
CORPORATE OVERVIEW SEPTEMBER 2020 EXHIBIT 99.2

Important Notices This presentation is provided for informational purposes only and has been prepared to assist interested parties in making their own evaluation with respect to the proposed business combination between Vincera Pharma, Inc. (“Vincera” or the “Company”) and LifeSci Acquisition Corp. (“LifeSci”) and related transactions (the “Proposed Transactions”) and for no other purpose. No representations or warranties, express or implied are given in, or in respect of, this presentation. To the fullest extent permitted by law in no circumstances will LifeSci, Vincera or any of their respective subsidiaries, stockholders, affiliates, representatives, partners, directors, officers, employees, advisers or agents be responsible or liable for any direct, indirect or consequential loss or loss of profit arising from the use of this presentation, its contents, its omissions, reliance on the information contained within it, or on opinions communicated in relation thereto or otherwise arising in connection therewith. Industry and market data used in this presentation have been obtained from third-party industry publications and sources as well as from research reports prepared for other purposes. Neither LifeSci nor Vincera has independently verified the data obtained from these sources and cannot assure you of the data’s accuracy or completeness. This data is subject to change. In addition, this presentation does not purport to be all-inclusive or to contain all of the information that may be required to make a full analysis of Vincera or the Proposed Transactions. Viewers of this presentation should each make their own evaluation of Vincera and of the relevance and adequacy of the information and should make such other investigations as they deem necessary. Additional Information In connection with the Proposed Transactions, LifeSci will file with the SEC a proxy statement on Schedule 14A (the “Proxy Statement”). A definitive proxy statement will be sent to holders of LifeSci’s common stock when it becomes available. Investors and security holders and other interested parties are urged to read the Proxy Statement, and any other documents filed with the SEC when they become available, carefully and in their entirety because they contain important information. Investors and security holders may obtain free copies of the preliminary proxy statement and definitive proxy statement (when available) and other documents filed with the SEC by LifeSci through the website maintained by the SEC at http://www.sec.gov, or by directing a request to: LifeSci Acquisition Corp., 250 W. 55th St., #3401, New York, NY 10019. Participants in the Solicitation LifeSci and Vincera and their respective directors and executive officers and other members of management and employees may be considered participants in the solicitation of proxies with respect to the Proposed Transactions. Information about the directors and executive officers of LifeSci and Vincera will be set forth in the definitive proxy statement and other relevant materials to be filed by LifeSci with the SEC regarding the Proposed Transactions. Stockholders, potential investors and other readers should read the definitive proxy statement carefully when it becomes available before making any voting or investment decisions. These documents can be obtained free of charge from the sources indicated above. No Offer or Solicitation This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act.

Forward-looking Statements This presentation includes certain statements that are not historical facts but are forward-looking statements for within the meaning of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook,” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to: statements regarding estimates and other financial and performance metrics; projections of market opportunity and expectations and average cost per patient; the Company’s mission and business strategy; preclinical and clinical development plan; expected product candidate pipeline and timing; timing of various business milestones, including preclinical and clinical trials and regulatory approval; expected impact and benefits of the Company’s PTEFb platform and bioconjugation platform; capital requirements and expected cash burn; expected use of proceeds; the Company’s ability to obtain and maintain intellectual property protection; LifeSci’s ability to consummate a transaction with the Company; and the expected timing of completion of the Proposed Transactions. These statements are based on various assumptions and on the current expectations of LifeSci’s and the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on as a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. These forward looking statements are subject to a number of risks and uncertainties, including: general economic, financial, legal, political and business conditions and changes in domestic and foreign markets; the potential effects of the COVID-19 pandemic; the inability of the parties to enter into definitive agreements or successfully or timely consummate the Proposed Transactions or to satisfy the other conditions to the closing of the Proposed Transactions, including the risk that the Bayer license agreement is not entered into, and the risk that any required regulatory approvals are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the combined company; risks associated with preclinical or clinical development conducted prior to the Company’s in-licensing; the risk that the approval of the stockholders of LifeSci for the Proposed Transactions is not obtained; failure to realize the anticipated benefits of the Proposed Transactions, including as a result of a delay in consummating the Proposed Transaction or difficulty in, or costs associated with, integrating the businesses of LifeSci and the Company; the amount of redemption requests made by LifeSci’s stockholders; the occurrence of events that may give rise to a right of one or both of LifeSci and the Company to terminate the Business Combination Agreement; risks related to the rollout of the Company’s business and the timing of expected business milestones; changes in the assumptions underlying the Company’s expectations regarding its future business or business model; the Company’s ability to develop and commercialize product candidates; the availability of capital; and the effects of competition on the Company’s future business. If the risks materialize or assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that neither LifeSci nor the Company presently do not know or that LifeSci and the Company currently believe are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. These forward-looking statements speak as of the date hereof, and LifeSci and the Company disclaim any obligation to update these forward-looking statements. Trademarks This presentation contains trademarks, service marks, trade names and copyrights of LifeSci, Vincera and other companies, which are the property of their respective owners.

OUR VISION We aspire to conquer cancer by addressing the unmet medical needs of our patients with paradigm-shifting therapeutics
Cohesive, accomplished management team Highly engaged scientific advisory board and chair Proven track record of successful drug development & approvals, company creation, fundraising and value creation MANAGEMENT TEAM Vincera Pharma Highlights Clinical small molecule: Best-in-class PTEFb [CDK9] inhibitors (oral and IV) in Phase 1; signs of clinical activity in double-hit DLBCL Preclinical bioconjugation platform: SMDC for solid tumors CXCR5 ADC for B-cell malignancies CD123 ADC for AML ASSETS BUSINESS STRATEGY Develop oncology therapies to address unmet patient needs with accelerated approval potential Bayer support in the start-up process Develop each asset to POC and optimize commercial value of each asset INNOVATIVE PROPRIETARY PLATFORMS First- & best-in-class assets Novel bioconjugation platform Small Molecule Drug Conjugate (SMDC) for solid tumors Next generation ADC with novel linker and warhead

Vincera Founders AHMED HAMDY, MD CEO Cofounder of Acerta Pharma Former CMO of Pharmacyclics, leading developer of Imbruvica® Proven track record for assembling experienced teams that deliver from INDs to NDAs RAQUEL IZUMI, PhD COO Cofounder of Acerta Pharma Former Sr Director of Clinical Development of Pharmacyclics Extensive drug development experience from preclinical stages through NDA submission STUART HWANG, PhD VP Business Development Biotech corporate development executive Leadership roles at Quadriga, Astex and Agilent in partnering, licensing & M&A Led drug and diagnostic R&D teams at SuperGen, Cor Tx / Millennium, Celera and UCSF/LBNL TOM THOMAS, JD Outside general counsel Extensive experience in venture, finance, M&A & IPO Partner, Pillsbury Winthrop JOHN BYRD, MD Scientific Advisor: World-renowned leader in drug development in hematologic malignancies (CLL, AML) D Warren Brown Chair of Leukemia Research at OSU, Comprehensive Cancer Center CMO, Beat AML LLC, Leukemia & Lymphoma Society
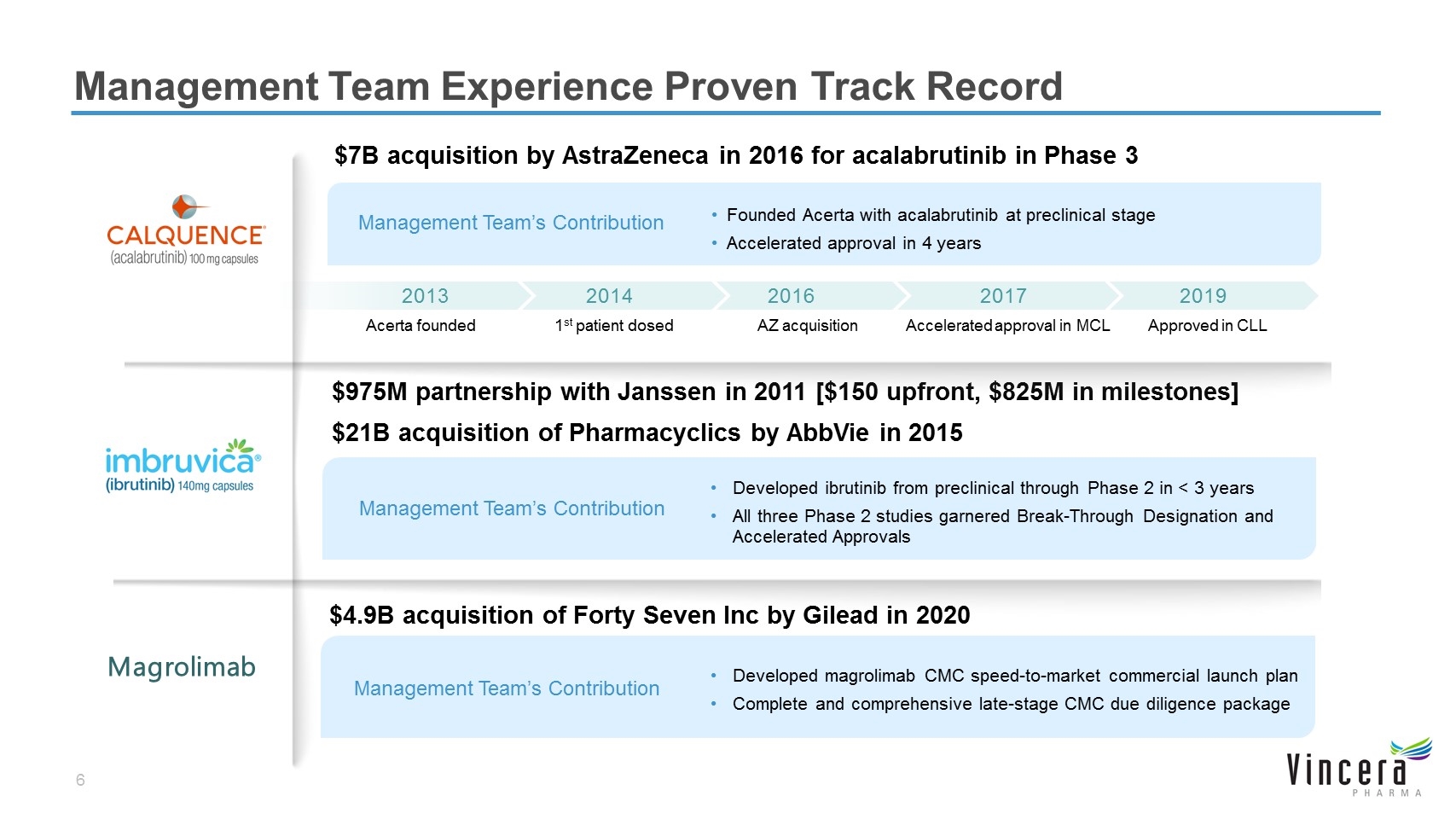
Management Team Experience Proven Track Record $7B acquisition by AstraZeneca in 2016 for acalabrutinib in Phase 3 $975M partnership with Janssen in 2011 [$150 upfront, $825M in milestones] $21B acquisition of Pharmacyclics by AbbVie in 2015 Approved in CLL 2019 Accelerated approval in MCL 2017 AZ acquisition 2016 1st patient dosed 2014 Acerta founded 2013 Developed ibrutinib from preclinical through Phase 2 in < 3 years All three Phase 2 studies garnered Break-Through Designation and Accelerated Approvals Management Team’s Contribution Management Team’s Contribution Founded Acerta with acalabrutinib at preclinical stage Accelerated approval in 4 years Magrolimab $4.9B acquisition of Forty Seven Inc by Gilead in 2020 Management Team’s Contribution Developed magrolimab CMC speed-to-market commercial launch plan Complete and comprehensive late-stage CMC due diligence package
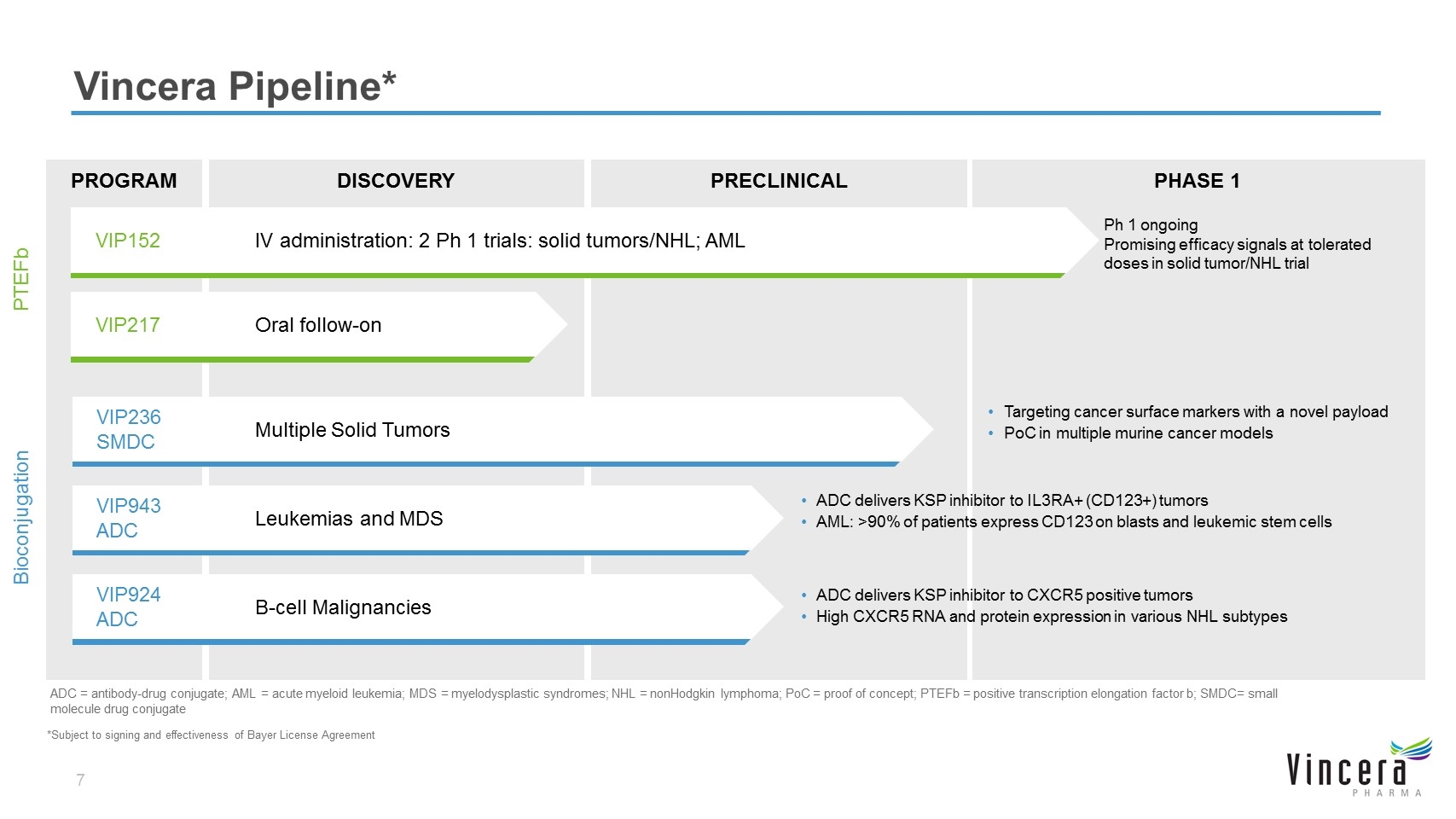
DISCOVERY PRECLINICAL PHASE 1 PROGRAM Vincera Pipeline* PTEFb Bioconjugation ADC = antibody-drug conjugate; AML = acute myeloid leukemia; MDS = myelodysplastic syndromes; NHL = nonHodgkin lymphoma; PoC = proof of concept; PTEFb = positive transcription elongation factor b; SMDC= small molecule drug conjugate Ph 1 ongoing Promising efficacy signals at tolerated doses in solid tumor/NHL trial VIP152IV administration: 2 Ph 1 trials: solid tumors/NHL; AML VIP217Oral follow-on Targeting cancer surface markers with a novel payload PoC in multiple murine cancer models VIP236 SMDC Multiple Solid Tumors ADC delivers KSP inhibitor to IL3RA+ (CD123+) tumors AML: >90% of patients express CD123 on blasts and leukemic stem cells VIP943 ADC Leukemias and MDS ADC delivers KSP inhibitor to CXCR5 positive tumors High CXCR5 RNA and protein expression in various NHL subtypes VIP924 ADC B-cell Malignancies *Subject to signing and effectiveness of Bayer License Agreement

PTEFb PROGRAM VIP152 IV (Phase 1) VIP217 Oral (Discovery)
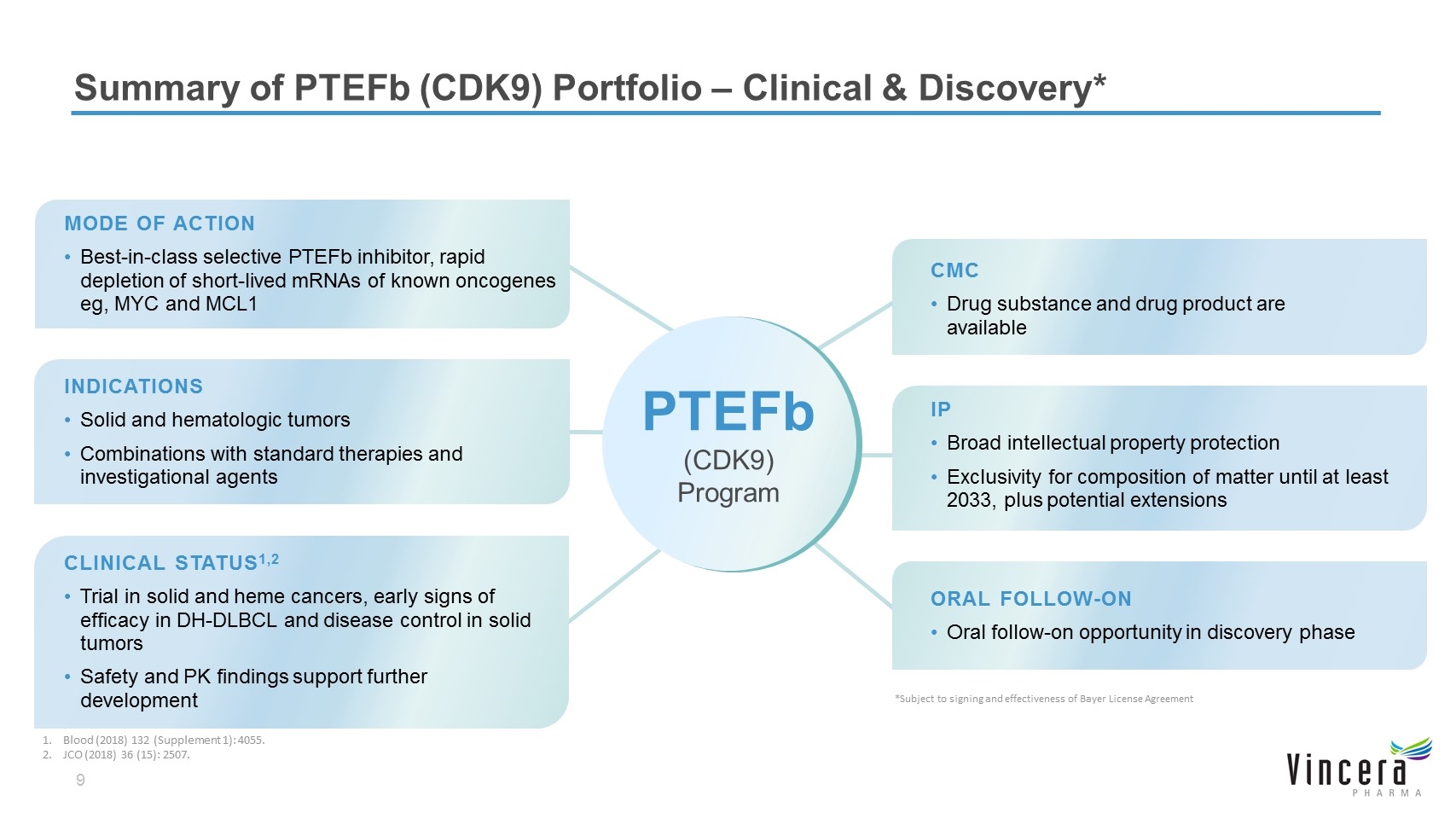
MODE OF ACTION Best-in-class selective PTEFb inhibitor, rapid depletion of short-lived mRNAs of known oncogenes eg, MYC and MCL1 CLINICAL STATUS1,2 Trial in solid and heme cancers, early signs of efficacy in DH-DLBCL and disease control in solid tumors Safety and PK findings support further development CMC Drug substance and drug product are available IP Broad intellectual property protection Exclusivity for composition of matter until at least 2033, plus potential extensions ORAL FOLLOW-ON Oral follow-on opportunity in discovery phase Summary of PTEFb (CDK9) Portfolio – Clinical & Discovery* INDICATIONS Solid and hematologic tumors Combinations with standard therapies and investigational agents PTEFb (CDK9) Program *Subject to signing and effectiveness of Bayer License Agreement Blood (2018) 132 (Supplement 1): 4055. JCO (2018) 36 (15): 2507.
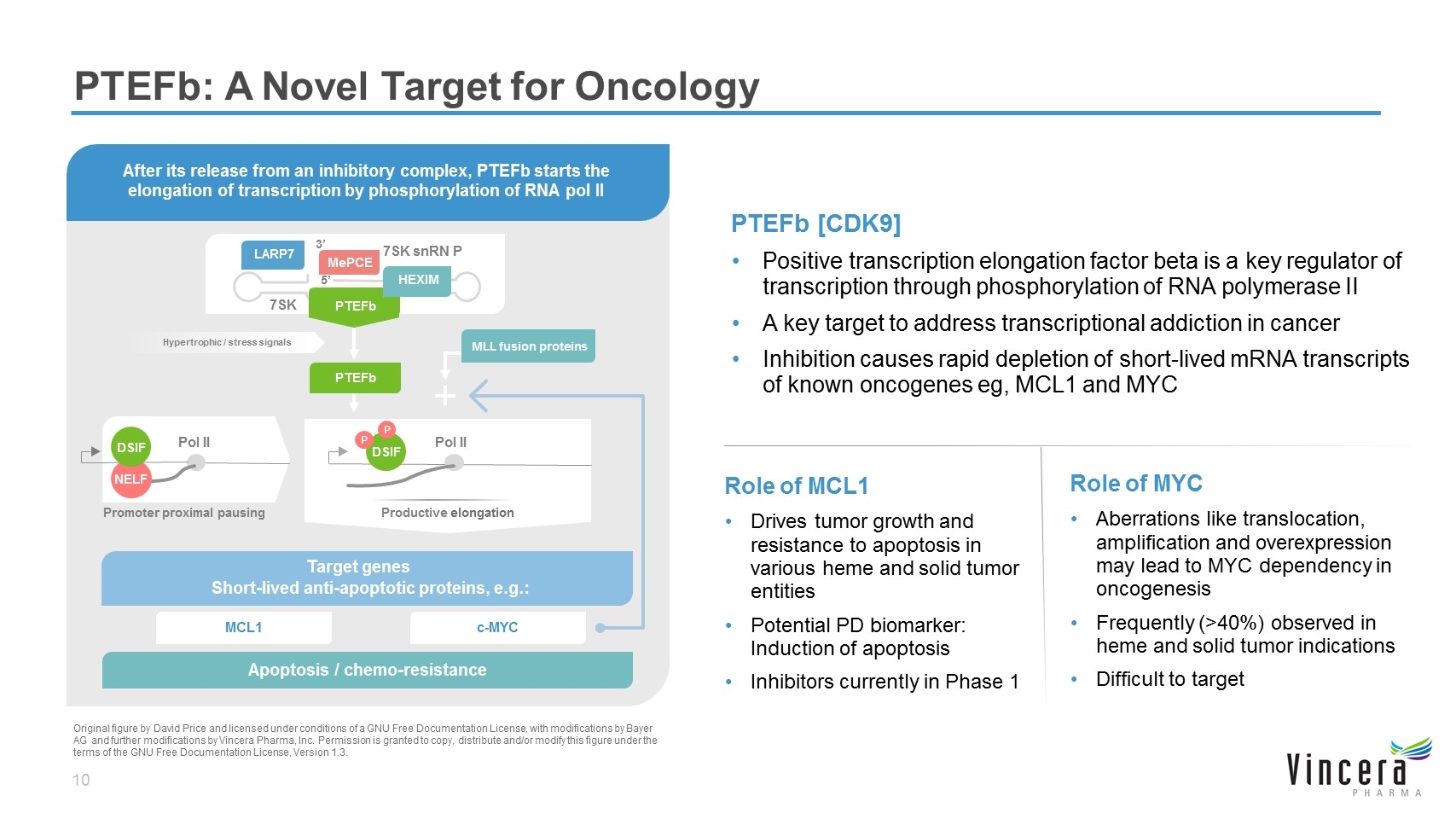
PTEFb [CDK9] Positive transcription elongation factor beta is a key regulator of transcription through phosphorylation of RNA polymerase II A key target to address transcriptional addiction in cancer Inhibition causes rapid depletion of short-lived mRNA transcripts of known oncogenes eg, MCL1 and MYC PTEFb: A Novel Target for Oncology Target genes Short-lived anti-apoptotic proteins, e.g.: Apoptosis / chemo-resistance Hypertrophic / stress signals Pol II Promoter proximal pausing Pol II PTEFb 7SK snRN P 7SK 5’ 3’ MCL1 c-MYC DSIF P P NELF DSIF MePCE LARP7 MLL fusion proteins HEXIM Original figure by David Price and licensed under conditions of a GNU Free Documentation License, with modifications by Bayer AG and further modifications by Vincera Pharma, Inc. Permission is granted to copy, distribute and/or modify this figure under the terms of the GNU Free Documentation License, Version 1.3. After its release from an inhibitory complex, PTEFb starts the elongation of transcription by phosphorylation of RNA pol II Productive elongation PTEFb Role of MYC Aberrations like translocation, amplification and overexpression may lead to MYC dependency in oncogenesis Frequently (>40%) observed in heme and solid tumor indications Difficult to target Role of MCL1 Drives tumor growth and resistance to apoptosis in various heme and solid tumor entities Potential PD biomarker: Induction of apoptosis Inhibitors currently in Phase 1
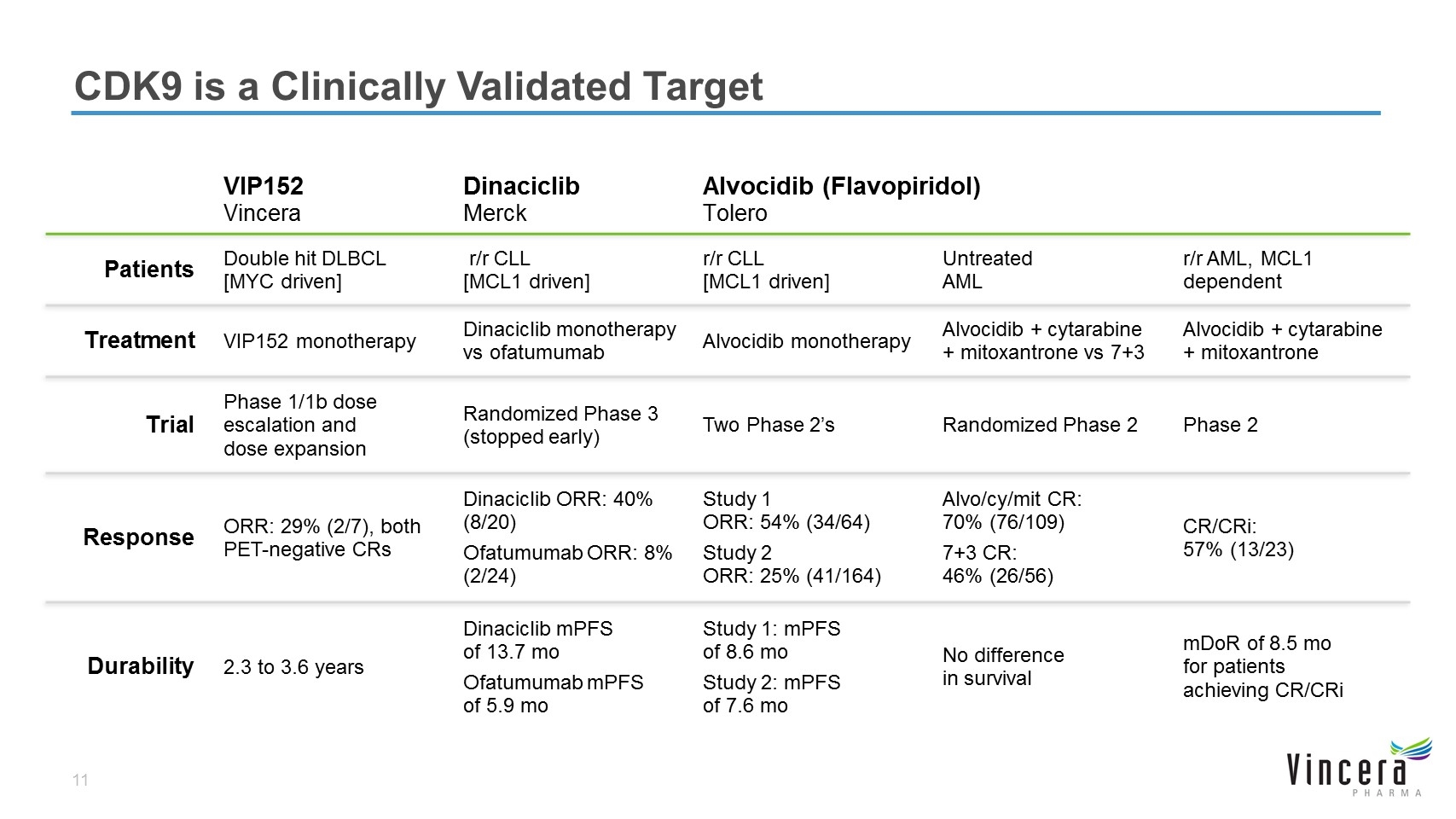
VIP152 Vincera Dinaciclib Merck Alvocidib (Flavopiridol) Tolero Patients Double hit DLBCL [MYC driven] r/r CLL [MCL1 driven] r/r CLL [MCL1 driven] Untreated AML r/r AML, MCL1 dependent Treatment VIP152 monotherapy Dinaciclib monotherapy vs ofatumumab Alvocidib monotherapy Alvocidib + cytarabine + mitoxantrone vs 7+3 Alvocidib + cytarabine + mitoxantrone Trial Phase 1/1b dose escalation and dose expansion Randomized Phase 3 (stopped early) Two Phase 2’s Randomized Phase 2 Phase 2 Response ORR: 29% (2/7), both PET-negative CRs Dinaciclib ORR: 40% (8/20) Ofatumumab ORR: 8% (2/24) Study 1 ORR: 54% (34/64) Study 2 ORR: 25% (41/164) Alvo/cy/mit CR: 70% (76/109) 7+3 CR: 46% (26/56) CR/CRi: 57% (13/23) Durability 2.3 to 3.6 years Dinaciclib mPFS of 13.7 mo Ofatumumab mPFS of 5.9 mo Study 1: mPFS of 8.6 mo Study 2: mPFS of 7.6 mo No difference in survival mDoR of 8.5 mo for patients achieving CR/CRi CDK9 is a Clinically Validated Target
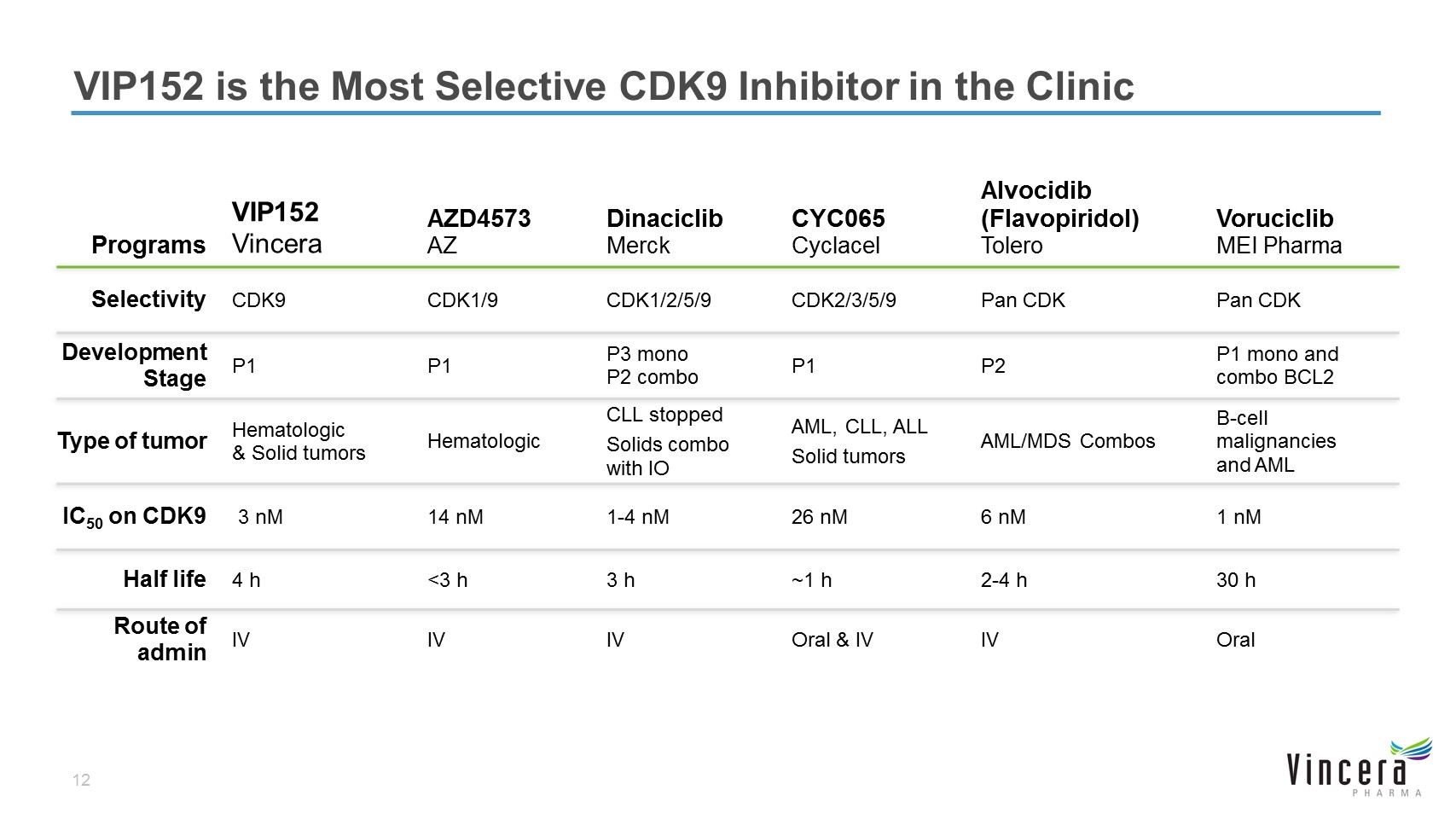
VIP152 is the Most Selective CDK9 Inhibitor in the Clinic Programs VIP152 Vincera AZD4573 AZ Dinaciclib Merck CYC065 Cyclacel Alvocidib (Flavopiridol) Tolero Voruciclib MEI Pharma Selectivity CDK9 CDK1/9 CDK1/2/5/9 CDK2/3/5/9 Pan CDK Pan CDK Development Stage P1 P1 P3 mono P2 combo P1 P2 P1 mono and combo BCL2 Type of tumor Hematologic & Solid tumors Hematologic CLL stopped Solids combo with IO AML, CLL, ALL Solid tumors AML/MDS Combos B-cell malignancies and AML IC50 on CDK9 3 nM 14 nM 1-4 nM 26 nM 6 nM 1 nM Half life 4 h <3 h 3 h ~1 h 2-4 h 30 h Route of admin IV IV IV Oral & IV IV Oral
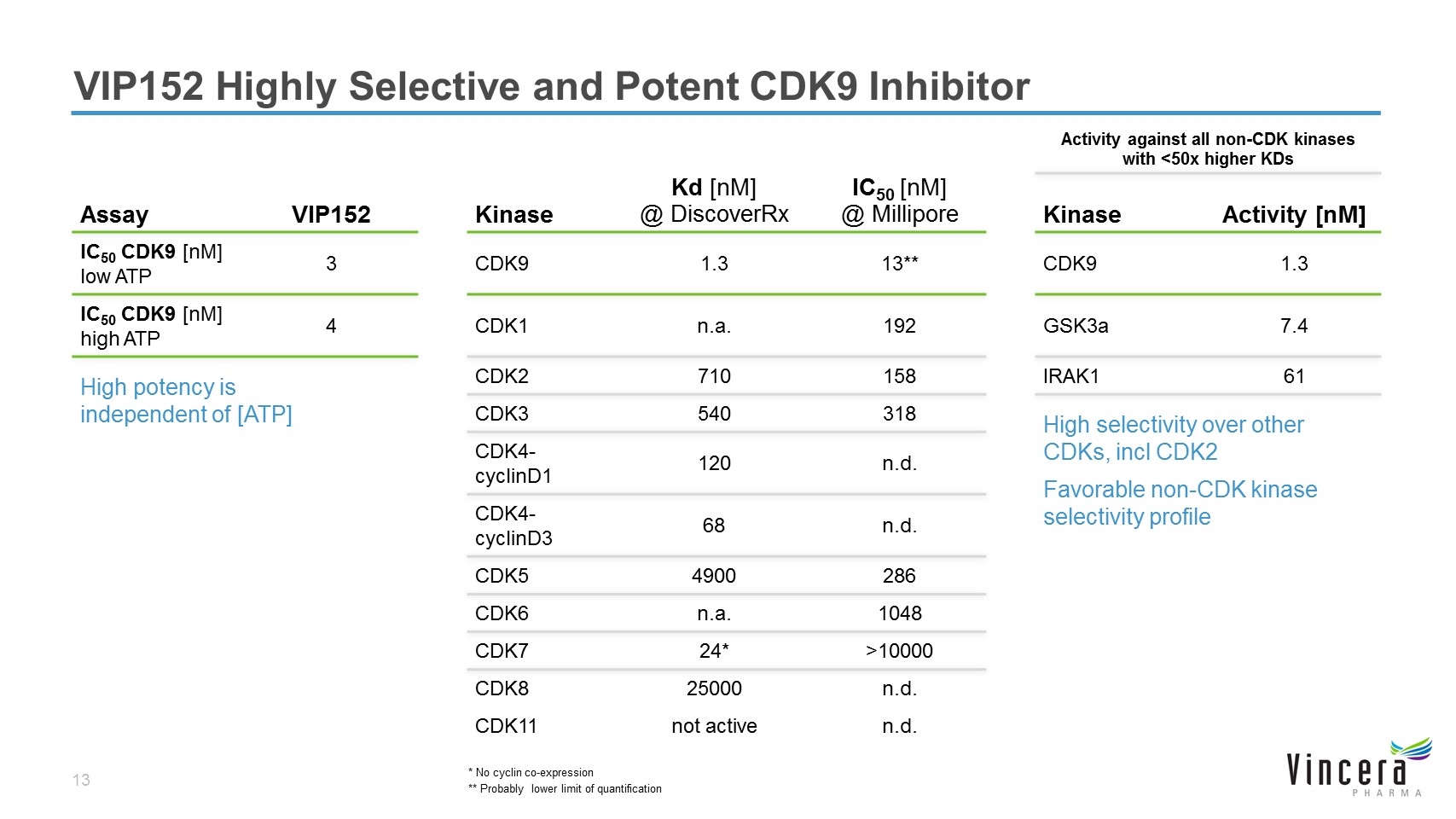
VIP152 Highly Selective and Potent CDK9 Inhibitor Activity against all non-CDK kinases with <50x higher KDs Assay VIP152 Kinase Kd [nM] @ DiscoverRx IC50 [nM] @ Millipore Kinase Activity [nM] IC50 CDK9 [nM] low ATP 3 CDK9 1.3 13** CDK9 1.3 IC50 CDK9 [nM] high ATP 4 CDK1 n.a. 192 GSK3a 7.4 High potency is independent of [ATP] CDK2 710 158 IRAK1 61 CDK3 540 318 High selectivity over other CDKs, incl CDK2 Favorable non-CDK kinase selectivity profile CDK4-cyclinD1 120 n.d. CDK4-cyclinD3 68 n.d. CDK5 4900 286 CDK6 n.a. 1048 CDK7 24* >10000 CDK8 25000 n.d. CDK11 not active n.d. * No cyclin co-expression ** Probably lower limit of quantification
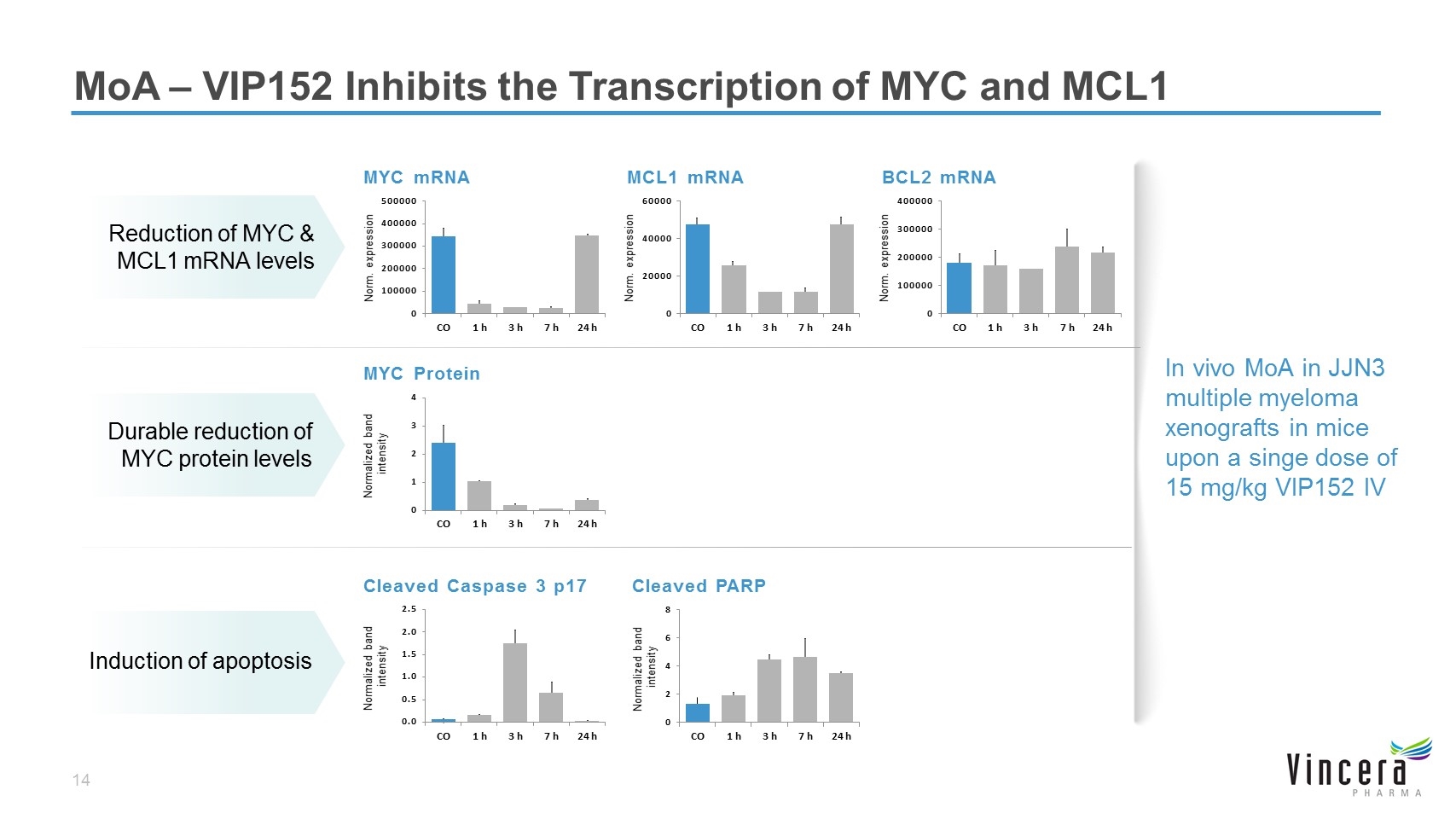
MoA – VIP152 Inhibits the Transcription of MYC and MCL1 Normalized band intensity MYC Protein Durable reduction of MYC protein levels In vivo MoA in JJN3 multiple myeloma xenografts in mice upon a singe dose of 15 mg/kg VIP152 IV Reduction of MYC & MCL1 mRNA levels Norm. expression MYC mRNA MCL1 mRNA Norm. expression BCL2 mRNA Norm. expression Induction of apoptosis Normalized band intensity Cleaved Caspase 3 p17 Cleaved PARP Normalized band intensity
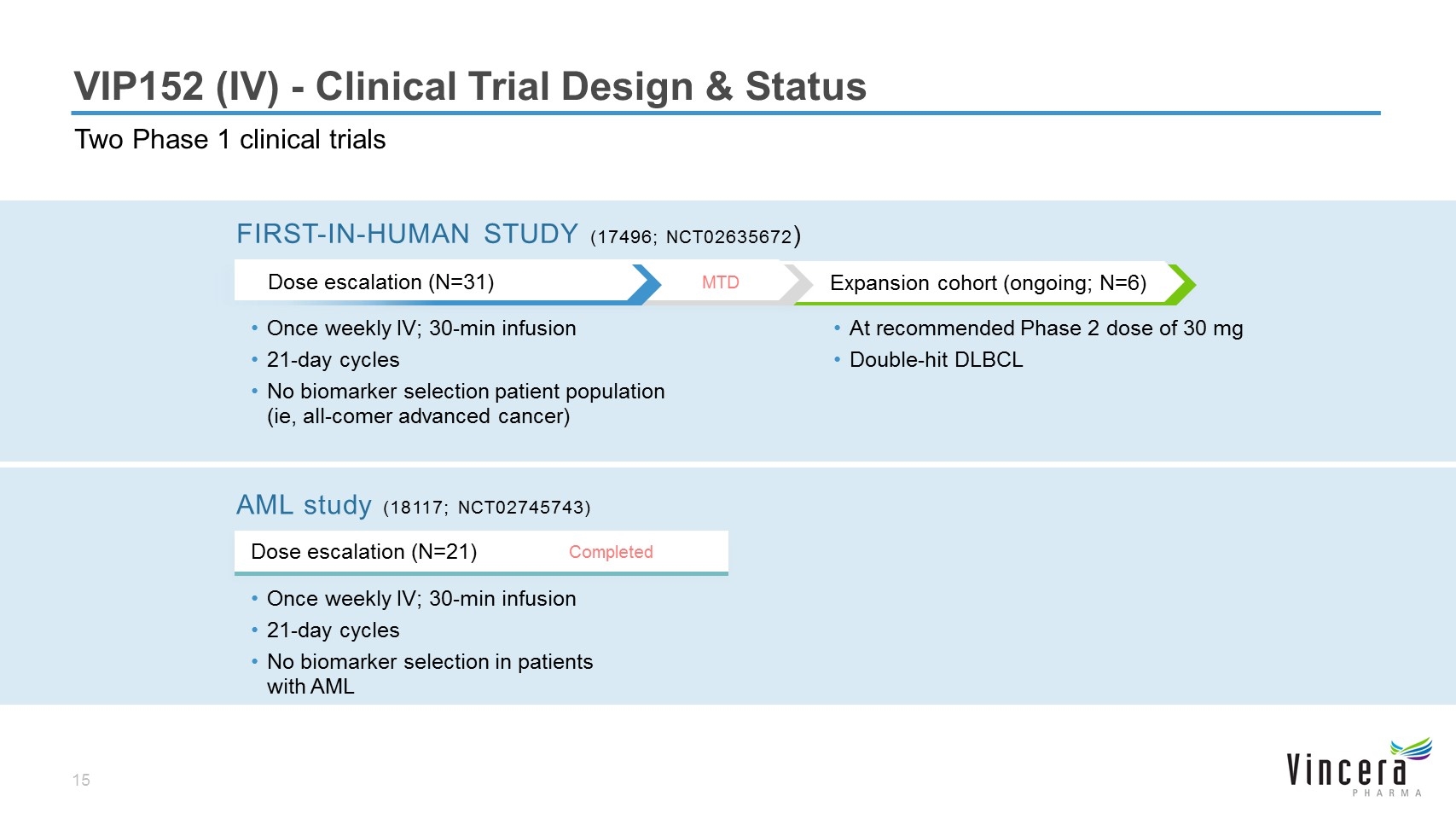
Expansion cohort (ongoing; N=6) VIP152 (IV) - Clinical Trial Design & Status Two Phase 1 clinical trials Once weekly IV; 30-min infusion 21-day cycles No biomarker selection patient population (ie, all-comer advanced cancer) At recommended Phase 2 dose of 30 mg Double-hit DLBCL MTD FIRST-IN-HUMAN STUDY (17496; NCT02635672) Once weekly IV; 30-min infusion 21-day cycles No biomarker selection in patients with AML Dose escalation (N=21) AML study (18117; NCT02745743) Completed Dose escalation (N=31)
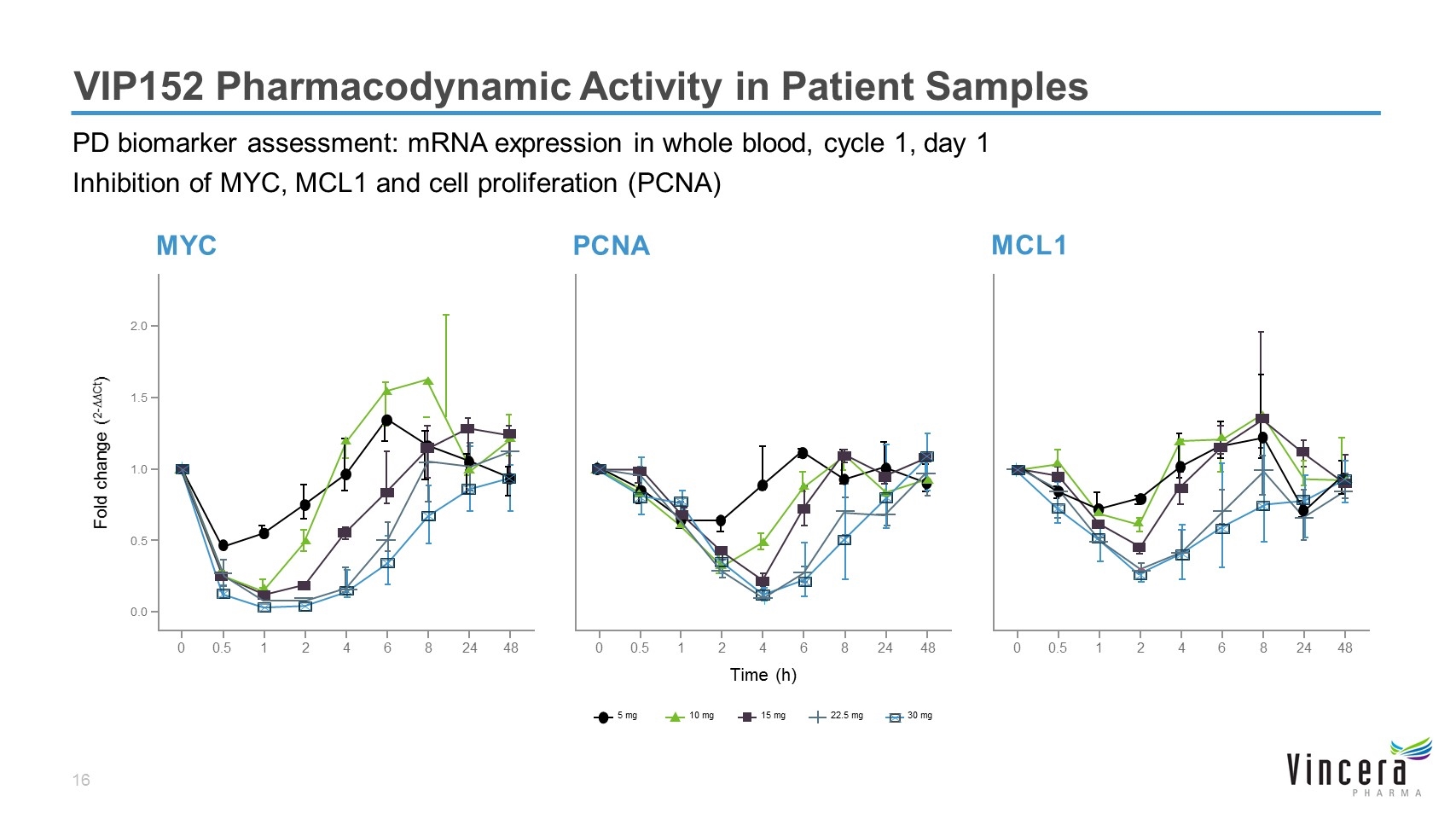
VIP152 Pharmacodynamic Activity in Patient Samples PD biomarker assessment: mRNA expression in whole blood, cycle 1, day 1 Inhibition of MYC, MCL1 and cell proliferation (PCNA) Time (h) Fold change (2-∆∆Ct) MYC PCNA MCL1 5 mg 10 mg 15 mg 22.5 mg 30 mg 0 0.5 1 2 4 6 8 24 48 0.0 0.5 1.0 1.5 2.0 0 0.5 1 2 4 6 8 24 48 0 0.5 1 2 4 6 8 24 48
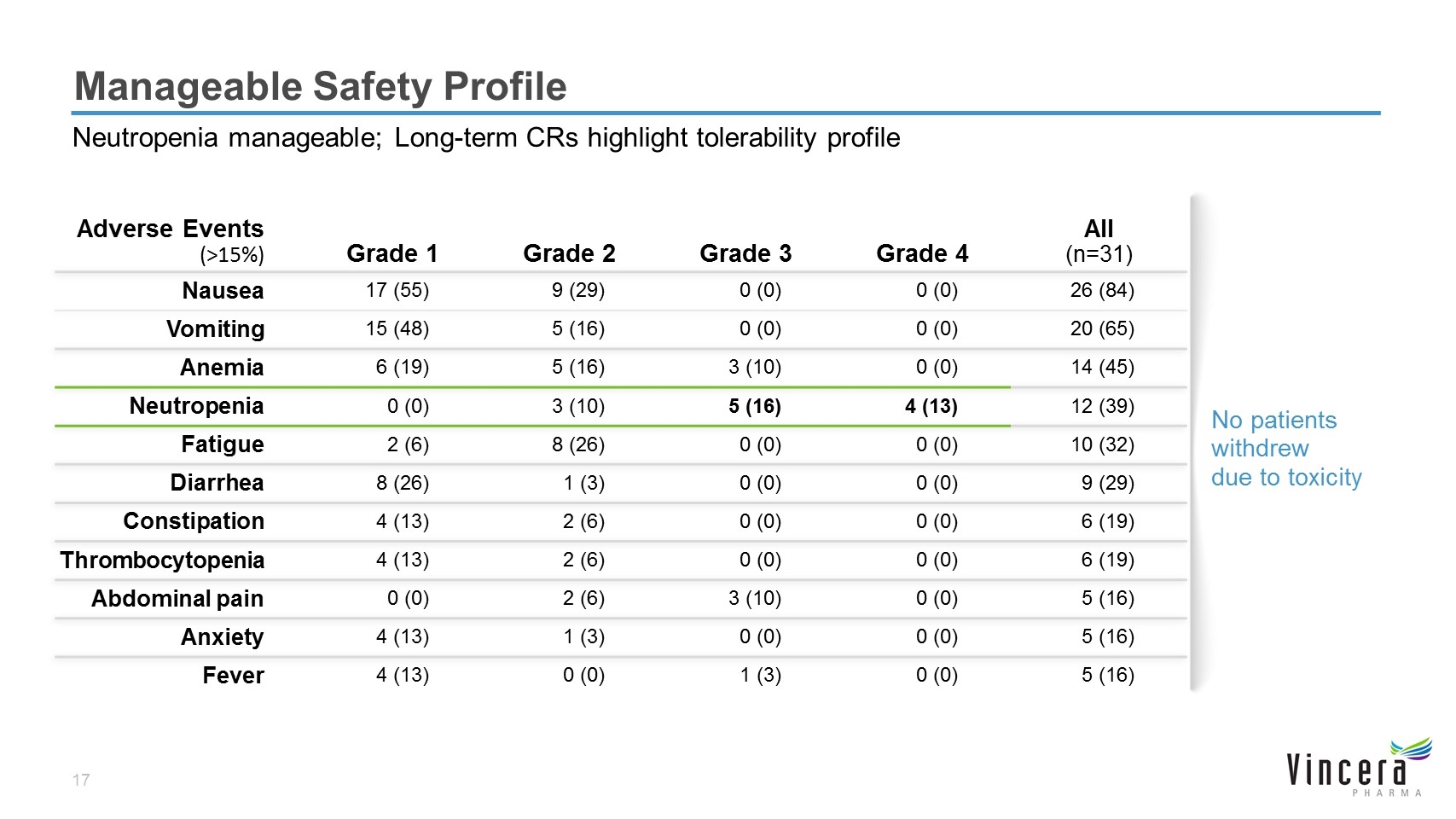
No patients withdrew due to toxicity Manageable Safety Profile Neutropenia manageable; Long-term CRs highlight tolerability profile Adverse Events (>15%) Grade 1 Grade 2 Grade 3 Grade 4 All (n=31) Nausea 17 (55) 9 (29) 0 (0) 0 (0) 26 (84) Vomiting 15 (48) 5 (16) 0 (0) 0 (0) 20 (65) Anemia 6 (19) 5 (16) 3 (10) 0 (0) 14 (45) Neutropenia 0 (0) 3 (10) 5 (16) 4 (13) 12 (39) Fatigue 2 (6) 8 (26) 0 (0) 0 (0) 10 (32) Diarrhea 8 (26) 1 (3) 0 (0) 0 (0) 9 (29) Constipation 4 (13) 2 (6) 0 (0) 0 (0) 6 (19) Thrombocytopenia 4 (13) 2 (6) 0 (0) 0 (0) 6 (19) Abdominal pain 0 (0) 2 (6) 3 (10) 0 (0) 5 (16) Anxiety 4 (13) 1 (3) 0 (0) 0 (0) 5 (16) Fever 4 (13) 0 (0) 1 (3) 0 (0) 5 (16)
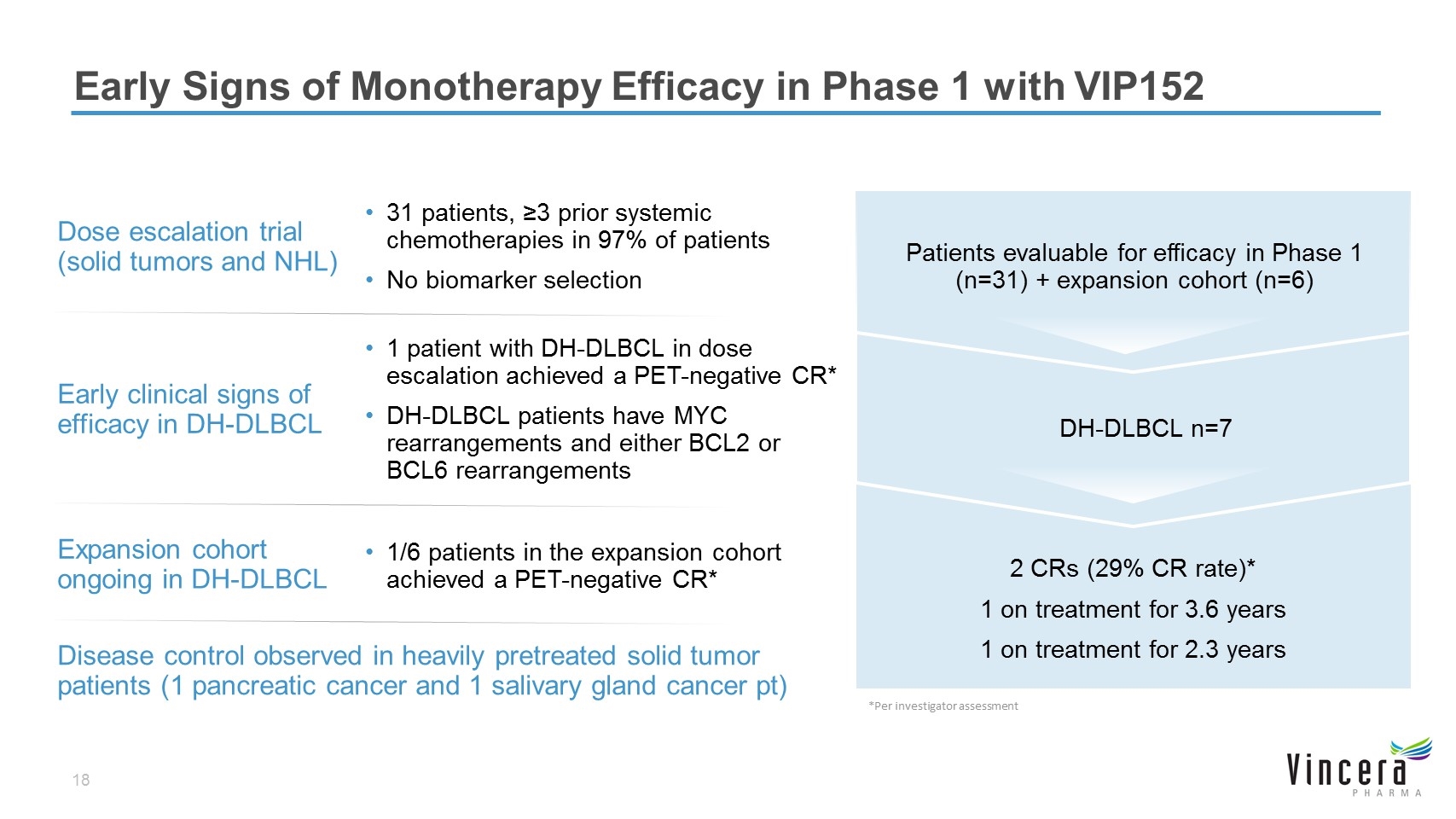
Early Signs of Monotherapy Efficacy in Phase 1 with VIP152 2 CRs (29% CR rate)* 1 on treatment for 3.6 years 1 on treatment for 2.3 years Disease control observed in heavily pretreated solid tumor patients (1 pancreatic cancer and 1 salivary gland cancer pt) Expansion cohort ongoing in DH-DLBCL 1/6 patients in the expansion cohort achieved a PET-negative CR* Patients evaluable for efficacy in Phase 1 (n=31) + expansion cohort (n=6) Dose escalation trial (solid tumors and NHL) 31 patients, ≥3 prior systemic chemotherapies in 97% of patients No biomarker selection DH-DLBCL n=7 Early clinical signs of efficacy in DH-DLBCL 1 patient with DH-DLBCL in dose escalation achieved a PET-negative CR* DH-DLBCL patients have MYC rearrangements and either BCL2 or BCL6 rearrangements *Per investigator assessment
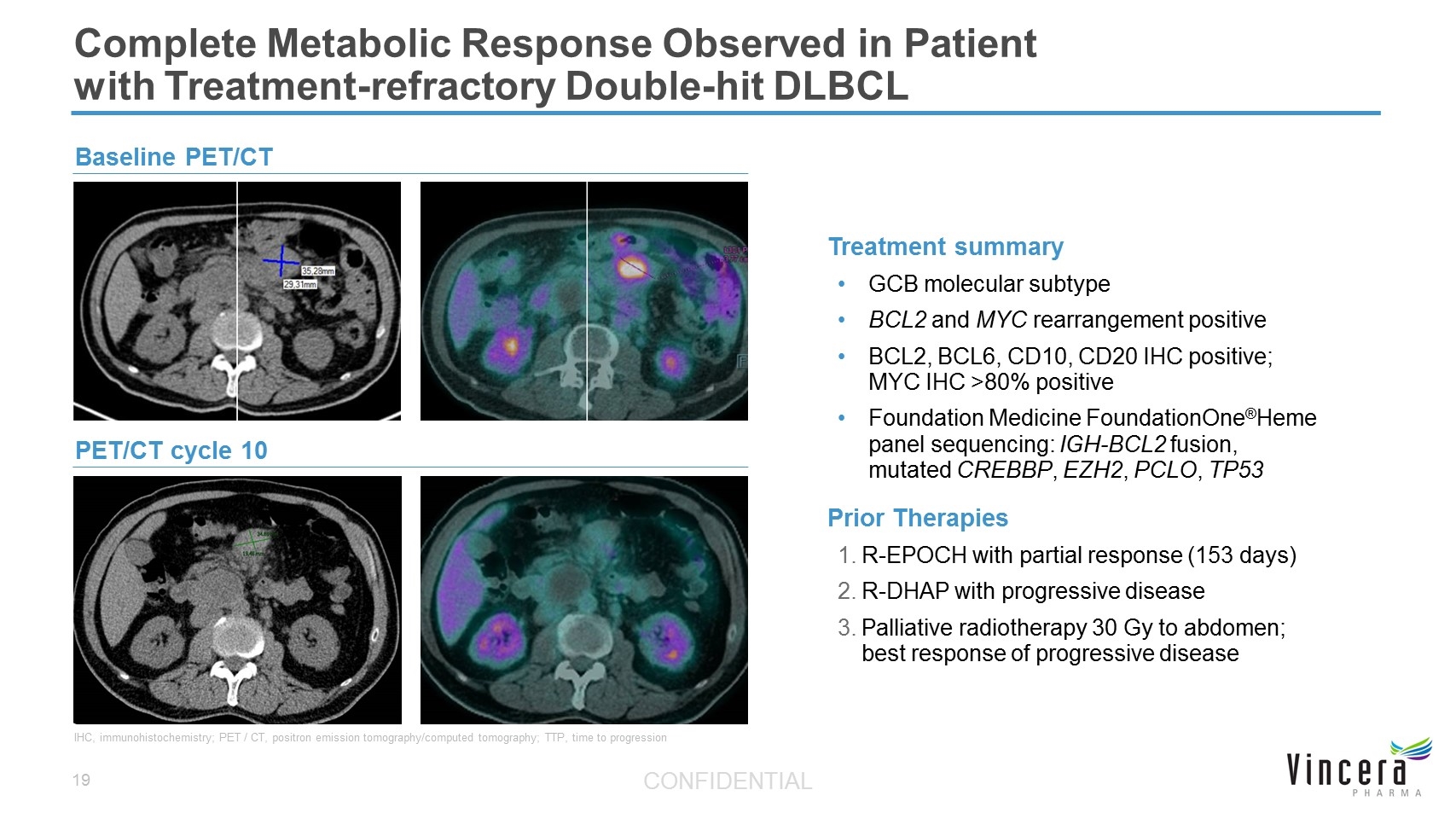
Complete Metabolic Response Observed in Patient with Treatment-refractory Double-hit DLBCL Treatment summary GCB molecular subtype BCL2 and MYC rearrangement positive BCL2, BCL6, CD10, CD20 IHC positive; MYC IHC >80% positive Foundation Medicine FoundationOne®Heme panel sequencing: IGH-BCL2 fusion, mutated CREBBP, EZH2, PCLO, TP53 Prior Therapies R-EPOCH with partial response (153 days) R-DHAP with progressive disease Palliative radiotherapy 30 Gy to abdomen; best response of progressive disease IHC, immunohistochemistry; PET / CT, positron emission tomography/computed tomography; TTP, time to progression PET/CT cycle 10 Baseline PET/CT CONFIDENTIAL
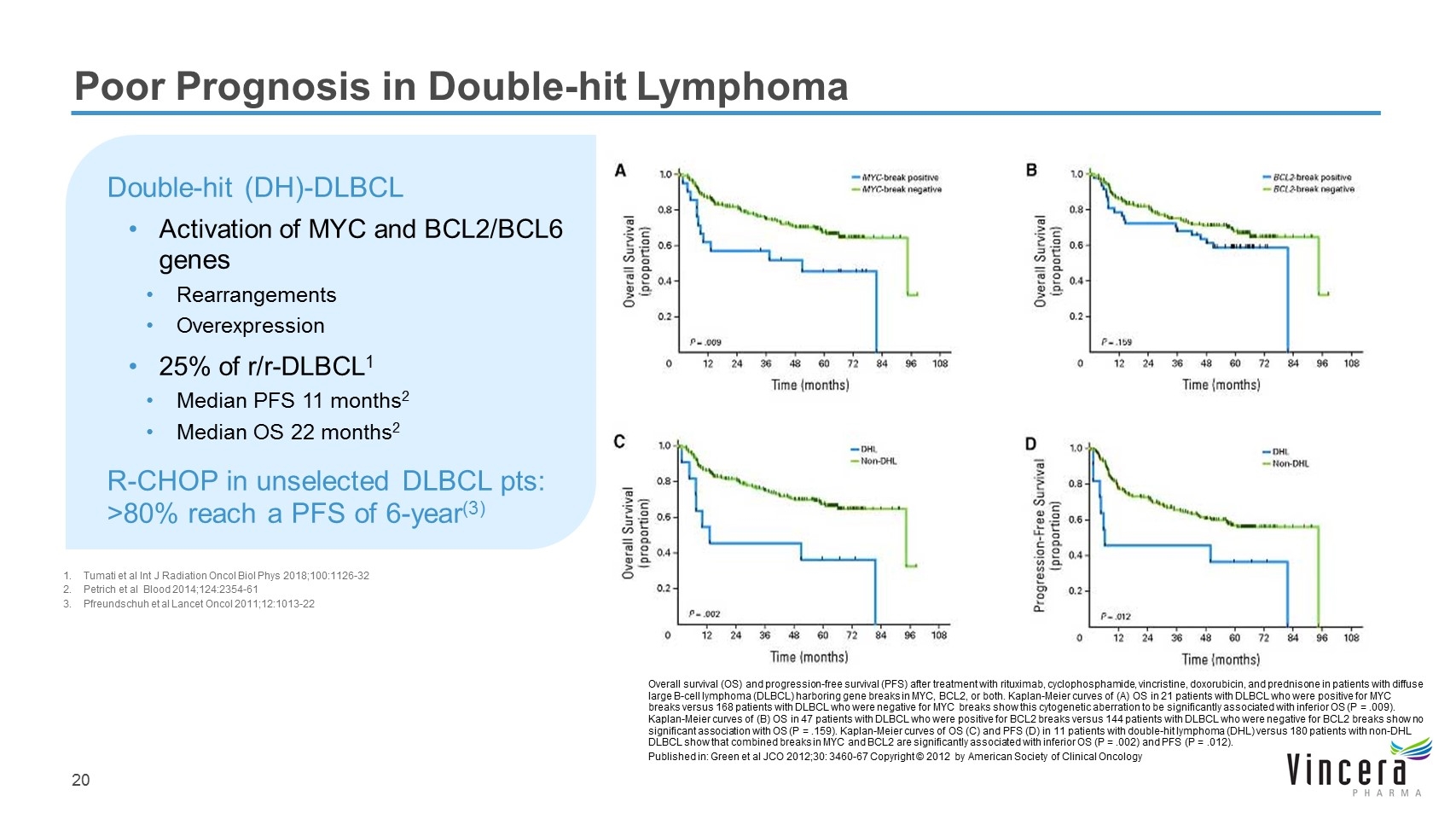
Tumati et al Int J Radiation Oncol Biol Phys 2018;100:1126-32 Petrich et al Blood 2014;124:2354-61 Pfreundschuh et al Lancet Oncol 2011;12:1013-22 Overall survival (OS) and progression-free survival (PFS) after treatment with rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone in patients with diffuse large B-cell lymphoma (DLBCL) harboring gene breaks in MYC, BCL2, or both. Kaplan-Meier curves of (A) OS in 21 patients with DLBCL who were positive for MYC breaks versus 168 patients with DLBCL who were negative for MYC breaks show this cytogenetic aberration to be significantly associated with inferior OS (P = .009). Kaplan-Meier curves of (B) OS in 47 patients with DLBCL who were positive for BCL2 breaks versus 144 patients with DLBCL who were negative for BCL2 breaks show no significant association with OS (P = .159). Kaplan-Meier curves of OS (C) and PFS (D) in 11 patients with double-hit lymphoma (DHL) versus 180 patients with non-DHL DLBCL show that combined breaks in MYC and BCL2 are significantly associated with inferior OS (P = .002) and PFS (P = .012). Published in: Green et al JCO 2012;30: 3460-67 Copyright © 2012 by American Society of Clinical Oncology Double-hit (DH)-DLBCL Activation of MYC and BCL2/BCL6 genes Rearrangements Overexpression 25% of r/r-DLBCL1 Median PFS 11 months2 Median OS 22 months2 R-CHOP in unselected DLBCL pts: >80% reach a PFS of 6-year(3) Poor Prognosis in Double-hit Lymphoma
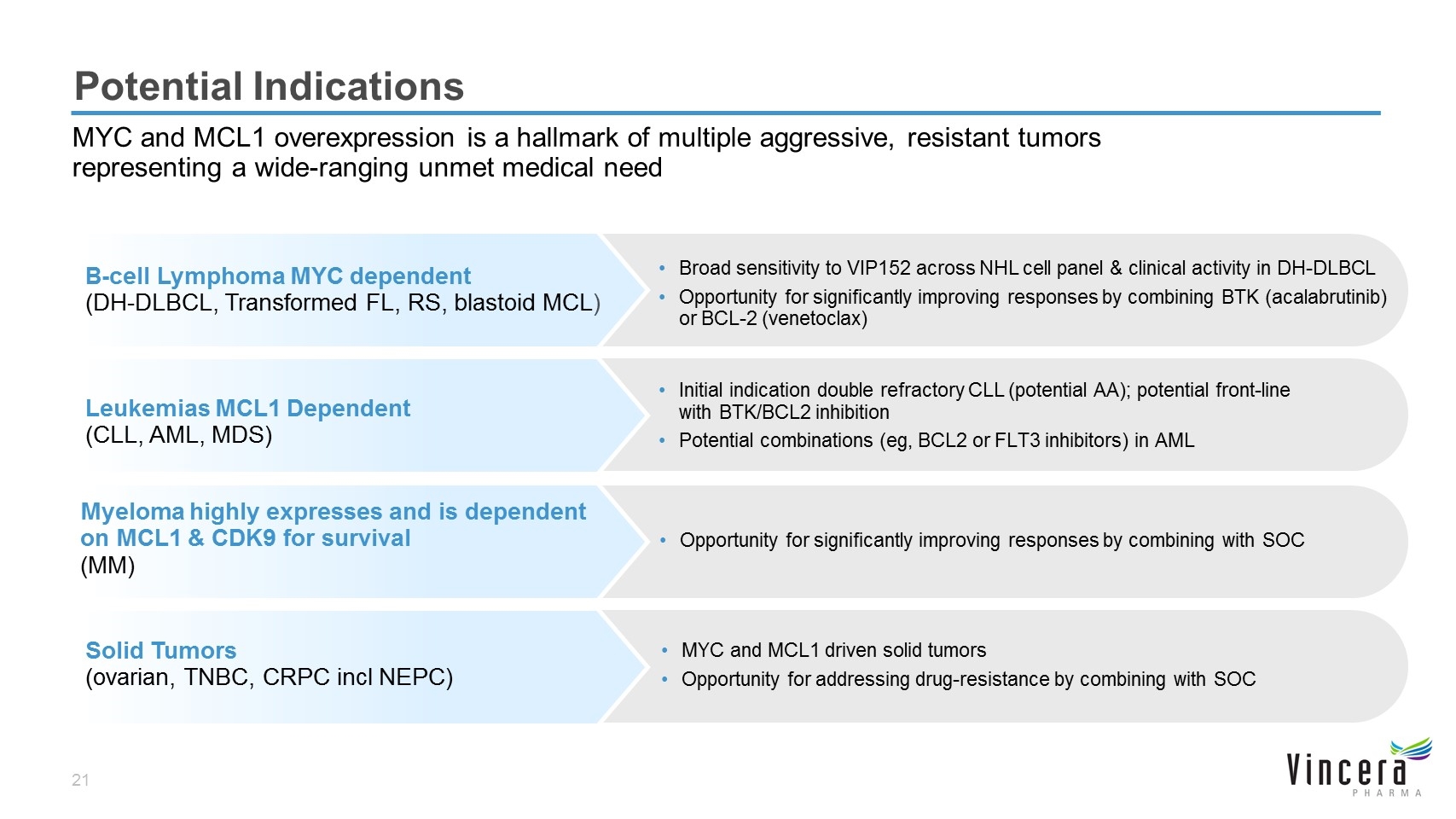
Potential Indications MYC and MCL1 overexpression is a hallmark of multiple aggressive, resistant tumors representing a wide-ranging unmet medical need B-cell Lymphoma MYC dependent (DH-DLBCL, Transformed FL, RS, blastoid MCL) Broad sensitivity to VIP152 across NHL cell panel & clinical activity in DH-DLBCL Opportunity for significantly improving responses by combining BTK (acalabrutinib) or BCL-2 (venetoclax) Leukemias MCL1 Dependent (CLL, AML, MDS) Initial indication double refractory CLL (potential AA); potential front-line with BTK/BCL2 inhibition Potential combinations (eg, BCL2 or FLT3 inhibitors) in AML Solid Tumors (ovarian, TNBC, CRPC incl NEPC) MYC and MCL1 driven solid tumors Opportunity for addressing drug-resistance by combining with SOC Myeloma highly expresses and is dependent on MCL1 & CDK9 for survival (MM) Opportunity for significantly improving responses by combining with SOC
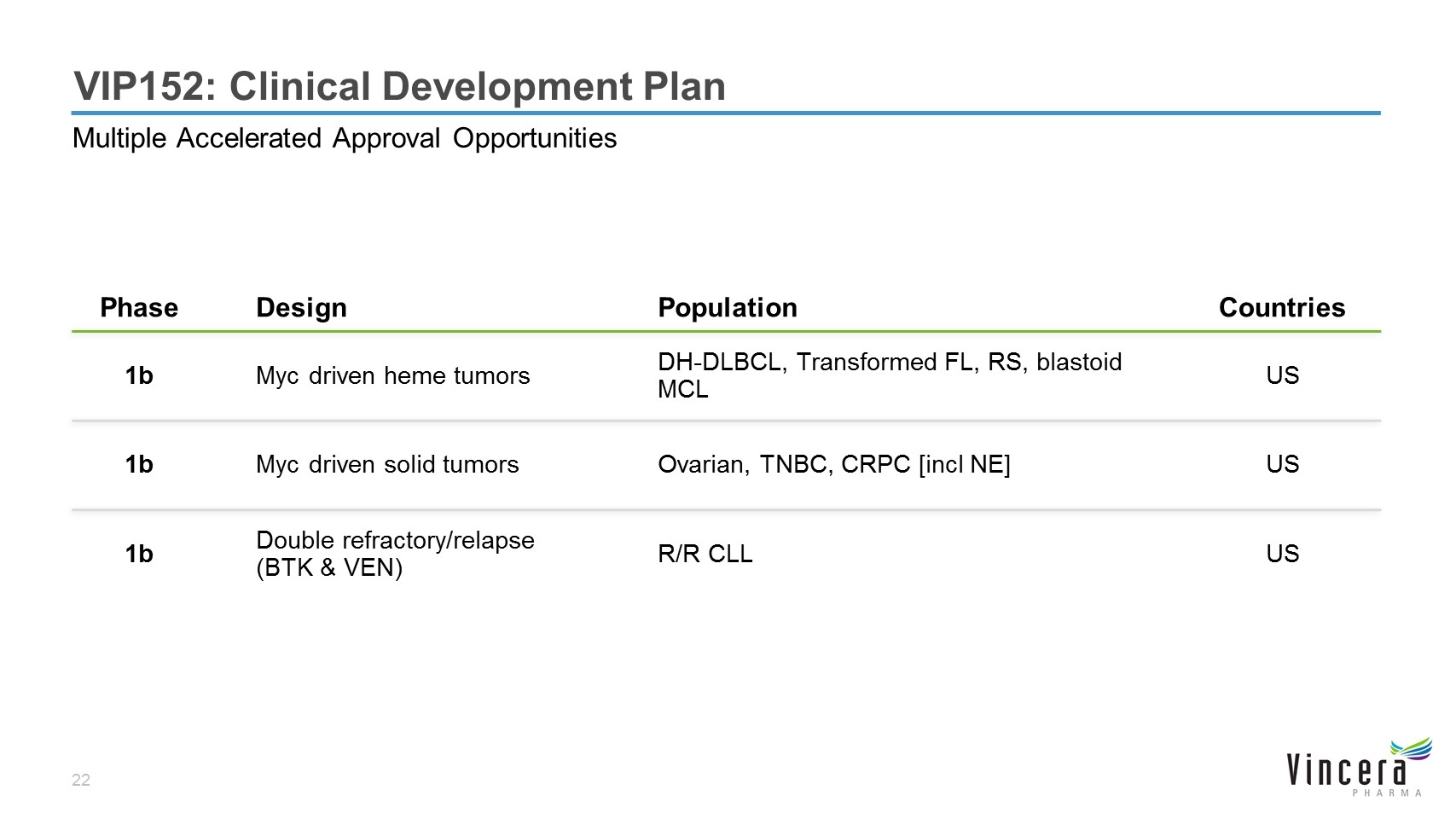
VIP152: Clinical Development Plan Multiple Accelerated Approval Opportunities Phase Design Population Countries 1b Myc driven heme tumors DH-DLBCL, Transformed FL, RS, blastoid MCL US 1b Myc driven solid tumors Ovarian, TNBC, CRPC [incl NE] US 1b Double refractory/relapse (BTK & VEN) R/R CLL US
Summary: PTEFb Portfolio* PTEFb INHIBITORS WITH BROAD CLINICAL POTENTIAL BEST-IN-CLASS PHARMACOLOGY AND PHARMACODYNAMIC PROFILE FAVORABLE ROBUST PRECLINICAL IN VIVO AND IN VITRO DATA CLEAR DEVELOPMENT PATHS IN HIGH UNMET MEDICAL NEEDS EARLY SIGNS OF SINGLE-AGENT CLINICAL EFFICACY SIGNIFICANT COMMERCIAL POTENTIAL ACROSS INDICATIONS IP PROTECTION UNTIL 2033 (POTENTIAL FOR EXTENSION) PTEFb Portfolio *Subject to signing and effectiveness of Bayer License Agreement
BIOCONJUGATION PLATFORM VIP236 (SMDC) VIP943 (CD123) VIP924 (CXCR5)
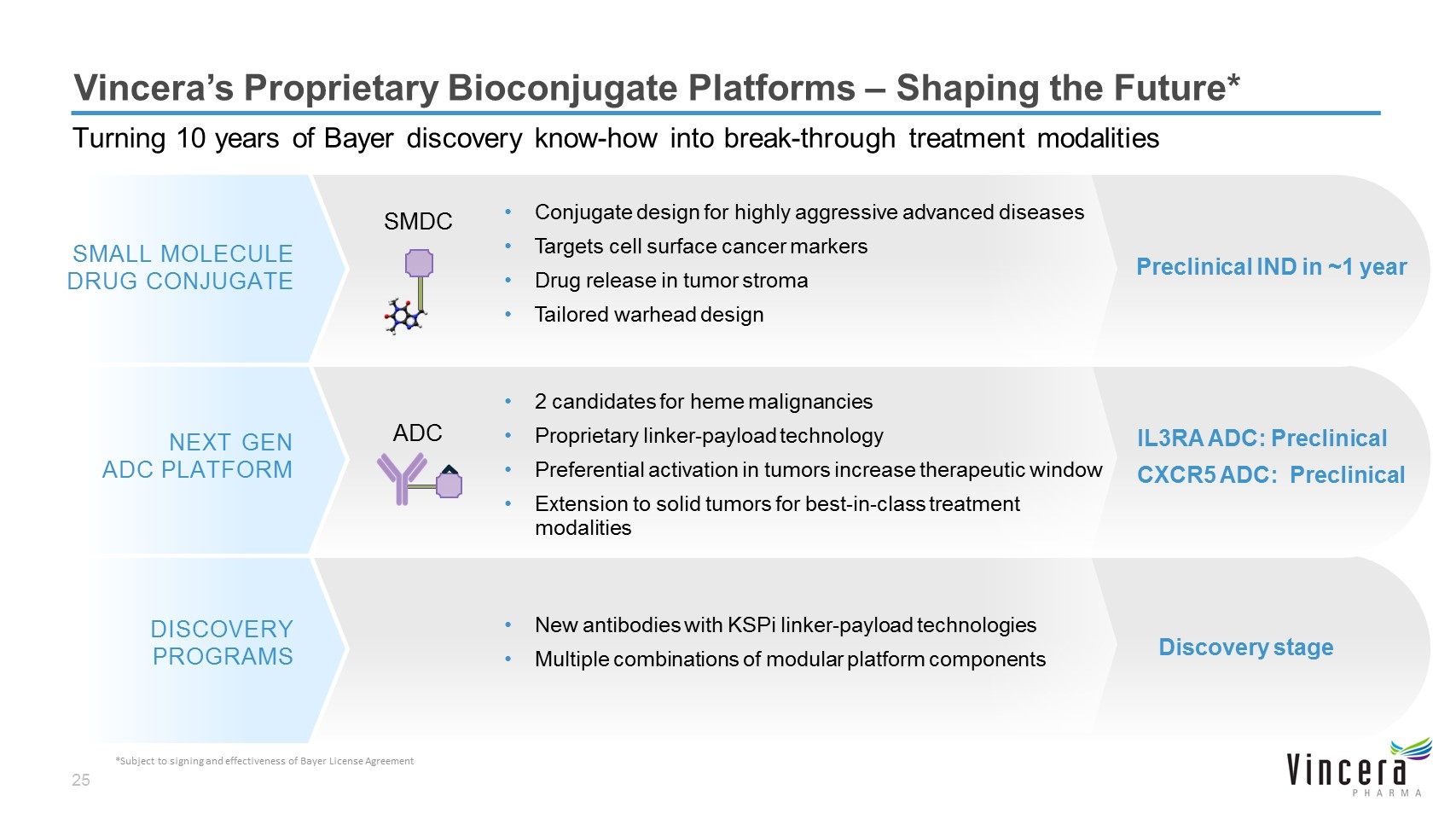
Vincera’s Proprietary Bioconjugate Platforms – Shaping the Future* Turning 10 years of Bayer discovery know-how into break-through treatment modalities NEXT GEN ADC PLATFORM IL3RA ADC: Preclinical CXCR5 ADC: Preclinical SMALL MOLECULE DRUG CONJUGATE Preclinical IND in ~1 year DISCOVERY PROGRAMS Discovery stage 2 candidates for heme malignancies Proprietary linker-payload technology Preferential activation in tumors increase therapeutic window Extension to solid tumors for best-in-class treatment modalities Conjugate design for highly aggressive advanced diseases Targets cell surface cancer markers Drug release in tumor stroma Tailored warhead design New antibodies with KSPi linker-payload technologies Multiple combinations of modular platform components ADC SMDC *Subject to signing and effectiveness of Bayer License Agreement

SMALL MOLECULE DRUG CONJUGATE (SMDC) VIP236
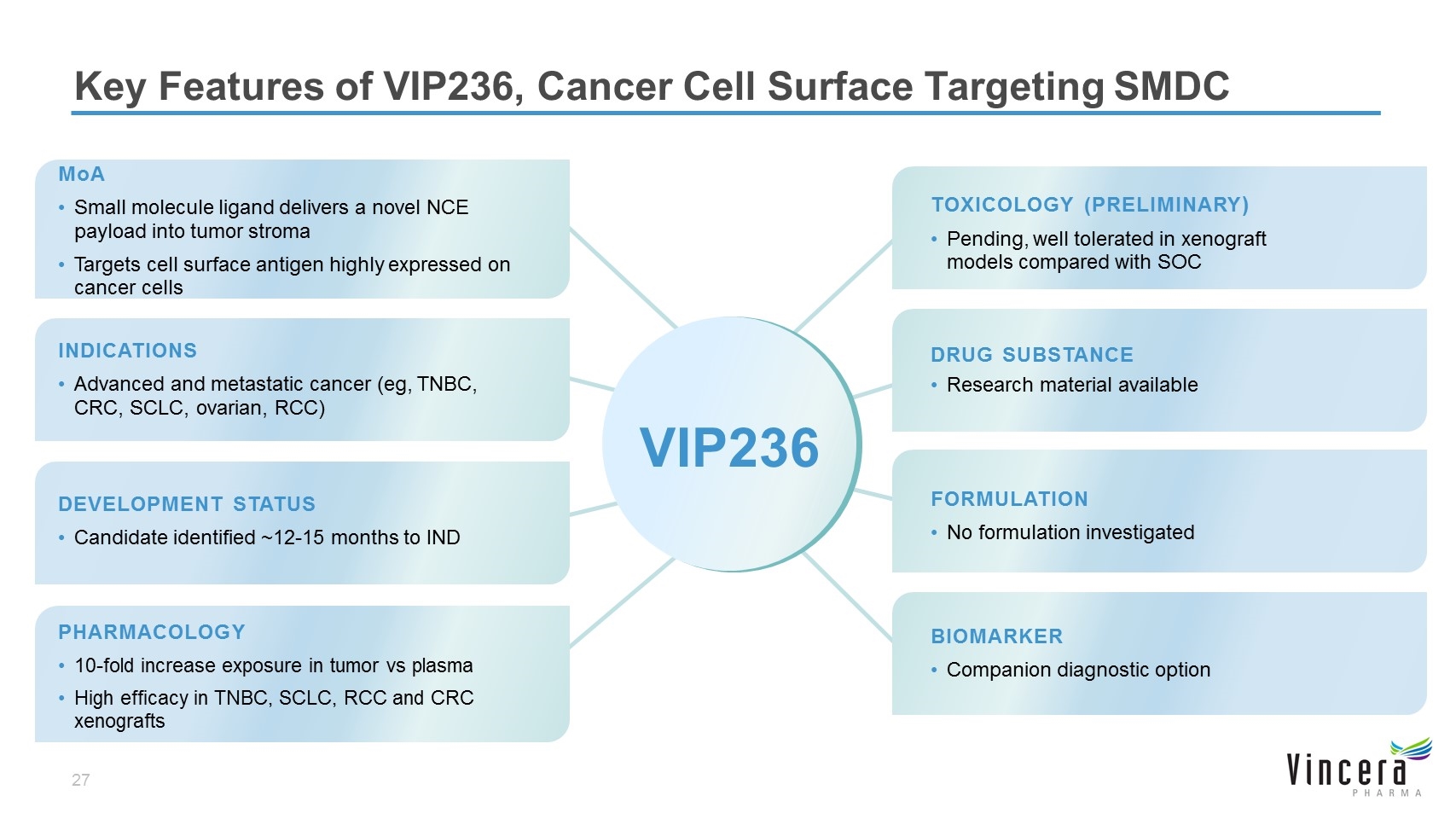
MoA Small molecule ligand delivers a novel NCE payload into tumor stroma Targets cell surface antigen highly expressed on cancer cells DEVELOPMENT STATUS Candidate identified ~12-15 months to IND TOXICOLOGY (PRELIMINARY) Pending, well tolerated in xenograft models compared with SOC DRUG SUBSTANCE Research material available FORMULATION No formulation investigated PHARMACOLOGY 10-fold increase exposure in tumor vs plasma High efficacy in TNBC, SCLC, RCC and CRC xenografts BIOMARKER Companion diagnostic option Key Features of VIP236, Cancer Cell Surface Targeting SMDC INDICATIONS Advanced and metastatic cancer (eg, TNBC, CRC, SCLC, ovarian, RCC) VIP236
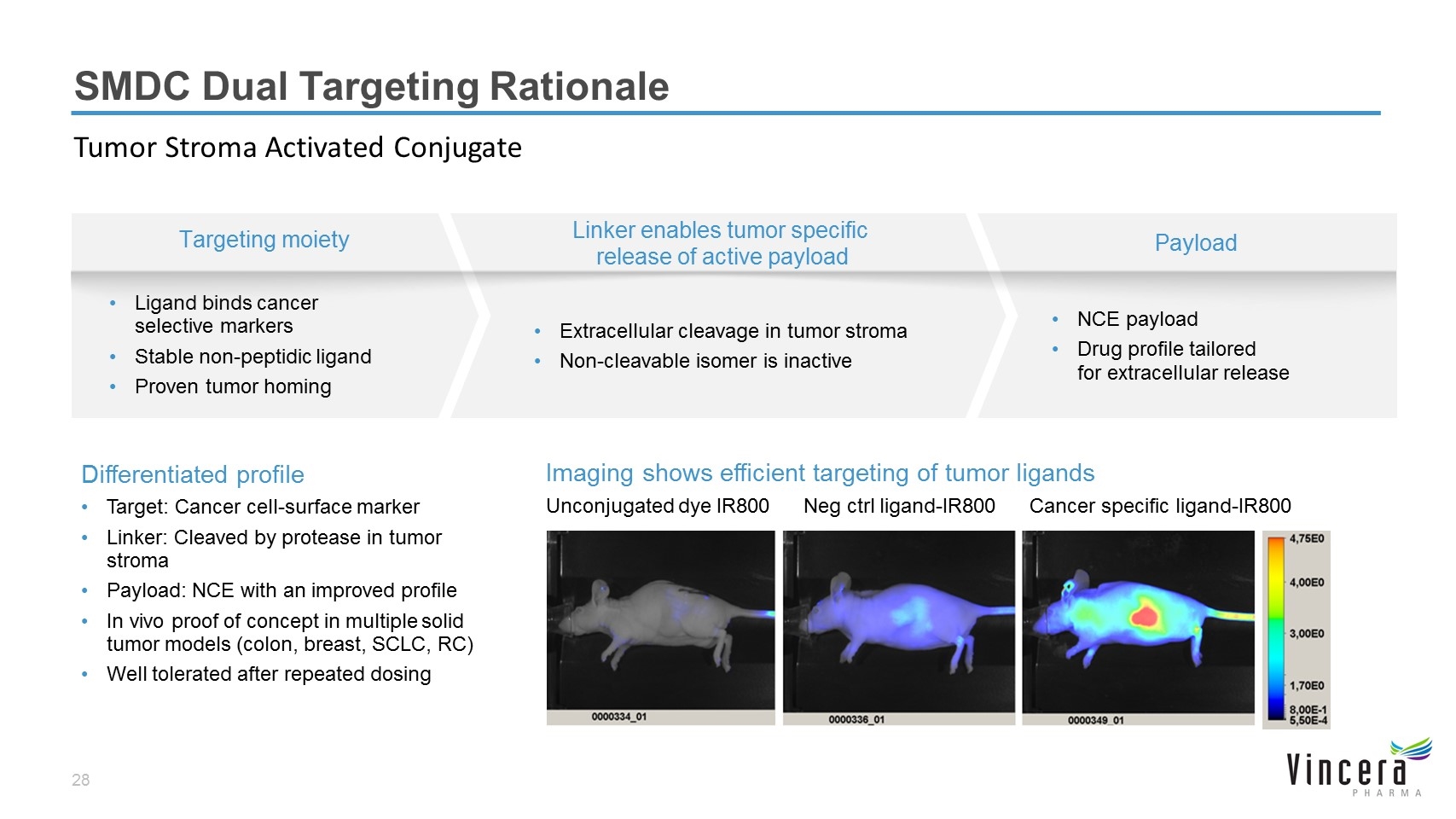
SMDC Dual Targeting Rationale Tumor Stroma Activated Conjugate Linker enables tumor specific release of active payload Extracellular cleavage in tumor stroma Non-cleavable isomer is inactive Targeting moiety Ligand binds cancer selective markers Stable non-peptidic ligand Proven tumor homing Payload NCE payload Drug profile tailored for extracellular release Unconjugated dye IR800 Neg ctrl ligand-IR800 Cancer specific ligand-IR800 Imaging shows efficient targeting of tumor ligands Differentiated profile Target: Cancer cell-surface marker Linker: Cleaved by protease in tumor stroma Payload: NCE with an improved profile In vivo proof of concept in multiple solid tumor models (colon, breast, SCLC, RC) Well tolerated after repeated dosing

NEXT GEN ADC PLATFORM VIP943 VIP924
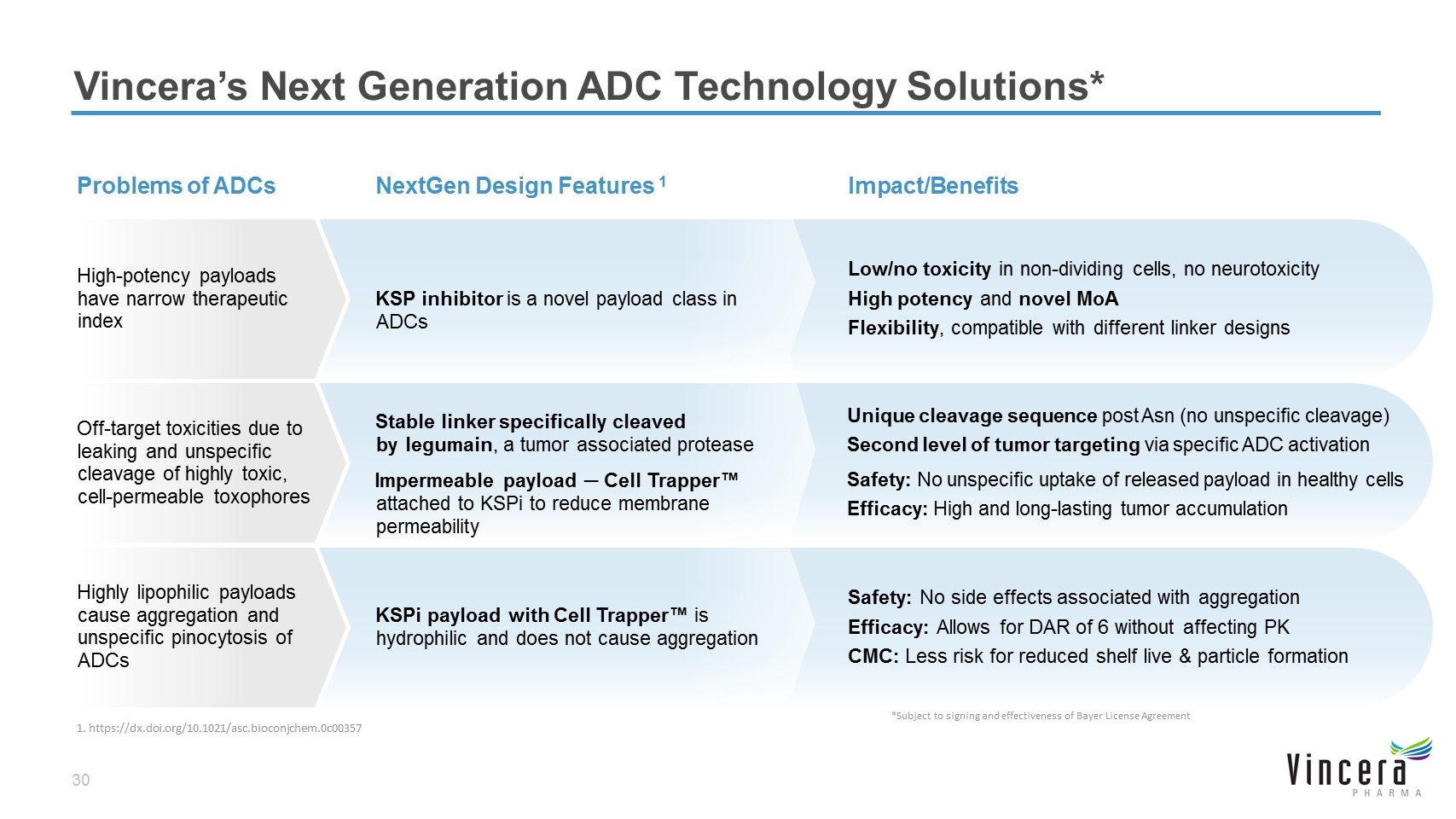
Vincera’s Next Generation ADC Technology Solutions* KSPi payload with Cell Trapper™ is hydrophilic and does not cause aggregation Safety: No side effects associated with aggregation Efficacy: Allows for DAR of 6 without affecting PK CMC: Less risk for reduced shelf live & particle formation Highly lipophilic payloads cause aggregation and unspecific pinocytosis of ADCs Stable linker specifically cleaved by legumain, a tumor associated protease Impermeable payload ─ Cell Trapper™ attached to KSPi to reduce membrane permeability Unique cleavage sequence post Asn (no unspecific cleavage) Second level of tumor targeting via specific ADC activation Safety: No unspecific uptake of released payload in healthy cells Efficacy: High and long-lasting tumor accumulation KSP inhibitor is a novel payload class in ADCs Low/no toxicity in non-dividing cells, no neurotoxicity High potency and novel MoA Flexibility, compatible with different linker designs High-potency payloads have narrow therapeutic index NextGen Design Features 1 Impact/Benefits Problems of ADCs Off-target toxicities due to leaking and unspecific cleavage of highly toxic, cell-permeable toxophores *Subject to signing and effectiveness of Bayer License Agreement 1. https://dx.doi.org/10.1021/asc.bioconjchem.0c00357
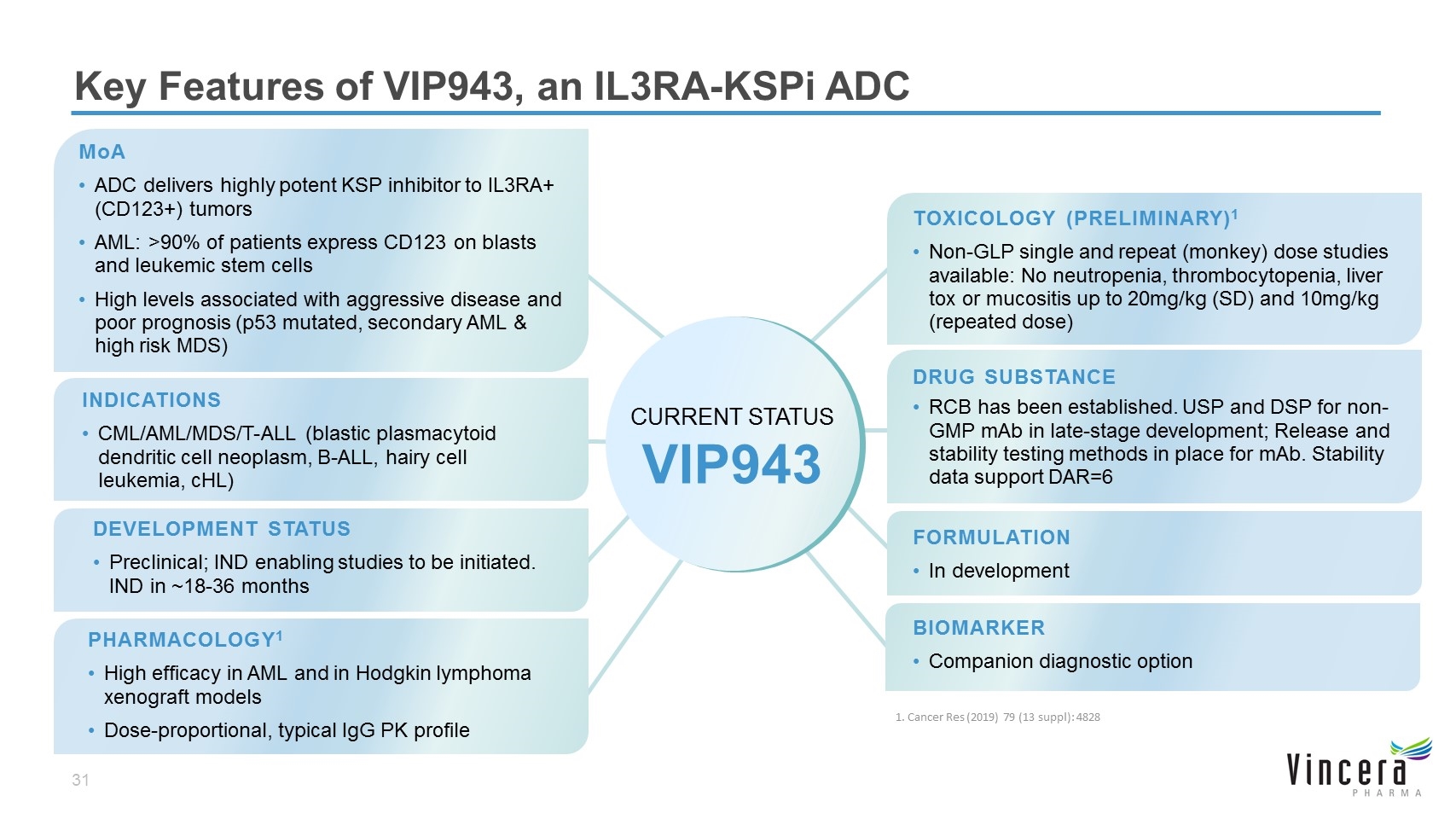
MoA ADC delivers highly potent KSP inhibitor to IL3RA+ (CD123+) tumors AML: >90% of patients express CD123 on blasts and leukemic stem cells High levels associated with aggressive disease and poor prognosis (p53 mutated, secondary AML & high risk MDS) TOXICOLOGY (PRELIMINARY)1 Non-GLP single and repeat (monkey) dose studies available: No neutropenia, thrombocytopenia, liver tox or mucositis up to 20mg/kg (SD) and 10mg/kg (repeated dose) DRUG SUBSTANCE RCB has been established. USP and DSP for non-GMP mAb in late-stage development; Release and stability testing methods in place for mAb. Stability data support DAR=6 FORMULATION In development BIOMARKER Companion diagnostic option Key Features of VIP943, an IL3RA-KSPi ADC INDICATIONS CML/AML/MDS/T-ALL (blastic plasmacytoid dendritic cell neoplasm, B-ALL, hairy cell leukemia, cHL) CURRENT STATUS VIP943 DEVELOPMENT STATUS Preclinical; IND enabling studies to be initiated. IND in ~18-36 months PHARMACOLOGY1 High efficacy in AML and in Hodgkin lymphoma xenograft models Dose-proportional, typical IgG PK profile 1. Cancer Res (2019) 79 (13 suppl): 4828
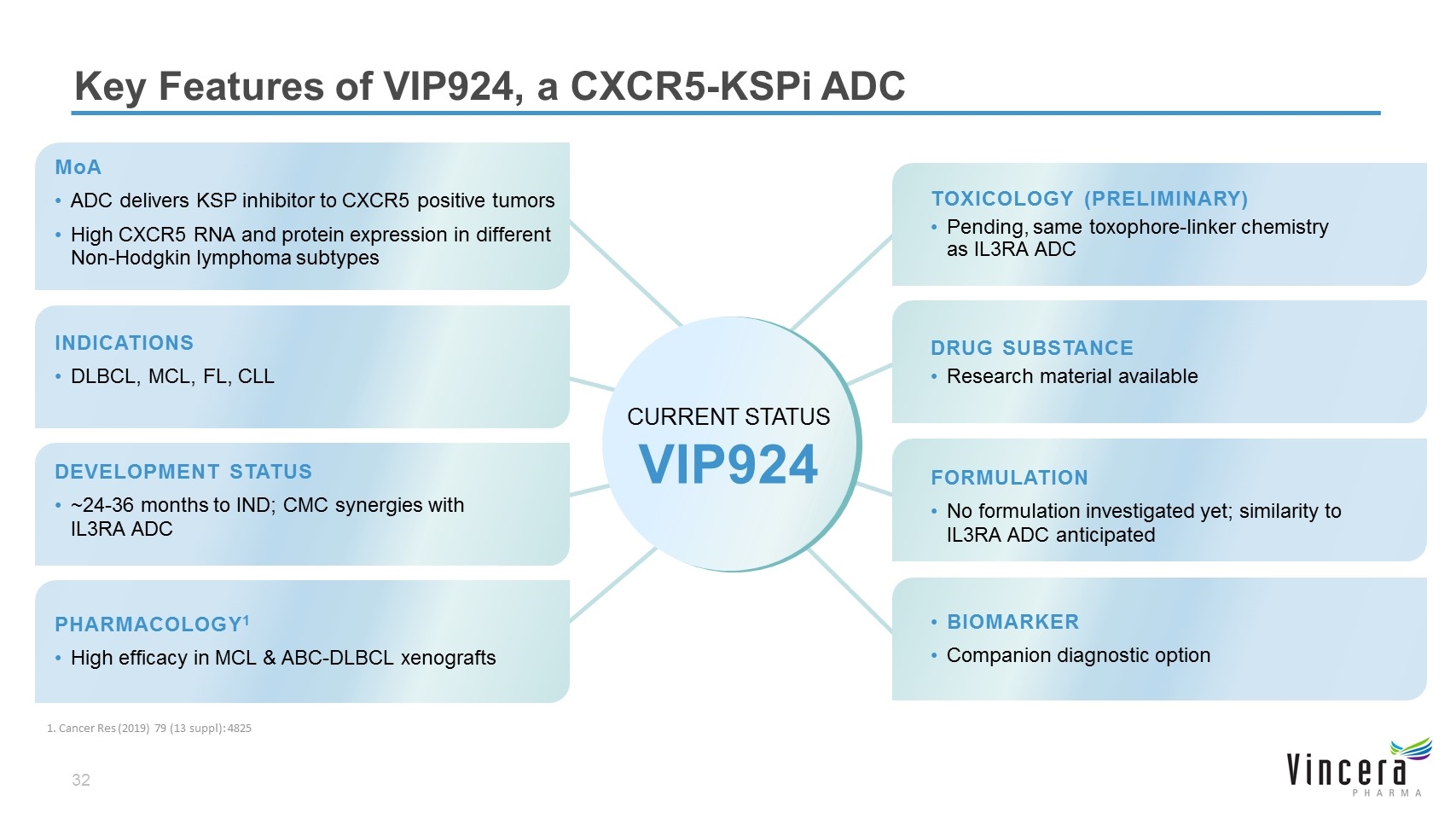
MoA ADC delivers KSP inhibitor to CXCR5 positive tumors High CXCR5 RNA and protein expression in different Non-Hodgkin lymphoma subtypes DEVELOPMENT STATUS ~24-36 months to IND; CMC synergies with IL3RA ADC TOXICOLOGY (PRELIMINARY) Pending, same toxophore-linker chemistry as IL3RA ADC DRUG SUBSTANCE Research material available FORMULATION No formulation investigated yet; similarity to IL3RA ADC anticipated PHARMACOLOGY1 High efficacy in MCL & ABC-DLBCL xenografts BIOMARKER Companion diagnostic option Key Features of VIP924, a CXCR5-KSPi ADC INDICATIONS DLBCL, MCL, FL, CLL CURRENT STATUS VIP924 1. Cancer Res (2019) 79 (13 suppl): 4825
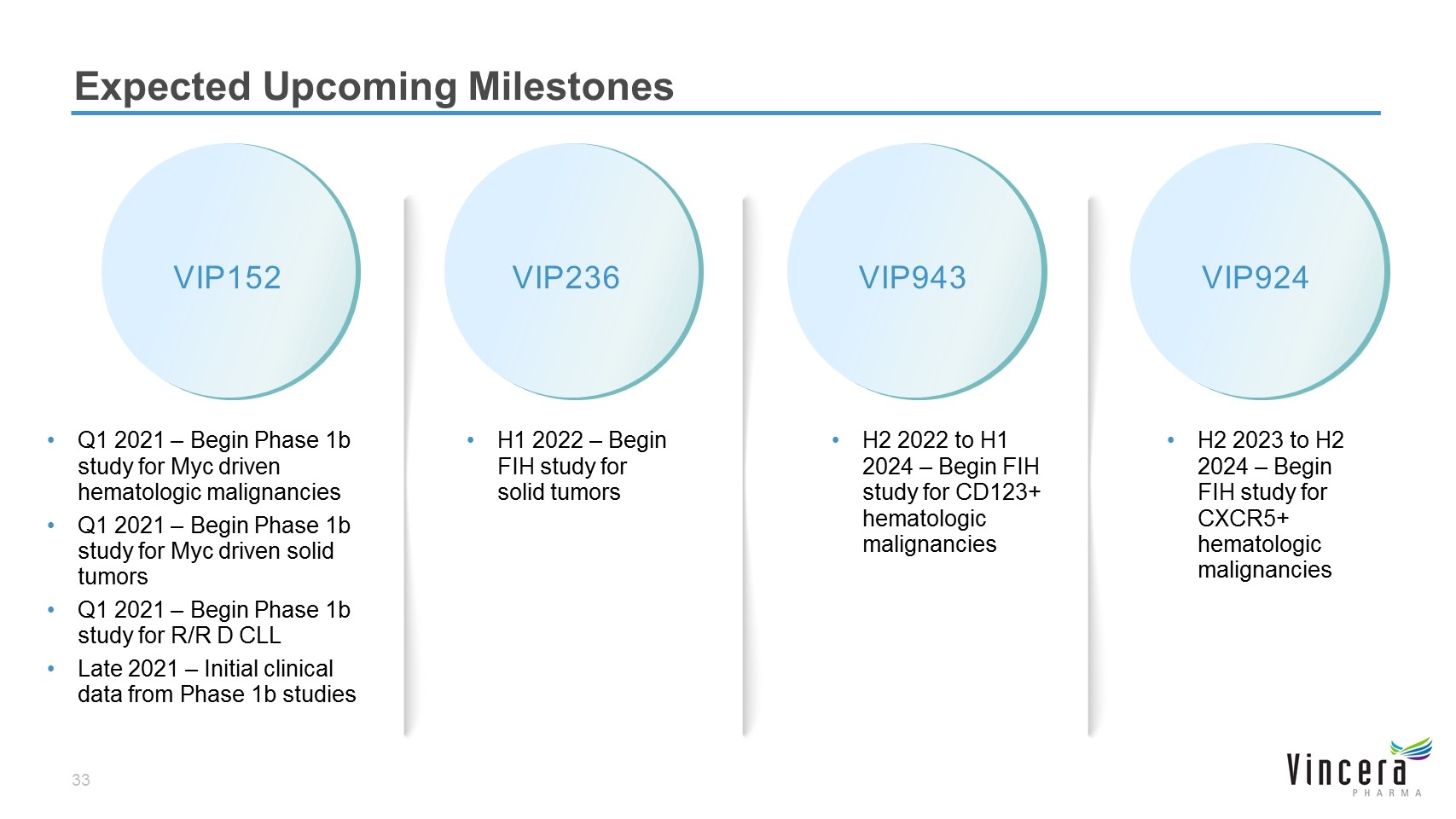
Expected Upcoming Milestones VIP152 Q1 2021 – Begin Phase 1b study for Myc driven hematologic malignancies Q1 2021 – Begin Phase 1b study for Myc driven solid tumors Q1 2021 – Begin Phase 1b study for R/R D CLL Late 2021 – Initial clinical data from Phase 1b studies VIP236 H1 2022 – Begin FIH study for solid tumors VIP924 H2 2023 to H2 2024 – Begin FIH study for CXCR5+ hematologic malignancies VIP943 H2 2022 to H1 2024 – Begin FIH study for CD123+ hematologic malignancies
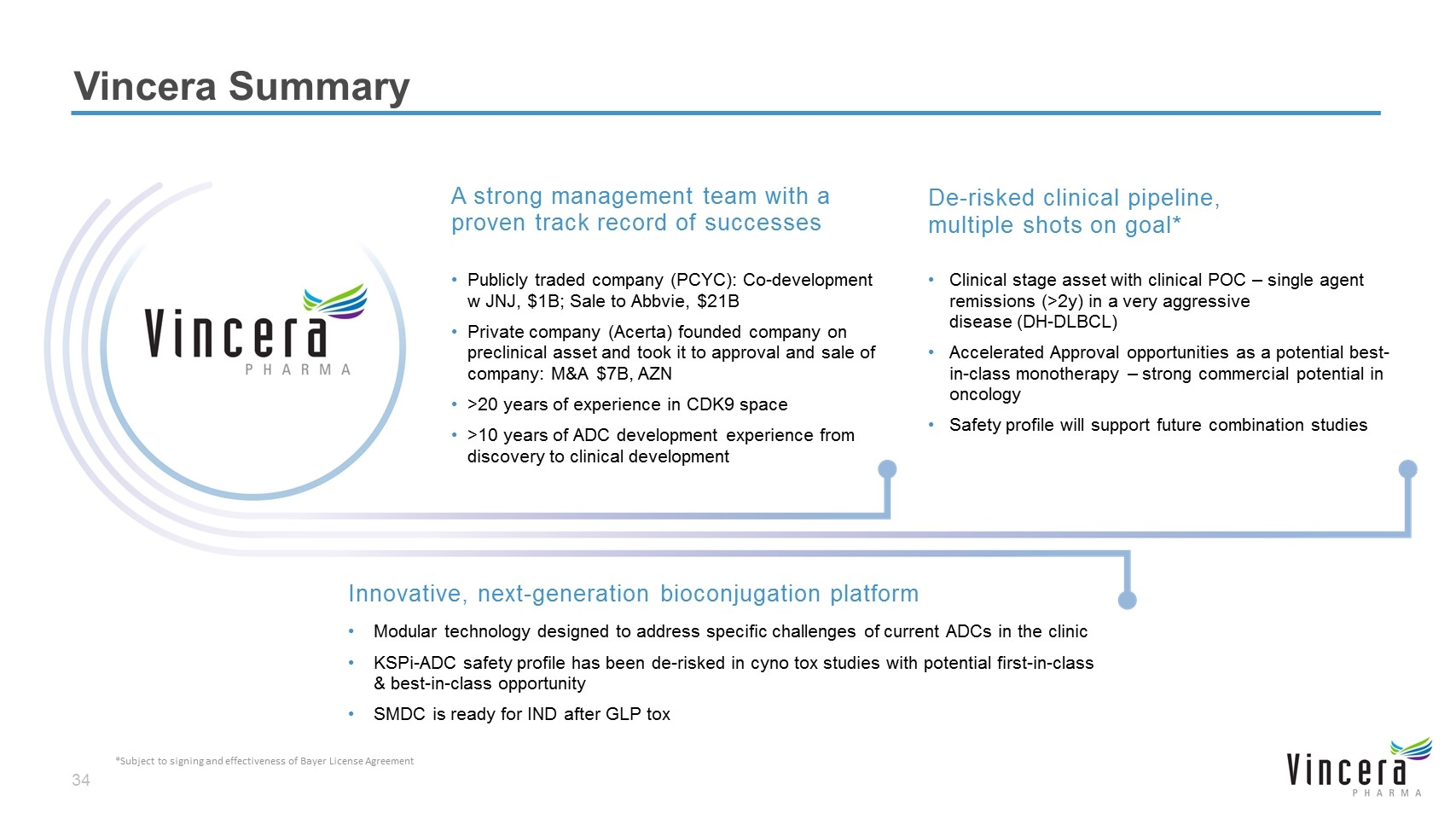
Vincera Summary A strong management team with a proven track record of successes Publicly traded company (PCYC): Co-development w JNJ, $1B; Sale to Abbvie, $21B Private company (Acerta) founded company on preclinical asset and took it to approval and sale of company: M&A $7B, AZN >20 years of experience in CDK9 space >10 years of ADC development experience from discovery to clinical development De-risked clinical pipeline, multiple shots on goal* Clinical stage asset with clinical POC – single agent remissions (>2y) in a very aggressive disease (DH-DLBCL) Accelerated Approval opportunities as a potential best-in-class monotherapy – strong commercial potential in oncology Safety profile will support future combination studies Innovative, next-generation bioconjugation platform Modular technology designed to address specific challenges of current ADCs in the clinic KSPi-ADC safety profile has been de-risked in cyno tox studies with potential first-in-class & best-in-class opportunity SMDC is ready for IND after GLP tox *Subject to signing and effectiveness of Bayer License Agreement


































