Exhibit 99.2

Delivering the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution. ACTengine® IMA203 TCR - T Monotherapy Targeting PRAME – Phase 1b Cohort A Interim Data Update Martin Wermke , Professor at the University Hospital Dresden and Coordinating Investigator of the ACTengine® IMA203 TCR - T trial Cedrik Britten , Chief Medical Officer, Immatics Harpreet Singh , Chief Executive Officer, Immatics May 02, 2023

Forward - Looking Statement This presentation (“Presentation”) is provided by Immatics N . V . (“Immatics” or the “Company”) for informational purposes only . The information contained herein does not purport to be all - inclusive and none of Immatics, any of its affiliates, any of its or their respective control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation . Forward - Looking Statements . Certain statements in this presentation may be considered forward - looking statements . Forward - looking statements generally relate to future events or the Company’s future financial or operating performance . For example, statements concerning timing of data read - outs for product candidates, the timing of IND or CTA filing for pre - clinical stage product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward - looking statements . In some cases, you can identify forward - looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology . Such forward - looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements . These forward - looking statements are based upon estimates and assumptions that, while considered reasonable, Immatics and its management, are inherently uncertain . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual report on Form 20 - F and other filings with the Securities and Exchange Commission (SEC) . Nothing in this presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will be achieved . You should not place undue reliance on forward - looking statements, which speak only as of the date they are made . The Company undertakes no duty to update these forward - looking statements . No Offer or Solicitation . This communication is for informational purposes only and does not constitute, or form a part of, an offer to sell or the solicitation of an offer to sell or an offer to buy or the solicitation of an offer to buy any securities, and there shall be no sale of securities, in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933 , as amended, or in an offering exempt from registration . Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source . All the scientific and clinical data presented within this presentation are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification . 2

Update on IMA203 TCR - T Monotherapy – Phase 1b Cohort A Delivering a Meaningful Benefit to Patients with an Unmet Need 3 Martin Wermke, MD Professor at the University Hospital Dresden, Coordinating Investigator of the ACTengine® IMA203 TCR - T trial Cedrik M. Britten, MD Chief Medical Officer Immatics Harpreet Singh, PhD Chief Executive Officer Immatics

The Multi - Cancer Opportunity of PRAME One of the Most Promising Solid Tumor Targets for TCR - based Therapies Known To Date 4 High prevalence High target density Homogeneous expression “Clean” expression profile Clinical proof - of - concept s q N S C L C Ovarian Cancer PRAME fulfills all properties of an ideal target for TCR - based therapies TUMOR CELL T CELL H LA - A * 02 : 01 PRAME TCR PRAME Pep ೦ de PRAME RNA detection in tumor samples (ISH) ISH: in situ hybridization, sqNSCLC: squamous non - small cell lung cancer
Unlocking Novel Treatments for Patients with Solid Cancers Key Pillars of Developing a Successful TCR - T Product Candidate 5 S a f e ty Anti - Tumor Activity Durability Product Quality Broad Reach
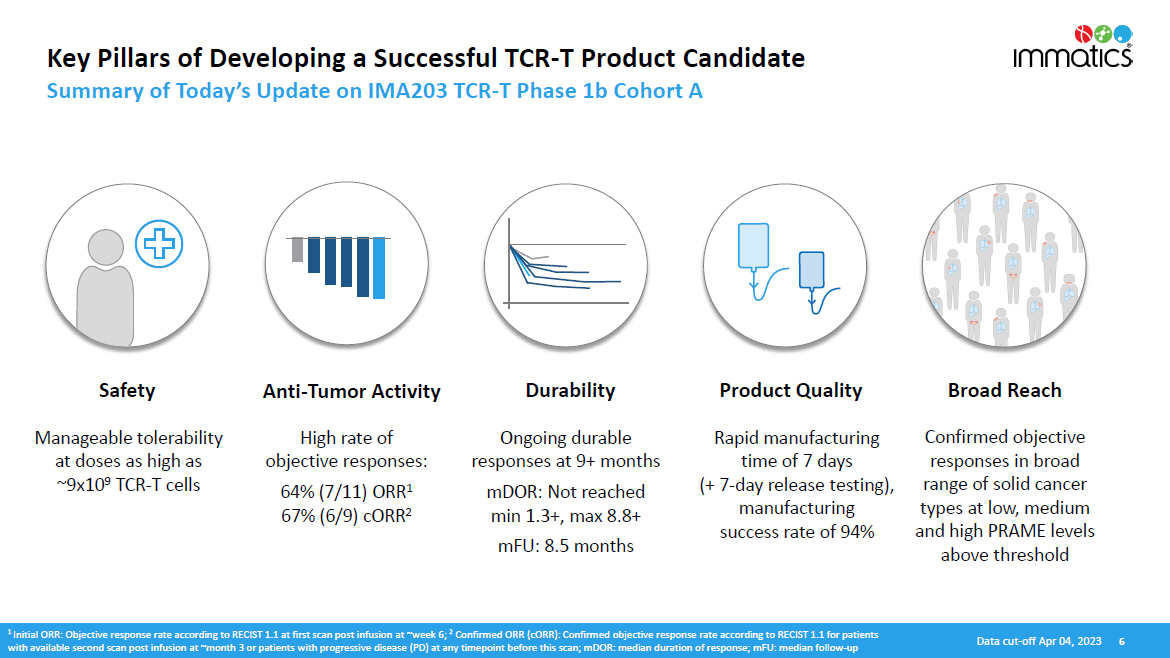
Key Pillars of Developing a Successful TCR - T Product Candidate Summary of Today’s Update on IMA203 TCR - T Phase 1b Cohort A S a f e ty Anti - Tumor Activity Durability Product Quality Broad Reach Manageable tolerability at doses as high as ~9x10 9 TCR - T cells High rate of objective responses: 64% (7/11) ORR 1 67% (6/9) cORR 2 Ongoing durable responses at 9+ months mDOR: Not reached min 1.3+, max 8.8+ mFU: 8.5 months Rapid manufacturing time of 7 days (+ 7 - day release testing), manufacturing success rate of 94% Confirmed objective responses in broad range of solid cancer types at low, medium and high PRAME levels above threshold 1 Initial ORR: Objective response rate according to RECIST 1.1 at first scan post infusion at ~week 6; 2 Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with available second scan post infusion at ~month 3 or patients with progressive disease (PD) at any timepoint before this scan; mDOR: median duration of response; mFU: median follow - up D ata c u t - o f f A p r 04 , 202 3 6
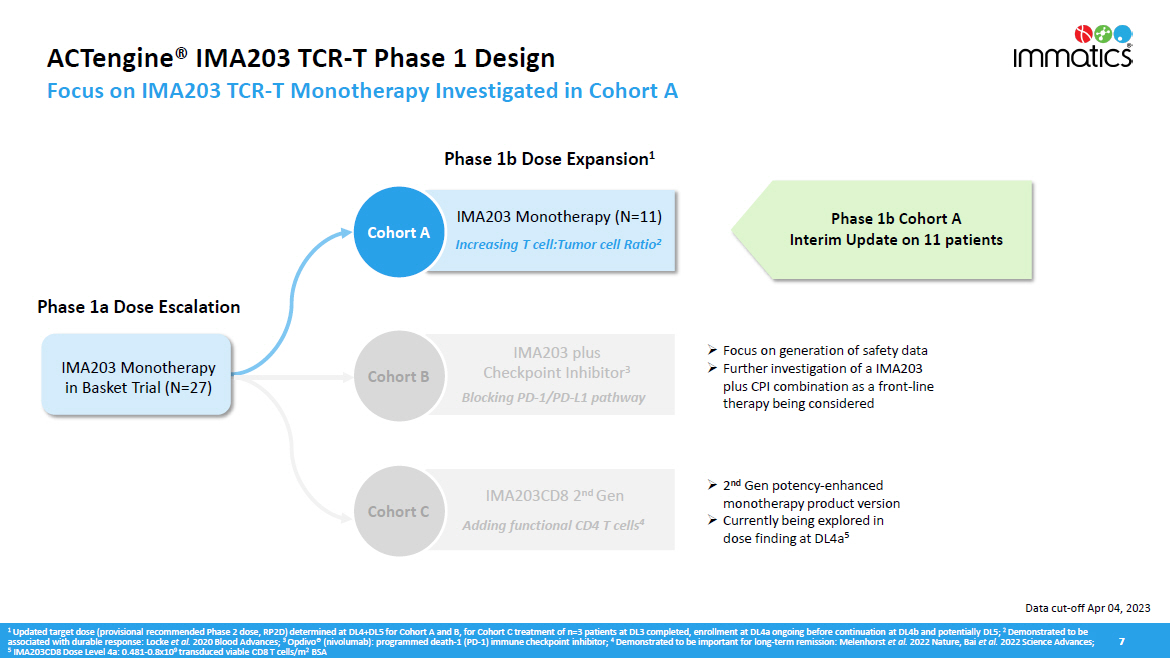
ACTengine® IMA203 TCR - T Phase 1 Design Focus on IMA203 TCR - T Monotherapy Investigated in Cohort A 7 Cohort C Phase 1b Cohort A Interim Update on 11 patients 1 Updated target dose (provisional recommended Phase 2 dose, RP2D) determined at DL4+DL5 for Cohort A and B, for Cohort C treatment of n=3 patients at DL3 completed, enrollment at DL4a ongoing before continuation at DL4b and potentially DL5; 2 Demonstrated to be associated with durable response: Locke et al. 2020 Blood Advances; 3 Opdivo® (nivolumab): programmed death - 1 (PD - 1) immune checkpoint inhibitor; 4 Demonstrated to be important for long - term remission: Melenhorst et al. 2022 Nature, Bai et al. 2022 Science Advances; 5 IMA203CD8 Dose Level 4a: 0.481 - 0.8x10 9 transduced viable CD8 T cells/m 2 BSA » Focus on generation of safety data » Further investigation of a IMA203 plus CPI combination as a front - line therapy being considered Adding functional CD4 T cells 4 nd I M A 203C D8 2 G en Phase 1b Dose Expansion 1 Phase 1a Dose Escalation Cohort A IMA203 Monotherapy (N=11) Increasing T cell:Tumor cell Ratio 2 IMA203 Monotherapy in Basket Trial (N=27) » 2 nd Gen potency - enhanced monotherapy product version » Currently being explored in dose finding at DL4a 5 Cohort B IMA203 plus Checkpoint Inhibitor 3 Blocking PD - 1/PD - L1 pathway Data cut - off Apr 04, 2023
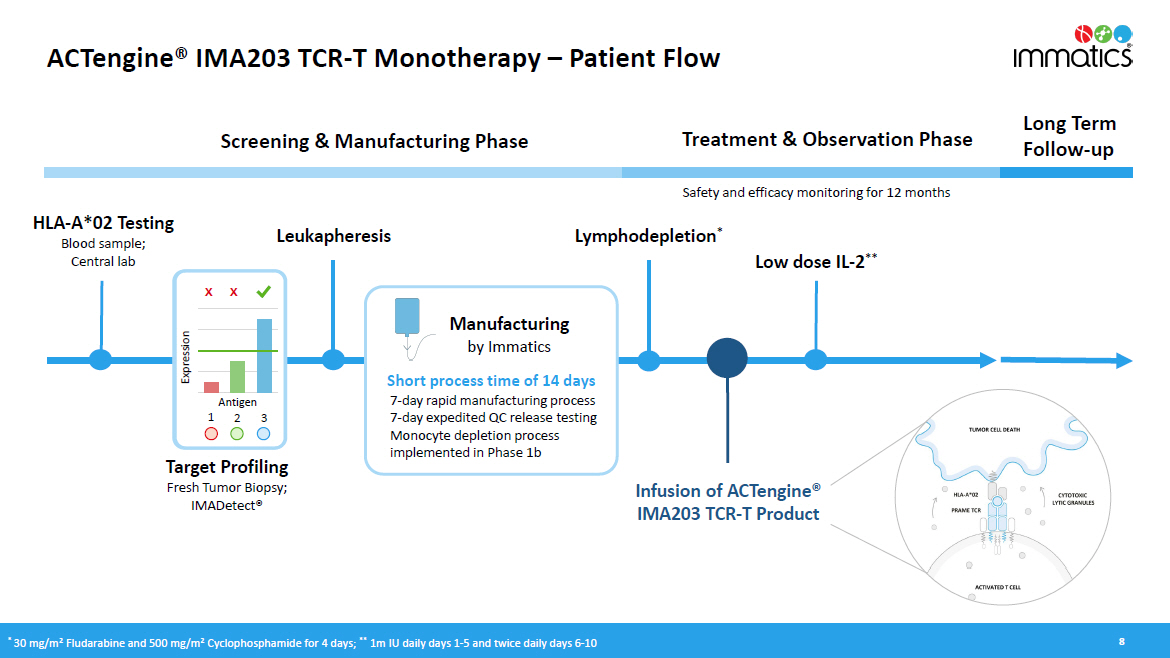
ACTengine® IMA203 TCR - T Monotherapy – Patient Flow 8 HLA - A*02 Testing Blood sample; Central lab Treatment & Observation Phase Long Term Follow - up Screening & Manufacturing Phase Manufacturing by Immatics Infusion of ACTengine® IMA203 TCR - T Product Lymphodepletion * Target Profiling Fresh Tumor Biopsy; IMADetect® Low dose IL - 2 ** Safety and efficacy monitoring for 12 months Leukapheresis x x E xpr e ss i on Antigen 1 2 3 * 30 mg/m 2 Fludarabine and 500 mg/m 2 Cyclophosphamide for 4 days; ** 1m IU daily days 1 - 5 and twice daily days 6 - 10 Short process time of 14 days 7 - day rapid manufacturing process 7 - day expedited QC release testing Monocyte depletion process implemented in Phase 1b
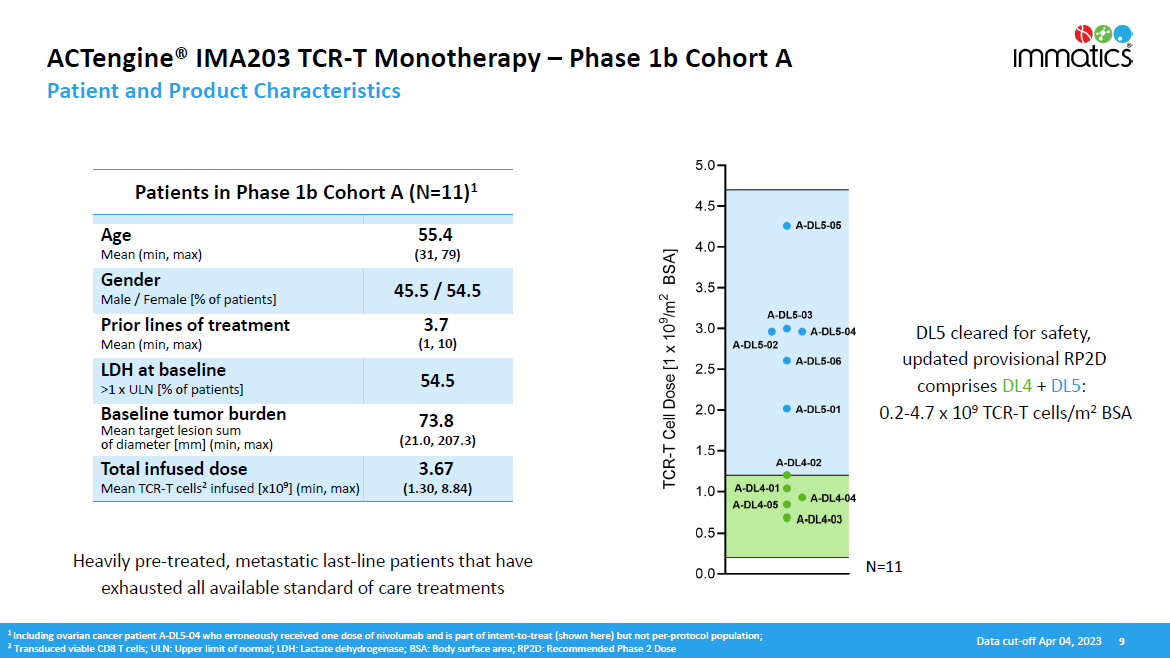
N =11 ACTengine® IMA203 TCR - T Monotherapy – Phase 1b Cohort A Patient and Product Characteristics D ata c u t - o f f A p r 04 , 202 3 9 Heavily pre - treated, metastatic last - line patients that have exhausted all available standard of care treatments 1 Including ovarian cancer patient A - DL5 - 04 who erroneously received one dose of nivolumab and is part of intent - to - treat (shown here) but not per - protocol population; 2 Transduced viable CD8 T cells; ULN: Upper limit of normal; LDH: Lactate dehydrogenase; BSA: Body surface area; RP2D: Recommended Phase 2 Dose DL5 cleared for safety, updated provisional RP2D comprises DL4 + DL5 : 0.2 - 4.7 x 10 9 TCR - T cells/m 2 BSA Patients in Phase 1b Cohort A (N=11) 1 Age Mean (min, max) 55.4 (31, 79) Gender Male / Female [% of patients] 45.5 / 54.5 Prior lines of treatment Mean (min, max) 3.7 (1, 10) LDH at baseline >1 x ULN [% of patients] 54.5 Baseline tumor burden Mean target lesion sum of diameter [mm] (min, max) 73.8 (21.0, 207.3) Total infused dose Mean TCR - T cells 2 infused [x10 9 ] (min, max) 3.67 (1.30, 8.84)

Most Frequent Adverse Events – Phase 1b Cohort A (N=11) Manageable Treatment - emergent Adverse Events (TEAEs) CRS and ICANS graded by CARTOX criteria (Neelapu et al ., 2018); 1 ICANS: Immune Effector Cell - Associated Neurotoxicity Syndrome • Expected cytopenia (Grade 1 - 4) associated with lymphodepletion in all patients • Low - moderate cytokine release syndrome (CRS) in 91% (10/11) of patients • 45% (5/11) of patients had Grade 1 CRS (3 in DL4, 2 in DL5) • 45% (5/11) of patients had Grade 2 CRS (2 in DL4, 3 in DL5) • No dose - dependent increase of CRS • No ICANS 1 • No Dose - limiting toxicity • For IMA203 TCR - T monotherapy tolerability profile including Phase 1a dose escalation, see appendix IMA203 TCR - T monotherapy shows managable tolerability at total doses as high as ~9x10 9 TCR - T cells Data cut - off Apr 04, 2023 10

Best Overall Response – Phase 1b Cohort A Deep Objective Responses Independent of Tumor Type 1 Ovarian cancer patient A - DL5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population; 2 Initial ORR: Objective response rate according to RECIST 1.1 at first scan post infusion at ~week 6; 3 Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with available second scan post infusion at ~month 3 or patients with progressive disease (PD) at any timepoint before this scan; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; BL: Baseline; BOR: Best Overall Response; NET: Neuroendocrine Tumor; CPI: Checkpoint Inhibitor ORR (at ~week 6) 2 64% (7/11) cORR (at ~month 3) 3 67% (6/9) Deep objective responses observed across multiple, heavily pre - treated tumor types • Responses observed in cutaneous and uveal melanoma, synovial sarcoma, head and neck cancer, and ovarian cancer • Initial responses at week 6 were confirmed in all 6 responders with available subsequent 3 - month scan • All cut. melanoma patients were CPI - refractory • All ovarian cancer patients were platinum - resistant Data cut - off Apr 04, 2023 11 1
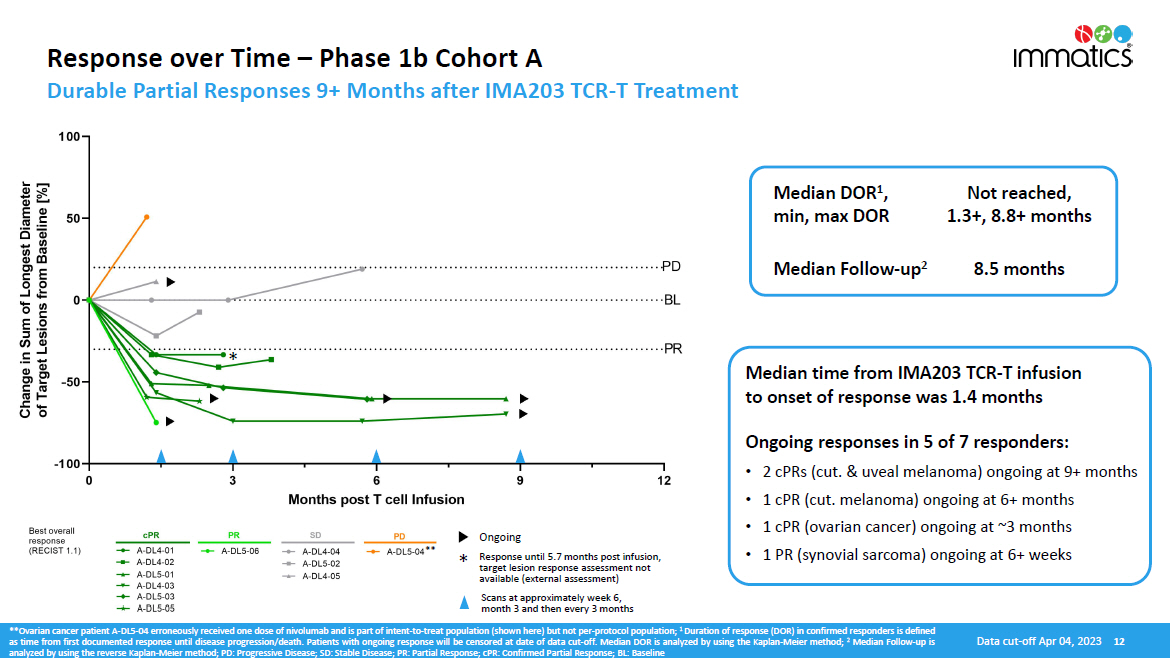
Response over Time – Phase 1b Cohort A Durable Partial Responses 9+ Months after IMA203 TCR - T Treatment **Ovarian cancer patient A - DL 5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population ; 1 Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death . Patients with ongoing response will be censored at date of data cut - off . Median DOR is analyzed by using the Kaplan - Meier method ; 2 Median Follow - up is analyzed by using the reverse Kaplan - Meier method ; PD : Progressive Disease ; SD : Stable Disease ; PR : Partial Response ; cPR : Confirmed Partial Response ; BL : Baseline Median time from IMA203 TCR - T infusion to onset of response was 1.4 months Ongoing responses in 5 of 7 responders: • 2 cPRs (cut. & uveal melanoma) ongoing at 9+ months • 1 cPR (cut. melanoma) ongoing at 6+ months • 1 cPR (ovarian cancer) ongoing at ~3 months • 1 PR (synovial sarcoma) ongoing at 6+ weeks Median DOR 1 , min, max DOR Not reached, 1.3+, 8.8+ months Median Follow - up 2 8.5 months * ** * Ongoing Response until 5.7 months post infusion, target lesion response assessment not available (external assessment) Scans at approximately week 6, month 3 and then every 3 months Data cut - off Apr 04, 2023 12
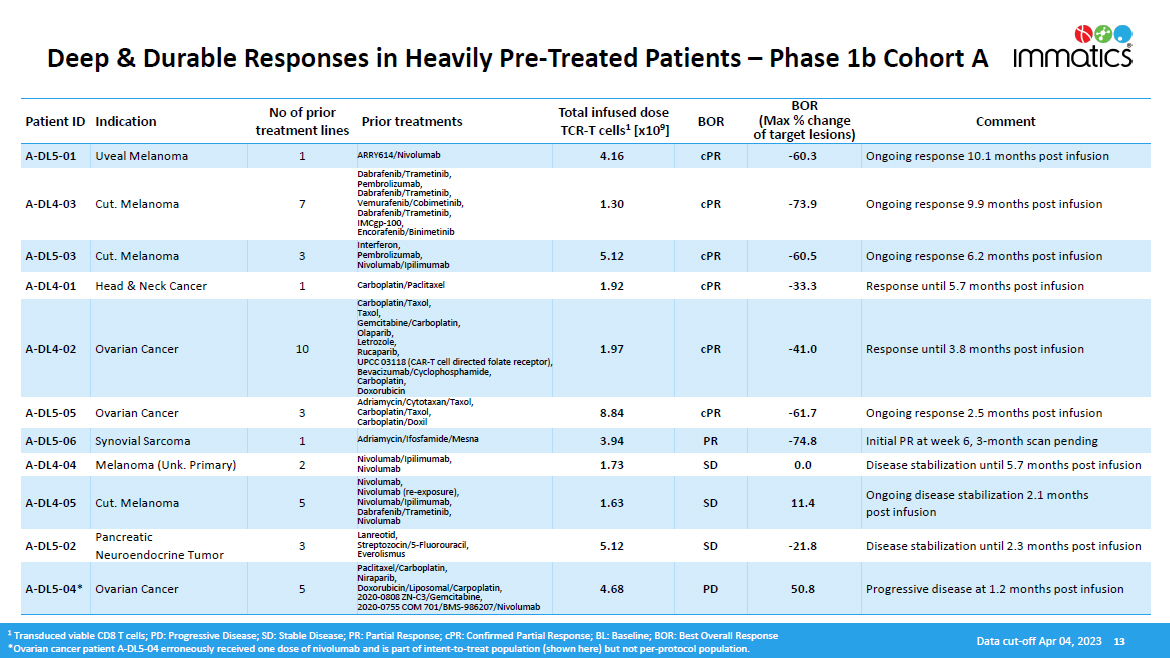
Deep & Durable Responses in Heavily Pre - Treated Patients – Phase 1b Cohort A Patient ID Indication No of prior treatment lines Prior treatments Total infused dose TCR - T cells 1 [x10 9 ] BOR BOR (Max % change of target lesions) Comment A - DL5 - 01 Uveal Melanoma 1 ARRY614/Nivolumab 4.16 cPR - 60.3 Ongoing response 10.1 months post infusion A - DL4 - 03 Cut. Melanoma 7 Dabrafenib/Trametinib, Pembrolizumab, Dabrafenib/Trametinib, Vemurafenib/Cobimetinib, Dabrafenib/Trametinib, IMCgp - 100, Encorafenib/Binimetinib 1.30 cPR - 73.9 Ongoing response 9.9 months post infusion A - DL5 - 03 Cut. Melanoma 3 Interferon, Pembrolizumab, Nivolumab/Ipilimumab 5.12 cPR - 60.5 Ongoing response 6.2 months post infusion A - DL4 - 01 Head & Neck Cancer 1 Carboplatin/Paclitaxel 1.92 cPR - 33.3 Response until 5.7 months post infusion A - DL4 - 02 Ovarian Cancer 10 Carboplatin/Taxol, Taxol, Ge m ci t abine /C arbopla t in , Olaparib, Letrozole, Rucaparib, UPCC 03118 (CAR - T cell directed folate receptor), Bevacizumab/Cyclophosphamide, Carboplatin, Doxorubicin 1.97 cPR - 41.0 Response until 3.8 months post infusion A - DL5 - 05 Ovarian Cancer 3 Adriamycin/Cytotaxan/Taxol, Carboplatin/Taxol, Carboplatin/Doxil 8.84 cPR - 61.7 Ongoing response 2.5 months post infusion A - DL5 - 06 Synovial Sarcoma 1 Adriamycin/Ifosfamide/Mesna 3.94 PR - 74.8 Initial PR at week 6, 3 - month scan pending A - DL4 - 04 Melanoma (Unk. Primary) 2 Nivolumab/Ipilimumab, Nivolumab 1.73 SD 0.0 Disease stabilization until 5.7 months post infusion A - DL4 - 05 Cut. Melanoma 5 Nivolumab, Nivolumab (re - exposure), Nivolumab/Ipilimumab, Dabrafenib/Trametinib, Nivolumab 1.63 SD 11.4 Ongoing disease stabilization 2.1 months post infusion A - DL5 - 02 Pancreatic Neuroendocrine Tumor 3 Lanreotid, Streptozocin/5 - Fluorouracil, Everolismus 5.12 SD - 21.8 Disease stabilization until 2.3 months post infusion A - DL5 - 04* Ovarian Cancer 5 P acli t axel /C arbopla t in , Niraparib, Doxorubicin/Liposomal/Carpoplatin, 2020 - 0808 ZN - C3/Gemcitabine, 2020 - 0755 COM 701/BMS - 986207/Nivolumab 4.68 PD 50.8 Progressive disease at 1.2 months post infusion 1 Transduced viable CD8 T cells; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; BL: Baseline; BOR: Best Overall Response *Ovarian cancer patient A - DL5 - 04 erroneously received one dose of nivolumab and is part of intent - to - treat population (shown here) but not per - protocol population. Data cut - off Apr 04, 2023 13
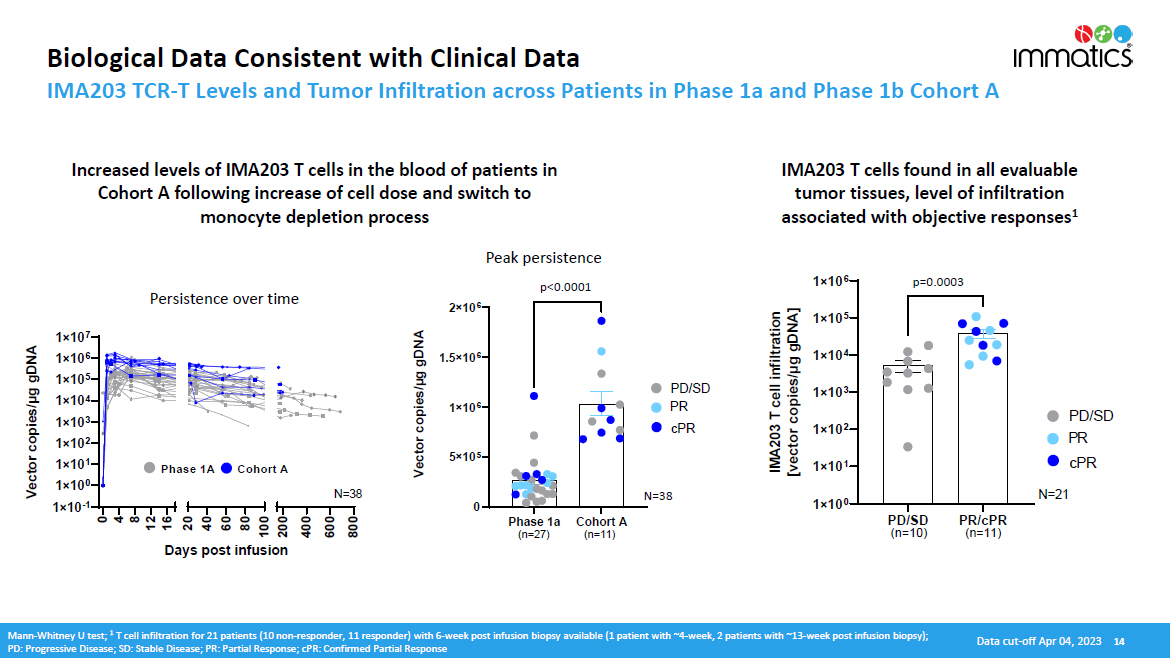
Biological Data Consistent with Clinical Data IMA203 TCR - T Levels and Tumor Infiltration across Patients in Phase 1a and Phase 1b Cohort A IMA203 T cells found in all evaluable tumor tissues, level of infiltration associated with objective responses 1 Increased levels of IMA203 T cells in the blood of patients in Cohort A following increase of cell dose and switch to monocyte depletion process Data cut - off Apr 04, 2023 14 Mann - Whitney U test; 1 T cell infiltration for 21 patients (10 non - responder, 11 responder) with 6 - week post infusion biopsy available (1 patient with ~4 - week, 2 patients with ~13 - week post infusion biopsy); PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response 1 î 10 0 1 î 10 1 1 î 10 2 1 î 10 3 1 î 10 4 1 î 10 5 1 î 10 6 IMA203 T cell infiltration [vector copies/µg gDNA] PD / S D (n=10) P R / cPR (n=11) P D /SD PR cPR 0. 0003 N = 2 1 p =0 . 0003 0 5 î 10 5 1 î 10 6 1.5 î 10 6 2 î 10 6 ) x a m C ( n o si n DNA] a exp g g k μ ea es / P i l p el co c [ T 3 20 MA I PD / SD PR cPR <0. 0 0 01 Cohort A (n=11) Phase 1a (n=27) Vector copies/µg gDNA p <0 . 0001 1 î 10 7 1 î 10 6 1 î 10 5 1 î 10 4 1 î 10 3 1 î 10 2 1 î 10 1 1 î 10 0 1 î 10 - 1 0 4 8 12 16 20 40 60 80 100 200 400 600 800 Days post infusion Vector copies/μg gDNA N = 3 8 Phase 1A Cohort A Persistence over time Peak persistence N =38
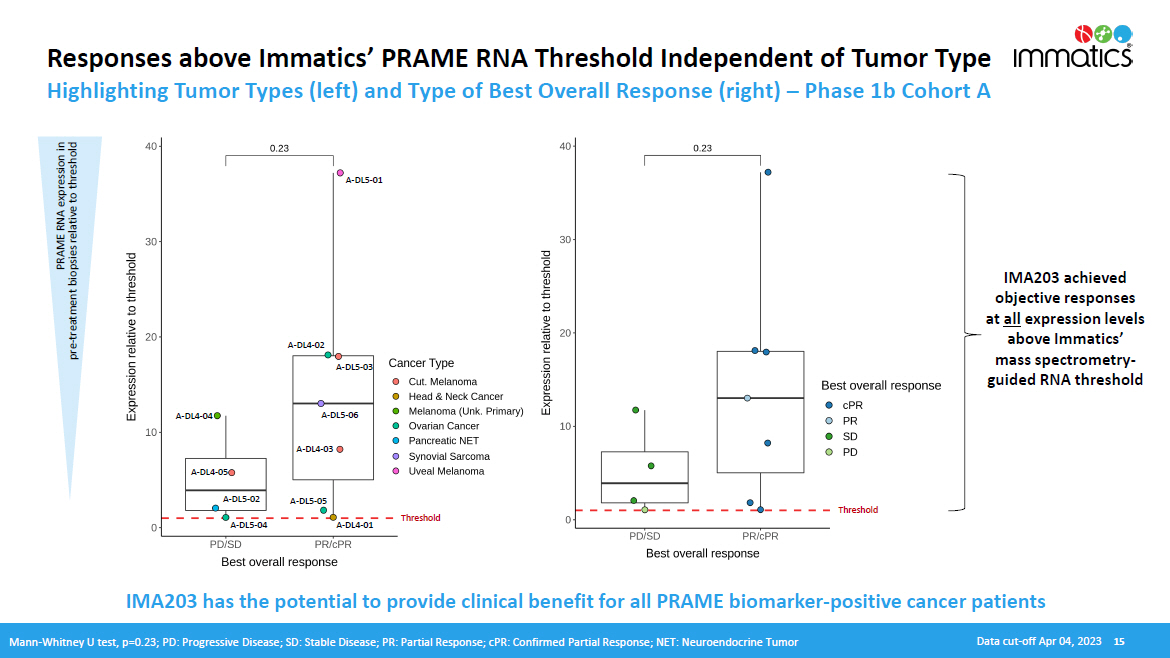
Responses above Immatics’ PRAME RNA Threshold Independent of Tumor Type Highlighting Tumor Types (left) and Type of Best Overall Response (right) – Phase 1b Cohort A PRAME RNA expression in pre - treatment biopsies relative to threshold IMA203 achieved objective responses at all expression levels above Immatics’ mass spectrometry - guided RNA threshold A - DL 5 - 01 A - DL 4 - 04 A - DL 4 - 05 A - DL 5 - 02 A - DL 5 - 04 A - DL 4 - 03 A - DL 5 - 05 A - DL 4 - 01 A - DL 5 - 03 A - DL 4 - 02 A - DL 5 - 06 IMA203 has the potential to provide clinical benefit for all PRAME biomarker - positive cancer patients Mann - Whitney U test, p=0.23; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; NET: Neuroendocrine Tumor Data cut - off Apr 04, 2023 15 T hreshold T hreshold

Potential of IMA203 in Additional Solid Cancer Indications Based on PRAME Expression in IMA203 TCR - T Responders – Phase 1b Cohort A Immatics’ proprietary mass spectrometry - guided mRNA threshold 100% 100% 95% 100% 50% (90% 2 ) 80% 60% 65% 25% % PRAME - positive patients 1 PRAME target expression distribution (blue histogram) based on TCGA RNAseq data, patient data (black dots) based on IMADetect® qPCR testing of screening biopsies; 1 PRAME target prevalence is based on TCGA RNAseq data combined with a proprietary MS - guided RNA expression threshold; 2 PRAME target prevalence in uveal melanoma based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=21) demonstrates substantial higher prevalence of 90% compared to prevalence based on TCGA data of 50%, TCGA: early & late - stage primary tumor samples, Immatics clinical trials: late - stage/metastatic tumor samples, Role of PRAME in metastasis of uveal melanoma: Field et al. 2016 Clinical Cancer Research; MS: mass spectrometry PRAME mRNA expression in Phase 1b Cohort A responders Data cut - off Apr 04, 2023 16 Selected indications A - DL4 - 03 A - DL5 - 03 A - DL5 - 06 A - DL5 - 01 A - DL4 - 02 A - DL5 - 05 A - DL4 - 01
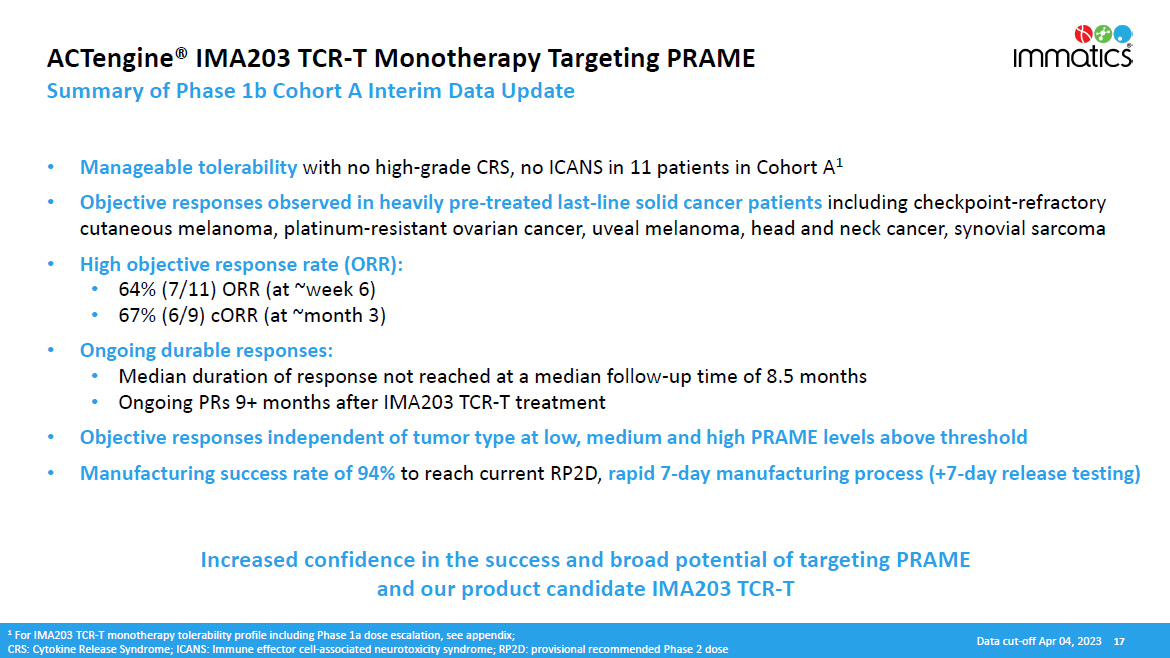
ACTengine® IMA203 TCR - T Monotherapy Targeting PRAME Summary of Phase 1b Cohort A Interim Data Update • Manageable tolerability with no high - grade CRS, no ICANS in 11 patients in Cohort A 1 • Objective responses observed in heavily pre - treated last - line solid cancer patients including checkpoint - refractory cutaneous melanoma, platinum - resistant ovarian cancer, uveal melanoma, head and neck cancer, synovial sarcoma • High objective response rate (ORR): • 64% (7/11) ORR (at ~week 6) • 67% (6/9) cORR (at ~month 3) • Ongoing durable responses: • Median duration of response not reached at a median follow - up time of 8.5 months • Ongoing PRs 9+ months after IMA203 TCR - T treatment • Objective responses independent of tumor type at low, medium and high PRAME levels above threshold • Manufacturing success rate of 94% to reach current RP2D, rapid 7 - day manufacturing process (+7 - day release testing) Increased confidence in the success and broad potential of targeting PRAME and our product candidate IMA203 TCR - T 1 For IMA203 TCR - T monotherapy tolerability profile including Phase 1a dose escalation, see appendix; CRS: Cytokine Release Syndrome; ICANS: Immune effector cell - associated neurotoxicity syndrome; RP2D: provisional recommended Phase 2 dose Data cut - off Apr 04, 2023 17

Immatics’ ACTengine® IMA203 TCR - T Development Strategy Two Pillared Strategy 18 Objective: Deliver best - in - class therapy in 1 - 2 last - line solid cancer types as fast as possible • Focus on indications with PRAME prevalence above 80% with available clinical PoC, such as cut. melanoma (potentially bundled with uveal melanoma) and ovarian cancer • Highly modular and scalable manufacturing facility expected to be operational in 2024 to support efforts to maximize speed to market • Planned start of a first Phase 2 trial in 1H 2024 – targeted to be already registration - directed Objective: Expand development to other cancer types • Signal finding in other cancer types with a broad patient reach, such as uterine cancer, lung cancer, breast cancer, head and neck cancer FAST & F O C US E D GO BROAD Update on all three IMA203 Phase 1b cohorts and clinical development path towards registration - directed trials and potential commercialization for PRAME TCR - T monotherapy is planned for 4Q 2023
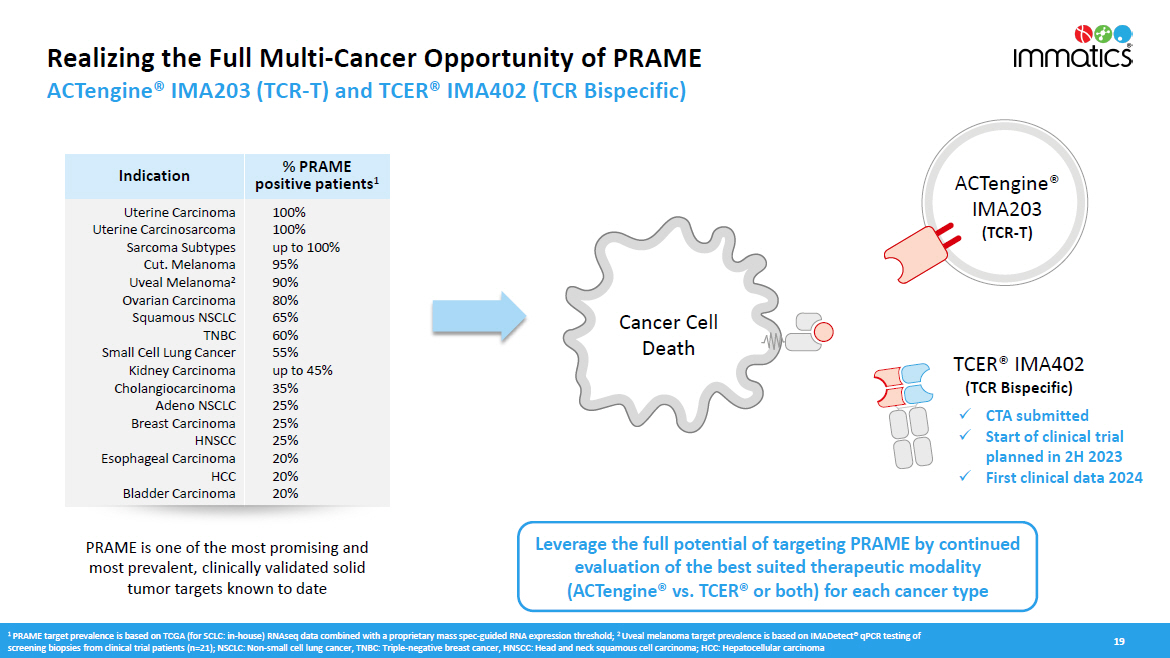
Realizing the Full Multi - Cancer Opportunity of PRAME ACTengine® IMA203 (TCR - T) and TCER® IMA402 (TCR Bispecific) 19 Indication % PRAME positive patients 1 Uterine Carcinoma Uterine Carcinosarcoma Sarcoma Subtypes Cut. Melanoma Uveal Melanoma 2 Ovarian Carcinoma Squamous NSCLC T N BC Small Cell Lung Cancer Kidney Carcinoma C h ola n g io c a r c i n oma Adeno NSCLC Breast Carcinoma H NS CC Esophageal Carcinoma HCC Bladder Carcinoma 100% 100% up to 100% 95% 90% 80% 65% 60% 55% up to 45% 35% 25% 25% 25% 20% 20% 20% TCER® IMA402 (TCR Bispecific) A C T eng i ne® IMA203 (TCR - T) Cancer Cell Death PRAME is one of the most promising and most prevalent, clinically validated solid tumor targets known to date Leverage the full potential of targeting PRAME by continued evaluation of the best suited therapeutic modality (ACTengine® vs. TCER® or both) for each cancer type x CTA submitted x Start of clinical trial planned in 2H 2023 x First clinical data 2024 1 PRAME target prevalence is based on TCGA (for SCLC: in - house) RNAseq data combined with a proprietary mass spec - guided RNA expression threshold; 2 Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=21); NSCLC: Non - small cell lung cancer, TNBC: Triple - negative breast cancer, HNSCC: Head and neck squamous cell carcinoma; HCC: Hepatocellular carcinoma
University Hospital Dresden University Hospital Bonn University Hospital Würzburg Columbia University University of Pittsburgh MD Anderson Cancer Center University Hospital Hamburg Dr. D. Araujo Dr. A. Tsimberidou Dr. A. Jazaeri Dr. W. Alsdorf Dr. T. Holderried Dr. M. Chatterjee Dr. J. Luke Ge rma n y United States Dr. M. Wermke … and the Investigators at the Clinical Sites Dr. R. Reshef 20 We are Immensely Grateful to the Patients, Their Families …

Delivering the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution. ww w .i mm a ti c s . c o m Appendix
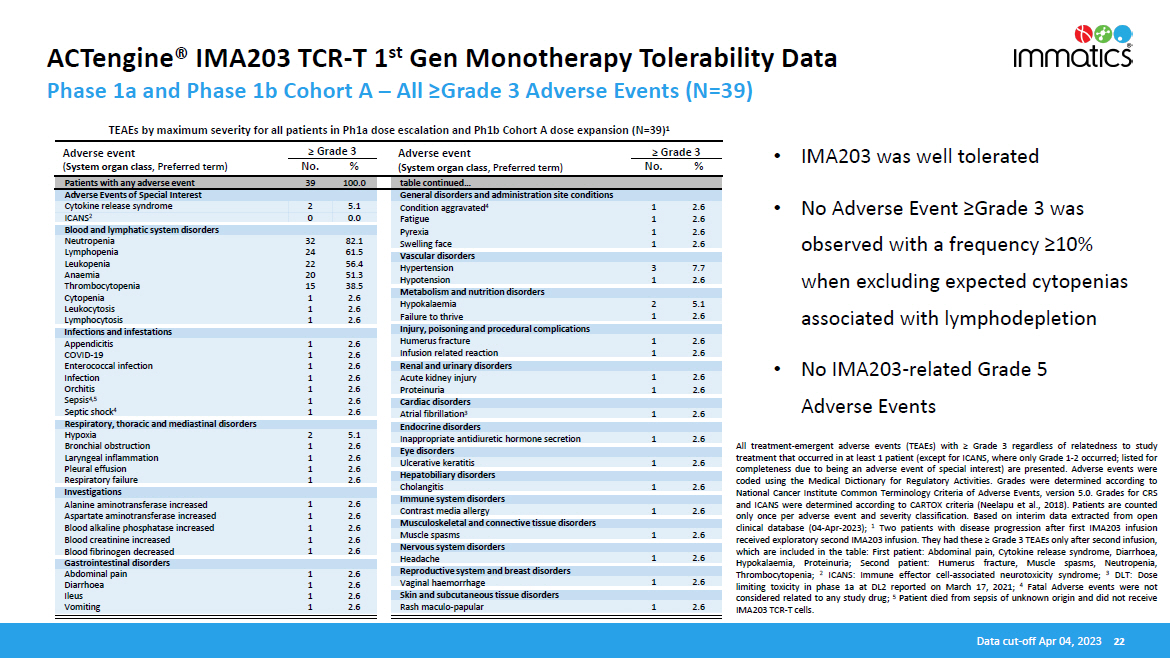
ACTengine® IMA203 TCR - T 1 st Gen Monotherapy Tolerability Data • IMA203 was well tolerated • No Adverse Event ≥Grade 3 was observed with a frequency ≥10% when excluding expected cytopenias associated with lymphodepletion • No IMA203 - related Grade 5 Adverse Events All treatment - emergent adverse events (TEAEs) with ≥ Grade 3 regardless of relatedness to study treatment that occurred in at least 1 patient (except for ICANS, where only Grade 1 - 2 occurred ; listed for completeness due to being an adverse event of special interest) are presented . Adverse events were coded using the Medical Dictionary for Regulatory Activities . Grades were determined according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 5 . 0 . Grades for CRS and ICANS were determined according to CARTOX criteria (Neelapu et al . , 2018 ) . Patients are counted only once per adverse event and severity classification . Based on interim data extracted from open clinical database ( 04 - Apr - 2023 ) ; 1 Two patients with disease progression after first IMA 203 infusion received exploratory second IMA 203 infusion . They had these ≥ Grade 3 TEAEs only after second infusion, which are included in the table : First patient : Abdominal pain, Cytokine release syndrome, Diarrhoea, Hypokalaemia, Proteinuria ; Second patient : Humerus fracture, Muscle spasms, Neutropenia, Thrombocytopenia ; 2 ICANS : Immune effector cell - associated neurotoxicity syndrome ; 3 DLT : Dose limiting toxicity in phase 1 a at DL 2 reported on March 17 , 2021 ; 4 Fatal Adverse events were not considered related to any study drug ; 5 Patient died from sepsis of unknown origin and did not receive IMA 203 TCR - T cells . Data cut - off Apr 04, 2023 22 Adverse event ( System organ class , Preferred term) No. % Adverse event ( System organ class , Preferred term) ≥ Grade 3 ≥ Grade 3 No. % Phase 1a and Phase 1b Cohort A – All ≥Grade 3 Adverse Events (N=39) TEAEs by maximum severity for all patients in Ph1a dose escalation and Ph1b Cohort A dose expansion (N=39) 1 Patients with any adverse event 39 100.0 table continued… Adverse Events of Special Interest General disorders and administration site conditions Cytokine release syndrome 2 5.1 ICANS 2 0 0.0 Condition aggravated 4 1 2.6 Fatigue 1 2.6 Pyrexia 1 2.6 Swelling face 1 2.6 Blood and lymphatic system disorders Neutropenia 32 82.1 Lymphopenia 24 61.5 Leukopenia 22 56.4 Anaemia 20 51.3 Thrombocytopenia 15 38.5 Cytopenia 1 2.6 Leukocytosis 1 2.6 Lymphocytosis 1 2.6 Vascular disorders Hypertension 3 7.7 Hypotension 1 2.6 Metabolism and nutrition disorders Hypokalaemia 2 5.1 Failure to thrive 1 2.6 Infections and infestations Injury, poisoning and procedural complications Appendicitis 1 2.6 COVID - 19 1 2.6 Enterococcal infection 1 2.6 Infection 1 2.6 Orchitis 1 2.6 Sepsis 4,5 1 2.6 Septic shock 4 1 2.6 Humerus fracture 1 2.6 Infusion related reaction 1 2.6 Renal and urinary disorders Acute kidney injury 1 2.6 Proteinuria 1 2.6 Cardiac disorders Atrial fibrillation 3 1 2.6 Respiratory, thoracic and mediastinal disorders Endocrine disorders Hypoxia 2 5.1 Bronchial obstruction 1 2.6 Laryngeal inflammation 1 2.6 Pleural effusion 1 2.6 Respiratory failure 1 2.6 Inappropriate antidiuretic hormone secretion 1 2.6 Eye disorders Ulcerative keratitis 1 2.6 Hepatobiliary disorders Investigations Cholangitis 1 2.6 Immune system disorders Alanine aminotransferase increased 1 2.6 Aspartate aminotransferase increased 1 2.6 Blood alkaline phosphatase increased 1 2.6 Blood creatinine increased 1 2.6 Blood fibrinogen decreased 1 2.6 Contrast media allergy 1 2.6 Musculoskeletal and connective tissue disorders Muscle spasms 1 2.6 Nervous system disorders Gastrointestinal disorders Headache 1 2.6 Abdominal pain 1 2.6 Diarrhoea 1 2.6 Ileus 1 2.6 Vomiting 1 2.6 Reproductive system and breast disorders Vaginal haemorrhage 1 2.6 Skin and subcutaneous tissue disorders Rash maculo - papular 1 2.6
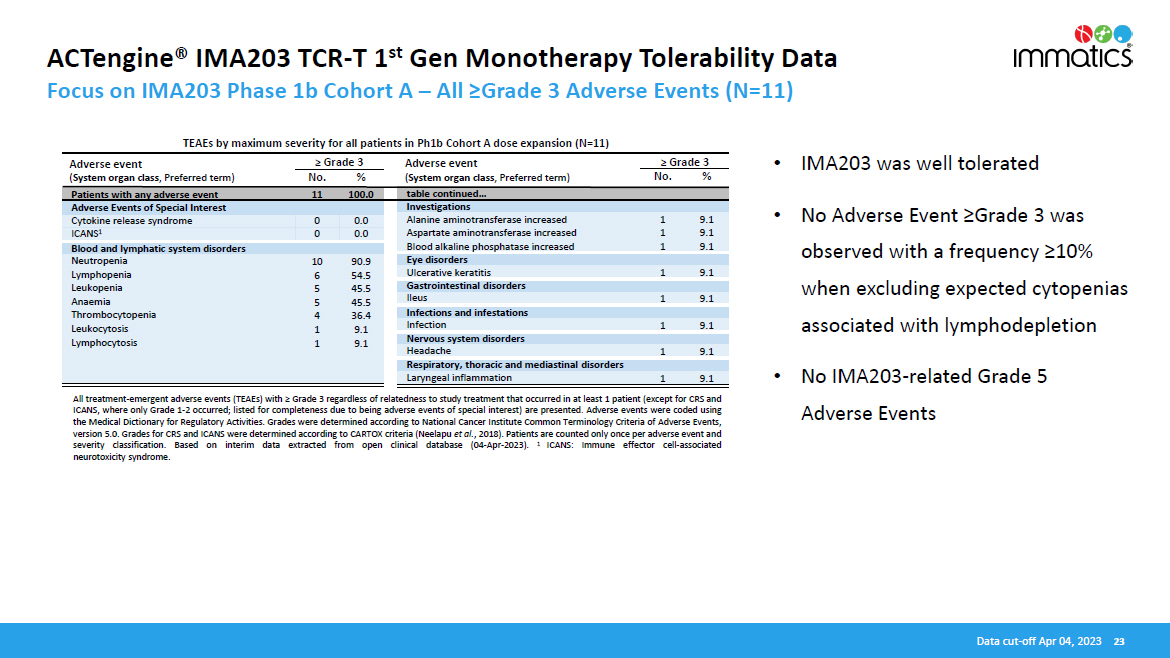
ACTengine® IMA203 TCR - T 1 st Gen Monotherapy Tolerability Data Focus on IMA203 Phase 1b Cohort A – All ≥Grade 3 Adverse Events (N=11) • IMA203 was well tolerated • No Adverse Event ≥Grade 3 was observed with a frequency ≥10% when excluding expected cytopenias associated with lymphodepletion • No IMA203 - related Grade 5 Adverse Events All treatment - emergent adverse events (TEAEs) with ≥ Grade 3 regardless of relatedness to study treatment that occurred in at least 1 patient (except for CRS and ICANS, where only Grade 1 - 2 occurred ; listed for completeness due to being adverse events of special interest) are presented . Adverse events were coded using the Medical Dictionary for Regulatory Activities . Grades were determined according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 5 . 0 . Grades for CRS and ICANS were determined according to CARTOX criteria (Neelapu et al . , 2018 ) . Patients are counted only once per adverse event and severity classification . Based on interim data extracted from open clinical database ( 04 - Apr - 2023 ) . 1 ICANS : Immune effector cell - associated neurotoxicity syndrome . Data cut - off Apr 04, 2023 23 Adverse event ( System organ class , Preferred term) ≥ Grade 3 No. % Adverse event ( System organ class , Preferred term) ≥ Grade 3 No. % TEAEs by maximum severity for all patients in Ph1b Cohort A dose expansion (N=11) Patients with any adverse event 11 100.0 table continued… Adverse Events of Special Interest Investigations Cytokine release syndrome 0 0.0 ICANS 1 0 0.0 Alanine aminotransferase increased 1 9.1 Aspartate aminotransferase increased 1 9.1 Blood alkaline phosphatase increased 1 9.1 Blood and lymphatic system disorders Neutropenia 10 90.9 Lymphopenia 6 54.5 Leukopenia 5 45.5 Anaemia 5 45.5 Thrombocytopenia 4 36.4 Leukocytosis 1 9.1 Lymphocytosis 1 9.1 Eye disorders Ulcerative keratitis 1 9.1 Gastrointestinal disorders Ileus 1 9.1 Infections and infestations Infection 1 9.1 Nervous system disorders Headache 1 9.1 Respiratory, thoracic and mediastinal disorders Laryngeal inflammation 1 9.1
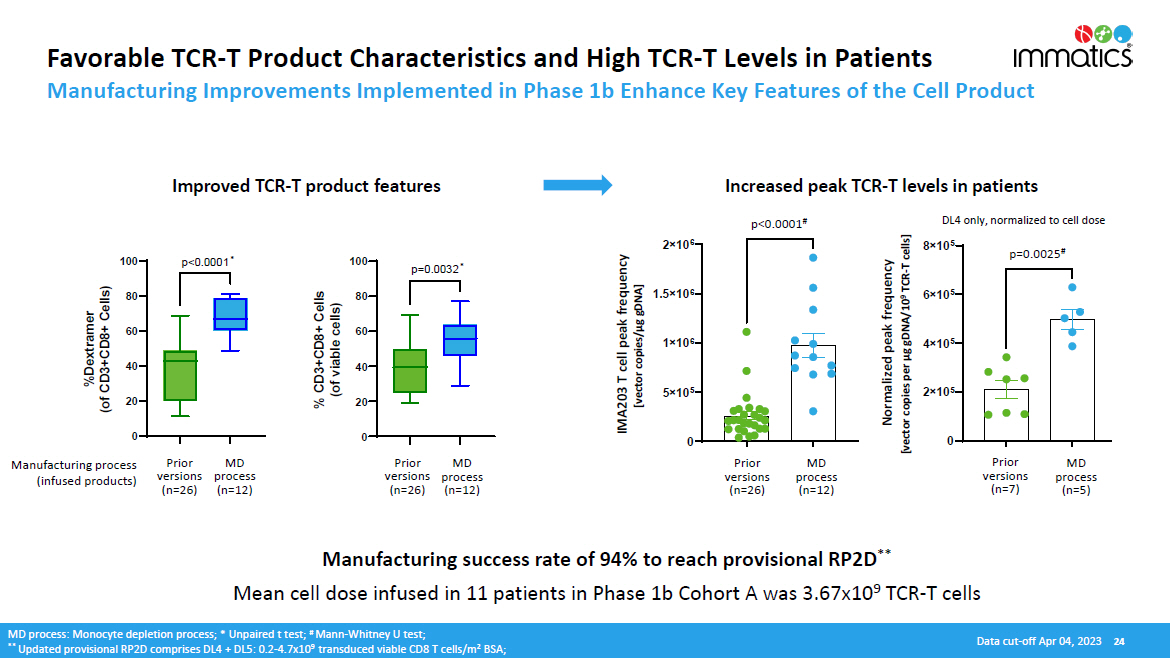
0 2 î 10 5 4 î 10 5 6 î 10 5 8 î 10 5 0. 0025 Favorable TCR - T Product Characteristics and High TCR - T Levels in Patients Manufacturing Improvements Implemented in Phase 1b Enhance Key Features of the Cell Product Manufacturing success rate of 94% to reach provisional RP2D ** Mean cell dose infused in 11 patients in Phase 1b Cohort A was 3.67x10 9 TCR - T cells Manufacturing process (infused products) MD pr o cess (n=12) Prior v e r s i o n s (n=26) Prior MD versions process (n=26) (n=12) MD process: Monocyte depletion process; * Unpaired t test; # Mann - Whitney U test; ** Updated provisional RP2D comprises DL4 + DL5: 0.2 - 4.7x10 9 transduced viable CD8 T cells/m 2 BSA; Increased peak TCR - T levels in patients Improved TCR - T product features 0 5 î 10 5 1 î 10 6 1.5 î 10 6 2 î 10 6 < 0 . 00 0 1 IMA203 T cell peak frequency [vector copies/µg gDNA] MD pr o cess (n=12) Prior v e r s i o n s (n=26) MD pr o cess (n=5) Prior v e r s i o n s (n=7) p<0.0001 # p=0.0025 # DL4 only, normalized to cell dose 0 20 40 60 80 100 % CD3+CD8+ Cells (of viable cells) 0 20 40 60 80 100 %Dextramer (of CD3+CD8+ Cells) Normalized peak frequency [vector copies per µg gDNA/10 9 TCR - T cells] Data cut - off Apr 04, 2023 24 p=0.0032 * p<0.0001 *
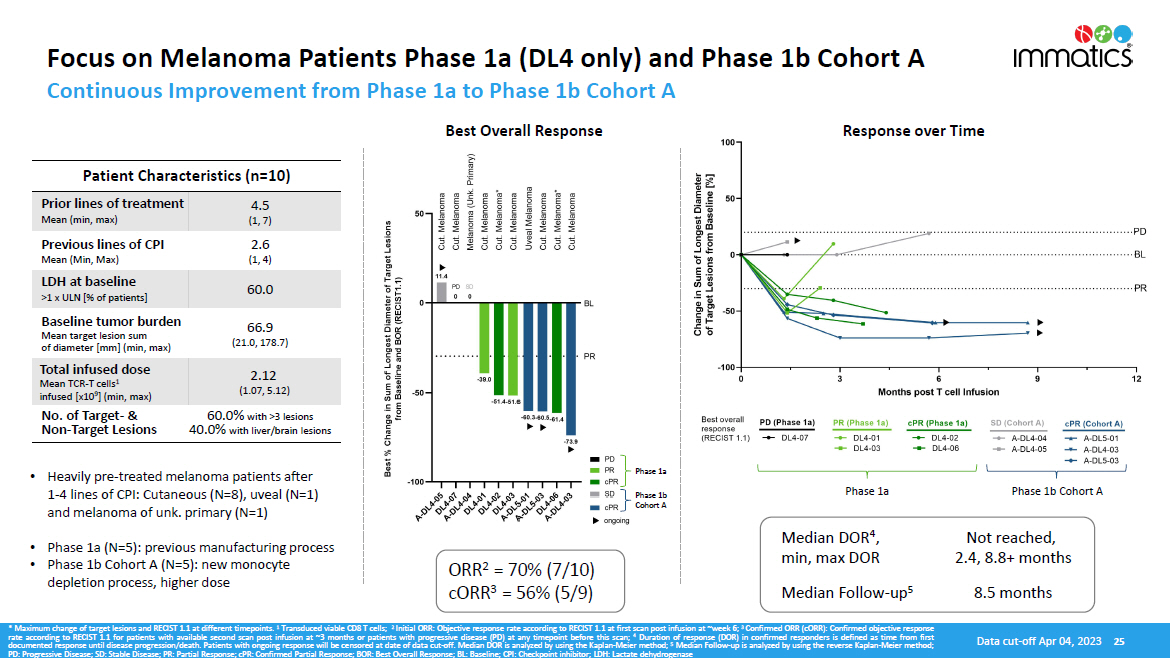
Focus on Melanoma Patients Phase 1a (DL4 only) and Phase 1b Cohort A Patient Characteristics (n=10) Prior lines of treatment Mean (min, max) 4.5 (1, 7) Previous lines of CPI Mean (Min, Max) 2.6 (1, 4) LDH at baseline >1 x ULN [% of patients] 60.0 Baseline tumor burden Mean target lesion sum of diameter [mm] (min, max) 66.9 (21.0, 178.7) Total infused dose Mean TCR - T cells 1 infused [x10 9 ] (min, max) 2.12 (1.07, 5.12) No. of Target - & Non - Target Lesions 60.0% with >3 lesions 40.0% with liver/brain lesions ORR 2 = 70% (7/10) cORR 3 = 56% (5/9) Median DOR 4 , min, max DOR Not reached, 2.4, 8.8+ months Median Follow - up 5 8.5 months * Maximum change of target lesions and RECIST 1 . 1 at different timepoints . 1 Transduced viable CD 8 T cells ; 2 Initial ORR : Objective response rate according to RECIST 1 . 1 at first scan post infusion at ~week 6 ; 3 Confirmed ORR (cORR) : Confirmed objective response rate according to RECIST 1 . 1 for patients with available second scan post infusion at ~ 3 months or patients with progressive disease (PD) at any timepoint before this scan ; 4 Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death . Patients with ongoing response will be censored at date of data cut - off . Median DOR is analyzed by using the Kaplan - Meier method ; 5 Median Follow - up is analyzed by using the reverse Kaplan - Meier method ; PD : Progressive Disease ; SD : Stable Disease ; PR : Partial Response ; cPR : Confirmed Partial Response ; BOR : Best Overall Response ; BL : Baseline ; CPI : Checkpoint inhibitor ; LDH : Lactate dehydrogenase Data cut - off Apr 04, 2023 25 • Heavily pre - treated melanoma patients after 1 - 4 lines of CPI: Cutaneous (N=8), uveal (N=1) and melanoma of unk. primary (N=1) • Phase 1a (N=5): previous manufacturing process • Phase 1b Cohort A (N=5): new monocyte depletion process, higher dose Phase 1a Phase 1b Cohort A Continuous Improvement from Phase 1a to Phase 1b Cohort A Best Overall Response Response over Time Phase 1a Phase 1b Cohort A

Delivering the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution. ww w .i mm a ti c s . c o m

























