Exhibit 99.1
Background:
CARTITUDE-4 is a global, phase 3, randomized, controlled trial (NCT04181827) of ciltacabtagene autoleucel (cilta-cel), a dual-binding, B-cell maturation antigen-targeting chimeric antigen receptor (CAR)-T cell therapy, versus (vs) standard of care (SOC; pomalidomide, bortezomib, and dexamethasone [PVd] or daratumumab, pomalidomide, and dexamethasone [DPd]) in lenalidomide-refractory patients.
Aims:
To report results of the first phase 3 study evaluating efficacy and safety of cilta-cel vs SOC in lenalidomide-refractory patients treated with 1-3 prior lines of therapy (LOT).
Methods:
Eligible patients had 1–3 prior LOT, including proteasome inhibitors (PI) and immunomodulatory drugs, and were lenalidomide-refractory. After apheresis, patients randomized to cilta-cel received PVd or DPd (physician’s choice) bridging therapy, then 1 cilta-cel infusion (target dose 0.75×106 CAR+ viable T cells/kg) 5–7 days after lymphodepletion. In the SOC group, patients received PVd or DPd (physician’s choice) until disease progression. The primary endpoint was progression-free survival (PFS), analyzed in the intent-to-treat (randomized) population. Informed consent was obtained prior to study entry.
Results:
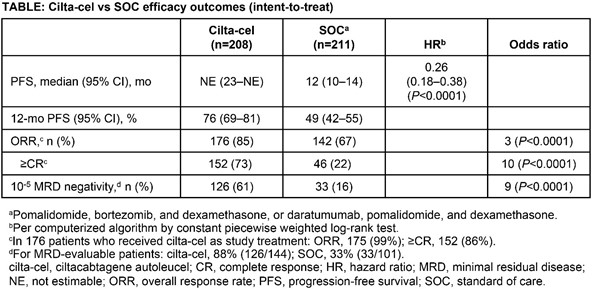
419 patients were randomized (cilta-cel, n=208; SOC, n=211 [PVd, n=28; DPd, n=183]). 176 patients received planned cilta-cel treatment, 20 more received cilta-cel after progressive disease (PD) during bridging therapy, and 208 received SOC treatment. There were no manufacturing failures. Baseline characteristics were balanced (cilta-cel vs SOC: 59% vs 63% cytogenetic high risk [including gain/amp 1q]; 50% vs 46% PI refractory; 24% vs 22% anti-CD38 refractory; 33% vs 32% had 1 prior LOT). Median dose of cilta-cel was 0.71×106 CAR+ viable T cells/kg. At Nov 1, 2022, data cut-off, median follow-up was 16 months (range, 0.1–27). The primary endpoint was met; cilta-cel reduced risk of progression/death by 74% (Hazard ratio [HR]=0.26; P-value [P] <0.0001). Patients in the cilta-cel group had significantly improved overall response rate, rate of complete response (CR) or better, and overall minimal residual disease (MRD) negativity rate compared to the SOC group (Table), with a positive trend in overall survival (HR, 0.78; 95% CI, 0.5–1.2). 97% and 94% of patients treated in the cilta-cel and SOC groups, respectively, had grade 3/4 adverse events, including infections (27% vs 25%) and cytopenias (94% vs 86%). In the cilta-cel and SOC groups, respectively, 39 and 46 patients died (14 and 30 due to PD). In patients who received cilta-cel as study treatment (n=176), 76% had cytokine release syndrome (1% grade 3; no grade 4/5) and 5% had immune effector cell associated neurotoxicity syndrome (all grade 1/2). A single case of movement and neurocognitive treatment-emergent adverse event was reported (grade 1).
Summary/Conclusion:
A single cilta-cel infusion significantly improved PFS vs SOC in lenalidomide-refractory patients with 1–3 prior LOT, with a favorable benefit/risk profile across patient populations. The 74% reduction in progression/death and high rates of CR and MRD-negativity highlight the potential for cilta-cel to become a key therapy for patients with multiple myeloma after first relapse.
Copyright © 2023. All rights reserved.