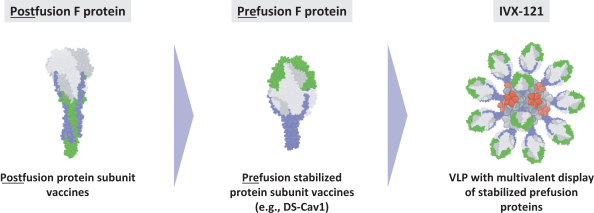
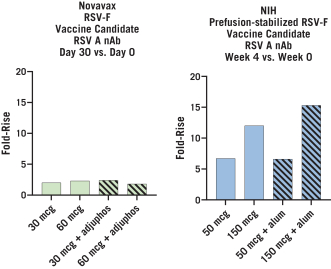
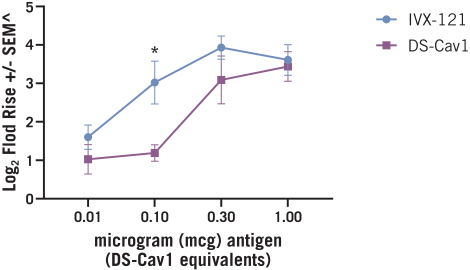
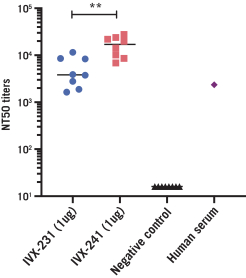
The UW License Agreement will terminate when all licensed rights have been terminated and all obligations due to UW have been fulfilled, or we may terminate the entirety of the agreement upon written notice thereof to UW.
During the year ended December 31, 2019, we paid $78,000 in fees associated with the license, which were expensed as incurred.
During the year ended December 31, 2020, we paid $5,000 in fees associated with the license, which were expensed as incurred.
During the three months ended March 31, 2020 and 2021, we did not make any payments associated with the license.
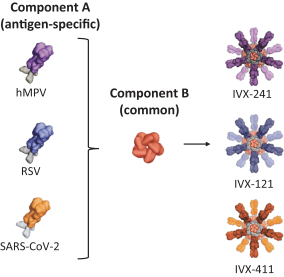
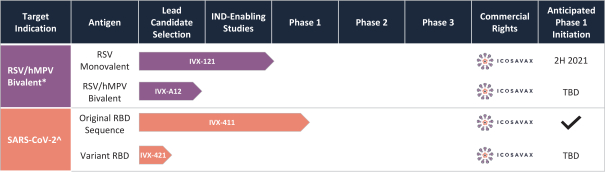
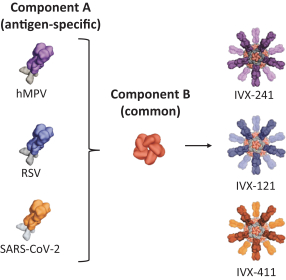
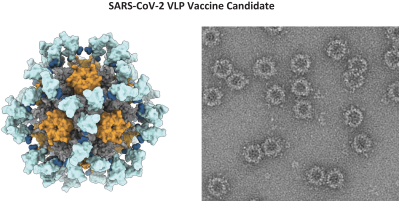
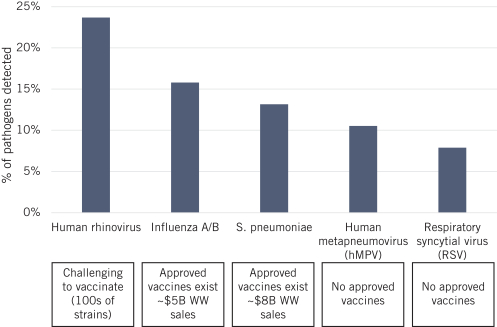
On July 2, 2020, we entered into a non-exclusive license agreement with respect to specified intellectual property with options for exclusivity in North America and Europe subject to the performance of certain development milestones, with UW (the Option and License Agreement). Under the Option and License Agreement we also received a non-exclusive, worldwide (excluding South Korea) license to use specific know-how, and rights to sublicense for computationally designed nanoparticles and vaccines; this license will become exclusive in the United States, Canada, Mexico and Europe (including Switzerland and the United Kingdom) in 2025. The Option and License Agreement was amended in August 2020 and in May 2021. Our rights and obligations under the Option and License Agreement are subject to certain U.S. government rights, certain global access commitment rights for humanitarian purposes to BMGF, certain rights to Howard Hughes Medical Institute, and certain other limited rights retained by the UW.
Under the Option and License Agreement, we are required to use commercially reasonable efforts to meet certain specified development, sales and regulatory milestones related to the licensed products within specified time periods. We have agreed to pay a royalty of a low single digit percentage on net sales of all products applicable to the license. However, we will not be required to pay royalties on net sales of any licensed product under the Option and License Agreement if we are required to pay royalties on net sales under the UW License Agreement. Additional royalties would be due in connection with sublicenses and milestones. Our royalty obligations continue for each licensed product for so long as licensed patent rights exist and have not expired, been revoked, lapsed, or held unenforceable.
The Option and License Agreement will terminate when all licensed rights have been terminated and all obligations due to the UW have been fulfilled, or we may terminate the entirety of the agreement upon written notice thereof to the UW.
During the year ended December 31, 2020, we reimbursed the UW for patent expenses under the UW License Agreement and the Option and License Agreement of $139,000, which were expensed as incurred.
During the years ended December 31, 2019 and 2020, we did not incur any other fees or make any payments associated with the Option and License Agreement.
During the three months ended March 31, 2020 and 2021, we reimbursed the University of Washington for patent expenses under the UW 2018 Agreement and UW 2020 Agreement as amended of $43,000 and $27,000, respectively, which were expensed as incurred. During the three months ended March 31, 2020 and 2021, we did not incur any other fees or make any payments associated with the UW 2020 Agreement as amended.
License Agreement with the University of Texas
In June 2021, we entered into an exclusive patent license agreement with an academic entity, the University of Texas at Austin (the “UT Agreement”). Under the UT Agreement, we obtained an exclusive, worldwide, royalty-bearing, sublicensable license under certain patent rights, to use licensed know-how for prevention, cure, amelioration or treatment of respiratory disease caused by metapneumovirus infection in all vaccine fields, excluding mRNA-based vaccines.
There are milestone payments due upon the completion of certain development, regulatory, and commercial milestones for licensed products in the future. We are obligated to pay aggregate potential milestone payments of up to $775,000 with respect to future development and regulatory based milestones, and up to $3.75 million with respect to future sales milestones following commercialization for each licensed product for so long as licensed patent rights exist and have not expired, been revoked, lapsed, or held unenforceable.
- 87 -