1. Nature of Operations
Passage Bio, Inc., or the Company, a Delaware corporation incorporated in July 2017, is a clinical stage genetic medicines company on a mission to improve the lives of patients with neurodegenerative diseases. The Company’s primary focus is the development and advancement of cutting-edge, one-time therapies designed to target critical underlying pathology in these conditions. The Company has a strategic research collaboration with the Trustees of the University of Pennsylvania’s, or Penn, Gene Therapy Program, or GTP.
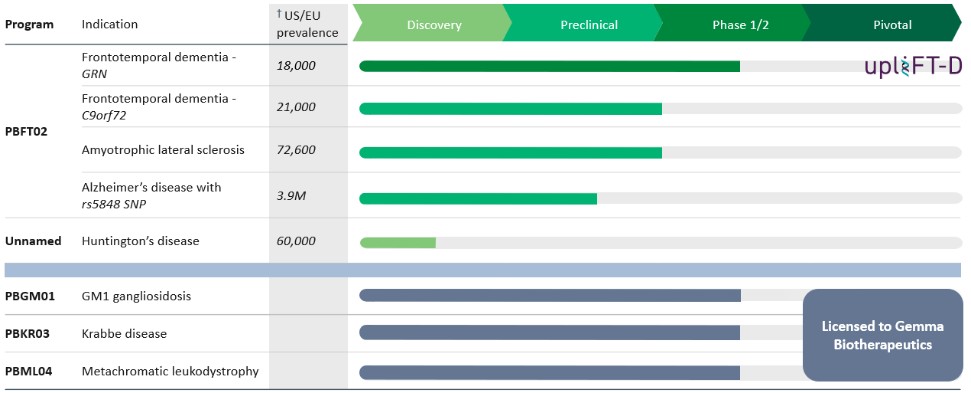
Through this collaboration, the Company has developed its lead clinical product candidate, PBFT02, for the treatment of frontotemporal dementia, or FTD, caused by progranulin deficiency, or FTD-GRN, which seeks to elevate progranulin levels to restore lysosomal function and slow disease progression.
2. Risks and Liquidity
The Company has incurred recurring losses and negative cash flows from operations since inception and had an accumulated deficit of $627.2 million as of June 30, 2024. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant sales of its product candidates currently in development. Substantial additional capital will be needed by the Company to fund its operations and to develop its product candidates.
The Company’s operations have consisted primarily of conducting preclinical studies, developing licensed technology, conducting clinical trials, and the development and manufacturing of clinical supply to support clinical trials. The Company faces risks associated with early-stage biotechnology companies whose product candidates are in development. Product candidates currently under development will require significant additional research and development efforts and establishing manufacturing capacity and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital for the Company to complete its research and development, achieve its research and development objectives, defend its intellectual property rights, and recruit and retain skilled personnel, and key members of management. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will realize revenue from product sales.
On March 5, 2021, the Company entered into a Sales Agreement, or the Sales Agreement, with Cowen and Company, LLC, or Cowen, relating to the applicable terms of at-the-market equity offerings, or the ATM Facility, pursuant to which the Company may, but is not obligated to, offer and sell, from time to time, shares of its common stock with an aggregate offering price up to $125.0 million through Cowen, as sales agent in the ATM Facility. The Company issued 6,000,000 shares of common stock under the ATM Facility, resulting in net proceeds of $8.7 million, after deducting offering costs of $0.3 million in March 2024. The Company is limited to $50 million in its capacity to offer and sell shares of its common stock under this sales agreement pursuant to its shelf registration statement on Form S-3, filed on March 4, 2024. As of June 30, 2024, $50 million of capacity remains available to be sold under the ATM Facility.
The Company plans to seek additional funding through public or private equity offerings, debt financings, other collaborations, strategic alliances and licensing arrangements. The Company may not be able to obtain financing on acceptable terms, or at all, and the Company may not be able to enter into strategic alliances or other arrangements on favorable terms, or at all. The terms of any financing may adversely affect the holdings or the rights of the Company’s stockholders. If the Company is unable to obtain funding or prospects of funding are unfavorable, the Company could be required to further delay, reduce or eliminate research and development programs, product portfolio expansion or future commercialization efforts, which could adversely affect its business prospects.
In accordance with Accounting Standards Update, or ASU, No. 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, the Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued. As of the issuance date of these financial statements, the Company expects that its cash, cash equivalents and marketable debt securities will be sufficient to fund