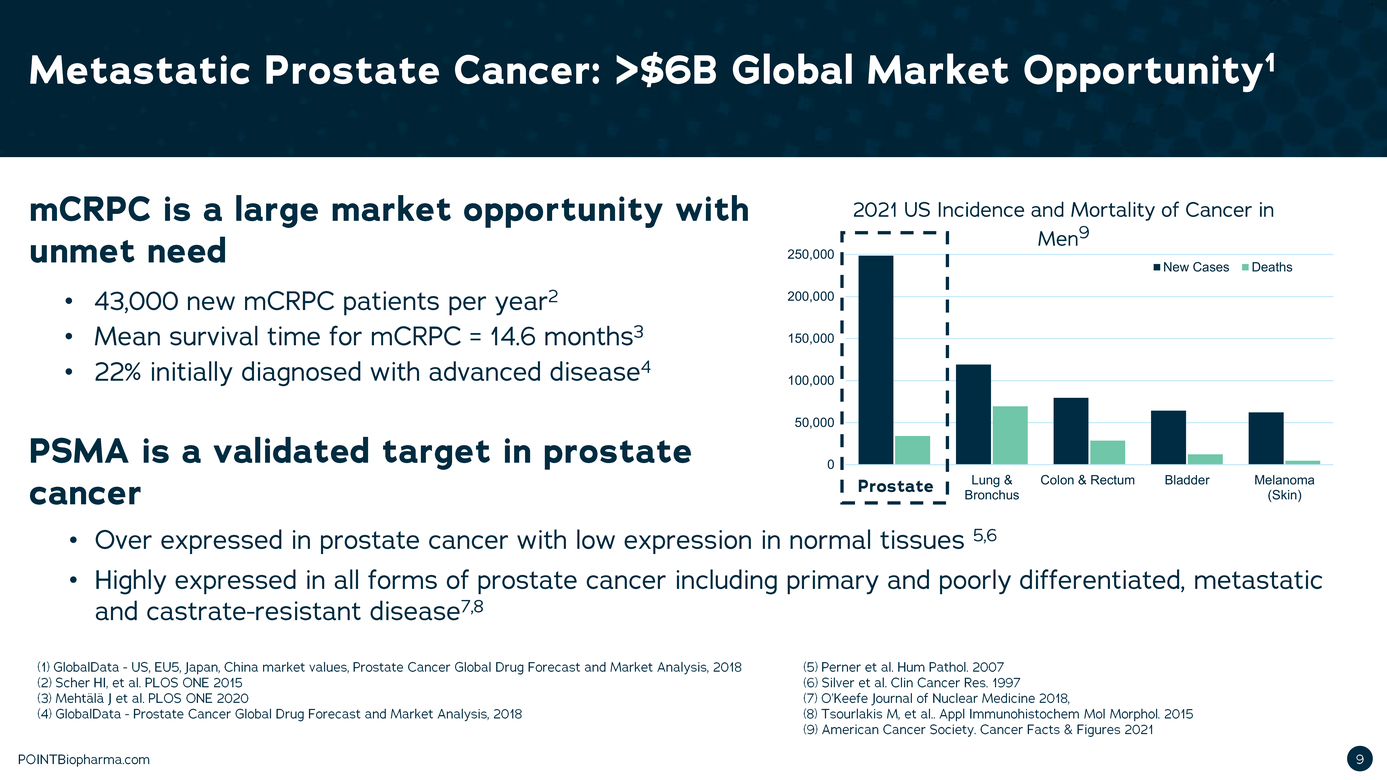
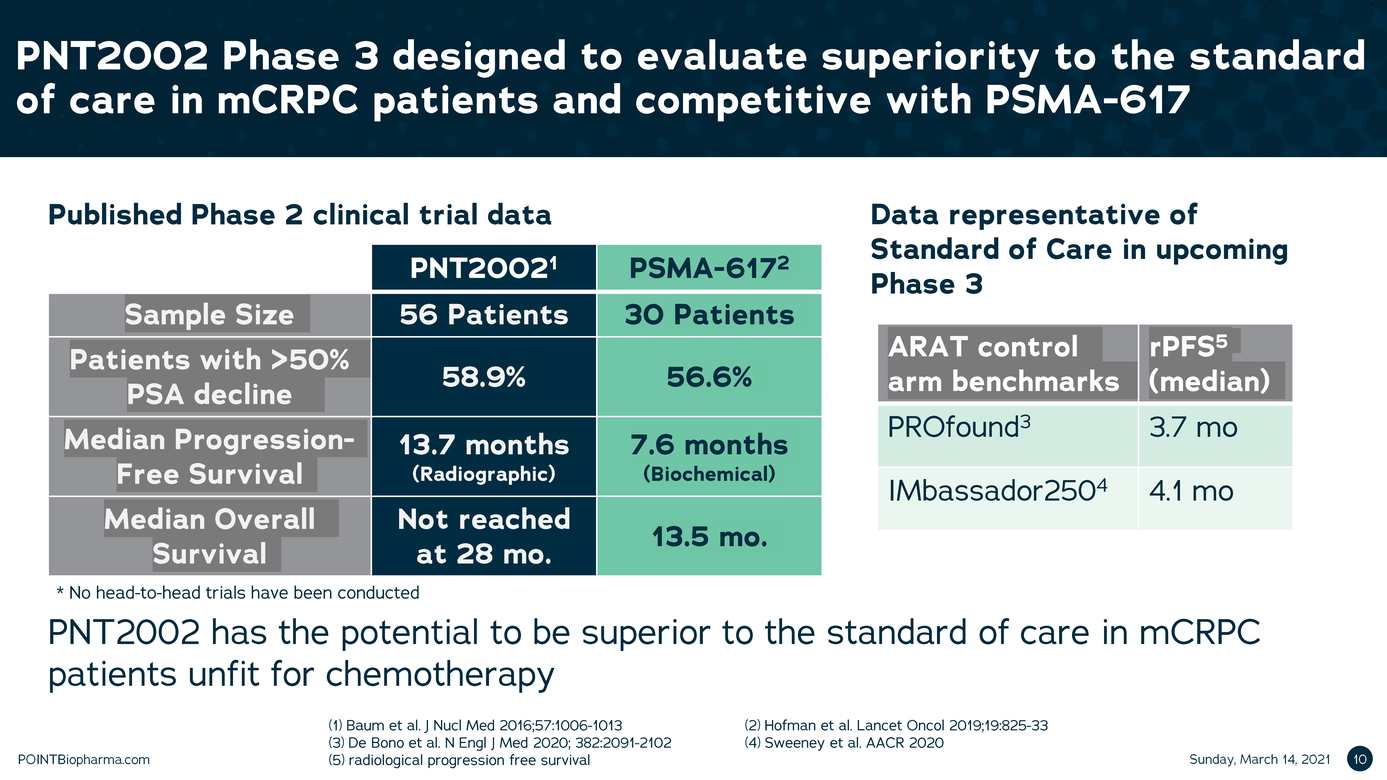
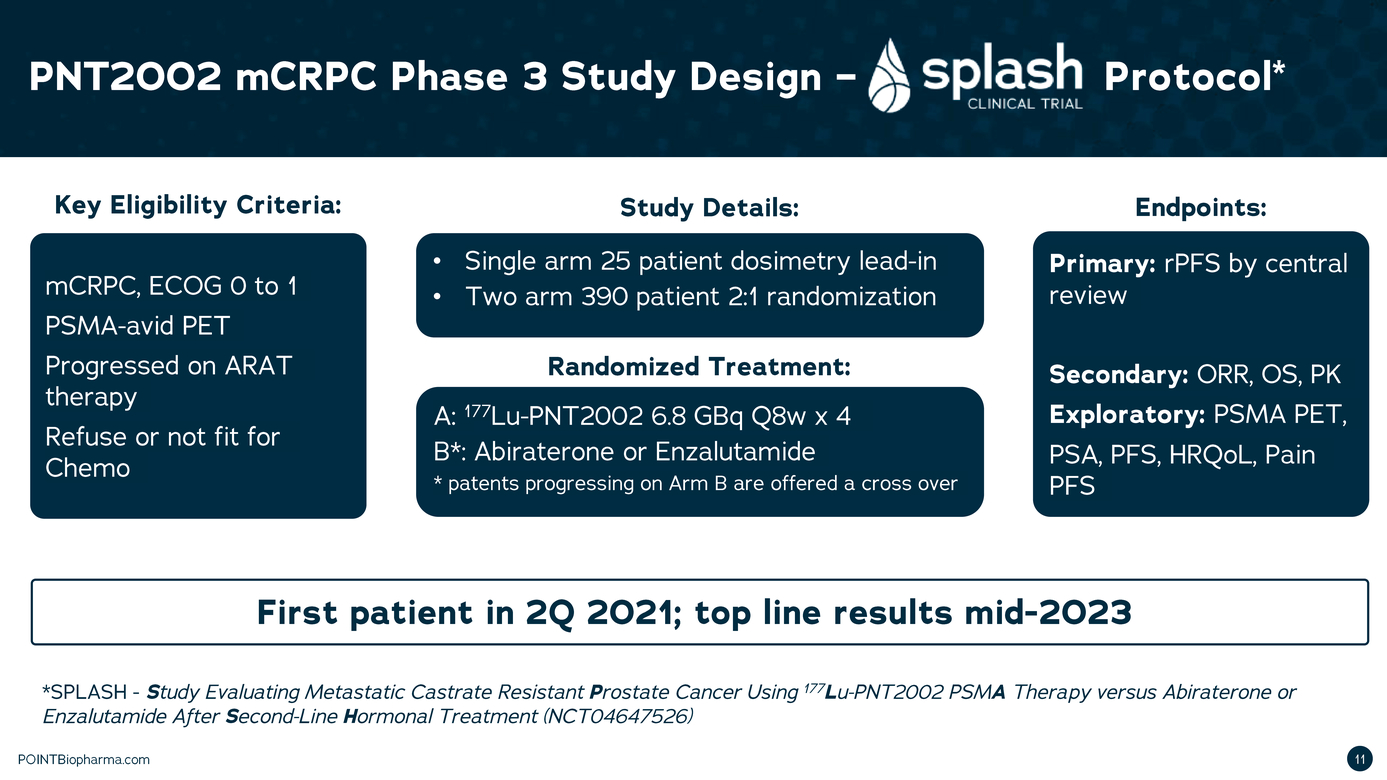
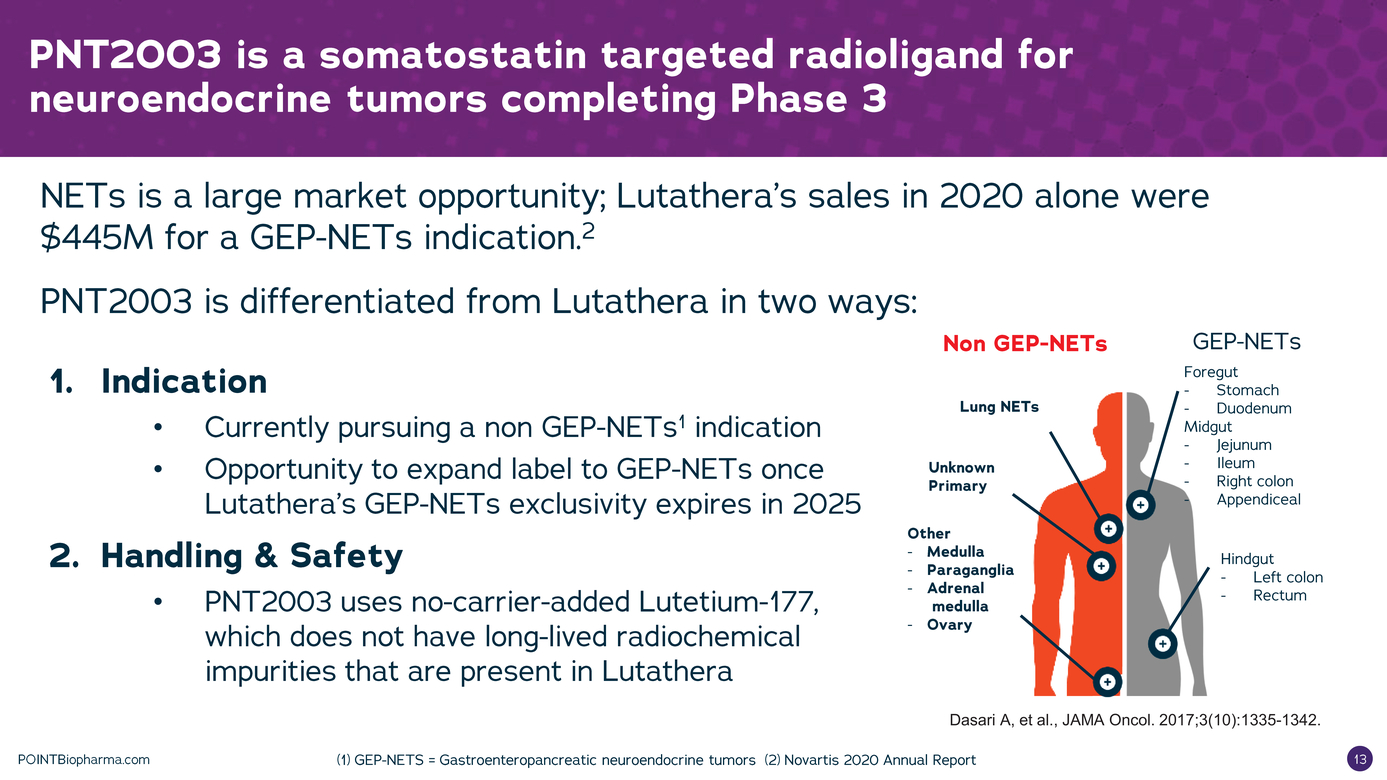
| Legal Disclaimer Except as otherwise indicated or unless the context otherwise requires, all references 1n this presentation to "we," "our," "us," "POINT," or the "Company " refer to POINT B1opharma Inc. The information contained in this presentation is submitted on a confidential basis solely for the recipient's use and the recipient agrees to maintain the confidentiality of t he information contained herein and not to reproduce or distribute any confidential information contained herein to any third party without our express written authorization T his information contained 1n this presentation does not constitute or form part of and should not be construed as, any offer for sale or subscription of, or any invitation to offer, buy or subscribe for, any securities, nor shall there be any offer, sol icitation or sale in any jurisd iction in which such offer, solic itation or sale would be unlawful. Forward Looking Statements. Certain statements in this presentation may be considered forward-look ng statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in t his presentation, we caution you that these statements are based on a combination of facts and fac tors currently known by us and our projections of t he future, about wh ich we cannot be certain. Forward-look ing statements generally relate to future events or the Company's future financial or operating performance. For example, statements concerning the following include forward-looking stateme nts: the success, cost and tim ng of product development activit ies, 1nclud ng timing of 1n1t1at1on, completion and data readouts for clinical trials and preclinical studies; the potential attributes and benefits of product candidates, 1nclud1ng with respect to radio igand select1v1ty, activity, side effect and tolerab1l1ty profile and relevant indications; ability to compete with other companies currently marketing or engaged in the development of treatments for relevant indicat ions; the Company's ex pected manufactur ing and commercial strategy; the size and growth potential of the markets for product candidates and ability to serve those markets; and the rate and degree of market acceptance of product candidates, if approved. In some c ases you can identify forw ard-look ing statements by terminology such as "may", "should", "expect", "intend", "will", "estimate", "anticipate", "believe","predict ", "potential", or "continue",or the negatives of these terms or variations of them or similar term inology. Such forward looking statements are subject to risks, uncertainties, and other factors which could cause actual results to d iffer materially from those ex pressed or imp ied by such forward-looking statements. These forward-look ing statements are based upon estimates and assumption that, while considered reasonable by POINT and its management, are inherently uncertain. New risks and uncerta nt1 es may emerge from t me to time,and it is not possible to predict all risks and uncerta1nt1es Factors that may cause actual results to differ materially from current expectations include, but are not l1m1ted to, factors associated with compan ies, such as the Company, that are engaged in clinical tr ials 1n the pharmaceutical industry, including uncertainty in the timing or results of clinical trials, product acceptance and/or receipt of regulatory approvals for product candidates, risks related to POINT's ability to successfully develop and launch a commercial product and achieve market acceptance, raise additional funds that may be necessary for the operatio ns of its business and product deve lopment, establish manufacturing capac ity, manage its operations and potential growth and scale its business, protect its intellectual property, and the potential impact of the COVID-19 pandemic, as well as var ious other factors beyond management's control, 1nclud1ng ge neral economic conditions Nothing n this presentation should be regarded as a representation by any person that the forward-looking statements set forth here in will be ac hieved or that any of the contemplated results of such forward-looking statements w i l be achieved. You should not place undue reliance on forward-looking statements 1n this presentation, whic h speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein The Company undertakes no duty to update these forward-looking statements. Important Information About the Business Combination and Where to Find It A full description of the terms of the business combination will be provided in a registration statement on Form S-4 to be filed with the SEC by Therapeutics Acquis ition Corp. d/ b/a/ Research Alliance Corp. L C'RACA"J that w ill inc ude a prospectus with respect to the Combined Company's securities to be issued in connection w ith the business combination and a prox y statement w ith respect to the share holder meeting of RAC A to vote on the business combinat ion. RACA urges its investors, shareholders and other interested persons to read, when available, the preliminary proxy statement/ prospectus as well as other documents filed with the SEC because these documents will contain important information about RACA, POINT and the business combination. After the registration statement is declared effective, t he definitive proxy statement/prospectus to be included in the registration statement w ill be mailed to shareholders of RACA as of a record date to be established for voting on the proposed business combination. Once available, shareholders will also be able to obtain a copy of the S-4, includ ng the prox y statement/prospectus, and other documents f iled with the SEC without charge, by directing a request to: Research Alliance Corp. I, Attn: Secretary, 200 Berkeley St, 18th f loor, Boston, MA 02116. The preliminary and definitive proxy statement/prospectus to be ncluded in the registrat ion statement, once available, can also be obtained, without charge, at the SEC's website (www.sec.govl. Participants in the Solicitation RACA and POINT and their respective directors and executive officers may be considered partic ipants in the solicitation of prox ies with respect to the proposed business combination described in this press release under the rules of the SEC Information about the directors and executive officers of RACA is set forth 1n RACA's final prospectus for nitial public offering filed with the SEC pursuant to Rule 424(b ) of the Securities Act of 1933, as amended (the "Securities Act") on July 9, 2020, and is available free of charge at the SEC's website at www.sec.gov or by directing a request to: Research Alliance Corp. L Attn: Secretary, 200 Berkeley St, 18th floor,Boston,MA 02116. Informat on regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of the RACA shareholders in connection with the proposed business combination w ill be set forth in the registration statement containing the proxy statement/prospectus for the proposed business combination when it is filed with the SEC These documents can be obtained free of c harge from the sources indicated above. Non-Solicitation This presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed business combination and sha l not const itute an offer to se l or a solicitation of an offer to buy any securities nor shall there be any sale of securities in any state or jurisdiction in w hich such offer, solicitation, or sale w ould be unlawful prior to registration or qualification under the secur it ies law s of any such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requireme nts of the Securities Act POINTBiopharma.com 8 |