
Exhibit 99.1 th Investor Relations Event at the 66 ASH Annual Meeting December 9, 2024

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts contained in this presentation, including, but not limited to, the following are forward-looking statements: statements regarding the attributes of the D-Domain and its potential benefits; the safety and efficacy profiles of anito-cel, its potential to be best-in-class, the speed, reliability and scalability of its manufacturing, the ability of patients to access anito-cel, and growth opportunities for anito-cel; enrollment in the iMMagine-3 study; our future financial condition, results, strategy, operations and prospects; plans and timelines for the preclinical and clinical development of our product candidates, including the therapeutic potential, clinical benefits and safety profiles; expectations regarding timing, success and data announcements of our preclinical studies and clinical trials, the initiation, timing, progress and results of our current and future preclinical studies and clinical trials; our plans to develop and commercialize our current and future product candidates, alone or with other parties; the plans and objectives of management; and the industry size and trends and market opportunities. In some cases, you can identify forward-looking statements by terminology such as “assume,” “believe,” “can,” “contemplate,” “continue,” “could,” “design,” “estimate,” “expect,” “imagine,” “intend,” “likely,” “may,” “might,” “objective,” “ongoing,” “plan,” “potential,” “predict,” “project,” “seek” “should,” “target,” “will” or “would,” or the negative of these terms or other similar expressions or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs, and these statements represent our views as of the date of this presentation. We may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Forward-looking statements are inherently subject to risks and uncertainties, including those set forth in Part II, Item 1A (Risk Factors) in the Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, filed with the Securities and Exchange Commission (SEC) on November 7, 2024, and the other documents that we may file from time to time with the SEC. New risk factors emerge from time to time and it is no possible for our management team to predict all risk factors or assess the impact of all factors on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. While we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. As a result of these risks and others, including those set forth in our filings with the Securities and Exchange Commission (SEC), actual results could vary significantly from those anticipated in this presentation, and our financial condition and results of operations could be materially adversely affected. This presentation discusses product candidates that are under preclinical or clinical evaluation and that have not yet been approved for marketing by the U.S. Food and Drug Administration or any other regulatory authority. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. The presentation also includes select interim and preliminary results from an ongoing clinical trial as of specific data cutoff dates. Such results should be viewed with caution as final results may differ as additional data becomes available. Until finalized in a clinical study report, clinical trial data presented herein remain subject to adjustment as a result of clinical site audits and other review processes. Cross-trial comparisons are not based on head-to-head studies and no direct comparisons can be made. Cross-trial data interpretation should be considered with caution as it is limited by differences in study population, design and other factors. This presentation also contains estimates and other statistical data made by independent parties or publicly available information, as well as other information based on our internal sources. These data involve a number of assumptions and limitations, and we have not independently verified the accuracy or completeness of the data contained in these industry publications and other publicly available information. Accordingly, we make no representations as to the accuracy or completeness of that data. 2 Arcellx | 2024 ASH IR Event

Agenda Opening Remarks 10 min Rami Elghandour Chairman and Chief Executive Officer, Arcellx iMMagine-1 Oral Presentation 20 min Ciara L. Freeman M.D., Ph.D iMMagine-1 Clinical Study Investigator Physician Panel Discussion 30 min Q&A 30 min 3 Arcellx | 2024 ASH IR Event

Potential best-in-class therapy partnered with Kite, the global leader in cell therapy. Scalable manufacturing and commercial footprint to support a Different Kind leadership in a $12B+ Multiple Myeloma cell therapy market. of Cell Therapy Company Sufficient capital to fund operations into 2027.

Anitocabtagene Autoleucel (anito-cel/CART-ddBCMA) 1,2 Autologous BCMA-directed CAR T-cell therapy using a novel, D-Domain binder Anito-cel attributes from novel D-Domain Small D-Domain construct Low total cell facilitates high transduction dose efficiency and CAR positivity, which permit a low total cell dose Rapid folding, lack of disulfide Lack of tonic bonds, and a hydrophobic core signaling enables D-Domain stability and 5,6 lack of tonic signaling 4 The D-Domain has a fast off-rate 3,4 Optimal and high CAR surface expression. tumor cell This combination may allow scFv Bivalent camelid VHH D-Domain killing optimal tumor cell killing without (~25 kDa) (~30 kDa) (~8 kDa) prolonged inflammation BCMA is B-cell Maturation Agent; CAR T is Chimeric Antigen Receptor T cell 1 2 3 4 5 Rotte, et al. Immuno-Oncology Insights 2022; 3(1), 13–24; Frigault, et al. Blood Adv. 2023; 7(5):768-777; Cante-Barrett, et al. BMC Res. Notes 2016; 9:13; Buonato, et al. Mol. Cancer Ther. 2022; 21(7):1171-1183; Zhu, et al. 6 5 Proc. Nat. Acad. Sci. 2003; 100(26): 15486-15491; Qin, et al. Mol. Ther. 2019; 27(7): 1262-1274 Arcellx | 2024 ASH IR Event
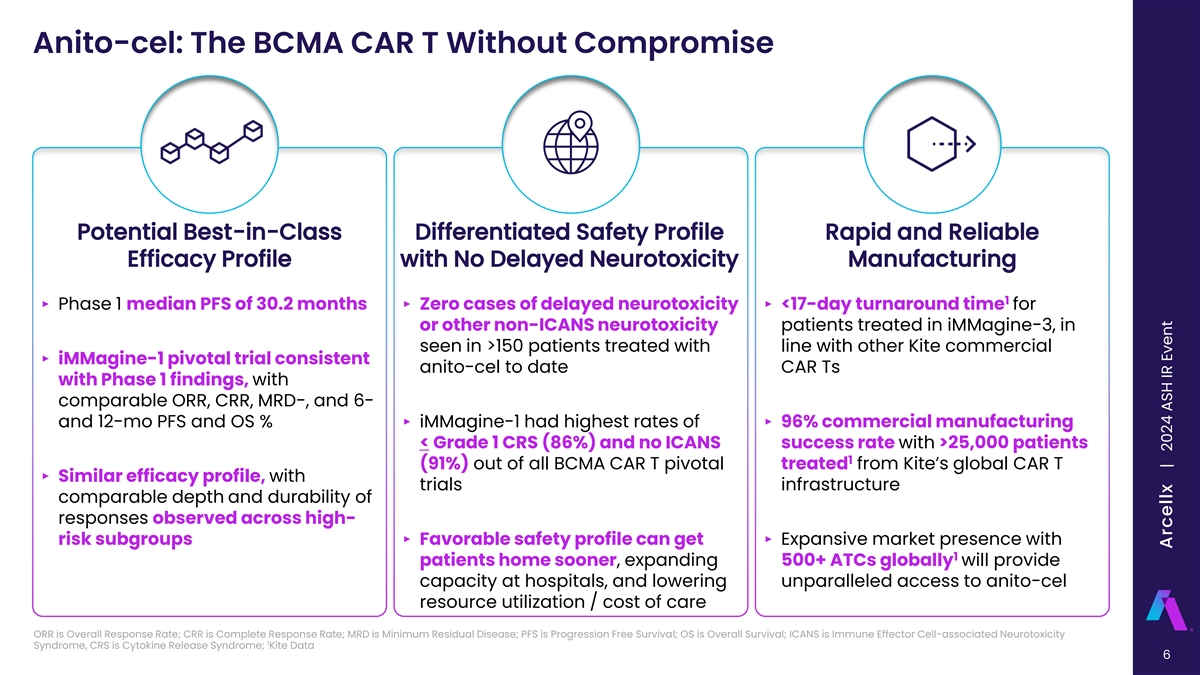
Anito-cel: The BCMA CAR T Without Compromise Potential Best-in-Class Differentiated Safety Profile Rapid and Reliable Efficacy Profile with No Delayed Neurotoxicity Manufacturing 1 ‣ Phase 1 median PFS of 30.2 months‣ Zero cases of delayed neurotoxicity ‣ <17-day turnaround time for or other non-ICANS neurotoxicity patients treated in iMMagine-3, in seen in >150 patients treated with line with other Kite commercial ‣ iMMagine-1 pivotal trial consistent anito-cel to date CAR Ts with Phase 1 findings, with comparable ORR, CRR, MRD-, and 6- and 12-mo PFS and OS %‣ iMMagine-1 had highest rates of ‣ 96% commercial manufacturing < Grade 1 CRS (86%) and no ICANS success rate with >25,000 patients 1 (91%) out of all BCMA CAR T pivotal treated from Kite’s global CAR T ‣ Similar efficacy profile, with trials infrastructure comparable depth and durability of responses observed across high- risk subgroups‣ Favorable safety profile can get ‣ Expansive market presence with 1 patients home sooner, expanding 500+ ATCs globally will provide capacity at hospitals, and lowering unparalleled access to anito-cel resource utilization / cost of care ORR is Overall Response Rate; CRR is Complete Response Rate; MRD is Minimum Residual Disease; PFS is Progression Free Survival; OS is Overall Survival; ICANS is Immune Effector Cell-associated Neurotoxicity 1 Syndrome, CRS is Cytokine Release Syndrome; Kite Data 6 Arcellx | 2024 ASH IR Event
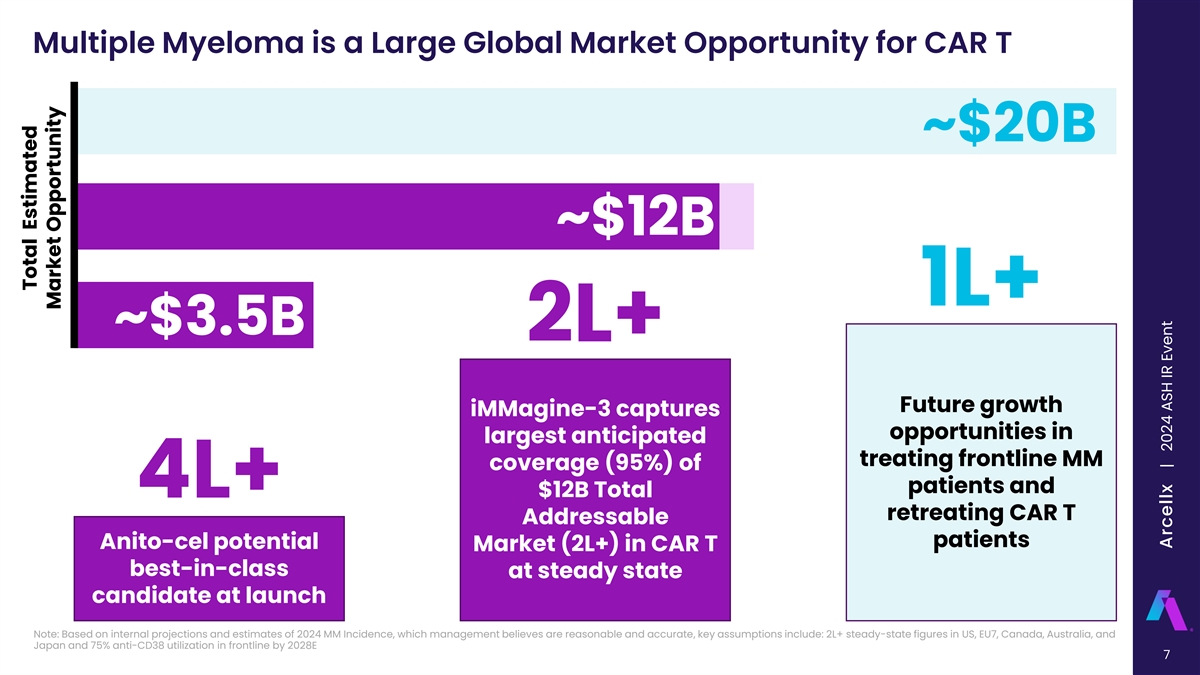
Multiple Myeloma is a Large Global Market Opportunity for CAR T ~$20B ~$12B 1L+ ~$3.5B 2L+ Future growth iMMagine-3 captures opportunities in largest anticipated treating frontline MM coverage (95%) of patients and 4L+ $12B Total retreating CAR T Addressable patients Anito-cel potential Market (2L+) in CAR T best-in-class at steady state candidate at launch Note: Based on internal projections and estimates of 2024 MM Incidence, which management believes are reasonable and accurate, key assumptions include: 2L+ steady-state figures in US, EU7, Canada, Australia, and Japan and 75% anti-CD38 utilization in frontline by 2028E 7 Total Estimated Market Opportunity Arcellx | 2024 ASH IR Event
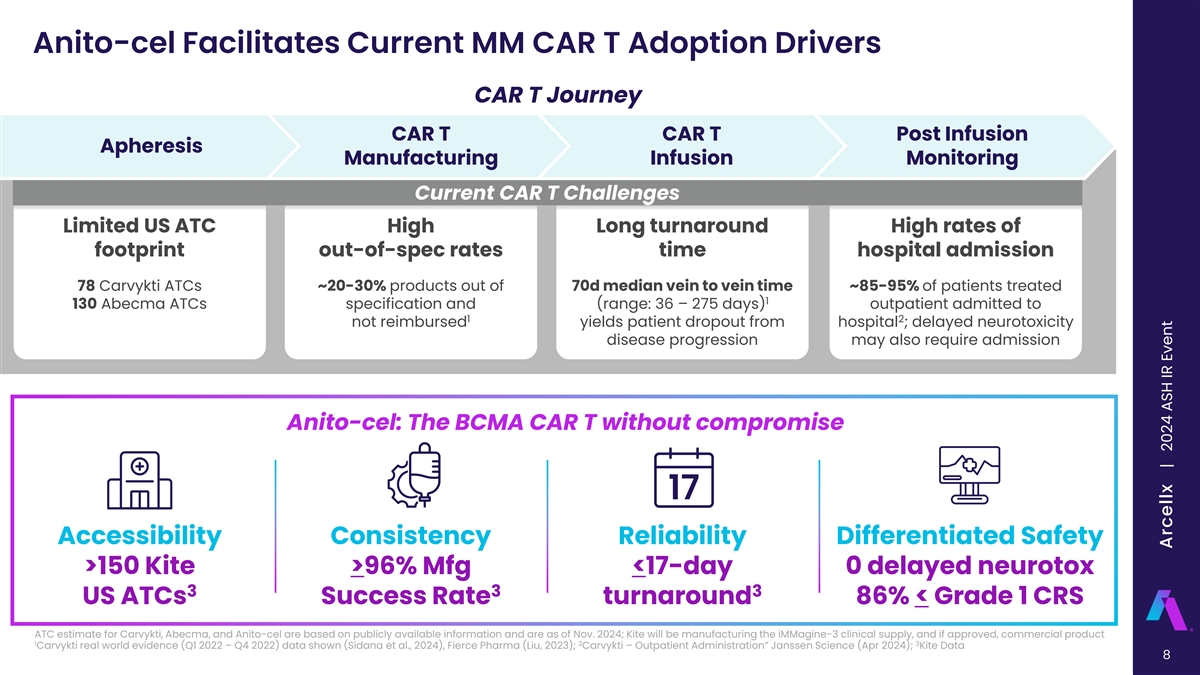
Anito-cel Facilitates Current MM CAR T Adoption Drivers CAR T Journey CAR T CAR T Post Infusion Apheresis Manufacturing Infusion Monitoring Current CAR T Challenges Limited US ATC High Long turnaround High rates of footprint out-of-spec rates time hospital admission 78 Carvykti ATCs ~20-30% products out of 70d median vein to vein time ~85-95% of patients treated 1 130 Abecma ATCs specification and (range: 36 – 275 days) outpatient admitted to 1 2 not reimbursed yields patient dropout from hospital ; delayed neurotoxicity disease progression may also require admission Anito-cel: The BCMA CAR T without compromise Accessibility Consistency Reliability Differentiated Safety >150 Kite >96% Mfg <17-day 0 delayed neurotox 3 3 3 US ATCs Success Rate turnaround 86% < Grade 1 CRS ATC estimate for Carvykti, Abecma, and Anito-cel are based on publicly available information and are as of Nov. 2024; Kite will be manufacturing the iMMagine-3 clinical supply, and if approved, commercial product 1 2 3 Carvykti real world evidence (Q1 2022 – Q4 2022) data shown (Sidana et al., 2024), Fierce Pharma (Liu, 2023); Carvykti – Outpatient Administration” Janssen Science (Apr 2024); Kite Data 8 Arcellx | 2024 ASH IR Event

Anito-cel Can Significantly Expand CAR T Access for MM Patients Anito-cel has seen 0 cases of Parkinsonism, Anito-cel CRS median time to onset ~4 days and Guillain-Barré syndrome, or cranial nerve palsies, median duration ~3 days, potentially limiting time in potentially driving patient volume in earlier lines of hospital and driving outpatient treatment therapy All other 100% 100% BCMA agents 86% (~7% of all cases of 80% 80% delayed neurotoxicity*) Carvykti 60% 60% (~93% of all cases of 40% 40% delayed 17% 20% neurotoxicity*) 20% 0% 0% iMM-1 No CRS iMM-1 Gr < 1 CRS Q1 - Q3 2024 ~92% of iMM-1 patients had no CRS symptoms 10 days Anito-cel has seen 0 cases of delayed or non-ICANS from infusion neurotoxicity to date >80% of CAR T cases have favorable reimbursement through case rate agreements or ASP+ *FDA FAERS Database Query was “CRANIAL NERVE PARALYSIS”, “CRANIAL NERVE PALSIES MULTIPLE”, “GUILLAN-BARRE SYNDROME”, “PARKINSONISM” Komodo claims analysis from 3/1/2022-3/1/2023; Freeman et al., Oral Presentation, ASH (Dec 2024); FDA FAERS Database 9 Arcellx | 2024 ASH IR Event

iMMagine-3 Global Phase 3 Trial with Kite Manufacturing, Currently Enrolling Multi-center, Global, Phase 3 Randomized Controlled Clinical Trial (RCT) for anti-CD38 mAb and IMiD exposed patients ‣ Largest percentage of second line (2L) patients as anti-CD38 mAbs become standard of care in front line (1L) ‣ Anticipate high physician interest in iMMagine-3 based on: o Potential best-in-class product profile o Relevant standard of care alternatives o Rapid and reliable turnaround time with Kite manufacturing ‣ Easy to identify patient population, expected to streamline access to anito-cel post approval ‣ Confirmatory RCT will include ~450 adult patients randomized 1:1 in US and Intl sites mAbs is monoclonal antibodies; IMiD is Immunomodulatory drugs 10 Arcellx | 2024 ASH IR Event

D-Domain Technology: Expansive Platform ARC-SparX Dosable CAR T ddCAR Classical Single Infusion CAR T ‣ Ability to leverage autologous or allogeneic strategies ‣ Therapeutic potential across liquid and solid tumors as well as non-oncology indications 11 CONFIDENTIAL Arcellx | 2024 ASH IR Event

A Rich Development Pipeline with Growth in Mind DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PARTNER Multiple Myeloma iMMagine-1: anito-cel (4L+) iMMagine-3: anito-cel earlier lines (2L+) ACLX-001: BCMA Arc-SparX Acute Myeloid Leukemia ACLX-002: CD123 Arc-SparX ACLX-003 ACLX-004 Non-Oncology Generalized Myasthenia Gravis: anito-cel Solid Tumors SCLC HCC 12 Arcellx | 2024 ASH IR Event

Agenda Opening Remarks 10 min Rami Elghandour Chairman and Chief Executive Officer, Arcellx iMMagine-1 Oral Presentation 20 min Ciara L. Freeman M.D., Ph.D iMMagine-1 Clinical Study Investigator Physician Panel Discussion 30 min Q&A 30 min 13 Arcellx | 2024 ASH IR Event
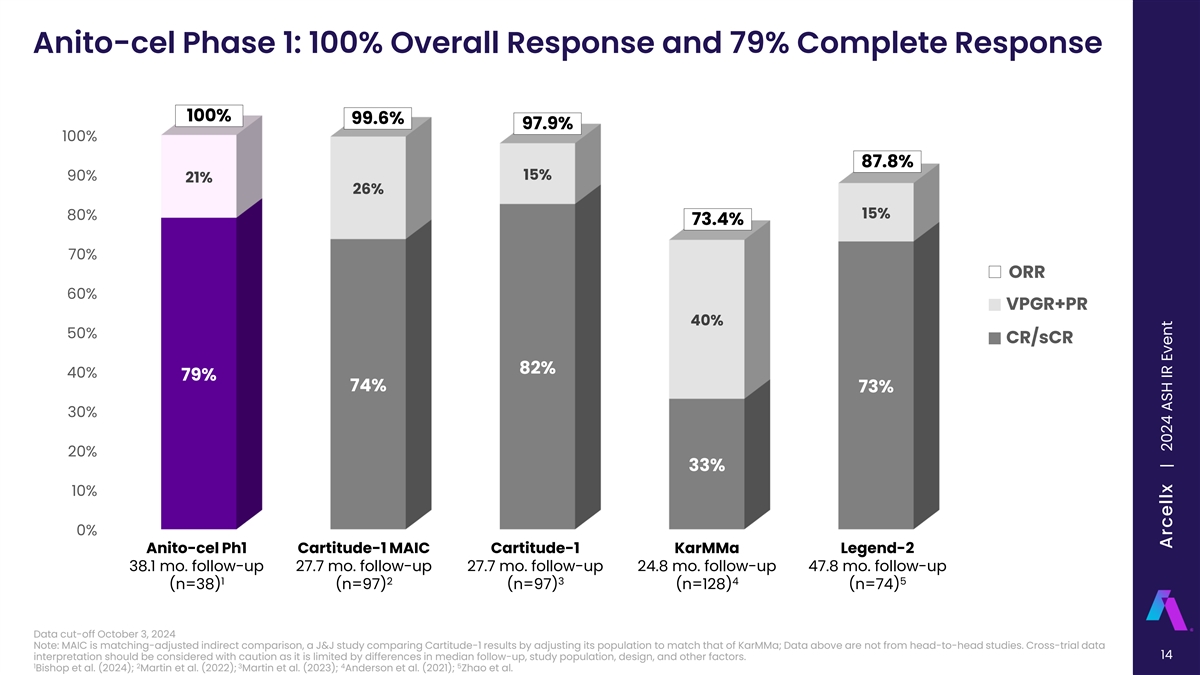
Anito-cel Phase 1: 100% Overall Response and 79% Complete Response 100% 99.6% 97.9% 100% 87.8% 15% 90% 21% 26% 15% 80% 73.4% 70% ORR 60% VPGR+PR 40% 50% CR/sCR 82% 40% 79% 74% 73% 30% 20% 33% 10% 0% Anito-cel Ph1 Cartitude-1 MAIC Cartitude-1 KarMMa Legend-2 38.1 mo. follow-up 27.7 mo. follow-up 27.7 mo. follow-up 24.8 mo. follow-up 47.8 mo. follow-up 1 2 3 4 5 (n=38) (n=97) (n=97) (n=128) (n=74) Data cut-off October 3, 2024 Note: MAIC is matching-adjusted indirect comparison, a J&J study comparing Cartitude-1 results by adjusting its population to match that of KarMMa; Data above are not from head-to-head studies. Cross-trial data 14 interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design, and other factors. 1 2 3 4 5 Bishop et al. (2024); Martin et al. (2022); Martin et al. (2023); Anderson et al. (2021); Zhao et al. Arcellx | 2024 ASH IR Event

Anito-cel Phase 1: Median PFS is 30.2 Months Anito-cel Phase 1 Median Follow-up 38.1 months Minimum Follow-up 25.0 months 95% Time PFS Confidence (months) Estimate (%) Interval (%) 6 92.1 (77.5, 97.4) 12 75.9 (58.7, 86.6) All 18 65.0 (47.5, 78.0) Subjects (n=38) 24 56.6 (39.2, 70.8) Time from Infusion (Months) All Subjects 38 34 28 24 19 12 6 3 2 1 0 30 50.3 (33.0, 65.3) With CR/sCR 30 28 26 23 19 12 6 3 2 1 0 Note: Data cut-off October 3, 2024 Bishop et al, American Society of Hematology 2024, Poster 4825 15 Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: Phase 2 Study Design Lymphodepleting chemotherapy 2 Cyclophosphamide 300 mg/m 2 Fludarabine 30 mg/m Leukapheresis Day -5, -4, -3 Anitocabtagene Anitocabtagene Response and safety autoleucel Long-term safety Screening autoleucel assessments infusion follow-up manufacturing (up to 24 months) Day 0 Bridging therapy if necessary Key Eligibility Criteria Primary Endpoint: • Prior IMiD, PI, and CD38-targeted therapy• ORR, per 2016 IMWG criteria • Received ≥3 prior lines of therapy Key Secondary Endpoints: • Refractory to the last line of therapy • ECOG PS of 0 or 1 • sCR/CR rate, per 2016 IMWG criteria • Evidence of measurable disease • ORR in patients limited to 3 prior LoT, per 2016 IMWG 6 Target Dose of 115 x 10 CAR+ T cells criteria Primary and key secondary endpoints to be assessed per Independent Review Committee (IRC); Investigator assessment of response per IMWG also permitted per protocol. CR, complete response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; IMiD, immunomodulatory drug; IMWG, International Myeloma Working Group; LoT, line of therapy; ORR, overall response rate; PI, 16 proteosome inhibitor; sCR, stringent complete response. Freeman et al., Oral Presentation, ASH (Dec 2024) | Freeman et al, American Society of Hematology 2024, Abstract 1031 Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: Overall Patient Disposition and Evaluable Populations Enrolled (Leukapheresed) Discontinued: n=11 n=129 • Withdrawal of Consent, n=4 • Disease Progression, n=4 • Intercurrent Illness, n=1 • Investigator Decision, n=1 Lymphodepletion • No longer eligible due to myocardial infarction, n=1 n=118 Discontinued: n=1 • Withdrawal of Consent, n=1 Total Dosed n=117 Safety Evaluable Efficacy Evaluable n=98 n=86 Subjects followed for ≥1 month by data Subjects followed for ≥2 months by cut-off of October 31, 2024 are data cut-off of October 31, 2024 are evaluable for safety analysis evaluable for efficacy analysis Anito-cel was successfully manufactured for 99% of patients enrolled Total Patients Dosed per clinical database as of presentation date [12/09/2024] Freeman et al., Oral Presentation, ASH (Dec 2024) 17 | Freeman et al, American Society of Hematology 2024, Abstract 1031 Arcellx | 2024 ASH IR Event
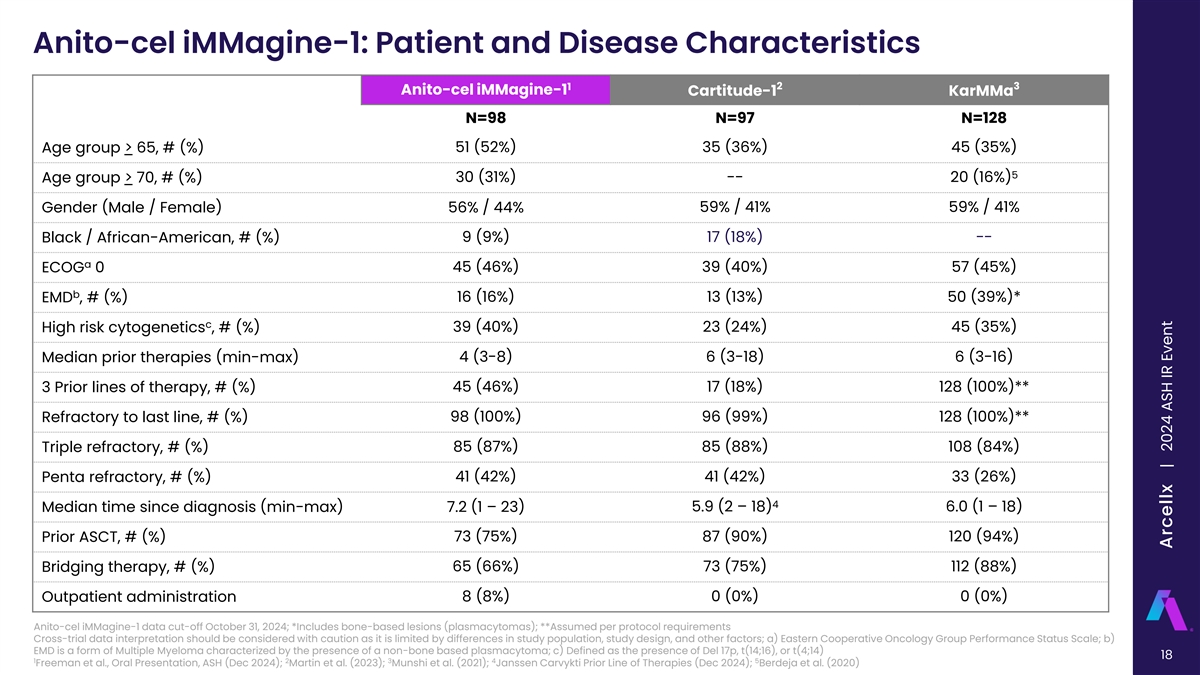
Anito-cel iMMagine-1: Patient and Disease Characteristics 1 2 3 Anito-cel iMMagine-1 Cartitude-1 KarMMa N=98 N=97 N=128 Age group > 65, # (%) 51 (52%) 35 (36%) 45 (35%) 5 Age group > 70, # (%) 30 (31%) -- 20 (16%) Gender (Male / Female) 56% / 44% 59% / 41% 59% / 41% Black / African-American, # (%) 9 (9%) 17 (18%) -- a ECOG 0 45 (46%) 39 (40%) 57 (45%) b EMD , # (%) 16 (16%) 13 (13%) 50 (39%)* c High risk cytogenetics , # (%) 39 (40%) 23 (24%) 45 (35%) Median prior therapies (min-max) 4 (3-8) 6 (3-18) 6 (3-16) 3 Prior lines of therapy, # (%) 45 (46%) 17 (18%) 128 (100%)** Refractory to last line, # (%) 98 (100%) 96 (99%) 128 (100%)** 85 (87%) 85 (88%) 108 (84%) Triple refractory, # (%) 41 (42%) 41 (42%) 33 (26%) Penta refractory, # (%) 4 5.9 (2 – 18) 6.0 (1 – 18) Median time since diagnosis (min-max) 7.2 (1 – 23) 73 (75%) 87 (90%) 120 (94%) Prior ASCT, # (%) 65 (66%) 73 (75%) 112 (88%) Bridging therapy, # (%) 8 (8%) 0 (0%) 0 (0%) Outpatient administration Anito-cel iMMagine-1 data cut-off October 31, 2024; *Includes bone-based lesions (plasmacytomas); **Assumed per protocol requirements Cross-trial data interpretation should be considered with caution as it is limited by differences in study population, study design, and other factors; a) Eastern Cooperative Oncology Group Performance Status Scale; b) EMD is a form of Multiple Myeloma characterized by the presence of a non-bone based plasmacytoma; c) Defined as the presence of Del 17p, t(14;16), or t(4;14) 18 1 2 3 4 5 Freeman et al., Oral Presentation, ASH (Dec 2024); Martin et al. (2023); Munshi et al. (2021); Janssen Carvykti Prior Line of Therapies (Dec 2024); Berdeja et al. (2020) Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: Overall Response Rate and MRD Negativity Efficacy Evaluable Patients (N=86) ORR=97% 15% At a median follow-up of 9.5 months, ORR was 97% and sCR/CR rate was 62% 20% 93.1% (n=54/58) of evaluable patients were -5 ≥VGPR MRD negative at minimum of 10 sensitivity 81% sCR/CR 62% 62% Evaluable Months Patients (min - max) Median time to first response 83 1.0 (0.9 - 7.3) -5 Median time to MRD negativity of ≤10 54 1.0 (0.9 - 6.4) Efficacy Evaluable Patients (N=86) Best Response: sCR/CR VGPR PR Responses are investigator assessed per IMWG criteria, ORR defined as partial response or better; MRD evaluable patients had an identifiable malignant clone in the baseline bone marrow sample and had a post- treatment bone marrow sample sufficient to assess MRD negativity; CR, complete response; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very 19 good partial response; Freeman et al., Oral Presentation, ASH (Dec 2024), Data cut-off October 31, 2024 | Freeman et al, American Society of Hematology 2024, Abstract 1031 Arcellx | 2024 ASH IR Event
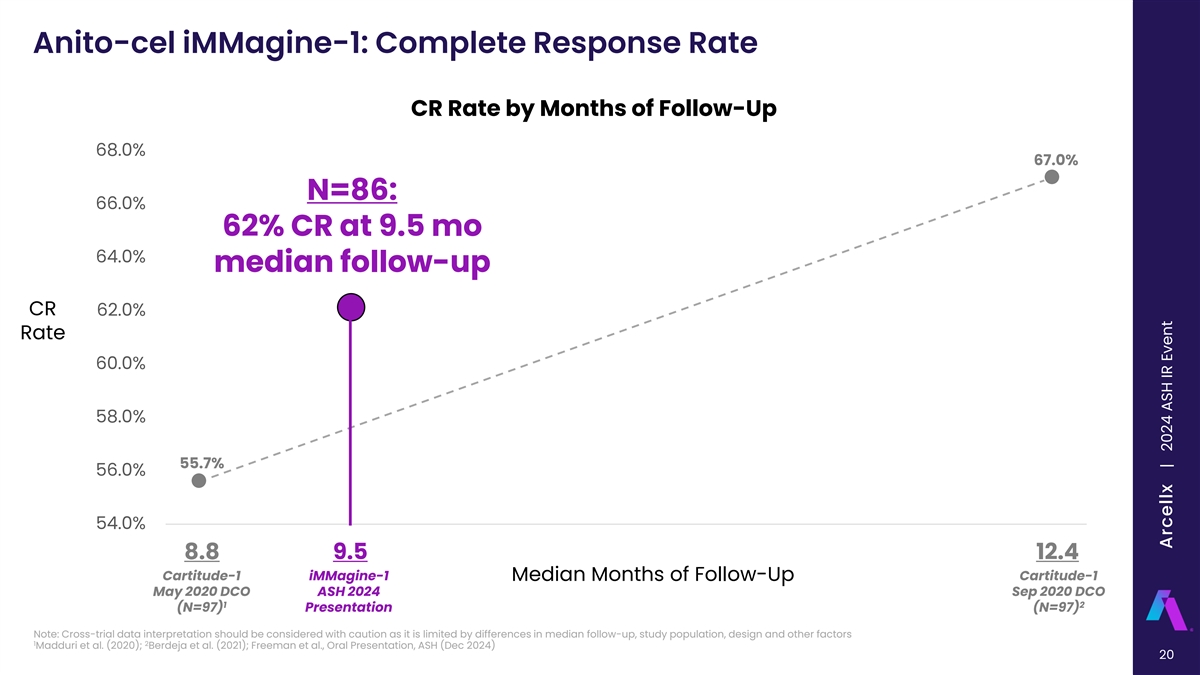
Anito-cel iMMagine-1: Complete Response Rate CR Rate by Months of Follow-Up 68.0% 67.0% N=86: 66.0% 62% CR at 9.5 mo 64.0% median follow-up CR 62.0% Rate 60.0% 58.0% 55.7% 56.0% 54.0% 8.8 9.1 9.4 9.7 10 10.3 10.6 10.9 11.2 11.5 11.8 12.1 12.4 8.8 9.5 12.4 Cartitude-1 iMMagine-1 Cartitude-1 Median Months of Follow-Up May 2020 DCO ASH 2024 Sep 2020 DCO 1 2 (N=97) Presentation (N=97) Note: Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design and other factors 1 2 Madduri et al. (2020); Berdeja et al. (2021); Freeman et al., Oral Presentation, ASH (Dec 2024) 20 Arcellx | 2024 ASH IR Event
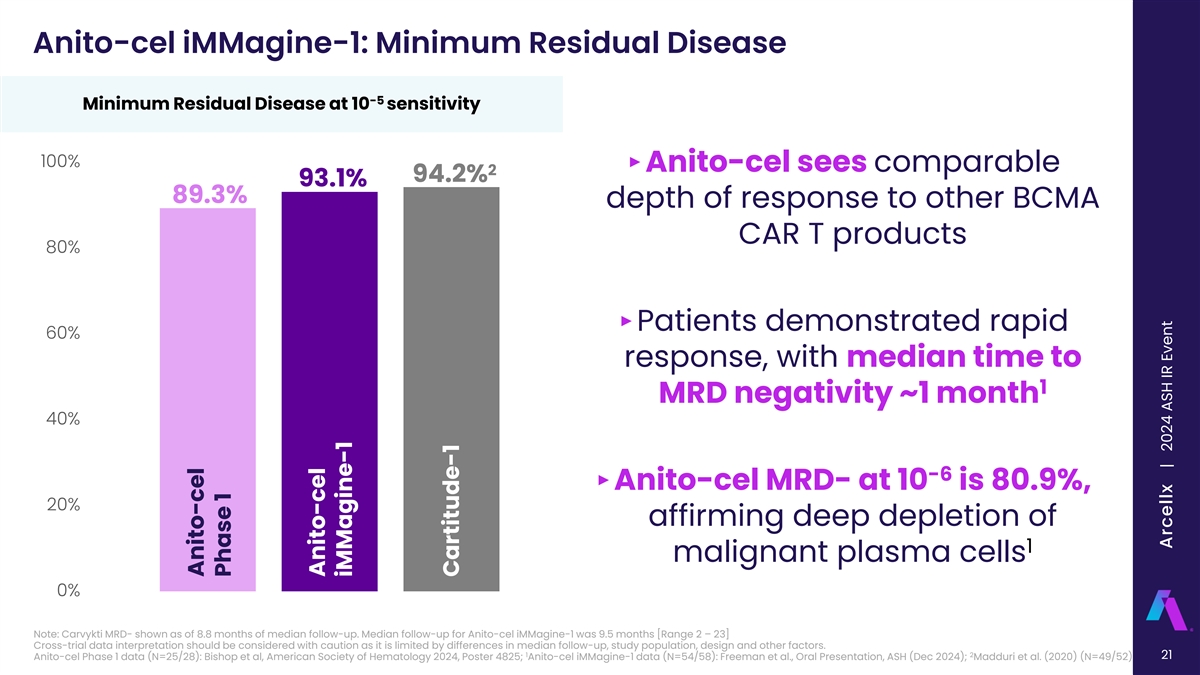
Anito-cel iMMagine-1: Minimum Residual Disease -5 Minimum Residual Disease at 10 sensitivity 100% ‣ Anito-cel sees comparable 2 94.2% 93.1% 89.3% depth of response to other BCMA CAR T products 80% ‣Patients demonstrated rapid 60% response, with median time to 1 MRD negativity ~1 month 40% -6 ‣ Anito-cel MRD- at 10 is 80.9%, 20% affirming deep depletion of 1 malignant plasma cells 0% Note: Carvykti MRD- shown as of 8.8 months of median follow-up. Median follow-up for Anito-cel iMMagine-1 was 9.5 months [Range 2 – 23] Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design and other factors. 1 2 21 Anito-cel Phase 1 data (N=25/28): Bishop et al, American Society of Hematology 2024, Poster 4825; Anito-cel iMMagine-1 data (N=54/58): Freeman et al., Oral Presentation, ASH (Dec 2024); Madduri et al. (2020) (N=49/52) Anito-cel Phase 1 Anito-cel iMMagine-1 Cartitude-1 Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: 6-mo PFS Rate is 93.3%, 12-mo PFS Rate is 78.5% 6-Month PFS Estimates (%) 12-Month PFS Estimates (%) 100% 100% 93.3% 92.1% 1 87.4% 1 78.5% 76.6% 80% 80% 75.9% 60% 60% 40% 40% 20% 20% 0% 0% Note: Carvykti 6-mo PFS at 8.8 months of median follow-up, 12-mo PFS at 12.4 months of median follow-up. Median follow-up for anito-cel iMMagine-1 was 9.5 months [Range 2 – 23] Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design and other factors. 1 22 Anito-cel Phase 1 data (N=38): Bishop et al, American Society of Hematology 2024, Poster 4825; Anito-cel iMMagine-1 data (N=86): Freeman et al., Oral Presentation, ASH (Dec 2024); Madduri et al. (2020) including supplementary materials (N=97) Anito-cel Phase 1 Anito-cel iMMagine-1 Cartitude-1 Anito-cel Phase 1 Anito-cel iMMagine-1 Cartitude-1 Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: Cytokine Release Syndrome Cytokine Release Syndrome (CRS) Safety Evaluable Patients Maximum CRS Grade (N=98) Per ASTCT criteria N=98 Median onset (min - max) 4 days (1 - 17 days) Median duration (min - max) 3 days (1 - 9 days) 67 (68%) Supportive Measures Tocilizumab 72% (71/98) Dexamethasone 65% (64/98) 17 Anakinra 8% (8/98) 13 (17%) (13%) Siltuximab 4% (4/98) 1 0 0 (1%) (0%) (0%) Vasopressor used 1% (1/98) No CRS Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 Intubation/mechanical ventilation 1% (1/98) ‣ CRS management per protocol was in line with standard medical practice • 83% (81/98) of patients had CRS of any Grade; the median onset was with no prophylactic administration of tocilizumab or dexamethasone 4 days • 86% (84/98) of patients had CRS Grade 1 or less, including 17% (17/98) ‒ For CRS onset in the first 48 hours, tocilizumab and dexamethasone were with no CRS protocol recommended • % of patients with either no CRS or CRS that resolved by: ‒ For CRS onset after the first 48 hours, if tocilizumab was administered at investigator discretion, dexamethasone was also recommended • ≤7 days of anito-cel infusion: 63% (62/98) • ≤10 days of anito-cel infusion: 92% (90/98)‣ 2/3 of all patients who received dexamethasone only ever received a single dose of 10 mg of dexamethasone • ≤14 days of anito-cel infusion: 98% (96/98) ‣ Grade 5 CRS occurred in a 76-year-old patient who had rapidly progressive ASTCT, American Society for Transplantation and Cellular Therapy disease between screening and baseline and did not receive bridging Freeman et al., Oral Presentation, ASH (Dec 2024), Data cut-off October 31, 2024 therapy 23 | Freeman et al, American Society of Hematology 2024, Abstract 1031 Arcellx | 2024 ASH IR Event
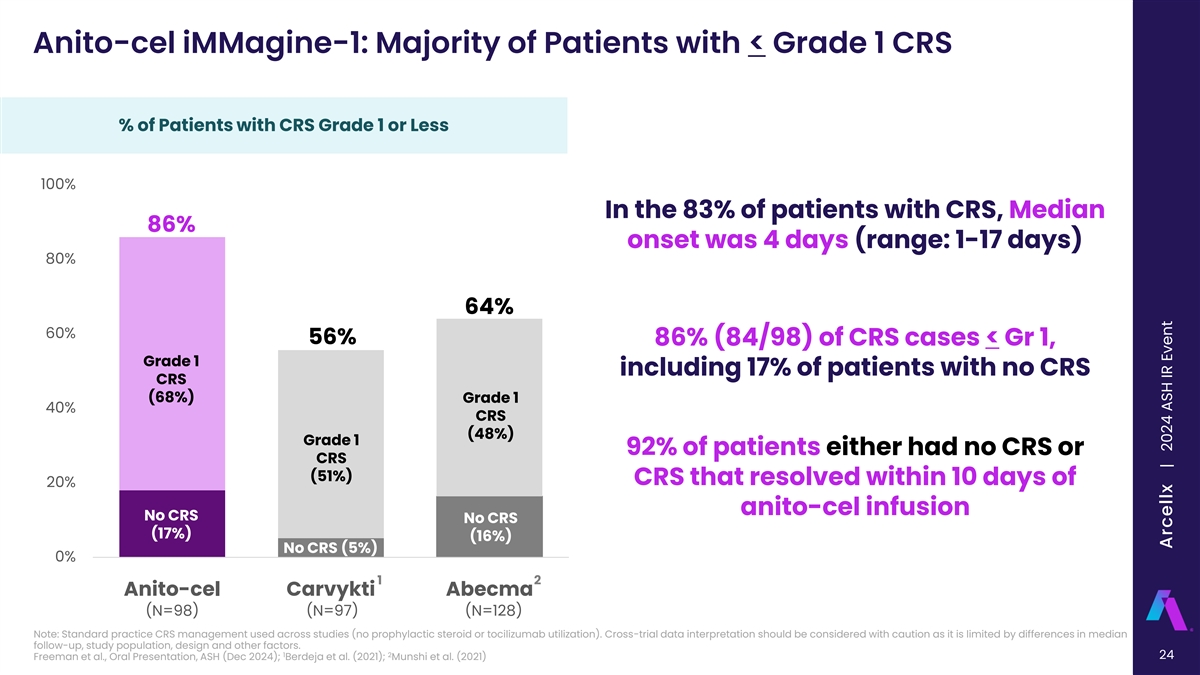
Anito-cel iMMagine-1: Majority of Patients with < Grade 1 CRS % of Patients with CRS Grade 1 or Less 100% In the 83% of patients with CRS, Median 86% onset was 4 days (range: 1-17 days) 80% 64% 60% 56% 86% (84/98) of CRS cases < Gr 1, Grade 1 including 17% of patients with no CRS CRS (68%) Grade 1 40% CRS (48%) Grade 1 92% of patients either had no CRS or CRS (51%) CRS that resolved within 10 days of 20% anito-cel infusion No CRS No CRS (17%) (16%) No CRS (5%) 0% 1 2 Anito-cel Carvykti Abecma (N=98) (N=97) (N=128) Note: Standard practice CRS management used across studies (no prophylactic steroid or tocilizumab utilization). Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design and other factors. 1 2 24 Freeman et al., Oral Presentation, ASH (Dec 2024); Berdeja et al. (2021); Munshi et al. (2021) Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: Majority of Patients with < Grade 1 CRS % of Patients with CRS Grade 2 or Less 99% 100% 95% 95% Grade 2 In the 83% of patients with CRS, Median CRS (13%) onset was 4 days (range: 1-17 days) Grade 2 80% Grade 2 CRS (30%) CRS (39%) 60% 86% (84/98) of CRS cases < Gr 1, Grade 1 including 17% of patients with no CRS CRS (68%) Grade 1 40% CRS (48%) Grade 1 92% of patients either had no CRS or CRS (51%) CRS that resolved within 10 days of 20% anito-cel infusion No CRS No CRS (17%) (16%) No CRS (5%) 0% 1 2 Anito-cel Carvykti Abecma (N=98) (N=97) (N=128) Note: Standard practice CRS management used across studies (no prophylactic steroid or tocilizumab utilization). Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design and other factors. 1 2 25 Freeman et al., Oral Presentation, ASH (Dec 2024); Berdeja et al. (2021); Munshi et al. (2021) Arcellx | 2024 ASH IR Event
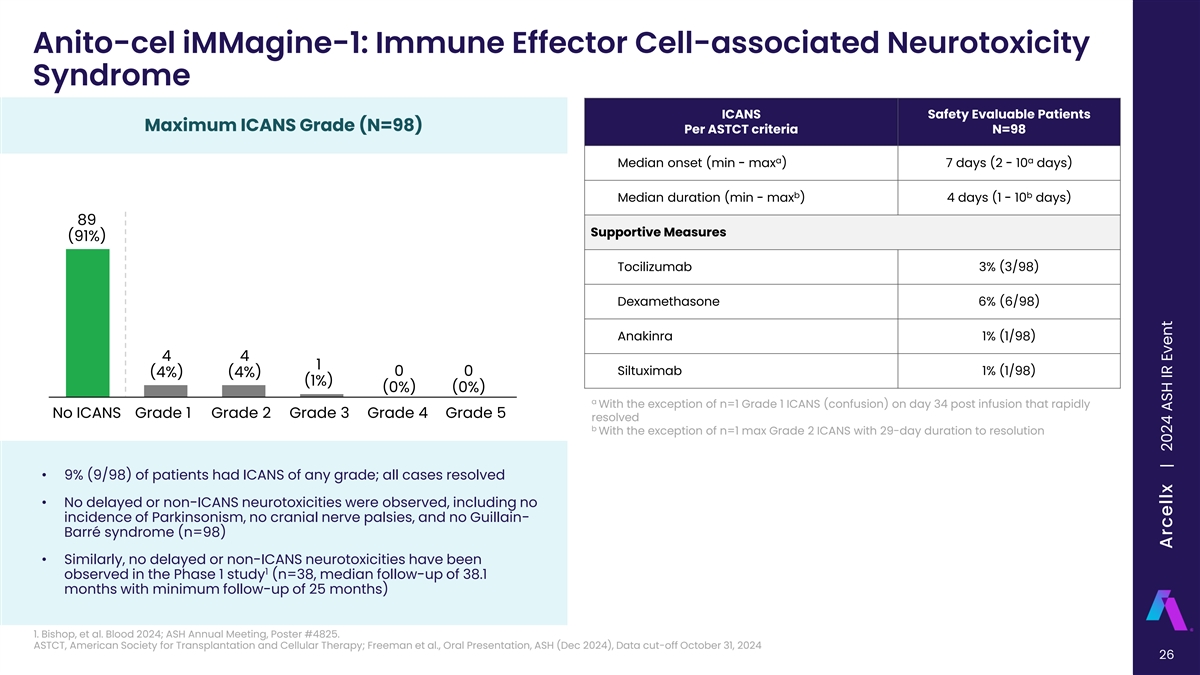
Anito-cel iMMagine-1: Immune Effector Cell-associated Neurotoxicity Syndrome ICANS Safety Evaluable Patients Maximum ICANS Grade (N=98) Per ASTCT criteria N=98 a a Median onset (min - max ) 7 days (2 - 10 days) b b Median duration (min - max ) 4 days (1 - 10 days) 89 Supportive Measures (91%) Tocilizumab 3% (3/98) Dexamethasone 6% (6/98) Anakinra 1% (1/98) 4 4 1 Siltuximab 1% (1/98) 0 0 (4%) (4%) (1%) (0%) (0%) a With the exception of n=1 Grade 1 ICANS (confusion) on day 34 post infusion that rapidly No ICANS Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 resolved b With the exception of n=1 max Grade 2 ICANS with 29-day duration to resolution • 9% (9/98) of patients had ICANS of any grade; all cases resolved • No delayed or non-ICANS neurotoxicities were observed, including no incidence of Parkinsonism, no cranial nerve palsies, and no Guillain- Barré syndrome (n=98) • Similarly, no delayed or non-ICANS neurotoxicities have been 1 observed in the Phase 1 study (n=38, median follow-up of 38.1 months with minimum follow-up of 25 months) 1. Bishop, et al. Blood 2024; ASH Annual Meeting, Poster #4825. ASTCT, American Society for Transplantation and Cellular Therapy; Freeman et al., Oral Presentation, ASH (Dec 2024), Data cut-off October 31, 2024 26 | Freeman et al, American Society of Hematology 2024, Abstract 1031 Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-1: Majority of Patients with No ICANS % of Patients with No ICANS % of Patients with Grade 1 or less CRS 100% 91% of patients did not have ICANS 91% 90% ICANS of any grade was observed in 9 83% 82%* patients (9%), of which 1 (1%) was 80% Grade 3, all cases resolved 70% No delayed or non-ICANS neurotoxicities were observed, including no incidence of 60% Parkinsonism, no cranial nerve palsies, and no Guillain-Barré 50% syndrome to date 1 2 Anito-cel Carvykti Abecma (N=98) (N=97) (N=128) *All neurotoxic events considered as ICANS and non-ICANS toxicity not separated Note: Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design, and other factors. 1 2 27 Freeman et al., Oral Presentation, ASH (Dec 2024), Data cut-off October 31, 2024; Berdeja et al. (2021); Munshi et al. (2021) Arcellx | 2024 ASH IR Event
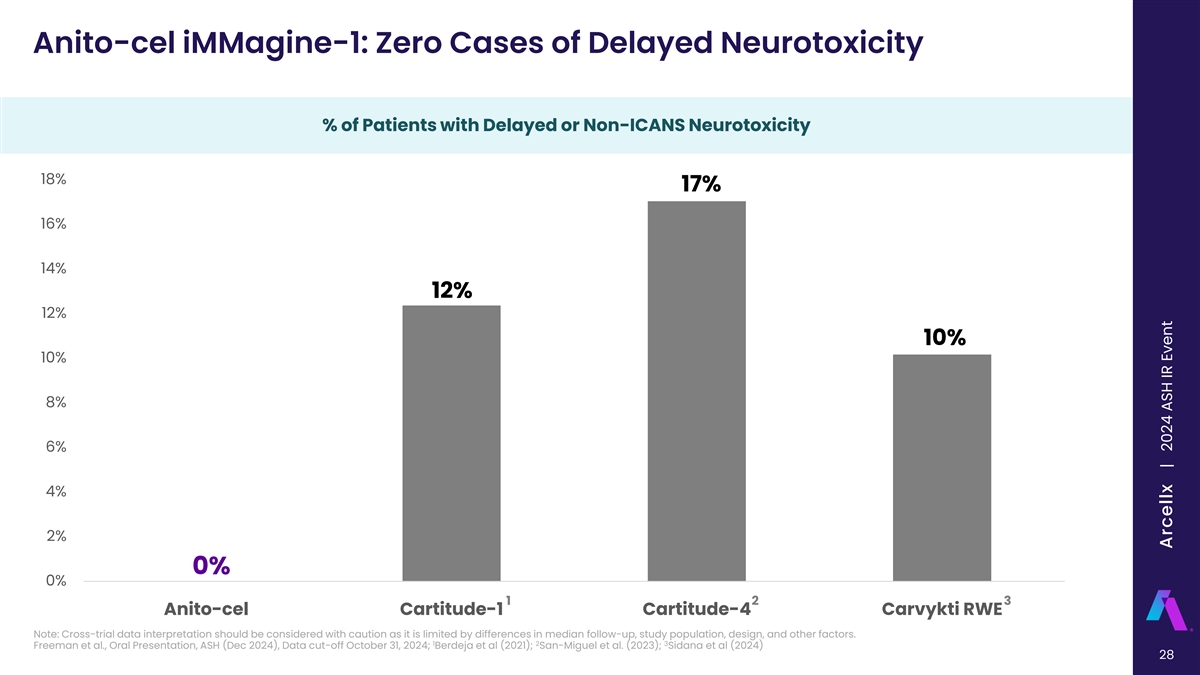
Anito-cel iMMagine-1: Zero Cases of Delayed Neurotoxicity % of Patients with Delayed or Non-ICANS Neurotoxicity % of Patients with Delayed Neurotoxicity 18% 17% 16% 14% 12% 12% 10% 10% 8% 6% 4% 2% 0% 0% 1 2 3 Anito-cel Cartitude-1 Cartitude-4 Carvykti RWE Note: Cross-trial data interpretation should be considered with caution as it is limited by differences in median follow-up, study population, design, and other factors. 1 2 3 Freeman et al., Oral Presentation, ASH (Dec 2024), Data cut-off October 31, 2024; Berdeja et al (2021); San-Miguel et al. (2023); Sidana et al (2024) 28 All Delayed Neurotoxicities Arcellx | 2024 ASH IR Event
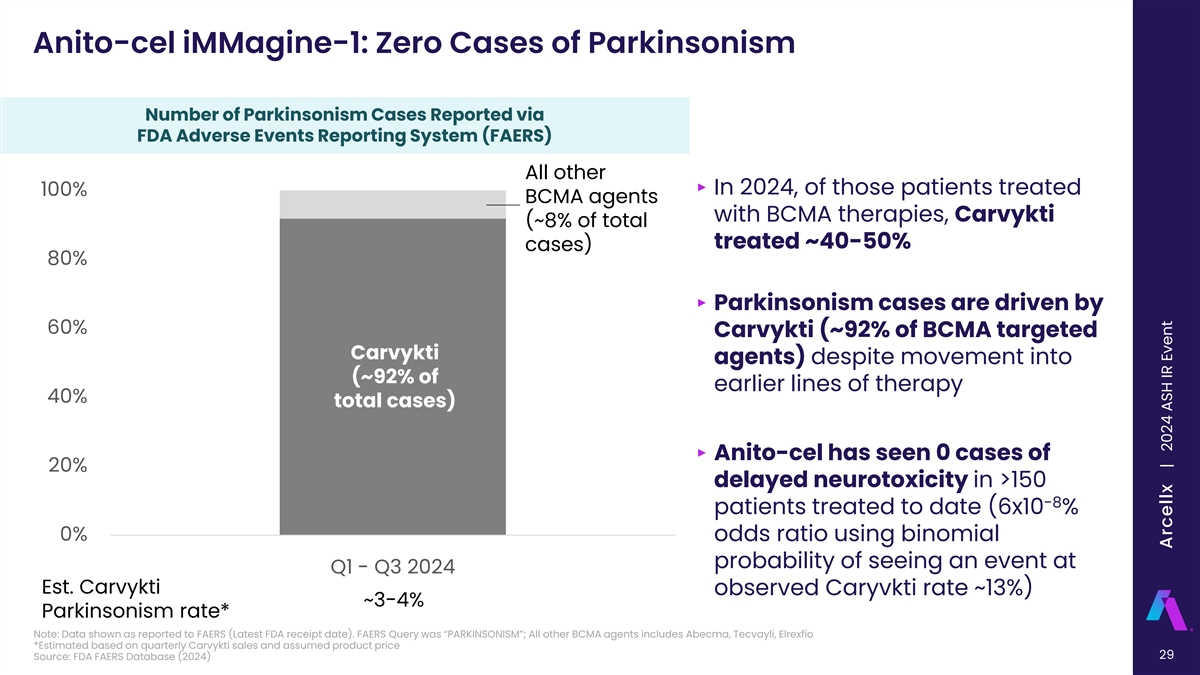
Anito-cel iMMagine-1: Zero Cases of Parkinsonism Number of Parkinsonism Cases Reported via FDA Adverse Events Reporting System (FAERS) All other ‣ In 2024, of those patients treated 100% BCMA agents with BCMA therapies, Carvykti (~8% of total treated ~40-50% cases) 80% ‣ Parkinsonism cases are driven by 60% Carvykti (~92% of BCMA targeted Carvykti agents) despite movement into (~92% of earlier lines of therapy 40% total cases) ‣ Anito-cel has seen 0 cases of 20% delayed neurotoxicity in >150 -8 patients treated to date (6x10 % 0% odds ratio using binomial probability of seeing an event at Q1 - Q3 2024 Est. Carvykti observed Caryvkti rate ~13%) ~3-4% Parkinsonism rate* Note: Data shown as reported to FAERS (Latest FDA receipt date). FAERS Query was “PARKINSONISM”; All other BCMA agents includes Abecma, Tecvayli, Elrexfio *Estimated based on quarterly Carvykti sales and assumed product price 29 Source: FDA FAERS Database (2024) Arcellx | 2024 ASH IR Event
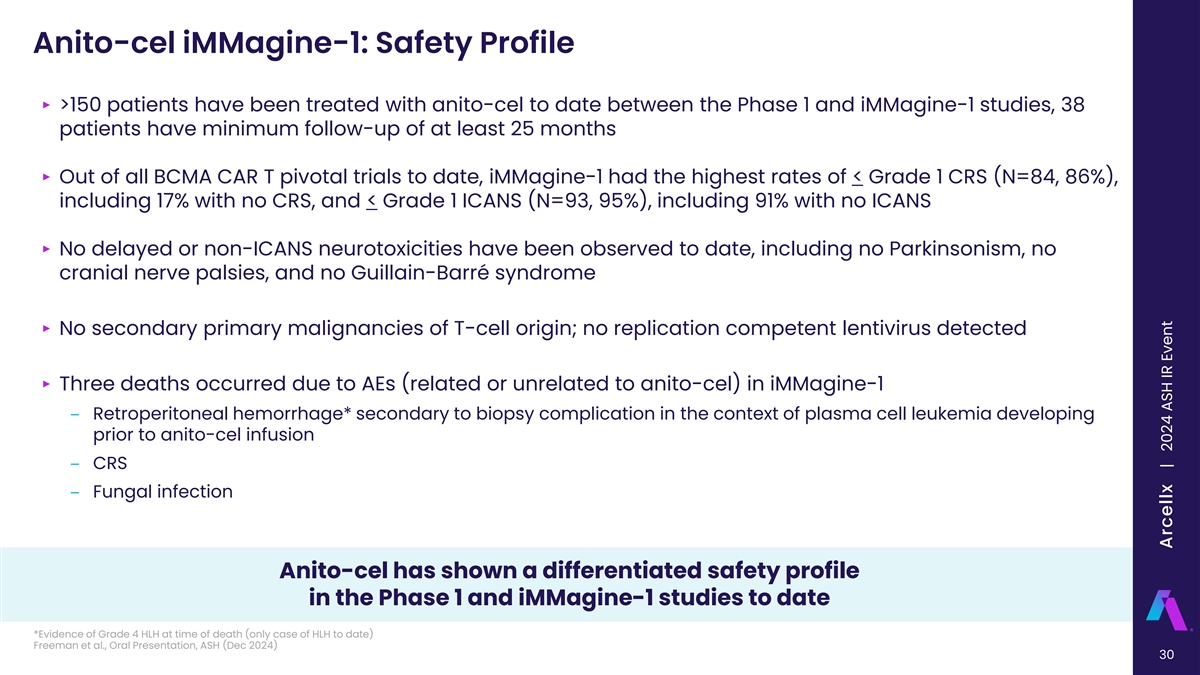
Anito-cel iMMagine-1: Safety Profile ‣ >150 patients have been treated with anito-cel to date between the Phase 1 and iMMagine-1 studies, 38 patients have minimum follow-up of at least 25 months ‣ Out of all BCMA CAR T pivotal trials to date, iMMagine-1 had the highest rates of < Grade 1 CRS (N=84, 86%), including 17% with no CRS, and < Grade 1 ICANS (N=93, 95%), including 91% with no ICANS ‣ No delayed or non-ICANS neurotoxicities have been observed to date, including no Parkinsonism, no cranial nerve palsies, and no Guillain-Barré syndrome ‣ No secondary primary malignancies of T-cell origin; no replication competent lentivirus detected ‣ Three deaths occurred due to AEs (related or unrelated to anito-cel) in iMMagine-1 ‒ Retroperitoneal hemorrhage* secondary to biopsy complication in the context of plasma cell leukemia developing prior to anito-cel infusion ‒ CRS ‒ Fungal infection Anito-cel has shown a differentiated safety profile in the Phase 1 and iMMagine-1 studies to date *Evidence of Grade 4 HLH at time of death (only case of HLH to date) Freeman et al., Oral Presentation, ASH (Dec 2024) 30 Arcellx | 2024 ASH IR Event
Anito-cel iMMagine-1: Conclusions ‣ Anito-cel utilizes a novel, synthetic, compact, and stable D-Domain binder ‒ D-Domain facilitates high transduction efficiency, CAR positivity, and CAR density on the T-cell surface and has a fast off-rate ‣ Anito-cel demonstrated deep and durable efficacy at a median follow-up of 9.5 months ‒ ORR was 97% and sCR/CR rate was 62%, per IMWG criteria -5 ‒ 93.1% of MRD evaluable patients (n=54/58) were MRD negative at 10 or lower ‒ Median PFS and OS not reached; 12-month PFS rate was 78.5% and OS rate was 96.5% ‣ The anito-cel safety profile is predictable and manageable ‒ No delayed or non-ICANS neurotoxicities to date, including no Parkinsonism, no cranial nerve palsies, and no Guillain-Barré syndrome reported across clinical trials ‒ 86% of patients did not have CRS or had a max Grade 1 CRS ‒ 91% of patients did not have ICANS ‣ More than 150 patients dosed across the anito-cel programs for RRMM Anito-cel demonstrated deep, durable responses in 4L+ RRMM with a manageable safety profile, including no delayed or non-ICANS neurotoxicities 31 | Freeman et al, American Society of Hematology 2024, Abstract 1031 Arcellx | 2024 ASH IR Event

Anito-cel iMMagine-3 (NCT06413498): Global Phase 3 Trial Currently Enrolling 1-3 prior LoT, including an anti-CD38 monoclonal antibody and an iMiD Anito-cel Arm 6 Target dose: 115 (± 10) x 10 CAR+ T cells Enrollment / Screening Follow-up Randomization b Standard of Care Arm KDd, PVd, DPd, Kd Study Design Study Endpoints • 1:1 Randomization • Primary Endpoint: PFS • n = Approximately 450, ~130 sites globally• Key Secondary Endpoints: CR rate, MRD, OS, safety a Optional Bridging therapy will be the SOC regimen selected prior to randomization b Cycles will continue until unacceptable toxicity, progression as per IMWG criteria, or patient withdrawal of consent 32 Leukapheresis Bridging a Therapy Lymphodepleting Chemotherapy Arcellx | 2024 ASH IR Event

References ‣ Anderson, Jr, L. D., Munshi, N. C., Shah, N., Jagannath, S., Berdeja, J. G., Lonial, S., Raje, N. S., Siegel, D. S., Lin, Y., Oriol, A., Moreau, P., Yakoub-Agha, I., Delforge, M., Petrocca, F., Patel, P., Huang, L., Campbell, T. B., Hege, K., & F. San-Miguel, J. (2021). Idecabtagene vicleucel (IDE-Cel, BB2121), a BCMA-directed car T cell therapy, in relapsed and refractory multiple myeloma: Updated KARMMA results. Journal of Clinical Oncology, 39(15_suppl), 8016–8016. https://doi.org/10.1200/jco.2021.39.15_suppl.8016 ‣ Berdeja, J. G., Raje, N. S., Siegel, D. S., Lin, Y., Anderson, L. D., Rodriguez-Otero, P., Manier, S., Einsele, H., Cavo, M., Truppel-Hartmann, A., Rowe, E., Sanford, J., Wang, J., Campbell, T. B., & Jagannath, S. (2021). Efficacy and safety of Idecabtagene Vicleucel (IDE-Cel, BB2121) in elderly patients with relapsed and refractory multiple myeloma: Karmma subgroup analysis. Transplantation and Cellular Therapy, 27(3). https://doi.org/10.1016/s2666-6367(21)00512-1 ‣ Berdeja, J. G., Madduri, D., Usmani, S. Z., Jakubowiak, A., Agha, M., Cohen, A. D., Stewart, A. K., Hari, P., Htut, M., Lesokhin, A., Deol, A., Munshi, N. C., O’Donnell, E., Avigan, D., Singh, I., Zudaire, E., Yeh, T.-M., Allred, A. J., Olyslager, Y., … Jagannath, S. (2021). Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (cartitude-1): A phase 1b/2 open-label study. The Lancet, 398(10297), 314–324. https://doi.org/10.1016/s0140-6736(21)00933-8 ‣ Bishop et al, American Society of Hematology 2024, Poster 4825 ‣ CARVYKTI® (ciltacabtagene autoleucel) HCP. CARTITUDE-4 Study | CARVYKTI® (ciltacabtagene autoleucel) HCP. (n.d.). https://www.carvyktihcp.com/cartitude-4-efficacy/ ‣ CARVYKTI® (ciltacabtagene autoleucel). janssen science wordmark. (n.d.). https://www.janssenscience.com/products/carvykti/medical-content/carvykti-outpatient-administration#biblioRef08 ‣ Freeman et al, American Society of Hematology 2024, Abstract 1031 ‣ Gong, Z., Umoru, G., Monge, J., Shah, N., Mohyuddin, G. R., Radhakrishnan, S. V., Chakraborty, R., Rasche, L., Schinke, C., D’Souza, A., & Mohan, M. (2024, March 5). Adverse effects and non-relapse mortality of BCMA directed T cell therapies in multiple myeloma: An faers database study. Nature News. https://www.nature.com/articles/s41408-024-01023-9 ‣ Liu, A. (2023, April 19). J&J, legend’s carvykti cut risk of progression or death by whopping 74% in earlier myeloma, leaked abstract shows. Fierce Pharma. https://www.fiercepharma.com/pharma/leaked- abstract-show-jj-legends-carvykti-reduce-progression-or-death-74-earlier-myeloma#:~:text=That’s%20significant%2C%20because%20current%20reports,or%205%20episodes%20were%20recorded. ‣ Madduri, D. (n.d.). Cartitude-1: Phase 1b/2 study of Ciltacabtagene Autoleucel, a B-cell maturation antigen–directed chimeric antigen receptor T cell therapy, in relapsed/refractory multiple myeloma. ash.confex.com. https://ash.confex.com/ash/2020/webprogram/Paper136307.html ‣ Martin, T., Usmani, S. Z., Schecter, J. M., Roccia, T., Jackson, C. C., Deraedt, W., … Samjoo, I. A. (2022). Updated results from a matching-adjusted indirect comparison of efficacy outcomes for ciltacabtagene autoleucel in CARTITUDE-1 versus idecabtagene vicleucel in KarMMa for the treatment of patients with relapsed or refractory multiple myeloma. Current Medical Research and Opinion, 39(1), 81–89. https://doi.org/10.1080/03007995.2022.2139052 ‣ Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, Avigan D, Deol A, Htut M, Lesokhin A, Munshi NC, O'Donnell E, Stewart AK, Schecter JM, Goldberg JD, Jackson CC, Yeh TM, Banerjee A, Allred A, Zudaire E, Deraedt W, Olyslager Y, Zhou C, Pacaud L, Madduri D, Jakubowiak A, Lin Y, Jagannath S. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J Clin Oncol. 2023 Feb 20;41(6):1265-1274. doi: 10.1200/JCO.22.00842. Epub 2022 Jun 4. PMID: 35658469; PMCID: PMC9937098. ‣ Munshi, N. C., Anderson, L. D., Shah, N., Madduri, D., Berdeja, J., Lonial, S., Raje, N., Lin, Y., Siegel, D., Oriol, A., Moreau, P., Yakoub-Agha, I., Delforge, M., Cavo, M., Einsele, H., Goldschmidt, H., Weisel, K., Rambaldi, A., Reece, D., … San-Miguel, J. (2021). Idecabtagene Vicleucel in relapsed and refractory multiple myeloma. New England Journal of Medicine, 384(8), 705–716. https://doi.org/10.1056/nejmoa2024850 ‣ Neurologic toxicities: ABECMA® (idecabtagene vicleucel). Neurologic Toxicities | ABECMA® (idecabtagene vicleucel). (n.d.). https://www.abecmahcp.com/safety/nt ‣ San-Miguel, J., Dhakal, B., Yong, K., Spencer, A., Anguille, S., Mateos, M.-V., Fernández de Larrea, C., Martínez-López, J., Moreau, P., Touzeau, C., Leleu, X., Avivi, I., Cavo, M., Ishida, T., Kim, S. J., Roeloffzen, W., van de Donk, N. W. C. J., Dytfeld, D., Sidana, S., … Einsele, H. (2023). CILTA-CEL or standard care in lenalidomide-refractory multiple myeloma. New England Journal of Medicine, 389(4), 335–347. https://doi.org/10.1056/nejmoa2303379 ‣ Sidana, S., Patel, K., Peres, L., Bansal, R., Kocoglu, M., Atrash, S., Dima, D., Smith, K., Ferreri, C., Midha, S., Dhakal, B., Herr, M., Nadeem, O., Reshef, R., Hashim, M., Kumar, A., Kalariya, N., Sborov, D., Richard, S., Khouri, J., Martin, T., Htut, Shune, L., Lin, Y., Hansen, D. (2024, September 25). Safety and Efficacy of Standard of Care Ciltacabtagene Autoleucel for Relapsed/Refractory Multiple Myeloma (RRMM): Real World Experience. IMS 2024. 33 Arcellx | 2024 ASH IR Event

Agenda Opening Remarks 10 min Rami Elghandour Chairman and Chief Executive Officer, Arcellx iMMagine-1 Oral Presentation 20 min Ciara L. Freeman M.D., Ph.D iMMagine-1 Clinical Study Investigator Physician Panel Discussion 30 min Q&A 30 min 34 Arcellx | 2024 ASH IR Event

Panel Discussion and Q&A Christopher Heery, Michael R. Bishop, Ciara L. Freeman, Matthew J. Frigault, M.D. M.D. M.D., Ph.D M.D., M.S. Chief Medical Officer, Arcellx iMMagine-1 Clinical Study iMMagine-1 Clinical Study iMMagine-1 and ACLX-001 Clinical Study Investigator; Investigator; Director, The Investigator; Assistant Clinical Director of the Cellular David and Etta Jonas Center Member, Department of for Cellular Therapy and Blood and Marrow Therapy Service at Mass Professor of Medicine at the Transplant and Cellular General Cancer Center and University of Chicago Immunotherapy, Moffitt Assistant Professor at Harvard Medical School Cancer Center

Thank You



































