Exhibit 99.2

1 Briquilimab is an investigative drug and is not approved for any indication Jasper Therapeutics: Preliminary BEACON Results NASDAQ: JSPR January 8 th , 2025

2 Briquilimab is an investigative drug and is not approved for any indication Safe Harbor Statements Forward - Looking Statements This investor presentation and any accompanying oral presentation (together, this “Presentation”) contain forward - looking statem ents. All statements other than statements of historical fact contained in this Presentation, including statements regarding the future opportunities an d p rospects of Jasper Therapeutics, Inc. (together with its subsidiary, "Jasper" or the "Company"), including milestones, potential regulatory fili ngs and the anticipated timing thereof, patient enrollment, future timelines, business strategy, and plans and objectives for future operations, are forward - lo oking statements. Jasper has based these forward - looking statements on its estimates and assumptions and its current expectations and projections about futur e events. These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those contained in the "Risk Fa cto rs" section of the Company's Annual Report on Form 10 - K for the year ended December 31, 2023, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K that the Company has subsequently filed or may subsequently file with the SEC. In light of these risks, uncertainties and assumptions, the forward - lo oking events and circumstances discussed in this Presentation are inherently uncertain and may not occur, and actual results could differ materially and adv ers ely from those anticipated or implied in the forward - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of fu ture events. Jasper undertakes no obligation to update publicly or revise any forward - looking statements for any reason after the date of this Prese ntation or to conform these statements to actual results or to changes in Jasper's expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither the Company nor its affiliates, adv ise rs or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or represen tat ives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the date of this Prese nta tion. Such data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use shou ld not be construed as an endorsement of the products or services of the Company.
3 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Today’s agenda and presenters Title (Affiliation) Presenter Topic Chief Executive Officer Ronald Martell Opening Remarks and Topline Summary Chief Medical Officer Edwin Tucker, MD, MRCP BEACON Preliminary Results Summary Prof of Medicine and Pediatrics, University of South Florida Thomas B Casale, MD Briquilimab for Chronic Urticaria Chief Executive Officer Ronald Martell Upcoming Milestones and Closing Remarks

4 Briquilimab is an investigative drug and is not approved for any indication 4 BEACON Preliminary Results Summary Edwin Tucker, MD, MRCP
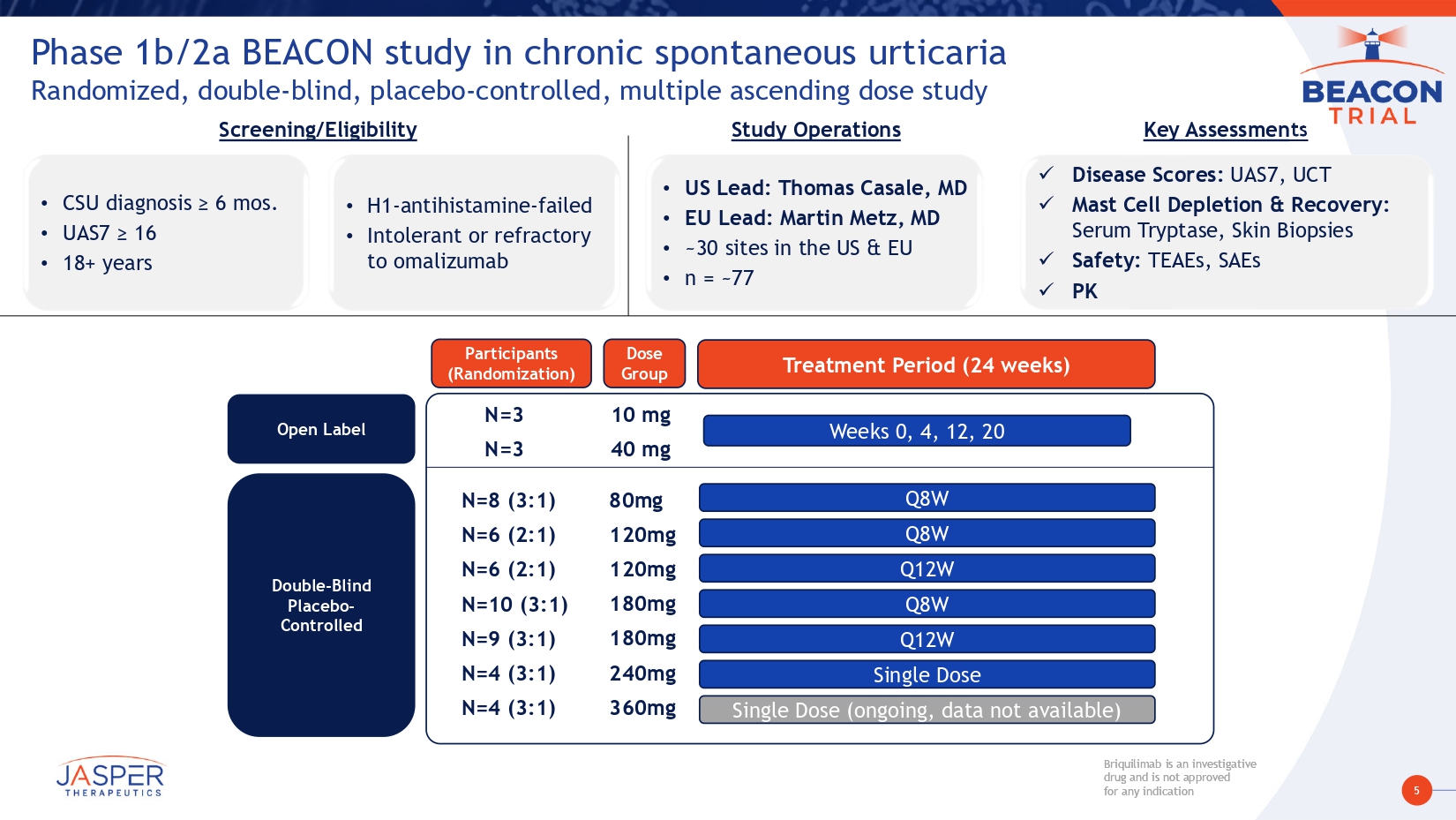
5 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication 80mg 120mg 120mg 180mg 180mg 240mg 360mg 10 mg 40 mg Open Label Double - Blind Placebo - Controlled Screening/Eligibility • CSU diagnosis ≥ 6 mos. • UAS7 ≥ 16 • 18+ years • H1 - antihistamine - failed • Intolerant or refractory to omalizumab Study Operations • US Lead: Thomas Casale , MD • EU Lead: Martin Metz, MD • ~30 sites in the US & EU • n = ~77 x Disease Scores: UAS7, UCT x Mast Cell Depletion & Recovery: Serum Tryptase, Skin Biopsies x Safety: TEAEs, SAEs x PK Key Assessments Weeks 0, 4, 12, 20 Phase 1b/2a BEACON study in chronic spontaneous urticaria Randomized, double - blind, placebo - controlled, multiple ascending dose study N=3 N=3 Participants ( Randomization ) N=8 (3:1) N=6 (2:1) N=6 (2:1) N=10 (3:1) N=9 (3:1) N=4 (3:1) N=4 (3:1) Treatment Period (24 weeks ) Dose Group Q8W Q8W Q8W Q12W Q12W Single Dose Single Dose (ongoing, data not available)
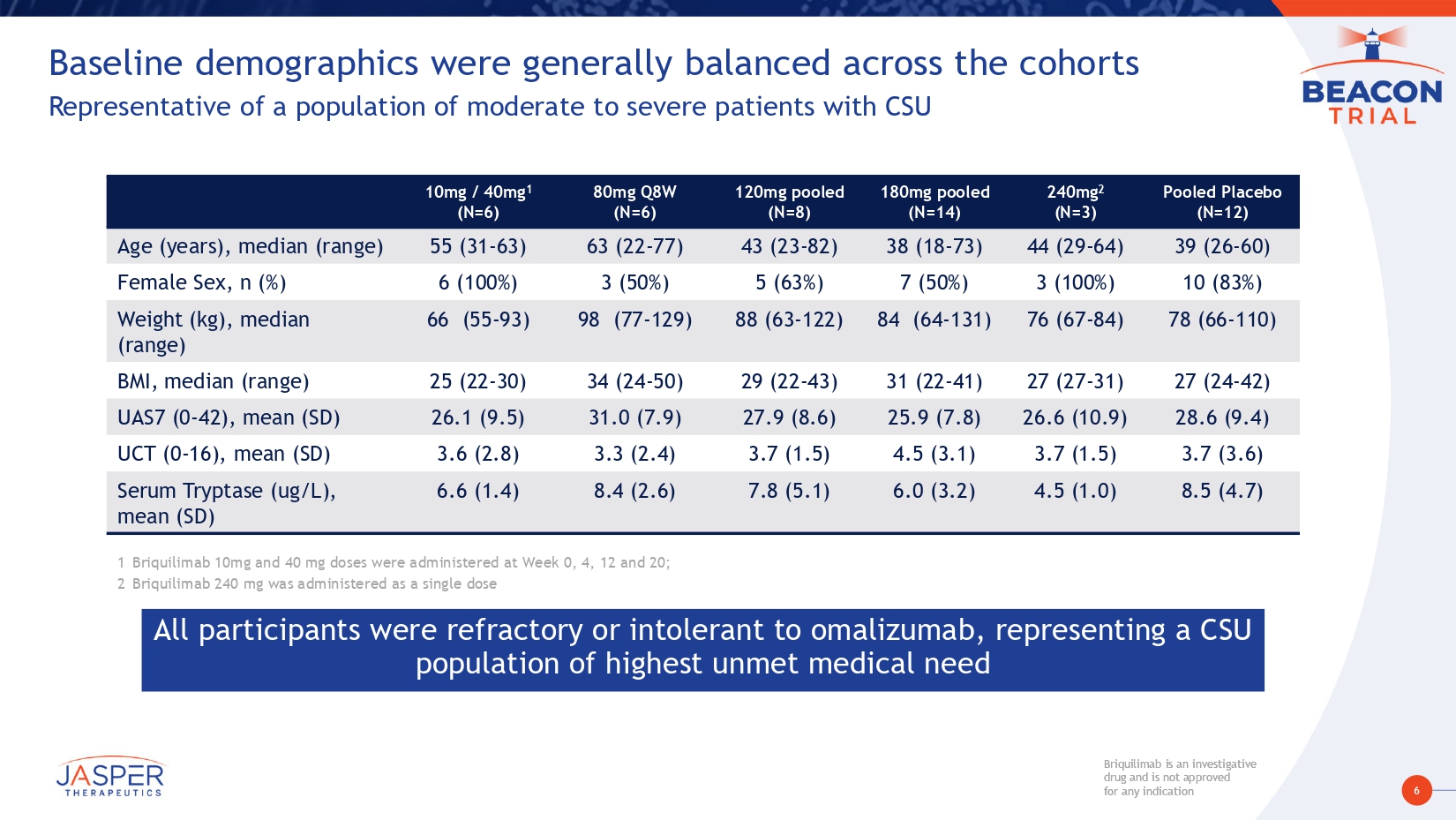
6 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Baseline demographics were generally balanced across the cohorts Representative of a population of moderate to severe patients with CSU Pooled Placebo (N=12) 240mg 2 (N=3) 180mg pooled (N=14) 120mg pooled (N=8) 80mg Q8W (N=6) 10mg / 40mg 1 (N=6) 39 (26 - 60) 44 (29 - 64) 38 (18 - 73) 43 (23 - 82) 63 (22 - 77) 55 (31 - 63) Age (years), median (range) 10 (83%) 3 (100%) 7 (50%) 5 (63%) 3 (50%) 6 (100%) Female Sex, n (%) 78 (66 - 110) 76 (67 - 84) 84 (64 - 131) 88 (63 - 122) 98 (77 - 129) 66 (55 - 93) Weight (kg), median (range) 27 (24 - 42) 27 (27 - 31) 31 (22 - 41) 29 (22 - 43) 34 (24 - 50) 25 (22 - 30) BMI, median (range) 28.6 (9.4) 26.6 (10.9) 25.9 (7.8) 27.9 (8.6) 31.0 (7.9) 26.1 (9.5) UAS7 (0 - 42), mean (SD) 3.7 (3.6) 3.7 (1.5) 4.5 (3.1) 3.7 (1.5) 3.3 (2.4) 3.6 (2.8) UCT (0 - 16), mean (SD) 8.5 (4.7) 4.5 (1.0) 6.0 (3.2) 7.8 (5.1) 8.4 (2.6) 6.6 (1.4) Serum Tryptase (ug/L), mean (SD) All participants were refractory or intolerant to omalizumab, representing a CSU population of highest unmet medical need 1 Briquilimab 10mg and 40 mg doses were administered at Week 0, 4, 12 and 20; 2 Briquilimab 240 mg was administered as a single dose
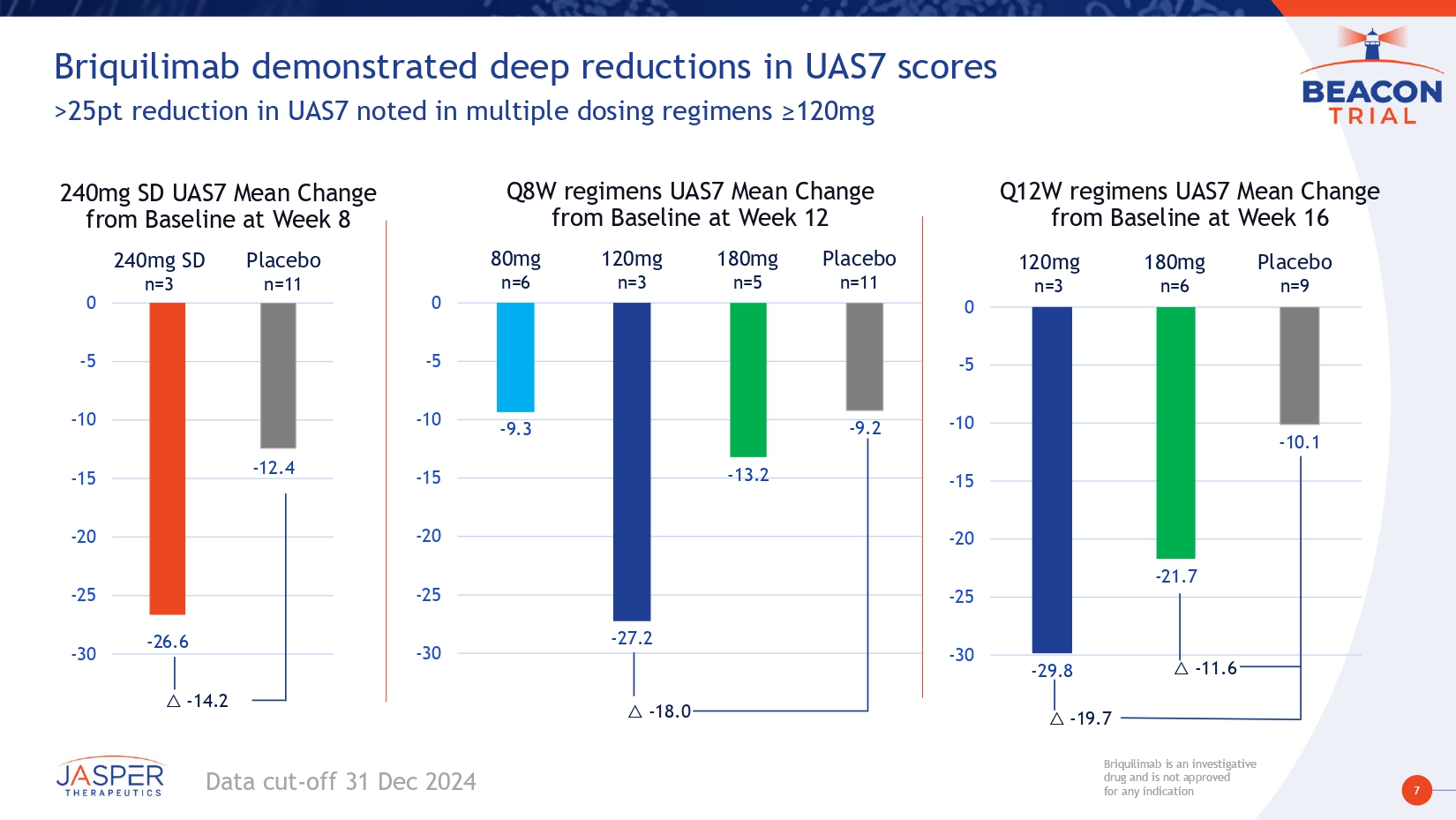
7 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Briquilimab demonstrated deep reductions in UAS7 scores >25pt reduction in UAS7 noted in multiple dosing regimens ≥120mg Q8W regimens UAS7 Mean Change from Baseline at Week 12 - 29.8 - 21.7 - 10.1 -30 -25 -20 -15 -10 -5 0 Q12W regimens UAS7 Mean Change from Baseline at Week 16 Placebo n=9 120mg n=3 180mg n=6 - 9.3 - 27.2 - 13.2 - 9.2 -30 -25 -20 -15 -10 -5 0 80mg n=6 Placebo n=11 120mg n=3 180mg n=5 △ - 18.0 △ - 19.7 △ - 11.6 240mg SD UAS7 Mean Change from Baseline at Week 8 - 26.6 - 12.4 -30 -25 -20 -15 -10 -5 0 Placebo n=11 240mg SD n=3 △ - 14.2 Data cut - off 31 Dec 2024
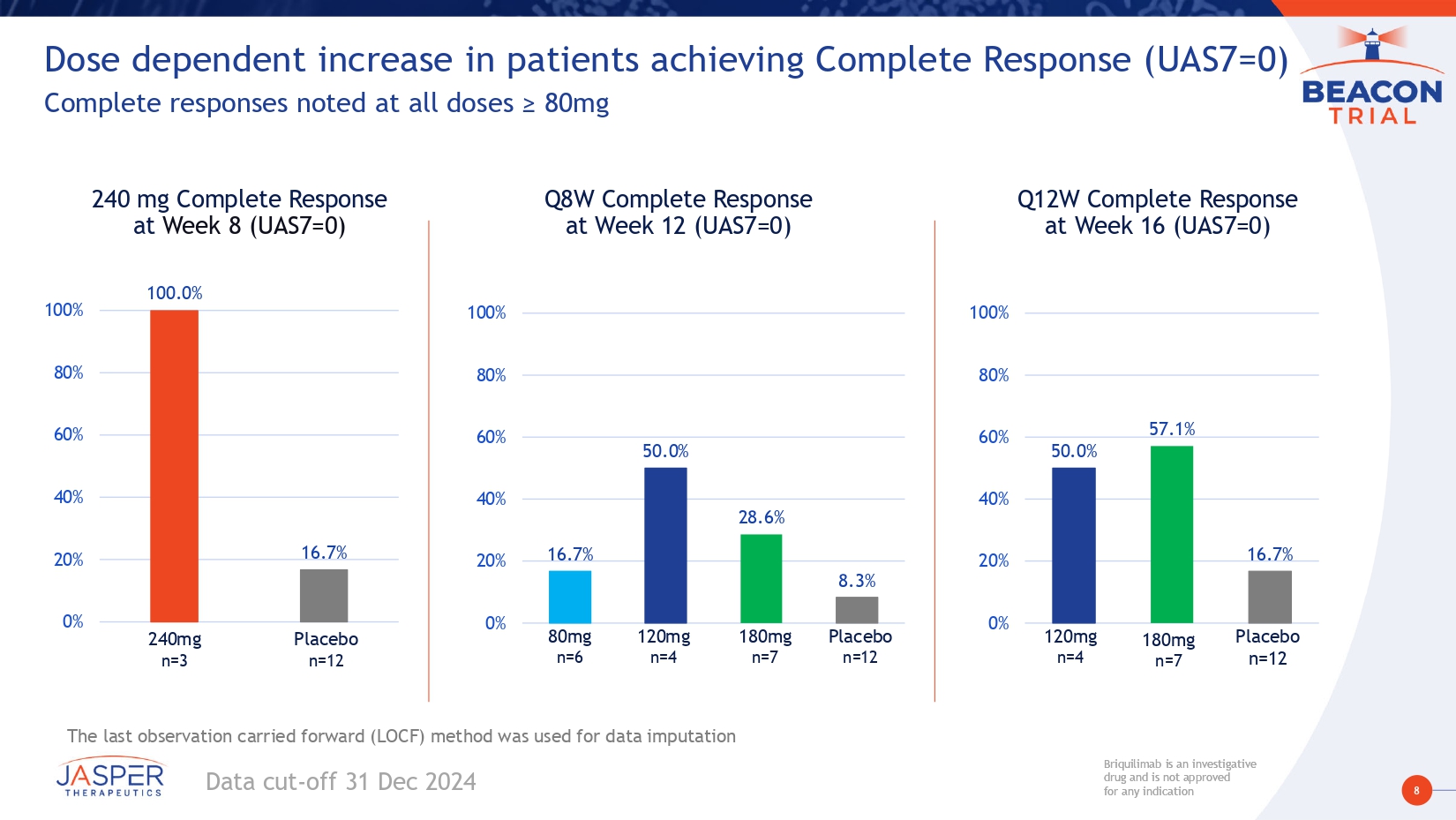
8 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Dose dependent increase in patients achieving Complete Response (UAS7=0) Complete responses noted at all doses ≥ 80mg Q8W Complete Response at Week 12 (UAS7=0) 16.7% 50.0% 28.6% 8.3% 0% 20% 40% 60% 80% 100% 80mg n =6 Placebo n =12 120mg n =4 180mg n =7 Q12W Complete Response at Week 16 (UAS7=0) 240 mg Complete Response at Week 8 (UAS7=0) 100.0% 16.7% 0% 20% 40% 60% 80% 100% Placebo n =12 240 mg n = 3 Data cut - off 31 Dec 2024 Placebo n=12 120mg n=4 180mg n=7 The last observation carried forward (LOCF) method was used for data imputation 50.0% 57.1% 16.7% 0% 20% 40% 60% 80% 100%
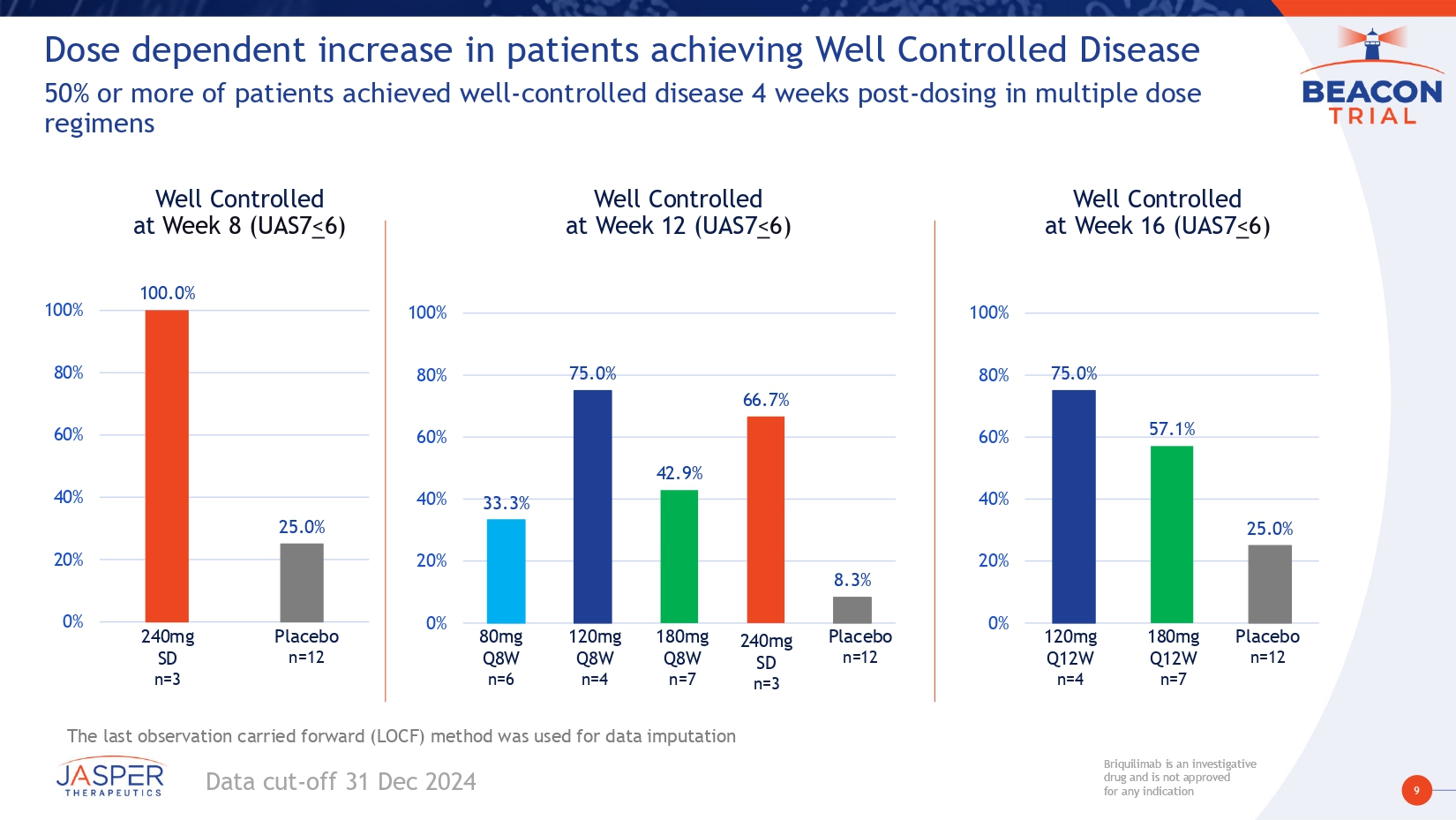
9 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Dose dependent increase in patients achieving Well Controlled Disease 50% or more of patients achieved well - controlled disease 4 weeks post - dosing in multiple dose regimens Well Controlled at Week 12 (UAS7 < 6) 33.3% 75.0% 42.9% 66.7% 8.3% 0% 20% 40% 60% 80% 100% 80mg Q8W n =6 Placebo n =12 120mg Q8W n =4 180mg Q8W n =7 Well Controlled at Week 16 (UAS7 < 6) Well Controlled at Week 8 (UAS7 < 6) Placebo n=12 120mg Q12W n=4 180mg Q12W n=7 The last observation carried forward (LOCF) method was used for data imputation 100.0% 25.0% 0% 20% 40% 60% 80% 100% Placebo n =12 240 mg SD n = 3 Data cut - off 31 Dec 2024 240 mg SD n =3 75.0% 57.1% 25.0% 0% 20% 40% 60% 80% 100%
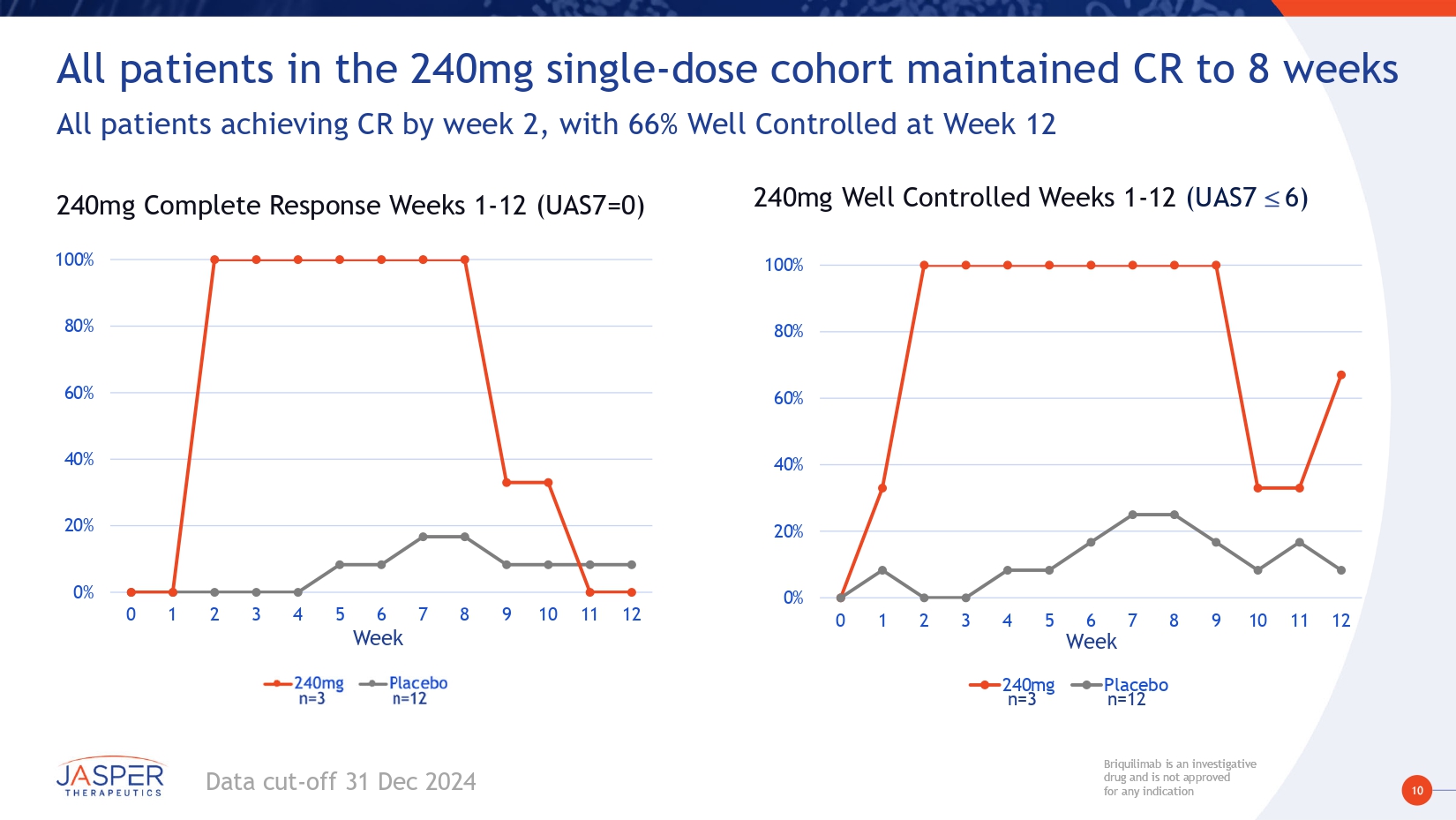
10 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication All patients in the 240mg single - dose cohort maintained CR to 8 weeks All patients achieving CR by week 2, with 66% Well Controlled at Week 12 0% 20% 40% 60% 80% 100% 0 1 2 3 4 5 6 7 8 9 10 11 12 Placebo 240mg Week 240mg Complete Response Weeks 1 - 12 (UAS7=0) 0% 20% 40% 60% 80% 100% 0 1 2 3 4 5 6 7 8 9 10 11 12 240mg Placebo Week 240mg Well Controlled Weeks 1 - 12 (UAS7 6) n=3 n=12 Data cut - off 31 Dec 2024

11 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Dose dependent UAS7 reductions observed over 26 weeks Deeper UAS7 reductions observed in subsequent doses 0 10 20 30 40 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 240mg SD 180mg Q8W 120mg Q8W Weeks Weekly UAS7 Score Placebo Data cut - off 31 Dec 2024
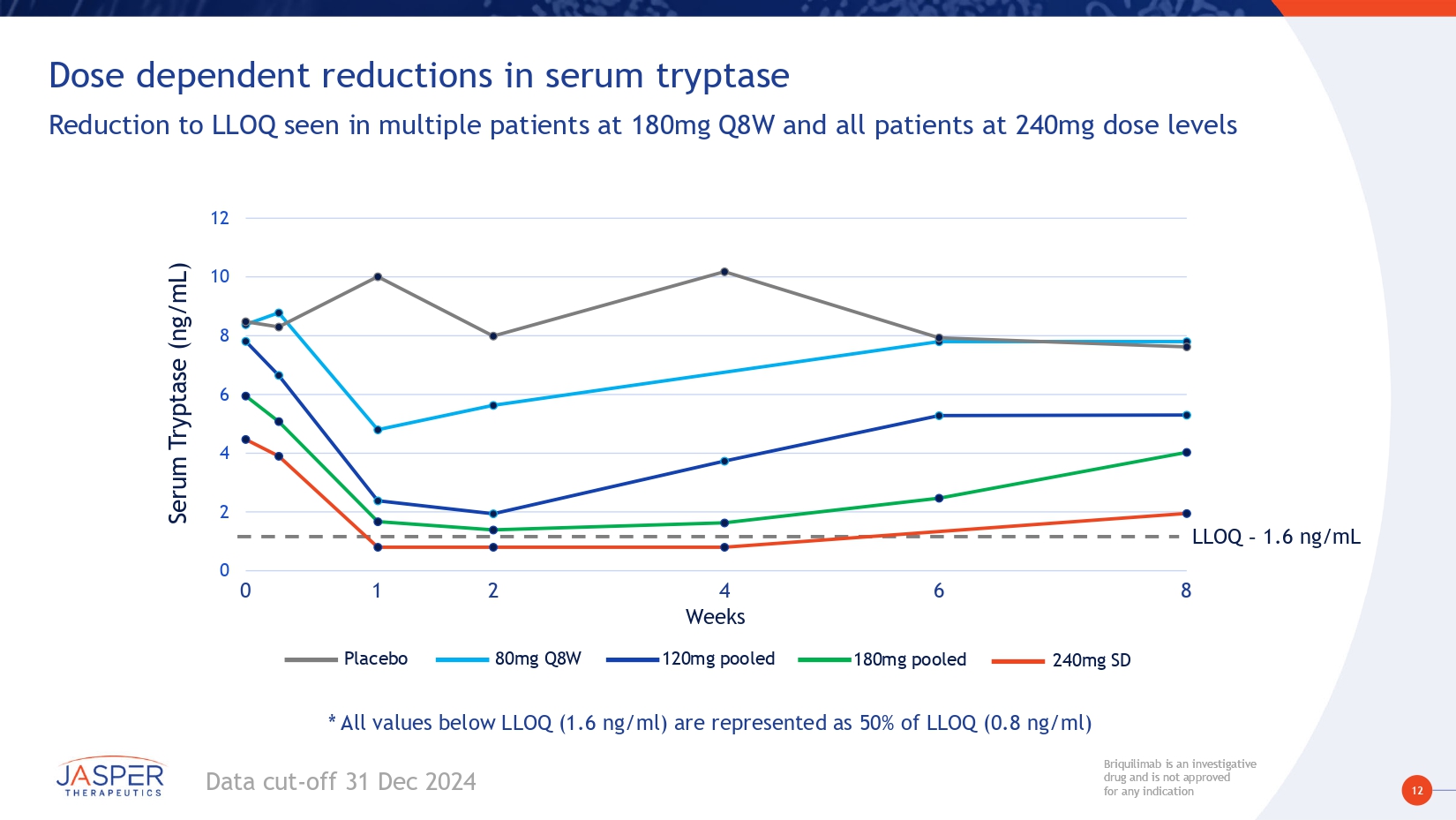
12 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Data cut - off 31 Dec 2024 Weeks Serum Tryptase ( n g/mL) 240mg SD 180mg pooled 120mg pooled Placebo 80mg Q8W LLOQ – 1.6 ng/mL 0 2 4 6 8 10 12 0 1 2 4 6 8 Dose dependent reductions in serum tryptase Reduction to LLOQ seen in multiple patients at 180mg Q8W and all patients at 240mg dose levels * All values below LLOQ (1.6 ng/ml) are represented as 50% of LLOQ (0.8 ng/ml)
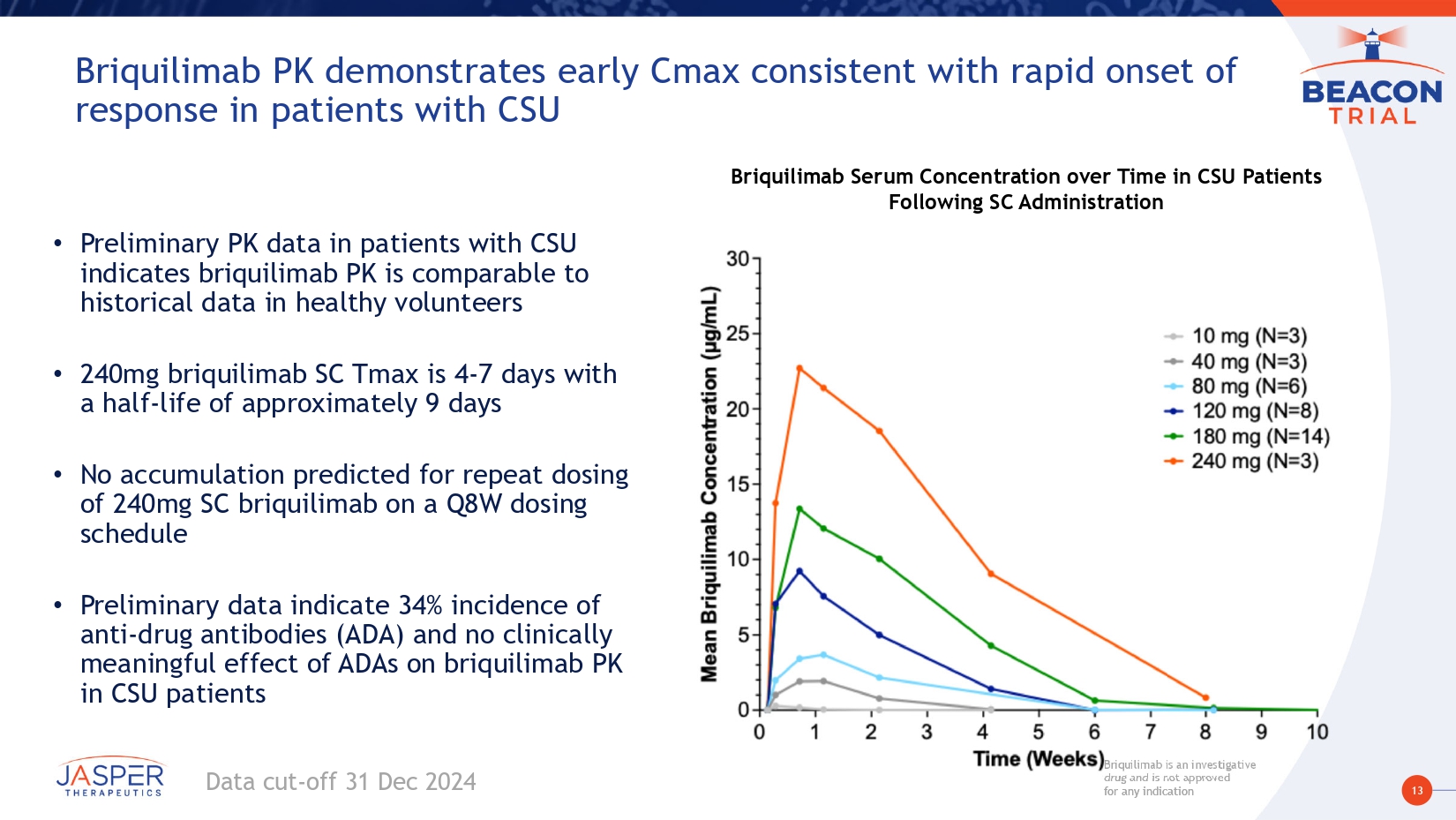
13 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Briquilimab PK demonstrates early Cmax consistent with rapid onset of response in patients with CSU • Preliminary PK data in patients with CSU indicates briquilimab PK is comparable to historical data in healthy volunteers • 240mg briquilimab SC Tmax is 4 - 7 days with a half - life of approximately 9 days • No accumulation predicted for repeat dosing of 240mg SC briquilimab on a Q8W dosing schedule • Preliminary data indicate 34% incidence of anti - drug antibodies (ADA) and no clinically meaningful effect of ADAs on briquilimab PK in CSU patients Briquilimab Serum Concentration over Time in CSU Patients Following SC Administration Data cut - off 31 Dec 2024
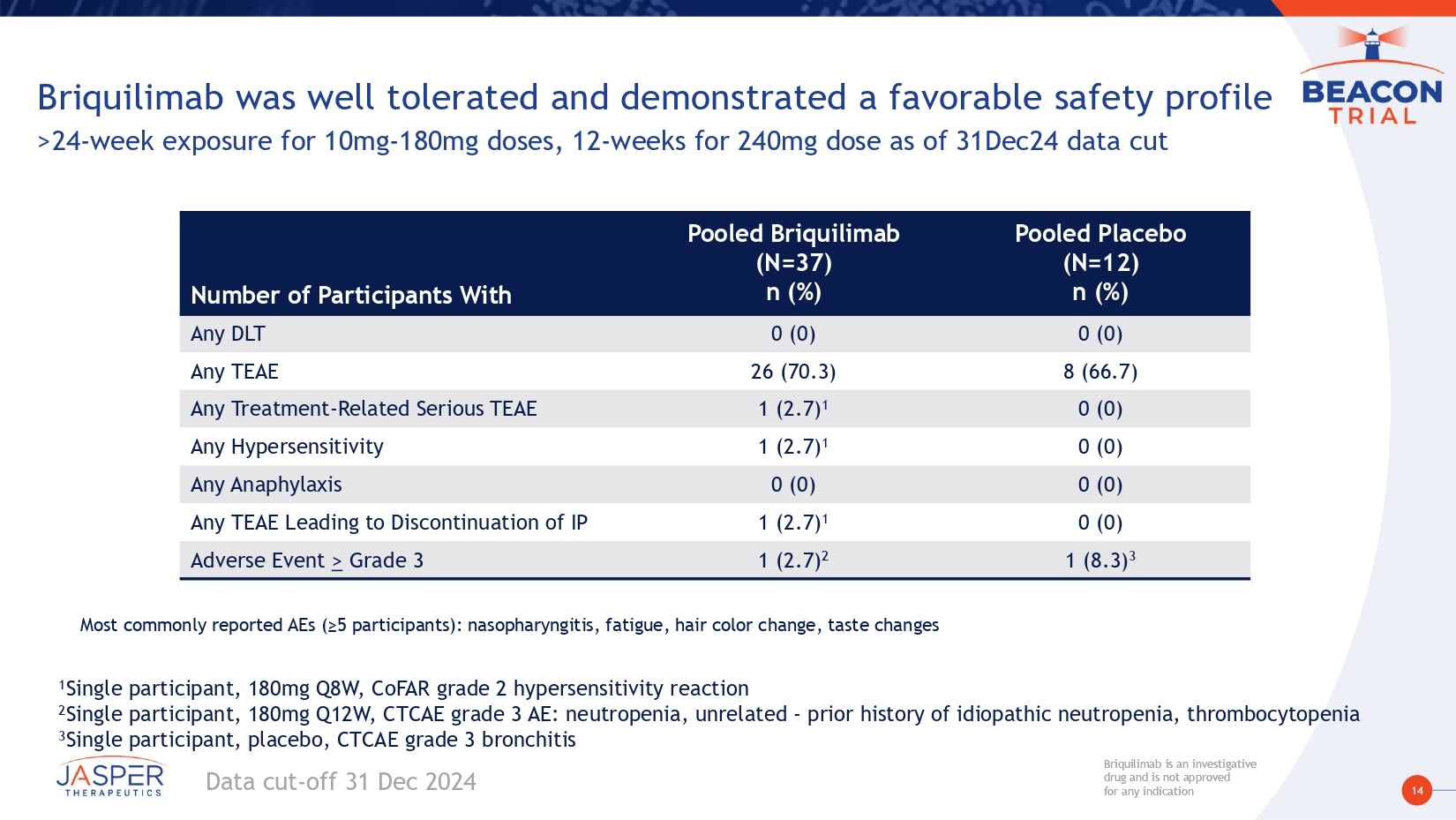
14 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Briquilimab was well tolerated and demonstrated a favorable safety profile >24 - week exposure for 10mg - 180mg doses, 12 - weeks for 240mg dose as of 31Dec24 data cut Pooled Placebo (N=12) n (%) Pooled Briquilimab (N=37) n (%) Number of Participants With 0 (0) 0 (0) Any DLT 8 (66.7) 26 (70.3) Any TEAE 0 (0) 1 (2.7) 1 Any Treatment - Related Serious TEAE 0 (0) 1 (2.7) 1 Any Hypersensitivity 0 (0) 0 (0) Any Anaphylaxis 0 (0) 1 (2.7) 1 Any TEAE Leading to Discontinuation of IP 1 (8.3) 3 1 (2.7) 2 Adverse Event > Grade 3 1 Single participant, 180mg Q8W, CoFAR grade 2 hypersensitivity reaction 2 Single participant, 180mg Q12W, CTCAE grade 3 AE: neutropenia, unrelated - prior history of idiopathic neutropenia, thrombocytopenia 3 Single participant , placebo, CTCAE grade 3 bronchitis Most commonly reported AEs (≥5 participants): nasopharyngitis, fatigue, hair color change, taste changes Data cut - off 31 Dec 2024
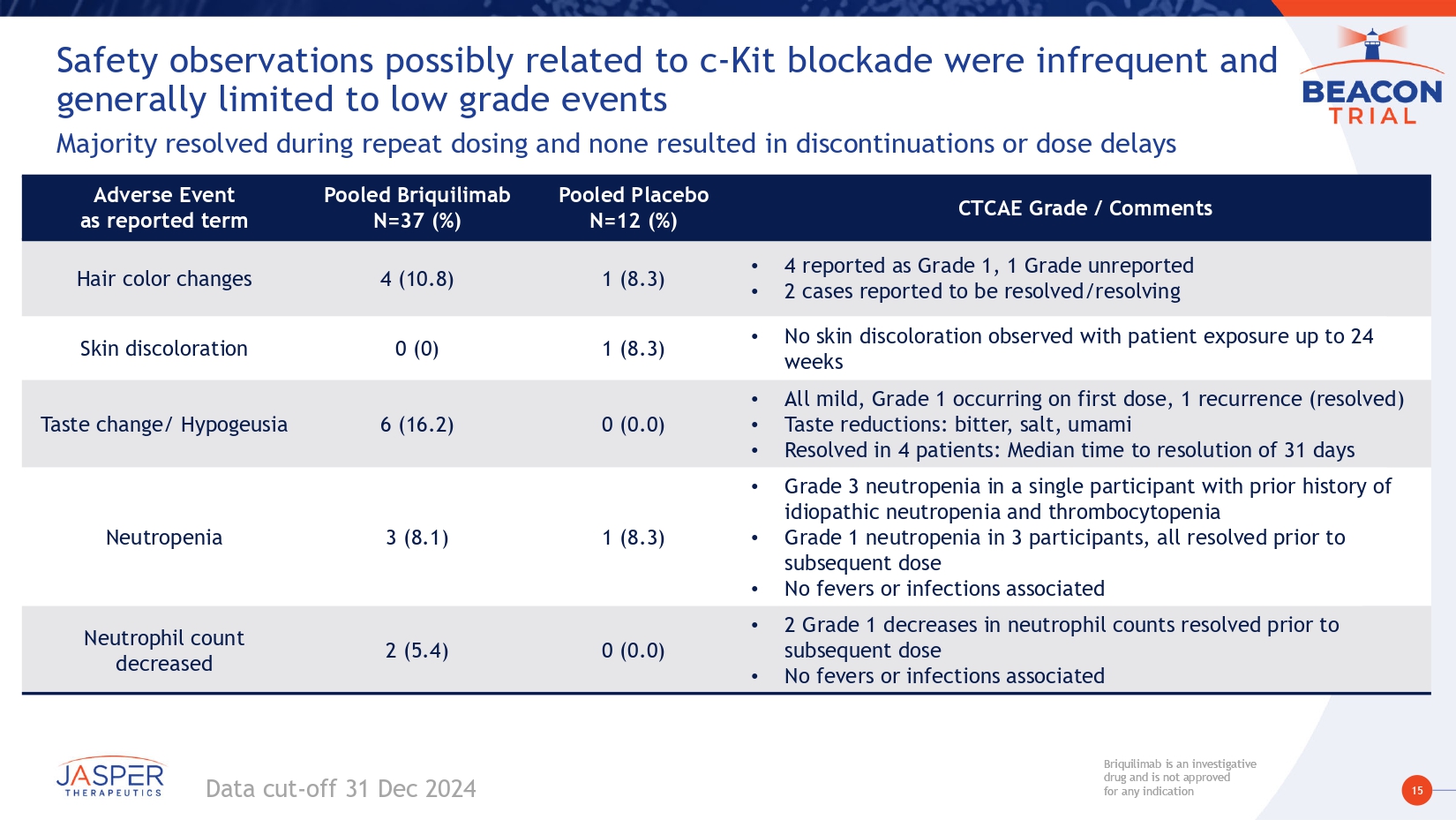
15 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Safety observations possibly related to c - Kit blockade were infrequent and generally limited to low grade events Majority resolved during repeat dosing and none resulted in discontinuations or dose delays Data cut - off 31 Dec 2024 CTCAE Grade / Comments Pooled Placebo N=12 (%) Pooled Briquilimab N=37 (%) Adverse Event as reported term • 4 reported as Grade 1, 1 Grade unreported • 2 cases reported to be resolved/resolving 1 (8.3) 4 (10.8) Hair color changes • No skin discoloration observed with patient exposure up to 24 weeks 1 (8.3) 0 (0) Skin discoloration • All mild, Grade 1 occurring on first dose, 1 recurrence (resolved) • Taste reductions: bitter, salt, umami • Resolved in 4 patients: Median time to resolution of 31 days 0 (0.0) 6 (16.2) Taste change/ Hypogeusia • Grade 3 neutropenia in a single participant with prior history of idiopathic neutropenia and thrombocytopenia • Grade 1 neutropenia in 3 participants, all resolved prior to subsequent dose • No fevers or infections associated 1 (8.3) 3 (8.1) Neutropenia • 2 Grade 1 decreases in neutrophil counts resolved prior to subsequent dose • No fevers or infections associated 0 (0.0) 2 (5.4) Neutrophil count decreased
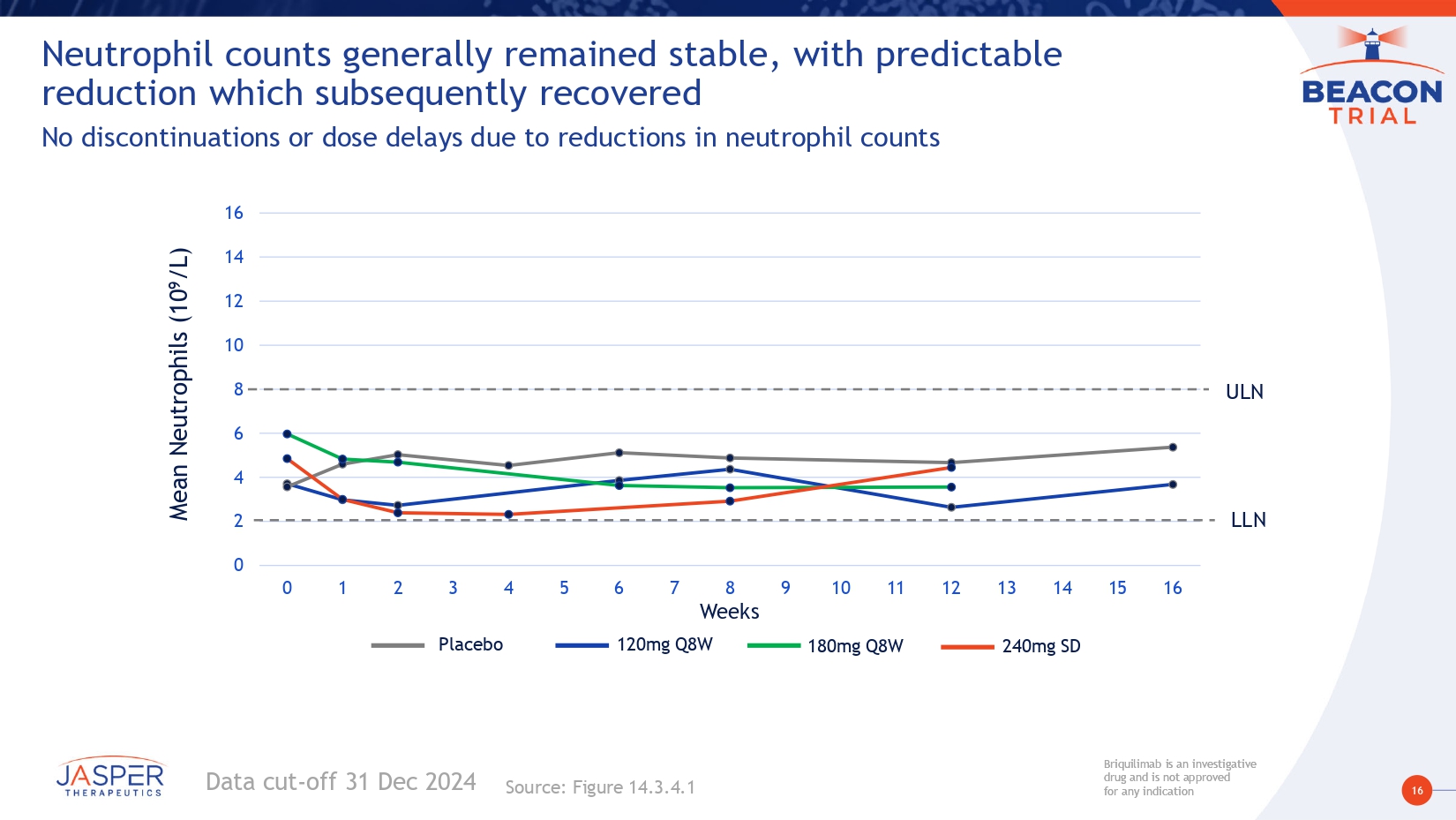
16 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication 0 2 4 6 8 10 12 14 16 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 240mg SD 180mg Q8W 120mg Q8W Weeks Mean Neutrophils (10 9 /L) Placebo Data cut - off 31 Dec 2024 Neutrophil counts generally remained stable, with predictable reduction which subsequently recovered No discontinuations or dose delays due to reductions in neutrophil counts Source: Figure 14.3.4.1 ULN LLN
17 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Preliminary BEACON study results demonstrate briquilimab achieved rapid, deep and durable responses in patients with moderate to severe CSU • Participants had moderate to severe CSU and were omalizumab experienced • Clinical responses in this hard - to - treat population are encouraging for a broader CSU population • Briquilimab demonstrated rapid and deep disease control • Rapid onset of effect – Clinical responses as early as 1 week post first dose • Deep dose - dependent response: – Multiple dosing regimens ≥120mg demonstrated UAS7 changes of more than - 25 points – Deepest responses shown of - 29 on the UAS7 scale • Complete and durable control demonstrated – Complete responses seen at all dose cohorts ≥ 80mg – 100% complete control with 240mg by week 2, durable to 8 weeks – Repeat dosing shows deepening clinical responses across multiple dose cohorts • Rapid, dose - dependent tryptase reductions correlated with early onset of clinical response • Reduction to LLOQ observed in multiple dose cohorts
18 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Efficacy, safety and PK informs an optimal biologic dose selection for briquilimab CSU registrational program expected to be initiated in 2H 2025 • Briquilimab was well tolerated and had a favorable safety profile in the study • Low and comparable frequency of Grade 1 hair color changes – in both active and placebo • Mild taste changes observed on first dose with majority resolving by a median of 31 days • No dose delays or discontinuations due to neutrophil reductions and no association with infection – Neutrophil recovery between doses • Single discontinuation due to AE • Registrational program in CSU expected to commence second half of 2025 • Deep and durable efficacy to 240mg dose, combined with the favorable safety profile observed support advancing into Phase 2b portion of a registrational program • Ongoing trials continue to generate clinical data to support registrational program • Expansion of 240mg and 360mg single dose cohorts ongoing • Additional cohorts of 240mg Q8W and 240mg induction dose followed by 180mg Q8W • Additional 180mg Q8W data from BEACON and SPOTLIGHT Open Label Extension study • These data will further inform final dose selection for the Phase 2b portion of our registrational program

19 Briquilimab is an investigative drug and is not approved for any indication 19 Briquilimab for Chronic Urticaria Thomas B Casale, MD Prof of Medicine and Pediatrics, University of South Florida
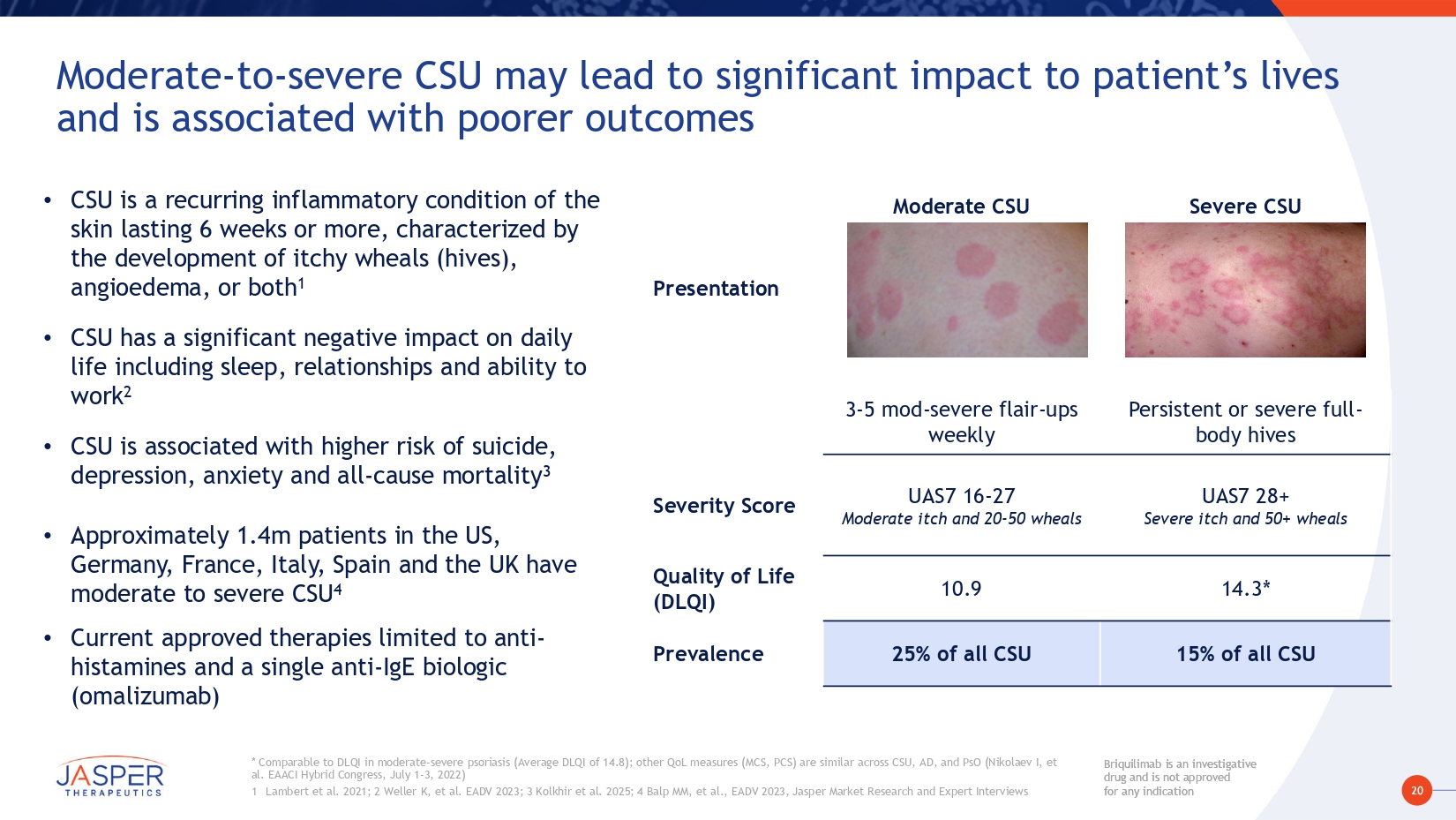
20 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Severe CSU Moderate CSU Presentation Persistent or severe full - body hives 3 - 5 mod - severe flair - ups weekly UAS7 28+ Severe itch and 50+ wheals UAS7 16 - 27 Moderate itch and 20 - 50 wheals Severity Score 14.3* 10.9 Quality of Life (DLQI) 15% of all CSU 25% of all CSU Prevalence Moderate - to - severe CSU may lead to significant impact to patient’s lives and is associated with poorer outcomes * Comparable to DLQI in moderate - severe psoriasis (Average DLQI of 14.8); other QoL measures (MCS, PCS) are similar across CSU, AD, and PsO (Nikolaev I, et al. EAACI Hybrid Congress, July 1 - 3, 2022) 1 Lambert et al. 2021; 2 Weller K, et al. EADV 2023; 3 Kolkhir et al. 2025; 4 Balp MM, et al., EADV 2023, Jasper Market Research and Expert Interviews • CSU is a recurring inflammatory condition of the skin lasting 6 weeks or more, characterized by the development of itchy wheals (hives), angioedema, or both 1 • CSU has a significant negative impact on daily life including sleep, relationships and ability to work 2 • CSU is associated with higher risk of suicide, depression, anxiety and all - cause mortality 3 • Approximately 1.4m patients in the US, Germany, France, Italy, Spain and the UK have moderate to severe CSU 4 • Current approved therapies limited to anti - histamines and a single anti - IgE biologic (omalizumab)
21 Briquilimab is an investigative drug and is not approved for any indication Preliminary BEACON data in CSU show that briquilimab leads to deep and durable efficacy with a favorable safety profile 1 Moller C et al. Blood (2005) 2 Hundley TR et al. Blood (2004) 3. Arnold JN et al. Annu Rev Immunol (2007) • Mast cell degranulation is the central pathogenic driver of chronic urticarias • Briquilimab blocks c - Kit signaling and may lead to depletion of mast cells • Preliminary BEACON efficacy results show multiple regimens associated with clinically meaningful declines in UAS7 • Preliminary efficacy results show rapid onset of action, with the 240mg dose showing high 100% CR, durable to 8 weeks • Preliminary BEACON safety results show low incidence of manageable AEs • Based on these data, briquilimab appears to a promising new therapy for moderate to severe CSU patients • Advancement to registrational trials is warranted

22 Briquilimab is an investigative drug and is not approved for any indication 22 Upcoming Milestones and Closing Remarks Ronald Martell
23 Briquilimab is an investigative drug and is not approved for any indication • Briquilimab demonstrated rapid onset of action with disease control observed as early as one week after initial dose • Complete responses were observed at doses as low as 80mg Q8W and 240mg dose demonstrated 100% Complete Responses with durability out to 8 weeks • Serum tryptase reductions below the lower limit of quantification were observed in multiple dose cohorts • Favorable safety profile observed in preliminary data support the potential of optimal biologic dosing o On target adverse events seen were low - grade and at low incidence rates o Many of the possible on - target AEs resolved between doses • Additional BEACON cohorts and the open label extension study will further inform final dose selection for Phase 2b study planned to commence 2H 2025 Positive preliminary BEACON study results support commencing briquilimab registrational program in CSU in 2H 2025

24 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Briquilimab has the potential to be a major immunology franchise by delivering control to millions of patients with mast - cell driven diseases • Eosinophilic Esophagitis • IBD • Food Allergies • Asthma • COPD • Chronic Rhinosinusitis with Nasal Polyps • Chronic Spontaneous Urticaria • Chronic Inducible Urticaria 2 million 20 million 30+ million Moderate - to - Severe Disease (US/EU 1,2 ) Chronic Atopic and Mast Cell Driven Diseases • Prurigo Nodularis • Atopic Dermatitis 1 EvaluatePharma ; 2 Databridge Market Research (allergic rhinitis)
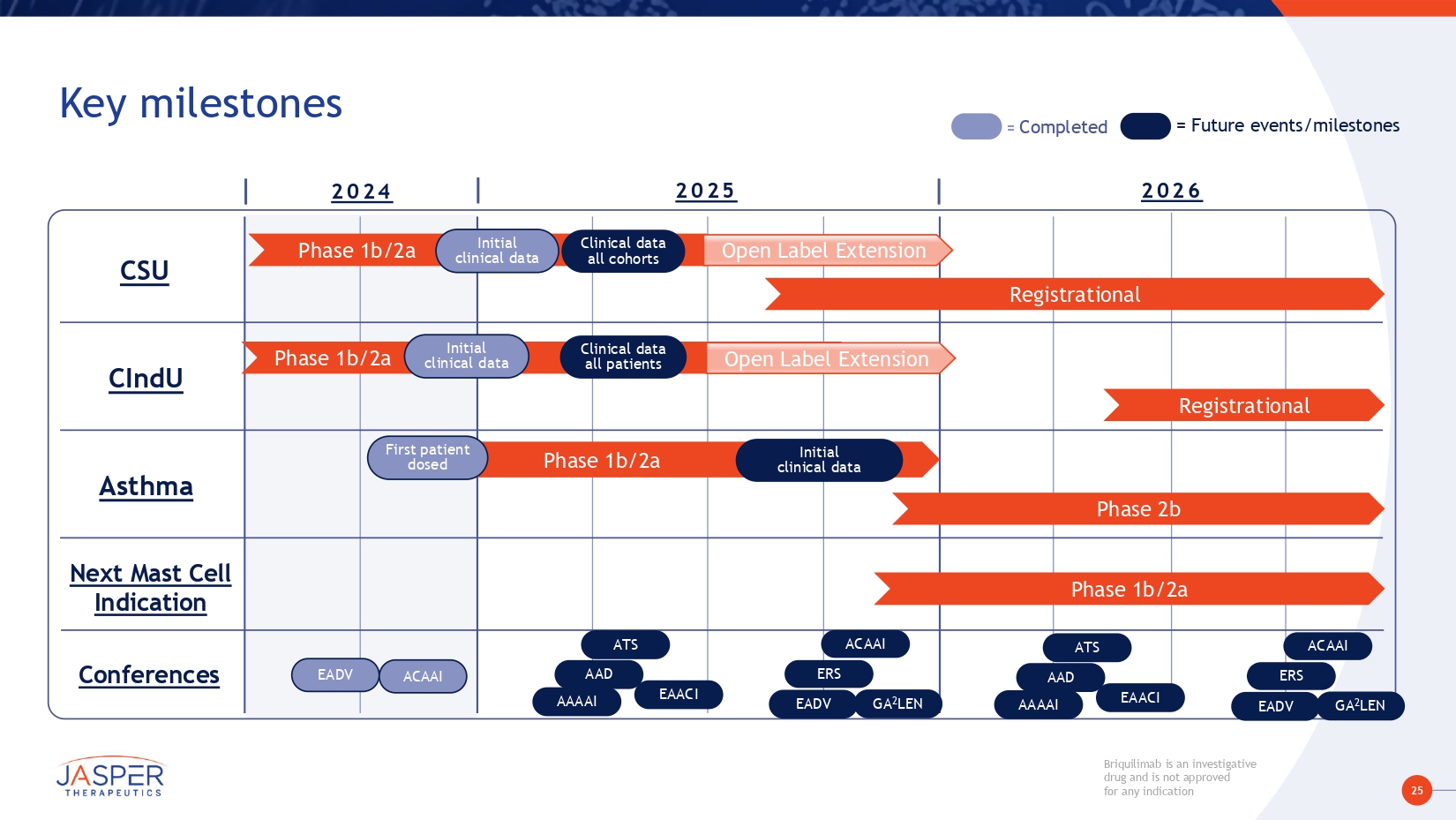
25 Briquilimab is an investigative drug and is not approved for any indication Key milestones 2024 CSU CIndU Asthma Conferences PHASE 1B/2A = Completed = Future events/milestones 2025 2026 Registrational Phase 1b/2a a. Initial clinical data Clinical data all cohorts Open Label Extension Phase 1b/2a Phase 1b/2aa. Open Label Extension Clinical data all patients Registrational Next Mast Cell Indication Initial clinical data Phase 1b/2a ase 1b/2aa. First patient dosed Initial clinical data Phase 2b Phase 1b/2a EADV ACAAI AAAAI EAACI EADV GA 2 LEN ATS ACAAI AAD AAAAI EAACI ATS AAD ERS EADV GA 2 LEN ACAAI ERS

26 Briquilimab is an investigative drug and is not approved for any indication Briquilimab is an investigative drug and is not approved for any indication Q&A

27 Briquilimab is an investigative drug and is not approved for any indication Jasper Therapeutics: Preliminary BEACON Results NASDAQ: JSPR January 8 th , 2025


























