PROSPECTUS SUMMARY
This summary highlights information contained in greater detail elsewhere in this prospectus. This summary does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including our consolidated financial statements and the related notes thereto and the information set forth under the sections titled “Risk Factors,” “Special Note Regarding Forward-Looking Statements,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in each case included in, or incorporated by reference into, this prospectus.
On November 1, 2018, Kymera Therapeutics, LLC, or Kymera LLC, a Delaware limited liability company, merged with and into Kymera Therapeutics, Inc., a Delaware corporation and the issuer of the shares of common stock offered by this prospectus, which we refer to as the Reorganization. As used in this prospectus, unless the context otherwise requires, references to “Kymera,” the “company,” “we,” “us” and “our” refer to (i) prior to the date of the Reorganization, Kymera LLC and its wholly owned, consolidated subsidiaries, or either or all of them as the context may require, and (ii) following the date of the Reorganization, Kymera Therapeutics, Inc., and its wholly owned, consolidated subsidiaries, or either or all of them as the context may require.
Company Overview
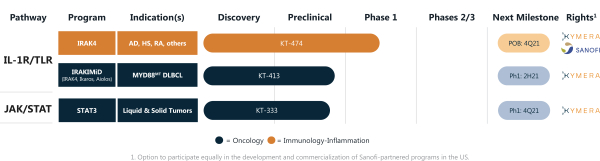
We are a biopharmaceutical company focused on discovering and developing novel small molecule therapeutics that selectively degrade disease-causing proteins by harnessing the body’s own natural protein degradation system. Our proprietary targeted protein degradation, or TPD, platform, which we refer to as Pegasus™, allows us to discover highly selective small molecule protein degraders with activity against disease-causing proteins throughout the body. We believe that our small molecule protein degraders have unique advantages over existing therapies and allow us to address a large portion of the human genome that was previously intractable with traditional modalities. We focus on biological pathways that have been clinically validated but where key biological nodes/proteins have not been drugged or inadequately drugged. To date, we have utilized our Pegasus™ platform to design novel protein degraders focused in the areas of immunology-inflammation and oncology, and continue to apply our platform’s capabilities to additional therapeutic areas. Our initial programs are IRAK4, IRAKIMiD, and STAT3, which each address high impact targets within the interleukin-1 receptor/toll-like receptor, or IL-1R/TLR, and janus kinase/signal transducers and activators of transcription, or JAK/STAT, pathways providing the opportunity to treat a broad range of immune-inflammatory diseases, hematologic malignancies, and solid tumors. Our programs exemplify our focus on addressing high impact targets that have been elusive to conventional modalities and that drive the pathogenesis of multiple serious diseases with significant unmet medical needs. With respect to our IRAK4 program, we are collaborating with Genzyme Corporation, a subsidiary of Sanofi S.A, or Sanofi, on the development of drug candidates targeting IRAK4 outside the oncology and immuno-oncology fields. We submitted an Investigational New Drug Application, or IND, to the U.S. Food and Drug Administration, or FDA, for KT-474 in 2020, and initiated the single ascending dose, or SAD, portion of our Phase 1 trial in adult healthy volunteers in February 2021. In June 2021, the FDA lifted the partial clinical hold on the multiple ascending dose, or MAD, portion of the Phase 1 trial of KT-474 following review of interim results from the SAD portion of the Phase 1 trial. As a result, in the second half of 2021, we expect to initiate enrollment in the MAD portion of the Phase 1 trial of KT-474, including healthy volunteers and a subsequent cohort of hidradenitis suppurativa, or HS, and atopic dermatitis, or AD, patients. We also expect to submit INDs for degraders KT-413 and KT-333 from our IRAKIMiD and STAT3 programs, respectively, in the second half of 2021, and if cleared, to initiate Phase 1 trials in patients thereafter.
Our initial programs are IRAK4, IRAKIMiD, and STAT3, which each focus on a single critical signaling node within the genetically and clinically validated IL-1R/TLR and JAK/STAT pathways. Our programs exemplify our focus on addressing high impact targets that have been elusive to conventional modalities and that drive the