Exhibit 99.2

T h a n k y o u f o r j o i n i n g . T h e p r e s e n ta t i o n w i l l be g i n s h o r t l y .

2 R e d e f i n i n g C e n t r a l N e r v o u s Sys t e m T h e r a p i e s N L S P i p e l i n e G o al s a n d Deve l o p m en t S t r a t e g ies J a n u a r y 31 , 202 3

Fo r w a r d - Lo o k i n g S t a t e m e n t s This presentation contains express or implied forward - looking statements within the Private Securities Litigation Reform Act of 1995 and other US Federal securities laws. For example, we are using forward - looking statements when we discuss the results of our clinical trials, the expected timing of our future clinical trials, the receipt of the results from clinical trials and obtaining regulatory approval for Quilience®; the potential for funding for its operations through 2025; its strategic priorities in 2023; its pipeline of product candidates for various indications; the various time horizon of its patent protections in various jurisdictions; the anticipated development timeline for Quilience; clinical data readout; proposed trials that may occur in the future; our ability to generate revenue from licensing agreements or in compassionate use programs; compounds or product candidates that we may seek to develop or add to our pipeline; and the potential benefits and impact our product candidates could have on improving patient health care. These forward - looking statements and their implications are based on the current expectations of our management only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward - looking statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing its clinical trials; our products may not be approved by regulatory agencies, our technology may not be validated as it progresses further and its methods may not be accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of its products; unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; our patents may not be sufficient; our products may harm recipients; changes in legislation may adversely impact us; inability to timely develop and introduce new technologies, products and applications; loss of market share and pressure on pricing resulting from competition, which could cause our actual results or performance to differ materially from those contemplated in such forward - looking statements. Except as otherwise required by law, we undertake no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting us is contained under the heading "Risk Factors" in our annual report on Form 20 - F for the year ended December 31, 2021 filed with the Securities and Exchange Commission (SEC), which is available on the SEC's website, www.sec.gov , and in subsequent filings made by us with the SEC. Our logo and some of our trademarks and tradenames are used or incorporated by reference in this presentation. This presentation also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks, tradenames and service marks referred to in this presentation may appear without the ®, TM and SM symbols, but those references are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensor to these trademarks, tradenames and service marks. We obtained the statistical data, market data and other industry data and forecasts described by reference in this presentation from market research, publicly available information and industry publications. Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information. Similarly, while we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information. 3

A l e x Zw y e r C h i e f E x e c u t i v e O ffi ce r & Co - F o u n d er

* M a r k e t D a t a a s o f Ja n ua r y 12 , 2023 ** ResearchAndMarkets.com press release (January 19, 2022) 5 N A S D A Q : N L S P , N L S P W M ar k e t C a p * : $ 59.02 M S ha r e s O u t s t a n d i n g: 32.43 M A D T V ( 3 m o . ) : 446.3K Bu s i n e s s H i g h l i g h t s NLS develops drug treatments for rare and co m p l e x C e n tr a l N e r v o u s S y s t e m ( C N S ) d i s o r d e rs • Quilience® has successfully completed a Phase 2 trial, including OLE, for narcolepsy treatment: $3.9B (2021) annual market** • O r p h a n D r u g D e s i g n a t i o n ( O D D ) g r a n t e d i n t h e U S an d E u r o p e • Pipeline progressed and expanded with long - dated IP protections in major markets • Secured funding for current programs and operations through 2025 • Named Patient Program for patients suffering from idiopathic hypersomnia l a u n c h e d i n t a r ge t m a r k e t s a c r o s s E u r o pe • Ke y E x e cu t i v e L e a d e r s h i p r o l e s f i ll e d

6 202 3 NL S St r at e g i c P r i o r i t i e s Focus on Rare and Complex CNS Disorders L e a d i ng e d g e s c i e nc e Wel l capitalize d – f u nd e d t h r o u g h e nd o f 2025 S e a s o n e d l e a d e r s hi p t e a m w i t h trac k record o f success M u l t i p l e s ho t s o n g o a l F l e x i b l e & ni m b l e b u s i n e s s model Develop Ma z indol I ni t i a t e P h a s e 3 p r o g r a m f o r treatment of EDS and cataplexy i n a d u l t p a t i e n t s w i t h narcolepsy A cce l e r a t e a pp r o v a l p a t h w a y Proo f - o f - Co nc e p t i n i d i op a t hi c hypersomnia P r o m i s i ng d a t a a c r o s s m u l t i p l e CN S i nd i c a t i o ns A dva nc e P ipeline L i c e n s e o r d e ve l o p s e c o n d m a z i nd o l - b a s e d pr o du c t candidate P r o g r e s s N L S - 4 ( l a u f l u m i d e ) f o r th e tr e a t m e n t o f C F S ( L o n g - C O V I D & c a n c e r f a t i g u e ) a n d o t h e r hy p e r s o m no l e nc e d i s or d e r s P r o g r e s s po r t f o l i o o f c o m po u nd s f o r f u r t h e r s t u d y i n r a r e C N S Disorders

7 N L S Le a d e r s h i p T e a m A l e x Z w y e r , M B A Chief Executive Officer & Co - Founder A co - f o un d e r of t he co m p a n y w i t h e x t en s i v e o p e r a t i o n a l , C - l e v e l p h a r m a ce u t i c a l e x p er i en c e . A s e r i a l entrepreneur and strong leader with a p r o v e n t r a c k - re c o r d t o g e t “d e a l s d o n e ” . S e r v e d as Ch i e f O p e r a t i n g O f f i ce r at Viforpharma AG, a global specialty p h a r m a ce u t i c a l c o m p a n y , a n d a re t i re d S w i ss a r m y C a pt a i n . Ge o r g e A p o s t o l , M D , MS Ch i e f M e d i c a l O f f i ce r Worked in pharma R&D organizations for mo r e t h a n 2 0 y ea r s. Br o a d dr u g de v e l o p m e n t e x p er t i s e ac r o ss e a r l y , mi d d l e a n d l at e p h a s e s o f de v e l o p m e n t at the Global R&D organizations of Eli Lilly, P fiz e r , A b b o tt , No v a r t i s , S h i r e a n d En d o . Received distinguished R&D awards and ac h i e v e d m u l t i p l e re g u l a t o ry ap p r o v a l s in U S , E U a n d J a p a n . Cha d He l l m a n n , M B A Ch i e f F i n a n c i a l O f f i ce r S e a s o n e d C - l e v e l e x e cu t i v e w i t h e x t en s i v e i n v e s t me n t e x p er i en c e i n b o t h the public and private markets. Fo un d e d B i s o n V e n t u r e s , a n e a r l y - s ta g e v e n t u r e ca p i t a l f u n d . S e r v e d on s e v e r a l b o a r d s of d i r ec t o r s o v e r t he l a s t 1 5 y ea r s i n c l u d i n g E T W a t e r , I n c . , Nuvision, Inc. (Chairman), and Vertebron, Inc. E r i c Ko n o f a l, M D , P h D Chief Scientific Officer & Co - Founder A drug - hunter and co - founder of the company wi t h a de e p k n o w l e d g e a n d ex p er i e n c e in cli n i ca l a n d sc i e n t i f i c re s e a r c h . H e is a s e n i o r me d ic a l co n s u l t a n t f o r t h e P e d i at r i c S l ee p D i s o r d e r s C e n t e r a t t h e Ro b e rt - D e b r e U n i v e r s i t y of Paris (APHP) and served as Principal Clinical I n v e s t i g a t o r a t t h e C l i n i c a l P h a r m ac o l o g y & P h a r m ac o g e n e t i c D e p a r t m e n t a t Ro b e rt - D e b r e U n i v e r s i t y o f Pa r i s ..

E x p e r i e n ce d Ma n a g e m e n t T e a m A ss e m b l e d AVERAGE 30+ YEARS 100+

9 R e d e f i n i n g C e n t r a l N e r v o u s Sys t e m T h e r a p i e s N L S P i p e l i n e U p d a te

G e o r g e A po s t o l , M D , MS C h i e f M e d i c a l O ffi ce r

11 Hi g h Unm e t M e d i cal Ne e d with Scientific Rationale Favorable Commercial Po t e n t ial w i t h S t ro ng I P Position Well Established Clinical D e v e l op m e nt Pathway NL S G u i d i ng Cons i d e r a t i ons f or E v a l u a t i ng P i pe l i n e P r o d uc t s
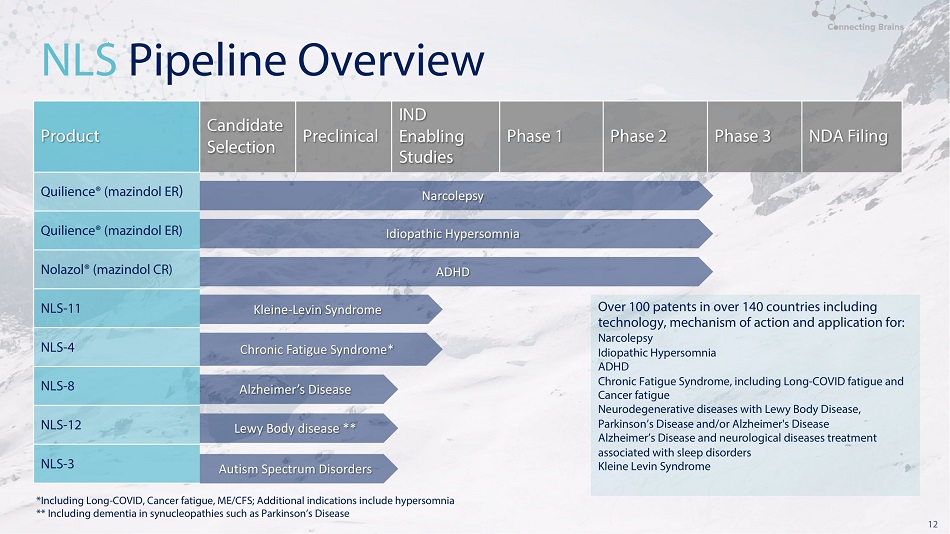
12 N L S P i p e l i n e O v e r v i ew *Including Long - COVID, Cancer fatigue, ME/CFS; Additional indications include hypersomnia * * I n c l u d i n g d e m e n t i a i n s y n u cl e op a t h i e s s u c h a s P a r k i ns o n ’ s D i s e a se : Product C a n d i da t e Selection Preclinical IND En a blin g Studies Ph a s e 1 Ph a s e 2 Ph a s e 3 NDA F i l ing Q u ilience ® ( ma z ind o l ER ) Narcolepsy Idiopathic Hypersomnia ADHD K leine - L e v in S ynd r o m e O v e r 10 0 pa tent s i n o v e r 14 0 c o u nt r ie s incl u ding technology, mechanism of action and application for Narcolepsy Ch r o nic F a t igue S ynd r o m e* I d i op a t h i c H y p e r s o mn i a ADHD Alzheimer’s Disease Chronic Fatigue Syndrome, including Long - COVID fatigue and C an c e r fa t i gue Ne u r o de g enera t i v e d i s ea s e s w i th Le wy Bo d y D i s ea s e , L e w y B o dy dise a se ** Par k i n so n ’s D i s ea se and /or Al zhe i mer' s D i s ea se Alzheimer’s Disease and neurological diseases treatment a ssoc i a t e d w i th sl ee p d i so rder s Au t ism S pe c t r um Dis o r de r s K l e i n e Lev i n Synd r o m e Q u ilience ® ( ma z ind o l ER ) N ol a z o l ® ( ma z ind o l C R ) NLS - 11 NLS - 4 NLS - 8 NLS - 12 NLS - 3

13 R e d e f i n i n g C e n t r a l N e r v o u s S y s t e m Therapies P r e - Cl i n i c a l Pro g r a m s

14 N L S - 11 N L S - 11 T a r g e t i n d i c a t i o n : Kl e i n e - Le v i n S y n d r o me Previous development (Schering) demonstrated a benefit on hypersomnia symptoms and exhaustion in adults As of today, there are no approved treatment available for KLS Promising results and extensive historical preclinical data, as well as preliminary clinical data, support continued development in KLS and other rare sleep disorders International Patents pending including the NLS - 11 mechanism of action (MoA) and its applications as treatment for KLS and sleep disorders (through 2041) P r e c l i n i c a l S t ud i es A number of animal studies showed NLS - 11 increased wakefulness similar to methylphenidate (MPH) but onset appeared slower and e f f e c t s l o n ge r l a s t i n g This effect occurred at doses of 5 mg/kg instead of 100 mg/kg as tested for MP It is assumed that NLS - 11 would exert similar CNS stimulant activity in humans, possibly with fewer side effects than described with MP Animal toxicity studies showed good safety and tolerability profile: less effect on cardiac and respiratory rates as well as on appetite than MP P r el i m i n a r y C l i n i c a l D a t a Do s e - e f f e c t f r o m 0 . 2 5 t o 1 . 0 mg/ d i n 3 3 a d ul t patients with exhaustion, depression or hypersomnia sy n d r o m e s tr e a t e d f o r 2 - 1 4 w ee k s As expected, NLS - 11 exerted similar CNS stimulant ac t i v i t y t o M PH b u t w i t h f e w e r - s i d e e ff ec t s Improvement in hypersomnia and exhaustion sy n d r o m e ( p e r s i s t e n t f a t i g u e , w h i c h i s r e l a t e d t o Chronic Fatigue Syndrome (CFS) and affecting daytime arousal and sleep) was also described clinically
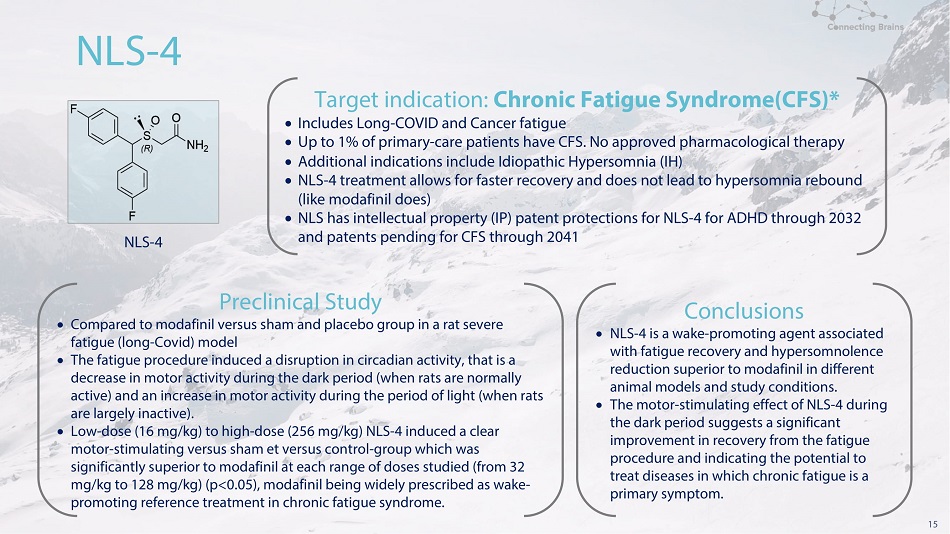
15 N L S - 4 N L S - 4 T a r g e t i n d i c a t i o n : C h r o n i c F a t i g u e S y n d r o m e (CF S )* I n c l u d e s L o n g - COV I D a nd C a n c e r f a t i g u e Up to 1% of primary - care patients have CFS. No approved pharmacological therapy Additional indications include Idiopathic Hypersomnia (IH) NLS - 4 treatment allows for faster recovery and does not lead to hypersomnia rebound (like modafinil does) NLS has intellectual property (IP) patent protections for NLS - 4 for ADHD through 2032 a n d p a te n t s p e nd i n g f o r CF S t h r o u g h 204 1 P r e c l i n i c a l S t ud y Compared to modafinil versus sham and placebo group in a rat severe f a t igu e (l on g - C o v i d ) m o d e l The fatigue procedure induced a disruption in circadian activity, that is a decrease in motor activity during the dark period (when rats are normally active) and an increase in motor activity during the period of light (when rats a r e la r g e l y i n ac t i v e ) . Low - do s e ( 1 6 m g / k g ) to h i g h - do s e ( 25 6 m g / k g ) N L S - 4 i n d u ce d a c l e a r mo t o r - s t i mu l a t i n g v e r s u s s h a m e t v e r s u s c o n tr o l - gr o u p w h i c h w a s significantly superior to modafinil at each range of doses studied (from 32 mg/kg to 128 mg/kg) (p<0.05), modafinil being widely prescribed as wake - pro m o t i n g re f e re n c e t re a t m e n t i n c h r o n i c f a t i g u e s y n d r o m e . Conclusions NLS - 4 is a wake - promoting agent associated wi t h f a t i g u e r ec o v e r y a n d h ype r s o m n o le n c e re d u c t i o n s u pe ri o r t o m o d a f i n i l i n d i ff e re n t an i m al m od e l s a n d s t u d y c on d i t i on s . The m o t o r - s t i mu l a t i n g e ff ec t o f N L S - 4 d u r i ng th e da r k p e r i o d s u gg e s ts a s i g n i f i ca n t imp r o v eme n t i n r e c o v er y fr o m t h e f a t igue pro ce d u r e a n d i n d i c a t i n g t h e p o t e n t i a l t o tr e a t d i s e a s e s i n w h i ch ch r o n i c f a t i g u e i s a pri m a r y s y m p t o m ..
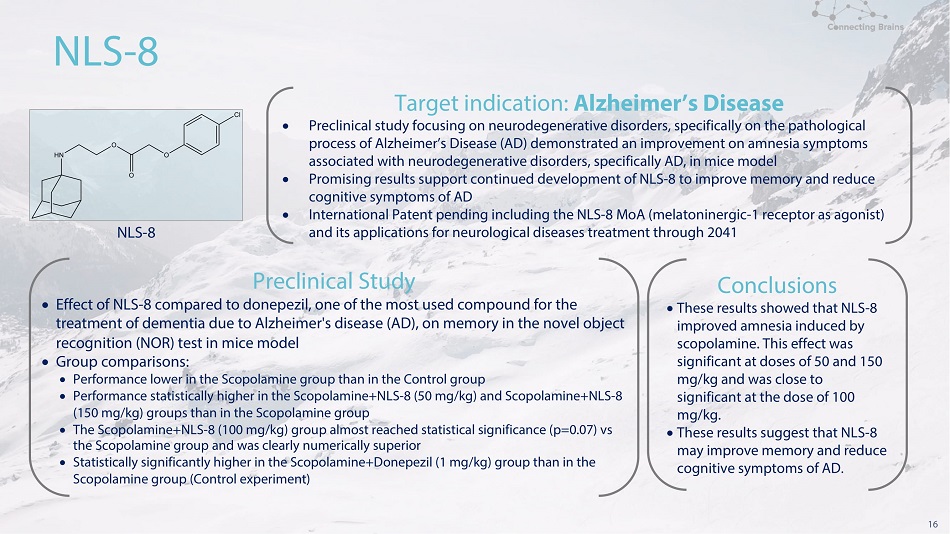
16 N L S - 8 N L S - 8 T a r g e t i n d i c a t i o n : Al z h e i m e r’s D i s e a s e Preclinical study focusing on neurodegenerative disorders, specifically on the pathological process of Alzheimer’s Disease (AD) demonstrated an improvement on amnesia symptoms associated with neurodegenerative disorders, specifically AD, in mice model Promising results support continued development of NLS - 8 to improve memory and reduce cognitive symptoms of AD International Patent pending including the NLS - 8 MoA (melatoninergic - 1 receptor as agonist) and its applications for neurological diseases treatment through 2041 P r e c l i n i c a l S t ud y Effect of NLS - 8 compared to donepezil, one of the most used compound for the treatment of dementia due to Alzheimer's disease (AD), on memory in the novel object recognition (NOR) test in mice model Gr ou p c o m p ari s o n s: Performance lower in the Scopolamine group than in the Control group Performance statistically higher in the Scopolamine+NLS - 8 (50 mg/kg) and Scopolamine+NLS - 8 (150 mg/kg) groups than in the Scopolamine group The Scopolamine+NLS - 8 (100 mg/kg) group almost reached statistical significance (p=0.07) vs the Scopolamine group and was clearly numerically superior Statistically significantly higher in the Scopolamine+Donepezil (1 mg/kg) group than in the Sc o p o l a m in e g r ou p ( C on t r o l e x p e r i m e n t ) Conclusions The s e r e s u l t s s ho w e d t ha t N LS - 8 imp r o v e d a mn e s i a i n d u ce d b y s c o p o l a m i n e . T h i s e ff ec t w a s s i g n i f i c a n t a t d o s e s o f 5 0 a nd 150 mg/ k g a n d w a s c l o s e t o s i g n i f i c a n t a t t h e d o s e o f 100 mg/kg. The s e r e s u l t s s u gg e s t t ha t N L S - 8 m a y imp r o v e mem o r y a nd r e d u c e co g n i t i ve s y m p to m s o f A D .
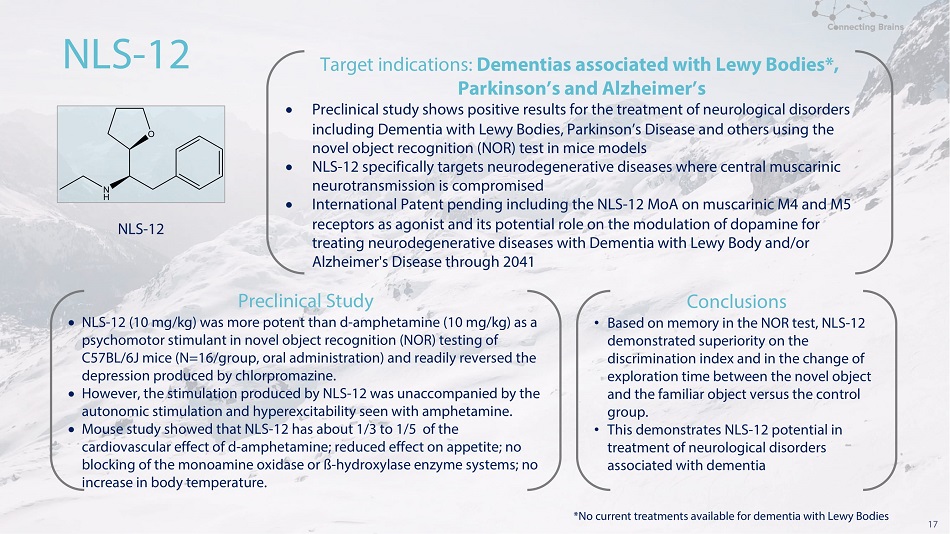
17 N L S - 12 N L S - 12 Tar g e t i n d i ca t i o n s : D e m e n t i a s a ssoc i a t e d w i t h L e w y B od i e s * , Pa r k i n s o n ’ s a n d A l z h e i m e r ’ s Preclinical study shows positive results for the treatment of neurological disorders including Dementia with Lewy Bodies, Parkinson’s Disease and others using the no v e l o b j e c t r e c o g n i t i on ( N O R ) t e s t i n m ic e m o d e l s NLS - 12 specifically targets neurodegenerative diseases where central muscarinic ne u r o t r a n s m i ss i on i s c o m p r o m i s e d International Patent pending including the NLS - 12 MoA on muscarinic M4 and M5 receptors as agonist and its potential role on the modulation of dopamine for treating neurodegenerative diseases with Dementia with Lewy Body and/or Al z h e i m e r ' s D i s e a s e th r ou g h 2041 Pr e c l i n i c a l S t ud y NLS - 12 (10 mg/kg) was more potent than d - amphetamine (10 mg/kg) as a psychomotor stimulant in novel object recognition (NOR) testing of C57BL/6J mice (N=16/group, oral administration) and readily reversed the d e p r e ss i o n p r o d u ce d b y c h l o r p r o m a z i n e . However, the stimulation produced by NLS - 12 was unaccompanied by the a u t o n om i c s t i mu l ati on a n d h y p e r e x c i t a b i l i t y s ee n w i t h a m ph e t a m i n e . Mouse study showed that NLS - 12 has about 1/3 to 1/5 of the cardiovascular effect of d - amphetamine; reduced effect on appetite; no blocking of the monoamine oxidase or ß - hydroxylase enzyme systems; no increase in body temperature. Conclusions • B a s e d o n me mo r y i n t h e N O R t e s t , N L S - 12 d e m on s tr at e d s u p e r i o r i ty o n t h e d i s c r i m i n a t i o n i n d e x a n d i n t h e c h a n g e o f exploration time between the novel object an d t h e f a m i l i ar o b j ec t v e r s u s t h e c o n tr ol group. • Th i s d e m on s tr at e s N L S - 1 2 p o t e n t i a l in treatment of neurological disorders a s s oc iat e d w i t h d e m e n t i a *No current treatments available for dementia with Lewy Bodies

18 N L S - 3 N L S - 3 Tar g e t i n d i ca t i o n s : Au t i s m S p e c t r u m D i s o r d e r s ( ASD ) , S u b s t a n c e U s e D i so r der ( SU D) Preclinical studies for NLS - 3 show potential for the treatment of ASD with improved efficacy, safety and tolerability versus MPH NLS has intellectual property (IP) patent protections for NLS - 3 in the U.S., J a p a n , C h i n a a n d E u r op e t h r o u g h 2032 Based on MoA, NLS - 3 also has potential for the treatment of Substance Use Disorder (SUD) Pr e c l i n i c a l & C l i n i c a l D a t a* Animal toxicology suggest multiple advantages over m e t h y l p h e n i d a t e a n d a mp h e t a m i n e Preclinical and clinical efficacy in ADHD symptoms and SUD as well as in severe psychiatric disorders, including depression, neurodevelopmental disorders, apathy, mood and psychotic s y m p t o m a t o l o g y D i d n o t e x h i b i t a dd i c t i o n o r w i t h d r a w a l s ym p t o m s L o w i n ci d e n c e o f s i d e e ff e c t s i n c h i l d r e n , a d o l e s c e n t s , a n d a d u l t s w i th AD H D i n p r e v i ou s cl i n i c a l s t u d i e s Conclusions N L S - 3 c o n s i s t e nt l y s h o w e d a s i g n i f i c a n t c l i n i c a l b e n e f i t w i t h fe we r s i d e e ffe c t s i n c h i ld re n , ad o l e s c e n t s , a n d ad u l t s w i t h A D H D - re l a t e d d i sord ers , d e p ress i o n , p s y c h o t i c d i s o r d e r s v e r s u s am p h e t am i n e a n d M P H Cl - N + H H O O *Review accepted for publication: https://pubmed.ncbi.nlm.nih.gov/36683369/

19 R e d e f i n i n g C e n t r a l N e r v o u s Sys t e m T h e r a p i e s N L S C l i n i cal P r o gr am

20 P O L A R I S : M a z i n d o l ER D e v e l o p m e n t P r o g r a m i n N a r c o l e p s y The Mazindol ER development program POLARIS currently consists o f t w o US c l i n i c a l tri a l s b o th a ppr o v e d by F D A/ W I RB * • NLS - 1021 Phase 2, A four - week double - blind, placebo controlled, randomized, US multi - center study of Mazindol ER 3 mg once daily vs. placebo (1:1) • NLS - 1022 Open Label Extension, An Open Label Extension (OLE) Study available for individuals following completion of the four - week NLS 1021 study. This OLE study offers participants the opportunity to take oral Mazindol ER once daily in the morning for up to six months. *US Food and Drug Administration and Western Institutional Review Board, Inc.
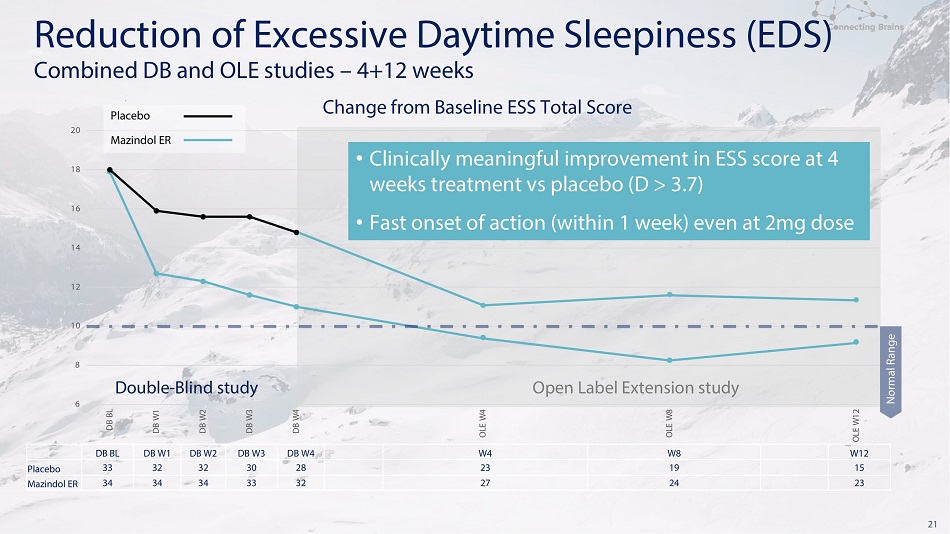
21 D B B L D B W1 D B W2 D B W3 D B W4 W4 W8 W12 Placebo 33 32 32 30 28 23 19 15 M a z i nd ol ER 34 34 34 33 32 27 24 23 6 8 10 12 14 16 18 20 DB B L DB W 1 DB W 2 DB W 3 DB W 4 OL E W 4 OL E W 8 OL E W 1 2 Change from Baseline ESS Total Score N o rma l R an ge Placebo Ma z i nd o l E R • C l i n i c a ll y m e an i n g f u l i m p r o v e m e n t i n E SS s co r e at 4 w e e k s t r e a t m e n t vs p l a ce b o ( D > 3 . 7 ) • Fa st o ns e t o f a c t i o n ( w i t h i n 1 w ee k ) e v e n a t 2 mg d o se D o u b l e - Bl i n d s t u d y Op e n L a b e l E x t e n s i on s t u dy R e d u c t i o n o f E xc e ss i v e Da y t i m e S l ee p i n e ss ( E D S ) Com b i n e d D B a n d O L E s t u d i e s – 4+1 2 w ee k s
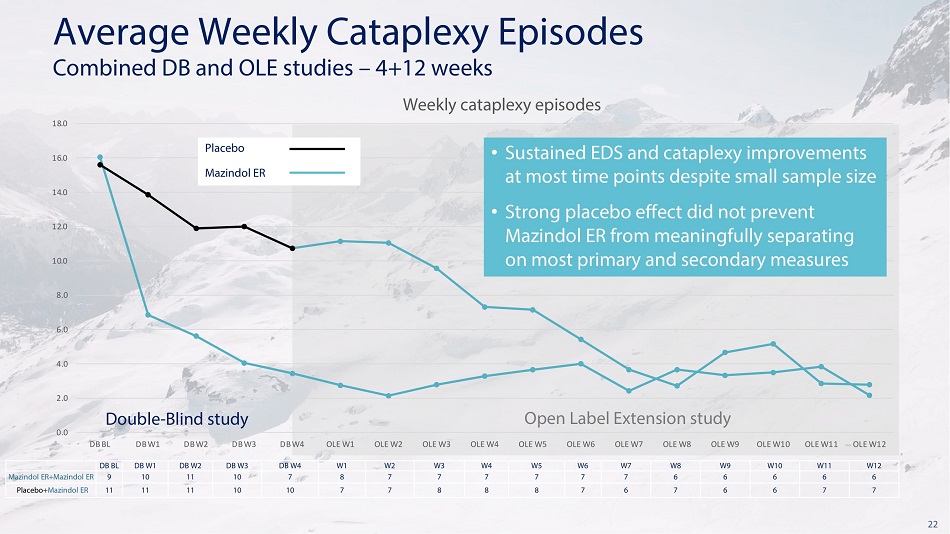
22 0 . 0 8 . 0 6 . 0 4 . 0 2 . 0 1 0 . 0 1 2 . 0 1 4 . 0 1 6 . 0 1 8 . 0 D B W 4 O L E W 1 O L E W 2 O L E W 3 O L E W 4 O L E W 1 0 O L E W 1 1 O L E W 1 2 Av e r a ge W e e k l y C a t a p l e x y E p is o d e s Com b i n e d D B a n d O L E s t u d i e s – 4+1 2 w ee k s Weekly cataplexy episodes Placebo Ma z i nd o l E R • Sustained EDS and cataplexy improvements a t m o s t t i m e po i n t s d es p i t e s m a l l s a m p l e s i z e • S t r ong p l ace b o e ff ec t d i d n ot p r e v e nt M a z i n d o l ER f r o m m ea n i n g f u ll y s e p a r a t i n g on m o s t p r i m a r y a nd s ec on d a r y m ea s u r e s DB BL DB W 1 DB W 2 DB W 3 DB W 4 W1 W2 W3 W4 W5 W6 W7 W8 W9 W10 W11 W12 M a z i n do l ER + M a z i n do l ER 9 10 11 10 7 8 7 7 7 7 7 7 6 6 6 6 6 Pl ac e b o + M a z i n do l ER 11 11 11 10 10 7 7 8 8 8 7 6 7 6 6 7 7 D o u b l e - Bl i n d s t u d y D B B L D B W 1 D B W 2 D B W 3 Op e n L a b e l E x t e n s i on s t u dy O L E W 5 O L E W 6 O L E W 7 O L E W 8 O L E W 9

23 P O LARI S : D B + O L E Co n c l u s i o n s Safety • Mazindol ER was well - tolerated with even the most common AEs (dry mouth, nausea, decreased appetite) being benign in nature and o c c u rr i n g i n l e ss t h an o n e i n f i v e p a t i e n t s • All adverse events where either mild or moderate • No s e v e r e , s e r i o u s , o f une x pe ct e d a d v e r s e e v e nt s

24 P O LARI S : D B + O L E Co n c l u s i o n s Efficacy • Mazindol ER demonstrated rapid, consistent, and clinically meaningful efficacy in the treatment of sleepiness in adult subjects with narcolepsy as well as a strong signal in treating cataplexy episodes • Patients treated with Mazindol ER continued to improve after 3 months of treatment • Previously placebo - treated group quickly reached ESS levels comparable to p r e v i o u sl y N L S - 2 - tr e a t e d g r o u p • Cataplexy episodes decreased to 2 - 4 episodes/week with some patients e x p e r i e n c i n g z e r o ca t a p l e x y e p i s od es • This data demonstrates further improvement in efficacy of mazindol ER in the treatment of excessive daytime sleepiness and cataplexy with longer treatment duration
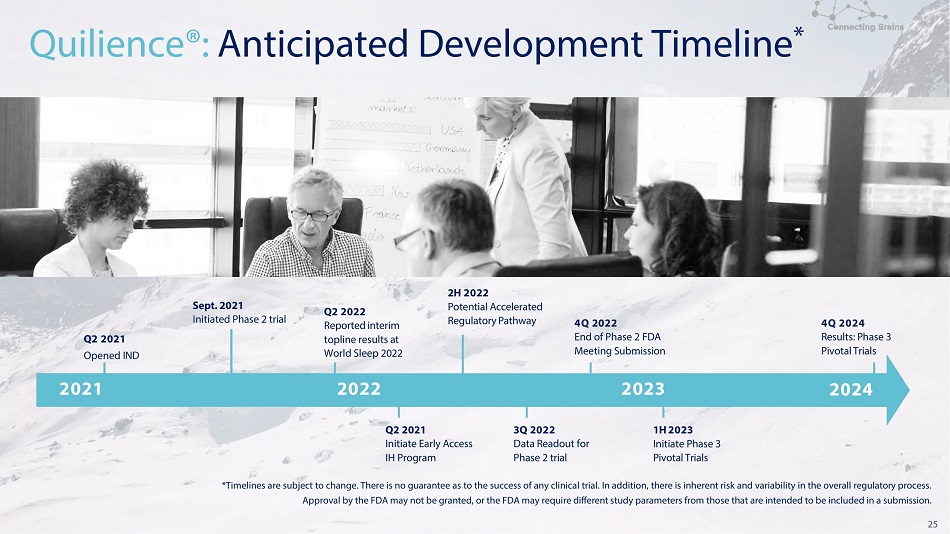
*Timelines are subject to change. There is no guarantee as to the success of any clinical trial. In addition, there is inherent risk and variability in the overall regulatory process. Approval by the FDA may not be granted, or the FDA may require different study parameters from those that are intended to be included in a submission. 25 Q 2 2021 I n i t i a t e Ea r l y A c c e s s I H P r o g r a m Q 2 2021 O p e n e d IN D 2021 2022 2023 Quilience®: Anticipated Development Timeline * Q 2 2022 R e p o r t e d i n t e r im to p l i n e r e s u l t s at W o r l d S l ee p 2022 4 Q 2022 E n d o f Ph a s e 2 F D A M e e t i n g Su b mi ss i o n 4 Q 2024 R e s u l t s : Ph a s e 3 P i v o t al T r i a l s 3Q 2022 Da t a R e a dou t f o r Ph a s e 2 t ri a l 2H 2022 P o t en t i al A c c e l e r a t e d R e g u l a t o r y P a t hw a y 1H 202 3 I n i t i a t e Ph a s e 3 P i v o t al T r i a l s Se p t. 2021 I n i t i a t e d Ph a s e 2 t ri al 2024

26 N L S - 1 3 ( maz i n d o l ) I d i op a t h i c H y p e r s o m n i a • O r p h a n D r u g D e s i g n a t i o n i n t h e U S an d E u r o p e • Phase 2 studies with Mazindol ER demonstrated efficacy in narcolepsy patients w i t h T y p e II d i s o r de r , w h i c h h a s s t r o n g s i m i l a r i t i e s t o I H • Promising results support continued development of NLS - 13 (mazindol) for IH • No approved treatment in Europe, 1 approved therapy in the US • Early access program under named - patient program has been initiated in Europe with the potential to generate short - term, non - dilutive revenues for NLSP • NLS has intellectual property (IP) patent protections for mazindol for use in treating attention deficit disorders, related deficit of alertness or decline of vigilance, or excessive daytime sleepiness (e.g., narcolepsy, idiopathic hypersomnia) in the US, EU and Canada through 2037

27 N L S - 2 0 ( maz i n d o l ) AD H D • NLS - sponsored Phase 2 trial in ADHD demonstrated efficacy comparable to first - line CII stimulants with favorable tolerability • T r i a l v a l i d a t e d o n c e - d a i l y f o r m u l at i o n • Studies suggest ADHD and narcolepsy are genetically related with possible co mm on u n d e rl y i n g b i o l o g i c m e ch a n i sm s • Approximately 1/3 of patients with narcolepsy have ADHD symptoms, making it an important comorbidity • Narcolepsy is often treated with the same stimulants approved for ADHD (methylphenidate, amphetamine products and others) • DEA Schedule IV classification vs. Schedule II for most stimulants; can reduce risk o f a b u s e , m i s u s e , a n d d i ve r s i on • NLS has intellectual property (IP) patent protections for mazindol use in ADHD in the US (2028), EU, CA, Australia, Israel & NZ through 2027

A l e x Zw y e r C h i e f E x e c u t i v e O ffi ce r & C o - F o u n d e r

29 A n t i c i p a t e d M i l e s t o n e s • Complete results for Quilience® in narcolepsy Open Label Extension (OLE) s t u d y a v a i l a bl e ( Q 1 / 2 023 ) • F D A E n d - o f - P h a s e 2 M ee t i n g ( Q 1 / 2023) • Potential for accelerated regulatory pathway designations (FastTrack, Br e a k t h r o u g h ) ( Q 2 / 2023) • Phase 3 trials start for Quilience® in narcolepsy anticipated (mid - 2023) • Continued penetration of Extended Access Program in Europe with potential f o r n o n d i l u t i v e r e v e nu e s ( o n g o i n g ) • I n it i a t i o n o f IN D - e n a b l i n g s t u d i e s f o r N LS - 4 ( H2 / 2023) • Potential for business development activity including license agreements or partnerships with other Pharma companies (ongoing)

30 W h y N L S ? F o c u se d G r o w t h i n 2023 • Experienced management team across pharmaceuticals, neuroscience, R&D and capital markets with a proven track record of bringing multiple drugs to market • Multiple innovative drug programs targeting rare and complex central nervous s y s t e m d i s o r d e r s w i t h h i g h u n me t me d i c a l n ee d s • Financed through 2025 to progress R&D pipeline with access to $30M+ in capital • Growing IP portfolio across 6 patent families to support clinical trials, M&A, and IP s t r a t e g i e s o f i nn ov a t i ve c om p o un d s • Solid opportunity for lead product Quilience ® (mazindol ER) through rigorous Phase 3 program • Partnerships with world - class scientists and Contract Research Organizations supporting R&D programs and further validating portfolio potential

Q u e s t i o n s f o r P a n e l

32

T h i s c o n c l u d e s o u r p r e s e n ta t i o n . T h a n k y o u f o r j o i n i n g .
































