



VOLUME 26 · NUMBER 20 · JULY 10 2008
| JOURNAL | OF CLINICAL ONCOLOGY | O | R | I | G | I | N | A | L | R | E | P | O | R | T |
From the Department of Medicine, Divi- sion of Hematology and Medical Oncol- ogy, Naval Medical Center San Diego, San Diego, CA; Department of Surgery, General Surgery Service, Brooke Army Medical Center, Fort Sam Houston; The University of Texas, M.D. Anderson Cancer Center, Houston, TX; Depart- ment of Medicine, Hematology and Medical Oncology Service, Walter Reed Army Medical Center, Washington, DC; Cancer Vaccine Development Program, United States Military Cancer Institute, Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda, MD; Joyce Murtha Breast Care Center, Windber Medical Center, Windber, PA; and Antigen Express, Worchester, MA.
Submitted December 15, 2007;
accepted March 25, 2008.
Supported by the United States Military Cancer Institute, Department of Surgery, Uniformed Services University of the Health Sciences; the Department of Clinical Investigation, Walter Reed Army Medical Center; and Antigen Express Inc.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, the Depart- ment of the Navy, or the Department
Results of the First Phase I Clinical Trial of the Novel Ii-Key Hybrid Preventive HER-2/neu Peptide (AE37) Vaccine
Jarrod P. Holmes, Linda C. Benavides, Jeremy D. Gates, Mark G. Carmichael, Matthew T. Hueman,
Elizabeth A. Mittendorf, James L. Murray, Asna Amin, Dianna Craig, Eric von Hofe, Sathibalan Ponniah, and George E. Peoples
A B S T R A C T
Purpose
HER-2/neu is overexpressed in breast cancer and is the source of immunogenic peptides. CD4+
T-helper peptides for HER-2/neu are being evaluated in vaccine trials. The addition of Ii-Key, a four–amino-acid LRMK modification, increases vaccine potency when compared with unmodified class II epitopes. We present the results of the first human phase I trial of the Ii-Key hybrid HER-2/neu peptide (AE37) vaccine in disease-free, node-negative breast cancer patients.
Patients and Methods
The dose escalation trial included five dose groups, to determine safety and optimal dose of the hybrid peptide (100 µg, 500 µg, 1,000 µg) and granulocyte-macrophage colony-stimulating factor (GM-CSF; range, 0 to 250 µg). In the event of significant local toxicity, GM-CSF (or peptide in absence of GM-CSF) was reduced by 50%. Immunologic response was monitored by delayed- type hypersensitivity and [3H]thymidine proliferative assays for both the hybrid AE37 (LRMK- positive HER-2/neu:776-790) and AE36 (unmodified HER-2/neu:776-790).
Results
All 15 patients completed the trial with no grade 3 to 5 toxicities. Dose reductions occurred in 47% of patients. In the second group (peptide, 500 µg; GM-CSF, 250 µg), all patients required dose reductions, prompting peptide-only inoculations in the third group. The vaccine induced dose- dependent immunologic responses in vitro and in vivo to AE37, as well as AE36.
Conclusion
The hybrid AE37 vaccine seems safe and well tolerated with minimal toxicity if properly dosed. AE37
is capable of eliciting HER-2/neu–specific immune responses, even without the use of an adjuvant. This trial represents the first human experience with the Ii-Key modification, and to our knowledge, AE37 is the first peptide vaccine to show potency in the absence of an immunoadjuvant.
J Clin Oncol 26:3426-3433. © 2008 by American Society of Clinical Oncology
2/neu–positive breast cancer.7,8 Thus far, they have
of Defense.
Authors’ disclosures of potential con- flicts of interest and author contribu- tions are found at the end of this article.
Corresponding author: COL George E. Peoples, MD, FACS, Department of Surgery, General Surgery Service, Brooke Army Medical Center, 3851 Roger Brooke Dr, Fort Sam Houston, TX, 78234; e-mail: george.peoples@ amedd.army.mil.
© 2008 by American Society of Clinical Oncology
0732-183X/08/2620-3426/$20.00 DOI: 10.1200/JCO.2007.15.7842
INTRODUCTION
Breast cancer is the most common cancer in women. Treatment is multimodal, including surgery, chem- otherapy, radiation, and immunotherapy, as indi- cated.1,2 Despite this intensive therapy, many women with high-risk features, such as overexpres- sion of HER-2/neu protein, will develop recurrent disease.3 HER-2/neu is a member of the epidermal growth factor receptor family, normally expressed during fetal development and overexpressed in 30% of breast cancer.4 This protein is also a source of immunogenic peptides.5,6
Immunogenic peptides of HER-2/neu stimu- late cytotoxic T lymphocytes (CTLs) to recognize and kill HER-2/neu– expressing cancer cells in vitro.5,6 Some of the peptides (E75 and GP2) are being used as clinical vaccines in patients with HER-
been shown to be safe and effective in stimulating antigen-specific immunity; more importantly, we have shown that the immunity conferred by E75 seems to have clinical benefit in decreasing breast cancer recurrence.9 Unfortunately, vaccine-induced immunity is not sustained without booster vaccina- tions.9,10 CD4+ T-helper peptides may be required to increase efficiency of induction and establishment of long-term immunity.11,12
CD4+ T-helper peptides for HER-2/neu have been described; the first was G89 (HER-2/neu: 777- 789) by Tuttle et al.13 A similar peptide (HER-2/neu: 776-790) was used in combination with two other peptides by Disis et al,14 producing encouraging im- munologic responses. A novel method to increase antigen-specific stimulation of T-helper cells has been the use of the invariant protein (Ii-Key). Spe- cifically, the addition of a four–amino-acid sequence
Ii-Key Hybrid HER-2/neu Peptide (AE37) Vaccine
(LRMK) added to T-helper peptides facilitates direct antigenic epitope charging of major histocompatibility complex (MHC) class II mole- cules at the cell surface.15,16 This enhanced epitope charging and concomitant increase in antigen presentation can increase potency 250 times or more compared with the unmodified class II epitope in vitro.17,18 Animal models have shown Ii-Key hybrid methodology to be highly efficient using melanoma peptides, but no human data exist to date applying this hybrid peptide.19 AE37 is the Ii-Key hybrid of HER-2/neu peptide 776 to 790 (AE36). We have performed a phase Ib trial of AE37 peptide vaccine in HER-2/neu–positive breast cancer patients to document safety and measure immunologic responses to escalating vaccine doses. The results of the first human trial of the Ii-Key hybrid technology are reported here.
PATIENTS AND METHODS
Patient Characteristics and Clinical Protocol
The trial was institutional review board approved and conducted at Walter Reed Army Medical Center (Washington, DC) under investigational new drug application #12,229. All patients had histologically confirmed, node- negative breast cancer and completed standard course of surgery, chemother- apy, and radiation (as required) before enrollment. Patients receiving hormonal therapy continued their regimens. After proper counseling/con- sent, breast cancer patients were enrolled and HLA typed (DNA genomic typing). Before vaccination, patients were skin tested with a panel of recall antigens (Mantoux test). Patients were considered immunocompetent if they reacted (> 5 mm) to two or more antigens.
Vaccine
The Ii-Key/HER-2/neu MHC class II peptide, AE37 (Ac- LRMKGVGSPYVSRLLGICL-NH2) was commercially produced in ac- cordance with federal guideline current good manufacturing practices by NeoMPS Inc (San Diego, CA). Peptide purity (> 95%) was verified by high- performance liquid chromatography and mass spectrometry. Sterility and general safety testing was carried out by the manufacturer. Lyophilized peptide was reconstituted in 0.5 mL of sterile saline at the following concentrations: 100 µg, 500 µg, and 1,000 µg. The peptide was mixed with granulocyte- macrophage colony-stimulating factor (GM-CSF) (Berlex, Seattle, WA) at varying concentrations in 0.5 mL (Table 1). The 1.0-mL inoculation was split and administered intradermally at two sites 5 cm apart in the same extremity.
Vaccination Series
The study was performed as a dose escalation trial to define vaccine and GM-CSF optimal dosing. Each dosing group consisted of three patients. The first three dose groups (patients A1-A9) received escalating amounts of AE37 peptide and fixed initial GM-CSF dose (Table 1). GM-CSF dose was chosen on the basis of our previous E75 trials. GM-CSF was reduced 50% in subsequent inoculations for local reactions of 100 mm or more or grade 2 systemic toxicities. The cutoff of 100 mm was determined from previous experience; at
Table 1. Dosing of Vaccine and Adjuvant Dose AE37 GM-CSF Initial Group* Patient No. Dose (µg) Dose (µg) Schedule |
| 100:250:6 | A1, A2, A3 | 100 | 250 | Monthly X6 |
| 500:250:6 | A4, A5, A6 | 500 | 250 | Monthly X6 |
| 1000:0:6 | A7, A8, A9 | 1,000 | 0 | Monthly X6 |
| 500:125:6 | A10, A11, A12 | 500 | 125 | Monthly X6 |
| 500:30:6 | A13, A14, A15 | 500 | 30 | Monthly X6 |
Abbreviation: GM-CSF, granulocyte-macrophage colony-stimulating factor. *Designated as peptide (µg):GM-CSF (µg): number of inoculations. |
100 mm and greater, the sites become confluent and local toxicity in- creases, and our goal is to minimize toxicity and prevent skin disruptions. All patients received six monthly inoculations. Because each patient in the 500:250:6 group (patients A4-A6) required multiple GM-CSF reductions, the 1,000:0:6 group (patients A7-A9) was inoculated without GM-CSF. One patient in this group (1,000:0:6) failed to respond with a local reaction during inoculations 1 to 3, so on the fourth inoculation, GM-CSF was reinstated to produce a local reaction. The two subsequent dose groups (patients A10-A15) received a fixed dose of AE37 (500 µg) with varying GM-CSF (Table 1).
Toxicity
Patients were observed 1 hour postvaccination for immediate hypersen- sitivity and returned 48 to 72 hours later to have injection sites measured and questioned in regard to local/systemic toxicities. Toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (reported on a 0-5 scale). Progression from one dose group to the next occurred only if no significant toxicity occurred in the lower dose group.
Control Peptides and Proteins
The AE37 peptide (Ac-LRMKGVGSPYVSRLLGICL-NH2) used in this study is a fusion of the Ii-Key peptide (LRMK) with the native HER- 2/neu peptide (aa776-790:GVGSPYVSRLLGICL). To assess whether mea- sured immune responses are representative of reactivity against native HER-2/neu peptide, we measured immune responses against the HER-2/neu MHC class II peptide, AE36 (aa776-790:Ac-GVGSPYVSRLLGICL-NH2). In
addition, a peptide (AEN) containing the Ii-Key peptide fused to a non–HER-
2/neu sequence (HIV/gag 164-181:YVDRFYKTLRAEQASQEV) was used as a negative control, and tetanus toxoid (TT; List Biologicals Inc, Campbell, CA) was used as a positive control antigen for the immune assays.
Peripheral-Blood Mononuclear Cell Isolation and Cultures
Blood was drawn before each inoculation and at 1 (postvaccine) and 6 months (long term) after vaccine series completion. Forty milliliters of blood was drawn and peripheral-blood mononuclear cells (PBMCs) were isolated. PBMCs were washed and resuspended in culture medium (RPMI-1640 –positive/10% FCS–positive/penicillin- and streptomycin– positive/L-glutamine) and used as a source of lymphocytes as previ- ously described.7,20,21
Proliferation Assay
PBMCs were used for monitoring of vaccine-specific proliferative activ- ity of T lymphocytes using a standard radioactive [3H]thymidine incorpora- tion assay. PBMCs were stimulated in absence or presence of peptide or antigen. Each of the peptides (AE36/AE37/AEN) or TT was added as triplicates to a 96 –round bottom well plate, and one set of wells had no stimulant added and served as control wells. The peptides were tested at two concentrations (1 and 10 µg/mL), whereas TT was used at 1 µg/mL. PBMCs were resuspended in culture medium and added at 3 X 105 cells/200µ/well. The plate was then incubated in a humidified CO2 incubator for 4 days. On day 3 of incubation, wells were pulsed with 1 uCi/well of radioactive [3H]thymidine, and plates returned to the incubator. On day 4, cells were collected using a cell harvester (Harvester96-MachIII; Tomtec, Orange, CT) on to a filter mat and counted using a scintillation counter (MicroBeta Trilux, Perkin Elmer, Norwalk, CT). Proliferation was measured by amount of thymidine incorporation, deter- mined as counts per minute. Average counts per minute were calculated for the triplicate cultures.
Delayed-Type Hypersensitivity
Delayed-type hypersensitivity (DTH) reaction was performed before vaccination and at 1 month after completion of vaccine series (long term). Intradermal injections, on the back or extremity (opposite side from vaccina- tion), of 100 µgof AE37 (without GM-CSF) in 0.5 mL of saline were compared with an equal volume control inoculum of saline. DTH reactions were mea- sured in two dimensions at 48 to 72 hours using the sensitive ballpoint pen method, and results were reported as an orthogonal mean.22
Holmes et al
Statistical Analysis
P values for clinicopathologic factors were calculated using Wilcoxon test, Fisher’s exact test, or x2 test as appropriate. P values for comparing pre- and postvaccine proliferative assays and DTH were calculated using the t test, paired or unpaired, as appropriate.
RESULTS
Patients
We enrolled and vaccinated 15 disease-free, node-negative breast cancer patients all expressing varying levels of HER-2/neu (ranging from IHC 1+ to 3+). No patient withdrew from this study. Patient demographics, prognostic factors, and treatment profiles are presented in Table 2.
Vaccine and Vaccination Series
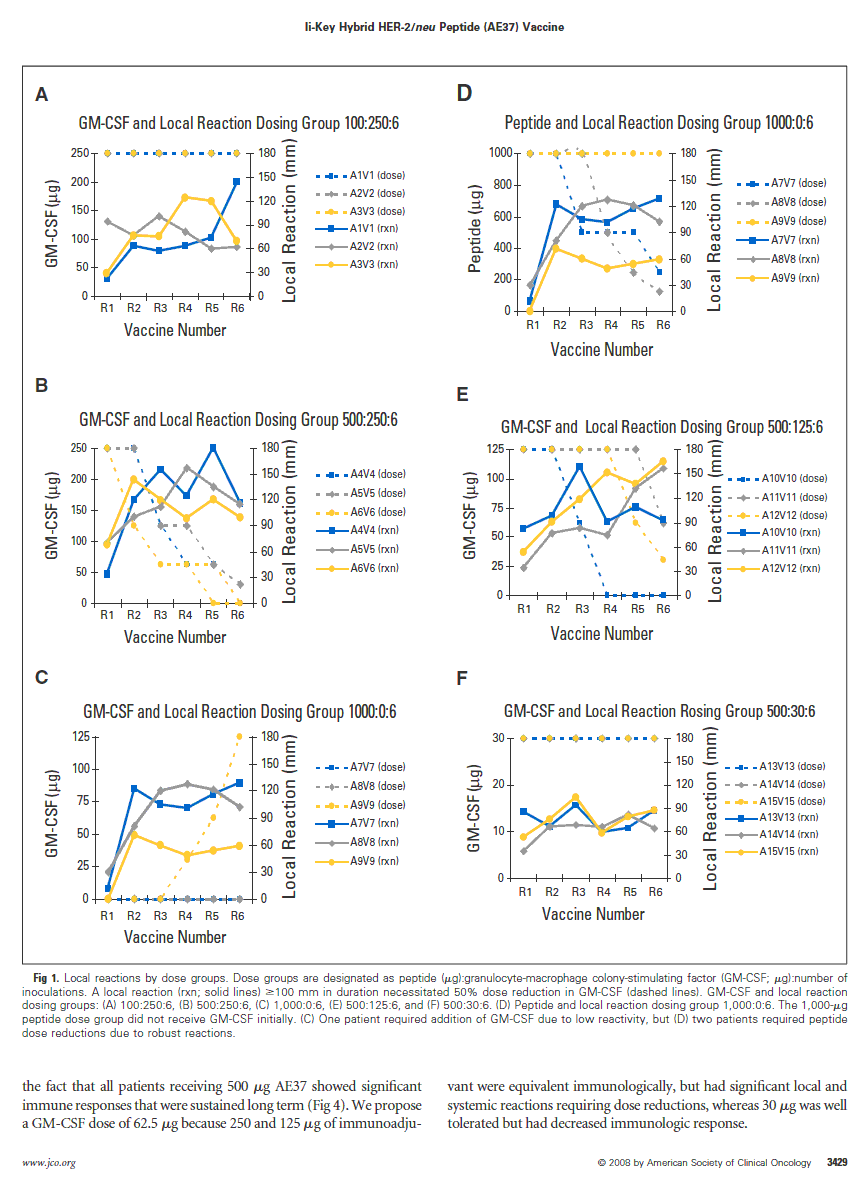
Table 1 provides the dose escalation strategy utilized, and Figure 1 illus- trates the robust local reactions to the hybrid vaccine. GM-CSF dose reduc- tions (or peptide if no GM-CSF), as dictated by local or systemic reactions, are depicted. Dose groups are designated as peptide(µg):GM-CSF(µg):number of inoculations. The first group (100:250:6) required no dose reductions. How- ever, all three patients in the second group (500:250:6) required GM-CSF dose reduction by the third vaccination. Given the significant local reactions, the third group (1,000:0:6) was initiated without GM-CSF; two of three patients in this group required reduction of peptide from 1,000 µg. Another patient in this group did not show a strong local response, and GM-CSF was added back to the patients’ vaccine schedule on the fourth inoculation in an escalating fashion. For the latter dose groups, a fixed amount of peptide (500 µg) was administered with varying GM-CSF doses. Dose reductions occurred in all patients in the 500:125:6 dose group, but later in the vaccination series com- pared with the 500:250:6 group. No reductions occurred in the patients in the 500:30:6 dose group.
Combined Dosing Group Results
There were no grade 3 to 5 toxicities among the 15 patients receiving a total of 90 doses of AE37 ± GM-CSF. Among all patients, maximum local toxicities were grade 1 (40%) or grade 2 (60%). Maximum systemic toxicity
was mild; grade 0 (13%), grade 1 (73%), and grade 2 (13%). Grade 1 toxicities
Table 2. Patient Demographics, Prognostic Factors, and Treatment Profiles
AE37-Vaccinated Patients (n = 15)
Characteristic No. % |
Age, years
Median Range | | | | | | | 57 44-70 | |
| Race | | | | | | | | |
| White | | | 9 | | | | | | | 60 |
| African American | | | 5 | | | | | | | 33 |
| Asian | | | 1 | | | | | | | 7 |
Tumor size > T2 (> 2 cm) | | | 1 | | | | | | | 7 |
| Histologic grade Grade III | | | 3 | | | | | | | 20 |
| HER-2/neu IHC 3+ or FISH+ | | | 5 | | | | | | | 33 |
| Hormone receptor negative | | | 4 | | | | | | | 27 |
| No chemotherapy | | | 12 | | | | | | | 80 |
| No RT | | | 4 | | | | | | | 27 |
| Hormonal therapy | | | 11 | | | | | | | 73 |
| Abbreviations: IHC, immunohistochemistry; FISH, fluorescent in situ hybrid- ization; RT, radiotherapy. |
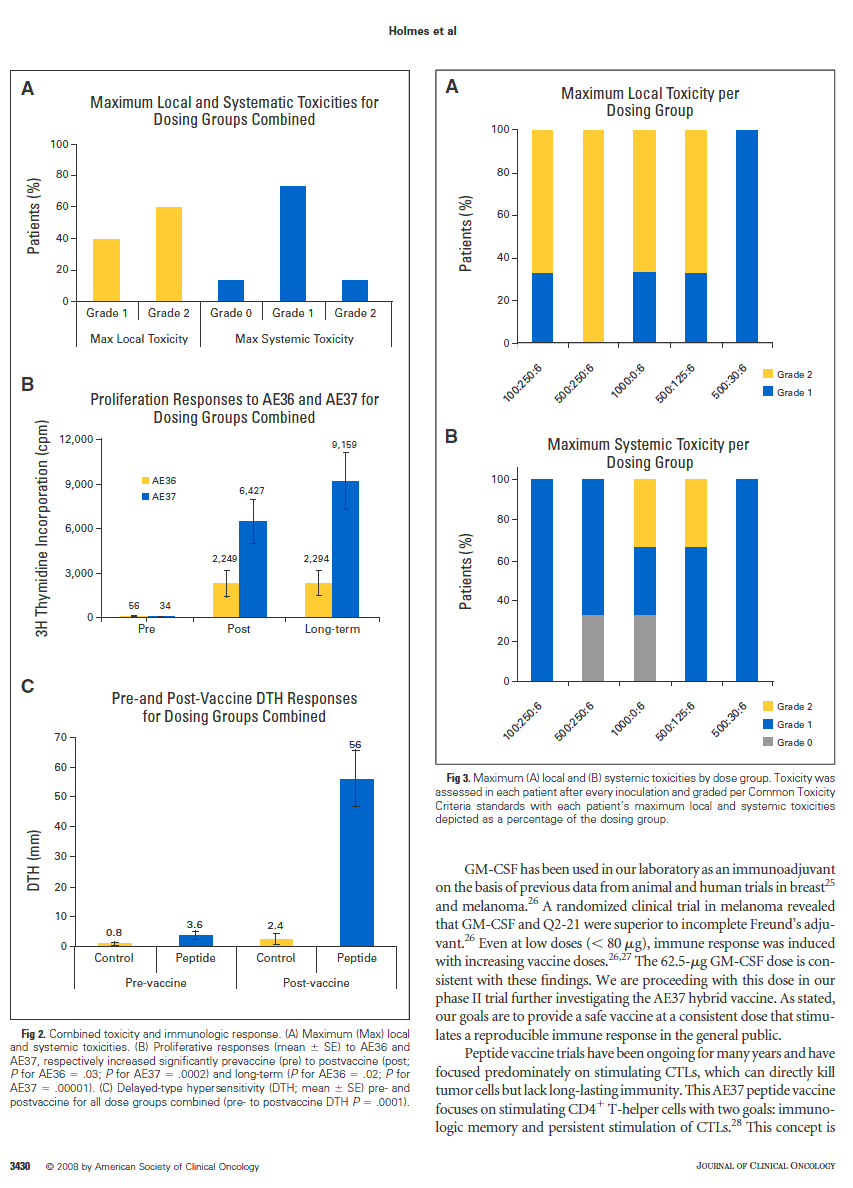
included fatigue, nausea, myalgias, rhinitis, diarrhea, headache, and cough. Grade 2 toxicities included joint pain and stiffness. Local reaction measure- ment of 100 mm or greater in duration or systemic grade 2 toxicity caused 47% of patients to undergo dose reductions. Taken as a whole, the vaccine is safe with minimal local and systemic toxicity, as depicted in Figure 2A.
The AE37 peptide vaccine was capable of eliciting an immune response both in vitro and in vivo. To assess in vitro immune response, we analyzed peptide-induced proliferation of T lymphocytes by [3H]thymidine incorpora- tion. The average proliferative response to AE36 and AE37 increased signifi- cantly pre- to postvaccine (1 month postcompletion), and prevaccine to long term (6 months postcompletion; Fig 2B). The vaccine’s in vivo effectiveness was demonstrated by significantly increased DTH comparing pre- with post- vaccination (1 month postcompletion) reactions (Fig 2C). Overall, all doses of AE37 were highly immunogenic.
Toxicity per Dosing Group
Local and systemic toxicities in each dosing group are depicted respec- tively in Figure 3A and 3B. Local toxicity was highest in the 500:250:6 dosing group, with 100% of the patients experiencing grade 2 toxicity. Systemic symptoms were predominately grade 1, with only two patients (one per dosing group: 1,000:0:6 and 500:125:6) experiencing grade 2 toxicity.
Immunologic Response per Dosing Group
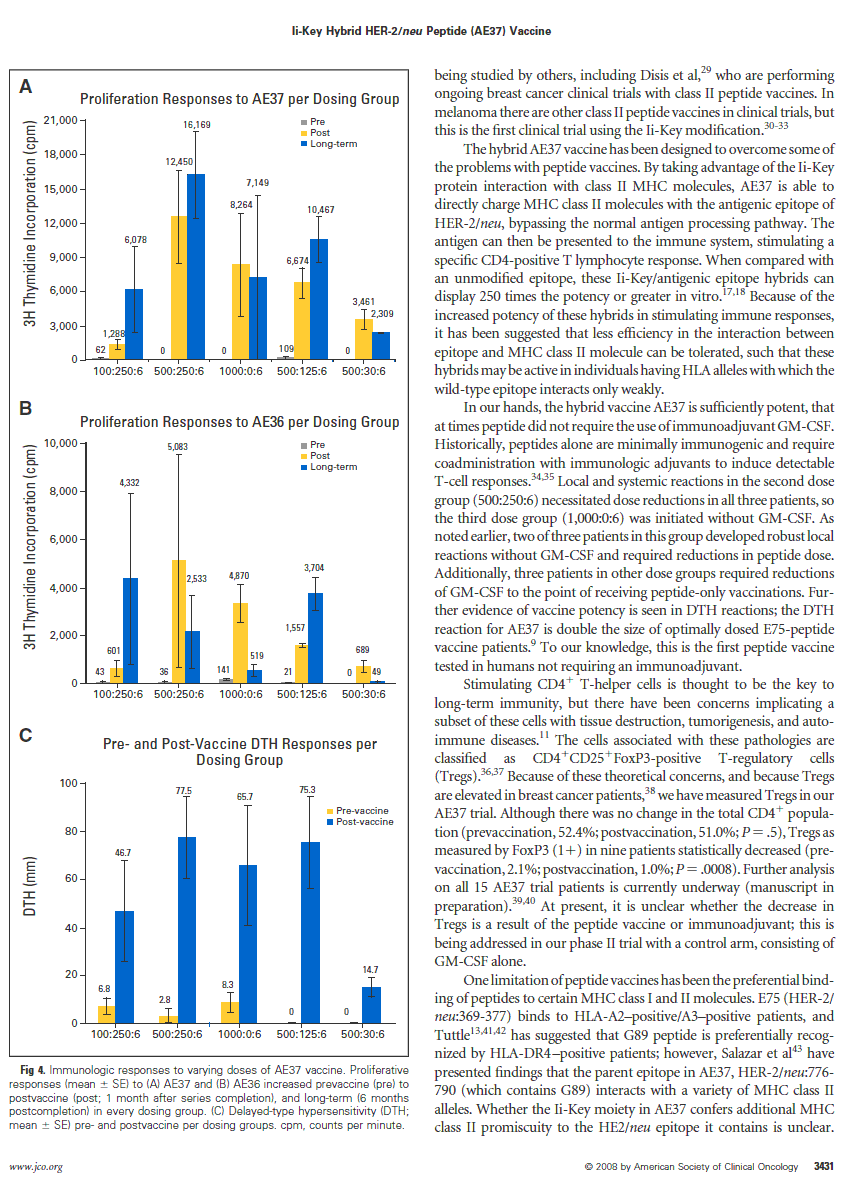
Proliferation assays. Mean proliferative responses to AE37 by dose group increased significantly pre- to postvaccine in all dose groups (Fig 4A). There was a significant increase in proliferation response at long-term follow-up compared with prevaccine in all of the 500-µg peptide dosing groups. The patients also responded to AE36, the wild-type peptide (Fig 4B). The 500:125:6 dosing group demonstrated the most consistent proliferative responses to both AE37 and AE36 in both short- and long-term postvaccine time points. Immunologic responses were decreased in the 500:30:6 dose group.
DTH. All of the dosing groups had statistically significant in- creases in their pre- to postvaccine DTH responses, and reaction measurements were enhanced as peptide dose increased (Fig 4C). There was negligible decrease in DTH reactions noted with GM-CSF reduction from 250 to 125 µg, but significant DTH decrease with GM-CSF of 30 µg.
DISCUSSION
In our phase I clinical trial, we have shown the AE37 peptide vaccine to be both safe and effective in raising HER-2/neu–specific immunity both in vitro and in vivo as long as the vaccine is dosed correctly. In preclinical models, the Ii-Key hybrid peptide AE37 is more immuno- genic than its wild-type counterpart23 and is more sensitive in detect- ing pre-existing HER-2/neu immunity.18,24 In our trial, dose reductions were required to the point of eliminating adjuvant GM- CSF altogether, demonstrating potency of the hybrid peptide vaccine. The goal of our phase I testing was to determine safety and optimal biologic dose (OBD) with an emphasis on the community; that is, striking a balance between stimulating as strong an immune response as possible without the need for dose reductions so the vaccine can be administered safely and effectively to a large population.
The OBD of the novel AE37 hybrid vaccine seems to be 500 µg of peptide with GM-CSF more than 30 µg and less than 125 µg. The OBD was established in two parts: the peptide dose and adjuvant dose. The optimal AE37 peptide dose (500 µg) is based on the fact that two of three patients receiving 1,000 µg of peptide required reduction in peptide to less than 250 µg by the end of their vaccine series, as well as
Ii-Key Hybrid HER-2/neu Peptide (AE37) Vaccine
A
GM-CSF and Local Reaction Dosing Group 100:250:6
D
Peptide and Local Reaction Dosing Group 1000:0:6
250
200
180
150
120
A1V1 (dose) A2V2 (dose)
1000
800
180
150
120
A7V7 (dose) A8V8 (dose)
150
100
A3V3 (dose)
90 A1V1 (rxn)
600
A9V9 (dose)
90 A7V7 (rxn)
60
50 30
A2V2 (rxn) A3V3 (rxn)
400
60 A8V8 (rxn)
0 0
R1 R2 R3 R4 R5 R6
Vaccine Number
B
200
0
E
30
0
R1 R2 R3 R4 R5 R6
Vaccine Number
A9V9 (rxn)
GM-CSF and Local Reaction Dosing Group 500:250:6
GM-CSF and Local Reaction Dosing Group 500:125:6
250
200
150
180
150
120
A4V4 (dose) A5V5 (dose) A6V6 (dose)
125
100
75
180
150
120
A10V10 (dose) A11V11 (dose) A12V12 (dose)
100
90 A4V4 (rxn) 50
60 A5V5 (rxn)
90
A10V10 (rxn)
60 A11V11 (rxn)
50 30
0 0
R1 R2 R3 R4 R5 R6
Vaccine Number
C
A6V6 (rxn)
25 30
0 0
R1 R2 R3 R4 R5 R6
Vaccine Number
F
A12V12 (rxn)
GM-CSF and Local Reaction Dosing Group 1000:0:6
GM-CSF and Local Reaction Rosing Group 500:30:6
125
100
75
180
150
120
30
A7V7 (dose)
A8V8 (dose) 20
A9V9 (dose)
180
150
120
90
A13V13 (dose) A14V14 (dose) A15V15 (dose)
90 A7V7 (rxn)
50 10
A13V13 (rxn)
60 A14V14 (rxn)
60 A8V8 (rxn)
30 A15V15 (rxn)
25 30
0 0
R1 R2 R3 R4 R5 R6
Vaccine Number
A9V9 (rxn)
0 0
R1 R2 R3 R4 R5 R6
Vaccine Number
Fig 1. Local reactions by dose groups. Dose groups are designated as peptide (µg):granulocyte-macrophage colony-stimulating factor (GM-CSF; µg):number of inoculations. A local reaction (rxn; solid lines) >100 mm in duration necessitated 50% dose reduction in GM-CSF (dashed lines). GM-CSF and local reaction dosing groups: (A) 100:250:6, (B) 500:250:6, (C) 1,000:0:6, (E) 500:125:6, and (F) 500:30:6. (D) Peptide and local reaction dosing group 1,000:0:6. The 1,000-µg peptide dose group did not receive GM-CSF initially. (C) One patient required addition of GM-CSF due to low reactivity, but (D) two patients required peptide dose reductions due to robust reactions.
the fact that all patients receiving 500 µg AE37 showed significant immune responses that were sustained long term (Fig 4). We propose a GM-CSF dose of 62.5 µg because 250 and 125 µg of immunoadju-
vant were equivalent immunologically, but had significant local and systemic reactions requiring dose reductions, whereas 30 µg was well tolerated but had decreased immunologic response.
Holmes et al
A Maximum Local and Systematic Toxicities for Dosing Groups Combined 100 80 60 40 20 0 Grade 1 Grade 2 Grade 0 Grade 1 Grade 2 Max Local Toxicity Max Systemic Toxicity B Proliferation Responses to AE36 and AE37 for Dosing Groups Combined 12,000 9,159 9,000 AE36 6,427 AE37 6,000 2,249 2,294 3,000 56 34 0 Pre Post Long-term C Pre-and Post-Vaccine DTH Responses for Dosing Groups Combined 70 56 60 50 40 30 20 10 3.6 2.4 0.8 0 Control Peptide Control Peptide Pre-vaccine Post-vaccine
| A Maximum Local Toxicity per Dosing Group 100 80 60 40 20 0 Grade 2 Grade 1 B Maximum Systemic Toxicity per Dosing Group 100 80 60 40 20 0 Grade 2 Grade 1 Grade 0 |
Fig 3. Maximum (A) local and (B) systemic toxicities by dose group. Toxicity was assessed in each patient after every inoculation and graded per Common Toxicity Criteria standards with each patient’s maximum local and systemic toxicities depicted as a percentage of the dosing group.
Fig 2. Combined toxicity and immunologic response. (A) Maximum (Max) local and systemic toxicities. (B) Proliferative responses (mean ± SE) to AE36 and AE37, respectively increased significantly prevaccine (pre) to postvaccine (post; P for AE36 = .03; P for AE37 = .0002) and long-term (P for AE36 = .02; P for AE37 = .00001). (C) Delayed-type hypersensitivity (DTH; mean ± SE) pre- and postvaccine for all dose groups combined (pre- to postvaccine DTH P = .0001).
GM-CSF has been used in our laboratory as an immunoadjuvant on the basis of previous data from animal and human trials in breast25 and melanoma.26 A randomized clinical trial in melanoma revealed that GM-CSF and Q2-21 were superior to incomplete Freund’s adju- vant.26 Even at low doses (< 80 µg), immune response was induced with increasing vaccine doses.26,27 The 62.5-µg GM-CSF dose is con- sistent with these findings. We are proceeding with this dose in our phase II trial further investigating the AE37 hybrid vaccine. As stated, our goals are to provide a safe vaccine at a consistent dose that stimu- lates a reproducible immune response in the general public.
Peptide vaccine trials have been ongoing for many years and have focused predominately on stimulating CTLs, which can directly kill tumor cells but lack long-lasting immunity. This AE37 peptide vaccine focuses on stimulating CD4+ T-helper cells with two goals: immuno- logic memory and persistent stimulation of CTLs.28 This concept is
Ii-Key Hybrid HER-2/neu Peptide (AE37) Vaccine
A Proliferation Responses to AE37 per Dosing Group 21,000 16,169 Pre Post Long-term 18,000 12,450 15,000 7,149 8,264 10,467 12,000 6,078 9,000 6,674 6,000 3,461 2,309 3,000 1,288 62 0 0 109 0 0 100:250:6 500:250:6 1000:0:6 500:125:6 500:30:6 B Proliferation Responses to AE36 per Dosing Group 10,000 5,083 Pre Post Long-term 4,332 8,000 6,000 3,704 2,533 4,870 4,000 1,557 2,000 601 519 689 43 36 141 21 0 49 0 100:250:6 500:250:6 1000:0:6 500:125:6 500:30:6 C Pre- and Post-Vaccine DTH Responses per Dosing Group 100 77.5 75.3 65.7 Pre-vaccine Post-vaccine 80 46.7 60 40 20 14.7 6.8 8.3 2.8 0 0 0 100:250:6 500:250:6 1000:0:6 500:125:6 500:30:6
being studied by others, including Disis et al,29 who are performing ongoing breast cancer clinical trials with class II peptide vaccines. In melanoma there are other class II peptide vaccines in clinical trials, but this is the first clinical trial using the Ii-Key modification.30-33
The hybrid AE37 vaccine has been designed to overcome some of the problems with peptide vaccines. By taking advantage of the Ii-Key protein interaction with class II MHC molecules, AE37 is able to directly charge MHC class II molecules with the antigenic epitope of HER-2/neu, bypassing the normal antigen processing pathway. The antigen can then be presented to the immune system, stimulating a specific CD4-positive T lymphocyte response. When compared with an unmodified epitope, these Ii-Key/antigenic epitope hybrids can display 250 times the potency or greater in vitro.17,18 Because of the increased potency of these hybrids in stimulating immune responses, it has been suggested that less efficiency in the interaction between epitope and MHC class II molecule can be tolerated, such that these hybrids may be active in individuals having HLA alleles with which the wild-type epitope interacts only weakly.
In our hands, the hybrid vaccine AE37 is sufficiently potent, that at times peptide did not require the use of immunoadjuvant GM-CSF. Historically, peptides alone are minimally immunogenic and require coadministration with immunologic adjuvants to induce detectable T-cell responses.34,35 Local and systemic reactions in the second dose group (500:250:6) necessitated dose reductions in all three patients, so the third dose group (1,000:0:6) was initiated without GM-CSF. As noted earlier, two of three patients in this group developed robust local reactions without GM-CSF and required reductions in peptide dose. Additionally, three patients in other dose groups required reductions of GM-CSF to the point of receiving peptide-only vaccinations. Fur- ther evidence of vaccine potency is seen in DTH reactions; the DTH reaction for AE37 is double the size of optimally dosed E75-peptide vaccine patients.9 To our knowledge, this is the first peptide vaccine tested in humans not requiring an immunoadjuvant.
Stimulating CD4+ T-helper cells is thought to be the key to long-term immunity, but there have been concerns implicating a subset of these cells with tissue destruction, tumorigenesis, and auto- immune diseases.11 The cells associated with these pathologies are classified as CD4+CD25+FoxP3-positive T-regulatory cells (Tregs).36,37 Because of these theoretical concerns, and because Tregs are elevated in breast cancer patients,38 we have measured Tregs in our AE37 trial. Although there was no change in the total CD4+ popula- tion (prevaccination, 52.4%; postvaccination, 51.0%; P = .5), Tregs as measured by FoxP3 (1+) in nine patients statistically decreased (pre- vaccination, 2.1%; postvaccination, 1.0%; P = .0008). Further analysis on all 15 AE37 trial patients is currently underway (manuscript in preparation).39,40 At present, it is unclear whether the decrease in Tregs is a result of the peptide vaccine or immunoadjuvant; this is being addressed in our phase II trial with a control arm, consisting of GM-CSF alone.
One limitation of peptide vaccines has been the preferential bind- ing of peptides to certain MHC class I and II molecules. E75 (HER-2/ neu:369-377) binds to HLA-A2–positive/A3–positive patients, and Tuttle13,41,42 has suggested that G89 peptide is preferentially recog- nized by HLA-DR4 –positive patients; however, Salazar et al43 have
Fig 4. Immunologic responses to varying doses of AE37 vaccine. Proliferative responses (mean ± SE) to (A) AE37 and (B) AE36 increased prevaccine (pre) to postvaccine (post; 1 month after series completion), and long-term (6 months postcompletion) in every dosing group. (C) Delayed-type hypersensitivity (DTH; mean ± SE) pre- and postvaccine per dosing groups. cpm, counts per minute.
presented findings that the parent epitope in AE37, HER-2/neu:776- 790 (which contains G89) interacts with a variety of MHC class II alleles. Whether the Ii-Key moiety in AE37 confers additional MHC class II promiscuity to the HE2/neu epitope it contains is unclear.
Holmes et al
Variability was seen within dose groups suggesting binding prefer- ences. Additional studies are being performed to determine optimal HLA-DR types for this specific vaccine.
There are numerous potential and theoretical advantages of HER-2/neu– directed vaccination therapies compared with other di- rected therapies like trastuzumab. These advantages include ease of administration, decreased systemic toxicity, broad applicability to all levels of HER-2/neu expression (1+ to 3+), and, most importantly, stimulation of broad immunologic response to include memory re- sponse with continued benefit after therapy is completed.
In conclusion, the hybrid AE37 vaccine seems safe and elicits a HER-2/neu–specific immune response, even without use of an adju- vant. A multicenter phase II trial of the AE37 vaccine is presently underway to further assess these intriguing findings. Eventually, our goal is to develop a combination multiepitope vaccine to stimulate antigen-specific CTL responses both directly and indirectly.
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked
with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Eric von Hofe, Antigen Express Inc (C) Consultant or Advisory Role: None Stock Ownership: Eric von Hofe, Antigen Express Inc Honoraria: None Research Funding: George
E. Peoples, Antigen Express Inc Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: George E. Peoples Financial support: Eric von Hofe Provision of study materials or patients: George E. Peoples Collection and assembly of data: Jarrod P. Holmes, Matthew T. Hueman, Asna Amin, Sathibalan Ponniah, George E. Peoples
Data analysis and interpretation: Jarrod P. Holmes, Linda C. Benavides, Jeremy D. Gates, Mark G. Carmichael, Matthew T. Hueman, Elizabeth A. Mittendorf, James L. Murray, Dianna Craig, Sathibalan Ponniah,
George E. Peoples
Manuscript writing: Jarrod P. Holmes, Linda C. Benavides, Jeremy D. Gates, Mark G. Carmichael, Sathibalan Ponniah, George E. Peoples Final approval of manuscript: George E. Peoples
REFERENCES
1. National Cancer Institute SEER Surveillance Epidemiology and End Results Cancer Statistics Review, 1975-2003: Summary Figures and Tables. 2006. http://seer.cancer.gov/csr/1975_2003/
2. Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al: Adjuvant docetaxol or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354:809-820, 2006
3. Pritchard KI, Shepherd LE, O’Malley FP, et al: HER2 and responsiveness of breast cancer to adju- vant chemotherapy. N Engl J Med 354:2103-2111, 2006
4. Slamon DJ, Godolphin W, Jones LA, et al: Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707-712, 1989
5. Fisk B, Blevins TL, Wharton JT, et al: Identifi- cation of immunodominant peptide of the HER2/neu proto-oncogene recognized by ovarian tumor- specific CTL lines. J Exp Med 181:2109-2117, 1995
6. Peoples GE, Goedegburre PS, Smith R, et al: Breast and ovarian cancer-specific cytotoxic T lym- phocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A 92:432-436, 1995
7. Peoples GE, Gurney JM, Hueman MT, et al: Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high risk breast cancer pa- tients. J Clin Oncol 23:7536-7545, 2005
8. Mittendorf E, Storrer C, Foley R: Evaluation of the HER2/neu-derived peptide GP2 for use in peptide-based breast cancer vaccine trial. Cancer 106:2309-2317, 2006
9. Peoples GE, Holmes JP, Hueman MT, et al: Combined clinical trial results of a HER2/neu (E75) vaccine for prevention of recurrence in high-risk breast cancer patients: United States Military Can- cer Institute Clinical Trials Group Study I-01 & I-02. Clin Cancer Res 14:797-803, 2008
10. Knutson KL, Schiffman K, Cheever MA, et al: Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369-377, results in short-lived peptide-specific immunity. Clin Cancer Res 8:1014- 1018, 2002
11. Knutson KL, Disis ML: Tumor antigen-specific T helper cells in cancer immunity and immunother- apy. Cancer Immunol Immunother 54:721-728, 2005
12. Knutson KL, Disis ML: Augmenting T helper cell immunity in cancer. Curr Drug Targets Immune Endocr Metabol Disord 5:365-371, 2005
13. Tuttle TM, Anderson BW, Thompson WE, et al: Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer pa- tients. Clin Cancer Res 4:2015-2024, 1998
14. Disis ML, Grabstein KH, Sleath PR, et al: Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res 5:1289-1297, 1999
15. Adams S, Humphreys RE: Invariant chain pep- tides enhancing or inhibiting the presentation of antigenic peptides by major histocompatibility com- plex class II molecules. Eur J Immun 25:1693-1702, 1995
16. Xu M, Lis J, Gulfo JV, et al: MHC class II allosteric site drugs: New immunotherapeutics for malignant, infectious and autoimmune disease. Scan J Immunol 54:39-44, 2001
17. Humphreys RE, Adams S, Koldzic G, et al: Increasing the potentcy of MHC class II-presented epitopes by linking to Ii-Key peptide. Vaccine 18: 2693-2697, 2000
18. Gillogly ME, Kallinteris NL, et al: Ii-Key/HER-2/ neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol Immunother 53:490-496, 2004
19. Kallinteris NL, Wu S, Lu X, et al: Enhanced CD4+ T-cell response in DR4-transgenic mice to a hybrid peptide linking the Ii-Key segment of the
invariant chain to the melanoma gp100(48-58) MHC class II epitope. J Immunother 28:352-358, 2005
20. Hueman MT, Dehqanzada ZA, Novak TE, et al: Phase I of a HER-2/neu peptide (E75) for the pre- vention of prostate-specific antigen recurrence in high-risk prostate cancer patients. Clin Cancer Res 11:7470-7479, 2005
21. Woll MM, Fisher CM, Ryan GB, et al: Direct measurement of peptide-specific CD8+ T cells us- ing HLA-A2:Ig dimmer for monitoring the in vivo immune response to a HER2/neu vaccine in breast and prostate cancer patients. J Clin Immunol 24: 449:461, 2004
22. Sokal JE: Measurement of delayed skin-test responses. N Engl J Med 293:501-502, 1975
23. Voutsas IF, Gritzapis AD, Mahaira LG, et al: Induction of potent CD4+ T cell-mediated antitumor responses by a helper HER-2/neu peptide linked to the Ii-Key moiety of the invariant chain. Int J Cancer 121:2031-2041, 2007
24. Sotiriadou NN, Kallinteris NL, Gritzapis AD, et al: Ii-Key/HER-2/neu(776-790) hybrid peptides in- duce more effective immunological responses over the native peptide in lymphocyte cultures from patients with HER-2/neu+ tumors. Cancer Immunol Immunother 56:601-613, 2007
25. Disis ML, Bernhard H, Shiota FM, et al: Granulocyte-macrophage colony-stimulating factor: An effective adjuvant for protein and peptide-based vaccines. Blood 88:202-210, 1996
26. Shaed SG, Klimek VM, Panageas KS, et al: T-cell responses against tyrosinase 368-376(370D) peptide in HLA*A0201+ melanoma patients: Ran- domized trial comparing incomplete Freund’s adju- vant, granulocyte macrophage colony-stimulating factors and QS-21 as immunological adjuvants. Clin Cancer Res 8:967-972, 2002
27. Parmiani G, Castelli C, Pilla L, et al: Opposite immune functions of GM-CSF administered as vac- cine adjuvant in cancer patients. Ann Oncol 18:226- 232, 2007
Ii-Key Hybrid HER-2/neu Peptide (AE37) Vaccine
28. Kalams SA, Walker BD: The critical need for CD4 help in maintaining effective cytotoxic T lym- phocyte responses. J Exp Med 188:2199-2204, 1998
29. Disis ML, Gooley TA, Rinn K, et al: Generation of T-cell immunity to the HER-2/neu protein after immunization with HER-2/neu peptide-based vac- cines. J Clin Oncol 20:2624-2632, 2002
30. Wong R, Lau R, Change J, et al: Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clin Cancer Res 10:5004-5013, 2004
31. Phan GQ, Touloukian CE, Yang JC, et al: Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother 26:349-356, 2003
32. Kobayashi H, Lu J, Celis E: Identification of helper T-cell epitopes that encompass or lie proxi- mal to cytotoxic T-cell epitopes in the gp100 mela- noma tumor antigen. Cancer Res 61:7577-7584, 2001
33. Kallinteris NL, Lu X, Wu S, et al: Ii-Key/MHC class II epitope hybrid peptide vaccines for HIV. Vaccine 21:4128-4132, 2003
34. Ja¨ eger E, Bernhard H, Romero P, et al: Gen- eration of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: Implications for tumor vaccines with melanoma-associated anti- gens. Int J Cancer 66:162-169, 1996
35. Marchand M, Van Baren N, Weynants P, et al: Tumor regressions observed in patients with meta- static melanoma treated with an antigenic peptide encoded by gen MAGE-3 and presented by HLA-A1. Int J Cancer 80:219-230, 1999
36. Sakaguchi S, Sakaguchi N, Asano M, et al: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor ex-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151-1164, 1995
37. Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330-336, 2003
38. Liyanage UK, Moore TT, Joo H, et al: Preva- lence of regulatory T cells is increased in peripheral blood and tumour microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756-2761, 2002
39. Hueman MT, Stojadinovic A, Storrer CE, et al: Levels of circulating regulatory CD4+CD25+ T cells are decreased in breast cancer patients after vacci- nation with a HER2/neu peptide (E75) and GM-CSF vaccine. Br Cancer Res Treat 98:17-29, 2006
40. Hueman MT, Holmes JP, Storrer CE, et al: Immune monitoring of CD4+CD25+FoxP3+ regu- latory T cells in a novel HLA Class-II HER2/neu peptide vaccine clinical trial in breast cancer pa- tients. Ann Surg Oncol S14:91, 2007 (suppl; abstr)
41. Parker KC, Bednarek MA, Coligan JE: Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side chains. J Immunol 152:163-175, 1994
42. Patil R, Holmes JP, Amin A, et al: Clinical and immunologic responses of HLA-A3+ breast cancer patients vaccinated with the HER2/neu-derived pep- tide vaccine, E75, in a phase I clinical trial. Br Cancer Res Treat 106:S123-S124, 2007 (suppl)
43. Salazar LG, Fikes J, Southwood S, et al: Immunization of cancer patients with HER-2/neu- derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res 9:5559- 5565, 2003
■ ■ ■







