
Exhibit 99.2 FPI-1434 Phase 1 Data Update June 27, 2023 Copyright © 2020 Fusion Pharma Inc. All Rights Reserved

Forward-Looking Statements and Legal Disclaimers This presentation contains express or implied forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements that are based on our management's belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as may, should, expects, intends, plans, anticipates, believes, estimates, predicts, potential, continue, or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other facts, which are, in some cases, beyond our control and which could materially affect results. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. These risks and uncertainties include, but are not limited to, uncertainties inherent in the drug development process, including Fusions’ programs’ early stage of development, the process of designing and conducting preclinical and clinical trials, the regulatory approval processes, the timing of regulatory filings, the challenges associated with manufacturing drug products, Fusion’s ability to successfully establish, protect and defend its intellectual property, risks relating to business interruptions resulting from the coronavirus (COVID-19) disease outbreak or similar public health crises and other matters that could affect the sufficiency of existing cash to fund operations. For a discussion of other risks and uncertainties, and other important factors, see the section entitled “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, as well as other risks detailed in the Company’s subsequent filings with the Securities and Exchange Commission. You should read this presentation and the documents that we reference in this presentation completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other information concerning our industry, our business and the markets for our product candidates. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other data from our own internal estimates and research as well as from reports, research, surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we are not aware of any misstatements regarding any third-party information presented in this presentation, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties and are subject to change based on various factors. Market data and industry information used throughout this presentation are based on management’s knowledge of the industry and the good faith estimates of management. We also relied, to the extent available, upon management’s review of independent industry surveys and publications and other publicly available information prepared by a number of third party sources. All of the market data and industry information used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Although we believe that these sources are reliable, we cannot guarantee the accuracy or completeness of this information, and we have not independently verified this information. While we believe the estimated market position, market opportunity and market size information included in this presentation are generally reliable, such information, which is derived in part from management’s estimates and beliefs, is inherently uncertain and imprecise. No representations or warranties are made by the Company or any of its affiliates as to the accuracy of any such statements or projections. Projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described above. These and other factors could cause results to differ materially from those expressed in our estimates and beliefs and in the estimates prepared by independent parties. 2 Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved

Unlocking the Potential of Radiopharmaceuticals in Novel Targets Fusion’s platform supports multiple development opportunities across both validated and novel targets, including the potential to create significant value creating next-generation antibody drug conjugates Preclinical/ Discovery Phase 1 Phase 2 Phase 3 IND Enabling mCRPC FPI-2265 FPI-1434 Solid Tumors Expressing IGF-1R Solid Tumors Expressing NTSR1 FPI-2059 FPI-1434 Solid Tumors Expressing IGF-1R Combination Solid Tumors Expressing EGFR-cMET FPI-2068 Discovery Multiple programs 3 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

FPI-1434 Phase 1 Trial: A First-of-its-Kind Targeted Alpha Therapy Study in Solid Tumors Using alpha emitters in place of chemotherapies or ADCs represents a significant yet untapped opportunity ■ Very limited precedent for industry sponsored development of antibody-based TATs Stepwise development strategy in all comers with IGF-1R tumor expression involved three parts: 1. Single ascending dose 2. Multiple ascending dose 3. Cold/hot dosing regimen Preliminary clinical data with cold/hot dosing regimen demonstrated the potential to improve the therapeutic index and increase tumor dose at lower injected total doses ■ Encouraging early results in the first cohort (15 kBq/kg) show potential to deliver multiple cycles of therapy at a dose equivalent to ~40 kBq/kg in hot only with improved safety profile ■ Despite being the first cohort, two heavily pre-treated patients received up to 5 cycles to date, with durable stable disease as their best response Currently dosing patients at 25 kBq/kg in cold/hot regimen (equivalent to 75 kBq/kg in the hot only study) 4 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Interim Trial Results: Improving the Therapeutic Index with Cold Antibody Pre-Dose Dmitri Bobilev, MD Chief Medical Officer Copyright © 2023 Fusion Pharma Inc. All Rights Reserved 5
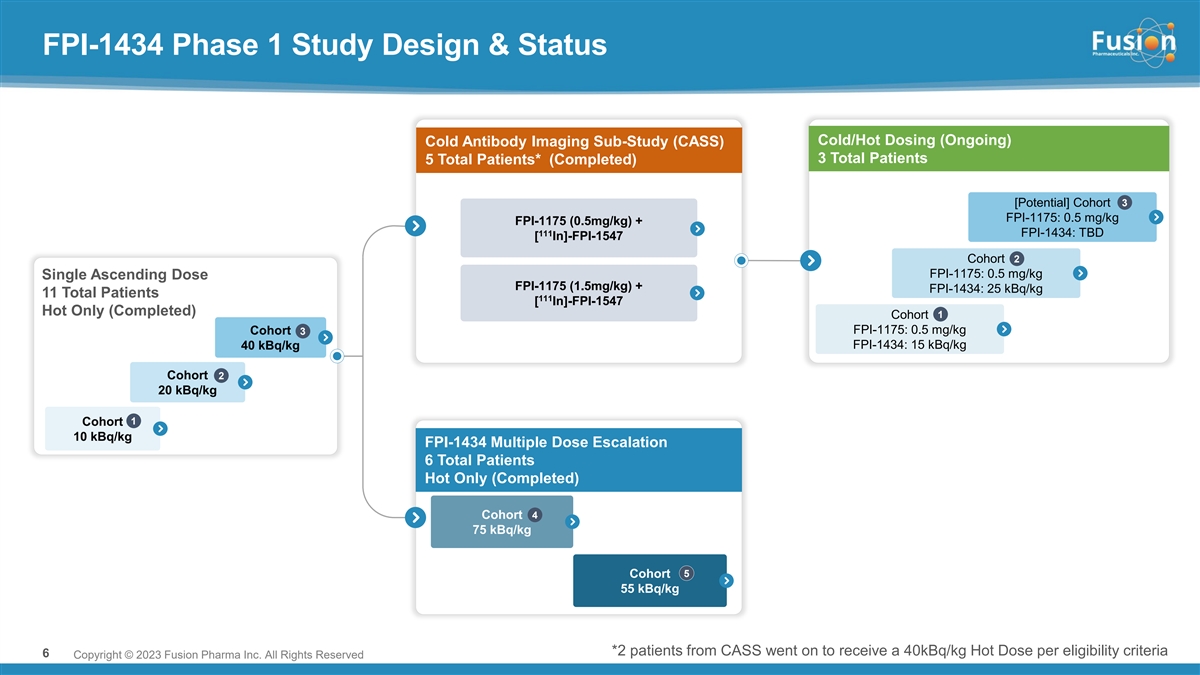
FPI-1434 Phase 1 Study Design & Status Cold/Hot Dosing (Ongoing) Cold Antibody Imaging Sub-Study (CASS) 3 Total Patients 5 Total Patients* (Completed) 3 [Potential] Cohort FPI-1175: 0.5 mg/kg FPI-1175 (0.5mg/kg) + 111 FPI-1434: TBD [ In]-FPI-1547 Cohort 2 FPI-1175: 0.5 mg/kg Single Ascending Dose FPI-1175 (1.5mg/kg) + FPI-1434: 25 kBq/kg 11 Total Patients 111 [ In]-FPI-1547 Hot Only (Completed) Cohort 1 FPI-1175: 0.5 mg/kg Cohort 3 FPI-1434: 15 kBq/kg 40 kBq/kg Cohort 2 20 kBq/kg 1 Cohort 10 kBq/kg FPI-1434 Multiple Dose Escalation 6 Total Patients Hot Only (Completed) Cohort 4 75 kBq/kg Cohort 5 55 kBq/kg *2 patients from CASS went on to receive a 40kBq/kg Hot Dose per eligibility criteria 6 Copyright © 2021 Fusion Pharma Inc. All Rights Reserved Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Prioritizing the Cold Antibody Pre-Dose Strategy Cold Antibody Sub Study: Results show ability to tune PK, increasing tumor uptake and decreasing dose to normal tissue Spleen Spleen 7 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Plasma Pharmacokinetics and Platelet Counts Cold/hot regimen demonstrates increased exposure but minimizes thrombocytopenia compared to hot only 225 Platelet Count Following [ Ac]-FPI-1434 Plasma Pharmacokinetics FPI-1434 Administration 10000 225 2.0 [ Ac]-FPI-1434 (10 kBq/kg, ~0.007 mg/kg) 225 [ Ac]-FPI-1434 (20 kBq/kg, ~0.013 mg/kg) 225 1000 1.5 [ Ac]-FPI-1434 (40 kBq/kg, ~0.026 mg/kg) 225 [ Ac]-FPI-1434 (55 kBq/kg, ~0.036 mg/kg) 225 [ Ac]-FPI-1434 (75 kBq/kg, ~0.049 mg/kg) 1.0 100 Cold+Hot Cohort 1 (15 kBq/kg + 0.5 mg/kg FPI-1175) 0.5 10 0.0 1 0 10 20 30 40 50 0 200 400 600 Days Post FPI-1434 Dose Time Post-Injection (hr) Cold+Hot Cohort 1 (15 kBq/kg + 0.5 mg/kg FPI-1175) 40 kBq/kg Hot Only 8 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved FPI-1434 Plasma Concentration (ng-Eq/g) Relative Platelet Count

FPI-1434 Phase 1 Trial Preliminary Safety Summary FPI-1434 (only) FPI-1434 (only) 40 kBq/kg n=9 FPI-1434 (only) FPI-1434 (15 kBq/kg) + All n=30 (%) 10-20 kBq/kg n=9 (incl. CASS=5) 55-75 kBq/kg n=9 FPI-1175 (0.5 mg/kg) n=3 Preferred Term Any Grade Grade 3-4 Any Grade Grade 3-4 Any Grade Grade 3-4 Any Grade Grade 3-4 Any Grade Grade 3-4 Thrombocytopenia 14 (47%) 5 (17%) 2 (22%) 0 5 (56%) 1 (11%) 6 (67%) 4 (44%) 1 (33%) 0 Anemia 6 (20%) 4 (13%) 0 0 1 (11%) 0 5 (56%) 4 (44%) 0 0 Leukopenia 8 (27%) 2 (7%) 1 (11%) 0 2 (22%) 1 (11%) 4 (44%) 1 (11%) 1 (33%) 0 Neutropenia 10 (33%) 5 (17%) 0 0 4 (44%) 1 (11%) 5 (56%) 4 (44%) 1 (33%) 0 Lymphopenia 5 (17%) 3 (10%) 1 (11%) 0 2 (22%) 1 (11%) 2 (22%) 2 (22%) 0 0 Fatigue 9 (30%) 0 2 (22%) 0 2 (22%) 0 4 (44%) 0 1 (33%) 0 Decreased appetite 4 (13%) 0 0 0 0 0 4 (44%) 0 0 0 Nausea 5 (17%) 0 2 (22%) 0 1 (11%) 0 2 (22%) 0 0 0 Diarrhea 3 (10%) 0 0 0 1 (11%) 0 2 (22%) 0 0 0 Blood count changes were the only dose-dependent adverse events ‒ No Dose-Limiting Toxicities (DLTs) were observed at dose levels of 10-40 kBq/kg (hot only) ‒ DLTs of Grade 4 thrombocytopenia were reported in 2 patients at dose levels of 55 and 75 kBq/kg (hot only) ‒ No DLTs were observed with cold/hot dosing regimen at 15kbq ̵ Grade 1 thrombocytopenia reported in 1 of 3 patients ̵ No significant effect on biomarkers related to IGF-1R pathway * Percentage is based on Safety Population that includes all patients who received at least one dose of FPI-1547 (FPI-1547 ± FPI-1434 ± FPI-1175) 9 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Organ Radiation Absorbed Doses FPI-1434 Program (CASS): mCRPC Radiation Absorbed Dose Estimates* Patient as own control Hot Only (N=19) Col d/Hot (N=5) Target Organ Mean (Gy) Mean (Gy) Kidneys 1.264 1.430 Liver 1.109 2.120 Lungs 0.753 0.850 Mean Cumulative Lesion Dose** at Dosimetric Limit Hot Only Cold/Hot 29.3 Gy 59.2 Gy Within prespecified critical organ limits, cold/hot regimen is estimated to deliver ~2x radiation dose compared to hot only regimen * Radiation doses estimated for 15 kBq cold/hot regimen and presented in units of Gy, based on relative biologic effectiveness (RBE) value of 3.4 for alpha emitters has been applied. ** RBE = 3.4 for alpha emissions applied; 70 kg body weight assumed 10 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Phase 1 Preliminary Data Repeat dosing at 15kBq/kg shows promising early safety results • Two of 3 patients reported stable disease and received 58 year old female with advanced repeat cycles of treatment with no Grade 3-4 AEs metastatic ovarian cancer • Patient heavily pre-treated with multiple lines of chemotherapy and PARP inhibitors Lesion • Stage IVb, neck, axillary, para-aortic, retroperitoneal, and cervical lymph node metastases Blood Pool in Heart • No DLTs or ≥ Grade 3 adverse events have been reported during therapy • Best response is Stable Disease per RECIST – First dose received October 2022; 5th cycle received in May 2023 • Key Dosimetry Results – Lesion Dose: 5.6 Gy/cycle (28 Gy cumulative) – Red Marrow Dose: 1.67 Gy/cycle (8.3 Gy cumulative) Exposure at 15kBq/kg is comparable to a 40kBq/kg dose in hot only regimen; Dose escalation is ongoing Copyright © 2020 Fusion Pharma Inc. All Rights Reserved 11 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Summary of Results The cold/hot regimen demonstrated: FPI-1434 Plasma AUC 150000 ■ Decreased rate of systemic clearance from plasma, leading to increased exposure (AUC) at 15 kBq/kg compared to the 40 kBq/kg 100000 hot only ■ No related DLTs, SAEs, or Grade ≥3 AEs at 15kBq/kg cold/hot 50000 compared to 40+kBq/kg in hot only The cold/hot regimen demonstrated potential to improve 0 therapeutic index 225 15 kBq/kg [ Ac]-FPI-1434 ■ No substantial change in organ absorbed dose (kidney, lung, bone + 0.5 mg/kg FPI-1175 225 marrow), except in liver (increased) and spleen (decreased) 40 kBq/kg [ Ac]-FPI-1434 ■ Per-cycle organ absorbed doses at 15kBq/kg levels were ≤7% of protocol-defined limits ■ Doubled the tumor absorbed dose Exploration of FPI-1434 at 25kBq/kg in cold/hot regimen is ongoing Copyright © 2023 Fusion Pharma Inc. All Rights Reserved 12 225 [ Ac]-FPI-1434 Plasma AUC (hr*ng/g)

Understanding the Mechanism and Benefits of Cold Antibody Pre-Dose Chris Leamon, Ph.D. Chief Scientific Officer Copyright © 2023 Fusion Pharma Inc. All Rights Reserved 13

For radiolabeled antibodies, sources of myelosuppression are likely a combination of non-specific and target mediated off-tumor binding Hypothesis: Cold antibody has the potential to reduce on-target and Fc mediated non-specific binding – pushing more activity in the Liver dose Spleen dose blood which may improve the safety profile and allow for greater tumor exposure Marrow dose Platelets/ Megakaryocytes Copyright © 2023 Fusion Pharma Inc. All Rights Reserved 14
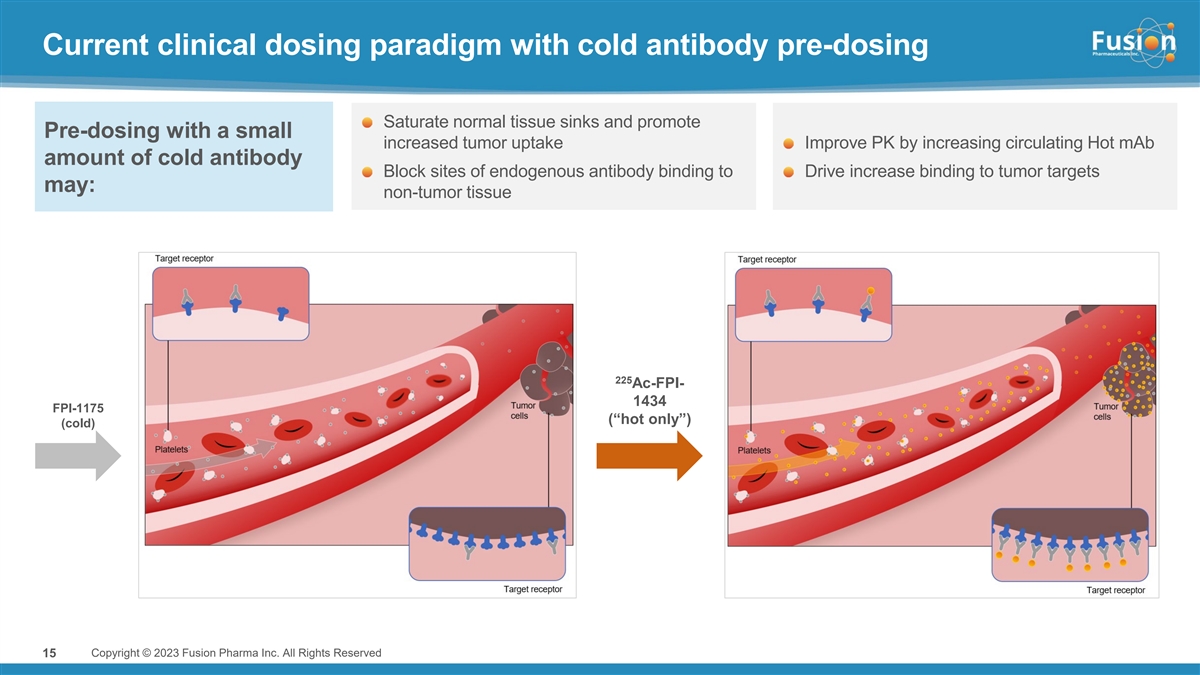
Current clinical dosing paradigm with cold antibody pre-dosing Saturate normal tissue sinks and promote Pre-dosing with a small increased tumor uptake Improve PK by increasing circulating Hot mAb amount of cold antibody Block sites of endogenous antibody binding to Drive increase binding to tumor targets may: non-tumor tissue 225 Ac-FPI- 1434 FPI-1175 (“hot only”) (cold) 15 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Hypothesis: Thrombocytopenia may be caused, in part, by targeting IGF-1R expressed on thrombocytes and/or megakaryocytes FPI-1434 binding to megakaryocytes can be blocked Megakaryocytes express significant IGF-1R + Cancer Cells > Megakaryocytes >> Thrombocytes > CD34 Stem Cells by addition of cold mAb (FPI-1175) 16000 100 80 Cold : Hot Molar ratio 60 8000 15718 40 8538 20 729 387 0 0 Analysis shows IGF-1R is expressed on megakaryocytes 16 IGFIR Receptor Number (Copies/cell) % Hot Binding

Would Cold Antibody Increase AUC and Observed Marrow Toxicity? NHP study: adding cold mAb enhanced AUC and marrow exposure with no additional toxicity Original NHP toxicity studies Tested Dose Levels Repeat NHP toxicity study Pushed dose to toxicity and saw Determined effect of added cold myelosuppression (reversible 37 kBq/kg (safe dose) Ab on myelosuppression and recoverable) 148 kBq/kg (toxic dose) Platelets (Female) Systemic Exposure 800 ‒ >10-fold greater exposure 150,000 600 110,000 ‒ Observed thrombocytopenia & 27x ↑ 100,000 400 myelosuppression 50,000 50,000 200 4,000 0 0 0 50 100 150 200 37 kBq/kg 37 kBq/kg 148 kBq/kg no cold with cold no cold Time Days Results Platelets (Male) 800 Platelet Levels Adding cold Ab 600 400 330 320 ‒ Increased exposure by nearly 400 300 30-fold 200 200 100 0 100 ‒ No toxicity 0 50 100 150 200 0 Time Days ‒ No reduction in platelets 37 kBq/kg 37 kBq/kg 148 kBq/kg no cold with cold no cold Vehicle Control CPD-1419 9.25 kBq/kg 37 kBq/kg 148 kBq/kg These results showed the opportunity to push to even higher tumor doses with cold antibody dosing 17 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved Platelets Platelets 3 3 (10 Cells/µL) (10 Cells/µL) 3 Platelets (10 /µL) AUC (ng-Eq*hr/g)

Cold mAb pre-dosing substantially increases systemic exposure of 111 patients to our TATs (FPI-1547 = In imaging analog of FPI-1434) 111 111 The cold antibody dosing regimen extends the In-FPI-1547 In-FPI-1547 Plasma half-life and increases AUC Concentration-Time Profile 10000 111 In-FPI-1547 PK Parameters (Mean ± SD) C AUC t 1000 max inf 1/2 (ng-eq/mL) (ng-eq*h/mL) (h) No FPI-1175 555 ± 155 11600 ± 5300 37 ± 8.0 100 0.5 mg/kg FPI-1175 999 ± 273 133000 ± 82700 104 ± 43 10 > 11-fold ↑ 1 111 PK profile of In-FPI-1547 is comparable at 0.5 mg/kg and 1.5 0 50 100 150 200 mg/kg FPI-1175 antibody Hours ‒ Doses ≥ 0.5 mg/kg saturate the antigen sink and linear PK is observed No FPI-1175 (cold) ‒ PK at doses ≥ 0.5 mg/kg is similar to the reported PK of the 0.5 mg/kg FPI-1175 (cold) parental mAb 18 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved 111 [ In]-FPI-1547 (ng-Eq/g)

Clinical Proof of Concept through imaging Pre-administration of cold Ab may enhance tumor delivery/dose of TATs Cold Ab = unconjugated/ Hot Ab = radiolabeled FPI-1434 CASS Study: Patient as own control unlabeled Ab conjugated Ab Typical mass ~ 0.5 mg/kg Typical mass ~0.01 mg/kg (40 mg, 80 kg patient) (0.8 mg, 80 kg patient) FPI-1434 Program: HR+, HER2 negative Breast Cancer Spleen Spleen TBR=19.8 (Left sacrum); Dose: 3.8 Gy/MBq 19

Summary We confirmed IGF-1R expression on thrombocytes and megakaryocytes ■ Cancer cells > megakaryocytes >>> thrombocytes > Cohort 1 Cold/Hot stem cells Regimen Megakaryocyte targeting in the bone marrow/spleen may contribute towards FPI-1434-related thrombocytopenia observed in hot only cohort Tumor Dose IGF-1R expression on megakaryocytes can be blocked by Spleen Dose cold mAb in vitro, preventing the binding of “hot” antibodies ■ Data supports the use of cold mAb addition during clinical trials as means to manage thrombocytopenia in patients Clinical advantages of cold antibody pre-dosing observed to date ■ > 11-fold increase in systemic exposure ■ Near 20-fold increase in TBR (imaging) ■ Data support continued dose escalation of FPI-1434 with pre-dosed cold mAb 20 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Next Steps Copyright © 2023 Fusion Pharma Inc. All Rights Reserved 21
FPI-1434 Phase 1 Study in Summary Encouraging results from cold/hot dosing regimen show potential to increase therapeutic index and supported ability to multi-dose patients (up to five cycles) Dosimetry data in key organs indicate there is the opportunity to further increase the injected dose Learnings related to the cold antibody approach have positive implications for our other programs, including FPI-2068 targeting EGFR-cMET We continue to believe TATs derived from antibodies hold the potential to be next generation ADCs Fusion intends to report results from Cohort 2 in the cold/hot dosing regimen around the end of 2023 22 Copyright © 2023 Fusion Pharma Inc. All Rights Reserved

Thank you Copyright © 2023 Fusion Pharma Inc. All Rights Reserved






















