
The world needs better vaccines. We’re striving to create them. CORPORATE OVERVIEW | APRIL 2022 Exhibit 99.1

Forward looking statements Statements contained in this presentation regarding matters that are not historical facts are forward-looking statements. The forward-looking statements are based on the company’s current beliefs and expectations and include, but are not limited to: the company’s goal to progress its preclinical and clinical programs, the timing of company milestone achievement, the company’s cash balance and the potential of the company’s VLP technology. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in the company’s business, including, without limitation: the early stage of the company’s development efforts; the company’s novel and unproven technology and the uncertainties associated with the development of the company’s novel candidates and their potential use as part of a pan respiratory vaccine; potential delays in the commencement, enrollment, and completion of clinical trials and preclinical studies; the company’s dependence on third parties in connection with manufacturing, research, and preclinical and clinical testing; unexpected adverse side effects or inadequate immunogenicity or efficacy of the company’s product candidates that may limit their development, regulatory approval, and/or commercialization as a monovalent vaccine or in a combination or pan-respiratory vaccine; results from preclinical studies or early clinical trials not necessarily being predictive of future results; the potential for the company’s IVX-411 drug product investigation to produce inconclusive results; the potential that, even if the investigation identifies a root cause or contributing factors for the lower than expected interim IVX-411 immunogenicity data, the company may be unable to resolve all ambiguity; the potential that any errors or other unknown factors that may have affected the interim immunogenicity data in the IVX-411-01 clinical trial may have impacted the safety data as well; the potential for the investigation into IVX-411 interim results to impact the results of the company’s ongoing trial for IVX-121; competing approaches limiting the commercial value of the company’s vaccine candidates; regulatory developments in the United States and other countries; the company’s ability to obtain and maintain intellectual property protection for its product candidates and maintain its rights under intellectual property licenses; the company’s ability to fund its operating plans with its current cash, cash equivalents, and investments; the company’s ability to maintain undisrupted business operations during the COVID-19 pandemic, including with respect to clinical trials, manufacturing, and supply chain; and other risks described in the company’s prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in the company’s annual report on Form 10-K for the year ended December 31, 2021 and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and the company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.
RSV hMPV COVID-19 Flu What if a SINGLE VACCINE could protect you from multiple viral respiratory infections? AND LAST BEYOND A SINGLE SEASON AND COVER EMERGING VARIANTS AND LIMIT UNWANTED SIDE EFFECTS
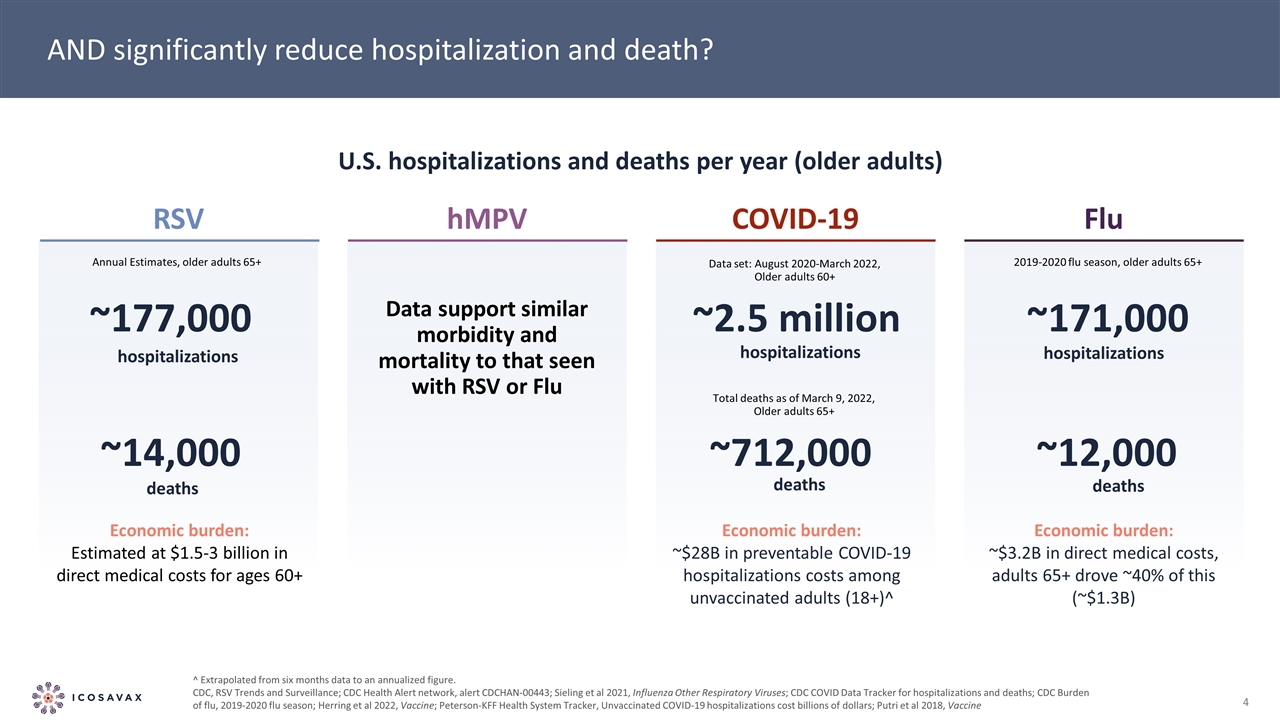
AND significantly reduce hospitalization and death? U.S. hospitalizations and deaths per year (older adults) ^ Extrapolated from six months data to an annualized figure. CDC, RSV Trends and Surveillance; CDC Health Alert network, alert CDCHAN-00443; Sieling et al 2021, Influenza Other Respiratory Viruses; CDC COVID Data Tracker for hospitalizations and deaths; CDC Burden of flu, 2019-2020 flu season; Herring et al 2022, Vaccine; Peterson-KFF Health System Tracker, Unvaccinated COVID-19 hospitalizations cost billions of dollars; Putri et al 2018, Vaccine RSV hMPV COVID-19 Flu hospitalizations ~177,000 deaths ~14,000 Economic burden: Estimated at $1.5-3 billion in direct medical costs for ages 60+ Annual Estimates, older adults 65+ Data support similar morbidity and mortality to that seen with RSV or Flu hospitalizations ~2.5 million deaths ~712,000 Economic burden: ~$28B in preventable COVID-19 hospitalizations costs among unvaccinated adults (18+)^ hospitalizations ~171,000 deaths ~12,000 Economic burden: ~$3.2B in direct medical costs, adults 65+ drove ~40% of this (~$1.3B) 2019-2020 flu season, older adults 65+ Data set: August 2020-March 2022, Older adults 60+ Total deaths as of March 9, 2022, Older adults 65+
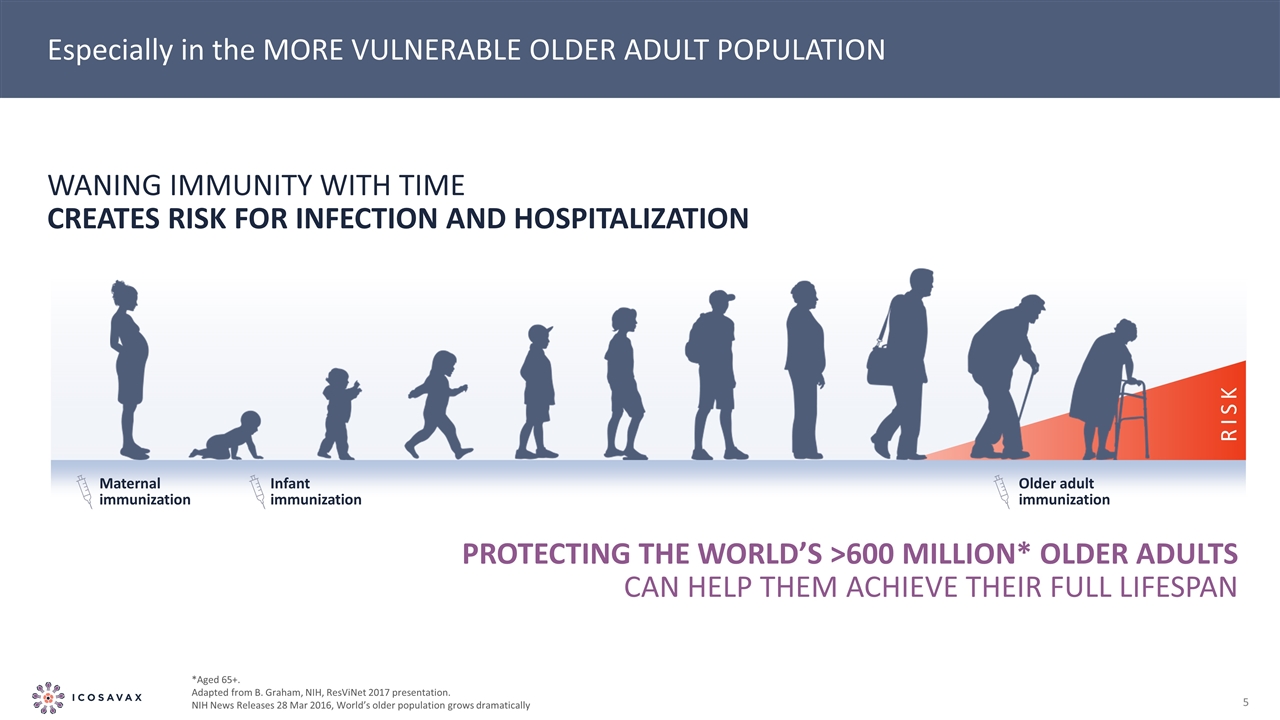
Especially in the MORE VULNERABLE OLDER ADULT POPULATION RISK Maternal immunization Infant immunization Older adult immunization Waning immunity with time creates risk for infection and hospitalization PROTECTING THE WORLD’s >600 MILLION* OLDER ADULTS CAN HELP THEM ACHIEVE THEIR FULL LIFESPAN *Aged 65+. Adapted from B. Graham, NIH, ResViNet 2017 presentation. NIH News Releases 28 Mar 2016, World’s older population grows dramatically
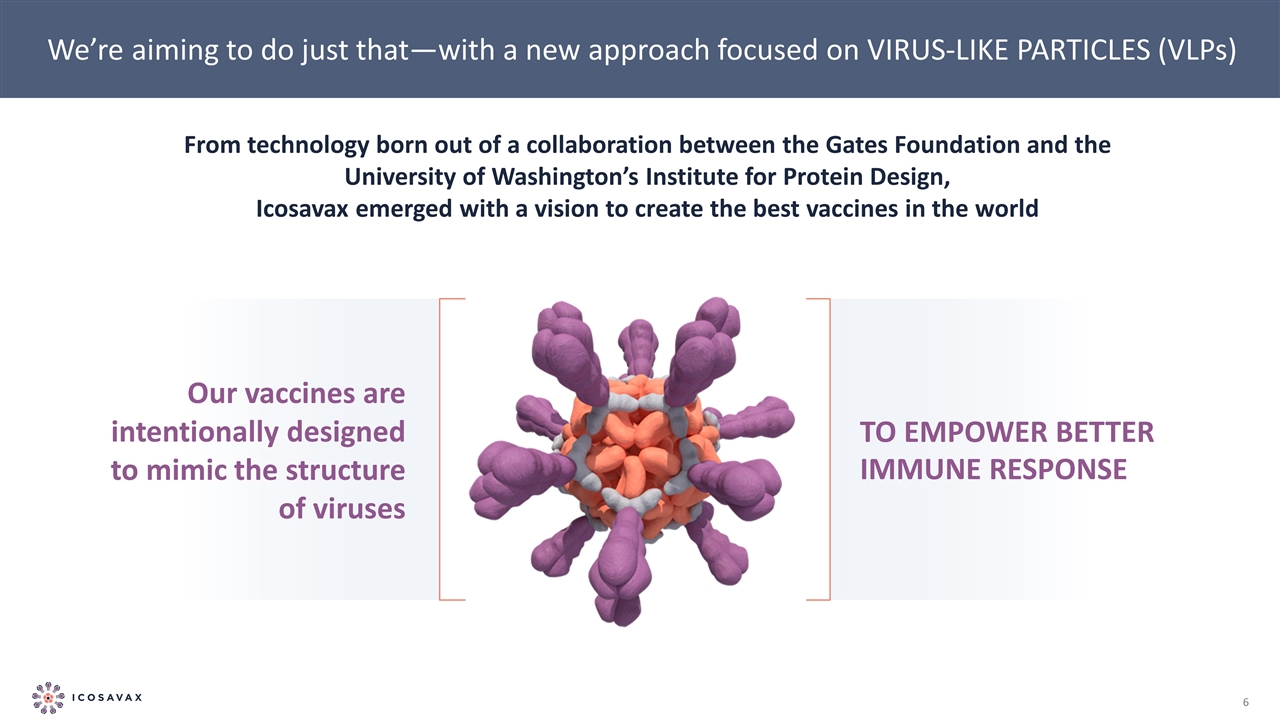
We’re aiming to do just that—with a new approach focused on VIRUS-LIKE PARTICLES (VLPs) From technology born out of a collaboration between the Gates Foundation and the University of Washington’s Institute for Protein Design, Icosavax emerged with a vision to create the best vaccines in the world Our vaccines are intentionally designed to mimic the structure of viruses TO EMPOWER BETTER IMMUNE RESPONSE
VLPs may offer one or more of these POTENTIAL BENEFITS Magnitude of response When compared to existing modalities, WE BELIEVE OUR VLP TECHNOLOGY HAS THE POTENTIAL TO IMPROVE UPON: Breadth of coverage Durability to counter immunosenescence that can occur in the elderly greater degree of protection against related viral strains and mutations; less customization for variants longer antibody persistence and requiring fewer boosters Tolerability/reactogenicity Manufacturing Combinability lower incidence of side effects and greater acceptability high productivity and scalability with process efficiencies, storage flexibility and stability ability to combine multiple VLPs in one vaccine
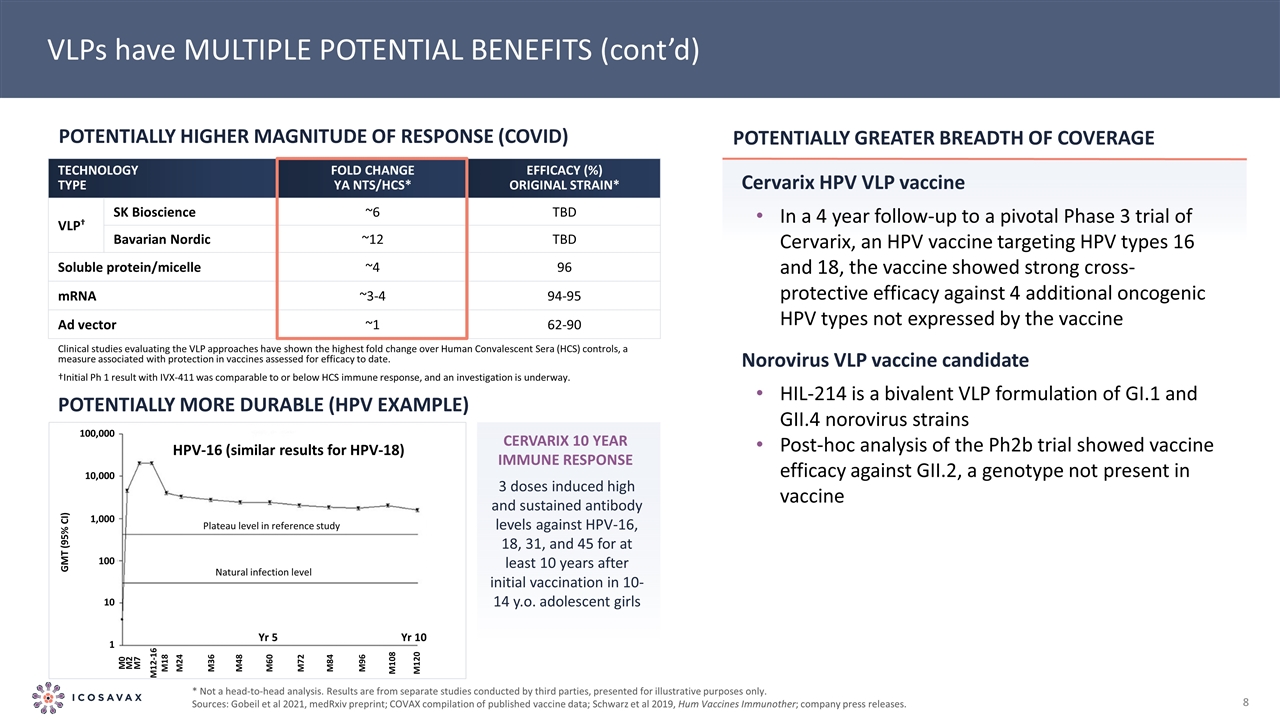
HPV-16 (similar results for HPV-18) Yr 10 Yr 5 VLPs have MULTIPLE POTENTIAL BENEFITS (cont’d) TECHNOLOGY TYPE FOLD CHANGE YA NTS/HCS* EFFICACY (%) ORIGINAL STRAIN* VLP† SK Bioscience ~6 TBD Bavarian Nordic ~12 TBD Soluble protein/micelle ~4 96 mRNA ~3-4 94-95 Ad vector ~1 62-90 Potentially Higher Magnitude of Response (COVID) Potentially More Durable (HPV example) * Not a head-to-head analysis. Results are from separate studies conducted by third parties, presented for illustrative purposes only. Sources: Gobeil et al 2021, medRxiv preprint; COVAX compilation of published vaccine data; Schwarz et al 2019, Hum Vaccines Immunother; company press releases. Potentially greater breadth of coverage Cervarix HPV VLP vaccine In a 4 year follow-up to a pivotal Phase 3 trial of Cervarix, an HPV vaccine targeting HPV types 16 and 18, the vaccine showed strong cross-protective efficacy against 4 additional oncogenic HPV types not expressed by the vaccine Norovirus VLP vaccine candidate HIL-214 is a bivalent VLP formulation of GI.1 and GII.4 norovirus strains Post-hoc analysis of the Ph2b trial showed vaccine efficacy against GII.2, a genotype not present in vaccine 3 doses induced high and sustained antibody levels against HPV-16, 18, 31, and 45 for at least 10 years after initial vaccination in 10-14 y.o. adolescent girls CeRvarix 10 year immune response 100,000 10,000 1,000 100 10 1 GMT (95% CI) M0 M2 M7 M12-16 M18 M24 M36 M48 M60 M72 M84 M96 M108 M120 Plateau level in reference study Natural infection level Clinical studies evaluating the VLP approaches have shown the highest fold change over Human Convalescent Sera (HCS) controls, a measure associated with protection in vaccines assessed for efficacy to date. †Initial Ph 1 result with IVX-411 was comparable to or below HCS immune response, and an investigation is underway.
RSV hMPV COVID-19 Flu PEDIATRICS Combination vaccines have been available for years – up to 6 in 1 shot OLDER ADULTS The time has come for combination vaccines for older adults - VLPs are an ideal modality to succeed with this vision as naturally occurring VLPs have already been utilized as combination vaccines MMRV Measles Mumps Rubella Chicken pox VLPs may also allow for COMBINATION VACCINES for older adults
Unlike soluble antigens, VLPs mimic the STRUCTURE of real viruses Natural virus Soluble antigen VLP-based antigen Traditionally manufactured or mRNA-derived
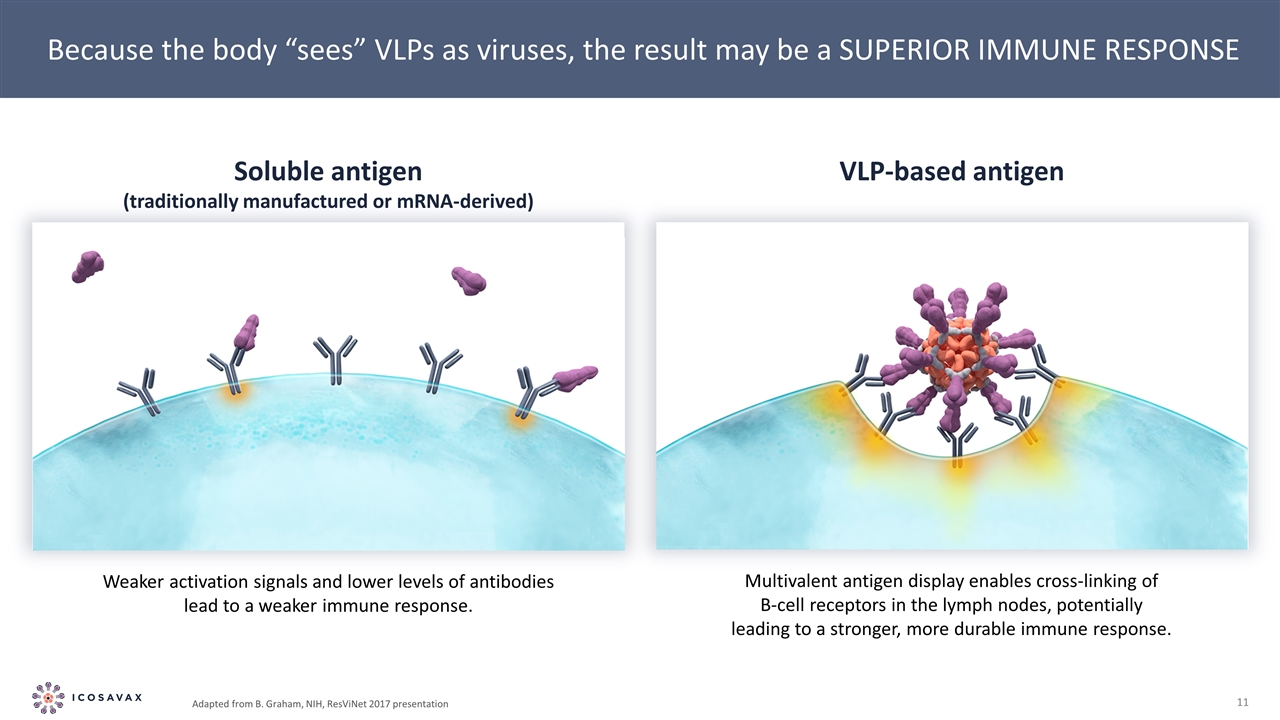
Because the body “sees” VLPs as viruses, the result may be a SUPERIOR IMMUNE RESPONSE Weaker activation signals and lower levels of antibodies lead to a weaker immune response. Multivalent antigen display enables cross-linking of B-cell receptors in the lymph nodes, potentially leading to a stronger, more durable immune response. Soluble antigen (traditionally manufactured or mRNA-derived) VLP-based antigen Adapted from B. Graham, NIH, ResViNet 2017 presentation
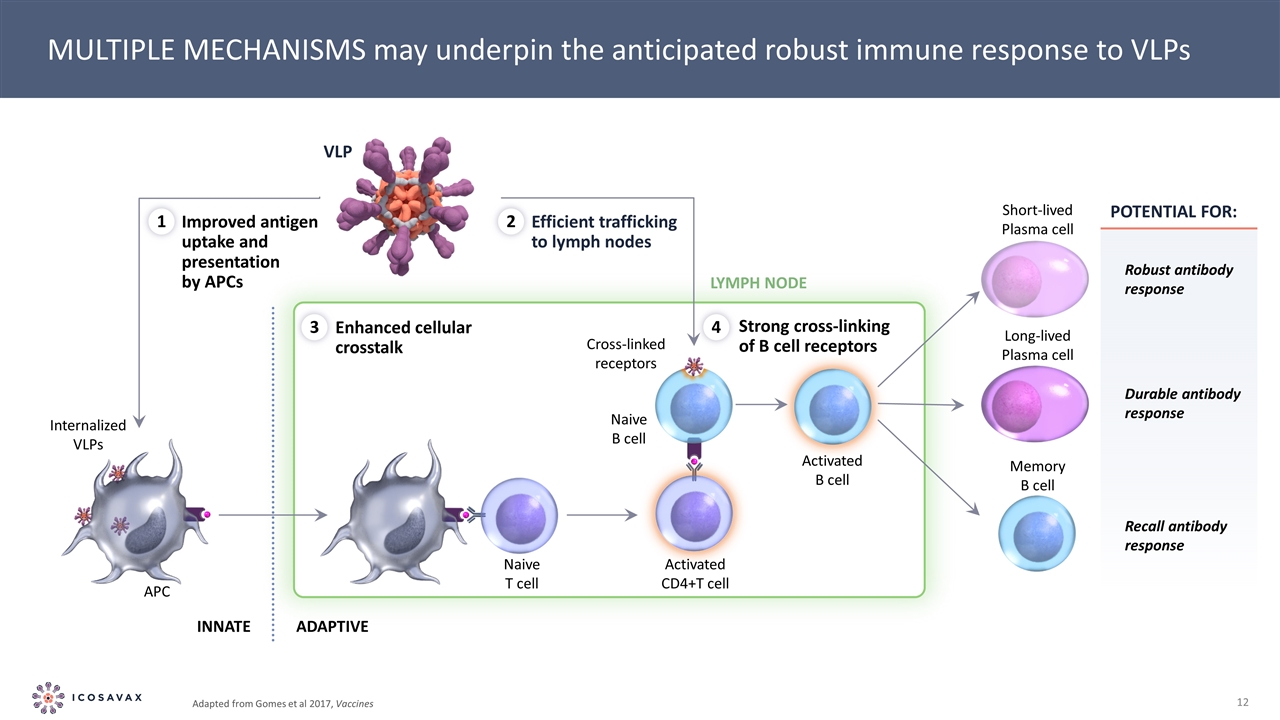
MULTIPLE MECHANISMS may underpin the anticipated robust immune response to VLPs Internalized VLPs VLP Improved antigen uptake and presentation by APCs Efficient trafficking to lymph nodes Enhanced cellular crosstalk Strong cross-linking of B cell receptors LYMPH NODE APC Naive T cell Activated CD4+T cell Naive B cell Cross-linked receptors Activated B cell Short-lived Plasma cell Long-lived Plasma cell Memory B cell Robust antibody response Durable antibody response Recall antibody response INNATE ADAPTIVE 1 2 3 4 POTENTIAL FOR: Adapted from Gomes et al 2017, Vaccines
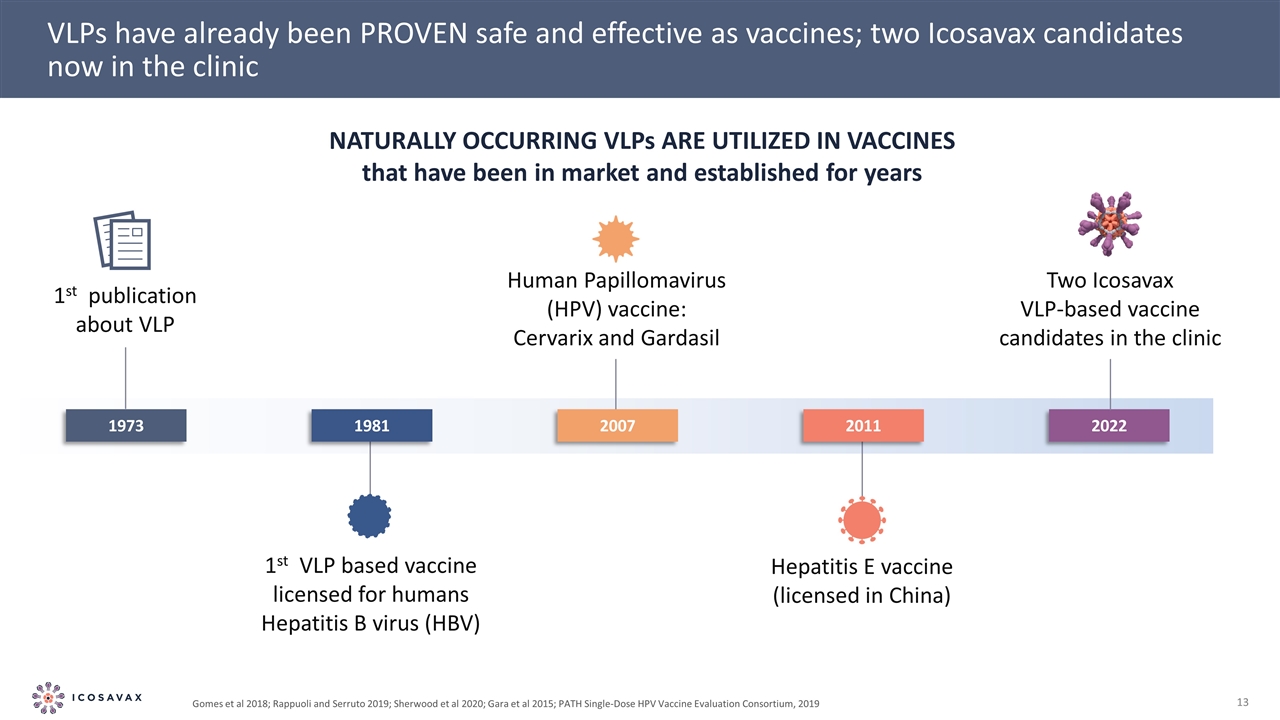
VLPs have already been PROVEN safe and effective as vaccines; two Icosavax candidates now in the clinic NATURALLY OCCURRING VLPs ARE UTILIZED IN VACCINES that have been in market and established for years Gomes et al 2018; Rappuoli and Serruto 2019; Sherwood et al 2020; Gara et al 2015; PATH Single-Dose HPV Vaccine Evaluation Consortium, 2019 1st publication about VLP 1st VLP based vaccine licensed for humans Hepatitis B virus (HBV) Human Papillomavirus (HPV) vaccine: Cervarix and Gardasil 1973 1981 Hepatitis E vaccine (licensed in China) 2011 2007 2022 Two Icosavax VLP-based vaccine candidates in the clinic
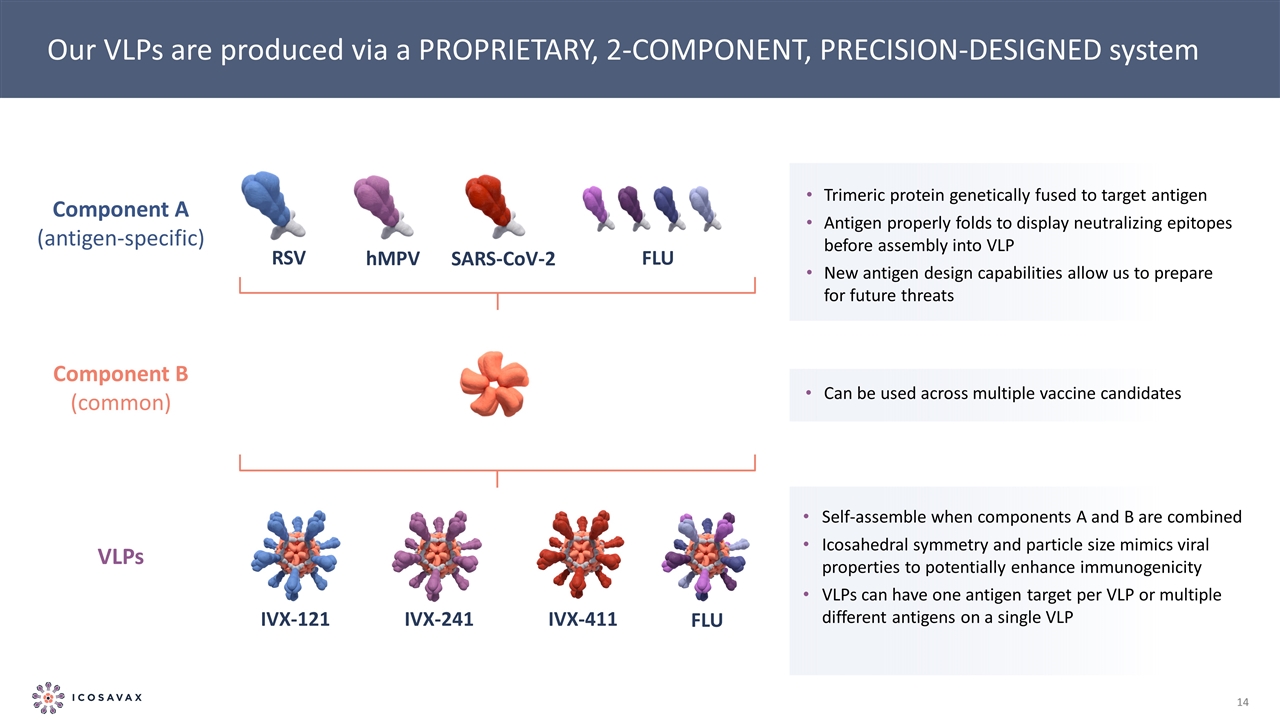
Our VLPs are produced via a PROPRIETARY, 2-COMPONENT, PRECISION-DESIGNED system IVX-121 IVX-241 FLU IVX-411 hMPV RSV SARS-CoV-2 FLU Trimeric protein genetically fused to target antigen Antigen properly folds to display neutralizing epitopes before assembly into VLP New antigen design capabilities allow us to prepare for future threats VLPs Component B (common) Can be used across multiple vaccine candidates Self-assemble when components A and B are combined Icosahedral symmetry and particle size mimics viral properties to potentially enhance immunogenicity VLPs can have one antigen target per VLP or multiple different antigens on a single VLP Component A (antigen-specific)
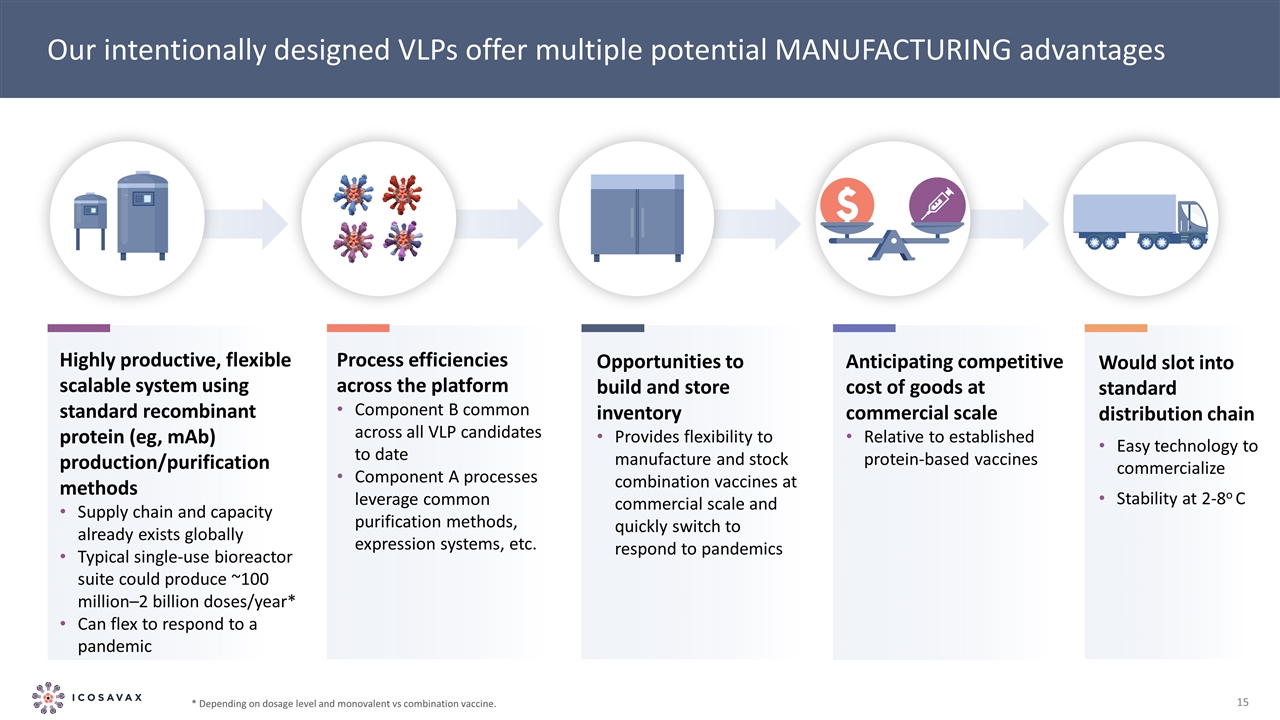
c c c c c Highly productive, flexible scalable system using standard recombinant protein (eg, mAb) production/purification methods Supply chain and capacity already exists globally Typical single-use bioreactor suite could produce ~100 million–2 billion doses/year* Can flex to respond to a pandemic Our intentionally designed VLPs offer multiple potential MANUFACTURING advantages Would slot into standard distribution chain Easy technology to commercialize Stability at 2-8o C Process efficiencies across the platform Component B common across all VLP candidates to date Component A processes leverage common purification methods, expression systems, etc. Opportunities to build and store inventory Provides flexibility to manufacture and stock combination vaccines at commercial scale and quickly switch to respond to pandemics * Depending on dosage level and monovalent vs combination vaccine. Anticipating competitive cost of goods at commercial scale Relative to established protein-based vaccines
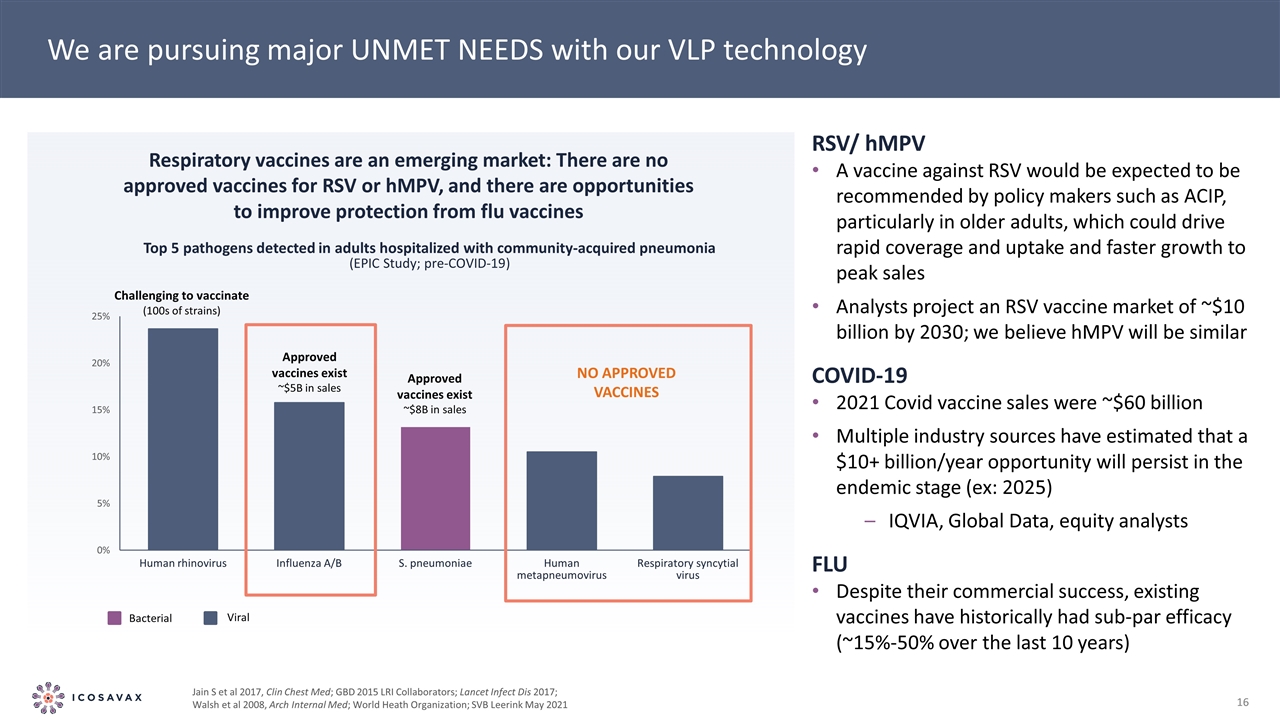
We are pursuing major UNMET NEEDS with our VLP technology Bacterial Viral Challenging to vaccinate (100s of strains) Approved vaccines exist ~$5B in sales Approved vaccines exist ~$8B in sales No approved vaccines Respiratory vaccines are an emerging market: There are no approved vaccines for RSV or hMPV, and there are opportunities to improve protection from flu vaccines RSV/ hMPV A vaccine against RSV would be expected to be recommended by policy makers such as ACIP, particularly in older adults, which could drive rapid coverage and uptake and faster growth to peak sales Analysts project an RSV vaccine market of ~$10 billion by 2030; we believe hMPV will be similar COVID-19 2021 Covid vaccine sales were ~$60 billion Multiple industry sources have estimated that a $10+ billion/year opportunity will persist in the endemic stage (ex: 2025) IQVIA, Global Data, equity analysts FLU Despite their commercial success, existing vaccines have historically had sub-par efficacy (~15%-50% over the last 10 years) Jain S et al 2017, Clin Chest Med; GBD 2015 LRI Collaborators; Lancet Infect Dis 2017; Walsh et al 2008, Arch Internal Med; World Heath Organization; SVB Leerink May 2021 Top 5 pathogens detected in adults hospitalized with community-acquired pneumonia (EPIC Study; pre-COVID-19)
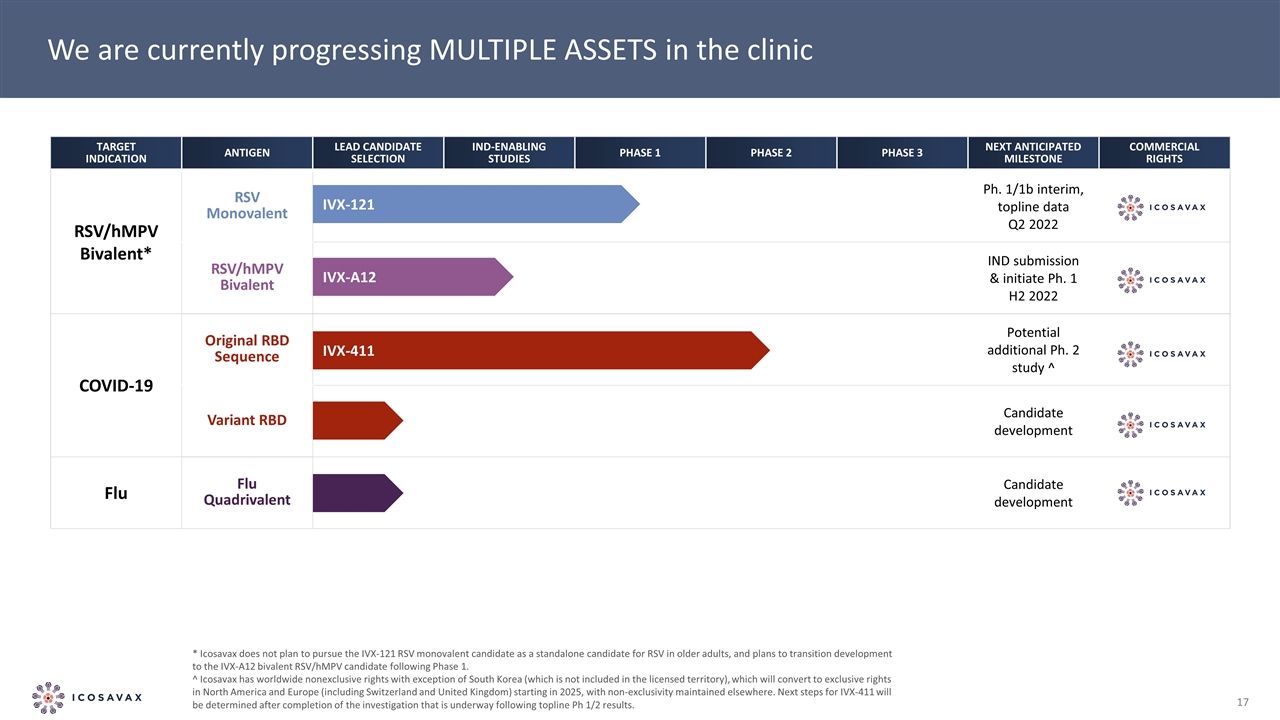
We are currently progressing MULTIPLE ASSETS in the clinic * Icosavax does not plan to pursue the IVX-121 RSV monovalent candidate as a standalone candidate for RSV in older adults, and plans to transition development to the IVX-A12 bivalent RSV/hMPV candidate following Phase 1. ^ Icosavax has worldwide nonexclusive rights with exception of South Korea (which is not included in the licensed territory), which will convert to exclusive rights in North America and Europe (including Switzerland and United Kingdom) starting in 2025, with non-exclusivity maintained elsewhere. Next steps for IVX-411 will be determined after completion of the investigation that is underway following topline Ph 1/2 results. Target Indication Antigen Lead Candidate Selection IND-Enabling Studies Phase 1 Phase 2 Phase 3 Next Anticipated Milestone Commercial Rights RSV/hMPV Bivalent* RSV Monovalent Ph. 1/1b interim, topline data Q2 2022 RSV/hMPV Bivalent IND submission & initiate Ph. 1 H2 2022 Covid-19 Original RBD Sequence Potential additional Ph. 2 study ^ Variant RBD Candidate development Flu Flu Quadrivalent Candidate development IVX-411 IVX-A12 IVX-121

RSV/hMPV Bivalent Vaccine Candidate (IVX-A12) CONFIDENTIAL
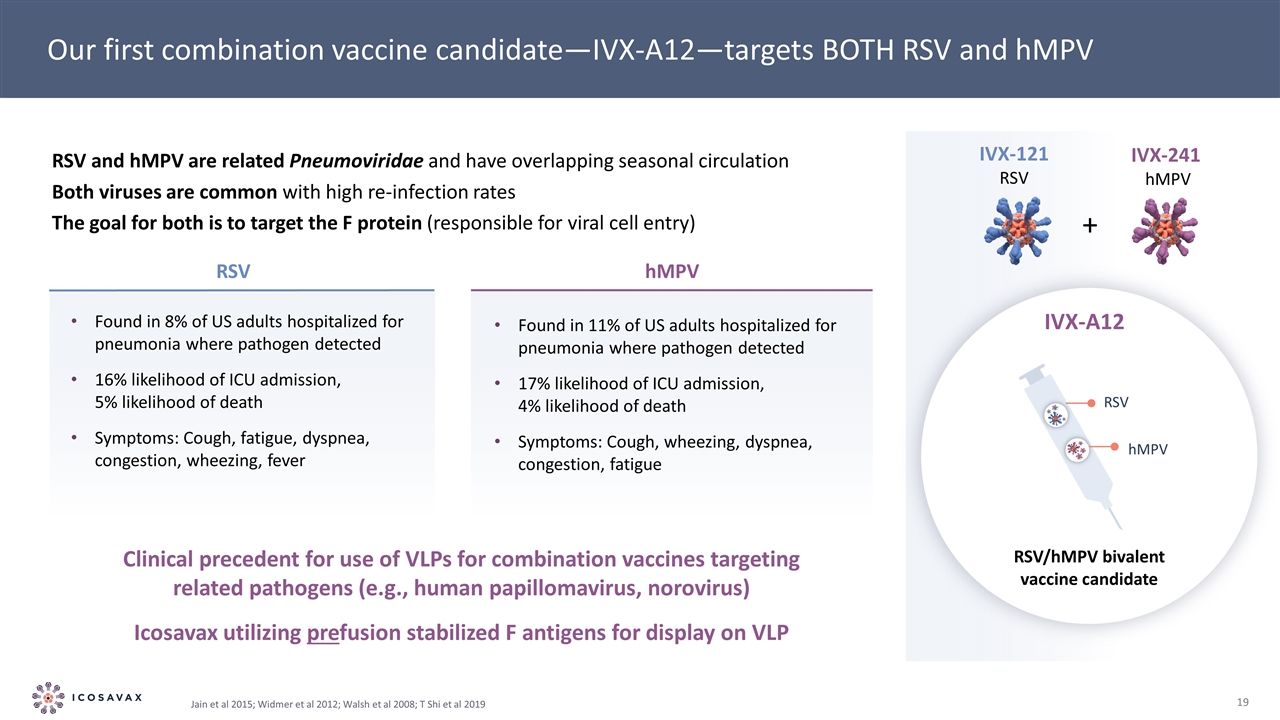
Our first combination vaccine candidate—IVX-A12—targets BOTH RSV and hMPV Found in 8% of US adults hospitalized for pneumonia where pathogen detected 16% likelihood of ICU admission, 5% likelihood of death Symptoms: Cough, fatigue, dyspnea, congestion, wheezing, fever RSV Found in 11% of US adults hospitalized for pneumonia where pathogen detected 17% likelihood of ICU admission, 4% likelihood of death Symptoms: Cough, wheezing, dyspnea, congestion, fatigue hMPV Clinical precedent for use of VLPs for combination vaccines targeting related pathogens (e.g., human papillomavirus, norovirus) Icosavax utilizing prefusion stabilized F antigens for display on VLP IVX-121 RSV IVX-241 hMPV + RSV and hMPV are related Pneumoviridae and have overlapping seasonal circulation Both viruses are common with high re-infection rates The goal for both is to target the F protein (responsible for viral cell entry) Jain et al 2015; Widmer et al 2012; Walsh et al 2008; T Shi et al 2019 RSV hMPV IVX-A12 RSV/hMPV bivalent vaccine candidate
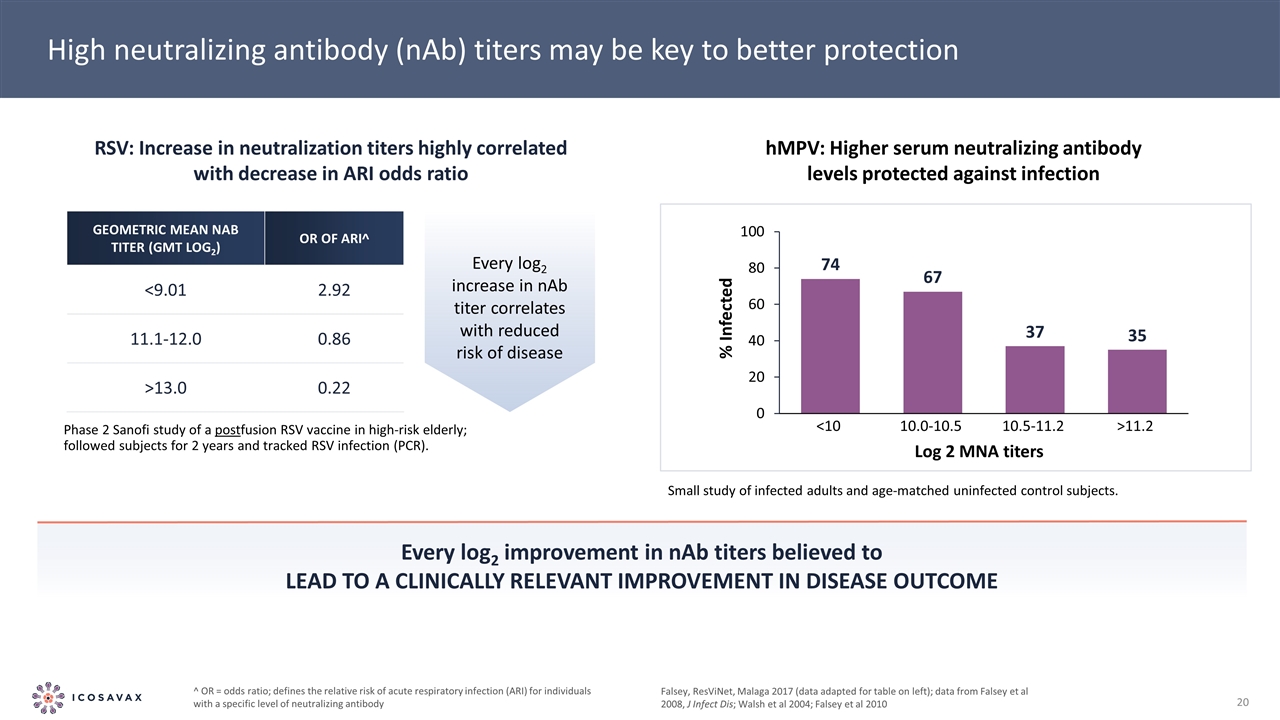
Every log2 improvement in nAb titers believed to lead to a clinically relevant improvement in disease outcome Falsey, ResViNet, Malaga 2017 (data adapted for table on left); data from Falsey et al 2008, J Infect Dis; Walsh et al 2004; Falsey et al 2010 High neutralizing antibody (nAb) titers may be key to better protection Phase 2 Sanofi study of a postfusion RSV vaccine in high-risk elderly; followed subjects for 2 years and tracked RSV infection (PCR). RSV: Increase in neutralization titers highly correlated with decrease in ARI odds ratio hMPV: Higher serum neutralizing antibody levels protected against infection Small study of infected adults and age-matched uninfected control subjects. Geometric Mean nAb Titer (GMT log2) OR of ARI^ <9.01 2.92 11.1-12.0 0.86 >13.0 0.22 ^ OR = odds ratio; defines the relative risk of acute respiratory infection (ARI) for individuals with a specific level of neutralizing antibody Every log2 increase in nAb titer correlates with reduced risk of disease % Infected Log 2 MNA titers
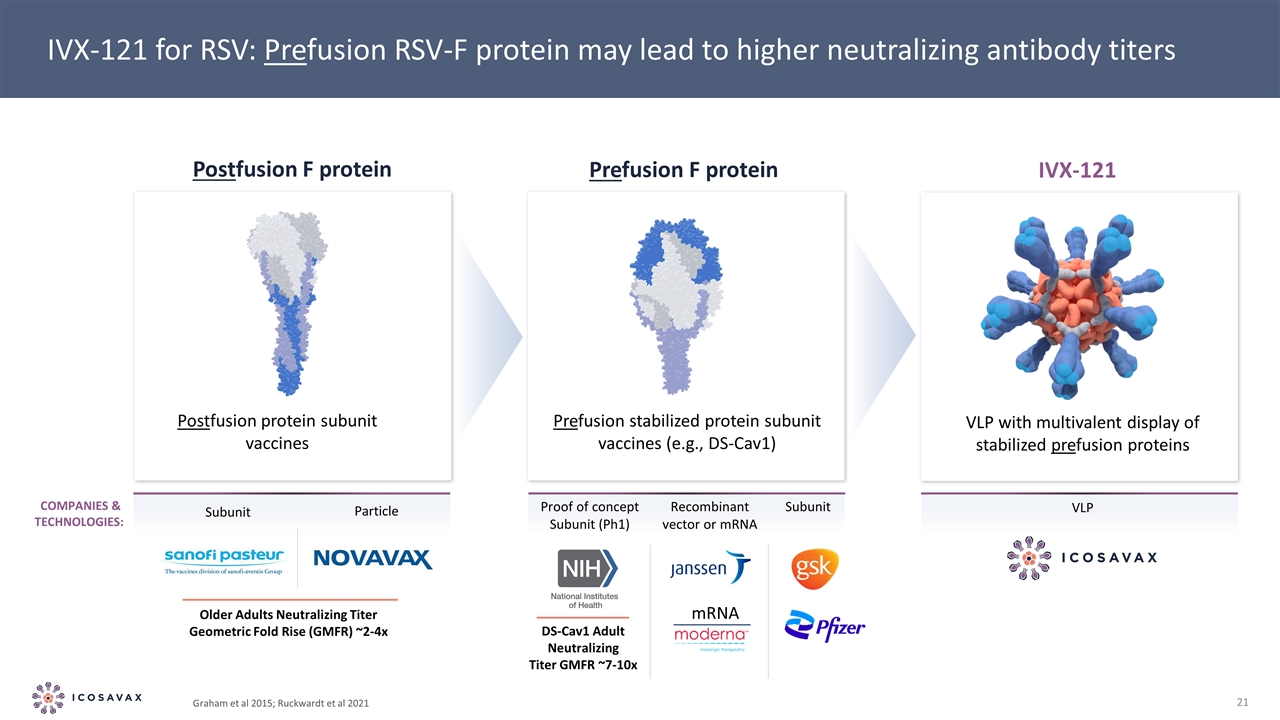
Graham et al 2015; Ruckwardt et al 2021 IVX-121 for RSV: Prefusion RSV-F protein may lead to higher neutralizing antibody titers Proof of concept Subunit (Ph1) Subunit Recombinant vector or mRNA VLP Companies & Technologies: mRNA DS-Cav1 Adult Neutralizing Titer GMFR ~7-10x Postfusion protein subunit vaccines Prefusion stabilized protein subunit vaccines (e.g., DS-Cav1) VLP with multivalent display of stabilized prefusion proteins Postfusion F protein Prefusion F protein IVX-121 Subunit Particle Older Adults Neutralizing Titer Geometric Fold Rise (GMFR) ~2-4x
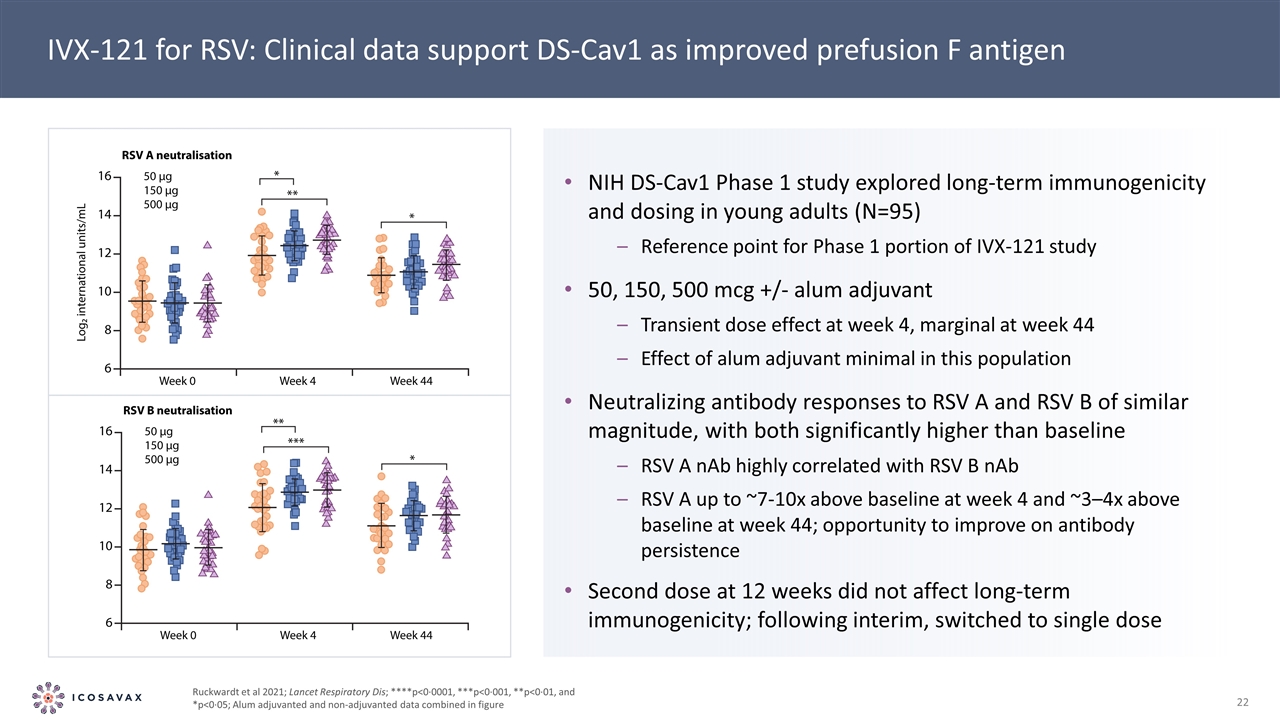
IVX-121 for RSV: Clinical data support DS-Cav1 as improved prefusion F antigen NIH DS-Cav1 Phase 1 study explored long-term immunogenicity and dosing in young adults (N=95) Reference point for Phase 1 portion of IVX-121 study 50, 150, 500 mcg +/- alum adjuvant Transient dose effect at week 4, marginal at week 44 Effect of alum adjuvant minimal in this population Neutralizing antibody responses to RSV A and RSV B of similar magnitude, with both significantly higher than baseline RSV A nAb highly correlated with RSV B nAb RSV A up to ~7-10x above baseline at week 4 and ~3–4x above baseline at week 44; opportunity to improve on antibody persistence Second dose at 12 weeks did not affect long-term immunogenicity; following interim, switched to single dose Ruckwardt et al 2021; Lancet Respiratory Dis; ****p<0·0001, ***p<0·001, **p<0·01, and *p<0·05; Alum adjuvanted and non-adjuvanted data combined in figure
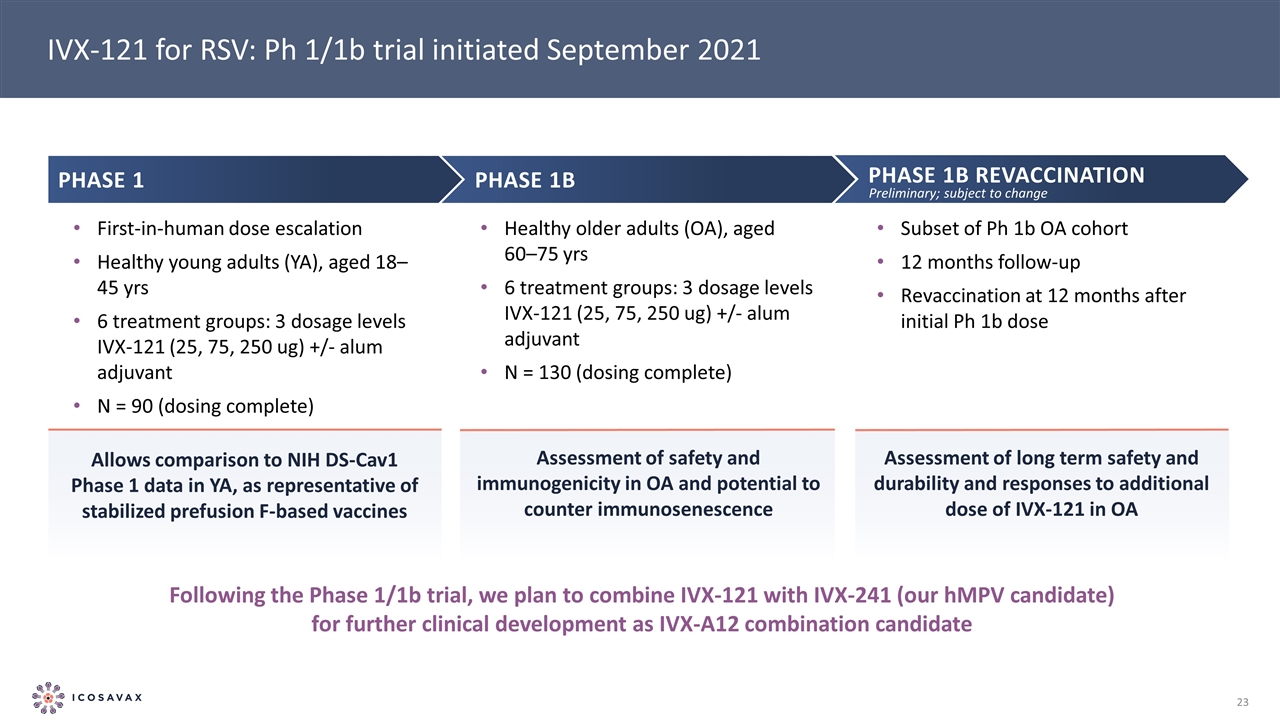
IVX-121 for RSV: Ph 1/1b trial initiated September 2021 Phase 1b revaccination Subset of Ph 1b OA cohort 12 months follow-up Revaccination at 12 months after initial Ph 1b dose Following the Phase 1/1b trial, we plan to combine IVX-121 with IVX-241 (our hMPV candidate) for further clinical development as IVX-A12 combination candidate Phase 1 First-in-human dose escalation Healthy young adults (YA), aged 18–45 yrs 6 treatment groups: 3 dosage levels IVX-121 (25, 75, 250 ug) +/- alum adjuvant N = 90 (dosing complete) Phase 1b Healthy older adults (OA), aged 60–75 yrs 6 treatment groups: 3 dosage levels IVX-121 (25, 75, 250 ug) +/- alum adjuvant N = 130 (dosing complete) Allows comparison to NIH DS-Cav1 Phase 1 data in YA, as representative of stabilized prefusion F-based vaccines Assessment of safety and immunogenicity in OA and potential to counter immunosenescence Assessment of long term safety and durability and responses to additional dose of IVX-121 in OA Preliminary; subject to change
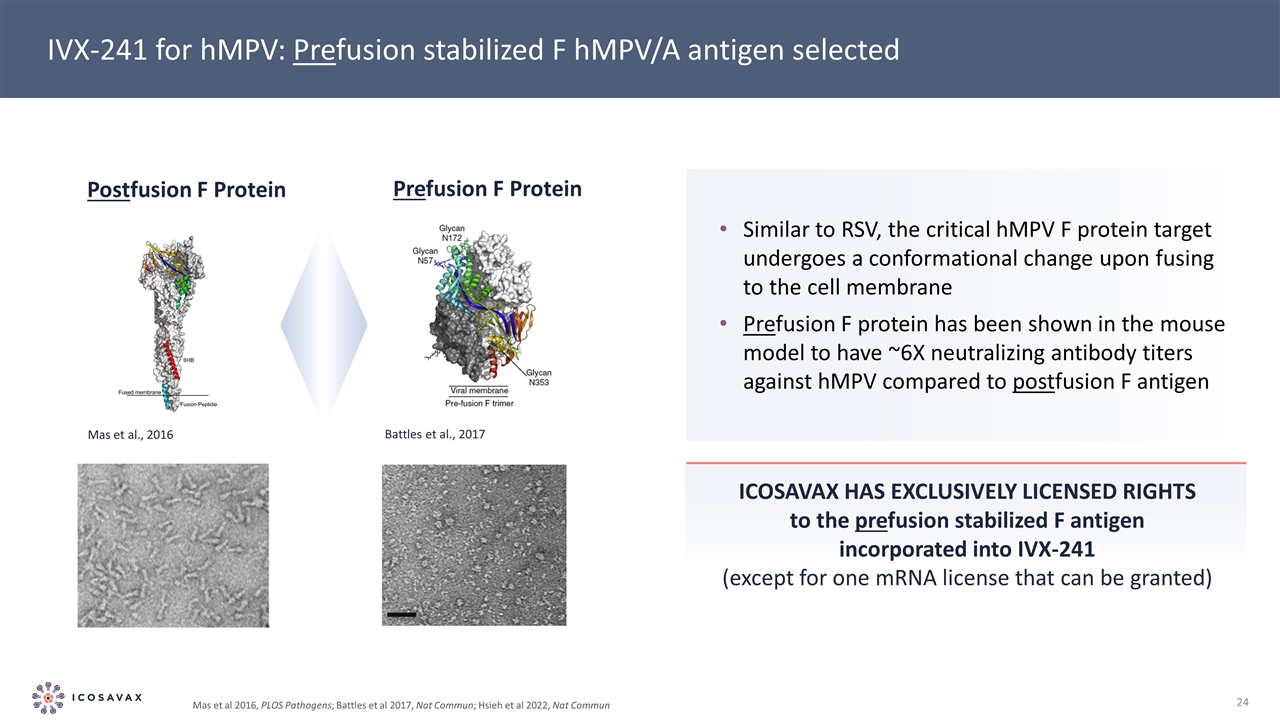
Icosavax has exclusively licensed rights to the prefusion stabilized F antigen incorporated into IVX-241 (except for one mRNA license that can be granted) IVX-241 for hMPV: Prefusion stabilized F hMPV/A antigen selected Similar to RSV, the critical hMPV F protein target undergoes a conformational change upon fusing to the cell membrane Prefusion F protein has been shown in the mouse model to have ~6X neutralizing antibody titers against hMPV compared to postfusion F antigen Prefusion F Protein Postfusion F Protein Battles et al., 2017 Mas et al., 2016 Mas et al 2016, PLOS Pathogens; Battles et al 2017, Nat Commun; Hsieh et al 2022, Nat Commun
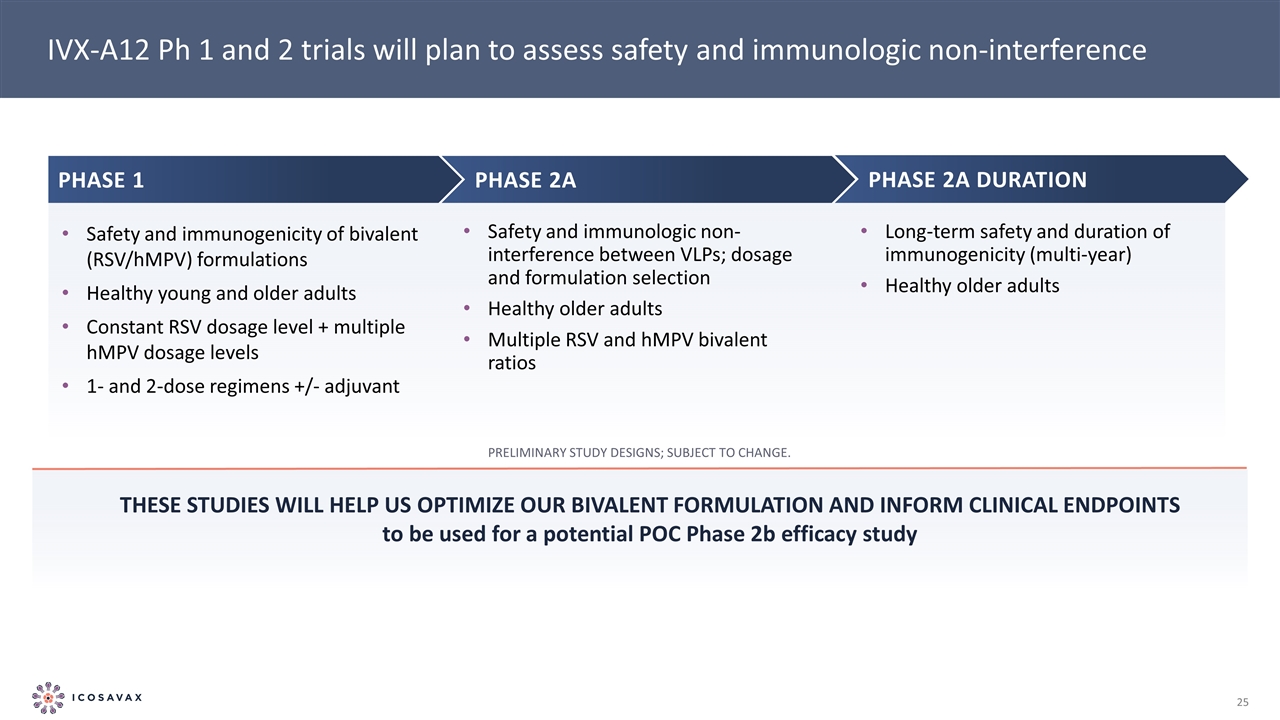
Safety and immunogenicity of bivalent (RSV/hMPV) formulations Healthy young and older adults Constant RSV dosage level + multiple hMPV dosage levels 1- and 2-dose regimens +/- adjuvant Phase 2A DURATION Phase 1 Phase 2a IVX-A12 Ph 1 and 2 trials will plan to assess safety and immunologic non-interference These studies will help us optimize our bivalent formulation and inform clinical endpoints to be used for a potential POC Phase 2b efficacy study Preliminary study designs; subject to change. Safety and immunologic non-interference between VLPs; dosage and formulation selection Healthy older adults Multiple RSV and hMPV bivalent ratios Long-term safety and duration of immunogenicity (multi-year) Healthy older adults
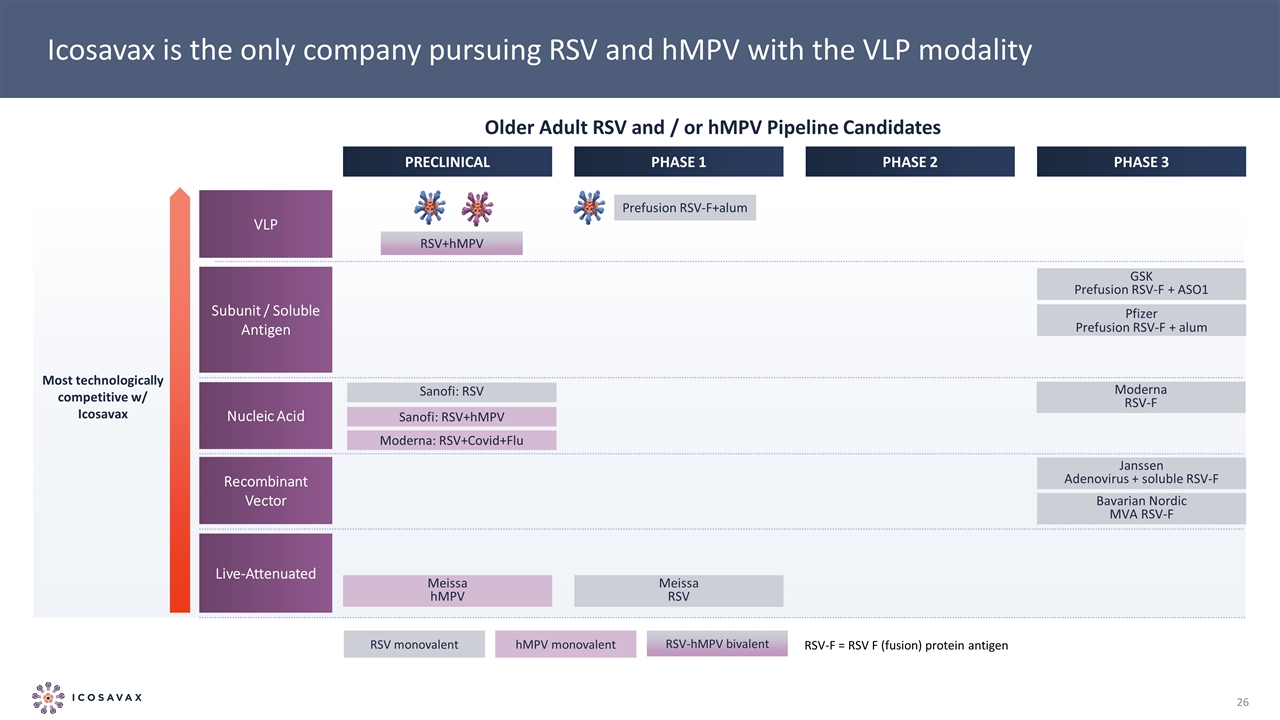
Icosavax is the only company pursuing RSV and hMPV with the VLP modality PRECLINICAL PHASE 1 PHASE 2 PHASE 3 VLP Subunit / Soluble Antigen Recombinant Vector GSK Prefusion RSV-F + ASO1 Pfizer Prefusion RSV-F + alum Janssen Adenovirus + soluble RSV-F Bavarian Nordic MVA RSV-F Live-Attenuated Meissa RSV Most technologically competitive w/ Icosavax Nucleic Acid Moderna RSV-F Meissa hMPV RSV monovalent hMPV monovalent RSV-hMPV bivalent RSV+hMPV Prefusion RSV-F+alum RSV-F = RSV F (fusion) protein antigen Sanofi: RSV Sanofi: RSV+hMPV Moderna: RSV+Covid+Flu Older Adult RSV and / or hMPV Pipeline Candidates
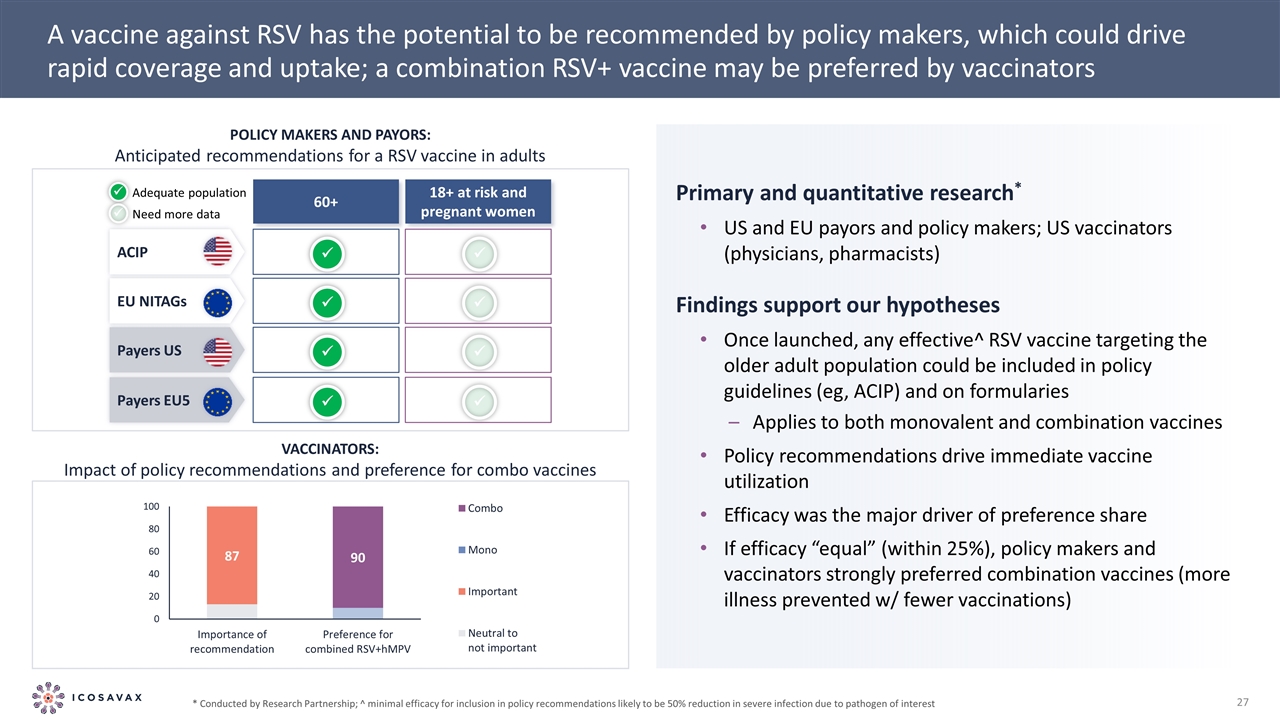
A vaccine against RSV has the potential to be recommended by policy makers, which could drive rapid coverage and uptake; a combination RSV+ vaccine may be preferred by vaccinators Policy makers and payors: Anticipated recommendations for a RSV vaccine in adults 18+ at risk and pregnant women 60+ ü ü Adequate population Need more data ACIP EU NITAGs Payers US Payers EU5 ü ü ü ü ü ü ü ü Primary and quantitative research* US and EU payors and policy makers; US vaccinators (physicians, pharmacists) Findings support our hypotheses Once launched, any effective^ RSV vaccine targeting the older adult population could be included in policy guidelines (eg, ACIP) and on formularies Applies to both monovalent and combination vaccines Policy recommendations drive immediate vaccine utilization Efficacy was the major driver of preference share If efficacy “equal” (within 25%), policy makers and vaccinators strongly preferred combination vaccines (more illness prevented w/ fewer vaccinations) * Conducted by Research Partnership; ^ minimal efficacy for inclusion in policy recommendations likely to be 50% reduction in severe infection due to pathogen of interest Vaccinators: Impact of policy recommendations and preference for combo vaccines

Covid-19 Vaccine Candidate (IVX-411)
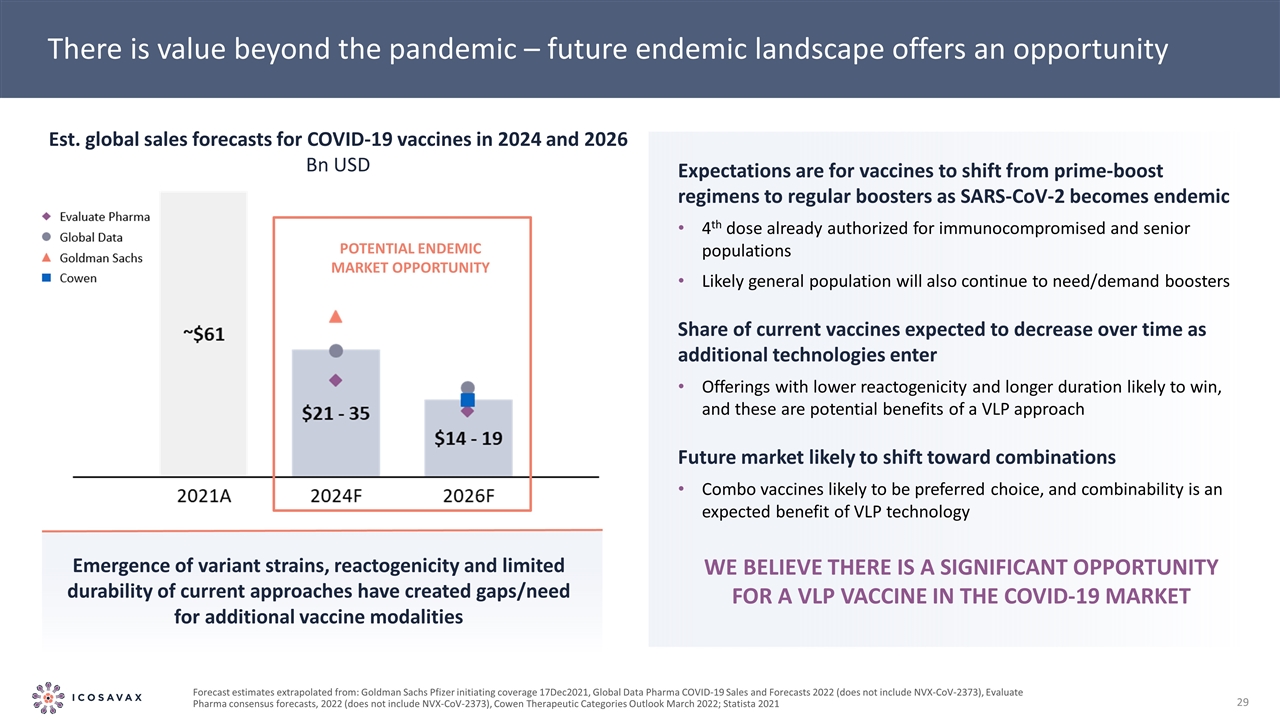
Potential endemic market opportunity There is value beyond the pandemic – future endemic landscape offers an opportunity Emergence of variant strains, reactogenicity and limited durability of current approaches have created gaps/need for additional vaccine modalities Expectations are for vaccines to shift from prime-boost regimens to regular boosters as SARS-CoV-2 becomes endemic 4th dose already authorized for immunocompromised and senior populations Likely general population will also continue to need/demand boosters Share of current vaccines expected to decrease over time as additional technologies enter Offerings with lower reactogenicity and longer duration likely to win, and these are potential benefits of a VLP approach Future market likely to shift toward combinations Combo vaccines likely to be preferred choice, and combinability is an expected benefit of VLP technology Est. global sales forecasts for COVID-19 vaccines in 2024 and 2026 Bn USD Forecast estimates extrapolated from: Goldman Sachs Pfizer initiating coverage 17Dec2021, Global Data Pharma COVID-19 Sales and Forecasts 2022 (does not include NVX-CoV-2373), Evaluate Pharma consensus forecasts, 2022 (does not include NVX-CoV-2373), Cowen Therapeutic Categories Outlook March 2022; Statista 2021 We believe there is a significant opportunity for a VLP vaccine in the COVID-19 market
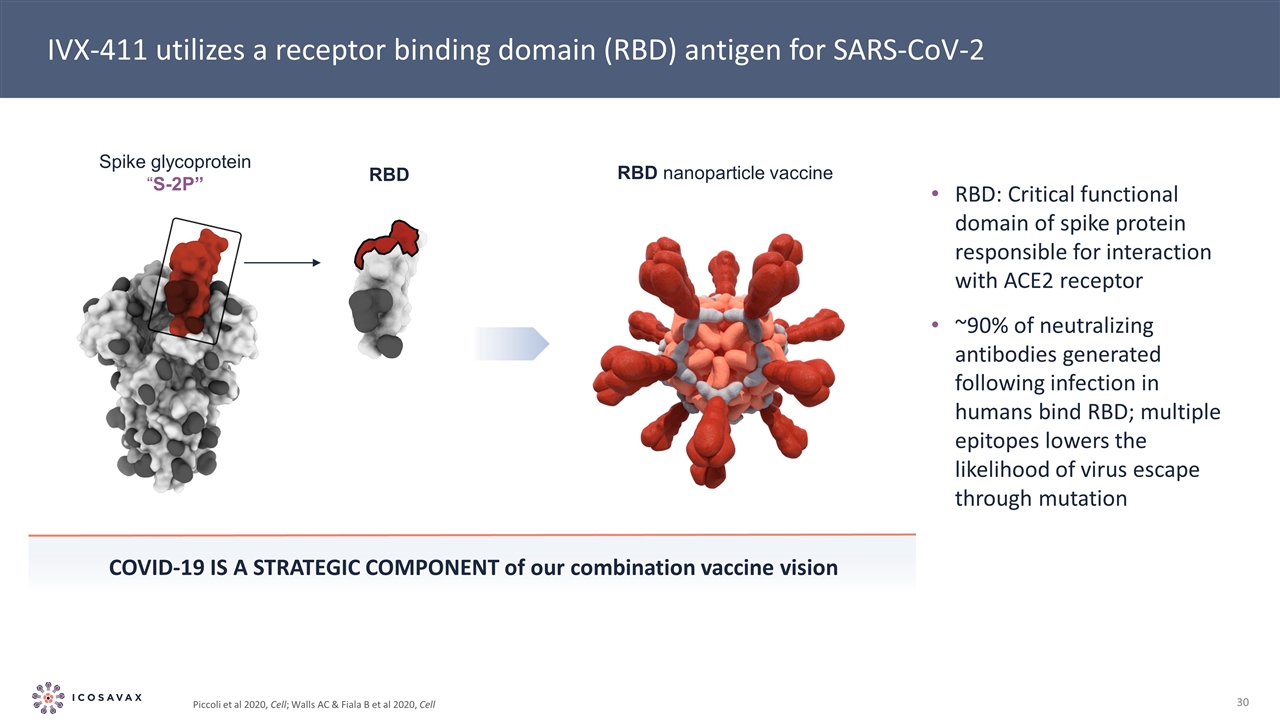
Spike glycoprotein “S-2P” RBD RBD nanoparticle vaccine IVX-411 utilizes a receptor binding domain (RBD) antigen for SARS-CoV-2 Piccoli et al 2020, Cell; Walls AC & Fiala B et al 2020, Cell RBD: Critical functional domain of spike protein responsible for interaction with ACE2 receptor ~90% of neutralizing antibodies generated following infection in humans bind RBD; multiple epitopes lowers the likelihood of virus escape through mutation Covid-19 is a strategic component of our combination vaccine vision
Study met primary safety and immunogenicity objectives: Phase 1 in primary setting (n = 84). 2 doses, 28 days apart: 5, 25, 125 ug IVX-411 dosage levels +/- adjuvant Phase 2 in booster setting (n = 84). 1 dose, 3-6 months after completion of primary regimen: 5, 25, 125 ug IVX-411 +/- adjuvant IVX-411 was generally safe and well tolerated Frequency of observed solicited and unsolicited adverse events (AEs) comparable with placebo Mild to moderate reactogenicity, none severe nor dose-limiting. No related serious AEs or AEs of special interest Immunogenicity shown in primary and booster vaccination Primary: Magnitude of nAb and IgG antibody titers for Wild-Type (WT) comparable to or below the Human Convalescent Sera (HCS) levels and above placebo in primary vaccination. High rates of seroconversion in adjuvanted groups Booster: Heterologous boosting after mRNA (3/4) and adeno (1/4) primary vaccination induced up to 5x rise from baseline for WT Variants: Immune responses seen across all variants of concern (beta, delta, omicron) in primary and booster vaccination context Key takeaways from IVX-411 Phase 1/2 topline data readout Immunogenicity inconsistent with expectations for RBD-VLP based on available preclinical data and VLP technology platforms Root cause investigation for low potency UNDERWAY, including antigen stability and characterization and in vivo potency
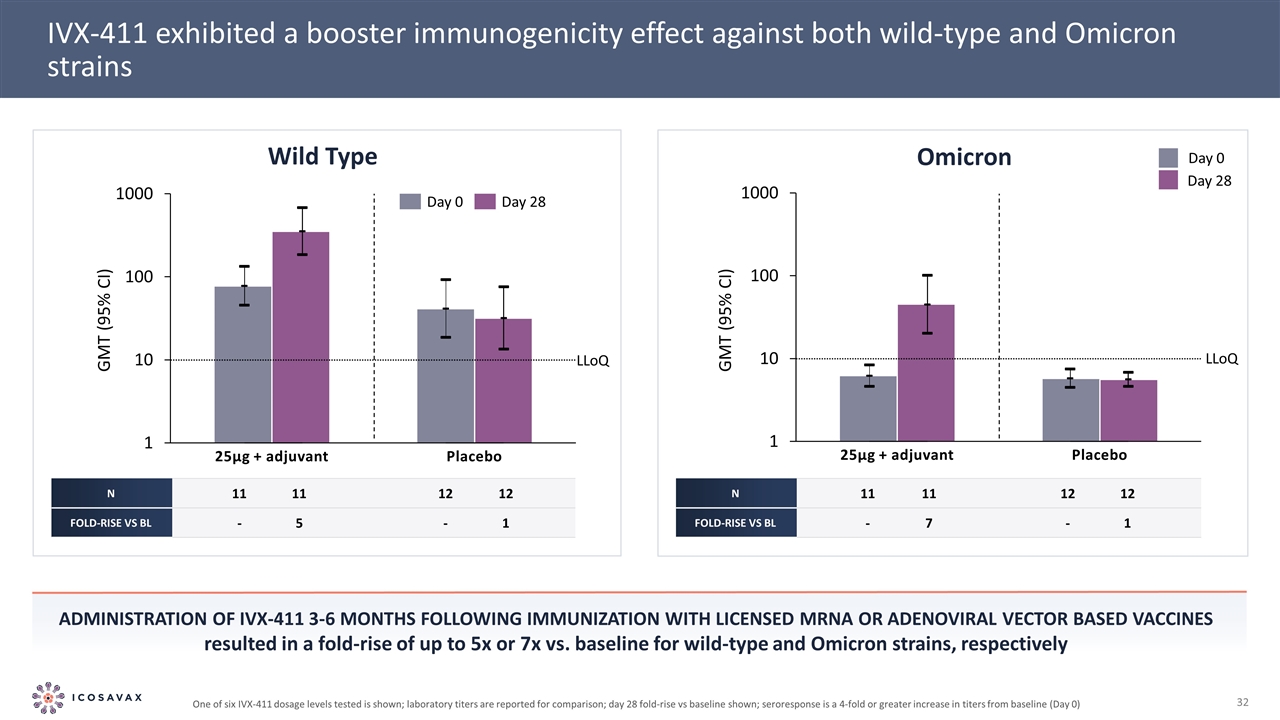
25µg + adjuvant IVX-411 exhibited a booster immunogenicity effect against both wild-type and Omicron strains Administration of IVX-411 3-6 months following immunization with licensed mRNA or Adenoviral vector based vaccines resulted in a fold-rise of up to 5x or 7x vs. baseline for wild-type and Omicron strains, respectively 25µg + adjuvant N 11 11 12 12 Fold-rise vs BL - 5 - 1 N 11 11 12 12 Fold-rise vs BL - 7 - 1 Omicron Wild Type GMT (95% CI) GMT (95% CI) LLoQ LLoQ One of six IVX-411 dosage levels tested is shown; laboratory titers are reported for comparison; day 28 fold-rise vs baseline shown; seroresponse is a 4-fold or greater increase in titers from baseline (Day 0)
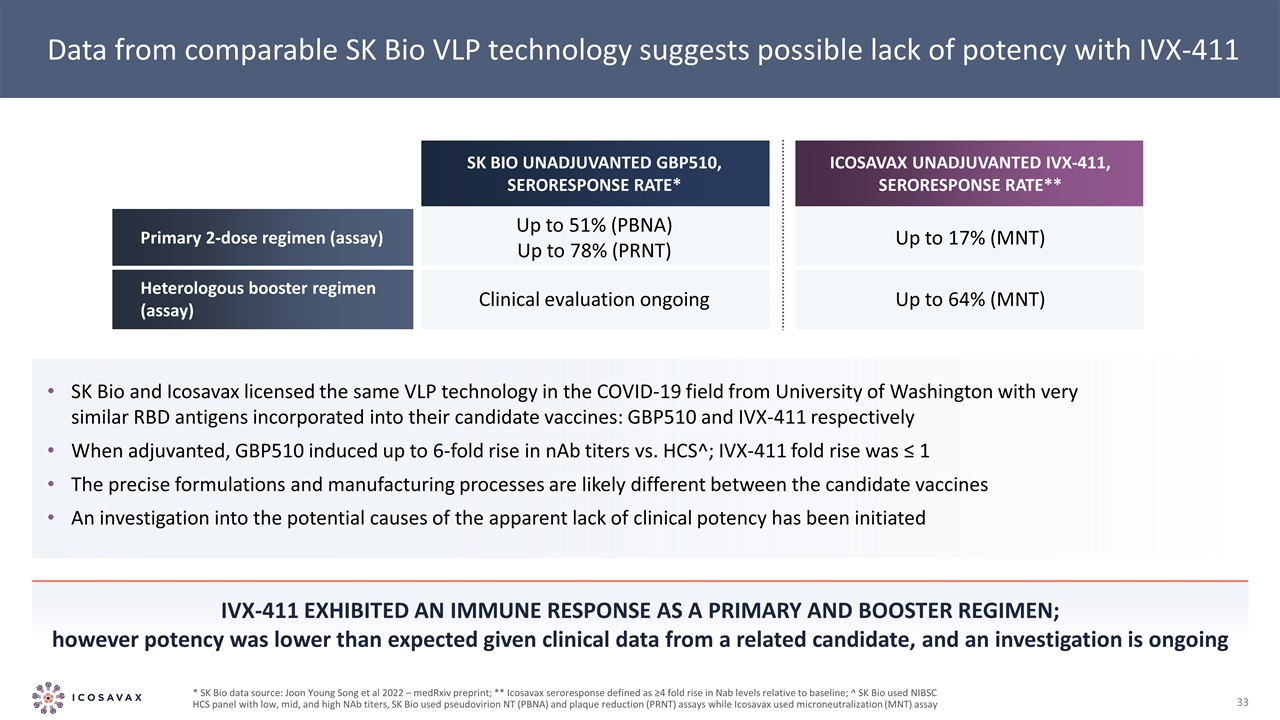
Data from comparable SK Bio VLP technology suggests possible lack of potency with IVX-411 SK Bio and Icosavax licensed the same VLP technology in the COVID-19 field from University of Washington with very similar RBD antigens incorporated into their candidate vaccines: GBP510 and IVX-411 respectively When adjuvanted, GBP510 induced up to 6-fold rise in nAb titers vs. HCS^; IVX-411 fold rise was ≤ 1 The precise formulations and manufacturing processes are likely different between the candidate vaccines An investigation into the potential causes of the apparent lack of clinical potency has been initiated IVX-411 exhibited aN immune response as a primary and booster regimen; however potency was lower than expected given clinical data from a related candidate, and an investigation is ongoing * SK Bio data source: Joon Young Song et al 2022 – medRxiv preprint; ** Icosavax seroresponse defined as ≥4 fold rise in Nab levels relative to baseline; ^ SK Bio used NIBSC HCS panel with low, mid, and high NAb titers, SK Bio used pseudovirion NT (PBNA) and plaque reduction (PRNT) assays while Icosavax used microneutralization (MNT) assay SK Bio unadjuvanted GBP510, seroresponse rate* Icosavax unadjuvanted IVX-411, seroresponse rate** Primary 2-dose regimen (assay) Up to 51% (PBNA) Up to 78% (PRNT) Up to 17% (MNT) Heterologous booster regimen (assay) Clinical evaluation ongoing Up to 64% (MNT)

Flu Program
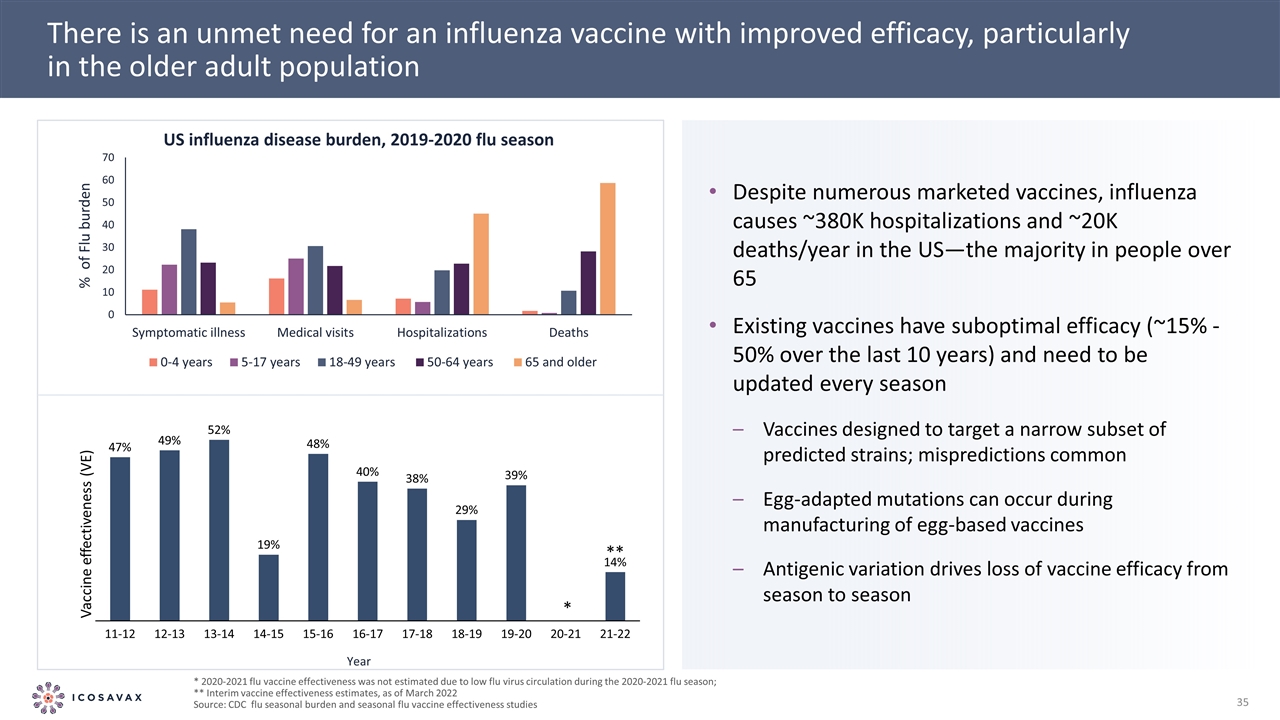
There is an unmet need for an influenza vaccine with improved efficacy, particularly in the older adult population * 2020-2021 flu vaccine effectiveness was not estimated due to low flu virus circulation during the 2020-2021 flu season; ** Interim vaccine effectiveness estimates, as of March 2022 Source: CDC flu seasonal burden and seasonal flu vaccine effectiveness studies Despite numerous marketed vaccines, influenza causes ~380K hospitalizations and ~20K deaths/year in the US—the majority in people over 65 Existing vaccines have suboptimal efficacy (~15% - 50% over the last 10 years) and need to be updated every season Vaccines designed to target a narrow subset of predicted strains; mispredictions common Egg-adapted mutations can occur during manufacturing of egg-based vaccines Antigenic variation drives loss of vaccine efficacy from season to season % of Flu burden Year * **
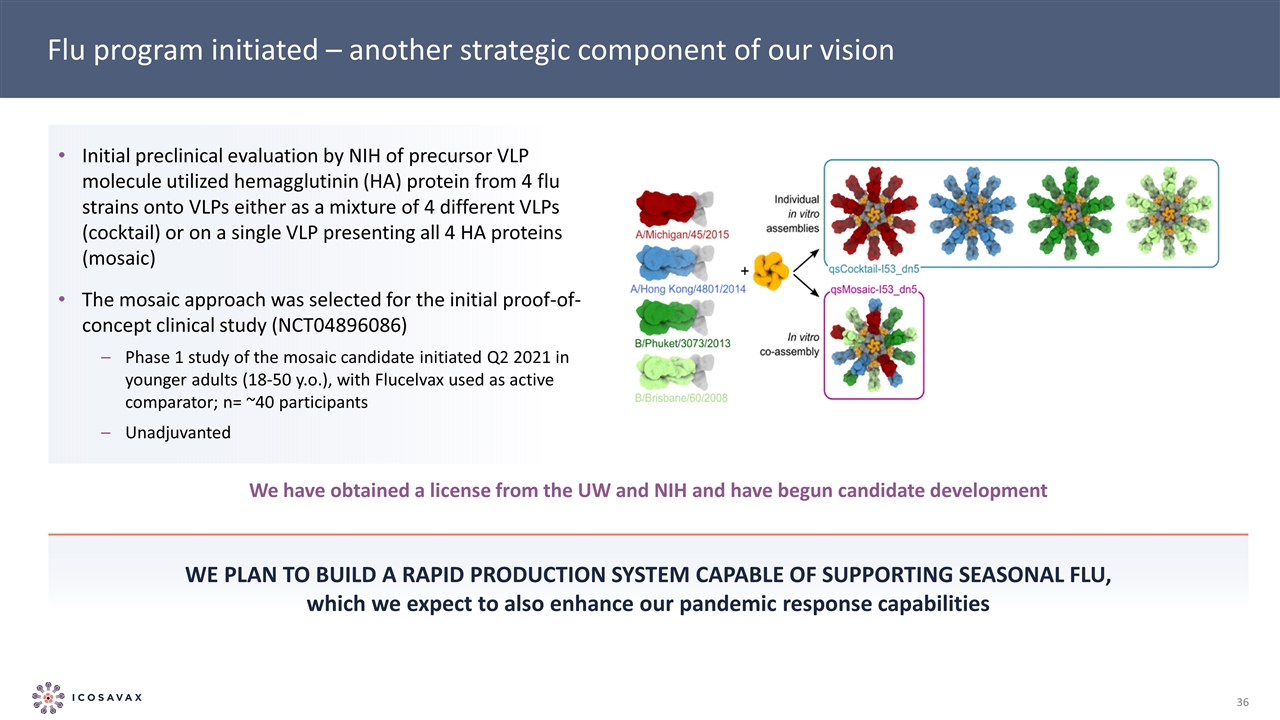
Flu program initiated – another strategic component of our vision We plan to build a rapid production system capable of supporting seasonal flu, which we expect to also enhance our pandemic response capabilities Initial preclinical evaluation by NIH of precursor VLP molecule utilized hemagglutinin (HA) protein from 4 flu strains onto VLPs either as a mixture of 4 different VLPs (cocktail) or on a single VLP presenting all 4 HA proteins (mosaic) The mosaic approach was selected for the initial proof-of-concept clinical study (NCT04896086) Phase 1 study of the mosaic candidate initiated Q2 2021 in younger adults (18-50 y.o.), with Flucelvax used as active comparator; n= ~40 participants Unadjuvanted We have obtained a license from the UW and NIH and have begun candidate development
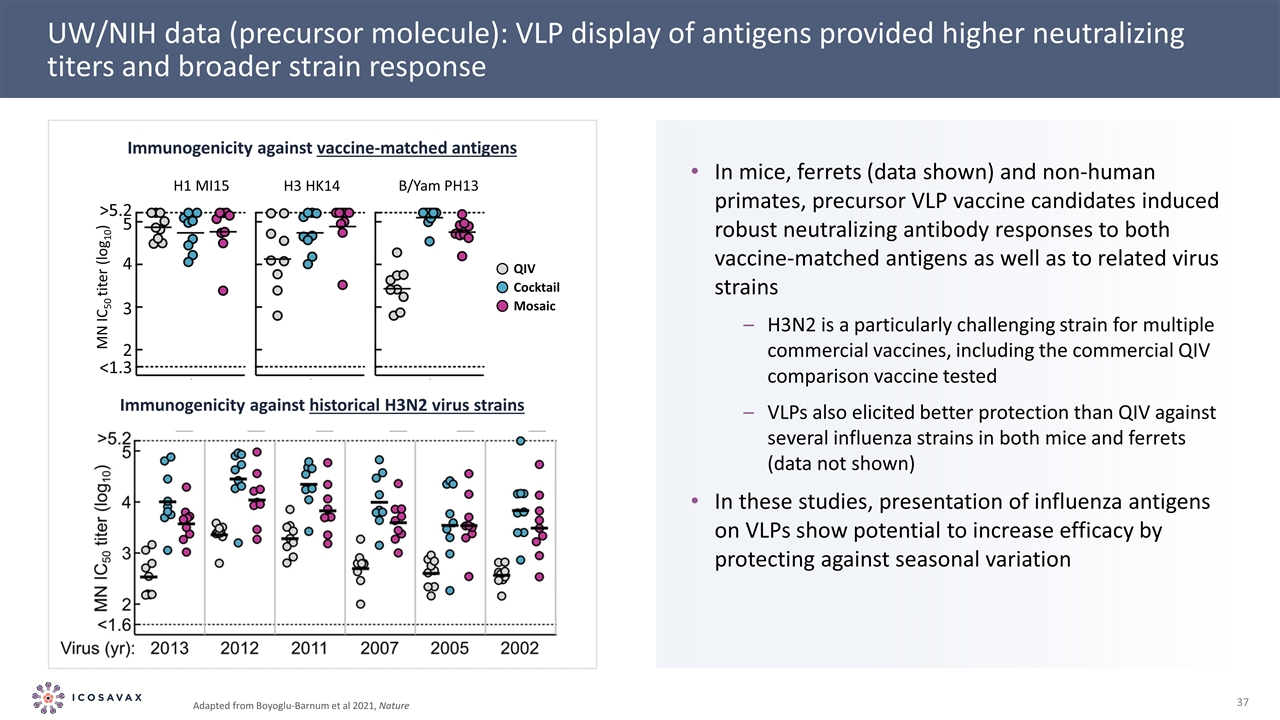
UW/NIH data (precursor molecule): VLP display of antigens provided higher neutralizing titers and broader strain response Immunogenicity against vaccine-matched antigens Immunogenicity against historical H3N2 virus strains Adapted from Boyoglu-Barnum et al 2021, Nature In mice, ferrets (data shown) and non-human primates, precursor VLP vaccine candidates induced robust neutralizing antibody responses to both vaccine-matched antigens as well as to related virus strains H3N2 is a particularly challenging strain for multiple commercial vaccines, including the commercial QIV comparison vaccine tested VLPs also elicited better protection than QIV against several influenza strains in both mice and ferrets (data not shown) In these studies, presentation of influenza antigens on VLPs show potential to increase efficacy by protecting against seasonal variation Cocktail QIV Mosaic H3 HK14 H1 MI15 B/Yam PH13 <1.3 2 3 4 5 >5.2 MN IC50 titer (log10)

In summary: Why Icosavax?

Executive Team Executive Team Adam Simpson Chief Executive Officer Doug Holtzman, Ph.D. MPH Chief Scientific Officer Niranjan Kanesa-thasan, M.D. MTMH Chief Medical Officer Tom Russo Chief Financial Officer Cassia Cearley, Ph.D. Chief Business Officer Elizabeth Bekiroğlu General Counsel Charles Richardson, Ph.D. SVP, Technical Operations Leadership Scientific Advisory Board Neil King, Ph.D. (Chair) Co-Founder David Baker, Ph.D. Co-Founder Ralf Clemens, M.D. Barney Graham, M.D. Ph.D. Christian Mandl, M.D. Ph.D. Jean-Paul Prieels, Ph.D. Robin Robinson, Ph.D. Board of Directors Terry Gould Adams Street Peter Kolchinsky, Ph.D. RA Capital Heidi Kunz Former EVP & CFO of Blue Shield of CA Mark McDade (Chair) Qiming Venture Partners John Shiver, Ph.D. IMG Biosciences; former Sanofi Pasteur, Merk, NIH Adam Simpson CEO Ann Veneman Former Executive Director, UNICEF

Developing VLP-based vaccines and combinations, with vision of pan-respiratory vaccines for older adults Unique modality intentionally designed to mimic the structure of viruses to empower better immune response VLPs have multiple potential benefits—and offer multiple manufacturing advantages and attractive commercial opportunities in major areas of unmet need RSV vaccine candidate IVX-121 currently in Phase 1/1b with topline data coming Q2’22; first combination vaccine candidate and lead program, IVX-A12 for RSV/hMPV planned to enter Phase 1 later this year COVID vaccine candidate IVX-411 has completed Phase 1/2; next steps to be determined following investigation Emerging flu program in preclinical development Continuing to expand capabilities, including new research team bringing state-of-the-art antigen design, optimizing speed in manufacturing, etc. Experienced team with extensive expertise in protein design and vaccine development supported by leading healthcare investors and distinguished Scientific Advisory Board Key highlights
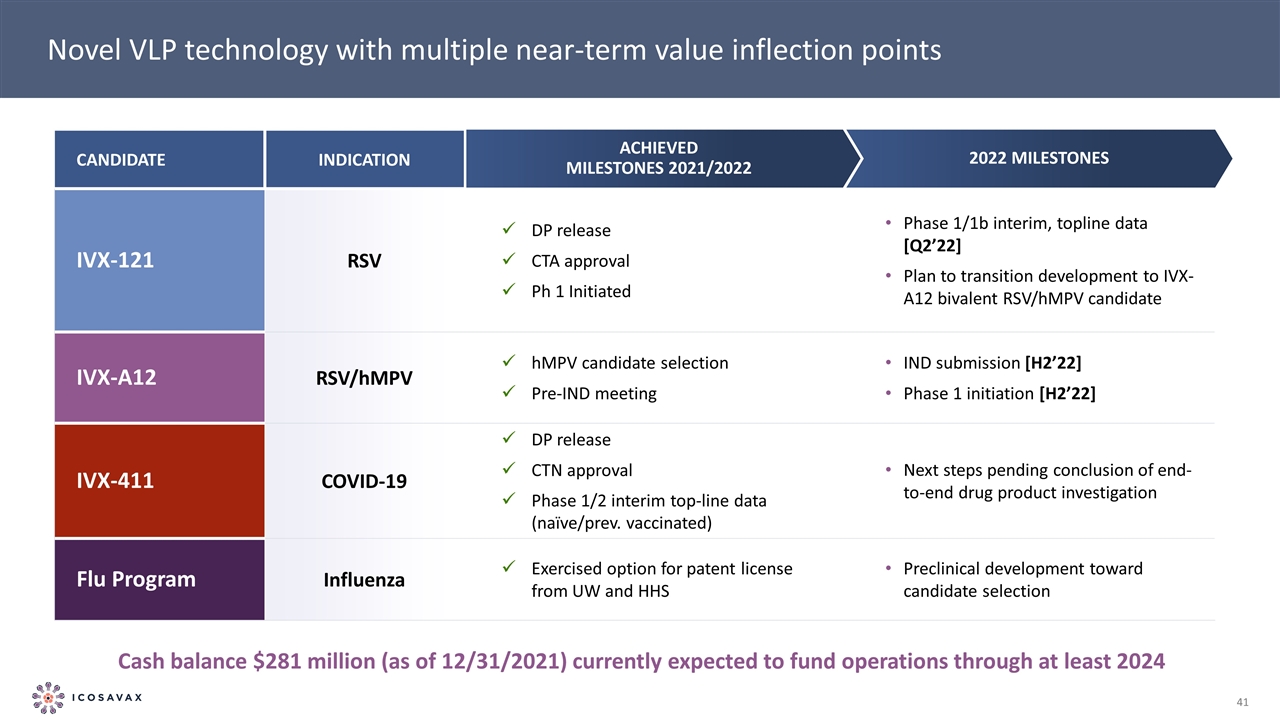
Novel VLP technology with multiple near-term value inflection points Candidate Indication IVX-121 RSV DP release CTA approval Ph 1 Initiated Phase 1/1b interim, topline data [Q2’22] Plan to transition development to IVX-A12 bivalent RSV/hMPV candidate IVX-A12 RSV/hMPV hMPV candidate selection Pre-IND meeting IND submission [H2’22] Phase 1 initiation [H2’22] IVX-411 Covid-19 DP release CTN approval Phase 1/2 interim top-line data (naïve/prev. vaccinated) Next steps pending conclusion of end-to-end drug product investigation Flu Program Influenza Exercised option for patent license from UW and HHS Preclinical development toward candidate selection Achieved Milestones 2021/2022 2022 Milestones Cash balance $281 million (as of 12/31/2021) currently expected to fund operations through at least 2024








































