CORPORATE OVERVIEW April 2024

Important Notice and Disclaimers This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “target,” “seek,” “predict,” “potential,” “continue” or the negative of these terms or other comparable terminology. Forward-looking statements in this presentation include, but are not limited to, statements about: the commercial success, cost of development, and timing of the approval of our clinical-stage product candidates; the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or clinical trials and related preparatory work, and the period during which the results of the trials will become available; our ability to submit, and obtain clearance of, any investigational new drug applications on our expected timelines, or at all; our ability to initiate, recruit, and enroll patients in and conduct our clinical trials at the pace that we project; our ability to obtain and maintain regulatory approval of our product candidates, and any related restrictions, limitations, or warnings in the label of any of our product candidates, if approved; our ability to compete with companies currently marketing therapies or developing product candidates with targets or indications similar to our product candidates’ targets or indications; our reliance on third parties to conduct our clinical trials and to manufacture drug substance and drug product for use in our clinical trials; the size and growth potential of the markets for any of our current and future product candidates, and our ability to serve those markets; our ability to identify and advance through clinical development any additional product candidates; the commercialization of our current and future product candidates, if approved, including our ability to successfully build a specialty sales force and commercial infrastructure to market our current and future product candidates; our ability to identify research priorities and apply a risk-mitigated strategy to efficiently discover and develop current and future product candidates; our ability to retain and recruit key personnel; our ability to obtain and maintain adequate intellectual property rights; our expectations regarding government and third-party payor coverage, pricing, and reimbursement; our estimates of our expenses, ongoing losses, capital requirements, the sufficiency of our current resources, and our needs for or ability to obtain additional financing; the milestone payments that we may receive from Taiho Pharmaceutical Co., Ltd.; potential investments in our pipeline and the potential for such product candidates; the potential benefits of strategic collaboration agreements, our ability to enter into additional strategic collaborations or arrangements, and our ability to attract collaborators with development, regulatory, and commercialization expertise; and developments and projections relating to our competitors or our industry. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Any forward-looking statements in this presentation are based on management's current expectations and beliefs of future events and are subject to known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any expressed or implied by the forward-looking statements. These risks include, but are not limited to, the following: uncertainty regarding the timing and results of regulatory submissions, including the investigational new drug application that we intend to file for CLN-978; success of our clinical trials and preclinical studies; risks related to our ability to protect and maintain our intellectual property position; risks related to manufacturing, supply, and distribution of our product candidates; the risk that any one or more of our product candidates, including those that are co-developed, will not be successfully developed and commercialized; the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies; success of any collaboration, partnership, license or similar agreements; and other important risks and uncertainties discussed in our filings with the Securities and Exchange Commission, including under the caption “Risk Factors” in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other filings that we make with the SEC from time to time. These risks could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change, except to the extent required by law. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither Cullinan nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements included in this presentation. Any forward-looking statement included in this presentation speaks only as of the date on which it was made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

Our Mission To create new standards of care for patients Pursuing modality-agnostic targeted therapeutics: Discovering and identifying high-impact targets Then applying the best approach to address each target Rigorously and rapidly advancing only highly differentiated molecules Yielding a diversified, robust portfolio of clinical-stage programs © CULLINAN THERAPEUTICS, INC. ALL RIGHTS RESERVED.

Our Approach We seek to achieve our mission through our rigorous �and differentiated approach to drug development Innovate without�borders Remain open to finding the best solutions in-house or through licensing Run early “thriller or killer” experiments Rapidly advance only potential first-in-class and/or best-in-class molecules Seek clear evidence of monotherapy activity Avoid uncertainty of early-stage�clinical combination studies Our Vision: �To become a commercial-stage biotech company creating new standards of care for patients © CULLINAN THERAPEUTICS, INC. ALL RIGHTS RESERVED.
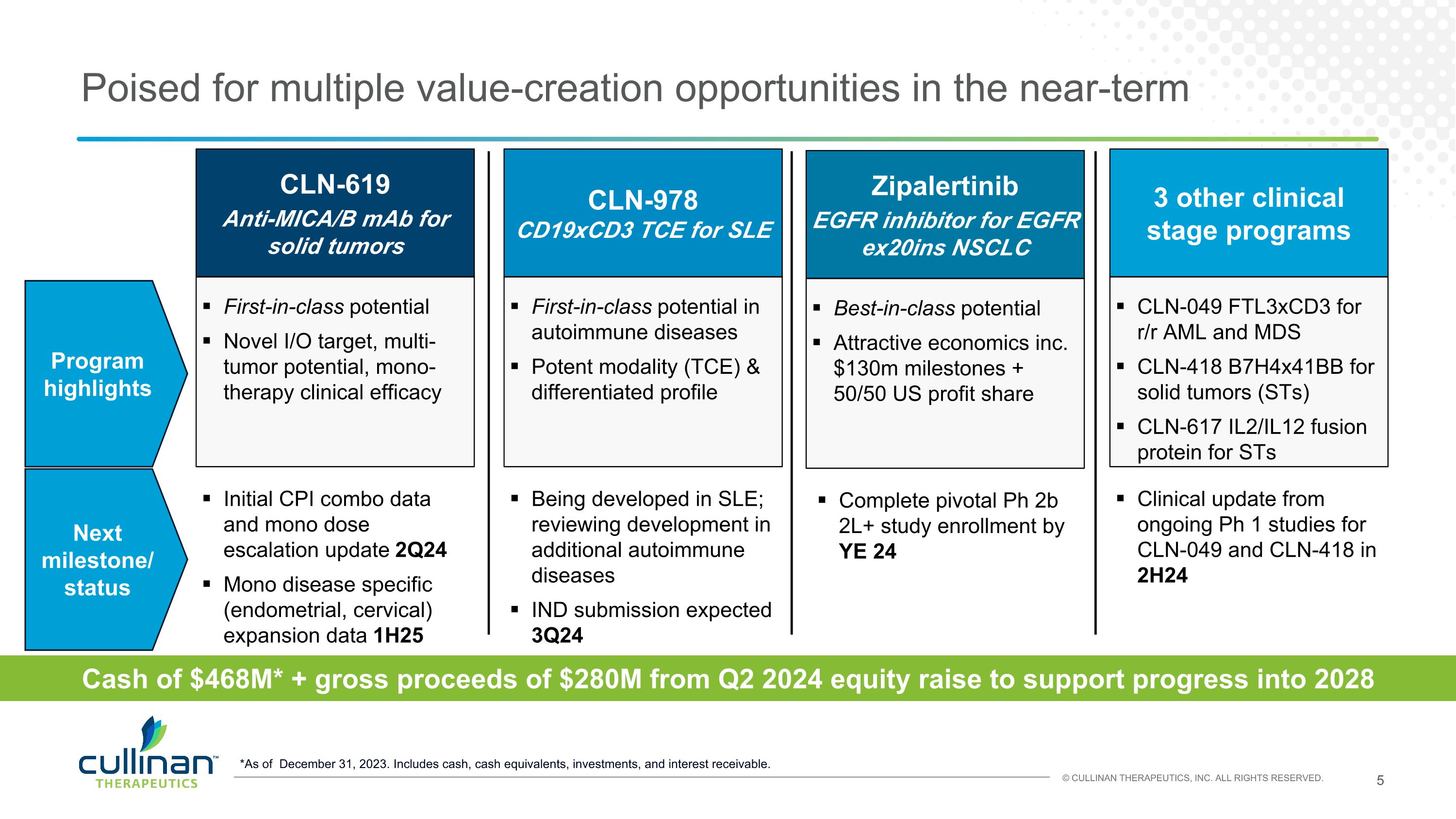
Cash of $468M* + gross proceeds of $280M from Q2 2024 equity raise to support progress into 2028 *As of December 31, 2023. Includes cash, cash equivalents, investments, and interest receivable. Poised for multiple value-creation opportunities in the near-term CLN-619 Anti-MICA/B mAb for solid tumors First-in-class potential Novel I/O target, multi-tumor potential, mono-therapy clinical efficacy Initial CPI combo data and mono dose escalation update 2Q24 Mono disease specific (endometrial, cervical) expansion data 1H25 Next milestone/ status Zipalertinib EGFR inhibitor for EGFR ex20ins NSCLC Best-in-class potential Attractive economics inc. $130m milestones + 50/50 US profit share Complete pivotal Ph 2b 2L+ study enrollment by YE 24 CLN-978 CD19xCD3 TCE for SLE First-in-class potential in autoimmune diseases Potent modality (TCE) & differentiated profile Being developed in SLE; reviewing development in additional autoimmune diseases IND submission expected 3Q24 3 other clinical stage programs CLN-049 FTL3xCD3 for r/r AML and MDS CLN-418 B7H4x41BB for solid tumors (STs) CLN-617 IL2/IL12 fusion protein for STs Clinical update from ongoing Ph 1 studies for CLN-049 and CLN-418 in 2H24 Program highlights

CLN-619�MICA/B-directed mAb
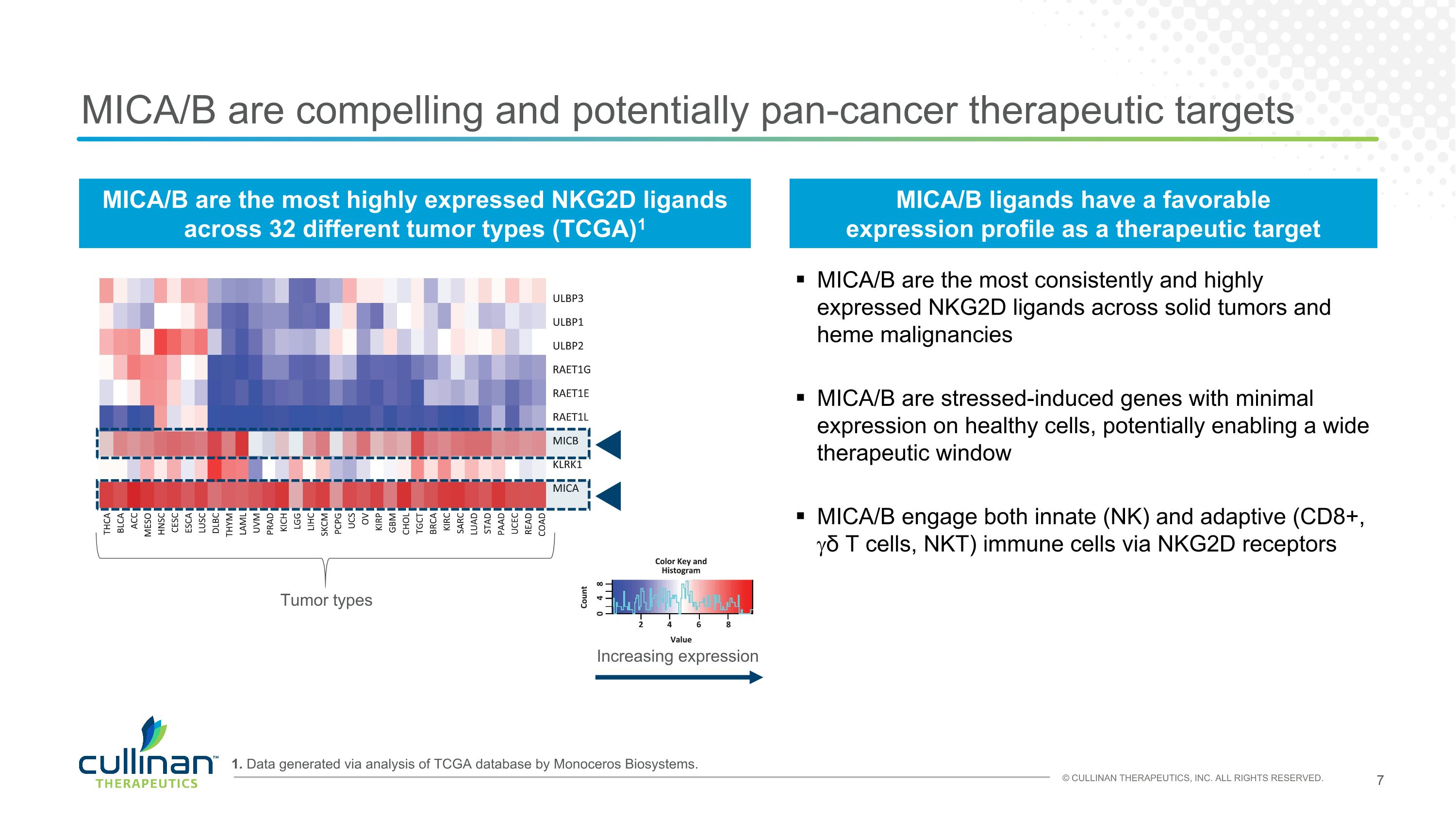
MICA/B are compelling and potentially pan-cancer therapeutic targets 1. Data generated via analysis of TCGA database by Monoceros Biosystems. MICA/B are the most highly expressed NKG2D ligands�across 32 different tumor types (TCGA)1 Increasing expression Tumor types MICA/B are the most consistently and highly expressed NKG2D ligands across solid tumors and heme malignancies MICA/B are stressed-induced genes with minimal expression on healthy cells, potentially enabling a wide therapeutic window MICA/B engage both innate (NK) and adaptive (CD8+, gδ T cells, NKT) immune cells via NKG2D receptors MICA/B ligands have a favorable expression profile as a therapeutic target
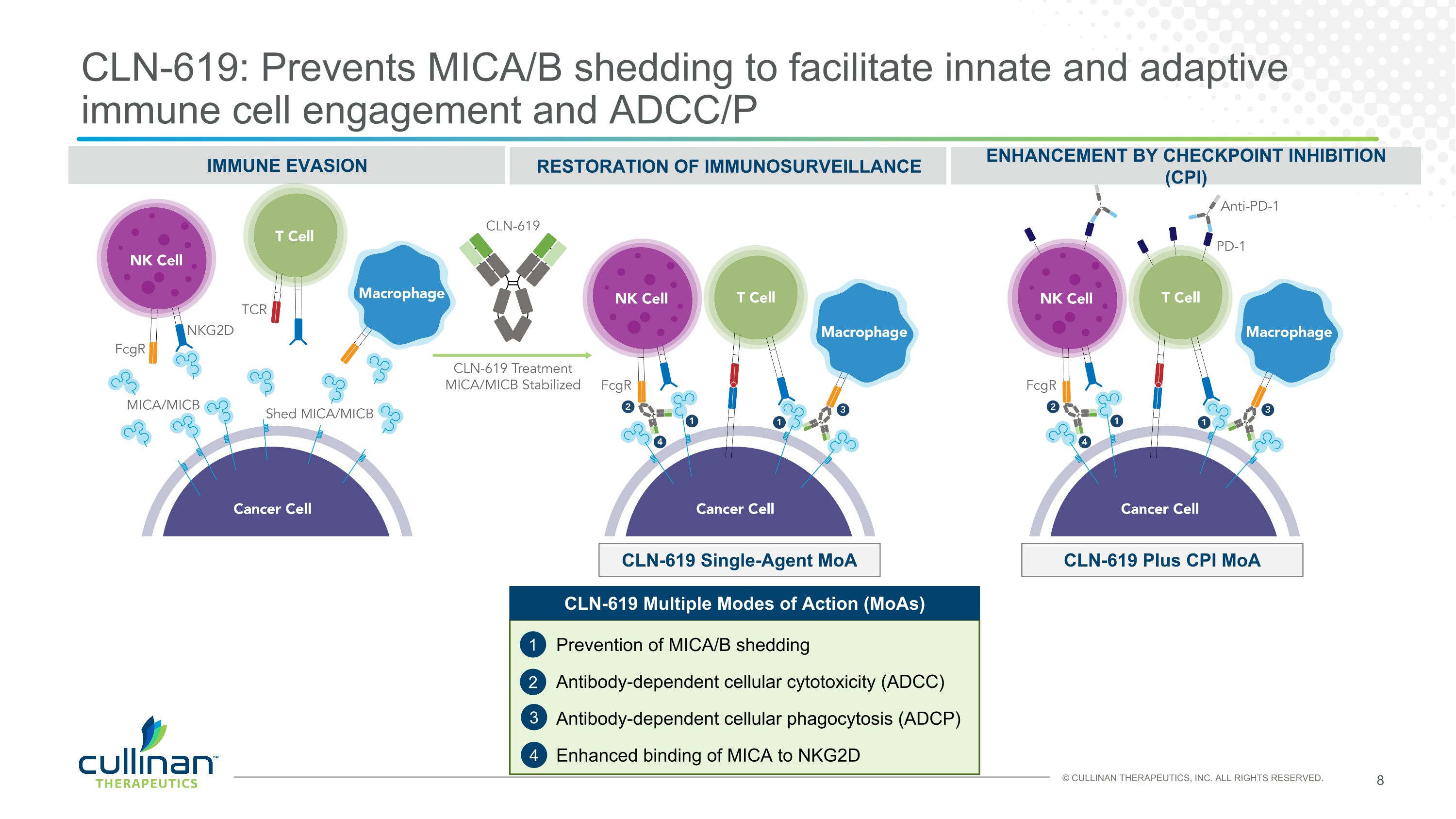
CLN-619: Prevents MICA/B shedding to facilitate innate and adaptive immune cell engagement and ADCC/P IMMUNE EVASION RESTORATION OF IMMUNOSURVEILLANCE Prevention of MICA/B shedding 1 2 Antibody-dependent cellular phagocytosis (ADCP) 3 4 ENHANCEMENT BY CHECKPOINT INHIBITION (CPI) CLN-619 Single-Agent MoA CLN-619 Plus CPI MoA CLN-619 Multiple Modes of Action (MoAs) Enhanced binding of MICA to NKG2D Antibody-dependent cellular cytotoxicity (ADCC)
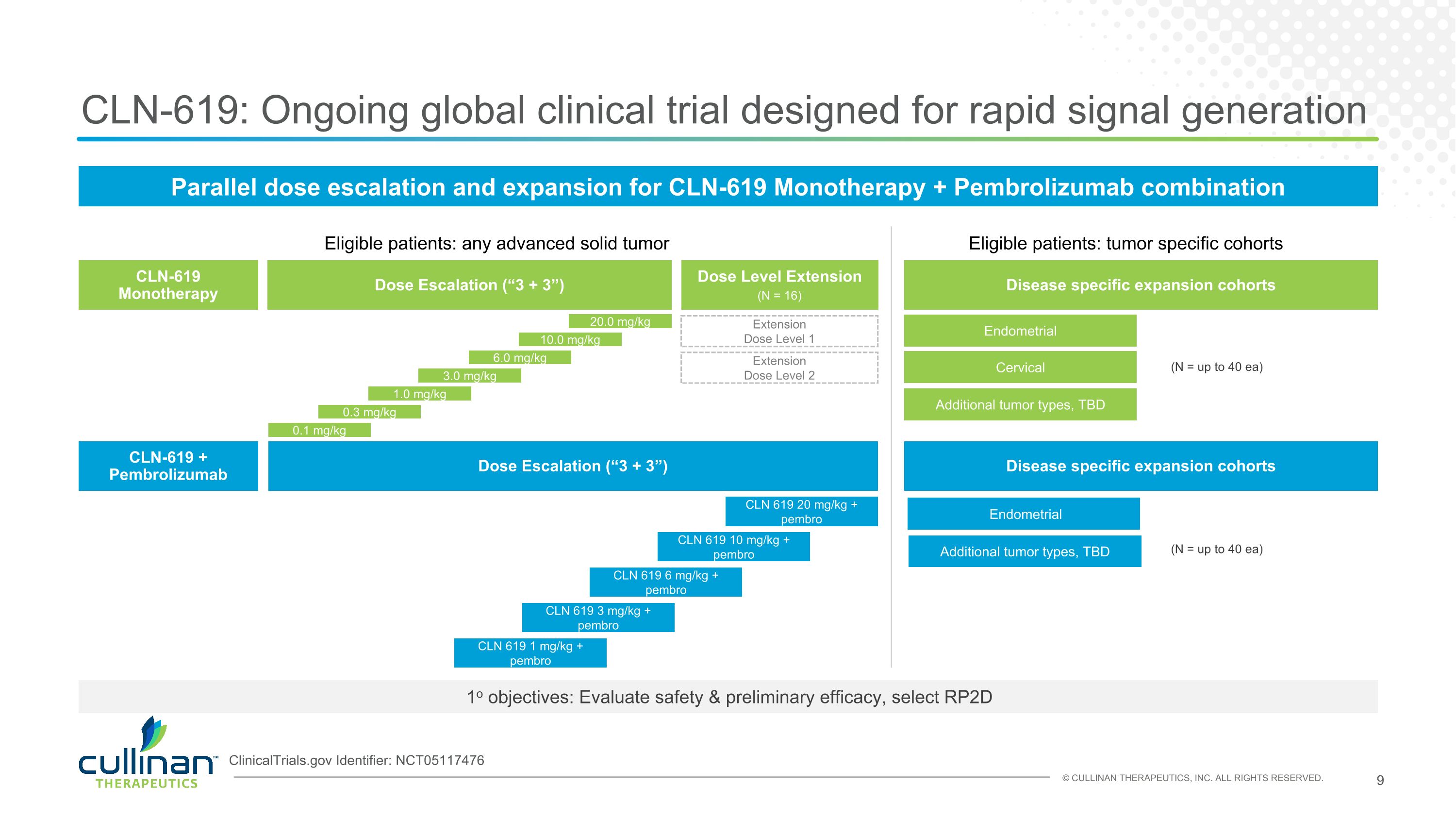
CLN-619: Ongoing global clinical trial designed for rapid signal generation Dose Escalation (“3 + 3”) 0.1 mg/kg Disease specific expansion cohorts Endometrial 1o objectives: Evaluate safety & preliminary efficacy, select RP2D CLN-619 Monotherapy Eligible patients: any advanced solid tumor Eligible patients: tumor specific cohorts Extension Dose Level 1 Dose Level Extension (N = up to 40 ea) Dose Escalation (“3 + 3”) CLN-619 + Pembrolizumab Disease specific expansion cohorts CLN 619 1 mg/kg + pembro 0.3 mg/kg 1.0 mg/kg 3.0 mg/kg 6.0 mg/kg 10.0 mg/kg Extension Dose Level 2 CLN 619 3 mg/kg + pembro CLN 619 6 mg/kg + pembro CLN 619 10 mg/kg + pembro Cervical (N = up to 40 ea) CLN 619 20 mg/kg + pembro 20.0 mg/kg ClinicalTrials.gov Identifier: NCT05117476 Parallel dose escalation and expansion for CLN-619 Monotherapy + Pembrolizumab combination (N = 16) Additional tumor types, TBD Additional tumor types, TBD Endometrial
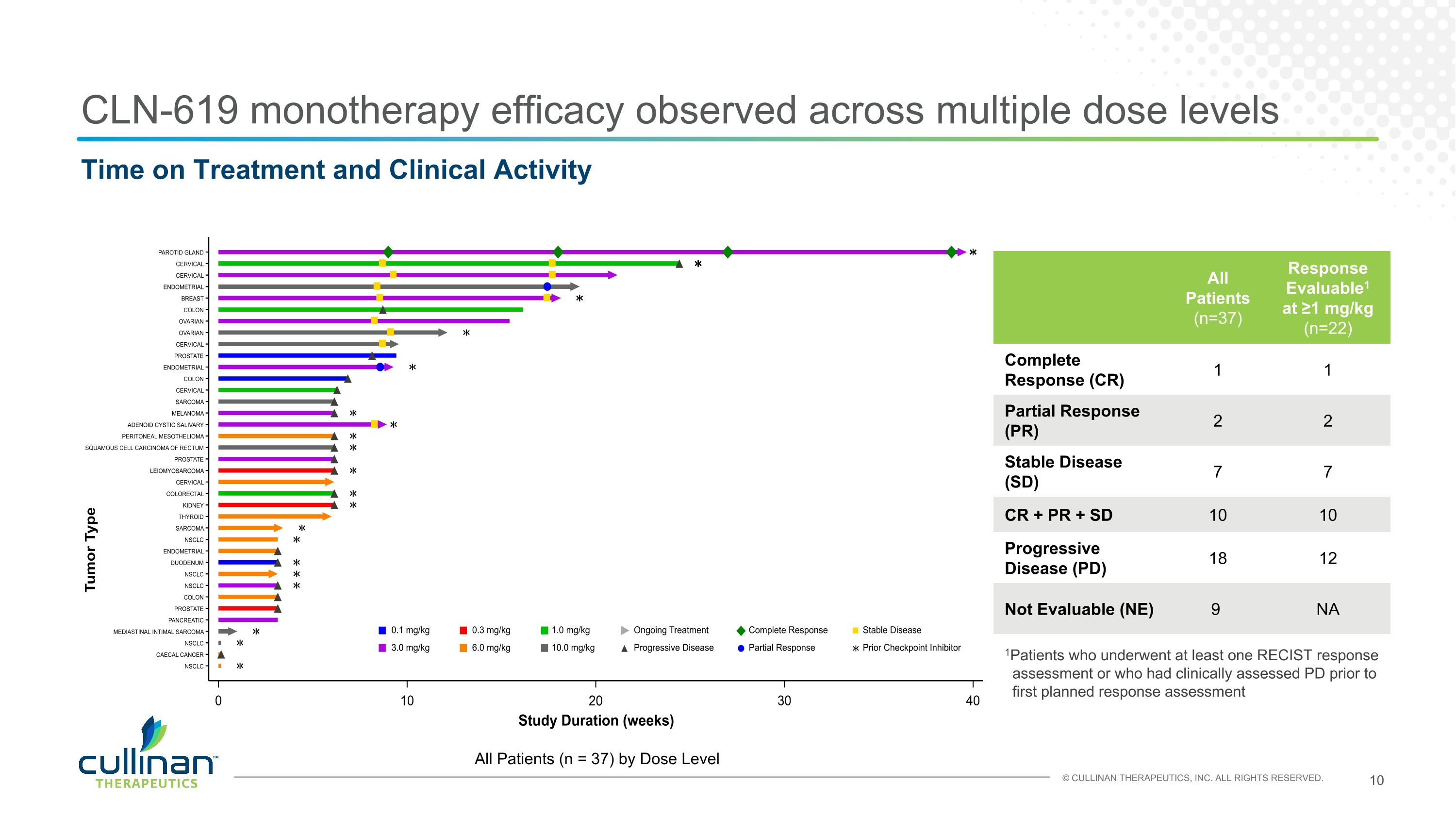
CLN-619 monotherapy efficacy observed across multiple dose levels Time on Treatment and Clinical Activity All Patients (n = 37) by Dose Level All Patients (n=37) Response Evaluable1 at ≥1 mg/kg (n=22) Complete Response (CR) 1 1 Partial Response (PR) 2 2 Stable Disease (SD) 7 7 CR + PR + SD 10 10 Progressive Disease (PD) 18 12 Not Evaluable (NE) 9 NA 1Patients who underwent at least one RECIST response assessment or who had clinically assessed PD prior to first planned response assessment
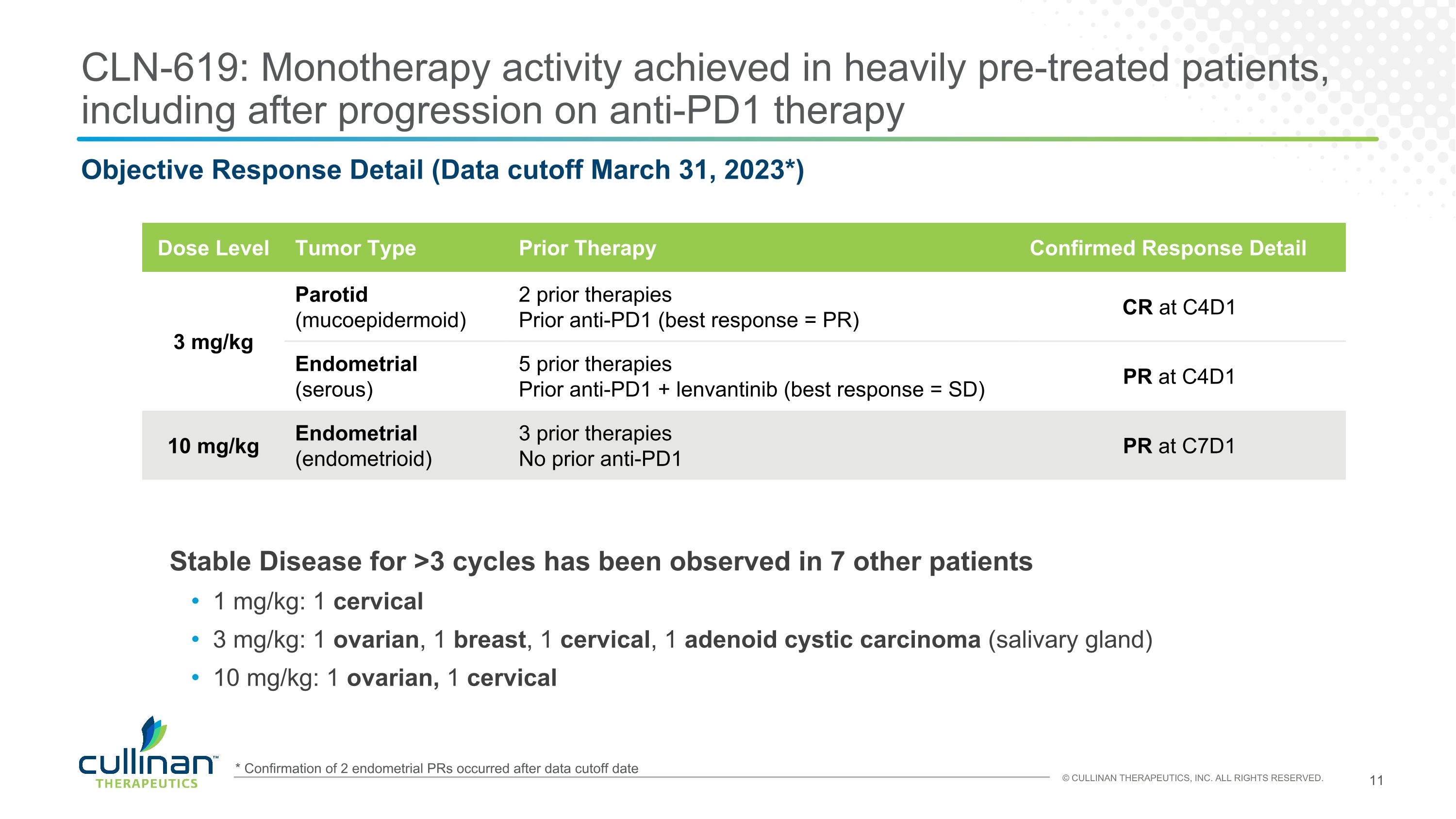
CLN-619: Monotherapy activity achieved in heavily pre-treated patients, including after progression on anti-PD1 therapy Dose Level Tumor Type Prior Therapy Confirmed Response Detail 3 mg/kg Parotid (mucoepidermoid) 2 prior therapies Prior anti-PD1 (best response = PR) CR at C4D1 3 mg/kg Endometrial (serous) 5 prior therapies Prior anti-PD1 + lenvantinib (best response = SD) PR at C4D1 10 mg/kg Endometrial (endometrioid) 3 prior therapies No prior anti-PD1 PR at C7D1 Stable Disease for >3 cycles has been observed in 7 other patients 1 mg/kg: 1 cervical 3 mg/kg: 1 ovarian, 1 breast, 1 cervical, 1 adenoid cystic carcinoma (salivary gland) 10 mg/kg: 1 ovarian, 1 cervical Objective Response Detail (Data cutoff March 31, 2023*) * Confirmation of 2 endometrial PRs occurred after data cutoff date
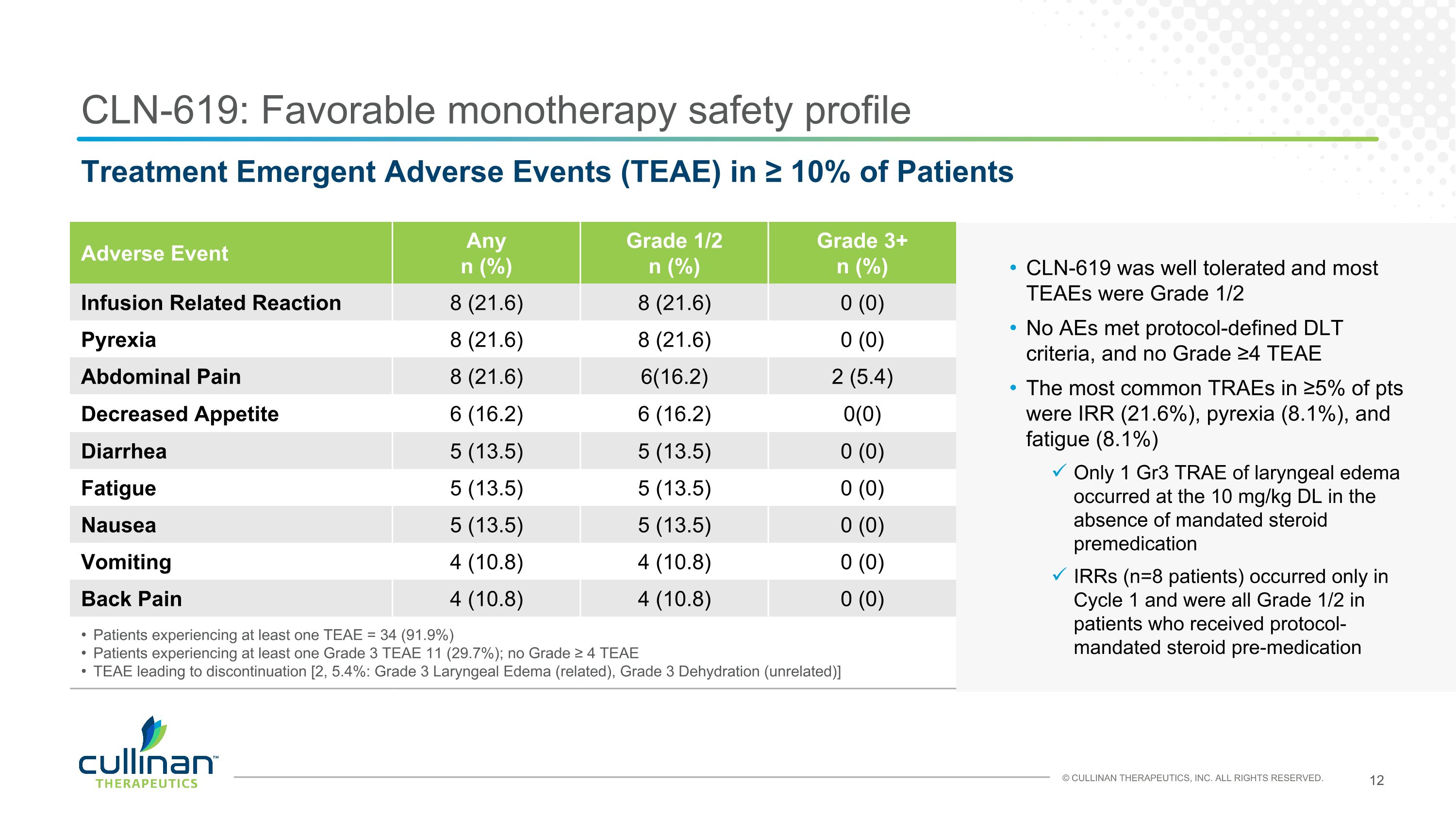
CLN-619: Favorable monotherapy safety profile Adverse Event Any n (%) Grade 1/2 n (%) Grade 3+ n (%) Infusion Related Reaction 8 (21.6) 8 (21.6) 0 (0) Pyrexia 8 (21.6) 8 (21.6) 0 (0) Abdominal Pain 8 (21.6) 6(16.2) 2 (5.4) Decreased Appetite 6 (16.2) 6 (16.2) 0(0) Diarrhea 5 (13.5) 5 (13.5) 0 (0) Fatigue 5 (13.5) 5 (13.5) 0 (0) Nausea 5 (13.5) 5 (13.5) 0 (0) Vomiting 4 (10.8) 4 (10.8) 0 (0) Back Pain 4 (10.8) 4 (10.8) 0 (0) Patients experiencing at least one TEAE = 34 (91.9%) Patients experiencing at least one Grade 3 TEAE 11 (29.7%); no Grade ≥ 4 TEAE TEAE leading to discontinuation [2, 5.4%: Grade 3 Laryngeal Edema (related), Grade 3 Dehydration (unrelated)] Treatment Emergent Adverse Events (TEAE) in ≥ 10% of Patients CLN-619 was well tolerated and most TEAEs were Grade 1/2 No AEs met protocol-defined DLT criteria, and no Grade ≥4 TEAE The most common TRAEs in ≥5% of pts were IRR (21.6%), pyrexia (8.1%), and fatigue (8.1%) Only 1 Gr3 TRAE of laryngeal edema occurred at the 10 mg/kg DL in the absence of mandated steroid premedication IRRs (n=8 patients) occurred only in Cycle 1 and were all Grade 1/2 in patients who received protocol-mandated steroid pre-medication
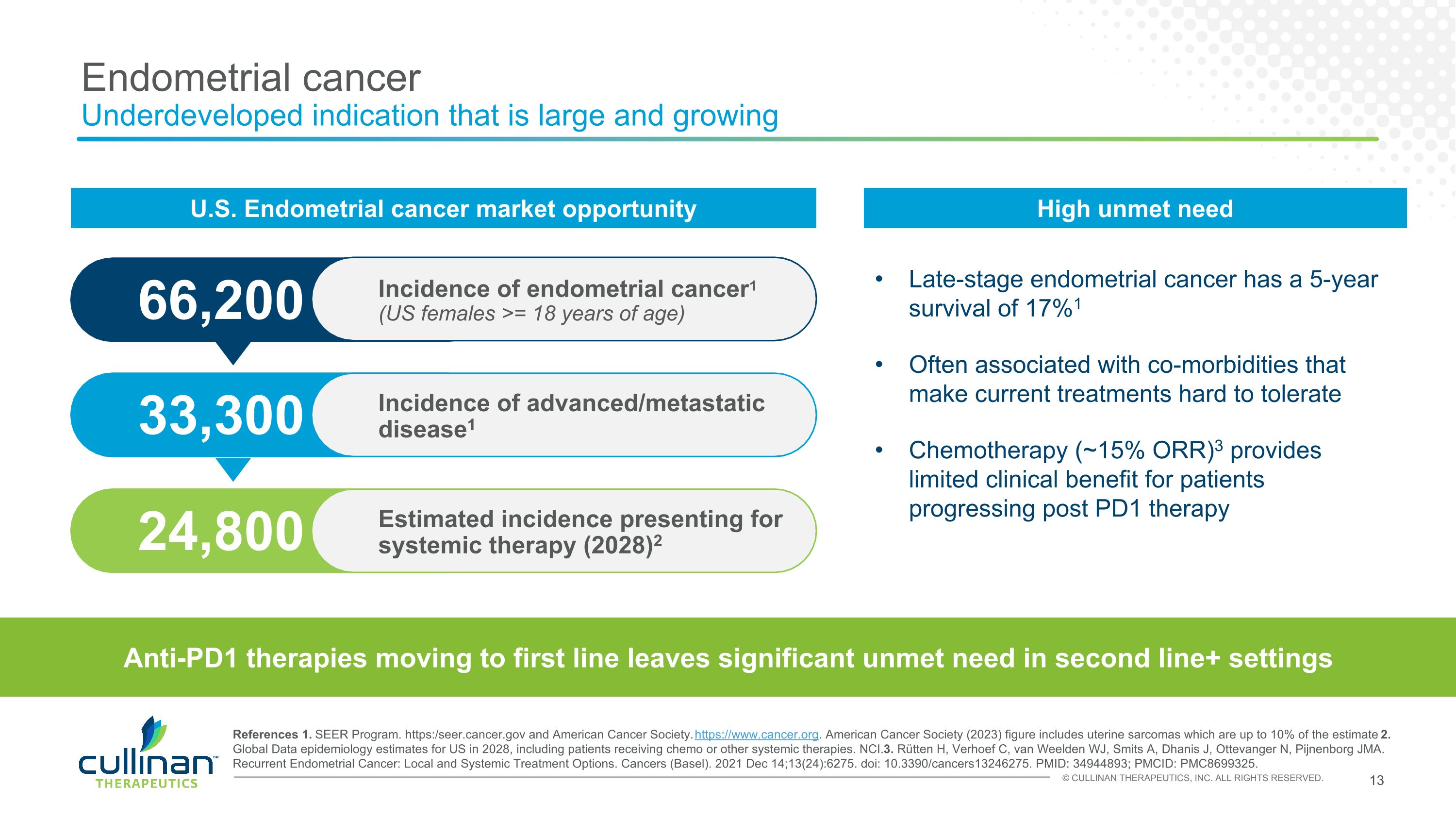
Endometrial cancer�Underdeveloped indication that is large and growing Late-stage endometrial cancer has a 5-year survival of 17%1 Often associated with co-morbidities that make current treatments hard to tolerate Chemotherapy (~15% ORR)3 provides limited clinical benefit for patients progressing post PD1 therapy References 1. SEER Program. https:/seer.cancer.gov and American Cancer Society. https://www.cancer.org. American Cancer Society (2023) figure includes uterine sarcomas which are up to 10% of the estimate 2. Global Data epidemiology estimates for US in 2028, including patients receiving chemo or other systemic therapies. NCI. 3. Rütten H, Verhoef C, van Weelden WJ, Smits A, Dhanis J, Ottevanger N, Pijnenborg JMA. Recurrent Endometrial Cancer: Local and Systemic Treatment Options. Cancers (Basel). 2021 Dec 14;13(24):6275. doi: 10.3390/cancers13246275. PMID: 34944893; PMCID: PMC8699325. 24,800 33,300 66,200 Estimated incidence presenting for systemic therapy (2028)2 Incidence of advanced/metastatic disease1 Incidence of endometrial cancer1�(US females >= 18 years of age) Anti-PD1 therapies moving to first line leaves significant unmet need in second line+ settings U.S. Endometrial cancer market opportunity High unmet need
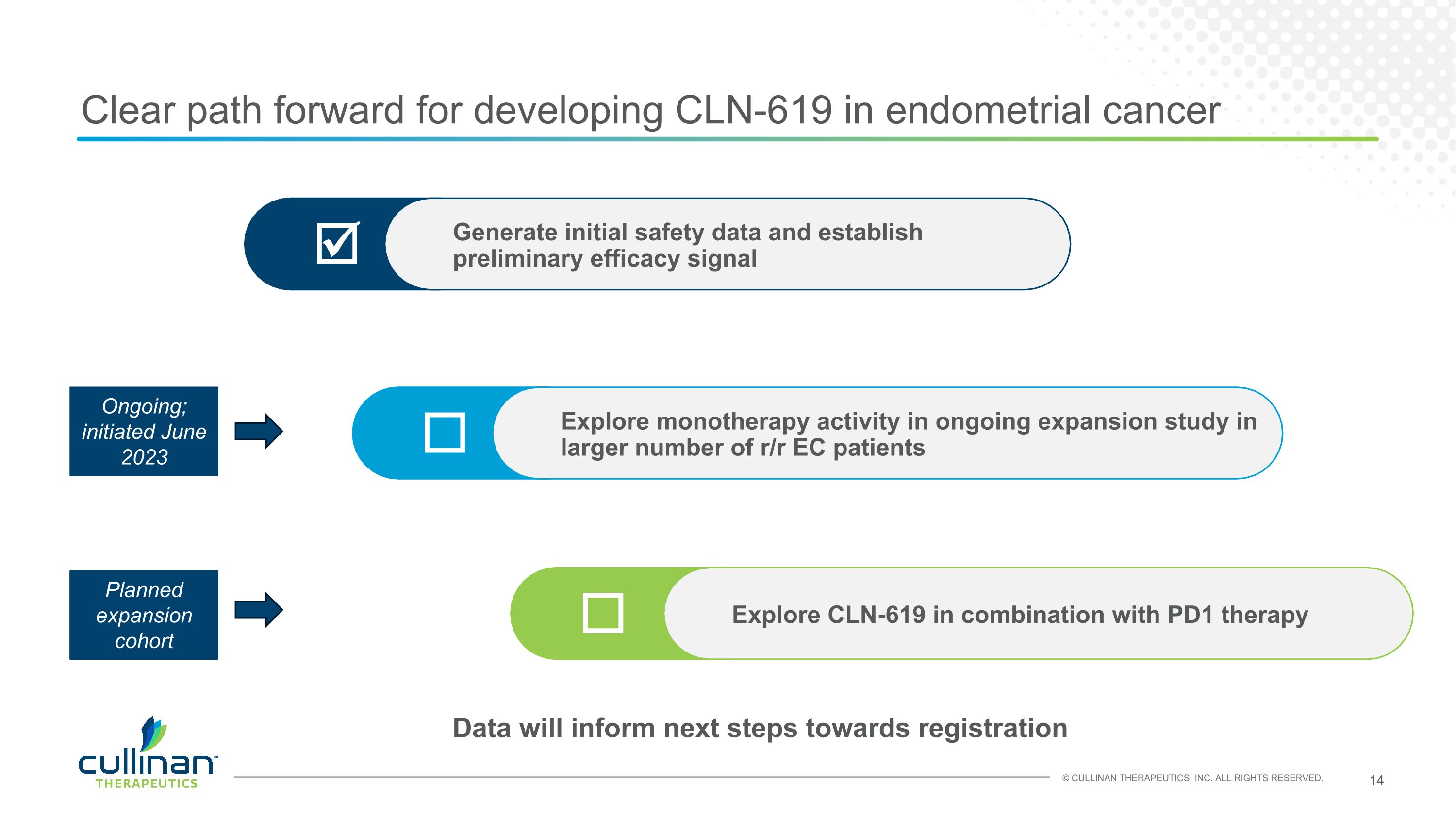
Clear path forward for developing CLN-619 in endometrial cancer Explore monotherapy activity in ongoing expansion study in larger number of r/r EC patients Generate initial safety data and establish preliminary efficacy signal Explore CLN-619 in combination with PD1 therapy Ongoing; initiated June 2023 Planned expansion cohort Data will inform next steps towards registration
CLN-619 status and next steps CLN-619 monotherapy was well tolerated Clinical benefit, including objective responses, observed across multiple tumor types, including after progression on checkpoint inhibitor therapy Notable monotherapy activity observed in gynecological malignancies Expansion cohorts in endometrial and cervical cancers are ongoing Clear path forward in endometrial cancer, a large and growing underdeveloped indication Additional expansion cohorts under evaluation based upon clinical activity observed in the current trial Upcoming clinical data milestones: 2Q 2024: Initial data from pembro combination dose escalation module and follow up data from patients in monotherapy dose escalation module 1H 2025: Initial data from disease specific cohorts
CLN-978�CD19xCD3 T cell Engager
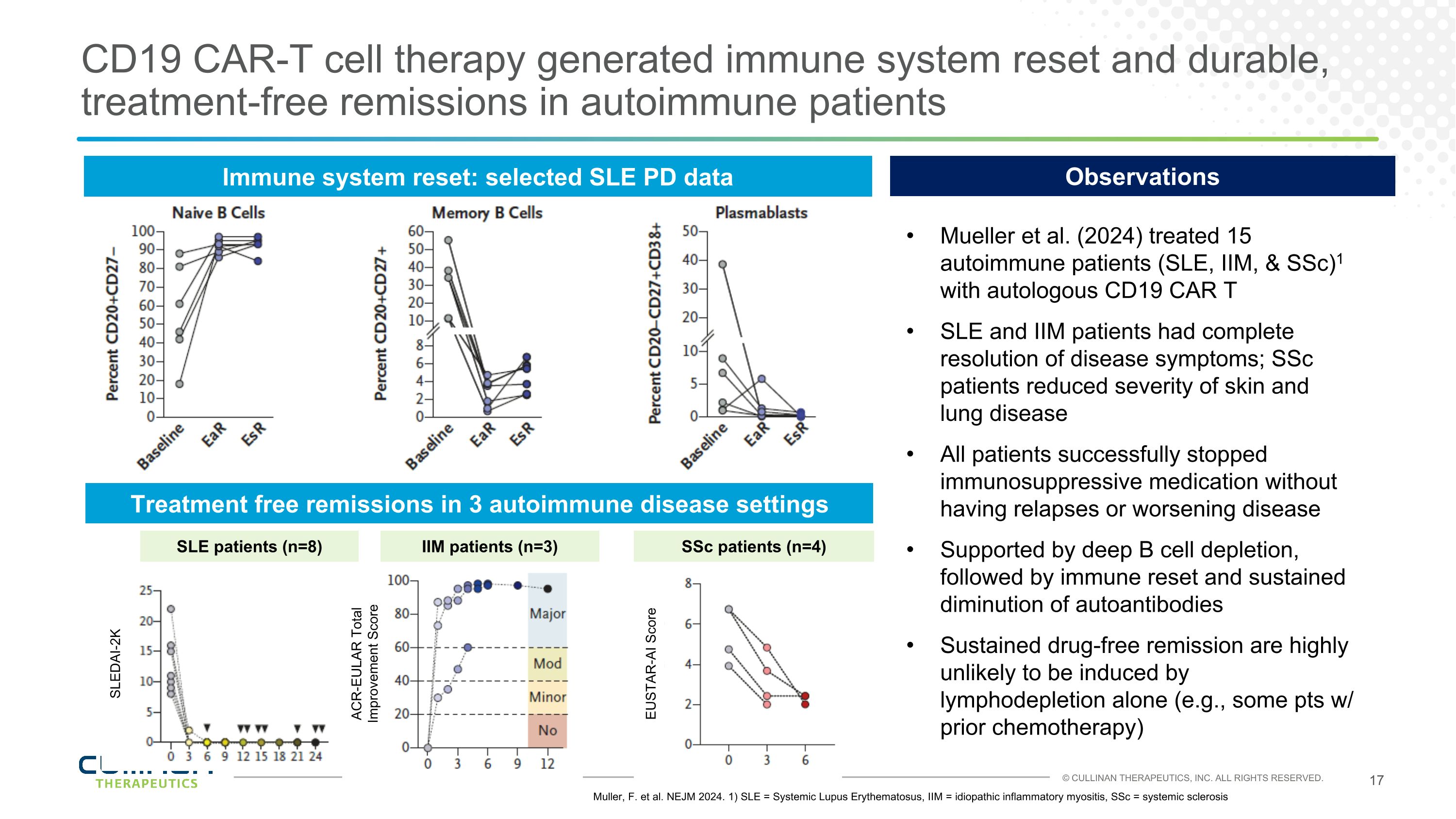
CD19 CAR-T cell therapy generated immune system reset and durable, treatment-free remissions in autoimmune patients Immune system reset: selected SLE PD data Muller, F. et al. NEJM 2024. 1) SLE = Systemic Lupus Erythematosus, IIM = idiopathic inflammatory myositis, SSc = systemic sclerosis Treatment free remissions in 3 autoimmune disease settings Mueller et al. (2024) treated 15 autoimmune patients (SLE, IIM, & SSc)1 with autologous CD19 CAR T SLE and IIM patients had complete resolution of disease symptoms; SSc patients reduced severity of skin and lung disease All patients successfully stopped immunosuppressive medication without having relapses or worsening disease Supported by deep B cell depletion, followed by immune reset and sustained diminution of autoantibodies Sustained drug-free remission are highly unlikely to be induced by lymphodepletion alone (e.g., some pts w/ prior chemotherapy) Observations SLE patients (n=8) SLEDAI-2K IIM patients (n=3) ACR-EULAR Total Improvement Score EUSTAR-AI Score SSc patients (n=4)

CAR T cell therapy: Promising outcomes but multiple challenges may limit broad uptake in autoimmune diseases Cell therapy limitations Available cellular therapies all require lymphodepleting chemotherapy, which has been associated with increased risk for infection, infertility, and secondary malignancies FDA has mandated boxed warnings across approved products highlighting the risk for secondary malignancies related to the CAR T cell therapy itself Complex manufacturing processes can introduce treatment delays for patients Reimbursement challenges continue to limit provider uptake for existing indications Treatment limited to specialized centers certified to provide CAR T cell therapies Prohibitive logistical and economic challenges will likely prevent retreatment upon relapse
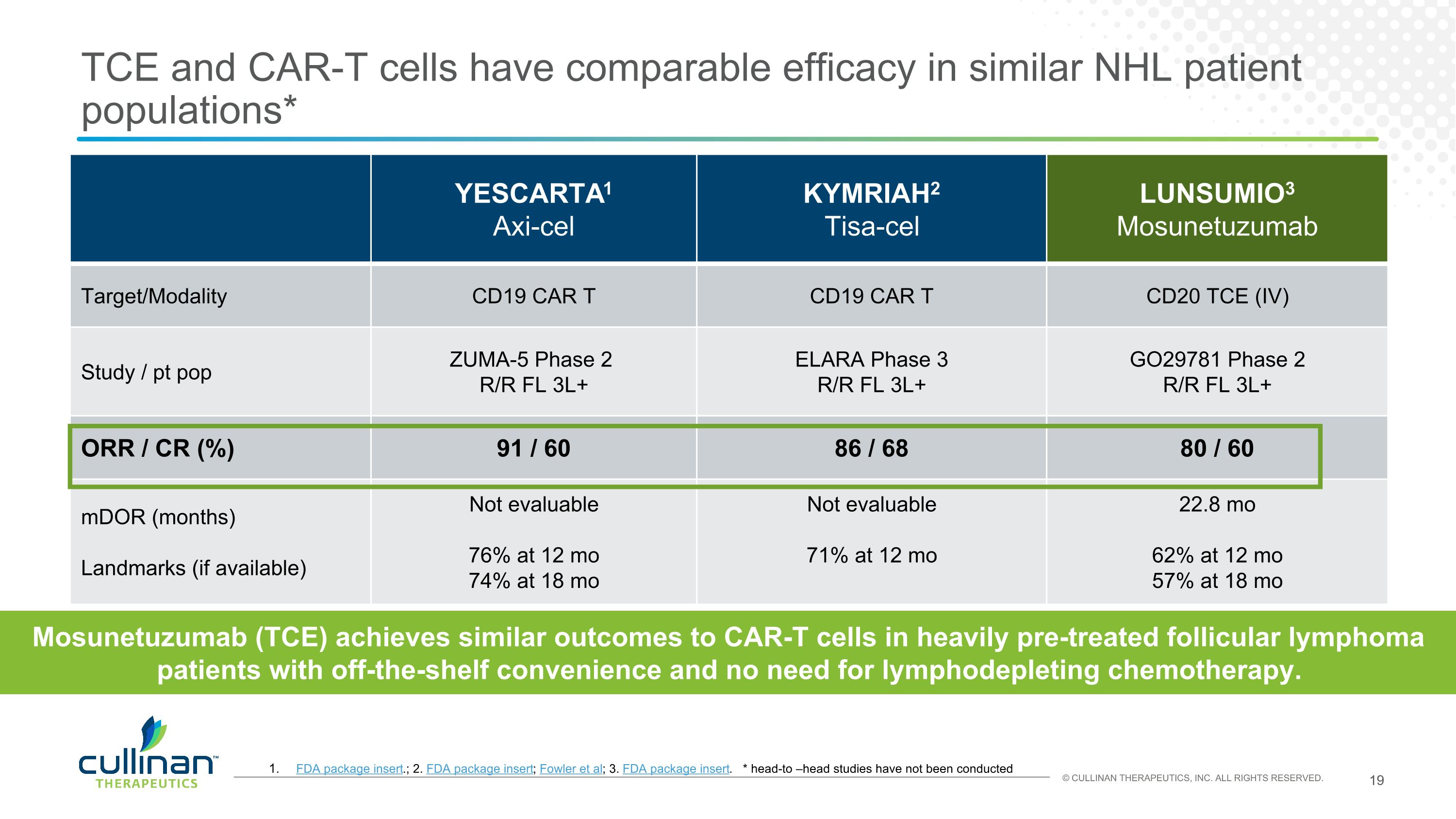
TCE and CAR-T cells have comparable efficacy in similar NHL patient populations* YESCARTA1 Axi-cel KYMRIAH2 Tisa-cel LUNSUMIO3 Mosunetuzumab Target/Modality CD19 CAR T CD19 CAR T CD20 TCE (IV) Study / pt pop ZUMA-5 Phase 2 R/R FL 3L+ ELARA Phase 3 R/R FL 3L+ GO29781 Phase 2 R/R FL 3L+ ORR / CR (%) 91 / 60 86 / 68 80 / 60 mDOR (months) Landmarks (if available) Not evaluable 76% at 12 mo 74% at 18 mo Not evaluable 71% at 12 mo 22.8 mo 62% at 12 mo 57% at 18 mo FDA package insert.; 2. FDA package insert; Fowler et al; 3. FDA package insert. * head-to –head studies have not been conducted Mosunetuzumab (TCE) achieves similar outcomes to CAR-T cells in heavily pre-treated follicular lymphoma patients with off-the-shelf convenience and no need for lymphodepleting chemotherapy.
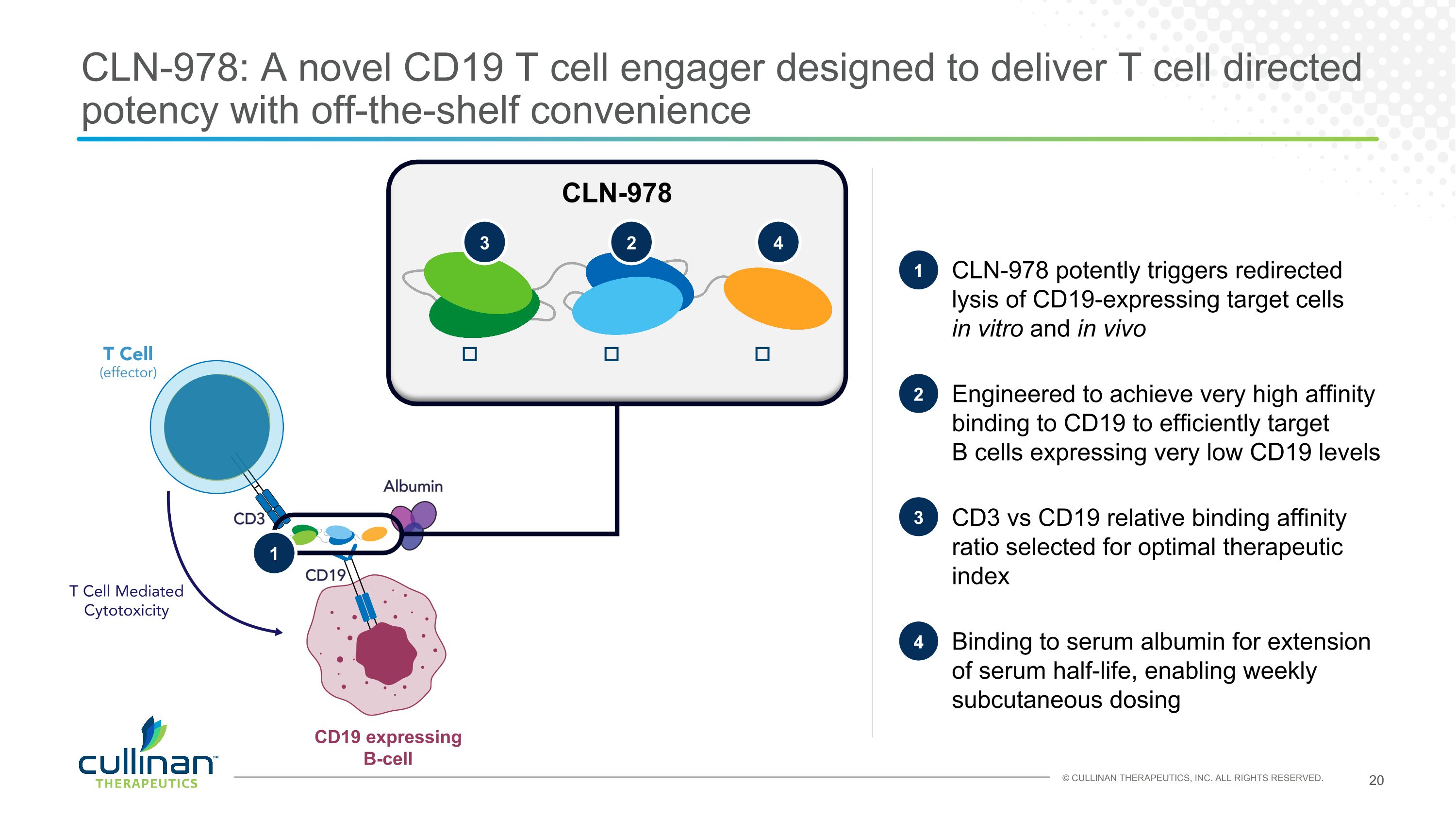
CLN-978: A novel CD19 T cell engager designed to deliver T cell directed potency with off-the-shelf convenience CLN-978 potently triggers redirected lysis of CD19-expressing target cells �in vitro and in vivo Engineered to achieve very high affinity binding to CD19 to efficiently target �B cells expressing very low CD19 levels CD3 vs CD19 relative binding affinity ratio selected for optimal therapeutic index Binding to serum albumin for extension of serum half-life, enabling weekly subcutaneous dosing 1 2 3 4 CD19 expressing B-cell 1 CLN-978 𝛼-HSA (VHH) 𝛼-CD19 (scFv) 𝛼-CD3 (scFv) 3 2 4
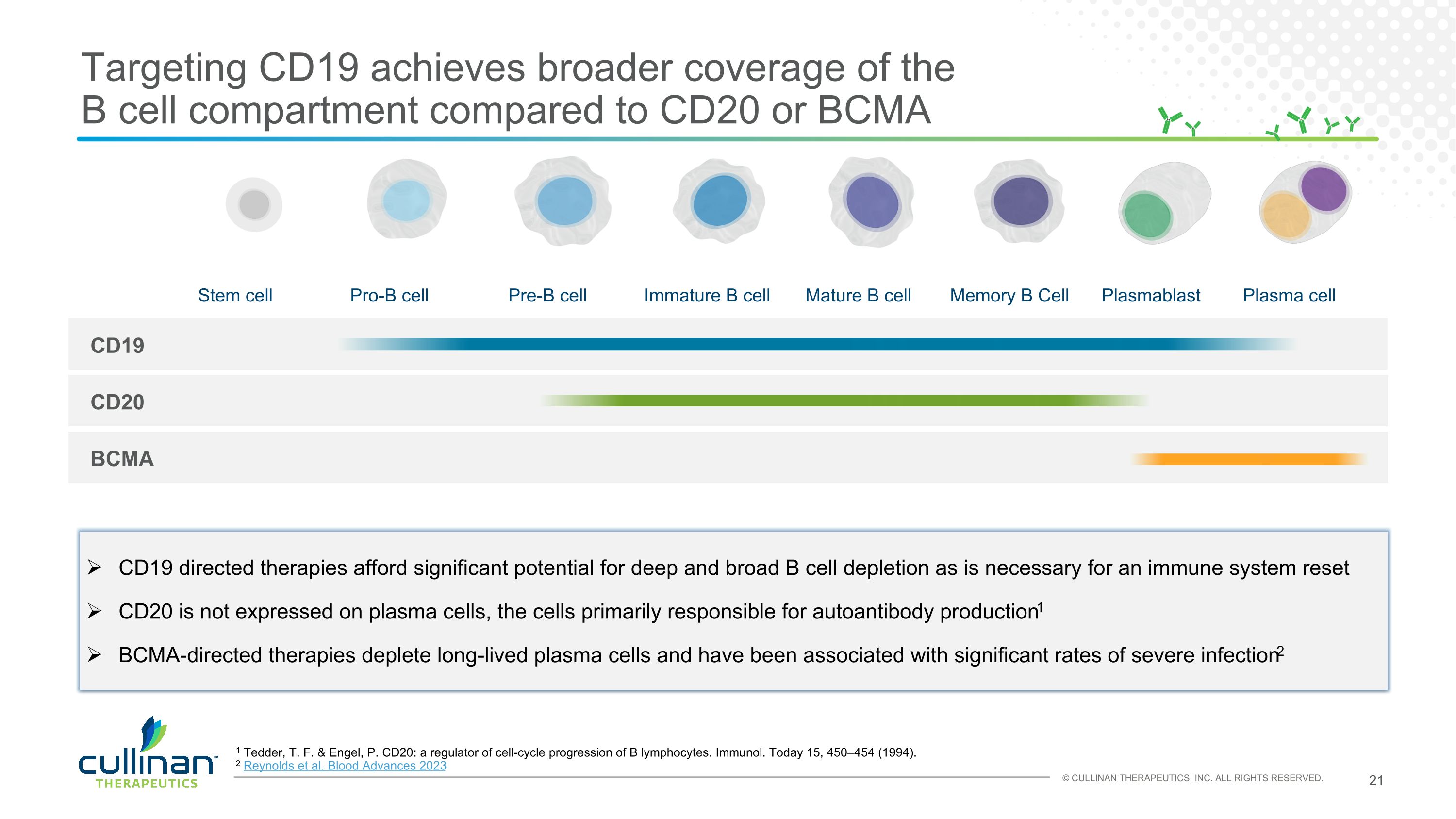
Targeting CD19 achieves broader coverage of the �B cell compartment compared to CD20 or BCMA Stem cell Pro-B cell Pre-B cell Immature B cell Mature B cell Memory B Cell Plasmablast Plasma cell CD19 CD20 BCMA CD19 directed therapies afford significant potential for deep and broad B cell depletion as is necessary for an immune system reset CD20 is not expressed on plasma cells, the cells primarily responsible for autoantibody production1 BCMA-directed therapies deplete long-lived plasma cells and have been associated with significant rates of severe infection2 1 Tedder, T. F. & Engel, P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol. Today 15, 450–454 (1994). 2 Reynolds et al. Blood Advances 2023
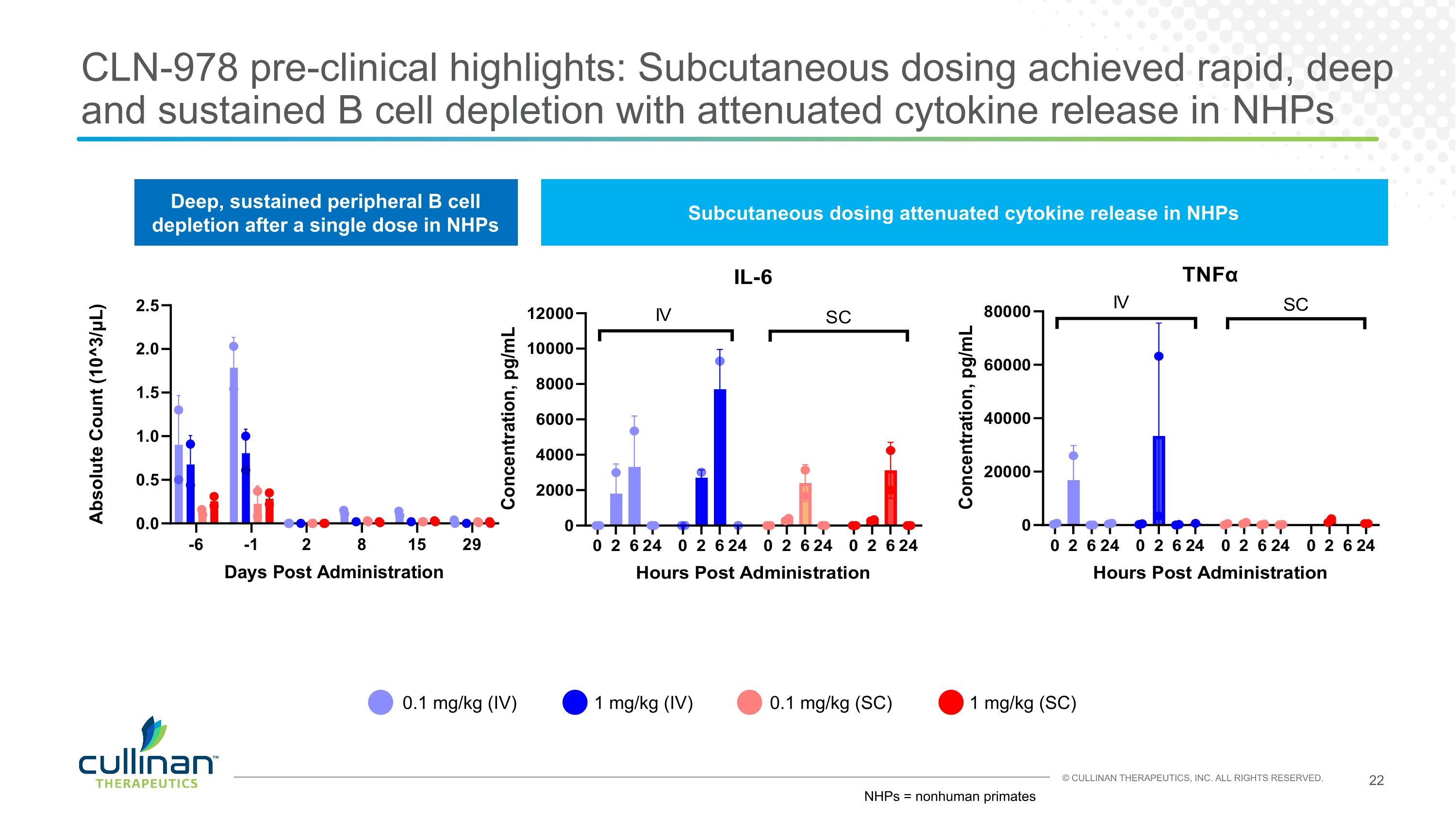
CLN-978 pre-clinical highlights: Subcutaneous dosing achieved rapid, deep and sustained B cell depletion with attenuated cytokine release in NHPs 0.1 mg/kg (IV) 1 mg/kg (IV) 0.1 mg/kg (SC) 1 mg/kg (SC) Deep, sustained peripheral B cell depletion after a single dose in NHPs Subcutaneous dosing attenuated cytokine release in NHPs NHPs = nonhuman primates
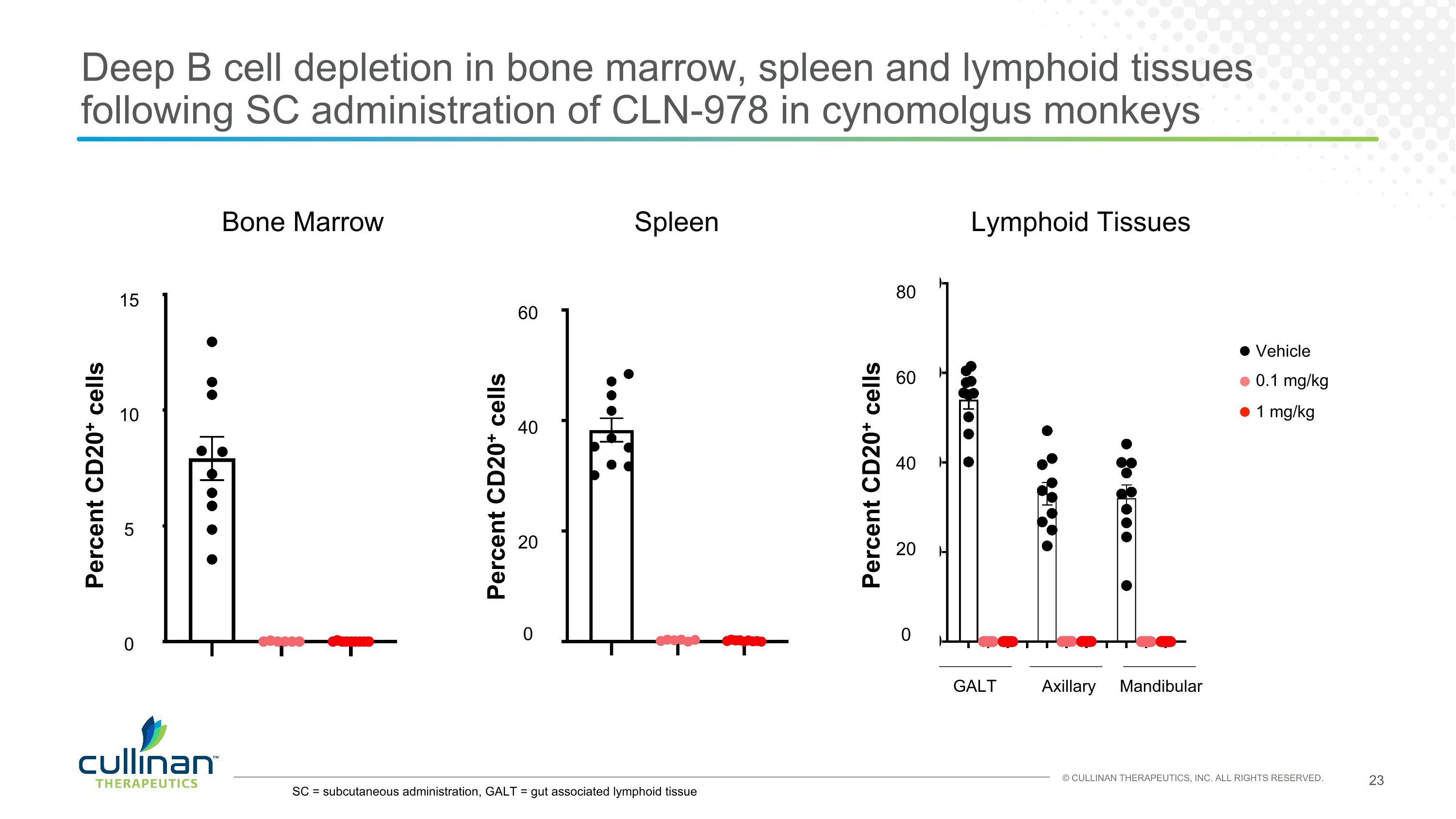
Bone marrow Spleen Lymphoid tissue GALT Axillary Vehicle Mandibular 0.1 mg/kg 1 mg/kg Percent CD20+ cells Percent CD20+ cells SC = subcutaneous administration, GALT = gut associated lymphoid tissue Deep B cell depletion in bone marrow, spleen and lymphoid tissues following SC administration of CLN-978 in cynomolgus monkeys 15 10 5 0 60 40 20 0 80 60 40 20 0 Percent CD20+ cells Bone Marrow Spleen Lymphoid Tissues
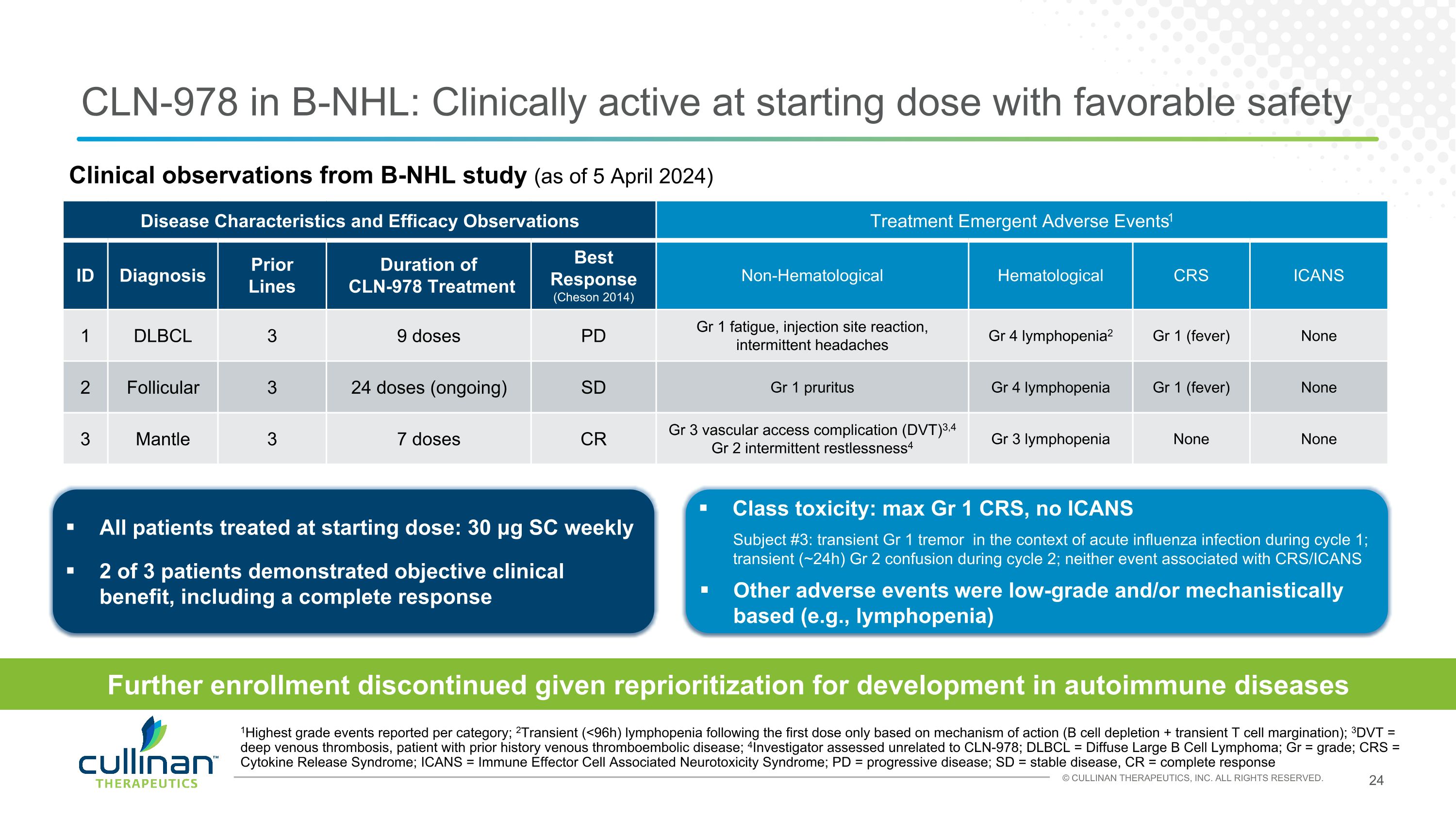
Clinical observations from B-NHL study (as of 5 April 2024) CLN-978 in B-NHL: Clinically active at starting dose with favorable safety Disease Characteristics and Efficacy Observations Treatment Emergent Adverse Events1 ID Diagnosis Prior Lines Duration of CLN-978 Treatment Best Response (Cheson 2014) Non-Hematological Hematological CRS ICANS 1 DLBCL 3 9 doses PD Gr 1 fatigue, injection site reaction, intermittent headaches Gr 4 lymphopenia2 Gr 1 (fever) None 2 Follicular 3 24 doses (ongoing) SD Gr 1 pruritus Gr 4 lymphopenia Gr 1 (fever) None 3 Mantle 3 7 doses CR Gr 3 vascular access complication (DVT)3,4 Gr 2 intermittent restlessness4 Gr 3 lymphopenia None None 1Highest grade events reported per category; 2Transient (<96h) lymphopenia following the first dose only based on mechanism of action (B cell depletion + transient T cell margination); 3DVT = deep venous thrombosis, patient with prior history venous thromboembolic disease; 4Investigator assessed unrelated to CLN-978; DLBCL = Diffuse Large B Cell Lymphoma; Gr = grade; CRS = Cytokine Release Syndrome; ICANS = Immune Effector Cell Associated Neurotoxicity Syndrome; PD = progressive disease; SD = stable disease, CR = complete response All patients treated at starting dose: 30 μg SC weekly 2 of 3 patients demonstrated objective clinical benefit, including a complete response Class toxicity: max Gr 1 CRS, no ICANS Subject #3: transient Gr 1 tremor in the context of acute influenza infection during cycle 1; transient (~24h) Gr 2 confusion during cycle 2; neither event associated with CRS/ICANS Other adverse events were low-grade and/or mechanistically based (e.g., lymphopenia) Further enrollment discontinued given reprioritization for development in autoimmune diseases
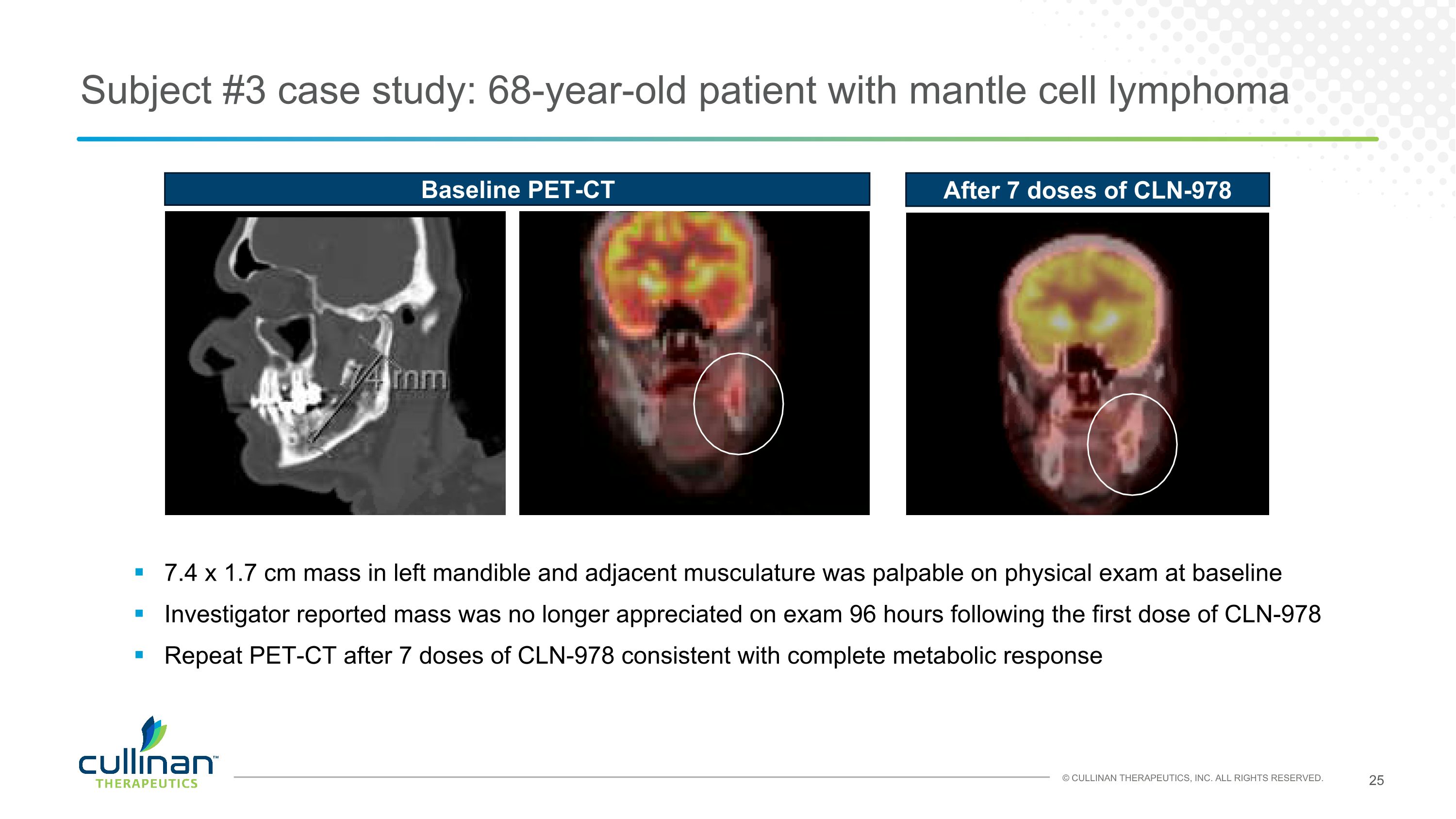
Subject #3 case study: 68-year-old patient with mantle cell lymphoma 7.4 x 1.7 cm mass in left mandible and adjacent musculature was palpable on physical exam at baseline Investigator reported mass was no longer appreciated on exam 96 hours following the first dose of CLN-978 Repeat PET-CT after 7 doses of CLN-978 consistent with complete metabolic response Baseline PET-CT After 7 doses of CLN-978
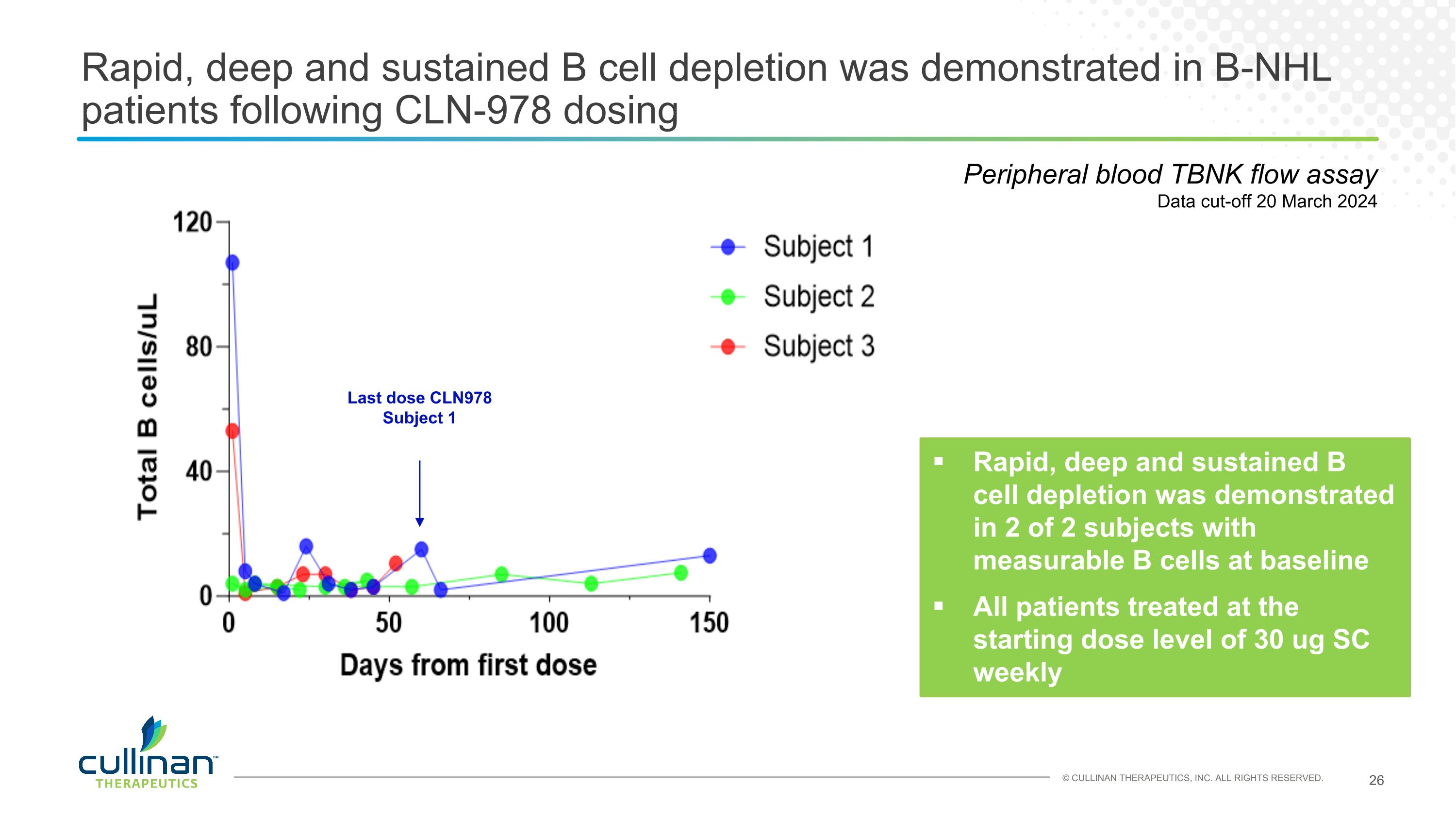
Rapid, deep and sustained B cell depletion was demonstrated in B-NHL patients following CLN-978 dosing Rapid, deep and sustained B cell depletion was demonstrated in 2 of 2 subjects with measurable B cells at baseline All patients treated at the starting dose level of 30 ug SC weekly Peripheral blood TBNK flow assay Data cut-off 20 March 2024 Last dose CLN978 Subject 1

CLN-978-SLE-001 planned study design Objectives Primary Objective: Safety of CLN-978 for treatment of active SLE Secondary Objectives: PK B cell kinetics Immunogenicity Preliminary efficacy PART A: DOSE ESCALATION PART B: DOSE EXPANSION PLANNED DESIGN FEATURES Step-wise escalation to determine target dose for further development Incorporation of step-up dosing to minimize risk for cytokine release syndrome and neurotoxicity Standard pre-medication including corticosteroids In-patient monitoring for 48 hours PLANNED DESIGN FEATURES Exploration of 2 or more dosing schedules in a larger number of SLE patients Study Population SLE patients One or more of the following SLE autoantibodies: anti-nucleosome anti-dsDNA anti-Smith SLEDAI-2K ≥ 8 No CNS disease IND submission planned 3Q:24
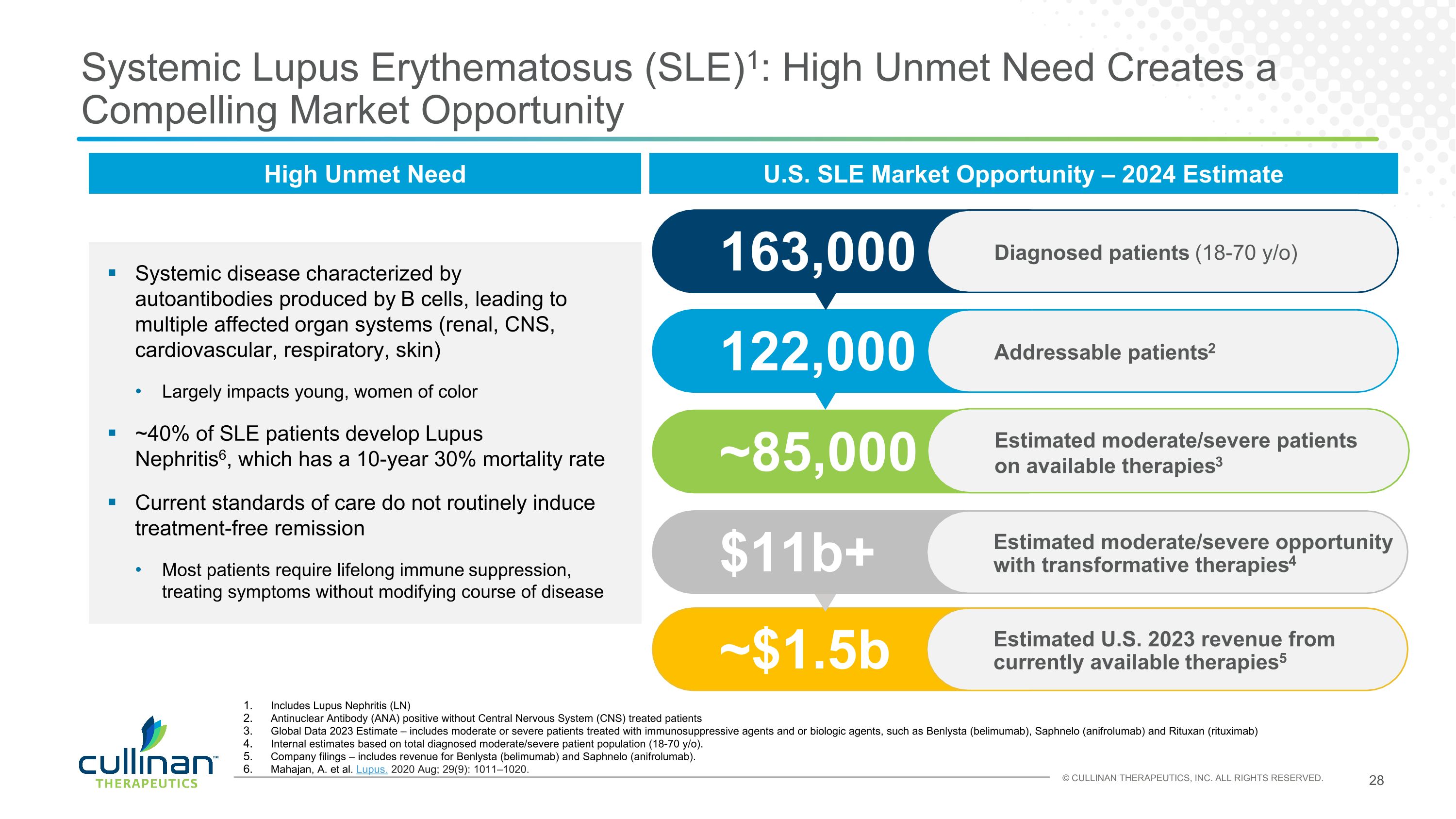
Systemic Lupus Erythematosus (SLE)1: High Unmet Need Creates a Compelling Market Opportunity High Unmet Need Systemic disease characterized by autoantibodies produced by B cells, leading to multiple affected organ systems (renal, CNS, cardiovascular, respiratory, skin) Largely impacts young, women of color ~40% of SLE patients develop Lupus Nephritis6, which has a 10-year 30% mortality rate Current standards of care do not routinely induce treatment-free remission Most patients require lifelong immune suppression, treating symptoms without modifying course of disease Includes Lupus Nephritis (LN) Antinuclear Antibody (ANA) positive without Central Nervous System (CNS) treated patients Global Data 2023 Estimate – includes moderate or severe patients treated with immunosuppressive agents and or biologic agents, such as Benlysta (belimumab), Saphnelo (anifrolumab) and Rituxan (rituximab) Internal estimates based on total diagnosed moderate/severe patient population (18-70 y/o). Company filings – includes revenue for Benlysta (belimumab) and Saphnelo (anifrolumab). Mahajan, A. et al. Lupus. 2020 Aug; 29(9): 1011–1020. 122,000 Addressable patients2 163,000 Diagnosed patients (18-70 y/o) U.S. SLE Market Opportunity – 2024 Estimate ~85,000 Estimated moderate/severe patients on available therapies3 ~$1.5b Estimated U.S. 2023 revenue from currently available therapies5 $11b+ Estimated moderate/severe opportunity with transformative therapies4
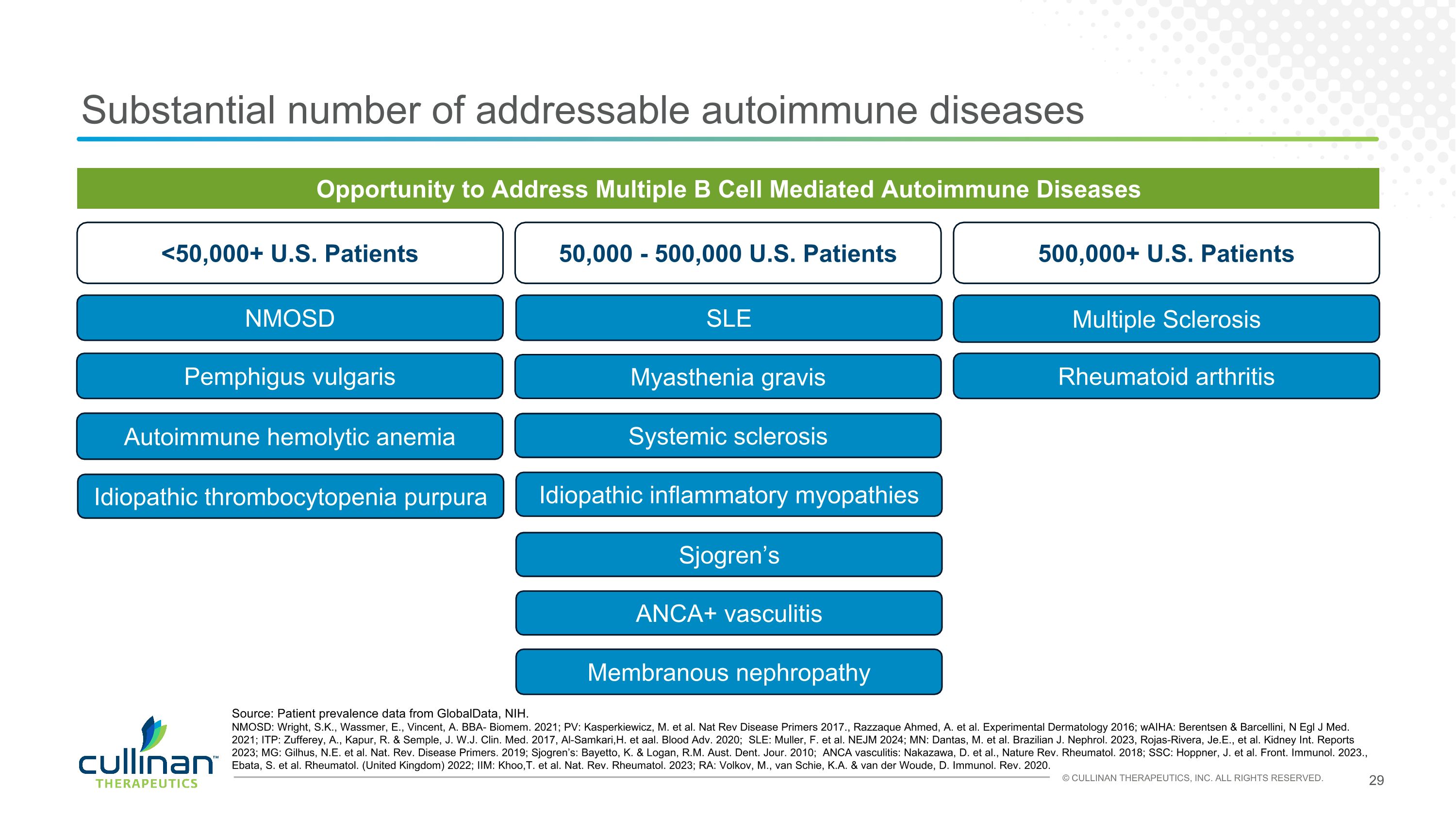
Substantial number of addressable autoimmune diseases Opportunity to Address Multiple B Cell Mediated Autoimmune Diseases 500,000+ U.S. Patients 50,000 - 500,000 U.S. Patients <50,000+ U.S. Patients NMOSD SLE Multiple Sclerosis Rheumatoid arthritis Membranous nephropathy Myasthenia gravis Pemphigus vulgaris Sjogren’s ANCA+ vasculitis Systemic sclerosis Idiopathic inflammatory myopathies Autoimmune hemolytic anemia Idiopathic thrombocytopenia purpura Source: Patient prevalence data from GlobalData, NIH. NMOSD: Wright, S.K., Wassmer, E., Vincent, A. BBA- Biomem. 2021; PV: Kasperkiewicz, M. et al. Nat Rev Disease Primers 2017., Razzaque Ahmed, A. et al. Experimental Dermatology 2016; wAIHA: Berentsen & Barcellini, N Egl J Med. 2021; ITP: Zufferey, A., Kapur, R. & Semple, J. W.J. Clin. Med. 2017, Al-Samkari,H. et aal. Blood Adv. 2020; SLE: Muller, F. et al. NEJM 2024; MN: Dantas, M. et al. Brazilian J. Nephrol. 2023, Rojas-Rivera, Je.E., et al. Kidney Int. Reports 2023; MG: Gilhus, N.E. et al. Nat. Rev. Disease Primers. 2019; Sjogren’s: Bayetto, K. & Logan, R.M. Aust. Dent. Jour. 2010; ANCA vasculitis: Nakazawa, D. et al., Nature Rev. Rheumatol. 2018; SSC: Hoppner, J. et al. Front. Immunol. 2023., Ebata, S. et al. Rheumatol. (United Kingdom) 2022; IIM: Khoo,T. et al. Nat. Rev. Rheumatol. 2023; RA: Volkov, M., van Schie, K.A. & van der Woude, D. Immunol. Rev. 2020.
CLN-978 program: Summary and next steps CLN-978 development refocused exclusively on autoimmune diseases CD19 is the optimal target for autoimmune diseases CLN-978 represents a potential first in class opportunity with the following differentiated benefits: Off-the-shelf convenience and subcutaneous delivery Potential disease-modifying treatment with a differentiated safety profile Flexible modality allowing for repeat dosing as needed In B-NHL patients, CLN-978 at the starting dose, has demonstrated: Rapid, deep and sustained B cell depletion Clinical activity including a complete response Favorable safety profile at a clinically active dose level SLE is the first indication with an IND submission planned for 3Q 2024 CLN-978 has the potential to address high unmet need and drive significant value as a disease-modifying treatment across a broad range of autoimmune diseases

ZIPALERTINIB�(CLN-081/TAS6417) EGFRex20ins inhibitor
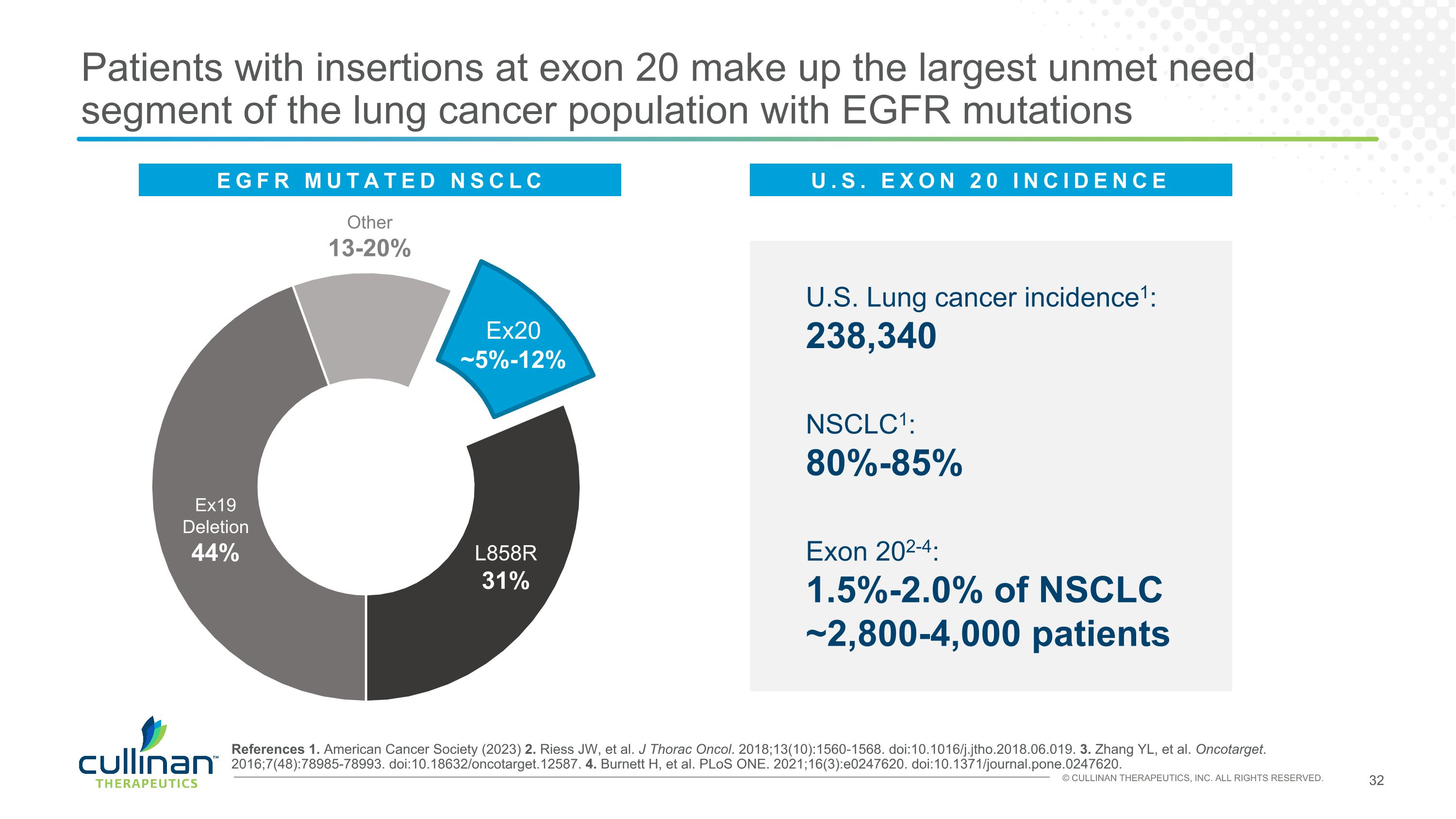
Patients with insertions at exon 20 make up the largest unmet need segment of the lung cancer population with EGFR mutations U.S. Lung cancer incidence1: 238,340 NSCLC1: 80%-85% Exon 202-4: 1.5%-2.0% of NSCLC�~2,800-4,000 patients Other 13-20% Ex19�Deletion 44% L858R 31% Ex20 ~5%-12% References 1. American Cancer Society (2023) 2. Riess JW, et al. J Thorac Oncol. 2018;13(10):1560-1568. doi:10.1016/j.jtho.2018.06.019. 3. Zhang YL, et al. Oncotarget. 2016;7(48):78985-78993. doi:10.18632/oncotarget.12587. 4. Burnett H, et al. PLoS ONE. 2021;16(3):e0247620. doi:10.1371/journal.pone.0247620. EGFR MUTATED NSCLC U.S. EXON 20 INCIDENCE
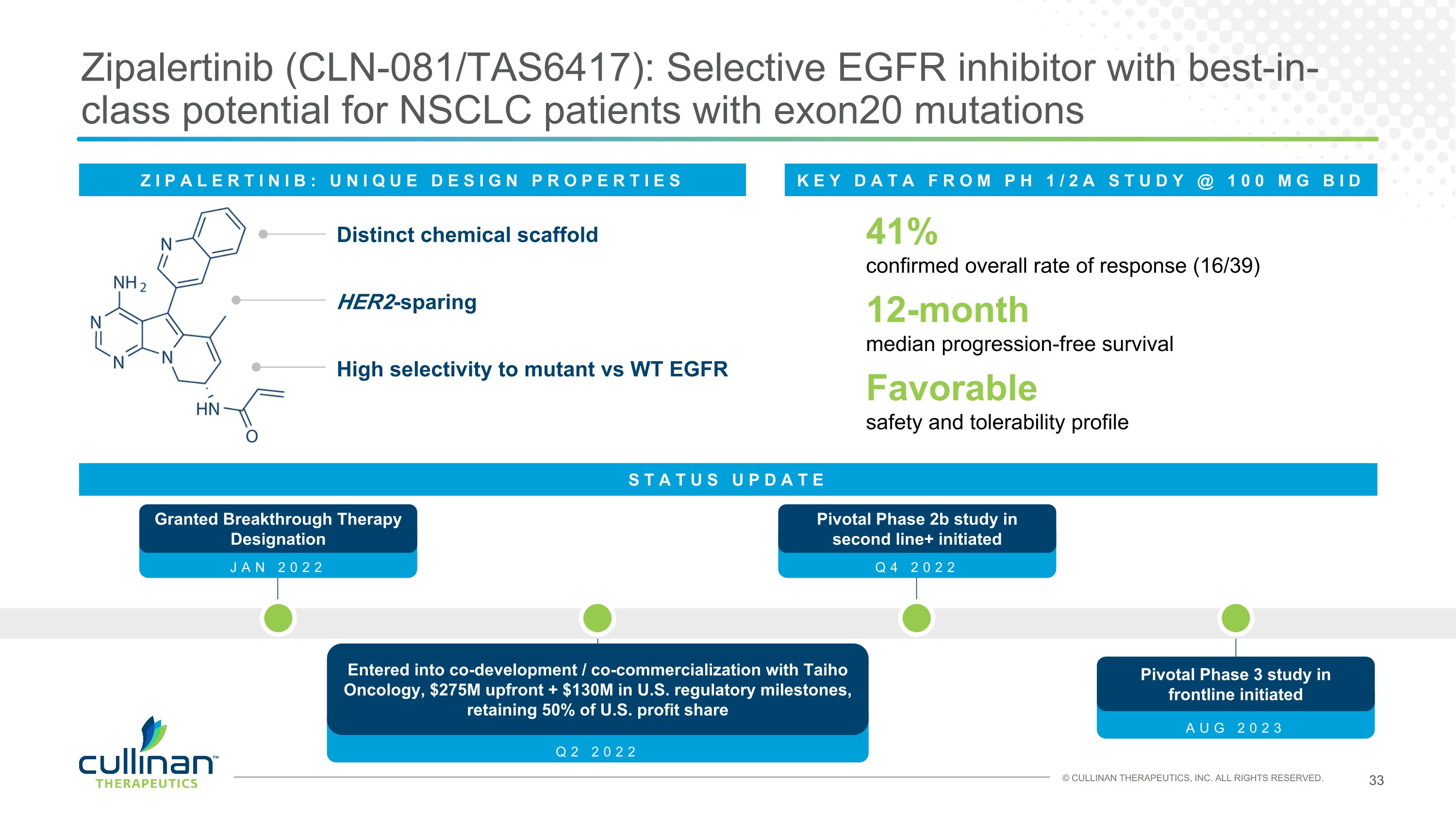
Zipalertinib (CLN-081/TAS6417): Selective EGFR inhibitor with best-in-class potential for NSCLC patients with exon20 mutations ZIPALERTINIB: UNIQUE DESIGN PROPERTIES Distinct chemical scaffold HER2-sparing High selectivity to mutant vs WT EGFR 41%�confirmed overall rate of response (16/39) 12-month�median progression-free survival Favorable�safety and tolerability profile KEY DATA FROM PH 1/2A STUDY @ 100 MG BID STATUS UPDATE AUG 2023 Pivotal Phase 3 study in frontline initiated Q2 2022 Entered into co-development / co-commercialization with Taiho Oncology, $275M upfront + $130M in U.S. regulatory milestones, retaining 50% of U.S. profit share Q4 2022 Pivotal Phase 2b study in second line+ initiated JAN 2022 Granted Breakthrough Therapy Designation
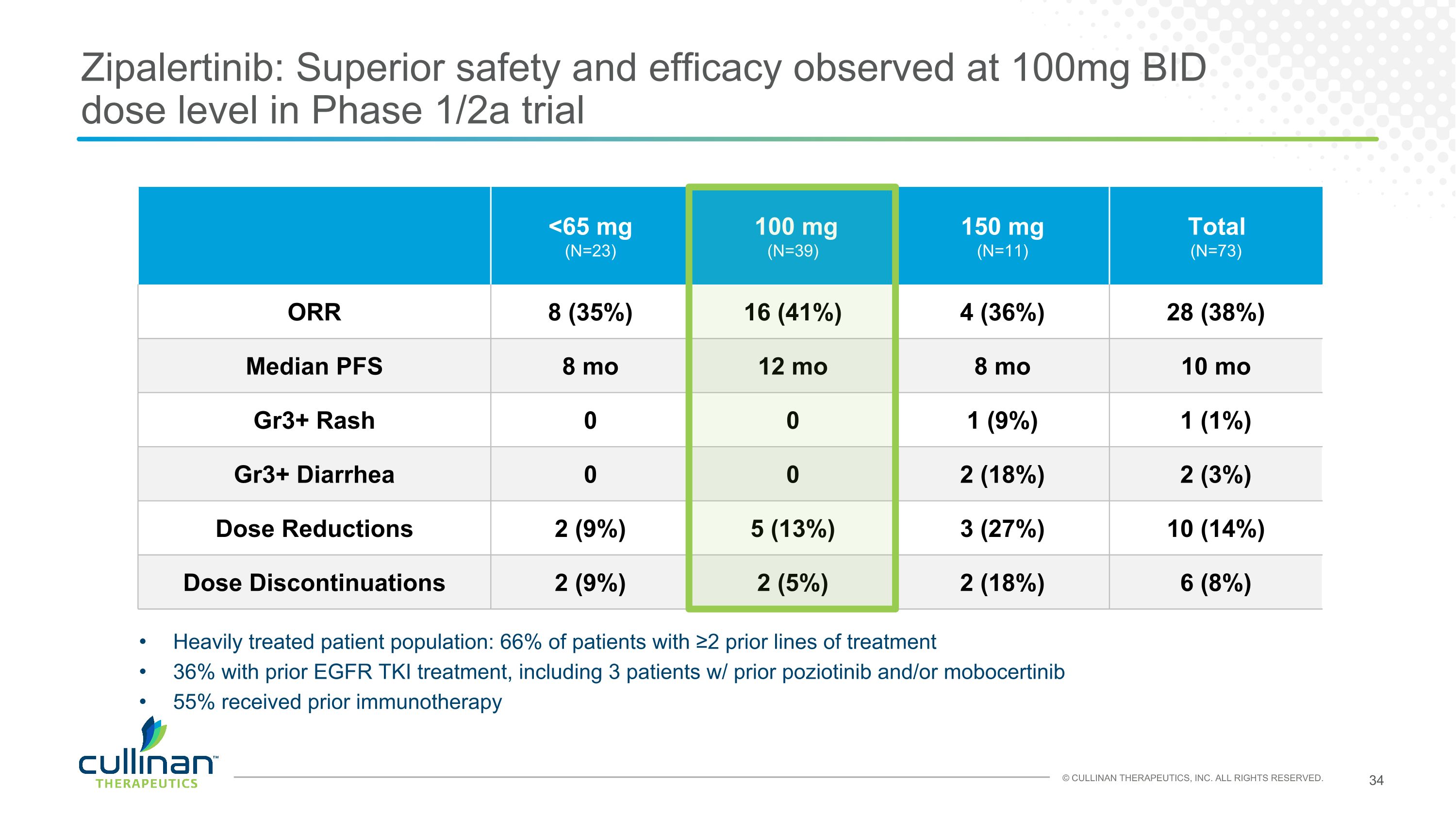
Zipalertinib: Superior safety and efficacy observed at 100mg BID dose level in Phase 1/2a trial <65 mg (N=23) 100 mg (N=39) 150 mg (N=11) Total (N=73) ORR 8 (35%) 16 (41%) 4 (36%) 28 (38%) Median PFS 8 mo 12 mo 8 mo 10 mo Gr3+ Rash 0 0 1 (9%) 1 (1%) Gr3+ Diarrhea 0 0 2 (18%) 2 (3%) Dose Reductions 2 (9%) 5 (13%) 3 (27%) 10 (14%) Dose Discontinuations 2 (9%) 2 (5%) 2 (18%) 6 (8%) Heavily treated patient population: 66% of patients with ≥2 prior lines of treatment 36% with prior EGFR TKI treatment, including 3 patients w/ prior poziotinib and/or mobocertinib 55% received prior immunotherapy
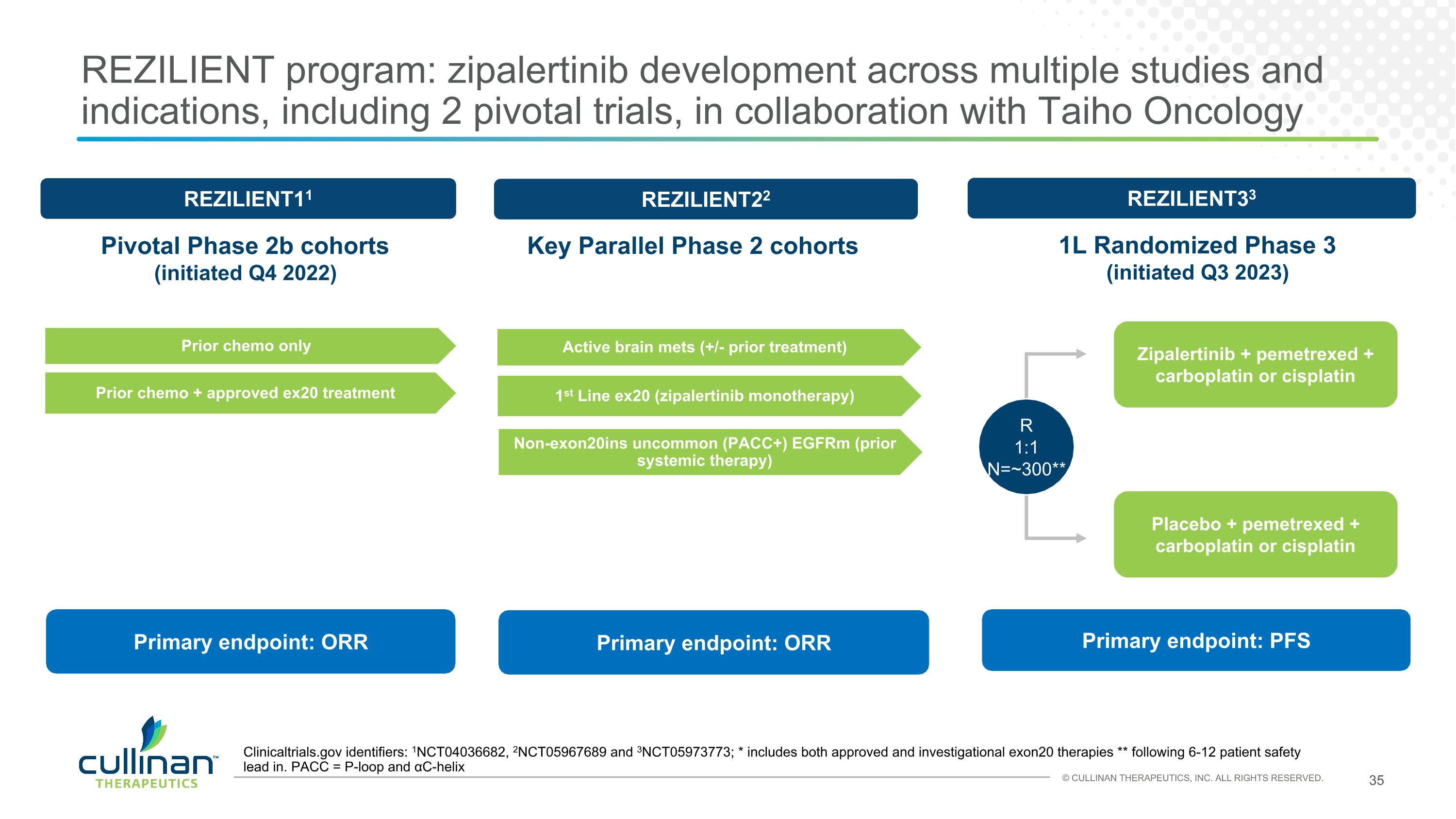
REZILIENT program: zipalertinib development across multiple studies and indications, including 2 pivotal trials, in collaboration with Taiho Oncology REZILIENT33 Clinicaltrials.gov identifiers: 1NCT04036682, 2NCT05967689 and 3NCT05973773; * includes both approved and investigational exon20 therapies ** following 6-12 patient safety lead in. PACC = P-loop and αC-helix Zipalertinib + pemetrexed + carboplatin or cisplatin Placebo + pemetrexed + carboplatin or cisplatin Primary endpoint: PFS R�1:1�N=~300** REZILIENT11 Prior chemo only Prior chemo + approved ex20 treatment REZILIENT22 1st Line ex20 (zipalertinib monotherapy) Active brain mets (+/- prior treatment) Non-exon20ins uncommon (PACC+) EGFRm (prior systemic therapy) Primary endpoint: ORR Primary endpoint: ORR Pivotal Phase 2b cohorts (initiated Q4 2022) Key Parallel Phase 2 cohorts 1L Randomized Phase 3 (initiated Q3 2023)
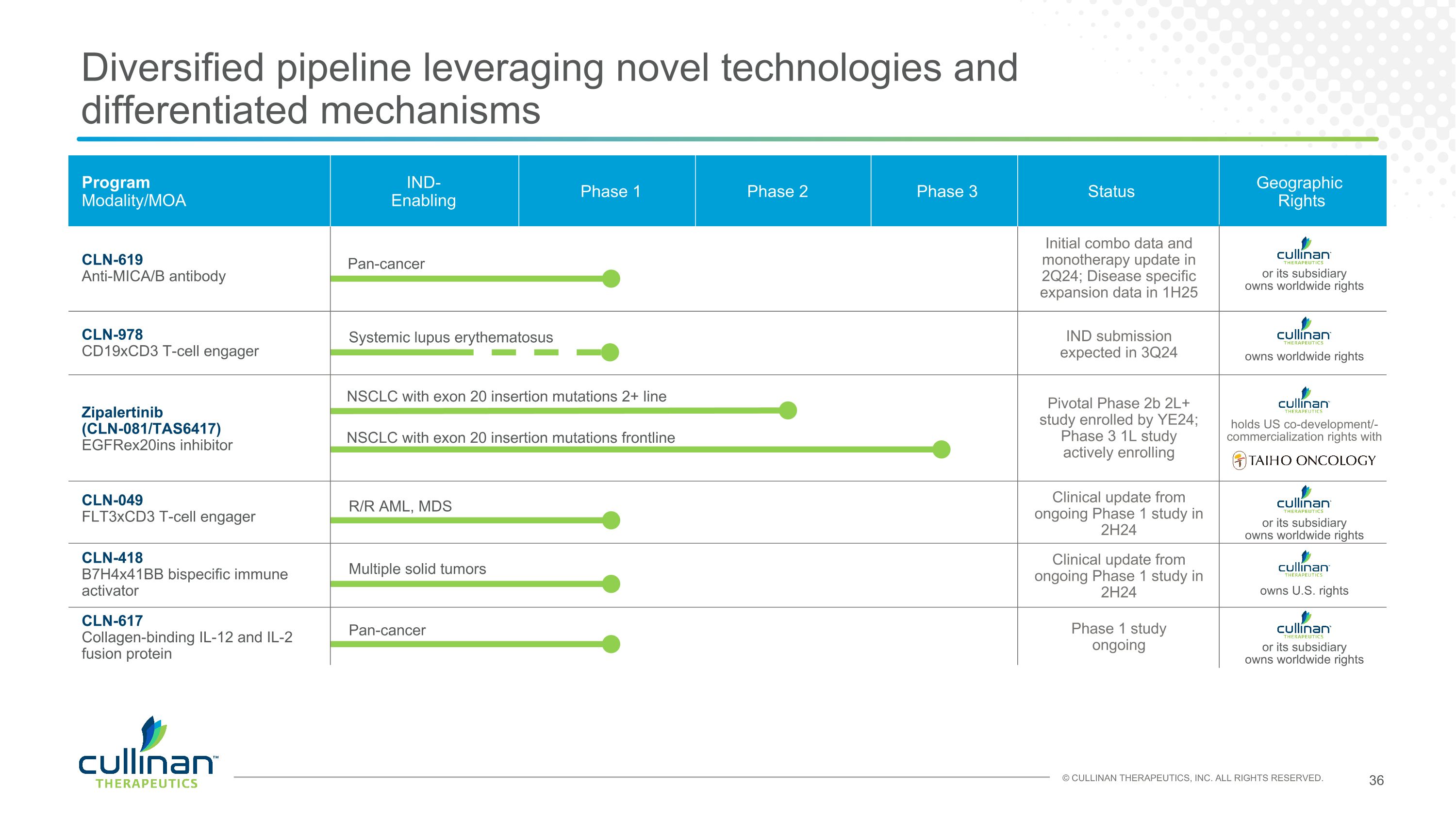
Diversified pipeline leveraging novel technologies and differentiated mechanisms Program�Modality/MOA IND-�Enabling Phase 1 Phase 2 Phase 3 Status Zipalertinib�(CLN-081/TAS6417) EGFRex20ins inhibitor CLN-049 FLT3xCD3 T-cell engager CLN-619 Anti-MICA/B antibody CLN-978 CD19xCD3 T-cell engager CLN-617 Collagen-binding IL-12 and IL-2�fusion protein NSCLC with exon 20 insertion mutations 2+ line R/R AML, MDS Pan-cancer Systemic lupus erythematosus Pan-cancer Pivotal Phase 2b 2L+ study enrolled by YE24; Phase 3 1L study actively enrolling Initial combo data and monotherapy update in 2Q24; Disease specific expansion data in 1H25 Clinical update from ongoing Phase 1 study in 2H24 IND submission expected in 3Q24 Phase 1 study ongoing CLN-418 B7H4x41BB bispecific immune activator Multiple solid tumors Early Programs holds US co-development/-commercialization rights with Geographic Rights owns U.S. rights NSCLC with exon 20 insertion mutations frontline or its subsidiary�owns worldwide rights or its subsidiary�owns worldwide rights owns worldwide rights or its subsidiary�owns worldwide rights Clinical update from ongoing Phase 1 study in 2H24