
Corporate Update Call: Data Presentations at AACR-NCI-EORTC Molecular Targets Conference October 2021 Exhibit 99.3

Disclaimer This presentation contains forward-looking statements and information about our current and future prospects and our operations and financial results, which are based on currently available information. All statements other than statements of historical facts contained in this presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ “opportunity,” ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include express or implied statements about the initiation, timing, progress and results of our current and future clinical trials, including the cohort expansion of our ongoing clinical trial for RLY-4008, the initiation of a clinical trial for RLY-2608 and additional data disclosures for RLY-4008 and RLY-2608, and current and future preclinical studies of our product candidates; the potential therapeutic benefits of our product candidates, including potential efficacy and tolerability, and combination potential of our product candidates; whether preliminary results from our preclinical or clinical trials will be predictive of the final results of the trials or any future clinical trials of our product candidates; the possibility that unconfirmed results from these trials will not be confirmed by additional data as the clinical trials progress; the market opportunities for our product candidates; the expected strategic benefits and potential receipt of payments under our collaborations; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; expectations regarding current and future interactions with the U.S. Food and Drug Administration (FDA); our ability to manufacture our product candidates in conformity with the FDA’s requirements; the capabilities and development of our DynamoTM platform; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; our plans to develop, manufacture and commercialize our current product candidates and any future product candidates; and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of risks and uncertainties. These and other risks, uncertainties and important factors are described in the section entitled "Risk Factors" in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2021, as well as any subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent our views only as of the date of this presentation and we undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or otherwise. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners.
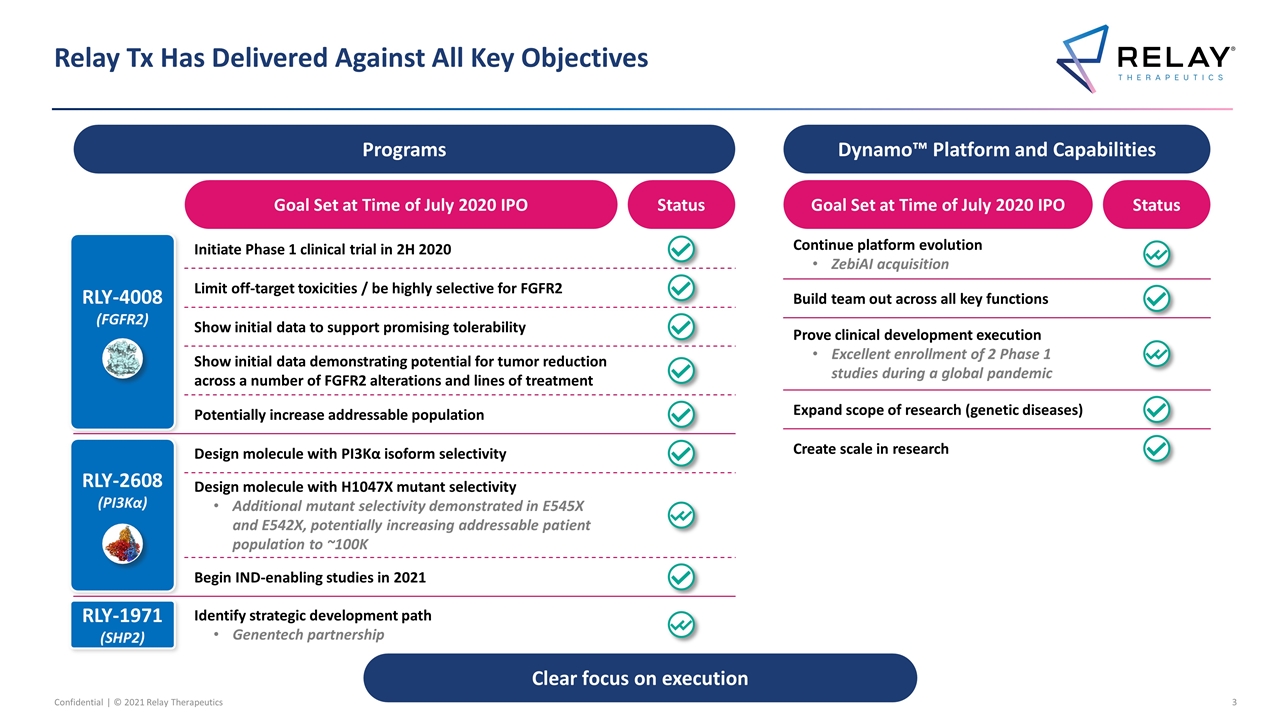
Relay Tx Has Delivered Against All Key Objectives Clear focus on execution Programs RLY-4008 (FGFR2) RLY-1971 (SHP2) RLY-2608 (PI3Kα) Goal Set at Time of July 2020 IPO Status Limit off-target toxicities / be highly selective for FGFR2 Show initial data to support promising tolerability Show initial data demonstrating potential for tumor reduction across a number of FGFR2 alterations and lines of treatment Initiate Phase 1 clinical trial in 2H 2020 Potentially increase addressable population Design molecule with H1047X mutant selectivity Additional mutant selectivity demonstrated in E545X and E542X, potentially increasing addressable patient population to ~100K Design molecule with PI3Kα isoform selectivity Begin IND-enabling studies in 2021 Identify strategic development path Genentech partnership Goal Set at Time of July 2020 IPO Status Dynamo™ Platform and Capabilities Continue platform evolution ZebiAI acquisition Prove clinical development execution Excellent enrollment of 2 Phase 1 studies during a global pandemic Expand scope of research (genetic diseases) Create scale in research Build team out across all key functions
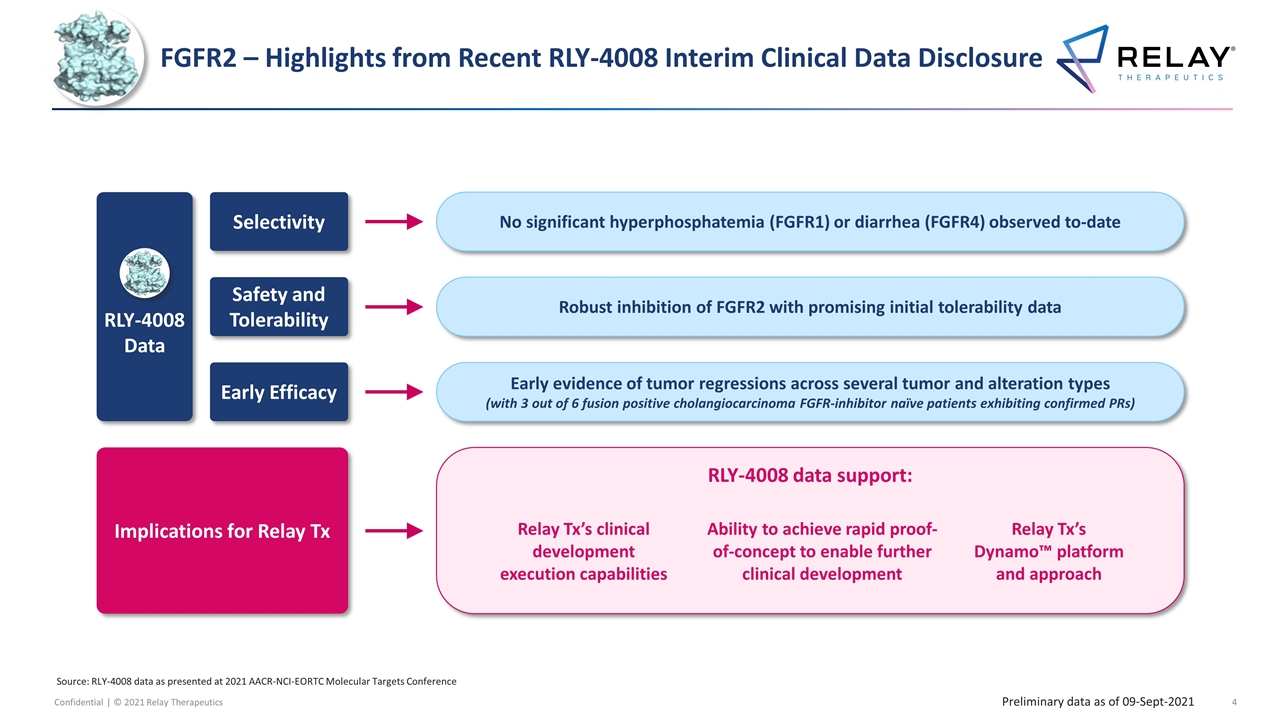
FGFR2 – Highlights from Recent RLY-4008 Interim Clinical Data Disclosure Robust inhibition of FGFR2 with promising initial tolerability data Safety and Tolerability Early evidence of tumor regressions across several tumor and alteration types (with 3 out of 6 fusion positive cholangiocarcinoma FGFR-inhibitor naïve patients exhibiting confirmed PRs) Early Efficacy No significant hyperphosphatemia (FGFR1) or diarrhea (FGFR4) observed to-date Selectivity RLY-4008 data support: Implications for Relay Tx Relay Tx’s Dynamo™ platform and approach Relay Tx’s clinical development execution capabilities Ability to achieve rapid proof-of-concept to enable further clinical development RLY-4008 Data Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Preliminary data as of 09-Sept-2021
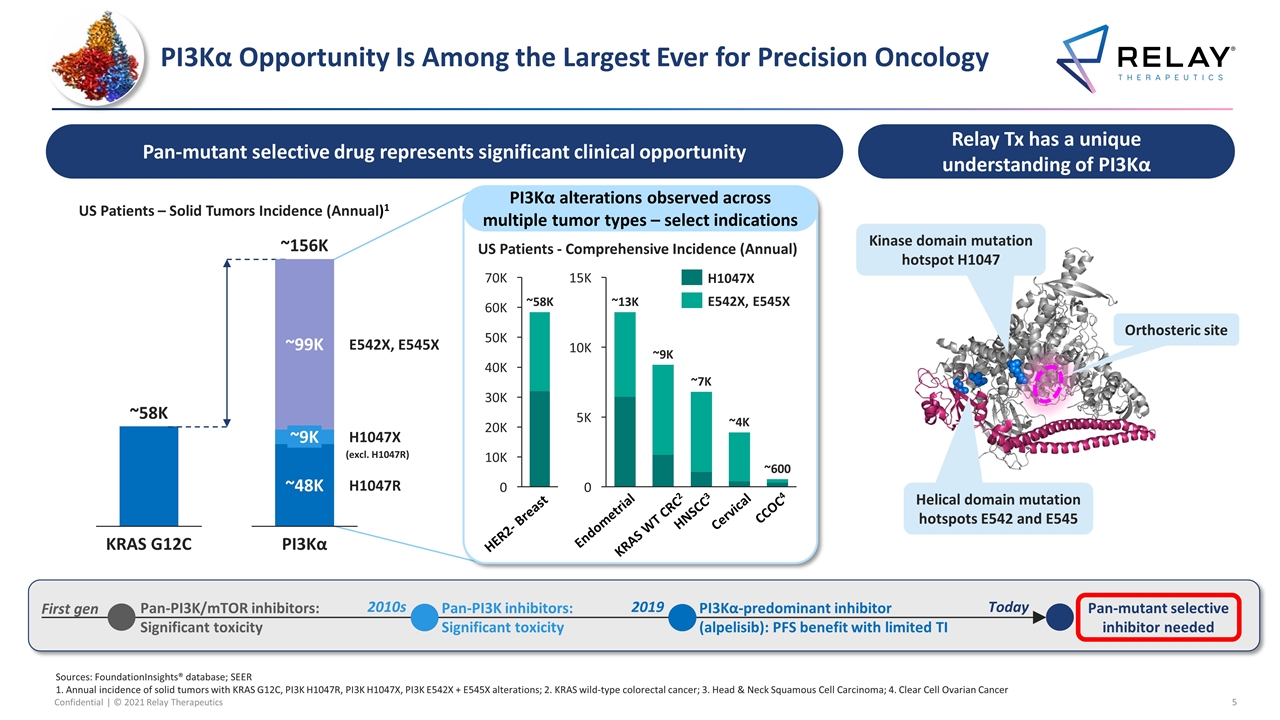
PI3Kα Opportunity Is Among the Largest Ever for Precision Oncology Sources: FoundationInsights® database; SEER 1. Annual incidence of solid tumors with KRAS G12C, PI3K H1047R, PI3K H1047X, PI3K E542X + E545X alterations; 2. KRAS wild-type colorectal cancer; 3. Head & Neck Squamous Cell Carcinoma; 4. Clear Cell Ovarian Cancer PI3Kα alterations observed across multiple tumor types – select indications Relay Tx has a unique understanding of PI3Kα Kinase domain mutation hotspot H1047 Helical domain mutation hotspots E542 and E545 Orthosteric site Pan-mutant selective drug represents significant clinical opportunity K K K ~600 CCOC4 HNSCC3 HER2- Breast Cervical Endometrial KRAS WT CRC2 US Patients - Comprehensive Incidence (Annual) 2010s Pan-PI3K inhibitors: Significant toxicity 2019 PI3Kα-predominant inhibitor (alpelisib): PFS benefit with limited TI Pan-mutant selective inhibitor needed Today K K 40K K K 20K K G12C ~156K (excl. H1047R) US Patients – Solid Tumors Incidence (Annual)1 Pan-PI3K/mTOR inhibitors: Significant toxicity First gen

PI3Kα – Highlights from Recent RLY-2608 Preclinical Data Disclosure Mutant-selective binding to novel allosteric site discovered by Dynamo™ platform Novel Mechanism Inhibited not only H1047X, but also E542X and E545X, potentially doubling the addressable population, while sparing WT PI3Kα Pan-Mutant Selectivity Achieved tumor regressions in vivo at doses projected to be clinically relevant with significantly reduced impact on glucose metabolism compared to non-mutant selective inhibitors Tolerability RLY-2608 represents the start of Relay Tx’s emerging PI3Kα franchise First-in-human study anticipated to start in 1H22* Approaching Clinic Implications for Relay Tx RLY-2608 Data *Subject to submission and acceptance of IND by the FDA
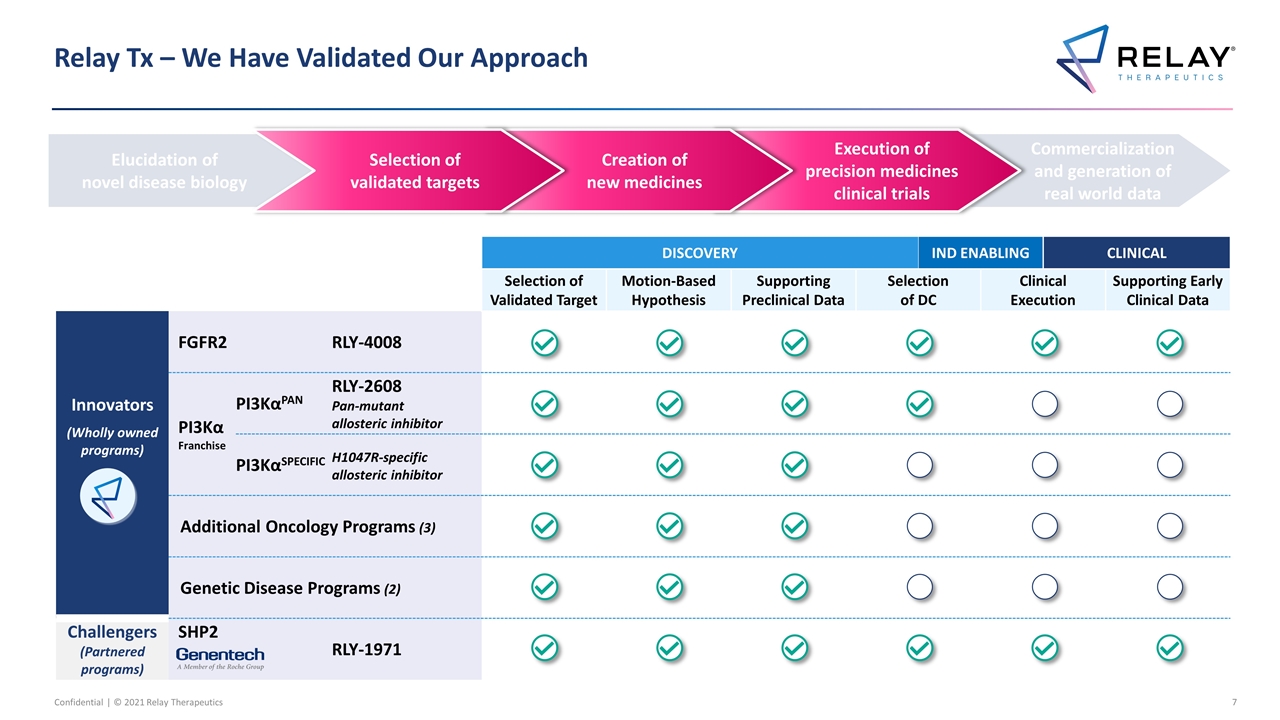
DISCOVERY DISCOVERY IND ENABLING CLINICAL Selection of Validated Target Motion-Based Hypothesis Supporting Preclinical Data Selection of DC Clinical Execution Supporting Early Clinical Data Innovators (Wholly owned programs) FGFR2 RLY-4008 RLY-4008 PI3Kα Franchise PI3KαPAN RLY-2608 Pan-mutant allosteric inhibitor PI3KαSPECIFIC H1047R-specific allosteric inhibitor Additional Oncology Programs (3) Add’l oncology programs (3) Genetic Disease Programs (2) Add’l genetic disease programs (2) Challengers (Partnered programs) SHP2 RLY-1971 RLY-1971 Relay Tx – We Have Validated Our Approach Elucidation of novel disease biology Commercialization and generation of real world data Execution of precision medicines clinical trials Creation of new medicines Selection of validated targets
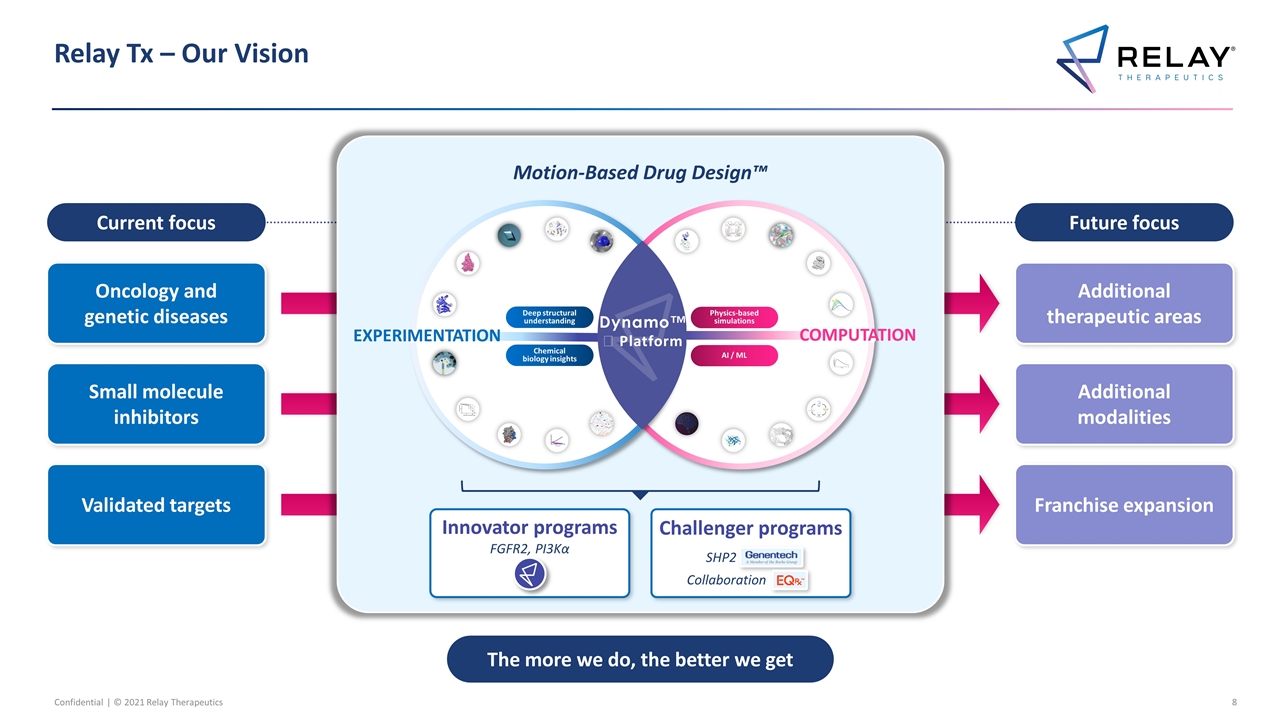
Relay Tx – Our Vision Current focus Future focus Additional therapeutic areas Additional modalities Franchise expansion Validated targets Small molecule inhibitors Oncology and genetic diseases The more we do, the better we get Motion-Based Drug Design™ Chemical biology insights Deep structural understanding Physics-based simulations AI / ML Dynamo™️ Platform EXPERIMENTATION COMPUTATION Innovator programs FGFR2, PI3Kα Challenger programs SHP2 Collaboration
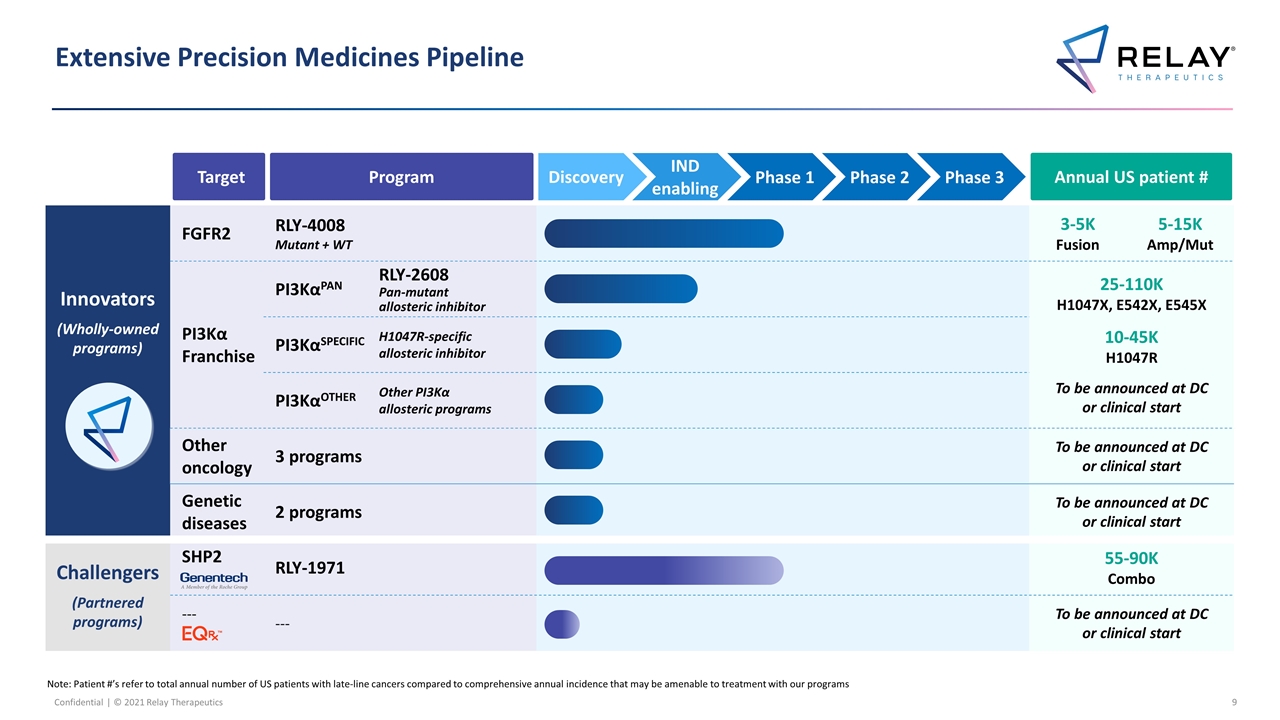
Innovators (Wholly-owned programs) FGFR2 RLY-4008 Mutant + WT 3-5K Fusion 5-15K Amp/Mut PI3Kα Franchise PI3KαPAN RLY-2608 Pan-mutant allosteric inhibitor RLY-2608 Pan-mutant allosteric inhibitor 25-110K H1047X, E542X, E545X 10-45K H1047R To be announced at DC or clinical start PI3KαSPECIFIC H1047R-specific allosteric inhibitor RLY-1047R H1047R allosteric inhibitor PI3KαOTHER Other PI3Kα allosteric programs Additional Other novel mutant selective mechanisms Other oncology 3 programs To be announced at DC or clinical start Genetic diseases 2 programs To be announced at DC or clinical start Challengers (Partnered programs) SHP2 RLY-1971 55-90K Combo --- --- To be announced at DC or clinical start Extensive Precision Medicines Pipeline Note: Patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs Target Program Annual US patient # IND enabling Phase 1 Phase 2 Phase 3 Discovery

Relay Tx Programs
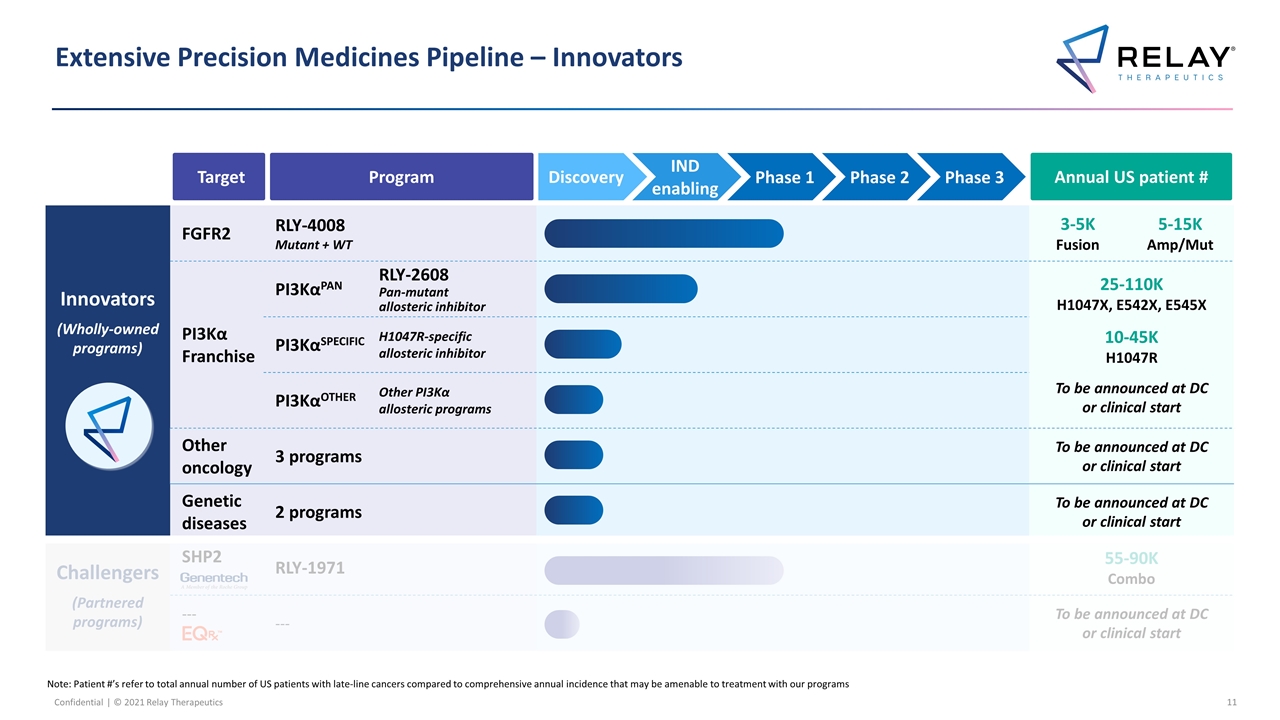
Extensive Precision Medicines Pipeline – Innovators Note: Patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs Innovators (Wholly-owned programs) FGFR2 RLY-4008 Mutant + WT 3-5K Fusion 5-15K Amp/Mut PI3Kα Franchise PI3KαPAN RLY-2608 Pan-mutant allosteric inhibitor RLY-2608 Pan-mutant allosteric inhibitor 25-110K H1047X, E542X, E545X 10-45K H1047R To be announced at DC or clinical start PI3KαSPECIFIC H1047R-specific allosteric inhibitor RLY-1047R H1047R allosteric inhibitor PI3KαOTHER Other PI3Kα allosteric programs Additional Other novel mutant selective mechanisms Other oncology 3 programs To be announced at DC or clinical start Genetic diseases 2 programs To be announced at DC or clinical start Challengers (Partnered programs) SHP2 RLY-1971 55-90K Combo --- --- To be announced at DC or clinical start Target Program Annual US patient # IND enabling Phase 1 Phase 2 Phase 3 Discovery
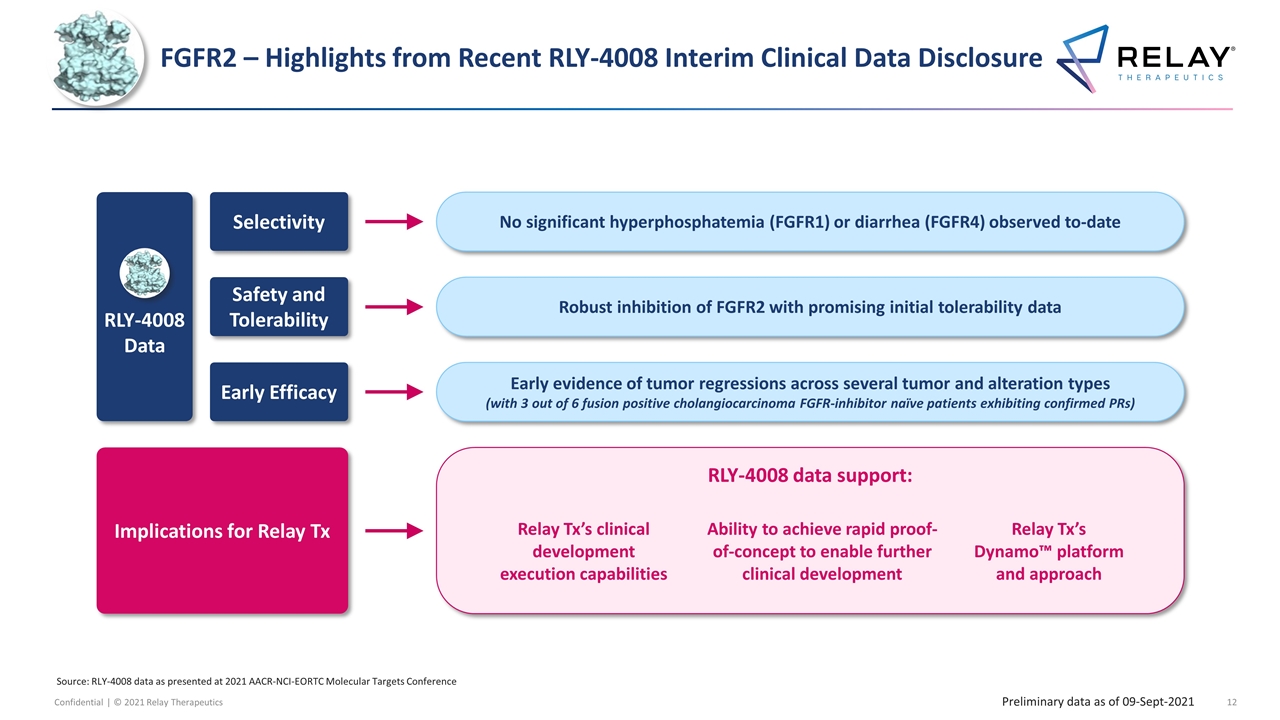
FGFR2 – Highlights from Recent RLY-4008 Interim Clinical Data Disclosure Robust inhibition of FGFR2 with promising initial tolerability data Safety and Tolerability Early evidence of tumor regressions across several tumor and alteration types (with 3 out of 6 fusion positive cholangiocarcinoma FGFR-inhibitor naïve patients exhibiting confirmed PRs) Early Efficacy No significant hyperphosphatemia (FGFR1) or diarrhea (FGFR4) observed to-date Selectivity RLY-4008 data support: Implications for Relay Tx Relay Tx’s Dynamo™ platform and approach Relay Tx’s clinical development execution capabilities Ability to achieve rapid proof-of-concept to enable further clinical development RLY-4008 Data Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Preliminary data as of 09-Sept-2021
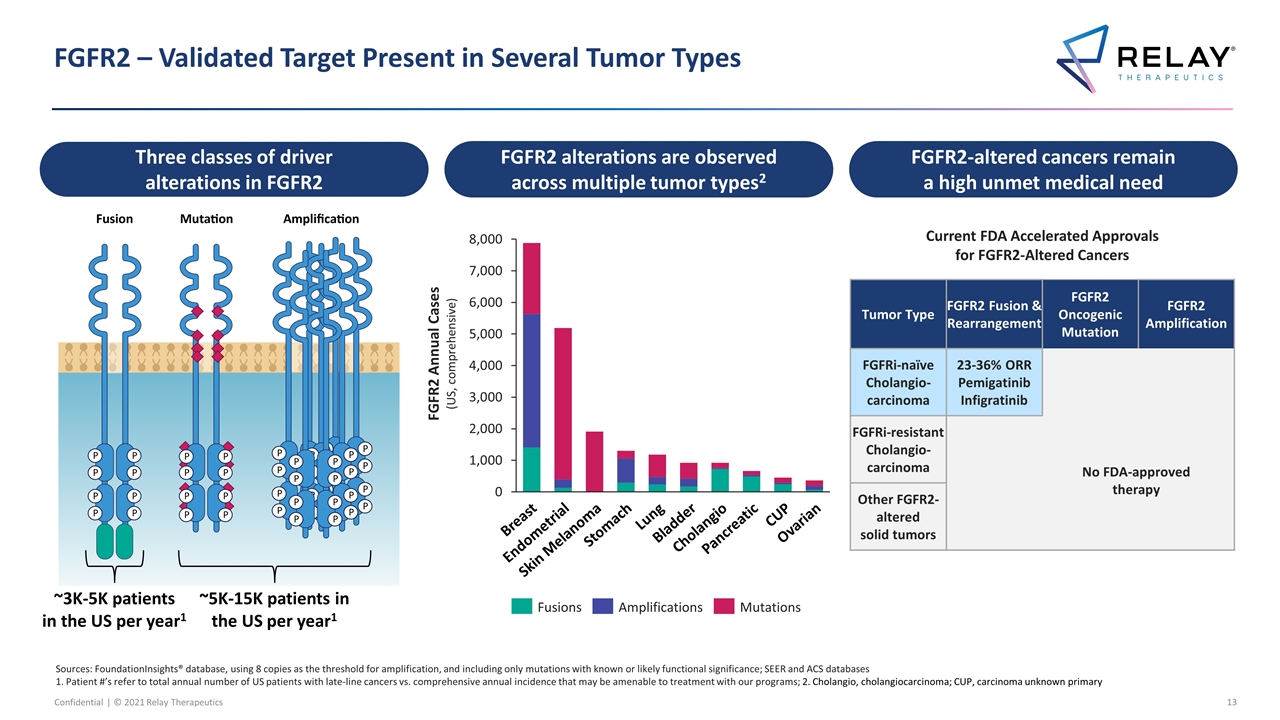
FGFR2 – Validated Target Present in Several Tumor Types FGFR2 Annual Cases (US, comprehensive) FGFR2-altered cancers remain a high unmet medical need Pancreatic Breast Endometrial Skin Melanoma Stomach Lung Bladder CUP Ovarian ~5K-15K patients in the US per year1 ~3K-5K patients in the US per year1 FGFR2 alterations are observed across multiple tumor types2 Three classes of driver alterations in FGFR2 Sources: FoundationInsights® database, using 8 copies as the threshold for amplification, and including only mutations with known or likely functional significance; SEER and ACS databases 1. Patient #’s refer to total annual number of US patients with late-line cancers vs. comprehensive annual incidence that may be amenable to treatment with our programs; 2. Cholangio, cholangiocarcinoma; CUP, carcinoma unknown primary Cholangio Current FDA Accelerated Approvals for FGFR2-Altered Cancers Tumor Type FGFR2 Fusion & Rearrangement FGFR2 Oncogenic Mutation FGFR2 Amplification FGFRi-naïve Cholangio-carcinoma 23-36% ORR Pemigatinib Infigratinib FGFRi-resistant Cholangio-carcinoma Other FGFR2-altered solid tumors No FDA-approved therapy
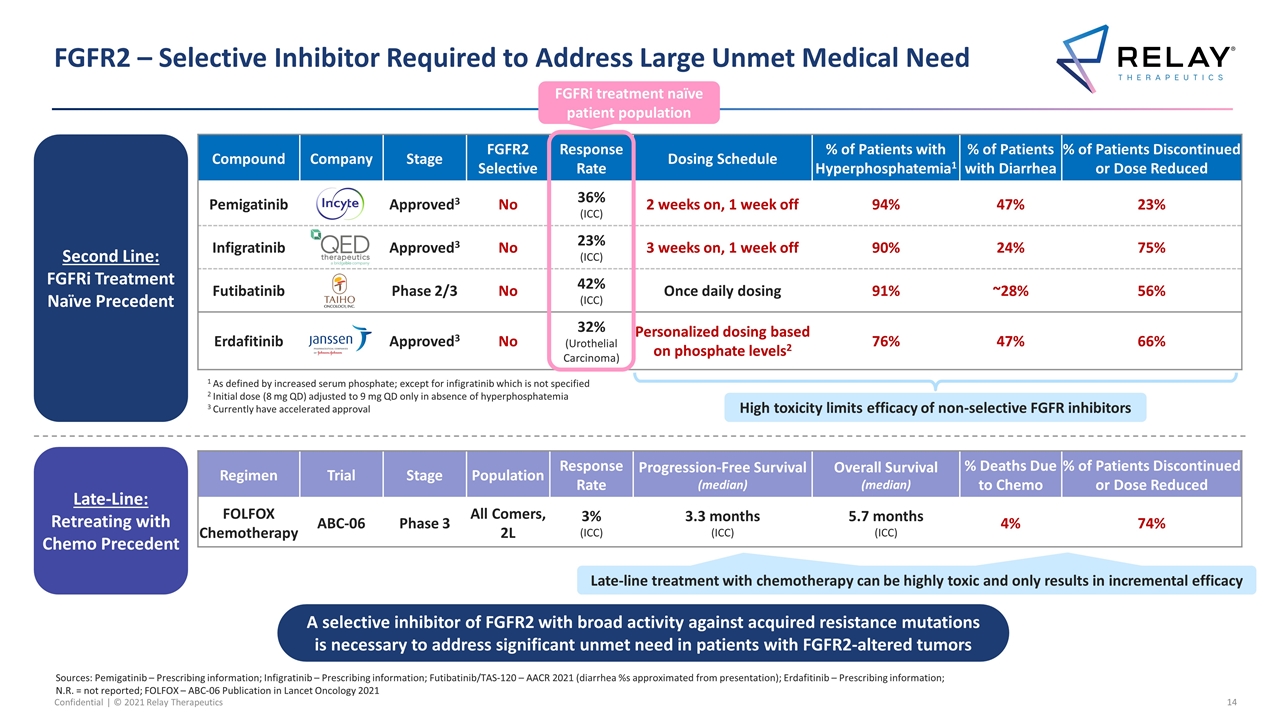
FGFR2 – Selective Inhibitor Required to Address Large Unmet Medical Need A selective inhibitor of FGFR2 with broad activity against acquired resistance mutations is necessary to address significant unmet need in patients with FGFR2-altered tumors Compound Company Stage FGFR2 Selective Response Rate Dosing Schedule % of Patients with Hyperphosphatemia1 % of Patients with Diarrhea % of Patients Discontinued or Dose Reduced Pemigatinib Approved3 No 36% (ICC) 2 weeks on, 1 week off 94% 47% 23% Infigratinib Approved3 No 23% (ICC) 3 weeks on, 1 week off 90% 24% 75% Futibatinib Phase 2/3 No 42% (ICC) Once daily dosing 91% ~28% 56% Erdafitinib Approved3 No 32% (Urothelial Carcinoma) Personalized dosing based on phosphate levels2 76% 47% 66% High toxicity limits efficacy of non-selective FGFR inhibitors Sources: Pemigatinib – Prescribing information; Infigratinib – Prescribing information; Futibatinib/TAS-120 – AACR 2021 (diarrhea %s approximated from presentation); Erdafitinib – Prescribing information; N.R. = not reported; FOLFOX – ABC-06 Publication in Lancet Oncology 2021 1 As defined by increased serum phosphate; except for infigratinib which is not specified 2 Initial dose (8 mg QD) adjusted to 9 mg QD only in absence of hyperphosphatemia 3 Currently have accelerated approval Second Line: FGFRi Treatment Naïve Precedent Late-Line: Retreating with Chemo Precedent Regimen Trial Stage Population Response Rate Progression-Free Survival (median) Overall Survival (median) % Deaths Due to Chemo % of Patients Discontinued or Dose Reduced FOLFOX Chemotherapy ABC-06 Phase 3 All Comers, 2L 3% (ICC) 3.3 months (ICC) 5.7 months (ICC) 4% 74% Late-line treatment with chemotherapy is highly toxic and only results in incremental efficacy Late-line treatment with chemotherapy can be highly toxic and only results in incremental efficacy FGFRi treatment naïve patient population
FGFR2 – Standard Approach to Discovery Has Had Limited Success Standard Approach FGFR1 FGFR2
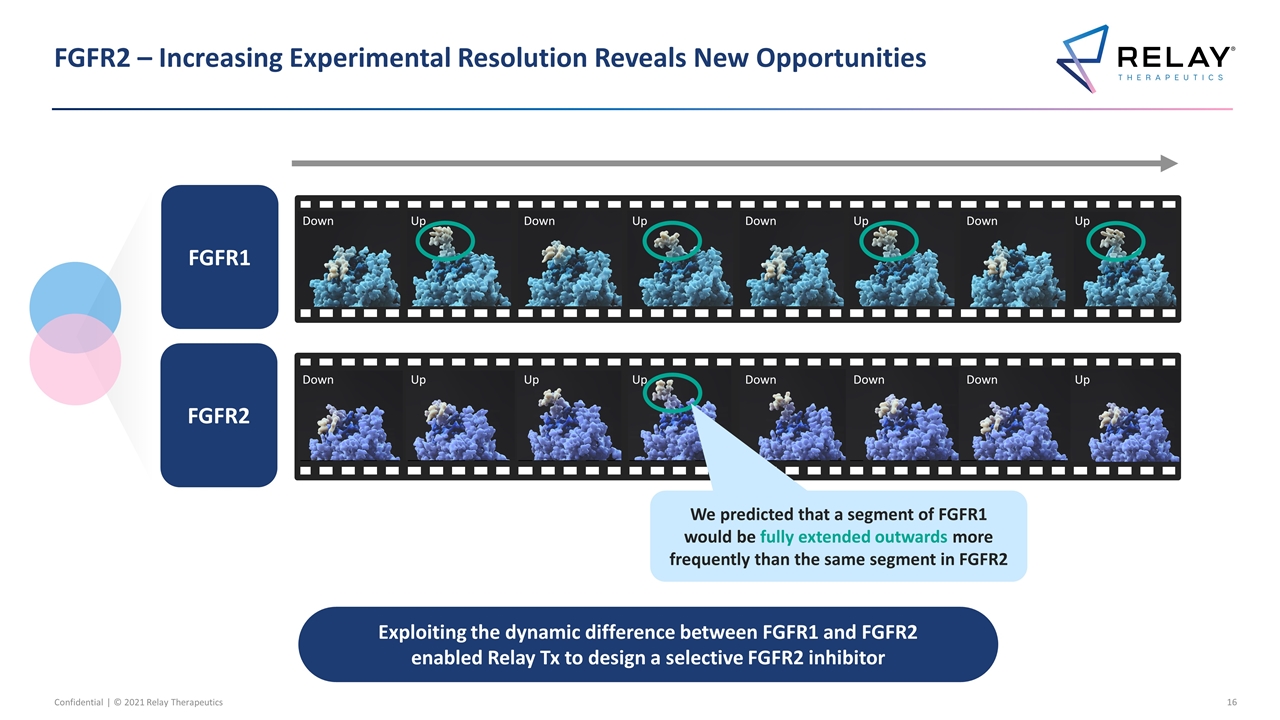
FGFR2 – Increasing Experimental Resolution Reveals New Opportunities FGFR1 FGFR2 Down Up Down Up Down Up Down Up Down Up Up Up Down Down Down Up Exploiting the dynamic difference between FGFR1 and FGFR2 enabled Relay Tx to design a selective FGFR2 inhibitor We predicted that a segment of FGFR1 would be fully extended outwards more frequently than the same segment in FGFR2
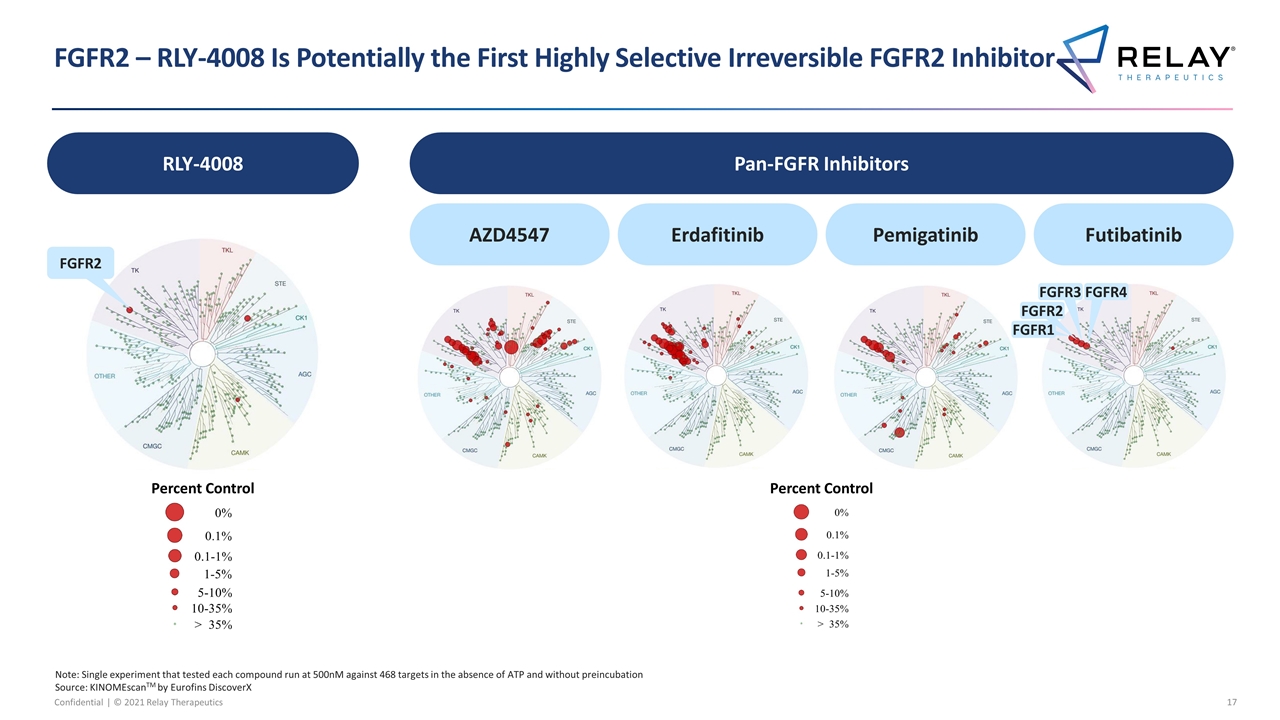
FGFR2 – RLY-4008 Is Potentially the First Highly Selective Irreversible FGFR2 Inhibitor Pan-FGFR Inhibitors RLY-4008 AZD4547 Erdafitinib Pemigatinib Futibatinib FGFR2 Note: Single experiment that tested each compound run at 500nM against 468 targets in the absence of ATP and without preincubation Source: KINOMEscanTM by Eurofins DiscoverX FGFR1 FGFR2 FGFR3 FGFR4 Percent Control Percent Control
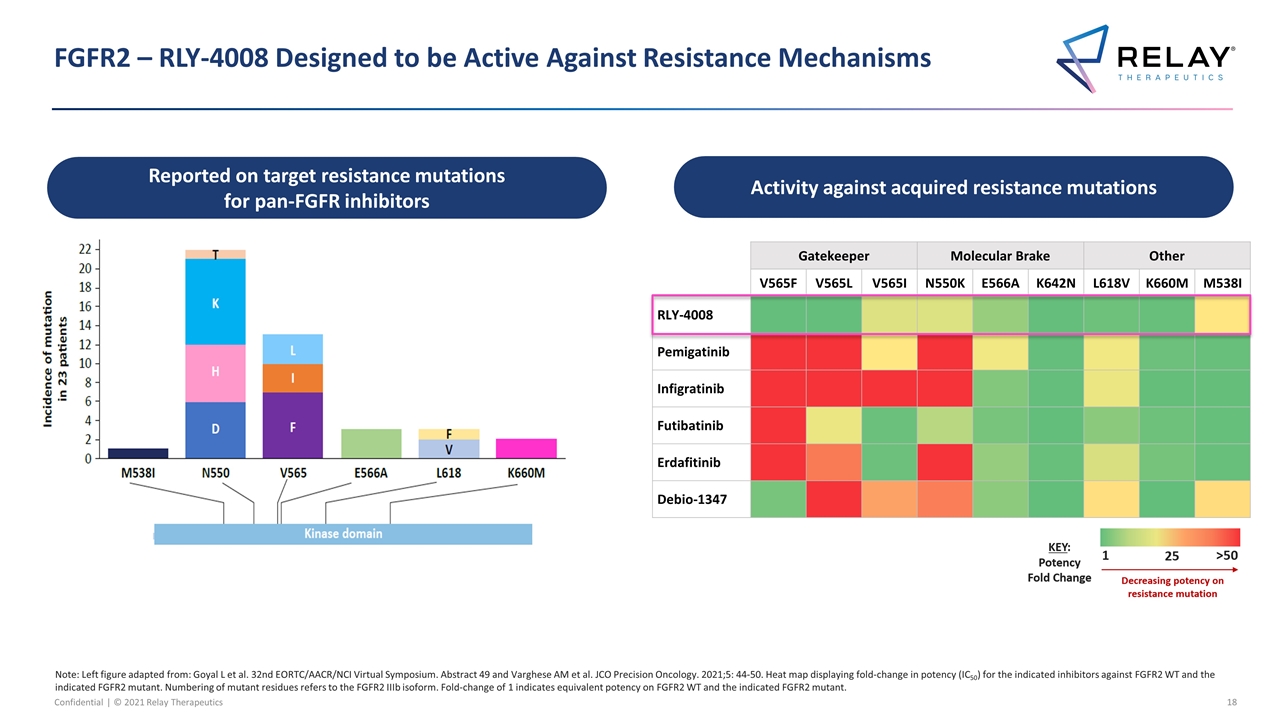
FGFR2 – RLY-4008 Designed to be Active Against Resistance Mechanisms Note: Left figure adapted from: Goyal L et al. 32nd EORTC/AACR/NCI Virtual Symposium. Abstract 49 and Varghese AM et al. JCO Precision Oncology. 2021;5: 44-50. Heat map displaying fold‑change in potency (IC50) for the indicated inhibitors against FGFR2 WT and the indicated FGFR2 mutant. Numbering of mutant residues refers to the FGFR2 IIIb isoform. Fold‑change of 1 indicates equivalent potency on FGFR2 WT and the indicated FGFR2 mutant. Activity against acquired resistance mutations Reported on target resistance mutations for pan-FGFR inhibitors Gatekeeper Molecular Brake Other V565F V565L V565I N550K E566A K642N L618V K660M M538I RLY-4008 Pemigatinib Infigratinib Futibatinib Erdafitinib Debio-1347
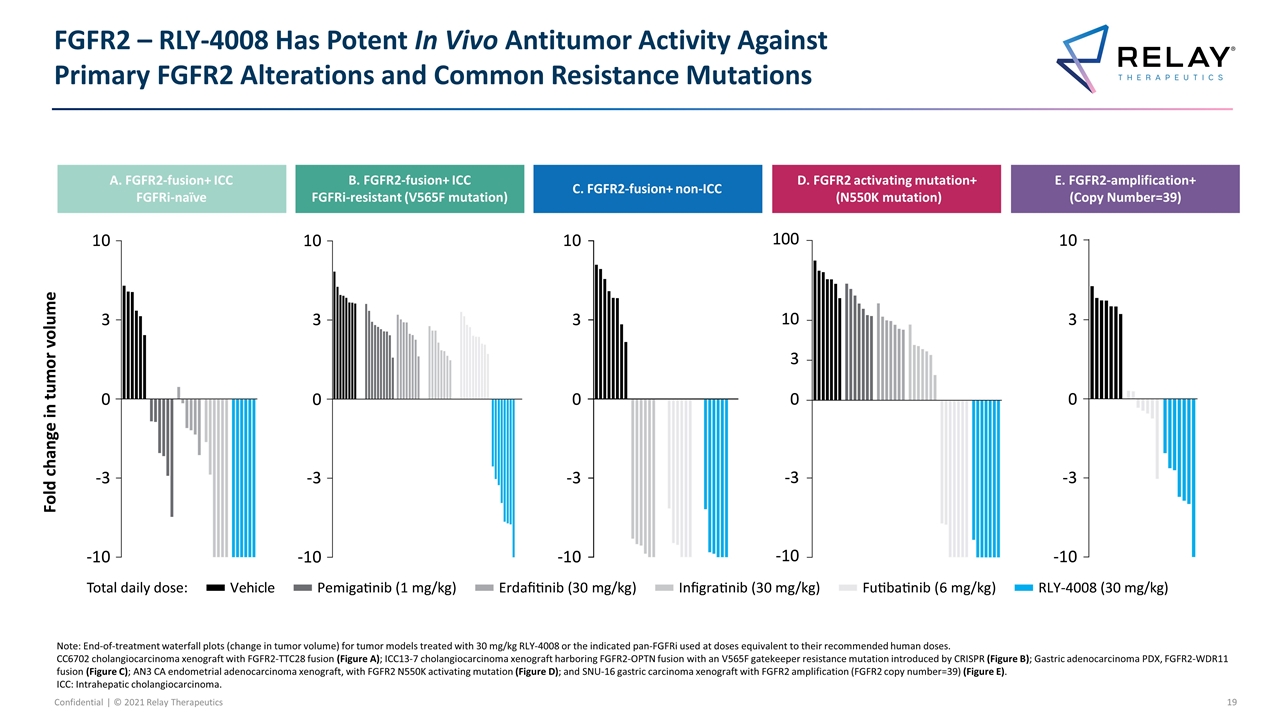
FGFR2 – RLY-4008 Has Potent In Vivo Antitumor Activity Against Primary FGFR2 Alterations and Common Resistance Mutations Note: End-of-treatment waterfall plots (change in tumor volume) for tumor models treated with 30 mg/kg RLY-4008 or the indicated pan-FGFRi used at doses equivalent to their recommended human doses. CC6702 cholangiocarcinoma xenograft with FGFR2-TTC28 fusion (Figure A); ICC13-7 cholangiocarcinoma xenograft harboring FGFR2-OPTN fusion with an V565F gatekeeper resistance mutation introduced by CRISPR (Figure B); Gastric adenocarcinoma PDX, FGFR2-WDR11 fusion (Figure C); AN3 CA endometrial adenocarcinoma xenograft, with FGFR2 N550K activating mutation (Figure D); and SNU-16 gastric carcinoma xenograft with FGFR2 amplification (FGFR2 copy number=39) (Figure E). ICC: Intrahepatic cholangiocarcinoma. A. FGFR2-fusion+ ICC FGFRi-naïve B. FGFR2-fusion+ ICC FGFRi-resistant (V564F mutation) C. FGFR2-fusion+ non-ICC D. FGFR2-mutation+ (N549K mutation) E. FGFR2-amplification+ (Copy number=38) A. FGFR2-fusion+ ICC FGFRi-naïve B. FGFR2-fusion+ ICC FGFRi-resistant (V565F mutation) C. FGFR2-fusion+ non-ICC D. FGFR2 activating mutation+ (N550K mutation) E. FGFR2-amplification+ (Copy Number=39)
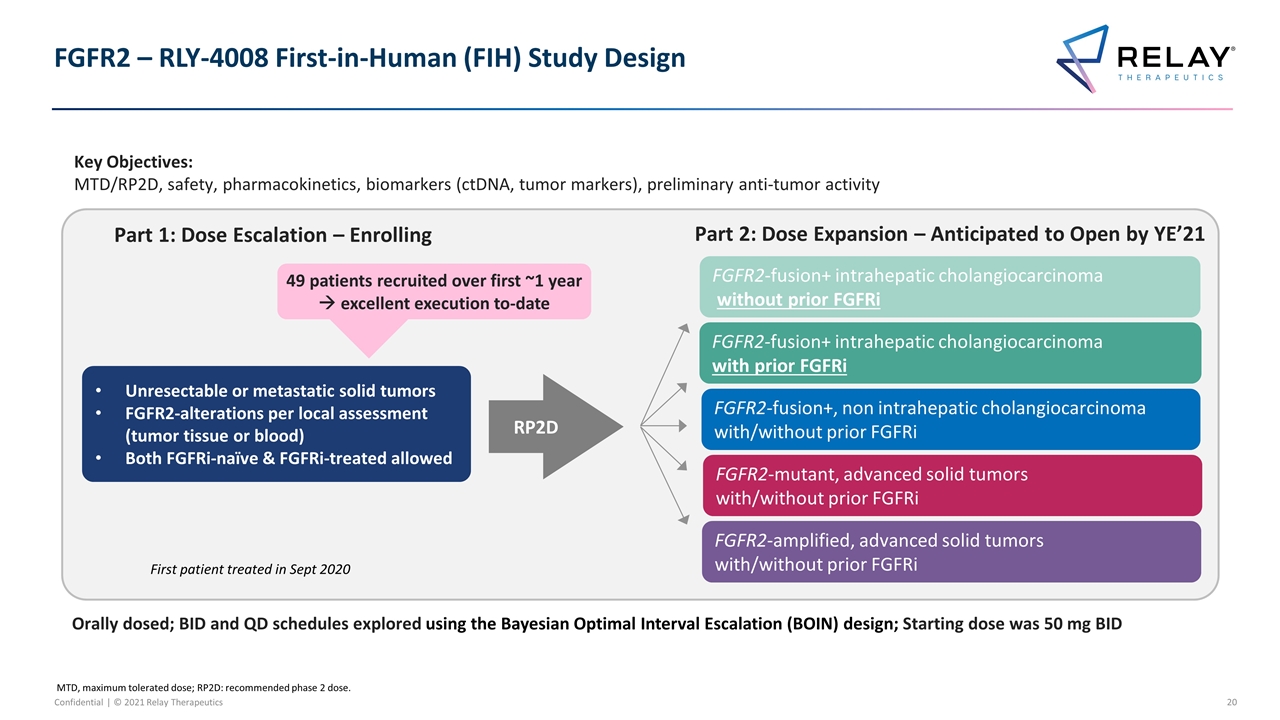
FGFR2 – RLY-4008 First-in-Human (FIH) Study Design Orally dosed; BID and QD schedules explored using the Bayesian Optimal Interval Escalation (BOIN) design; Starting dose was 50 mg BID Key Objectives: MTD/RP2D, safety, pharmacokinetics, biomarkers (ctDNA, tumor markers), preliminary anti-tumor activity MTD, maximum tolerated dose; RP2D: recommended phase 2 dose. Unresectable or metastatic solid tumors FGFR2-alterations per local assessment (tumor tissue or blood) Both FGFRi-naïve & FGFRi-treated allowed Part 1: Dose Escalation – Enrolling RP2D Part 2: Dose Expansion – Anticipated to Open by YE’21 FGFR2-fusion+ intrahepatic cholangiocarcinoma with prior FGFRi FGFR2-fusion+, non intrahepatic cholangiocarcinoma with/without prior FGFRi FGFR2-amplified, advanced solid tumors with/without prior FGFRi FGFR2-mutant, advanced solid tumors with/without prior FGFRi FGFR2-fusion+ intrahepatic cholangiocarcinoma without prior FGFRi First patient treated in Sept 2020 49 patients recruited over first ~1 year à excellent execution to-date
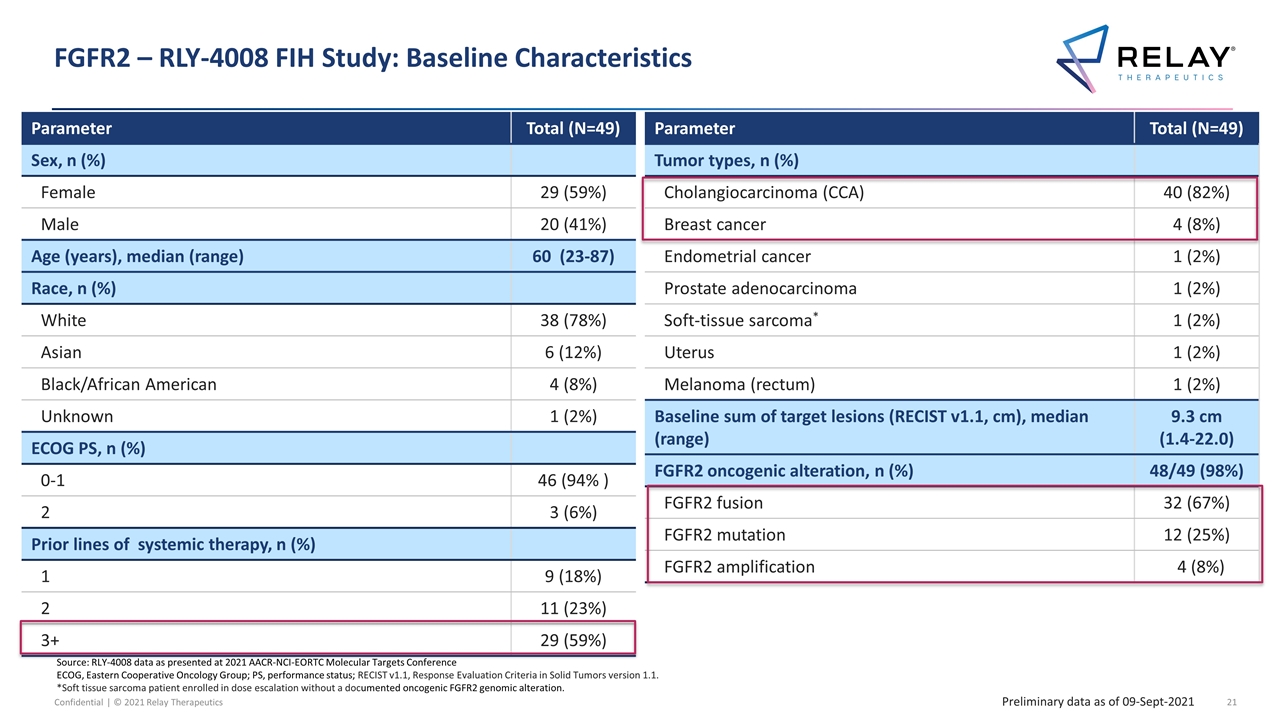
FGFR2 – RLY-4008 FIH Study: Baseline Characteristics Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference ECOG, Eastern Cooperative Oncology Group; PS, performance status; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1. *Soft tissue sarcoma patient enrolled in dose escalation without a documented oncogenic FGFR2 genomic alteration. Parameter Total (N=49) Sex, n (%) Female 29 (59%) Male 20 (41%) Age (years), median (range) 60 (23-87) Race, n (%) White 38 (78%) Asian 6 (12%) Black/African American 4 (8%) Unknown 1 (2%) ECOG PS, n (%) 0-1 46 (94% ) 2 3 (6%) Prior lines of systemic therapy, n (%) 1 9 (18%) 2 11 (23%) 3+ 29 (59%) Parameter Total (N=49) Tumor types, n (%) Cholangiocarcinoma (CCA) 40 (82%) Breast cancer 4 (8%) Endometrial cancer 1 (2%) Prostate adenocarcinoma 1 (2%) Soft-tissue sarcoma* 1 (2%) Uterus 1 (2%) Melanoma (rectum) 1 (2%) Baseline sum of target lesions (RECIST v1.1, cm), median (range) 9.3 cm (1.4-22.0) FGFR2 oncogenic alteration, n (%) 48/49 (98%) FGFR2 fusion 32 (67%) FGFR2 mutation 12 (25%) FGFR2 amplification 4 (8%) Preliminary data as of 09-Sept-2021
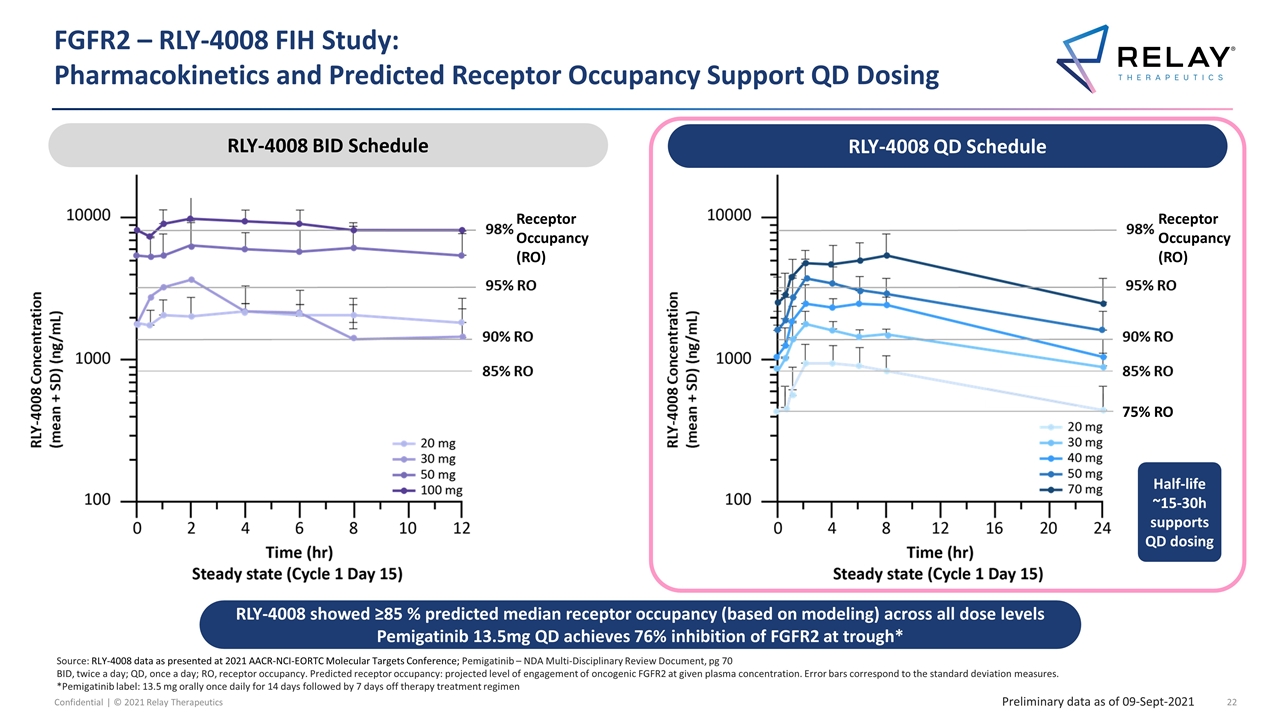
Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference; Pemigatinib – NDA Multi-Disciplinary Review Document, pg 70 BID, twice a day; QD, once a day; RO, receptor occupancy. Predicted receptor occupancy: projected level of engagement of oncogenic FGFR2 at given plasma concentration. Error bars correspond to the standard deviation measures. *Pemigatinib label: 13.5 mg orally once daily for 14 days followed by 7 days off therapy treatment regimen FGFR2 – RLY-4008 FIH Study: Pharmacokinetics and Predicted Receptor Occupancy Support QD Dosing Preliminary data as of 09-Sept-2021 Receptor Occupancy (RO) RLY-4008 showed ≥85 % predicted median receptor occupancy (based on modeling) across all dose levels Pemigatinib 13.5mg QD achieves 76% inhibition of FGFR2 at trough* Receptor Occupancy (RO) RLY-4008 QD Schedule RLY-4008 BID Schedule 75% RO Half-life ~15-30h supports QD dosing
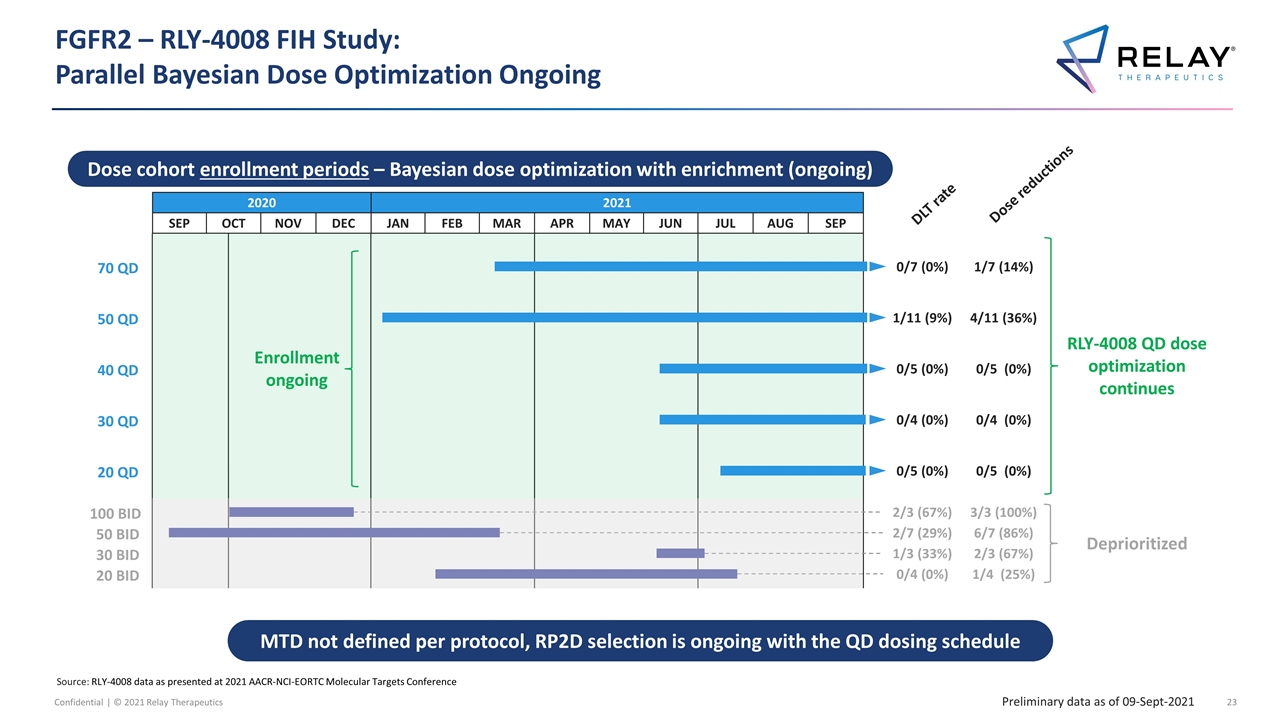
FGFR2 – RLY-4008 FIH Study: Parallel Bayesian Dose Optimization Ongoing MTD not defined per protocol, RP2D selection is ongoing with the QD dosing schedule Preliminary data as of 09-Sept-2021 Dose reductions 2020 2021 SEP OCT NOV DEC JAN FEB MAR APR MAY JUN JUL AUG SEP Dose cohort enrollment periods – Bayesian dose optimization with enrichment (ongoing) DLT rate Deprioritized 6/7 (86%) 2/7 (29%) 50 BID 3/3 (100%) 2/3 (67%) 100 BID 2/3 (67%) 1/3 (33%) 30 BID 1/4 (25%) 0/4 (0%) 20 BID RLY-4008 QD dose optimization continues 1/7 (14%) 0/7 (0%) 70 QD 4/11 (36%) 1/11 (9%) 50 QD 0/4 (0%) 0/4 (0%) 30 QD 0/5 (0%) 0/5 (0%) 20 QD 0/5 (0%) 0/5 (0%) 40 QD Enrollment ongoing Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference
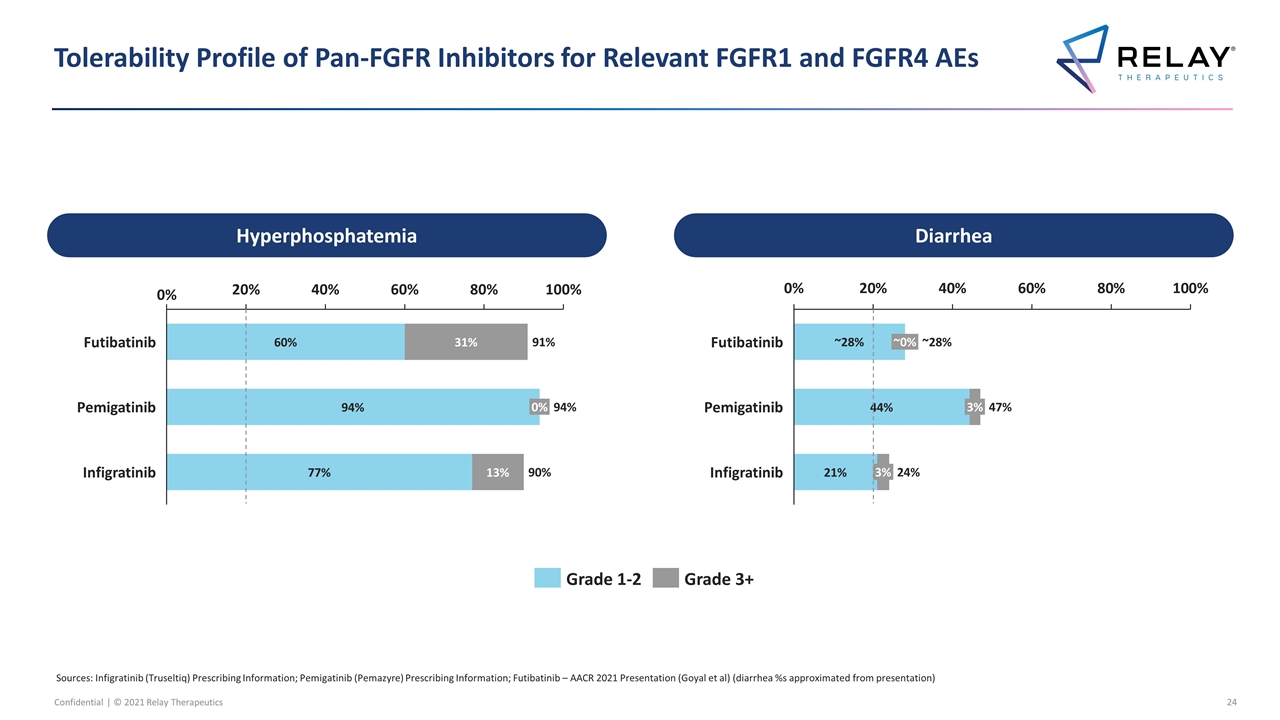
Tolerability Profile of Pan-FGFR Inhibitors for Relevant FGFR1 and FGFR4 AEs Sources: Infigratinib (Truseltiq) Prescribing Information; Pemigatinib (Pemazyre) Prescribing Information; Futibatinib – AACR 2021 Presentation (Goyal et al) (diarrhea %s approximated from presentation) ~ ~ ~ Hyperphosphatemia Diarrhea
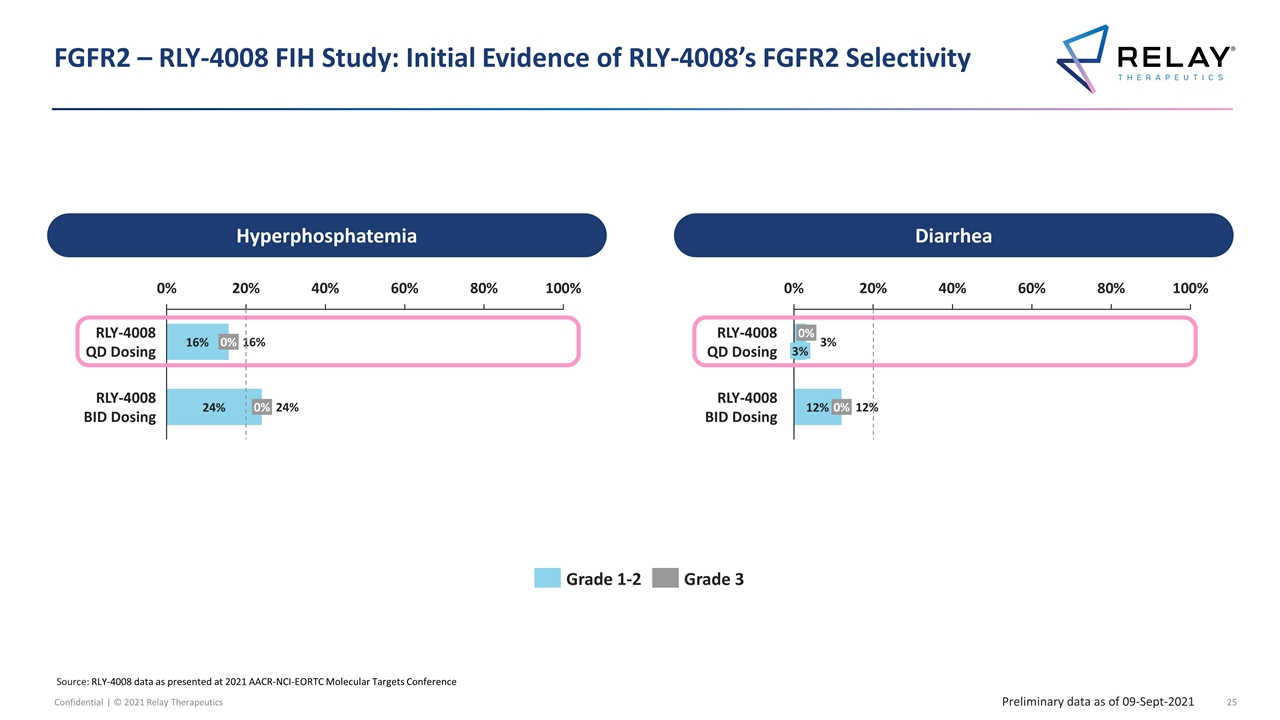
FGFR2 – RLY-4008 FIH Study: Initial Evidence of RLY-4008’s FGFR2 Selectivity Hyperphosphatemia Diarrhea Preliminary data as of 09-Sept-2021 Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference
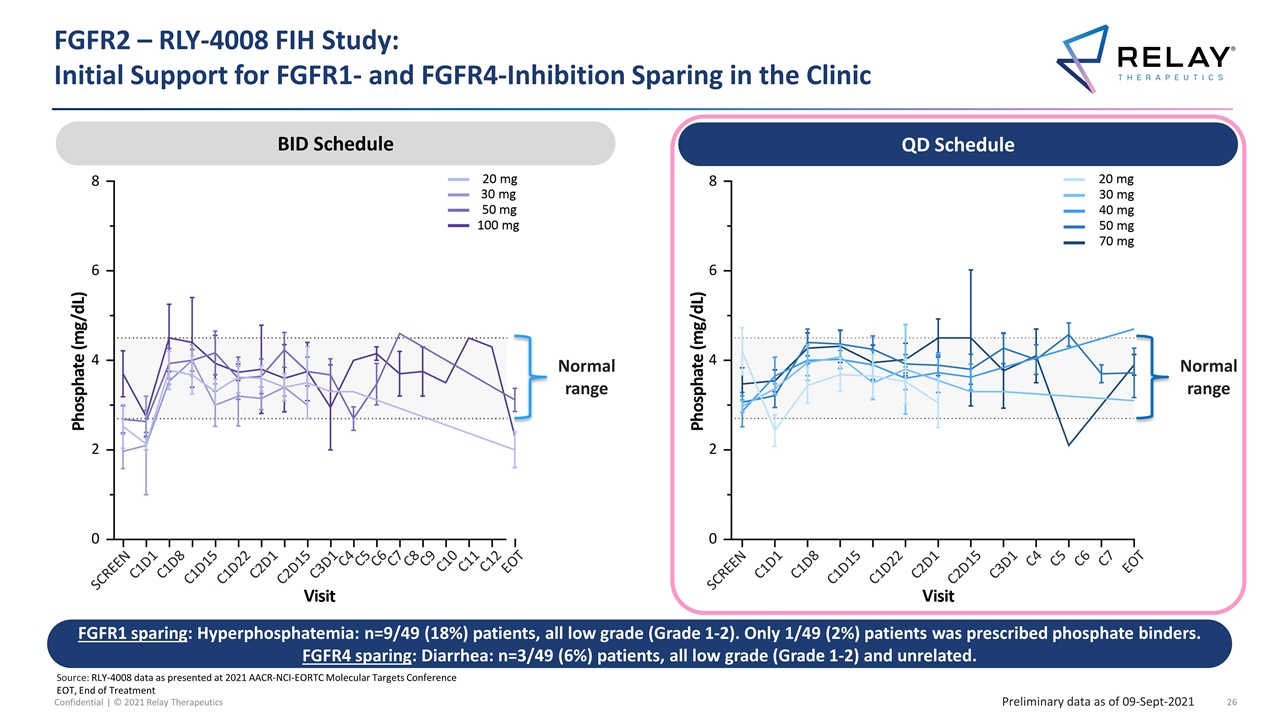
FGFR2 – RLY-4008 FIH Study: Initial Support for FGFR1- and FGFR4-Inhibition Sparing in the Clinic Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference EOT, End of Treatment Normal range BID Schedule BID Schedule FGFR1 sparing: Hyperphosphatemia: n=9/49 (18%) patients, all low grade (Grade 1-2). Only 1/49 (2%) patients was prescribed phosphate binders. FGFR4 sparing: Diarrhea: n=3/49 (6%) patients, all low grade (Grade 1-2) and unrelated. QD Schedule Normal range QD Schedule Preliminary data as of 09-Sept-2021
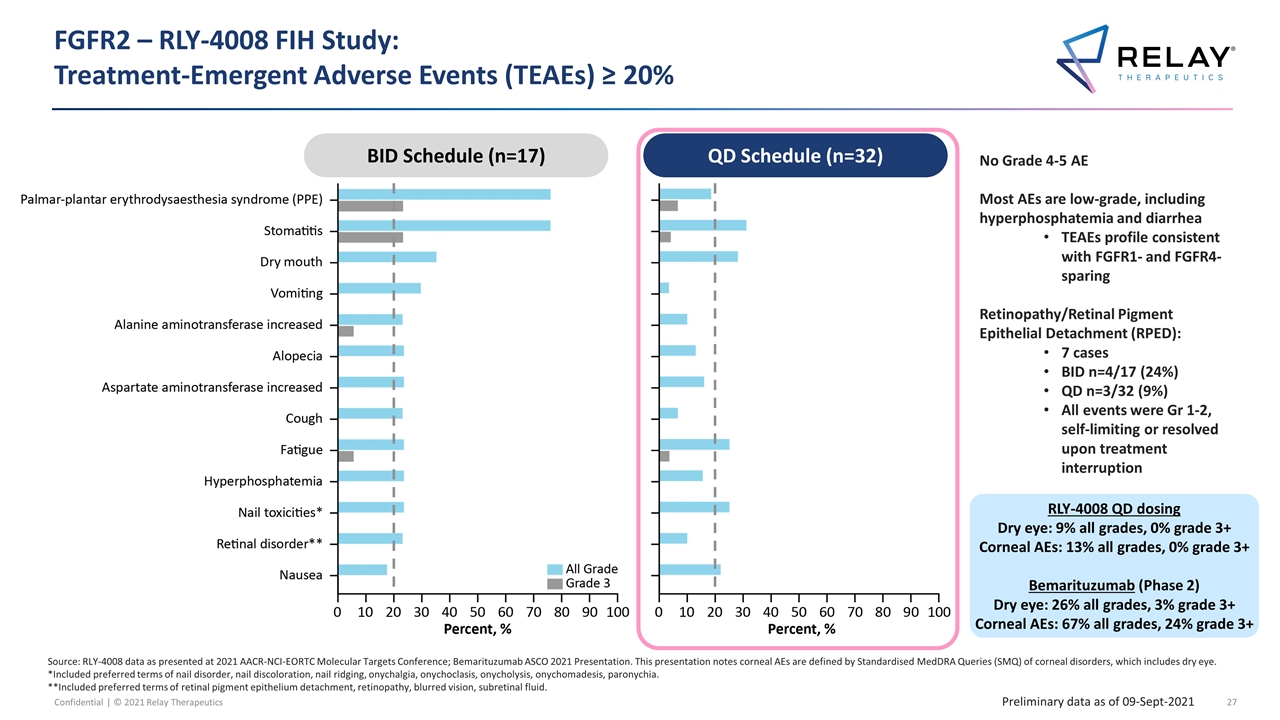
FGFR2 – RLY-4008 FIH Study: Treatment-Emergent Adverse Events (TEAEs) ≥ 20% No Grade 4-5 AE Most AEs are low-grade, including hyperphosphatemia and diarrhea TEAEs profile consistent with FGFR1- and FGFR4-sparing Retinopathy/Retinal Pigment Epithelial Detachment (RPED): 7 cases BID n=4/17 (24%) QD n=3/32 (9%) All events were Gr 1-2, self-limiting or resolved upon treatment interruption QD Schedule (n = 32) Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference; Bemarituzumab ASCO 2021 Presentation. This presentation notes corneal AEs are defined by Standardised MedDRA Queries (SMQ) of corneal disorders, which includes dry eye. *Included preferred terms of nail disorder, nail discoloration, nail ridging, onychalgia, onychoclasis, onycholysis, onychomadesis, paronychia. **Included preferred terms of retinal pigment epithelium detachment, retinopathy, blurred vision, subretinal fluid. Preliminary data as of 09-Sept-2021 BID Schedule (n=17) QD Schedule (n=32) RLY-4008 QD dosing Dry eye: 9% all grades, 0% grade 3+ Corneal AEs: 13% all grades, 0% grade 3+ Bemarituzumab (Phase 2) Dry eye: 26% all grades, 3% grade 3+ Corneal AEs: 67% all grades, 24% grade 3+
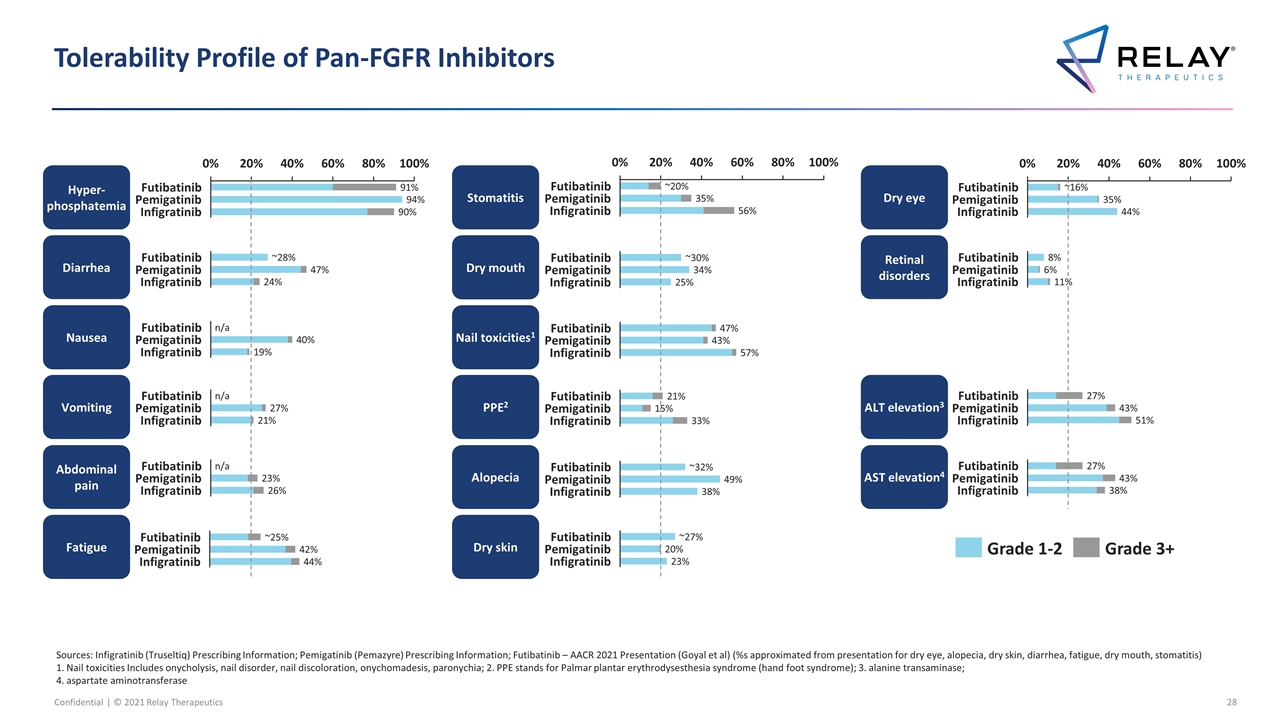
Tolerability Profile of Pan-FGFR Inhibitors Sources: Infigratinib (Truseltiq) Prescribing Information; Pemigatinib (Pemazyre) Prescribing Information; Futibatinib – AACR 2021 Presentation (Goyal et al) (%s approximated from presentation for dry eye, alopecia, dry skin, diarrhea, fatigue, dry mouth, stomatitis) 1. Nail toxicities Includes onycholysis, nail disorder, nail discoloration, onychomadesis, paronychia; 2. PPE stands for Palmar plantar erythrodysesthesia syndrome (hand foot syndrome); 3. alanine transaminase; 4. aspartate aminotransferase Nausea Vomiting Abdominal pain Nail toxicities1 Dry skin Alopecia Dry mouth Stomatitis AST elevation4 PPE2 Dry eye Retinal disorders Fatigue ALT elevation3 Hyper-phosphatemia Diarrhea ~ n/a n/a n/a ~ ~ ~ ~ ~ ~
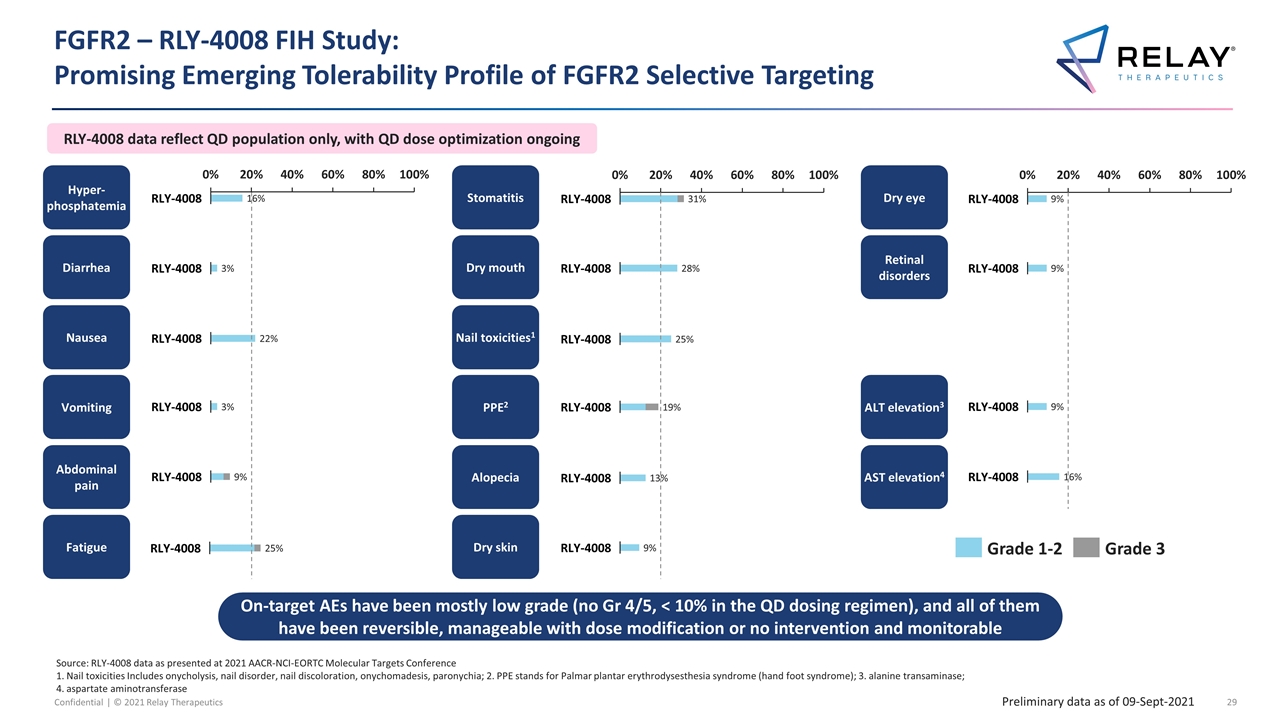
FGFR2 – RLY-4008 FIH Study: Promising Emerging Tolerability Profile of FGFR2 Selective Targeting Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference 1. Nail toxicities Includes onycholysis, nail disorder, nail discoloration, onychomadesis, paronychia; 2. PPE stands for Palmar plantar erythrodysesthesia syndrome (hand foot syndrome); 3. alanine transaminase; 4. aspartate aminotransferase Preliminary data as of 09-Sept-2021 On-target AEs have been mostly low grade (no Gr 4/5, < 10% in the QD dosing regimen), and all of them have been reversible, manageable with dose modification or no intervention and monitorable Nausea Vomiting Abdominal pain Nail toxicities1 Dry skin Alopecia Dry mouth Stomatitis AST elevation4 PPE2 Dry eye Retinal disorders Fatigue ALT elevation3 Hyper-phosphatemia Diarrhea RLY-4008 data reflect QD population only, with QD dose optimization ongoing
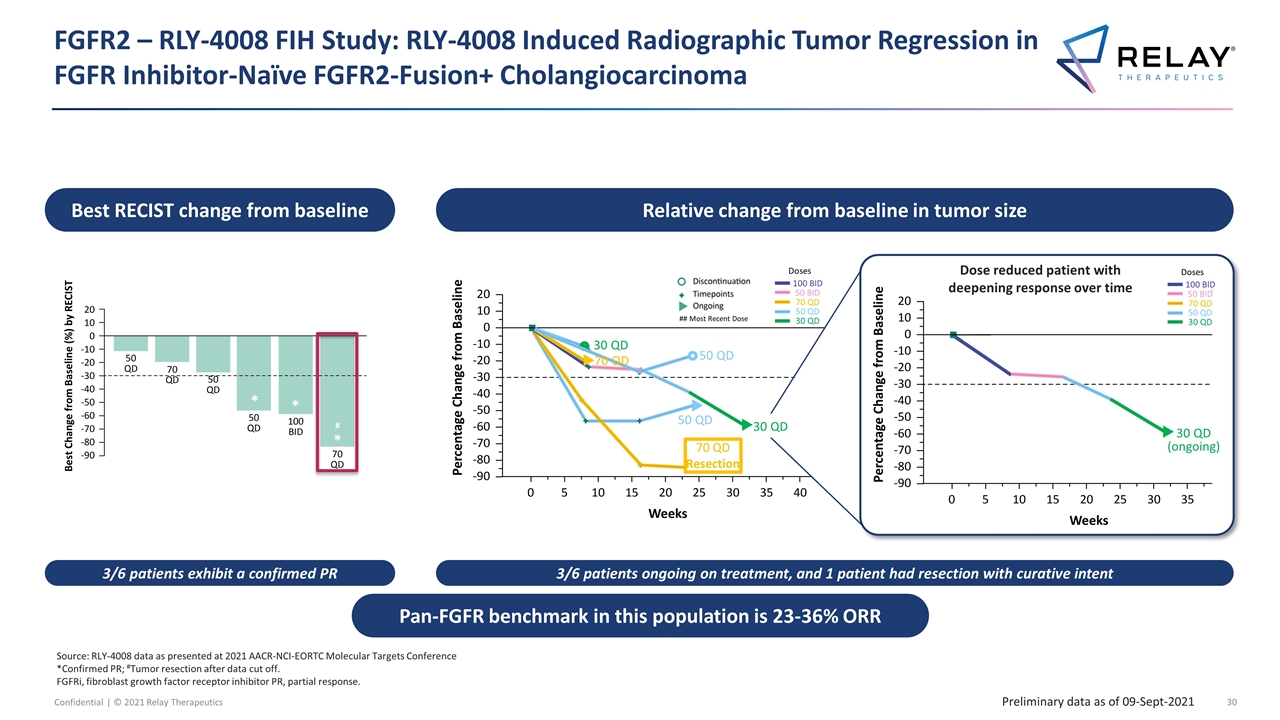
FGFR2 – RLY-4008 FIH Study: RLY-4008 Induced Radiographic Tumor Regression in FGFR Inhibitor-Naïve FGFR2-Fusion+ Cholangiocarcinoma Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference *Confirmed PR; #Tumor resection after data cut off. FGFRi, fibroblast growth factor receptor inhibitor PR, partial response. Preliminary data as of 09-Sept-2021 Best RECIST change from baseline 3/6 patients exhibit a confirmed PR 3/6 patients ongoing on treatment, and 1 patient had resection with curative intent Relative change from baseline in tumor size Pan-FGFR benchmark in this population is 23-36% ORR * * * # 70 QD Resection 50 QD 30 QD ## Most Recent Dose 50 QD Doses 100 BID 50 BID 70 QD 50 QD 30 QD 70 QD 30 QD 30 QD (ongoing) Dose reduced patient with deepening response over time Doses 100 BID 50 BID 70 QD 50 QD 30 QD
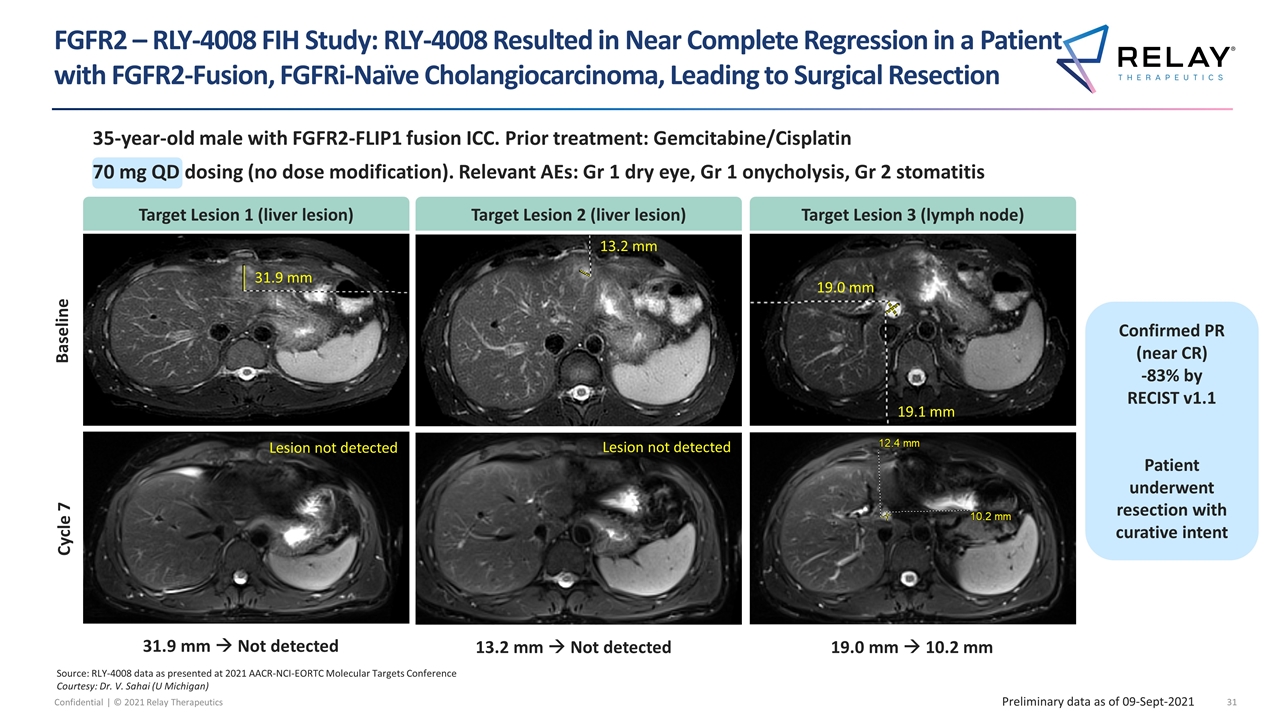
35-year-old male with FGFR2-FLIP1 fusion ICC. Prior treatment: Gemcitabine/Cisplatin 70 mg QD dosing (no dose modification). Relevant AEs: Gr 1 dry eye, Gr 1 onycholysis, Gr 2 stomatitis Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Courtesy: Dr. V. Sahai (U Michigan) Baseline Cycle 7 31.9 mm à Not detected 13.2 mm à Not detected 19.0 mm à 10.2 mm 31.9 mm 13.2 mm 19.0 mm 19.1 mm Lesion not detected Lesion not detected FGFR2 – RLY-4008 FIH Study: RLY-4008 Resulted in Near Complete Regression in a Patient with FGFR2-Fusion, FGFRi-Naïve Cholangiocarcinoma, Leading to Surgical Resection Target Lesion 1 (liver lesion) Target Lesion 2 (liver lesion) Target Lesion 3 (lymph node) Preliminary data as of 09-Sept-2021 Confirmed PR (near CR) -83% by RECIST v1.1 Patient underwent resection with curative intent
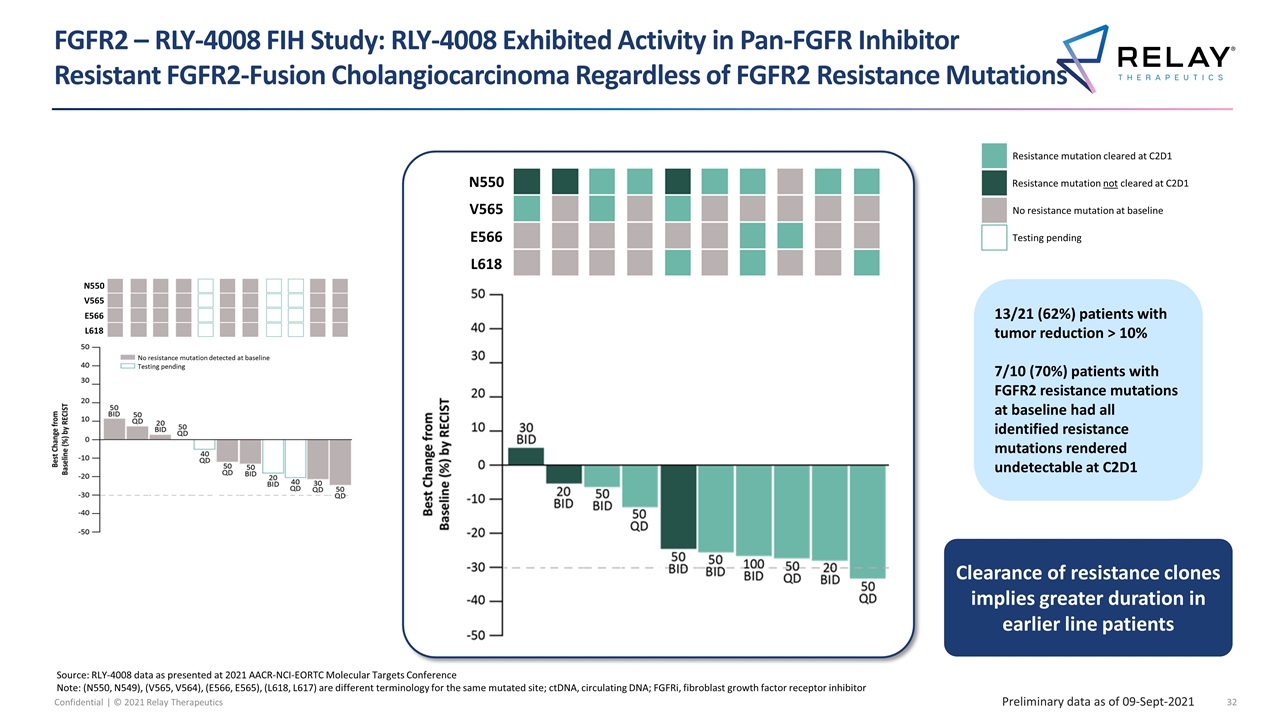
FGFR2 – RLY-4008 FIH Study: RLY-4008 Exhibited Activity in Pan-FGFR Inhibitor Resistant FGFR2-Fusion Cholangiocarcinoma Regardless of FGFR2 Resistance Mutations Preliminary data as of 09-Sept-2021 Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Note: (N550, N549), (V565, V564), (E566, E565), (L618, L617) are different terminology for the same mutated site; ctDNA, circulating DNA; FGFRi, fibroblast growth factor receptor inhibitor Testing pending No resistance mutation at baseline Resistance mutation not cleared at C2D1 Resistance mutation cleared at C2D1 Clearance of resistance clones implies greater duration in earlier line patients 13/21 (62%) patients with tumor reduction > 10% 7/10 (70%) patients with FGFR2 resistance mutations at baseline had all identified resistance mutations rendered undetectable at C2D1 No resistance mutation detected at baseline Testing pending N550 V565 E566 L618 ≥ 1 resistance mutation detected at baseline, and all rendered undetectable at C2D1 ≥ 1 resistance mutation detected at baseline and C2D1 N550 V565 E566 L618

FGFR2 – RLY-4008 FIH Study: RLY-4008 Produced Tumor Regression in a Patient with FGFR2-Fusion+ Cholangiocarcinoma Pretreated with Futibatinib 51-year-old female with FGFR2-CIT fusion ICC. Prior treatments: Gemcitabine/Cisplatin, Futibatinib Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Note: E566 and E565 are different terminology for the same mutated site Courtesy: Dr. L. Goyal (Mass. General Hospital) 60 x 46 mm 42 x 35 mm ctDNA: Baseline FGFR2-E566V mutation is undetectable at C2D1 Sustained tumor reduction at Cycle 7 - 21% by RECIST v1.1 Antitumor activity: Sustained tumor reduction at C7 (-21% per RECIST v1.1) Safety and tolerability: No dose interruption or modification RLY-4008 treatment is ongoing (50 mg QD) Baseline Cycle 7 Pelvic lesions Preliminary data as of 09-Sept-2021 FGFR2 E566V AID1A Q781fs AID1A Q782T HNF1A G288G 53 x 27 mm 23 x 22 mm
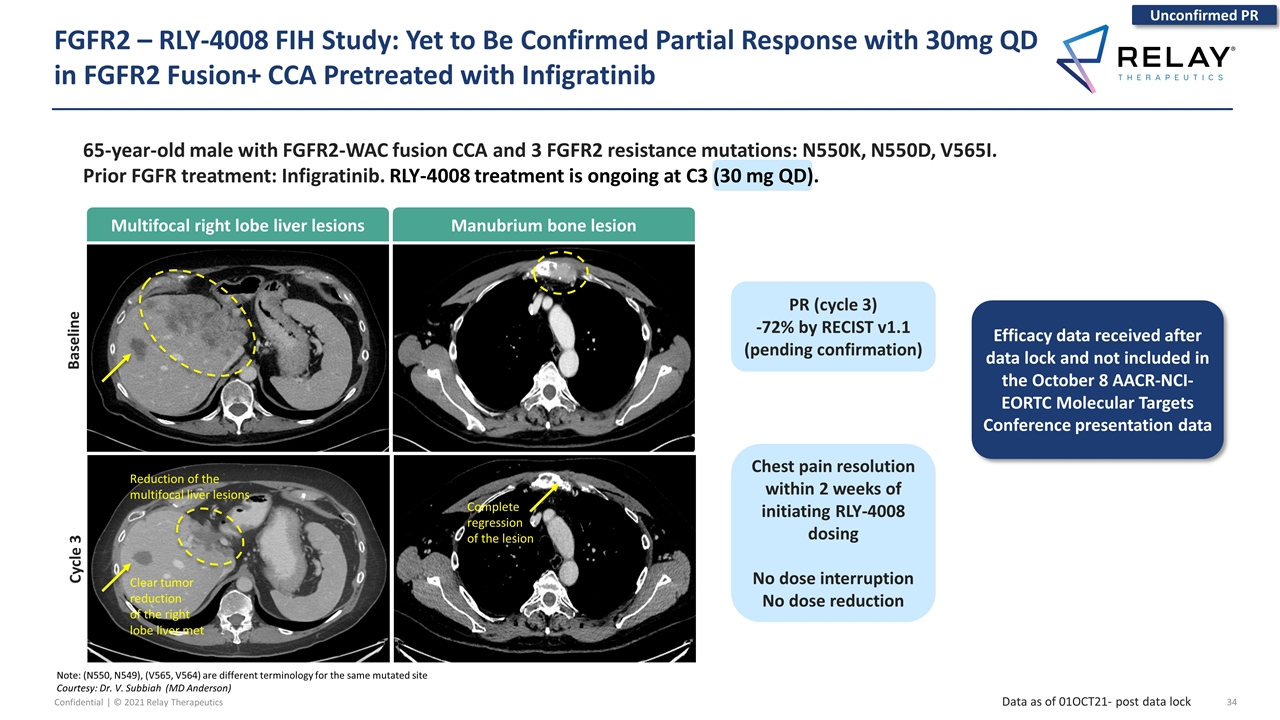
65-year-old male with FGFR2-WAC fusion CCA and 3 FGFR2 resistance mutations: N550K, N550D, V565I. Prior FGFR treatment: Infigratinib. RLY-4008 treatment is ongoing at C3 (30 mg QD). Data as of 01OCT21- post data lock Note: (N550, N549), (V565, V564) are different terminology for the same mutated site Courtesy: Dr. V. Subbiah (MD Anderson) Unconfirmed PR Efficacy data received after data lock and not included in the October 8 AACR-NCI-EORTC Molecular Targets Conference presentation data Multifocal right lobe liver lesions Manubrium bone lesion Baseline Cycle 3 Complete regression of the lesion Clear tumor reduction of the right lobe liver met Reduction of the multifocal liver lesions Chest pain resolution within 2 weeks of initiating RLY-4008 dosing No dose interruption No dose reduction PR (cycle 3) -72% by RECIST v1.1 (pending confirmation) FGFR2 – RLY-4008 FIH Study: Yet to Be Confirmed Partial Response with 30mg QD in FGFR2 Fusion+ CCA Pretreated with Infigratinib
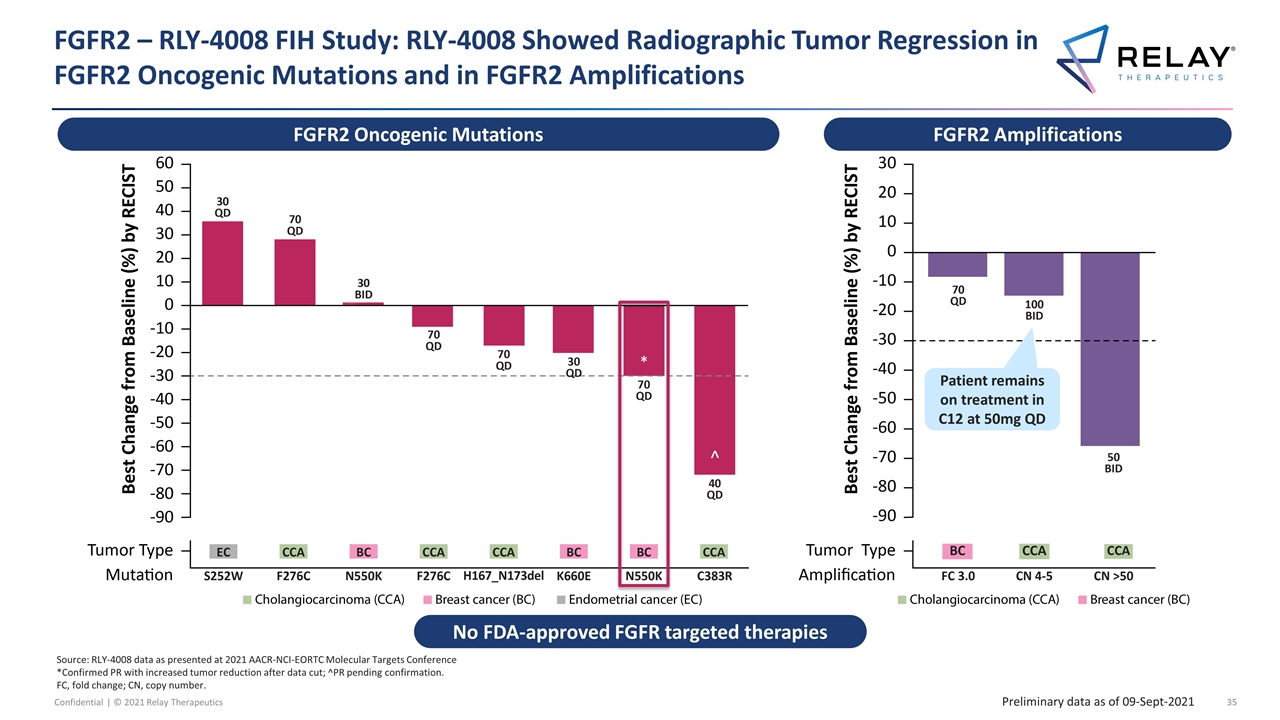
FGFR2 – RLY-4008 FIH Study: RLY-4008 Showed Radiographic Tumor Regression in FGFR2 Oncogenic Mutations and in FGFR2 Amplifications Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference *Confirmed PR with increased tumor reduction after data cut; ^PR pending confirmation. FC, fold change; CN, copy number. Preliminary data as of 09-Sept-2021 * ^ ^ FGFR2 Oncogenic Mutations FGFR2 Amplifications No FDA-approved FGFR targeted therapies Patient remains on treatment in C12 at 50mg QD
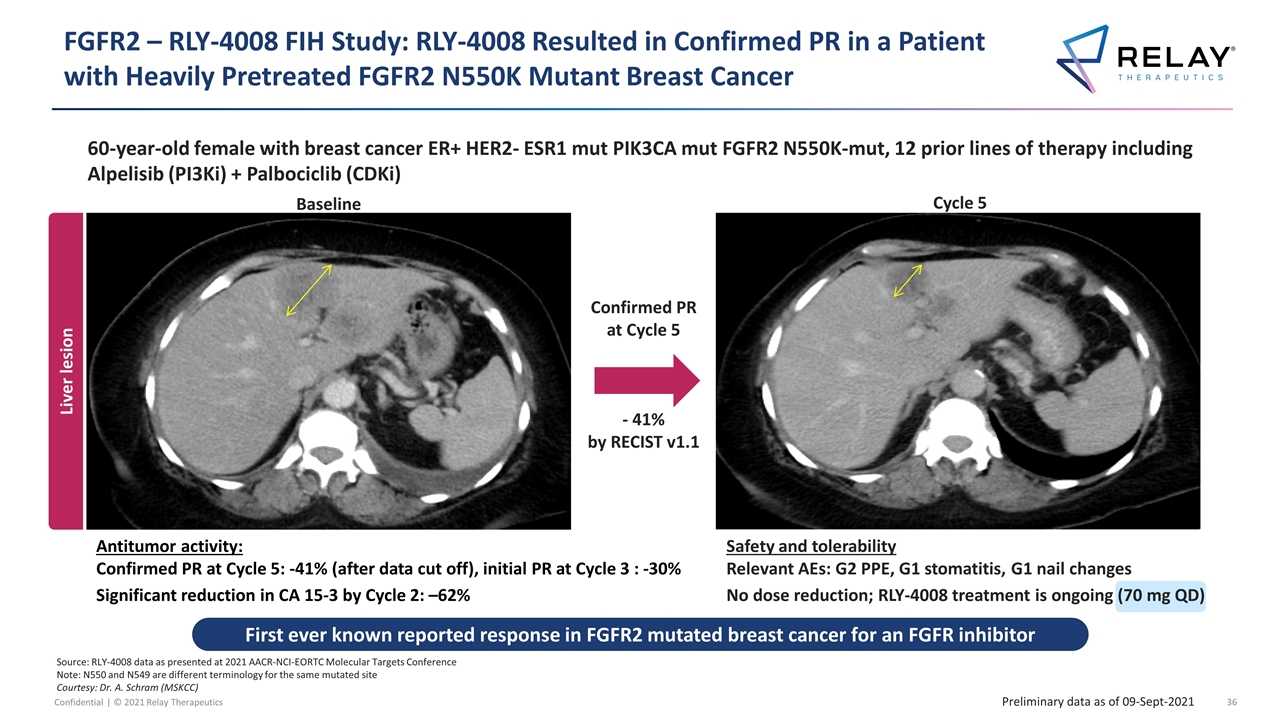
Confirmed PR at Cycle 5 - 41% by RECIST v1.1 Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference Note: N550 and N549 are different terminology for the same mutated site Courtesy: Dr. A. Schram (MSKCC) 60-year-old female with breast cancer ER+ HER2- ESR1 mut PIK3CA mut FGFR2 N550K-mut, 12 prior lines of therapy including Alpelisib (PI3Ki) + Palbociclib (CDKi) FGFR2 – RLY-4008 FIH Study: RLY-4008 Resulted in Confirmed PR in a Patient with Heavily Pretreated FGFR2 N550K Mutant Breast Cancer Antitumor activity: Confirmed PR at Cycle 5: -41% (after data cut off), initial PR at Cycle 3 : -30% Significant reduction in CA 15-3 by Cycle 2: –62% C3D1 Baseline Cycle 5 Liver lesion Preliminary data as of 09-Sept-2021 First ever known reported response in FGFR2 mutated breast cancer for an FGFR inhibitor Safety and tolerability Relevant AEs: G2 PPE, G1 stomatitis, G1 nail changes No dose reduction; RLY-4008 treatment is ongoing (70 mg QD)
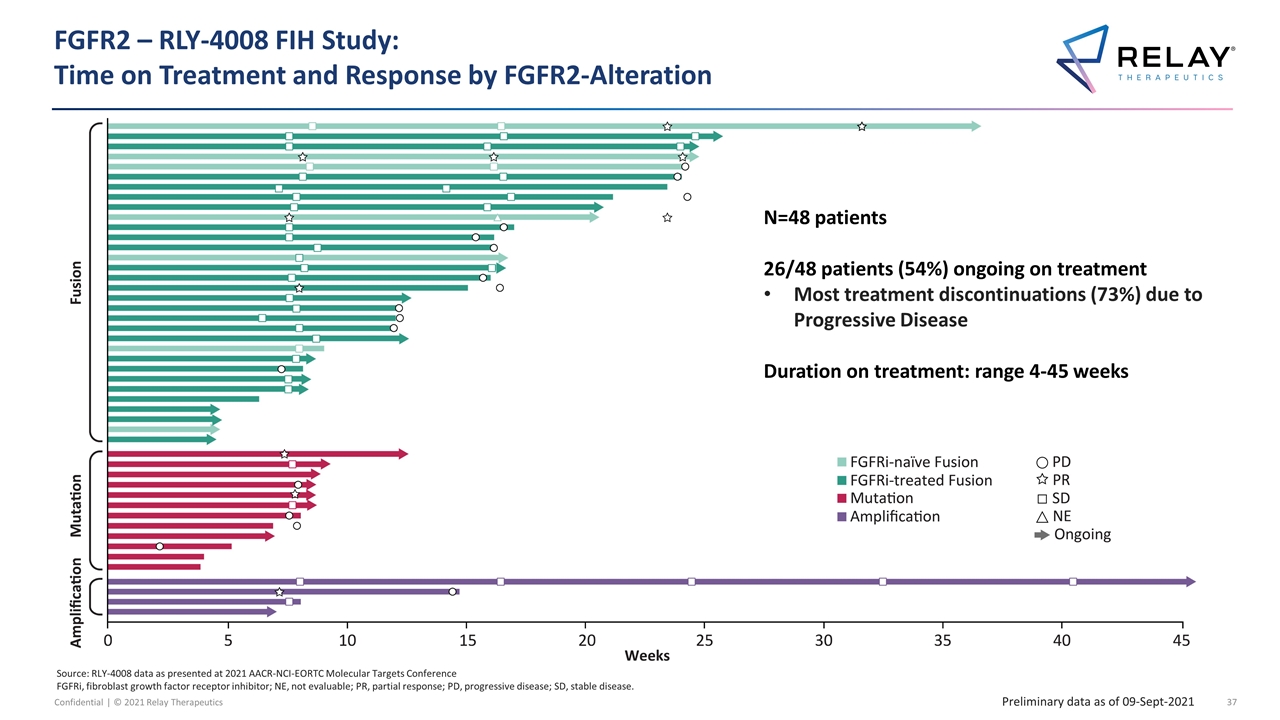
FGFR2 – RLY-4008 FIH Study: Time on Treatment and Response by FGFR2-Alteration N=48 patients 26/48 patients (54%) ongoing on treatment Most treatment discontinuations (73%) due to Progressive Disease Duration on treatment: range 4-45 weeks Preliminary data as of 09-Sept-2021 Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference FGFRi, fibroblast growth factor receptor inhibitor; NE, not evaluable; PR, partial response; PD, progressive disease; SD, stable disease.
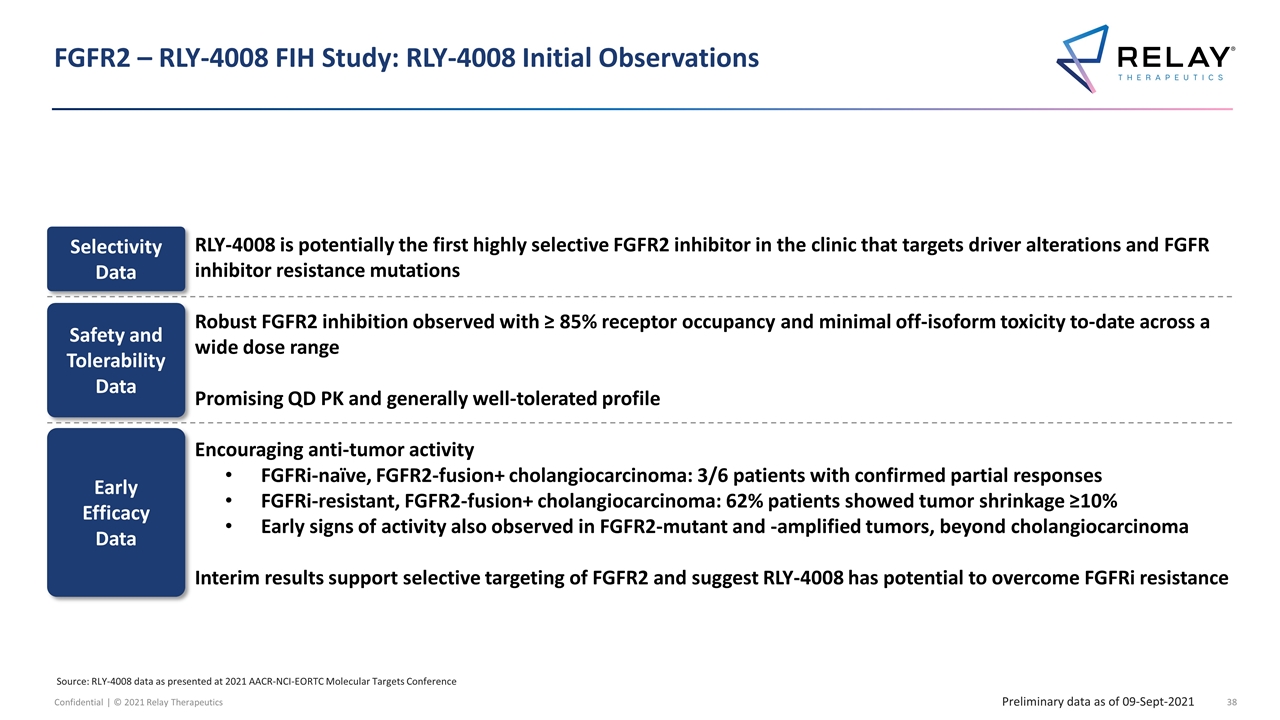
FGFR2 – RLY-4008 FIH Study: RLY-4008 Initial Observations RLY-4008 is potentially the first highly selective FGFR2 inhibitor in the clinic that targets driver alterations and FGFR inhibitor resistance mutations Robust FGFR2 inhibition observed with ≥ 85% receptor occupancy and minimal off-isoform toxicity to-date across a wide dose range Promising QD PK and generally well-tolerated profile Encouraging anti-tumor activity FGFRi-naïve, FGFR2-fusion+ cholangiocarcinoma: 3/6 patients with confirmed partial responses FGFRi-resistant, FGFR2-fusion+ cholangiocarcinoma: 62% patients showed tumor shrinkage ≥10% Early signs of activity also observed in FGFR2-mutant and -amplified tumors, beyond cholangiocarcinoma Interim results support selective targeting of FGFR2 and suggest RLY-4008 has potential to overcome FGFRi resistance Preliminary data as of 09-Sept-2021 Selectivity Data Safety and Tolerability Data Early Efficacy Data Source: RLY-4008 data as presented at 2021 AACR-NCI-EORTC Molecular Targets Conference
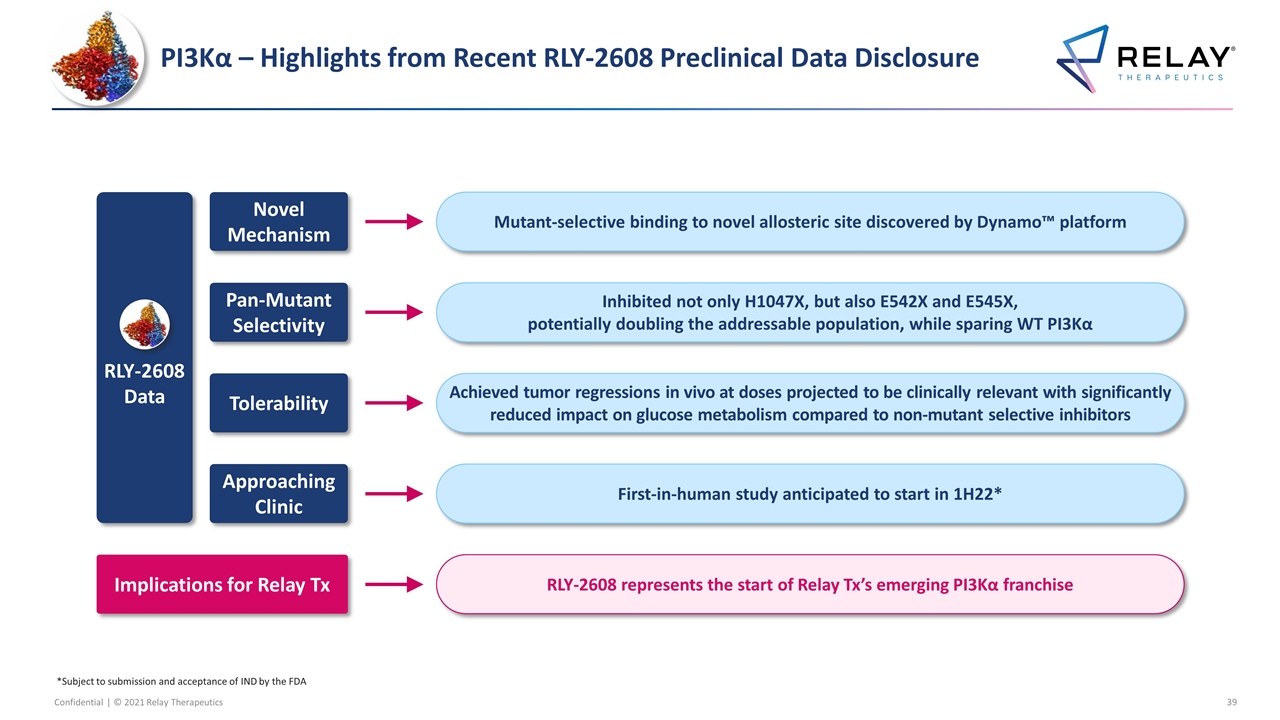
PI3Kα – Highlights from Recent RLY-2608 Preclinical Data Disclosure Mutant-selective binding to novel allosteric site discovered by Dynamo™ platform Novel Mechanism Inhibited not only H1047X, but also E542X and E545X, potentially doubling the addressable population, while sparing WT PI3Kα Pan-Mutant Selectivity Achieved tumor regressions in vivo at doses projected to be clinically relevant with significantly reduced impact on glucose metabolism compared to non-mutant selective inhibitors Tolerability RLY-2608 represents the start of Relay Tx’s emerging PI3Kα franchise First-in-human study anticipated to start in 1H22* Approaching Clinic Implications for Relay Tx RLY-2608 Data *Subject to submission and acceptance of IND by the FDA
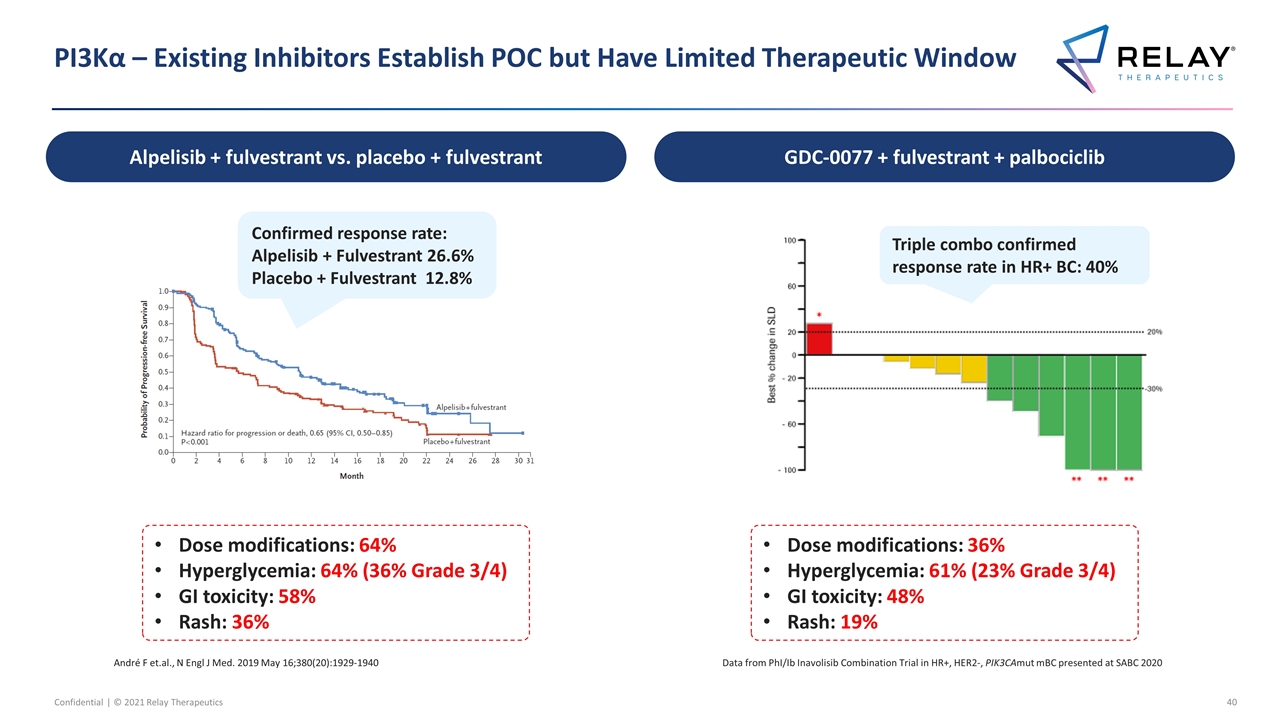
PI3Kα – Existing Inhibitors Establish POC but Have Limited Therapeutic Window Data from PhI/Ib Inavolisib Combination Trial in HR+, HER2-, PIK3CAmut mBC presented at SABC 2020 Dose modifications: 36% Hyperglycemia: 61% (23% Grade 3/4) GI toxicity: 48% Rash: 19% Dose modifications: 64% Hyperglycemia: 64% (36% Grade 3/4) GI toxicity: 58% Rash: 36% André F et.al., N Engl J Med. 2019 May 16;380(20):1929-1940 Confirmed response rate: Alpelisib + Fulvestrant 26.6% Placebo + Fulvestrant 12.8% Triple combo confirmed response rate in HR+ BC: 40% Alpelisib + fulvestrant vs. placebo + fulvestrant GDC-0077 + fulvestrant + palbociclib
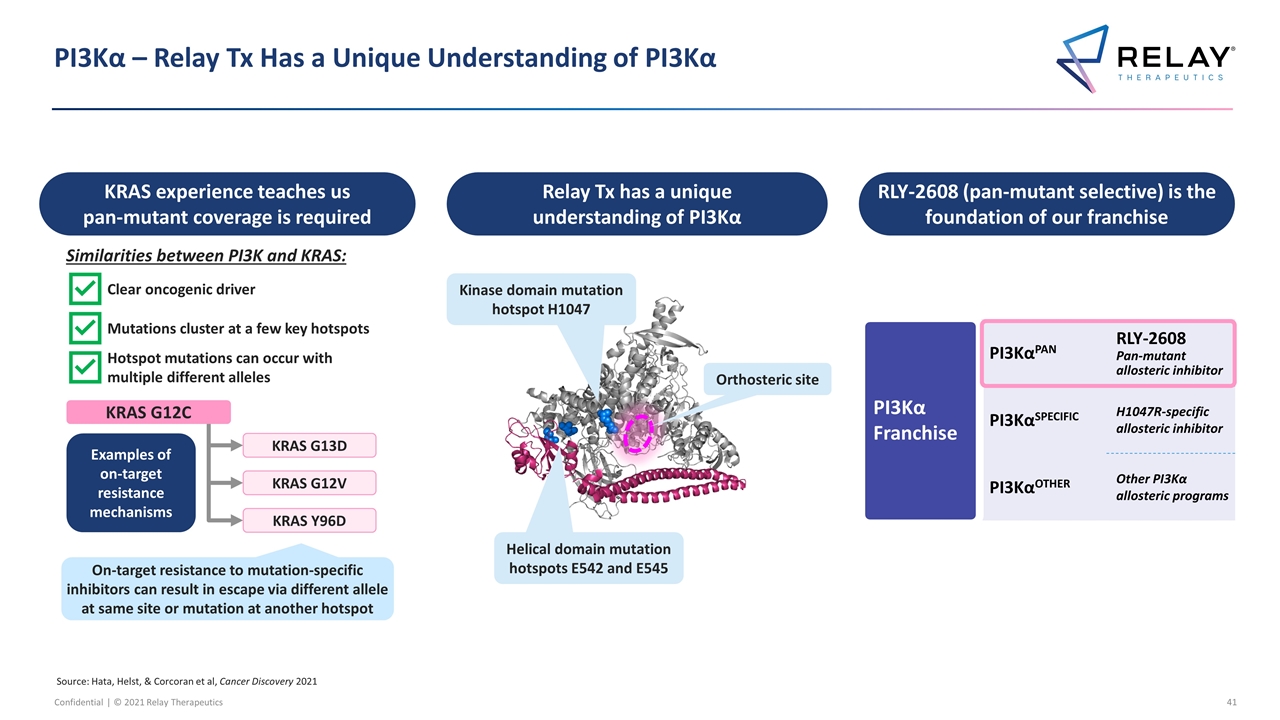
PI3Kα – Relay Tx Has a Unique Understanding of PI3Kα PI3Kα Franchise PI3KαPAN RLY-2608 Pan-mutant allosteric inhibitor PI3KαSPECIFIC H1047R-specific allosteric inhibitor PI3KαOTHER Other PI3Kα allosteric programs Relay Tx has a unique understanding of PI3Kα RLY-2608 (pan-mutant selective) is the foundation of our franchise Kinase domain mutation hotspot H1047 Helical domain mutation hotspots E542 and E545 Orthosteric site KRAS experience teaches us pan-mutant coverage is required Similarities between PI3K and KRAS: Clear oncogenic driver Mutations cluster at a few key hotspots Hotspot mutations can occur with multiple different alleles On-target resistance to mutation-specific inhibitors can result in escape via different allele at same site or mutation at another hotspot Examples of on-target resistance mechanisms KRAS G13D KRAS G12V KRAS Y96D KRAS G12C Source: Hata, Helst, & Corcoran et al, Cancer Discovery 2021

PI3Kα – Proprietary Insights Unlock Additional Approaches A differentiated understanding of the structure of PI3Kα and its relationship to function equips Relay Tx to design optimal mutant-selective inhibitors of PI3Kα Designed mutant selective PI3Kα inhibitor Discovered novel allosteric pocket favored in mutant protein Solved first full-length structures of PI3Kα (mutant and wild-type) Mutant PI3Kα Orthosteric Site
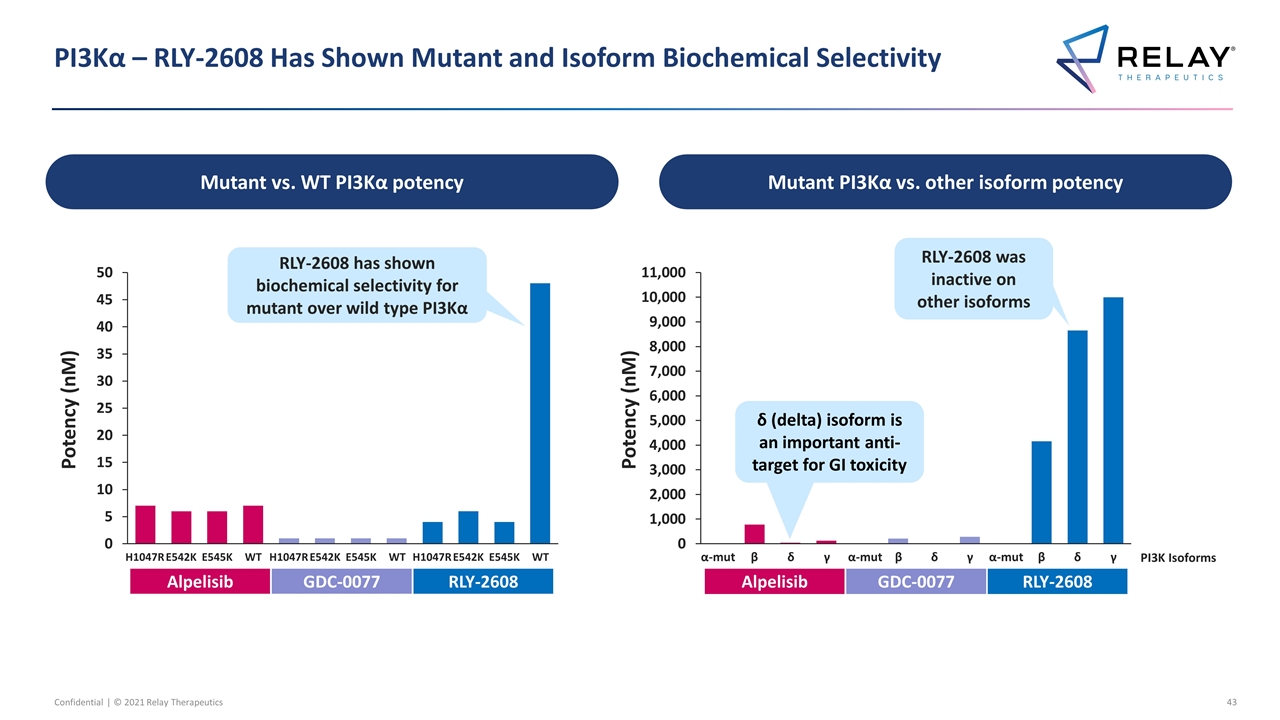
PI3Kα – RLY-2608 Has Shown Mutant and Isoform Biochemical Selectivity Potency (nM) H1047R E542K E545K WT Alpelisib GDC-0077 RLY-2608 RLY-2608 has shown biochemical selectivity for mutant over wild type PI3Kα α-mut Potency (nM) β δ Alpelisib RLY-2608 GDC-0077 RLY-2608 was inactive on other isoforms γ α-mut β δ γ α-mut β δ γ PI3K Isoforms Mutant vs. WT PI3Kα potency Mutant PI3Kα vs. other isoform potency δ (delta) isoform is an important anti-target for GI toxicity H1047R E542K E545K WT H1047R E542K E545K WT
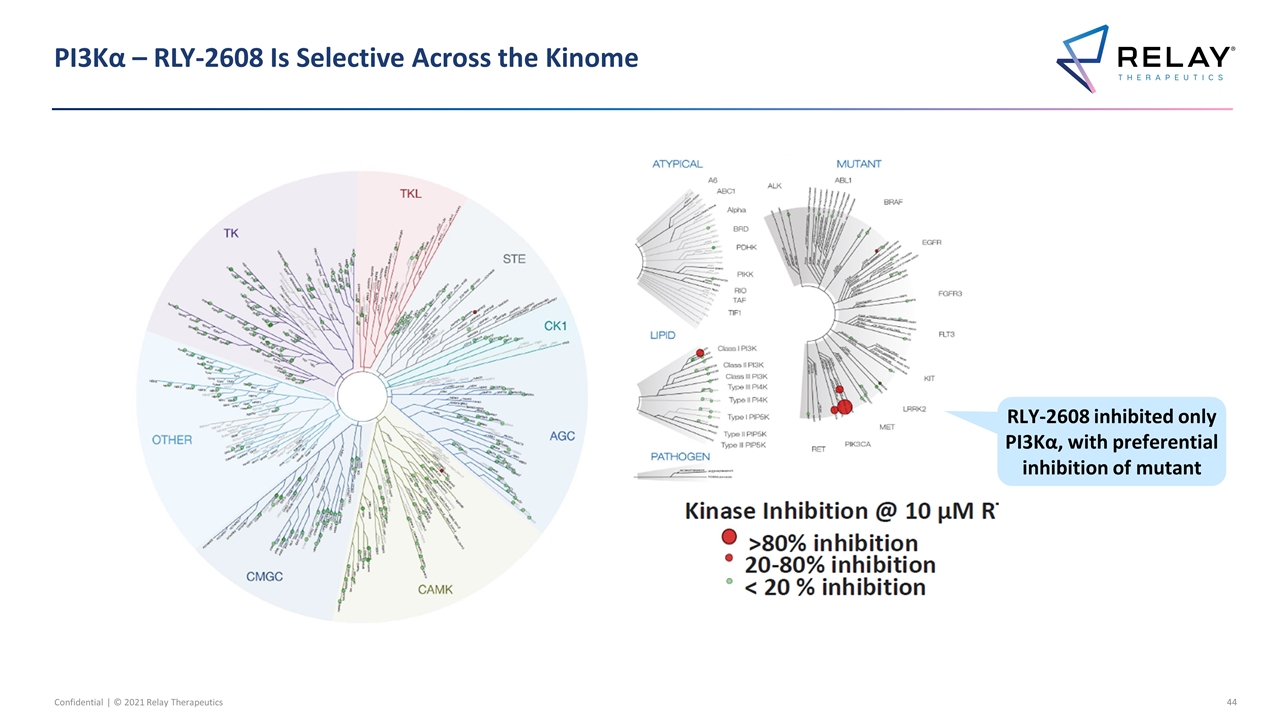
PI3Kα – RLY-2608 Is Selective Across the Kinome RLY-2608 inhibited only PI3Kα, with preferential inhibition of mutant
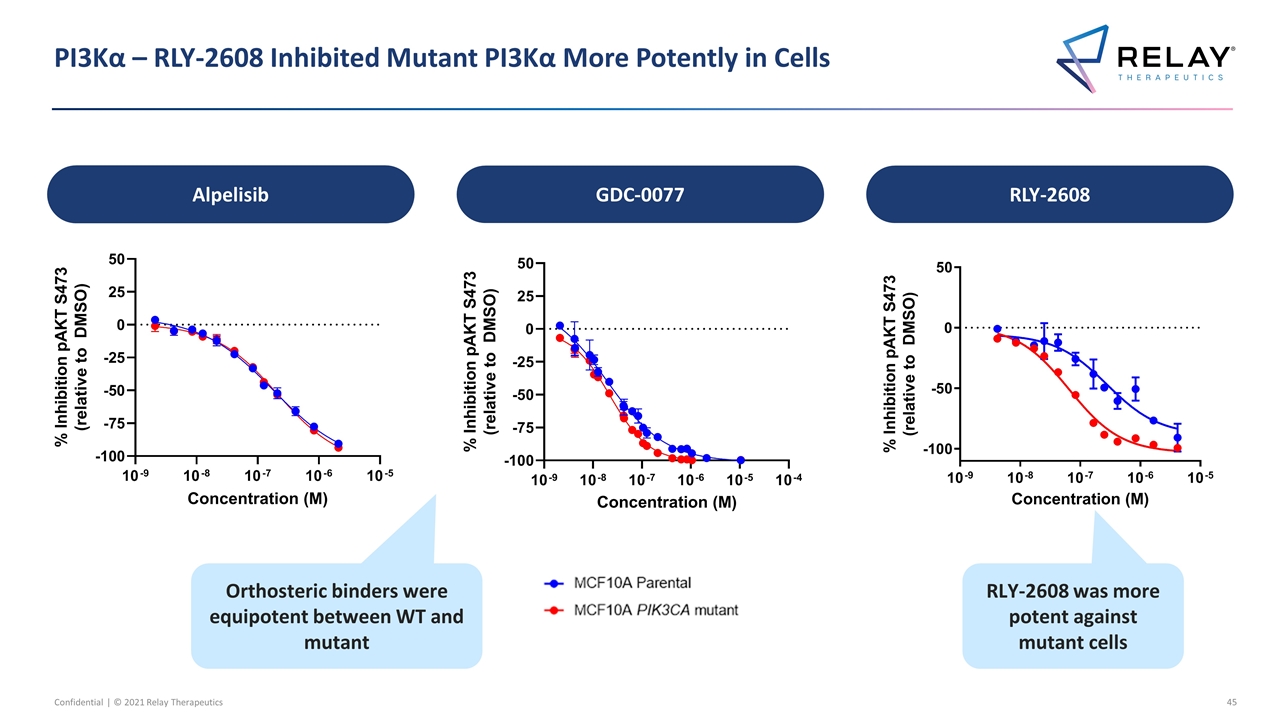
PI3Kα – RLY-2608 Inhibited Mutant PI3Kα More Potently in Cells RLY-2608 was more potent against mutant cells Orthosteric binders were equipotent between WT and mutant Alpelisib GDC-0077 RLY-2608
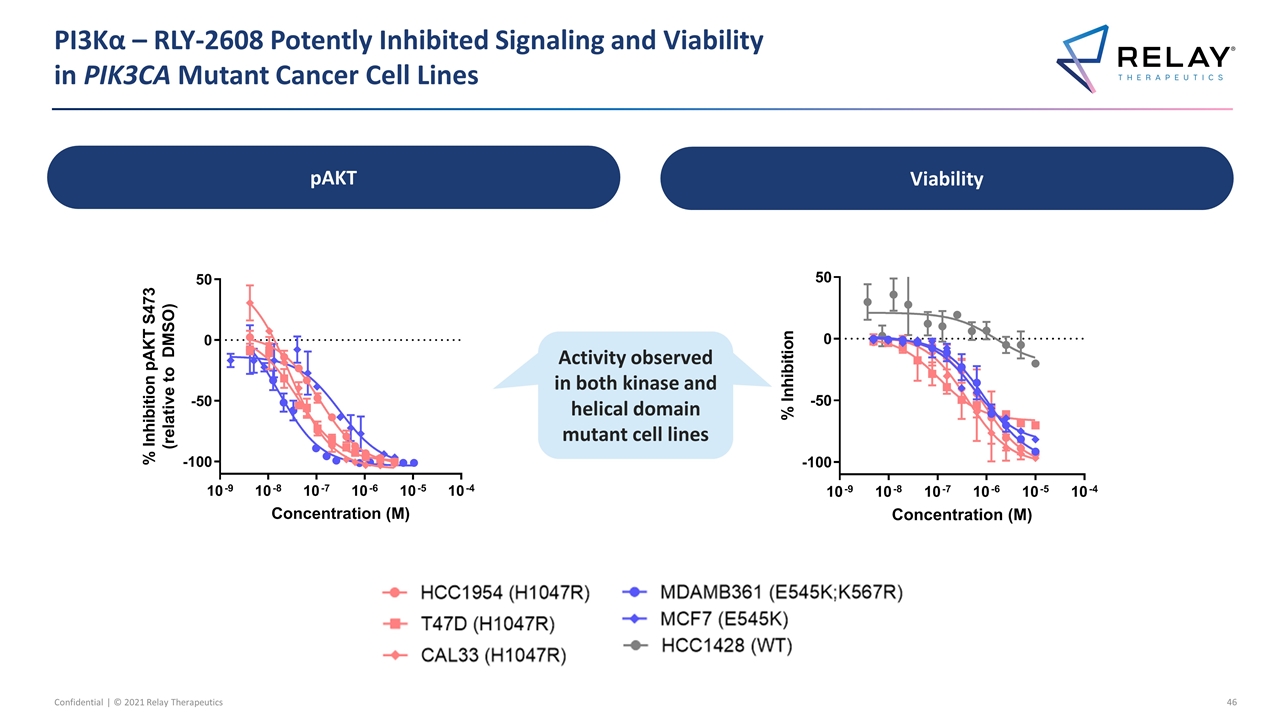
PI3Kα – RLY-2608 Potently Inhibited Signaling and Viability in PIK3CA Mutant Cancer Cell Lines Activity observed in both kinase and helical domain mutant cell lines Activity observed in both kinase and helical domain mutant cell lines pAKT Viability
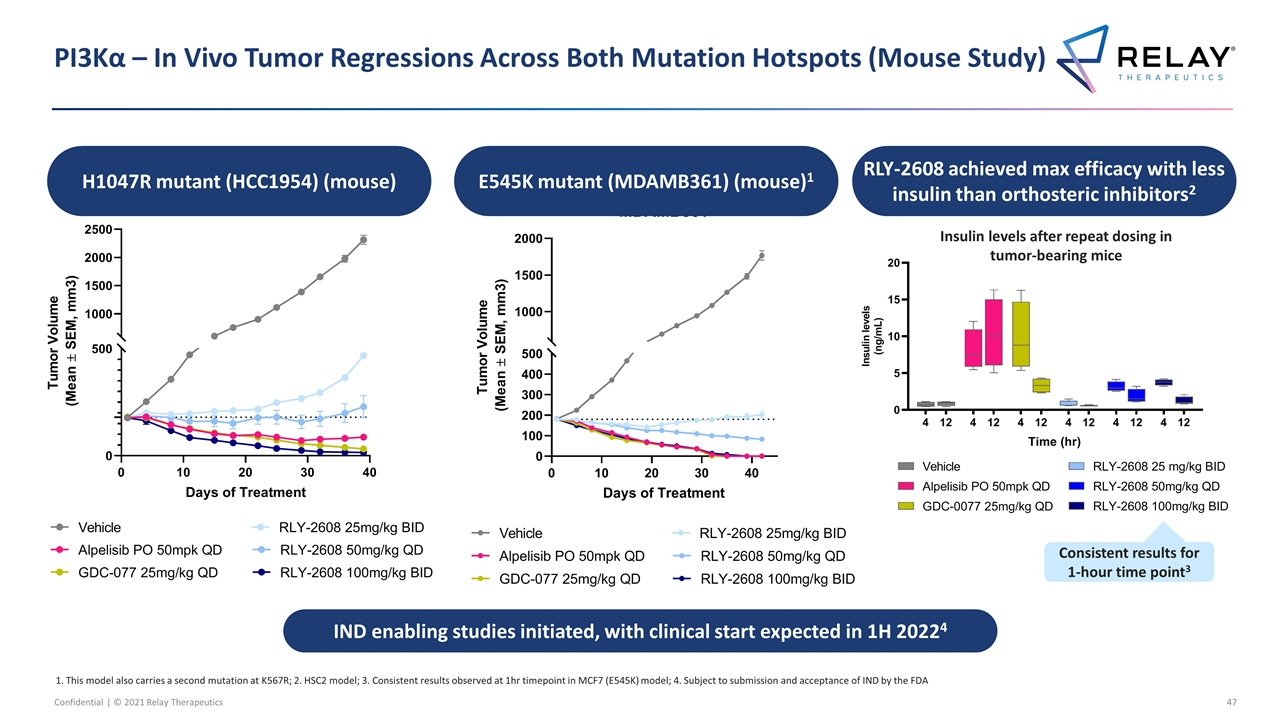
PI3Kα – In Vivo Tumor Regressions Across Both Mutation Hotspots (Mouse Study) H1047R mutant (HCC1954) (mouse) 1. This model also carries a second mutation at K567R; 2. HSC2 model; 3. Consistent results observed at 1hr timepoint in MCF7 (E545K) model; 4. Subject to submission and acceptance of IND by the FDA E545K mutant (MDAMB361) (mouse)1 RLY-2608 achieved max efficacy with less insulin than orthosteric inhibitors2 IND enabling studies initiated, with clinical start expected in 1H 20224 Insulin levels after repeat dosing in tumor-bearing mice Consistent results for 1-hour time point3
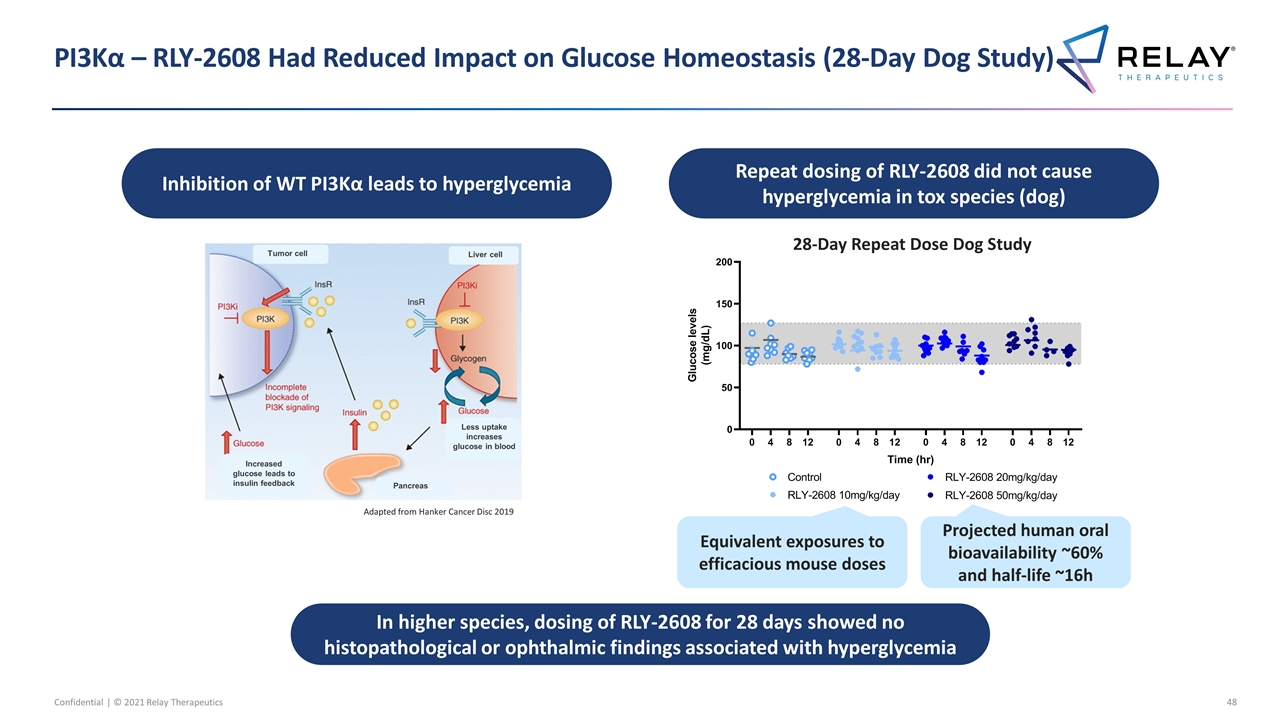
In higher species, dosing of RLY-2608 for 28 days showed no histopathological or ophthalmic findings associated with hyperglycemia PI3Kα – RLY-2608 Had Reduced Impact on Glucose Homeostasis (28-Day Dog Study) Adapted from Hanker Cancer Disc 2019 Less uptake increases glucose in blood Pancreas Liver cell Tumor cell Increased glucose leads to insulin feedback Inhibition of WT PI3Kα leads to hyperglycemia Repeat dosing of RLY-2608 did not cause hyperglycemia in tox species (dog) 28-Day Repeat Dose Dog Study Equivalent exposures to efficacious mouse doses Projected human oral bioavailability ~60% and half-life ~16h
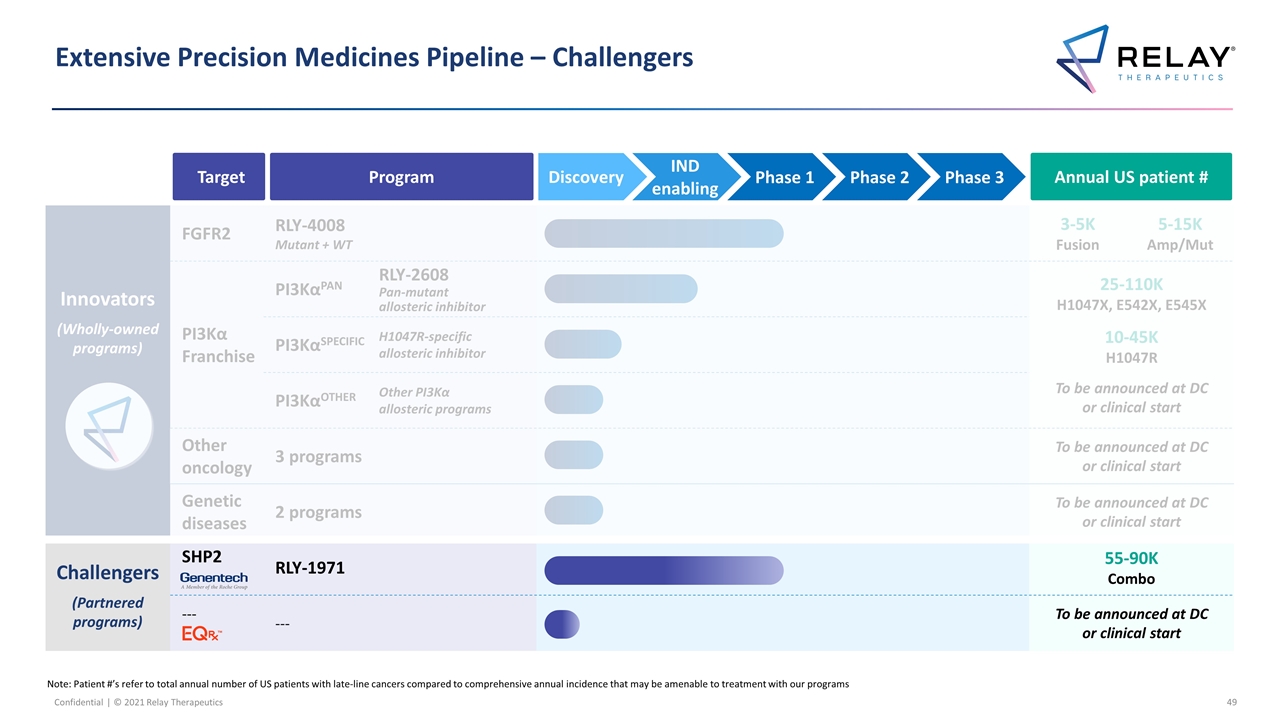
Extensive Precision Medicines Pipeline – Challengers Note: Patient #’s refer to total annual number of US patients with late-line cancers compared to comprehensive annual incidence that may be amenable to treatment with our programs Innovators (Wholly-owned programs) FGFR2 RLY-4008 Mutant + WT 3-5K Fusion 5-15K Amp/Mut PI3Kα Franchise PI3KαPAN RLY-2608 Pan-mutant allosteric inhibitor RLY-2608 Pan-mutant allosteric inhibitor 25-110K H1047X, E542X, E545X 10-45K H1047R To be announced at DC or clinical start PI3KαSPECIFIC H1047R-specific allosteric inhibitor RLY-1047R H1047R allosteric inhibitor PI3KαOTHER Other PI3Kα allosteric programs Additional Other novel mutant selective mechanisms Other oncology 3 programs To be announced at DC or clinical start Genetic diseases 2 programs To be announced at DC or clinical start Challengers (Partnered programs) SHP2 RLY-1971 55-90K Combo --- --- To be announced at DC or clinical start Target Program Annual US patient # IND enabling Phase 1 Phase 2 Phase 3 Discovery
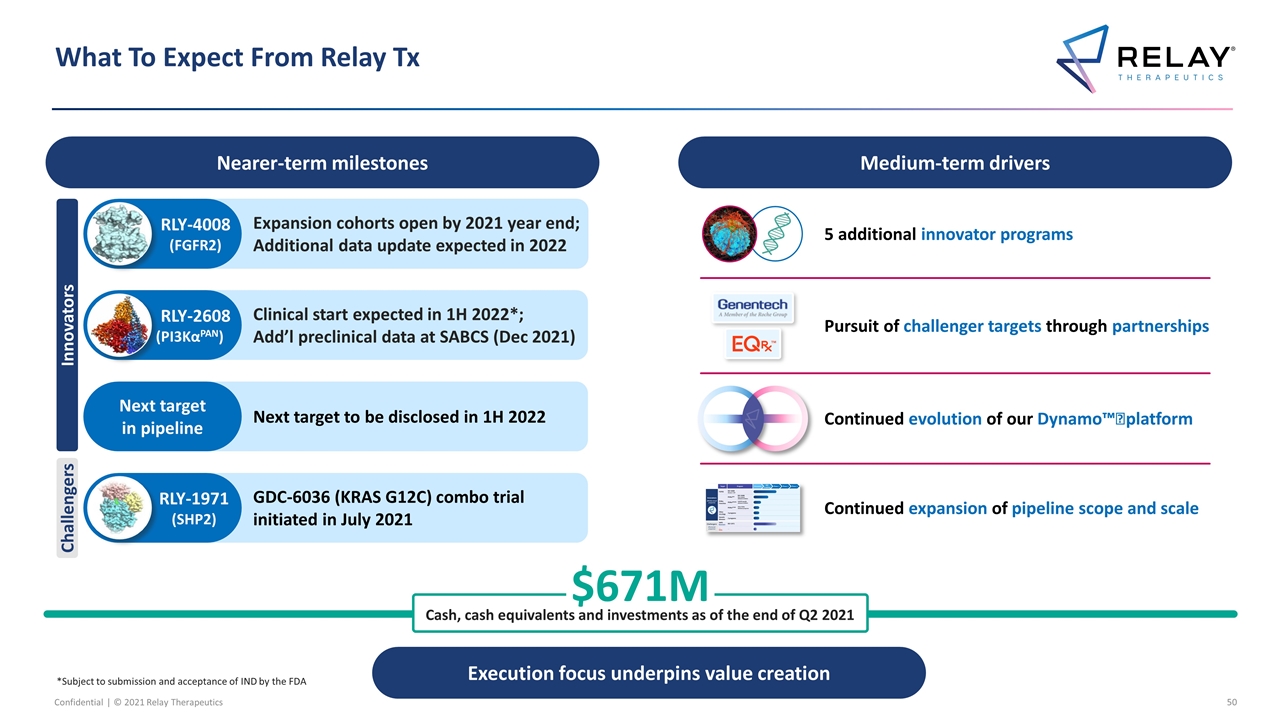
What To Expect From Relay Tx Nearer-term milestones Medium-term drivers $671M Cash, cash equivalents and investments as of the end of Q2 2021 Continued evolution of our Dynamo™️ platform 5 additional innovator programs Pursuit of challenger targets through partnerships Execution focus underpins value creation Clinical start expected in 1H 2022*; Add’l preclinical data at SABCS (Dec 2021) RLY-2608 (PI3KαPAN) Expansion cohorts open by 2021 year end; Additional data update expected in 2022 RLY-4008 (FGFR2) Next target to be disclosed in 1H 2022 Next target in pipeline GDC-6036 (KRAS G12C) combo trial initiated in July 2021 RLY-1971 (SHP2) Innovators Challengers Continued expansion of pipeline scope and scale *Subject to submission and acceptance of IND by the FDA



















































