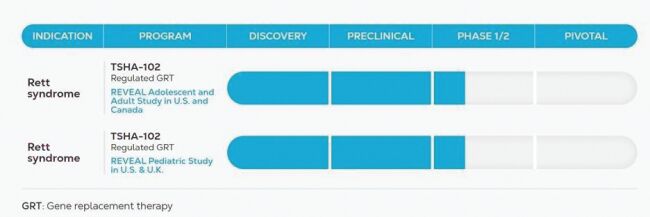
cohort 2 evaluating the 1x1015 total vg dose, with dosing to occur following IDMC review of the 42-day safety data from the patient treated with the high dose of TSHA-102 in the adolescent and adult trial. We expect to dose the first pediatric patient in cohort 2 in the third quarter of 2024. We expect to report initial available safety and efficacy data from cohort 2 of both the adolescent and adult trial and the pediatric trial in the second half of 2024.
The maximum tolerated dose, or MTD, or maximum administered dose, or MAD, established in Part A will be administered during dose expansion in Part B. Data from Part A will be assessed by regulatory agencies and the IDMC to determine key elements of Part B of the study, including efficacy endpoints, study duration and the MTD or MAD.
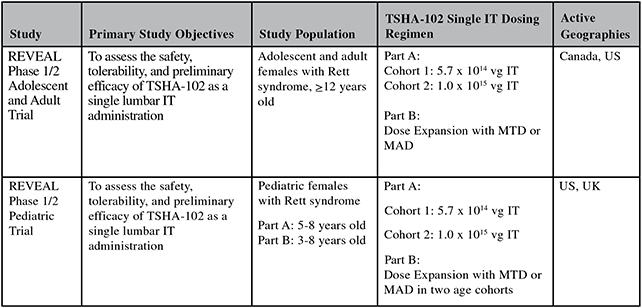
TSHA-102 REVEAL Phase 1/2 Adolescent / Adult Trial Safety and Efficacy Summary
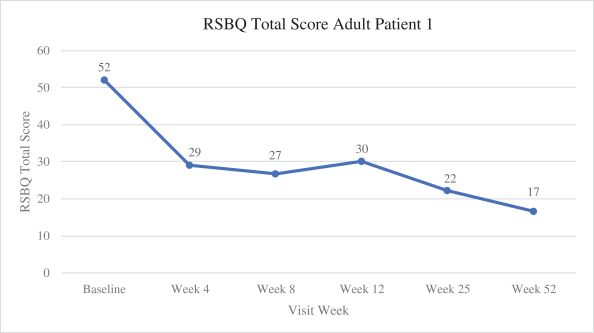
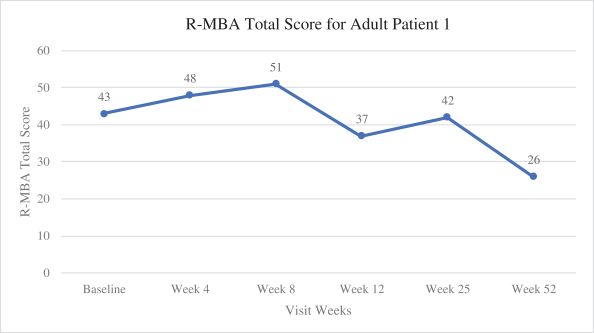
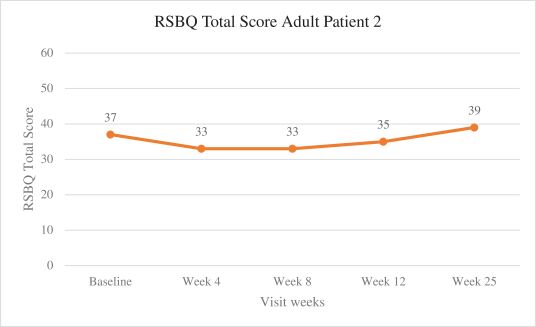
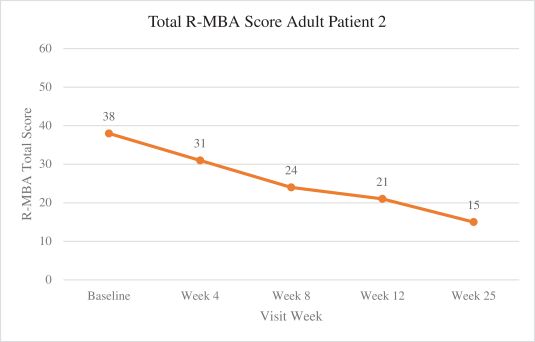
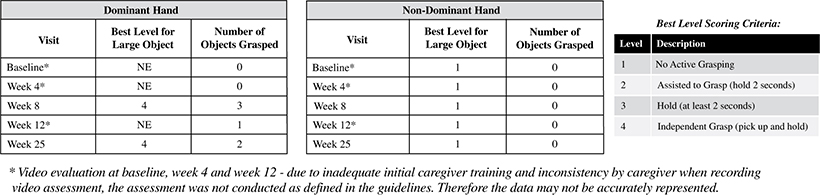
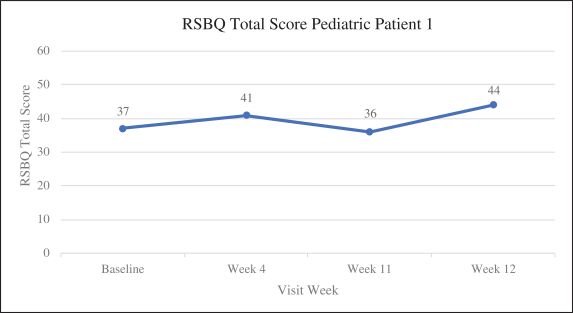
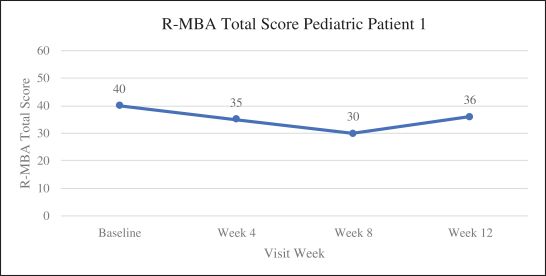
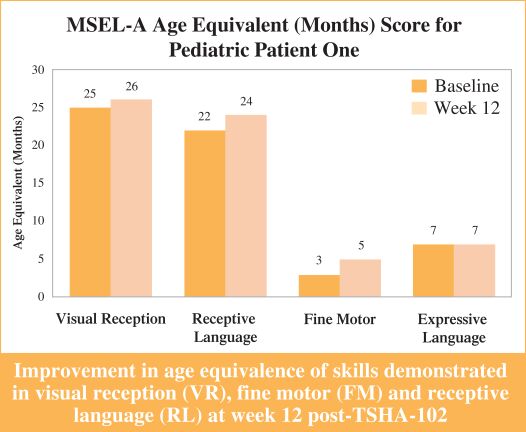
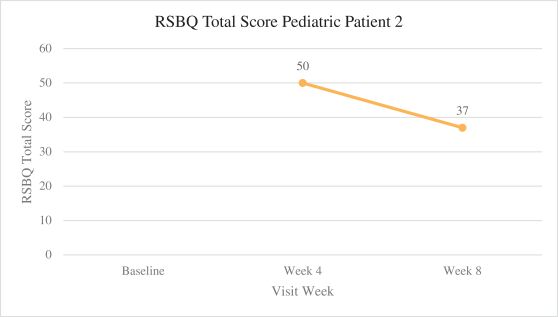
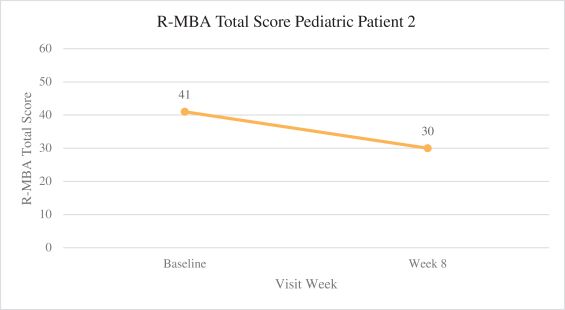
Efficacy endpoints include patient assessments performed by clinicians, including the Clinical Global Impressions Scale – Improvement, or CGI-I, the Clinical Global Impressions Scale – Severity, or CGI-S, Rett Syndrome Hand Function Scale, or RSHFS, and Revised Motor Behavior Assessment, or R-MBA. Additional efficacy endpoints also include patient assessments by caregivers, including Parental Global Impressions Improvement, or PGI-I, the Rett Syndrome Behavior Questionnaire, or RSBQ, and seizure diaries.
Overview of Cohort 1 Patients and Results
The first adult patient, a 20-year-old female at the time of dosing, has the most advanced stage of Rett syndrome, Stage IV, with a genetic change consisting of a large deletion within the MECP2 gene that is known to cause Rett syndrome. This patient’s phenotypic manifestation is severe. She lost the ability to walk, stand, and sit without support by age eight (non-ambulatory, wheelchair bound, limited movements of her lower extremities), lost fine motor and hand function by age six (unable to grasp and hold objects of any size, with essentially no function in non-dominant hand) and speak around age six (non-verbal, minimal vocalizations). She experienced frequent apnea and hyperventilation episodes by age three and has a history of seizures since the age of five. Per the Principal Investigator, or PI, the first adult patient’s baseline reported seizure frequency was approximately two to four seizures per year.
In the first adult patient, TSHA-102 was generally well-tolerated with no SAEs related to TSHA-102 or DLTs as of the 52-week assessment post-treatment. Per the protocol, prophylactic immunosuppressant therapy began seven days prior to TSHA-102 administration. The first adult patient’s steroid taper was completed by week 36, and her sirolimus taper was completed by week 43. At week 52 post-treatment, the first adult patient demonstrated sustained and new improvements across multiple clinical domains compared to baseline after the completion of her immunosuppression taper. Specifically, the PI reported that the patient sustained improvements in motor function, with the gained ability to kick her legs against gravity and sit unassisted for the first time in over a decade, and sustained improvements in fine motor and hand function with gained function in her non-dominant hand. Additionally, she could open her hands, dissociate her fingers, scratch her nose and touch a screen through week 52 post-treatment. The PI reported sustained improvements in communication and socialization at week 52 post-treatment as the patient was more alert and socially interactive, with increased communication using vocalizations. Caregivers reported an enhanced ability to use an eye-gaze driven communication device at week 25, which she had not expressed interest in before treatment. The first adult patient also demonstrated sustained improvements in autonomic function at week 52 post-treatment, including improved breathing patterns with infrequent hyperventilation and fewer breath holding spells compared to before treatment, normalized sleep/night-time behaviors with the ability to sleep through the night for the first time in 20 years, and improved circulation with the patient’s hands and feet restored to normal temperature and color (whereas before treatment, her hands and feet were usually cold and blue). The PI also reported that the first patient’s seizures were overall well-controlled through week 52 following treatment at lower levels of anti-seizure medication, relative to baseline, and the patient no longer experienced unprovoked seizures. The PI’s clinical observations are supported by clinical, caregiver and video evidence.
4