
IVX-121 Ph 1/1b Six-Month Durability Update December 13, 2022 Exhibit 99.1

Forward looking statements Statements contained in this presentation regarding matters that are not historical facts are forward-looking statements. The forward-looking statements are based on the company’s current beliefs and expectations and include but are not limited to: the potential for the company’s VLP platform to result in safe and effective vaccines against infectious diseases including IVX-121 for RSV and IVX-A12 for hMPV and RSV, and to be well-suited for combination vaccines; and the company’s ability to advance its development programs and achieve the noted development milestones in 2023. Actual results or developments may differ from those set forth in this presentation due to the risks and uncertainties inherent in the company’s business, including, without limitation: the early stage of the company’s development efforts; the risk that results of a clinical trial at a particular time point may not predict final results and that an outcome may materially change as follow-up of subjects continues and following more comprehensive reviews of the data; the possibility of disappointing results in later clinical trials despite promising results in earlier preclinical research or clinical trials; potential unexpected adverse side effects or inadequate immunogenicity or efficacy of IXV-121 or IVX-A12 that may limit their development, regulatory approval, and/or commercialization; the company’s approach to the development of vaccine candidates, including its IVX-A12 combination bivalent RSV/hMPV VLP vaccine candidate, which is a novel and unproven approach; potential delays in the development process including without limitation in the enrollment, conduct of, and receipt of data from, clinical trials; the company’s dependence on third parties in connection with manufacturing, research, and clinical testing; the potential for challenges encountered in the manufacturing and scale up process; competing approaches limiting the commercial value of the company’s vaccine candidates; and other risks described in the company’s prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in the company’s quarterly report on Form 10-Q for the quarter ended September 30, 2022 and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and the company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995.

Positive IVX-121 Phase 1/1b Topline Interim Results and Six-Month Immunogenicity Update As reported in June and December 2022, respectively Initial indication of the company’s differentiated VLP platform technology High RSV-A and RSV-B neutralizing antibody titers seen from the lowest dose tested Positive topline data from the Phase 1/1b trial of IVX-121 suggest a competitive initial profile in RSV Similarly robust responses in older versus young adults, favorable tolerability, suitability for combination Opportunities to further differentiate RSV profile; durability to be assessed in Phase 1b extension out to 12 months Proceeded to combination with proprietary hMPV VLP in a differentiated bivalent vaccine candidate IVX-A12 (RSV/hMPV) for older adults Tolerability profile up to maximum dose tested in Phase 1 (250 µg) and immunogenicity down to 25 µg gives room for multivalent combinations Six-month immunogenicity update showed durability of RSV-A and RSV-B neutralizing antibody titers up to 180 days after vaccination Geometric mean titers (GMT) against RSV-A through day 180 persisting at 64-98% of the GMTs at day 28 in older adults First clinical evidence of potential differentiation on durability with company’s VLP platform technology No safety concerns, IVX-121 continued to be generally well tolerated Next step: IVX-121 (RSV) Phase 1b 12-month data and IVX-A12 (RSV/hMPV) Phase 1 topline data expected mid-2023
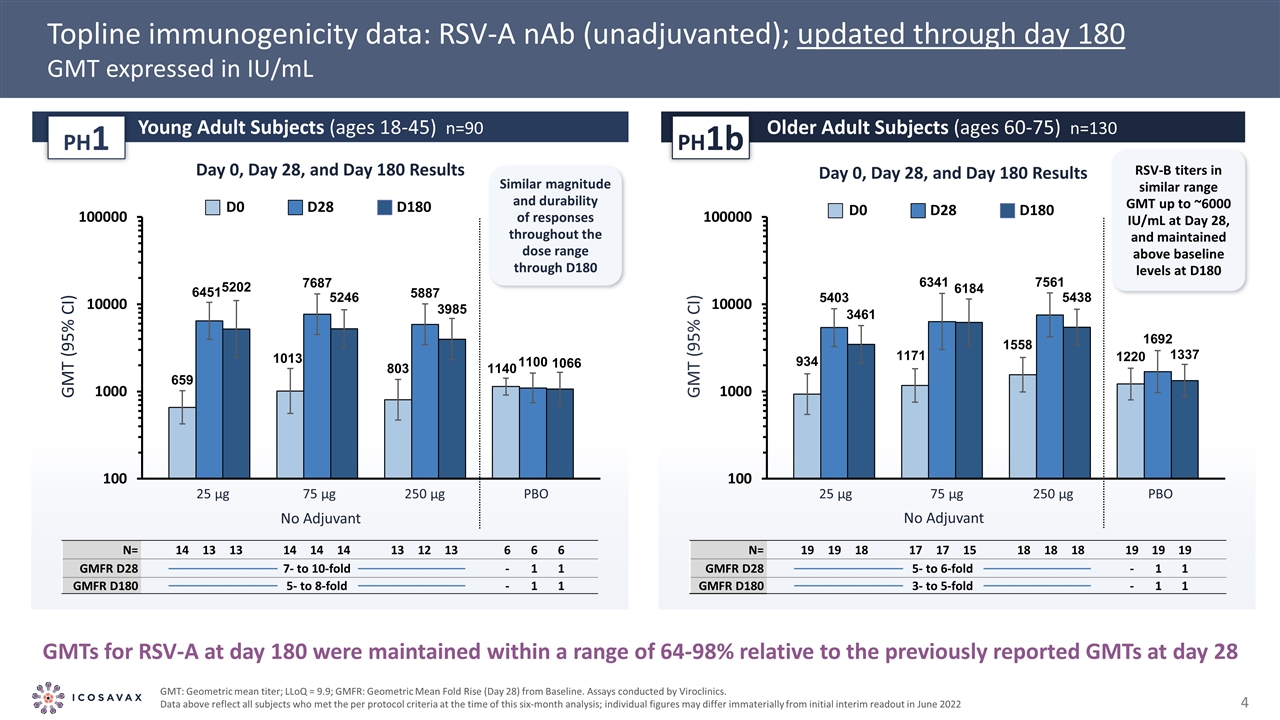
Topline immunogenicity data: RSV-A nAb (unadjuvanted); updated through day 180 GMT expressed in IU/mL GMTs for RSV-A at day 180 were maintained within a range of 64-98% relative to the previously reported GMTs at day 28 GMT: Geometric mean titer; LLoQ = 9.9; GMFR: Geometric Mean Fold Rise (Day 28) from Baseline. Assays conducted by Viroclinics. Data above reflect all subjects who met the per protocol criteria at the time of this six-month analysis; individual figures may differ immaterially from initial interim readout in June 2022 GMT (95% CI) No Adjuvant PBO GMT (95% CI) 25 µg 75 µg 250 µg Young Adult Subjects (ages 18-45) n=90 PH1 Older Adult Subjects (ages 60-75) n=130 PH1b No Adjuvant PBO 25 µg 75 µg 250 µg Similar magnitude and durability of responses throughout the dose range through D180 Day 0, Day 28, and Day 180 Results D0 D28 D180 Day 0, Day 28, and Day 180 Results D0 D28 D180 RSV-B titers in similar range GMT up to ~6000 IU/mL at Day 28, and maintained above baseline levels at D180 N= 14 13 13 14 14 14 13 12 13 6 6 6 GMFR D28 7- to 10-fold 92 77 - 57 64 - 67 69 - 1 1 GMFR D180 5- to 8-fold 10 8 - 8 5 - 7 5 - 1 1 N= 19 19 18 17 17 15 18 18 18 19 19 19 GMFR D28 5- to 6-fold 58 39 - 65 53 - 44 33 - 1 1 GMFR D180 3- to 5-fold 6 4 - 5 5 - 5 3 - 1 1
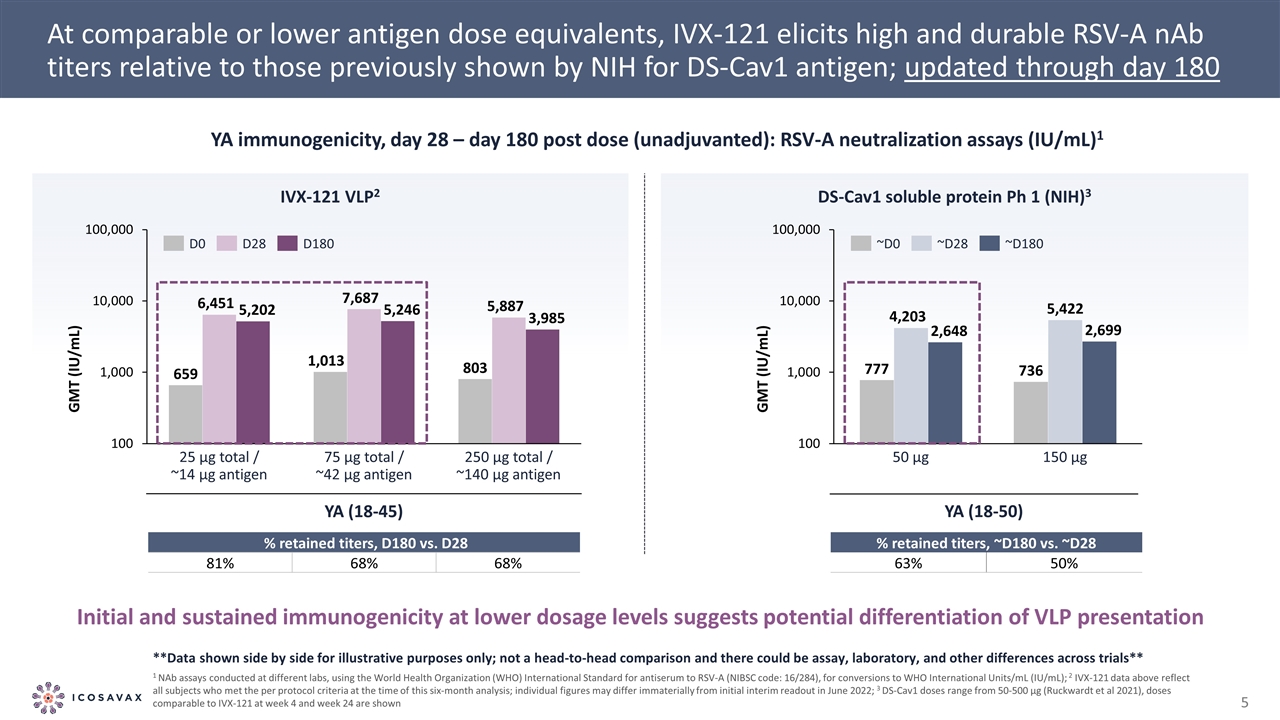
At comparable or lower antigen dose equivalents, IVX-121 elicits high and durable RSV-A nAb titers relative to those previously shown by NIH for DS-Cav1 antigen; updated through day 180 DS-Cav1 soluble protein Ph 1 (NIH)3 YA immunogenicity, day 28 – day 180 post dose (unadjuvanted): RSV-A neutralization assays (IU/mL)1 IVX-121 VLP2 YA (18-50) YA (18-45) GMT (IU/mL) GMT (IU/mL) % retained titers, D180 vs. D28 81% 68% 68% % retained titers, ~D180 vs. ~D28 63% 50% **Data shown side by side for illustrative purposes only; not a head-to-head comparison and there could be assay, laboratory, and other differences across trials** 1 NAb assays conducted at different labs, using the World Health Organization (WHO) International Standard for antiserum to RSV-A (NIBSC code: 16/284), for conversions to WHO International Units/mL (IU/mL); 2 IVX-121 data above reflect all subjects who met the per protocol criteria at the time of this six-month analysis; individual figures may differ immaterially from initial interim readout in June 2022; 3 DS-Cav1 doses range from 50-500 µg (Ruckwardt et al 2021), doses comparable to IVX-121 at week 4 and week 24 are shown Initial and sustained immunogenicity at lower dosage levels suggests potential differentiation of VLP presentation
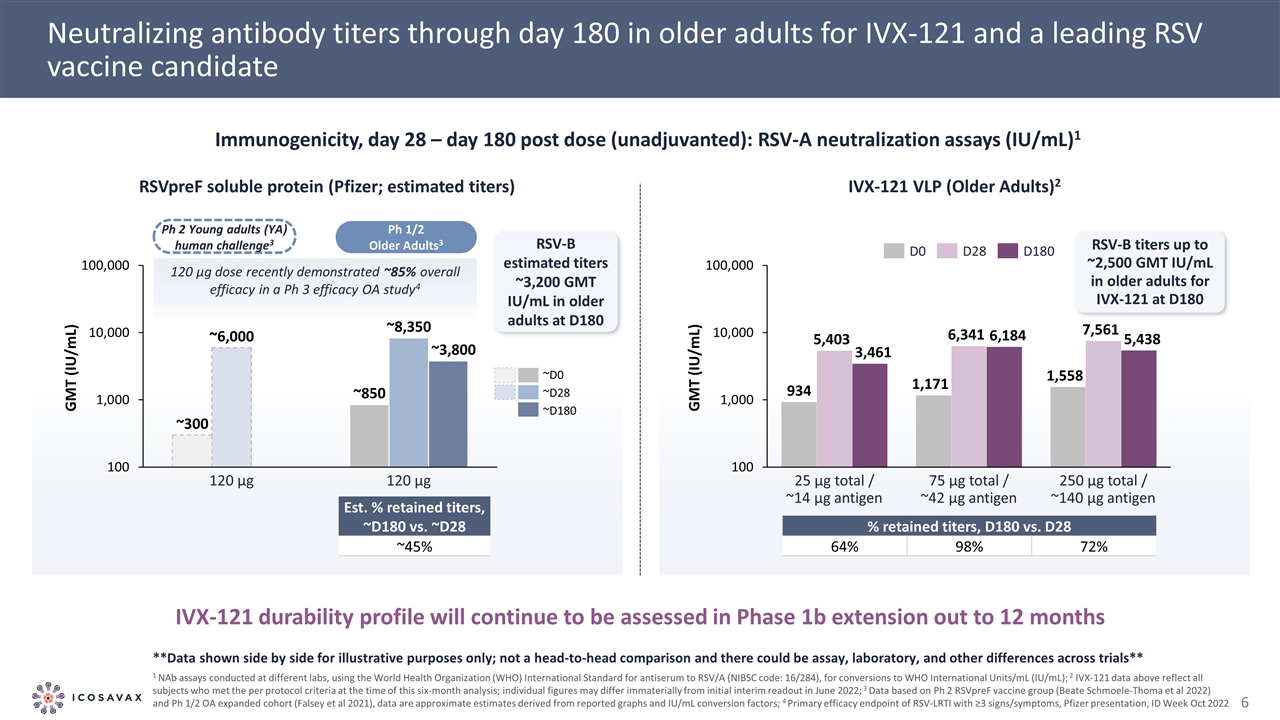
120 µg dose recently demonstrated ~85% overall efficacy in a Ph 3 efficacy OA study4 Neutralizing antibody titers through day 180 in older adults for IVX-121 and a leading RSV vaccine candidate RSVpreF soluble protein (Pfizer; estimated titers) IVX-121 VLP (Older Adults)2 **Data shown side by side for illustrative purposes only; not a head-to-head comparison and there could be assay, laboratory, and other differences across trials** 1 NAb assays conducted at different labs, using the World Health Organization (WHO) International Standard for antiserum to RSV/A (NIBSC code: 16/284), for conversions to WHO International Units/mL (IU/mL); 2 IVX-121 data above reflect all subjects who met the per protocol criteria at the time of this six-month analysis; individual figures may differ immaterially from initial interim readout in June 2022; 3 Data based on Ph 2 RSVpreF vaccine group (Beate Schmoele-Thoma et al 2022) and Ph 1/2 OA expanded cohort (Falsey et al 2021), data are approximate estimates derived from reported graphs and IU/mL conversion factors; 4 Primary efficacy endpoint of RSV-LRTI with ≥3 signs/symptoms, Pfizer presentation, ID Week Oct 2022 Immunogenicity, day 28 – day 180 post dose (unadjuvanted): RSV-A neutralization assays (IU/mL)1 GMT (IU/mL) RSV-B estimated titers ~3,200 GMT IU/mL in older adults at D180 IVX-121 durability profile will continue to be assessed in Phase 1b extension out to 12 months RSV-B titers up to ~2,500 GMT IU/mL in older adults for IVX-121 at D180 % retained titers, D180 vs. D28 64% 98% 72% Ph 1/2 Older Adults3 Ph 2 Young adults (YA) human challenge3 GMT (IU/mL) Est. % retained titers, ~D180 vs. ~D28 ~45%
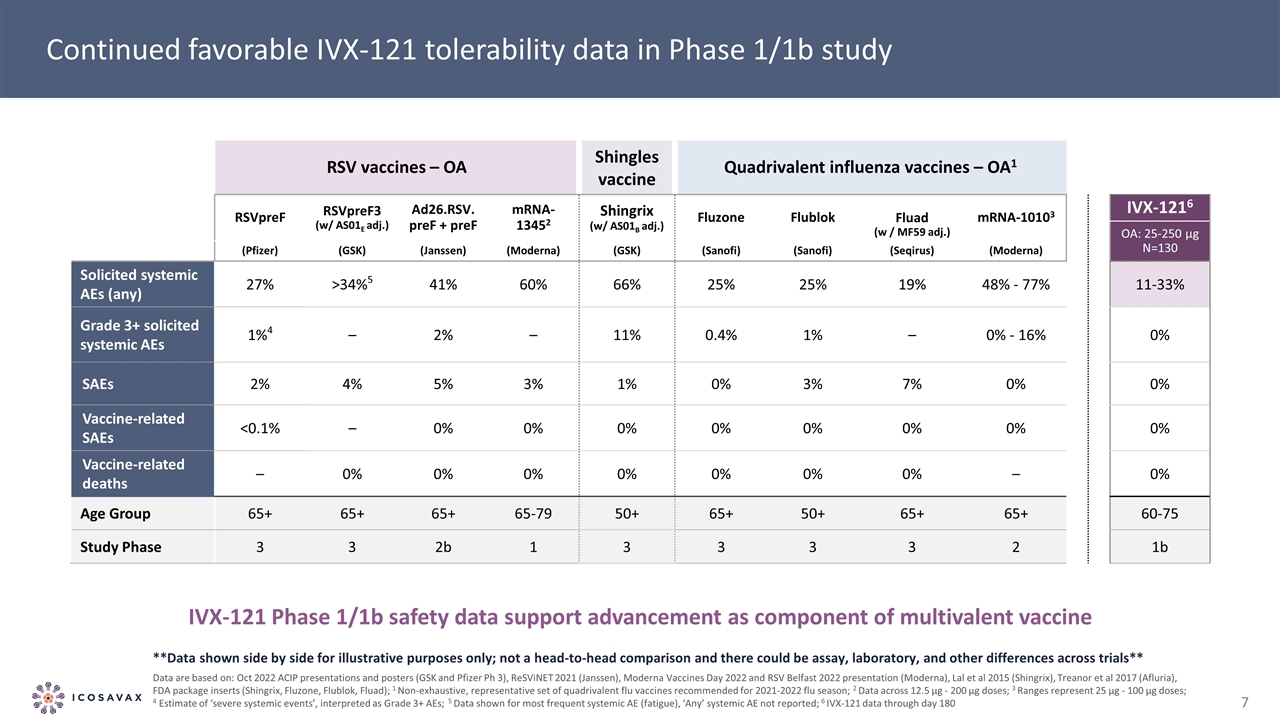
Continued favorable IVX-121 tolerability data in Phase 1/1b study RSV vaccines – OA Shingles vaccine Quadrivalent influenza vaccines – OA1 RSVpreF RSVpreF3 (w/ AS01E adj.) Ad26.RSV. preF + preF mRNA-13452 Shingrix (w/ AS01B adj.) Fluzone Flublok Fluad (w / MF59 adj.) mRNA-10103 IVX-1216 OA: 25-250 µg N=130 (Pfizer) (GSK) (Janssen) (Moderna) (GSK) (Sanofi) (Sanofi) (Seqirus) (Moderna) Solicited systemic AEs (any) 27% >34%5 41% 60% 66% 25% 25% 19% 48% - 77% 11-33% Grade 3+ solicited systemic AEs 1%4 – 2% – 11% 0.4% 1% – 0% - 16% 0% SAEs 2% 4% 5% 3% 1% 0% 3% 7% 0% 0% Vaccine-related SAEs <0.1% – 0% 0% 0% 0% 0% 0% 0% 0% Vaccine-related deaths – 0% 0% 0% 0% 0% 0% 0% – 0% Age Group 65+ 65+ 65+ 65-79 50+ 65+ 50+ 65+ 65+ 60-75 Study Phase 3 3 2b 1 3 3 3 3 2 1b **Data shown side by side for illustrative purposes only; not a head-to-head comparison and there could be assay, laboratory, and other differences across trials** Data are based on: Oct 2022 ACIP presentations and posters (GSK and Pfizer Ph 3), ReSViNET 2021 (Janssen), Moderna Vaccines Day 2022 and RSV Belfast 2022 presentation (Moderna), Lal et al 2015 (Shingrix), Treanor et al 2017 (Afluria), FDA package inserts (Shingrix, Fluzone, Flublok, Fluad); 1 Non-exhaustive, representative set of quadrivalent flu vaccines recommended for 2021-2022 flu season; 2 Data across 12.5 µg - 200 µg doses; 3 Ranges represent 25 µg - 100 µg doses; 4 Estimate of ‘severe systemic events’, interpreted as Grade 3+ AEs; 5 Data shown for most frequent systemic AE (fatigue), ‘Any’ systemic AE not reported; 6 IVX-121 data through day 180 IVX-121 Phase 1/1b safety data support advancement as component of multivalent vaccine

Appendix
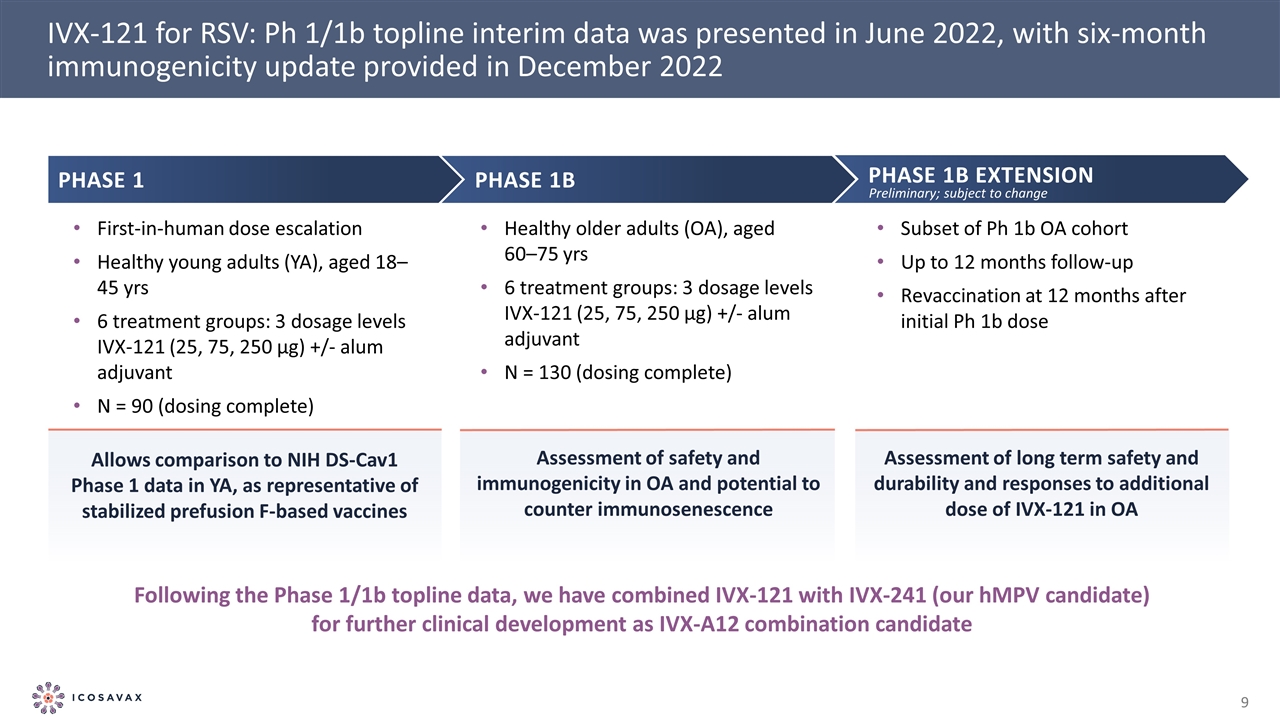
IVX-121 for RSV: Ph 1/1b topline interim data was presented in June 2022, with six-month immunogenicity update provided in December 2022 Phase 1b EXTENSION Subset of Ph 1b OA cohort Up to 12 months follow-up Revaccination at 12 months after initial Ph 1b dose Following the Phase 1/1b topline data, we have combined IVX-121 with IVX-241 (our hMPV candidate) for further clinical development as IVX-A12 combination candidate Phase 1 First-in-human dose escalation Healthy young adults (YA), aged 18–45 yrs 6 treatment groups: 3 dosage levels IVX-121 (25, 75, 250 µg) +/- alum adjuvant N = 90 (dosing complete) Phase 1b Healthy older adults (OA), aged 60–75 yrs 6 treatment groups: 3 dosage levels IVX-121 (25, 75, 250 µg) +/- alum adjuvant N = 130 (dosing complete) Allows comparison to NIH DS-Cav1 Phase 1 data in YA, as representative of stabilized prefusion F-based vaccines Assessment of safety and immunogenicity in OA and potential to counter immunosenescence Assessment of long term safety and durability and responses to additional dose of IVX-121 in OA Preliminary; subject to change

IVX-121 GMT ranges for RSV-A and RSV-B in older adults GMTs for RSV-B showed greater variability but were maintained above baseline through day 180 Day 28 Day 180 GMT (95% CI) IVX-121 Day 28 and Day 180 Results (Unadjuvanted) Older Adult Subjects (ages 60-75) PH1b 25 µg 75 µg 250 µg PBO RSV-A GMT (95% CI) 25 µg 75 µg 250 µg PBO RSV-B % retained titers, D180 vs. D28 64% 98% 72% % retained titers, D180 vs. D28 40% 53% 41% Day 28 Day 180
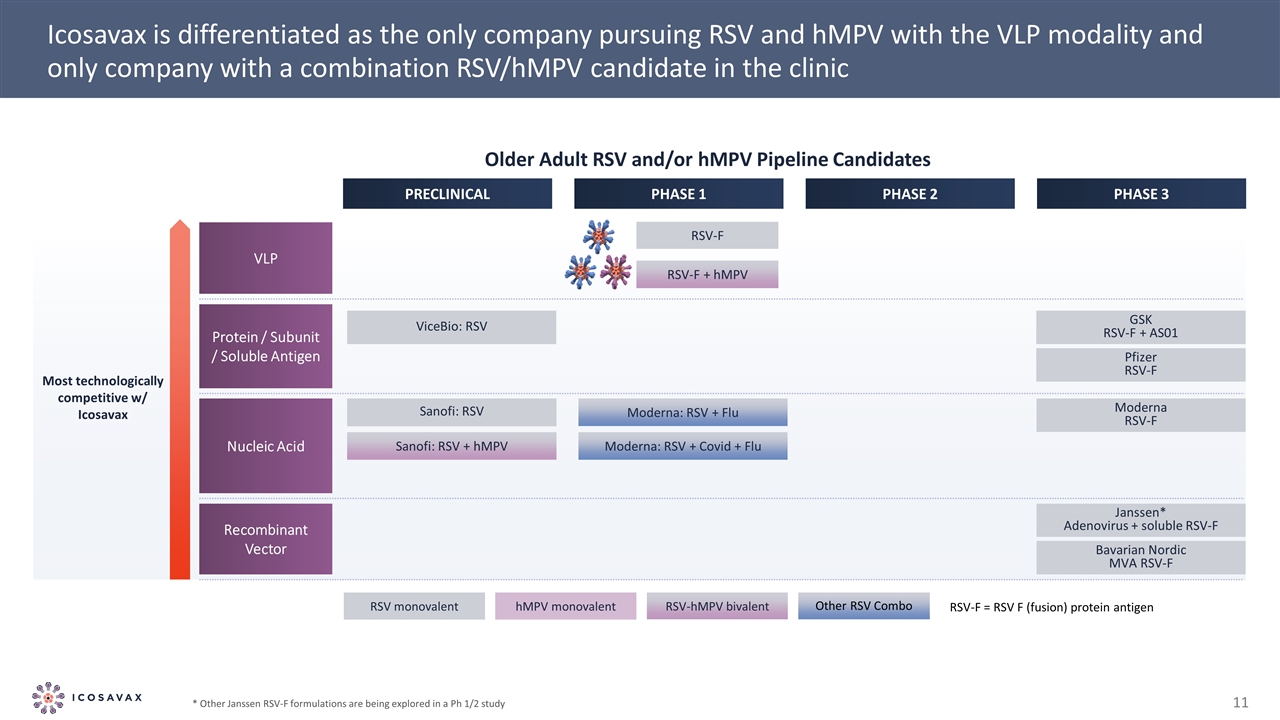
VLP Recombinant Vector Janssen* Adenovirus + soluble RSV-F Bavarian Nordic MVA RSV-F Most technologically competitive w/ Icosavax Nucleic Acid Moderna RSV-F Sanofi: RSV Sanofi: RSV + hMPV RSV-F + hMPV RSV-F Protein / Subunit / Soluble Antigen GSK RSV-F + AS01 Pfizer RSV-F ViceBio: RSV Moderna: RSV + Covid + Flu Moderna: RSV + Flu Icosavax is differentiated as the only company pursuing RSV and hMPV with the VLP modality and only company with a combination RSV/hMPV candidate in the clinic PRECLINICAL PHASE 1 PHASE 2 PHASE 3 RSV monovalent hMPV monovalent RSV-hMPV bivalent Other RSV Combo RSV-F = RSV F (fusion) protein antigen Older Adult RSV and/or hMPV Pipeline Candidates * Other Janssen RSV-F formulations are being explored in a Ph 1/2 study










