Exhibit 99.2

CONFIDENTIAL Enabling Cures with Hematopoietic Stem Cell Transplants April 2021

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Safe Harbor Statement 2 About this Presentation This investor presentation (this “Presentation”) is for informational purposes only to assist interested parties in making th eir own evaluation with respect to the proposed business combination (the “Proposed Business Combination”) between Amplitude Heal th care Acquisition Corp. ("AMHC") and Jasper Therapeutics, Inc. (together with its subsidiaries, “Jasper Therapeutics” or the “Company”) and for no other purpose. The information contained her ein does not purport to be all - inclusive and none of AMHC, the Company or their respective affiliates makes any representation o r warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation. Viewers of this presentation should make their own evaluation of the Company and of the relevance and accuracy of the information and should make such other investigations as they deem neces sar y. This Presentation does not constitute (i) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Proposed Business Combination or (ii) an offer to sell, a solicitation of an offer to buy, or a recommendat ion to purchase any security of AMHC, the Company, or any of their respective affiliates, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unl awful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities sh all be made expect by means of a prospectus meeting the requirements of the U.S. Securities Act of 1933, as amended (the “Securities Act”). You should not construe the contents of this Presentation as legal , t ax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors a s t o legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any d eci sion. The distribution of this Presentation may also be restricted by law and persons into whose possession this Presentation comes sh ould inform themselves about and observe any such restrictions. The recipient acknowledges that it is (a) aware that the Unit ed States securities laws prohibit any person who has material, non - public information concerning a company from purchasing or selling securities of such company or from communicating such info rma tion to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sel l such securities, and (b) familiar with the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder (collectively, the "Exchange Act"), and that the recip ien t will neither use, nor cause any third party to use, this Presentation or any information contained herein in contravention of the Exchange Act, including, without limitation, Rule 10b - 5 thereunder. This Presentation and information contained herein constitutes confidential information and is provided to you on the conditi on that you agree that you will hold it in strict confidence and not reproduce, disclose, forward or distribute it in whole or i n p art without the prior written consent of AMHC and the Company and is intended for the recipient hereof only. Additional Information The Company intends to file with the SEC a proxy statement / prospectus on Form S - 4 relating to the Proposed Business Combinatio n, which will be mailed to AMHC’s shareholders once definitive. This Presentation does not contain all the information that s hou ld be considered concerning the Proposed Business Combination and is not intended to form the basis of any investment decision or any other decision in respect of the Proposed Business Co mbi nation. AMHC’s shareholders and other interested persons are advised to read, when available, the preliminary proxy statement / prospectus and the amendments thereto and the proxy statement / prospectus and other documents filed in connection with the Proposed Business Combination, as these materials will contain im por tant information about the Company, AMHC and the Proposed Business Combination. When available, the proxy statement / prospec tus and other relevant materials for the Proposed Business Combination will be mailed to shareholders of AMHC as of a record date to be established for voting on the Proposed Business Com bination. Shareholders will also be able to obtain copies of the preliminary proxy statement / prospectus, the definitive pro xy statement / prospectus and other documents filed with the SEC, without charge, once available, at the SEC’s website at www.sec.gov, or by directing a request to Jasper Therapeutics at Jasp er Therapeutics, Inc., 2200 Bridge Pkwy Suit #102, Redwood City, CA 94065 or to AMHC at Amplitude Healthcare Acquisition Corp., 117 7 Avenue of the Americas, Fl 40, New York, NY 10036. Participants in the Solicitation AMHC and its directors and executive officers may be deemed participants in the solicitation of proxies from AMHC’s sharehold ers with respect to the Proposed Business Combination. A list of the names of those directors and executive officers and a descri pt ion of their interests in AMHC is contained in AMHC’s Registration Statement on Form S - 1, as effective on November 19, 2019, which was filed with the SEC and is available free of charge at the SE C’s web site at www.sec.gov, or by directing a request to AMHC at Amplitude Healthcare Acquisition Corp., 1177 Avenue of the Ame ricas, Fl 40, New York, NY 10036. Additional information regarding the interests of such participants will be contained in the proxy statement / prospectus for the Proposed Business Combinatio n w hen available. The Company and its directors and executive officers may also be deemed to be participants in the solicitation of proxies fro m t he shareholders of AMHC in connection with the Proposed Business Combination. A list of the names of such directors and execu tiv e officers and information regarding their interests in the Proposed Business Combination will be included in the proxy statement / prospectus for the Proposed Business Combination when availabl e. Private Placement The PIPE financing described herein has not been and will not be registered under the Securities Act, or any applicable state se curities laws. This Presentation is being furnished solely in reliance on applicable exemptions from the registration require men ts under the Securities Act. If the Proposed Business Combination is entered into, the PIPE financing will be offered and sold only to "qualified institutional buyers" (as defined in Rule 144A u nde r the Securities Act) and institutional "accredited investors" (as defined in Rule 501(a)(1), (2),(3) or (7) promulgated unde r t he Securities Act) upon the consummation of the Proposed Business Combination. This presentation does not constitute an offer to sell or a solicitation of an offer to buy the securities that sha ll constitute the PIPE financing described herein, nor shall there be any offer, solicitation, or sale of any such securities in any jurisdiction in which such offer, solicitation, or sale would be unlawful. Before you invest you should undertake your own diligence regarding the Proposed Business Combination. Forward - Looking Statements This Presentation contains forward - looking statements. All statements other than statements of historical fact contained in this Presentation, including statements regarding the future financial position of Jasper Therapeutics, including financial target s, business strategy, and plans and objectives for future operations, are forward - looking statements. Jasper Therapeutics has based these forward - looking statements on its estimates and assumptions and its current expectations and projections about future events. These forward - looking statements are subject to a number of risks, uncertainties and assumptions. In light of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this Presentation are inherently uncert ain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forw ard - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of future events. Jasper Therapeutics undertakes no obligation to update publicly or revise an y forward - looking statements for any reason after the date of this Presentation or to conform these statements to actual results or to changes in Jasper Therapeutics’ expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither the Company nor its affiliates, adv ise rs or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affili ate s, advisers or representatives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the date of this Presentation. S uch data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use shou ld not be construed as an endorsement of the products or services of the Company.
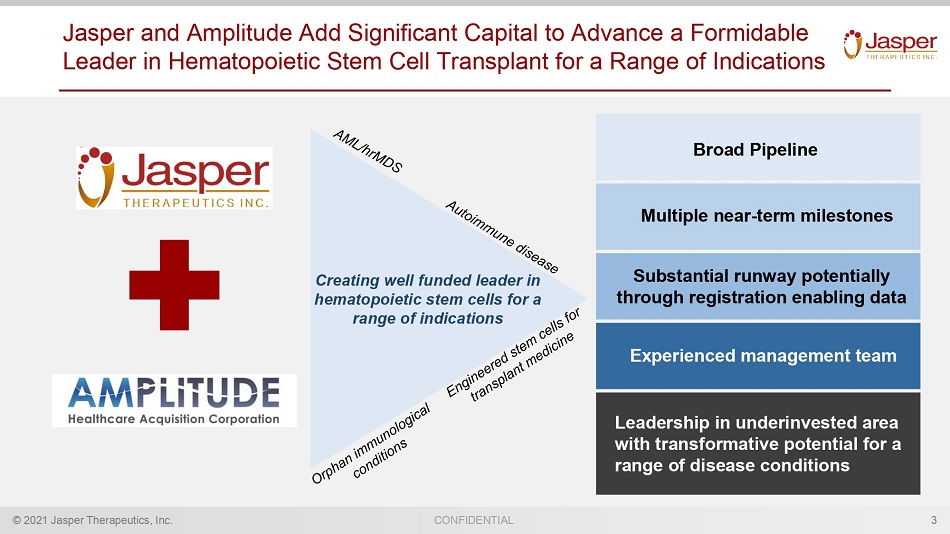
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Jasper and Amplitude Add Significant Capital to Advance a Formidable Leader in Hematopoietic Stem Cell Transplant for a Range of Indications 3 Creating well funded leader in hematopoietic stem cells for a range of indications Multiple near - term milestones Experienced management team Broad Pipeline Substantial runway potentially through registration enabling data Leadership in underinvested area with transformative potential for a range of disease conditions
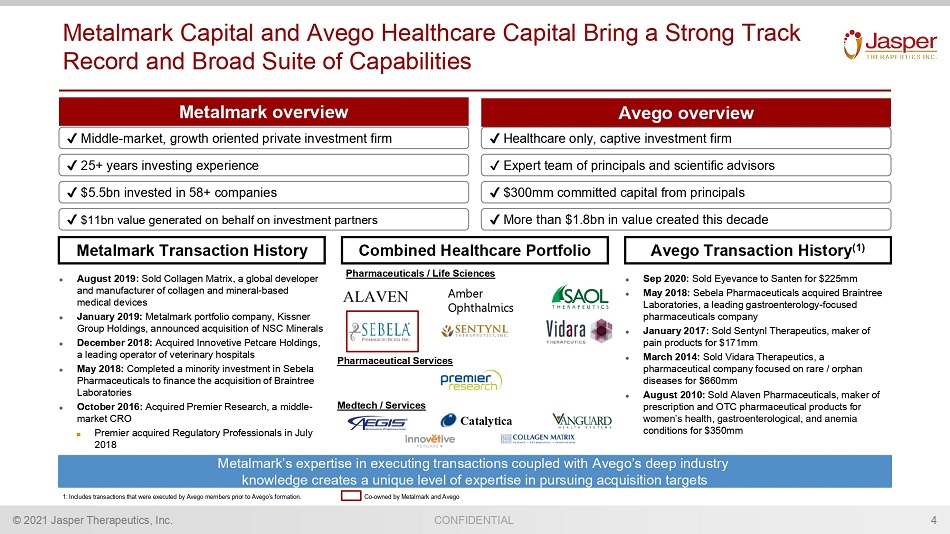
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Metalmark Capital and Avego Healthcare Capital Bring a Strong Track Record and Broad Suite of Capabilities 4 Metalmark’s expertise in executing transactions coupled with Avego’s deep industry knowledge creates a unique level of expertise in pursuing acquisition targets Metalmark overview Avego overview Combined Healthcare Portfolio Metalmark Transaction History Avego Transaction History (1) ● August 2019: Sold Collagen Matrix, a global developer and manufacturer of collagen and mineral - based medical devices ● January 2019: Metalmark portfolio company, Kissner Group Holdings, announced acquisition of NSC Minerals ● December 2018: Acquired Innovetive Petcare Holdings, a leading operator of veterinary hospitals ● May 2018: Completed a minority investment in Sebela Pharmaceuticals to finance the acquisition of Braintree Laboratories ● October 2016: Acquired Premier Research, a middle - market CRO ■ Premier acquired Regulatory Professionals in July 2018 ● Sep 2020: Sold Eyevance to Santen for $225mm ● May 2018: Sebela Pharmaceuticals acquired Braintree Laboratories, a leading gastroenterology - focused pharmaceuticals company ● January 2017: Sold Sentynl Therapeutics, maker of pain products for $171mm ● March 2014: Sold Vidara Therapeutics, a pharmaceutical company focused on rare / orphan diseases for $660mm ● August 2010: Sold Alaven Pharmaceuticals, maker of prescription and OTC pharmaceutical products for women’s health, gastroenterological, and anemia conditions for $350mm Pharmaceuticals / Life Sciences Medtech / Services Pharmaceutical Services Catalytica Amber Ophthalmics 1: Includes transactions that were executed by Avego members prior to Avego’s formation. Co - owned by Metalmark and Avego Middle - market, growth oriented private investment firm $5.5bn invested in 58+ companies $11bn value generated on behalf on investment partners Healthcare only, captive investment firm Expert team of principals and scientific advisors 25+ years investing experience $300mm committed capital from principals More than $1.8bn in value created this decade
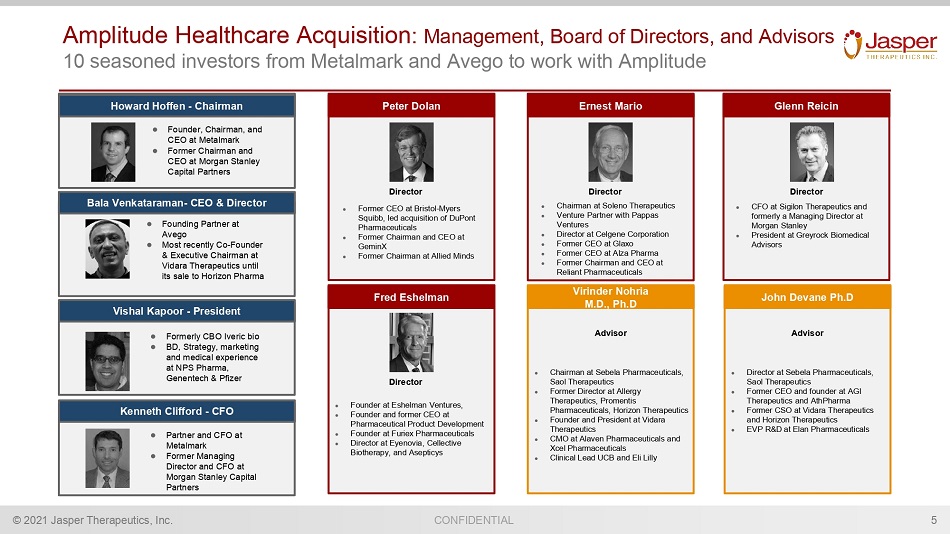
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Amplitude Healthcare Acquisition : Management, Board of Directors, and Advisors 10 seasoned investors from Metalmark and Avego to work with Amplitude 5 Bala Venkataraman - CEO & Director ● Founding Partner at Avego ● Most recently Co - Founder & Executive Chairman at Vidara Therapeutics until its sale to Horizon Pharma Ernest Mario ● Chairman at Soleno Therapeutics ● Venture Partner with Pappas Ventures ● Director at Celgene Corporation ● Former CEO at Glaxo ● Former CEO at Alza Pharma ● Former Chairman and CEO at Reliant Pharmaceuticals Director Peter Dolan ● Former CEO at Bristol - Myers Squibb, led acquisition of DuPont Pharmaceuticals ● Former Chairman and CEO at GeminX ● Former Chairman at Allied Minds Director Glenn Reicin ● CFO at Sigilon Therapeutics and formerly a Managing Director at Morgan Stanley ● President at Greyrock Biomedical Advisors Director Fred Eshelman ● Founder at Eshelman Ventures, ● Founder and former CEO at Pharmaceutical Product Development ● Founder at Furiex Pharmaceuticals ● Director at Eyenovia , Cellective Biotherapy, and Asepticys Director Howard Hoffen - Chairman ● Founder, Chairman, and CEO at Metalmark ● Former Chairman and CEO at Morgan Stanley Capital Partners 1 Kenneth Clifford - CFO ● Partner and CFO at Metalmark ● Former Managing Director and CFO at Morgan Stanley Capital Partners Virinder Nohria M.D., Ph.D ● Chairman at Sebela Pharmaceuticals, Saol Therapeutics ● Former Director at Allergy Therapeutics, Promentis Pharmaceuticals, Horizon Therapeutics ● Founder and President at Vidara Therapeutics ● CMO at Alaven Pharmaceuticals and Xcel Pharmaceuticals ● Clinical Lead UCB and Eli Lilly Advisor John Devane Ph.D ● Director at Sebela Pharmaceuticals, Saol Therapeutics ● Former CEO and founder at AGI Therapeutics and AthPharma ● Former CSO at Vidara Therapeutics and Horizon Therapeutics ● EVP R&D at Elan Pharmaceuticals Advisor ● Formerly CBO Iveric bio ● BD, Strategy, marketing and medical experience at NPS Pharma, Genentech & Pfizer Vishal Kapoor - President

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Jasper Expected Milestones • Q2 2021 – JSP191 AML/MDS Phase 1a top line 90 - day data • Q2 2021 – JSP191 AML/MDS open enrollment of Phase 1b expansion • Q4 2021 – JSP191 Autoimmune IND filing for Phase 1a pilot study • Q4 2021 – Engineered Stem Cell Platform in - vivo Proof - of - Concept • Q1 2022 – JSP191 Investigator Sponsored Studies preliminary data from Fanconi’s Anemia and Sickle Cell • 1H 2022 – JSP191 Gene Therapy first collaboration data • 1H 2022 – JSP191 AML/MDS expansion cohort top line data • 2H 2022 – JSP191 SCID Phase I/II complete study enrollment • Q4 2022 – JSP191 Autoimmune pilot study interim data • Q4 2022 – Engineered Stem Cell Platform first IND filing 6
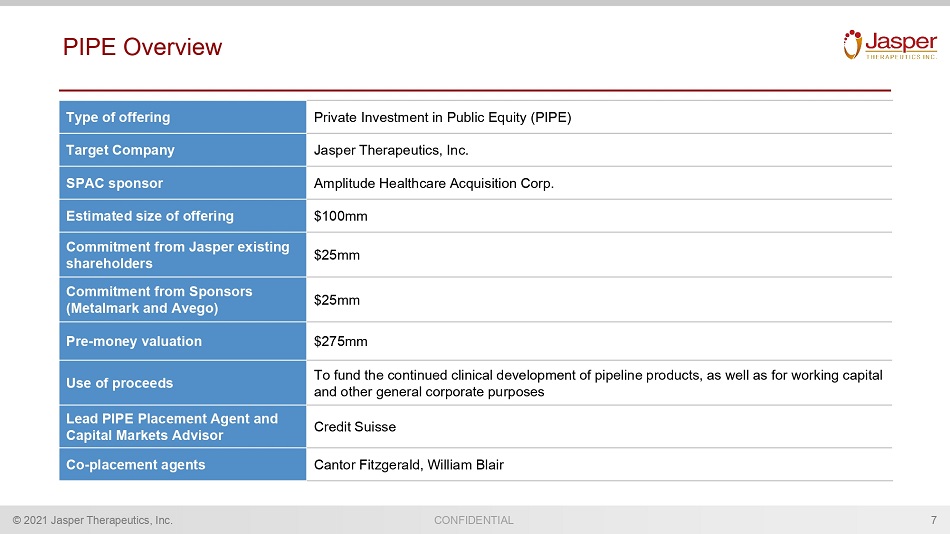
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. PIPE Overview 7 Type of offering Private Investment in Public Equity (PIPE) Target Company Jasper Therapeutics, Inc. SPAC sponsor Amplitude Healthcare Acquisition Corp. Estimated size of offering $100mm Commitment from Jasper existing shareholders $25mm Commitment from Sponsors (Metalmark and Avego ) $25mm Pre - money valuation $275mm Use of proceeds To fund the continued clinical development of pipeline products, as well as for working capital and other general corporate purposes Lead PIPE Placement Agent and Capital Markets Advisor Credit Suisse Co - placement agents Cantor Fitzgerald, William Blair
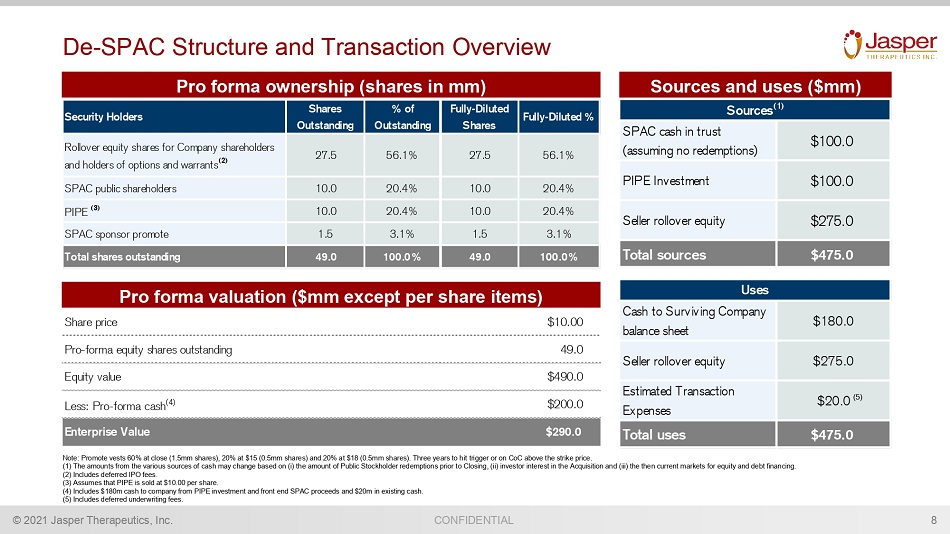
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. De - SPAC Structure and Transaction Overview 8 Sources and uses ($mm) Pro forma ownership (shares in mm) Note: Promote vests 60% at close (1.5mm shares), 20% at $15 (0.5mm shares) and 20% at $18 (0.5mm shares). Three years to hit tri gger or on CoC above the strike price. (1) The amounts from the various sources of cash may change based on (i) the amount of Public Stockholder redemptions prior t o C losing, (ii) investor interest in the Acquisition and (iii) the then current markets for equity and debt financing. (2) Includes deferred IPO fees. (3) Assumes that PIPE is sold at $10.00 per share. (4) Includes $180m cash to company from PIPE investment and front end SPAC proceeds and $20m in existing cash. (5) Includes deferred underwriting fees. Pro forma valuation ($mm except per share items) Security Holders Shares Outstanding % of Outstanding Fully-Diluted Shares Fully-Diluted % Rollover equity shares for Company shareholders and holders of options and warrants (2) 27.5 56.1% 27.5 56.1% SPAC public shareholders 10.0 20.4% 10.0 20.4% PIPE (3) 10.0 20.4% 10.0 20.4% SPAC sponsor promote 1.5 3.1% 1.5 3.1% Total shares outstanding 49.0 100.0% 49.0 100.0% Share price $10.00 Pro-forma equity shares outstanding 49.0 Equity value Less: Pro-forma cash (4) $200.0 Enterprise Value $290.0 $490.0 (5)
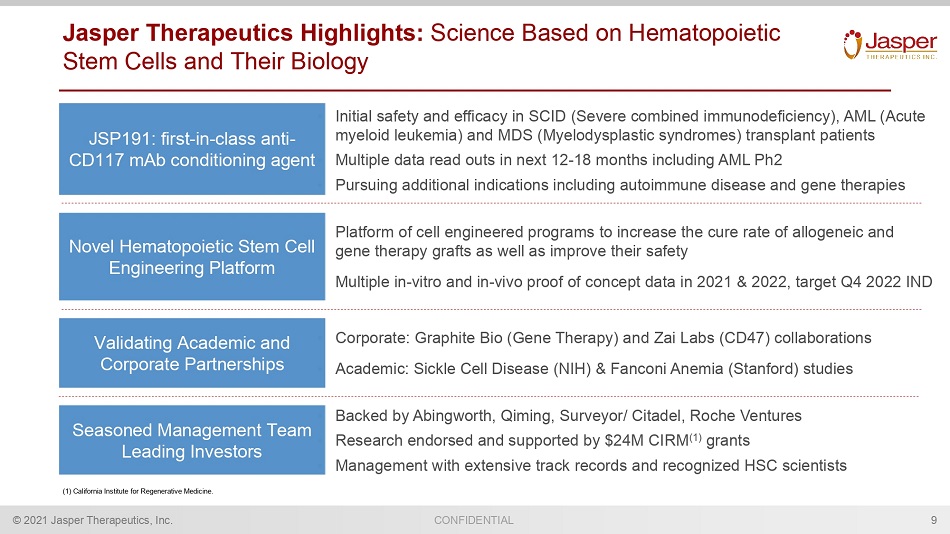
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Jasper Therapeutics Highlights: Science Based on Hematopoietic Stem Cells and Their Biology 9 • Platform of cell engineered programs to increase the cure rate of allogeneic and gene therapy grafts as well as improve their safety • Multiple in - vitro and in - vivo proof of concept data in 2021 & 2022, target Q4 2022 IND Novel Hematopoietic Stem Cell Engineering Platform Seasoned Management Team Leading Investors • Backed by Abingworth , Qiming , Surveyor/ Citadel, Roche Ventures • Research endorsed and supported by $24M CIRM (1) grants • Management with extensive track records and recognized HSC scientists JSP191: first - in - class anti - CD117 mAb conditioning agent • Initial safety and efficacy in SCID (Severe combined immunodeficiency), AML (Acute myeloid leukemia) and MDS (Myelodysplastic syndromes) transplant patients • Multiple data read outs in next 12 - 18 months including AML Ph2 • Pursuing additional indications including autoimmune disease and gene therapies Validating Academic and Corporate Partnerships • Corporate: Graphite Bio (Gene Therapy) and Zai Labs (CD47) collaborations • Academic: Sickle Cell Disease (NIH) & Fanconi Anemia (Stanford) studies (1) California Institute for Regenerative Medicine.

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Management Team and Scientific Advisory Board: Drug Development & Company Building Track Record and Experts in the Field 10 MANAGEMENT William Lis , Executive Chair & CEO Kevin N. Heller , Executive Vice President, Research and Development Jeet Mahal , Chief Financial Officer Carol Zoltowski , Senior Vice President Regulatory & Quality Craig Burns , Vice President Program Management Janet Hurt , Vice President Clinical Operations Luca DiNoto , Vice President Technical Operations Wendy Pang , Vice President, Research & Translational Medicine SCIENTIFIC ADVISORY BOARD Judith Shizuru (Chair), Co - founder, Professor of Medicine and Pediatrics Fredrick Appelbaum , Exec Vice President and Deputy Director Lori Kunkel , Independent Director Harry Malech , Chief Genetic Immunotherapy Section NIAID Jeff Ravetch , Professor Molecular Genetics and Immunology Arthur Weiss , Professor, Departments of Medicine, Microbiology and Immunology; Investigator Howard Hughes Medical Institute

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Unmet Medical Need: Hematopoietic Stem Cell Transplants (HSCT) Most Powerful Form of Disease Cure, Yet Remain Underutilized 11 Jasper Therapeutics could exponentially expand the eligible patient population for both allogeneic and autologous gene edited hematopoietic stem cell therapy Only a minority of patients receive a transplant Those who do not receive a transplant are left with life threatening disease Limitations of Conditioning (prepare patient’s bone marrow) • Old SOC agents are genotoxic • Major Toxicities and AEs: – Treatment related Cancer – Veno - occlusive Disease – Bacteremia – Pulmonary Fibrosis – Infertility • Mortality Risk • Hospitalization in isolation Limitations of Transplant Grafts • Clinical Relapse • Failed or Poor Engraftment • Graft vs. Host Disease (GvHD) • Long - term Immunosuppression
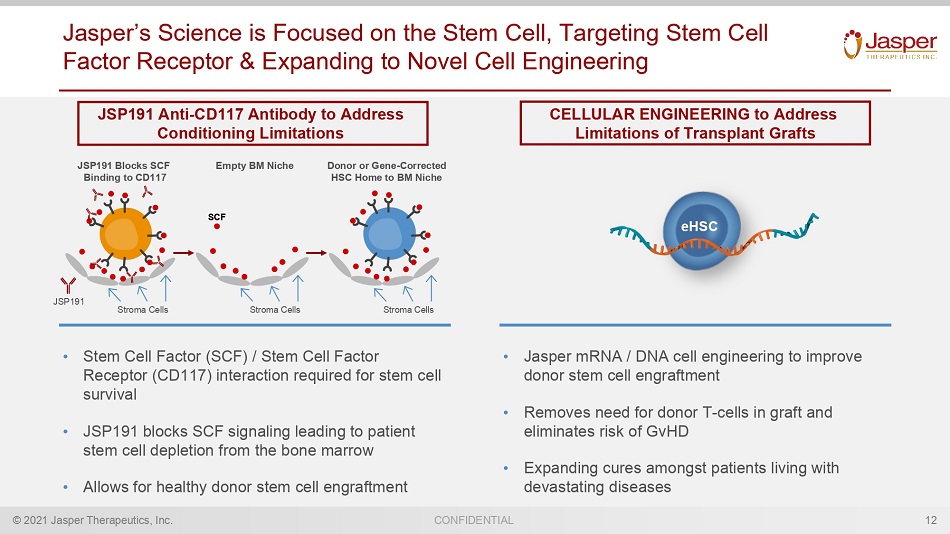
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Jasper’s Science is Focused on the Stem Cell, Targeting Stem Cell Factor Receptor & Expanding to Novel Cell Engineering 12 • Stem Cell Factor (SCF) / Stem Cell Factor Receptor (CD117) interaction required for stem cell survival • JSP191 blocks SCF signaling leading to patient stem c ell depletion from the bone marrow • Allows for healthy donor stem cell engraftment • Jasper mRNA / DNA cell engineering to improve donor stem cell engraftment • Removes need for donor T - cells in graft and eliminates risk of GvHD • Expanding cures amongst patients living with devastating diseases JSP191 Stroma Cells Stroma Cells Stroma Cells JSP191 Blocks SCF Binding to CD117 Empty BM Niche Donor or Gene - Corrected HSC Home to BM Niche JSP191 Anti - CD117 Antibody to Address Conditioning Limitations CELLULAR ENGINEERING to Address Limitations of Transplant Grafts eHSC SCF
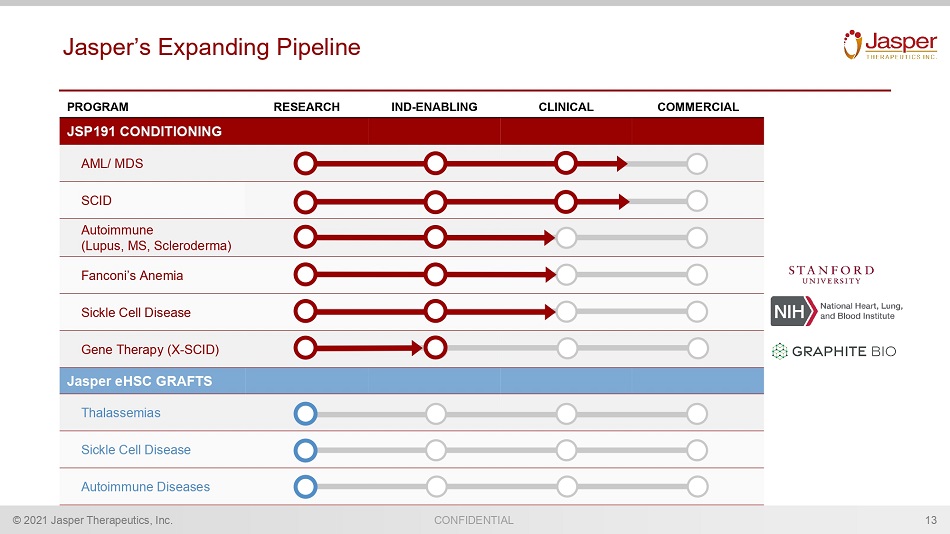
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Jasper’s Expanding Pipeline 13 PROGRAM RESEARCH IND - ENABLING CLINICAL COMMERCIAL JSP191 CONDITIONING AML/ MDS SCID Autoimmune (Lupus, MS, Scleroderma) Fanconi’s Anemia Sickle Cell Disease Gene Therapy (X - SCID) Jasper eHSC GRAFTS Thalassemias Sickle Cell Disease Autoimmune Diseases

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Unique and Differentiated JSP191 Properties Compared to All Other CD117 Antibodies Only anti - CD117 antibody that inhibits stem cell factor survival signal resulting in targeted depletion • Only JSP191 shows in - vivo single agent depletion • JSP191 SCF signal inhibition sensitizes stem cells for synergistic combination therapy (radiation, 5 - azacytidine, CD47) Only JSP191 is aglycosylated and designed to remove all effector cell function and mast cell activation • No mast cell related anaphylaxis • No reported treatment related SAEs No toxic payload that may lead to off - target effects based on normal CD117 expression • CD117 also expressed on mast cells, germ cells, Cajal (GI) cells, melanocytes 14 JSP191 Blocks SCF Binding to CD117 Receptor Inhibition of Stem Cell Survival Signal Targeted Stem Cell Depletion Toxic Payload Binds to all CD117 Expressing Cells No CD117 Signal Inhibition Toxin Internalized on Mast Cells, Germ Cells, Melanocytes, etc. SCF CD117
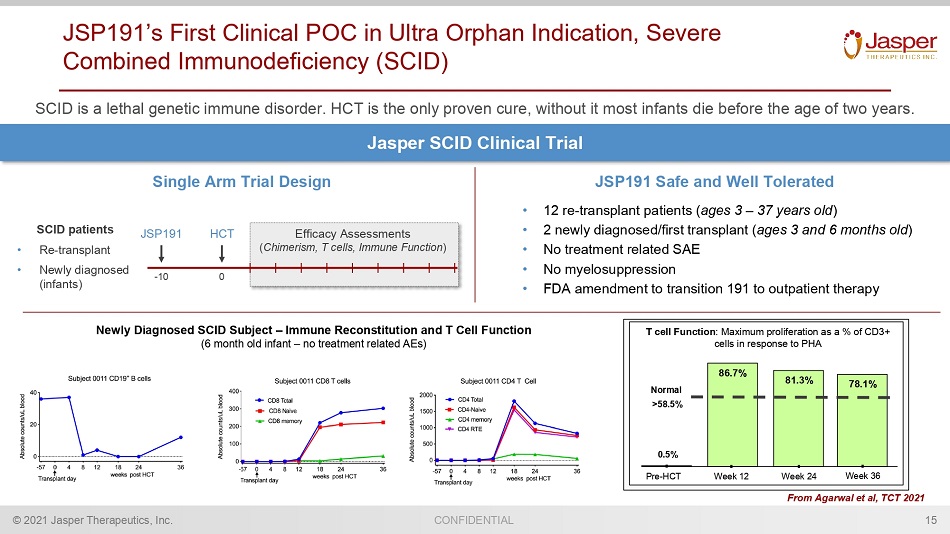
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191’s First Clinical POC in Ultra Orphan Indication, Severe Combined Immunodeficiency (SCID) SCID is a lethal genetic immune disorder. HCT is the only proven cure, without it most infants die before the age of two year s. 15 Jasper SCID Clinical Trial Single Arm Trial Design JSP191 HCT 0 - 10 Efficacy Assessments ( Chimerism, T cells, Immune Function ) SCID patients • Re - transplant • Newly diagnosed (infants) JSP191 Safe and Well Tolerated • 12 re - transplant patients ( ages 3 – 37 years old ) • No treatment related SAE • No myelosuppression • FDA amendment to transition 191 to outpatient therapy From Agarwal et al, TCT 2021 Pre - HCT 86.7% 0.5% Week 12 81.3% Week 24 Normal >58.5% T cell Function : Maximum proliferation as a % of CD3+ cells in response to PHA 78.1% Week 36 Newly Diagnosed SCID Subject – Immune Reconstitution and T Cell Function (6 month old infant – no treatment related AEs)

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 Conditioning in SCID HCT Demonstrates Durable Naive T - cell Production and Immune System Reconstitution 16 Naïve CD4 T cell production post - cell infusion A. No Conditioning (Matched Cohort Patient) B. JSP191 Conditioning *85 /µL *Expected Level for Clinical Benefit Weeks Post Boost 0 4 8 12 18 24 29 36 52 78 104 115

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 is Advancing in AML and MDS, a Large Opportunity for Curative HCT with Non - Toxic Conditioning 17 AML and MDS are clonal myeloid malignancies and diseases of elderly with high mortality HCT is a SOC curative procedure: ~11,000 G7 HCTs in 2019, but up 30,000 could be eligible Jasper JSP191 strategy: • Synergistic combination with low dose radiation • Start with elderly patients not eligible for HCT for expedited development path • Expand HCT use broadly in AML/MDS AML/MDS Disease Overview “MDS is curable by transplant, but only few go through the transplant” - Leading Academic KOL
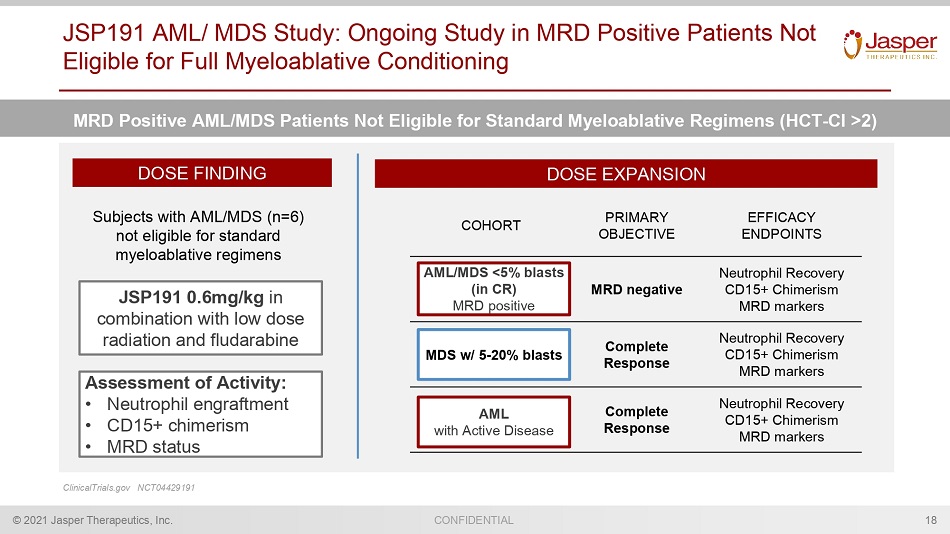
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 AML/ MDS Study: Ongoing Study in MRD Positive Patients Not Eligible for Full Myeloablative Conditioning 18 MRD Positive AML/MDS Patients Not Eligible for Standard Myeloablative Regimens (HCT - Cl >2) ClinicalTrials.gov NCT04429191 DOSE FINDING DOSE EXPANSION Subjects with AML/MDS (n=6) n ot eligible for standard myeloablative regimens JSP191 0.6mg/kg in combination with low dose radiation and fludarabine COHORT PRIMARY OBJECTIVE EFFICACY ENDPOINTS MRD negative Neutrophil Recovery CD15+ Chimerism MRD markers Complete Response Neutrophil Recovery CD15+ Chimerism MRD markers Complete Response Neutrophil Recovery CD15+ Chimerism MRD markers MDS w/ 5 - 20% blasts AML /MDS <5% blasts ( in CR ) MRD positive AML with Active Disease Assessment of Activity: • Neut rophil engraftment • CD15+ chimerism • MRD status
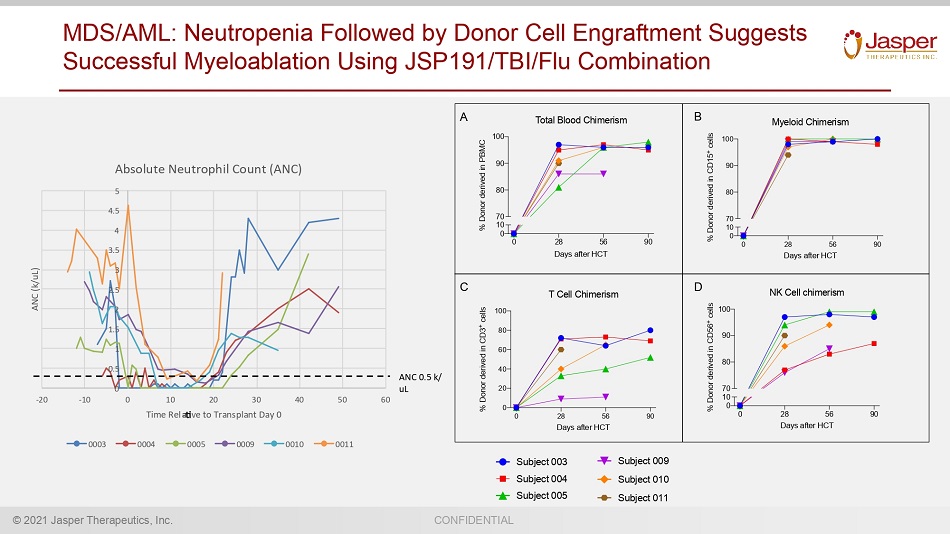
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL MDS/AML: Neutropenia Followed by Donor Cell Engraftment Suggests Successful Myeloablation Using JSP191/TBI/Flu Combination 0 28 56 90 0 10 70 80 90 100 Days after HCT % D o n o r d e r i v e d i n P B M C Total Blood Chimerism 0 28 56 90 0 20 40 60 80 100 Days after HCT % D o n o r d e r i v e d i n C D 3 + c e l l s T Cell Chimerism 0 28 56 90 0 10 70 80 90 100 Days after HCT % D o n o r d e r i v e d i n C D 1 5 + c e l l s Myeloid Chimerism 0 28 56 90 0 10 70 80 90 100 Days after HCT % D o n o r d e r i v e d i n C D 5 6 + c e l l s NK Cell chimerism A B C D Subject 003 Subject 004 Subject 005 Subject 009 Subject 010 Subject 011 0 0 . 5 1 1 . 5 2 2 . 5 3 3 . 5 4 4 . 5 5 - 2 0 - 1 0 0 1 0 2 0 3 0 4 0 5 0 6 0 A N C ( k / u L ) T i m e R e l a v e t o T r a n s p l a n t D a y 0 A b s o l u t e N e u t r o p h i l C o u n t ( A N C ) 0 0 0 3 0 0 0 4 0 0 0 5 0 0 0 9 0 0 1 0 0 0 1 1 A N C 0 . 5 k / u L
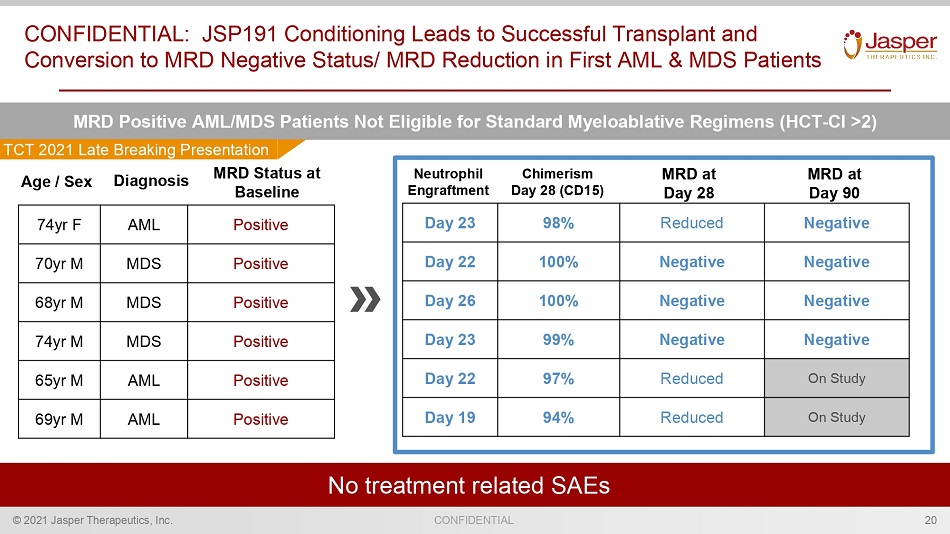
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. CONFIDENTIAL: JSP191 Conditioning Leads to Successful Transplant and Conversion to MRD Negative Status/ MRD Reduction in First AML & MDS Patients 20 N o treatment related SAEs MRD Positive AML/MDS Patients Not Eligible for Standard Myeloablative Regimens (HCT - Cl >2) MRD MRD at Day 28 Chimerism Day 28 (CD15) Neutrophil Engraftment 74yr F AML Positive 70yr M MDS Positive 68yr M MDS Positive 74yr M MDS Positive 65yr M AML Positive 69yr M AML Positive Day 23 98% Reduced Negative Day 22 100% Negative Negative Day 26 100% Negative Negative Day 23 99% Negative Negative Day 22 97% Reduced On Study Day 19 94% Reduced On Study Age / Sex Diagnosis MRD at Day TCT 2021 Late Breaking Presentation
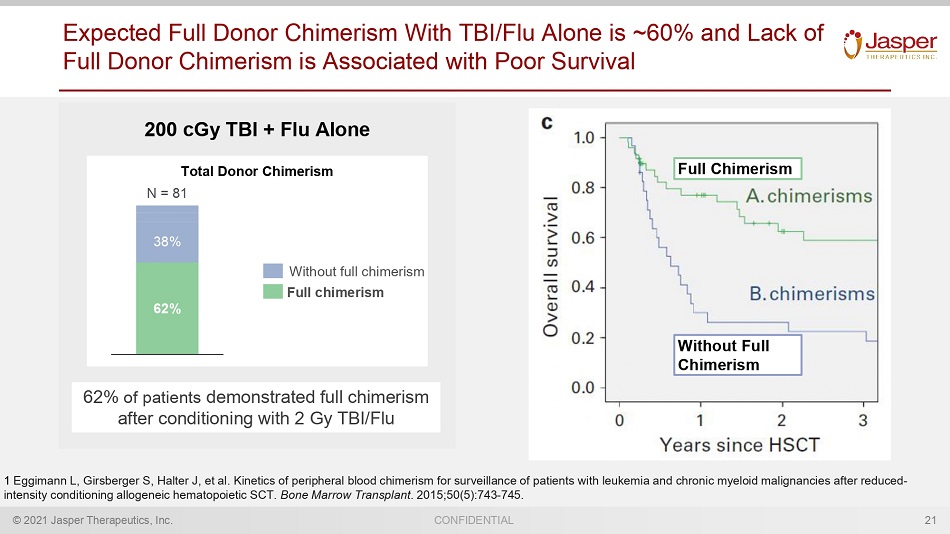
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Expected Full Donor Chimerism With TBI/Flu Alone is ~60% and Lack of Full Donor Chimerism is Associated with Poor Survival 21 200 cGy TBI + Flu Alone 62% of patients demonstrated full chimerism after conditioning with 2 Gy TBI/Flu 1 Eggimann L, Girsberger S, Halter J, et al. Kinetics of peripheral blood chimerism for surveillance of patients with leukemia and chronic myeloid mal ig nancies after reduced - intensity conditioning allogeneic hematopoietic SCT. Bone Marrow Transplant . 2015;50(5):743 - 745. 62% 38% N = 81 Without full chimerism Full chimerism Total Donor Chimerism Full Chimerism Without Full Chimerism
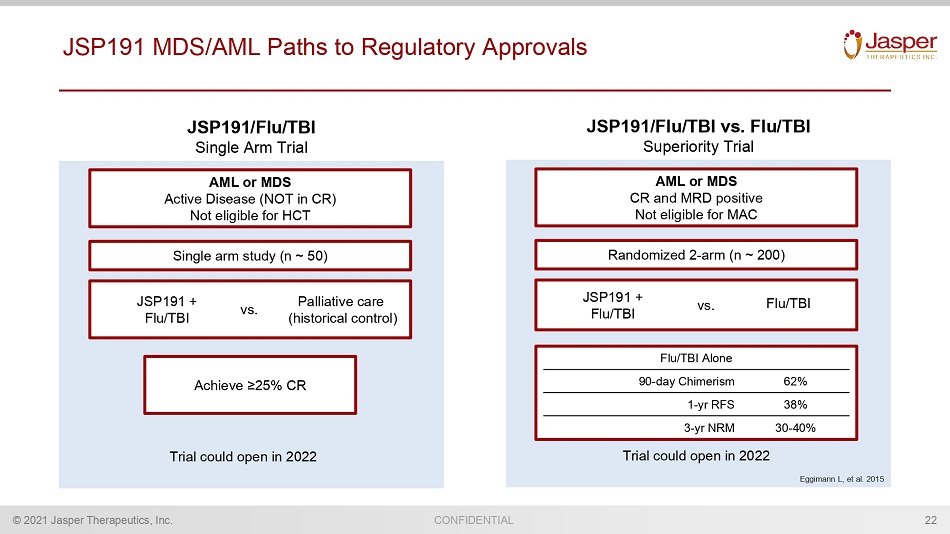
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 MDS/AML Paths to Regulatory Approvals 22 AML or MDS CR and M RD positive Not eligible for MAC Randomized 2 - arm (n ~ 200) JSP191 + Flu/TBI Flu/TBI vs. Trial could open in 2022 JSP191/Flu/TBI vs. Flu/TBI Superiority Trial Eggimann L, et al. 2015 AML or MDS Active Disease (NOT in CR) Not eligible for HCT Achieve ≥ 25% CR Single arm study (n ~ 50) Trial could open in 2022 JSP191/Flu/TBI Single Arm Trial JSP191 + Flu/TBI Palliative care (historical control) vs. Flu/TBI Alone 90 - day Chimerism 62% 1 - yr RFS 38% 3 - yr NRM 30 - 40%
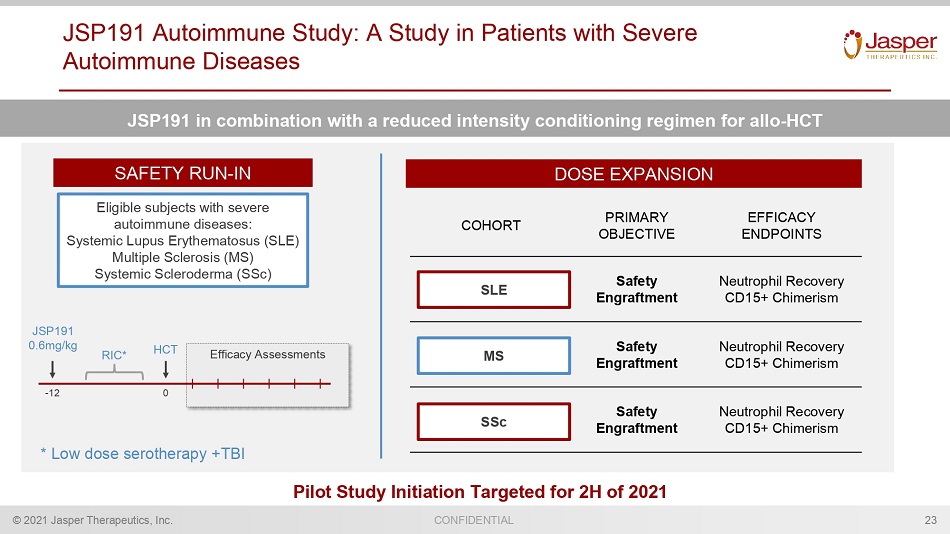
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 Autoimmune Study: A Study in Patients with Severe Autoimmune Diseases 23 JSP191 in combination with a reduced intensity conditioning regimen for allo - HCT SAFETY RUN - IN DOSE EXPANSION COHORT PRIMARY OBJECTIVE EFFICACY ENDPOINTS Safety Engraftment Neutrophil Recovery CD15+ Chimerism Safety Engraftment Neutrophil Recovery CD15+ Chimerism Safety Engraftment Neutrophil Recovery CD15+ Chimerism MS SLE SSc Eligible subjects with severe autoimmune diseases: Systemic Lupus Erythematosus (SLE) Multiple Sclerosis (MS) Systemic Scleroderma ( SSc ) JSP191 0.6mg/kg HCT 0 - 12 Efficacy Assessments * L ow dose serotherapy +TBI RIC* Pilot Study Initiation Targeted for 2H of 2021
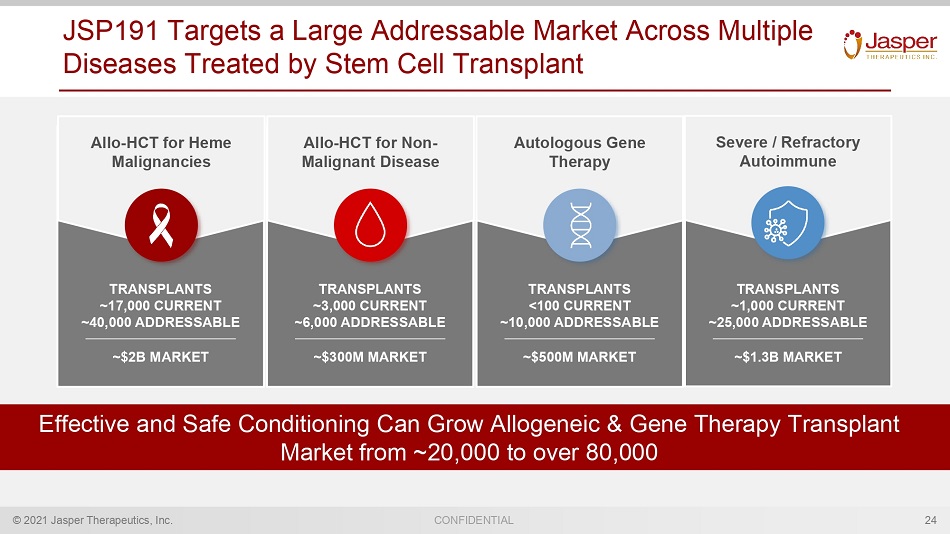
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Allo - HCT for Heme Malignancies Allo - HCT for Non - Malignant Disease Autologous Gene Therapy JSP191 Targets a Large Addressable Market Across Multiple Diseases Treated by Stem Cell Transplant 24 Severe / Refractory Autoimmune ~$2B MARKET TRANSPLANTS ~17,000 CURRENT ~40,000 ADDRESSABLE Effective and Safe Conditioning Can Grow Allogeneic & Gene Therapy Transplant Market from ~20,000 to over 80,000 ~$300M MARKET TRANSPLANTS ~3,000 CURRENT ~6,000 ADDRESSABLE ~$500M MARKET TRANSPLANTS <100 CURRENT ~10,000 ADDRESSABLE ~$1.3B MARKET TRANSPLANTS ~1,000 CURRENT ~25,000 ADDRESSABLE
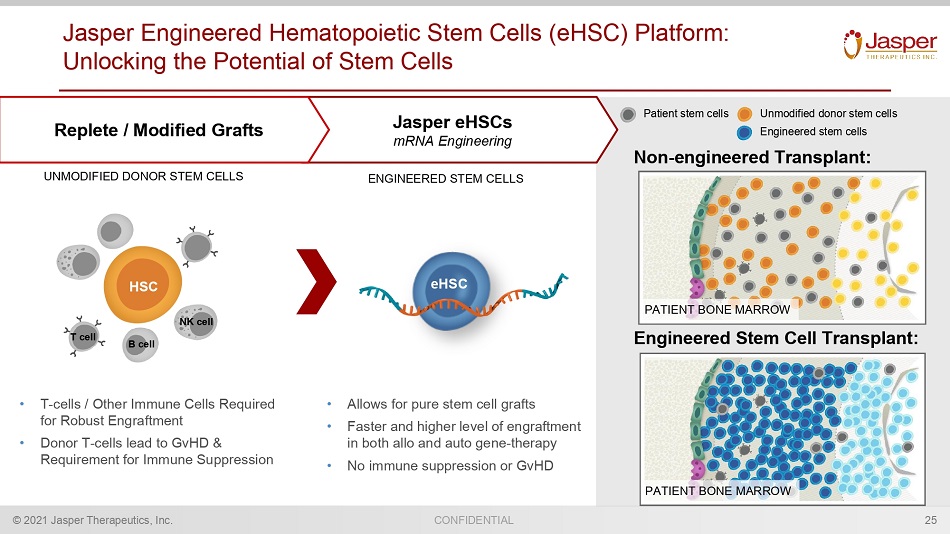
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Jasper Engineered Hematopoietic Stem Cells ( eHSC ) Platform: Unlocking the Potential of Stem Cells Jasper eHSCs mRNA Engineering Replete / Modified Grafts Patient stem cells Unmodified donor stem cells Engineered stem cells UNMODIFIED DONOR STEM CELLS ENGINEERED STEM CELLS • T - cells / Other Immune Cells Required for Robust Engraftment • Donor T - cells lead to GvHD & Requirement for Immune Suppression • Allows for pure stem cell grafts • Faster and higher level of engraftment in both allo and auto gene - therapy • No immune suppression or GvHD eHSC HSC Non - engineered Transplant: Engineered Stem Cell Transplant: PATIENT BONE MARROW PATIENT BONE MARROW 25
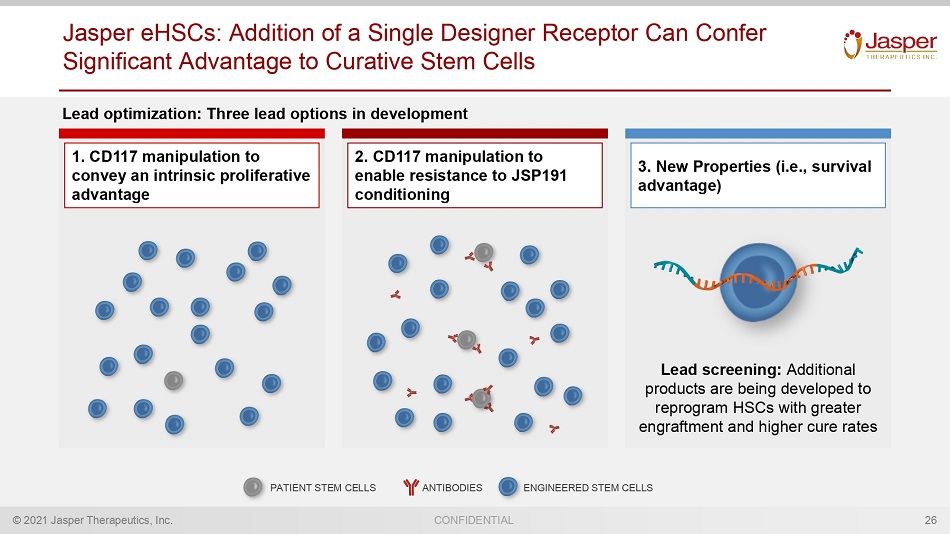
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Jasper eHSCs : Addition of a Single Designer Receptor Can Confer Significant Advantage to Curative Stem Cells 26 1. CD117 manipulation to convey an intrinsic proliferative advantage 2. CD117 manipulation to enable resistance to JSP191 conditioning 3. New Properties (i.e., survival advantage) ANTIBODIES PATIENT STEM CELLS ENGINEERED STEM CELLS Lead screening: Additional products are being developed to reprogram HSCs with greater engraftment and higher cure rates Lead optimization: Three lead options in development
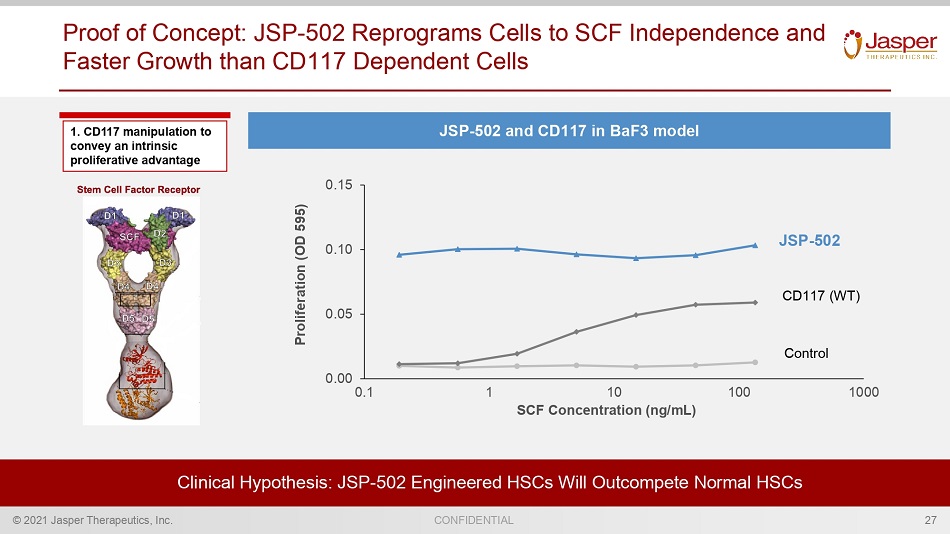
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL 1. CD117 manipulation to convey an intrinsic proliferative advantage Proof of Concept: JSP - 502 Reprograms Cells to SCF Independence and Faster Growth than CD117 Dependent Cells Clinical Hypothesis: JSP - 502 Engineered HSCs Will Outcompete Normal HSCs 0.00 0.05 0.10 0.15 0.1 1 10 100 1000 SCF Concentration (ng/mL) JSP - 502 CD117 (WT) Control 27 JSP - 502 and CD117 in BaF3 model Proliferation (OD 595)
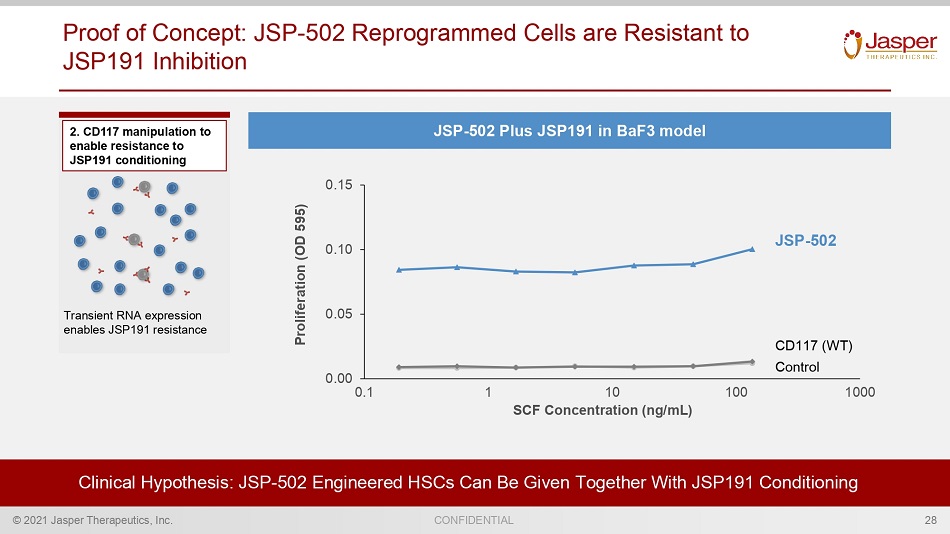
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL 2. CD117 manipulation to enable resistance to JSP191 conditioning Transient RNA expression enables JSP191 resistance Proof of Concept: JSP - 502 Reprogrammed Cells are Resistant to JSP191 Inhibition Clinical Hypothesis: JSP - 502 Engineered HSCs Can Be Given Together With JSP191 Conditioning 0.00 0.05 0.10 0.15 100 1 0.1 10 1000 SCF Concentration (ng/mL) Proliferation (OD 595) JSP - 502 CD117 (WT) Control 28 JSP - 502 Plus JSP191 in BaF3 model
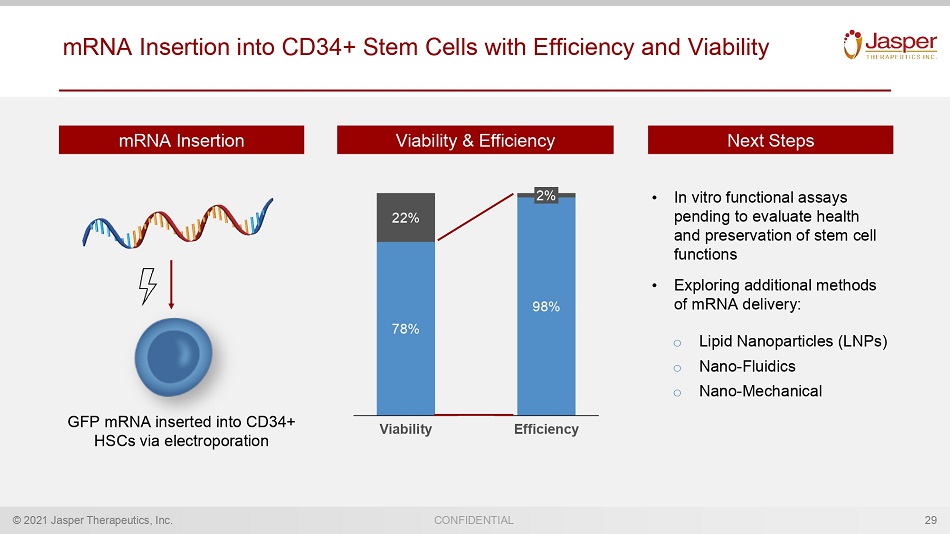
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Viability & Efficiency mRNA Insertion into CD34+ Stem Cells with Efficiency and Viability 29 78% 22% Viability 98% 2% Efficiency Next Steps mRNA Insertion GFP mRNA inserted into HSCs via electroporation • In vitro functional assays pending to evaluate health and preservation of stem cell functions • Exploring additional methods of mRNA delivery: o Lipid Nanoparticles (LNPs) o N

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Allo - HCT for non - malignant heme Auto gene therapy for beta - thal + SCD Severe / refractory autoimmune eHSCs Aim to be Broadly Applicable Across Patient Populations with Unmet Need and Large Addressable Markets 30 x Improved safety x Improved engraftment x Greater cures x Synergy with gene therapy x Improved safety & efficacy x Greater cures x Transformative safety & efficacy vs. DMARDs x Enable cures for Millions Solid organ transplant tolerance x Improved safety & engraftment x Eliminate lifelong immuno - suppression ~$1.5B MARKET TRANSPLANTS ~3,000 CURRENT ~6,000 ADDRESSABLE ~$2.5B MARKET TRANSPLANTS <100 CURRENT ~10,000 ADDRESSABLE ~$6B MARKET TRANSPLANTS ~1,000 CURRENT ~25,000 ADDRESSABLE ~$2.5B MARKET ~10,000 CURRENT Living Donor Kidney Transplants ~10,000 ADDRESSABLE

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Jasper Expected Milestones • Q2 2021 – JSP191 AML/MDS Phase 1a top line 90 - day data • Q2 2021 – JSP191 AML/MDS open enrollment of Phase 1b expansion • Q4 2021 – JSP191 Autoimmune IND filing for Phase 1a pilot study • Q4 2021 – Engineered Stem Cell Platform in - vivo Proof - of - Concept • Q1 2022 – JSP191 Investigator Sponsored Studies preliminary data from Fanconi’s Anemia and Sickle Cell • 1H 2022 – JSP191 Gene Therapy first collaboration data • 1H 2022 – JSP191 AML/MDS expansion cohort top line data • 2H 2022 – JSP191 SCID Phase I/II complete study enrollment • Q4 2022 – JSP191 Autoimmune pilot study interim data • Q4 2022 – Engineered Stem Cell Platform first IND filing 31

CONFIDENTIAL Appendix April 2021

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 HCT Conditioning Poised to Transform HCT to Cure Severe Autoimmune Diseases 33 JSP191 + Modified Graft LUPUS 15,000 + SCLERODERMA 50,000+ MS 50,000+ Disease Modification vs. DMARDs Pilot Study Initiation Targeted for 2H of 2021 JSP191 therapy has potential to achieve the efficacy of auto - HCT without the toxicity that prohibits its use. Stem Cell Transplant (Autologous) in Multiple Sclerosis Severe Autoimmune Diseases Population (G7)

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Hematopoietic Stem Cell Transplantation is a Multi - Step Procedure Resulting in Curative Cellular Replacement 34 Patient Conditioning Before Transplant Healthy Donor or Gene Therapy Stem Cells Transplanted into Patient • Conditioning with radiation and chemotherapy is required to prepare the bone marrow to accept new donor cells • Cures for: o AML/ MDS o SCID o Severe Autoimmune Diseases o Sickle Cell Disease o Many other diseases Stem Cells Harvested from PATIENT or DONOR Allogeneic Autologous Gene Therapy Gene - modified self transplant Donor Transplant
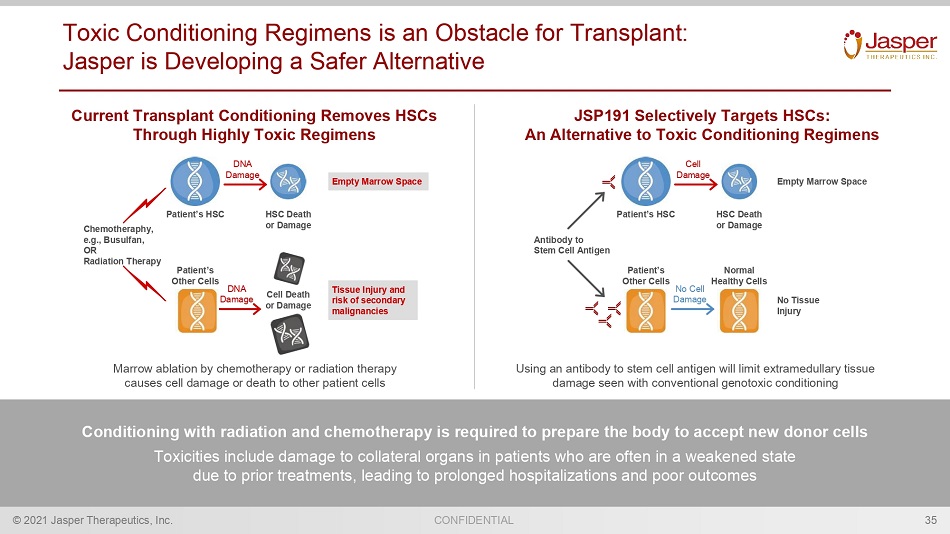
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Toxic Conditioning Regimens is an Obstacle for Transplant: Jasper is Developing a Safer Alternative 35 Conditioning with radiation and chemotherapy is required to prepare the body to accept new donor cells Toxicities include damage to collateral organs in patients who are often in a weakened state due to prior treatments, leading to prolonged hospitalizations and poor outcomes JSP191 Selectively Targets HSCs: An Alternative to Toxic Conditioning Regimens Marrow ablation by chemotherapy or radiation therapy causes cell damage or death to other patient cells Using an antibody to stem cell antigen will limit extramedullary tissue damage seen with conventional genotoxic conditioning Current Transplant Conditioning Removes HSCs Through Highly Toxic Regimens Empty Marrow Space No Tissue Injury Patient’s HSC HSC Death or Damage Patient’s Other Cells Normal Healthy Cells Cell Damage No Cell Damage Antibody to Stem Cell Antigen Tissue Injury and risk of secondary malignancies Patient’s HSC HSC Death or Damage Patient’s Other Cells Cell Death or Damage DNA Damage DNA Damage Chemotheraphy , e.g., Busulfan , OR Radiation Therapy Empty Marrow Space
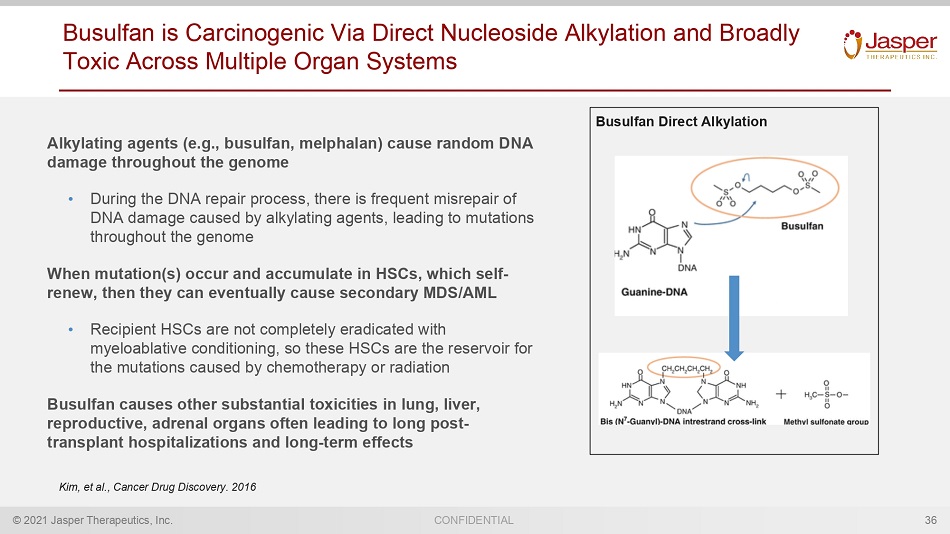
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Busulfan is Carcinogenic Via Direct Nucleoside Alkylation and Broadly Toxic Across Multiple Organ Systems Alkylating agents (e.g., busulfan, melphalan) cause random DNA damage throughout the genome • During the DNA repair process, there is frequent misrepair of DNA damage caused by alkylating agents, leading to mutations throughout the genome When mutation(s) occur and accumulate in HSCs, which self - renew, then they can eventually cause secondary MDS/AML • Recipient HSCs are not completely eradicated with myeloablative conditioning, so these HSCs are the reservoir for the mutations caused by chemotherapy or radiation Busulfan causes other substantial toxicities in lung, liver, reproductive, adrenal organs often leading to long post - transplant hospitalizations and long - term effects 36 Kim, et al., Cancer Drug Discovery. 2016 Busulfan Direct Alkylation
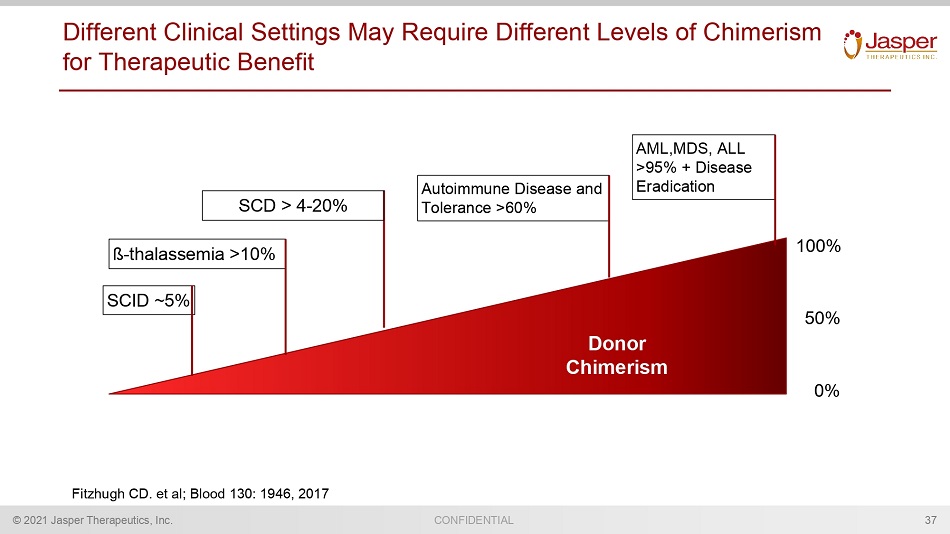
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Different Clinical Settings May Require Different Levels of Chimerism for Therapeutic Benefit 37 Donor Chimerism SCID ~5% ß - thalassemia >10% Autoimmune Disease and Tolerance >60% AML,MDS, ALL >95% + Disease Eradication 100% 50% 0% SCD > 4 - 20% Fitzhugh CD. et al; Blood 130: 1946, 2017
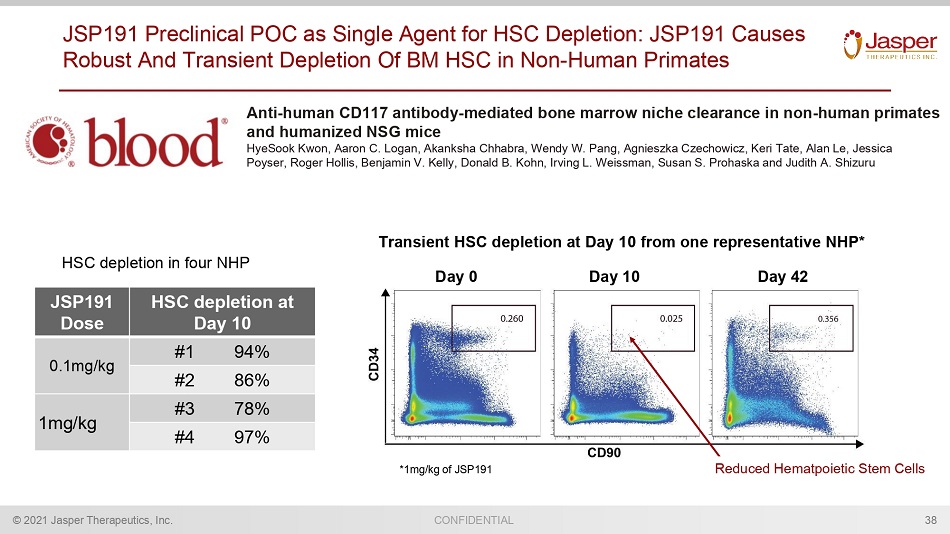
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 Preclinical POC as Single Agent for HSC Depletion: JSP191 Causes Robust And Transient Depletion Of BM HSC in Non - Human Primates 38 Anti - human CD117 antibody - mediated bone marrow niche clearance in non - human primates and humanized NSG mice HyeSook Kwon, Aaron C. Logan, Akanksha Chhabra, Wendy W. Pang, Agnieszka Czechowicz , Keri Tate, Alan Le, Jessica Poyser , Roger Hollis, Benjamin V. Kelly, Donald B. Kohn, Irving L. Weissman, Susan S. Prohaska and Judith A. Shizuru Transient HSC depletion at Day 10 from one representative NHP* Day 10 Day 42 Day 0 JSP191 Dose HSC depletion at Day 10 0.1mg/kg #1 94% #2 86% 1mg/kg #3 78% #4 97% HSC depletion in four NHP *1mg/kg of JSP191 Reduced Hematpoietic Stem Cells
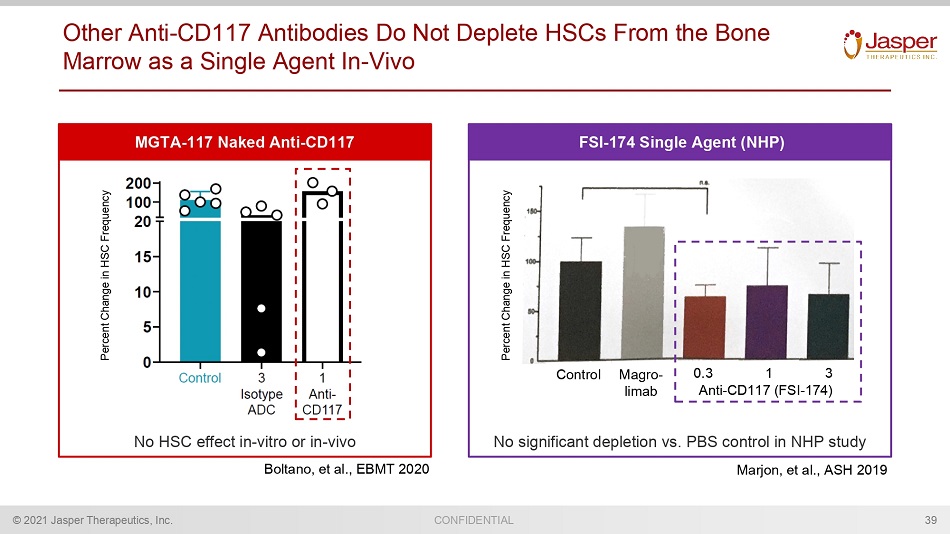
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Other Anti - CD117 Antibodies Do Not Deplete HSCs From the Bone Marrow as a Single Agent In - Vivo 39 Control Magro - limab Anti - CD117 (FSI - 174) 0.3 1 3 Percent Change in HSC Frequency Percent Change in HSC Frequency MGTA - 117 Naked Anti - CD117 FSI - 174 Single Agent (NHP) Boltano , et al., EBMT 2020 Marjon , et al., ASH 2019 No HSC effect in - vitro or in - vivo No significant depletion vs. PBS control in NHP study
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 Validated in Multiple Disease Models Where HSC is Standard Curative Therapy 40 Demonstrated robust pre - clinical data in multiple models of transplant and disease • Disease: SCID, AML, MDS, Sickle Cell • Transplant: Mouse, Non - human primate Consistent JSP191 stem cell depletion followed by successful transplant and disease modification • JSP191 alone and various combinations Benign safety profile supporting use in infants, elderly and other fragile populations Severe combined immunodeficiency (SCID) Hematologic Cancers (AML, MDS) Sickle Cell Disease
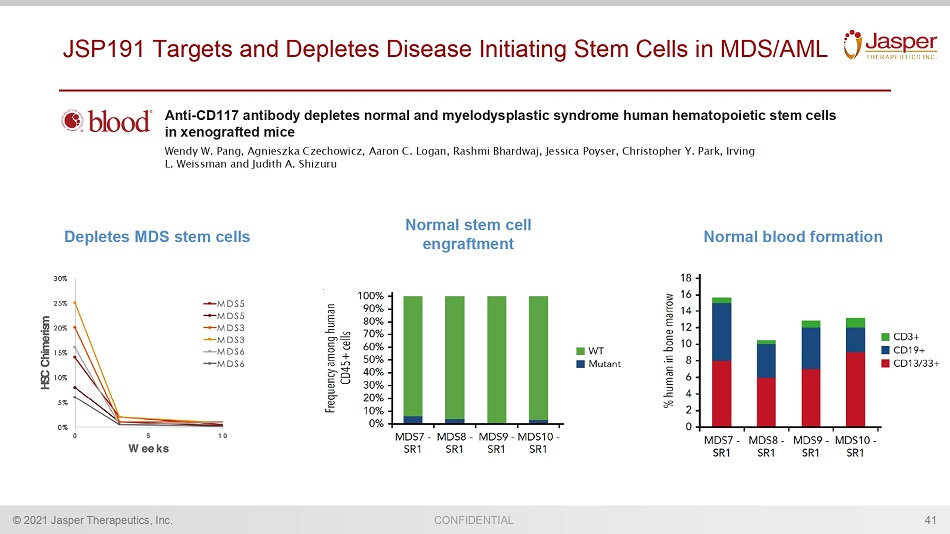
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. 41 JSP191 Targets and Depletes Disease Initiating Stem Cells in MDS/AML Anti - CD117 antibody depletes normal and myelodysplastic syndrome human hematopoietic stem cells in xenografted mice Wendy W. Pang, Agnieszka Czechowicz , Aaron C. Logan, Rashmi Bhardwaj, Jessica Poyser , Christopher Y. Park, Irving L. Weissman and Judith A. Shizuru Normal blood formation Depletes MDS stem cells Normal stem cell engraftment
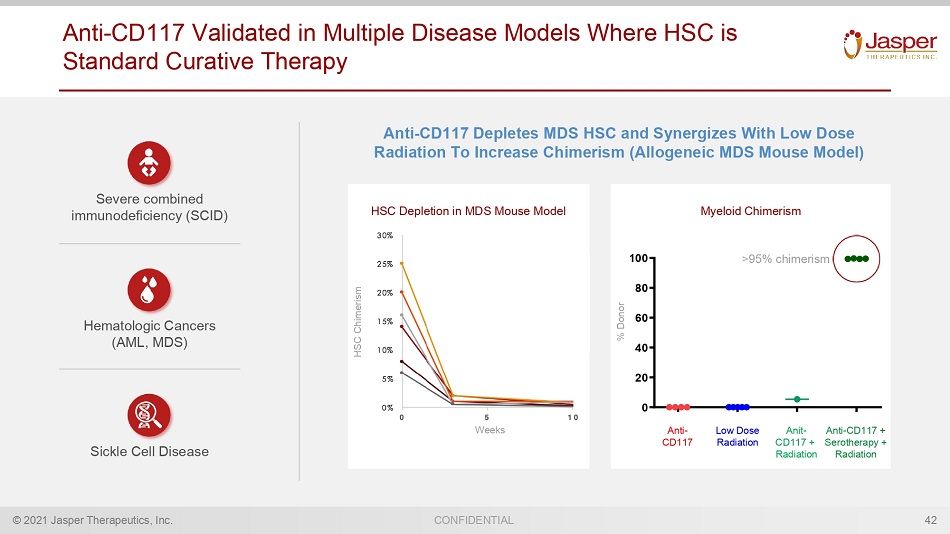
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Anti - CD117 Validated in Multiple Disease Models Where HSC is Standard Curative Therapy 42 Severe combined immunodeficiency (SCID) Hematologic Cancers (AML, MDS) Sickle Cell Disease Anti - CD117 Depletes MDS HSC and Synergizes With Low Dose Radiation To Increase Chimerism (Allogeneic MDS Mouse Model) 0 20 40 60 80 100 % D o n o r Anti - CD117 Low Dose Radiation Anit - CD117 + Radiation Anti - CD117 + Serotherapy + Radiation >95% chimerism HSC Depletion in MDS Mouse Model HSC Chimerism Weeks % Donor Myeloid Chimerism
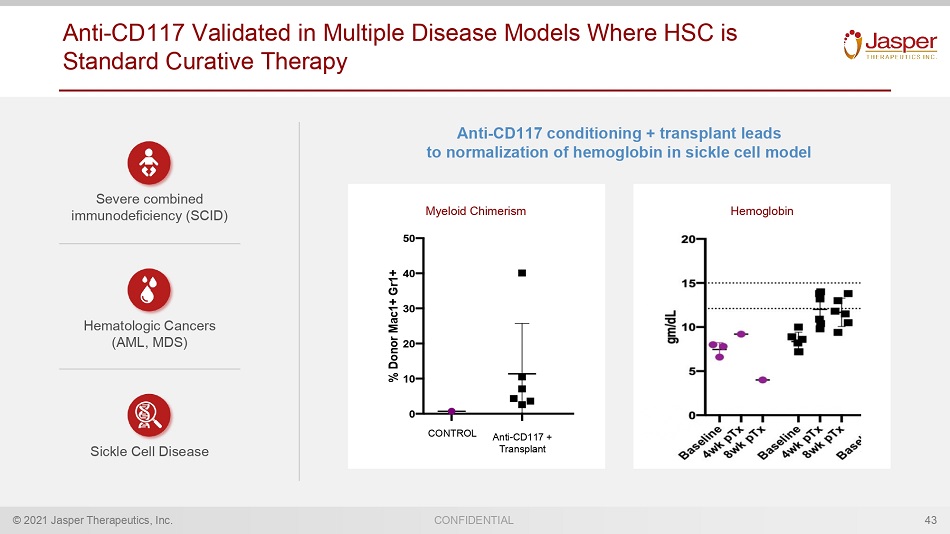
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Anti - CD117 Validated in Multiple Disease Models Where HSC is Standard Curative Therapy 43 Anti - CD117 conditioning + transplant leads to normalization of hemoglobin in sickle cell model Myeloid Chimerism Hemoglobin 0 10 20 30 40 50 % D o n o r M a c 1 + G r 1 + 8wk Myeloid Donor (blood) 200ug ACK2 + 200cGy TBI +aCD4/8 (300ug IPx3) 200ug ACK2 + 200cGy TBI +aCD4/8 (300ug IPx3) + transfusion 300ug ACK2 + 200cGy TBI + aCD4/8 (300ug IPx3) 300ug ACK2 + 200cGy TBI + aCD4/8 (300ug IPx3) + transfusion CONTROL Anti - CD117 + Transplant Severe combined immunodeficiency (SCID) Hematologic Cancers (AML, MDS) Sickle Cell Disease
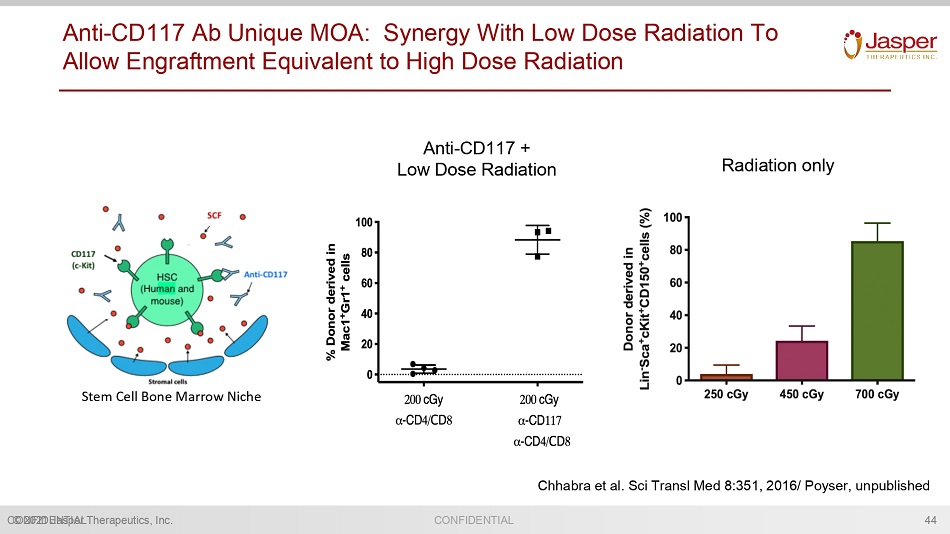
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Anti - CD117 Ab Unique MOA: Synergy With Low Dose Radiation To Allow Engraftment Equivalent to High Dose Radiation 44 Chhabra et al. Sci Transl Med 8:351, 2016/ Poyser , unpublished Radiation only Anti - CD117 + Low Dose Radiation Stem Cell Bone Marrow Niche
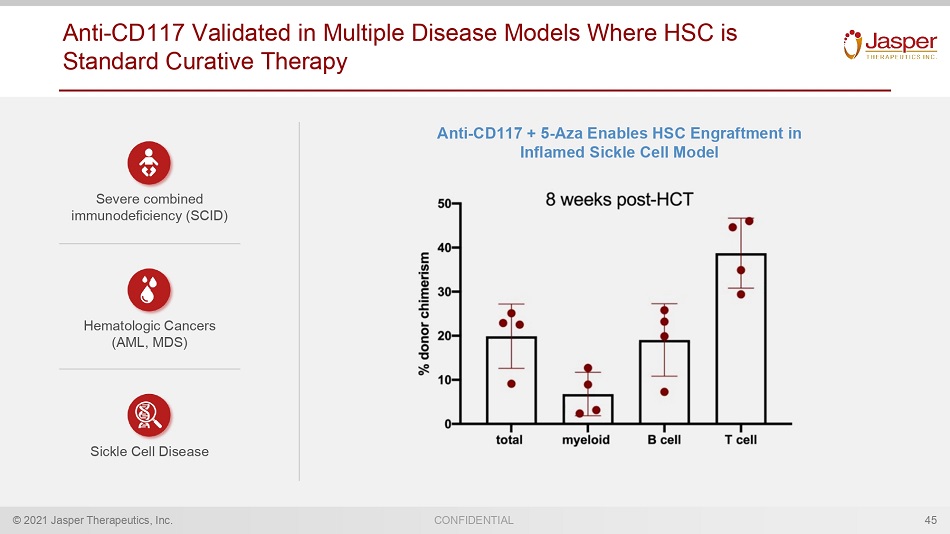
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Anti - CD117 Validated in Multiple Disease Models Where HSC is Standard Curative Therapy 45 Anti - CD117 + 5 - Aza Enables HSC E ngraftment in Inflamed Sickle Cell Model Severe combined immunodeficiency (SCID) Hematologic Cancers (AML, MDS) Sickle Cell Disease

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 + 5 - Azacytidine Combination For Deep Non - Radiation Based Outpatient Conditioning 46 ▪ New data showing synergistic effect of SCF blockade + 5 - azacytidine on stem cell engraftment (Stanford) ▪ Widely available Sub - q / IV generic currently used to treat MDS ▪ JSP191 + Single cycle of 5 - aza expected to be safe outpatient conditioning ▪ Expected combination dose of 0.3 - 0.6mg/kg JSP191 + 5 - aza 75 mg/m 2 for 5 - 7 days CD117 blockade + 5 - Aza Synergistic Effect on Congenic Stem Cell Engraftment in Immune Competent Mice CONFIDENTIAL
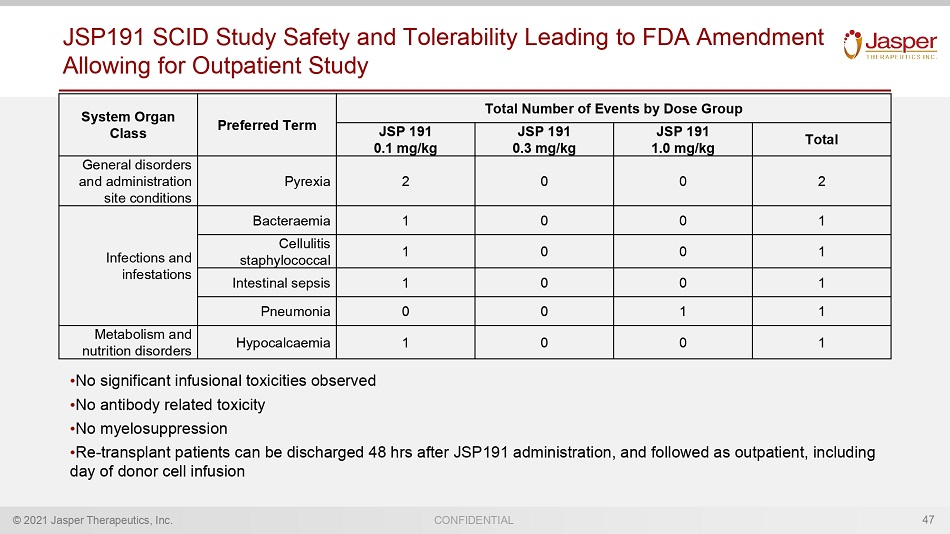
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 SCID Study Safety and Tolerability Leading to FDA Amendment Allowing for Outpatient Study • No significant infusional toxicities observed • No antibody related toxicity • No myelosuppression • Re - transplant p atients can be discharged 48 hrs after JSP191 administration , and followed as outpatient , including day of donor cell infusion 47 System Organ Class Preferred Term Total Number of Events by Dose Group JSP 191 0.1 mg/kg JSP 191 0.3 mg/kg JSP 191 1.0 mg/kg Total General disorders and administration site conditions Pyrexia 2 0 0 2 Infections and infestations Bacteraemia 1 0 0 1 Cellulitis staphylococcal 1 0 0 1 Intestinal sepsis 1 0 0 1 Pneumonia 0 0 1 1 Metabolism and nutrition disorders Hypocalcaemia 1 0 0 1
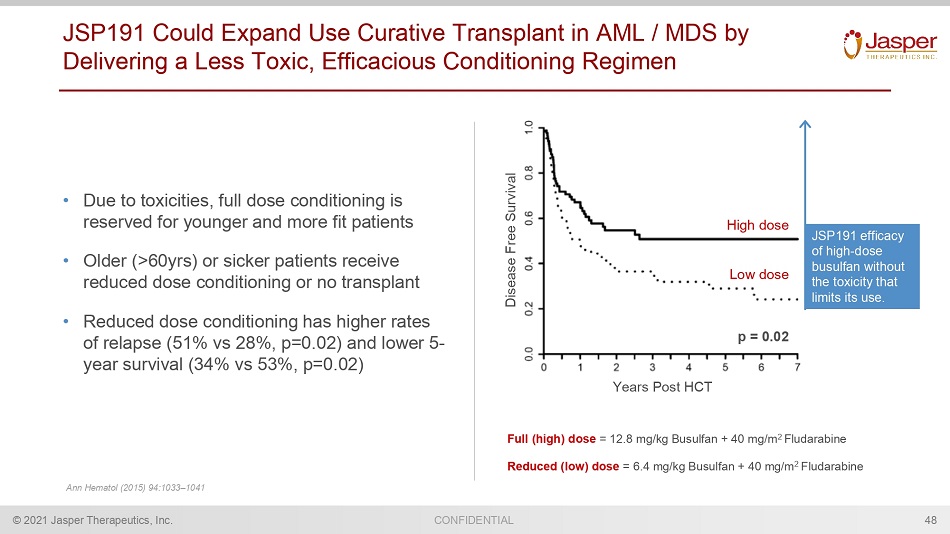
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Years Post HCT Low dose High dose Disease Free Survival p = 0.02 JSP191 Could Expand Use Curative Transplant in AML / MDS by Delivering a Less Toxic, Efficacious Conditioning Regimen • Due to toxicities, full dose conditioning is reserved for younger and more fit patients • Older (>60yrs) or sicker patients receive reduced dose conditioning or no transplant • Reduced dose conditioning has higher rates of relapse (51% vs 28%, p=0.02) and lower 5 - year survival (34% vs 53%, p=0.02) JSP191 efficacy of high - dose busulfan without the toxicity that limits its use. Full (high) dose = 12.8 mg/kg Busulfan + 40 mg/m 2 Fludarabine Reduced (low) dose = 6.4 mg/kg Busulfan + 40 mg/m 2 Fludarabine Ann Hematol (2015) 94:1033 – 1041 48
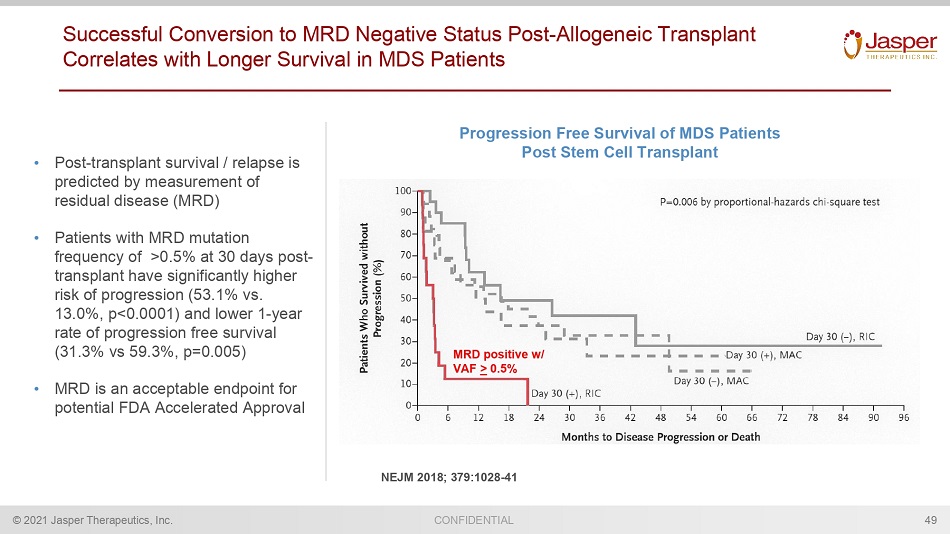
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Successful Conversion to MRD Negative Status Post - Allogeneic Transplant Correlates with Longer Survival in MDS Patients • Post - transplant survival / relapse is predicted by measurement of residual disease (MRD) • Patients with MRD mutation frequency of >0.5% at 30 days post - transplant have significantly higher risk of progression (53.1% vs. 13.0%, p<0.0001) and lower 1 - year rate of progression free survival (31.3% vs 59.3%, p=0.005) • MRD is an acceptable endpoint for potential FDA Accelerated Approval Progression Free Survival of MDS Patients Post Stem Cell Transplant 49 MRD positive w/ VAF > 0.5% NEJM 2018; 379:1028 - 41

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. MDS/AML Subject Demographics (detailed) 50 Subject Number Age Sex Diagnosis Prior Therapy for MDS or AML Med History Donor 003 74F T - AML Azacitidine / Venetoclax • Breast Cancer with recurrence treated with Chemotherapy and Radiation therapy • Hypothyroidism • Osteopenia • Depression/anxiety • Arrythmias (treated with propranolol) • MPN (JAK2+) Matched unrelated 004 70M MDS Erythropoietin • Pre - diabetic • Hypertension Matched related 005 68M MDS Azacitidine • Atrial Fibrillation • Hypertension • Hypertrophic Cardiomyopathy • Supraventricular tachycardia Matched unrelated 009 74M MDS None • Umbilical hernia repair • No significant PMx Matched unrelated 010 65M AML + FLT3 Idarubicin/ Midostaurin Azacitidine / Venetoclax • Type - 2 Diabetes • Peripheral neuropathy • Hyperlipidemia • Hypertension Matched unrelated 011 69M AML Cytarabine/ Daunorubicin (7+3) Cytarabine/ Daunorubicin (5+2) • Type - 2 Diabetes • Hypertension • Diverticulitis Matched related Muffly et al, TCT2021 Late Breaking abstract (#LBA5)

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 PK at 0.6 mg/kg was Observed to be Consistent Among AML/MDS Subjects (N = 6) 51 71.0 hours Observed half - life 10 - 12 days Clearance time to donor graft infusion Muffly et al, TCT2021 Late Breaking abstract (#LBA5)
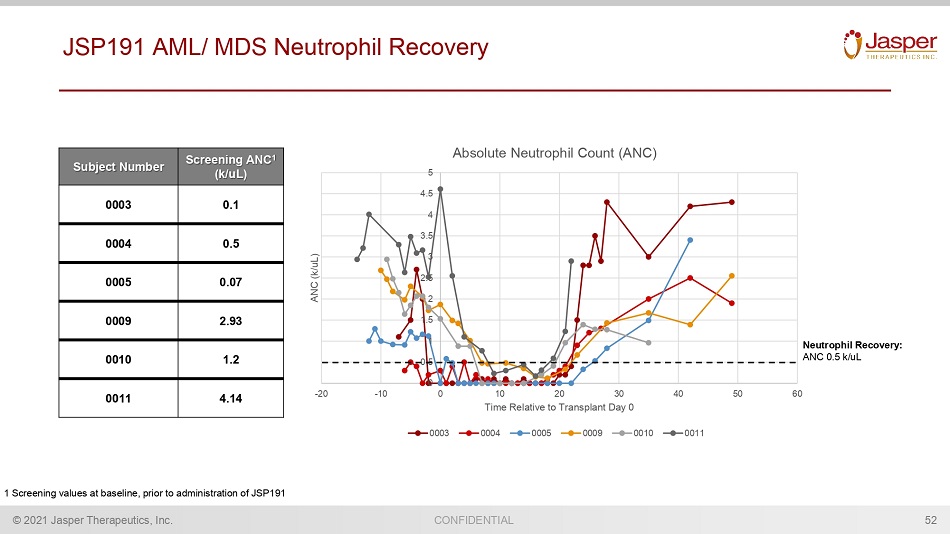
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 AML/ MDS Neutrophil Recovery 52 Subject Number Screening ANC 1 (k/ uL ) 0003 0.1 0004 0.5 0005 0.07 0009 2.93 0010 1.2 0011 4.14 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 -20 -10 0 10 20 30 40 50 60 ANC (k/uL) Time Relative to Transplant Day 0 Absolute Neutrophil Count (ANC) 0003 0004 0005 0009 0010 0011 1 Screening values at baseline, prior to administration of JSP191

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 AML / MDS Study Chimerism to 90days 53 Table data reflected in graphs: A : Total cell chimerism, B: CD15+ Myeloid Cell Chimerism, C: CD3+ T Cell Chimerism, D: CD56 NK Cell Chimerism. Subject Number Donor Chimerism at TD+28 Donor Chimerism at TD+56 Donor Chimerism at TD+90 Total CD15 CD3 CD56 Total CD15 CD3 CD56 Total CD15 CD3 CD56 003 97% 98% 72% 97% 96% 99% 64% 98% 96% 100% 80% 97% 004 95% 100% 71% 77% 97% 99% 73% 83% 95% 98% 69% 87% 005 81% 100% 33% 94% 96% 100% 40% 99% 98% 100% 52% 99% 009 86% 99% 9% 78% 86% 99% 11% 85% 87% 97% 10% 87% 010 91% 97% 40% 86% 96% 100% 65% 94% Subject still on study 011 90% 94% 60% 90% Subject still on study – assessments TBD
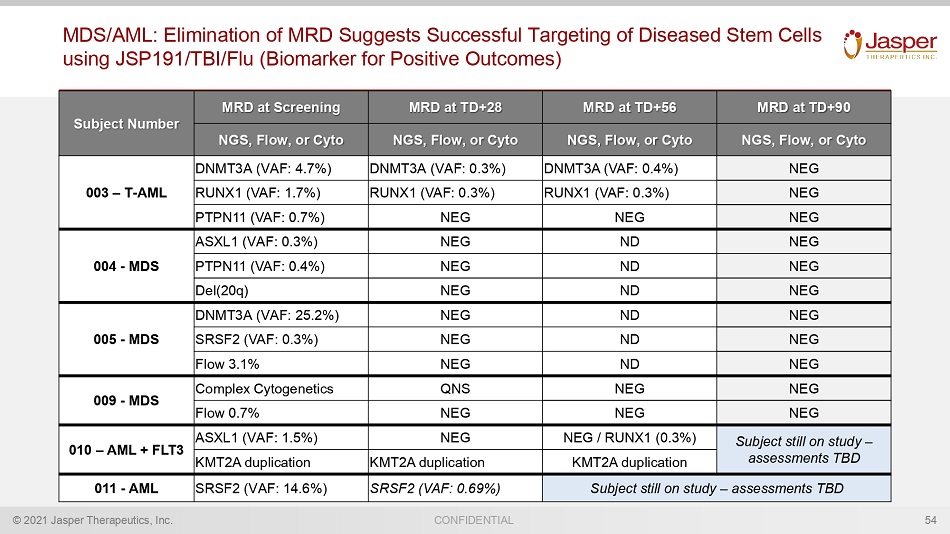
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL MDS/AML: Elimination of MRD Suggests Successful Targeting of Diseased Stem Cells using JSP191/TBI/Flu (Biomarker for Positive Outcomes) Subject Number MRD at Screening MRD at TD+28 MRD at TD+56 MRD at TD+90 NGS, Flow, or Cyto NGS, Flow, or Cyto NGS, Flow, or Cyto NGS, Flow, or Cyto 003 – T - AML DNMT3A (VAF: 4.7%) DNMT3A (VAF: 0.3%) DNMT3A (VAF: 0.4%) NEG RUNX1 (VAF: 1.7%) RUNX1 (VAF: 0.3%) RUNX1 (VAF: 0.3%) NEG PTPN11 (VAF: 0.7%) NEG NEG NEG 004 - MDS ASXL1 (VAF: 0.3%) NEG ND NEG PTPN11 (VAF: 0.4%) NEG ND NEG Del(20q) NEG ND NEG 005 - MDS DNMT3A (VAF: 25.2%) NEG ND NEG SRSF2 (VAF: 0.3%) NEG ND NEG Flow 3.1% NEG ND NEG 009 - MDS Complex Cytogenetics QNS NEG NEG Flow 0.7% NEG NEG NEG 010 – AML + FLT3 ASXL1 (VAF: 1.5%) NEG NEG / RUNX1 (0.3%) Subject still on study – assessments TBD KMT2A duplication KMT2A duplication KMT2A duplication 011 - AML SRSF2 (VAF: 14.6%) SRSF2 (VAF: 0.69%) Subject still on study – assessments TBD 54
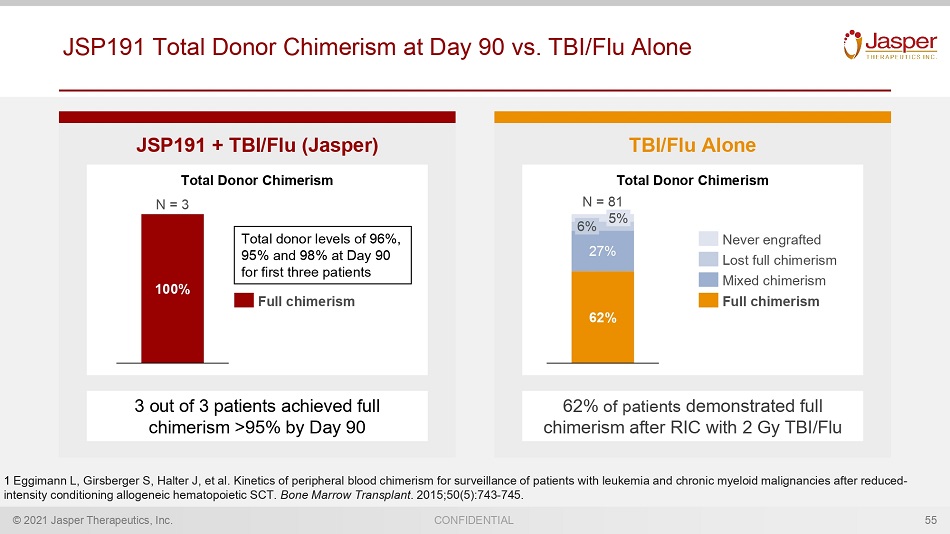
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 Total Donor Chimerism at Day 90 vs. TBI/Flu Alone 55 JSP191 + TBI/Flu (Jasper) TBI/Flu Alone 62% of patients demonstrated full chimerism after RIC with 2 Gy TBI/Flu 1 Eggimann L, Girsberger S, Halter J, et al. Kinetics of peripheral blood chimerism for surveillance of patients with leukemia and chronic myeloid mal ig nancies after reduced - intensity conditioning allogeneic hematopoietic SCT. Bone Marrow Transplant . 2015;50(5):743 - 745. 3 out of 3 patients achieved full chimerism >95% by Day 90 Total donor levels of 96%, 95% and 98% at Day 90 for first three patients 62% 27% 5% 6% N = 81 Never engrafted Lost full chimerism Full chimerism Mixed chimerism 100% N = 3 Full chimerism Total Donor Chimerism Total Donor Chimerism

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL After RIC with TBI/Flu, 38% Patients Fail to Demonstrate Full Chimerism, Which Predicts Significantly Worse Survival 56 Source: Eggimann L, Girsberger S, Halter J, et al. Kinetics of peripheral blood chimerism for surveillance of patients with leukemia and chronic myeloid mal ig nancies after reduced - intensity conditioning allogeneic hematopoietic SCT. Bone Marrow Transplant . 2015;50(5):743 - 745. Chimerism is universally used to monitor engraftment after hematopoietic SCT (HSCT) • It is valuable for predicting graft failure, rejection as well as relapses in malignant diseases For patients achieving full donor chimerism, a significant benefit in survival was observed • 3 - year OS was 63% vs. 23% for patients who did not reach or lost full donor chimerism (P < 0.0001) 27 of 77 patients (38%) failed to demonstrate full chimerism after RIC with 2 Gy TBI + Fludarabine • Full chimerism defined as >95% in peripheral blood during the follow - up Full Chimerism Mixed / Absent Chimerism
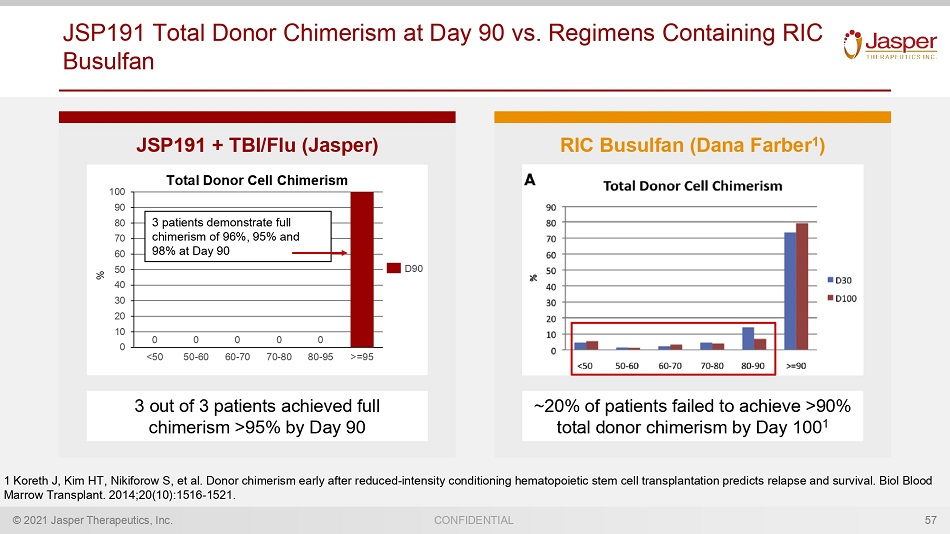
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 Total Donor Chimerism at Day 90 vs. Regimens Containing RIC Busulfan 57 JSP191 + TBI/Flu (Jasper) RIC Busulfan (Dana Farber 1 ) ~20% of patients failed to achieve >90% total donor chimerism by Day 100 1 1 Koreth J, Kim HT, Nikiforow S, et al. Donor chimerism early after reduced - intensity conditioning hematopoietic stem cell transplantation predicts relapse a nd survival. Biol Blood Marrow Transplant. 2014;20(10):1516 - 1521. 3 out of 3 patients achieved full chimerism >95% by Day 90 0 10 20 30 40 50 60 70 80 90 100 0 0 60 - 70 <50 50 - 60 70 - 80 0 80 - 95 >=95 0 0 D90 Total Donor Cell Chimerism % 3 patients demonstrate full chimerism of 96%, 95% and 98% at Day 90
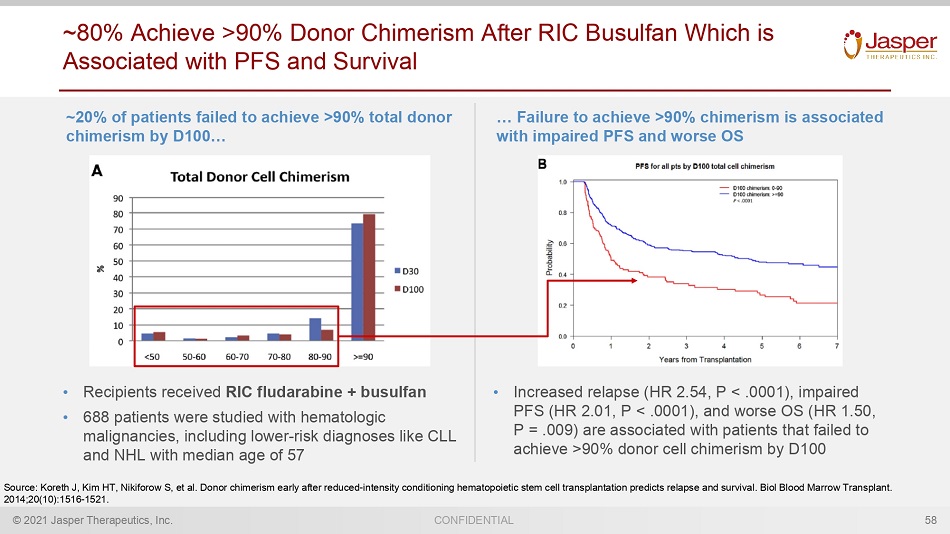
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL ~80% Achieve >90% Donor Chimerism After RIC Busulfan Which is Associated with PFS and Survival 58 Source: Koreth J, Kim HT, Nikiforow S, et al. Donor chimerism early after reduced - intensity conditioning hematopoietic stem cell transplantation predicts relapse a nd survival. Biol Blood Marrow Transplant. 2014;20(10):1516 - 1521. • Increased relapse (HR 2.54, P < .0001), impaired PFS (HR 2.01, P < .0001), and worse OS (HR 1.50, P = .009) are associated with patients that failed to achieve >90% donor cell chimerism by D100 … Failure to achieve >90% chimerism is associated with impaired PFS and worse OS ~20% of patients failed to achieve >90% total donor chimerism by D100… • Recipients received RIC fludarabine + busulfan • 688 patients were studied with hematologic malignancies, including lower - risk diagnoses like CLL and NHL with median age of 57
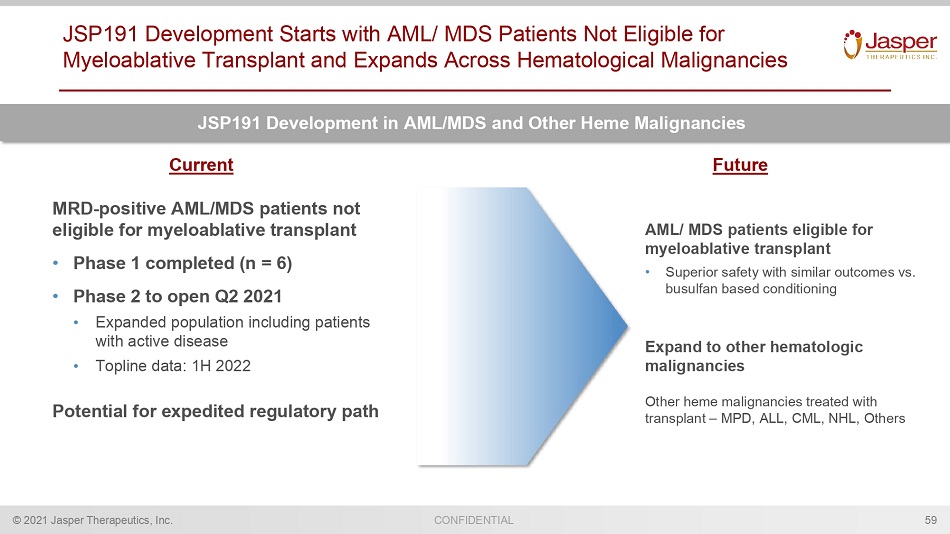
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 Development Starts with AML/ MDS Patients Not Eligible for Myeloablative Transplant and Expands Across Hematological Malignancies 59 JSP191 Development in AML/MDS and Other Heme Malignancies MRD - positive AML/MDS patients not eligible for myeloablative transplant • Phase 1 completed (n = 6) • Phase 2 to open Q2 2021 • Expanded population including patients with active disease • Topline data: 1H 2022 Potential for expedited regulatory path AML/ MDS patients eligible for myeloablative transplant • Superior safety with similar outcomes vs. busulfan based conditioning Expand to other hematologic malignancies Other heme malignancies treated with transplant – MPD, ALL, CML, NHL, Others Current Future
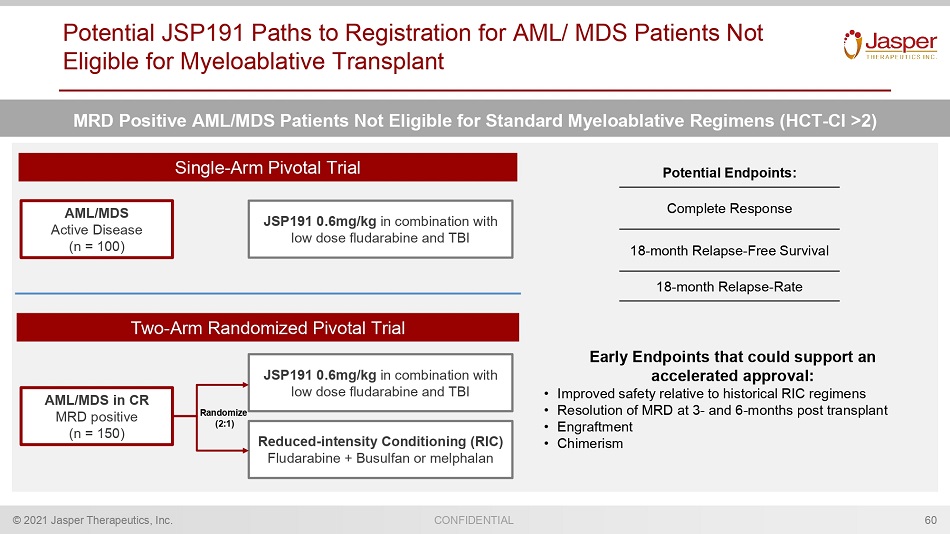
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Potential JSP191 Paths to Registration for AML/ MDS Patients Not Eligible for Myeloablative Transplant 60 JSP191 0.6mg/kg in combination with low dose fludarabine and TBI Potential Endpoints: Complete Response 18 - month Relapse - Free Survival 18 - month Relapse - Rate MRD Positive AML/MDS Patients Not Eligible for Standard Myeloablative Regimens (HCT - Cl >2) Single - Arm Pivotal Trial AML/MDS Active Disease (n = 100) JSP191 0.6mg/kg in combination with low dose fludarabine and TBI Two - Arm Randomized Pivotal Trial AML/MDS in CR MRD positive (n = 150) Reduced - intensity Conditioning (RIC) Fludarabine + Busulfan or melphalan Randomize (2:1) Early Endpoints that could support an accelerated approval: • Improved safety relative to historical RIC regimens • Resolution of MRD at 3 - and 6 - months post transplant • Engraftment • Chimerism
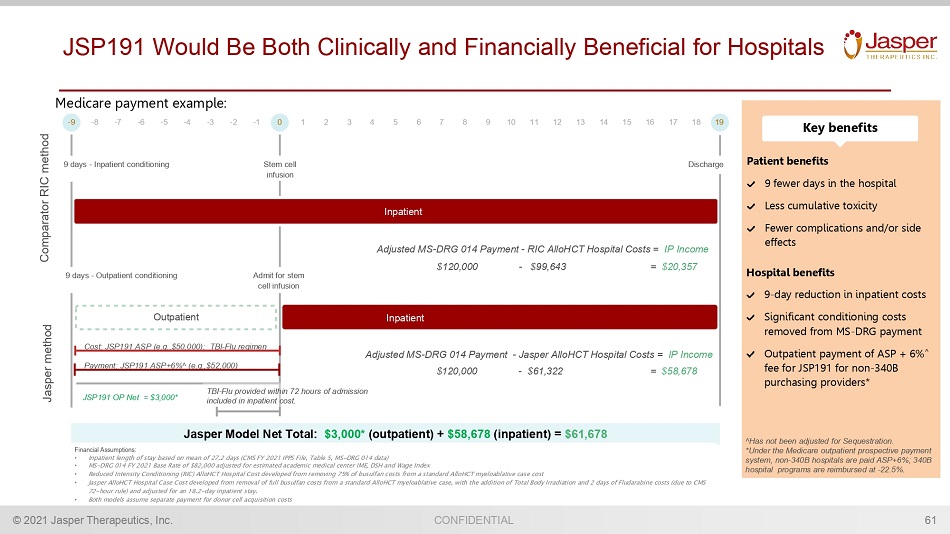
CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. JSP191 Would Be Both Clinically and Financially Beneficial for Hospitals 61 Adjusted MS - DRG 014 Payment - Jasper AlloHCT Hospital Costs = IP Income - 9 - 8 - 7 - 6 - 5 - 4 - 3 - 2 - 1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Jasper method Comparator RIC method Stem cell infusion Outpatient Inpatient Inpatient Adjusted MS - DRG 014 Payment - RIC AlloHCT Hospital Costs = IP Income JSP191 OP Net = $ 3 ,000 * Cost: JSP191 ASP (e.g.,$50,000) ; TBI - Flu regimen Payment: JSP191 ASP +6 % ^ (e.g.,$5 2 ,000) Admit for stem cell infusion 9 days - Inpatient conditioning Discharge 9 day s - Outpatient conditioning $120,000 - $61,322 = $58,678 Jasper Model Net Total: $3,000* (outpatient) + $58,678 (inpatient) = $61,678 Financial Assumptions: • Inpatient length of stay based on mean of 27.2 days (CMS FY 2021 IPPS File, Table 5, MS - DRG 014 data) • MS - DRG 014 FY 2021 Base Rate of $82,000 adjusted for estimated academic medical center IME, DSH and Wage Index • Reduced Intensity Conditioning (RIC) AlloHCT Hospital Cost developed from removing 75% of busulfan costs from a standard Allo HCT myeloablative case cost • Jasper AlloHCT Hospital Case Cost developed from removal of full busulfan costs from a standard AlloHCT myeloablative case, w ith the addition of Total Body Irradiation and 2 days of Fludarabine costs (due to CMS 72 - hour rule) and adjusted for an 18.2 - day inpatient stay. • Both models assume separate payment for donor cell acquisition costs TBI - Flu provided within 72 hours of admission included in inpatient cost. Medicare payment example: Patient benefit s 9 fewer days in the hospital Less cumulative toxicity Fewer complications and/or side effects Hospital benefit s 9 - day reduction in inpatient costs Significant c onditioning costs removed from MS - DRG payment Outpatient payment of ASP + 6% ^ fee for JSP191 for non - 340B purchasing providers* Key benefits ^Has not been adjusted for Sequestration. *Under the Medicare outpatient prospective payment system, non - 340B hospitals are paid ASP+6% ; 340B hospital program s are reimbursed at - 22.5%. $120,000 - $99,643 = $20,357
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 Intellectual Property and Exclusivity 62 • Amgen: WW license on molecule and COM IP • Stanford: IP on use as conditioning agent, combinations, dosing • Regulatory exclusivity expected to 2036

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL JSP191 CMC 63 • CMC process successfully transferred from Amgen to Lonza • First Lonza drug substance batch run at commercial scale and released at GMP • Initial pilot scale biochemical comparability complete, commercial scale comparability ongoing • Validation activities started
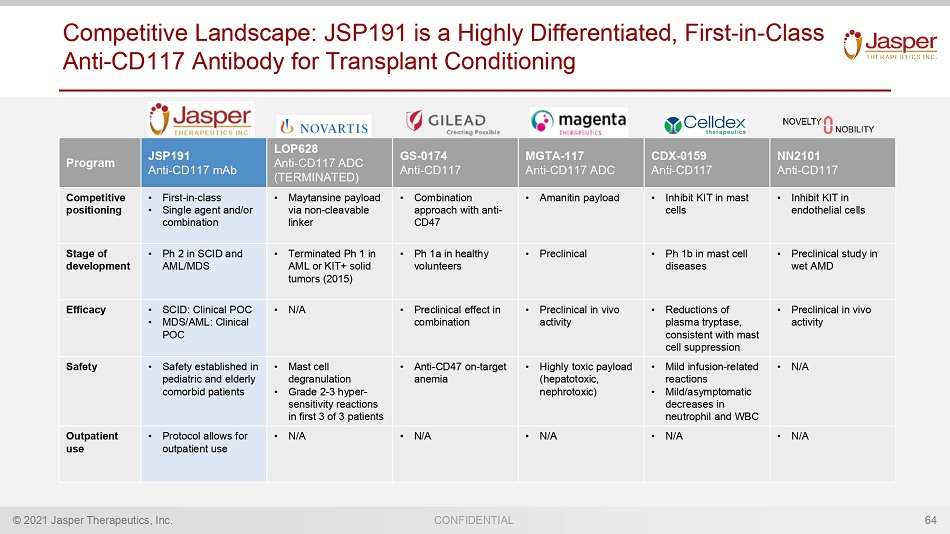
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Competitive Landscape: JSP191 is a Highly Differentiated, First - in - Class Anti - CD117 Antibody for Transplant Conditioning 64 Program JSP191 Anti - CD117 mAb LOP628 Anti - CD117 ADC (TERMINATED) GS - 0174 Anti - CD117 MGTA - 117 Anti - CD117 ADC CDX - 0159 Anti - CD117 NN2101 Anti - CD117 Competitive positioning • First - in - class • Single agent and/or combination • Maytansine payload via non - cleavable linker • Combination approach with anti - CD47 • Amanitin payload • Inhibit KIT in mast cells • Inhibit KIT in endothelial cells Stage of development • Ph 2 in SCID and AML/MDS • Terminated Ph 1 in AML or KIT+ solid tumors (2015) • Ph 1a in healthy volunteers • Preclinical • Ph 1b in mast cell diseases • Preclinical study in wet AMD Efficacy • SCID: Clinical POC • MDS/AML: Clinical POC • N/A • Preclinical effect in combination • Preclinical in vivo activity • Reductions of plasma tryptase, consistent with mast cell suppression • Preclinical in vivo activity Safety • Safety established in pediatric and elderly comorbid patients • Mast cell degranulation • Grade 2 - 3 hyper - sensitivity reactions in first 3 of 3 patients • Anti - CD47 on - target anemia • Highly toxic payload (hepatotoxic, nephrotoxic) • Mild infusion - related reactions • Mild/asymptomatic decreases in neutrophil and WBC • N/A Outpatient use • Protocol allows for outpatient use • N/A • N/A • N/A • N/A • N/A

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Targeted Busulfan Conditioning Still Results in Significant Toxicities • No demonstrated benefit in infertility – “infertility risk from Busulfan in gene therapy continues to be studied” • median 74.4 mg*h/L 1 65
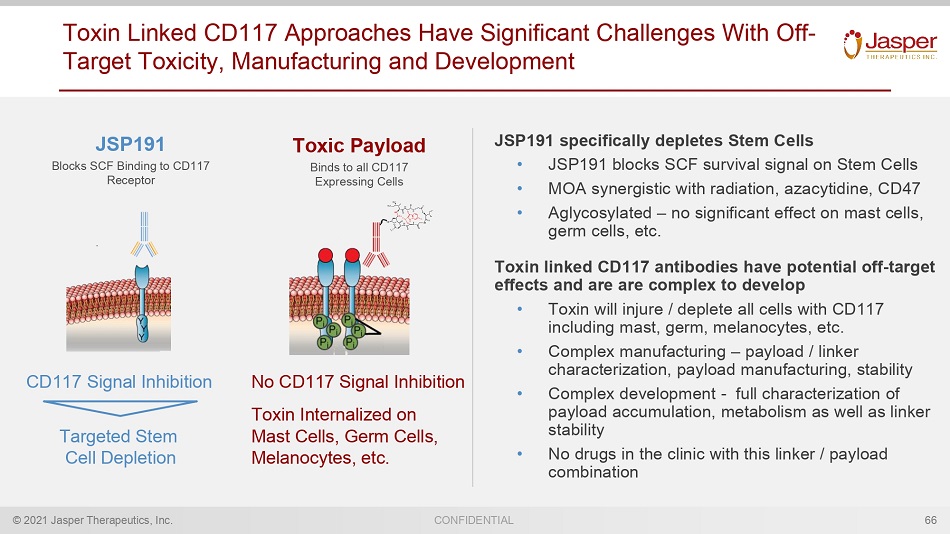
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Toxin Linked CD117 Approaches Have Significant Challenges With Off - Target Toxicity, Manufacturing and Development JSP191 specifically depletes Stem Cells • JSP191 blocks SCF survival signal on Stem Cells • MOA synergistic with radiation, azacytidine, CD47 • Aglycosylated – no significant effect on mast cells, germ cells, etc. Toxin linked CD117 antibodies have potential off - target effects and are are complex to develop • Toxin will injure / deplete all cells with CD117 including mast, germ, melanocytes, etc. • Complex manufacturing – payload / linker characterization, payload manufacturing, stability • Complex development - full characterization of payload accumulation, metabolism as well as linker stability • No drugs in the clinic with this linker / payload combination 66 JSP191 Blocks SCF Binding to CD117 Receptor CD117 Signal Inhibition Toxic Payload Binds to all CD117 Expressing Cells No CD117 Signal Inhibition Toxin Internalized on Mast Cells, Germ Cells, Melanocytes, etc. Targeted Stem Cell Depletion
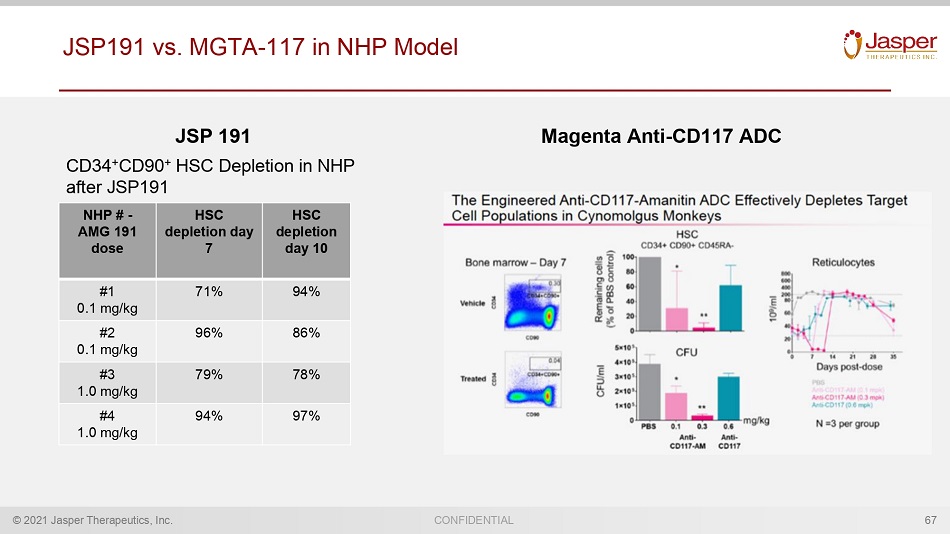
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL NHP # - AMG 191 dose HSC depletion day 7 HSC depletion day 10 #1 0.1 mg/kg 71% 94% #2 0.1 mg/kg 96% 86% #3 1.0 mg/kg 79% 78% #4 1.0 mg/kg 94% 97% CD34 + CD90 + HSC Depletion in NHP after JSP191 JSP 191 Magenta Anti - CD117 ADC JSP191 vs. MGTA - 117 in NHP Model 67

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL MGTA - 117 Will Require Additional Therapies for Allogeneic Transplant 68 Depletion of patient’s T - cells is required for allogeneic transplant • Patient’s T - cells will attack donor graft, leading to graft failure • Current strategies include: TBI + flu, Bu+Flu , Mel+flu , TLI MGTA - 117 does not deplete T - cells and will require combination therapy for allogeneic transplant • TBI, TLI, Busulfan and Melphalan affect lymphocytes • Unclear if fludarabine alone can sufficiently deplete patient T - cells prior to transplant
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL We Believe Jasper’s Engineered Hematopoietic Stem Cells ( eHSCs ) Will Drive Cures, Decrease Toxicity, and Eliminate Post Transplant Immune Suppression 69 • Primary human CD34+ cell experiments with multiple engineering approaches • Preclinical proof of concept in xenograft mouse model • GLP production for IND enabling studies and GMP scale - up • Clinical proof of concept (allogeneic + gene - therapy) • Expansion to additional patient populations Next Steps for eHSCs Future Development

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Jasper Engineered Hematopoietic Stem Cells ( eHSCs ) Improve Donor Stem Cell Engraftment From Donation to Recovery 70 1. Stem Cells Harvested 2. Conditioning 3. Transplant The picture can't be displayed. 4. Post - HCST Recovery x Increased utility for haplo / mis - matched grafts x Expand population x Less intense/ toxic conditioning x No immune suppression or GvHD x Reduced blood cancer relapse x Pure stem cell grafts x Faster & more consistent engraftment

© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Stem Cell Engineering: Only Jasper eHSC Designed to Increase Stem Cell Engraftment and Proliferation 71 Program Jasper eHSCs VOR33 (CD33) Hypoimmune Technology In Vivo Gene Therapy Competitive positioning • Cellular engineering leading to improved donor cell engraftment • Treatment - resistant marrow cells that enable CD33 targeted therapy • Hypoimmune cells designed to evade rejection • In vivo gene therapy for stem cells Stage of development • In - Vitro POC • Preclinical • Preclinical • Preclinical Efficacy • Jasper CD117 eHSCs show higher and more consistent proliferation vs. WT • CD33del shows in vitro and in vivo resistance to CD33 • NHP: iPSCs do not elicit adaptive or innate response • In vivo POC in mice with bio - luminescence Safety • N/A • Tox from myeloablative conditioning regimen (VOD, mucositis, secondary malignancy) • Not yet validated with human iPSCs or differentiated cells • Multiple rounds of toxic alkylators for conditioning • AAV cytokine activation Outpatient use • Potential for outpatient use with non - toxic conditioning • N/A • N/A • N/A Ensoma
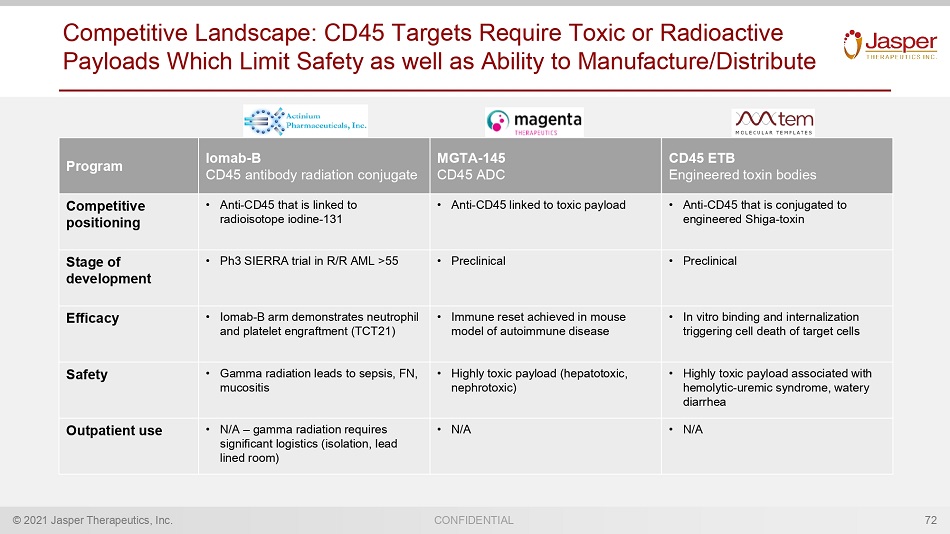
© 2021 Jasper Therapeutics, Inc. CONFIDENTIAL Competitive Landscape: CD45 Targets Require Toxic or Radioactive Payloads Which Limit Safety as well as Ability to Manufacture/Distribute 72 Program Iomab - B CD45 antibody radiation conjugate MGTA - 145 CD45 ADC CD45 ETB Engineered toxin bodies Competitive positioning • Anti - CD45 that is linked to radioisotope iodine - 131 • Anti - CD45 linked to toxic payload • Anti - CD45 that is conjugated to engineered Shiga - toxin Stage of development • Ph3 SIERRA trial in R/R AML >55 • Preclinical • Preclinical Efficacy • Iomab - B arm demonstrates neutrophil and platelet engraftment (TCT21) • Immune reset achieved in mouse model of autoimmune disease • In vitro binding and internalization triggering cell death of target cells Safety • Gamma radiation leads to sepsis, FN, mucositis • Highly toxic payload (hepatotoxic, nephrotoxic) • Highly toxic payload associated with hemolytic - uremic syndrome, watery diarrhea Outpatient use • N/A – gamma radiation requires significant logistics (isolation, lead lined room) • N/A • N/A

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Jasper’s Success May Lead to Routine Hematopoietic Stem Cell Transplants For Hundreds of Thousands of Patients 73 2021 TODAY 2028 JSP191 + Jasper eHSCs • JSP191 safe outpatient conditioning • Jasper eHSCs for improved transplant without hospitalization or GvHD • Routine cure for hundreds of thousands of patients • Safe, outpatient conditioning • Potential for less immunogenic grafts • Expanded transplant population • Curative • Highly toxic conditioning • Graft failures & GvHD • Limited population 2024 JSP191

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Risk Factors 74 The list below of risk factors has been prepared solely for purposes of the proposed private placement transaction (the “Priv ate Placement”) as part of the proposed business combination (the “Business Combination”) of Amplitude Healthcare Acquisition Cor p. (“AMHC”) and Jasper Therapeutics, Inc. (“Jasper”), and solely for potential investors in the Private Placement, and not for any other purpose. T he risks presented below are certain of the general risks related to the businesses of Jasper, the Private Placement and the Bus ine ss Combination, and such list is not exhaustive. The list below is qualified in its entirety by disclosures contained in future documents filed or furnished by J asp er and AMHC, with the U.S. Securities and Exchange Commission (“SEC”), including the documents filed or furnished in connecti on with the proposed transactions between Jasper and AMHC. The risks presented in such filings will be consistent with those that would be required for a publ ic company in its SEC filings, including with respect to the business and securities of Jasper and AMHC and the proposed transac tio ns between Jasper and AMHC, and may differ significantly from and be more extensive than those presented below. Investing in securities (the “Securities”) to be issued in connection with the Business Combination involves a high degree of ri sk. Investors should carefully consider the risks and uncertainties inherent in an investment in Jasper and in the Securitie s, including those described below, before subscribing for the Securities. If either Jasper cannot address any of the following risks and uncertainties effectiv ely , or any other risks and difficulties that may arise in the future, Jasper’s business, financial condition or results of oper ati ons could be materially and adversely affected. The risks described below are not the only ones Jasper faces. Additional risks that Jasper currently does not kno w a bout or that Jasper currently believes to be immaterial may also impair its business, financial condition or results of opera tio ns. You should review the investors’ presentation and perform your own due diligence, prior to making an investment in AMHC or Jasper. Risks Related to Jasper’s Financial Position and Capital Requirements Jasper has incurred significant net losses since its inception. Jasper expects to incur net losses for the foreseeable future an d may never achieve or maintain profitability. Jasper will need substantial additional funding. If Jasper is unable to raise capital when needed, it would be forced to dela y, reduce or eliminate its research and product development programs or future commercialization efforts. Jasper has a limited operating history and no history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for its future viability. Jasper has never generated revenue from product sales and may never be profitable. Risks Related to the Development of Jasper’s Product Candidates Jasper is early in its development efforts. If Jasper is unable to advance its product candidates to obtain regulatory approv al and ultimately commercialize its product candidates, or experiences significant delays in doing so, its business will be mate ria lly harmed. Results of preclinical studies and early clinical trials may not be predictive of results of future clinical trials, and such re sults do not guarantee approval of a product candidate by regulatory authorities. In addition, Jasper’s clinical trials to da te have been limited in scope and results received to date may not be replicated in expanded or additional future clinical trials. Clinical development involves a lengthy and expensive process, with an uncertain outcome. Jasper may incur additional costs o r e xperience delays in completing, or ultimately be unable to complete, the development and commercialization of any product can did ates. Jasper may not be successful in its efforts to identify, develop and commercialize additional product candidates. If these ef for ts are unsuccessful, Jasper may never become a commercial stage company or generate any revenues. Jasper may expend its limited resources to pursue a particular product candidate or indication and fail to capitalize on prod uct candidates or indications that may be more profitable or for which there is a greater likelihood of success. Jasper faces significant competition in an environment of rapid technological change, and there is a possibility that its com pet itors may achieve regulatory approval before Jasper or develop therapies that are safer or more advanced or effective than Ja spe r’s, which may harm Jasper’s financial condition and its ability to successfully market or commercialize its product candidates. If any of Jasper’s product candidates causes serious adverse events, undesirable side effects or unexpected characteristics, suc h events, side effects or characteristics could delay or prevent regulatory approval of the product candidate, limit its comm erc ial potential or result in significant negative consequences following any potential marketing approval.

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Risk Factors (cont’d) 75 Risks Related to the Regulatory Regime for Jasper’s Product Candidates Jasper has no experience as a company in obtaining regulatory approval for a drug. The regulatory landscape that will govern Jasper’s product candidates is uncertain; regulations relating to more established cel lular therapy products are still developing, and changes in regulatory requirements could result in delays or discontinuation of development of its product candidates or unexpected costs in obtaining regulatory approval. The FDA and other governing bodies may disagree with Jasper’ s r egulatory plan and it may fail to obtain regulatory approval of its product candidates. Jasper’s product candidates are complex and difficult to manufacture. Jasper could experience delays in satisfying regulatory au thorities or production problems that result in delays in its development or commercialization programs, limit the supply of its product candidates, or otherwise harm its business. If clinical trials of Jasper’s product candidates it may identify and develop fail to demonstrate safety and efficacy to the sat isfaction of regulatory authorities or do not otherwise produce positive results, Jasper may incur additional costs or experi enc e delays in completing, or ultimately be unable to complete, the development and commercialization of such product candidates. Even if Jasper completes the necessary clinical trials, it cannot predict when, or if, it will obtain regulatory approval to com mercialize its product candidates in the United States or any other jurisdiction, and any such approval may be for a more nar row indication than Jasper seeks. Interim “top - line” and preliminary results from Jasper’s clinical trials that it may announce or publish from time to time may c hange as more patient data become available and are subject to audit and verification procedures that could result in materia l c hanges in the final data. If Jasper experiences delays or difficulties in the enrollment of patients in clinical trials, the cost of developing product ca ndidates could increase and its receipt of necessary regulatory approvals could be delayed or prevented. Jasper may never obtain FDA approval for any of its product candidates in the U.S., and even if it does, Jasper may never obt ain approval for or commercialize any of its product candidates in any other jurisdiction, which would limit Jasper’s ability to re alize their full market potential. Risks Related to Jasper’s Dependence on Third Parties Jasper relies on third parties to conduct its preclinical and clinical trials and will rely on them to perform other tasks fo r i t. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regu lat ory requirements, Jasper may not be able to obtain regulatory approval for or commercialize its product candidates and its business could be substantially harmed. Jasper is highly dependent on intellectual property licensed from third parties and termination of any of these licenses coul d r esult in the loss of significant rights, which would harm its business. Jasper currently relies on a single manufacturer for its clinical supply of its product candidates. In the event of a loss o f t his manufacturer, or a failure by such manufacturer to comply with FDA regulations, Jasper may not be able to find an alterna tiv e source on commercially reasonable terms, or at all. In addition, third - party manufacturers and any third - party collaborators may be unable to successfully scale - u p manufacturing of Jasper’s current or future product candidates in sufficient quality and quantity, which would delay or pre ven t Jasper from developing its product candidates and commercializing approved products, if any.

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Risk Factors (cont’d) 76 Risks Related to Jasper’s Intellectual Property Jasper’s commercial success depends on its ability to obtain, maintain and protect its intellectual property and proprietary tec hnology. The patent protection Jasper obtains for its product candidates may not be sufficient enough to provide it with any competiti ve advantage or its patents may be challenged. Patent terms may be inadequate to protect Jasper’s competitive position on its product candidates for an adequate amount of t ime , and the lives of its patents may not be sufficient to effectively protect its product candidates and business. In addition, ch anges to patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing Jasper’s ability to p rot ect its product candidates. If Jasper is unable to protect the confidentiality of its trade secrets, its business and competitive position may be harmed. Third - party claims of intellectual property infringement, misappropriation or other violations may prevent or delay Jasper’s pro duct discovery and development efforts and have a material adverse effect on its business. Jasper may become involved in lawsuits to protect or enforce its patents or other intellectual property, which could be expen siv e, time - consuming and unsuccessful. Jasper may not be able to protect its intellectual property rights throughout the world. Other Risk Factors Related to Jasper The COVID - 19 pandemic has caused, and could continue to cause, severe disruptions in the U.S., regional and global economies and could seriously harm Jasper’s development efforts, increase its costs and expenses and have a material adverse effect on Jasp er ’s business, financial condition and results of operations. Jasper’s internal computer systems, or those of its third - party vendors, collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of its product development programs, compromise se nsitive information related to its business or prevent Jasper from accessing critical information, potentially exposing it to liability or otherwise adverse ly affecting its business. Jasper and its management have a limited track record as an operating company. Failures in the operational execution of the exp ected business plans may have a material impact on Jasper’s commercial prospects. Further, if Jasper is not able to attract and retain highly - qualified personnel, it may not be able to successfully implement its business strategy. If Jasper loses key management personnel, or if it fails to recruit additional highly skilled personnel, Jasper’s ability to con tinue developing and identify and develop new or next generation product candidates will be impaired, which could result in d ela ys in the development process, loss of market opportunities, make Jasper less competitive and have a material adverse effect on Jasper’s business, financial cond iti on and results of operations. Jasper may be adversely affected by existing or future laws and regulations. Jasper is subject to the laws and regulations of th e federal government and of various state, local and provincial government entities. These laws and regulations set very stri nge nt requirements for the business. In addition, such laws and regulations are subject to change and amendment at any time. Jasper may incur significant expenses re lated to compliance with such laws and regulations and it may need to adjust rapidly to address changes in the regulatory fra mew ork applicable to its business. Jasper may fail to comply with federal, state, local and international regulations in its area of operation, and fu tur e regulations may impose additional requirements on its business. Jasper’s business is subject to possible scrutiny from regu lat ors, who may enforce existing or future regulations that impact the viability or attractiveness of its assets. Jasper currently has limited marketing personnel. If Jasper is unable to establish effective marketing and sales capabilities or enter into agreements with third parties to market and sell its product candidates, if approved, Jasper may not be able to ef fe ctively market and sell its product candidates, if approved, or generate product revenues Jasper’s commercial success depends upon attaining significant market acceptance of its product candidates, if approved, amon g p hysicians, patients, healthcare payers and operators of major clinics. Jasper’s business will ultimately depend on its ability to successfully generate revenues from its product candidates, if app rov ed. Reimbursement for such products is subject to different regulatory regimes in different jurisdictions. If any of Jasper ’s product candidates is approved, an unfavorable reimbursement determination in any of the major markets could have a material impact on Jasper. Further, an unfa vor able change in such regimes (e.g., price controls) could have a material impact on Jasper.

CONFIDENTIAL © 2021 Jasper Therapeutics, Inc. Risk Factors (cont’d) 77 Risks Related to the Private Placement AMHC may be unable to raise sufficient capital in the Private Placement or otherwise obtain additional financing to complete the Business Combination or to fund the operations and growth of the combined company following the Business Combination (the “Co mb ined Company”). The issuance of shares of the Combined Company’s securities in connection with the Private Placement will dilute substantiall y t he voting power of Combined Company's stockholders. AMHC may issue shares of its Class A common stock upon the conversion of its Class B common stock at a ratio greater than one - to - one at the closing of the Business Combination as a result of the anti - dilution provisions contained in its amended and restate d certificate of incorporation. Any such issuance would dilute the interest of the Combined Company’s stockholders and likely present other ri sks . Risks Related to the Business Combination Each of AMHC and Jasper will incur significant transaction costs in connection with the Business Combination. The consummation of the Business Combination is subject to a number of conditions and if those conditions are not satisfied o r w aived, the Business Combination agreement may be terminated in accordance with its terms and the Business Combination may not be completed. The ability to successfully effect the Business Combination and the Combined Company’s ability to successfully operate the bu sin ess thereafter will be largely dependent upon the efforts of certain key personnel of Jasper. The loss of such key personnel co uld negatively impact the operations and financial results of the combined business. Section 404 of the Sarbanes - Oxley Act will be applicable to the Combined Company after the Business Combination is consummated, and Jasper is only now beginning the costly and challenging process of compiling the system and processing documentation nece ssa ry to perform the evaluation of its internal control over financial reporting needed to comply with Section 404 of the Sarbanes - Oxley Act. The Com bined Company’s failure to timely and effectively implement controls and procedures required by Section 404 of the Sarbanes - Oxle y Act could have a material adverse effect on its business. There is no assurance that a stockholder’s decision whether to redeem its shares for a pro rata portion of AMHC’s trust accou nt will put the stockholder in a better future economic position. If the Business Combination’s benefits do not meet the expectations of investors or securities analysts, the market price of AMH C’s securities or, following the consummation of the Business Combination, the Combined Company’s securities, may decline. A market for the Combined Company’s securities may not develop, which would adversely affect the liquidity and price of such sec urities. There can be no assurance that the Combined Company’s securities will be approved for listing on the Nasdaq Global Market (“N asd aq”) or that the Combined Company will be able to comply with the continued listing standards of Nasdaq. Directors of AMHC have potential conflicts of interest in recommending that AMHC’s stockholders vote in favor of the adoption of the Business Combination. AMHC may redeem unexpired warrants prior to their exercise at a time that is disadvantageous to the holders of AMHC warrants, th ereby making such warrants worthless. Further, even if the Business Combination is completed, there can be no assurance that AM HC’s warrants will be in the money during their exercise period, and they may expire worthless. If AMHC seeks stockholder approval of the Business Combination, its sponsor, directors, officers, advisors and their affiliat es may elect to purchase shares or warrants from public stockholders, which may influence a vote on the Business Combination and re duce the public “float” of AMHC’s Class A common stock or warrants. If AMHC seeks stockholder approval of the Business Combination, its sponsor, officers and directors have agreed to vote in fa vor of such Business Combination, regardless of how its public stockholders vote. The ability of AMHC’s public stockholders to exercise redemption rights with respect to a large number of its shares could in cre ase the probability that the Business Combination would be unsuccessful. AMHC is not required to obtain an opinion from an independent investment banking firm or from an independent accounting firm, an d consequently, its stockholders may have no assurance from an independent source that the price it is paying for the busines s i s fair to AMHC from a financial point of view. Legal proceedings in connection with the Business Combination, the outcomes of which are uncertain, could delay or prevent th e c ompletion of the Business Combination. The Business Combination or Combined Company may be materially adversely affected by the recent COVID - 19 outbreak. Changes in laws or regulations, or a failure to comply with any laws and regulations, may adversely affect AMHC’s and the Com bin ed Company’s business, including AMHC’s and the Combined Company’s ability to consummate the Business Combination, and result s o f operations.












































































