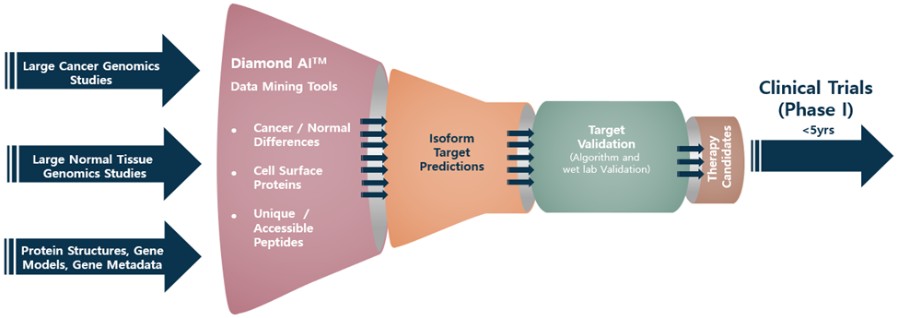
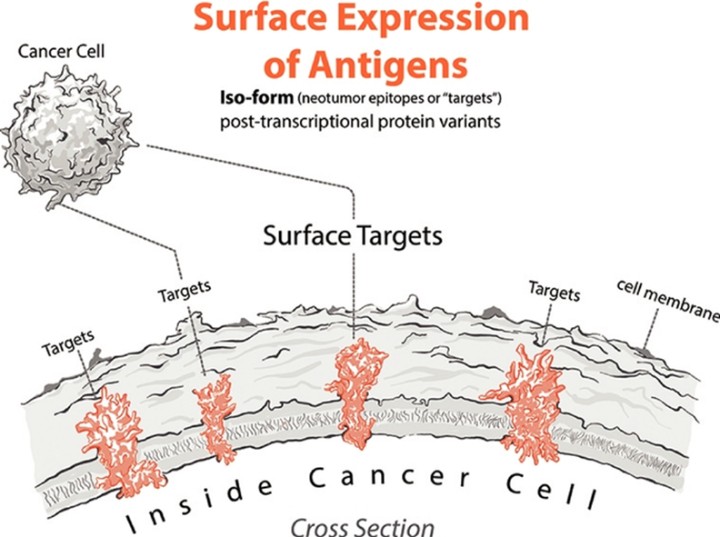
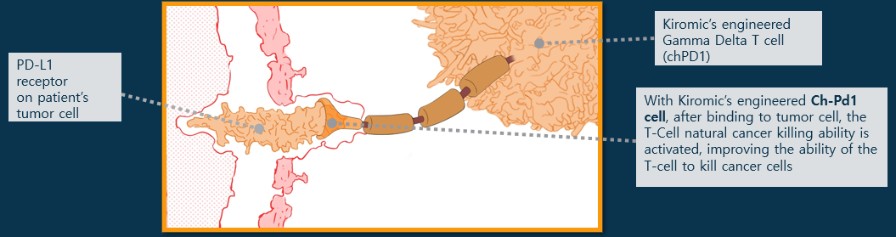
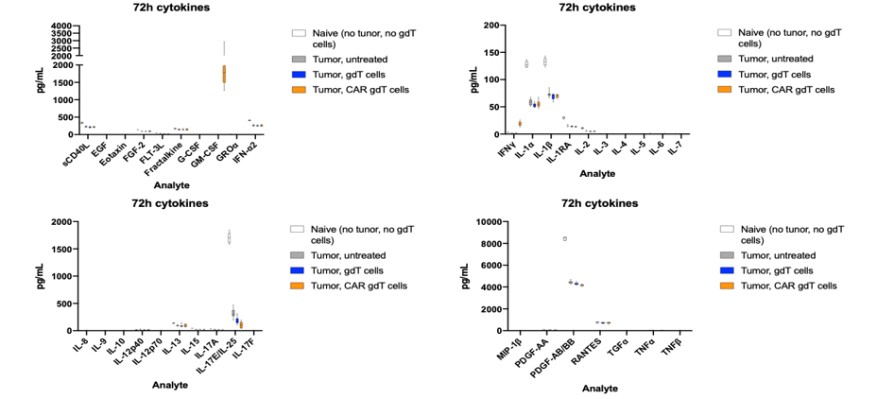
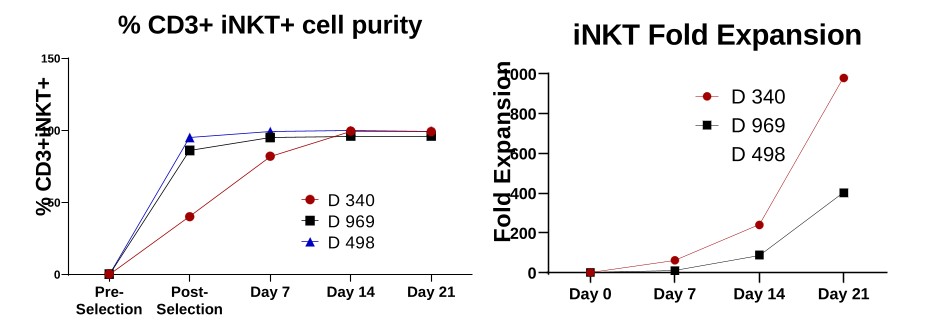
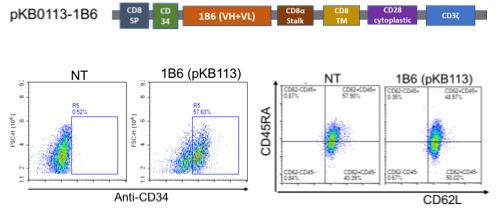
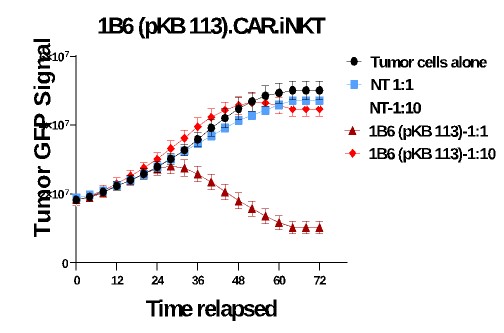
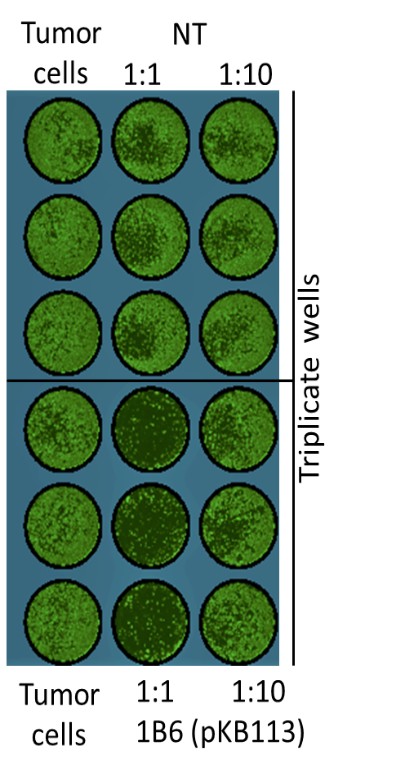
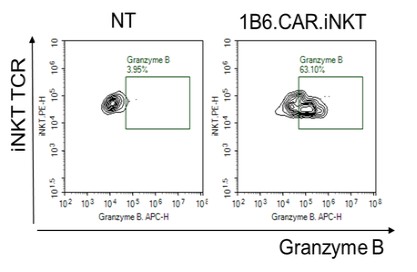
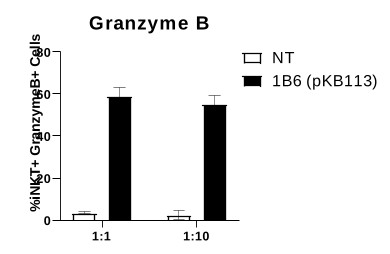
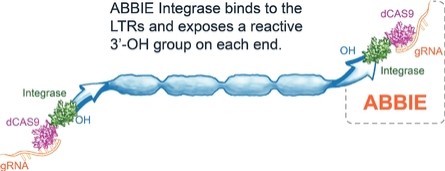
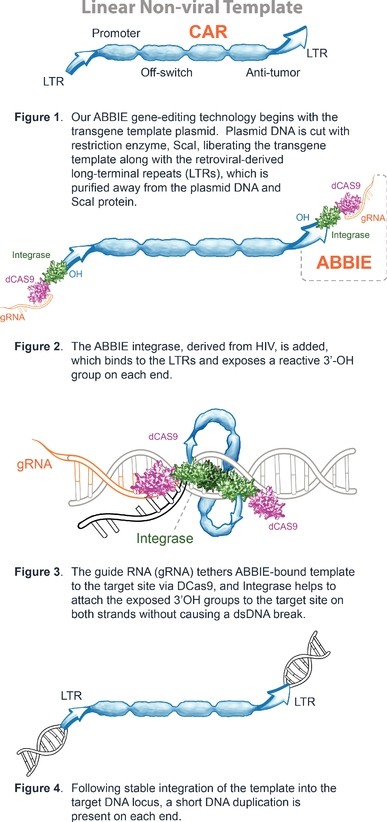
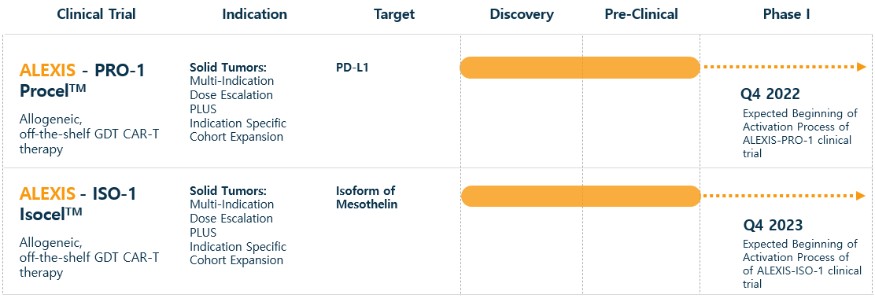
Our Research and Development Collaborations
University of Texas MD Anderson Cancer Center Grant
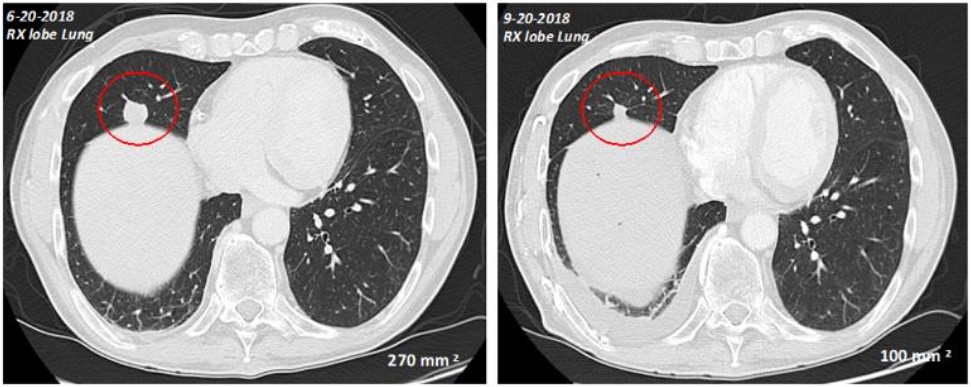
On April 8, 2021, we entered into a letter of intent (the “Letter of Intent”) with the University of Texas MD Anderson Cancer Center (“MD Anderson”) pursuant to which MD Anderson shall receive a research grant from the Company titled, “Validation of biomarker isomeso for pancreatic cancer,” which is aimed at discovering new cancer-specific antigen targets (the “Grant”). The total costs to the Company to be paid in connection with the Grant was $300,000. Pursuant to the Letter of Intent, the Grant commenced on April 1, 2021 and was initially scheduled to end on March 31, 2022. The Company extended the Grant until March 31, 2023 at no additional cost.
Molipharma Agreement
On April 3, 2020, we entered into a joint venture agreement ("Joint Venture") with Molipharma, S.R.L. ("Molipharma") to collaborate in (1) a clinical trial program in oncology development ("Oncology") and (2) a clinical trial program in COVID-19 Vaccine ("COVID-19 Vaccine").
With respect to Oncology, we will grant a low single digit royalty to Molipharma for turnover of the marketing of ovarian cancer research in Europe. With respect to COVID-19 Vaccine, economic rights in Europe will transfer to Molipharma, and economic rights in the U.S. will transfer to us. Molipharma agreed to financially support the research program for COVID-19 and we agreed to financially support the research program in oncology. The Joint Venture has a duration of five years, extendable for a further five years, unless notice of non-renewal is sent one year before the expiration date. The parties may withdraw from the Joint Venture only for serious and justified reasons or by mutual consent.
Our Competition
Our products will compete with novel therapies developed by biopharmaceutical companies, academic research institutions, governmental agencies and public and private research institutions, in addition to standard of care treatments.
In 2017, two autologous anti-CD19 CAR-T cell therapies, Kymriah, developed by Novartis International AG, and Yescarta, developed by Kite Pharma, Inc., were approved by the FDA for the treatment of relapsing/remitting B cell precursor acute lymphoblastic leukemia and relapsing/remitting large B cell lymphoma, respectively.
Due to the promising therapeutic effect of T cell therapies in clinical trials, we anticipate increasing competition from existing and new companies developing these therapies, as well as in the development of allogenic T cell therapies.
Potential cell therapy competitors include:
• Autologous T cell therapy competition: Adaptimmune Therapeutics PLC, Amgen Inc., Autolus Therapeutics plc, bluebird, Gilead (acquired Kite), Novartis International AG, Celgene (acquired Juno), Tmunity Therapeutics, Inc. and Unum Therapeutics Inc.
• Allogenic T cell therapy competition: Atara Biotherapeutics, Inc., Celyad S.A., CRISPR Therapeutics AG, Fate Therapeutics Inc., Intellia Therapeutics, Inc., Gilead (acquired Kite), Allogene Therapeutics, Inc., Poseida Therapeutics, Inc., Precision Biosciences, Inc., Sangamo Therapeutics, Inc., and Cytomed Therapeutics Pte, Ltd.
Competition will also arise from non-cell based immune and other pursued by small-cap biotechnology and large-cap pharmaceutical companies including Amgen Inc., AstraZeneca plc, Bristol-Myers Squibb Company, Incyte Corporation, Merck & Co., Inc., and F. Hoffmann-La Roche AG. For instance, we may experience competition from companies, such as Amgen Inc., Regeneron Pharmaceuticals, Inc., Xencor Inc., MacroGenics, Inc., GlaxoSmithKline plc and F. Hoffmann-La Roche AG, that are pursuing bispecific antibodies, which target both the cancer antigen and TCR, thus bringing both cancer cells and T cells in close proximity to maximize the likelihood of an immune response to the cancer cells. Additionally, companies, such as Amgen Inc., GlaxoSmithKline plc and Seattle Genetics, Inc., are pursuing