- REVB Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Revelation Biosciences (REVB) 8-KOther Events
Filed: 11 Feb 25, 4:05pm
Rebalancing inflammation to optimize health NASDAQ: REVB www.revbiosciences.com

This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words "anticipate", "believe", "expect", "estimate", "plan", "outlook", and "project" and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions investors not to place undue reliance on any such forward-looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the risk that our preclinical studies will not demonstrate sufficient positive data to support commencement of clinical trials; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for Gemini or any other product candidates; potential indications for which product candidates may be developed; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation’s balances will fund its operations; the ability of Revelation to obtain further financing and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation. Forward-Looking Statements
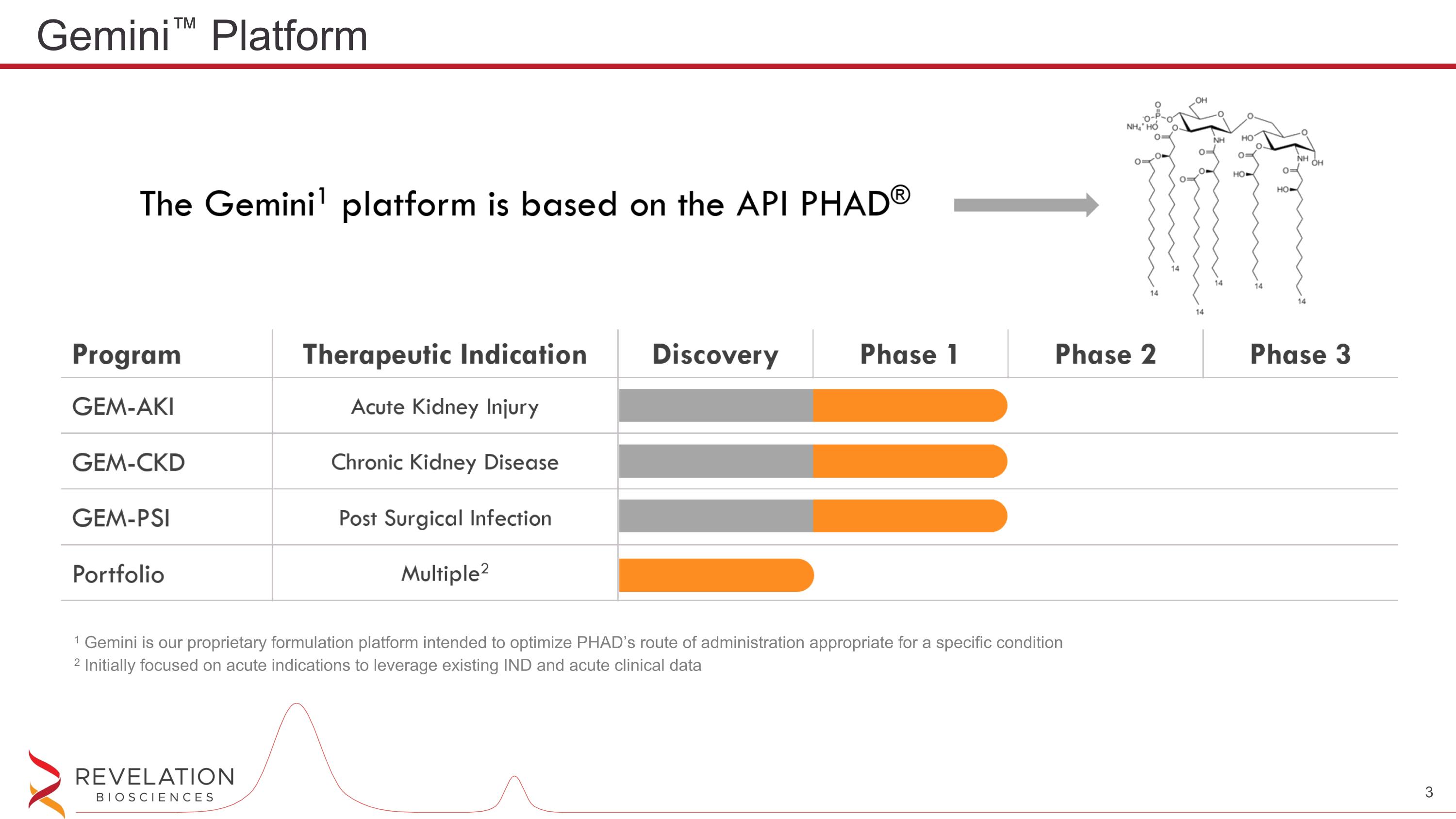
Gemini™ Platform 1 Gemini is our proprietary formulation platform intended to optimize PHAD’s route of administration appropriate for a specific condition 2 Initially focused on acute indications to leverage existing IND and acute clinical data
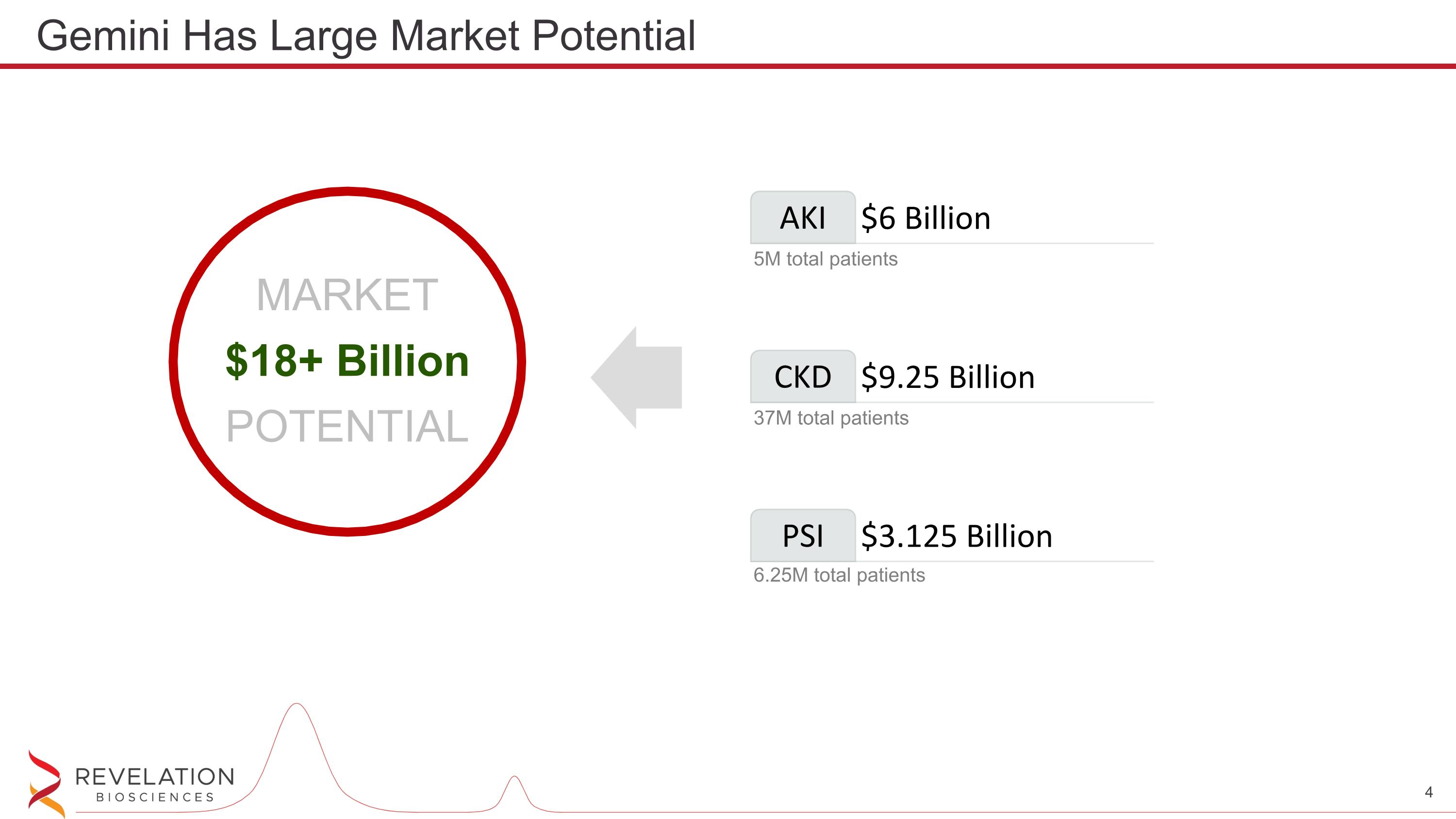
Gemini Has Large Market Potential AKI $6 Billion 5M total patients CKD $9.25 Billion 37M total patients PSI $3.125 Billion 6.25M total patients MARKET $18+ Billion POTENTIAL
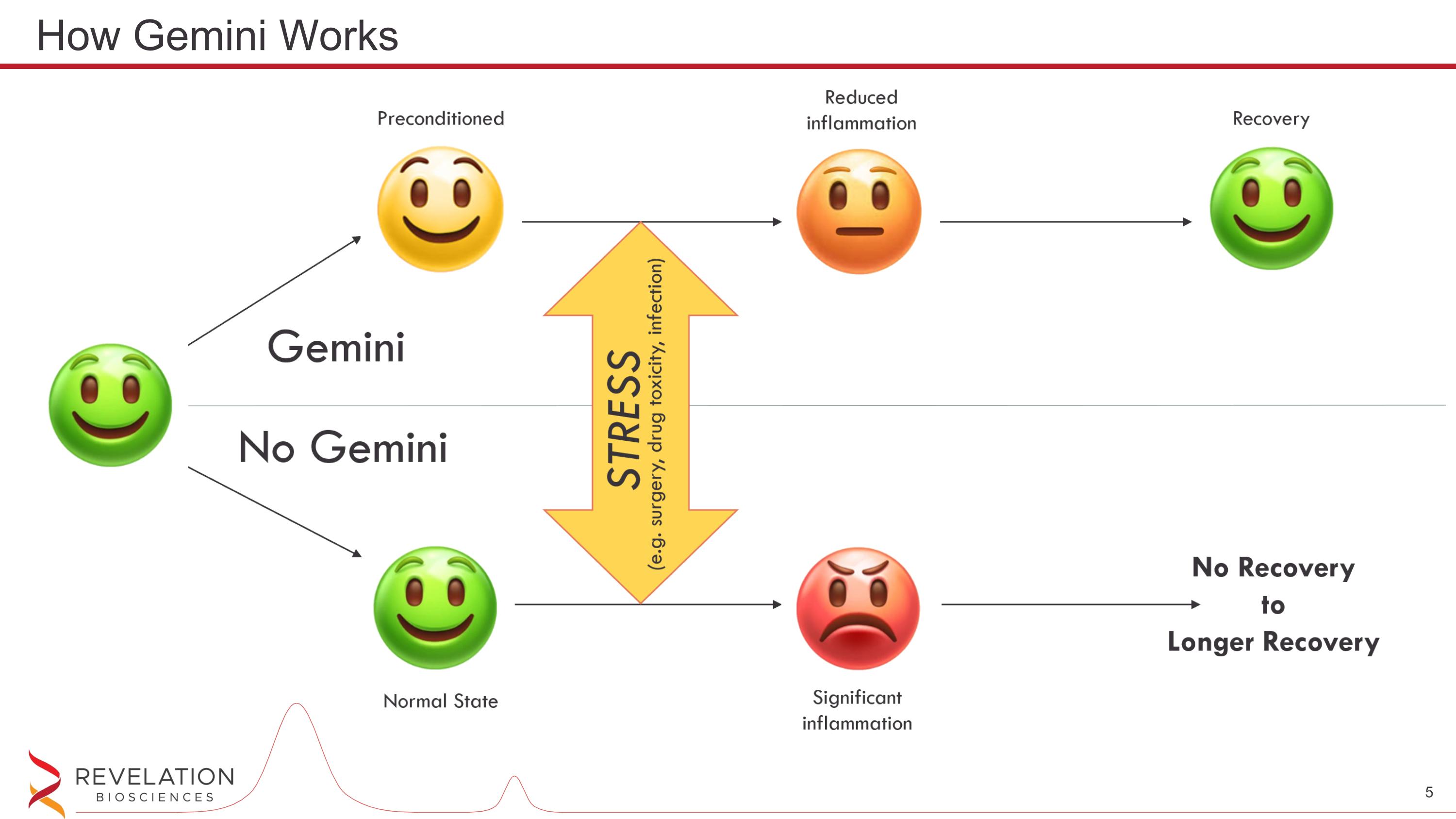
How Gemini Works

GEM-AKI and GEM-CKD Gemini For Acute Kidney Injury and Chronic Kidney Disease 6
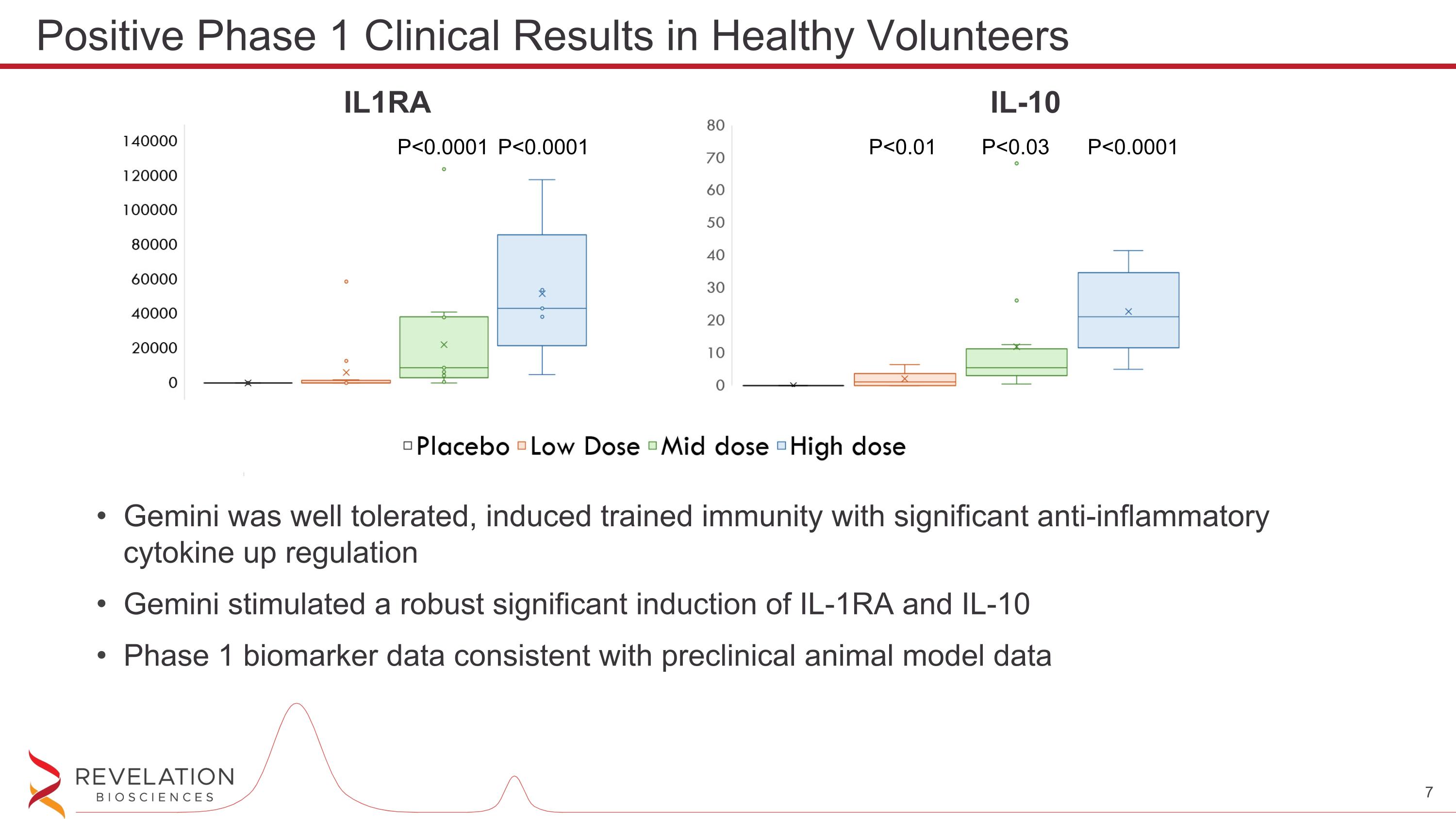
Gemini was well tolerated, induced trained immunity with significant anti-inflammatory cytokine up regulation Gemini stimulated a robust significant induction of IL-1RA and IL-10 Phase 1 biomarker data consistent with preclinical animal model data Positive Phase 1 Clinical Results in Healthy Volunteers P<0.0001 P<0.0001 P<0.0001 P<0.03 P<0.01 IL1RA IL-10
PRIME – PRotective IMmunostimulatory Evaluation A Phase 1b, Randomized, Placebo Controlled, Single Blind, Single-Ascending Dose in Stage 3 and Stage 4 CKD Patients Design Single dose, dose escalation 5 cohorts, 8 subjects per cohort 1:4 placebo vs drug (up to 40 patients) Follow for 7 days Readouts Safety, tolerability, PK, and activity biomarkers Key biomarkers of activity: IL-10, IL-1RA, and TGF-b Demonstration of attenuated immune cell response to second stress Status Study Initiated Jan 2025 Topline data 1st half 2025 PRIME Phase 1b Clinical Study in CKD Patients
GEM-PSI Gemini for Post-Surgical Infection 9
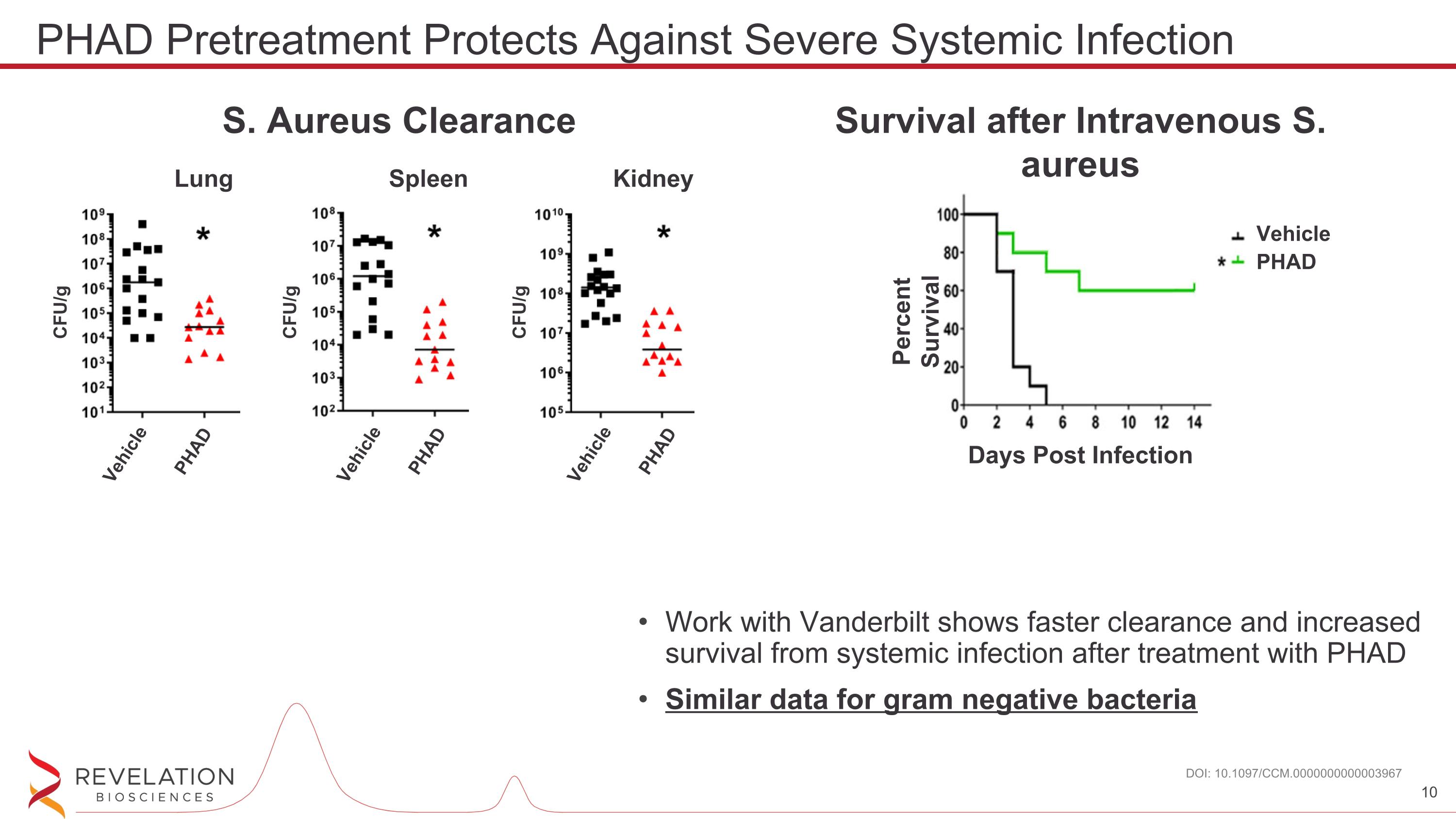
Work with Vanderbilt shows faster clearance and increased survival from systemic infection after treatment with PHAD Similar data for gram negative bacteria DOI: 10.1097/CCM.0000000000003967 PHAD Pretreatment Protects Against Severe Systemic Infection S. Aureus Clearance Lung Kidney Spleen CFU/g CFU/g CFU/g Vehicle PHAD Vehicle PHAD Vehicle PHAD Survival after Intravenous S. aureus Percent Survival Days Post Infection Vehicle PHAD

Financial Overview 11
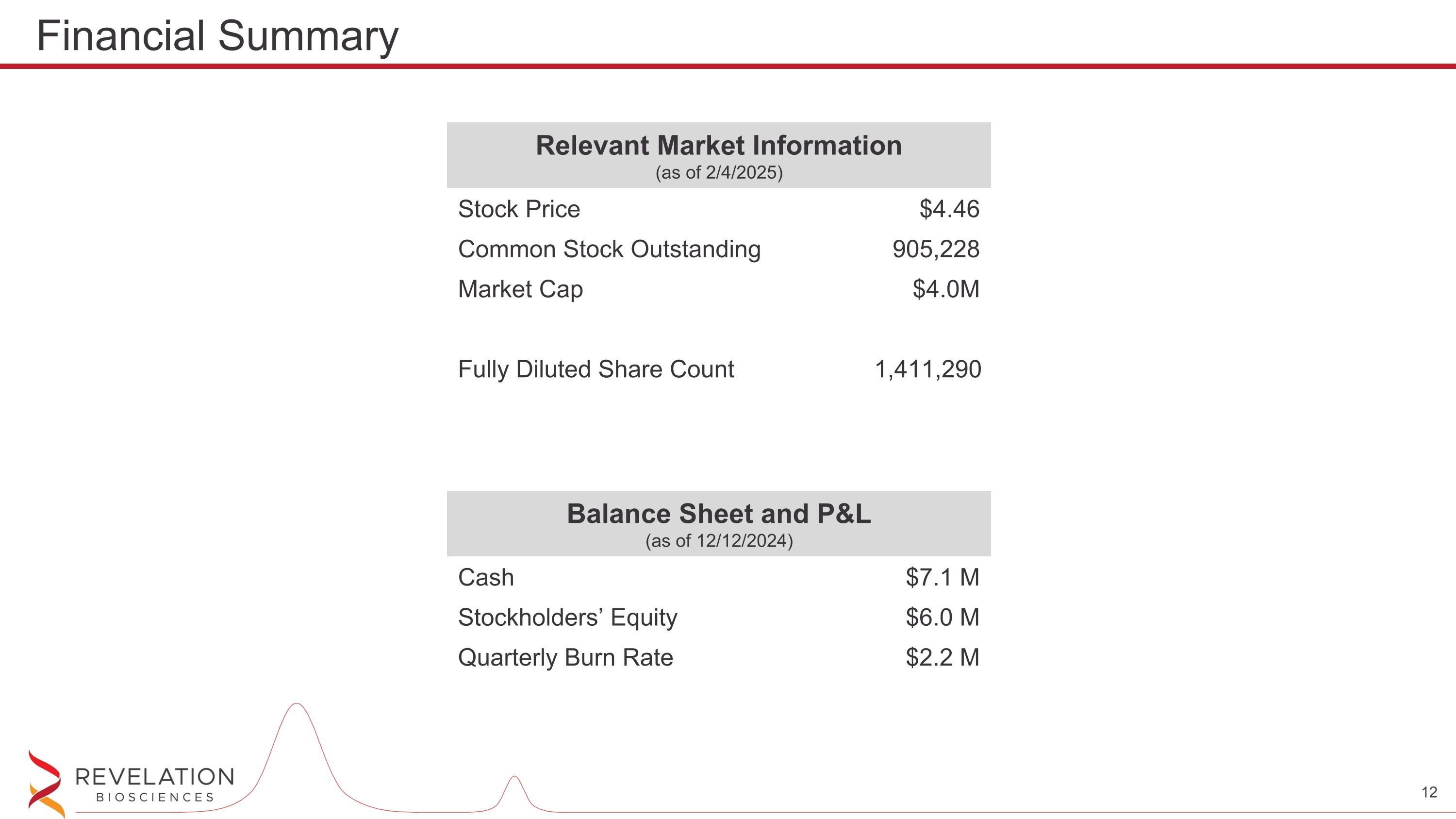
Relevant Market Information (as of 2/4/2025) Stock Price $4.46 Common Stock Outstanding 905,228 Market Cap $4.0M Fully Diluted Share Count 1,411,290 Financial Summary Balance Sheet and P&L (as of 12/12/2024) Cash $7.1 M Stockholders’ Equity $6.0 M Quarterly Burn Rate $2.2 M
Enormous market potential ($18+ Billion) Experienced Management with multiple FDA approvals (8 approvals) Rapidly derisking the Gemini platform Product candidates that can significantly help many people Why You Should Invest in REVB

Thank You! For more information, please visit www.revbiosciences.com 14

Supplemental Information 15
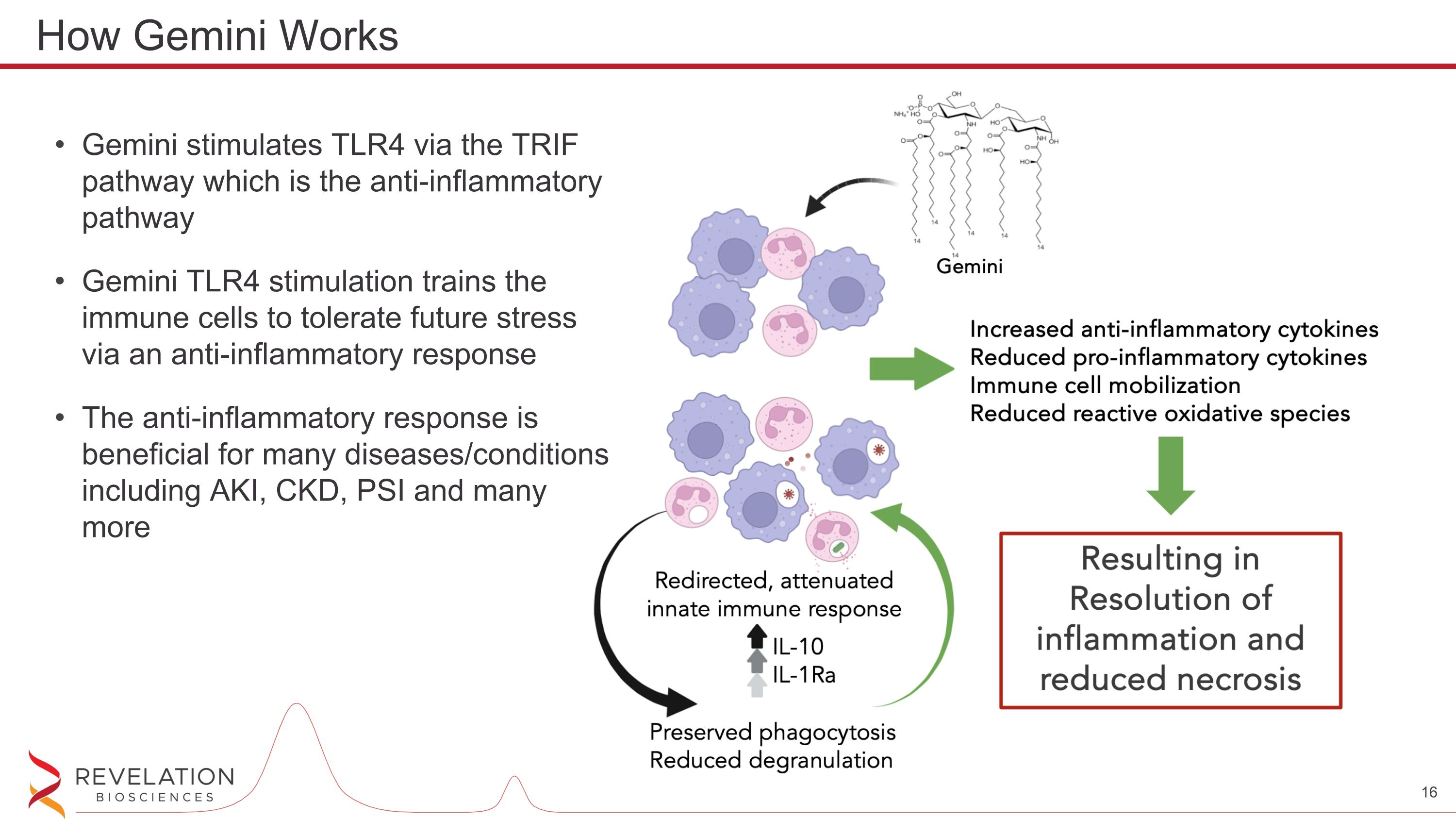
Gemini stimulates TLR4 via the TRIF pathway which is the anti-inflammatory pathway Gemini TLR4 stimulation trains the immune cells to tolerate future stress via an anti-inflammatory response The anti-inflammatory response is beneficial for many diseases/conditions including AKI, CKD, PSI and many more How Gemini Works
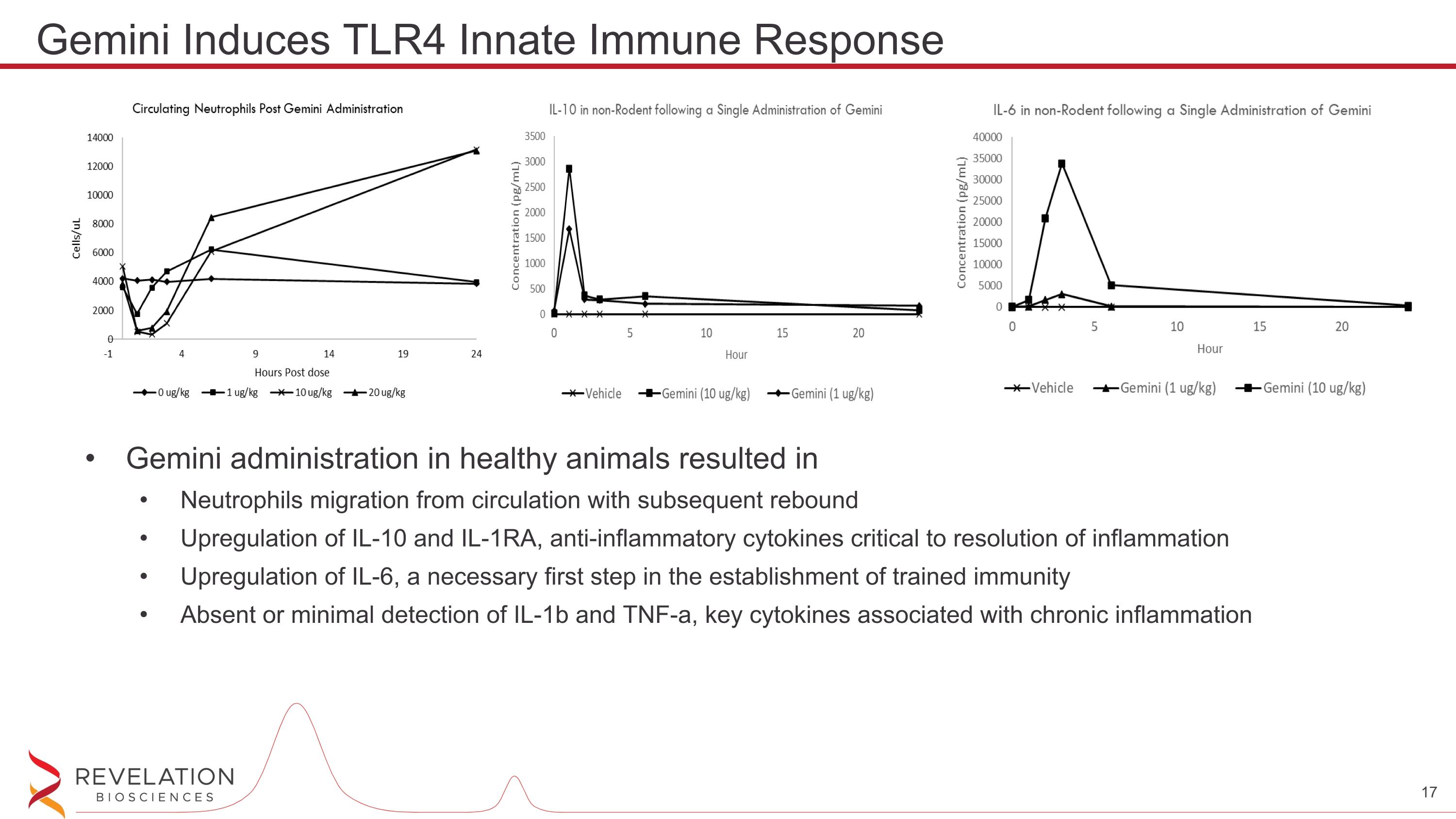
Gemini administration in healthy animals resulted in Neutrophils migration from circulation with subsequent rebound Upregulation of IL-10 and IL-1RA, anti-inflammatory cytokines critical to resolution of inflammation Upregulation of IL-6, a necessary first step in the establishment of trained immunity Absent or minimal detection of IL-1b and TNF-a, key cytokines associated with chronic inflammation Gemini Induces TLR4 Innate Immune Response
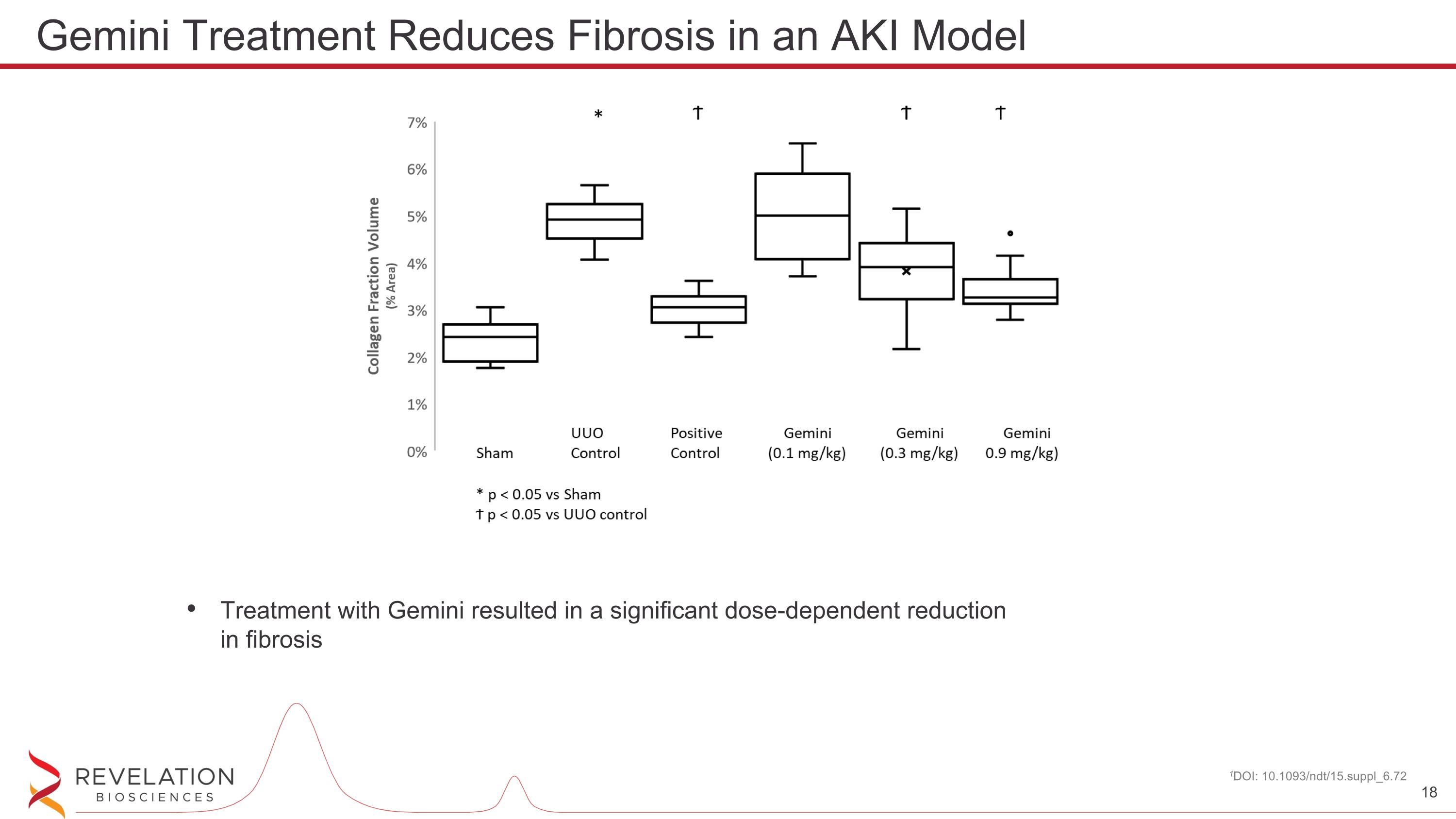
Treatment with Gemini resulted in a significant dose-dependent reduction in fibrosis 1DOI: 10.1093/ndt/15.suppl_6.72 Gemini Treatment Reduces Fibrosis in an AKI Model
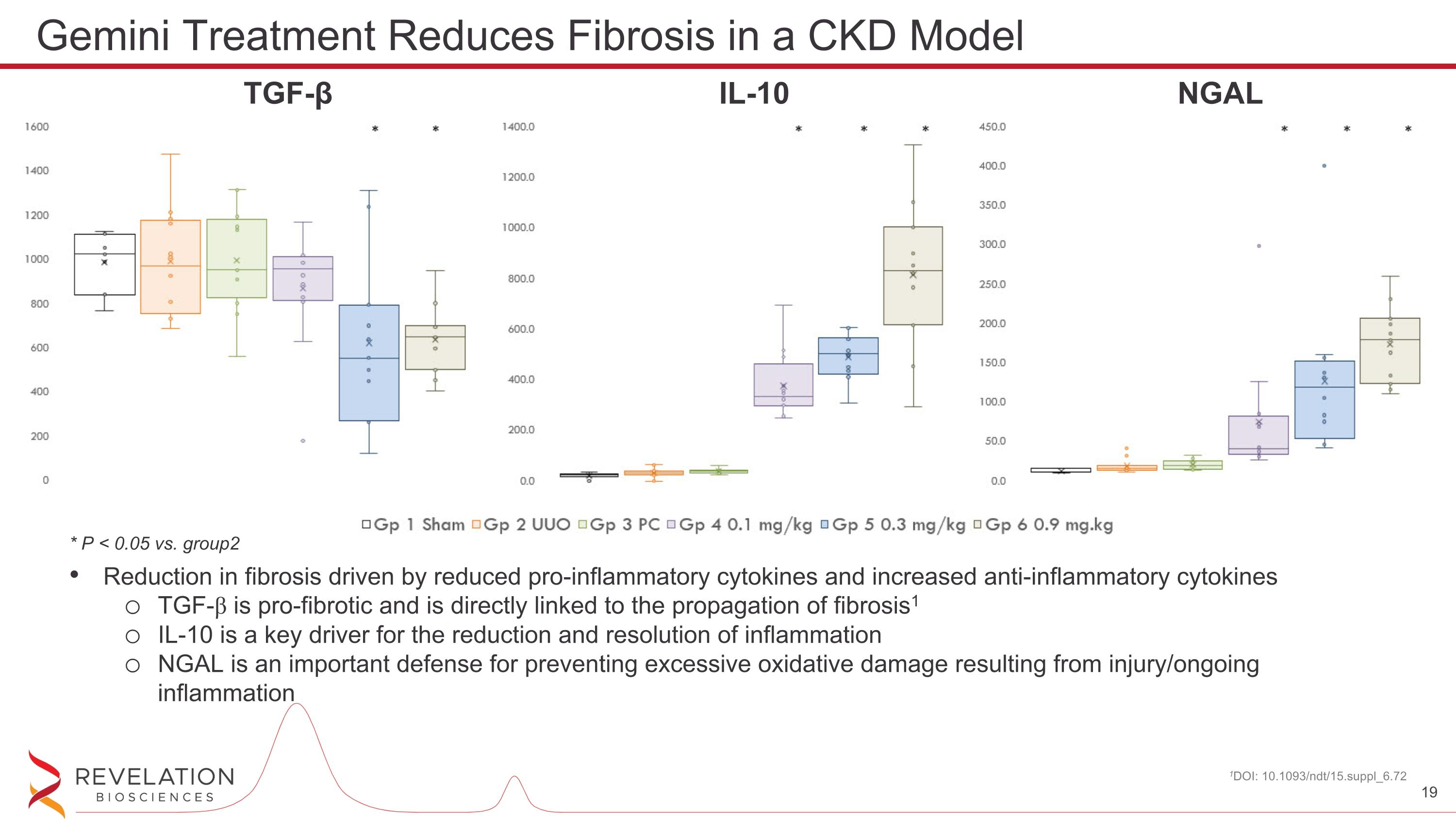
Reduction in fibrosis driven by reduced pro-inflammatory cytokines and increased anti-inflammatory cytokines TGF-β is pro-fibrotic and is directly linked to the propagation of fibrosis1 IL-10 is a key driver for the reduction and resolution of inflammation NGAL is an important defense for preventing excessive oxidative damage resulting from injury/ongoing inflammation 1DOI: 10.1093/ndt/15.suppl_6.72 Gemini Treatment Reduces Fibrosis in a CKD Model * P < 0.05 vs. group2 TGF-β IL-10 NGAL
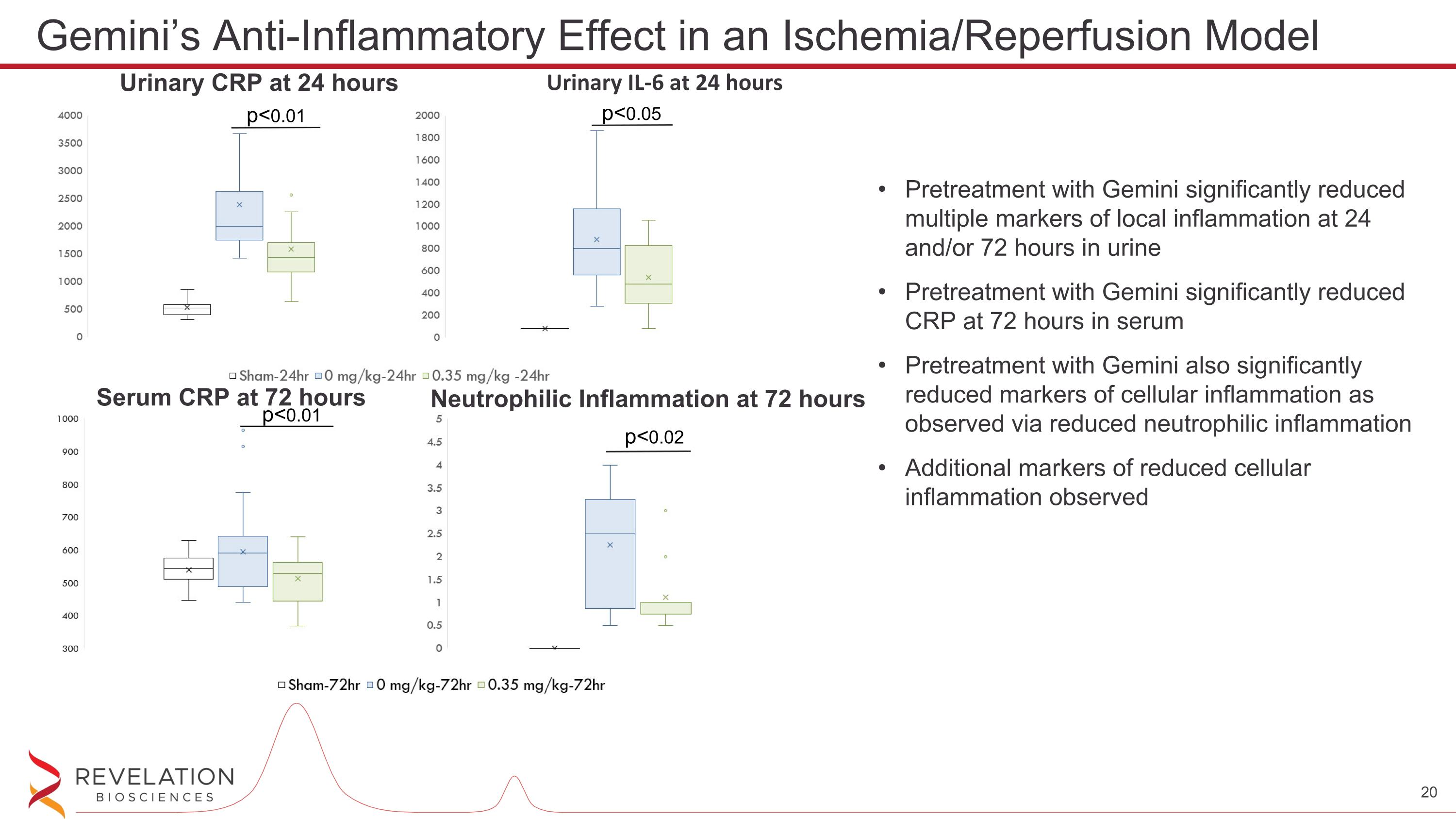
Pretreatment with Gemini significantly reduced multiple markers of local inflammation at 24 and/or 72 hours in urine Pretreatment with Gemini significantly reduced CRP at 72 hours in serum Pretreatment with Gemini also significantly reduced markers of cellular inflammation as observed via reduced neutrophilic inflammation Additional markers of reduced cellular inflammation observed Gemini’s Anti-Inflammatory Effect in an Ischemia/Reperfusion Model Urinary CRP at 24 hours Urinary IL-6 at 24 hours p<0.01 p<0.05 Neutrophilic Inflammation at 72 hours Serum CRP at 72 hours p<0.01 p<0.02
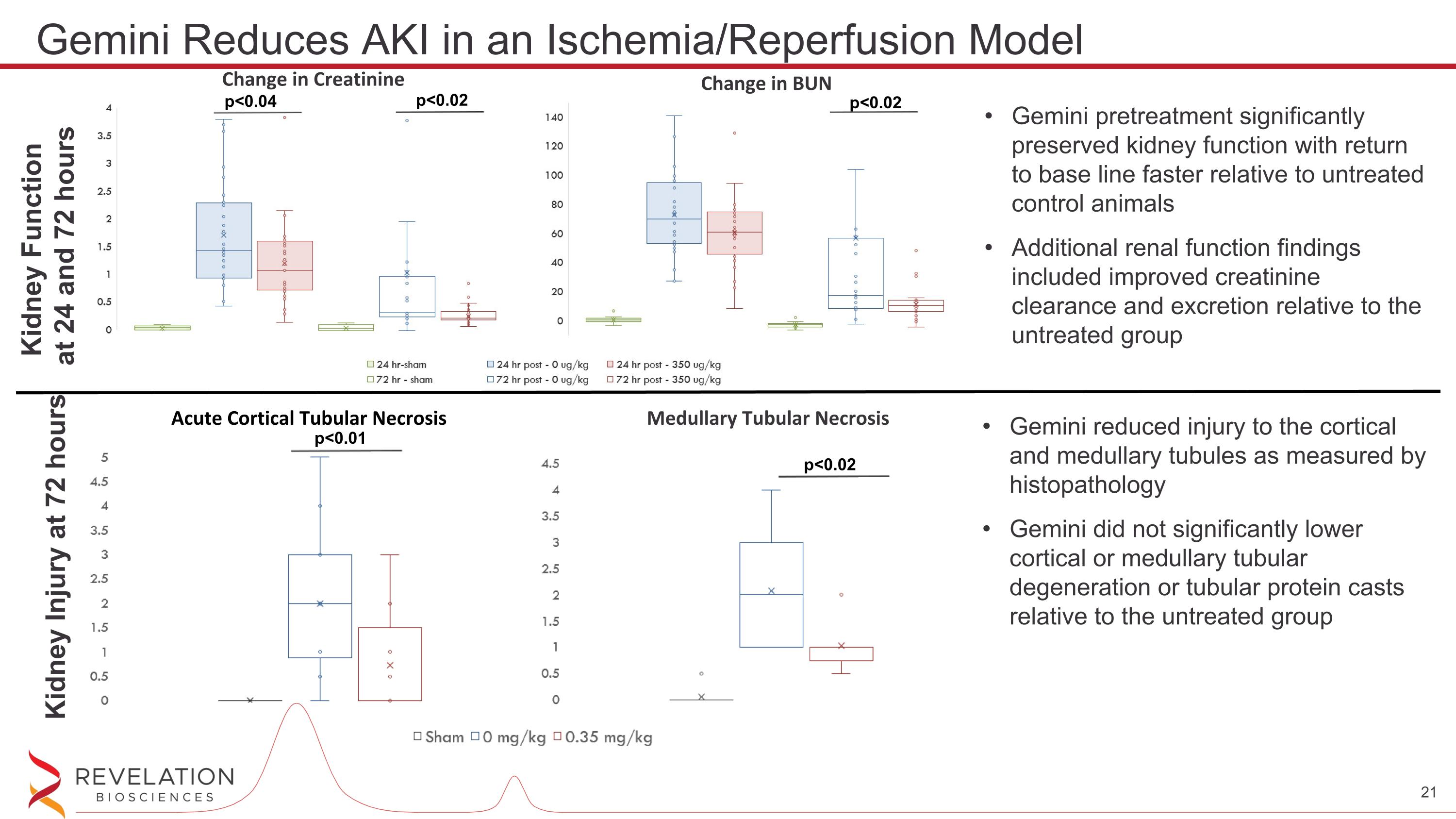
Kidney Function at 24 and 72 hours Kidney Injury at 72 hours Gemini pretreatment significantly preserved kidney function with return to base line faster relative to untreated control animals Additional renal function findings included improved creatinine clearance and excretion relative to the untreated group Gemini reduced injury to the cortical and medullary tubules as measured by histopathology Gemini did not significantly lower cortical or medullary tubular degeneration or tubular protein casts relative to the untreated group Change in BUN Change in Creatinine p<0.02 p<0.02 p<0.04 Acute Cortical Tubular Necrosis Medullary Tubular Necrosis p<0.02 p<0.01 Gemini Reduces AKI in an Ischemia/Reperfusion Model
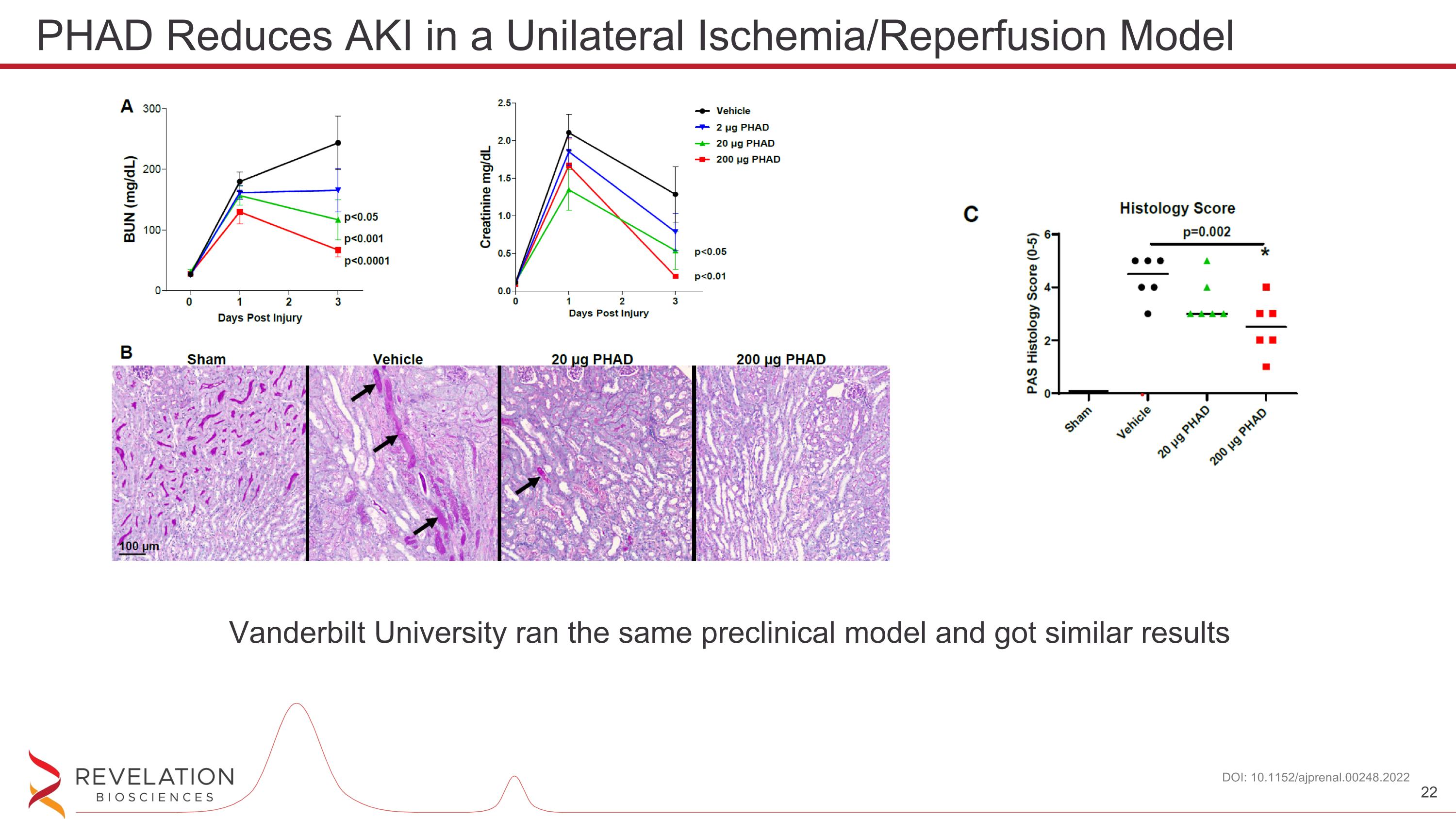
PHAD Reduces AKI in a Unilateral Ischemia/Reperfusion Model DOI: 10.1152/ajprenal.00248.2022 Vanderbilt University ran the same preclinical model and got similar results
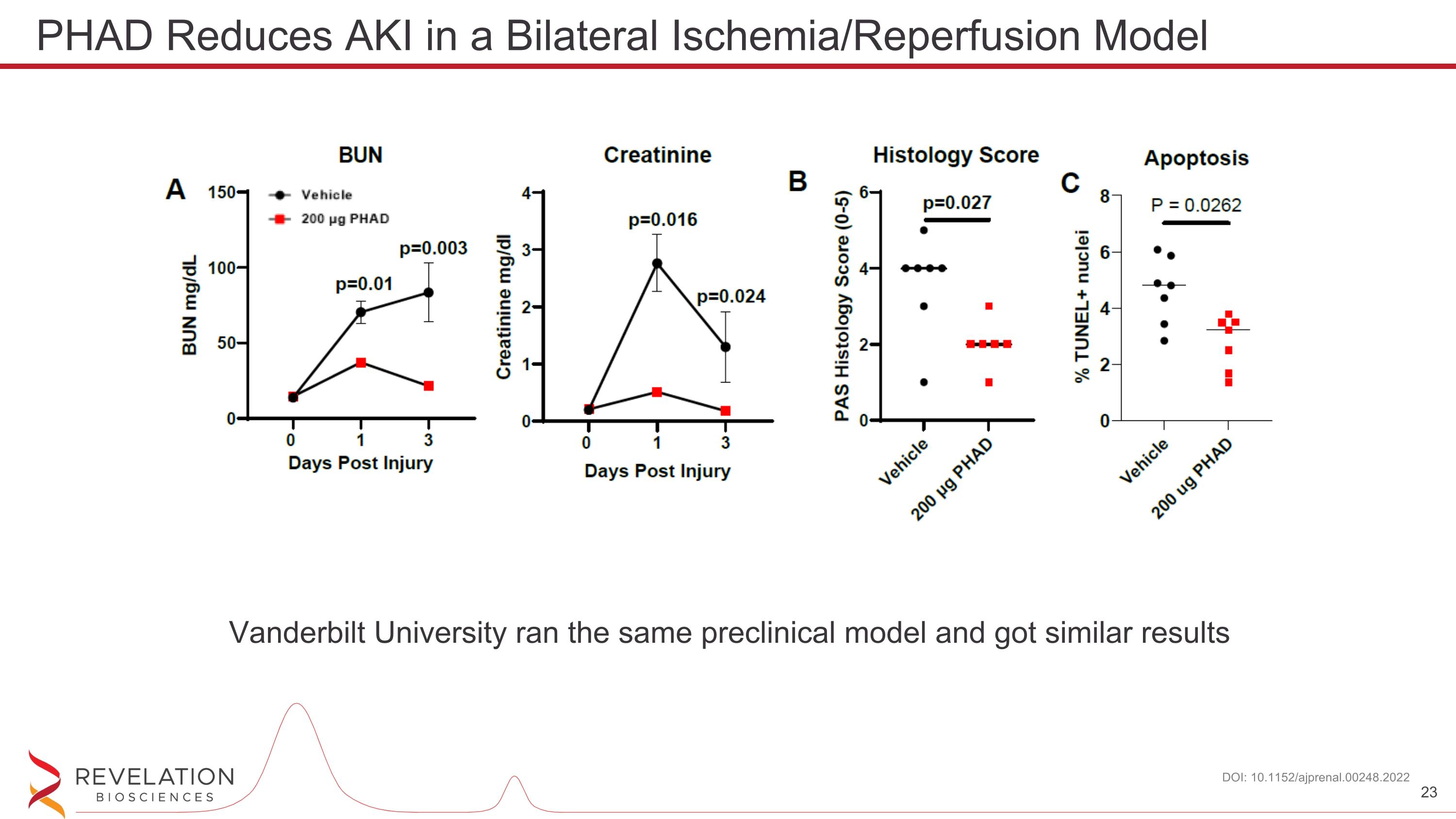
PHAD Reduces AKI in a Bilateral Ischemia/Reperfusion Model DOI: 10.1152/ajprenal.00248.2022 Vanderbilt University ran the same preclinical model and got similar results