
Developing a Differentiated FGF21 for NASH and SHTG April 2020 NASDAQ: ETNB Exhibit 99.2

Cautionary Note Regarding Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, which statements are subject to substantial risks and uncertainties and are based on estimates and assumptions. Other than statements of historical facts, all statements included in this presentation are forward-looking statements, including statements concerning our plans, objectives, goals, strategies, future events, future revenues or performance, financing needs, plans or intentions relating to product candidates, estimates of market size, business trends, the anticipated timing, costs, design and conduct of our planned clinical trials for BIO89-100, our only product candidate, the association of preclinical data with potential clinical benefit, the timing of anticipated milestones, the effect of the COVID-19 pandemic on our clinical trials and business operations, the timing and likelihood of regulatory filings and approvals for BIO89-100, our ability to commercialize BIO89-100, if approved, the pricing and reimbursement of BIO89-100, if approved, the potential to develop future product candidates, our ability to scale up manufacturing, the potential benefits of strategic collaborations and our intent to enter into any strategic arrangements, the timing and likelihood of success, plans and objectives of management for future operations and future results of anticipated product development efforts. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms, and similar expressions intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the forward-looking statements expressed or implied in this presentation including those described more fully our most recent Form 10-K under the caption “Risk Factors” and elsewhere is such report. We cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. Forward-looking statements are not historical facts, and reflect our current views with respect to future events. Given the significant uncertainties, you should evaluate all forward-looking statements made in this presentation in the context of these risks and uncertainties and not place undue reliance on these forward-looking statements as predictions of future events. All forward-looking statements in this presentation apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this presentation. We disclaim any intent to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances, except as required by law. We obtained the industry, market and competitive position data used throughout this presentation from our own internal estimates and research, as well as from industry and general publications, and research, surveys and studies conducted by third parties. Internal estimates are derived from publicly available information released by industry analysts and third-party sources, our internal research and our industry experience, and are based on assumptions made by us based on such data and our knowledge of the industry and market, which we believe to be reasonable. In addition, while we believe the industry, market and competitive position data included in this presentation is reliable and based on reasonable assumptions, we have not independently verified any third-party information, and all such data involve risks and uncertainties and are subject to change based on various factors. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the forward-looking statements expressed or implied in this presentation, including those described more fully in our most recent Form 10-K under the caption “Risk Factors” and elsewhere in such report. Disclaimer

89bio – Investment Highlights Targeting large and growing unmet need in Non-Alcoholic Steatohepatitis (NASH) and Severe Hypertriglyceridemia (SHTG) Significant Commercial Opportunities BIO89-100 is a glycoPEGylated analog of FGF21 with compelling early human data FGF21 has the potential to become mainstay of NASH therapy – addresses key liver pathologies and underlying metabolic dysregulation Potentially Differentiated Asset Targeting Clinically Validated Mechanism in NASH Two trials with BIO89-100: (i) ongoing Phase 1b/2a trial in NASH (enrollment complete and topline data anticipated in 2H20); (ii) planned Phase 2 trial in SHTG (initiation in 1H20 delayed due to the COVID-19 pandemic) Potential to transition to Phase 2b trial in NASH in 1H21 Anticipated Near-term Catalysts Established manufacturing process in place for near-term clinical supplies Issued composition of matter patent expected to expire in 2038 Established Manufacturing Expertise; Long IP Protection Robust reduction in triglyceride levels seen in animal and human studies Established development and regulatory path offering a potentially quicker path to market for BIO89-100 Potentially Differentiated Therapy for SHTG CONFIDENTIAL

OPPORTUNITY IN NASH
BIO89-100 is A Compelling Drug Candidate in NASH BIO89-100 HAS THE POTENTIAL TO BE A DIFFERENTIATED FGF21 ANALOG Robust clinical and preclinical data with an upcoming Anticipated efficacy readout Proprietary glycoPEGylation technology may offer robust biologic effects, favorable tolerability profile and a longer dosing interval NASH IS A SERIOUS LIVER CONDITION Large market size with significant economic burden and no FDA-approved treatment options Complicated disease with significant co-morbidities FGF21 HAS THE POTENTIAL TO BE MAINSTAY OF THERAPY GIVEN ITS BROAD-BASED EFFECTS Addresses steatosis and fibrosis and underlying metabolic issues Phase 1a trial showed favorable tolerability, PK and PD markers building on strong preclinical package Phase 1b/2a trial in NASH has closed enrollment with 98% patients enrolled (81/83 patients) due to impact of the COVID-19 pandemic; topline data expected in 2H20 CONFIDENTIAL
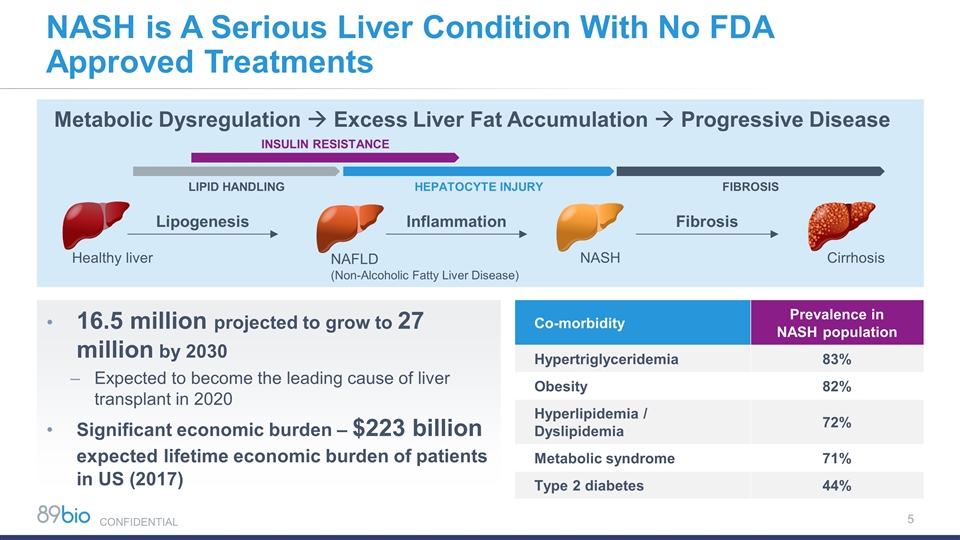
NASH is A Serious Liver Condition With No FDA Approved Treatments 16.5 million projected to grow to 27 million by 2030 Expected to become the leading cause of liver transplant in 2020 Significant economic burden – $223 billion expected lifetime economic burden of patients in US (2017) Co-morbidity Prevalence in NASH population Hypertriglyceridemia 83% Obesity 82% Hyperlipidemia / Dyslipidemia 72% Metabolic syndrome 71% Type 2 diabetes 44% Healthy liver NAFLD (Non-Alcoholic Fatty Liver Disease) NASH Cirrhosis Lipogenesis Inflammation Fibrosis Metabolic Dysregulation à Excess Liver Fat Accumulation à Progressive Disease INSULIN RESISTANCE HEPATOCYTE INJURY FIBROSIS LIPID HANDLING CONFIDENTIAL
Key Attributes for Successful NASH Therapies NASH Therapeutics - Market Dynamics Similarities to multi-billion diabetes and dyslipidemia markets Potent injectables have the potential to be a preferred treatment option for some patient populations (e.g. GLP-1 agonists achieved ~$9 billion in sales in 2018*) Robust efficacy with respect to liver pathologies Ability to address underlying co-morbidities Well tolerated 1 Potential for Multiple Winners 2 * Includes Victoza, Trulicity, Ozempic, Saxenda, Xultophy, Bydureon, Soliqua and Adlyxin. CONFIDENTIAL
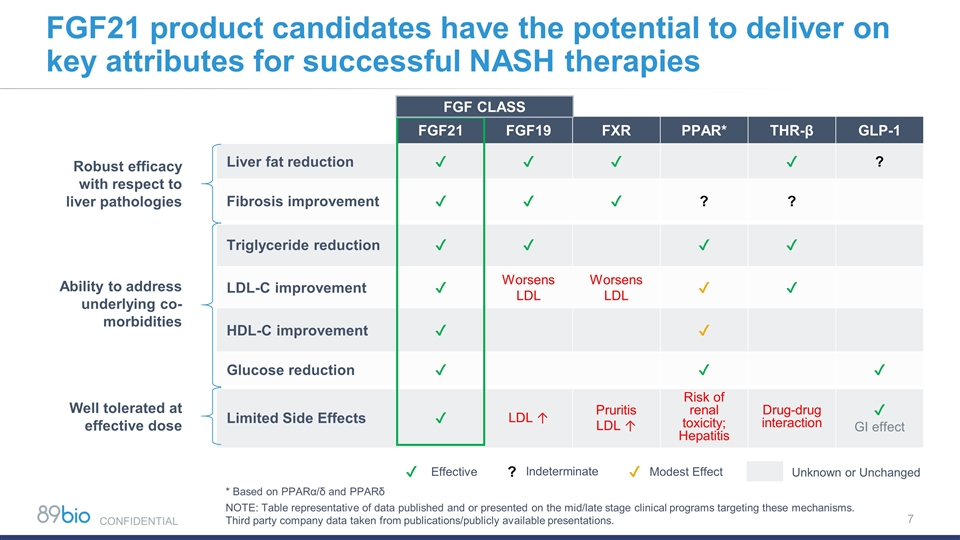
FGF21 FGF19 FXR PPAR* THR-β GLP-1 Liver fat reduction ✔ ✔ ✔ ✔ ? Fibrosis improvement ✔ ✔ ✔ ? ? Triglyceride reduction ✔ ✔ ✔ ✔ LDL-C improvement ✔ Worsens LDL Worsens LDL ✔ ✔ HDL-C improvement ✔ ✔ Glucose reduction ✔ ✔ ✔ Limited Side Effects ✔ LDL ↑ Pruritis LDL ↑ Risk of renal toxicity; Hepatitis Drug-drug interaction ✔ GI effect Ability to address underlying co-morbidities Robust efficacy with respect to liver pathologies Well tolerated at effective dose FGF21 product candidates have the potential to deliver on key attributes for successful NASH therapies * Based on PPARα/δ and PPARδ NOTE: Table representative of data published and or presented on the mid/late stage clinical programs targeting these mechanisms. Third party company data taken from publications/publicly available presentations. Effective Indeterminate Modest Effect ✔ ? ✔ Unknown or Unchanged FGF CLASS CONFIDENTIAL
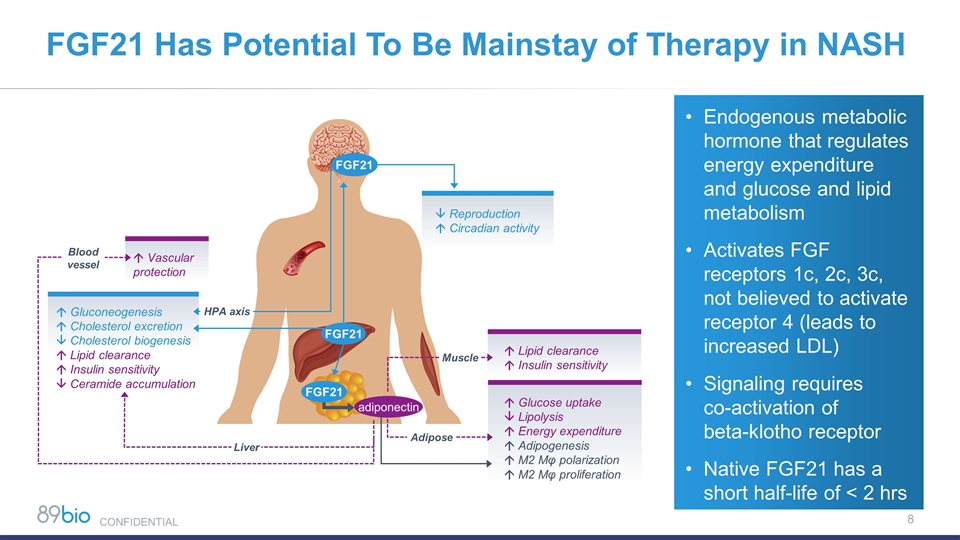
Endogenous metabolic hormone that regulates energy expenditure and glucose and lipid metabolism Activates FGF receptors 1c, 2c, 3c, not believed to activate receptor 4 (leads to increased LDL) Signaling requires co-activation of beta-klotho receptor Native FGF21 has a short half-life of < 2 hrs á Lipid clearance á Insulin sensitivity FGF21 á Glucose uptake â Lipolysis á Energy expenditure á Adipogenesis á M2 Mφ polarization á M2 Mφ proliferation â Reproduction á Circadian activity Adipose Muscle á Gluconeogenesis á Cholesterol excretion â Cholesterol biogenesis á Lipid clearance á Insulin sensitivity â Ceramide accumulation Liver á Vascular protection Blood vessel HPA axis FGF21 adiponectin FGF21 FGF21 Has Potential To Be Mainstay of Therapy in NASH CONFIDENTIAL
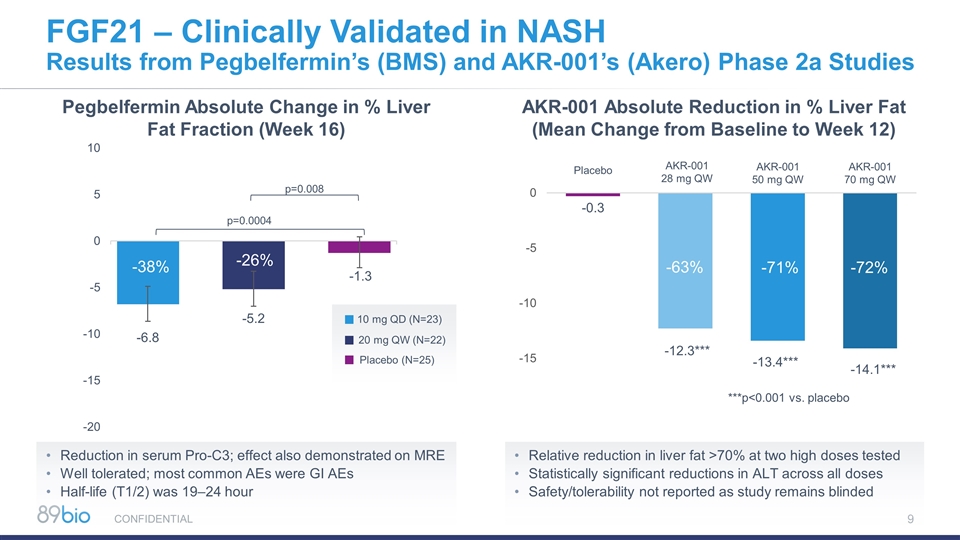
Pegbelfermin Absolute Change in % Liver Fat Fraction (Week 16) FGF21 – Clinically Validated in NASH Results from Pegbelfermin’s (BMS) and AKR-001’s (Akero) Phase 2a Studies 10 mg QD (N=23) 20 mg QW (N=22) Placebo (N=25) AKR-001 Absolute Reduction in % Liver Fat (Mean Change from Baseline to Week 12) -38% -26% -0.3 -12.3*** -13.4*** -14.1*** Placebo AKR-001 28 mg QW AKR-001 50 mg QW AKR-001 70 mg QW Reduction in serum Pro-C3; effect also demonstrated on MRE Well tolerated; most common AEs were GI AEs Half-life (T1/2) was 19–24 hour Relative reduction in liver fat >70% at two high doses tested Statistically significant reductions in ALT across all doses Safety/tolerability not reported as study remains blinded CONFIDENTIAL ***p<0.001 vs. placebo -63% -71% -72%
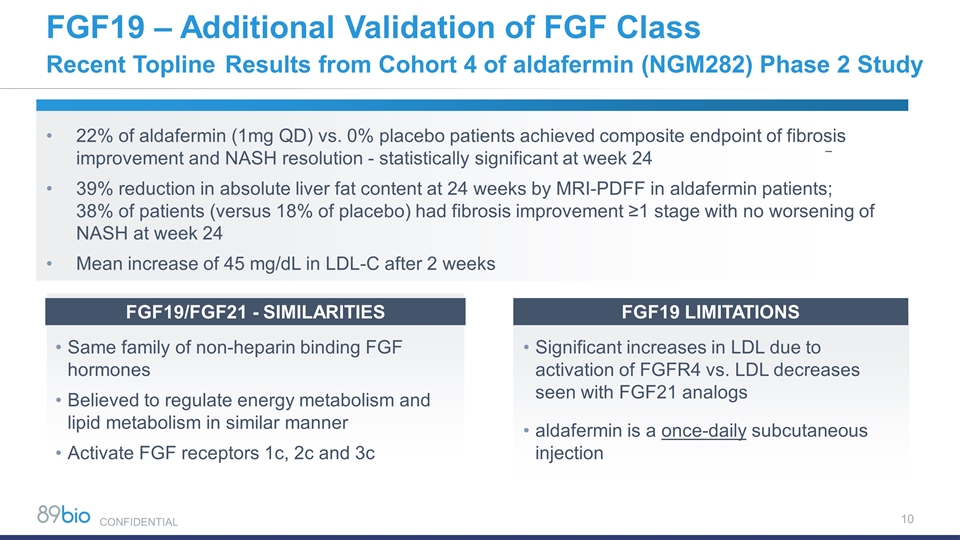
22% of aldafermin (1mg QD) vs. 0% placebo patients achieved composite endpoint of fibrosis improvement and NASH resolution - statistically significant at week 24 39% reduction in absolute liver fat content at 24 weeks by MRI-PDFF in aldafermin patients; 38% of patients (versus 18% of placebo) had fibrosis improvement ≥1 stage with no worsening of NASH at week 24 Mean increase of 45 mg/dL in LDL-C after 2 weeks FGF19 – Additional Validation of FGF Class Recent Topline Results from Cohort 4 of aldafermin (NGM282) Phase 2 Study Same family of non-heparin binding FGF hormones Believed to regulate energy metabolism and lipid metabolism in similar manner Activate FGF receptors 1c, 2c and 3c FGF19/FGF21 - SIMILARITIES Significant increases in LDL due to activation of FGFR4 vs. LDL decreases seen with FGF21 analogs aldafermin is a once-daily subcutaneous injection FGF19 LIMITATIONS CONFIDENTIAL
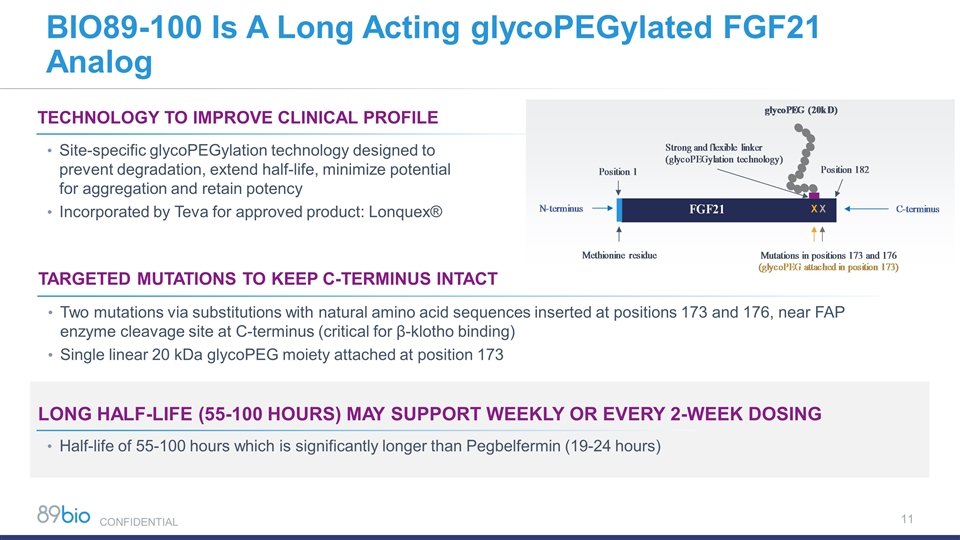
BIO89-100 Is A Long Acting glycoPEGylated FGF21 Analog LONG Half-life (55-100 hours) MAY SUPPORT WEEKLY OR EVERY 2-WEEK DOSING Technology TO IMPROVE CLINICAL PROFILE Site-specific glycoPEGylation technology designed to prevent degradation, extend half-life, minimize potential for aggregation and retain potency Incorporated by Teva for approved product: Lonquex® Targeted Mutations TO KEEP C-TERMINUS INTACT Two mutations via substitutions with natural amino acid sequences inserted at positions 173 and 176, near FAP enzyme cleavage site at C-terminus (critical for β-klotho binding) Single linear 20 kDa glycoPEG moiety attached at position 173 Half-life of 55-100 hours which is significantly longer than Pegbelfermin (19-24 hours) CONFIDENTIAL
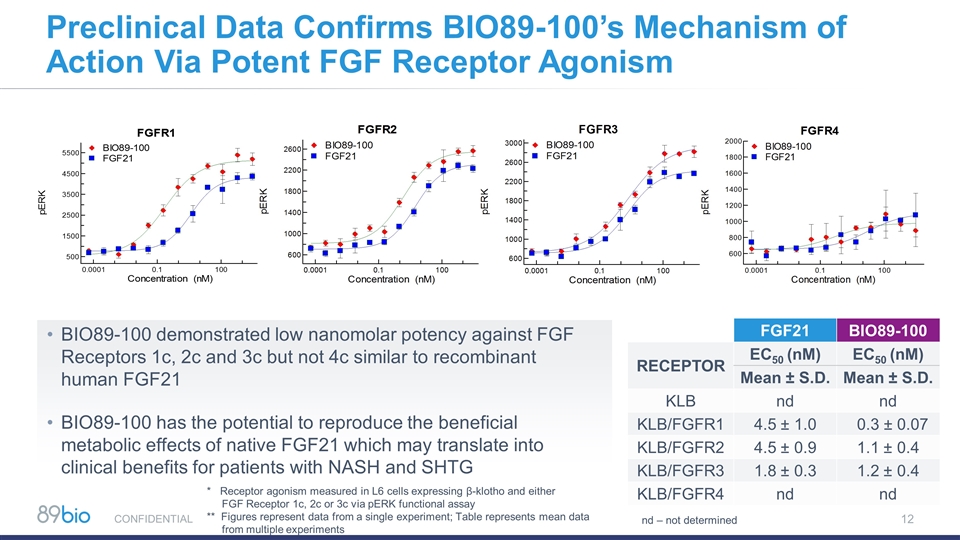
12 Preclinical Data Confirms BIO89-100’s Mechanism of Action Via Potent FGF Receptor Agonism CONFIDENTIAL BIO89-100 demonstrated low nanomolar potency against FGF Receptors 1c, 2c and 3c but not 4c similar to recombinant human FGF21 BIO89-100 has the potential to reproduce the beneficial metabolic effects of native FGF21 which may translate into clinical benefits for patients with NASH and SHTG FGF21 BIO89-100 RECEPTOR EC50 (nM) EC50 (nM) Mean ± S.D. Mean ± S.D. KLB nd nd KLB/FGFR1 4.5 ± 1.0 0.3 ± 0.07 KLB/FGFR2 4.5 ± 0.9 1.1 ± 0.4 KLB/FGFR3 1.8 ± 0.3 1.2 ± 0.4 KLB/FGFR4 nd nd nd – not determined * Receptor agonism measured in L6 cells expressing β-klotho and either FGF Receptor 1c, 2c or 3c via pERK functional assay ** Figures represent data from a single experiment; Table represents mean data from multiple experiments
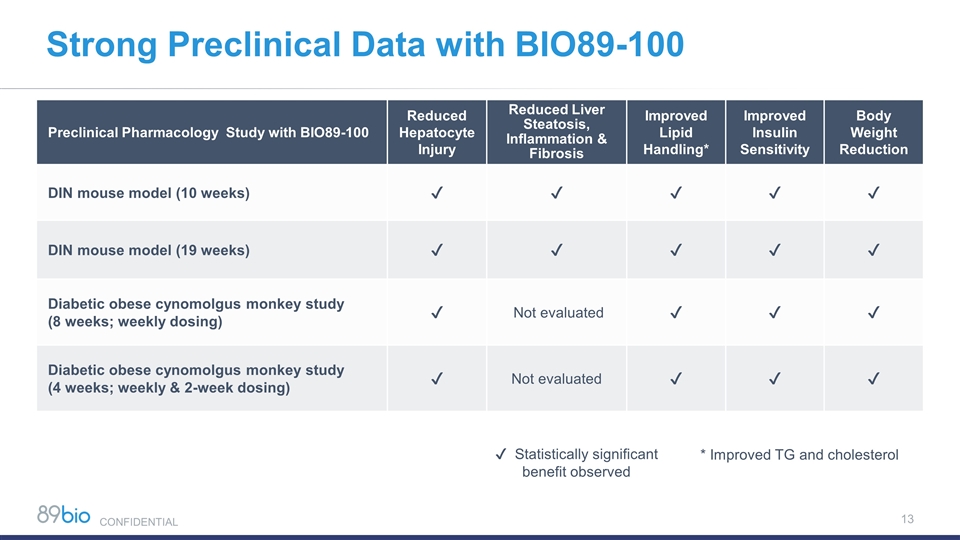
Strong Preclinical Data with BIO89-100 Preclinical Pharmacology Study with BIO89-100 Reduced Hepatocyte Injury Reduced Liver Steatosis, Inflammation & Fibrosis Improved Lipid Handling* Improved Insulin Sensitivity Body Weight Reduction DIN mouse model (10 weeks) ✔ ✔ ✔ ✔ ✔ DIN mouse model (19 weeks) ✔ ✔ ✔ ✔ ✔ Diabetic obese cynomolgus monkey study (8 weeks; weekly dosing) ✔ Not evaluated ✔ ✔ ✔ Diabetic obese cynomolgus monkey study (4 weeks; weekly & 2-week dosing) ✔ Not evaluated ✔ ✔ ✔ ✔ Statistically significant benefit observed * Improved TG and cholesterol CONFIDENTIAL
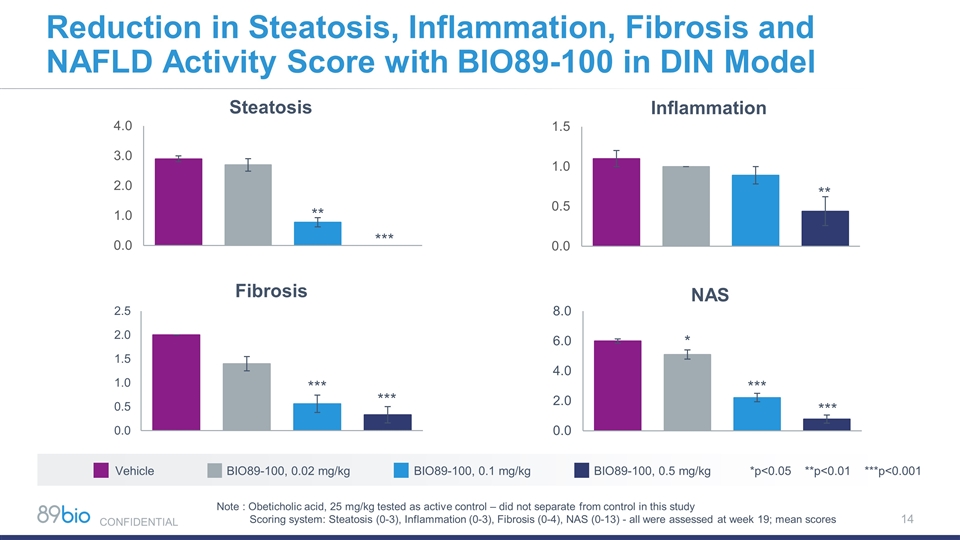
Note : Obeticholic acid, 25 mg/kg tested as active control – did not separate from control in this study Scoring system: Steatosis (0-3), Inflammation (0-3), Fibrosis (0-4), NAS (0-13) - all were assessed at week 19; mean scores *** *** Vehicle BIO89-100, 0.02 mg/kg BIO89-100, 0.1 mg/kg BIO89-100, 0.5 mg/kg *p<0.05 **p<0.01 ***p<0.001 Reduction in Steatosis, Inflammation, Fibrosis and NAFLD Activity Score with BIO89-100 in DIN Model * CONFIDENTIAL
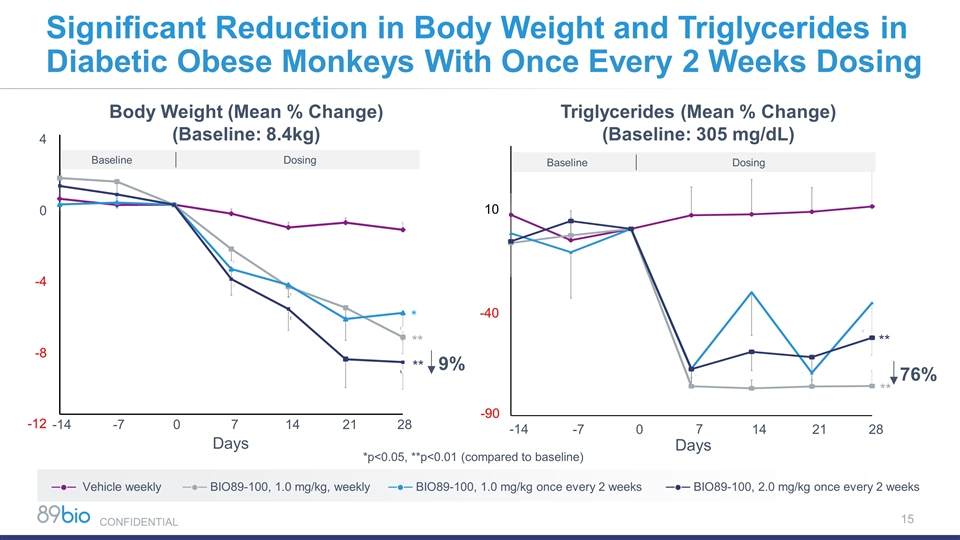
Triglycerides (Mean % Change) (Baseline: 305 mg/dL) Dosing Baseline -14 -7 0 7 14 21 28 10 -40 -90 Significant Reduction in Body Weight and Triglycerides in Diabetic Obese Monkeys With Once Every 2 Weeks Dosing Baseline Dosing Body Weight (Mean % Change) (Baseline: 8.4kg) 4 0 -4 -8 -12 Days -14 -7 0 7 14 21 28 *p<0.05, **p<0.01 (compared to baseline) * ** ** 9% ─●─ Vehicle weekly ─●─ BIO89-100, 1.0 mg/kg, weekly ─●─ BIO89-100, 1.0 mg/kg once every 2 weeks ─●─ BIO89-100, 2.0 mg/kg once every 2 weeks Days 76% ** ** CONFIDENTIAL
BIO89-100 Demonstrated a Favorable Clinical Profile in Phase 1a Study Well tolerated Most commonly observed treatment related AEs (in > 2 subjects) were injection site reaction and headache, all of which were reported as mild PK was dose proportional; half-life of 55–100 hours Significant improvements in key lipid parameters at 8 and 15 days after single dose (baseline values were in normal range)* Triglycerides reduction up to 51% LDL-C reduction up to 37% HDL-C increase up to 36% Supports weekly and once every 2-week dosing regimen Trial Design: Double-blind, placebo-controlled Single Ascending Dose (SAD); 58 healthy volunteers; 7 dose cohorts * Mean changes versus baseline CONFIDENTIAL
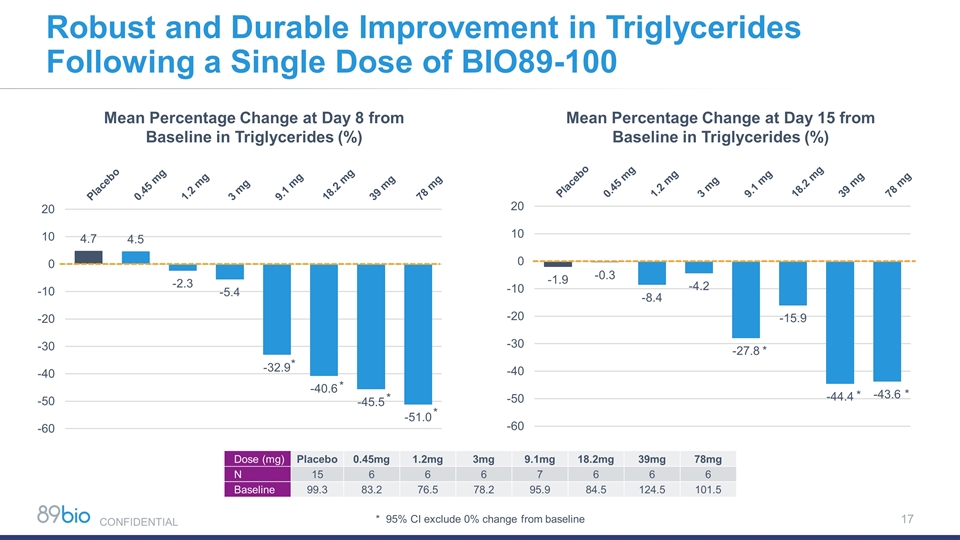
Robust and Durable Improvement in Triglycerides Following a Single Dose of BIO89-100 * * * * * * * Mean Percentage Change at Day 8 from Baseline in Triglycerides (%) Mean Percentage Change at Day 15 from Baseline in Triglycerides (%) * 95% CI exclude 0% change from baseline Dose (mg) Placebo 0.45mg 1.2mg 3mg 9.1mg 18.2mg 39mg 78mg N 15 6 6 6 7 6 6 6 Baseline 99.3 83.2 76.5 78.2 95.9 84.5 124.5 101.5 CONFIDENTIAL
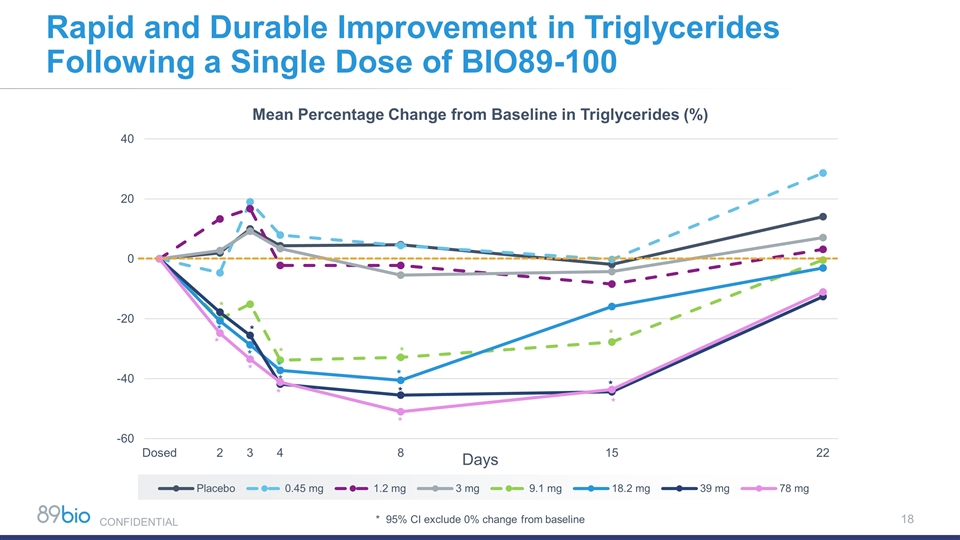
Mean Percentage Change from Baseline in Triglycerides (%) Days * 95% CI exclude 0% change from baseline * * * * * * * * * * * * * * * * Rapid and Durable Improvement in Triglycerides Following a Single Dose of BIO89-100 CONFIDENTIAL
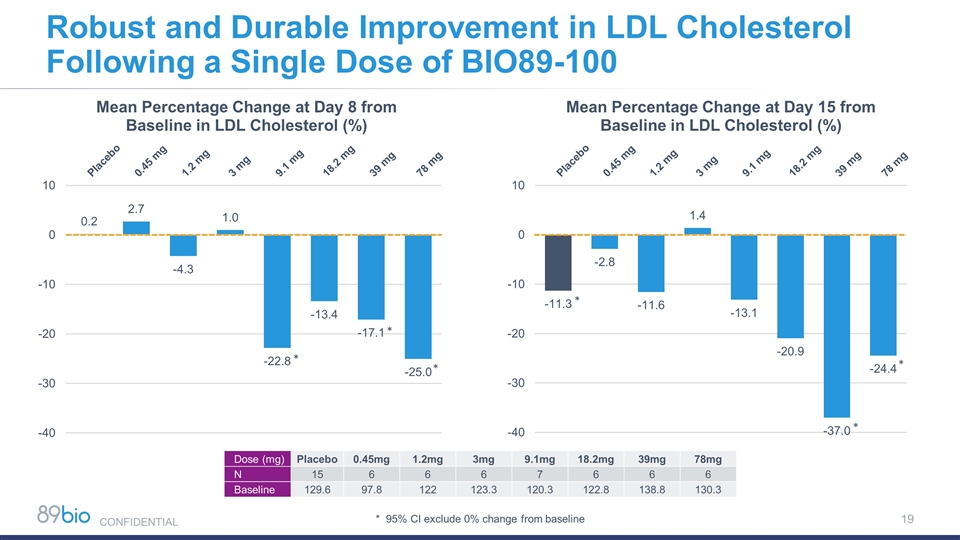
Robust and Durable Improvement in LDL Cholesterol Following a Single Dose of BIO89-100 * * * * * * Dose (mg) Placebo 0.45mg 1.2mg 3mg 9.1mg 18.2mg 39mg 78mg N 15 6 6 6 7 6 6 6 Baseline 129.6 97.8 122 123.3 120.3 122.8 138.8 130.3 * 95% CI exclude 0% change from baseline CONFIDENTIAL
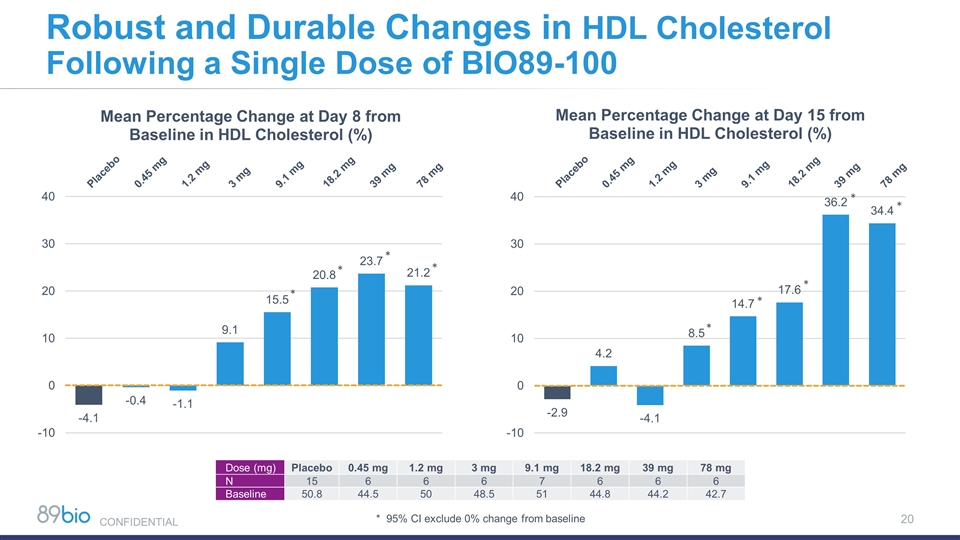
Robust and Durable Changes in HDL Cholesterol Following a Single Dose of BIO89-100 * * * * * * * * * Dose (mg) Placebo 0.45 mg 1.2 mg 3 mg 9.1 mg 18.2 mg 39 mg 78 mg N 15 6 6 6 7 6 6 6 Baseline 50.8 44.5 50 48.5 51 44.8 44.2 42.7 * 95% CI exclude 0% change from baseline CONFIDENTIAL
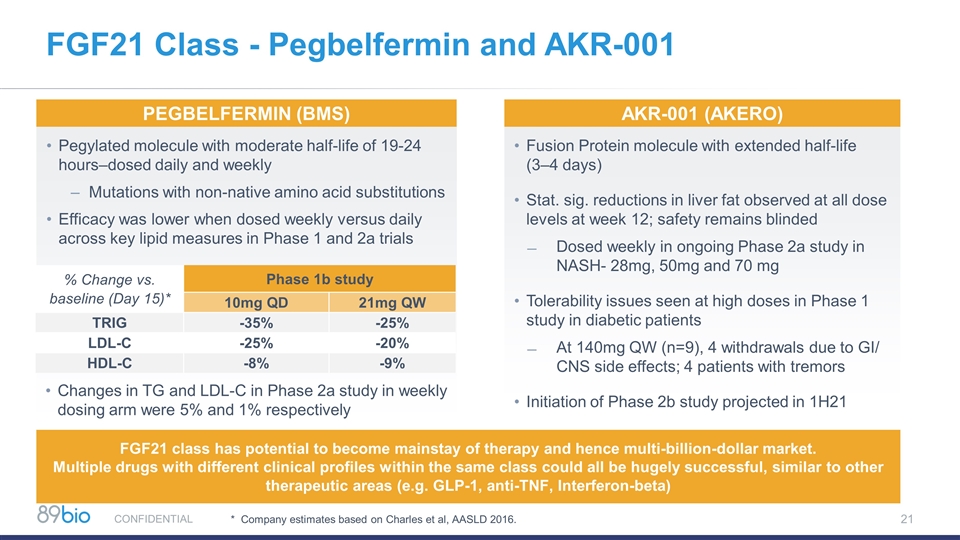
FGF21 Class - Pegbelfermin and AKR-001 Pegylated molecule with moderate half-life of 19-24 hours–dosed daily and weekly Mutations with non-native amino acid substitutions Efficacy was lower when dosed weekly versus daily across key lipid measures in Phase 1 and 2a trials Fusion Protein molecule with extended half-life (3–4 days) Stat. sig. reductions in liver fat observed at all dose levels at week 12; safety remains blinded Dosed weekly in ongoing Phase 2a study in NASH- 28mg, 50mg and 70 mg Tolerability issues seen at high doses in Phase 1 study in diabetic patients At 140mg QW (n=9), 4 withdrawals due to GI/ CNS side effects; 4 patients with tremors Initiation of Phase 2b study projected in 1H21 PEGBELFERMIN (BMS) Akr-001 (aKERO) % Change vs. baseline (Day 15)* Phase 1b study 10mg QD 21mg QW TRIG -35% -25% LDL-C -25% -20% HDL-C -8% -9% Changes in TG and LDL-C in Phase 2a study in weekly dosing arm were 5% and 1% respectively * Company estimates based on Charles et al, AASLD 2016. FGF21 class has potential to become mainstay of therapy and hence multi-billion-dollar market. Multiple drugs with different clinical profiles within the same class could all be hugely successful, similar to other therapeutic areas (e.g. GLP-1, anti-TNF, Interferon-beta) CONFIDENTIAL 21
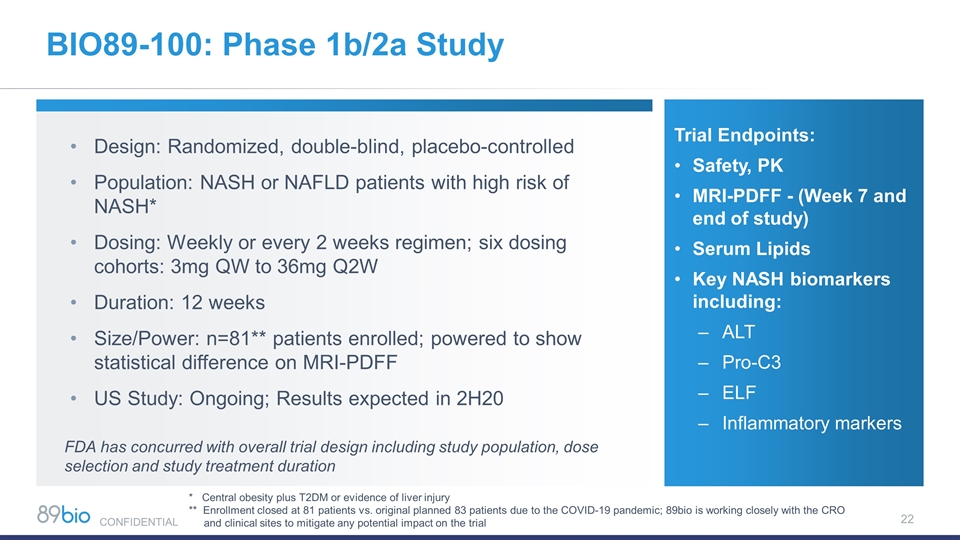
BIO89-100: Phase 1b/2a Study Design: Randomized, double-blind, placebo-controlled Population: NASH or NAFLD patients with high risk of NASH* Dosing: Weekly or every 2 weeks regimen; six dosing cohorts: 3mg QW to 36mg Q2W Duration: 12 weeks Size/Power: n=81** patients enrolled; powered to show statistical difference on MRI-PDFF US Study: Ongoing; Results expected in 2H20 Trial Endpoints: Safety, PK MRI-PDFF - (Week 7 and end of study) Serum Lipids Key NASH biomarkers including: ALT Pro-C3 ELF Inflammatory markers * Central obesity plus T2DM or evidence of liver injury ** Enrollment closed at 81 patients vs. original planned 83 patients due to the COVID-19 pandemic; 89bio is working closely with the CRO and clinical sites to mitigate any potential impact on the trial FDA has concurred with overall trial design including study population, dose selection and study treatment duration CONFIDENTIAL

OPPORTUNITY IN SHTG

BIO89-100: A Compelling Drug Candidate For SHTG Showed significant reduction in triglycerides in preclinical and clinical studies (up to 78% in monkey study and up to 51% after a single human dose) Potential for use as monotherapy or in combination with existing drugs or those in development Large patient population who are unable to achieve treatment goals with existing therapies Increased interest in lowering TGs to reduce residual CV risk SIGNIFICANT COMMERCIAL OPPORTUNITY BIO89-100 offers a POTENT and differentiated profile relative to existing therapies Established regulatory path; POTENTIAL FOR RELATIVELY Smaller and faster TRIALS Removes lipids from circulation, increases lipid catabolism and has the potential to address multiple co-morbidities (dyslipidemia, diabetes) FGF21 is a highly promising mechanism of action for treatment of SHTG Potential to be faster to market than NASH CONFIDENTIAL
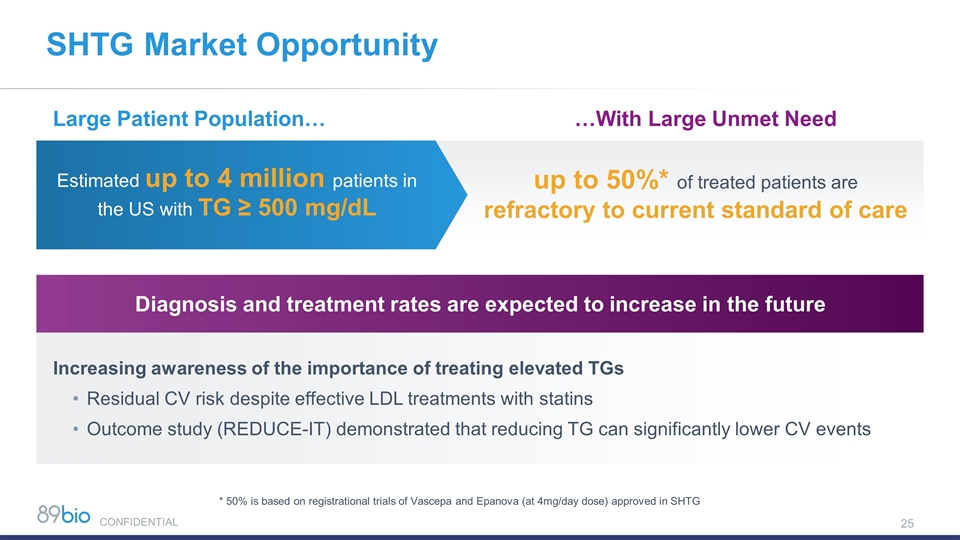
SHTG Market Opportunity Large Patient Population… …With Large Unmet Need Increasing awareness of the importance of treating elevated TGs Residual CV risk despite effective LDL treatments with statins Outcome study (REDUCE-IT) demonstrated that reducing TG can significantly lower CV events Diagnosis and treatment rates are expected to increase in the future Estimated up to 4 million patients in the US with TG ≥ 500 mg/dL up to 50%* of treated patients are refractory to current standard of care * 50% is based on registrational trials of Vascepa and Epanova (at 4mg/day dose) approved in SHTG CONFIDENTIAL

Key Attributes for Successful SHTG Therapies SHTG Therapeutics – Key Attributes Robust TG reduction Address multiple co-morbidities of patients (dyslipidemia and insulin resistance) Need to have favorable benefit/risk profile Support compliance with effective dosing CONFIDENTIAL
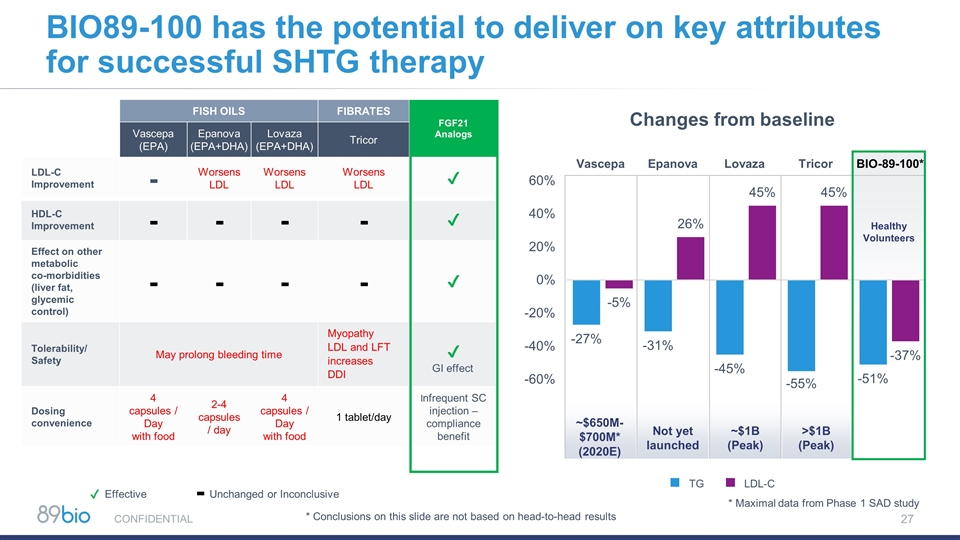
FISH OILS FIBRATES FGF21 Analogs Vascepa (EPA) Epanova (EPA+DHA) Lovaza (EPA+DHA) Tricor LDL-C Improvement - Worsens LDL Worsens LDL Worsens LDL ✔ HDL-C Improvement - - - - ✔ Effect on other metabolic co-morbidities (liver fat, glycemic control) - - - - ✔ Tolerability/ Safety May prolong bleeding time Myopathy LDL and LFT increases DDI ✔ Dosing convenience 4 capsules / Day with food 2-4 capsules / day 4 capsules / Day with food 1 tablet/day Infrequent SC injection – compliance benefit BIO89-100 has the potential to deliver on key attributes for successful SHTG therapy TG LDL-C Changes from baseline CONFIDENTIAL Vascepa Epanova Lovaza Tricor BIO-89-100* Healthy Volunteers ~$1B (Peak) >$1B (Peak) Not yet launched ~$650M-$700M* (2020E) GI effect Effective ✔ Unchanged or Inconclusive - * Maximal data from Phase 1 SAD study * Conclusions on this slide are not based on head-to-head results
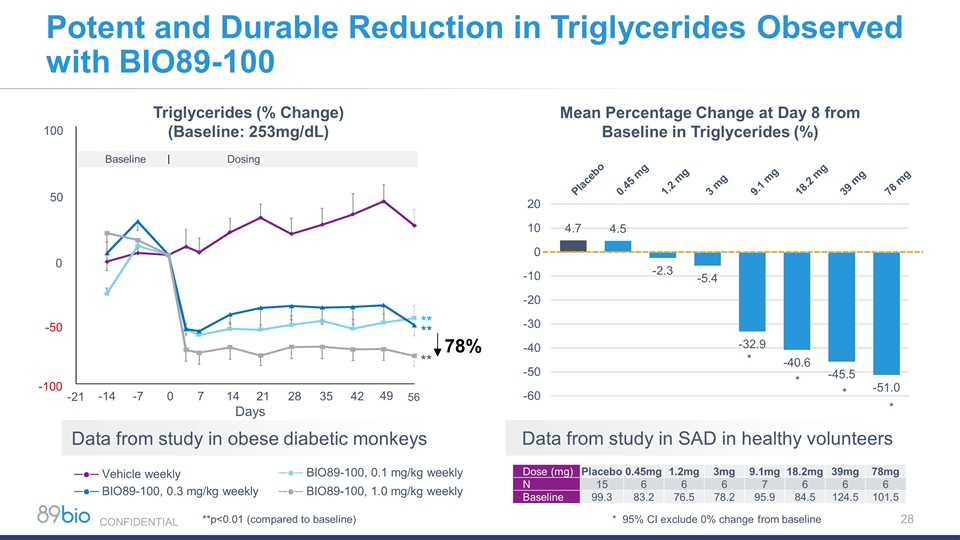
Potent and Durable Reduction in Triglycerides Observed with BIO89-100 Baseline Dosing Days -14 -7 100 50 0 -50 -100 0 7 14 21 28 35 42 49 ** ** ** Triglycerides (% Change) (Baseline: 253mg/dL) * * * * Mean Percentage Change at Day 8 from Baseline in Triglycerides (%) Data from study in SAD in healthy volunteers 56 -21 * 95% CI exclude 0% change from baseline Dose (mg) Placebo 0.45mg 1.2mg 3mg 9.1mg 18.2mg 39mg 78mg N 15 6 6 6 7 6 6 6 Baseline 99.3 83.2 76.5 78.2 95.9 84.5 124.5 101.5 78% **p<0.01 (compared to baseline) ─●─ Vehicle weekly ─●─ BIO89-100, 0.3 mg/kg weekly ─●─ BIO89-100, 1.0 mg/kg weekly Data from study in obese diabetic monkeys ─●─ BIO89-100, 0.1 mg/kg weekly CONFIDENTIAL

Study Design Phase 2 Study* Adults with TG ≥ 500 on no background TG lowering therapy or on stable dose of statin and/or prescription fish oil; N = ~90 Multiple doses vs placebo Primary endpoint: Reduction from baseline in TG Secondary endpoints: Other lipids, hsCRP, glucose, body weight, safety, PK Study duration: Topline data would be expected approximately a year or so after dosing our first patient Registrational Trial* Patients with TG ≥ 500mg/dL; Endpoint = % reduction of TG from baseline SHTG Clinical and Regulatory Path is Defined and May Represent a Quicker Path to Market US approval in SHTG has been granted based on demonstration of TG reduction from baseline; clinical outcome study was not required for certain third-party approvals Phase 3 studies for Vascepa and Epanova were single, 12-week trials with 75–100 patients per treatment arm * Phase 2 study initiation delayed due to the COVID-19 pandemic; Study designs and development plan to be confirmed with regulatory feedback BIO89-100 Anticipated Development Plan* 1 2 CONFIDENTIAL

Financial Position Summary Completed successful upsized IPO on November 13, 2019 Raised gross proceeds of $97.6 million Priced within range at $16.00/share Cash Position $93.3 million (as of March 31, 2020); On April 7, 2020, 89bio entered into a debt facility with Silicon Valley Bank for a tranched secured term loan of up to $15.0 million CONFIDENTIAL

Recent and Upcoming Anticipated Clinical Milestones BIO89-100 in NASH: BIO89-100 in SHTG: 2020 2021 1H 2H 1H 2H Topline data from Phase 1b/2a study Initiation of Phase 2b study Initiation of Phase 2 subject to conducive external environment* * Delayed due to the COVID-19 pandemic; Study designs and development plan to be confirmed with regulatory feedback CONFIDENTIAL

CEO, CCO experience Avanir, Medivation, J&J Commercial, strategy, and R&D experience Management Team 20 years of operations, BD, and strategy experience VP of strategy and transformation, Teva R&D VP of business development, XTL bio Rohan Palekar, CEO Ram Waisbourd COO and CBO Hank Mansbach, MD CMO 20+ years biopharma and R&D leadership in clinical development and medical affairs Ultragenyx, Medivation, Valeant, GSK 20+ years biopharma and leadership in technical operations, product supply, and quality Aduro, Bayer, Novartis, Chiron, BioMarin Quoc Le-Nguyen CTO & Head of Quality Ryan Martins, CFO CFO, Strategy/IR, finance, sell-side experience Revolution Medicines, Ultragenyx, Chiron, Jefferies, Lazard, Barclays/Lehman Brothers CONFIDENTIAL

89bio – Investment Highlights Targeting large and growing unmet need in Non-Alcoholic Steatohepatitis (NASH) and Severe Hypertriglyceridemia (SHTG) Significant Commercial Opportunities BIO89-100 is a glycoPEGylated analog of FGF21 with compelling early human data FGF21 has the potential to become mainstay of NASH therapy – addresses key liver pathologies and underlying metabolic dysregulation Potentially Differentiated Asset Targeting Clinically Validated Mechanism in NASH Two trials with BIO89-100: (i) ongoing Phase 1b/2a trial in NASH (enrollment complete and topline data anticipated in 2H20); (ii) planned Phase 2 trial in SHTG (initiation in 1H20 delayed due to the COVID-19 pandemic) Potential to transition to Phase 2b trial in NASH in 1H21 Anticipated Near-term Catalysts Established manufacturing process in place for near-term clinical supplies Issued composition of matter patent expected to expire in 2038 Established Manufacturing Expertise; Long IP Protection Robust reduction in triglyceride levels seen in animal and human studies Established development and regulatory path offering a potentially quicker path to market for BIO89-100 Potentially Differentiated Therapy for SHTG CONFIDENTIAL

APPENDIX
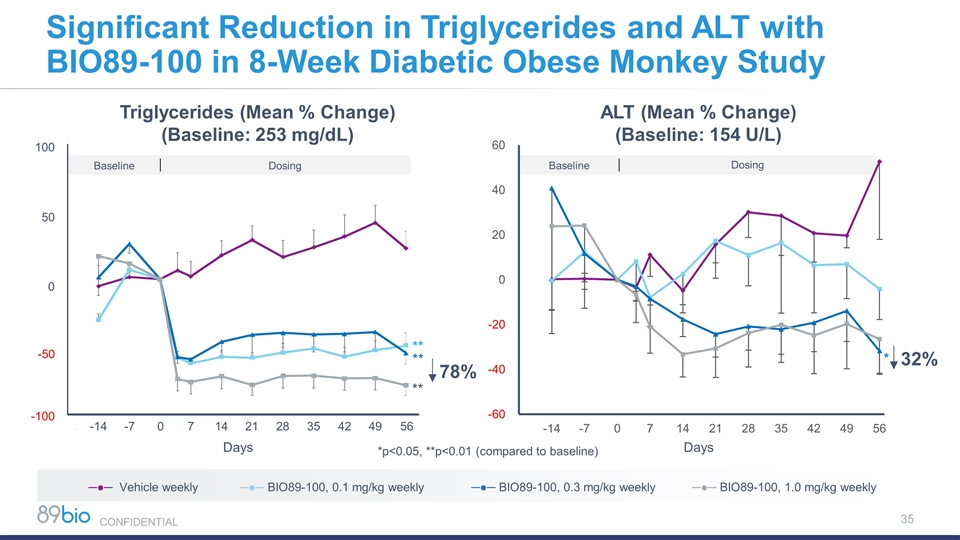
Significant Reduction in Triglycerides and ALT with BIO89-100 in 8-Week Diabetic Obese Monkey Study Baseline Dosing -14 -7 100 50 0 -50 -100 0 7 14 21 28 35 42 49 ** ** ** Triglycerides (Mean % Change) (Baseline: 253 mg/dL) 78% *p<0.05, **p<0.01 (compared to baseline) ALT (Mean % Change) (Baseline: 154 U/L) * 32% ─●─ Vehicle weekly ─●─ BIO89-100, 0.1 mg/kg weekly ─●─ BIO89-100, 0.3 mg/kg weekly ─●─ BIO89-100, 1.0 mg/kg weekly 56 Baseline Dosing Days Days CONFIDENTIAL
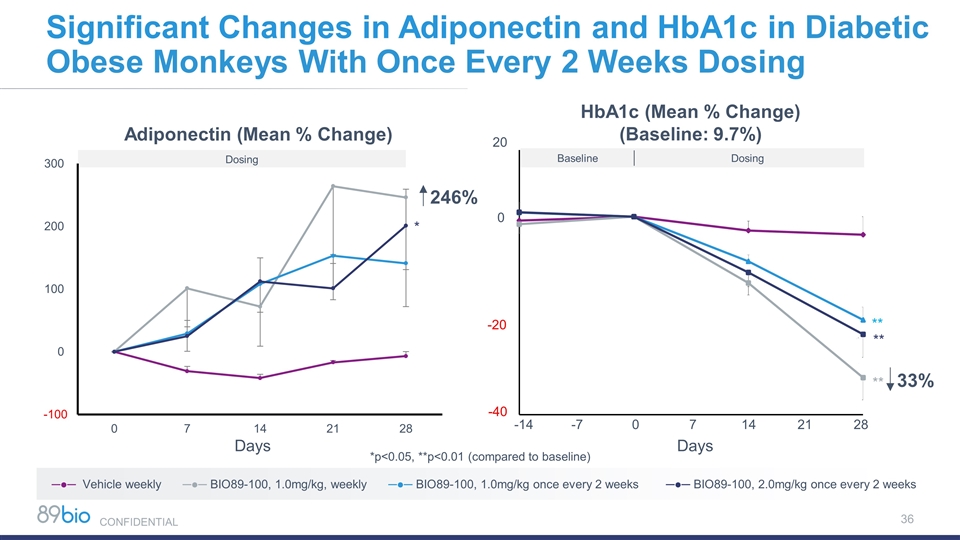
HbA1c (Mean % Change) (Baseline: 9.7%) Dosing Baseline 20 0 -20 -40 -14 -7 0 7 14 21 28 Days 33% ** ** ** ─●─ Vehicle weekly ─●─ BIO89-100, 1.0mg/kg, weekly ─●─ BIO89-100, 1.0mg/kg once every 2 weeks ─●─ BIO89-100, 2.0mg/kg once every 2 weeks Significant Changes in Adiponectin and HbA1c in Diabetic Obese Monkeys With Once Every 2 Weeks Dosing *p<0.05, **p<0.01 (compared to baseline) Days Adiponectin (Mean % Change) 246% * Dosing CONFIDENTIAL
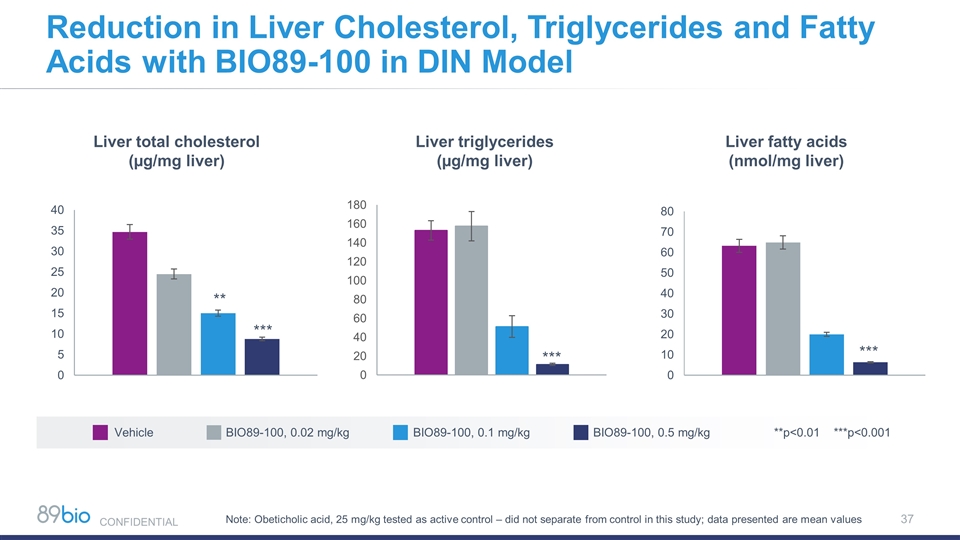
Liver total cholesterol (µg/mg liver) Liver triglycerides (µg/mg liver) Liver fatty acids (nmol/mg liver) Note: Obeticholic acid, 25 mg/kg tested as active control – did not separate from control in this study; data presented are mean values Vehicle BIO89-100, 0.02 mg/kg BIO89-100, 0.1 mg/kg BIO89-100, 0.5 mg/kg **p<0.01 ***p<0.001 Reduction in Liver Cholesterol, Triglycerides and Fatty Acids with BIO89-100 in DIN Model CONFIDENTIAL
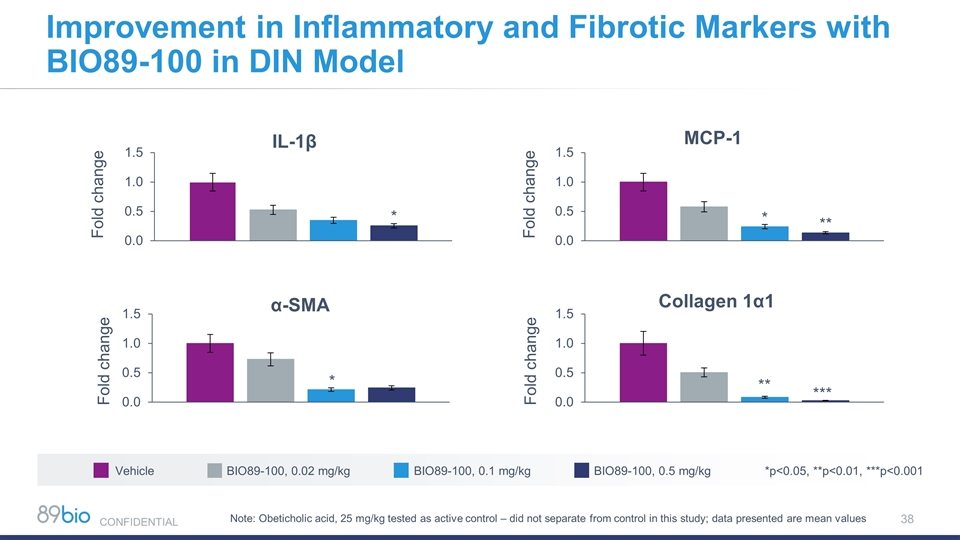
Improvement in Inflammatory and Fibrotic Markers with BIO89-100 in DIN Model Vehicle BIO89-100, 0.02 mg/kg BIO89-100, 0.1 mg/kg BIO89-100, 0.5 mg/kg *p<0.05, **p<0.01, ***p<0.001 *** ** * * Note: Obeticholic acid, 25 mg/kg tested as active control – did not separate from control in this study; data presented are mean values CONFIDENTIAL
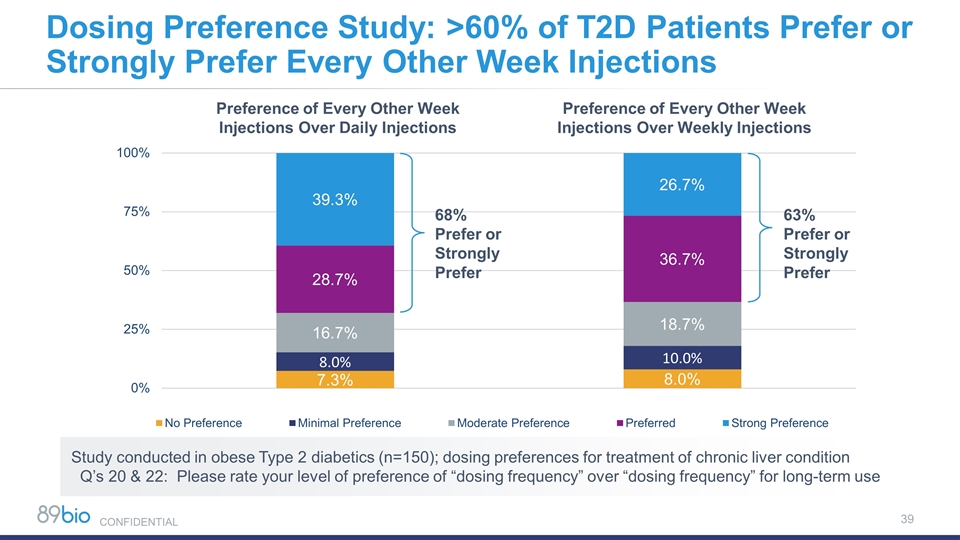
Dosing Preference Study: >60% of T2D Patients Prefer or Strongly Prefer Every Other Week Injections 68% Prefer or Strongly Prefer 63% Prefer or Strongly Prefer Preference of Every Other Week Injections Over Daily Injections Preference of Every Other Week Injections Over Weekly Injections Study conducted in obese Type 2 diabetics (n=150); dosing preferences for treatment of chronic liver condition Q’s 20 & 22: Please rate your level of preference of “dosing frequency” over “dosing frequency” for long-term use CONFIDENTIAL







































