
Analyst & Investor Event June 27, 2022 Exhibit 99.2

Disclaimer This presentation contains forward-looking statements and information about our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ “opportunity,” ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include express or implied statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates; the timing of disclosures regarding our pipeline and additional clinical data for RLY-4008 and initial clinical data for RLY-2608; the potential therapeutic benefits of our product candidates, including potential efficacy and tolerability, and combination potential of our product candidates; whether preliminary results from our preclinical or clinical trials will be predictive of the final results of the trials or any future clinical trials of our product candidates; the possibility that unconfirmed results from these trials will not be confirmed by additional data as the clinical trials progress; the competitive landscape and market opportunities for our product candidates; the expected strategic benefits under our collaborations; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; expectations regarding current and future interactions with the U.S. Food and Drug Administration (FDA); our ability to manufacture our product candidates in conformity with the FDA’s requirements; the capabilities and development of our DynamoTM platform; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; our plans to develop, manufacture and commercialize our current product candidates and any future product candidates; and the implementation of our business model and operating plans as well as the strategic plans for our business, current product candidates and any future product candidates. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our most recent Annual Report on Form 10-K or most recent Quarterly Report on Form 10-Q, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners.
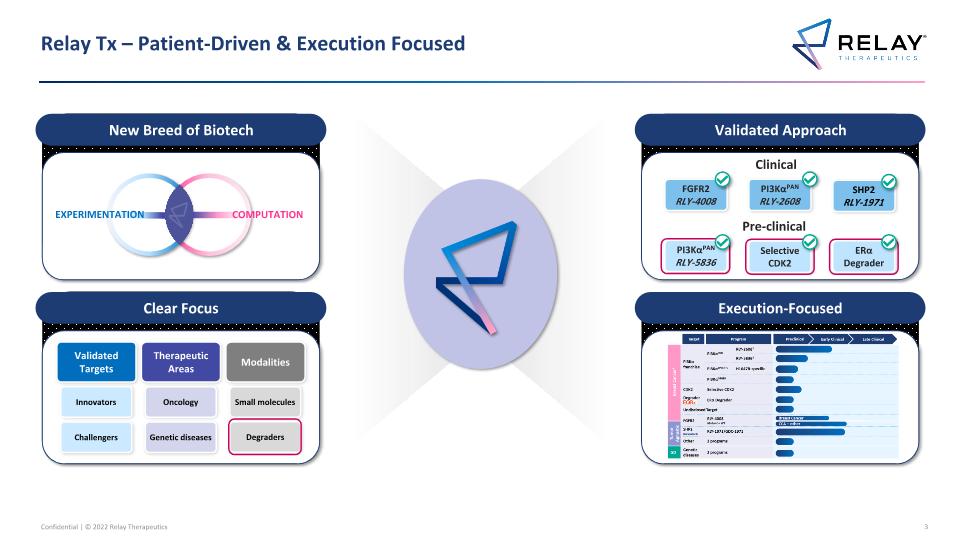
Relay Tx – Patient-Driven & Execution Focused Clear Focus Validated Targets Therapeutic Areas Modalities Small molecules Oncology Genetic diseases Challengers Innovators Validated Approach PI3KαPAN RLY-2608 FGFR2 RLY-4008 PI3KαPAN RLY-5836 Execution-Focused Selective CDK2 SHP2 RLY-1971 ERα Degrader Degraders New Breed of Biotech EXPERIMENTATION COMPUTATION Clinical Pre-clinical
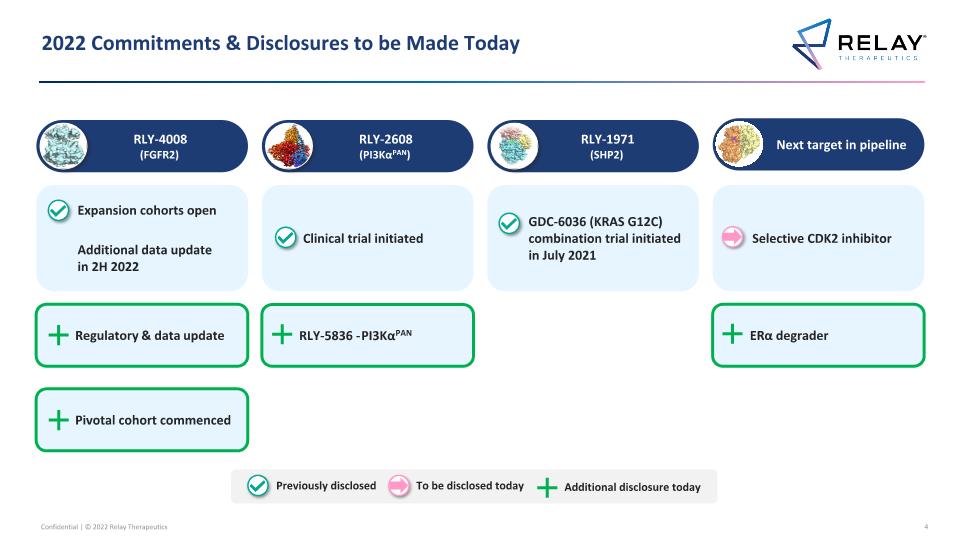
2022 Commitments & Disclosures to be Made Today RLY-2608 (PI3KαPAN) RLY-4008 (FGFR2) Next target in pipeline RLY-1971 (SHP2) Selective CDK2 inhibitor Expansion cohorts open Additional data update in 2H 2022 Clinical trial initiated GDC-6036 (KRAS G12C) combination trial initiated in July 2021 ERα degrader Regulatory & data update Previously disclosed To be disclosed today Additional disclosure today RLY-5836 - PI3KαPAN Pivotal cohort commenced
Future Guidance and Q&A 4 Agenda Overview of DynamoTM Platform 3 Breast Cancer Portfolio 2 RLY-4008 Regulatory Update 1
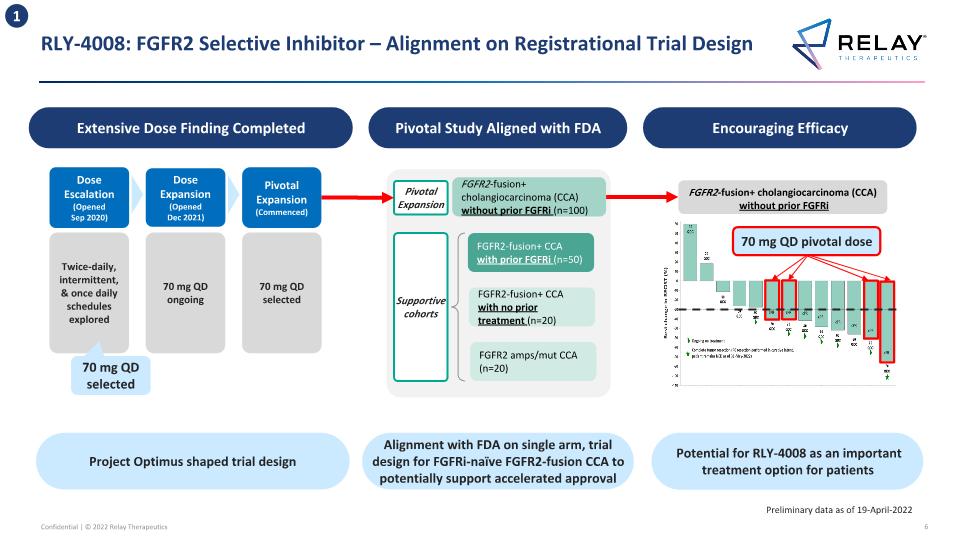
RLY-4008: FGFR2 Selective Inhibitor – Alignment on Registrational Trial Design Preliminary data as of 19-April-2022 Potential for RLY-4008 as an important treatment option for patients Alignment with FDA on single arm, trial design for FGFRi-naïve FGFR2-fusion CCA to potentially support accelerated approval Encouraging Efficacy Pivotal Study Aligned with FDA FGFR2-fusion+ cholangiocarcinoma (CCA) without prior FGFRi (n=100) FGFR2-fusion+ CCA with prior FGFRi (n=50) FGFR2-fusion+ CCA with no prior treatment (n=20) FGFR2 amps/mut CCA (n=20) Supportive cohorts Pivotal Expansion Extensive Dose Finding Completed Project Optimus shaped trial design FGFR2-fusion+ cholangiocarcinoma (CCA) without prior FGFRi 70 mg QD pivotal dose 1 Dose Escalation (Opened Sep 2020) Dose Expansion (Opened Dec 2021) Pivotal Expansion (Commenced) Twice-daily, intermittent, & once daily schedules explored 70 mg QD ongoing 70 mg QD selected 70 mg QD selected
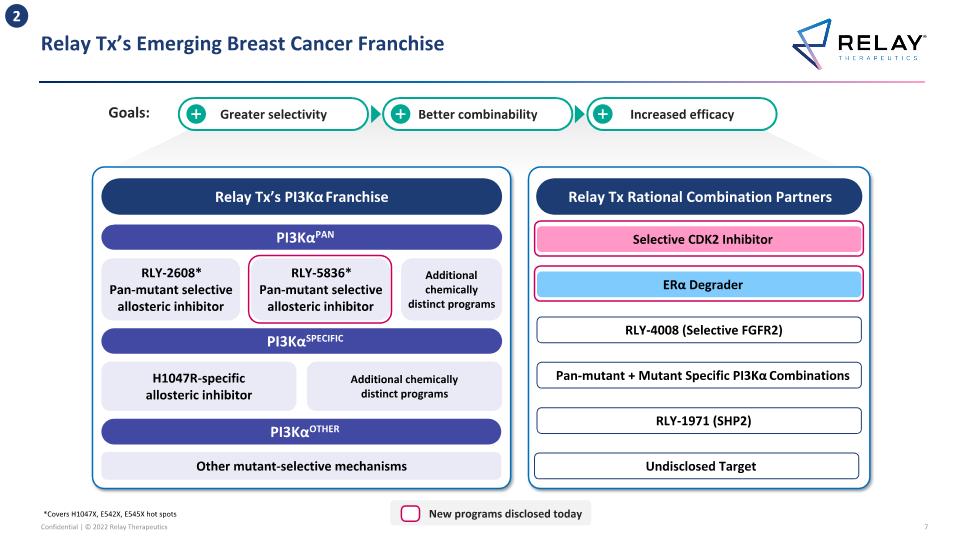
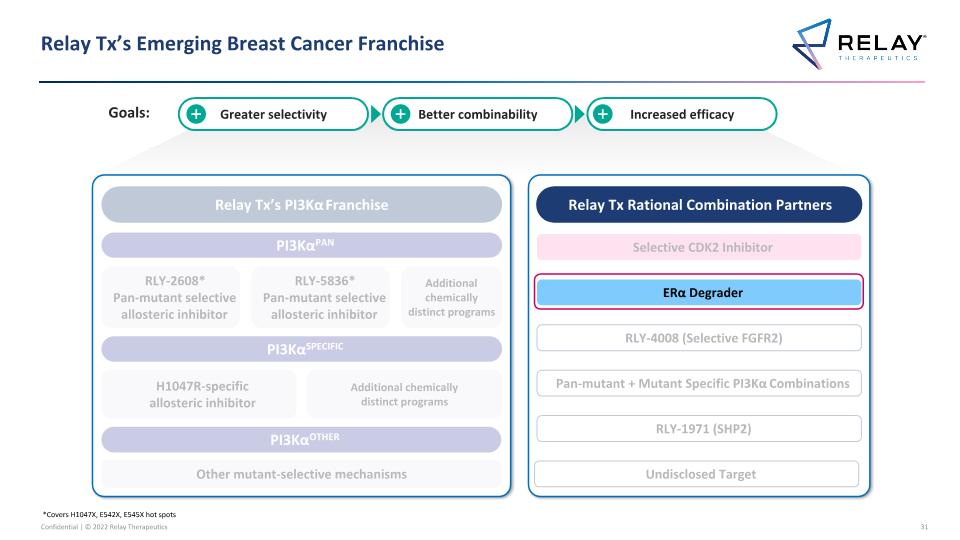
Relay Tx’s Emerging Breast Cancer Franchise *Covers H1047X, E542X, E545X hot spots 2 Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 Inhibitor ERα Degrader RLY-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Greater selectivity Better combinability Increased efficacy Undisclosed Target New programs disclosed today Goals:
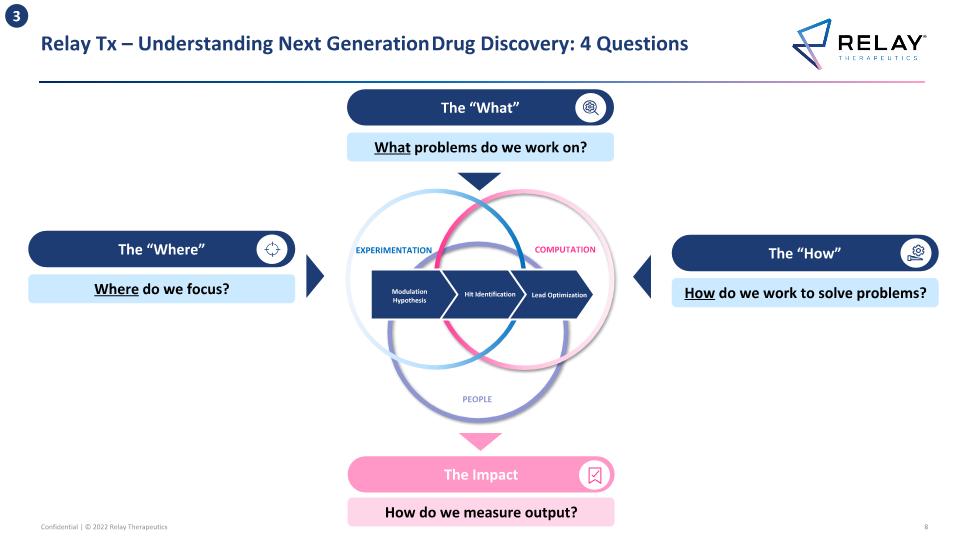
Relay Tx – Understanding Next Generation Drug Discovery: 4 Questions EXPERIMENTATION COMPUTATION PEOPLE Modulation Hypothesis Hit Identification Lead Optimization The “How” How do we work to solve problems? The “What” What problems do we work on? The “Where” Where do we focus? The Impact How do we measure output? 3
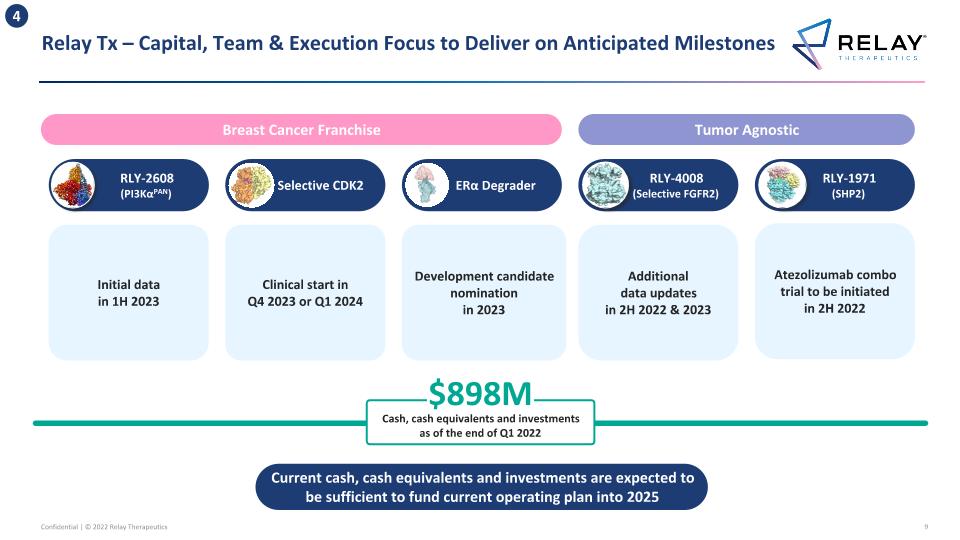
Relay Tx – Capital, Team & Execution Focus to Deliver on Anticipated Milestones 4 RLY-4008 (Selective FGFR2) Clinical start in Q4 2023 or Q1 2024 Selective CDK2 RLY-1971 (SHP2) Atezolizumab combo trial to be initiated in 2H 2022 RLY-2608 (PI3KαPAN) $898M Cash, cash equivalents and investments as of the end of Q1 2022 Development candidate nomination in 2023 ERα Degrader Tumor Agnostic Breast Cancer Franchise Additional data updates in 2H 2022 & 2023 Current cash, cash equivalents and investments are expected to be sufficient to fund current operating plan into 2025 Initial data in 1H 2023
Future Guidance and Q&A 4 Agenda Overview of DynamoTM Platform 3 Breast Cancer Portfolio 2 RLY-4008 Regulatory Update 1
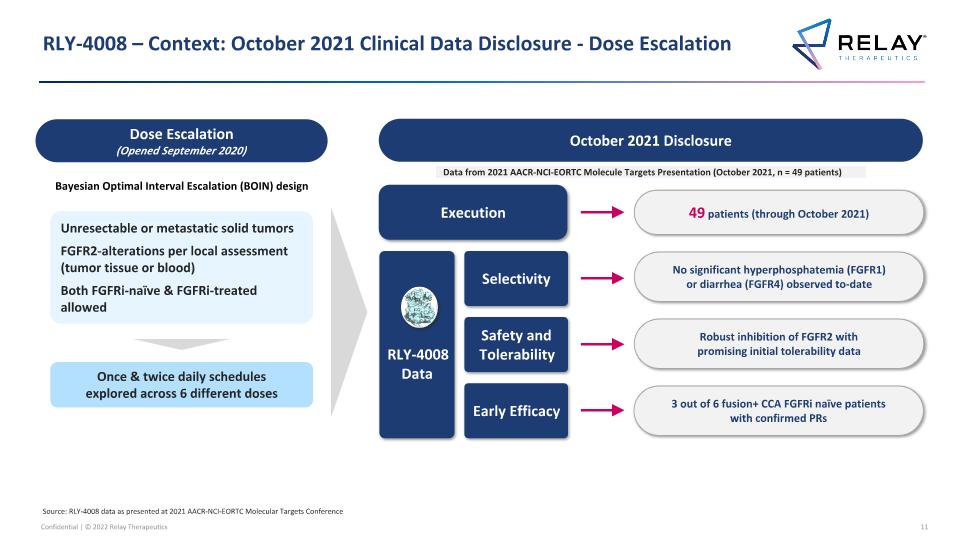
RLY-4008 – Context: October 2021 Clinical Data Disclosure - Dose Escalation Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Data from 2021 AACR-NCI-EORTC Molecule Targets Presentation (October 2021, n = 49 patients) Execution 49 patients (through October 2021) October 2021 Disclosure Robust inhibition of FGFR2 with promising initial tolerability data Safety and Tolerability 3 out of 6 fusion+ CCA FGFRi naïve patients with confirmed PRs Early Efficacy No significant hyperphosphatemia (FGFR1) or diarrhea (FGFR4) observed to-date Selectivity RLY-4008 Data Unresectable or metastatic solid tumors FGFR2-alterations per local assessment (tumor tissue or blood) Both FGFRi-naïve & FGFRi-treated allowed Bayesian Optimal Interval Escalation (BOIN) design Dose Escalation (Opened September 2020) Once & twice daily schedules explored across 6 different doses
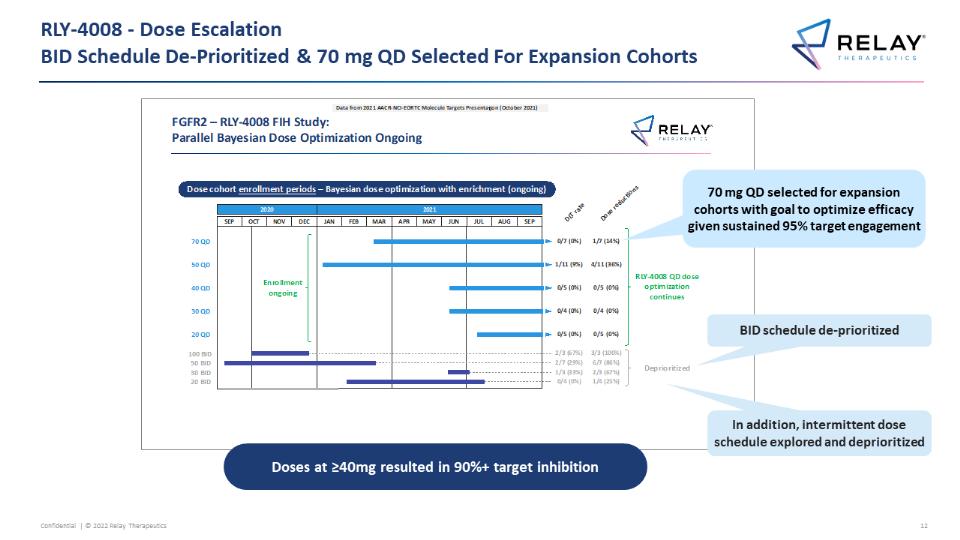
RLY-4008 - Dose Escalation�BID Schedule De-Prioritized & 70 mg QD Selected For Expansion Cohorts Data from 2021 AACR NCI-EOR TC Molecule Targets Presentation (October 2021) FGFR2 - RLY-4008 FIH Study: Parallel Bayesian Dose Optimization Ongoing Dose cohort enrollment periods - Bayesian dose optimization with enrichment (ongoing) 2020 2021 DLT rate Dose reductions SEP OCT NOV DEC JAN FEB MAR APR MAY JUN JUL AUG SEP 70 QD 50 QD 40 QD 30 QD 20 QD 100 BID 50 BID 30 BID 20 BID Enrollment ongoing 0/7 (0%) 1/7(14%) 1/11 (9%) 4/11 (36%) 0/5 (0%) 0/5 (0%) 0/4 (0%) 0/4 (0%) 0/5 (0%) 0/5 (0%) 2/3 (67%) 3/3 (100%) 2/7 (29%) 6/7 (86%) 1/3 (33%) 2/3 (67%) 0/4 (0%) 1/4 (25%) RLY-4008 QD dose optimization continues Deprioritized 70 mg QD selected for expansion cohorts with goal to optimize efficacy given sustained 95% target engagement BID schedule de-prioritized In addition, intermittent dose schedule explored and deprioritized Doses at ≥40mg resulted in 90%+ target inhibition
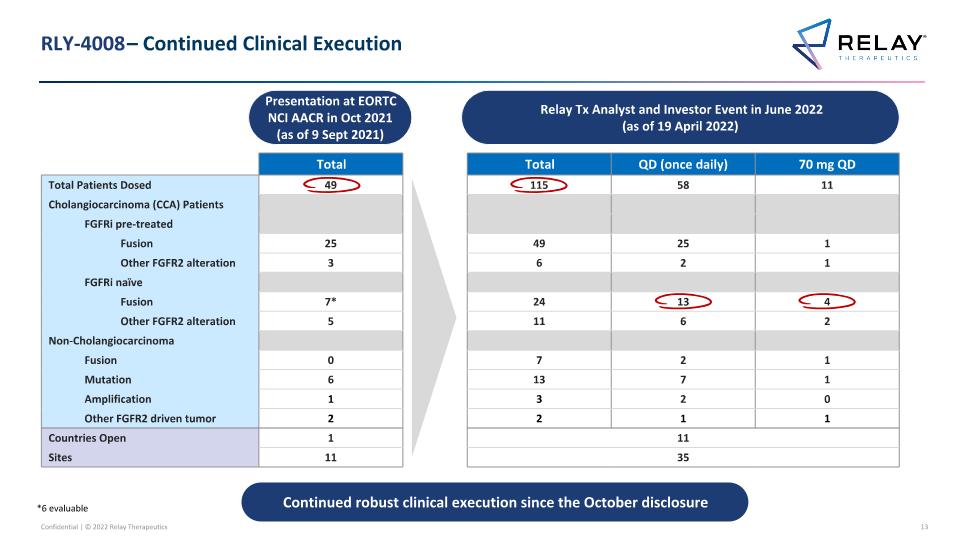
RLY-4008 – Continued Clinical Execution Presentation at EORTC NCI AACR in Oct 2021 (as of 9 Sept 2021) Total Total QD (once daily) 70 mg QD Total Patients Dosed 49 115 58 11 Cholangiocarcinoma (CCA) Patients FGFRi pre-treated Fusion 25 49 25 1 Other FGFR2 alteration 3 6 2 1 FGFRi naïve Fusion 7* 24 13 4 Other FGFR2 alteration 5 11 6 2 Non-Cholangiocarcinoma Fusion 0 7 2 1 Mutation 6 13 7 1 Amplification 1 3 2 0 Other FGFR2 driven tumor 2 2 1 1 Countries Open 1 11 Sites 11 35 Relay Tx Analyst and Investor Event in June 2022 (as of 19 April 2022) Continued robust clinical execution since the October disclosure *6 evaluable
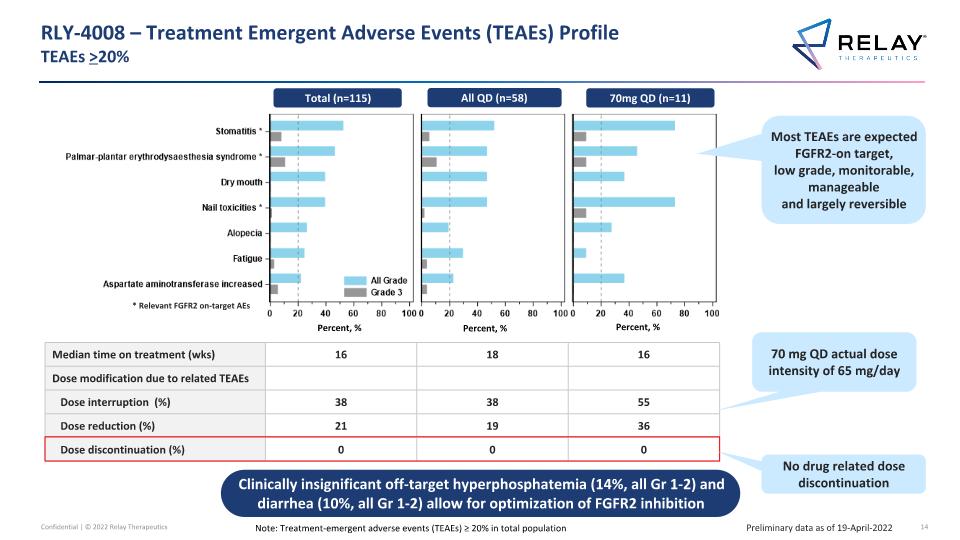
RLY-4008 – Treatment Emergent Adverse Events (TEAEs) Profile�TEAEs >20% Preliminary data as of 19-April-2022 Note: Treatment-emergent adverse events (TEAEs) ≥ 20% in total population Median time on treatment (wks) 16 18 16 Dose modification due to related TEAEs Dose interruption (%) 38 38 55 Dose reduction (%) 21 19 36 Dose discontinuation (%) 0 0 0 0 40 0 40 0 40 No drug related dose discontinuation 70 mg QD actual dose intensity of 65 mg/day Most TEAEs are expected FGFR2-on target, low grade, monitorable, manageable and largely reversible Percent, % Percent, % Percent, % * Relevant FGFR2 on-target AEs Total (n=115) All QD (n=58) 70mg QD (n=11) Clinically insignificant off-target hyperphosphatemia (14%, all Gr 1-2) and diarrhea (10%, all Gr 1-2) allow for optimization of FGFR2 inhibition
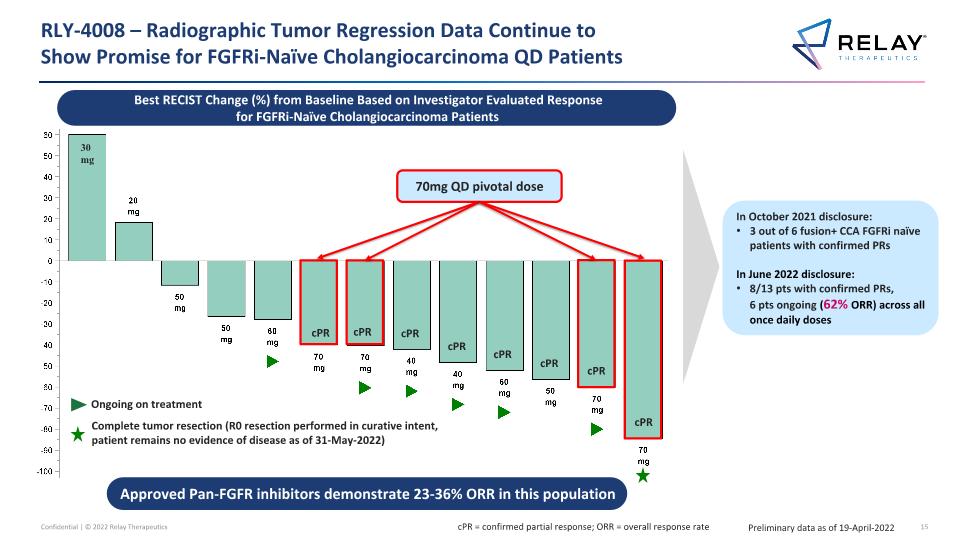
RLY-4008 – Radiographic Tumor Regression Data Continue to �Show Promise for FGFRi-Naïve Cholangiocarcinoma QD Patients Preliminary data as of 19-April-2022 In October 2021 disclosure: 3 out of 6 fusion+ CCA FGFRi naïve patients with confirmed PRs In June 2022 disclosure: 8/13 pts with confirmed PRs, 6 pts ongoing (62% ORR) across all once daily doses Best RECIST Change (%) from Baseline Based on Investigator Evaluated Response for FGFRi-Naïve Cholangiocarcinoma Patients 70mg QD pivotal dose 30 mg Ongoing on treatment Complete tumor resection (R0 resection performed in curative intent, patient remains no evidence of disease as of 31-May-2022) cPR cPR cPR cPR cPR cPR cPR cPR Approved Pan-FGFR inhibitors demonstrate 23-36% ORR in this population cPR = confirmed partial response; ORR = overall response rate
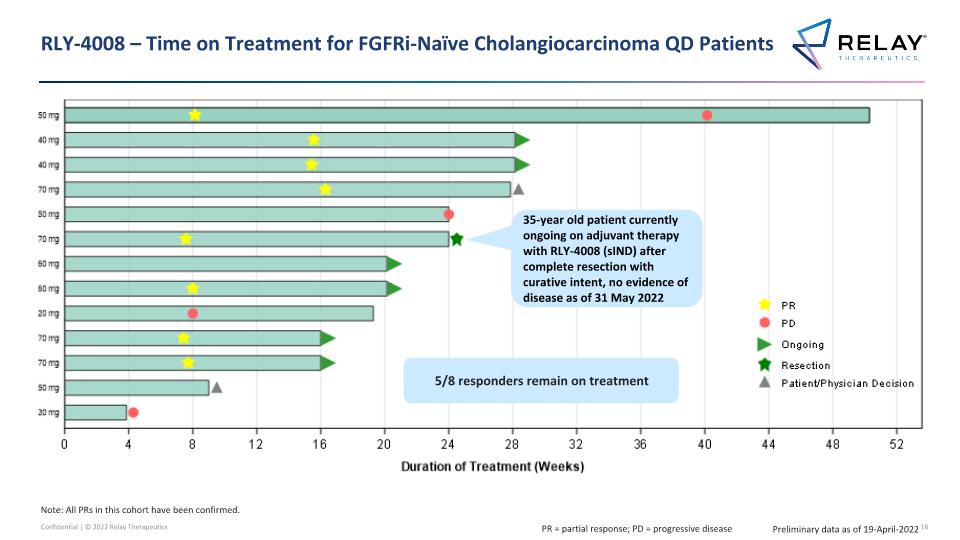
RLY-4008 – Time on Treatment for FGFRi-Naïve Cholangiocarcinoma QD Patients 35-year old patient currently ongoing on adjuvant therapy with RLY-4008 (sIND) after complete resection with curative intent, no evidence of disease as of 31 May 2022 Preliminary data as of 19-April-2022 5/8 responders remain on treatment Note: All PRs in this cohort have been confirmed. PR = partial response; PD = progressive disease
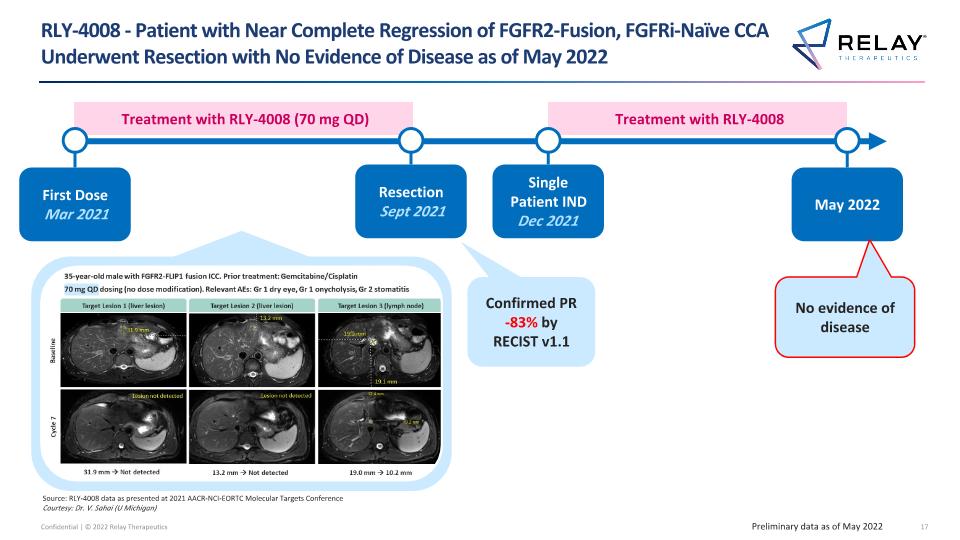
Treatment with RLY-4008 RLY-4008 - Patient with Near Complete Regression of FGFR2-Fusion, FGFRi-Naïve CCA Underwent Resection with No Evidence of Disease as of May 2022 Preliminary data as of May 2022 Treatment with RLY-4008 (70 mg QD) First Dose Mar 2021 Resection Sept 2021 Single Patient IND Dec 2021 May 2022 Confirmed PR -83% by RECIST v1.1 No evidence of disease Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Courtesy: Dr. V. Sahai (U Michigan)
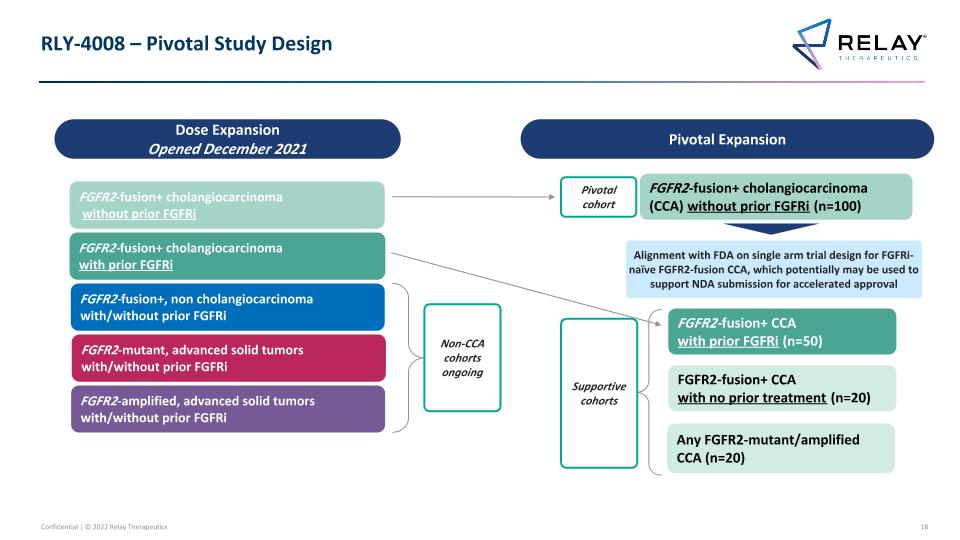
RLY-4008 – Pivotal Study Design FGFR2-fusion+ cholangiocarcinoma (CCA) without prior FGFRi (n=100) FGFR2-fusion+ CCA with prior FGFRi (n=50) FGFR2-fusion+ CCA with no prior treatment (n=20) Any FGFR2-mutant/amplified CCA (n=20) Pivotal Expansion Supportive cohorts Pivotal cohort FGFR2-fusion+ cholangiocarcinoma with prior FGFRi FGFR2-fusion+, non cholangiocarcinoma with/without prior FGFRi FGFR2-amplified, advanced solid tumors with/without prior FGFRi FGFR2-mutant, advanced solid tumors with/without prior FGFRi FGFR2-fusion+ cholangiocarcinoma without prior FGFRi Dose Expansion Opened December 2021 Alignment with FDA on single arm trial design for FGFRi-naïve FGFR2-fusion CCA, which potentially may be used to support NDA submission for accelerated approval Non-CCA cohorts ongoing
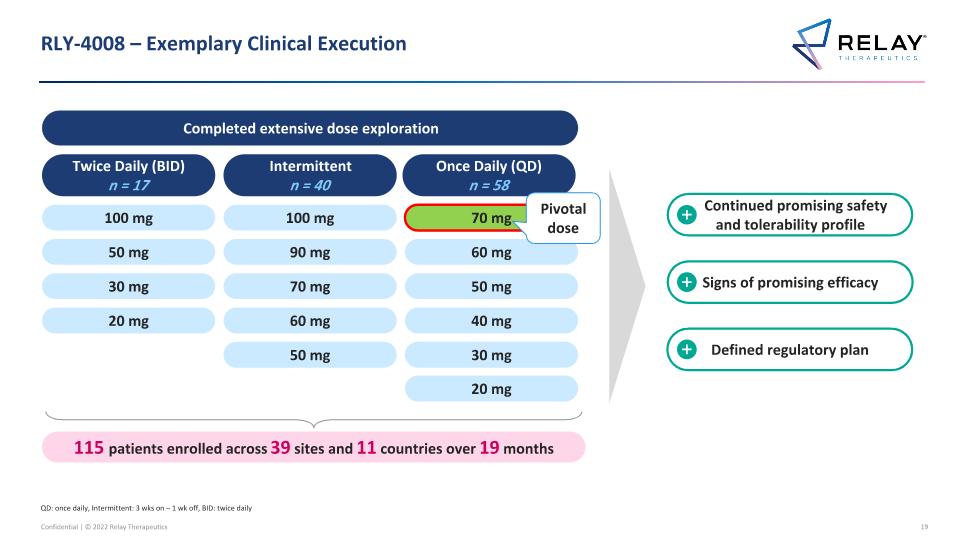
RLY-4008 – Exemplary Clinical Execution Completed extensive dose exploration Continued promising safety and tolerability profile Signs of promising efficacy Defined regulatory plan Twice Daily (BID) n = 17 Intermittent n = 40 100 mg 50 mg 30 mg 20 mg Once Daily (QD) n = 58 115 patients enrolled across 39 sites and 11 countries over 19 months 100 mg 90 mg 70 mg 60 mg 50 mg 70 mg 60 mg 50 mg 40 mg 30 mg 20 mg QD: once daily, Intermittent: 3 wks on – 1 wk off, BID: twice daily Pivotal dose
Future Guidance and Q&A 4 Agenda Overview of DynamoTM Platform 3 Breast Cancer Portfolio 2 RLY-4008 Regulatory Update 1
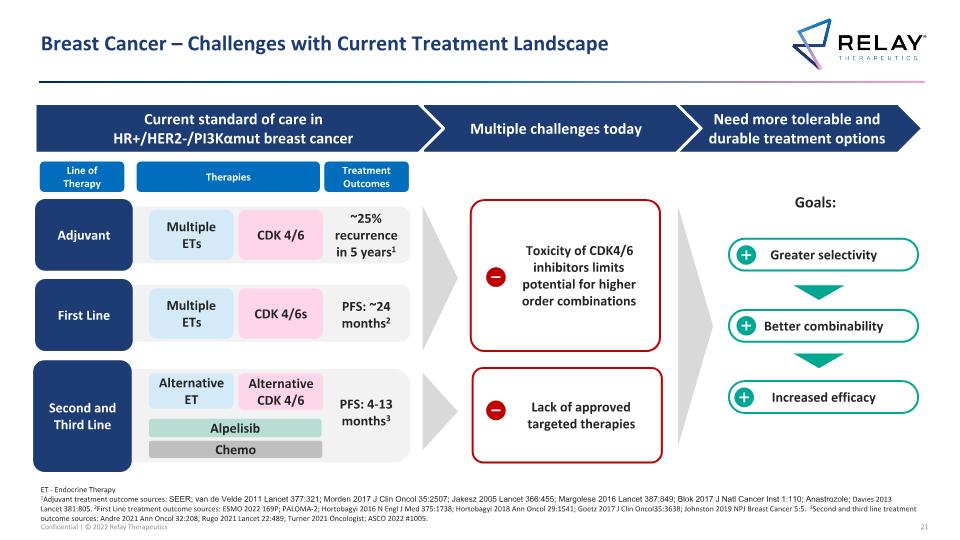
Breast Cancer – Challenges with Current Treatment Landscape ET - Endocrine Therapy 1Adjuvant treatment outcome sources: SEER; van de Velde 2011 Lancet 377:321; Morden 2017 J Clin Oncol 35:2507; Jakesz 2005 Lancet 366:455; Margolese 2016 Lancet 387:849; Blok 2017 J Natl Cancer Inst 1:110; Anastrozole; Davies 2013 Lancet 381:805. 2First Line treatment outcome sources: ESMO 2022 169P; PALOMA-2; Hortobagyi 2016 N Engl J Med 375:1738; Hortobagyi 2018 Ann Oncol 29:1541; Goetz 2017 J Clin Oncol35:3638; Johnston 2019 NPJ Breast Cancer 5:5. 3Second and third line treatment outcome sources: Andre 2021 Ann Oncol 32:208; Rugo 2021 Lancet 22:489; Turner 2021 Oncologist; ASCO 2022 #1005. First Line Second and Third Line PFS: ~24 months2 Multiple ETs CDK 4/6s Alternative ET PFS: 4-13 months3 Adjuvant Multiple ETs CDK 4/6 ~25% recurrence in 5 years1 Alpelisib Chemo Alternative CDK 4/6 Toxicity of CDK4/6 inhibitors limits potential for higher order combinations Lack of approved targeted therapies Greater selectivity Better combinability Increased efficacy Need more tolerable and durable treatment options Multiple challenges today Current standard of care in HR+/HER2-/PI3Kαmut breast cancer Line of Therapy Therapies Treatment Outcomes Goals:
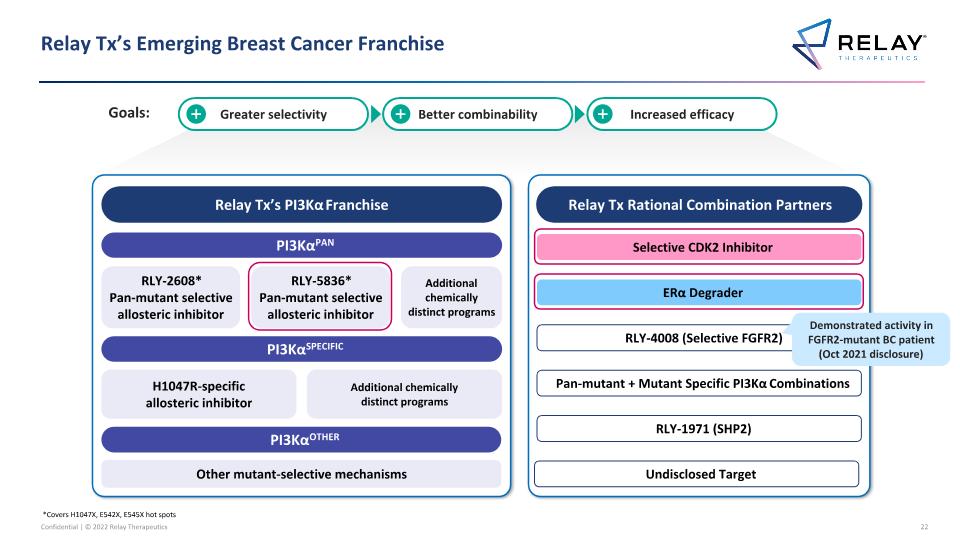
Relay Tx’s Emerging Breast Cancer Franchise *Covers H1047X, E542X, E545X hot spots Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 Inhibitor ERα Degrader RLY-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Greater selectivity Better combinability Increased efficacy Undisclosed Target Demonstrated activity in FGFR2-mutant BC patient (Oct 2021 disclosure) Goals:
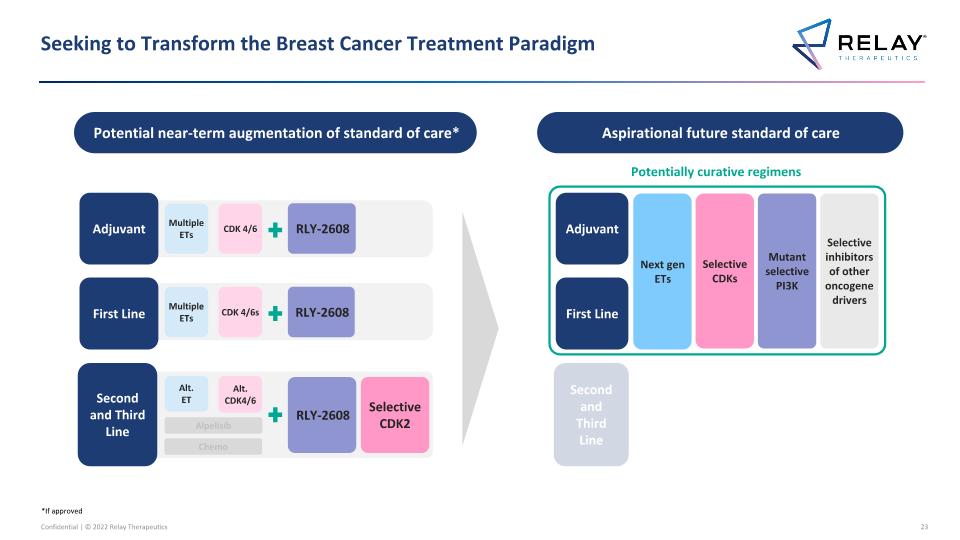
Seeking to Transform the Breast Cancer Treatment Paradigm Potential near-term augmentation of standard of care* Aspirational future standard of care First Line Second and Third Line Multiple ETs CDK 4/6s Alt. ET Adjuvant Multiple ETs CDK 4/6 Alpelisib Chemo Alt. CDK4/6 RLY-2608 RLY-2608 RLY-2608 Selective CDK2 First Line Second and Third Line Adjuvant Potentially curative regimens Next gen ETs Selective CDKs Mutant selective PI3K Selective inhibitors of other oncogene drivers *If approved
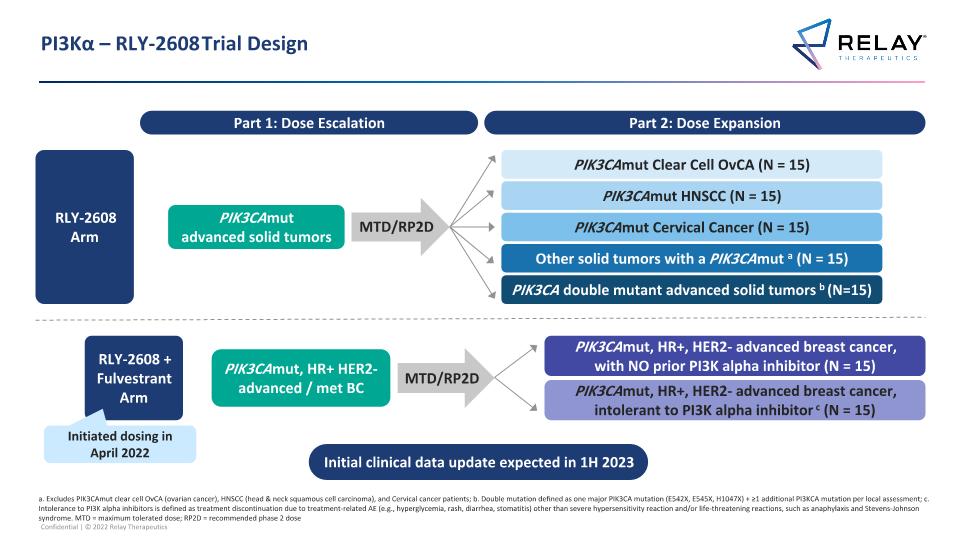
PI3Kα – RLY-2608 Trial Design Part 1: Dose Escalation a. Excludes PIK3CAmut clear cell OvCA (ovarian cancer), HNSCC (head & neck squamous cell carcinoma), and Cervical cancer patients; b. Double mutation defined as one major PIK3CA mutation (E542X, E545X, H1047X) + ≥1 additional PI3KCA mutation per local assessment; c. Intolerance to PI3K alpha inhibitors is defined as treatment discontinuation due to treatment-related AE (e.g., hyperglycemia, rash, diarrhea, stomatitis) other than severe hypersensitivity reaction and/or life-threatening reactions, such as anaphylaxis and Stevens-Johnson syndrome. MTD = maximum tolerated dose; RP2D = recommended phase 2 dose Part 2: Dose Expansion MTD/RP2D PIK3CAmut advanced solid tumors PIK3CA double mutant advanced solid tumors b (N=15) PIK3CAmut Clear Cell OvCA (N = 15) PIK3CAmut HNSCC (N = 15) PIK3CAmut Cervical Cancer (N = 15) Other solid tumors with a PIK3CAmut a (N = 15) RLY-2608 Arm PIK3CAmut, HR+ HER2- advanced / met BC MTD/RP2D PIK3CAmut, HR+, HER2- advanced breast cancer, with NO prior PI3K alpha inhibitor (N = 15) PIK3CAmut, HR+, HER2- advanced breast cancer, intolerant to PI3K alpha inhibitor c (N = 15) RLY-2608 + Fulvestrant Arm Initial clinical data update expected in 1H 2023 Initiated dosing in April 2022
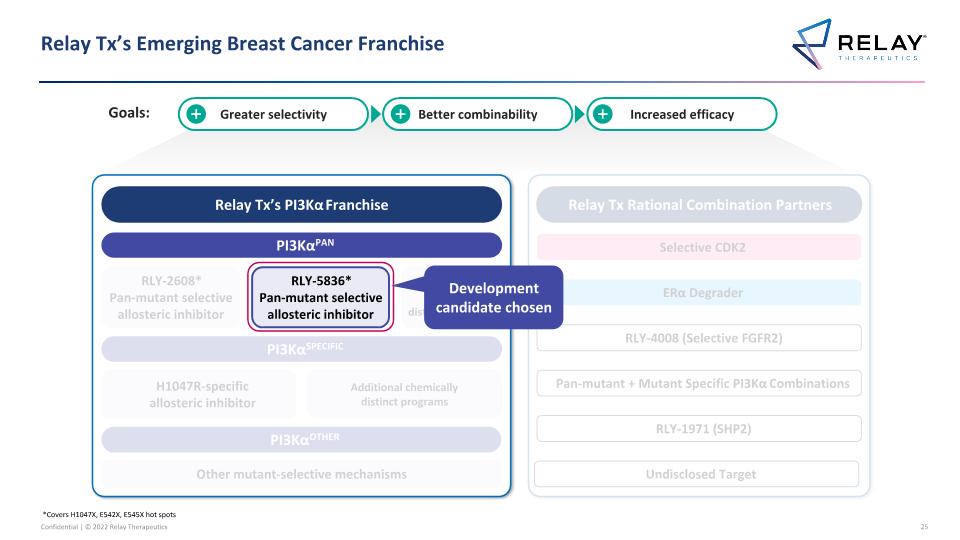
Relay Tx’s Emerging Breast Cancer Franchise *Covers H1047X, E542X, E545X hot spots Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 ERα Degrader RLY-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Greater selectivity Better combinability Increased efficacy Undisclosed Target Development candidate chosen Goals:
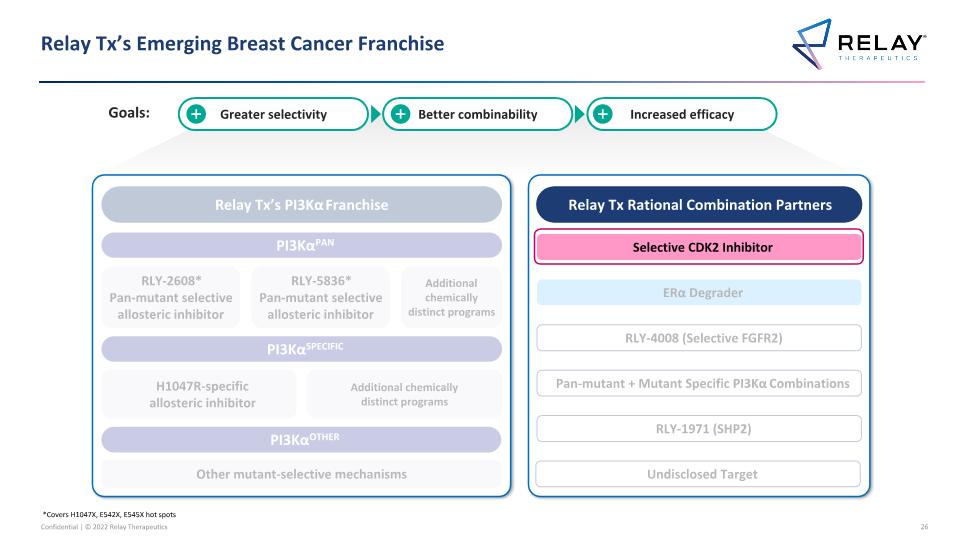
Relay Tx’s Emerging Breast Cancer Franchise *Covers H1047X, E542X, E545X hot spots Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 Inhibitor ERα Degrader RLY-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Greater selectivity Better combinability Increased efficacy Undisclosed Target Goals:
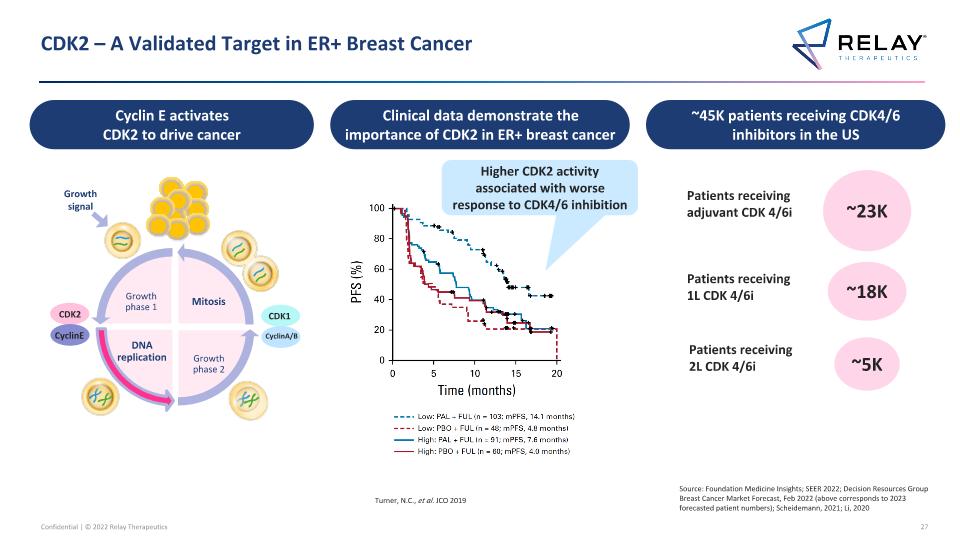
CDK2 – A Validated Target in ER+ Breast Cancer Cyclin E activates CDK2 to drive cancer Clinical data demonstrate the importance of CDK2 in ER+ breast cancer Turner, N.C., et al. JCO 2019 Higher CDK2 activity associated with worse response to CDK4/6 inhibition Growth phase 1 DNA replication Growth phase 2 Mitosis CDK2 CyclinE CDK1 CyclinA/B Growth signal ~45K patients receiving CDK4/6 inhibitors in the US ~23K ~18K Patients receiving adjuvant CDK 4/6i Patients receiving 1L CDK 4/6i ~5K Patients receiving 2L CDK 4/6i Source: Foundation Medicine Insights; SEER 2022; Decision Resources Group Breast Cancer Market Forecast, Feb 2022 (above corresponds to 2023 forecasted patient numbers); Scheidemann, 2021; Li, 2020
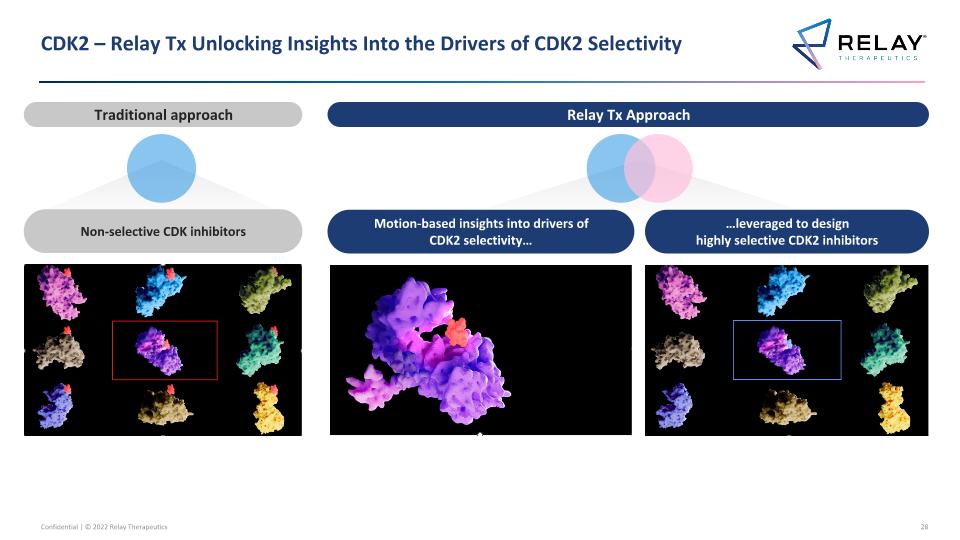
CDK2 – Relay Tx Unlocking Insights Into the Drivers of CDK2 Selectivity Relay Tx Approach Motion-based insights into drivers of CDK2 selectivity… …leveraged to design highly selective CDK2 inhibitors Traditional approach Non-selective CDK inhibitors
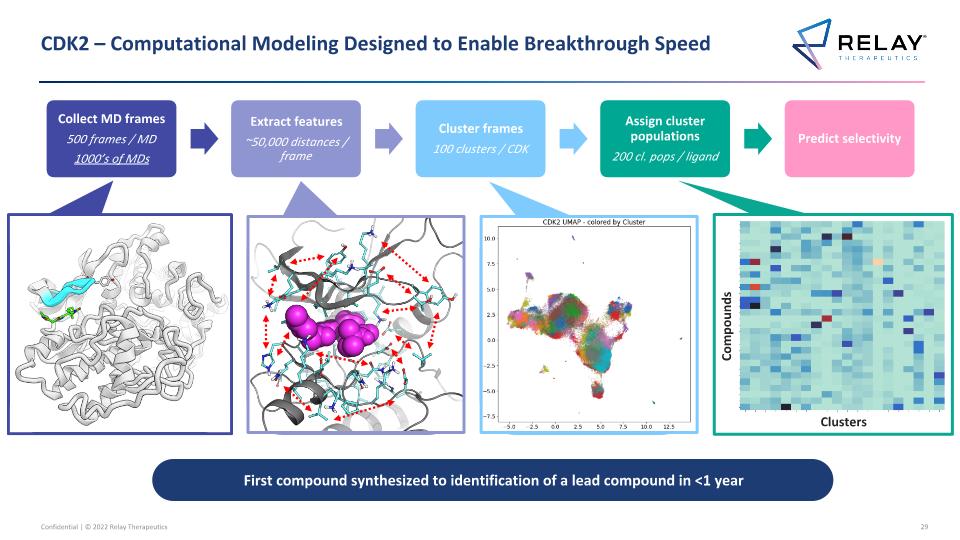
CDK2 – Computational Modeling Designed to Enable Breakthrough Speed Collect MD frames 500 frames / MD 1000’s of MDs Extract features ~50,000 distances / frame Cluster frames 100 clusters / CDK Assign cluster populations 200 cl. pops / ligand Predict selectivity Clusters Compounds First compound synthesized to identification of a lead compound in <1 year
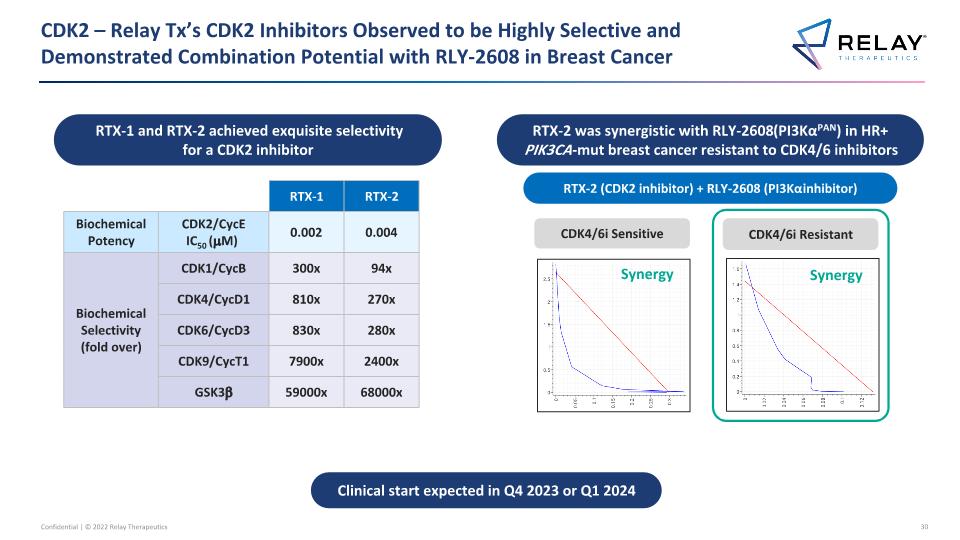
CDK2 – Relay Tx’s CDK2 Inhibitors Observed to be Highly Selective and Demonstrated Combination Potential with RLY-2608 in Breast Cancer RTX-1 and RTX-2 achieved exquisite selectivity for a CDK2 inhibitor Clinical start expected in Q4 2023 or Q1 2024 RTX-2 was synergistic with RLY-2608 (PI3KαPAN) in HR+ PIK3CA-mut breast cancer resistant to CDK4/6 inhibitors CDK4/6i Sensitive CDK4/6i Resistant RTX-2 (CDK2 inhibitor) + RLY-2608 (PI3Kα inhibitor) Synergy Synergy RTX-1 RTX-2 Biochemical Potency CDK2/CycE IC50 (mM) 0.002 0.004 Biochemical Selectivity (fold over) CDK1/CycB 300x 94x CDK4/CycD1 810x 270x CDK6/CycD3 830x 280x CDK9/CycT1 7900x 2400x GSK3b 59000x 68000x
Relay Tx’s Emerging Breast Cancer Franchise *Covers H1047X, E542X, E545X hot spots Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 Inhibitor ERα Degrader RLY-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Greater selectivity Better combinability Increased efficacy Undisclosed Target Goals:
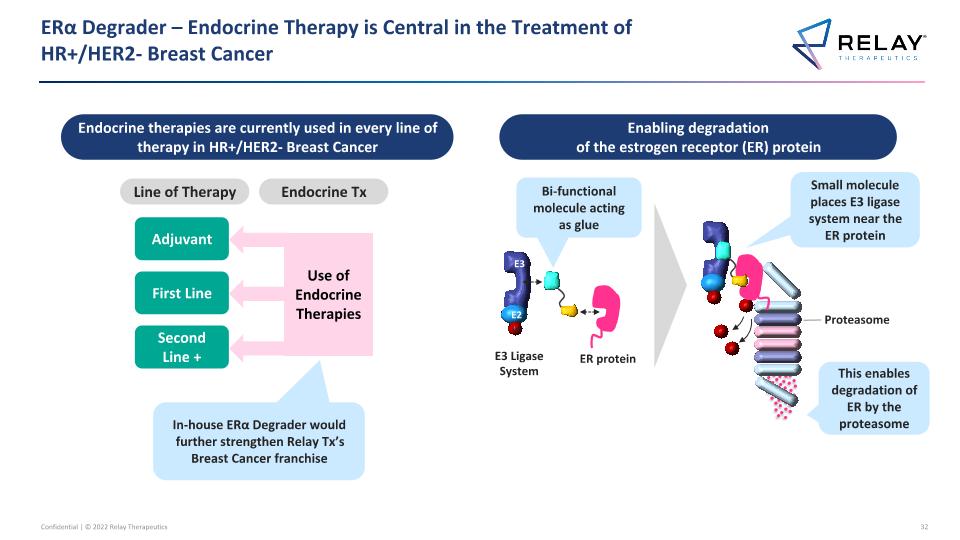
ERα Degrader – Endocrine Therapy is Central in the Treatment of �HR+/HER2- Breast Cancer Endocrine therapies are currently used in every line of therapy in HR+/HER2- Breast Cancer Enabling degradation of the estrogen receptor (ER) protein E3 E2 Ub ER protein E3 Ligase System This enables degradation of ER by the proteasome Bi-functional molecule acting as glue Small molecule places E3 ligase system near the ER protein Second Line + First Line Adjuvant Line of Therapy Use of Endocrine Therapies Endocrine Tx In-house ERα Degrader would further strengthen Relay Tx’s Breast Cancer franchise Proteasome
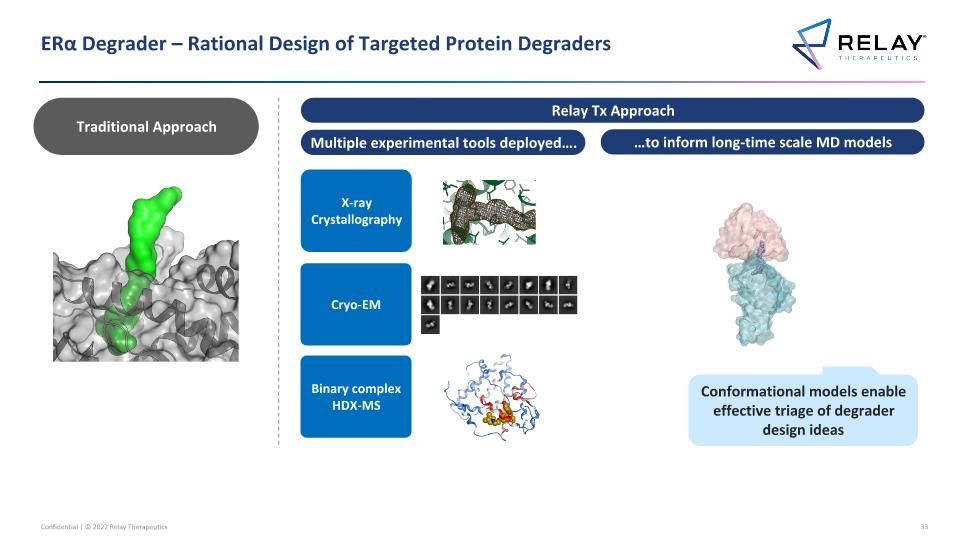
ERα Degrader – Rational Design of Targeted Protein Degraders Multiple experimental tools deployed…. …to inform long-time scale MD models X-ray Crystallography Cryo-EM Conformational models enable effective triage of degrader design ideas Traditional Approach Relay Tx Approach Binary complex HDX-MS
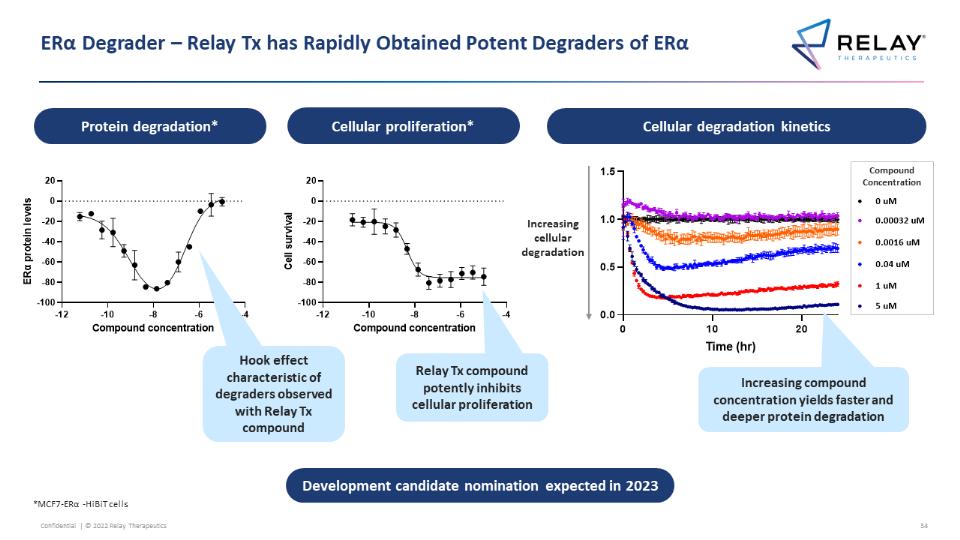
ERα Degrader – Relay Tx has Rapidly Obtained Potent Degraders of ERα Protein degradation* Cellular proliferation* Cellular degradation kinetics ERa protein levels 20 0 -20 -40 -60 -80 -100 -12 -10 -8 -6 -4 Compound concentration Cell survival 20 0 -20 -40 -60 -80 -100 -12 -10 -8 -6 -4 Compound concentration Increasing cellular degradation 1.5 1.0 0.5 0.0 0 10 20 Time (hr) Compound Concentration 0 uM 0.00032 uM 0.0016 uM 0.04 uM 1 uM 5 uM Hook effect characteristic of degraders observed with Relay Tx compound Relay Tx compound potently inhibits cellular proliferation Increasing compound concentration yields faster and deeper protein degradation Development candidate nomination expected in 2023 *MCF7-ERα -HiBiT cells
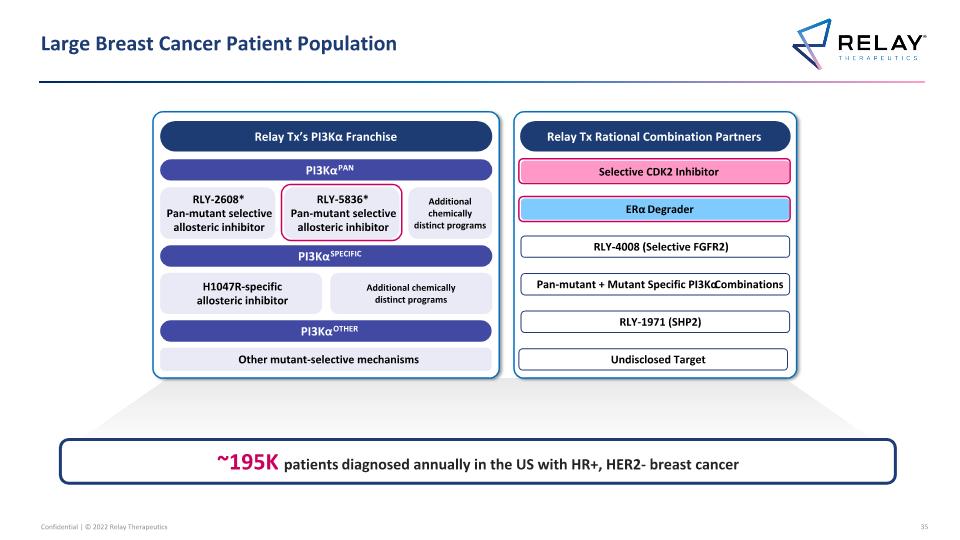
Large Breast Cancer Patient Population Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 Inhibitor ERα Degrader RLY-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Undisclosed Target ~195K patients diagnosed annually in the US with HR+, HER2- breast cancer
Future Guidance and Q&A 4 Agenda Overview of DynamoTM Platform 3 Breast Cancer Portfolio 2 RLY-4008 Regulatory Update 1
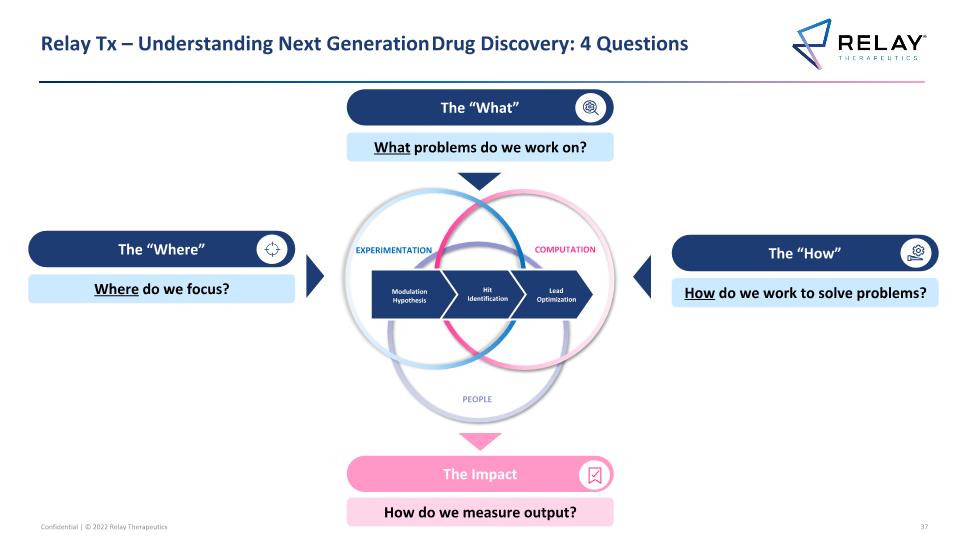
Relay Tx – Understanding Next Generation Drug Discovery: 4 Questions EXPERIMENTATION COMPUTATION PEOPLE Modulation Hypothesis Hit Identification Lead Optimization The “How” How do we work to solve problems? The “What” What problems do we work on? The “Where” Where do we focus? The Impact How do we measure output?
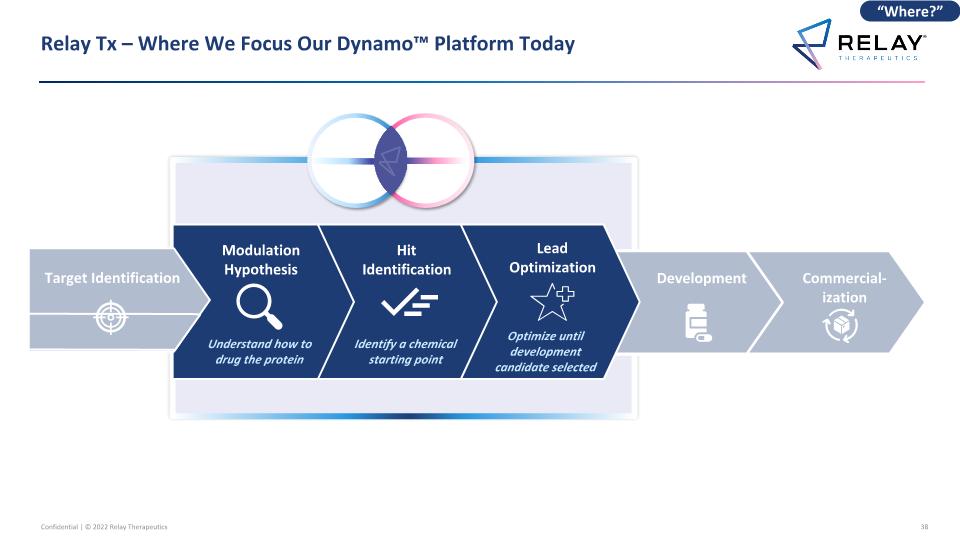
Commercial-ization Relay Tx – Where We Focus Our Dynamo™ Platform Today Development “Where?” Understand how to drug the protein Modulation Hypothesis Hit Identification Lead Optimization Identify a chemical starting point Optimize until development candidate selected Target Identification
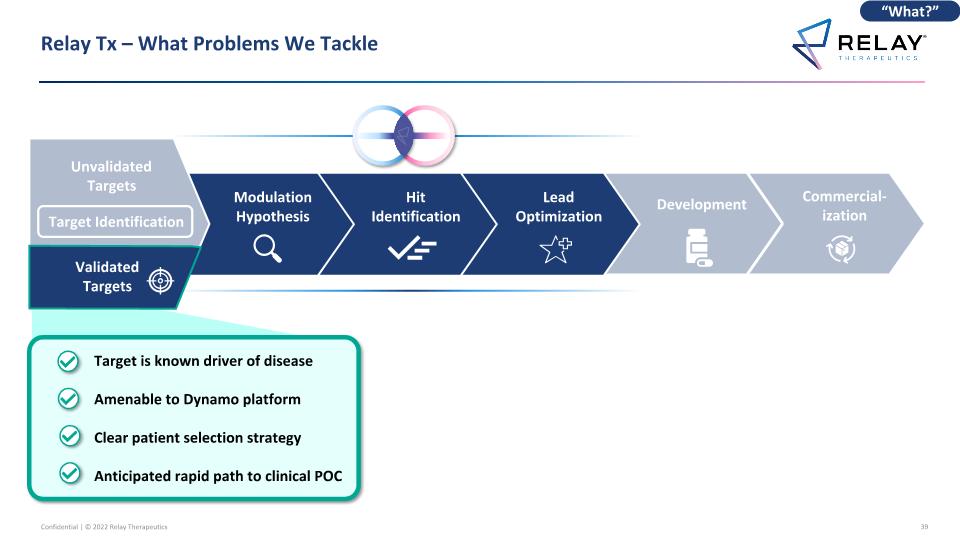
Commercial-ization Relay Tx – What Problems We Tackle Development Lead Optimization Hit Identification Modulation Hypothesis Target is known driver of disease Amenable to Dynamo platform Clear patient selection strategy Anticipated rapid path to clinical POC “What?” Unvalidated Targets Validated Targets Target Identification
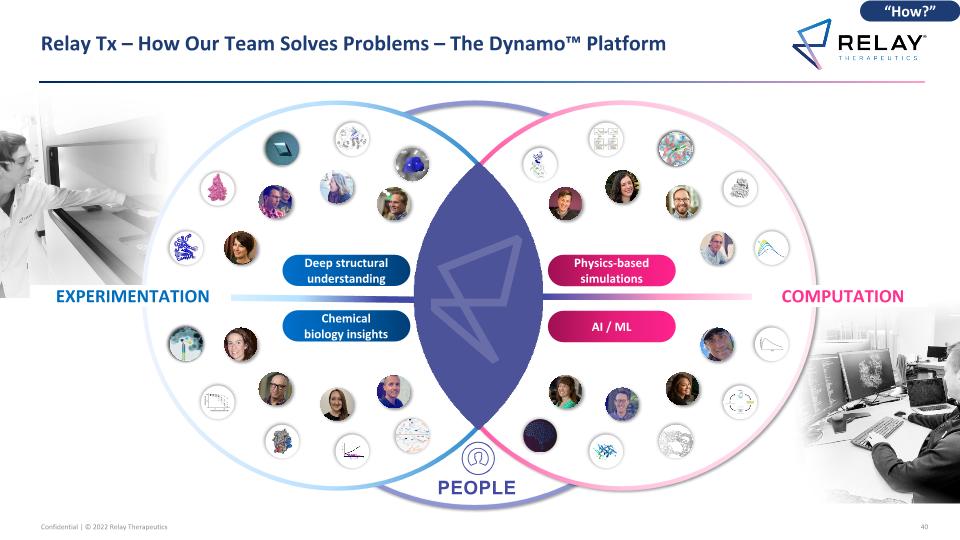
Relay Tx – How Our Team Solves Problems – The Dynamo™ Platform Chemical biology insights Deep structural understanding Physics-based simulations AI / ML PEOPLE EXPERIMENTATION COMPUTATION “How?”
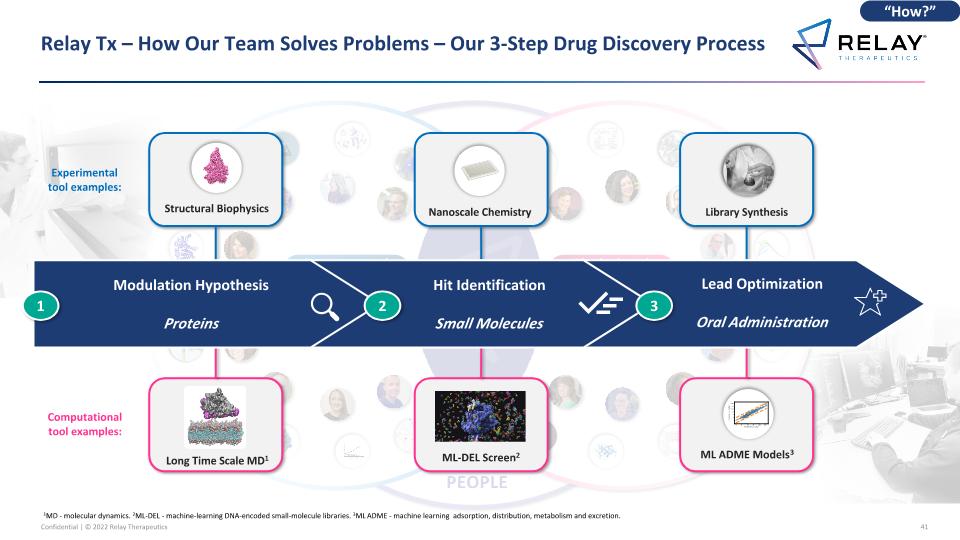
Relay Tx – How Our Team Solves Problems – Our 3-Step Drug Discovery Process Chemical biology insights Deep structural understanding Physics-based simulations AI / ML PEOPLE EXPERIMENTATION COMPUTATION Experimental tool examples: Computational tool examples: Lead Optimization Oral Administration Hit Identification Small Molecules Modulation Hypothesis Proteins 2 3 1 Structural Biophysics Long Time Scale MD1 ML ADME Models3 ML-DEL Screen2 Nanoscale Chemistry Library Synthesis “How?” 1MD - molecular dynamics. 2ML-DEL - machine-learning DNA-encoded small-molecule libraries. 3ML ADME - machine learning adsorption, distribution, metabolism and excretion.

Relay Tx – How Our Team Solves Problems – Using Industry-Leading Expertise We believe the Relay Tx Team is leading the field of Automated Chemical Design (ACD) ACD Framework describes automated small molecule design systems “How?”
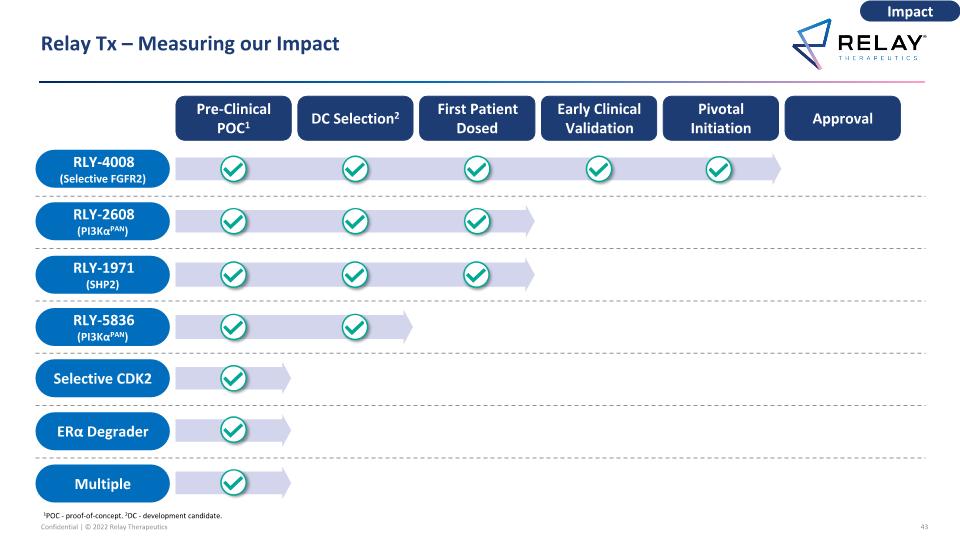
Multiple Relay Tx – Measuring our Impact RLY-4008 (Selective FGFR2) RLY-2608 (PI3KαPAN) RLY-1971 (SHP2) RLY-5836 (PI3KαPAN) Selective CDK2 Pre-Clinical POC1 DC Selection2 First Patient Dosed Early Clinical Validation Approval ERα Degrader Pivotal Initiation Impact 1POC - proof-of-concept. 2DC - development candidate.
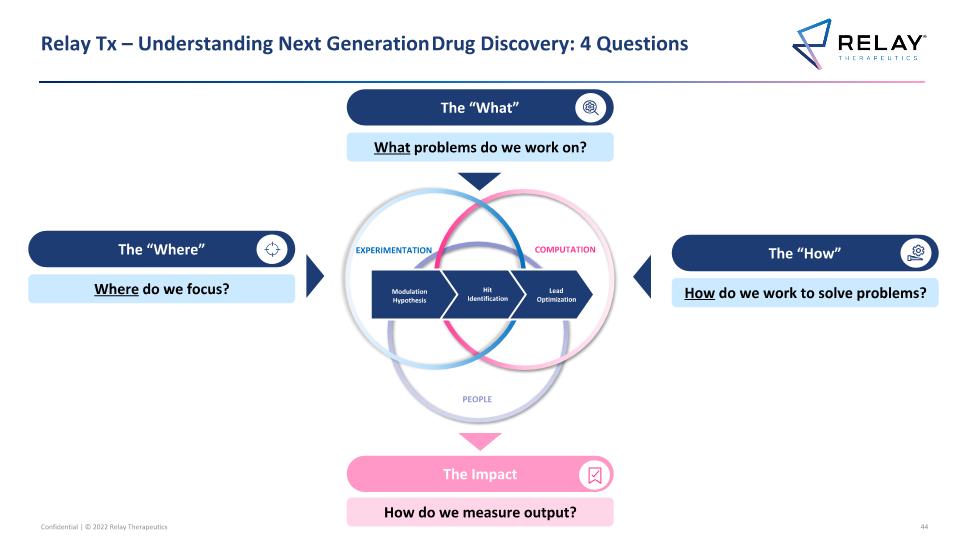
Relay Tx – Understanding Next Generation Drug Discovery: 4 Questions EXPERIMENTATION COMPUTATION PEOPLE Modulation Hypothesis Hit Identification Lead Optimization The “How” How do we work to solve problems? The “What” What problems do we work on? The “Where” Where do we focus? The Impact How do we measure output?
Future Guidance and Q&A 4 Agenda Overview of DynamoTM Platform 3 Breast Cancer Portfolio 2 RLY-4008 Regulatory Update 1
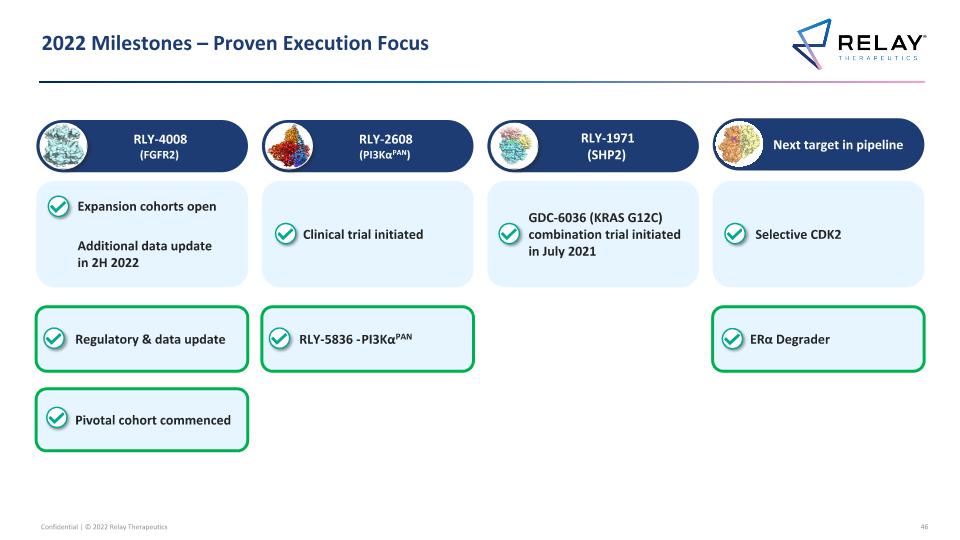
2022 Milestones – Proven Execution Focus RLY-2608 (PI3KαPAN) RLY-4008 (FGFR2) Next target in pipeline RLY-1971 (SHP2) Selective CDK2 Expansion cohorts open Additional data update in 2H 2022 Clinical trial initiated GDC-6036 (KRAS G12C) combination trial initiated in July 2021 ERα Degrader Regulatory & data update RLY-5836 - PI3KαPAN Pivotal cohort commenced
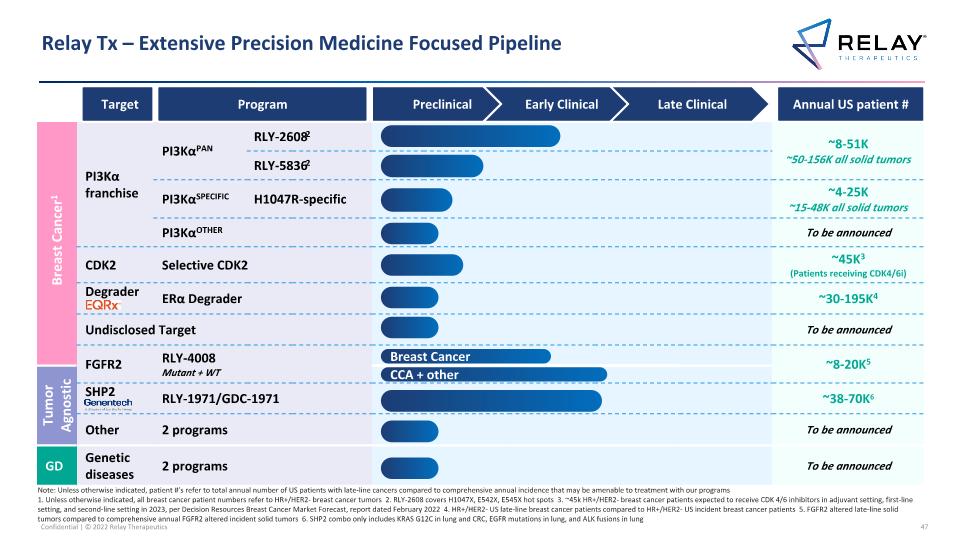
Breast Cancer1 PI3Kα franchise PI3KαPAN RLY-26082 RLY-2608 Pan-mutant allosteric inhibitor ~8-51K ~50-156K all solid tumors RLY-58362 PI3KαSPECIFIC H1047R-specific RLY-1047R H1047R allosteric inhibitor ~4-25K ~15-48K all solid tumors PI3KαOTHER Additional Other novel mutant selective mechanisms To be announced Challengers CDK2 Selective CDK2 ~45K3 (Patients receiving CDK4/6i) Challengers Degrader ERα Degrader ~30-195K4 Undisclosed Target To be announced FGFR2 RLY-4008 Mutant + WT ~8-20K5 Tumor Agnostic SHP2 RLY-1971/GDC-1971 ~38-70K6 Other 2 programs To be announced GD Genetic diseases 2 programs To be announced Relay Tx – Extensive Precision Medicine Focused Pipeline Target Program Annual US patient # CCA + other Preclinical Early Clinical Late Clinical Breast Cancer Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors 2. RLY-2608 covers H1047X, E542X, E545X hot spots 3. ~45k HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2023, per Decision Resources Breast Cancer Market Forecast, report dated February 2022 4. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients 5. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors 6. SHP2 combo only includes KRAS G12C in lung and CRC, EGFR mutations in lung, and ALK fusions in lung
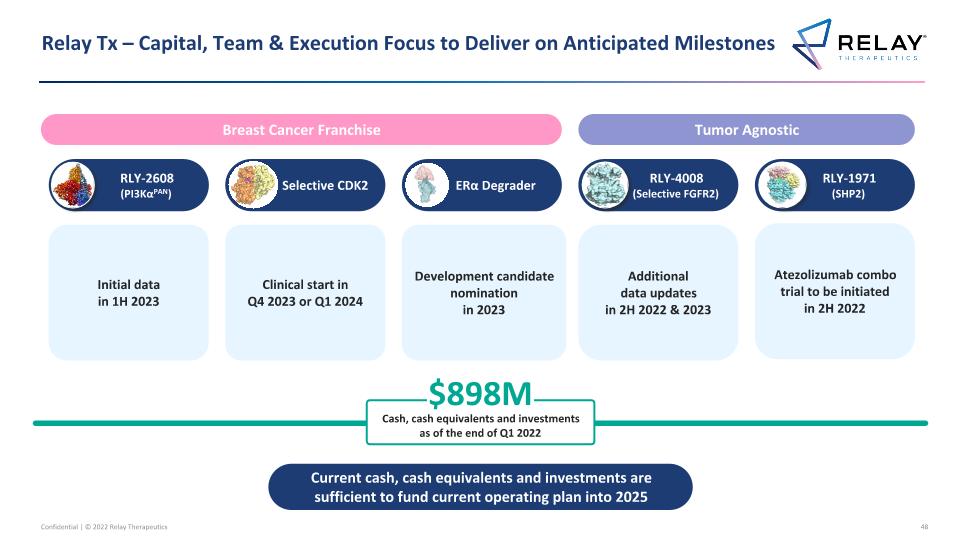
Relay Tx – Capital, Team & Execution Focus to Deliver on Anticipated Milestones RLY-4008 (Selective FGFR2) Selective CDK2 RLY-1971 (SHP2) RLY-2608 (PI3KαPAN) $898M Cash, cash equivalents and investments as of the end of Q1 2022 ERα Degrader Tumor Agnostic Breast Cancer Franchise Current cash, cash equivalents and investments are sufficient to fund current operating plan into 2025 Clinical start in Q4 2023 or Q1 2024 Atezolizumab combo trial to be initiated in 2H 2022 Initial data in 1H 2023 Development candidate nomination in 2023 Additional data updates in 2H 2022 & 2023

Q&A


















































