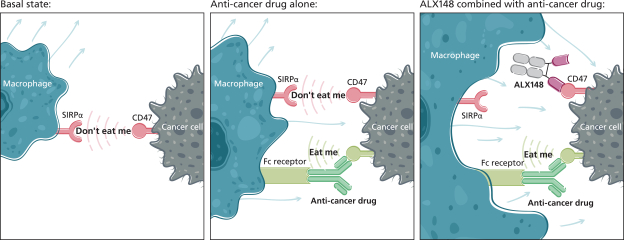
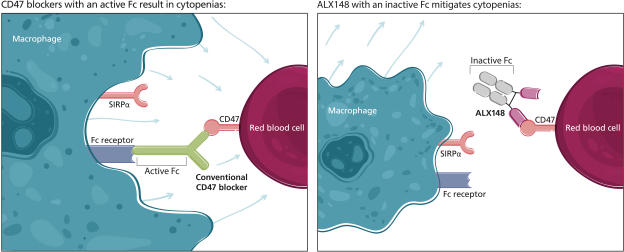
trade secrets, knowhow, continuing technological innovation and confidential information to develop and maintain our proprietary position.
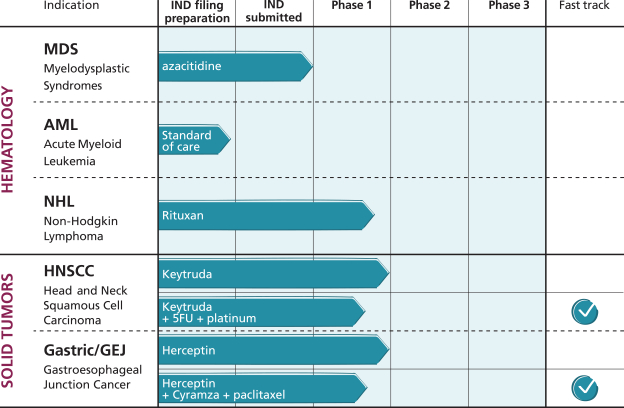
As of July 1, 2020, we own two issued U.S. patents, seven pending U.S. nonprovisional patent applications, six pending U.S. provisional patent applications and a portfolio of national patent application filings in a variety of non-U.S. jurisdictions, including Europe, Hong Kong, Brazil, Mexico, New Zealand, Japan, Australia, Canada, China, India, Israel, Republic of Korea, Singapore and Russia. Of these patents and patent applications, the following relate to ALX148: two issued U.S. patents, four pending U.S. nonprovisional patent applications, six pending U.S. provisional patent applications and a portfolio of national patent application filings in a variety of non-U.S. jurisdictions, including Europe, Hong Kong, Brazil, Mexico, New Zealand, Japan, Australia, Canada, China, India, Israel, Republic of Korea, Singapore and Russia.
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. Our two U.S. issued patents and, if issued as U.S. patents, our seven U.S. nonprovisional patent applications and six U.S. provisional patent applications are expected to expire between August 2036 and November 2040, excluding any additional term for patent term adjustments or patent term extensions, with an expiration of between August 2036 and November 2040 with respect to our patent and patent applications related to ALX148, excluding any additional term for patent term extensions.
We obtained a worldwide, royalty-bearing, sublicensable license from the Board of Trustees of the Leland Stanford Junior University, or Stanford, under certain patents relating to high-affinity SIRPa variant polypeptides, to develop, manufacture and commercialize products for use in certain licensed fields, the scope of which would include the application of the licensed intellectual property in oncology. Our portfolio of exclusively licensed patents from Stanford includes five issued patents (one of which is in the United States) and applications are pending in six jurisdictions (including the United States and the European Patent Office). For more information regarding our license agreement with Stanford, please see “—Exclusive (Equity) Agreement with The Board of Trustees of the Leland Stanford Junior University.”
Our patent portfolio exclusively licensed from Stanford contains patent families relating to high-affinity SIRPa variant polypeptides, which is comprised of one issued patent in each of U.S., Australia, China, Europe, Hong Kong and Japan, one pending U.S. patent application and one pending European patent application. The European patent has been validated as national patents in 37 different European countries. These patents and patent applications are subject to retained rights by Stanford to allow academic and nonprofit research institutions to practice the licensed technology and patents for noncommercial purposes. In addition, these patents are subject to certain pre-existing rights that Stanford has granted to two third parties. These patents are expected to expire in 2033 excluding any extension of patent term that may be available.
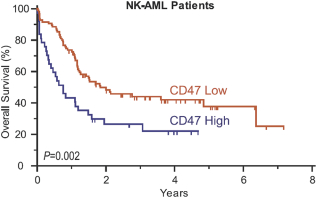
We are aware of a revoked European patent (EP 2 429 574) owned by UHN and The Hospital for Sick Children that may encompass certain therapies for the treatment of cancer using polypeptides comprising soluble human SIRPa, or a CD47-binding fragment thereof. This revoked patent related to the treatment of cancer with polypeptides comprising soluble human SIRPa, or a CD47-binding fragment thereof. This patent was revoked by the European Patent Office and UHN and The Hospital for Sick Children have appealed the decision. The U.S. counterpart is not yet granted. If UHN and the Hospital for Sick Children win their appeal of the European Patent Office decision revoking their European patent, or if the U.S. counterpart grants them a patent, the resulting patent claims could potentially limit our ability to pursue ALX148 in certain new indications or geographies in the future.
For more information regarding the risks related to our intellectual property, including the above referenced intellectual property proceedings, see “Risk Factors—Risks Related to Our Intellectual Property.”
Exclusive (Equity) Agreement with The Board of Trustees of the Leland Stanford Junior University
In March 2015, we entered into a license agreement, or the Stanford Agreement, with Stanford under which we obtained a worldwide, royalty-bearing, sublicensable license under certain patents relating to our current product candidates, to develop, manufacture and commercialize products for use in certain licensed fields, the scope of which would include the application of the licensed intellectual property in oncology. The license granted to us in
111