research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. Our estimates of the potential market opportunities for our product candidates include a number of key assumptions based on our industry knowledge, industry publications and third-party research, surveys and studies, which may be based on a small sample size and fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. If any of our assumptions or estimates, or these publications, research, surveys or studies prove to be inaccurate, then the actual market for any of our product candidates may be smaller than we expect, and as a result our revenues from product sales may be limited and it may be more difficult for us to achieve or maintain profitability.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug or biologic products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of many of the disease indications for which we are developing our product candidates. Some of these competitive products and therapies are based on scientific approaches that are similar to our approach, and others are based on entirely different approaches. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
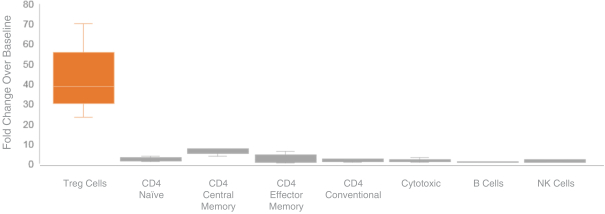
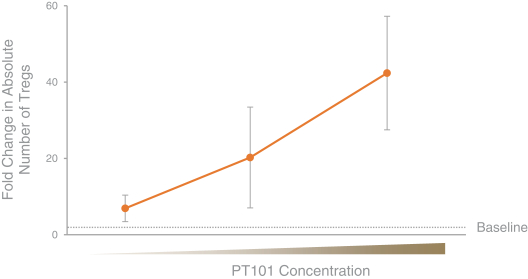
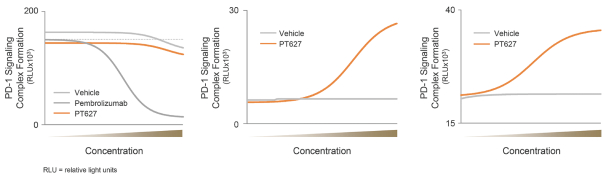
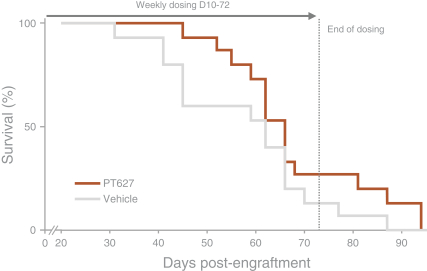
We are aware of several other companies developing programs that utilize IL-2 for the selective expansion of regulatory T cells, including Amgen Inc., Nektar Therapeutics (in partnership with Eli Lilly & Company, or Eli Lilly), Roche Holding AG, or Roche, and Celgene Corporation, or Celgene. We are also aware of other companies with research or preclinical-stage programs in this area, including Synthorx, Inc., Moderna, Inc. and Xencor, Inc. We are also aware of other companies with PD-1 agonist programs for the treatment of autoimmune diseases, including AnaptysBio, Inc., Celgene and Eli Lilly.
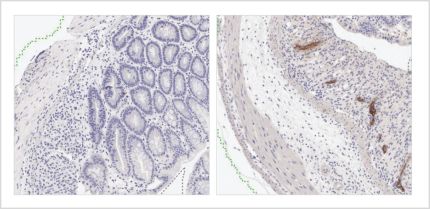
If approved for the treatment of patients with moderate-to-severe UC who are nonresponsive or intolerant to corticosteroids, PT101 would compete with Entyvio, which is an a4b7 integrin antibody marketed by Takeda Pharmaceutical Company Ltd, Humira, which is a TNF antibody marketed by AbbVie, Stelara, which is an IL-12/IL-23 antibody marketed by Johnson & Johnson, Xeljanz, which is a JAK1 inhibitor marketed by Pfizer Inc., and Simponi, which is a TNF antibody marketed by Johnson & Johnson.
We are aware of several companies with product candidates for the treatment of patients with UC, including Rinvoq, which is a JAK1 inhibitor being developed in Phase 3 clinical trials by AbbVie, ozanimod, which is a S1P inhibitor being developed in Phase 3 clinical trials by Celgene, etrolizumab, which is a b7 integrin being developed in Phase 3 clinical trials by Roche, mirikizumab, which is an anti-IL-23 antibody being developed in Phase 3 clinical trials by Eli Lilly and filgotinib, a JAK1 inhibitor being developed in Phase 3 clinical trials by Gilead Sciences, Inc. We are also aware of additional product candidates in clinical trials by AbbVie, Abivax SA, Amgen Inc., Arena Pharmaceuticals, Inc. Boehringer Ingelheim, Bristol-Myers Squibb Company, Celgene, Gilead Sciences, Inc., GlaxoSmithKline plc, Gossamer Bio, Inc., Incyte Corp., Janssen Pharmaceutica N.V., Landos Biopharma, Inc., Pfizer Inc., Protagonist Therapeutics, Inc., and Theravance Biopharma, Inc.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing,
48