | | | |
| Total unrecognized pension cost, end of period | | $ | 18,698 | | | $ | 11,254 | |
The weighted average of the key assumptions used to compute the benefit obligations were as follows:
| | | 2019 | | | 2018 | |
| Discount rate | | | 0.35 | % | | | 0.80 | % |
| Rate of increase in compensation level | | | 0.00 | % | | | 0.00 | % |
| Interest credit rate on savings accounts | | | 1.00 | % | | | 1.00 | % |
| Expected long-term rate of return on plan assets | | | 2.00 | % | | | 2.00 | % |
| Inflation rate | | | 0.60 | % | | | 0.60 | % |
The assumption of the expected long-term rate of return on plan assets was based on the long-term historical rates of returns for the different investment categories which were adjusted, where appropriate, to reflect financial market developments.
The accumulated benefit obligation as of December 31, 2019 and 2018 amounted to $386,064 and $385,448, respectively.
The investment risk is borne by the insurer and the reinsurer respectively, and the investment decision is taken by the board of trustees of the collective insurance.
Note 10
License Revenues:
In February 2019, the Company entered into the EF License Agreement, to develop and commercialize its product candidate, Nolazol, in Latin American countries with Eurofarma, a Brazilian pharmaceutical company. The EF License Agreement covers the grant of non-transferable licenses, without the right to sublicense, to Eurofarma to develop and commercialize Nolazol in Latin America. The EF License Agreement also specifies the Company’s obligation to advance development activities with respect to Nolazol in the United States. A joint steering committee will oversee the development and regulatory activities directed towards marketing approval, manufacturing and commercialization phases. The Company believes its participation in the joint steering committee is not of material significance to the licenses in the context of the EF License Agreement on the whole and, as such, management has excluded these activities in the determination of its performance obligation(s) under the EF License Agreement.
The EF License Agreement provides that the parties shall enter into a separate manufacturing and supply agreement during the term of the EF License Agreement.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Under the EF License Agreement, the Company received a non-refundable, upfront payment, of $2,500,000 and is further eligible to receive non-refundable milestone payments of up to $16,000,000, based on the achievement of milestones related to regulatory filings, regulatory approvals and the commercialization of Nolazol. The achievement and timing of the milestones depend on the success of development, approval and sales progress, if any, of Nolazol in the future. In addition, the Company is also eligible for tiered royalty payments.
The Company identified the licenses granted to Eurofarma and its obligation to advance development activities with respect to Nolazol in the United States as the material promises under the EF License Agreement. For purposes of identifying the Company’s performance obligations under the EF License Agreement, management believes that while the exclusive licenses were granted to Eurofarma at the outset of the EF License Agreement, the grant of those licenses does not singularly result in the transfer of the Company’s broader obligation to Eurofarma under the EF License Agreement.
The Company is obligated under the EF License Agreement to advance its development activities in the United States and those activities precede Eurofarma’s necessary regulatory approvals for commercialization of Nolazol in Latin American countries. The Company intends to apply its proprietary know-how to the planned development activities in the United States involving its intellectual property relating to Nolazol. These development activities are specific to the Company and the Company believes they are not capable of being distinct in the context of the EF License Agreement on the whole.
The licenses provided to Eurofarma are not transferable and without the right to sublicense; therefore, Eurofarma is not presently able to monetize its investment in Nolazol as clinical development in the United States or any Latin American countries has yet to be completed and Eurofarma has yet to seek or obtain regulatory approval in any Latin American country. The licenses to Eurofarma represent rights to use the Company’s intellectual property with respect to Nolazol for which revenue is recognized at a point in time which is when Eurofarma is able to use and benefit from the licenses. The licenses are considered of limited value without the Company’s development activities with respect to Nolazol in the United States. As such, the licenses are not capable of being distinct until after successful clinical development and regulatory approval and alone do not have standalone functionality to Eurofarma. Management has determined that the licenses, while capable of being distinct, are not distinct as they do not have stand-alone value to Eurofarma without the Company’s planned development activities in the United States and the approval for sale in Latin America.
Bundled together with the Company’s development activities of Nolazol in the United States, the licenses granted under the EF License Agreement will enable Eurofarma to seek regulatory approvals and ultimately seek to commercialize Nolazol in Latin America. Therefore, management believes the licenses bundled together with the Company’s development activities in the United States constitute a single distinct performance obligation under the EF License Agreement for accounting purposes (the “License Performance Obligation”).
The Company has initially estimated a total transaction price of $2,500,000, consisting of the fixed upfront payment determined to be an advance on the License Performance Obligation. Upon execution of the EF License Agreement and as of December 31, 2019, variable consideration consisting of milestone payments has been constrained and excluded from the transaction price given the significant uncertainty of achievement of the development and regulatory milestones.
The Company has allocated the transaction price entirely to the single License Performance Obligation and recorded the $2,500,000 as deferred revenue that is expected to be recognized upon Brazilian or other Latin American market approval or, in the event marketing approval in the United States and/or Latin America is not achieved, whether by failure in clinical development or otherwise, when the Company’s performance obligations are contractually complete or the EF License Agreement is terminated.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Amounts expected to be recognized as revenue within the 12 months following the balance sheet date are classified as a current portion of deferred revenue in the accompanying balance sheets. Amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion. As of December 31, 2019, the Company has long-term deferred revenues of $2,500,000, which will be recognized when the development services of Nolazol are completed and the product candidate receives applicable regulatory approval in Latin America that allows Eurofarma to commence commercialization of Nolazol in accordance with the EF License Agreement.
Note 11
Commitments and Contingencies:
Commitments
In December 2019, the Company entered into a feasibility development agreement (the “Development Agreement”) with Adare Pharmaceuticals, Inc. (“Adare”), pursuant to which the Company intends to utilize Adare’s proprietary modified release technologies in the development of mazindol for use in the Company’s product candidates used for the treatment of narcolepsy and ADHD. The formulation to be developed by Adare under this Development Agreement is intended to be owned solely by the Company. Upon completion of certain milestones, as defined in the Development Agreement, the Company is obligated to pay Adare up to $840,000. As the project hadn’t begun in 2019, the Company recorded no liability for the year ended December 31, 2019.
Litigation
The Company may become involved in miscellaneous litigation and legal actions, including product liability, consumer, commercial, tax and governmental matters, which can arise from time to time in the ordinary course of the Company’s business. Litigation and legal actions are inherently unpredictable, and excessive verdicts can result in such situations. The Company is not currently involved in any such matters.
COVID-19
In December 2019, a novel strain of coronavirus (“COVID-19”) emerged in Wuhan, China. Since then, it has spread to several other countries and infections have been reported around the world. On March 11, 2020, the World Health Organization declared the outbreak of COVID-19 a global pandemic. In response to the outbreak, governmental authorities in the United States, Canada and internationally, including in Switzerland, have introduced various recommendations and measures to try to limit the pandemic, including travel restrictions, border closures, non-essential business closures, quarantines, self-isolations, shelters-in-place and social distancing. The COVID-19 outbreak and the response of governmental authorities to try to limit it are having a significant impact on the private sector and individuals, including unprecedented business, employment and economic disruptions. The continued spread of COVID-19 in the United States, Canada and globally, including in Switzerland, could have an adverse impact on our business, operations and financial results, including through disruptions in our cultivation and processing activities, supply chains, as well as a deterioration of general economic conditions including a possible national or global recession. Shelter-in-place orders and social distancing practices designed to limit the spread of COVID-19 may affect our ability to conduct clinical studies. Due to the speed with which the COVID-19 situation is developing and the uncertainty of its magnitude, outcome and duration, it is not possible to estimate its impact on our business, operations or financial results; however, the impact could be material.
Note 12
Stockholders’ Deficit:
As of December 31, 2019, the Company had 6,960,000 registered and issued common shares.
In March 2019, the Company issued a total of 580,000 of its common shares to creditors in exchange for payment of $5,131,481 of the Initial Credit Facility, $2,000,000 of the Bridge Loan and $529,769 of convertible promissory notes. See notes 5, 6 and 7 above for further information.
In July 2018, the Company issued 260,000 of its common shares to the creditors in exchange for payment of $3,418,519 due under the Initial Credit Facility. See note 7 for further information.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
Note 13
Income Taxes:
The Company has Swiss tax loss carryforwards of $11.5 million as of December 31, 2019 (December 31, 2018: $9.7 million) of which $7.1 million will expire within the next five years, and $4.4 million will expire between 2025 - 2026.
The significant components of net deferred taxes as of December 31, 2019 and 2018 are shown in the following table:
| | | 2019 | | | 2018 | |
| Deferred tax assets: | | | | | | |
| Net benefit from tax loss carryforwards | | $ | 1,031,524 | | | $ | 873,878 | |
| Deferred revenues | | | 224,997 | | | | - | |
| Other, net | | | (15,065 | ) | | | 42,006 | |
| Valuation allowance | | | (1,241,456 | ) | | | (915,884 | ) |
| | | | | | | | | |
| Net deferred taxes | | $ | - | | | $ | - | |
The Company recorded a valuation allowance in 2019 and 2018 to reduce the net deferred taxes, as the Company deemed it to be more likely than not that the future deferred tax assets would not be realized in the future based on the lack of sufficient positive evidence in the jurisdictions related to the realization of the deferred tax assets.
The effective tax rate was 0% for the years ended December 31, 2019 and 2018. The following table shows the income taxes in 2019 and 2018:
| | | 2019 | | | 2018 | |
| | | | | | | |
| Current tax | | $ | - | | | $ | - | |
| Deferred income tax (benefit) | | | (325,572 | ) | | | (289,462 | ) |
| | | | (325,572 | ) | | | (289,462 | ) |
| Change in valuation allowance | | | 325,572 | | | | 289,462 | |
| Total income tax expense | | $ | - | | | $ | - | |
The Company files income tax returns in Switzerland. The Company’s income tax position in Switzerland is finally assessed up to the year ended December 31, 2018, so only the year ended December 31, 2019 is open for examination. Currently the Company does not have any open tax assessments. The following table shows the reconciliation between expected and effective tax rate:
| | | 2019 | | | 2018 | |
| | | | | | | |
| Statutory tax rate | | | 9.0 | % | | | 9.0 | % |
| Effect of permanent differences | | | (2.3 | )% | | | (3.7 | )% |
| Change in valuation allowance on deferred tax assets | | | (6.7 | )% | | | (5.3 | )% |
| | | | | | | | | |
| Effective tax rate | | | 0.0 | % | | | 0.0 | % |
The Company had generated approximately $9,700,000 of net operating losses (“NOLs”) prior to the Merger, in which the Company’s preliminary analysis indicates that such NOLs would not be subject to significant limitations pursuant to applicable income tax regulations.
NLS PHARMACEUTICS LTD.
NOTES TO THE FINANCIAL STATEMENTS
As of December 31, 2019 and 2018, there were no unrecognized tax benefits. If such matters were to arise, the Company would recognize interest and penalties related to income tax matters in income tax expense. The Company did not incur any material interest or penalties in connection with income taxes during the years ended December 31, 2019 and 2018.
Note 14
Subsequent Events:
In August 2020, the Company received the first tranche of CHF 300,000 ($309,780) pursuant to a bridge loan (the “Bridge Loan”). Pursuant to the Bridge Loan, the Company may borrow up to an aggregate of CHF 500,000 ($516,300), or an additional CHF 200,000 ($206,520), upon successful filing of its Form F-1. As of the date of filing of these financial statements, the terms and agreement for this Bridge Loan have not been finalized.
In April 2020, in response to the COVID-19 pandemic, the Swiss Federal Council issued the COVID-19 Ordinance on Joint and Several Guarantees, according to which companies domiciled in Switzerland that are economically affected by the COVID-19 pandemic can apply for financial support in the form of emergency bank loans of up to 10% of their 2019 revenues or a maximum of CHF 20 million (the “COVID-19 Loan”). This COVID-19 Loan is secured by the Swiss Confederation. The lending banks receive collateral in the form of joint and several guarantees. The Company received a COVID-19 Loan from its bank for CHF 248,400 ($256,498), which carries a 0% interest rate and is due in 60 months. The loan can be drawn down over several installments and to date the Company has drawn down the entire amount. Pursuant to the terms of the COVID-19 Loan, the Company may not pay any dividends until it repays such loan in full.
In February, March and June 2020, the Company entered into five additional convertible loans, on terms similar to those of the Convertible Loans, including one with a related party, for a total of CHF 315,822 ($326,117). Each Convertible Loan carries an interest rate of 10% per year, compounded annually and matures as of June 30, 2020 (with respect to the related party Convertible Loan) and between August 31, 2021 and January 31, 2022, unless converted into common shares or repaid by the Company prior to then. If the Company defaults on the Convertible Loans, it will be subject to a default interest rate of 15% per year. Conversion of the Convertible Loans is at the discretion of each lender at a conversion price of CHF 9 ($10) per common share.
In January, February and July 2020, the following loans and notes were amended such that the maturity date of each instrument is currently scheduled to be September 30, 2020:
| | ● | $85,737 under Credit Facility D; |
| | ● | $150,000 under the Second Credit Facility; |
| | ● | CHF 500,000 ($516,300) under the four convertible promissory notes from January 2019, each of which carries an interest rate of 10% per year; |
| | ● | CHF 23,020 ($23,770) under the Convertible Note with MRI, which carries an interest rate of 10% per year; and |
| | ● | CHF 150,000 ($154,890) under the Convertible Loan with a related party, which carries an interest rate of 10% per year. |
3,636,363 Units consisting of
3,636,363 Common Shares and Warrants to Purchase up to 3,636,363 Common Shares

NLS Pharmaceutics Ltd.
PROSPECTUS
Book Running Manager
Maxim Group LLC
Co-Manager
Brookline Capital Markets
a division of Arcadia Securities, LLC
, 2020
Until and including, , 2020 (25 days after the date of this prospectus), all dealers that buy, sell, or trade the common shares, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors, Officers and Employees
Under Swiss law, a corporation may indemnify its directors or officers against losses and expenses (except for such losses and expenses arising from willful misconduct or negligence, although legal scholars advocate that at least gross negligence be required; however, some scholars also advocate that with any breach of duty, indemnification by the Company is not permissible), including attorney’s fees, judgments, fines and settlement amounts actually and reasonably incurred in a civil or criminal action, suit or proceeding by reason of having been the representative of, or serving at the request of, the corporation.
Subject to Swiss law, our articles of association provide for indemnification of the existing and former members of our board of directors, executive management, and their heirs, executors and administrators, against liabilities arising in connection with the performance of their duties in such capacity, and permit us to advance the expenses of defending any act, suit or proceeding to members of our board of directors and executive management.
In addition, under general principles of Swiss employment law, an employer may be required to indemnify an employee against losses and expenses incurred by such employee in the proper execution of his or her duties under the employment agreement with the company.
We intend to enter into indemnification agreements with each of the members of our board of directors and executive officers in the form to be filed as an exhibit to this registration statement upon the completion of this offering. Irrespective of our entering or not entering into indemnification agreements with our board of directors and executive officers, prior to or shortly after the completion of this offering, we intend to take out directors’ and officers’ liability insurance to cover certain actions undertaken by our board of directors and executive officers.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, may be permitted to directors, officers and controlling persons of the Company, the Company has been advised that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
Item 7. Recent Sales of Unregistered Securities
Set forth below are the sales of all securities by the Company since November 2017, which were not registered under the Securities Act. The Company believes that each of such issuances was exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, Rule 701 and/or Regulation S under the Securities Act.
On September 14, 2020, our shareholders approved an amendment to our articles of association, which reflects a 5,000 for 1 stock split of our common shares, effective as of September 14, 2020, or the Share Split. Information in this Item 7 reflects the Share Split.
On July 17, 2018, we issued an aggregate of 260,000 of our common shares to the Shareholders, in connection with the conversion of an aggregate amount of $3,418,519 borrowed by us pursuant to a $7.1 million credit facility, or the Credit Facility, at a conversion price of $13 per common share.
On March 12, 2019, we issued an aggregate of 280,000 of our common shares to the Shareholders, in connection with the conversion of the remaining $3,681,481 borrowed by us pursuant to the Credit Facility, at a conversion price of $13 per common share.
Also on March 12, 2019, we issued an aggregate of an additional 40,000 of our common shares to Magnetic Rock Investment AG in connection with the conversion CHF 526,979.84 ($526,769) of a CHF 550,000 ($557,920) convertible promissory note at a conversion price of CHF 13 (approximately $13) per common share.
On March 12, 2019, there was a reorganization of our Company, whereby NLS-0 Pharma AG and NLS Pharma AG merged into the Company, or the Merger, and as a result thereof, and in connection therewith, we issued and subsequently assigned an aggregate of 745,000 of our common shares to those holders of NLS-0 and NLS Pharma common shares, respectively.
On March 12, 2019, simultaneously with the closing of the Merger, we issued an aggregate of 260,000 of our common shares to the Shareholders, in exchange for the consideration of the conversion of a bridge loan in the amount of $2 million and a credit facility, of which we had drawn $1.45 million, at a conversion price of $13 per common share.
Item 8. Exhibits and Financial Statement Schedules
Exhibits:
| Exhibit Number | | Exhibit Description |
| | | |
| 1.1^ | | Form of Underwriting Agreement by and among NLS Pharmaceutics Ltd. and the underwriters named therein. |
| 3.1^ | | Form of Amended and Restated Articles of Association of NLS Pharmaceutics Ltd. |
| 4.1^ | | Form of Representative’s Warrant (included in Exhibit 1.1) |
| 4.2^ | | Form of Warrant Agent Agreement. |
| 4.3^ | | Form of Warrant. |
| 5.1^ | | Opinion of Wenger & Vieli AG, Swiss counsel to NLS Pharmaceutics Ltd. |
| 5.2^ | | Opinion of Sullivan & Worcester LLP, U.S. counsel to NLS Pharmaceutics Ltd. |
| 10.1^ | | Merger Agreement dated March 12, 2019 between NLS-1 Pharma AG, NLS-0 Pharma AG and NLS Pharma AG. |
| 10.2^ | | Convertible Promissory Note dated January 18, 2019 between Magnetic Rock Investment AG and the Company. |
| 10.3^ | | Addendum to Convertible Promissory Note dated January 18, 2019 between Magnetic Rock Investment AG and the Company. |
| 10.4^ | | Addendum Number 2 to Convertible Promissory Note dated January 18, 2019 between Magnetic Rock Investment AG and the Company. |
| 10.5^ | | Form of Promissory Note dated January 2019 between certain investors and the Company. |
| 10.6^ | | Form of Addendum to Promissory Note dated January 2019 between certain investors and the Company. |
| 10.7^ | | Form of Addendum Number 2 to Promissory Note dated January 2019 between certain investors and the Company. |
| 10.8^ | | Credit Facility dated August 31, 2015 between NLS Pharma AG and the Lenders named therein. |
| 10.9^ | | Addendum to Credit Facility dated August 31, 2015 between NLS Pharma AG and the Lenders named therein. |
| 10.10^ | | Addendum Number 2 to Credit Facility dated August 31, 2015 between NLS Pharma AG and the Lenders named therein. |
| 10.11^ | | Credit Facility dated August 31, 2015 between NLS-1 Pharma AG and the Lenders named therein. |
| 10.12^ | | Addendum Number 1 to Credit Facility dated August 31, 2015 between NLS-1 Pharma AG and the Lenders named therein. |
| 10.13^ | | Addendum Number 2 to Credit Facility dated August 31, 2015 between NLS-1 Pharma AG and the Lenders named therein. |
| 10.14^ | | License Agreement dated February 2019 between Eurofarma Laboratorios S.A. and the Company. |
| 10.15^ | | Assignment and Transfer Agreement dated August 31, 2015 between NLS-1 AG and NeuroLife Sciences SAS. |
| 10.16^ | | First Amendment to Assignment and Transfer Agreement dated August 31, 2015 between NLS-1 AG and NeuroLife Sciences SAS. |
| 10.17^ | | Second Amendment to Assignment and Transfer Agreement dated August 31, 2015 between NLS-1 AG and NeuroLife Sciences SAS. |
| 10.18^ | | Form of Convertible Loan Agreement between NLS Pharmaceutics Ltd. and certain lenders. |
| 10.19^ | | Amendment Number 1 to Convertible Loan Agreement dated February 21, 2020 between NLS Pharmaceutics Ltd. and Alexander Zwyer. |
| 10.20^ | | Amendment Number 2 to Convertible Loan Agreement dated February 21, 2020 between NLS Pharmaceutics Ltd. and Alexander Zwyer. |
| 10.21^ | | Amendment Number 3 to Convertible Loan Agreement dated February 21, 2020 between NLS Pharmaceutics Ltd. and Alexander Zwyer. |
| 10.22^ | | Bridge Loan Agreement between NLS Pharmaceutics Ltd. and Magnetic Rock Investment AG. |
| 10.23^ | | Amendment to Bridge Loan Agreement between NLS Pharmaceutics Ltd. and Magnetic Rock Investment AG. |
| 10.24^ | | Form of Amendment to Convertible Loan Agreement between NLS Pharmaceutics Ltd. and certain lenders. |
| 10.25^ | | Form of Addendum Number 3 to Promissory Note dated January 2019 between certain investors and the Company. |
| 10.26^ | | Addendum Number 3 to Credit Facility dated August 31, 2015 between NLS Pharma AG and the Lenders named therein. |
| 10.27^ | | Addendum Number 3 to Credit Facility dated August 31, 2015 between NLS-1 Pharma AG and the Lenders named therein. |
| 10.28^ | | Addendum Number 3 to Convertible Promissory Note dated January 18, 2019 between Magnetic Rock Investment AG and the Company. |
| 23.1* | | Consent of Marcum LLP. |
| 23.2^ | | Consent of Wenger & Vieli AG, Swiss counsel to NLS Pharmaceutics Ltd. (included in Exhibit 5.1). |
| 23.3^ | | Consent of Sullivan & Worcester LLP, U.S. counsel to NLS Pharmaceutics Ltd. (included in Exhibit 5.2). |
| 24.1^ | | Power of Attorney (included on the signature page of this Registration Statement). |
| 99.1^ | | Consent of Stig Løkke Pedersen. |
| 99.2^ | | Consent of Myoung-Ok Kwon. |
| * | Filed herewith. |
| ^ | Previously filed. |
Financial Statement Schedules:
All financial statement schedules have been omitted because either they are not required, are not applicable or the information required therein is otherwise set forth in the Company’s financial statements and related notes thereto.
Item 9. Undertakings
(a) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6 hereof, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(b) The undersigned registrant hereby undertakes that:
(1) That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement on Form F-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Stans, Switzerland on November 17, 2020.
| | NLS Pharmaceutics Ltd. |
| | | |
| | By: | /s/ Alexander Zwyer |
| | | Alexander Zwyer |
| | | Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, this registration statement on Form F-1 has been signed by the following persons in the capacities and on the dates indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Alexander Zwyer | | Chief Executive Officer | | November 17, 2020 |
| Alexander Zwyer | | (Principal Executive Officer) | | |
| | | | | |
| /s/ Robert Dickey | | Interim Chief Financial Officer | | November 17, 2020 |
| Robert Dickey | | (Principal Financial and Accounting Officer) | | |
| | | | | |
| /s/ * | | Chairman of the Board of Directors | | November 17, 2020 |
| Ronald Hafner | | | | |
| | | | | |
| /s/ * | | Director | | November 17, 2020 |
| Pascal Brenneisen | | | |
| * By: | /s/ Alexander Zwyer | |
| | Alexander Zwyer | |
| | Attorney-in-fact | |
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, Puglisi & Associates Donald Puglisi, the duly authorized representative in the United States of NLS Pharmaceutics Ltd., has signed this registration statement on November 17, 2020.
| | Puglisi & Associates, |
| | | |
| | By: | /s/ Donald J. Puglisi |
| | Name: | Donald J. Puglisi |
| | Title: | Managing Director |
II-6













