March 2024 ENGINEERING Natural Killer Cells for next generation treatment of autoimmune diseases and cancer ON DEMAND CONFIDENTIAL Exhibit 99.1

Forward-looking statements This presentation contains forward‐looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, regarding future events and the future results of the company that are based on current expectations, estimates, forecasts, and projections about the industry in which the company operates and the future of our business, future plans and strategies, projections, anticipated trends and events, the economy, and other future conditions, and the beliefs and assumptions of the management of the company. Words such as “address,” “anticipate,” “believe,” “consider,” “continue,” “develop,” “estimate,” “expect,” “further,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “seek,” “should,” “target,” “will,” variations of such words, and similar expressions are intended to identify such forward-looking statements. Such statements reflect the current views of the company and its management with respect to future events and are subject to inherent risks, uncertainties, and changes in circumstances that are difficult to predict and may be outside our control. Therefore, you should not rely on any of these forward-looking statements. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, the company's actual results, performance, or achievements could differ materially from the results expressed in, or implied by, these forward-looking statements. Please see section entitled “Risk Factors” in our annual, quarterly and other filings with the Securities and Exchange Commission for a description of these risks and uncertainties. This presentation has been prepared by the company based on information it has obtained from sources it believes to be reliable. Summaries of documents contained in this presentation may not be complete. The company does not represent that the information herein is complete. The information in this presentation is current only as of the date on the cover, and the company's business or financial condition and other information in this presentation may change after that date. The company undertakes no obligation to update any forward‐looking statements in order to reflect any event or circumstance occurring after the date of this presentation or currently unknown facts or conditions. Interim data from clinical trials are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more data on existing patients become available. The clinical trial programs are ongoing, and the final results may be materially different from those reflected in any interim data the company reports. Further, others, including regulatory agencies, may not accept or agree with the company’s assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and the value of the company in general. In addition, the information the company chooses to publicly disclose regarding a particular study or clinical trial is typically a summary of extensive information, and you or others may not agree with what the company determines is the material or otherwise appropriate information to include in its disclosure, and any information the company determines not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or business. No Offer or Solicitation This presentation is for informational purpose only and does not constitute an offer or solicitation for the sale or purchase of the securities, assets or business described herein or a commitment of the company with respect to any of the foregoing, and this presentation shall not form the basis of any contract. There shall be no sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. This presentation contains selected proprietary and confidential information of the company. The disclosure of the contents of this presentation to any person without the prior consent of the company is prohibited. Each recipient agrees to be bound by the foregoing limitations and conditions and, in particular, will be deemed to have represented, warranted and undertaken that such recipient has read and agreed to comply with the contents of this disclaimer including, without limitation, the obligation to maintain the confidentiality of this presentation and all information that is contained in this presentation and not already in the public domain.
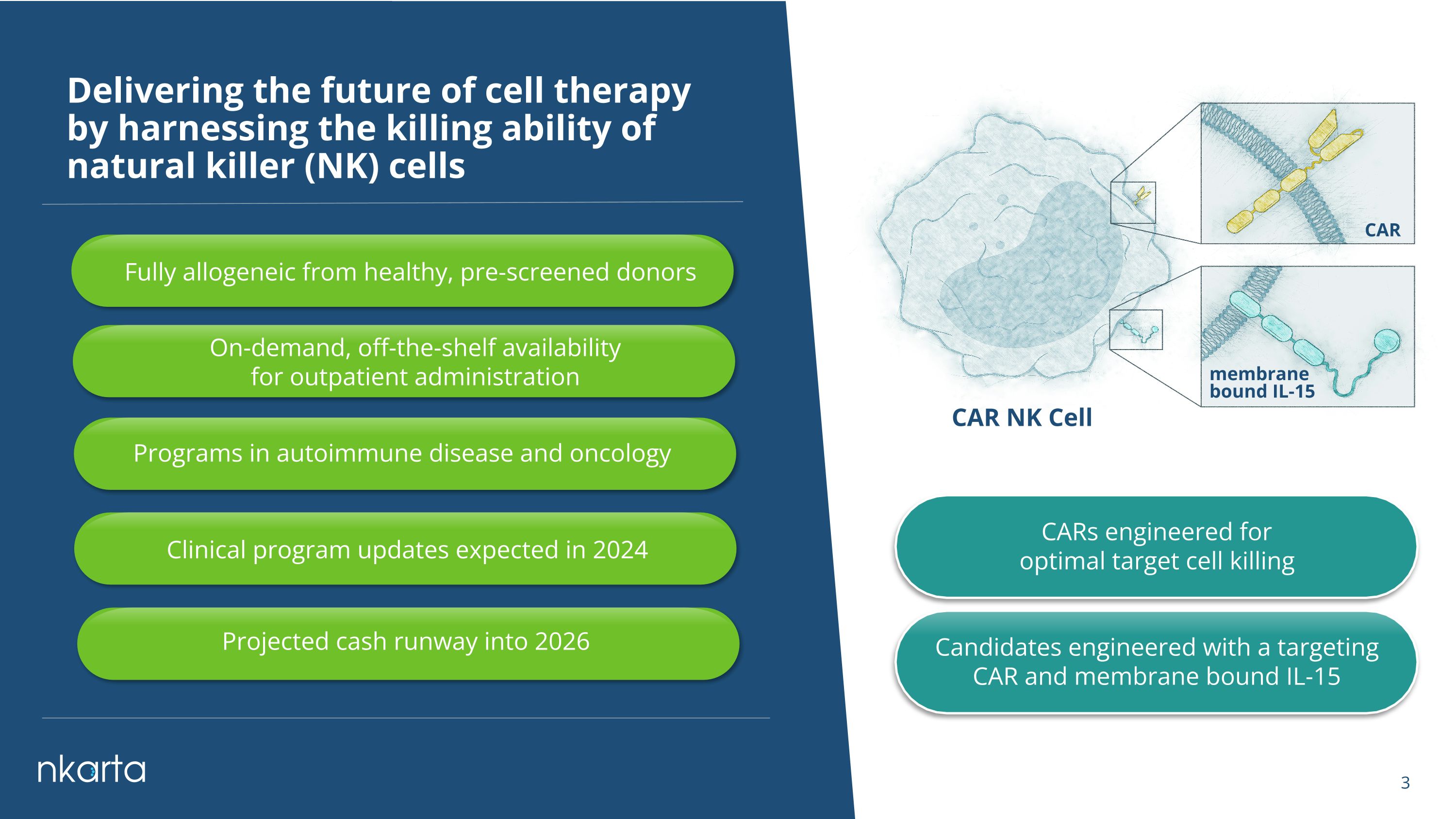
Delivering the future of cell therapy by harnessing the killing ability of natural killer (NK) cells CARs engineered for optimal target cell killing CAR NK Cell CAR membrane bound IL-15 Candidates engineered with a targeting CAR and membrane bound IL-15 Fully allogeneic from healthy, pre-screened donors Programs in autoimmune disease and oncology On-demand, off-the-shelf availability for outpatient administration Clinical program updates expected in 2024 Projected cash runway into 2026
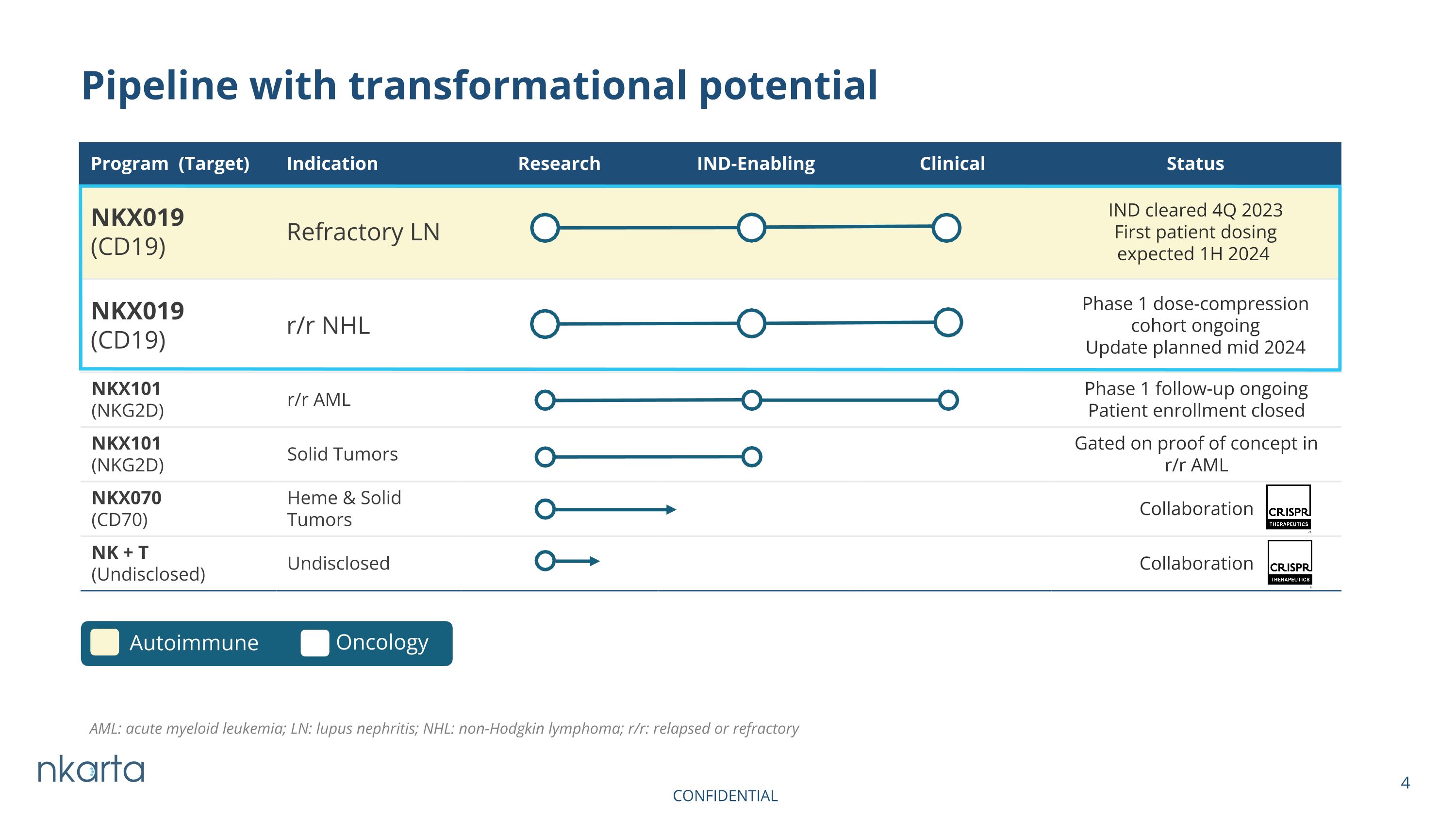
NKX101 (NKG2D) r/r AML Phase 1 follow-up ongoing Patient enrollment closed NKX101 (NKG2D) Solid Tumors Gated on proof of concept in r/r AML NKX070 (CD70) Heme & Solid Tumors Collaboration NK + T (Undisclosed) Undisclosed Collaboration Pipeline with transformational potential Program (Target) Indication Research IND-Enabling Clinical Status NKX019 (CD19) Refractory LN IND cleared 4Q 2023 First patient dosing expected 1H 2024 NKX019 (CD19) r/r NHL Phase 1 dose-compression cohort ongoing Update planned mid 2024 Autoimmune Oncology AML: acute myeloid leukemia; LN: lupus nephritis; NHL: non-Hodgkin lymphoma; r/r: relapsed or refractory

NKX019 in Autoimmune Disease
Autoimmune disease is a major unmet need Estimated 7 million patients in U.S. with a form of B-cell mediated autoimmune disease1 Pathogenic B cells can drive systemic diseases via combination of intrinsic and extrinsic factors Effectiveness of current therapies is inadequate and often consists of lifelong immune suppression CD19-directed cell therapy has challenged the treatment paradigm for autoimmune diseases Drug-free remissions after a single treatment in academic trials2 Cell therapy offers a promise of a disease-modifying option for patients with refractory autoimmune disease Tsokos, N Engl J Med 2011; 365:2110-2121. 1: Canaccord Genuity, 14 Nov 2023. 2: Mackensen et al. Nature Med. 28 Oct 22. 2124–2132.
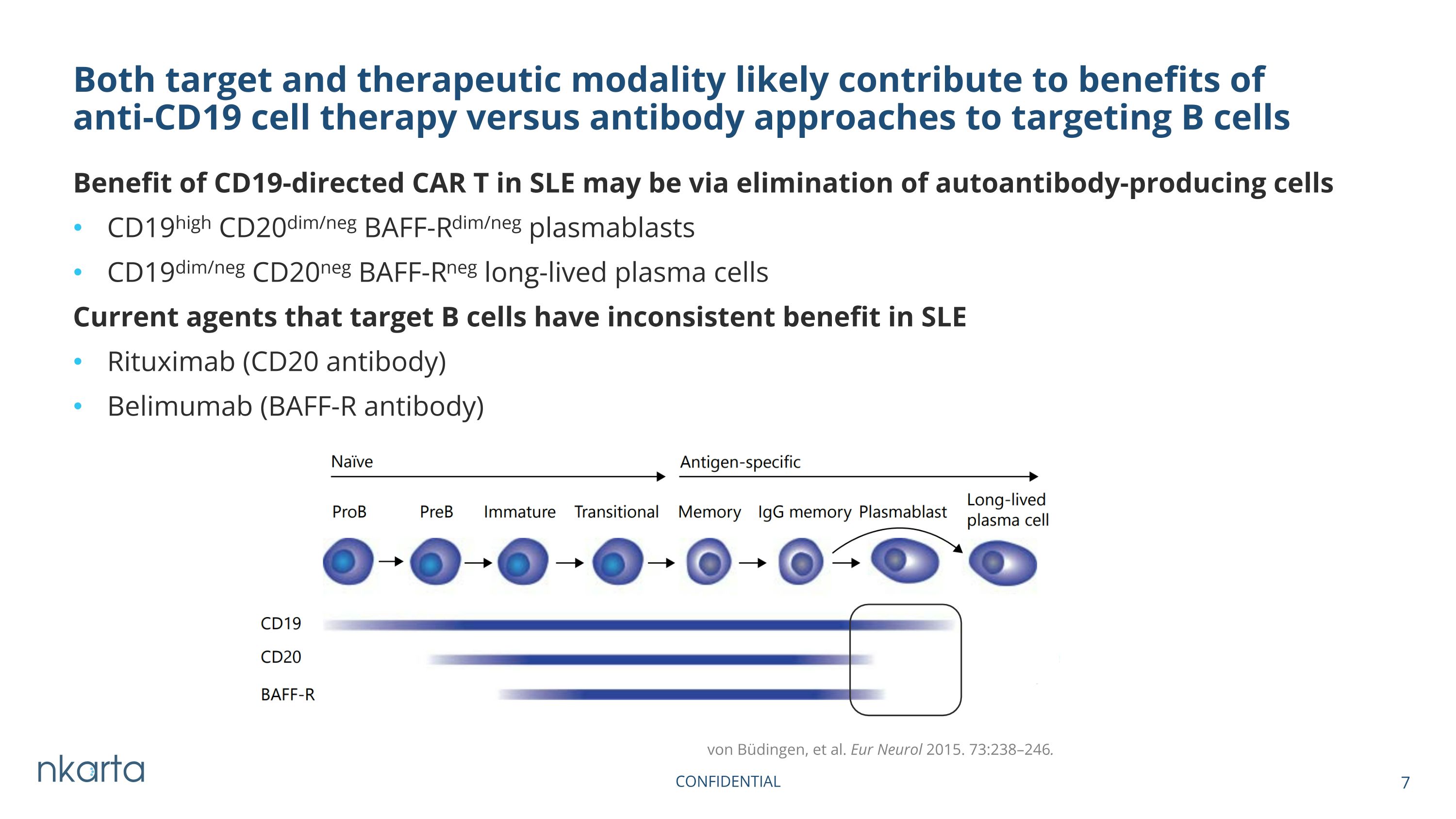
Benefit of CD19-directed CAR T in SLE may be via elimination of autoantibody-producing cells CD19high CD20dim/neg BAFF-Rdim/neg plasmablasts CD19dim/neg CD20neg BAFF-Rneg long-lived plasma cells Current agents that target B cells have inconsistent benefit in SLE Rituximab (CD20 antibody) Belimumab (BAFF-R antibody) Both target and therapeutic modality likely contribute to benefits of anti-CD19 cell therapy versus antibody approaches to targeting B cells von Büdingen, et al. Eur Neurol 2015. 73:238–246.
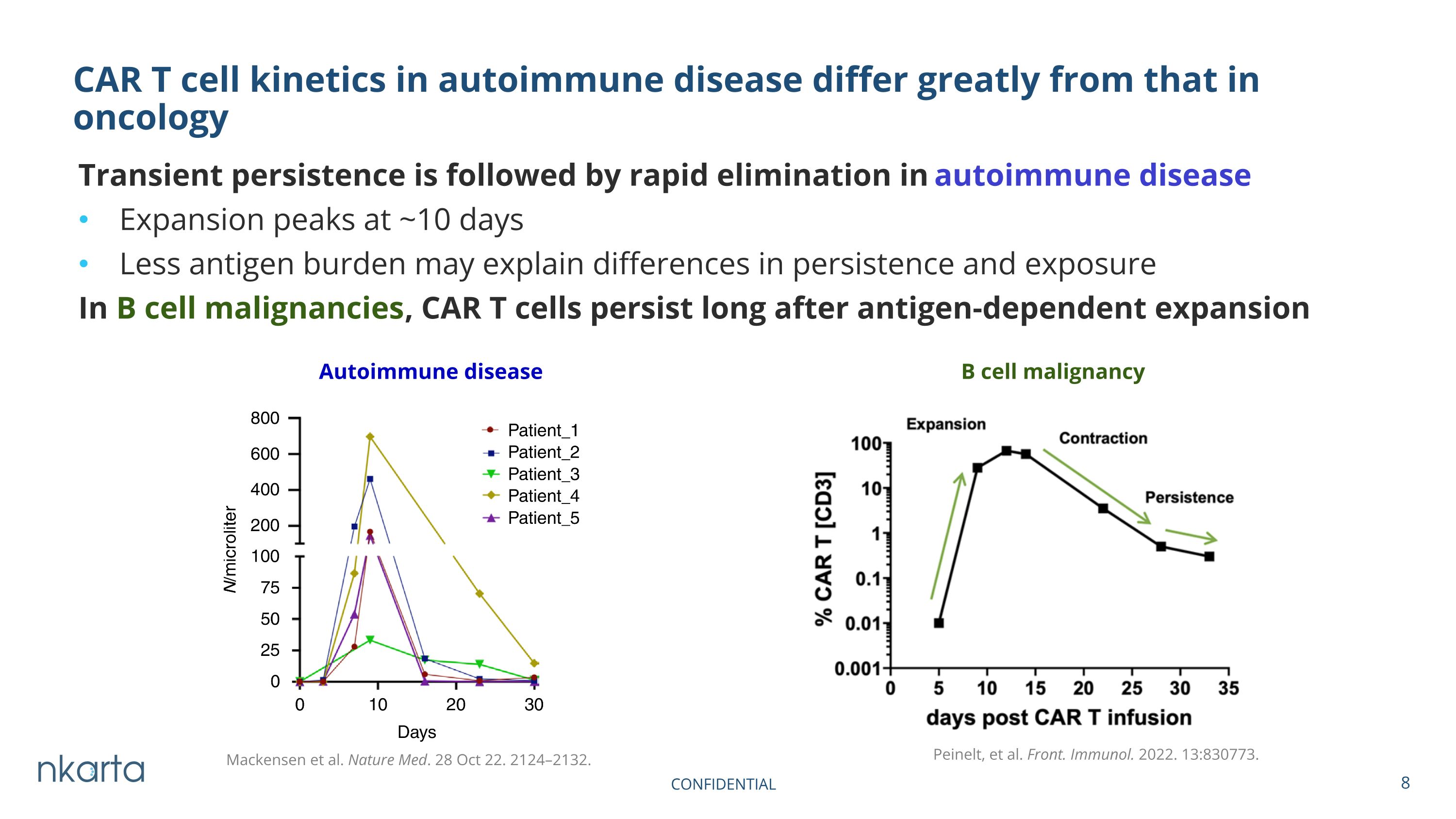
Transient persistence is followed by rapid elimination in autoimmune disease Expansion peaks at ~10 days Less antigen burden may explain differences in persistence and exposure In B cell malignancies, CAR T cells persist long after antigen-dependent expansion CAR T cell kinetics in autoimmune disease differ greatly from that in oncology Peinelt, et al. Front. Immunol. 2022. 13:830773. Autoimmune disease B cell malignancy Mackensen et al. Nature Med. 28 Oct 22. 2124–2132.
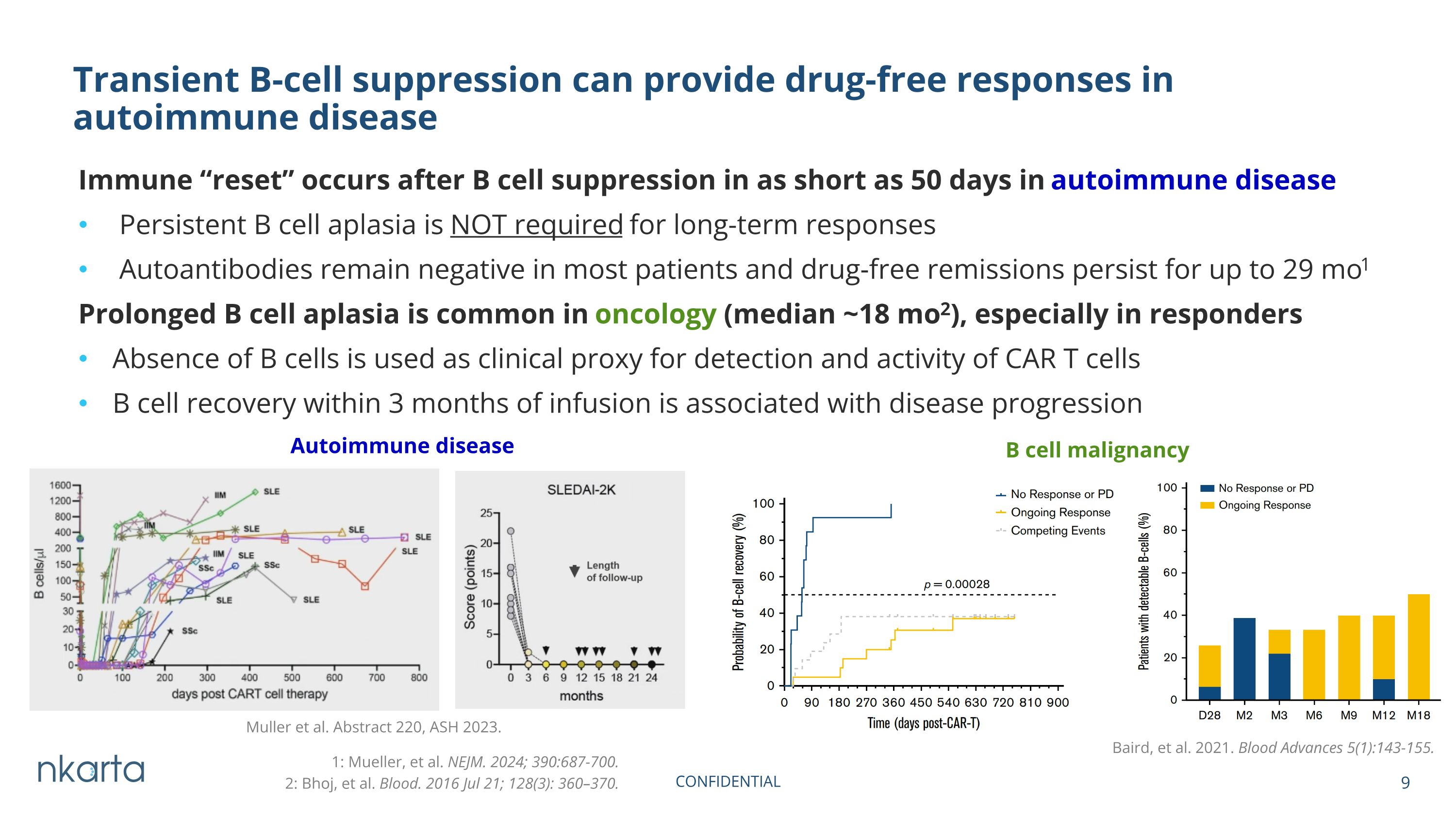
Immune “reset” occurs after B cell suppression in as short as 50 days in autoimmune disease Persistent B cell aplasia is NOT required for long-term responses Autoantibodies remain negative in most patients and drug-free remissions persist for up to 29 mo1 Prolonged B cell aplasia is common in oncology (median ~18 mo2), especially in responders Absence of B cells is used as clinical proxy for detection and activity of CAR T cells B cell recovery within 3 months of infusion is associated with disease progression Transient B-cell suppression can provide drug-free responses in autoimmune disease Muller et al. Abstract 220, ASH 2023. 1: Mueller, et al. NEJM. 2024; 390:687-700. Autoimmune disease B cell malignancy Baird, et al. 2021. Blood Advances 5(1):143-155. 2: Bhoj, et al. Blood. 2016 Jul 21; 128(3): 360–370.
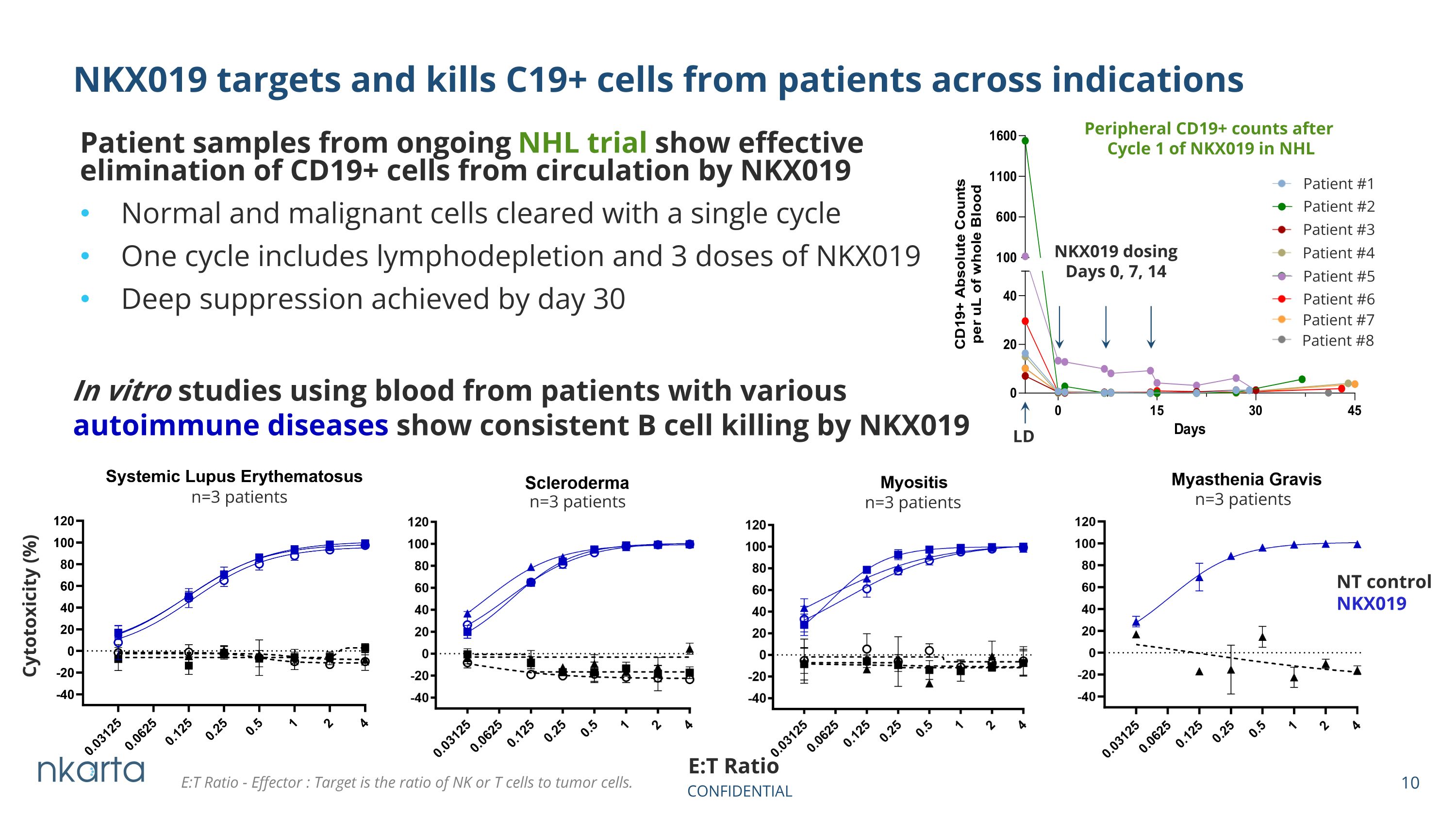
Patient samples from ongoing NHL trial show effective elimination of CD19+ cells from circulation by NKX019 Normal and malignant cells cleared with a single cycle One cycle includes lymphodepletion and 3 doses of NKX019 Deep suppression achieved by day 30 NKX019 targets and kills C19+ cells from patients across indications In vitro studies using blood from patients with various autoimmune diseases show consistent B cell killing by NKX019 n=3 patients Cytotoxicity (%) n=3 patients n=3 patients n=3 patients NT control NKX019 E:T Ratio LD NKX019 dosing Days 0, 7, 14 Patient #1 Peripheral CD19+ counts after Cycle 1 of NKX019 in NHL Patient #2 Patient #3 Patient #4 Patient #5 Patient #6 Patient #7 Patient #8 E:T Ratio - Effector : Target is the ratio of NK or T cells to tumor cells.
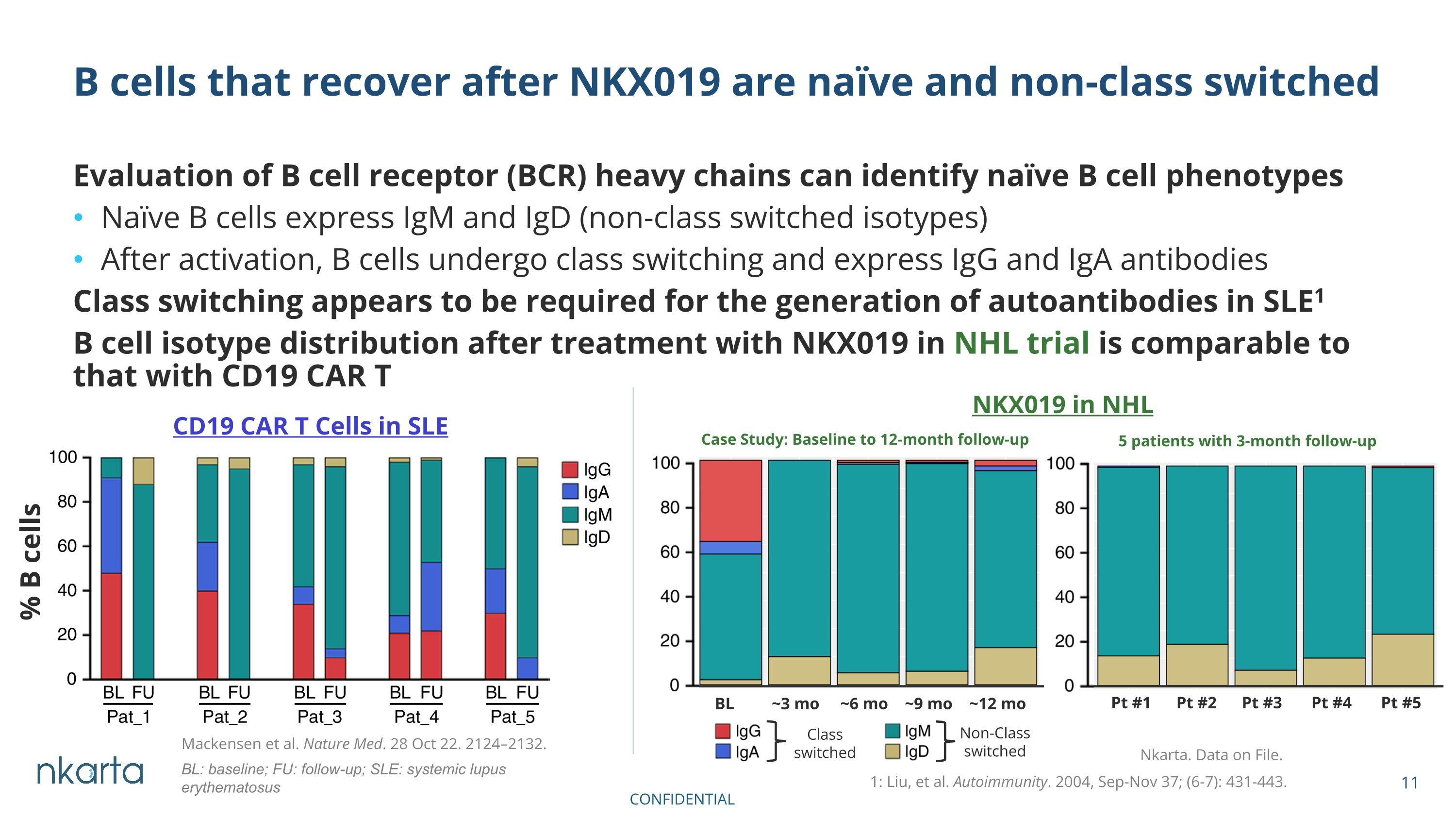
Evaluation of B cell receptor (BCR) heavy chains can identify naïve B cell phenotypes Naïve B cells express IgM and IgD (non-class switched isotypes) After activation, B cells undergo class switching and express IgG and IgA antibodies Class switching appears to be required for the generation of autoantibodies in SLE1 B cell isotype distribution after treatment with NKX019 in NHL trial is comparable to that with CD19 CAR T B cells that recover after NKX019 are naïve and non-class switched CD19 CAR T Cells in SLE Case Study: Baseline to 12-month follow-up % B cells 5 patients with 3-month follow-up NKX019 in NHL Nkarta. Data on File. BL ~3 mo ~6 mo ~9 mo ~12 mo 1: Liu, et al. Autoimmunity. 2004, Sep-Nov 37; (6-7): 431-443. BL: baseline; FU: follow-up; SLE: systemic lupus erythematosus Pt #1 Pt #2 Pt #3 Pt #4 Pt #5 Mackensen et al. Nature Med. 28 Oct 22. 2124–2132. Class switched Non-Class switched CONFIDENTIAL
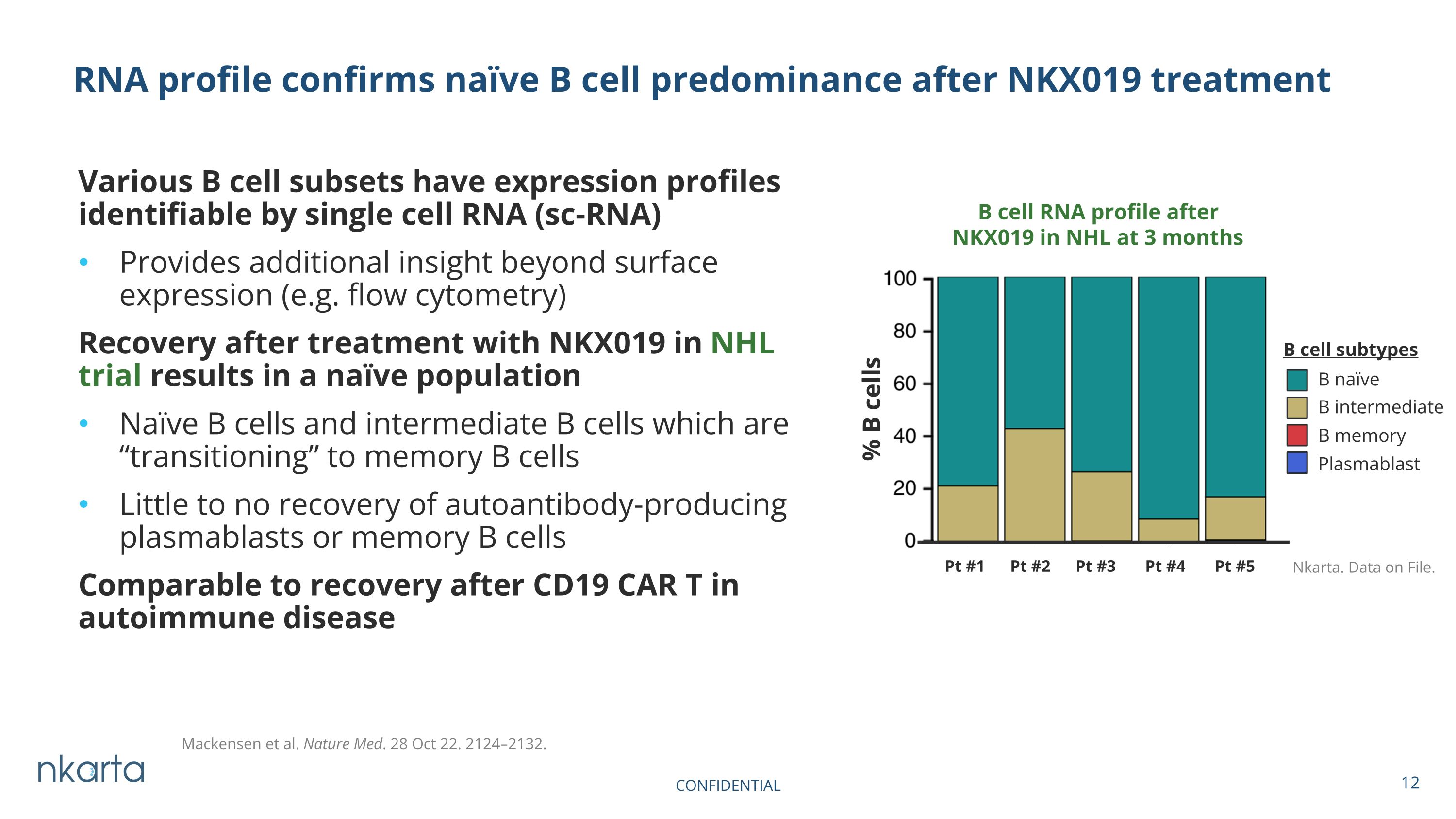
Various B cell subsets have expression profiles identifiable by single cell RNA (sc-RNA) Provides additional insight beyond surface expression (e.g. flow cytometry) Recovery after treatment with NKX019 in NHL trial results in a naïve population Naïve B cells and intermediate B cells which are “transitioning” to memory B cells Little to no recovery of autoantibody-producing plasmablasts or memory B cells Comparable to recovery after CD19 CAR T in autoimmune disease RNA profile confirms naïve B cell predominance after NKX019 treatment CONFIDENTIAL B cell RNA profile after NKX019 in NHL at 3 months Pt #1 Pt #2 Pt #3 Pt #4 Pt #5 Mackensen et al. Nature Med. 28 Oct 22. 2124–2132. % B cells B cell subtypes B naïve B intermediate B memory Plasmablast Nkarta. Data on File.
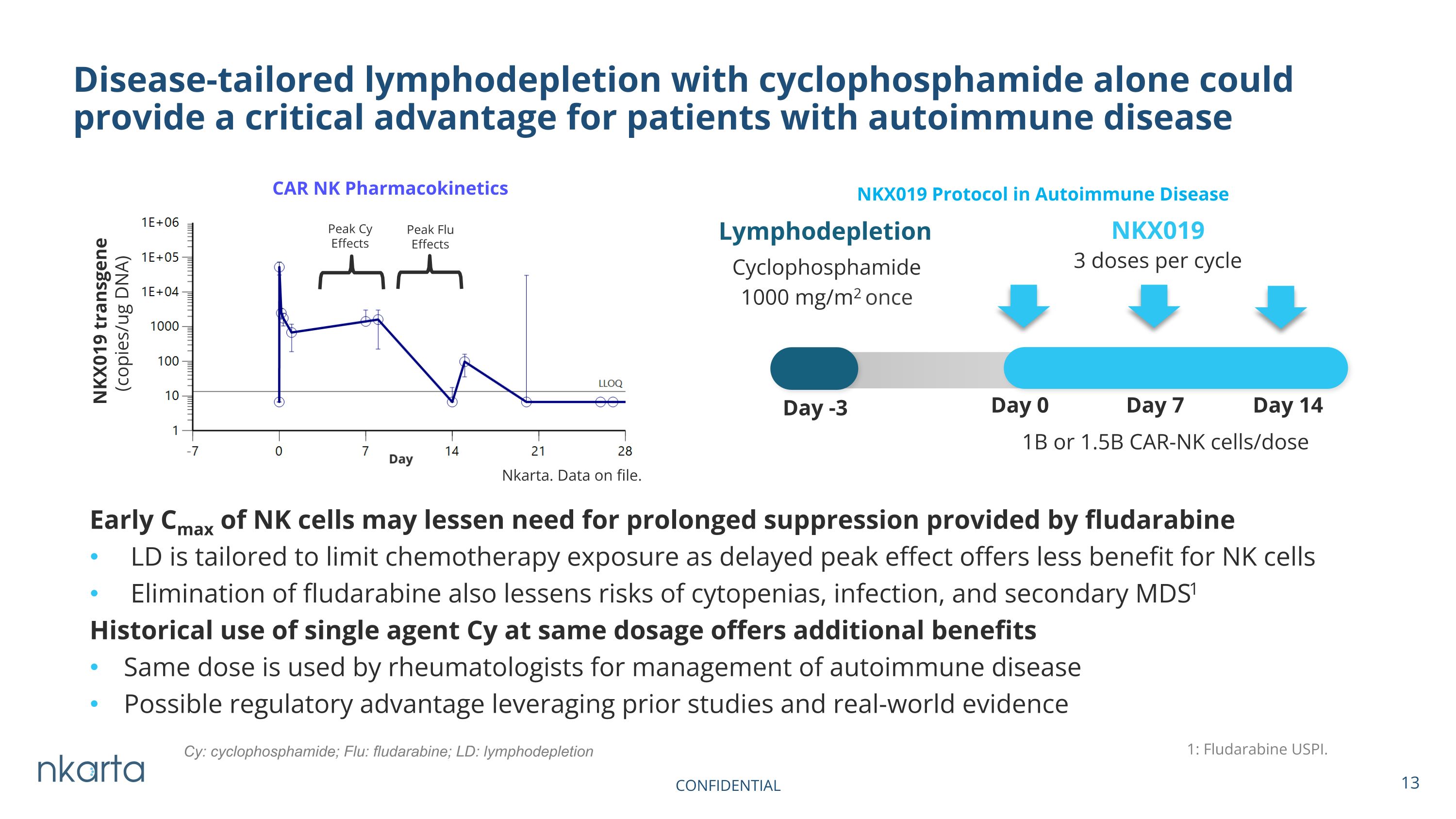
Early Cmax of NK cells may lessen need for prolonged suppression provided by fludarabine LD is tailored to limit chemotherapy exposure as delayed peak effect offers less benefit for NK cells Elimination of fludarabine also lessens risks of cytopenias, infection, and secondary MDS1 Historical use of single agent Cy at same dosage offers additional benefits Same dose is used by rheumatologists for management of autoimmune disease Possible regulatory advantage leveraging prior studies and real-world evidence Disease-tailored lymphodepletion with cyclophosphamide alone could provide a critical advantage for patients with autoimmune disease CAR NK Pharmacokinetics 1: Fludarabine USPI. Nkarta. Data on file. NKX019 1B or 1.5B CAR-NK cells/dose Lymphodepletion Cyclophosphamide 1000 mg/m2 once Day -3 Day 0 Day 7 Day 14 3 doses per cycle Peak Cy Effects Peak Flu Effects NKX019 Protocol in Autoimmune Disease Cy: cyclophosphamide; Flu: fludarabine; LD: lymphodepletion CONFIDENTIAL NKX019 transgene (copies/ug DNA)
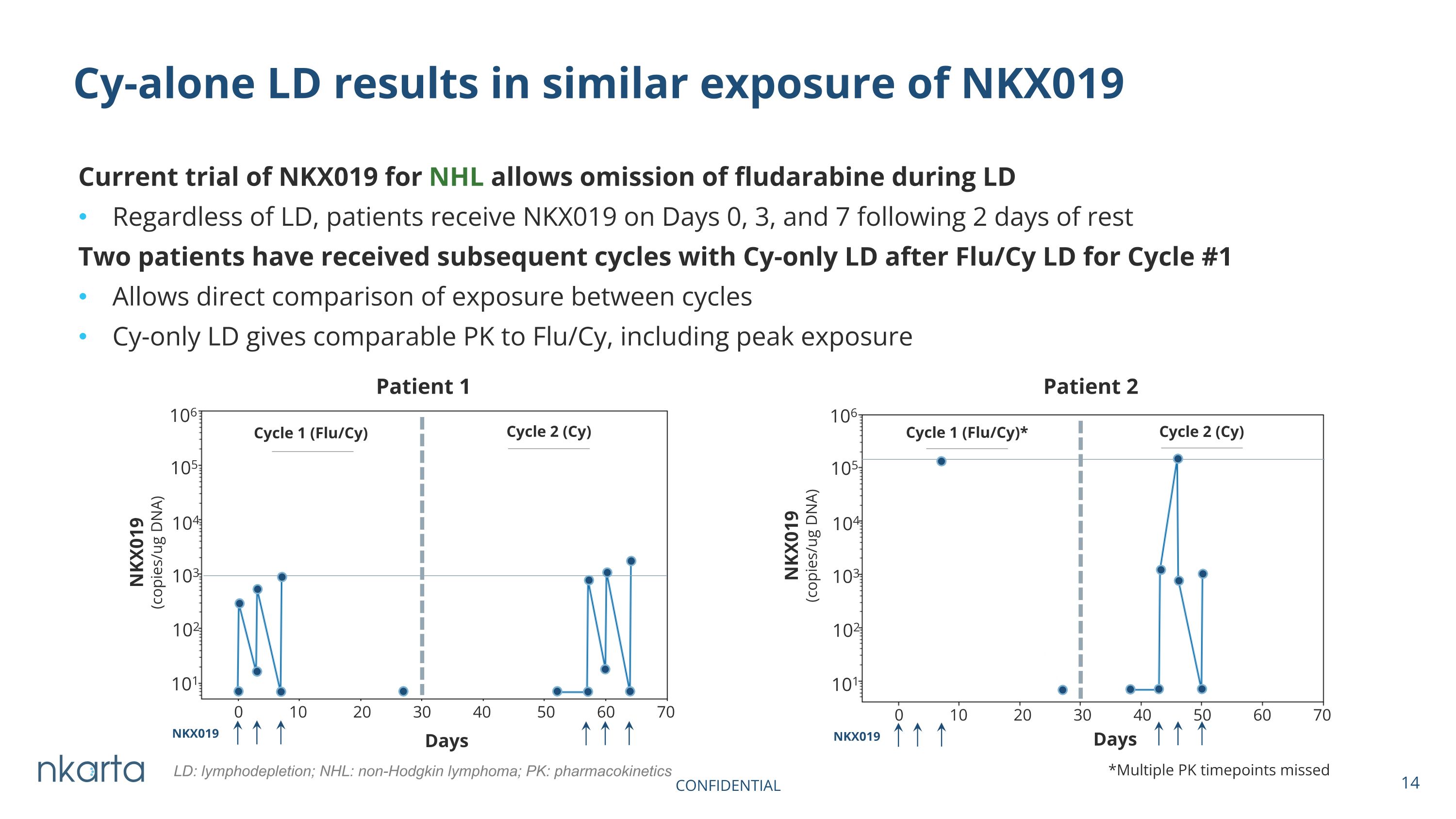
Days Days 0 10 20 30 40 50 60 70 0 10 20 30 40 50 60 70 NKX019 (copies/ug DNA) 106 105 104 103 102 101 106 105 104 103 102 101 Cycle 1 (Flu/Cy) Patient 1 Patient 2 Current trial of NKX019 for NHL allows omission of fludarabine during LD Regardless of LD, patients receive NKX019 on Days 0, 3, and 7 following 2 days of rest Two patients have received subsequent cycles with Cy-only LD after Flu/Cy LD for Cycle #1 Allows direct comparison of exposure between cycles Cy-only LD gives comparable PK to Flu/Cy, including peak exposure Cy-alone LD results in similar exposure of NKX019 *Multiple PK timepoints missed Cycle 1 (Flu/Cy)* Cycle 2 (Cy) Cycle 2 (Cy) NKX019 (copies/ug DNA) LD: lymphodepletion; NHL: non-Hodgkin lymphoma; PK: pharmacokinetics CONFIDENTIAL NKX019 NKX019
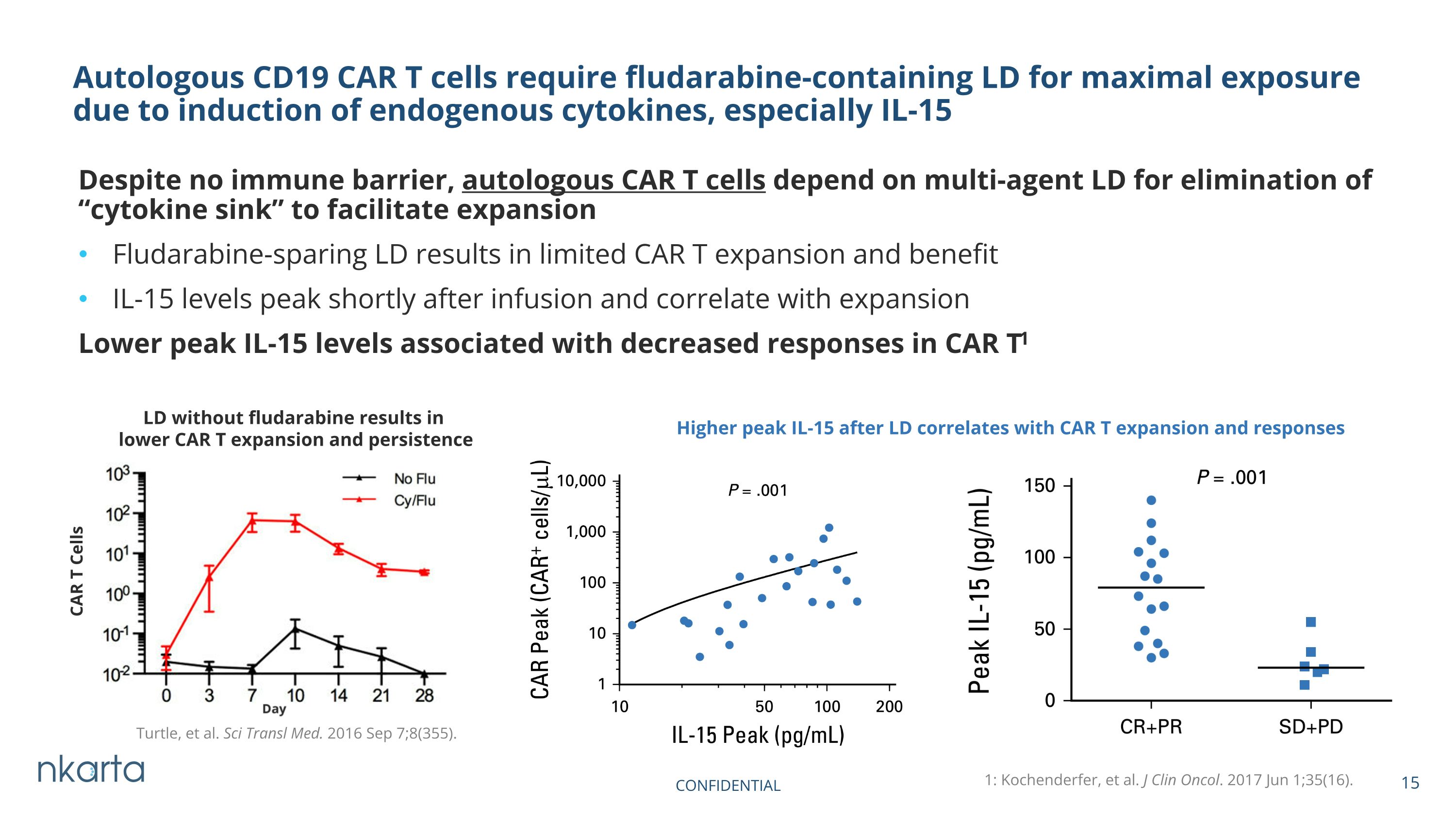
Despite no immune barrier, autologous CAR T cells depend on multi-agent LD for elimination of “cytokine sink” to facilitate expansion Fludarabine-sparing LD results in limited CAR T expansion and benefit IL-15 levels peak shortly after infusion and correlate with expansion Lower peak IL-15 levels associated with decreased responses in CAR T1 Autologous CD19 CAR T cells require fludarabine-containing LD for maximal exposure due to induction of endogenous cytokines, especially IL-15 Turtle, et al. Sci Transl Med. 2016 Sep 7;8(355). CAR T Cells 1: Kochenderfer, et al. J Clin Oncol. 2017 Jun 1;35(16). Higher peak IL-15 after LD correlates with CAR T expansion and responses LD without fludarabine results in lower CAR T expansion and persistence CONFIDENTIAL
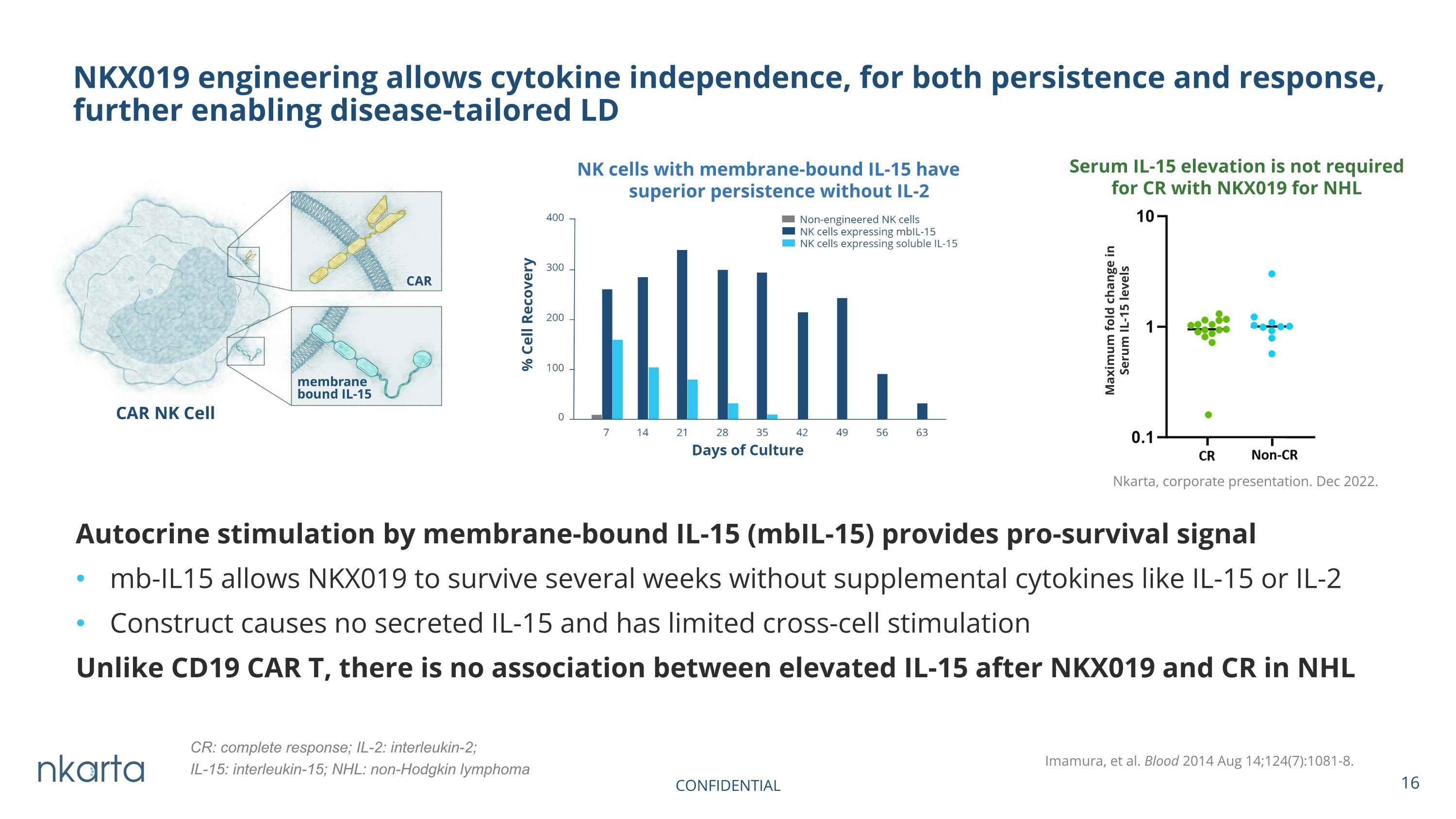
Autocrine stimulation by membrane-bound IL-15 (mbIL-15) provides pro-survival signal mb-IL15 allows NKX019 to survive several weeks without supplemental cytokines like IL-15 or IL-2 Construct causes no secreted IL-15 and has limited cross-cell stimulation Unlike CD19 CAR T, there is no association between elevated IL-15 after NKX019 and CR in NHL NKX019 engineering allows cytokine independence, for both persistence and response, further enabling disease-tailored LD NK cells with membrane-bound IL-15 have superior persistence without IL-2 Imamura, et al. Blood 2014 Aug 14;124(7):1081-8. Serum IL-15 elevation is not required for CR with NKX019 for NHL Nkarta, corporate presentation. Dec 2022. Maximum fold change in Serum IL-15 levels CR: complete response; IL-2: interleukin-2; IL-15: interleukin-15; NHL: non-Hodgkin lymphoma CONFIDENTIAL
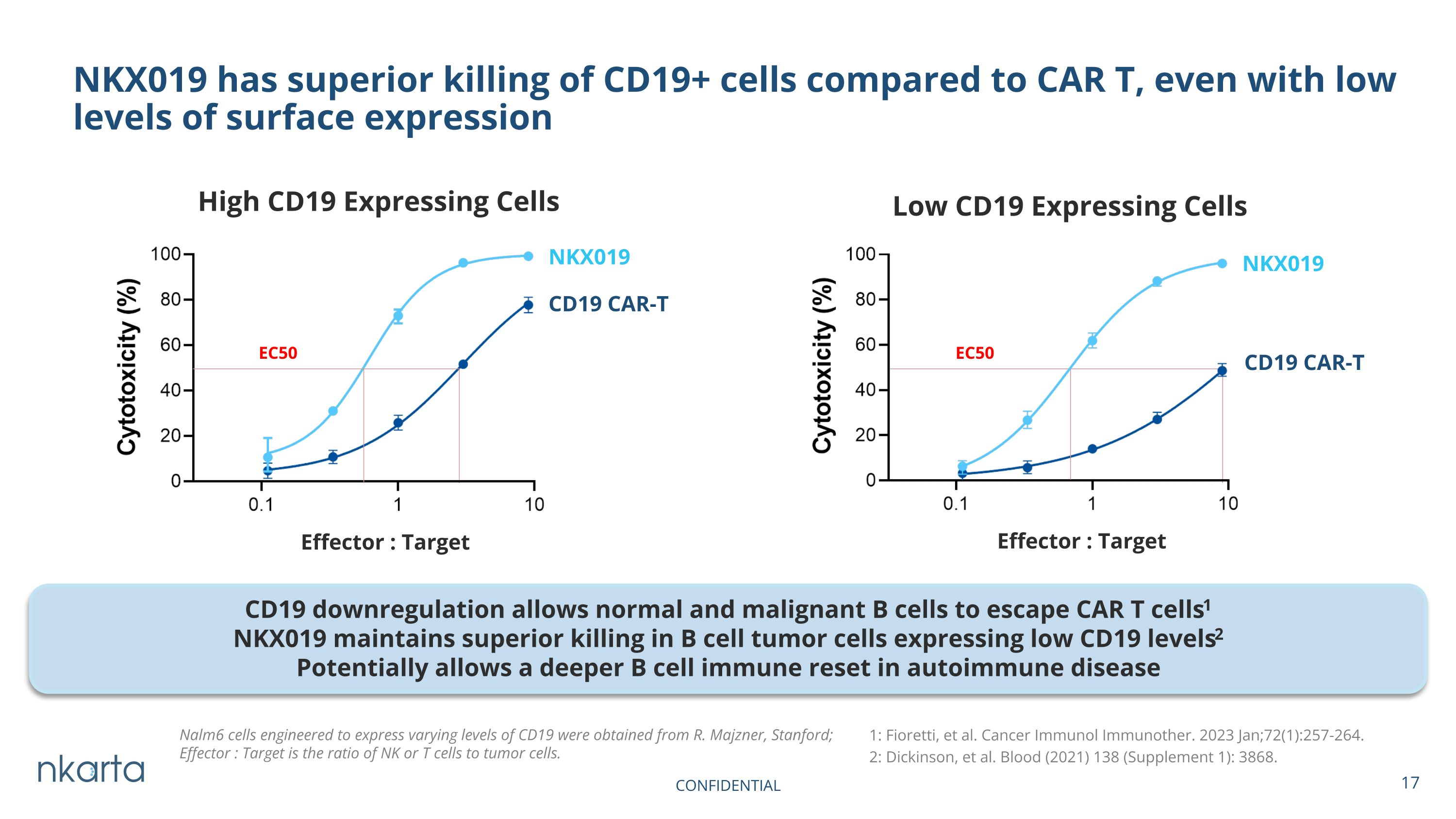
NKX019 has superior killing of CD19+ cells compared to CAR T, even with low levels of surface expression NKX019 Low CD19 Expressing Cells High CD19 Expressing Cells NKX019 CD19 CAR-T CD19 CAR-T Effector : Target Effector : Target Nalm6 cells engineered to express varying levels of CD19 were obtained from R. Majzner, Stanford; Effector : Target is the ratio of NK or T cells to tumor cells. CD19 downregulation allows normal and malignant B cells to escape CAR T cells1 NKX019 maintains superior killing in B cell tumor cells expressing low CD19 levels2 Potentially allows a deeper B cell immune reset in autoimmune disease NKX019 1: Fioretti, et al. Cancer Immunol Immunother. 2023 Jan;72(1):257-264. 2: Dickinson, et al. Blood (2021) 138 (Supplement 1): 3868. EC50 EC50 CONFIDENTIAL
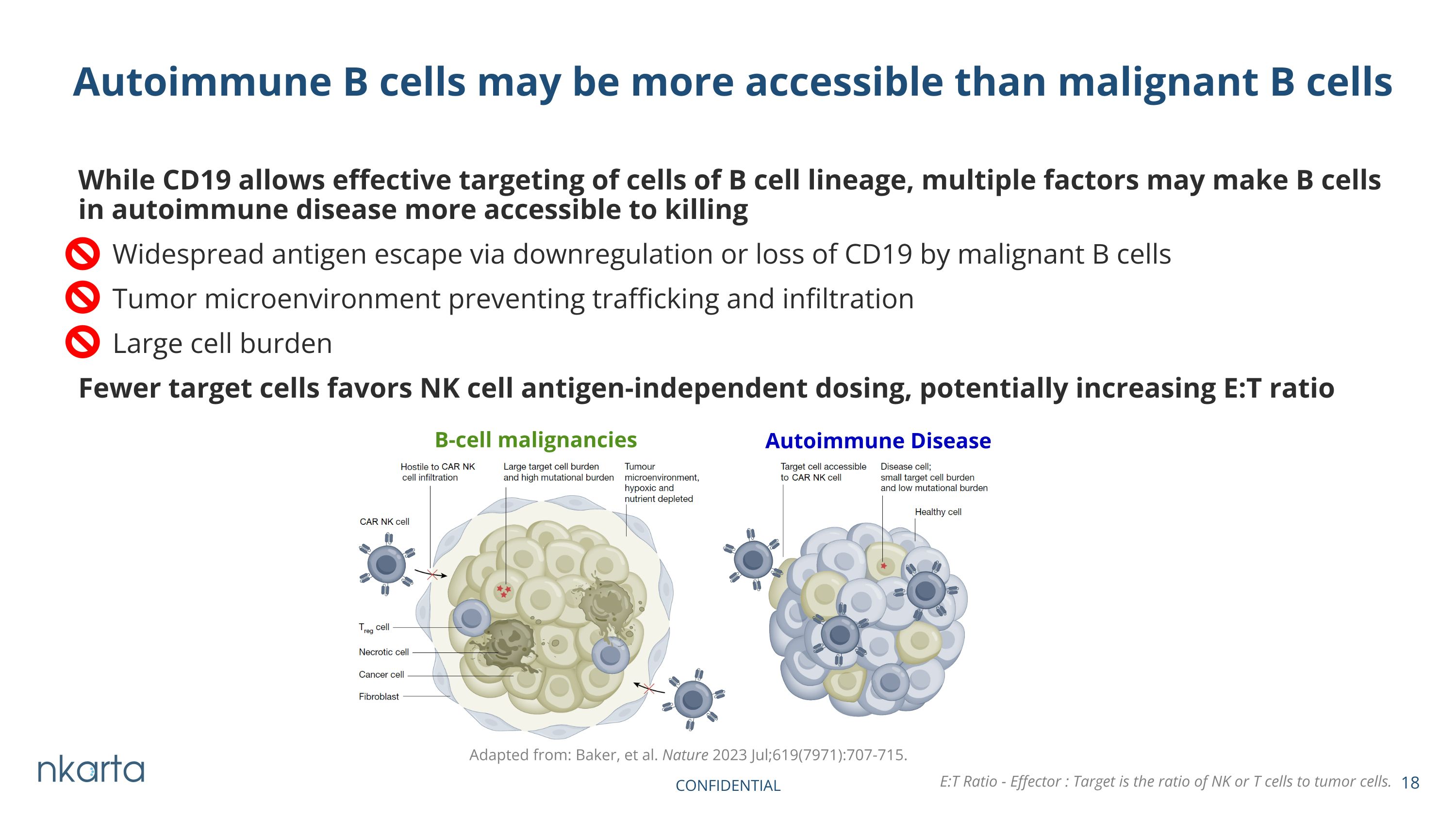
While CD19 allows effective targeting of cells of B cell lineage, multiple factors may make B cells in autoimmune disease more accessible to killing Widespread antigen escape via downregulation or loss of CD19 by malignant B cells Tumor microenvironment preventing trafficking and infiltration Large cell burden Fewer target cells favors NK cell antigen-independent dosing, potentially increasing E:T ratio Autoimmune B cells may be more accessible than malignant B cells Adapted from: Baker, et al. Nature 2023 Jul;619(7971):707-715. Autoimmune Disease B-cell malignancies CONFIDENTIAL E:T Ratio - Effector : Target is the ratio of NK or T cells to tumor cells.
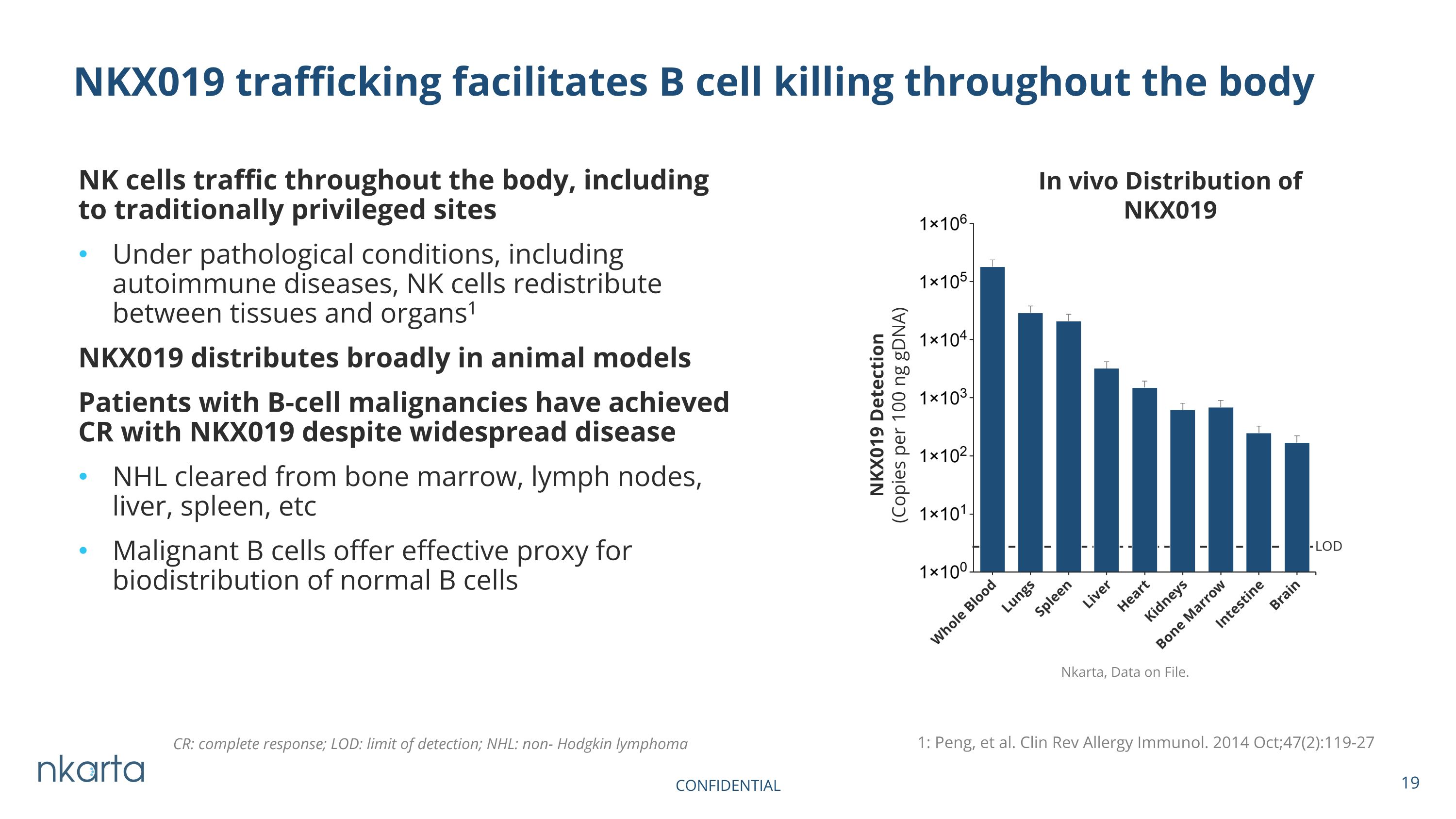
NK cells traffic throughout the body, including to traditionally privileged sites Under pathological conditions, including autoimmune diseases, NK cells redistribute between tissues and organs1 NKX019 distributes broadly in animal models Patients with B-cell malignancies have achieved CR with NKX019 despite widespread disease NHL cleared from bone marrow, lymph nodes, liver, spleen, etc Malignant B cells offer effective proxy for biodistribution of normal B cells NKX019 trafficking facilitates B cell killing throughout the body CONFIDENTIAL In vivo Distribution of NKX019 NKX019 Detection (Copies per 100 ng gDNA) 1: Peng, et al. Clin Rev Allergy Immunol. 2014 Oct;47(2):119-27 CR: complete response; LOD: limit of detection; NHL: non- Hodgkin lymphoma Nkarta, Data on File.
NK cells reach peak activity at infusion for rapid target activity Maximal immediate effect without in vivo expansion T cells require expansion and necessitate a different LD approach Allogeneic NK cells are cleared by host immunity Low risk of prolonged B-cell aplasia which is not required for response Long-lived CAR T cells have FDA-issued risk of T-cell malignancy1 Superior safety and accessibility in non-malignant setting On-demand availability without need for cumbersome infrastructure at treatment centers Low risk of expansion-related toxicities including CRS and ICANS CD19 CAR NK cells may be ideally suited for autoimmune disease 1: Nelson. Lancet. Vol 402. 2181. December 9, 2023.
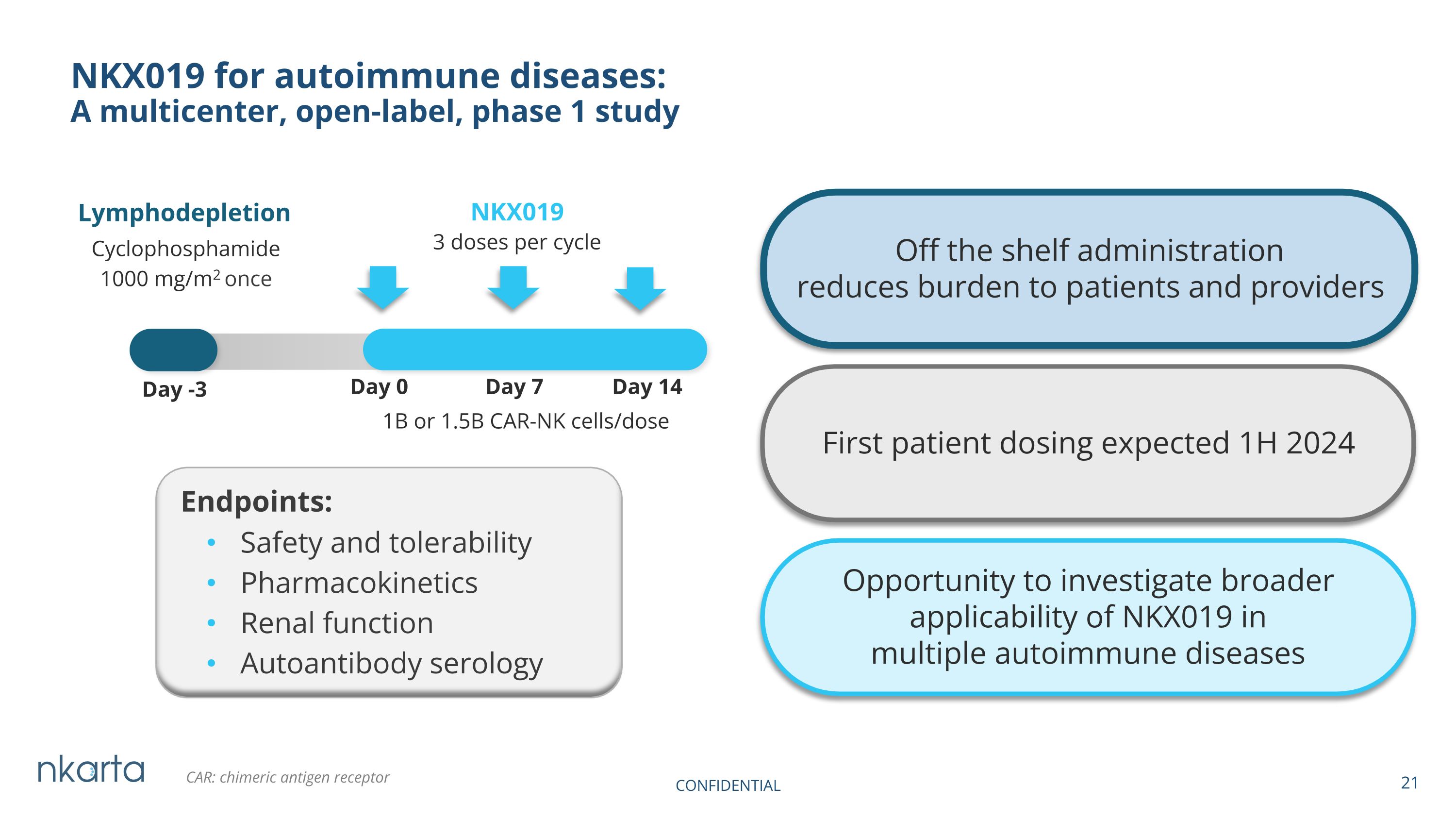
NKX019 for autoimmune diseases: A multicenter, open-label, phase 1 study NKX019 1B or 1.5B CAR-NK cells/dose Lymphodepletion Cyclophosphamide 1000 mg/m2 once Day -3 Day 0 Day 7 Day 14 First patient dosing expected 1H 2024 CAR: chimeric antigen receptor Endpoints: 3 doses per cycle Opportunity to investigate broader applicability of NKX019 in multiple autoimmune diseases Off the shelf administration reduces burden to patients and providers Safety and tolerability Pharmacokinetics Renal function Autoantibody serology CONFIDENTIAL
NKX019 in Oncology
CAR T-cell therapy is not broadly accessible Only 20-30% of patients with LBCL who could benefit from CAR T receive it Patients often need to change providers and receive bridging chemotherapy Potential toxicity requires proximity to a specialized inpatient treatment center Over 25% of patients require ICU care Grade 3+ CRS: 13 to 49%, Grade 3+ ICANS / neurotoxicity: 18 to 31% Only 30-40% of patients with LBCL treated with CAR T-cell therapy have 6-month CR No ability to re-dose for incomplete response Outcomes among those that relapse are poor Autologous CAR T-cell therapy has set the bar for cellular therapies in r/r NHL but has limitations YESCARTA USPI; KYMRIAH USPI; BREYANZI USPI; Azoulay et al, 2020; Tomas, et al. 2022. CAR, chimeric antigen receptor; CR, complete response; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; ICU, intensive care unit; LBCL, large B-cell lymphoma; NHL, non-Hodgkin lymphoma. NKX019 CONFIDENTIAL
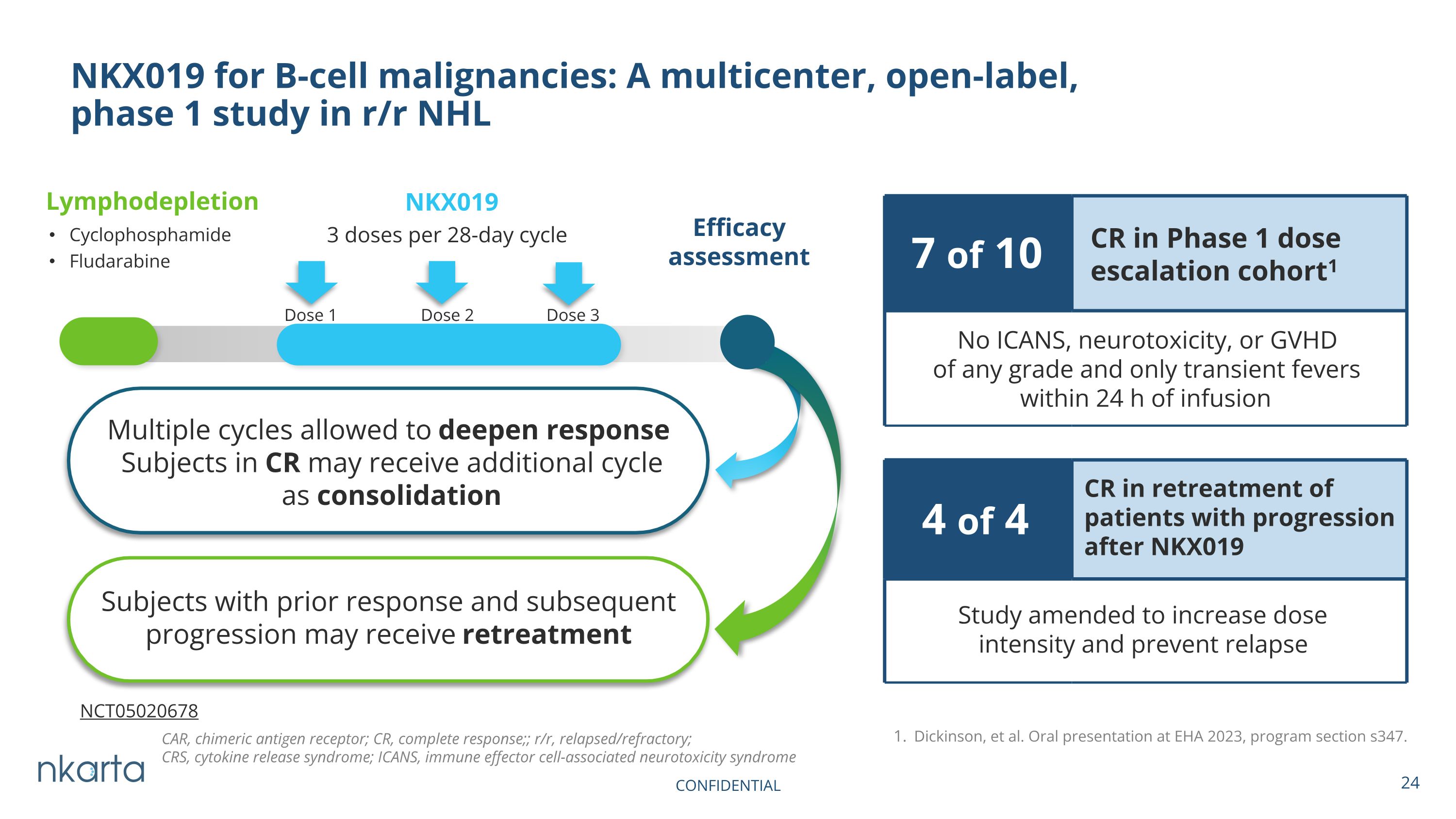
NKX019 for B-cell malignancies: A multicenter, open-label, phase 1 study in r/r NHL NKX019 3 doses per 28-day cycle Lymphodepletion Cyclophosphamide Fludarabine Efficacy assessment Dose 1 Dose 2 Dose 3 Dickinson, et al. Oral presentation at EHA 2023, program section s347. 4 of 4 CR in retreatment of patients with progression after NKX019 Study amended to increase dose intensity and prevent relapse 7 of 10 CR in Phase 1 dose escalation cohort1 No ICANS, neurotoxicity, or GVHD of any grade and only transient fevers within 24 h of infusion Multiple cycles allowed to deepen response Subjects in CR may receive additional cycle as consolidation Subjects with prior response and subsequent progression may receive retreatment CAR, chimeric antigen receptor; CR, complete response;; r/r, relapsed/refractory; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome NCT05020678 CONFIDENTIAL
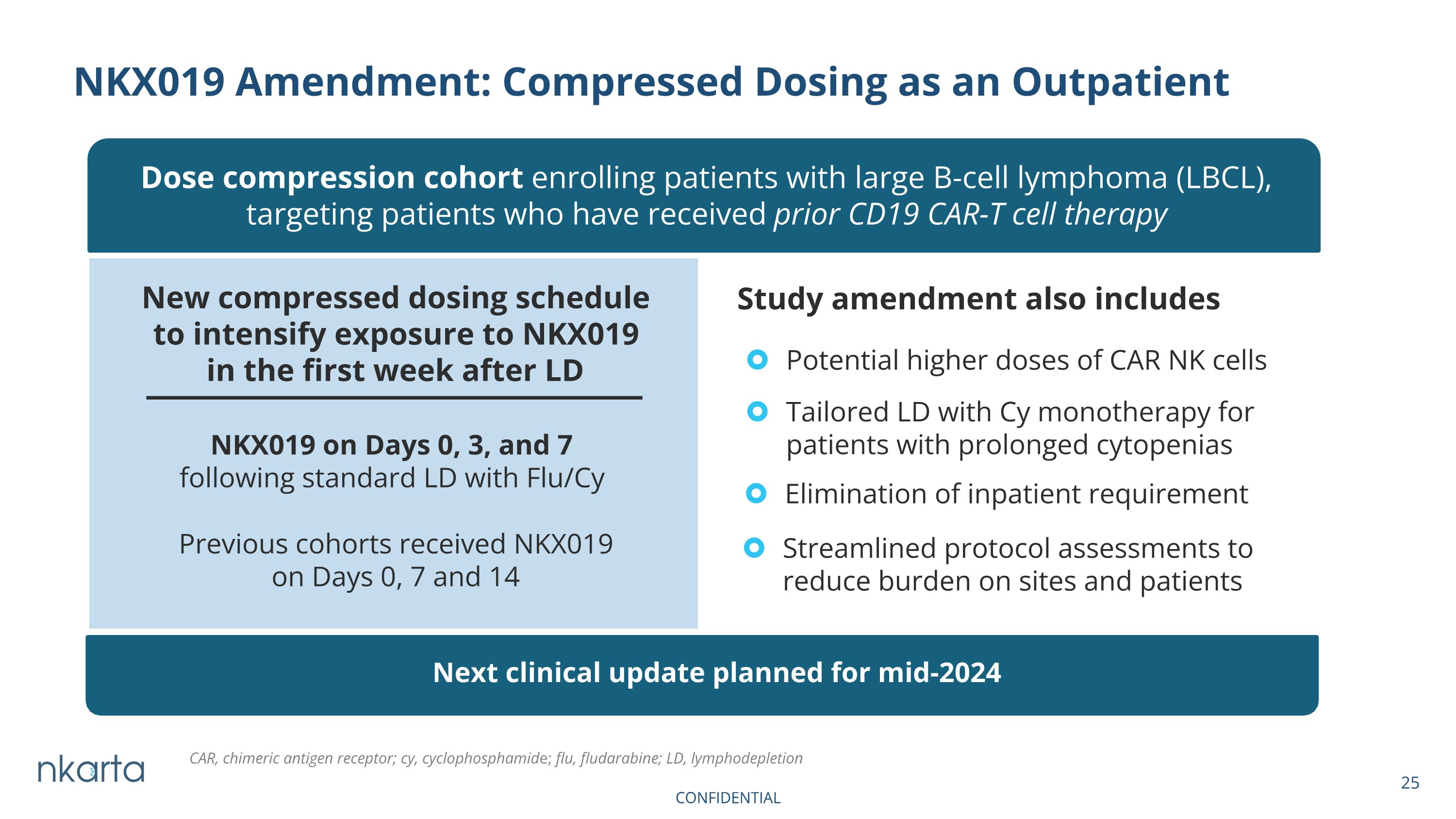
NKX019 Amendment: Compressed Dosing as an Outpatient Dose compression cohort enrolling patients with large B-cell lymphoma (LBCL), targeting patients who have received prior CD19 CAR-T cell therapy New compressed dosing schedule to intensify exposure to NKX019 in the first week after LD Previous cohorts received NKX019 on Days 0, 7 and 14 NKX019 on Days 0, 3, and 7 following standard LD with Flu/Cy Potential higher doses of CAR NK cells Tailored LD with Cy monotherapy for patients with prolonged cytopenias Elimination of inpatient requirement Streamlined protocol assessments to reduce burden on sites and patients Study amendment also includes Next clinical update planned for mid-2024 CAR, chimeric antigen receptor; cy, cyclophosphamide; flu, fludarabine; LD, lymphodepletion
Summary
1H 2024 NKX019 in lupus nephritis Dose first patient and program update Autoimmune expansion | 2024 updates | Cash runway Anticipated 2024 clinical milestones in autoimmune Pipeline prioritization focuses on NKX019 development in autoimmune disease Disease-tailored lymphodepletion leverages NK cell biology and supports differentiated safety/accessibility profile Further investment in NKX019 oncology gated by clinical signals from next data update $250.9 M in cash and cash equivalents as of 31 Dec 2023*; projected cash runway into 2026 CONFIDENTIAL * Includes short-term investments and restricted cash