Exhibit 99.2

TCR Bispecific Molecule TCER® IMA401 Targeting MAGEA4/8 - Phase 1 Dose Escalation Clinical Data Update September 16, 2024 Oral presentation by Martin Wermke at the European Society of Medical Oncology Congress 2024 on September 16, 2024 Data cut - off Jul 23, 2024 Delivering the Power of T cells to Cancer Patients © Immatics. Not for further reproduction or distribution.

Forward - Looking Statement This presentation (“Presentation”) is provided by Immatics N . V . (“Immatics” or the “Company”) for informational purposes only . The information contained herein does not purport to be all - inclusive and none of Immatics, any of its affiliates, any of its or their respective control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation . Forward - Looking Statements . Certain statements in this presentation may be considered forward - looking statements . Forward - looking statements generally relate to future events or the Company’s future financial or operating performance . For example, statements concerning timing of data read - outs for product candidates, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration - enabling), the timing of IND or CTA filing for pre - clinical stage product candidates, estimated market opportunities of product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward - looking statements . In some cases, you can identify forward - looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology . Such forward - looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements . These forward - looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual Report on Form 20 - F and other filings with the Securities and Exchange Commission (SEC) . Nothing in this presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will be achieved . You should not place undue reliance on forward - looking statements, which speak only as of the date they are made . The Company undertakes no duty to update these forward - looking statements . No Offer or Solicitation . This communication is for informational purposes only and does not constitute, or form a part of, an offer to sell or the solicitation of an offer to sell or an offer to buy or the solicitation of an offer to buy any securities, and there shall be no sale of securities, in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933 , as amended, or in an offering exempt from registration . Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source . All the scientific and clinical data presented within this presentation are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification . 2
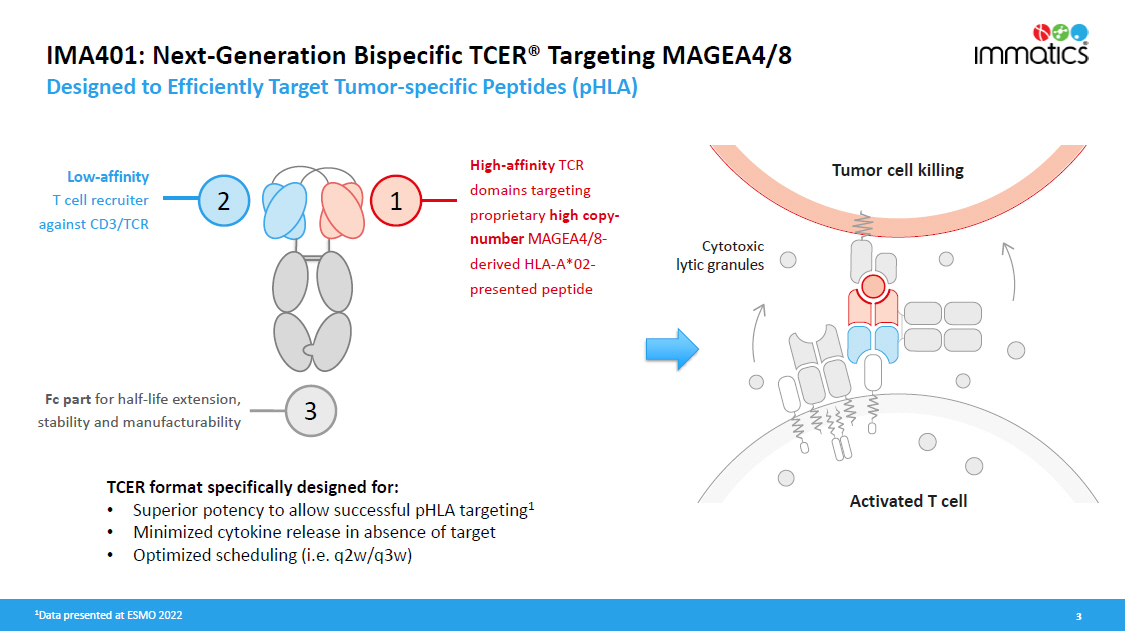
IMA401: Next - Generation Bispecific TCER® Targeting MAGEA4/8 Designed to Efficiently Target Tumor - specific Peptides (pHLA) 1 Data presented at ESMO 2022 3 High - affinity TCR domains targeting proprietary high copy - number MAGEA4/8 - derived HLA - A*02 - presented peptide L o w - a ffi n ity T cell recruiter against CD3/TCR Fc part for half - life extension, stability and manufacturability 2 1 3 C y t o t o x ic lytic granules Tumor cell killing Activated T cell TCER format specifically designed for: • Superior potency to allow successful pHLA targeting 1 • Minimized cytokine release in absence of target • Optimized scheduling (i.e. q2w/q3w)

Potency of Our Proprietary TCR Bispecific Format TCER® 4 • Seven different TCR Bispecific formats were evaluated with a pHLA targeting TCR and the identical T cell recruiting antibody • TCER® format had higher combination of potency and specificity 2 than six alternative TCR Bispecific format designs evaluated Flexible Plug - and - play platform: TCER® format successfully validated for different TCRs & different T cell recruiting antibodies T C E R ® 2+1 TCR bispecific format: High potency was linked to a significantly reduced specificity profile 2 Killing of target - positive cells by different TCR Bispecifics 1 1 Data presented at SITC 2022; 2 Preclinical data on specificity not shown

TCER® IMA401 Targeting MAGEA4/8 Higher Target Density of MAGEA4/8 Peptide 5 MAGEA4 protein detection in tumor samples (IHC) 1 MAGEA4/8 target prevalences are based on TCGA and in - house data combined with a XPRESIDENT® - determined target individual MS - based mRNA expression threshold; qPCR - threshold for patient screening; 2 Students paired T test; 3 Copy number per tumor cell (CpC) measured on a paired - sample basis by AbsQuant®, i.e. comparing MAGEA4 vs. MAGEA4/8 peptide presentation on same sample p<0.001 2 MAGEA4/8 target is presented at >5 - fold higher target density 3 than a commonly used MAGEA4 target peptide HNSCC sq. NSCLC 100 µm MAGEA4/8 target prevalence in selected cancer indications Number of addressable patients* Target prevalence 1 [%] Indications 22k 52% Squamous non - small cell lung carcinoma 7k 36% Head and neck squamous cell carcinoma 9k 29% Bladder carcinoma 4k 23% Ovarian carcinoma 3k 23% Esophageal carcinoma 4k 21% Small cell lung cancer 2k 20% Triple - negative breast cancer 3k 14% Gastric adenocarcinoma 2k 18% Cutaneous melanoma 6k 9% Non - small cell lung adenocarcinoma *1L+ Unresectable or Metastatic Addressable Patient Populations (US, UK, EU4 in 2025), total MAGE A4/A8+ and HLA - A*02+

6 180 µg 540 µg 1800 µg 2500 µg Key Eligibility Criteria Object iv es Primary: • Determine MTD and/or RP2D Secondary: • Tolerability • Pharmacokinetics • Initial anti - tumor activity • Recurrent and/or refractory solid tumors • HLA - A*02:01 positive • MAGEA4/8 - positive as confirmed by mRNA - based assay 3 • ECOG status 0 - 2 • Received or not eligible for all available indicated standard of care treatments 60 µg 1200 µg 20 µg 6.6 µg • MTD not yet determined • Dose escalation ongoing to optimize dosing intervals and schedule Total safety population (N=35) • MABEL - based starting dose • Dose escalation based on cohorts of 1 - 6 patients using adaptive design (BLRM model) • Four initial q1w step dosings 1 up to target dose, q2w after reaching target dose 2 Trial Design – IMA401 - 101 Phase 1a Dose Escalation First - in - Human Basket Trial Targeting the MAGEA4/8 Peptide in Solid Tumors 1 Step dosing with 300 µg and 600 µg introduced at DL6; Low - dose dexamethasone pre - medication used at higher dose levels as used with other approved bispecific products has been implemented as preventive measure for continued dose escalation; Patients can increase their dose to previously cleared dose levels; 2 q2w: once every two weeks, weekly (q1w) dosing was applied up to DL5; 3 IMADetect®: proprietary mRNA - based assay using Immatics’ MS - guided threshold; BLRM: Bayesian logistic regression model; MTD: Maximum tolerated dose. D L1 D L2 D L3 D L4 D L5 D L6a D L7 D L6 t bd 2000 µg D L6b Data cut - off Jul 23, 2024

Baseline Characteristics Heavily Pre - treated Patients with a Broad Range of Tumor Types 7 1 Efficacy Analysis Set (EAS) prospectively defined in the study protocol: patients who received at least four IMA401 infusions and had at least one post - baseline efficacy assessment or clinical progression. Three patients did not receive all four infusions due to clinical progression and three patients awaiting their first scans as of the data cut - off date are not included in the EAS; 2 Patients in this analysis had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold. LDH: Lactate dehydrogenase; ULN: Upper limit of normal. Data cut - off Jul 23, 2024 Patients with relevant IMA401 doses and MAGEA4/8 high levels 2 N=17 Efficacy - evaluable Population 1 N=29 Safety Population N=35 Characteristic 64 (35, 82) 63 (35, 82) 62 (19, 82) Age Median (min, max) 3 [17.6] 12 [70.6] 2 [11.8] 6 [20.7] 21 [72.4] 2 [6.9] 10 [28.6] 23 [65.7] 2 [5.7] ECOG performance status 0 - n [%] 1 - n [%] 2 - n [%] 4 (2, 8) 3 (2, 8) 4 (2, 8) Prior lines of systemic treatment Median (min, max) 41.2 58.8 0.0 55.2 41.4 3.4 51.4 40.0 8.6 LDH at baseline ≤ 1xULN [%] 1 - 2xULN [%] > 2xULN [%] 84 (18, 202.8) 80 (15, 202.8) 74 (15, 202.8) Baseline tumor burden Median target lesion sum of diameter [mm] (min, max) 3 (1, 6) 3 (1, 6) 3 (1, 6) Number of organs with metastases Median (min, max) 47.1 41.4 40.0 Liver/ Brain Lesions [% of patients]

8 IMA401 Demonstrates Manageable Tolerability in N=35 Patients Most Frequent Related AEs were Lymphopenia, CRS and Neutropenia ≥ Grade 3 All Grades TEAEs, n [%] 26 [74] 32 [91] Any 19 [54] 28 [80] Treatment - related ≥ Grade 3 All Grades Treatment - related AEs 1 , n [%] 11 [31] 12 [34] Lymphopenia 0 11 [31] Cytokine release syndrome 5 [14] 8 [23] Neutropenia 2 [6] 6 [17] Facial pain 4 [11] 5 [14] Anaemia 2 [6] 5 [14] Thrombocytopenia 1 [3] 5 [14] Headache 2 [6] 4 [11] Hypertension 2 [6] 4 [11] Leukopenia 0 4 [11] Fatigue 0 3 [9] Nausea 1 [3] 2 [6] Hypoxia 1[3] 1 [3] Aspartate aminotransferase increased 1[3] 1 [3] Febrile neutropenia 1[3] 1 [3] Pneumonia 1[3] 1 [3] Sinus tachycardia • Overall manageable tolerability profile • Most frequent/relevant related AEs were • transient lymphopenia, • mild to moderate CRS (23% Grade 1, 9% Grade 2, no Grade ≥ 3 ), majority at first dose • neutropenia 2 occurred mostly at initial target dose and fully resolved in all cases except one (see below) • one possibly related death (pneumonia in the context of lung tumor progression and concurrent neutropenia) as previously reported 3 • MTD not reached based on the BLRM 1 All treatment - emergent adverse events (TEAEs) at least possibly related to IMA401 infusion with grade 1 - 2 occurring in at least 9% of patients and all events with grade 3 - 5; 2 with three dose - limiting events at 2.5 mg (DLT), neutropenia observed in patients with and without dexamethasone pre - medication; 3 reported in Annual Report 2023, patient did not receive dexamethasone pre - medication; CRS: Cytokine release syndrome; BLRM: Bayesian logistic regression model; MTD: Maximum tolerated dose. Data cut - off Jul 23, 2024
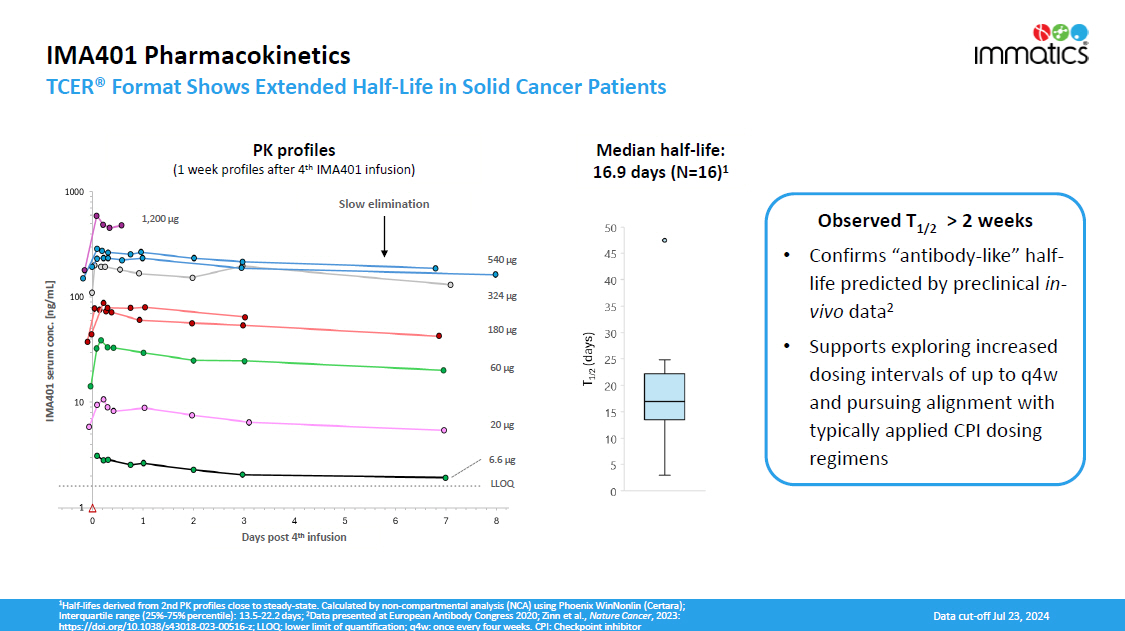
IMA401 Pharmacokinetics TCER® Format Shows Extended Half - Life in Solid Cancer Patients 1 Half - lifes derived from 2nd PK profiles close to steady - state. Calculated by non - compartmental analysis (NCA) using Phoenix WinNonlin (Certara); Interquartile range (25% - 75% percentile): 13.5 - 22.2 days; 2 Data presented at European Antibody Congress 2020; Zinn et al., Nature Cancer , 2023: https://doi.org/10.1038/s43018 - 023 - 00516 - z; LLOQ: lower limit of quantification; q4w: once every four weeks. CPI: Checkpoint inhibitor Median half - life: 16.9 days (N=16) 1 Slow elimination Days post 4 th infusion IMA401 serum conc. [ng/mL] 6.6 µg 20 µg 60 µg 180 µg LL OQ 324 µg 540 µg 1,200 µg PK profiles (1 week profiles after 4 th IMA401 infusion) Observed T 1 / 2 > 2 weeks • Confirms “antibody - like” half - life predicted by preclinical in - vivo data 2 • Supports exploring increased dosing intervals of up to q4w and pursuing alignment with typically applied CPI dosing regimens Data cut - off Jul 23, 2024

IMA401 Demonstrates Initial Anti - Tumor Activity in Multiple Tumor Types Phase 1a Dose Escalation Across All Dose and Target Levels (DL1 - 7; N=29 * ) 10 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available. BOR for one cut. melanoma patient is presented as SD as per iRECIST while BOR per RECIST1.1 was PD, as there was a site error in imaging baseline non - target lesions. 1 includes confirmed and unconfirmed PR; BL: Baseline ; BOR: Best overall response; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease. Data cut - off Jul 23, 2024 • Responses in HNSCC, neuroendocrine tumor, cut . and muc . melanoma • Durable responses in 3 of 4 confirmed responses ongoing at 13 +, 8 + and 3 + months • Disease control in a number of relevant tumor types including sqNSCLC, ovarian carcinoma, TNBC, gastric adenocarcinoma, and gallbladder adenocarcinoma • All confirmed responses in patients who had received infusions at ≥ 1 mg Cancer Indications: Cut.: Cutaneous; HNSCC: Head & Neck Squamous Cell Carcinoma; LCNEC: Large Cell Neuroendocrine Carcinoma; Muc.: Mucosal; NET CUP: Neurodendocrine Tumor, Cancer of Unknown Primary; SCLC: Small Cell Lung Cancer; sqNSCLC: Squamous Non - small Cell Lung Cancer; TNBC: Triple Negative Breast Cancer. Overall response rate 1 : 21% (6/29) Cancer indications Ongoing treatment Timepoint of PD according to RECIST 1.1 BOR (RECIST 1.1)
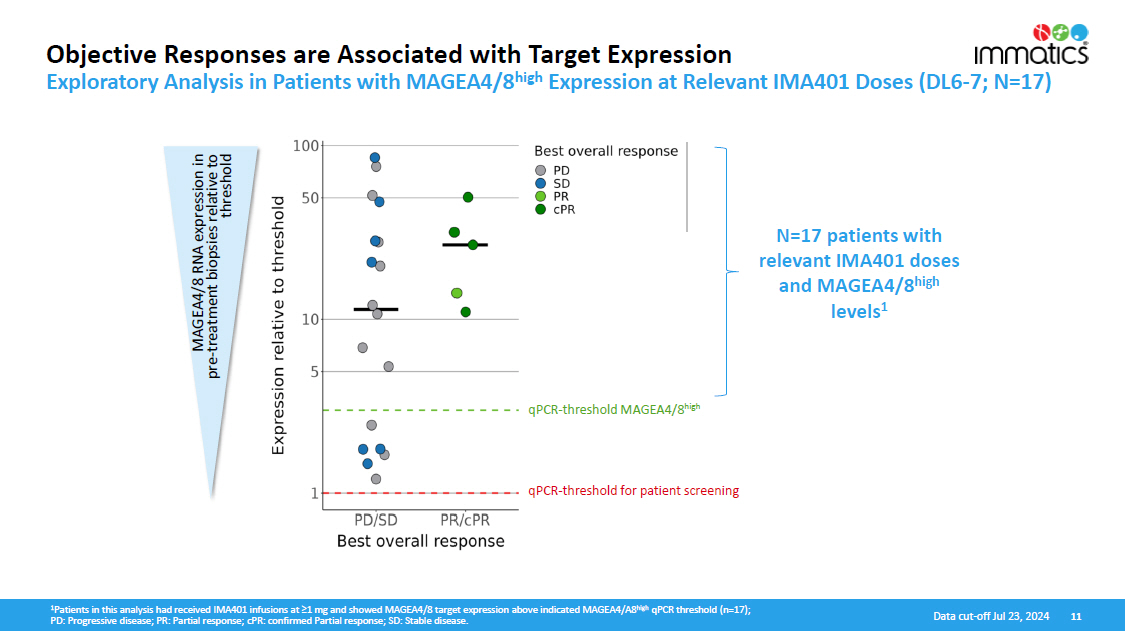
1 Patients in this analysis had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression above indicated MAGEA4/A8 high qPCR threshold (n=17); PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease. 11 Data cut - off Jul 23, 2024 Objective Responses are Associated with Target Expression Exploratory Analysis in Patients with MAGEA4/8 high Expression at Relevant IMA401 Doses (DL6 - 7; N=17) qPCR - threshold MAGEA4/8 high qPCR - threshold for patient screening MAGEA4/8 RNA expression in pre - treatment biopsies relative to t h r es ho ld N=17 patients with relevant IMA401 doses and MAGEA4/8 high levels 1

12 *Patients in this analysis are part of the efficacy analysis set with at least one post - treatment tumor assessment and had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold (n=17); Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with at least two available post infusion scans or patients with progressive disease (PD) at any prior timepoint; two patients not included in tumor shrinkage calculation or shown in the figures as they had clinical progression and post - treatment tumor assessment is not available; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease. Data cut - off Jul 23, 2024 11 12 13 14 - 1 0 0 - 5 0 0 50 1 0 0 Change in Sum of Longest Diameter of Target Lesions from Baseline [%] ⯈ BL PR PD ⯈ ⯈ ⯈ ⯈ ⯈ Target Lesion r e s e c t e d ⯈ ⯈ IMA401 Demonstrates Initial Anti - Tumor Activity in Multiple Tumor Types Exploratory Analysis in Patients with MAGEA4/8 high Expression at Relevant IMA401 Doses (DL6 - 7; N=17*) 29% (5/17) ORR 25% (4/16) cORR 53% (9/17) DCR 53% (8/15) Tumor s h rin k a g e 0 1 2 3 4 5 6 7 8 9 10 Months post First IMA401 Infusion Cancer Indications: Cut.: Cutaneous; HNSCC: Head & Neck Squamous Cell Carcinoma; LCNEC: Large Cell Neuroendocrine Carcinoma; Muc.: Mucosal; NET CUP: Neurodendocrine Tumor, Cancer of Unknown Primary; SCLC: Small Cell Lung Cancer; sqNSCLC: Squamous Non - small Cell Lung Cancer; TNBC: Triple Negative Breast Cancer. BOR (RECIST 1.1) Ongoing treatment

Tumor Shrinkage and Disease Control Induced by IMA401 Associated with Prolonged Overall Survival Analysis Across All Doses and Target Levels (DL1 - 7) 13 12.7 months median OS across multiple tumor types and all dose levels (n=29) Tumor shrinkage (12/27 patients) and disease control (16/29) associate with long - term outcome: » Significantly longer OS in these groups of patients (mOS not reached vs. 4.3 months or 3.2 months, respectively) Data cut - off Jul 23, 2024 Overall Survival (OS) censored at data - cut; *two patients with clinical progression prior to first tumor assessment not included; mOS: median overall survival. 0 6 1 2 1 8 0 5 0 1 0 0 OS - All Patients (n=27*) OS [months] OS [%] Tumor shrinkage (n=12) No tumor shrinkage (n=15) Log - rank test p - value = 0.0093 mOS = not reached mOS = 4.3 months 0 6 1 2 1 8 0 5 0 1 0 0 OS - All Patients (n=29) OS [months] OS [%] Disease control (n=16) Progressive disease (n=13) Log - rank test p - value <0.0001 mOS = not reached mOS = 3.2 months OS in patients with and without tumor shrinkage (N=27*) OS in patients with disease control and progressive disease (N=29)

Clinical Activity in Heavily Pre - Treated Cancer Patients 14 CT and MRI scans courtesy of treating physicians (Dr. Manik Chatterjee, University Hospital Wuerzburg and Dr. Max - Felix Häring, Eberhard Karls University Tue bingen); HNSCC: Head and neck squamous cell carcinoma; NET CUP: Neuroendocrine tumor - cancer of unknown primary; LA: Long axis; cPR: confirmed Partial response; BOR: Best overall response Baseline MRI Follow Up Week 13 Outcomes Patient Characteristics cPR - 56% reduction (BOR: - 58.6%) NET CUP cPR ongoing at week 36 post - treatment start Lesions in liver, lung, bone, pancreas, adrenal gland, lymph nodes 4 prior lines of therapy: Two lines of radiopharmaceuticals, chemotherapy, mTOR inhibitor 60 - year - old female, NET CUP, MAGEA4/8 high 63 - year - old male, HNSCC, MAGEA4/8 high Outcomes Patient Characteristics cPR - 59% reduction HNSCC, Hypopharynx cPR ongoing at week 12 post - treatment start Lesions in lung 3 prior lines of therapy: Platinum chemotherapy, anti - PD - 1/chemotherapy, anti - EGFR/chemotherapy LA: 18mm LA: 21mm LA: 70mm LA: 34mm Data cut - off Jul 23, 2024 Baseline CT Follow Up Week 13 Lung right Lung left LA: 6mm LA: 10mm

First - in - human Data of IMA401 TCER® Targeting MAGEA4/8 • Tolerability : Most common treatment - related AEs are low - grade CRS, transient lymphopenia and neutropenia • Pharmacokinetics : Median terminal half - life of 16.9 days supporting potential further flexibility in future dosing schedules incl. combination with CPI and increased dosing intervals up to q4w • Initial anti - tumor activity in heavily pre - treated patients • Objective responses in HNSCC, neuroendocrine tumor of unknown origin, cutaneous and mucosal melanoma including durable ongoing PRs of up to 13+ months • Deep responses (tumor shrinkage of ≥ 50%) in four patients including deepening of responses over time • Objective responses are associated with target expression and IMA401 dose: ORR 29%, cORR 25%, and tumor shrinkage in 53% of patients with relevant IMA401 doses and MAGEA4/8 high target levels • Dose escalation ongoing 15 AE: Adverse Event; CRS: Cytokine Release Syndrome; CPI: checkpoint inhibitors; q4w: once every four weeks; HNSCC: Head and neck squamous cell carcinoma; PR: Partial response
Special Thanks to the Patients, their Families 16 B o n n Dresden Tuebingen Heidelberg Ulm Wuerzburg Erlangen Mun i c h Reg en s bu rg Duesseldorf M a i n z Chemnitz B e rlin Leipz i g Freib u rg Nuremberg Muenster K i e l Sponsor: Immatics …and the IMA401 Investigators at the Clinical Sites Dresden: Prof. M. Wermke Berlin: Prof. S. Ochsenreither Wuerzburg: Dr. M. Chatterjee Duesseldorf: Dr. S. Gröpper Tuebingen: Dr. M. - F. Häring Regensburg: Dr. D. Heudobler Heidelberg: Prof. D. Jäger Muenster: Prof. A. Bleckmann Erlangen: Dr. S. Spörl Nuremberg: Prof. S. Knop Bonn: Dr. T. Holderried Munich: Dr. J. Hecker Freiburg: Prof. H. Becker Chemnitz: Dr. M. Hänel Mainz: Dr. M. Fried Leipzig: Dr. G. Stocker Ulm: Dr. A. Babiak Kiel: Prof. A. Letsch

A p pen d ix Confidential 17

IMA401 Demonstrates Initial Anti - Tumor Activity in Multiple Tumor Types Phase 1a Dose Escalation Across All Dose and Target Levels (DL1 - 7; N=29 * ) 18 # of Patients Safety (Efficacy - evaluable) Population 16 Different Indications 7 (7) Cut. Melanoma 1 (1) Muc. Melanoma 6 (3) Synovial Sarcoma 4 (3) TNBC 4 (4) HNSCC 2 (2) SCLC 2 (2) Ovarian Carcinoma 1 (1) sqNSCLC 1 (1) AdNSCLC 1 (1) NET CUP 1 (1) Gastric Adenocarcinoma 1 (1) LCNEC Esophageal 1 (1) LCNEC Lung 1 (1) Gallbladder Adenocarcinoma 1 (0) Bladder carcinoma 1 (0) Testicular GCT * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown ; two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available . BOR for one cut . melanoma patient is presented as SD as per iRECIST while BOR per RECIST 1 . 1 was PD, as there was a site error in imaging baseline non - target lesions . Cancer Indications : Cut .: Cutaneous ; HNSCC : Head & Neck Squamous Cell Carcinoma ; LCNEC: Large Cell Neuroendocrine Carcinoma; Muc.: Mucosal; NET CUP: Neu ro d e n d ocr i n e T u m o r, Can c e r of Unknown Primary ; SCLC : Small Cell Lung Cancer ; sqNSCLC : Squamous Non - small Cell Lung Cancer ; TNBC : Triple Negative Breast Cancer . BL : Baseline ; BOR : Best overall response ; PD : Progressive disease ; SD : Stable disease ; PR : Partial response ; cPR : confirmed Partial response . BOR (RECIST 1.1) Ongoing treatment Data cut - off Jul 23, 2024

19 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; Two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available. BOR for one cut. melanoma patient is presented as SD as per iRECIST while BOR per RECIST1.1 was PD, as there was a site error in imaging baseline non - target lesions. BOR: Best overall response; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease. Data cut - off Jul 23, 2024 IMA401 Demonstrates Initial Anti - Tumor Activity in Multiple Tumor Types Patients at Relevant IMA401 Doses (DL6 - 7; N=23*) Cancer indications BOR (RECIST 1.1) 0 1 2 3 4 11 12 13 14 - 1 0 0 - 5 0 0 50 1 0 0 5 6 7 8 9 10 Months post First IMA401 Infusion Change in Sum of Longest Diameter of Target Lesions from Baseline [%] ⯈ B L PR PD ⯈ ⯈ ⯈ ⯈ ⯈ ⯈ ⯈ Target Lesion resected ⯈ Cancer Indications: Cut.: Cutaneous; HNSCC: Head & Neck Squamous Cell Carcinoma; LCNEC: Large Cell Neuroendocrine Carcinoma; Muc.: Mucosal; NET CUP: Neurodendocrine Tumor, Cancer of Unknown Primary; SCLC: Small Cell Lung Cancer; sqNSCLC: Squamous Non - small Cell Lung Cancer; TNBC: Triple Negative Breast Cancer.

1 Patients in this analysis had received IMA 401 infusions at ≥ 1 mg and showed MAGEA 4 / 8 target expression higher than the MAGEA 4 / 8 qPCR threshold (N= 17 ) ; DCR : Disease Control Rate ; ORR : Objective Response Rate ; Confirmed objective response rate (cORR) according to RECIST 1 . 1 for patients with at least two available post infusion scans or patients with progressive disease (PD) at any prior timepoint ; two patients not included in tumor shrinkage calculation as they had clinical progression and post - treatment tumor assessment is not available . 20 Data cut - off Jul 23, 2024 21% (6/29) 29% (5/17) ORR 14% (4/28) 25% (4/16) cORR 55% (16/29) 53% (9/17) DCR 44% (12/27) 53% (8/15) Tumor shrinkage Patients with relevant IMA401 doses and MAGEA4/8 high levels 1 (N=17) Overall efficacy - evaluable population across all dose and target levels (N=29) Objective Responses are Associated with Target Expression Exploratory Analysis in Patients with MAGEA4/8 high Expression at Relevant IMA401 Doses (DL6 - 7; N=17)
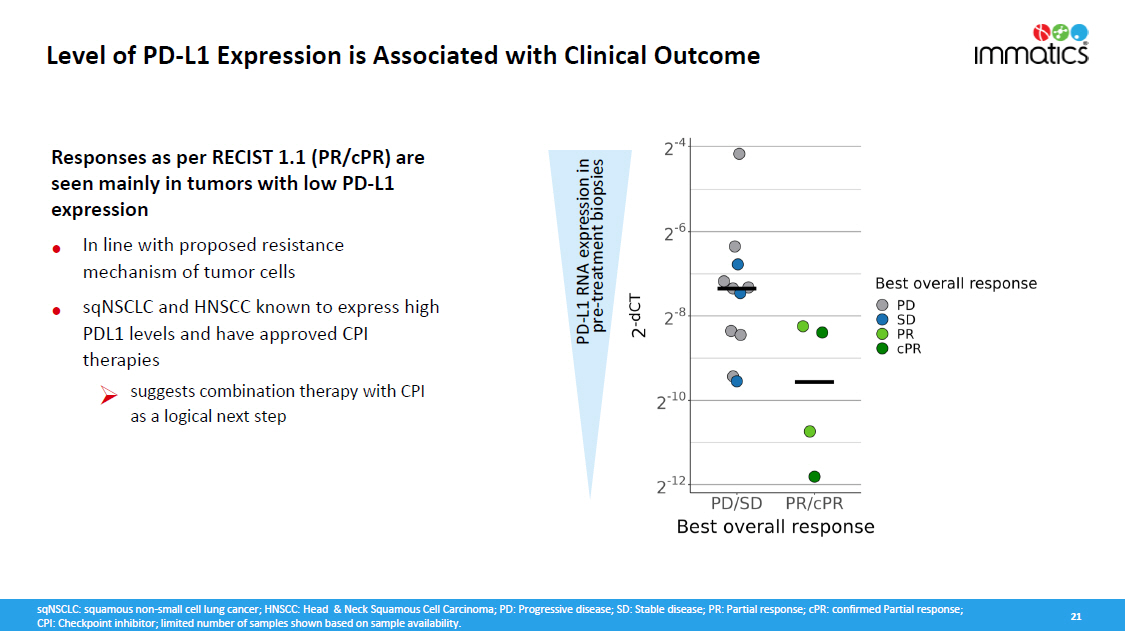
21 Level of PD - L1 Expression is Associated with Clinical Outcome Responses as per RECIST 1.1 (PR/cPR) are seen mainly in tumors with low PD - L1 expression • • In line with proposed resistance mechanism of tumor cells sqNSCLC and HNSCC known to express high PDL1 levels and have approved CPI therapies » suggests combination therapy with CPI as a logical next step PD - L1 RNA expression in pre - treatment biopsies sqNSCLC: squamous non - small cell lung cancer; HNSCC: Head & Neck Squamous Cell Carcinoma; PD: Progressive disease; SD: Stable disease; PR: Partial response; cPR: confirmed Partial response; CPI: Checkpoint inhibitor; limited number of samples shown based on sample availability.

MAGEA4/8 Target Expression Profiles Across Selected Tumor Types 22 Data cut - off Jul 23, 2024 qPCR - threshold MAGEA4/8 high qPCR - threshold for patient screening 31% 36% 41% 52% 15% 18% Target Prevalences 1 MAGEA4/8 target expression distribution (blue histogram) based on TCGA RNAseq data; 1 MAGEA4/8 target prevalence is based on TCGA RNAseq data combined with a proprietary MS - guided RNA expression threshold.

23 Initial Anti - Tumor Activity – Subanalysis of Melanoma Patients Phase 1a Dose Escalation Across All Dose and Target Levels (DL1 - DL7; N=8*) 0 1 2 3 4 10 11 12 13 14 - 1 0 0 - 5 0 0 50 1 0 0 5 6 7 8 9 Months post First IMA401 Infusion Change in Sum of Longest Diameter of Target Lesions from Baseline [%] ⯈ B L P R P D ⯈ ⯈ ⯈ ⯈ Target Lesion resected ⯈ Cancer indications BOR (RECIST 1.1) Ongoing treatment Timepoint of PD according to RECIST 1.1 50% (4/8) 60% (3/5) ORR 25% (2/8) 40% (2/5) cORR 75% (6/8) 60% (3/5) DCR 75% (6/8) 80% (4/5) Tumor shrinkage Patients with relevant IMA401 doses and MAGEA4/8 high levels 1 Overall efficacy - evaluable population across all dose and target levels Data cut - off Jul 23, 2024 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; BOR for one cut. melanoma patient is presented as SD as per iRECIST while BOR per RECIST1.1 was PD, as there was a site error in imaging baseline non - target lesions. 1 Patients in this analysis had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold (N=5). DCR: Disease Control Rate; ORR: Objective Response Rate; Confirmed objective response rate (cORR) according to RECIST 1.1 for patients with at least two available post infusion scans or patients with progressive disease (PD) at any prior timepoint; two patients not included in tumor shrinkage calculation as they had clinical progression and post - treatment tumor assessment is not available. BL: Baseline; BOR: Best overall response; Cut: Cutaneous; Muc: Mucosal; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease.

Baseline Characteristics – Subanalysis of Melanoma Patients Heavily Pre - treated Melanoma Patients 24 Data cut: 23 - Jul - 2024 Safety Population: All Melanoma Patients (N=8) Characteristic Cut. Melanoma 7/8 [87.5] Muc. Melanoma 1/8 [12.5] Indications n [%] 76.5 (62, 82) Age Median (min, max) 1 [12.5] 7 [87.5] 0 [0.0] ECOG performance status 0 - n [%] 1 - n [%] 2 - n [%] 4 (2, 5) Prior lines of systemic treatment Median (min, max) 2 (1, 3) Prior lines of CPI treatment Median (min, max) 100.0 87.5 25.0 25.0 Thereof patients treated with Anti - PD1 Therapy [%] Ipilimumab [%] BRAF Inhibitors [%] Experimental Therapies [%] 62.5 37.5 0.0 LDH at baseline ≤ 1xULN [%] 1 - 2xULN [%] >2xULN [%] 71.5 (15, 178) Baseline tumor burden Median target lesion sum of diameter [mm] (min, max) 3.5 (1, 5) Number of organs with metastases Median (min, max) 25.0 Liver/ Brain Lesions [% of patients] LDH: Lactate dehydrogenase; ULN: Upper limit of normal.

Clinical Activity in Heavily Pre - Treated Melanoma Patients 25 CT scans courtesy of treating physicians (Dr. S. Ochsenreither, Charité Berlin and Dr. M. Wermke, TU Dresden); SD: Stable disease, cPR: confirmed Partial response. Outcomes Patient Characteristics cPR - 42.6% reduction Cutaneous Melanoma Deepening response from SD to cPR over 44 weeks post - treatment start Lesions in lymph nodes, peritoneum, soft tissue gluteal, subcutaneous 2 prior lines of therapy: Anti - PD - 1, anti - CTLA - 4/anti - PD - 1 78 - year - old male, cut. melanoma, MAGEA4/8 high 75 - year - old male, cut. melanoma, MAGEA4/8 high Outcomes Patient Characteristics cPR - 65.1% reduction Cutaneous Melanoma cPR ongoing at week 58 post - treatment start Lesions in lymph nodes, chest wall, liver, spleen 5 prior lines of therapy: Anti - PD - 1, RAF kinase inhibitors, MEK kinase inhibitor, oncolytic virus, Anti CTLA - 4 Baseline CT Follow Up Month 8 Follow Up Month 3 Baseline CT Follow Up M3 Follow Up M6 Data cut - off Jul 23, 2024

IMA401: Initial Anti - Tumor Activity in Heavily Pretreated Patients Phase 1a Dose Escalation Across All Dose and Target Levels, Efficacy - evaluable Population (N=29*) 26 Data cut - off Jul 23, 2024 BOR (Max % change of target lesions) B OR Baseline Tumor Burden [mm] Highest DL received List of prior treatment lines No of prior treatment lines MAGEA4/8 high 1 Indication #N 29.1 PD 55 DL1 Doxorubicin/ Ifosfamide Trabectedin D oc e ta x el/Ge mc i ta b in e 3 Yes Syn. Sarcoma 1 - 3.6 SD 57 DL3 Letrozole Cape c i ta b in e Gemcitabine 3 Yes TNBC 2 9.2 SD 65 DL3 Melphalan/ Tumor Necrosis Factor Alpha Doxorubicin/ Ifosfamide 2 Yes Syn. Sarcoma 3 14.3 SD 112 DL5 Fluorouracil/ Carboplatin/ Pembrolizumab Cetuximab/ Docetaxel 2 Yes HNSCC 4 - 65.1 cPR 106 DL6a Nivolumab Trametinib/ Dabrafenib Binimetinib/ Encorafenib Talimogene Laherparepvec Ipilimumab 5 Yes Cut. Melanoma 5 8.3 PD 48 DL5 Cisplatin Carboplatin/ Fluorouracil/ Folic Acid/ Pembrolizumab Cisplatin/ Fluorouracil/ Cetuximab 3 Yes HNSCC, Tonsil 6 - 42.6 cPR 61 DL6a Pembrolizumab Ipilimumab/ Nivolumab 2 Yes Cut. Melanoma 7 - 30.6 PR 111 DL5 Pembrolizumab Ipilimumab/ Nivolumab/ Talimogene Laherparepvec Dacarbazine Citrate Ipilimumab/ Nivolumab Trametinib 5 Yes Cut. Melanoma 8 40.4 PD 52 DL6 Cyclophosphamide/ Epirubicin/ Paclitaxel Paclitaxel Nanoparticle Albumin - bound/ Atezolizumab Eribulin/ Sacituzumab Govitecan/ Gemcitabine/ Carboplatin Eribulin Trastuzumab Deruxtecan Cisplatin/ Gemcitabine 6 Yes TNBC 9 20.2 PD 114 DL6 Carboplatin/ Paclitaxel/ Bevacizumab/ Niraparib Pegylated Liposomal Doxorubicin Hydrochloride 2 Yes Ovarian Cancer 10 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available. 1 Patients showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold. BOR: Best overall response; DL: Dose level; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease.
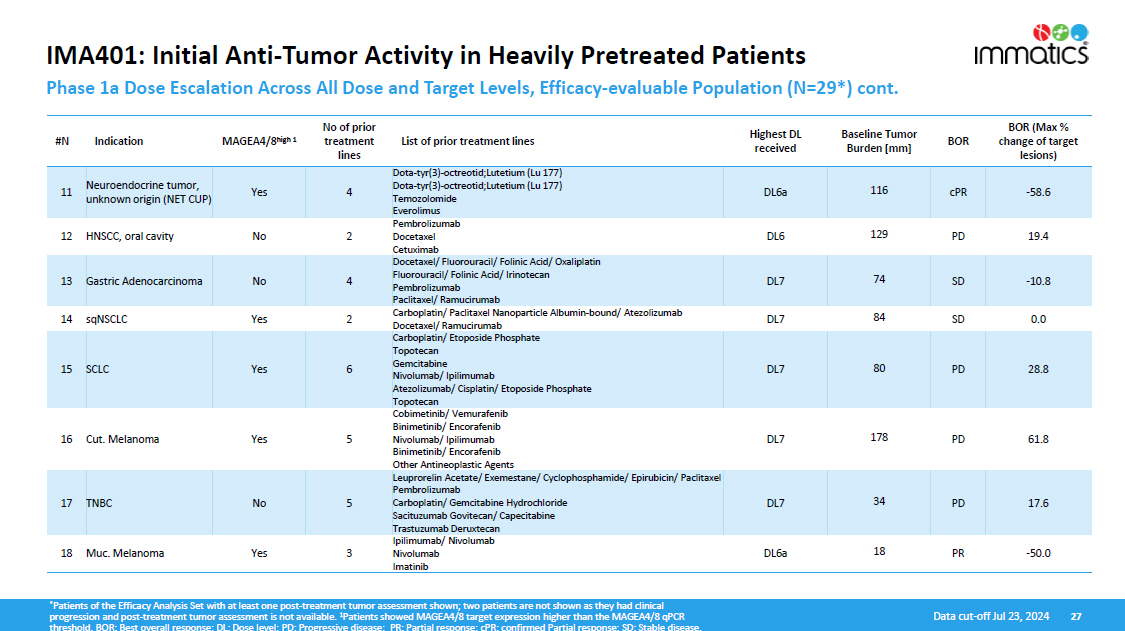
IMA401: Initial Anti - Tumor Activity in Heavily Pretreated Patients Phase 1a Dose Escalation Across All Dose and Target Levels, Efficacy - evaluable Population (N=29*) cont. 27 Data cut - off Jul 23, 2024 BOR (Max % change of target lesions) BOR Baseline Tumor Burden [mm] Highest DL received List of prior treatment lines No of prior treatment lines MAGEA4/8 high 1 Indication #N - 58.6 cPR 116 DL6a Dota - tyr(3) - octreotid;Lutetium (Lu 177) Dota - tyr(3) - octreotid;Lutetium (Lu 177) Temozolomide Everolimus 4 Yes Neuroendocrine tumor, unknown origin (NET CUP) 11 19.4 PD 129 DL6 Pembrolizumab Docetaxel Ce t ux i mab 2 No HNSCC, oral cavity 12 - 10.8 SD 74 DL7 Docetaxel/ Fluorouracil/ Folinic Acid/ Oxaliplatin Fluorouracil/ Folinic Acid/ Irinotecan Pembrolizumab Paclitaxel/ Ramucirumab 4 No Gastric Adenocarcinoma 13 0.0 SD 84 DL7 Carboplatin/ Paclitaxel Nanoparticle Albumin - bound/ Atezolizumab Docetaxel/ Ramucirumab 2 Yes sqNSCLC 14 28.8 PD 80 DL7 Carboplatin/ Etoposide Phosphate Topotecan Gemcitabine Nivolumab/ Ipilimumab Atezolizumab/ Cisplatin/ Etoposide Phosphate Topotecan 6 Yes SCLC 15 61.8 PD 178 DL7 Cobimetinib/ Vemurafenib Binimetinib/ Encorafenib Nivolumab/ Ipilimumab Binimetinib/ Encorafenib Other Antineoplastic Agents 5 Yes Cut. Melanoma 16 17.6 PD 34 DL7 Leuprorelin Acetate/ Exemestane/ Cyclophosphamide/ Epirubicin/ Paclitaxel Pembrolizumab Carboplatin/ Gemcitabine Hydrochloride Sacituzumab Govitecan/ Capecitabine Trastuzumab Deruxtecan 5 No TNBC 17 - 50.0 PR 18 DL6a Ipilimumab/ Nivolumab Ni v o lu mab Imatinib 3 Yes Muc. Melanoma 18 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available. 1 Patients showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold. BOR: Best overall response; DL: Dose level; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease.

IMA401: Initial Anti - Tumor Activity in Heavily Pretreated Patients Phase 1a Dose Escalation Across All Dose and Target Levels, Efficacy - evaluable Population (N=29*) cont. 28 Data cut - off Jul 23, 2024 BOR (Max % change of target lesions) BOR Baseline Tumor Burden [mm] Highest DL received List of prior treatment lines No of prior treatment lines MAGEA4/8 high 1 Indication #N - 19.6 SD 202.8 DL6a Carboplatin/ Gemcitabine/ Paclitaxel Carboplatin/ Paclitaxel/ Bevacizumab Carboplatin/ Doxorubicin/ Niraparib Letrozole Bevacizumab/ Carboplatin/ Paclitaxel Trametinib Carboplatin/ Paclitaxel Sacituzumab Govitecan 8 Yes Ovarian Cancer 19 - 14.6 PD (iSD) 2 82 DL6a Pembrolizumab Ipilimumab/ Nivolumab 2 No Cut. Melanoma 20 95.9 PD 99.4 DL6a Carboplatin/ Etoposide Calcium Folinate;Fluorouracil;Irinotecan Hydrochloride Avelumab/ Cabozantinib 3 Yes LCNEC, Esophageal 21 13.3 SD 15 DL6a Pembrolizumab Ipilimumab/ Nivolumab Dacarbazine Citrate Ipilimumab/ Nivolumab 4 No Cut. Melanoma 22 - 59.0 cPR 39 DL6a Cisplatin/ Carboplatin Carboplatin/ Fluorouracil/ Pembrolizumab Docetaxel/ Cetuximab 3 Yes HNSCC, Hypopharynx 23 0.0 SD 23 DL6a Carboplatin/ Atezolizumab/ Etoposide Carboplatin/ Paclitaxel 2 Yes LCNEC, Lung 24 - 6.2 SD 193 DL7 Capecitabine Cisplatin/ Gemcitabine Fluorouracil/ Folinic Acid/ Oxaliplatin Fluorouracil/ Folinic Acid/ Irinotecan Cisplatin/ Gemcitabine Hydrochloride/ Durvalumab Pembrolizumab/ Lenvatinib 6 Yes Gallbladder Ad e nocarcino m a 25 55.6 PD 81 DL7 Carboplatin/ Etoposide Atezolizumab Carboplatin/ Etoposide 3 No SCLC 26 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available. 1 Patients showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold. 2 BOR is SD as per iRECIST while BOR per RECIST1.1 was PD, as there was a site error in imaging baseline non - target lesions. BOR: Best overall response; DL: Dose level; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease.

IMA401: Initial Anti - Tumor Activity in Heavily Pretreated Patients Phase 1a Dose Escalation Across All Dose and Target Levels, Efficacy - evaluable Population (N=29*) cont. 29 Data cut - off Jul 23, 2024 BOR (Max % change of target lesions) BOR Baseline Tumor Burden [mm] Highest DL received List of prior treatment lines No of prior treatment lines MAGEA4/8 high 1 Indication #N NA PD 169.4 DL6a Doxorubicin/ Ifosfamide Doxorubicin/ Ifosfamide Trofosfamide Pazopanib 4 Yes Syn. Sarcoma 27 - 5.9 PD 34 DL6a Bempegaldesleukin/ Nivolumab Talimogene Laherparepvec ICT 01/ Pembrolizumab Other Antineoplastic Agents 4 Yes Cut. Melanoma 28 NA PD 66 DL6a Carboplatin/ Ipilimumab/ Nivolumab/ Pemetrexed Cyclophosphamide/ Interleukin - 2/ Tumor - infiltrating Lymphocytes/ Fludarabine Docetaxel/ Nintedanib Cyclophosphamide/ Fludarabine/ T - cells + Interleukin - 2 4 Yes AdNSCLC 29 * Patients of the Efficacy Analysis Set with at least one post - treatment tumor assessment shown; two patients are not shown as they had clinical progression and post - treatment tumor assessment is not available. 1 Patients showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold. BOR: Best overall response; DL: Dose level; PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease.

www.immatics.com Please contact us via partnering@immatics.com to learn more about partnering and licensing opportunities utilizing our platform technologies XPRESIDENT®, XCEPTOR®, IMADetect®, AbsQuant® and TCR Scout®. Thank you





























