
ESMO Disclosure Call Materials September 2022 Exhibit 99.2

Disclaimer This presentation contains forward-looking statements and information about our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ “opportunity,” ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include express or implied statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates; the timing of disclosures regarding our pipeline and additional clinical data for RLY-4008 and initial clinical data for RLY-2608; the potential therapeutic benefits of our product candidates, including potential efficacy and tolerability, and combination potential of our product candidates; whether preliminary results from our preclinical or clinical trials will be predictive of the final results of the trials or any future clinical trials of our product candidates; the possibility that unconfirmed results from these trials will not be confirmed by additional data as the clinical trials progress; the competitive landscape and market opportunities for our product candidates; the potential strategic benefits under our collaborations; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; expectations regarding current and future interactions with the U.S. Food and Drug Administration (FDA); our ability to manufacture our product candidates in conformity with the FDA’s requirements; the capabilities and development of our DynamoTM platform; our financial performance; our plans to develop, manufacture and commercialize our current product candidates and any future product candidates; and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our most recent Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities of the Company.
Relay Tx – New Breed of Biotech Chemical biology insights Deep structural understanding Physics-based simulations AI / ML PEOPLE EXPERIMENTATION COMPUTATION
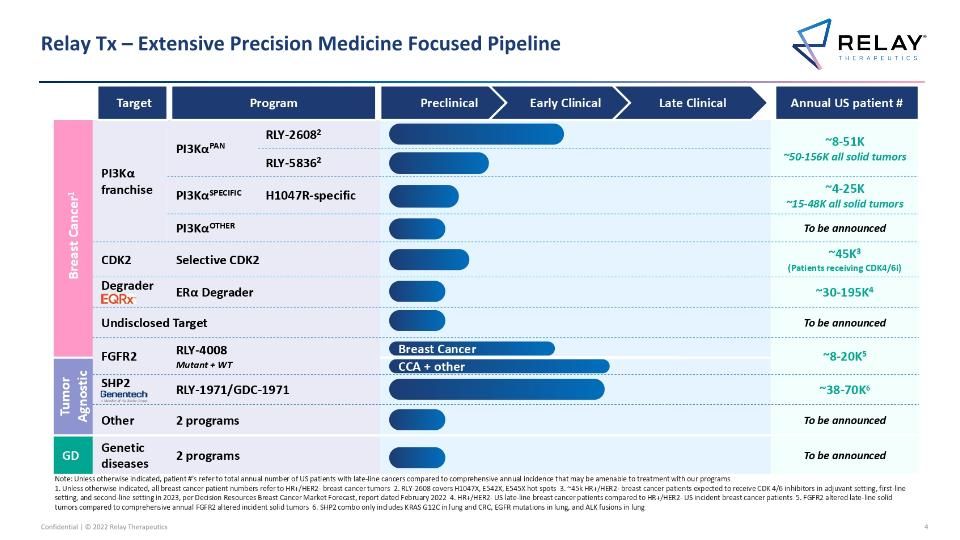
Relay Tx – Extensive Precision Medicine Focused Pipeline Breast Cancer1 PI3Kα franchise PI3KαPAN RLY-26082 RLY-2608 Pan-mutant allosteric inhibitor ~8-51K ~50-156K all solid tumors RLY-58362 PI3KαSPECIFIC H1047R-specific RLY-1047R H1047R allosteric inhibitor ~4-25K ~15-48K all solid tumors PI3KαOTHER Additional Other novel mutant selective mechanisms To be announced Challengers CDK2 Selective CDK2 ~45K3 (Patients receiving CDK4/6i) Challengers Degrader ERα Degrader ~30-195K4 Undisclosed Target To be announced FGFR2 RLY-4008 Mutant + WT ~8-20K5 Tumor Agnostic SHP2 RLY-1971/GDC-1971 ~38-70K6 Other 2 programs To be announced GD Genetic diseases 2 programs To be announced Relay Tx – Extensive Precision Medicine Focused Pipeline Target Program Annual US patient # CCA + other Preclinical Early Clinical Late Clinical Breast Cancer Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors 2. RLY-2608 covers H1047X, E542X, E545X hot spots 3. ~45k HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2023, per Decision Resources Breast Cancer Market Forecast, report dated February 2022 4. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients 5. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors 6. SHP2 combo only includes KRAS G12C in lung and CRC, EGFR mutations in lung, and ALK fusions in lung
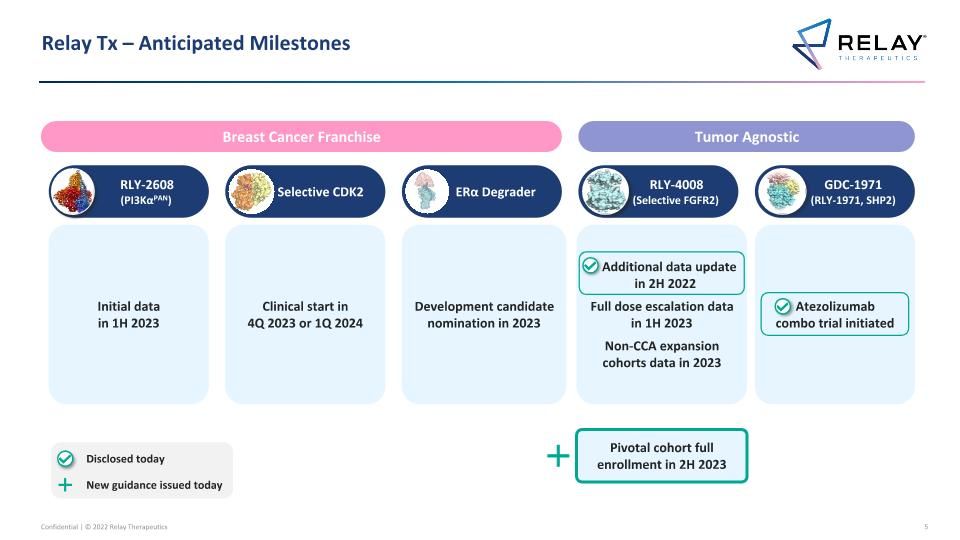
Relay Tx – Anticipated Milestones RLY-4008 (Selective FGFR2) Clinical start in 4Q 2023 or 1Q 2024 Selective CDK2 GDC-1971 (RLY-1971, SHP2) Atezolizumab combo trial initiated RLY-2608 (PI3KαPAN) Development candidate nomination in 2023 ERα Degrader Tumor Agnostic Breast Cancer Franchise Additional data update in 2H 2022 Full dose escalation data in 1H 2023 Non-CCA expansion cohorts data in 2023 Initial data in 1H 2023 Pivotal cohort full enrollment in 2H 2023 Disclosed today New guidance issued today
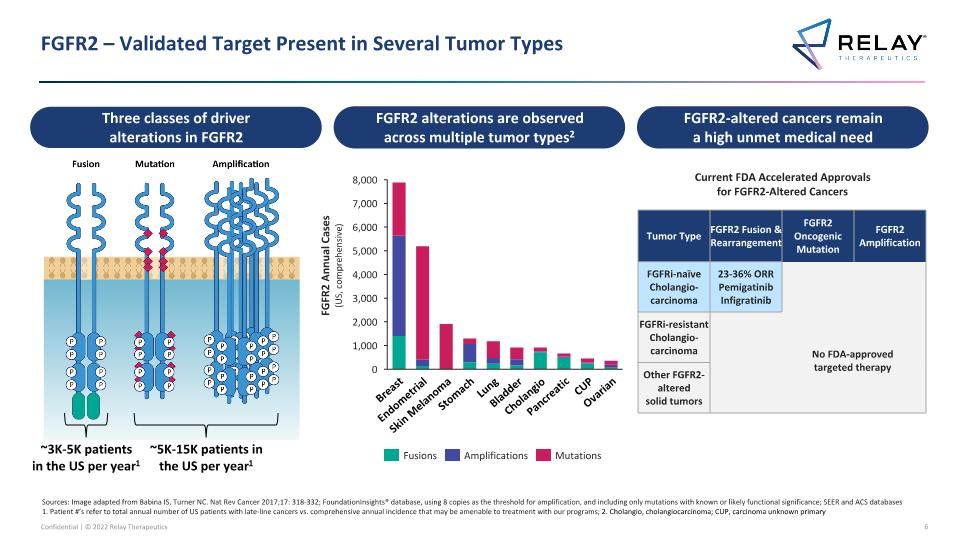
FGFR2 – Validated Target Present in Several Tumor Types Fusions Amplifications Mutations FGFR2 Annual Cases�(US, comprehensive) FGFR2-altered cancers remain a high unmet medical need Pancreatic Breast Endometrial Skin Melanoma Stomach Lung Bladder CUP Ovarian ~5K-15K patients in the US per year1 ~3K-5K patients in the US per year1 FGFR2 alterations are observed across multiple tumor types2 Three classes of driver alterations in FGFR2 Sources: Image adapted from Babina IS, Turner NC. Nat Rev Cancer 2017;17: 318-332; FoundationInsights® database, using 8 copies as the threshold for amplification, and including only mutations with known or likely functional significance; SEER and ACS databases 1. Patient #’s refer to total annual number of US patients with late-line cancers vs. comprehensive annual incidence that may be amenable to treatment with our programs; 2. Cholangio, cholangiocarcinoma; CUP, carcinoma unknown primary Cholangio Current FDA Accelerated Approvals for FGFR2-Altered Cancers Tumor Type FGFR2 Fusion & Rearrangement FGFR2 Oncogenic Mutation FGFR2 Amplification FGFRi-naïve Cholangio-carcinoma 23-36% ORR Pemigatinib Infigratinib FGFRi-resistant Cholangio-carcinoma Other FGFR2-altered solid tumors No FDA-approved targeted therapy
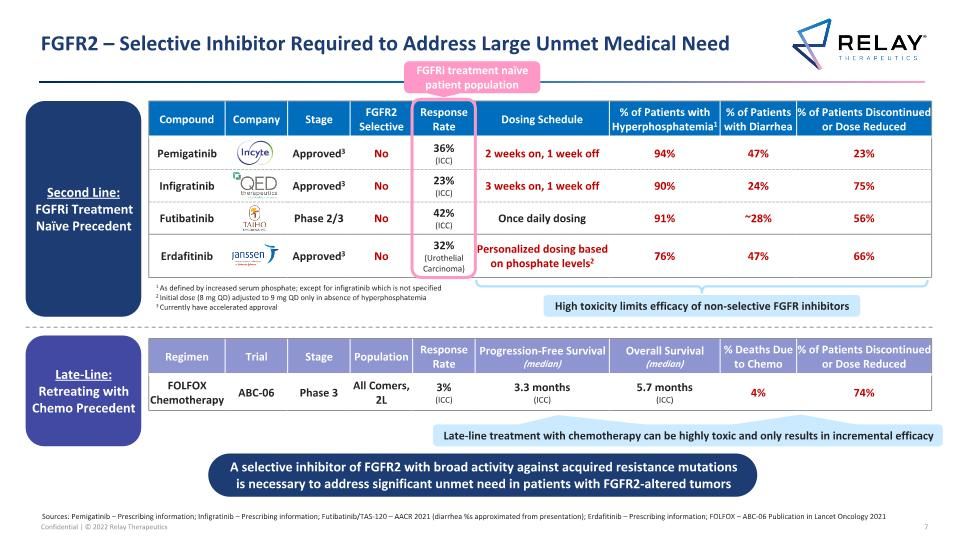
FGFR2 – Selective Inhibitor Required to Address Large Unmet Medical Need A selective inhibitor of FGFR2 with broad activity against acquired resistance mutations is necessary to address significant unmet need in patients with FGFR2-altered tumors Compound Company Stage FGFR2 Selective Response Rate Dosing Schedule % of Patients with Hyperphosphatemia1 % of Patients with Diarrhea % of Patients Discontinued or Dose Reduced Pemigatinib Approved3 No 36% (ICC) 2 weeks on, 1 week off 94% 47% 23% Infigratinib Approved3 No 23% (ICC) 3 weeks on, 1 week off 90% 24% 75% Futibatinib Phase 2/3 No 42% (ICC) Once daily dosing 91% ~28% 56% Erdafitinib Approved3 No 32% (Urothelial Carcinoma) Personalized dosing based on phosphate levels2 76% 47% 66% High toxicity limits efficacy of non-selective FGFR inhibitors Sources: Pemigatinib – Prescribing information; Infigratinib – Prescribing information; Futibatinib/TAS-120 – AACR 2021 (diarrhea %s approximated from presentation); Erdafitinib – Prescribing information; FOLFOX – ABC-06 Publication in Lancet Oncology 2021 1 As defined by increased serum phosphate; except for infigratinib which is not specified 2 Initial dose (8 mg QD) adjusted to 9 mg QD only in absence of hyperphosphatemia 3 Currently have accelerated approval Second Line: FGFRi Treatment Naïve Precedent Late-Line: Retreating with Chemo Precedent Regimen Trial Stage Population Response Rate Progression-Free Survival (median) Overall Survival (median) % Deaths Due to Chemo % of Patients Discontinued or Dose Reduced FOLFOX Chemotherapy ABC-06 Phase 3 All Comers, 2L 3% (ICC) 3.3 months (ICC) 5.7 months (ICC) 4% 74% Late-line treatment with chemotherapy is highly toxic and only results in incremental efficacy Late-line treatment with chemotherapy can be highly toxic and only results in incremental efficacy FGFRi treatment naïve patient population
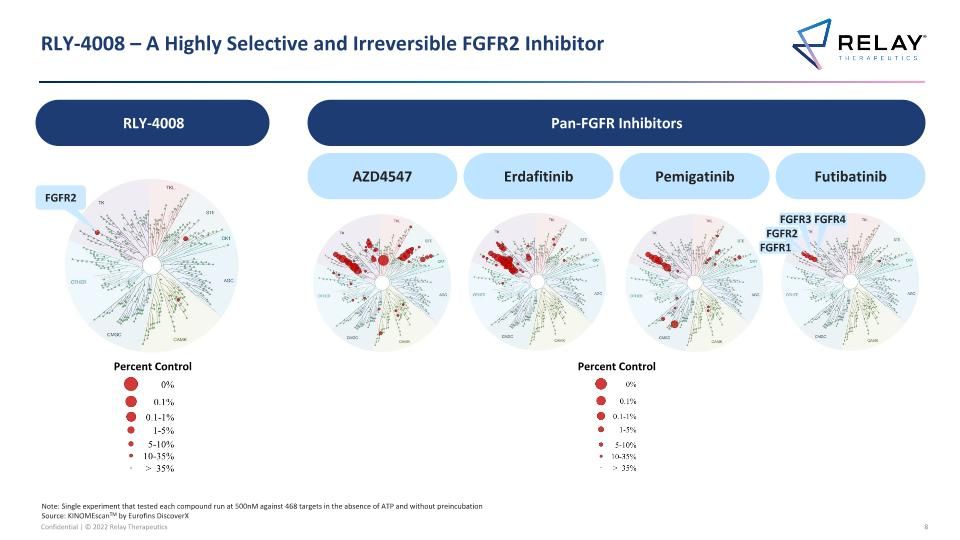
RLY-4008 – A Highly Selective and Irreversible FGFR2 Inhibitor Pan-FGFR Inhibitors RLY-4008 AZD4547 Erdafitinib Pemigatinib Futibatinib FGFR2 Note: Single experiment that tested each compound run at 500nM against 468 targets in the absence of ATP and without preincubation Source: KINOMEscanTM by Eurofins DiscoverX FGFR1 FGFR2 FGFR3 FGFR4 Percent Control Percent Control
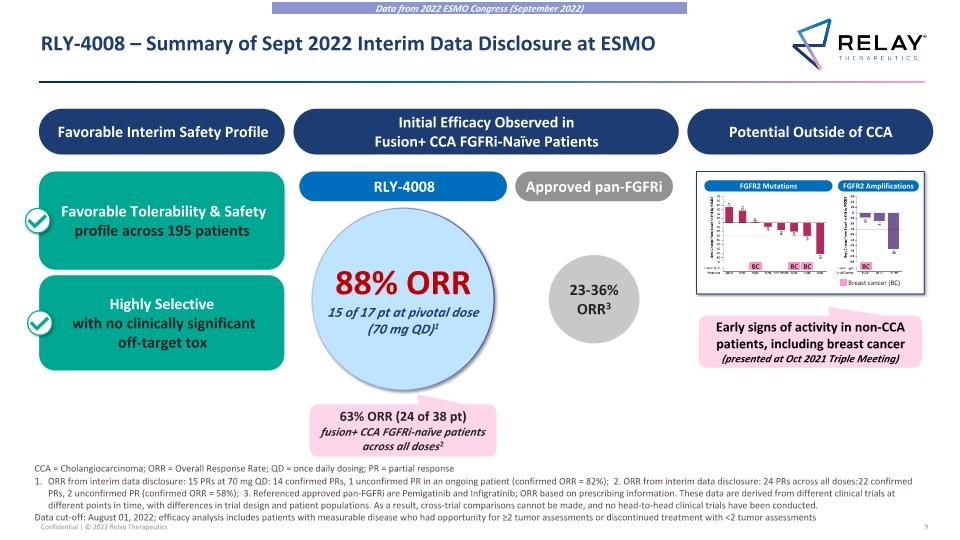
RLY-4008 – Summary of Sept 2022 Interim Data Disclosure at ESMO Favorable Interim Safety Profile Potential Outside of CCA Favorable Tolerability & Safety profile across 195 patients Highly Selective with no clinically significant off-target tox Early signs of activity in non-CCA patients, including breast cancer (presented at Oct 2021 Triple Meeting) Initial Efficacy Observed in Fusion+ CCA FGFRi-Naïve Patients RLY-4008 88% ORR 15 of 17 pt at pivotal dose (70 mg QD)1 63% ORR (24 of 38 pt) fusion+ CCA FGFRi-naïve patients across all doses2 Approved pan-FGFRi 23-36% ORR3 FGFR2 Mutations FGFR2 Amplifications BC BC BC Breast cancer (BC) BC ORR from interim data disclosure: 15 PRs at 70 mg QD: 14 confirmed PRs, 1 unconfirmed PR in an ongoing patient (confirmed ORR = 82%); 2. ORR from interim data disclosure: 24 PRs across all doses: 22 confirmed PRs, 2 unconfirmed PR (confirmed ORR = 58%); 3. Referenced approved pan-FGFRi are Pemigatinib and Infigratinib; ORR based on prescribing information. These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments CCA = Cholangiocarcinoma; ORR = Overall Response Rate; QD = once daily dosing; PR = partial response Data from 2022 ESMO Congress (September 2022)
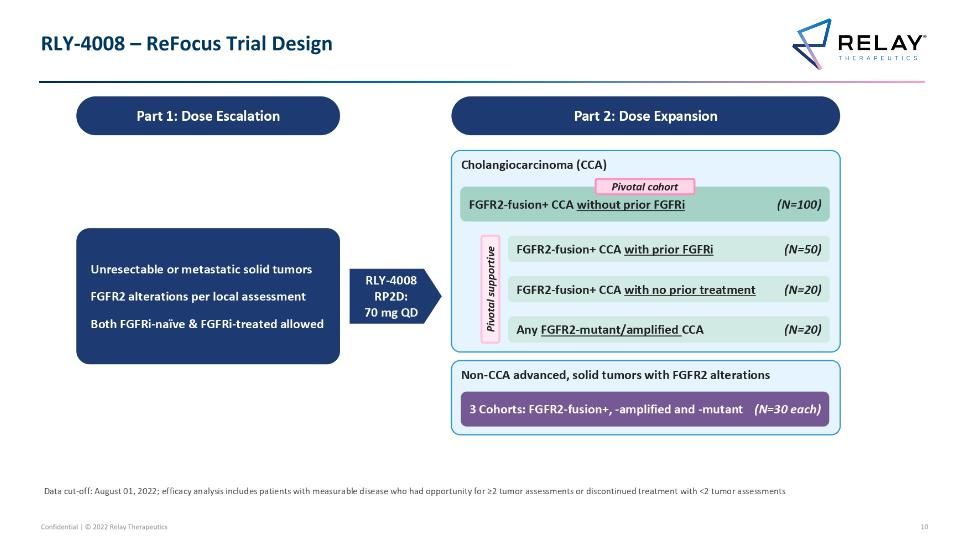
RLY-4008 – ReFocus Trial Design RLY-4008 – ReFocus Trial Design Part 1: Dose Escalation Unresectable or metastatic solid tumors FGFR2 alterations per local assessment Both FGFRi-naïve & FGFRi-treated allowed RLY-4008 RP2D: 70 mg QD Part 2: Dose Expansion Non-CCA advanced, solid tumors with FGFR2 alterations 3 Cohorts: FGFR2-fusion+, -amplified and -mutant (N=30 each) Cholangiocarcinoma (CCA) FGFR2-fusion+ CCA with no prior treatment (N=20) Any FGFR2-mutant/amplified CCA (N=20) FGFR2-fusion+ CCA with prior FGFRi (N=50) Pivotal supportive Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments FGFR2-fusion+ CCA without prior FGFRi (N=100) Pivotal cohort
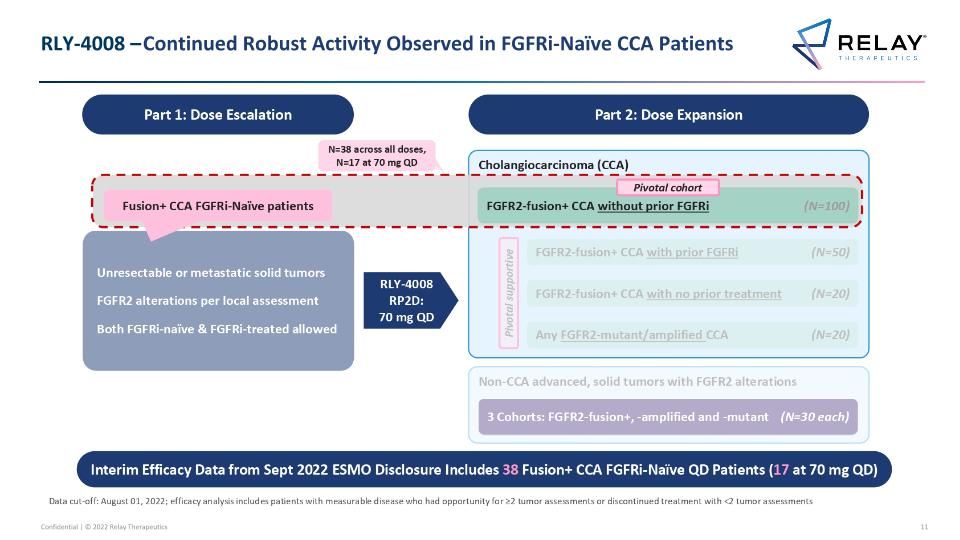
RLY-4008 – Continued Robust Activity Observed in FGFRi-Naïve CCA Patients Unresectable or metastatic solid tumors FGFR2 alterations per local assessment Both FGFRi-naïve & FGFRi-treated allowed RLY-4008 RP2D: 70 mg QD RLY-4008 – Continued Robust Activity Observed in FGFRi-Naïve CCA Patients Part 1: Dose Escalation Part 2: Dose Expansion Non-CCA advanced, solid tumors with FGFR2 alterations 3 Cohorts: FGFR2-fusion+, -amplified and -mutant (N=30 each) Cholangiocarcinoma (CCA) FGFR2-fusion+ CCA with no prior treatment (N=20) Any FGFR2-mutant/amplified CCA (N=20) FGFR2-fusion+ CCA with prior FGFRi (N=50) Pivotal supportive Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments N=38 across all doses, N=17 at 70 mg QD FGFR2-fusion+ CCA without prior FGFRi (N=100) Pivotal cohort Fusion+ CCA FGFRi-Naïve patients Interim Efficacy Data from Sept 2022 ESMO Disclosure Includes 38 Fusion+ CCA FGFRi-Naïve QD Patients (17 at 70 mg QD)
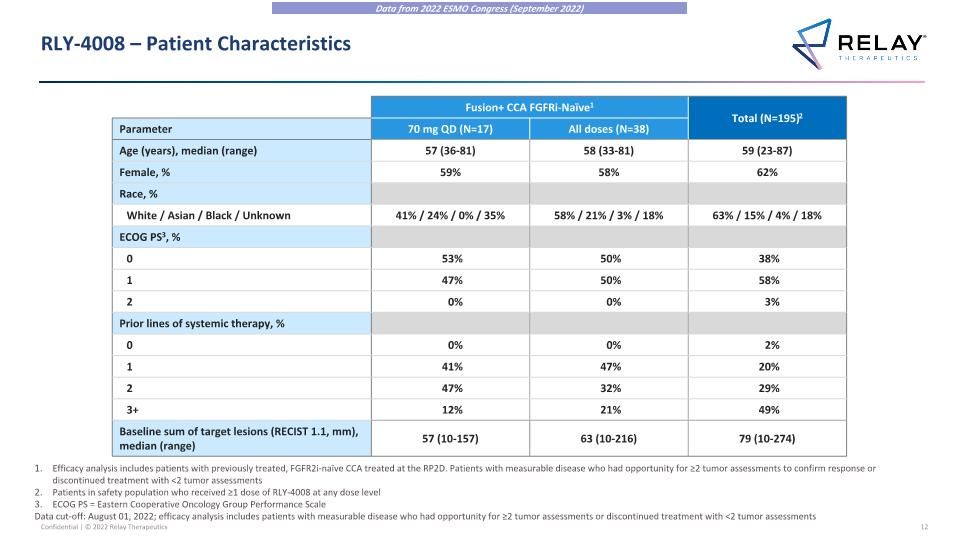
RLY-4008 – Patient Characteristics Fusion+ CCA FGFRi-Naïve1 Total (N=195)2 Parameter 70 mg QD (N=17) All doses (N=38) Age (years), median (range) 57 (36-81) 58 (33-81) 59 (23-87) Female, % 59% 58% 62% Race, % White / Asian / Black / Unknown 41% / 24% / 0% / 35% 58% / 21% / 3% / 18% 63% / 15% / 4% / 18% ECOG PS3, % 0 53% 50% 38% 1 47% 50% 58% 2 0% 0% 3% Prior lines of systemic therapy, % 0 0% 0% 2% 1 41% 47% 20% 2 47% 32% 29% 3+ 12% 21% 49% Baseline sum of target lesions (RECIST 1.1, mm), median (range) 57 (10-157) 63 (10-216) 79 (10-274) Efficacy analysis includes patients with previously treated, FGFR2i-naïve CCA treated at the RP2D. Patients with measurable disease who had opportunity for ≥2 tumor assessments to confirm response or discontinued treatment with <2 tumor assessments Patients in safety population who received ≥1 dose of RLY-4008 at any dose level ECOG PS = Eastern Cooperative Oncology Group Performance Scale Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments Data from 2022 ESMO Congress (September 2022)
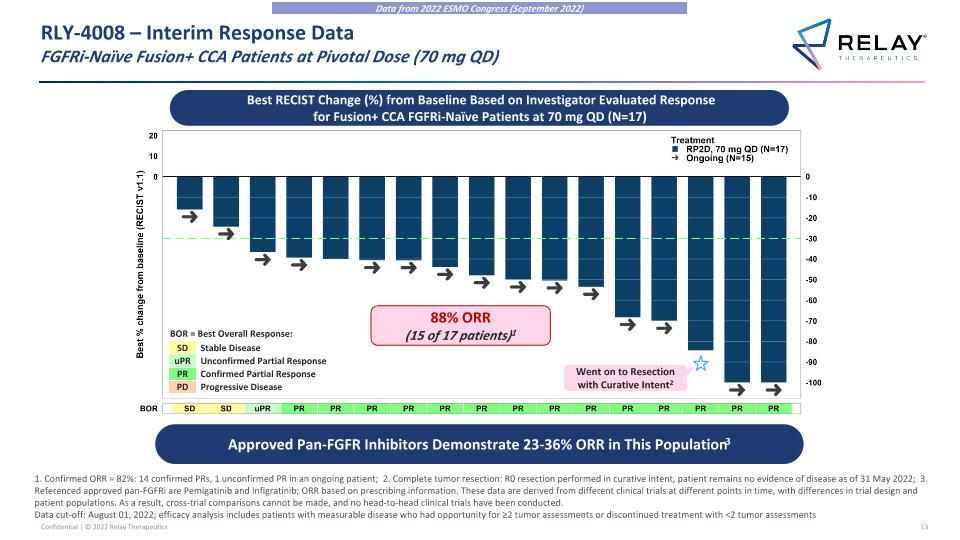
Approved Pan-FGFR Inhibitors Demonstrate 23-36% ORR in This Population3 RLY-4008 – Interim Response Data�FGFRi-Naïve Fusion+ CCA Patients at Pivotal Dose (70 mg QD) Best RECIST Change (%) from Baseline Based on Investigator Evaluated Response for Fusion+ CCA FGFRi-Naïve Patients at 70 mg QD (N=17) 1. Confirmed ORR = 82%: 14 confirmed PRs, 1 unconfirmed PR in an ongoing patient; 2. Complete tumor resection: R0 resection performed in curative intent, patient remains no evidence of disease as of 31 May 2022; 3. Referenced approved pan-FGFRi are Pemigatinib and Infigratinib; ORR based on prescribing information. These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments SD uPR PR Stable Disease Unconfirmed Partial Response Confirmed Partial Response Progressive Disease PD BOR = Best Overall Response: 88% ORR (15 of 17 patients)1 Went on to Resection with Curative Intent2 Data from 2022 ESMO Congress (September 2022)
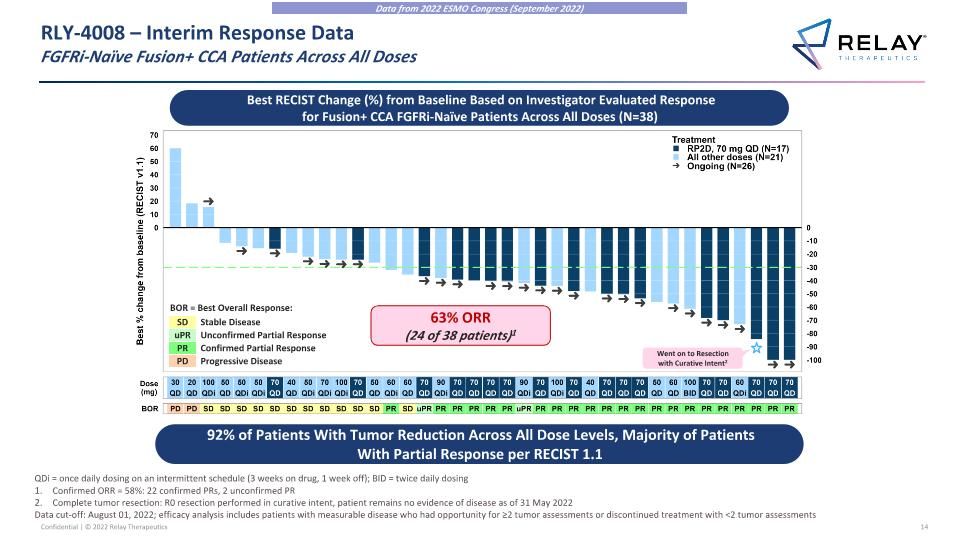
RLY-4008 – Interim Response Data�FGFRi-Naïve Fusion+ CCA Patients Across All Doses 92% of Patients With Tumor Reduction Across All Dose Levels, Majority of Patients With Partial Response per RECIST 1.1 Best RECIST Change (%) from Baseline Based on Investigator Evaluated Response for Fusion+ CCA FGFRi-Naïve Patients Across All Doses (N=38) Confirmed ORR = 58%: 22 confirmed PRs, 2 unconfirmed PR Complete tumor resection: R0 resection performed in curative intent, patient remains no evidence of disease as of 31 May 2022 Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments Went on to Resection with Curative Intent2 SD uPR PR Stable Disease Unconfirmed Partial Response Confirmed Partial Response Progressive Disease PD BOR = Best Overall Response: 63% ORR (24 of 38 patients)1 Data from 2022 ESMO Congress (September 2022) QDi = once daily dosing on an intermittent schedule (3 weeks on drug, 1 week off); BID = twice daily dosing
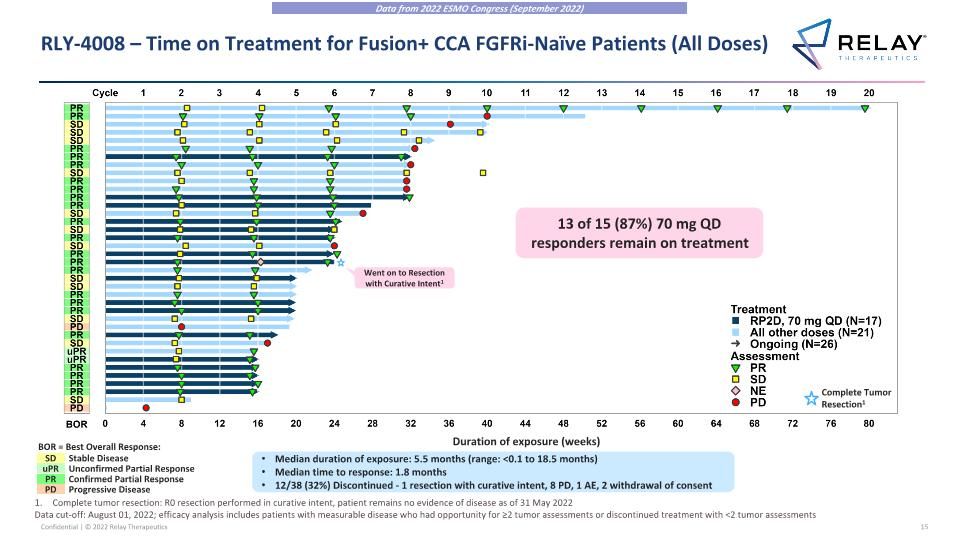
RLY-4008 – Time on Treatment for Fusion+ CCA FGFRi-Naïve Patients (All Doses) Complete tumor resection: R0 resection performed in curative intent, patient remains no evidence of disease as of 31 May 2022 Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments SD uPR PR Stable Disease Unconfirmed Partial Response Confirmed Partial Response Progressive Disease PD BOR = Best Overall Response: 13 of 15 (87%) 70 mg QD responders remain on treatment Complete Tumor Resection1 Duration of exposure (weeks) Median duration of exposure: 5.5 months (range: <0.1 to 18.5 months) Median time to response: 1.8 months 12/38 (32%) Discontinued - 1 resection with curative intent, 8 PD, 1 AE, 2 withdrawal of consent Went on to Resection with Curative Intent1 Data from 2022 ESMO Congress (September 2022)
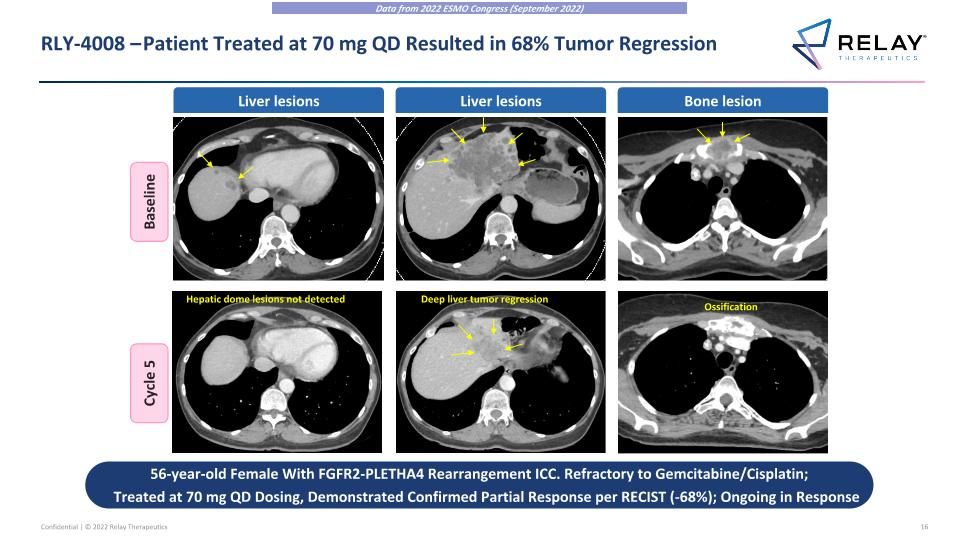
RLY-4008 – Patient Treated at 70 mg QD Resulted in 68% Tumor Regression Baseline Cycle 5 Liver lesions Liver lesions Bone lesion Hepatic dome lesions not detected Deep liver tumor regression Ossification 56-year-old Female With FGFR2-PLETHA4 Rearrangement ICC. Refractory to Gemcitabine/Cisplatin; Treated at 70 mg QD Dosing, Demonstrated Confirmed Partial Response per RECIST (-68%); Ongoing in Response Data from 2022 ESMO Congress (September 2022)
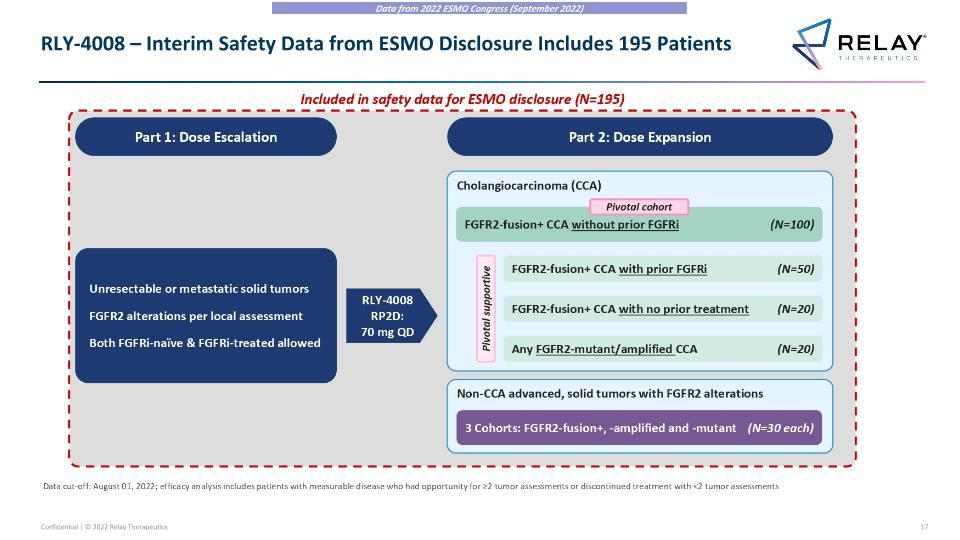
RLY-4008 – Interim Safety Data from ESMO Disclosure Includes 195 Patients Data from 2022 ESMO Congress (September 2022) RLY-4008 – Interim Safety Data from ESMO Disclosure Includes 195 Patients Part 1: Dose Escalation Part 2: Dose Expansion Non-CCA advanced, solid tumors with FGFR2 alterations 3 Cohorts: FGFR2-fusion+, -amplified and -mutant (N=30 each) Cholangiocarcinoma (CCA) FGFR2-fusion+ CCA with no prior treatment (N=20) Any FGFR2-mutant/amplified CCA (N=20) FGFR2-fusion+ CCA with prior FGFRi (N=50) Pivotal supportive Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments FGFR2-fusion+ CCA without prior FGFRi (N=100) Pivotal cohort Included in safety data for ESMO disclosure (N=195) Unresectable or metastatic solid tumors FGFR2 alterations per local assessment Both FGFRi-naïve & FGFRi-treated allowed RLY-4008 RP2D: 70 mg QD Data from 2022 ESMO Congress (September 2022)
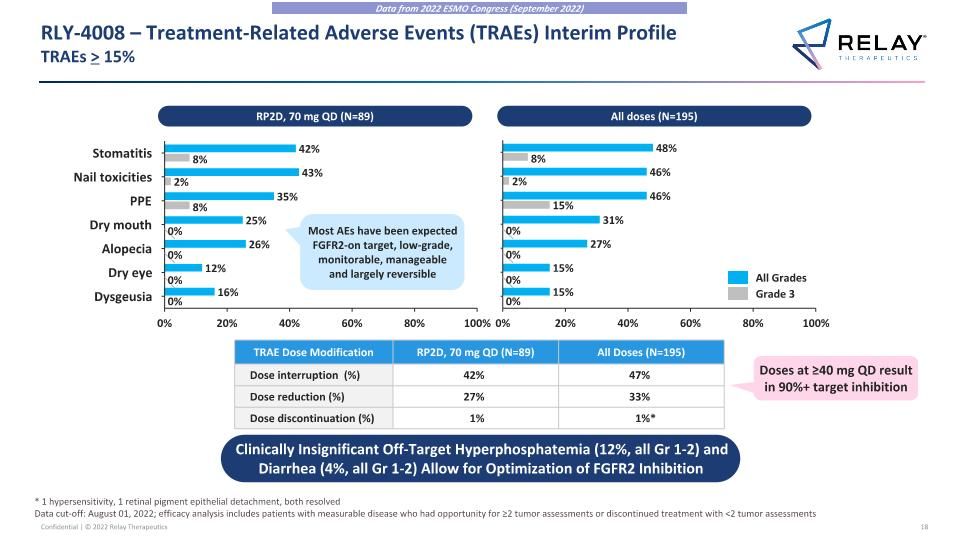
RLY-4008 – Treatment-Related Adverse Events (TRAEs) Interim Profile�TRAEs > 15% TRAE Dose Modification RP2D, 70 mg QD (N=89) All Doses (N=195) Dose interruption (%) 42% 47% Dose reduction (%) 27% 33% Dose discontinuation (%) 1% 1%* Clinically Insignificant Off-Target Hyperphosphatemia (12%, all Gr 1-2) and Diarrhea (4%, all Gr 1-2) Allow for Optimization of FGFR2 Inhibition Doses at ≥40 mg QD result in 90%+ target inhibition Most AEs have been expected FGFR2-on target, low-grade, monitorable, manageable and largely reversible RP2D, 70 mg QD (N=89) All doses (N=195) Data from 2022 ESMO Congress (September 2022) All Grades Grade 3 * 1 hypersensitivity, 1 retinal pigment epithelial detachment, both resolved Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments
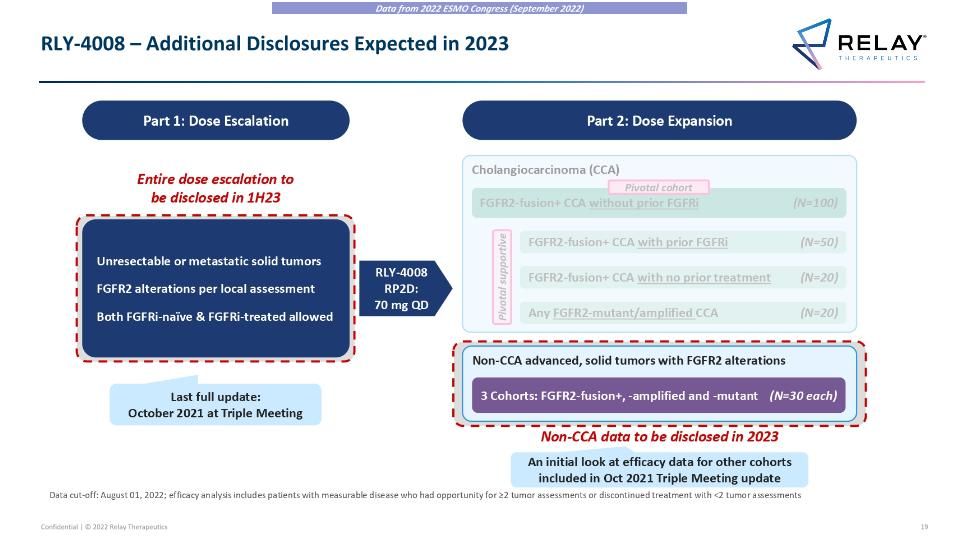
RLY-4008 – Additional Disclosures Expected in 2023 Data from 2022 ESMO Congress (September 2022) Unresectable or metastatic solid tumors FGFR2 alterations per local assessment Both FGFRi-naïve & FGFRi-treated allowed RLY-4008 RP2D: 70 mg QD Entire dose escalation to be disclosed in 1H23 Last full update: October 2021 at Triple Meeting Non-CCA advanced, solid tumors with FGFR2 alterations RLY-4008 – Additional Disclosures Expected in 2023 Part 1: Dose Escalation Part 2: Dose Expansion 3 Cohorts: FGFR2-fusion+, -amplified and -mutant (N=30 each) Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments Cholangiocarcinoma (CCA) FGFR2-fusion+ CCA with no prior treatment (N=20) Any FGFR2-mutant/amplified CCA (N=20) FGFR2-fusion+ CCA with prior FGFRi (N=50) Pivotal supportive FGFR2-fusion+ CCA without prior FGFRi (N=100) Pivotal cohort Non-CCA data to be disclosed in 2023 An initial look at efficacy data for other cohorts included in Oct 2021 Triple Meeting update Data from 2022 ESMO Congress (September 2022)
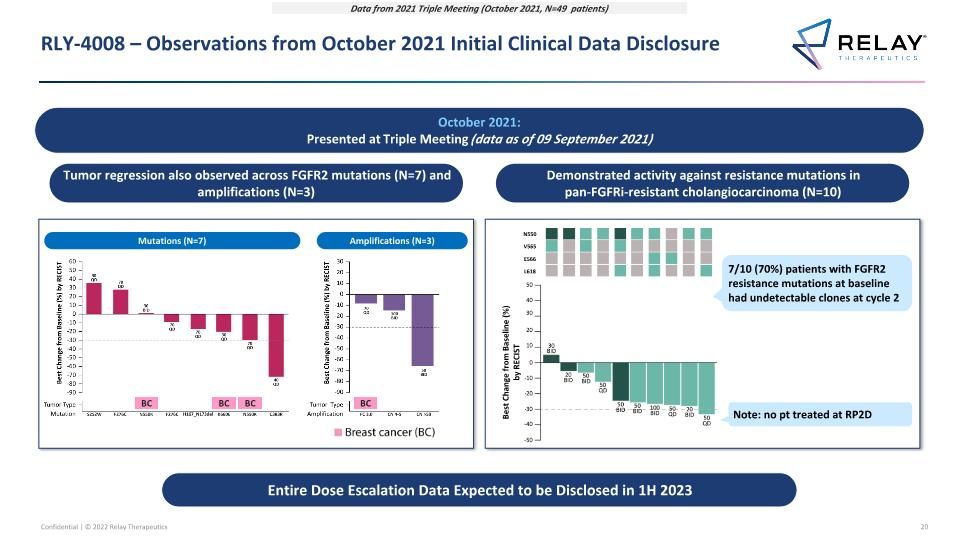
RLY-4008 – Observations from October 2021 Initial Clinical Data Disclosure Data from 2021 Triple Meeting (October 2021, N=49 patients) Tumor regression also observed across FGFR2 mutations (N=7) and amplifications (N=3) Mutations (N=7) Amplifications (N=3) BC BC BC BC Demonstrated activity against resistance mutations in pan-FGFRi-resistant cholangiocarcinoma (N=10) ≥ 1 resistance mutation detected at baseline, and all rendered undetectable at C2D1 ≥ 1 resistance mutation detected at baseline and C2D1 N550 V565 E566 L618 Best Change from Baseline (%) by RECIST 7/10 (70%) patients with FGFR2 resistance mutations at baseline had undetectable clones at cycle 2 Note: no pt treated at RP2D October 2021: Presented at Triple Meeting (data as of 09 September 2021) Entire Dose Escalation Data Expected to be Disclosed in 1H 2023
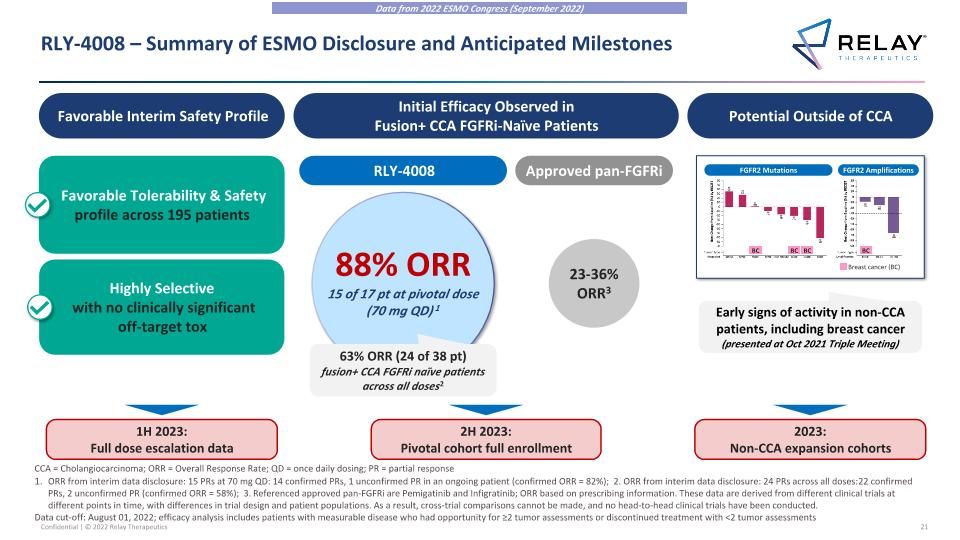
RLY-4008 – Summary of ESMO Disclosure and Anticipated Milestones Data from 2022 ESMO Congress (September 2022) Favorable Interim Safety Profile Potential Outside of CCA Favorable Tolerability & Safety profile across 195 patients Highly Selective with no clinically significant off-target tox Initial Efficacy Observed in Fusion+ CCA FGFRi-Naïve Patients RLY-4008 88% ORR 15 of 17 pt at pivotal dose (70 mg QD) 1 63% ORR (24 of 38 pt) fusion+ CCA FGFRi naïve patients across all doses2 Approved pan-FGFRi 23-36% ORR3 1H 2023: Full dose escalation data 2H 2023: Pivotal cohort full enrollment 2023: Non-CCA expansion cohorts Early signs of activity in non-CCA patients, including breast cancer (presented at Oct 2021 Triple Meeting) FGFR2 Mutations FGFR2 Amplifications BC BC BC Breast cancer (BC) BC ORR from interim data disclosure: 15 PRs at 70 mg QD: 14 confirmed PRs, 1 unconfirmed PR in an ongoing patient (confirmed ORR = 82%); 2. ORR from interim data disclosure: 24 PRs across all doses: 22 confirmed PRs, 2 unconfirmed PR (confirmed ORR = 58%); 3. Referenced approved pan-FGFRi are Pemigatinib and Infigratinib; ORR based on prescribing information. These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Data cut-off: August 01, 2022; efficacy analysis includes patients with measurable disease who had opportunity for ≥2 tumor assessments or discontinued treatment with <2 tumor assessments CCA = Cholangiocarcinoma; ORR = Overall Response Rate; QD = once daily dosing; PR = partial response
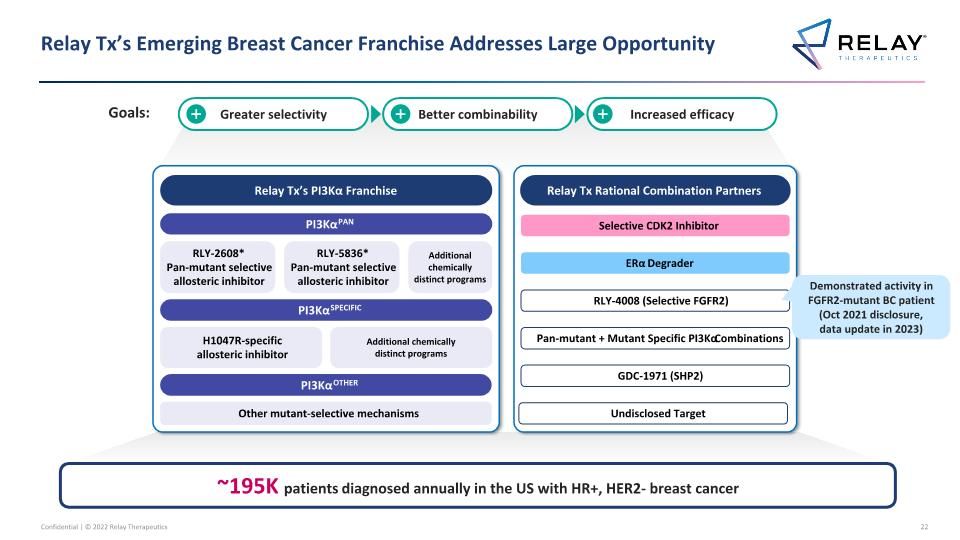
Relay Tx’s Emerging Breast Cancer Franchise Addresses Large Opportunity Relay Tx’s PI3Kα Franchise PI3KαPAN PI3KαSPECIFIC PI3KαOTHER RLY-2608* Pan-mutant selective allosteric inhibitor H1047R-specific allosteric inhibitor Other mutant-selective mechanisms Additional chemically distinct programs Relay Tx Rational Combination Partners Selective CDK2 Inhibitor ERα Degrader GDC-1971 (SHP2) RLY-4008 (Selective FGFR2) RLY-5836* Pan-mutant selective allosteric inhibitor Pan-mutant + Mutant Specific PI3Kα Combinations Additional chemically distinct programs Undisclosed Target ~195K patients diagnosed annually in the US with HR+, HER2- breast cancer Greater selectivity Better combinability Increased efficacy Demonstrated activity in FGFR2-mutant BC patient (Oct 2021 disclosure, data update in 2023) Goals:
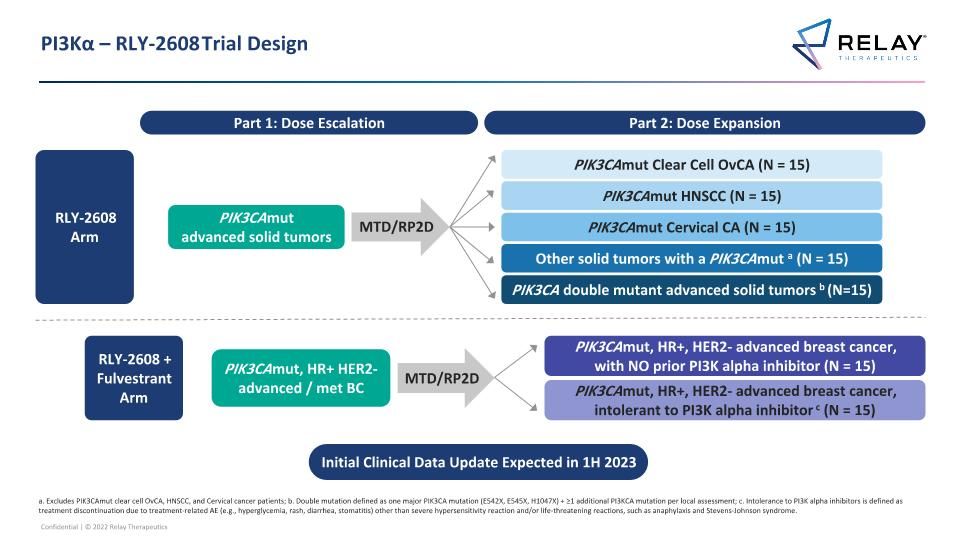
PI3Kα – RLY-2608 Trial Design Part 1: Dose Escalation a. Excludes PIK3CAmut clear cell OvCA, HNSCC, and Cervical cancer patients; b. Double mutation defined as one major PIK3CA mutation (E542X, E545X, H1047X) + ≥1 additional PI3KCA mutation per local assessment; c. Intolerance to PI3K alpha inhibitors is defined as treatment discontinuation due to treatment-related AE (e.g., hyperglycemia, rash, diarrhea, stomatitis) other than severe hypersensitivity reaction and/or life-threatening reactions, such as anaphylaxis and Stevens-Johnson syndrome. Part 2: Dose Expansion MTD/RP2D PIK3CAmut advanced solid tumors PIK3CA double mutant advanced solid tumors b (N=15) PIK3CAmut Clear Cell OvCA (N = 15) PIK3CAmut HNSCC (N = 15) PIK3CAmut Cervical CA (N = 15) Other solid tumors with a PIK3CAmut a (N = 15) RLY-2608 Arm PIK3CAmut, HR+ HER2- advanced / met BC MTD/RP2D PIK3CAmut, HR+, HER2- advanced breast cancer, with NO prior PI3K alpha inhibitor (N = 15) PIK3CAmut, HR+, HER2- advanced breast cancer, intolerant to PI3K alpha inhibitor c (N = 15) RLY-2608 + Fulvestrant Arm Initial Clinical Data Update Expected in 1H 2023
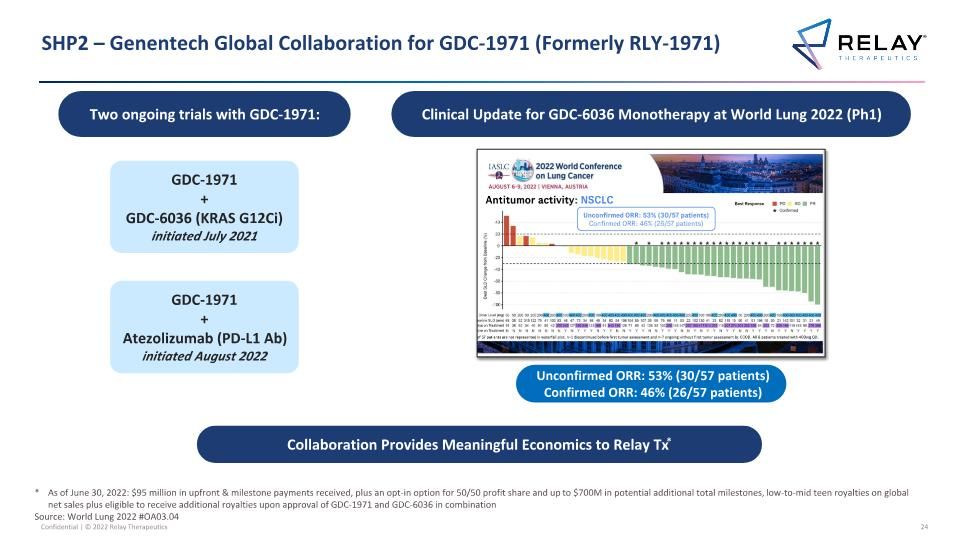
SHP2 – Genentech Global Collaboration for GDC-1971 (Formerly RLY-1971) GDC-1971 + Atezolizumab (PD-L1 Ab) initiated August 2022 Collaboration Provides Meaningful Economics to Relay Tx* * As of June 30, 2022: $95 million in upfront & milestone payments received, plus an opt-in option for 50/50 profit share and up to $700M in potential additional total milestones, low-to-mid teen royalties on global net sales plus eligible to receive additional royalties upon approval of GDC-1971 and GDC-6036 in combination Source: World Lung 2022 #OA03.04 GDC-1971 + GDC-6036 (KRAS G12Ci) initiated July 2021 Unconfirmed ORR: 53% (30/57 patients) Confirmed ORR: 46% (26/57 patients) Two ongoing trials with GDC-1971: Clinical Update for GDC-6036 Monotherapy at World Lung 2022 (Ph1)
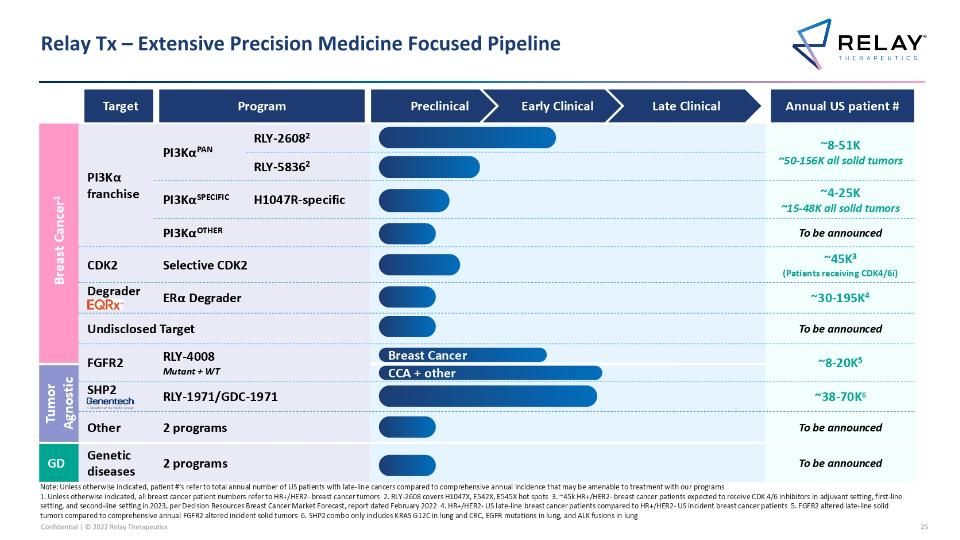
Relay Tx – Extensive Precision Medicine Focused Pipeline Breast Cancer1 PI3Kα franchise PI3KαPAN RLY-26082 RLY-2608 Pan-mutant allosteric inhibitor ~8-51K ~50-156K all solid tumors RLY-58362 PI3KαSPECIFIC H1047R-specific RLY-1047R H1047R allosteric inhibitor ~4-25K ~15-48K all solid tumors PI3KαOTHER Additional Other novel mutant selective mechanisms To be announced Challengers CDK2 Selective CDK2 ~45K3 (Patients receiving CDK4/6i) Challengers Degrader ERα Degrader ~30-195K4 Undisclosed Target To be announced FGFR2 RLY-4008 Mutant + WT ~8-20K5 Tumor Agnostic SHP2 RLY-1971/GDC-1971 ~38-70K6 Other 2 programs To be announced GD Genetic diseases 2 programs To be announced Relay Tx – Extensive Precision Medicine Focused Pipeline Target Program Annual US patient # CCA + other Preclinical Early Clinical Late Clinical Breast Cancer Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors 2. RLY-2608 covers H1047X, E542X, E545X hot spots 3. ~45k HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2023, per Decision Resources Breast Cancer Market Forecast, report dated February 2022 4. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients 5. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors 6. SHP2 combo only includes KRAS G12C in lung and CRC, EGFR mutations in lung, and ALK fusions in lung
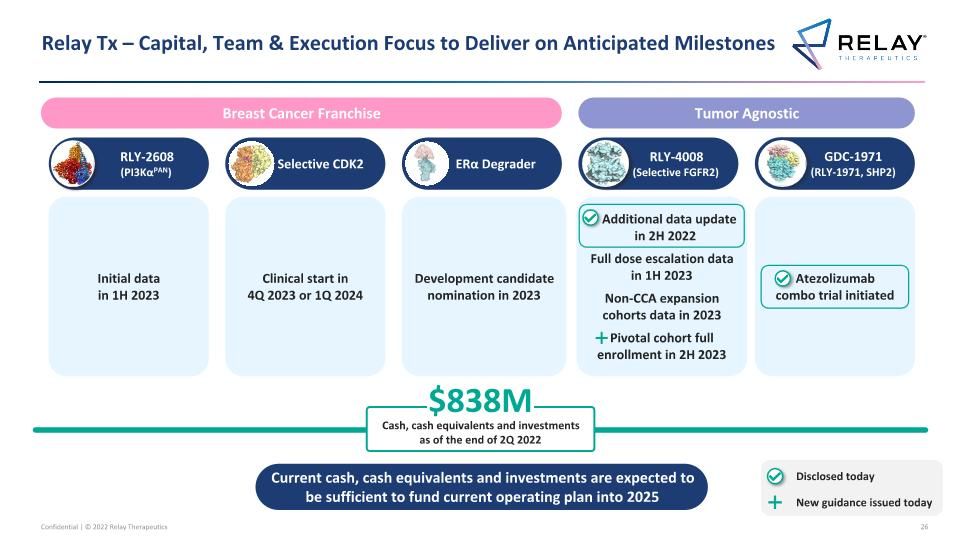
GDC-1971 (RLY-1971, SHP2) Relay Tx – Capital, Team & Execution Focus to Deliver on Anticipated Milestones RLY-4008 (Selective FGFR2) Clinical start in 4Q 2023 or 1Q 2024 Selective CDK2 Atezolizumab combo trial initiated RLY-2608 (PI3KαPAN) $838M Cash, cash equivalents and investments as of the end of 2Q 2022 Development candidate nomination in 2023 ERα Degrader Tumor Agnostic Breast Cancer Franchise Additional data update in 2H 2022 Full dose escalation data in 1H 2023 Non-CCA expansion cohorts data in 2023 Pivotal cohort full enrollment in 2H 2023 Current cash, cash equivalents and investments are expected to be sufficient to fund current operating plan into 2025 Initial data in 1H 2023 Disclosed today New guidance issued today



























