
J.P. Morgan Conference Presentation January 2024 Exhibit 99.1

Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, implied and express statements regarding the progress and timing of the clinical development of the programs across our portfolio, including the expected therapeutic benefits of our programs, timing of enrollment completion, and potential efficacy and tolerability; the timing of clinical data updates across our pipeline; the possibility that unconfirmed results from these trials will not be confirmed by additional data as our clinical trials progress; the potential of our product candidates to address a major unmet medical need; expectations regarding our pipeline, operating plan, use of capital, expenses and other financial results; our cash runway projection; the competitive landscape and potential market opportunities for our product candidates; the expected strategic benefits under our collaborations; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; expectations regarding current and future interactions with the U.S. Food and Drug Administration (FDA); our ability to manufacture our product candidates in conformity with the FDA’s requirements; the capabilities and development of our DynamoTM platform; our plans to develop, manufacture and commercialize our current product candidates and any future product candidates; and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. The words “may,” “might,” “will,” “could,” “would,” “should,” “plan,” “anticipate,” “intend,” “believe,” “expect,” “estimate,” “seek,” “predict,” “future,” “project,” “potential,” “continue,” “target” and similar words or expressions, or the negative thereof, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this presentation are based on management's current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, risks associated with: the impact of global economic uncertainty, geopolitical instability, or public health epidemics or outbreaks of an infectious disease on countries or regions in which we have operations or do business, as well as on the timing and anticipated results of our clinical trials, strategy, future operations and profitability; the delay of any current or planned clinical trials or the development of our drug candidates; the risk that the preliminary results of our preclinical or clinical trials may not be predictive of future or final results in connection with future clinical trials of our product candidates; our ability to successfully demonstrate the safety and efficacy of our drug candidates; the timing and outcome of our planned interactions with regulatory authorities; and obtaining, maintaining and protecting our intellectual property. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners.
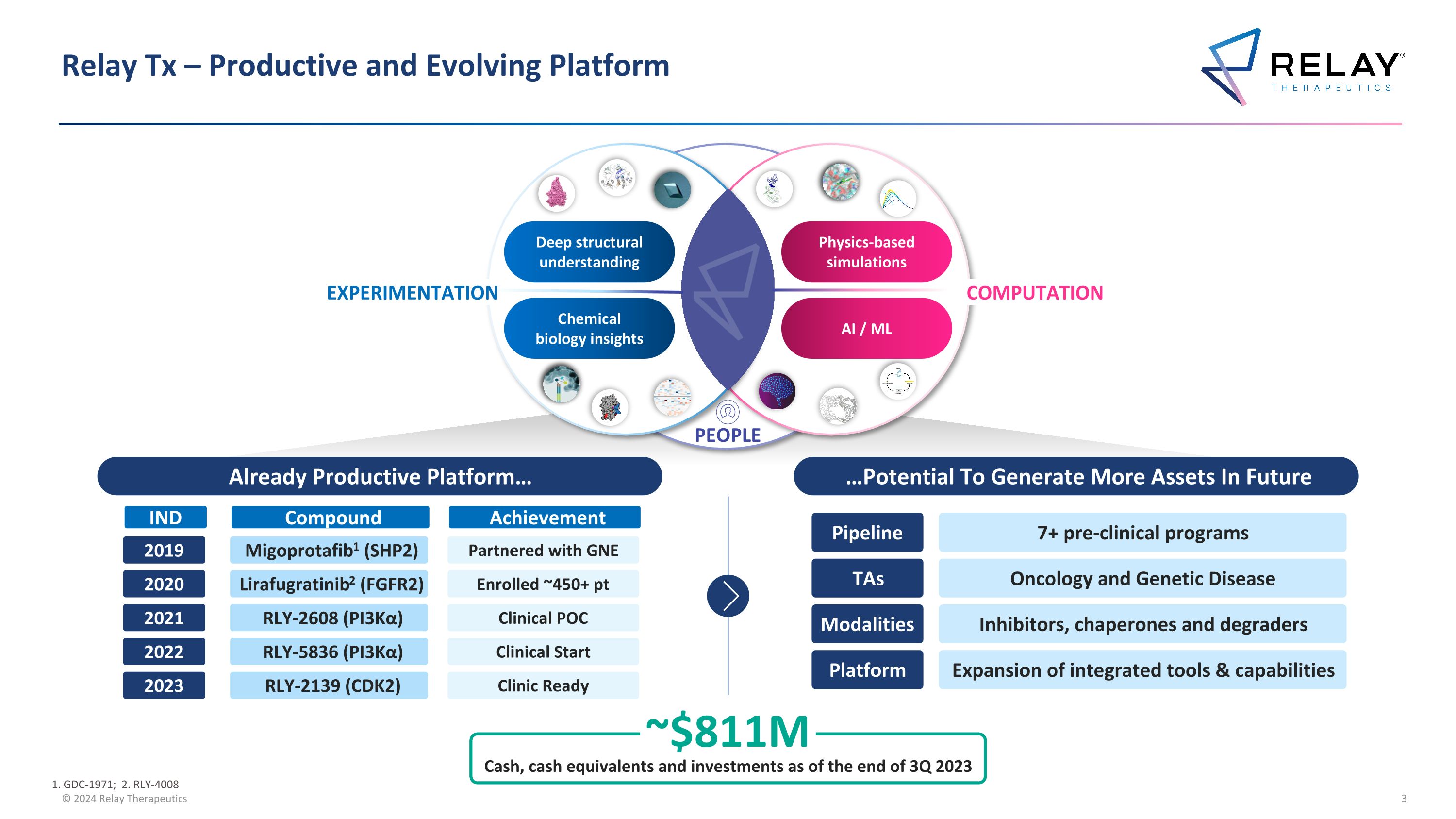
Relay Tx – Productive and Evolving Platform PEOPLE Chemical biology insights Deep structural understanding Physics-based simulations AI / ML EXPERIMENTATION COMPUTATION PEOPLE ~$811M Cash, cash equivalents and investments as of the end of 3Q 2023 Pipeline 7+ pre-clinical programs TAs Oncology and Genetic Disease Modalities Inhibitors, chaperones and degraders Platform Expansion of integrated tools & capabilities …Potential To Generate More Assets In Future Already Productive Platform… 2019 2020 2021 2022 2023 Migoprotafib1 (SHP2) Lirafugratinib2 (FGFR2) RLY-2608 (PI3Kα) RLY-5836 (PI3Kα) RLY-2139 (CDK2) Partnered with GNE Enrolled ~450+ pt Clinical POC Clinical Start Clinic Ready IND Compound Achievement 1. GDC-1971; 2. RLY-4008
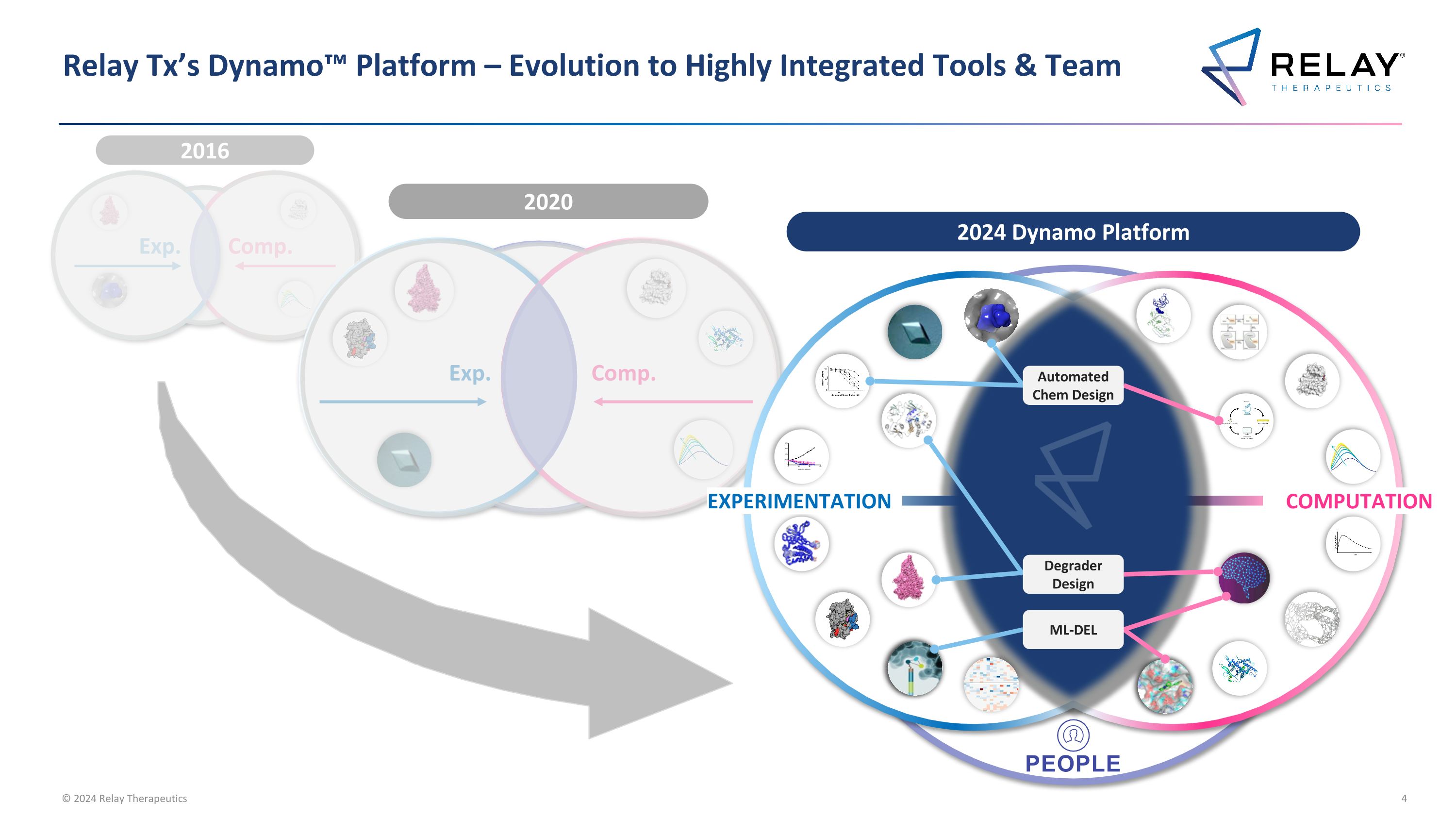
2016 Exp. Comp. Relay Tx’s Dynamo™ Platform – Evolution to Highly Integrated Tools & Team 2020 Exp. Comp. 2024 Dynamo Platform COMPUTATION PEOPLE EXPERIMENTATION Degrader Design ML-DEL Automated Chem Design
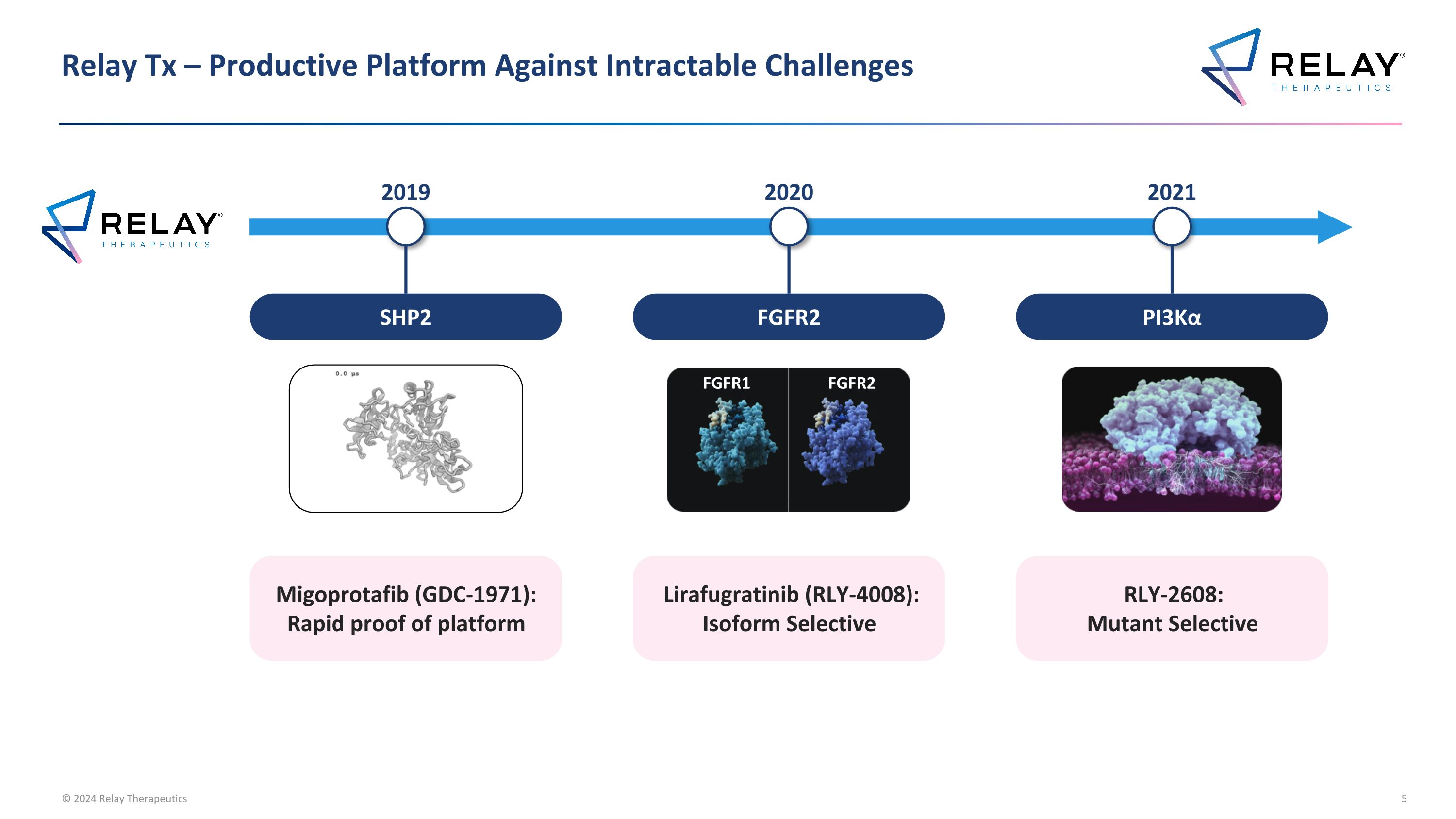
Relay Tx – Productive Platform Against Intractable Challenges FGFR2 FGFR1 FGFR2 Lirafugratinib (RLY-4008): Isoform Selective 2020 PI3Kα RLY-2608: Mutant Selective 2021 Migoprotafib (GDC-1971): Rapid proof of platform SHP2 2019
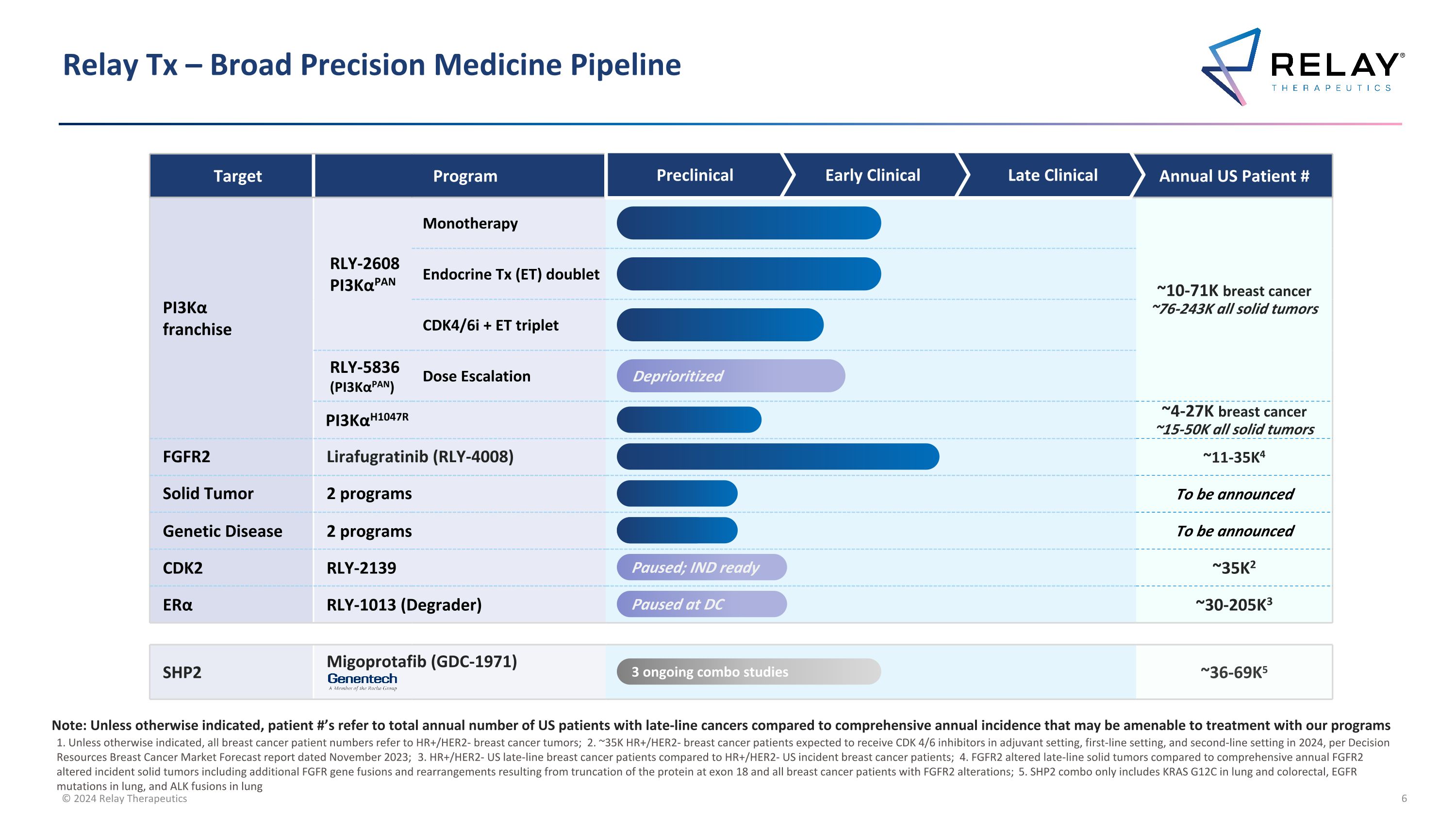
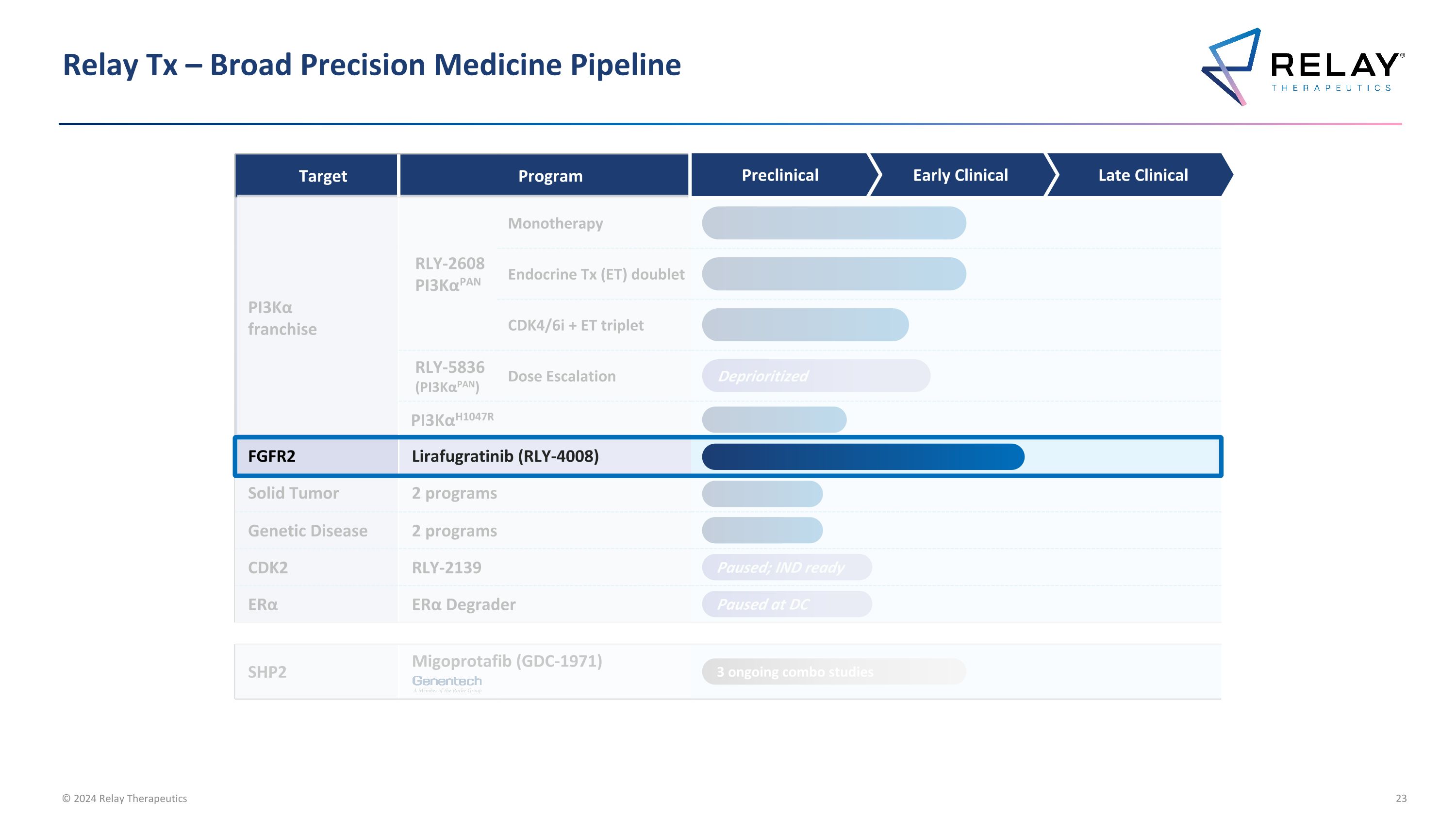
Relay Tx – Broad Precision Medicine Pipeline Target Program Annual US Patient # PI3Kα franchise RLY-2608 PI3KαPAN Monotherapy ~10-71K breast cancer ~76-243K all solid tumors Endocrine Tx (ET) doublet CDK4/6i + ET triplet RLY-5836 (PI3KαPAN) Dose Escalation PI3KαH1047R ~4-27K breast cancer ~15-50K all solid tumors FGFR2 Lirafugratinib (RLY-4008) ~11-35K4 Solid Tumor 2 programs To be announced Genetic Disease 2 programs To be announced CDK2 RLY-2139 ~35K2 ERα RLY-1013 (Degrader) ~30-205K3 SHP2 Migoprotafib (GDC-1971) ~36-69K5 Preclinical Early Clinical Late Clinical Paused; IND ready Paused at DC 3 ongoing combo studies 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors; 2. ~35K HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2024, per Decision Resources Breast Cancer Market Forecast report dated November 2023; 3. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients; 4. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors including additional FGFR gene fusions and rearrangements resulting from truncation of the protein at exon 18 and all breast cancer patients with FGFR2 alterations; 5. SHP2 combo only includes KRAS G12C in lung and colorectal, EGFR mutations in lung, and ALK fusions in lung Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs Deprioritized
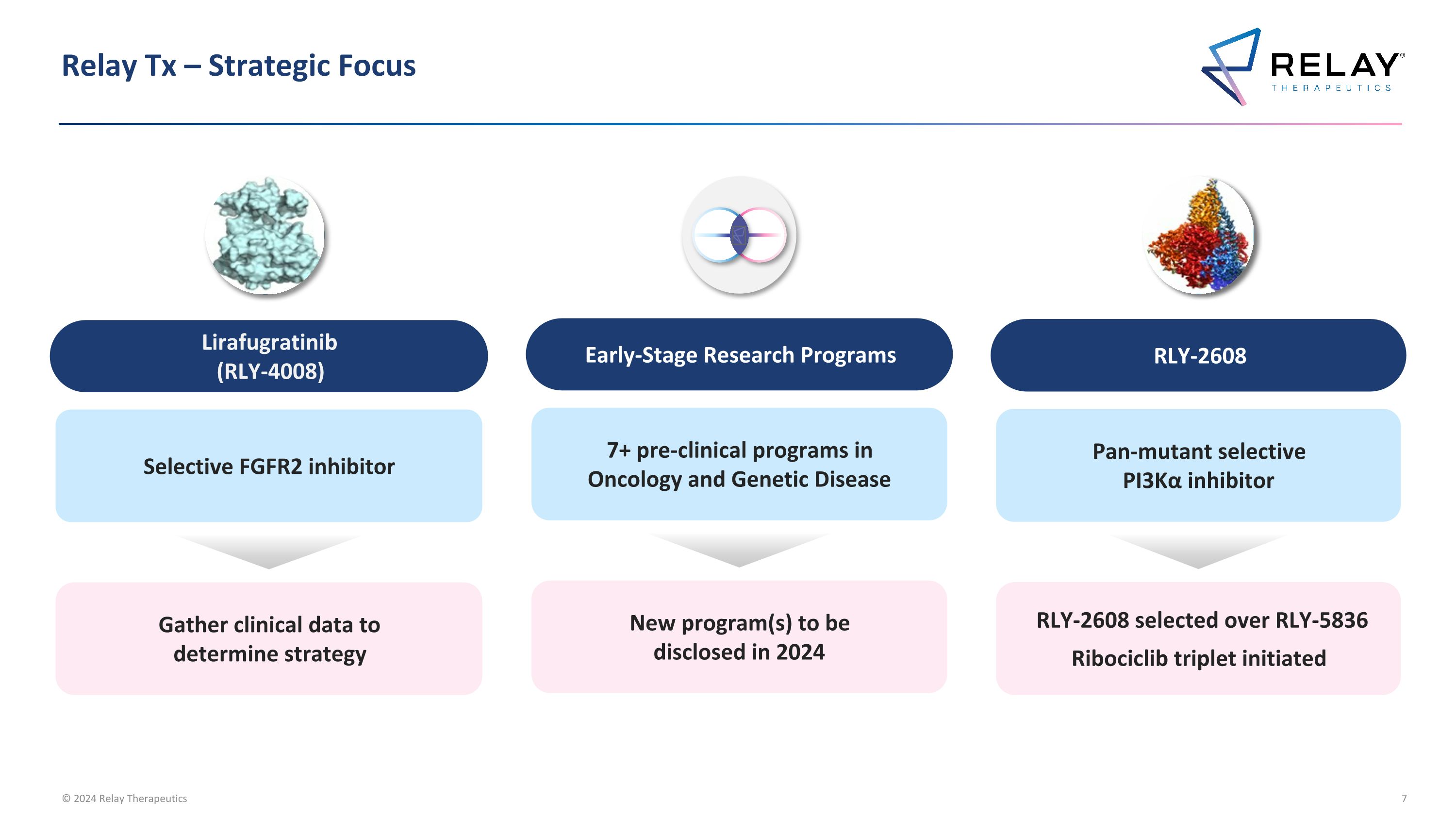
Relay Tx – Strategic Focus Early-Stage Research Programs 7+ pre-clinical programs in Oncology and Genetic Disease New program(s) to be disclosed in 2024 RLY-2608 Pan-mutant selective PI3Kα inhibitor RLY-2608 selected over RLY-5836 Ribociclib triplet initiated Lirafugratinib (RLY-4008) Selective FGFR2 inhibitor Gather clinical data to determine strategy
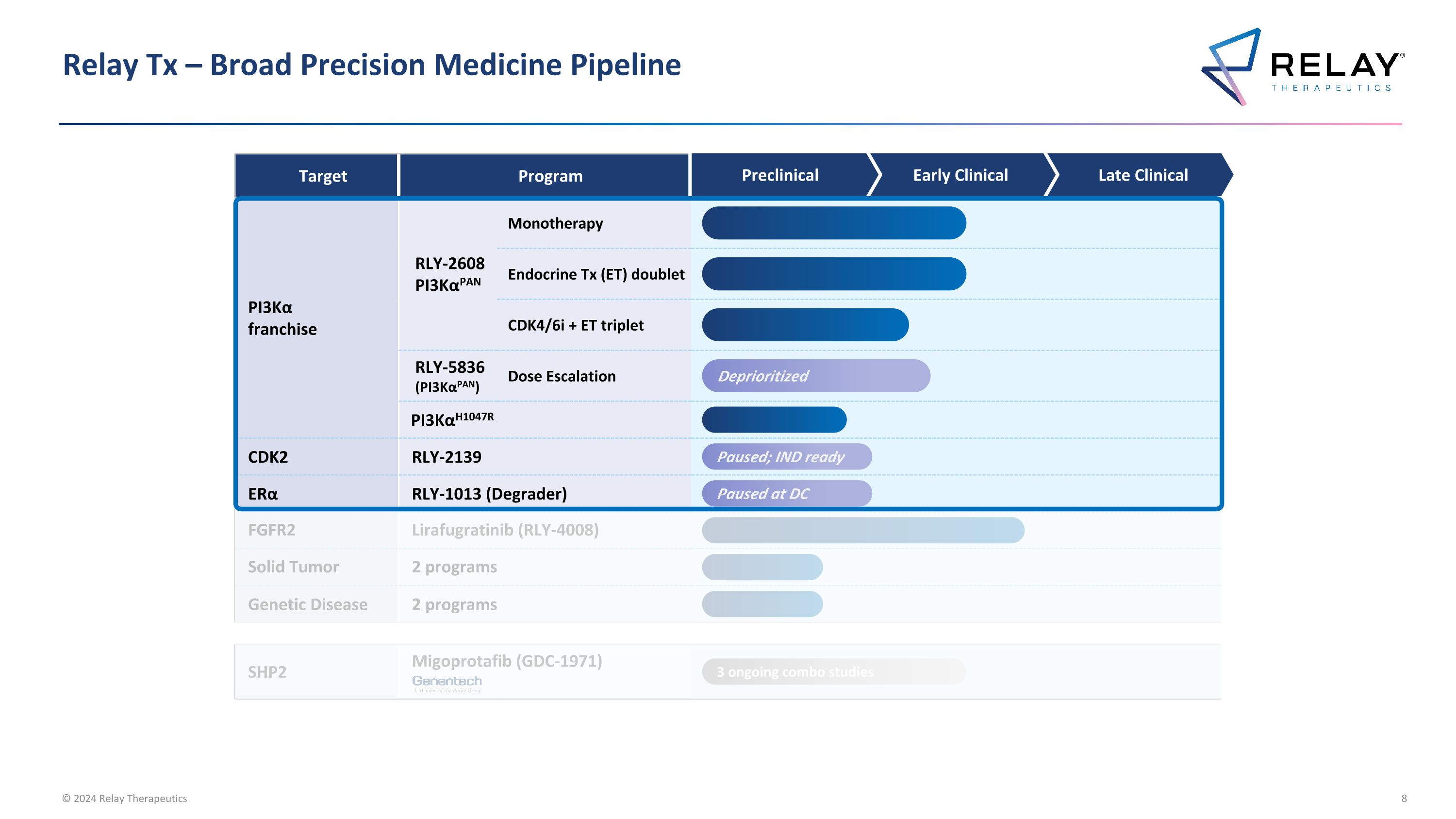
Relay Tx – Broad Precision Medicine Pipeline Target Program PI3Kα franchise RLY-2608 PI3KαPAN Monotherapy Endocrine Tx (ET) doublet CDK4/6i + ET triplet RLY-5836 (PI3KαPAN) Dose Escalation PI3KαH1047R CDK2 RLY-2139 ERα RLY-1013 (Degrader) FGFR2 Lirafugratinib (RLY-4008) Solid Tumor 2 programs Genetic Disease 2 programs SHP2 Migoprotafib (GDC-1971) Preclinical Early Clinical Late Clinical Paused; IND ready Paused at DC 3 ongoing combo studies Deprioritized
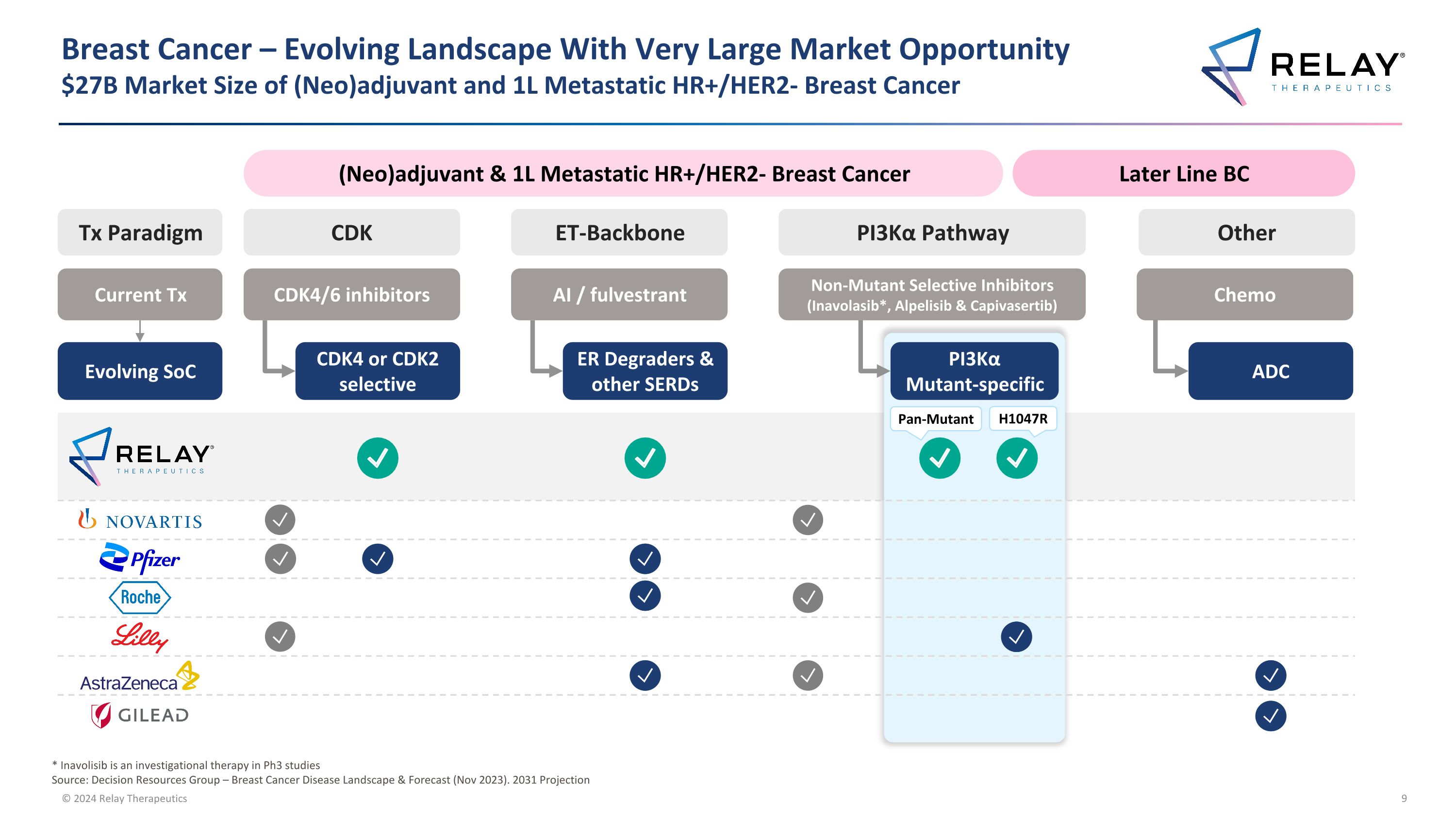
Breast Cancer – Evolving Landscape With Very Large Market Opportunity�$27B Market Size of (Neo)adjuvant and 1L Metastatic HR+/HER2- Breast Cancer (Neo)adjuvant & 1L Metastatic HR+/HER2- Breast Cancer Later Line BC Current Tx Evolving SoC * Inavolisib is an investigational therapy in Ph3 studies Source: Decision Resources Group – Breast Cancer Disease Landscape & Forecast (Nov 2023). 2031 Projection Tx Paradigm CDK4/6 inhibitors CDK4 or CDK2 selective CDK AI / fulvestrant ER Degraders & other SERDs ET-Backbone Chemo ADC Other PI3Kα Pathway PI3Kα Mutant-specific Non-Mutant Selective Inhibitors (Inavolasib*, Alpelisib & Capivasertib) Pan-Mutant H1047R
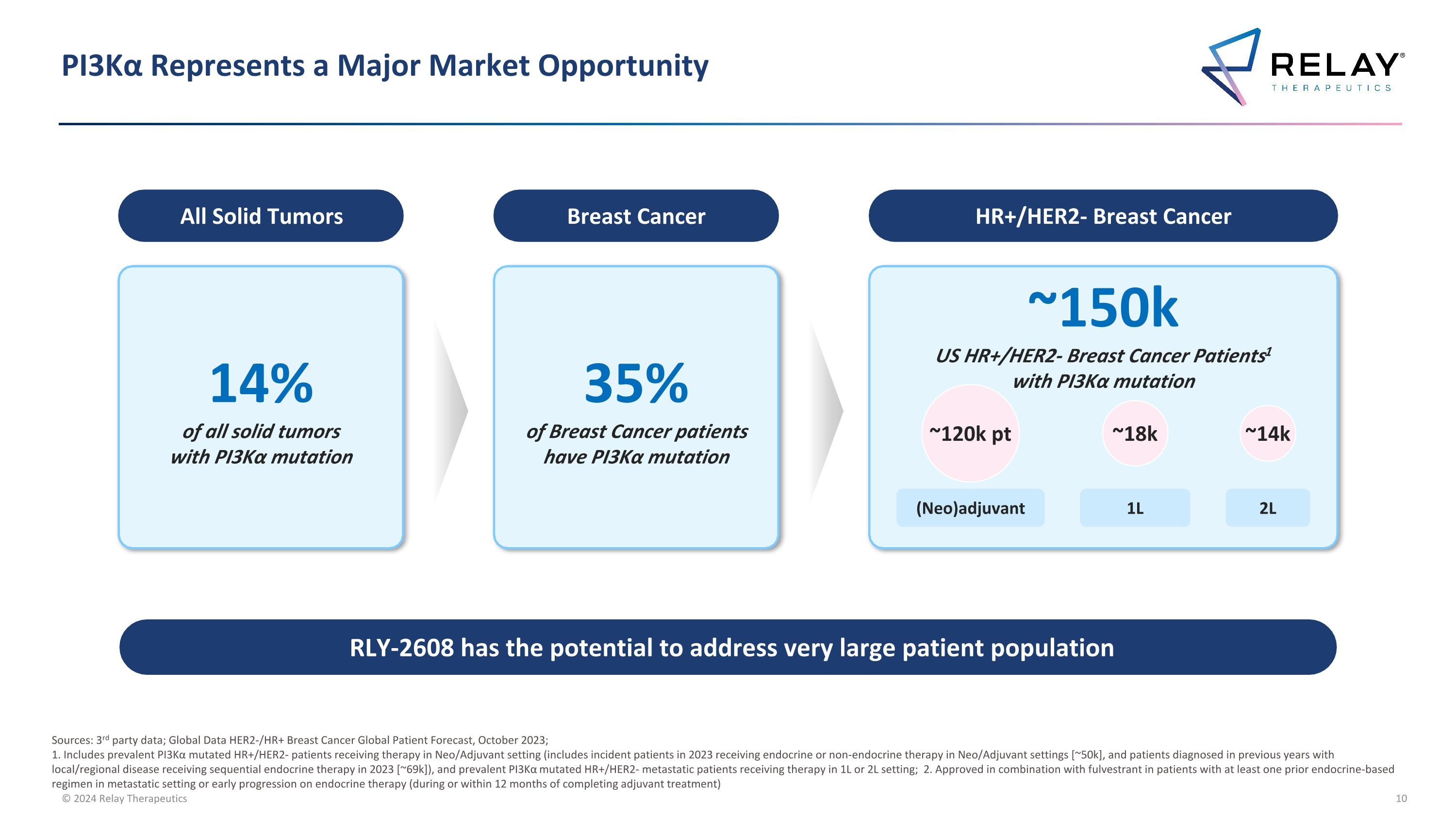
PI3Kα Represents a Major Market Opportunity 14% of all solid tumors with PI3Kα mutation All Solid Tumors 35% of Breast Cancer patients have PI3Kα mutation Breast Cancer ~150k US HR+/HER2- Breast Cancer Patients1 with PI3Kα mutation HR+/HER2- Breast Cancer ~14k ~18k ~120k pt 1L 2L (Neo)adjuvant RLY-2608 has the potential to address very large patient population Sources: 3rd party data; Global Data HER2-/HR+ Breast Cancer Global Patient Forecast, October 2023; 1. Includes prevalent PI3Kα mutated HR+/HER2- patients receiving therapy in Neo/Adjuvant setting (includes incident patients in 2023 receiving endocrine or non-endocrine therapy in Neo/Adjuvant settings [~50k], and patients diagnosed in previous years with local/regional disease receiving sequential endocrine therapy in 2023 [~69k]), and prevalent PI3Kα mutated HR+/HER2- metastatic patients receiving therapy in 1L or 2L setting; 2. Approved in combination with fulvestrant in patients with at least one prior endocrine-based regimen in metastatic setting or early progression on endocrine therapy (during or within 12 months of completing adjuvant treatment)
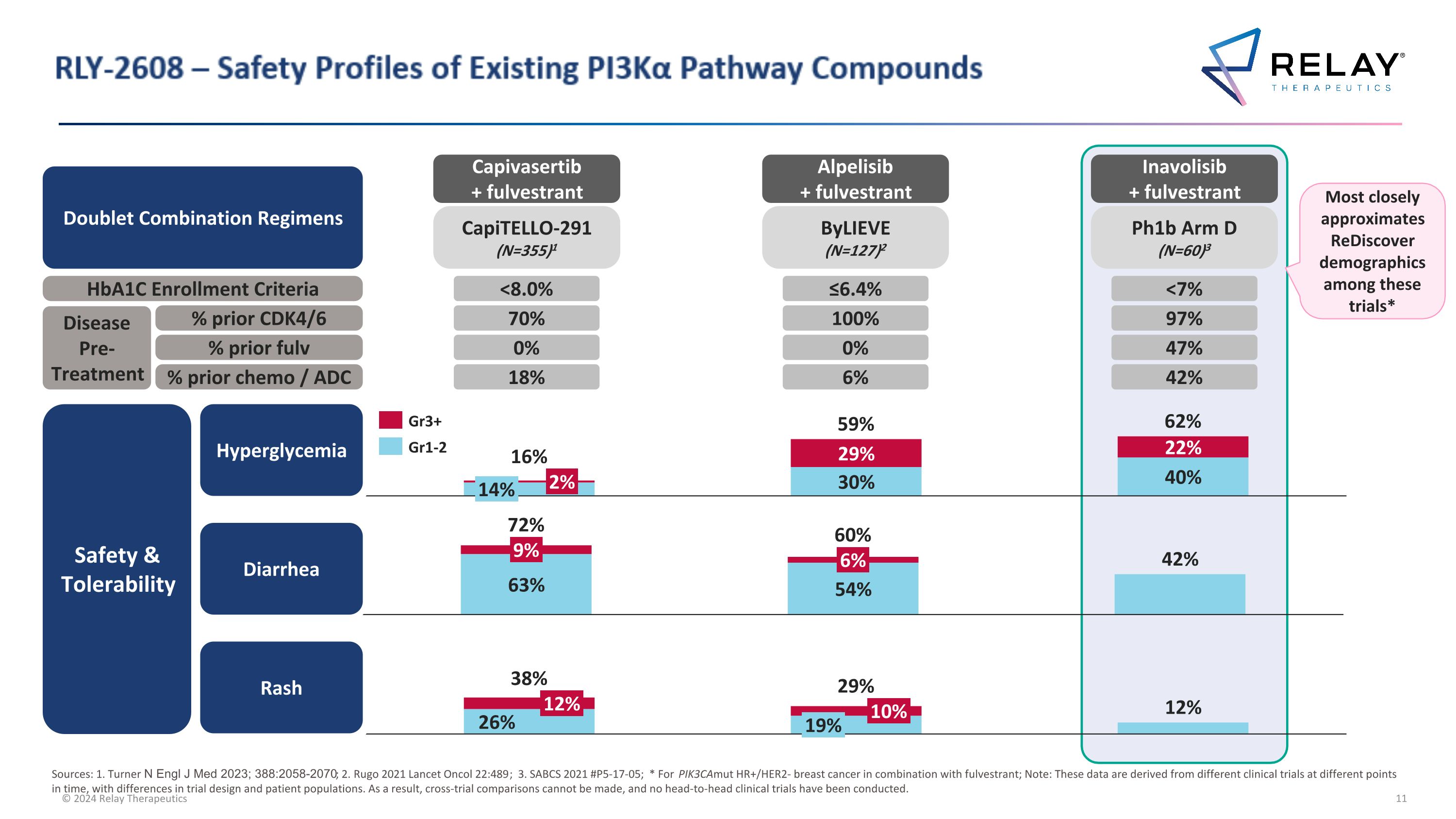
RLY-2608 – Safety Profiles of Existing PI3Kα Pathway Compounds 9% 6% 72% 60% 12% 10% 19% 38% 29% 2% 14% 16% 59% 62% Gr3+ Gr1-2 Diarrhea Rash Hyperglycemia Safety & Tolerability Sources: 1. Turner N Engl J Med 2023; 388:2058-2070; 2. Rugo 2021 Lancet Oncol 22:489; 3. SABCS 2021 #P5-17-05; * For PIK3CAmut HR+/HER2- breast cancer in combination with fulvestrant; Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Most closely approximates ReDiscover demographics among these trials* 97% 47% 42% 100% 0% 6% 70% 0% 18% Ph1b Arm D (N=60)3 ByLIEVE (N=127)2 CapiTELLO-291 (N=355)1 Inavolisib + fulvestrant Alpelisib + fulvestrant Capivasertib + fulvestrant Doublet Combination Regimens HbA1C Enrollment Criteria Disease Pre-Treatment % prior CDK4/6 % prior fulv % prior chemo / ADC <7% ≤6.4% <8.0%
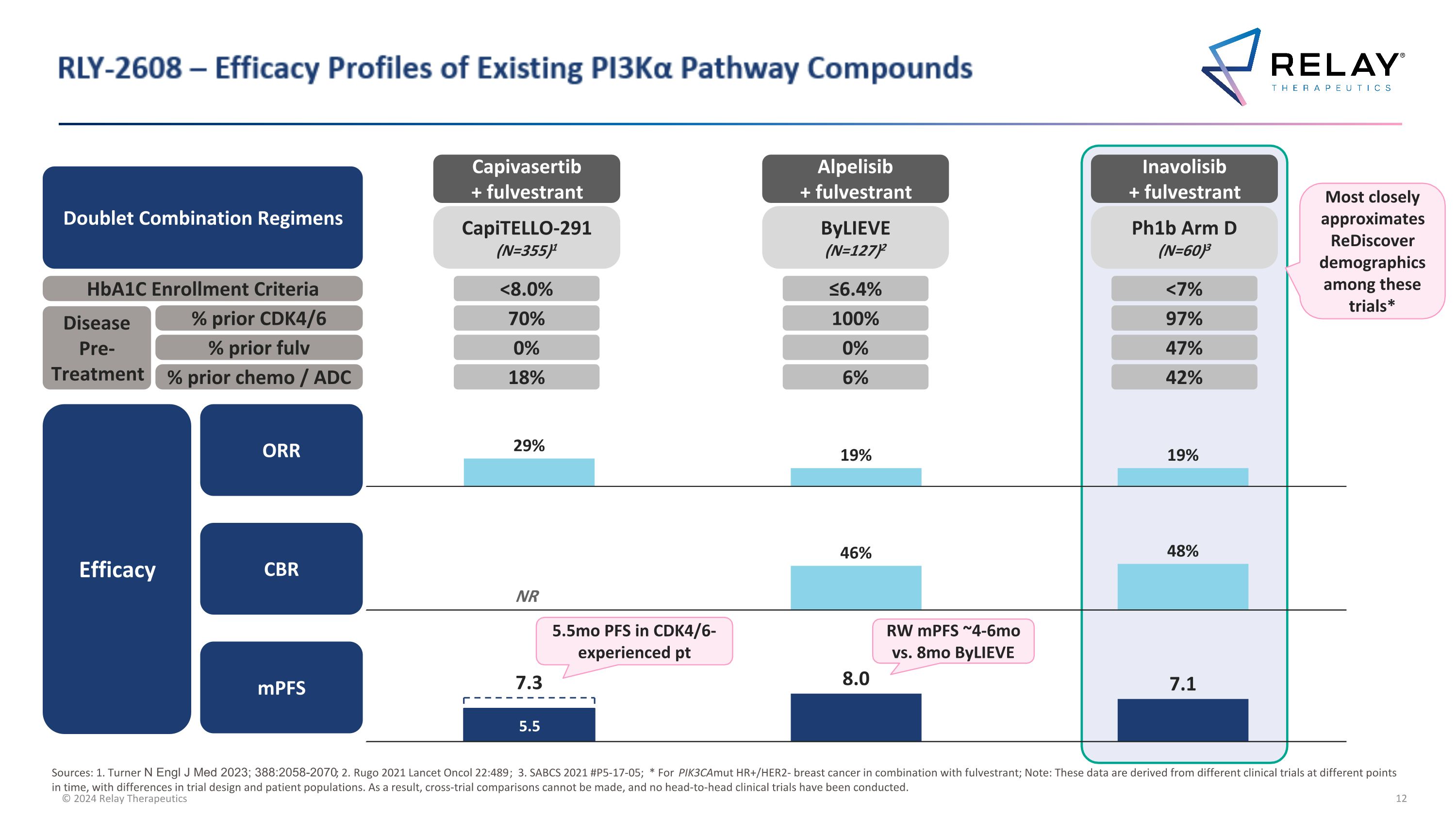
RLY-2608 – Efficacy Profiles of Existing PI3Kα Pathway Compounds 7.3 97% 47% 42% 100% 0% 6% 70% 0% 18% RW mPFS ~4-6mo vs. 8mo ByLIEVE 5.5mo PFS in CDK4/6-experienced pt Sources: 1. Turner N Engl J Med 2023; 388:2058-2070; 2. Rugo 2021 Lancet Oncol 22:489; 3. SABCS 2021 #P5-17-05; * For PIK3CAmut HR+/HER2- breast cancer in combination with fulvestrant; Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. NR CBR mPFS ORR Efficacy Ph1b Arm D (N=60)3 ByLIEVE (N=127)2 CapiTELLO-291 (N=355)1 Inavolisib + fulvestrant Alpelisib + fulvestrant Capivasertib + fulvestrant Most closely approximates ReDiscover demographics among these trials* Doublet Combination Regimens HbA1C Enrollment Criteria Disease Pre-Treatment % prior CDK4/6 % prior fulv % prior chemo / ADC <7% ≤6.4% <8.0%
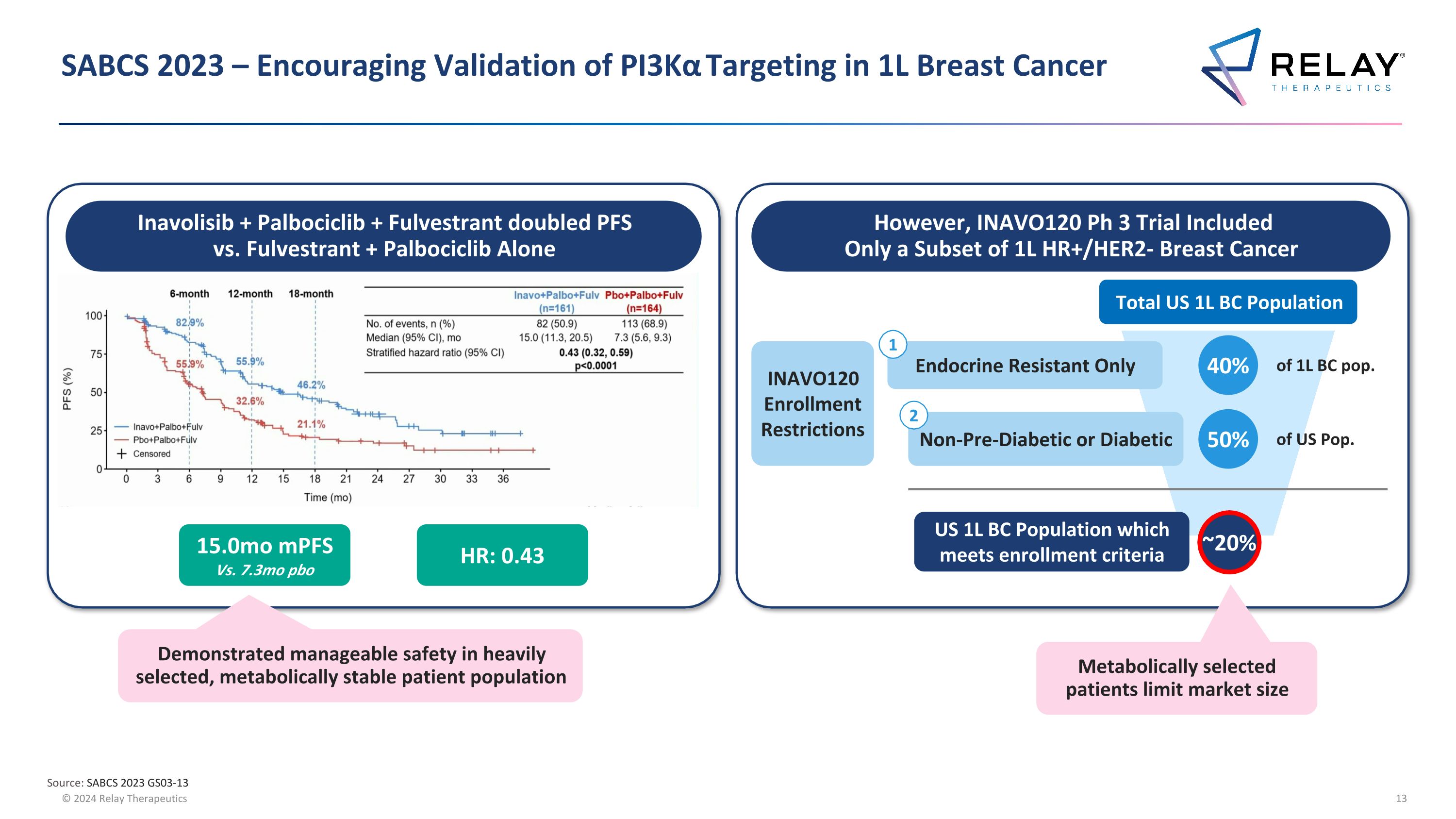
SABCS 2023 – Encouraging Validation of PI3Kα Targeting in 1L Breast Cancer Source: SABCS 2023 GS03-13 Inavolisib + Palbociclib + Fulvestrant doubled PFS vs. Fulvestrant + Palbociclib Alone 15.0mo mPFS Vs. 7.3mo pbo HR: 0.43 However, INAVO120 Ph 3 Trial Included Only a Subset of 1L HR+/HER2- Breast Cancer Total US 1L BC Population Endocrine Resistant Only 40% of 1L BC pop. Non-Pre-Diabetic or Diabetic 50% of US Pop. US 1L BC Population which meets enrollment criteria ~20% INAVO120 Enrollment Restrictions 1 2 Demonstrated manageable safety in heavily selected, metabolically stable patient population Metabolically selected patients limit market size
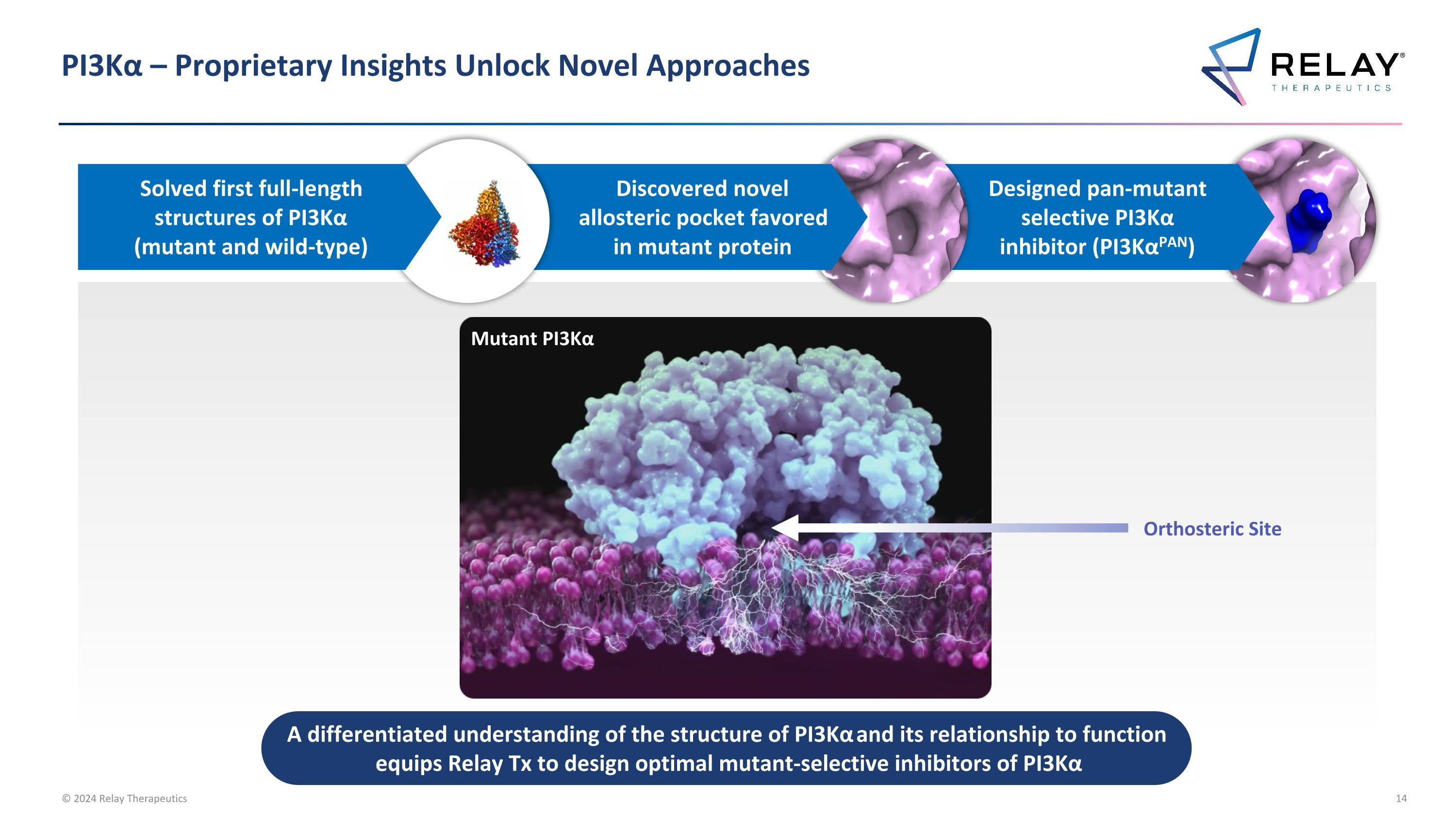
PI3Kα – Proprietary Insights Unlock Novel Approaches A differentiated understanding of the structure of PI3Kα and its relationship to function equips Relay Tx to design optimal mutant-selective inhibitors of PI3Kα Designed pan-mutant selective PI3Kα inhibitor (PI3KαPAN) Discovered novel allosteric pocket favored in mutant protein Solved first full-length structures of PI3Kα (mutant and wild-type) Mutant PI3Kα Orthosteric Site
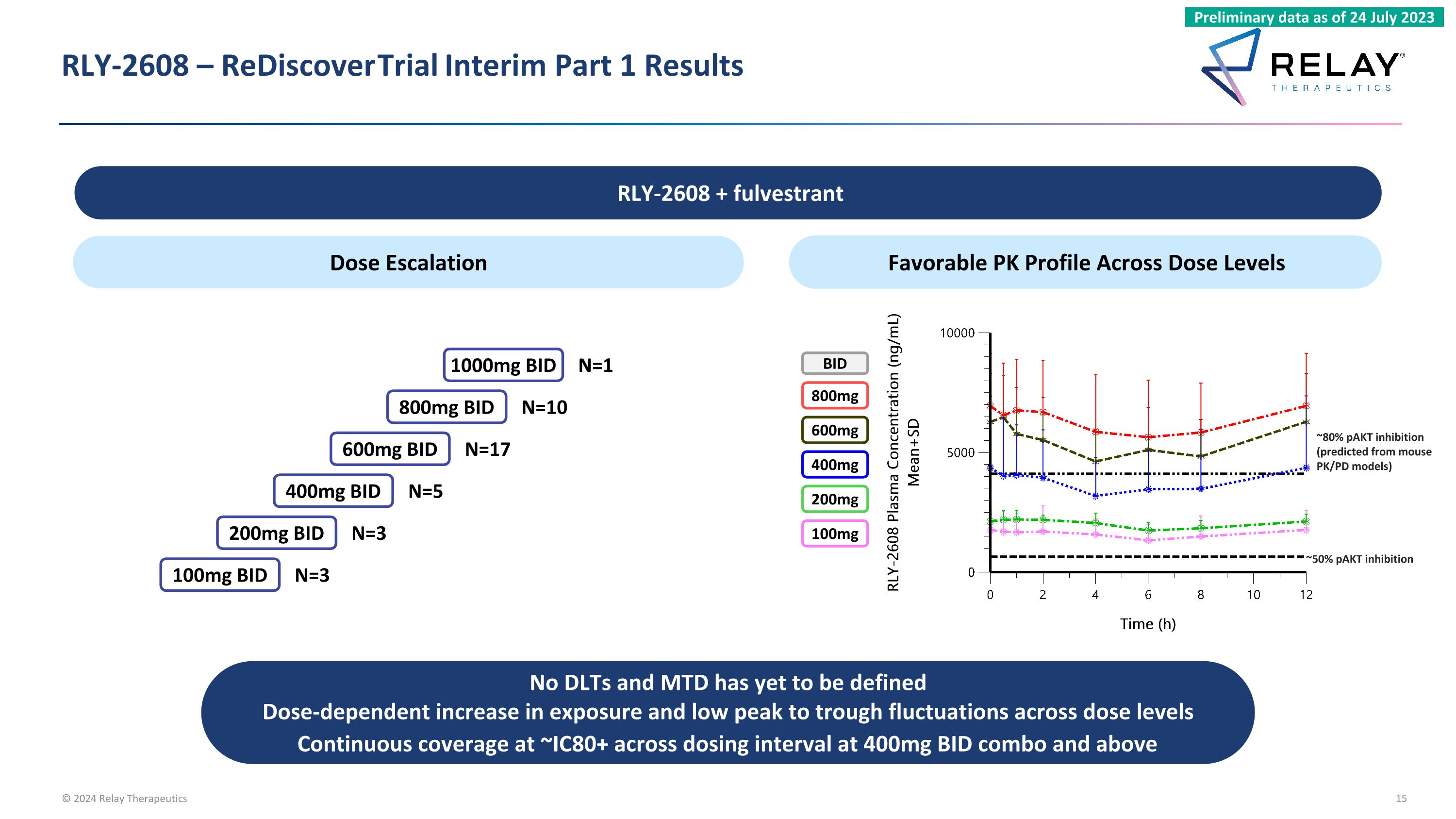
RLY-2608 – ReDiscover Trial Interim Part 1 Results Dose Escalation Favorable PK Profile Across Dose Levels ~50% pAKT inhibition 400mg 200mg 100mg 800mg 600mg BID ~80% pAKT inhibition (predicted from mouse PK/PD models) ~50% pAKT inhibition No DLTs and MTD has yet to be defined Dose-dependent increase in exposure and low peak to trough fluctuations across dose levels Continuous coverage at ~IC80+ across dosing interval at 400mg BID combo and above RLY-2608 + fulvestrant Preliminary data as of 24 July 2023 400mg BID 200mg BID 100mg BID 800mg BID 600mg BID 1000mg BID N=17 N=10 N=3 N=3 N=5 N=1
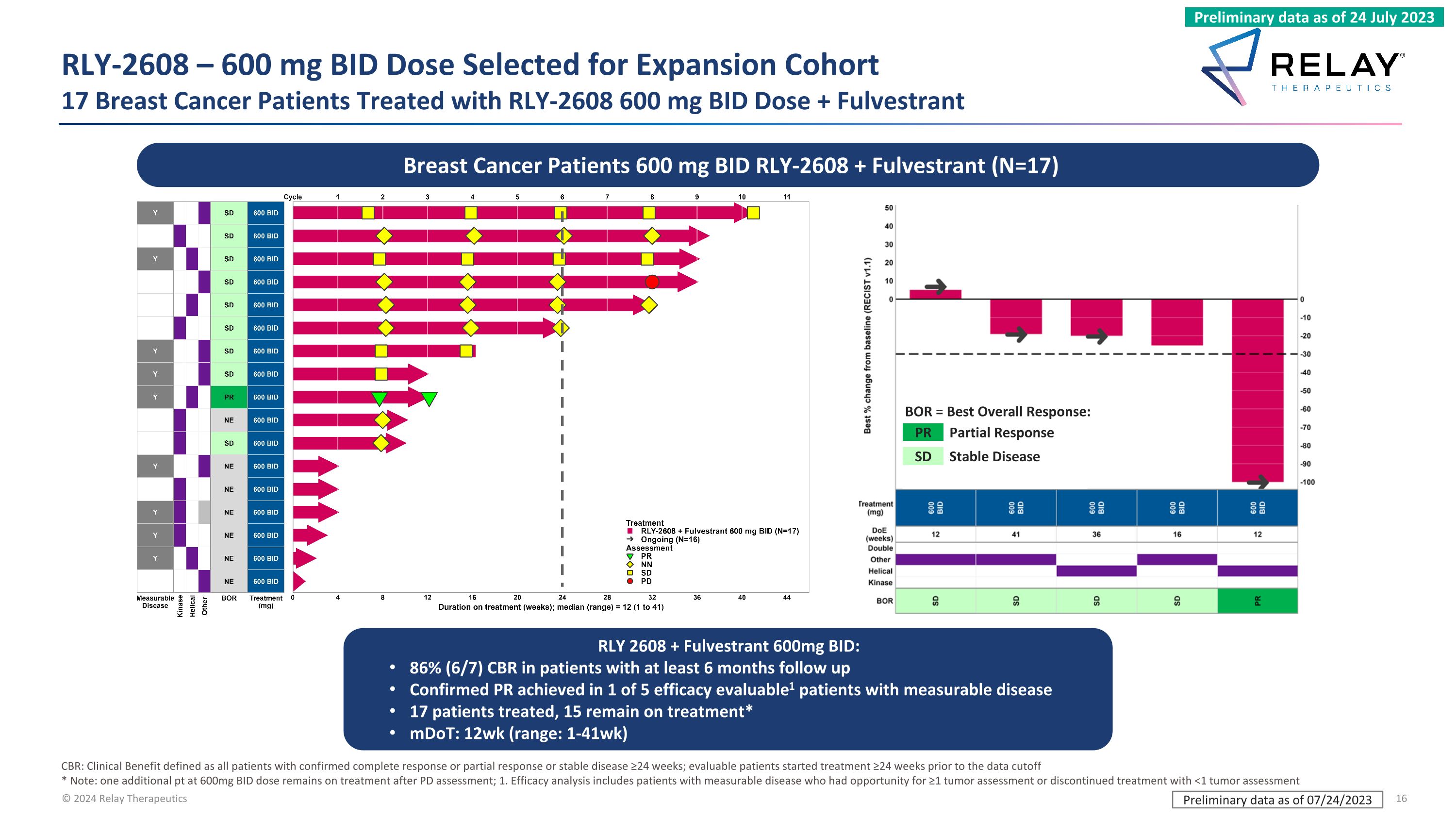
RLY-2608 – 600 mg BID Dose Selected for Expansion Cohort�17 Breast Cancer Patients Treated with RLY-2608 600 mg BID Dose + Fulvestrant Preliminary data as of 07/24/2023 Breast Cancer Patients 600 mg BID RLY-2608 + Fulvestrant (N=17) RLY 2608 + Fulvestrant 600mg BID: 86% (6/7) CBR in patients with at least 6 months follow up Confirmed PR achieved in 1 of 5 efficacy evaluable1 patients with measurable disease 17 patients treated, 15 remain on treatment* mDoT: 12wk (range: 1-41wk) SD Stable Disease BOR = Best Overall Response: Partial Response PR CBR: Clinical Benefit defined as all patients with confirmed complete response or partial response or stable disease ≥24 weeks; evaluable patients started treatment ≥24 weeks prior to the data cutoff * Note: one additional pt at 600mg BID dose remains on treatment after PD assessment; 1. Efficacy analysis includes patients with measurable disease who had opportunity for ≥1 tumor assessment or discontinued treatment with <1 tumor assessment Preliminary data as of 24 July 2023
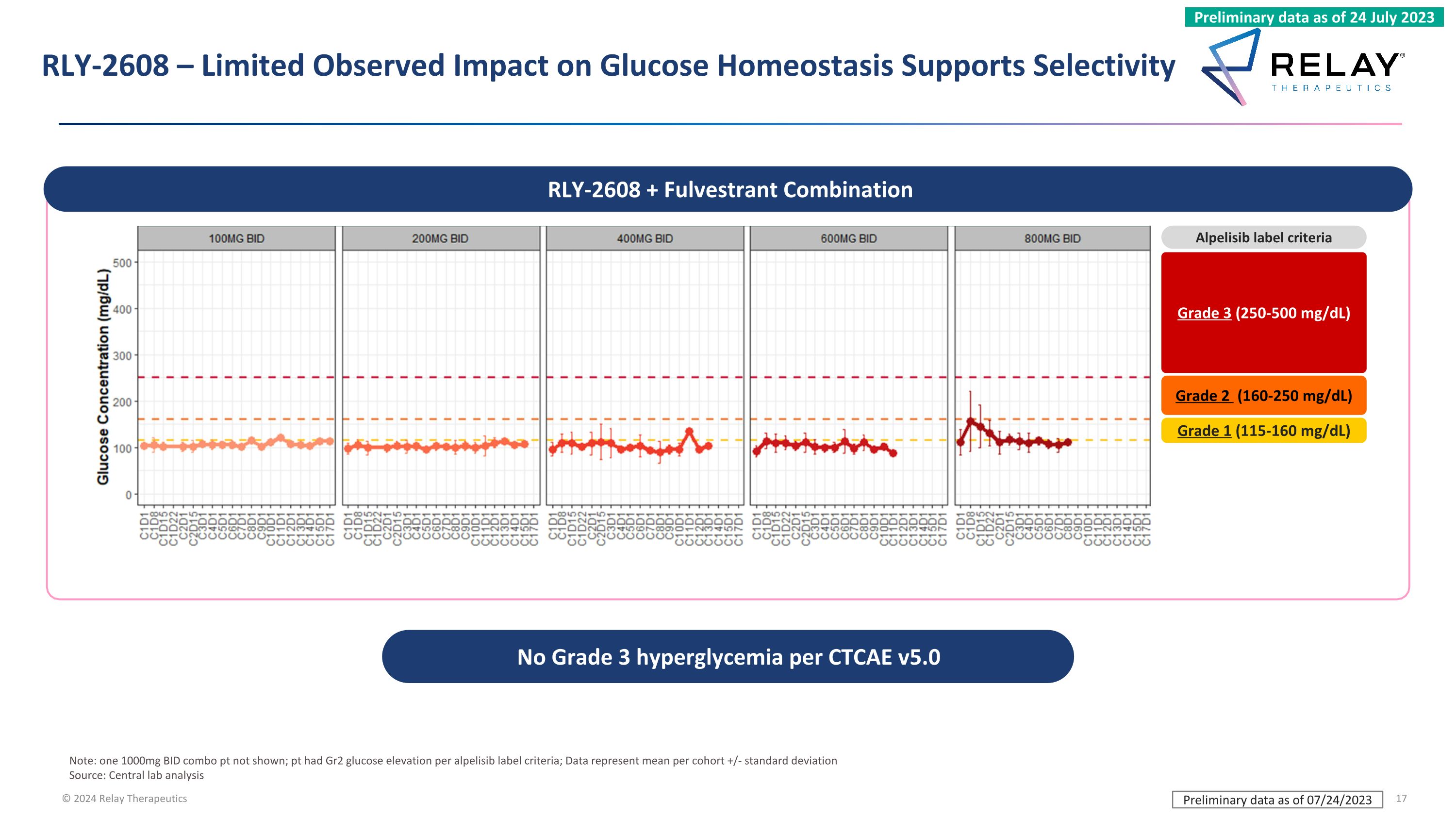
Note: one 1000mg BID combo pt not shown; pt had Gr2 glucose elevation per alpelisib label criteria; Data represent mean per cohort +/- standard deviation Source: Central lab analysis RLY-2608 – Limited Observed Impact on Glucose Homeostasis Supports Selectivity Grade 3 (250-500 mg/dL) Grade 2 (160-250 mg/dL) Grade 1 (115-160 mg/dL) Alpelisib label criteria RLY-2608 + Fulvestrant Combination No Grade 3 hyperglycemia per CTCAE v5.0 Preliminary data as of 07/24/2023 Preliminary data as of 24 July 2023
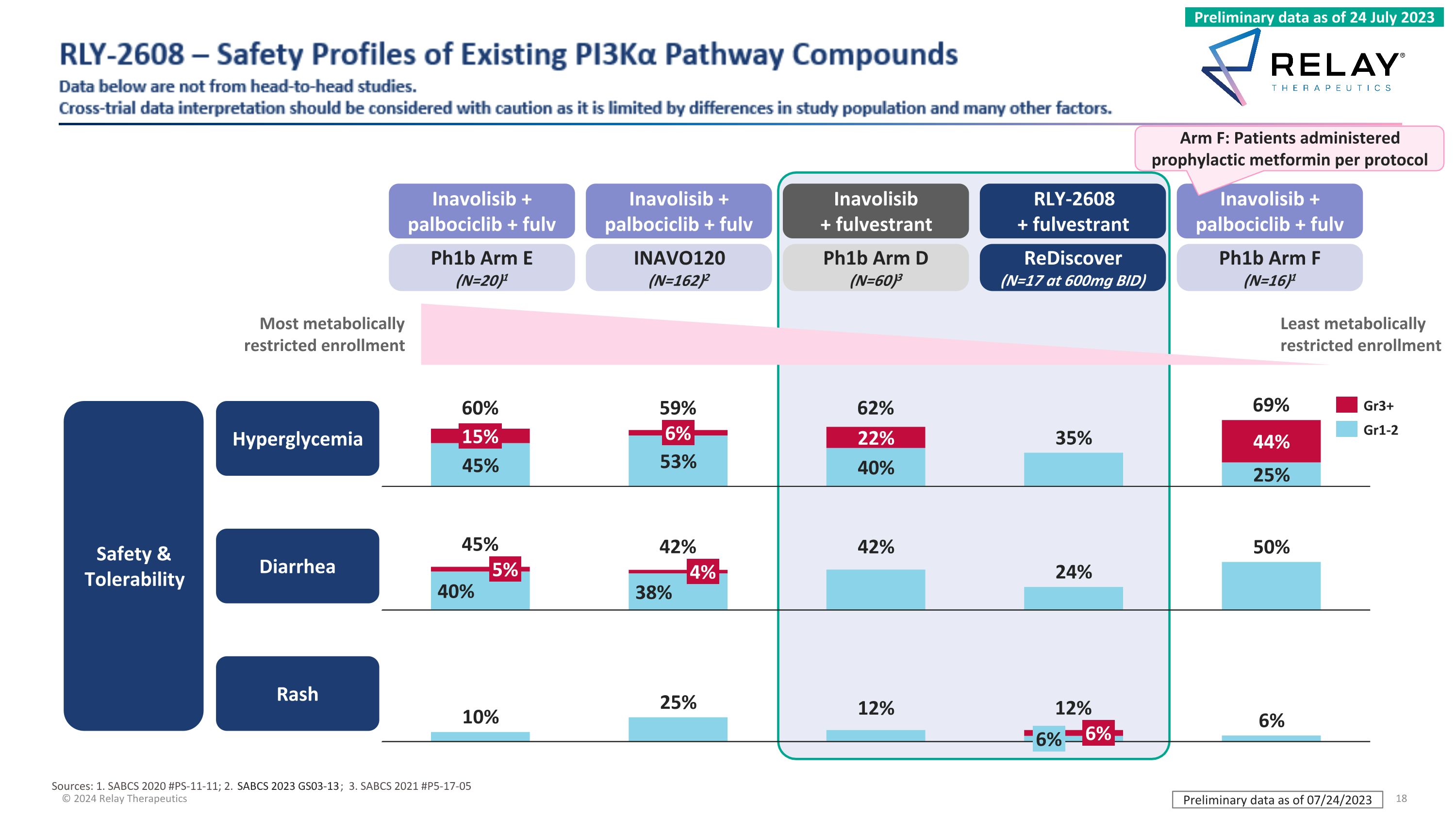
RLY-2608 – Safety Profiles of Existing PI3Kα Pathway Compounds Data below are not from head-to-head studies. Cross-trial data interpretation should be considered with caution as it is limited by differences in study population and many other factors. Ph1b Arm D (N=60)3 Inavolisib + fulvestrant Ph1b Arm F (N=16)1 Inavolisib + palbociclib + fulv 15% 6% 60% 59% 62% 69% 5% 4% 45% 42% 6% 6% 12% Gr3+ Gr1-2 Diarrhea Rash Hyperglycemia Safety & Tolerability Inavolisib + palbociclib + fulv INAVO120 (N=162)2 Ph1b Arm E (N=20)1 Inavolisib + palbociclib + fulv ReDiscover (N=17 at 600mg BID) RLY-2608 + fulvestrant Most metabolically restricted enrollment Arm F: Patients administered prophylactic metformin per protocol Sources: 1. SABCS 2020 #PS-11-11; 2. SABCS 2023 GS03-13; 3. SABCS 2021 #P5-17-05 Preliminary data as of 07/24/2023 Preliminary data as of 24 July 2023 Least metabolically restricted enrollment
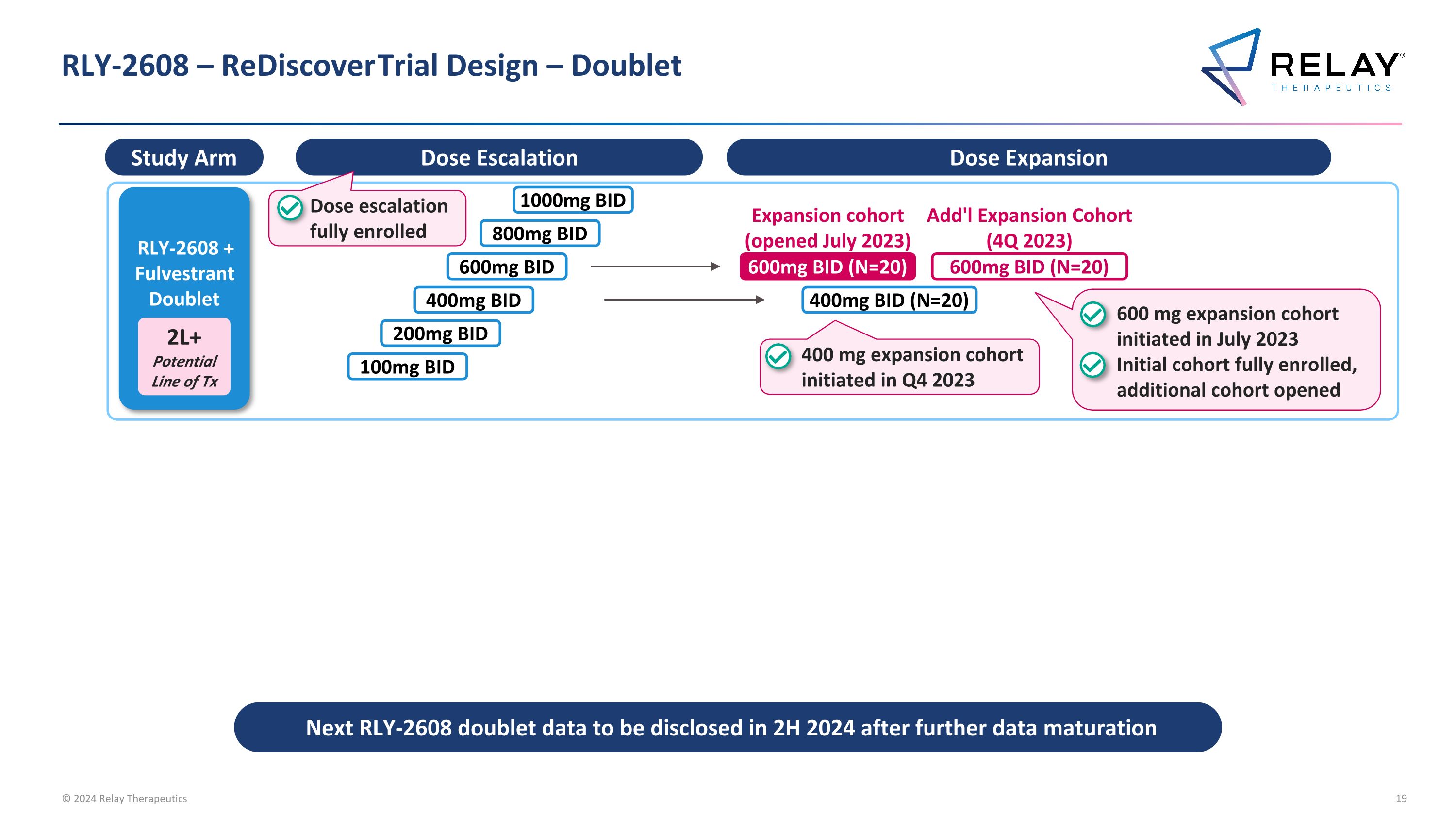
RLY-2608 – ReDiscover Trial Design – Doublet Dose Escalation Dose Expansion RLY-2608 + Fulvestrant Doublet 400mg BID 200mg BID 100mg BID 800mg BID 600mg BID 1000mg BID Expansion cohort (opened July 2023) Add'l Expansion Cohort (4Q 2023) 600mg BID (N=20) 600mg BID (N=20) 400mg BID (N=20) Dose escalation fully enrolled 600 mg expansion cohort initiated in July 2023 Initial cohort fully enrolled, additional cohort opened 2L+ Potential Line of Tx Next RLY-2608 doublet data to be disclosed in 2H 2024 after further data maturation 400 mg expansion cohort initiated in Q4 2023 Study Arm
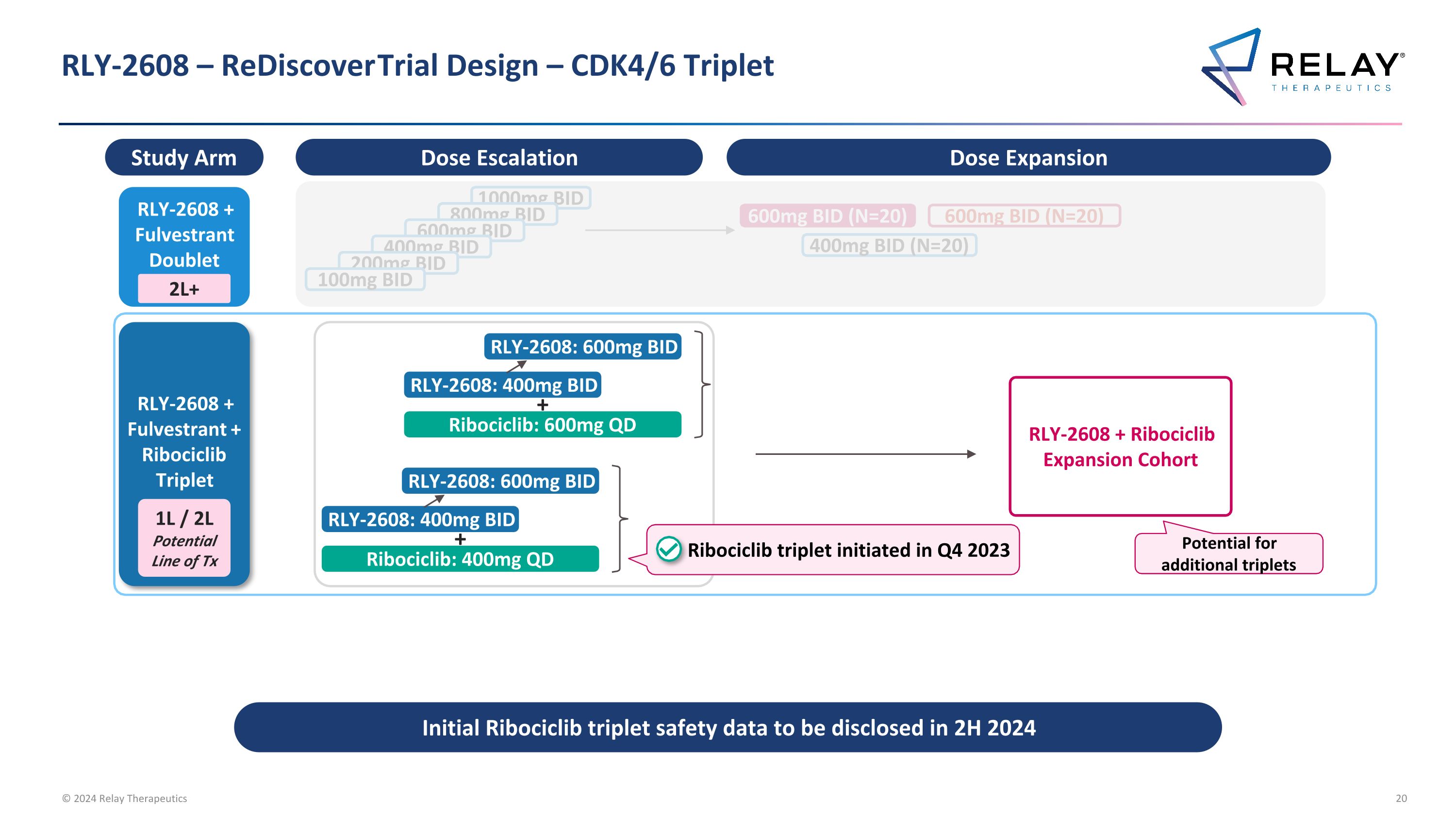
RLY-2608 – ReDiscover Trial Design – CDK4/6 Triplet Initial Ribociclib triplet safety data to be disclosed in 2H 2024 RLY-2608 + Fulvestrant Doublet 600mg BID (N=20) 400mg BID (N=20) 2L+ 1000mg BID 800mg BID 600mg BID 400mg BID 200mg BID 100mg BID RLY-2608 + Fulvestrant + Ribociclib Triplet RLY-2608: 400mg BID RLY-2608: 600mg BID Ribociclib: 600mg QD + RLY-2608: 400mg BID RLY-2608: 600mg BID Ribociclib: 400mg QD + RLY-2608 + Ribociclib Expansion Cohort 1L / 2L Potential Line of Tx Ribociclib triplet initiated in Q4 2023 Dose Escalation Dose Expansion 600mg BID (N=20) Study Arm Potential for additional triplets
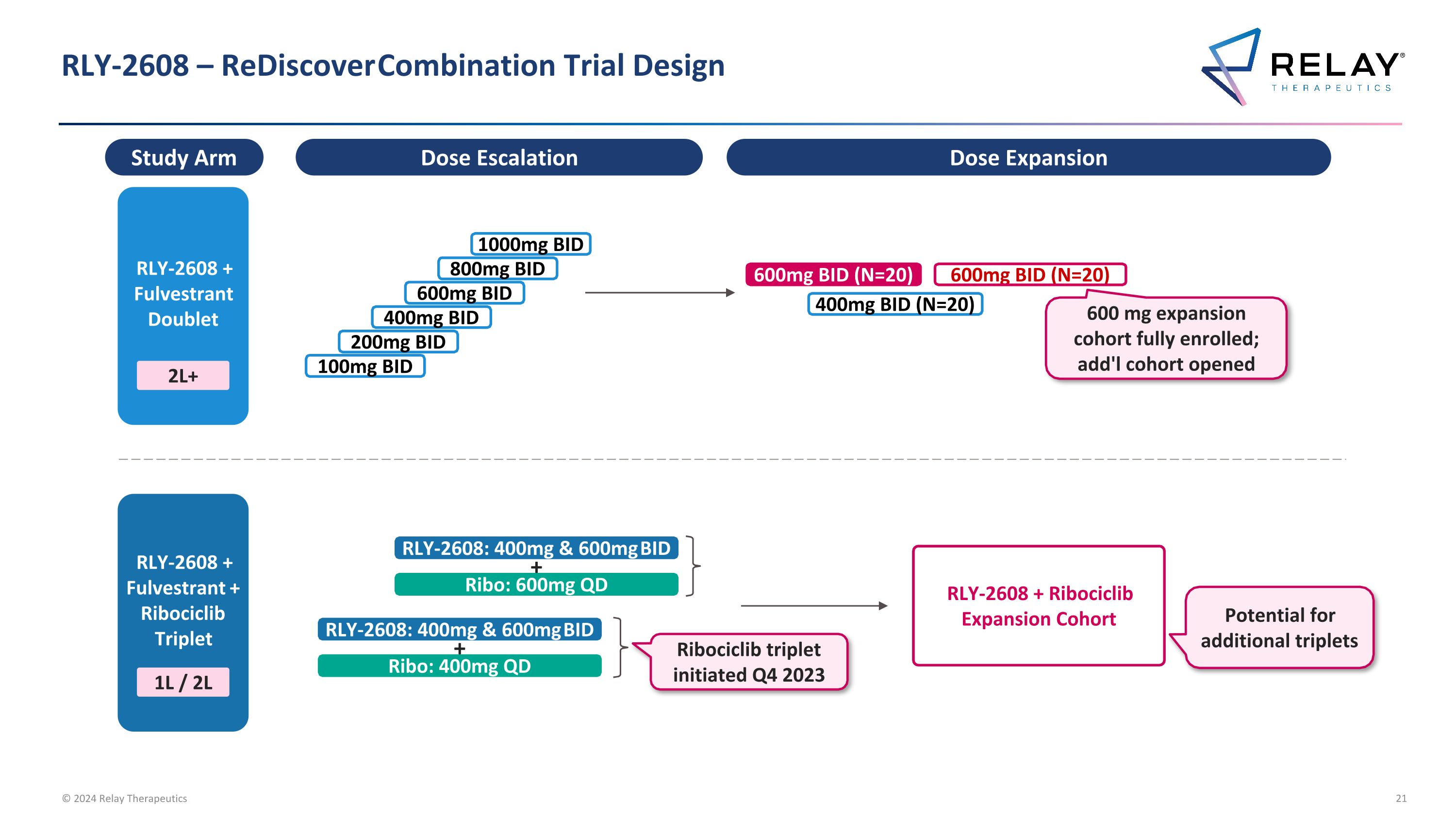
RLY-2608 – ReDiscover Combination Trial Design Dose Escalation Dose Expansion RLY-2608 + Fulvestrant Doublet 2L+ RLY-2608 + Fulvestrant + Ribociclib Triplet 1L / 2L 600mg BID (N=20) 400mg BID (N=20) 1000mg BID 800mg BID 600mg BID 400mg BID 200mg BID 100mg BID 600mg BID (N=20) 600 mg expansion cohort fully enrolled; add'l cohort opened Study Arm RLY-2608 + Ribociclib Expansion Cohort RLY-2608: 400mg & 600mg BID Ribo: 400mg QD + RLY-2608: 400mg & 600mg BID Ribo: 600mg QD + Ribociclib triplet initiated Q4 2023 Potential for additional triplets
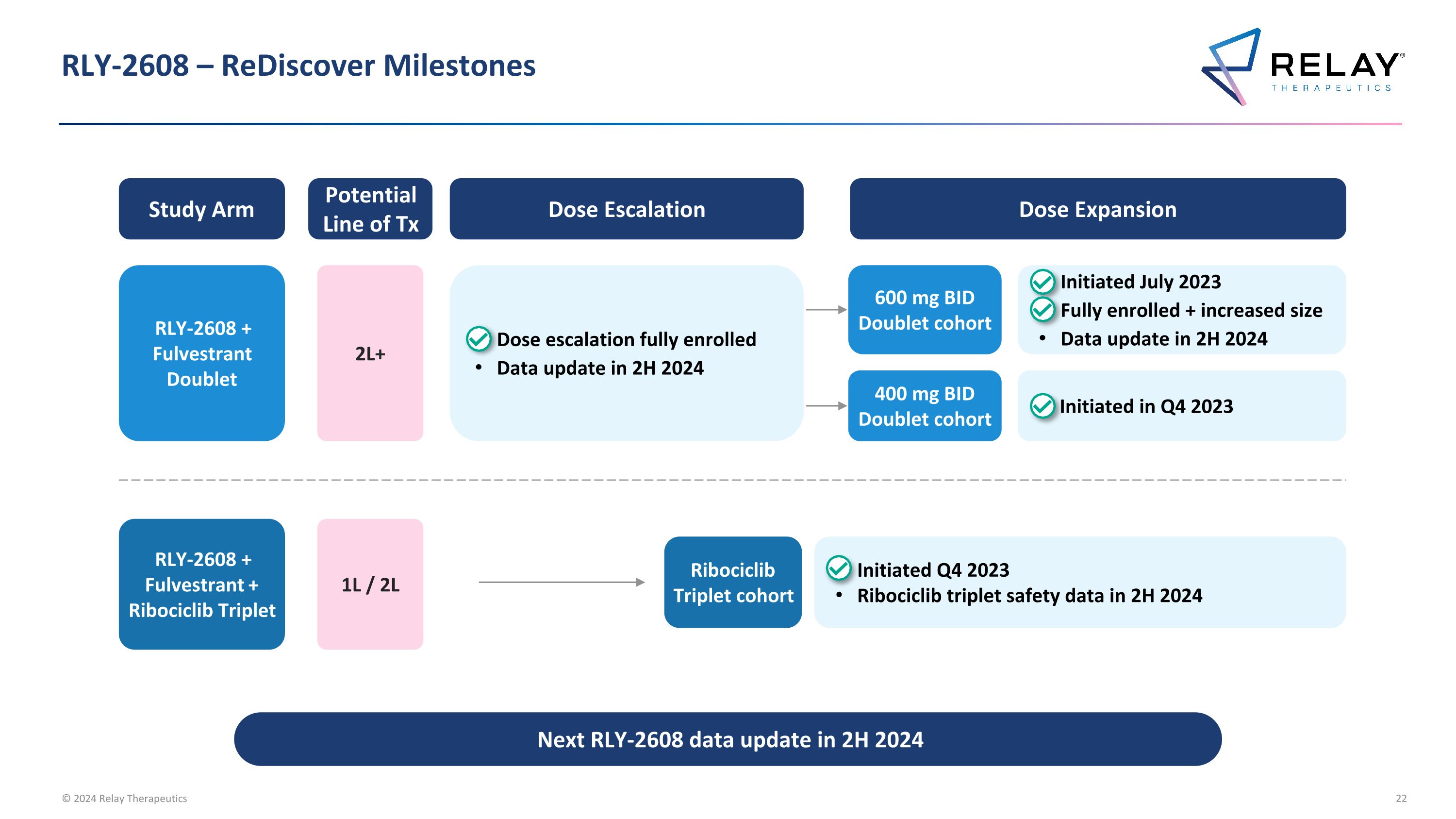
RLY-2608 – ReDiscover Milestones Next RLY-2608 data update in 2H 2024 Study Arm Dose Escalation Dose Expansion RLY-2608 + Fulvestrant + Ribociclib Triplet Potential Line of Tx 1L / 2L Ribociclib Triplet cohort Initiated Q4 2023 Ribociclib triplet safety data in 2H 2024 RLY-2608 + Fulvestrant Doublet 2L+ Dose escalation fully enrolled Data update in 2H 2024 600 mg BID Doublet cohort Initiated July 2023 Fully enrolled + increased size Data update in 2H 2024 400 mg BID Doublet cohort Initiated in Q4 2023
Relay Tx – Broad Precision Medicine Pipeline Target Program PI3Kα franchise RLY-2608 PI3KαPAN Monotherapy Endocrine Tx (ET) doublet CDK4/6i + ET triplet RLY-5836 (PI3KαPAN) Dose Escalation PI3KαH1047R FGFR2 Lirafugratinib (RLY-4008) Solid Tumor 2 programs Genetic Disease 2 programs CDK2 RLY-2139 ERα ERα Degrader SHP2 Migoprotafib (GDC-1971) Paused; IND ready Paused at DC 3 ongoing combo studies Deprioritized Preclinical Early Clinical Late Clinical
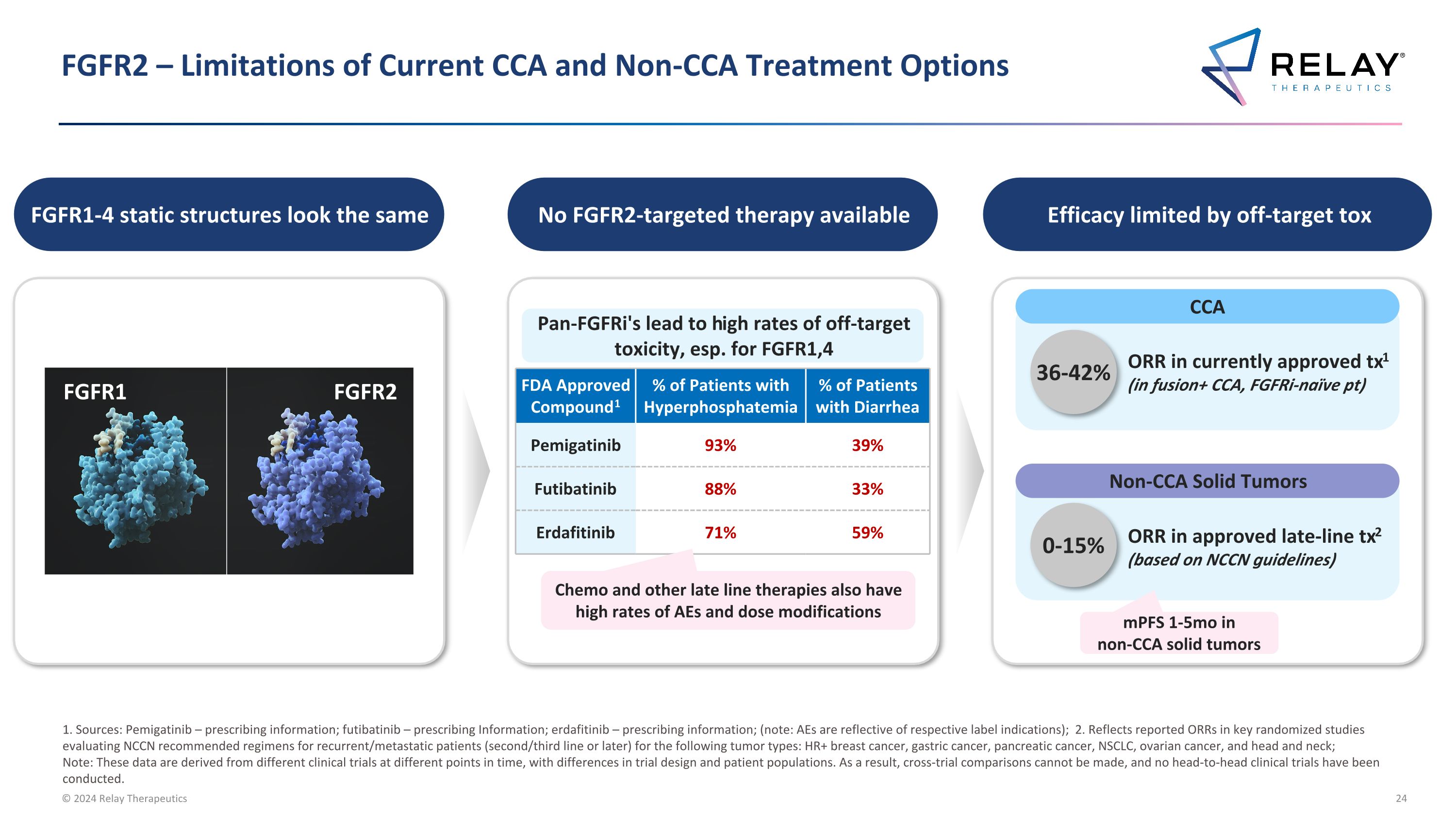
FGFR2 – Limitations of Current CCA and Non-CCA Treatment Options 1. Sources: Pemigatinib – prescribing information; futibatinib – prescribing Information; erdafitinib – prescribing information; (note: AEs are reflective of respective label indications); 2. Reflects reported ORRs in key randomized studies evaluating NCCN recommended regimens for recurrent/metastatic patients (second/third line or later) for the following tumor types: HR+ breast cancer, gastric cancer, pancreatic cancer, NSCLC, ovarian cancer, and head and neck; Note: These data are derived from different clinical trials at different points in time, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. FDA Approved Compound1 % of Patients with Hyperphosphatemia % of Patients with Diarrhea Pemigatinib 93% 39% Futibatinib 88% 33% Erdafitinib 71% 59% 36-42% ORR in currently approved tx1 (in fusion+ CCA, FGFRi-naïve pt) Pan-FGFRi's lead to high rates of off-target toxicity, esp. for FGFR1,4 CCA ORR in approved late-line tx2 (based on NCCN guidelines) Non-CCA Solid Tumors mPFS 1-5mo in non-CCA solid tumors Chemo and other late line therapies also have high rates of AEs and dose modifications 0-15% FGFR1-4 static structures look the same FGFR1 FGFR2 No FGFR2-targeted therapy available Efficacy limited by off-target tox
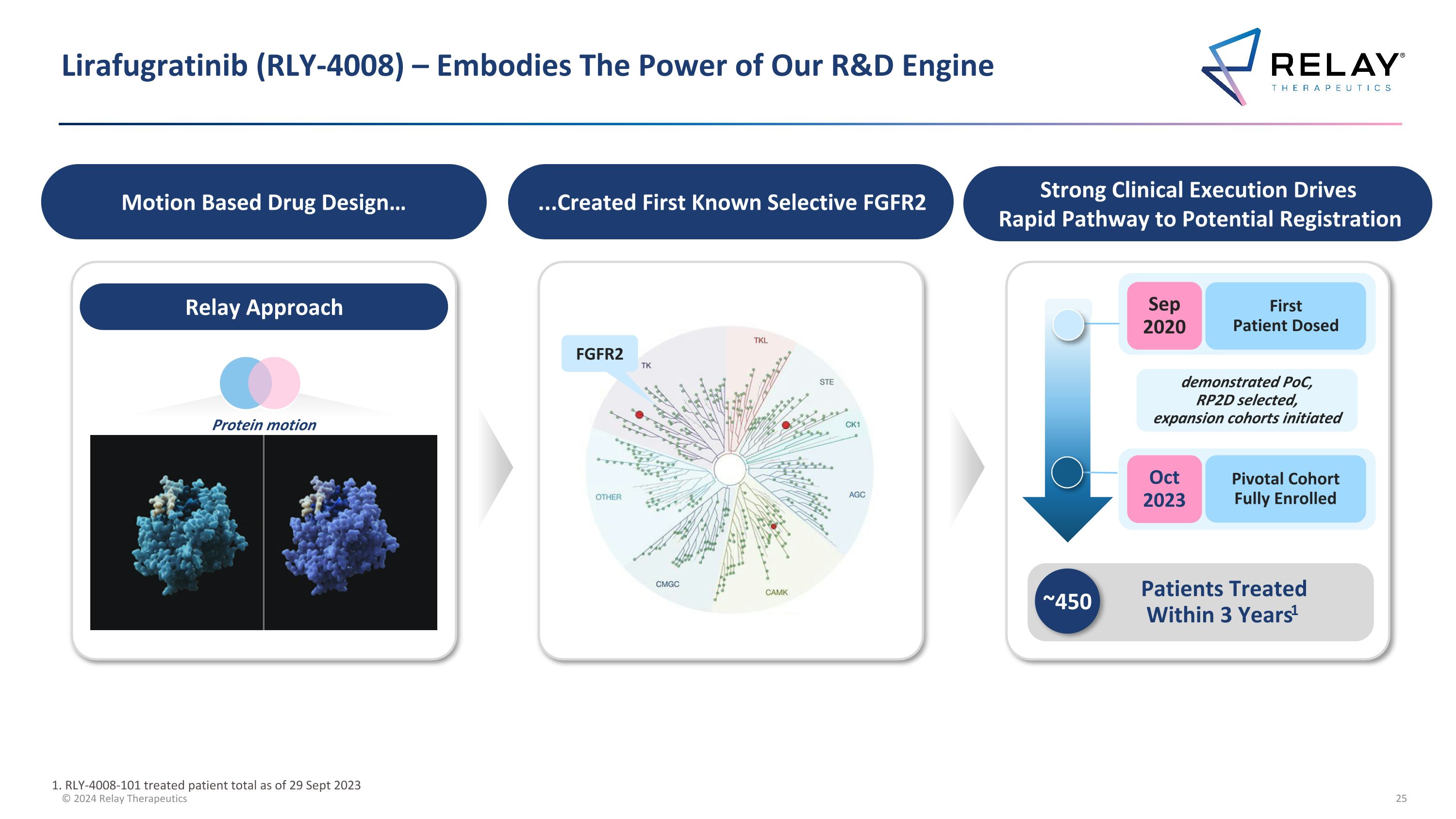
Lirafugratinib (RLY-4008) – Embodies The Power of Our R&D Engine Motion Based Drug Design… ...Created First Known Selective FGFR2 Strong Clinical Execution Drives Rapid Pathway to Potential Registration FGFR2 Relay Approach First Patient Dosed Sep 2020 Oct 2023 Patients Treated Within 3 Years1 ~450 Pivotal Cohort Fully Enrolled demonstrated PoC, RP2D selected, expansion cohorts initiated 1. RLY-4008-101 treated patient total as of 29 Sept 2023 Protein motion
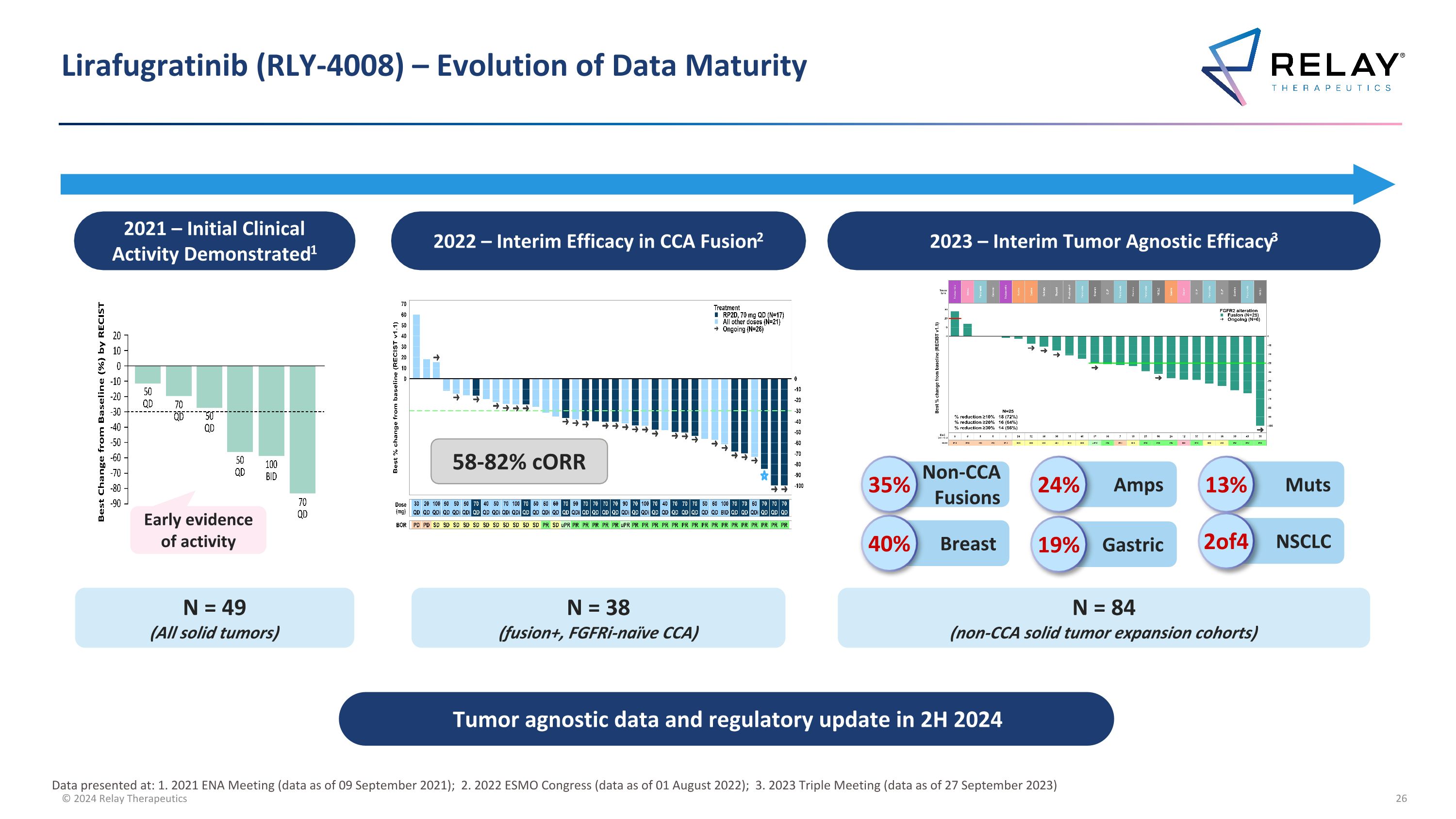
Lirafugratinib (RLY-4008) – Evolution of Data Maturity 2021 – Initial Clinical Activity Demonstrated1 2022 – Interim Efficacy in CCA Fusion2 2023 – Interim Tumor Agnostic Efficacy3 Non-CCA Fusions 35% Amps 24% Muts 13% Breast 40% Gastric 19% NSCLC 2of4 N = 49 (All solid tumors) N = 38 (fusion+, FGFRi-naïve CCA) N = 84 (non-CCA solid tumor expansion cohorts) 58-82% cORR Early evidence of activity Tumor agnostic data and regulatory update in 2H 2024 Data presented at: 1. 2021 ENA Meeting (data as of 09 September 2021); 2. 2022 ESMO Congress (data as of 01 August 2022); 3. 2023 Triple Meeting (data as of 27 September 2023)
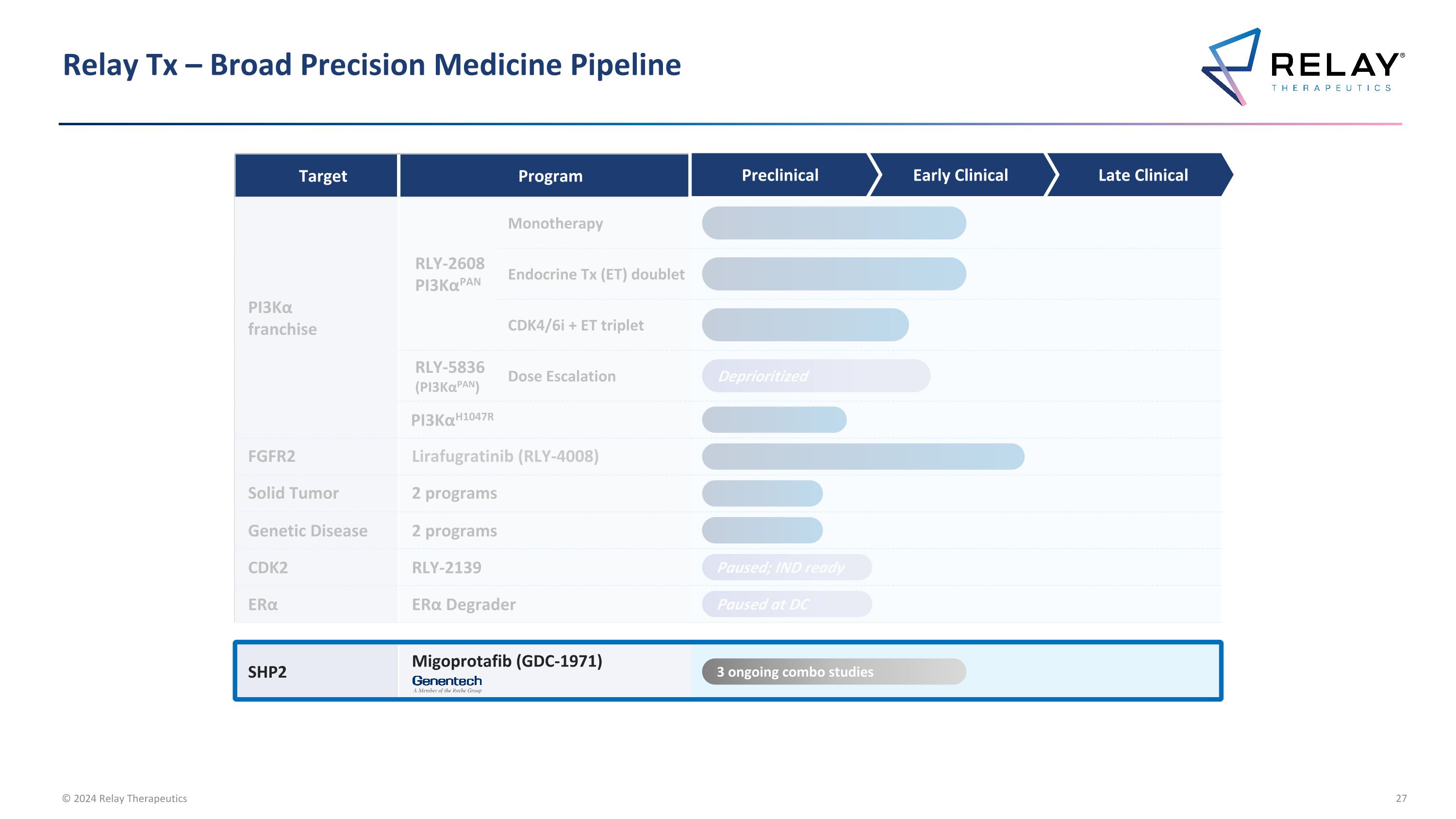
Relay Tx – Broad Precision Medicine Pipeline Target Program PI3Kα franchise RLY-2608 PI3KαPAN Monotherapy Endocrine Tx (ET) doublet CDK4/6i + ET triplet RLY-5836 (PI3KαPAN) Dose Escalation PI3KαH1047R FGFR2 Lirafugratinib (RLY-4008) Solid Tumor 2 programs Genetic Disease 2 programs CDK2 RLY-2139 ERα ERα Degrader SHP2 Migoprotafib (GDC-1971) Paused; IND ready Paused at DC 3 ongoing combo studies Deprioritized Preclinical Early Clinical Late Clinical
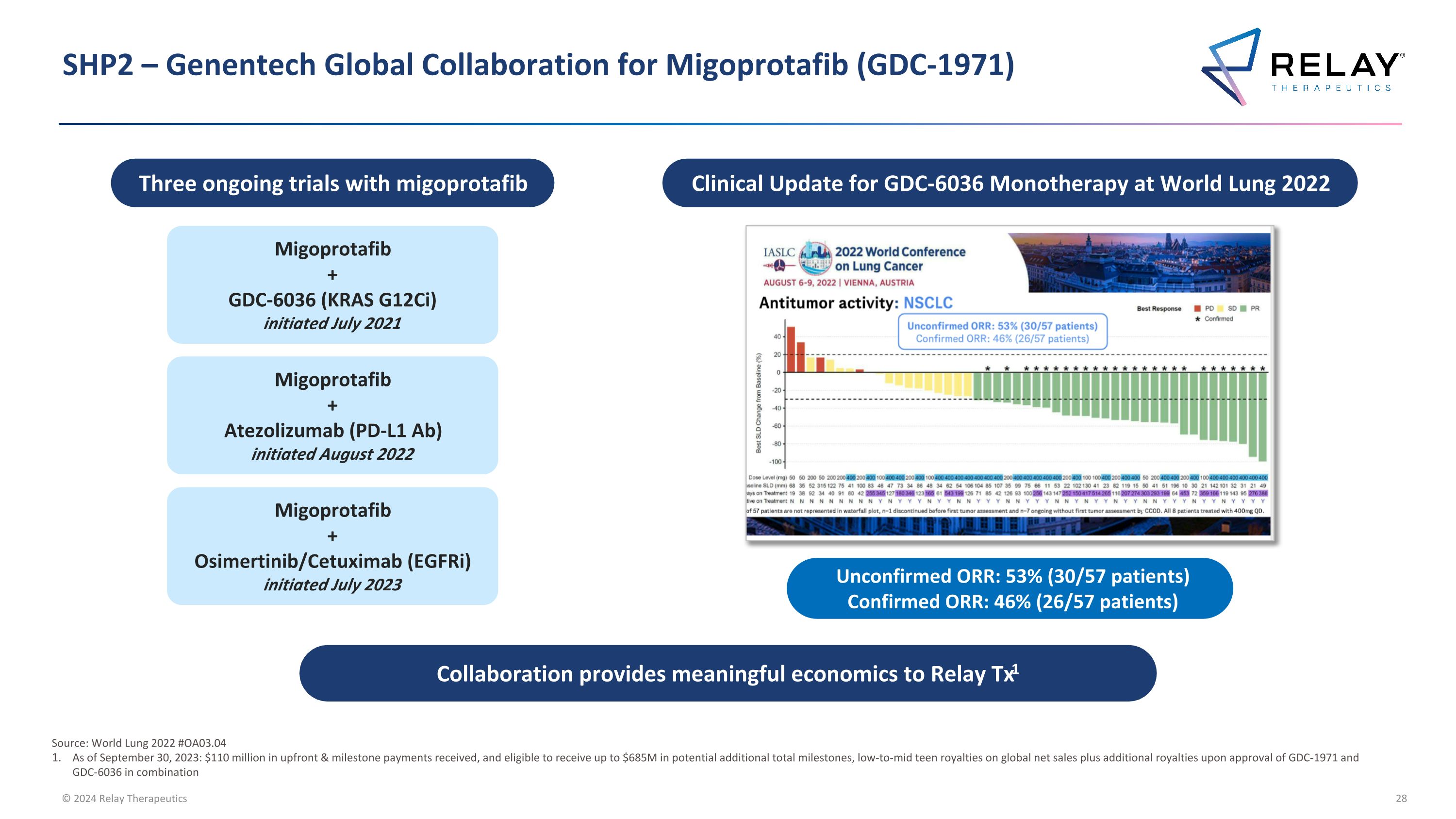
SHP2 – Genentech Global Collaboration for Migoprotafib (GDC-1971) Collaboration provides meaningful economics to Relay Tx1 Migoprotafib + Atezolizumab (PD-L1 Ab) initiated August 2022 Migoprotafib + GDC-6036 (KRAS G12Ci) initiated July 2021 Unconfirmed ORR: 53% (30/57 patients) Confirmed ORR: 46% (26/57 patients) Three ongoing trials with migoprotafib Clinical Update for GDC-6036 Monotherapy at World Lung 2022 Migoprotafib + Osimertinib/Cetuximab (EGFRi) initiated July 2023 Source: World Lung 2022 #OA03.04 As of September 30, 2023: $110 million in upfront & milestone payments received, and eligible to receive up to $685M in potential additional total milestones, low-to-mid teen royalties on global net sales plus additional royalties upon approval of GDC-1971 and GDC-6036 in combination
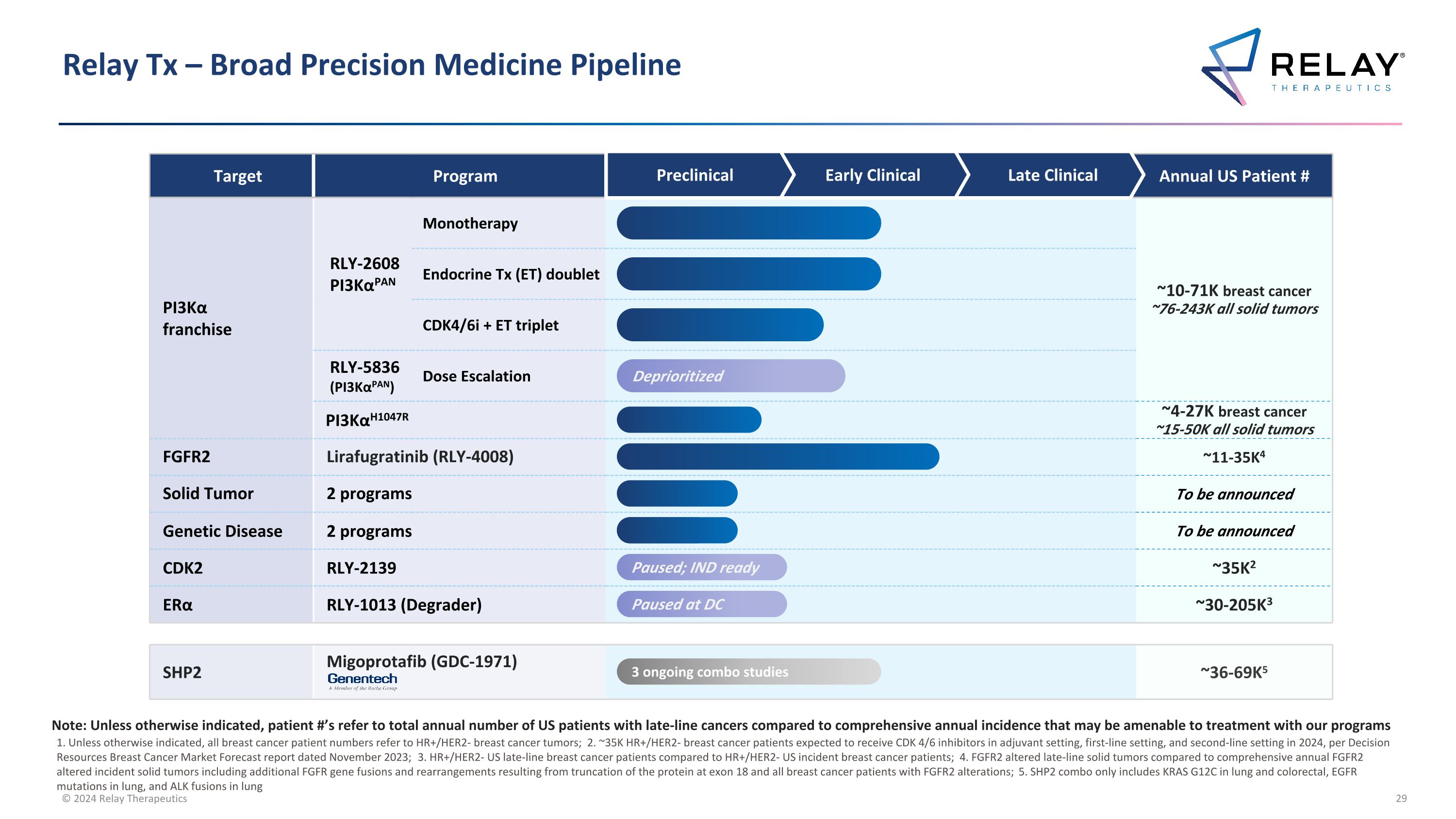
Relay Tx – Broad Precision Medicine Pipeline Target Program Annual US Patient # PI3Kα franchise RLY-2608 PI3KαPAN Monotherapy ~10-71K breast cancer ~76-243K all solid tumors Endocrine Tx (ET) doublet CDK4/6i + ET triplet RLY-5836 (PI3KαPAN) Dose Escalation PI3KαH1047R ~4-27K breast cancer ~15-50K all solid tumors FGFR2 Lirafugratinib (RLY-4008) ~11-35K4 Solid Tumor 2 programs To be announced Genetic Disease 2 programs To be announced CDK2 RLY-2139 ~35K2 ERα RLY-1013 (Degrader) ~30-205K3 SHP2 Migoprotafib (GDC-1971) ~36-69K5 Preclinical Early Clinical Late Clinical Paused; IND ready Paused at DC 3 ongoing combo studies 1. Unless otherwise indicated, all breast cancer patient numbers refer to HR+/HER2- breast cancer tumors; 2. ~35K HR+/HER2- breast cancer patients expected to receive CDK 4/6 inhibitors in adjuvant setting, first-line setting, and second-line setting in 2024, per Decision Resources Breast Cancer Market Forecast report dated November 2023; 3. HR+/HER2- US late-line breast cancer patients compared to HR+/HER2- US incident breast cancer patients; 4. FGFR2 altered late-line solid tumors compared to comprehensive annual FGFR2 altered incident solid tumors including additional FGFR gene fusions and rearrangements resulting from truncation of the protein at exon 18 and all breast cancer patients with FGFR2 alterations; 5. SHP2 combo only includes KRAS G12C in lung and colorectal, EGFR mutations in lung, and ALK fusions in lung Note: Unless otherwise indicated, patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs Deprioritized
Relay Tx’s Execution Focus Continuing to achieve the goals we set JPM Conference 2021 JPM Conference 2023 JPM Conference 2022 JPM Conference 2020
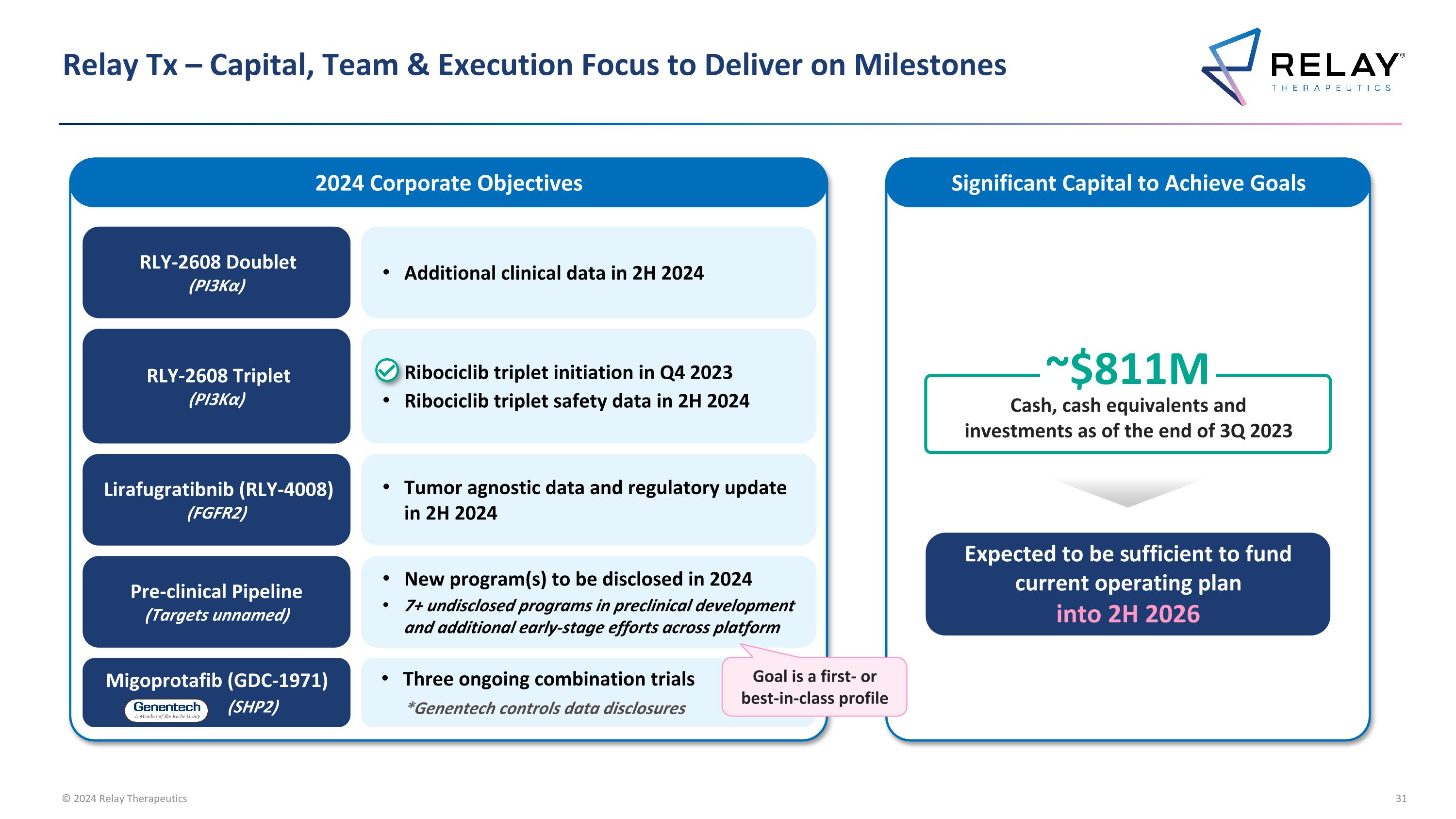
Relay Tx – Capital, Team & Execution Focus to Deliver on Milestones 2024 Corporate Objectives Cash, cash equivalents and investments as of the end of 3Q 2023 ~$811M Expected to be sufficient to fund current operating plan into 2H 2026 Significant Capital to Achieve Goals RLY-2608 Doublet (PI3Kα) Additional clinical data in 2H 2024 Migoprotafib (GDC-1971) (SHP2) Three ongoing combination trials *Genentech controls data disclosures Lirafugratibnib (RLY-4008) (FGFR2) Tumor agnostic data and regulatory update in 2H 2024 Pre-clinical Pipeline (Targets unnamed) New program(s) to be disclosed in 2024 7+ undisclosed programs in preclinical development and additional early-stage efforts across platform RLY-2608 Triplet (PI3Kα) Ribociclib triplet initiation in Q4 2023 Ribociclib triplet safety data in 2H 2024 Goal is a first- or best-in-class profile
































