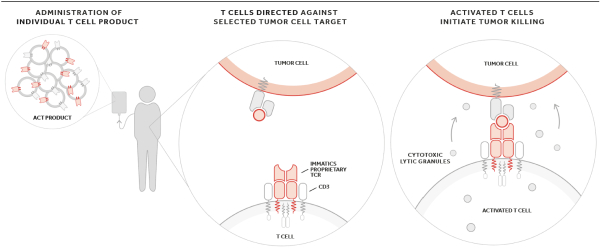
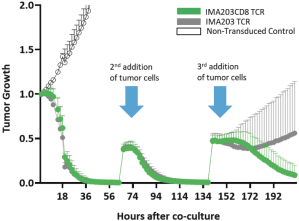
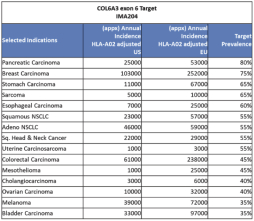
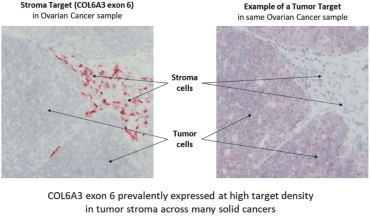
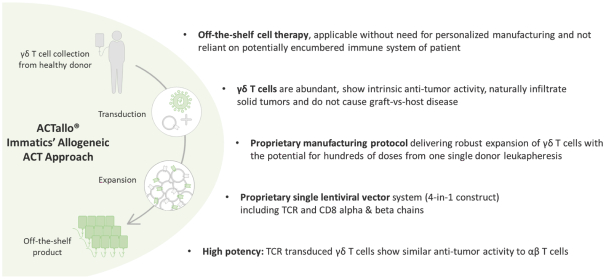
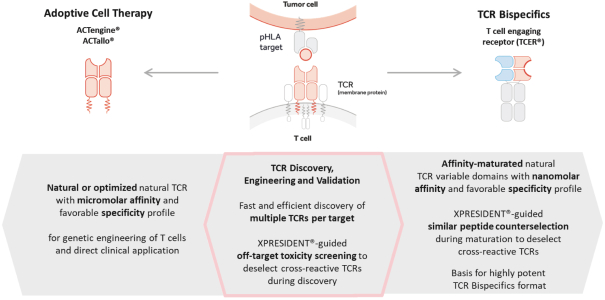
In August 2019, we and Celgene Corporation, a wholly owned subsidiary of BMS, entered into a strategic collaboration and license agreement to develop novel adoptive cell therapies targeting multiple cancers. Under the agreement, we may develop
TCR-T
programs against solid tumor targets discovered by our XPRESIDENT technology. We will utilize proprietary TCRs identified by our XCEPTOR TCR discovery and engineering platform. We will be responsible for the development of these programs through the lead candidate stage, at which time BMS may exercise its option to exclusively license one or more programs, thereby assuming sole responsibility for further worldwide development, manufacturing and commercialization of the
TCR-T
cell therapies. We retain certain early stage
co-development
and
co-funding
rights for selected
TCR-T
cell therapies arising from the collaboration.
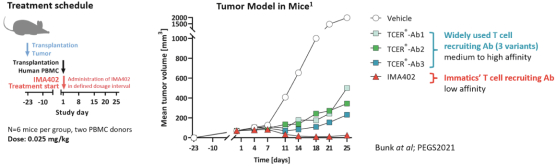
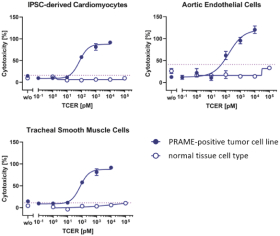
On December 10, 2021, we entered into a License, Development and Commercialization Agreement BMS relating to our TCR Bispecific candidate, IMA401. Pursuant to the agreement, we granted to BMS an exclusive, worldwide, sublicensable license to develop, manufacture, and commercialize IMA401 and certain other bispecific and multispecific molecules that bind to a MAGEA4/A8 peptide and engage and activate endogenous
T-cells
or other immune cells for any diagnostic, prophylactic or therapeutic uses, excluding cell therapy and cell therapy products. BMS granted us a
non-exclusive,
perpetual, worldwide, sublicensable, royalty-free license to certain BMS Company patents and
know-how
that are improvements to our platform technology that may be generated by Bristol-Myers Squibb in the performance of activities under the agreement. In consideration for such licenses, we received an upfront payment of $150 million and will be eligible to receive milestone payments of up to $770 million upon the achievement of certain development, regulatory and commercial milestones. In addition, during the royalty term, we will be eligible to receive tiered, low double-digit percentage royalties on worldwide net sales of licensed products. We have the option in certain instances to
co-fund
the development of the licensed products for the United States. If exercised, we will be responsible for a portion of the U.S. development expenses incurred by BMS and will be eligible to receive tiered, low double-digit percentage royalties on U.S. net sales of licensed products that are higher than those if we did not exercise its U.S. development
co-funding
option. The royalty percentages described above are subject to reduction in a given country under certain circumstances, including, but not limited to, the introduction of biosimilar products. In addition, we have the option to
co-promote
approved licensed products in the United States. Under the agreement, we will be responsible for, and will bear the cost of, the first Phase 1 clinical trial in Germany for the first licensed product and for performing certain related preclinical studies and
CMC-related
development activities. BMS will be responsible for, and will bear the cost of, performing all other development and commercialization activities, subject to our U.S. development
co-funding
option and U.S.
co-promote
option described above. The Agreement will expire upon expiration of the last royalty term contemplated by the agreement. A royalty term with respect to a licensed product in a given country begins upon the first commercial sale of such licensed product in such country and terminates upon certain events or at the end of certain time periods relevant to such licensed product, including, but not limited to: the expiration of regulatory exclusivity,