- PPD Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
-
ETFs
- Insider
- Institutional
- Shorts
-
DRS Filing
PPD (PPD) DRSDraft registration statement
Filed: 20 Jul 20, 12:00am
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
As confidentially submitted to the Securities and Exchange Commission on July 20, 2020
This draft registration statement has not been filed with the Securities and Exchange Commission and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
PPD, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 8731 | 45-3806427 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
929 North Front Street
Wilmington, North Carolina 28401
(910) 251-0081
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
B. Judd Hartman
Executive Vice President, General Counsel and Chief Administrative Officer
PPD, Inc.
929 North Front Street
Wilmington, North Carolina 28401
(910) 251-0081
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
William B. Brentani Simpson Thacher & Bartlett LLP 2475 Hanover Street Palo Alto, California 94304 Tel: (650) 251-5000 Fax: (650) 251-5002 | Patrick H. Shannon Jason M. Licht Latham & Watkins, LLP 555 Eleventh Street, NW—Suite 1000 Washington, D.C. 20004 Tel: (202) 637-2200 Fax: (202) 637-2201 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| ||||||||
| Title of Each Class of Securities to be Registered | Amount to be Registered(1) | Proposed Maximum Offering Price Per Share | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee | ||||
Common stock, $0.01 par value per share | $ | $ | $ | |||||
| ||||||||
| ||||||||
| (1) | Includes shares that the underwriters have the option to purchase. See “Underwriting.” |
| (2) | Estimated solely for the purpose of computing the amount of the registration fee. In accordance with Rule 457(c) under the Securities Act of 1933, as amended, the maximum offering price per share and maximum aggregate offering price are based on the average of the $ (high) and $ (low) sale price of the registrant’s common stock as reported on The Nasdaq Global Select Market on , 2020, which date is within five business days prior to filing this Registration Statement. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The information in this prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the Registration Statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and the selling stockholders are not soliciting offers to buy the securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus dated July 20, 2020
PROSPECTUS
Shares
PPD, Inc.
Common Stock
This is a public offering of shares of common stock of PPD, Inc.
The selling stockholders identified in this prospectus are offering shares of our common stock. We are not selling any shares of common stock under this prospectus and will not receive any proceeds from the sale of the shares by the selling stockholders.
Our common stock is listed and traded on The Nasdaq Global Select Market (“Nasdaq”) under the symbol “PPD.” On July 17, 2020, the last reported sale price of our common stock on Nasdaq was $30.12 per share.
Investing in our common stock involves risks that are described in the “Risk Factors” section beginning on page 19 of this prospectus and the risk factors in the documents incorporated by reference in this prospectus.
| Per Share | Total | |||||||
Public offering price | $ | $ | ||||||
Underwriting discount(1) | $ | $ | ||||||
Proceeds, before expenses, to selling stockholders | $ | $ | ||||||
| (1) | See “Underwriting” for a description of the compensation payable to the underwriters. |
The selling stockholders have granted the underwriters an option exercisable for 30 days after the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of shares from the selling stockholders at the public offering price less underwriting discounts and commissions.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The shares will be ready for delivery on or about , 2020.
The date of this prospectus is , 2020.
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83

OUR PURPOSE
Improve Health
OUR MISSION
Help Our Customers Deliver
Life-Changing Therapies
OUR STRATEGY
Bend the Cost and Time Curve of
Drug Development and Optimize
Value for Our Customers
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| Page | ||||
| 1 | ||||
| 19 | ||||
| 43 | ||||
| 46 | ||||
| 47 | ||||
| 48 | ||||
| 49 | ||||
| 53 | ||||
| 62 | ||||
Certain United States Federal Income and Estate Tax Consequences to Non-U.S. Holders | 64 | |||
| 67 | ||||
| 75 | ||||
| 75 | ||||
We, the selling stockholders and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses that we have prepared. We, the selling stockholders and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of the shares. Our business, financial condition, results of operations and prospects may have changed since the date on the front cover of this prospectus.
For investors outside the United States: We, the selling stockholders and the underwriters have not done anything that would permit a public offering of the shares of our common stock or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside of the United States.
i
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Trademarks and Service Marks
All trademarks, trade names, product names, graphics and logos of PPD contained herein or incorporated by reference are trademarks or registered trademarks of PPD, Inc. or its subsidiaries, as applicable, in the United States and/or other countries. All other party trademarks, trade names, product names, graphics and logos contained herein or incorporated by reference are the property of their respective owners. The use or display of other parties’ trademarks, trade names, product names, graphics or logos is not intended to imply, and should not be construed to imply, a relationship with, or endorsement or sponsorship of PPD, Inc. or its subsidiaries by, such other party. Solely for convenience, we may refer to trademarks in this prospectus and in the information incorporated by reference without the TM and ® symbols. Such references are not intended to indicate, in any way, that we or the respective owners will not assert, to the fullest extent permitted by law, our or their rights to such trademarks, as applicable.
Industry and Market Data
Market data used throughout this prospectus and in the information incorporated by reference is based on management’s knowledge of the industry and the good faith estimates of management. All of management’s estimates presented and incorporated by reference herein are based on industry sources, including analyst reports and management’s knowledge. We also relied, to the extent available, upon management’s review of independent industry surveys and publications prepared by a number of sources and other publicly available information. We are responsible for all of the disclosure in this prospectus and the information incorporated by reference herein and while we believe that each of the publications, studies and surveys used throughout this prospectus and the information incorporated by reference are prepared by reputable sources and are generally reliable, we have not independently verified market and industry data from third-party sources. All of the market data used in this prospectus and the information incorporated by reference involves a number of assumptions and limitations and therefore is inherently uncertain and imprecise, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” in this prospectus, PPD’s Annual Report on Form 10-K for the fiscal year ended December 31, 2019 (“PPD’s 2019 10-K”) and PPD’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2020 (“PPD’s 2020 Q1 10-Q”). These and other factors could cause results to differ materially from those expressed in our estimates and beliefs and in the estimates prepared by independent parties.
Basis of Presentation
Unless otherwise indicated or the context otherwise requires, references in this prospectus to:
| • | the term “2025 Notes” means the 4.625% Senior Notes due 2025 issued by Jaguar Holding Company II and PPD Development, L.P.; |
| • | the term “2028 Notes” means the 5.000% Senior Notes due 2028 issued by Jaguar Holding Company II and PPD Development, L.P.; |
| • | the term “Company” or references to “PPD,” “we,” “us” and “our” means PPD, Inc. and its consolidated subsidiaries; |
| • | the term “Majority Sponsors” means those certain investment funds of The Carlyle Group Inc. and its affiliates (“Carlyle”) and Hellman & Friedman LLC and its affiliates (“Hellman & Friedman”); |
| • | the term “Notes” means the 2025 Notes and the 2028 Notes; |
| • | the term “Senior Secured Credit Facilities” means the senior secured term loan (the “Term Loan”), of which $3.088 billion was outstanding as of March 31, 2020, and revolving credit facility (the “Revolving Credit Facility”), of which $150.0 million was outstanding as of March 31, 2020, under the Credit Agreement, dated as of August 18, 2015 (as amended, the “Credit Agreement”), among Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG, Cayman Islands Branch, as Administrative Agent, Collateral Agent and L/C Issuer, and each lender from time to time party thereto, as amended; and |
ii
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | the term “Sponsors” means, collectively, the Majority Sponsors, Blue Spectrum ZA 2015 LP, a Cayman Islands exempted limited partnership, an investment vehicle of the Abu Dhabi Investment Authority, and its successors and permitted assigns, and Clocktower Investment Pte Ltd., a Singapore private limited company, and its successors and permitted assigns. |
Where You Can Find Additional Information
We have filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. This prospectus is a part of the registration statement and does not contain all of the information set forth in the registration statement and its exhibits and schedules, portions of which have been omitted as permitted by the rules and regulations of the SEC. For further information about us and our common stock, you should refer to the registration statement and its exhibits and schedules.
We file reports and other information with the SEC. Our SEC filings are available to the public at the SEC’s website at http://www.sec.gov as well as the PPD Investor Relations website at https://investors.ppd.com/investor-relations; however, information on, or accessible through, our website is not part of this prospectus.
Incorporation by Reference
The rules of the SEC allow us to incorporate by reference information we file with the SEC. This means that we are disclosing important information to you by referring to other documents. The information incorporated by reference is considered to be part of this prospectus. To the extent there are inconsistencies between the information contained in this prospectus and the information contained in the documents filed with the SEC prior to the date of this prospectus and incorporated by reference, the information in this prospectus shall be deemed to supersede the information in such incorporated documents. We incorporate by reference the documents listed below (other than any portions thereof, which under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and applicable SEC rules, are not deemed “filed” under the Exchange Act):
| • | PPD’s 2019 10-K as superseded by, and solely to the extent set forth in, our first Current Report on Form 8-K filed on May 21, 2020 (“PPD’s May 2020 8-K”); |
| • | PPD’s 2020 Q1 10-Q; and |
| • | our Current Reports on Form 8-K, filed on February 11, 2020, February 18, 2020, May 21, 2020 and June 5, 2020. |
If we have incorporated by reference any statement or information in this prospectus and we subsequently modify that statement or information with information contained in this prospectus, the statement or information previously incorporated in this prospectus is also modified or superseded in the same manner.
We will provide without charge to each person to whom a copy of this prospectus has been delivered, a copy of any and all of these filings. You may request a copy of these filings by writing to us at:
Investor Relations
929 North Front Street
Wilmington, NC 28401
e-mail: Investors@ppd.com
Exhibits to any documents incorporated by reference in this prospectus will not be sent, however, unless those exhibits have been specifically referenced in this prospectus.
iii
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
This prospectus summary highlights selected information contained or incorporated by reference in this prospectus and may not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read the entire prospectus, including the information incorporated by reference. For a more complete understanding of our company and this offering and before making any investment decision, you should read this entire prospectus and the information incorporated by reference, including “Risk Factors.”
Our Company
We are a leading provider of drug development services to the biopharmaceutical industry, focused on helping our customers bring their new medicines to patients around the world. We have been in the drug development business for more than 30 years, providing a comprehensive suite of clinical development and laboratory services to pharmaceutical, biotechnology, medical device, government organizations and other industry participants. We have deep experience across a broad range of rapidly growing areas of drug development and engage with customers through a variety of commercial models, including both full-service and functional service partnerships and other offerings tailored to address the specific needs of our customers. Our company is currently organized and managed around two reportable segments, clinical development services (“Clinical Development Services”) and laboratory services (“Laboratory Services”).
Our purpose and mission are to improve health by helping our customers deliver life-changing therapies. We pursue our purpose and mission through our clinical development and laboratory services and our strategy to bend the cost and time curve of drug development and optimize value for our customers.
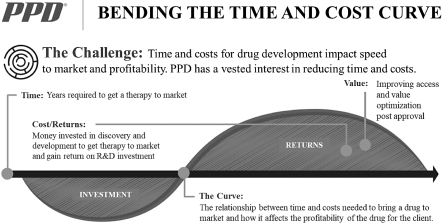
Our customers benefit from accelerated time to market because it results in lengthened periods of market exclusivity, and our real-world evidence solutions support the superior efficacy and value of their novel therapies. We believe our medical, scientific and drug development expertise, along with our innovative technologies and knowledge of global regulatory requirements, help our customers accelerate the development of safe and effective therapeutics and maximize returns on their research and development (“R&D”) investments.
Our service offerings include both clinical development and laboratory services. Our clinical development services include all phases of development (i.e., Phase I-IV), peri- and post-approval and site and patient access services. Our laboratory services offer a range of high-value, advanced testing services, including bioanalytical, biomarker, vaccine, good manufacturing practice (“GMP”) and central laboratory services. We have deep
1
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
experience across a broad range of rapidly growing areas of drug development and engage with customers through a variety of commercial models, including both full-service and functional service partnerships and other offerings tailored to address the specific needs of our customers.
We have developed significant expertise in the design and execution of complex global clinical trials, a result of conducting studies on global, national, regional and local levels across a wide spectrum of therapeutic areas for more than 30 years and in over 100 countries. Our customers entrust us to design, execute and deliver results on some of the most critical aspects of the drug development process for the key assets in their pipelines. As of March 31, 2020, we had approximately 24,000 employees worldwide, approximately 5,100 of whom hold advanced degrees, and we had 100 offices in 46 countries. Over the last five years, we have conducted more than 2,100 clinical trials, and our laboratory scientists have completed more than 57,000 pharmaceutical development projects and worked with more than 7,600 compounds.
Our deep understanding of the drug development process has allowed us to effectively invest in, and evolve our service offerings to meet the needs of our customers. Examples of some of our recent initiatives and investments include:
| • | Innovative site and patient access. We have developed differentiated capabilities that meaningfully address two of the biggest challenges that our customers face: patient enrollment and site performance. |
| • | Purpose-built PPD Biotech offering. Over the past five years, we pioneered the development of a new model to better serve the specific needs of the biotechnology customer segment. |
| • | Advanced laboratory services. Over the last five years, in response to strong customer demand for our services, we have invested over $200 million to significantly increase the size and operating capacity of our laboratory facilities, acquire innovative laboratory equipment, expand our test menus and build out differentiated IT systems and laboratory automation. |
| • | Innovative peri-and post-approval studies. We have significantly expanded our capabilities in this growing area, providing our customers with service offerings in areas such as (i) market access, (ii) health economics modeling and (iii) patient-centered research. |
| • | Targeted geographic expansion. We maintain a strong presence of experienced professionals in all key regions and countries necessary to support our customers’ global drug development programs. In response to the growing importance of conducting global studies that include cohorts in Japan and China and the opportunity to serve local customers in those geographies with their global drug development needs, we have significantly increased the size and scale of our operations in those countries while maintaining the quality and operating standards demanded by our customers and regulatory authorities alike. |
We believe these investments in our businesses and our innovative solutions have enhanced the strength of our clinical development and laboratory services and further differentiated our offerings from other clinical development organizations, providing us with meaningful competitive advantages and growth opportunities.
Our Markets
The drug development process involves the testing of drug candidates to demonstrate safety and efficacy in order to meet regulatory requirements. Developing new drugs for the treatment of human disease is an extremely expensive, complex, high-risk and time-consuming process. It is estimated that bringing a new drug or medical device to market can take up to 15 years and cost $2.5 billion or more.
The drug development process consists of two stages: pre-clinical and clinical. The clinical stage is the most time-consuming and expensive part of the drug development process. During the clinical stage, the drug
2
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
candidate undergoes a series of tests in humans, including healthy volunteers, as well as participants with the targeted disease or condition. Human trials usually start on a small scale to assess safety, efficacy and dosage (Phase I–II) and then expand to larger trials (Phase III) to test efficacy and safety in the target population. Phase IV, or post-approval trials, involve monitoring or verifying the risks and benefits of a drug product.
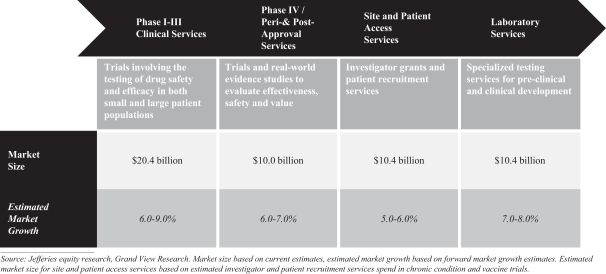
As of December 31, 2019, our total addressable market was greater than $51 billion, consisting of clinical development services, including peri- and post-approval services and site and patient enrollment services, and laboratory services. We believe the clinical development services (Phase I–III), or clinical research organization (“CRO”), market to be an approximately $20.4 billion market as of December 31, 2019 and expect this market to continue to grow at an average annual growth rate of approximately 6-9%. We have expanded our capabilities in the $10.0 billion Phase IV and peri- and post-approval services market, which we anticipate will grow at an annual growth rate of approximately 6-7%. Our Accelerated Enrollment Solutions (“AES”) delivery model has allowed us to participate in the economics and growth of the investigator and patient recruitment market that otherwise would represent pass-through revenues, as it does for most other CROs. We expect this to be an approximately $10.4 billion market and anticipate it to grow at an annual growth rate of approximately 5-6%. In addition to competing in the CRO market, through our strategic investments we have strengthened our position in the laboratory services market and expanded our addressable market to include the markets for investigator and patient recruitment and peri- and post-approval services. In laboratory services, in addition to the $4.3 billion central laboratory market, we compete in the $6.0 billion market for advanced laboratory testing, which we anticipate will grow at an annual growth rate of 7-8%.
We believe there are five key trends affecting our end markets that will create increasing demand for our offering of services:
| • | Growth in R&D spending. Biopharmaceutical companies must continually invest in drug development in order to create innovative new therapies or use existing drugs to treat new indications, to address unmet medical needs and to replace lost revenues when their currently marketed drugs lose patent protection. From 2008 to 2018, R&D spending increased approximately 3.3% annually, driven by long-term secular fundamentals including a 30% increase in active INDs and an approximately 80% increase in average annual FDA approvals from 2008 to 2018. |
| • | Increased levels of outsourcing by biopharmaceutical companies. As biopharmaceutical companies continue to seek ways to reduce clinical development costs and focus resources on core competencies, |
3
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
we believe they will continue to increase the amount of clinical development work they outsource to CROs. Outsourcing penetration as a percentage of total development spending by biopharmaceutical companies increased from approximately 36% in 2007 to approximately 49% in 2018. |
| • | Increased complexity in clinical development. Clinical trials continue to increase in complexity due to a confluence of factors including, but not limited to, (i) new therapeutic modalities, (ii) the collection of more clinical trial endpoints, (iii) more specific patient inclusion/exclusion criteria, (iv) ever-changing regulatory requirements and (v) an expansion of evidence generation methods, such as electronic patient-reported outcomes and virtual clinical trials. All of these factors result in more complex trial design, challenges in enrolling protocol-eligible patients, longer duration of clinical trials and greater overall clinical trial cost. As a result, we expect biopharmaceutical companies to increasingly seek partners that have the experience and expertise to conduct cost-effective clinical studies. In particular, we believe large CROs who possess scale, geographic reach and differentiated capabilities to manage the complexity of clinical trials will continue to grow at a higher rate and take market share versus the overall industry. |
| • | Biotechnology sector growth. The U.S. biotechnology sector has grown rapidly over the last decade and has emerged as a key customer segment for the drug development services industry. The rate of biotechnology companies’ R&D spending growth has been higher than that of traditional pharmaceutical companies in recent years, and we believe that over the last five years, innovative biotechnology companies have accounted for approximately 40% of new drug approvals (“NDAs”). This has largely been fueled by a robust funding environment, both public and private, with over $150 billion of capital raised for biotechnology companies in the last three years. Today, we believe the majority of biotechnology companies have enough cash on hand to fund R&D expenditures for two to three years. Many biotechnology companies are smaller, discovery research-focused organizations that do not find it economically attractive to invest in the infrastructure and personnel necessary to conduct their clinical development programs on their own, and we believe they will continue to rely on CROs, like us, for their global drug development needs. |
| • | Increasing importance to prove value of new therapies. As participants in the healthcare industry are increasingly focused on managing costs, biopharmaceutical companies need to find alternatives to align market constituents on the value of their treatments. The ability to perform peri- and post-approval studies to transform real-world data (such as medical claims data or electronic medical records) into real-world evidence provides biopharmaceutical companies a solution to quantify the value of new therapies to market constituents. Real-world data and evidence enable biopharmaceutical companies to develop better therapies and optimize the commercial potential of their new therapies. With increased R&D activity and competition among newly approved therapies in similar indications, we anticipate the continued adoption of real-world data and evidence to demonstrate the value of new medicines. |
Our Competitive Strengths
We believe we are well-positioned to serve the global biopharmaceutical industry in obtaining the approval for, and maximizing the market access and value of, their new medicines. We differentiate ourselves from others in our industry through our competitive strengths, which include:
Leading Drug Development Expertise with Scale and a Long Track Record of Excellence
We are one of the world’s largest providers of clinical development services, with the scale to leverage investments in capabilities and innovative solutions to serve the increasingly complex and diverse needs across our extensive customer base. We have developed our scale, capabilities and track record of quality and
4
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
innovation over a more than 30-year history, earning us a reputation as a leading global partner to the most sophisticated biopharmaceutical companies. We believe the combination of our scale, expertise, track record and innovative offerings positions us to continue to grow and take market share within the industry.
Differentiated Clinical Development Services
Building on our solid foundation, we have invested heavily in recent years to further strengthen our competitive position through differentiated clinical development solutions designed to address our customers’ needs and bend the time and cost curve of their clinical trials. Our key clinical development investments improve trial feasibility, shorten study start-up timelines, accelerate enrollment, improve site performance, reduce the time and cost of monitoring trial sites and establish the value of new medicines.
Comprehensive and Growing Laboratory Services
We own and operate an integrated and scaled suite of laboratory services and offer a range of high-value, advanced testing services. We believe we are differentiated from other laboratory providers by our global scale and the comprehensiveness of our service offering. We believe we are one of the leading providers in each of the GMP, bioanalytical and central laboratory services sectors as well as in the growing vaccines market.
Large and Growing Diversified Customer Base
Over the past five years, we have provided services to all of the top 50 biopharmaceutical companies in the world, as ranked by 2018 R&D spending, small and mid-size pharmaceutical companies and over 300 biotechnology customers as well as government, academic and non-profit organizations. We have long-standing relationships with our customers as demonstrated by having provided services for a decade or more to each of our top ten customers by revenue for the year ended December 31, 2019. We have also strategically positioned ourselves to benefit from the rapid growth of the biotechnology market through the formation and build-out of PPD Biotech. As a result of our diversified customer base, no one customer accounted for more than 10% of our 2019 revenue.
Experienced, Highly Technical Organization with a Culture of Excellence and Industry-Leading Retention
We are led by an experienced and talented team of individuals who collectively have extensive experience in the CRO and biopharmaceutical industries and understand the challenges our customers face. We believe the technical and therapeutic expertise of our dedicated employees provides us with a competitive advantage—of our approximately 24,000 employees as of March 31, 2020, approximately 5,100 hold advanced, masters or equivalent degrees, including 1,100 MDs and PhDs. In recent years, we have made significant investments to build capabilities to effectively recruit, train, develop and retain talented individuals and teams. Our consistent focus on talent and culture has contributed to both overall retention and retention in key operational roles, such as project managers, that is significantly ahead of industry averages.
Disciplined Operational and Financial Approach
We have strategically oriented our business towards the largest and highest growth areas of the drug development services market, including key therapeutic areas, the biotechnology end market and peri- and post-approval services, in order to position ourselves to win high value-add business. Our operating model is focused on providing our customers with a mix of full-service contracts and select functional service provider commercial arrangements in differentiated value-add areas. We have also leveraged our track record of operational discipline and expertise around contract pricing and backlog policy to create a highly visible and stable revenue base. Furthermore, we have focused our operations on key initiatives, including optimal utilization
5
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
of billable staff and prudent cost management. Our positive historical operating results have allowed us to deploy significant capital into our business through strategic investments and acquisitions while also returning capital to our stockholders. We believe our strong financial profile demonstrates the quality and efficiency of our operating model and positions us for continued growth.
Our Growth Strategy
The key elements of our growth strategy to help our customers bend the cost and time curve of drug development include:
Further Strengthen Our Offerings in Existing and New Markets
Our global footprint, scale, integrated systems and deep scientific expertise enable us to conduct complex, multi-center clinical trials simultaneously throughout the world. We have a well-established presence in all of the major biopharmaceutical markets, including the United States, Europe and Asia, with nearly 3,800 professionals in the latter region and scale and differentiation in Japan and China, two countries of increasingly strategic importance for drug development programs. We plan to further strengthen our leadership position by investing in geographies that are critical to address the needs of our customers and their drug development pipelines.
Expand Leading Therapeutic Expertise in Existing and Novel Areas
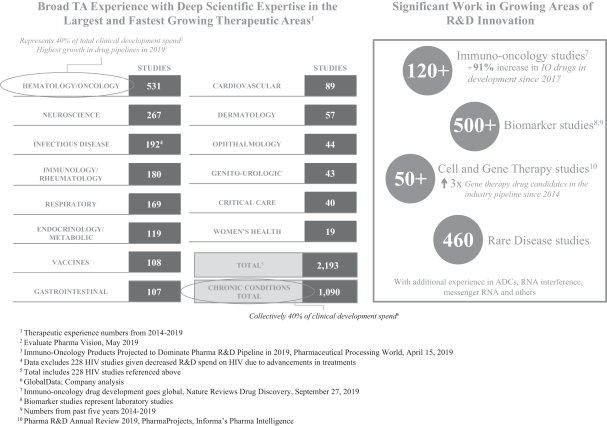
We have amassed deep scientific expertise in the largest and fastest growing therapeutic areas. In addition, we have developed specific capabilities in disciplines that cross therapeutic areas, such as rare diseases, vaccines and a broad array of chronic conditions. Over 75% of total R&D spend on late stage clinical trials conducted from 2015 through 2018 related to hematology/oncology and chronic conditions. Over the last five years, we have performed a significant amount of work in both of these areas, having provided services in over 500 hematology/oncology studies and over 1,000 chronic condition studies in the last five years.
We are also conducting significant work in growing areas of R&D innovation, such as immuno-oncology, which has experienced a 91% increase in the number of drugs in development since 2017, and cell and gene therapy, for which the industry pipeline of drugs has more than tripled since 2014. In addition, customers are hiring us to run their programs in other areas of innovative R&D, such as ADC’s, ribonucleic acid (“RNA”) interference, messenger RNA and others. We intend to continue investing in our scientific and operational capabilities to further strengthen our leadership position in key therapeutic areas and position ourselves to take advantage of the evolving trends in the biopharmaceutical industry.
6
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Build Upon Our Existing Dedicated Biotech Offering
Over the last five years, innovative biotechnology companies focused on new and complex therapies have accounted for approximately 40% of new drug approvals and have driven significant growth in related R&D spending. Large biopharmaceutical companies have had to fill gaps in their pipelines through strategic collaborations with, and acquisitions of, biotechnology companies, further increasing growth in the number of innovative, complex and global clinical trials. We were at the forefront of realizing these trends and formed our dedicated PPD Biotech model in 2014. We believe that our track record of serving biotechnology companies through our PPD Biotech model has earned us a reputation as the strategic partner of choice.
Increase Use of Our Innovative Site Network and Patient Enrollment Platform
Through our AES delivery model, we have developed an approach to directly serve our customers’ needs by addressing patient enrollment and site performance challenges, which are two of the biggest challenges our customers face in clinical development. We believe our integrated strategy of using technology and identified and consented data, our global site network and support for leading independent sites, is the ideal approach to serving our customers. To date, AES has played a critical role in completing some of the most important and complex clinical trials for our customers. We plan to continue to build out our AES capabilities and further strengthen the value propositions we offer and deliver to our customers through this differentiated model.
In addition to providing us with a competitively advantaged asset, our AES delivery model is financially attractive as it allows us to participate in the economics and growth of the market for investigator and patient recruitment services that otherwise would represent pass-through revenues, as is the case for most other CROs.
7
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Capitalize on our Growing Laboratory Segment
Our laboratory services offering is focused on the high-growth, innovative segment of laboratory services through its diverse range of high-value, advanced testing services. As an example, we have developed a significant and growing number of assays to address the testing needs of gene therapy. Our Laboratory Services segment represents approximately 16.0% and 18.8% of our revenues for the three months ended March 31, 2020 and March 31, 2019, respectively, and increased approximately 19.6% and 30.6%, respectively, for the year ended December 31, 2019 and three months ended March 31, 2020, as compared to the same periods in 2018 and 2019, respectively. It also affords us significant operating leverage and diversification, and provides higher backlog visibility and related conversion rates. Our Laboratory Services segment allows us to provide integrated offerings to customers that need both clinical development and laboratory services.
Continue to Invest in Innovation
We have consistently been and are committed to spending our time and resources on adding to and improving on our capabilities and service offerings. We continually assess the need to add new and innovative capabilities to reduce the cost and time required to generate evidence for our customers’ product candidates. We believe that the biopharmaceutical industry is constantly evolving, and we are focused on evaluating opportunities in a disciplined manner that is both capital efficient and flexible in approach. We are adept at successfully identifying and executing on acquisitions, joint ventures and strategic venture investments to pursue and amplify nascent technologies and capabilities for our customers’ benefit, as evidenced by our investments in Science 37, Inc. and Medable, Inc.
Risks Related to Our Business
Investing in our common stock involves a high degree of risk. You should carefully consider these risks before investing in our common stock, including the risks related to our business and industry described under “Risk Factors” elsewhere in this prospectus and in PPD’s 2019 10-K and PPD’s 2020 Q1 10-Q. In particular, the following considerations, among others, may offset our competitive strengths or have a negative effect on our business strategy, which could cause a decline in the price of our common stock and result in a loss of all or a portion of your investment:
| • | current and uncertain future impact from the novel coronavirus disease pandemic (the “COVID-19 pandemic”) on our business; |
| • | the fragmented and highly competitive nature of the drug development services industry; |
| • | changes in trends in the biopharmaceutical industry, including decreases in R&D spending and outsourcing; |
| • | our ability to keep pace with rapid technological changes that could make our services less competitive or obsolete; |
| • | the termination, delay or reduction in scope by our customers of our contracts with them; |
| • | the failure to successfully manage our business; |
| • | our inability to recruit, retain and motivate key personnel; |
| • | the significant influence of the Majority Sponsors over us; |
| • | our ability to generate cash flow to service our substantial debt obligations; and |
| • | other factors set forth under “Risk Factors” in this prospectus, PPD’s 2019 10-K and PPD’s 2020 Q1 10-Q. |
8
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Recent Developments
COVID-19
In March 2020, the World Health Organization declared COVID-19 a global pandemic that has resulted in travel and business disruption and volatile conditions in the capital and credit markets and overall economy. Globally, governments have implemented travel bans, stay at home or total lock-down mandates and other social distancing measures to combat the spread of COVID-19. In response to the global pandemic, we have created a pandemic response committee of company leaders, including our chief medical officer, to help manage our response to the pandemic focused on (1) the health and safety of our employees and patients and (2) business continuity, preserving the integrity of the work we do for our customers, including support for vaccines and anti-viral therapies for COVID-19. To implement social distancing measures and maximize work productivity, we have limited personnel in our facilities, with remote-capable employees throughout our company working remotely in all jurisdictions. We have also significantly limited domestic and international travel of our employees.
To date, the COVID-19 pandemic has impacted our business across both our Clinical Development Services and Laboratory Services segments. This includes the ability of our employees to visit hospitals and other clinical trial sites to conduct monitoring and other critical site visits and patient recruitment and enrollment activities as part of services offered within our Clinical Development Services segment, as well as a temporary shutdown of our Phase I clinics. Furthermore, we have had customers delay new studies and/or pause ongoing studies or certain activities thereof, such as patient recruitment, patient enrollment, site visits and site monitoring. These delays have impacted and will continue to impact the timing and extent to which backlog has and will convert to revenue. Additionally, our Laboratory Services segment has experienced limited reductions in central lab services due to delays in clinical trial activity.
In response to the COVID-19 pandemic, we have taken measures to mitigate the impact of the aforementioned factors across both of our segments by leveraging the geographical and operational diversification of our business activities. Such mitigation activities include, but are not limited to, winning new authorizations and services to help our customers treat or combat the spread of COVID-19 with anti-viral therapies and vaccines, as well as cost reduction strategies we have implemented, including reducing travel and related expenses, limiting increases in employee headcount, voluntary and limited temporary involuntary employee furloughs and reduced working hours. We may also implement other cost mitigation or reduction measures in the future, depending on a number of factors related to the progression of the COVID-19 pandemic. In addition, we borrowed $150.0 million under our Revolving Credit Facility as a precautionary measure in order to further strengthen our cash position and to preserve financial flexibility due to the uncertainty in the global credit and capital markets as a result of the COVID-19 pandemic.
We do not yet know the full extent of the impacts of the COVID-19 pandemic on our business, financial condition, results of operations or the global economy as a whole, as the ultimate impact of the pandemic is highly uncertain and subject to change. While the financial impact from the COVID-19 pandemic has not been material to our results of operations as of March 31, 2020, in part due to the mitigation activities discussed above, the financial impact could become material in the future due to the significant uncertainty as to the magnitude, continued duration, geographic reach, ongoing impact on the global economy and capital and credit markets and current travel and other restrictions relating to the COVID-19 pandemic. Additionally, federal, state and local governments have implemented economic and other stimulus measures to support individuals and businesses impacted by the COVID-19 pandemic, and while we intend to utilize such measures where appropriate and applicable, there can be no assurance that such measures will benefit us or otherwise offset any or all of the financial impacts from the COVID-19 pandemic. If the pandemic continues for an extended period or was to worsen and/or governments’ actions to contain the spread of COVID-19 are ineffective, these factors could result
9
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
in a material negative impact on our business, growth, reputation, prospects, financial condition, results of operations (including components of our financial results), cash flows and liquidity. Such impact could include, but is not limited to, additional customer delays or cancellations of awarded services, customers may reduce their R&D drug development pipeline, which could result in lower growth to our industry, additional costs related to restructuring activities, non-cash impairments of goodwill and other long-lived assets, decreases in the value of our investments, loss of hedge accounting and restrictions on our ability to obtain additional financing, if needed, or refinance our Senior Secured Credit Facilities or other debt.
We are closely monitoring the changing landscape with respect to the COVID-19 pandemic and taking actions to manage our business and support our employees, patients and customers. We will continue to evaluate the nature and extent of the impact to our business, results of operations, financial condition and liquidity. For more information regarding the potential impact of the COVID-19 pandemic on our business and operations see the section titled “Risk Factors,” included elsewhere in this prospectus and the information incorporated by reference.
Notes Offering
On June 5, 2020, the Company’s indirect wholly owned subsidiaries, Jaguar Holding Company II and PPD Development, L.P. (collectively, the “Issuers”), issued and sold $1.2 billion of senior notes consisting of the 2025 Notes and 2028 Notes, in each case, under an Indenture dated as of June 5, 2020, by and among the Issuers, the Company, as a guarantor, the other guarantors party thereto and Wilmington Trust, National Association, as trustee.
The Notes were offered and sold in a private placement sale within the United States to persons reasonably believed to be qualified institutional buyers in reliance on Rule 144A under the Securities Act and outside the United States to non-U.S. persons in reliance on Regulation S under the Securities Act.
The Company used the net proceeds of the offering of the Notes to redeem all of the existing 6.375% Senior Notes due 2023 issued by Jaguar Holding Company II and Pharmaceutical Product Development, LLC, an indirect wholly owned subsidiary of the Company.
Our Sponsors
Hellman & Friedman is a leading private equity investment firm with offices in San Francisco, New York City and London. Founded in 1984, Hellman & Friedman currently has $45 billion of assets under management. The firm focuses on investing in outstanding business franchises and serving as a value-added partner to management in select industries including healthcare, software, internet & media, financial services, business & information services, industrials & energy and retail & consumer.
Select Hellman & Friedman healthcare investments include Multiplan, Inc. (one of the largest third-party providers of cost-containment solutions to U.S. health plans), Change Healthcare (formerly Emdeon, a provider of revenue and payment cycle management solutions connecting payers, providers and patients in the U.S. healthcare system), Sheridan Healthcare, Inc. (a multi-specialty physician practice management company that provides outsourced physician staffing services to hospitals and ambulatory surgery centers), Sedgwick Inc. (a provider of technology-enabled risk, benefits and integrated business solutions) and Mitchell International, Inc. (a provider of medical claims software).
Founded in 1987, Carlyle is a global alternative asset manager and one of the world’s largest global private equity firms with approximately $217 billion of assets under management across 392 investment vehicles as of March 31, 2020. Carlyle invests across four segments—Corporate Private Equity, Real Assets, Global Credit,
10
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
and Investment Solutions. Carlyle has expertise in various industries, including aerospace, defense & government services, consumer & retail, energy, financial services, healthcare, industrials & transportation, technology & business services and telecommunications & media. Carlyle employs more than 1,775 employees, including more than 675 investment professionals, in 32 offices across six continents.
Carlyle is one of the leading private equity investors in the healthcare sector, having completed 65 total healthcare transactions representing approximately $12.3 billion in equity invested since inception. Recent transactions include Sedgwick Inc., One Medical (a technology-enabled primary care organization), Millicent Pharma Limited (a pharmaceutical company), MedRisk Holdco, LLC (a physical therapy-focused workers’ compensation solutions company), Albany Molecular Research, Inc. (a contract research and drug manufacturing organization), WellDyneRx, LLC (an independent pharmacy benefit manager), Rede D’Or São Luiz S.A. (a hospital provider in Brazil), Ortho-Clinical Diagnostics (a global provider of in vitro diagnostic solutions for screening, diagnosing, monitoring and confirming diseases), Healthscope Limited (a hospital in Australia) and PPD.
Corporate Information
PPD, Inc. was formed as a corporation in Delaware on April 13, 2017. PPD’s principal executive offices are located at 929 North Front Street, Wilmington, North Carolina 28401. PPD’s telephone number is (910) 251-0081. PPD’s website address is www.ppd.com. Information contained on, or that can be accessed through, PPD’s website does not constitute part of this prospectus and inclusion of our website address in this prospectus and the information incorporated by reference is intended to be an inactive textual reference only.
11
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The Offering
Common stock offered by the selling stockholders | shares. |
Common stock to be outstanding immediately after this offering | shares, which assumes shares issued in connection with the exercise of options by certain selling stockholders in connection with this offering. Except as provided in the immediately preceding sentence, the number of shares of common stock outstanding will not change as a result of this offering. |
Option to purchase additional shares | The selling stockholders have granted the underwriters a 30-day option to purchase up to an additional shares of common stock from the selling stockholders at the public offering price, less the underwriting discounts and commissions. |
Use of proceeds | The selling stockholders will receive all of the net proceeds from the sale of shares of common stock in this offering. We will not receive any proceeds from the sale of shares of common stock by the selling stockholders or if the underwriters exercise their option to purchase additional shares. See “Use of Proceeds.” |
Risk factors | See “Risk Factors” and the other information included in this prospectus and incorporated by reference for a discussion of the factors you should consider carefully before deciding to invest in our common stock. |
Dividend policy | We currently do not intend to declare any dividends on our common stock in the foreseeable future. Our ability to pay dividends on our common stock is limited by the Credit Agreement governing our Senior Secured Credit Facilities and the indenture governing the Notes. See “Dividend Policy.” |
Nasdaq symbol | “PPD” |
Except as otherwise indicated, all information in this prospectus regarding the number of shares of common stock that will be outstanding immediately after this offering is based on 348,583,743 shares of common stock outstanding as of March 31, 2020, and:
| • | excludes 6,030 shares of common stock underlying restricted stock awards that were outstanding as of March 31, 2020; |
| • | excludes shares of common stock issuable upon the exercise of time-based options to purchase shares of our common stock outstanding as of March 31, 2020 with a weighted average exercise price of $ per share, which excludes those being exercised by certain selling stockholders in connection with this offering; |
| • | excludes 8,925,607 shares of common stock issuable upon the exercise of performance-based options to purchase shares of our common stock outstanding as of March 31, 2020 with a weighted average exercise price of $13.22 per share; |
| • | excludes 2,384,541 shares of common stock issuable upon the exercise of liquidity/realization event-based options to purchase shares of our common stock outstanding as of March 31, 2020 with a |
12
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
weighted average exercise price of $10.88 per share, and which have not previously vested, will not vest upon the consummation of this offering and are eligible to vest only if and when the Majority Sponsors have achieved specified internal rates of return with respect to its investment in the Company; and |
| • | does not reflect 39,053,663 shares of common stock available for future issuance under the Company’s 2020 Omnibus Incentive Plan (the “2020 Incentive Plan”). |
13
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Summary Consolidated Financial Data
The following table sets forth the summary consolidated financial data of PPD for the periods and dates indicated.
On January 1, 2018, PPD adopted Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”), which outlines a single comprehensive model for entities to use in accounting for revenue from contracts with customers. PPD adopted ASC 606 using the modified retrospective method for all contracts not completed as of the date of adoption. PPD’s consolidated financial data for the periods beginning January 1, 2018 and thereafter are presented in accordance with ASC 606. Prior to January 1, 2018, PPD applied the accounting guidance from the application of ASC Topic 605, Revenue Recognition (“ASC 605”).
The balance sheet data as of March 31, 2020 and the statements of operations and cash flow data for the three months ended March 31, 2020 and 2019 have been derived from PPD’s unaudited condensed consolidated financial statements incorporated by reference from PPD’s 2020 Q1 10-Q. The balance sheet data as of December 31, 2019 and the statement of operations and cash flow data for the years ended December 31, 2019, 2018 and 2017 have been derived from PPD’s audited consolidated financial statements incorporated by reference from PPD’s May 2020 8-K. The statement of operations and cash flow data for the years ended December 31, 2016 and 2015 have been derived from PPD’s audited consolidated financial statements not included or incorporated by reference in this prospectus.
The summary consolidated financial data set forth below should be read in conjunction with “Risk Factors” and “Capitalization” in this prospectus, PPD’s 2019 10-K (as superseded by, and solely to the extent set forth in, PPD’s May 2020 8-K) and PPD’s 2020 Q1 10-Q, including PPD’s audited consolidated financial statements and unaudited interim financial statements incorporated by reference in this prospectus.
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||||||||||||||||||
| 2020(1) | 2019(1) | 2019(1)(2) | 2018(1) | 2018(1) | 2017(1),(2) | 2016(1),(2) | 2015(1),(2) | |||||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||
Statement of operations data: | ||||||||||||||||||||||||||||||||
Revenue: | ||||||||||||||||||||||||||||||||
Revenue(3) | $ | 1,072,462 | $ | 963,738 | $ | 4,031,017 | $ | 3,748,971 | $ | 2,837,810 | $ | 2,767,476 | $ | 2,467,941 | $ | 2,073,484 | ||||||||||||||||
Reimbursed revenue(4) | — | — | — | — | 222,224 | 233,574 | 211,624 | 178,350 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Total revenue | 1,072,462 | $ | 963,738 | 4,031,017 | 3,748,971 | 3,060,034 | 3,001,050 | 2,679,565 | 2,251,834 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Operating costs and expenses: | ||||||||||||||||||||||||||||||||
Direct costs, exclusive of depreciation and amortization | 414,439 | 367,202 | 1,484,258 | 1,333,812 | 1,327,500 | 1,302,983 | 1,175,051 | 965,098 | ||||||||||||||||||||||||
Reimbursed costs | 250,850 | 225,019 | 924,634 | 940,913 | 222,224 | 233,574 | 211,624 | 178,350 | ||||||||||||||||||||||||
Selling, general and administrative expenses | 247,776 | 218,380 | 938,806 | 813,035 | 816,659 | 809,333 | 718,139 | 652,900 | ||||||||||||||||||||||||
Recapitalization costs | — | — | — | — | — | 114,766 | — | — | ||||||||||||||||||||||||
Depreciation and amortization | 66,315 | 65,418 | 264,830 | 258,974 | 258,974 | 279,066 | 260,487 | 262,871 | ||||||||||||||||||||||||
Goodwill and long-lived asset impairment | — | — | 1,284 | 29,626 | 29,626 | 43,459 | 28,101 | 13,686 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Total operating costs and expenses | 979,380 | 876,019 | 3,613,812 | 3,376,360 | 2,654,983 | 2,783,181 | 2,393,402 | 2,072,905 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Income from | 93,082 | 87,719 | 417,205 | 372,611 | 405,051 | 217,869 | 286,163 | 178,929 | ||||||||||||||||||||||||
Interest expense, net | (64,710 | ) | (66,523 | ) | (311,744 | ) | (263,618 | ) | (263,618 | ) | (253,891 | ) | (203,294 | ) | (228,084 | ) | ||||||||||||||||
(Loss) gain on investments | (26,872 | ) | (14,100 | ) | (19,043 | ) | 15,936 | 15,936 | 92,750 | 61,576 | 19,525 | |||||||||||||||||||||
Loss on extinguishment of debt | (50,065 | ) | — | — | — | — | — | — | (131,755 | ) | ||||||||||||||||||||||
Other income (expense), net | 29,294 | (24,301 | ) | (27,143 | ) | 21,701 | 21,701 | (40,259 | ) | 22,448 | 19,462 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
14
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||||||||||||||||||
| 2020(1) | 2019(1) | 2019(1)(2) | 2018(1) | 2018(1) | 2017(1),(2) | 2016(1),(2) | 2015(1),(2) | |||||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||
(Loss) income before (benefit from) provision for income taxes | $ | (19,271 | ) | $ | (17,205 | ) | $ | 59,275 | $ | 146,630 | $ | 179,070 | $ | 16,469 | $ | 166,893 | $ | (141,923 | ) | |||||||||||||
(Benefit from) provision from income taxes | (7,717 | ) | (3,299 | ) | 2,957 | 39,579 | 48,444 | (284,360 | ) | (15,961 | ) | 2,173 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
(Loss) income before equity in losses of unconsolidated affiliates | (11,554 | ) | (13,906 | ) | 56,318 | 107,051 | 130,626 | 300,829 | 182,854 | (144,096 | ) | |||||||||||||||||||||
Equity in losses of unconsolidated affiliates, net of income taxes | (1,566 | ) | (328 | ) | (3,563 | ) | (186 | ) | (186 | ) | — | — | — | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Net (loss) income | (13,120 | ) | (14,234 | ) | 52,755 | 106,865 | 130,440 | 300,829 | 182,854 | (144,096 | ) | |||||||||||||||||||||
Loss from discontinued operations, net of taxes | — | — | — | — | — | — | — | (4,139 | ) | |||||||||||||||||||||||
Net (income) loss attributable to noncontrolling interests | (2,718 | ) | (861 | ) | (4,934 | ) | (2,679 | ) | (2,679 | ) | (4,802 | ) | 241 | 1,678 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Net (loss) income attributable to PPD, Inc. | (15,838 | ) | (15,095 | ) | 47,821 | 104,186 | 127,761 | 296,027 | 183,095 | (146,557 | ) | |||||||||||||||||||||
Recapitalization investment portfolio consideration | 20,062 | 10,628 | 6,846 | (7,849 | ) | (7,849 | ) | (97,136 | ) | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Net income (loss) attributable to common stockholders of PPD, Inc. | $ | 4,224 | $ | (4,467 | ) | $ | 54,667 | $ | 96,337 | $ | 119,912 | $ | 198,891 | $ | 183,095 | $ | (146,557 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||||||||||||||
| 2020(1) | 2019(1) | 2019(1)(2) | 2018(1) | 2017(1),(2) | 2016(1),(2) | 2015(1),(2) | ||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||
| (shares in thousands, except per share data) | ||||||||||||||||||||||||||||
Per share data: | ||||||||||||||||||||||||||||
Earnings (loss) per share attributable to common stockholders: | ||||||||||||||||||||||||||||
Basic | $ | 0.01 | $ | (0.02 | ) | $ | 0.20 | $ | 0.34 | $ | 0.68 | $ | 0.59 | $ | (0.46 | ) | ||||||||||||
Diluted | $ | 0.01 | $ | (0.02 | ) | $ | 0.19 | $ | 0.34 | $ | 0.68 | $ | 0.58 | $ | (0.46 | ) | ||||||||||||
Weighted average common shares outstanding: | ||||||||||||||||||||||||||||
Basic | 318,221 | 279,086 | 279,285 | 279,238 | 291,027 | 312,065 | 311,874 | |||||||||||||||||||||
Diluted | 322,424 | 279,086 | 280,693 | 279,317 | 293,826 | 316,553 | 311,874 | |||||||||||||||||||||
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||||||||||||||||||
| 2020(1) | 2019(1) | 2019(1)(2) | 2018(1) | 2018(1) | 2017(1),(2) | 2016(1),(2) | 2015(1),(2) | |||||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||||||||||||
Cash flow data: | ||||||||||||||||||||||||||||||||
Net cash provided by (used in): | ||||||||||||||||||||||||||||||||
Operating activities | $ | 19,373 | $ | 13,595 | $ | 432,946 | $ | 423,406 | $ | 423,406 | $ | 359,079 | $ | 407,995 | $ | 416,288 | ||||||||||||||||
Investing activities | (43,220 | ) | (51,390 | ) | (233,228 | ) | (90,525 | ) | (90,525 | ) | (92,743 | ) | (519,746 | ) | (253,542 | ) | ||||||||||||||||
Financing activities | 451,858 | (6,004 | ) | (422,039 | ) | (166,942 | ) | (166,942 | ) | (249,393 | ) | 130,465 | (44,629 | ) | ||||||||||||||||||
Cash paid for interest | (96,872 | ) | (74,428 | ) | (300,528 | ) | (262,921 | ) | (262,921 | ) | (238,826 | ) | (191,084 | ) | (154,060 | ) | ||||||||||||||||
Cash paid for capital expenditures | (42,768 | ) | (24,761 | ) | (125,928 | ) | (116,145 | ) | (116,145 | ) | (105,135 | ) | (90,258 | ) | (63,797 | ) | ||||||||||||||||
Other financial data: | ||||||||||||||||||||||||||||||||
Net authorizations(5) | $ | 1,063,571 | $ | 977,845 | $ | 3,827,291 | $ | 3,420,954 | $ | 3,420,954 | $ | 2,485,419 | $ | 3,051,596 | $ | 2,491,584 | ||||||||||||||||
Backlog (at end of period)(5) | $ | 7,312,193 | $ | 6,535,964 | $ | 7,066,254 | $ | 6,313,710 | $ | 6,313,710 | $ | 5,730,568 | $ | 6,006,644 | $ | 5,192,054 | ||||||||||||||||
Backlog conversion(5) | 11.6 | % | 12.0 | % | 11.9 | % | 11.9 | % | 11.9 | % | 11.7 | % | 11.4 | % | 10.6 | % | ||||||||||||||||
Net book-to-bill(5) | 1.3x | 1.3x | 1.2x | 1.2x | 1.2x | 0.9x | 1.2x | 1.2x | ||||||||||||||||||||||||
Adjusted EBITDA(6)(7)(8) | $ | 196,858 | $ | 167,840 | $ | 776,945 | $ | 707,406 | $ | 739,846 | $ | 711,124 | $ | 631,491 | $ | 531,201 | ||||||||||||||||
Adjusted Net Income(6)(7)(8) | $ | 76,508 | $ | 55,853 | $ | 286,819 | $ | 257,559 | $ | 281,134 | $ | 320,043 | $ | 236,886 | $ | 196,037 | ||||||||||||||||
| Last Twelve Months Ended March 31, 2020(1) | As of December 31, 2019(1)(2) | |||||||
Debt ratios: | ||||||||
Consolidated total net debt ratio(9) | 4.5x | 6.9x | ||||||
Consolidated senior secured net debt ratio(10) | 3.1x | 3.5x | ||||||
15
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| As of March 31, 2020(1) | As of December 31, 2019(1)(2) | |||||||
| (in thousands) | ||||||||
Balance sheet data: | ||||||||
Cash and cash equivalents | $ | 738,364 | $ | 345,187 | ||||
Property and equipment, net | 455,439 | 458,845 | ||||||
Working capital | 212,321 | (288,059 | ) | |||||
Total assets | 5,814,788 | 5,556,246 | ||||||
Gross debt(11) | 4,395,363 | 5,705,862 | ||||||
Total stockholders’ deficit | (1,079,898 | ) | (2,698,148 | ) | ||||
| (1) | Financial data as of March 31, 2020, for the last twelve months ended March 31, 2020, for the three months ended March 31, 2020 and 2019 and for the year ended December 31, 2019 is reported in accordance with ASC 606. Our financial data for the year ended December 31, 2018 has been presented on both an ASC 606 and ASC 605 basis to provide greater comparability of our operating results during 2018. Financial data as of and for the years ended December 31, 2017, 2016 and 2015 is reported in accordance with ASC 605. |
| (2) | We acquired Synarc, Inc. on September 3, 2019, Medimix International on July 1, 2019, Optimal Research, LLC on September 1, 2017, Evidera Holdings, Inc. on September 1, 2016, Synexus Clinical Research Topco Limited on May 31, 2016, CRA Intermediate Holdings, Inc. on May 12, 2015 and the clinical research division of SNBL, subsequently renamed PPD-SNBL, on April 1, 2015. We own 60% of PPD-SNBL. The financial results of these entities have been included as of and since the dates of each acquisition. |
| (3) | Under ASC 606, revenue is comprised of direct, third-party pass-through and out-of-pocket revenue from providing services to our customers. Direct revenue represents revenue associated with the direct services provided under our contracts. Third-party pass-through and out-of-pocket revenue represents the reimbursement by customers of third-party pass-through and out-of-pocket costs incurred by us under our contracts. Under ASC 605, revenue is comprised of direct revenue only. Our consolidated financial data for the year ended December 31, 2018 has been presented on both an ASC 606 and ASC 605 basis to provide greater comparability of our operating results during 2018. |
| (4) | Represents out-of-pocket revenues and related costs reimbursed by our customers at cost when we are the principal (and not the agent) in the relationship in accordance with ASC 605 for the years ended December 31, 2017, 2016 and 2015. Our consolidated financial data for the year ended December 31, 2018 has been presented on both an ASC 606 and ASC 605 basis to provide greater comparability of our operating results during 2018. |
| (5) | Net authorizations represent new business awards, net of award or contract modifications, contract cancellations, foreign currency fluctuations and other adjustments. Backlog for all periods represents anticipated direct revenue for work not yet completed or performed (i) under signed contracts, letters of intent and, in some cases, awards that are supported by other forms of written communication and (ii) where there is sufficient or reasonable certainty about the customer’s ability and intent to fund and commence the services within six months. Backlog and net authorizations exclude the impact of net authorizations from anticipated third-party pass-through and out-of-pocket revenue. Backlog conversion represents the quarterly average of direct revenue for the period divided by opening backlog for that period. Net book-to-bill represents the amount of net authorizations for the period divided by direct revenue recognized in that period. All amounts related to 2018 and later periods presented on an ASC 606 basis, including those derived or calculated from such amounts, exclude the impact of ASC 606 on direct revenue. |
| (6) | In addition to the financial measures prepared in accordance with generally accepted accounting principles in the United States (“GAAP”), this prospectus contains certain non-GAAP financial measures, including Adjusted EBITDA, Adjusted Net Income, consolidated total net debt ratio and consolidated senior secured net debt ratio. A non-GAAP financial measure is generally defined as a numerical measure of a company’s financial performance or financial position that excludes or includes amounts so as to be different than the most directly comparable measure calculated and presented in accordance with GAAP. Other companies in our industry may calculate adjusted EBITDA, Adjusted Net Income, consolidated total net debt ratio and consolidated senior secured net debt ratio differently than we do. As a result, these non-GAAP financial measures have limitations as analytical and comparative tools and should not be considered in isolation, or as a substitute for analysis of our results as reported under GAAP. Adjusted EBITDA, Adjusted Net Income, consolidated total net debt ratio and consolidated senior secured net debt ratio should not be considered as measures of discretionary cash available to us to invest in the growth of our business. In calculating these performance and liquidity financial measures, we make certain adjustments that are based on assumptions and estimates that may prove to have been inaccurate. Our presentation of Adjusted EBITDA, Adjusted Net Income, consolidated total net debt ratio and consolidated senior secured net debt ratio should not be construed as an inference that our future results and financial position will be unaffected by unusual items. |
| (7) | Adjusted EBITDA consists of net income (loss) attributable to common stockholders of PPD, adjusted for changes in recapitalization investment portfolio consideration and net (income) loss attributable to noncontrolling interest and before interest expense, net, provision for (benefit from) income taxes and depreciation and amortization and eliminates (i) non-operating income or expense and (ii) impacts of certain non-cash, unusual or other items that are included in net income (loss) that we do not consider indicative of our ongoing operating performance. Adjusted Net Income consists of net income attributable to common stockholders of PPD, Inc. before amortization and the elimination of (i) non-operating income or expense and (ii) impacts of certain non-cash, unusual or other items that are included in net income (loss) that we do not consider indicative of our ongoing operating performance. In the case of Adjusted EBITDA and Adjusted Net Income, we believe that making such adjustments provides management and investors meaningful information to understand our operating performance and ability to analyze financial and business trends on a period-to-period basis. Although we exclude amortization of acquired intangible assets from our non-GAAP expenses, we note that revenue generated from such intangibles is included within revenue in determining net income (loss) attributable to common stockholders of PPD. |
16
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| (8) | The following table reconciles net income (loss) attributable to common stockholders of PPD, Inc. to Adjusted EBITDA and net income (loss) attributable to common stockholders of PPD, Inc. to Adjusted Net Income. |
| Last Twelve Months Ended March 31, 2020(a) | Three Months Ended March 31,(a) | Year Ended December 31,(a) | ||||||||||||||||||||||||||||||||||
| 2020 | 2019 | 2019 | 2018 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||||||
Reconciliation to Adjusted EBITDA: | ||||||||||||||||||||||||||||||||||||
Net income (loss) attributable to common stockholders of PPD, Inc. | $ | 63,358 | $ | 4,224 | $ | (4,467 | ) | $ | 54,667 | $ | 96,337 | $ | 119,912 | $ | 198,891 | $ | 183,095 | $ | (146,557 | ) | ||||||||||||||||
Recapitalization investment portfolio consideration | (16,280 | ) | (20,062 | ) | (10,628 | ) | (6,846 | ) | 7,849 | 7,849 | 97,136 | — | — | |||||||||||||||||||||||
Net income attributable to noncontrolling interests | 6,791 | 2,718 | 861 | 4,934 | 2,679 | 2,679 | 4,802 | (241 | ) | (1,678 | ) | |||||||||||||||||||||||||
Loss from discontinued operations, net of taxes | — | — | — | — | — | — | — | — | 4,139 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Net income (loss) | 53,869 | (13,120 | ) | (14,234 | ) | 52,755 | 106,865 | 130,440 | 300,829 | 182,854 | (144,096 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Interest expense, net | 309,931 | 64,710 | 66,523 | 311,744 | 263,618 | 263,618 | 253,891 | 203,294 | 228,084 | |||||||||||||||||||||||||||
(Benefit from) provision for income taxes | (1,461 | ) | (7,717 | ) | (3,299 | ) | 2,957 | 39,579 | 48,444 | (284,360 | ) | (15,961 | ) | 2,173 | ||||||||||||||||||||||
Depreciation and amortization | 265,727 | 66,315 | 65,418 | 264,830 | 258,974 | 258,974 | 279,066 | 260,487 | 262,871 | |||||||||||||||||||||||||||
Stock-based compensation expense | 17,170 | 5,272 | 3,734 | 15,632 | 18,265 | 18,265 | 22,570 | 8,770 | 9,154 | |||||||||||||||||||||||||||
Option holder special bonuses(b) | 20,979 | 2,105 | — | 18,874 | — | — | 1,993 | 25,979 | 23,768 | |||||||||||||||||||||||||||
Other (income) expense, net | (26,452 | ) | (29,294 | ) | 24,301 | 27,143 | (21,701 | ) | (21,701 | ) | 40,259 | (22,448 | ) | (19,462 | ) | |||||||||||||||||||||
Goodwill and long-lived asset impairments | 1,284 | — | — | 1,284 | 29,626 | 29,626 | 43,459 | 28,101 | 13,686 | |||||||||||||||||||||||||||
Recapitalization Cost | — | — | — | — | — | — | 114,766 | — | — | |||||||||||||||||||||||||||
Sponsor fees and related costs(c) | 3,320 | 448 | 933 | 3,805 | 3,569 | 3,569 | 3,337 | 2,709 | 2,367 | |||||||||||||||||||||||||||
Severance and charges for other cost reduction activities(d) | 8,540 | 754 | 2,612 | 10,398 | �� | 7,938 | 7,938 | 10,461 | 6,311 | 22,053 | ||||||||||||||||||||||||||
Loss on extinguishment of debt | 50,065 | 50,065 | — | — | — | — | — | — | 131,755 | |||||||||||||||||||||||||||
Transaction-related costs(e) | 22,584 | 3,625 | 3,991 | 22,950 | 2,938 | 2,938 | 4,078 | 9,197 | 12,237 | |||||||||||||||||||||||||||
Loss (gain) on investments(f) | 31,815 | 26,872 | 14,100 | 19,043 | (15,936 | ) | (15,936 | ) | (92,750 | ) | (61,576 | ) | (19,525 | ) | ||||||||||||||||||||||
Other adjustments(g) | 48,592 | 26,823 | 3,761 | 25,530 | 13,671 | 13,671 | 13,525 | 3,774 | 6,136 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Adjusted EBITDA | $ | 805,963 | $ | 196,858 | $ | 167,840 | $ | 776,945 | $ | 707,406 | $ | 739,846 | $ | 711,124 | $ | 631,491 | $ | 531,201 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
| Three Months Ended March 31,(a) | Year Ended December 31,(a) | |||||||||||||||||||||||||||||||
| 2020 | 2019 | 2019 | 2018 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||
Reconciliation to Adjusted Net Income: |
| |||||||||||||||||||||||||||||||
Net income (loss) attributable to common stockholders of PPD, Inc. | $ | 4,224 | $ | (4,467 | ) | $ | 54,667 | $ | 96,337 | $ | 119,912 | $ | 198,891 | $ | 183,095 | $ | (146,557 | ) | ||||||||||||||
Recapitalization investment portfolio consideration | (20,062 | ) | (10,628 | ) | (6,846 | ) | 7,849 | 7,849 | 97,136 | — | — | |||||||||||||||||||||
Net income (loss) attributable to noncontrolling interests | 2,718 | 816 | 4,934 | 2,679 | 2,679 | 4,802 | (241 | ) | (1,678 | ) | ||||||||||||||||||||||
Loss from discontinued operations, net of taxes | — | — | — | — | — | — | — | 4,139 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Net (loss) income | (13,120 | ) | (14,234 | ) | 52,755 | 106,865 | 130,440 | 300,829 | 182,854 | (144,096 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Amortization of intangible assets | 39,697 | 40,743 | 162,121 | 168,639 | 168,639 | 183,421 | 171,647 | 182,167 | ||||||||||||||||||||||||
Amortization of debt issuance and modification costs and debt discount | 3,857 | 2,575 | 17,768 | 10,082 | 10,082 | 9,001 | 6,479 | 16,880 | ||||||||||||||||||||||||
Amortization of accumulated other comprehensive income on derivative instruments | (2,342 | ) | (2,410 | ) | (9,523 | ) | (5,269 | ) | (5,269 | ) | — | — | — | |||||||||||||||||||
Stock-based compensation expense | 5,272 | 3,734 | 15,632 | 18,265 | 18,265 | 22,570 | 8,770 | 9,154 | ||||||||||||||||||||||||
Option holder special bonuses(b) | 2,105 | — | 18,874 | — | — | 1,993 | 25,979 | 23,768 | ||||||||||||||||||||||||
Other (income) expense, net | (29,294 | ) | 24,301 | 27,143 | (21,701 | ) | (21,701 | ) | 40,259 | (22,448 | ) | (19,462 | ) | |||||||||||||||||||
17
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| Three Months Ended March 31,(a) | Year Ended December 31,(a) | |||||||||||||||||||||||||||||||
| 2020 | 2019 | 2019 | 2018 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||||||||||||
| ASC 606 | ASC 605 | |||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||
Goodwill and other asset impairments | — | — | 1,284 | 29,626 | 29,626 | 43,459 | 28,101 | 13,686 | ||||||||||||||||||||||||
Loss on extinguishment of debt | 50,065 | — | — | — | — | — | — | 131,755 | ||||||||||||||||||||||||
Recapitalization costs | — | — | — | — | — | 114,766 | — | — | ||||||||||||||||||||||||
Sponsor fees and related costs(c) | 448 | 933 | 3,805 | 3,569 | 3,569 | 3,337 | 2,709 | 2,367 | ||||||||||||||||||||||||
Severance and charges for other cost reduction activities(d) | 754 | 2,612 | 10,398 | 7,938 | 7,938 | 10,461 | 6,311 | 22,053 | ||||||||||||||||||||||||
Transaction-related costs(e) | 3,625 | 3,991 | 22,950 | 2,938 | 2,938 | 4,078 | 9,197 | 12,237 | ||||||||||||||||||||||||
Loss (gain) on investments(f) | 26,872 | 14,100 | 19,043 | (15,936 | ) | (15,936 | ) | (92,750 | ) | (61,576 | ) | (19,525 | ) | |||||||||||||||||||
Other adjustments(g) | 26,823 | 3,761 | 25,530 | 13,671 | 13,671 | 13,525 | 3,774 | 6,136 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Total adjustments | 127,882 | 94,340 | 315,025 | 211,822 | 211,822 | 354,120 | 178,943 | 381,216 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Tax effect of adjustments(h) | (33,478 | ) | (24,253 | ) | (80,961 | ) | (54,226 | ) | (54,226 | ) | (132,795 | ) | (69,967 | ) | (146,768 | ) | ||||||||||||||||
Other tax adjustments(h) | (4,776 | ) | — | — | (6,902 | ) | (6,902 | ) | (202,111 | ) | (54,944 | ) | 105,685 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Adjusted Net Income | $ | 76,508 | $ | 55,853 | $ | 286,819 | $ | 257,559 | $ | 281,134 | $ | 320,043 | $ | 236,886 | $ | 196,037 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
| (a) | Financial data for the twelve months ended March 31, 2020, three months ended March 31, 2020 and 2019 and for the year ended December 31, 2019 is reported in accordance with ASC 606. Our financial data for the year ended December 31, 2018 has been presented on both an ASC 606 and ASC 605 basis to provide greater comparability of our operating results during 2018. Financial data for the years ended December 31, 2017, 2016 and 2015 is reported in accordance with ASC 605. |
| (b) | Represents PPD’s costs associated with special cash bonuses paid to PPD’s option holders. |
| (c) | Represents management fees incurred under consulting services agreements with certain investment funds of the Majority Sponsors. These consulting services agreements terminated upon consummation of PPD’s initial public offering. |
| (d) | Represents employee separation costs, exit and disposal costs with the full or partial exit of certain leased facilities, costs associated with planned employee reorganizations and other contract termination costs from various cost-reduction activities. |
| (e) | Represents integration and transaction costs incurred with completed or contemplated acquisitions, costs incurred in connection with PPD’s initial public offering, other transaction costs and costs associated with PPD’s public company transition. |
| (f) | Represents the fair value accounting gains or losses primarily from PPD’s investments in Auven Therapeutics Holdings L.P. (“Auven”) and venBio Global Strategic Fund, L.P (“venBio”). |
| (g) | Other adjustments include amounts that management believes are not representative of our operating performance. These adjustments include implementation costs associated with a new enterprise resource planning application, one-time costs incurred in 2020 associated with the termination of a long-term incentive program which is being replaced by a traditional stock-based program in 2020, advisory costs associated with the adoption of new accounting standards and other unusual charges or income. |
| (h) | Non-GAAP adjustments were tax effected at an estimated blended effective tax rate of between 38% and 39% for the years ended December 31, 2017, 2016 and 2015 and 26% for all periods starting January 1, 2018 and forward, excluding the change in recapitalization investment portfolio consideration. A non-recurring net benefit of $(4.8) million was recognized for the three months ending March 31, 2020 and non-recurring gains associated with the Tax Cuts and Jobs Act of 2017 were $(6.9) million and $(202.1) million for the years ending December 31, 2018 and 2017, respectively, and are reflected as adjustments as they are not representative of our operating performance. In addition, $(54.9) million and $105.7 million were reflected as adjustments for the years ending December 31, 2016 and 2015, respectively. The $(54.9) million adjustment for the year ending December 31, 2016 relates to a release of a deferred tax liability on foreign earnings previously considered not permanently reinvested, and the $105.7 million adjustment for the year ending December 31, 2015 relates primarily to a change in assertion related to certain unremitted earnings on foreign operations. |
| (9) | Consolidated total net debt ratio is a non-GAAP financial measure used in key financial covenants contained in the indenture that governs the Notes, which is a material facility supporting our capital structure and providing liquidity to our business. Consolidated total net debt ratio is defined as the ratio of (a) gross debt less, cash and cash equivalents that would be stated on the balance sheet of PPD and its restricted subsidiaries and held by PPD and its restricted subsidiaries, as determined in accordance with GAAP, to (b) Adjusted EBITDA of PPD and its restricted subsidiaries for the period of the most recently ended four fiscal quarters for which internal financial statements are available, in each case with certain pro forma adjustments permitted in calculating covenant compliance under our debt instruments. The impact of ASC 606 on Adjusted EBITDA is excluded from the consolidated total net debt ratio and the consolidated senior secured net debt ratio as defined in the Credit Agreement governing the Senior Secured Credit Facilities. |
| (10) | Consolidated senior secured net debt ratio is a non-GAAP financial measure used in key financial covenants contained in the indenture that governs the Notes and the Credit Agreement that governs our Senior Secured Credit Facilities, which are the material facilities supporting our capital structure and providing liquidity to our business. Consolidated senior secured net debt ratio is defined as the ratio of (a) the consolidated indebtedness of PPD and its restricted subsidiaries that is secured by liens, less cash and cash equivalents that would be stated on the balance sheet of PPD and its restricted subsidiaries and held by PPD and its restricted subsidiaries, as determined in accordance with GAAP, to (b) Adjusted EBITDA of PPD and its restricted subsidiaries for the period of the most recently ended four fiscal quarters for which internal financial statements are available, in each case with certain pro forma adjustments permitted in calculating covenant compliance under our debt instruments. The impact of ASC 606 on Adjusted EBITDA is excluded from the consolidated total net debt ratio and the consolidated senior secured net debt ratio as defined in the Credit Agreement governing the Senior Secured Credit Facilities. |
| (11) | Gross debt is a non-GAAP financial measure used in key financial covenants contained in the indenture governing the Notes, which is a material facility supporting our capital structure and providing liquidity to our business. Gross debt is defined as the consolidated indebtedness of PPD and its restricted subsidiaries excluding unamortized debt discounts and unamortized debt issuance costs. |
18
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors together with all of the other information included or incorporated by reference in this prospectus, including the risks described under “Risk Factors” in Part I, Item 1A of PPD’s 2019 10-K and PPD’s 2020 Q1 10-Q and our consolidated financial statements and related notes incorporated by reference in this prospectus, before deciding whether to invest in shares of our common stock. Additional risks and uncertainties that we are unaware of or that we currently believe are not material may also become important factors that materially and adversely affect our business. The occurrence of any of the events described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the trading price of our common stock may decline and you may lose all or part of your investment.
Risks Related to our Business
Our operations might be affected by the occurrence of a natural disaster, pandemics, such as the COVID-19 pandemic, or other catastrophic events.
We depend on our customers, investigators, laboratories and other facilities for the continued operation of our business. While we maintain disaster recovery and business continuity plans, they might not adequately protect us. Despite any precautions we take for natural disasters or other catastrophic events, these events, including terrorist attack, hurricanes, fires, floods, ice and snowstorms and pandemics, such as the COVID-19 pandemic, may result in interruptions in our ability to provide services to our customers. Disruptions in the infrastructure, laboratory, clinic or office closures, mandatory stay at home orders or other social distancing measures caused by events such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism or pandemics, such as the COVID-19 pandemic, or other “acts of god,” particularly involving countries and cities in which we have laboratories, clinics or offices, could adversely affect our businesses. Although we carry business interruption insurance policies and typically have provisions in our contracts that protect us from certain events, our coverage might not respond or be adequate to compensate us for all losses that may occur, including those relating to the COVID-19 pandemic. Any natural disaster or catastrophic event, such as the COVID-19 pandemic affecting us or our customers, investigators or payers could have a significant negative impact on our operations or financial performance.
The COVID-19 pandemic has impacted our ability to carry out critical services relating to our clinical studies on a timely basis, including the ability of our employees to visit hospitals and other clinical trial sites to conduct monitoring and other critical site visits and patient recruitment and enrollment activities as part of services offered within our Clinical Development Services segment, as well as a temporary shutdown of our Phase I clinics. Additionally, our Laboratory Services segment has experienced limited reductions in central lab services due to delays in clinical trial activity. Furthermore, we have had customers delay new studies and/or pause ongoing studies or certain activities thereof, such as patient recruitment, patient enrollment, site visits and site monitoring. These delays have impacted and will continue to impact the timing and extent to which backlog will convert to revenue.
We do not yet know the full extent of the impacts of the COVID-19 pandemic on our business, results of operations, financial condition or the global economy as a whole, as the ultimate impact of the pandemic is highly uncertain and subject to change. While the financial impact from the COVID-19 pandemic has not been material to our results of operations as of March 31, 2020, in part due to the mitigation activities discussed in “Prospectus Summary—Recent Developments—COVID-19,” the financial impact could become material in the future due to the significant uncertainty as to the magnitude, continued duration, geographic reach, ongoing impact on the global economy and capital and credit markets and current travel and other restrictions relating to the COVID-19 pandemic. Additionally, federal, state and local governments have implemented economic and other stimulus measures to support individuals and businesses impacted by the COVID-19 pandemic, and while we intend to utilize such measures where appropriate and applicable, there can be no assurance that such
19
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
measures will benefit us or otherwise offset any or all of the financial impacts from the COVID-19 pandemic. If the pandemic continues for an extended period or was to worsen and/or governments’ actions to contain the spread of COVID-19 were to be ineffective, these factors could result in a material negative impact on our business, growth, reputation, prospects, financial condition, results of operations (including components of our financial results), cash flows and liquidity. Such impact could include, but is not limited to, additional customer delays or cancellations of awarded services, customers may reduce their R&D drug development pipeline, which could result in lower growth to the CRO industry, additional costs related to restructuring activities, non-cash impairments of goodwill and other long-lived assets, decreases in the value of our investments, loss of hedge accounting and restrictions on our ability to obtain additional financing, if needed, or refinance our Senior Secured Credit Facilities or other debt. To the extent the COVID-19 pandemic adversely affects our business, operations, financial condition and operating results, it may also have the effect of heightening many of the other risks described in this “Risk Factors” section (including those described in PPD’s 2019 10-K and PPD’s 2020 Q1 10-Q, which are incorporated by reference herein), such as those relating to our high level of indebtedness, our need to generate sufficient cash flows to service our indebtedness, and our ability to comply with the covenants contained in the agreements that govern our indebtedness.
Our business is subject to international and U.S. economic, currency, political and other risks, including those caused by the global COVID-19 pandemic, that could negatively affect our business, results of operations, financial condition and/or cash flows.
We provide services globally and have business operations in numerous countries throughout the world. Because we provide our services worldwide, our business is subject to risks associated with doing business internationally, including risks associated with a global pandemic such as the COVID-19 pandemic. Our revenue from our non-U.S. operations represented approximately 47.1% of our revenue for the year ended December 31, 2019. We anticipate that we will continue to perform a significant portion of our services through our international operations. Our U.S. and international operations are subject to risk and uncertainties inherent in operating in these regions, including:
| • | conducting a clinical trial in multiple countries is complex, and issues in one country can affect the progress of the trial in other countries and result in delays or cancellation of contracts, including delays and other issues caused by the COVID-19 pandemic; |
| • | the United States or foreign countries could enact legislation or impose regulations, including unfavorable labor regulations, tax policies or economic sanctions, that could have an adverse effect on our ability to conduct business in or expatriate profits from the countries in which we operate; |
| • | the complexities of operating within multiple tax jurisdictions, including potentially negative consequences from changes in tax laws or from current and future tax examinations; |
| • | foreign countries are expanding or might expand their regulatory framework with respect to patient informed consent or other aspects of the conduct of clinical trials, which could delay or inhibit our ability to conduct trials in such countries, including changes that may be enacted or result from the COVID-19 pandemic; |
| • | the regulatory or judicial authorities of foreign countries might not enforce legal rights and recognize business procedures in a manner to which we are accustomed or would reasonably expect; |
| • | changes in political and economic conditions might lead to changes in the business environment in which we operate, such as the current changes caused by the COVID-19 pandemic, including those to protect the general population and patient safety in clinical trials; |
| • | actual or perceived risk of infection relating to the COVID-19 pandemic or otherwise might impact the willingness of patients to enroll in clinical trials and to visit clinics, hospitals and other clinical research sites for procedures associated with the conduct of clinical trials, which in turn could have an adverse effect on our ability to conduct business in relevant countries and regions; |
20
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | changes in foreign currency exchange rates, including the impact of contractual provisions that shift the risk of unfavorable movement in certain exchange rates to us; |
| • | potential violations of existing or newly enacted laws may cause difficulties in staffing and managing international operations; |
| • | customers in foreign countries may have longer payment cycles, and it may be more difficult to collect receivables in those countries; |
| • | political unrest could interrupt our services, endanger our personnel or cause project delays or loss of clinical trial material or results; and |
| • | any failure by us to comply with foreign regulations or restrictions or become aware of and acknowledge changes in foreign regulations or restrictions, which could result in the delay of a clinical trial, including changes in foreign regulations or restrictions due to the COVID-19 pandemic. |
These risks and uncertainties could negatively impact our ability to, among other things, perform large, global projects for our customers. Furthermore, our ability to manage these risks and uncertainties could be affected by U.S. laws and could have an adverse impact on our business, results of operations, financial condition and/or cash flows.
Difficult and volatile conditions in the capital and credit markets and in the overall economy, including those caused by the global COVID-19 pandemic, could materially adversely affect our business, financial position, results of operations and/or cash flows.
Our business, financial position, results of operations and/or cash flows could be materially adversely affected by difficult conditions and volatility in the capital and credit markets and changes in national or global economic conditions including, but not limited to, inflation, interest rates, the negative impacts caused by pandemics, including the COVID-19 pandemic, and the effects of governmental initiatives to manage economic conditions. Difficult conditions in these markets and the overall economy affect our business and operations in a number of ways. For example:
| • | as a result of the COVID-19 pandemic, customers may delay or cancel clinical trials (i) to protect patient safety, (ii) as a result of government restrictions, (iii) to limit impacts on healthcare systems and (iv) due to concerns regarding the ability of hospitals and other clinical trial sites to conduct clinical trials safely, efficiently and effectively; |
| • | if a significant percentage of our workforce is unable to work, including because of illness or travel or government restrictions in connection with the COVID-19 pandemic, our operations may be negatively impacted; |
| • | as a result of the COVID-19 pandemic, we have temporarily shut down our Phase I clinics. Depending on the future duration, severity and impacts of the COVID-19 pandemic, we may have to extend the shutdown of our Phase I clinics and also shut down one or more of our laboratories, other clinics or offices due to patient safety, government restrictions, illness or other impacts in connection with the COVID-19 pandemic; |
| • | market conditions, including those caused by the COVID-19 pandemic, could result in our key customers experiencing financial difficulties and/or electing to limit spending or delay payment of invoices, or become unable to pay invoices, which in turn could result in decreased revenues, cash flows and earnings for us; |
| • | under difficult market conditions there can be no assurance that borrowings under our Senior Secured Credit Facilities would be available or sufficient, and in such a case, we might not be able to successfully obtain additional financing or refinancing of our Senior Secured Credit Facilities or other debt on reasonable terms and within a reasonable time period acceptable to us, or at all, which could also impact our hedge accounting on our variable rate debt; and |
21
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | in order to respond to market conditions, we may need to seek waivers of various provisions in the Credit Agreement governing our Senior Secured Credit Facilities, and we might not be able to obtain such waivers on reasonable terms, if at all. |
If we are unable to recruit, retain and motivate key personnel, our business could be adversely affected.
Our success depends on the collective performance, contribution and expertise of our senior management team and other key personnel throughout our businesses, including qualified management, professional, operational, scientific, technical and business development personnel. There is significant competition for qualified personnel in the biopharmaceutical and related services industries, particularly personnel with advanced degrees and those with significant experience and expertise. The loss of any key executive, including if he or she becomes seriously ill with COVID-19 or otherwise, or our inability to continue to recruit, retain and motivate key personnel and replace departed personnel in a timely fashion, may adversely impact our ability to compete effectively and grow our business and negatively affect our ability to meet our short and long-term financial and operational objectives.
We depend on third parties for critical goods and support services.
We depend on third parties for a variety of goods and support services that are critical to us. These third-party providers include, but are not limited to, software and other technology providers, third-party transportation and travel providers, suppliers of study drugs for clinical trials, couriers, customs brokers, drug depots and distributors, suppliers of licensing agreements, investigator meeting planners, suppliers of kits, reagents, contractors and other supplies used by our laboratory segments and equipment maintenance providers. The failure of any of these third parties to adequately provide goods or services to us or to comply with relevant laws and regulations could have a material adverse effect on our reputation, business, results of operations, financial condition and/or cash flows. While we have not seen an adverse impact from the COVID-19 pandemic on the third parties that we rely on to provide goods and services, there can be no guarantee that a significant impact to our third-party providers will not occur in the future.
Our backlog might not accurately predict our future revenue, and we might not realize all or any part of the anticipated revenue reflected in our backlog.
Our backlog represents anticipated direct revenue for work not yet completed or performed (i) under signed contracts, letters of intent and, in some cases, awards that are supported by other forms of written communication and (ii) where there is sufficient or reasonable certainty about the customer’s ability and intent to fund and commence the services within six months. Our backlog excludes anticipated third-party pass-through and out-of-pocket revenue and, for amounts related to 2018 and later periods presented on an ASC 606 basis (including those derived or calculated from such amounts), exclude the impact of ASC 606 on direct revenue. Once work begins, we recognize direct revenue over the life of the contract based on our performance of services under the contract. Contracts may be terminated or delayed by our customers or regulatory authorities for reasons beyond our control. To the extent projects are delayed, the anticipated timing of our direct revenue could be materially affected.
In the event a customer terminates a contract, we are generally entitled to be paid for services rendered through the termination date and for services provided in winding down the project. However, we are generally not entitled to receive the full amount of direct revenue reflected in our backlog in the event of a contract termination. The duration of the projects in our backlog, and the related revenue recognition, ranges from several months to many years. A number of factors may affect backlog and the direct revenue generated from our backlog, including:
| • | the size, complexity and duration of projects; |
| • | the cancellation or delay of projects; and |
| • | changes in the scope of work during the course of a project. |
22
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Our backlog at March 31, 2020 was $7,312.2 million compared to a backlog of $6,536.0 million at March 31, 2019. Although an increase in backlog will generally result in an increase in future direct revenue to be recognized over time (depending on future contract modifications, contract cancellations and other adjustments), an increase in backlog at a particular point in time does not necessarily correspond to an increase in direct revenues during a particular period. The timing and extent to which backlog will result in direct revenue depends on many factors, including the timing of commencement of work, the rate at which we perform services, scope changes, cancellations, delays, receipt of regulatory approvals and the nature, duration, size, complexity and phase of the studies. In addition, delayed projects remain in backlog until they are canceled. As a result of these factors, our backlog is not necessarily a reliable indicator of future direct revenue and we might not realize all or any part of the direct revenue from the authorizations in backlog as of any point in time.
The majority of our customers’ contracts can be terminated, delayed or reduced in scope upon short notice or no notice.
Most of our contracts may be terminated by the customer upon 30 to 90 days’ notice. Customers terminate, delay or reduce the scope of their contracts for a variety of reasons, including but not limited to:
| • | lack of available funding or financing; |
| • | mergers or acquisitions involving the customer; |
| • | a change in customer priorities; |
| • | products being tested fail to satisfy safety requirements or efficacy criteria; |
| • | products have undesirable preclinical or clinical results; |
| • | the customer decides to forgo a particular study; |
| • | inability to enroll enough patients in a particular study; |
| • | inability to recruit enough investigators for a particular study; |
| • | the customer decides to shift business to a competitor or to use internal resources; |
| • | manufacturing problems that cause shortages of the study drug; |
| • | actions by regulatory authorities; and |
| • | performance failures. |
As a result, contract terminations, delays and reductions in scope occur regularly in the normal course of our business. However, the delay, loss or reduction in scope of a large contract or multiple smaller contracts could result in under-utilization of our personnel, a decline in revenue and profitability and adjustments to our backlog, any or all of which could have a material adverse effect on our business, results of operations, financial condition and/or cash flows. Further, we believe the risk of termination or delay of multiple contracts may be higher where we have strategic partnership arrangements with biopharmaceutical companies and a large backlog of work for those companies.
We may be adversely affected by industry, customer or therapeutic concentration.
We provide services to biopharmaceutical, biotechnology, medical device and government organizations, as well as other industry participants that sponsor clinical trials, and our revenue is dependent upon expenditures by these customers. Accordingly, our business could be materially adversely affected by mergers, consolidations, business failures, distress in the financial markets or other factors resulting in a decrease in the number of potential customers or therapeutic products being developed through the drug development process. In the last few years, biopharmaceutical consolidation has been accelerating. If the number of our potential customers were to decline in the future, they might be able to negotiate price discounts or other terms for services that are less
23
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
favorable to us than they have historically. Although we did not have any one customer that represented more than 10% of our total revenue for the years ended December 31, 2019, 2018 and 2017 or for the three months ended March 31, 2020, we have experienced customer concentration in the past and could again in the future. For example, our top 10 customers accounted for approximately 47.9% and 48.7% of our total revenue for the year ended December 31, 2019 and three months ended March 31, 2020, respectively. The loss of business from a significant customer could have a material adverse effect on our business, results of operations, financial condition and/or cash flows.
At times, we conduct multiple clinical studies for different customers in a single therapeutic area involving drugs with similar effects or to treat the same specific condition. As a result, our business could be adversely affected if some or all of the clinical studies are canceled due to newly discovered scientific information or regulatory decisions that affect the drugs within a particular class or for the treatment of a specific condition.
Our financial results may be adversely impacted if we underprice our contracts, overrun our cost estimates or fail to receive approval for or experience delays in documenting change orders.
The majority of our service contracts are based on fixed prices or fixed unit prices for those services, and therefore have set limits on the amounts we can charge for our direct and indirect services. As a result, variations in the timing and progress of large contracts may materially adversely affect our results of operations. In addition, we bear the risk of cost overruns unless the scope of activity is revised from the contract specifications and we are able to negotiate a contract modification with the customer shifting the additional cost to the customer. If we fail to adequately price our contracts for direct and indirect services in total or at the unit level or if we experience significant cost overruns (including direct and indirect costs such as pass-through costs), or are delayed in, or fail to, execute contract modifications with customers increasing the scope of activity, our results of operations could be materially adversely affected. From time to time, we have had to commit unanticipated resources to complete projects, resulting in lower margins and profitability on those projects. We might experience similar situations in the future, which could have a material adverse impact on our results of operations and cash flows.
Our business depends on the efficient and uninterrupted operation of our information and communication systems, including systems we use to deliver services to our customers, and failures in, breach of, or unauthorized access to or use of these systems or data contained therein may materially limit our operations and result in significant harm to our business.
Our success depends on the security and efficient and uninterrupted operation of our information and communication systems, including information and communication systems maintained by third parties on our behalf, and we expect to increase our reliance on these and similar systems over time. As the breadth, complexity and reliance on information systems grows, we will be increasingly exposed to the risks inherent in the development, deployment, operation, use and reliance on these systems, including:
| • | disruption, impairment or failure of data centers, telecommunications facilities or other key infrastructure platforms; |
| • | security breaches of, cyber-attacks on and other failures or malfunctions in our critical application systems or their associated hardware; and |
| • | excessive costs, delays or other deficiencies in systems development and deployment. |
The occurrence of these risks could impede the processing of data, the delivery of services to our customers and the day-to-day management and operation of our business and could result in the corruption, loss, disclosure or unauthorized access to proprietary, confidential or other data, which in turn could result in diminished internal and external reporting capabilities, impaired ability to process transactions, harm to our control environment, diminished employee productivity and unanticipated increases in costs.
24
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
While we maintain disaster recovery plans, they might not adequately protect us. Despite any precautions we take, damage from cybersecurity attacks, computer viruses, fire, floods, hurricanes, power loss, telecommunications failures, break-ins and similar events at our facilities or those of our suppliers could result in interruptions in the flow of data to our servers and from our servers to our customers. Corruption or loss of data could result in the need to repeat a trial at no cost to our customer, but at significant cost to us, and may result in the termination of a contract and/or damage to our reputation. Additionally, significant delays in system enhancements and improvements, or inadequate performance of the systems once they are completed, could damage our reputation and harm our business. Although we carry insurance, our coverage might not respond or be adequate to compensate us for all losses that may occur.
Unauthorized disclosure of or access to sensitive or confidential data, including confidential information of our customers, whether through third-party attack, system failure, employee negligence, fraud or misappropriation, could significantly damage our business. We have been, and expect we will continue to be, subject to attempts to gain unauthorized access to or through our information systems, whether by our employees or third parties, including by cyber-attack from computer programmers or hackers who deploy viruses, worms or other malicious software programs. To date, these attacks have not had a material impact on our operations or financial results. However, attacks in the future could result in fines, negative publicity, significant remediation costs, liability and/or damage to our reputation, and could have a material adverse effect on our business, results of operations, financial condition and/or cash flows. In addition, any insurance coverage we have might not respond or be sufficient to cover us against claims or penalties imposed by the federal government or state governments related to security breaches, cyber-attacks and other related breaches.
We are in the process of upgrading our existing human capital management, financial management and general ledger systems to an integrated enterprise resource planning system. We expect this upgrade to be substantially complete in 2020. Our ability to serve customers effectively depends on the reliability of our technology network. We depend on information systems to perform many critical business needs. Any disruption to these information systems could adversely impact our business. Despite extensive planning, we could experience disruptions in our business operations because of the project’s complexity. The potential consequences could include project and other delays, loss of information, diminished internal and external reporting capabilities, impaired ability to process transactions, harm to our control environment, diminished employee productivity and unanticipated increases in costs, all of which could result in material adverse effects on our business, results of operations, financial condition and/or cash flows.
If we fail to perform our services in accordance with contractual requirements, regulatory standards and ethical considerations, we could be liable for significant costs or penalties and our reputation could be harmed.
The clinical development and laboratory services we provide to biopharmaceutical companies and other entities are complex and subject to contractual requirements, regulatory standards and ethical considerations. For example, we must adhere to regulatory requirements from the FDA governing our activities relating to preclinical studies and clinical trials, including good clinical practices, good laboratory practice and GMP requirements. We are accredited by certain professional bodies, such as the College of American Pathologists. We are also subject to regulation by the U.S. Drug Enforcement Administration which regulates the distribution, recordkeeping, handling, security and disposal of controlled substances. If we fail to perform our services in accordance with these requirements, regulatory agencies have in the past and may in the future take action against us or our customers for failure to comply with applicable regulations governing clinical trials and the development and testing of therapeutic products. Such actions may include sanctions, such as warning or untitled letters, injunctions or failure of such regulatory authorities to grant marketing approval of products, delay, suspension or withdrawal of approvals, license revocation, loss of accreditation, product seizures or recalls, operational restrictions, civil or criminal penalties or prosecutions, damages or fines. Customers may also bring claims against us for breach of our contractual obligations in clinical trials, may terminate their contracts with us and/or may choose not to award further work to us, and patients involved in the clinical trials or taking drugs approved
25
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
on the basis of those trials may bring personal injury claims against us. Any such action could have a material adverse effect on our reputation, business, results of operations, financial condition and/or cash flows.
Such consequences could arise if, among other things, the following occur:
Failure or inadequate performance of our services. The performance of clinical development and laboratory services is complex and time-consuming. For example, we might make mistakes in conducting a clinical trial or providing laboratory services that could negatively impact or obviate the usefulness of the trial or the data generated from it or cause the results of the trial to be reported improperly. If the trial results are compromised, we could be subject to significant costs or liability, which could have a material adverse impact on our business, reputation and ability to perform our services. Examples include:
| • | non-compliance generally could result in the termination of ongoing clinical trials or the disqualification of data for submission to regulatory authorities, or enforcement action from regulators; |
| • | compromise of data from a particular trial, such as our failure to verify that informed consents were obtained from patients, could require us to repeat the trial under the terms of our contract at no further cost to our customer, but at a substantial cost to us; |
| • | improperly conducting or reporting laboratory results could affect medical decisions for the patient in the trial as well as the clinical trial data and create liability for personal injury and breach of contract for us; and |
| • | breach of a contractual term could result in liability for damages and/or termination of the contract. |
Large clinical trials can cost hundreds of millions of dollars and, while we endeavor to contractually limit our exposure to such risks and maintain insurance coverage, improper performance of our services could have a material adverse effect on our financial condition, damage our reputation and result in the cancellation of current contracts by or failure to obtain future contracts from the affected customer and other customers.
Interactive Response Technology (“IRT”) malfunction. Our IRT is critical because it enables the randomization of patients in a given clinical trial to different treatment arms and regulates the supply of an investigational drug, all by means of interactive voice response and interactive web response systems. If these systems malfunction or our personnel make mistakes in the provision of these services and, as a result, patients are incorrectly randomized or misdosed during the course of the clinical trial, then we could be subject to claims for significant damages for any resulting personal injury or death and/or breach of contract claims by our customers, as well as face potential regulatory enforcement. Furthermore, we could suffer from negative publicity associated with any such malfunctions or failures that could have a material adverse effect on our business and reputation. Additionally, errors in randomization may require us to repeat the trial at no further cost to our customer, but a substantial cost to us.
Inspections/Investigations of customers. From time to time, our customers are inspected or investigated by regulatory authorities or enforcement agencies with respect to regulatory compliance of their clinical trials. In these situations, we have often provided services to our customers with respect to the clinical trials being inspected or investigated, and we are called upon to respond to requests for information by the authorities and agencies. There is a risk that either our customers or regulatory authorities could claim that we performed our services improperly or that we were responsible for clinical trial non-compliance. If our customers or regulatory authorities make such claims against us, we could be subject to material damages, fines, penalties or other liabilities. In addition, negative publicity regarding compliance of our customers’ clinical trials, programs or drugs could have an adverse effect on our business and reputation.
26
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
If we encounter difficulties or delays in attracting suitable investigators and enrolling a sufficient number of patients for our customers’ clinical trials, our Clinical Development Services segment may be adversely affected.
The recruitment of investigators and patients is essential for the clinical research studies we run for our customers. Investigators are typically located at hospitals, clinics or other sites, including sites we own, and supervise administration of the study drug to patients during the course of a clinical trial. Patients generally are people from the communities in which clinical trials are conducted and may be difficult to locate and enroll in trials, particularly for rare or acute indications, or if the trial protocol requires patients who have not taken other treatments or have failed other treatments for the relevant condition. If we are unable to attract suitable and willing investigators or recruit, enroll and retain patients for clinical trials, our Clinical Development Services segment could be materially adversely affected. For example, if we are unable to recruit sufficient investigators to conduct clinical trials as planned or enroll the required number of patients, we may need to incur additional costs to meet the recruitment or enrollment targets or cause a delay or modification to the clinical trial plans. Delays in patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to fulfill our obligations to our customers. Any such difficulties or delays could result in additional costs to us and materially adversely affect our business, results of operations, financial condition and/or cash flows and reputation in the industry.
We are subject to numerous privacy and data security laws and our failure to comply with those laws could cause us significant harm.
In the normal course of our business, we collect, process, use and disclose individual personal data, including patient-specific medical and other clinical trial data, as well as personal data relating to health professionals and our employees. The collection, processing, use, disclosure, disposal and protection of this information and personal data is highly regulated both in the United States and other jurisdictions we are subject to, including but not limited to, applicable regulations arising from the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and the Privacy, Security and Breach Notification Rules, 45 C.F.R. Parts 160-164, that implement those laws; U.S. state privacy, security and breach notification and healthcare information laws; the E.U. General Data Protection Directive (the “GDPR”); other European privacy laws and other privacy laws that are increasingly being adopted in other regions globally. These laws and regulations include varied and sometimes inconsistent requirements, increasing legal risk and the costs and risks of compliance.
These regulations often govern the use, handling and disclosure of personally identifiable medical information and require the use of standard transactions, privacy and security standards and other administrative simplification provisions by covered entities, which include many healthcare providers, health plans, and healthcare clearinghouses. Although certain aspects of our businesses are subject to HIPAA, we do not consider our service offerings generally to cause us to be subject to HIPAA as a directly covered entity; however, there are extremely limited circumstances where we enter into business associate agreements. However, we endeavor to embrace sound identity protection practices and have implemented processes and systems in order to comply with these laws and continue to monitor and enhance them. If we improperly process personal information, fail to protect the confidentiality and security of this information or otherwise breach applicable privacy laws, regulations or duties relating to the use, privacy or security of personal data, we could be subject to civil liability or criminal prosecution, be forced to alter our business practices and we could suffer significant financial, reputational and other harm and our business, results of operations, financial condition and/or cash flows could be materially adversely affected.
The GDPR became enforceable on May 25, 2018. The GDPR includes sanctions for violations up to the greater of €20 million or 4.0% of worldwide gross annual revenue and applies to services providers such as us. Other privacy laws, including HIPAA and HITECH, provide for potentially large fines for violations. Were we to
27
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
be subject to any such sanction, it could result in a material adverse effect on our reputation, business, results of operations, financial condition and/or cash flows.
In connection with some clinical trials that we conduct in the European Union on behalf of our customers, we serve as the customer’s E.U. data privacy representative under the GDPR. As the customer’s representative, we could in certain circumstances be liable for the customer’s failure to comply with the GDPR. We believe we maintain adequate processes and systems to ensure our and our customers’ compliance with the requirements of the GDPR, but it is possible that we could fail to comply or that we could incur liability due to the acts or omissions of our customers. Our contracts for these services include indemnification provisions intended to protect us from a customers’ failure to comply with the GDPR, but it might not cover all our losses in the event of a failure to comply. In the event we are not able to secure indemnification or the indemnification and any insurance coverage is inadequate to cover our losses, we could suffer significant financial, operational, reputational and other harm and our business, results of operations, financial condition and/or cash flows could be materially adversely affected.
The United States, the European Union, and other jurisdictions where we operate continue to issue new, and enhance existing, privacy and data security protection regulations related to the collection, use, disclosure, disposal and protection of personal data and medical information, such as the recently enacted California Consumer Protection Act. Privacy and data security laws are rapidly evolving both in the United States and internationally, and the future interpretation of those laws is somewhat uncertain. For example, we do not know how E.U. regulators will interpret or enforce many aspects of the GDPR and some regulators may do so in an inconsistent manner. In the United States, privacy and data security is an area of emphasis for some but not all state regulators, and new legislation has been and likely will continue to be introduced at the state and/or federal level. Additional legislation or regulation might, among other things, require us to implement new security measures and processes or bring within the legislation or regulation de-identified health or other personal data, each of which may require substantial expenditures or limit our ability to offer some of our services.
Our business could be harmed if we are unable to effectively manage our growth.
We believe that sustained growth places a strain on human, operational and financial resources. To manage our organic and inorganic growth and increasing complexity of our business, we must continue to attract and retain qualified management, professional, scientific, technical and business development personnel and improve our operating and administrative systems. We believe that maintaining and enhancing both personnel and our systems at reasonable cost are instrumental to our success. We cannot assure you that we will be able to enhance our current technology or obtain new technology that will enable our systems to keep pace with industry developments and the sophisticated needs of our customers. The nature and pace of our organic and inorganic growth introduces risks associated with quality control and customer dissatisfaction due to delays in performance or other problems. In addition, non-U.S. operations involve the additional risks of assimilating differences in non-U.S. business practices, hiring and retaining qualified personnel and overcoming language barriers. If we are unable to manage our growth effectively, we could incur losses.
Changes in accounting standards issued by the Financial Accounting Standards Board (“FASB”), including ASC 606, or other standard-setting bodies may adversely affect trends and comparability of our financial results.
We are required to prepare our financial statements in accordance with U.S. GAAP, which is periodically revised and/or expanded. From time to time, we are required to adopt new or revised accounting standards issued by recognized authoritative bodies, including the FASB and the SEC. It is possible that future accounting standards we are required to adopt may require additional changes to the current accounting treatment that we apply to our financial statements and may result in significant changes to our results, disclosures and supporting reporting systems. Such changes could result in a material adverse impact on our results of operations and financial condition.
28
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
For example, effective January 1, 2018, we were required to adopt ASC 606, which outlines a single comprehensive model for entities to use in accounting for revenue from contracts with customers. Under ASC 606, third-party pass-through costs and reimbursed costs are included in our measurement of progress. This change in revenue recognition requires significant estimates of project costs that are updated and adjusted on a regular basis. These updates and adjustments may result in variability in our revenue recognition from period to period that may cause unexpected variability in our operating results. Additionally, effective January 1, 2019, we were required to adopt ASC Topic 842, Leases (“ASC 842”), which required us to recognize certain operating leases in our consolidated balance sheet. See Note 1, Note 3 and Note 11 to our audited consolidated financial statements incorporated by reference from PPD’s May 2020 8-K for more information regarding ASC 606 and ASC 842.
We operate in many different countries and are subject to the U.S. Foreign Corrupt Practices Act (the “FCPA”), the Bribery Act and anti-corruption laws and regulations in other countries, as well as laws and regulations relating to trade compliance and economic sanctions. Violations of these laws and regulations could harm our reputation and business, or materially adversely affect our business, results of operations, financial condition and/or cash flows.
We are subject to various U.S. and non-U.S. anti-corruption laws, including the FCPA and the U.K. Bribery Act 2010 (the “Bribery Act”). The FCPA and the Bribery Act prohibit us and our officers, directors, employees and third parties acting on our behalf, including agents, from corruptly offering, promising, authorizing or providing anything of value to a “foreign official” for the purposes of influencing official decisions or obtaining or retaining business or otherwise obtaining favorable treatment. The FCPA further requires us to make and keep books, records and accounts that accurately reflect transactions and dispositions of assets and to maintain a system of adequate internal accounting controls. The Bribery Act also prohibits “commercial” bribery and accepting bribes.
Our global business operations also must be conducted in compliance with applicable export controls and economic sanctions laws and regulations, including those administered by the U.S. Department of the Treasury’s (the “U.S. Treasury”) Office of Foreign Assets Control, the U.S. Department of State, the U.S. Department of Commerce, the United Nations Security Council, the European Union, Her Majesty’s Treasury and other relevant sanctions authorities.
Our internal policies and procedures require strict compliance with these anti-corruption and economic sanctions laws. Despite our training and compliance efforts, we cannot assure that our policies and procedures will protect us from liability for violations of anti-corruption or economic sanctions laws committed by persons associated with us, including our employees or third parties acting on our behalf. Our continued expansion outside the United States, including in countries that are known to have an increased prevalence of corruption, could increase such risks in the future. Violations of these anti-corruption laws or economic sanctions, or even allegations of such violations, could disrupt our business and result in a material adverse effect on our reputation, business, results of operations, financial condition and/or cash flows. For example, violations may result in criminal or civil penalties, disgorgement of profits, related stockholder lawsuits and other remedial measures, and companies that violate these laws can be debarred by the U.S. government and lose U.S. export privileges. Future changes in anti-corruption or economic sanctions laws and enforcement could also result in increased compliance requirements and related costs which could have a material adverse effect on our business, results of operations, financial condition and/or cash flows.
The competition between our existing and potential customers may adversely impact the extent to which those customers use our services, which may materially adversely affect our business, results of operations, financial condition and/or cash flows.
We regularly provide services to biopharmaceutical companies that compete against each other and we sometimes provide services to customers that are developing competing drugs. Therefore, the existing or future
29
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
business we receive from a customer might discourage a competing customer or potential customer from requesting our services. Also, in connection with the negotiation of a contract, a customer might require that we agree to limit the scope of services we provide to other customers or other restrictive covenants that might limit our ability to provide services to others. The loss of, or reduction in, business we receive from a customer or limits on our ability to service other customers may have a material adverse effect on our business, results of operations, financial condition and/or cash flows.
We face risks associated with business restructurings and the integration of new businesses, which, if not properly managed, could materially affect our business.
In the past few years, we have adopted and implemented restructuring plans and cost-saving initiatives designed to, among other things, improve our operating efficiencies, match our capacity with market demand and reduce costs. At the same time, we have made strategic investments by acquiring businesses that we believe complement our existing portfolio of services. Restructurings and the integration of new businesses present potential risks that could materially adversely affect our business. Restructurings could result in a decline in employee morale, an increase in employment claims, the failure to achieve the stated operational objectives and/or targeted costs savings and the failure to meet customer requirements. Conversely, the success of any acquisition will depend upon, among other things, our ability to effectively integrate the acquired business operations, personnel, services and technologies into our organization, retain and motivate personnel key to the future success of the acquired business and retain customers. If we fail to identify and effectively manage these potential risks, our reputation, business, results of operations, financial condition and/or cash flows could be materially adversely affected.
Our business exposes us to potential liability that could affect our reputation, business, results of operations, financial condition and/or cash flows.
Our business involves the testing of new drugs on humans participating in clinical trials and, if marketing approval is received, the availability of these drugs to be prescribed to patients. Our provision of clinical trial services and involvement in the drug development process exposes us to the risk of liability for personal injury or death from, among other things, improper administration of a drug during testing and adverse reactions to the drug administered during testing and after the drug has been approved for sale by regulatory authorities. For example, we have in the past been sued by individuals alleging personal injury due to their participation in a clinical trial. In addition, we have also been sued by individuals alleging personal injury and death caused by the ingestion of drugs approved for sale by regulatory authorities due to our participation in a clinical trial of the drug prior to its approval. In each of these suits, the individuals were seeking monetary damages under a variety of legal theories. If we are required to pay damages or incur defense costs in connection with any personal injury claim that is outside the scope of indemnification agreements we have with customers, if any indemnification agreement is not performed in accordance with its terms or if our liability exceeds the amount of any applicable indemnification limits or available insurance coverage, our reputation, business, results of operations, financial condition and/or cash flows could be materially adversely impacted. We also might not be able to procure adequate insurance for these risks in the future upon terms acceptable to us, if at all.
In the normal course of providing clinical trial services for our customers, we contract with physicians who serve as investigators to administer the protocols and conduct the trials. In addition, we currently own and operate a global site network and employ physicians who serve as investigators on clinical trials. In either case, if an investigator errs during a clinical trial resulting in harm to a patient, claims for personal injury or product liability damages may result. Additionally, trial data may be compromised and our customer may seek damages from us or require us to repeat the trial at our cost. If we were liable for claims related to a physician’s conduct, such liability could have a material adverse impact on our business, results of operations, financial condition and/or cash flows.
From time to time we act as legal representative, importer of record or in a similar capacity on behalf of our customers in certain countries or regions, either as a result of being directly engaged to do so or being deemed to
30
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
take on such role by virtue of providing associated services. Acting in this capacity exposes us to increased risk, including potential liability to patients and regulatory authorities for the action and/or inaction of the customer. As a condition to providing such services, we generally require specific indemnification and insurance from the customer, however any such insurance coverage might not respond or be sufficient to cover us against claims or penalties imposed, and in the event that we seek to enforce an indemnification provision, the indemnifying party may not have sufficient resources to fully satisfy its indemnification obligations or may otherwise not comply with its contractual obligations. In these circumstances, we could suffer significant financial, operational, reputational and other harm and our business, results of operations, financial condition and/or cash flows could be materially adversely affected.
The operation of our early development Phase I clinics and our AES offering involves direct interaction with clinical trial volunteers, and exposes us to potential liability for personal injury or death that could materially adversely affect our reputation and business.
We operate early development clinics, which involve direct interaction by us with clinical trial volunteers, and we also have strategic alliances with other early development clinics that serve as subcontractors for us. We also own and operate a global site network, which involves direct interaction with clinical trial volunteers. As a part of our early development and our AES operations, we employ and contract with physicians, nurses and other trained health care professionals who conduct the protocol and testing directly on individuals, which may involve administration of the investigational drug, drawing of blood and other medical procedures required under the protocol. Any personal injury to, or death of, a person participating in a clinical trial caused by the medical malpractice or negligence of our physicians, nurses or other staff, or those of our subcontractors, may result in liability to us and have a material adverse effect on our reputation, business, results of operations, financial condition and/or cash flows.
Our insurance might not cover all of our liabilities, including indemnification obligations, associated with the operation of our business and provision of services.
We procure and maintain insurance for ordinary risks associated with the operation of our business, including our indemnification obligations. This insurance coverage under the policies we procure might not be sufficient to cover all of our liabilities or may be contested by our carriers. If our insurance is not adequate or available to cover our liabilities, including our indemnification obligations, or if insurance is not available in the future upon terms acceptable to us, if at all, or if the cost of our insurance is far in excess of historical amounts, our business, results of operations, financial condition and/or cash flows may be materially adversely harmed.
Our business uses biological and hazardous materials, which are regulated by various laws. As such, we are exposed to liabilities for violations of those laws and claims for personal injury or death that could materially adversely affect our business.
Our drug development activities involve the use of biological materials, hazardous materials, chemicals and various radioactive compounds. We are subject to various laws and regulations governing the use, storage, handling and disposal of these materials. In the event we violate these laws, we could be liable for costs and expenses for cleanup and remediation, statutory fines and penalties and other civil and criminal penalties. In addition, if there are changes in these laws or regulations or new laws or regulations are enacted, we might be required to incur significant costs to bring our operations into compliance with any new requirements. Furthermore, in the event of an incident involving these materials, we may be subject to claims for personal injury, death or property damage, all of which could materially adversely impact our business, results of operations, financial condition and/or cash flows.
Tax reform in the United States could materially affect our business, results of operations, financial condition and/or cash flows.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act of 2017 (the “Tax Act”). The Tax Act made broad and complex changes to the U.S.
31
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
tax code including, but not limited to, (i) reducing the corporate statutory income tax rate from 35% to 21%, effective for 2018 and thereafter, (ii) amending the limitations on deductions for interest and (iii) transitioning U.S. international taxation in part from a worldwide system to a territorial system, inclusive of a one-time mandatory transition tax on accumulated unremitted foreign earnings as of December 31, 2017. Although we have adopted the applicable portions of the Tax Act as required, certain amounts recorded represent our best estimate based on regulatory guidance and information available at the time of recording. The ultimate impact from applying the Tax Act may differ materially from amounts recognized, due to, among other things, additional regulatory guidance that may be issued and actions we take because of the Tax Act. We continue to assess the impact of the Tax Act, and are awaiting further guidance from the Internal Revenue Service and the U.S. Treasury relating to interpretation and application of the Tax Act. Our accounting for the Tax Act could have a material effect on our business, results of operations, financial condition and/or cash flows.
On March 27, 2020, the U.S. government passed the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) in response to the COVID-19 pandemic. The CARES Act provides wide-ranging economic relief, including significant changes to U.S. business tax provisions. These changes include, in summary, (i) modifications to limitations on the deductibility of net operating losses, (ii) modifications to limitations on the deductibility of business interest, (iii) alternative minimum tax credit acceleration and (iv) the expensing of qualified improvement property. The most significant impact to us from the CARES Act relates to the modification to limitations on the deductibility of business interest and the expensing of qualified improvement property. We have accounted for the impact of the CARES Act on prior tax years within the period ended March 31, 2020 and accounted for the impact on the 2020 tax year in our annual effective tax rate and benefit from income taxes for the three months ended March 31, 2020. We are continuing to assess the income tax impact of the CARES Act and other legislative changes enacted and being considered by governments around the world in response to the COVID-19 pandemic.
Our cash taxes paid and effective tax rate have and will continue to fluctuate from time to time, and increases in either may adversely affect our business, results of operations, financial condition and/or cash flows.
Our cash taxes paid and effective income tax rate are influenced by our projected and actual profitability in the taxing jurisdictions in which we operate as well as changes in income tax rates. Additionally, changes in the distribution of profits and losses among taxing jurisdictions may have a significant impact on our cash taxes paid and effective income tax rate. Factors that may affect our cash taxes paid and/or effective income tax rate include, but are not limited to:
| • | the requirement to exclude from our quarterly worldwide effective income tax calculations losses in jurisdictions where no income tax benefit can be recognized; |
| • | actual and projected full year pre-tax income; |
| • | changes in existing tax laws and rates in various taxing jurisdictions; |
| • | examinations or audits by taxing authorities; |
| • | the use of foreign tax credits, and restrictions therein; |
| • | changes in our capital structure; |
| • | the establishment of valuation allowances against deferred income tax assets if we determine that it is more likely than not that future income tax benefits will not be realized; and |
| • | other provisions of the Tax Act, including (i) base erosion and anti-abuse tax, if applicable, (ii) taxation of foreign-derived intangible income and global intangible low-taxed income and (iii) limitations on deductions for interest, among others. |
These factors could have a material adverse effect on our business, results of operations, financial condition and/or cash flows.
32
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Additionally, we rely upon generally accepted interpretations of tax laws and regulations in the countries in which we operate and cannot be certain that these interpretations are accurate or that the responsible taxing authority is in agreement with our views. We currently have open examinations with various tax authorities. If a satisfactory resolution cannot be achieved with the tax authorities, the ultimate tax outcome may have a material adverse effect on our results of operations, financial condition and/or cash flows.
Economic conditions and regulatory changes relating to the United Kingdom’s exit from the European Union could negatively affect our business, results of operations, financial condition and/or cash flows.
We have operations in multiple countries, including the United Kingdom, and have transactions in multiple currencies, including the Pound Sterling. We also employ nationals of E.U. countries in the United Kingdom and U.K. nationals in our E.U. businesses. During the second quarter of 2016, the United Kingdom voted by referendum to exit the European Union, commonly referred to as “Brexit.” On January 31, 2020, the U.K. ceased to be part of the European Union. The impact of the United Kingdom’s departure from, and future relationship with, the European Union are uncertain. Brexit has and continues to create general economic uncertainty in the United Kingdom and European Union. The effects of Brexit could have an adverse impact on our business, results of operations, financial condition, and/or cash flows.
Our inability to adequately protect our intellectual property rights could adversely affect our business.
Our success is dependent, in part, on our ability to develop, use and protect our proprietary methodologies, software, compositions, processes, procedures, systems, technologies and other intellectual property. To protect our intellectual property rights, we primarily rely upon trade secret law, confidentiality agreements and policies, invention assignments and other contractual arrangements, along with patent, copyright and trademark laws. Existing laws of the countries outside of the United States in which we provide services offer only limited protection, and these are subject to change at any time. In addition, the agreements upon which we rely to protect our intellectual property might be breached, or might not be fully enforceable. Our intellectual property rights might not prevent our competitors from independently developing intellectual property that is similar to or duplicative of ours. Also, enforcing our intellectual property rights might also require substantial time, money and oversight, and we might not be successful in enforcing our rights.
Any patents that we own or license might not provide adequate protection in the future for the covered technology or inventions. Any patent applications we file might not result in the issuance of valid patents or the scope of our issued patents might not provide meaningful competitive advantages. Also, any patent protection might not prevent others from developing competitive products using related or other technology that does not infringe our patent rights. The scope and enforceability of patents can be highly uncertain and often involves complex legal and factual questions and proceedings, which could be expensive, last several years and either prevent issuance of additional patents to us or result in a significant reduction in the scope or invalidation of our patents. Moreover, the issuance of a patent in one country does not assure the issuance of a patent with similar claim scope in another country, and claim interpretation and infringement laws vary among countries, so we are unable to predict the extent of patent protection in any country.
We cannot be certain that the conduct of our business does not and will not infringe the intellectual property or other proprietary rights of others. Any claim of infringement by a third party, even one without merit, could cause us to incur substantial costs to defend against such claim, could distract our management and employees, and generally interfere with our business.
Our investments in third parties are illiquid and subject to loss which could materially adversely affect our financial condition.
We have made investments and commitments to invest in other companies and investment vehicles. Most of our investments are as a limited partner in investment partnerships and are not directly in individual companies.
33
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
In many cases, there is no public market for these investments and we might not be able to sell them on terms acceptable to us, if at all. In addition, if these funds or companies encounter financial difficulties, we might lose all or part of our investment. We account for the majority of these equity method investments at fair value, utilizing the fair value option, in accordance with GAAP. These investments could have a significant impact on our operating results due to changes in fair market value of their respective investment portfolios or changes in the valuation assumptions by management. We have recorded a liability for additional consideration estimated to be payable related to the recapitalization of the Company in 2017. The contingent additional consideration is based primarily on changes in the fair value of Auven and venBio, net of taxes and other expenses related to such investments. For more information see Note 7, “Investments,” to our audited consolidated financial statements incorporated by reference from PPD’s May 2020 8-K.
We may need to recognize impairment charges related to goodwill, definite-lived intangible assets and/or fixed assets.
We have substantial balances of goodwill and definite-lived intangible assets as a result of being taken private by our Majority Sponsors in 2011 as well as our other acquisitions. As of March 31, 2020, our goodwill and intangible assets totaled $1,723.3 million and $835.3 million, respectively. We are required to test goodwill for possible impairment on the same date each year and on an interim basis if there are indicators of a possible impairment. We are also required to evaluate amortizable intangible assets for impairment if there are indicators of a possible impairment.
There is significant judgment required in the analysis of a potential impairment of goodwill and intangible assets. As a result of a general economic slowdown, deterioration in one or more of the markets in which we operate or in our financial performance and/or future outlook of reporting units with assigned goodwill or intangible assets, we may determine that impairment of our goodwill or intangible assets exists. An impairment charge would be determined based on the estimated fair value of the reporting unit’s assigned goodwill and estimated fair value of intangible assets and any such impairment charge could have a material adverse effect on our results of operations and financial condition. We perform our annual impairment test of goodwill during the fourth quarter of each year, or more frequently if impairment indicators arise, which requires significant judgment. As of the date of our 2019 annual goodwill impairment test, of our nine reporting units with goodwill allocated, one reporting unit’s estimate of the fair value did not exceed its respective carrying value by a substantial margin. This reporting unit had recorded goodwill of $30.0 million as of the goodwill impairment testing date. The percentage by which the reporting unit’s estimated fair value exceeded carrying value was 22.0%. Certain portions of this reporting unit were negatively impacted by the COVID-19 pandemic and to the extent that the impacts are prolonged or that they result in a change to long-term outlook, such could trigger an impairment to this reporting unit in the future. Future goodwill or other long-lived asset impairment, if any, could have a material impact on our results of operations or financial condition. For example, for the years ended December 31, 2018 and 2017, we recognized goodwill impairment charges of $29.6 million and $38.4 million, respectively. For the year ended December 31, 2019 and the three months ended March 31, 2020, we did not recognize any goodwill impairment charges. See Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates” of PPD’s 2019 10-K for additional information on the goodwill impairment recognized.
Risks Associated with Our Indebtedness
Our substantial indebtedness could materially adversely affect our financial condition and our ability to operate our business, react to changes in the economy or industry, repay our debts and meet our obligations under the agreements governing our debt arrangements, and could divert our cash flow from operations to debt payments.
We have a significant amount of indebtedness. As of March 31, 2020, our total borrowings under our Senior Secured Credit Facilities was $3,283.3 million, including $150.0 million of Revolving Credit Facility
34
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
borrowings, and we had $1,125.0 million outstanding of 6.375% Senior Notes due 2023 issued by Jaguar Holding Company II and Pharmaceutical Product Development, LLC (the “OpCo Notes”), which were redeemed in full on June 5, 2020 with the proceeds of the Notes issued on June 5, 2020. In addition, as of March 31, 2020, we had a $300.0 million Revolving Credit Facility under which we had $150.0 million of availability (not giving effect to $1.6 million of outstanding letters of credit). In addition, subject to restrictions in the agreements governing our Senior Secured Credit Facilities and the indenture governing our Notes, we may incur additional debt.
Our substantial debt could have important consequences, including the following:
| • | it may be difficult for us to satisfy our obligations, including debt service requirements under our outstanding debt, resulting in possible defaults on and acceleration of such indebtedness; |
| • | our ability to obtain additional financing for working capital, capital expenditures, debt service requirements or other general corporate purposes may be impaired; |
| • | a substantial portion of cash flow from operations may be dedicated to the payment of principal and interest on our debt, therefore reducing our ability to use our cash flow to fund our operations, capital expenditures, future business opportunities, acquisitions and other purposes; |
| • | we are more vulnerable to economic downturns and adverse industry conditions and our flexibility to plan for, or react to, changes in our business or industry may be more limited; |
| • | our ability to capitalize on business opportunities and to react to competitive pressures, as compared to our competitors, may be compromised; and |
| • | our ability to borrow additional funds or to refinance debt may be limited. |
Furthermore, all of our debt under our Senior Secured Credit Facilities bears interest at variable rates based on LIBOR. If these rates were to increase significantly, whether because of an increase in market interest rates or a decrease in our creditworthiness, our ability to borrow additional funds may be reduced and the risks related to our substantial debt would intensify. Each quarter-point increase in the LIBOR would have increased the interest expense on our current variable rate debt by approximately $7.8 million during 2019.
In addition, on July 27, 2017, the Chief Executive of the U.K. Financial Conduct Authority, which regulates LIBOR, announced that it intends to stop persuading or compelling banks to submit rates for the calibration of LIBOR to the administrator of LIBOR after 2021. If LIBOR ceases to exist, the method and rate used to calculate our interest rates and/or payments on our Senior Secured Credit Facilities in the future may result in interest rates and/or payments that are higher than, lower than or that do not otherwise correlate over time with the interest rates and/or payments that would have been applicable to our obligations if LIBOR was available in its current form. There is currently no definitive information regarding the future utilization of LIBOR or of any particular replacement rate. As such, the potential effect of any such event is uncertain, but were it to occur, our cost of capital, financial results, cash flows and results of operations may be adversely affected.
Servicing our debt requires a significant amount of cash. Our ability to generate sufficient cash depends on numerous factors beyond our control, and we may be unable to generate sufficient cash flow from operating activities to service our debt obligations. Our ability to make payments on and to refinance our debt and to fund planned capital expenditures depends on our ability to generate cash in the future. To some extent, this is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. If we are unable to generate sufficient cash flow from operations to service our debt and meet our other commitments, we may need to refinance all or a portion of our debt, sell material assets or operations, delay capital expenditures or raise additional debt or equity capital. We may not be able to effect any of these actions on a timely basis, on commercially reasonable terms or at all, and these actions may not be sufficient to meet our capital requirements. In addition, the terms of our existing or future debt agreements may restrict us from pursuing any of these alternatives.
35
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Restrictive covenants in the Credit Agreement governing our Senior Secured Credit Facilities and the indenture governing our Notes may restrict our ability to pursue our business strategies, and failure to comply with any of these restrictions could result in acceleration of our debt.
The operating and financial restrictions and covenants in the Credit Agreement governing our Senior Secured Credit Facilities and the indenture governing our Notes may materially adversely affect our ability to distribute monies to our stockholders, finance future operations or capital needs or engage in other business activities. Such agreements limit our ability, among other things, to:
| • | incur additional indebtedness and guarantee indebtedness; |
| • | pay dividends on or make distributions in respect of our common stock or make other restricted payments; |
| • | make loans and investments; |
| • | sell or otherwise dispose of assets; |
| • | incur liens; |
| • | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; |
| • | enter into agreements restricting our subsidiaries’ ability to pay dividends; |
| • | enter into certain transactions with our affiliates; and |
| • | designate our subsidiaries as unrestricted subsidiaries. |
In addition, the restrictive covenants in the Credit Agreement governing our Senior Secured Credit Facilities require us to maintain a specified first lien net leverage ratio when a certain percentage of our Revolving Credit Facility commitments are borrowed and outstanding as of the end of each fiscal quarter. In certain circumstances, our ability to meet this financial covenant may be affected by events beyond our control.
A breach of the covenants under the Credit Agreement governing our Senior Secured Credit Facilities or the indenture governing our Notes could result in an event of default under the applicable indebtedness. Such a default might allow the creditors to accelerate the related debt and might result in the acceleration of any other debt to which a cross-acceleration or cross-default provision applies. In addition, an event of default under the Credit Agreement governing our Senior Secured Credit Facilities would permit the lenders under our Senior Secured Credit Facilities to terminate all commitments to extend further credit under our Senior Secured Credit Facilities. Furthermore, if we were unable to repay the amounts due and payable under our Senior Secured Credit Facilities, those lenders could proceed against the collateral granted to them to secure that indebtedness. In the event our lenders or noteholders accelerate the repayment of our borrowings, we and our subsidiaries may not have sufficient assets to repay that indebtedness.
As a result of all of these restrictions, we and/or our subsidiaries, as applicable, may be:
| • | limited in how we conduct our business; |
| • | unable to raise additional debt or equity financing to operate during general economic or business downturns; or |
| • | unable to compete effectively or to take advantage of new business opportunities. |
These restrictions might hinder our ability to service our indebtedness or grow in accordance with our business strategy. Furthermore, the terms of any future indebtedness we may incur could have further additional restrictive covenants. We may not be able to maintain compliance with these covenants in the future, and in the event that we are not able to maintain compliance, we cannot assure you that we will be able to obtain waivers from the lenders or amend the covenants.
36
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Despite current debt levels, we and our subsidiaries may still be able to incur substantially more debt. This could further exacerbate the risks associated with our substantial leverage.
We and our subsidiaries may be able to incur substantial additional debt in the future. Although the Credit Agreement governing our Senior Secured Credit Facilities and the indenture governing our Notes contain restrictions on the incurrence of additional debt, these restrictions are subject to a number of qualifications and exceptions, and the debt incurred in compliance with these restrictions could be substantial. Additionally, we may successfully obtain waivers of these restrictions. If we incur additional debt above the levels currently in effect, the risks associated with our leverage, including those described above, would increase. In addition, we had a $300.0 million Revolving Credit Facility under which we had $150.0 million of availability as of March 31, 2020 (not giving effect to $1.6 million of outstanding letters of credit).
Risks Related to this Offering and Ownership of Our Common Stock
The market price of our common stock has been volatile and may continue to fluctuate substantially, which could result in substantial losses for purchasers of our common stock.
The trading price of our common stock has been and is likely to continue to be volatile. The stock market has experienced extreme volatility. This volatility often has been unrelated or disproportionate to the operating performance of particular companies. Since shares of our common stock were sold in our initial public offering in February 2020 at a price of $27.00 per share, our stock price has ranged from $10.61 to $33.23 through July 17, 2020. The market price of our common stock has been highly volatile and may continue to fluctuate substantially due to a number of factors such as those listed in “—Risks Related to Our Business” and the following:
| • | results of operations that vary from the expectations of securities analysts and investors; |
| • | results of operations that vary from those of our competitors; |
| • | changes in expectations as to our future financial performance, including financial estimates and investment recommendations by securities analysts and investors; |
| • | declines in the market prices of stocks generally; |
| • | the current and uncertain future impact from the COVID-19 pandemic on our business, growth, reputation, prospects, financial condition, results of operations (including components of our financial results), cash flows and liquidity; |
| • | strategic actions by us or our competitors; |
| • | announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments; |
| • | changes in general economic or market conditions or trends in our industry or markets; |
| • | changes in business or regulatory conditions; |
| • | additions or departures of key management personnel; |
| • | future sales of our common stock or other securities by us or our existing stockholders, or the perception of such future sales; |
| • | investor perceptions of the investment opportunity associated with our common stock relative to other investment alternatives; |
| • | the public’s response to press releases or other public announcements by us or third parties, including our filings with the SEC; |
| • | announcements relating to litigation; |
| • | guidance, if any, that we provide to the public, any changes in this guidance or our failure to meet this guidance; |
37
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | the development and sustainability of an active trading market for our stock; |
| • | changes in accounting principles; and |
| • | other events or factors, including those resulting from natural disasters, war, acts of terrorism or responses to these events. |
These broad market and industry fluctuations may materially adversely affect the market price of our common stock, regardless of our actual operating performance. In addition, price volatility may be greater if the public float and trading volume of our common stock are low.
In the past, following periods of market volatility, stockholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
Our quarterly operating results fluctuate and may fall short of prior periods, our projections or the expectations of securities analysts or investors, which could materially adversely affect our stock price.
Our operating results have fluctuated from quarter to quarter at points in the past, and they may do so in the future. Therefore, results of any one fiscal quarter are not a reliable indication of results to be expected for any other fiscal quarter or for any year. If we fail to increase our results over prior periods, to achieve our projected results or to meet the expectations of securities analysts or investors, our stock price may decline, and the decrease in the stock price may be disproportionate to the shortfall in our financial performance. Results may be affected by various factors, including those described in these risk factors. We maintain a forecasting process that seeks to align expenses to backlog conversion. If we do not control costs or appropriately adjust costs to actual results, or if actual results differ significantly from our forecast, our financial performance could be materially adversely affected.
We are a holding company with no operations and rely on our operating subsidiaries to provide us with funds necessary to meet our financial obligations.
We are a holding company with no material direct operations. Our principal assets are the shares of common stock of Eagle Holding Company II, LLC (“Eagle II”) that we hold. Eagle II is the indirect parent of Pharmaceutical Product Development, LLC which, together with its subsidiaries, owns substantially all of our operating assets. As a result, we are dependent on loans, dividends and other payments from our subsidiaries to generate the funds necessary to meet our financial obligations. Our subsidiaries are legally distinct from us and may be prohibited or restricted from paying dividends or otherwise making funds available to us, including restrictions under the covenants of the Credit Agreement governing our Senior Secured Credit Facilities and the indenture governing our Notes. If we are unable to obtain funds from our subsidiaries, we may be unable to meet our financial obligations.
We currently do not intend to declare dividends on our common stock in the foreseeable future and, as a result, your only opportunity to achieve a return on your investment is if the price of our common stock appreciates.
We currently do not expect to declare any dividends on our common stock in the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used to provide working capital, to pay interest and principal on our debt, to support our operations and to finance the growth and development of our business. Any determination to declare or pay dividends in the future will be at the discretion of our board of directors, subject to applicable laws and dependent upon a number of factors, including our earnings, capital requirements and overall financial conditions. In addition, our ability to pay dividends on our common stock is currently limited by the covenants of our Senior Secured Credit Facilities and Notes and may be further restricted by the terms of any future debt or preferred securities. Accordingly, your only opportunity to achieve a return on
38
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
your investment in our company may be if the market price of our common stock appreciates and you sell your shares at a profit. The market price for our common stock may never exceed, and may fall below, the price that you pay for such common stock.
If securities analysts do not publish research or reports about our business or if they downgrade our stock or our sector, our stock price and trading volume could decline.
The trading market for our common stock relies in part on the research and reports that industry or financial analysts publish about us or our business or industry. We do not control these analysts. Furthermore, if one or more of the analysts who do cover us downgrade our stock or our industry, or the stock of any of our competitors, or publish inaccurate or unfavorable research about our business or industry, the price of our stock could decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, we could lose visibility in the market, which in turn could cause our stock price or trading volume to decline.
Future sales, or the perception of future sales, by us or our existing stockholders in the public market following this offering could cause the market price for our common stock to decline.
After this offering, the sale of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
Our directors and certain of our executive officers and significant equity holders have entered into lock-up agreements in connection with this offering, on substantially similar terms, which expire days from the date of this prospectus. Upon completion of this offering, based on the number of shares outstanding on March 31, 2020, shares of our common stock will be restricted from sale as a result of lock-up agreements with the underwriters through the date that is days from the date of this prospectus.
After this offering, the holders of an aggregate of shares of our outstanding common stock immediately following this offering (assuming no exercise of the underwriters’ option to purchase additional shares), will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or our stockholders. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by our affiliates as defined in Rule 144 under the Securities Act. See “Shares Eligible for Future Sale.”
As restrictions on resale end or if these stockholders exercise their registration rights, the market price of our shares of common stock could drop significantly if the holders of these shares sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our shares of common stock or other securities.
In addition, the shares of our common stock reserved for future issuance under our 2020 Incentive Plan (as defined elsewhere in this prospectus) will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements, lock-up agreements and Rule 144 under the Securities Act, as applicable. A total of 39,053,663 shares of common stock have been reserved for future issuance under our 2020 Incentive Plan.
In the future, we may also issue our securities in connection with investments or acquisitions. The amount of shares of our common stock issued in connection with an investment or acquisition could constitute a material portion of our then-outstanding shares of our common stock. Any issuance of additional securities in connection with investments or acquisitions may result in additional dilution to you.
39
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Provisions in our organizational documents could delay or prevent a change of control.
Certain provisions of our amended and restated certificate of incorporation, amended and restated bylaws and second amended and restated stockholders agreement may have the effect of delaying or preventing a merger, acquisition, tender offer, takeover attempt or other change of control transaction that a stockholder might consider to be in its best interest, including attempts that might result in a premium over the market price of our common stock.
These provisions provide for, among other things:
| • | the division of our board of directors into three classes, as nearly equal in size as possible, with directors in each class serving three-year terms and with terms of the directors of only one class expiring in any given year; |
| • | that at any time when the Majority Sponsors and certain of their respective affiliates beneficially own, in the aggregate, less than 40% in voting power of the stock of our company entitled to vote generally in the election of directors, directors may only be removed for cause, and only by the affirmative vote of the holders of at least two-thirds in voting power of all the then-outstanding shares of stock entitled to vote thereon, voting together as a single class; |
| • | the ability of our board of directors to issue one or more series of preferred stock with voting or other rights or preferences that could have the effect of impeding the success of an attempt to acquire us or otherwise effect a change of control; |
| • | advance notice for nominations of directors by stockholders and for stockholders to include matters to be considered at stockholder meetings; |
| • | the right of the Majority Sponsors and certain of their respective affiliates to nominate the majority of the members of our board of directors and the obligation of certain of our other pre-IPO stockholders to support such nominees; |
| • | certain limitations on convening special stockholder meetings; and |
| • | that certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws may be amended only by the affirmative vote of the holders of at least two-thirds in voting power of all the then-outstanding shares of our stock entitled to vote thereon, voting together as a single class, if the Majority Sponsors and certain of their respective affiliates beneficially own, in the aggregate, less than 40% in voting power of our stock entitled to vote generally in the election of directors. |
These provisions could make it more difficult for a third party to acquire us, even if the third-party’s offer may be considered beneficial by many of our stockholders. As a result, our stockholders may be limited in their ability to obtain a premium for their shares. See “Description of Capital Stock.”
The Majority Sponsors will continue to hold a significant percentage of our outstanding common stock after this offering and their interests may be different than the interests of other holders of our securities.
Upon the completion of this offering, the Majority Sponsors will own approximately % of our outstanding common stock, or approximately % if the underwriters exercise in full their option to purchase additional shares. As a result, the Majority Sponsors are able to control or influence actions to be taken by us, including future issuances of our common stock or other securities, the payment of dividends, if any, on our common stock, amendments to our organizational documents and the approval of significant corporate transactions, including mergers, sales of substantially all of our assets, distributions of our assets, the incurrence of indebtedness and any incurrence of liens on our assets.
The interests of the Majority Sponsors may be materially different than the interests of our other stakeholders. In addition, the Majority Sponsors may have an interest in pursuing acquisitions, divestitures and
40
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
other transactions that, in their judgment, could enhance their investment, even though such transactions might involve risks to you. For example, the Majority Sponsors may cause us to take actions or pursue strategies that could impact our ability to make payments under our Senior Secured Credit Facilities and the Notes or that could cause a change of control. In addition, to the extent permitted by agreements governing our Senior Secured Credit Facilities and the Notes, the Majority Sponsors may cause us to pay dividends rather than make capital expenditures or repay debt. The Majority Sponsors are in the business of making investments in companies and may from time to time acquire and hold interests in businesses that compete directly or indirectly with us. Our amended and restated certificate of incorporation provides that none of the Majority Sponsors, any of their respective affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his director and officer capacities) or his or her affiliates will have any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. The Majority Sponsors also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us.
So long as the Majority Sponsors continue to own a significant amount of our outstanding common stock, even if such amount is less than 50%, they will continue to be able to strongly influence or effectively control our decisions and, so long as each of the Majority Sponsors continues to own shares of our outstanding common stock, they will have the ability to nominate individuals to our board of directors pursuant to the second amended and restated stockholders agreement. See Part III, Item 13, “Certain Relationships and Related Party Transactions, and Director Independence—Second Amended and Restated Stockholders Agreement” of PPD’s 2019 10-K for additional information. In addition, the Majority Sponsors, acting together, will be able to determine the outcome of all matters requiring stockholder approval and will be able to cause or prevent a change of control of our company or a change in the composition of our board of directors and could preclude any unsolicited acquisition of our company. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of our company and ultimately might affect the market price of our common stock.
Failure to comply with requirements to design, implement and maintain effective internal controls could have a material adverse effect on our business and stock price.
As a privately-held company, we were not required to evaluate our internal control over financial reporting in a manner that meets the standards of publicly traded companies required by Section 404(a) of the Sarbanes-Oxley Act of 2002 (“Section 404” and the “Sarbanes-Oxley Act,” respectively). As a public company, we have significant requirements for enhanced financial reporting and internal controls. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. If we are unable to establish or maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations on a timely basis, result in material misstatements in our consolidated financial statements and harm our results of operations. In addition, we are required, pursuant to Section 404, to furnish a report by management on, among other things, the effectiveness of our internal controls over financial reporting in our annual report for the year ended December 31, 2020. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. The rules governing the standards that must be met for our management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation. Testing and maintaining internal controls may divert our management’s attention from other matters that are important to our business.
In connection with the implementation of the necessary procedures and practices related to internal control over financial reporting, we may identify deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. In addition, we may encounter problems or delays in completing the remediation of any deficiencies identified by our independent registered public accounting firm in connection with the issuance of their attestation report.
41
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. A material weakness in internal controls could result in our failure to detect a material misstatement of our annual or quarterly consolidated financial statements or disclosures. We may not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. If we are unable to conclude that we have effective internal controls over financial reporting, investors could lose confidence in our reported financial information, which could have a material adverse effect on the trading price of our common stock.
Our amended and restated bylaws provide, subject to limited exceptions, that the Court of Chancery of the State of Delaware and, to the extent enforceable, the federal district courts of the United States will be the sole and exclusive forums for certain stockholder litigation matters, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated bylaws provide, subject to limited exceptions, that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for any (i) derivative action or proceeding brought on behalf of our company, (ii) action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of our company to the Company or our stockholders, (iii) action asserting a claim against the Company or any director, officer or other employee of the Company arising pursuant to any provision of the Delaware General Corporation Law, or the DGCL, or our amended and restated certificate of incorporation or our amended and restated bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (iv) action asserting a claim against the Company or any director, officer or other employee of the Company governed by the internal affairs doctrine. These provisions shall not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction and our stockholders cannot waive compliance with federal securities laws and the rules and regulations thereunder. Unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act, subject to and contingent upon a final adjudication in the State of Delaware of the enforceability of such exclusive forum provision. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum provisions in our amended and restated bylaws.
These choice of forum provisions may limit a stockholder’s ability to bring a claim in a different judicial forum, including one that it may find favorable or convenient for disputes with us or any of our directors, officers or other employees which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provisions that are contained in our amended and restated bylaws to be inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, operating results and financial condition.
Our board of directors is authorized to issue and designate shares of our preferred stock in additional series without stockholder approval.
Our amended and restated certificate of incorporation authorizes our board of directors, without the approval of our stockholders, to issue 100,000,000 shares of our preferred stock, subject to limitations prescribed by applicable law, rules and regulations and the provisions of our amended and restated certificate of incorporation, as shares of preferred stock in series, to establish from time to time the number of shares to be included in each such series and to fix the designation, powers, preferences and rights of the shares of each such series and the qualifications, limitations or restrictions thereof. The powers, preferences and rights of these additional series of preferred stock may be senior to or on parity with our common stock, which may reduce its value.
42
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements made herein or incorporated by reference in this prospectus contain forward-looking statements within the meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act. Such forward-looking statements reflect, among other things, our current expectations and anticipated results of operations, all of which are subject to known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements, market trends, or industry results to differ materially from those expressed or implied by such forward-looking statements. Therefore, any statements contained and incorporated by reference herein that are not statements of historical fact may be forward-looking statements and should be evaluated as such. These statements often include words such as “anticipate,” “expect,” “suggest,” “plan,” “believe,” “intend,” “project,” “forecast,” “estimates,” “targets,” “projections,” “should,” “could,” “would,” “may,” “might,” “will” and other similar expressions, and include forward-looking statements about the impact from the COVID-19 pandemic. These forward-looking statements are contained or incorporated by reference throughout this prospectus and the information incorporated by reference.
We base these forward-looking statements on our current expectations, plans and assumptions that we have made in light of our experience in the industry, as well as our perceptions of historical trends, current conditions, expected future developments and other factors we believe are appropriate under the circumstances at this time, including the impact from the COVID-19 pandemic. As you read and consider this prospectus and the information incorporated by reference, you should understand that these statements are not guarantees of performance or results. The forward-looking statements contained and incorporated by reference herein are subject to and involve risks, uncertainties and assumptions and you should not place undue reliance on these forward-looking statements. Although we believe that these forward-looking statements are based on reasonable assumptions at the time they are made, actual results might differ materially from those expressed in the forward-looking statements. Factors that might materially affect such forward-looking statements and projections include:
| • | the magnitude, continued duration, geographic reach and ongoing impact on the global economy and capital and credit markets of the COVID-19 pandemic; |
| • | the current and uncertain future impact from the COVID-19 pandemic on our business, growth, reputation, prospects, financial condition, results of operations (including components of our financial results), cash flows and liquidity; |
| • | the fragmented and highly competitive nature of the drug development services industry; |
| • | changes in trends in the biopharmaceutical industry, including decreases in research and development spending and outsourcing; |
| • | our ability to keep pace with rapid technological changes that could make our services less competitive or obsolete; |
| • | the United States and international healthcare industry is subject to political, economic and/or regulatory influences and changes, such as healthcare reform, all of which could adversely affect both our customers’ and our businesses; |
| • | any failure of our backlog to accurately predict or convert into future revenue; |
| • | the fact that our customers can terminate, delay or reduce the scope of our contracts with them upon short notice or with no notice; |
| • | the impact of industry, customer and therapeutic area concentration; |
| • | our ability to accurately price our contracts and manage our costs associated with performance of such contracts; |
| • | any failures in our information and communication systems, including cybersecurity breaches, impacting us or our customers, clinical trial participants or employees; |
43
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | any failure to perform services in accordance with contractual requirements, regulatory standards and ethical standards; |
| • | our ability to recruit, retain and motivate key personnel, including the loss of any key executive who becomes severely ill with COVID-19; |
| • | our ability to attract suitable investigators or enroll a sufficient number of patients for our customers’ clinical trials; |
| • | any failure by us to comply with numerous privacy laws; |
| • | our dependence on third parties for critical goods and support services, including a significant impact from the COVID-19 pandemic to our suppliers; |
| • | our dependence on our technology network, and the impact from upgrades to the network; |
| • | any violation of laws, including laws governing the conduct of clinical trials or other biopharmaceutical research, and anti-corruption laws, such as the U.S. Foreign Corrupt Practices Act and the U.K. Bribery Act of 2010; |
| • | competition between our existing and potential customers and the potential negative impact on our business; |
| • | our management of business restructuring transactions and the integration of acquisitions; |
| • | risks related to the drug development services industry that could result in potential liability that could affect our business, reputation and financial condition; |
| • | any failure of our insurance to cover the potential liabilities, including indemnification obligations, associated with the operation of our business and provision of services; |
| • | our use of biological and hazardous materials, which could violate law or cause injury or death, resulting in liability; |
| • | disruptions to our operations by the occurrence of a natural disaster, pandemic (such as the COVID-19 pandemic), or other catastrophic events; |
| • | international or U.S. economic, currency, political and other risks, such as those from the COVID-19 pandemic; |
| • | economic conditions and regulatory changes relating to the United Kingdom’s exit from the European Union; |
| • | any inability to adequately protect our intellectual property or the security of our systems and the data stored therein; |
| • | consolidation amongst our customers, and the potential for rationalization of the combined drug development pipeline, resulting in fewer products in clinical development; |
| • | any patent or other intellectual property litigation we might be involved in; |
| • | changes in tax laws, such as U.S. tax reform, or interpretations of existing tax laws; |
| • | our investments in third parties, which are illiquid and subject to loss; |
| • | the substantial value of our goodwill and intangible assets, which we might not fully realize, resulting in impairment losses; |
| • | difficult and volatile conditions in the capital and credit markets and in the overall economy, including those caused by the COVID-19 pandemic; |
| • | risks related to our indebtedness; |
| • | the significant influence certain stockholders have over us; |
44
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | other factors beyond our control; and |
| • | the other factors disclosed or incorporated by reference in this prospectus. |
These cautionary statements should not be construed by you to be exhaustive and speak only as of the date the statements are made. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. For further discussion of the risks relating to our business, see the section titled “Risk Factors.”
45
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
We are filing the registration statement of which this prospectus is a part to permit holders of the shares of our common stock included in the section entitled “Principal and Selling Stockholders” to resell such shares. The selling stockholders will receive all of the net proceeds from the sale of shares of common stock in this offering. We are not selling any shares of common stock under this prospectus and will not receive any proceeds from the sale of shares by the selling stockholders or if the underwriters exercise their option to purchase additional shares.
46
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
We currently do not expect to declare any dividends on our common stock in the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used to provide working capital, to support our operations, to finance the growth and development of our business and to reduce our long-term debt. Any determination to declare dividends in the future will be at the discretion of our board of directors, subject to applicable laws, and will be dependent on a number of factors, including our earnings, capital requirements and overall financial condition. We are controlled by the Majority Sponsors, who have the ability to nominate a majority of the members of our board of directors and therefore control the payment of dividends. In addition, because we are a holding company, our ability to pay dividends on our common stock may be limited by restrictions on our ability to obtain sufficient funds through dividends from subsidiaries, including restrictions under the covenants of the Credit Agreement governing our Senior Secured Credit Facilities and the indenture governing our Notes, and may be further restricted by the terms of any future debt or preferred securities. See Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness” of PPD’s 2019 10-K and our Current Report on Form 8-K filed on June 5, 2020, which are incorporated by reference in this prospectus, for more information about our Senior Secured Credit Facilities and our Notes.
In May and November 2019, we paid special dividends to our stockholders of $1,086.0 million and $160.0 million, respectively.
47
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2020.
This table should be read in conjunction with the information presented under the captions “Prospectus Summary—Summary Consolidated Financial Data” and “Use of Proceeds” in this prospectus, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in the condensed consolidated financial statements and the related notes thereto included in PPD’s 2020 Q1 10-Q and our Current Report on Form 8-K filed on June 5, 2020, which are incorporated by reference in this prospectus.
| As of March 31, 2020 | ||||
| (in millions) | ||||
Cash and cash equivalents | $ | 738.4 | ||
|
| |||
Debt (including current portion): | ||||
Senior Secured Credit Facilities, consisting of the following: | ||||
Term Loan | $ | 3,088.3 | ||
Revolving Credit Facility | 150.0 | |||
6.375% Senior Notes due 2023(1) | 1,125.0 | |||
Other debt | 3.8 | |||
Finance lease obligations | 28.2 | |||
|
| |||
Total debt(2) | 4,395.3 | |||
|
| |||
Total stockholders’ deficit | (1,079.9 | ) | ||
|
| |||
Total capitalization | $ | 3,315.4 | ||
|
| |||
| (1) | On June 5, 2020, we issued (a) $500.0 million in aggregate principal amount of the 2025 Notes and (b) $700.0 million in aggregate principal amount of the 2028 Notes, the proceeds of which were used to redeem all of the 6.375% Senior Notes due 2023. |
| (2) | Excludes debt discounts and debt issuance costs. |
48
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
PRINCIPAL AND SELLING STOCKHOLDERS
The following table and accompanying footnotes set forth information with respect to the beneficial ownership of the common stock of PPD, Inc. as of July 20, 2020 by:
| • | each person known by us to own beneficially 5% or more of our outstanding shares of common stock; |
| • | each of our directors; |
| • | each of our named executive officers; |
| • | our directors and executive officers as a group; and |
| • | all selling stockholders. |
The number of shares and percentages of beneficial ownership prior to this offering set forth below are based on the number of shares of our common stock to be issued and outstanding immediately prior to the consummation of this offering. The number of shares and percentages of beneficial ownership after this offering set forth below are based on the number of shares of our common stock to be issued and outstanding immediately after the consummation of this offering.
Beneficial ownership for the purposes of the following table is determined in accordance with the rules and regulations of the SEC. A person is a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of the security, or “investment power,” which includes the power to dispose of or to direct the disposition of the security or has the right to acquire such powers within 60 days.
Unless otherwise noted in the footnotes to the following table, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to their beneficially owned common stock.
Except as otherwise indicated in the footnotes below, the address of each beneficial owner is c/o PPD, Inc., 929 North Front Street, Wilmington, North Carolina 28401.
| Shares to be Sold in this Offering | Shares Beneficially Owned After the Offering | |||||||||||||||||||||||||||||||||||||||
| Shares Beneficially Owned Prior to the Offering | If Underwriters’ Option to Purchase Additional Shares is Not Exercised | If Underwriters’ Option to Purchase Additional Shares is Exercised in Full | If Underwriters’ Option to Purchase Additional Shares is Not Exercised | If Underwriters’ Option to Purchase Additional Shares is Exercised in Full | ||||||||||||||||||||||||||||||||||||
Name of Beneficial Owner | Shares | % | Shares | % | Shares | % | Shares | % | Shares | % | ||||||||||||||||||||||||||||||
5% Stockholders: | ||||||||||||||||||||||||||||||||||||||||
H&F Investors(1) | 158,426,641 | 45.4 | % | |||||||||||||||||||||||||||||||||||||
Carlyle Investors(2) | 66,454,994 | 19.1 | ||||||||||||||||||||||||||||||||||||||
Blue Spectrum Investor(3) | 25,585,173 | 7.3 | ||||||||||||||||||||||||||||||||||||||
GIC Investor(4) | 25,585,173 | 7.3 | ||||||||||||||||||||||||||||||||||||||
Directors and Named Executive Officers: | ||||||||||||||||||||||||||||||||||||||||
David Simmons(5) | 3,424,848 | * | ||||||||||||||||||||||||||||||||||||||
Joe Bress(6) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Stephen Ensley(7) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Maria Teresa Hilado(8) | 33,471 | * | * | |||||||||||||||||||||||||||||||||||||
Colin Hill(9) | 12,160 | * | * | |||||||||||||||||||||||||||||||||||||
Jeffrey B. Kindler(10) | 112,095 | * | * | |||||||||||||||||||||||||||||||||||||
49
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| Shares to be Sold in this Offering | Shares Beneficially Owned After the Offering | |||||||||||||||||||||||||||||||||||||||
| Shares Beneficially Owned Prior to the Offering | If Underwriters’ Option to Purchase Additional Shares is Not Exercised | If Underwriters’ Option to Purchase Additional Shares is Exercised in Full | If Underwriters’ Option to Purchase Additional Shares is Not Exercised | If Underwriters’ Option to Purchase Additional Shares is Exercised in Full | ||||||||||||||||||||||||||||||||||||
Name of Beneficial Owner | Shares | % | Shares | % | Shares | % | Shares | % | Shares | % | ||||||||||||||||||||||||||||||
P. Hunter Philbrick(7) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Allen R. Thorpe(7) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Stephen H. Wise(6) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
William J. Sharbaugh(11) | 1,001,400 | * | ||||||||||||||||||||||||||||||||||||||
Christopher G. Scully(12) | 277,523 | * | ||||||||||||||||||||||||||||||||||||||
Anshul Thakral(13) | 215,678 | * | ||||||||||||||||||||||||||||||||||||||
B. Judd Hartman(14) | 290,209 | * | ||||||||||||||||||||||||||||||||||||||
All directors and executive officers as a group (18 persons)(15) | 6,060,914 | 1.7 | % | |||||||||||||||||||||||||||||||||||||
Other Selling Stockholders: | ||||||||||||||||||||||||||||||||||||||||
All additional selling stockholders(16) | ||||||||||||||||||||||||||||||||||||||||
| * | Indicates beneficial ownership of less than 1%. |
| (1) | Reflects (i) 63,069,561 shares directly held by Hellman & Friedman Capital Partners VII, L.P., 24,143,479 shares directly held by Hellman & Friedman Capital Partners VII (Parallel), L.P., 4,330,024 shares directly held by HFCP VII (Parallel-A), L.P. and 428,587 shares directly held by H&F Executives VII, L.P. (collectively, the “H&F VII Funds”) and (ii) 42,483,348 shares directly held by Hellman & Friedman Capital Partners VIII, L.P., 19,066,602 shares directly held by Hellman & Friedman Capital Partners VIII (Parallel), L.P., 3,603,189 shares directly held by HFCP VIII (Parallel-A), L.P., 1,114,449 shares directly held by H&F Executives VIII, L.P. and 187,402 shares directly held by H&F Associates VIII, L.P. (collectively, the “H&F VIII Funds” and, together with the H&F VII Funds, the “H&F Investors”). Hellman & Friedman Investors VII, L.P. (“H&F Investors VII”) is the general partner of the H&F VII Funds. H&F Corporate Investors VII, Ltd. (“H&F VII”) is the general partner of H&F Investors VII. As the general partner of H&F Investors VII, H&F VII may be deemed to have beneficial ownership of the shares of common stock beneficially owned by H&F Investors VII. Hellman & Friedman Investors VIII, L.P. (“H&F Investors VIII”) is the general partner of the H&F VIII Funds. H&F Corporate Investors VIII, Ltd. (“H&F VIII”) is the general partner of H&F Investors VIII. As the general partner of H&F Investors VIII, H&F VIII may be deemed to have beneficial ownership of the shares of common stock beneficially owned by H&F Investors VIII. Voting and investment determinations with respect to shares of common stock held by H&F Investors VII and H&F Investors VIII are made by the boards of directors of H&F VII and H&F VIII, respectively. The board of directors of each of H&F VII and H&F VIII consists of Philip U. Hammarskjold, David R. Tunnell and Allen R. Thorpe. Each of the members of the boards of directors of H&F VII and H&F VIII disclaims beneficial ownership of such shares of our common stock. The address of each entity named in this footnote is c/o Hellman & Friedman LLC, 415 Mission Street, Suite 5700, San Francisco, California 94105. |
| (2) | Reflects shares directly held by Carlyle Partners VI Holdings II, L.P. (the “Carlyle Investor”). Carlyle Group Management L.L.C. holds an irrevocable proxy to vote a majority of the shares of The Carlyle Group Inc., which is a publicly traded entity listed on Nasdaq. The Carlyle Group Inc. is the sole member of |
50
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| Carlyle Holdings II GP L.L.C., which is the managing member of Carlyle Holdings II L.L.C., which, with respect to the securities reported herein, is the managing member of CG Subsidiary Holdings L.L.C., which is the general partner of TC Group Cayman Investment Holdings, L.P., which is the general partner of TC Group Cayman Investment Holdings Sub L.P., which is the sole member of TC Group VI, L.L.C., which is the general partner of TC Group VI, L.P., which is the general partner of the Carlyle Investor. Voting and investment determinations with respect to the common shares held by the Carlyle Investor are made by an investment committee of TC Group VI, L.P. comprised of Allan Holt, William Conway, Jr., Daniel D’Aniello, David Rubenstein, Peter Clare, Kewsong Lee, Norma Kuntz, Sandra Horbach and Marco De Benedetti as a non-voting observer. Accordingly, each of the foregoing entities and individuals may be deemed to share beneficial ownership of the securities held of record by Carlyle Partners VI Holdings II, L.P. Each of them disclaims beneficial ownership of such securities. The address of each of TC Group Cayman Investment Holdings, L.P. and TC Group Cayman Investment Holdings Sub L.P. is c/o Walkers, Cayman Corporate Center, 27 Hospital Road, George Town, Grand Cayman KY1-9008, Cayman Islands. The address of each of the other entities named in this footnote is c/o The Carlyle Group Inc., 1001 Pennsylvania Avenue, NW, Suite 220 South, Washington, D.C. 20004. |
| (3) | Reflects shares directly held by Blue Spectrum ZA 2015 L.P. (the “Blue Spectrum Investor”). The general partner of the Blue Spectrum Investor is Procific, a Cayman Island exempted company with limited liability and a wholly owned subsidiary of ADIA. By reason of its ownership of Procific and pursuant to the rules and regulations of the SEC, ADIA may be deemed to share investment and voting power over and, therefore, beneficial ownership of, the shares held directly by Blue Spectrum. The address for each of the Blue Spectrum Investor and Procific is c/o Collas Crill Corporate Services Limited, Willow House, Cricket Square, PO Box 709, Grand Cayman, KY1-1107, Cayman Islands. The address for ADIA is 211 Corniche Street, P.O. Box 3600, Abu Dhabi, United Arab Emirates. |
| (4) | Reflects shares directly held by Clocktower Investment Pte Ltd. (the “GIC Investor”). The GIC Investor shares the power to vote and the power to dispose of these shares with GIC Special Investments Pte. Ltd. (“GIC SI”), and GIC, both of which are private limited companies incorporated in Singapore. GIC SI is wholly owned by GIC and is the private equity investment arm of GIC. GIC is wholly owned by the Government of Singapore and was set up with the sole purpose of managing Singapore’s foreign reserves. The Government of Singapore disclaims beneficial ownership of these shares. The business address for the GIC Investor is 168 Robinson Road, #37-01 Capital Tower, Singapore 068912. |
| (5) | Includes 603,000 shares held by 2015 Simmons Family Gift Trust U/A dated June 18, 2015 of which Mr. Simmons is a Trustee, 867,759 shares held by David S. Simmons Revocable Trust dated November 13, 2009 of which Mr. Simmons is a Trustee and 1,914,089 shares issuable upon the exercise of options exercisable within 60 days following July 20, 2020. |
| (6) | The address of each of Messrs. Bress and Wise is c/o The Carlyle Group Inc., 1001 Pennsylvania Avenue, NW, Suite 2200 South, Washington, D.C. 20004. |
| (7) | The address of each of Messrs. Ensley, Philbrick and Thorpe is c/o Hellman & Friedman LLC, 415 Mission Street, Suite 5700, San Francisco, California 94105. |
| (8) | Includes 1,508 shares of restricted common stock. |
| (9) | Includes 1,508 shares of restricted common stock. |
| (10) | Includes 1,508 shares of restricted common stock. |
| (11) | Includes 90,000 shares held by William J. Sharbaugh, III 2020 Grantor Retained Annuity Trust u/a 01/15/2020 of which Mr. Sharbaugh is a Trustee, 332,485 shares held by William J. Sharbaugh and 548,915 shares issuable upon the exercise of options exercisable within 60 days following July 20, 2020. |
| (12) | Includes 251,523 shares issuable upon the exercise of options exercisable within 60 days following July 20, 2020. |
| (13) | Includes 181,452 shares issuable upon the exercise of options exercisable within 60 days following July 20, 2020. |
| (14) | Includes 196,043 shares issuable upon the exercise of options exercisable within 60 days following July 20, 2020. |
51
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| (15) | Includes 3,643,601 shares issuable upon the exercise of options exercisable within 60 days following July 20, 2020 and 4,524 shares of restricted common stock held by our current executive officers and directors. |
| (16) | Additional selling stockholders include . |
52
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
General
The following description summarizes the material terms of, and is qualified in its entirety by, our amended and restated certificate of incorporation and amended and restated bylaws. For a complete description of our capital stock, you should refer to our amended and restated certificate of incorporation, amended and restated bylaws and the applicable provisions of Delaware laws.
Our purpose is to engage in any lawful act or activity for which corporations may now or hereafter be organized under the General Corporation Law of the State of Delaware (the “DGCL”). Our authorized capital stock consists of two billion shares of common stock, par value $0.01 per share, and one hundred million shares of preferred stock, par value $0.01 per share. No shares of preferred stock will be issued or outstanding immediately after the offering contemplated by this prospectus. Unless our board of directors determines otherwise, we will issue all shares of our common stock in uncertificated form.
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors. The holders of our common stock do not have cumulative voting rights in the election of directors.
Upon our liquidation, dissolution or winding up and after payment in full of all amounts required to be paid to creditors and subject to the rights of the holders of one or more outstanding series of preferred stock having liquidation preferences, if any, the holders of our common stock are entitled to receive pro rata our remaining assets available for distribution. Holders of our common stock do not have preemptive, subscription, redemption sinking fund or conversion rights. The common stock is not subject to further calls or assessment by us. All shares of our common stock that will be outstanding at the time of the completion of the offering will be fully paid and non-assessable. The rights, powers, preferences and privileges of holders of our common stock are subject to those of the holders of any shares of our preferred stock or any series or class of stock we may authorize and issue in the future.
Preferred Stock
Our amended and restated certificate of incorporation authorizes our board of directors to establish one or more series of preferred stock (including convertible preferred stock). Unless required by law or by the Nasdaq rules, the authorized shares of preferred stock are available for issuance without further action by you, and holders of our common stock are not entitled to vote on any amendment to our amended and restated certificate of incorporation that relates solely to the terms of any outstanding shares of preferred stock, if the holders of such shares of preferred stock are entitled to vote thereon. Our board of directors is authorized to determine, with respect to any series of preferred stock, the powers (including voting powers), preferences and relative, participating, optional and other special rights, and the qualifications, limitations or restrictions thereof, including, without limitation:
| • | the designation of the series; |
| • | the number of shares of the series, which our board of directors may, except where otherwise provided in the preferred stock designation, increase (but not above the total number of authorized shares of the class of stock) or decrease (but not below the number of shares then outstanding); |
| • | whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series; |
| • | the dates at which dividends, if any, will be payable; |
53
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | redemption rights and price or prices, if any, for shares of the series; |
| • | the terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series; |
| • | the amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of the affairs of our company; |
| • | whether the shares of the series will be convertible into shares of any other class or series of the stock of our company, or any other security of our company or any other entity, and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate adjustments, the date or dates as of which the shares will be convertible and all other terms and conditions upon which the conversion may be made; |
| • | restrictions on the issuance of shares of the same series or of any other class or series of our capital stock; and |
| • | the voting rights, if any, of the holders of the series. |
We could issue a series of preferred stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of the holders of our common stock might believe to be in their best interests or in which the holders of our common stock might receive a premium for their common stock over the market price of the common stock. Additionally, the issuance of preferred stock may adversely affect the holders of our common stock, including, without limitation, by restricting dividends on the common stock, diluting the voting power of the common stock or subordinating the liquidation rights of the common stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our common stock.
Dividends
Holders of our common stock are entitled to receive dividends when, as and if declared by our board of directors out of funds legally available therefor, subject to any statutory or contractual restrictions on the payment of dividends and to the rights of the holders or one or more outstanding series of our preferred stock.
The DGCL permits a corporation to declare and pay dividends out of “surplus” or, if there is no “surplus,” out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. “Surplus” is defined as the excess of the net assets of the corporation over the amount determined to be the capital of the corporation by the board of directors. The capital of the corporation is typically calculated to be (and cannot be less than) the aggregate par value of all issued shares of capital stock. Net assets equal the fair value of the total assets minus total liabilities. The DGCL also provides that dividends may not be paid out of net profits if, after the payment of the dividend, remaining capital would be less than the capital represented by the outstanding stock of all classes having a preference upon the distribution of assets.
Declaration and payment of any dividend is subject to the discretion of our board of directors. The time and amount of such dividends, if any, will be dependent upon our financial condition, operations, compliance with applicable law, cash requirements and availability, debt repayment obligations, capital expenditure needs and restrictions in our debt instruments, contractual restrictions, business prospects, industry trends, the provisions of Delaware law affecting the payment of distributions to stockholders and any other factors our board of directors may consider relevant.
We do not expect to declare or pay any dividends on our common stock in the foreseeable future. In addition, our ability to pay dividends on our common stock is limited by the covenants of our Senior Secured Credit Facilities and the Notes and may be further restricted by the terms of any future debt or preferred securities. See “Dividend Policy,” Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Credit Facilities” of PPD’s 2019 10-K and our Current Report on Form 8-K filed on June 5, 2020.
54
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Annual Stockholder Meetings
Our amended and restated certificate of incorporation and our amended and restated bylaws provide that annual stockholder meetings will be held at a date, time and place, if any, as exclusively selected by our board of directors. To the extent permitted under applicable law, we may conduct meetings by remote communications, including by webcast.
Effects of Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws and Certain Provisions of Delaware Law
Our amended and restated certificate of incorporation and amended and restated bylaws and the DGCL contain provisions, which are summarized in the following paragraphs, that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of our board of directors to maximize stockholder value in connection with any unsolicited offer to acquire us. However, these provisions may have the effect of delaying, deterring or preventing a merger or acquisition of our company by means of a tender offer, a proxy contest or other takeover attempt that a stockholder might consider in its best interest, including attempts that might result in a premium over the prevailing market price for the shares of common stock held by stockholders.
Authorized but Unissued Capital Stock
Delaware law does not require stockholder approval for any issuance of authorized shares. However, the listing requirements of Nasdaq, which apply so long as our common stock remains listed on Nasdaq, require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of common stock. Additional shares that may be used in the future may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
Our board of directors may generally issue one or more series of preferred shares on terms calculated to discourage, delay or prevent a change of control of our company or the removal of our management. Moreover, our authorized but unissued shares of preferred stock are available for future issuances in one or more series without stockholder approval and could be utilized for a variety of corporate purposes, including future offerings to raise additional capital, to facilitate acquisitions and employee benefit plans.
One of the effects of the existence of authorized and unissued and unreserved common stock or preferred stock may be to enable our board of directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of our Company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Classified Board of Directors
Our amended and restated certificate of incorporation provides that, subject to the right of holders of any series of preferred stock, our board of directors will be divided into three classes of directors, with the classes to be as nearly equal in number as possible, and with the directors serving staggered three-year terms, with only one class of directors being elected at each annual meeting of stockholders. As a result, approximately one-third of our board of directors will be elected each year. The classification of directors has the effect of making it more difficult for stockholders to change the composition of our board of directors. Our amended and restated certificate of incorporation and amended and restated bylaws provide that, subject to any rights of holders of
55
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
preferred stock to elect additional directors under specified circumstances, the number of directors will be fixed from time to time exclusively pursuant to a resolution adopted by the board of directors; however, if at any time the Majority Sponsors own at least 40% in voting power of the stock of our Company entitled to vote generally in the election of directors, the stockholders may also fix the number of directors.
Business Combinations
We have opted out of Section 203 of the DGCL; however, our amended and restated certificate of incorporation contains similar provisions providing that we may not engage in certain “business combinations” with any “interested stockholder” for a three-year period following the time that the stockholder became an interested stockholder, unless:
| • | prior to such time, our board of directors approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding certain shares; |
| • | at or subsequent to that time, the business combination is approved by our board of directors and by the affirmative vote of holders of at least 66 2/3% of our outstanding voting stock that is not owned by the interested stockholder; or |
| • | the stockholder became an interested stockholder inadvertently and (i) as soon as practicable divested itself of sufficient ownership to cease to be an interested stockholder and (ii) had not been an interested stockholder but for the inadvertent acquisition of ownership within three years of the business combination. |
Generally, a “business combination” includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with that person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of our outstanding voting stock. For purposes of this section only, “voting stock” has the meaning given to it in Section 203 of the DGCL.
Under certain circumstances, this provision will make it more difficult for a person who would be an “interested stockholder” to effect various business combinations with our company for a three-year period. This provision may encourage companies interested in acquiring our Company to negotiate in advance with our board of directors because the stockholder approval requirement would be avoided if our board of directors approves either the business combination or the transaction which results in the stockholder becoming an interested stockholder. These provisions also may have the effect of preventing changes in our board of directors and may make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Our amended and restated certificate of incorporation provides that the Majority Sponsors, and any of their respective direct or indirect transferees and any group as to which such persons or entities are a party, do not constitute “interested stockholders” for purposes of this provision.
Removal of Directors; Vacancies
Under the DGCL, unless otherwise provided in our amended and restated certificate of incorporation, directors serving on a classified board may be removed by the stockholders only for cause. Our amended and restated certificate of incorporation provides that, other than directors elected by holders of our preferred stock, if any, directors may be removed with or without cause upon the affirmative vote of a majority in voting power of
56
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
all outstanding shares of stock entitled to vote thereon, voting together as a single class; provided, however, at any time when the Majority Sponsors beneficially own less than 40% in voting power of the stock of our company entitled to vote generally in the election of directors, directors may only be removed for cause, and only by the affirmative vote of holders of at least 66 2/3% in voting power of all the then-outstanding shares of stock of our company entitled to vote thereon, voting together as a single class. In addition, our amended and restated certificate of incorporation provides that, subject to the rights granted to one or more series of preferred stock then outstanding, any newly created directorship on the board of directors or the rights granted pursuant to the second amended and restated stockholders agreement that results from an increase in the number of directors and any vacancies on our board of directors will be filled only by the affirmative vote of a majority of the remaining directors, even if less than a quorum, or by a sole remaining director or by the stockholders; provided, however, at any time when the Majority Sponsors beneficially own less than 40% in voting power of the stock of our company entitled to vote generally in the election of directors, any newly created directorship on the board of directors that results from an increase in the number of directors and any vacancy occurring in the board of directors may only be filled by a majority of the directors then in office, although less than a quorum, or by a sole remaining director (and not by the stockholders). Our amended and restated certificate of incorporation provides that the board of directors may increase the number of directors by the affirmative vote of a majority of the directors or, at any time when the Majority Sponsors beneficially own at least 40% of the voting power of the stock of our Company entitled to vote generally in the election of directors, of the stockholders.
No Cumulative Voting
Under Delaware law, the right to vote cumulatively does not exist unless the certificate of incorporation specifically authorizes cumulative voting. Our amended and restated certificate of incorporation does not authorize cumulative voting. Therefore, stockholders holding a majority in voting power of the shares of our stock entitled to vote generally in the election of directors are able to elect all of our directors.
Special Stockholder Meetings
Our amended and restated certificate of incorporation provides that special meetings of our stockholders may be called at any time only by or at the direction of the board of directors or the chairman of the board of directors; provided, however, at any time when the Majority Sponsors beneficially own, in the aggregate, at least 40% in voting power of the stock of our company entitled to vote generally in the election of directors, special meetings of our stockholders shall also be called by the board of directors or the chairman of the board of directors at the request of any of the Majority Sponsors. Our amended and restated bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of our Company.
Requirements for Advance Notification of Director Nominations and Stockholder Proposals
Our amended and restated bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors. In order for any matter to be properly brought before a meeting of our stockholders, a stockholder will have to comply with advance notice requirements and provide us with certain information. Generally, to be timely, a stockholder’s notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the immediately preceding annual meeting of stockholders. Our amended and restated bylaws also specify requirements as to the form and content of a stockholder’s notice. Our amended and restated bylaws allow the chairman of the meeting at a meeting of the stockholders to adopt rules and regulations for the conduct of meetings, which may have the effect of precluding the conduct of certain business at a meeting if the rules and
57
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
regulations are not followed. These provisions may also deter, delay or discourage a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to influence or obtain control of our company.
Stockholder Action by Written Consent
Pursuant to Section 228 of the DGCL, any action required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice and without a vote if a consent or consents in writing, setting forth the action so taken, is signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of our stock entitled to vote thereon were present and voted, unless our amended and restated certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation precludes stockholder action by written consent at any time when the Majority Sponsors beneficially own less than 40% in voting power of the stock of our Company entitled to vote generally in the election of directors, other than certain rights that holders of our preferred stock may have to act by written consent.
Supermajority Provisions
Our amended and restated certificate of incorporation and amended and restated bylaws provide that the board of directors is expressly authorized to make, alter, amend, change, add to, rescind or repeal, in whole or in part, our bylaws without a stockholder vote in any matter not inconsistent with Delaware law or our amended and restated certificate of incorporation. In addition, for as long as the Majority Sponsors beneficially own at least 40% in voting power of the stock of our company entitled to vote generally in the election of directors, any amendment, alteration, rescission or repeal of our bylaws by our stockholders requires the affirmative vote of a majority in voting power of the outstanding shares of our stock present in person or represented by proxy at the meeting of stockholders and entitled to vote on such amendment, alteration, change, addition, rescission, change, addition or repeal. At any time when the Majority Sponsors beneficially own less than 40% in voting power of all outstanding shares of the stock of our company entitled to vote generally in the election of directors, any amendment, alteration, rescission, change, addition or repeal of our bylaws by our stockholders will require the affirmative vote of the holders of at least 66 2/3% in voting power of all the then-outstanding shares of stock of our Company entitled to vote thereon, voting together as a single class.
The DGCL provides generally that the affirmative vote of a majority of the outstanding shares entitled to vote thereon, voting together as a single class, is required to amend a corporation’s certificate of incorporation, unless the certificate of incorporation requires a greater percentage.
Our amended and restated certificate of incorporation provides that at any time when the Majority Sponsors beneficially own less than 40% in voting power of the stock of our Company entitled to vote generally in the election of directors, then, in addition to any vote required by applicable law or our amended and restated certificate of incorporation, any amendment, alteration, repeal or rescission of the following provisions in our amended and restated certificate of incorporation require the affirmative vote of the holders of at least 66 2/3% in voting power of all the then-outstanding shares of stock of our Company entitled to vote thereon, voting together as a single class:
| • | the provision requiring a 66 2/3% supermajority vote for stockholders to amend our amended and restated bylaws; |
| • | the provisions providing for a classified board of directors (the election and term of our directors); |
| • | the provisions regarding resignation and removal of directors; |
| • | the provisions regarding competition and corporate opportunities; |
58
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
| • | the provisions regarding Section 203 of the DGCL and entering into business combinations with interested stockholders; |
| • | the provisions regarding stockholder action by written consent; |
| • | the provisions regarding calling annual or special meetings of stockholders; |
| • | the provisions regarding filling vacancies on our board of directors and newly created directorships; |
| • | the provisions eliminating monetary damages for breaches of fiduciary duty by a director; and |
| • | the amendment provision requiring that the above provisions be amended only with a 66 2/3% supermajority vote. |
The combination of the classification of our board of directors, the lack of cumulative voting and the supermajority voting requirements will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Because our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management.
These provisions may have the effect of deterring hostile takeovers or delaying or preventing changes in control of our management or our company, such as a merger, reorganization or tender offer. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. These provisions are also intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in management of our company.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, our stockholders will have appraisal rights in connection with a merger or consolidation with our Company. Pursuant to the DGCL, stockholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of our stockholders may bring an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of our shares at the time of the incident to which the action relates or such stockholder’s stock thereafter devolved by operation of law.
Exclusive Forum
Our amended and restated bylaws provide that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for any (i) derivative action or proceeding brought on behalf of our company, (ii) action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of our company to our company or our company’s stockholders, (iii) action asserting a claim against our company or any director, officer or other employee of our company arising pursuant to any provision of the DGCL or our amended and
59
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
restated certificate of incorporation or our amended and restated bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware or (iv) action asserting a claim against our company or any director, officer or other employee of our company governed by the internal affairs doctrine. These provisions shall not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Unless the Company consents in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act, subject to and contingent upon a final adjudication in the State of Delaware of the enforceability of such exclusive forum provision. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock of our company shall be deemed to have notice of and consented to the forum provisions in our amended and restated bylaws. However, it is possible that a court could find our forum selection provisions to be inapplicable or unenforceable.
Conflicts of Interest
Delaware law permits corporations to adopt provisions renouncing any interest or expectancy in certain opportunities that are presented to the corporation or its officers, directors or stockholders. Our amended and restated certificate of incorporation, to the maximum extent permitted from time to time by Delaware law, renounces any interest or expectancy that we have in, or right to be offered an opportunity to participate in, any business opportunities that are from time to time presented to our officers, directors or stockholders or their respective affiliates, other than those officers, directors, stockholders or affiliates who are our or our subsidiaries’ employees. Our amended and restated certificate of incorporation provides that, to the fullest extent permitted by law, none of the Majority Sponsors or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his director and officer capacities) or his or her affiliates will have any duty to refrain from (i) engaging in a corporate opportunity in the same or similar business activities or lines of business in which we or our affiliates now engage or propose to engage or (ii) otherwise competing with us or our affiliates. In addition, to the fullest extent permitted by law, in the event that either of the Majority Sponsors or any non-employee director acquires knowledge of a potential transaction or other business opportunity which may be a corporate opportunity for itself, himself or herself, or its, his or her, affiliates or for us or our affiliates, such person will have no duty to communicate or offer such transaction or business opportunity to us or any of our affiliates and they may take any such opportunity for themselves or offer it to another person or entity. Our amended and restated certificate of incorporation does not renounce our interest in any business opportunity that is expressly offered to a non-employee director solely in his or her capacity as a director or officer of our company. To the fullest extent permitted by law, no business opportunity will be deemed to be a potential corporate opportunity for us unless we would be permitted to undertake the opportunity under our amended and restated certificate of incorporation, we have sufficient financial resources to undertake the opportunity and the opportunity would be in line with our business.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. Our amended and restated certificate of incorporation includes a provision that eliminates the personal liability of directors for monetary damages for any breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions is to eliminate the rights of us and our stockholders, through stockholders’ derivative suits on our behalf, to recover monetary damages from a director for breach of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation does not apply to any director if the director has acted in bad faith, knowingly or intentionally violated a law during the performance of his or her duties, fiduciary or otherwise, owed to us, authorized illegal dividends, repurchases or redemptions or derived an improper benefit from his or her actions as a director.
60
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Our amended and restated bylaws provide that we must indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We also are expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe that these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, indemnification and advancement provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. In addition, any investment in our common stock may be adversely affected to the extent we pay the costs of settlement and damages awards against directors and officers pursuant to these indemnification provisions.
We have entered into an indemnification agreement with each of our directors and officers. These agreements require us to indemnify these individuals to the fullest extent permitted under the DGCL against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.
There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Broadridge Corporate Issuer Solutions, Inc.
Listing
Our common stock is listed on The Nasdaq Global Select Market under the symbol “PPD.”
61
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SHARES ELIGIBLE FOR FUTURE SALE
General
We cannot predict what effect, if any, market sales of shares of common stock or the availability of shares of common stock for sale will have on the market price of our common stock prevailing from time to time. Nevertheless, sales of substantial amounts of common stock, including shares issued upon the exercise of outstanding options, in the public market, or the perception that such sales could occur, could materially and adversely affect the market price of our common stock and could impair our future ability to raise capital through the sale of our equity or equity-related securities at a time and price that we deem appropriate. See “Risk Factors—Risks Related to this Offering and Ownership of Our Common Stock—Future sales, or the perception of future sales, by us or our existing stockholders in the public market following this offering could cause the market price for our common stock to decline.”
As of March 31, 2020, we had a total of 348,583,743 shares of common stock outstanding. All shares sold in this offering will be freely tradable without registration under the Securities Act and without restriction, except for shares held by our “affiliates” (as defined under Rule 144). The shares of common stock held by the Majority Sponsors and certain of our directors, officers and employees after this offering will be “restricted” securities under the meaning of Rule 144 and may not be sold in the absence of registration under the Securities Act, unless an exemption from registration is available, including the exemptions pursuant to Rule 144 under the Securities Act.
Pursuant to Rule 144, the restricted shares held by our affiliates will be available for sale in the public market at various times after the date of this prospectus following the expiration of the applicable lock-up period.
In addition, a total of 39,053,663 shares of our common stock have been reserved for issuance under the 2020 Incentive Plan (subject to adjustments for stock splits, stock dividends and similar events), which will equal approximately % of the shares of our common stock outstanding immediately following this offering. We filed a registration statement on Form S-8 under the Securities Act to register common stock issued or reserved for issuance under the 2020 Incentive Plan, which automatically became effective upon filing. Accordingly, shares registered under such registration statement are available for sale in the open market, unless such shares are subject to vesting restrictions or the lock-up restrictions described below.
Rule 144
In general, under Rule 144 of the Securities Act, as currently in effect, a person (or persons whose shares are deemed aggregated) who is not deemed to be or have been one of our affiliates for purposes of the Securities Act at any time during 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than an affiliate, is entitled to sell such shares without registration, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of a prior owner other than an affiliate, then such person is entitled to sell such shares without complying with any of the requirements of Rule 144.
Under Rule 144, our affiliates or persons selling shares on behalf of our affiliates, who have met the six-month holding period for beneficial ownership of “restricted shares” of our common stock, are entitled to sell within any three-month period, a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or |
| • | the average reported weekly trading volume of our common stock on Nasdaq during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
62
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us. The sale of these shares, or the perception that sales will be made, could adversely affect the price of our common stock after this offering because an increased supply of shares would be, or would be perceived to be, available for sale in the public market.
Rule 701
In general, under Rule 701 of the Securities Act, as currently in effect, any of our employees, consultants or advisors who received shares from us in connection with a compensatory stock or option plan or other written agreement in a transaction in reliance on Rule 701 that was completed before the effective date of the registration statement on Form S-1 for our initial public offering are now eligible to resell such shares in reliance on Rule 144, but without compliance with certain restrictions, including the holding period, contained in Rule 144. Such sales may, however, remain subject to the restrictions related to the lock-up agreements discussed below.
Registration Rights
Our Sponsors have certain registration rights with respect to our common stock pursuant to the stockholders agreement. See “Certain Relationships and Related Person Transactions—Second Amended and Restated Stockholders Agreement” in PPD’s 2019 10-K, which is incorporated by reference in this prospectus.
Lock-Up Agreements
In connection with this offering, we, our directors and certain of our executive officers and significant equity holders have agreed with the underwriters, subject to certain exceptions, not to sell, dispose of or hedge any shares of our common stock or securities convertible into or exchangeable for shares of common stock during the period ending days after the date of this prospectus, except with the prior written consent of the representatives of the underwriters.
Immediately following the consummation of this offering, equity holders subject to lock-up agreements will hold shares of our common stock, representing approximately % of our then outstanding shares of common stock, or approximately % if the underwriters exercise in full their option to purchase additional shares.
We have agreed not to issue, sell or otherwise dispose of any shares of our common stock during the -day period following the date of this prospectus. We may, however, grant options to purchase shares of common stock, issue shares of common stock upon the exercise of outstanding options, issue shares of common stock in connection with certain acquisitions or business combinations or an employee stock purchase plan and in certain other circumstances.
63
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
CERTAIN UNITED STATES FEDERAL INCOME AND ESTATE TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following is a summary of certain United States federal income and estate tax consequences of the purchase, ownership and disposition of our common stock as of the date hereof. Except where noted, this summary deals only with common stock that is held as a capital asset by a non-U.S. holder (as defined below).
A “non-U.S. holder” means a beneficial owner of our common stock (other than an entity treated as a partnership for United States federal income tax purposes) that is not, for United States federal income tax purposes, any of the following:
| • | an individual citizen or resident of the United States; |
| • | a corporation (or any other entity treated as a corporation for United States federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate the income of which is subject to United States federal income taxation regardless of its source; or |
| • | a trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable United States Treasury regulations to be treated as a United States person. |
This summary is based upon provisions of the Internal Revenue Code of 1986, as amended (the “Code”), and regulations, rulings and judicial decisions as of the date hereof. Those authorities may be changed, perhaps retroactively, so as to result in United States federal income and estate tax consequences different from those summarized below. This summary does not address all aspects of United States federal income and estate taxes and does not deal with foreign, state, local or other tax considerations that may be relevant to non-U.S. holders in light of their particular circumstances. In addition, it does not represent a detailed description of the United States federal income and estate tax consequences applicable to you if you are subject to special treatment under the United States federal income tax laws (including if you are a United States expatriate, foreign pension fund, “controlled foreign corporation,” “passive foreign investment company” or a partnership or other pass-through entity for United States federal income tax purposes). We cannot assure you that a change in law will not alter significantly the tax considerations that we describe in this summary.
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) holds our common stock, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. If you are a partner of a partnership holding our common stock, you should consult your tax advisors.
If you are considering the purchase of our common stock, you should consult your own tax advisors concerning the particular United States federal income and estate tax consequences to you of the purchase, ownership and disposition of our common stock, as well as the consequences to you arising under other United States federal tax laws and the laws of any other taxing jurisdiction.
Dividends
In the event that we make a distribution of cash or other property (other than certain pro rata distributions of our stock) in respect of our common stock, the distribution generally will be treated as a dividend for United States federal income tax purposes to the extent it is paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. Any portion of a distribution that exceeds our current and accumulated earnings and profits generally will be treated first as a tax-free return of capital, causing
64
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
a reduction in the adjusted tax basis of a non-U.S. holder’s common stock, and to the extent the amount of the distribution exceeds a non-U.S. holder’s adjusted tax basis in our common stock, the excess will be treated as gain from the disposition of our common stock (the tax treatment of which is discussed below under “ —Gain on Disposition of Common Stock”).
Dividends paid to a non-U.S. holder generally will be subject to withholding of United States federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. However, dividends that are effectively connected with the conduct of a trade or business by the non-U.S. holder within the United States (and, if required by an applicable income tax treaty, are attributable to a United States permanent establishment) are not subject to the withholding tax, provided certain certification and disclosure requirements are satisfied. Instead, such dividends are subject to United States federal income tax on a net income basis in the same manner as if the non-U.S. holder were a United States person as defined under the Code. Any such effectively connected dividends received by a foreign corporation may be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A non-U.S. holder who wishes to claim the benefit of an applicable treaty rate and avoid backup withholding, as discussed below, for dividends will be required (a) to provide the applicable withholding agent with a properly executed Internal Revenue Service, or IRS, Form W-BEN or Form W-8BEN-E (or other applicable form) certifying under penalty of perjury that such holder is not a United States person as defined under the Code and is eligible for treaty benefits or (b) if our common stock is held through certain foreign intermediaries, to satisfy the relevant certification requirements of applicable United States Treasury regulations. Special certification and other requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals.
A non-U.S. holder eligible for a reduced rate of United States federal withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
Gain on Disposition of Common Stock
Subject to the discussion of backup withholding and FATCA below, any gain realized by a non-U.S. holder on the sale or other disposition of our common stock generally will not be subject to United States federal income or withholding tax unless:
| • | the gain is effectively connected with a trade or business of the non-U.S. holder in the United States (and, if required by an applicable income tax treaty, is attributable to a United States permanent establishment of the non-U.S. holder); |
| • | the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of that disposition, and certain other conditions are met; or |
| • | we are or have been a “United States real property holding corporation” for United States federal income tax purposes and certain other conditions are met. |
A non-U.S. holder described in the first bullet point immediately above will be subject to tax on the gain derived from the sale or other disposition in the same manner as if the non-U.S. holder were a United States person as defined under the Code. In addition, if any non-U.S. holder described in the first bullet point immediately above is a foreign corporation, the gain realized by such non-U.S. holder may be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. An individual non-U.S. holder described in the second bullet point immediately above will be subject to a 30% (or such lower rate as may be specified by an applicable income tax treaty) tax on the gain derived from the sale or other disposition, which gain may be offset by United States source capital losses even though the individual is not considered a resident of the United States.
65
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Generally, a corporation is a “United States real property holding corporation” if the fair market value of its United States real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests and its other assets used or held for use in a trade or business (all as determined for United States federal income tax purposes). We believe we are not and do not anticipate becoming a “United States real property holding corporation” for United States federal income tax purposes.
Federal Estate Tax
Common stock held by an individual non-U.S. holder at the time of death will be included in such holder’s gross estate for United States federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding
Distributions paid to a non-U.S. holder and the amount of any tax withheld with respect to such distributions generally will be reported to the IRS. Copies of the information returns reporting such distributions and any withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty.
A non-U.S. holder will not be subject to backup withholding on dividends received if such holder certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that such holder is a United States person as defined under the Code), or such holder otherwise establishes an exemption.
Information reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale or other disposition of our common stock made within the United States or conducted through certain United States-related financial intermediaries, unless the beneficial owner certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that the beneficial owner is a United States person as defined under the Code), or such owner otherwise establishes an exemption.
Backup withholding is not an additional tax and any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against a non-U.S. holder’s United States federal income tax liability provided the required information is timely furnished to the IRS.
Additional Withholding Requirements
Under Sections 1471 through 1474 of the Code (such Sections commonly referred to as “FATCA”), a 30% United States federal withholding tax may apply to any dividends paid on our common stock to (i) a “foreign financial institution” (as specifically defined in the Code) which does not provide sufficient documentation, typically on IRS Form W-8BEN-E, evidencing either (x) an exemption from FATCA, or (y) its compliance (or deemed compliance) with FATCA (which may alternatively be in the form of compliance with an intergovernmental agreement with the United States) in a manner which avoids withholding, or (ii) a “non-financial foreign entity” (as specifically defined in the Code) which does not provide sufficient documentation, typically on IRS Form W-8BEN-E, evidencing either (x) an exemption from FATCA, or (y) adequate information regarding certain substantial United States beneficial owners of such entity (if any). If a dividend payment is both subject to withholding under FATCA and subject to the withholding tax discussed above under “—Dividends,” the withholding under FATCA may be credited against, and therefore reduce, such other withholding tax. FATCA withholding may also apply to payments of gross proceeds of dispositions of our common stock, although under proposed regulations (the preamble to which specifies that taxpayers are permitted to rely on them pending finalization), no withholding will apply on payments of gross proceeds. You should consult your own tax advisors regarding these requirements and whether they may be relevant to your ownership and disposition of our common stock.
66
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The selling stockholders are offering the shares of common stock described in this prospectus through a number of underwriters. and are acting as joint book-running managers and representatives of each of the underwriters named below. We and the selling stockholders have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, the selling stockholders have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from the selling stockholders, the number of shares of common stock listed next to its name in the following table:
Name | Number of Shares | |||
|
| |||
Total | ||||
The underwriters are committed to purchase all the common shares offered by the selling stockholders if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The representatives have advised us and the selling stockholders that the underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The underwriters have an option to buy up to additional shares of common stock from the selling stockholders to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional shares. If any shares are purchased with this option to purchase additional shares, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to the selling stockholders per share of common stock. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without option to purchase additional shares exercise | With full option to purchase additional shares exercise | |||||||
Per Share. | $ | $ | ||||||
Total | $ | $ | ||||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $ million. We have agreed to reimburse the underwriters for expenses relating to clearance of this offering with the Financial Industry Regulatory Authority up to $ . The underwriters have agreed to reimburse the selling stockholders for certain expenses incurred in connection with this offering.
67
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make internet distributions on the same basis as other allocations. We have agreed that we will not, subject to certain limited exceptions, (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, or submit to, or file with, the Securities and Exchange Commission a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exercisable or exchangeable for any shares of our common stock, or publicly disclose the intention to undertake any of the foregoing, or (ii) enter into any swap or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise), in each case without the prior written consent of for a period of days after the date of this prospectus, other than the shares of our common stock to be sold hereunder and any shares of our common stock issued upon the exercise of options granted under our existing equity incentive plans.
Our directors and certain of our executive officers and significant equity holders have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of days after the date of this prospectus, may not, subject to certain limited exceptions, without the prior written consent of , (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including, without limitation, common stock or such other securities which may be deemed to be beneficially owned by such directors, executive officers, managers and members in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant), or publicly disclose the intention to undertake any of the foregoing, (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the common stock or such other securities (regardless of whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or such other securities, in cash or otherwise), or (3) make any demand for or exercise any right with respect to the registration of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock.
We and the selling stockholders have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
Our common stock is listed on The Nasdaq Global Select Market under the symbol “PPD.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional
68
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock, and, as a result, the price of our common stock may be higher than the price that otherwise might exist in the open market. The underwriters may carry out these transactions on Nasdaq, in the over the counter market or otherwise.
Neither we, the selling stockholders, nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we, the selling stockholders, nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Other than in the United States, no action has been taken by us, the selling stockholders, or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Certain of the underwriters and their affiliates may provide from time to time certain commercial banking, financial advisory, investment banking and other services for us and our affiliates in the ordinary course of their business, for which they may receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments.
Notice to Prospective Investors in Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
69
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to Prospective Investors in the European Economic Area and United Kingdom
In relation to each Member State of the European Economic Area and the United Kingdom (each a “Relevant State”), no shares have been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that offers of shares may be made to the public in that Relevant State at any time under the following exemptions under the Prospectus Regulation:
| a) | to any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
| b) | to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of the underwriters; or |
| c) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and the Company that it is a “qualified investor” within the meaning of Article 2(e) of the Prospectus Regulation. In the case of any shares being offered to a financial intermediary as that term is used in the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters have been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer to the public” in relation to shares in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Regulation) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as
70
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
“relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the shares in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in Japan
The shares have not been and will not be registered pursuant to Article 4, Paragraph 1 of the Financial Instruments and Exchange Act. Accordingly, none of the shares nor any interest therein may be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any “resident” of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan in effect at the relevant time.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (the “SFO”) of Hong Kong and any rules made thereunder; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong) (the “CO”) or which do not constitute an offer to the public within the meaning of the CO. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made thereunder.
Notice to Prospective Investors in Singapore
Each underwriter has acknowledged that this prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, each underwriter has represented and agreed that it has not
71
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
offered or sold any shares or caused the shares to be made the subject of an invitation for subscription or purchase and will not offer or sell any shares or cause the shares to be made the subject of an invitation for subscription or purchase, and has not circulated or distributed, nor will it circulate or distribute, this prospectus or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares, whether directly or indirectly, to any person in Singapore other than:
| a) | to an institutional investor (as defined in Section 4A of the Securities and Futures Act (Chapter 289) of Singapore, as modified or amended from time to time (the “SFA”)) pursuant to Section 274 of the SFA; |
| b) | to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA; or |
| c) | otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA. |
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, securities or securities-based derivatives contracts (each term as defined in Section 2(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except: |
| i. | to an institutional investor or to a relevant person, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| ii. | where no consideration is or will be given for the transfer; |
| iii. | where the transfer is by operation of law; |
| iv. | as specified in Section 276(7) of the SFA; or |
| v. | as specified in Regulation 37A of the Securities and Futures (Offers of Investments) (Securities and Securities-based Derivatives Contracts) Regulations 2018. |
Notice to Prospective Investors in China
This prospectus will not be circulated or distributed in the PRC and the shares will not be offered or sold, and will not be offered or sold to any person for re-offering or resale directly or indirectly to any residents of the PRC except pursuant to any applicable laws and regulations of the PRC. Neither this prospectus nor any advertisement or other offering material may be distributed or published in the PRC, except under circumstances that will result in compliance with applicable laws and regulations.
Notice to Prospective Investors in Mexico
The shares have not been registered with the National Securities Registry (Registro Nacional de Valores) or reviewed or authorized by the National Banking and Securities Commission (Comisión Nacional Bancaria y de Valores) of Mexico or listed in any Mexican securities exchange. Any Mexican investor who acquires shares does so at his or her own risk. The shares will be initially placed with less than 100 persons in Mexico. Once placed, the shares can be resold exclusively to persons that qualify as qualified investors or institutional investors pursuant to applicable provisions of Mexican law.
72
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Notice to Prospective Investors in India
This prospectus will not be circulated or distributed in India and the shares will not be offered or sold, and this prospectus does not constitute a public offer or offer to sell to the public in India. This prospectus is only directed to the intended recipient and not meant to be transferred or circulated in any manner.
Notice to Prospective Investors in Serbia
The shares will neither be offered nor sold in Serbia within the meaning of Article 2 point 37 of the Serbian Capital Markets Law. This prospectus has not been registered or approved by the Serbian Securities Commission (Komisija za hartije od vrednosti Republike Srbije).
Notice to Prospective Investors in Australia
This prospectus has been prepared for information purposes only and the provision of this prospectus to any person in Australia does not constitute an offer of the shares to that person or an invitation to that person to apply for the issue of the shares unless the recipient is a person to whom an offer of securities may be made in Australia without the need for disclosure under Part 6D.2 of the Corporations Act 2001 (Cth) (“Corporations Act”) because of section 708(8) (sophisticated investors) or section 708(11) (professional investors) and/or the recipient is a person who is a “wholesale client” as defined in section 761G of the Corporations Act (together a “Wholesale Client”). In no circumstances may this prospectus be made available to a person who is not a Wholesale Client, and only Wholesale Clients may hold the shares.
The Company is not, and is not required to be, registered with the Australian Securities and Investments Commission (“ASIC”) as a managed investment scheme or a foreign company under the Corporations Act. The Company does not carry on a business in Australia and is not, and is not required to be, registered in Australia as a foreign company under Part 5B.2 of the Corporations Act. The Company does not hold an Australian Financial Services Licence (“AFSL”) and is not licensed under the Corporations Act to provide financial product advice or other regulated financial services in relation to the shares. There is no cooling-off regime applicable in respect of an acquisition of the shares.
If any ‘financial service’ is provided by any other person, it will only be provided to the extent that the person holds an AFSL, relies on an AFSL exemption under the Corporations Act or an ASIC class order (for the purposes of this Notice, each a “Class Order”). Persons relying on an AFSL exemption of a Class Order do not hold an AFSL. Where a person is relying on a Class Order, they will be regulated by the regulatory body relevant to them under laws of the applicable Class Order jurisdiction, which differ from Australian laws. This prospectus has not been prepared specifically for Australian investors. They may contain references to dollar amounts which are not Australian dollars, may contain financial information which is not prepared in accordance with Australian law or practices and may not address risks associated with investment in foreign currency denominated investments.
Notice to Prospective Investors in Peru
The shares will not be subject to a public offering in Peru, as this offer does not qualify as a public offering under Article 5 of the “Ley de Mercado de Valores,” whose Uniformed Ordered Text has been approved by the Decreto Supremo N°093-2002-EF, as amended (the “Peruvian Securities Market Law”); and, Article 6.d) of the “Reglamento de Oferta Pública Primara y de Venta de Valores Mobiliarios,” approved by the Resolución CONASEV N°141-98-EF-94.10, as amended (the “Public Offering Rules”). Therefore, this prospectus and the shares have not been, and will not be, registered with or approved by the Peruvian Superintendency of the Securities Market (Superintendencia del Mercado de Valores – SMV) or the Lima Stock Exchange. Accordingly, the shares cannot be offered or sold in Peru, except if such offering is considered a private offering under the securities laws and regulations of Peru.
73
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The Peruvian Securities Market Law and the Public Offering Rules establish that any particular offer may qualify as private, if it is directed exclusively to investors that do not qualify as a public segment of Peruvian market (“Segmento del Público”), as defined thereunder. The investors at which this private offering of the Shares is exclusively directed, are employees of the Company, and in total, they do not exceed of the number of 100, therefore, they do not qualify as a “Segmento del Público.”
The investors which should be employees of the Company, must rely on their own examination of the Company and the terms of the offering of the shares in order to determine their legal ability to invest in the shares.
This prospectus and other offering materials relating to the offer of the shares are being supplied only to those investors, referred before, who have expressly requested it. They are strictly confidential and may not be distributed to any person or entity other than the intended recipients hereof, which are exclusively employees of the Issuer.
Notice to Prospective Investors in the Dubai International Financial Centre (“DIFC”)
This document relates to an Exempt Offer in accordance with the Markets Rules 2012 of the Dubai Financial Services Authority (“DFSA”). This document is intended for distribution only to persons of a type specified in the Markets Rules 2012 of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus supplement nor taken steps to verify the information set forth herein and has no responsibility for this document. The securities to which this document relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this document you should consult an authorized financial advisor.
In relation to its use in the DIFC, this document is strictly private and confidential and is being distributed to a limited number of investors and must not be provided to any person other than the original recipient, and may not be reproduced or used for any other purpose. The interests in the securities may not be offered or sold directly or indirectly to the public in the DIFC.
74
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
The validity of the shares of common stock offered by this prospectus will be passed upon for us by Simpson Thacher & Bartlett LLP, Palo Alto, California. Certain other legal matters will be passed upon for the underwriters by Latham & Watkins LLP, Washington, District of Columbia.
The consolidated financial statements, and the related financial statement schedule, incorporated in this prospectus by reference from the Company’s Current Report on Form 8-K filed on May 21, 2020, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report, which is incorporated herein by reference. Such financial statements and financial statement schedule have been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
75
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83

PPD is a leading provider of drug development services to the biopharmaceutical industry, focused on
helping our customers bring their new medicines to patients around the world. We have been in the
drug development services business for more than 30 years, providing a comprehensive suite of clinical
development and laboratory services. We pursue our purpose and mission – to improve health by helping
our customers deliver life-changing therapies – through our strategy to bend the cost and time curve of
drug development and optimize value for our customers.
HELPING DELIVER LIFE-CHANGING THERAPIES
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83

CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than the underwriting discount, payable by the registrant and the selling stockholders in connection with the sale and distribution of the securities being registered. All amounts are estimated except the Securities and Exchange Commission (“SEC”) registration fee and the Financial Industry Regulatory Authority (“FINRA”) filing fee.
| Amount to be paid | ||||
SEC Registration Fee | $ | * | ||
FINRA Filing Fee | * | |||
Legal Fees and Expenses | * | |||
Accounting Fees and Expenses | * | |||
Printing Fees and Expenses | * | |||
Transfer Agent and Registrar Fees | * | |||
Miscellaneous Expenses | * | |||
|
| |||
Total | $ | * | ||
| * | To be provided by amendment. |
Item 14. Indemnification of Directors and Officers
Section 102(b)(7) of the Delaware General Corporation Law (the “DGCL”) allows a corporation to provide in its certificate of incorporation that a director of the corporation will not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our amended and restated certificate of incorporation provides for this limitation of liability.
Section 145 of the DGCL, provides, among other things, that a Delaware corporation may indemnify any person who was, is or is threatened to be made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful. A Delaware corporation may indemnify any persons who were or are a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests, provided further that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses (including attorneys’ fees) which such officer or director has actually and reasonably incurred.
II-1
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation would otherwise have the power to indemnify such person under Section 145.
Our amended and restated bylaws provide that we must indemnify and advance expenses to our directors and officers to the full extent authorized by the DGCL.
We have entered into indemnification agreements with each of our directors and executive officers. Such agreements may require us, among other things, to advance expenses and otherwise indemnify our executive officers and directors against certain liabilities that may arise by reason of their status or service as executive officers or directors, to the fullest extent permitted by law. We intend to enter into indemnification agreements with any new directors and executive officers in the future.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, any provision of our amended and restated certificate of incorporation, our amended and restated bylaws, agreement, vote of stockholders or disinterested directors or otherwise. Notwithstanding the foregoing, we shall not be obligated to indemnify a director or officer in respect of a proceeding (or part thereof) instituted by such director or officer, unless such proceeding (or part thereof) has been authorized by the board of directors pursuant to the applicable procedure outlined in the amended and restated bylaws.
Section 174 of the DGCL provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption, may be held jointly and severally liable for such actions. A director who was either absent when the unlawful actions were approved or dissented at the time may avoid liability by causing his or her dissent to such actions to be entered in the books containing the minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful acts.
We maintain and expect to maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers.
The underwriting agreement provides for indemnification by the underwriters of us and our officers and directors, and by us of the underwriters, for certain liabilities arising under the Securities Act or otherwise in connection with this offering.
Item 15. Recent Sales of Unregistered Securities
Since June 30, 2017, we or our predecessor, Jaguar Holding Company I, have granted under our equity incentive plans (1) stock options to purchase an aggregate of 15,659,435 shares of our common stock, which options had exercise prices per share ranging between $9.89 and $21.70 when issued and (2) an aggregate of 32,874 shares of restricted common stock to our employees and directors. On August 14, 2017, we issued and sold an aggregate of 496,184 shares of our non-voting common stock to certain of our employees for a price of $15.05 per share. On February 19, 2018, we issued and sold an aggregate of 31,889 shares of our non-voting common stock to certain of our directors for a price of $15.05 per share. In addition, on July 1, 2019, we issued an aggregate of 268,054 shares to a seller of Medimix International in connection with our acquisition of such company. The issuances of these stock options and shares of common stock were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an
II-2
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. None of the foregoing transactions involved any underwriters, underwriting discounts or commissions or any public offering.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits. See Exhibit Index immediately preceding the signature page hereto, which is incorporated by reference as if fully set forth herein.
(b) Financial Statement Schedules. Schedule I—Registrant’s Condensed Financial Statements, incorporated by reference to Schedule I of Exhibit 99.3 of the Registrant’s first Current Report on Form 8-K filed on May 21, 2020.
Item 17. Undertakings
(1) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(2) The undersigned registrant hereby undertakes that:
(a) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus as filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(b) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
EXHIBIT INDEX
Exhibit | Description | |
| 1.1† | Form of Underwriting Agreement | |
| 3.1 | Amended and Restated Certificate of Incorporation of PPD, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K (File No. 001-39212) filed with the SEC on February 10, 2020) | |
| 3.2 | Amended and Restated Bylaws of PPD, Inc. (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K (File No. 001-39212) filed with the SEC on February 10, 2020) | |
| 4.1 | Form of Stock Certificate (incorporated by reference to Exhibit 4.1 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 4.2 | Description of PPD, Inc.’s Securities (incorporated by reference to Exhibit 4.5 to the Company’s Current Report on Form 10-K (File No. 001-39212) filed with the SEC on March 5, 2020) | |
| 4.3 | Indenture, dated as of August 18, 2015, between Jaguar Holding Company II and Pharmaceutical Product Development, LLC and Wilmington Trust, National Association, as trustee (incorporated by reference to Exhibit 4.2 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 4.4 | Indenture, dated as of May 11, 2017, between Eagle Holding Company II, LLC and Wilmington Trust, National Association, as trustee (incorporated by reference to Exhibit 4.3 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 4.5 | Indenture, dated as of May 14, 2019, between Eagle Holding Company II, LLC and Wilmington Trust, National Association, as trustee (incorporated by reference to Exhibit 4.4 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 4.6 | Indenture, dated as of June 5, 2020 by and among, Jaguar Holding Company II, PPD Development, L.P, the guarantors time to time party thereto and Wilmington Trust, National Association, as trustee (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K (File No. 001-39212) filed with the SEC on June 5, 2020) | |
| 5.1† | Opinion of Simpson Thacher & Bartlett LLP | |
| 10.1 | Second Amended and Restated Stockholders Agreement by and among PPD, Inc. and the other parties named therein (incorporated by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K (File No. 001-39212) filed by PPD, Inc. on February 10, 2020) | |
| 10.2* | Side Letter to the Stockholders Agreement, dated as of May 11, 2017, by and among David Simmons, Hellman & Friedman Capital Partners VIII, L.P., Hellman & Friedman Capital Partners VII, L.P., Carlyle Partners VI Holdings II, L.P., and Eagle Holding Company I (incorporated by reference to Exhibit 10.2 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.3* | Side Letter to the Stockholders Agreement, dated as of May 2, 2018, by and among Christopher G. Scully, Hellman & Friedman Capital Partners VIII, L.P., Hellman & Friedman Capital Partners VII, L.P., Carlyle Partners VI Holdings II, L.P., and Eagle Holding Company I (incorporated by reference to Exhibit 10.3 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
II-4
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Exhibit | Description | |
| 10.4* | Side Letter to the Stockholders Agreement, dated as of May 11, 2017, by and among William Sharbaugh, Hellman & Friedman Capital Partners VIII, L.P., Hellman & Friedman Capital Partners VII, L.P., Carlyle Partners VI Holdings II, L.P., and Eagle Holding Company I (incorporated by reference to Exhibit 10.4 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.5* | Side Letter to the Stockholders Agreement, dated as of May 11, 2017, by and among B. Judd Hartman, Hellman & Friedman Capital Partners VIII, L.P., Hellman & Friedman Capital Partners VII, L.P., Carlyle Partners VI Holdings II, L.P., and Eagle Holding Company I (incorporated by reference to Exhibit 10.5 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.6* | Side Letter to the Stockholders Agreement, dated as of May 11, 2017, by and among Anshul Thakral, Hellman & Friedman Capital Partners VIII, L.P., Hellman & Friedman Capital Partners VII, L.P., Carlyle Partners VI Holdings II, L.P., and Eagle Holding Company I (incorporated by reference to Exhibit 10.6 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.7 | Credit Agreement, dated as of August 18, 2015, between Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG, Cayman Islands Branch, as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto (incorporated by reference to Exhibit 10.7 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.8 | Amendment No. 1 to the Credit Agreement, dated as of May 31, 2016, between Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG, Cayman Islands Branch, as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto (incorporated by reference to Exhibit 10.8 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.9 | Amendment No. 2 to the Credit Agreement, dated as of November 10, 2016, between Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto (incorporated by reference to Exhibit 10.9 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.10 | Amendment No. 3 to the Credit Agreement, dated as of May 30, 2017, between Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG, Cayman Islands Branch, as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto (incorporated by reference to Exhibit 10.10 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.11 | Amendment No. 4 to the Credit Agreement, dated as of March 29, 2018, between Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG, Cayman Islands Branch, as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto (incorporated by reference to Exhibit 10.11 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
II-5
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Exhibit | Description | |
| 10.12 | Amendment No. 5 to the Credit Agreement, dated as of April 23, 2019, between Jaguar Holding Company II, Pharmaceutical Product Development, LLC, Jaguar Holding Company I, LLC, Credit Suisse AG, Cayman Islands Branch, as Administrative Agent, Collateral Agent and L/C Issuer and each lender from time to time party thereto (incorporated by reference to Exhibit 10.12 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.13 | Holdings Guaranty, dated as of August 18, 2015, between Jaguar Holding Company I, as Guarantor, and Credit Suisse AG, Cayman Islands Branch, as Administrative Agent (incorporated by reference to Exhibit 10.13 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.14 | Subsidiary Guaranty, dated as of August 18, 2015, the Guarantors, as defined therein, the Additional Guarantors as defined therein, and Credit Suisse AG, Cayman Islands Branch, as Administrative Agent and Collateral Agent (incorporated by reference to Exhibit 10.14 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.15 | Security Agreement, dated as of August 18, 2015, between the Grantors, as defined therein, and Credit Suisse AG, Cayman Islands Branch, as Collateral Agent (incorporated by reference to Exhibit 10.15 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.16* | Form of Indemnification Agreement between PPD, Inc. and directors and executive officers of PPD, Inc. (incorporated by reference to Exhibit 10.16 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.17* | Employment Agreement, dated as of May 17, 2012, by and among David Simmons, Pharmaceutical Product Development, LLC, and Jaguar Holding Company I (the “Simmons Employment Agreement”) (incorporated by reference to Exhibit 10.17 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.18* | Assignment and Assumption Agreement, dated as of May 11, 2017, by and among Jaguar Holding Company I, Eagle Holding Company I, Pharmaceutical Product Development, LLC, and David Simmons (incorporated by reference to Exhibit 10.18 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.19* | Amendment No. 1 to the Simmons Employment Agreement, dated as of April 1, 2018 (incorporated by reference to Exhibit 10.19 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.20* | Employment Agreement, dated as of May 2, 2018, between Christopher G. Scully, Pharmaceutical Product Development, LLC, and, solely for purposes of Sections 1(c), 2(h), 9(m), and (9)(n) thereof, Eagle Holding Company I (incorporated by reference to Exhibit 10.20 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.21* | Employment Agreement, dated as of April 10, 2012, between William J. Sharbaugh, Pharmaceutical Product Development, LLC, and, solely for purposes of Sections 9(l)(vii) and (9)(n) thereof, Jaguar Holding Company I (the “Sharbaugh Employment Agreement”) (incorporated by reference to Exhibit 10.22 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.22* | Amendment No. 1 to the Sharbaugh Employment Agreement, dated as of February 10, 2016 (incorporated by reference to Exhibit 10.22 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
II-6
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Exhibit | Description | |
| 10.23* | Assignment and Assumption Agreement, dated as of May 11, 2017, by and among Jaguar Holding Company I, Eagle Holding Company I, Pharmaceutical Product Development, LLC, and William Sharbaugh (incorporated by reference to Exhibit 10.23 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.24* | Amendment No. 2 to the Sharbaugh Employment Agreement, dated as of March 1, 2019 (incorporated by reference to Exhibit 10.24 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.25* | Employment Agreement, dated as of April 10, 2012, between B. Judd Hartman, Pharmaceutical Product Development, LLC, and, solely for purposes of Section 9(n) thereof, Jaguar Holding Company I (the “Hartman Employment Agreement”) (incorporated by reference to Exhibit 10.25 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.26* | Amendment No. 1 to the Hartman Employment Agreement, dated as of February 10, 2016 (incorporated by reference to Exhibit 10.26 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.27* | Assignment and Assumption Agreement, dated as of May 11, 2017, by and among Jaguar Holding Company I, Eagle Holding Company I, Pharmaceutical Product Development, LLC, and B. Judd Hartman (incorporated by reference to Exhibit 10.27 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.28* | Amendment No. 2 to the Hartman Employment Agreement, dated as of April 1, 2018 (incorporated by reference to Exhibit 10.28 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.29* | Amendment No. 3 to the Hartman Employment Agreement, dated as of December 18, 2019 (incorporated by reference to Exhibit 10.29 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.30* | Amended and Restated Employment Agreement, effective as of November 1, 2019, between Anshul Thakral, Pharmaceutical Product Development, LLC, and, solely for purposes of Sections 2(g), 9(m), and (9)(n) thereof, Eagle Holding Company I (incorporated by reference to Exhibit 10.30 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.31* | Eagle Holding Company I 2017 Equity Incentive Plan (incorporated by reference to Exhibit 10.33 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.32* | Form of Nonqualified Stock Option Agreement under the Eagle Holding Company I 2017 Equity Incentive Plan (David Simmons) (incorporated by reference to Exhibit 10.2 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.33* | Form of Nonqualified Stock Option Agreement under the Eagle Holding Company I 2017 Equity Incentive Plan (Christopher G. Scully, William J. Sharbaugh, and B. Judd Hartman) (incorporated by reference to Exhibit 10.35 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.34* | Form of Nonqualified Stock Option Agreement under the Eagle Holding Company I 2017 Equity Incentive Plan (Anshul Thakral for 2017 and 2018) (incorporated by reference to Exhibit 10.36 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
II-7
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Exhibit | Description | |
| 10.35* | Form of Nonqualified Stock Option Agreement under the Eagle Holding Company I 2017 Equity Incentive Plan (Anshul Thakral for 2019) (incorporated by reference to Exhibit 10.37 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 16, 2020) | |
| 10.36* | PPD, Inc. 2020 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.38 to Amendment No. 2 to the Company’s Registration Statement on Form S-2 (File No. 333-235860) filed with the SEC on January 27, 2020) | |
| 10.37* | Form of Option Grant Notice and Agreement under the PPD, Inc. 2020 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.39 to Amendment No. 2 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 27, 2020) | |
| 10.38* | Form of Restricted Stock Grant Notice and Agreement under the PPD, Inc. 2020 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.40 to Amendment No. 2 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 27, 2020) | |
| 10.39* | Form of Restricted Stock Unit Grant Notice and Agreement for Directors under the PPD, Inc. 2020 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.41 to Amendment No. 2 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 27, 2020) | |
| 10.40* | Form of Restricted Stock Unit Grant Notice and Agreement for Employees under the PPD, Inc. 2020 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.42 to Amendment No. 2 to the Company’s Registration Statement on Form S-1 (File No. 333-235860) filed with the SEC on January 27, 2020) | |
| 21.1 | Subsidiaries of the Registrant (incorporated by reference to Exhibit 21.1 to the Company’s Annual Report on Form 10-K (File No. 001-39212) filed with the SEC on March 5, 2020) | |
| 23.1† | Consent of Simpson Thacher & Bartlett LLP (included in Exhibit 5.1) | |
| 23.2† | Consent of Deloitte & Touche LLP | |
| 24.1† | Power of Attorney (included in the signature page to the Registration Statement) | |
| † | To be filed by amendment. |
| * | Management contract or compensatory plan or arrangement. |
II-8
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
SIGNATURES
Pursuant to the requirements of the Securities Act, we have duly caused this registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, North Carolina, on , 2020.
| PPD, Inc. | ||
| By: | ||
| Name: | David Simmons | |
| Title: | Chief Executive Officer | |
The undersigned directors and officers of PPD, Inc. hereby constitute and appoint David Simmons, Christopher G. Scully and B. Judd Hartman and each of them, any of whom may act without joinder of the other, the individual’s true and lawful attorneys in fact and agents, with full power of substitution and resubstitution, for the person and in his or her name, place and stead, in any and all capacities, to sign this registration statement and any or all amendments, including post effective amendments to the Registration Statement, including a prospectus or an amended prospectus therein and any registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act, and all other documents in connection therewith to be filed with the SEC, granting unto said attorneys in fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys in fact as agents or any of them, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereto.
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities indicated on , 2020.
Signature | Title | |
David Simmons | Chief Executive Officer and Chairman (Principal Executive Officer) | |
Christopher G. Scully | Chief Financial Officer (Principal Financial Officer) | |
Glen Donovan | Chief Accounting Officer (Principal Accounting Officer) | |
Joe Bress | Director | |
Stephen Ensley | Director | |
Maria Teresa Hilado | Director | |
Colin Hill | Director | |
Jeffrey B. Kindler | Director | |
II-9
CONFIDENTIAL TREATMENT REQUESTED
PURSUANT TO 17 C.F.R. SECTION 200.83
Signature | Title | |
P. Hunter Philbrick | Director | |
Allen R. Thorpe | Director | |
Stephen H. Wise | Director | |
II-10