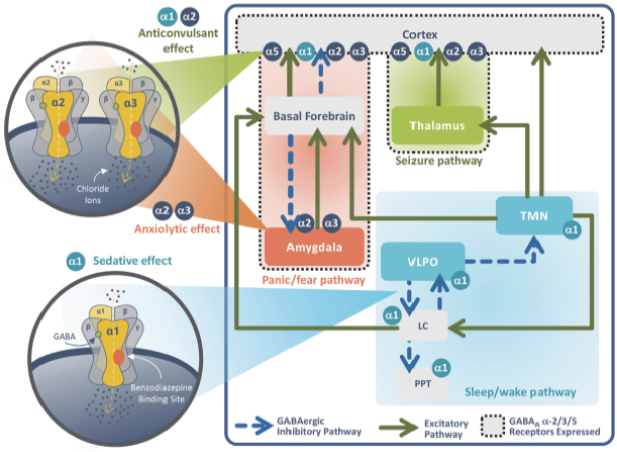
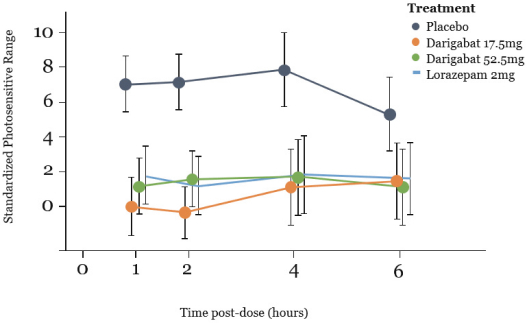
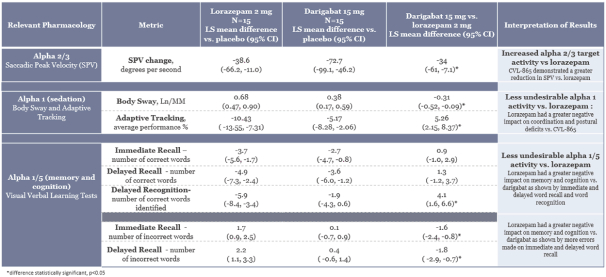
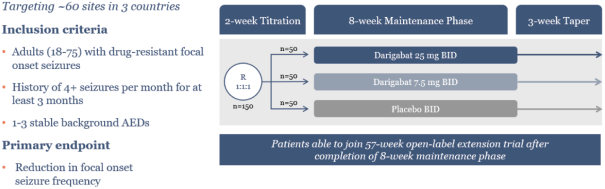
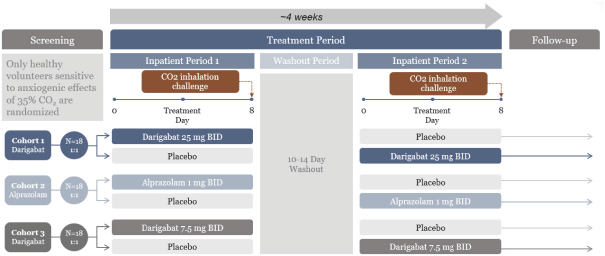
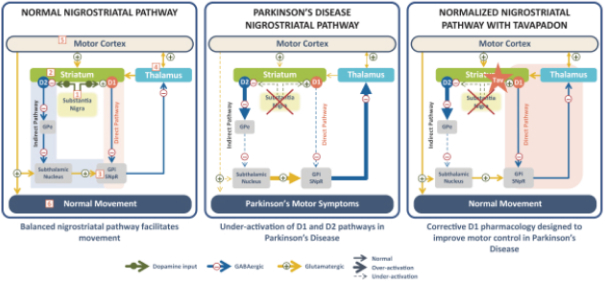
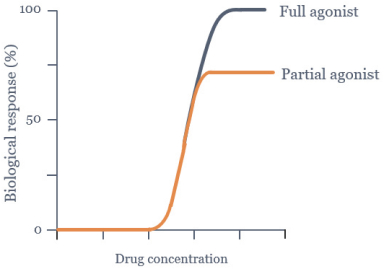
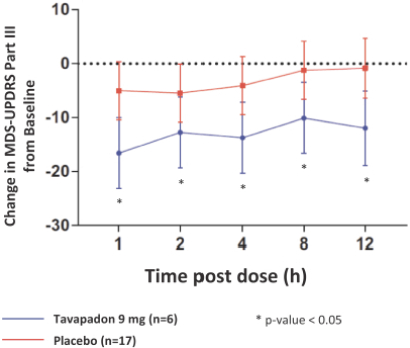
epilepsy such as Lennox-Gastaut syndrome and Dravet Syndrome that could have efficacy in broader epileptic populations, including fenfluramine from Zogenix and cannabinoid-based therapies from GW Pharmaceuticals.
Parkinson’s Disease
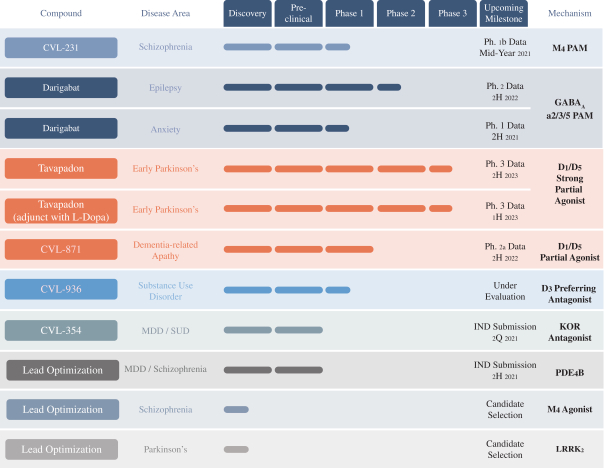
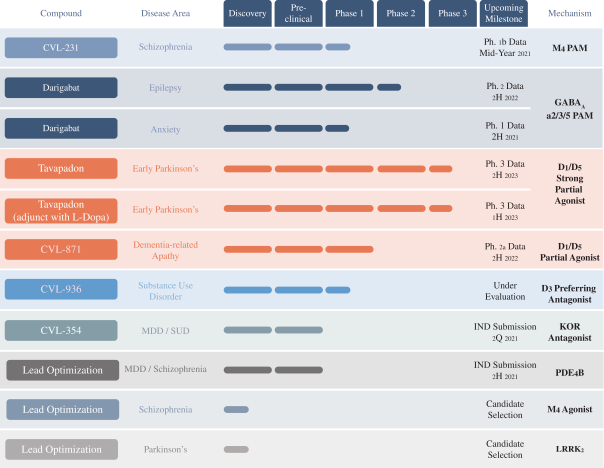
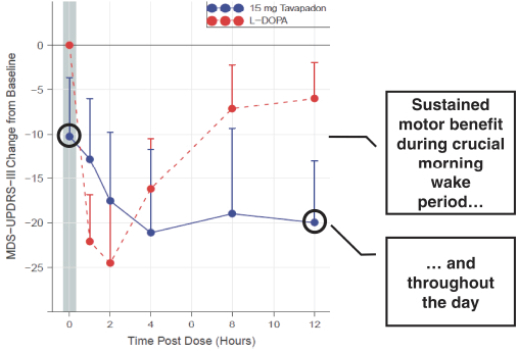
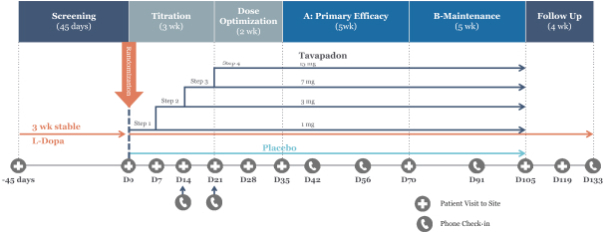
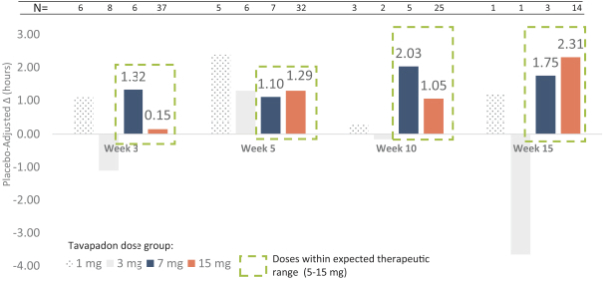
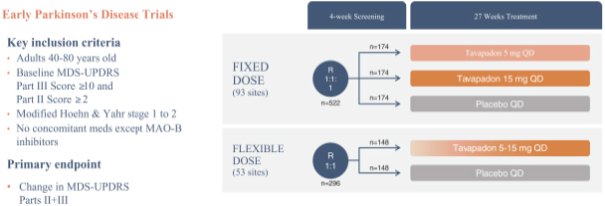
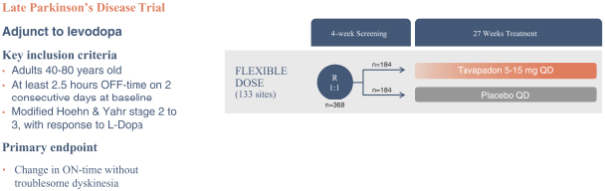
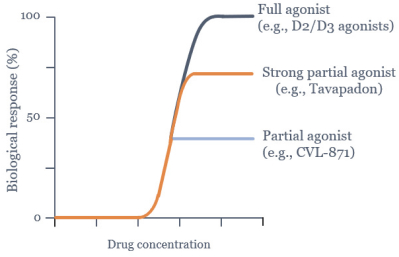
We are developing tavapadon for the treatment of early- and late-stage Parkinson’s. We may face competition from currently available treatments for both stages of disease, such as L-dopa, D2/D3-preferring agonists and MAO-B inhibitors as monotherapy or in combination, as well as deep brain stimulation devices by Medtronic Inc. and St. Jude Medical Inc., among others, for the later stages of disease. Additionally, we are aware of several potential therapeutics being developed by other pharmaceutical and biotechnology companies, including Denali, Prothena, Roche, Voyager Therapeutics, Prevail Therapeutics, Sage Therapeutics, Neurocrine Biosciences, Eli Lilly, AstraZeneca, and IRLAB Therapeutics, that are in various stages of clinical development. These companies are employing a variety of therapeutic modalities, including gene therapy and gene editing, in addition to small molecule chemistry, to address Parkinson’s.
Substance Use Disorder
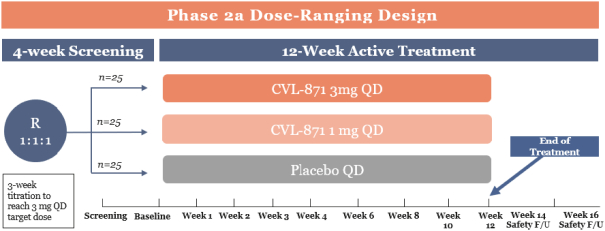
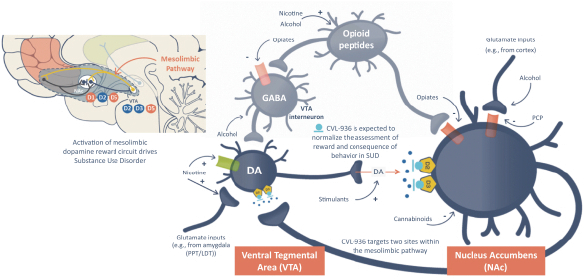
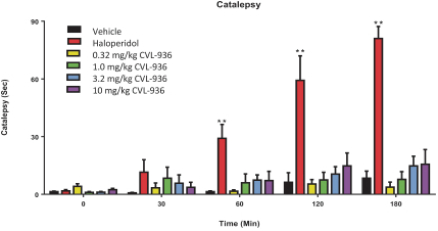
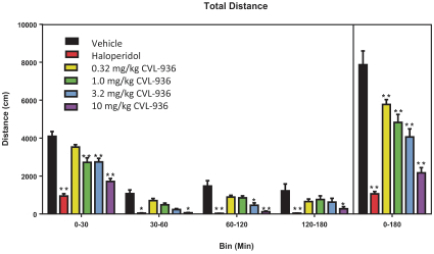
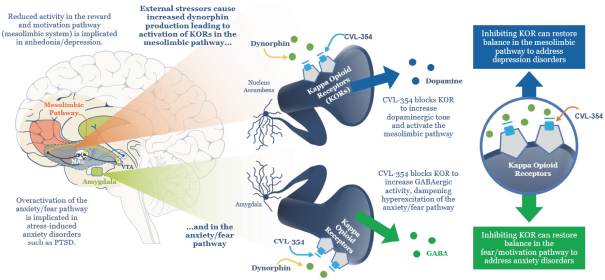
We are developing CVL-936 for the treatment of SUD, with an initial focus on OUD. In the treatment of OUD, we may face competition from manufacturers of oral buprenorphine products, including Indivior, which markets Suboxone and Subutex brands, and Braeburn, which markets Brixadi. We may also face competition from manufacturers of naloxone, naltrexone and methadone, including Emergent BioSolutions, which markets Narcan, BioDelivery Sciences, which markets Bunavail, and Alkermes, which markets Vivitrol. Other products are marketed or in development by companies such as Eli Lilly and GlaxoSmithKline.
Pfizer License Agreement
In August 2018, we entered into the Pfizer License Agreement pursuant to which we were granted an exclusive, sublicensable, worldwide license under certain Pfizer patent rights, and a non-exclusive, sublicensable, worldwide license under certain Pfizer know-how, to develop, manufacture and commercialize certain compounds and products, which currently constitute the product candidates included in the table in the section entitled “–Our Pipeline”, in the field of treatment, prevention, diagnosis, control and maintenance of all diseases and disorders in humans, subject to the terms and conditions of the Pfizer License Agreement. The license excludes the field of treatment, prevention, diagnosis, control and maintenance of inflammatory bowel diseases and disorders in humans by compounds or products exerting a therapeutic effect on the LRRK2 target, which is retained by Pfizer. Under the terms of the Pfizer License Agreement, Pfizer is granted a non-exclusive, sublicensable, royalty-free, worldwide license under intellectual property we develop during the term of the agreement for all purposes in the LRRK2 field retained by Pfizer. Additionally, Pfizer has an exclusive right of first negotiation in the event that we seek to enter into any significant transaction with a third party with respect to a product either globally or in certain designated countries. Significant transactions include exclusive licenses, assignments, sales, exclusive co-promotion arrangements, and other transfers of all commercial rights to a product globally or in certain designated countries, as well as exclusive distribution agreements globally or in certain designated countries.
Under the Pfizer License Agreement, we are solely responsible for the development, manufacture, regulatory approval and commercialization of compounds and products in the field. We are required to use commercially reasonable efforts to develop and seek regulatory approval for a product that contains or incorporates one of certain scheduled compounds to exert a therapeutic effect on certain targets, in each of the following countries: United Kingdom, Germany, France, Italy, Spain, China, Japan and the United States, each a major market country. We are also required to use commercially reasonable efforts to commercialize each such product, if approved, in each major market country in which regulatory approval for such product has been obtained. The Pfizer License Agreement requires Pfizer to transfer certain know-how and data, regulatory filings
139