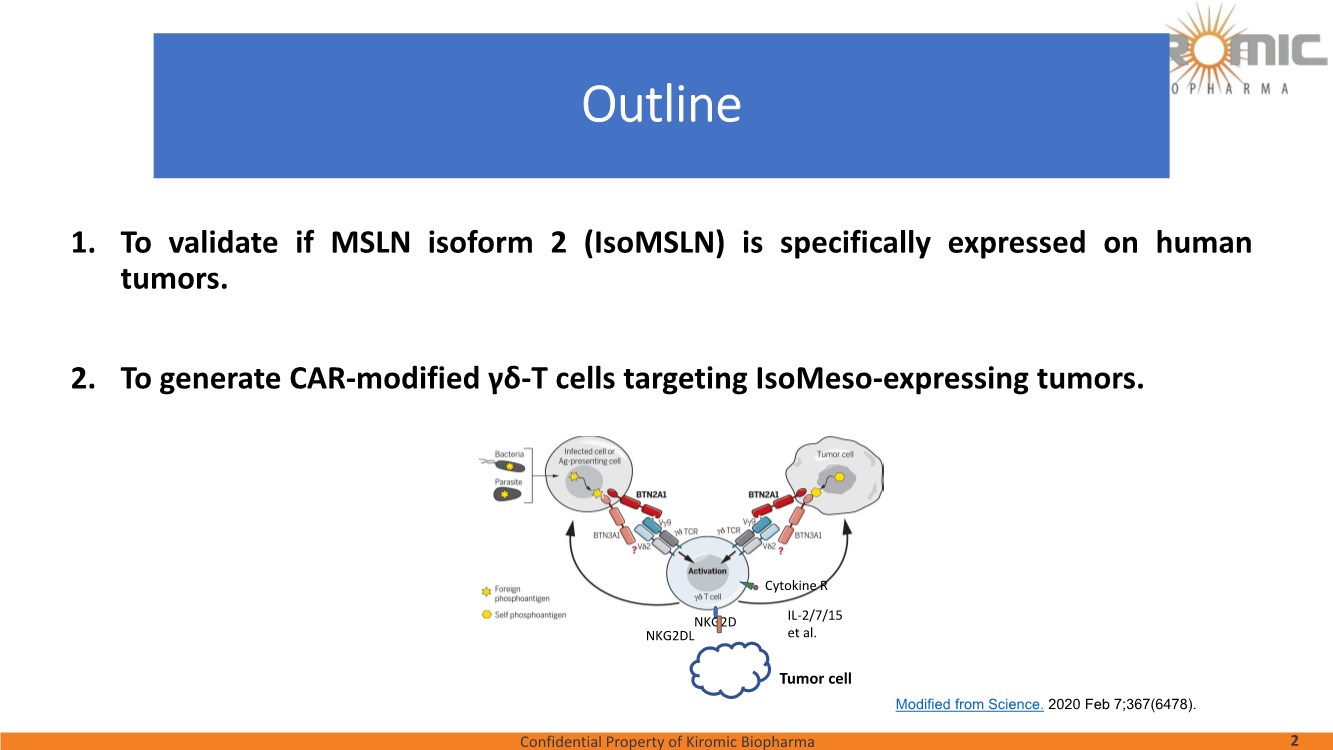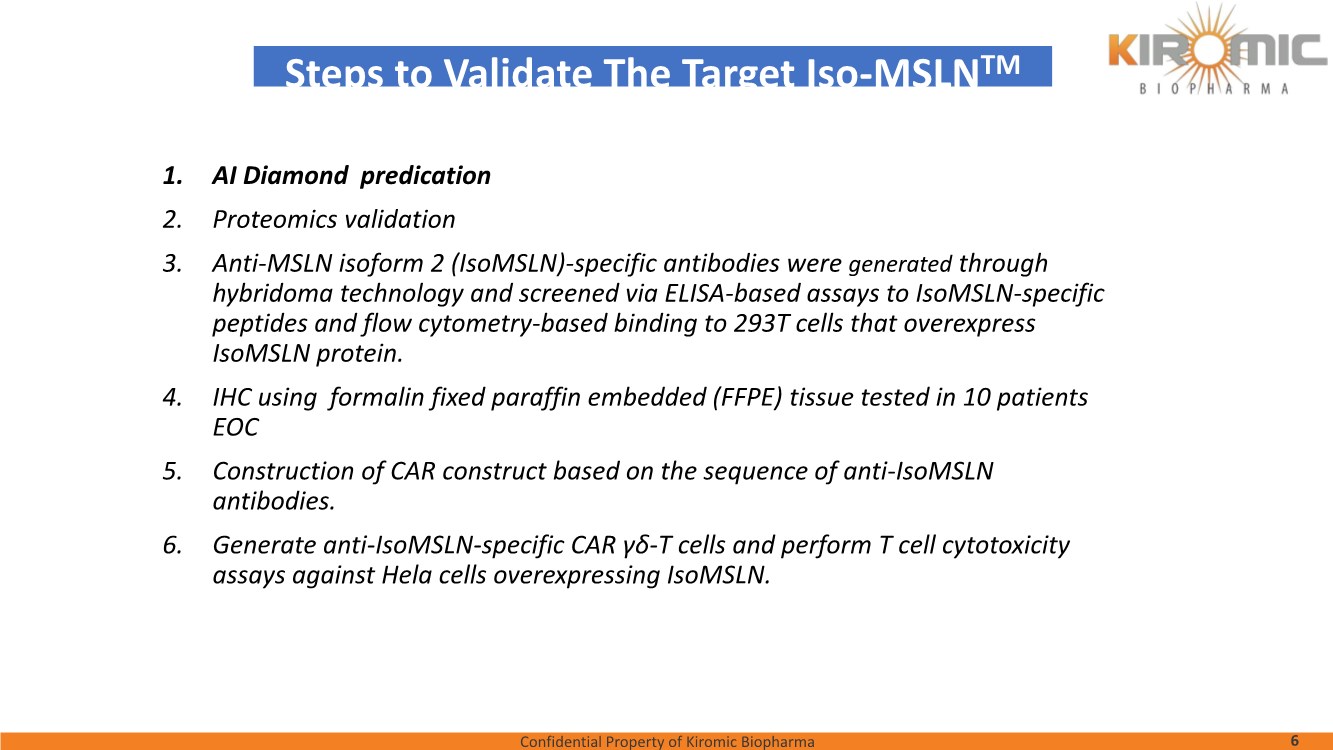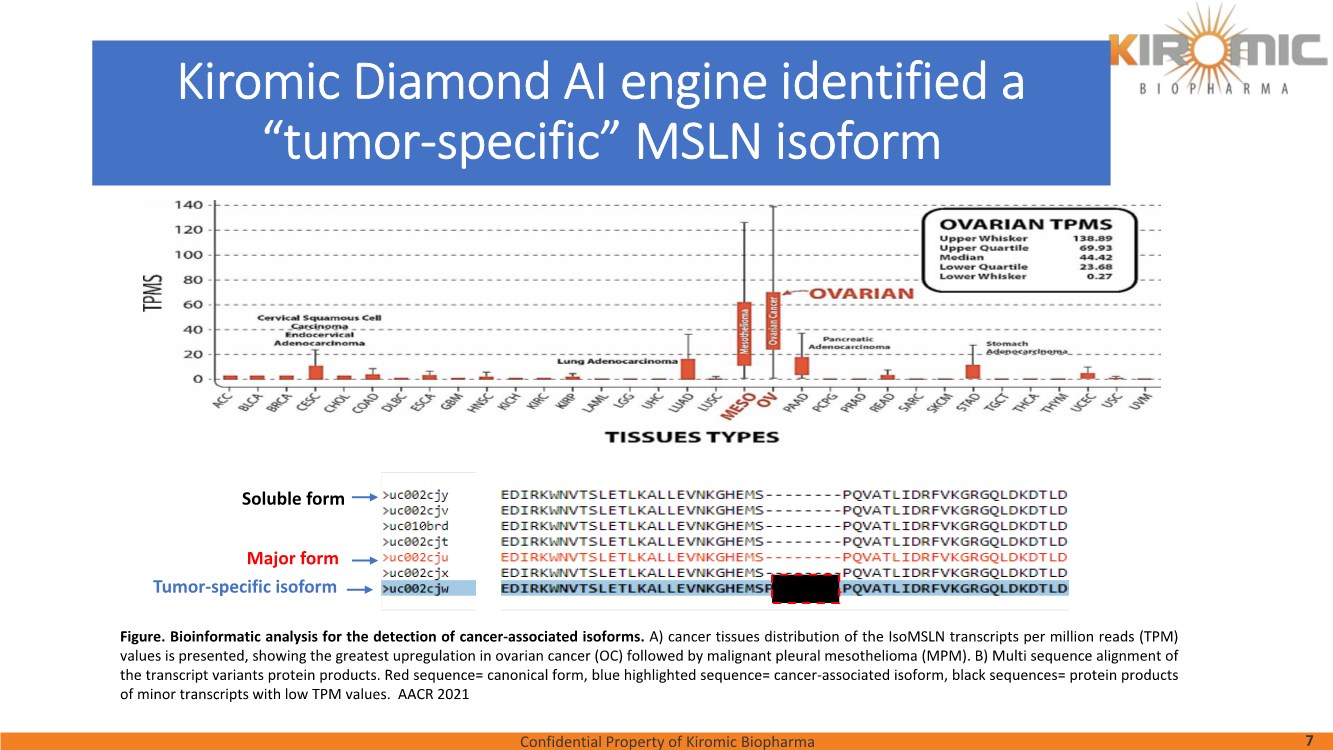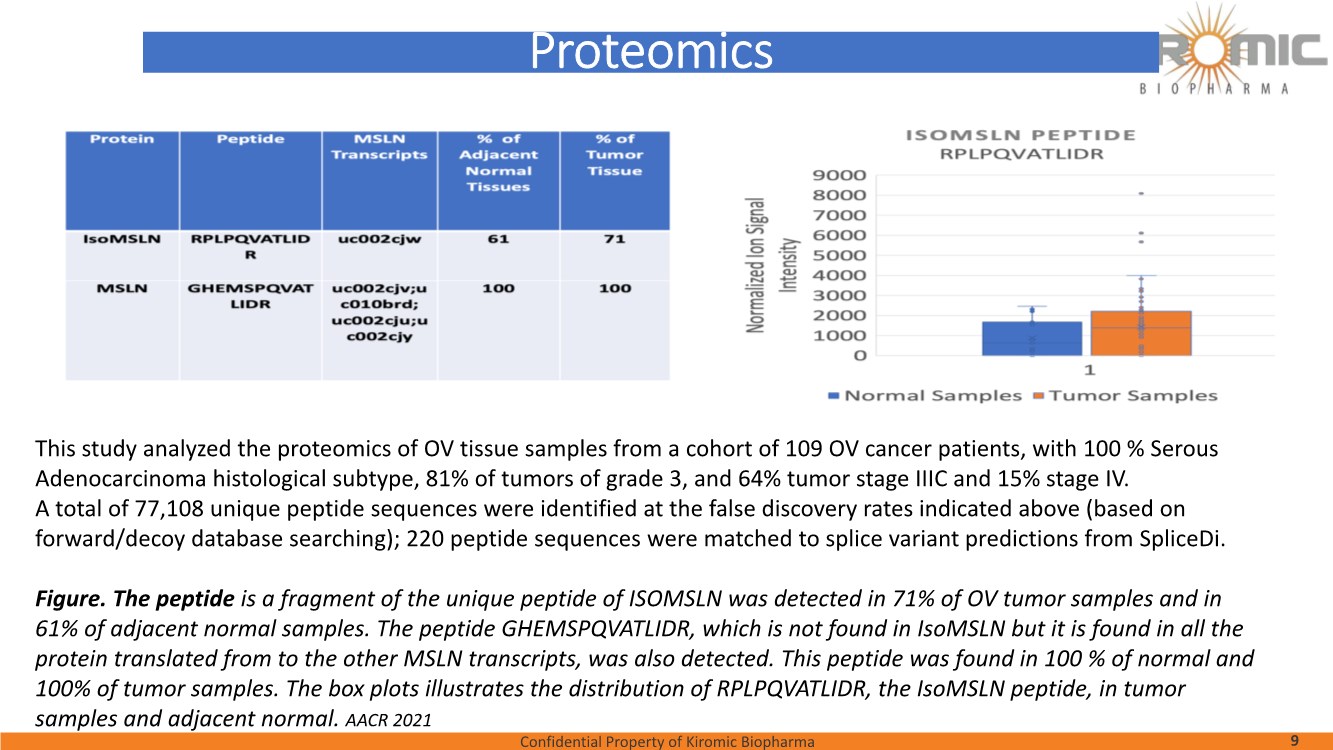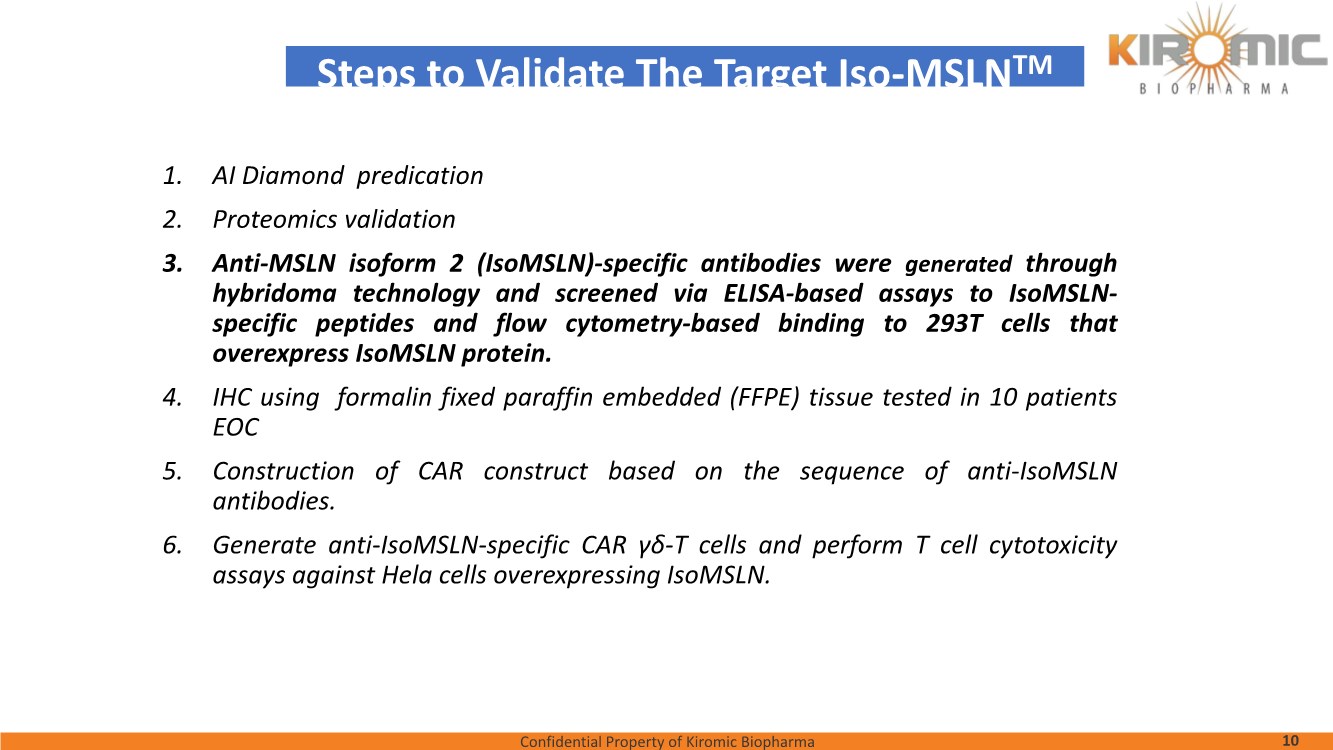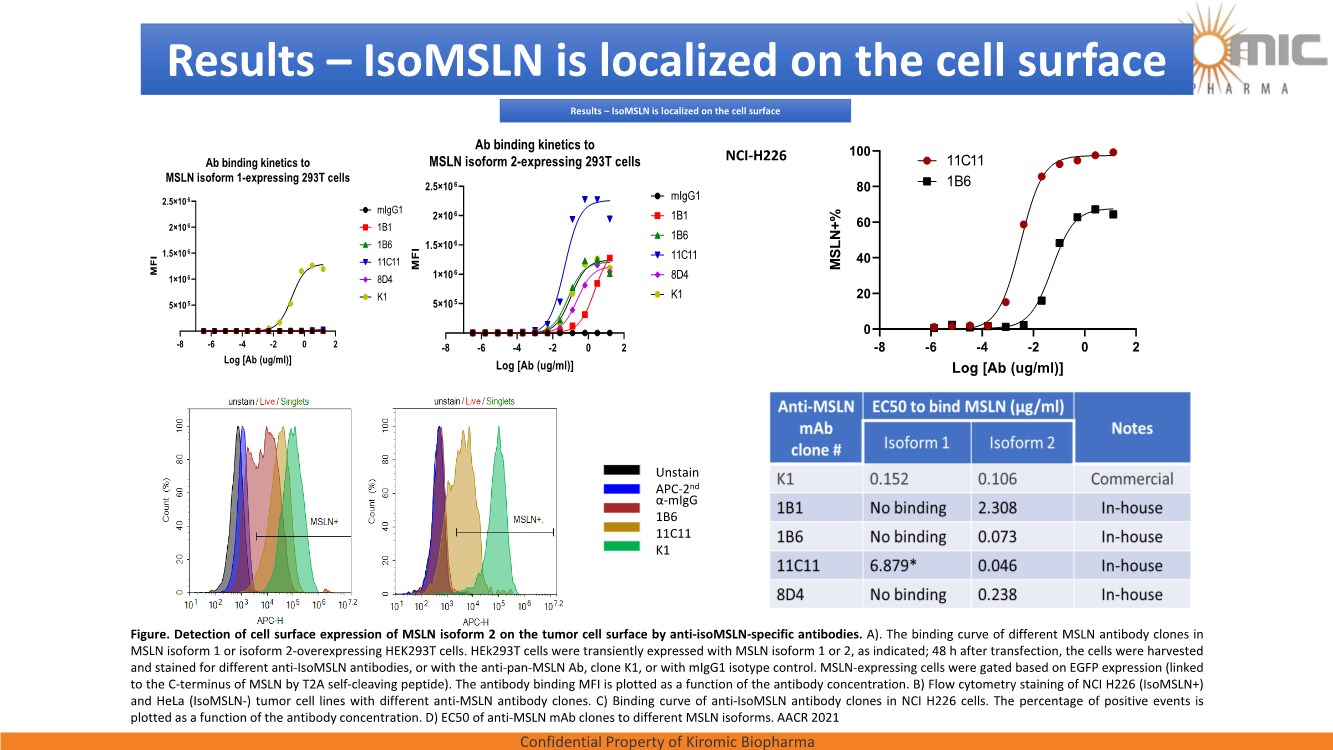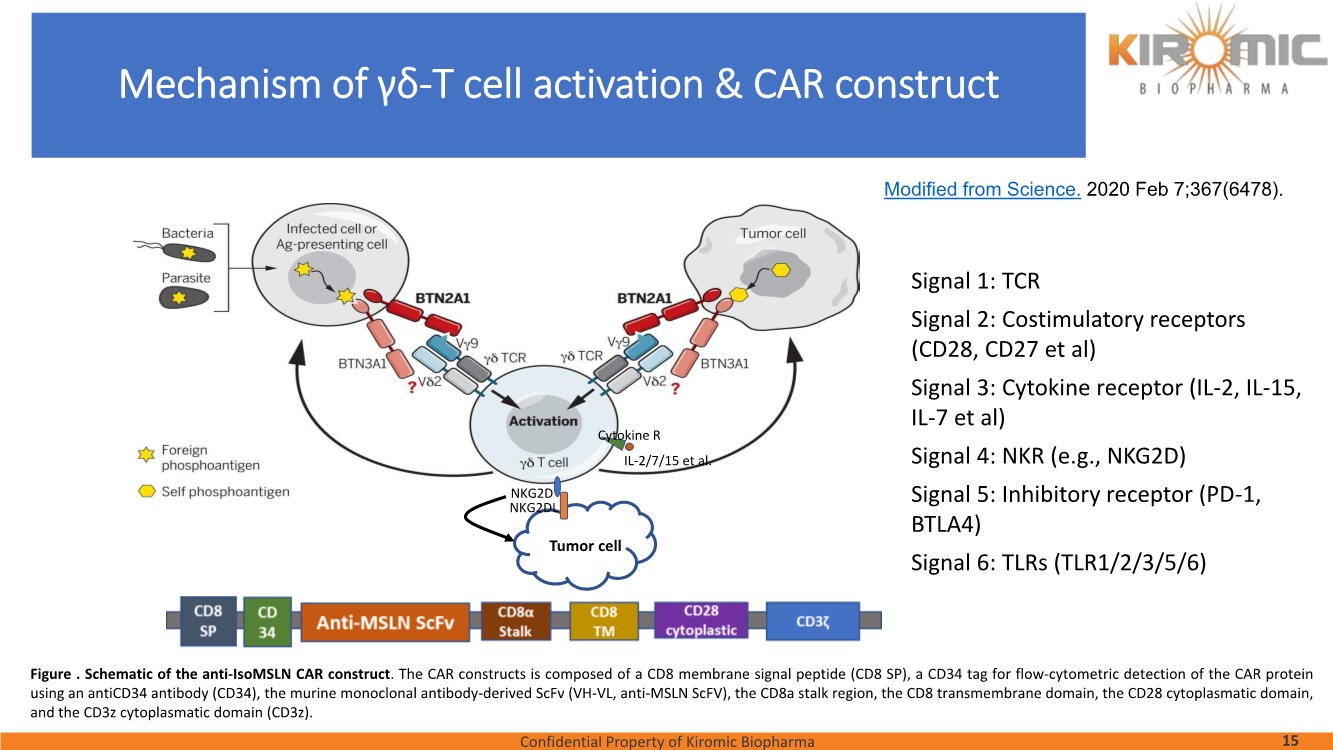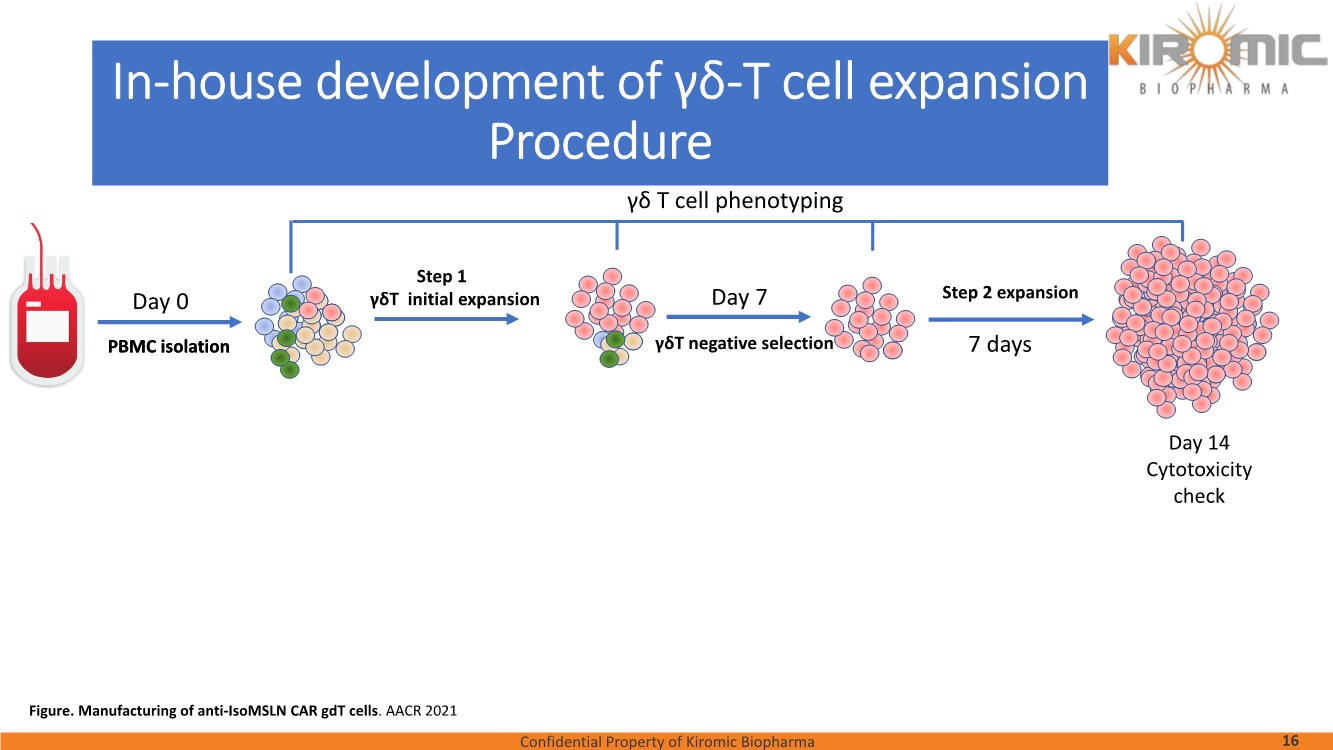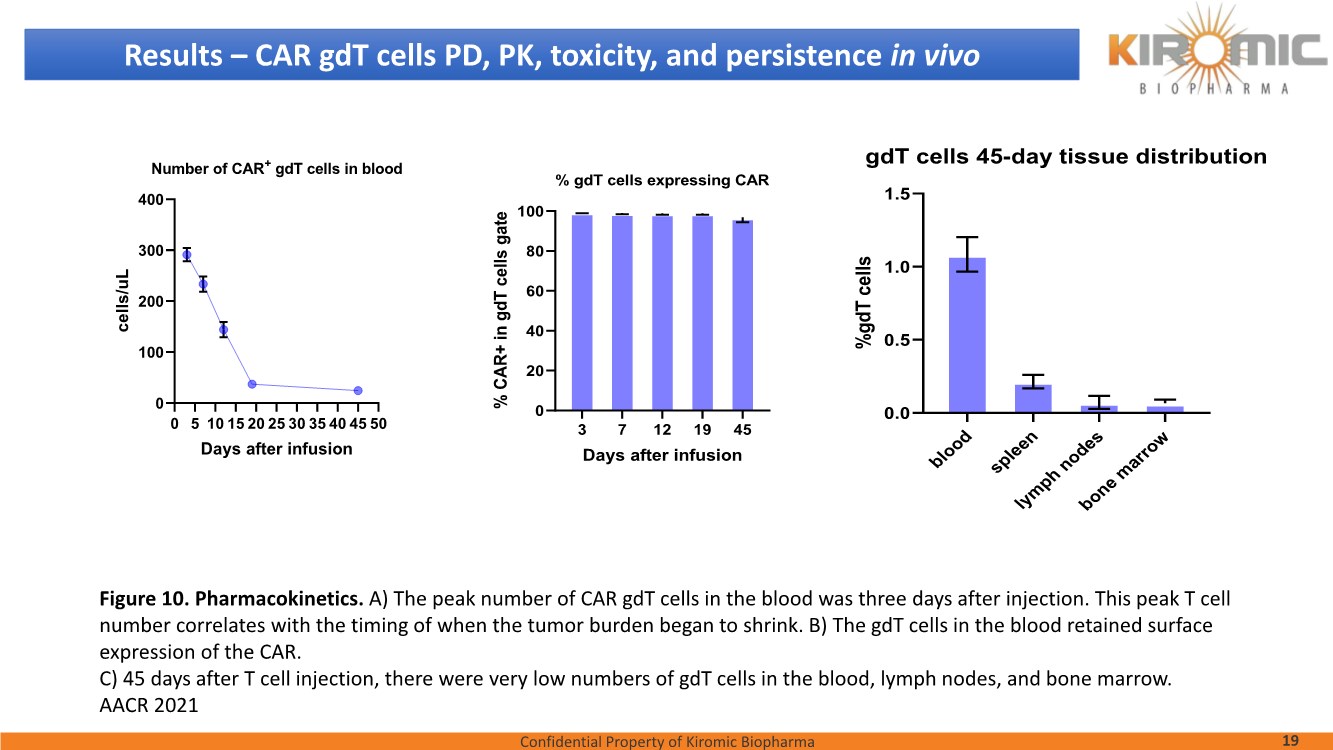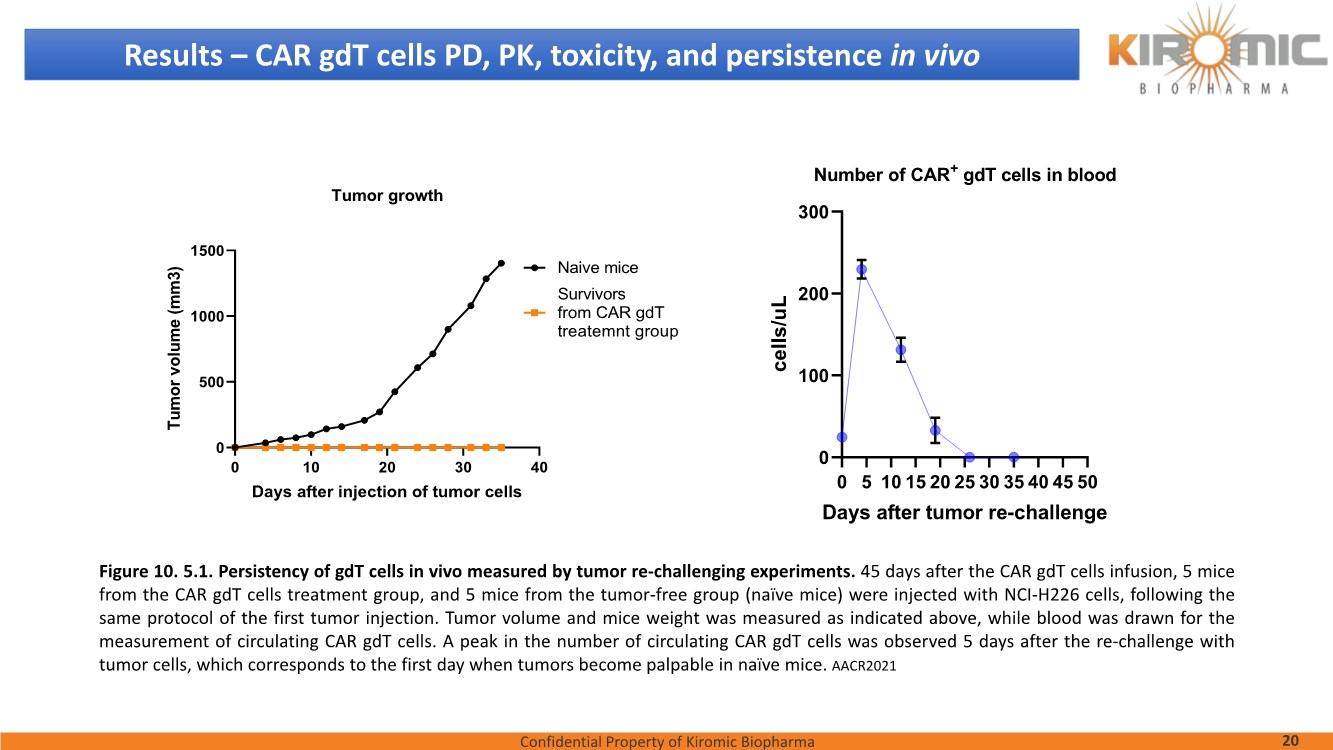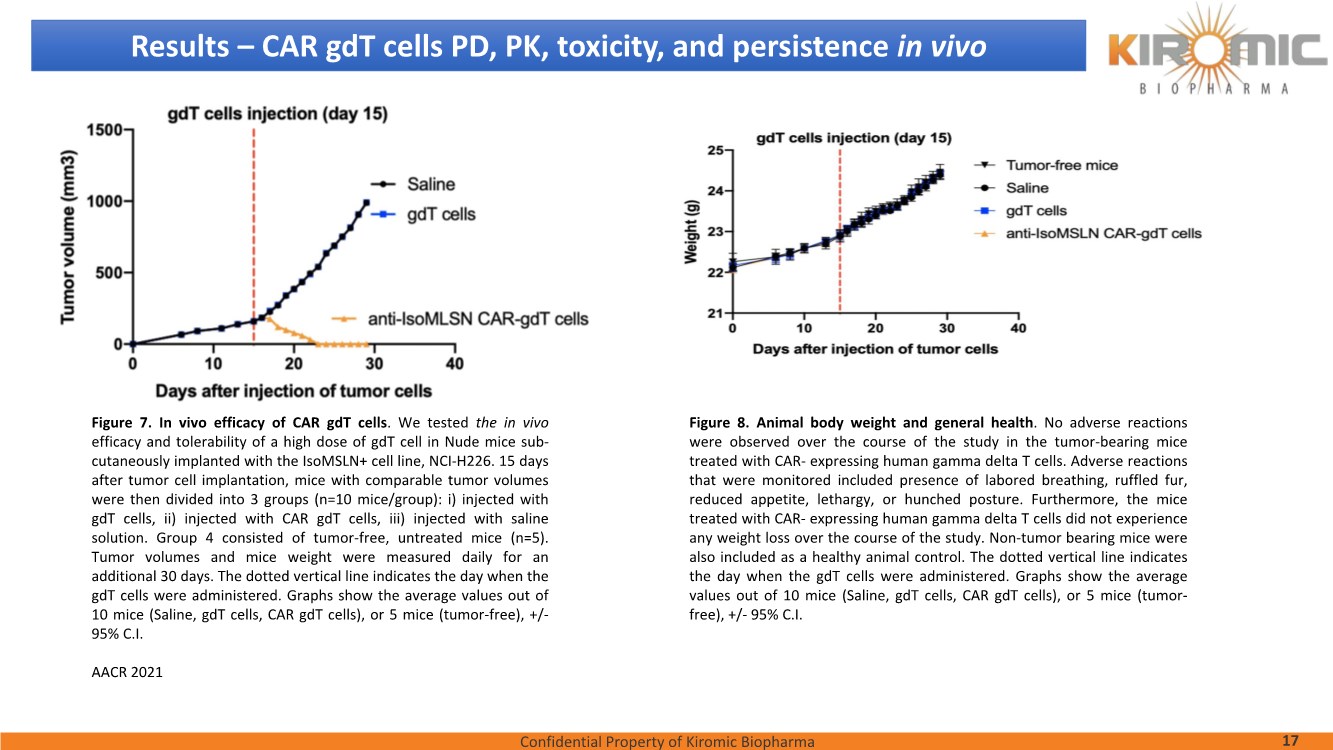
| Confidential Property of Kiromic Biopharma 17 Results – CAR gdT cells PD, PK, toxicity, and persistence in vivo Figure 8. Animal body weight and general health. No adverse reactions were observed over the course of the study in the tumor-bearing mice treated with CAR- expressing human gamma delta T cells. Adverse reactions that were monitored included presence of labored breathing, ruffled fur, reduced appetite, lethargy, or hunched posture. Furthermore, the mice treated with CAR- expressing human gamma delta T cells did not experience any weight loss over the course of the study. Non-tumor bearing mice were also included as a healthy animal control. The dotted vertical line indicates the day when the gdT cells were administered. Graphs show the average values out of 10 mice (Saline, gdT cells, CAR gdT cells), or 5 mice (tumor- free), +/- 95%C.I. Figure 7. In vivo efficacy of CAR gdT cells. We tested the in vivo efficacy and tolerability of a high dose of gdT cell in Nude mice sub- cutaneously implanted with the IsoMSLN+ cell line, NCI-H226. 15 days after tumor cell implantation, mice with comparable tumor volumes were then divided into 3 groups (n=10 mice/group): i) injected with gdT cells, ii) injected with CAR gdT cells, iii) injected with saline solution. Group 4 consisted of tumor-free, untreated mice (n=5). Tumor volumes and mice weight were measured daily for an additional 30 days. The dotted vertical line indicates the day when the gdT cells were administered. Graphs show the average values out of 10 mice (Saline, gdT cells, CAR gdT cells), or 5 mice (tumor-free), +/- 95%C.I. AACR 2021 |