BB-301
is a
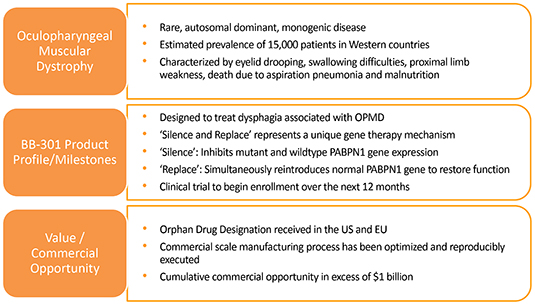
genetic medicine employing the “silence and replace” approach for the treatment of OPMD. OPMD is an insidious, autosomal- dominant, late-onset, degenerative muscle disorder that typically presents in patients at
years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1 gene (PABPN1). OPMD is a rare disease, however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified and significant geographical clustering has been noted for patients with this disorder, both of which could simplify clinical development and global commercialization efforts for
BB-301.
PABPN1 is a ubiquitous factor that promotes the interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage. The characteristic genetic mutation underlying OPMD results in trinucleotide repeat expansion(s) within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the
N-terminal
end of PABPN1. The mutation generates a protein with an
N-terminal
expanded poly-alanine tract of up to 18 contiguous alanine residues, and the mutant protein is prone to the formation of intranuclear aggregates designated as “intranuclear inclusions” (INIs). The INIs that sequester wildtype PABPN1 could also contribute to the “loss of function” phenotype associated with OPMD.
No therapeutic agents are approved for the treatment of OPMD. Additionally, there are no surgical interventions available with the capacity to alter the long-term deterioration of swallowing function which comprises the key element of the natural history of OPMD.
BB-301
has received Orphan Drug Designation in the United States and the European Union and, upon achievement of regulatory approval for
BB-301
in these respective jurisdictions, the Orphan Drug Designations would provide commercial exclusivity independent of intellectual property protection. While OPMD is a rare medical disorder, we believe the commercial opportunity for a safe and efficacious therapeutic agent in this clinical indication exceeds $1 billion over the course of the commercial life of the product.
Benitec has previously outlined the core
CTA-enabling
and
IND-enabling
studies required by global regulatory agencies to support the initiation of
BB-301
clinical trials in OPMD patients, and these studies include a
BB-301
Pilot Dosing Study (the “Pilot Dosing Study”) in large animals and a classical
12-week
GLP Toxicology and Biodistribution Study for
BB-301.
BB-301
is directly injected into the pharyngeal muscles known to underlie the morbidity and mortality which characterizes the natural history of OPMD.
As referenced above, the
BB-301
Pilot Dosing Study in large animals was the first of two
CTA-enabling
and
IND-enabling
studies conducted by Benitec. This study was carried out under the guidance of the scientific team at Benitec, with key elements of the study design and execution conducted in close collaboration with a team of leading experts in both medicine and surgery that have been deeply engaged in the treatment of OPMD patients for several decades. The
BB-301
Pilot Dosing Study and the GLP Toxicology and Biodistribution Study for
BB-301
were conducted in canine subjects in order to:
| | • | | Support the validation and optimization of the newly designed route and method of BB-301 administration, |
| | • | | Confirm the efficiency of vector transduction and transgene expression in the key tissue compartments underlying the natural history of OPMD, |
| | • | | Confirm the optimal BB-301 doses in advance of initiation of human clinical studies, |
| | • | | Facilitate the observation of key toxicological data-points. |
The
BB-301
Pilot Dosing Study was designed as an
8-week
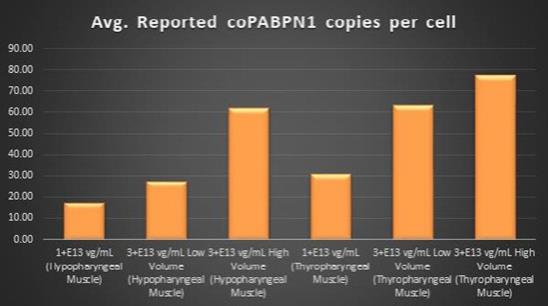
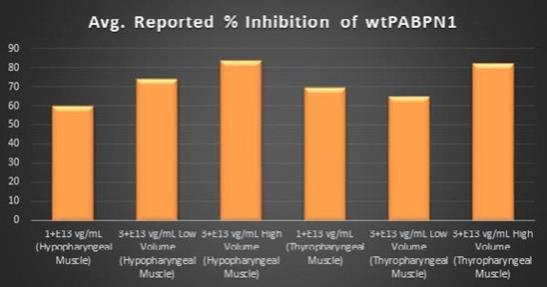
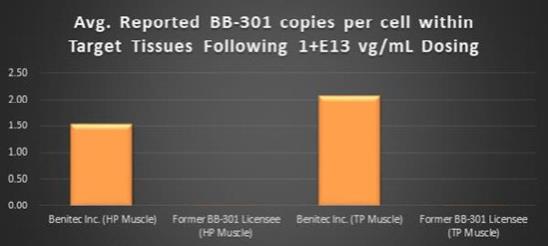
study in Beagle dogs to confirm the transduction efficiency of
BB-301
upon administration via direct intramuscular injection into specific anatomical regions of the pharynx through the use of an open surgical procedure. This new method and route of
BB-301
administration was developed in collaboration with key surgical experts in the field of Otolaryngology, and this novel method of
BB-301
dosing will significantly enhance the ability of treating physicians to accurately administer the AAV-based investigational agent to the muscles that underlie the characteristic deficits associated with disease progression in OPMD. It is important to note that prior
BB-301
non-clinical
studies have reproducibly validated the robust biological activity achieved following direct intramuscular injection of the
AAV-based
agent. As an example, direct injection of
BB-301
into the tibialis anterior muscles of A17 mice facilitated robust transduction of the targeted skeletal muscle cells and supported complete remission of the OPMD disease phenotype in this animal model.