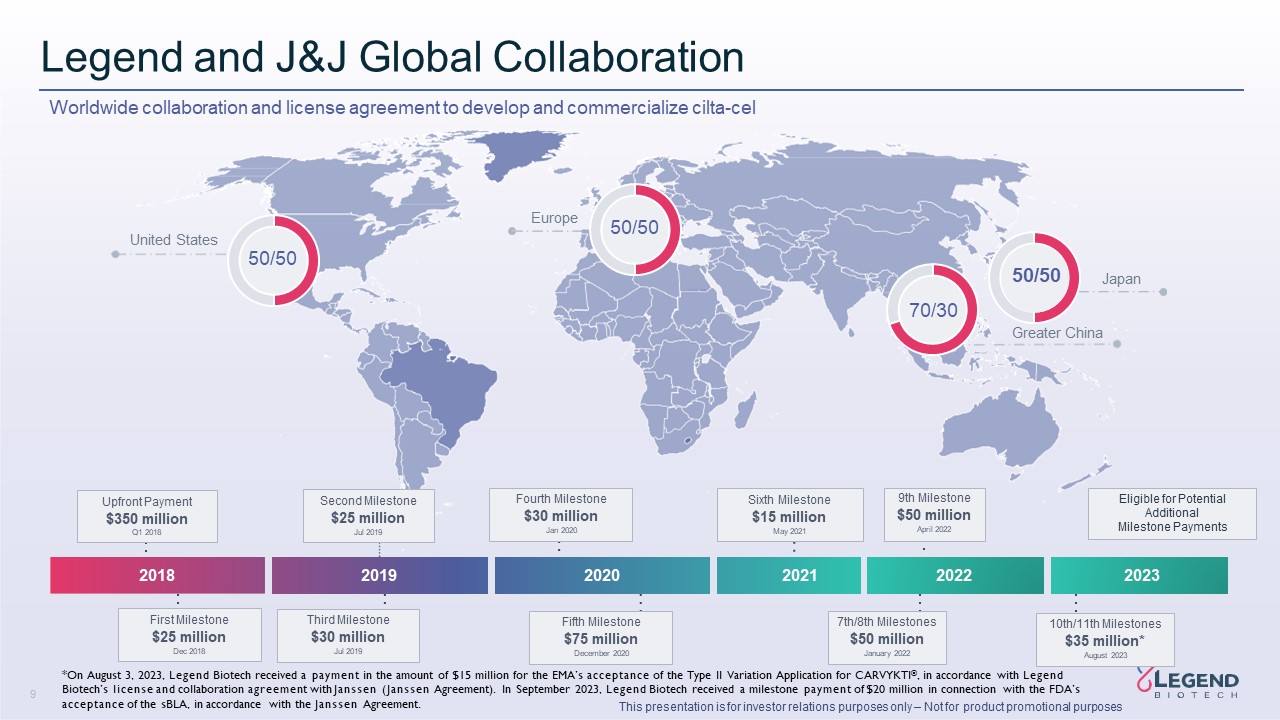
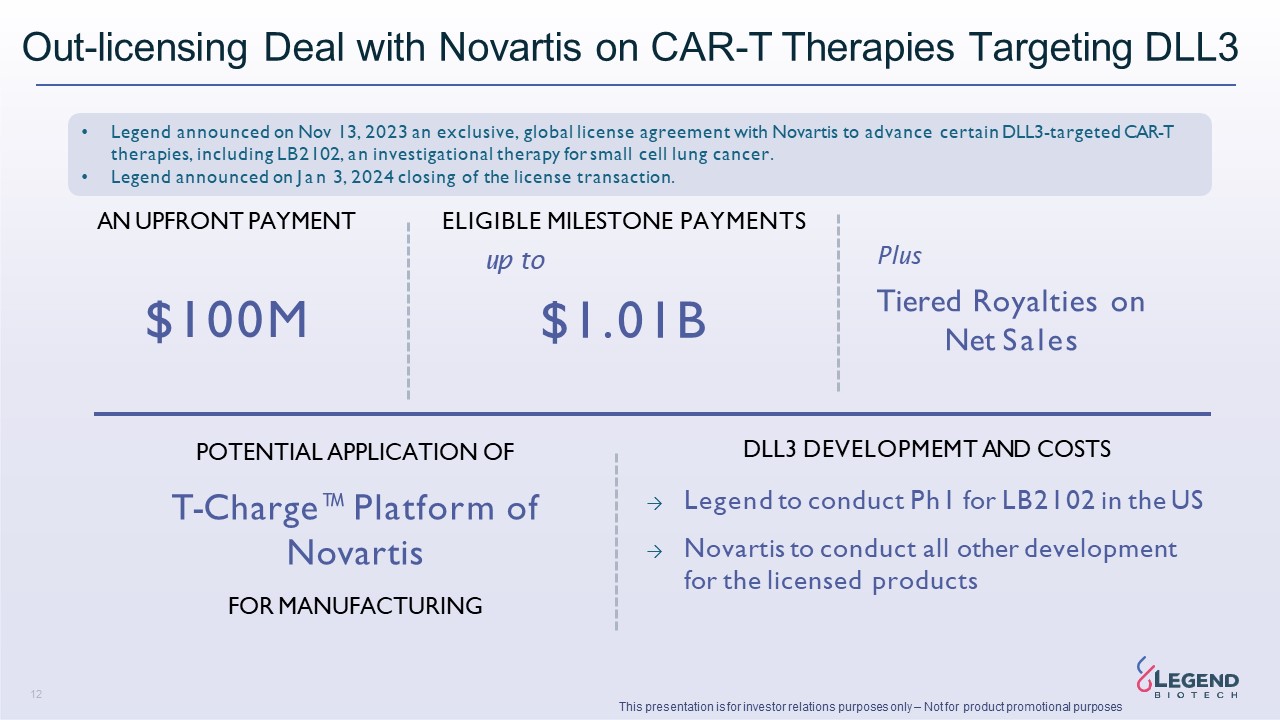
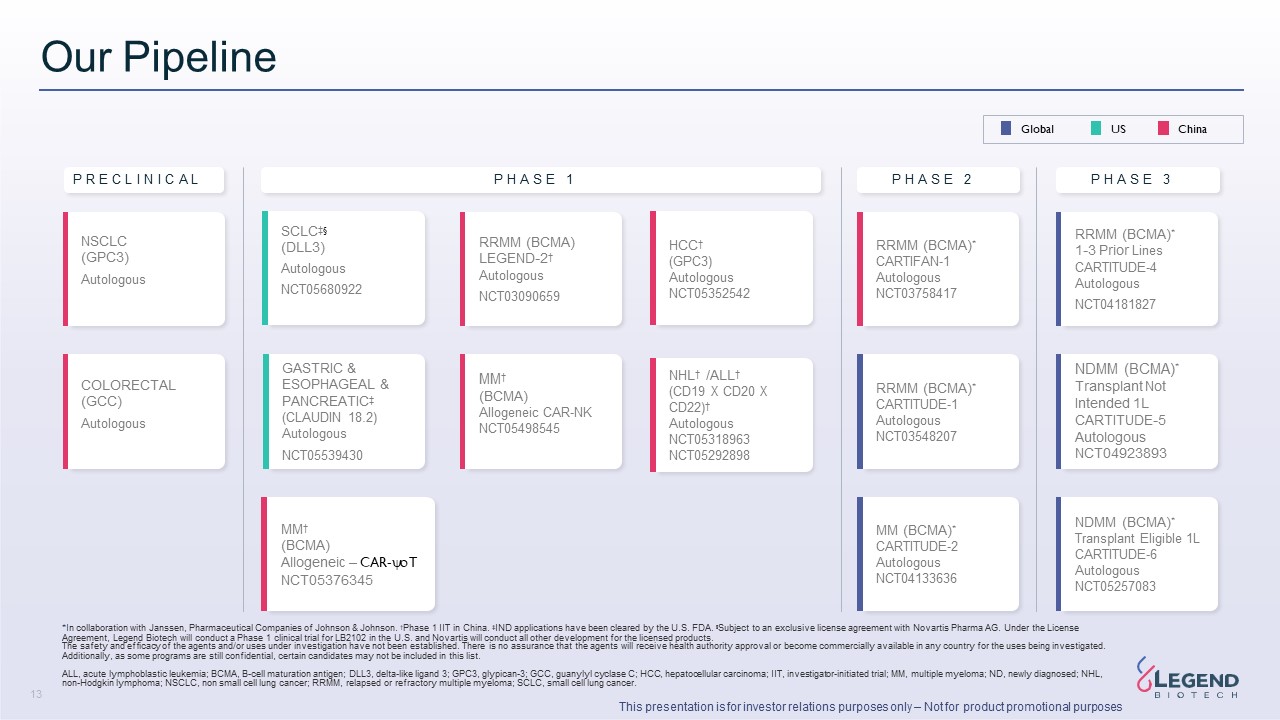
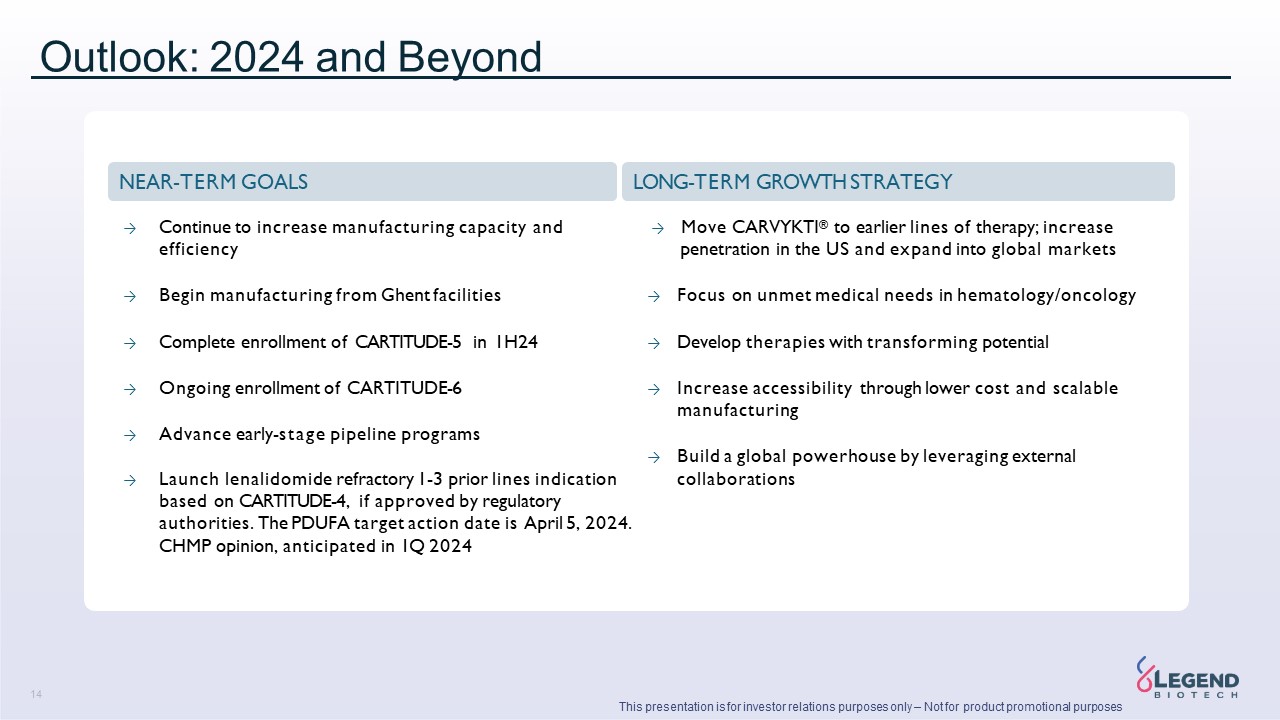
Disclaimer 3 This presentation is for investor relations purposes only – Not for product promotional purposes This presentation has been prepared by Legend Biotech Corporation (“Legend Biotech” or the “Company”) solely for information purpose and does not contain all relevant information relating to the Company. The safety and efficacy of the agents and/or uses under investigation discussed in this presentation have not been established, except to the extent specifically provided by marketing authorizations previously received from relevant health authorities. Further, for investigational agents and/or uses, the Company cannot guarantee health authority approval or that such agents and/or uses will become commercially available in any country. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and Legend Biotech's own internal estimates and research. While Legend Biotech believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Legend Biotech believes its internal research is reliable, such research has not been verified by any independent source. Statements in this presentation about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to Legend Biotech’s strategies and objectives; statements relating to CARVYKTI®, including Legend Biotech’s expectations for CARVYKTI®, including manufacturing expectations for CARVYKTI®; and statements about regulatory submissions for CARVYKTI®, and the progress of such submissions with the FDA, the EMA and other regulatory authorities; and expected results and timing of clinical trials; Legend Biotech’s expectations for LB2102 and its potential benefits; Legend Biotech’s ability to close the licensing transaction with Novartis and potential benefits of the transaction; Legend Biotech’s expectations on advancing their pipeline and product portfolio; and the potential benefits of Legend Biotech’s product candidates. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward- looking statements, although not all forward-looking statements contain these identifying words. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Legend Biotech’s expectations could be affected by, among other things, uncertainties involved in the development of new pharmaceutical products; unexpected clinical trial results, including as a result of additional analysis of existing clinical data or unexpected new clinical data; unexpected regulatory actions or delays, including requests for additional safety and/or efficacy data or analysis of data, or government regulation generally; unexpected delays as a result of actions undertaken, or failures to act, by our third party partners; uncertainties arising from challenges to Legend Biotech’s patent or other proprietary intellectual property protection, including the uncertainties involved in the U.S. litigation process; competition in general; government, industry, and general product pricing and other political pressures; the duration and severity of the COVID-19 pandemic and governmental and regulatory measures implemented in response to the evolving situation; as well as the other factors discussed in the “Risk Factors” section of Legend Biotech’s Annual Report on Form 20-F filed with the Securities and Exchange Commission (SEC) on March 30, 2023 and Legend Biotech’s other filings with the SEC. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in this presentation as anticipated, believed, estimated or expected. Any forward-looking statements contained in this presentation speak only as of the date of this presentation. Legend Biotech specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.