| Exhibit 99.1 |

June 2021

Disclaimer This presentation has been prepared by Legend Biotech Corporation (“Legend Biotech” or the “Company”) solely for information purpose and does not contain all relevant information relating to the Company.The safety and efficacy of the agents and/or uses under investigation discussed in this presentation have not been established. There is no guarantee that the agents will receive health authority approval or become commercially available in any country for the uses being investigated.Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and Legend Biotech's own internal estimates and research. While Legend Biotech believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Legend Biotech believes its internal research is reliable, such research has not been verified by any independent source. Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, statements relating to the Company’s strategies and objectives; the anticipated timing of, and ability to progress, clinical trials; the ability to make, and the timing of, regulatory submissions in the United States, Europe and Asia, including Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) for ciltacabtagene autoleucel (cilta-cel) for relapsed or refractory multiple myeloma (RRMM), the submission of a marketing authorisation application (MAA) for cilta-cel to the European Medicines Agency (EMA), and the submission of an Investigational New Drug (IND) for LB1901 in relapsed or refractory T-Cell Lymphoma (TCL); the ability to generate, analyze and present data from clinical trials; patient enrollment; anticipated timing regarding regulatory approvals by the FDA, EMA or Center for Drug Evaluation (CDE); and the potential benefits of Legend Biotech’s product candidates. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Legend Biotech’s expectations could be affected by, among other things, uncertainties involved in the development of new pharmaceutical products; unexpected clinical trial results, including as a result of additional analysis of existing clinical data or unexpected new clinical data; unexpected regulatory actions or delays, including requests for additional safety and/or efficacy data or analysis of data, or government regulation generally; unexpected delays as a result of actions undertaken, or failures to act, by our third party partners; uncertainties arising from challenges to Legend Biotech’s patent or other proprietary intellectual property protection, including the uncertainties involved in the US litigation process; competition in general; government, industry, and general public pricing and other political pressures; the duration and severity of the COVID-19 pandemic and governmental and regulatory measures implemented in response to the evolving situation; as well as the other factors discussed in the “Risk Factors” section of the Company’s Annual Report filed with the Securities and Exchange Commission on April 2, 2021. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this presentation as anticipated, believed, estimated or expected. Any forward-looking statements contained in this presentation speak only as of the date of this presentation. None of the Company nor any of its affiliates, advisers, or representatives has any obligation and does not undertake to update any forward-looking statements to reflect future events or circumstances. 2

Cell Therapy Platform Overview
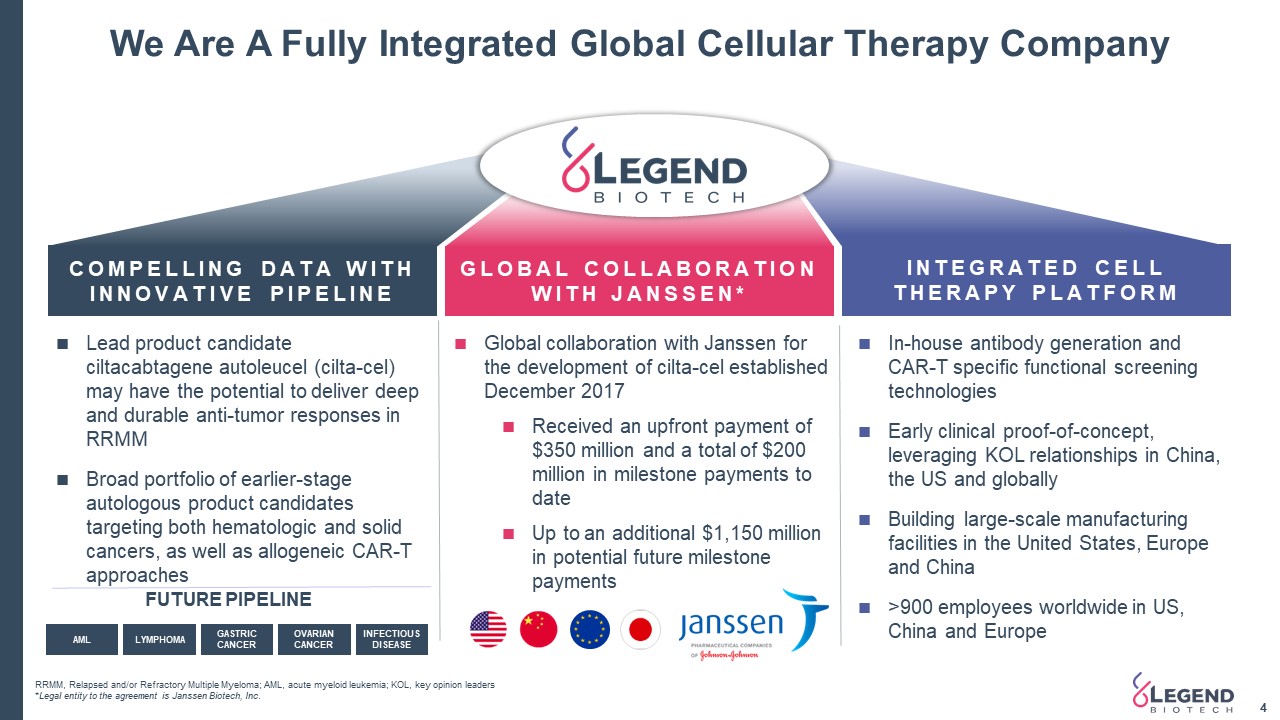
FUTURE PIPELINE Global collaboration with Janssen for the development of cilta-cel established December 2017Received an upfront payment of $350 million and a total of $200 million in milestone payments to dateUp to an additional $1,150 million in potential future milestone payments We Are A Fully Integrated Global Cellular Therapy Company 4 RRMM, Relapsed and/or Refractory Multiple Myeloma; AML, acute myeloid leukemia; KOL, key opinion leaders*Legal entity to the agreement is Janssen Biotech, Inc. COMPELLING DATA WITHINNOVATIVE PIPELINE GLOBAL COLLABORATION WITH JANSSEN* INTEGRATED CELL THERAPY PLATFORM In-house antibody generation and CAR-T specific functional screening technologiesEarly clinical proof-of-concept, leveraging KOL relationships in China, the US and globallyBuilding large-scale manufacturing facilities in the United States, Europe and China>900 employees worldwide in US, China and Europe Lead product candidate ciltacabtagene autoleucel (cilta-cel) may have the potential to deliver deep and durable anti-tumor responses in RRMMBroad portfolio of earlier-stage autologous product candidates targeting both hematologic and solid cancers, as well as allogeneic CAR-T approaches AML LYMPHOMA GASTRIC CANCER OVARIAN CANCER INFECTIOUS DISEASE
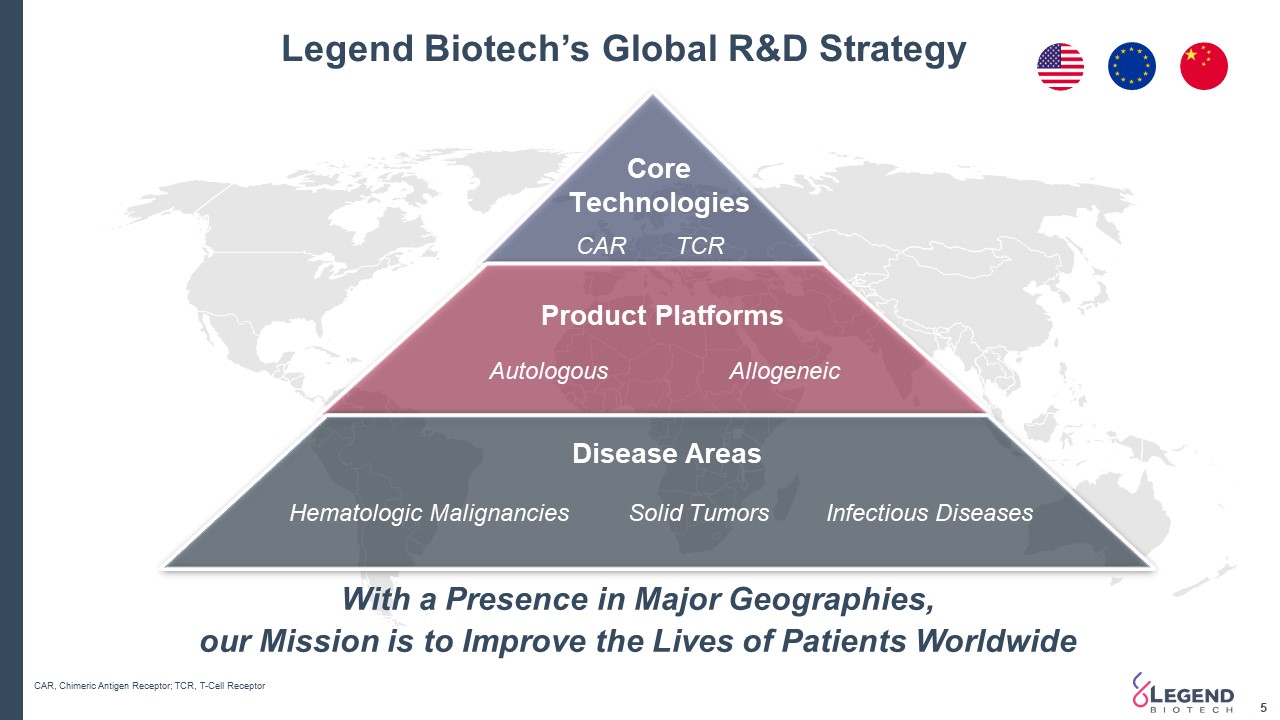
Legend Biotech’s Global R&D Strategy 5 With a Presence in Major Geographies, our Mission is to Improve the Lives of Patients Worldwide CAR, Chimeric Antigen Receptor; TCR, T-Cell Receptor CoreTechnologies Disease Areas Product Platforms Hematologic Malignancies CAR Autologous Allogeneic TCR Solid Tumors Infectious Diseases
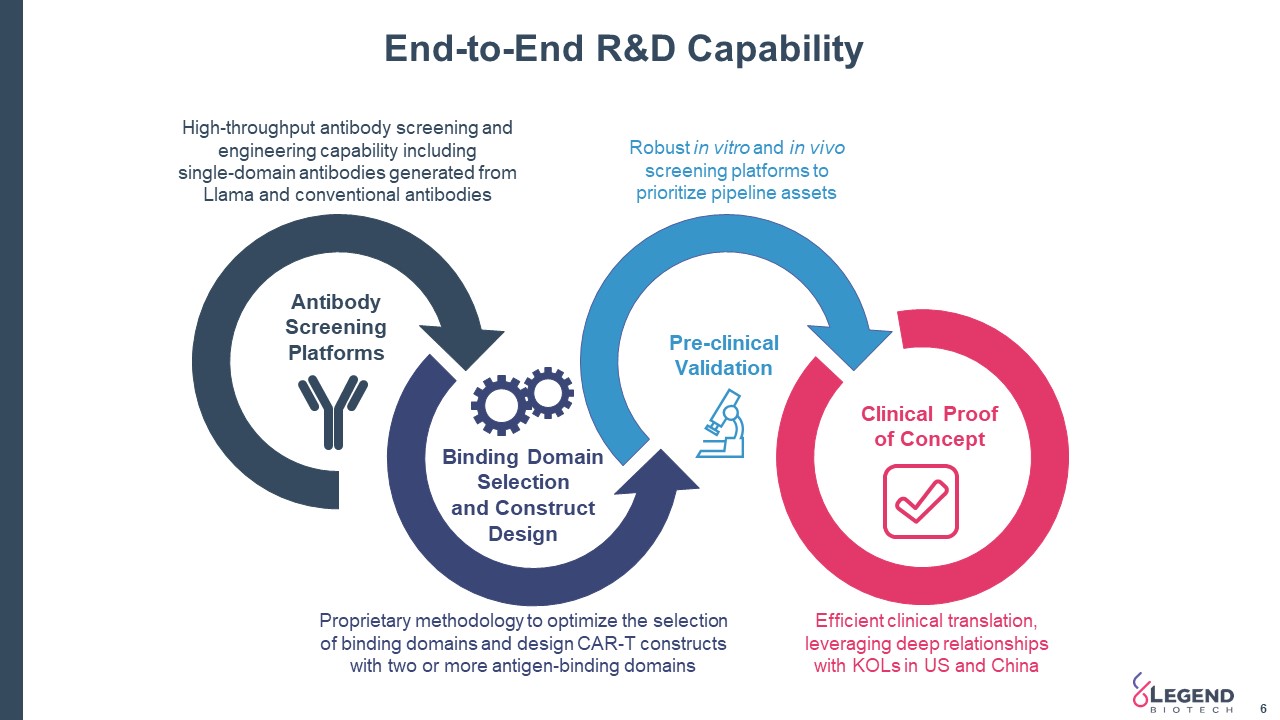
End-to-End R&D Capability 6 Proprietary methodology to optimize the selection of binding domains and design CAR-T constructs with two or more antigen-binding domains Robust in vitro and in vivo screening platforms to prioritize pipeline assets High-throughput antibody screening and engineering capability including single-domain antibodies generated from Llama and conventional antibodies Binding Domain Selection and Construct Design Pre-clinical Validation Antibody Screening Platforms Clinical Proof of Concept Efficient clinical translation, leveraging deep relationships with KOLs in US and China
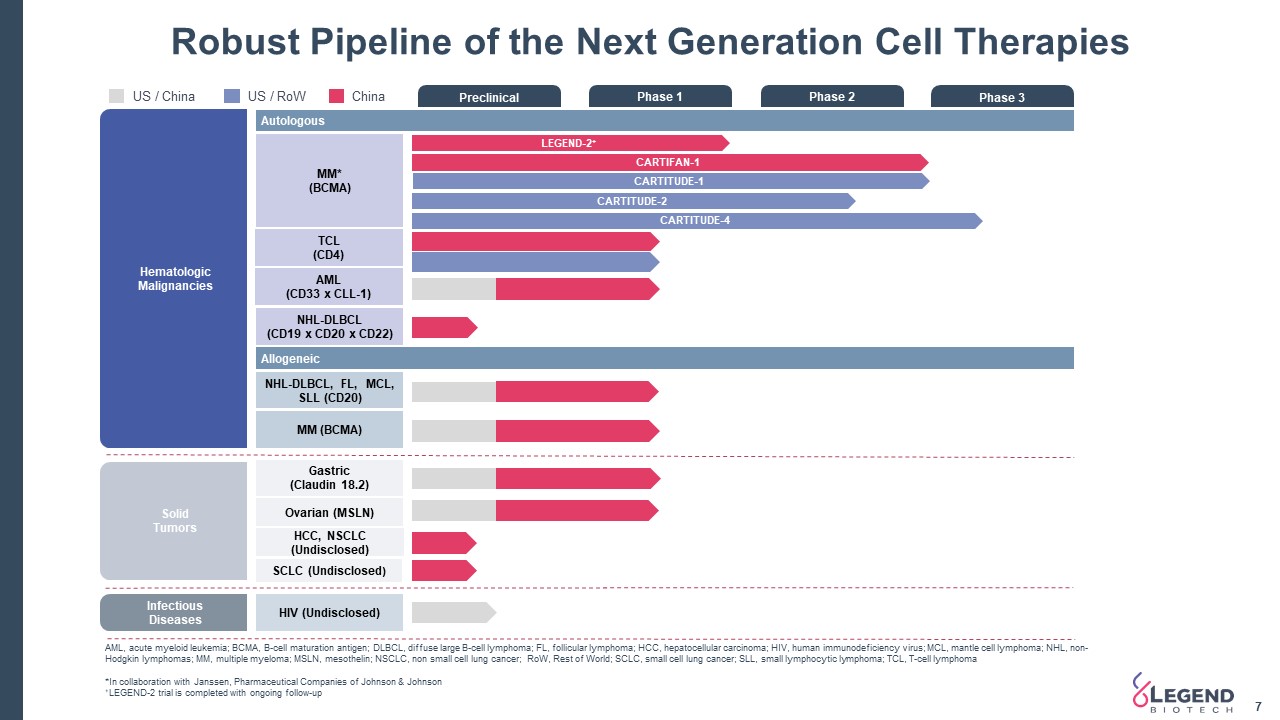
InfectiousDiseases SolidTumors Preclinical Phase 1 Phase 2 Phase 3 Autologous TCL(CD4) AML(CD33 x CLL-1) NHL-DLBCL(CD19 x CD20 x CD22) MM*(BCMA) Allogeneic NHL-DLBCL, FL, MCL, SLL (CD20) HIV (Undisclosed) LEGEND-2+ CARTITUDE-4 Gastric(Claudin 18.2) Ovarian (MSLN) Hematologic Malignancies CARTIFAN-1 CARTITUDE-2 US / RoW China AML, acute myeloid leukemia; BCMA, B-cell maturation antigen; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphomas; MM, multiple myeloma; MSLN, mesothelin; NSCLC, non small cell lung cancer; RoW, Rest of World; SCLC, small cell lung cancer; SLL, small lymphocytic lymphoma; TCL, T-cell lymphoma*In collaboration with Janssen, Pharmaceutical Companies of Johnson & Johnson+LEGEND-2 trial is completed with ongoing follow-up MM (BCMA) Robust Pipeline of the Next Generation Cell Therapies US / China HCC, NSCLC (Undisclosed) SCLC (Undisclosed) CARTITUDE-1 7
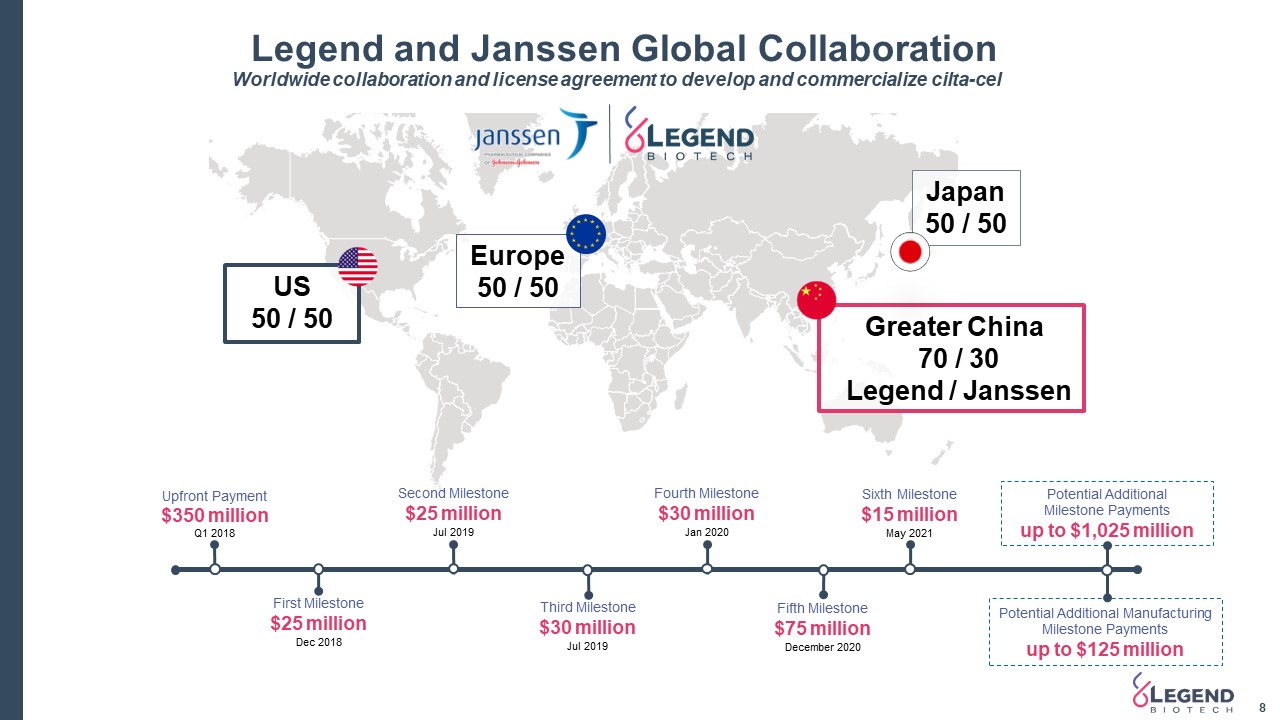
Legend and Janssen Global Collaboration 8 Worldwide collaboration and license agreement to develop and commercialize cilta-cel US 50 / 50 Europe 50 / 50 Greater China 70 / 30 Legend / Janssen Japan50 / 50 Potential Additional Milestone Paymentsup to $1,025 million Potential Additional Manufacturing Milestone Paymentsup to $125 million First Milestone$25 millionDec 2018 Fourth Milestone$30 millionJan 2020 Third Milestone$30 millionJul 2019 Second Milestone$25 millionJul 2019 Upfront Payment$350 millionQ1 2018 Fifth Milestone$75 millionDecember 2020 Sixth Milestone$15 millionMay 2021

Highly Experienced Management Team 9 CHINA US FRANK FANChief Scientific Officer & Co-Founder ELIZABETH GOSENGlobal Manufacturing YING HUANG Chief Executive Officer/ Chief Financial Officer Lida PacaudClinical Development TRACY LUOClinical Development CHONG YANGCommercial Development SIMON WUResearch & Development YUHONG QIUGlobal Regulatory STEVE GAVELCommercial Development ALAN KICKGlobal Quality MEETA CHATTERJEEGlobal Business Development DONG GENGEarly-stage Drug Development Lori MacomberFinance

Cilta-celClinical Development
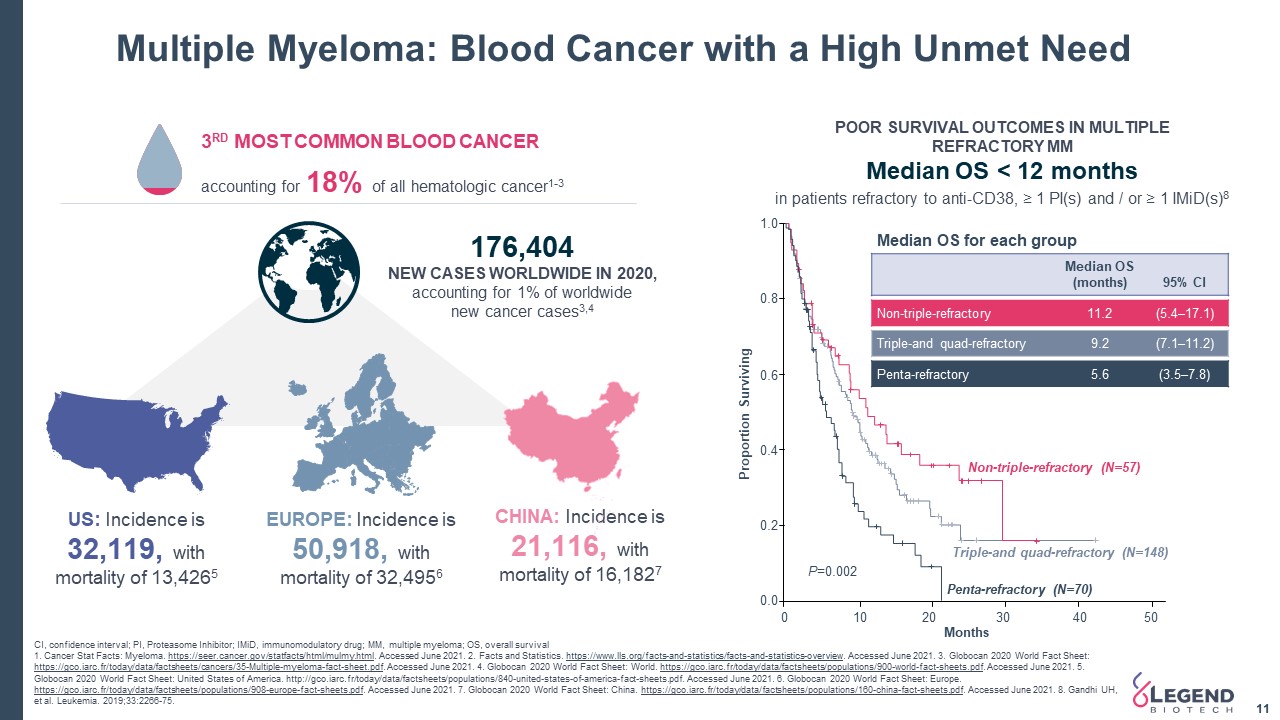
Multiple Myeloma: Blood Cancer with a High Unmet Need 11 CI, confidence interval; PI, Proteasome Inhibitor; IMiD, immunomodulatory drug; MM, multiple myeloma; OS, overall survival 1. Cancer Stat Facts: Myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed June 2021. 2. Facts and Statistics. https://www.lls.org/facts-and-statistics/facts-and-statistics-overview. Accessed June 2021. 3. Globocan 2020 World Fact Sheet: https://gco.iarc.fr/today/data/factsheets/cancers/35-Multiple-myeloma-fact-sheet.pdf. Accessed June 2021. 4. Globocan 2020 World Fact Sheet: World. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf. Accessed June 2021. 5. Globocan 2020 World Fact Sheet: United States of America. http://gco.iarc.fr/today/data/factsheets/populations/840-united-states-of-america-fact-sheets.pdf. Accessed June 2021. 6. Globocan 2020 World Fact Sheet: Europe. https://gco.iarc.fr/today/data/factsheets/populations/908-europe-fact-sheets.pdf. Accessed June 2021. 7. Globocan 2020 World Fact Sheet: China. https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf. Accessed June 2021. 8. Gandhi UH, et al. Leukemia. 2019;33:2266-75. US: Incidence is 32,119, with mortality of 13,4265 EUROPE: Incidence is 50,918, with mortality of 32,4956 CHINA: Incidence is 21,116, with mortality of 16,1827 176,404 NEW CASES WORLDWIDE IN 2020,accounting for 1% of worldwidenew cancer cases3,4 1.0 0.8 0.6 0.4 0.2 0.0 0 10 20 30 40 50 Months Proportion Surviving Non-triple-refractory (N=57) Triple-and quad-refractory (N=148) Penta-refractory (N=70) P=0.002 POOR SURVIVAL OUTCOMES IN MULTIPLE REFRACTORY MMMedian OS < 12 months 3RD MOST COMMON BLOOD CANCER accounting for 18% of all hematologic cancer1-3 in patients refractory to anti-CD38, ≥ 1 PI(s) and / or ≥ 1 IMiD(s)8 Median OS for each group Median OS(months) 95% Cl Non-triple-refractory 11.2 (5.4–17.1) Triple-and quad-refractory 9.2 (7.1–11.2) Penta-refractory 5.6 (3.5–7.8)
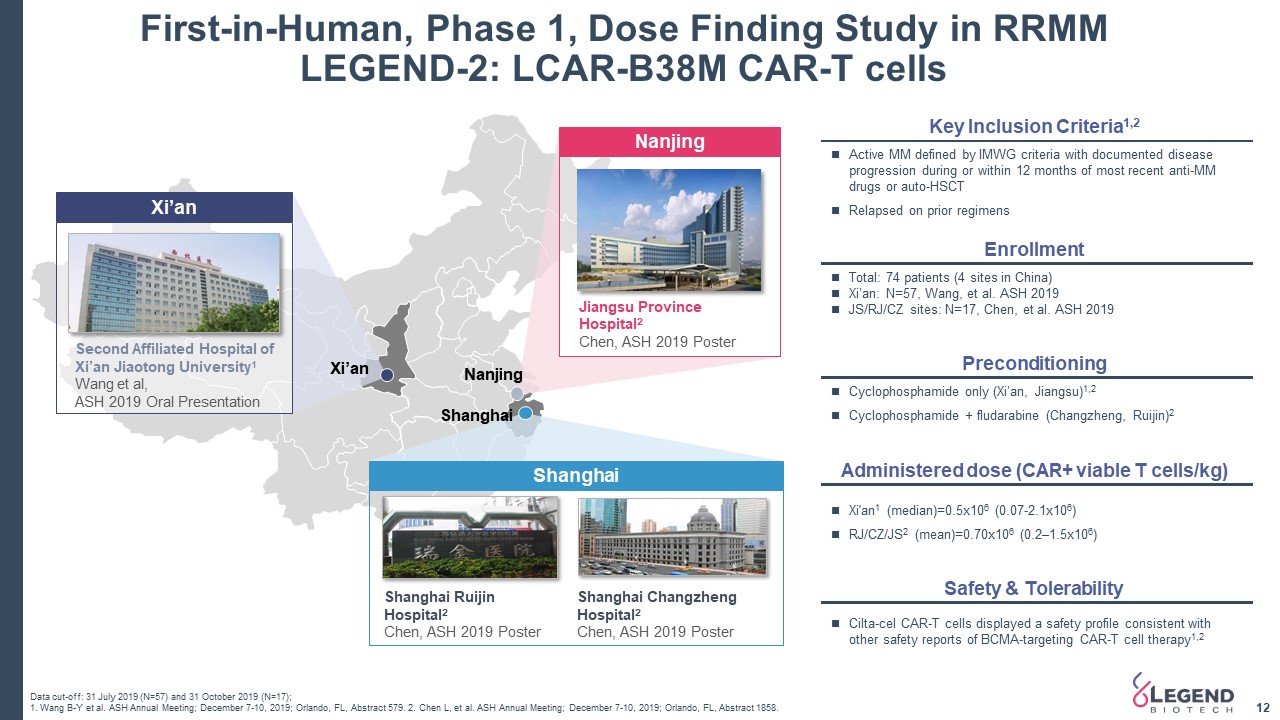
First-in-Human, Phase 1, Dose Finding Study in RRMM LEGEND-2: LCAR-B38M CAR-T cells 12 Shanghai Jiangsu Province Hospital2 Chen, ASH 2019 Poster Nanjing Second Affiliated Hospital of Xi’an Jiaotong University1 Wang et al, ASH 2019 Oral Presentation Xi’an Data cut-off: 31 July 2019 (N=57) and 31 October 2019 (N=17);1. Wang B-Y et al. ASH Annual Meeting; December 7-10, 2019; Orlando, FL, Abstract 579. 2. Chen L, et al. ASH Annual Meeting; December 7-10, 2019; Orlando, FL, Abstract 1858. Shanghai Ruijin Hospital2Chen, ASH 2019 Poster Shanghai Changzheng Hospital2Chen, ASH 2019 Poster Xi’an Nanjing Shanghai Enrollment Total: 74 patients (4 sites in China) Xi’an: N=57, Wang, et al. ASH 2019JS/RJ/CZ sites: N=17, Chen, et al. ASH 2019 Active MM defined by IMWG criteria with documented disease progression during or within 12 months of most recent anti-MM drugs or auto-HSCTRelapsed on prior regimens Key Inclusion Criteria1,2 Administered dose (CAR+ viable T cells/kg) Xi'an1 (median)=0.5x106 (0.07-2.1x106)RJ/CZ/JS2 (mean)=0.70x106 (0.2–1.5x106) Preconditioning Cyclophosphamide only (Xi’an, Jiangsu)1,2Cyclophosphamide + fludarabine (Changzheng, Ruijin)2 Safety & Tolerability Cilta-cel CAR-T cells displayed a safety profile consistent with other safety reports of BCMA-targeting CAR-T cell therapy1,2
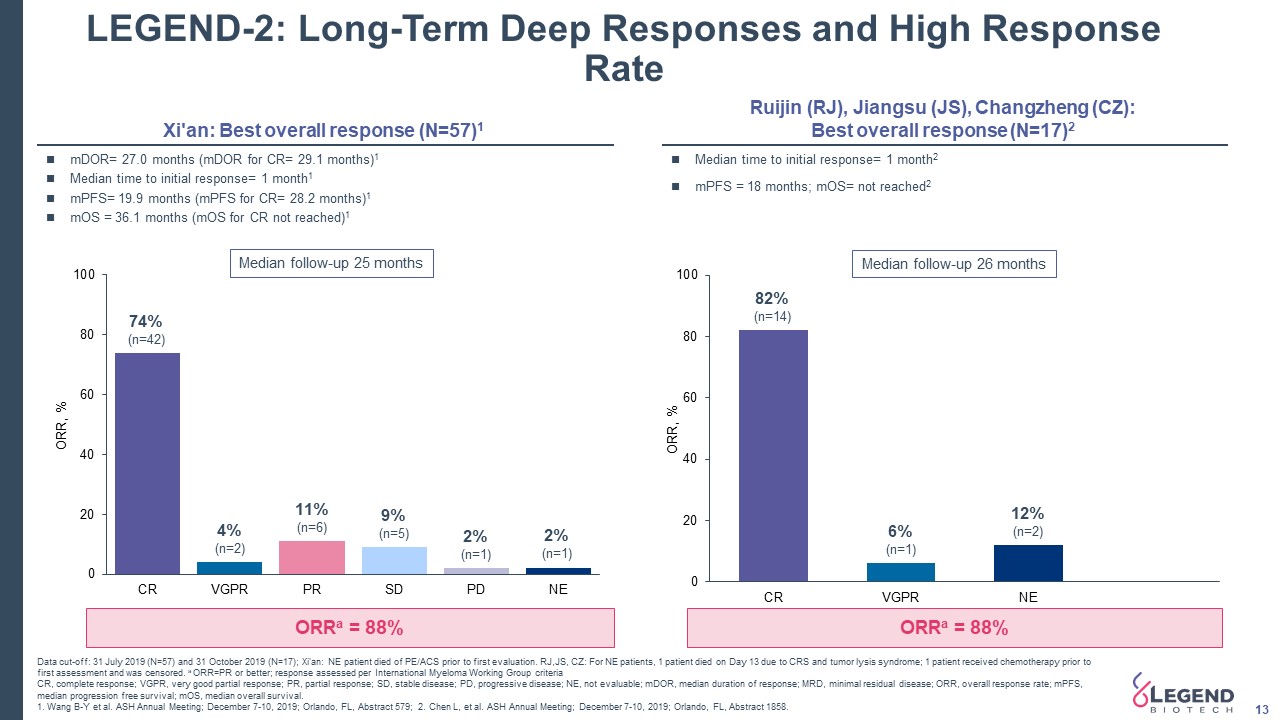
LEGEND-2: Long-Term Deep Responses and High Response Rate 13 ORRa = 88% 74%(n=42) 4%(n=2) 11%(n=6) 9%(n=5) 2%(n=1) 2%(n=1) 82%(n=14) 6%(n=1) 12%(n=2) Xi'an: Best overall response (N=57)1 ORRa = 88% Median time to initial response= 1 month2mPFS = 18 months; mOS= not reached2 mDOR= 27.0 months (mDOR for CR= 29.1 months)1Median time to initial response= 1 month1mPFS= 19.9 months (mPFS for CR= 28.2 months)1 mOS = 36.1 months (mOS for CR not reached)1 Ruijin (RJ), Jiangsu (JS), Changzheng (CZ): Best overall response (N=17)2 Median follow-up 25 months Median follow-up 26 months Data cut-off: 31 July 2019 (N=57) and 31 October 2019 (N=17); Xi'an: NE patient died of PE/ACS prior to first evaluation. RJ,JS, CZ: For NE patients, 1 patient died on Day 13 due to CRS and tumor lysis syndrome; 1 patient received chemotherapy prior to first assessment and was censored. a ORR=PR or better; response assessed per International Myeloma Working Group criteriaCR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; mDOR, median duration of response; MRD, minimal residual disease; ORR, overall response rate; mPFS, median progression free survival; mOS, median overall survival.1. Wang B-Y et al. ASH Annual Meeting; December 7-10, 2019; Orlando, FL, Abstract 579; 2. Chen L, et al. ASH Annual Meeting; December 7-10, 2019; Orlando, FL, Abstract 1858.
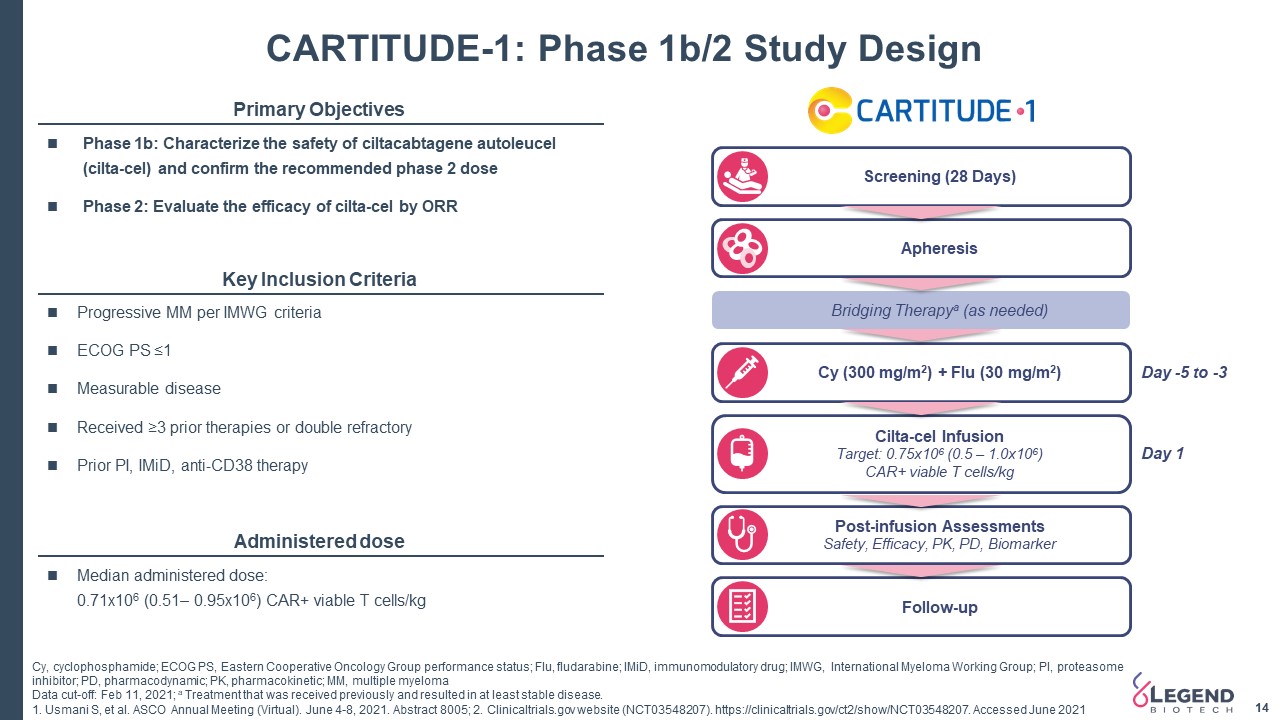
Post-infusion AssessmentsSafety, Efficacy, PK, PD, Biomarker Screening (28 Days) Apheresis Bridging Therapya (as needed) Cy (300 mg/m2) + Flu (30 mg/m2) Cilta-cel InfusionTarget: 0.75x106 (0.5 – 1.0x106) CAR+ viable T cells/kg Follow-up CARTITUDE-1: Phase 1b/2 Study Design 14 14 Day -5 to -3 Day 1 Primary Objectives Key Inclusion Criteria Administered dose Median administered dose: 0.71x106 (0.51– 0.95x106) CAR+ viable T cells/kg Progressive MM per IMWG criteriaECOG PS ≤1 Measurable diseaseReceived ≥3 prior therapies or double refractoryPrior PI, IMiD, anti-CD38 therapy Phase 1b: Characterize the safety of ciltacabtagene autoleucel (cilta-cel) and confirm the recommended phase 2 dosePhase 2: Evaluate the efficacy of cilta-cel by ORR Cy, cyclophosphamide; ECOG PS, Eastern Cooperative Oncology Group performance status; Flu, fludarabine; IMiD, immunomodulatory drug; IMWG, International Myeloma Working Group; PI, proteasome inhibitor; PD, pharmacodynamic; PK, pharmacokinetic; MM, multiple myelomaData cut-off: Feb 11, 2021; a Treatment that was received previously and resulted in at least stable disease.1. Usmani S, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8005; 2. Clinicaltrials.gov website (NCT03548207). https://clinicaltrials.gov/ct2/show/NCT03548207. Accessed June 2021
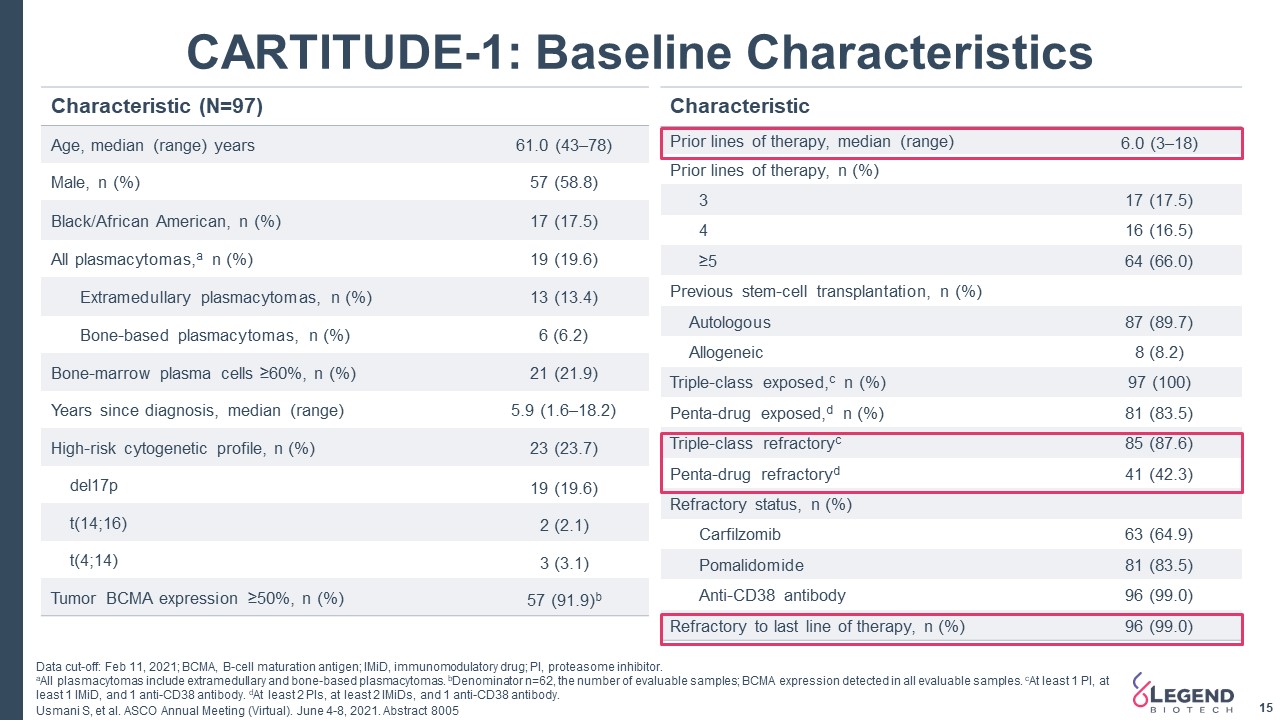
15 CARTITUDE-1: Baseline Characteristics Characteristic (N=97) Age, median (range) years 61.0 (43–78) Male, n (%) 57 (58.8) Black/African American, n (%) 17 (17.5) All plasmacytomas,a n (%) 19 (19.6) Extramedullary plasmacytomas, n (%) 13 (13.4) Bone-based plasmacytomas, n (%) 6 (6.2) Bone-marrow plasma cells ≥60%, n (%) 21 (21.9) Years since diagnosis, median (range) 5.9 (1.6–18.2) High-risk cytogenetic profile, n (%) 23 (23.7) del17p 19 (19.6) t(14;16) 2 (2.1) t(4;14) 3 (3.1) Tumor BCMA expression ≥50%, n (%) 57 (91.9)b Characteristic Prior lines of therapy, median (range) 6.0 (3–18) Prior lines of therapy, n (%) 3 17 (17.5) 4 16 (16.5) ≥5 64 (66.0) Previous stem-cell transplantation, n (%) Autologous 87 (89.7) Allogeneic 8 (8.2) Triple-class exposed,c n (%) 97 (100) Penta-drug exposed,d n (%) 81 (83.5) Triple-class refractoryc 85 (87.6) Penta-drug refractoryd 41 (42.3) Refractory status, n (%) Carfilzomib 63 (64.9) Pomalidomide 81 (83.5) Anti-CD38 antibody 96 (99.0) Refractory to last line of therapy, n (%) 96 (99.0) Data cut-off: Feb 11, 2021; BCMA, B-cell maturation antigen; IMiD, immunomodulatory drug; PI, proteasome inhibitor.aAll plasmacytomas include extramedullary and bone-based plasmacytomas. bDenominator n=62, the number of evaluable samples; BCMA expression detected in all evaluable samples. cAt least 1 PI, at least 1 IMiD, and 1 anti-CD38 antibody. dAt least 2 PIs, at least 2 IMiDs, and 1 anti-CD38 antibody. Usmani S, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8005
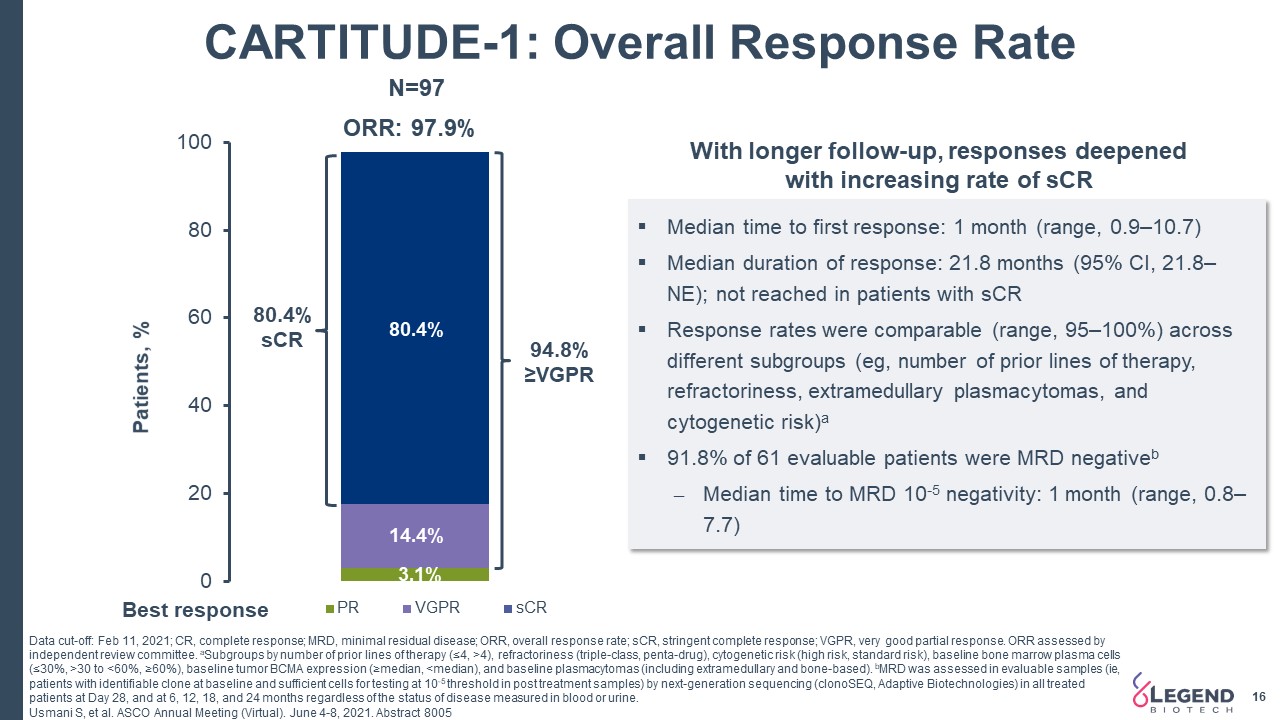
CARTITUDE-1: Overall Response Rate Data cut-off: Feb 11, 2021; CR, complete response; MRD, minimal residual disease; ORR, overall response rate; sCR, stringent complete response; VGPR, very good partial response. ORR assessed by independent review committee. aSubgroups by number of prior lines of therapy (≤4, >4), refractoriness (triple-class, penta-drug), cytogenetic risk (high risk, standard risk), baseline bone marrow plasma cells (≤30%, >30 to <60%, ≥60%), baseline tumor BCMA expression (≥median, <median), and baseline plasmacytomas (including extramedullary and bone-based). bMRD was assessed in evaluable samples (ie, patients with identifiable clone at baseline and sufficient cells for testing at 10-5 threshold in post treatment samples) by next-generation sequencing (clonoSEQ, Adaptive Biotechnologies) in all treated patients at Day 28, and at 6, 12, 18, and 24 months regardless of the status of disease measured in blood or urine. Usmani S, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8005 Median time to first response: 1 month (range, 0.9–10.7)Median duration of response: 21.8 months (95% CI, 21.8–NE); not reached in patients with sCRResponse rates were comparable (range, 95–100%) across different subgroups (eg, number of prior lines of therapy, refractoriness, extramedullary plasmacytomas, and cytogenetic risk)a91.8% of 61 evaluable patients were MRD negativebMedian time to MRD 10-5 negativity: 1 month (range, 0.8–7.7) N=97 80.4%sCR 94.8% ≥VGPR With longer follow-up, responses deepened with increasing rate of sCR 16
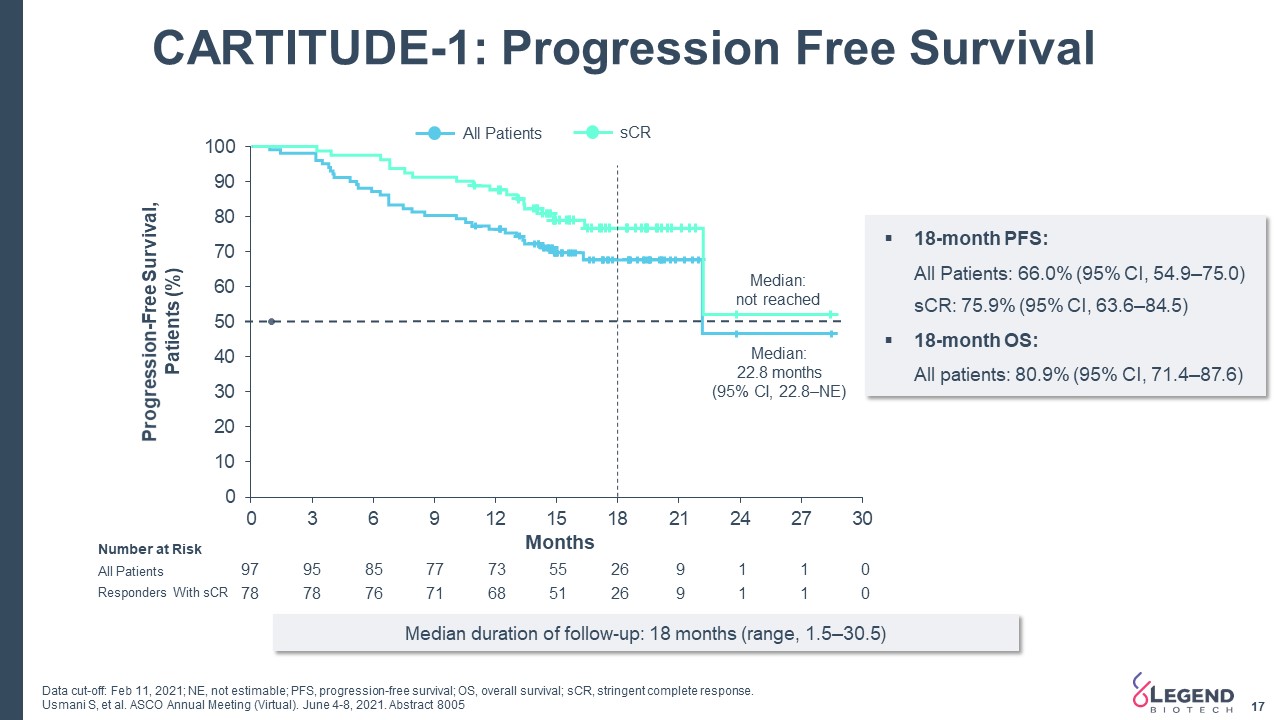
CARTITUDE-1: Progression Free Survival 17 Progression-Free Survival, Patients (%) Months All Patients sCR Median: 22.8 months (95% CI, 22.8–NE) Median: not reached 18-month PFS:All Patients: 66.0% (95% CI, 54.9–75.0)sCR: 75.9% (95% CI, 63.6–84.5)18-month OS:All patients: 80.9% (95% CI, 71.4–87.6) 97 95 85 77 73 55 26 9 1 1 0 78 78 76 71 68 51 26 9 1 1 0 All Patients Responders With sCR Number at Risk Data cut-off: Feb 11, 2021; NE, not estimable; PFS, progression-free survival; OS, overall survival; sCR, stringent complete response.Usmani S, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8005 Median duration of follow-up: 18 months (range, 1.5–30.5)
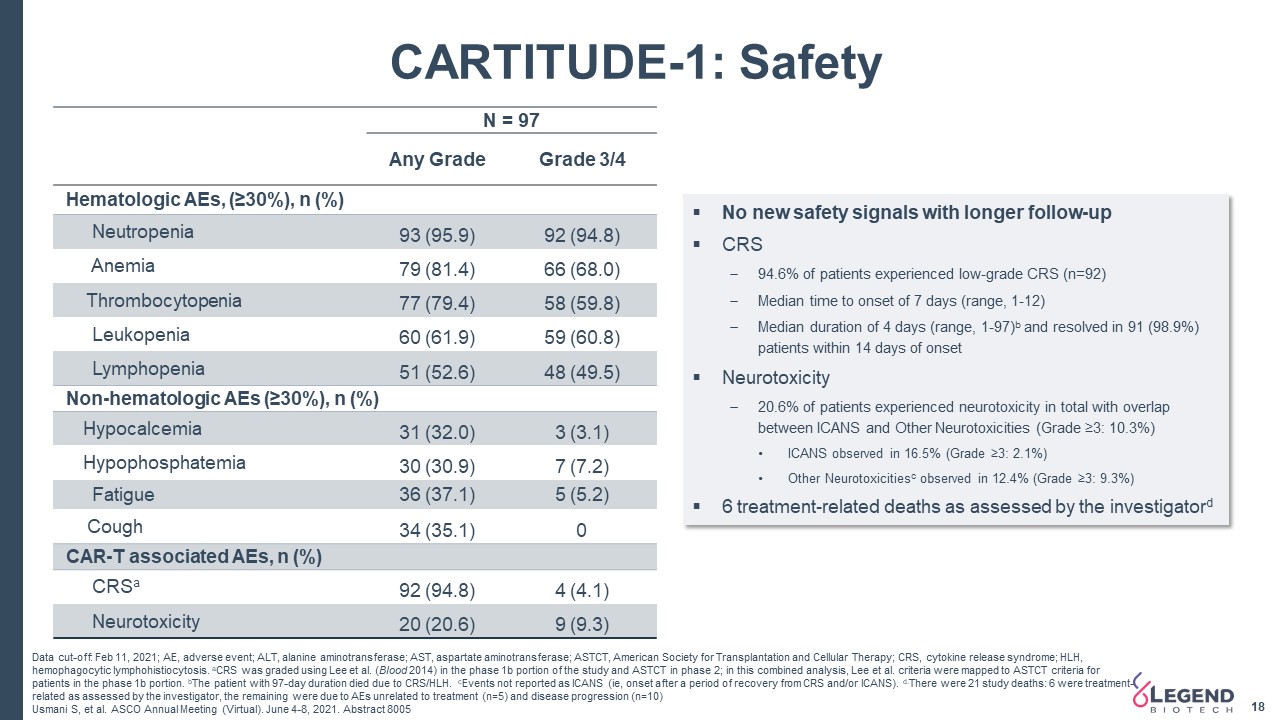
18 CARTITUDE-1: Safety Data cut-off: Feb 11, 2021; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ASTCT, American Society for Transplantation and Cellular Therapy; CRS, cytokine release syndrome; HLH, hemophagocytic lymphohistiocytosis. aCRS was graded using Lee et al. (Blood 2014) in the phase 1b portion of the study and ASTCT in phase 2; in this combined analysis, Lee et al. criteria were mapped to ASTCT criteria for patients in the phase 1b portion. bThe patient with 97-day duration died due to CRS/HLH. cEvents not reported as ICANS (ie, onset after a period of recovery from CRS and/or ICANS). d.There were 21 study deaths: 6 were treatment-related as assessed by the investigator, the remaining were due to AEs unrelated to treatment (n=5) and disease progression (n=10)Usmani S, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8005 No new safety signals with longer follow-upCRS94.6% of patients experienced low-grade CRS (n=92)Median time to onset of 7 days (range, 1-12)Median duration of 4 days (range, 1-97)b and resolved in 91 (98.9%) patients within 14 days of onsetNeurotoxicity20.6% of patients experienced neurotoxicity in total with overlap between ICANS and Other Neurotoxicities (Grade ≥3: 10.3%)ICANS observed in 16.5% (Grade ≥3: 2.1%)Other Neurotoxicitiesc observed in 12.4% (Grade ≥3: 9.3%)6 treatment-related deaths as assessed by the investigatord N = 97 Any Grade Grade 3/4 Hematologic AEs, (≥30%), n (%) Neutropenia 93 (95.9) 92 (94.8) Anemia 79 (81.4) 66 (68.0) Thrombocytopenia 77 (79.4) 58 (59.8) Leukopenia 60 (61.9) 59 (60.8) Lymphopenia 51 (52.6) 48 (49.5) Non-hematologic AEs (≥30%), n (%) Hypocalcemia 31 (32.0) 3 (3.1) Hypophosphatemia 30 (30.9) 7 (7.2) Fatigue 36 (37.1) 5 (5.2) Cough 34 (35.1) 0 CAR-T associated AEs, n (%) CRSa 92 (94.8) 4 (4.1) Neurotoxicity 20 (20.6) 9 (9.3)
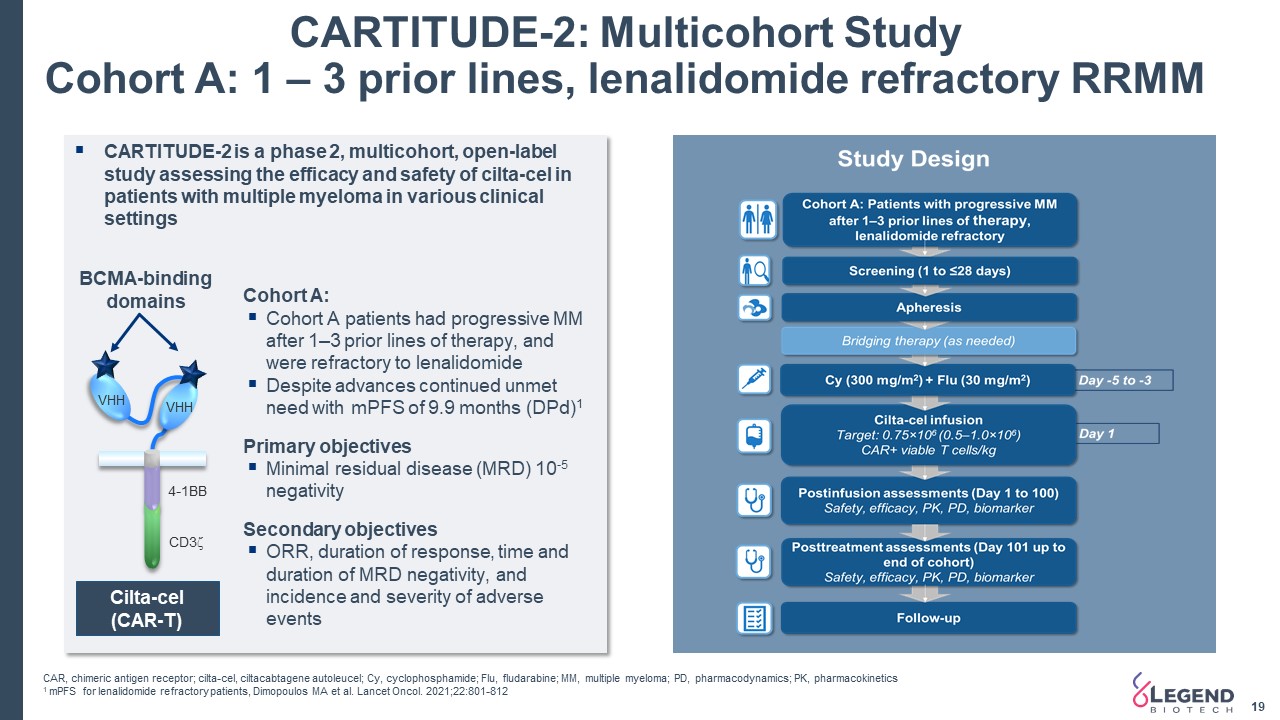
CARTITUDE-2: Multicohort StudyCohort A: 1 – 3 prior lines, lenalidomide refractory RRMM 19 CARTITUDE-2 is a phase 2, multicohort, open-label study assessing the efficacy and safety of cilta-cel in patients with multiple myeloma in various clinical settingsCohort A:Cohort A patients had progressive MM after 1–3 prior lines of therapy, and were refractory to lenalidomideDespite advances continued unmet need with mPFS of 9.9 months (DPd)1 Primary objectivesMinimal residual disease (MRD) 10-5 negativitySecondary objectivesORR, duration of response, time and duration of MRD negativity, and incidence and severity of adverse events VHH VHH BCMA-binding domains CD3z 4-1BB Cilta-cel(CAR-T) CAR, chimeric antigen receptor; cilta-cel, ciltacabtagene autoleucel; Cy, cyclophosphamide; Flu, fludarabine; MM, multiple myeloma; PD, pharmacodynamics; PK, pharmacokinetics1 mPFS for lenalidomide refractory patients, Dimopoulos MA et al. Lancet Oncol. 2021;22:801-812
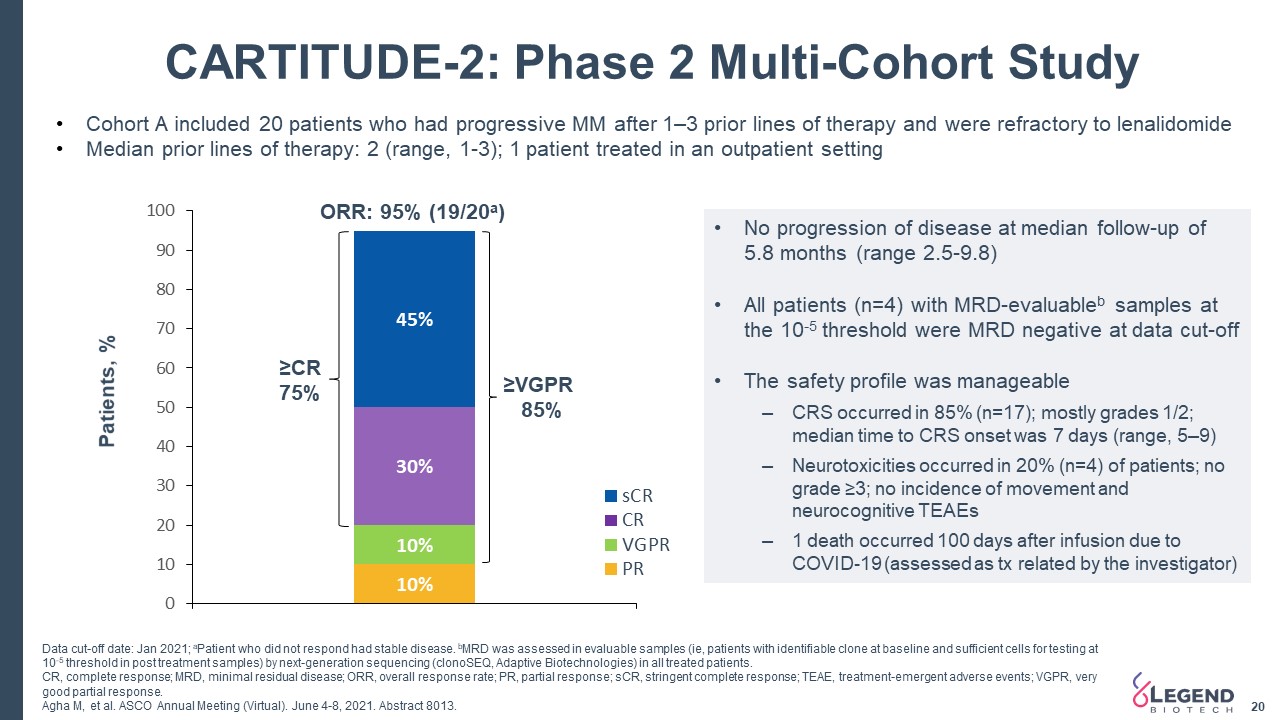
20 CARTITUDE-2: Phase 2 Multi-Cohort Study Cohort A included 20 patients who had progressive MM after 1–3 prior lines of therapy and were refractory to lenalidomideMedian prior lines of therapy: 2 (range, 1-3); 1 patient treated in an outpatient setting ≥VGPR 85% ≥CR 75% ORR: 95% (19/20a) Patients, % No progression of disease at median follow-up of 5.8 months (range 2.5-9.8)All patients (n=4) with MRD-evaluableb samples at the 10-5 threshold were MRD negative at data cut-offThe safety profile was manageableCRS occurred in 85% (n=17); mostly grades 1/2; median time to CRS onset was 7 days (range, 5–9)Neurotoxicities occurred in 20% (n=4) of patients; no grade ≥3; no incidence of movement and neurocognitive TEAEs1 death occurred 100 days after infusion due to COVID-19 (assessed as tx related by the investigator) Data cut-off date: Jan 2021; aPatient who did not respond had stable disease. bMRD was assessed in evaluable samples (ie, patients with identifiable clone at baseline and sufficient cells for testing at 10-5 threshold in post treatment samples) by next-generation sequencing (clonoSEQ, Adaptive Biotechnologies) in all treated patients. CR, complete response; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; sCR, stringent complete response; TEAE, treatment-emergent adverse events; VGPR, very good partial response.Agha M, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8013.
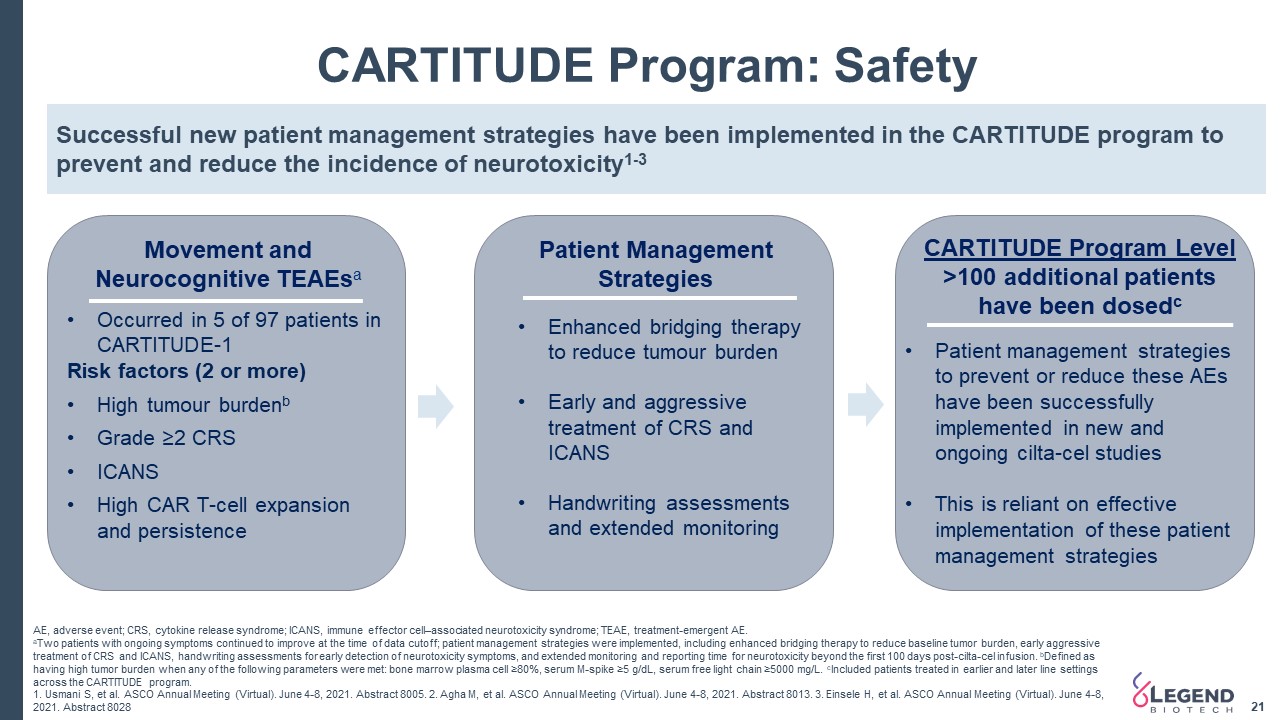
21 AE, adverse event; CRS, cytokine release syndrome; ICANS, immune effector cell–associated neurotoxicity syndrome; TEAE, treatment-emergent AE. aTwo patients with ongoing symptoms continued to improve at the time of data cutoff; patient management strategies were implemented, including enhanced bridging therapy to reduce baseline tumor burden, early aggressive treatment of CRS and ICANS, handwriting assessments for early detection of neurotoxicity symptoms, and extended monitoring and reporting time for neurotoxicity beyond the first 100 days post-cilta-cel infusion. bDefined as having high tumor burden when any of the following parameters were met: bone marrow plasma cell ≥80%, serum M-spike ≥5 g/dL, serum free light chain ≥5000 mg/L. cIncluded patients treated in earlier and later line settings across the CARTITUDE program. 1. Usmani S, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8005. 2. Agha M, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8013. 3. Einsele H, et al. ASCO Annual Meeting (Virtual). June 4-8, 2021. Abstract 8028 CARTITUDE Program: Safety Movement andNeurocognitive TEAEsa Patient Management Strategies CARTITUDE Program Level>100 additional patientshave been dosedc Successful new patient management strategies have been implemented in the CARTITUDE program to prevent and reduce the incidence of neurotoxicity1-3 Occurred in 5 of 97 patients in CARTITUDE-1Risk factors (2 or more)High tumour burdenbGrade ≥2 CRSICANSHigh CAR T-cell expansion and persistence Enhanced bridging therapy to reduce tumour burdenEarly and aggressive treatment of CRS and ICANSHandwriting assessments and extended monitoring Patient management strategies to prevent or reduce these AEs have been successfully implemented in new and ongoing cilta-cel studiesThis is reliant on effective implementation of these patient management strategies
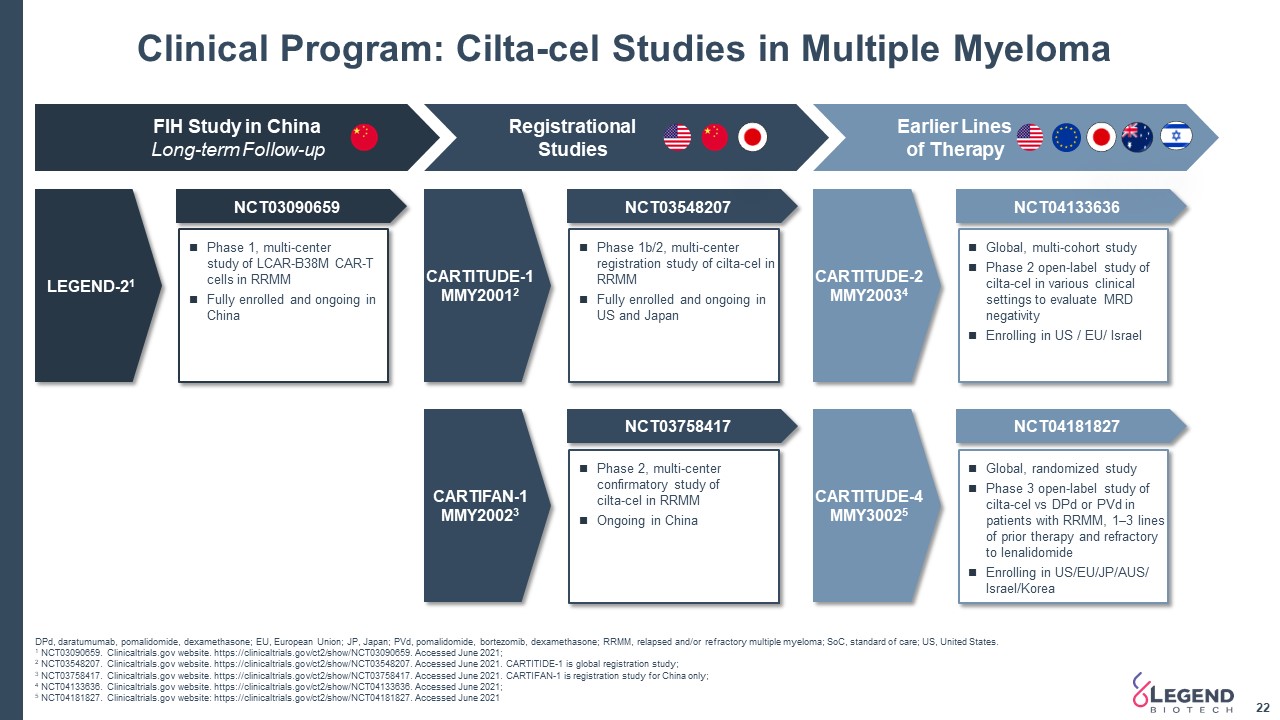
Clinical Program: Cilta-cel Studies in Multiple Myeloma 22 DPd, daratumumab, pomalidomide, dexamethasone; EU, European Union; JP, Japan; PVd, pomalidomide, bortezomib, dexamethasone; RRMM, relapsed and/or refractory multiple myeloma; SoC, standard of care; US, United States. 1 NCT03090659. Clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT03090659. Accessed June 2021; 2 NCT03548207. Clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT03548207. Accessed June 2021. CARTITIDE-1 is global registration study;3 NCT03758417. Clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT03758417. Accessed June 2021. CARTIFAN-1 is registration study for China only; 4 NCT04133636. Clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT04133636. Accessed June 2021; 5 NCT04181827. Clinicaltrials.gov website: https://clinicaltrials.gov/ct2/show/NCT04181827. Accessed June 2021 FIH Study in ChinaLong-term Follow-up Registrational Studies Earlier Lines of Therapy LEGEND-21 NCT03090659 NCT03548207 NCT03758417 NCT04133636 NCT04181827 Phase 1, multi-centerstudy of LCAR-B38M CAR-T cells in RRMMFully enrolled and ongoing in China Phase 2, multi-center confirmatory study ofcilta-cel in RRMMOngoing in China Phase 1b/2, multi-center registration study of cilta-cel in RRMMFully enrolled and ongoing in US and Japan Global, multi-cohort studyPhase 2 open-label study of cilta-cel in various clinical settings to evaluate MRD negativityEnrolling in US / EU/ Israel Global, randomized studyPhase 3 open-label study of cilta-cel vs DPd or PVd in patients with RRMM, 1–3 lines of prior therapy and refractory to lenalidomideEnrolling in US/EU/JP/AUS/ Israel/Korea CARTITUDE-1MMY20012 CARTIFAN-1MMY20023 CARTITUDE-2MMY20034 CARTITUDE-4MMY30025
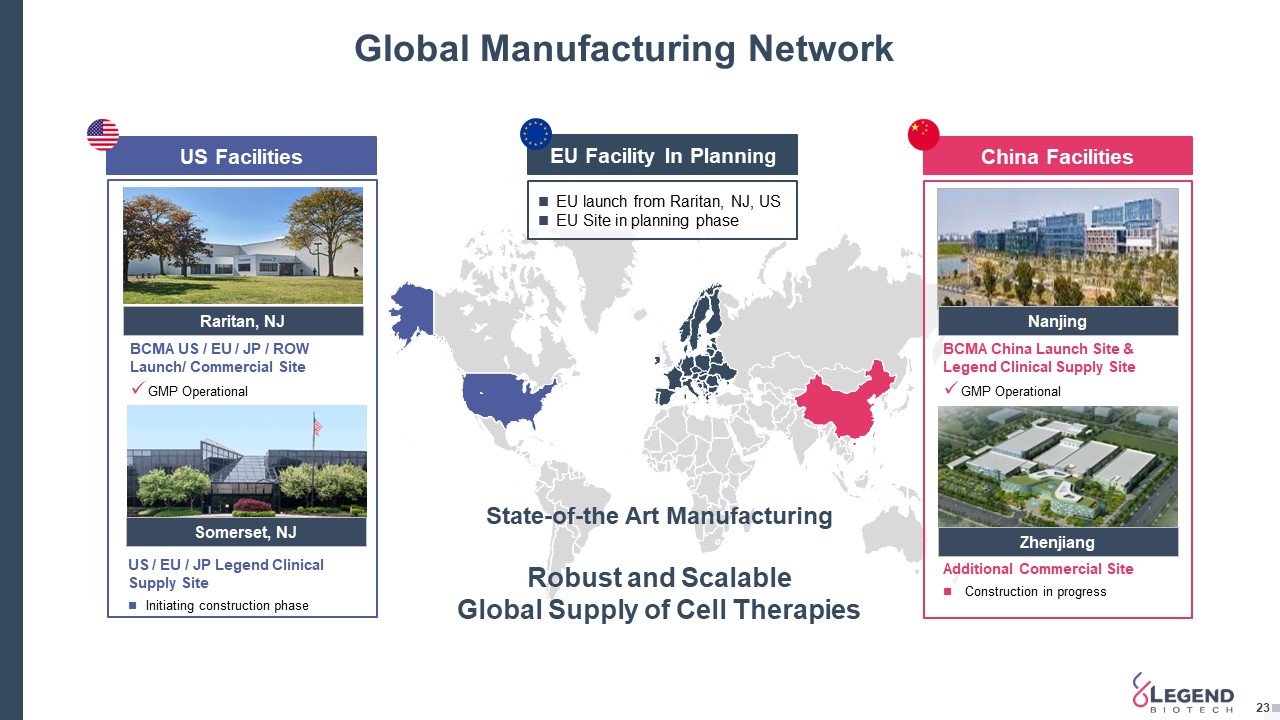
Global Manufacturing Network 23 EU Facility In Planning US Facilities China Facilities Raritan, NJ Nanjing Zhenjiang Somerset, NJ BCMA US / EU / JP / ROW Launch/ Commercial SiteGMP Operational BCMA China Launch Site & Legend Clinical Supply SiteGMP Operational Additional Commercial Site Construction in progress US / EU / JP Legend Clinical Supply SiteInitiating construction phase State-of-the Art Manufacturing Robust and Scalable Global Supply of Cell Therapies EU launch from Raritan, NJ, USEU Site in planning phase
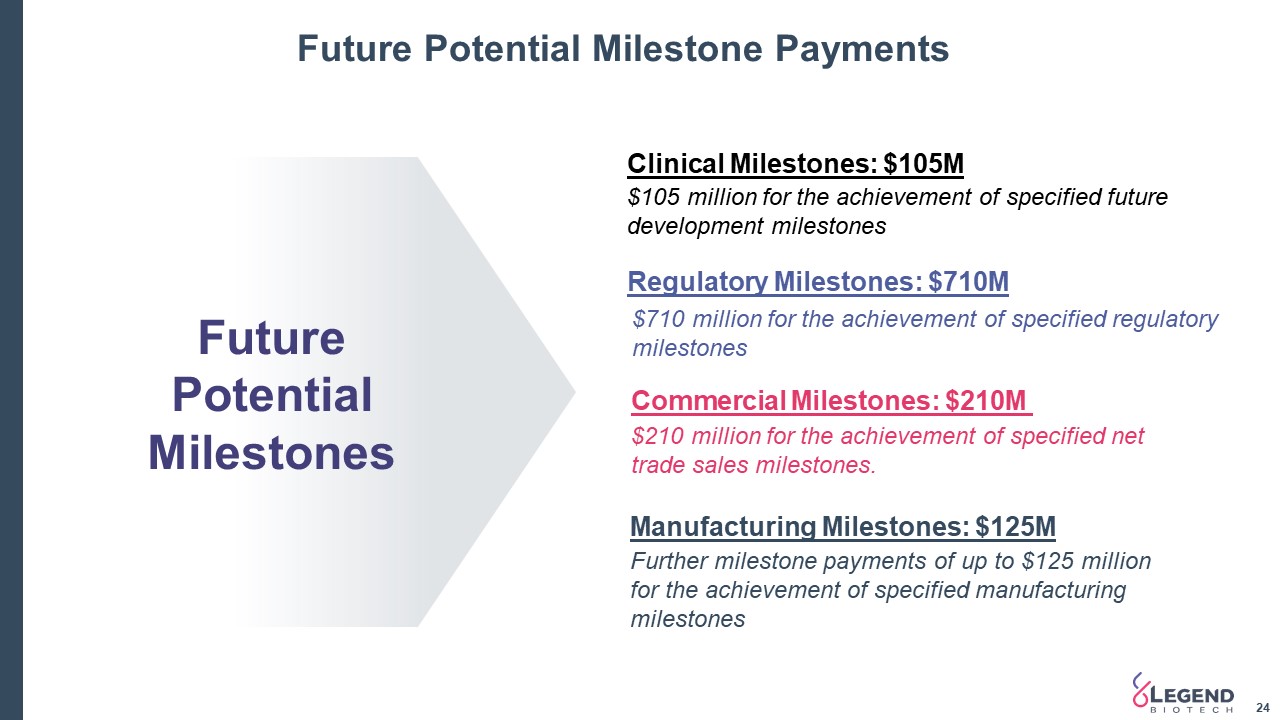
Future Potential Milestone Payments 24 Clinical Milestones: $105M $105 million for the achievement of specified future development milestones $710 million for the achievement of specified regulatory milestones Regulatory Milestones: $710M Further milestone payments of up to $125 million for the achievement of specified manufacturing milestones Manufacturing Milestones: $125M $210 million for the achievement of specified net trade sales milestones. Commercial Milestones: $210M Future Potential Milestones

Program Areas of DevelopmentLegend Biotech is utilizing the extensive cell therapy experience of our leadership and R&D staff, global clinical partners, and expanding research facilities to realize the potential of cell therapy to treat diseases that are thought to be incurable, such as hematologic malignancies, solid tumors and infectious diseases.
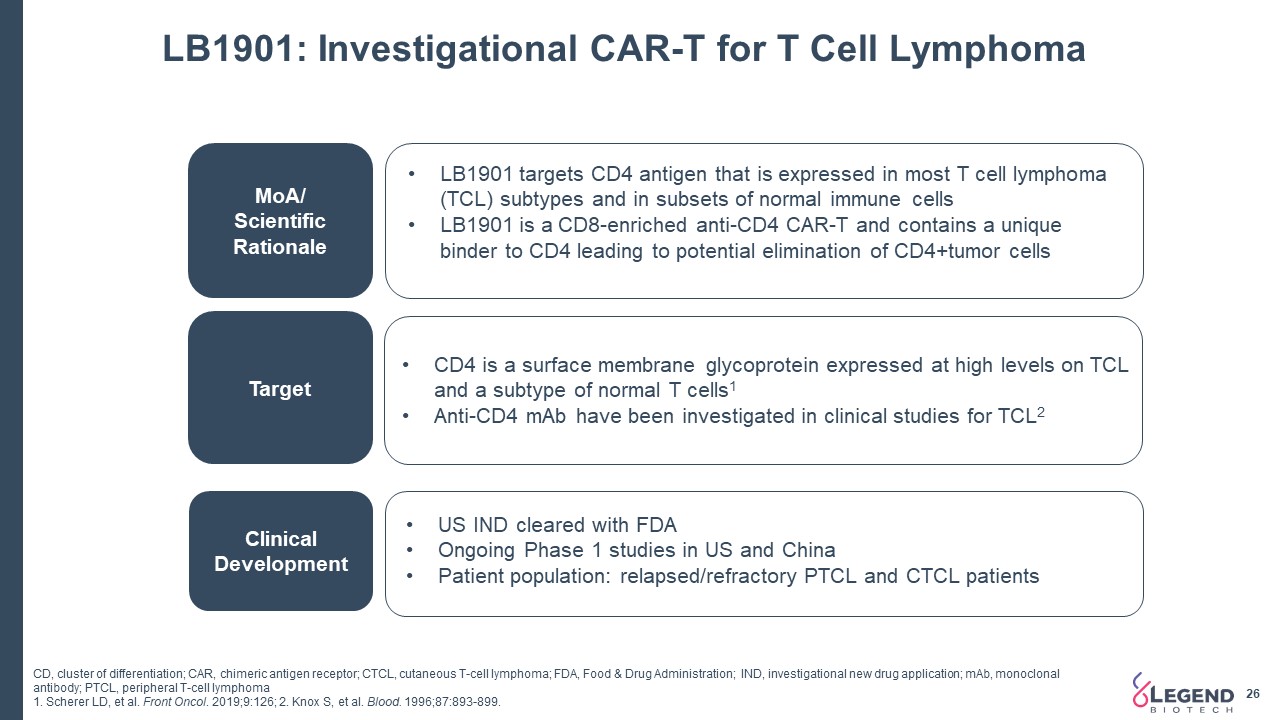
LB1901: Investigational CAR-T for T Cell Lymphoma 26 MoA/ Scientific Rationale Target Clinical Development LB1901 targets CD4 antigen that is expressed in most T cell lymphoma (TCL) subtypes and in subsets of normal immune cellsLB1901 is a CD8-enriched anti-CD4 CAR-T and contains a unique binder to CD4 leading to potential elimination of CD4+tumor cells CD4 is a surface membrane glycoprotein expressed at high levels on TCL and a subtype of normal T cells1Anti-CD4 mAb have been investigated in clinical studies for TCL2 US IND cleared with FDA Ongoing Phase 1 studies in US and China Patient population: relapsed/refractory PTCL and CTCL patients CD, cluster of differentiation; CAR, chimeric antigen receptor; CTCL, cutaneous T-cell lymphoma; FDA, Food & Drug Administration; IND, investigational new drug application; mAb, monoclonal antibody; PTCL, peripheral T-cell lymphoma1. Scherer LD, et al. Front Oncol. 2019;9:126; 2. Knox S, et al. Blood. 1996;87:893-899.
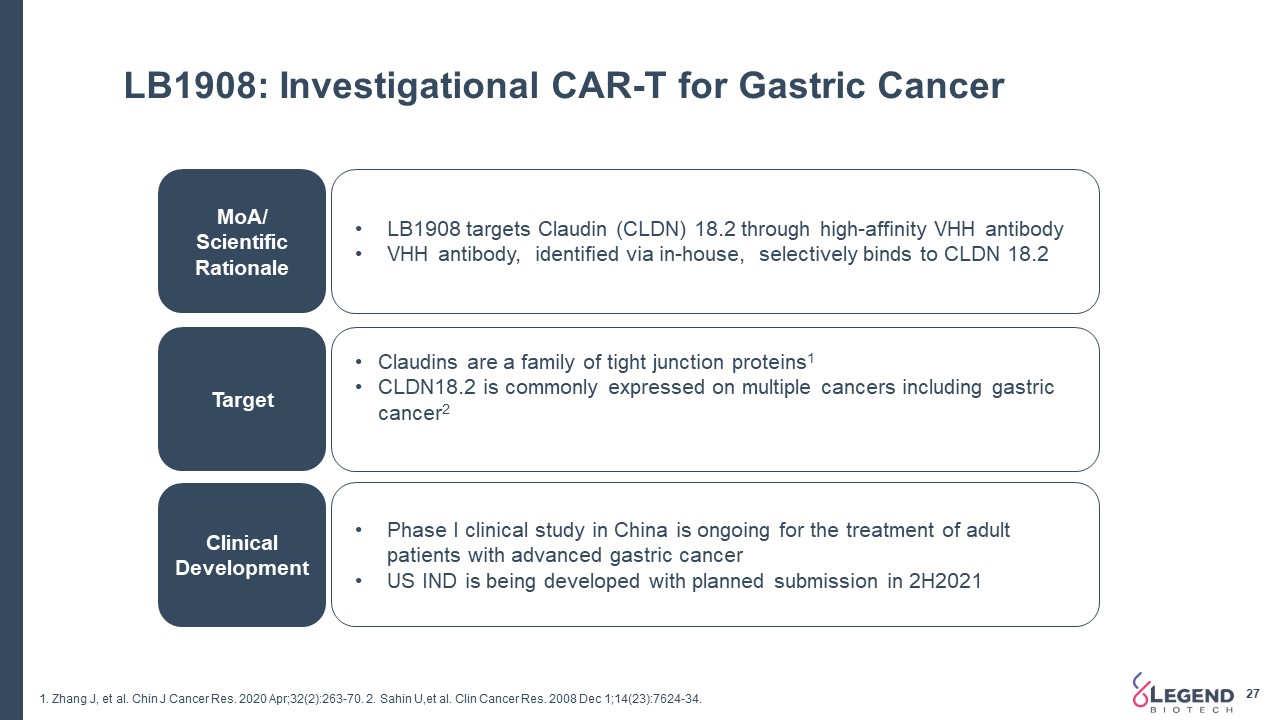
LB1908: Investigational CAR-T for Gastric Cancer 27 MoA/ Scientific Rationale Target Clinical Development LB1908 targets Claudin (CLDN) 18.2 through high-affinity VHH antibodyVHH antibody, identified via in-house, selectively binds to CLDN 18.2 Claudins are a family of tight junction proteins1CLDN18.2 is commonly expressed on multiple cancers including gastric cancer2 Phase I clinical study in China is ongoing for the treatment of adult patients with advanced gastric cancerUS IND is being developed with planned submission in 2H2021 1. Zhang J, et al. Chin J Cancer Res. 2020 Apr;32(2):263-70. 2. Sahin U,et al. Clin Cancer Res. 2008 Dec 1;14(23):7624-34.
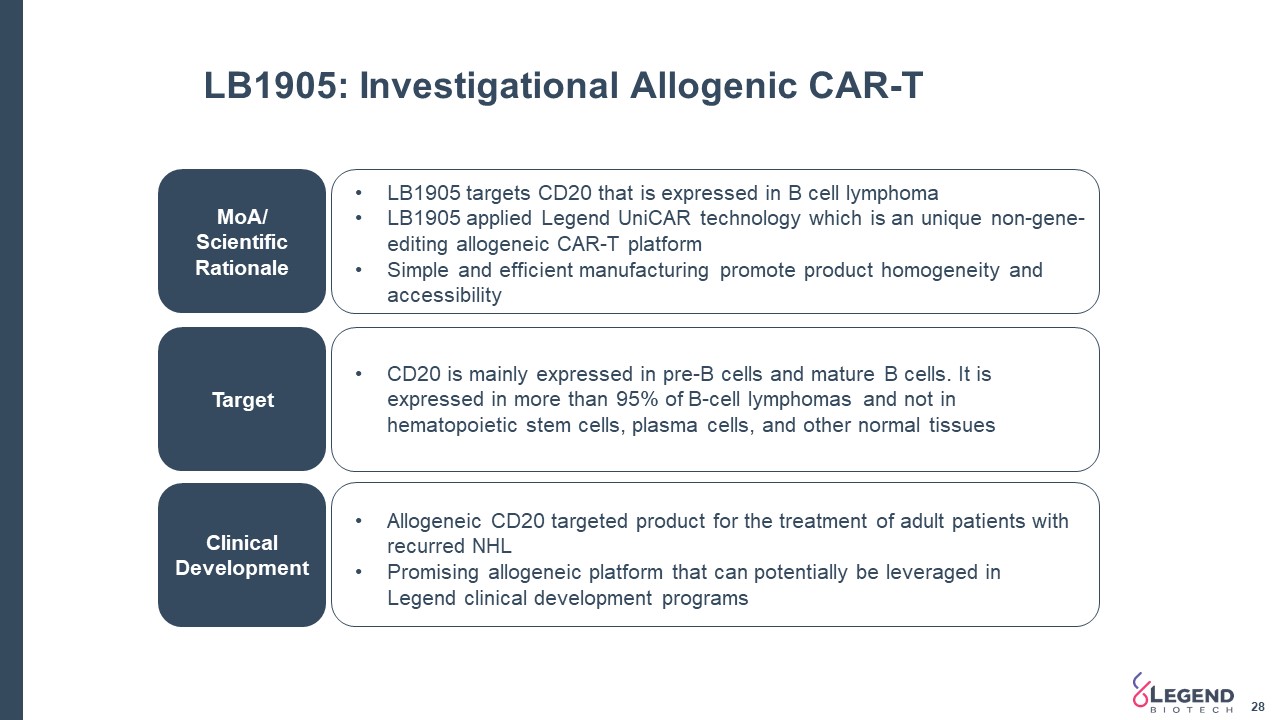
LB1905: Investigational Allogenic CAR-T MoA/ Scientific Rationale Target Clinical Development LB1905 targets CD20 that is expressed in B cell lymphoma LB1905 applied Legend UniCAR technology which is an unique non-gene-editing allogeneic CAR-T platformSimple and efficient manufacturing promote product homogeneity and accessibility CD20 is mainly expressed in pre-B cells and mature B cells. It is expressed in more than 95% of B-cell lymphomas and not in hematopoietic stem cells, plasma cells, and other normal tissues Allogeneic CD20 targeted product for the treatment of adult patients with recurred NHLPromising allogeneic platform that can potentially be leveraged in Legend clinical development programs 28
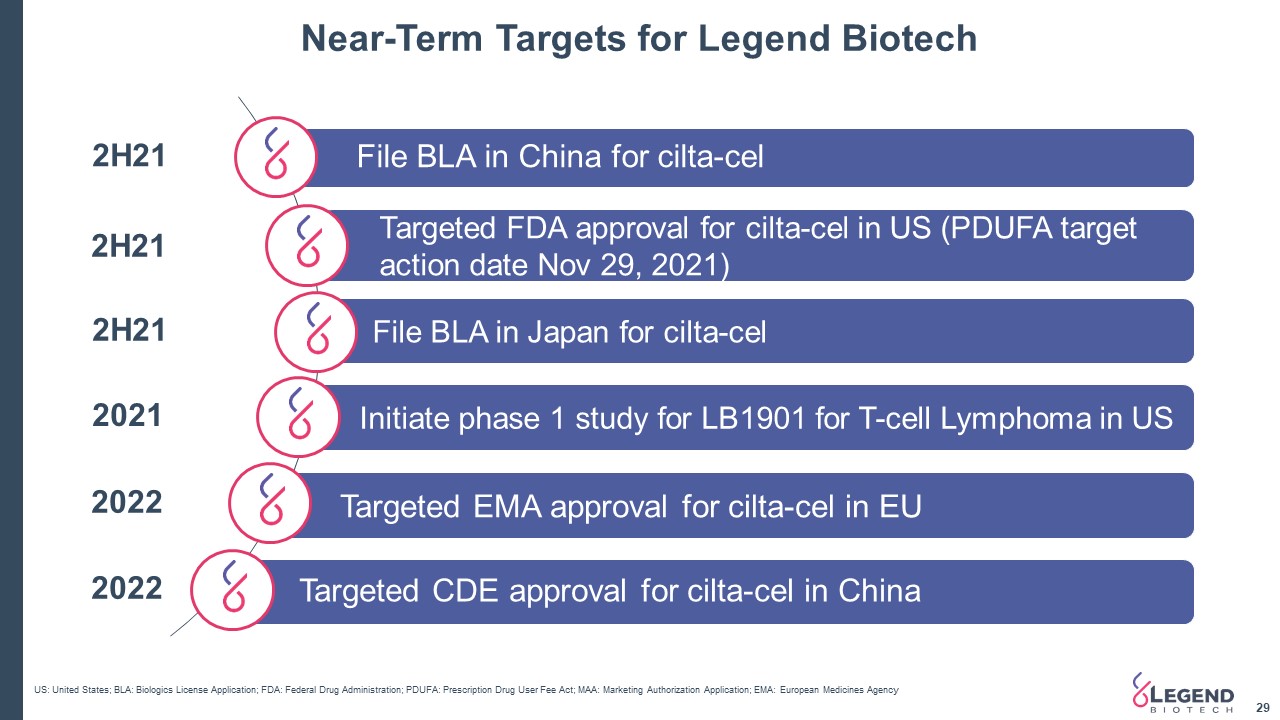
Near-Term Targets for Legend Biotech 29 File BLA in China for cilta-cel Targeted FDA approval for cilta-cel in US (PDUFA target action date Nov 29, 2021) 2H21 US: United States; BLA: Biologics License Application; FDA: Federal Drug Administration; PDUFA: Prescription Drug User Fee Act; MAA: Marketing Authorization Application; EMA: European Medicines Agency Targeted CDE approval for cilta-cel in China 2022 Targeted EMA approval for cilta-cel in EU 2022 Initiate phase 1 study for LB1901 for T-cell Lymphoma in US 2021 2H21 File BLA in Japan for cilta-cel 2H21
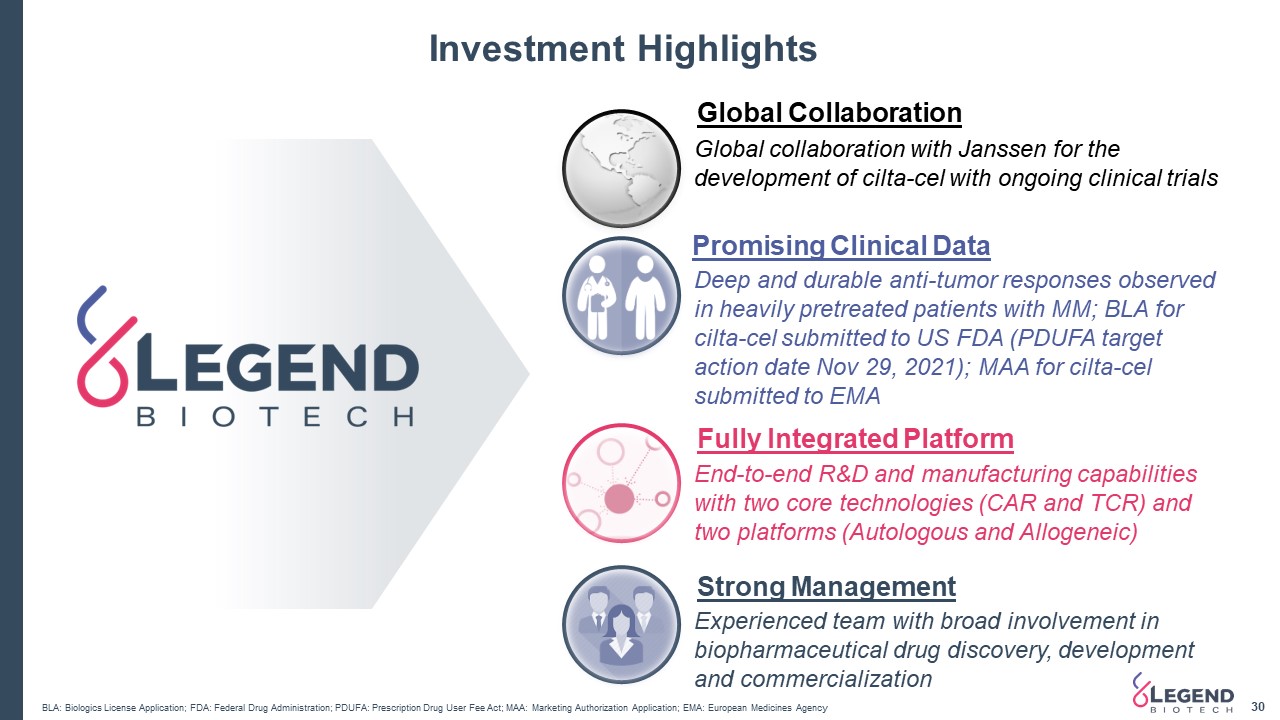
Investment Highlights 30 Global Collaboration Global collaboration with Janssen for the development of cilta-cel with ongoing clinical trials Deep and durable anti-tumor responses observed in heavily pretreated patients with MM; BLA for cilta-cel submitted to US FDA (PDUFA target action date Nov 29, 2021); MAA for cilta-cel submitted to EMA Promising Clinical Data End-to-end R&D and manufacturing capabilities with two core technologies (CAR and TCR) and two platforms (Autologous and Allogeneic) Experienced team with broad involvement in biopharmaceutical drug discovery, development and commercialization Fully Integrated Platform Strong Management BLA: Biologics License Application; FDA: Federal Drug Administration; PDUFA: Prescription Drug User Fee Act; MAA: Marketing Authorization Application; EMA: European Medicines Agency


Thank You !