
Exhibit 99.2 Clinical Program Update 25 April 2022 Clinical Data as of 21 April 2022 1

Forward looking statements This presentation contains forward-looking statements, within the meaning of the Private Securities This presentation has been prepared by the company based on information it has obtained from Litigation Reform Act of 1995, regarding future events and the future results of the company that are sources it believes to be reliable. Summaries of documents contained in this presentation may not based on current expectations, estimates, forecasts, and projections about the industry in which the be complete. The company does not represent that the information herein is complete. The company operates and the future of our business, future plans and strategies, projections, information in this presentation is current only as of the date on the cover, and the company's anticipated trends and events, the economy, and other future conditions, and the beliefs and business or financial condition and other information in this presentation may change after that assumptions of the management of the company. Words such as “address,” “anticipate,” date. The company undertakes no obligation to update any forward-looking statements in order to “believe,” “consider,” “continue,” “develop,” “estimate,” “expect,” “further,” “goal,” “intend,” reflect any event or circumstance occurring after the date of this presentation or currently unknown “may,” “plan,” “potential,” “project,” “seek,” “should,” “target,” “will,” variations of such words, facts or conditions. and similar expressions are intended to identify such forward-looking statements. Such statements reflect the current views of the company and its management with respect to future events and are Interim data from clinical trials are subject to the risk that one or more of the clinical outcomes may subject to inherent risks, uncertainties, and changes in circumstances that are difficult to predict and materially change as patient enrollment continues and more data on existing patients become may be outside our control. Therefore, you should not rely on any of these forward-looking available. The clinical trial program is ongoing, and the final results may be materially different from statements. Should one or more of these risks or uncertainties materialize, or should underlying those reflected in any interim data the company reports. Further, others, including regulatory assumptions prove incorrect, the company's actual results, performance, or achievements could agencies, may not accept or agree with the company’s assumptions, estimates, calculations, differ materially from the results expressed in, or implied by, these forward-looking statements. conclusions or analyses or may interpret or weigh the importance of data differently, which could Please see section entitled “Risk Factors” in our annual, quarterly and other filings with the Securities impact the value of the particular program, the approvability or commercialization of the particular and Exchange Commission for a description of these risks and uncertainties. product candidate or product and the value of the company in general. In addition, the information the company chooses to publicly disclose regarding a particular study or clinical trial is typically a summary of extensive information, and you or others may not agree with what the company determines is the material or otherwise appropriate information to include in its disclosure, and any information the company determines not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or business. 2
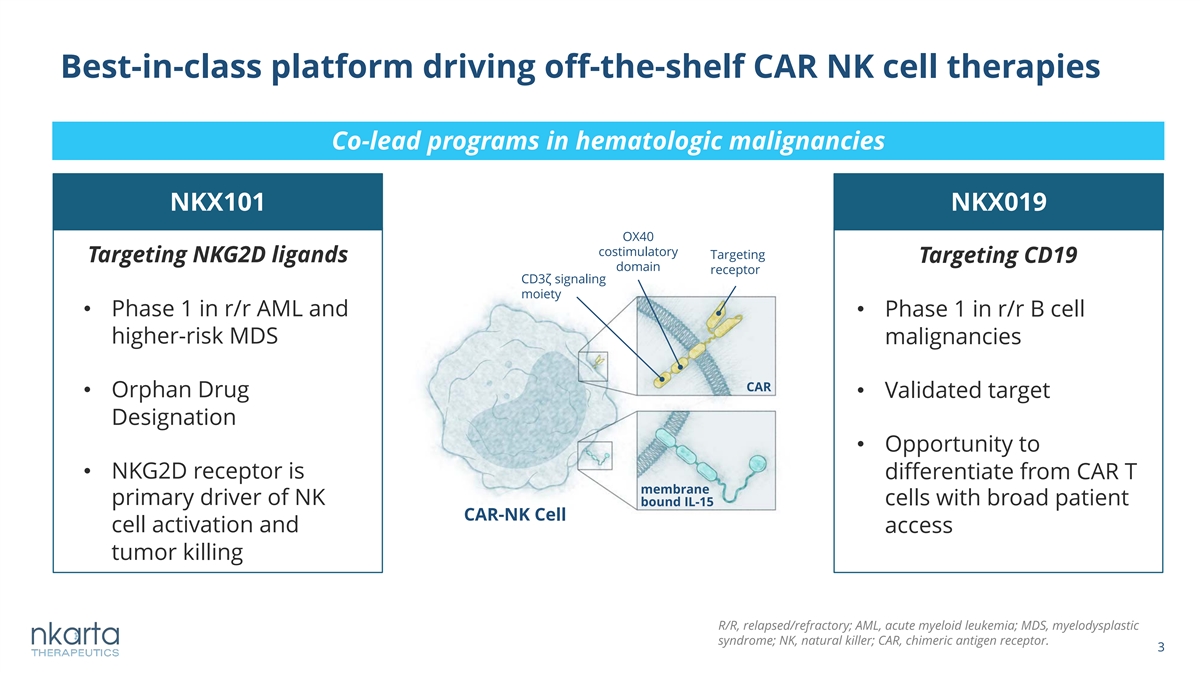
Best-in-class platform driving off-the-shelf CAR NK cell therapies Co-lead programs in hematologic malignancies NKX101 NKX019 OX40 costimulatory Targeting Targeting NKG2D ligands Targeting CD19 domain receptor CD3ζ signaling moiety • Phase 1 in r/r AML and • Phase 1 in r/r B cell higher-risk MDS malignancies CAR • Orphan Drug • Validated target Designation • Opportunity to • NKG2D receptor is differentiate from CAR T membrane primary driver of NK cells with broad patient bound IL-15 CAR-NK Cell cell activation and access tumor killing R/R, relapsed/refractory; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NK, natural killer; CAR, chimeric antigen receptor. 3
NKX101 and NKX019: Well tolerated and highly active in heavily pre-treated r/r AML and r/r NHL patients, respectively • NKX101 - No DLTs or cases of CRS, GvHD or neurotoxicity - 3 of 5 patients achieved CR (60%) in r/r AML at highest two dose levels in 3 dose regimen - 2 out of 3 CRs were MRD negative - Responses and blast reduction observed across dose levels • NKX019 - No DLTs or cases of CRS, GvHD or neurotoxicity - 5 of 6 patients responded (83%) and 3 of 6 patients achieved complete response (50%) in NHL at 1B cells x 3 - Complete responses observed in multiple NHL histologies including DLBCL Based on interim data from open clinical database as of 21 Apr 2022 DLT, dose-limiting toxicity; CRS, cytokine release syndrome; GvHD, graft-versus-host disease; MRD-, minimal residual disease negative; DLBCL, diffuse large B cell lymphoma. 4

NKX101 for the Treatment of Relapsed/Refractory AML and Higher-Risk MDS 5
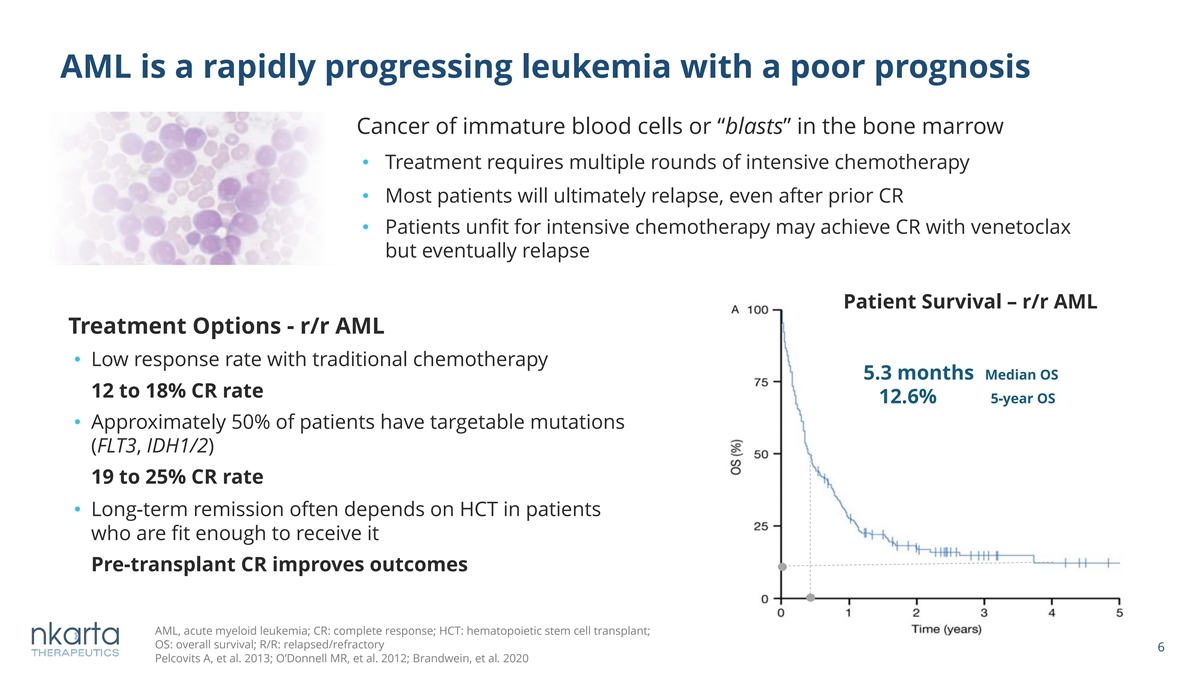
AML is a rapidly progressing leukemia with a poor prognosis Cancer of immature blood cells or “blasts” in the bone marrow • Treatment requires multiple rounds of intensive chemotherapy • Most patients will ultimately relapse, even after prior CR • Patients unfit for intensive chemotherapy may achieve CR with venetoclax but eventually relapse Patient Survival – r/r AML Treatment Options - r/r AML • Low response rate with traditional chemotherapy 5.3 months Median OS 12 to 18% CR rate 12.6% 5-year OS • Approximately 50% of patients have targetable mutations (FLT3, IDH1/2) 19 to 25% CR rate • Long-term remission often depends on HCT in patients who are fit enough to receive it Pre-transplant CR improves outcomes AML, acute myeloid leukemia; CR: complete response; HCT: hematopoietic stem cell transplant; OS: overall survival; R/R: relapsed/refractory 6 Pelcovits A, et al. 2013; O’Donnell MR, et al. 2012; Brandwein, et al. 2020
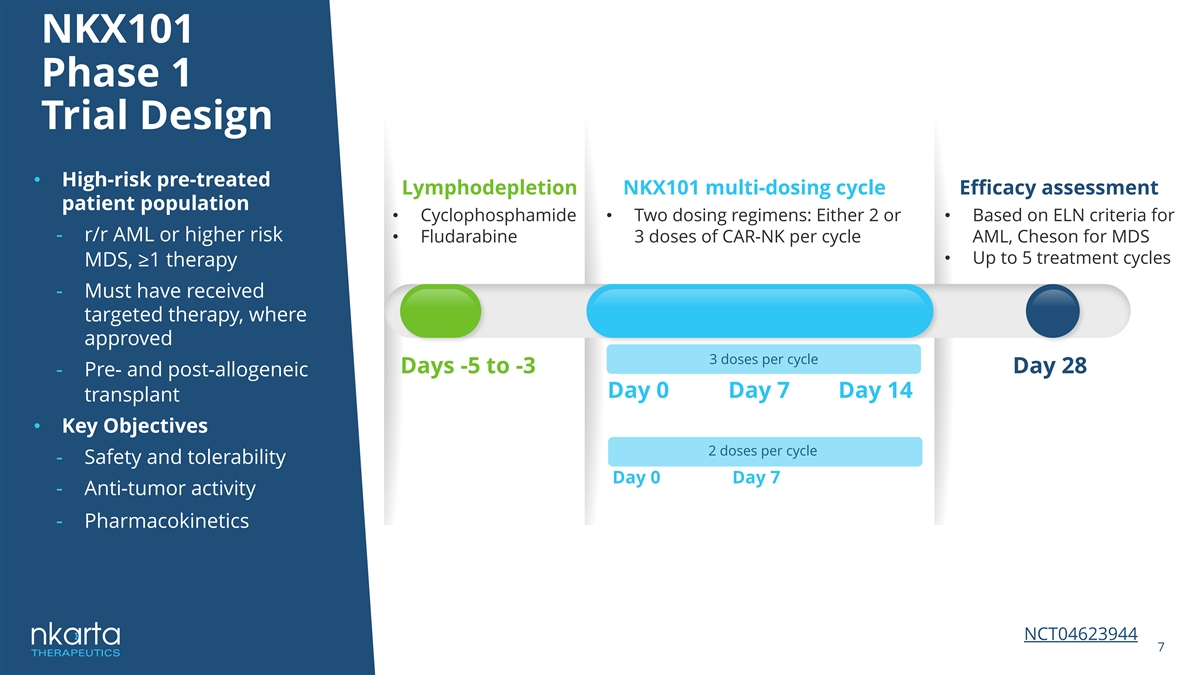
NKX101 Phase 1 Trial Design • High-risk pre-treated Lymphodepletion NKX101 multi-dosing cycle Efficacy assessment patient population • Cyclophosphamide • Two dosing regimens: Either 2 or • Based on ELN criteria for - r/r AML or higher risk • Fludarabine 3 doses of CAR-NK per cycle AML, Cheson for MDS • Up to 5 treatment cycles MDS, ≥1 therapy - Must have received targeted therapy, where approved 3 doses per cycle Days -5 to -3 Day 28 - Pre- and post-allogeneic Day 0 Day 7 Day 14 transplant • Key Objectives 2 doses per cycle - Safety and tolerability Day 0 Day 7 - Anti-tumor activity - Pharmacokinetics NCT04623944 7
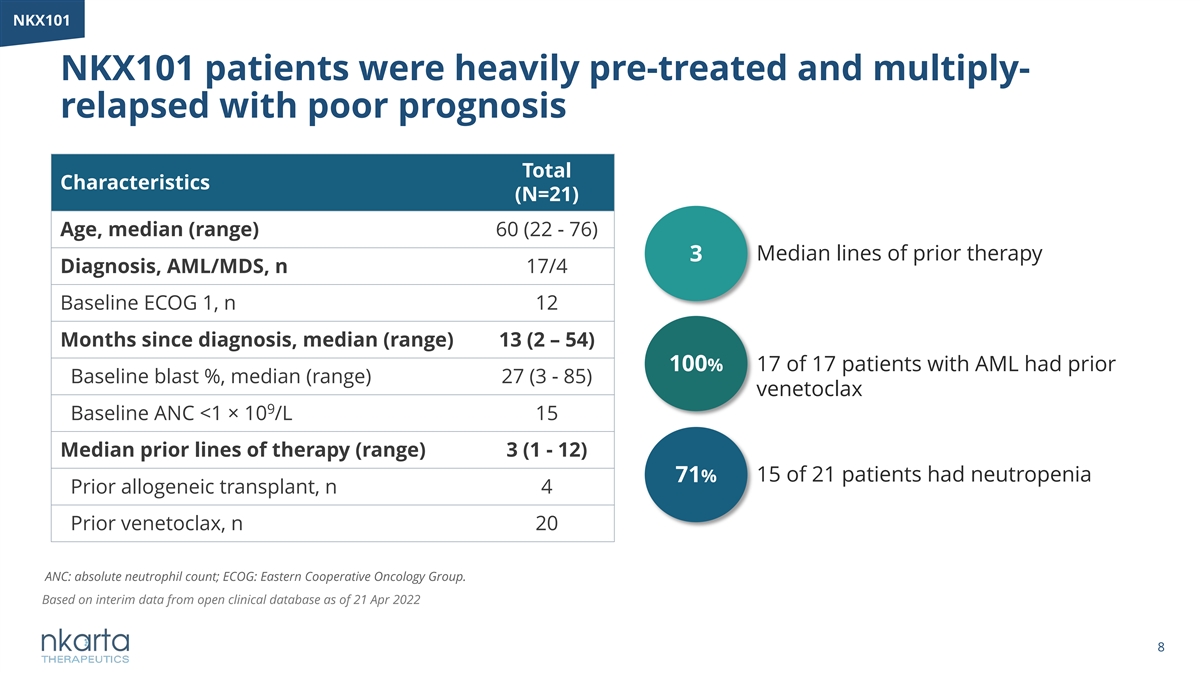
NKX101 NKX101 patients were heavily pre-treated and multiply- relapsed with poor prognosis Total Characteristics (N=21) Age, median (range) 60 (22 - 76) 3 Median lines of prior therapy Diagnosis, AML/MDS, n 17/4 Baseline ECOG 1, n 12 Months since diagnosis, median (range) 13 (2 – 54) 100% 17 of 17 patients with AML had prior Baseline blast %, median (range) 27 (3 - 85) venetoclax 9 Baseline ANC <1 × 10 /L 15 Median prior lines of therapy (range) 3 (1 - 12) 15 of 21 patients had neutropenia 71% Prior allogeneic transplant, n 4 Prior venetoclax, n 20 ANC: absolute neutrophil count; ECOG: Eastern Cooperative Oncology Group. Based on interim data from open clinical database as of 21 Apr 2022 8
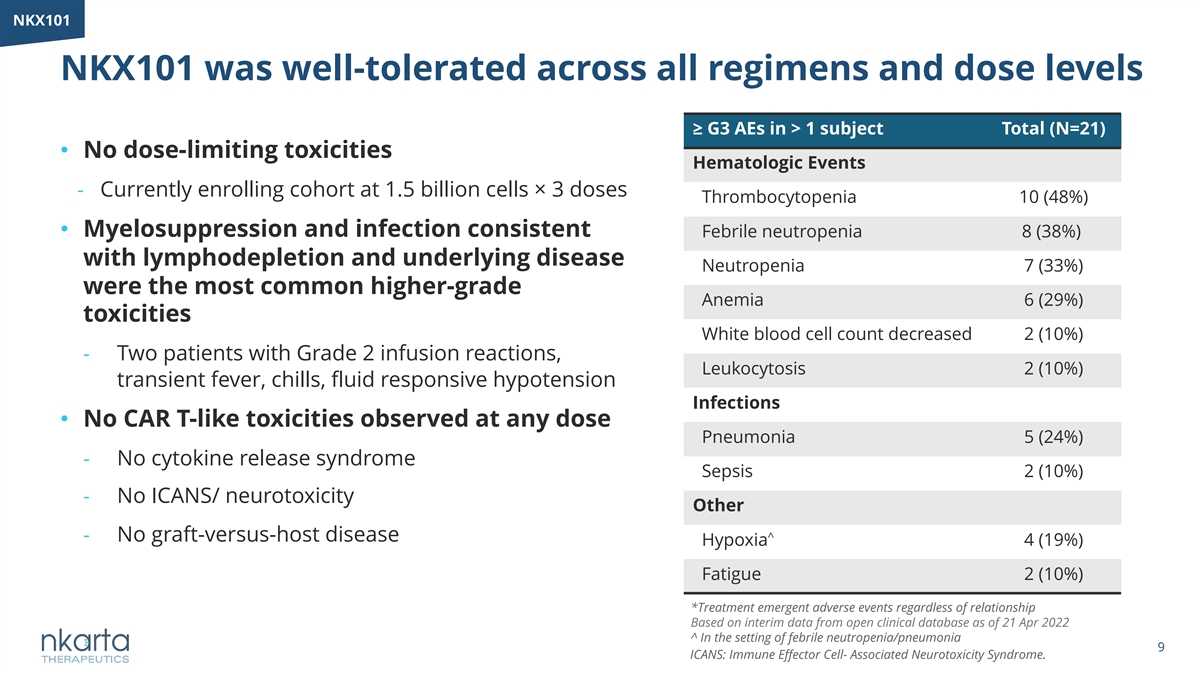
NKX101 NKX101 was well-tolerated across all regimens and dose levels ≥ G3 AEs in > 1 subject Total (N=21) • No dose-limiting toxicities Hematologic Events - Currently enrolling cohort at 1.5 billion cells× 3 doses Thrombocytopenia 10 (48%) • Myelosuppression and infection consistent Febrile neutropenia 8 (38%) with lymphodepletion and underlying disease Neutropenia 7 (33%) were the most common higher-grade Anemia 6 (29%) toxicities White blood cell count decreased 2 (10%) - Two patients with Grade 2 infusion reactions, Leukocytosis 2 (10%) transient fever, chills, fluid responsive hypotension Infections • No CAR T-like toxicities observed at any dose Pneumonia 5 (24%) - No cytokine release syndrome Sepsis 2 (10%) - No ICANS/ neurotoxicity Other - No graft-versus-host disease ^ Hypoxia 4 (19%) Fatigue 2 (10%) *Treatment emergent adverse events regardless of relationship Based on interim data from open clinical database as of 21 Apr 2022 ^ In the setting of febrile neutropenia/pneumonia 9 ICANS: Immune Effector Cell- Associated Neurotoxicity Syndrome.
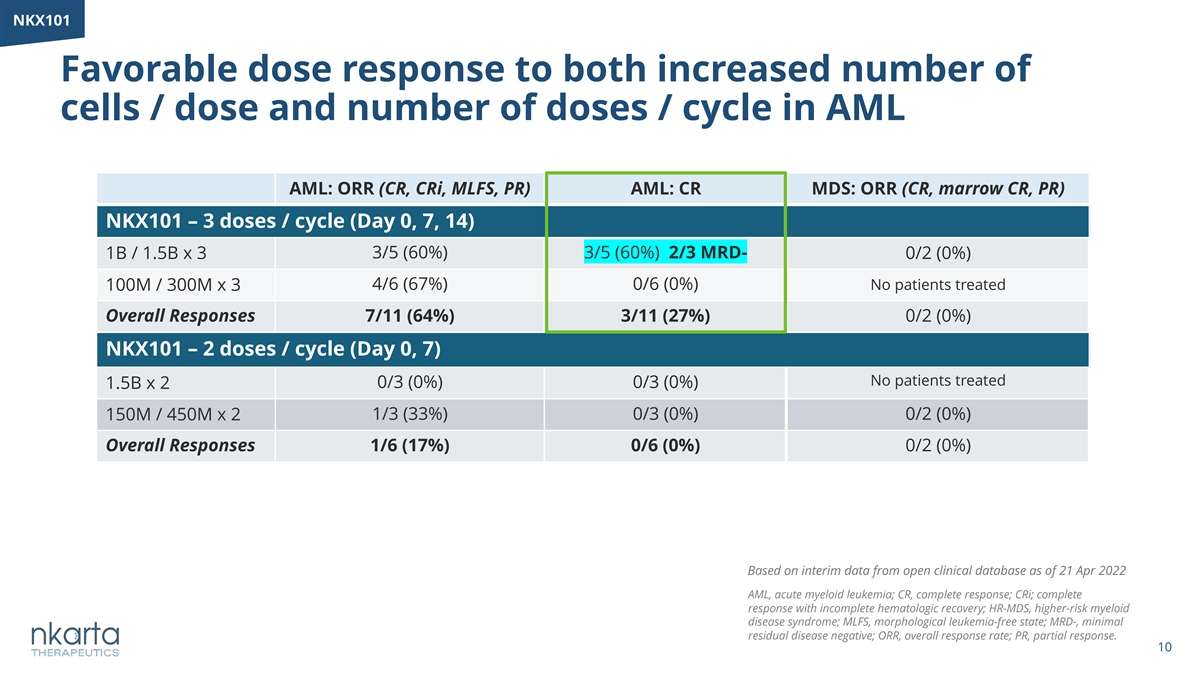
NKX101 Favorable dose response to both increased number of cells / dose and number of doses / cycle in AML AML: ORR (CR, CRi, MLFS, PR) AML: CR MDS: ORR (CR, marrow CR, PR) NKX101 – 3 doses / cycle (Day 0, 7, 14) 1B / 1.5B x 3 3/5 (60%) 3/5 (60%) 2/3 MRD- 0/2 (0%) 4/6 (67%) 0/6 (0%) No patients treated 100M / 300M x 3 Overall Responses 7/11 (64%) 3/11 (27%) 0/2 (0%) NKX101 – 2 doses / cycle (Day 0, 7) No patients treated 1.5B x 2 0/3 (0%) 0/3 (0%) 150M / 450M x 2 1/3 (33%) 0/3 (0%) 0/2 (0%) Overall Responses 1/6 (17%) 0/6 (0%) 0/2 (0%) Based on interim data from open clinical database as of 21 Apr 2022 AML, acute myeloid leukemia; CR, complete response; CRi; complete response with incomplete hematologic recovery; HR-MDS, higher-risk myeloid disease syndrome; MLFS, morphological leukemia-free state; MRD-, minimal residual disease negative; ORR, overall response rate; PR, partial response. 10

NKX101 NKX101 drives AML blast reduction at all dose levels in 3 dose regimen with some patients achieving MRD- Dose Baseline Best post-NK # Level marrow blasts response 1.5B × 3 13% PD 3/5 CR 8% CR (MRD-) at highest CR (MRD+) doses in 16% MLFS end of Cycle 1, 1B × 3 go-forward CR end of Cycle 2 3-dose 10% PD regimen 10% CR (MRD-) 35% MLFS 300M × 3 37% MLFS 47% PD 30% PR 100M × 3 56% PD 3% MLFS MLFS, morphologic leukemia free state; MRD-, minimal residual disease negative; MRD+, minimal residual disease positive; PR, partial response; PD, progressive disease. # Baseline is the most recent available data prior to first dose * PD, Peripheral blast progression Based on interim data from open clinical database as of 21 Apr 2022 11
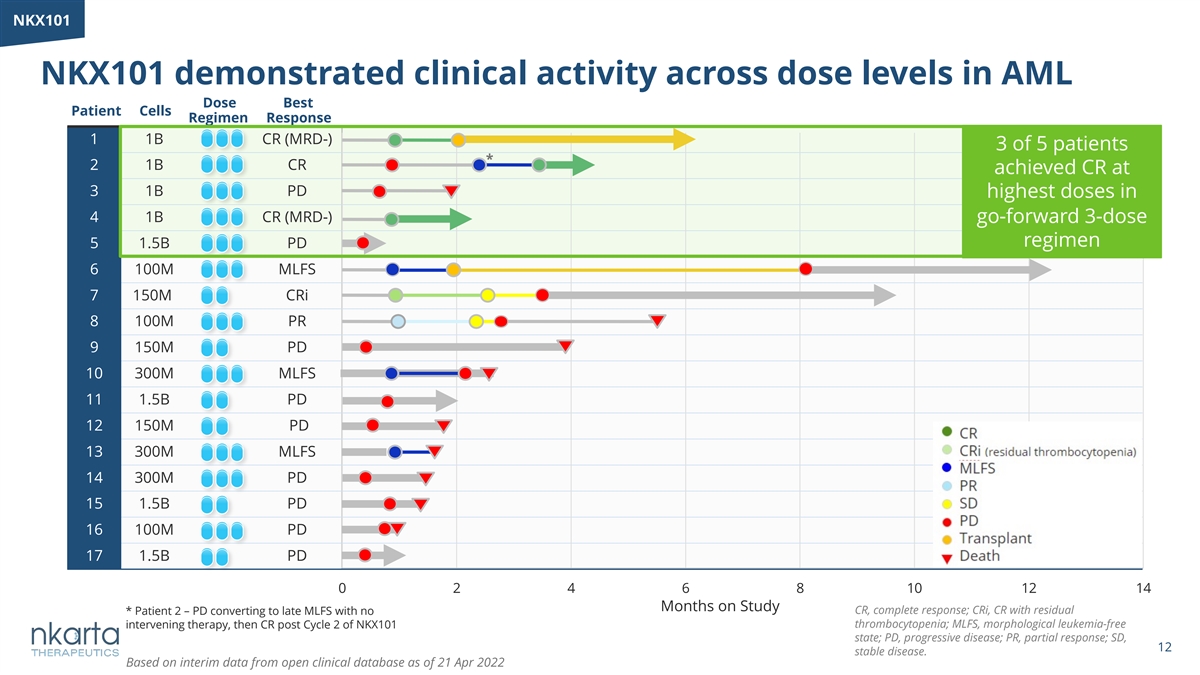
NK NKX1 X10 01 1 NKX101 demonstrated clinical activity across dose levels in AML Dose Best Patient Cells Regimen Response Months on Study 1 1B CR (MRD-) 3 of 5 patients * 2 1B CR achieved CR at 3 1B PD highest doses in 4 1B CR (MRD-) go-forward 3-dose regimen 5 1.5B PD 6 100M MLFS 7 150M CRi 8 100M PR 9 150M PD 10 300M MLFS 11 1.5B PD 12 150M PD 13 300M MLFS 14 300M PD 15 1.5B PD 16 100M PD 17 1.5B PD 0 2 4 6 8 10 12 14 Months on Study CR, complete response; CRi, CR with residual * Patient 2 – PD converting to late MLFS with no thrombocytopenia; MLFS, morphological leukemia-free intervening therapy, then CR post Cycle 2 of NKX101 state; PD, progressive disease; PR, partial response; SD, 12 stable disease. Based on interim data from open clinical database as of 21 Apr 2022
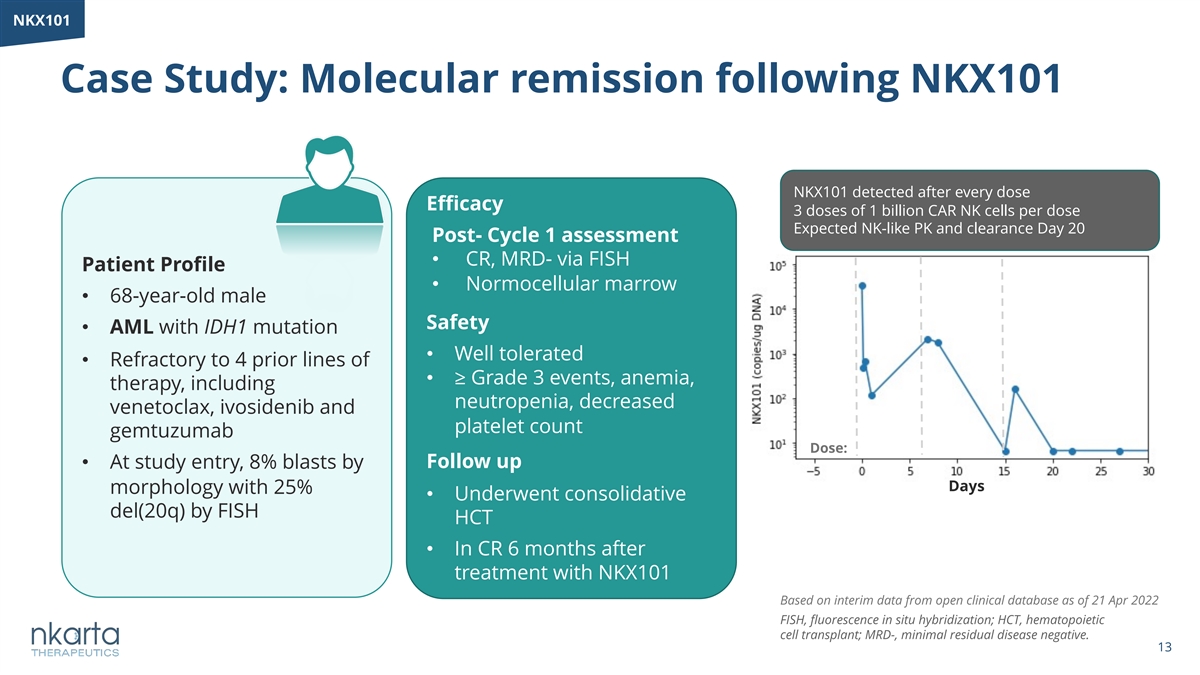
NKX101 Case Study: Molecular remission following NKX101 NKX101 detected after every dose Efficacy 3 doses of 1 billion CAR NK cells per dose Expected NK-like PK and clearance Day 20 Post- Cycle 1 assessment • CR, MRD- via FISH Patient Profile • Normocellular marrow • 68-year-old male Safety • AML with IDH1 mutation • Well tolerated • Refractory to 4 prior lines of • ≥ Grade 3 events, anemia, therapy, including neutropenia, decreased venetoclax, ivosidenib and platelet count gemtuzumab Dose: • At study entry, 8% blasts by Follow up Days morphology with 25% • Underwent consolidative del(20q) by FISH HCT • In CR 6 months after treatment with NKX101 Based on interim data from open clinical database as of 21 Apr 2022 FISH, fluorescence in situ hybridization; HCT, hematopoietic cell transplant; MRD-, minimal residual disease negative. 13
NKX101 Summary: NKX101 was well-tolerated and highly active in heavily pre-treated AML patients • No DLTs or cases of CRS, GvHD or neurotoxicity • 3 of 5 patients achieved CR (60%) in r/r AML at highest two dose levels in 3-dose regimen - 2 out of 3 CRs were MRD negative • Responses and blast reduction observed across dose levels - Dose response - Deepening of response with additional cycle • Next steps - Dosing of AML patients at 1.5B cells x 3 in dose escalation study - Potential for approval in r/r AML with single arm expansion cohort data - Potential to move to earlier lines in combination - Next data update 2H 2022 Based on interim data from open clinical database as of 21 Apr 2022 14
NKX019 for the Treatment of Relapsed/Refractory B-Cell Malignancies 15
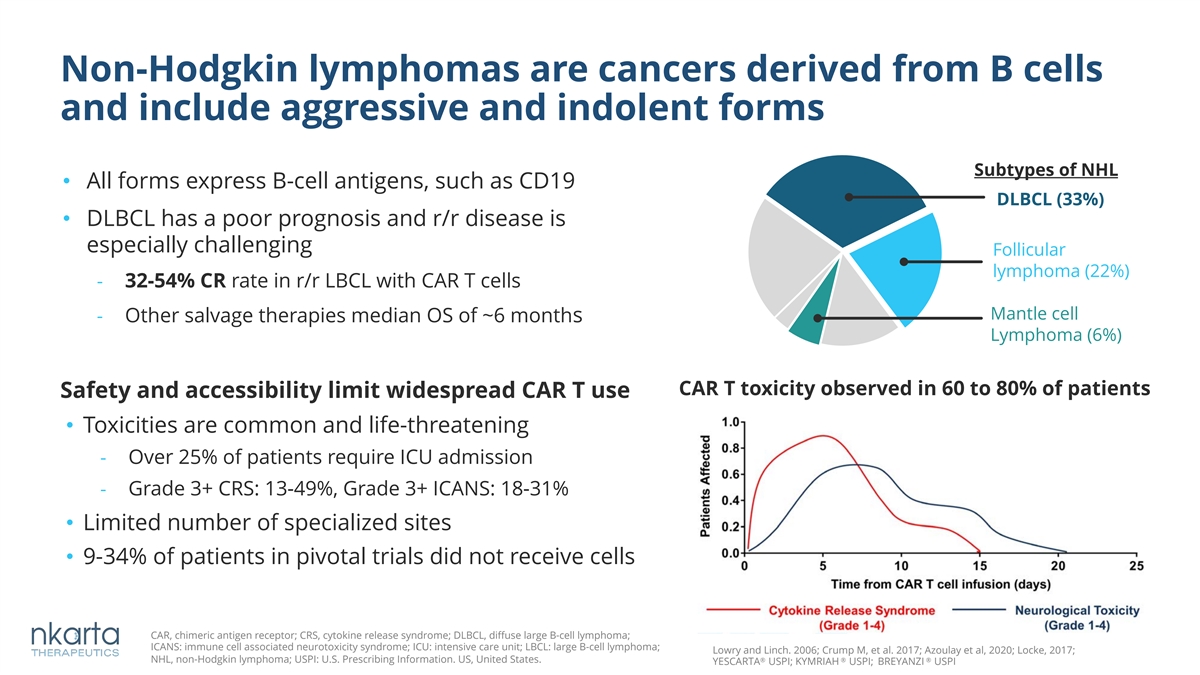
Non-Hodgkin lymphomas are cancers derived from B cells and include aggressive and indolent forms Subtypes of NHL • All forms express B-cell antigens, such as CD19 DLBCL (33%) • DLBCL has a poor prognosis and r/r disease is especially challenging Follicular lymphoma (22%) - 32-54% CR rate in r/r LBCL with CAR T cells Mantle cell - Other salvage therapies median OS of ~6 months Lymphoma (6%) CAR T toxicity observed in 60 to 80% of patients Safety and accessibility limit widespread CAR T use • Toxicities are common and life-threatening - Over 25% of patients require ICU admission - Grade 3+ CRS: 13-49%, Grade 3+ ICANS: 18-31% • Limited number of specialized sites • 9-34% of patients in pivotal trials did not receive cells CAR, chimeric antigen receptor; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; ICANS: immune cell associated neurotoxicity syndrome; ICU: intensive care unit; LBCL: large B-cell lymphoma; Lowry and Linch. 2006; Crump M, et al. 2017; Azoulay et al, 2020; Locke, 2017; NHL, non-Hodgkin lymphoma; USPI: U.S. Prescribing Information. US, United States. ® ® ® YESCARTA USPI; KYMRIAH USPI; BREYANZI USPI

NKX019 Phase 1 Trial Design • High-risk, high-need patient population Lymphodepletion NKX019 multi-dosing cycle Efficacy assessment • Cyclophosphamide • 3 doses of CAR-NK per cycle • Based on Lugano criteria - r/r NHL, CLL and B-ALL • Fludarabine for NHL, NCCN for B-ALL - ≥2 prior therapies • Up to 5 treatment cycles - CAR T naïve • Key Objectives - Safety and tolerability Days -5 to -3 Day 0 Day 7 Day 14 Day 28 Dose 1 Dose 2 Dose 3 - Anti-tumor activity - Pharmacokinetics NCT05020678 CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; B-ALL, B cell acute lymphocytic leukemia; NHL, non-Hodgkin lymphoma. 17
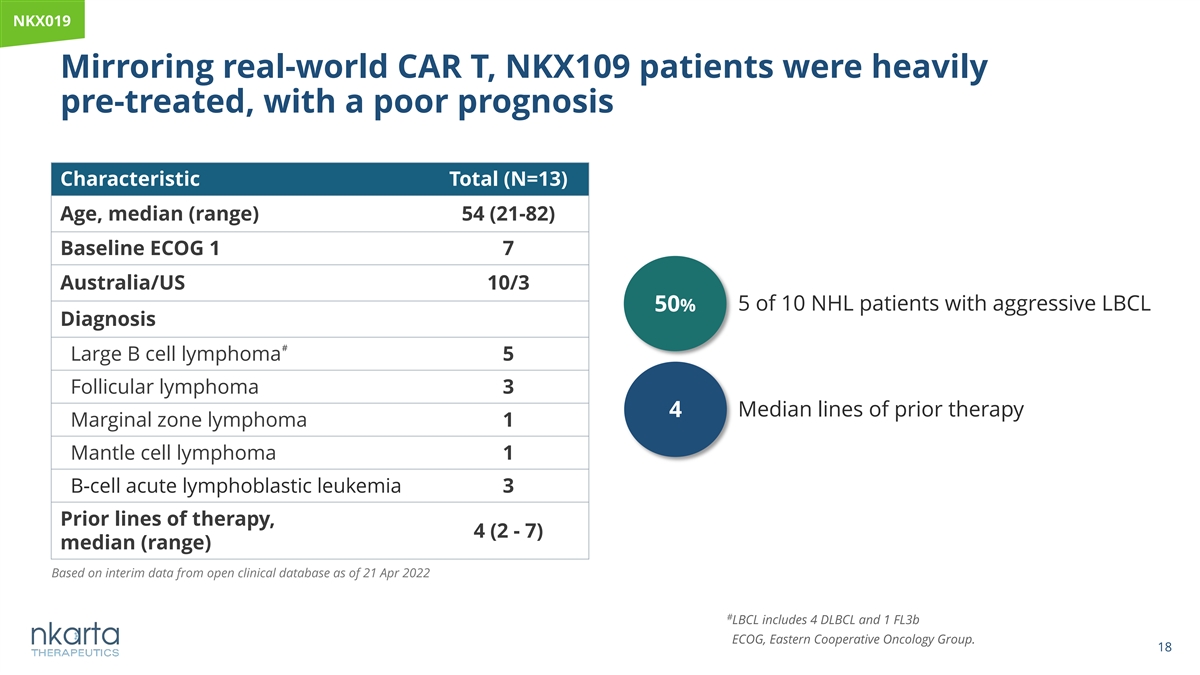
NK NKX0 X01 19 9 Mirroring real-world CAR T, NKX109 patients were heavily pre-treated, with a poor prognosis Characteristic Total (N=13) Age, median (range) 54 (21-82) Baseline ECOG 1 7 Australia/US 10/3 50% 5 of 10 NHL patients with aggressive LBCL Diagnosis # Large B cell lymphoma 5 Follicular lymphoma 3 Median lines of prior therapy 4 Marginal zone lymphoma 1 Mantle cell lymphoma 1 B-cell acute lymphoblastic leukemia 3 Prior lines of therapy, 4 (2 - 7) median (range) Based on interim data from open clinical database as of 21 Apr 2022 # LBCL includes 4 DLBCL and 1 FL3b ECOG, Eastern Cooperative Oncology Group. 18
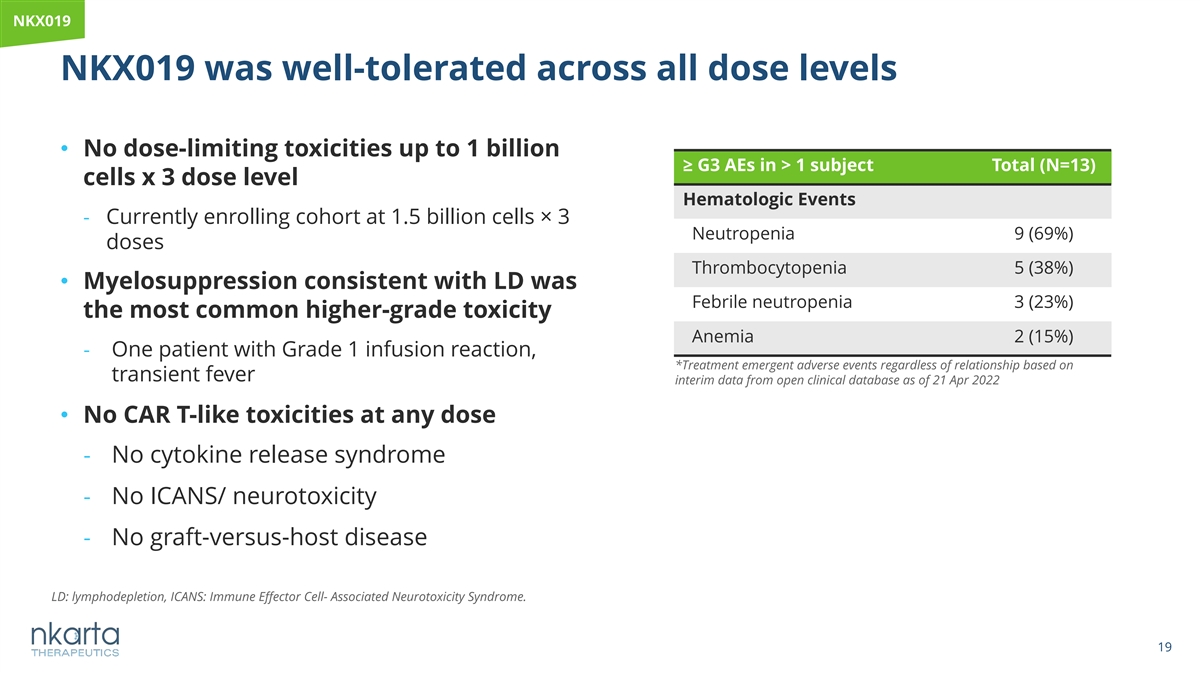
NKX019 NKX019 was well-tolerated across all dose levels • No dose-limiting toxicities up to 1 billion ≥ G3 AEs in > 1 subject Total (N=13) cells x 3 dose level Hematologic Events - Currently enrolling cohort at 1.5 billion cells× 3 Neutropenia 9 (69%) doses Thrombocytopenia 5 (38%) • Myelosuppression consistent with LD was Febrile neutropenia 3 (23%) the most common higher-grade toxicity Anemia 2 (15%) - One patient with Grade 1 infusion reaction, *Treatment emergent adverse events regardless of relationship based on transient fever interim data from open clinical database as of 21 Apr 2022 • No CAR T-like toxicities at any dose - No cytokine release syndrome - No ICANS/ neurotoxicity - No graft-versus-host disease LD: lymphodepletion, ICANS: Immune Effector Cell- Associated Neurotoxicity Syndrome. 19
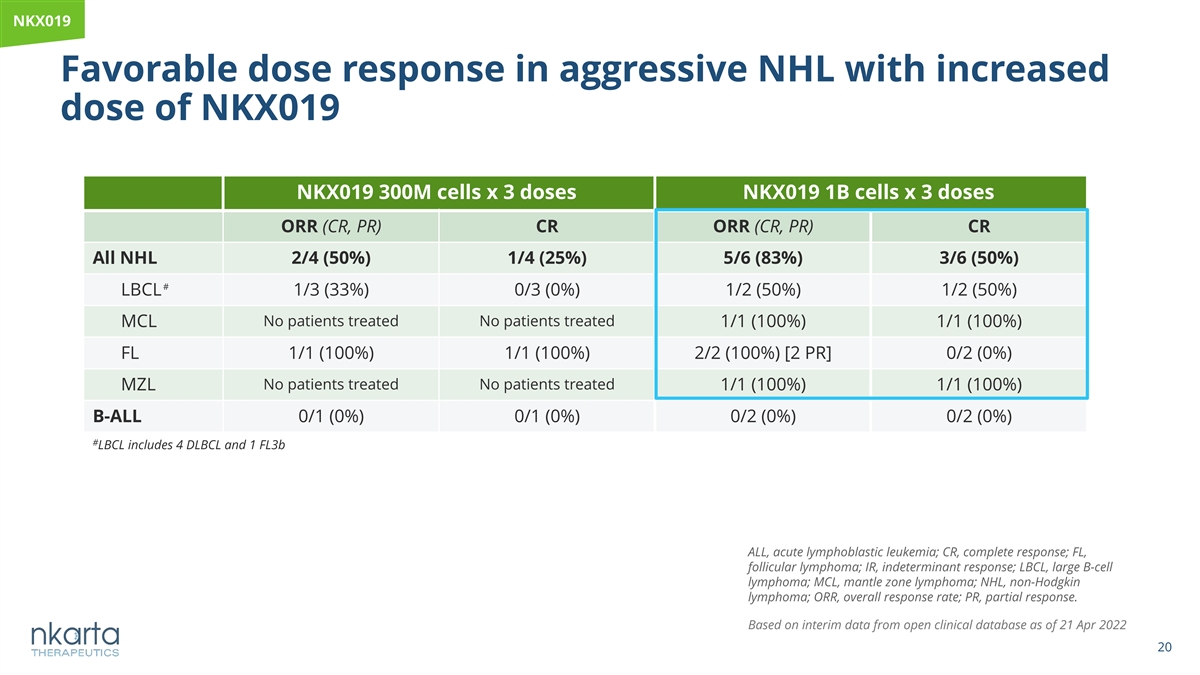
NKX019 Favorable dose response in aggressive NHL with increased dose of NKX019 NKX019 300M cells x 3 doses NKX019 1B cells x 3 doses ORR (CR, PR) CR ORR (CR, PR) CR All NHL 2/4 (50%) 1/4 (25%) 5/6 (83%) 3/6 (50%) # LBCL 1/3 (33%) 0/3 (0%) 1/2 (50%) 1/2 (50%) No patients treated No patients treated MCL 1/1 (100%) 1/1 (100%) FL 1/1 (100%) 1/1 (100%) 2/2 (100%) [2 PR] 0/2 (0%) MZL No patients treated No patients treated 1/1 (100%) 1/1 (100%) B-ALL 0/1 (0%) 0/1 (0%) 0/2 (0%) 0/2 (0%) # LBCL includes 4 DLBCL and 1 FL3b ALL, acute lymphoblastic leukemia; CR, complete response; FL, follicular lymphoma; IR, indeterminant response; LBCL, large B-cell lymphoma; MCL, mantle zone lymphoma; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PR, partial response. Based on interim data from open clinical database as of 21 Apr 2022 20
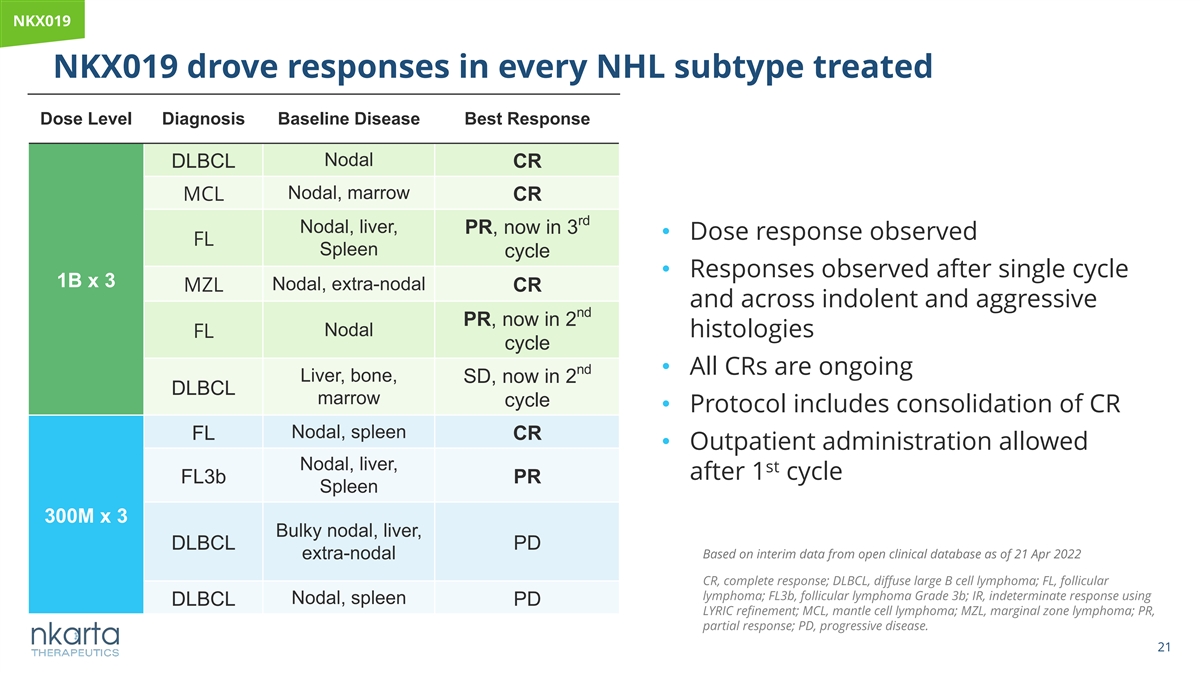
NKX019 NKX019 drove responses in every NHL subtype treated Dose Level Diagnosis Baseline Disease Best Response Nodal DLBCL CR Nodal, marrow MCL CR rd Nodal, liver, PR, now in 3 • Dose response observed FL Spleen cycle • Responses observed after single cycle 1B x 3 Nodal, extra-nodal MZL CR and across indolent and aggressive nd PR, now in 2 Nodal FL histologies cycle nd • All CRs are ongoing Liver, bone, SD, now in 2 DLBCL marrow cycle • Protocol includes consolidation of CR Nodal, spleen FL CR • Outpatient administration allowed Nodal, liver, st after 1 cycle FL3b PR Spleen 300M x 3 Bulky nodal, liver, DLBCL PD Based on interim data from open clinical database as of 21 Apr 2022 extra-nodal CR, complete response; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; FL3b, follicular lymphoma Grade 3b; IR, indeterminate response using Nodal, spleen DLBCL PD LYRIC refinement; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; PR, partial response; PD, progressive disease. 21
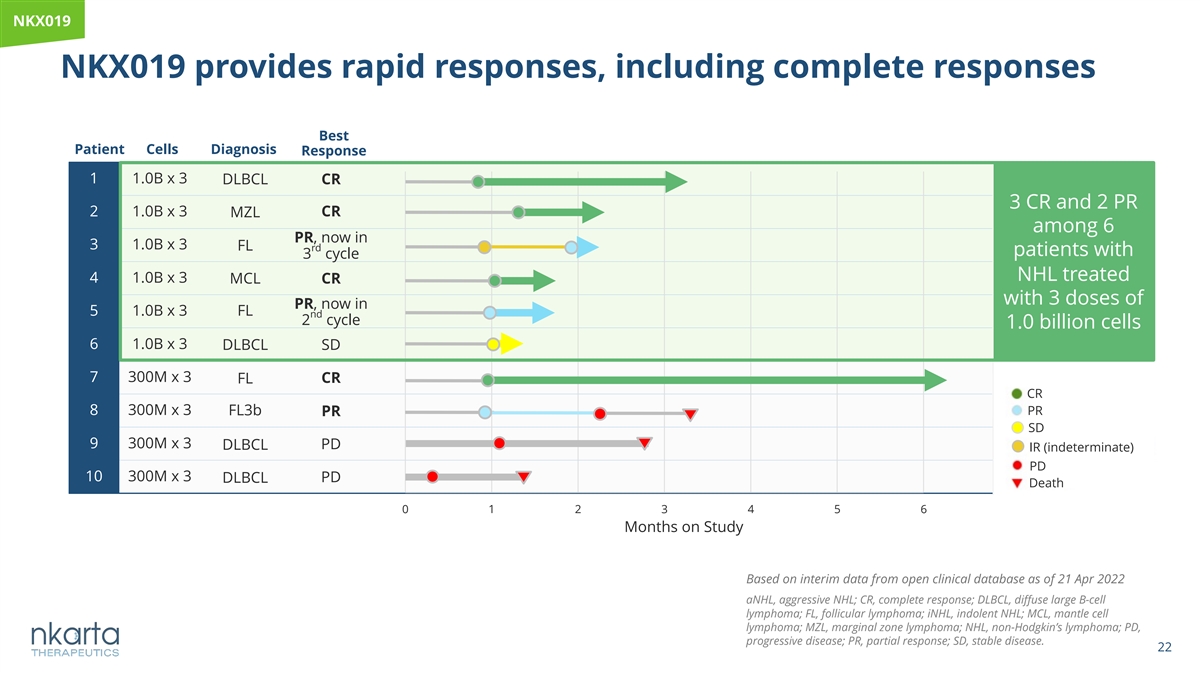
NKX019 NKX019 provides rapid responses, including complete responses Best Patient Cells Diagnosis Response 1 1.0B x 3 DLBCL CR 3 CR and 2 PR 2 1.0B x 3 CR MZL among 6 PR, now in 3 1.0B x 3 FL rd patients with 3 cycle NHL treated 4 1.0B x 3 MCL CR with 3 doses of PR, now in 5 1.0B x 3 FL nd 2 cycle 1.0 billion cells 6 1.0B x 3 DLBCL SD 7 300M x 3 CR FL CR 8 300M x 3 FL3b PR PR SD 9 300M x 3 DLBCL PD IR (indeterminate) PD 10 300M x 3 DLBCL PD Death 0 1 2 3 4 5 6 Months on Study Based on interim data from open clinical database as of 21 Apr 2022 aNHL, aggressive NHL; CR, complete response; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; iNHL, indolent NHL; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non-Hodgkin’s lymphoma; PD, progressive disease; PR, partial response; SD, stable disease. 22
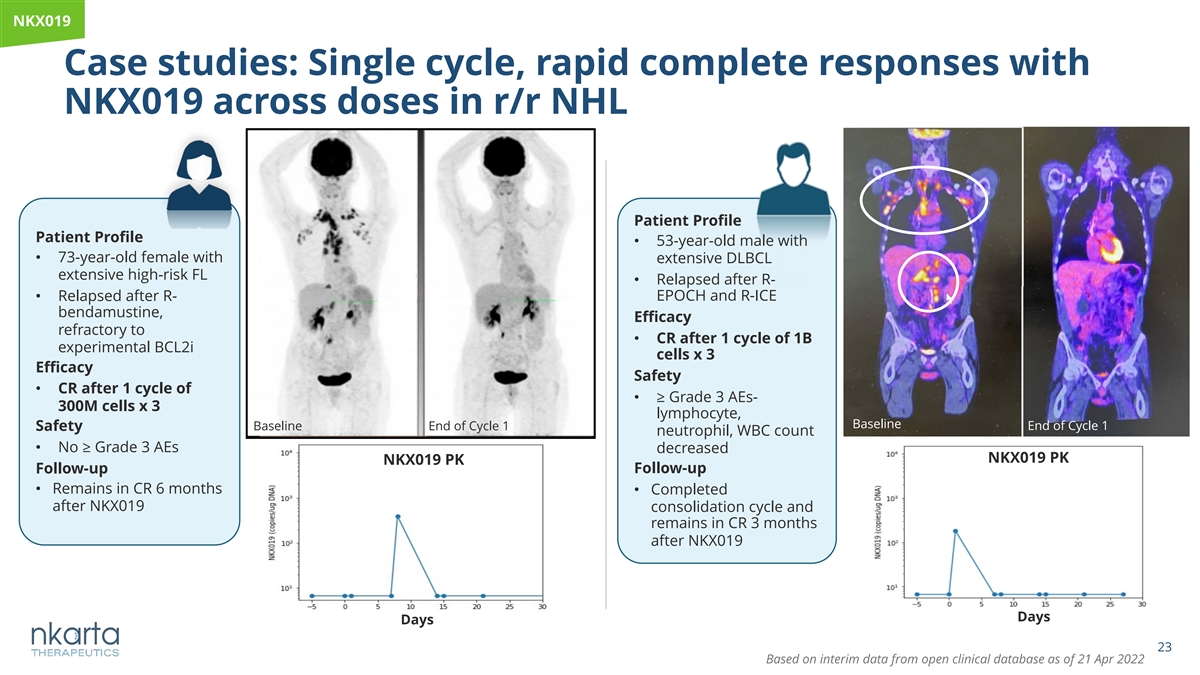
NKX019 Case studies: Single cycle, rapid complete responses with NKX019 across doses in r/r NHL Patient Profile Patient Profile • 53-year-old male with • 73-year-old female with extensive DLBCL extensive high-risk FL • Relapsed after R- • Relapsed after R- EPOCH and R-ICE bendamustine, Efficacy refractory to • CR after 1 cycle of 1B experimental BCL2i cells x 3 Efficacy Safety • CR after 1 cycle of • ≥ Grade 3 AEs- 300M cells x 3 lymphocyte, Baseline Baseline End of Cycle 1 End of Cycle 1 Safety neutrophil, WBC count • No ≥ Grade 3 AEs decreased NKX019 PK NKX019 PK Follow-up Follow-up • Remains in CR 6 months • Completed after NKX019 consolidation cycle and remains in CR 3 months after NKX019 Days Days 23 Based on interim data from open clinical database as of 21 Apr 2022
NKX019 NKX019 shows compelling preliminary activity and safety in NHL with the potential to address multiple unmet needs • No DLTs or cases of CRS, GvHD or neurotoxicity • 5 of 6 patients responded (83% ORR) and 3 of 6 patients achieved complete response (50% CR) in NHL at 1B cells× 3 dose level - Complete responses observed in multiple NHL histologies including DLBCL • Durability of at least 5 months with one patient at lowest dose of 300M cells x 3 • Next steps - Potential to improve and deepen responses with higher dose of 1.5B cells x 3 - Planned expansion cohorts in both CAR T treated and CAR T naïve LBCL - Potential combination studies in expansion cohorts - Next data update 2H 2022 Based on interim data from open clinical database as of 21 Apr 2022 24
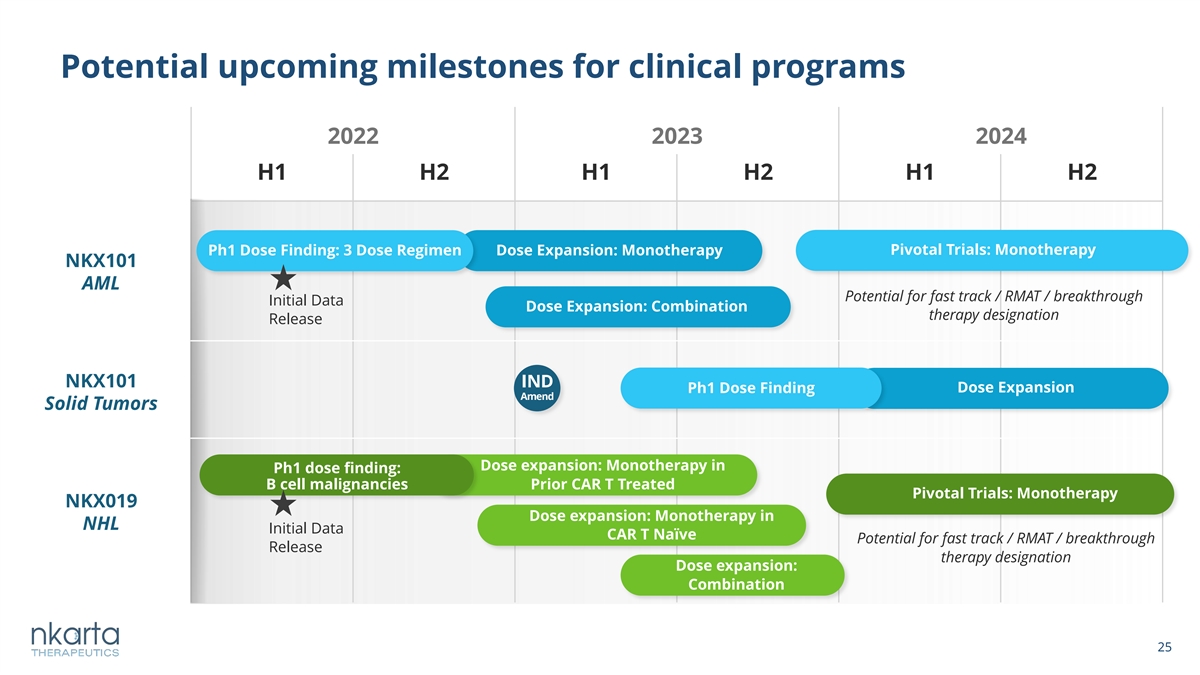
Potential upcoming milestones for clinical programs 2022 2023 2024 H1 H2 H1 H2 H1 H2 Ph1 Dose Finding: 3 Dose Regimen Dose Expansion: Monotherapy Pivotal Trials: Monotherapy NKX101 AML Potential for fast track / RMAT / breakthrough Initial Data Dose Expansion: Combination therapy designation Release NKX101 IND Dose Expansion Ph1 Dose Finding Amend Solid Tumors Dose expansion: Monotherapy in Ph1 dose finding: B cell malignancies Prior CAR T Treated Pivotal Trials: Monotherapy NKX019 Dose expansion: Monotherapy in NHL Initial Data CAR T Naïve Potential for fast track / RMAT / breakthrough Release therapy designation Dose expansion: Combination 25 25

ü Promising preliminary activity with favorable safety profiles Emerging clinical ü Allogeneic and off-the-shelf therapies data from both available on demand NKX101 and ü Potential to pursue accelerated regulatory NKX019 programs pathway options, earlier line combination validate the Nkarta regimens for both programs platform ü Significant progress toward fulfilling the mission to: Discover, develop and deliver novel off-the-shelf NK cell therapy product candidates that have a profound impact on cancer patients 26

























