UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_______________________________________
FORM 10-K
| | | | | |
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
or
| | | | | |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from______to______
Commission File Number: 001-39486
QUANTUM-SI INCORPORATED
(Exact name of registrant as specified in its charter)
_______________________________________
| | | | | | | | |
| Delaware | | 85-1388175 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | | | | | | |
| 29 Business Park Drive | | |
| Branford, Connecticut | | 06405 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code: (866) 688-7374
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbols(s) | | Name of each exchange on which registered |
| Class A common stock, $0.0001 per share | | QSI | | The Nasdaq Stock Market LLC |
| Redeemable warrants, each whole warrant exercisable for one share of Class A common stock, each at an exercise price of $11.50 per share | | QSIAW | | The Nasdaq Stock Market LLC |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | o | Accelerated filer | o |
| Non-accelerated filer | x | Smaller reporting company | x |
| | | Emerging growth company | o |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the registrant’s voting and non-voting equity held by non-affiliates of the registrant (without admitting that any person whose securities are not included in such calculation is an affiliate) computed by reference to the price at which the Class A common stock was last sold as of June 30, 2024, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $106.4 million.
As of February 26, 2025, the registrant had 163,202,105 shares of Class A common stock outstanding and 19,937,500 shares of Class B common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated by reference from the Registrant’s Proxy Statement for the 2025 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission.
QUANTUM-SI INCORPORATED
FORM 10-K
For the fiscal year ended December 31, 2024
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that relate to future events, our future operations or financial performance, or our plans, strategies and prospects. These statements are based on the beliefs and assumptions of our management team. Although we believe that our plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, we cannot assure that we will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or performance, are forward-looking statements. The actual results may differ from its expectations, estimates, and projections and, consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, our expectations with respect to future performance and development and commercialization of products and services. The forward-looking statements are based on projections prepared by, and are the responsibility of, management and involve significant risks and uncertainties that could cause the actual results to differ materially from those discussed in the forward-looking statements. Most of these factors are outside our control and are difficult to predict. Forward-looking statements contained in this Annual Report on Form 10-K include, but are not limited to, statements about:
•the impact of international conflicts, pandemics or epidemics on our business;
•maintaining the listing of our Class A common stock on The Nasdaq Stock Market LLC;
•changes in applicable laws or regulations;
•our ability to raise financing in the future;
•the success, cost and timing of our product development and commercialization activities;
•the commercialization and adoption of our existing products, including our Platinum® line of sequencing instruments, our consumable kits and the success of any product we may offer in the future;
•our ability to obtain and maintain regulatory approval for its products, and any related restrictions and limitations of any approved product;
•our ability to identify, in-license or acquire additional technology;
•our ability to maintain our existing lease, license, manufacture and supply agreements;
•our ability to compete with other companies currently marketing or engaged in the development or commercialization of products and services that serve customers engaged in proteomic analysis, many of which have greater financial and marketing resources than us;
•the size and growth potential of the markets for our products and services, and its ability to serve those markets once commercialized, either alone or in partnership with others;
•our estimates regarding future expenses, future revenue, capital requirements and needs for additional financing; and
•our financial performance.
These forward-looking statements are based on information available as of the date of this report, and current expectations, forecasts and assumptions, and involve a number of judgments, risks and uncertainties. Important factors could cause actual results, performance or achievements to differ materially from those indicated or implied by forward-looking statements such as those described in Part I, Item 1A, “Risk Factors” in this Annual Report on Form 10-K as filed with the Securities and Exchange Commissions (the “SEC”). The risks described under the heading “Risk Factors” are not exhaustive. New risk factors emerge from time to time, and it is not possible to predict all such risk factors, nor can we assess the impact of all such risk factors on our business or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements, which speak only as of the date hereof. All forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the foregoing cautionary statements. We undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
SUMMARY OF RISK FACTORS
We are providing the following summary of the risk factors contained in this Annual Report on Form 10-K to enhance the readability and accessibility of our risk factor disclosures. We encourage you to carefully review the full risk factors contained in this Annual Report on Form 10-K in their entirety for additional information regarding the material factors that make an investment in our securities speculative or risky. References in the summary below to “we”, “us”, “our” the “Company” and “Quantum-Si” refer to Quantum-Si Incorporated and its subsidiaries.
These risks and uncertainties include, but are not limited to, the following:
Risks Related to Our Financial Condition and Capital Requirements
•We are an early-stage life sciences technology company with a history of net losses and negative cash flow, which we expect to continue, and we may not be able to generate meaningful revenues or achieve and sustain profitability or positive cash flow in the future.
•We have a limited operating history, which may make it difficult to evaluate the prospects for our future viability and predict our future performance. As such, you cannot rely upon our historical operating performance to make an investment or voting decision regarding us.
•We may need to raise additional capital to fund ongoing research and development, operating activities, and commercialization activities.
Risks Related to Our Business and Industry
•We may not gain commercial traction for our current products and we may not be able to successfully commercially launch other future products.
•If we are unable to establish superior sales and marketing capabilities, we may not be successful in commercializing our products.
•The size of the markets for our products may be smaller than estimated, and new market opportunities may not develop as quickly as we expect, or at all, limiting our ability to successfully sell our products.
•Unfavorable global economic conditions, retaliatory economic policies, or other geopolitical conditions associated with intra-country economics and policies could adversely affect our business, financial condition or results of operations.
•If we do not sustain or successfully manage our anticipated growth, our business and prospects will be harmed.
•Recently and in the past, we have undergone leadership transitions and an internal restructuring, and we depend on our key personnel and other highly qualified personnel, and if we are unable to recruit, train and retain our personnel in the future, we may not achieve our goals.
•Our business will depend significantly on research and development spending by academic institutions and other research institutions, and any reduction in spending, driven by these customers or other third party funding sources such as the National Institutes of Health, could limit demand for our products and adversely affect our business, results of operations, financial condition and prospects.
•We rely on certain contract manufacturers to manufacture and supply our instruments, components of our instruments, and certain components of our consumable offerings. If these manufacturers should fail or not perform satisfactorily, our ability to commercialize and supply our instruments and consumable offerings would be adversely affected.
•Our internal manufacturing equipment is specialized with limited vendor options and long lead times. If these pieces of equipment were to stop working and be unable to be repaired in a timely manner or at all, our ability to manufacturer our semiconductor chips would be adversely affected.
•A portion of our revenue is generated through a number of key channel partners, and the loss of any such channel partner could adversely impact our business and our results of operations could suffer.
•If we do not successfully develop and maintain our Platinum Analysis Software service, our commercialization efforts and therefore business and results of operations could suffer.
•Commercializing our products outside of the United States could expose us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
•We have limited experience producing and supplying our products, and we may be unable to consistently manufacture or source our instruments and consumables to the necessary specifications or in quantities necessary to meet demand on a timely basis and at acceptable performance and cost levels.
•We rely on third-party foundries to produce silicon wafers, which when packaged and tested internally, lead to our supply of semiconductor chips. If these third-party foundries should fail or not perform satisfactorily, our ability to supply semiconductor chips would be negatively and adversely affected.
•The life sciences technology market is highly competitive. If we fail to compete effectively, our business and results of operations will suffer.
•We may acquire other companies or technologies which could divert our management’s attention, result in additional dilution to our stockholders and otherwise disrupt our operations and harm our operating results.
•If our facilities or our third-party manufacturers’ facilities become unavailable or inoperable, our research and development program and commercialization launch plan could be adversely impacted and manufacturing of our instruments and consumables could be interrupted.
•If we experience a significant disruption in our information technology systems, including our Platinum Analysis Software services, or cybersecurity incidents, our business could be adversely affected.
Risks Related to Government Regulation
•Our research use only (“RUO”) products could become subject to government regulation as medical devices by the U.S. Food and Drug Administration (“FDA”) and other regulatory agencies even if we do not elect to seek regulatory authorization to market our products for diagnostic purposes, which would adversely impact our ability to market and sell our products and harm our business.
•Our reagents may be used by clinical laboratories to create Laboratory-Developed Tests (“LDTs”), which could, in the future, become subject to some form of FDA or other regulatory requirements, which could materially and adversely affect our business and results of operations.
•We may be subject to certain federal, state and foreign fraud and abuse laws, health information privacy and security laws and physician payment transparency laws, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
•We are currently subject to, and may in the future become subject to, both U.S. federal and state laws and regulations as well as international laws imposing obligations on how we collect, store and process personal information. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our business and future customer base, and thereby decrease our revenue.
Risks Related to Our Intellectual Property
•If we are unable to obtain and maintain and enforce sufficient intellectual property protection for our products and technology, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products may be impaired.
•If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, and our business could be harmed.
•Patent terms may be inadequate to protect our competitive position on our products for an adequate amount of time.
•We may become involved in lawsuits to defend against third-party claims of infringement, misappropriation or other violations of intellectual property or to protect or enforce our intellectual property, any of which could be expensive, time consuming and unsuccessful, and may prevent or delay our development and commercialization efforts.
Risks Related to Our Securities and to Being a Public Company
•Our outstanding warrants became exercisable for our Class A common stock in September 2021, which increased the number of shares eligible for future resale in the public market and could result in dilution to our stockholders if exercised.
•We have in the past experienced material weaknesses in our internal control over financial reporting, and if we experience such material weaknesses in our internal control over financial reporting in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to report our financial condition, results of operations or cash flows accurately or in a timely manner, which may adversely affect investor confidence in us and, as a result, materially and adversely affect our business and the value of our Class A common stock.
•Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
•Because we are a “controlled company” within the meaning of the Nasdaq rules, our stockholders may not have certain corporate governance protections that are available to stockholders of companies that are not controlled companies.
•We could fail to maintain the listing of our Class A common stock on the Nasdaq Stock Market LLC, which could seriously harm the liquidity of our shares and our ability to raise capital or complete a strategic transaction.
•The dual class structure of our common stock has the effect of concentrating voting power with our Founder, who is also on our Board of Directors, which will limit an investor’s ability to influence the outcome of important transactions, including a change in control.
PART I
ITEM 1. BUSINESS
Overview
Quantum-Si Incorporated (including its subsidiaries, the “Company” or “Quantum-Si”) was incorporated in Delaware on June 10, 2020 as HighCape Capital Acquisition Corp. (“HighCape”). The Company’s legal name became Quantum-Si Incorporated following a business combination on June 10, 2021 between the Company and Q-SI Operations Inc. (formerly Quantum-Si Incorporated) (the “Business Combination”), which was founded in 2013.
We are a life sciences company focused on the development and commercialization of proteomics research tools, with the mission to bring single-molecule proteomics analysis to every lab, everywhere. We have developed a proprietary universal single-molecule detection platform that we are initially applying to proteomics to enable Next-Generation Protein SequencingTM (“NGPS”). Traditionally, proteomic workflows to sequence proteins have several limitations including low throughput (only one protein at a time), requires the use of hazardous chemicals, is time consuming and labor intensive requiring days or weeks of a scientist’s time to complete. NGPS delivers single amino acid level resolution, using a low cost, low maintenance instrument and includes a suite of automated data analysis tools so that any lab, with modest scientific expertise, can easily implement protein sequencing into their workflows. Our products include instrumentation (Platinum and Platinum Pro), consumable kits for library preparation and sequencing for use with our instruments and a suite of automated data analysis tools. We began a controlled launch of the Platinum instrument and started to take orders in December 2022, and subsequently began shipping Platinum in January 2023. We moved to a full commercial launch of Platinum beginning in the second quarter of 2024. In January of 2025, we announced the launch of Platinum Pro with first shipments expected to occur by the end of the first quarter of 2025.
In November 2024, we presented a technology and product roadmap that we believe positions us to be a leader in proteomics. The roadmap includes continued updates and enhancements to our current platform including instrumentation, consumable kits and software tools, and a next generation platform called Proteus. This new Proteus platform is expected to significantly scale the sequencing output per sample, sample throughput per run and increase workflow automation. In addition, during our presentation in November 2024, we provided data demonstrating the wide range of proteomics applications that are addressable with our proprietary single molecule, kinetic detection technology. We intend to execute on this roadmap through a combination of internal development programs and external partnerships to ultimately bring to market the most comprehensive proteomics platform in our industry.
We believe that our core technology can address the broadest range of applications in the rapidly evolving proteomics tools market. Our current and future platforms aim to address many of the key challenges and bottlenecks with legacy proteomic solutions, such as mass spectrometry (“MS”), which include high instrument costs both in terms of acquisition and ownership, and complexity with data analysis, which together limit broad adoption. We believe our platform, which offers single molecule, amino acid level resolution at a lower instrument cost and with greater automation than legacy proteomic solutions, could allow our products to have wide utility across the study of the proteome. For example, our platform could be used for biomarker discovery and disease detection, pathway analysis, immune response, vaccine development, quality assurance and quality control, among other applications.
According to 2021 SVB Leerink research report, proteomics represents a $75 billion market opportunity spanning from life science research through diagnostics. Within this, proteomics research represented a $20 billion opportunity, while proteomic diagnostics and personalized medicine represented the remaining $55 billion. Within the $20 billion current addressable research market, we are focusing on an initial target market of approximately $8 billion that includes protein identification (approximately $3 billion), protein expression and quantification (approximately $3 billion) and proteoforms and post translational modifications (approximately $1.5 billion).
Our team has decades of cumulative experience in developing, commercializing and scaling tools in the life sciences industry. Our management team has previously employed similar approaches at other companies to launch other disruptive technologies, including market leading next generation DNA sequencing technologies. We believe this experience will allow us to introduce our platform in a structured manner to demonstrate its use, value and practicality, while working directly with our customers, to help ensure a positive experience.
We began a controlled launch of Platinum in December 2022, operated under this controlled commercial launch in 2023 and through the first quarter of 2024, at which point we began a full commercial launch at the beginning of the second
quarter of 2024. Revenue for the years ended December 31, 2024 and 2023 was $3.1 million and $1.1 million, respectively, and there was no revenue for the year ended December 31, 2022. We incurred net losses of $101.0 million, $96.0 million and $132.4 million for the years ended December 31, 2024, 2023 and 2022, respectively.
Importance of Proteomics
The human proteome is diverse, complex and dynamic, with multiple protein variants derived from each gene due to multiple biological steps required to generate the functional proteome, including transcription, translation and post-translational modifications (“PTMs”). While our genomes contain approximately 20,000 genes, current estimates are that these genes ultimately code for more than 1,000,000 different protein variants called proteoforms. Thus, most of the diversity that exists in our cells comes from proteins, which are organic compounds made up of amino acids. Aside from water, the majority of the molecules in our bodies are comprised of proteins, which play a central role in the body’s biological processes, from the immune system response, signaling pathways to transporting oxygen molecules and providing our cells with structure. Proteins or a group of interacting proteins are responsible for virtually every biological function within a living organism. Unlike the genome, the proteome is in constant flux depending on the state of the cell. However, even with the knowledge of the proteome’s influence, the proteome remains largely unexplored relative to the genome. Over the past decades, genomics has ushered in a greater understanding of human biology and disease through the decoding of the human genome, providing a greater understanding of the genes that lay out the instructions for the function, development and reproduction of organisms. While genomics has allowed the interrogation of genetic variation, protein variants hold information yet to be explored or connected to the network of genomic knowledge to better understand cellular function and disease. The protein’s elaborate structure, complicated composition, and vast number of variants, provide a dynamic look into the functions they provide. For example, proteins function as antibodies that bind to specific particles like viruses to protect the body; they act as enzymes to carry out chemical reactions in cells; they act as messengers like hormones to transmit signals; they exist as structural components; and form the basis for storage to carry additional molecules throughout the body.
Proteomic discovery provides insight into what is immediately happening biologically. This insight may be based on both genetic and environmental factors that influence protein structure and function. Given their dynamic nature, proteins, while complex structures, are an excellent indicator that we believe can be used to track therapeutic response, disease progression and a person’s overall health. In a sense, DNA tells us “what could happen” and proteins tell us “what is happening.”
While our products are limited to RUO applications, we note that proteomics tools have been broadly used across a wide range of applications, including:
•Systems biology: system-wide investigations of disease pathways to identify biomarkers, drug action, toxicity, efficacy and resistance;
•Drug discovery and development: identification of drug candidates, novel drug delivery systems, and aid in drug development;
•Biomarker discovery: identification of protein markers for disease identification and management;
•Personalized medicine: tailoring of disease treatment based real-time proteomic data;
•Industry / agriculture: bioproduction and study of plant-pathogen interaction (e.g. crop engineering for drought resistance); and
•Food science: identification of allergies, understanding an improvement of nutritional values and food quality and safety control.
Limitations of Legacy Proteomic Technologies
There is higher diversity and level of complexity related to proteins than genes. Depending on the combination of genes, specific proteins are built to perform specialized functions in the body. A single gene can encode multiple proteoforms depending on the role the protein will ultimately play in the cell. Protein synthesis happens in two stages. First is transcription, where DNA is converted into messenger RNA. Second is translation, where a cell’s ribosomes read the RNA instructions to assemble the protein. An increase in the complexity of the proteome is facilitated by post translational modifications (“PTMs”) where pieces of the protein are modified to either activate or inactivate the protein as part of a
signaling pathway to localize the protein to a certain cellular compartment. Legacy proteomic techniques can be grouped into three general categories: Mass Spectrometry, Affinity-based methods and Sequencing via Edman Degradation.
•Mass Spectrometry. MS is a method for the mass determination and characterization of proteins and for more than a decade, has been the dominant tool for unbiased protein analysis. MS workflows allow for the interrogation of individual peptides and protein sequences; however, these techniques are generally complex, lengthy, utilize expensive equipment, and require extensive data analysis by specially trained staff. MS instruments can cost $1,000,000 or more per new instrument and given the technical staff required for performing the process and analyzing the resulting data, the use of MS is often constrained to large, centralized core laboratories. In addition, current sensitivity and dynamic range restrictions of MS also make it difficult to use with liquid samples and restrict the ability to analyze at single molecule resolution or the ability to deeply integrate a protein. Taken together, these factors limit the broad scale adoption of MS in the market.
•Affinity-Based Methods. Affinity-based methods are effective when specific protein(s) or epitopes of interest are known. Affinity-based methods use a variety of molecules, such as antibodies or aptamers, which bind to specific regions, rather than individual amino acids, and therefore may not detect the presence of a protein variant. For instance, the average binding site of an affinity reagent is an epitope with a length of 5 to 8 amino acids, whereas the average length of a human protein is approximately 470 amino acids. Changes or modifications to the protein may prevent the affinity reagent from binding, resulting in missed identification or false negative results. In addition, affinity reagents do not recognize differences in protein structure outside of the targeted binding site making them ineffective at differentiating protein variants. These fundamental challenges limit the ability of affinity-based methods to accurately survey the full complexity of the proteome. Furthermore, affinity-based methods when applied to highly multiplexed analysis of proteins, requires the use of expensive DNA sequencing instrumentation and reagents to resolve barcodes necessary to complete the analysis process. This additional step and instrumentation requirement often limits the use of these high-plex methods to large, core laboratories that have the required infrastructure and data analysis capabilities in their laboratories.
•Sequencing via Edman Degradation. Edman degradation is a method for sequencing of single amino acids from a peptide by alternating acidic and alkaline conditions to cleave the N terminal amino acid from a peptide with the resulting cleaved amino acid being detected using chromatography or electrophoresis. Edman degradation requires the use of hazardous chemicals, is labor and time intensive, and is subject to failures due to naturally occurring chemical modifications of amino acids such as acetylation. While Edman Degradation offers single amino acid level resolution, the technical challenges and limitations of the procedure limit its usefulness outside of a select number of research laboratories.
Our Market Opportunity
Proteomics represents a large and growing market opportunity. According to a 2021 SVB Leerink research report, proteomics represented a $75 billion market opportunity spanning from life science research through diagnostics. Within this, the proteomics research market represents a $20 billion addressable market, and we are focused on an initial target market of approximately $8 billion that includes protein identification (approximately $3 billion), protein expression and quantification (approximately $3 billion) and proteoforms and post translational modifications (approximately $1.5 billion).
Proteomics Landscape
Given the complexity of analyzing the proteome, the market today, is highly fragmented with a mix of legacy and new technologies that address specific requirements of proteomic researchers with no single solution addressing the full range of requirements. When evaluating and purchasing new proteomics research instrumentation, customers must make tradeoffs across a range of product attributes and capabilities including the breadth of coverage (proteins) versus depth of resolution (amino acid, PTM), sample throughput versus instrument costs, biased (affinity-based methods) versus unbiased (protein sequencing, mass spectrometry) amongst others. In addition, many of the legacy technologies also involve a complex, manual workflow and specialized staff to perform data analysis. The combination of these factors means that proteomics research tool adoption remains constrained to specialty facilities, often called core laboratories. For researchers outside of these core laboratories, they must send out their proteomics analysis work increasing the cost and extending the time to complete their research studies. Additionally, in many countries outside of the United States or Western Europe, there are few to no core laboratories to send work to, so the availability of advanced proteomics tools is extremely limited.
Today, our protein sequencing platforms, Platinum and Platinum Pro, address these market challenges in two distinct ways. First, our single molecule, amino acid level resolution capability allows researchers to deeply characterize proteins including protein isoforms, variants and PTMs, types of analysis that are either very difficult to perform or not feasible at all using affinity-based methods or MS. This makes Platinum and Platinum Pro a valuable complement to current technologies used in proteomics core laboratories. Second, our instrument has a low capital cost and includes automated data analysis, easing adoption in both core laboratories and smaller research laboratories looking to insource their proteomics work saving time and budget compared to sending samples to core laboratories. For countries with limited or no core laboratory infrastructure, our platform in some cases, represents the only advanced proteomics instrument local researchers can implement.
Looking to the future, we believe that our Proteus instrument and core technology will be able to address a broad range of proteomics analysis methods, thereby limiting the need for a laboratory to own multiple, specialized platforms. Proteus will provide single molecule, amino acid level resolution as we do with Platinum and Platinum Pro, while also offer significantly higher sequencing output per sample, increased sample throughout per run, automation of the sequencing workflow and automated data analysis. We believe that the Proteus platform will enable any lab to perform a broad range of proteomics analysis methods on a single instrument.
Our protein sequencing platforms are currently intended for research use only or “RUO”. In the future, it is possible that our products may be used for clinical purposes. If our products are used for clinical purposes, they may require regulatory authorization.
Our Products
Our products include instruments, consumables and software used together as a platform for protein analysis. Our customers use commercially available products to prepare their proteins which is the starting material for the protein sequencing process as depicted below.
Overview of the Protein Sequencing Process
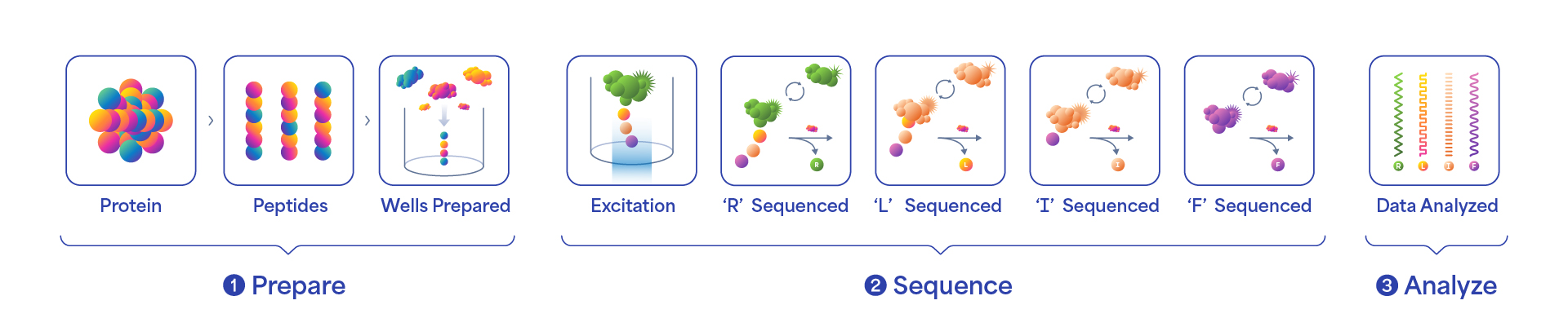
The protein sequencing process starts with a step called library prep. The first step in library prep is to digest the proteins into smaller fragments called peptides. In the second part of the library prep process, a linker is attached to the end of the peptide. This linker is designed to attach to the bottom of the reaction well in our sequencing consumable. Once the library prep process is complete, the user then sets up the sequencing step. In this step, the prepared library is combined with sequencing reagents and introduced into a chamber in our sequencing consumable. The sequencing consumable contains millions of features, each capable of performing a single molecule sequencing reaction. The sequencing reagents contain amino acid recognizers, enzymes that remove terminal amino acids and other buffers. A single recognizer is capable of uniquely identifying more than one amino acid. Our technology is designed to accurately determine the recognizer by its unique kinetic signature. After removing the terminal amino acid, the recognition process repeats until the full peptide chain is sequenced. While traditional single-molecule platforms rely on a single measurement for the detection of an event, the advantage of our approach is that our technology can obtain tens to hundreds of data points for each amino acid. Cumulatively, we expect the multiple measurements to deliver high amino acid call accuracy. We believe we are the first company to have successfully commercialized a NGPS product.
Common biological questions that researchers use NGPS to answer include the following:
•What protein is present? Amino acid resolution can provide insight into more than just whether a protein is present or absent. The sequence information could also indicate what version of the protein is present and how it has been changed from the normal version.
•How much of the protein is present? Relative quantification provides information about protein abundance relative to other proteins or protein variants present in the sample.
•How has the protein been modified? Single-molecule sensitivity could show how the protein has been post-translationally modified thus providing greater insights to its role in the context of biological processes within the cell.
Our Current Product Offerings Consists of the Platinum and Platinum Pro Instruments, Library Preparation and Barcoding Kits, Sequencing Kit and Platinum Analysis Software
Instruments
Platinum - NGPS Instrument
We believe Platinum was the first to market the NGPS instrument. While traditional instruments like MS can cost up to $1,000,000 or more per new instrument, our Platinum device is currently priced at approximately $85,000. Platinum is designed to provide single molecule, amino acid level resolution with a streamlined workflow, including automated data analysis, making it accessible to researchers in all laboratory types.
Platinum Pro - NGPS Instrument
In January of 2025, we announced the launch of Platinum Pro with first shipments expected to occur by the end of the first quarter of 2025. Platinum Pro will provide the same technology capabilities of Platinum (single molecule, amino acid resolution) and includes an enhanced user interface, cloud or on instrument data analysis and an available Pro Mode, an option for customers who want to build custom analysis methods utilizing the power of our single molecule, kinetic detection technology. Our Platinum Pro device is currently priced at approximately $120,000.
Consumables
We expect to derive recurring revenue from the sale of consumables that are required to run samples on the Platinum and Platinum Pro instruments or future generations of sequencing platforms that we may launch. Current consumable kits consist of library preparation kits and sequencing kits. These kits are designed for use only with our instruments.
Library Preparation
Our library preparation kit is designed to prepare a customer’s protein sample for sequencing. This kit includes the reagents required to digest the protein(s) into peptides and attach a linker that allows the peptide to bind to the bottom of the reaction features on our sequencing consumable.
Barcoding Kit
Our barcoding kit is a type of library preparation kit, that is specifically designed to optimize the workflow and performance of our technology when applied to protein barcoding applications. This kit includes the reagents required to prepare the barcodes for sequencing and the associated protocol provides information about barcode sequence design and performance.
Sequencing Kit
Our sequencing kits contain the reagents and consumables used to perform NGPS on the Platinum or Platinum Pro instrument. In the currently marketed kit, the consumable is a semiconductor chip with two million features.
Platinum Analysis Software
Our Platinum Analysis Software is a cloud-based solution that automates data analysis workflows and provides a user-friendly interface and visualization of the sequencing results. Our software suite includes tools to map peptides and visualize the amino acid coverage, align peptides to proteins, including a protein inference workflow and a variant caller workflow that is specifically designed to aid users in the identification and relative quantification of variants including single amino acid changes or PTMs.
Going forward, with the launch of our Platinum Pro instrument, we plan to offer a combination of cloud-based and on-instrument software and data analysis tools to address situations where customers have limited or no ability to access to the cloud environment.
Our Competitive Strengths
We believe that our competitive strengths include the following:
•Differentiated technology with broad applicability across a range of proteomics analysis methods. At the core, our technology provides single molecule, kinetic detection of individual amino acids. By enabling single molecule detection, we are not reliant on ensemble measurements, which can often vary from sample to sample and even run to run. Amino acid level resolution allows researchers to deeply characterize proteins including protein isoforms, variants and PTM’s, all types of analysis that are either very difficult to perform or not feasible at all using affinity-based methods or MS. Finally, our ability to detect the kinetic signatures of amino acid recognizers can be extended to other detection molecules including engineered proteins, nanobodies or antibodies. Looking to the future, we believe that our core technology will be able to address a broad range of proteomics analysis methods, thereby limiting the need for a laboratory to own multiple, specialized platforms.
•Platinum Analysis Software provides automated, user-friendly data analysis and visualization workflows. Our Platinum Analysis Software is a suite of tools that can map peptides and visualize the amino acid coverage, align peptides to proteins, including a protein inference workflow and a variant caller workflow that is specifically designed to aid users in the identification and relative quantification of variants including single amino acid changes or PTMs. These automated tools allow researchers, regardless of their laboratory infrastructure or staff specialization, to perform NGPS.
•Business model that leverages growing installed base of instruments each utilizing an increasing number of consumables over time. As part of our commercialization efforts, we aim to grow our installed base of instruments globally while in parallel, expanding the range of applications that customers can perform with our technology through continued launch of new instruments, consumable kits and software workflows. From this process and the increase of the installed base over time, we expect to grow a substantial base of recurring revenues from our customers purchasing a greater number of consumables per instrument over time.
•Platform to enable expanded access to proteomics tools. Our instrument has a low capital cost and includes automated data analysis, easing adoption in both core laboratories and smaller research laboratories looking to insource their proteomics work saving time and budget compared to sending samples to core laboratories. For countries with limited core laboratory infrastructure, our platform in some cases represents the only advanced proteomics instrument local researchers can implement.
•Robust patent protection. We have a strong intellectual property strategy in which we have 396 issued patents and 598 pending applications as of December 31, 2024. In addition, we believe many of our pending and issued patents include foundational technology in the proteomics field.
•Experienced Life Science Management team and Board of Directors with significant experience in healthcare. We have a world-class management team and Board of Directors, including our chairman, executive officers, and other senior management, with decades of cumulative experience in the healthcare and life sciences end-markets. We believe this leadership team positions us as a potentially disruptive force in creating a new market of next generation protein sequencing.
Our Strategies
Our strategies include the following:
•First to market using a phased approach to broad commercialization and adoption. We began a controlled launch of the Platinum instrument and started to take orders in December 2022, and subsequently began commercial shipments of Platinum in January 2023, moving to a full commercial launch at the beginning of second quarter 2024. Members of our team have previously utilized a similar phased launch approach to successfully launch and drive long term adoption of other disruptive technologies. We believe this approach allow us to introduce our platform in a structured manner to demonstrate its use and practicality, while working directly with our key potential customers and industry thought leaders to help ensure a positive experience. Our leadership team has decades of cumulative experience working directly in the life sciences industry with many of the companies and research centers that have the potential to become customers.
•Build our commercial infrastructure globally. We are continuing to build our commercial and operational infrastructure to sell and support our platform as we gain traction throughout the world. Presently, we have a direct sales force in the United States, with a combined direct and distributor approach in Europe, and distributor relationships in certain key markets in the rest of the world. Further, in November 2024 we announced a North American distribution agreement with Avantor to distribute the Platinum Pro and related consumables. We formally began accepting orders through Avantor in the first quarter of 2025 and anticipating ramping activity through this relationship throughout 2025.
•Invest in scientific affairs and market development activities to drive evidence generation and increase the awareness of the importance NGPS. We believe that our platform has the capability to enable users to generate a depth of proteomic information that until our launch, was not available. We believe the utility of our platform spans basic research, drug discovery and development, translational research and quality control testing across multiple market segments including academic research, biopharma, contract development and manufacturing organization (“CDMOs”), government and industrial. We plan to invest in scientific affairs and market development activities and partnerships to generate the scientific evidence of the importance and utility of NGPS and to expand the awareness and demand for our products.
•Continued technical innovation to drive product enhancements, new products, and additional applications. Our leadership team has deep expertise in technology development and commercialization in the life sciences and diagnostics markets. Since the launch of our Platinum instrument, we have delivered a steady cadence of new products including sequencing kits, library prep kits, a barcoding kit and software workflows including protein inference and variant caller. In January of 2025, we announced the launch of Platinum Pro with first shipments expected to occur by the end of the first quarter of 2025. We aim to continually innovate and deliver new products, product enhancements, applications, workflows, and other tools to enable our customers to leverage the power of NGPS at scale. See “Product Roadmap” below for further information.
•Lead with accessibility. Our mission is to bring NGPS to every lab, everywhere. Our instrument has a low capital cost and includes automated data analysis, easing adoption in both core laboratories and smaller research laboratories looking to insource their proteomics work saving time and budget compared to sending samples to core laboratories. For countries with limited core laboratory infrastructure, our platform in some cases represents the only advanced proteomics instrument local researchers can implement. As we develop new platforms, we aim to expand the capability of our core technology and further increase the level of workflow automation while continuing to offer our platform at a price point that is advantageous to our customers compared to the capabilities and cost of many legacy proteomics technologies. Our ability to develop our platforms in such a way may allow proteomic analysis to reach new markets and new users, potentially enabling and accelerating innovative discoveries.
•Build an ecosystem that delivers platform consolidation and a streamlined customer experience. Given the complexity of analyzing the proteome, customers often own and must manage many specialized instruments each addressing a subset of their research needs. Many of the associated laboratory workflows are also highly manual and require specialized facilities and staff. We believe that our core technology can address the broadest range of proteomics analysis methods in the market and through partnerships and internal development programs (Proteus), we will be able to simplify the customer experience through platform consolidation and greater workflow automation.
•Maintain a strong intellectual property portfolio for existing and new technologies. We have a broad and deep patent protection strategy, which includes 396 issued patents and 598 pending applications as of December 31, 2024, including certain foundational IP around proteomics. Protection of our intellectual property is a strategic priority for the business; we have taken, and will continue to take, steps to protect our current and future intellectual property and proprietary technology. We believe our broad patent portfolio and continued rigorous patent protection strategy will help to allow us to focus on our key priorities of commercializing our platform, continuing to innovate with new technologies, and preventing fast-followers.
Commercial Strategy
As we continue to commercialize our platforms, we plan to build out our commercial infrastructure to sell and support our products, across a growing number of market segments and geographies. Presently, we have a direct sales force in the United States, with a combined direct and distributor approach in Europe, and distributor relationships in certain key
markets in the rest of the world. In November 2024, we announced a North American distribution agreement with Avantor to distribute the Platinum Pro platform and related consumables.
Our leadership team has decades of experience bring new technologies to the market and working directly with many of the companies and research centers that have the potential to become customers. Our commercial strategy includes the following areas of focus.
1.Continue to build our direct sales and support infrastructure in the U.S. and Western Europe. We plan to continue to build our commercial and operational infrastructure to sell and support our platform as we seek to grow adoption in these regions. In addition to our direct investments, we will continue to evaluate opportunities to partner with leading companies to augment our commercial footprint while minimizing the level of direct investment required.
2.Commercial partnerships. In November 2024, we announced a North American distribution agreement with Avantor to distribute the Platinum Pro and related consumables. Avantor is a leading distributor of life sciences products with established relationships in our target market segments. We formally began accepting orders through Avantor in the first quarter of 2025.
3.Geographic partnerships. We exited 2024 with 18 international distribution partners spanning many countries across Europe, Middle East, Africa, Asia Pacific and South America. Each of our international partners brings a depth of local expertise in the fields of genomics and proteomics and have experience bringing new technologies to market. We expect to continue to expand this international network in 2025.
4.Technical and scientific support. We believe that a key to successfully commercializing a new technology like NGPS, is providing high quality technical and scientific support to customers. We use a mix of field based and in house scientific staff to support our customers through all phases of the commercial process from pre-sales, to training and onboarding and through post-sales support. We will continue to invest in this area in both our direct markets and regionally to support our geographic partners as appropriate.
5.Key opinion leaders (“KOL”) and evidence generation. We believe that our platform has the capability to enable users to generate a depth of proteomic information that until our launch, was not available. We work closely with KOLs in the development and commercialization of our products including supporting research studies that lead to the presentation and/or publishing of data using our technology. We plan to continue to invest in research studies and scientific collaborations to generate scientific evidence that supports the importance and utility of NGPS in proteomics research.
6.Technology access program. We believe that our core technology has broad utility across the field of proteomics research. While our internal R&D focus is on the development and commercialization of NGPS, we believe there would be interests from potential customers and strategic partners, to leverage our single molecule, kinetic detection capabilities for other proteomics applications. As part of the launch of Platinum Pro, we are offering Pro Mode, which enables a customer to access our technology for custom application development. To support that work, we initiated a Technology Access Program that allows customers to engage directly with our R&D scientists to explore custom applications of our core technology in support of their on-going research efforts.
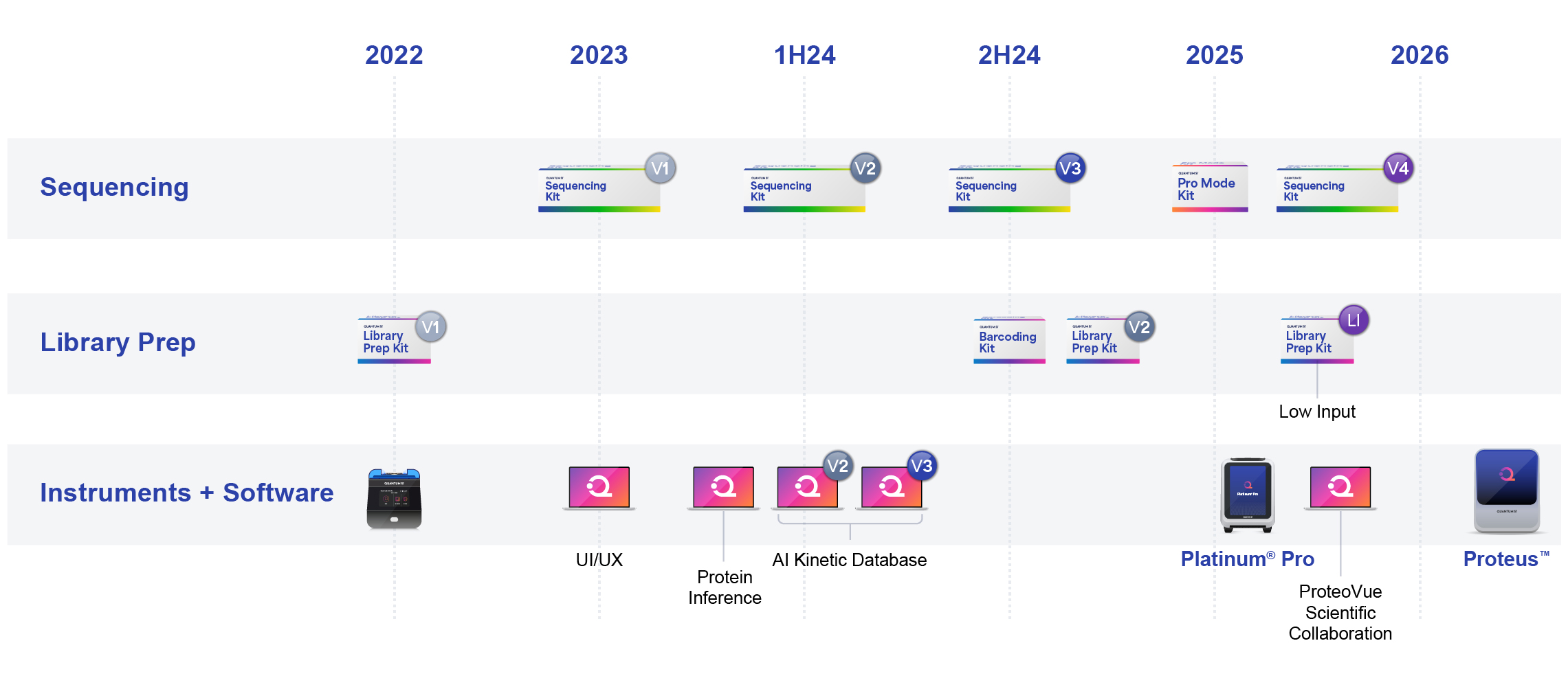
Product Roadmap
We believe that our product and technology roadmap and internal development processes position us as a leader in the proteomics market. Since the launch of Platinum in December 2022, we have executed on numerous product launches across instruments, library prep kits, sequencing kits and software tools. This steady cadence of technology advancements ensures our customers have a continuous flow of new capabilities that enable them to pursue their research interests to the fullest. The following is our actual product launches through February 2025, and estimated timing of product launches after February 2025:
At an investor and analyst day on November 20, 2024, we laid out a long-term product and technology roadmap that we believe is capable of delivering a platform (Proteus) and core technology that can address the broadest range of proteomics applications in the market today. The roadmap includes innovations across all areas of our technology.
1.Sequencing Instrumentation and Consumables. In January 2025, we launched Platinum Pro, a new generation of instrumentation that utilizes our current technology architecture, namely a semiconductor chip. Looking to the future, we are developing a new platform called Proteus, which introduces a new instrument and consumable architecture that is being designed to scale the sequencing output per sample from two million features per consumable today, to billions of features per consumable in the future. The first generation of Proteus and associated sequencing consumables, which is estimated to be ready for launch in the second half of 2026, is being designed to have 80 million features per consumable and 160 million features per instrument run when using two consumables.
2.Library Preparation. In December 2024, we launched a version 2 of our library preparation kit which lowered the input quantity of protein required and increased the sample success rate across a broad range of proteins tested. In addition, we also launched a barcoding kit which is designed specifically for the efficient preparation of protein barcodes for NGPS. We expect to continue to innovate in library preparation with key areas of focus being sample input concentration and complex sample preparation amongst other parameters or features.
3.Sequencing Chemistry. Our current sequencing kit recognizes 13 of the 20 known, naturally occurring, amino acids accounting for up to 69% of amino acids in the proteome. We are continuing to evolve our sequencing chemistry with key areas of focus being amino acid coverage, sequence speed and depth, amongst other parameters. We believe that recognition of all 20 amino acids is feasible over time.
4.Software. Our core technology is single molecule, kinetic detection at the amino acid level. Each of our amino acid recognizers exhibits a unique kinetic signature. Utilizing artificial intelligence, we can continuously train our models and improve the accuracy of the database used to interpret these kinetic signatures, resulting in higher data output and accuracy. We expect to continue to evolve this aspect of our software in addition to continuing to evolve our analysis workflows, data visualizers and other software tools that add value to our customers.
Platinum Pro offers a consumable chip with 2 million features. Proteus 1.0 is anticipated to launch with the capability of accepting two consumable chips, each with 80 million features, creating a total feature range from 80-160 million. The Proteus 2.0 feature set of 10 billion features is estimated based on long-term development initiatives.
Suppliers and Manufacturing
Our products are built using both custom-made and off-the-shelf components supplied by outside manufacturers and vendors located in Asia, Europe, and the United States. These products and product components include our custom-made disposable semiconductor chip, our proprietary mode-locked laser as well as proprietary enzymes, recognizers and buffers used for protein sequencing. The majority of other components for our platform are off-the-shelf.
We purchase some of our components and materials used in manufacturing, including the underlying wafers for our semiconductor chip as well as other critical manufacturing steps, from single source suppliers. We believe that alternatives would be available; however, it will likely take a significant amount of time to identify and validate replacement components, which could negatively affect our ability to supply our products on a timely basis. To mitigate this future risk, we and our third-party contractors attempt to carry a significant inventory of our critical components. However, any transaction disruptions in these suppliers and potential associated ramp up time of a new supplier would result in a supply disruption that could impact our business. In addition, any strategic decision to slow, pause, or stop the manufacturing of any core supply based on assumptions of adequate safety stock that subsequently proves to be an incorrect estimate of supply needs could result in a supply disruption due to the inability to ramp up or ramp down processes.
Our instruments are developed and designed by us but have historically been manufactured by a third-party manufacturing partner. Overall, we believe that our manufacturing strategy is efficient and conserves capital. However, we do not have long-term supply or manufacturing commitments from all our suppliers or manufacturers, and some of our products and components are currently supplied on a purchase order basis. In addition, we will need to increase the supply and manufacturing of our products as we continue to grow. If we are unable to maintain manufacturing at our contract manufacturing partners, it will affect our ability to produce instruments which would harm our research and development efforts and commercial operations. In the event that it becomes necessary to utilize a different contract manufacturer or suppliers for our products, now or in the future, we may experience additional costs, delays and difficulties in doing so, and our business could be harmed.
Certain processes related to our semiconductor chip technology are developed by us but manufactured by a third-party partner. Throughout 2024, we were in a process of transitioning a portion of key activities to a new partner, which has resulted in completed wafers with surface coatings that are usable for commercial purposes, but not fully optimized for long-term production or a fully sustainable process. If we determine finalizing this process is economically appropriate, we believe we can complete final steps by late-2025. However, transitioning these processes could take more time than anticipated and may ultimately prove to be unsuccessful. If we are unable to begin consistently manufacturing our semiconductor chip surface coating process at this new contract manufacturer in a sustainable fashion, it will affect our ability to supply semiconductor chips, affecting the commercial availability of our sequencing kit and our ability to complete development activities that allow us to improve the throughput of our platforms, which could ultimately harm our ability to deliver consumable sequencing kits to our customers, both of which would harm our research and development efforts and commercial operations.
Human Capital Management
Our people are a key reason for our success, and we have structured our organization to maximize productivity and performance. Our future success largely depends upon our continued ability to attract and retain highly-skilled employees.
We believe in attracting, developing, and retaining diverse talent and each individual, regardless of their role, makes a difference and impacts our progress. As of December 31, 2024, we employed 143 full-time employees in the United States and six full-time employees internationally. In addition, we utilize Professional Employment Organizations (“PEOs”) to provide labor for certain key activities outside the United States. None of our employees are covered by collective bargaining agreements. We understand that our success depends on our highly talented employees, and our human capital management practices focus on attracting and retaining an engaged workforce.
Mission and Core Values. Our mission is to put the groundbreaking power of protein sequencing in the hands of every scientist, every lab, everywhere. We are committed to pioneering a new generation of technology to democratize protein sequencing so that scientists can generate deeper insights faster. Employees are made aware of our values - Embrace Change, Stand Up, Speak Up, Never Settle, and Succeed Together. These values are the basis of our actions and decisions.
Employee Engagement. We have established an annual employee survey process to gather feedback from our employees. The feedback received allows us to grow stronger as a company and allows us to create an environment where employee contributions matter and employees feel valued.
Training and Development. We listen to our employees to understand their training needs. Employees are encouraged to take advantage of our training platform which has a plethora of online learning courses. We conduct monthly seminars to update employees on what is happening throughout our Company.
Compensation and Benefits. Life sciences companies, both large and small, compete for a limited number of qualified applicants to fill specialized positions. To attract qualified applicants and retain employees, we offer a total rewards package consisting of base salary, cash bonus, and equity compensation. Bonus opportunity and equity compensation increase as a percentage of total compensation based on level of responsibility. In addition, we provide a comprehensive benefits package inclusive of medical, dental, and vision healthcare coverage, including company-paid contributions into a Health Savings Account for those employees that enroll in our High Deductible Medical Plan option. Additional employee benefits include, life insurance and disability coverage, 401(k) investment plans, tax advantaged savings account, generous paid time off and leave of absence policies, employee assistance programs, and wellness programs.
Employee Health and Safety. We have training programs for general, chemical and biological safety. We are continuously evaluating the guidance from federal and local authorities and have created strict policies and guidelines that put our employees’ health and safety first.
Competition
We face significant competition in the life sciences technology market. We currently compete with life sciences technology and diagnostic companies that are supplying components, products and services that serve customers engaged in proteomics analysis. These companies include Agilent Technologies, Bio-Rad Laboratories, Danaher, Luminex, Merck KGaA (and its subsidiary MilliporeSigma) and Thermo Fisher Scientific.
We also may compete with a number of emerging growth companies that have developed, or are developing, proteomic products and solutions, such as Nautilus Biotechnology, Olink Proteomics (acquired by Thermo Fisher Scientific), Quanterix, Seer and Standard BioTools. In addition, there are a number of privately-held entities working on similar technologies as ours.
We believe there are currently no commercially available NGPS platforms beyond our Platinum and Platinum Pro systems. The legacy proteomics market today is largely served by companies that offer a variety of analytical instruments, such as MS and associated reagents and consumables. There are also a number of companies that provide proteomic analysis services and have developed or are developing novel proteomic technologies. Additional competing products may emerge from various sources, including life sciences tools, diagnostics, pharmaceutical and biotechnology companies, third-party service providers, academic research institutions, governmental agencies and/or public and private research institutions, among others. Many of the companies with which we compete have substantially greater financial, operational and sales channel resources than we have.
The broader life science instrumentation industry is highly competitive and expected to grow more competitive with the increasing knowledge gained from ongoing research and development. Given the potential market opportunity and
scientific importance of proteomic analysis, we expect increased competition and competitor technologies to emerge in the future. We believe the principal competitive factors in our target markets include:
•the scale required to address the complexity and dynamic range of the proteome;
•resolution and sensitivity;
•accuracy and reproducibility of results;
•cost of instruments and consumables;
•efficiency and speed of workflows;
•throughput to meet lab testing volume;
•reputation among customers and key thought leaders;
•innovation in product offerings;
•strength of intellectual property portfolio;
•operational and manufacturing footprint;
•customer support infrastructure; and
•a leadership and commercial team with extensive execution and scientific background.
We believe that there are currently no other commercially available products that provide the same level of analysis at the same scale and sensitivity that our platform provides. We have continued to enhance our position through our ongoing product development, commercial strategy, deployment of new and updated products as well as ongoing collaborations and partnerships with key thought leaders.
Intellectual Property
Protection of our intellectual property is a strategic priority for our business. We rely on a combination of patents, trademark, copyright, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies.
Patented Technologies
The patents owned and in-licensed by us provide comprehensive coverage of our peptide sequencing and nucleic acid sequencing processes and are directed to aspects including instrument and laser light source architecture, pixel design, waveguide architecture, lifetime discrimination methods, machine learning, and surface chemistry. We have developed a portfolio of issued patents and pending patent applications directed to commercial products and technologies for potential development. We believe that our intellectual property is a core strength of our business, and our strategy includes the continued development of our patent portfolio.
Patent Portfolio
As of December 31, 2024, we own 396 issued patents and 598 pending patent applications. Of our 396 issued patents, 98 were issued U.S. utility patents. These issued patents have expected expiration dates ranging between 2034 and 2043.
As of December 31, 2024, of our 598 pending patent applications, 128 were pending U.S. utility patent applications, 9 of which were allowed. In addition, we own 298 issued patents in foreign jurisdictions, including Australia, Brazil, China, Europe, Hong Kong, India, Japan, Korea, Mexico, and Taiwan, and 470 pending patent applications in foreign
jurisdictions, including Australia, Canada, China, Europe, Hong Kong, India, Israel, Japan, Korea, Malaysia, Mexico, Singapore, Taiwan, and Thailand, 13 of which were allowed.
Going forward, we will continue to evaluate the potential for filing patents for new intellectual property, as well as strategic adjustments of our existing portfolio to maximize our market position, as well as effective deployment of capital to maintain our existing portfolio.
Trademark Portfolio
We also protect important marks through trademark registrations. As of December 31, 2024, we owned 68 trademark registrations and 94 trademark applications, of which 16 are U.S. trademark applications. Twelve of the U.S. trademark applications have been allowed.
Other Intellectual Property
In addition to patents, we also rely on trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. We seek to protect our proprietary information and other intellectual property by taking appropriate measures including, for example, generally requiring our employees, consultants, contractors, suppliers, outside scientific collaborators and other advisors to execute non-disclosure and assignment of invention agreements on commencement of their employment or engagement. Agreements with our employees also forbid them from using or incorporating the proprietary rights of third parties during their engagement with us.
We also generally require confidentiality or material transfer agreements from third parties that receive our confidential data or materials.
Licensed Intellectual Property
We have entered into exclusive and non-exclusive licenses in the ordinary course of business relating to our technologies or other intellectual property rights or assets.
Government Regulation
Life Sciences Research Use Only Technologies
Our protein sequencing products are currently intended for RUO applications, although the systems may provide data to customers and other third parties that are themselves engaged in the research and development of potential diagnostic and therapeutic products and services for which they may later pursue clearance, authorization or approval from regulatory authorities, such as the U.S. Food and Drug Administration (“FDA”). All our products are labeled “For Research Use Only,” and will be sold to academic and research life sciences institutions that conduct basic and translational research, and biopharmaceutical and biotechnology companies for non-diagnostic and non-clinical purposes.
Under a long-standing FDA regulation, products that are intended for RUO and are labeled as RUO are not regulated by the FDA as in vitro diagnostic (“IVD”) devices and are not subject to the regulatory requirements discussed below for medical devices. RUO products may therefore be used or distributed for research use without obtaining FDA clearance or approval. Such products must bear the statement: “For Research Use Only. Not for Use in Diagnostic Procedures.” RUO products also cannot make any claims related to safety, effectiveness or diagnostic utility, and they cannot be intended for human clinical diagnostic use.
Accordingly, a product labeled RUO but intended or promoted for clinical diagnostic use may be viewed by the FDA as adulterated and misbranded under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and subject to FDA enforcement action. The FDA will consider the totality of the circumstances surrounding distribution and use of an RUO product, including how the product is marketed and to whom, when determining its intended use. If the FDA disagrees with a company’s RUO status for its product, the company may be subject to FDA enforcement activities, including, without limitation, requiring the company to seek clearance, authorization or approval for the product.
FDA and FTC Regulation of Medical Devices in the United States
In the United States, medical devices are subject to extensive regulation by the FDA under the FDCA and its implementing regulations, and other federal and state statutes and regulations. The laws and regulations govern, among other things, medical device design and development, non-clinical and clinical testing, pre-market clearance, authorization or approval, establishment registration and product listing, product manufacturing, product packaging and labeling, product storage, advertising and promotion, product distribution, recalls and field actions, servicing and post-market clinical surveillance. A number of U.S. states also impose licensing and compliance regimes on companies that manufacture or distribute prescription devices into or within the state.
The Federal Trade Commission (“FTC”) also oversees the advertising and promotion of our current and future products pursuant to its broad authority to police deceptive advertising for goods or services within the United States. Under the Federal Trade Commission Act, the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. In the context of performance claims for products, compliance with the FTC Act includes ensuring that there is scientific data to substantiate the claims being made, that the advertising is neither false nor misleading, and that any user testimonials or endorsements disseminated related to the goods or services comply with disclosure and other regulatory requirements. In addition, with respect to products that are marketed as in vitro diagnostic or clinical products, FDA’s regulations applicable to medical device products prohibit them from being promoted for uses not within the scope of a given product’s intended use(s), among other promotional and labeling rules applicable to products subject to the FDCA.
The FDCA and FDA’s implementing regulations define a medical device as an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent or other similar or related article, including any component part or accessory, which is (i) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or (ii) intended to affect the structure or any function of the body of man or other animals and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. IVDs are a type of medical device and include reagents and instruments used in the diagnosis or detection of diseases, conditions or infections, including, without limitation, the presence of certain chemicals, genetic information or other biomarkers. Predictive, prognostic, and screening tests can also be IVDs.
Medical devices, including IVD products, must undergo pre-market review by, and receive clearance or approval from, the FDA prior to commercialization, unless the device is of a type exempted from such review by statute, regulation, or an FDA exercise of enforcement discretion. The FDA classifies medical devices into three classes based on risk with Class I being the lowest risk and Class III being the highest risk. The FDA generally must clear or approve the commercial sale of most new medical devices that fall within product categories designated as Class II and III. Commercial sales of most Class II and III medical devices within the United States must be preceded either by pre-market notification and FDA clearance pursuant to Section 510(k) of the FDCA (Class II) or by the granting of a pre-market approval (“PMA”) (Class III), after a pre-market application is submitted. Both 510(k) notifications and PMA applications must be submitted to FDA with significant user fees, although reduced fees for small businesses are available. Class I devices are generally exempt from pre-market review and notification, as are some moderate-risk Class II devices. Manufacturers of all classes of devices must comply with FDA’s QSR, establishment registration, medical device listing, labeling requirements, and medical device reporting (“MDR”) regulations, which are collectively referred to as medical device general controls. Class II devices may also be subject to special controls such as performance standards, post-market surveillance, FDA guidelines, or particularized labeling. Some Class I and Class II devices may be exempted by regulation from the requirement of compliance with substantially all of the QSR.
Moreover, as electronic and digital medical devices have become increasingly connected to the Internet, hospital networks, and other medical devices to provide features that improve health care and patient accessibility, FDA and other regulatory authorities have recognized that those same features also increase the risk of cybersecurity threats. These types of medical devices may be vulnerable to cybersecurity incidents that could potentially impact the safety and effectiveness of the device, and device manufacturers are responsible for identifying cybersecurity risks and hazards associated with our products. In recent years, the FDA has increased its scrutiny of this issue as part of the review and marketing authorization process for new medical devices; the agency also monitors reports of cybersecurity incidents as part of its post-marketing device surveillance activities. In addition, as part of the Consolidated Appropriations Act for 2023, signed into law on December 29, 2022 (P.L. 117-328), Congress created new pre-market requirements for developers of “cyber devices,”
defined as medical devices that include software, connect to the Internet, and contain any technological features that could be vulnerable to cybersecurity threats.
Ongoing Post-Market Regulatory Requirements and FDA Enforcement
If our products were deemed to be “medical devices” by the FDA, then our company would be subject to general controls which include:
•establishment registration and device listing;
•the QSR, which requires manufacturers, including third-party manufacturers, to follow design, testing, control, storage, supplier/contractor selection, complaint handling, documentation and other quality assurance procedures;
•labeling regulations, which govern the mandatory elements of the device labels and packaging (including Unique Device Identifier markings for certain categories of products);
•the FDA’s prohibitions against the promotion of products for uncleared, unapproved or “off-label” uses and other requirements related to promotional activities;
•the MDR regulations, which require that manufacturers report to the FDA if a device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur;
•voluntary and mandatory device recalls addressing problems when a device is defective and/or could be a risk to health;
•correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; and
•post-market surveillance regulations, which apply to certain Class II or III devices when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
Additionally, certain Class II and Class III devices are subject to special controls. To ensure compliance with regulatory requirements, medical device manufacturers are subject to market surveillance and periodic, pre-scheduled and unannounced inspections by the FDA and certain state authorities. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may lead to any of the following sanctions:
•Warning Letters or Untitled Letters that require corrective action;
•fines and civil penalties;
•unanticipated expenditures;
•delays in approving/clearing or refusal to approve/clear any of our future products;
•FDA refusal to issue certificates to foreign governments needed to export our products for sale in other countries;
•suspension or withdrawal of FDA approval or clearance (as may be applicable);
•product recall or seizure;
•partial suspension or total shutdown of production;
•operating restrictions;
•injunctions or consent decrees; and
•civil or criminal prosecution.
A company, any contract manufacturers, and some suppliers of components or device accessories would also be required to manufacture medical device products in compliance with current Good Manufacturing Practice requirements set forth in the QSR, unless explicitly exempted by regulation, should we develop and seek regulatory authorization for one or more diagnostic intended uses for our products. The QSR requires a quality system for the design, manufacture, packaging, labeling, storage, installation and servicing of marketed devices, and includes extensive requirements with respect to quality management and organization, device design, buildings, equipment, purchase and handling of components or services, production and process controls, packaging and labeling controls, device evaluation, distribution, installation, complaint handling, servicing, and record keeping. The FDA recently issued a final rule that amends its implementing
regulations in order to harmonize the QSR with ISO 13485:2016; the rule changes will become effective on February 2, 2026. Although the harmonization process is not expected to have a significant impact on the quality system compliance operations of device manufacturers because most requirements described in the QSR correspond to requirements set forth in ISO 13485:2016, device manufacturers will likely need to revise certain quality system procedures to ensure compliance with the harmonized regulations. Any failure to make such revisions or adapt to the harmonized regulations, once they become effective, may result in observations of non-compliance during facility inspections by the FDA or comparable regulatory authorities.
The FDA evaluates compliance with the QSR, as well as other applicable device regulatory requirements, through periodic pre-scheduled or unannounced inspections that may include registered manufacturing facilities. Following such inspections, FDA may issue reports known as Forms FDA 483 or Notices of Inspectional Observations, which list instances where the FDA inspector believes the manufacturer has failed to comply with applicable regulations and/or procedures. If the observations are sufficiently serious or the manufacturer fails to respond appropriately, the FDA may issue Warning Letters, which are notices of intended enforcement actions against the manufacturer. For less serious violations that may not rise to the level of regulatory significance, FDA may issue Untitled Letters. The FDA may take more significant administrative or legal action if a manufacturer continues to be in substantial noncompliance with applicable regulations.
For example, if the FDA believes a medical device developer or any of its contract manufacturers or regulated suppliers are not in compliance with these requirements and patients are being subjected to serious risks, the agency can shut down manufacturing operations, require recalls of medical device products, refuse to approve new marketing applications for future products, initiate legal proceedings to detain or seize products, enjoin future violations, or assess civil and criminal penalties against a manufacturer or its officers or other employees.
U.S. Fraud and Abuse Laws and Other Compliance Requirements
FCPA and Other Anti-Bribery and Anti-Corruption Laws. The U.S. Foreign Corrupt Practices Act (“FCPA”) prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA would include interactions with certain healthcare professionals or organizations in many countries. Our present and future business has been and will continue to be subject to various other U.S. and foreign laws, rules and/or regulations.
U.S. and European Data Security and Data Privacy Laws
All U.S. states have enacted legislation protecting the privacy and security of “personal information” such as identifiable financial or health information, social security numbers, credit card information and other personally identifiable information. These laws overlap and apply simultaneously with federal privacy and security requirements and regulated entities must comply with all of them. The California Consumer Privacy Act (“CCPA”) went into effect January 1, 2020, and is one of the most restrictive state privacy laws, protecting a wide variety of personal information and granting significant rights to California residents with respect to their personal information. Regulations under CCPA have been modified several times and continue to be modified. Additionally, a new privacy law, the California Privacy Rights Act, (“CPRA”) was approved by California voters in the election of November 3, 2020 and went into effect in January of 2023. The CPRA modified the CCPA significantly, and may result in further uncertainty, additional costs and expenses stemming from efforts to comply with this law, and increases the potential for harm and liability for failure to comply. Among other things, the CPRA established a new regulatory authority, the California Privacy Protection Agency, which is enacting new regulations and has expanded enforcement authority. Other states in the U.S. have implemented or are considering privacy laws similar to CCPA. Colorado, Connecticut, Delaware, Florida, Indiana, Iowa, Montana, New Jersey, Oregon, Tennessee, Texas, Utah, and Virginia have enacted similar data protection laws to California and other U.S. states have proposals under consideration, increasing the regulatory compliance risk. In dealing with health information for the development of our technology or for commercial purposes, we will be indirectly affected by HIPAA and state-imposed health information privacy and cybersecurity laws because these laws regulate the ability of our potential customers and research collaborators to share health information with us. Additionally, we must identify and comply with all applicable state laws for the protection of personal information with respect to personal information that we collect.
In the European Union, increasingly stringent data protection and privacy rules that have and will continue to have substantial impact on the use of personal and patient data across the healthcare industry became stronger in May 2018. The General Data Protection Regulation (“GDPR”) applies across the European Union and includes, among other things, a
requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR fine framework can be up to 20 million euros, or up to 4% of the company’s total global turnover of the preceding fiscal year, whichever is higher. The GDPR sets out a number of requirements that must be complied with when handling the personal data of such European Union based data subjects including: providing expanded disclosures about how their personal data will be used; higher standards for organizations to demonstrate that they have obtained valid consent or have another legal basis in place to justify their data processing activities; the obligation to appoint data protection officers in certain circumstances; new rights for individuals to be “forgotten” and rights to data portability, as well as enhanced current rights (e.g., access requests); the principal of accountability and demonstrating compliance through policies, procedures, training and audit; and the new mandatory data breach regime. In particular, medical or health data, genetic data and biometric data where the latter is used to uniquely identify an individual are all classified as “special category” data under the GDPR and are afforded greater protection and require additional compliance obligations. Noncompliance could result in the imposition of fines, penalties, or orders to stop noncompliant activities. We are subject to GDPR as we undertake and expand operations in the EU, offer products or services to individuals in the EU, or monitor the behavior of individuals within the EU.
We could also be subject to evolving European Union laws on data export, for transfers of data outside the EU to us, group companies or third parties. The GDPR only permits exports of data outside the EU to jurisdictions that ensure an adequate level of data protection. The United States has not been deemed to offer an adequate level of protection, so in order for us to transfer personal data from the EU to the United States, we must identify a legal basis for data transfer (e.g., the European Union Commission approved Standard Contractual Clauses or certification under the recently-adopted EU-U.S. Data Privacy Framework). On July 16, 2020, the Court of Justice of the European Union or the CJEU, issued a landmark opinion in the case Maximilian Schrems vs. Facebook (Case C-311/18), called Schrems II. This decision (a) calls into question commonly relied upon data transfer mechanisms as between the EU member states and the U.S. (such as the Standard Contractual Clauses) and (b) invalidates the EU-U.S. Privacy Shield on which many companies had relied as an acceptable mechanism for transferring such data from the EU to the U.S. The CJEU is the highest court in Europe and the Schrems II decision heightens the burden on data importers to assess U.S. national security laws on their business and future actions of EU data protection authorities are difficult to predict. Consequently, there is some risk of data transfers from the EU being halted. While the recently-adopted EU-U.S. Data Privacy Framework is intended to address the issues and concerns raised by the CJEU in Schrems II and provide an approved method for cross-border data transfer from the EU to the U.S., it will likely be subject to future legal challenges and we have not yet certified to participate in the EU-U.S. Data Privacy Framework. If we have to rely on third parties to carry out services for us, including the processing of personal data on our behalf, we are required under the GDPR to enter into contractual arrangements to help ensure that these third parties only process such data according to our instructions and have sufficient security measures in place. Any security breach or non-compliance with our contractual terms or breach of applicable law by such third parties could result in enforcement actions, litigation, fines and penalties or adverse publicity and could cause customers to lose trust in us, which would have an adverse impact on our reputation and business.
Further, the United Kingdom’s decision to leave the European Union, often referred to as Brexit, has created uncertainty with regard to data protection regulation in the United Kingdom. In particular, while the Data Protection Act of 2018 that “implements” and complements the GDPR achieved Royal Assent on May 23, 2018 and is now effective in the United Kingdom, it is still unclear whether transfer of data from the European Economic Area to the United Kingdom will remain lawful under GDPR.
Other Governmental Regulation
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials. For example, the U.S. Occupational Safety and Health Administration (“OSHA”) has established extensive requirements relating specifically to workplace safety for employers in the United States. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, including preventing or minimizing any exposure through needle stick injuries. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the United States Postal Service and the International Air Transport Association. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste and radioactive materials that we may use during our research.
International Laws and Regulations for IVD Products
Whether or not we obtain FDA marketing authorization for a clinical diagnostic product in the future, we must still obtain the requisite approvals from regulatory authorities in non-U.S. countries prior to the marketing of any product for clinical diagnostic use in those countries. The regulations in other jurisdictions vary from those in the United States and may be easier or more difficult to satisfy and are subject to change. For example, the European Commission published Regulation (EU) 2024/1860 in July 2024. This new regulation amended Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostics (IVD Regulation). Under Regulation (EU) 2024/1860, the transitional periods for certain IVDs have been extended, the implementation of the EUDAMED database will be rolled out in phases, and manufacturers are required to provide notification to the competent authority and healthcare institutions before the interruption or discontinuation of supply of certain medical devices and IVDs.
Outside of the European Union, regulatory authorization needs to be sought on a country-by-country basis in order for us to market any clinical diagnostic products. Some countries have adopted medical device regulatory regimes, such as the Medical Device Administrative Control System (“MDACS”) published by the Hong Kong Department of Health, the Health Sciences Authority of Singapore regulation of medical devices under the Health Products Act and Health Products (Medical Devices) Regulations, and Health Canada’s risk-based classification system for devices, among others, that incorporate IVD products like the FDA’s current system. Each country may have its own processes and requirements for IVD licensing, approval/clearance, and regulation, therefore requiring us to seek any regulatory approvals on a country- by-country basis.
Corporate Information
Quantum-Si Incorporated was originally incorporated under the laws of the State of Delaware on June 24, 2013. Our principal executive offices are located at 29 Business Park Drive, Branford, Connecticut 06405, and our telephone number is (866) 688-7374.
Information Available on the Internet
Our internet address is https://www.quantum-si.com, to which we regularly post copies of our press releases as well as additional information about us. Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports, will be available to you free of charge through the Investor Relations section of our website as soon as reasonably practicable after such materials have been electronically filed with, or furnished to the SEC. The SEC maintains an internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. We include our web site address in this Annual Report on Form 10-K only as an inactive textual reference. The information contained in our website does not constitute a part of this report or our other filings with the SEC.
ITEM 1A. RISK FACTORS
Careful consideration should be given to the following risk factors, in addition to the other information set forth in this Annual Report, including the section of this Annual Report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our Consolidated Financial Statements and related Notes, and in other documents that we file with the SEC, in evaluating our company and our business. Investing in our securities involves a high degree of risk. If any of the events described in the following risk factors actually occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected, and the trading price of our securities could decline. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Annual Report on Form 10-K.
Unless the context otherwise requires, references in this section to “we”, “us”, “our,”, the “Company” and “Quantum-Si” refer to Quantum-Si Incorporated and its subsidiaries following the Business Combination, or to Legacy Quantum-Si or HighCape prior to the Business Combination, as the case may be.
Risks Related to Our Financial Condition and Capital Requirements
We are an early-stage life sciences technology company with a history of net losses and negative cash flow, which we expect to continue, and we may not be able to generate meaningful revenues or achieve and sustain profitability or positive cash flow in the future.
We are an early-stage life sciences technology company and have incurred significant losses since Quantum-Si was formed in 2013, and expect to continue to incur losses in the future. We incurred net losses of $101.0 million, $96.0 million and $132.4 million in the years ended December 31, 2024, 2023 and 2022, respectively. As of December 31, 2024, we had an accumulated deficit of $596.6 million. These losses and accumulated deficit were primarily due to the substantial investments made to develop and improve our technology. Over the next several years, we expect to continue to devote substantially all of our resources towards development and commercialization of our products and research and development efforts for additional products. These efforts may prove more costly than we currently anticipate. In December 2022, we initiated a controlled launch of Platinum for RUO, and to date we have generated limited product revenue and may never generate revenue sufficient to offset our expenses or produce enough cash to sustain operations. Accordingly, we cannot assure you that we will achieve profitability or positive cash flow production in the future or that, if we do become profitable and cash flow positive, we will sustain these levels.
We have a limited operating history, which may make it difficult to evaluate the prospects for our future viability and predict our future performance. As such, you cannot rely upon our historical operating performance to make an investment or voting decision regarding us.
We recently commercialized our first product and have generated limited revenue to date. Even with our Platinum product launch, our operations to date have been primarily limited to developing our technology and products. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. We have not yet produced our products at scale, established sales models, or conducted sales and marketing activities necessary for widespread product commercialization. Consequently, predictions about our future success or viability are highly uncertain and may not be as accurate as they could be if we had a longer operating history or a company history of successfully developing and commercializing products.
In addition, as a business with a limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown obstacles. We are transitioning from a company that previously had a sole focus on research and development to a company capable of supporting commercial activities and we may not be successful in such a transition. We have encountered in the past, and we expect to encounter in the future, risks and uncertainties frequently experienced by growing companies with limited operating histories in emerging and rapidly changing industries. If our assumptions regarding these risks and uncertainties, which we use to plan and operate our business, are incorrect or change, or if we do not address these risks successfully, our results of operations could differ materially from our expectations, and our business, financial condition, results of operations and cash flows could be adversely affected.
Our operating results may fluctuate significantly in the future, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we or other third parties may provide.
Our quarterly and annual operating results may fluctuate significantly, which makes it difficult for us to predict our future operating results. These fluctuations may occur due to a variety of factors, many of which are outside of our control, including, but not limited to:
•the timing and amount of expenditures that we may incur to develop, commercialize or acquire additional products and technologies or for other purposes, such as the expansion of our facilities;
•changes in governmental funding of life sciences research and development or changes that impact budgets or budget cycles;
•seasonal spending patterns of our customers;
•the timing of when we recognize any revenues;
•future accounting pronouncements or changes in our accounting policies;
•the outcome of any future litigation or governmental investigations involving us, our industry or both;
•higher than anticipated service, replacement and warranty costs;
•the impact of past or future epidemics or pandemics on the economy, investment in life sciences and research industries, our business operations, and resources and operations of our suppliers, distributors and potential customers; and
•general industry, economic and market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
The cumulative effects of the factors discussed above could result in large fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparing our operating results on a period-to-period basis may not be meaningful.
This variability and unpredictability could also result in us failing to meet the expectations of industry or financial analysts or investors for any period. If we are unable to realize our objectives associated with commercializing our products, or if our operating results fall below the expectations of analysts or investors or below any guidance we may provide, or if any guidance we provide is below the expectations of analysts or investors, it could cause the market price of our Class A common stock to decline.
We may need to raise additional capital to fund ongoing research and development, operating activities, and commercialization activities.
Our operations have consumed substantial amounts of cash since inception. We expect to spend substantial additional amounts to continue the commercialization of our products and to develop new products. We expect to use our funds on hand to further develop and commercialize our products, develop new products, and for working capital and general corporate purposes. As of December 31, 2024, we had cash and cash equivalents and investments in marketable securities totaling $209.6 million. We expect our cash and cash equivalents and investments in marketable securities will be able to fund our operations for at least the next twelve months. However, this does not reflect the possibility that we may not be able to access a portion of our existing cash and cash equivalents and investments in marketable securities due to market conditions.
We may require additional capital to develop and commercialize our products and to develop new products. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned.
We cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any future financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our Class A common stock to decline. The incurrence of indebtedness could result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating
restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable, and we may be required to relinquish rights to some of our technologies or products or otherwise agree to terms that are unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. In addition, raising additional capital through the issuance of equity or debt securities would cause dilution to holders of our equity securities and/or increased fixed payment obligations, and may affect the rights of then-existing holders of our equity securities. Furthermore, these securities may have rights senior to those of our Class A common stock and could contain covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects. Even if we believe that we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Risks Related to Our Business and Industry
We may not gain commercial traction for our current products and we may not be able to successfully commercially launch other future products.
We commercially launched our first product, Platinum for RUO in December 2022. We are following a three-phase launch plan for commercialization, which includes an early access limited release phase, the current controlled commercial launch phase, and a broad commercial availability phase. Our commercial launch plan may not progress as planned due to:
•the inability to establish the capabilities and value proposition of our products with key opinion leaders in a timely fashion;
•the potential need or desire to modify aspects of our products throughout our commercial launch plan;
•changing industry or market conditions, customer requirements or competitor offerings over the span of our commercial launch plan;
•delays in building out our sales, customer support and marketing organization as needed for each of the phases of our commercial launch plan; and
•delays in ramping up manufacturing, either internally or through our suppliers to meet the expected demand in each of the phases of our commercial launch plan.
To the extent our commercial launch plan is unsuccessful, our financial results will be adversely impacted.
Our success depends on broad scientific and market acceptance, which we may fail to achieve.
Our ability to achieve and maintain scientific and commercial market acceptance of our products depends and will depend on a number of factors. Our products are and will be subject to market forces and adoption curves common to other new technologies. The market for proteomics and genomics technologies and products is in its early stages of development and if widespread adoption of our products takes longer than anticipated, we will continue to experience operating losses.
The success of life sciences products is due, in large part, to acceptance by the scientific community and their adoption of certain products in the applicable field of research. The life sciences scientific community is often led by a small number of early adopters and key opinion leaders who significantly influence the rest of the community through publications in peer-reviewed journals. In such journal publications, the researchers will describe not only their discoveries, but also the methods, and typically the products used, to fuel such discoveries. Mentions in peer-reviewed journal publications is a driver for the general acceptance of life sciences products, such as our products. During the early access limited release phase of our commercialization launch plan, we collaborated with a small number of key opinion leaders who are highly skilled at evaluating novel technologies and whose feedback helped us solidify our commercialization plans and processes. Ensuring that early adopters and key opinion leaders publish research involving the use of our products during the early access limited release phase is critical to ensuring our products gain widespread scientific acceptance. In addition, continuing collaborative relationships with such key opinion leaders will be vital to maintaining any market acceptance we achieve. If too few researchers describe the use of our products, too many researchers shift to a competing product and publish research outlining their use of that product or too many researchers negatively describe the use of our products in publications, it may drive customers away from our products and it may delay our progression towards the broad commercial release phase of our commercialization plan.
Other factors in achieving commercial market acceptance, include:
•our ability to market and increase awareness of the capabilities of our products;
•the ability of our products to demonstrate comparable performance in intended use applications broadly in the hands of customers consistent with the early access limited release phase of our commercialization plan;
•our potential customers’ willingness to adopt new products and workflows;
•our product’s ease of use and whether it reliably provides advantages over other alternative technologies;
•the rate of adoption of our products by academic institutions, laboratories, biopharmaceutical companies and others;
•the prices we charge for our products;
•our ability to develop new products and workflows and solutions for customers;
•if competitors develop and commercialize products that perform similar functions as our products; and
•the impact of our investments in product innovation and commercial growth.
We may not be successful in addressing each of these criteria or other criteria that might affect the market acceptance of Platinum and any other products we commercialize. If we are unsuccessful in achieving and maintaining market acceptance of our products, our business, financial condition, results of operations and cash flows will be adversely affected.
If we are unable to establish superior sales and marketing capabilities, we may not be successful in commercializing our products.
We have limited experience as a company in sales and marketing and our ability to achieve revenue growth depends on us being able to attract customers for our products. Although members of our management team have considerable industry experience, we will be required to expand our sales, marketing, distribution and customer service and support capabilities with the appropriate technical expertise to gain market share and revenue growth. To perform sales, marketing, distribution, and customer service and support successfully, we will face a number of risks, including:
•our ability to attract, retain and manage the sales, marketing and customer service and support force necessary to commercialize and gain market acceptance of our products;
•the time and cost of establishing a specialized sales, marketing and customer service and support force; and
•our sales, marketing and customer service and support force may be unable to initiate and execute successful commercialization activities.
We may seek to enlist certain third parties to assist with sales, distribution and customer service and support globally or in certain regions of the world. There is no guarantee, if we do seek to enter into such arrangements, that we will be successful in attracting desirable sales and distribution partners or that we will be able to enter into such arrangements on an ongoing basis on favorable terms. If our sales and marketing efforts, or those of any third-party sales and distribution partners, are not successful, our products may not gain market acceptance, which could materially impact our business operations.
The size of the markets for our products may be smaller than estimated, and new market opportunities may not develop as quickly as we expect, or at all, limiting our ability to successfully sell our products.
The market for proteomics and genomics technologies and products is evolving, making it difficult to predict with any accuracy the size of the markets for our current and future products. Our estimates of the total addressable market for our current and future products are based on a number of internal and third-party estimates and assumptions. In particular, our estimates are based on our expectations that researchers in the market for certain life sciences research tools and technologies will view our products as competitive alternatives to, or better options than, existing tools and technologies. We also expect researchers will recognize the ability of our products to complement, enhance and enable new applications of their current tools and technologies. We expect them to recognize the value proposition offered by our products, enough to purchase our products in addition to the tools and technologies they already own. Underlying each of these expectations are a number of estimates and assumptions that may be incorrect, including the assumptions that government or other sources of funding will continue to be available to life sciences researchers at times and in amounts necessary to allow them to purchase our products and that researchers have sufficient samples and an unmet need for performing proteomics
studies at scale across thousands of samples. In addition, sales of new products into new market opportunities may take years to develop and mature and we cannot be certain that these market opportunities will develop as we expect. New life sciences technology may not be adopted until the consistency and accuracy of such technology, method or device has been proven. As a result, the sizes of the annual total addressable market for new markets and new products are even more difficult to predict. Our products are innovative new products, and while we draw comparisons between the evolution and growth of the genomics and proteomics markets, the proteomics market may develop more slowly or differently. While we believe our assumptions and the data underlying our estimates of the total addressable market for our products are reasonable, these assumptions and estimates may not be correct and the conditions supporting our assumptions or estimates, or those underlying the third-party data it has used, may change at any time, thereby reducing the accuracy of our estimates. As a result, our estimates of the total addressable market for our products may be incorrect.
Epidemics, pandemics or other public health crises could adversely affect our business.
Our operations could be significantly adversely affected by the effects of a widespread outbreak of epidemics, pandemics or other health crises. We cannot accurately predict the impact of epidemics and pandemics would have on our operations and the ability of third-parties to meet their obligations under contracts or arrangements with us, including uncertainties relating to the ultimate geographic spread of epidemics and pandemics, the severity of the underlying diseases, the duration of outbreaks, and the length of travel and quarantine restrictions imposed by governments of affected countries. In addition, a significant outbreak of contagious diseases in the human population could result in a widespread health crisis that could adversely affect the economies and financial markets of many countries, resulting in an economic downturn that could further affect our operations and ability to finance our operations.
Environmental, social and governance matters may impact our business and reputation.
Increasingly, companies are being judged by their performance on a variety of environmental, social and governance (“ESG”) matters, which are considered to contribute to the long-term sustainability of companies’ performance.
A variety of organizations measure the performance of companies on such ESG topics, and the results of these assessments are widely publicized. In addition, investment in funds that specialize in companies that perform well in such assessments are becoming increasingly popular, and major institutional investors have publicly emphasized the importance of such ESG measures to their investment decisions. Topics taken into account in such assessments include, among others, the company’s efforts and impacts on climate change and human rights, ethics and compliance with law, and the role of the company’s board of directors in supervising various sustainability issues.
The severity and frequency of weather-related natural disasters has been amplified, and is expected to continue to be amplified, by global climate change. Such natural disasters have caused, and in the future may cause, damage to and/or disrupt our operations, which may result in a material adverse effect on our business and results of operations. Our suppliers, vendors and business partners also face similar risks, and any disruption to their operations could have an adverse effect on our supply and manufacturing chain.
Climate change has had significant legislative and regulatory effects on a global basis, and there are expected to be additional changes to the regulations in these areas. These changes could directly increase the cost of energy, which may have an impact on the way we manufacture products or utilize energy to produce our products. In addition, any new regulations or laws in the environmental area might increase the cost of raw materials we use in our products and the cost of compliance. Other regulations in the environmental area may require us to continue to monitor and ensure proper disposal or recycling of our products.
In light of investors’ increased focus on ESG matters, there can be no certainty that we will manage such issues successfully, or that we will successfully meet society’s expectations as to our proper role. Any failure or perceived failure by us in this regard could have a material adverse effect on our reputation and on our business, share price, financial condition, results of operations or cash flows, including the sustainability of our business over time.
Unfavorable global economic conditions, retaliatory economic policies, or other geopolitical conditions associated with intra-country economics and policies could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets, including changes in inflation, interest rates, tariffs, retaliatory trade policies including limitations of shipments of products, and overall economic conditions and uncertainties. For instance, we have experienced pricing
increases from our suppliers. To the extent inflation or other factors increase our business costs, it may not be feasible to pass price increases on to our customers or offset higher costs through manufacturing efficiencies. Inflation or economic policies could also adversely affect the ability of our customers to purchase our products. An economic downturn could result in a variety of risks to our business, including weakened demand for our products and our inability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also result in further constraints on our suppliers or cause future customers to delay making payments for our products. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
On February 1, 2025, the President of the United States issued executive orders directing the United States to impose new tariffs on imports from Canada, Mexico and China. Although a portion of these new tariffs have been temporarily suspended, other parts of these new tariffs are now in effect, and it is unclear for how long and to what extent such suspensions will remain in effect. The U.S. has also announced new tariffs on foreign steel and aluminum, with such tariffs taking effect in early March. The U.S. has further raised the possibility of new tariffs on imports from additional countries, including those in Europe. The new tariffs likely will increase the cost of the products we source from these international jurisdictions and affect future shipments from any of our foreign suppliers. We may not be able to pass along increases in tariffs and freight charges, and any alterations we may make to our business strategy or operations to adapt to the foregoing, including sourcing products from suppliers in other countries, would be time consuming and expensive and could adversely impact our business.
Geopolitical conflicts could potentially affect our sales and disrupt our operations and could have a material adverse impact on our business.
Geopolitical conflicts, including the ongoing conflicts in Ukraine and Israel and Gaza, could adversely impact our operations or those of our suppliers, manufacturers or customers. The extent to which these events impact our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence. If the uncertainty surrounding geopolitical conflicts and in the global marketplace continues, or if we, or any of our suppliers, manufacturers or customers encounter any disruptions to our or their respective operations or facilities, then we or they may be prevented or delayed from effectively operating our or their business, respectively, and the marketing and sale of our products and our financial results could be adversely affected.
If we do not sustain or successfully manage our anticipated growth, our business and prospects will be harmed.
Our anticipated growth will place significant strains on our management, operational and manufacturing systems and processes, sales and marketing team, financial systems and internal controls and other aspects of our business. As of December 31, 2024, we had 143 full-time employees in the United States and six full-time employees internationally. Our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements and effectively manage these growth activities. We may face challenges integrating, developing and motivating our employee base. To effectively manage our growth, we must continue to improve our operational and manufacturing systems and processes, our financial systems and internal controls and other aspects of our business and continue to effectively expand, train and manage our personnel. If we do not successfully manage our anticipated growth, our business, results of operations, financial condition and prospects will be harmed.
Recently and in the past, we have undergone leadership transitions and an internal restructuring, and we depend on our key personnel and other highly qualified personnel, and if we are unable to recruit, train and retain our personnel in the future, we may not achieve our goals.
In January and August 2023, and November 2024, we committed to organizational restructurings designed to decrease our costs and create a more streamlined organization to support our business. In connection with these activities, we reduced our workforce by approximately 12%, 16% and 23% effective in January and August 2023, and November 2024, respectively. We believe this re-prioritized strategic focus is the best way to optimize our financial and other resources to advance our goal of developing and commercializing our products and services. There can be no assurance that our restructuring will achieve the cost savings, operating efficiencies or other benefits that we may initially expect. Restructuring activities may also result in a loss of continuity, accumulated knowledge and inefficiency during transitional periods and thereafter. In addition, internal restructurings can require a significant amount of time and focus from management and other employees, which may divert attention from operations. Further, our restructuring may result in unexpected expenses or liabilities and/or write-offs. If our restructuring fails to achieve some or all of the expected benefits
therefrom, our cash resources may not last as long as estimated and our business, results of operations and financial condition could be materially and adversely affected.
Additionally, our future success depends upon our ability to recruit, train, retain and motivate key personnel, including our senior management team, as well as our research and development team and manufacturing and sales and marketing personnel. Our senior management team is critical to our vision, strategic direction, product development and commercialization efforts. The departure of one or more of our executive officers, senior management team members, or other key employees could be disruptive to our business until we are able to hire qualified successors. We do not maintain “key person” life insurance on our senior management team.
Our continued growth and ability to successfully transition from a company primarily focused on research and development to commercialization depends, in part, on attracting, retaining and motivating qualified personnel, including highly-trained sales personnel with the necessary scientific background and ability to understand our products and systems at a technical level to effectively identify and sell to potential new customers. New hires require significant training and, in most cases, take significant time before they achieve full productivity. Our failure to successfully integrate these key personnel into our business could adversely affect our business. In addition, competition for qualified personnel is intense. We compete for qualified scientific and information technology personnel with other life science and information technology companies as well as academic institutions and research institutions. Some of our scientific personnel are qualified foreign nationals whose ability to live and work in the United States is contingent upon the continued availability of appropriate visas. Due to the competition for qualified personnel in our industry, we may continue to utilize foreign nationals to fill part of our recruiting needs. As a result, changes to U.S. immigration policies could restrain the flow of technical and professional talent into the United States and may inhibit our ability to hire qualified personnel.
We do not maintain fixed term employment contracts with any of our employees. As a result, our employees could leave the Company with little or no prior notice and may be free to work for a competitor. Due to the complex and technical nature of our products and technology and the dynamic market in which we compete, any failure to attract, train, retain and motivate qualified personnel could materially harm our business, results of operations, financial condition and prospects.
We expect to be dependent upon revenue generated from the sales of our initial products from the time they are commercialized through the foreseeable future.
In December 2022, we initiated a controlled launch of Platinum for RUO, and subsequently initiated a full commercial launch at the beginning of the second quarter 2024. If we are able to successfully commercialize our products, we expect that we will generate substantially all of our revenue from the sale of our instruments and consumables. There can be no assurance that we will be able to successfully commercialize our products, design other products that will meet the expectations of our customers or that any of our future products will become commercially viable. As technologies change in the future for life sciences research tools in general and in proteomics and genomics technologies specifically, we will be expected to upgrade or adapt our products in order to keep up with the latest technology. To date, we have limited experience simultaneously designing, testing, manufacturing and selling products and there can be no assurance we will be able to do so. Our sales expectations are based in part on the assumption that our products will increase study sizes for our future customers and their associated purchases of our consumables. If sales of our instruments fail to materialize, so will the related consumable sales and associated revenue.
In our development and commercialization plans for our products, we may forego other opportunities that may provide greater revenue or be more profitable. If our research and product development efforts do not result in commercially viable products within the anticipated timelines, or at all, our business and results of operations will be adversely affected. Any delay or failure by us to develop and release our products or product enhancements would have a substantial adverse effect on our business and results of operations.
Our business will depend significantly on research and development spending by academic institutions and other research institutions, and any reduction in spending, driven by these customers or other third party funding sources such as the National Institutes of Health, could limit demand for our products and adversely affect our business, results of operations, financial condition and prospects.
We expect that a large portion of all of our revenue in the near term could be generated from sales of RUO protein sequencing products to academic institutions and other research institutions. Much of these customers’ funding will be, in turn, provided by various state, federal and international government agencies. As a result, the demand for our products
will depend upon the research and development budgets of these customers, which are impacted by factors beyond our control, such as:
•decreases or freezes in government funding of research and development;
•changes to programs that provide funding to research laboratories and institutions, including changes in the amount of funds allocated to different areas of research or changes that have the effect of increasing the length of the funding process;
•macroeconomic conditions and the political climate;
•potential changes in the regulatory environment;
•differences in budgetary cycles, especially government or grant-funded customers, whose cycles often coincide with government fiscal year ends;
•competitor product offerings or pricing;
•market-driven pressures to consolidate operations and reduce costs; and
•market acceptance of relatively new technologies.
In addition, various state, federal and international agencies that provide grants and other funding may be subject to stringent budgetary constraints that could result in spending reductions, reduced grant making, reduced allocations or budget cutbacks, which could jeopardize the ability of these customers, or the customers to whom they provide funding, to purchase our products. A decrease in the amount of, or delay in the approval of, appropriations to National Institutes of Health (“NIH”) or other similar U.S. or international organizations, such as the Medical Research Council in the United Kingdom, could result in fewer grants benefiting life sciences research. For example, on February 7, 2025, the NIH imposed a standard indirect rate of 15% across all NIH grants for indirect costs, defined as “facilities” and “administration,” in lieu of a separately negotiated rate for indirect costs in every grant. Indirect costs represent more than 25% of total grant dollars in 2023 awarded by the NIH. This limitation may impact research institution’s ability to govern or even accept NIH grants, thereby affecting total spending on products such as ours. Further, as of February 2025, much of the current period NIH funding allotments have either been slowed or frozen. These reductions or delays could also result in a decrease in the aggregate amount of grants awarded for life sciences research or the redirection of existing funding to other projects or priorities, any of which in turn could cause our potential customers to reduce or delay purchases of our products.
If we use biological and hazardous materials in a manner that causes injury or violates laws or regulations, we could be liable for damages or subject to enforcement actions.
Our research and product development activities currently require the controlled use of potentially harmful biological and hazardous materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state, and local laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste and radioactive materials that we may use during our research. The cost of compliance with these laws and regulations may become significant and could have a material adverse effect on our financial condition, results of operations and cash flows.
We rely on certain contract manufacturers to manufacture and supply our instruments, components of our instruments, and certain components of our consumable offerings. If these manufacturers should fail or not perform satisfactorily, our ability to commercialize and supply our instruments and consumable offerings would be adversely affected.
We rely on certain contract manufacturers to manufacture and supply our instruments, components of our instruments, and certain components of our consumable offerings. Since most of our contracts with these manufacturers do not commit them to carry inventory or make available any particular quantities, these manufacturers may give other customers’ needs higher priority than ours, and we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms. Further, if these manufacturers are unable to obtain critical components used in our instruments or supply our instruments on the timelines we require, our business and commercialization efforts would be harmed. Further, any strategic decision to slow, pause, or stop any core supply based on assumptions of adequate safety stock that subsequently
proves to be an incorrect estimate of supply needs could result in a supply disruption based on in ability to ramp up or slowness in ramp down processes.
In the event it becomes necessary to utilize different contract manufacturers for our products or components of our products, we would experience additional costs, delays and difficulties in doing so as a result of identifying and entering into an agreement with a new manufacturer as well as preparing such new manufacturer to meet the logistical requirements associated with manufacturing our instruments and consumable offerings, and our business would suffer. In addition, if our products are authorized for use by the FDA as medical devices, we will need to contract with FDA-registered device establishments that are able to comply with current Good Manufacturing Practice requirements that are set forth in the Quality System Regulations (“QSR”), unless explicitly exempted by regulation.
In addition, certain of the components and consumables used in our instruments and consumable offerings are sourced from a limited number, or sole source suppliers. If we were to lose such a supplier, there can be no assurance that we will be able to identify or enter into an agreement with an alternative supplier on a timely basis on acceptable terms, if at all. An interruption in our ability to sell and deliver instruments or consumable offerings to customers could occur if we encounter delays or difficulties in securing these components or consumables, or if the quality of the components or consumables supplied do not meet specifications, or if we cannot then obtain an acceptable substitute. In the past, our suppliers have been impacted by global pandemics and epidemics, such as the COVID-19 pandemic, and we have experienced supply delays for critical hardware and instrumentation as a result. If any of these events occur, our business, results of operations, financial condition and prospects could be harmed.
Our internal manufacturing equipment is specialized with limited vendor options and long lead times. If these pieces of equipment were to stop working and be unable to be repaired in a timely manner or at all, our ability to manufacturer our semiconductor chips would be adversely affected.
Our internal manufacturing equipment is specialized with limited vendor options and long lead times. If these pieces of equipment were to stop working and be unable to be repaired in a timely manner or at all, our ability to manufacturer our semiconductor chips could be adversely affected.
In the event it becomes necessary to utilize other equipment for our semiconductor chip manufacturing, we would experience additional costs, delays and difficulties in manufacturing our semiconductor chips, and our business would suffer. There can be no assurance that we would be able to obtain alternative equipment on a timely basis on acceptable terms, if at all. An interruption in our ability to manufacture our semiconductor chips could occur if we encounter delays or difficulties in securing this equipment or if we cannot then obtain an acceptable substitute. If any of these events occur, our business, results of operations, financial condition and prospects could be harmed.
A portion of our revenue is generated through a number of key channel partners, and the loss of any such channel partner could adversely impact our business and our results of operations could suffer.
We are dependent on our channel partners for a portion of our revenue. Our contracts with our channel partners allow them to exercise significant discretion in the placement and promotion of our products, and such contracts do not contain any long-term volume commitments. Further, revenue from our channel partners also depends on a number of factors outside our control and may vary from period to period. If one or more of our channel partners do not effectively market and sell our products or they promote other products over ours, the volume of our products sold to customers could decrease, and our business and results of operations could therefore be significantly harmed.
If we do not successfully develop and maintain our Platinum Analysis Software service, our commercialization efforts and therefore business and results of operations could suffer.
The success of our products depends, in part, on our ability to design and deploy our Platinum Analysis Software service in a manner that enables the integration with potential customers’ systems and accommodates potential customers’ needs. Without our software, the depth of the analysis provided for data generated by our system could be limited and utilization of our products could be hindered.
We have and will continue to spend significant amounts of effort developing our software, and potential enhanced versions over time, to meet our potential customers’ evolving needs. There is no assurance that the development or deployment of our software, or any potential enhancements, will be compelling to our customers. In addition, we may experience delays in our release dates of our software, and there can be no assurance that our software will be released according to schedule. If
our software development and deployment plan does not accurately anticipate customer demands or if we fail to develop our software in a manner that satisfies customer preferences in a timely and cost-effective manner, our products may fail to gain market acceptance.
Commercializing our products outside of the United States could expose us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
Engaging in international business inherently involves a number of difficulties and risks, including:
•required compliance with existing and changing foreign regulatory requirements and laws that are or may be applicable to our business in the future, such as the GDPR and other data privacy requirements, labor and employment regulations, anti-competition regulations, the U.K. Bribery Act of 2010 and other anti-corruption laws, regulations relating to the use of certain hazardous substances or chemicals in commercial products, and require the collection, reuse, and recycling of waste from products we manufacture;
•required compliance with U.S. laws such as the Foreign Corrupt Practices Act, and other U.S. federal laws and regulations established by the Office of Foreign Assets Control of the U.S. Department of the Treasury;
•export requirements and import or trade restrictions;
•laws and business practices favoring local companies;
•foreign currency exchange, longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;
•changes in social, economic, and political conditions or in laws, regulations and policies governing foreign trade, manufacturing, research and development, and investment both domestically as well as in the other countries and jurisdictions in which we operate and into which it may sell our products including as a result of the separation of the United Kingdom from the European Union (“Brexit”);
•potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements, and other trade barriers;
•difficulties and costs of staffing and managing foreign operations; and
•difficulties protecting, maintaining, enforcing or procuring intellectual property rights.
If one or more of these risks occurs, it could require us to dedicate significant resources to remedy such occurrence, and if we are unsuccessful in finding a solution, our financial results will suffer.
We have limited experience producing and supplying our products, and we may be unable to consistently manufacture or source our instruments and consumables to the necessary specifications or in quantities necessary to meet demand on a timely basis and at acceptable performance and cost levels.
Our products provide a solution with many different components that work together. As such, a quality defect in a single component can compromise the performance of the entire solution. In order to successfully generate revenue from our products, we need to supply our customers with products that meet their expectations for quality and functionality in accordance with established specifications on a timely basis. Certain of our products or components of our products are manufactured by a third-party contract manufacturer at our facility using complex processes, sophisticated equipment and strict adherence to specifications and quality systems procedures. Given the complexity of our devices, individual units may occasionally require additional installation and service time prior to becoming available for customer use.
We and our manufacturing partners procure certain components of our instruments and consumables from third-party manufacturers, which includes the commonly-available raw materials needed for manufacturing as well as proprietary components. These manufacturing processes are complex. If we are not able to repeatedly produce our kits at commercial scale or source them from third-party suppliers, or encounter unexpected difficulties in packaging our consumables, our business will be adversely impacted.
As we continue to scale commercially and develop new products, and as our products incorporate increasingly sophisticated technology, it will be increasingly difficult to ensure our products are produced in the necessary quantities without sacrificing quality. There is no assurance that we will be able to continue to manufacture our instruments so that we consistently achieve the product specifications and produce results with acceptable quality. Our kits, semiconductor chips, and other consumables have a limited shelf life, after which their performance is not ensured. Shipment of
consumables that effectively expire early or shipment of defective instruments or consumables to customers may result in recalls and warranty replacements, which would increase our costs, and depending upon current inventory levels and the availability and lead time for additional inventory, could lead to availability issues. Any future design issues, unforeseen manufacturing problems, such as contamination of our or our manufacturers’ facilities, equipment malfunctions, aging components, quality issues with components and materials sourced from third-party suppliers, or failures to strictly follow procedures or meet specifications, may have a material adverse effect on our brand, business, results of operations and financial condition and could result in our or our third-party manufacturers losing International Organization for Standardization (“ISO”) quality management certifications. If our third-party manufacturers fail to maintain ISO quality management certifications, customers might choose not to purchase products from us.
In addition, as we commercialize our Platinum Analysis Software service, we will also need to make corresponding improvements to other operational functions, such as our customer support, service and billing systems, compliance programs and our internal quality assurance programs. As we develop additional products, we may need to bring new equipment online, implement new systems, technology, controls and procedures and hire personnel with different qualifications.
An inability to manufacture products and components that consistently meet specifications, in necessary quantities, at commercially acceptable costs and without significant delays, may have a material adverse effect on our business, results of operations, financial condition and prospects.
We rely on third-party foundries to produce silicon wafers, which when packaged and tested internally, lead to our supply of semiconductor chips. If these third-party foundries should fail or not perform satisfactorily, our ability to supply semiconductor chips would be negatively and adversely affected.
We currently rely on third-party foundries for the production of wafers, and we may not be able to obtain adequate supplies in a timely manner or on commercially reasonable terms. If any of these third parties were not able to supply our wafers, our semiconductor chip supply would be negatively impacted and our business would be harmed.
In the event it becomes necessary to utilize a different third party for the production of wafers, we would experience additional costs and significant delays, including identifying and entering into an agreement with a new foundry partner as well as preparing such new foundry partner to meet the logistical requirements associated with producing our wafers, which would further harm our business.
In addition, if we were to lose such third-party foundries, there can be no assurance that we will be able to identify or enter into agreements with alternative foundries on a timely basis on acceptable terms, if at all. An interruption in our ability to sell and deliver semiconductor chips to customers could occur if we encounter delays or difficulties in securing these wafers, if the quality of the wafers supplied do not meet specifications, or if we cannot then obtain an acceptable substitute. If any of these events occur, our business and operating results could be harmed.
Our products could have defects or errors, which may give rise to claims against us and adversely affect our business, financial condition, results of operations and cash flows.
Our products utilize novel and complex technology and may develop or contain undetected defects or errors. Material performance problems, defects, or errors may arise, and as we commercialize our products, these risks may increase. We expect to provide warranties that our products will meet performance expectations and will be free from defects. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating margins.
In manufacturing our products, we depend upon third parties for the supply of our instruments and various components, many of which require a significant degree of technical expertise to produce. If our suppliers fail to produce our products and components to specification or provide defective products to us and our quality control tests and procedures fail to detect such errors or defects, or if we or our suppliers use defective materials or workmanship in the manufacturing process, the reliability and performance of our products will be compromised.
If our products contain defects, we may experience:
•a failure to achieve market acceptance for our products or expansion of our product sales;
•loss of customer orders and delay in order fulfillment;
•damage to our brand reputation;
•loss of revenue;
•increased warranty and customer service and support costs due to product repair or replacement;
•product recalls or replacements;
•inability to attract new customers;
•diversion of resources from our manufacturing and research and development team into our service team; and
•legal claims against us, including product liability claims, which could be costly and time consuming to defend and result in substantial damages.
The life sciences technology market is highly competitive. If we fail to compete effectively, our business and results of operations will suffer.
We face significant competition in the life sciences technology market. We currently compete with life sciences technology and the diagnostic companies that are supplying components, products and services that serve customers engaged in proteomics analysis. These companies include but are not limited to: Agilent Technologies, Bio-Rad Laboratories, Danaher, Luminex, Merck (and its subsidiary MilliporeSigma) and Thermo Fisher Scientific. We also compete with a number of emerging growth companies that have developed, or are developing, proteomic products and solutions, such as: Nautilus Biotechnology, Olink Proteomics, Quanterix, Seer and Standard BioTools (including its recent acquisition of SomaLogic). Finally, we compete with a number of privately held companies that are developing technology that may compete with our products.
Some of our current competitors are large publicly-traded companies, or are divisions of large publicly-traded companies, and may enjoy a number of competitive advantages over us, including:
•greater name and brand recognition;
•greater financial and human resources;
•broader product lines;
•larger sales forces and more established distributor networks;
•substantial intellectual property portfolios;
•larger and more established customer bases and relationships; and
•better established, larger scale and lower cost manufacturing capabilities.
We also face competition from researchers developing their own products. The area in which we compete involves rapid innovation and some of our customers have in the past, and more may in the future, elect to create their own assays rather than rely on a third-party supplier such as the Company. This is particularly true for the largest research centers and laboratories that are continually testing and trying new technologies, whether from a third-party vendor or developed internally. We will also compete for the resources our customers allocate for purchasing a wide range of products used to analyze the proteome, some of which may be additive to or complementary with our own but not directly competitive.
Our products may not compete favorably, and we may not be successful in the face of increasing competition from products and technologies introduced by our existing or future competitors, companies entering our markets or developed by our customers internally. In addition, our competitors may have or may develop products or technologies that currently or in the future will enable them to produce competitive products with greater capabilities or at lower costs than ours or that are able to run comparable experiments at a lower total experiment cost. Any failure to compete effectively could materially and adversely affect our business, financial condition, results of operations and cash flows.
We are party to Technology and Services Exchange Agreements by and among us and certain affiliated companies, pursuant to which the parties agreed to share personnel and certain non-core technologies. The sharing arrangements under the agreements may prevent us from fully utilizing our personnel and/or the technologies shared under the agreements. Furthermore, if these agreements were to terminate, or if we were to lose access to these technologies and services, our business could be adversely affected.
We have entered into Technology and Services Exchange Agreements (the “TSEAs”) by and among us and other participant companies controlled by the Rothberg family, consisting of Butterfly Network, Inc., OrphAI Therapeutics, Inc.,
Hyperfine, Inc., 4Bionics LLC, identifeye HEALTH Inc. (f/k/a Tesseract Health, Inc.), Liminal Sciences, Inc. and Detect, Inc. The TSEA with Butterfly Network, Inc. was signed in November 2020, and the TSEA with the remaining participant companies was signed in February 2021 and became effective upon the closing of the Business Combination. Under the TSEAs, we and the other participant companies may, in our or their discretion, permit the use of certain non-core technologies, which include any technologies, information or equipment owned or otherwise controlled by the participant company that are not specifically related to the core business area of the participant, such as software, hardware, electronics, fabrication and supplier information, vendor lists and contractor lists, with the other participant companies. The TSEAs provide that ownership of each non-core technology shared by us or another participant company will remain with the company that originally shared the non-core technology. In addition, any participant company (including us) may, in its discretion, permit its personnel to be engaged by another participant company to perform professional, technical or consulting services for such participant. Unless otherwise agreed to by us and the other participant company, all rights, title and interest in and to any inventions, works-of-authorship, idea, data or know-how invented, made, created or developed by the personnel (employees, contractors or consultants) in the course of conducting services for a participant company (“Created IP”) will be owned by the participant company for which the work was performed, and the recipient participant company grants to the party that had its personnel provide the services that resulted in the creation of the Created IP a royalty-free, perpetual, limited, worldwide, non-exclusive, sub-licensable (and with respect to software, sub-licensable in object code only) license to utilize the Created IP only in the core business field of the originating participant company, including a license to create and use derivative works based on the Created IP in the originating participant’s core business field, subject to any agreed upon restrictions.
The technology and personnel-sharing arrangements under the TSEAs may prevent us from fully utilizing our personnel if such personnel are also being used by the other participant companies and may also cause our personnel to enter into agreements with or provide services to other companies that interfere with their obligations to us. Created IP under the TSEAs may be relevant to our business and created by our personnel but owned by the other participant companies. Furthermore, if the TSEAs were to terminate, or if we were to lose access to the technologies and services available pursuant to the TSEAs, our business could be adversely affected.
We may acquire other companies or technologies which could divert our management’s attention, result in additional dilution to our stockholders and otherwise disrupt our operations and harm our operating results.
We may in the future seek to acquire or invest in businesses, applications or technologies that we believe could complement or expand our existing or future products, enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various costs and expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated. We may not be able to identify desirable acquisition targets or be successful in entering into an agreement with any particular target or obtain the expected benefits of any acquisition or investment.
Other than the acquisition of Majelac, to date, the growth of our operations has been organic, and we have limited experience in acquiring other businesses or technologies. We may not be able to successfully integrate acquired personnel, operations and technologies, or effectively manage the combined business following an acquisition. Acquisitions could also result in dilutive issuances of equity securities, the use of our available cash, or the incurrence of debt, which could harm our operating results. In addition, if an acquired business fails to meet our expectations, our operating results, business and financial condition may suffer.
We may seek to enter into strategic collaborations and licensing arrangements with third parties, but we may not be successful in establishing or maintaining such arrangements.
We may seek to enter into strategic collaborations and licensing agreements with third parties to develop products, such as the creation and identification of content and development of new applications. However, there is no assurance that we will be successful in doing so. Establishing collaborations and licensing arrangements is difficult and time-consuming, and discussions may not lead to collaborations or licenses on favorable terms, if at all. Even if we establish such relationships, if our partners do not prioritize and commit sufficient resources to develop and sell products, they may never result in the successful development or commercialization of products.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
As of December 31, 2024, we had federal net operating loss carryforwards (“NOLs”) to offset future taxable income of approximately $388.3 million, of which $65.5 million will begin to expire in 2033 if not utilized. A lack of future taxable
income would adversely affect our ability to utilize these NOLs. In addition, under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change NOLs and other pre-change tax attributes (such as research tax credits) to offset post-change taxable income. For these purposes, an ownership change generally occurs where the equity ownership of one or more stockholders or groups of stockholders who owns at least 5% of a corporation’s stock increases its ownership by more than 50 percentage points over its lowest ownership percentage within a three-year period (calculated on a rolling basis). We have completed an analysis through December 31, 2024 and no such ownership change has occurred. Future changes in our stock ownership, including future offerings, as well as other changes that may be outside of our control, could result in additional ownership changes under Section 382 of the Code. Our NOLs may also be impaired under similar provisions of state law. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future benefits of those assets.
In addition to the limitations discussed above under Sections 382 of the Code, the utilization of NOLs incurred in taxable years beginning after December 31, 2017, are subject to limitations adopted by the Tax Cuts and Jobs Act, as modified by the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”). Under the TCJA, in general, NOLs generated in taxable years beginning after December 31, 2017 may offset no more than 80 percent of such year’s taxable income and there is no ability for such NOLs to be carried back to a prior taxable year. The CARES Act modifies the TCJA with respect to the TCJA’s limitation on the deduction of NOLs and provides that NOLs arising in taxable years beginning after December 31, 2017 and before January 1, 2021, may be carried back to each of the five taxable years preceding the tax year of such loss, but NOLs arising in taxable years beginning after December 31, 2020 may not be carried back. In addition, the CARES Act eliminates the limitation on the deduction of NOLs to 80 percent of current year taxable income for taxable years beginning before January 1, 2021. As a result of such limitation, we may be required to pay federal income tax in some future year notwithstanding that we had a net loss for all years in the aggregate.
If our facilities or our third-party manufacturers’ facilities become unavailable or inoperable, our research and development program and commercialization launch plan could be adversely impacted and manufacturing of our instruments and consumables could be interrupted.
Our Branford, Connecticut facilities primarily house our corporate headquarters as well as research and development activities. In addition, we have a product development and operations facility located in San Diego, California, and a semiconductor chip manufacturing location in Garnet Valley, Pennsylvania. Our instruments are manufactured at our third-party manufacturer’s facilities in the United States and internationally, and our consumables are manufactured at various locations in the United States including at our facilities in San Diego, California and Garnet Valley, Pennsylvania, and internationally.
Our facilities in Branford, Garnet Valley, San Diego and those of our third-party manufacturers are vulnerable to natural disasters, public health crises and catastrophic events. If any disaster, public health crisis or catastrophic event were to occur, our ability to operate our business would be seriously, or potentially completely, impaired. If our facilities or our third-party manufacturer’s facilities become unavailable for any reason, we cannot provide assurances that we will be able to secure alternative manufacturing facilities with the necessary capabilities and equipment on acceptable terms, if at all. We may encounter particular difficulties in replacing our facilities given the specialized equipment housed within them. The inability to manufacture our instruments or consumables, combined with limited inventory of manufactured instruments and consumables, may result in the loss of future customers or harm our reputation, and we may be unable to re-establish relationships with those customers in the future.
If our research and development program or commercialization program were disrupted by a disaster or catastrophe, the launch of new products and the timing of improvements to our products could be significantly delayed and could adversely impact our ability to compete with other available products and solutions. If we or our third-party manufacturer’s capabilities are impaired, we may not be able to manufacture and ship our products in a timely manner, which would adversely impact our business. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
If we experience a significant disruption in our information technology systems, including our Platinum Analysis Software services, or cybersecurity incidents, our business could be adversely affected.
We rely, and will continue to rely on, information technology systems to keep financial and employment records, facilitate our research and development initiatives, manage our manufacturing operations, maintain quality control, fulfill customer
orders, maintain corporate records, communicate with staff, provide our Platinum Analysis Software services and operate other critical functions. Our information technology systems and those of our vendors and partners are potentially vulnerable to disruption due to breakdown, malicious intrusion and computer viruses or other disruptive events, including, but not limited to, natural disasters and catastrophes. Cyberattacks and other malicious internet-based activity continue to increase, and cloud-based platform providers of services have been and are expected to continue to be targeted, especially in the health care industry. Methods of attacks on information technology systems and data security breaches change frequently, are increasingly complex and sophisticated, including deployment of harmful malware and key loggers, ransomware, a malicious website, social engineering and phishing scams, and other means to affect the confidentiality, integrity and availability of our technology systems and data, and can originate from a wide variety of sources. In addition to traditional computer “hackers,” malicious code, such as viruses and worms, denial-of-service attacks and sophisticated nation-state and nation-state supported actors present a constant threat, including advanced persistent threat intrusions. Cyberattacks may also be due to employee error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and our system redundancy and other disaster recovery planning may be ineffective or inadequate in preventing or responding to any of these circumstances. Techniques used in cybersecurity attacks to obtain unauthorized access, disable or sabotage information technology systems are evolving rapidly with data breaches and other cybersecurity incidents becoming commonplace. Despite our efforts to create security barriers to such threats, it is virtually impossible for us to completely mitigate these risks. In August 2020, we discovered ransomware on a server and engaged third-party forensics experts and outside counsel for incident response. We did not pay ransom to the attacker because the documents that were encrypted by the attacker were sufficiently backed up and the investigation further confirmed that no employee data or other personal information was accessed. We implemented a number of security enhancements as the incident unfolded and continue to implement short- and long-term security enhancements to further secure our network.
If our security measures, or those of our vendors and partners, are compromised due to any cybersecurity attacks or incidents, including as a result of third-party action, employee or customer error, malfeasance, stolen or fraudulently obtained log-in credentials, power outages, hardware failures, telecommunication or utility failures, catastrophes, other unforeseen events or otherwise, our reputation could be damaged, our business may be harmed, we could become subject to litigation and we could incur significant expense and liability. If we were to experience a prolonged system disruption in our information technology systems or those of certain of our vendors and partners, it could adversely impact our business and operations, and could result in financial, legal, operational or reputational harm to us, loss of competitive advantage or loss of consumer confidence. If our operations are disrupted, it may cause a material disruption in our business if we do not restore functionality in an acceptable timeframe. In addition, cybersecurity incidents could lead to the loss of trade secrets or other intellectual property, or could lead to the unauthorized access or loss of personal information, including sensitive personal information, of our employees, customers and other third parties, any of which could have a material adverse effect on our business and result in financial, legal, operational or reputational harm to us, loss of competitive advantage or loss of consumer confidence.
In addition, cybersecurity incidents could result in legal claims or proceedings, including class action lawsuits, regulatory investigations or actions, and other types of liability under laws that protect the privacy and security of personal information, including federal, state and foreign data protection and privacy rules and regulations, violations of which could result in material penalties and fines. In addition, although we seek to detect and investigate all cybersecurity incidents, bad actors have become increasingly proficient at operating undetected within an information system, making cybersecurity incidents involving unauthorized access to our information technology systems and data difficult to detect and any delay in identifying such incidents may lead to increased harm and legal exposure of the type described above. Moreover, we may be required to make public announcements regarding any cybersecurity incidents involving the Company and any steps we take to respond to or remediate such incidents, and if securities analysts or investors perceive these announcements to be negative, it could, among other things, have a material adverse effect on the price of our Class A common stock.
The cost of protecting against, investigating, mitigating and responding to potential breaches of information technology systems and cybersecurity incidents and complying with applicable breach notification obligations to individuals, regulators, partners and others can be significant. As cybersecurity incidents and regulatory requirements continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any security vulnerabilities. The inability to implement, maintain and upgrade adequate safeguards could have a material effect on our business, financial condition, results of operations and ability to operate the business as a going concern . While we currently maintain cybersecurity insurance, our insurance policies may not provide adequate coverage to compensate us for the potential costs and other losses arising from such disruptions, failures or security incidents. In addition, such insurance may not be available to us in the future on economically
reasonable terms, or at all, and it is possible that an insurer may deny coverage as to any future claim. The successful assertion of one or more large claims against us that exceed available insurance coverage, or the occurrence of changes in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could have a material adverse effect on our business, and result in financial, legal, operational or reputational harm to us, loss of competitive advantage or loss of consumer confidence.
We could become subject to various litigation claims and legal proceedings.
We, as well as certain of our directors and officers, may become subject to claims or lawsuits during the ordinary course of business. If any such claim or lawsuit was brought, regardless of the outcome, such claim or lawsuit could result in significant legal fees and expenses and could divert management’s time and other resources. If any such claims or lawsuits are successfully asserted against us, or any other party we have agreed to indemnify, we could be liable for damages or reimbursement of damages and be required to alter or cease certain of our business practices. Any of these outcomes could cause our business, financial performance and cash position to be negatively impacted.
Risks Related to Government Regulation
If we elect to label and promote any of our products as clinical diagnostics or medical devices, we would be required to obtain prior marketing authorization from the FDA, which would take significant time and expense and could fail to result in FDA marketing authorization of the device for the intended use or uses we believe are commercially attractive.
Our protein sequencing products are currently labeled, promoted, and sold primarily to academic and research institutions and research companies as RUO products. They are not currently designed, or intended to be used, for clinical diagnostic purposes or as medical devices. If we elect to label and market our products for use as, or in the performance of, clinical diagnostics in the United States, thereby subjecting them to FDA regulation as medical devices, we would be required to obtain pre-market authorization from the FDA, unless an exception applies.
In the future, if we choose to develop and market our products for clinical or diagnostic uses in the United States, we will be required to comply with FDA’s regulations for in vitro diagnostic (“IVD”) medical devices. Complying with FDA’s medical device regulations may be expensive, time-consuming, and subject us to significant and/or unanticipated delays. There can be no guarantee that we will be able to obtain the appropriate marketing authorization for our protein sequencing products that may be developed for clinical or diagnostic intended uses in the future.
We may in the future register with the FDA as a specification developer and list some of our ancillary products with the FDA as Class I general purpose laboratory equipment, subjecting us to ongoing inspections by the FDA. While this regulatory classification is exempt from certain FDA requirements, such as the need to submit a pre-market notification commonly known as a 510(k), and some of the requirements of the FDA’s QSR, those device products would be subject to mandatory general controls that apply to all classes of medical devices. In addition to establishment registration, device listing and compliance with applicable QSR, general controls include compliance with FDA regulations for labeling, reporting adverse events or malfunctions for the products, and general prohibitions against misbranding and adulteration.
There can be no assurance that future products for which we may seek pre-market clearance or approval will be approved or cleared by FDA or a comparable foreign regulatory authority on a timely basis, if at all, nor can there be assurance that labeling claims will be consistent with our anticipated claims or adequate to support continued adoption of such products. Compliance with FDA or comparable foreign regulatory authority regulations will require substantial costs, and subject us to heightened scrutiny by regulators and substantial penalties for failure to comply with such requirements or the inability to market our products. The lengthy and unpredictable pre-market clearance or approval process, as well as the unpredictability of the results of any required clinical studies, may result in us failing to obtain regulatory clearance or approval to market such products, which would significantly harm our business, results of operations, reputation, and prospects.
If we sought and received regulatory marketing authorization for certain of our protein sequencing products, we would be subject to ongoing FDA obligations and continued regulatory oversight and review, including the general controls listed above. In addition, we could be required to obtain a new clearance or approval before we could introduce subsequent modifications or improvements to such products. We could also be subject to additional FDA post-marketing obligations for such products, any or all of which would increase our costs and divert resources away from other projects. If we sought and received regulatory clearance or approval and are not able to maintain regulatory compliance with applicable laws, we could be prohibited from marketing our products for use as, or in the performance of, clinical diagnostics and/or could be
subject to enforcement actions, including Warning Letters and adverse publicity, fines, injunctions, and civil penalties; recall or seizure of products; operating restrictions; and criminal prosecution.
In addition, we could decide to seek regulatory clearance or approval for certain of our future clinical diagnostic products in countries outside of the United States. Sales of such products outside the United States will likely be subject to foreign regulatory requirements, which can vary greatly from country to country. As a result, the time required to obtain clearances or approvals outside the United States may differ from that required to obtain FDA marketing authorization and we may not be able to obtain foreign regulatory approvals on a timely basis or at all. In Europe, we would need to comply with the new Medical Device Regulation 2017/745 and In Vitro Diagnostic Regulation 2017/746, which became effective May 26, 2017, with application dates of May 26, 2021 (postponed from 2020) and May 26, 2022, respectively. This will increase the difficulty of regulatory approvals in Europe in the future. In addition, the FDA regulates exports of medical devices. Failure to comply with these regulatory requirements or obtain and maintain required approvals, clearances and certifications could impair our ability to commercialize our products for diagnostic use outside of the United States.
Our research use only (“RUO”) products could become subject to government regulation as medical devices by the U.S. Food and Drug Administration (“FDA”) and other regulatory agencies even if we do not elect to seek regulatory authorization to market our products for diagnostic purposes, which would adversely impact our ability to market and sell our products and harm our business.
Although our current protein sequencing products are labeled, promoted, and sold as RUO products that are therefore not regulated as IVD medical devices, the FDA or comparable agencies of other countries could disagree with our conclusion that our products are intended for RUO or deem our sales, marketing and promotional efforts as being inconsistent with the criteria for RUO products. For example, our customers may independently elect to use our RUO labeled products in their own lab-developed tests (“LDTs”) for clinical diagnostic uses, which could subject our products to government regulation, and regulatory requirements related to marketing, selling, and distribution of RUO products could change or be uncertain, even if clinical uses of our RUO products by our customers were done without our consent. FDA reviews the totality of the circumstances when it comes to evaluating whether equipment and testing components are properly labeled as RUO and takes the position that merely including a labeling statement that the product is for research purposes only will not necessarily render the device exempt from the FDA’s device regulatory requirements if the circumstances surrounding the distribution, marketing and promotional practices indicate that the manufacturer knows its products are, or intends for its products to be, used for clinical diagnostic purposes. If the FDA or other regulatory authorities assert that any of our RUO products are subject to regulatory clearance or approval, our business, financial condition, results of operations or cash flows could be adversely affected.
For a number of years, the FDA has exercised its regulatory enforcement discretion not to regulate LDTs as medical devices if the tests are created and used within a single laboratory. However, in October 2023, the FDA issued a proposed rule aimed at regulating LDTs under the current medical device framework and proposing to phase out its existing enforcement discretion policy for this category of diagnostic tests; the public comment period ended in early December 2023. The proposal envisions that the LDT enforcement policy phase-out process would occur in gradual stages over a total period of four years, although more details are expected to be provided with the upcoming final rule. The likelihood of the FDA finalizing the proposed rule in April 2024 (as currently projected), as well as potential litigation challenging the agency’s authority to take such action, is uncertain at this time. Affected entities continue to press for a comprehensive legislative solution instead of implementation of the proposed FDA administrative action, which may be disruptive to the industry and to patient access to certain diagnostic tests, and litigation against FDA is expected to be initiated following issuance of the final rule. Separately, federal legislators have been working with stakeholders for several years on a possible bill to reform the regulation of in vitro clinical tests including LDTs. For example, as drafted and re-introduced for consideration by the current Congress, the Verifying Accurate, Leading-edge IVCT Development Act (“VALID Act”) would codify into law the term “in vitro clinical test” (“IVCT”) to create new medical product category separate from medical devices that includes products currently regulated as IVDs as well as LDTs. The VALID Act would also create a new system for labs and hospitals to use to submit their tests electronically to the FDA for approval, which is aimed at reducing the amount of time it takes for the agency to approve such tests, and establish a new program to expedite the development of diagnostic tests that can be used to address a current unmet need for patients.
Any legislative or administrative rule making or new federal oversight of LDTs, if and when finalized, may impact the sales of our products and how customers use our products, and may require us to change our business model in order to maintain compliance with these laws. We cannot predict how these various efforts will be resolved, how Congress or the FDA will regulate LDTs in the future, or how that modernized regulatory system will impact our business. Changes to the current regulatory framework, including the imposition of additional or new regulations, including regulation of our
products, could arise at any time during the development or marketing of our products, which may negatively affect our ability to obtain or maintain FDA or comparable regulatory approval of our products, if required. Further, sales of devices for diagnostic purposes may subject us to additional healthcare regulation and enforcement by the applicable government agencies. Such laws include, without limitation, state and federal anti-kickback or anti-referral laws, healthcare fraud and abuse laws, false claims laws, the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), the Physician Payments Sunshine Act and related transparency and manufacturer reporting laws, and other laws and regulations applicable to medical device manufacturers.
Our reagents may be used by clinical laboratories to create Laboratory-Developed Tests (“LDTs”), which could, in the future, become subject to some form of FDA or other regulatory requirements, which could materially and adversely affect our business and results of operations.
We may in the future register with the FDA as a specification developer and list ancillary products such as customized reagents with the FDA as Class I general purpose laboratory equipment and reagents. A clinical laboratory could potentially use our custom-manufactured reagents to create what is called an LDT. LDTs are diagnostic tests that are developed, validated and performed by a single clinical laboratory operating in compliance with the Clinical Laboratory Improvement Amendments (“CLIA”), and under the oversight of the Centers for Medicare & Medicaid Services (“CMS”). Historically, FDA has generally exercised enforcement discretion not to regulate LDTs as medical devices, although as discussed above in October 2023 it issued a proposed rule to regulate LDTs under the current medical device framework and phase out its existing enforcement discretion policy for LDTs. The FDA initiated this rulemaking following several years of inaction by Congress and in light of public health concerns the agency perceives to exist with certain marketed LDTs, in part as a result of the growth in the volume and complexity of testing services utilizing LDTs, such as genetic testing services over the past four decades Congress also continues to face pressure from stakeholders to enact a comprehensive legislative solution to create a harmonized paradigm for oversight of LDTs by both the FDA and CMS. Any legislative or administrative rule making or new federal oversight of LDTs, if and when finalized, could decrease demand for our reagents by affecting how customers can use those products. Additionally, compliance with additional regulatory burdens could be time consuming and costly for our customers. We cannot predict how these various efforts will be resolved, how Congress or the FDA will regulate LDTs in the future, or how that modernized regulatory system will impact our business.
Further, the FDA may disagree that such products are Class 1 medical devices and require us to obtain pre-market clearance or approval before we can continue to sell our reagent products to certain customers.
We may be subject to certain federal, state and foreign fraud and abuse laws, health information privacy and security laws and physician payment transparency laws, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
There are numerous U.S. federal and state, as well as foreign, laws pertaining to healthcare fraud and abuse, including anti-kickback, false claims and physician transparency laws. Our business practices and relationships with providers and hospitals are subject to scrutiny under these laws. We may also be subject to patient information privacy and security regulation by both the federal government and the states and foreign jurisdictions in which we conduct our business. The healthcare laws and regulations of concern as we develop and begin to commercialize products include:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual or furnishing or arranging for a good or service, for which payment may be made, in whole or in part, under federal healthcare programs, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation;
•the federal civil and criminal false claims laws, including the federal civil False Claims Act, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other federal healthcare programs that are false or fraudulent. Private individuals can bring False Claims Act “qui tam” actions, on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in amounts paid by the entity to the government in fines or settlement.
•the federal Civil Monetary Penalties Law, which prohibits, among other things, offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the
beneficiary’s decision to order or receive items or services reimbursable by the government from a particular provider or supplier;
•HIPAA, which created additional federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
•the federal Physician Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, to report annually to CMS, information related to payments and other transfers of value to physicians (defined broadly to include doctors, dentists, optometrists, podiatrists and chiropractors), teaching hospitals and certain advanced non-physician healthcare practitioners, as well as ownership interests held by physicians and their immediate family members; and
•analogous state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers or patients.
These laws and regulations, among other things, constrain our business, marketing and other promotional activities by limiting the kinds of financial arrangements we may have with hospitals, physicians or other developers or potential purchasers of our products.
If our operations are found to be in violation of any of the healthcare laws or regulations described above or any other healthcare regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, contractual damages, reputational harm, disgorgement and the curtailment or restructuring of our operations.
In addition, members of our management and companies with which they are affiliated or have been affiliated with in the past, have been, and may in the future be, involved in investigations, prosecutions, convictions or settlements in the healthcare industry. For example, Kevin Rakin, a member of our board of directors (the “Board”), was named as a defendant in United States ex rel. Webb v. Advanced BioHealing, Inc. (“ABH”), a whistleblower suit relating to sales methods employed by sales representatives of ABH, a biotechnology company for which Mr. Rakin served as its chief executive officer. All claims in the lawsuit were dismissed with prejudice pursuant to a settlement agreement, in which Mr. Rakin expressly denied that he engaged in any wrongful conduct, and Mr. Rakin agreed to pay to the United States $2.5 million. Any investigations, prosecutions, convictions or settlements involving members of our management and companies with which they are or have been affiliated may be detrimental to our reputation and could negatively affect our business, financial condition, results of operations and cash flows.
We are currently subject to, and may in the future become subject to, both U.S. federal and state laws and regulations as well as international laws imposing obligations on how we collect, store and process personal information. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our business and future customer base, and thereby decrease our revenue.
In the ordinary course of our business, we currently, and in the future will, collect, store, transfer, use or process sensitive data, including personally identifiable information of employees. The secure processing, storage, maintenance, and transmission of this critical information are vital to our operations and business strategy. We are, and may increasingly become, subject to various laws and regulations, as well as contractual obligations, relating to data privacy and security in the jurisdictions in which we operate. The regulatory environment related to data privacy and security is increasingly rigorous, with new and constantly changing requirements applicable to our business, and enforcement practices are likely to remain uncertain for the foreseeable future. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible that they will be interpreted and applied in ways that may have a material adverse effect on our business, financial condition, results of operations and prospects.
In the United States, various federal and state regulators, including governmental agencies like the Consumer Financial Protection Bureau and the Federal Trade Commission, have adopted, or are considering adopting, laws and regulations concerning personal information and data security. Certain state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to personal information than federal, international or other state laws, and such laws may differ from each other, all of which complicates compliance efforts. For example, the California Consumer Privacy Act (“CCPA”), which increases privacy rights for California residents and imposes obligations on companies that process
their personal information, came into effect on January 1, 2020. Among other things, the CCPA requires covered companies to provide disclosures regarding information practices to California consumers and provide such consumers new data protection and privacy rights, including the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information. This private right of action may increase the likelihood of, and risks associated with, data breach litigation. Additionally, the California Privacy Rights Act (“CPRA”), was approved by California voters in the election of November 3, 2020 and went into effect in January of 2023 modifying the CCPA significantly, potentially resulting in further uncertainty and requiring us to incur additional costs and expenses in an effort to comply. In addition, similar laws and regulations in other U.S. states, such as Colorado, Connecticut, New Jersey, Delaware, Utah, Virginia, Oregon, Indiana, Iowa, Tennessee, Montana, Florida and Texas and other international jurisdictions have been applied to protect individuals’ privacy (including laws regarding unfair and deceptive practices in the United States and GDPR in the European Union) and may be subject to evolving interpretations or applications. Furthermore, defending a suit for the wrongful use or disclosure of health or personal information, regardless of its merit, could be costly, divert management’s attention and harm our reputation. Laws in all 50 U.S. states require businesses to provide notice to consumers whose personal information has been disclosed as a result of a data breach. State laws are changing rapidly and there is discussion in the U.S. Congress of a new comprehensive federal data privacy law to which we would become subject if it is enacted.
At the federal level, regulations promulgated pursuant to HIPAA as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”) establish privacy and security standards that limit the use and disclosure of individually identifiable health information (known as “protected health information” when protected under HIPAA) and require the implementation of administrative, physical and technological safeguards to protect the privacy and security of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. Determining HIPAA applicability to our operations as our operations evolve, obligations under applicable privacy standards and our contractual obligations may require complex factual and regulatory analyses and may be subject to differing or changing interpretations. Although we take measures to protect sensitive data from unauthorized access, use or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or accessed due to employee error, malfeasance or other malicious or inadvertent disruptions. Any such breach, incident or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, manipulated, acquired, publicly disclosed, lost or stolen. Any such access or other loss of information could result in legal claims or proceedings, and liability for us or our customers under international or U.S. federal or state laws that protect the privacy of health information, such as HIPAA, and regulatory penalties. Notice of certain incidents may be required to be provided to affected individuals, the Secretary of the Department of Health and Human Services, and for extensive breaches, notice may also need to be made to the media. Additionally, state law may require notice to the applicable state Attorney General. Such notices could result in financial, legal, operational or reputational harm to us, loss of competitive advantage or loss of consumer confidence.
We continue to evaluate our compliance obligations, but do not currently have in place formal policies and procedures related to the storage, collection and processing of information, and have not conducted any internal or external data privacy audits, to ensure our compliance with all applicable data protection laws and regulations. Additionally, we do not currently have policies and procedures in place for assessing our third-party vendors’ compliance with applicable data protection laws and regulations. All of these evolving compliance and operational requirements impose significant costs, such as costs related to organizational changes, implementing additional protection technologies, training employees and engaging consultants, which are likely to increase over time. In addition, such requirements may require us to modify our data processing and cybersecurity practices and any policies that we have implemented, distract management or divert resources from other initiatives and projects, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Any failure or perceived failure by us or our third-party vendors, collaborators, contractors and consultants to comply with any applicable federal, state or international laws and regulations relating to data privacy and security, could result in damage to our reputation, as well as proceedings or litigation by governmental agencies or other third parties, including class action litigation in certain jurisdictions, which would subject us to significant expense, as well as potential fines, sanctions, awards, penalties or judgments, all of which could have a material adverse effect on our business, financial condition, reputation, results of operations and prospects.
We could be adversely affected by alleged violations of the Federal Trade Commission Act or other truth-in-advertising and consumer protection laws.
Our advertising for current and future products is subject to federal truth-in-advertising laws enforced by the Federal Trade Commission (“FTC”), as well as comparable state consumer protection laws. Under the Federal Trade Commission Act (“FTC Act”), the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or
deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which we would be able to market services or products in the future, or criminal prosecution. In the context of performance claims for products such as our goods and services, compliance with the FTC Act includes ensuring that there is scientific data to substantiate the claims being made, that the advertising is neither false nor misleading, and that any user testimonials or endorsements we or our agents disseminate related to the goods or services comply with disclosure and other regulatory requirements. Any actual or perceived non-compliance with those laws could lead to an investigation by the FTC or a comparable state agency or could lead to allegations of misleading advertising by private plaintiffs. Any such action against us could disrupt our business operations, cause damage to our reputation, and result in material adverse effects on our business.
In addition, with respect to any of our future products that are marketed as in vitro diagnostic or clinical products, FDA’s regulations applicable to medical device products prohibit them from being promoted for uses not within the scope of a given product’s intended use(s), among other promotional and labeling rules applicable to products subject to the Federal Food, Drug, and Cosmetic Act (“FDCA”).
Medical product manufacturers’ use of social media platforms presents new risks.
Our potential customer base for future clinical diagnostic applications of our protein sequencing technologies may be active on social media. We intend to engage through those platforms to elevate our national marketing presence, both for our RUO product offerings and any future medical device product offerings. Social media practices in the medical device and biopharmaceutical industries are evolving, which creates uncertainty and risk of non-compliance with regulations applicable to our business. For example, there is a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us or our products on any social networking website. If these events were to occur or we otherwise fail to comply with any applicable regulations, we could incur liability, face restrictive regulatory actions or experience other harm to our business.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain and enforce sufficient intellectual property protection for our products and technology, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products may be impaired.
We rely on patent protection as well as trademark, copyright, trade secret and other intellectual property right protection and contractual restrictions to protect our proprietary products and technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. If we fail to obtain, maintain and sufficiently enforce our intellectual property, third parties may be able to compete more effectively against us. In addition, we may incur substantial litigation costs in our attempts to recover damages or restrict use of our intellectual property.
To the extent our intellectual property offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct competition. If our intellectual property does not provide adequate coverage against our competitors’ products, our competitive position could be adversely affected, as could our business, financial condition, results of operations and prospects. Both the patent application process and the process of managing patent and other intellectual property disputes can be time-consuming and expensive.
Our success depends in large part on our and our licensors’ ability to obtain and maintain protection of the intellectual property we may own solely or jointly with, or license from, third parties, particularly patents, in the United States and other countries directed to our products and technologies. We apply for patents covering our products and technologies and uses thereof, as we deem appropriate. However, obtaining and enforcing patents is costly, time-consuming and complex, and we may fail to apply for patents on important products and technologies in a timely fashion or at all, or we may fail to apply for patents in potentially relevant jurisdictions. We may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents that may be issued from such patent applications, at a reasonable cost or in a timely manner or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, we may not develop
additional proprietary products, methods and technologies that are patentable. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed from or to third parties. Therefore, these patents and applications may not be prosecuted, obtained and enforced by such third parties in a manner consistent with the best interests of our business.
In addition, the patent position of life sciences technology companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries or regions may diminish the value of our intellectual property. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights presents a reasonably limited degree of uncertainty. It is possible that some of our pending patent applications will not result in issued patents in a timely fashion or at all, and even if patents are granted, they may not provide a basis for intellectual property protection of commercially viable products or services, may not provide any competitive advantages, or may be challenged, narrowed and/or invalidated by third parties. There exists some degree of uncertainty over the breadth of claims that may be allowed or enforced in our patents or in third-party patents. It is possible that third parties will attempt to design around our current or future patents such that we cannot prevent such third parties from using similar technologies and commercializing similar products to compete with us. Some of our owned or licensed patents or patent applications may be challenged at a future point in time and we may not be successful in defending any such challenges made against our patents or patent applications. Any successful third-party challenge to our patents could result in the narrowing, unenforceability or invalidity of such patents and increased competition to our business. The outcome of patent litigation or other proceedings can be uncertain, and any attempt by us to enforce our patent rights against others or to challenge the patent rights of others may not be successful, or, regardless of success, may take substantial time and result in substantial cost, and may divert our efforts and attention from other aspects of our business. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and cash flows.
The U.S. law relating to the patentability of certain inventions in the life sciences technology industry is uncertain and rapidly changing, which may adversely impact our existing patents or our ability to obtain patents in the future.
Changes in either the patent laws or interpretation of the patent laws in the United States or in other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. For instance, under the Leahy-Smith America Invents Act (the “America Invents Act”), enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application is entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. These changes include allowing third-party submission of prior art to the United States Patent and Trademark Office (“USPTO”) during patent prosecution and additional procedures to challenge the validity of a patent through USPTO administered post-grant proceedings, including post-grant review, inter partes review and derivation proceedings. The America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Various courts, including the U.S. Supreme Court, have rendered decisions that impact the scope of patentability of certain inventions or discoveries relating to life sciences technology. Specifically, these decisions stand for the proposition that patent claims that recite laws of nature are not themselves patentable unless those patent claims have sufficient additional features that provide practical assurance that the processes are genuine inventive applications of those laws rather than patent drafting efforts designed to monopolize the law of nature itself. What constitutes a “sufficient” additional feature is somewhat uncertain. Furthermore, in view of these decisions, since December 2014, the USPTO has published and continues to publish revised guidelines for patent examiners to apply when examining process claims for patent eligibility.
In addition, U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to some degree of uncertainty with regard to our ability to obtain patents in the future, this combination of events has created a degree of uncertainty with respect to the value of patents, once obtained. Depending on relevant laws enacted by the U.S. Congress, and decisions by the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that may have a material adverse effect on our ability to obtain new patents and to defend and enforce our existing patents and patents that we might obtain in the future.
Our patent portfolio may be negatively impacted by current uncertainties in the state of the law, new court rulings or changes in guidance or procedures issued by the USPTO or other similar patent offices around the world. From time to
time, the U.S. Supreme Court, other federal courts, the U.S. Congress or the USPTO may change the standards of patentability, scope and validity of patents within the life sciences technology and any such changes, or any similar adverse changes in the patent laws of other jurisdictions, could have a negative impact on our business, financial condition, prospects and results of operations.
We may not be able to protect our intellectual property rights throughout the world.
The laws of some foreign countries do not offer intellectual property rights to the same extent as the laws of the United States, and we and our licensors may encounter difficulties in obtaining, enforcing and defending such rights in foreign jurisdictions. Consequently, we and our licensors may not be able to prevent third parties from practicing our or our licensors’ inventions in some or all countries outside the United States, or from selling or importing products made using our or our licensors’ inventions in other jurisdictions. Competitors and other third parties may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and technologies and may also export infringing products to territories where we have patent protection, but enforcement practices or laws are not as strong as those in the United States. These products may compete with our products. We and our licensors’ patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. In addition, certain countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to other parties. Furthermore, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of any patents.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain other countries are not as favorable as the United States in the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the misappropriation or other violations of our intellectual property rights including infringement of our patents in such countries. The legal systems in certain countries may also favor state-sponsored entities or companies headquartered in particular jurisdictions over our first-in-time patents and other intellectual property protection. The absence of harmonized intellectual property protection laws and effective enforcement makes it difficult to ensure consistent respect for patents, trade secrets, and other intellectual property rights on a worldwide basis. As a result, it is possible that we will not be able to enforce our rights against third parties that misappropriate our proprietary technology in those countries.
Proceedings to enforce our or our licensors’ patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business, could put us and our licensors’ patents at risk of being invalidated or interpreted narrowly and our licensors’ patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We and our licensors may not prevail in any lawsuits that we or our licensors initiate, or that are initiated against us or our licensors, and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our products, services and other technologies and the enforcement of intellectual property. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
Issued patents covering our products could be found invalid or unenforceable if challenged.
Our owned and licensed patents and patent applications may be subject to validity, enforceability and priority disputes. The issuance of a patent is not conclusive as to our inventorship, scope, validity or enforceability. Some of our patents or patent applications (including licensed patents and patent applications) may be challenged at a future point in time in opposition, derivation, reexamination, inter partes review, post-grant review or interference or other similar proceedings. Any successful third-party challenge to our patents in this or any other proceeding could result in the unenforceability or invalidity of such patents, which may lead to increased competition to our business, which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, if we or our licensors initiate legal proceedings against a third party to enforce a patent covering our products, the defendant could counterclaim that such patent covering our products, as applicable, is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. There are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent intentionally
withheld relevant information from the relevant patent office, or knowingly made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include ex parte re-examination, inter partes review, post-grant review, derivation and equivalent proceedings in non-U.S. jurisdictions, such as opposition proceedings. Such proceedings could result in revocation of or amendment to our patents in such a way that they no longer cover and protect our products. With respect to the validity of our patents, for example, we cannot be certain that there is no invalidating prior art of which we, our licensors, our patent counsel and the patent examiner were unaware during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. If a defendant or other third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on certain aspects of our products and technologies, which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license intellectual property or develop or commercialize current or future products.
We may not be aware of all third-party intellectual property rights potentially relating to our products. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until approximately 18 months after filing or, in some cases, not until such patent applications issue as patents. We might not have been the first to make the inventions covered by each of our pending patent applications and we might not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings, derivation proceedings or other post-grant proceedings declared by the USPTO, or other similar proceedings in non-U.S. jurisdictions that could result in substantial cost to us and the loss of valuable patent protection. The outcome of such proceedings is uncertain. No assurance can be given that other patent applications will not have priority over our patent applications. In addition, changes to the patent laws of the United States allow for various post-grant opposition proceedings that have not been extensively tested, and their outcome is therefore uncertain. Furthermore, if third parties bring these proceedings against our patents, regardless of the merit of such proceedings and regardless of whether they are successful, we could experience significant costs and our management may be distracted. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected, and our business could be harmed.
We rely heavily on trade secrets and confidentiality agreements to protect our unpatented know-how, technology and other proprietary information, and to maintain our competitive position. However, trade secrets and know-how can be difficult to protect. In particular, we anticipate that with respect to our technologies, these trade secrets and know-how will over time be disseminated within the industry through independent development, the publication of journal articles describing the methodology, and the movement of personnel from academic to industry scientific positions.
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into agreements, including confidentiality agreements, non-disclosure agreements and intellectual property assignment agreements, with our employees, consultants, academic institutions, corporate partners and, when needed, our advisers. However, we cannot be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors or other third parties will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure, which could adversely impact our ability to establish or maintain a competitive advantage in the market, and our business, financial condition, results of operations and prospects.
Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had wrongfully obtained and was using our trade secrets, it would be expensive and time-consuming, it could distract our personnel, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
We also seek to preserve the integrity and confidentiality of our confidential proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is
possible that these security measures could be breached. If any of our confidential proprietary information were to be lawfully obtained or independently developed by a competitor or other third party, absent patent protection, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. Competitors or third parties could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts, design around our protected technology, develop their own competitive technologies that fall outside the scope of our intellectual property rights or independently develop our technologies without reference to our trade secrets. If any of our trade secrets were to be disclosed to or independently discovered by a competitor or other third party, it could materially and adversely affect our business, financial condition, results of operations and prospects.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest in our owned or in-licensed patents, trade secrets or other intellectual property as an inventor or co-inventor. For example, we or our licensors may have inventorship disputes arise from alleged inventors such as employees, consultants or others who are involved in developing our products, some of whom may have conflicting IP ownership obligations. In addition, counterparties to our consulting, sponsored research, software development and other agreements may assert that they have an ownership interest in intellectual property developed under such arrangements. In particular, certain software development agreements pursuant to which certain third parties have developed parts of our proprietary software may not include provisions that expressly assign to us ownership of all intellectual property developed for us by such third parties. Furthermore, certain of our sponsored research agreements pursuant to which we provide certain research services for third parties do not assign to us all intellectual property developed under such agreements. As such, we may not have the right to use all such developed intellectual property under such agreements, we may be required to obtain licenses from third parties and such licenses may not be available on commercially reasonable terms or at all, or may be non-exclusive. If we are unable to obtain such licenses and such licenses are necessary for the development, manufacture and commercialization of our products and technologies, we may need to cease the development, manufacture and commercialization of our products and technologies. Litigation may be necessary to defend against these and other claims challenging inventorship of our or our licensors’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. In such an event, we may be required to obtain licenses from third parties and such licenses may not be available on commercially reasonable terms or at all, or may be non-exclusive. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture and commercialization of our products and technologies. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees, and certain customers or partners may defer engaging with us until the particular dispute is resolved. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may not be able to protect and enforce our trademarks and trade names, or build name recognition in our markets of interest thereby harming our competitive position.
The registered or unregistered trademarks or trade names that we own may be challenged, infringed, circumvented, declared generic, lapsed or determined to be infringing on or dilutive of other marks. We may not be able to protect our rights in these trademarks and trade names, which we need in order to build name recognition. In addition, third parties have filed, and may in the future file, for registration of trademarks similar or identical to our trademarks, thereby impeding our ability to build brand identity and possibly leading to market confusion. If such third parties were to succeed in registering or developing common law rights in any other trademarks that are similar or identical to our trademarks, and if we are not successful in challenging such rights and defending against challenges to our trademarks, we may not be able to use such trademarks to develop brand recognition of our technologies, products or services. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Further, we have and may in the future enter into agreements with owners of such third-party trade names or trademarks to avoid potential trademark litigation which may limit our ability to use our trade names or trademarks in certain fields of business. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business, financial condition, results of operations and prospects may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Patent terms may be inadequate to protect our competitive position on our products for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a utility patent is generally 20 years from its earliest U.S. non-provisional filing date. While extensions may be available, the life of a patent, and the protection it affords, is limited. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent or may be shortened if a patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. Even if patents covering our products are obtained, once the patent life has expired, we may be open to additional competition from competitive products. If one of our products requires extended development, testing and/or regulatory review, patents protecting such products might expire before or shortly after such products are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to our products, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed to us alleged trade secrets of their other clients or former employers, which could subject us to costly litigation.
As is common in the life sciences industry, we engage the services of consultants and independent contractors to assist us in the development of our products. Many of these consultants and independent contractors were previously employed at, or may have previously or may be currently providing consulting or other services to, universities or other technology, biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may become subject to claims that we, a consultant or an independent contractor inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. We may similarly be subject to claims stemming from similar actions of an employee, such as one who was previously employed by another company, including a competitor or potential competitor. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management team. If we are not successful, we could lose access or exclusive access to valuable intellectual property.
We may become involved in lawsuits to defend against third-party claims of infringement, misappropriation or other violations of intellectual property or to protect or enforce our intellectual property, any of which could be expensive, time consuming and unsuccessful, and may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our ability and the ability of future collaborators to develop, manufacture, market and sell our products and use our products and technologies without infringing, misappropriating or otherwise violating the intellectual property rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the life sciences technology sector, as well as administrative proceedings for challenging patents, including interference, derivation, inter partes review, post grant review, and reexamination proceedings before the USPTO, or oppositions and other comparable proceedings in foreign jurisdictions. We may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our products, manufacturing methods, software and/or technologies infringe, misappropriate or otherwise violate their intellectual property rights. Numerous issued patents and pending patent applications that are owned by third parties exist in the fields in which we are developing our products and technologies. It is not always clear to industry participants, including us, the claim scope that may be issued from pending patent applications owned by third parties or which patents cover various types of products, technologies or their methods of use or manufacture. Thus, because of the large number of patents issued and patent applications filed in our fields, there may be a risk that third parties, including our competitors, may allege they have patent rights encompassing our products, technologies or methods and that we are employing their proprietary technology without authorization.
If third parties, including our competitors, believe that our products or technologies infringe, misappropriate or otherwise violate their intellectual property, such third parties may seek to enforce against us their intellectual property, including patents, by filing against us an intellectual property-related lawsuit, including a patent infringement lawsuit. Even if we believe third-party intellectual property claims are without merit, there is no assurance that a court would find in our favor on questions of infringement, validity, enforceability, or priority. If any third parties were to assert these or any other patents against us and we are unable to successfully defend against any such assertions, we may be required, including by court order, to cease the development and commercialization of the infringing products or technology and we may be required to redesign such products and technologies so they do not infringe such patents, which may not be possible or may require substantial monetary expenditures and time. We could also be required to pay damages, which could be significant, including treble damages and attorneys’ fees if we are found to have willfully infringed such patents. We could also be
required to obtain a license to such patents in order to continue the development and commercialization of the infringing product or technology. However, such a license may not be available on commercially reasonable terms or at all, including because certain of these patents may be held by or exclusively licensed to our competitors. Even if such license is available, it may require substantial payments or cross-licenses under our intellectual property rights, and it may only be available on a nonexclusive basis, in which case third parties, including our competitors, could use the same licensed intellectual property to compete with us. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations or prospects.
We may choose to challenge, including in connection with any allegation of patent infringement by a third party, the patentability, validity, ownership or enforceability of any third-party patent that we believe may have applicability in our field, and any other third-party patent that may at some future time possibly be asserted against us. Such challenges may be brought either in court or by requesting that the USPTO, European Patent Office (“EPO”), or other foreign patent offices review the patent claims, such as in an ex-parte reexamination, inter partes review, post-grant review proceeding or opposition proceeding. However, there can be no assurance that any such challenge by us or any third party will be successful. Even if such proceedings are successful, these proceedings are expensive and may consume our time or other resources, distract our management and technical personnel, and the costs of these opposition proceedings could be substantial. There can be no assurance that our defenses of non-infringement, invalidity or unenforceability will succeed.
Third parties, including our competitors, could be infringing, misappropriating or otherwise violating our solely owned and/or in-licensed intellectual property rights. Monitoring unauthorized use of intellectual property is difficult and costly. We may not be able to detect unauthorized use of, or take appropriate steps to enforce, our intellectual property rights. From time to time, we seek to analyze our competitors’ products and services, and may in the future seek to enforce our rights based on potential infringement, misappropriation or violation of our intellectual property. However, the steps we will take to protect our intellectual property rights may not be adequate to enforce our rights as against such infringement, misappropriation or violation of our intellectual property. Any inability to meaningfully enforce our intellectual property rights could harm our ability to compete and reduce demand for our products and technologies.
Litigation proceedings may be necessary for us to enforce our patent and other intellectual property rights. In any such proceeding, a court may refuse to stop the other party from using the technology at issue on the grounds that our owned and in-licensed patents do not cover the technology in question. Further, in such a proceeding, the defendant could counterclaim that our intellectual property is invalid or unenforceable and the court may agree, in which case we could lose valuable intellectual property rights, which could allow third parties to commercialize technology or products similar to ours and compete directly with us, without payment to us. Alternatively or additionally, such proceeding could result in requiring us to obtain license rights from the prevailing party in order to be able to manufacture or commercialize our products without infringing such party’s intellectual property rights, and if we are unable to obtain such a license, we may be required to cease commercialization of our products and technologies, any of which could have a material adverse effect on our business, financial condition, results of operations and prospects. The outcome in any such proceeding is unpredictable.
Regardless of whether we are defending against or asserting an intellectual property-related claim in an intellectual property-related proceeding that may be necessary in the future, and regardless of outcome, substantial costs and diversion of resources may result which could have a material adverse effect on our business, financial condition, results of operations and prospects. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our Class A common stock. Some of our competitors and other third parties may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. We may not have sufficient financial or other resources to adequately conduct these types of litigation or proceedings. Any of the foregoing, or any uncertainties resulting from the initiation and continuation of any litigation, could have a material adverse effect on our business, financial condition, results of operations and prospects. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar adverse effect on our business, financial condition, results of operations and prospects.
Obtaining and maintaining our patent protection depends on compliance with various required procedures, document submissions, fee payments and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States at several stages over the lifetime of the patents and/or applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In certain circumstances, we rely on our licensors to pay these fees due to the U.S. and non-U.S. patent agencies and to take the necessary action to comply with these requirements with respect to our licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors may be able to enter the market without infringing our patents and this circumstance could have a material adverse effect on our business, financial condition, results of operations and prospects.
We currently rely on licenses from third parties, and in the future may rely on additional licenses from other third parties, and if we lose any of these licenses, then we may be subjected to future litigation.
We are, and may in the future become, a party to license agreements that grant us rights to use certain intellectual property, including patents and patent applications, typically in certain specified fields of use. We may need to obtain additional licenses from others to advance our research, development and commercialization activities.
Our success may depend in part on the ability of our licensors and any future licensors to obtain, maintain and enforce patent protection for our licensed intellectual property. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products and technologies for sale, which could materially adversely affect our competitive business position and harm our business prospects, financial condition, results of operations or cash flows.
Our current license agreements impose, and future agreements may impose, various diligence, commercialization, milestone payment, royalty, insurance and other obligations on us and require us to meet development timelines, or to exercise commercially reasonable efforts to develop and commercialize licensed products, in order to maintain the licenses. If we fail to comply with these obligations, our licensor(s) may have the right to terminate our license, in which event we would not be able to develop or market products or technology covered by the licensed intellectual property. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
Moreover, disputes may also arise between us and our licensors regarding intellectual property subject to a license agreement, including:
•the scope of rights granted under the license agreement and other interpretation-related issues;
•our financial or other obligations under the license agreement;
•whether, and the extent to which, our products, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
•our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
•the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensor(s); and
•the priority of invention of patented technology.
If we do not prevail in such disputes, we may lose any or all of our rights under such license agreements, experience significant delays in the development and commercialization of our products and technologies, or incur liability for damages, any of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. In addition, we may seek to obtain additional licenses from our licensor(s) and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensor(s), including by agreeing to terms that could enable third parties, including our competitors, to receive licenses to a portion of the intellectual property that is subject to our existing licenses and to compete with our products.
In addition, the agreements under which we currently and in the future license intellectual property or technology from third parties are complex and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize any affected products or services, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Absent the license agreements, we may infringe patents subject to those agreements, and if the license agreements are terminated, we may be subject to litigation by the licensor. Litigation could result in substantial costs and distract our management. If we do not prevail, we may be required to pay damages, including treble damages, attorneys’ fees or costs and expenses and royalties, which could adversely affect our ability to offer products or services, our ability to continue operations and our business, financial condition, results of operations and prospects.
If we cannot license rights to use technologies on reasonable terms, we may not be able to commercialize new products in the future.
We may identify third-party technology that we may need to license or acquire in order to develop or commercialize our products or technologies. However, we may be unable to secure such licenses or acquisitions. The licensing or acquisition of third-party intellectual property rights is a competitive area, and several more established companies may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us.
We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment or at all. In return for the use of a third party’s technology, we may agree to pay the licensor royalties based on sales of our products or services. Royalties are a component of cost of products or technologies and affect the margins on our products. We may also need to negotiate licenses to patents or patent applications before or after introducing a commercial product. We may not be able to obtain necessary licenses to patents or patent applications, and our business may suffer if we are unable to enter into the necessary licenses on acceptable terms or at all, if any necessary licenses are subsequently terminated, if the licensor fails to abide by the terms of the license or fails to prevent infringement by third parties, or if the licensed intellectual property rights are found to be invalid or unenforceable.
Certain of our in-licensed patents are, and our future owned and in-licensed patents may be, subject to a reservation of rights by one or more third parties, including government march-in rights, that may limit our ability to exclude third parties from commercializing products similar or identical to ours.
In addition, our owned and in-licensed patents may be subject to a reservation of rights by one or more third parties. For example, the U.S. government has certain rights, including march-in rights, to patent rights and technology funded by the U.S. government and licensed to us from Boreal and the University of British Columbia. When new technologies are developed with government funding, in order to secure ownership of such patent rights, the recipient of such funding is required to comply with certain government regulations, including timely disclosing the inventions claimed in such patent rights to the U.S. government and timely electing title to such inventions. Any failure to timely elect title to such inventions may permit the U.S. government to, at any time, take title in such inventions. Additionally, the U.S. government generally obtains certain rights in any resulting patents, including a non-exclusive license authorizing the government to use the invention or to have others use the invention on its behalf. If the government decides to exercise these rights, it is not required to engage us as our contractor in connection with doing so. These rights may permit the U.S. government to disclose our confidential information to third parties and to exercise march-in rights to use or allow third parties to use our licensed technology. The U.S. government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, our rights in such inventions may be subject to certain requirements to manufacture products embodying such inventions in the United States. Any exercise by the government of any of the foregoing rights could have a material adverse effect on our business, financial condition, results of operations and prospects.
Our products contain third-party open-source software components and failure to comply with the terms of the underlying open-source software licenses could restrict our ability to sell our products and provide third parties access to our proprietary software.
Our products may contain software licensed by third parties under open-source software licenses. Use and distribution of open-source software may entail greater risks than use of third-party commercial software, as open-source software licensors generally do not provide warranties or other contractual protections regarding infringement claims or the quality of the code. Some open-source software licenses contain requirements that the licensee make its source code publicly available if the licensee creates modifications or derivative works using the open-source software, depending on the type of open-source software the licensee uses and how the licensee uses it. If we combine our proprietary software with open-source software in a certain manner, we could, under certain open-source software licenses, be required to release the source code of our proprietary software to the public for free. This would allow our competitors and other third parties to create similar products with less development effort and time and ultimately could result in a loss of our product sales and revenue, which could have a material adverse effect on our business, financial condition, results of operations and prospects. In addition, some companies that use third-party open-source software have faced claims challenging their use of such open-source software and their compliance with the terms of the applicable open-source license. We may be subject to suits by third parties claiming ownership of what they believe to be open-source software or claiming non-compliance with the applicable open-source licensing terms. Use of open-source software may also present additional security risks because the public availability of such software may make it easier for hackers and other third parties to compromise or attempt to compromise our technology platform and systems.
Although we review our use of open-source software to avoid subjecting our proprietary software to conditions we do not intend, the terms of many open source-software licenses have not been interpreted by U.S. courts, and there is a risk that these licenses could be construed in a way that could impose unanticipated conditions or restrictions on our ability to commercialize our products and proprietary software. Moreover, our processes for monitoring and controlling our use of open-source software in our products may not be effective. If we are held to have breached the terms of an open-source software license, we could be subject to damages, required to seek licenses from third parties to continue offering our products on terms that are not economically feasible, to re-engineer our products, to discontinue the sale of our products if re-engineering could not be accomplished on a timely basis, or to make generally available, in source code form, our proprietary code, any of which could adversely affect our business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to make products that are similar to products and technologies we may develop or utilize similar technology that are not covered by the claims of the patents that we own or license now or in the future;
•we, or our licensor(s), might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or may own in the future;
•we, or our licensor(s), might not have been the first to file patent applications covering certain of our or their inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing, misappropriating or otherwise violating our owned or licensed intellectual property rights;
•it is possible that our pending licensed patent applications or those that we may own in the future will not lead to issued patents;
•issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors;
•our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable;
•the patents of others may harm our business; and
•we may choose not to file a patent for certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property.
If any of these events occur, they could materially adversely affect our business, financial condition, results of operations and prospects.
Risks Related to Our Securities and to Being a Public Company
Our outstanding warrants became exercisable for our Class A common stock in September 2021, which increased the number of shares eligible for future resale in the public market and could result in dilution to our stockholders if exercised.
Following the Business Combination, there were 3,833,319 outstanding warrants issued in connection with the initial public offering of HighCape (the “Public Warrants”) to purchase 3,833,319 shares of our Class A common stock at an exercise price of $11.50 per share, which warrants became exercisable on September 9, 2021. In addition, there are 135,000 private placement warrants (the “Private Warrants”) to purchase 135,000 shares of our Class A common stock at an exercise price of $11.50 per share. In certain circumstances, the Public Warrants and Private Warrants may be exercised on a cashless basis. To the extent such warrants are exercised, additional shares of our Class A common stock will be issued, which will result in dilution to the holders of our Class A common stock and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market could adversely affect the market price of our Class A common stock, the impact of which is increased as the value of our stock price increases.
Our warrants are accounted for as liabilities and changes in the value of our warrants could have a material effect on our financial results.
On April 12, 2021, the Acting Director of the Division of Corporation Finance and Acting Chief Accountant of the SEC together issued a statement regarding the accounting and reporting considerations for warrants issued by special purpose acquisition companies entitled “Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies” (“SPACs”) (the “SEC Statement”). Specifically, the SEC Statement focused on certain settlement terms and provisions related to certain tender offers following a de-SPAC process such as our Business Combination, which terms are similar to those contained in the warrant agreement governing our warrants. As a result of the SEC Statement, HighCape reevaluated the accounting treatment of its Public Warrants and Private Warrants and determined to classify the warrants as derivative liabilities measured at fair value, with changes in fair value each period reported in earnings.
As a result, included on our balance sheets as of December 31, 2024 and December 31, 2023, are derivative liabilities related to our warrants. Accounting Standards Codification 815, Derivatives and Hedging (“ASC 815”), provides for the remeasurement of the fair value of such derivatives at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value being recognized in earnings in the statement of operations. As a result of the recurring fair value measurement, our Consolidated Financial Statements and results of operations may fluctuate quarterly, based on factors that are outside of our control. Due to the recurring fair value measurement, it is expected that we will recognize non-cash gains or losses on the warrants each reporting period and that the amount of such gains or losses could be material.
There can be no assurance that the warrants will be in the money prior to their expiration, and they may expire worthless.
The exercise price for our outstanding warrants is $11.50 per share of our Class A common stock. There can be no assurance that the warrants will be in the money prior to their expiration, and as such, the warrants may expire worthless.
There are currently outstanding an aggregate of 3,968,319 warrants to acquire shares of our Class A common stock, which comprise 135,000 Private Warrants held by HighCape’s initial stockholders at the time of HighCape’s initial public offering and 3,833,319 Public Warrants. Each of our outstanding whole warrants is exercisable as of September 9, 2021, for one share of our Class A common stock in accordance with its terms. Therefore, as of December 31, 2024, if we assume that each outstanding whole warrant is exercised and one share of HighCape Class A common stock is issued as a result of such exercise, with payment of the exercise price of $11.50 per share, our fully-diluted share capital would increase by a total of 3,968,319 shares, with approximately $45.6 million paid to us to exercise the warrants.
We have in the past experienced material weaknesses in our internal control over financial reporting, and if we experience such material weaknesses in our internal control over financial reporting in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to report our financial condition, results of operations or cash flows accurately or in a timely manner, which may adversely affect investor confidence in us and, as a result, materially and adversely affect our business and the value of our Class A common stock.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected and corrected on a timely basis.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. Material weaknesses could result in material misstatements to our annual or interim financial statements that might not be prevented or detected on a timely basis, or in delayed filing of required periodic reports. If we are unable to assert that our internal control over financing reporting is effective, or if our independent registered public accounting firm is unable to express an unqualified opinion as to the effectiveness of the internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reporting, the market price of our Class A common stock could be adversely affected and we could become subject to litigation or investigations by Nasdaq, the SEC, or other regulatory authorities, which could require additional financial and management resources.
If we identify any material weaknesses in the future, any such newly identified material weakness could limit our ability to prevent or detect a misstatement of our accounts or disclosures that could result in a material misstatement of our annual or interim financial statements. In such case, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, investors may lose confidence in our financial reporting and our stock price may decline as a result. We cannot assure you that the measures we have taken to date, or any measures that may be taken in the future, will be sufficient to avoid potential future material weaknesses.
In addition, we may face potential for litigation or other disputes which may include, among others, claims invoking the federal and state securities laws, contractual claims or other claims arising from the restatement and material weaknesses in our internal control over financial reporting and the preparation of our Consolidated Financial Statements. We can provide no assurance that such litigation or dispute will not arise in the future. Any such litigation or dispute, whether successful or not, could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. We design our disclosure controls and procedures to reasonably assure that information we are required to disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple errors or mistakes. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosure due to error or fraud may occur and we may not detect them.
Any failure to maintain effective internal controls and procedures over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows.
Because we are a “controlled company” within the meaning of the Nasdaq rules, our stockholders may not have certain corporate governance protections that are available to stockholders of companies that are not controlled companies.
So long as more than 50% of the voting power for the election of our directors is held by an individual, a group or another company, we will qualify as a “controlled company” within the meaning of the Nasdaq listing rules. As of February 26, 2025, Dr. Rothberg controlled 73.4% of the voting power of our outstanding capital stock, including our Class A common stock and Class B common stock. As a result, we are a “controlled company” within the meaning of the Nasdaq corporate governance standards and are not subject to the requirements that would otherwise require us to have: (i) a majority of independent directors; (ii) a compensation committee comprised solely of independent directors; and (iii) director nominees
selected, or recommended for our Board’s selection, either by a majority of the independent directors or a nominating committee comprised solely of independent directors.
Dr. Rothberg may have his interest in us diluted due to future equity issuances or his own actions in selling shares of our Class B common stock, in each case, which could result in a loss of the “controlled company” exemption under the Nasdaq listing rules. We would then be required to comply with those provisions of the Nasdaq listing requirements.
We could fail to maintain the listing of our Class A common stock on the Nasdaq Stock Market LLC, which could seriously harm the liquidity of our shares and our ability to raise capital or complete a strategic transaction.
Nasdaq has established continued listing requirements, including a requirement to maintain a minimum closing bid price of at least $1.00 per share. On November 4, 2024, we received written notice from Nasdaq notifying us that, because the closing bid price for our Class A common stock had fallen below $1.00 per share for 30 consecutive business days, and we had no longer met the minimum bid price requirement for continued inclusion on The Nasdaq Global Market pursuant to Nasdaq Listing Rule 5450(a)(1) (the “Bid Price Requirement”). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we had been provided an initial period of 180 calendar days, or until May 5, 2025 (the “Compliance Date”), to regain compliance with the Bid Price Requirement. To regain compliance, the closing bid price of our Class A common stock must be at least $1.00 per share for a minimum of 10 consecutive business days during this 180-calendar day period. If we did not regain compliance with the Bid Price Requirement by the Compliance Date, we may have been eligible for an additional 180-calendar day compliance period. To qualify, we would need to transfer the listing of our Class A common stock to The Nasdaq Capital Market and meet the continued listing requirement for the market value of publicly held shares and all other initial listing standards, with the exception of the Bid Price Requirement. To effect such a transfer, we would also need to pay an application fee to Nasdaq and would need to provide written notice to the Staff of our intention to cure the deficiency during the additional compliance period. On December 5, 2024, we were notified by NASDAQ that we had regained compliance with the closing bid price of $1.00 effective December 4, 2024.
Despite regaining compliance on December 4, 2024, there can be no assurance that we will be able to maintain compliance with the Bid Price Requirement or maintain compliance with other Nasdaq requirements in the future. If we are not able to maintain compliance with Nasdaq requirements, our Class A common stock may be delisted from Nasdaq, which could have a material adverse effect on us and our stockholders, including by reducing the liquidity of our shares and having a material adverse effect on our ability to raise capital or complete a strategic transaction.
The dual class structure of our common stock has the effect of concentrating voting power with our Founder, who is also on our Board of Directors, which will limit an investor’s ability to influence the outcome of important transactions, including a change in control.
Shares of our Class B common stock have 20 votes per share, while shares of our Class A common stock have one vote per share. Dr. Rothberg and his affiliates hold all of the issued and outstanding shares of our Class B common stock, and as of February 26, 2025, Dr. Rothberg and his affiliates held 73.4% of the voting power of our capital stock, including our Class A common stock and Class B common stock and is able to control matters submitted to our stockholders for approval, including the election of directors, amendments to our organizational documents and any merger, consolidation, sale of all or substantially all of our assets or other major corporate transactions. Dr. Rothberg may have interests that differ from yours and may vote in a way with which you disagree, and which may be adverse to your interests. This concentrated control may have the effect of delaying, preventing or deterring a change in control of us, could deprive our stockholders of an opportunity to receive a premium for their capital stock as part of a sale of us, and might ultimately affect the market price of shares of our Class A common stock. If additional shares of our Class B common stock are issued, your shares and your votes may be significantly diluted.
We cannot predict the impact our dual class structure may have on the stock price of our Class A common stock.
We cannot predict whether our dual class structure will result in a lower or more volatile market price of our Class A common stock or in adverse publicity or other adverse consequences. For example, certain index providers have announced restrictions on including companies with multiple-class share structures in certain of their indexes. Under these policies, our dual class capital structure would make us ineligible for inclusion in certain indices, and as a result, mutual funds, exchange-traded funds and other investment vehicles that attempt to passively track those indices will not be investing in our stock. It is unclear what effect, if any, these policies will have on the valuations of publicly traded companies excluded from such indices, but it is possible that they may depress valuations, as compared to similar companies that are included. As a result, the market price of shares of our Class A common stock could be adversely affected.
Delaware law and provisions in our certificate of incorporation and bylaws could make a takeover proposal more difficult.
Our organizational documents are governed by Delaware law. Certain provisions of Delaware law and of our certificate of incorporation and bylaws could discourage, delay, defer or prevent a merger, tender offer, proxy contest or other change of control transaction that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares of our Class A common stock held by our stockholders. These provisions provide for, among other things:
•the ability of our Board to issue one or more series of preferred stock;
•stockholder action by written consent only until the first time when Dr. Rothberg ceases to beneficially own a majority of the voting power of our capital stock;
•certain limitations on convening special stockholder meetings;
•advance notice for nominations of directors by stockholders and for stockholders to include matters to be considered at our annual meetings;
•amendment of certain provisions of the organizational documents only by the affirmative vote of (i) a majority of the voting power of our capital stock so long as Dr. Rothberg beneficially owns shares representing a majority of the voting power of our capital stock and (ii) at least two-thirds of the voting power of the capital stock from and after the time that Dr. Rothberg ceases to beneficially own shares representing a majority of our voting power; and
•a dual-class common stock structure with 20 votes per share of our Class B common stock, the result of which is that Dr. Rothberg has the ability to control the outcome of matters requiring stockholder approval, even though Dr. Rothberg owns less than a majority of the outstanding shares of our capital stock.
These anti-takeover provisions as well as certain provisions of Delaware law could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many of our stockholders. As a result, our stockholders may be limited in their ability to obtain a premium for their shares. If prospective takeovers are not consummated for any reason, we may experience negative reactions from the financial markets, including negative impacts on the price of our Class A common stock. These provisions could also discourage proxy contests and make it more difficult for our stockholders to elect directors of their choosing and to cause us to take other corporate actions that our stockholders desire.
Our certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings and the federal district courts as the sole and exclusive forum for other types of actions and proceedings, in each case, that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain what such stockholders believe to be a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our certificate of incorporation provides that, unless we consent to the selection of an alternative forum, any (i) derivative action or proceeding brought on behalf of us; (ii) action asserting a claim of breach of a fiduciary duty owed by, or any other wrongdoing by, any current or former director, officer or other employee or stockholder of ours; (iii) action asserting a claim against us or any director or officer arising pursuant to any provision of the General Corporation Law of the State of Delaware (“DGCL”) or our certificate of incorporation or our bylaws; or (iv) action to interpret, apply, enforce, or determine the validity of any provisions in the certificate of incorporation of bylaws; or (v) action asserting a claim against us or any director or officer of ours governed by the internal affairs doctrine, shall, to the fullest extent permitted by law, be exclusively brought in the Court of Chancery of the State of Delaware or, if such court does not have subject matter jurisdiction thereof, the federal district court of the State of Delaware. Subject to the foregoing, the federal district courts of the United States are the exclusive forum for the resolution of any action, suit or proceeding asserting a cause of action under the Securities Act. The exclusive forum provision does not apply to suits brought to enforce any liability or duty created by the Exchange Act. Any person or entity purchasing or otherwise acquiring an interest in any shares of our capital stock shall be deemed to have notice of and to have consented to the forum provisions in our certificate of incorporation. These choice-of-forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that he, she or it believes to be favorable for disputes with us or our directors, officers or other employees or stockholders, which may discourage such lawsuits. We note that there is uncertainty as to whether a court would enforce these provisions and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act creates concurrent jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder.
Alternatively, if a court were to find these provisions of our certificate of incorporation inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could materially adversely affect our business, financial condition, results of operations and cash flows and result in a diversion of the time and resources of our management and Board.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
ITEM 1C. CYBERSECURITY
Creating and maintaining a secure information infrastructure is of paramount importance to Quantum-Si, and we have invested, and will continue to invest significant resources to ensure we maintain the most secure environment reasonably possible to protect our assets, customers and employees. Our Board of Directors, through our Audit Committee, is actively involved in oversight of our risk management activities, and cybersecurity represents an important element of our overall approach to risk management. Our cybersecurity policies, standards, processes and practices are based on recognized frameworks established by the National Institute of Standards and Technology, (“NIST”) and other applicable industry standards. We address cybersecurity risks through a comprehensive, cross-functional approach that is focused on preserving the confidentiality, security and availability of the information we maintain by identifying, preventing and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
Risk Management and Strategy
We face a variety of risks related to cybersecurity, such as unauthorized access, cybersecurity attacks and other security incidents, including as perpetrated by hackers and unintentional damage or disruption to hardware and software systems, loss of data, and misappropriation of confidential information. To identify and assess material risks from cybersecurity threats, we maintain a cybersecurity program to ensure our systems are effective and prepared for information security risks, including regular oversight of our programs for security monitoring for internal and external threats to ensure the confidentiality and integrity of our information assets. We employ a range of tools and services, including regular network and endpoint monitoring utilizing leading market monitoring tools, audits and vulnerability assessments including penetration testing to inform our risk identification and assessment. Our Audit Committee of the Board of Directors provides oversight of our cybersecurity risk management and strategy processes, which is led by our Chief Financial Officer.
We also identify our cybersecurity threat risks by comparing our processes to standards set by NIST as well as by engaging experts to attempt to penetrate our information systems. To provide for the availability of critical data and systems, manage our material risks from cybersecurity threats, and protect against and respond to cybersecurity incidents, we undertake the following activities:
•monitor emerging data protection laws and implement changes to our processes that are designed to comply with such laws;
•through our policies, practices and contracts (as applicable), require employees, as well as third parties that provide services on our behalf, to treat confidential information and data with care;
•employ technical safeguards that are designed to protect our information systems from cybersecurity threats, including firewalls, intrusion prevention and detection systems, anti-malware functionality and access controls, which are evaluated and improved through vulnerability assessments and cybersecurity threat intelligence;
•provide regular, mandatory training for our employees and contractors regarding cybersecurity threats as a means to equip them with effective tools to address cybersecurity threats, and to communicate our evolving information security policies, standards, processes and practices;
•conduct regular phishing email simulations for all employees and contractors with access to our email systems to enhance awareness and responsiveness to possible threats;
•leverage the NIST incident handling framework to help us identify, protect, detect, respond and recover when there is an actual or potential cybersecurity incident; and
•carry information security risk insurance that provides protection against the potential losses arising from a cybersecurity incident.
Our incident response plan coordinates the activities we take to prepare for, detect, respond to and recover from cybersecurity incidents, which include processes to triage, assess severity for, escalate, contain, investigate and remediate the incident, as well as to comply with potentially applicable legal obligations and mitigate damage to our business and reputation.
As part of the above processes, we regularly engage with consultants, auditors and other third parties in assisting in review our cybersecurity program to help identify areas for continued focus, improvement and compliance.
We describe whether and how risks from identified cybersecurity threats, including as a result of any previous cybersecurity incidents, have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition, under the heading “If we experience a significant disruption in our information technology systems, including our Platinum Analysis Software services, or cybersecurity incidents, our business could be adversely affected”, which disclosures are incorporated by reference herein.
Despite our efforts to create security barriers to such threats, it is virtually impossible for us to completely mitigate these risks. In August 2020, we discovered ransomware on a server and engaged third-party forensics experts and outside counsel for incident response. We did not pay ransom to the attacker because the documents that were encrypted by the attacker were sufficiently backed up and the investigation further confirmed that no employee data or other personal information was accessed. We implemented a number of security enhancements as the incident unfolded and continue to implement short- and long-term security enhancements to further secure our network.
Cybersecurity Governance; Management
Cybersecurity is an important part of our broader risk management processes and an area of focus for our Board of Directors and management. In general, our Board of Directors oversees risk management activities designed and implemented by our management, and considers specific risks, including, for example, risks associated with our strategic plan, business operations, and capital structure. Our Board of Directors executes its oversight responsibility for risk management both directly and through delegating oversight of certain of these risks to its committees, and our Board of Directors has authorized our Audit Committee to oversee risks from cybersecurity threats.
We plan for our Chief Financial Officer to provide our Audit Committee with quarterly general risk assessment updates, which shall cover cyber risk topics such as data security posture, results from third-party assessments, progress towards predetermined risk-mitigation-related goals, our incident response plan, and material cybersecurity threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. Our Audit Committee is also involved in the reporting function of our incident response plan which includes communication of any cybersecurity incident that meets our reporting thresholds, as well as ongoing updates regarding any such incident, until such incident has been resolved.
Our cybersecurity risk management and strategy processes, which are discussed in greater detail above, are led by our Chief Financial Officer in conjunction with our General Counsel and our Head of Information Technology and report to our Audit Committee. In addition, we maintain an Information Technology Steering Committee, which is comprised of several senior members of the Company. Our Chief Financial Officer has over 10 years of experience in leading Information Technology departments at various companies. Our General Counsel has over 10 years of experience related to general legal matters, including in the capacity of understanding and guiding companies on Information Technology matters and the related risks. Our Head of Information Technology has over 30 years of relevant experience, including managing information security, developing cybersecurity strategy, implementing effective information and cybersecurity programs. These management team members are informed about and monitor the prevention, mitigation, detection and remediation of cybersecurity incidents through their management of, and participation in, the cybersecurity risk management and strategy processes described above, including the operation of our incident response plan.
ITEM 2. PROPERTIES
We currently lease our executive office at 29 Business Park Drive, Branford, Connecticut 06405. We also lease office, laboratory and manufacturing space in San Diego, California and office space in Santa Clara, California and New Haven, Connecticut. Our semiconductor chip assembly and packaging business is located in Garnet Valley, Pennsylvania. Our leases expire at various dates through 2032. We believe that our current office, laboratory and assembly and packaging locations are sufficient to meet our needs at existing volume levels for the foreseeable future. For further information, please refer to Note 8. Leases, in the accompanying notes to our Consolidated Financial Statements included elsewhere in the Annual Report on Form 10-K.
ITEM 3. LEGAL PROCEEDINGS
We are involved in various legal proceedings, claims, investigations and litigation that arise in the ordinary course of our business. For further information regarding our legal proceedings, please refer to Note 18. Commitments and Contingencies of the Notes to the Consolidated Financial Statements (Part II, Item 8) included in this report, which is incorporated by reference herein. ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our Class A common stock and warrants to purchase Class A common stock are listed on Nasdaq under the symbols “QSI” and “QSIAW”, respectively.
Stockholders
As of February 26, 2025, we had 163,202,105 shares of Class A common stock issued and outstanding held of record by 303 holders, 19,937,500 shares of Class B common stock issued and outstanding held of record by two holders, and 3,833,319 public warrants held of record by two holders and 135,000 private placement warrants issued in connection with HighCape’s initial public offering held of record by three holders, each exercisable for one share of Class A Common Stock at a price of $11.50 per share.
There is no public market for our Class B common stock.
Dividends
We have not paid any cash dividends on our Class A common stock or Class B common stock to date, and we do not anticipate paying any cash dividends in the foreseeable future. The payment of cash dividends is subject to the discretion of our board of directors and may be affected by various factors, including our future earnings, financial condition, capital requirements, share repurchase activity, current and future planned strategic growth initiatives, levels of indebtedness, and other considerations our board of directors deem relevant.
Unregistered Sales of Equity Securities
During the year ended December 31, 2024, the Company granted (i) an option to purchase 543,271 shares of the Company’s common stock with an exercise price of $0.98 per share and (ii) 899,305 restricted stock units to certain of our employees as an “inducement” grant under our 2023 Inducement Equity Incentive Plan pursuant to Rule 5635(c)(4) of the Nasdaq Listing Rules. We intend to file a registration statement on a Form S-8 to register the shares of common stock underlying these inducement awards prior to the time at which the awards become exercisable or settleable, as applicable.
Issuer Purchases of Equity Securities
Not applicable.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis provides information which management believes is relevant to an assessment and understanding of our consolidated results of operations and financial condition. The discussion should be read in conjunction with the Consolidated Financial Statements and Notes thereto contained in this Annual Report on Form 10-K. This discussion contains forward looking statements and involves numerous risks and uncertainties, including, but not limited to, those described in the “Risk Factors” section of this Annual Report on Form 10-K. Actual results may differ materially from those contained in any forward-looking statements. For a discussion on forward-looking statements, see the information set forth in the introductory note to this Annual Report under the caption “Cautionary Note Regarding Forward Looking Statements,” which information is incorporated herein by reference. Unless the context otherwise requires, references to “we”, “us”, “our”, the “Company” or “Quantum-Si” are intended to mean the business and operations of Quantum-Si Incorporated and its consolidated subsidiaries. For discussion and analysis pertaining to the year ended December 31, 2023 overview and highlights as compared to the year ended December 31, 2022, please refer to the Company’s Annual Report on Form 10-K, as filed with the SEC on February 29, 2024.
Overview
Quantum-Si Incorporated (including its subsidiaries, the “Company” or “Quantum-Si”) was incorporated in Delaware on June 10, 2020 as HighCape Capital Acquisition Corp. (“HighCape”). The Company’s legal name became Quantum-Si Incorporated following a business combination on June 10, 2021 between the Company and Q-SI Operations Inc. (formerly Quantum-Si Incorporated) (the “Business Combination”), which was founded in 2013.
We are a life sciences company focused on proteomics research, with the mission of transforming single-molecule analysis and democratizing its use by providing researchers and clinicians access to the proteome, the set of proteins expressed within a cell. We have developed a proprietary universal single-molecule detection platform that we are applying to proteomics to enable Next-Generation Protein SequencingTM (“NGPS”), to sequence proteins in a massively parallel fashion (rather than sequentially, one at a time), which can also be used for the study of nucleic acids. We believe that the ability to sequence proteins in a massively parallel fashion and offer a fast analysis time provides NGPS with the potential to unlock significant biological information through improved resolution and unbiased access to the proteome at a speed and scale that is not available today. Traditionally, proteomic workflows to sequence proteins required days or weeks to complete. Our platform includes our Platinum NGPS instrument, Platinum Analysis Software, and consumable kits for use with our Platinum instrument. In 2021, we introduced our Platinum early access program to sites with participation from leading academic centers and key industry partners. The early access program introduced the Platinum single-molecule sequencing system to key opinion leaders across the globe for both expansion and development of applications and workflows. We began a controlled launch of the Platinum instrument and started to take orders in December 2022, and subsequently began a controlled commercial launch of Platinum in January 2023, and then moved to a full commercial launch of Platinum beginning the second quarter of 2024.
Going forward, we intend to follow a systematic, phased approach to continue to successfully launch updates and enhancements to our platform which can include improvements to our hardware, software and chemistry that works together to produce the overall platform.
We believe that our platform offers a differentiated solution in a rapidly evolving proteomics tools market. Within our initial focus market of proteomics, our platform is designed to provide users a seamless opportunity to gain key insights into the immediate state of biological pathways and cell state. Our platform aims to address many of the key challenges and bottlenecks with legacy proteomic solutions, such as mass spectrometry (“MS”), which include high instrument costs both in terms of acquisition and ownership, and complexity with data analysis, which together limit broad adoption. We believe our platform, which is designed to streamline sequencing and data analysis at a lower instrument cost and with greater automation than legacy proteomic solutions, could allow our product to have wide utility across the study of the proteome. For example, our platform could be used for biomarker discovery and disease detection, pathway analysis, immune response, vaccine development, quality assurance and quality control, among other applications.
Global Developments
The macroeconomic climate has experienced, and is continuing to experience, pressure from global developments such as high levels of inflation, global supply chain disruptions and international geopolitical conflicts. We continue to monitor these supply chain, inflation and interest rate factors, as well as the uncertainty resulting from the overall economic
environment. Although we do not expect to be significantly impacted by geopolitical conflicts, we have experienced some constraints in product and material availability and increasing costs required to obtain some materials and supplies as a result of these conflicts on the global economy. As geopolitical conflicts continue or worsen, it may impact our business, financial condition or results of operations. We have taken and will continue to take actions to help mitigate the impact of these economic challenges, but there can be no assurance as to the effectiveness of our efforts going forward.
Restructuring
On November 21, 2024, we announced that we committed to an organizational restructuring program designed to streamline and focus our overall corporate resources, as well as align required resources to focus on future product development objectives, including its recently announced Proteus™ platform. As a result, we terminated approximately 23% of our 187 employee workforce. In connection with the restructuring, we recognized one-time cash charges related to severance and other benefits of approximately $2.3 million in 2024 and expect to recognize $0.7 million in the first six months of 2025. In addition, we recognized non-cash expense of approximately $0.1 million in the fourth quarter of 2024 related to stock option modifications. The severance and other benefits related charge, as well as the expense related to stock option modifications are subject to a number of assumptions, and actual results may differ materially. We may also incur additional costs not currently contemplated due to events that may occur as a result of, or that are associated with, the restructuring. We substantially completed the restructuring in the fourth quarter of 2024. For further information regarding our restructuring activities, please refer to Note 14. Restructuring. Equity Transactions
On August 11, 2023, we filed a universal shelf registration statement on Form S-3 (the “Shelf Registration Statement”), which became effective on August 22, 2023, covering the offering of Class A common stock, preferred stock, debt securities, warrants, rights and units.
On December 11, 2024, we entered into an Equity Distribution Agreement (the “Sales Agreement”) with Canaccord Genuity LLC (“Canaccord”) to sell shares of our Class A common stock, par value $0.0001, having an aggregate offering price of up to $75.0 million, from time to time through an “at-the-market” offering program under which Canaccord will act as sales agent (the “ATM Offering”). We have no obligation to sell any shares under the Sales Agreement and may at any time suspend solicitation and offers under the EDA. The ATM Offering is being made pursuant to our shelf registration and a prospectus supplement related to the ATM Offering dated December 11, 2024. During the year ended December 31, 2024, we sold and issued 23,425,650 shares of our Class A common stock under the ATM Offering, resulting in gross proceeds of $36.2 million. Net proceeds were $34.8 million after commissions and issuance costs of $1.4 million.
Also on December 11, 2024, we exercised its right to terminate the Equity Distribution Agreement (the “Equity Distribution Agreement”), dated August 11, 2023, by and between the Company and Evercore Group L.L.C. (“Evercore”), as sales agent. The Equity Distribution Agreement previously established an “at-the-market” offering program through which we had the right to sell, from time to time, through Evercore, up to an aggregate of $75.0 million of our Class A Common Stock. We sold no shares under the Equity Distribution Agreement.
On January 3, 2025, we entered into a securities purchase agreement with certain institutional investors pursuant to which we agreed to issue and sell, in a registered direct offering (the “Registered Direct Offering”) an aggregate of 15,625,000 shares of our Class A common stock at a price of $3.20 per Share. The gross proceeds from the Registered Direct Offering were $50.0 million. After deducting estimated placement agents’ fees and other offering expenses payable by the Company, net proceeds were approximately $46.9 million.
In connection with the Registered Direct Offering, we entered into a placement agency agreement with A.G.P./Alliance Global Partners (the “Placement Agent”), pursuant to which the Placement Agent agreed to serve as the sole placement agent for the Company, on a reasonable best efforts basis. We agreed to pay the Placement Agent an aggregate cash fee equal to 6.0% of the gross proceeds received in the Registered Direct Offering.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our Consolidated Financial Statements, which have been prepared in accordance with U.S. GAAP. The preparation of these Consolidated Financial Statements requires us to make estimates and assumptions that affect the reported amounts of assets and
liabilities and the disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements, as well as expenses incurred during the reporting periods. Our estimates are based on historical experience and various other factors we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about items not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Revenue Recognition
Revenue is derived from sales of products and services. Product revenue is primarily generated from the sales of instruments and consumables used in protein sequencing and analysis. Service revenue is primarily generated from service maintenance contracts including access to analysis software and advanced training for instrument use. We recognize revenue when or as a customer obtains control of the promised goods and services. The amount of revenue recognized reflects the consideration to which we expect to be entitled in exchange for these goods and services. This process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the contract price, allocating the contract price to the distinct performance obligations in the contract, and recognizing revenue as the performance obligations have been satisfied. We have made the accounting policy election allowed for under ASC 606-10-32-2A to exclude all sales taxes from transaction price. Revenue recognition for contracts with multiple deliverables is based on the separate satisfaction of each distinct performance obligation within the contract. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. We allocate transaction price to the performance obligations in a contract with a customer based on the relative standalone selling price of each performance obligation. We determine standalone selling price based on the price at which the performance obligation is sold separately. If the standalone selling price is not observable through past transactions, we estimate the standalone selling price taking into account available information and specific factors such as competitive positioning, internal costs, profit objectives, and internally approved pricing guidelines related to the performance obligation.
We consider performance obligation for sales of products satisfied upon shipment of the goods to the customer in accordance with the shipping terms (either upon shipment or delivery), which is when control of the product is deemed to be transferred; this includes instruments and consumables. Customers generally do not have a right to return products, except for defective or damaged products during the warranty period or unless prior written consent is provided. In instances where right of payment or transfer of title is contingent upon the customer’s acceptance of the product, revenue is deferred until all acceptance criteria have been met. Revenues for service maintenance contracts, which start after the first year of purchase and are considered as service type warranties that effectively extend the standard first-year service coverage at the customer’s option are recognized ratably over the contract service period as these services are performed evenly over time. Revenues for advanced training is recognized at a point in time upon satisfaction of the underlying performance obligation. We typically provide a standard one-year warranty which covers defects in materials, workmanship and manufacturing or performance conditions under normal use and service. The first year of the warranty of the products is considered an assurance-type warranty and is recorded as Cost of revenue within the Consolidated Statements of Operations and Comprehensive Loss. We have determined this standard first-year warranty is not a distinct performance obligation.
Stock-based Compensation
Stock-based compensation expense for stock option grants with only service conditions is recognized on a straight-line basis over the requisite service period of the individual grants, which is generally the vesting period, based on the estimated grant date fair values. Stock-based compensation expense for stock option grants subject to non-financing event performance conditions on an accelerated basis is recognized as though each vesting portion of the award was, in substance, a separate award.
Prior to the Business Combination, the fair value of the shares of common stock underlying stock options had been determined by the Board, with input from management and contemporaneous third-party valuations, as there was no public market for the common stock. Given the absence of a public trading market for our common stock, the Board exercised reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of the fair value of our common stock at each option grant date.
After the completion of the Business Combination, we measure compensation expense for stock-based awards to employees, non-employees and directors based upon the awards’ initial grant-date fair values. Stock-based compensation
expense for stock options, restricted stock units and performance awards is recorded over the requisite service period. For awards with only a service condition, we expense stock-based compensation using the straight-line method over the requisite service period for the entire award. For awards with a market condition, we expense the grant date fair value at the target over the vesting period regardless of the value the award recipients ultimately receive. The fair value of restricted stock without a market condition is estimated using the current market price of our common stock on the date of grant. The fair value of stock option grants with a market condition is estimated at the date of grant using the Monte Carlo simulation model (“Monte Carlo”). The fair values of stock option grants with only a service condition are estimated as of the date of grant by applying the Black-Scholes option valuation model (“Black-Scholes model”). The Black-Scholes model and Monte Carlo models incorporate assumptions as to stock price volatility, the expected life of options or restricted stock, a risk-free interest rate and dividend yield. The effect of forfeiture in compensation costs is recognized based on actual forfeitures when they occur.
The Black-Scholes model is affected by the stock price on the date of the grant as well as assumptions regarding a number of variables. These variables include the expected term of the option, expected risk-free interest rate, the expected volatility of common stock, and expected dividend yield; each of which is described below. The assumptions for expected term of the option and expected volatility of common stock are the two assumptions that significantly affect the grant date fair value.
•Expected Term: The expected term is calculated using the weighted-average period that the stock options are expected to be outstanding prior to being exercised. We determine expected term based on historical exercise patterns and our expectation of the time that it will take for employees to exercise options still outstanding. We estimate non-employees’ options based on the contractual term.
•Risk-free Interest Rate: The risk-free interest rate for periods within the expected term of the awards is based on the U.S. Treasury yield curve in effect at the time of the grant.
•Expected Stock Price Volatility: We determined expected annual equity volatility based on the combination of the historical volatility of our common stock and the historical volatility of the common stock comparable to our common stock.
•Dividend Yield: Because we have never paid a dividend and do not expect to begin doing so in the foreseeable future, we assume no dividend yield in valuing the stock-based awards.
•Exercise Price: The exercise price is taken directly from the grant notice issued to employees and non-employees.
Stock options granted to non-employees are accounted for based on their fair value on the measurement date using the Black-Scholes model. For further information regarding our stock-based compensation and equity incentive plans, please refer to Note 2. Summary of Significant Accounting Policies, and Note 11. Stock-based Compensation, in the accompanying notes to our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. Inventory
Inventory is stated at the lower of cost or net realizable value with cost determined using the first-in, first-out method. Materials that may be utilized for either commercial or, alternatively, for research and development purposes, are classified as inventory. Amounts in inventory used for research and development purposes are charged to research and development expense when the product enters the research and development process and can no longer be used for commercial purposes and, therefore, does not have an “alternative future use” as defined in authoritative guidance. During the years ended December 31, 2024 and 2023 we identified $3.2 million and $3.4 million, respectively, of product that no longer had an alternative future use and therefore was included as part of research and development expense. There was no such expense identified for the year ended December 31, 2022.
An assessment of the recoverability of capitalized inventory is performed during each reporting period and, if needed, we record a reserve for any excess and obsolete inventory to record inventory at its estimated net realizable value in the period it is identified. Inventory excess and obsolescence reserves related to cost of revenue were immaterial for the years ended December 31, 2024 and 2023.
For further information regarding our significant accounting policies and estimates, please refer to Note 2. Summary of Significant Accounting Policies, in the accompanying notes to our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. Warrant Liability
Outstanding warrants include Public Warrants which were issued as one-third of one redeemable warrant per unit during HighCape’s initial public offering on September 9, 2020, and Private Warrants sold to the Sponsor. The Public Warrants and Private Warrants meet the definition of a derivative and we recorded these warrants as long-term liabilities in the Consolidated Balance Sheets at fair value upon the closing of the Business Combination, with subsequent changes in their respective fair values recognized in the Consolidated Statements of Operations and Comprehensive Loss at each reporting date. For further information regarding our warrants, please refer to Note 12. Warrant Liabilities. Recently Issued Accounting Pronouncements
For a discussion of recently adopted accounting pronouncements and accounting pronouncements pending adoption, please refer to Note 2. Summary of Significant Accounting Policies, in the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. Results of Operations for the Year Ended December 31, 2024 as Compared to the Year Ended December 31, 2023
The following table summarizes the results of our operations for the years ended December 31, 2024 and 2023 (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | $ Change | | % Change |
| Revenue | | | | | | | |
| Product | $ | 2,925 | | | $ | 1,031 | | | $ | 1,894 | | | 183.7 | % |
| Service | 133 | | | 51 | | | 82 | | | 160.8 | % |
| Total revenue | 3,058 | | | 1,082 | | | 1,976 | | | 182.6 | % |
| | | | | | | |
| Cost of revenue | 1,458 | | | 594 | | | 864 | | | 145.5 | % |
| | | | | | | |
| Gross profit | 1,600 | | | 488 | | | 1,112 | | | 227.9 | % |
| Operating expenses: | | | | | | | |
| Research and development | 59,641 | | | 67,025 | | | (7,384) | | | (11.0) | % |
| Selling, general and administrative | 50,535 | | | 44,634 | | | 5,901 | | | 13.2 | % |
| Total operating expenses | 110,176 | | | 111,659 | | | (1,483) | | | (1.3) | % |
| Loss from operations | (108,576) | | | (111,171) | | | 2,595 | | | (2.3) | % |
| Dividend and interest income | 11,366 | | | 9,536 | | | 1,830 | | | 19.2 | % |
| Unrealized gain on trading securities | — | | | 10,690 | | | (10,690) | | | (100.0) | % |
| Realized loss on trading securities | — | | | (5,103) | | | 5,103 | | | 100.0 | % |
| Change in fair value of warrant liabilities | (3,722) | | | (278) | | | (3,444) | | | 1238.8 | % |
| Other (expense) income, net | (19) | | | 366 | | | (385) | | | (105.2) | % |
| Loss before provision for income taxes | (100,951) | | | (95,960) | | | (4,991) | | | 5.2 | % |
| Provision for income taxes | (56) | | | - | | | (56) | | | nm(1) |
| Net loss | $ | (101,007) | | | $ | (95,960) | | | $ | (5,047) | | | 5.3 | % |
(1)“nm” indicates amount is not meaningful.
Revenue, Cost of Revenue and Gross Profit
Revenue is derived from sales of products and services. Product revenue is generated from the following sources: (i) sales of our Platinum® instrument, (ii) consumables, which consist of sales of our library preparation kits, sequencing kit (which includes sequencing reagents and semiconductor chips), and (iii) freight revenue, which is recognized upon shipment. Service revenue is generated from service maintenance contracts including Platinum Analysis Software access, and advanced training for instrument use.
Cost of revenue primarily consists of product and service costs including material costs, personnel costs and benefits, inbound and outbound freight, packaging, warranty replacement costs, royalty costs, facilities costs, depreciation and amortization expense, and inventory write-offs.
Revenue, Cost of revenue and Gross profit for the years ended December 31, 2024 and 2023 are as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Total revenue | $ | 3,058 | | | $ | 1,082 | | | $ | 1,976 | | | 182.6 | % |
| Cost of revenue | 1,458 | | | 594 | | | 864 | | | 145.5 | % |
| Gross profit | $ | 1,600 | | | $ | 488 | | | $ | 1,112 | | | 227.9 | % |
| Gross profit margin | 52.3 | % | | 45.1 | % | | | | |
Total revenue for the sale of Platinum instruments, related reagent kits and service maintenance contracts increased $2.0 million, or 182.6% as compared to the same period in 2023.
Cost of revenue increased $0.9 million, or 145.5% as compared to the same period in 2023.
Gross profit increased $1.1 million, or 227.9% as compared to the same period in 2023.
We began a controlled launch of the Platinum instrument and started to take orders in December 2022, and subsequently began a controlled commercial launch of Platinum in January 2023, and then moved to a full commercial launch of Platinum beginning in the second quarter of 2024.
Research and Development Expenses
Research and development expenses primarily consist of personnel costs and benefits, stock-based compensation, lab supplies, consulting and professional services, fabrication services, charges related to product without an alternative future use, facilities costs, software, and other outsourced expenses. Research and development expenses are recognized as incurred. Our research and development expenses are primarily related to developing new products and services.
Research and development expenses for the years ended December 31, 2024 and 2023 are as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Research and development | $ | 59,641 | | | $ | 67,025 | | | $ | (7,384) | | | (11.0) | % |
Research and development expenses decreased by $7.4 million, or 11.0%, for the year ended December 31, 2024 as compared to the same period in 2023. This decrease was primarily due to a $7.3 million decrease in payroll and payroll-related costs, which includes a $0.3 million decrease in stock-based compensation, a $2.2 million decrease in fabrication and outsourced services and a $0.8 million net decrease of other research and development expenses. The decrease in payroll and payroll-related costs were primarily driven by restructuring activities and an increase in personnel costs that were capitalized during the year ended December 31, 2024. These decreases were partially offset by a $2.9 million increase in laboratory supplies expense.
Selling, General and Administrative Expenses
Selling, general and administrative expenses primarily consist of personnel costs and benefits, stock-based compensation, patent and filing fees, consulting and professional services, legal and accounting services, facilities costs, depreciation and amortization expense, insurance and office expenses, product advertising and marketing.
Selling, general and administrative expenses for the years ended December 31, 2024 and 2023 are as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Selling, general and administrative | $ | 50,535 | | | $ | 44,634 | | | $ | 5,901 | | | 13.2 | % |
Selling, general and administrative expenses increased by $5.9 million, or 13.2%, for the year ended December 31, 2024 as compared to the same period in 2023. The increase was primarily due to a $4.1 million increase in payroll and payroll-related costs, which includes a $0.7 million increase in stock-based compensation, a $2.1 million increase in legal fees, a $1.0 million increase in outsourced services, a $0.7 million increase in trade show and other marketing-related expenses, a $0.6 million increase in non-income tax expenses and a $0.9 million net increase in other selling, general and administrative expenses. The increase in payroll and payroll-related costs are primarily related to on-going investments made in commercial operations. These increases were partially offset by a decrease of $2.8 million in professional services and consulting fees and a $0.7 million decrease in insurance costs from lower market premiums.
Dividend and Interest Income
For the year ended December 31, 2024, dividend and interest income is derived primarily from fixed income securities and money market mutual funds. For the year ended December 31, 2023, dividend and interest income was derived from mutual funds.
Dividend and interest income for the years ended December 31, 2024 and 2023 is as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Dividend and interest income | $ | 11,366 | | | $ | 9,536 | | | $ | 1,830 | | | 19.2 | % |
Dividend and interest income increased by $1.8 million, or 19.2% for the year ended December 31, 2024 as compared to the same period in 2023. This increase was a result of higher dividends earned on invested balances in marketable securities resulting from higher market interest rates.
Unrealized Gain on Trading Securities
Unrealized gain on trading securities for the years ended December 31, 2024 and 2023 is as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Unrealized gain on trading securities | $ | — | | | $ | 10,690 | | | $ | (10,690) | | | (100.0) | % |
There was no Unrealized gain on trading securities for the year ended December 31, 2024 as compared to a gain of $10.7 million for the same period in 2023. The prior year gain was primarily related to market adjustments of investments in trading securities, which consisted of fixed income mutual funds.
Realized loss on Trading Securities
Realized loss on trading securities for the years ended December 31, 2024 and 2023 is as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Realized loss on trading securities | $ | — | | | $ | (5,103) | | | $ | 5,103 | | | 100.0 | % |
There was no Realized loss on trading securities for the year ended December 31, 2024 as compared to a loss of $5.1 million for the same period in 2023. The prior year losses were primarily related to market adjustments of investments in trading securities, which consisted of fixed income mutual funds.
Change in Fair Value of Warrant Liabilities
The warrant liabilities were recorded at fair value as part of the Business Combination. Change in fair value of warrant liabilities primarily consists of the change in the fair value of our Public Warrants and Private Warrants.
Change in warrant liabilities for the years ended December 31, 2024 and 2023 is as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | $ Change | | % Change |
| Change in fair value of warrant liabilities | $ | (3,722) | | | $ | (278) | | | $ | (3,444) | | | 1238.8 | % |
The change in fair value of warrant liabilities increased by $3.4 million, or 1238.8% for the year ended December 31, 2024 as compared to the same period in 2023. Change in fair value of warrant liabilities increased $3.7 million for the year ended December 31, 2024 as compared to an increase of $0.3 million for the same period in 2023. This increase was primarily driven by the change in the underlying trading price of our Class A common stock during the periods reported.
Other income, net
Other income, net, for the years ended December 31, 2024 and 2023 is as follows (dollars in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | $ Change | | % Change |
| Other (expense) income, net | $ | (19) | | | $ | 366 | | | $ | (385) | | | (105.2) | % |
Other (expense) income, net, decreased by $0.4 million, or 105.2%, for the year ended December 31, 2024 as compared to the same period in 2023. For the year ended December 31, 2023, Other income, net, included a $0.4 million gain for the write off of contingent consideration related to the Majelac acquisition. For further details regarding the acquisition of Majelac, please refer to Note 3. Acquisition, in the accompanying notes to the Consolidated Financial Statements included elsewhere in the Annual Report on Form 10-K. Liquidity and Capital Resources
The following table presents a summary of our consolidated cash flows for operating, investing, and financing activities for the years ended December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | |
| | 2024 | | 2023 |
| Net cash (used in) provided by: | | | |
| Net cash used in operating activities | $ | (87,795) | | | $ | (94,036) | |
| Net cash (used in) provided by investing activities | (32,675) | | | 143,428 | |
| Net cash provided by financing activities | 35,876 | | | 149 | |
| Effect of exchange rate changes on cash and cash equivalents | (25) | | | — | |
| Net (decrease) increase in cash and cash equivalents | $ | (84,619) | | | $ | 49,541 | |
Net cash used in operating activities
For the year ended December 31, 2024, net cash used in operating activities of $87.8 million was primarily due to a net loss of $101.0 million resulting from continued spend on research and development and commercialization efforts, accretion on marketable securities of $8.4 million and a $3.2 million write-down of inventory partially offset by stock-based compensation of $8.9 million, a net increase in cash provided by operating assets and liabilities of $4.6 million due to timing of cash receipts and disbursements, depreciation and amortization of $4.6 million, a change in fair value of warrant liabilities of $3.7 million, and non-cash lease expense of $2.4 million.
For the year ended December 31, 2023, the net cash used in operating activities of $94.0 million was primarily due to a net loss of $96.0 million resulting from the continued ramp-up of research and development and commercialization efforts, unrealized net gains on trading securities of $10.7 million, net cash outflows from changes in operating assets and liabilities of $3.2 million due to timing of cash receipts and disbursements and a $3.4 million write-down of inventory, partially offset by stock-based compensation of $8.5 million, realized net losses on trading securities of $5.1 million, depreciation and amortization of $4.2 million and non-cash lease expense of $1.4 million.
Net cash (used in) provided by investing activities
For the year ended December 31, 2024, net cash used in investing activities was $32.7 million as compared to net cash provided by investing activities in $143.4 million for the same period in 2023. The increase in cash used was due primarily to an increase of purchases of marketable securities of $245.6 million partially offset by a $68.9 million increase in proceeds from the sales and maturities of marketable securities.
Net cash provided by financing activities
For the year ended December 31, 2024, net cash provided by financing activities was $35.9 million as compared to $0.1 million for the same period in 2023. This increase in cash provided was due primarily to $34.8 million of net proceeds from the issuance of common stock under the ATM Offering. Further information regarding the ATM Offering can be found below.
Liquidity Outlook
Since our inception, we have funded our operations primarily with proceeds from the issuance of equity to private investors, as well as with the proceeds received from the closing of the Business Combination. Additionally, we began to generate revenue during 2023 from commercial sales of our Platinum instrument. Our primary uses of liquidity have been operating expenses, capital expenditures and our acquisition of certain assets. Cash flows from operations have been historically negative as we continue to invest in the development of our technology in NGPS. Going forward, we anticipate debt or equity offerings will be the primary source of funds to support our operating needs and capital expenditures until we reach scale of our commercial operations. We expect to incur negative operating cash flows on an annual basis for the foreseeable future until such time that we can scale our revenue growth.
We expect that our existing cash and cash equivalents and investments in marketable securities, together with revenue from the sale of our products and services, will be sufficient to meet our liquidity, capital expenditure, and anticipated working capital requirements and fund our operations for at least the next 12 months. We expect to use our cash and cash equivalents and investments in marketable securities and funds from revenue generated to invest in our continued commercialization efforts, to further invest in research and development, for other operating expenses, business acquisitions and for working capital and general corporate purposes.
As of December 31, 2024, we had cash and cash equivalents and investments in marketable securities totaling $209.6 million. Our future capital requirements may vary from those currently planned and will depend on various factors including the pace and success of product commercialization.
Our ongoing commercialization of Platinum and Platinum Pro as well as our continuing research and development efforts to enhance our instruments may require an accelerated amount of spending to enhance the sales and marketing teams, continue to drive development, and build inventory. Other factors that could accelerate cash needs include: (i) delays in achieving scientific and technical milestones, (ii) unforeseen capital expenditures and fabrication costs related to manufacturing for commercialization, (iii) changes we may make in our business or commercialization strategy, (iv) costs of running a public company, (v) other items affecting our forecasted level of expenditures and use of cash resources, including potential acquisitions, and (vi) increased product and service costs.
On August 11, 2023, we filed a universal shelf registration statement on Form S-3 (the “Shelf Registration Statement”), which became effective on August 22, 2023, covering the offering of Class A common stock, preferred stock, debt securities, warrants, rights and units.
On December 11, 2024, we entered into an Equity Distribution Agreement (the “Sales Agreement”) with Canaccord Genuity LLC (“Canaccord”) to sell shares of our Class A common stock, par value $0.0001, having an aggregate offering price of up to $75.0 million, from time to time through an “at-the-market” offering program under which Canaccord will
act as sales agent. We have no obligation to sell any shares under the Sales Agreement and may at any time suspend solicitation and offers under the EDA. The ATM Offering is being made pursuant to our shelf registration and a prospectus supplement related to the ATM Offering dated December 11, 2024. During the year ended December 31, 2024, we sold and issued 23,425,650 shares of our Class A common stock under the ATM Offering, resulting in gross proceeds of $36.2 million. Net proceeds were $34.8 million after commissions and issuance costs of $1.4 million.
Also on December 11, 2024, we exercised its right to terminate the Equity Distribution Agreement (the “Equity Distribution Agreement”), dated August 11, 2023, by and between the Company and Evercore Group L.L.C. (“Evercore”), as sales agent. The Equity Distribution Agreement previously established an “at-the-market” offering program through which we had the right to sell, from time to time, through Evercore, up to an aggregate of $75.0 million of our Class A common stock. We sold no shares under the Equity Distribution Agreement.
On January 3, 2025, we entered into a securities purchase agreement with certain institutional investors pursuant to which we agreed to issue and sell, in a registered direct offering (the “Registered Direct Offering”) an aggregate of 15,625,000 shares of our Class A common stock at a price of $3.20 per Share. The gross proceeds from the Registered Direct Offering were $50.0 million. After deducting estimated placement agents’ fees and other offering expenses payable by the Company, net proceeds were approximately $46.9 million.
In connection with the Registered Direct Offering, we entered into a placement agency agreement with A.G.P./Alliance Global Partners (the “Placement Agent”), pursuant to which the Placement Agent agreed to serve as the sole placement agent for the Company, on a reasonable best efforts basis. We agreed to pay the Placement Agent an aggregate cash fee equal to 6.0% of the gross proceeds received in the Registered Direct Offering.
In the future, we may be unable to obtain any required additional financing on terms favorable to us, if at all. If adequate funds are not available to us on acceptable terms or otherwise, we may be unable to successfully develop or enhance products and services, respond to competitive pressure or take advantage of acquisition opportunities, any of which could have a material adverse effect on our business, financial condition, operating results and cash flows.
Related Party Transactions
For a description of our related party transactions, please refer to Note 17. Related Party Transactions, in the accompanying notes to the Consolidated Financial Statements included elsewhere in the Annual Report on Form 10-K. Capital Expenditures
During the year ended December 31, 2024, capital expenditures were $4.6 million. We forecast capital expenditures in order to execute on our business plan and maintain growth; however, the actual amount and timing of such capital expenditures will ultimately be determined by the volume of business. We anticipate our future capital expenditures will be at approximately the same level as compared to the year ended December 31, 2024. We have funded and plan to continue funding these capital expenditures with cash and financing.
Contractual Obligations
We lease certain facilities and equipment under noncancellable lease agreements that expire at various dates through 2032. As of December 31, 2024, the future lease payments, before adjustments for tenant incentives, were approximately $26.7 million.
Licenses related to certain intellectual property
We license certain intellectual property, some of which may be utilized in our current or future product offerings. To preserve the right to use such intellectual property, we are required to make annual minimum fixed payments totaling approximately $0.2 million as well as royalties based on net sales if the royalties exceed annual minimum fixed payments.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Inflation risk
We believe inflation can and has had an impact on the underlying cost of our supplies and manufacturing components related to our business. To the extent our costs are impacted by general inflationary pressures, we may not be able to fully offset such higher costs through price increases or manufacturing efficiencies. Our inability or failure to do so could harm our business, financial condition, results of operations or cash flows.
Interest rate risk
As of December 31, 2024, our marketable securities are comprised primarily of investments in money market funds backed by U.S. government issued securities, U.S. Treasury bills, and high-quality corporate commercial paper. The primary objective of our investments is the preservation of capital to fulfill liquidity needs. We do not enter into investments for trading or speculative purposes. Based on the short-term nature of our holdings, future interest rate changes are not expected to have a material impact on our marketable securities.
Foreign currency risk
Presently, we operate our business primarily within the United States, with limited sales outside the United States. To date, we have executed the majority of our transactions in U.S. dollars. In the future, we anticipate expanding into Europe and other locations outside the United States. This expansion may include transacting business in currencies other than the U.S. Dollar. Despite this, we anticipate conducting limited activity outside the U.S. Dollar in the near term, and therefore foreign currency translation risk is not expected to have a material impact on our Consolidated Financial Statements. However, the growth of our operations, scope of transactions outside the United States, and the use of currencies other than the U.S. Dollar may grow in the future, at which point it is possible foreign currency translation will have a material effect on our operations. To date, we have not entered into any hedging arrangements with respect to foreign currency risk. As our international operations grow, we will continue to reassess our approach to managing our risk relating to fluctuations in currency rates.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Quantum-Si Incorporated
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Quantum-Si Incorporated and its subsidiaries (the “Company”) as of December 31, 2024, and the related consolidated statements of operations and comprehensive loss, of changes in stockholders’ equity (deficit) and of cash flows for the year then ended, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Valuation of Stock Option Grants
As described in Notes 2 and 11 to the consolidated financial statements, the Company measures compensation expense for stock-based awards to employees, non-employees and directors based upon the awards’ initial grant-date fair values. Stock-based compensation expense for stock options is recorded over the requisite service period. The fair values of stock option grants with only a service condition are estimated as of the date of grant by applying the Black-Scholes option valuation model (“Black-Scholes model”). The Black-Scholes model incorporates assumptions as to stock price volatility, the expected life of options, a risk-free interest rate, and dividend yield. The Black-Scholes model is affected by the stock price on the date of the grant, as well as assumptions regarding a number of variables. These variables include the expected term of the option, expected risk-free interest rate, expected volatility of common stock, and expected dividend yield. The
assumptions for the expected term of the option and expected volatility of common stock are the two assumptions that significantly affect the grant date fair value. The Company’s stock-based compensation expense related to stock options for the year ended December 31, 2024 was $6.4 million.
The principal considerations for our determination that performing procedures relating to the valuation of stock option grants is a critical audit matter are (i) the significant judgment by management when developing the fair value estimate of the stock option grants and (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumption related to expected volatility.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included, among others (i) testing management’s process for developing the fair value estimate of the stock option grants; (ii) evaluating the appropriateness of the Black-Scholes model; (iii) testing the completeness and accuracy of the underlying data used in the estimate of the fair value of the stock option grants; (iv) testing stock-based compensation expense for a sample of stock options granted by obtaining and inspecting source documents, such as grant award letters and Board of Director minutes; and (v) evaluating the reasonableness of the significant assumption used by management related to expected volatility. Evaluating management’s assumption related to expected volatility involved considering (i) the historical stock prices and volatilities of the Company; (ii) the historical stock prices and volatilities of the peer group data by utilizing data obtained from an independent-third party source; and (iii) whether the assumption was consistent with evidence obtained in other areas of the audit.
/s/ Pricewaterhouse Coopers LLP
San Diego, California
March 3, 2025
We have served as the Company’s auditor since 2024.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of Quantum-Si Incorporated:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Quantum-Si Incorporated and subsidiaries (the “Company”) as of December 31, 2023, the related consolidated statements of operations and comprehensive loss, changes in convertible preferred stock and stockholders’ equity (deficit), and cash flows, for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte & Touche LLP
New York, New York February 29, 2024 (March 3, 2025, as to the effects of the adoption of ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, described in Note 2)
We began serving as the Company’s auditor in 2020. In 2024 we became the predecessor auditor.
QUANTUM-SI INCORPORATED
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and par value amounts)
| | | | | | | | | | | |
| | December 31,
2024 | | December 31,
2023 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 49,241 | | | $ | 133,860 | |
| Marketable securities | 160,362 | | | 123,876 | |
| Accounts receivable, net of allowance of $124 and $0, respectively | 1,333 | | | 368 | |
| Inventory, net | 4,067 | | | 3,945 | |
| Prepaid expenses and other current assets | 3,006 | | | 4,261 | |
| Total current assets | 218,009 | | | 266,310 | |
| Property and equipment, net | 15,993 | | | 16,275 | |
| Internally developed software, net | — | | | 532 | |
| Operating lease right-of-use assets | 13,061 | | | 14,438 | |
| Other assets | 808 | | | 695 | |
| Total assets | $ | 247,871 | | | $ | 298,250 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 1,931 | | | $ | 1,766 | |
| Accrued payroll and payroll-related costs | 5,331 | | | 4,943 | |
| Accrued contracted services | 2,379 | | | 1,519 | |
| Accrued expenses and other current liabilities | 4,848 | | | 1,815 | |
| Current portion of operating lease liabilities | 3,698 | | | 1,566 | |
| Total current liabilities | 18,187 | | | 11,609 | |
| Warrant liabilities | 4,995 | | | 1,274 | |
| Operating lease liabilities | 9,250 | | | 13,737 | |
| Other long-term liabilities | 19 | | | 11 | |
| Total liabilities | 32,451 | | | 26,631 | |
| Commitments and contingencies (Note 18) | | | |
| Stockholders’ equity: | | | |
| Class A Common stock, $0.0001 par value; 600,000,000 shares authorized as of December 31, 2024 and December 31, 2023; 146,953,271 and 121,832,417 shares issued and outstanding as of December 31, 2024 and December 31, 2023, respectively | 16 | | | 12 | |
| Class B Common stock, $0.0001 par value; 27,000,000 shares authorized as of December 31, 2024 and December 31, 2023; 19,937,500 shares issued and outstanding as of December 31, 2024 and December 31, 2023 | 2 | | | 2 | |
| Additional paid-in capital | 811,998 | | | 767,239 | |
| Accumulated other comprehensive income | 45 | | | - | |
| Accumulated deficit | (596,641) | | | (495,634) | |
| Total stockholders’ equity | 215,420 | | | 271,619 | |
| Total liabilities and stockholders’ equity | $ | 247,871 | | | $ | 298,250 | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
QUANTUM-SI INCORPORATED
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except per share amounts)
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Revenue | | | | | |
| Product | $ | 2,925 | | | $ | 1,031 | | | $ | — | |
| Service | 133 | | | 51 | | | — | |
| Total revenue | 3,058 | | | 1,082 | | | — | |
| | | | | |
| Cost of revenue | 1,458 | | | 594 | | | — | |
| | | | | |
| Gross profit | 1,600 | | | 488 | | | — | |
| Operating expenses: | | | | | |
| Research and development | 59,641 | | | 67,025 | | | 72,062 | |
| Selling, general and administrative | 50,535 | | | 44,634 | | | 42,296 | |
| Goodwill impairment | — | | | — | | | 9,483 | |
| Total operating expenses | 110,176 | | | 111,659 | | | 123,841 | |
| Loss from operations | (108,576) | | | (111,171) | | | (123,841) | |
| Dividend and interest income | 11,366 | | | 9,536 | | | 5,301 | |
| Unrealized gain (loss) on trading securities | - | | | 10,690 | | | (16,162) | |
| Realized loss on trading securities | - | | | (5,103) | | | (4,441) | |
| Change in fair value of warrant liabilities | (3,722) | | | (278) | | | 6,243 | |
| Other (expense) income, net | (19) | | | 366 | | | 458 | |
| Loss before provision for income taxes | (100,951) | | | (95,960) | | | (132,442) | |
| Provision for income taxes | (56) | | | — | | | — | |
| Net loss | $ | (101,007) | | | $ | (95,960) | | | $ | (132,442) | |
| | | | | |
| Net loss per common share attributable to common stockholders, basic and diluted | $ | (0.71) | | | $ | (0.68) | | | $ | (0.95) | |
| Weighted-average shares used to compute net loss per share attributable to common stockholders, basic and diluted | 143,196 | | 141,300 | | 139,255 |
| | | | | |
| Other comprehensive gain (loss): | | | | | |
| Net unrealized gain on marketable securities, net of tax | $ | 70 | | | $ | — | | | $ | — | |
| Foreign currency translation adjustment | (25) | | | — | | | — | |
| Total other comprehensive gain, net of tax | 45 | | | — | | | — | |
| Comprehensive loss | $ | (100,962) | | | $ | (95,960) | | | $ | (132,442) | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
QUANTUM-SI INCORPORATED
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Class A common stock | | Class B common stock | | Additional
paid-in capital | | Accumulated other comprehensive income (loss) | | Accumulated
deficit | | Total
stockholders’
equity (deficit) |
| Shares | | Amount | | Shares | | Amount | | | | |
| Balance - January 1, 2022 | 118,025,410 | | $ | 12 | | | 19,937,500 | | $ | 2 | | | $ | 744,252 | | | $ | — | | | $ | (267,232) | | | $ | 477,034 | |
| Common stock issued upon exercise of stock options | 1,921,824 | | — | | — | | — | | 2,757 | | — | | — | | 2,757 |
| | | | | | | | | | | | | | | |
| Majelac Technologies LLC Acquisition | 59,523 | | — | | — | | — | | 151 | | — | | — | | 151 |
| Stock-based compensation | — | | — | | — | | — | | 11,206 | | — | | — | | 11,206 |
| Net loss | — | | — | | — | | — | | — | | — | | (132,442) | | (132,442) |
| Balance - December 31, 2022 | 120,006,757 | | 12 | | 19,937,500 | | 2 | | 758,366 | | — | | (399,674) | | 358,706 |
| Common stock issued upon exercise of stock options | 1,825,660 | | — | | — | | — | | 357 | | — | | — | | 357 |
| | | | | | | | | | | | | | | |
| Stock-based compensation | — | | — | | — | | — | | 8,516 | | — | | — | | 8,516 |
| Net loss | — | | — | | — | | — | | — | | — | | (95,960) | | (95,960) |
| Balance - December 31, 2023 | 121,832,417 | | 12 | | 19,937,500 | | 2 | | 767,239 | | — | | (495,634) | | 271,619 |
| Common stock issued upon exercise of stock options | 598,689 | | 1 | | — | | — | | 1,071 | | — | | — | | 1,072 |
| Common stock issued upon vesting of restricted stock units | 1,096,515 | | 1 | | — | | — | | — | | — | | — | | 1 |
| Common stock issued from at-the-market offering, net of commissions and issuance costs | 23,425,650 | | 2 | | — | | — | | 34,787 | | — | | — | | 34,789 |
| Stock-based compensation | — | | — | | — | | — | | 8,887 | | — | | — | | 8,887 |
| Net unrealized gain on marketable securities, net of tax | — | | — | | — | | — | | — | | 70 | | — | | 70 |
| Refund of issuance costs | — | | — | | — | | — | | 14 | | — | | — | | 14 |
| Foreign currency translation | — | | — | | — | | — | | — | | (25) | | — | | (25) |
| Net loss | — | | — | | — | | — | | — | | — | | (101,007) | | (101,007) |
| Balance - December 31, 2024 | 146,953,271 | | $ | 16 | | | 19,937,500 | | $ | 2 | | | $ | 811,998 | | | $ | 45 | | | $ | (596,641) | | | $ | 215,420 | |
| | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
QUANTUM-SI INCORPORATED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2024 | | 2023 | | 2022 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (101,007) | | | $ | (95,960) | | | $ | (132,442) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 4,600 | | | 4,156 | | | 2,584 | |
| Allowance for credit losses | 124 | | | — | | | — | |
| Non-cash lease expense | 2,424 | | | 1,402 | | | 1,249 | |
| Unrealized (gain) loss on trading securities, net | — | | | (10,690) | | | 16,162 | |
| Realized loss on trading securities, net | — | | | 5,103 | | | 4,441 | |
| Accretion on marketable securities | (8,405) | | | — | | | — | |
| Loss on disposal of fixed assets | 425 | | | 136 | | | 91 | |
| Write-down of inventory | (3,229) | | | (3,382) | | | — | |
| Goodwill impairment | — | | | — | | | 9,483 | |
| Change in fair value of warrant liabilities | 3,721 | | | 278 | | | (6,243) | |
| Change in fair value of contingent consideration | — | | | (400) | | | 176 | |
| Change in fair value of stock consideration | — | | | — | | | (320) | |
| Stock-based compensation | 8,887 | | | 8,516 | | | 11,206 | |
| Other | 22 | | | — | | | — | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable, net | (1,089) | | | (368) | | | — | |
| Inventory, net | 4,342 | | | 286 | | | — | |
| Prepaid expenses and other current assets | 486 | | | 2,159 | | | (1,005) | |
| Operating lease right-of-use assets | — | | | (83) | | | (10,033) | |
| Other assets | (113) | | | 2 | | | (7) | |
| Accounts payable | 390 | | | (1,220) | | | 721 | |
| Accrued expenses and other current liabilities | 4,021 | | | (1,835) | | | 4,009 | |
| Other long-term liabilities | 7 | | | 7 | | | — | |
| Operating lease liabilities | (3,401) | | | (2,143) | | | 9,368 | |
| Net cash used in operating activities | (87,795) | | | (94,036) | | | (90,560) | |
| Cash flows from investing activities: | | | | | |
| Purchases of property and equipment | (4,583) | | | (4,510) | | | (10,741) | |
| Internally developed software - capitalized costs | (59) | | | (763) | | | — | |
| Purchases of marketable securities | (369,433) | | | (123,809) | | | (834) | |
| Sales of marketable securities | 341,400 | | | 272,510 | | | 148,760 | |
| Net cash (used in) provided by investing activities | (32,675) | | | 143,428 | | | 137,185 | |
| Cash flows from financing activities: | | | | | |
| Proceeds from exercise of stock options | 1,072 | | | 357 | | | 2,757 | |
| Proceeds from issuance of common stock from “at-the-market” offering, net of commissions and issuance costs | 34,789 | | | — | | | — | |
| Proceeds from vesting of restricted stock | 1 | | | — | | | — | |
| Deferred offering costs | — | | | (208) | | | — | |
| Payment of contingent consideration - business acquisition | — | | | — | | | (348) | |
| Payment of deferred consideration - business acquisition | — | | | — | | | (500) | |
| Refund of issuance costs | 14 | | | — | | | — | |
| Net cash provided by financing activities | 35,876 | | | 149 | | | 1,909 | |
| Effect of exchange rate changes on cash and cash equivalents | (25) | | | — | | | — | |
| Net (decrease) increase in cash and cash equivalents | (84,619) | | | 49,541 | | | 48,534 | |
| Cash and cash equivalents at beginning of period | 133,860 | | | 84,319 | | | 35,785 | |
| Cash and cash equivalents at end of period | $ | 49,241 | | | $ | 133,860 | | | $ | 84,319 | |
| | | | | | | | | | | | | | | | | |
| Supplemental disclosure of cash flow information: | | | | | |
| Cash received from exchange of research and development tax credits | $ | — | | | $ | 1,523 | | | $ | — | |
| Supplemental disclosure of non-cash investing and financing activities: | | | | | |
| Property and equipment purchased but not paid | $ | 337 | | | $ | 302 | | | $ | 1,260 | |
| Deferred offering costs payable | $ | 553 | | | $ | 105 | | | $ | — | |
| Noncash equity issuance - business acquisition | $ | — | | | $ | — | | | $ | 151 | |
The accompanying notes are an integral part of these Consolidated Financial Statements.
QUANTUM-SI INCORPORATED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Description of Business and Basis of Presentation
Background
Quantum-Si Incorporated (including its subsidiaries, the “Company” or “Quantum-Si”) was incorporated in Delaware on June 10, 2020 as HighCape Capital Acquisition Corp. (“HighCape”). The Company’s legal name became Quantum-Si Incorporated following a business combination on June 10, 2021 between the Company and Q-SI Operations Inc. (formerly Quantum-Si Incorporated) (the “Business Combination”), which was founded in 2013.
The Company is a life sciences company focused on proteomics research, with the mission of transforming single-molecule analysis and democratizing its use by providing researchers and clinicians access to the proteome, the set of proteins expressed within a cell. The Company has developed a proprietary universal single-molecule detection platform that it is applying to proteomics to enable Next-Generation Protein SequencingTM (“NGPS”), to sequence proteins in a massively parallel fashion (rather than sequentially, one at a time), which can also be used for the study of nucleic acids. The Company believes that the ability to sequence proteins in a massively parallel fashion and offer a fast analysis time provides NGPS with the potential to unlock significant biological information through improved resolution and unbiased access to the proteome at a speed and scale that is not available today. Traditionally, proteomic workflows to sequence proteins required days or weeks to complete. Our platform includes our Platinum NGPS instrument, Platinum Analysis Software, and consumable kits for use with our Platinum instrument.
Liquidity and Capital Resources
The Company has historically financed its operations primarily with proceeds from the issuance of equity to private investors, as well as with the proceeds received from the closing of the Business Combination. The Company has incurred significant losses and negative cash flows from operations in all periods since inception and had an accumulated deficit of $596.6 million as of December 31, 2024. The Company has incurred significant operating losses, including net losses of $101.0 million, $96.0 million and $132.4 million for the years ended December 31, 2024, 2023 and 2022 respectively. As of December 31, 2024, the Company had cash, cash equivalents and investments in marketable securities of $209.6 million. Management believes that the Company’s current cash, cash equivalents and marketable securities, together with revenue from the sales of its products and services, will be sufficient to fund its planned operations for at least the next twelve months from the date of the issuance of the accompanying Consolidated Financial Statements.
Until such time as the Company can generate significant revenue from product sales, if ever, it expects to finance its operations through private and public equity offerings, debt financings, and/or potential future collaboration, license and development agreements. However, there can be no assurance that the Company will be able to complete any such transactions on acceptable terms or otherwise, and the Company may be unable to obtain sufficient additional capital when needed. The inability to raise capital as and when needed would have a negative impact on the Company’s financial condition and its ability to pursue its business strategy. The Company will need to generate significant revenue to achieve profitability and it may never do so.
Global Developments
Sustained levels of high inflation caused the U.S. Federal Reserve and other central banks to increase interest rates throughout 2022 and 2023. Although the U.S. Federal Reserve lowered interest rates slightly in 2024, it is not known whether additional action will be taken to lower interest rates and if this decrease, and any other decreases, will have an impact on inflation. While these rate fluctuations have not had a significant adverse impact on the Company to date, the impact of such rate increases on the overall financial markets and the economy may adversely impact the Company in the future. In addition, the global economy has experienced and is continuing to experience high levels of inflation and global supply chain disruptions. The Company continues to monitor these supply chain, inflation and interest rate factors, as well as the uncertainty resulting from the overall economic environment.
Although the Company was not significantly impacted by geopolitical conflicts, such as those in Ukraine or Israel and Gaza, the Company has experienced some constraints in product and material availability and increasing costs required to obtain some materials and supplies as a result of these conflicts on the global economy. To date, the business has not been
materially impacted by the conflicts, however, as the conflicts continue or worsen, it may impact the business, financial condition, results of operations and cash flows.
Basis of Presentation and Principles of Consolidation
The accompanying Consolidated Financial Statements include the accounts of the Company and have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the accounting disclosure rules and regulations of the Securities and Exchange Commission (the “SEC”). All intercompany transactions are eliminated.
Reclassifications
Certain prior year amounts have been reclassified for consistency with the current year’s presentation.
Note 2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of the Consolidated Financial Statements in conformity with U.S. GAAP requires the Company to make estimates and assumptions about future events that affect the amounts recorded in its Consolidated Financial Statements and accompanying notes. Future events and their effects cannot be determined with certainty. On an ongoing basis, management evaluates these estimates and assumptions. Significant estimates and assumptions include:
•inventory valuation;
•valuation for acquisitions;
•valuation of goodwill;
•assumptions used for leases;
•valuation allowances with respect to deferred tax assets;
•valuation of warrant liabilities;
•assumptions associated with revenue recognition; and
•assumptions underlying the fair value used in the calculation of the stock-based compensation.
The Company bases these estimates on historical and anticipated results and trends and on various other assumptions the Company believes are reasonable under the circumstances, including assumptions as to future events. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates, and any such differences may be material to the Consolidated Financial Statements.
Concentration of Business Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash and cash equivalents and marketable securities. As of December 31, 2024 and 2023, the Company’s marketable securities consist of mutual funds, U.S. Treasury securities and commercial paper. For further information regarding marketable securities, please refer to Note 4. Investments in Marketable Securities. The Company also maintains balances in certain operating accounts above federally insured limits and, as a result, the Company is exposed to credit risk in the event of default by the financial institutions to the extent account balances exceed the amount insured by the Federal Deposit Insurance Corporation. The Company sources certain key materials and components utilized in the Company’s products from single or limited suppliers. Historically, the Company has not experienced significant issues sourcing these materials and components. However, if these suppliers were not able to supply the requested amount of materials or components, it could take a considerable length of time to obtain alternative sources, which could affect the Company’s development efforts and commercial operations.
Segment Reporting
The Company’s Chief Operating Decision Maker (the “CODM”), its Chief Executive Officer, reviews financial information presented on a consolidated basis for purposes of assessing financial performance, making operating decisions and allocating resources. Accordingly, the Company has determined that it operates as a single reportable segment. For further discussion related to segment reporting, please refer to Note 16. Segment Information. Foreign Currency Translation and Transactions
For the Company’s international operations, the local currency has been determined to be the functional currency. The results of its non-U.S. dollar-based operations are translated to U.S. dollars at the average exchange rates during the period. Assets and liabilities are translated at the rate of exchange prevailing on the balance sheet date. Equity is translated at the prevailing rate of exchange at the date of the equity transaction. The translational effects of revaluing non-functional currency assets and liabilities were immaterial for the years ended December 31, 2024, 2023 and 2022.
The Company realizes foreign currency gains/(losses) in the normal course of business based on movement in the applicable exchange rates. As of December 31, 2024 and 2023 and for the years ended December 31, 2024, 2023 and 2022, the effect of foreign currency translation and transactions on the Company’s Consolidated Financial Statements was immaterial.
Cash Equivalents
All highly liquid investments purchased with a maturity of three months or less are cash equivalents. As of December 31, 2024 and 2023, cash and cash equivalents consist of a mix of cash and short-term money market accounts, U.S. Treasury securities and commercial paper.
Accounts Receivable, Net
Accounts receivable, net are stated at the amount the Company expects to collect from customers based on their outstanding invoices. The Company reviews accounts receivable regularly to determine if any receivable may not be collectible. The Company estimates the amount of the allowance for doubtful accounts necessary to reduce accounts receivable to its estimated net realizable value by analyzing the status of significant past due receivables and current and historical bad debt trends. The Company writes off accounts receivable against the allowance when it determines a balance is uncollectible and ceases collection efforts. As of the December 31, 2024, the Company recorded a $0.1 million allowance against accounts receivable. There was no such allowance as of December 31, 2023. The Company did not write off any accounts receivable balances during the years ended December 31, 2024 and 2023.
Marketable Securities
The Company considers all of its investments in marketable securities as available for use in current operations and therefore classifies these securities within current assets in the Consolidated Balance Sheets.
During the year ended December 31, 2023, the Company’s investments in marketable securities were classified as trading securities and consisted of ownership interests in fixed income mutual funds. As of December 31, 2024 and 2023 the Company’s marketable securities were classified as available-for-sale securities, carried at fair value, with the unrealized holding gains/(losses), net of income taxes, reflected in accumulated other comprehensive income/(loss) until realized. Regardless of the intent to sell a security, the Company performs additional analysis on all securities with unrealized losses to evaluate losses associated with the creditworthiness of the security. Credit losses are recorded when the Company does not expect to receive cash flows sufficient to recover the amortized cost basis of a security. For the purposes of computing realized and unrealized gains and losses, cost and fair value are determined on a specific identification basis. Purchase premiums and discounts on marketable debt securities are amortized or accreted into the cost basis over the life of the related security as adjustments to the yield using the effective-interest method.
Dividends on marketable securities are recognized as income when declared and are recorded as Dividend income in the Consolidated Statements of Operations and Comprehensive Loss. Interest income is recognized when earned.
Inventory, Net
Inventory is stated at the lower of cost or net realizable value with cost determined using the first-in, first-out method. Materials that may be utilized for either commercial or, alternatively, for research and development purposes, are classified as inventory. Amounts in inventory used for research and development purposes are charged to research and development expense when the product enters the research and development process and can no longer be used for commercial purposes and, therefore, does not have an “alternative future use” as defined in authoritative guidance.
Inventory valuation is established based on a number of factors including, but not limited to, finished goods not meeting product specifications, product excess and obsolescence, or application of the lower of cost or net realizable value concepts. The determination of events requiring the establishment of inventory valuation, together with the calculation of the amount of such adjustments may require judgment. The Company performs an assessment of the recoverability of capitalized inventory during each reporting period and, if needed, records a write-down of inventory to its estimated net realizable value in the period it is identified. For further discussion related to inventory, please refer to Note 6. Inventory, Net. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets include amounts paid in advance for operating expenses as well as monies to be received from the State of Connecticut for research and development tax credits. These research and development tax credits are exchanged for a cash refund and are typically collected within one year from the date the tax return is filed with the state. The credits are recognized as an offset to research and development expenses in the Consolidated Statements of Operations and Comprehensive Loss in the annual period the corresponding expenses were incurred.
Property and Equipment, Net
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation expense is computed using the straight-line method over the estimated useful lives of the related assets. Leasehold improvements are amortized over the shorter of the asset’s useful life or the life of the lease term.
Estimated useful lives of property and equipment are as follows:
| | | | | | | | |
| Property and equipment, net | | Estimated useful life |
| Laboratory and production equipment | | 3-5 years |
| Computer equipment | | 3-5 years |
| Software | | 3 years |
| Furniture and fixtures | | 3-7 years |
Expenditures for major renewals and improvements are capitalized. Expenditures for repairs and maintenance are expensed as incurred. Costs for property and equipment not yet placed into service have been recorded as construction in process and will be depreciated in accordance with the above guidelines once placed into service. When assets are retired or otherwise disposed of, the cost of these assets and related accumulated depreciation and amortization is eliminated from the balance sheet, and any resulting gains or losses are included in the Consolidated Statements of Operations and Comprehensive Loss in the period of disposal.
Capitalized Software Development Costs
The Company capitalizes certain internal use software development costs related to its SaaS platform incurred during the application development stage when management with the relevant authority authorizes and commits to the funding of the project, it is probable the project will be completed, and the software will be used as intended. The Company also capitalizes costs related to specific upgrades and enhancements when it is probable the expenditure will result in additional functionality. Costs related to preliminary project activities and to post-implementation activities are expensed as incurred. Internal use software is amortized on a straight-line basis over its estimated useful life, which is generally two years. Management evaluates the useful lives of these assets on an annual basis and tests for impairment whenever events or changes in circumstances occur that could impact the recoverability of the assets. Capitalized costs are recorded as Internally developed software in the Consolidated Balance Sheets. Amortization expense related to internally developed
software was $0.6 million and $0.2 million for the years ended December 31, 2024 and 2023, respectively. There was no internally developed software or related amortization expense recorded for the year ended December 31, 2022.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment when the Company determines a triggering event has occurred. When a triggering event has occurred, each impairment test is based on a comparison of the future expected undiscounted cash flows to the recorded value of the asset. If the recorded value of the asset is less than the undiscounted cash flows, the asset is written down to its estimated fair value. No impairments were recorded for the years ended December 31, 2024, 2023, and 2022.
Leases
The Company’s leases generally do not have a readily determinable implicit discount rate. As such, the Company uses an incremental borrowing rate based on the information available at the lease commencement date to determine the present value of the lease payments. The Company’s incremental borrowing rate is the estimated rate that would be required to pay for a collateralized borrowing equal to the total lease payment over the lease term. The Company measures right-of-use (“ROU”) assets based on the corresponding lease liability adjusted for (i) payments made to the lessor at or before the commencement date, (ii) initial direct costs incurred and (iii) tenant incentives under the lease. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that it will exercise that option. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term for operating leases. Finance leases will result in a front-loaded expense pattern. With respect to finance leases, amortization of the ROU asset is presented separately from interest expense related to the finance lease liability. In addition, the Company does not have significant residual value guarantees or restrictive covenants in the lease portfolio.
Certain of the Company’s lease agreements contain tenant improvement incentives and allowances, rent holidays, or rent escalation clauses. For tenant improvement incentives, if the incentive is determined to be a leasehold improvement owned by the lessee and the Company is reasonably certain to use the incentive, the Company generally records the incentive as a reduction to the fixed lease payments liability as a reduction to lease cost. Reimbursable construction costs incurred are recorded as leasehold improvements and are amortized over the term of the lease. The Company records rental expense related to rent holidays and rent escalation clauses on a straight-line basis over the term of the lease. The Company uses the date of initial possession as the commencement date for lease incentives, which is generally when the Company is given right of access to the space and begins to make improvements in preparation for intended use.
Upon inception, leases are evaluated and classified as operating or finance for financial reporting purposes. Operating leases that are short term in nature and have month-to-month payment terms are expensed as incurred in Research and development expenses or in Selling, general and administrative expenses in the Consolidated Statements of Operations and Comprehensive Loss. The Company’s lease agreements contain variable lease costs for common area maintenance, utilities, taxes and insurance, which are expensed as incurred.
Goodwill
The Company reviews goodwill for possible impairment annually during the fourth quarter as of October 1 or when events or circumstances indicate the carrying amount may not be recoverable.
In order to test goodwill for impairment, an entity is permitted to first assess qualitative factors to determine whether a quantitative assessment of goodwill is necessary. The qualitative factors considered by the Company may include, but are not limited to, general economic conditions, the Company’s outlook, market performance of the Company’s industry and recent and forecasted financial performance. If a quantitative assessment is required, the Company determines the fair value of its reporting unit using a combination of the income and market approaches. If the net book value of the reporting unit exceeds its fair value, the Company recognizes a goodwill impairment charge for the reporting unit equal to the lesser of (i) the total goodwill allocated to that reporting unit and (ii) the amount by which that reporting unit’s carrying amount exceeds its fair value.
Majelac Technologies LLC (“Majelac”) was fully integrated into the Company after the acquisition. As a result, the Company operates as a single reporting unit. Accordingly, all of the goodwill is associated with the entire Company. The Company performed its annual goodwill impairment test during the fourth quarter of 2022 quantitatively evaluating its reporting unit. The Company determined the fair value of its reporting unit using a combination of the income and market
approaches. The Company placed a 100% weighting on the market approach method, as the results of the income approach were not representative of the fair value of the Company. The determination of fair value using a market approach requires management to make significant assumptions related to the determination of an appropriate group of peer companies, and market revenue multiples from within the selected group of peer companies. As of October 1, 2022, the carrying value of the net asset of the Company’s reporting unit exceeded its enterprise wide fair value and the Company recognized a goodwill impairment charge, fully impairing goodwill of $9.5 million in the Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2022. There was no impairment of goodwill recorded for the years ended December 31, 2024 or 2023.
Warrant Liabilities
The Company’s outstanding warrants include publicly traded warrants (the “Public Warrants”) which were issued as one-third of one redeemable warrant per unit issued during HighCape’s initial public offering on September 9, 2020, and warrants sold in a private placement (the “Private Warrants”) to HighCape’s sponsor, HighCape Capital Acquisition LLC (the “Sponsor”). The Public Warrants and Private Warrants meet the definition of a derivative and the Company recorded these warrants as long-term liabilities in the Consolidated Balance Sheets at fair value upon the closing of the Business Combination, with subsequent changes in their respective fair values recognized in the Consolidated Statements of Operations and Comprehensive Loss at each reporting date. For further discussion related to the Public Warrants and Private Warrants, please refer to Note 12. Warrant Liabilities. Warranty
The Company provides a free one-year assurance-type warranty to customers with the initial purchase of a Platinum instrument. The cost of the warranty is accrued upon the initial sale of an instrument in Accrued expenses and other current liabilities in the Consolidated Balance Sheets.
Revenue Recognition
The Company’s revenue is derived from sales of products and services. Product revenue is primarily generated from the sales of instruments and consumables used in protein sequencing and analysis. Service revenue is primarily generated from service maintenance contracts including access to analysis software and advanced training for instrument use. The Company recognizes revenue when or as a customer obtains control of the promised goods and services. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled in exchange for these goods and services. This process involves identifying the contract with a customer, determining the performance obligations in the contract, determining the contract price, allocating the contract price to the distinct performance obligations in the contract, and recognizing revenue as the performance obligations have been satisfied. The Company has made the accounting policy election allowed for under ASC 606-10-32-2A to exclude all sales taxes from transaction price. Revenue recognition for contracts with multiple deliverables is based on the separate satisfaction of each distinct performance obligation within the contract. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. The Company allocates transaction price to the performance obligations in a contract with a customer based on the relative standalone selling price of each performance obligation. The Company determines standalone selling price based on the price at which the performance obligation is sold separately. If the standalone selling price is not observable through past transactions, the Company estimates the standalone selling price taking into account available information and specific factors such as competitive positioning, internal costs, profit objectives, and internally approved pricing guidelines related to the performance obligation.
The Company considers performance obligation for sales of products satisfied upon shipment of the goods to the customer in accordance with the shipping terms (either upon shipment or delivery), which is when control of the product is deemed to be transferred; this includes instruments and consumables. Customers generally do not have a right to return products, except for defective or damaged products during the warranty period or unless prior written consent is provided. In instances where right of payment or transfer of title is contingent upon the customer’s acceptance of the product, revenue is deferred until all acceptance criteria have been met. Revenues for service maintenance contracts, which start after the first year of purchase and are considered as service type warranties that effectively extend the standard first-year service coverage at the customer’s option are recognized ratably over the contract service period as these services are performed evenly over time. Revenues for advanced training is recognized at a point in time upon satisfaction of the underlying performance obligation. The Company typically provides a standard one-year warranty which covers defects in materials, workmanship and manufacturing or performance conditions under normal use and service. The first year of the warranty of
the products is considered an assurance-type warranty and is recorded as Cost of revenue within the Consolidated Statements of Operations and Comprehensive Loss. The Company has determined the standard first-year warranty is not a distinct performance obligation.
The Company disaggregates revenue from contracts with customers by type of revenue. The Company believes product revenue and service revenue aggregate the payor types by nature, amount, timing and uncertainty of its revenue streams. Total revenue generated from domestic and international sales was $1.2 million and $1.8 million, respectively, for the year ended December 31, 2024. Total revenue generated from domestic and international sales was $0.8 million and $0.3 million, respectively, for the year ended December 31, 2023. There was no revenue for the year ended December 31, 2022.
Deferred Revenue
Deferred revenue primarily consists of billings and payments received in advance of revenue recognition from service maintenance contracts including software subscription and advanced training, and is reduced as the revenue recognition criteria are met. Deferred revenue also includes advanced training provided to customers until the service has been performed. Deferred revenue that will be recognized as revenue within the succeeding 12-month period is recorded as current and is included within Accrued expenses and other current liabilities in the Company’s Consolidated Balance Sheets. The portion of deferred revenue where revenue is expected to be recognized beyond twelve months from the reporting date is recorded as non-current deferred revenue and is included in Other long-term liabilities in the Company’s Consolidated Balance Sheets.
As of December 31, 2024, the Company had deferred revenue of $0.2 million included within Accrued expenses and other current liabilities in the Company’s Consolidated Balance Sheets. As of December 31, 2024, amounts included within Other long-term liabilities in the Company’s Consolidated Balance Sheets were immaterial. The Company expects to recognize approximately 90% of its remaining performance obligations as revenue during the year ending December 31, 2025.
Shipping and Handling Costs
Shipping and handling costs associated with outbound freight after control of a product has transferred to a customer are accounted for as fulfillment costs and are included in Cost of revenue in the Consolidated Statements of Operations and Comprehensive Loss. Shipping and handling costs billed to customers are considered part of the transaction price and are recognized as revenue with the underlying product sales.
Research and Development
Research and development expenses primarily consist of personnel costs and benefits, stock-based compensation, lab supplies, consulting and professional services, fabrication services, facilities costs, software, and other outsourced expenses. Research and development expenses are expensed as incurred and are primarily related to the development of new products and services.
Selling, General and Administrative
Selling, general and administrative expenses primarily consist of personnel costs and benefits, stock-based compensation, patent and filing fees, consulting and professional services, legal and accounting services, facilities costs, depreciation and amortization expense, insurance and office expenses, product advertising and marketing. Advertising costs are expensed as incurred. Advertising expenses were immaterial for the years ended December 31, 2024, 2023 and 2022.
Net Loss per Share
Basic net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of Class A and Class B common stock of the Company outstanding during the period, without consideration of potentially dilutive securities.
Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of Class A and Class B common shares plus the common equivalent shares of the period, including any dilutive effect from such shares. The Company’s diluted net loss per share is the same as basic net loss per share for all
periods presented, since the effect of potentially dilutive securities is anti-dilutive. For further discussion, please refer to Note 13. Net Loss Per Share. Stock-Based Compensation
Stock-based compensation expense for restricted stock and stock option grants with only service conditions is recognized on a straight-line basis over the requisite service period of the individual grants, which is generally the vesting period, based on the estimated grant date fair values. Stock-based compensation expense for stock option grants subject to non-financing event performance conditions on an accelerated basis is recognized as though each vesting portion of the award was, in substance, a separate award.
The fair value of restricted stock is estimated using the current market price of the Company’s common stock on the date of grant.
Prior to the Business Combination, the fair value of the shares of common stock underlying stock options had historically been determined by the Board, with input from management and contemporaneous third-party valuations, as there was no public market for the common stock. Given the absence of a public trading market for the Company’s common stock, the Board exercised reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of the fair value of the Company’s common stock at each option grant date.
After the completion of the Business Combination, the Company measures compensation expense for stock-based awards to employees, non-employees and directors based upon the awards’ initial grant-date fair values. Stock-based compensation expense for stock options, restricted stock units and performance awards is recorded over the requisite service period. For awards with only a service condition, the Company expenses stock-based compensation using the straight-line method over the requisite service period for the entire award. For awards with a market condition, the Company expenses the grant date fair value at the target over the vesting period regardless of the value the award recipients ultimately receive. The fair value of restricted stock without a market condition is estimated using the current market price of the Company’s common stock on the date of grant. The fair value of stock option grants with a market condition is estimated at the date of grant using the Monte Carlo simulation model (“Monte Carlo”). The fair values of stock option grants with only a service condition are estimated as of the date of grant by applying the Black-Scholes option valuation model (“Black-Scholes model”). The Black-Scholes model and Monte Carlo models incorporate assumptions as to stock price volatility, the expected life of options or restricted stock, a risk-free interest rate and dividend yield. The effect of forfeiture in compensation costs is recognized based on actual forfeitures when they occur.
The Black-Scholes model is affected by the stock price on the date of the grant as well as assumptions regarding a number of variables. These variables include the expected term of the option, expected risk-free interest rate, the expected volatility of common stock, and expected dividend yield; each of which is described below. The assumptions for expected term of the option and expected volatility of common stock are the two assumptions that significantly affect the grant date fair value.
•Expected Term: The expected term is calculated using the weighted-average period that the stock options are expected to be outstanding prior to being exercised. The Company determines expected term based on historical exercise patterns and its expectation of the time that it will take for employees to exercise options still outstanding. The Company estimates non-employees’ options based on the contractual term.
•Risk-free Interest Rate: The risk-free interest rate for periods within the expected term of the awards is based on the U.S. Treasury yield curve in effect at the time of the grant.
•Expected Stock Price Volatility: The Company determined expected annual equity volatility based on the combination of the historical volatility of its common stock and the historical volatility of the common stock comparable to the Company’s common stock.
•Dividend Yield: Because the Company has never paid a dividend and does not expect to begin doing so in the foreseeable future, the Company assumes no dividend yield in valuing the stock-based awards.
•Exercise Price: The exercise price is taken directly from the grant notice issued to employees and non-employees.
Income Taxes
The Company utilizes the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of assets and liabilities using the enacted statutory tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. A valuation allowance is established against net deferred tax assets if, based on the weight of available evidence, it is more likely than not some or all of the net deferred tax assets will not be realized.
The Company accounts for uncertainty in income taxes recognized in the financial statements by applying a two-step process to determine the amount of tax benefit to be recognized. First, the tax position must be evaluated to determine the likelihood it will be sustained upon external examination by the taxing authorities. If the tax position is deemed more-likely-than-not-to be sustained, the tax position is then assessed to determine the amount of benefit to recognize in the financial statements. The amount of the benefit that may be recognized is the largest amount that has a greater than 50% likelihood of being realized upon ultimate settlement. The provision for income taxes includes the effects of any resulting tax reserves or unrecognized tax benefits that are considered appropriate, as well as the related net interest and penalties, as applicable. As of both December 31, 2024 and 2023, no accrued interest and penalties associated with any uncertain tax positions were recorded in the Consolidated Balance Sheets. The Company recognized an immaterial amount of interest and penalties in the Statements of Operations and Comprehensive Income for the year ended December 31, 2024. There were no interest and penalties recognized in the Statements of Operations and Comprehensive Income for the years ended December 31, 2023 and 2022.
Recently Issued Accounting Pronouncements
In November 2024, the FASB issued ASU No. 2024-03, Disaggregation of Income Statement Expenses (“DISE”), which requires additional disclosure of the nature of expenses included in the income statement. The ASU requires disclosures about specific types of expenses included in the expense captions presented on the face of the income statement as well as disclosures about selling expenses. The amendments in this update are effective for annual reporting periods beginning after December 15, 2026 and interim reporting periods beginning after December 15, 2027, with early adoption permitted. The ASU is required to be applied prospectively with the option for retrospective application. The Company is currently evaluating the impact ASU 2024-03 may have on its Consolidated Financial Statements and disclosures.
In March 2024, the FASB issued ASU No. 2024-02, Codification Improvements - Amendments to Remove References to the Concepts Statements, which contains amendments to the Codification that remove references to various Concepts Statements. The amendments in ASU 2024-02 are not intended to result in significant accounting changes for most entities. The amendments in this update are effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact ASU 2024-02 may have on its Consolidated Financial Statements and disclosures.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which expands income tax disclosure requirements to include additional information related to the rate reconciliation of effective tax rates to statutory rates, as well as additional disaggregation of taxes paid in both U.S. and foreign jurisdictions. The amendments in ASU 2023-09 also remove disclosures related to certain unrecognized tax benefits and deferred taxes. The amendments are effective for fiscal years beginning after December 31, 2024, with early adoption permitted. The Company did not early adopt the new standard. The new standard is expected to apply prospectively, but retrospective application is permitted. The Company is currently evaluating the impact ASU 2023-09 may have on its Consolidated Financial Statements and disclosures.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires enhanced disclosures about significant segment expenses. In addition, the ASU clarified that single reportable segment entities must apply Topic 280 in its entirety. The ASU does not change how an entity identifies its operating segments, aggregates those operating segments, or applies the quantitative thresholds to determine its reportable segments. The Company adopted ASU 2023-07 during the year ended December 31, 2024. The impact of ASU 2023-07 was not material to the Company’s Consolidated Financial Statements and disclosures. For further details regarding the Company’s reportable segment, please refer to Note 16. Segment Information.
Note 3. Acquisition
Majelac Technologies LLC
Pursuant to the terms and conditions of an Asset Purchase Agreement by and among the Company, Majelac Technologies LLC (“Majelac”), and certain other parties, on November 5, 2021 (the “Majelac Closing Date”), the Company acquired certain assets and assumed certain liabilities of Majelac, a privately-owned company providing semiconductor chip assembly and packaging capabilities located in Pennsylvania. The acquisition brought semiconductor chip assembly and packaging capabilities in-house to secure the Company’s supply chain and support its commercialization efforts. Prior to the acquisition, Majelac was a vendor of the Company.
The total purchase price of $10.4 million consists of $4.6 million in cash and 535,715 shares of Class A common stock, valued at $4.2 million, issued to Majelac at the close of the acquisition; approximately $0.5 million additional cash paid six months after the Majelac Closing Date; additional 59,523 shares of Class A common stock valued at $0.5 million were issued to Majelac 12 months after the Majelac Closing Date; additional milestone-based consideration of up to $0.8 million, which was fair valued at $0.5 million at the Majelac Closing Date. On May 4, 2022, the Company paid Majelac $0.4 million (fair value of $0.3 million at the Majelac Closing Date) for the first of two milestones that was met.
Of the total purchase price, $9.5 million was attributable to goodwill. During the fourth quarter ended December 31, 2022, the Company concluded the goodwill from the Majelac acquisition was fully impaired and recorded a charge of $9.5 million in the Consolidated Statements of Operations and Comprehensive Loss.
During the second quarter of 2023, the Company determined the estimated fair value of the contingent consideration was de minimis as the probability of the second milestone being met by November 1, 2023 was remote. There was no subsequent change in the third or fourth quarter of 2023. As a result, the Company recorded a gain of $0.4 million during the year ended December 31, 2023 in Other income, net, in the Consolidated Statements of Operations and Comprehensive Loss.
Note 4. Investments in Marketable Securities
For the year ended December 31, 2022 the Company’s investments in marketable securities were determined to be trading securities and were comprised of fixed income mutual funds, reported at fair value, with gains or losses resulting from changes in fair value recognized within Unrealized gain on trading securities and Realized gain (loss) on trading securities in the Consolidated Statements of Operations and Comprehensive Loss. In the fourth quarter of 2023, these trading securities were sold. The Company reinvested the funds received from the sale in marketable securities deemed to be available-for-sale securities. Gross unrealized gains or losses resulting from changes in available-for-sale securities for the year ended December 31, 2024 were $0.1 million. Gross unrealized gains or losses resulting from changes in available-for-sale securities for the year ended December 31, 2023 were deemed immaterial.
Dividend and interest income from marketable securities related to the Company’s available-for-sale securities for the years ended December 31, 2024 and 2023, and unrealized gain (loss) on trading securities and realized loss on trading securities related to the Company’s trading securities for the years ended December 31, 2023 and 2022 were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Dividend income from marketable securities | $ | 1,728 | | | $ | 9,077 | | | $ | 5,301 | |
| Interest income from marketable securities | $ | 9,638 | | | $ | 459 | | | $ | — | |
| Unrealized gain (loss) on trading securities | $ | — | | | $ | 10,690 | | | $ | (16,162) | |
| Realized loss on trading securities | $ | — | | | $ | (5,103) | | | $ | (4,441) | |
The following is a summary of the Company’s available-for-sale securities recorded within Marketable securities in the Consolidated Balance Sheets as of December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2024 |
| Amortized
Costs | | Gross
Realized
Net Gains | | Gross
Unrealized
Net Gains | | Fair
Value |
| Financial assets: | | | | | | | |
| Short-term marketable securities: | | | | | | | |
| U.S. Treasury securities | $ | 108,047 | | | $ | — | | | $ | 63 | | | $ | 108,110 | |
| Commercial paper | 52,243 | | | — | | | 9 | | | 52,252 | |
| Total | $ | 160,290 | | | $ | — | | | $ | 72 | | | $ | 160,362 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| Amortized
Costs | | Gross
Realized
Net Gains | | Gross
Unrealized
Net Gains | | Fair
Value |
| Financial assets: | | | | | | | |
| Short-term marketable securities: | | | | | | | |
| U.S. Treasury securities | $ | 82,625 | | | $ | — | | | $ | 15 | | | $ | 82,640 | |
| Commercial paper | 41,229 | | | — | | | 7 | | | 41,236 | |
| Total | $ | 123,854 | | | $ | — | | | $ | 22 | | | $ | 123,876 | |
The fair values of the Company’s available-for-sale securities included within Marketable securities in the Consolidated Balance Sheets as of December 31, 2024 and 2023, by remaining contractual maturity, are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2024 |
| One Year
or Less | | Over
One Year
Through
Five Years | | Over
Five Years | | Total |
| Financial Assets: | | | | | | | |
| Short-term marketable securities: | | | | | | | |
| U.S. Treasury securities | $ | 108,110 | | | $ | — | | | $ | — | | | $ | 108,110 | |
| Commercial paper | 52,252 | | | — | | | — | | | 52,252 | |
| Total | $ | 160,362 | | | $ | — | | | $ | — | | | $ | 160,362 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| One Year
or Less | | Over
One Year
Through
Five Years | | Over
Five Years | | Total |
| Financial Assets: | | | | | | | |
| Short-term marketable securities: | | | | | | | |
| U.S. Treasury securities | $ | 82,640 | | | $ | — | | | $ | — | | | $ | 82,640 | |
| Commercial paper | 41,236 | | | — | | | — | | | 41,236 | |
| Total | $ | 123,876 | | | $ | — | | | $ | — | | | $ | 123,876 | |
Note 5. Fair Value of Financial Instruments
Fair value estimates of financial instruments are made at a specific point in time, based on relevant information about financial markets and specific financial instruments. As these estimates are subjective in nature, involving uncertainties and matters of significant judgment, they cannot be determined with precision. Changes in assumptions can significantly affect estimated fair value.
The Company measures fair value as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The Company utilizes a three-tier hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
•Level 1: Valuations based on quoted prices in active markets for identical assets or liabilities that an entity has the ability to access.
•Level 2: Valuations based on quoted prices for similar assets or liabilities, quoted prices for identical assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially the full term of the assets or liabilities.
•Level 3: Valuations based on inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The carrying value of cash and cash equivalents, accounts payable and accrued expenses and other current liabilities approximates their fair values due to the short-term or on demand nature of these instruments. At December 31, 2024 and 2023, the Company’s investment portfolio included available-for-sale securities which were comprised of money market funds, U.S. treasury bills and commercial paper. The Company has U.S. Treasury bills and commercial papers that are classified as Level 2 due to the fair value for these instruments being determined by utilizing observable inputs in similar assets or identical assets in non-active markets. The fair value of certain of the U.S. Treasury bills transferred to Level 2 from Level 1 of the fair value hierarchy due to trading activity, observability and accessibility of the pricing information from the most active market of the investment.
Warrants are recorded as Warrant liabilities in the Consolidated Balance Sheets. The warrant liabilities are measured at fair value at inception and on a recurring basis, with changes in fair value presented as Change in fair value of warrant liabilities in the Consolidated Statements of Operations and Comprehensive Loss.
The Public Warrants and Private Warrants were carried at fair value as of December 31, 2024 and 2023. The Public Warrants were valued using Level 1 inputs as they are traded in an active market. The Private Warrants were valued using a binomial lattice model, which results in a Level 3 fair value measurement. The primary unobservable input utilized in determining the fair value of the Private Warrants was the expected volatility of the Company’s Class A common stock. The expected volatility was based on consideration of the implied volatility from the Company’s own public warrant pricing and on the historical volatility observed at guideline public companies. As of December 31, 2024, the significant assumptions used in preparing the binomial lattice model for valuing the Private Warrants liability include (i) volatility of 194.3%, (ii) risk-free interest rate of 4.20%, (iii) strike price of $11.50, (iv) fair value of common stock of $2.70, and (v) expected life of 1.4 years. As of December 31, 2023, the significant assumptions used in preparing the binomial lattice model for valuing the Private Warrants liability include (i) volatility of 92.1%, (ii) risk-free interest rate of 4.10%, (iii) strike price of $11.50, (iv) fair value of common stock of $2.01, and (v) expected life of 2.4 years.
The following tables set forth the Company’s fair value hierarchy for assets and liabilities measured at fair value as of December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2024 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Financial Assets: | | | | | | | |
| Cash equivalents: | | | | | | | |
| Money market funds | $ | 20,340 | | | $ | — | | | $ | — | | | $ | 20,340 | |
| | | | | | | |
| Commercial paper | 16,919 | | | — | | | — | | | 16,919 | |
| Marketable securities: | | | | | | | |
| U.S. Treasury securities | — | | | 108,110 | | | — | | | 108,110 | |
| Commercial paper | — | | | 52,252 | | | — | | | 52,252 | |
| Total assets at fair value on a recurring basis | $ | 37,259 | | | $ | 160,362 | | | $ | — | | | $ | 197,621 | |
| | | | | | | |
| Liabilities: | | | | | | | |
| Public Warrants | $ | 4,792 | | | $ | — | | | $ | — | | | $ | 4,792 | |
| Private Warrants | — | | | — | | | 203 | | | 203 | |
| Total liabilities at fair value on a recurring basis | $ | 4,792 | | | $ | — | | | $ | 203 | | | $ | 4,995 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Financial Assets: | | | | | | | |
| Cash equivalents: | | | | | | | |
| Money market funds | $ | 50,226 | | | $ | — | | | $ | — | | | $ | 50,226 | |
| U.S. Treasury securities | 59,654 | | | — | | | — | | | 59,654 | |
| Commercial paper | — | | | 19,436 | | | — | | | 19,436 | |
| Marketable securities: | | | | | | | |
| U.S. Treasury securities | 82,640 | | | — | | | — | | | 82,640 | |
| Commercial paper | — | | | 41,236 | | | — | | | 41,236 | |
| Total assets at fair value on a recurring basis | $ | 192,520 | | | $ | 60,672 | | | $ | — | | | $ | 253,192 | |
| | | | | | | |
| Liabilities: | | | | | | | |
| Public Warrants | $ | 1,227 | | | $ | — | | | $ | — | | | $ | 1,227 | |
| Private Warrants | — | | | — | | | 47 | | | 47 | |
| Total liabilities at fair value on a recurring basis | $ | 1,227 | | | $ | — | | | $ | 47 | | | $ | 1,274 | |
Note 6. Inventory, Net
Inventory, net, consists of the following as of December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Raw materials | $ | 1,290 | | | $ | 1,608 | |
| Work in progress | 2,212 | | | 779 | |
| Finished goods | 565 | | | 1,558 | |
| Total inventory, net | $ | 4,067 | | | $ | 3,945 | |
For the years ended December 31, 2024 and 2023, the Company recorded charges for inventory write-downs of $3.2 million and $3.4 million, respectively, in Research and Development expenses in the Consolidated Statements of Operations and Comprehensive Loss. There were no such inventory write-downs for the year ended December 31, 2022.
Note 7. Property and Equipment, Net
Property and equipment, net, consists of the following as of December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Laboratory and production equipment | $ | 13,412 | | | $ | 14,727 | |
| Computer equipment | 1,724 | | | 1,707 | |
| Purchased software | 57 | | | 188 | |
| Furniture and fixtures | 321 | | | 310 | |
| Leasehold improvements | 7,226 | | | 6,948 | |
| Construction in process | 4,960 | | | 2,438 | |
| Subtotal | 27,700 | | | 26,318 | |
| Less: Accumulated depreciation and amortization | (11,707) | | | (10,043) | |
| Property and equipment, net | $ | 15,993 | | | $ | 16,275 | |
Depreciation and amortization expense is included within Cost of revenue, Research and development and Selling, general and administrative expenses in the Consolidated Statements of Operations and Comprehensive Loss. For the years ended December 31, 2024, 2023 and 2022, depreciation and amortization expense was $4.6 million, $4.2 million and $2.6 million, respectively. For the year ended December 31, 2024, the Company recorded losses on disposals of $0.4 million relating to property and equipment of $3.2 million with accumulated depreciation and amortization of $2.8 million. For each of the years ended December 31, 2023 and 2022, the company recorded losses on disposals of $0.1 million relating to property and equipment of $0.3 million with accumulated depreciation and amortization of $0.2 million.
Note 8. Leases
Lease-related costs for the years ended December 31, 2024 and 2023 are as follows (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Operating lease cost | $ | 3,518 | | | $ | 3,478 | |
| Variable lease cost | 1,786 | | | 1,678 | |
| Total lease cost | $ | 5,304 | | | $ | 5,156 | |
As of December 31, 2024, the maturities of the operating lease liabilities and a reconciliation to the present value of lease liabilities were as follows (dollars in thousands):
| | | | | |
| | Remaining
Lease Payments |
| 2025 | $ | 4,527 | |
| 2026 | 4,585 | |
| 2027 | 4,549 | |
| 2028 | 2,975 | |
| 2029 | 2,806 | |
| Thereafter | 7,247 | |
| Total remaining undiscounted lease payments | $ | 26,689 | |
| Less: Imputed interest | (4,637) | |
Less: Lease incentives (1) | (9,104) | |
| Total operating lease liabilities | 12,948 | |
| Less: current portion | (3,698) | |
| Long-term operating lease liabilities | $ | 9,250 | |
| Weighted-average remaining lease term (in years) | 5.6 |
| Weighted-average discount rate | 7.9 | % |
(1)Includes lease incentives that are estimated to be realized in 2026 and 2027 for the costs of leasehold improvements.
The following table provides certain cash flow and supplemental cash flow information related to the Company’s right-of-use assets and lease liabilities for the years ended December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | |
| | 2024 | | 2023 |
| Operating cash paid to settle operating lease liabilities | $ | 4,436 | | | $ | 4,298 | |
Right-of-use assets obtained in exchange for lease liabilities(2) | $ | 1,047 | | | $ | 83 | |
(2) The year ended December 31, 2024 includes an increase in right-of-use assets due to the change in estimated timing of receipt of reimbursements for tenant improvements related to our New Haven, Connecticut lease. On September 13, 2022, the Company filed a lawsuit against the landlord related to these tenant improvements. For further details regarding this lawsuit, please refer to Note 18. Commitments and Contingencies. As of December 31, 2024 and 2023, the Company has incurred and recognized total leasehold improvements of approximately $1.2 million and $1.6 million, respectively, related to reimbursable construction costs included in construction in progress within Property and equipment, net, in the Consolidated Balance Sheets.
Note 9. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following as of December 31, 2024 and 2023 (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Restructuring costs | $ | 679 | | | $ | 519 | |
| | | |
| Legal fees | 2,166 | | | 979 | |
| Royalties | 150 | | | 123 | |
| Sales tax payable | 1,339 | | | 7 | |
| Other | 514 | | | 187 | |
| Total accrued expenses and other current liabilities | $ | 4,848 | | | $ | 1,815 | |
Note 10. Equity Transactions
At-the-Market Equity Offering Programs
On August 11, 2023, the Company filed a universal shelf registration statement on Form S-3 (the “Shelf Registration Statement”), which became effective on August 22, 2023, covering the offering of Class A common stock, preferred stock, debt securities, warrants, rights and units.
On December 11, 2024, the Company entered into an Equity Distribution Agreement (the “Sales Agreement”) with Canaccord Genuity LLC (“Canaccord”) to sell shares of the Company’s Class A common stock, par value $0.0001, having an aggregate offering price of up to $75.0 million, from time to time through an “at-the-market” offering program under which Canaccord will act as sales agent (the “ATM Offering”). The Company has no obligation to sell any shares under the Sales Agreement and may at any time suspend solicitation and offers under the EDA. The ATM Offering is being made pursuant to the Company’s Shelf Registration Statement and a prospectus supplement related to the ATM Offering dated December 11, 2024. During the year ended December 31, 2024, the Company sold and issued 23,425,650 shares of the Company’s Class A common stock under the ATM Offering, resulting in gross proceeds of $36.2 million. Net proceeds were $34.8 million after commissions and issuance costs of $1.4 million.
On December 11, 2024, the Company also exercised its right to terminate the Equity Distribution Agreement (the “Equity Distribution Agreement”), dated August 11, 2023, by and between the Company and Evercore Group L.L.C. (“Evercore”), as sales agent. The Equity Distribution Agreement previously established an “at-the-market” offering program through which the Company had the right to sell, from time to time, through Evercore, up to an aggregate of $75.0 million of the Company’s Class A common stock. The Company sold no shares under the Equity Distribution Agreement.
Class A Common stock
As of December 31, 2024 and 2023, the Company had authorized 600,000,000 shares of Class A common stock at $0.0001 par value per share, of which a total of 146,953,271 and 121,832,417 shares were outstanding, respectively.
Voting Rights
Holders of Class A common stock will be entitled to cast one vote per Class A share. Generally, holders of all classes of common stock vote together as a single class, and an action is approved by stockholders if a majority of votes cast affirmatively or negatively on the action are cast in favor of the action, while directors are elected by a plurality of the votes cast. Holders of Class A common stock will not be entitled to cumulate their votes in the election of directors.
Dividend Rights
With limited exceptions in the case of certain stock dividends or disparate dividends approved by the affirmative vote of the holders of a majority of the Class A common stock and Class B common stock, each voting separately as a class, holders of Class A common stock will share ratably (based on the number of shares of Class A common stock held), together with each holder of Class B common stock, if and when any dividend is declared by the Board out of funds legally available therefore, subject to restrictions, whether statutory or contractual (including with respect to any outstanding indebtedness), on the declaration and payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock or any class or series of stock having a preference over, or the right to participate with, the Class A common stock with respect to the payment of dividends.
Class B Common stock
As of December 31, 2024 and 2023, the Company had authorized 27,000,000 shares of Class B common stock at $0.0001 par value per share, of which a total of 19,937,500 shares were outstanding for both years.
Voting Rights
Holders of Class B common stock will be entitled to cast 20 votes per share of Class B common stock. Generally, holders of all classes of common stock vote together as a single class, and an action is approved by stockholders if a majority of votes cast affirmatively or negatively on the action are cast in favor of the action, while directors are elected by a plurality of the votes cast. Holders of Class B common stock will not be entitled to cumulate their votes in the election of directors.
Dividend Rights
With limited exceptions in the case of certain stock dividends or disparate dividends approved by the affirmative vote of the holders of a majority of the Class A common stock and Class B common stock, each voting separately as a class, holders of Class B common stock will share ratably (based on the number of shares of Class B common stock held), together with each holder of Class A common stock, if and when any dividend is declared by the Board out of funds legally available therefor, subject to restrictions, whether statutory or contractual (including with respect to any outstanding indebtedness), on the declaration and payment of dividends and to any restrictions on the payment of dividends imposed by the terms of any outstanding preferred stock or any class or series of stock having a preference over, or the right to participate with, the Class B common stock with respect to the payment of dividends.
Preferred Stock
As of December 31, 2024 and 2023, the Company had authorized 1,000,000 shares of preferred stock at $0.0001 par value per share. There were no preferred shares outstanding for both years.
Preferred stock may be issued from time to time in one or more series. Any shares of preferred stock which may be redeemed, purchased or acquired by the Company may be reissued except as otherwise provided by law.
Note 11. Stock-based Compensation
Equity Incentive Plan
The Quantum-Si Incorporated 2021 Equity Incentive Plan (the “2021 Plan”) provides for grants of stock options, stock appreciation rights, restricted stock, restricted stock units, and other stock or cash-based awards. Directors, officers and other employees of the Company and its subsidiaries, as well as others performing consulting or advisory services for the Company, are eligible for grants under the 2021 Plan. As of December 31, 2024, there were 14,699,942 shares available for future grant under the 2021 Plan.
Inducement Equity Incentive Plan
On May 8, 2023, the Company adopted the 2023 Inducement Equity Incentive Plan (the “2023 Inducement Plan”) to reserve 3,000,000 shares of its Class A common stock to be used exclusively for grants of awards to individuals that were not previously employees or directors of the Company as a material inducement to such individuals’ entry into employment with the Company within the meaning of Rule 5635(c)(4) of the Nasdaq Listing Rules. On August 23, 2024, the Company amended the 2023 Inducement Plan to reserve an additional 3,000,000 shares of its Class A common stock under the 2023 Inducement Plan. The terms and conditions of the 2023 Inducement Plan, as amended, are substantially similar to those of the 2021 Plan. As of December 31, 2024, there were 1,645,515 shares remaining available for issuance under the 2023 Inducement Plan.
Stock options
The Company granted an aggregate of 2,282,600 stock option awards to participants during the year ended December 31, 2024, with vesting subject to the participant’s continued employment with or continued service provided to the Company through the applicable vesting dates.
During the year ended December 31, 2023, the Company granted an aggregate of 10,138,730 stock option awards to participants, with vesting subject to the participant’s continued employment with or continued service provided to the Company through the applicable vesting dates and, in specific instances, certain market conditions. These stock option awards included 2,000,000 stock options granted to the Chief Financial Officer which are subject to service and/or certain market conditions. The stock option awards granted to the Chief Financial Officer included 1,000,000 stock options issued from the 2021 Plan and 1,000,000 inducement stock options issued from the 2023 Inducement Plan which are subject to a service condition and certain market conditions. The service condition requires the Chief Financial Officer’s continued employment with the Company through the applicable vesting dates. The market conditions require the Company’s Class A common stock trade above a specified level for a defined period of time. The fair value of awards with market conditions was estimated at the grant date using the Monte Carlo simulation model.
During the year ended December 31, 2022, the Company granted 14,271,330 stock option awards to participants with vesting subject to the participant’s continued employment with the Company through the applicable vesting dates and, in specific instances, certain market conditions. These stock option awards included 6,950,000 stock options granted to the Chief Executive Officer which are subject to service and/or certain market conditions and 2,000,000 stock options granted to the prior President and Chief Operating Officer, of which 1,708,334 were forfeited on August 31, 2023 upon the termination of his employment with the Company. The stock options granted to the Chief Executive Officer include 4,170,000 stock options issued from the 2021 Plan and 2,780,000 inducement stock options granted outside of the 2021 Plan. The service condition requires the Chief Executive Officer’s continued employment with the Company through the applicable vesting dates. The market conditions require the Company’s Class A common stock trade above a specified level for a defined period of time. The fair value of awards with market conditions was estimated at the grant date using the Monte Carlo simulation model.
Stock-based compensation related to stock options for the years ended December 31, 2024, 2023 and 2022 was $6.4 million, $7.1 million and $7.3 million, respectively. The Company estimates and records the compensation cost associated with the grants described above with an offsetting entry to paid-in capital. The Company utilized the Black-Scholes model for determining the estimated fair value for service or performance-based stock-based awards. The Black-Scholes model requires the use of subjective assumptions which determine the fair value of stock-based awards. The assumptions used to value option grants to employees and non-employees for the years ended December 31, 2024, 2023 and 2022 were as follows:
| | | | | | | | | | | | | | | | | | | | |
| | 2024 | | 2023 | | 2022 |
| Expected term (in years) | | 4.6 – 5.0 | | 5.0 – 6.2 | | 5.5 – 6.4 |
| Risk-free interest rate | | 3.4% – 5.2% | | 3.4% – 4.4% | | 1.7% – 4.2% |
| Expected volatility | | 82% - 91% | | 62% - 64% | | 58% - 64% |
| Expected dividend yield | | — | | — | | — |
| Weighted average fair value/share at grant date | $ | 1.03 | $ | 1.01 | $ | 1.51 |
A summary of the stock option activity is presented in the table below:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number
of Options | | Weighted Average
Exercise Price
(per share) | | Weighted Average
Remaining
Contractual Life
(in years) | | Aggregate
Intrinsic Value
(in thousands) |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Outstanding at December 31, 2023 | 22,511,900 | | $ | 2.79 | | | 8.2 | | $ | 3,194 | |
| Granted | 2,282,600 | | 1.51 | | | | | |
| Exercised | (598,689) | | 1.79 | | | | | $ | 547 | |
| Forfeited | (3,277,407) | | 3.23 | | | | | |
| Expired | (115,262) | | 2.45 | | | | | |
| Outstanding at December 31, 2024 | 20,803,142 | | $ | 2.61 | | | 7.8 | | $ | 11,507 | |
| Exercisable at December 31, 2024 | 9,014,076 | | $ | 6.72 | | | 6.7 | | $ | 3,468 | |
| Vested and expected to vest at December 31, 2024 | 17,936,135 | | $ | 7.68 | | | 7.7 | | $ | 9,552 | |
The total fair value of stock options that vested during the years ended December 31, 2024 and 2023 was approximately $6.4 million and $7.8 million, respectively.
As of December 31, 2024 total unrecognized stock-based compensation related to stock options was $11.4 million, which is expected to be recognized over a remaining weighted average vesting period of 2.3 years.
Modification of Performance Stock Options
As described above, in November 2022 and May 2023, the Company granted 2,780,000 and 1,000,000 performance-based stock option awards to its Chief Executive Officer and Chief Financial Officer, respectively. The vesting of these awards
are subject to continued service to the Company and certain market conditions. The market conditions require the Company’s Class A common stock trade above specified levels for certain periods of time. The fair values of the awards were estimated at the grant date using the Monte Carlo simulation model.
On March 15, 2024, the market conditions that trigger the vesting of these performance-based stock option awards were modified. The modified market conditions require the Company’s Class A common stock to trade above specified levels for certain defined periods of time that are different from the original awards. The Company accounted for the modifications as modifications of market conditions. The total incremental stock-based compensation expense to be recognized for these awards is approximately $2.4 million within Selling, general and administrative operating expenses in the Consolidated Statements of Operations and Comprehensive Loss. Incremental stock-based compensation expense for the year ended December 31, 2024 was $0.6 million. There were no such modifications to performance-based stock option awards for the years ended December 31, 2023 and 2022.
Restricted stock units
During the year ended December 31, 2024, the Company granted 9,216,559 restricted stock unit (“RSU”) awards.
During the year ended December 31, 2023, the Company granted 786,938 restricted stock unit (“RSU”) awards.
During the year ended December 31, 2022, the Company granted 66,666 restricted stock unit (“RSU”) awards. On February 8, 2022, John Stark, the Company’s former Chief Executive Officer and member of its board of directors, stepped down from all of his positions with the Company. As a result of Mr. Stark not meeting the service conditions of certain awards previously granted to him, 1,731,371 RSU awards were forfeited, resulting in a reversal of stock-based compensation for the year ended December 31, 2022 of $4.7 million.
Stock-based compensation related to RSU awards for the years ended December 31, 2024, 2023 and 2022 was $2.4 million, $1.4 million and $3.9 million, respectively.
The number of shares and weighted average grant date fair values of restricted non-vested common stock at the beginning and end of 2024, as well as restricted stock units granted, vested, and forfeited during the year were as follows:
| | | | | | | | | | | |
| Number of Shares
Underlying RSUs | | Weighted Average
Grant-Date Fair
Value (per share) |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Non-vested RSUs at December 31, 2023 | 847,169 | | $ | 2.68 | |
| Granted | 9,216,559 | | 1.45 |
| Vested | (1,096,515) | | 2.42 |
| Forfeited | (1,788,204) | | 1.69 |
| Non-vested RSUs at December 31, 2024 | 7,179,009 | | $ | 1.39 | |
The total fair value of restricted stock vested during the years ended December 31, 2024 and 2023 was approximately $2.6 million and $14.4 million, respectively.
Stock-based compensation is allocated to Research and development and Selling, general and administrative operating expenses in the Consolidated Statements of Operations and Comprehensive Loss. Stock-based compensation expense for the years ended December 31, 2024, 2023 and 2022 is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Research and development | $ | 2,659 | | | $ | 2,961 | | | $ | 4,548 | |
| Selling, general and administrative | 6,228 | | | 5,555 | | | 6,658 | |
| Total stock-based compensation | $ | 8,887 | | | $ | 8,516 | | | $ | 11,206 | |
No related tax benefits of the stock-based compensation expense have been recognized and no related tax benefits have been realized from the exercise of stock options due to the Company’s net operating loss carryforwards.
As of December 31, 2024 total unrecognized stock-based compensation related to restricted stock was $9.3 million, which is expected to be recognized over the remaining weighted average vesting period of 3.3 years.
Note 12. Warrant Liabilities
Public Warrants
As of December 31, 2024 and 2023, there was an aggregate of 3,833,319 outstanding Public Warrants which entitle the holder to acquire Class A common stock. Each whole warrant entitles the registered holder to purchase one share of Class A common stock at an exercise price of $11.50 per share, subject to adjustment as discussed below, beginning on September 9, 2021. The warrants will expire on June 10, 2026 or earlier upon redemption or liquidation.
Redemptions
At any time while the warrants are exercisable, the Company may redeem not less than all of the outstanding Public Warrants:
•in whole and not in part;
•at a price of $0.01 per warrant;
•upon not less than 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder; and
•if, and only if, the closing price of the Company’s common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending three business days before the Company sends the notice of redemption to the warrant holders.
If the foregoing conditions are satisfied and the Company issues a notice of redemption of the Public Warrants at $0.01 per warrant, each holder of Public Warrants will be entitled to exercise his, her or its Public Warrants prior to the scheduled redemption date.
If the Company calls the Public Warrants for redemption for $0.01 as described above, the Company’s Board of Directors may elect to require any holder that wishes to exercise his, her or its Public Warrants to do so on a “cashless basis.” If the Company’s Board of Directors makes such election, all holders of Public Warrants would pay the exercise price by surrendering their warrants for the number of shares of Class A common stock equal to the quotient obtained by dividing (x) the product of the number of shares of Class A common stock underlying the warrants, multiplied by the excess of the “fair market value” over the exercise price of the warrants by (y) the “fair market value.” For purposes of the redemption provisions of the warrants, the “fair market value” means the average last reported sale price of the Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
The Public Warrants do not meet the criteria to be classified in stockholders’ equity as the exercise of the warrants may be settled in cash upon the occurrence of a tender offer or exchange offer in which the maker of the tender offer or exchange offer, upon completion of the tender offer or exchange offer, beneficially owns more than 50% of the outstanding shares of the Company’s Class A common stock, even if it would not result in a change of control of the Company. This provision precludes the Public Warrants from being classified in equity and thus they are classified as long-term liabilities in the Consolidated Balance Sheets.
Private Warrants
There were 135,000 Private Warrants outstanding as of December 31, 2024 and 2023. The Private Warrants are identical to the Public Warrants, except that so long as they are held by the Sponsor or any of its permitted transferees, (i) the Private Warrants and the shares of Class A common stock issuable upon the exercise of the Private Warrants were not transferable, assignable or saleable until 30 days after the completion of the Business Combination, (ii) the Private Warrants will be exercisable for cash or on a cashless basis, at the holder’s option, and (iii) the Private Warrants are not subject to the Company’s redemption option at the price of $0.01 per warrant. The Private Warrants are subject to the Company’s redemption option at the price of $0.01 per warrant, provided the other conditions of such redemption are met, as described above. If the Private Warrants are held by a holder other than the Sponsor or any of its permitted transferees, the Private
Warrants will be redeemable by the Company in all redemption scenarios applicable to the Public Warrants and exercisable by such holders on the same basis as the Public Warrants.
The Private Warrants do not meet the criteria to be classified in stockholders’ equity as the terms of the warrants provide for potential changes to the settlement amounts depending upon the characteristics of the warrant holder, and, because the holder of a warrant is not an input into the pricing of a fixed-for-fixed option on equity shares. This provision precludes the Private Warrants from being classified in equity and thus they are classified as long-term liabilities in the Consolidated Balance Sheets.
The fair value of warrant liabilities was $5.0 million and $1.3 million as of December 31, 2024 and 2023, respectively. The Company recognized a loss of $3.7 million and $0.3 million for the years ended December 31, 2024 and 2023, respectively, and a gain of $6.2 million for the year ended December 31, 2022, as a Change in fair value of warrant liabilities in the Consolidated Statements of Operations and Comprehensive Loss. There were no exercises or redemptions of the Public Warrants or Private Warrants during the years ended December 31, 2024, 2023 or 2022.
Note 13. Net Loss Per Share
The Company presents both basic earnings per share (“EPS”) and diluted EPS. Basic and diluted net loss per share was the same for each period presented as the inclusion of all common share equivalents would have been anti-dilutive.
The following table presents the calculations for the years ended December 31, 2024, 2023 and 2022 of basic and diluted net loss per share for the Company’s common stock (in thousands, except per share amounts):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Numerator | | | | | |
| Net loss | $ | (101,007) | | | $ | (95,960) | | | $ | (132,442) | |
| Numerator for basic and diluted EPS - loss attributable to common stockholders | $ | (101,007) | | | $ | (95,960) | | | $ | (132,442) | |
| Denominator | | | | | |
| Common stock | 143,196 | | 141,300 | | 139,255 |
| Denominator for basic and diluted EPS - weighted-average common stock | 143,196 | | 141,300 | | 139,255 |
| Basic and diluted net loss per share | $ | (0.71) | | | $ | (0.68) | | | $ | (0.95) | |
Net loss per share attributable to Class A and Class B common stockholders was the same on a basic and diluted basis, as the inclusion of all potential common equivalent shares outstanding would have been anti-dilutive.
The following potential dilutive shares were excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive for the years ended December 31, 2024, 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Outstanding options to purchase common stock | 20,803,142 | | 22,511,900 | | 19,427,755 |
| Outstanding restricted stock units | 7,179,009 | | 847,169 | | 2,018,449 |
| Outstanding warrants | 3,968,319 | | 3,968,319 | | 3,968,319 |
| 31,950,470 | | 27,327,388 | | 25,414,523 |
Note 14. Restructuring
The Company committed to organizational restructurings during the first and third quarters of 2023 and the fourth quarter of 2024, designed to decrease its costs and create a more streamlined organization to support its business. Restructuring liabilities were $0.7 million and $0.5 million as of December 31, 2024 and 2023, respectively. These liabilities are included in Accrued expenses and other current liabilities in the Consolidated Balance Sheets.
The Company’s restructuring costs, primarily for cash severance and other severance costs, are allocated to the following operating expense categories as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Research and
Development | | Selling,
general and
administrative | | Total |
| Balance as of December 31, 2023 | $ | 513 | | | $ | 6 | | | $ | 519 | |
Restructuring charges incurred(1) | 1,551 | | | 702 | | | 2,253 | |
Cash payments and other adjustments(1) | (1,551) | | | (542) | | | (2,093) | |
| Balance as of December 31, 2024 | $ | 513 | | | $ | 166 | | | $ | 679 | |
| Current liabilities | | | | | $ | 679 | |
| Long-term liabilities | | | | | — | |
| Total liabilities as of December 31, 2024 | | | | | $ | 679 | |
(1) Restructuring charges incurred and Cash payments and other adjustments include non-cash charges related to stock-based compensation expenses.
The Company’s restructuring activities were complete as of December 31, 2024. The Company does not expect to incur material additional charges associated with these activities.
Note 15. Income Taxes
The components of (loss) income before provision for income taxes for the years ended December 31, 2024, 2023 and 2022 are as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| U.S. loss before provision for income taxes | $ | (101,108) | | | $ | (95,977) | | | $ | (132,442) | |
| Foreign income before provision for income taxes | 157 | | | 17 | | | — | |
| Loss before provision for income taxes | $ | (100,951) | | | $ | (95,960) | | | $ | (132,442) | |
For purposes of reconciling the Company’s provision for income taxes at the statutory rate and the Company’s (provision) benefit for income taxes at the effective rate, a notional 21% tax rate was applied as follows:
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Federal income tax at statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State income tax, net of federal benefit | 3.21 | | | 7.81 | | | 4.1 | |
| Uncertain tax positions | (0.15) | | | (1.45) | | | — | |
| Tax credits generated in current year | (3.41) | | | 0.68 | | | 1.9 | |
| Return to provision adjustments | — | | | (3.25) | | | — | |
| Stock-based compensation | (1.01) | | | (3.17) | | | (0.5) | |
| Other | 0.51 | | | (0.10) | | | 0.9 | |
| Tax rate change | (3.41) | | | - | | | — | |
| Valuation allowance change | (16.79) | | | (21.52) | | | (27.4) | |
| Effective income tax rate | (0.05 | %) | | 0.0 | % | | 0.0 | % |
The difference between the statutory federal income tax rate in 2024, 2023 and 2022 is primarily attributable to the effect of losses sustained which required a valuation allowance.
The provision (benefit) for income taxes for the years ended December 31, 2024, 2023 and 2022 consists of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Current: | | | | | |
| Federal | $ | — | | | $ | — | | | $ | — | |
| State | — | | | — | | | — | |
| Foreign | 56 | | | — | | | — | |
| Total current | 56 | | | — | | | — | |
| Deferred: | | | | | |
| Federal | — | | | — | | | — | |
| State | — | | | — | | | — | |
| Foreign | — | | | — | | | — | |
| Total deferred | — | | | — | | | — | |
| Income tax provision | $ | 56 | | | $ | — | | | $ | — | |
The tax effects of temporary differences that give rise to significant portions of the Company’s deferred tax assets and liabilities as of December 31, 2024 and 2023 related to the following (in thousands):
| | | | | | | | | | | |
| 2024 | | 2023 |
| Deferred income tax assets: | | | |
| Net operating loss carryforwards | $ | 101,115 | | | $ | 89,525 | |
| Tax credit carryforwards | 10,576 | | | 9,855 | |
| Stock-based compensation | 2,997 | | | 4,528 | |
| Operating lease liabilities | 3,258 | | | 3,385 | |
| Loss on marketable securities | 3,963 | | | 2,195 | |
| Capitalized research and development | 32,220 | | | 30,878 | |
| Other | 5,687 | | | 2,938 | |
| Total deferred income tax assets | $ | 159,816 | | | $ | 143,304 | |
| | | | |
| Deferred income tax liabilities: | | | |
| Operating lease right-of-use assets | $ | (3,287) | | | $ | (215) | |
| Property and equipment | (36) | | | (3,558) | |
| Total deferred income tax liabilities | (3,323) | | | (3,773) | |
| Valuation allowance | (156,493) | | | (139,531) | |
| Net deferred tax assets (liabilities) | $ | — | | | $ | — | |
A valuation allowance is recorded to reduce deferred tax assets to the amount that is more likely than not to be realized based on an assessment of positive and negative evidence, including estimates of future taxable income necessary to realize future deductible amounts. A significant piece of objective negative evidence evaluated was the cumulative loss incurred over the three-year period ended December 31, 2024. Such objective evidence limits the ability to consider other subjective evidence such as its projections for future growth. On the basis of this evaluation, as of December 31, 2024, 2023 and 2022, the Company recorded a valuation allowance of $156.5 million, $139.5 million, and $119.0 million, respectively. The valuation allowance increased by $17.0 million, $20.5 million, and $36.3 million, for the years ended December 31, 2024, 2023 and 2022, respectively, primarily due to changes in capitalized R&D expenditures, net operating loss carry forwards, research and development credits, and capitalization of certain intangibles.
As of December 31, 2024, the Company has accumulated federal and state net operating loss (“NOL”) carryforwards of $388.3 million and $331.2 million, respectively. Of the $388.3 million of federal NOL carryforwards, $65.5 million was generated before January 1, 2018 and is subject to the 20-year carryover period (“pre-Tax Act losses”) and begin to expire
in 2033. The remaining $322.8 million can be carried forward indefinitely but is subject to the 80% taxable income limitation. Of the $331.2 million of state NOL carryforwards, $11.4 million can be carried forward indefinitely with the remaining beginning to expire in 2028.
In addition, the Company has federal and Connecticut research and development credit carryforwards totaling $8.1 million and $4.5 million, respectively. The federal research and development credits begin to expire in 2033 unless previously utilized. The Connecticut research and development credits do not expire.
Pursuant to Sections 382 and 383 of the Internal Revenue Code (“IRC”), annual use of the Company’s NOL and credit carryforwards may be limited in the event a cumulative change of ownership of more than 50% occurs within a three-year period. Since the Company’s formation, the Company has raised capital through the issuance of capital stock, which on its own or combined with the purchasing stockholders’ subsequent disposition of those shares, may have resulted in such an ownership change, or could result in an ownership change.
Upon the occurrence of an ownership change under Section 382 as outlined above, utilization of the Company’s NOL and research and development credit carryforwards are subject to an annual limitation, which is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term tax-exempt rate, which could be subject to additional adjustments, as required. Any limitation may result in expiration of a portion of the NOL or research and development credit carryforwards before utilization. The Company has completed an analysis through December 31, 2024 and no such ownership change has occurred however an ownership change may occur in the future.
The Company recognizes liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the tax position will be sustained on audit, including resolution of related appeals or litigation processes if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement. While the Company believes it has appropriate support for the positions taken on its tax returns, the Company regularly assesses the potential outcome of examinations by tax authorities in determining the adequacy of its provision for income taxes.
The following table summarizes the activity related to the Company’s gross unrecognized tax benefits as of December 31, 2024, 2023 and 2022 (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Beginning balance | $ | 1,379 | | | $ | — | | | $ | — | |
| Increases related to prior year tax positions | 136 | | | 1,310 | | | — | |
| Increases related to current year tax positions | 170 | | | 69 | | | — | |
| Ending balance | $ | 1,685 | | | $ | 1,379 | | | $ | — | |
As of December 31, 2024, the Company had gross unrecognized tax benefits of $1.7 million, which would affect the effective tax rate if recognized subject to valuation allowance. The Company does not anticipate any significant changes in its unrecognized tax benefits over the next 12 months. The Company’s policy is to recognize the interest expense and/or penalties related to income tax matters as a component of income tax expense. As of both December 31, 2024 and 2023, no accrued interest and penalties associated with any uncertain tax positions were recorded in the Consolidated Balance Sheets. The Company recognized an immaterial amount of interest and penalties in the Statements of Operations and Comprehensive Income for the year ended December 31, 2024. There were no interest and penalties recognized in the Statements of Operations and Comprehensive Income for the years ended December 31, 2023 and 2022.
The Company is subject to taxation in the United States, various states within the United States and France. The Company was notified in November 2024 that the Internal Revenue Service will be examining their December 31, 2022 Federal income tax return. The audit had not commenced as of December 31, 2024. The Company has not been notified that it is under audit by any state or foreign taxing authorities, however, due to the presence of NOL carryforwards, all of the income tax years remain open for examination in each of these jurisdictions.
Additionally, as a result of legislation in the state of Connecticut, companies have the opportunity to exchange certain research and development tax credit carryforwards for a cash payment of 65% of the research and development tax credit. The research and development expenses that qualify for Connecticut credits are limited to those costs incurred within
Connecticut. The Company has elected to participate in the exchange program and, as a result, has recognized net benefits of $0.2 million for each of the years ended December 31, 2024, 2023 and 2022, which are included in Research and development in the Consolidated Statements of Operations and Comprehensive Loss. As of each of the years ended December 31, 2024 and 2023, the Company recorded $0.2 million of the research and development tax credit receivables in Prepaid expenses and other current assets on the Company’s Consolidated Balance Sheets. As of December 31, 2024, all refunds for years prior to 2024 have been received by the company. As of December 31, 2024 the company recorded a receivable in the amount of $0.2 million related to refunds for the year ended December 31, 2024.
Deferred income taxes are not required for undistributed earnings of the Company’s foreign subsidiary given the federal election to treat the entity as a United States Subsidiary. Additionally, no withholding taxes are due under the current treaty.
Note 16. Segment Information
The Company is a life sciences company focused on proteomics research, with the mission of transforming single-molecule analysis and democratizing its use by providing researchers and clinicians access to the proteome, the set of proteins expressed within a cell. Our platform includes our Platinum NGPS instrument, Platinum Analysis Software, and consumable kits for use with our Platinum instrument.
The Company’s Chief Operating Decision Maker (the “CODM”), its Chief Executive Officer, utilizes the Company’s long-range plan, which includes product development roadmaps and long-range financial models, as a key input to resource allocation. The CODM makes decisions on resource allocation, assesses the performance of the business, and monitors budget versus actual results on a consolidated basis using loss from operations as reported on the Consolidated Statements of Operations and Comprehensive Loss. Net loss and the change in cash and cash equivalents and marketable securities are also measures that are considered in monitoring budget versus actual results.
Significant expenses within loss from operations, as well as within net loss, include research and development, and selling, general and administrative expenses, which are each separately presented on the Company’s Consolidated Statements of Operations and Comprehensive Loss.
The Company’s revenue is derived from sales of products and services. Product revenue is primarily generated from the sales of instruments and consumables used in protein sequencing and analysis. Service revenue is primarily generated from service maintenance contracts including access to analysis software and advanced training for instrument use.
Total revenue generated from domestic and international sales for the years ended December 31, 2024, 2023 and 2022 is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2024 | | 2023 | | 2022 |
| Domestic | $ | 1,235 | | | $ | 788 | | | $ | — | |
| International | 1,823 | | | 294 | | | — | |
| Total revenue | $ | 3,058 | | | $ | 1,082 | | | $ | — | |
Note 17. Related Party Transactions
The Company was a party to the Amended and Restated Technology Services Agreement (the “ARTSA”), most recently amended on November 11, 2020, by and among 4Catalyzer Corporation (“4C”), the Company and other participant companies controlled by Dr. Jonathan Rothberg, the Chairman of the Company’s Board of Directors. The Company entered into a First Addendum to the ARTSA on February 17, 2021 pursuant to which the Company agreed to terminate its participation under the ARTSA no later than immediately prior to the effective time of the Business Combination, resulting in the termination of the Company’s participation under the ARTSA on June 10, 2021. In connection with the termination of the Company’s participation under the ARTSA, the Company terminated its lease agreement with 4C and negotiated an arm’s length lease agreement. Under the ARTSA, the Company and the other participant companies had agreed to share certain non-core technologies, which means any technologies, information or equipment owned or otherwise controlled by the participant company that are not specifically related to the core business area of the participant and subject to certain restrictions on use. The ARTSA also provided for 4C to perform certain services for the Company and each other participant company such as monthly administrative, management and technical consulting services to the Company which were pre-funded approximately once per quarter.
The Company incurred expenses of $0.3 million, $0.5 million and $0.6 million, which included $0.1 million, $0.1 million and $0.2 million under month-to-month sublease arrangements for office and laboratory spaces from 4C, during the years ended December 31, 2024, 2023 and 2022, respectively. These amounts are included in Selling, general and administrative expenses in the Consolidated Statements of Operations and Comprehensive Loss. Amounts advanced and due to 4C were deemed immaterial as of December 31, 2024. There were no such amounts as of December 31, 2023. There were no amounts advanced and due from 4C as of December 31, 2024. Amounts advanced and due from 4C were deemed immaterial as of December 31, 2023.
The ARTSA also provided for the participant companies to provide other services to each other. The Company also had transactions with other entities under common ownership, which included payments made to third parties on behalf of the Company and payments made by the Company to third parties on behalf of the other entities. There were no amounts payable to or from the Company as of December 31, 2024 and 2023.
On September 20, 2021, the Company entered into a Binders Collaboration (the “Collaboration”) with Protein Evolution, Inc. (“PEI”) to develop technology and methods in the field of nanobodies and potentially other binders to produce novel biological reagents and related data. The Collaboration was made pursuant to and governed by the Technology and Services Exchange Agreement, effective as of June 10, 2021, by and among the Company and the participants named therein, including PEI. Dr. Rothberg serves as Chairman of the Board of Directors of PEI and the Rothberg family are controlling stockholders of PEI. Effective March 31, 2022, the Collaboration with PEI was terminated, and the Company recorded a final payment of $1.1 million under the Collaboration for all services rendered included in Selling, general and administrative expenses in the Consolidated Statements of Operations and Comprehensive Loss. There were no such amounts recorded for the years ended December 31, 2024 and 2023.
Effective October 1, 2022, the Company entered into a Protein Engineering Collaboration (the “New Collaboration”) with Protein Evolution, Inc. (“PEI”) to develop technology and methods in the field of nanobodies and potentially other binders to produce novel biological reagents and related data. Dr. Rothberg serves as a member of the board of directors of PEI and the Rothberg family are controlling stockholders of PEI. As of December 31, 2024, there was no amount due from PEI to the Company related to the New Collaboration. As of December 31, 2023, the amount due to the Company was $0.3 million. The New Collaboration was terminated effective May 1, 2024.
Effective November 1, 2022, the Company entered into an Advisory Agreement with Dr. Rothberg (the “Advisory Agreement”), pursuant to which Dr. Rothberg previously served as Chairman of the Board, advises the Chief Executive Officer and the Board on strategic matters, and provides consulting, business development and similar services on matters relating to the Company’s current, future and potential scientific and strategic initiatives and such other consulting services reasonably requested from time to time. Pursuant to the Advisory Agreement, as compensation for the services provided, in March 2023, the Company granted Dr. Rothberg an option to purchase 250,000 shares of Class A common stock pursuant to the 2021 Plan. In connection with the Advisory Agreement, Dr. Rothberg’s title was changed from Executive Chairman to Chairman of the Board. Subsequently, in May 2024, Dr. Rothberg’s title was changed from Chairman of the Board to Director.
Note 18. Commitments and Contingencies
Commitments
Licenses related to certain intellectual property:
The Company licenses certain intellectual property, some of which may be utilized in its current or future product offerings. To preserve the right to use such intellectual property, the Company is required to make annual minimum fixed payments totaling approximately $0.2 million as well as royalties based on net sales if the royalties exceed annual minimum fixed payments. As of December 31, 2024 and 2023, the Company recorded $0.2 million and $0.1 million related to royalties in Accrued expenses and other current liabilities in the Consolidated Balance Sheets.
Other commitments:
The Company sponsors a 401(k) defined contribution plan covering all eligible U.S. employees. Contributions to the 401(k) plan are discretionary. The Company did not make any matching contributions to the 401(k) plan for the years ended December 31, 2024 and 2023.
Contingencies
The Company is subject to claims in the ordinary course of business. Except as discussed below, the Company is not currently a party to any pending or threatened litigation, the outcome of which would be expected to have a material adverse effect on its financial condition, results of operations, or cash flows. The Company accrues contingent liabilities to the extent the liability is probable and estimable.
On May 16, 2024, a punitive class action lawsuit was filed in the Delaware Court of Chancery, styled Farzad v. HighCape Capital, et al. (the “Delaware Stockholder Litigation”). The Delaware Stockholder Litigation asserts breach of fiduciary duty claims against the former officers and directors of HighCape, including Kevin Rakin, Matt Zuga, David Colpman, Robert Taub and Antony Loebel, HighCape Capital Acquisition LLC and HighCape Capital L.P., aiding and abetting breach of fiduciary duty claims against Foresite Capital Management, LLC and Dr. Rothberg, and unjust enrichment claims against all defendants related to the Business Combination. The Delaware Stockholder Litigation complaint alleges that the transactions contemplated by the Business Combination were a product of an unfair process which was allegedly impacted by conflicts of interest, resulting in mispricing of the Business Combination. The complaint seeks, among other things, unspecified damages and attorneys’ fees and costs. On July 29, 2024, the defendants filed motions to dismiss the Delaware Stockholder Litigation complaint. On October 8, 2024, the plaintiff filed a motion to compel discovery from the HighCape defendants. Then, on October 25, 2024, the plaintiff and the HighCape defendants met and conferred in an attempt to resolve the motion to compel. The plaintiff’s counsel also indicated they would be filing an amended complaint in response to the motions to dismiss. Quantum-Si, as part of the Business Combination, had previously agreed to identify certain of the defendants related to actions such as the Delaware Stockholder Litigation. There is no assurance that defendants will be successful in the defense of the litigation or that insurance will be available or adequate to fund any potential settlement or judgment or the litigation costs of the action. Further, since the Quantum-Si has indemnified certain of the defendants, there is no guarantee that insurance available to Quantum-Si will be available or adequate to fund any potential settlement or judgment or related costs associated with the indemnified parties. At the time of this filing, the outcome of this matter is not estimable or probable.
In April 2023, the Company informed the contract manufacturer that had manufactured the Platinum and another development product, Carbon™, that it intended to wind down the relationship and transition to a different contract manufacturer. In October 2023, the former contract manufacturer filed a complaint against the Company in the State of Texas alleging breach of contract and made claims for economic damage and attorney costs. In January 2024, the suit was withdrawn and refiled in the State of Minnesota alleging similar claims. The Company denied all liability and countersued alleging claims of negligence. In February 2025, the court ordered mandatory non-binding settlement conference to take place on June 11, 2025. Although it is not possible to determine the potential financial exposure associated with the alleged claim at this time given its early stage, the Company believes it has a meritorious defense and intends to vigorously defend against all claims asserted in the complaint. At the time of this filing, the outcome of this matter is not estimable or probable.
In December 2021, the Company signed a 10-year lease for approximately 67,000 square feet of space in New Haven, Connecticut. The lease commenced on January 8, 2022 with rent payments beginning on July 7, 2022. Under the lease, the landlord agreed to reimburse the Company for up to $9.1 million in improvements to the space, to be used for such improvements as the Company deems “necessary or desirable.” On September 13, 2022, the Company filed a lawsuit against the landlord, alleging that the landlord has: (i) refused to reimburse the Company for costs related to improvements already incurred and submitted; (ii) delayed the Company’s completion of improvements, in order to avoid reimbursing the costs of those improvements; and (iii) improperly rejected the Company’s proposed improvement plans. On January 10, 2025, the landlord filed a Motion for Summary Judgment, and the Company submitted a brief in opposition on February 14, 2025. Oral argument on the motion for summary judgment will be held in March 2025, and the trial is expected to commence in September 2025. The Company accounted for these lease incentives as an offset to the lease liability recorded at the inception of the lease. In September 2024, the Company determined there was a change in the estimated timing of receipt of reimbursements for the improvements. This resulted in an increase of the carrying value of the right-of-use asset and the corresponding lease liabilities of $1.0 million. Although the Company believes it is contractually entitled to the $9.1 million of lease incentives, based on the current status of the litigation, the Company cannot determine the likely outcome or estimate the impact on such carrying values.
The Company enters into agreements that contain indemnification provisions with other parties in the ordinary course of business, including business partners, investors, contractors, and the Company’s officers, directors and certain employees. The Company has agreed to indemnify and defend the indemnified party claims and related losses suffered or incurred by the indemnified party from actual or threatened third-party claims because of the Company’s activities or non-compliance
with certain representations and warranties made by the Company. It is not possible to determine the maximum potential loss under these indemnification provisions due to the Company’s limited history of prior indemnification claims and the unique facts and circumstances involved in any particular case. To date, losses recorded in the Consolidated Statements of Operations and Comprehensive Loss in connection with the indemnification provisions have not been material.
Note 19. Subsequent Event
On January 3, 2025, the Company entered into a securities purchase agreement with certain institutional investors pursuant to which the Company agreed to issue and sell, in a registered direct offering (the “Registered Direct Offering”) an aggregate of 15,625,000 shares of the Company’s Class A common stock at a price of $3.20 per Share. The gross proceeds to the Company from the Registered Direct Offering were $50.0 million. After deducting estimated placement agents’ fees and other offering expenses payable by the Company, net proceeds were approximately $46.9 million.
In connection with the Registered Direct Offering, the Company entered into a placement agency agreement with A.G.P./Alliance Global Partners (the “Placement Agent”), pursuant to which the Placement Agent agreed to serve as the sole placement agent for the Company, on a reasonable best efforts basis. The Company agreed to pay the Placement Agent an aggregate cash fee equal to 6.0% of the gross proceeds received in the Registered Direct Offering.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
As previously disclosed on the Company’s Current Report on Form 8-K filed on June 7, 2024, on June 4, 2024, the Audit Committee of the Company’s Board of Directors dismissed Deloitte & Touche LLP (“Deloitte”) and appointed PricewaterhouseCoopers LLP (“PwC”) as the Company’s independent registered public accounting firm for the fiscal year ended December 31, 2024.
During the fiscal years ended December 31, 2023 and 2022 and the subsequent interim period through June 4, 2024, there were no disagreements (as defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions to Item 304 of Regulation S-K) with Deloitte on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Deloitte, would have caused Deloitte to make reference to the subject matter of the disagreements in its audit reports on the Company’s consolidated financial statements for such years.
During the fiscal years ended December 31, 2023 and 2022 and in the subsequent interim period through June 4, 2024, there were no reportable events (as defined in Item 304(a)(1)(v) of Regulation S-K), except as follows:
•The following material weaknesses in our internal control over financial reporting were disclosed in our 2022 filings with the Securities and Exchange Commission (1) related to inaccurate accounting for the Public Warrants and Private Warrants issued in connection with HighCape’s initial public offering and (2) Legacy Quantum-Si outsourced its accounting and financial reporting to a third-party service provider and did not have its own finance function or finance or accounting professionals that had the requisite experience or were in a position to appropriately perform the supervision and review of the information received from that third-party service provider.
These reportable events were discussed among the Audit Committee and Deloitte. Deloitte has been authorized by the Company to respond fully to the inquiries of PwC, the successor independent registered public accounting firm, concerning these reportable events.
During the fiscal years ended December 31, 2023 and 2022 and the subsequent interim period through June 4, 2024, neither the Company nor anyone on its behalf has consulted with PwC with respect to either (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s consolidated financial statements, and neither a written report nor oral advice was provided to the Company that PwC concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a disagreement (as defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions to Item 304 of Regulation S-K) or a reportable event (as defined in Item 304(a)(1)(v) of Regulation S-K).
For more information, please refer to the Company’s Current Report on Form 8-K filed on June 7, 2024.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act.
Disclosure controls and procedures are controls and other procedures designed to ensure information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include controls and procedures designed to ensure information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure. Based on the evaluation of our disclosure controls and procedures, our Chief Executive Officer and Chief Financial Officer concluded our disclosure controls and procedures were effective as of December 31, 2024.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2024 based on the guidelines established in the Internal Control—Integrated Framework (2013 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Internal control over financial reporting includes policies and procedures that provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles.
Based on the results of its evaluation, management concluded our internal control over financial reporting was effective as of December 31, 2024.
Internal control over financial reporting has inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements will not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
This Annual Report does not include an attestation report of the Company’s independent registered public accounting firm because we are a “non-accelerated filer,” and may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are accelerated filers, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2024 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
10b5-1 Trading Arrangements
From time to time, our officers (as defined in Rule 16a-1(f) of the Exchange Act) and directors may enter into Rule 10b5-1 or non-Rule 10b5-1 trading arrangements (as each such term is defined in Item 408 of Regulation S-K). During the three months ended December 31, 2024, none of our officers or directors adopted, modified or terminated any such trading arrangements.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The response to this item is incorporated by reference from the discussion responsive thereto under the headings “Management and Corporate Governance” and “Code of Conduct and Ethics” in our proxy statement for the 2025 annual meeting of stockholders (the “2025 Proxy Statement”).
ITEM 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto under the heading “Executive Compensation” in our 2025 Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item is incorporated by reference from the discussion responsive thereto under the headings “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our 2025 Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The response to this item is incorporated by reference from the discussion responsive thereto under the headings “Certain Relationships and Related Person Transactions” and “Management and Corporate Governance” in our 2025 Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The response to this item is incorporated by reference from the discussion responsive thereto under the heading “Ratification of Appointment of Independent Registered Public Accounting Firm” in our 2025 Proxy Statement.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)The following documents are filed as part of this Annual Report on Form 10-K:
(i)Financial Statements (included in Item 8 of this Annual Report on Form 10-K):
The following Consolidated Financial Statements of the Company and the Reports of PricewaterhouseCoopers LLP and Deloitte & Touche LLP, Independent Registered Public Accounting Firms, are included in Part II, Item 8 of this Annual Report on Form 10-K:
1.Reports of Independent Registered Public Accounting Firms - Pricewaterhouse Coopers LLC and Deloitte & Touche LLP
2.Consolidated Balance Sheets as of December 31, 2024 and 2023
3.Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2024, 2023 and 2022
4.Consolidated Statements of Changes in Stockholders’ Equity (Deficit) for the years ended December 31, 2024, 2023 and 2022
5.Consolidated Statements of Cash Flows for the years ended December 31, 2024, 2023 and 2022
6.Notes to the Consolidated Financial Statements
(ii)Financial Statement Schedules.
All schedules are omitted because they are not applicable or the required information is shown in the Consolidated Financial Statements or the Notes thereto.
(iii)Exhibits.
The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
(b)Exhibits.
The following exhibits are filed herewith or are incorporated by reference to exhibits filed with the SEC:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Exhibit Description | | Filed
Herewith | | Incorporated
by
Reference
Herein from
Form or
Schedule | | Filing
Date |
| | Business Combination Agreement, dated as of February 18, 2021, by and among Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.), Clay Merger Sub, Inc., and Q-SI Operations Inc. (formerly Quantum-Si Incorporated) | | | | Form 8-K
(Exhibit 2.1) | | 2/18/2021 |
| | Second Amended and Restated Certificate of Incorporation of Quantum-Si Incorporated, as amended. | | | | Form 10-Q
(Exhibit 3.1) | | 8/7/2024 |
| | Certificate of Amendment to the Second Amended and Restated Certificate of Incorporation of the Registrant, as filed with the Secretary of State of the State of Delaware on May 12, 2023. | | | | Form 8-K
(Exhibit 3.1) | | 5/16/2023 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Amended and Restated Bylaws of Quantum-Si Incorporated | | | | Form 10-K
(Exhibit 3.2) | | 3/1/2021 |
| | Description of Securities | | | | Form 10-K
(Exhibit 4.1) | | 2/29/2024 |
| | Specimen Class A Common Stock Certificate | | | | Form S-4/A
(Exhibit 4.1) | | 5/11/2021 |
| | Warrant Agreement, dated as of September 3, 2020, by and between Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.) and Continental Stock Transfer & Trust Company | | | | Form 8-K
(Exhibit 4.1) | | 9/9/2020 |
| | Form of PIPE Investor Subscription Agreement for institutional investors, dated as of February 18, 2021, by and between Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.) and the subscriber parties thereto | | | | Form 8-K
(Exhibit 10.1) | | 2/18/2021 |
| | Form of PIPE Investor Subscription Agreement for accredited investors, dated as of February 18, 2021, by and between Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.) and the subscriber parties thereto | | | | Form 8-K/A
(Exhibit 10.2) | | 2/19/2021 |
| | Form of Subscription Agreement, dated as of February 18, 2021, by and between Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.) and the Foresite Funds | | | | Form 8-K/A
(Exhibit 10.3) | | 2/19/2021 |
| | Transaction Support Agreement, dated as of February 19, 2021, by and among Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.), and certain supporting stockholders of Q-SI Operations Inc. (formerly Quantum-Si Incorporated) | | | | Form 8-K
(Exhibit 10.1) | | 2/22/2021 |
| | Sponsor Letter Agreement, dated as of February 18, 2021, by and among HighCape Capital Acquisition LLC, Deerfield Partners, L.P., Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.) and Q-SI Operations Inc. (formerly Quantum-Si Incorporated) | | | | Form 8-K
(Exhibit 10.4) | | 2/18/2021 |
| | Advisory Agreement, dated as of November 1, 2022, by and between Quantum-Si Incorporated and Jonathan M. Rothberg, Ph.D. | | | | Form 10-K
(Exhibit 10.6+) | | 3/17/2023 |
| | Offer Letter of Employment, dated as of October 2, 2022, by and between Quantum-Si Incorporated and Jeffrey Hawkins | | | | Form 8-K
(Exhibit 10.1) | | 10/4/2022 |
| | Offer Letter of Employment, dated as of April 27, 2023, by and between Quantum-Si Incorporated and Jeffry Keyes | | | | Form 8-K
(Exhibit 10.1) | | 5/2/2023 |
| | Offer Letter of Employment, dated as of November 4, 2020, by and between Q-SI Operations Inc. (formerly Quantum-Si Incorporated) and Christian LaPointe, Ph.D., as supplemented by the Letter Agreement, dated as of February 16, 2021, by and between Q-SI Operations Inc. and Christian LaPointe, Ph.D. | | | | Form 10-K
(Exhibit 10.12+) | | 3/1/2022 |
| | Technology and Services Exchange Agreement, dated as of February 17, 2021, by and among Q-SI Operations Inc. (formerly Quantum-Si Incorporated) and the participants named therein | | | | Form 10-Q
(Exhibit 10.1) | | 11/15/2021 |
| | Protein Engineering Collaboration Agreement, dated as of March 13, 2023, by and between Quantum-Si Incorporated and Protein Evolution, Inc. | | | | Form 10-K
(Exhibit 10.14) | | 3/17/2023 |
| | Quantum-Si Incorporated 2021 Equity Incentive Plan | | | | Form 8-K
(Exhibit 10.13.1) | | 6/15/2021 |
| | Form of Stock Option Agreement under 2021 Equity Incentive Plan | | | | Form 8-K
(Exhibit 10.13.2) | | 6/15/2021 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Form of Restricted Stock Unit Agreement under 2021 Equity Incentive Plan | | | | Form 10-K
(Exhibit 10.13.3+) | | 2/29/2024 |
| | Q-SI Operations Inc. 2013 Employee, Director and Consultant Equity Incentive Plan, as amended | | | | Form 8-K
(Exhibit 10.14.1) | | 6/15/2021 |
| | Form of Stock Option Agreement under 2013 Employee, Director and Consultant Equity Incentive Plan, as amended | | | | Form 8-K
(Exhibit 10.14.2) | | 6/15/2021 |
| | Form of Restricted Stock Unit Agreement under 2013 Employee, Director and Consultant Equity Incentive Plan, as amended | | | | Form 8-K
(Exhibit 10.14.3) | | 6/15/2021 |
| | Form of Performance-Based Non-Qualified Stock Option Agreement | | | | Form S-8
(Exhibit 99.1) | | 11/10/2022 |
| | Nonemployee Director Compensation Policy | | | | Form 10-K
(Exhibit 10.16+) | | 2/29/2024 |
| | Form of Indemnification Agreement | | | | Form 8-K
(Exhibit 10.16) | | 6/15/2021 |
| | Amended and Restated Registration Rights Agreement, dated as of June 10, 2021, by and among Quantum-Si Incorporated (formerly HighCape Capital Acquisition Corp.) and certain of its securityholders | | | | Form 8-K
(Exhibit 10.17) | | 6/15/2021 |
| | Lease Agreement between Quantum-Si Incorporated and BP3-SD5 5510 Morehouse Drive LLC, dated June 18, 2021 | | | | Form 8-K
(Exhibit 10.1) | | 6/24/2021 |
| | Lease Agreement between Quantum-Si Incorporated and Winchester Office LLC, dated December 28, 2021 | | | | Form 8-K
(Exhibit 10.1) | | 1/24/2022 |
| | Quantum-Si Incorporated Executive Severance Plan, effective November 1, 2024 | | X | | | | |
| | 2023 Inducement Equity Incentive Plan, as amended | | | | Form 10-Q
(Exhibit 10.3+) | | 11/12/2024 |
| | Form of Restricted Stock Agreement under the 2023 Inducement Equity Incentive Plan | | | | Form 10-Q
(Exhibit 10.3.1+) | | 11/12/2024 |
| | Form of Stock Option Agreement under the 2023 Inducement Equity Incentive Plan | | | | Form S-8
(Exhibit 99.2) | | 7/20/2023 |
| | Letter of Employment, dated as of August 12, 2024, by and between Quantum-Si Incorporated and John Vieceli | | | | Form 10-Q
(Exhibit 10.1+) | | 11/12/2024 |
| | Offer Letter of Employment, dated as of August 22, 2024, by and between Quantum-Si Incorporated and Todd Bennett | | | | Form 10-Q
(Exhibit 10.2+) | | 11/12/2024 |
| | Sales Agreement, dated December 11, 2024, by and between Quantum-Si Incorporated and Canaccord Genuity LLC | | | | Form 8-K
(Exhibit 10.1) | | 12/11/2024 |
| | Form of Securities Purchase Agreement, by and among the Company and the Purchasers | | | | Form 8-K
(Exhibit 10.1) | | 1/6/2025 |
| | Placement Agency Agreement between the Company and the Placement Agent | | | | Form 8-K
(Exhibit 10.2) | | 1/6/2025 |
| | Letter from Deloitte & Touche LLP, dated June 7, 2024. | | | | Form 10-K
(Exhibit 16.1) | | 6/7/2024 |
| | Insider Trading Policy, effective February 2025 | | X | | | | |
| | List of Subsidiaries | | | | Form 10-K
(Exhibit 21.1) | | 3/17/2023 |
| | Consent of PricewaterhouseCoopers LLP | | X | | | | |
| | Consent of Deloitte & Touche LLP | | X | | | | |
| | Power of Attorney (included in the signature page) | | X | | | | |
| | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | X | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Certification of the Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | X | | | | |
| | Certifications of the Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | X | | | | |
| | Clawback Policy, effective as of August 3, 2023 | | | | Form 10-K
(Exhibit 97) | | 2/29/2024 |
| | | | | | | | |
| 101.INS | | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document) | | X | | | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document | | X | | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | X | | | | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document | | X | | | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document | | X | | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | X | | | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) | | X | | | | |
†Certain of the exhibits and schedules to this Exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Registrant agrees to furnish a copy of all omitted exhibits and schedules to the SEC upon its request.
+Management contract or compensatory plan or arrangement.
*The certifications attached as Exhibit 32 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Quantum-Si Incorporated under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of such Form 10-K), irrespective of any general incorporation language contained in such filing.
ITEM 16. FORM 10-K SUMMARY
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| QUANTUM-SI INCORPORATED |
| March 3, 2025 | | |
| By: | /s/ Jeffrey Hawkins |
| | Jeffrey Hawkins |
| | President and Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Jeffrey Hawkins and Jeffry Keyes his or her true and lawful attorney-in-fact and agent, with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his or her substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
IN WITNESS WHEREOF, each of the undersigned has executed this Power of Attorney as of the date indicated opposite his or her name.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
| | | | | | | | |
| Name | Title | Date |
| | |
| /s/ Jeffrey Hawkins | President, Chief Executive Officer and Director | March 3, 2025 |
| Jeffrey Hawkins | (Principal Executive Officer) | |
| | |
| /s/ Jeffry Keyes | Chief Financial Officer and Treasurer | March 3, 2025 |
| Jeffry Keyes | (Principal Financial and Accounting Officer) | |
| | |
| /s/ Charles Kummeth | Chairman of the Board | March 3, 2025 |
| Charles Kummeth | | |
| | |
| /s/ Paula Dowdy | Director | March 3, 2025 |
| Paula Dowdy | | |
| | |
| /s/ Ruth Fattori | Director | March 3, 2025 |
| Ruth Fattori | | |
| | |
| /s/ Amir Jafri | Director | March 3, 2025 |
| Amir Jafri | | |
| | |
| /s/ John Patrick Kenny | Director | March 3, 2025 |
| John Patrick Kenny | | |
| | |
| /s/ Brigid A. Makes | Director | March 3, 2025 |
| Brigid A. Makes | | |
| | |
| /s/ Scott Mendel | Director | March 3, 2025 |
| Scott Mendel | | |
| | |
| /s/ Kevin Rakin | Director | March 3, 2025 |
| Kevin Rakin | | |
| | |
| /s/ Jonathan M. Rothberg, Ph.D. | Director | March 3, 2025 |
| Jonathan M. Rothberg, Ph.D. | | |