
KOL Event December 10, 2024 Exhibit 99.2

Disclaimers and forward-looking statements This presentation and the accompanying discussion contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding Tscan Therapeutics, Inc. (the “Company” or “TScan”)’s plans, progress, and timing relating to the Company’s hematologic malignancies programs, solid tumor programs and the presentation of data, the Company’s current and future research and development plans or expectations, the structure, timing and success of the Company’s planned preclinical development, submission of INDs, and clinical trials, the potential benefits of any of the Company’s proprietary platforms, multiplexing, or current or future product candidates in treating patients, and the Company’s goals and strategy. TScan intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan,” “on track,” or similar expressions or the negative of those terms. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. The express or implied forward-looking statements included in this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: the beneficial characteristics, safety, efficacy, therapeutic effects and potential advantages of TScan’s TCR-T therapy candidates; TScan’s expectations regarding its preclinical studies being predictive of clinical trial results; the timing of the initiation, progress and expected results of TScan’s preclinical studies, clinical trials and its research and development programs; TScan’s plans relating to developing and commercializing its TCR-T therapy candidates, if approved, including sales strategy; estimates of the size of the addressable market for TScan’s TCR-T therapy candidates; TScan’s manufacturing capabilities and the scalable nature of its manufacturing process; TScan’s estimates regarding expenses, future milestone payments and revenue, capital requirements and needs for additional financing; TScan’s expectations regarding competition; TScan’s anticipated growth strategies; TScan’s ability to attract or retain key personnel; TScan’s ability to establish and maintain development partnerships and collaborations; TScan’s expectations regarding federal, state and foreign regulatory requirements; TScan’s ability to obtain and maintain intellectual property protection for its proprietary platform technology and our product candidates; the sufficiency of TScan’s existing capital resources to fund its future operating expenses and capital expenditure requirements; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of TScan’s most recent Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and any other filings that TScan has made or may make with the SEC in the future. Any forward-looking statements contained in this presentation represent TScan’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, TScan explicitly disclaims any obligation to update any forward-looking statements.

Agenda Ran Reshef, M.D., M.Sc., Director of Translational Research, Blood and Marrow Transplantation Program, Columbia University Irving Medical Center Results from ALLOHA™ Phase 1 trial study of TSC-100 and TSC-101 in patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS) Gavin MacBeath, Ph.D., Chief Executive Officer Pivotal trial design and heme development strategy Market opportunity Expansion opportunities Q&A Solid tumor program update and strategy for 2025 Q&A

Monzr M. Al Malki, MD1, Alla Keyzner, MD2, Uday Popat, MD3, Yi-Bin Chen, MD, MS4,Hyung C Suh, MD, PhD5, Tania Jain, MD6, Melhem M. Solh, MD7, Anson Snow, MD8, Saar Gill, MD, PhD9, Lohith Gowda, MD10, Joseph Uberti, MD, PhD11, Erica Buonomo, PhD12, Yun Wang, PhD12, Nancy Nabilsi, PhD12, Timothy White12, Cuong Nguyen13, Jim Murray12, Gavin MacBeath, PhD12, Chrystal Louis, MD, MPH12, Shrikanta Chattopadhyay, MD12, Michelle Matzko, MD, PhD12 and Ran Reshef, MD, MSc14 (1)City of Hope, Duarte, CA, (2)Icahn School of Medicine at Mount Sinai, New York, NY, (3)MD Anderson Cancer Center, Houston, TX, (4)Massachusetts General Hospital, Boston, MA, (5)John Theurer Cancer Center, Hackensack University Medical Center, Hackensack, NJ, (6)Johns Hopkins University, Baltimore, MD, (7)Northside Hospital Cancer Institute, Atlanta, GA, (8)Lineberger Comprehensive Cancer Center, University of Carolina at Chapel Hill, Chapel Hill, NC, (9)Abramson Cancer Center and Hospital of the University of Pennsylvania, Philadelphia, PA, (10)Yale Cancer Center and Yale School of Medicine, New Haven, CT, (11)Karmanos Cancer Center/ Wayne State University, Detroit, MI, (12)TScan Therapeutics, Waltham, MA, (13)Biostatistical Consulting, Lexington, MA, (14)Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY TSC-100 and TSC-101 Demonstrate the Potential to Reduce Relapse Rates and Increase Relapse-free Survival in Patients with AML, ALL, or MDS Undergoing Allogeneic HCT with Reduced Intensity Conditioning (RIC): Preliminary Results from the Phase 1 ALLOHA Trial
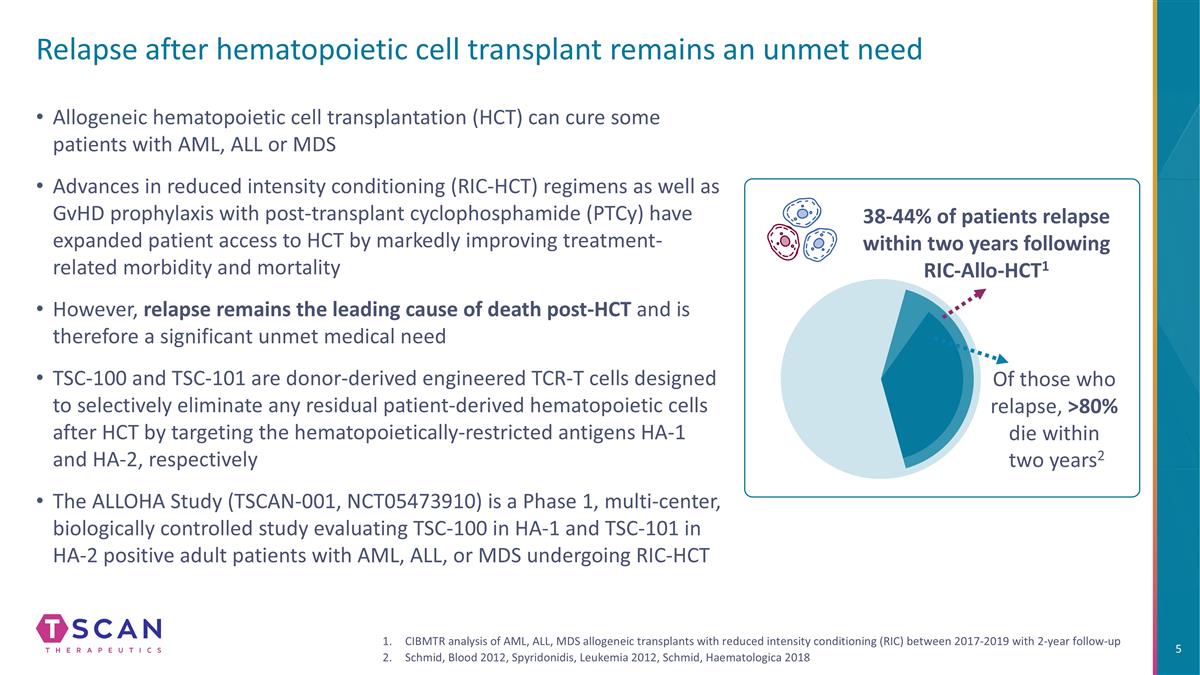
Allogeneic hematopoietic cell transplantation (HCT) can cure some patients with AML, ALL or MDS Advances in reduced intensity conditioning (RIC-HCT) regimens as well as GvHD prophylaxis with post-transplant cyclophosphamide (PTCy) have expanded patient access to HCT by markedly improving treatment-related morbidity and mortality However, relapse remains the leading cause of death post-HCT and is therefore a significant unmet medical need TSC-100 and TSC-101 are donor-derived engineered TCR-T cells designed to selectively eliminate any residual patient-derived hematopoietic cells after HCT by targeting the hematopoietically-restricted antigens HA-1 and HA-2, respectively The ALLOHA Study (TSCAN-001, NCT05473910) is a Phase 1, multi-center, biologically controlled study evaluating TSC-100 in HA-1 and TSC-101 in HA-2 positive adult patients with AML, ALL, or MDS undergoing RIC-HCT Of those who relapse, >80% die within two years2 38-44% of patients relapse within two years following RIC-Allo-HCT1 CIBMTR analysis of AML, ALL, MDS allogeneic transplants with reduced intensity conditioning (RIC) between 2017-2019 with 2-year follow-up Schmid, Blood 2012, Spyridonidis, Leukemia 2012, Schmid, Haematologica 2018 Relapse after hematopoietic cell transplant remains an unmet need
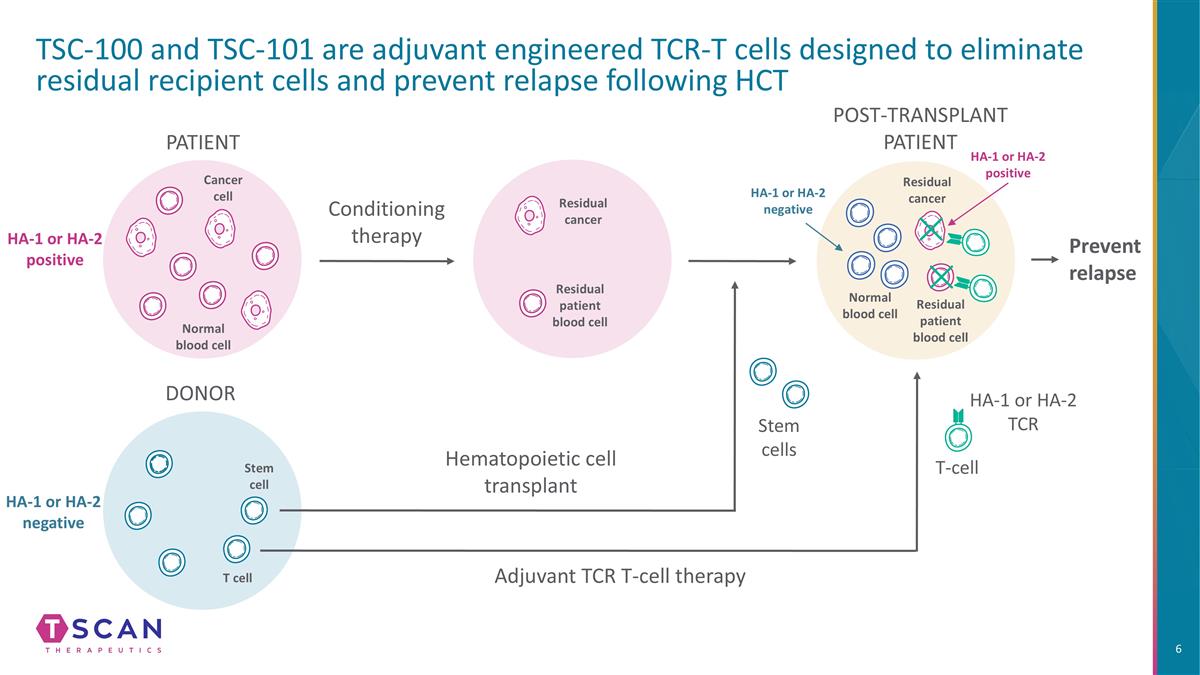
TSC-100 and TSC-101 are adjuvant engineered TCR-T cells designed to eliminate residual recipient cells and prevent relapse following HCT Conditioning therapy PATIENT DONOR Hematopoietic cell transplant Stem cells Prevent relapse Adjuvant TCR T-cell therapy T-cell HA-1 or HA-2 TCR Cancer cell Normal blood cell Stem cell T cell HA-1 or HA-2 positive HA-1 or HA-2 negative Residual cancer HA-1 or HA-2 negative HA-1 or HA-2 positive Residual cancer Normal blood cell Residual patient blood cell Residual patient blood cell POST-TRANSPLANT PATIENT
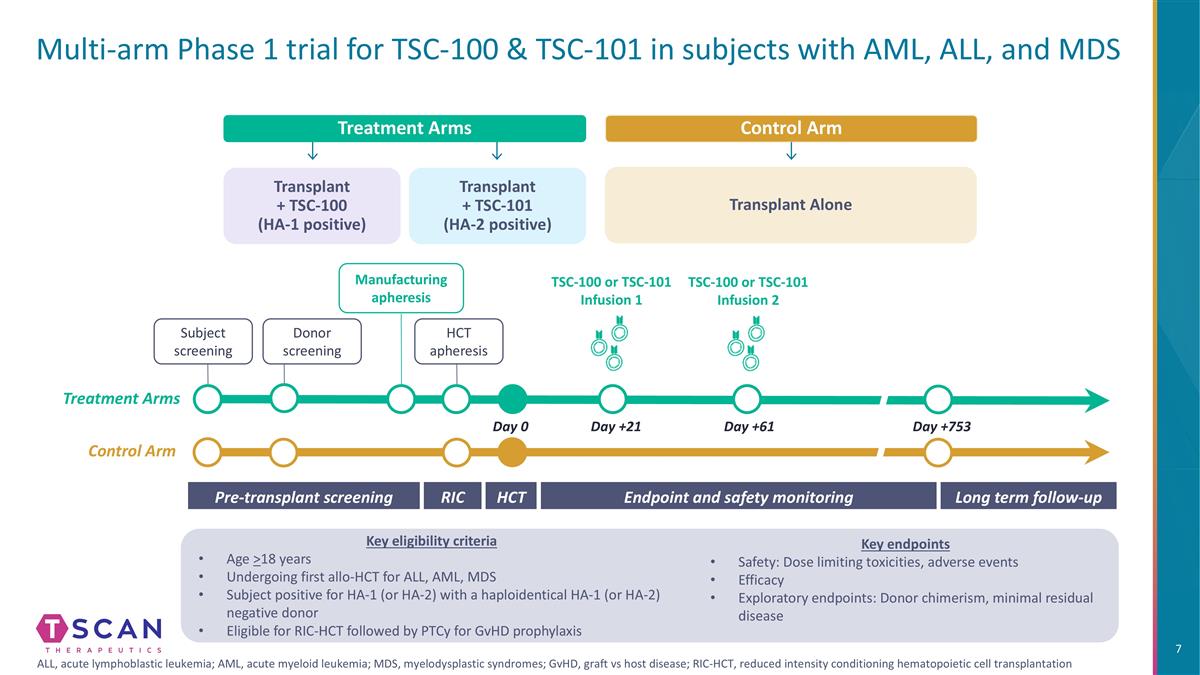
Multi-arm Phase 1 trial for TSC-100 & TSC-101 in subjects with AML, ALL, and MDS Transplant + TSC-100 (HA-1 positive) Treatment Arms Transplant + TSC-101 (HA-2 positive) Control Arm Transplant Alone Long term follow-up HCT Pre-transplant screening Endpoint and safety monitoring RIC Day 0 Day +21 Day +61 Day +753 Donor screening Manufacturing apheresis HCT apheresis TSC-100 or TSC-101 Infusion 1 TSC-100 or TSC-101 Infusion 2 Subject screening Control Arm Treatment Arms Key eligibility criteria Age >18 years Undergoing first allo-HCT for ALL, AML, MDS Subject positive for HA-1 (or HA-2) with a haploidentical HA-1 (or HA-2) negative donor Eligible for RIC-HCT followed by PTCy for GvHD prophylaxis Key endpoints Safety: Dose limiting toxicities, adverse events Efficacy Exploratory endpoints: Donor chimerism, minimal residual disease ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; GvHD, graft vs host disease; RIC-HCT, reduced intensity conditioning hematopoietic cell transplantation
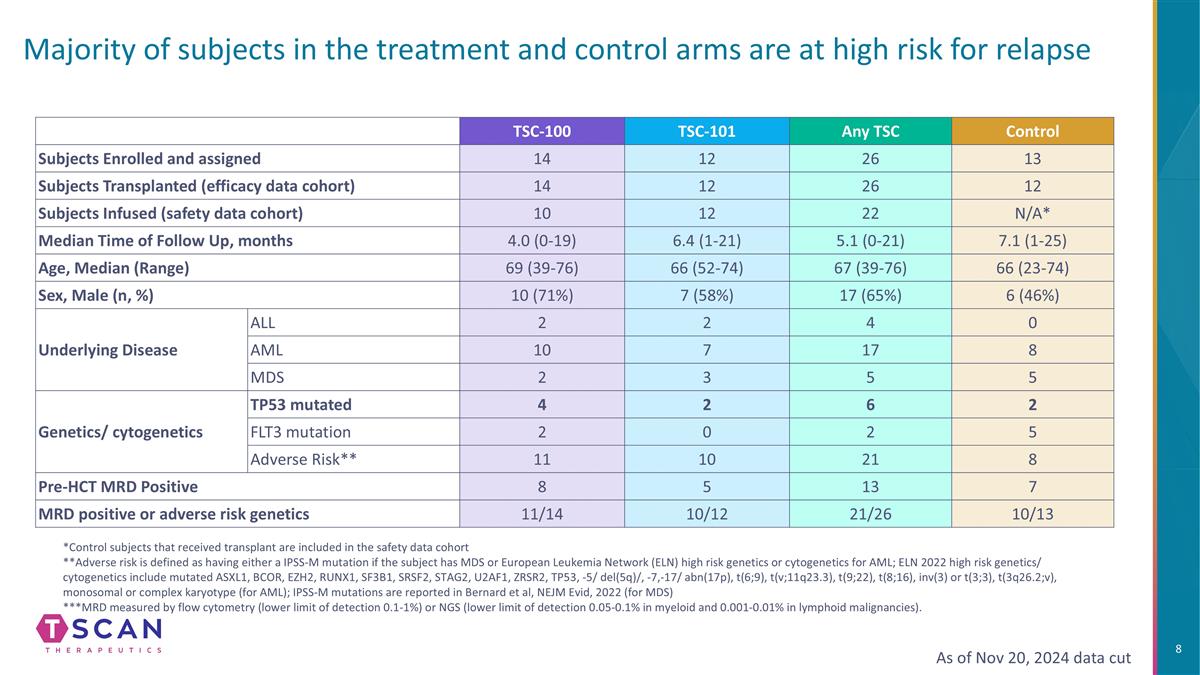
Majority of subjects in the treatment and control arms are at high risk for relapse As of Nov 20, 2024 data cut TSC-100 TSC-101 Any TSC Control Subjects Enrolled and assigned 14 12 26 13 Subjects Transplanted (efficacy data cohort) 14 12 26 12 Subjects Infused (safety data cohort) 10 12 22 N/A* Median Time of Follow Up, months 4.0 (0-19) 6.4 (1-21) 5.1 (0-21) 7.1 (1-25) Age, Median (Range) 69 (39-76) 66 (52-74) 67 (39-76) 66 (23-74) Sex, Male (n, %) 10 (71%) 7 (58%) 17 (65%) 6 (46%) Underlying Disease ALL 2 2 4 0 AML 10 7 17 8 MDS 2 3 5 5 Genetics/ cytogenetics TP53 mutated 4 2 6 2 FLT3 mutation 2 0 2 5 Adverse Risk** 11 10 21 8 Pre-HCT MRD Positive 8 5 13 7 MRD positive or adverse risk genetics 11/14 10/12 21/26 10/13 *Control subjects that received transplant are included in the safety data cohort **Adverse risk is defined as having either a IPSS-M mutation if the subject has MDS or European Leukemia Network (ELN) high risk genetics or cytogenetics for AML; ELN 2022 high risk genetics/ cytogenetics include mutated ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2, TP53, -5/ del(5q)/, -7,-17/ abn(17p), t(6;9), t(v;11q23.3), t(9;22), t(8;16), inv(3) or t(3;3), t(3q26.2;v), monosomal or complex karyotype (for AML); IPSS-M mutations are reported in Bernard et al, NEJM Evid, 2022 (for MDS) ***MRD measured by flow cytometry (lower limit of detection 0.1-1%) or NGS (lower limit of detection 0.05-0.1% in myeloid and 0.001-0.01% in lymphoid malignancies).
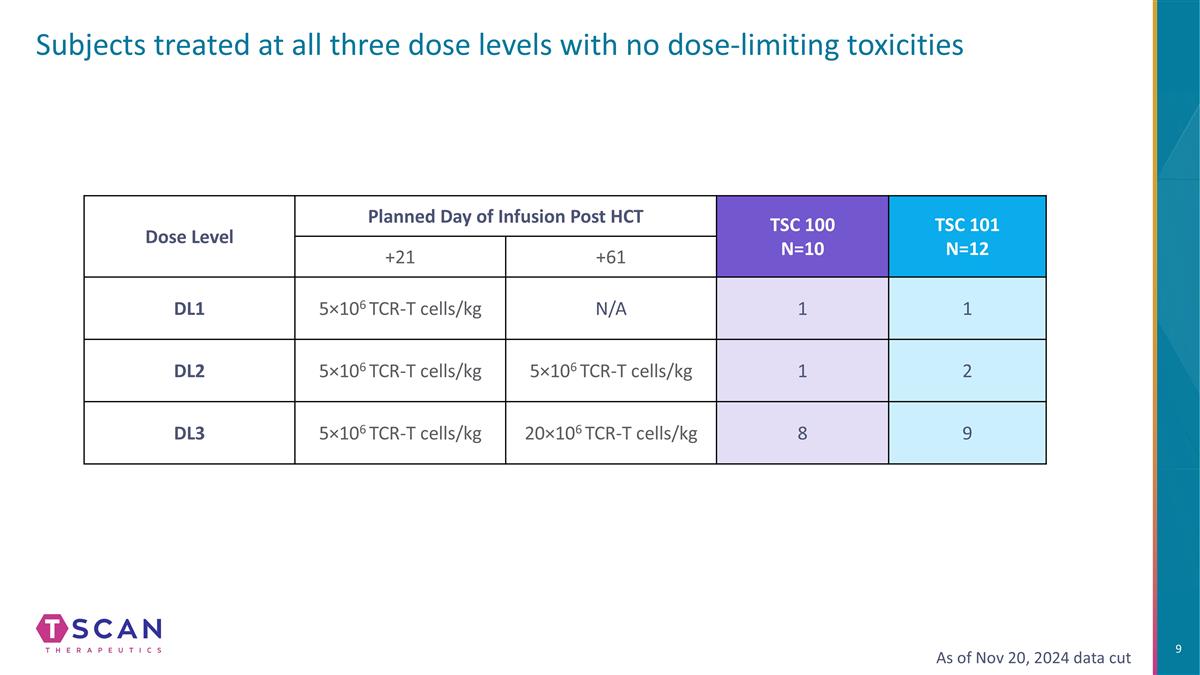
Subjects treated at all three dose levels with no dose-limiting toxicities Dose Level Planned Day of Infusion Post HCT TSC 100 N=10 TSC 101 N=12 +21 +61 DL1 5×106 TCR-T cells/kg N/A 1 1 DL2 5×106 TCR-T cells/kg 5×106 TCR-T cells/kg 1 2 DL3 5×106 TCR-T cells/kg 20×106 TCR-T cells/kg 8 9 As of Nov 20, 2024 data cut
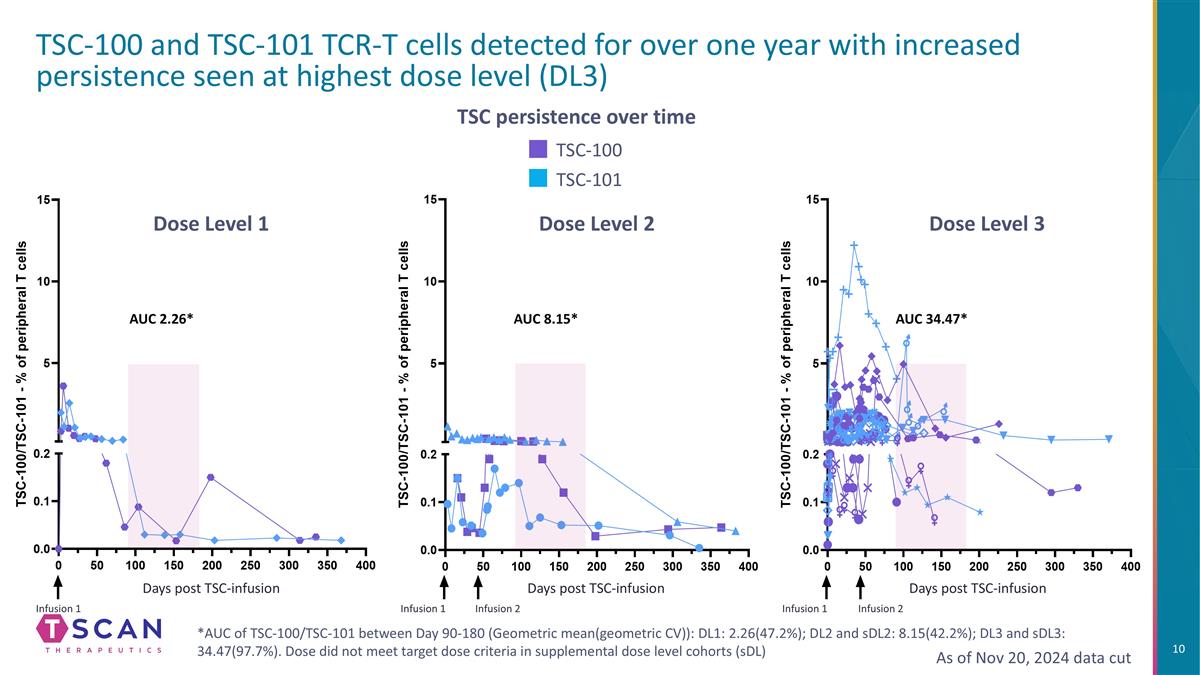
TSC-100 and TSC-101 TCR-T cells detected for over one year with increased persistence seen at highest dose level (DL3) TSC persistence over time TSC-101 TSC-100 *AUC of TSC-100/TSC-101 between Day 90-180 (Geometric mean(geometric CV)): DL1: 2.26(47.2%); DL2 and sDL2: 8.15(42.2%); DL3 and sDL3: 34.47(97.7%). Dose did not meet target dose criteria in supplemental dose level cohorts (sDL) Dose Level 1 Dose Level 2 Dose Level 3 Infusion 1 Infusion 1 Infusion 2 Infusion 1 Infusion 2 Days post TSC-infusion Days post TSC-infusion Days post TSC-infusion AUC 2.26* AUC 8.15* AUC 34.47* As of Nov 20, 2024 data cut
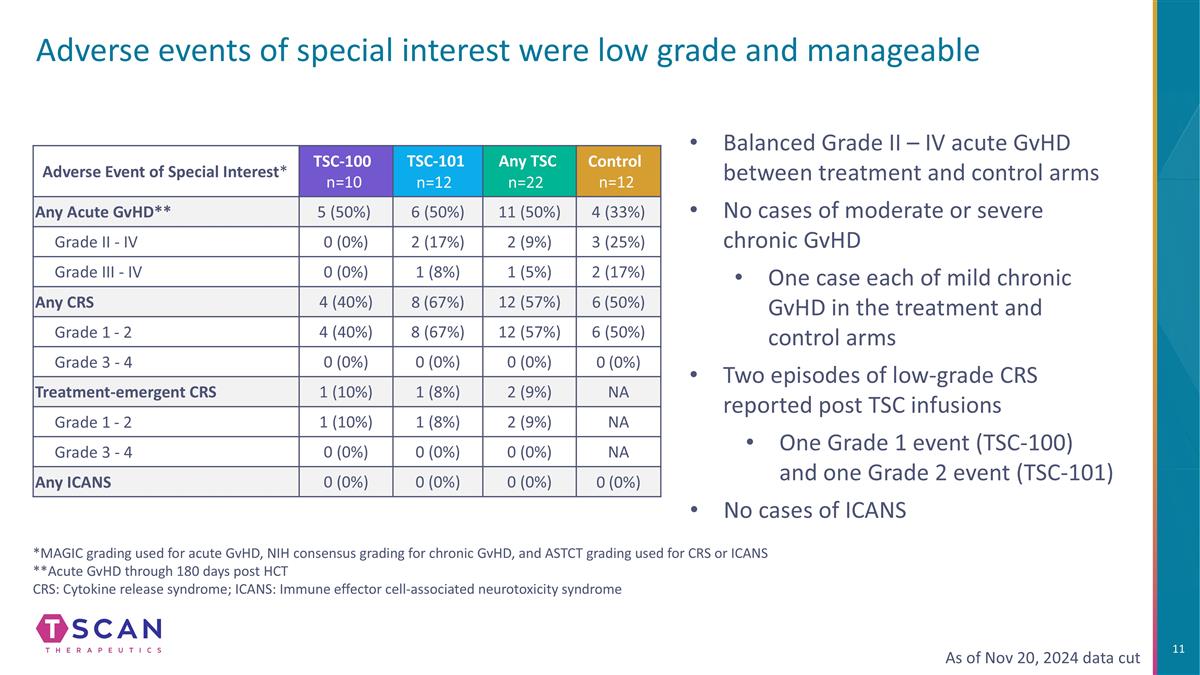
Adverse events of special interest were low grade and manageable Adverse Event of Special Interest* TSC-100 n=10 TSC-101 n=12 Any TSC n=22 Control n=12 Any Acute GvHD** 5 (50%) 6 (50%) 11 (50%) 4 (33%) Grade II - IV 0 (0%) 2 (17%) 2 (9%) 3 (25%) Grade III - IV 0 (0%) 1 (8%) 1 (5%) 2 (17%) Any CRS 4 (40%) 8 (67%) 12 (57%) 6 (50%) Grade 1 - 2 4 (40%) 8 (67%) 12 (57%) 6 (50%) Grade 3 - 4 0 (0%) 0 (0%) 0 (0%) 0 (0%) Treatment-emergent CRS 1 (10%) 1 (8%) 2 (9%) NA Grade 1 - 2 1 (10%) 1 (8%) 2 (9%) NA Grade 3 - 4 0 (0%) 0 (0%) 0 (0%) NA Any ICANS 0 (0%) 0 (0%) 0 (0%) 0 (0%) As of Nov 20, 2024 data cut Balanced Grade II – IV acute GvHD between treatment and control arms No cases of moderate or severe chronic GvHD One case each of mild chronic GvHD in the treatment and control arms Two episodes of low-grade CRS reported post TSC infusions One Grade 1 event (TSC-100) and one Grade 2 event (TSC-101) No cases of ICANS *MAGIC grading used for acute GvHD, NIH consensus grading for chronic GvHD, and ASTCT grading used for CRS or ICANS **Acute GvHD through 180 days post HCT CRS: Cytokine release syndrome; ICANS: Immune effector cell-associated neurotoxicity syndrome
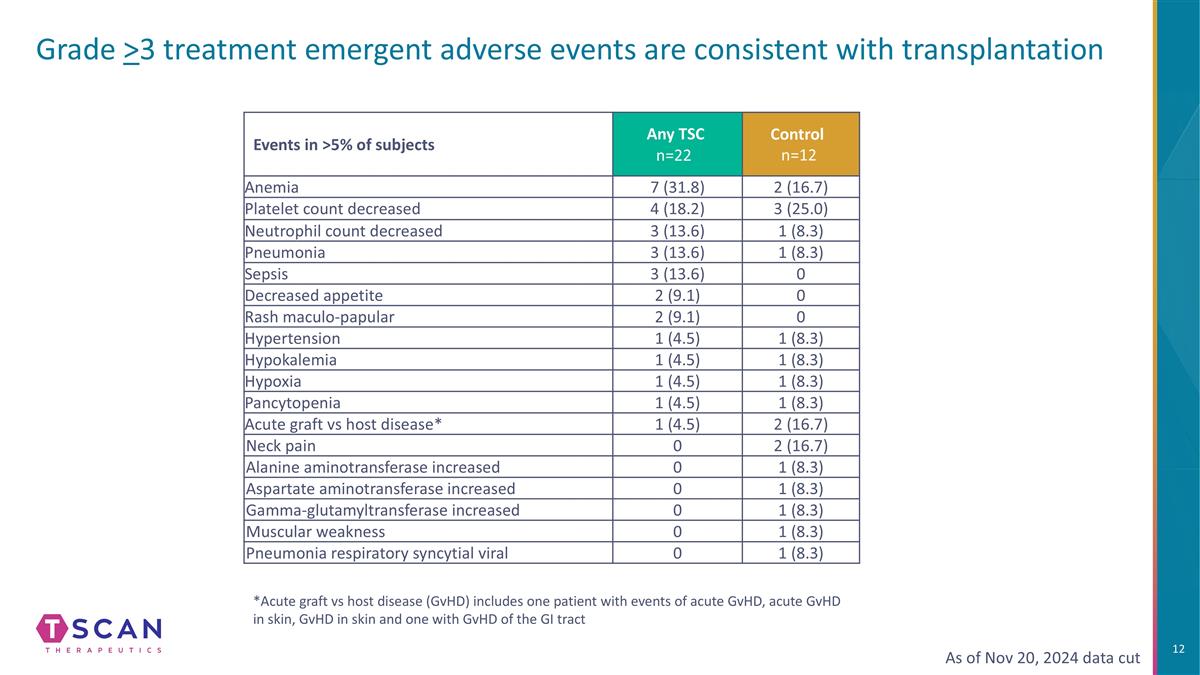
Grade >3 treatment emergent adverse events are consistent with transplantation Events in >5% of subjects Any TSC n=22 Control n=12 Anemia 7 (31.8) 2 (16.7) Platelet count decreased 4 (18.2) 3 (25.0) Neutrophil count decreased 3 (13.6) 1 (8.3) Pneumonia 3 (13.6) 1 (8.3) Sepsis 3 (13.6) 0 Decreased appetite 2 (9.1) 0 Rash maculo-papular 2 (9.1) 0 Hypertension 1 (4.5) 1 (8.3) Hypokalemia 1 (4.5) 1 (8.3) Hypoxia 1 (4.5) 1 (8.3) Pancytopenia 1 (4.5) 1 (8.3) Acute graft vs host disease* 1 (4.5) 2 (16.7) Neck pain 0 2 (16.7) Alanine aminotransferase increased 0 1 (8.3) Aspartate aminotransferase increased 0 1 (8.3) Gamma-glutamyltransferase increased 0 1 (8.3) Muscular weakness 0 1 (8.3) Pneumonia respiratory syncytial viral 0 1 (8.3) As of Nov 20, 2024 data cut *Acute graft vs host disease (GvHD) includes one patient with events of acute GvHD, acute GvHD in skin, GvHD in skin and one with GvHD of the GI tract
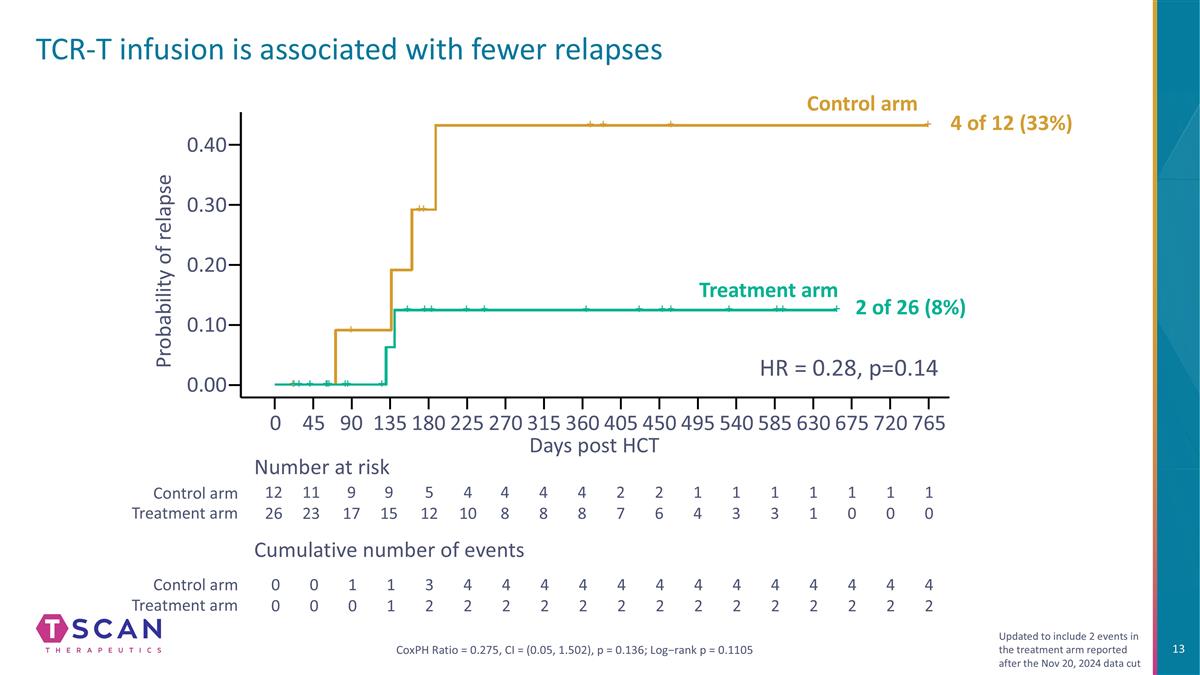
TCR-T infusion is associated with fewer relapses Probability of relapse 0.40 0.20 0.30 0.00 0 90 135 180 270 360 450 540 585 Days post HCT 12 26 11 23 9 17 9 15 5 12 4 10 4 8 1 4 1 3 Number at risk Cumulative number of events 0 0 0 0 1 0 1 1 3 2 4 2 4 2 Control arm Treatment arm Control arm Treatment arm 1 0 1 0 675 765 CoxPH Ratio = 0.275, CI = (0.05, 1.502), p = 0.136; Log−rank p = 0.1105 45 225 315 405 495 630 720 1 0 1 1 1 3 4 8 2 7 4 2 2 6 4 8 4 2 4 2 4 2 4 2 4 2 4 2 4 2 4 2 4 2 4 2 0.10 HR = 0.28, p=0.14 Updated to include 2 events in the treatment arm reported after the Nov 20, 2024 data cut Control arm Treatment arm 4 of 12 (33%) 2 of 26 (8%)
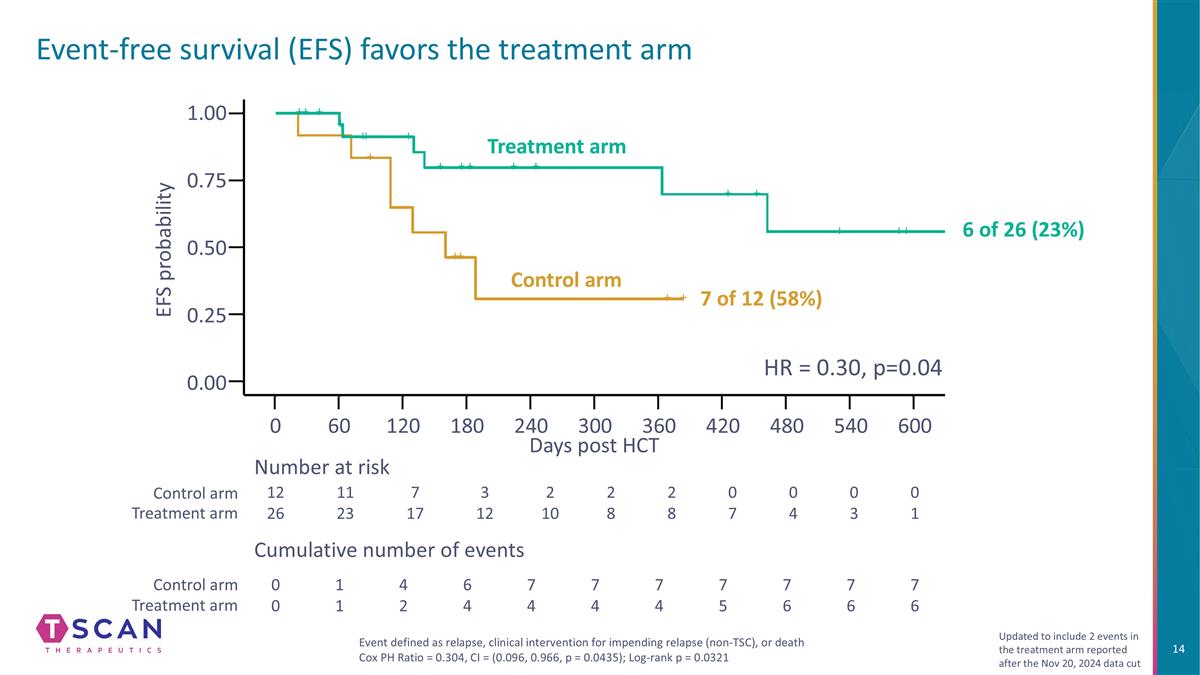
Event-free survival (EFS) favors the treatment arm 1.00 0.75 0.25 0.50 0.00 0 60 120 180 240 300 360 420 480 Days post HCT EFS probability 12 26 11 23 7 17 3 12 2 10 2 8 2 8 0 7 0 4 Number at risk Cumulative number of events 0 0 1 1 4 2 6 4 7 4 7 4 7 4 7 5 7 6 Control arm Treatment arm Control arm Treatment arm 0 3 0 1 7 6 7 6 540 600 Event defined as relapse, clinical intervention for impending relapse (non-TSC), or death Cox PH Ratio = 0.304, CI = (0.096, 0.966, p = 0.0435); Log-rank p = 0.0321 HR = 0.30, p=0.04 Updated to include 2 events in the treatment arm reported after the Nov 20, 2024 data cut Control arm Treatment arm 7 of 12 (58%) 6 of 26 (23%)
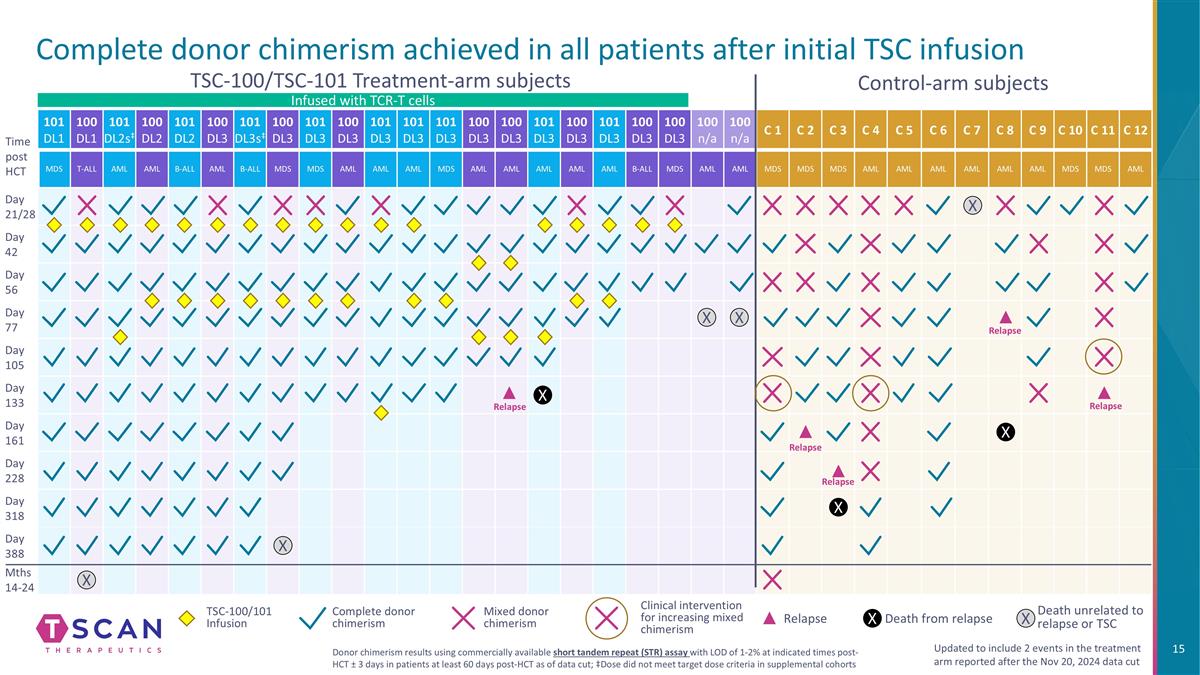
Complete donor chimerism achieved in all patients after initial TSC infusion 101 DL1 100 DL1 101 DL2s‡ 100 DL2 101 DL2 100 DL3 101 DL3s‡ 100 DL3 101 DL3 100 DL3 101 DL3 101 DL3 101 DL3 100 DL3 100 DL3 101 DL3 100 DL3 101 DL3 100 DL3 100 DL3 100 n/a 100 n/a C 1 C 2 C 3 C 4 C 5 C 6 C 7 C 8 C 9 C 10 C 11 C 12 MDS T-ALL AML AML B-ALL AML B-ALL MDS MDS AML AML AML MDS AML AML AML AML AML B-ALL MDS AML AML MDS MDS MDS AML AML AML AML AML AML MDS MDS AML Day 21/28 Day 42 Day 56 Day 77 Day 105 Day 133 Day 161 Day 228 Day 318 Day 388 Mths 14-24 Control-arm subjects TSC-100/TSC-101 Treatment-arm subjects Time post HCT X X X X Relapse Relapse Relapse Relapse X X X Mixed donor chimerism Complete donor chimerism Clinical intervention for increasing mixed chimerism Updated to include 2 events in the treatment arm reported after the Nov 20, 2024 data cut Donor chimerism results using commercially available short tandem repeat (STR) assay with LOD of 1-2% at indicated times post-HCT ± 3 days in patients at least 60 days post-HCT as of data cut; ‡Dose did not meet target dose criteria in supplemental cohorts Death from relapse Death unrelated to relapse or TSC X Relapse TSC-100/101 Infusion X Infused with TCR-T cells X Relapse
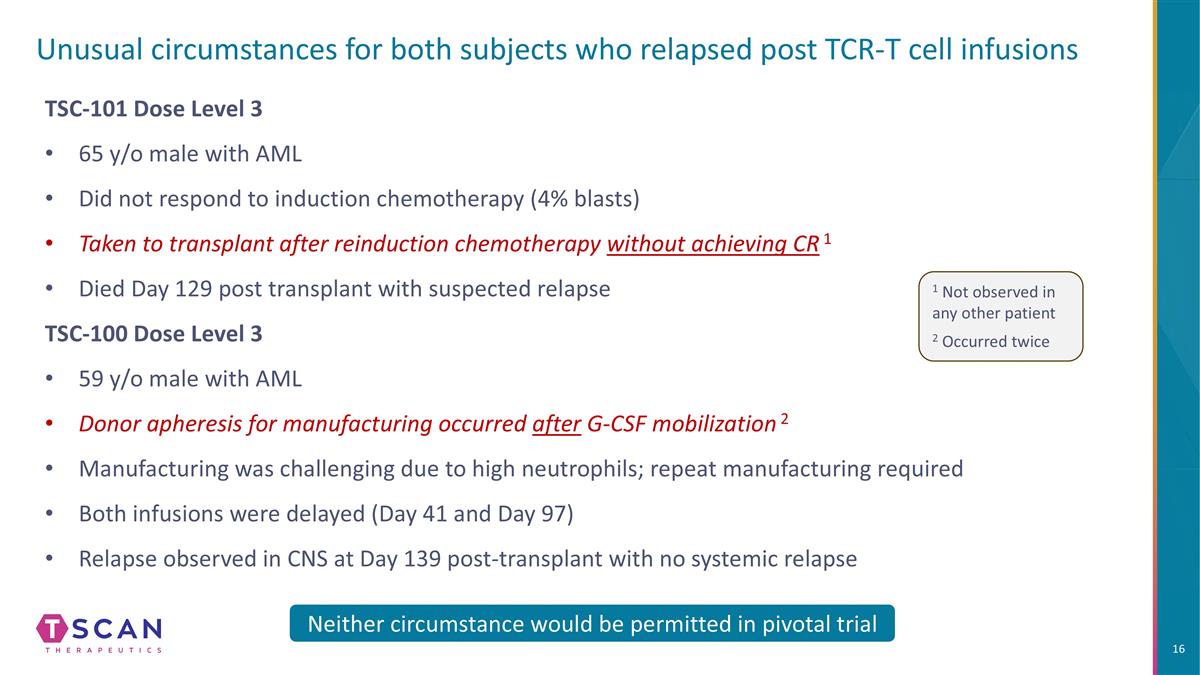
Unusual circumstances for both subjects who relapsed post TCR-T cell infusions TSC-101 Dose Level 3 65 y/o male with AML Did not respond to induction chemotherapy (4% blasts) Taken to transplant after reinduction chemotherapy without achieving CR 1 Died Day 129 post transplant with suspected relapse TSC-100 Dose Level 3 59 y/o male with AML Donor apheresis for manufacturing occurred after G-CSF mobilization 2 Manufacturing was challenging due to high neutrophils; repeat manufacturing required Both infusions were delayed (Day 41 and Day 97) Relapse observed in CNS at Day 139 post-transplant with no systemic relapse Neither circumstance would be permitted in pivotal trial 1 Not observed in any other patient 2 Occurred twice
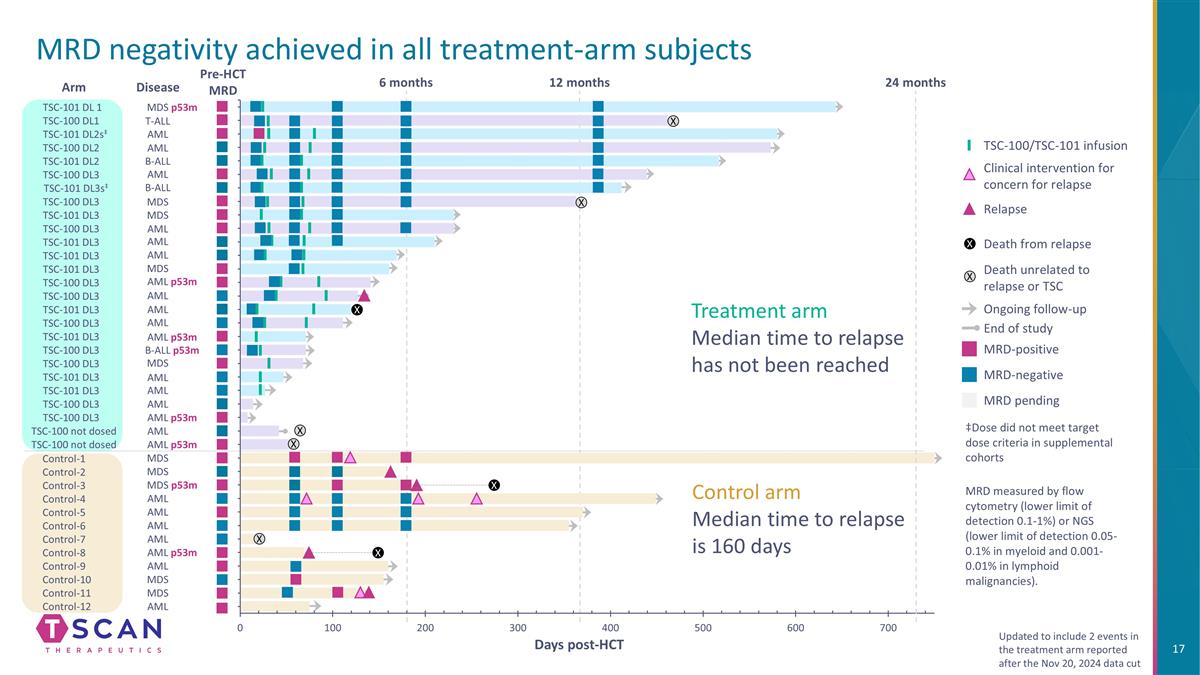
Pre-HCT MRD Control-1 Control-2 Control-3 Control-7 Control-8 Control-5 Control-4 Control-6 Control-9 Control-10 Control-11 Control-12 Arm ‡Dose did not meet target dose criteria in supplemental cohorts MRD-positive MRD-negative MRD pending TSC-100/TSC-101 infusion Clinical intervention for concern for relapse Ongoing follow-up Death from relapse Death unrelated to relapse or TSC Relapse End of study X X TSC-101 DL 1 TSC-100 DL1 TSC-101 DL2s‡ TSC-100 DL2 TSC-101 DL2 TSC-100 DL3 TSC-101 DL3s‡ TSC-100 DL3 TSC-101 DL3 TSC-100 DL3 TSC-101 DL3 TSC-101 DL3 TSC-101 DL3 TSC-100 DL3 TSC-100 DL3 TSC-101 DL3 TSC-100 DL3 TSC-101 DL3 TSC-100 DL3 TSC-101 DL3 TSC-100 DL3 TSC-101 DL3 TSC-100 DL3 TSC-100 DL3 TSC-100 not dosed TSC-100 not dosed X X X Days post-HCT 24 months X X X 12 months 6 months Control arm Median time to relapse is 160 days Treatment arm Median time to relapse has not been reached MRD measured by flow cytometry (lower limit of detection 0.1-1%) or NGS (lower limit of detection 0.05-0.1% in myeloid and 0.001-0.01% in lymphoid malignancies). MRD negativity achieved in all treatment-arm subjects X Updated to include 2 events in the treatment arm reported after the Nov 20, 2024 data cut X MDS MDS MDS p53m AML AML AML AML AML AML p53m MDS MDS AML MDS T-ALL AML AML AML AML p53m AML AML AML B-ALL p53m MDS AML AML p53m B-ALL B-ALL MDS MDS AML AML AML AML p53m AML AML AML MDS p53m Disease AML p53m
Summary Infusions with TSC-100 and TSC-101 were well-tolerated with no DLTs and adverse events consistent with HCT TSC-100 and TSC-101 TCR-T cells have been detected >1 year post infusion and have a clear dose-persistence relationship 2 of 26 (8%) treatment-arm subjects relapsed as compared to 4 of 12 (33%) control-arm subjects Median time to relapse was not evaluable in TCR-T-treated subjects vs 160 days in the control arm Event-free survival strongly favors the treatment arm (HR=0.30) These data support the continued evaluation of TSC-100 and TSC-101 as adjuvant TCR-T cells to treat residual disease and prevent relapse in subjects with AML, ALL, or MDS post RIC-HCT

Pivotal trial design and heme development strategy
Highly collaborative RMAT meeting with FDA provided clear feedback on a path to registration Proposed patient population is acceptable: AML, MDS, and ALL undergoing allo-HCT with haploidentical or MMUD donors Analytical comparability is sufficient to support a commercial-ready process Proposed potency assays are sufficient to support a pivotal study CMC Clinical MUD: Matched unrelated donor; MMUD: Mismatched unrelated donor CIBMTR: Center for International Blood and Marrow Transplant Research Relapse-free survival (RFS) is an appropriate primary end-point to support full approval Use of an external control arm using data from CIBMTR is acceptable to support full approval
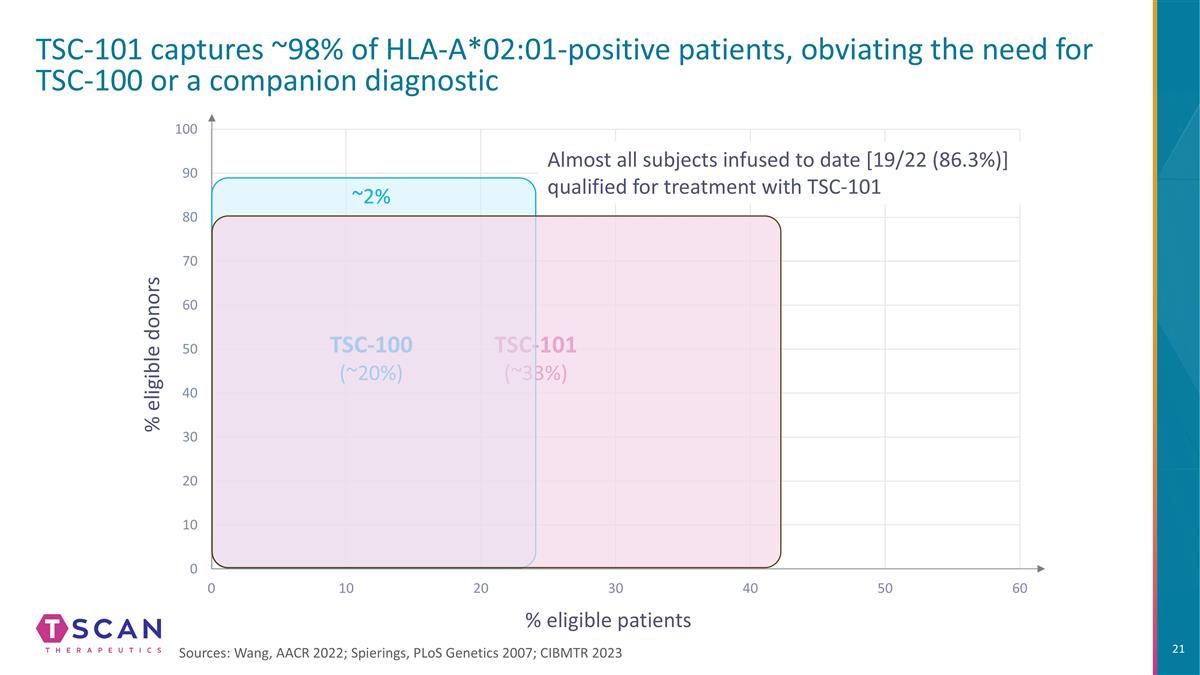
% eligible donors % eligible patients TSC-101 captures ~98% of HLA-A*02:01-positive patients, obviating the need for TSC-100 or a companion diagnostic TSC-101 (~33%) Almost all subjects infused to date [19/22 (86.3%)] qualified for treatment with TSC-101 Sources: Wang, AACR 2022; Spierings, PLoS Genetics 2007; CIBMTR 2023 TSC-100 (~20%) ~2%
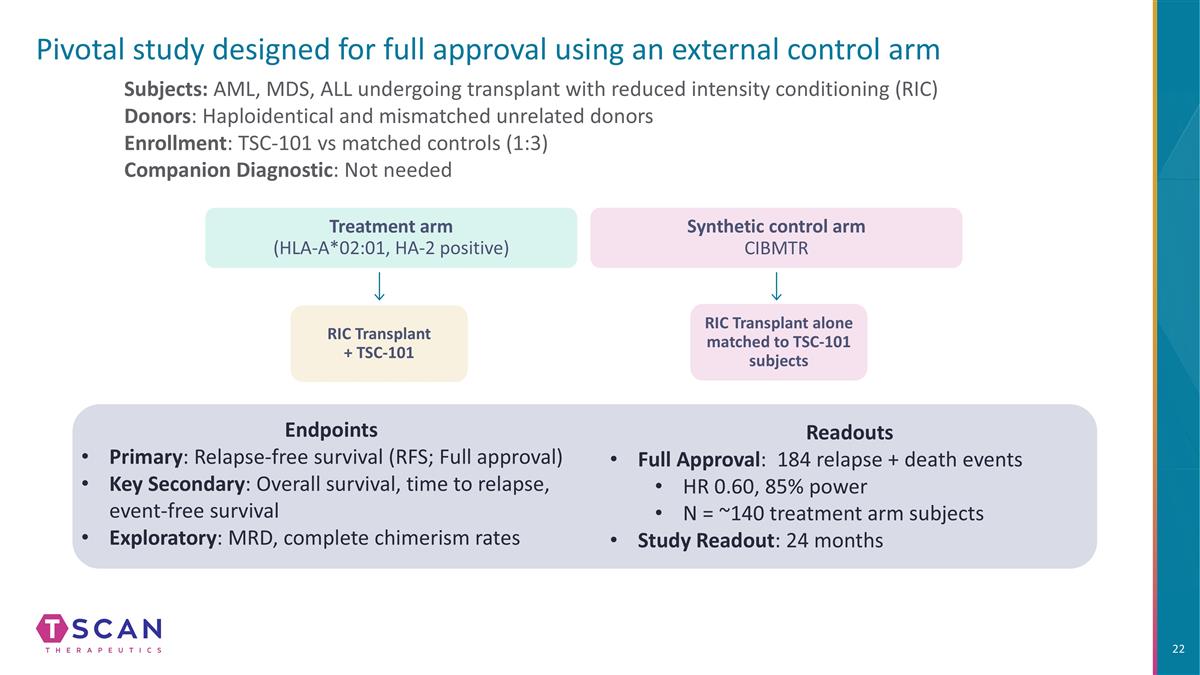
Pivotal study designed for full approval using an external control arm Subjects: AML, MDS, ALL undergoing transplant with reduced intensity conditioning (RIC) Donors: Haploidentical and mismatched unrelated donors Enrollment: TSC-101 vs matched controls (1:3) Companion Diagnostic: Not needed Endpoints Primary: Relapse-free survival (RFS; Full approval) Key Secondary: Overall survival, time to relapse, event-free survival Exploratory: MRD, complete chimerism rates Treatment arm (HLA-A*02:01, HA-2 positive) RIC Transplant + TSC-101 Synthetic control arm CIBMTR RIC Transplant alone matched to TSC-101 subjects Readouts Full Approval: 184 relapse + death events HR 0.60, 85% power N = ~140 treatment arm subjects Study Readout: 24 months
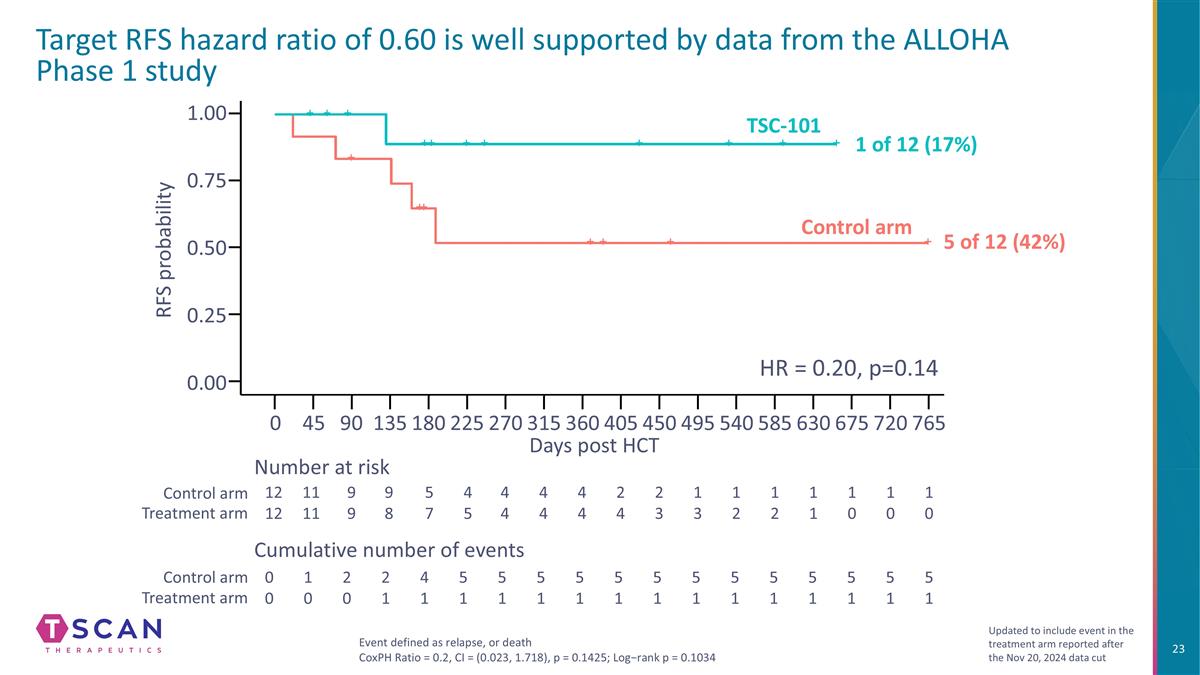
Target RFS hazard ratio of 0.60 is well supported by data from the ALLOHA Phase 1 study 1.00 0.75 0.25 0.50 0.00 Days post HCT RFS probability Number at risk Cumulative number of events Control arm Treatment arm Control arm Treatment arm Event defined as relapse, or death CoxPH Ratio = 0.2, CI = (0.023, 1.718), p = 0.1425; Log−rank p = 0.1034 Updated to include event in the treatment arm reported after the Nov 20, 2024 data cut 0 90 135 180 270 360 450 540 585 675 765 45 225 315 405 495 630 720 12 12 11 11 9 9 9 8 5 7 4 5 4 4 1 3 1 2 0 0 1 0 2 0 2 1 4 1 5 1 1 0 1 0 1 0 1 1 1 2 4 4 2 4 2 3 4 4 5 1 5 1 5 1 5 1 5 1 5 1 5 1 5 1 5 1 5 1 5 1 5 1 HR = 0.20, p=0.14 Control arm TSC-101 1 of 12 (17%) 5 of 12 (42%)

Market opportunity
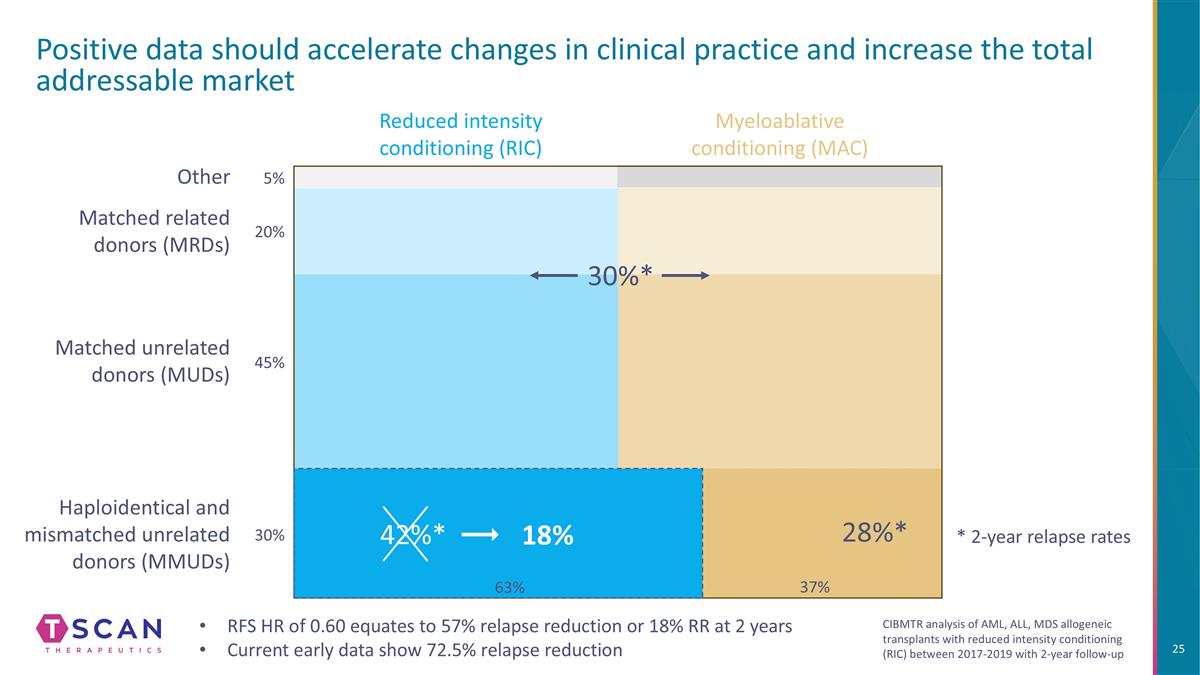
Haploidentical and mismatched unrelated donors (MMUDs) Reduced intensity conditioning (RIC) Positive data should accelerate changes in clinical practice and increase the total addressable market Myeloablative conditioning (MAC) Matched unrelated donors (MUDs) Matched related donors (MRDs) 28%* Other 5% 20% 45% 30% 37% 30%* RFS HR of 0.60 equates to 57% relapse reduction or 18% RR at 2 years Current early data show 72.5% relapse reduction 42%* 18% 63% * 2-year relapse rates CIBMTR analysis of AML, ALL, MDS allogeneic transplants with reduced intensity conditioning (RIC) between 2017-2019 with 2-year follow-up
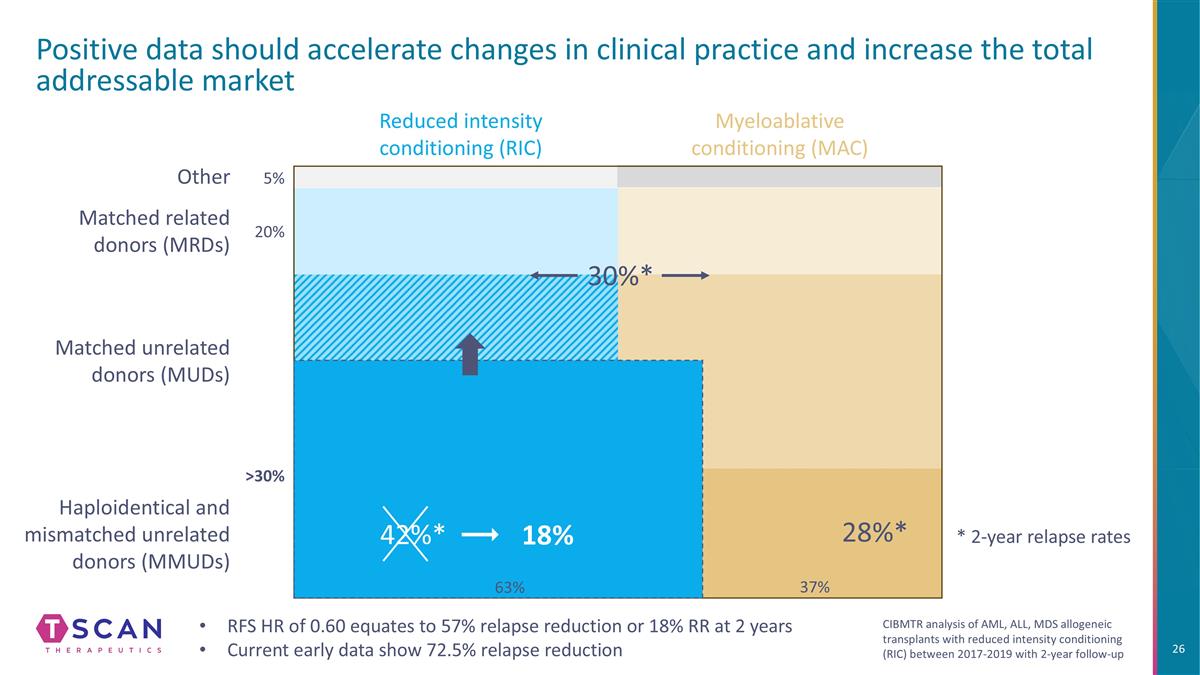
Haploidentical and mismatched unrelated donors (MMUDs) Reduced intensity conditioning (RIC) Positive data should accelerate changes in clinical practice and increase the total addressable market Myeloablative conditioning (MAC) Matched unrelated donors (MUDs) Matched related donors (MRDs) 28%* Other 5% 20% >30% 37% 30%* 42%* 18% 63% RFS HR of 0.60 equates to 57% relapse reduction or 18% RR at 2 years Current early data show 72.5% relapse reduction * 2-year relapse rates CIBMTR analysis of AML, ALL, MDS allogeneic transplants with reduced intensity conditioning (RIC) between 2017-2019 with 2-year follow-up
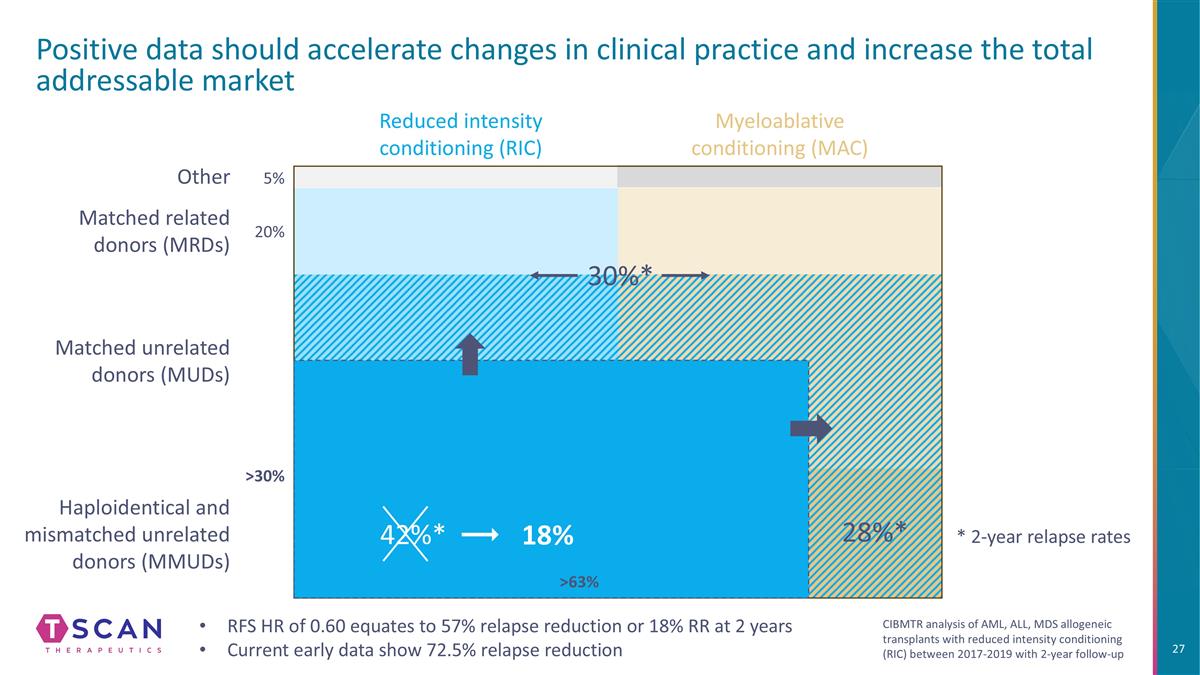
Haploidentical and mismatched unrelated donors (MMUDs) Reduced intensity conditioning (RIC) Positive data should accelerate changes in clinical practice and increase the total addressable market Myeloablative conditioning (MAC) Matched unrelated donors (MUDs) Matched related donors (MRDs) 28%* Other 5% 20% 30%* 42%* 18% >30% >63% RFS HR of 0.60 equates to 57% relapse reduction or 18% RR at 2 years Current early data show 72.5% relapse reduction * 2-year relapse rates CIBMTR analysis of AML, ALL, MDS allogeneic transplants with reduced intensity conditioning (RIC) between 2017-2019 with 2-year follow-up
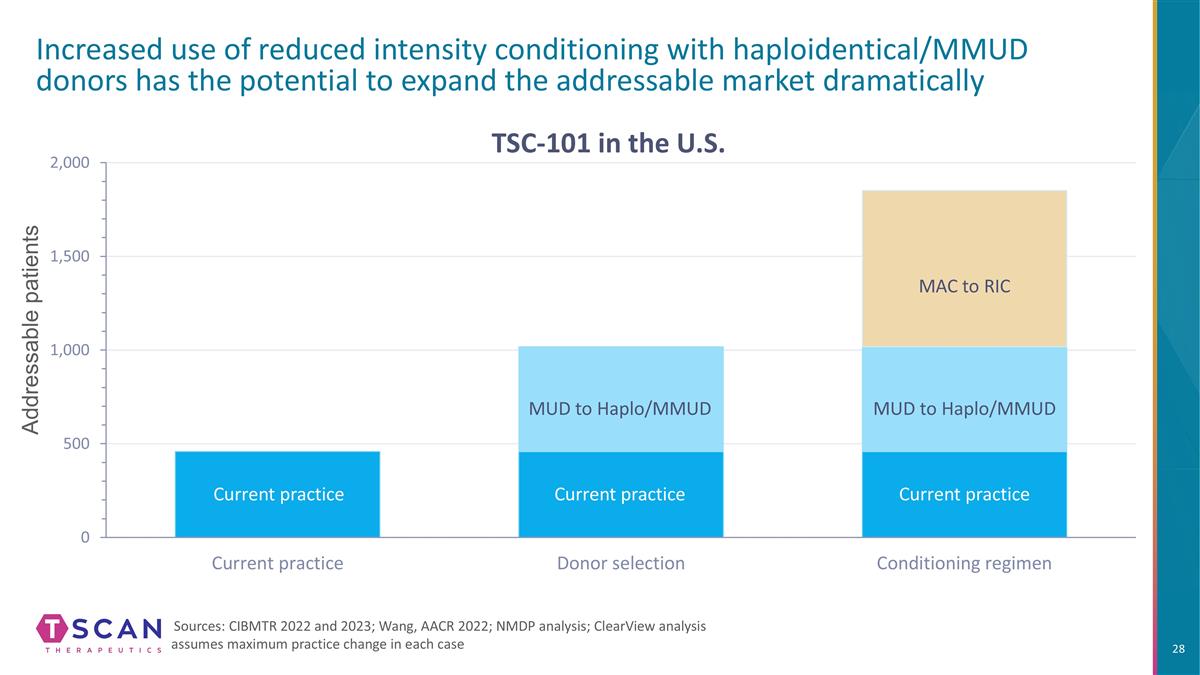
Addressable patients Increased use of reduced intensity conditioning with haploidentical/MMUD donors has the potential to expand the addressable market dramatically Sources: CIBMTR 2022 and 2023; Wang, AACR 2022; NMDP analysis; ClearView analysis assumes maximum practice change in each case MUD to Haplo/MMUD MUD to Haplo/MMUD MAC to RIC Current practice Current practice Current practice TSC-101 in the U.S.
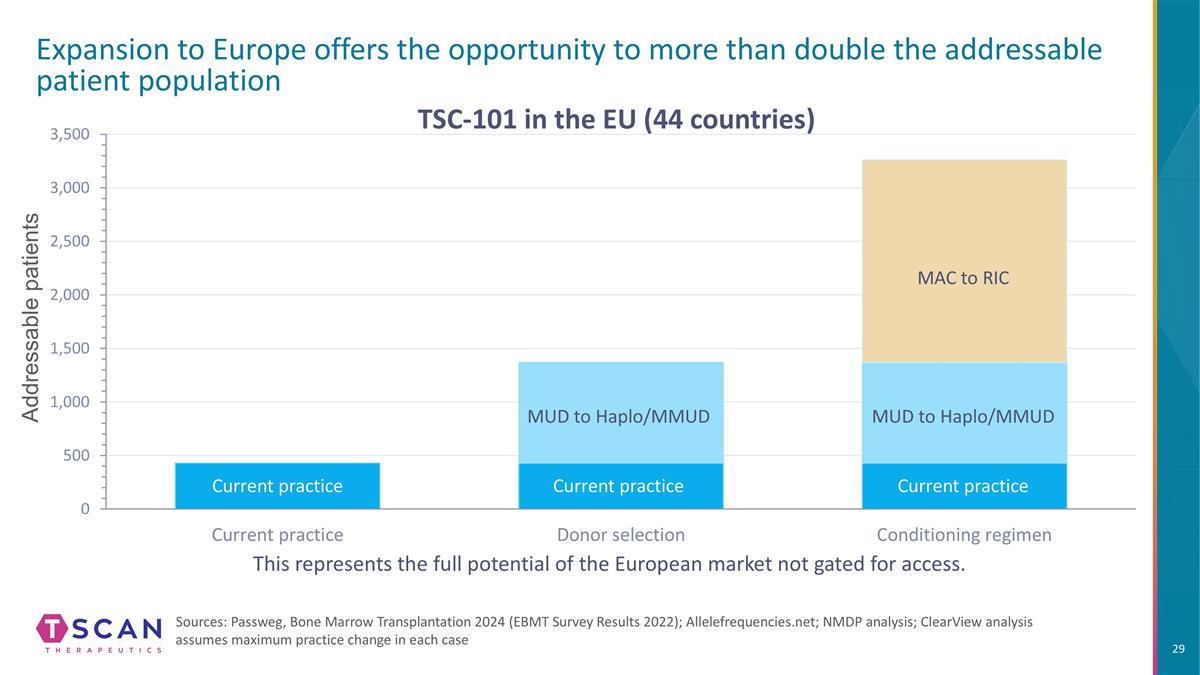
Addressable patients Expansion to Europe offers the opportunity to more than double the addressable patient population Sources: Passweg, Bone Marrow Transplantation 2024 (EBMT Survey Results 2022); Allelefrequencies.net; NMDP analysis; ClearView analysis assumes maximum practice change in each case This represents the full potential of the European market not gated for access. TSC-101 in the EU (44 countries) MUD to Haplo/MMUD MUD to Haplo/MMUD MAC to RIC Current practice Current practice Current practice
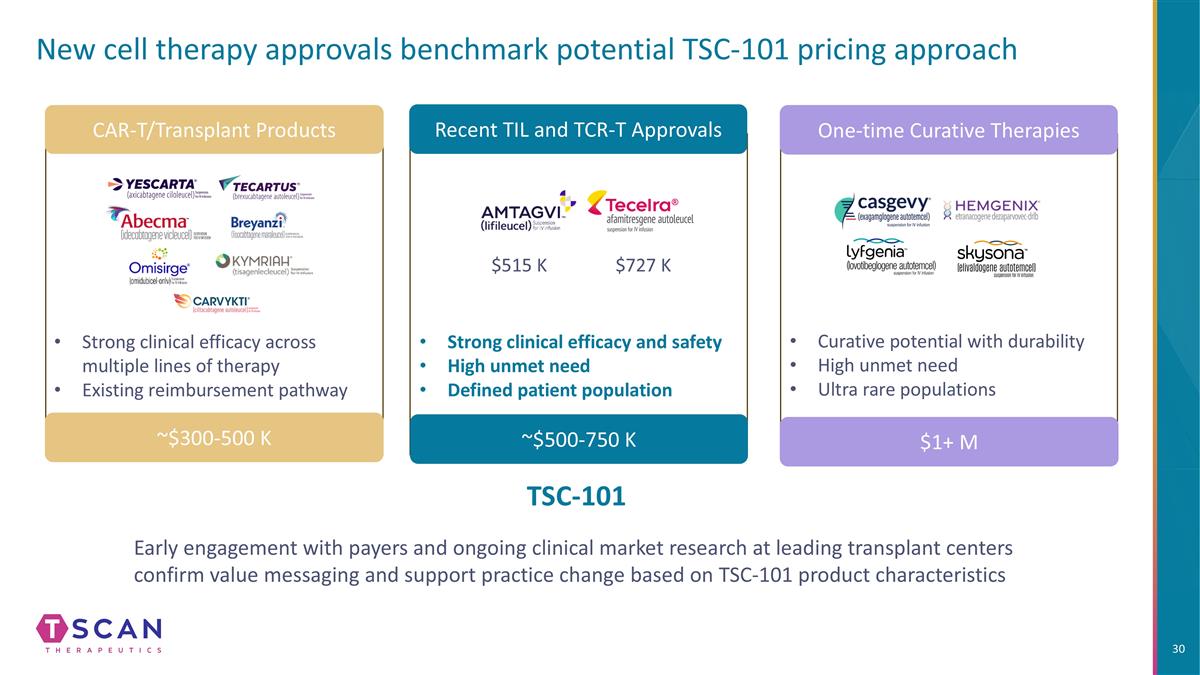
New cell therapy approvals benchmark potential TSC-101 pricing approach CAR-T/Transplant Products Recent TIL and TCR-T Approvals One-time Curative Therapies Strong clinical efficacy across multiple lines of therapy Existing reimbursement pathway ~$300-500 K ~$500-750 K $1+ M Strong clinical efficacy and safety High unmet need Defined patient population $515 K $727 K Curative potential with durability High unmet need Ultra rare populations Early engagement with payers and ongoing clinical market research at leading transplant centers confirm value messaging and support practice change based on TSC-101 product characteristics TSC-101

Expansion opportunities
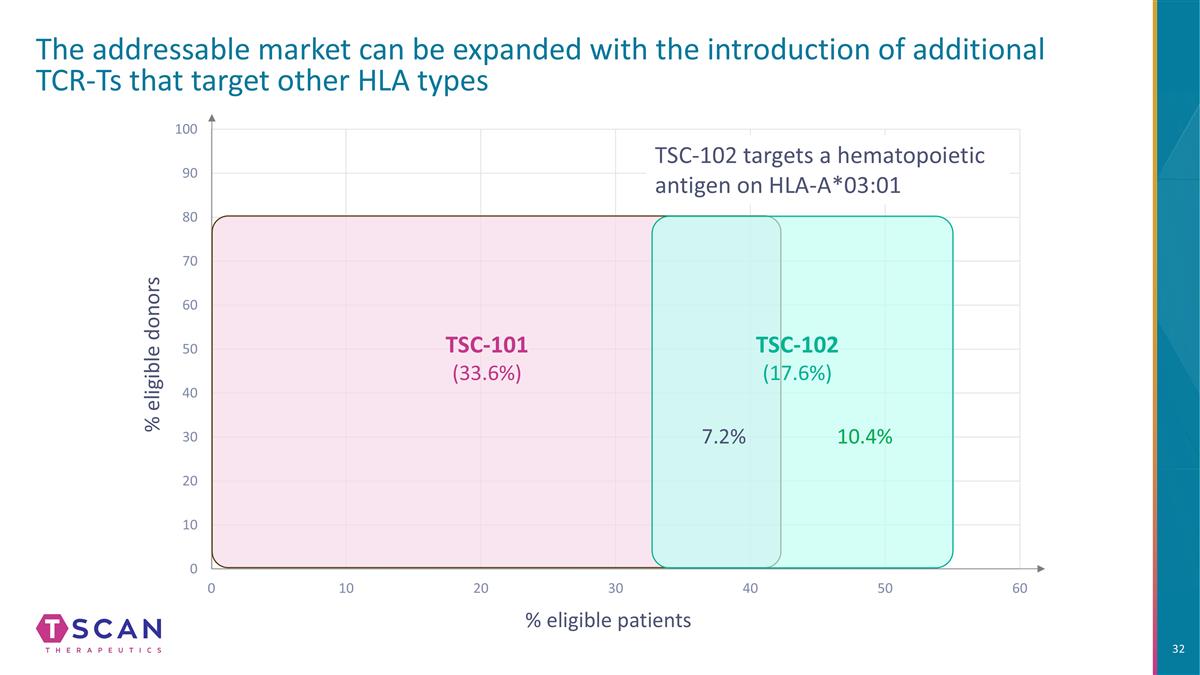
% eligible donors % eligible patients The addressable market can be expanded with the introduction of additional TCR-Ts that target other HLA types TSC-101 (33.6%) TSC-102 targets a hematopoietic antigen on HLA-A*03:01 10.4% 7.2% TSC-102 (17.6%)
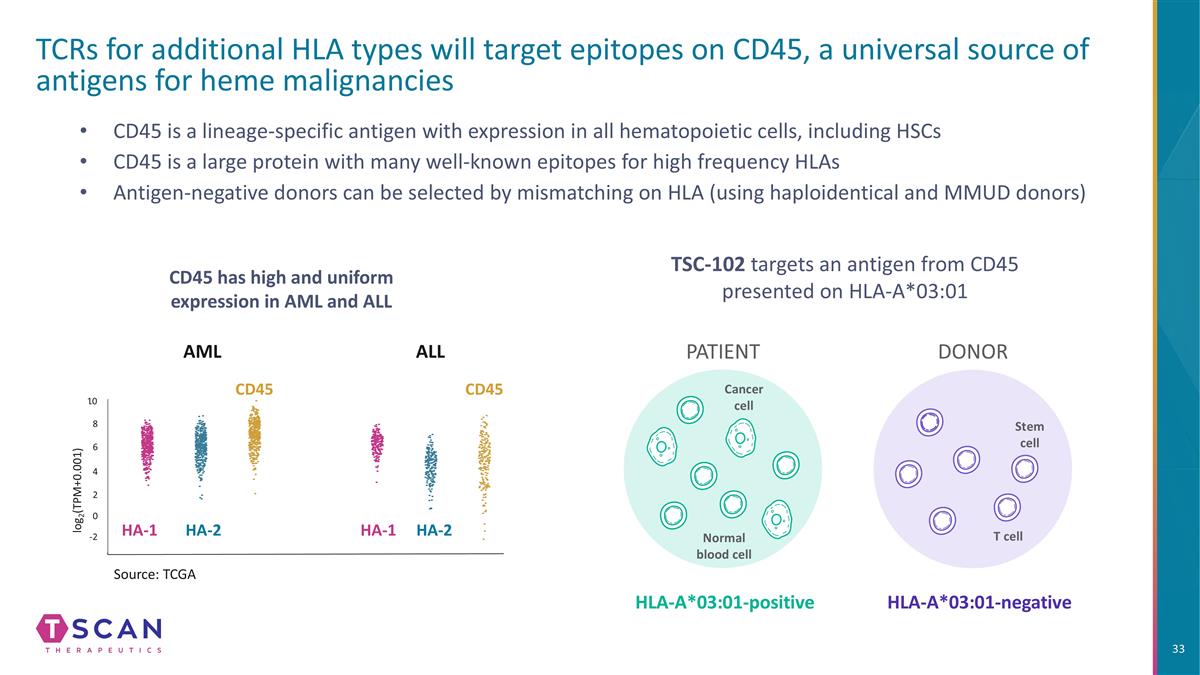
AML ALL -2 0 2 4 6 8 10 log2(TPM+0.001) Source: TCGA CD45 is a lineage-specific antigen with expression in all hematopoietic cells, including HSCs CD45 is a large protein with many well-known epitopes for high frequency HLAs Antigen-negative donors can be selected by mismatching on HLA (using haploidentical and MMUD donors) TCRs for additional HLA types will target epitopes on CD45, a universal source of antigens for heme malignancies CD45 has high and uniform expression in AML and ALL HA-1 HA-1 HA-2 HA-2 CD45 PATIENT DONOR HLA-A*03:01-positive HLA-A*03:01-negative Stem cell T cell Cancer cell Normal blood cell CD45 TSC-102 targets an antigen from CD45 presented on HLA-A*03:01
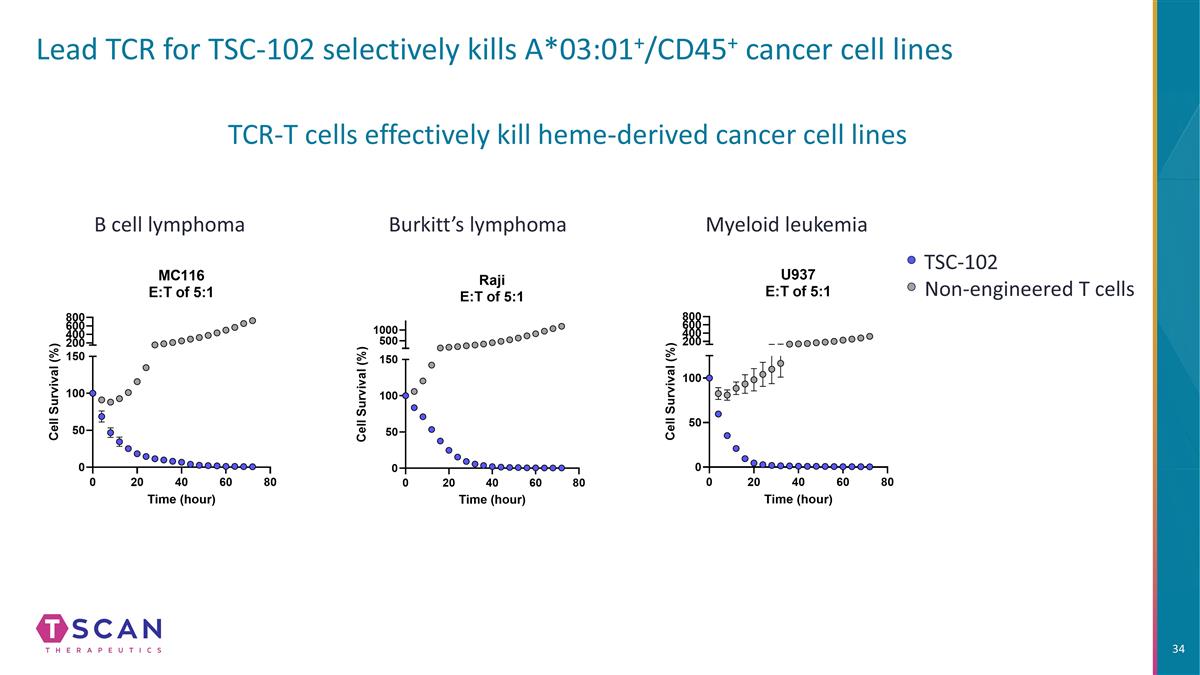
Lead TCR for TSC-102 selectively kills A*03:01+/CD45+ cancer cell lines Burkitt’s lymphoma Myeloid leukemia B cell lymphoma TCR-T cells effectively kill heme-derived cancer cell lines TSC-102 Non-engineered T cells
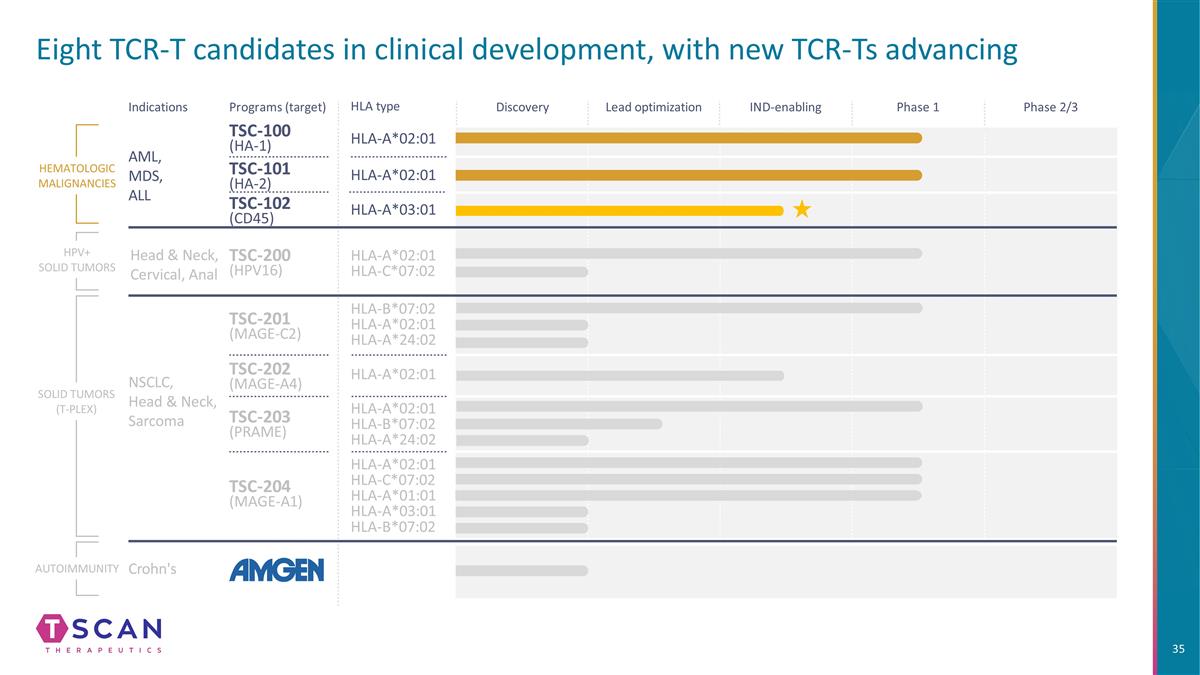
Eight TCR-T candidates in clinical development, with new TCR-Ts advancing HLA type Indications Programs (target) TSC-100 (HA-1) HLA-A*02:01 HLA-A*02:01 HLA-A*02:01 HLA-C*07:02 HLA-B*07:02 HLA-A*02:01 HLA-A*24:02 HLA-A*02:01 HLA-C*07:02 HLA-A*01:01 HLA-A*03:01 HLA-B*07:02 HLA-A*02:01 HLA-B*07:02 HLA-A*24:02 TSC-101 (HA-2) TSC-200 (HPV16) TSC-201 (MAGE-C2) TSC-202 (MAGE-A4) TSC-203 (PRAME) TSC-204 (MAGE-A1) HEMATOLOGIC MALIGNANCIES SOLID TUMORS (T-PLEX) AML, MDS, ALL NSCLC, Head & Neck, Sarcoma HLA-A*02:01 Autoimmunity Crohn's Discovery Lead optimization IND-enabling Phase 1 Phase 2/3 HPV+ SOLID TUMORS Head & Neck, Cervical, Anal HLA-A*03:01 TSC-102 (CD45)
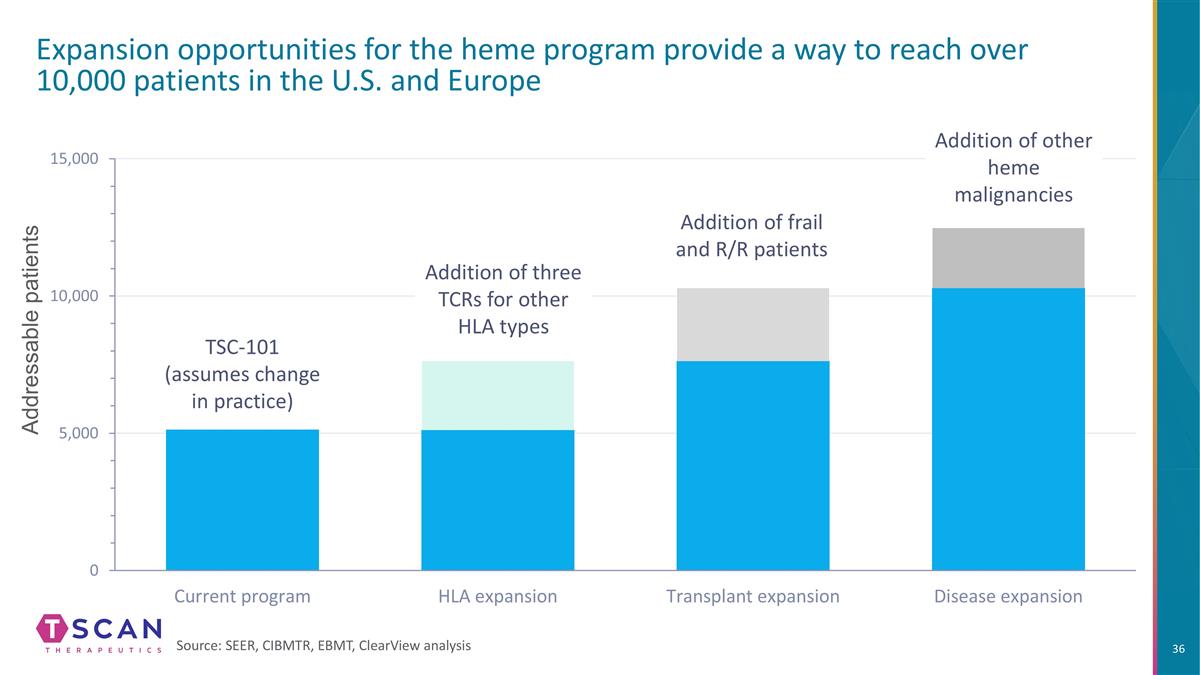
Addressable patients Expansion opportunities for the heme program provide a way to reach over 10,000 patients in the U.S. and Europe Source: SEER, CIBMTR, EBMT, ClearView analysis TSC-101 (assumes change in practice) Addition of three TCRs for other HLA types Addition of frail and R/R patients Addition of other heme malignancies
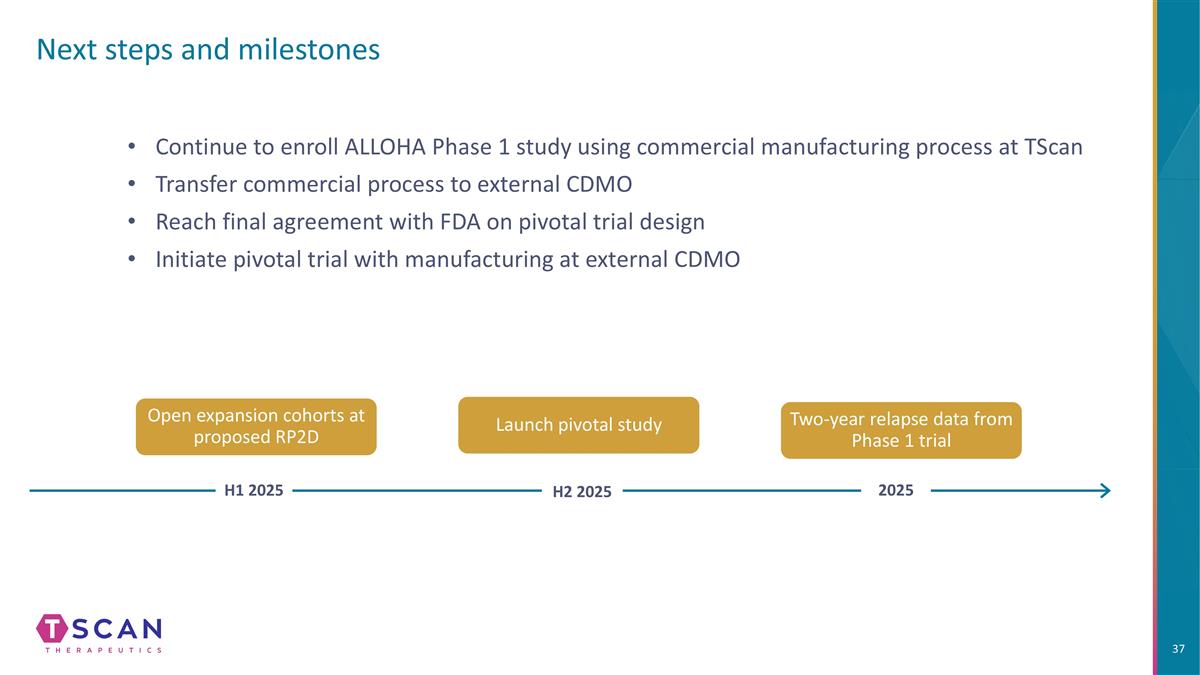
Next steps and milestones Launch pivotal study Two-year relapse data from Phase 1 trial Open expansion cohorts at proposed RP2D H1 2025 H2 2025 2025 Continue to enroll ALLOHA Phase 1 study using commercial manufacturing process at TScan Transfer commercial process to external CDMO Reach final agreement with FDA on pivotal trial design Initiate pivotal trial with manufacturing at external CDMO

Q&A

Solid tumor program update
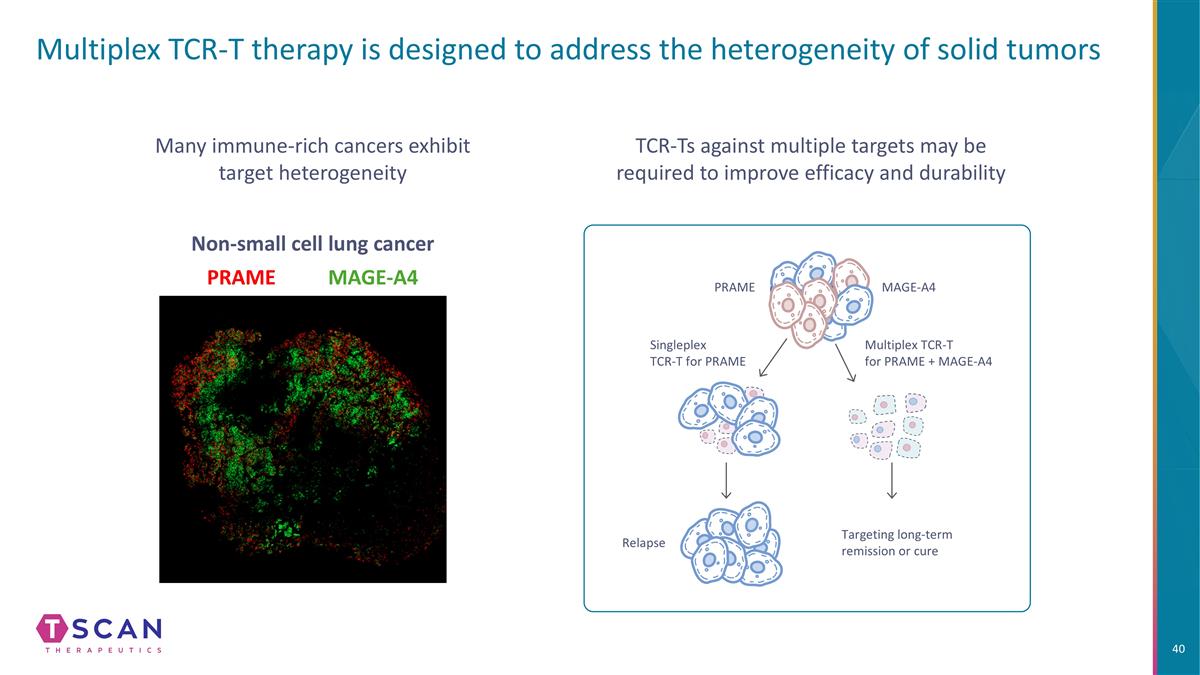
Multiplex TCR-T therapy is designed to address the heterogeneity of solid tumors Many immune-rich cancers exhibit target heterogeneity MAGE-A4 PRAME Non-small cell lung cancer TCR-Ts against multiple targets may be required to improve efficacy and durability PRAME MAGE-A4 Relapse Targeting long-term remission or cure Singleplex TCR-T for PRAME Multiplex TCR-T for PRAME + MAGE-A4
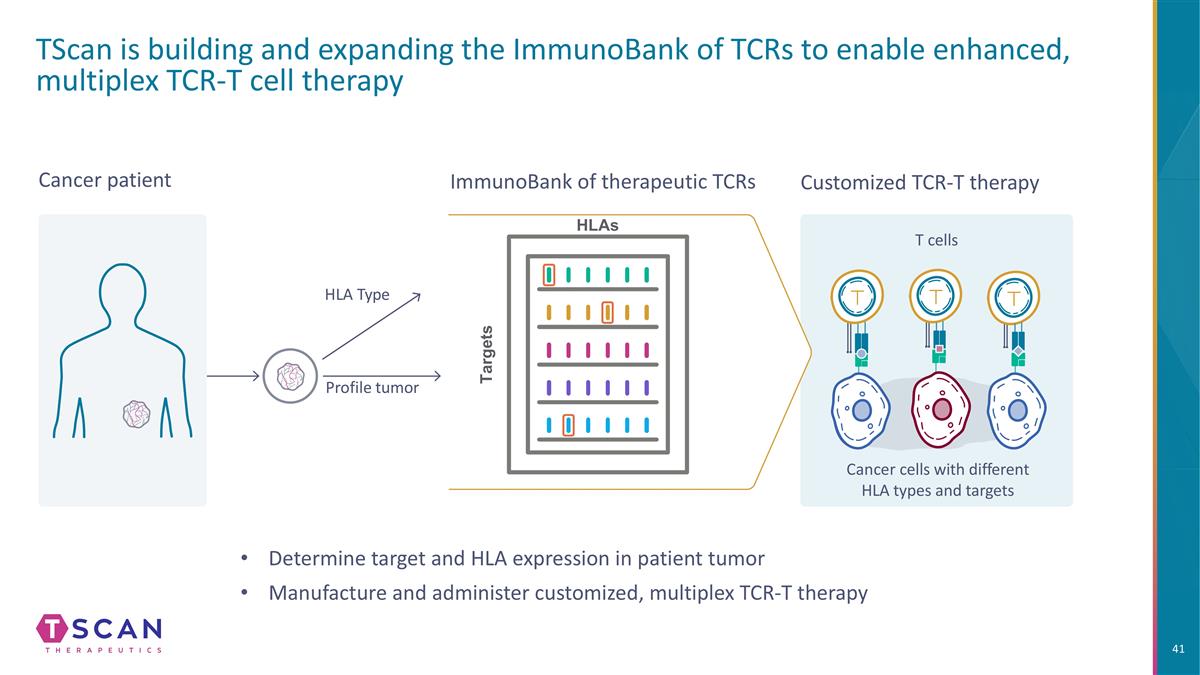
TScan is building and expanding the ImmunoBank of TCRs to enable enhanced, multiplex TCR-T cell therapy Customized TCR-T therapy Cancer cells with different HLA types and targets T cells Cancer patient Profile tumor HLA Type ImmunoBank of therapeutic TCRs HLAs Targets Determine target and HLA expression in patient tumor Manufacture and administer customized, multiplex TCR-T therapy
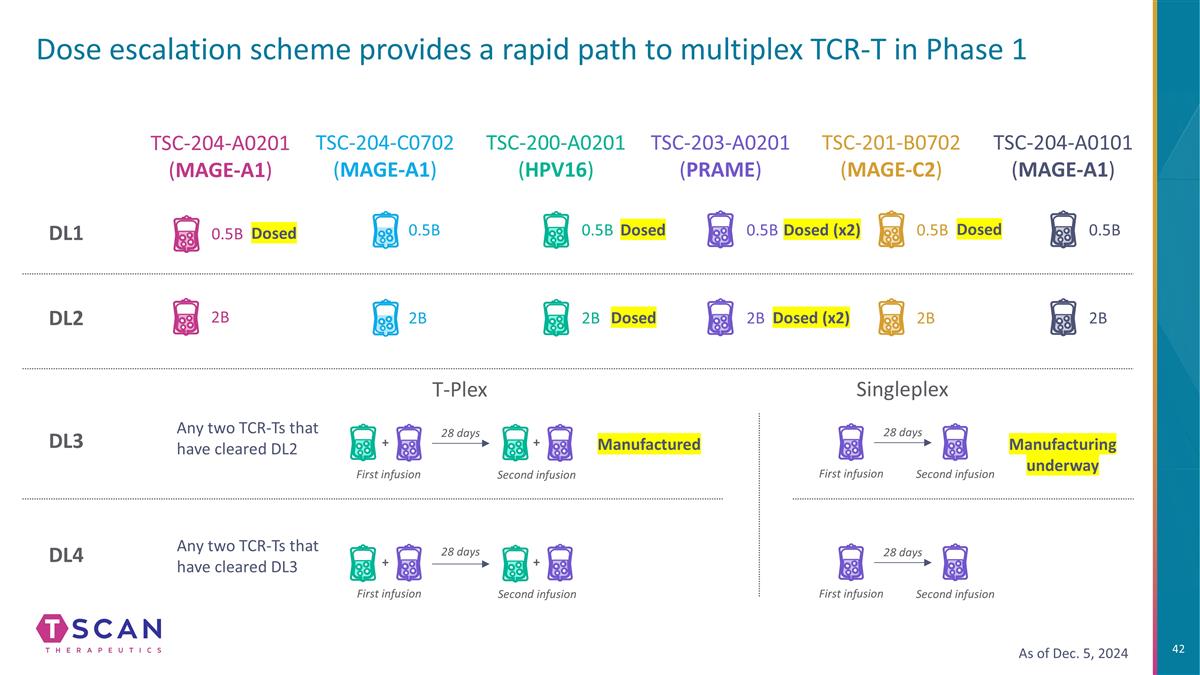
Dose escalation scheme provides a rapid path to multiplex TCR-T in Phase 1 TSC-204-A0201 (MAGE-A1) TSC-204-C0702 (MAGE-A1) DL3 DL4 DL1 DL2 TSC-200-A0201 (HPV16) TSC-203-A0201 (PRAME) TSC-201-B0702 (MAGE-C2) TSC-204-A0101 (MAGE-A1) 2B 0.5B 2B 0.5B 2B 0.5B 2B 0.5B 2B 0.5B 2B T-Plex 28 days Any two TCR-Ts that have cleared DL2 Any two TCR-Ts that have cleared DL3 + + + 28 days First infusion Second infusion First infusion + Second infusion Dosed Dosed (x2) As of Dec. 5, 2024 0.5B Dosed Dosed (x2) Dosed Dosed Manufactured Singleplex 28 days First infusion Second infusion Manufacturing underway 28 days First infusion Second infusion
Enroll study efficiently Progressing multiple TCRs through early dose levels sets us up to investigate multiplexed therapy in 2025 Manufacture successfully Manufacture successfully Progress through dose escalation to T-Plex Early signs of anti-tumor activity

Strategy for 2025
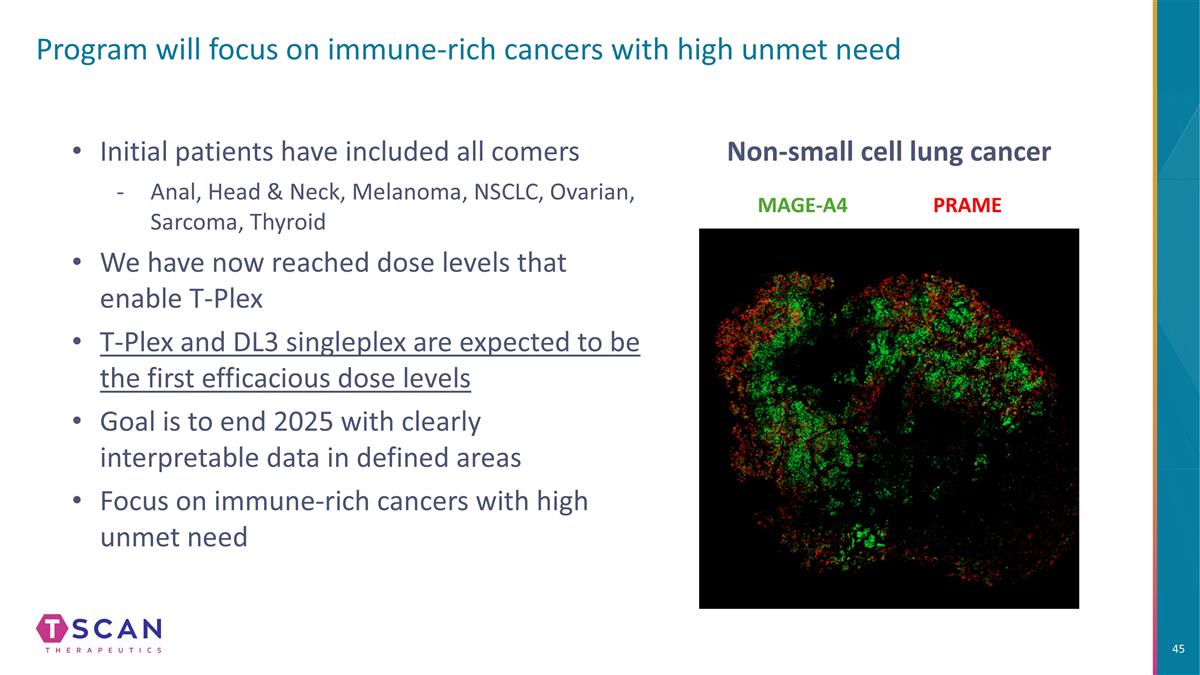
Program will focus on immune-rich cancers with high unmet need Initial patients have included all comers Anal, Head & Neck, Melanoma, NSCLC, Ovarian, Sarcoma, Thyroid We have now reached dose levels that enable T-Plex T-Plex and DL3 singleplex are expected to be the first efficacious dose levels Goal is to end 2025 with clearly interpretable data in defined areas Focus on immune-rich cancers with high unmet need MAGE-A4 PRAME Non-small cell lung cancer
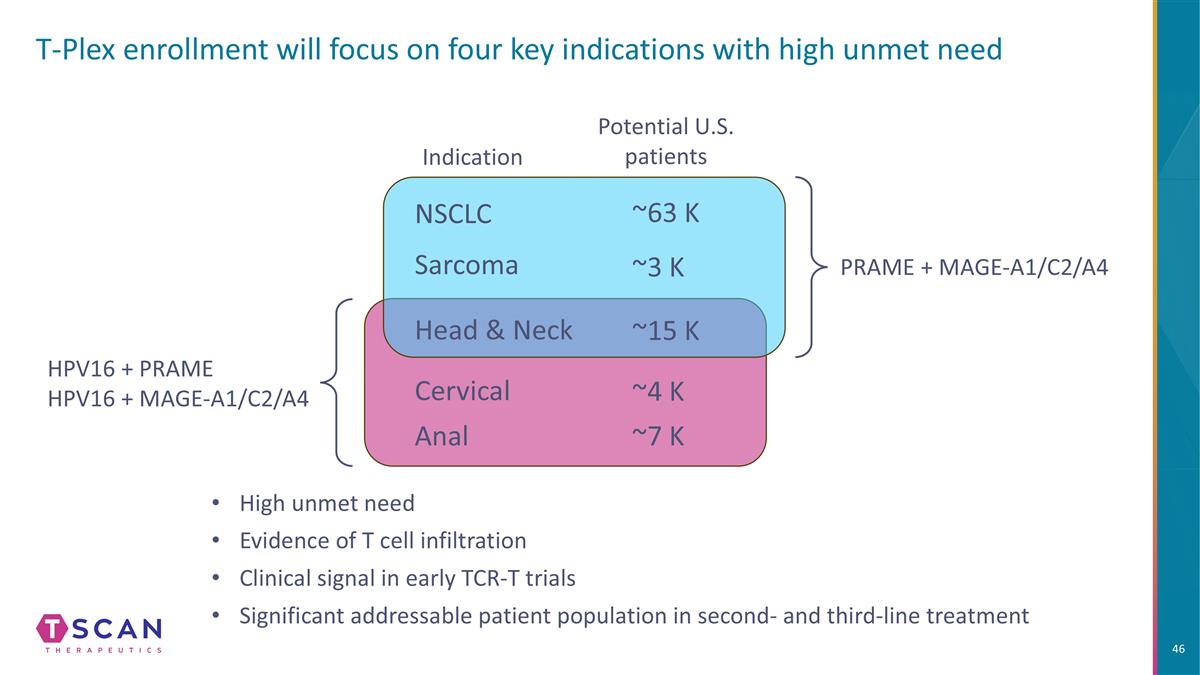
T-Plex enrollment will focus on four key indications with high unmet need NSCLC Sarcoma Head & Neck Anal PRAME + MAGE-A1/C2/A4 HPV16 + PRAME HPV16 + MAGE-A1/C2/A4 High unmet need Evidence of T cell infiltration Clinical signal in early TCR-T trials Significant addressable patient population in second- and third-line treatment Indication Potential U.S. patients Cervical ~63 K ~3 K ~15 K ~4 K ~7 K
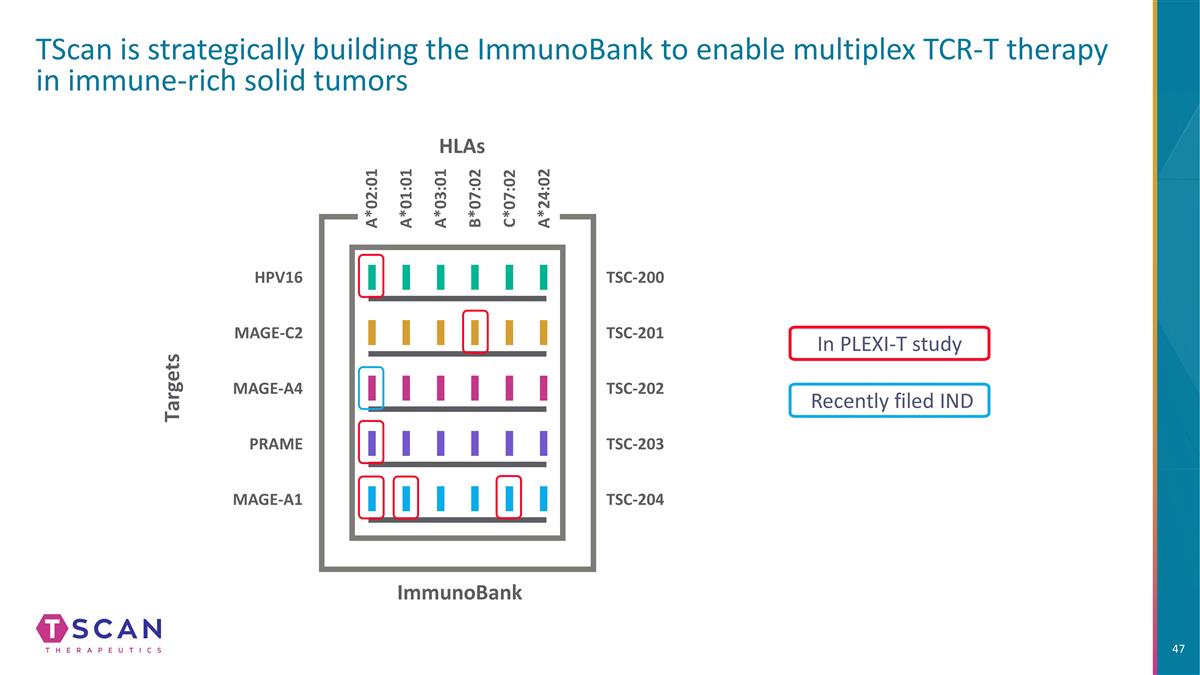
TScan is strategically building the ImmunoBank to enable multiplex TCR-T therapy in immune-rich solid tumors In PLEXI-T study HLAs Targets ImmunoBank TSC-200 TSC-203 TSC-202 TSC-204 A*02:01 A*01:01 A*03:01 B*07:02 C*07:02 TSC-201 A*24:02 HPV16 MAGE-A4 MAGE-C2 PRAME MAGE-A1 Recently filed IND
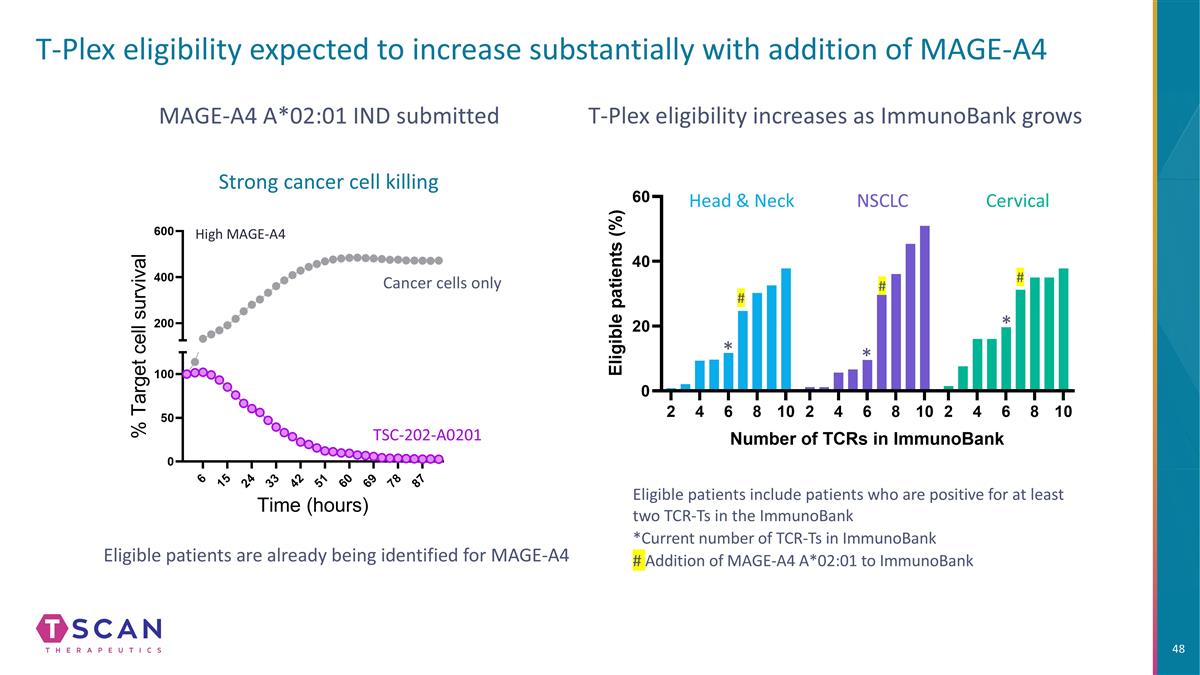
T-Plex eligibility expected to increase substantially with addition of MAGE-A4 T-Plex eligibility increases as ImmunoBank grows Eligible patients include patients who are positive for at least two TCR-Ts in the ImmunoBank *Current number of TCR-Ts in ImmunoBank # Addition of MAGE-A4 A*02:01 to ImmunoBank Head & Neck NSCLC Cervical * * * Eligible patients are already being identified for MAGE-A4 MAGE-A4 A*02:01 IND submitted High MAGE-A4 # # # Strong cancer cell killing TSC-202-A0201 Cancer cells only
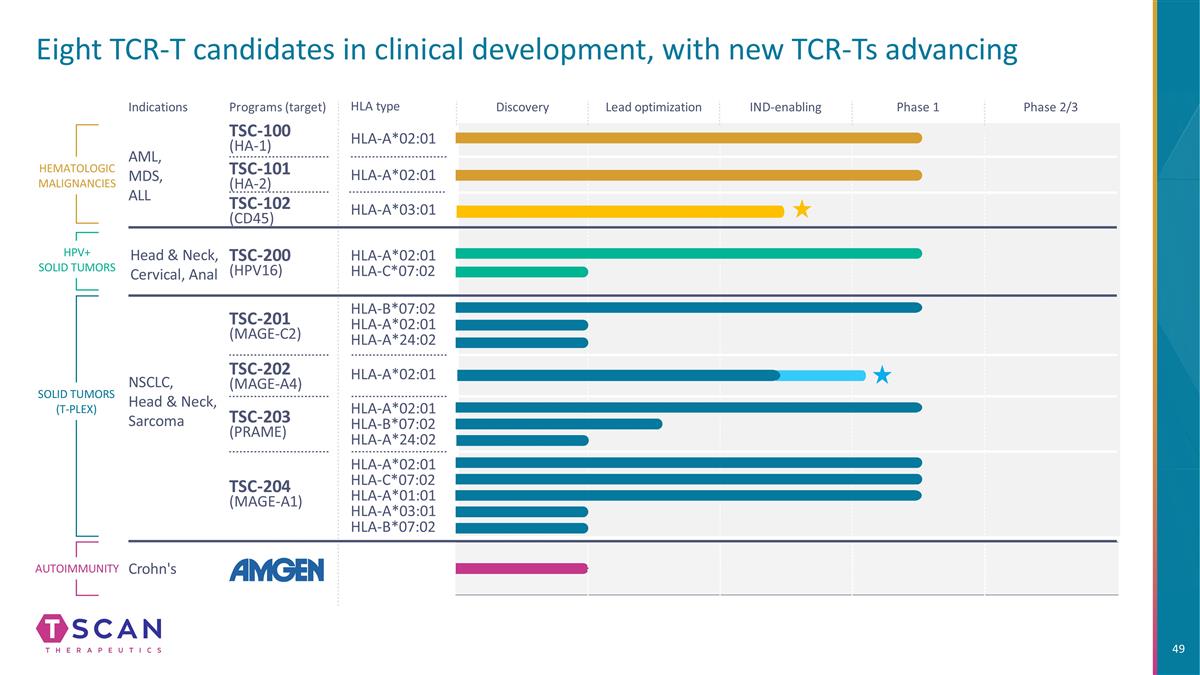
Eight TCR-T candidates in clinical development, with new TCR-Ts advancing HLA type Indications Programs (target) TSC-100 (HA-1) HLA-A*02:01 HLA-A*02:01 HLA-A*02:01 HLA-C*07:02 HLA-B*07:02 HLA-A*02:01 HLA-A*24:02 HLA-A*02:01 HLA-C*07:02 HLA-A*01:01 HLA-A*03:01 HLA-B*07:02 HLA-A*02:01 HLA-B*07:02 HLA-A*24:02 TSC-101 (HA-2) TSC-200 (HPV16) TSC-201 (MAGE-C2) TSC-202 (MAGE-A4) TSC-203 (PRAME) TSC-204 (MAGE-A1) HEMATOLOGIC MALIGNANCIES SOLID TUMORS (T-PLEX) AML, MDS, ALL NSCLC, Head & Neck, Sarcoma HLA-A*02:01 Autoimmunity Crohn's Discovery Lead optimization IND-enabling Phase 1 Phase 2/3 HPV+ SOLID TUMORS Head & Neck, Cervical, Anal HLA-A*03:01 TSC-102 (CD45)
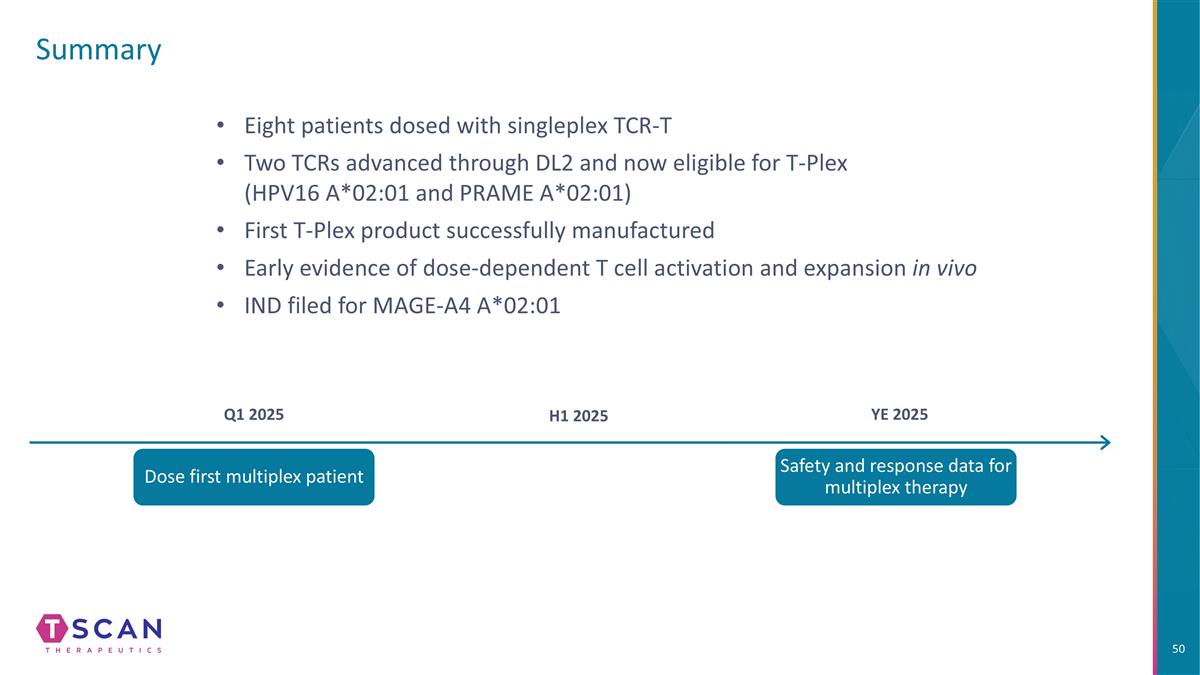
Summary Q1 2025 H1 2025 YE 2025 Dose first multiplex patient Safety and response data for multiplex therapy Eight patients dosed with singleplex TCR-T Two TCRs advanced through DL2 and now eligible for T-Plex (HPV16 A*02:01 and PRAME A*02:01) First T-Plex product successfully manufactured Early evidence of dose-dependent T cell activation and expansion in vivo IND filed for MAGE-A4 A*02:01

Q&A


















































