operate without infringing on the proprietary rights of others; and to prevent others from infringing our proprietary rights. Our strategy is to seek to protect our proprietary position by, among other methods, filing or
in-licensing
U.S. and foreign patents and patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trademarks, trade secrets,
know-how,
continuing technological innovation, and potential
in-licensing
opportunities to develop and maintain our proprietary position.
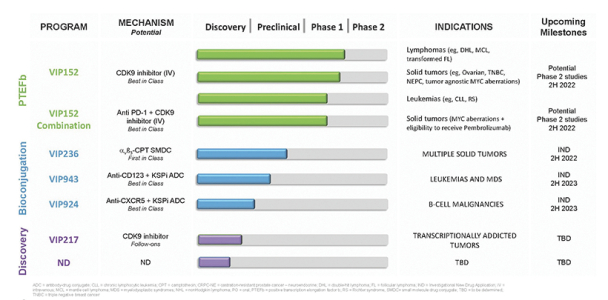
We have a license to patents and other intellectual property relating to VIP152, VIP217, VIP943, VIP924, VIP236, and our other current product candidates from Bayer on an exclusive, worldwide basis under the Bayer License Agreement. The portfolio as of December 31, 2021 includes 24 issued U.S. patents, 14 pending U.S. patent applications, 274 issued patents in various jurisdictions outside of the United States and approximately 134 pending patent applications in various jurisdictions outside of the United States. The Bayer License Agreement is described more fully below.
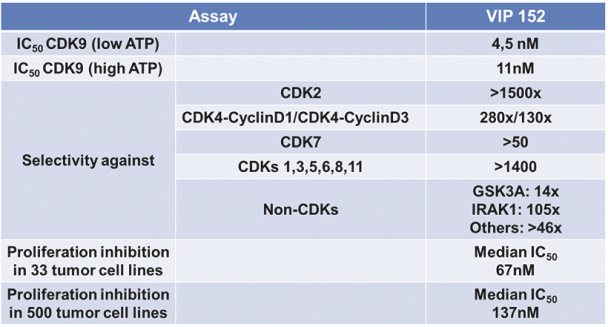
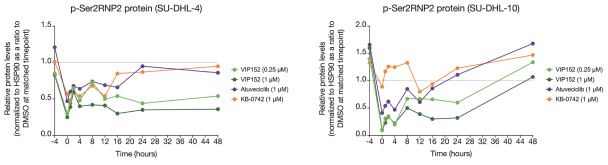
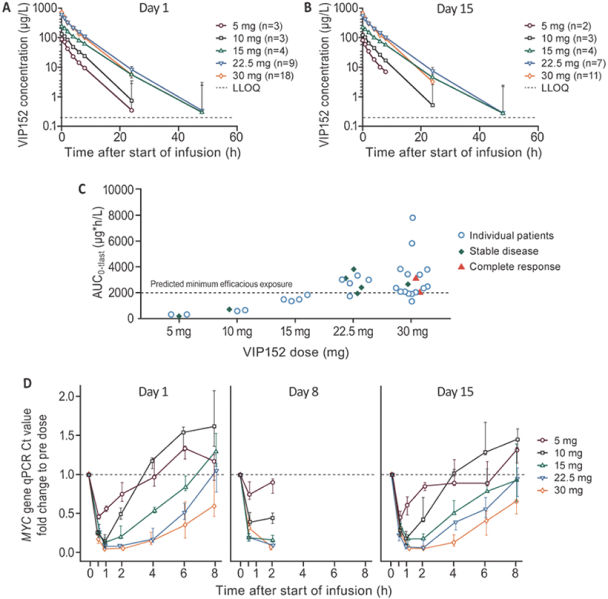
Our patent portfolio covering VIP152 consists of issued patents in the U.S., Europe, China, Japan, India and Mexico, along with issued patents and pending applications in other markets. The issued U.S. patent covering the composition of matter of VIP152 is expected to expire in November 2033, absent any patent term extensions for regulatory delay. With respect to VIP943 and VIP924, we have pending applications in the U.S., Europe, China, Japan, India, Argentina, Brazil and Mexico, along with an issued patent and pending applications in other markets covering the composition of matter of VIP943 and VIP924. Any patent that may issue from our pending patent applications related to VIP943 and VIP924 are expected to expire in December 2037, absent any patent term adjustments or extensions. With respect to VIP236, we have pending applications in the U.S., Europe, China, Japan, India, Argentina, Brazil, Mexico and other markets covering the composition of matter of VIP236. Any patent that may issue from our pending patent applications related to VIP236 is expected to expire in October 2039, absent any patent term adjustments or extensions. In addition, our patent portfolio covering VIP217 consists of issued patents in the U.S., Europe, China, Japan, India and Mexico, along with issued patents and pending applications in other markets. The issued U.S. patent covering the composition of matter of VIP217 is expected to expire in April 2035, absent any patent term extensions for regulatory delay. With respect to our product candidates and processes we intend to develop and commercialize in the normal course of business, we intend to pursue patent protection covering, when possible, compositions, methods of use, dosing and formulations. We may also pursue patent protection with respect to manufacturing and drug development processes and technologies.
We also rely upon trade secrets and
know-how
and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, by using confidentiality and invention assignment agreements with our commercial partners, collaborators, employees and consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting
know-how
and inventions.
On October 7, 2020, we entered into the Bayer License Agreement, pursuant to which we have been granted an exclusive, worldwide, royalty-bearing, worldwide license under certain Bayer patents and
know-how
to develop, use, manufacture, commercialize, sublicense and distribute, for all uses in the cure, mitigation, treatment or prevention of diseases or disorders in humans or animals, (i) a clinical-stage small molecule drug platform, including VIP152 (formerly known as BAY 1251152), a PTEFb/CDK9 inhibitor compound, and (ii) a preclinical stage bioconjugation platform, including VIP924 (formerly
BAY-924),
and VIP943 (formerly known as
BAY-943),
next-generation ADC compounds, and VIP236, a SMDC compound. These platforms currently