
Corporate Overview February 28, 2023 Clinical stage biopharmaceutical company focused �on the development of first-in-class therapeutics for �patients with autoimmune disease. Exhibit 99.3

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Annual Report on Form 10-K for the year ended December 31, 2021. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this prospectus involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. NX-13 PH. 1b in UC Forward Looking Statements

SINGULAR FOCUS on advancing clinical development of NX-13 in Ulcerative Colitis (UC) NX-13 is a potential GAMECHANGER, with a novel mechanism of action (MOA), impressive safety profile, once-daily dosing with promising and early signals of clinical improvement (as soon as 2 weeks in patients’ symptoms and 4 weeks by endoscopy in exploratory endpoints) NX-13 IS CLINIC READY NOW and expected to enter Phase 2 in Q2’23; top-line data expected in Q4’24 Cash to fund planned operations into first half of 2025 Significant OPTIONALITY portfolio-wide for partnerships, development, and investment in the future Strong IP position EXPERIENCED management team with significant immunology & gastroenterology expertise Landos’ Path Forward Note: Cash includes cash, cash equivalents and marketable securities
Landos’ Novel �Immunology Portfolio 2 8 4 Novel target (NLRX1 and PLXDC2) libraries of immunometabolic modulation pathways Potentially first-in-class, once-daily, oral therapeutics upstream of multiple canonical inflammatory/ regulatory pathways Indications in the immunology space targeted by broadly applicable MOAs

Broad Portfolio of Clinical & �Pre-clinical Programs NX-13 (entering Phase 2 for UC): Novel, oral, gut-selective NLRX1 agonist in development for the treatment of Ulcerative Colitis and Crohn’s Disease as a once-daily treatment LABP-66 (pre-clinical): Novel, oral, once-daily, product candidate targeting NLRX1 in development for Multiple Sclerosis and Alzheimer’s Disease LABP-73 (pre-clinical): Novel, oral, once-daily, product candidate targeting NLRX1 in development for Asthma and COPD LABP-69 (pre-clinical): Novel, oral, once-daily, product candidate targeting PLXDC2 in development for Rheumatoid Arthritis and Diabetic Nephropathy Our Focus: Advancing NX-13 Clinical Development in UC
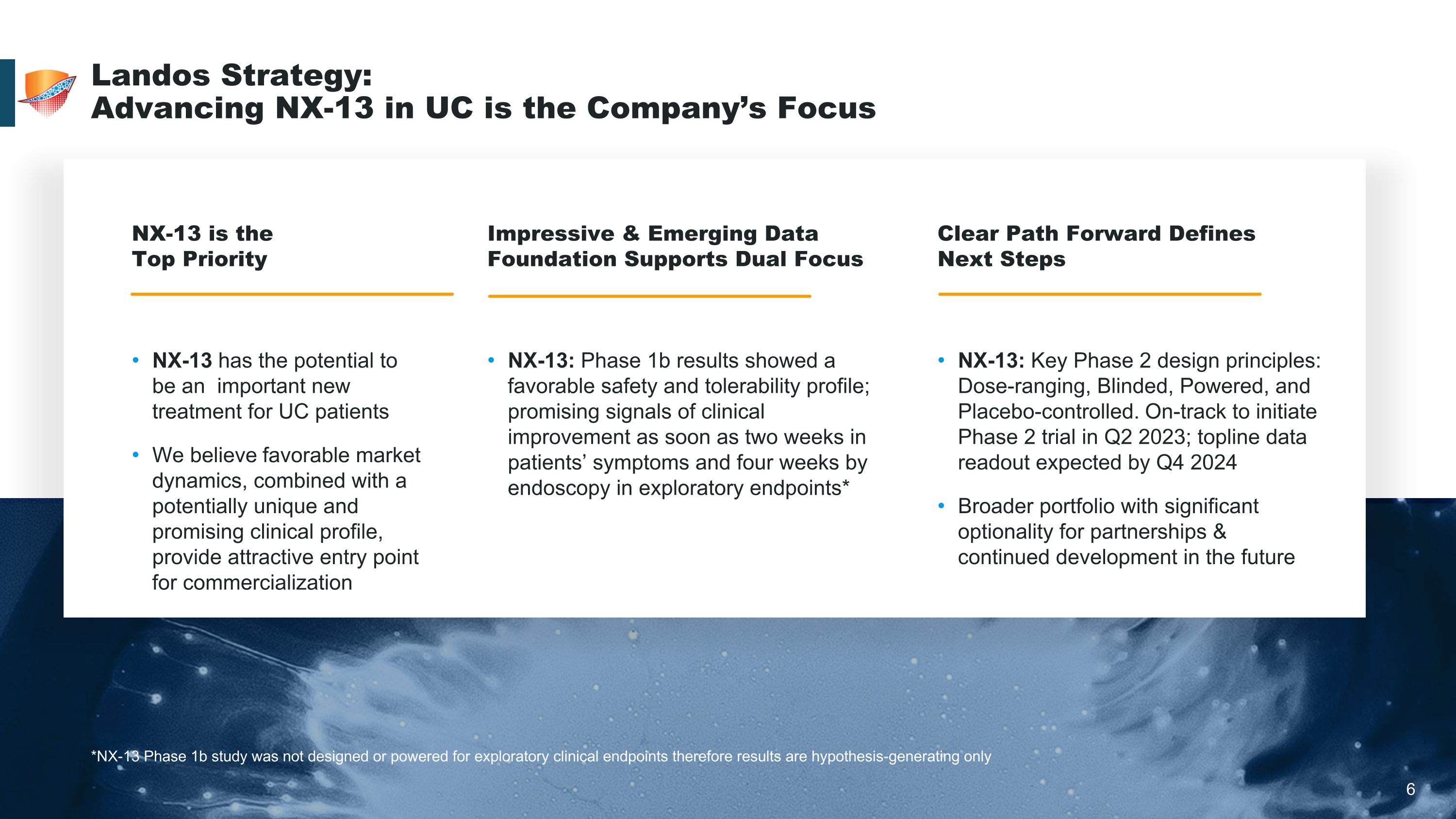
6 Landos Strategy: �Advancing NX-13 in UC is the Company’s Focus *NX-13 Phase 1b study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only NX-13 is the �Top Priority Impressive & Emerging Data Foundation Supports Dual Focus Clear Path Forward Defines �Next Steps NX-13 has the potential to be an important new treatment for UC patients We believe favorable market dynamics, combined with a potentially unique and promising clinical profile, provide attractive entry point for commercialization NX-13: Phase 1b results showed a favorable safety and tolerability profile; promising signals of clinical improvement as soon as two weeks in patients’ symptoms and four weeks by endoscopy in exploratory endpoints* NX-13: Key Phase 2 design principles: Dose-ranging, Blinded, Powered, and Placebo-controlled. On-track to initiate Phase 2 trial in Q2 2023; topline data readout expected by Q4 2024 Broader portfolio with significant optionality for partnerships & continued development in the future
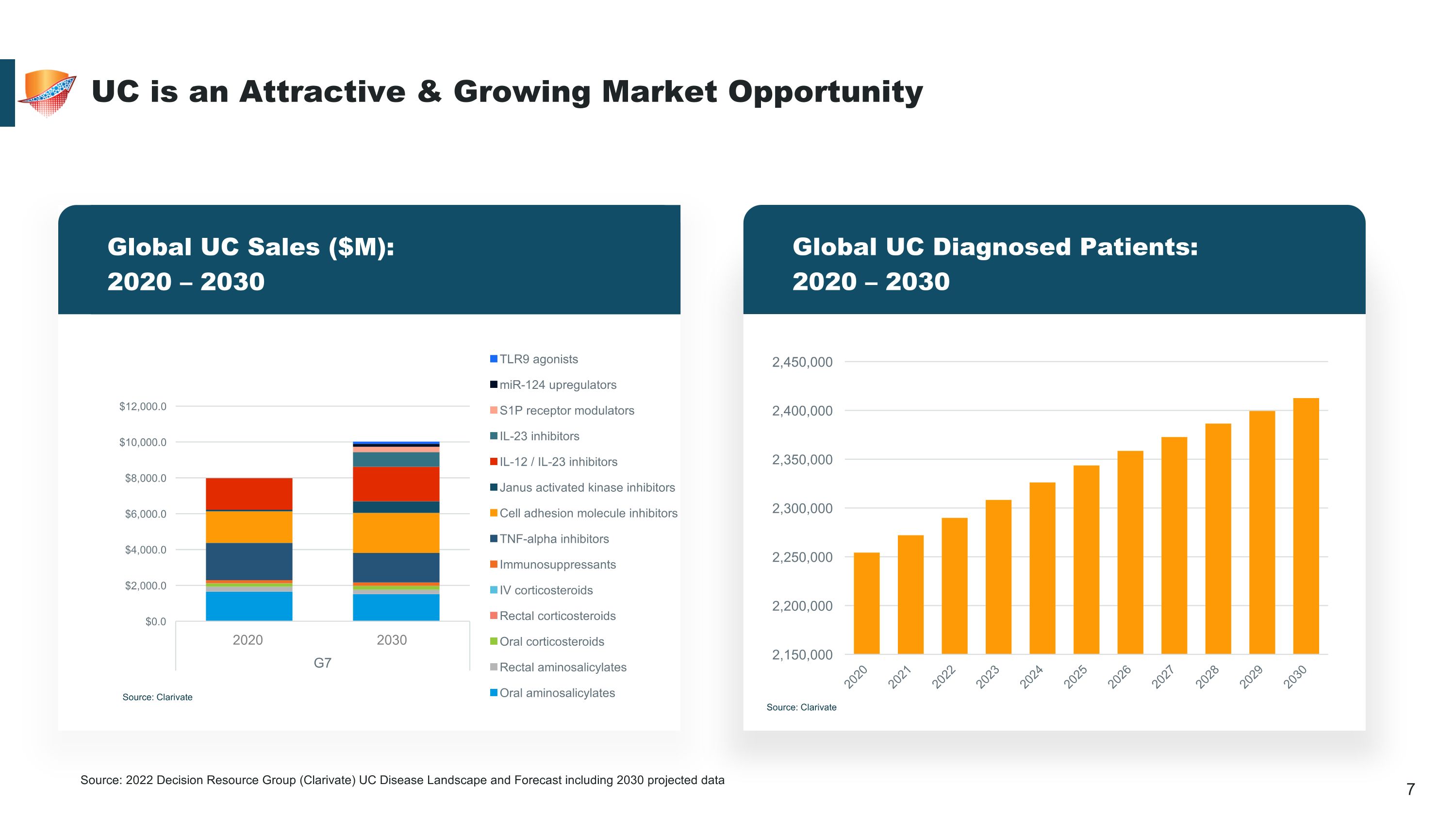
Global Sales ($M): 2020 and 2030 UC is an Attractive & Growing Market Opportunity Source: Clarivate 2020 2030 Global UC Diagnosed Patients: �2020 – 2030 Source: Clarivate Source: 2022 Decision Resource Group (Clarivate) UC Disease Landscape and Forecast including 2030 projected data Global UC Sales ($M): �2020 – 2030
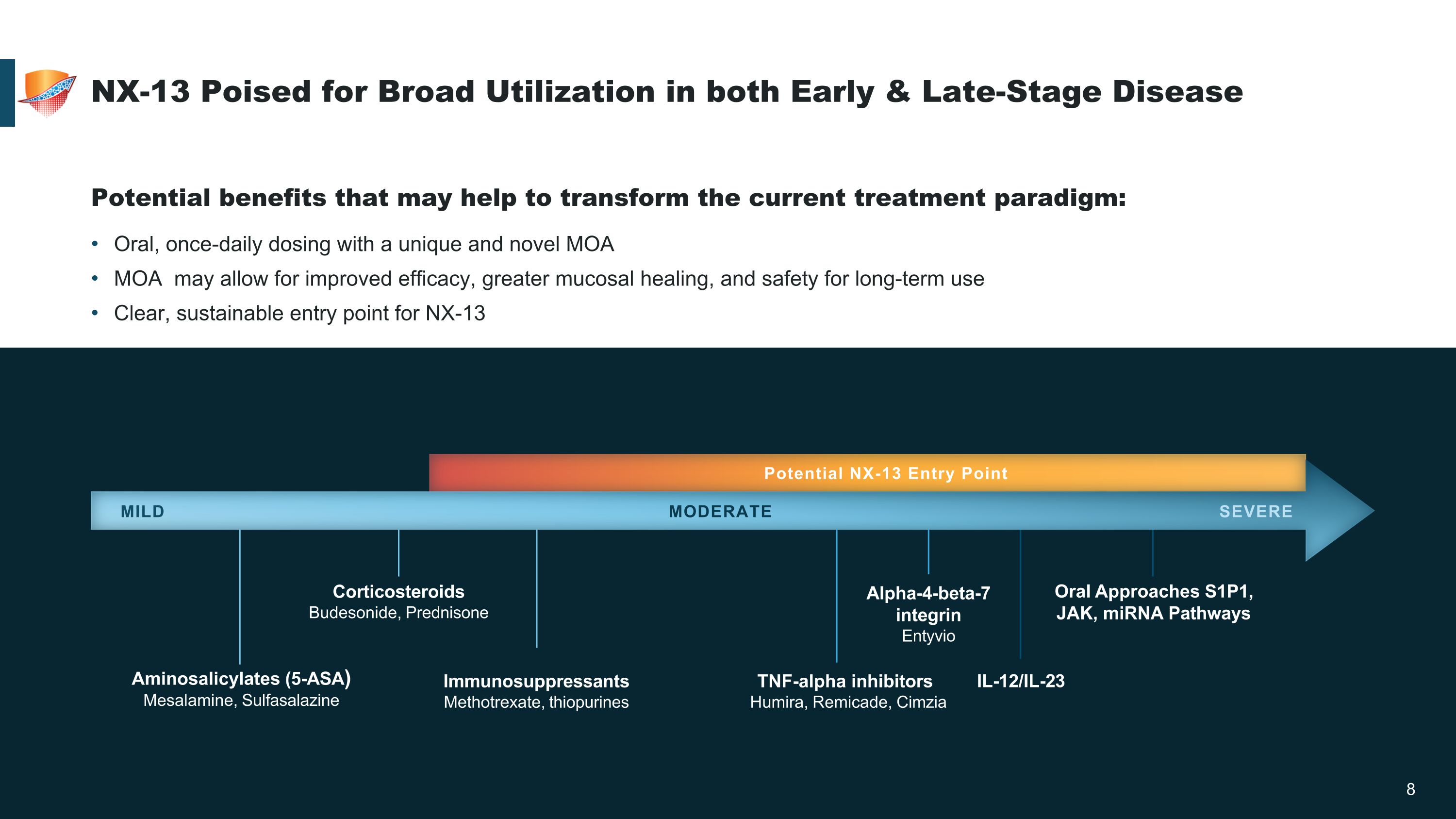
Aminosalicylates (5-ASA) Mesalamine, Sulfasalazine Immunosuppressants Methotrexate, thiopurines Corticosteroids Budesonide, Prednisone Alpha-4-beta-7 �integrin Entyvio TNF-alpha inhibitors Humira, Remicade, Cimzia IL-12/IL-23 Oral Approaches S1P1, �JAK, miRNA Pathways MILD MODERATE SEVERE NX-13 Poised for Broad Utilization in both Early & Late-Stage Disease Potential benefits that may help to transform the current treatment paradigm: Oral, once-daily dosing with a unique and novel MOA MOA may allow for improved efficacy, greater mucosal healing, and safety for long-term use Clear, sustainable entry point for NX-13 Potential NX-13 Entry Point

NLRX1 �Library
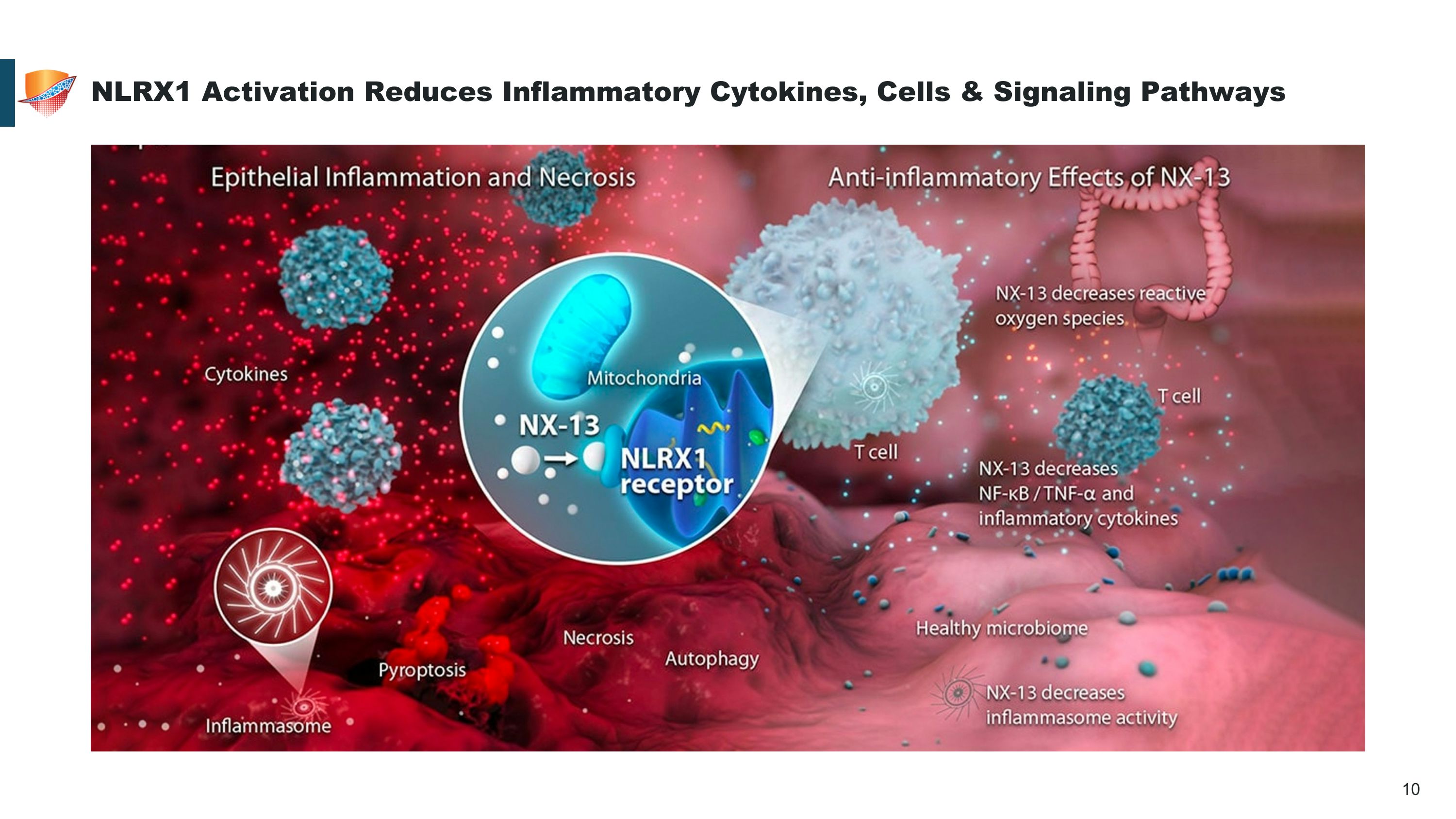
NLRX1 Activation Reduces Inflammatory Cytokines, Cells & Signaling Pathways
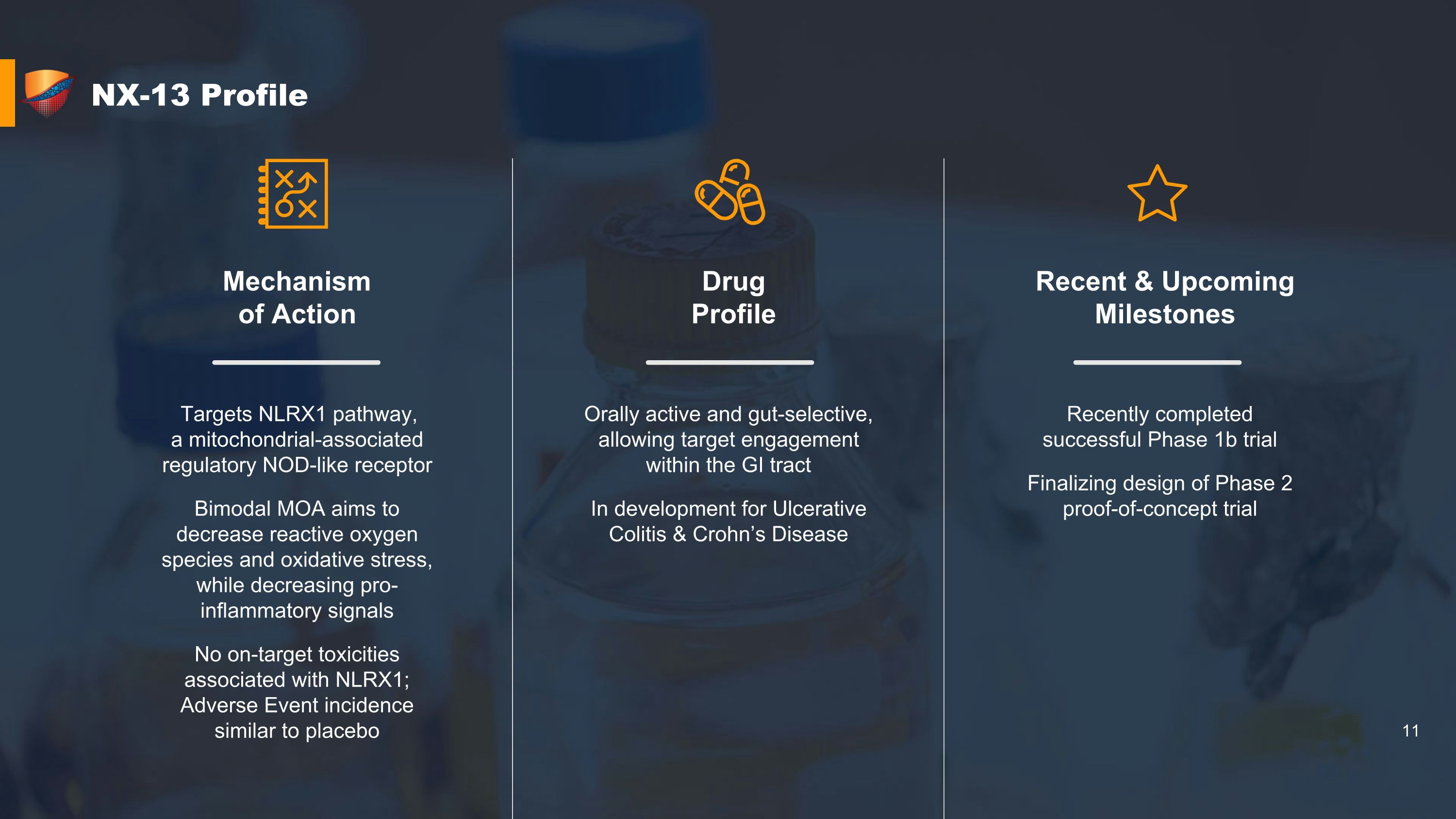
NX-13 Profile Mechanism �of Action Drug �Profile Recent & Upcoming Milestones Targets NLRX1 pathway, �a mitochondrial-associated regulatory NOD-like receptor Bimodal MOA aims to decrease reactive oxygen species and oxidative stress, while decreasing pro-inflammatory signals No on-target toxicities associated with NLRX1; Adverse Event incidence similar to placebo Orally active and gut-selective, allowing target engagement within the GI tract In development for Ulcerative Colitis & Crohn’s Disease Recently completed successful Phase 1b trial Finalizing design of Phase 2 proof-of-concept trial
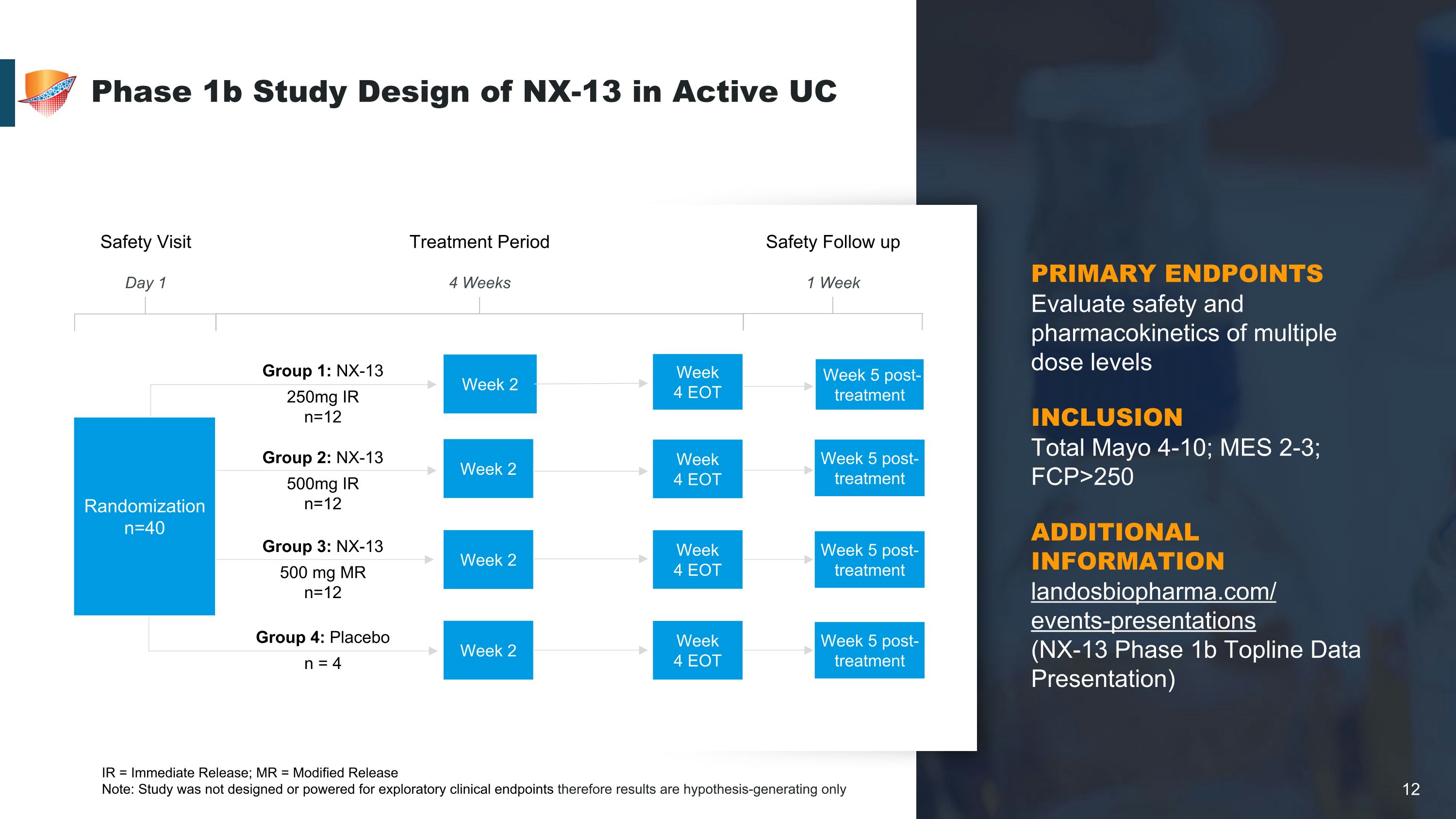
Phase 1b Study Design of NX-13 in Active UC IR = Immediate Release; MR = Modified Release Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only Group 1: NX-13 250mg IR n=12 Group 4: Placebo n = 4 Week 2 Week �4 EOT Group 3: NX-13 500 mg MR n=12 Week 5 post-treatment Week 2 Week �4 EOT Week 5 post-treatment Week 2 Week �4 EOT Week 5 post-treatment Week 2 Week �4 EOT Week 5 post-treatment Group 2: NX-13 500mg IR n=12 Safety Follow up 1 Week Treatment Period 4 Weeks Safety Visit Day 1 Randomization n=40 PRIMARY ENDPOINTS Evaluate safety and pharmacokinetics of multiple dose levels INCLUSION�Total Mayo 4-10; MES 2-3; FCP>250 ADDITIONAL INFORMATION�landosbiopharma.com/�events-presentations �(NX-13 Phase 1b Topline Data Presentation)
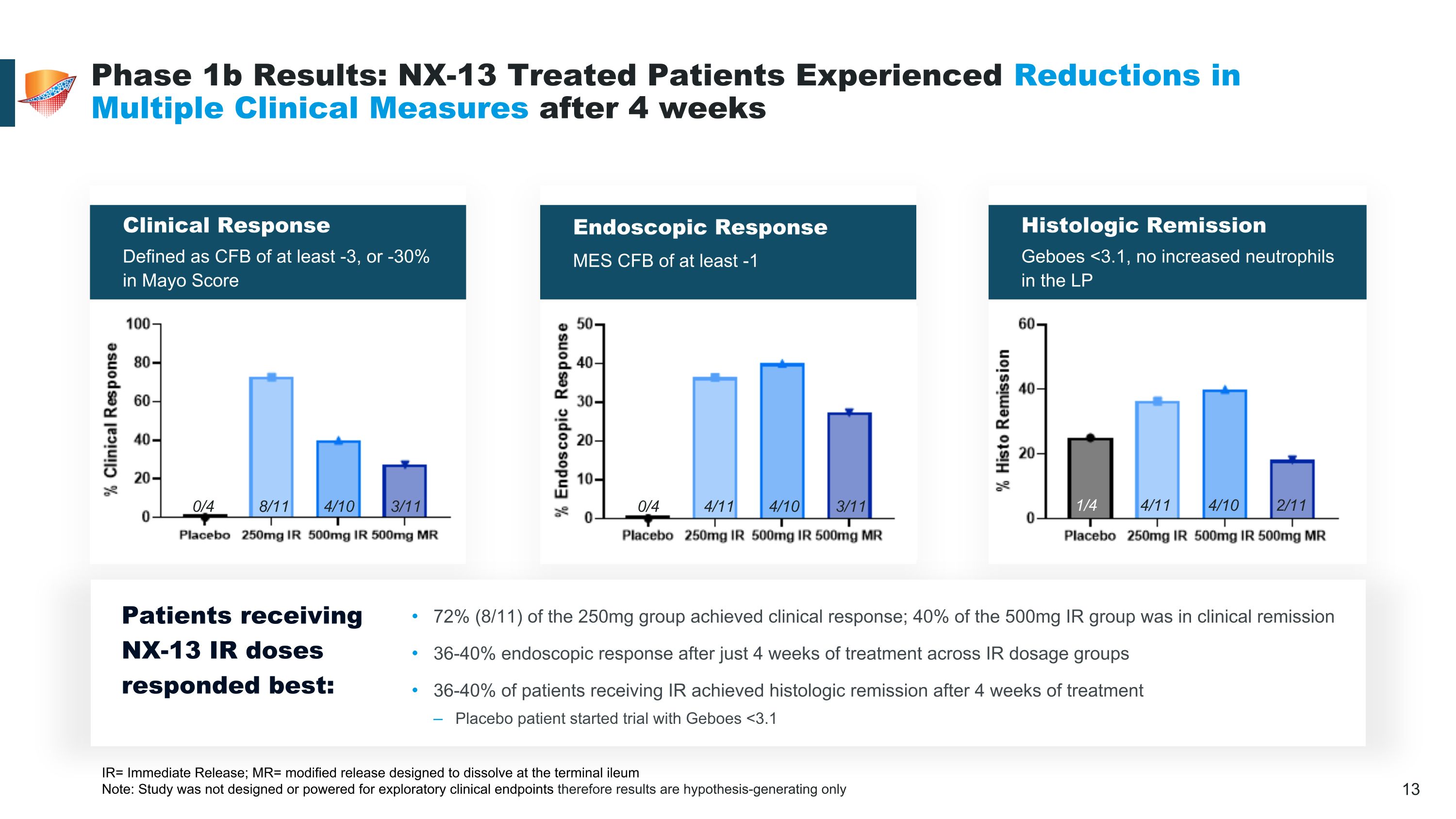
Clinical Response Defined as CFB of at least -3, or -30% in Mayo Score Histologic Remission Geboes <3.1, no increased neutrophils in the LP Endoscopic Response MES CFB of at least -1 Phase 1b Results: NX-13 Treated Patients Experienced Reductions in Multiple Clinical Measures after 4 weeks IR= Immediate Release; MR= modified release designed to dissolve at the terminal ileum Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only 0/4 8/11 4/10 3/11 0/4 4/11 4/10 3/11 1/4 4/11 4/10 2/11 Patients receiving NX-13 IR doses responded best: 72% (8/11) of the 250mg group achieved clinical response; 40% of the 500mg IR group was in clinical remission 36-40% endoscopic response after just 4 weeks of treatment across IR dosage groups 36-40% of patients receiving IR achieved histologic remission after 4 weeks of treatment Placebo patient started trial with Geboes <3.1
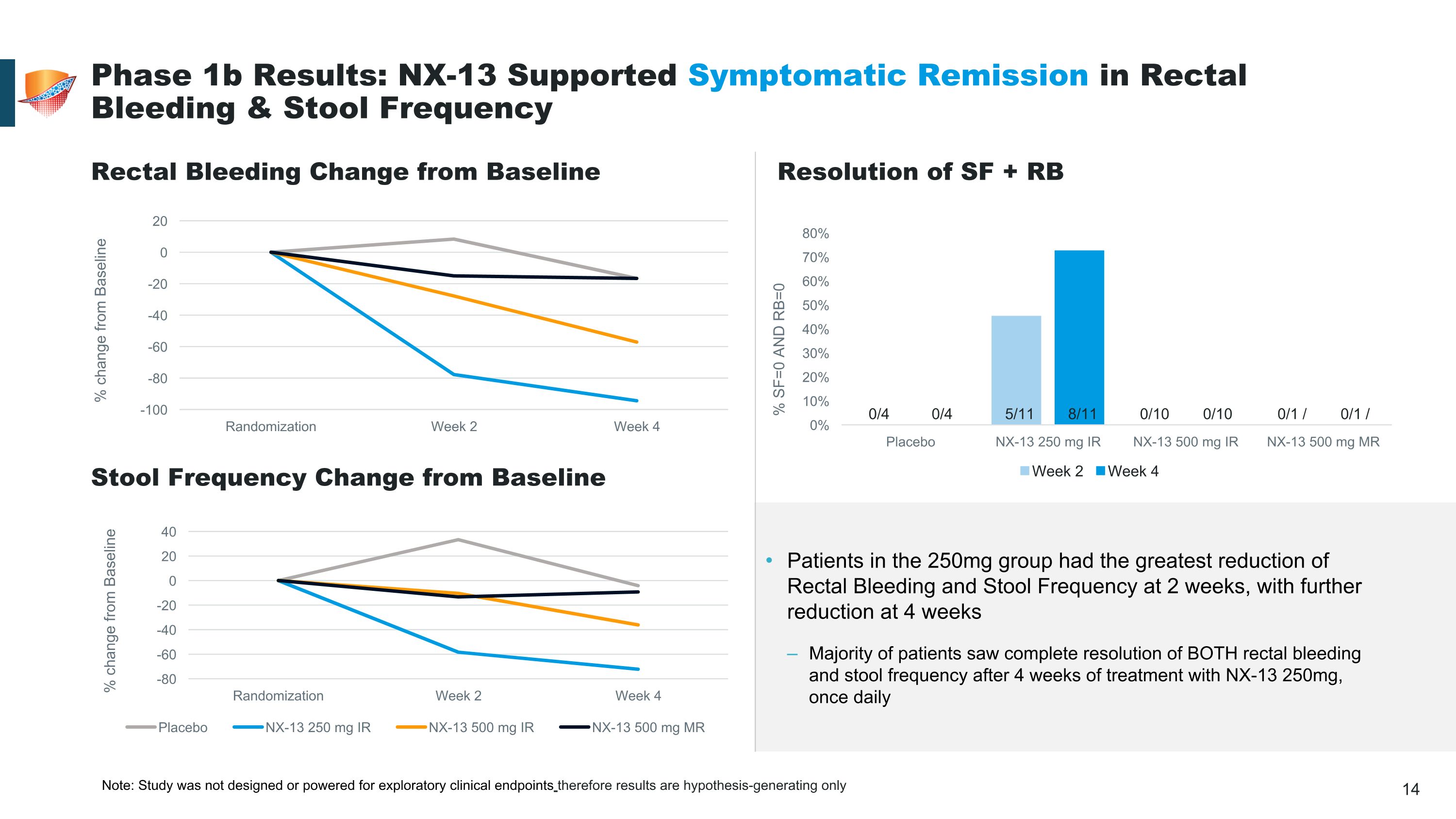
Patients in the 250mg group had the greatest reduction of Rectal Bleeding and Stool Frequency at 2 weeks, with further reduction at 4 weeks Majority of patients saw complete resolution of BOTH rectal bleeding and stool frequency after 4 weeks of treatment with NX-13 250mg, once daily Phase 1b Results: NX-13 Supported Symptomatic Remission in Rectal Bleeding & Stool Frequency Rectal Bleeding Change from Baseline Stool Frequency Change from Baseline Resolution of SF + RB Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only
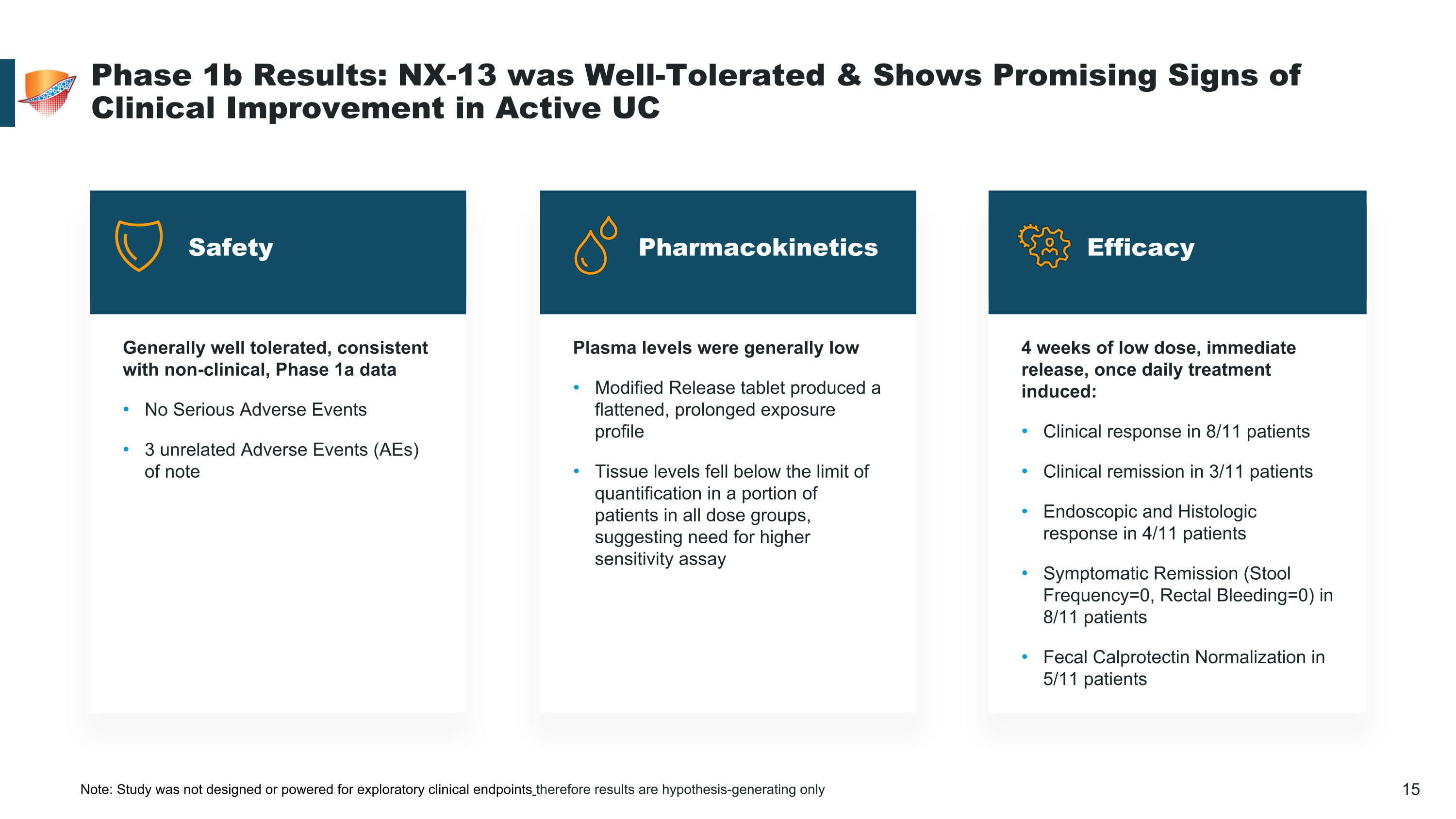
Generally well tolerated, consistent with non-clinical, Phase 1a data No Serious Adverse Events 3 unrelated Adverse Events (AEs) of note 4 weeks of low dose, immediate release, once daily treatment induced: Clinical response in 8/11 patients Clinical remission in 3/11 patients Endoscopic and Histologic response in 4/11 patients Symptomatic Remission (Stool Frequency=0, Rectal Bleeding=0) in 8/11 patients Fecal Calprotectin Normalization in 5/11 patients Plasma levels were generally low Modified Release tablet produced a flattened, prolonged exposure profile Tissue levels fell below the limit of quantification in a portion of patients in all dose groups, suggesting need for higher sensitivity assay Phase 1b Results: NX-13 was Well-Tolerated & Shows Promising Signs of Clinical Improvement in Active UC Safety Efficacy Pharmacokinetics Note: Study was not designed or powered for exploratory clinical endpoints therefore results are hypothesis-generating only

What’s Next: Study Design for NX-13 Phase 2 Proof of Concept Trial GOAL | Evaluate the safety, efficacy and pharmacokinetics of NX-13 in moderate to severe UC patients. TIMING | On-track to initiate Phase 2 trial in Q2 2023; Expecting to report topline data by Q4 2024 ADDITIONAL PHASE 2 LEARNINGS | Dose-Exposure-Response relationships and PK/PD relationships (including site and mechanism of action) Key Design Principles: Dose-Ranging Placebo Controlled Powered Blinded
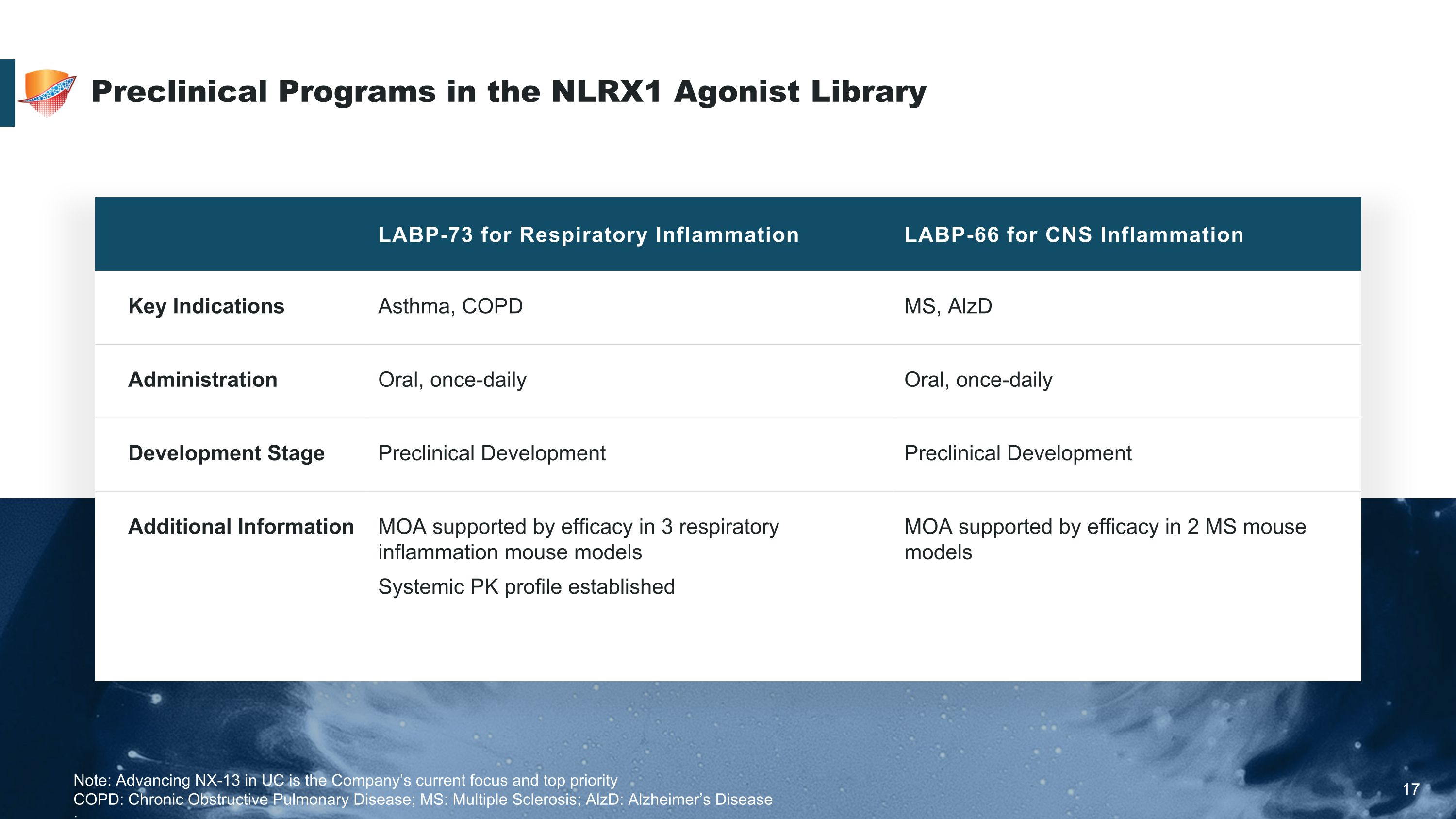
Preclinical Programs in the NLRX1 Agonist Library 17 LABP-73 for Respiratory Inflammation LABP-66 for CNS Inflammation Key Indications Asthma, COPD MS, AlzD Administration Oral, once-daily Oral, once-daily Development Stage Preclinical Development Preclinical Development Additional Information MOA supported by efficacy in 3 respiratory inflammation mouse models Systemic PK profile established MOA supported by efficacy in 2 MS mouse models Note: Advancing NX-13 in UC is the Company’s current focus and top priority COPD: Chronic Obstructive Pulmonary Disease; MS: Multiple Sclerosis; AlzD: Alzheimer’s Disease ;

PLXDC2 �Library
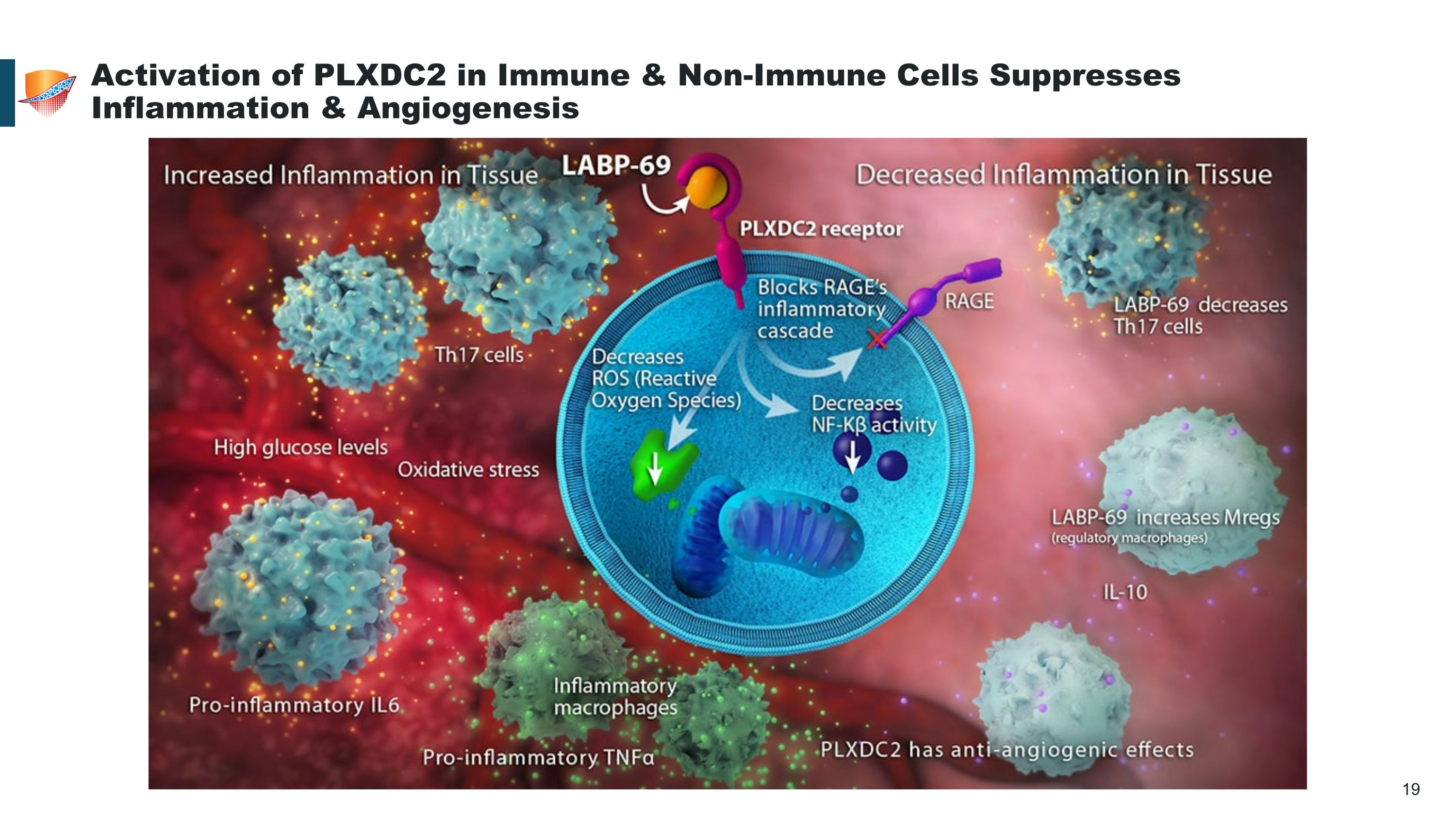
Activation of PLXDC2 in Immune & Non-Immune Cells Suppresses Inflammation & Angiogenesis

Preclinical Programs in the PLXDC2 Agonist Library 20 LABP-69 Key Indications Rheumatoid Arthritis (“RA”), Diabetic Nephropathy Administration Oral, once-daily Development Stage Preclinical Development Additional Information MOA: Designed to decrease reactive oxygen species, oxidative stress, pro-inflammatory signals & angiogenesis MOA: Evidence in 2 RA rodent models Note: Advancing NX-13 in UC is the Company’s current focus and top priority





















