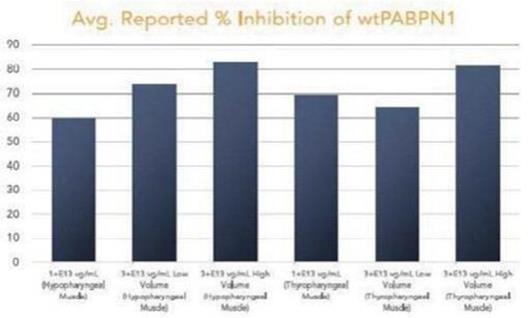
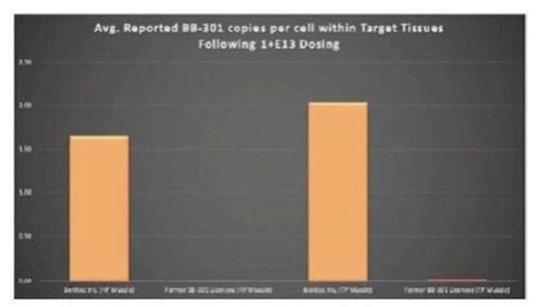
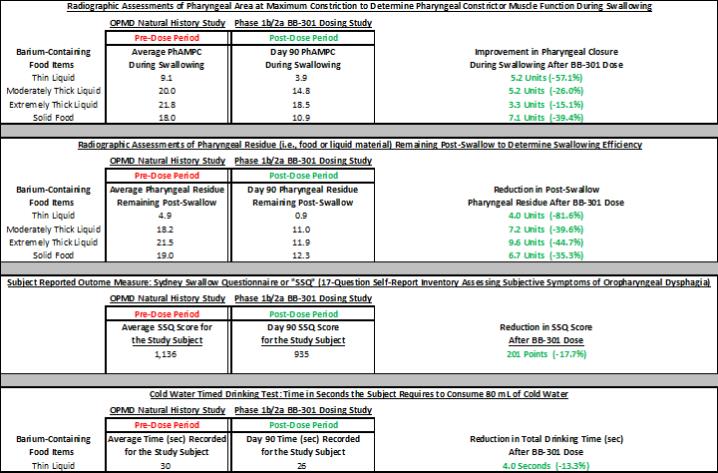
Government Regulation
As a pharmaceutical and biological product company that wishes to conduct clinical trials and ultimately obtain marketing approval in the United States, we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, the Public Health Service Act, or PHS Act, and their implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. A failure to comply explicitly with any requirements during the product development, approval, or post-approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB, of a suspension on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for the testing and marketing of our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in the European Union are addressed in a centralized way through the EMA, but country-specific regulation remains essential in many respects.
Government regulation may delay or prevent testing or marketing of our products and impose costly procedures upon our activities. The testing and marketing approval process, and the subsequent compliance with appropriate statutes and regulations, requires substantial time, effort, and financial resources, and we cannot be certain that the FDA or any other regulatory agency will grant marketing approvals for our products or any future products on a timely basis, if at all. The FDA’s or any other regulatory agency’s policies may change and additional governmental regulations may be enacted that could prevent or delay regulatory approval of our products or any future products or approval of new indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative, judicial, or administrative action, either in the United States or abroad.
Recent Developments in Regulation of Gene Therapy
Government Regulation in the United States
The FDA has provided guidance for the development of gene therapy products. For example, the FDA has established the Office of Tissues and Advanced Therapies (formerly Office of Cellular, Tissue and Gene Therapies) within the Center for Biologics Evaluation and Research (CBER), to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its reviews. In addition, the FDA has issued a growing body of clinical guidelines, chemical, manufacturing and control, or CMC, guidelines, regenerative medicine guidelines and other guidelines, all of which are intended to facilitate industry’s development of gene therapy products.
In 2016, Section 3033 of the 21st Century Cures Act created a new product category called “regenerative medicine advanced therapy”, or the RMAT designation. The RMAT designation gives the sponsor of a new investigational biologic access to increased meeting opportunities with the FDA, in a manner comparable to those offered to sponsors of therapies designated as “breakthrough therapies” by the FDA. Because the designated products meet the criteria for unmet medical need in the treatment of a serious condition, they may be eligible for priority review, in which the initial assessment of the BLA is reduced from 12 months to eight months, and accelerated approval, which bases approval on an effect on a predictive surrogate endpoint or an intermediate clinical endpoint. RMATs qualifying for such accelerated approval may be able to satisfy licensing requirements through commitment to post-approval clinical studies as well as real-world data such as patient registries and health record analysis. The eligibility of the RMAT-designated product for these expedited
47