
Pioneering Novel IO Therapies Focused on Key Mechanisms of Immunosuppression November 2020 Exhibit 99.3
Disclaimer This Presentation has been prepared by iTeos Therapeutics, Inc. (“we,” “us,” our “our”) and contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and future conditions. All statements, other than statements of historical facts, contained in this Presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates, including our clinical trials of EOS-850, our clinical trials of EOS-448 and of our research and development programs; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; our ability to manufacture our product candidates, including EOS-850 and EOS-448, or any other product candidate in conformity with the Food and Drug Administration’s requirements and to scale up manufacturing of our product candidates to commercial scale, if approved; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; and our plans to develop and commercialize our current product candidates and any future product candidates and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. These statements are based on management’s current expectations and beliefs and are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: uncertainties inherent in clinical studies and the availability and timing of data from ongoing clinical studies; whether interim results from a clinical trial will be predictive of the final results of a trial; that the results from our clinical trials for EOS-850 and EOS-448 may not support further development and marketing approval; the risk that we may be unable to gain approval for our product candidates on a timely basis, if at all; the risk that the current COVID-19 pandemic will impact our clinical trials and operations; and other risks set forth under the caption ‘Risk Factors’ in our most recent Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, as filed with the SEC on November 12, 2020, and in our future filings with the SEC available at the SEC’s website at www.sec.gov. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. You should not place undue reliance on any forward-looking statements, which speak only as of the date they are made. Certain information contained in this Presentation and statements made orally during this Presentation relate to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party studies, publications, surveys and other data to be reliable as of the date of the Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent sources has evaluated the reasonableness or accuracy of the Company’s internal estimates or research and no reliance should be made on any information or statements made in this Presentation relating to or based on such internal estimates and research. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.
Key Investment Highlights Developing next generation IO therapeutics, targeting key mechanisms of immunosuppression Multiple near-term clinical and pipeline milestones EOS-850 is a potential best-in-class selective A2AR antagonist with two confirmed PRs in Phase 1 single agent dose escalation Pipeline of complementary programs enabling intra-portfolio combinations EOS-448 is a clinical stage anti-TIGIT antibody designed to have high affinity and to actively engage FcγR Highly experienced management team with significant expertise in oncology drug development

Our Leadership Team Matt Call Chief Operating Officer Joanne Lager, M.D. Chief Medical Officer Michel Detheux, Ph.D. President & CEO Matthew Gall Chief Financial Officer Leadership Team Yvonne McGrath, Ph.D. Vice President, R&D Board of Directors David Hallal, Chair CEO Elevate Bio Michel Detheux CEO iTeos Priyanka Belawat HBM Detlev Biniszkiewicz MPM Capital Aaron Davis CEO, Boxer Capital Derek DiRocco RA Capital Matt Roden Executive Partner, MPM Capital Tim Van Hauwermeiren CEO argenx Ann Rhoads Former CFO, Forty Seven Philippe Brantegem Vice President, HR
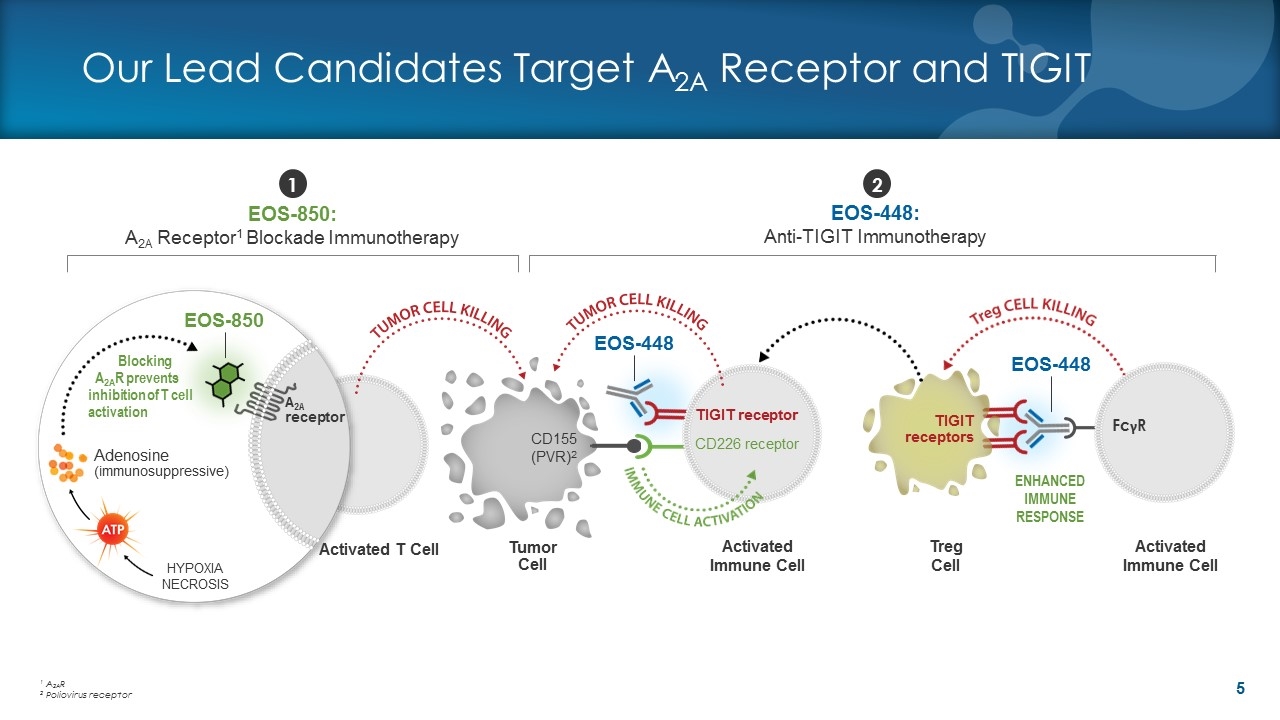
Activated Immune Cell Our Lead Candidates Target A2A Receptor and TIGIT EOS-448: Anti-TIGIT Immunotherapy 1 2 EOS-448 CD226 receptor TIGIT receptor FcγR Treg Cell TIGIT receptors ENHANCED IMMUNE RESPONSE Blocking A2AR prevents inhibition of T cell activation EOS-850: A2A Receptor1 Blockade Immunotherapy EOS-850 Tumor Cell CD155 (PVR)2 Adenosine (immunosuppressive) HYPOXIA NECROSIS A2A receptor Activated T Cell Activated Immune Cell EOS-448 1 A2AR 2 Poliovirus receptor
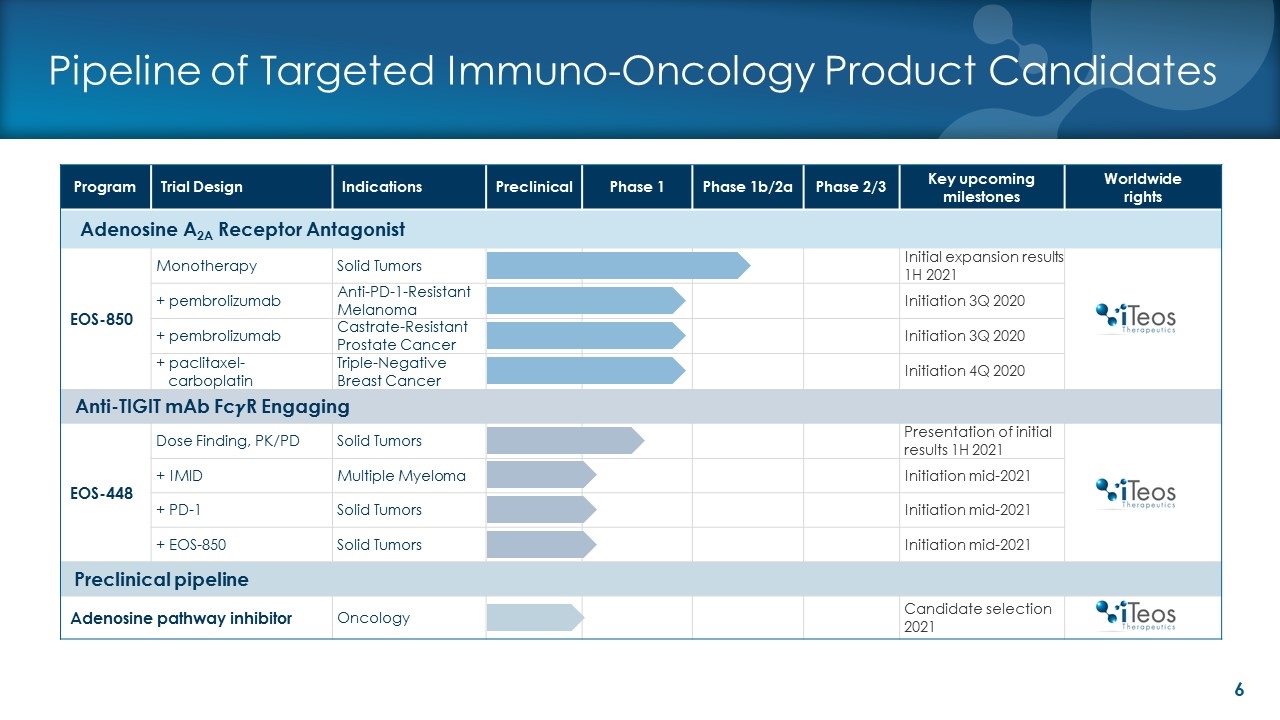
Pipeline of Targeted Immuno-Oncology Product Candidates Program Trial Design Indications Preclinical Phase 1 Phase 1b/2a Phase 2/3 Key upcoming milestones Worldwide rights Adenosine A2A Receptor Antagonist EOS-850 Monotherapy Solid Tumors Initial expansion results 1H 2021 + pembrolizumab Anti-PD-1-Resistant Melanoma Initiation 3Q 2020 + pembrolizumab Castrate-Resistant Prostate Cancer Initiation 3Q 2020 + paclitaxel- carboplatin Triple-Negative Breast Cancer Initiation 4Q 2020 Anti-TIGIT mAb Fc��R Engaging EOS-448 Dose Finding, PK/PD Solid Tumors Presentation of initial results 1H 2021 + IMID Multiple Myeloma Initiation mid-2021 + PD-1 Solid Tumors Initiation mid-2021 + EOS-850 Solid Tumors Initiation mid-2021 Preclinical pipeline Adenosine pathway inhibitor Oncology Candidate selection 2021

Phase 1/2 Program with Early Single Agent Activity EOS-850 Potentially Best-in-Class Adenosine Receptor Antagonist
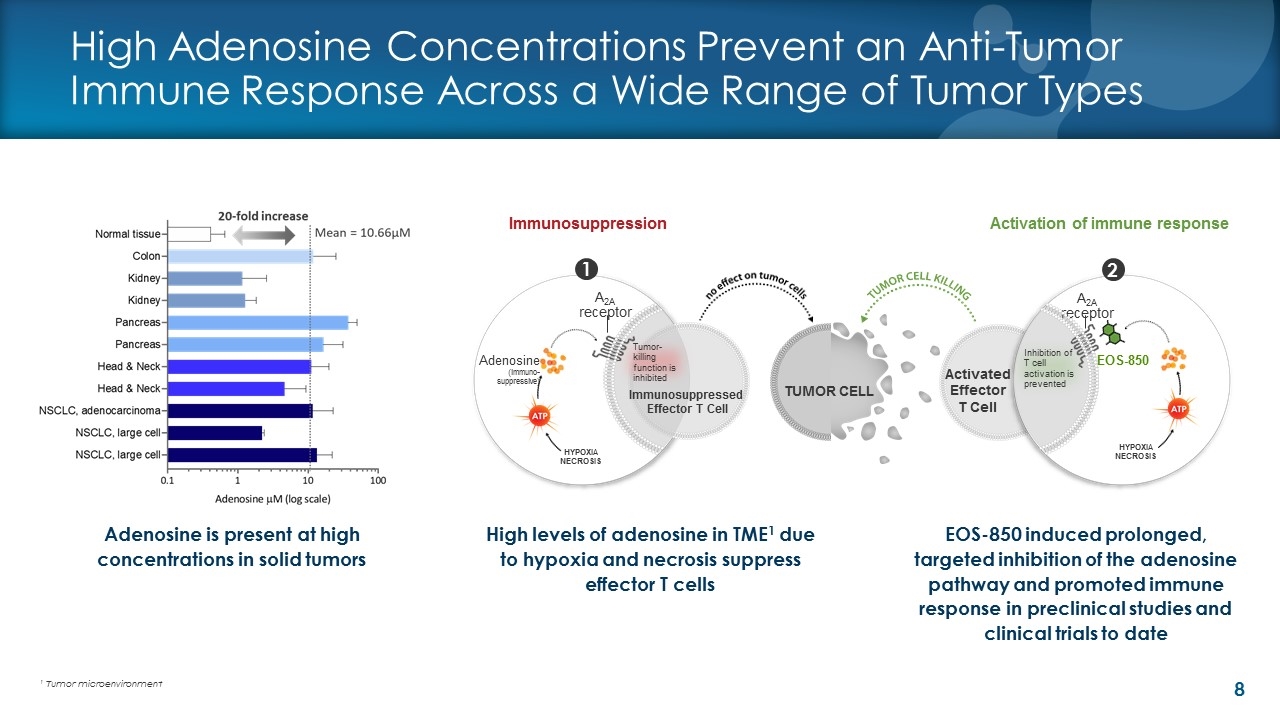
High Adenosine Concentrations Prevent an Anti-Tumor Immune Response Across a Wide Range of Tumor Types High levels of adenosine in TME1 due to hypoxia and necrosis suppress effector T cells Immunosuppression 1 2 Activation of immune response Activated Effector T Cell EOS-850 TUMOR CELL A2A receptor Adenosine (immuno- suppressive) HYPOXIA NECROSIS Inhibition of T cell activation is prevented A2A receptor HYPOXIA NECROSIS Immunosuppressed Effector T Cell Tumor-killing function is inhibited Adenosine is present at high concentrations in solid tumors EOS-850 induced prolonged, targeted inhibition of the adenosine pathway and promoted immune response in preclinical studies and clinical trials to date 1 Tumor microenvironment
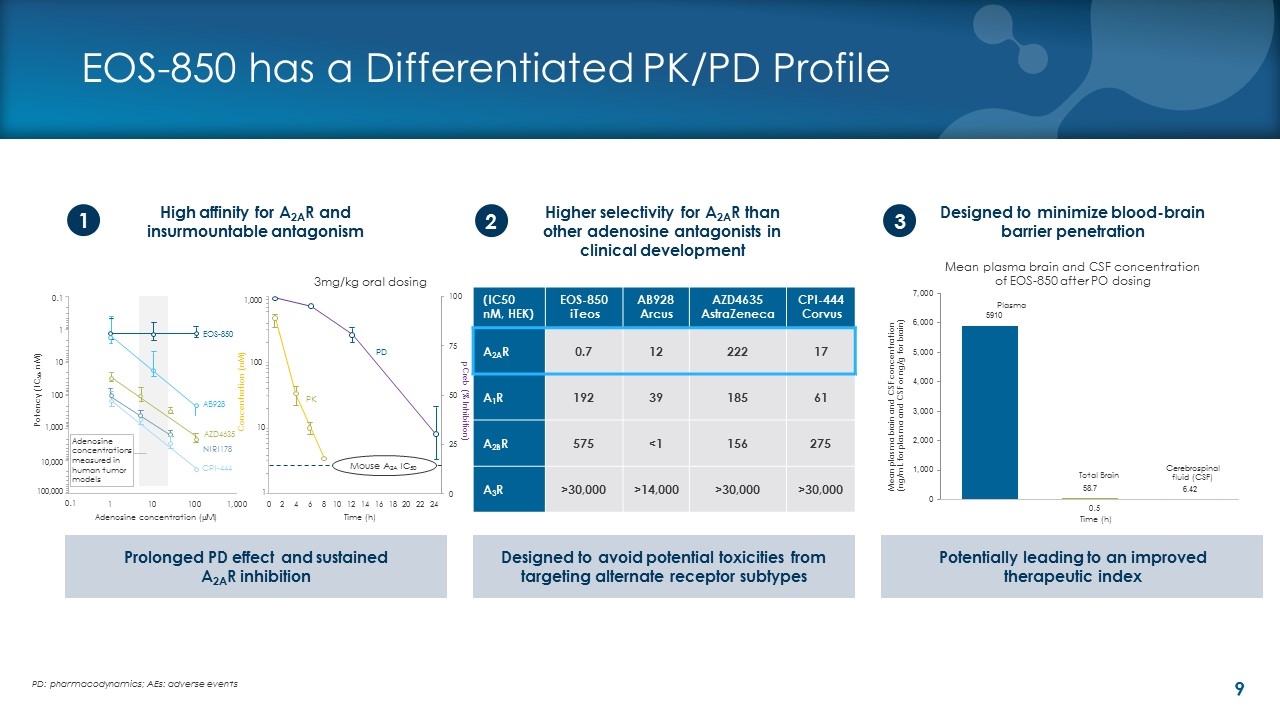
EOS-850 has a Differentiated PK/PD Profile 3mg/kg oral dosing High affinity for A2AR and insurmountable antagonism Designed to minimize blood-brain barrier penetration 1 3 Prolonged PD effect and sustained A2AR inhibition Designed to avoid potential toxicities from targeting alternate receptor subtypes Potentially leading to an improved therapeutic index 2 Higher selectivity for A2AR than other adenosine antagonists in clinical development PD: pharmacodynamics; AEs: adverse events (IC50 nM, HEK) EOS-850 iTeos AB928 Arcus AZD4635 AstraZeneca CPI-444 Corvus A2AR 0.7 12 222 17 A1R 192 39 185 61 A2BR 575 <1 156 275 A3R >30,000 >14,000 >30,000 >30,000 0.1 1 10 100 1,000 10,000 100,000 Potency (IC50, nM) EOS-850 AB928 AZD4635 NIRI178 CPI-444 Adenosine concentrations measured in human tumor models 1,000 Concentration (nM) 100 10 1 Mouse A2A IC50 Adenosine concentration (mM) Time (h) PK PD 0.1 Mean plasma brain and CSF concentration (ng/mL for plasma and CSF or ng/g for brain) Mean plasma brain and CSF concentration of EOS-850 after PO dosing Plasma Total Brain Cerebrospinal fluid (CSF) Time (h) 0.5 pCreb (% Inhibition)

EOS-850 has a Potential Best-in-Class Therapeutic Profile Generally well tolerated at all dose levels with no DLT1 observed Preliminary evidence of clinical benefit in 7 patients: 2 ongoing confirmed PRs2 Sustained inhibition of A2AR and prolonged PD activity Ongoing tumor profiling and biomarker identification, including via biopsies 80 mg BID selected as recommended Phase 2 dose Advanced solid tumor patients (n=21) Phase 1 Dose Escalation (Single Agent) Preliminary Results 1: DLT: Dose Limiting Toxicity 2: as of September 9, 2020

EOS-850 Monotherapy Demonstrated Preliminary Evidence of Clinical Benefit in Heavily Pretreated Patients As of 10 Jun 2020 Notes: 1 Once daily doses 2 Twice daily doses CRC: colorectal cancer; NSCLC: non-small-cell lung carcinoma; TCC: transitional cell carcinoma; CRPC: castrate resistant prostate cancer; SCPC: small cell prostate cancer; TNBC: triple-negative breast cancer BID: Twice daily dosing Best Response QD1 doses (n=6), n (%) BID2 doses (n=15), n (%) Total (n=21), n (%) Complete Response 0% 0% 0% Partial Response 0% 2 (13%) 2 (9.5%) Stable Disease 1 (16.5%) 4 (27%) 5 (24%) Progressive Disease 4 (67%) 8 (53%) 12 (57%) Not Assessed 1 (16.5%) 1 (7%) 2 (9.5%) Initial findings indicate a disease control rate of 40% (PR + SD) for BID doses
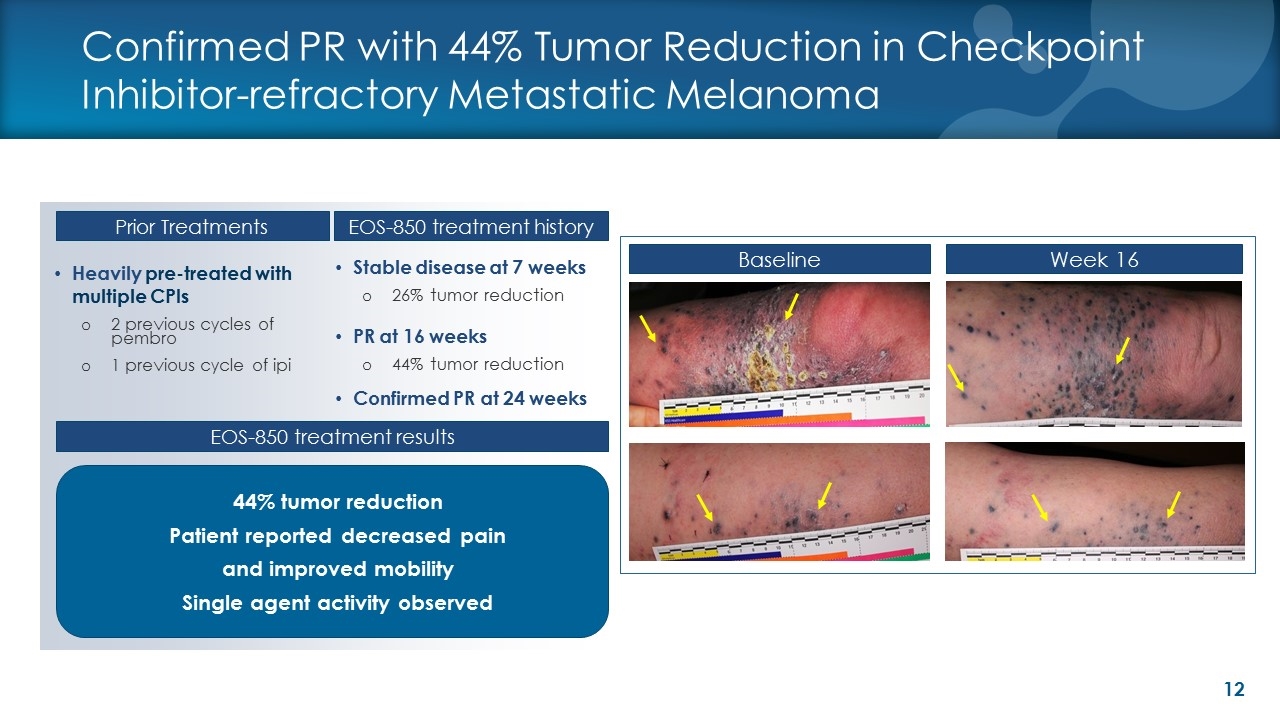
Heavily pre-treated with multiple CPIs 2 previous cycles of pembro 1 previous cycle of ipi Stable disease at 7 weeks 26% tumor reduction PR at 16 weeks 44% tumor reduction Confirmed PR at 24 weeks Baseline Week 16 Prior Treatments Confirmed PR with 44% Tumor Reduction in Checkpoint Inhibitor-refractory Metastatic Melanoma EOS-850 treatment history 44% tumor reduction Patient reported decreased pain and improved mobility Single agent activity observed EOS-850 treatment results
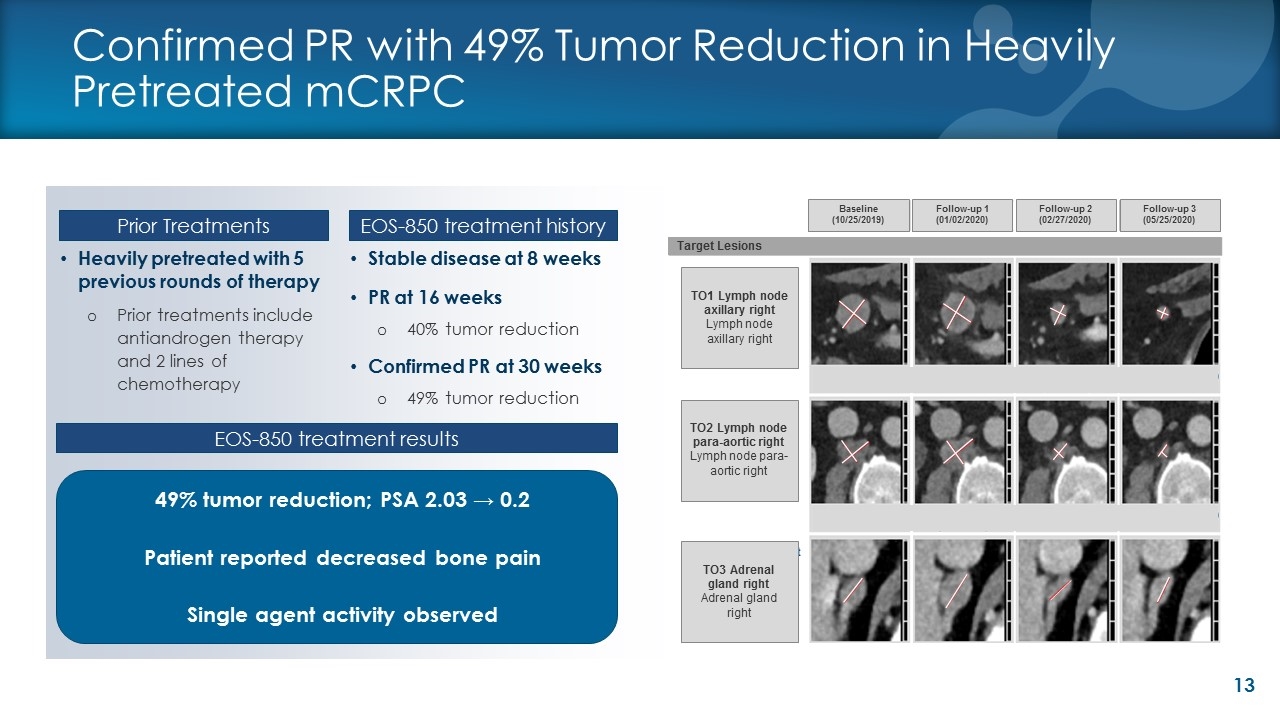
Confirmed PR with 49% Tumor Reduction in Heavily Pretreated mCRPC Stable disease at 8 weeks PR at 16 weeks 40% tumor reduction Confirmed PR at 30 weeks 49% tumor reduction Heavily pretreated with 5 previous rounds of therapy Prior treatments include antiandrogen therapy and 2 lines of chemotherapy Follow-up 3 (05/25/2020) Baseline (10/25/2019) Follow-up 1 (01/02/2020) Follow-up 2 (02/27/2020) TO1 Lymph node axillary right Lymph node axillary right TO2 Lymph node para-aortic right Lymph node para-aortic right TO3 Adrenal gland right Adrenal gland right Target Lesions 49% tumor reduction; PSA 2.03 → 0.2 Patient reported decreased bone pain Single agent activity observed Prior Treatments EOS-850 treatment history EOS-850 treatment results
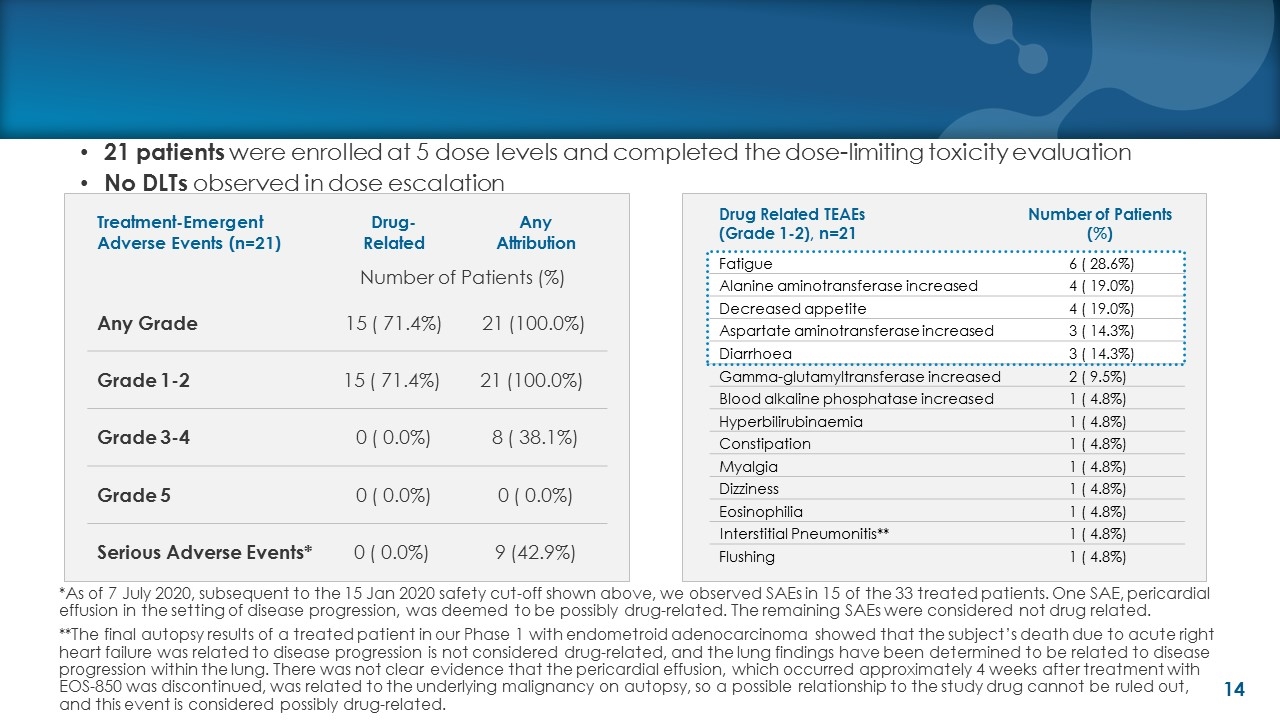
21 patients were enrolled at 5 dose levels and completed the dose-limiting toxicity evaluation No DLTs observed in dose escalation Treatment-Emergent Adverse Events (n=21) Drug- Related Any Attribution Number of Patients (%) Any Grade 15 ( 71.4%) 21 (100.0%) Grade 1-2 15 ( 71.4%) 21 (100.0%) Grade 3-4 0 ( 0.0%) 8 ( 38.1%) Grade 5 0 ( 0.0%) 0 ( 0.0%) Serious Adverse Events* 0 ( 0.0%) 9 (42.9%) Drug Related TEAEs (Grade 1-2), n=21 Number of Patients (%) Fatigue 6 ( 28.6%) Alanine aminotransferase increased 4 ( 19.0%) Decreased appetite 4 ( 19.0%) Aspartate aminotransferase increased 3 ( 14.3%) Diarrhoea 3 ( 14.3%) Gamma-glutamyltransferase increased 2 ( 9.5%) Blood alkaline phosphatase increased 1 ( 4.8%) Hyperbilirubinaemia 1 ( 4.8%) Constipation 1 ( 4.8%) Myalgia 1 ( 4.8%) Dizziness 1 ( 4.8%) Eosinophilia 1 ( 4.8%) Interstitial Pneumonitis** 1 ( 4.8%) Flushing 1 ( 4.8%) *As of 7 July 2020, subsequent to the 15 Jan 2020 safety cut-off shown above, we observed SAEs in 15 of the 33 treated patients. One SAE, pericardial effusion in the setting of disease progression, was deemed to be possibly drug-related. The remaining SAEs were considered not drug related. **The final autopsy results of a treated patient in our Phase 1 with endometroid adenocarcinoma showed that the subject’s death due to acute right heart failure was related to disease progression is not considered drug-related, and the lung findings have been determined to be related to disease progression within the lung. There was not clear evidence that the pericardial effusion, which occurred approximately 4 weeks after treatment with EOS-850 was discontinued, was related to the underlying malignancy on autopsy, so a possible relationship to the study drug cannot be ruled out, and this event is considered possibly drug-related.
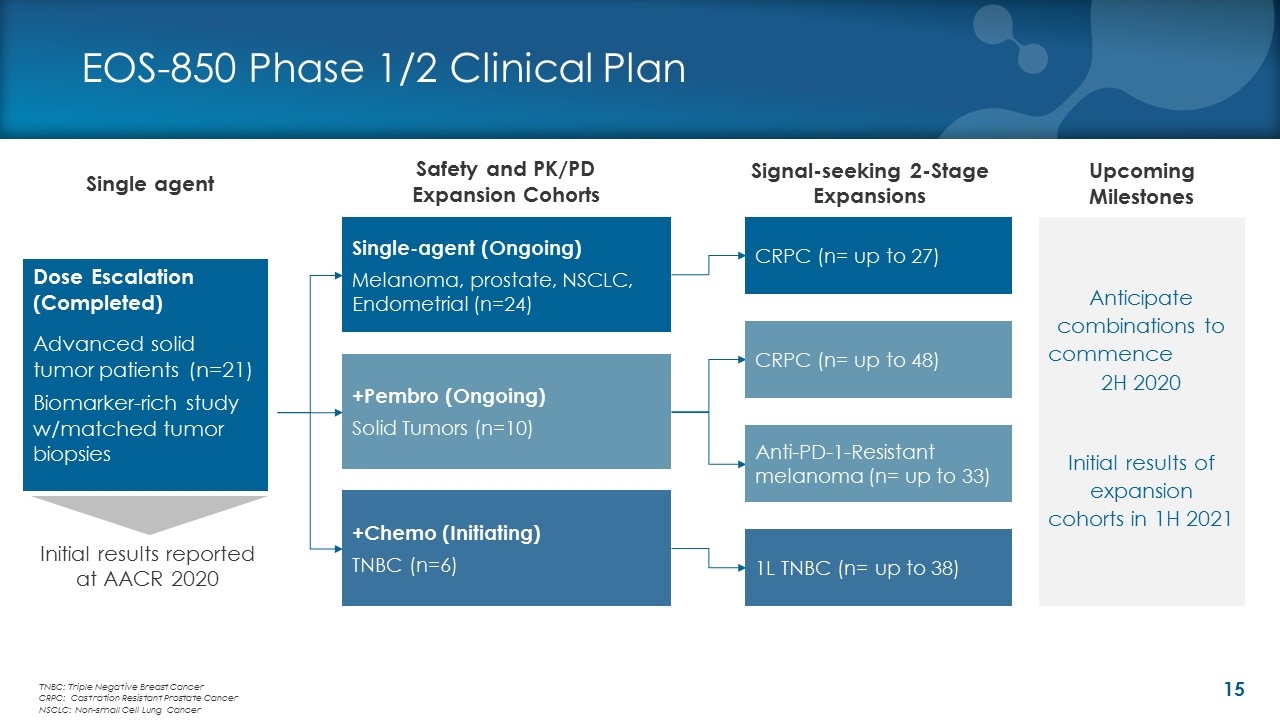
EOS-850 Phase 1/2 Clinical Plan Single agent Safety and PK/PD Expansion Cohorts Dose Escalation (Completed) Advanced solid tumor patients (n=21) Biomarker-rich study w/matched tumor biopsies +Pembro (Ongoing) Solid Tumors (n=10) +Chemo (Initiating) TNBC (n=6) 1L TNBC (n= up to 38) Anti-PD-1-Resistant melanoma (n= up to 33) Single-agent (Ongoing) Melanoma, prostate, NSCLC, Endometrial (n=24) Signal-seeking 2-Stage Expansions TNBC: Triple Negative Breast Cancer CRPC: Castration Resistant Prostate Cancer NSCLC: Non-small Cell Lung Cancer CRPC (n= up to 27) CRPC (n= up to 48) Initial results reported at AACR 2020 Anticipate combinations to commence 2H 2020 Initial results of expansion cohorts in 1H 2021 Upcoming Milestones
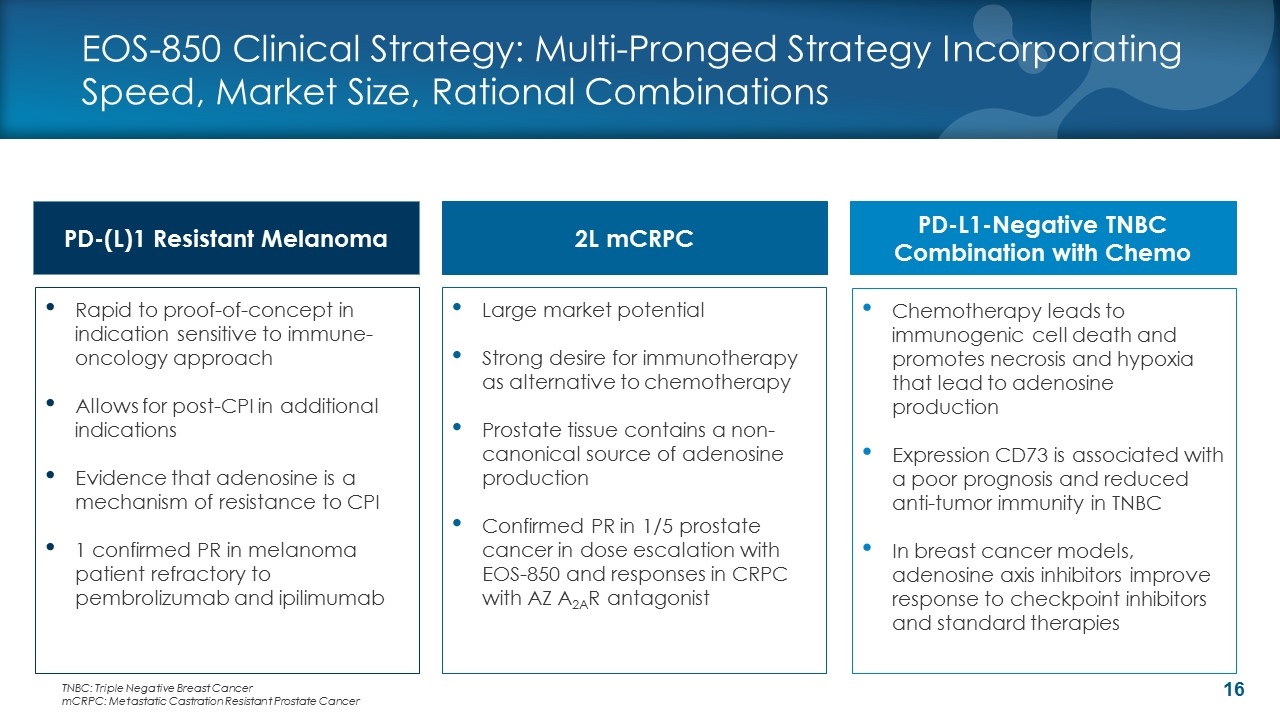
EOS-850 Clinical Strategy: Multi-Pronged Strategy Incorporating Speed, Market Size, Rational Combinations PD-L1-Negative TNBC Combination with Chemo 2L mCRPC PD-(L)1 Resistant Melanoma Chemotherapy leads to immunogenic cell death and promotes necrosis and hypoxia that lead to adenosine production Expression CD73 is associated with a poor prognosis and reduced anti-tumor immunity in TNBC In breast cancer models, adenosine axis inhibitors improve response to checkpoint inhibitors and standard therapies Large market potential Strong desire for immunotherapy as alternative to chemotherapy Prostate tissue contains a non-canonical source of adenosine production Confirmed PR in 1/5 prostate cancer in dose escalation with EOS-850 and responses in CRPC with AZ A2AR antagonist Rapid to proof-of-concept in indication sensitive to immune-oncology approach Allows for post-CPI in additional indications Evidence that adenosine is a mechanism of resistance to CPI 1 confirmed PR in melanoma patient refractory to pembrolizumab and ipilimumab TNBC: Triple Negative Breast Cancer mCRPC: Metastatic Castration Resistant Prostate Cancer

Currently in Dose Escalation Phase 1/2 Trial EOS-448 Fc��R-engaging Anti-TIGIT Antibody
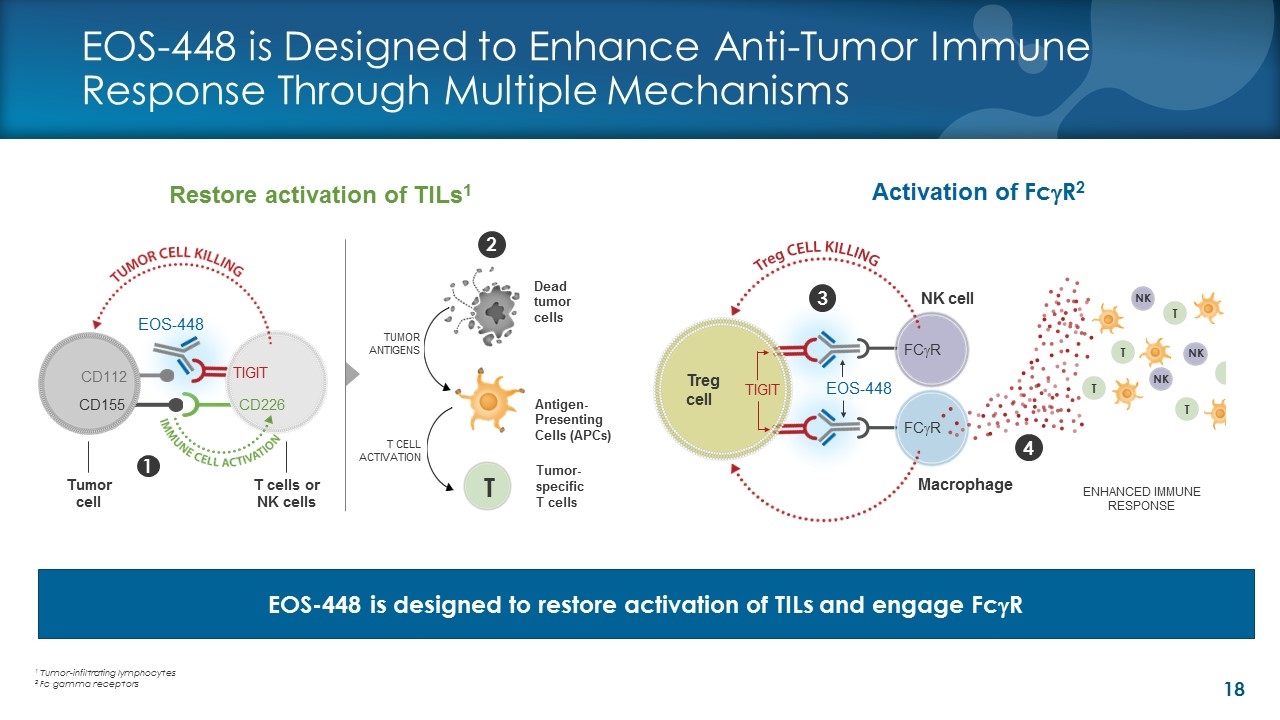
EOS-448 is Designed to Enhance Anti-Tumor Immune Response Through Multiple Mechanisms 4 2 3 T cells or NK cells 1 Tumor cell EOS-448 CD112 CD155 CD226 TIGIT FCgR Treg cell NK cell TUMOR ANTIGENS Tumor- specific T cells Macrophage FCgR EOS-448 TIGIT Dead tumor cells Antigen-Presenting Cells (APCs) T CELL ACTIVATION ENHANCED IMMUNE RESPONSE T T NK T NK T NK T Activation of FcgR2 Restore activation of TILs1 EOS-448 is designed to restore activation of TILs and engage FcgR 1 Tumor-infiltrating lymphocytes 2 Fc gamma receptors
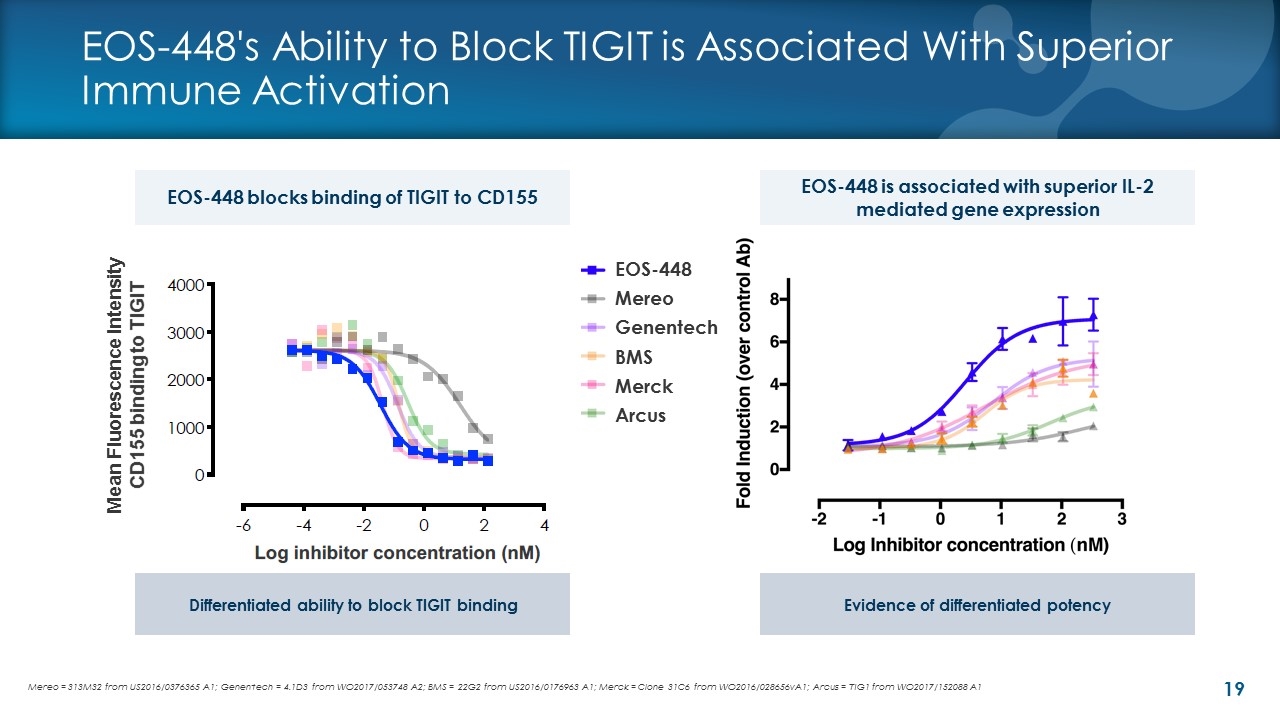
EOS-448's Ability to Block TIGIT is Associated With Superior Immune Activation EOS-448 is associated with superior IL-2 mediated gene expression Mereo = 313M32 from US2016/0376365 A1; Genentech = 4.1D3 from WO2017/053748 A2; BMS = 22G2 from US2016/0176963 A1; Merck = Clone 31C6 from WO2016/028656vA1; Arcus = TIG1 from WO2017/152088 A1 Log inhibitor concentration (nM) Mean Fluorescence Intensity CD155 binding to TIGIT EOS-448 blocks binding of TIGIT to CD155 EOS-448 Mereo Genentech BMS Merck Arcus Differentiated ability to block TIGIT binding Evidence of differentiated potency Mean Fluorescence Intensity CD155 binding to TIGIT
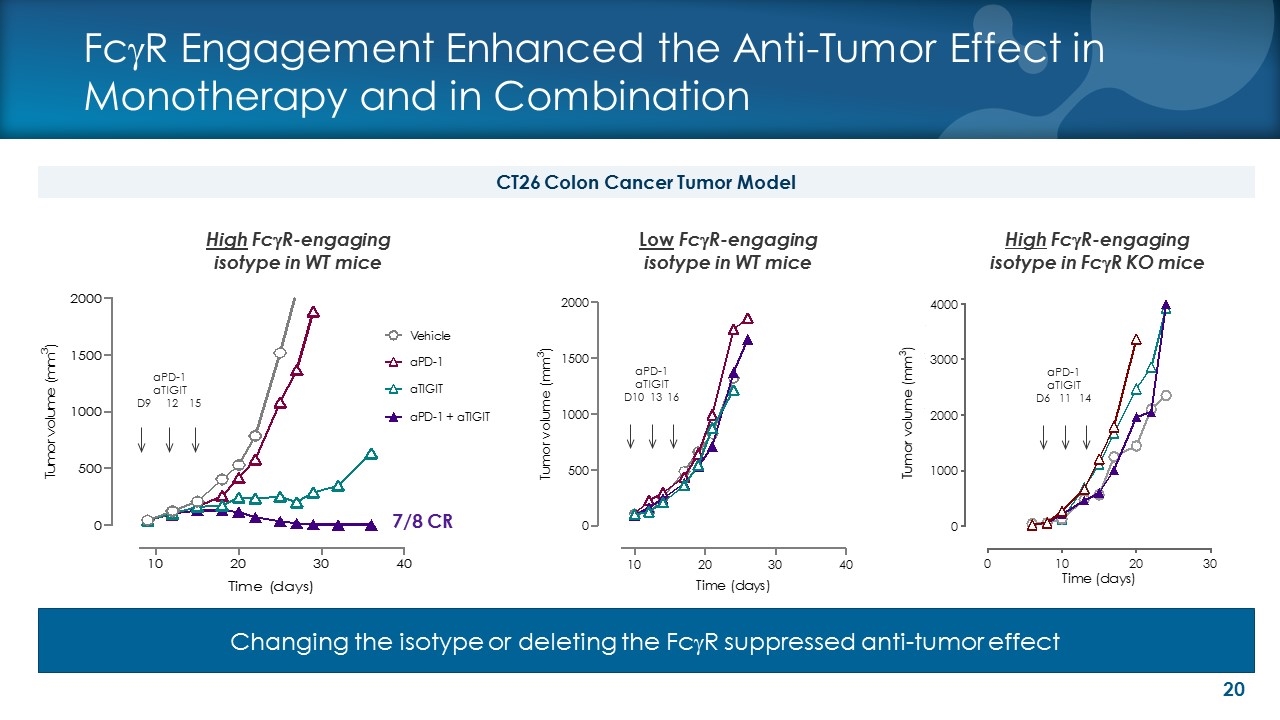
High FcgR-engaging isotype in WT mice Low FcgR-engaging isotype in WT mice aPD-1 aTIGIT D10 13 16 aPD-1 aTIGIT D9 12 15 7/8 CR FcgR Engagement Enhanced the Anti-Tumor Effect in Monotherapy and in Combination CT26 Colon Cancer Tumor Model High FcgR-engaging isotype in FcgR KO mice Changing the isotype or deleting the FcgR suppressed anti-tumor effect aPD-1 aTIGIT D6 11 14
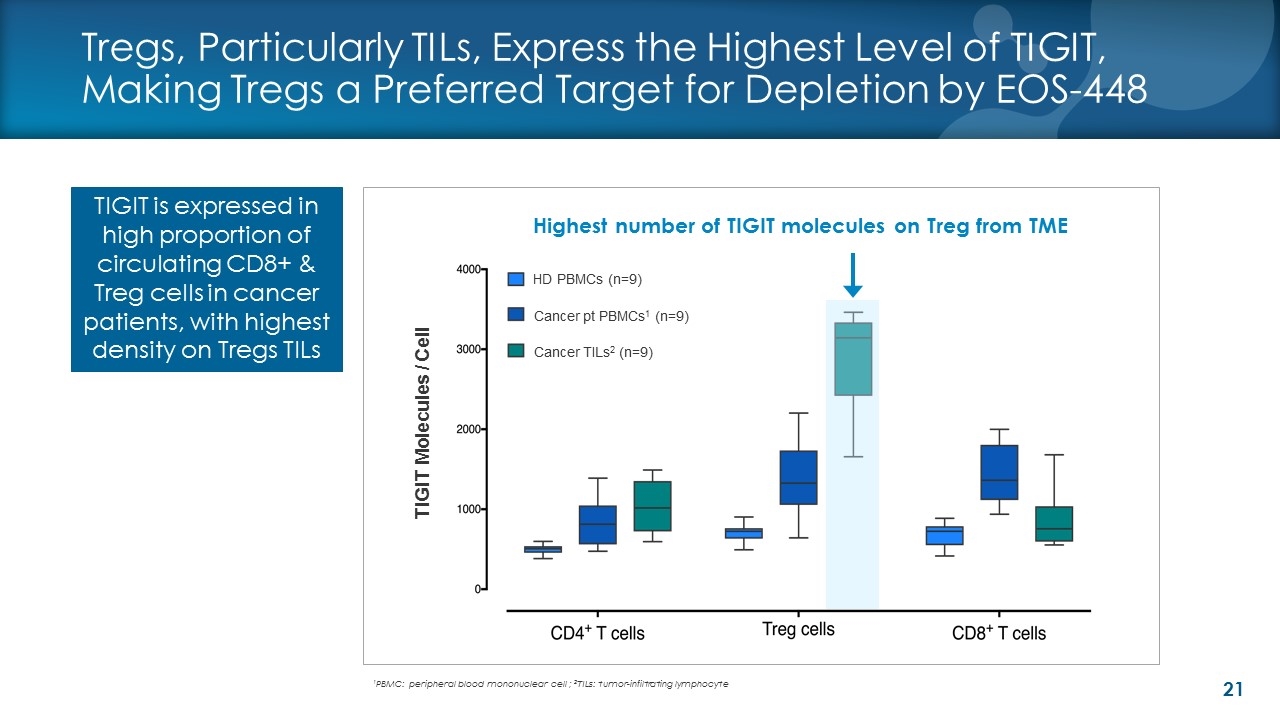
TIGIT is expressed in high proportion of circulating CD8+ & Treg cells in cancer patients, with highest density on Tregs TILs Tregs, Particularly TILs, Express the Highest Level of TIGIT, Making Tregs a Preferred Target for Depletion by EOS-448 Highest number of TIGIT molecules on Treg from TME TIGIT Molecules / Cell HD PBMCs (n=9) 1PBMC: peripheral blood mononuclear cell ; 2TILs: tumor-infiltrating lymphocyte Cancer TILs2 (n=9) Cancer pt PBMCs1 (n=9)
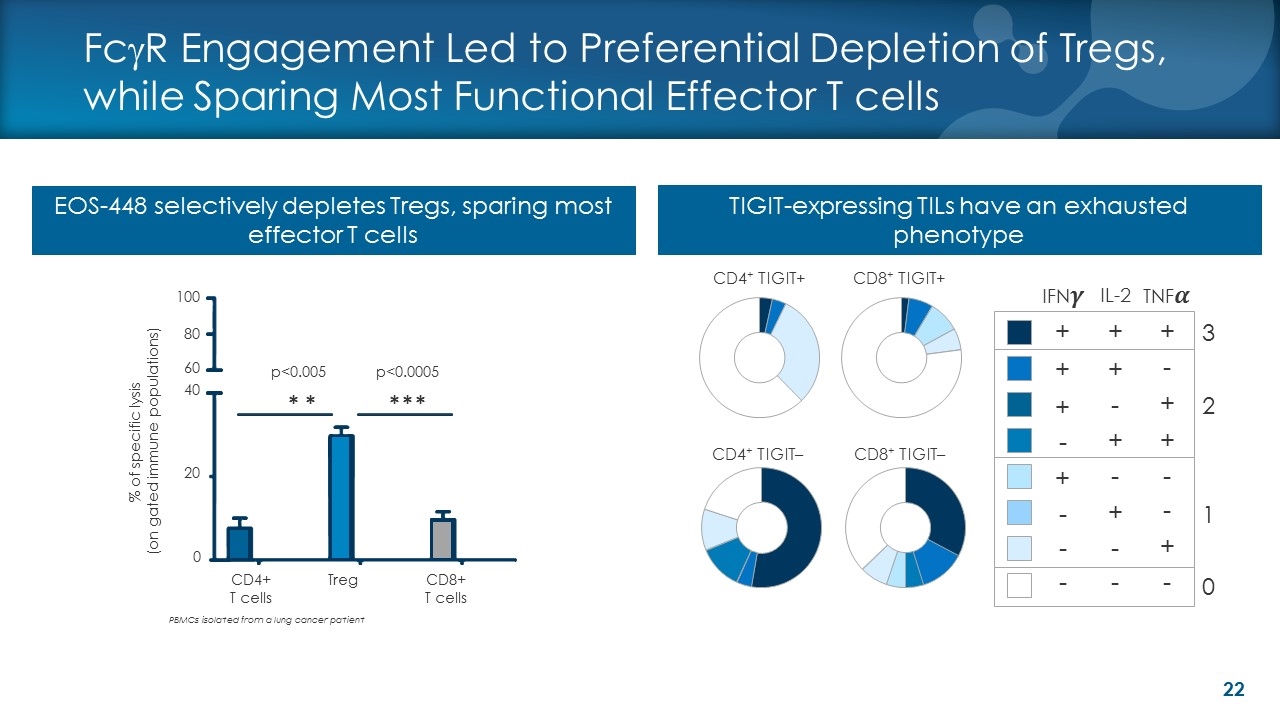
FcgR Engagement Led to Preferential Depletion of Tregs, while Sparing Most Functional Effector T cells PBMCs isolated from a lung cancer patient TIGIT-expressing TILs have an exhausted phenotype EOS-448 selectively depletes Tregs, sparing most effector T cells Þ Þ Þ Þ 0 20 40 60 p<0.005 p<0.0005 % of specific lysis (on gated immune populations) 80 100 CD4+ T cells Treg CD8+ T cells Þ Fill level 1 + + + + - - - - + + - + + - - - + + + - - + - CD4+ TIGIT+ CD8+ TIGIT+ CD4+ TIGIT– CD8+ TIGIT– - 3 0 2 1 IFN�� IL-2 TNF��
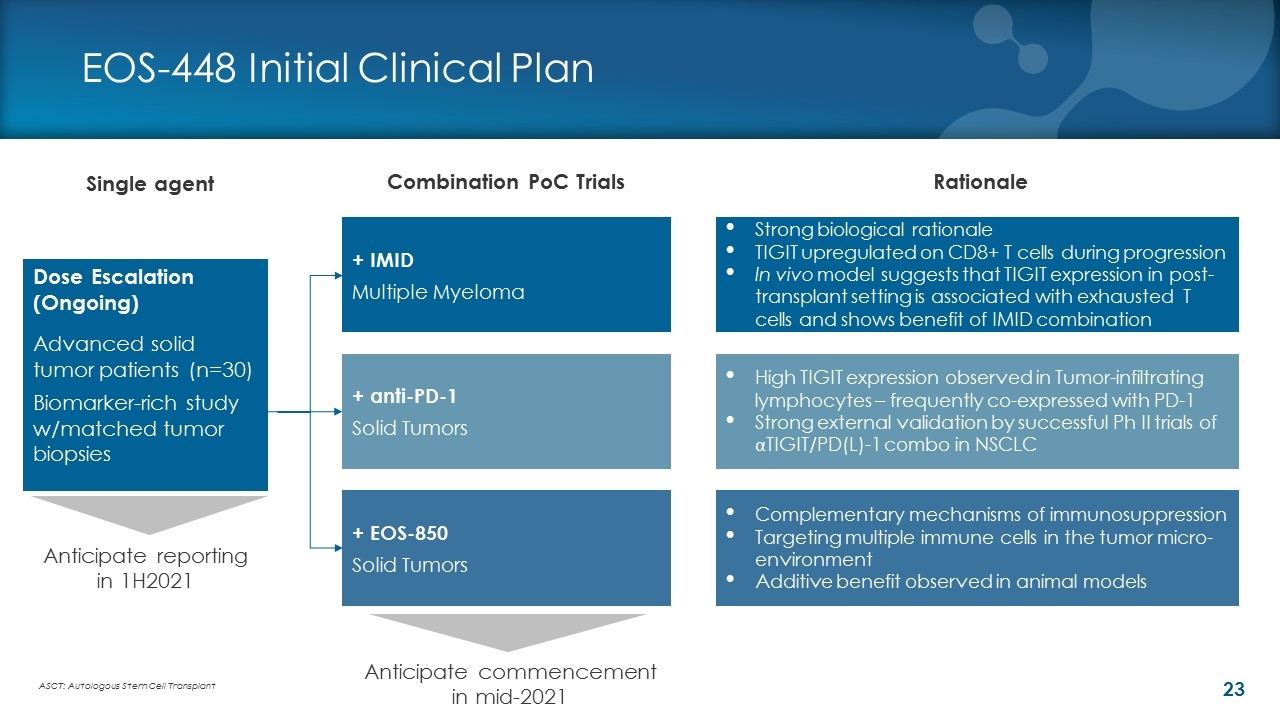
EOS-448 Initial Clinical Plan Single agent Combination PoC Trials Dose Escalation (Ongoing) Advanced solid tumor patients (n=30) Biomarker-rich study w/matched tumor biopsies + anti-PD-1 Solid Tumors + EOS-850 Solid Tumors Complementary mechanisms of immunosuppression Targeting multiple immune cells in the tumor micro-environment Additive benefit observed in animal models + IMID Multiple Myeloma ASCT: Autologous Stem Cell Transplant Strong biological rationale TIGIT upregulated on CD8+ T cells during progression In vivo model suggests that TIGIT expression in post-transplant setting is associated with exhausted T cells and shows benefit of IMID combination High TIGIT expression observed in Tumor-infiltrating lymphocytes – frequently co-expressed with PD-1 Strong external validation by successful Ph II trials of αTIGIT/PD(L)-1 combo in NSCLC Anticipate reporting in 1H2021 Anticipate commencement in mid-2021 Rationale

Investment Forecast and Near-term Catalysts iTeos Financials
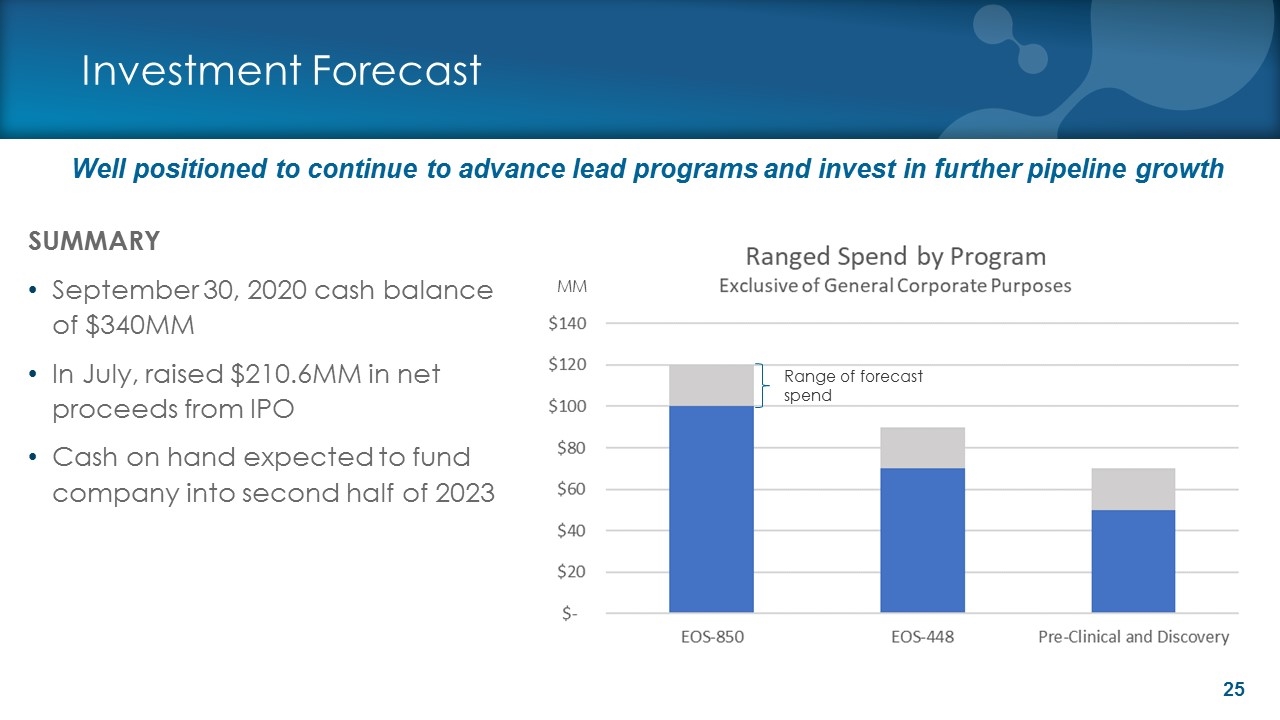
Investment Forecast SUMMARY September 30, 2020 cash balance of $340MM In July, raised $210.6MM in net proceeds from IPO Cash on hand expected to fund company into second half of 2023 Range of forecast spend MM Well positioned to continue to advance lead programs and invest in further pipeline growth
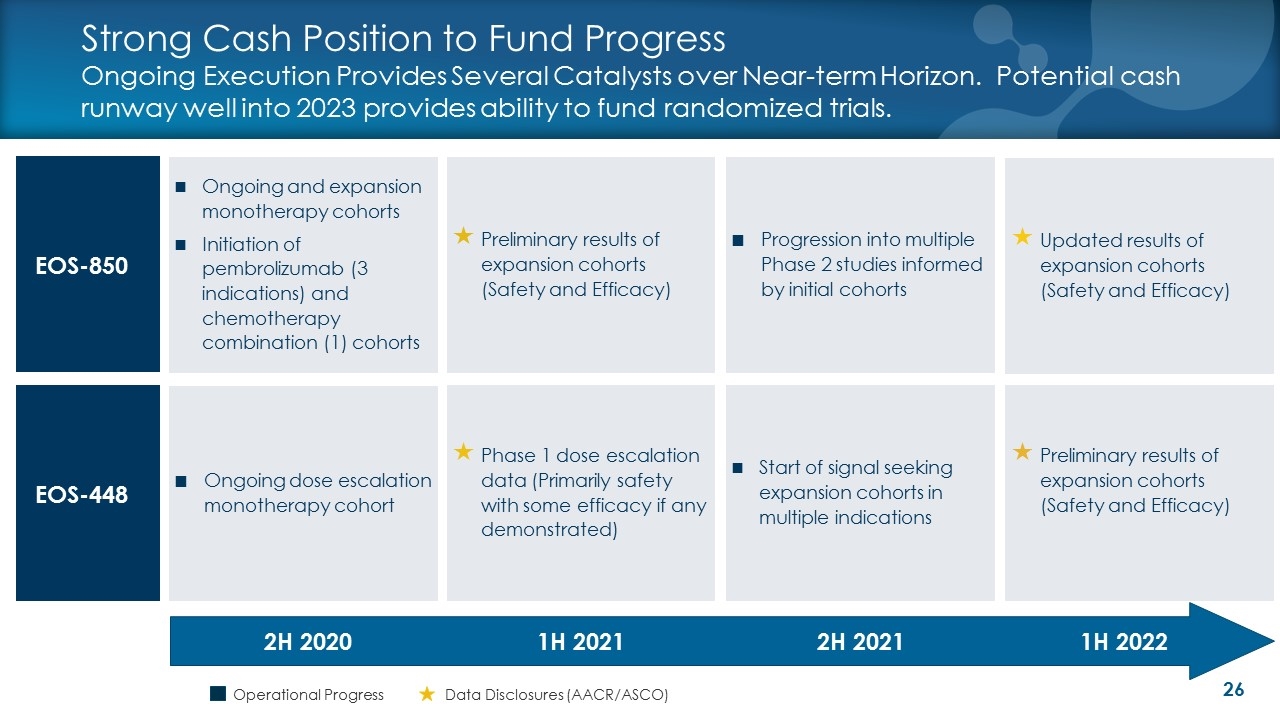
Strong Cash Position to Fund Progress Ongoing Execution Provides Several Catalysts over Near-term Horizon. Potential cash runway well into 2023 provides ability to fund randomized trials. EOS-850 EOS-448 Preliminary results of expansion cohorts (Safety and Efficacy) Phase 1 dose escalation data (Primarily safety with some efficacy if any demonstrated) 2H 2020 1H 2021 Progression into multiple Phase 2 studies informed by initial cohorts Start of signal seeking expansion cohorts in multiple indications 2H 2021 Ongoing and expansion monotherapy cohorts Initiation of pembrolizumab (3 indications) and chemotherapy combination (1) cohorts Preliminary results of expansion cohorts (Safety and Efficacy) Updated results of expansion cohorts (Safety and Efficacy) 1H 2022 Ongoing dose escalation monotherapy cohort Operational Progress Data Disclosures (AACR/ASCO)
Key Investment Highlights Developing next generation IO therapeutics, targeting key mechanisms of immunosuppression Multiple near-term clinical and pipeline milestones EOS-850 is a potential best-in-class selective A2AR antagonist with two confirmed PRs in Phase 1 single agent dose escalation Pipeline of complementary programs enabling intra-portfolio combinations EOS-448 is a clinical stage anti-TIGIT antibody designed to have high affinity and to actively engage FcγR Highly experienced management team with significant expertise in oncology drug development


























